- 1Centre for Nutraceuticals, University of Westminster, London, United Kingdom
- 2Centre for Better Living, Anglia Ruskin University, Cambridge, United Kingdom
- 3Centre for Health and Life Sciences (CHLS), Coventry University, Coventry, United Kingdom
Background: Chronic stress is a prevalent issue among university students, negatively affecting both mental and physiological health. Palmitoylethanolamide (PEA), particularly in the Levagen+® formulation, has been investigated for its potential stress-modulating effects through its anti-inflammatory and neuroprotective properties. This study aimed to assess the effects of 6 weeks of Levagen+® PEA supplementation on physiological and subjective markers of stress in moderately stressed female university students.
Methods: A double-blind, placebo-controlled crossover trial was conducted with 16 female participants who met the inclusion criteria based on the Perceived Stress Scale (PSS). Participants were randomly assigned to receive either 6 weeks of PEA supplementation (600 mg/day) or a placebo, with a six-week washout period. Stress responses were assessed through heart rate variability (HRV), subjective stress and mood measures (PSS, PANAS), and salivary cortisol levels. To enhance ecological validity, assessments were conducted in real-life settings rather than laboratory environments.
Results: PEA supplementation significantly increased the Standard Deviation of Normal-to-Normal (SDNN), a key HRV marker associated with autonomic resilience to stress (+9.70 ± 6.02 ms) compared to placebo (−5.72 ± 3.14 ms, p = 0.024), suggesting enhanced physiological stress regulation. While there was a trend of increased Root Mean Square Successive Difference (RMSSD) with PEA, it did not significantly change between conditions (p = 0.087). Similarly, a trend toward reduced self-reported stress was observed, though it did not reach statistical significance. No significant changes were detected in positive (p = 0.78) or negative (p = 0.95) emotions experienced. Salivary cortisol levels remained unchanged between conditions (p = 0.70).
Conclusion: This exploratory study demonstrates for the first time that PEA supplementation may enhance physiological resilience to stress as indicated by improved HRV. While subjective stress and emotional measures did not show significant changes, the observed trend suggests potential benefits in individuals experiencing moderate stress. Given PEA’s role in the endocannabinoid system and its influence on inflammation, further research is warranted in larger and more diverse populations, including individuals with higher baseline stress levels. These preliminary findings contribute to the growing body of evidence supporting PEA as a promising dietary intervention for stress management.
Clinical trial registration: https://clinicaltrials.gov, NCT06225440.
Introduction
The human body is intricately programmed to maintain a predefined steady state fundamental to normal life and well-being, termed homeostasis. This state of optimal equilibrium is normally maintained through an elegant interplay between various physiological systems despite constant intrinsic and extrinsic challenges described as stressors (1). Although stress is a broad term not commonly described by a consensus definition, in the biological sense, it is defined as a maladaptive state of threatened homeostasis triggered by stressors that may be physical, psychological, behavioural, or combinations of these (1). A complex network involving the hypothalamic–pituitary–adrenal (HPA) axis and the autonomic nervous system (ANS) acting in concert with the key centres in the central nervous system (CNS) and peripheral tissues are all mobilised to generate an adaptive, coping response to stress. Acute stress arises from immediate challenges or stressors with the body mobilising the classic ‘fight or flight’ responses mediated by sympathetic nervous system (SNS) activation. This initiates a cascade of hormonal secretions including catecholamine release, and subsequent physiological changes such as increased arterial pressure and blood flow to muscles (2). Mobilisation of acute responses does not necessarily place a health burden on an individual and provides positive or rewarding stimuli (3). However, prolonged exposure to stressors and sustained mobilisation of adaptive stress responses can lead to maladaptive physiological function and physical, psychological and behavioural impairments contributing to disease (4). For example, psychosocial stress has been shown to be a significant risk factor in the development of anxiety disorders, a major health concern globally and one of the most diagnosed categories of mental health (5). Several studies have shown sex differences in stress responses, for example, in an academic context, research has identified a gender gap, with females more likely to experience greater levels of stress (6, 7). These effects may be compounded by lifestyle choices, including adherence to a Western-style diet, which has been linked to elevated stress levels in female college students, potentially due to its pro-inflammatory properties (8). Consequently, interventions aimed at reducing inflammation may help mitigate stress within this population (9).
Palmitoylethanolamide (N -palmitoylethanolamine or PEA) is an endogenous fatty acid amide belonging to the family of N-acylethanolamines (NAEs) that is ubiquitously produced in the body (10). NAEs include the endogenous cannabinoid receptor ligand anandamide (AEA, arachidonoylethanolamide) and the satiety agent oleoylethanolamide (OEA). However, despite structural similarities, PEA does not act as a classical endocannabinoid and has a rather complex and varied pharmacological profile (11). Originally referred to as ALIAamide (ALIA - autacoid local inflammation antagonist) on the basis of observations that it reduced mast cell degranulation, PEA has since been shown to interact with a range of receptor and non-receptor targets to produce a host of biological effects, including anti-inflammatory, analgesic, antiviral and neuroprotective (10). PEA’s most well-documented actions are mediated via the nuclear transcription factor peroxisome proliferator-activated receptor alpha (PPAR-α), via which it is shown to exert anti-inflammatory actions (12, 13). Although not a direct ligand of the cannabinoid receptors (CB1 and CB2), PEA has been shown to act indirectly by potentiating the actions of endocannabinoids such as AEA via an ‘entourage effect’ (13, 14). In this model, PEA increases receptor affinity for AEA by reducing its enzymatic degradation via the enzyme fatty acid amide hydroxylase (FAAH) by serving as an alternative substrate, thereby enhancing its effects attributed to AEA (13).
In addition to its endogenous expression, PEA is also available exogenously through the diet and is present in various foods such as soy lecithin, palm oil, peanut meal and egg yolk. However, the available levels are relatively low, giving rise to the utilisation of exogenous PEA in the form of dietary supplements. However, issues persist with exogenous PEA administration due to its poor pharmacokinetic profile, which leads to the application of strategies such as micronisation and carrier-based delivery systems to enhance its bioaccesibility (11). Levagen+® is a formulated form of PEA utilising a cold-water dispersible (CWD) technology (LipiSperse®) previously shown to have enhanced bioavailability (15). Recent clinical studies have shown Levagen+® PEA to improve sleep quality as well as parameters of cognitive function (16, 17). Given the close interplay between these functionalities, the observed beneficial effects of supplemental PEA on markers of wellbeing and cognition raises intriguing possibilities for exploring its role in mitigating stress. Based on this rationale, the current study aimed to address the research question: Does 6 weeks of supplementation with Levagen+® (a formulated PEA) improve physiological and subjective markers of stress in moderately stressed female university students compared to placebo?
Methods
Study design
A randomised, double-blinded, placebo-controlled cross-over approach was utilised to examine the impact of PEA supplementation on stress, mood and heart rate variability in university students. This approach was selected to reduce selection and allocation biases, control for potential confounding factors, and ensure that every participant received both PEA supplementation and a placebo in a counterbalanced order. Participants were asked to self-record measurements at home on four occasions across two academic semesters, with follow-up assessments scheduled during periods of assignments and exams to capture periods that have been shown to elicit stress in university student populations (18). The day before the collection period, participants attended the laboratory and were given an at-home salivary collection kit and fitted with a heart rate variability monitor (described below). HRV, morning salivary cortisol, and subjective measures (Perceived Stress Scale and PANAS) were all recorded during the first hour after waking, on the morning following the laboratory visit. Twenty-four hours before collection, participants were asked to abstain from alcohol and stimulant consumption, extensive training, sauna use, sleep deficit, exposure to excessive noise and illuminance and maintain hydration status to minimise potential confounding factors. The manuscript was written in accordance with the Consolidated Standards of Reporting Trials (CONSORT).
Participants
A subset of female participants from a larger randomised controlled trial (ClinicalTrials.gov Identifier: NCT06225440) were invited to take part in this exploratory sub-study. Selection was based on scoring in the moderate range of perceived stress (PSS > 13), as this population has not been specifically investigated in the context of PEA supplementation. In addition, due to logistical considerations, only a defined number of participants could be provided with validated HRV monitoring devices capable of capturing reliable morning recordings. This approach allowed for a focused analysis of HRV responses in a moderately stressed cohort while maintaining feasibility within the operational constraints of the larger study.
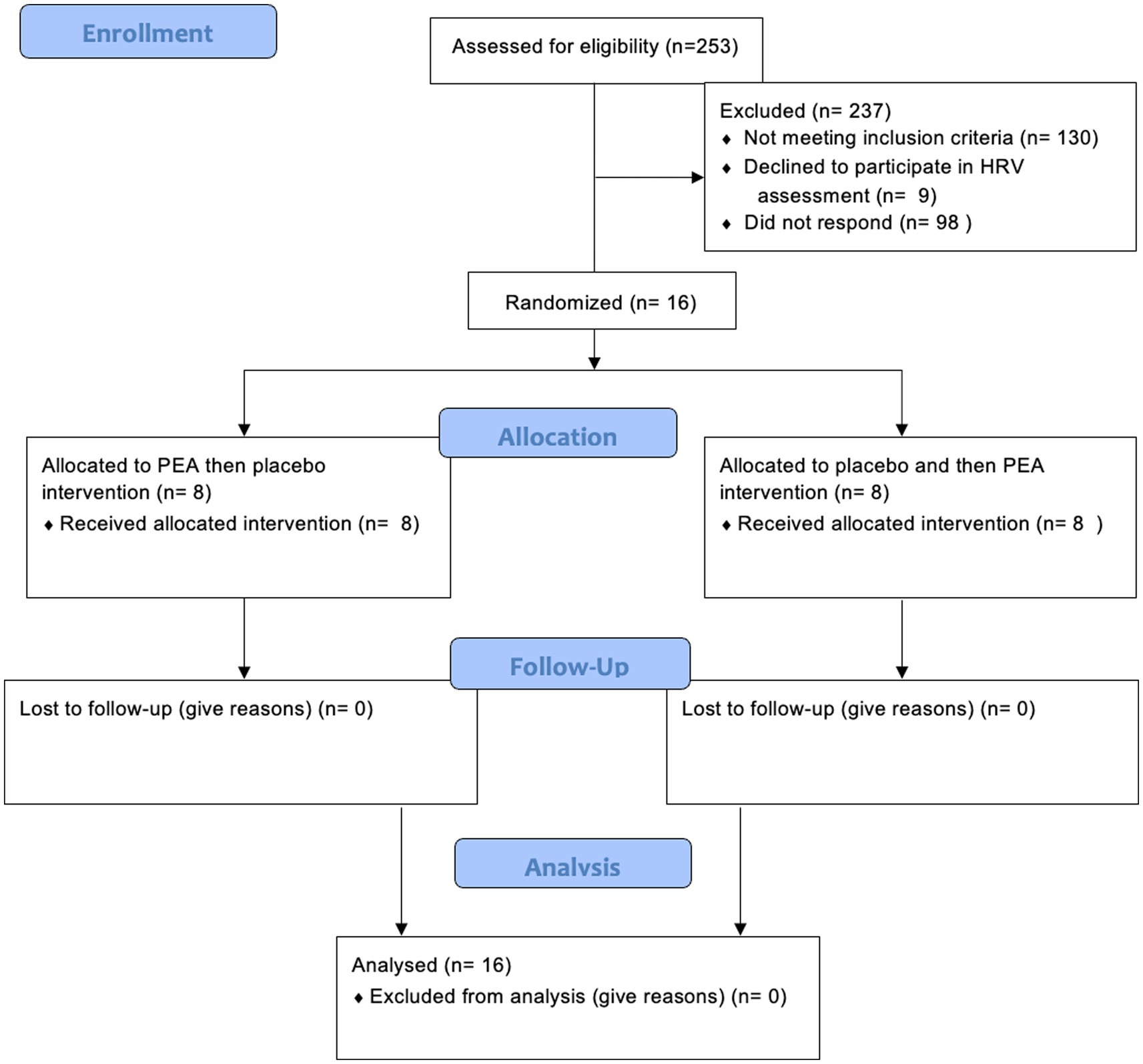
Sixteen female participants were recruited for this part of the study (mean ± SD age: 22 ± 2.4 years; Figure 1). They were required to be fully enrolled in their courses and committed to completing the entire intervention. Female participants who reported being moderately stressed were recruited, as determined by the Perceived Stress Scale (PSS; described below). Individuals with a score greater than 13 were determined to have moderate stress levels (19). Moderately stressed individuals (PSS > 13) were selected to reflect a non-clinical population that may benefit from early lifestyle or nutraceutical interventions before progression to chronic stress-related disorders. This approach also aligns with ethical considerations of conducting supplementation trials in otherwise healthy participants and avoids confounding effects related to pharmacological treatment or psychiatric comorbidities commonly seen in severely stressed or clinical populations. Additionally, recruiting moderately stressed individuals improves generalisability to broader populations, such as university students, where subclinical stress is highly prevalent.
The exclusion criteria comprised individuals with chronic health conditions, learning disabilities, smoking, and excessive alcohol consumption (more than 14 units per week), as well as individuals with neurological disorders or on medication, including those using dietary supplements and herbal remedies, were excluded from participation. Additional exclusions included individuals who are obese, pregnant, breastfeeding, attempting conception, or undergoing/planning medical, dental, or orthodontic procedures. Online recruitment via social media was used to target students in higher education at any local London university. Interested volunteers completed a survey to screen against the eligibility criteria. The first on-site visit involved measuring participants’ body mass index (BMI) and blood pressure, with eligibility limited to those having a BMI between 20 and 30 and blood pressure below 140/90 mmHg. Prospective participants were briefed on the study, which included reviewing the Participant Information Sheet and detailing the study’s objectives, procedures, and potential risks and benefits. Following this detailed explanation, each participant provided a signed consent form, signifying their willingness to partake in the study. The faculty ethics committee at the University of Westminster granted ethical approval for the study (Application ID: ETH2122-1031).
Intervention
Participants were randomly assigned to one of two treatment sequences in a double-blind, placebo-controlled crossover design: (1) Levagen+® (formulated PEA) followed by placebo or (2) Placebo followed by Levagen+®. Randomisation was conducted using an unrestricted probability sampling approach via a computer-generated sequence,1 ensuring equal allocation across conditions. Each treatment phase lasted 6 weeks, separated by a two-week washout period to minimise carryover effects. This design is consistent with previous crossover trials involving PEA supplementation (15, 16).
Participants received daily doses of either Levagen+® (Gencor Pacific Limited, Lantau Island, Hong Kong) or placebo (microcrystalline cellulose). The active supplement (Levagen+®) delivered 600 mg of PEA per day (two capsules containing not less than 300 mg PEA each) using LipiSperse® dispersion technology (Pharmako Biotechnologies Pty Ltd., Sydney, Australia) to enhance bioavailability. Capsules in both conditions were identical in size, shape, and colour, and were manufactured by Power Health Products Ltd. (York, United Kingdom). Participants were instructed to take both capsules simultaneously each day for the six-week period. Regular researcher contact helped support adherence, and no adverse effects were reported.
The selected dose of 600 mg/day of palmitoylethanolamide (PEA) in the present study is based on the bioavailability and efficacy profile of the formulation used—Levagen+®, which incorporates LipiSperse®, a delivery system that enhances water dispersion and has been shown to improve PEA bioavailability and uptake in humans (15). Several studies have demonstrated favourable outcomes across a range of clinical applications and dosages. For example, 300 mg/day of Levagen+® over 8 weeks significantly reduced sleep onset latency and improved next-day cognition (17). A daily dose of 450 mg/day was associated with a reduction in headache duration and faster resolution of severe headaches compared to 400 mg/day of ibuprofen (20). Notably, 600 mg/day of Levagen+® led to greater headache resolution at 2 and 8 h, lower pain scores at 1.5 and 4 h, and reduced reliance on rescue medication in individuals with recurrent migraines, compared to placebo (21). In the context of mental health, 600 mg/day over 8 weeks also resulted in significantly reduced symptoms of depression and anxiety (22). Taken together, these findings support the selection of 600 mg/day for the current study, aligning with prior evidence on both the safety and efficacy of this dose and its relevance to the targeted outcome measures.
Heart rate variability
Short-term HRV was recorded and analysed at home during a 5-min rest period on waking in the morning. Short-term HRV measurement is the most commonly used assessment of HRV (23) and is posited to be a useful biomarker to assess the effect of dietary interventions on physical and mental health (24). Participants in the study were equipped with The Firstbeat Bodyguard 2 (BG2, Firstbeat Technologies Oy, Finland), a sensor designed for short and long-term HRV measurements. This lightweight (Weight: 24 grams, Dimensions: 47 mm x 63 mm x 10.6 mm) and easy-to-use device, affixed directly to the skin with two chest electrodes, automatically initiated data recording. The researcher demonstrated correct application in person, and further written instructions were provided at home. Participants were asked to wear the HRV upon waking so that initial resting measurements could be taken. Data obtained from the Firstbeat Bodyguard 2 (BG2) devices were extracted using the offline mode of the Firstbeat uploader software (version 3.4.4.0). Subsequently, the extracted data were imported into Kubios HRV Scientific (version 4.1.0). Kubios was used to determine HRV parameters, encompassing time and frequency domain measures. Time domain measures included standard deviation of normal-to-normal intervals (SDNN), root mean square of successive differences (RMSSD), and the proportion of adjacent RR intervals differing by more than 50 ms (pNN50). Frequency domain measures of low-frequency (LF) and high-frequency (HF) ratios were also analysed.
Subjective assessment of stress and mood
The Perceived Stress Scale (PSS) was used to monitor stress at the beginning and end of each experimental arm. The PSS has been widely used to assess the effect of dietary interventions on stress (25–27) and is a valid tool to assess stress in a university study cohort (28). Similarly, the Positive and Negative Affect Schedule (PANAS) was completed pre- and post-experimental treatment arms (29). The Positive and Negative Affect Schedule (PANAS) is a widely used 20-item scale that measures positive and negative emotions through two distinct subscales. The Positive Affect (PA) scale assesses feelings of engagement and enthusiasm, while the Negative Affect (NA) scale captures distressing emotions such as fear and anger. The PANAS has demonstrated strong psychometric properties, including excellent reliability (α = 0.89 for PA and α = 0.85 for NA) (30).
Salivary cortisol collection and analysis
Saliva samples were collected using a non-invasive method that involved the participant inserting a Sarstedt Salivette® (Sarstedt, Germany) cotton bud containing a saliva preservative inside their mouth for 2 min. To ensure appropriate collection techniques, participants completed a supervised collection during each visit and were asked to replicate this the following day on waking (between 7 and 8 am). Participants were instructed to keep the collected samples in their home fridge before returning them to the laboratory, where they were aliquoted and stored at −80°C until analysis. Cortisol levels were determined using a competitive enzyme-linked immunosorbent assay kit (Salimetrics, Carlsbad, CA).
Statistical analysis
As initial assumptions and tests of normality were met, a paired t-test was used to compare the change score of all outcome variables between baseline and follow-up for the placebo and PEA conditions. A significance level of p < 0.05 was applied to determine statistical significance. All analysis was conducted on SPSS 19 (IBM, Chicago, IL, United States) and Microsoft Excel (Microsoft, Redmond, WA, United States). All descriptive data are reported as mean and SEM.
Results
Heart rate variability measurement
Six weeks of supplementation with Levagen+® PEA significantly affected SDNN (Figure 1). SDNN saw a significant increase of 9.70 ± 6.01 ms in the PEA treatment arm compared to a reduction of −5.72 ± 3.14 ms in the placebo arm (p = 0.024). Although there was a trend for an increase in other time domain measurement of RMSDD with PEA (+10.60 ± 6.08 ms) compared to placebo (−3.86 ± 4.62 ms; p = 0.087; Figure 2). However, there were no differences in the low-to-high frequency ratio between placebo and PEA (p = 0.965). Furthermore, no order effect was found across these HRV variables (p > 0.05).
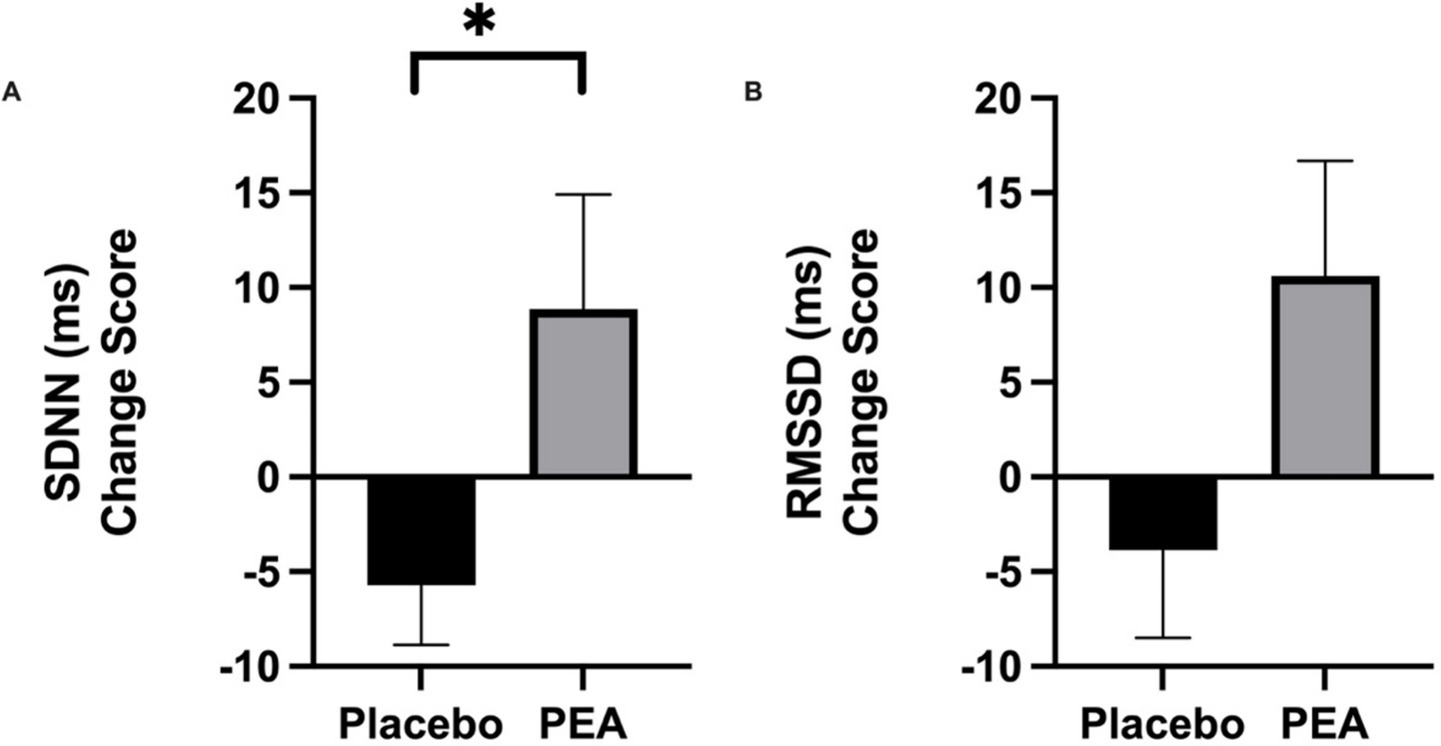
Figure 2. Intervention pre-post change scores comparing placebo and PEA Levagen+® for HRV variable (a) Standard Deviation of Normal-to-Normal (SDNN) and (b) Root mean square of successive differences (RMSSD). *represent statiscally signifcant difference p < 0.05.
Subjective stress and mood
At the start of both treatment arms, participants were moderately stressed, averaging 21.09 ± 1.70 (PEA) and 18.27 ± 2.23 (placebo). While there was a trend in the reduction following PEA supplementation (14.82 ± 1.32) compared to placebo (17.64 ± 1.35), there were no significant differences between conditions (p = 0.10; Figure 3). Equally, no difference in positive (p = 0.78) or negative (p = 0.95) emotions was observed between the two conditions (Figure 3). No order effect was found across stress and mood variables (p > 0.05).
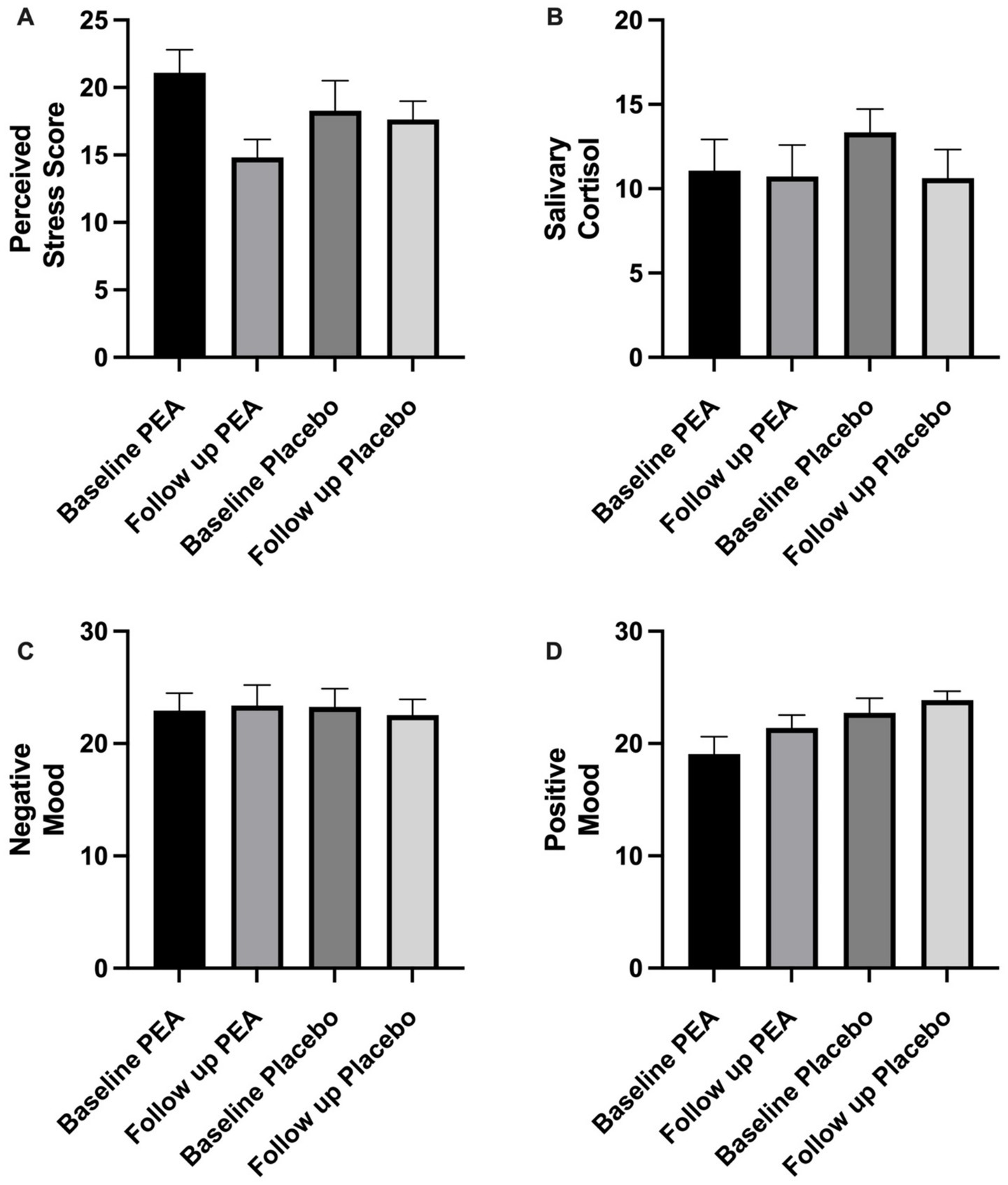
Figure 3. Showing pre and post-intervention comparing placebo and PEA Levagen+® for (a) perceived stress score, (b) salivary cortisol, (c) negative mood and (d) positive mood. No significant differences were observed between conditions.
Salivary cortisol
Salivary cortisol values did not change between PEA and placebo treatment arms. Baseline values were 11.07 ± 1.84 nmoL/L and 13.35 ± 1.37 nmoL/L, which were not significantly different at the end of the intervention period, with values of 10.73 ± 1.86 nmoL/L and 10.63 ± 1.69 nmoL/L for PEA (p = 0.64) and placebo (p = 0.41), respectively (Figure 3). Change scores between PEA (−1.06 ± 0.97 nmoL/L) and placebo (−2.72 ± 2.13) did not differ (p = 0.49). No order effect was found for salivary cortisol (p > 0.05).
Discussion
This study aimed to assess the effects of 6 weeks of Levagen+® formulated PEA supplementation in moderately stressed female participants on markers of objective physiological stress (HRV and salivary cortisol) and subjective reports of stress and emotions. Using a double-blind, randomised controlled trial, participants were asked to report markers of stress on waking at the participant’s home to give the study a higher degree of ecological validity. This meant participants were not exposed to artificial acute stress from laboratory visits, which is seldom experienced in daily life. The study found an increase in the HRV parameter of SDNN and a trend toward reducing self-reported stress. Measurements of morning salivary cortisol and self-reported emotions did not change. As such, this study demonstrates the potential of PEA to mitigate physiological stress in female university students, and it may be a plausible dietary intervention in moderately stressed individuals.
Heart rate variability has been posited as a biomarker to assess a nutritional intervention’s physiological and psychological effects due to the consistent relationship reported between HRV and health outcomes (24). In accordance with this, the main finding from the study was the reduction in SDNN following 6 weeks of Levagen+® PEA supplementation. This aligns with previous research that has demonstrated the influence of dietary interventions in increasing SDNN measurements following omega-3 (31, 32) and polyphenol-rich treatment (33). Like these nutrients, PEA possesses anti-inflammatory properties that may explain the effect on HRV (34). Indeed, there is a negative correlation between C-reactive protein (CRP), a marker that indicates pro-inflammatory conditions, and SDNN (35), suggesting PEA may contribute to lower levels of inflammation and result in a higher SDNN. High-frequency (HF) components of HRV are also linked with CRP, with reports that lower HF-HRV predicted a higher CRP 4 years later (36). The current study did not observe a link between HF-HRV and PEA supplementation. However, this may be due to the short-term intervention used, and extended intervention periods may be required to determine whether PEA has benefits for HRV. It should be acknowledged that an unexpected reduction in both SDNN and RMSSD was observed in the placebo condition. This may be attributed to elevated stress during the follow-up assessment period, which coincided with academic deadlines. While this aligns with previous evidence showing HRV is impacted by stress (37), the absence of this decline in the PEA group was a novel finding in the current study. The increased SDNN observed may suggest cardiovascular benefits, with a meta-analysis reporting that a 1% increase in SDNN is associated with a 1% risk reduction in cardiovascular disease risk (38).
Despite improvements in HRV parameters in a self-reported stressed cohort of female students, we did not find any significant effect on the subjective reports following PEA supplementation. SDNN is suggested to be an index of physiological reserve against stress (37), which may explain the trend toward reduced stress following PEA. Previous research has reported a link between SDNN and self-reported work stress, with greater stress associated with a lower SDNN (39, 40). Equally, in university students, the stress caused during examination periods was found to have a lower SDNN (41). The observations in the current study do not align with this theory, as the increased SDNN following PEA supplementation did not result in significantly lower self-reported stress. It should be noted that larger-scale ecological studies conducted with workers in situ suggested that the significant association between SDNN and stress was small (42). As such, the smaller sample size in the current study may contribute to the lack of significance observed in subject stress.
This study also reported no differences in positive or negative emotions with the PEA intervention. This is in contrast with previous research that reported 6 weeks of PEA supplementation has been shown to reduce depression in patients with major depressive disorder (43), which is also supported in pre-clinical mice models (44). In a retrospective analysis of COVID-19 patients, it was reported that those taking PEA reported fewer depressive symptoms, with authors suggesting that individuals who were identified to have pre-treatment fatigue or report poor subjective well-being were more likely to benefit from a PEA intervention (45). Furthermore, recent reviews have proposed that plasma PEA may emerge as a biomarker for psychosis, and supplementation could be seen as a therapeutic treatment (46). Therefore, it is likely that the stress and low emotions experienced in the sample recruited for this study were not severe enough to report improvements in subjective mental health parameters. There remained a plausible effect from PEA in the general population, and further research may consider PEA interventions on individuals who report high-stress levels and depressive symptoms, which are classed as sub-clinical.
Salivary cortisol did not respond to PEA intervention in the current study, which aligns with previous research assessing dietary intervention. While there have been examples of anti-inflammatory supplements reducing salivary cortisol, such as dark chocolate polyphenols and omega-3 (47–49), much of the research has shown a high degree of variability (50). Pre-clinical mice models have suggested that PEA may act on the HPA axis by reducing the expression of hypothalamic corticotropin-releasing hormone and its type 1 receptor (51). This hormone is the primary driver of the body’s stress response, so there remains promise for PEA. Cortisol measurements demonstrate widespread diurnal variability (52), rendering it challenging to determine an intervention effect. This may explain the inconsistent findings across nutritional intervention research (50) and that observed in the current study. Future investigations may consider a more comprehensive assessment of cortisol response, including multiple daily measurements and assessments following acute artificial stress in laboratory settings.
The endocannabinoid system (ECS) is central in modulating stress reactions and adaptations to chronic stress via the recruitment of the endocannabinoid AEA and the related NAEs PEA and OEA (53). It regulates cardiovascular function, including heart rate and blood pressure, which are key elements of HRV, with AEA implicated as the key actor (W-SV Ho). Indeed, a recent study examining endocannabinoid-HRV interactions in response to stress reported that AEA, PEA and OEA were noticeably increased in hair samples of individuals partaking in intense, prolonged exercise (54). Taken together, these observations support a model wherein AEA and PEA may play a central role in adaptive responses to stress via modulation of HRV, and in this scenario, supplementation with bioavailable, exogenous PEA would further sustain and amplify these effects as observed in this study.
The conclusion drawn from this study should be viewed in the context of certain limitations. Due to logistical challenges, the sample from this study was recruited from a subset of a larger study (17). Post Hoc analysis shows the power of the primary outcome was 57%; however, previous studies examining the positive effects of dietary interventions on HRV use similar sample sizes (55, 56). While the smaller sample size may increase the risk of a Type II error, the findings from this study provide early insights into the role of PEA in modulating autonomic function. The lack of effect on secondary variables from the PSS and PANAS shows a disconnect between physiological HRV measurements and subjective markers. It is likely that these perceptual variables are not sensitive enough to detect changes within the current sample size. Alternative scales should also be considered, such as the State–Trait Anxiety Inventory (STAI), to capture nuanced emotions. Future studies could use Ecological Momentary Assessment (EMA) to overcome the limitations of retrospective mood and stress assessments, which can be confounded by daily stressors and the dynamic fluctuations of emotional states. The use of at-home assessments was a novel aspect of this study, enhancing ecological validity; however, unsupervised data collection introduces potential variability due to environmental factors and participant compliance. To mitigate these concerns, participants received detailed instructions, supervised practice sessions, written guidance, and regular check-ins by researchers throughout the study. Furthermore, the present study intentionally focused on female participants due to their greater vulnerability to stress-related disorders; the absence of male participants limits the generalizability of our findings. To further clarify potential sex-specific responses to PEA supplementation, future research should incorporate male comparator groups. Furthermore, menstrual cycle phase and hormonal contraceptive use were not recorded in this study. While these factors can influence salivary cortisol concentrations, HRV has been shown to be relatively unaffected across the menstrual cycle (57). Nevertheless, we acknowledge this as a limitation in interpreting endocrine-related measures and recommend that future studies account for reproductive hormone status. Lastly, several potential confounding factors such as acute daily stressors, academic workload fluctuations, social support, and socioeconomic status were not systematically controlled or assessed in this study. These variables are known to influence both subjective and physiological stress responses and may have contributed to the variability and limited number of significant findings. Although the study design aimed to enhance ecological validity by assessing participants in their usual environments, this approach also introduced variation in daily experiences that could not be fully accounted for. Future studies should consider including contextual data collection methods, such as daily diaries, EMA, or indicators of socioeconomic background, to better control for these influences.
To better address these limitations, future studies could integrate real-time, continuous measures such as EMA for subjective stress, multiple diurnal cortisol samplings, and laboratory-based stress induction procedures. Additionally, assessments of inflammatory biomarkers (e.g., C-reactive protein, interleukin-6) should be included to elucidate potential underlying mechanisms linking PEA supplementation with observed HRV improvements. Alternatively, laboratory-based studies could introduce acute stressors previously validated to induce physiological and psychological stress responses measurable via HRV. Employing such controlled protocols would allow for a clearer understanding of how physiological, biochemical, and subjective stress responses interact and respond to nutraceutical interventions like PEA supplementation.
Conclusion
PEA has been relatively well studied in the context of inflammation and immune health; however, its putative benefits on stress and cognition have been explored less. This preliminary study evaluated the effects of PEA supplementation on markers of stress in females moderately stressed at onset, demonstrating for the first time an increase in the HRV parameter of SDNN and a trend toward reducing self-reported stress. HRV assessments indicate stress in various situations and are frequently used as a sensitive digital assessment tool in this regard, with SDNN regarded as an index of physiological resilience against stress (58). Our data, therefore, provide a meaningful rationale for using PEA supplementation in stress management. The sample size and the specificity of the study’s inclusion criteria may have contributed to the lack of significance observed in other measures; the work, therefore, also serves as a foundation for more comprehensive examinations to further clarify the findings.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by University of Westminster Institutional Ethics Commitee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SD: Writing – original draft, Writing – review & editing. NK: Writing – review & editing. BP: Writing – review & editing. DR: Writing – review & editing. MZ: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by Knowledge Exchange funding from Gencor Pacific Ltd. (Lantau Island, Hong Kong) (UT724540). The study design, data collection, statistical analysis, and interpretation were performed independently of the study funder, Gencor.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Tsigos, C, Kyrou, I, Kassi, E, and Chrousos, GP. Stress: Endocrine Physiology and Pathophysiology In: C Tsigos, editor. Endotext. South Dartmouth, MA: MDText.com, Inc. (2020)
2. Chu, B, Marwaha, K, Sanvictores, T, Awosika, AO, and Ayers, D. Physiology, Stress Reaction In: B Chu, editor. StatPearls. Treasure Island, FL: StatPearls Publishing (2024)
3. Dorn, LD, and Chrousos, GP. The endocrinology of stress and stress system disorders in adolescence. Endocrinol Metab Clin N Am. (1993) 22:685–700. doi: 10.1016/S0889-8529(18)30158-0
4. Campkin, M. Stress management in primary care. Fam Pract. (2000) 17:98. doi: 10.1093/fampra/17.1.98--a
5. Wright, EC, Luo, PX, Zakharenkov, HC, Godoy, AS, Lake, AA, Prince, ZD, et al. Sexual differentiation of neural mechanisms of stress sensitivity during puberty. Proc Natl Acad Sci U S A. (2023) 120:e2306475120. doi: 10.1073/pnas.2306475120
6. Montolio, D, and Taberner, PA. Gender differences under test pressure and their impact on academic performance: a quasi-experimental design. J Econ Behav Organ. (2021) 191:1065–90. doi: 10.1016/j.jebo.2021.09.021
7. Koehl-Hackert, N, Schultz, J-H, Nikendei, C, Möltner, A, Gedrose, B, van den Bussche, H, et al. Burdened into the job -- final-year students’ empathy and burnout. Z Evid Fortbild Qual Gesundhwes. (2012) 106:116–24. doi: 10.1016/j.zefq.2012.02.020
8. Alfreeh, L, Abulmeaty, MMA, Abudawood, M, Aljaser, F, Shivappa, N, Hebert, JR, et al. Association between the inflammatory potential of diet and stress among female college students. Nutrients. (2020) 12:389. doi: 10.3390/nu12082389
9. Pizarro Meléndez, GP, Valero-Jara, V, Acevedo-Hernández, P, and Thomas-Valdés, S. Impact of polyphenols on stress and anxiety: a systematic review of molecular mechanisms and clinical evidence. Crit Rev Food Sci Nutr. (2024) 64:2340–57. doi: 10.1080/10408398.2022.2122925
10. Keppel Hesselink, JM, Kopsky, DJ, and Witkamp, RF. Palmitoylethanolamide (PEA)—‘promiscuous’ anti-inflammatory and analgesic molecule at the interface between nutrition and pharma. PharmaNutrition. (2014) 2:19–25. doi: 10.1016/j.phanu.2013.11.127
11. Valenza, M, Facchinetti, R, Steardo, L, and Scuderi, C. Palmitoylethanolamide and White Matter Lesions: Evidence for Therapeutic Implications. Biomolecules. (2022) 12:1191. doi: 10.3390/biom12091191
12. LoVerme, J, Russo, R, La Rana, G, Fu, J, Farthing, J, Mattace-Raso, G, et al. Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-α. J Pharmacol Exp Ther. (2006) 319:1051–61. doi: 10.1124/jpet.106.111385
13. Petrosino, S, and Di Marzo, V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br J Pharmacol. (2017) 174:1349–65. doi: 10.1111/bph.13580
14. Ho, WSV, Barrett, DA, and Randall, MD. Entourage effects of N-palmitoylethanolamide and N-oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors. Br J Pharmacol. (2008) 155:837–46. doi: 10.1038/bjp.2008.324
15. Briskey, D, Mallard, AR, and Rao, A. Increased absorption of Palmitoylethanolamide using a novel dispersion technology system (LipiSperse). J Nutr Food Sci. (2020) 5:2. doi: 10.36648/nutraceuticals.5.2.3
16. Kim, N, Parolin, B, Renshaw, D, Deb, SK, and Zariwala, MG. Formulated palmitoylethanolamide supplementation improves parameters of cognitive function and BDNF levels in Young, healthy adults: a randomised cross-over trial. Nutrients. (2024) 16:489. doi: 10.3390/NU16040489
17. Rao, A, Ebelt, P, Mallard, A, and Briskey, D. Palmitoylethanolamide for sleep disturbance. A double-blind, randomised, placebo-controlled interventional study. Sleep Sci Pract. (2021) 5:1–8. doi: 10.1186/S41606-021-00065-3
18. Barbayannis, G, Bandari, M, Zheng, X, Baquerizo, H, Pecor, KW, and Ming, X. Academic stress and mental well-being in college students: correlations, affected groups, and COVID-19. Front Psychol. (2022) 13:886344. doi: 10.3389/fpsyg.2022.886344
19. Cohen, S, Kamarck, T, and Mermelstein, R. A global measure of perceived stress. J Health Soc Behav. (1983) 24:385–96. doi: 10.2307/2136404
20. Briskey, D, Ebelt, P, Steels, E, Subah, S, Bogoda, N, Rao, A, et al. Efficacy of palmitoylethanolamide (Levagen+TM) compared to ibuprofen for reducing headache pain severity and duration in healthy adults: a double-blind, parallel, randomized clinical trial. Food Nutr Sci. (2022) 13:690–701. doi: 10.4236/fns.2022.137050
21. Briskey, D, Skinner, R, Smith, C, and Rao, A. Effectiveness of Palmitoylethanolamide (Levagen+) compared to a placebo for reducing pain, duration, and medication use during migraines in otherwise healthy participants-a double-blind randomised controlled study. Pharmaceuticals (Basel). (2024) 17:145. doi: 10.3390/ph17020145
22. Steels, E, Venkatesh, R, Steels, E, Vitetta, G, and Vitetta, L. A double-blind randomized placebo controlled study assessing safety, tolerability and efficacy of palmitoylethanolamide for symptoms of knee osteoarthritis. Inflammopharmacology. (2019) 27:475–85. doi: 10.1007/S10787-019-00582-9
23. Shaffer, F, and Ginsberg, JP. An overview of heart rate variability metrics and norms. Front Public Health. (2017) 5:290215. doi: 10.3389/fpubh.2017.00258, /PDF
24. Young, HA, and Benton, D. Heart-rate variability: a biomarker to study the influence of nutrition on physiological and psychological health? Behav Pharmacol. (2018) 29:140–51. doi: 10.1097/fbp.0000000000000383
25. McCabe, D, Bednarz, J, Lockwood, C, and Barker, TH. Specific nutrient intake via diet and/or supplementation in relation to female stress: a cross-sectional study. Womens Health Rep. (2020) 1:241–51. doi: 10.1089/whr.2020.0035
26. Camfield, DA, Wetherell, MA, Scholey, AB, Cox, KHM, Fogg, E, White, DJ, et al. The effects of multivitamin supplementation on diurnal cortisol secretion and perceived stress. Nutrients. (2013) 5:4429–50 2013) 5:4429–4450. doi: 10.3390/NU5114429
27. Cicero, AF, Bove, M, Colletti, A, Rizzo, M, Fogacci, F, Giovannini, M, et al. Short-term impact of a combined nutraceutical on cognitive function, perceived stress and depression in young elderly with cognitive impairment: a pilot, double-blind, randomized clinical trial. J Prev Alzheimers Dis. (2017) 4:12–5. doi: 10.14283/jpad.2016.10
28. Anwer, S, Manzar, MD, Alghadir, AH, Salahuddin, M, and Hameed, UA. Psychometric analysis of the perceived stress scale among healthy university students. Neuropsychiatr Dis Treat. (2020) 16:2389–96. doi: 10.2147/NDT.s268582
29. Magyar-Moe, JL. Therapist’s guide to positive psychological interventions. Cambridge, MA: Academic Press (2009).
30. Crawford, JR, and Henry, JD. The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. Br J Clin Psychol. (2004) 43:245–65. doi: 10.1348/0144665031752934
31. Radaelli, A, Cazzaniga, M, Viola, A, Balestri, G, Janetti, MB, Signorini, MG, et al. Enhanced baroreceptor control of the cardiovascular system by polyunsaturated fatty acids in heart failure patients. J Am Coll Cardiol. (2006) 48:1600–6. doi: 10.1016/j.jacc.2006.05.073
32. Madsen, T, Schmidt, EB, and Christensen, JH. The effect of n-3 fatty acids on C-reactive protein levels in patients with chronic renal failure. J Ren Nutr. (2007) 17:258–63. doi: 10.1053/j.jrn.2007.03.003
33. Janszky, I, Ericson, M, Blom, M, Georgiades, A, Magnusson, JO, Alinagizadeh, H, et al. Wine drinking is associated with increased heart rate variability in women with coronary heart disease. Heart. (2005) 91:314–8. doi: 10.1136/hrt.2004.035105
34. de, SMCI, and Castor, MGM. Therapeutic use of Palmitoylethanolamide as an anti-inflammatory and Immunomodulator. Future Pharmacol. (2023) 3:951–77. doi: 10.3390/futurepharmacol3040058
35. Madsen, T, Christensen, JH, Toft, E, and Schmidt, EB. C-reactive protein is associated with heart rate variability. Ann Noninvasive Electrocardiol. (2007) 12:216–22. doi: 10.1111/j.1542-474x.2007.00164.x
36. Jarczok, MN, Koenig, J, Mauss, D, Fischer, JE, and Thayer, JF. Lower heart rate variability predicts increased level of C-reactive protein 4 years later in healthy, nonsmoking adults. J Intern Med. (2014) 276:667–71. doi: 10.1111/joim.12295
37. Kim, HG, Cheon, EJ, Bai, DS, Lee, YH, and Koo, BH. Stress and heart rate variability: a Meta-analysis and review of the literature. Psychiatry Investig. (2018) 15:235–45. doi: 10.30773/pi.2017.08.17
38. Hillebrand, S, Gast, KB, De Mutsert, R, Swenne, CA, Jukema, JW, Middeldorp, S, et al. Heart rate variability and first cardiovascular event in populations without known cardiovascular disease: meta-analysis and dose-response meta-regression. Europace. (2013) 15:742–9. doi: 10.1093/europace/eus341
39. Kang, MG, Koh, SB, Cha, BS, Park, JK, Woo, JM, and Chang, SJ. Association between job stress on heart rate variability and metabolic syndrome in shipyard male workers. Yonsei Med J. (2004) 45:838–46. doi: 10.3349/ymj.2004.45.5.838
40. Chandola, T, Britton, A, Brunner, E, Hemingway, H, Malik, M, Kumari, M, et al. Work stress and coronary heart disease: what are the mechanisms? Eur Heart J. (2008) 29:640–8. doi: 10.1093/eurheartj/ehm584
41. Hammoud, S, Karam, R, Mourad, R, Saad, I, and Kurdi, M. Stress and Heart Rate Variability during University Final Examination among Lebanese Students. Behav Sci. (2019) 9:3. doi: 10.3390/bs9010003
42. Martinez, GJ, Grover, T, Mattingly, SM, Mark, G, D’Mello, S, Aledavood, T, et al. Alignment between heart rate variability from fitness trackers and perceived stress: perspectives from a large-scale in situ longitudinal study of information workers. JMIR Hum Factors. (2022) 9:e33754. doi: 10.2196/33754
43. Ghazizadeh-Hashemi, M, Ghajar, A, Shalbafan, MR, Ghazizadeh-Hashemi, F, Afarideh, M, Malekpour, F, et al. Palmitoylethanolamide as adjunctive therapy in major depressive disorder: a double-blind, randomized and placebo-controlled trial. J Affect Disord. (2018) 232:127–33. doi: 10.1016/j.jad.2018.02.057
44. Lama, A, Pirozzi, C, Annunziata, C, Morgese, MG, Senzacqua, M, Severi, I, et al. Palmitoylethanolamide counteracts brain fog improving depressive-like behaviour in obese mice: possible role of synaptic plasticity and neurogenesis. Br J Pharmacol. (2021) 178:845–59. doi: 10.1111/bph.15071
45. Merolla, A, De Lorenzo, R, Paolazzi, G, Critelli, S, Palladini, M, Damanti, S, et al. Micronized/ultramicronized palmitoylethanolamide improves depression and fatigue in coronavirus disease 2019 (COVID-19) survivors. Int Clin Psychopharmacol. (2024) 39:361–8. doi: 10.1097/yic.0000000000000537
46. Bortoletto, R, Piscitelli, F, Candolo, A, Bhattacharyya, S, Balestrieri, M, and Colizzi, M. Questioning the role of palmitoylethanolamide in psychosis: a systematic review of clinical and preclinical evidence. Front Psych. (2023) 14:1231710. doi: 10.3389/fpsyt.2023.1231710
47. Madison, AA, Belury, MA, Andridge, R, Renna, ME, Rosie Shrout, M, Malarkey, WB, et al. Omega-3 supplementation and stress reactivity of cellular aging biomarkers: an ancillary substudy of a randomized, controlled trial in midlife adults. Mol Psychiatry. (2021) 26:3034–42. doi: 10.1038/s41380-021-01077-2
48. Noreen, EE, Sass, MJ, Crowe, ML, Pabon, VA, Brandauer, J, and Averill, LK. Effects of supplemental fish oil on resting metabolic rate, body composition, and salivary cortisol in healthy adults. J Int Soc Sports Nutr. (2010) 7:2783. doi: 10.1186/1550-2783-7-31
49. Tsang, C, Hodgson, L, Bussu, A, Farhat, G, and Al-Dujaili, E. Effect of polyphenol-rich dark chocolate on salivary cortisol and mood in adults. Antioxidants. (2019) 8:149. doi: 10.3390/antiox8060149
50. Lopresti, AL, Smith, SJ, and Drummond, PD. Modulation of the hypothalamic-pituitary-adrenal (HPA) axis by plants and phytonutrients: a systematic review of human trials. Nutr Neurosci. (2022) 25:1704–30. doi: 10.1080/1028415X.2021.1892253
51. Lama, A, Pirozzi, C, Severi, I, Morgese, MG, Senzacqua, M, Annunziata, C, et al. Palmitoylethanolamide dampens neuroinflammation and anxiety-like behavior in obese mice. Brain Behav Immun. (2022) 102:110–23. doi: 10.1016/j.bbi.2022.02.008
52. Norton, SA, Baranger, DA, Young, ES, Voss, M, Hansen, I, Bondy, E, et al. Reliability of diurnal salivary cortisol metrics: a meta-analysis and investigation in two independent samples. Compr Psychoneuroendocrinol. (2023) 16:100191. doi: 10.1016/j.cpnec.2023.100191
53. Wilker, S, Pfeiffer, A, Elbert, T, Ovuga, E, Karabatsiakis, A, Krumbholz, A, et al. Endocannabinoid concentrations in hair are associated with PTSD symptom severity. Psychoneuroendocrinology. (2016) 67:198–206. doi: 10.1016/j.psyneuen.2016.02.010
54. Mourtakos, S, Vassiliou, G, Kontoangelos, K, Philippou, A, Tzavellas, E, Tornero-Aguilera, JF, et al. Endocannabinoids and heart rate variability alterations after exposure to prolonged intensive physical exercise of the Hellenic navy SEALs. Int J Environ Res Public Health. (2021) 19:28. doi: 10.3390/ijerph19010028
55. Van Steenwijk, HP, Osch, FHM, Troost, FJ, Bast, A, de Boer, A, and Semen, KO. Heart rate variability correlates with the effect of sulforaphane on calorie-induced inflammation in healthy participants: a randomized placebo-controlled study. Clin Nutr Open Sci. (2023) 49:140–56. doi: 10.1016/j.nutos.2023.05.002
56. Hansen, AL, Dahl, L, Bakke, L, Frøyland, L, and Thayer, JF. Fish consumption and heart rate variability. J Psychophysiol. (2010) 24:41–7. doi: 10.1027/0269-8803/A000005
57. Teixeira, AL, Ramos, PS, Vianna, LC, and Ricardo, DR. Heart rate variability across the menstrual cycle in young women taking oral contraceptives. Psychophysiology. (2015) 52:1451–5. doi: 10.1111/psyp.12510
Keywords: palmitoylethanolamide, stress, heart rate variability, university students, autonomic regulation, salivary cortisol, mood
Citation: Deb SK, Kim N, Parolin B, Renshaw D and Zariwala MG (2025) The effects of formulated palmitoylethanolamide supplementation on indicators of stress and heart rate variability in female university students: a randomised cross-over trial. Front. Nutr. 12:1586409. doi: 10.3389/fnut.2025.1586409
Edited by:
Gianpiero Greco, University of Bari Aldo Moro, ItalyReviewed by:
Hector A. Cabrera-Fuentes, Imam Abdulrahman bin Faisal University, Saudi ArabiaBoris C. Rodríguez-Martín, International University of La Rioja, Spain
Copyright © 2025 Deb, Kim, Parolin, Renshaw and Zariwala. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammed Gulrez Zariwala, TS5aYXJpd2FsYUB3ZXN0bWluc3Rlci5hYy51aw==
†These authors have contributed equally to this work
 Sanjoy K. Deb
Sanjoy K. Deb Nadia Kim
Nadia Kim Brenda Parolin
Brenda Parolin Derek Renshaw3
Derek Renshaw3 Mohammed Gulrez Zariwala
Mohammed Gulrez Zariwala