- Clinical Nutrition, The General Hospital of Western Theater Command, Chengdu, China
Background: Pediatric asthma, a prevalent chronic disease with rising global incidence, imposing substantial healthcare and socioeconomic burdens. Emerging evidence highlights the gut-lung axis as a pivotal therapeutic target, with microbiota dysbiosis implicated in immune dysregulation and airway hyperresponsiveness. This systematic review evaluated the efficacy and safety of probiotics, prebiotics, synbiotics, and postbiotics in pediatric asthma management.
Methods: A comprehensive search of PubMed, Cochrane library, Web of Science, and Embase was conducted up to 2nd January 2025. Inclusion criteria encompassed randomized controlled trials (RCTs) evaluating the therapeutic use of probiotics, prebiotics, synbiotics, or postbiotics in children and/or adolescents (<18 years) with asthma.
Results: Eighteen studies (13 RCTs, n = 2,419 participants) were analyzed, focusing on children aged < 18 years. Probiotic interventions, predominantly Lactobacillus (5 studies) and Bifidobacterium (5 studies), demonstrated significant reductions in asthma exacerbations and improved pulmonary function, with strain-specific effects linked to Th2 cytokine suppression and gut-lung axis modulation. Postbiotics, including bacterial lysates (OM-85 BV, PMBL®), attenuated airway hyperresponsiveness and systemic inflammation. Synbiotics reduced viral respiratory infections and healthcare utilization. However, there is still a lack of direct RCTs to explore the therapeutic effects of prebiotics on pediatric asthma. Key limitations include methodological heterogeneity (dosing: 108–1010 CFU/day; duration: 8 weeks−12 months) and risk of bias (3 low-risk, 12 with concerns).
Conclusion: Our findings underscored the potential of microbiota-targeted therapies but highlight the need for standardized protocols, strain-specific trials, and pediatric prebiotic research. Future studies should integrate multi-omics to elucidate mechanisms and optimize personalized interventions.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42025641318, identifier: CRD42025641318.
1 Introduction
Asthma is a chronic inflammatory airway disease characterized by airway hyperresponsiveness, reversible airflow obstruction, and heterogeneous clinical phenotypes, affecting over 300 million individuals globally (1, 2). In children and adolescents, asthma remains the most prevalent chronic respiratory condition, according to the World Health Organization (WHO), ~14% of children worldwide experience asthma-related symptoms, with higher prevalence rates observed in high-income countries compared to low- and middle-income nations (3). Studies have shown that in 2019, the incidence of asthma in children aged 1–4 years, children aged 5–9 years, children aged 10–14 years and adolescents aged 15–19 years was 1,884.6/100,000, 980.3/100,000, 587.0/100,000, and 387.9/100,000, respectively (4). The burden of pediatric asthma is further compounded by its association with recurrent exacerbations, reduced quality of life, and significant healthcare costs (5, 6). Despite advances in pharmacotherapy, a subset of patients remains suboptimally controlled, underscoring the need for novel therapeutic strategies targeting (7).
Existing evidence highlights the gut-lung axis's multifactorial involvement in asthma pathogenesis, including immune modulation, metabolic (8–10), neuroendocrine (11, 12), and epithelial barrier mechanisms (13). Gut microbiota dysbiosis in pediatric asthma manifests not only as reduced diversity and altered Firmicutes/Bacteroidetes (14) ratio but also as disruption of microbial metabolite production, particularly short-chain fatty acids (SCFAs) like propionate and butyrate (8). These metabolites regulate pulmonary inflammation through G-protein coupled receptor-dependent pathways (9) and histone deacetylase inhibition (10), while their deficiency exacerbates Th17-mediated airway remodeling (15). Concurrently, gut barrier dysfunction permits bacterial lipopolysaccharide (LPS) translocation, activating TLR4/NF-κB signaling in alveolar macrophages and promoting neutrophilic infiltration. Notably, vagus nerve-mediated cholinergic anti-inflammatory pathways (11) and microbial-tryptophan metabolism derivatives (e.g., indole-3-aldehyde) (12) further modulate IL-22-dependent mucosal repair. Epigenetic reprogramming via microbial-derived microRNAs may additionally influence DNA methylation patterns in airway epithelial cells, predisposing to bronchial hyperresponsiveness (16). This interconnected microbial-immune-metabolic network establishes microbial homeostasis as a potential therapeutic target across asthma (17).
In recent years, various gut health interventions, including probiotics, prebiotics, synbiotics, and postbiotics, have been explored for their potential therapeutic roles in asthma management. Probiotics, such as Lactobacillus and Bifidobacterium strains, have shown immunomodulatory effects that may help reduce airway inflammation and asthma exacerbations (18). Synbiotics combining probiotics and prebiotics enhance microbial diversity and reduce wheezing episodes in high-risk infants and children (19). Postbiotics, which refer to the metabolic by-products of probiotic bacteria, may also play a role in modulating immune responses and providing protection against inflammatory conditions like asthma (20). However, existing evidence remains heterogeneous due to variations in strain specificity, dosing regimens, and outcome measures (21). A systematic synthesis of current data is critical to clarify the efficacy, safety, and mechanistic underpinnings of microbiota-targeted therapies in pediatric asthma, informing clinical guidelines and future research priorities.
2 Materials and methods
The research protocol has been registered with the International Prospective Register of Systematic Reviews (PROSPERO), under identifier CRD42025641318. To ensure methodological rigor and transparency, the review process strictly followed the PRISMA 2020 framework, with all checklist criteria systematically implemented to standardize reporting quality and minimize bias in evidence synthesis.
2.1 Sources and search strategy
The literature search was conducted from inception to 2nd January 2025 in the following electronic databases including PUBMED, EMBASE, Web of Science, and Cochrane Library. The main search string included the following words: probiotics, prebiotics, synbiotics, postbiotic, asthma and children, the detailed search strategy is provided in Supplementary material. The search was limited to human studies published in English.
2.2 Eligibility criteria
The inclusion criteria for eligible trials were defined as follows: (1) Trials investigating the therapeutic use of probiotics, prebiotics, synbiotics, or postbiotics in children and/or adolescents (<18 years) with asthma. (2) Studies employing a randomized controlled clinical trial design (parallel or crossover). (3) Control groups receiving placebo, usual care, conventional therapy, or no intervention. (4) Trials reporting at least one of the following outcomes: asthma exacerbation, relapse rate, symptomatic improvement (e.g., cough, wheezing), quality of life scores (validated questionnaires), Asthma Control Test (ACT) or Childhood Asthma Control Test (C-ACT), pulmonary function parameters [(e.g., the forced expiratory volume(FEV1), forced vital capacity(FVC), FEV1/FVC ratio, peak expiratory flow rate(PEFR)], inflammatory biomarkers (e.g., TNF-α, IL-10, IL-13, IFN-γ, IL-12), incidence of respiratory infections, immunological indices (e.g., IgE, IgG, CD3+ percentage, CD4+ percentage, CD4+/CD8+ ratio, NK cell activity).
The following types of studies were excluded: (1) non-human experimental models, animal studies, systematic reviews, meta-analyses, commentaries, study protocols, trial registrations, case reports. (2) Trials utilizing combined therapeutic regimens (e.g., synbiotics co-administered with vitamin D or acupuncture) that compromised intervention specificity. (3) Original full text not found.
2.3 Study selection and data extraction
Two investigators (D.F. and J.H.) performed duplicate independent screening of titles/abstracts followed by full-text evaluation against predefined inclusion/exclusion criteria. Discrepancies in inclusion judgments were reconciled through iterative discussion, with unresolved conflicts adjudicated by a third reviewer (N.L.). Researchers recorded the author, year of publication, country, sample size, population characteristics (age, sex, diagnosis), study design, intervention (including dosage and duration), outcome variables, and main findings from each paper, in an Excel spreadsheet.
2.4 Risk of bias assessment
Methodological quality appraisal was independently conducted by two investigators (D.F. and J.H.) utilizing the Cochrane RoB2 tool. Inter-rater discrepancies in bias risk classification underwent iterative adjudication by a third reviewer (N.L.) until full consensus was attained. Methodological rigor was assessed across seven domains defined by the Cochrane RoB2 framework: sequence randomization procedures, allocation concealment implementation, blinding protocols, outcome assessment objectivity, outcome data integrity, reporting transparency (selective outcome disclosure), ancillary bias sources. Domains were rated using standardized criteria (low/unclear/high risk) with explicit justification based on trial documentation.
3 Results
3.1 Literature search and selection
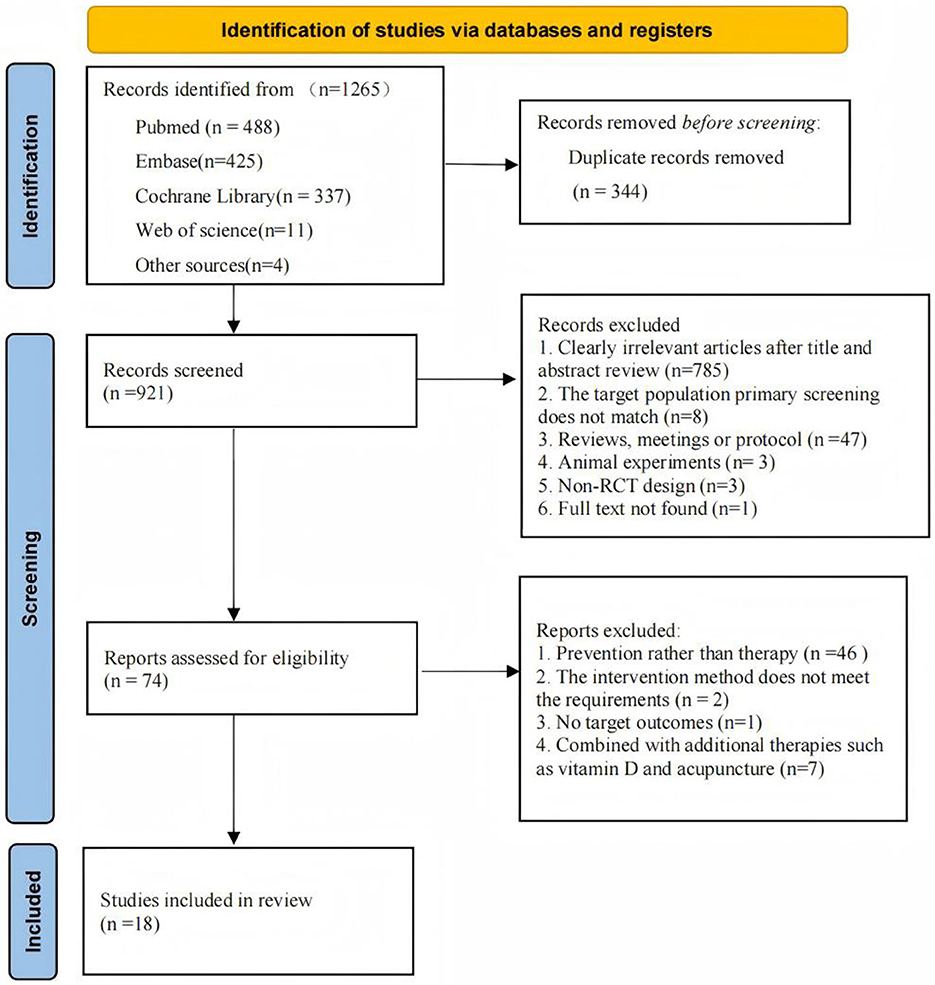
A systematic search identified 1265 potentially relevant articles, with 921 retained following duplicate removal. Title and abstract screening excluded 785 records, leaving 74 articles for full-text evaluation. Following rigorous application of inclusion criteria, 18 studies met eligibility requirements for final analysis. The study selection process is detailed in Figure 1.
3.2 Study characteristics
A systematic review of the 18 included investigations (22–39), comprising 13 randomized controlled trials (RCTs) and encompassing 2,419 participants, predominantly focused on pediatric populations. The majority studies exhibited a mean age range spanning childhood to preadolescence (2–11 years), only one investigation targeted infant cohorts (mean age <2 years). The male-to-female ratio approximated 1.4:1 (58.7% male, 41.3% female), reflecting a balanced yet slightly male-predominant demographic distribution. Among the 18 included studies evaluating microbial interventions: probiotics were investigated in 10 studies, with 4 deriving data from a single RCT (NCT04289441); postbiotics were assessed in 6 studies, including 2 sharing a common RCT dataset (NCT02541331); synbiotics were examined in 2 studies; no studies focused on prebiotics. Intervention durations spanned 8 weeks to 12 months. Heterogeneity in study populations included variations in asthma severity (intermittent to moderate persistent) and comorbid allergic rhinitis.
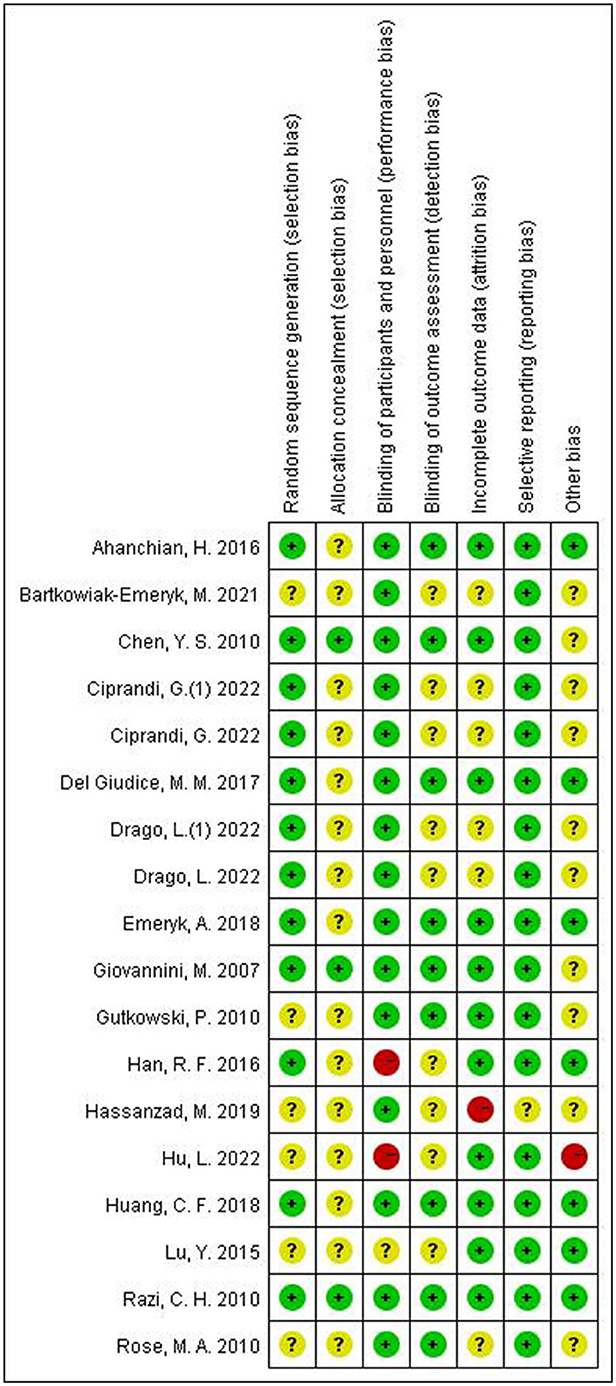
Risk of bias assessments for each included study are presented in Figure 2, 3 studies showed a low risk of bias, 3 studies showed a high risk of bias and 12 studies had some concerns.
3.3 Probiotics in the treatment of pediatric asthma
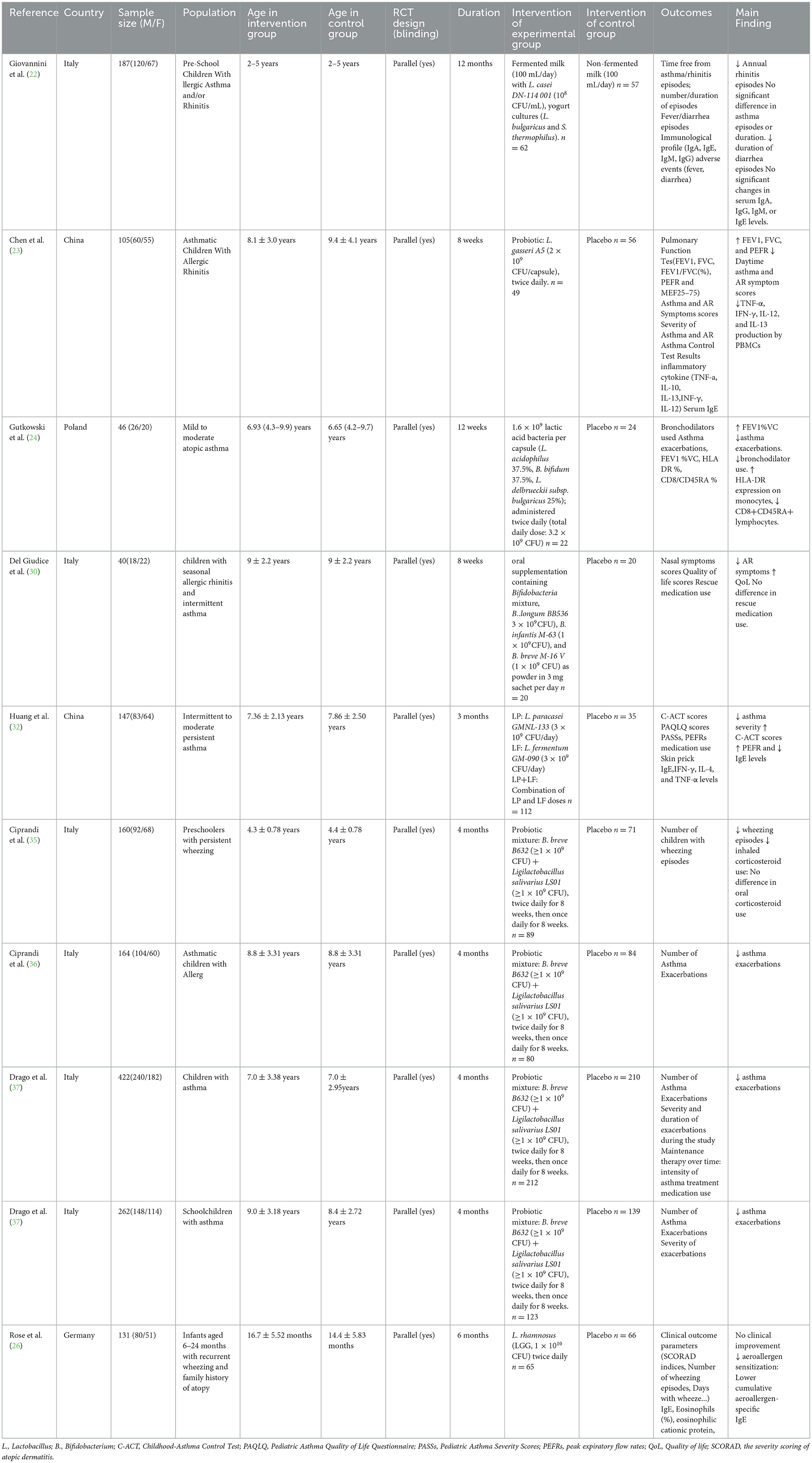
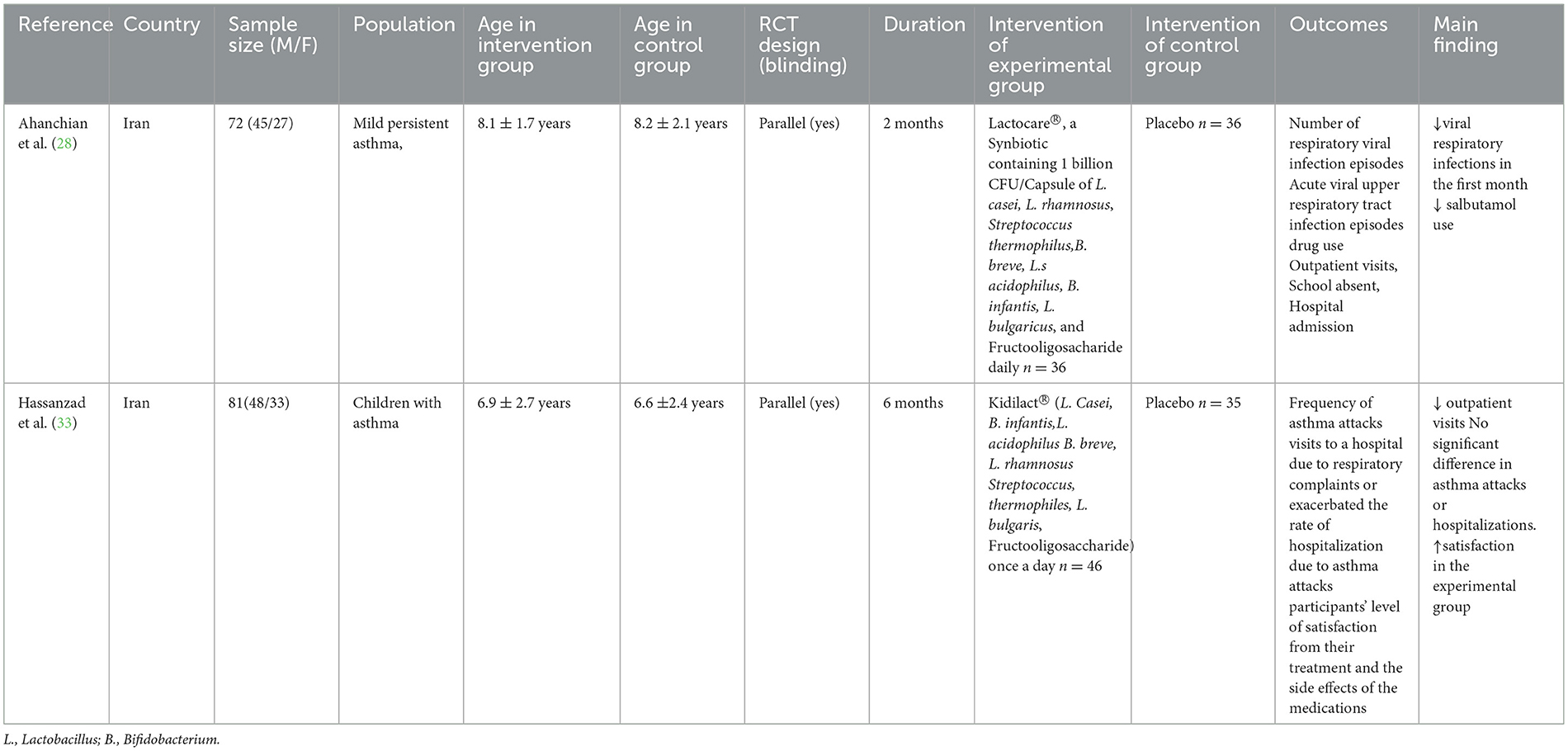
A total of 10 studies investigating the therapeutic efficacy of probiotics in pediatric asthma management were included. The studies included reports on Lactobacillus (n = 5), Bifidobacterium (n = 5), among the five studies investigating Bifidobacterium, four studies originated from a same RCT (NCT04289441). All of studies compared probiotics with control or placebo. Primary outcome measures comprised asthma exacerbations, pulmonary function, and immunological biomarkers. The characteristics and details of included studies were shown in Table 1.
3.3.1 Asthma exacerbation
Seven studies (22, 24, 26, 35–38) evaluated the effects of probiotics on asthma exacerbations. Five of these investigations demonstrated significant reductions in asthma exacerbations following probiotic administration. One study reported a markedly lower incidence of asthma exacerbations in the probiotic group (9%) compared to placebo (23.8%), with an odds ratio (OR) of 3.17 (p < 0.001) (37). Conversely, one study observed no statistically significant difference in asthma episode frequency or duration between intervention and control groups (P > 0.05) (22). Furthermore, a longitudinal analysis evaluating exacerbation patterns showed no statistically significant differences in wheezing episodes or days with wheeze during the 6-month intervention period and the subsequent 6-month follow-up phase (P > 0.05) (26).
3.3.2 Pulmonary function
Among all included studies on probiotic interventions, three investigated pulmonary function outcomes (23, 24, 32). Pooled data of these trials demonstrated significant improvements in pulmonary function indices within the probiotic intervention groups. Specifically, one trial documented enhanced lung function parameters [FEV1, FVC, and FEV1/FVC (%)] in the probiotic cohort compared to the control group (P < 0.05) (23). Another study showed improved PEFR in Lactobacillus paracasei GMNL-133 + Lactobacillus fermentum GM-090 group (32).
3.3.3 C-ACT scores
Two randomized trials evaluated the impact of probiotics vs. placebo on Childhood Asthma Control Test (C-ACT) scores. Both studies established significant C-ACT score enhancements with probiotic supplementation. Specifically, One study reported a significantly higher proportion of patients achieving improvements in C-ACT scores within the probiotic cohort (67.3%, 33/49) compared to the control group (58.9%, 33/56; P < 0.05) (23). Another study employed generalized estimating equations (GEE) with age-sex adjustments, demonstrating clinically relevant C-ACT improvements across probiotic arms (L. paracasei [LP], L. fermentum [LF], and LP+LF) (P < 0.05) (32).
3.3.4 Immune biomarker levels
Among the included studies, five investigated immune biomarker profiles, including IgE, IgG, TNF-α, IL-10, IL-13, IFN-γ, and IL-12. Regarding IgE-specific parameters, one trial documented significantly reduced total serum IgE levels in the L. paracasei (LP) + Lactobacillus fermentum (LF) intervention group post-treatment (P < 0.05) (32), while another study observed decreased cumulative aeroallergen-specific IgE in the probiotic cohort compared to controls (P = 0.027 post-intervention; P = 0.03 at follow-up) (26). In contrast, two other trials found no statistically significant intergroup differences in IgE levels (22, 23). For inflammatory mediators, PBMC analysis in one study revealed significant reductions in TNF-α, IFN-γ, IL-12, and IL-13 production following probiotic administration (23). Conversely, serum concentrations of IFN-γ, IL-4, and TNF-α remained unchanged in another investigation (32).
3.4 Postbiotics in the treatment of pediatric asthma
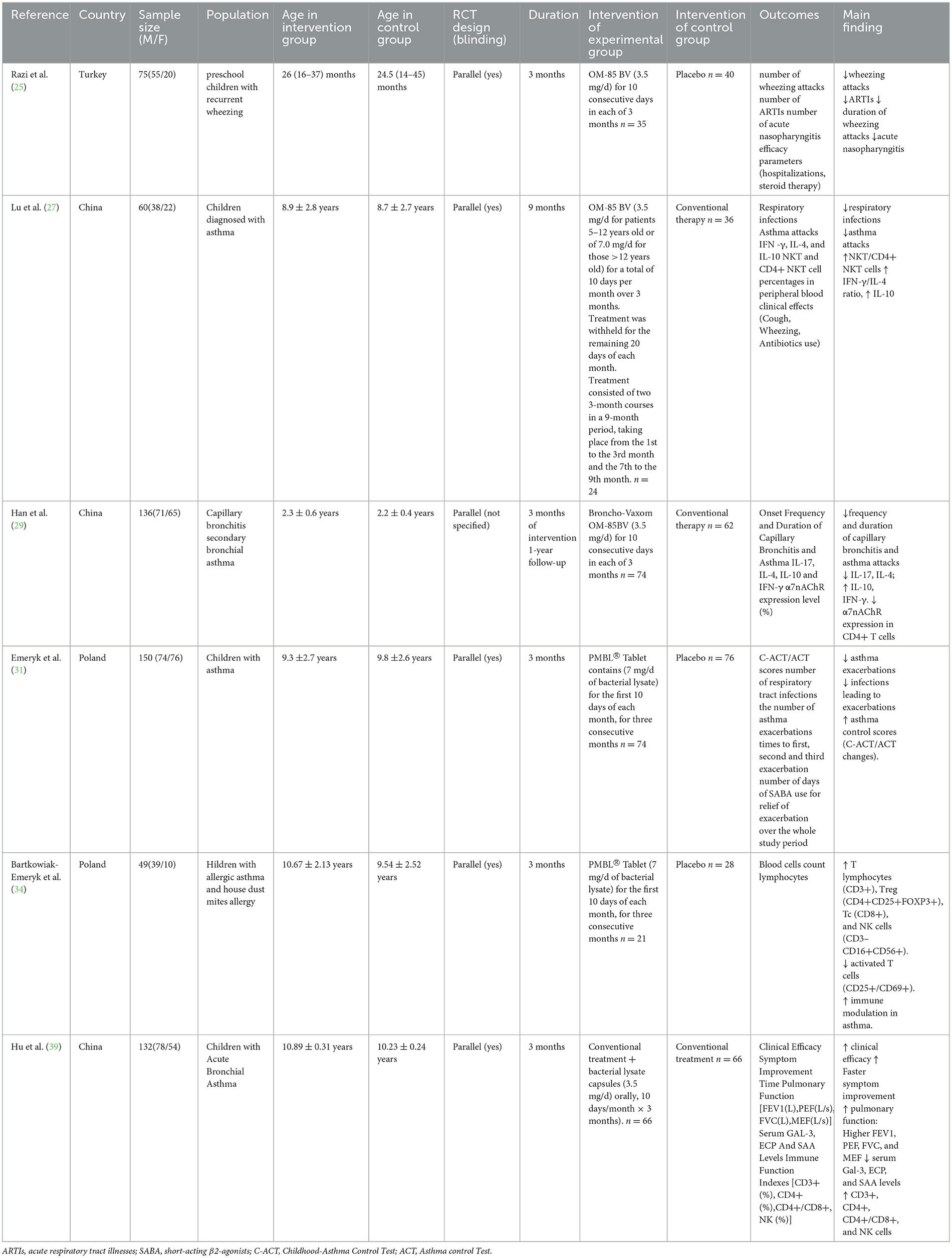
The efficacy of postbiotics was also explored in six studies (25, 27, 29, 31, 34, 39). The intervention used in the six studies was bacterial lysate at a dose of 3.5–7mg/day. All of studies compared bacterial lysate with conventional treatment or placebo. Primary outcome measures comprised asthma exacerbations, acute respiratory tract illnesses (ARTIs), pulmonary function, and immunological biomarkers. The details of included studies were shown in Table 2.
3.4.1 Asthma attacks
Four studies (25, 27, 29, 31) assessed the therapeutic impact of postbiotics on asthma exacerbations, with all trials demonstrating the efficacy of bacterial lysates in reducing attack frequency. Notably, one randomized controlled trial (RCT) observed a 37.9% decrease in wheezing episodes with OM-85 BV vs. placebo (P < 0.001) (25). Another study revealed superior asthma control through bacterial lysate administration compared to standard inhaled corticosteroids (ICS), evidenced by significantly fewer exacerbations (0.9 vs. 1.8 attacks; P = 0.01) in the OM-85 BV cohort (27). Furthermore, a comparative study documented enhanced clinical efficacy when bacterial lysate was combined with ICS (95.45% vs. 84.85% improvement rate; P < 0.05) (39).
3.4.2 ARTIs
Among all included studies, three trials evaluated bacterial lysate interventions for reducing acute respiratory tract infections (ARTIs), with all demonstrating significant efficacy. A RCT documented a 31.4% reduction in ARTI incidence with OM-85 BV vs. placebo (P < 0.001) (25). Another comparative study reported lower respiratory infection rates in the OM-85 BV cohort compared to conventional inhaled corticosteroid therapy (mean episodes: 3.1 vs. 7.4; P < 0.01) (27). Furthermore, a third study revealed fewer asthma-exacerbating infections in the PMBL® group relative to controls (10 vs. 21 cases; P = 0.002) (31).
3.4.3 Immune biomarker levels
Among the included studies, four investigated immune biomarker profiles, with all demonstrating the efficacy of bacterial lysates in enhancing immunological parameters. Regarding immune cell modulation: one study reported increased NKT/CD4+ NKT cells in experimental group (P < 0.01) (27), one study reported increased T lymphocytes (CD3+), Treg (CD4+CD25+FOXP3+), Tc (CD8+), and NK cells (CD3-CD16+CD56+), and decreased activated T cells (CD25+/CD69+) in experimental group (34). Hu et al. (39) showed increased CD3+, CD4+, CD4+/CD8+, and NK cells in experimental group (P < 0.001). Regarding inflammatory markers, Lu et al. (27) demonstrated increased IFN-γ/IL-4 ratio, IL-10 in OM-85 BV group (P < 0.01). Han et al. (29) confirmed that IL-17and IL-4 decreased in the experimental group, while IL-10 and IFN-γ increased.
3.4.4 Pulmonary function
Only one study, Hu et al. (39), reported the effect of bacterial lysate on pulmonary function. The study showed improved pulmonary function: Higher FEV1, PEF, FVC, and MEF in the bacterial lysate plus conventional inhaled corticosteroid compared with conventional treatment (P < 0.001).
3.5 Synbiotics in the treatment of pediatric asthma
As shown in Table 3, a total of 2 studies investigating the therapeutic efficacy of synbiotics in pediatric asthma management were included. The two studies used interventions Lactocare® and Kidilact®, respectively, with placebo in the control group. Ahanchian et al. (28) reported reduced viral respiratory infections (0.44 vs. 0.74 episodes; P = 0.007) and lower salbutamol use in the synbiotics group (P = 0.017). Hassanzad et al. (33) reported reduced outpatient visits (19 vs. 55; P = 0.001) and higher satisfaction in the synbiotics group (78.3% vs. 25.7%), while no significant difference in asthma attacks or hospitalizations.
3.6 Safety and adverse events
Across all studies, probiotics, postbiotics, and synbiotics were generally well-tolerated. Most studies reported no severe adverse effects. Mild and transient adverse events, such as abdominal symptoms and fever, were noted in some trials (22, 25, 27, 33) but occurred at similar rates in both intervention and control groups.
4 Discussion
This systematic review synthesizes evidence from 18 randomized controlled trials (RCTs) to evaluate the efficacy and safety of microbiome-targeted interventions—probiotics, synbiotics, postbiotics, and prebiotics—in pediatric asthma management. By adhering to PRISMA guidelines, we analyzed 2,419 participants aged <18 years, with a focus on clinically relevant endpoints such as asthma exacerbations, pulmonary function, and immune modulation. The included studies predominantly investigated Lactobacillus and Bifidobacterium strains (10 RCTs), bacterial lysates (6 RCTs), and synbiotic formulations (2 RCTs), while identifying a critical gap in prebiotics research.
4.1 Effect of probiotics, prebiotics, synbiotics and postbiotics on pediatric asthma
The current synthesis underscores the potential of probiotics in modulating asthma outcomes, albeit with notable heterogeneity across studies. Overall, probiotics demonstrated efficacy in reducing asthma exacerbations (35, 37), improving pulmonary function (32), and modulating immune profiles (23). However, heterogeneity persisted across studies: Lactobacillus monotherapy (e.g., L. gasseri A5 in Chen et al. (23)) improved lung function but had limited impact on asthma exacerbations, while Bifidobacterium strains (e.g., B. breve B632 in Ciprandi et al. (35)) reduced inhaled corticosteroid use. Multi-strain formulations, such as L. paracasei + L. fermentum in Huang et al. (32), showed synergistic benefits, enhancing PEFR improvement and IgE reduction effects compared to single strains. Emerging evidence suggests that the therapeutic effect of probiotics in pediatric asthma involves multifactorial pathways. Probiotics, particularly Lactobacillus and Bifidobacterium strains, restore the Th1/Th2 balance by suppressing Th2 cytokines (IL-4, IL-5, IL-13) and enhancing Th1 cytokines (IFN-γ), thereby counteracting allergic inflammation (40–42). Concurrently, they induce regulatory T cells (Tregs) to secrete anti-inflammatory IL-10 and TGF-β, dampening airway hyperresponsiveness (43, 44). Study shown that Bifidobacterium-derived acetate and propionate likely enhance Treg differentiation, suppressing eosinophilic inflammation (8), while Lactobacillus strains modulate dendritic cell tolerance, reducing allergen-specific IgE (45). By modulating gut microbiota, probiotics promote the production of metabolites such as short-chain fatty acids (SCFAs) and tryptophan. SCFAs inhibits histone deacetylases (HDACs), reducing airway inflammation epigenetically (46). Tryptophan metabolites (e.g., Indole-3-Lactic Acid) suppress TNF-α and IL-6 release via AhR signaling in alveolar macrophages (47, 48). Additionally, probiotics strengthen mucosal barriers in the gut and lungs via upregulation of tight-junction proteins (occludin, claudin), limiting allergen/pathogen translocation (49–51). Probiotics prime airway epithelial cells to upregulate pattern-recognition receptors and boost type I interferons (IFN-α/β), enhancing antiviral responses and reducing viral-triggered exacerbations (52).
Bacterial lysates (e.g., OM-85 BV and PMBL®), classified here as postbiotics, demonstrated consistent efficacy in reducing asthma attacks and ARTIs across six studies (25, 27, 29, 31, 34, 39). Their immunomodulatory effects, including enhanced NK cell activity and IFN-γ/IL-4 ratio shifts (27). Mechanistically, these lysates contain microbial components (e.g., peptidoglycans, lipoteichoic acids) that act as pathogen-associated molecular patterns (PAMPs), engaging Toll-like receptors (TLRs) and NOD-like receptors (NLRs) to activate innate immunity (53). TLR2/4 signaling primes dendritic cells to promote a Th1-skewed response, evidenced by elevated IFN-γ/IL-4 ratio (27), while suppressing eosinophilic inflammation via IL-10 upregulation (29). This aligns with the paradigm of microbial training of innate immunity, where bacterial lysates induce epigenetic reprogramming in epithelial cells, enhancing antiviral defense (54).
Limited evidence from two RCTs suggests that synbiotics interventions may reduce healthcare utilization in pediatric asthma, including decreased outpatient visits (33), and attenuate viral respiratory infections (28). Notably, however, one study found no significant reduction in asthma exacerbation frequency with synbiotics, underscoring the need for large-scale RCTs to validate their therapeutic role in childhood asthma (33). Moreover, both studies exhibit methodological limitations in intervention details. The original trial failed to explicitly report quantitative dosage parameters of the synbiotic formulation, including CFU counts per strain and precise prebiotic content, which may affect the reproducibility of the findings and comparability with other clinical studies. Synbiotics—combinations of probiotics (live beneficial microorganisms) and prebiotics (non-digestible substrates that selectively promote microbial growth)—leverage synergistic interactions to enhance gut microbiota modulation. Prebiotics such as fructooligosaccharides (FOS) and galactooligosaccharides (GOS) act as fermentable substrates for probiotic strains (e.g., Bifidobacterium and Lactobacillus), improving their survival, colonization, and metabolic activity in the gut lumen. This synergy may theoretically strengthen mucosal immunity by promoting microbial diversity and metabolic output (55). Contrasting these mechanistic insights, a 3-arm crossover RCT involving 17 patients with stable asthma demonstrated that inulin alone led to significant improvements in the Asthma Control Questionnaire scores, reduced sputum eosinophil percentage, and downregulated sputum histone deacetylase 9 mRNA expression. Intriguingly, these benefits were absent in the combined inulin-probiotic group (56). Consequently, whether probiotics and prebiotics exert additive or synergistic effects in asthma management remains unresolved, necessitating further rigorously designed trials.
This review included 0 studies on prebiotics in the treatment of childhood asthma. Emerging evidence from adult asthma RCTs suggests potential benefits of prebiotics. In adults, interventions with galactooligosaccharides (GOS) and inulin have demonstrated improving asthma. In an RCT, adults with asthma receiving 5.5 g/day Bimuno-galactooligosaccharide (B-GOS) for 3 weeks exhibited a 40% attenuation in post-hyperpnoea FEV1 decline alongside reduced systemic inflammation markers, including TNF-α and CCL17, demonstrating prebiotic modulation of airway hyperresponsiveness and inflammation in asthma (57). Another trial using soluble fiber (inulin 12 g/day) in adults with stable asthma reported improvements in airway inflammation, asthma control and gut microbiome composition (56). Observational data demonstrated a link between low dietary fiber intake and pediatric asthma severity (58). Current RCTs on prebiotics in children primarily focus on evaluating the preventive effects of prebiotic supplementation against allergies, atopic dermatitis, and respiratory infections in healthy children or those at high risk of atopy (59–63). However, evidence on the therapeutic efficacy of prebiotics in children with asthma remains scarce, highlighting a critical gap in current research.
4.2 Implications for practice
Included studies in this review have corroborated the beneficial effects of probiotics and synbiotics in the treatment of pediatric asthma, thereby emphasizing their potential as adjunctive therapeutic modalities. Notwithstanding, a notable limitation in current research is the scarcity of direct comparisons among different strains. This scarcity makes it challenging for clinicians to make informed decisions regarding the most effective strain or combination of strains for individual patients. Furthermore, the substantial variability in dosing (ranging from 108 to 1010 CFU/day) and intervention durations (spanning from 8 weeks to 12 months) of probiotics across studies accentuates the exigency for standardized guidelines. Pending the establishment of such guidelines for prebiotic use in children with asthma, clinicians could determine their medication regimen based on other pediatric probiotic guidelines (64–67), as well as available evidence, and closely monitor patient efficacy and safety.
Bacterial lysates, which have demonstrated efficacy in ameliorating asthma symptoms, reducing acute respiratory tract illnesses, and enhancing immunity, presents a viable option for clinicians. Therapies based on bacterial lysates, such as OM-85 BV or PMBL®, could be considered for incorporation into treatment plans, particularly for patients with recurrent exacerbations.
Although probiotics, synbiotics, and postbiotics generally manifested favorable safety profiles in the studies included in this review, mild and transient adverse events were nonetheless reported. Clinicians should remain vigilant in monitoring patients for any adverse effects, including abdominal discomfort, rash, and fever, especially within high-risk subgroups such as immunocompromised children.
In summary, while microbiota—targeted interventions hold great promise for the management of pediatric asthma, the current body of evidence is circumscribed by research gaps. Clinicians should exercise caution, stay updated on emerging research, and consider referring patients to well—designed clinical trials to further advance the field.
4.3 Implications for research
Based on some limitations of the studies included in this paper, suggestions for future research directions are proposed to promote the development of the field of microbiome targeted intervention in pediatric asthma.
Research on probiotics for the treatment of childhood asthma has the following limitations. Firstly, Bifidobacterium strains from a single RCT dominated the evidence base (NCT04289441), which limited generalizability and highlighted the need for diversification in probiotic research. Secondly, heterogeneous dosing (108 - 1010 CFU/day), intervention durations (8 weeks−12 months) and outcome indexs failed to standardize the optimal optimal protocol and reduced comparability, which echoing concerns raised by Cuello-Garcia et al. (68). Thirdly, 90% of studies focused on Lactobacillus and Bifidobacterium, neglecting other emerging bacteria that may have immunomodulatory effects. Future researches should prioritize head-to-head comparative studies of single- vs. multi-strain formulations and distinct strains, explore novel microbial species, and integrate multi-omics approaches to optimize therapeutic strategies.
Current researches on postbiotics for pediatric asthma treatment primarily utilizes bacterial lysates. However, the classification of bacterial lysates as postbiotics remains contentious, with ISAPP defining postbiotics as “preparation of inanimate microorganisms and/or their components that confers a health benefit on the host”, and excluding bacterial lysates from postbiotics classification (69). While there are still numerous contemporary studies continue to categorize bacterial lysates as postbiotics (20, 70, 71). Despite this ambiguity, the bacterial lysates incorporated in the our study exemplify the inanimate nature and health benefit properties characteristic of postbiotics. Further standardization of postbiotic definitions is critical to harmonize research and clinical applications. Standardizing the definition would enable more accurate comparison of studies and facilitate the development of evidence—based guidelines for the use of postbiotics in pediatric asthma treatment.
Existing research frameworks lack robust evidence regarding the clinical therapeutic value of prebiotic formulations in children with diagnosed asthma. This gap highlights the urgent need for pediatric trials to validate prebiotics as a viable therapeutic strategy inpediatric asthma. Future research should prioritize the design and implementation of well—controlled pediatric RCTs of therapeutic efficacy of prebiotics in children with asthma.
Regarding synbiotics, there remains a pressing need for more standardized and large-scale clinical trials to verify their effects on the treatment of childhood asthma. These trials should assess multiple endpoints, including asthma exacerbation, pulmonary function improvement, immunological biomarker modulation, and quality of life.
4.4 Strengths and limitations
This review represents the first systematic synthesis of evidence on the therapeutic roles of probiotics, synbiotics, prebiotics, and postbiotics in pediatric asthma. Its novelty and comprehensiveness provide a critical foundation for guiding clinical practice and future research directions in microbiome-targeted interventions for pediatric asthma.
However, our study is subject to several limitations mainly stemming from the current state of research in this field. Firstly, heterogeneity in outcome measures across studies was unavoidable, such as asthma remission rates, exacerbation frequency, number of exacerbation and symptom scores, which precluded quantitative meta-analysis, restricting conclusions to qualitative synthesis. Secondly, while the inclusion criteria targeted individuals aged <18 years, the enrolled population predominantly comprised children aged 2–11 years, with only one study including infants and toddlers (<2 years). Despite initial plans to perform subgroup analyses to differentiate effects across developmental stages (infants, children, and adolescents), the limited age diversity hindered planned subgroup analyses to assess age-specific effects. Thirdly, the heterogeneity including strain specificity, dosage variability and intervention durations among observed probiotic or synbiotic formulations complicated the identification of optimal regimen, resulting in uncertainty in formulating clinical recommendations. Lastly, included studies were predominantly conducted in Europe (Italy, Poland, Germany) and Asia (China, Iran, Turkey), with no representation from North/South America, Africa, or Oceania. This regional skew may limit the generalizability of findings, as genetic, environmental, and socioeconomic factors influencing asthma and gut microbiota vary globally.
5 Conclusion
This systematic review synthesizes emerging evidence supporting the therapeutic potential of probiotics, postbiotics, and synbiotics in pediatric asthma management. Probiotic interventions, particularly Lactobacillus and Bifidobacterium strains, demonstrate efficacy in reducing asthma exacerbations and improving pulmonary function, likely through Th1/Th2 immune balance restoration and gut-lung axis modulation. Postbiotics, such as bacterial lysates, show promise in reducing airway hyperresponsiveness and systemic inflammation, though their classification remains debated. Synbiotics further enhance clinical outcomes by reducing viral respiratory infections and healthcare utilization. However, significant heterogeneity in strain selection, dosing, and outcome measures, coupled with methodological limitations, constrain definitive conclusions. Notably, there is a conspicuous absence of direct RCTs dedicated to exploring the therapeutic effects of prebiotics in pediatric asthma. Future research could prioritize strain-specific trials, standardized protocols, and mechanistic studies integrating multi-omics to validate gut microbiota-targeted therapies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
DF: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. JH: Data curation, Investigation, Methodology, Writing – review & editing. NL: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Nutritional Science Discipline Booster Fund (41C42E8).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1586129/full#supplementary-material
References
1. Reddel HK, Bacharier LB, Bateman ED, Brightling CE, Brusselle GG, Buhl R, et al. Global Initiative for Asthma Strategy 2021: executive summary and rationale for key changes. Eur Respir J. (2022) 59:2102730. doi: 10.1183/13993003.02730-2021
2. Wang Z, Li Y, Gao Y, Fu Y, Lin J, Lei X, et al. Global, regional, and national burden of asthma and its attributable risk factors from 1990 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. Respir Res. (2023) 24:169. doi: 10.1186/s12931-023-02475-6
3. Global Global burden of 369 diseases and injuries in 204 countries and territories 1990-2019: 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
4. Zhang D, Zheng J. The Burden of Childhood Asthma by Age Group, 1990-2019: A Systematic Analysis of Global Burden of Disease 2019 Data. Front Pediatr. (2022) 10:823399. doi: 10.3389/fped.2022.823399
5. Shipp CL, Gergen PJ, Gern JE, Matsui EC, Guilbert TW. Asthma management in children. J Allergy Clin Immunol Pract. (2023) 11:9–18. doi: 10.1016/j.jaip.2022.10.031
6. Perry R, Braileanu G, Palmer T, Stevens P. The economic burden of pediatric asthma in the united states: literature review of current evidence. Pharmacoeconomics. (2019) 37:155–67. doi: 10.1007/s40273-018-0726-2
7. Agache I, Eguiluz-Gracia I, Cojanu C, Laculiceanu A, Del Giacco S, Zemelka-Wiacek M, et al. Advances and highlights in asthma in 2021. Allergy. (2021) 76:3390–407. doi: 10.1111/all.15054
8. Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. (2014) 20:159–66. doi: 10.1038/nm.3444
9. Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. (2013) 504:451–5. doi: 10.1038/nature12726
10. Thio CL, Chi PY, Lai AC, Chang YJ. Regulation of type 2 innate lymphoid cell-dependent airway hyperreactivity by butyrate. J Allergy Clin Immunol. (2018) 142:1867–83.e12. doi: 10.1016/j.jaci.2018.02.032
11. Bonaz B, Bazin T, Pellissier S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front Neurosci. (2018) 12:49. doi: 10.3389/fnins.2018.00049
12. Gury-BenAri M, Thaiss CA, Serafini N, Winter DR, Giladi A, Lara-Astiaso D, et al. The spectrum and regulatory landscape of intestinal innate lymphoid cells are shaped by the microbiome. Cell. (2016) 166:1231–46.e13. doi: 10.1016/j.cell.2016.07.043
13. Losol P, Sokolowska M, Hwang YK, Ogulur I, Mitamura Y, Yazici D, et al. Epithelial barrier theory: the role of exposome, microbiome, and barrier function in allergic diseases. Allergy Asthma Immunol Res. (2023) 15:705–24. doi: 10.4168/aair.2023.15.6.705
14. Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. (2016) 22:1187–91. doi: 10.1038/nm.4176
15. Wang J, Zhu N, Su X, Gao Y, Yang R. Gut-microbiota-derived metabolites maintain gut and systemic immune homeostasis. Cells. (2023) 12:793. doi: 10.3390/cells12050793
16. Liu S, da Cunha AP, Rezende RM, Cialic R, Wei Z, Bry L, et al. The host shapes the gut microbiota via fecal MicroRNA. Cell Host Microbe. (2016) 19:32–43. doi: 10.1016/j.chom.2015.12.005
17. Huang YJ. The respiratory microbiome and innate immunity in asthma. Curr Opin Pulm Med. (2015) 21:27–32. doi: 10.1097/MCP.0000000000000124
18. Chiu CJ, Huang MT. Asthma in the precision medicine era: biologics and probiotics. Int J Mol Sci. (2021) 22:4528. doi: 10.3390/ijms22094528
19. Meirlaen L, Levy EI, Vandenplas Y. Prevention and management with pro-, pre and synbiotics in children with asthma and allergic rhinitis: a narrative review. Nutrients. (2021) 13:934. doi: 10.3390/nu13030934
20. Wegrzyn K, Jasińska A, Janeczek K, Feleszko W. The role of postbiotics in asthma treatment. Microorganisms. (2024) 12:1642. doi: 10.3390/microorganisms12081642
21. Balan D, Baral T, Manu MK, Mohapatra AK, Miraj SS. Efficacy of probiotics as adjuvant therapy in bronchial asthma: a systematic review and meta-analysis. Allergy Asthma Clin Immunol. (2024) 20:60. doi: 10.1186/s13223-024-00922-7
22. Giovannini M, Agostoni C, Riva E, Salvini F, Ruscitto A, Zuccotti GV, et al. A randomized prospective double blind controlled trial on effects of long-term consumption of fermented milk containing Lactobacillus casei in pre-school children with allergic asthma and/or rhinitis. Pediatr Res. (2007) 62:215–20. doi: 10.1203/PDR.0b013e3180a76d94
23. Chen YS, Jan RL, Lin YL, Chen HH, Wang JY. Randomized placebo-controlled trial of lactobacillus on asthmatic children with allergic rhinitis. Pediatr Pulmonol. (2010) 45:1111–20. doi: 10.1002/ppul.21296
24. Gutkowski P, Madaliński K, Grek M, Dmeńska H, Syczewska M, Michałkiewicz J. Effect of orally administered probiotic strains Lactobacillus and Bifidobacterium in children with atopic asthma. Central-Eur J Immunol. (2010) 35:233–8.
25. Razi CH, Harmanci K, Abaci A, Özdemir O, Hizli S, Renda R, et al. The immunostimulant OM-85 BV prevents wheezing attacks in preschool children. J Allergy Clin Immunol. (2010) 126:763–9. doi: 10.1016/j.jaci.2010.07.038
26. Rose MA, Stieglitz F, Köksal A, Schubert R, Schulze J, Zielen S. Efficacy of probiotic Lactobacillus GG on allergic sensitization and asthma in infants at risk. Clin Exp Allergy. (2010) 40:1398–405. doi: 10.1111/j.1365-2222.2010.03560.x
27. Lu Y, Li Y, Xu L, Xia M, Cao L. Bacterial lysate increases the percentage of natural killer T cells in peripheral blood and alleviates asthma in children. Pharmacology. (2015) 95:139–44. doi: 10.1159/000377683
28. Ahanchian H, Jafari SA, Ansari E, Ganji T, Kiani MA, Khalesi M, et al. A multi-strain Synbiotic may reduce viral respiratory infections in asthmatic children: a randomized controlled trial. Electron Physician. (2016) 8:2833–9. doi: 10.19082/2833
29. Han RçF, Li HY, Wang JW, Cong XJ. Study on clinical effect and immunologic mechanism of infants capillary bronchitis secondary bronchial asthma treated with bacterial lysates Broncho-Vaxom. Eur Rev Med Pharmacol Sci. (2016) 20:2151–5.
30. Del Giudice MM, Indolfi C, Capasso M, Maiello N, Decimo F, Ciprandi G. Bifidobacterium mixture (B longum BB536, B infantis M-63, B breve M-16V) treatment in children with seasonal allergic rhinitis and intermittent asthma. Ital J Pediatr. (2017) 43:25. doi: 10.1186/s13052-017-0340-5
31. Emeryk A, Bartkowiak-Emeryk M, Raus Z, Braido F, Ferlazzo G, Melioli G. Mechanical bacterial lysate administration prevents exacerbation in allergic asthmatic children-The EOLIA study. Pediatr Allergy Immunol. (2018) 29:394–401. doi: 10.1111/pai.12894
32. Huang CF, Chie WC, Wang IJ. Efficacy of lactobacillus administration in school-age children with asthma: a randomized, placebo-controlled trial. Nutrients. (2018) 10:1678. doi: 10.3390/nu10111678
33. Hassanzad M, Maleki Mostashari K, Ghaffaripour H, Emami H, Rahimi Limouei S, Velayati AA. Synbiotics and treatment of asthma: a double-blinded, randomized, placebo-controlled clinical trial. Galen Med J. (2019) 8:e1350. doi: 10.31661/gmj.v8i0.1350
34. Bartkowiak-Emeryk M, Emeryk A, Rolinski J, Wawryk-Gawda E, Markut-Miotla E. Impact of Polyvalent Mechanical Bacterial Lysate on lymphocyte number and activity in asthmatic children: a randomized controlled trial. Allergy Asthma Clin Immunol. (2021) 17:10. doi: 10.1186/s13223-020-00503-4
35. Ciprandi G, Cioffi L, Giuliano M, Pane M, Drago L. The PRObiotics in Pediatric Asthma Management (PROPAM) study: a post hoc analysis in preschoolers. Pediatr Pulmonol. (2022) 57:1355–7. doi: 10.1002/ppul.25878
36. Ciprandi G, Schiavetti I, Cioffi L, Pane M, Drago L. The Probiotics in Pediatric Asthma Management (PROPAM) study: A Post Hoc analysis in allergic children. Ann Allergy Asthma Immunol. (2022) 129:111–3. doi: 10.1016/j.anai.2022.04.026
37. Drago L, Cioffi L, Giuliano M, Pane M, Amoruso A, Schiavetti I, et al. The Probiotics in Pediatric Asthma Management (PROPAM) Study in the Primary Care Setting: a Randomized, Controlled, Double-Blind Trial with Ligilactobacillus salivarius LS01 (DSM 22775) and Bifidobacterium breve B632 (DSM 24706). J Immunol Res. (2022) 2022:3837418. doi: 10.1155/2022/3837418
38. Drago L, Cioffi L, Giuliano M, Pane M, Ciprandi G, Brunese FP, et al. A post hoc analysis on the effects of a probiotic mixture on asthma exacerbation frequency in schoolchildren. ERJ Open Res. (2022) 8:00020-2022. doi: 10.1183/23120541.00020-2022
39. Hu L, Wen H. Efficacy of bacterial lysate capsules in adjuvant treatment of children with acute bronchial asthma based on changes in pulmonary function and serum cytokine levels. Revista de psiquiatria clinica. (2022) 49:44–8. doi: 10.15761/0101-60830000000406
40. Chen X, Yong SB Yii CY, Feng B, Hsieh KS Li Q. Intestinal microbiota and probiotic intervention in children with bronchial asthma. Heliyon. (2024) 10:e34916. doi: 10.1016/j.heliyon.2024.e34916
41. Wang W, Luo X, Zhang Q, He X, Zhang Z, Wang X. Bifidobacterium infantis relieves allergic asthma in mice by regulating Th1/Th2. Med Sci Monit Int Med J Exp Clin Res. (2020) 26:e920583. doi: 10.12659/MSM.920583
42. Fujimura KE, Lynch SV. Microbiota in allergy and asthma and the emerging relationship with the gut microbiome. Cell Host Microbe. (2015) 17:592–602. doi: 10.1016/j.chom.2015.04.007
43. Strickland DH, Holt PG, T. regulatory cells in childhood asthma. Trends Immunol. (2011) 32:420–7. doi: 10.1016/j.it.2011.06.010
44. Abbasi-Dokht T, Sadrifar S, Forouzandeh S, Malek F, Hemmati M, Kokhaei P, et al. Multistrain probiotics supplement alleviates asthma symptoms via increasing Treg cells population: a randomized, double-blind, placebo-controlled trial. Int Arch Allergy Immunol. (2023) 184:291–301. doi: 10.1159/000526739
45. Fujimura KE, Demoor T, Rauch M, Faruqi AA, Jang S, Johnson CC, et al. House dust exposure mediates gut microbiome Lactobacillus enrichment and airway immune defense against allergens and virus infection. Proc Natl Acad Sci U S A. (2014) 111:805–10. doi: 10.1073/pnas.1310750111
46. Singh N, Thangaraju M, Prasad PD, Martin PM, Lambert NA, Boettger T, et al. Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)-dependent inhibition of histone deacetylases. J Biol Chem. (2010) 285:27601–8. doi: 10.1074/jbc.M110.102947
47. Yu K, Li Q, Sun X, Peng X, Tang Q, Chu H, et al. Bacterial indole-3-lactic acid affects epithelium-macrophage crosstalk to regulate intestinal homeostasis. Proc Natl Acad Sci U S A. (2023) 120:e2309032120. doi: 10.1073/pnas.2309032120
48. Ehrlich AM, Pacheco AR, Henrick BM, Taft D, Xu G, Huda MN, et al. Indole-3-lactic acid associated with Bifidobacterium-dominated microbiota significantly decreases inflammation in intestinal epithelial cells. BMC Microbiol. (2020) 20:357. doi: 10.1186/s12866-020-02023-y
49. Tan JK, Macia L, Mackay CR. Dietary fiber and SCFAs in the regulation of mucosal immunity. J Allergy Clin Immunol. (2023) 151:361–70. doi: 10.1016/j.jaci.2022.11.007
50. McKenzie C, Tan J, Macia L, Mackay CR. The nutrition-gut microbiome-physiology axis and allergic diseases. Immunol Rev. (2017) 278:277–95. doi: 10.1111/imr.12556
51. Paone P, Cani PD. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut. (2020) 69:2232–43. doi: 10.1136/gutjnl-2020-322260
52. Lehtoranta L, Pitkäranta A, Korpela R. Probiotics in respiratory virus infections. Eur J Clin microbial Infect Dis. (2014) 33:1289–302. doi: 10.1007/s10096-014-2086-y
53. Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. (2001) 2:675–80. doi: 10.1038/90609
54. Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, et al. Trained immunity: a program of innate immune memory in health and disease. Science. (2016) 352:aaf1098. doi: 10.1126/science.aaf1098
55. Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. (2017) 14:491–502. doi: 10.1038/nrgastro.2017.75
56. McLoughlin R, Berthon BS, Rogers GB, Baines KJ, Leong LEX, Gibson PG, et al. Soluble fibre supplementation with and without a probiotic in adults with asthma: A 7-day randomised, double blind, three way cross-over trial. EBioMedicine. (2019) 46:473–85. doi: 10.1016/j.ebiom.2019.07.048
57. Williams NC, Johnson MA, Shaw DE, Spendlove I, Vulevic J, Sharpe GR, et al. A prebiotic galactooligosaccharide mixture reduces severity of hyperpnoea-induced bronchoconstriction and markers of airway inflammation. Br J Nutr. (2016) 116:798–804. doi: 10.1017/S0007114516002762
58. Vaccaro JA, Niego J, Huffman FGJCsHC. Dietary factors, body weight, and screen time in U.S. children with and without asthma. (2016) 45:22–38. doi: 10.1080/02739615.2014.948165
59. Kukkonen K, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, et al. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: A randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. (2007) 119:192–8. doi: 10.1016/j.jaci.2006.09.009
60. Palmer DJ, Cuthbert AR, Sullivan TR, Pretorius RA, Garssen J, Rueter K, et al. Effects of pregnancy and lactation prebiotics supplementation on infant allergic disease: a randomized controlled trial. J Allergy Clin Immunol. (2025) 155:144–52. doi: 10.1016/j.jaci.2024.08.009
61. Vandenplas Y, Żołnowska M, Berni Canani R, Ludman S, Tengelyi Z, Moreno-Álvarez A, et al. Effects of an extensively hydrolyzed formula supplemented with two human milk oligosaccharides on growth, tolerability, safety and infection risk in infants with cow's milk protein allergy: a randomized, multi-center trial. Nutrients. (2022) 14:530. doi: 10.3390/nu14030530
62. Ranucci G, Buccigrossi V, Borgia E, Piacentini D, Visentin F, Cantarutti L, et al. Galacto-oligosaccharide/polidextrose enriched formula protects against respiratory infections in infants at high risk of atopy: a randomized clinical trial. Nutrients. (2018) 10:286. doi: 10.3390/nu10030286
63. Scalabrin D, Ribeiro T, De Matto AP, Zhuang W, Stolz S, Pontes M, et al. Formula with docosahexaenoic acid, prebiotics, and beta-glucan supports respiratory and skin health in children. FASEB J. (2014) 28:382–6. doi: 10.1096/fasebj.28.1_supplement.382.6
64. Su GL, Ko CW, Bercik P, Morgan RL, Weizman AV, Kashyap P, et al. Spotlight: Probiotics Guidelines. Gastroenterology. (2020) 159:707. doi: 10.1053/j.gastro.2020.07.025
65. Depoorter L, Vandenplas Y. Probiotics in Pediatrics. A Review and Practical Guide. Nutrients. (2021) 13:2176. doi: 10.3390/nu13072176
66. Szajewska H, Berni Canani R, Domellöf M, Guarino A, Hojsak I, Indrio F, et al. Probiotics for the management of pediatric gastrointestinal disorders: position paper of the ESPGHAN special interest group on gut microbiota and modifications. J Pediatr Gastroenterol Nutr. (2023) 76:232–47. doi: 10.1097/MPG.0000000000003633
67. van den Akker CHP, van Goudoever JB, Shamir R, Domellöf M, Embleton ND, Hojsak I, et al. Probiotics and preterm infants: a position paper by the european society for paediatric gastroenterology hepatology and Nutrition Committee on Nutrition and the European Society for Paediatric Gastroenterology Hepatology and Nutrition Working Group for Probiotics and Prebiotics. J Pediatr Gastroenterol Nutr. (2020) 70:664–80. doi: 10.1097/MPG.0000000000002655
68. Cuello-Garcia CA, Brożek JL, Fiocchi A, Pawankar R, Yepes-Nuñez JJ, Terracciano L, et al. Probiotics for the prevention of allergy: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. (2015) 136:952–61. doi: 10.1016/j.jaci.2015.04.031
69. Salminen S, Collado MC, Endo A, Hill C, Lebeer S, Quigley EMM, et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. (2021) 18:649–67. doi: 10.1038/s41575-021-00440-6
70. Mi XJ, Tran THM, Park HR, Xu XY, Subramaniyam S, Choi HS, et al. Immune-enhancing effects of postbiotic produced by Bacillus velezensis Kh2-2 isolated from Korea Foods. Food Res Int. (2022) 152:110911. doi: 10.1016/j.foodres.2021.110911
Keywords: pediatric asthma, probiotics, prebiotics, synbiotics, postbiotics, systematic review
Citation: Fan D, Hu J and Lin N (2025) Effects of probiotics, prebiotics, synbiotics and postbiotics on pediatric asthma: a systematic review. Front. Nutr. 12:1586129. doi: 10.3389/fnut.2025.1586129
Received: 02 March 2025; Accepted: 07 April 2025;
Published: 25 April 2025.
Edited by:
Ke Chen, University of Electronic Science and Technology of China, ChinaReviewed by:
Shanshan Geng, Nanjing Medical University, ChinaLirong Shen, Zhejiang University, China
Jing Chen, Shanghai University of Traditional Chinese Medicine, China
Copyright © 2025 Fan, Hu and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Lin, aGVsZW5tZWRpY0B5ZWFoLm5ldA==
 Die Fan
Die Fan Jilei Hu
Jilei Hu Ning Lin*
Ning Lin*