- 1College of Food Science and Technology, Northwest University, Xi'an, China
- 2Xi'an Xida Institute of Life Sciences and Health Research Co., Ltd., Xi'an, China
- 3Shaanxi Functional Food Engineering Center Co., Ltd., Xi'an, China
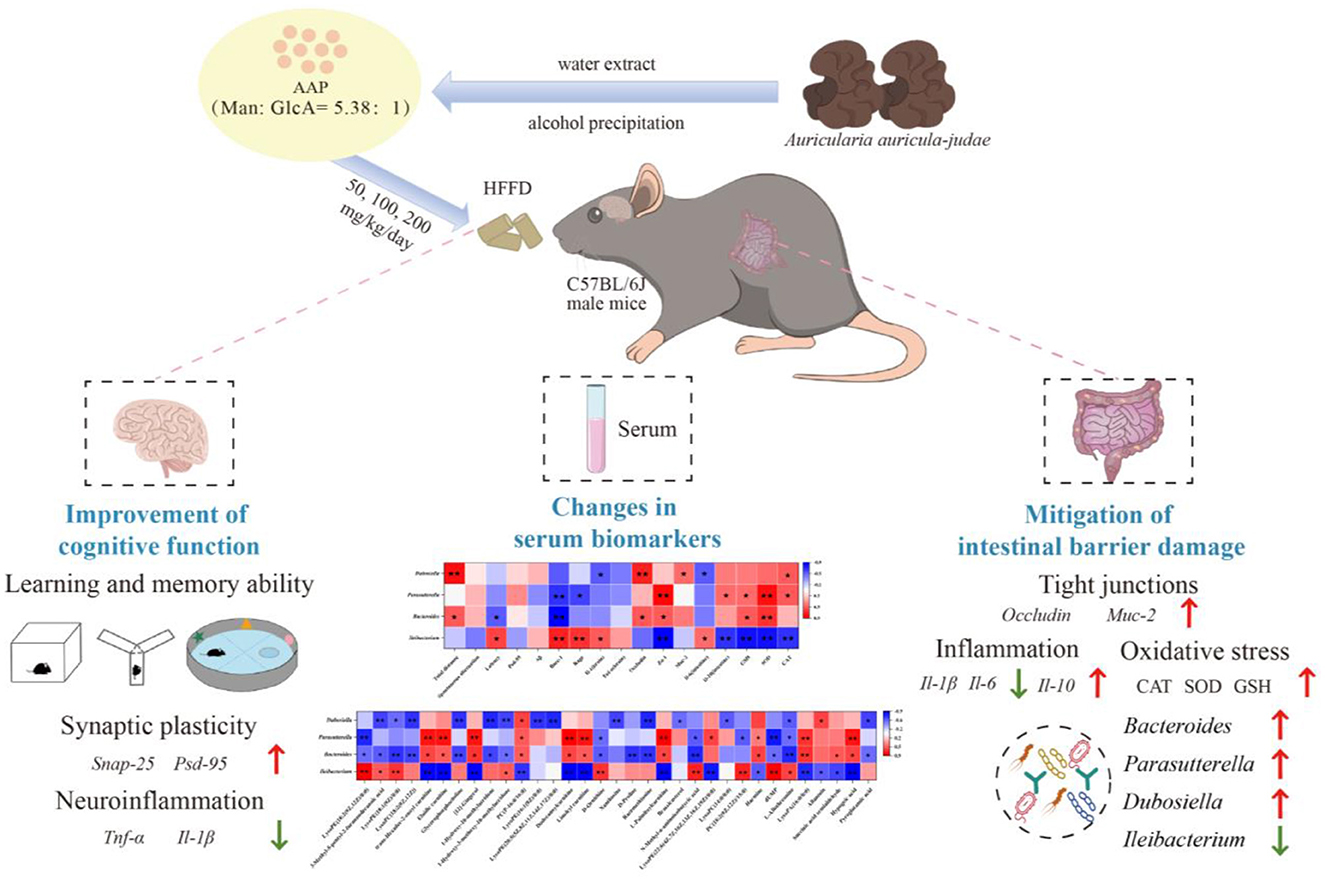
As a major nutraceutical component of a traditional edible fungus Auricularia auricula-judae, Auricularia auricula-judae polysaccharide (AAP) has been well-documented due to its outstanding hypolipidemic and hypoglycemic bioactivities. This study investigated the effects of AAP on hypercaloric diet-induced cognitive dysfunction in mice and the underlying mechanisms. Behavioral and histological results demonstrated that AAP could ameliorate high-fat and high-fructose diets (HFFD)-induced memory impairment and neuronal loss. AAP significantly inhibited inflammatory responses and balanced oxidative stress states in mice brain and colon tissues. AAP dietary supplements remarkably reshaped gut bacteria composition. The abundance of Dubosiella, Bacteroides, and Parasutterella were significantly increased. Differential bacteria abundance showed a strong correlation with behavioral related indicators, inflammatory factors, antioxidant enzymes and serum metabolites levels. These results suggest that AAP is able to ameliorate high-calorie diet-induced cognitive dysfunction in mice, as well as modulate the regulation of gut flora homeostasis and serum metabolites in mice. And these results are of positive significance for promoting the utilization of Auricularia auricula-judae resources and for the development of nutraceutical products in the brain with AAP as the primary active component.
1 Introduction
Numerous researches have identified a substantial correlation between the intake of foods rich in saturated fats (high-fat diet, HFD) and development of cognitive impairment (1, 2). Both short- (1 week) and long-term (10 months) HFD interventions induce learning and memory decline in mice (3–5). The HFD modulates processes including oxidative stress, neuroinflammation, insulin resistance, synaptic plasticity dysfunction, blood-cerebrospinal fluid barrier dysfunction, and impaired cerebral blood flow that have been identified as key pathophysiological mechanisms leading to cognitive impairment (6, 7). There are strong correlations between intestinal flora and cognition. Altering the composition of the host gut microorganisms by using antibiotic or fecal transplants can modulate host behaviors such as learning, memory, mood, anxiety, depression, and stress (8, 9). Promoting homeostasis of the gut flora-gut-brain axis has emerged an effective measure to improve brain nutritional status and cognitive function.
Auricularia Auricula-judae, which is rich in gelatin, is a common and delicious health food that is widely available, is referred to as “black treasure”, and is known to be an “intestinal scavenger”(10). Auricularia Auricula-judae polysaccharide (AAP) is a crucial nutraceutical component in the Auricularia Auricula-judae fruiting body, for it can promote intestinal peristalsis, lower blood glucose and blood lipids, and exert antioxidant, anti-thrombosis, and immunity-enhancing effects (11, 12). The main components of AAP are glucose, mannose, and galactose under hot water extraction conditions (13, 14); the structural backbone of AAP involves a (1 → 4)-D-glucopyranosyl group and an O6 glucopyranose side group, with a glucuronic acid structure extracted by 70% ethanol solution (15). Several researches suggest that AAP may have a beneficial effect on brain health. Xiong et al. found that AAP can increase the number of hippocampal neurons surviving in brain tissue, and significantly increase superoxide dismutase (SOD) activity, which can protect against ischemic brain damage in neonatal rats to a certain degree (16). The wood ear mushroom-containing polyherbal GCJ attenuated cerebral ischemia-reperfusion injury-induced cognitive deficits in MetS rats, potentially linked to its modulation of AChE activity and activation of eNOS, BDNF, and pERK/ERK pathways in memory-related brain regions (17). Oral administration of Auricularia polytricha aqueous extract (AEAP, 400/600 mg/kg) significantly attenuated haloperidol-induced catalepsy in rats, which may be attributed to the modulation of oxidative stress by AEAP, such as the levels of superoxide dismutase, catalase (CAT), and glutathione (GSH) (18). Two hundreds mg/kg/day of purified fraction AAP I-a obtained by ultrasound-assisted extraction method administered by gavage for 35 days significantly reduced malondialdehyde (MDA, a lipid peroxidation product) levels in mouse brains, increased SOD activity and GSH content, and significantly inhibited D-galactose-induced aging in mice (19).
While accumulating evidence has substantiated the therapeutic potential of AAP in nutritional management, critical knowledge gaps persist regarding its mechanistic interplay with cognitive dysfunctions induced by obesogenic diets, particularly in elucidating how specific bioactive constituents counteract neural metabolic disturbances. This study aimed to examine the interventional impacts of AAP on cognitive dysfunction caused by high-calorie diets in mice and analyze the mechanism based on the intestinal flora-gut-brain axis using metabolomics and intestinal microbiomics techniques. The results will lay a theoretical foundation for further studies of intervention in high-calorie diet-induced nutritional health disorders using natural functional food polysaccharide components, and provide new ideas to guide individuals and societies in establishing reasonable dietary structures and addressing the issue of overnutrition.
2 Materials and methods
2.1 Preparation of AAP
A 30 g weighed sample of powdered Auricularia Auricula-judae (purchased from Shaanxi Tianmei Green Industry Co., Ltd.) was extracted by adding distilled water at 100°C to a material-liquid ratio of 1:50 (g:mL) for 2 h. The extraction solution was centrifuged at 2,800 × g for 15 min and the supernatant was collected and concentrated. Then, anhydrouas ethanol was added at a ratio of 1:4 (v/v), for ethanol precipitation. After 12 h of ethanol precipitation, the sample was centrifuged at 2,800 × g for 15 min for 10 min, and the filtered residue was retained and dissolved in water. Proteins were removed via the Sevag method by adding a reagent (dichloromethane:n-butanol = 4:1, v/v) to the solution, stirring for 20 min, and centrifuging at 2,800 × g for 15 min. The supernatant was removed, the precipitant and organic layers were discarded, and the procedure was repeated 4–5 times. AAP was obtained by dialysis in flowing tap water for 3 d using cellulose dialysis bags with a molecular cut-off of 3,000 Da, followed by concentration, freeze-drying, weighing, and bagging. The carbohydrate content, protein content, and uronic acid content of AAP were 51.24 ± 4.52%, 4.18 ± 0.36%, and 12.31 ± 3.26%, respectively. Mannose and glucuronic acid are the main monosaccharide existed in AAP, and the relative molar ration of the two is 5.38:1 (Supplementary Figure S1). Calculated according to the calibration curve, the average molecular weight of AAP was 1.91 × 103 kDa (Supplementary Figure S2).
2.2 Animal treatments
Animal experiment protocols were approved by the Animal Ethics Committee of the Laboratory Animal Center of Northwest University (NWU-AWC-20200401M). Following a week of acclimation, 60 C57BL/6J mouse (SPF, 7-week-old, male, purchased from Xi'an Jiaotong University) were split into six groups randomly. A cognitive impairment model was induced by chronic exposure to 45% HFD combined with 10% high-fructose solution in drinking water. Mice received AAP via ad libitum dietary supplementation at doses of 50, 100, and 200 mg/kg/day. The experimental design detailing animal groupings and treatment parameters is documented in Supplementary Table S1, with the overall study timeline illustrated in Figure 1A.
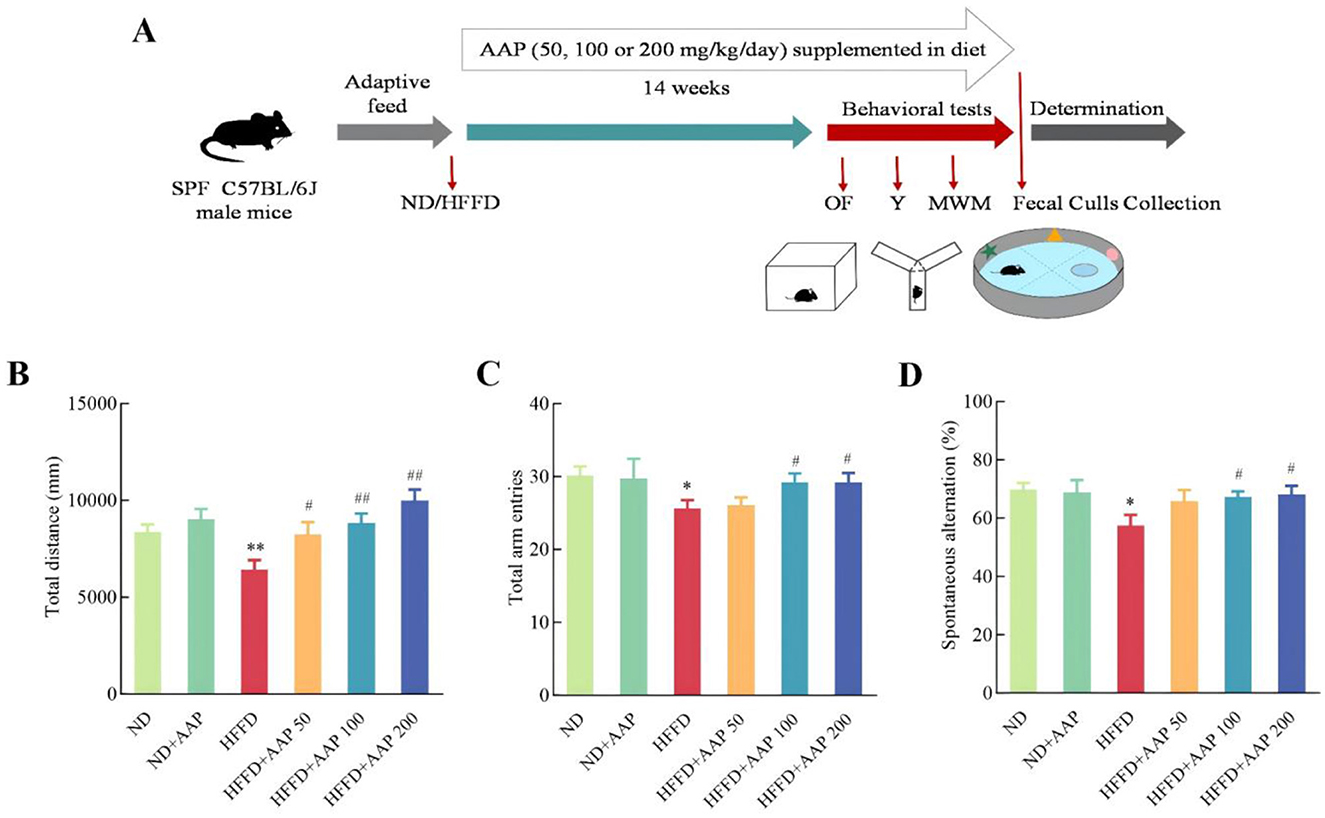
Figure 1. Timeline illustrating AAP treatment and evaluations of mouse cognitive functions. (A) Experimental scheme for effects of AAP on mouse memory loss induced by HFFD; (B) Total movement distance in open-field trials; (C) Total number of arm entries and (D) percentage of alternations in the Y maze. The data are presented as means ± SEM, n = 10. *p < 0.05, **p < 0.01, vs. ND group, #p < 0.05, ##p < 0.01 vs. HFFD group.
2.3 Animal behavioral experiments
Voluntary activity of mice was assessed using an open-field test, while the Y-maze test was primarily utilized to evaluate rodents' spatial working memory (20). The Morris water maze test is a classic experiment used to assess learning and memory in rodents (21). Following acclimation training, positioning cruise experiments were performed four times daily over a 5-days period. The results of the positioning cruise experiments on Days 1, 3, and 5 were recorded. In these experiments mice were placed into a pool at four random entry points facing the pool wall, and the total distance traveled by the mice and the time required after entering the water to find a hidden platform and stand on it (i.e., evasion latency) were recorded automatically using the Super Maze Animal Behavioral Trajectory Analysis System. On the second day after the localization cruise experiment, the platform was removed and the spatial exploration experiment was carried out. Over a 60 s interval, the frequency of mouse crossings over the original platform was documented, while the distance traveled within the target quadrant and the total distance covered were systematically quantified.
2.4 Serum metabolite extraction and detection
At the end of the experiment, the mice were then placed in an induction chamber and exposed to 4% isoflurane-oxygen mixture until they were unconscious, as confirmed by the lack of a pedal withdrawal reflex. Blood was collected from the eyeballs of anesthetized mice and centrifuged at 1,000 × g for 20 min to separate the supernatant, resulting in serum. Serum metabolic analyses in mice were conducted following established laboratory protocols (22). Substance annotation was performed by matching the self-built secondary mass spectrometry database BiotreeDB (V2.1). Combined with multivariate statistical analysis, the original data were converted to mzXML format using ProteoWizard software to screen metabolic markers.
2.5 Histopathological and morphological observations
Paraffin sections of brain and colon tissues were subjected to xylene and gradient ethanol for dewaxing and rehydration. After washing with phosphate buffered solution (0.1 mol/L pH 7.4), nuclei were stained with hematoxylin for 6 min, followed by 1% hydrochloric acid alcohol differentiation, indirect rinsing in tap water for 20 min, counterblueing with 1% dilute ammonia, cytoplasm staining with eosin for 3 min, conventional ethanol dehydration, and xylene exposure for clarification. The slices were hermetically sealed using a neutral resin and then imaged using a light microscope after being allowed to dry in the air.
2.6 RT-qPCR
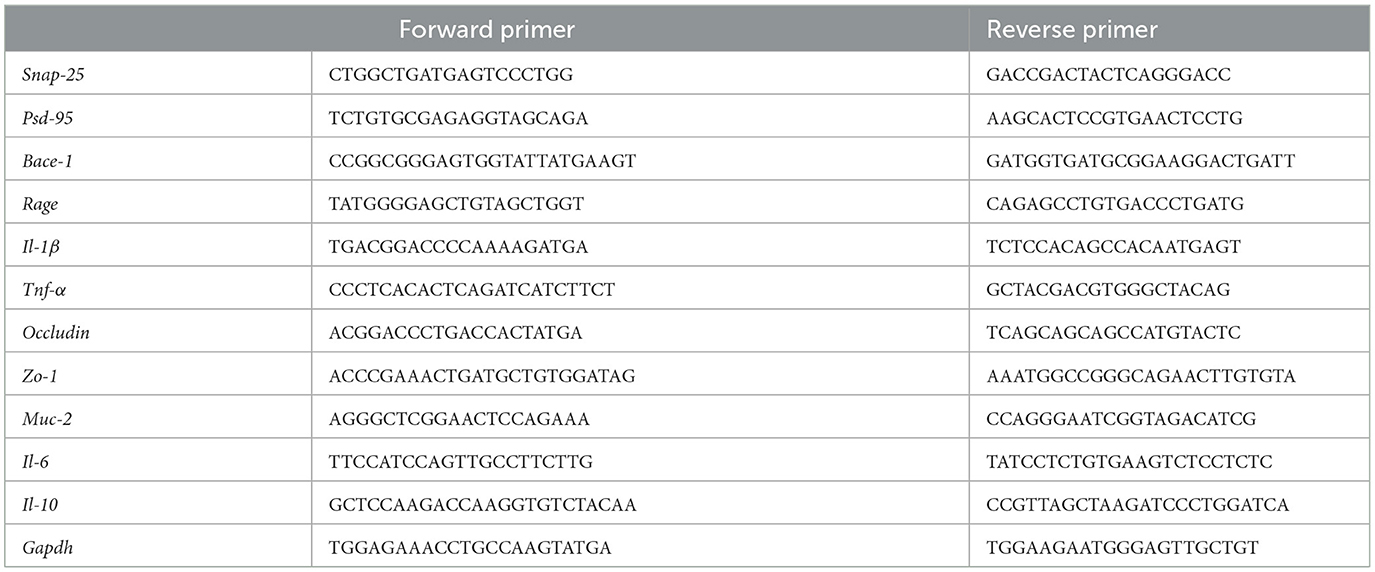
According to the reference (23), RNAiso Plus (Total RNA Extraction Reagent) kits were utilized to extract RNA from the colon tissues and brain of mice. Reverse transcribe RNA to cDNA following the instructions provided with the reverse transcription kit (RT mix with DNase, All-in-One). Gene mRNA levels were detected by real-time quantitative PCR using a Light Cycler 480 Real-Time System (EMCLAB, Germany). Mouse-specific primers are listed in Table 1.
2.7 Detection of amyloid content in brain tissue and oxidative stress in colonic tissues
β amyloid in mouse brain tissue was determined according to the instructions of Aβ Amyloid ELISA kit (Shanghai Xinle Biotechnology Co., Ltd., China). MDA content, CAT, SOD, and GSH activities in mouse colon tissues were detected using Nanjing Jiancheng Kit (Nanjing Jiancheng Bioengineering Institute, China).
2.8 Fecal 16s rRNA sequencing analyses
Fecal 16S rRNA sequencing analyses were conducted following established laboratory methods (22). BIOTREE was entrusted with conducting high-throughput sequencing and bioinformatic analysis targeting the hypervariable V3–V4 region of the 16S rRNA gene. Following sequencing completion, raw paired-end reads underwent rigorous processing through quality control and filtering procedures to obtain high-quality sequences. Subsequent bioinformatic processing involved clustering of Operational Taxonomic Units (OTUs) at 97% similarity threshold followed by taxonomic classification using reference databases. To elucidate β-diversity patterns among distinct sample groups, multivariate statistical approaches including Principal Coordinates Analysis (PCoA) and Principal Component Analysis (PCA) were employed for dimensionality reduction visualization. Differential analysis was performed using Student's t-test complemented by LEfSe (Linear Discriminant Analysis Effect Size) algorithm to identify statistically significant variations in microbial composition and community structure across experimental groups. Furthermore, bivariate correlation analysis (Spearman's rank correlation) was implemented to investigate potential associations between discriminant bacterial taxa and host physiological parameters, including serum metabolite profiles and relevant clinical indices.
2.9 Statistical analyses
All data are expressed as means ± SEM (n ≥ 6) and were analyzed by Tukey's one-way ANOVA using SPSS 19.0. p < 0.05 was considered to be statistically significant difference.
3 Results
3.1 Effects of AAP dietary supplementation on mice learning and memory impairments induced by HFFD
Animal behavioral experiments have gradually become the most commonly used models for evaluating animal learning and memory. Results from the open-field (Figure 1B) and Y-maze (Figures 1C, D) experiments showed no significant difference in total arm-in distance, total number of arm-ins, or percentage of alternations between ND and ND + AAP groups, that is, AAP did not significantly affect the mice's voluntary movement ability. Compared with the ND group, the overall distance traveled and the proportion of mice entering the three different arms consecutively were considerably lower in the HFFD group, indicating that motor and working memory abilities were notably impaired. The impairment was significantly alleviated by dietary supplementation with 100 and 200 mg/kg/day of AAP.
To systematically investigate the therapeutic potential of AAP intervention for learning and cognitive impairments, the Morris Water Maze behavioral assay was employed in the present study (Figure 2), with particular focus on spatial memory acquisition and retention capacities. The mice in the HFFD group had a significantly longer escape latency compared to the ND group. It is worth noting that dietary supplementation with different concentrations of AAP effectively rescued this phenotype and reduced the platform-finding time. Figure 2B also showed a notable disparity in the evasion distances of the mice in each group. Again, AAP effectively reduced HFFD evasion distance. Figures 2C–E showed the results of spatial exploration experiments, in which the mice in the AAP intervention group displayed a significantly increased number of times they traversed their platforms, increased time spent in the quadrant containing the platforms, and increased distance traveled, i.e., AAP effectively ameliorated mice learning and cognitive deficits induced by HFFD.
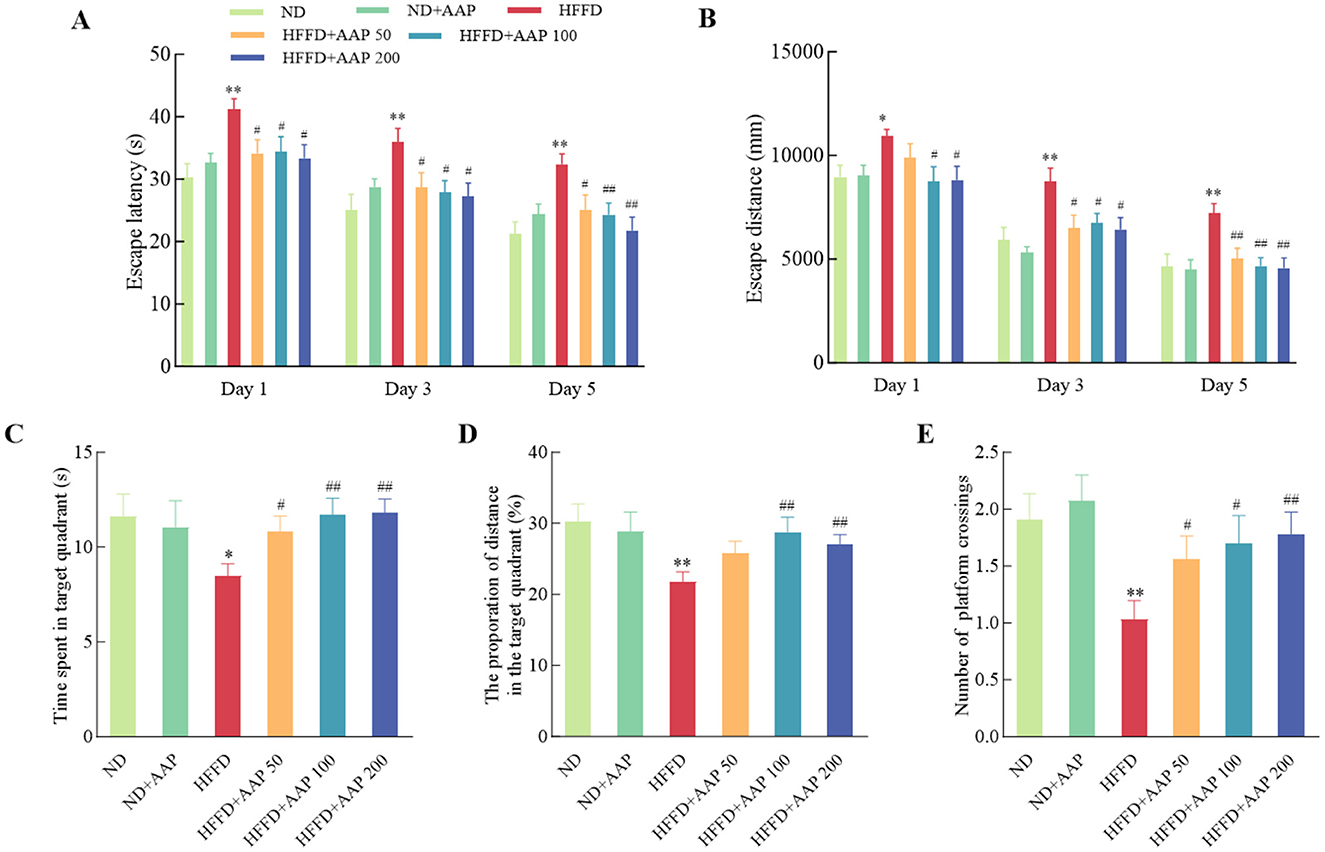
Figure 2. Suppression of HFFD-induced memory loss by AAP administration measured in a water maze test. Escape latency (A), escape distance during the place navigation test (B), time spent in the target quadrant (C), percentage of total distance traveled within the target quadrant (D), and number of platform crossings (E) during the probe trial were recorded. Data are presented as means ± SEM, n = 10. *p < 0.05, **p < 0.01, vs. ND group, #p < 0.05, ##p < 0.01 vs. HFFD group.
3.2 Effects of AAP intervention on HFFD-induced neuronal damage and inflammatory responses in the mouse brain
To study the effect of dietary supplementation with AAP on HFFD-induced neuronal damage, the HE staining method was applied to observe neuronal cell morphology in the cortical area of the mouse brain (Figure 3A). Compared with the ND group, the neuronal cells in the brains of HFFD-induced mice were arranged in a loose and disorganized manner, and the neurons vanished or suffered nuclear solidification morphological changes. AAP supplementation obviously rescued neuron morphological abnormalities in the cortices of HFFD-induced mice. Aβ amyloid deposition is a primary cause of Alzheimer's disease. To investigate whether AAP would reduce HFFD-induced Aβ amyloid deposition and improve learning memory function, Aβ expression in the cerebral cortex was measured by ELISA. Figure 3B ELISA results demonstrated that the content of Aβ amyloid deposits was dramatically higher in the HFFD group, and dietary AAP supplementation substantially reduced Aβ content in the brains of HFFD-fed mice by approximately 25.32%.
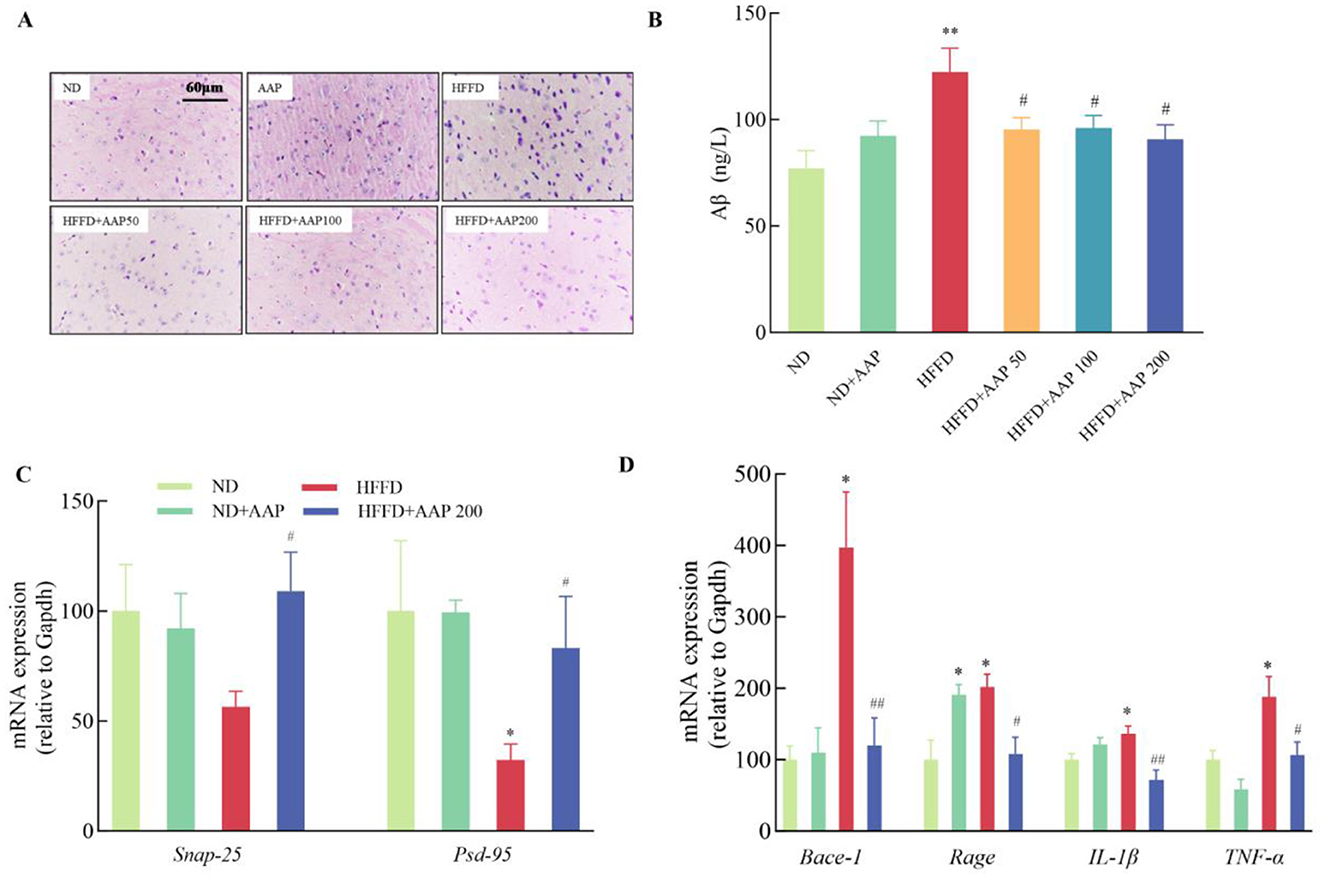
Figure 3. Effects of AAP on HFFD-induced neuronal damage and inflammatory responses in mouse brain. (A) H&E staining of mouse cerebral cortex (400×); (B) Aβ amyloid content; mRNA expression levels of genes related to (C) synaptic plasticity; (D) Aβ amyloid synthesis and inflammatory response in the mouse brain. Data are presented as means ± SEM, *p < 0.05, **p < 0.01, vs. ND group, #p < 0.05, ##p < 0.01 vs. HFFD group.
Furthermore, Real-time quantitative PCR results (Figure 3C) showed that supplementation with 50, 100 or 200 mg/kg body weight/day AAP significantly increased Snap-25 and Psd-95 mRNA levels and enhanced synaptic plasticity compared with the HFFD. For in-depth analysis of pathological mechanisms, the HFFP+APP 200 model was systematically selected based on dose-response characteristics. Remarkable downregulation of HFFD-induced mRNA expression was observed in AAP-treated specimens, specifically in β-site amyloid precursor protein cleaving enzyme-1 (Bace-1) and receptor for advanced glycation end products (Rage) transcripts (Figure 3D), with reduction rates of 69.77 ± 9.68% and 46.29 ± 11.59%, respectively (p < 0.05), establishing a molecular basis for attenuated Aβ deposition. Inflammation is also thought to be an important factor in Aβ plaque accumulation, and thus in disease. Glial cell overactivation will lead to release of large amounts of pro-inflammatory factors (13). As shown in Figure 3D, AAP treatment significantly suppressed the mRNA expression of inflammatory mediators Il-1β and Tnf-α by 47.31 ± 10.24% and 43.41 ± 9.57%, respectively.
3.3 Equations effects of AAP on mice intestinal barrier damage induced by HFFD
Intestinal barrier damage causes changes in intestinal permeability, inducing a series of related tissue injuries caused by inflammatory responses, and aggravating disease development (24). In contrast to the ND group, colon morphology and structure in HFFD group mice showed varied microvillus lengths accompanied by rupture of villi, nuclear solidification, disrupted tight junctions, and disorganized cell morphology. Dietary supplementation with AAP improved the quality of tight junctions and morphology of microvilli (Figure 4A). These results indicated that AAP intervention reduced histomorphological changes in HFFD-exposed colonic tissues in mice. To investigate the ameliorative effect of dietary AAP supplementation on intestinal barrier damage, the mRNA levels of tight junction protein-related genes (Occludin and Zo-1) and mucosal protein-related genes (Muc-2) in the intestinal tissues of mice were detected using qRT-PCR (25). As demonstrated in Figure 4B, colonic tissues from HFFD + AAP-treated mice showed a 2.85 ± 0.31-fold increase in Occludin mRNA expression (p < 0.01) and a 5.34 ± 0.52-fold increase in Muc-2 (p < 0.01) compared to the HFFD group.
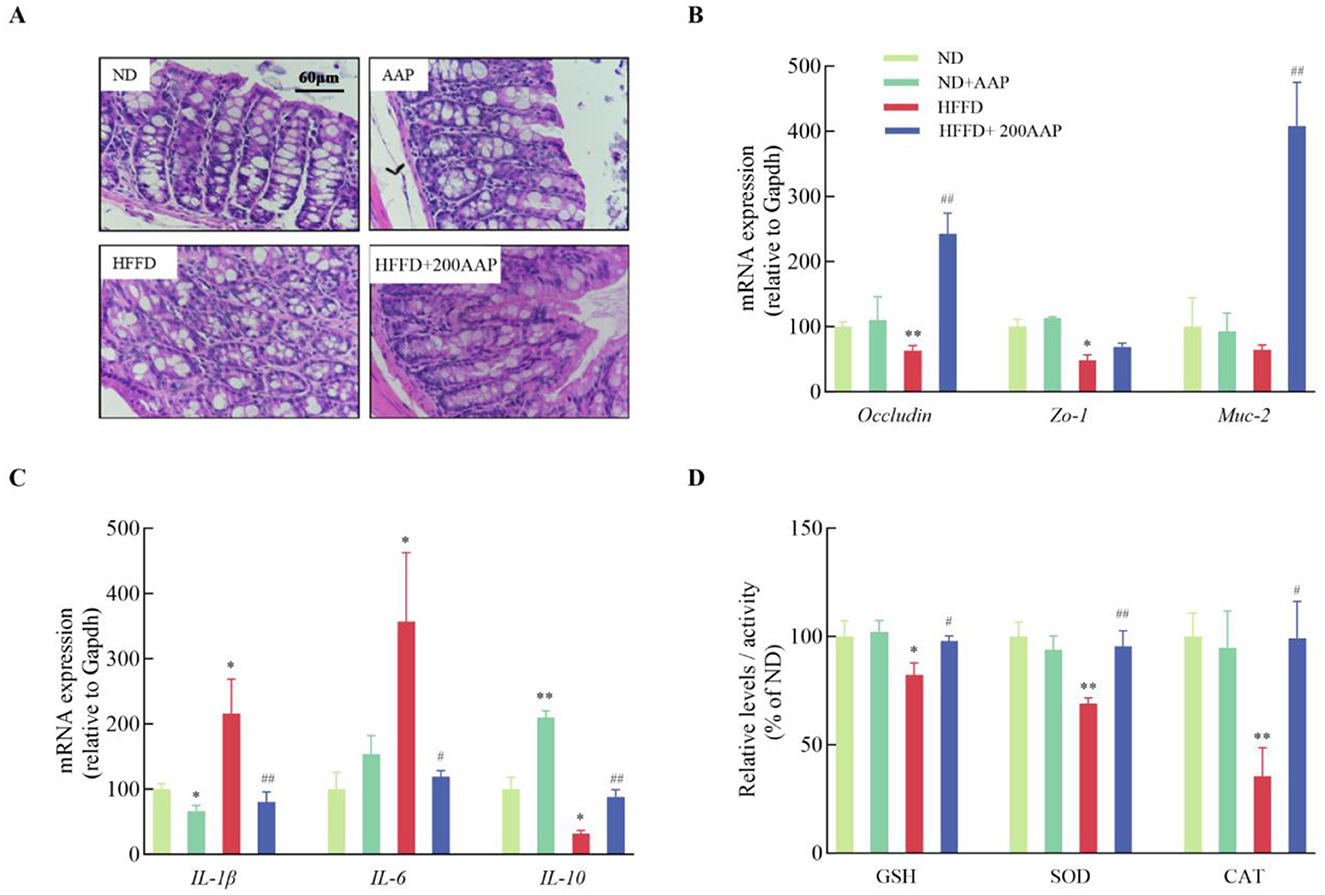
Figure 4. Effect of AAP on HFFD-induced intestinal barrier damage in mice. (A) H&E stained images of colon tissue morphology (400×); mRNA levels of (B) tight junction protein and (C) inflammation-related genes; (D) expressions of oxidative stress-related enzymes in mouse colon tissue. Data are presented as means ± SEM, *p < 0.05, **p < 0.01, vs. ND group, #p < 0.05, ##p < 0.01 vs. HFFD group.
Additionally, in the colonic tissues of mice in the HFFD group, the expression levels of Il-1β and Il-6 mRNA were significantly elevated, while Il-10 mRNA levels were markedly reduced compared to the ND group (Figure 4C). This suggests that HFFD exacerbates intestinal inflammatory responses, in agreement with previous research (26). On the contrary, dietary supplementation with AAP significantly ameliorated the inflammation. Antioxidant levels in intestinal tissues are shown in Figure 4D, which shows that the HFFD + AAP group significantly up-regulated CAT and SOD enzyme activities, and increased GSH content in mouse colon tissues compared with these levels in HFFD group mice. These results indicated that AAP exerts a significant ameliorating effect on the imbalance in the inflammatory response and oxidative stress state in the colon tissues of HFFD-treated mice.
3.4 Effects of AAP on HFFD-induced intestinal flora in mice
The Venn diagrams in Figure 5A were employed to evaluate the similarity and overlap in OTU composition among the various groups. There are 89, 108, and 116 OTUs in ND, HFFD, and HFFD + AAP group separately. Shannon index is a measure of species richness and evenness, and Simpson index mainly reflects the evenness of the distribution of species in the community. Both Shannon index and Simpson index of the HFFD + AAP group were higher than those of the HFFD group, suggesting that the intestinal flora of the mice were more enriched and even after dietary supplementation with AAP (Supplementary Figure S3). Furthermore, PCoA at the OTU level demonstrated that all ND, HFFD, and HFFD + AAP samples each clustered together, and their structural profiles could be separated, indicating that the intestinal flora structure varied significantly among the groups and that dietary AAP supplementation induced a change in the β-diversity of intestinal microorganisms in mice on an HFFD (Figure 5B). At the phylum level, the microbial community structure of mouse feces is illustrated in Figure 5C, which was primarily composed of Firmicutes, Bacteroidota, Actinobacteriota, Campilobacterota, and Deferribacteres, consistent with the results of previous studies (27). AAP supplementation strikingly repressed the increase in Firmicutes abundance and the downregulation of Bacteroidota abundance induced by HFFD. It also significantly increased the abundance of Deferribacteres, a phylum of Defertilobacteria, which has been reported to correlate positively with memory, as compared with the HFFD group (28).
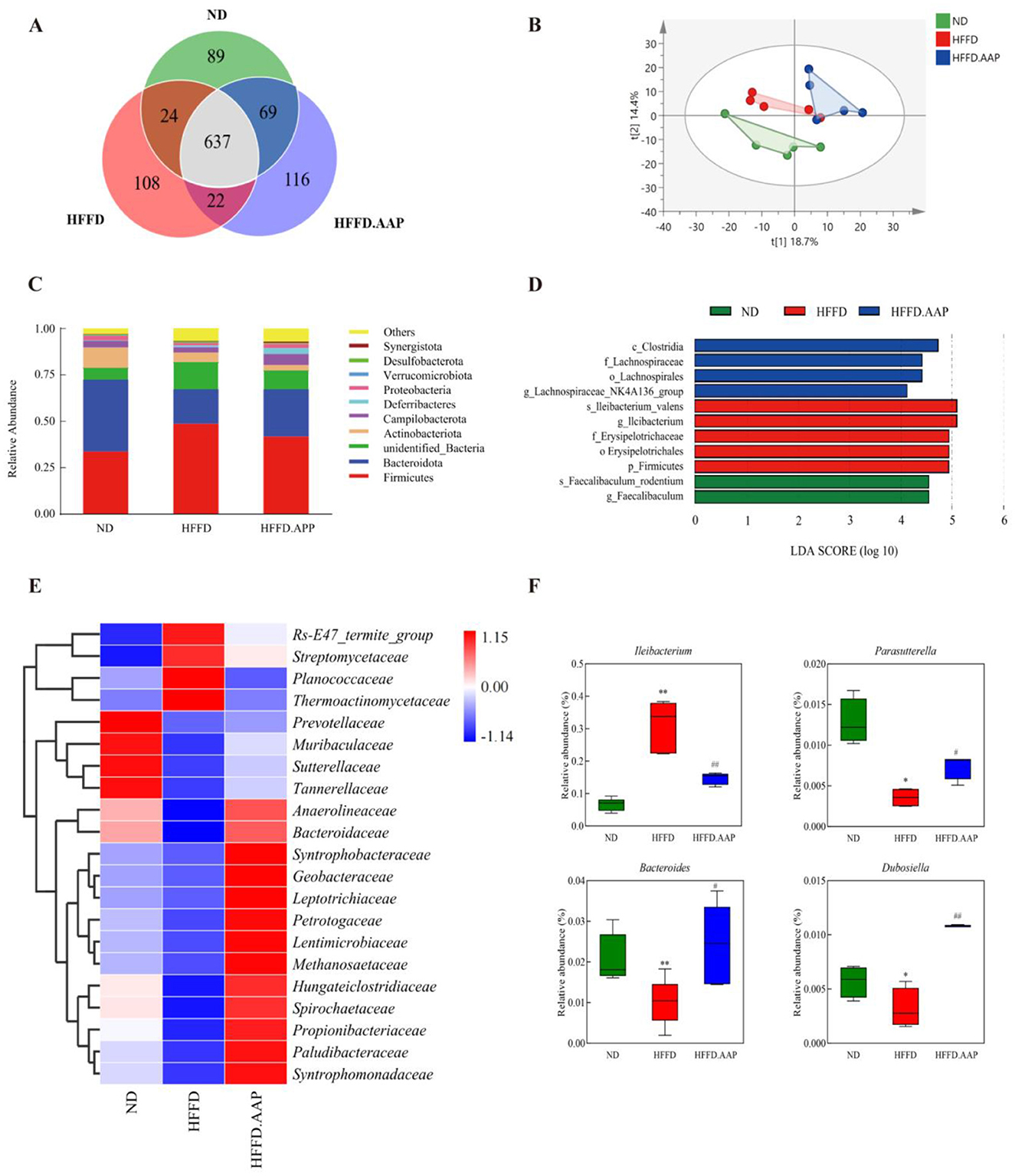
Figure 5. Effects of AAP on the intestinal microbiota of HFFD-induced mice. (A) Venn diagram; (B) PCoA; (C) Gut microbial structure at the phylum level; (D) Histogram of LDA discrimination; (E) Heat map of intestinal flora at the family level for each group of mice; (F) Abundance of differentially present bacteria. Data are presented as means ± SEM, *p < 0.05, **p < 0.01, vs. ND group, # p < 0.05, ## p < 0.01 vs. HFFD group.
Due to differences in mouse fecal flora composition and structure across treatment groups and to understand information on species with significant differences, LEFSe used linear discriminant analysis (LDA) to identify specific altered bacterial phenotypes and key species with significant differences between groups. As shown in Figure 5D, LEFSe results showed that a total of 11 species information with significant differences between groups were found, of which the ND group had four categories of key species with significant differences, namely, c_Clostridia, f_Lachnospiraceae, o_Lachnospirales, and g_Lachnospiraceae_NK4A136_group; the HFFD had five categories of significantly different key species, s_lleibacterium valens, g_lleibacterium, f_Erysipelotrichaceae, o_Erysipelotrichales and p_Firmicutes; the HFFD + AAP group had two categories of significantly different key species, s_Faecalibaculum_rodentium and g_Faecalibaculum.
To systematically evaluate intergroup disparities in intestinal microbiota, fecal microbial composition was analyzed through hierarchical clustering visualization at the taxonomic family level. As illustrated in Figure 5E, AAP supplementation markedly boosted the relative abundance of Syntrophomonadaceae, Paludibacteraceae, Propionibacteriaceae, Spirochaetaceae, Hungateiclostridiaceae, and downregulated Planococcaceae, Thermoactinomycetaceae, Streptomycetaceae, Rs-E47_termite_group relative abundance compared to the HFFD model group. Strains with significant differences were identified using an intergroup t-test. As shown in Figure 5F, dietary AAP supplementation significantly modulated gut microbiota composition in comparison to the HFFD group, with a marked increase in the relative abundance of Parasutterella (1.09-fold, p < 0.05), Bacteroides (1.38-fold, p < 0.05), and Dubosiella (2.38-fold, p < 0.01), alongside a significant reduction in Ileibacterium levels (p < 0.01).
3.5 Changes in serum biomarkers in the HFFD-fed mice after AAP consumption
The OPLS-DA score plot (Figures 6A, B) clearly demonstrates a highly significant differentiation between the serum samples of mice in the HFFD and HFFD + AAP groups. As illustrated in Figures 6C, D, the model exhibits good predictability without overfitting and can be analyzed in the next step. The differential metabolite screening results were visualized as volcano plots, shown in Figures 6E, F. Substances meeting the following requirements were identified as differential metabolites: (1) Variable Importance Projection (VIP) value >1; (2) Significant difference p-value < 0.01; and (3) Exclusion of heterologous metabolites in the context of the literature. A total of 81 differential metabolites with potential as biomarkers were identified in the serum of HFFD-fed mice after AAP consumption (Supplementary Table S2). Among these, metabolites 1–55 were acquired using positive ion mode, while metabolites 56–81 were acquired by negative ion mode scan. The compounds L-Proline, L-Lysine, Citrulline, L-Phenylalanine, (±)- Tryptophan, and L-Norleucine were detected in both positive and negative ionization modes.
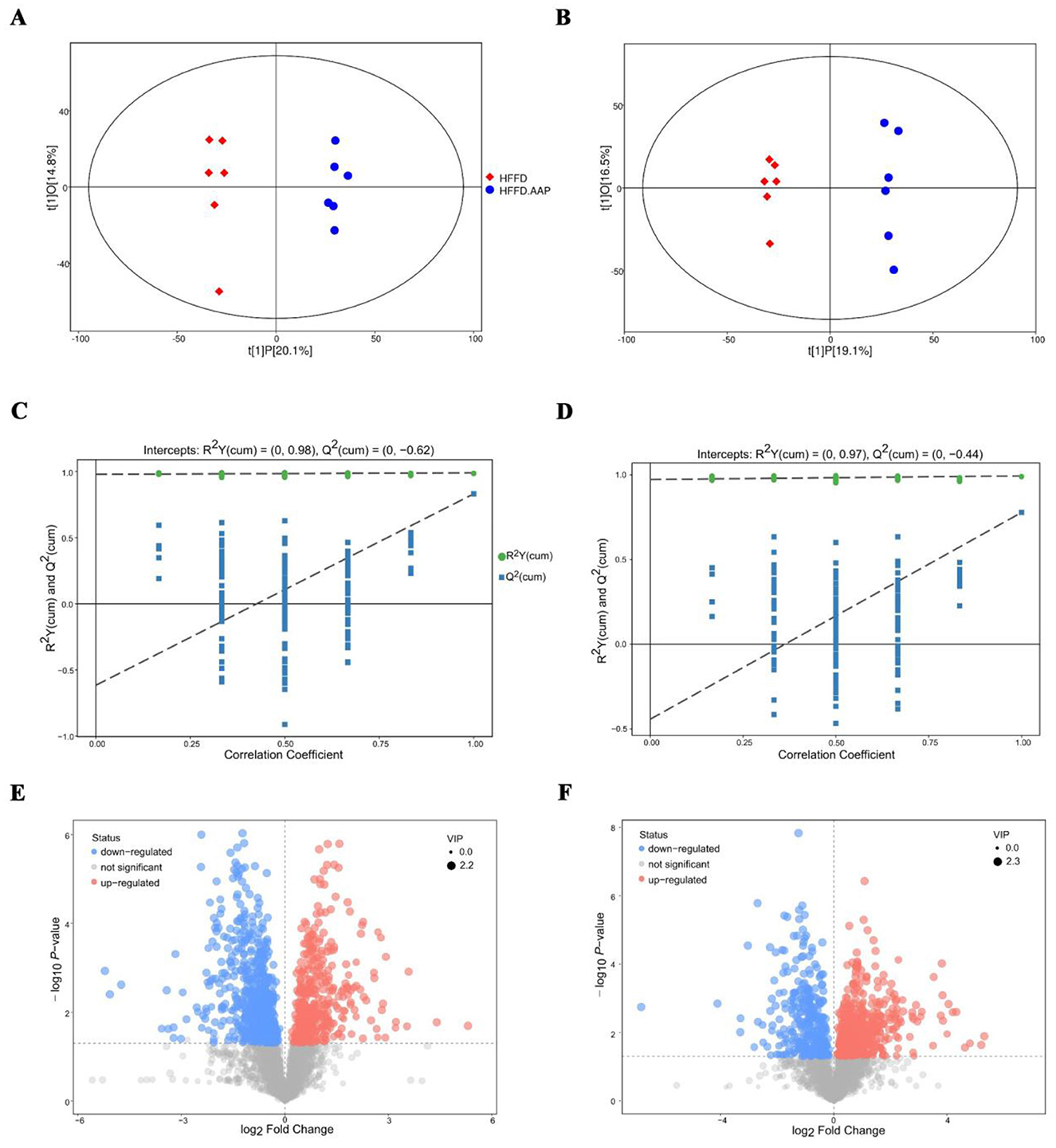
Figure 6. Biomarker identification in AAP treated mice using serum metabolomics. OPLS-DA score plot in (A) positive ion mode and (B) negative ion mode; OPLS-DA replacement test (200 times) in (C) positive ion mode and (D) negative ion mode; volcano map of differential metabolite screening in (E) positive ion mode and (F) negative ion mode.
3.6 Relevance analysis
To elucidate multidimensional interactions within the gut-brain axis, Spearman's rank correlation analysis was systematically conducted based on experimental datasets encompassing intestinal microbiota profiles, serum metabolomic signatures, and neurotrophic parameters. Figure 7A reveals significant positive correlations between Dubosiella abundance and both locomotor activity indices (total distance) and intestinal barrier integrity markers (Occludin). Bacteroides demonstrated synergistic associations with antioxidant enzymes (SOD, GSH) and tight junction proteins (Zo-1), whereas inverse correlations were observed with Bace-1 expression levels. There was a strong negative correlation with Bace-1 and a notably positive correlation with SOD and Zo-1 for Parasutterella. Ileibacterium exhibited a significant positive association with Bace-1 and Rage, and significant negative correlations with SOD, Il-10, CAT, GSH, and Zo-1. Metabolite levels with p-values ≤ 0.001 were heat mapped against the abundance of colonies that showed a significant difference in presence. The results showed that significantly different flora abundances correlated with serum metabolites, the intestinal flora may regulate body metabolism by regulating flora metabolites (Figure 7B).

Figure 7. Correlation analysis. (A) Correlation analysis between characteristic bacteria and basic indices, and (B) Correlation analysis between characteristic bacteria and differential serum metabolites. Deeper color represents greater correlation. ⋆ indicates p < 0.05, ⋆⋆ indicates p < 0.01.
4 Discussion
A strong relationship between diet and risk factors for neurodegenerative disorders has been demonstrated. Excessive saturated fatty acid intake enhances neurodegeneration in Alzheimer's disease (AD) and Parkinson's disease (PD) by exacerbating oxidative stress and lipid peroxidation, and high-calorie intake is positively associated with early onset of Hyperactivity disorder (HD) (29). Increased saturated fat intake can also cause an inflammatory response, which can lead to peripheral immune cell entry into the central nervous system (30), which may be an exacerbating factor in neurological disease symptoms pursuant to poor dietary intake. Numerous studies have found that dietary intake of nutrients and their metabolites can regulate neuroinflammation and exert beneficial effects on neurological functions and metabolic disorders (31). The United Nations has recommended “one meat, one vegetable, and one mushroom” as the dietary pattern for the twenty-first century (32, 33). Hericium, Ganoderma, Lentinula, and Psilocöm are a few of the mushrooms that can be used to improve brain health and treat cognitive decline. Proactive substances such as cordycepin from Cordyceps, lentinan from Lentinula, and hericenones from mushrooms and mycelia of lion mane can reduce stress, improve sleep quality, and improve memory by stimulating the brain and reducing cortical neuron shrinkage (34–36). In the current investigation, it was discovered that learning and memory in mice were markedly enhanced by active polysaccharides isolated from Auricularia Auricula-judae, and that prolongation of escape latency and reduction in the percentage of total distance traveled within the target quadrant in the water maze induced by a high-caloric-energy diet were significantly inhibited.
The gut, as the body's largest immunological organ, and the microorganisms that live there are essential for regulating metabolism and endocrinology. Macrogenomic data suggest that extrinsic factors, including diet, greatly outweigh the contribution of genetic factors in regulating the structure and function of the intestinal flora (37). Customized diets have the potential to alter an organism's internal metabolism, which can have an impact on health (38, 39). Animal experiments and population studies have shown that changes in microbiota composition in response to a hypercaloric diet include an increase in the proportion of thick-walled bacilli and mycobacteriophages, and increased abundance of Dentinobacterium, Bacillus, and Clostridium (all of which belong to the thick-walled bacilli phylum). Compared to animal-based diets, plant-based diets promote gut microbiota complexity, increase the abundance of dietary fiber-fermenting microbiota, and induce increases in metabolites including SCFA in the gut and circulating blood (40). Zhang et al. conducted a comparative study on the regulatory effects of Auricularia auricula and AAP on the intestinal microbiota of rats with hyperlipidemia. They found that AAP strongly stimulated certain SCFA-producing bacteria with lower abundance, such as Flavonifractor and Clostridium IV (41). Our previous study found that supplementation with AAP (from Qinba mountains) significantly changed mouse intestinal flora composition compared to the normal C57BL/6J mouse group. The relative abundances of Lactobacillus johnsonii, Weissella cibaria, Kosakonia cowanii, Enterococcus faecalis, Bifidobacterium animalis, and Bacteroides uniformis were remarkably enhanced, whereas Firmicutes bacterium M10-2 was downregulated (22). In the present study, AAP supplementation significantly boosted the abundance of Dubosiella in the intestinal tract of HFFD mice, upregulated the abundance of Bacteroides and Parasutterella, and markedly reduced the abundance of Ileibacterium to levels similar to those in normal C57BL/6J mice (Figure 5F).
It has been found that Dubosiella can exert an inhibitory effect on AD development through the synthesis of palmitoleic acid (42). In addition, Dubosiella can regulate the homeostasis of intestinal flora, e.g., SCFAs produced by Dubosiella newyorkensis can regulate intestinal immune homeostasis by activating the AhR-IDO1-Kyn metabolic circuit (43). Bacteroides has a wide range of health benefits and is one of the most abundant genera in the human gut flora. Previous studies have shown that Bacteroides uniformis improves locomotor performance in mice and humans (44). Key features associated with Aβ loading include a reduction in Bacteroides, while the addition of Bacteroides ovatus was effective in improving AD symptoms (45). Additionally, Autism spectrum disorder (ASD)-like behaviors can be improved by regulating intestinal amino acid transport protein levels and serum glutamine levels through supplementation with Bacteroides uniformis (46). Bacteroides possesses 20% of the genome to regulate the degradation of polysaccharides, e.g., it can degrade complex arabinoxylans by manipulating the polysaccharide-utilization loci while releasing ferulic acid, which in turn exerts immunomodulatory effects (47, 48). Parasutterell is associated with a high calorie diet-induced inflammation of the hypothalamus, which controls appetite by regulating the secretion of multiple hormones, and dysregulation of appetite and satiety can lead to weight gain (49). Furthermore, its ability to regulate the process of bile acid synthesis by altering bile acid metabolites has a potential therapeutic effect on hypercaloric diets (50). Correlation analysis is the starting point for gut-brain axis research. However, correlation analyses have limitations, including the inability to target specific strains of bacteria and the inability to determine whether gut-brain interactions show a threshold effect or a dynamic equilibrium (e.g., a critical abundance of a particular group of bacteria is required to affect neurotransmitters). These limitations need to be compensated for by causal inference experiments, refined study design and integration of multidisciplinary approaches (e.g., fecal microbiota transplantation assays, targeted colony editing, etc.). Further verification is still needed to prove that the characteristic bacteria in the mice intestine play a key role in cognitive intervention after dietary supplementation with AAP.
A lot of studies have demonstrated that the intestinal flora can achieve bidirectional information exchange with the central and enteric nervous systems via direct and indirect pathways involving multiple systems, including neural, endocrine, and immune systems, forming the flora-gut-brain axis (51). Intestinal flora effects on the nervous system include direct interactions with enterocytes and enteric neurons, metabolite-mediated inflammation, vagal activation, HPA axis modulation, and immune system regulation (52–54). Ma et al. demonstrated that Lactobacillus, Akkermansia, Oscillospira, etc. were associated with cognitive decline by fecal microbiota transplantation (55). Akkermansia has also been shown in earlier studies to be a beneficial bacterium that improves cognitive deficits (56). Current research suggests that gut flora has a causal effect on cognitive function through metabolites, immune modulation and gut-brain axis signaling. However, this conclusion is mainly based on animal model intervention experiments (flora transplantation, direct intervention with metabolites) and human intervention studies (probiotic/prebiotic trials, dietary interventions). Gut microecological changes induced by high-fat, high-sugar diets are important factors in the development of cognitive dysfunction and anxiety-like behaviors. Non-digestible polysaccharides cannot be digested or utilized by the body. One of their key biological roles is to maintain intestinal flora homeostasis (57). Polysaccharides improve intestinal integrity; alleviate intestinal mucosal damage; increase the activity of carbohydrate-active enzymes; promote SCFAs production; decrease endotoxin levels; downregulate inflammatory factor expression; upregulate tight junction protein expression; and regulate microglial cell maturation (58). Although structure-function relationships have not been clarified, some structural properties of these polysaccharides, including molecular weight, composition of monosaccharides, and the way their molecular chains are linked, may be crucial for their brain nutritional properties (59). The study demonstrated that AAP from the Qinba Mountains exerted a significant modulatory effect on serum metabolite profiles in mice fed a hypercaloric diet, with the differentiated genes mainly enriched in the glycerophosphate metabolism pathway. AAP up-regulated synaptic plasticity-related genes expressions, such as Snap-25 and Psd-95, and suppressed Il-1β and Tnf-α inflammatory mediators levels, effectively ameliorating high-caloric diet-induced cognitive decline in mice. This polysaccharide was found to be primarily composed of mannose (>60%) and glucuronic acid in our earlier investigation (60). In a 5xFAD transgenic AD mice model, LIU et al. discovered that an 8-week therapy with Mannan oligosaccharide (MOS, 0.12% w/v) dramatically enhanced cognitive performance and spatial memory, along with decreased anxiety- and obsessive-like behaviors. Furthermore, MOS improved HPA axis disorders by upregulating norepinephrine expression and lowering corticosterone and corticotropin-releasing hormone levels (61). To what extent the brain health-promoting effects of AAP are dependent on their mannose content, their effector molecules, and the relationship between fine structure and function needs to be further investigated. This study demonstrates the potential of edible mushroom polysaccharides in improving cognitive function based on animal models; however, human clinical trials have not yet been conducted. The effective dosage, feasibility of translating mechanisms of action to humans, and long-term safety profiles still require systematic validation.
5 Conclusion
The therapeutic promise of AAP in nutritional interventions has been documented, whereas the mechanistic connections between its bioactive properties and high calorie diets-induced cognitive deterioration require further elucidation. In the present investigation, the capacity of AAP to mitigate HFFD-associated cognitive dysfunction was mechanistically linked to its coordinated regulation of intestinal microbiota composition and serum metabolic signatures in murine models. These results advance the current understanding of plant-derived polysaccharides as multifunctional modulators of the microbiota-metabolite axis, establishing AAP as a candidate for targeted dietary strategies against neurocognitive complications. Additionally, the mechanistic framework delineated herein supports the rational design of nutritional guidelines to counteract health risks associated with excessive caloric intake.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by the Animal Ethics Committee of the Laboratory Animal Center of Northwest University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YF: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. JZ: Data curation, Formal analysis, Investigation, Writing – original draft. SY: Data curation, Investigation, Visualization, Writing – original draft. YL: Investigation, Visualization, Writing – original draft. XW: Project administration, Validation, Writing – original draft. MW: Project administration, Validation, Writing – original draft. HZ: Supervision, Writing – review & editing. QL: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Key research and development plans of Shaanxi province (No. 2023-ZDLNY-30); Innovation ability support plan of Shaanxi province-young science and technology nova talent project (2024ZC-KJXX-071); National Key Research and Development Program (2021YFD1600400); and Xi'an Science and Technology Plan Project (24ZHFH0009).
Conflict of interest
XW, MW, and QL were employed by Xi'an Xida Institute of Life Sciences and Health Research Co., Ltd. and Shaanxi Functional Food Engineering Center Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1585778/full#supplementary-material
References
1. Gannon OJ, Robison LS, Salinero AE, Abi-Ghanem C, Mansour FM, Kelly RD, et al. High-fat diet exacerbates cognitive decline in mouse models of Alzheimer's disease and mixed dementia in a sex-dependent manner. J Neuroinflammation. (2022) 19:110. doi: 10.1186/s12974-022-02466-2
2. Valentin-Escalera J, Leclerc M, Calon F. High-fat diets in animal models of alzheimer's disease: how can eating too much fat increase Alzheimer's disease risk? J Alzheimers Dis. (2024) 97:977–1005. doi: 10.3233/JAD-230118
3. Gainey SJ, Kwakwa KA, Bray JK, Pillote MM, Tir VL, Towers AE, et al. Short-term high-fat diet (hfd) induced anxiety-like behaviors and cognitive impairment are improved with treatment by glyburide. Front Behav Neurosci. (2016) 10:156. doi: 10.3389/fnbeh.2016.00156
4. Mota B, Ramos M, Marques SI, Silva A, Pereira PA, Madeira MD, et al. Effects of high-fat and high-fat high-sugar diets in the anxiety, learning and memory, and in the hippocampus neurogenesis and neuroinflammation of aged rats. Nutrients. (2023) 15:1370. doi: 10.3390/nu15061370
5. Liang Z, Gong X, Ye R, Zhao Y, Yu J, Zhao Y, et al. Long-term high-fat diet consumption induces cognitive decline accompanied by tau hyper-phosphorylation and microglial activation in aging. Nutrients. (2023) 15:250. doi: 10.3390/nu15010250
6. Tan BL, Norhaizan ME. Effect of high-fat diets on oxidative stress, cellular inflammatory response and cognitive function. Nutrients. (2019) 11:2579. doi: 10.3390/nu11112579
7. Freeman LR, Haley-Zitlin V, Rosenberger DS, Granholm AC. Damaging effects of a high-fat diet to the brain and cognition: a review of proposed mechanisms. Nutr Neurosci. (2014) 17:241–51. doi: 10.1179/1476830513Y.0000000092
8. Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. (2011) 141:599–U701. doi: 10.1053/j.gastro.2011.04.052
9. Collins SM, Kassam Z, Bercik P. The adoptive transfer of behavioral phenotype via the intestinal microbiota: experimental evidence and clinical implications. Curr Opin Microbiol. (2013) 16:240–5. doi: 10.1016/j.mib.2013.06.004
10. Islam T, Ganesan K, Xu BJ. Insights into health-promoting effects of jew's ear (Auricularia Auricula-Judae). Trends Food Sci Technol. (2021) 114:552–69. doi: 10.1016/j.tifs.2021.06.017
11. Zhou Y, Jia Y, Xu N, Tang L, Chang Y. Auricularia Auricula-Judae (Bull) polysaccharides improve obesity in mice by regulating gut microbiota and Tlr4/Jnk signaling pathway. Int J Biol Macromol. (2023) 250:126172. doi: 10.1016/j.ijbiomac.2023.126172
12. Shu Y, Huang Y, Dong W, Fan X, Sun Y, Chen G, et al. The polysaccharides from auricularia auricula alleviate non-alcoholic fatty liver disease via modulating gut microbiota and bile acids metabolism. Int J Biol Macromol. (2023) 246:125662. doi: 10.1016/j.ijbiomac.2023.125662
13. Choi SB, Kwon S, Kim JH, Ahn NH, Lee JH, Yang SH. The molecular mechanisms of neuroinflammation in Alzheimer's disease, the consequence of neural cell death. Int J Mol Sci. (2023) 24:11757. doi: 10.3390/ijms241411757
14. Miao J, Regenstein JM, Qiu J, Zhang J, Zhang X, Li H, et al. Isolation, structural characterization and bioactivities of polysaccharides and its derivatives from auricularia-a review. Int J Biol Macromol. (2020) 150:102–13. doi: 10.1016/j.ijbiomac.2020.02.054
15. Ma Z, Wang J, Zhang L. Structure and chain conformation of beta-glucan isolated from auricularia auricula-judae. Biopolymers. (2008) 89:614–22. doi: 10.1002/bip.20971
16. Xiong QP, Zhu LJ, Zhang FM Li HL, Wu J, Liang J, et al. Protective activities of polysaccharides from Cipangopaludina Chinensis against high-fat-diet-induced atherosclerosis via regulating gut microbiota in apoe-deficient mice. Food Funct. (2019) 10:6644–54. doi: 10.1039/C9FO01530B
17. Nakyam T, Wattanathorn J, Thukham-Mee W, Muchimapura S. The polyherbal functional ingredient containing ginger, chinese date, and wood ear mushroom protects against dementia following metabolic syndrome. Biomed Res Int. (2023) 2023:9911397.
18. Liu X, Sharma RK, Mishra A, Chinnaboina GK, Gupta G, Singh M. Role of aqueous extract of the wood ear mushroom, Auricularia polytricha (Agaricomycetes), in avoidance of haloperidol-induced catalepsy via oxidative stress in rats. Int J Med Mushrooms. (2019) 21:323–30. doi: 10.1615/IntJMedMushrooms.2019030351
19. Zhang H, Wang ZY, Zhang Z, Wang X. Purified Auricularia Auricular-Judae polysaccharide (Aap I-a) prevents oxidative stress in an ageing mouse model. Carbohydr Polym. (2011) 84:638–48. doi: 10.1016/j.carbpol.2010.12.044
20. Liu XN Li X, Xia B, Jin X, Zou QH, Zeng ZH, et al. High-fiber diet mitigates maternal obesity-induced cognitive and social dysfunction in the offspring via gut-brain axis. Cell Metab. (2021) 33:923–8. doi: 10.1016/j.cmet.2021.02.002
21. Tang JJ, Huang LF, Deng JL, Wang YM, Guo C, Peng XN, et al. Cognitive enhancement and neuroprotective effects of oabl, a sesquiterpene lactone in 5xfad Alzheimer's disease mice model. Redox Biol. (2022) 50:102229. doi: 10.1016/j.redox.2022.102229
22. Liu Q, An X, Chen Y, Deng Y, Niu H, Ma R, et al. Effects of auricularia auricula polysaccharides on gut microbiota and metabolic phenotype in mice. Foods. (2022) 11:2700. doi: 10.3390/foods11172700
23. Liu Q, Ma R, Li S, Fei Y, Lei J, Li R, et al. Dietary supplementation of auricularia auricula-judae polysaccharides alleviate nutritional obesity in mice via regulating inflammatory response and lipid metabolism. Foods. (2022) 11:942. doi: 10.3390/foods11070942
24. Lee SH. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest Res. (2015) 13:11–8. doi: 10.5217/ir.2015.13.1.11
25. Shi H, Yu Y, Lin D, Zheng P, Zhang P, Hu M, et al. Beta-glucan attenuates cognitive impairment via the gut-brain axis in diet-induced obese mice. Microbiome. (2020) 8:143. doi: 10.1186/s40168-020-00920-y
26. Apaijit K, Pakdeechote P, Maneesai P, Meephat S, Prasatthong P, Bunbupha S. Hesperidin alleviates vascular dysfunction and remodelling in high-fat/high-fructose diet-fed rats by modulating oxidative stress, inflammation, adipor1, and enos expression. Tissue Cell. (2022) 78:101901. doi: 10.1016/j.tice.2022.101901
27. Lecomte V, Kaakoush NO, Maloney CA, Raipuria M, Huinao KD, Mitchell HM, et al. Changes in gut microbiota in rats fed a high fat diet correlate with obesity-associated metabolic parameters. PLoS ONE. (2015) 10:e0126931. doi: 10.1371/journal.pone.0126931
28. Mao J-H, Kim Y-M, Zhou Y-X, Hu D, Zhong C, Chang H, et al. Genetic and metabolic links between the murine microbiome and memory. Microbiome. (2020) 8:53. doi: 10.1186/s40168-020-00870-5
29. Studzinski CM Li F, Bruce-Keller AJ, Fernandez-Kim SO, Zhang L, Weidner AM, et al. Effects of short-term western diet on cerebral oxidative stress and diabetes related factors in app X Ps1 knock-in mice. J Neurochem. (2009) 108:860–6. doi: 10.1111/j.1471-4159.2008.05798.x
30. Buckman LB, Hasty AH, Flaherty DK, Buckman CT, Thompson MM, Matlock BK, et al. Obesity induced by a high-fat diet is associated with increased immune cell entry into the central nervous system. Brain Behav Immun. (2014) 35:33–42. doi: 10.1016/j.bbi.2013.06.007
31. Decandia D, Gelfo F, Landolfo E, Balsamo F, Petrosini L, Cutuli D. Dietary protection against cognitive impairment, neuroinflammation and oxidative stress in Alzheimer's disease animal models of lipopolysaccharide-induced inflammation. Int J Mol Sci. (2023) 24:5921. doi: 10.3390/ijms24065921
32. Rani M, Mondal SM, Kundu P, Thakur A, Chaudhary A, Vashistt J, et al. Edible mushroom: occurrence, management and health benefits. Food Mat Res. (2023) 3:21. doi: 10.48130/FMR-2023-0021
33. Thomas L, Mago P. Unearthing the Therapeutic Benefits of Culinary-Medicinal Mushrooms for Humans: Emerging Sustainable Bioresources of 21st Century. J Basic Microbiol (2024) 64:e2400127. doi: 10.1002/jobm.202400127
34. Venturella G. Biology, cultivation and applications of mushrooms. Q Rev Biol. (2024) 99:57–8. doi: 10.1086/729235
35. Su AX, Yang WJ, Zhao LY, Pei F, Yuan B, Zhong L, et al. Flammulina Velutipes polysaccharides improve scopolamine-induced learning and memory impairment in mice by modulating gut microbiota composition. Food Funct. (2018) 9:1424–32. doi: 10.1039/C7FO01991B
36. Arya C. “Potential uses of mushrooms as dietary supplement to enhance memory,” In: Biology, Cultivation and Applications of Mushrooms. Singapore: Springer Singapore (2022), 387–402.
37. Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. (2018) 555:210–5. doi: 10.1038/nature25973
38. Bashiardes S, Godneva A, Elinav E, Segal E. Towards utilization of the human genome and microbiome for personalized nutrition. Curr Opin Biotechnol. (2018) 51:57–63. doi: 10.1016/j.copbio.2017.11.013
39. Verma M, Hontecillas R, Tubau-Juni N, Abedi V, Bassaganya-Riera J. Challenges in personalized nutrition and health. Front Nutr. (2018) 5:117. doi: 10.3389/fnut.2018.00117
40. Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. (2011) 334:105–8. doi: 10.1126/science.1208344
41. Zhang TT, Zhao WY, Xie BZ, Liu H. Effects of Auricularia Auricula and its polysaccharide on diet-induced hyperlipidemia rats by modulating gut microbiota. J Funct Foods. (2020) 72:104038. doi: 10.1016/j.jff.2020.104038
42. Chen Y, Li Y, Fan Y, Chen S, Chen L, Chen Y, et al. Gut microbiota-driven metabolic alterations reveal gut-brain communication in Alzheimer's disease model mice. Gut Microbes. (2024) 16:2302310. doi: 10.1080/19490976.2024.2302310
43. Zhang Y, Tu S, Ji X, Wu J, Meng J, Gao J, et al. Dubosiella newyorkensis modulates immune tolerance in colitis via the L-lysine-activated Ahr-Ido1-Kyn pathway. Nat Commun. (2024) 15:1333. doi: 10.1038/s41467-024-45636-x
44. Morita H, Kano C, Ishii C, Kagata N, Ishikawa T, Hirayama A, et al. Bacteroides uniformis and its preferred substrate, alpha-cyclodextrin, enhance endurance exercise performance in mice and human males. Sci Adv. (2023) 9:eadd2120. doi: 10.1126/sciadv.add2120
45. Zha X, Liu X, Wei M, Huang H, Cao J, Liu S, et al. Microbiota-derived lysophosphatidylcholine alleviates Alzheimer's disease pathology via suppressing ferroptosis. Cell Metab. (2025) 37:169–86 e9. doi: 10.1016/j.cmet.2024.10.006
46. Yu Y, Zhang B, Ji P, Zuo Z, Huang Y, Wang N, et al. Changes to gut amino acid transporters and microbiome associated with increased E/I ratio in Chd8(+/–) mouse model of Asd-like behavior. Nat Commun. (2022) 13:1151. doi: 10.1038/s41467-022-28746-2
47. Pereira GV, Abdel-Hamid AM, Dutta S, D'Alessandro-Gabazza CN, Wefers D, Farris JA, et al. Degradation of complex arabinoxylans by human colonic bacteroidetes. Nat Commun. (2021) 12:459. doi: 10.1038/s41467-020-20737-5
48. Schwalm ND, Groisman EA. Navigating the gut buffet: control of polysaccharide utilization in Bacteroides Spp. Trends Microbiol. (2017) 25:1005–15. doi: 10.1016/j.tim.2017.06.009
49. Kreutzer C, Peters S, Schulte DM, Fangmann D, Tuerk K, Wolff S, et al. Hypothalamic inflammation in human obesity is mediated by environmental and genetic factors. Diabetes. (2017) 66:2407–15. doi: 10.2337/db17-0067
50. Ju T, Kong JY, Stothard P, Willing BP. Defining the role of parasutterella, a previously uncharacterized member of the core gut microbiota. ISME J. (2019) 13:1520–34. doi: 10.1038/s41396-019-0364-5
51. Long-Smith C, O'Riordan KJ, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota-gut-brain axis: new therapeutic opportunities. Ann Rev Pharmacol Toxicol. (2020) 60:477–502. doi: 10.1146/annurev-pharmtox-010919-023628
52. Wu Y, Hang Z, Lei T, Du H. Intestinal flora affect Alzheimer's disease by regulating endogenous hormones. Neurochem Res. (2022) 47:3565–82. doi: 10.1007/s11064-022-03784-w
53. Agirman G, Yu KB, Hsiao EY. Signaling inflammation across the gut-brain axis. Science. (2021) 374:1087–92. doi: 10.1126/science.abi6087
54. Rutsch A, Kantsjö JB, Ronchi F. The gut-brain axis: how microbiota and host inflammasome influence brain physiology and pathology. Front Immunol. (2020) 11:604179. doi: 10.3389/fimmu.2020.604179
55. Ma X, Liu J, Jiang L, Gao Z, Shi Z, Zhang N, et al. Dynamic changes in the gut microbiota play a critical role in age-associated cognitive dysfunction via scfas and lps synthesis metabolic pathways during brain aging. Int J Biol Macromol. (2025) 304:140945. doi: 10.1016/j.ijbiomac.2025.140945
56. Gu X, Bi N, Wang T, Huang C, Wang R, Xu Y, et al. Probiotic Lactobacillus rhamnosus Gr-1 supplementation attenuates pb-induced learning and memory deficits by reshaping the gut microbiota. Front Nutr. (2022) 9:934118. doi: 10.3389/fnut.2022.934118
57. Sun QY, Cheng L, Zeng XX, Zhang X, Wu ZF, Weng PF. The Modulatory effect of plant polysaccharides on gut flora and the implication for neurodegenerative diseases from the perspective of the microbiota-gut-brain axis. Int J Biol Macromol. (2020) 164:1484–92. doi: 10.1016/j.ijbiomac.2020.07.208
58. Erny D, de Angelis ALH, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the Cns. Nat Neurosci. (2015) 18:965–77. doi: 10.1038/nn.4030
59. Rang YF, Liu H, Liu CH. Potential for non-starch polysaccharides in the prevention and remediation of cognitive impairment: a comprehensive review. Int J Biol Macromol. (2022) 208:182–95. doi: 10.1016/j.ijbiomac.2022.03.065
60. Liu Q, Zhang X, Wang Y, Wang A, Zhao Y, Long Z, et al. Physicochemical properties and antioxidant activities in vitro of auricularia auricula polysaccharides in Qinba Mountain Area. Food Ferment Industries. (2021) 47:91–7. doi: 10.13995/j.cnki.11-1802/ts.027141
Keywords: Auricularia auricula-judae, polysaccharides, behavioral disorders, hypercaloric diet, gut microbiota
Citation: Fei Y, Zhang J, Yuan S, Liu Y, Wang X, Wang M, Zhao H and Liu Q (2025) Ameliorating potential of Auricularia auricula-judae polysaccharides in mitigating hypercaloric diet-induced behavioral disorders through gut microbiota regulation. Front. Nutr. 12:1585778. doi: 10.3389/fnut.2025.1585778
Received: 01 March 2025; Accepted: 07 May 2025;
Published: 29 May 2025.
Edited by:
Dionisio Pedro Amorim-Neto, Campinas State University, BrazilReviewed by:
Mahmoud Yolmeh, Campinas State University, BrazilEdem E. Edem, Afe Babalola University, Nigeria
Copyright © 2025 Fei, Zhang, Yuan, Liu, Wang, Wang, Zhao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian Liu, bGl1cWlhbjIwMTdAbnd1LmVkdS5jbg==
 Yujie Fei
Yujie Fei Jingwen Zhang
Jingwen Zhang Sijia Yuan
Sijia Yuan Ye Liu
Ye Liu Xiaoru Wang2,3
Xiaoru Wang2,3 Qian Liu
Qian Liu