- 1Ningxia Regional Key Laboratory of Integrated Traditional Chinese and Western Medicine for Prevention and Treatment of Regional High Incidence Disease, Ningxia Medical University, Yinchuan, China
- 2Key Laboratory of Ningxia Ethnomedicine Modernization, Ministry of Education, Ningxia Medical University, Yinchuan, China
- 3Department of Nephrology, Affiliated Hospital of Traditional Chinese Medicine, Ningxia Medical University, Yinchuan, China
Background: Non-alcoholic fatty liver disease (NAFLD) has become a public health issue worldwide. Dietary polyphenols are naturally occurring plant active ingredients and are widely employed in the treatment of NAFLD. However, the therapeutic effect is still controversial. In this study, a network meta-analysis (NMA) was performed to appraise the effects of various polyphenols on metabolic indices of NAFLD.
Methods: PubMed, Embase, the Cochrane Library, and Web of Science were retrieved for English studies on dietary polyphenols in the treatment of NAFLD. Outcome measures were extracted from the included studies and compared using a Bayesian NMA model, encompassing body mass index (BMI), alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and necrosis factor alpha (TNF-α).
Results: In total, 54 randomized controlled trials (RCTs) were included in this study, including 3,132 participants. It involved 13 single (or combined) dietary polyphenols. Naringenin could reduce serum TC (surface under the cumulative ranking curve: 94.59%) and TG (99.00%) in NAFLD patients. Catechin could decrease BMI (77.74%) and serum ALT (94.21%), AST (93.56%), TC (92.26%), and increase HDL-C (93.72%). Dihydromyricetin (DHM) was effective in reducing serum LDL-C (73.22%), and quercetin decreased serum TNF-α (99.47%).
Conclusion: Catechin may be the most appropriate dietary polyphenol supplement for NAFLD. Future studies should incorporate more RCTs to further validate the efficacy of dietary polyphenols (like DHM and quercetin), which are limited in sample sizes, in treating NAFLD. On the other hand, it is essential to investigate improvements in the bioavailability of these dietary polyphenols and to clarify their safety profiles.
1 Introduction
Non-alcoholic fatty liver disease (NAFLD) is the condition of excessive liver fat accumulation, excluding secondary causes and excessive alcohol intake, including two pathologies with various prognoses: non-alcoholic fatty liver and non-alcoholic steatohepatitis (NASH) (1). NAFLD is generally believed to be associated with an unhealthy lifestyle, including a high-fat, high-sugar diet and sedentary behavior (1). Its pathogenesis is based on the “multiple hit” hypothesis, i.e., it is the result of multiple injuries (such as oxidative stress, lipotoxicity, and inflammatory state) (2). NAFLD is the most widespread cause of chronic liver disease across the globe. Between 1990 and 2019, the global prevalence of NAFLD was 30.1%, with 16.02% for NASH (3). Over the last 30 years, NAFLD prevalence has grown by 50.4% (3). During the 1999–2019 survey period in Asia, Mainland China had the highest NAFLD incidence rate, at 63 cases per 1,000 person-years (4). NAFLD patients are 1.9 times more likely to develop cancer than the general population (5). Extrahepatic cancers like uterine and breast cancer have an incidence rate more than eight times higher than that of hepatocellular carcinoma (HCC) (6). In the U.S., NASH is the foremost cause of end-stage liver disease and the second most frequent indication for liver transplantation (7). Since the onset of NAFLD is closely linked to cardiometabolic risk factors, such as type 2 diabetes mellitus (T2DM) and obesity, it has gained widespread attention (8). NAFLD is now a global public health issue and burden, and this burden is escalating quickly (3, 9).
An encouraging development is that in the first half of 2024, the U.S. Food and Drug Administration (FDA) granted approval for resmetirom (Rezdiffra™) as a treatment for adults with non-cirrhotic NASH and liver fibrosis (10), offering new hope for NAFLD treatment. The FDA states that this medication should be used together with diet and exercise, meaning that lifestyle changes remain the key approach in treating NAFLD. Combining the Mediterranean diet (MD), caloric restriction, and moderate- to high-intensity aerobic exercise/resistance training has proven effective in improving metabolic measures of NAFLD (11). However, the absence of long-term studies on their impact on the natural progression of NAFLD has prompted the exploration of alternative treatment approaches. Natural products are chemical substances with pharmacological or biological properties naturally produced by living organisms like plants, insects, animals, aquatic organisms and microorganisms (12), which are currently a hot research area for the treatment of diseases. Polyphenols are a class of plant-derived compounds, polyphenolic substances or isolated polyphenol monomers extracted from dietary substances or traditional medicines, which belong to the natural product paradigm. Dietary polyphenols, a class of plant-derived compounds found in fruits, tea, and vegetables, exhibit anti-inflammatory and antioxidant properties that can aid in improving the metabolic disturbances in NAFLD (13). A meta-analysis has examined the therapeutic effects of eight common dietary polyphenols, including curcumin, on NAFLD (14), providing guidance for the clinical selection of dietary polyphenols in NAFLD treatment. However, due to the wide variety of dietary polyphenols, most randomized controlled trials (RCTs) have primarily focused on comparing the efficacy differences between various polyphenols and placebos, without direct comparisons among polyphenols. This gap in evidence can be bridged through network meta-analysis (NMA).
The NMA is able to simultaneously compare multiple interventions not directly compared in RCTs and rank the effectiveness of the interventions (15). Hence, this study intends to compare the efficacy of various single (or combined) dietary polyphenol supplements in the treatment of NAFLD, like curcumin, resveratrol, anthocyanin, silymarin, catechin, chlorogenic acid (CA), ellagic acid (EA), genistin/genistein, naringenin, dihydromyricetin (DHM), hesperidin, quercetin, and gallic acid (GA) and CA, providing evidence-based guidance for both clinicians and patients in selecting appropriate dietary polyphenol therapies for NAFLD.
2 Methods
2.1 Registration
This study was in line with the preferred reporting items for systematic reviews and meta-analyses extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions (16) and was registered in the international prospective register of systematic reviews1 with the registration number CRD42024591985.
2.2 Literature search strategy
Studies were retrieved from four electronic databases (PubMed, Embase, the Cochrane Library, and Web of Science) up to May 14, 2024. The keywords for the search were “polyphenol,” “phenolic acid,” “silymarin,” “anthocyanidin,” “hesperidin,” “resveratrol,” “nonalcoholic fatty liver,” “non-alcoholic fatty liver disease,” and “NAFLD.” The search formula employed was [(polyphenol) OR (phenolic acid) OR (silymarin) OR (anthocyanidin) OR (hesperidin) OR (resveratrol)] AND [(nonalcoholic fatty liver) OR (non-alcoholic fatty liver disease) OR (NAFLD)]. The detailed search strategy is available in Supplementary material 1.
2.3 Inclusion criteria
Population (P): (i) Sex was not limited; (ii) age ≥18 years; (iii) diagnosis of NAFLD confirmed by imaging or liver tissue biopsy.
Intervention (I): The intervention was to employ at least one dietary polyphenol available in the Phenol-Explorer 3.6 database (database on polyphenol content in foods).2
Comparison (C): The control intervention was placebo or any of the dietary polyphenols available in the Phenol-Explorer 3.6 database.
Outcomes (O): At least one of the following outcomes must be included: anthropometric measures, including weight, body mass index (BMI), waist circumference (WC), hip circumference (HC), waist-to-hip ratio (WHR), systolic blood pressure (SBP), and diastolic blood pressure (DBP); liver function measures, such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and gamma-glutamyl transferase (GGT); blood lipid metabolism measures, like triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C); insulin resistance (IR) measures, including fasting blood glucose (FBG), insulin, and homeostasis model assessment of IR; inflammation markers, like necrosis factor alpha (TNF-α).
Study design (S): RCTs.
The language was limited to English.
2.4 Exclusion criteria
(i) Studies with duplicate publications, repeated populations, and unavailable outcome measures.
(ii) Studies focusing on NAFLD with diabetes mellitus (DM) and other types of chronic liver diseases, such as viral hepatitis.
(iii) Studies where the intervention involved a particular food but did not clearly define the dietary polyphenol active ingredients, or where the list of these ingredients was too extensive for mechanism analysis; or studies that included dietary polyphenol supplements alongside western drugs (such metformin) or antioxidants (such as vitamin E).
(iv) Studies excluding the outcome measures covered in this study.
(v) Studies with insufficient data.
(vi) Animal or cell experiments, case reports, scientific experiment programs, reviews, letters, editorials, and conference papers.
2.5 Data extraction
Study screening and management were carried out by means of Endnote X9 software. Two authors (XiaW and LS) initially excluded studies that did not meet the inclusion criteria based on the titles and abstracts, then further searched for and read the full texts to make a final decision on inclusion. Data extraction was independently carried out by the two authors using a pre-created standardized electronic form, where the following key data were extracted and recorded: dietary polyphenols, title, first author, region, publication year, sample size, sex ratio, age, intervention, treatment duration, and outcome measures. To guarantee the accuracy of the data, all extracted data were input into Excel. Any disputed issues during the data extraction process were resolved through discussion between the two authors, with the involvement of a third author (XinW) when necessary.
2.6 Quality evaluation
Using the Cochrane risk of bias tool (ROB 2.0), the quality of each RCT included was appraised sequentially in five areas (17): (i) bias from the randomization process, (ii) bias from the intended intervention-intervention allocation, (iii) bias for missing outcome data, (iv) bias for outcome measurement, and (v) bias for selective reporting of results. The relevant response options were selected for each domain namely: yes, probably yes, probably no, no, and no information. These options were distinctly categorized as low risk of bias (ROB), moderate ROB, and high ROB. In the final quality evaluation, a study was deemed to have a low risk of bias (ROB) if all five domains indicated low ROB. If no more than three domains showed some ROB, it was classified as having a moderate ROB. A study with any domain identified as high ROB was considered to have a high ROB. The work was independently done by two authors (XiaW and XinW), and any differences were settled with a third author (LS).
2.7 Statistical analysis
All variables were expressed as mean ± standard deviation, with effect sizes measured by means of weighted mean difference (WMD) or standardized mean difference. When a study employed the same dietary polyphenol intervention with varying doses, only noticeable efficacy data was included in the NMA. When a study had two dietary polyphenol interventions, they were considered separate studies and included in the NMA. If certain studies were potentially or definitively derived from the same clinical trial registry, they may be included in the NMA, provided that duplicate outcome measures were excluded.
In this study, the Markov Chain Monte Carlo method was leveraged to create a Bayesian NMA model (18), which was iterated to estimate the relative efficacy of various dietary polyphenols. During testing, the model chain was set to 4, annealing to 10,000, iterations to 50,000, with a step size of 1 and an initial value of 2.5. This procedure was designed to obtain the posterior distribution.
To choose an appropriate effect model, heterogeneity among the included studies was appraised employing the I2 statistic. If I2 ≤ 50%, indicating moderate heterogeneity across studies (19), the Bayesian fixed-effect NMA model was employed. In contrast, the Bayesian random-effects NMA model was applied (20). In this way, the overall effect size could be estimated more accurately. Since the study results did not form a closed loop, an inconsistency test was not executed.
Simultaneously, a network diagram was plotted to intuitively display the links among various polyphenol interventions for NAFLD. Finally, the surface under the cumulative ranking curve (SUCRA) was calculated (21), allowing for a ranking of the therapeutic effects of each polyphenol. Additionally, a publication bias test was executed. Funnel plots were leveraged to appraise the potential for publication bias (22), thereby ensuring the impartiality and thoroughness of the analysis results.
R (version 4.3.2) was leveraged to build the Bayesian NMA model, perform heterogeneity tests, and calculate SUCRA. Network diagrams and funnel plots were created by means of Stata (version 15.1). p < 0.05 was deemed statistically significant.
3 Results
3.1 Study selection
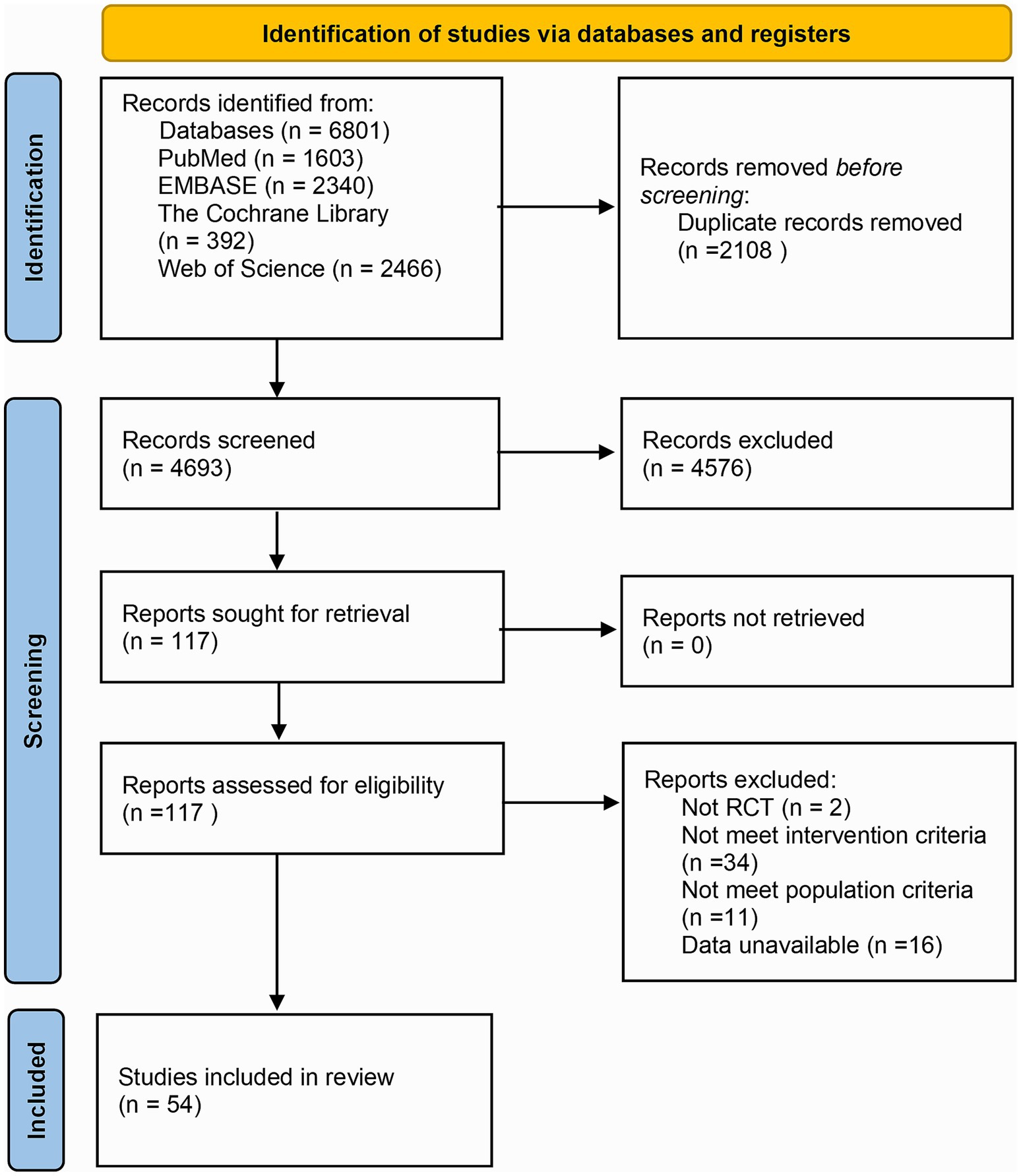
In total, 6,801 articles were retrieved from the above databases. Endnote X9 was leveraged to search for study titles with terms such as “animal” “mouse” “cell” “review” “abstract” and “network pharmacology” to exclude non-RCTs. Through examining the abstracts, other studies that did not meet the inclusion criteria were excluded, leaving 117 articles. Upon reading the entire text, further screening was executed on the PICOS framework. Among the 117 articles, 2 were non-RCTs, 34 had interventions in the treatment or control groups that did not meet the inclusion criteria, 11 involved participants with NAFLD combined with DM or pediatric NAFLD, and 16 had no available outcome measures. Ultimately, 54 articles were included in the NMA, and the detailed screening procedure is depicted in Figure 1.
3.2 Basic characteristics and quality assessment of the included studies
The basic characteristics of the studies included are available in Supplementary material 2. In total, 54 RCTs were included (23–76), involving 3,132 participants aged 18 to 70 years. Males represented approximately 56.84%, and females accounted for approximately 45.56%. Forty-four studies were conducted in Iran, 3 in China, 2 in India, and 1 each in Australia, Denmark, Japan, Malaysia, and Pakistan. The type of intervention was dietary polyphenol supplements or diets containing certain dietary polyphenol active ingredients. Curcumin was employed in 18 studies, resveratrol in 8, anthocyanin in 5, silymarin in 6, catechin in 4, CA in 4, EA in 2, genistin/genistein in 2, naringenin in 2, DHM in 1, hesperidin in 1, quercetin in 1, and GA and CA in 1.
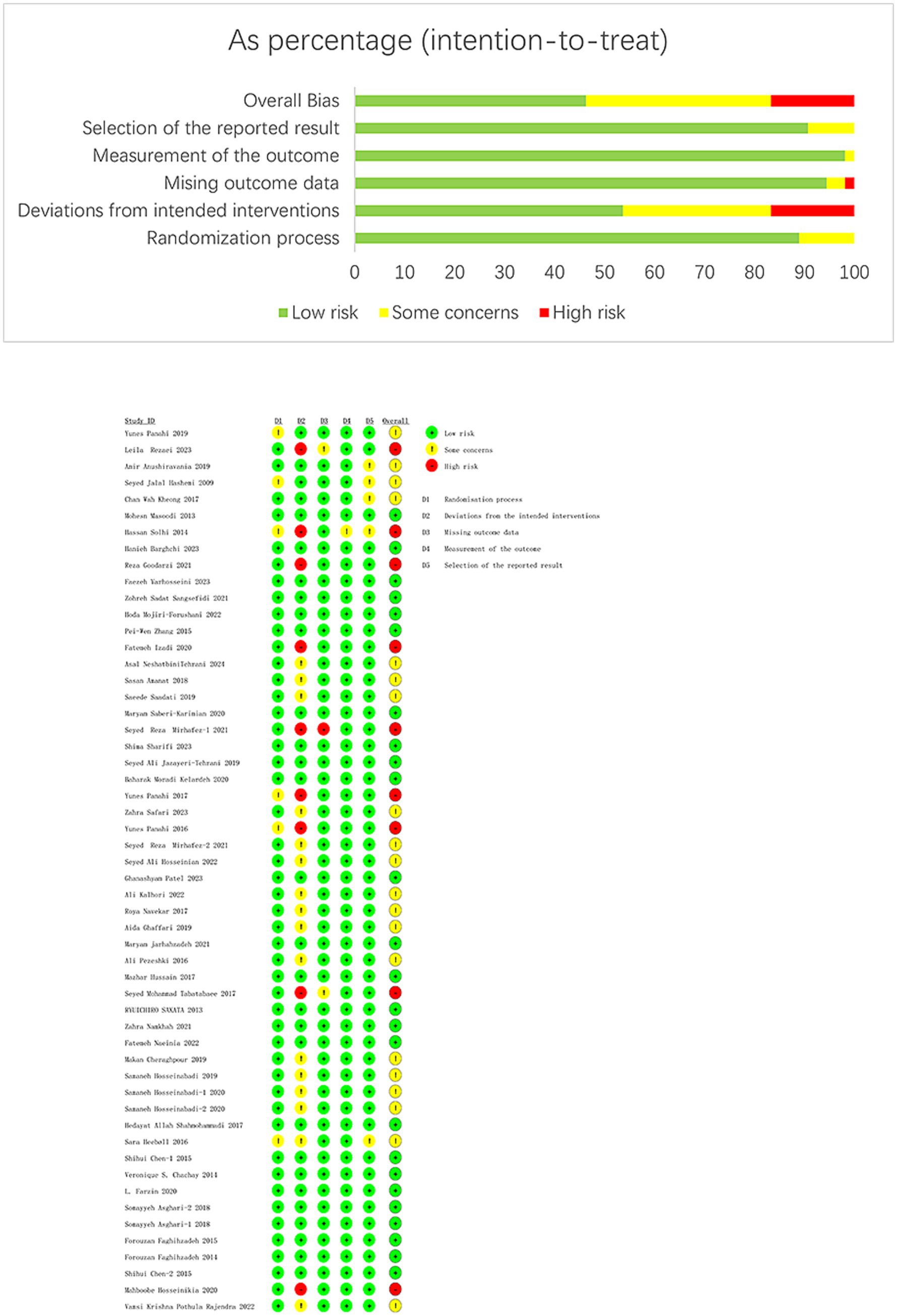
The results of the quality evaluation are available in Figure 2. Out of the studies, 9 (16.66%) were high risk, 25 (46.30%) were low risk, and 20 (37.04%) had some risk. Among the nine high-risk studies, six had a dropout rate over 10% (27, 29, 50, 62, 67, 75), and three had a dropout rate over 10% and did not employ a double-blind approach (32, 33, 56).
3.3 NMA results
3.3.1 Network diagram
Figure 3 displays the network diagrams of outcome measures (such as BMI) in the treatment of NAFLD with various dietary polyphenols. Each node in the diagram represented a polyphenol or placebo, and the connecting line between two nodes represented a direct comparison of two polyphenols or any polyphenol with a placebo. The size of each node and the width of the connecting line were proportional to the number of studies (22). The network diagrams of other outcome measures can be found in Supplementary material 3.
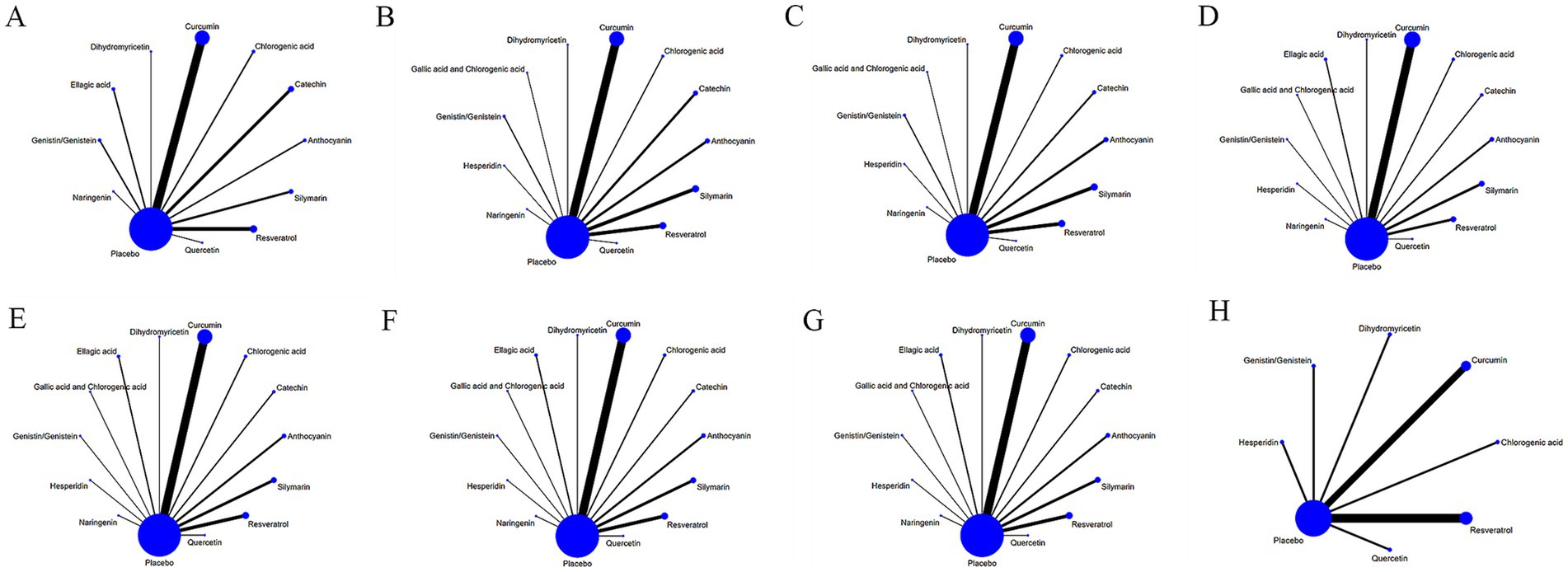
Figure 3. Network relationship diagram of different outcome indicators in NAFLD patients treated with different dietary polyphenol supplements. (A) BMI; (B) ALT; (C) AST; (D) TC; (E) TG; (F) HDL-C; (G) LDL-C; (H) TNF-α.
3.3.2 SUCRA plot
The SUCRA varied from 0 to 100%. A larger SUCRA indicated that the dietary polyphenol was ranked higher compared to other polyphenols and was more effective in treating NAFLD. Figure 4 displays the SUCRAs for outcome measures, including BMI, following the treatment of NAFLD with various dietary polyphenol supplements. The SUCRAs for other outcome measures are provided in Supplementary material 3.
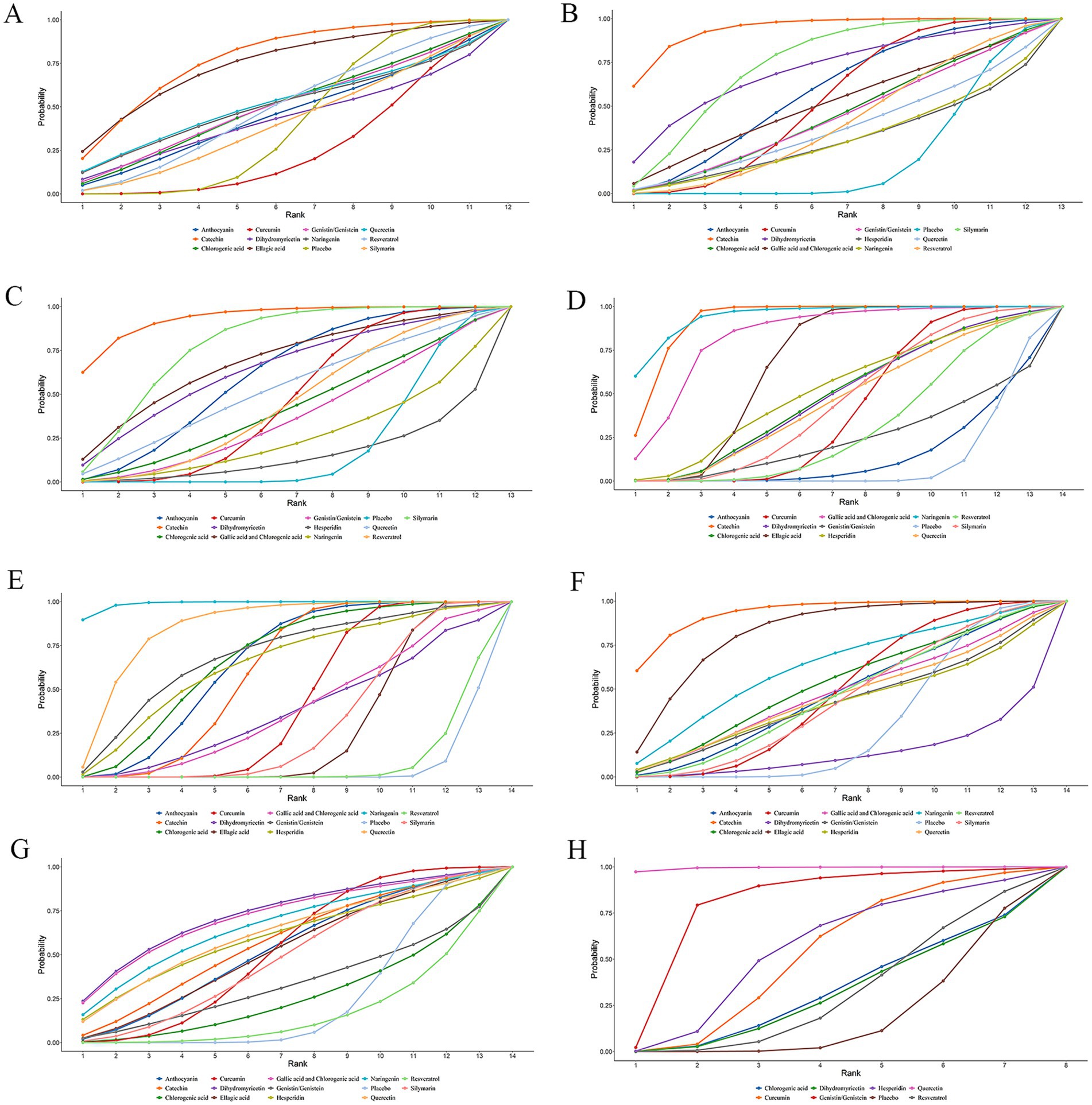
Figure 4. SUCRA diagram of changes in different outcome indicators caused by different dietary polyphenol supplements in the treatment of NAFLD. (A) BMI; (B) ALT; (C) AST; (D) TC; (E) TG; (F) HDL-C; (G) LDL-C; (H) TNF-α.
3.3.3 Anthropometric outcome measures
The anthropometric outcome measures included weight, BMI, WHR, WC, HC, SBP, and DBP, with 34 studies reporting BMI. The NMA results of weight, WC, HC, WHR, SBP, and DBP are available in Supplementary material 3.
The NMA results exhibited no marked differences in BMI changes induced by these dietary polyphenols (Table 1). According to the SUCRA probability ranking for BMI reduction, catechin (77.74%) was ranked highest, followed by EA (74.29%) and quercetin (51.58%) (Figure 4).
3.3.4 Liver function-related outcome measures
Liver function-related outcome measures included ALT, AST, ALP, and GGT. ALT was reported in 42 studies, and AST in 41 studies. The NMA results of ALP and GGT can be found in Supplementary material 3.
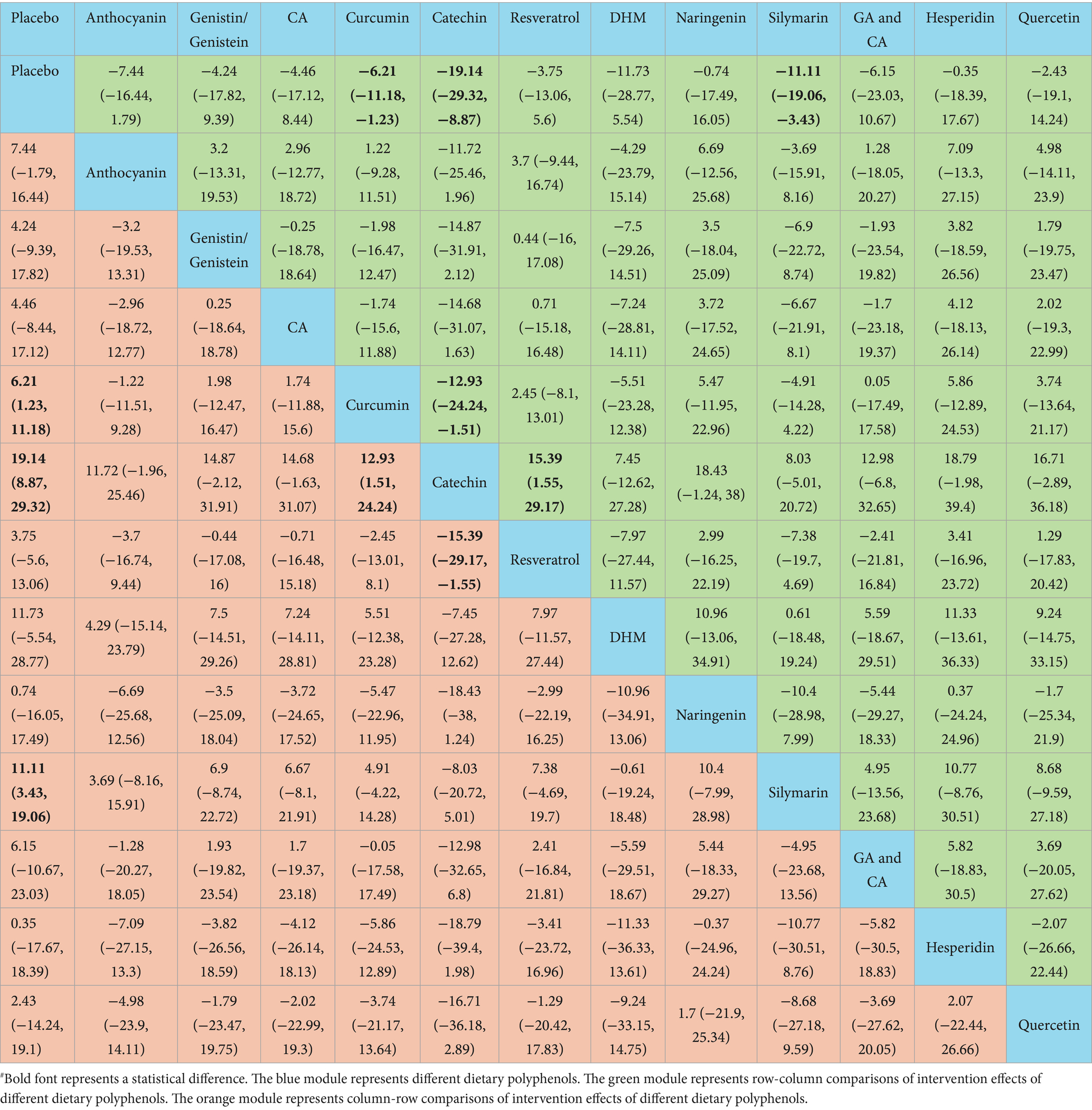
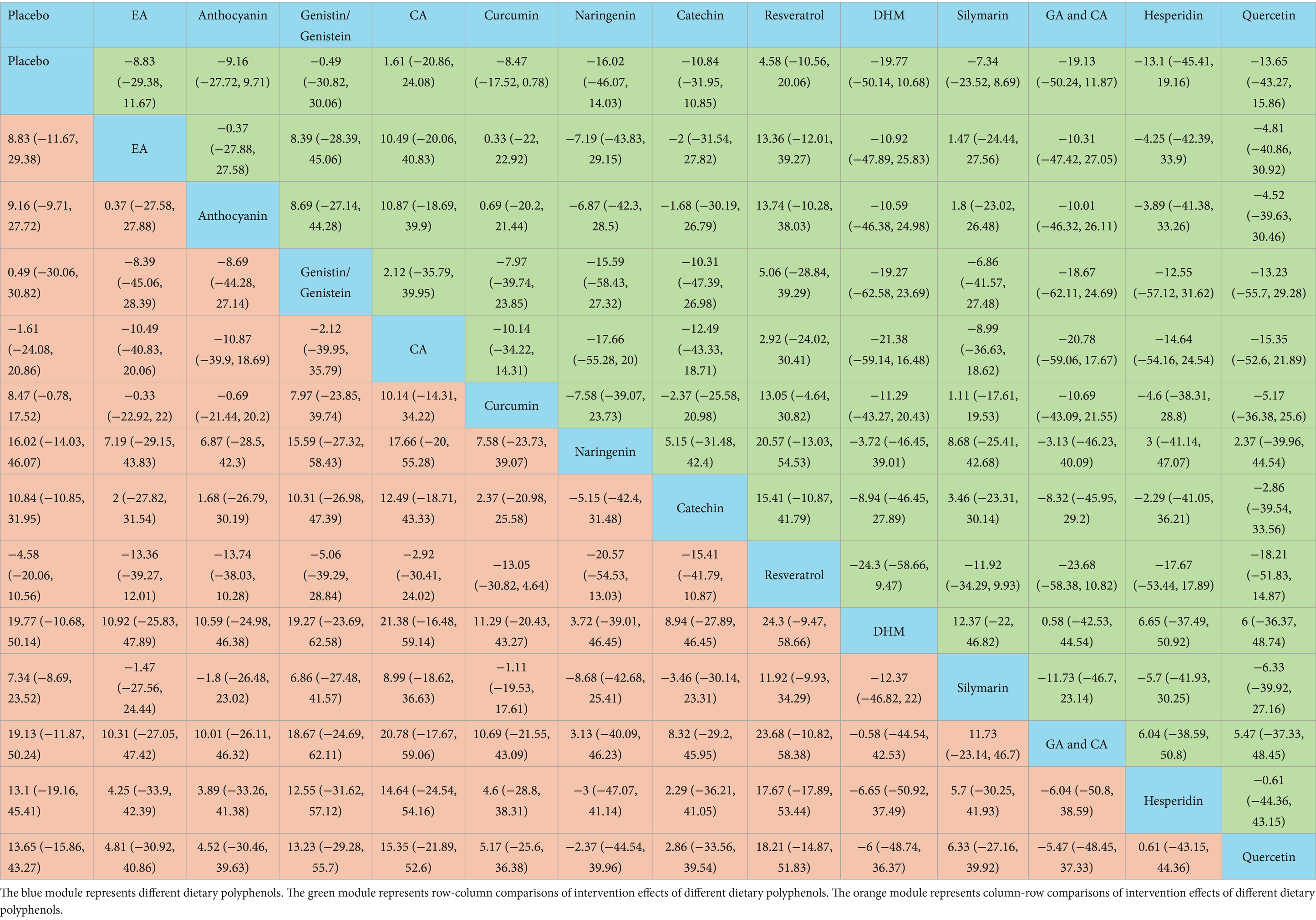
The NMA results revealed that catechin was more effective than placebo [WMD = 19.14, 95% confidence interval (CI) = (8.87, 29.32)], curcumin [WMD = 12.93, 95% CI = (1.51, 24.24)], and resveratrol [WMD = −15.39, 95% CI = (−29.17, −1.55)] in reducing ALT. Additional results with differences are provided in Table 2. The SUCRA probability ranking for reducing serum ALT revealed that catechin (94.21%) was the most effective, followed by silymarin (74.76%) and DHM (70.86%) (Figure 4).
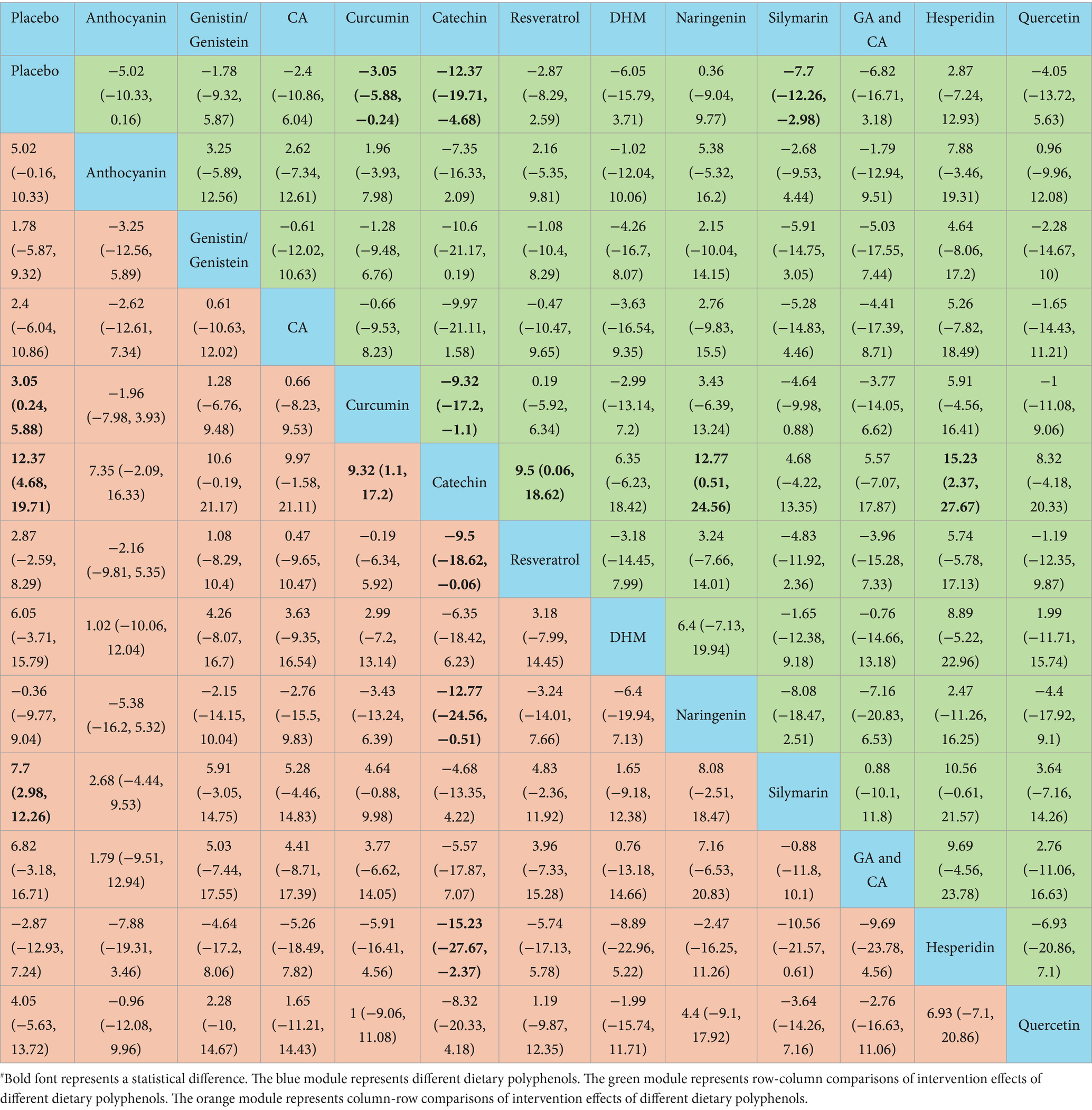
Catechin demonstrated superior efficacy in lowering AST relative to placebo [WMD = 12.37, 95% CI = (4.68, 19.71)], curcumin [WMD = 9.32, 95% CI = (1.1, 17.2)], resveratrol [WMD = −9.5, 95% CI = (−18.62, −0.06)], naringenin [WMD = −12.77, 95% CI = (−24.56, −0.51)], and hesperidin [WMD = −15.23, 95% CI = (−27.67, −2.37)]. Additional results with differences are provided in Table 3. For lowering serum AST, the SUCRA probability ranking showed catechin (93.56%) as the highest, followed by silymarin (78.37%) and GA and CA (68.49%) (Figure 4).
3.3.5 Blood lipid-related outcome measures
Blood lipid-related outcome measures involved TC, TG, HDL-C, and LDL-C. Thirty-five studies reported TC, TG, HDL-C, and LDL-C each.
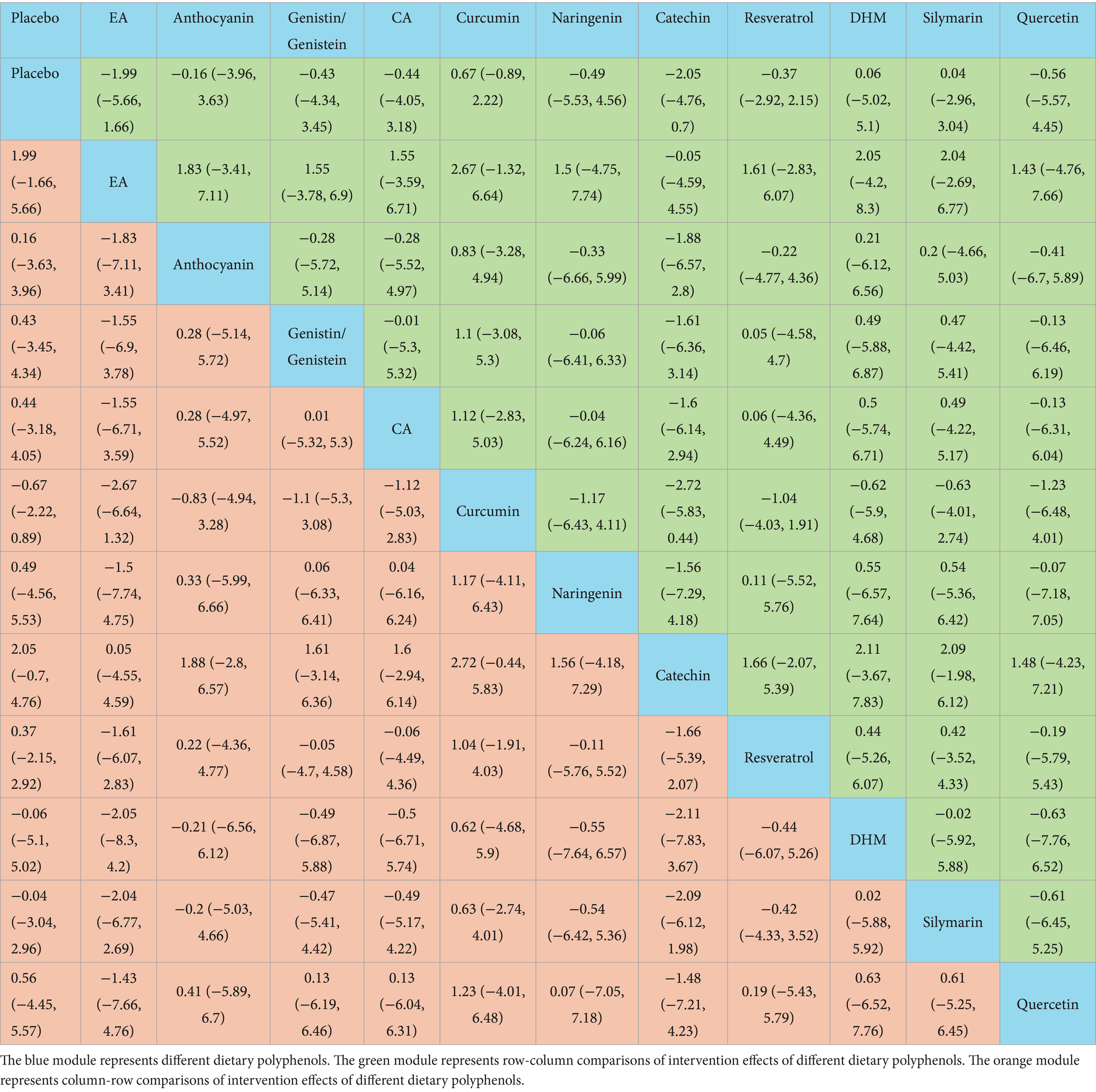
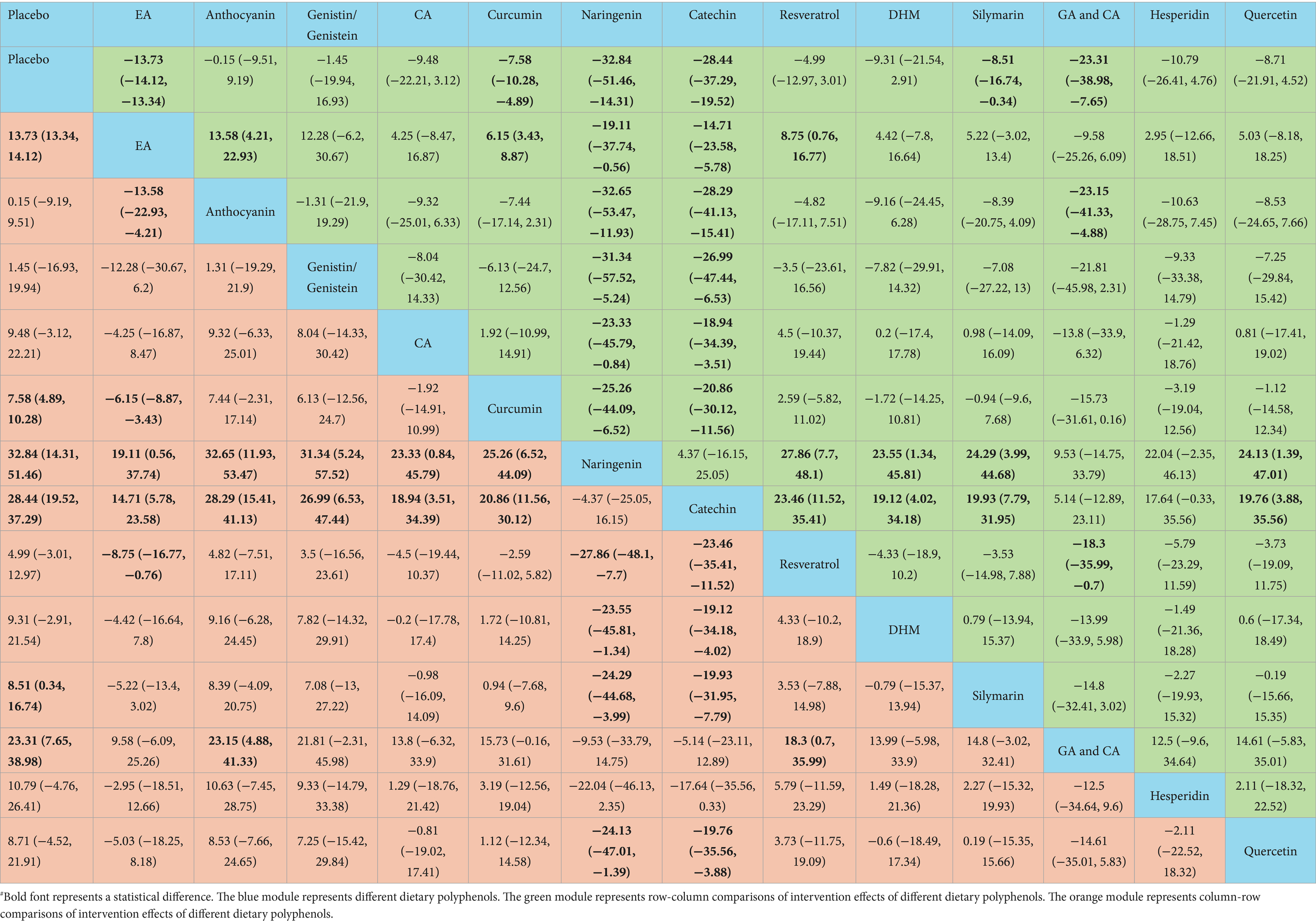
According to the NMA results, naringenin outperformed placebo [WMD = 32.84, 95% CI = (14.31, 51.46)], EA [WMD = 19.11, 95% CI = (0.56, 37.74)], anthocyanin [WMD = 32.65, 95% CI = (11.93, 53.47)], genistin/genistein [WMD = 31.34, 95% CI = (5.24, 57.52)], CA [WMD = 23.33, 95% CI = (0.84, 45.79)], curcumin [WMD = 25.26, 95% CI = (6.52, 44.09)], resveratrol [WMD = −27.86, 95% CI = (−48.1, −7.7)], DHM [WMD = −23.55, 95% CI = (−45.81, −1.34)], silymarin [WMD = −24.29, 95% CI = (−44.68, −3.99)], and quercetin [WMD = −24.13, 95% CI = (−47.01, −1.39)] in reducing TC. Catechin exhibited superior efficacy in lowering TC relative to placebo [WMD = 28.44, 95% CI = (19.52, 37.29)], EA [WMD = 14.71, 95% CI = (5.78, 23.58)], anthocyanin [WMD = 28.29, 95% CI = (15.41, 41.13)], genistin/genistein [WMD = 26.99, 95% CI = (6.53, 47.44)], CA [WMD = 18.94, 95% CI = (3.51, 34.39)], curcumin [WMD = 20.86, 95% CI = (11.56, 30.12)], resveratrol [WMD = −23.46, 95% CI = (−35.41, −11.52)], DHM [WMD = −19.12, 95% CI = (−34.18, −4.02)], silymarin [WMD = −19.93, 95% CI = (−31.95, −7.79)], and quercetin [WMD = −19.76, 95% CI = (−35.56, −3.88)]. More results with differences are presented in Table 4. The SUCRA probability ranking for lowering serum TC demonstrated that naringenin (94.59%) was the most effective, followed by catechin (92.26%) and GA and CA (83.52%) (Figure 4).
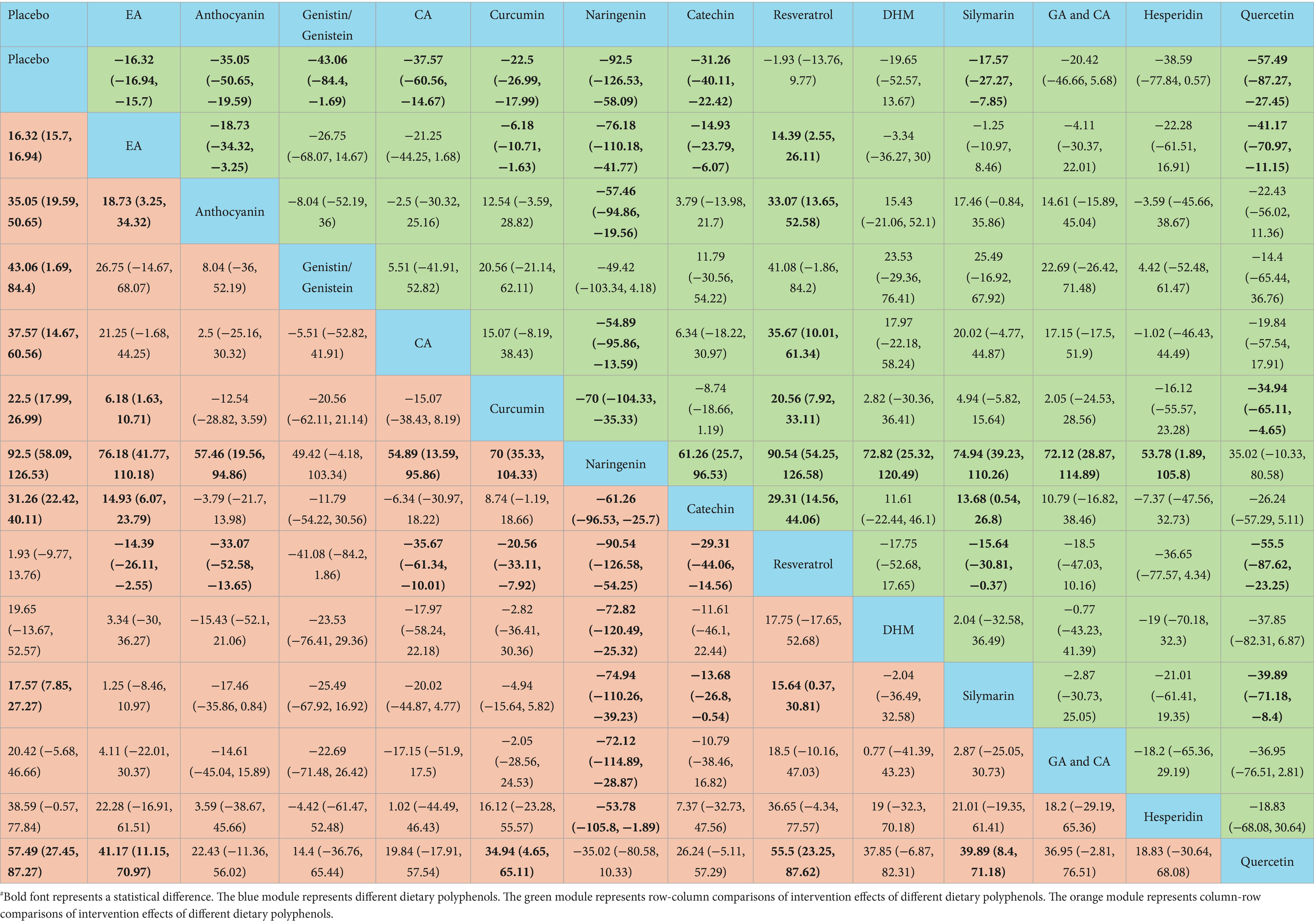
Naringenin outperformed placebo [WMD = 92.5, 95% CI = (58.09, 126.53)], EA [WMD = 76.18, 95% CI = (41.77, 110.18)], anthocyanin [WMD = 57.46, 95% CI = (19.56, 94.86)], CA [WMD = 54.89, 95% CI = (13.59, 95.86)], curcumin [WMD = 70, 95% CI = (35.33, 104.33)], catechin [WMD = −61.26, 95% CI = (−96.53, −25.7)], resveratrol [WMD = −90.54, 95% CI = (−126.58, −54.25)], DHM [WMD = −72.82, 95% CI = (−120.49, −25.32)], silymarin [WMD = −74.94, 95% CI = (−110.26, −39.23)], GA and CA [WMD = −72.12, 95% CI = (−114.89, −28.87)], and hesperidin [WMD = −53.78, 95% CI = (−105.8, −1.89)] in lowering TG. More results with differences are presented in Table 5. In terms of lowering serum TG, the SUCRA probability ranking results showed that naringenin (99.00%) was ranked highest, followed by quercetin (85.73%) and genistin/genistein (69.22%) (Figure 4).
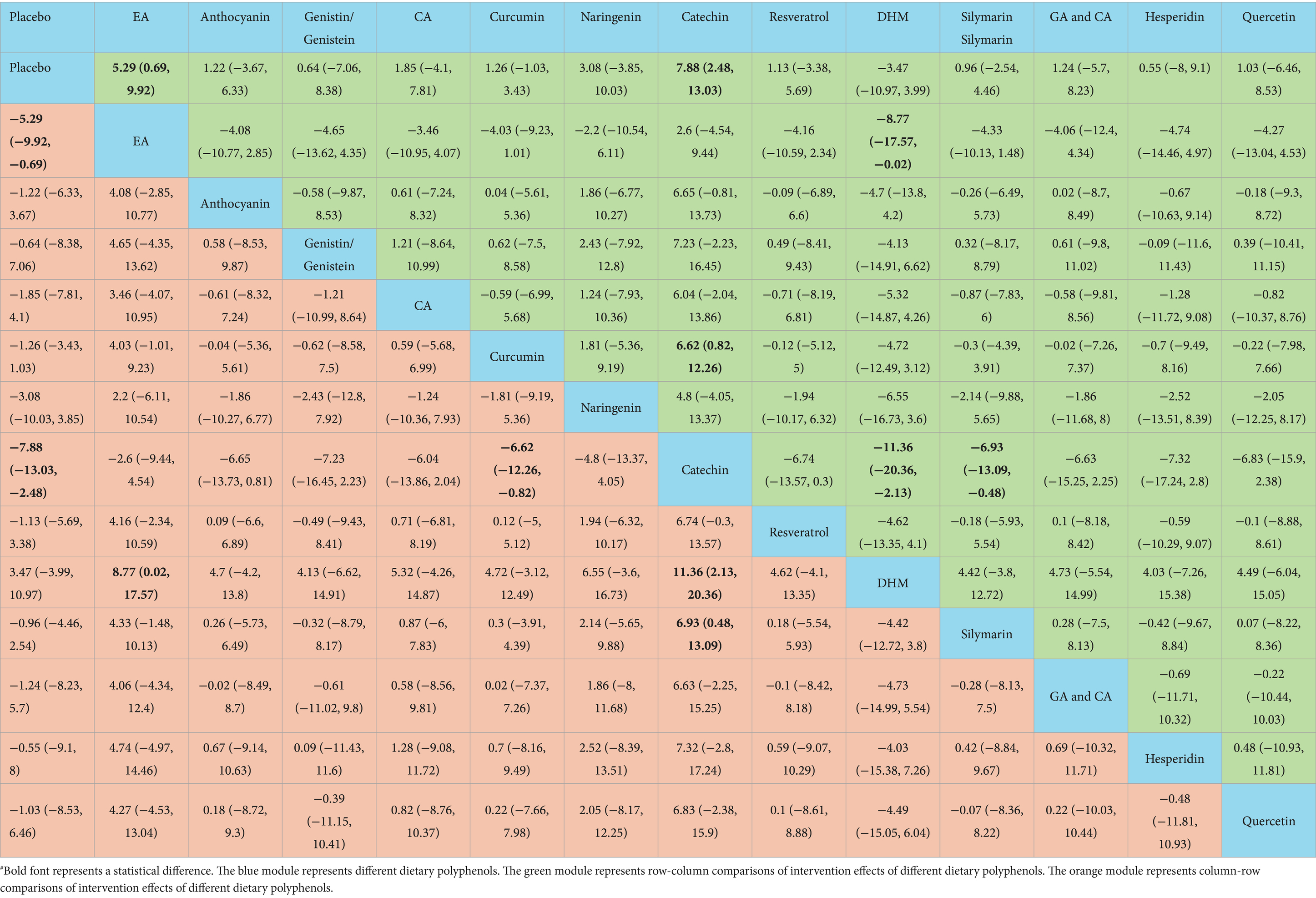
Catechin was more effective in increasing HDL-C than placebo [WMD = −7.88, 95% CI = (−13.03, −2.48)], curcumin [WMD = −6.62, 95% CI = (−12.26, −0.82)], DHM [WMD = 11.36, 95% CI = (2.13, 20.36)], and silymarin [WMD = 6.93, 95% CI = (0.48, 13.09)]. Additional results with differences are presented in Table 6. According to the SUCRA probability ranking for increasing serum HDL-C, catechin (93.72%) was ranked highest, followed by EA (82.69%) and naringenin (63.05%) (Figure 4).
No statistical differences were noted in the impact of these dietary polyphenols on LDL-C levels (Table 7). The SUCRA probability ranking results for lowering serum LDL-C levels indicated that DHM (73.22%) was more effective than GA and CA (71.95%), followed by naringenin (66.44%) (Figure 4).
3.3.6 Inflammation-related outcome measures
Twelve studies reported serum TNF-α levels after dietary polyphenol treatment for NAFLD.
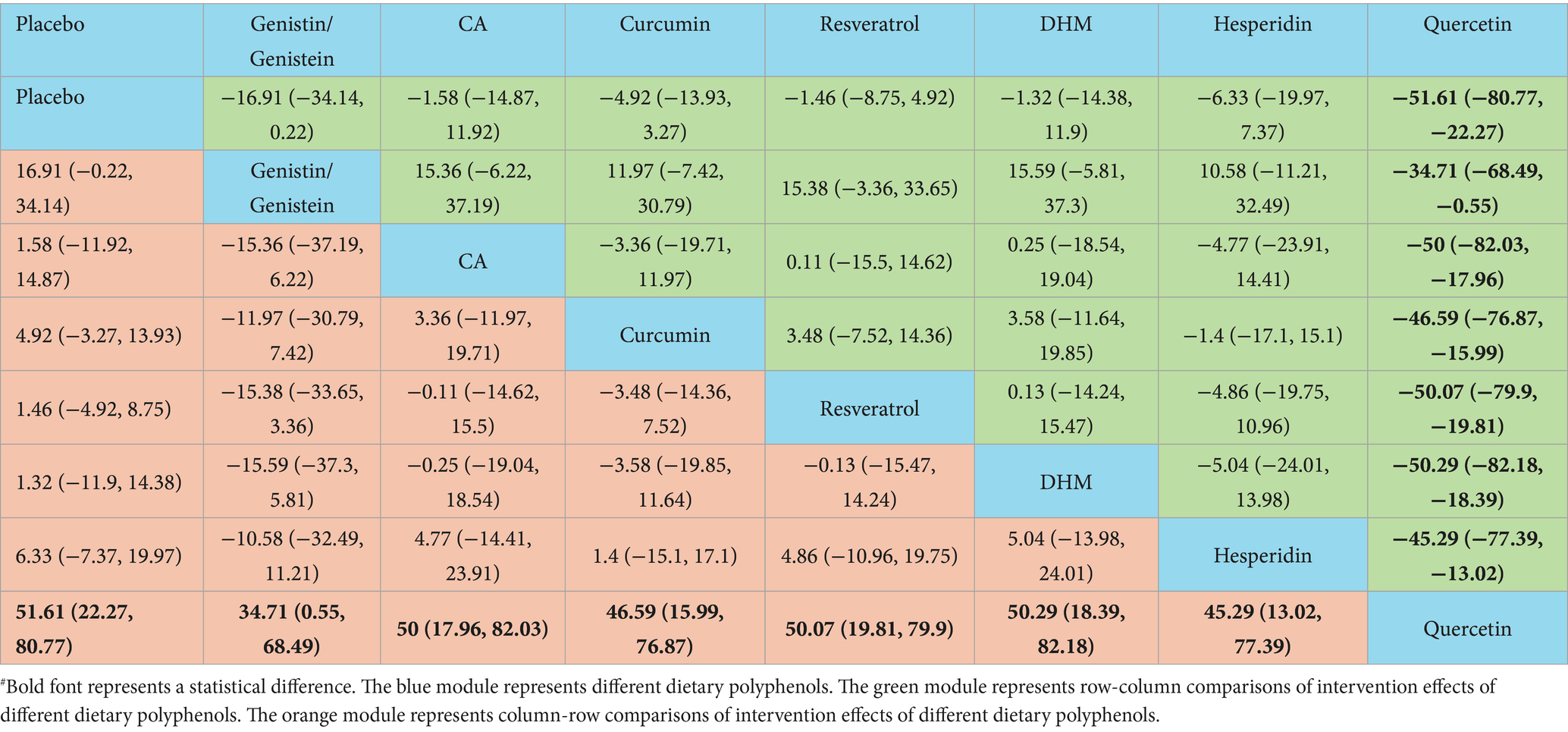
The NMA results demonstrated that quercetin was superior to placebo [WMD = 51.61, 95% CI = (22.27, 80.77)], genistin/genistein [WMD = 34.71, 95% CI = (0.55, 68.49)], CA [WMD = 50, 95% CI = (17.96, 82.03)], curcumin [WMD = 46.59, 95% CI = (15.99, 76.87)], resveratrol [WMD = 50.07, 95% CI = (19.81, 79.9)], DHM [WMD = 50.29, 95% CI = (18.39, 82.18)], and hesperidin [WMD = 45.29, 95% CI = (13.02, 77.39)] in reducing TNF-α (Table 8). According to the SUCRA probability ranking for lowering serum TNF-α levels, quercetin (99.47%) was ranked highest, followed by genistin/genistein (79.74%) and hesperidin (55.45%) (Figure 4).
3.4 Publication bias
A corrected comparison funnel plot was created for each outcome measure to appraise publication bias. Upon visual inspection of the funnel plot, no notable publication bias was detected (Figure 5). Funnel plots for publication bias of the other measures are provided in Supplementary material 3.
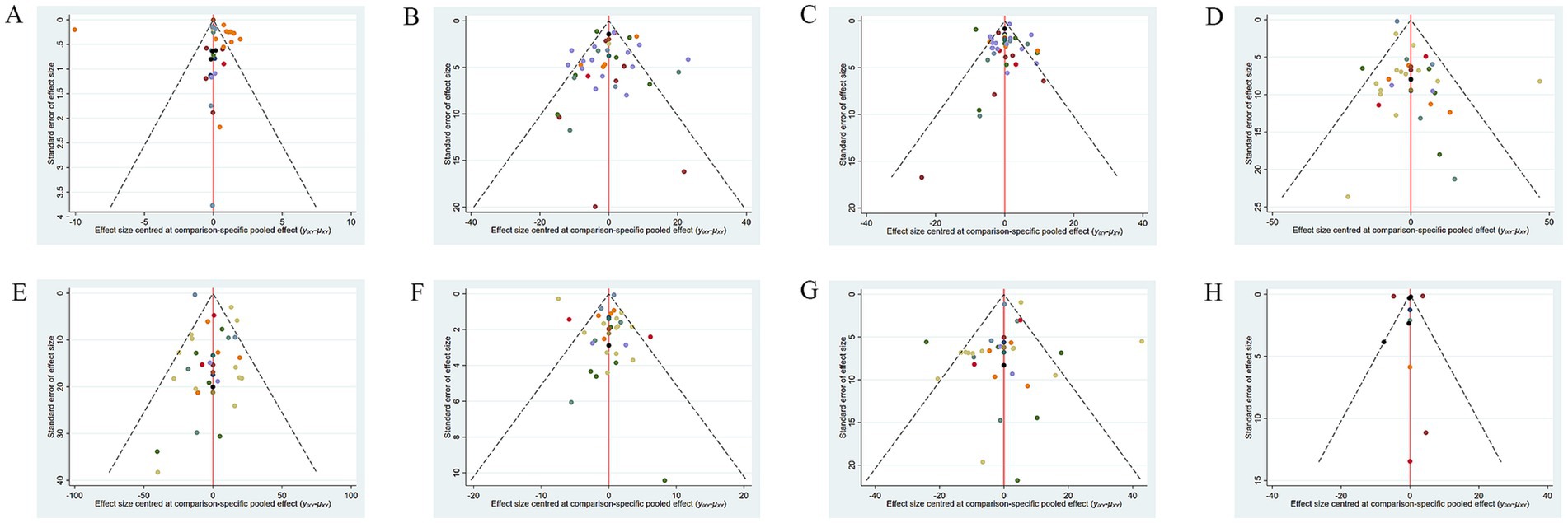
Figure 5. Funnel plot of different outcome indicators of NAFLD patients treated with different dietary polyphenol supplements. (A) BMI; (B) ALT; (C) AST; (D) TC; (E) TG; (F) HDL-C; (G) LDL-C; (H) TNF-α.
4 Discussion
The effects of 13 various single (or combined) dietary polyphenol supplements on NAFLD treatment are compared in this study. The results indicate that naringenin is the most effective polyphenol for lowering serum TC and TG, catechin for reducing BMI and ALT, AST, ALP (98.65%) and raising HDL-C, DHM for reducing LDL-C, FBG (82.80%), and insulin levels (77.34%) and quercetin for lowering TNF-α. In conclusion, our findings suggest that catechin may be the most appropriate dietary polyphenol supplement for treating patients with NAFLD.
Catechin is a naturally occurring polyphenolic compound found in dried tea leaves and is the main bioactive ingredient in green tea extracts. The results of this study imply that catechin is the most effective in reducing BMI, consistent with previous studies (14, 77). BMI is a common measure of overweight/obesity, and individuals with these conditions are more likely to develop NAFLD (78). Epigallocatechin gallate (EGCG) is the most abundant (60.89%) effective catechin in green tea (79). Animal studies (80, 81) indicate that EGCG reduces body weight by (i) decreasing intestinal absorption of lipids and proteins, reducing caloric intake, and (ii) activating adenosine monophosphate-activated protein kinase (AMPK), which regulates lipid, cholesterol, and glucose metabolism in the liver, skeletal muscles, and white adipose tissue, thereby promoting lipid and glucose catabolism (82).
Besides reducing BMI, catechin can regulate serum lipids in NAFLD patients by lowering TC levels and raising HDL-C levels. The liver is the primary organ responsible for regulating lipid metabolism. High TC levels are positively tied to NAFLD onset (83), while low HDL-C levels are a risk factor for both NAFLD and severe liver diseases (83, 84). Moreover, the TC/HDL-C ratio serves as a more reliable predictor of NAFLD. A higher ratio indicates a greater risk of BMI-related NAFLD (83, 85). Catechin can increase HDL-C levels in overweight/obese individuals (86). In addition, the consumption of green tea drinks or extracts can notably reduce serum TC levels in adults (87), demonstrating the role of catechins in lipid metabolism regulation. An animal experiment suggests that EGCG can inhibit hepatic cholesterol synthesis by modulating the sterol regulatory element-binding protein 2/silent information regulator 1 (SIRT1)/forkhead box transcription factor O1 signaling pathway, thereby affecting lipid metabolism in hyperlipidemic rats (88). Moreover, oxidative stress is a core mechanism in the progression of NAFLD. Nuclear factor erythroid 2-related factor 2 (NRF2) is a key factor that limits oxidative stress through its transcriptional activity and can regulate the expression of lipid metabolism-related genes, while EGCG can notably inhibit oxidative stress via the NRF2 signaling pathway (89).
ALT, AST, and ALP serve as traditional blood biomarkers for evaluating liver cell damage. Elevated levels of AST and ALT indicate hepatocellular necrosis, while elevated ALP levels suggest damage to biliary epithelial cells or tubular membranes (90). All three may be linked to the risk of HCC development (91). Multiple animal studies have confirmed that EGCG intervention can reduce serum liver enzyme levels in NAFLD/NASH model animals. The action mechanism is multifaceted, including the improvement of insulin sensitivity, facilitation of glucose metabolism in liver tissue, and regulation of the expression of inflammatory mediators and related pathological changes (89, 92, 93). Moreover, catechin is a natural iron chelator that can restrict iron absorption, potentially helping to prevent liver iron accumulation and slow the progression of NASH (59, 62, 94).
This study includes four RCTs on catechin treatment for NAFLD. One study notes mild bloating in NAFLD patients who take green tea extract. However, it does not specify whether this is linked to the green tea extract (61). Another article states that taking a high dose of catechin (1,080 mg/700 mL) does not induce any side effects (59). Although high-dose catechin may offer more benefits in reducing patients’ weight, BMI, and ALT (59, 95), it may also lead to mild nausea and stomach pain, particularly when taken on an empty stomach (89). Basic research also reveals that high doses of EGCG can result in mild liver damage in mice (96) and concentration-dependent hepatocyte death (97). Hence, the safety dosage of catechin still requires further research to be determined. Furthermore, the bioavailability of catechins is influenced by various processes, such as chemical degradation, microbial metabolism, and hepatic metabolism. Consequently, only a small fraction of the catechins that enter the intestine after tea consumption are absorbed (98). This issue warrants further attention.
High levels of TC and TG are connected to the development of NAFLD (83, 84). Naringenin is a natural citrus flavonoid that is widely present in grapefruit and oranges. It is the most effective dietary polyphenol in this study for decreasing serum TC and TG, aligning with prior research results (77). According to basic research results, the mechanisms through which naringenin reduces TC and TG levels in NAFLD/NASH rodent models are outlined below: (i) The key regulatory enzyme that inhibits cholesterol synthesis, 3-hydroxy-3-methylglutaryl-coenzyme A reductase is involved in lipid metabolism (99). (ii) By inhibiting the activation of the NLR family pyrin domain containing 3 inflammasome in hepatocytes, inflammation and lipid accumulation are alleviated (100). (iii) By activating the SIRT1-mediated signaling cascade, naringenin regulates the expression of lipid metabolism-related genes (fatty acid synthase, stearoyl-CoA desaturase 1, peroxisome proliferator-activated receptor α, carnitine palmitoyltransferase 1α) and clears reactive oxygen species and lipid peroxides in the body (101). (iv) By downregulating the liver X receptor in the liver and activating AMPK, naringenin stimulates fatty acid oxidation and inhibits lipogenesis (102). Additionally, since gut bacteria can influence host lipid metabolism, naringenin can lower TC and TG levels in NASH mice by regulating the gut microbiota (103). Two included RCTs on naringenin for NAFLD show no side effects (71, 72). An animal study indicates that different concentrations of naringenin (1 mM, 10 mM, 100 mM) do not cause intestinal damage in rats (104). However, there are contradictory research results (105). Consequently, the safety of naringenin must be explored in more detail. Moreover, although naringenin can be rapidly absorbed after oral administration, its bioavailability is only 15%. This low bioavailability is attributed to its very low affinity for water and the prolonged metabolic time during intestinal passage (106, 107).
DHM is the primary flavonoid substance in the edible medicinal plant Ampelopsis grossedentata. In this study, it is the most effective dietary polyphenol in reducing serum LDL-C, FBG, and insulin levels. High serum levels of LDL-C are the primary cause of the development of atherosclerosis (108). NAFLD is prevalent in T2DM patients (109). Chen et al. (73) propose that DHM lowers LDL-C, FBG, and insulin by regulating fibroblast growth factor 21 (FGF21) to improve IR. FGF21 is expressed in the liver, adipose tissue, and pancreas, playing a key role in the regulation of glucose and lipid metabolism. It may potentially develop into a biomarker for monitoring NAFLD/NASH (73, 110). Basic research indicates that DHM is a potential agonist of peroxisome proliferator-activated receptor γ (PPARγ) (112), an important metabolic regulatory factor for targeted treatment of NAFLD (111). DHM improves IR by activating PPARγ and subsequently modulating the FGF21-AMPK pathway (112). No toxicity has been reported for DHM within the typical dosage range. The only included RCT on DHM treatment for NAFLD shows that participants taking 600mg of DHM capsules daily experience no adverse effects (73). A study demonstrates that mice with NAFLD receive oral doses of DHM at 500 mg/kg/day, 750 mg/kg/day, and 1000 mg/kg/day for 8 weeks, with no adverse reactions reported (113). Moreover, an acute toxicity test on DHM reveals that its toxicity is very slight, the highest tolerance level for oral gavage in rats is 5g/kg body weight (114). The maximum safe dose for mice is around 16 g/kg, while for adults, it is approximately 1.6 g/kg, based on the body surface area standardization method (115). This could serve as a reference for determining the safe dosage of DHM. The absolute bioavailability of DHM in rats is less than 10%, which may be due to its poor solubility in water, as well as its metabolism and elimination within the intestinal tract (116).
Quercetin, a natural flavonoid found abundantly in various foods (like apples, onions, and tomatoes), possesses potent anti-inflammatory capabilities. It has the greatest effect in reducing TNF-α in NAFLD patients. Inflammation is regarded as a critical driver of NAFLD progression and can promote hepatic steatosis (100). Furthermore, TNF-α can mediate inflammation by activating mitogen-activated protein kinases (MAPK) signaling pathways, like extracellular signal-regulated kinase, c-Jun N-terminal kinase, nuclear factor kappa-B (NF-κB), and activator protein 1 (AP-1) (75). Nevertheless, quercetin can block the TNF-α-induced inflammatory cascade by inhibiting MAPK or enhancing PPARγ activity, thus antagonizing NF-κB or AP-1 activation and indirectly reducing inflammation (117, 118). The sole RCT included on quercetin treatment for NAFLD involves patients taking 500 mg of quercetin daily for 12 weeks, and no adverse effects are observed (75). Other clinical studies report minimal adverse effects of quercetin. Nonetheless, the safety of long-term (>12 weeks) high-dose (≥1,000 mg) supplementation needs further evaluation (119). A pharmacokinetic study indicates that the bioavailability of a single oral dose of quercetin is very low (approximately 2%). This may be ascribed to factors like reduced absorption rates, extensive metabolism, and/or rapid elimination (117).
The MD has been proven to be a healthy eating pattern that can improve blood lipids and liver enzymes, and reverse IR in patients with NAFLD (120–122). The MD is marked by food diversity, including fruits, vegetables, legumes, spices, and herbs. The health benefits are attributed to its rich content of dietary polyphenols with anti-inflammatory and antioxidant effects (123, 124). Given the absence of consistent evidence on the effective dosage and safety of isolated dietary polyphenol monomers, it is recommended for NAFLD patients to follow the MD, which is a safer, more affordable, and easier-to-maintain adjunctive therapy. However, before starting the MD, it is still important to seek the advice and guidance of a nutritionist or doctor based on one’s health condition to ensure safety and effectiveness.
This study compared the effects of different dietary polyphenol supplements on NAFLD, including 54 RCTs with 3,132 patients, offering a large sample size. Nonetheless, this study also presents certain limitations. Firstly, the network diagram has not formed a loop, and there is insufficient evidence for direct comparisons of various dietary polyphenols. Secondly, although this study shows that DHM and quercetin have markable effects, the number of samples available for research is very limited. Finally, the literature included in this study exhibits an imbalance in regional distribution. There are differences among various regions regarding healthcare systems, methods for diagnosing and assessing NAFLD, as well as laboratory testing indicators. The above limitations could impact the reliability of the findings in this study.
5 Conclusion
This study indicates that catechin, naringenin, DHM, and quercetin are dietary polyphenols that demonstrate notable efficacy in improving metabolic and inflammatory markers in patients with NAFLD. Among these, catechin appears to comprehensively enhance BMI, liver enzymes, and blood lipids linked to NAFLD, suggesting it may be the most suitable dietary polyphenol supplement. These findings provide preliminary recommendations and reliable evidence for healthcare professionals or nutritionists advocating for the use of dietary polyphenol supplements or polyphenol-rich diets as adjunctive therapies for NAFLD. The focus of future studies may, on the one hand, still be based on well-designed and rigorous RCTs to provide adequate sample sizes that can be employed for analyses. This is particularly important for dietary polyphenols that the current study has identified as most efficacious but which are limited by small sample sizes (like DHM and quercetin). On the other hand, given the uncertainties surrounding the safety of dietary polyphenols and their relatively low bioavailability, it may be beneficial to utilize RCTs to examine the incidence of adverse reactions tied to these compounds in order to better understand their safety profiles. Or, conducting a meta-analysis of clinical studies related to specific dietary polyphenols could help summarize effective strategies for maximizing their bioavailability in humans, determining the lowest effective doses, and addressing long-term administration safety concerns.
Author contributions
X-cW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. LS: Conceptualization, Data curation, Investigation, Supervision, Validation, Visualization, Writing – review & editing. X-hW: Formal analysis, Investigation, Methodology, Resources, Software, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the University-Level Research Project of Ningxia Medical University (No. XZ2022007) and the Special Talent Startup Project of Ningxia Medical University (No. XT2022016).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1582861/full#supplementary-material
Footnotes
References
1. European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. (2016) 64:1388–402. doi: 10.1016/j.jhep.2015.11.004
2. Buzzetti, E, Pinzani, M, and Tsochatzis, EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. (2016) 65:1038–48. doi: 10.1016/j.metabol.2015.12.012
3. Younossi, ZM, Golabi, P, Paik, JM, Henry, A, Van Dongen, C, and Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. (2023) 77:1335–47. doi: 10.1097/HEP.0000000000000004
4. Li, J, Zou, B, Yeo, YH, Feng, Y, Xie, X, Lee, DH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2019) 4:389–98. doi: 10.1016/S2468-1253(19)30039-1
5. Allen, AM, Hicks, SB, Mara, KC, Larson, JJ, and Therneau, TM. The risk of incident extrahepatic cancers is higher in non-alcoholic fatty liver disease than obesity—a longitudinal cohort study. J Hepatol. (2019) 71:1229–36. doi: 10.1016/j.jhep.2019.08.018
6. Thomas, JA, Kendall, BJ, Dalais, C, Macdonald, GA, and Thrift, AP. Hepatocellular and extrahepatic cancers in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Eur J Cancer. (2022) 173:250–62. doi: 10.1016/j.ejca.2022.06.051
7. Cholankeril, G, Wong, RJ, Hu, M, Perumpail, RB, Yoo, ER, Puri, P, et al. Liver transplantation for nonalcoholic steatohepatitis in the US: temporal trends and outcomes. Dig Dis Sci. (2017) 62:2915–22. doi: 10.1007/s10620-017-4684-x
8. European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J Hepatol. (2024) 81:492–542. doi: 10.1016/j.jhep.2024.04.031
9. Paik, JM, Golabi, P, Younossi, Y, Mishra, A, and Younossi, ZM. Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of NAFLD. Hepatology. (2020) 72:1605–16. doi: 10.1002/hep.31173
11. Younossi, ZM, Zelber-Sagi, S, Henry, L, and Gerber, LH. Lifestyle interventions in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. (2023) 20:708–22. doi: 10.1038/s41575-023-00800-4
12. Zhu, Y, Ouyang, Z, Du, H, Wang, M, Wang, J, Sun, H, et al. New opportunities and challenges of natural products research: when target identification meets single-cell multiomics. Acta Pharm Sin B. (2022) 12:4011–39. doi: 10.1016/j.apsb.2022.08.022
13. Abenavoli, L, Larussa, T, Corea, A, Procopio, AC, Boccuto, L, Dallio, M, et al. Dietary polyphenols and non-alcoholic fatty liver disease. Nutrients. (2021) 13:494. doi: 10.3390/nu13020494
14. Yang, K, Chen, J, Zhang, T, Yuan, X, Ge, A, Wang, S, et al. Efficacy and safety of dietary polyphenol supplementation in the treatment of non-alcoholic fatty liver disease: a systematic review and meta-analysis. Front Immunol. (2022) 13:949746. doi: 10.3389/fimmu.2022.949746
15. Watt, J, and Del Giovane, C. Network meta-analysis. Methods Mol Biol. (2022) 2345:187–201. doi: 10.1007/978-1-0716-1566-9_12
16. Hutton, B, Salanti, G, Caldwell, DM, Chaimani, A, Schmid, CH, Cameron, C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/M14-2385
17. Sterne, JAC, Savović, J, Page, MJ, Elbers, RG, Blencowe, NS, Boutron, I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
18. Turner, RM, Jackson, D, Wei, Y, Thompson, SG, and Higgins, JP. Predictive distributions for between-study heterogeneity and simple methods for their application in Bayesian meta-analysis. Stat Med. (2015) 34:984–98. doi: 10.1002/sim.6381
19. Melsen, WG, Bootsma, MC, Rovers, MM, and Bonten, MJ. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect. (2014) 20:123–9. doi: 10.1111/1469-0691.12494
20. Jackson, D, Riley, R, and White, IR. Multivariate meta-analysis: potential and promise. Stat Med. (2011) 30:2481–98. doi: 10.1002/sim.4172
21. Salanti, G, Ades, AE, and Ioannidis, JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. (2011) 64:163–71. doi: 10.1016/j.jclinepi.2010.03.016
22. Chaimani, A, Higgins, JP, Mavridis, D, Spyridonos, P, and Salanti, G. Graphical tools for network meta-analysis in STATA. PLoS One. (2013) 8:e76654. doi: 10.1371/journal.pone.0076654
23. Saadati, S, Hatami, B, Yari, Z, Shahrbaf, MA, Eghtesad, S, Mansour, A, et al. The effects of curcumin supplementation on liver enzymes, lipid profile, glucose homeostasis, and hepatic steatosis and fibrosis in patients with non-alcoholic fatty liver disease. Eur J Clin Nutr. (2019) 73:441–9. doi: 10.1038/s41430-018-0382-9
24. Patel, G, Dudhatra, N, and Chhaya, G. Evaluation of the safety and efficacy of CurcuVail® in patients with non-alcoholic fatty liver disease—a prospective randomized, double blind, placebo controlled, parallel group study. Int J Green Pharm. (2023) 17:218–25. doi: 10.22377/ijgp.v17i03.3472
25. Panahi, Y, Valizadegan, G, Ahamdi, N, Ganjali, S, Majeed, M, and Sahebkar, A. Curcuminoids plus piperine improve nonalcoholic fatty liver disease: a clinical trial. J Cell Biochem. (2019) 120:15989–96. doi: 10.1002/jcb.28877
26. Saberi-Karimian, M, Keshvari, M, Ghayour-Mobarhan, M, Salehizadeh, L, Rahmani, S, Behnam, B, et al. Effects of curcuminoids on inflammatory status in patients with non-alcoholic fatty liver disease: a randomized controlled trial. Complement Ther Med. (2020) 49:102322. doi: 10.1016/j.ctim.2020.102322
27. Mirhafez, SR, Dehabeh, M, Hariri, M, Farimani, AR, Movahedi, A, Naderan, RD, et al. Curcumin and piperine combination for the treatment of patients with non-alcoholic fatty liver disease: a double-blind randomized placebo-controlled trial. Adv Exp Med Biol. (2021) 1328:11–9. doi: 10.1007/978-3-030-73234-9_2
28. Sharifi, S, Bagherniya, M, Khoram, Z, Ebrahimi Varzaneh, A, Atkin, SL, Jamialahmadi, T, et al. Efficacy of curcumin plus piperine co-supplementation in moderate-to-high hepatic steatosis: a double-blind, randomized, placebo-controlled clinical trial. Phytother Res. (2023) 37:2217–29. doi: 10.1002/ptr.7764
29. Rezaei, L, Ebrahimi, M, Shafaghi, A, and Hojati, A. Benefits of Pilates training and nano-curcumin supplementation for overweight and obese females with NAFLD: a pilot study. Forum Nutr. (2023) 48:49. doi: 10.1186/s41110-023-00236-5
30. Jazayeri-Tehrani, SA, Rezayat, SM, Mansouri, S, Qorbani, M, Alavian, SM, Daneshi-Maskooni, M, et al. Nano-curcumin improves glucose indices, lipids, inflammation, and Nesfatin in overweight and obese patients with non-alcoholic fatty liver disease (NAFLD): a double-blind randomized placebo-controlled clinical trial. Nutr Metab. (2019) 16:8. doi: 10.1186/s12986-019-0331-1
31. Kelardeh, BM, Rahmati-Ahmadabad, S, Farzanegi, P, Helalizadeh, M, and Azarbayjani, MA. Effects of non-linear resistance training and curcumin supplementation on the liver biochemical markers levels and structure in older women with non-alcoholic fatty liver disease. J Bodyw Mov Ther. (2020) 24:154–60. doi: 10.1016/j.jbmt.2020.02.021
32. Panahi, Y, Kianpour, P, Mohtashami, R, Jafari, R, Simental-Mendía, LE, and Sahebkar, A. Curcumin lowers serum lipids and uric acid in subjects with nonalcoholic fatty liver disease: a randomized controlled trial. J Cardiovasc Pharmacol. (2016) 68:223–9. doi: 10.1097/FJC.0000000000000406
33. Panahi, Y, Kianpour, P, Mohtashami, R, Jafari, R, Simental-Mendía, LE, and Sahebkar, A. Efficacy and safety of phytosomal curcumin in non-alcoholic fatty liver disease: a randomized controlled trial. Drug Res. (2017) 67:244–51. doi: 10.1055/s-0043-100019
34. Mirhafez, SR, Azimi-Nezhad, M, Dehabeh, M, Hariri, M, Naderan, RD, Movahedi, A, et al. The effect of curcumin phytosome on the treatment of patients with non-alcoholic fatty liver disease: a double-blind, randomized, placebo-controlled trial. Adv Exp Med Biol. (2021) 1308:25–35. doi: 10.1007/978-3-030-64872-5_3
35. Hosseinian, SA, Mehrzad, J, Mirhafez, SR, Saeedi, J, Zhiani, R, and Sahebkar, A. Evaluation of the effect of phytosomal curcuminoids on oxidative stress and inflammatory markers in NAFLD: a randomized double-blind placebo-controlled trial. J Funct Foods. (2022) 96:105202. doi: 10.1016/j.jff.2022.105202
36. Safari, Z, Bagherniya, M, Khoram, Z, Ebrahimi Varzaneh, A, Heidari, Z, Sahebkar, A, et al. The effect of curcumin on anthropometric indices, blood pressure, lipid profiles, fasting blood glucose, liver enzymes, fibrosis, and steatosis in non-alcoholic fatty livers. Front Nutr. (2023) 10:1163950. doi: 10.3389/fnut.2023.1163950
37. Navekar, R, Rafraf, M, Ghaffari, A, Asghari-Jafarabadi, M, and Khoshbaten, M. Turmeric supplementation improves serum glucose indices and leptin levels in patients with nonalcoholic fatty liver diseases. J Am Coll Nutr. (2017) 36:261–7. doi: 10.1080/07315724.2016.1267597
38. Ghaffari, A, Rafraf, M, Navekar, R, Sepehri, B, Asghari-Jafarabadi, M, and Ghavami, SM. Turmeric and chicory seed have beneficial effects on obesity markers and lipid profile in non-alcoholic fatty liver disease (NAFLD). Int J Vitam Nutr Res. (2019) 89:293–302. doi: 10.1024/0300-9831/a000568
39. Jarhahzadeh, M, Alavinejad, P, Farsi, F, Husain, D, and Rezazadeh, A. The effect of turmeric on lipid profile, malondialdehyde, liver echogenicity and enzymes among patients with nonalcoholic fatty liver disease: a randomized double blind clinical trial. Diabetol Metab Syndr. (2021) 13:112. doi: 10.1186/s13098-021-00731-7
40. Kalhori, A, Rafraf, M, Navekar, R, Ghaffari, A, and Jafarabadi, MA. Effect of turmeric supplementation on blood pressure and serum levels of Sirtuin 1 and adiponectin in patients with nonalcoholic fatty liver disease: a double-blind, randomized, placebo-controlled trial. Prev Nutr Food Sci. (2022) 27:37–44. doi: 10.3746/pnf.2022.27.1.37
41. Chachay, VS, Macdonald, GA, Martin, JH, Whitehead, JP, O’Moore-Sullivan, TM, Lee, P, et al. Resveratrol does not benefit patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. (2014) 12:2092–2103.e6. doi: 10.1016/j.cgh.2014.02.024
42. Chen, S, Zhao, X, Ran, L, Wan, J, Wang, X, Qin, Y, et al. Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: a randomized controlled trial. Dig Liver Dis. (2015) 47:226–32. doi: 10.1016/j.dld.2014.11.015
43. Heebøll, S, Kreuzfeldt, M, Hamilton-Dutoit, S, Kjær Poulsen, M, Stødkilde-Jørgensen, H, Møller, HJ, et al. Placebo-controlled, randomised clinical trial: high-dose resveratrol treatment for non-alcoholic fatty liver disease. Scand J Gastroenterol. (2016) 51:456–64. doi: 10.3109/00365521.2015.1107620
44. Farzin, L, Asghari, S, Rafraf, M, Asghari-Jafarabadi, M, and Shirmohammadi, M. No beneficial effects of resveratrol supplementation on atherogenic risk factors in patients with nonalcoholic fatty liver disease. Int J Vitam Nutr Res. (2020) 90:279–89. doi: 10.1024/0300-9831/a000528
45. Faghihzadeh, F, Adibi, P, Rafiei, R, and Hekmatdoost, A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr Res. (2014) 34:837–43. doi: 10.1016/j.nutres.2014.09.005
46. Faghihzadeh, F, Adibi, P, and Hekmatdoost, A. The effects of resveratrol supplementation on cardiovascular risk factors in patients with non-alcoholic fatty liver disease: a randomised, double-blind, placebo-controlled study. Br J Nutr. (2015) 114:796–803. doi: 10.1017/S0007114515002433
47. Asghari, S, Asghari-Jafarabadi, M, Somi, MH, Ghavami, SM, and Rafraf, M. Comparison of calorie-restricted diet and resveratrol supplementation on anthropometric indices, metabolic parameters, and serum Sirtuin-1 levels in patients with nonalcoholic fatty liver disease: a randomized controlled clinical trial. J Am Coll Nutr. (2018) 37:223–33. doi: 10.1080/07315724.2017.1392264
48. Asghari, S, Rafraf, M, Farzin, L, Asghari-Jafarabadi, M, Ghavami, SM, and Somi, MH. Effects of pharmacologic dose of resveratrol supplementation on oxidative/antioxidative status biomarkers in nonalcoholic fatty liver disease patients: a randomized, double-blind, placebo-controlled trial. Adv Pharm Bull. (2018) 8:307–17. doi: 10.15171/apb.2018.036
49. Zhang, PW, Chen, FX, Li, D, Ling, WH, and Guo, HH. A CONSORT-compliant, randomized, double-blind, placebo-controlled pilot trial of purified anthocyanin in patients with nonalcoholic fatty liver disease. Medicine. (2015) 94:e758. doi: 10.1097/MD.0000000000000758
50. Izadi, F, Farrokhzad, A, Tamizifar, B, Tarrahi, MJ, and Entezari, MH. Effect of sour tea supplementation on liver enzymes, lipid profile, blood pressure, and antioxidant status in patients with non-alcoholic fatty liver disease: a double-blind randomized controlled clinical trial. Phytother Res. (2021) 35:477–85. doi: 10.1002/ptr.6826
51. Sangsefidi, ZS, Yarhosseini, F, Hosseinzadeh, M, Ranjbar, A, Akhondi-Meybodi, M, Fallahzadeh, H, et al. The effect of (Cornus mas L.) fruit extract on liver function among patients with nonalcoholic fatty liver: a double-blind randomized clinical trial. Phytother Res. (2021) 35:5259–68. doi: 10.1002/ptr.7199
52. Mojiri-Forushani, HHA, Khanzadeh, A, and Zahedi, A. Effectiveness of grape seed extract in patients with nonalcoholic fatty liver: a randomized double-blind clinical study. Hepat Mon. (2022) 22:22. doi: 10.5812/hepatmon-132309
53. Yarhosseini, F, Sangouni, AA, Sangsefidi, ZS, Hosseinzadeh, M, Akhondi-Meybodi, M, Ranjbar, A, et al. Effect of Cornus mas L. fruit extract on blood pressure, anthropometric and body composition indices in patients with non-alcoholic fatty liver disease: a double-blind randomized controlled trial. Clin Nutr ESPEN. (2023) 56:18–24. doi: 10.1016/j.clnesp.2023.04.018
54. Hashemi, SJ, Eskandar, H, and Sardabi, EH. A placebo-controlled trial of silymarin in patients with nonalcoholic fatty liver disease. Hepat Mon. (2009) 9:265–70.
55. Masoodi, MRA, Panahian, M, and Vojdanian, M. Effects of silymarin on reducing liver aminotransferases in patients with nonalcoholic fatty liver diseases. GOVARESH. (2013) 18:181–5.
56. Solhi, H, Ghahremani, R, Kazemifar, AM, and Hoseini, YZ. Silymarin in treatment of non-alcoholic steatohepatitis: a randomized clinical trial. Caspian J Intern Med. (2014) 5:9–12.
57. Wah Kheong, C, Nik Mustapha, NR, and Mahadeva, S. A randomized trial of Silymarin for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. (2017) 15:1940–9.e8. doi: 10.1016/j.cgh.2017.04.016
58. Anushiravani, A, Haddadi, N, Pourfarmanbar, M, and Mohammadkarimi, V. Treatment options for nonalcoholic fatty liver disease: a double-blinded randomized placebo-controlled trial. Eur J Gastroenterol Hepatol. (2019) 31:613–7. doi: 10.1097/MEG.0000000000001369
59. Sakata, R, Nakamura, T, Torimura, T, Ueno, T, and Sata, M. Green tea with high-density catechins improves liver function and fat infiltration in non-alcoholic fatty liver disease (NAFLD) patients: a double-blind placebo-controlled study. Int J Mol Med. (2013) 32:989–94. doi: 10.3892/ijmm.2013.1503
60. Pezeshki, A, Safi, S, Feizi, A, Askari, G, and Karami, F. The effect of green tea extract supplementation on liver enzymes in patients with nonalcoholic fatty liver disease. Int J Prev Med. (2016) 7:28. doi: 10.4103/2008-7802.173051
61. Hussain, M, Habib Ur, R, and Akhtar, L. Therapeutic benefits of green tea extract on various parameters in non-alcoholic fatty liver disease patients. Pak J Med Sci. (2017) 33:931–6. doi: 10.12669/pjms.334.12571
62. Tabatabaee, SM, Alavian, SM, Ghalichi, L, Miryounesi, SM, Mousavizadeh, K, Jazayeri, S, et al. Green tea in non-alcoholic fatty liver disease: a double blind randomized clinical trial. Hepat Mon. (2017) 17:6. doi: 10.5812/hepatmon.14993
63. Shahmohammadi, HA, Hosseini, SA, Hajiani, E, Malehi, AS, and Alipour, M. Effects of green coffee bean extract supplementation on patients with non-alcoholic fatty liver disease: a randomized clinical trial. Hepat Mon. (2017) 63:e12299. doi: 10.5812/hepatmon.45609
64. Hosseinabadi, S, Rafraf, M, and Asgbari-Jafarabadi, M. Effects of green coffee extract supplementation on blood pressure and antioxidants status in patients with non-alcoholic fatty liver disease. Prog Nutr. (2019) 21:180–9. doi: 10.23751/pn.v21i2-S.7267
65. Hosseinabadi, S, Rafraf, M, Asghari, S, Asghari-Jafarabadi, M, and Vojouhi, S. Effect of green coffee extract supplementation on serum adiponectin concentration and lipid profile in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Complement Ther Med. (2020) 49:102290. doi: 10.1016/j.ctim.2019.102290
66. Hosseinabadi, S, Rafraf, M, Mahmoodzadeh, A, Asghari-Jafarabadi, M, and Asghari, S. Effects of green coffee extract supplementation on glycemic indexes, leptin, and obesity values in patients with non-alcoholic fatty liver disease. J Herb Med. (2020) 22:100340. doi: 10.1016/j.hermed.2020.100340
67. Goodarzi, R, Jafarirad, S, Mohammadtaghvaei, N, Dastoorpoor, M, and Alavinejad, P. The effect of pomegranate extract on anthropometric indices, serum lipids, glycemic indicators, and blood pressure in patients with nonalcoholic fatty liver disease: a randomized double-blind clinical trial. Phytother Res. (2021) 35:5871–82. doi: 10.1002/ptr.7249
68. Barghchi, H, Milkarizi, N, Belyani, S, Norouzian Ostad, A, Askari, VR, Rajabzadeh, F, et al. Pomegranate (Punica granatum L.) peel extract ameliorates metabolic syndrome risk factors in patients with non-alcoholic fatty liver disease: a randomized double-blind clinical trial. Nutr J. (2023) 22:40. doi: 10.1186/s12937-023-00869-2
69. Amanat, S, Eftekhari, MH, Fararouei, M, Bagheri Lankarani, K, and Massoumi, SJ. Genistein supplementation improves insulin resistance and inflammatory state in non-alcoholic fatty liver patients: a randomized, controlled trial. Clin Nutr. (2018) 37:1210–5. doi: 10.1016/j.clnu.2017.05.028
70. Neshatbini Tehrani, A, Hatami, B, Helli, B, Yari, Z, Daftari, G, Salehpour, A, et al. The effect of soy isoflavones on non-alcoholic fatty liver disease and the level of fibroblast growth factor-21 and fetuin A. Sci Rep. (2024) 14:5134. doi: 10.1038/s41598-024-55747-6
71. Namkhah, Z, Naeini, F, Mahdi Rezayat, S, Mehdi, Y, Mansouri, S, and Javad Hosseinzadeh-Attar, M. Does naringenin supplementation improve lipid profile, severity of hepatic steatosis and probability of liver fibrosis in overweight/obese patients with NAFLD? A randomised, double-blind, placebo-controlled, clinical trial. Int J Clin Pract. (2021) 75:e14852. doi: 10.1111/ijcp.14852
72. Naeini, F, Namkhah, Z, Tutunchi, H, Rezayat, SM, Mansouri, S, Yaseri, M, et al. Effects of naringenin supplementation on cardiovascular risk factors in overweight/obese patients with nonalcoholic fatty liver disease: a pilot double-blind, placebo-controlled, randomized clinical trial. Eur J Gastroenterol Hepatol. (2022) 34:345–53. doi: 10.1097/MEG.0000000000002323
73. Chen, S, Zhao, X, Wan, J, Ran, L, Qin, Y, Wang, X, et al. Dihydromyricetin improves glucose and lipid metabolism and exerts anti-inflammatory effects in nonalcoholic fatty liver disease: a randomized controlled trial. Pharmacol Res. (2015) 99:74–81. doi: 10.1016/j.phrs.2015.05.009
74. Cheraghpour, M, Imani, H, Ommi, S, Alavian, SM, Karimi-Shahrbabak, E, Hedayati, M, et al. Hesperidin improves hepatic steatosis, hepatic enzymes, and metabolic and inflammatory parameters in patients with nonalcoholic fatty liver disease: a randomized, placebo-controlled, double-blind clinical trial. Phytother Res. (2019) 33:2118–25. doi: 10.1002/ptr.6406
75. Hosseinikia, M, Oubari, F, Hosseinkia, R, Tabeshfar, Z, Salehi, MG, Mousavian, Z, et al. Quercetin supplementation in non-alcoholic fatty liver disease a randomized, double-blind, placebo-controlled clinical trial. Nutr Food Sci. (2020) 50:1279–93. doi: 10.1108/NFS-10-2019-0321
76. Rajendra, VKP, Kurapati, S, Balineni, SK, and Gogineni, NTT. A blend of Sphaeranthus indicus flower head and Terminalia chebula fruit extracts reduces fatty liver and improves liver function in non-alcoholic, overweight adults. Funct Foods Health D. (2022) 12:361–79. doi: 10.31989/ffhd.v12i7.958
77. Liu, H, Li, Y, Jin, Y, Li, X, Wang, D, Yu, X, et al. Effects of different natural products in patients with non-alcoholic fatty liver disease-a network meta-analysis of randomized controlled trials. Phytother Res. (2024) 38:3801–24. doi: 10.1002/ptr.8182
78. Le, MH, Le, DM, Baez, TC, Wu, Y, Ito, T, Lee, EY, et al. Global incidence of non-alcoholic fatty liver disease: a systematic review and meta-analysis of 63 studies and 1,201,807 persons. J Hepatol. (2023) 79:287–95. doi: 10.1016/j.jhep.2023.03.040
79. Isemura, M. Catechin in human health and disease. Molecules. (2019) 24:528. doi: 10.3390/molecules24030528
80. Yang, CS, Zhang, J, Zhang, L, Huang, J, and Wang, Y. Mechanisms of body weight reduction and metabolic syndrome alleviation by tea. Mol Nutr Food Res. (2016) 60:160–74. doi: 10.1002/mnfr.201500428
81. Li, F, Gao, C, Yan, P, Zhang, M, Wang, Y, Hu, Y, et al. EGCG reduces obesity and white adipose tissue gain partly through AMPK activation in mice. Front Pharmacol. (2018) 9:1366. doi: 10.3389/fphar.2018.01366
82. Chang, YC, Chan, MH, Yang, YF, Li, CH, and Hsiao, M. Glucose transporter 4: insulin response mastermind, glycolysis catalyst and treatment direction for cancer progression. Cancer Lett. (2023) 563:216179. doi: 10.1016/j.canlet.2023.216179
83. Ren, XY, Shi, D, Ding, J, Cheng, ZY, Li, HY, Li, JS, et al. Total cholesterol to high-density lipoprotein cholesterol ratio is a significant predictor of nonalcoholic fatty liver: Jinchang cohort study. Lipids Health Dis. (2019) 18:47. doi: 10.1186/s12944-019-0984-9
84. Jarvis, H, Craig, D, Barker, R, Spiers, G, Stow, D, Anstee, QM, et al. Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of population-based observational studies. PLoS Med. (2020) 17:e1003100. doi: 10.1371/journal.pmed.1003100
85. Lu, S, Xie, Q, Kuang, M, Hu, C, Li, X, Yang, H, et al. Lipid metabolism, BMI and the risk of nonalcoholic fatty liver disease in the general population: evidence from a mediation analysis. J Transl Med. (2023) 21:192. doi: 10.1186/s12967-023-04047-0
86. Wang, Y, Xia, H, Yu, J, Sui, J, Pan, D, Wang, S, et al. Effects of green tea catechin on the blood pressure and lipids in overweight and obese population-a meta-analysis. Heliyon. (2023) 9:e21228. doi: 10.1016/j.heliyon.2023.e21228
87. Zheng, XX, Xu, YL, Li, SH, Liu, XX, Hui, R, and Huang, XH. Green tea intake lowers fasting serum total and LDL cholesterol in adults: a meta-analysis of 14 randomized controlled trials. Am J Clin Nutr. (2011) 94:601–10. doi: 10.3945/ajcn.110.010926
88. Li, Y, and Wu, S. Epigallocatechin gallate suppresses hepatic cholesterol synthesis by targeting SREBP-2 through SIRT1/FOXO1 signaling pathway. Mol Cell Biochem. (2018) 448:175–85. doi: 10.1007/s11010-018-3324-x
89. Tang, G, Xu, Y, Zhang, C, Wang, N, Li, H, and Feng, Y. Green tea and epigallocatechin gallate (EGCG) for the management of nonalcoholic fatty liver diseases (NAFLD): insights into the role of oxidative stress and antioxidant mechanism. Antioxidants. (2021) 10:1076. doi: 10.3390/antiox10071076
90. Tamber, SS, Bansal, P, Sharma, S, Singh, RB, and Sharma, R. Biomarkers of liver diseases. Mol Biol Rep. (2023) 50:7815–23. doi: 10.1007/s11033-023-08666-0
91. Stepien, M, Fedirko, V, Duarte-Salles, T, Ferrari, P, Freisling, H, Trepo, E, et al. Prospective association of liver function biomarkers with development of hepatobiliary cancers. Cancer Epidemiol. (2016) 40:179–87. doi: 10.1016/j.canep.2016.01.002
92. Chen, C, Liu, Q, Liu, L, Hu, YY, and Feng, Q. Potential biological effects of (-)-epigallocatechin-3-gallate on the treatment of nonalcoholic fatty liver disease. Mol Nutr Food Res. (2018) 62:1700483. doi: 10.1002/mnfr.201700483
93. Abunofal, O, and Mohan, C. Salubrious effects of green tea catechins on fatty liver disease: a systematic review. Medicines. (2022) 9:20. doi: 10.3390/medicines9030020
94. Bessone, F, Razori, MV, and Roma, MG. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell Mol Life Sci. (2019) 76:99–128. doi: 10.1007/s00018-018-2947-0
95. Chen, IJ, Liu, CY, Chiu, JP, and Hsu, CH. Therapeutic effect of high-dose green tea extract on weight reduction: a randomized, double-blind, placebo-controlled clinical trial. Clin Nutr. (2016) 35:592–9. doi: 10.1016/j.clnu.2015.05.003
96. Saleh, IG, Ali, Z, Abe, N, Wilson, FD, Hamada, FM, Abd-Ellah, MF, et al. Effect of green tea and its polyphenols on mouse liver. Fitoterapia. (2013) 90:151–9. doi: 10.1016/j.fitote.2013.07.014
97. Kucera, O, Mezera, V, Moravcova, A, Endlicher, R, Lotkova, H, Drahota, Z, et al. In vitro toxicity of epigallocatechin gallate in rat liver mitochondria and hepatocytes. Oxid Med Cell Longev. (2015) 2015:476180. doi: 10.1155/2015/476180
98. Cai, ZY, Li, XM, Liang, JP, Xiang, LP, Wang, KR, Shi, YL, et al. Bioavailability of tea catechins and its improvement. Molecules. (2018) 23:2346. doi: 10.3390/molecules23092346
99. Naeini, F, Namkhah, Z, Ostadrahimi, A, Tutunchi, H, and Hosseinzadeh-Attar, MJ. A comprehensive systematic review of the effects of naringenin, a citrus-derived flavonoid, on risk factors for nonalcoholic fatty liver disease. Adv Nutr. (2021) 12:413–28. doi: 10.1093/advances/nmaa106
100. Wang, Q, Ou, Y, Hu, G, Wen, C, Yue, S, Chen, C, et al. Naringenin attenuates non-alcoholic fatty liver disease by down-regulating the NLRP3/NF-κB pathway in mice. Br J Pharmacol. (2020) 177:1806–21. doi: 10.1111/bph.14938
101. Hua, YQ, Zeng, Y, Xu, J, and Xu, XL. Naringenin alleviates nonalcoholic steatohepatitis in middle-aged Apoe−/− mice: role of SIRT1. Phytomedicine. (2021) 81:153412. doi: 10.1016/j.phymed.2020.153412
102. Ren, B, Qin, W, Wu, F, Wang, S, Pan, C, Wang, L, et al. Apigenin and naringenin regulate glucose and lipid metabolism, and ameliorate vascular dysfunction in type 2 diabetic rats. Eur J Pharmacol. (2016) 773:13–23. doi: 10.1016/j.ejphar.2016.01.002
103. Cao, P, Yue, M, Cheng, Y, Sullivan, MA, Chen, W, Yu, H, et al. Naringenin prevents non-alcoholic steatohepatitis by modulating the host metabolome and intestinal microbiome in MCD diet-fed mice. Food Sci Nutr. (2023) 11:7826–40. doi: 10.1002/fsn3.3700
104. Surampalli, G, Nanjwade, BK, and Patil, PA. Safety evaluation of naringenin upon experimental exposure on rat gastrointestinal epithelium for novel optimal drug delivery. Drug Deliv. (2016) 23:512–24. doi: 10.3109/10717544.2014.923957
105. Hernández-Aquino, E, and Muriel, P. Beneficial effects of naringenin in liver diseases: molecular mechanisms. World J Gastroenterol. (2018) 24:1679–707. doi: 10.3748/wjg.v24.i16.1679
106. Arafah, A, Rehman, MU, Mir, TM, Wali, AF, Ali, R, Qamar, W, et al. Multi-therapeutic potential of naringenin (4′,5,7-trihydroxyflavonone): experimental evidence and mechanisms. Plants. (2020) 9:1784. doi: 10.3390/plants9121784
107. Joshi, R, Kulkarni, YA, and Wairkar, S. Pharmacokinetic, pharmacodynamic and formulations aspects of naringenin: an update. Life Sci. (2018) 215:43–56. doi: 10.1016/j.lfs.2018.10.066
108. Qiao, YN, Zou, YL, and Guo, SD. Low-density lipoprotein particles in atherosclerosis. Front Physiol. (2022) 13:931931. doi: 10.3389/fphys.2022.931931
109. Younossi, ZM, Golabi, P, de Avila, L, Paik, JM, Srishord, M, Fukui, N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. (2019) 71:793–801. doi: 10.1016/j.jhep.2019.06.021
110. Tillman, EJ, and Rolph, T. FGF21: an emerging therapeutic target for non-alcoholic steatohepatitis and related metabolic diseases. Front Endocrinol. (2020) 11:601290. doi: 10.3389/fendo.2020.601290
111. Chen, H, Tan, H, Wan, J, Zeng, Y, Wang, J, Wang, H, et al. PPAR-γ signaling in nonalcoholic fatty liver disease: pathogenesis and therapeutic targets. Pharmacol Ther. (2023) 245:108391. doi: 10.1016/j.pharmthera.2023.108391
112. Zhou, Y, Wu, Y, Qin, Y, Liu, L, Wan, J, Zou, L, et al. Ampelopsin improves insulin resistance by activating PPARγ and subsequently up-regulating FGF21-AMPK signaling pathway. PLoS One. (2016) 11:e0159191. doi: 10.1371/journal.pone.0159191
113. Kang, L, Ma, X, Yu, F, Xu, L, and Lang, L. Dihydromyricetin alleviates non-alcoholic fatty liver disease by modulating gut microbiota and inflammatory signaling pathways. J Microbiol Biotechnol. (2024) 34:2637–47. doi: 10.4014/jmb.2406.06048
114. Xu, J-J, Yao, M-J, and Wu, M-C. Study on biological efficacy of dihydromyricetin. Food Science. (2008) 29:622–5.
115. Zeng, T, Song, Y, Qi, S, Zhang, R, Xu, L, and Xiao, P. A comprehensive review of vine tea: Origin, research on Materia Medica, phytochemistry and pharmacology. J Ethnopharmacol. (2023) 317:116788. doi: 10.1016/j.jep.2023.116788
116. Liu, D, Mao, Y, Ding, L, and Zeng, XA. Dihydromyricetin: a review on identification and quantification methods, biological activities, chemical stability, metabolism and approaches to enhance its bioavailability. Trends Food Sci Technol. (2019) 91:586–97. doi: 10.1016/j.tifs.2019.07.038
117. Li, Y, Yao, J, Han, C, Yang, J, Chaudhry, MT, Wang, S, et al. Quercetin, inflammation and immunity. Nutrients. (2016) 8:167. doi: 10.3390/nu8030167
118. Chen, T, Zhang, X, Zhu, G, Liu, H, Chen, J, Wang, Y, et al. Quercetin inhibits TNF-α induced HUVECs apoptosis and inflammation via downregulating NF-kB and AP-1 signaling pathway in vitro. Medicine. (2020) 99:e22241. doi: 10.1097/MD.0000000000022241
119. Andres, S, Pevny, S, Ziegenhagen, R, Bakhiya, N, Schäfer, B, Hirsch-Ernst, KI, et al. Safety aspects of the use of quercetin as a dietary supplement. Mol Nutr Food Res. (2018) 62. doi: 10.1002/mnfr.201700447
120. Del Bo, C, Perna, S, Allehdan, S, Rafique, A, Saad, S, AlGhareeb, F, et al. Does the Mediterranean diet have any effect on lipid profile, central obesity and liver enzymes in non-alcoholic fatty liver disease (NAFLD) subjects? A systematic review and meta-analysis of randomized control trials. Nutrients. (2023) 15:2250. doi: 10.3390/nu15102250
121. Haigh, L, Kirk, C, El Gendy, K, Gallacher, J, Errington, L, Mathers, JC, et al. The effectiveness and acceptability of Mediterranean diet and calorie restriction in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis. Clin Nutr. (2022) 41:1913–31. doi: 10.1016/j.clnu.2022.06.037
122. Mirabelli, M, Chiefari, E, Arcidiacono, B, Corigliano, DM, Brunetti, FS, Maggisano, V, et al. Mediterranean diet nutrients to turn the tide against insulin resistance and related diseases. Nutrients. (2020) 12:1066. doi: 10.3390/nu12041066
123. Gantenbein, KV, and Kanaka-Gantenbein, C. Mediterranean diet as an antioxidant: the impact on metabolic health and overall wellbeing. Nutrients. (2021) 13. doi: 10.3390/nu13061951
Keywords: non-alcoholic fatty liver disease, dietary polyphenol, network meta-analysis, randomized controlled trials, metabolic indices, catechin
Citation: Wang X-c, Song L and Wang X-h (2025) Efficacy of dietary polyphenol supplement in patients with non-alcoholic fatty liver disease: a network meta-analysis. Front. Nutr. 12:1582861. doi: 10.3389/fnut.2025.1582861
Edited by:
Arpita Mukhopadhyay, St. John’s Research Institute, IndiaReviewed by:
Stefan Kabisch, Charité, University Medicine Berlin, GermanyXiangcheng Fan, Zhejiang University, China
Vlad Pădureanu, University of Medicine and Pharmacy of Craiova, Romania
Copyright © 2025 Wang, Song and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-cui Wang, d2FuZ3hjMTIxNUAxNjMuY29t
 Xiao-cui Wang
Xiao-cui Wang Li Song
Li Song Xin-han Wang
Xin-han Wang