- 1Leonard Davis School of Gerontology, University of Southern California, Los Angeles, CA, United States
- 2Department of Clinical, Internal, Anesthesiologic and Cardiovascular Sciences, Sapienza University of Rome, Rome, Italy
- 3Department of Human, Philosophical and Educational Sciences, University of Salerno, Salerno, Italy
- 4Department of Translational and Precision Medicine, Sapienza University of Rome, Rome, Italy
Background: Insulin-like Growth Factor I (IGF-I) and Vitamin D are crucial for growth and metabolism, with their levels declining with age. However, their mutual interactions and contributions to body composition remain unclear.
Objectives: To examine the relationships between IGF-I, Vitamin D, and body composition in geriatric outpatients, and to test the mediational role of IGF-I in the association between Vitamin D and Fat-Free Mass (FFM).
Methods: One hundred thirty patients were eligible at the Geriatric Outpatient Clinic at the Policlinico Umberto I, Sapienza University of Rome, Italy. Multimorbidity was evaluated with the Cumulative Illness Rating scale for Geriatrics (CIRS-G). Body composition was measured using bioelectrical impedance analysis. Complete blood count, metabolic panel, IGF-I, and 25(OH) Vitamin D were assessed.
Results: Ninety-one patients were included in the analysis. Mean age was 74.4 ± 7.2 years; 50.5% female. Mean BMI was 28 kg/m2 ± 3.9. Mean CIRS-G total score was 14.14 ± 4.1, and Severity Index (SI) was 1.16 ± 0.32. Median IGF-I was 122.0 ng/mL (IQR, 69.8) with higher levels in males compared to females (p = 0.0096). Mean 25(OH) Vitamin D was 27.04 ng/mL ± 14.69 with no significant sex difference. Level of 25(OH) Vitamin D positively correlated with IGF-I (ρ = 0.317, p = 0.003), while no correlation was found between Vitamin D and body composition parameters. Patients with higher IGF-I exhibited higher Total Body Water (TBW) (p = 0.024), Intracellular Water (ICW) (p = 0.018), FFM (p = 0.022), and Muscle Mass (MM) (p = 0.017), Body Cell Mass (BCM) (p = 0.046). Linear regression analysis showed that IGF-I and male sex predicted FFM (B = 13.933, p < 0.001; B = 0.040; p = 0.034; respectively). The mediation analysis confirmed no significant direct effect of Vitamin D on FFM (direct effect, B = −0.058, p = 0.319, 95% CI: −0.175, 0.058); however, the effect was significant when mediated by IGF-I (indirect effect, B = 0.039, SE = 0.022, 95% CI: 0.005, 0.091).
Conclusion: These findings provide further evidence of a positive correlation between IGF-I and lean body mass and suggest that IGF-I may mediate the physiological effect of Vitamin D on FFM, highlighting their potential roles in assessing pre-frailty and personalizing nutrition interventions.
1 Introduction
Aging is a complex phenomenon characterized by the dynamic interplay among multiple biological processes, including alterations in mitochondrial function, loss of proteostasis, and dysregulated nutrient-sensing (1, 2). Over time, these mechanisms contribute to phenotypic changes such as modifications in body composition, which may increase the risk for sarcopenia and frailty (3–6). Reduced physical activity and age-related changes to metabolic and hormonal responses may further promote the loss of muscle mass (7–11). Insulin-like Growth Factor I (IGF-I) is a key regulator of growth and development and plays an essential role in the anabolic pathways (12, 13). IGF-I is primarily produced in the liver, where it mediates the actions of Growth Hormone (GH) (14, 15). Furthermore, IGF-I exerts autocrine and paracrine effects in muscle cells, promoting cell differentiation, survival, and repair (16–18). Upon binding to its transmembrane receptors, which consist of two extracellular α-subunits and two transmembrane β-subunits with intrinsic tyrosine kinase activity, similar to other hormones (19), IGF-I modulates several intracellular signaling cascades (20, 21). The PI3K/Akt and MAPK/ERK pathways modulate protein synthesis (22), muscle hypertrophy (23), and satellite cell activation (24, 25), contributing to muscle homeostasis.
IGF-I levels fluctuate throughout the lifespan, beginning with low concentrations in infancy, peaking during adolescence, and tending to decline in adulthood (26, 27). However, the biological role of IGF-I in the aging process is complex, with its effects on health outcomes influenced by metabolic changes and the presence of chronic illnesses (28–30). For example, low IGF-I has been associated with low HDL cholesterol (31), and reduced insulin sensitivity (32), both of which are known risk factors for cardiovascular disease (CVD) and type 2 diabetes mellitus (T2DM) (33–35). Additionally, low serum IGF-I has been linked to sarcopenia in both animal models and human studies (36, 37). In contrast, higher IGF-I levels have been associated with an increased risk of certain forms of cancer (28, 38).
A large prospective study involving over 7,000 individuals, stratified by age, described a U-shaped relationship between IGF-I levels and the risk of cancer, CVD, and all-cause mortality, suggesting that both low and high circulating IGF-I levels may increase the risk of such conditions (39). IGF-I levels are also influenced by factors such as physical activity and nutrient intake, further contributing to variability in research findings (40–44). In this regard, previous studies suggest a correlation between Vitamin D and IGF-I (45–48), although randomized controlled trials have yielded inconsistent results (49–51). Furthermore, the mechanisms underlying these observations remain elusive. Indeed, hepatocytes do not consistently express the nuclear Vitamin D receptor (VDR), while non-parenchymal liver cells have been shown to express VDR (52), suggesting a potential role of Vitamin D in modulating IGF-I bioavailability (53). Moreover, evidence suggests that IGF-I may stimulate the enzyme 1-α-hydroxylase in the kidneys, which converts 25-hydroxyvitamin D (25 OH Vitamin D) into its active form, calcitriol (1,25-dihydroxyvitamin D) (54, 55). In addition to the described effect of IGF-I on body composition, Vitamin D deficiency has been associated with depressive symptoms (56), frailty (57), muscle weakness (58), and an increased risk for falls (59, 60). Given the age-associated decline of both IGF-I and Vitamin D (27, 61), this study aimed to (i) examine the relationships between IGF-I, Vitamin D, and parameters of body composition; (ii) explore the clinical variables associated with IGF-I levels; and (iii) test IGF-I as a mediator in the relationship between Vitamin D and FFM in geriatric outpatients.
2 Materials and methods
2.1 Study design and criteria
This cross-sectional study was conducted at the Geriatric Outpatient Clinic at Policlinico Umberto I University Hospital, Sapienza University of Rome, Italy. Participants with the following criteria were considered eligible: age ≥65 years, written informed consent to participate in the study, no contraindications to body composition analysis according to the device manufacturer instructions, and independence in both the activities of daily living (ADL) and the instrumental activities of daily living (IADL). Exclusion criteria were: a body mass index (BMI) > 40 kg/m2, a history of alcohol misuse (more than 7 standard drinks per week for women and more than 14 standard drinks per week for men), alterations in liver enzymes such as alanine aminotransferase (ALT) and aspartate transaminase (AST) beyond the normal reference range, an estimated Glomerular Filtration Rate (eGFR) < 50 mL/min/1.73 m2, reported weight fluctuation in the past 3 months (>10% of total body weight), poorly controlled T2DM or individuals receiving insulin therapy, and history of cancer treated with radiation or chemotherapy in the past 5 years. Patients with acute medical conditions were excluded. A total of 130 patients were consecutively enrolled. IGF-I measurements were completed for 92 patients due to an unexpected shortage of reagents in the laboratories. One patient was excluded for not meeting the study criteria, leading to 91 patients in the final analysis.
2.2 Assessments
A Comprehensive Geriatric Assessment (CGA) was performed for each participant, which included a detailed clinical history, physical examination, electrocardiogram, and a review of all relevant diagnostics. The Cumulative Illness Rating Scale for Geriatrics (CIRS-G) was then completed and reviewed by two physicians. Any discrepancies in the CIRS-G scores were resolved with a third physician. For the analysis, the CIRS-G was calculated excluding the psychiatric item (item 14), resulting in a 13-item scale. Physical activity levels were evaluated using the self-reported Physical Activity Scale for the Elderly (PASE). All patients provided their informed consent, and the study was approved by the local board of Sapienza University of Rome and conformed to the ethical guidelines of the Declaration of Helsinki.
2.2.1 Body composition
After the initial visit, enrolled participants returned to the clinic for anthropometric measurements (i.e., weight, height, and waist circumference). Body composition was assessed using bioimpedance analysis (BIA 101, AKERN, Italy), conducted between 8:30 a.m. and 11:00 a.m. with participants fasted overnight. BIA was performed following a 5-min rest in the supine position, with the participant’s upper limbs abducted at a 30° angle and lower limbs at a 45° angle. Bioelectrical data and body composition parameters were then collected, and reported as follows: Total Body Water (TBW) in liters, Intra- and Extracellular Water (ICW and ECW, respectively) in liters, Fat-Free mass (FFM) in kilograms, Muscle Mass (MM) in kilograms, Body Cell Mass (BCM) in kilograms, and Fat Mass (FM) in kilograms.
2.2.2 Blood tests and biomarkers
Blood samples were collected from the participants and sent to a local laboratory. All samples were handled and analyzed according to standard practices for measuring hematological parameters, including complete blood count, serum electrolytes, metabolic panel, albumin, folate, Vitamin B12, 25(OH) Vitamin D, IGF-I, and C-reactive protein (CRP).
2.3 Statistical analysis
Statistical analysis was performed using SPSS software (version 29.0.2; IBM, Armonk, NY, United States). Descriptive statistics were used to summarize the data. Categorical variables were reported as frequencies or percentages, while continuous variables were presented as means and standard deviations (SD) or medians and interquartile ranges (IQR), depending on the distribution of the data. Spearman’s rank correlation was used to assess associations between variables. To compare numerical data between groups, either Student’s t-test or the Mann–Whitney U test was used, based on the normality of the data distribution. All tests were two-tailed, and a p-values less than 0.05 was considered statistically significant. To investigate the factors independently associated with FFM, a multivariate linear regression model was constructed using the variables of interest. Prior to analysis, the fundamental assumptions of linear regression were assessed. To test the hypothesis of IGF-I as a mediator in the relationship between Vitamin D and FFM, a mediation analysis was conducted using the model described by Preacher and Hayes (62). Specifically, we employed Model 4 of the SPSS PROCESS Macro to test the indirect effect of the independent variable [25(OH) Vitamin D] on the dependent variable (FFM) through the mediator (IGF-I) (63). The analysis utilized 5,000 bootstrap samples to estimate the indirect effect and construct 95% confidence intervals (CIs) for the mediation effect. Original figures were prepared using GraphPad Prism (version 10.4.1 for Mac, GraphPad Software, Boston, Massachusetts, United States) and BioRender.
3 Results
3.1 Patient characteristics
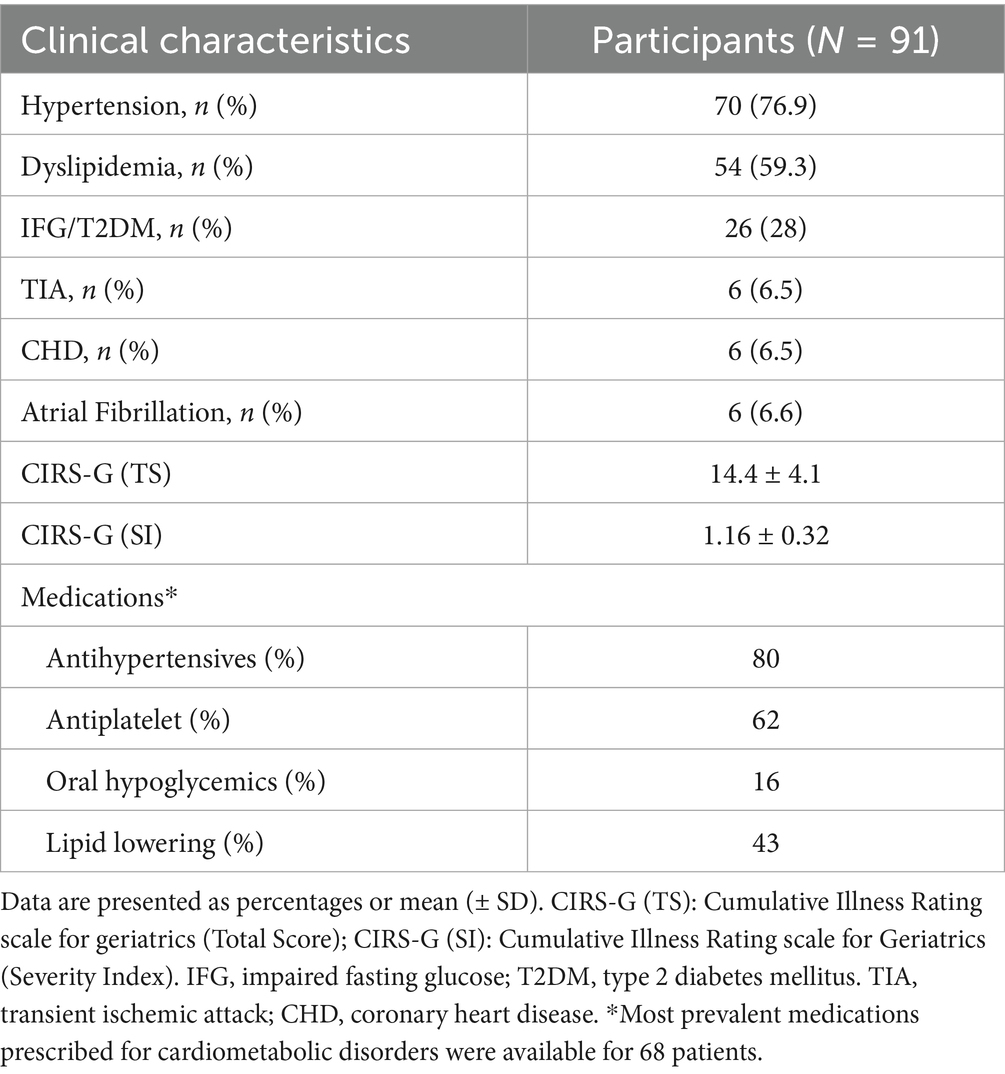
The analysis included 91 patients (50.5% women). The mean age of participants was 74.4 ± 7.2 years, and the mean BMI was 28.0 ± 3.9 kg/m2. The prevalence of hypertension was 76.9%, while dyslipidemia and Impaired Fasting Glucose (IFG)/T2DM were present in 59.3 and 28% of the participants, respectively. Atrial fibrillation, Coronary Heart Disease (CHD), and history of Transient Ischemic Attacks (TIA) were each present in 6.5% of participants. The mean CIRS-G score was 14.4 ± 4.1 while the severity index was 1.16 ± 0.32, suggesting a moderate level of multimorbidity. Clinical characteristics of patients are reported in Tables 1, 2.
3.2 Relationship between IGF-I, vitamin D, and parameters of body composition
The median IGF-I level was 122.0 ng/mL (IQR, 69.8), while the mean 25(OH) Vitamin D level was 27.04 ng/mL (±14.69 SD). IGF-I levels were significantly higher in males compared to females (p = 0.0096), whereas no significant sex differences were observed for the 25(OH) Vitamin D (Figures 1A,B). Levels of 25(OH) Vitamin D positively correlated with IGF-I (ρ = 0.317, p = 0.003) (Figure 1C), while no significant correlations were found between 25(OH) Vitamin D and body composition parameters. As expected, parameters of muscularity were higher in males compared to females (ICW: p < 0.001; FFM: p < 0.001; MM: p < 0.001; BCM: p < 0.001), while no significant sex differences were observed for FM (p = 0.272). In an exploratory sex-stratified analysis, a positive trend was observed between IGF-I and BCM in females only (ρ = 0.256, p = 0.086). Additionally, a direct correlation between FM and CRP was found in the overall cohort (ρ = 0.387, p < 0.001).
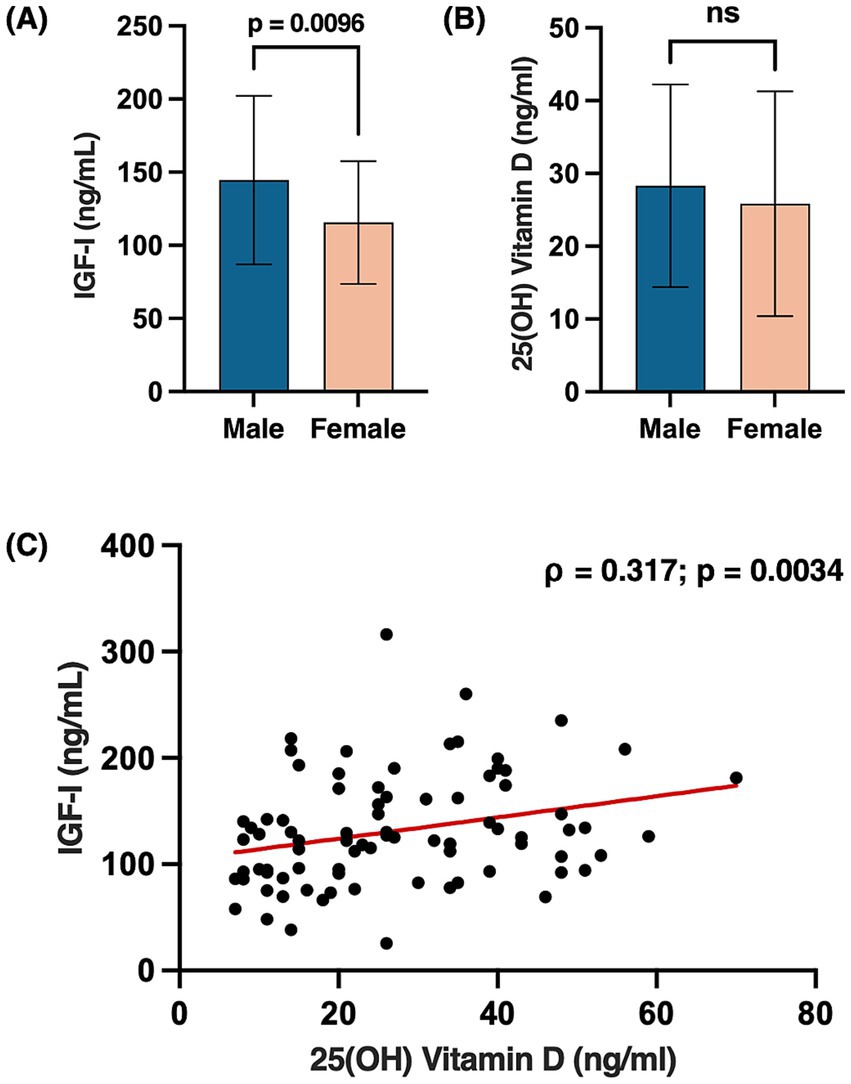
Figure 1. Sex differences in circulating levels of IGF-I (A) and 25(OH) Vitamin D (B). Bars represent mean ± SD. Scatter plot showing the Spearman correlation between 25(OH) Vitamin D and IGF-I levels, with a fitted regression line (C); p-values < 0.05 were considered statistically significant. Created with BioRender.com. Vicinanza, R. (2025) https://BioRender.com/l25t663.
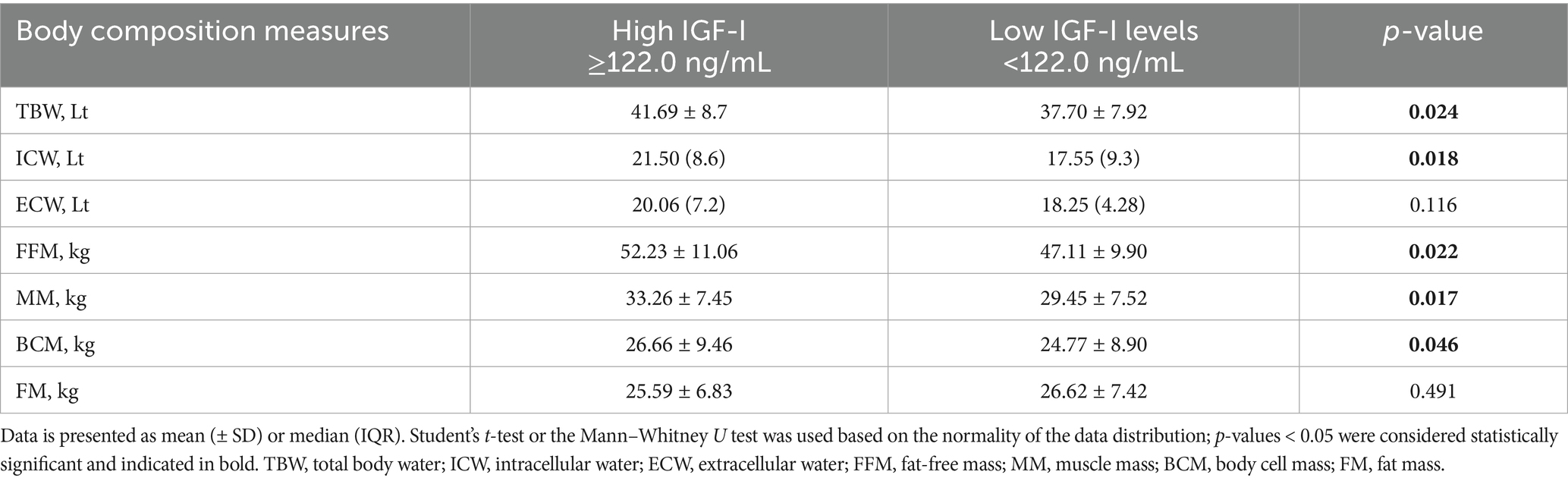
Given the lack of consistent sex-specific associations between IGF-I and parameters of muscularity, the cohort was subsequently dichotomized based on the median IGF-I level, resulting in two subgroups: low (IGF-I ≤ 122.0 ng/mL) and high (IGF-I > 122.0 ng/mL). As summarized in Table 3, significant clinical differences between the two groups were observed for number of males (high IGF-I vs. low IGF-I, 29 vs. 18; p = 0.017) and 25(OH) Vitamin D levels (high IGF-I vs. low IGF-I, 30.37 ± 15.04 vs. 23.45 ± 2.14 ng/mL; p = 0.031).
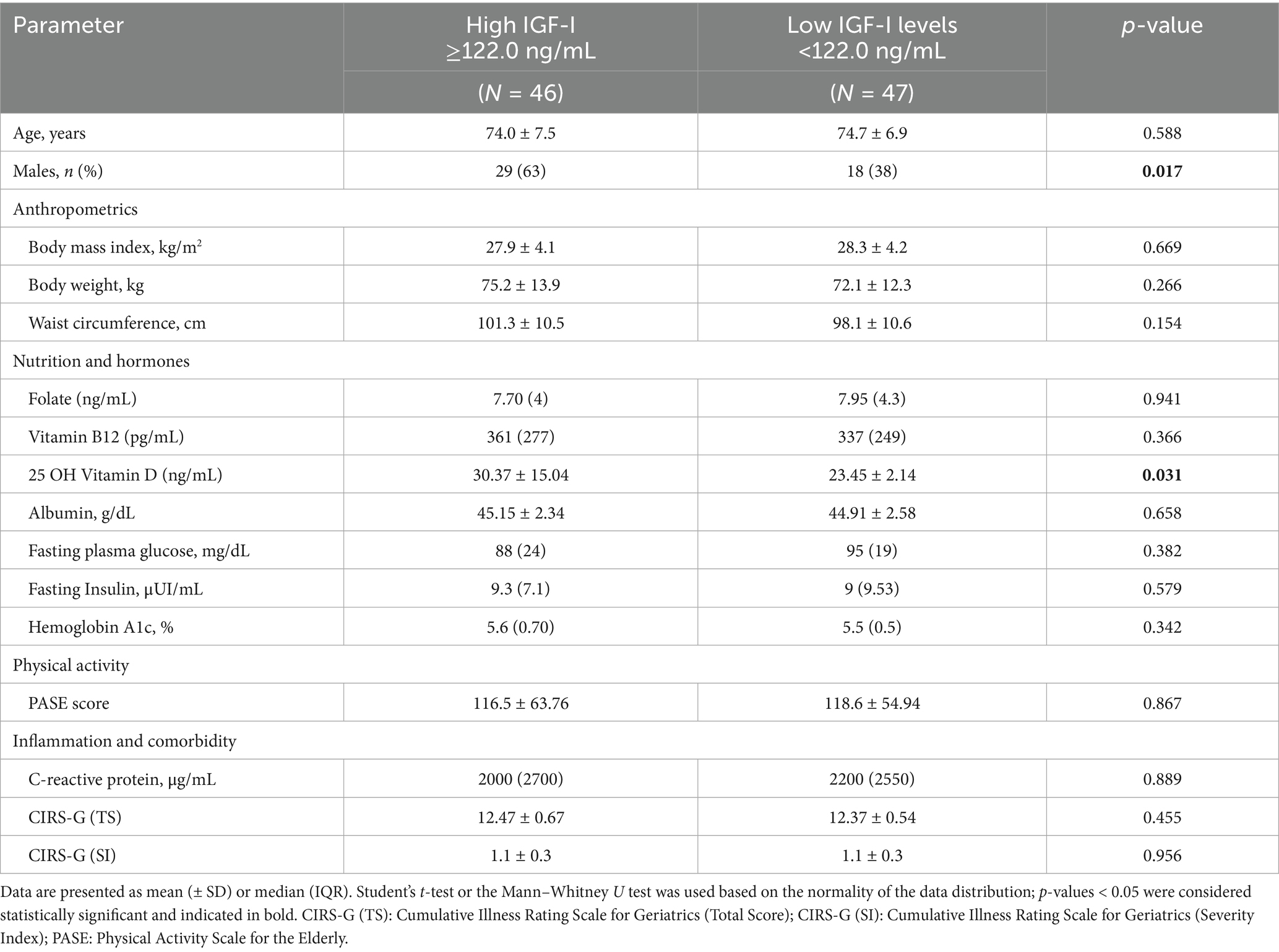
Table 3. Clinical variables according to high (≥122.0 ng/mL) and low (<122.0 ng/mL) levels of IGF-I.
In terms of body composition, patients with higher IGF-I exhibited significantly greater values, compared to low IGF-I, for the following parameters: TBW (41.69 ± 8.7 Lt vs. 37.70 ± 7.92 Lt; p = 0.024), ICW (21.50 [IQR, 8.6] Lt vs. 17.55 [IQR, 9.3] Lt; p = 0.018), FFM (52.23 ± 11.06 kg vs. 47.11 ± 9.90 kg; p = 0.022), MM (33.26 ± 7.45 kg vs. 29.45 ± 7.52 kg, p = 0.017) and BCM (26.66 ± 9.46 kg vs. 24.77 ± 8.90 kg; p = 0.046). No significant difference in FM was observed between the two groups (Table 4).
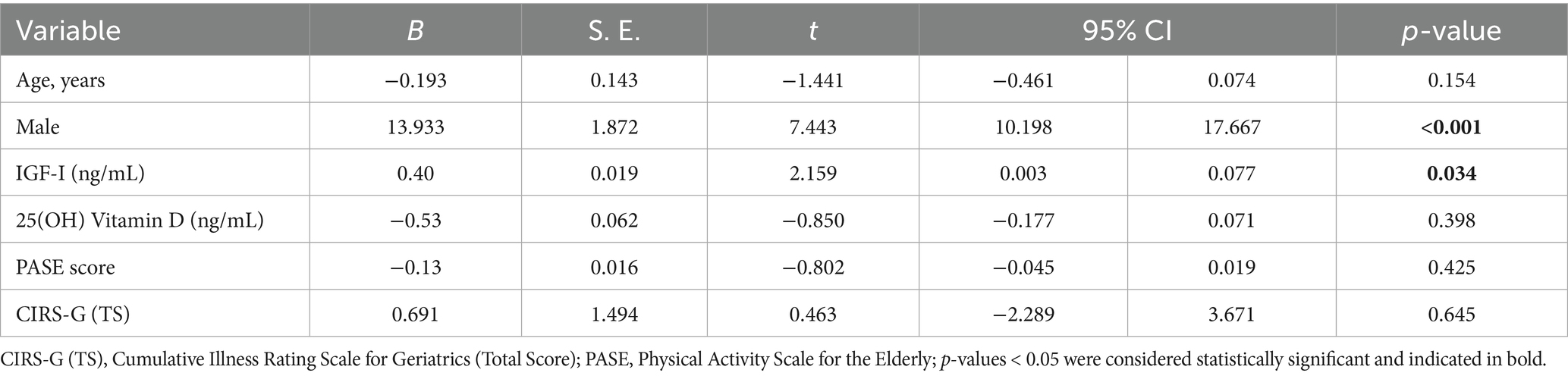
Linear regression analysis was performed to identify factors independently associated with FFM. The analysis revealed that IGF-I levels (B = 0.40, p = 0.034) and male sex (B = 13.933, p < 0.001) were independently and significantly associated with FFM. No significant associations were found for age (p = 0.154), 25(OH) Vitamin D (p = 0.398), levels of physical activity (PASE score) (p = 0.425), and level of multimorbidity (CIRS-G score) (p = 0.645) (Table 5).
3.3 Mediation analysis
Although our analysis found no significant associations between Vitamin D and FFM, we hypothesized that the effect of Vitamin D on FFM could be mediated by IGF-I. To test this hypothesis, we conducted a mediation analysis, using 25(OH) Vitamin D as the independent variable, FFM as the dependent variable, and IGF-I as the mediator, adjusting for age and sex. The results of the mediation analysis showed a significant positive effect of Vitamin D on IGF-I (B = 0.951; SE = 0.374, p = 0.013). In turn, IGF-I was positively associated with FFM (B = 0.041; SE = 0.017, p = 0.017). As expected, the direct effect of Vitamin D on FFM was not significant (B = −0.058; SE = 0.058, p = 0.319), nor was the total effect of Vitamin D on FFM (B = −0.019; SE = 0.058, 95% CI: −0.134, 0.096, p = 0.738). However, the indirect effect of Vitamin D on FFM through IGF-I was significant (B = 0.039; SE = 0.022, 95% CI: 0.005, 0.091) with male sex being a significant covariate in the model (B = 12.555, p < 0.001). Results of the mediation analysis are reported in Figure 2, integrated with the associated graphical representation of the theoretical framework.
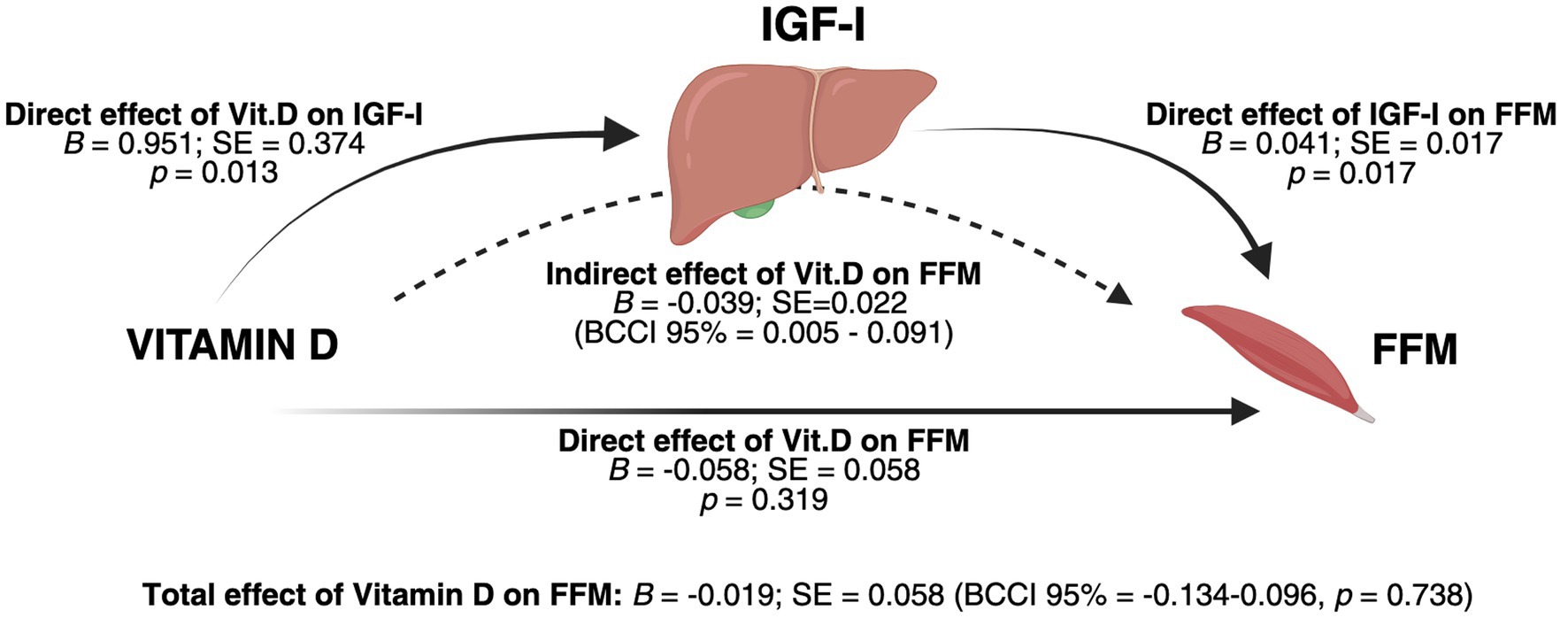
Figure 2. Integrated graphical representation of the mediation model and conceptual framework. Results of the mediation analysis were obtained after controlling for age and sex. The analysis revealed a significant indirect effect of 25(OH) Vitamin D on fat-free mass (FFM) through IGF-I as a mediator, suggesting a physiological regulatory pathway in which Vitamin D influences FFM by modulating IGF-I in the liver. Male sex was a significant covariate in the model (B = 12.555, p < 0.001). BCCI: Bias-corrected confidence intervals; SE = Standard Error. Created with BioRender.com. Vicinanza, R. (2025) https://BioRender.com/c27j696.
4 Discussion
The present study investigated the relationships between IGF-I, Vitamin D, and body composition parameters in geriatric outpatients with moderate multimorbidity. Our findings showed that (i) IGF-I positively and significantly correlated with Vitamin D, (ii) higher IGF-I, but not Vitamin D, was significantly associated with parameters of muscularity, and along with male sex, was an independent predictor of FFM, (iii) IGF-I was an indirect mediator of the effect of Vitamin D on FFM, although no significant direct or total effects were found.
The observed relationship between Vitamin D and IGF-I was consistent with previous findings (46, 64–66), including a Mendelian randomization study demonstrating a positive association between IGF-I and 25(OH) Vitamin D levels (45). Additionally, a 12-week intervention study with oral Vitamin D3 supplementation resulted in a dose-dependent increase of IGF-I in adults (67), and similar results were found with 6-month supplementation in overweight patients (68). However, a systematic review and meta-analysis of clinical trials yielded mixed results (49). In animal models, VDR knockout mice exhibited reduced IGF-I levels (69), although the precise mechanisms through which Vitamin D may modulate IGF-I production and bioavailability remain unclear (70). In vitro studies suggested that non-parenchymal liver cells (e.g., stellate cells, sinusoidal endothelial cells), rather than hepatocytes, express the VDR (52). In turn, the binding of the VDR to the Vitamin D Responsive Element (VDRE) has been shown to activate the promoter regions of the Insulin-like Growth Factor Binding Protein 3 (IGFBP-3) gene, the primary binding protein for IGF-I, in several cell types (71, 72). On the other hand, IGF-I has been shown to stimulate 1α-hydroxylase enzyme in renal cells, while treatment with IGF-I has been reported to significantly increase Vitamin D levels in healthy individuals (55). Taken together, these studies support the existence of a mutual nutrient-hormone interaction that should be considered in clinical practice, particularly in the assessment of Vitamin D deficiency, opening new windows of opportunity to explore treatment strategies.
Regarding the second aim of the study, consistent with previous observations (10, 37), we found that higher IGF-I levels were associated with TBW, ICW, MM, BCM and FFM. Supporting the role of IGF-I in maintaining healthy body composition, particularly in older adults, the InChianti study demonstrated that lower IGF-I concentrations were associated with an increased risk of sarcopenia (73). Other studies have shown that reduced serum concentrations of IGF-I correlated with increased frailty measures (74), decreased functional outcomes (75–78), and a reduced number of motor units (79). However, the exploratory analysis of variables found no significant association between IGF-I and the PASE scores, likely due to the low levels of physical activity within the cohort (80, 81). As expected, CRP was associated with FM (82, 83).
The relationship between IGF-I and clinical outcomes is complex, as both low and high IGF-I concentrations have been associated with increased risk of cancer, CVD, and all-cause mortality (39, 84). Rahmani et al. suggested that IGF-I levels between 120 and 160 ng/mL were associated with positive clinical outcomes and reduced mortality risk (85). Notably, consistent with this observation, in our cohort the median IGF-I value was 122 ng/mL, and no significant associations were found between IGF-I and levels of multimorbidity evaluated with the CIRS-G. Moreover, only participants with no history of cancer in the past 5 years were included, allowing this research to focus primarily on the role of IGF-I on body composition.
Although no associations were found between Vitamin D and parameters of muscularity in our cohort, such evidence has been observed in both human studies and animal models (24, 58, 86, 87). Therefore, we hypothesized a nutrient-hormone interaction where Vitamin D stimulates IGF-I in the liver, which in turn could mediate the effect of Vitamin D on FFM. Using a mediation analysis, previously implemented in the context of multimorbidity (88), we found that IGF-I was an indirect-only mediator for the effect of Vitamin D on FFM, while no significant direct or total effects were observed. Although speculative, these findings suggest a potential regulatory pathway through which Vitamin D may influence lean body mass via IGF-I. Thus, considering this pathway in the clinical setting could further support the evaluation of nutritional status and the assessment of pre-frailty.
Regarding the methods used for this statistical model, we acknowledge that in a typical mediation analysis, a significant relationship between the independent and dependent variable is generally considered a prerequisite for examining the indirect effect (89). Nevertheless, according to Rucker et al. and Preacher and Hayes, the absence of a significant direct or total effect does not preclude the investigation into the indirect effect if the theoretical framework or the rationale behind the analysis supports its plausibility (90, 91). Therefore, considering the described roles of both IGF-I and Vitamin D on lean body mass, we developed an integrated model to illustrate the mediation analysis results alongside the proposed physiological regulatory pathway that may underlie the observed associations (Figure 2).
However, this study has several limitations. First, the cross-sectional design did not allow for the determination of causality or the directionality of the observed associations. Second, the relatively small sample size constrained our ability to perform statistically robust stratifications by age and sex, limiting further investigation of subgroup differences without the loss of statistical power. Consequently, we conducted exploratory sex-stratified analyses with a limited number of participants and low intra-group variance, which may reduce the generalizability and reproducibility of our findings compared to larger studies. Furthermore, although free IGF-I is widely used to measure its serum levels (92), a more comprehensive evaluation of IGF-I bioavailability should also include IGFBP-3. Another limitation is the reliance on a self-administered questionnaire to evaluate physical activity levels, although we inferred the physical function of participants from indices of muscularity, measured with the BIA, and combined with assessment of ADLs and IADLs. Additionally, dietary intake was only partially collected with medical history, and more detailed information about the intake of macronutrients should be addressed. On the other hand, when the participants were divided by IGF-I levels, no significant clinical differences were found between the two groups aside from the variables of interest, indicating a relatively homogeneous sample. Moreover, examining the role of IGF-I in patients with multimorbidity offered a more real-life approach, particularly in geriatric medicine, since research in this population is often underrepresented.
In conclusion, these findings provide additional evidence of (i) a positive correlation between IGF-I and Vitamin D and (ii) the positive effect of IGF-I on parameters of muscularity. Finally, the mediation model suggests that IGF-I may contribute to the physiological effect of Vitamin D on FFM, with possible implications for assessing pre-frailty and personalizing nutrition interventions in this demographic.
Further research is needed to explore the underlying mechanisms and establish causality of these relationships.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics committee of the Policlinico Umberto I Hospital, Sapienza University of Rome. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
RV: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. AF: Data curation, Investigation, Writing – review & editing. JP: Formal analysis, Writing – original draft, Writing – review & editing. VM: Data curation, Investigation, Writing – review & editing. MM: Investigation, Writing – review & editing. GI: Formal analysis, Writing – review & editing. PP: Writing – review & editing. EE: Writing – review & editing. MB: Investigation, Methodology, Writing – review & editing. AM: Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors acknowledge Flaminia Crisciotti, Sciaila Bernardini, Elisa D’Ottavio, for their contributions to the organization and collection of clinical data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. López-Otín, C, Blasco, MA, Partridge, L, Serrano, M, and Kroemer, G. Hallmarks of aging: an expanding universe. Cell. (2023) 186:243–78. doi: 10.1016/j.cell.2022.11.001
2. Tenchov, R, Sasso, JM, Wang, X, and Zhou, QA. Aging hallmarks and progression and age-related diseases: a landscape view of research advancement. ACS Chem Neurosci. (2024) 15:1–30. doi: 10.1021/acschemneuro.3c00531
3. Larsson, L, Degens, H, Li, M, Salviati, L, Lee, Y, Thompson, W, et al. Sarcopenia: aging-related loss of muscle mass and function. Physiol Rev. (2019) 99:427–511. doi: 10.1152/physrev.00061.2017
4. Roubenoff, R. Sarcopenia: effects on body composition and function. J Gerontol A Biol Sci Med Sci. (2003) 58:M1012–7. doi: 10.1093/gerona/58.11.M1012
5. Billot, M, Calvani, R, Urtamo, A, Sánchez-Sánchez, JL, Ciccolari-Micaldi, C, Chang, M, et al. Preserving mobility in older adults with physical frailty and sarcopenia: opportunities, challenges, and recommendations for physical activity interventions. Clin Interv Aging. (2020) 15:1675–90. doi: 10.2147/CIA.S253535
6. Zhou, H, Ding, X, and Luo, M. The association between sarcopenia and functional disability in older adults. J Nutr Health Aging. (2024) 28:100016. doi: 10.1016/j.jnha.2023.100016
7. Goodpaster, BH, Park, SW, Harris, TB, Kritchevsky, SB, Nevitt, M, Schwartz, AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. (2006) 61:1059–64. doi: 10.1093/gerona/61.10.1059
8. Hajek, A, and König, HH. Longitudinal predictors of functional impairment in older adults in Europe – evidence from the survey of health, ageing and retirement in Europe. PLoS One. (2016) 11:e0146967. doi: 10.1371/journal.pone.0146967
9. Palmer, AK, and Jensen, MD. Metabolic changes in aging humans: current evidence and therapeutic strategies. J Clin Invest. (2022) 132:e158451. doi: 10.1172/JCI158451
10. Baumgartner, RN, Waters, DL, Gallagher, D, Morley, JE, and Garry, PJ. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev. (1999) 107:123–36. doi: 10.1016/S0047-6374(98)00130-4
11. Houston, DK, Nicklas, BJ, Ding, J, Harris, TB, Tylavsky, FA, Newman, AB, et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the health, aging, and body composition (health ABC) study. Am J Clin Nutr. (2008) 87:150–5. doi: 10.1093/ajcn/87.1.150
12. Barclay, RD, Burd, NA, Tyler, C, Tillin, NA, and Mackenzie, RW. The role of the IGF-1 signaling cascade in muscle protein synthesis and anabolic resistance in aging skeletal muscle. Front Nutr. (2019) 6:146. doi: 10.3389/fnut.2019.00146
13. Velloso, CP. Regulation of muscle mass by growth hormone and IGF-I. Br J Pharmacol. (2008) 154:557–68. doi: 10.1038/bjp.2008.153
14. Ohlsson, C, Mohan, S, Sjögren, K, Tivesten, Å, Isgaard, J, Isaksson, O, et al. The role of liver-derived insulin-like growth factor-I. Endocr Rev. (2009) 30:494. doi: 10.1210/er.2009-0010
15. Takahashi, Y. The role of growth hormone and insulin-like growth factor-I in the liver. Int J Mol Sci. (2017) 18:1447. doi: 10.3390/ijms18071447
16. Jennische, E, and Hansson, HA. Regenerating skeletal muscle cells express insulin-like growth factor I. Acta Physiol Scand. (1987) 130:327–32. doi: 10.1111/j.1748-1716.1987.tb08144.x
17. Canicio, J, Gallardo, E, Illa, I, Testar, X, Palacín, M, Zorzano, A, et al. p70 S6 kinase activation is not required for insulin-like growth factor-induced differentiation of rat, mouse, or human skeletal muscle cells. Endocrinology. (1998) 139:5042–9. doi: 10.1210/endo.139.12.6360
18. Coolican, SA, Samuel, DS, Ewton, DZ, McWade, FJ, and Florini, JR. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J Biol Chem. (1997) 272:6653–62. doi: 10.1074/jbc.272.10.6653
19. Vicinanza, R, Coppotelli, G, Malacrino, C, Nardo, T, Buchetti, B, Lenti, L, et al. Oxidized low-density lipoproteins impair endothelial function by inhibiting non-genomic action of thyroid hormone–mediated nitric oxide production in human endothelial cells. Thyroid. (2013) 23:231–8. doi: 10.1089/thy.2011.0524
20. Laviola, L, Natalicchio, A, and Giorgino, F. The IGF-I signaling pathway. Curr Pharm Des. (2007) 13:663–9. doi: 10.2174/138161207780249146
21. Hakuno, F, and Takahashi, SI. 40 years of IGF1: IGF1 receptor signaling pathways. (2018). Available online at: https://jme.bioscientifica.com/view/journals/jme/61/1/JME-17-0311.xml (Accessed September 30, 2024).
22. Ahmad, SS, Ahmad, K, Lee, EJ, Lee, YH, and Choi, I. Implications of insulin-like growth Factor-1 in skeletal muscle and various diseases. Cells. (2020) 9:1773. doi: 10.3390/cells9081773
23. Yoshida, T, and Delafontaine, P. Mechanisms of IGF-1-mediated regulation of skeletal muscle hypertrophy and atrophy. Cells. (2020) 9:1970. doi: 10.3390/cells9091970
24. Barton-Davis, ER, Shoturma, DI, and Sweeney, HL. Contribution of satellite cells to IGF-I induced hypertrophy of skeletal muscle. Acta Physiol Scand. (1999) 167:301–5. doi: 10.1046/j.1365-201x.1999.00618.x
25. Clemmons, DR. Role of IGF-I in skeletal muscle mass maintenance. Trends Endocrinol Metab. (2009) 20:349–56. doi: 10.1016/j.tem.2009.04.002
26. Löfqvist, C, Andersson, E, Gelander, L, Rosberg, S, Blum, WF, and Wikland, KA. Reference values for IGF-I throughout childhood and adolescence: a model that accounts simultaneously for the effect of gender, age, and puberty. J Clin Endocrinol Metab. (2001) 86:5870–6. doi: 10.1210/jcem.86.12.8117
27. Janssen, JAMJL. IGF-I and the endocrinology of aging. Curr Opin Endocr Metab Res. (2019) 5:1–6. doi: 10.1016/j.coemr.2018.11.001
28. Svensson, J, Carlzon, D, Petzold, M, Karlsson, MK, Ljunggren, Ö, Tivesten, Å, et al. Both low and high serum IGF-I levels associate with Cancer mortality in older men. J Clin Endocrinol Metab. (2012) 97:4623–30. doi: 10.1210/jc.2012-2329
29. Hawkes, CP, and Grimberg, A. Insulin-like growth factor-I is a marker for the nutritional state. Pediatr Endocrinol Rev. (2015) 13:499–511.
30. Milman, S, Huffman, DM, and Barzilai, N. The Somatotropic Axis in human aging: framework for the current state of knowledge and future research. Cell Metab. (2016) 23:980–9. doi: 10.1016/j.cmet.2016.05.014
31. Liang, S, Hu, Y, Liu, C, Qi, J, and Li, G. Low insulin-like growth factor 1 is associated with low high-density lipoprotein cholesterol and metabolic syndrome in Chinese nondiabetic obese children and adolescents: a cross-sectional study. Lipids Health Dis. (2016) 15:112. doi: 10.1186/s12944-016-0275-7
32. Succurro, E, Andreozzi, F, Marini, MA, Lauro, R, Hribal, ML, Perticone, F, et al. Low plasma insulin-like growth factor-1 levels are associated with reduced insulin sensitivity and increased insulin secretion in nondiabetic subjects. Nutr Metab Cardiovasc Dis. (2009) 19:713–9. doi: 10.1016/j.numecd.2008.12.011
33. van Bunderen, CC, van Nieuwpoort, IC, van Schoor, NM, Deeg, DJH, Lips, P, and Drent, ML. The Association of Serum Insulin-like Growth Factor-I with mortality, cardiovascular disease, and Cancer in the elderly: a population-based study. J Clin Endocrinol Metab. (2010) 95:4616–24. doi: 10.1210/jc.2010-0940
34. Sanders, JL, Guo, W, O’Meara, ES, Kaplan, RC, Pollak, MN, Bartz, TM, et al. Trajectories of IGF-I predict mortality in older adults: the cardiovascular health study. J Gerontol A Biol Sci Med Sci. (2018) 73:953–9. doi: 10.1093/gerona/glx143
35. Kubo, H, Sawada, S, Satoh, M, Asai, Y, Kodama, S, Sato, T, et al. Insulin-like growth factor-1 levels are associated with high comorbidity of metabolic disorders in obese subjects; a Japanese single-center, retrospective-study. Sci Rep. (2022) 12:20130. doi: 10.1038/s41598-022-23521-1
36. Ascenzi, F, Barberi, L, Dobrowolny, G, Villa Nova Bacurau, A, Nicoletti, C, Rizzuto, E, et al. Effects of IGF-1 isoforms on muscle growth and sarcopenia. Aging Cell. (2019) 18:e12954. doi: 10.1111/acel.12954
37. Bian, A, Ma, Y, Zhou, X, Guo, Y, Wang, W, Zhang, Y, et al. Association between sarcopenia and levels of growth hormone and insulin-like growth factor-1 in the elderly. BMC Musculoskelet Disord. (2020) 21:214. doi: 10.1186/s12891-020-03236-y
38. Knuppel, A, Fensom, GK, Watts, EL, Gunter, MJ, Murphy, N, Papier, K, et al. Circulating insulin-like growth factor-I concentrations and risk of 30 cancers: prospective analyses in UK biobank. Cancer Res. (2020) 80:4014–21. doi: 10.1158/0008-5472.CAN-20-1281
39. Mukama, T, Srour, B, Johnson, T, Katzke, V, and Kaaks, R. IGF-1 and risk of morbidity and mortality from Cancer, cardiovascular diseases, and all causes in EPIC-Heidelberg. J Clin Endocrinol Metab. (2023) 108:e1092–105. doi: 10.1210/clinem/dgad212
40. Kim, JH, Kim, SJ, Lee, J, Shin, CH, and Seo, JY. Factors affecting IGF-I level and correlation with growth response during growth hormone treatment in LG growth study. PLoS One. (2021) 16:e0252283. doi: 10.1371/journal.pone.0252283
41. Maggio, M, De Vita, F, Lauretani, F, Buttò, V, Bondi, G, Cattabiani, C, et al. IGF-1, the cross road of the nutritional, inflammatory and hormonal pathways to frailty. Nutrients. (2013) 5:4184–205. doi: 10.3390/nu5104184
42. Moran, S, Chen, Y, Ruthie, A, and Nir, Y. Alterations in IGF-I affect elderly: role of physical activity. Eur Rev Aging Phys Act. (2007) 4:77–84. doi: 10.1007/s11556-007-0022-1
43. Watts, EL, Perez-Cornago, A, Appleby, PN, Albanes, D, Ardanaz, E, Black, A, et al. The associations of anthropometric, behavioural and sociodemographic factors with circulating concentrations of IGF-I, IGF-II, IGFBP-1, IGFBP-2 and IGFBP-3 in a pooled analysis of 16,024 men from 22 studies. Int J Cancer. (2019) 145:3244–56. doi: 10.1002/ijc.32276
44. Arturi, F, Succurro, E, Procopio, C, Pedace, E, Mannino, GC, Lugarà, M, et al. Nonalcoholic fatty liver disease is associated with low circulating levels of insulin-like growth factor-I. J Clin Endocrinol Metab. (2011) 96:E1640–4. doi: 10.1210/jc.2011-1227
45. Gou, Z, Li, F, Qiao, F, Maimaititusvn, G, and Liu, F. Causal associations between insulin-like growth factor 1 and vitamin D levels: a two-sample bidirectional Mendelian randomization study. Front Nutr. (2023) 10:1162442. doi: 10.3389/fnut.2023.1162442
46. Bogazzi, F, Rossi, G, Lombardi, M, Tomisti, L, Sardella, C, Manetti, L, et al. Vitamin D status may contribute to serum insulin-like growth factor I concentrations in healthy subjects. J Endocrinol Investig. (2011) 34:e200–3. doi: 10.3275/7228
47. Ciresi, A, and Giordano, C. Vitamin D across growth hormone (GH) disorders: from GH deficiency to GH excess. Growth Hormon IGF Res. (2017) 33:35–42. doi: 10.1016/j.ghir.2017.02.002
48. Gómez, JM, Maravall, FJ, Gómez, N, Navarro, MA, Casamitjana, R, and Soler, J. Relationship between 25-(OH) D3, the IGF-I system, leptin, anthropometric and body composition variables in a healthy, randomly selected population. Horm Metab Res. (2004) 36:48–53. doi: 10.1055/s-2004-814103
49. Kord-Varkaneh, H, Rinaldi, G, Hekmatdoost, A, Fatahi, S, Tan, SC, Shadnoush, M, et al. The influence of vitamin D supplementation on IGF-1 levels in humans: a systematic review and meta-analysis. Ageing Res Rev. (2020) 57:100996. doi: 10.1016/j.arr.2019.100996
50. Meshkini, F, Abdollahi, S, Clark, CCT, and Soltani, S. The effect of vitamin D supplementation on insulin-like growth factor-1: a systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. (2020) 50:102300. doi: 10.1016/j.ctim.2020.102300
51. Trummer, C, Schwetz, V, Pandis, M, Grübler, MR, Verheyen, N, Gaksch, M, et al. Effects of vitamin D supplementation on IGF-1 and calcitriol: a randomized-controlled trial. Nutrients. (2017) 9:623. doi: 10.3390/nu9060623
52. Gascon-Barré, M, Demers, C, Mirshahi, A, Néron, S, Zalzal, S, and Nanci, A. The normal liver harbors the vitamin D nuclear receptor in non-parenchymal and biliary epithelial cells. Hepatology. (2003) 37:1034–42. doi: 10.1053/jhep.2003.50176
53. Moreno-Santos, I, Castellano-Castillo, D, Lara, MF, Fernandez-Garcia, JC, Tinahones, FJ, and Macias-Gonzalez, M. IGFBP-3 interacts with the vitamin D receptor in insulin signaling associated with obesity in visceral adipose tissue. Int J Mol Sci. (2017) 18:2349. doi: 10.3390/ijms18112349
54. Kamenický, P, Mazziotti, G, Lombès, M, Giustina, A, and Chanson, P. Growth hormone, insulin-like growth Factor-1, and the kidney: pathophysiological and clinical implications. Endocr Rev. (2014) 35:234–81. doi: 10.1210/er.2013-1071
55. Bianda, T, Hussain, MA, Glatz, Y, Bouillon, R, Froesch, ER, and Schmid, C. Effects of short-term insulin-like growth factor-I or growth hormone treatment on bone turnover, renal phosphate reabsorption and 1,25 dihydroxyvitamin D3 production in healthy man. J Intern Med. (1997) 241:143–50. doi: 10.1046/j.1365-2796.1997.94101000.x
56. Bersani, FS, Ghezzi, F, Maraone, A, Vicinanza, R, Cavaggioni, G, Biondi, M, et al. The relationship between vitamin D and depressive disorders. Riv Psichiatr. (2019) 54:229–34. doi: 10.1708/3281.32541
57. Marcos-Pérez, D, Sánchez-Flores, M, Proietti, S, Bonassi, S, Costa, S, Teixeira, JP, et al. Low vitamin D levels and frailty status in older adults: a systematic review and Meta-analysis. Nutrients. (2020) 12:2286. doi: 10.3390/nu12082286
58. Aspell, N, Laird, E, Healy, M, Lawlor, B, and O’Sullivan, M. Vitamin D deficiency is associated with impaired muscle strength and physical performance in community-dwelling older adults: findings from the English longitudinal study of ageing. Clin Interv Aging. (2019) 14:1751–61. doi: 10.2147/CIA.S222143
59. Tan, L, He, R, and Zheng, X. Effect of vitamin D, calcium, or combined supplementation on fall prevention: a systematic review and updated network meta-analysis. BMC Geriatr. (2024) 24:390. doi: 10.1186/s12877-024-05009-x
60. Murad, MH, Elamin, KB, Abu Elnour, NO, Elamin, MB, Alkatib, AA, Fatourechi, MM, et al. The effect of vitamin D on falls: a systematic review and Meta-analysis. J Clin Endocrinol Metab. (2011) 96:2997–3006. doi: 10.1210/jc.2011-1193
61. Haitchi, S, Moliterno, P, and Widhalm, K. Prevalence of vitamin D deficiency in seniors – a retrospective study. Clin Nutr ESPEN. (2023) 57:691–6. doi: 10.1016/j.clnesp.2023.07.005
62. Andrew, FH, Michael, DS, Leslie, BS, Preacher, KJ, and Hayes, AF. SAGE research methods – the SAGE sourcebook of advanced data analysis methods for communication research – contemporary approaches to assessing mediation in communication research. (2025). Available online at: https://methods.sagepub.com/book/edvol/sage-srcbk-adv-data-analysis-methods-comm-research/chpt/contemporary-approaches-assessing-mediation-communication (Accessed February 5, 2025).
63. Hayes, AF. Introduction to mediation, moderation, and conditional process analysis, second edition: a regression-based approach. 2nd ed. New York: The Guilford Press (2018).
64. Li, W, and Yu, T. Relationship between 25-hydroxyvitamin D and IGF1: a cross-sectional study of the third National Health and nutrition examination survey participants. J Health Popul Nutr. (2023) 42:35. doi: 10.1186/s41043-023-00374-6
65. Hyppönen, E, Boucher, BJ, Berry, DJ, and Power, C. 25-Hydroxyvitamin D, IGF-1, and metabolic syndrome at 45 years of age: a cross-sectional study in the 1958 British birth cohort. Diabetes. (2008) 57:298–305. doi: 10.2337/db07-1122
66. Mortensen, C, Mølgaard, C, Hauger, H, Kristensen, M, and Damsgaard, CT. Winter vitamin D3 supplementation does not increase muscle strength, but modulates the IGF-axis in young children. Eur J Nutr. (2019) 58:1183–92. doi: 10.1007/s00394-018-1637-x
67. Ameri, P, Giusti, A, Boschetti, M, Bovio, M, Teti, C, Leoncini, G, et al. Vitamin D increases circulating IGF1 in adults: potential implication for the treatment of GH deficiency. Eur J Endocrinol. (2013) 169:767–72. doi: 10.1530/EJE-13-0510
68. Al-Daghri, NM, Yakout, SM, Wani, K, Khattak, MNK, Garbis, SD, Chrousos, GP, et al. IGF and IGFBP as an index for discrimination between vitamin D supplementation responders and non-responders in overweight Saudi subjects. Medicine. (2018) 97:e0702. doi: 10.1097/MD.0000000000010702
69. Song, Y, Fleet, JC, and Kato, S. Vitamin D receptor (VDR) knockout mice reveal VDR-independent regulation of intestinal calcium absorption and ECaC2 and Calbindin D9k mRNA. J Nutr. (2003) 133:374–80. doi: 10.1093/jn/133.2.374
70. Ameri, P, Giusti, A, Boschetti, M, Murialdo, G, Minuto, F, and Ferone, D. Interactions between vitamin D and IGF-I: from physiology to clinical practice. Clin Endocrinol. (2013) 79:457–63. doi: 10.1111/cen.12268
71. Peng, L, Malloy, PJ, and Feldman, D. Identification of a functional vitamin D response element in the human insulin-like growth factor binding Protein-3 promoter. Mol Endocrinol. (2004) 18:1109–19. doi: 10.1210/me.2003-0344
72. Matilainen, M, Malinen, M, Saavalainen, K, and Carlberg, C. Regulation of multiple insulin-like growth factor binding protein genes by 1alpha,25-dihydroxyvitamin D3. Nucleic Acids Res. (2005) 33:5521–32. doi: 10.1093/nar/gki872
73. Volpato, S, Bianchi, L, Cherubini, A, Landi, F, Maggio, M, Savino, E, et al. Prevalence and clinical correlates of sarcopenia in community-dwelling older people: application of the EWGSOP definition and diagnostic algorithm. J Gerontol A Biol Sci Med Sci. (2014) 69:438–46. doi: 10.1093/gerona/glt149
74. Doi, T, Makizako, H, Tsutsumimoto, K, Hotta, R, Nakakubo, S, Makino, K, et al. Association between insulin-like growth factor-1 and frailty among older adults. J Nutr Health Aging. (2018) 22:68–72. doi: 10.1007/s12603-017-0916-1
75. van Nieuwpoort, IC, Vlot, MC, Schaap, LA, Lips, P, and Drent, ML. The relationship between serum IGF-1, handgrip strength, physical performance and falls in elderly men and women. Eur J Endocrinol. (2018) 179:73–84. doi: 10.1530/EJE-18-0076
76. Onder, G, Liperoti, R, Russo, A, Soldato, M, Capoluongo, E, Volpato, S, et al. Body mass index, free insulin-like growth factor I, and physical function among older adults: results from the ilSIRENTE study. Am J Physiol-Endocrinol Metab. (2006) 291:E829–34. doi: 10.1152/ajpendo.00138.2006
77. Cappola, AR, Bandeen-Roche, K, Wand, GS, Volpato, S, and Fried, LP. Association of IGF-I levels with muscle strength and mobility in older women. J Clin Endocrinol Metab. (2001) 86:4139–46. doi: 10.1210/jcem.86.9.7868
78. Barbieri, M, Ferrucci, L, Ragno, E, Corsi, A, Bandinelli, S, Bonafè, M, et al. Chronic inflammation and the effect of IGF-I on muscle strength and power in older persons. Am J Physiol Endocrinol Metab. (2003) 284:E481–7. doi: 10.1152/ajpendo.00319.2002
79. Jarmusch, S, Baber, L, Bidlingmaier, M, Ferrari, U, Hofmeister, F, Hintze, S, et al. Influence of IGF-I serum concentration on muscular regeneration capacity in patients with sarcopenia. BMC Musculoskelet Disord. (2021) 22:807. doi: 10.1186/s12891-021-04699-3
80. Washburn, RA, Smith, KW, Jette, AM, and Janney, CA. The physical activity scale for the elderly (PASE): development and evaluation. J Clin Epidemiol. (1993) 46:153–62. doi: 10.1016/0895-4356(93)90053-4
81. Washburn, RA, McAuley, E, Katula, J, Mihalko, SL, and Boileau, RA. The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol. (1999) 52:643–51. doi: 10.1016/s0895-4356(99)00049-9
82. Visser, M, Bouter, LM, McQuillan, GM, Wener, MH, and Harris, TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. (1999) 282:2131–5. doi: 10.1001/jama.282.22.2131
83. Zuliani, G, Volpato, S, Galvani, M, Blè, A, Bandinelli, S, Corsi, AM, et al. Elevated C-reactive protein levels and metabolic syndrome in the elderly: the role of central obesity data from the InChianti study. Atherosclerosis. (2009) 203:626–32. doi: 10.1016/j.atherosclerosis.2008.07.038
84. Zhang, WB, Ye, K, Barzilai, N, and Milman, S. The antagonistic pleiotropy of insulin-like growth factor 1. Aging Cell. (2021) 20:e13443. doi: 10.1111/acel.13443
85. Rahmani, J, Montesanto, A, Giovannucci, E, Zand, H, Barati, M, Kopchick, JJ, et al. Association between IGF-1 levels ranges and all-cause mortality: a meta-analysis. Aging Cell. (2022) 21:e13540. doi: 10.1111/acel.13540
86. Kirwan, R, Isanejad, M, Davies, IG, and Mazidi, M. Genetically determined serum 25-Hydroxyvitamin D is associated with Total, trunk, and arm fat-free mass: a Mendelian randomization study. J Nutr Health Aging. (2022) 26:46–51. doi: 10.1007/s12603-021-1696-1
87. Sun, X, Tanisawa, K, Zhang, Y, Ito, T, Oshima, S, Higuchi, M, et al. Effect of vitamin D supplementation on body composition and physical fitness in healthy adults: a double-blind, randomized controlled trial. Ann Nutr Metab. (2019) 75:231–7. doi: 10.1159/000504873
88. Vicinanza, R, Bersani, FS, D’Ottavio, E, Murphy, M, Bernardini, S, Crisciotti, F, et al. Adherence to Mediterranean diet moderates the association between multimorbidity and depressive symptoms in older adults. Arch Gerontol Geriatr. (2020) 88:104022. doi: 10.1016/j.archger.2020.104022
89. Baron, RM, and Kenny, DA. The moderator–mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. (1986) 51:1173–82. doi: 10.1037/0022-3514.51.6.1173
90. Rucker, DD, Preacher, KJ, Tormala, ZL, and Petty, RE. Mediation analysis in social psychology: current practices and new recommendations. Soc Personal Psychol Compass. (2011) 5:359–71. doi: 10.1111/j.1751-9004.2011.00355.x
91. Preacher, KJ, and Hayes, AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. (2008) 40:879–91. doi: 10.3758/BRM.40.3.879
Keywords: aging, body composition, fat-free mass, frailty, IGF-I, insulin-like growth factor-I, multimorbidity, vitamin D
Citation: Vicinanza R, Frizza A, Pollard JA, Mazza V, De Martino MU, Imbimbo G, Pignatelli P, Ettorre E, Del Ben M and Molfino A (2025) Aging and IGF-I: relationships with vitamin D and body composition. A mediation analysis. Front. Nutr. 12:1585696. doi: 10.3389/fnut.2025.1585696
Edited by:
Carlo Pedrolli, Azienda Provinciale per i Servizi Sanitari (APSS), ItalyReviewed by:
Elena Succurro, University of Magna Graecia, ItalyFrancesco S. Celi, UConn Health, United States
Benjamin Trujillo-Hernandez, University of Colima, Mexico
Copyright © 2025 Vicinanza, Frizza, Pollard, Mazza, De Martino, Imbimbo, Pignatelli, Ettorre, Del Ben and Molfino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roberto Vicinanza, Um9iZXJ0by52aWNpbmFuemFAdXNjLmVkdQ==
 Roberto Vicinanza
Roberto Vicinanza Alessandro Frizza2
Alessandro Frizza2 Julie A. Pollard
Julie A. Pollard Massimo Ulderico De Martino
Massimo Ulderico De Martino Pasquale Pignatelli
Pasquale Pignatelli Alessio Molfino
Alessio Molfino