- Department of Intensive Care Unit, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin, Tianjin's Clinical Research Center for Cancer, Tianjin, China
Background: The prognostic nutritional index (PNI) has been proven to represent a biomarker for predicting prognosis in many groups of patients with severe diseases. However, few studies have investigated the association between PNI and mortality in Japan older people with dysphagia patients.
Objective: This retrospective cohort study aimed to assess the prognostic value of PNI in older Japanese patients with dysphagia.
Methods: We analyzed data from 248 patients diagnosed with dysphagia at a single center between January 2014 and January 2017. According to PNI score, all patients were divided into normal nutrition group (PNI ≥ 38), moderate malnutrition group (35 ≤ PNI < 38) and severe malnutrition group (PNI < 35). Cox regression analysis was used to compare the mortality rates among the three groups. Subgroup analyses were conducted, and Kaplan-Meier curves were used to determine the median survival times.
Results: The mean age of the patients was 83.0 ± 9.3 years, with a male-to-female ratio of 0.64:1. Of the patients, 180 received percutaneous endoscopic gastrostomy (PEG) and 68 received total parenteral nutrition (TPN). After adjusting for all covariates, the multivariable Cox regression analysis revealed a significant association between PNI and the risk of mortality (HR = 0.94, 95% CI: 0.92–0.97, P < 0.001). Compared with the normal nutrition group, the mortality rate of severe malnutrition group was significantly higher (P = 0.007). The adjusted hazard ratios for the severe and moderate malnutrition groups were 1.83 (95%CI: 1.18–2.84, P = 0.007) and 1.39 (95%CI: 0.81–2.4, P = 0.234), respectively. Kaplan-Meier curves indicated median survival times of 189, 447, and 864 days for severe malnutrition group, moderate malnutrition group, and normal nutrition group, respectively.
Conclusion: PNI was negatively associated with mortality in older Japanese patients with dysphagia. There was no interaction for the subgroup analysis. The result was stable.
1 Introduction
Dysphagia, a prevalent condition among older adults, has been found in 10%−27% of people in the community, with a prevalence of up to 50% of care home residents and elderly hospitalized patients (1). Dysphagia is frequently observed among stroke patients, with its prevalence varying from 37% to 78% (2). It is more prevalent among the elderly diagnosed with neurological disorders [the incidence of dysphagia is approximately 80% in patients with Alzheimer's disease and around 60% in those with Parkinson's disease (3)]. Patients diagnosed with dysphagia may encounter malnutrition, pneumonia, and dehydration, which subsequently contribute to an elevation of both long-term hospitalization rates and mortality. In a large cross-sectional study targeting nursing home residents, it was revealed that the 6-month mortality of residents experiencing dysphagia was 24.7%, compared with 11.9% in those without dysphagia (P < 0.001) (4). In the hospital setting, patients diagnosed with dysphagia exhibited a 1.7-times (95%CI: 1.67 to 1.74 times) (5) in the likelihood of mortality when compared to those without dysphagia. Moreover, dysphagia is correlated with a longer hospital length of stay (LOS). A study based on a large sample database of hospitalized patients in the United States from 2009 to 2013 demonstrated that the mean hospital LOS for patients with dysphagia was 3.8 days longer than that for those without dysphagia (8.8 days vs. 5.0 days; P < 0.001) (5). The average total hospitalization cost for patients with dysphagia increased by $6,243 ($19,244 vs. $13,001; P < 0.001) (5). Consequently, it is of crucial importance to detect dysphagia at an early stage and conduct active interventions to prevent patients from suffering more severe consequences.
The Prognostic Nutritional Index (PNI) was first proposed in 1984, calculated by combining serum albumin (ALB) levels and total lymphocyte count (6). Initially, it was used to evaluate the preoperative surgical risks of digestive system tumors (7). In recent years, it has been demonstrated to possess favorable predictive value across a diverse range of diseases, especially in chronic diseases and malignant tumors (8–10). Lymphocytes play a crucial role in the host immune response. Lymphocytopenia indicates a decline in the ability to resist infections. ALB, an acute-phase protein with a long half-life, is regarded as an indicator of nutritional status and inflammatory activity. A low ALB level suggests impaired capacity for protein synthesis and a malnourished state. Overall, a low PNI indicates hypoalbuminemia and lymphocytopenia, reflecting the nutritional status and overall health status of patients, which is associated with increased mortality and poor prognosis (11).
However, a comprehensive guideline for the management of dysphagia in the elderly is still lacking. Early assessment and prediction of the adverse prognosis associated with dysphagia are crucial. While previous studies have identified the prognostic value of PNI in various diseases, the research evidence regarding the correlation between PNI and mortality in patients with dysphagia is limited. Factors predicting mortality in patients with dysphagia remain under investigation. Therefore, the present study aims to investigate whether PNI is independently associated with mortality in elderly patients with dysphagia residing in Japan.
2 Materials and methods
2.1 Data source
The data utilized in this study were obtained from the Dryad Digital Repository (12), which represents a platform affording users unfettered access to and retrieval of the original data. All authors have waived copyright to the original research data. Therefore, these data were used for secondary analysis without infringing on the authors' rights. Since all the data used were extracted from online data resources, no institutional ethics committee approval was required.
2.2 Study design
This retrospective cohort study was carried out at a single center, with a particular focus on older patients afflicted with dysphagia and who underwent percutaneous endoscopic gastrostomy (PEG) or total parenteral nutrition (TPN), including implantable central venous ports (PORT), non-tunneled central venous catheters (NT-CVC), and peripherally inserted central catheters (PICC) during the period from January 2014 to January 2017. In this dataset, the evaluation of dysphagia was performed clinically by a doctor, a nurse, and a speech-language pathologist, in conjunction with assessments via video fluoroscopy. Subsequently, it was clearly demonstrated that each patient exhibited severe dysphagia. Patients with terminal cancer, those necessitating PEG for gastric decompression, and individuals who had undergone PEG prior to January 2014 were excluded from the present study.
The following clinical information was collected and processed for secondary analysis, including age, gender, underlying diseases such as cerebrovascular disease, severe dementia, neuromuscular diseases, aspiration pneumonia, ischemic heart disease (IHD), chronic heart failure, chronic lung disease, chronic liver disease, chronic kidney disease (CKD), the presence of PORT, NT-CVC, PICC, PEG, oral intake recovery, blood test results, body mass index, daily calorie intake, discharge to home, severe pneumonia, and sepsis, etc. The blood test results were performed within 7 days before the start of PEG feeding or TPN (12). In addition, the survival status and follow-up duration of each patient with dysphagia were also determined. Grouping was carried out according to PNI, with PNI > 38 reflecting the normal nutritional status group, 35–38 as the moderate malnutrition group, and < 35 as the severe malnutrition group (13).
Data anonymization eliminated the need for informed consent, and all methodologies were in strict accordance with the relevant guidelines and regulations. This retrospective study was approved by the Ethical Review Board of Miyanomori Memorial Hospital, which dispensed with the requirement for informed consent.
2.3 Statistical analysis
Continuous variables are presented as mean (SD) or median (IQR), while categorical variables are expressed in the form of percentages (%). To appraise the baseline characteristics, a one-way analysis of variance (ANOVA) was implemented for continuous variables, and a chi-square test was carried out to scrutinize the statistical disparities among the normal nutritional status group, the moderate malnutrition group, and the severe malnutrition group. The correlation between PNI and mortality in patients with dysphagia was investigated using Cox proportional hazards model. Survival curves were constructed using the Kaplan-Meier and log-rank analyses. The likelihood ratio test was employed to probe into the interactions among the subgroups. A two-sided significance level of P < 0.05 (two-sided) was taken into account, and all reported p-values were < 0.05. All statistical analyses were executed with Free Statistics software version 1.9.
3 Results
3.1 Baseline characteristics of the study population
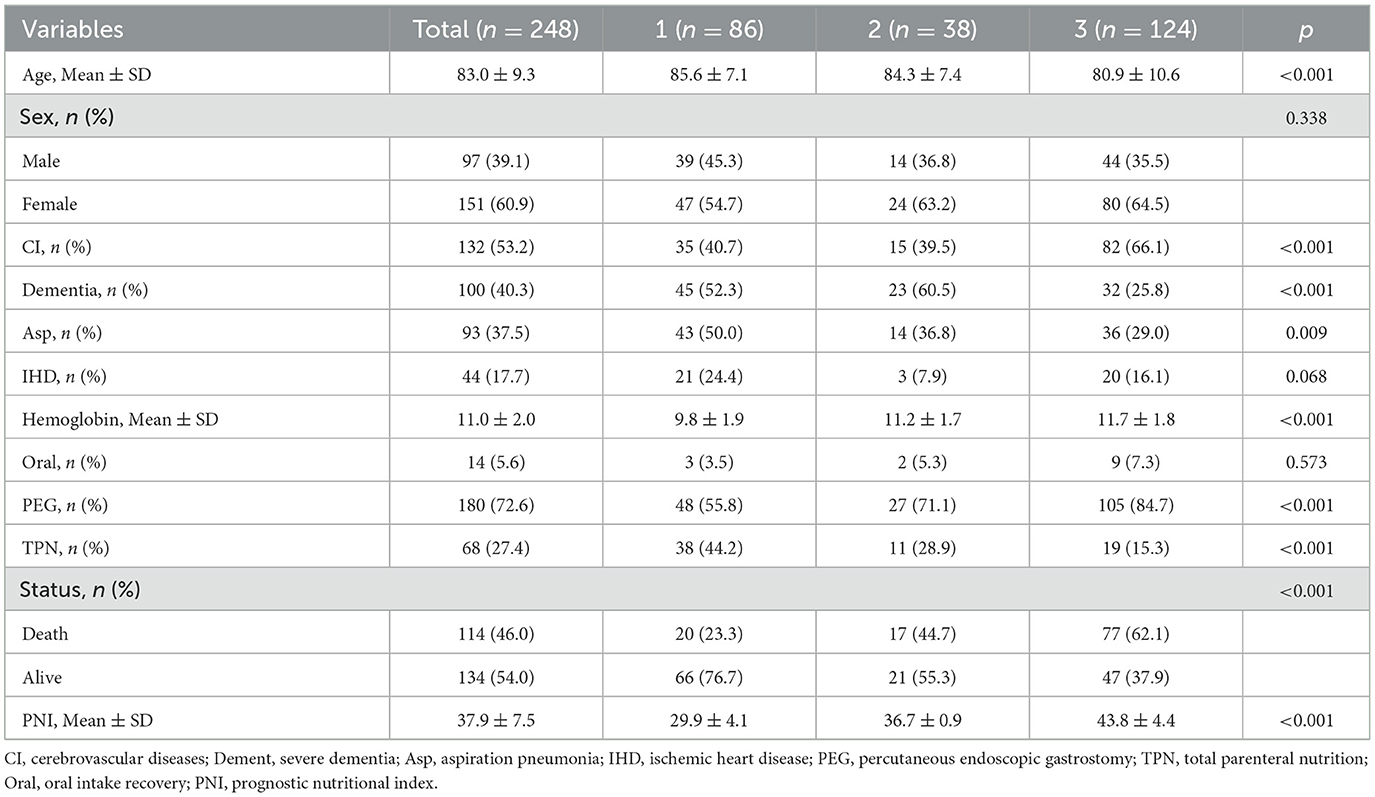
Table 1 presents the essential characteristics of the cohort of 248 (97 male and 151 female) patients. The average age of the patients was 83.0 years, with a standard deviation of 9.3. Among them, 180 cases were fed via PEG, and 68 cases were fed via TPN, including 26 cases via PORT, 23 cases via NT-CVC, and 19 cases via PICC. Among the total patient cohort, 86 cases (34.7%) were classified into the severe malnutrition group, 38 cases (15.3%) into the moderate malnutrition group, and 124 cases (50.0%) into the normal nutritional status group. As PNI declined, there was a concomitant increase in the patients' age and a downward tendency in the hemoglobin level (p < 0.001). Statistically significant differences were observed among the three groups with respect to cerebrovascular diseases, severe dementia, aspiration pneumonia, PEG, and TPN (P < 0.05). In contrast, no remarkable statistical difference was detected in the oral intake recovery (p = 0.573).
3.2 Kaplan-Meier curve
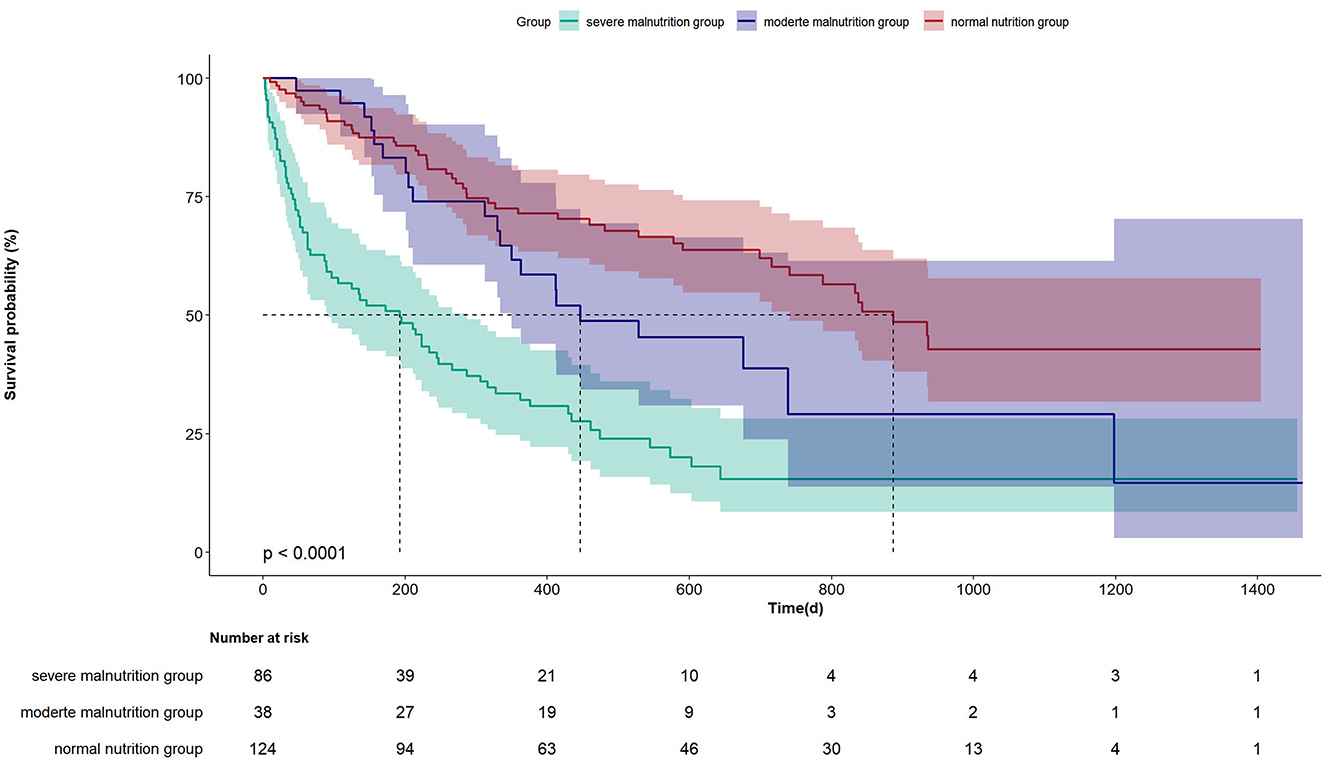
The Kaplan-Meier curve in Figure 1 indicates that there is a statistical difference in the prognosis among the three groups of patients. The median survival duration of the severe malnutrition group is significantly shorter compared to the other two groups (P < 0.0001). The respective values for median survival times were 189, 447, and 864 days for the severe malnutrition group, the moderate malnutrition group, and the normal nutrition status group.
3.3 Association between PNI and mortality in various models
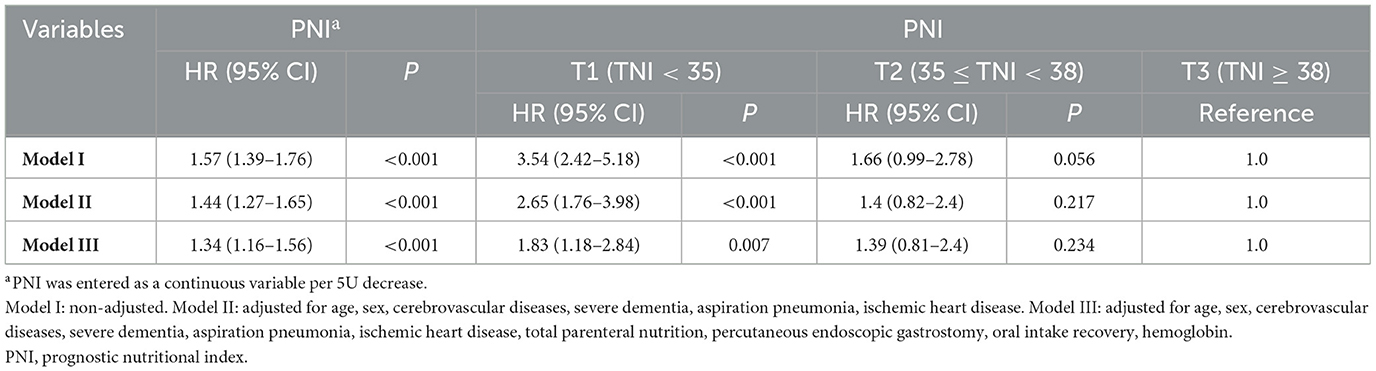
Table 2 presents the hazard ratio (HR) and 95% confidence intervals (95% Cl) related to the risk of mortality in patients with dysphagia based on PNI. Four distinct models were formulated, considering PNI as continuous and categorical variables. In model I, no variables were elected for adjustment. For model II, Variables of demographics (age, sex) and past medical history (cerebrovascular diseases, severe dementia, aspiration pneumonia, IHD) were designated for adjustment. In model III, all of the variables (age, sex, IHD, cerebrovascular diseases, severe dementia, aspiration pneumonia, PEG, hemoglobin, TPN, and oral intake recovery) were subjected to adjustment. The results of these models were shown in Table 2. The Univariable Cox regression analysis showed that when PNI was analyzed as a continuous variable, it was negatively correlated with mortality, with a 57% increase in the risk of mortality for every 5-point decrease in PNI (HR = 1.57, 95% CI: 1.39–1.76, P < 0.001). Upon adjusting for all covariates in the multivariable Cox regression analysis, the HR was 1.34 (95% CI: 1.16–1.56, P < 0.001). Compared to the normal nutrition status group (PNI ≥ 38), the severe malnutrition group (PNI < 35) had a significantly higher mortality, with a statistically significant difference (P = 0.007). The adjusted HR values for PNI and mortality in the severe malnutrition group and moderate malnutrition group (35 ≤ PNI < 38) were 1.83 (95% CI: 1.18–2.84, P = 0.007) and 1.39 (95% CI: 0.81–2.4, P = 0.234), respectively.
3.4 Subgroup analyses
Subgroup and interaction analyses were conducted to evaluate the consistency of the correlation between PNI and the mortality related to patients with dysphagia (Figure 2). Following the adjustment for all the covariates encompassed in this study, namely age, sex, IHD, cerebrovascular diseases, severe dementia, aspiration pneumonia, PEG, hemoglobin, TPN, and recovery of oral intake, and during the process of subgroup analysis, in case the subgroup analysis variable was a categorical variable, it is excluded from the analysis. No interaction was identified among the eight subgroups. The result was consistent and is depicted in Figure 2.
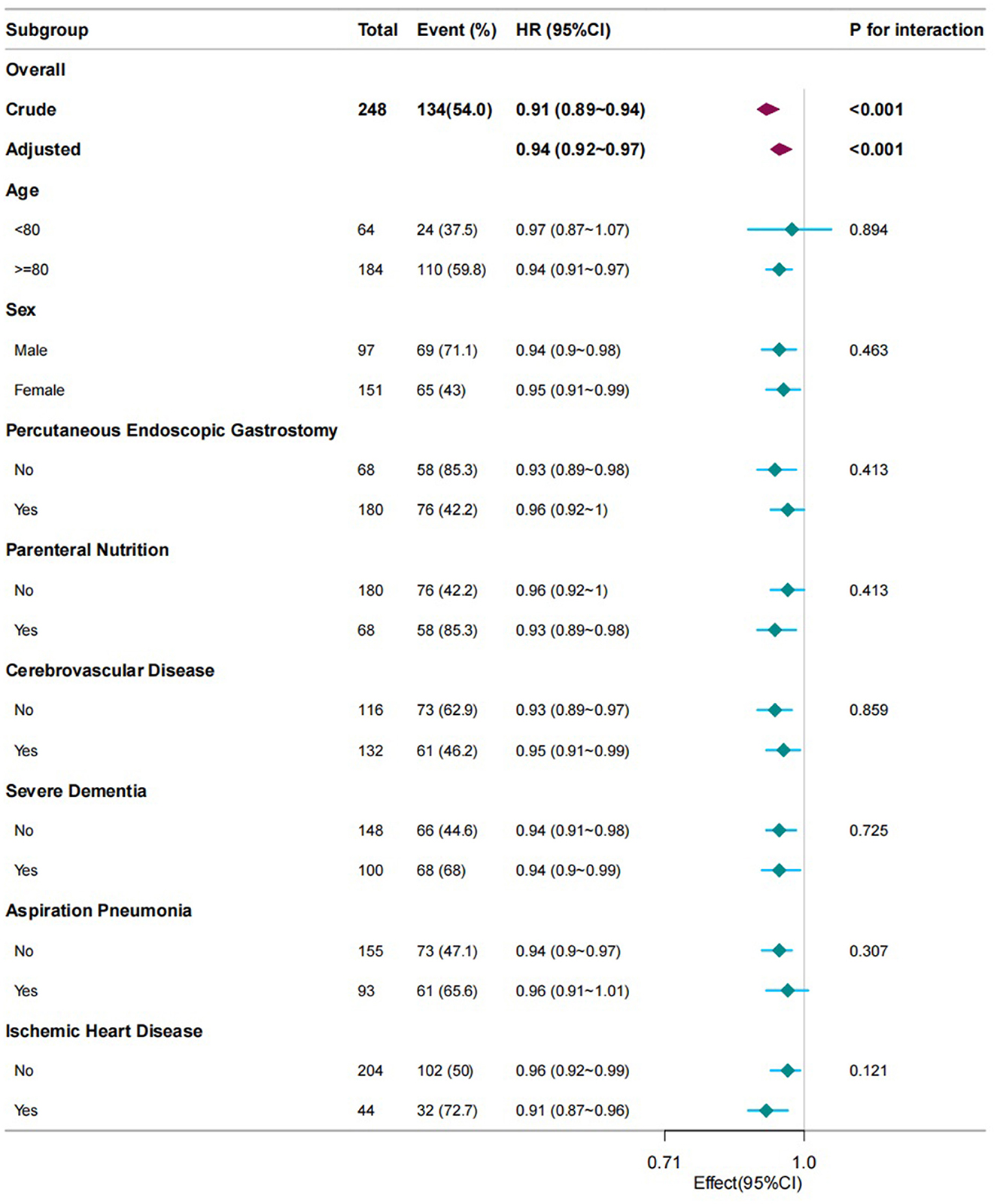
Figure 2. Subgroup analyses of PNI associated with mortality. Hazard ratios (HRs) were adjusted for age, sex, cerebrovascular diseases, severe dementia, aspiration pneumonia, ischemic heart disease, total parenteral nutrition, percutaneous endoscopic gastrostomy, oral intake recovery, and hemoglobin. PNI, prognostic nutritional index.
4 Discussion
Dysphagia is a complex process that requires good coordination among multiple tissues and organs. It is regulated and controlled by several regions within the central nervous system and depends on the normal functions of numerous muscles-striated and smooth muscles, in addition to the soft tissues and skeletal anatomy (14). Pathological damage at any point in the swallowing pathway can lead to dysphagia. The three main populations at risk of dysphagia include: people with stroke, patients with neurodegenerative diseases (including Alzheimer's disease, Parkinson's disease, multiple sclerosis, and amyotrophic lateral sclerosis), and patients with head and neck cancers (including oral cancer, nasopharyngeal cancer, and laryngeal cancer) (15, 16). Besides recognizable disease states, the process of healthy aging is also associated with an increased prevalence of dysphagia (15). In the elderly population, existing evidence shows that 9% of care home residents in the Netherlands and 11.4% of community residents in the UK have symptoms of dysphagia (17, 18). Multiple studies in Europe have found that the incidence of dysphagia among community-dwelling elderly is 11.4%18, 17.3% in care homes (19), and 47.4% in hospitals (20). Dysphagia in the elderly can lead to serious complications and has a great impact on patients' health, nutritional status, and quality of life. Developed dysphagia or inefficient nutritional intake leads to malnutrition and/or dehydration, while impaired swallowing safety results in aspiration, respiratory infections, and an increased risk of readmission. All these complications will increase the mortality among this group of people (15, 21–23).
PNI represents a reliable and novel instrument for prognostic assessment, which can assist in predicting patient outcomes in various disease states (6, 24, 25). Our study evaluated the prognosis of elderly patients with dysphagia using PNI calculated based on ALB and total lymphocyte count. It was found that there was a significant negative correlation between PNI and mortality. Specifically, when PNI was analyzed as a continuous variable, for every 5-point decrease in PNI, the mortality increased by 57% (HR = 1.57, 95% CI: 1.39–1.76, P < 0.001). Even after adjusting for age, gender, underlying diseases, nutritional intake methods, and hematological parameters, the difference remained statistically significant, with an HR of 1.34 (95% CI: 1.16–1.56) (P < 0.001). Further, patients were grouped according to PNI to compare the prognostic differences between the normal nutrition status group and the moderate and severe malnutrition groups. The results showed that in both univariate and multivariate COX regression analyses, PNI was significantly associated with mortality. In the model without adjustment for confounding factors, the mortality of the severe malnutrition group was 3.5 times that of the group with normal nutrition status. After adjusting for all covariates, the multivariate analysis results indicated that the statistical significance of the correlation between PNI and the mortality of dysphagia still existed. The all-cause mortality of the severe malnutrition group was 1.83 times that of the group with normal nutrition status (p = 0.007).
PNI was initially used to assess the surgical risks of digestive system tumors (7), and is now being used for prognostic assessment of various tumors, such as early-stage colorectal cancer, renal cancer, cervical cancer, and malignant melanoma (26–28). In recent years, an increasing number of studies have revealed its association with the prognoses of multiple inflammatory diseases. Some scholars have found that among patients with community-acquired pneumonia, a higher PNI is related to a lower mortality (24). Wu et al. (6) investigated the relationship between PNI and all-cause mortality in patients with sepsis and discovered that PNI is an independent risk factor for mortality. In other diseases, including peritoneal dialysis and COVID-19, a decrease in PNI is associated with a poor prognosis (6, 25). Our study has similar findings. For elderly patients with dysphagia, PNI is an independent risk factor for mortality. Particularly in patients with severe malnutrition, the mortality is significantly increased. It is noteworthy that there was no statistically significant difference in mortality between the moderate malnutrition group and the severe malnutrition group as well as the group with normal nutrition status. The possible reason for this might be the relatively small number of cases in the moderate malnutrition group. Further clarification is required with more robust large-scale data studies. However, according to the Kaplan-Meier curve in Figure 1, there was a statistically significant difference in mortality among the three groups (P < 0.0001). The median survival duration of the severe malnutrition group, the moderate malnutrition group, and the normal nutrition group were 189 days, 447 days, and 864 days respectively.
Previous studies have found that patients with residual dysphagia after cerebrovascular diseases have a poor prognosis (11, 29), which is related to varying degrees of inflammatory responses (30). The inflammatory process contributes to the mechanisms of injury and repair. Therefore, it is very important to consider the inflammatory state for the prognostic assessment of such patients. PNI combines the level of ALB and the total lymphocyte count. The total lymphocyte count reflects the function of the immune system, and a low level may indicate the presence of immunodeficiency. It has been reported that PNI is associated with inflammatory states such as diabetic nephropathy and infections (31, 32). Wang et al. also found that in young stroke patients, a low PNI was associated with a high level of inflammatory response and was an independent risk factor for poor prognosis at 90 days (33). This correlation emphasizes the potential utility of PNI in studying the inflammatory process and nutritional status of patients with dysphagia. In addition, some scholars have proposed that malnutrition caused by dysphagia will further aggravate the swallowing disorder in patients, creating a vicious cycle, and leading to a poor prognosis (34). Malnutrition restricts the normal function of immune cells, reduces the body's ability to resist infections and regulate inflammatory responses, and ultimately has an adverse impact on the function of the immune system (35). Moreover, malnutrition will promote oxidative stress responses and the production of inflammatory mediators, trigger excessive inflammatory responses, and exacerbate the severity of various diseases (36, 37). Good nutritional status can accelerate recovery, reduce the risk of infection, and improve the overall prognosis of patients with dysphagia. Since the clinical trials by Seltzer et al. (38) ALB has been widely used as a marker of nutritional status. Although the role of ALB in the assessment of nutritional status has been challenged recently, studies have shown that patients with low ALB levels are at risk of malnutrition. One study has determined that ALB < 3.5 g/L is an independent risk factor for predicting a poor prognosis in patients with dysphagia (39). PNI combines the ALB and the total lymphocyte count. These two components, when used together to evaluate the overall nutritional and inflammatory status of patients, have more advantages in predicting patient prognosis compared to a single indicator.
There is a gap in the current research literature regarding the application of PNI to evaluate the prognosis of elderly patients with dysphagia. Our study has found that the mortality of patients with severe malnutrition is significantly increased. Considering this situation, stratifying the nutritional status of elderly patients with dysphagia based on PNI and providing sufficient nutrition to enhance immune function and tissue integrity as well as reduce the incidence of inflammation would prove beneficial for improving the prognosis. The use of nutritional supplements via enteral or intravenous routes constitutes a potential risk factor for infection and may have implications for the outcome (12). Subgroup analyses were conducted separately on patients with PEG and TPN, and the results remained stable. Among patients with dysphagia, regardless of whether nutritional supplements are administered through enteral or intravenous means, PNI serves as an independent risk factor for the mortality. A study focusing on cancer patients revealed that those with a lower PNI already required alternative feeding pathways even in the pre-treatment stage of the tumor (40). Another European study regarding head and neck tumors found that 89.2% of patients with dysphagia and malnutrition were unable to utilize the oral route for nutrition. Research reports indicated that one-fifth of such patients needed enteral feeding or stoma creation. When this option is unavailable, it becomes necessary to provide nutritional support through intravenous or enteral feeding (41). We also carried out subgroup analyses on age, gender, IHD, cerebrovascular disease, severe dementia, and aspiration pneumonia, and the results were all stable with no interaction detected. This suggests that even in the presence of multiple comorbidities, monitoring PNI, and providing adequate nutritional support according to the nutritional status may enhance patient survival. Future research should further refine the exploration of the impact of the dynamic changes of PNI on the mortality.
A true advantage of this study is that ALB and total lymphocyte count are commonly used clinical blood testing indicators, with rapid detection that does not impose additional economic burden on patients. According to our research, this may be an important prognostic indicator and supports wider implementation in the clinical setting. However, it is important to acknowledge the limitations of this study. Firstly, this study was a retrospective study conducted at a single center in Japan, which may introduce bias and inaccuracy in data collection and recording and further robust multi-center prospective studies in different countries are needed for comparison. Secondly, only differences in mortality were evaluated, and other complications were not assessed. Thirdly, in this data, all patients receiving PEG or TPN had clinically evaluated severe dysphagia, while that on alternative enteral nutrition or with milder illnesses were not evaluated. Lastly, this was a secondary analysis of data from a pre-existing database. We did not design or control the data collection methods.
5 Conclusion
PNI is negatively correlated with the mortality of elderly patients with dysphagia. The mortality of patients with severe malnutrition is significantly increased. Subgroup analysis showed no interaction, and the results were stable.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The studies involving humans were approved by Ethical Review Board of Miyanomori Memorial Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
RP: Conceptualization, Data curation, Formal analysis, Methodology, Resources, Software, Validation, Writing – original draft, Writing – review & editing. DW: Conceptualization, Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-009A).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dziewas R, Beck AM, Clave P, Hamdy S, Heppner HJ, Langmore SE, et al. Recognizing the importance of dysphagia: stumbling blocks and stepping stones in the twenty-first century. Dysphagia. (2017) 32:78–82. doi: 10.1007/s00455-016-9746-2
2. Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. (2005) 36:2756–63. doi: 10.1161/01.STR.0000190056.76543.eb
3. Clavé P, Shaker R. Dysphagia: current reality and scope of the problem. Nat Rev Gastroenterol Hepatol. (2015) 12:259–70. doi: 10.1038/nrgastro.2015.49
4. Wirth R, Pourhassan M, Streicher M, Hiesmayr M, Schindler K, Sieber CC, et al. The impact of dysphagia on mortality of nursing home residents: results from the nutrition Day project. J Am Med Dir Assoc. (2018) 19:775–8. doi: 10.1016/j.jamda.2018.03.016
5. Patel DA, Krishnaswami S, Steger E, Conover E, Vaezi MF, Ciucci MR, et al. Economic and survival burden of dysphagia among inpatients in the united states. Dis Esophagus Off J Int Soc Dis Esophagus. (2018) 31:1–7. doi: 10.1093/dote/dox131
6. Wu H, Zhou C, Kong W, Zhang Y, Pan D. Prognostic nutrition index is associated with the all-cause mortality in sepsis patients: a retrospective cohort study. J Clin Lab Anal. (2022) 36:e24297. doi: 10.1002/jcla.24297
7. Sun K, Chen S, Xu J, Li G, He Y. The prognostic significance of the prognostic nutritional index in cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol. (2014) 140:1537–49. doi: 10.1007/s00432-014-1714-3
8. Ishiguro T, Aoyama T, Ju M, Kazama K, Fukuda M, Kanai H, et al. Prognostic nutritional index as a predictor of prognosis in postoperative patients with gastric cancer. Vivo Athens Greece. (2023) 37:1290–6. doi: 10.21873/invivo.13207
9. Zhang X, Liu Y, Mu D. Influence of prognostic nutritional index on the surveillance after surgery-based systematic therapy for breast cancer. Am Surg. (2023) 89:6157–71. doi: 10.1177/00031348231191200
10. Okui M, Horio H, Asakawa A, Yamamichi T, Harada M. The prognostic nutritional index in resected high-grade pulmonary neuroendocrine carcinoma. Gen Thorac Cardiovasc Surg. (2020) 68:43–8. doi: 10.1007/s11748-019-01150-2
11. Ustaalioğlu İ, Umaç GA. The role of the prognostic nutritional index in predicting mortality in stroke patients. Rev Assoc Médica Bras. (2024) 70:e20240714. doi: 10.1590/1806-9282.20240714
12. Masaki S, Kawamoto T. Comparison of long-term outcomes between enteral nutrition via gastrostomy and total parenteral nutrition in older persons with dysphagia: a propensity-matched cohort study. PLoS ONE. (2019) 14:e0217120. doi: 10.1371/journal.pone.0217120
13. Scrutinio D, Lanzillo B, Guida P, Passantino A, Spaccavento S, Battista P. Association between malnutrition and outcomes in patients with severe ischemic stroke undergoing rehabilitation. Arch Phys Med Rehabil. (2020) 101:852–60. doi: 10.1016/j.apmr.2019.11.012
14. Jean, A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. (2001) 81:929–69. doi: 10.1152/physrev.2001.81.2.929
15. Baijens LW, Clavé P, Cras P, Ekberg O, Forster A, Kolb G, et al. European society for swallowing disorders – European union geriatric medicine society white paper: oropharyngeal dysphagia as a geriatric syndrome. Clin Interv Aging. (2016) 11:1403–28. doi: 10.2147/CIA.S107750
16. Zhang M, Li C, Zhang F, Han X, Yang Q, Lin T, et al. Prevalence of dysphagia in China: an epidemiological survey of 5943 participants. Dysphagia. (2021) 36:339–50. doi: 10.1007/s00455-020-10138-7
17. van der Maarel-Wierink CD, Meijers JMM, De Visschere LMJ, de Baat C, Halfens RJG, Schols JMGA. Subjective dysphagia in older care home residents: a cross-sectional, multi-centre point prevalence measurement. Int J Nurs Stud. (2014) 51:875–81. doi: 10.1016/j.ijnurstu.2013.10.016
18. Holland G, Jayasekeran V, Pendleton N, Horan M, Jones M, Hamdy S. Prevalence and symptom profiling of oropharyngeal dysphagia in a community dwelling of an elderly population: a self-reporting questionnaire survey. Dis Esophagus Off J Int Soc Dis Esophagus. (2011) 24:476–80. doi: 10.1111/j.1442-2050.2011.01182.x
19. Irles Rocamora JA, Sánchez-Duque MJ, de Valle Galindo PB, Bernal López E, Fernández Palacín A, Almeida González C, et al. A prevalence study of dysphagia and intervention with dietary counselling in nursing home from seville. Nutr Hosp. (2009) 24:498–503.
20. Carrión S, Cabré M, Monteis R, Roca M, Palomera E, Serra-Prat M, et al. Oropharyngeal dysphagia is a prevalent risk factor for malnutrition in a cohort of older patients admitted with an acute disease to a general hospital. Clin Nutr Edinb Scotl. (2015) 34:436–42. doi: 10.1016/j.clnu.2014.04.014
21. Rofes L, Arreola V, Almirall J, Cabré M, Campins L, García-Peris P, et al. Diagnosis and management of oropharyngeal dysphagia and its nutritional and respiratory complications in the elderly. Gastroenterol Res Pract. (2011) 2011:818979. doi: 10.1155/2011/818979
22. Clavé P, Rofes L, Carrión S, Ortega O, Cabré M, Serra-Prat M, et al. Pathophysiology, relevance and natural history of oropharyngeal dysphagia among older people. Nestle Nutr Inst Workshop Ser. (2012) 72:57–66. doi: 10.1159/000339986
23. Rofes L, Arreola V, Romea M, Palomera E, Almirall J, Cabré M, et al. Pathophysiology of oropharyngeal dysphagia in the frail elderly. Neurogastroenterol Motil. (2010) 22:851. doi: 10.1111/j.1365-2982.2010.01521.x
24. De Rose L, Sorge J, Blackwell B, Benjamin M, Mohamed A, Roverts T, et al. Determining if the prognostic nutritional index can predict outcomes in community acquired bacterial pneumonia. Respir Med. (2024) 226:107626. doi: 10.1016/j.rmed.2024.107626
25. Shang S, Huang Y, Zhan X, Peng F, Wang X, Wen Y, et al. The relationship between the prognostic nutritional index and new-onset pneumonia in peritoneal dialysis patients. Int Urol Nephrol. (2022) 54:3017–24. doi: 10.1007/s11255-022-03233-1
26. Maruyama T, Shimoda M, Hakoda H, Sako A, Ueda K, Suzuki S. Preoperative prognostic nutritional index predicts risk of recurrence after curative resection for stage IIA colon cancer. Am J Surg. (2021) 222:179–85. doi: 10.1016/j.amjsurg.2020.10.032
27. Gangopadhyay A. Prognostic nutritional index and clinical response in locally advanced cervical cancer. Nutr Cancer. (2020) 72:1438–42. doi: 10.1080/01635581.2020.1729820
28. Mirili C, Yilmaz A, Demirkan S, Bilici M, Basol Tekin S. Clinical significance of prognostic nutritional index (PNI) in malignant melanoma. Int J Clin Oncol. (2019) 24:1301–10. doi: 10.1007/s10147-019-01461-7
29. Cabre M, Serra-Prat M, Palomera E, Almirall J, Pallares R, Clavé P. Prevalence and prognostic implications of dysphagia in elderly patients with pneumonia. Age Ageing. (2010) 39:39–45. doi: 10.1093/ageing/afp100
30. Simats A, Liesz A. Systemic inflammation after stroke: Implications for post-stroke comorbidities. EMBO Mol Med. (2022) 14:e16269. doi: 10.15252/emmm.202216269
31. Aktas G. Association between the prognostic nutritional index and chronic microvascular complications in patients with type 2 diabetes mellitus. J Clin Med. (2023) 12:5952. doi: 10.3390/jcm12185952
32. Demirkol ME, Aktas G, Alisik M, Yis OM, Kaya M, Kocadag D. Is the prognostic nutritional index a predictor of covid-19 related hospitalizations and mortality? Malawi Med J J Med Assoc Malawi. (2023) 35:15–21. doi: 10.4314/mmj.v35i1.4
33. Wang X, Kang Z, Wang Y, Zheng Y, Wei Y. Application of prognostic nutritional index in the predicting of prognosis in young adults with acute ischemic stroke. World Neurosurg. (2023) 178:e292–9. doi: 10.1016/j.wneu.2023.07.045
34. Saito T, Hayashi K, Nakazawa H, Yagihashi F, Oikawa LO, Ota T, et al. significant association of malnutrition with dysphagia in acute patients. Dysphagia. (2018) 33:258–65. doi: 10.1007/s00455-017-9855-6
35. Hao X, Li D, Zhang N. Geriatric nutritional risk index as a predictor for mortality: a meta-analysis of observational studies. Nutr Res. (2019) 71:8–20. doi: 10.1016/j.nutres.2019.07.005
36. Keusch GT. The history of nutrition: malnutrition, infection and immunity. J Nutr. (2003) 133:336S−40S. doi: 10.1093/jn/133.1.336S
37. de Sire A, Ferrillo M, Lippi L, Agostini F, de Sire R, Ferrara PE, et al. Sarcopenic dysphagia, malnutrition, and oral frailty in elderly: a comprehensive review. Nutrients. (2022) 14:982. doi: 10.3390/nu14050982
38. Seltzer MH, Bastidas JA, Cooper DM, Engler P, Slocum B, Fletcher HS. Instant nutritional assessment. JPEN J Parenter Enteral Nutr. (1979) 3:157–9. doi: 10.1177/014860717900300309
39. Byun SE, Kwon KB, Kim SH, Lim SJ. The prevalence, risk factors and prognostic implications of dysphagia in elderly patients undergoing hip fracture surgery in Korea. BMC Geriatr. (2019) 19:356. doi: 10.1186/s12877-019-1382-x
40. Chang PH, Hsieh JCH, Yeh KY, Chen EYC, Yang SW, Huang JS, et al. Prognostic nutritional index relevance in chemoradiotherapy for advanced oral cavity, oropharyngeal and hypopharyngeal cancer. Asia Pac J Clin Nutr. (2018) 27:996–1001.
Keywords: dysphagia, mortality, albumin, total lymphocyte count, prognostic nutritional index
Citation: Pei R and Wang D (2025) Prognostic nutritional index negative associated with mortality in older Japanese patients with dysphagia. Front. Nutr. 12:1586248. doi: 10.3389/fnut.2025.1586248
Received: 02 March 2025; Accepted: 17 April 2025;
Published: 08 May 2025.
Edited by:
Carlo Pedrolli, Azienda Provinciale per i Servizi Sanitari (APSS), ItalyReviewed by:
Claudia Venturini, National Institute of Science and Health for Aging (IRCCS), ItalyXiuli Yan, First Affiliated Hospital of Jilin University, China
Copyright © 2025 Pei and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Donghao Wang, d2FuZ2RvbmdoYW9AdGptdWNoLmNvbQ==
 Ruijun Pei
Ruijun Pei Donghao Wang
Donghao Wang