- 1Department of Radiation Oncology, The First Affiliated Hospital of Bengbu Medical University, Bengbu, Anhui, China
- 2Joint Research Center for Regional Diseases of IHM, Bengbu Medical University, Bengbu, Anhui, China
- 3Joint Research Center for Regional Diseases of IHM, The First Affiliated Hospital of Bengbu Medical University, Bengbu, Anhui, China
- 4Anhui Provincial Key Laboratory of Tumor Evolution and Intelligent Diagnosis and Treatment, Bengbu Medical University, Bengbu, Anhui, China
- 5Department of Radiation Oncology (Key Laboratory of Cancer Prevention and Intervention, China National Ministry of Education, Key Laboratory of Molecular Biology in Medical Sciences, Zhejiang, China), The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China
- 6Anhui Hospital, The Second Affiliated Hospital of Zhejiang University School of Medicine, Bengbu, Anhui, China
Background: Metabolic and nutritional status are recognized as prognostic and predictive biomarkers in cancer treatment. However, there is limited research on oligometastatic non-small cell lung cancer (NSCLC) patients undergoing radiotherapy. We aimed to explore the independent and synergistic effects of body composition and immune-nutritional status on survival outcomes in these patients.
Methods: Patients with oligometastatic NSCLC who underwent radiotherapy between 2017 and 2022 were retrospectively included. The evaluated outcomes were overall survival (OS) and progression-free survival (PFS). The skeletal muscle index (SMI), subcutaneous fat index (SFI), and pericardial fat index (PFI) were obtained using computed tomography (CT) and normalized by height squared. Laboratory biomarkers were utilized to assess immune-nutritional status. Univariate chi-square tests and multivariate logistic regression analyses were employed to determine correlations between hematological parameters and body composition. We conducted K-M analyses, along with univariate and multivariate Cox regression analyses to evaluate survival outcomes.
Results: 102 patients [mean age 61.44 years, 51 males (50%)] were included. Compared to non-responders, responders exhibited a significantly lower prevalence of sarcopenia (44.93 vs. 55.07%, P = 0.007) and demonstrated relatively better immune-nutritional scores. Logistic regression analyses revealed that a low Prognostic Nutritional Index (PNI) was a risk factor for SMI, SFI, and PFI. Multivariate Cox analyses revealed that C reactive protein-to-albumin ratio (CAR) (HR = 0.26; 95% CI 0.11–0.61; P = 0.002) and PFI (HR = 2.73; 95% CI 1.06–7.01; P = 0.037) were predictive factors for OS. CAR (HR = 0.34, P = 0.001) and SFI (HR = 1.82, P = 0.049) were independent prognostic markers for PFS. K-M analyses indicated that the group with high PNI and high SFI exhibited markedly improved OS and PFS, as well as the one with high PNI and without sarcopenia (P < 0.001).
Conclusion: Among oligometastatic NSCLC patients receiving radiotherapy, higher skeletal muscle and fat content, along with better immune-nutritional status, correlated with improved survival outcomes.
1 Introduction
Lung cancer remains a leading cause for cancer-related mortality globally, with non-small cell lung cancer (NSCLC) accounting for about 85% of diagnoses (1). Unfortunately, most NSCLC patients are diagnosed at advanced stages, frequently accompanied by metastases, resulting in poor prognoses (2). Oligometastatic NSCLC represents a specific subtype, characterized by limited and organ-site-specific metastasis, suggesting that enhancing standard treatment with targeted local therapies may improve survival outcomes (3). A randomized controlled trial has demonstrated that radiotherapy significantly improves overall survival (OS) compared to standard palliative care alone (4). However, predictive markers for therapeutic efficacy remain largely unexplored.
Recent studies have highlighted a significant correlation between body composition and cancer prognosis, particularly regarding skeletal muscle and adipose tissue (5–7). Some longitudinal studies indicate that in cancer patients, obesity serves as an independent risk factor for unfavorable prognosis, and elevated visceral fat is associated with disease progression and decreased postoperative survival (8–10). Additionally, sarcopenia is thought to be associated with poorer outcomes, as well as an increased risk of recurrence and metastasis (11). These impacts are believed to be related to immune and inflammatory responses (12). Infiltration of immune cells, including M1-polarized macrophages and T cells, causes metabolic inflammation in excess visceral adipose tissue, prompting adipose tissue to secrete pro-inflammatory cytokines. Such an inflammatory environment may impair the immune response in obese patients, exacerbating their prognosis (13). For example, as a marker of visceral fat, pericardial fat is linked to the prognosis of NSCLC (14). Moreover, macrophage infiltration within adipose tissue can initiate systemic inflammation, a hallmark of cancer. Systemic inflammatory response might promote catabolic pathways, contributing to muscle wasting in cancer patients (15). Numerous studies have demonstrated associations between systemic inflammatory markers and sarcopenia, emphasizing the profound impact of sarcopenia on survival outcomes, particularly when associated with an elevated systemic inflammatory response (SIR) (16–18). Additionally, a low skeletal muscle index (SMI), indicative of sarcopenia, is correlated with frailty and immobility in NSCLC patients (7).
Nonetheless, the influence of adipose tissue on survival remains contentious. Some studies report that visceral fat levels do not affect prognosis and may even show a positive impact on long-term survival (19). These discrepancies may arise from the heterogeneity of study populations, variations in data analysis methodologies, differing thresholds, and changes in clinical settings. Furthermore, few studies have investigated the impact of body composition and systemic inflammation in oligometastatic NSCLC patients receiving radiotherapy, and the generalizability of the existing conclusions across diverse populations remains underexplored. Currently, computed tomography (CT) provides a definitive and non-invasive method of quantifying skeletal muscle and adipose tissue (20). Therefore, we aimed to comprehensively analyze the impact of body composition and immune-nutritional status on survival of oligometastatic NSCLC patients undergoing radiotherapy, identify patients at risk, and promote more targeted and patient-centered treatment strategies.
2 Materials and methods
2.1 Patients
We retrospectively included patients at the First Affiliated Hospital of Bengbu Medical College who were diagnosed with oligometastatic NSCLC and underwent radiotherapy between December 2017 and December 2022. The inclusion criteria were the following: (a) a pathological diagnosis of oligometastatic NSCLC; (b) administration of radiotherapy; (c) comprehensive medical history and follow-up data; (d) accessible CT scans taken before treatment. After excluding patients who did not complete radiation therapy or suffered from chronic conditions such as hematological or autoimmune diseases, as well as those with other concurrent malignancies, 102 patients were included, and all had available baseline imaging (Figure 1). This retrospective study adhered to the Declaration of Helsinki and was approved by the Ethics Committee of the First Affiliated Hospital of Bengbu Medical University.
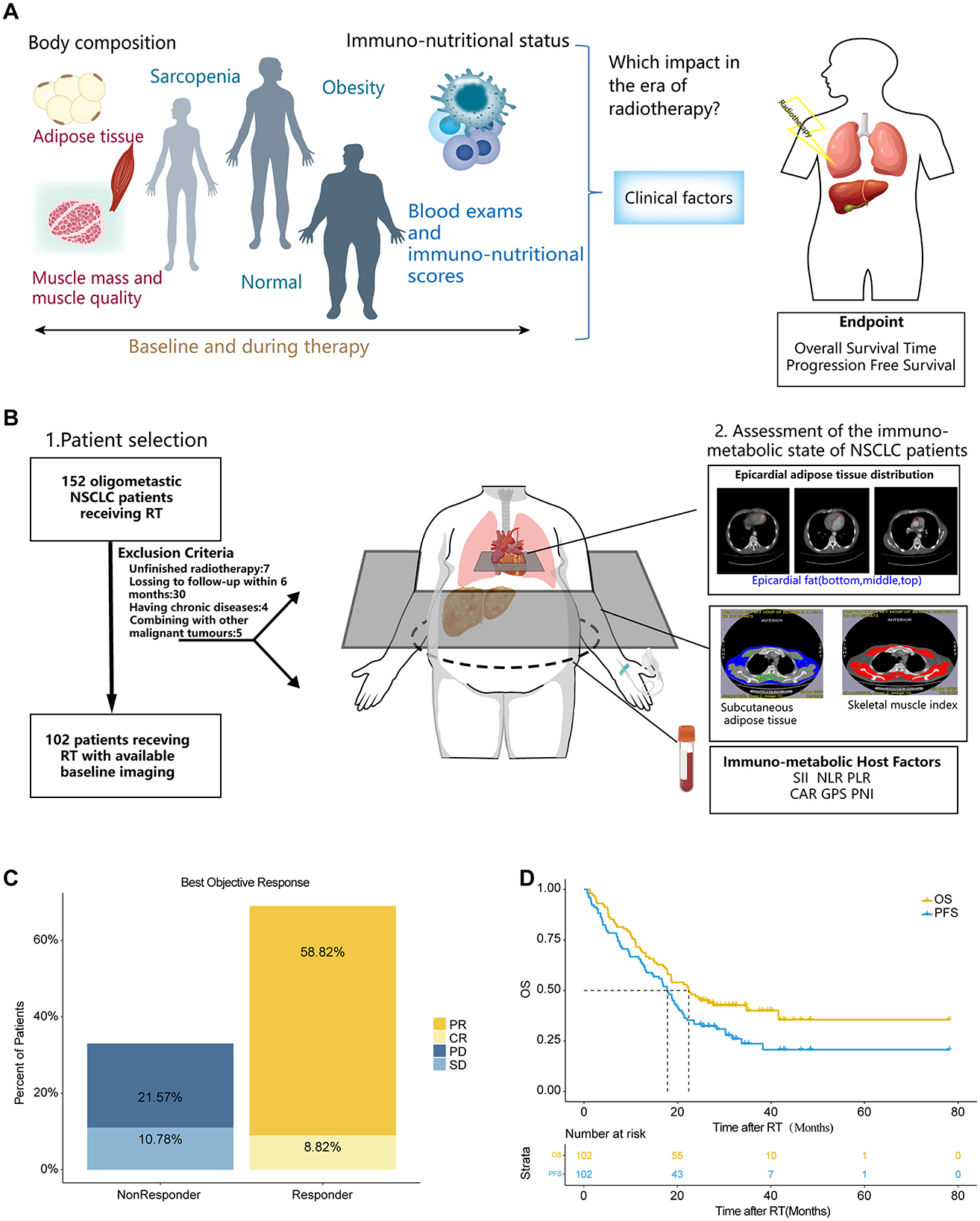
Figure 1. The study cohort demonstrates representative real-world clinical outcomes. (A) Graphical Abstract. (B) Schematic representation outlining from left to right: the study cohort with key exclusion criteria, methods for body composition measurements and assessment of the immuno-nutritional status, and the study endpoints. (C) Best objective response rate (ORR) at day 90, determined according to RECIST 1.1 criteria. (D) Kaplan-Meier estimates of PFS and OS for the entire study cohort. Median survival times are depicted in months. CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; OS, overall survival; PFS, progression free survival; SII, systemic immune-inflammation index; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; CAR, C reactive protein-to-albumin ratio; PNI, the Prognostic Nutritional Index; GPS, the Glasgow Prognostic Score; SFI, subcutaneous fat index; PFI, Pericardial Fat Index; SMI, Skeletal muscle index.
2.2 Data collection
We collected general information from the patients, including age, sex, BMI, the presence of chronic diseases, smoking history, tumor histological type, the number of metastatic organs, oligometastatic state, the Eastern Cooperative Oncology Group (ECOG) performance status and laboratory test indicators such as low-density lipoprotein (LDL), high-density lipoprotein (HDL), cholesterol, triglycerides, albumin, and C-reactive protein (CRP). Immuno-nutritional scores were derived from specific laboratory biomarkers. CRP and albumin levels were used to determine the Glasgow prognostic score (GPS) (21): a CRP ≤ 10 mg/dL scored 0; a CRP > 10 mg/dL with albumin > 3.5 g/dL scored 1; a CRP > 10 mg/dL with albumin < 3.5 g/dL scored 2. The C reactive protein-to-albumin ratio (CAR) was calculated by dividing CRP (mg/L) by albumin (g/dL) (22). The systemic immune-inflammation index (SII) was calculated as the product of neutrophil count and platelet count divided by lymphocyte count (109/L) (23). The neutrophil-to-lymphocyte ratio (NLR) was determined by dividing the absolute neutrophil count by the total lymphocyte count (24). The prognostic nutritional index (PNI) was determined using the following equation: albumin (g/dL) plus 0.005 times the total lymphocyte count (25). The platelet-to-lymphocyte ratio (PLR) was calculated by dividing the platelet count (× 109/L) by the lymphocyte count (× 109/L) (23). Further details can be found in Supplementary Table 1. All these blood biomarkers reflecting metabolic or nutritional status were obtained within 14 days prior to the initiation of radiotherapy.
2.3 CT image analysis
Although skeletal muscle is typically measured at the third lumbar (L3) level using CT scans, chest CT is more commonly utilized in clinical practice, especially in regions like China, where CT lung cancer screening is routinely performed. Therefore, quantifying pectoralis central muscle using chest CT can provide a more effective method for routine body composition analysis, avoiding additional radiation exposure and complicated measurements (26).
We evaluated body composition characteristics based on the best response criteria derived from pre-lymphodepletion imaging studies. The outer margins of the bilateral pectoralis major and minor muscles on non-contrast chest CT images were manually delineated at the level of the fourth thoracic vertebra (Th4) by a radiologist with experience in chest imaging, who was unaware of patient information. The pre-processed scans were analyzed using sliceOmatic (Tomovision V.5.0) following established methods, and we obtained skeletal muscle area (cm2). From the same image, we measured the subcutaneous fat area (cm2) extending from the pectoral muscles to the pectoralis major. The Hounsfield unit threshold for skeletal muscle and subcutaneous fat were −29 to 150 and −190 to −30, respectively. Pericardial fat was identified as the area extending vertically from the right pulmonary artery to the diaphragm and horizontally from the left edge of the heart apex to the right edge of the atrium, using CT density within the range of −190 to −30 HU. Once pericardial fat was identified, its volume (cm3) was automatically calculated. Sarcopenia is assessed by normalizing the cross-sectional area of skeletal muscle (cm2) by height squared (m2), resulting in SMI (cm2/m2) (7). The subcutaneous fat index (SFI) (cm2/m2) was calculated by normalizing the subcutaneous fat area to height squared (m2) (27). The pericardial fat index (PFI) (cm3/m2) was derived by dividing the pericardial fat volume by body surface area using the Mosteller formula (14).
2.4 Endpoints
We define the time from the commencement of therapy until death as OS and the duration from the commencement of therapy to disease progression or death as Progression-Free Survival (PFS). Efficacy assessment was performed 90 days following the completion of radiotherapy, at which point optimal therapeutic outcomes are expected. We assessed the tumor response according to the RECIST 1.1 criteria, classifying responses as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). Patients who achieved PR or CR were categorized as responders, whereas those with SD or PD were non-responders.
2.5 Statistical analysis
We assessed normal distribution using the d'Agostino-Pearson test. Continuous variables were represented by their median and interquartile range (IQR), unless stated otherwise. Categorical variables were expressed as numbers with percentages. Comparisons of continuous variables between groups were conducted using the Mann-Whitney U-test or Student's t-test, while categorical variables were analyzed using Fisher's exact test or chi-square test. We used the Spearman test to evaluate correlations between parameters. Chi-square tests and logistic regression were used to clarify the associations between hematological parameters and body composition. Following univariate logistic regression analyses, variables yielding a p-value < 0.1 were selected for further multivariate logistic regression. Both univariate and multivariate Cox proportional hazard models were used to calculate hazard ratios (HRs) for mortality and to identify predictive factors for death outcomes. Cut-off values were established through a survival-based software package fitting a Cox proportional hazards model. Nonetheless, given the small sample sizes and the absence of statistical significance in most Cox proportional hazards models, we chose to use values previously reported in the literature or established normal laboratory values (PNI, NLR, PLR). K-M curves facilitated the comparison of survival distributions between groups using time-dependent tests. Two-sided P-values < 0.05 are considered statistically significant. R 4.1.3 was used to analyze all data.
3 Results
3.1 Patient characteristics
There are 102 patients with oligometastatic NSCLC, comprising half male participants. The average age of participants was 61.44 years. Histological examination revealed that adenocarcinoma was the predominant subtype (n = 67, 65.69%). 66.67% (n = 68) of the patients presented with single-organ metastases. 63.73% (n = 65) of the patients had hypertension, and 78.43% (n = 80) were diagnosed with diabetes mellitus. A total of 61 patients (59.8%) underwent radiotherapy targeting the brain, while 19 (18.63%) to bone, and 8 (7.84%) to lung. Other locations accounted for 13.73%. The ECOG performance status was significantly lower in responders (P < 0.001), and baseline CRP levels were markedly higher (5.83 vs. 2.8 mg/dL, P = 0.002) in non-responders. Detailed information is shown in Table 1. The overall response rate at 90 days post-radiotherapy was 67.64%, with a complete response rate of 8.82% (Figure 1C). After a median follow-up of 22.4 months, the median PFS (mPFS) and median OS (mOS) were 17.8 months and 22.37 months, respectively (Figure 1D).
3.2 Responders exhibit sarcopenia and altered immuno-nutritional scores
Using CT imaging and blood biomarkers, we calculated and compared the body composition and immuno-nutritional scores of the participants (Table 2). A significant difference in BMI was observed between responders and non-responders (P < 0.05). Specifically, the prevalence of overweight individuals was higher among responders, while a greater proportion of underweight individuals was found in the non-responder group. Indicators assessing adipose and muscle tissue distribution, including SFI, PFI, did not reveal statistically significant differences. However, the prevalence of sarcopenia was markedly elevated among non-responders (75.76 vs. 44.93%, P = 0.006). Additionally, we examined established immuno-nutritional scores, such as GPS, CAR, and PNI. Responders exhibited lower GPS scores (GPS = 0: 68.12 vs. 45.45%, P = 0.048), reduced CAR (CAR > 1.44: 14.49 vs. 51.52%, P < 0.001), and higher PNI (PNI > 45: 84.06 vs. 48.48%, P < 0.001). Between the two groups, no significant differences were observed in the inflammatory markers, including NLR, PLR, and SII. These results suggested that sarcopenia, nutritional status, and inflammation may serve as prognostic indicators for early responses to radiotherapy.
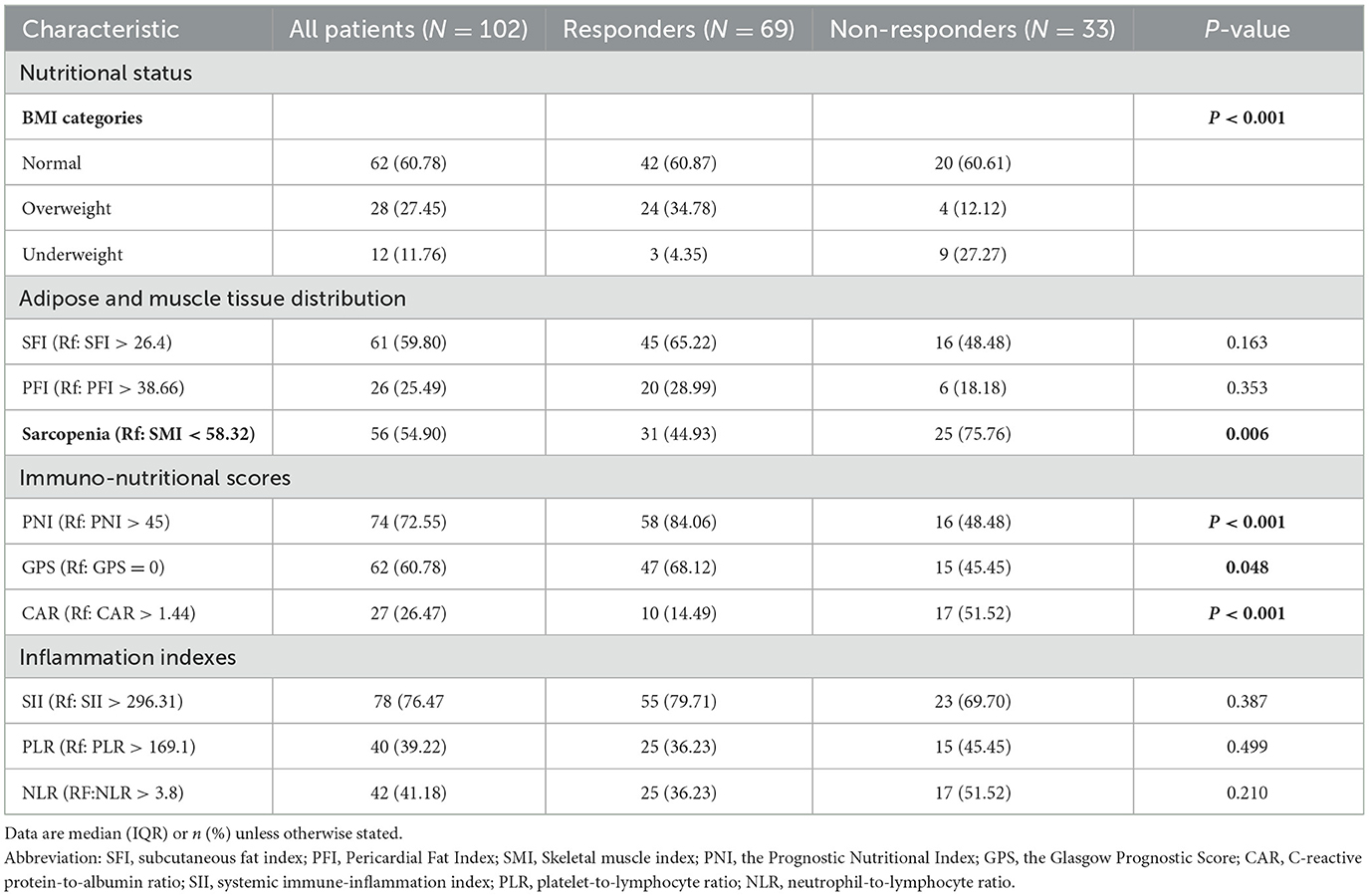
Table 2. Distribution of body composition and immuno-nutritional status between responders and non-responders.
3.3 Body composition and prognosis nutritional index are predictors for survival in oligometastatic NSCLC receiving radiotherapy
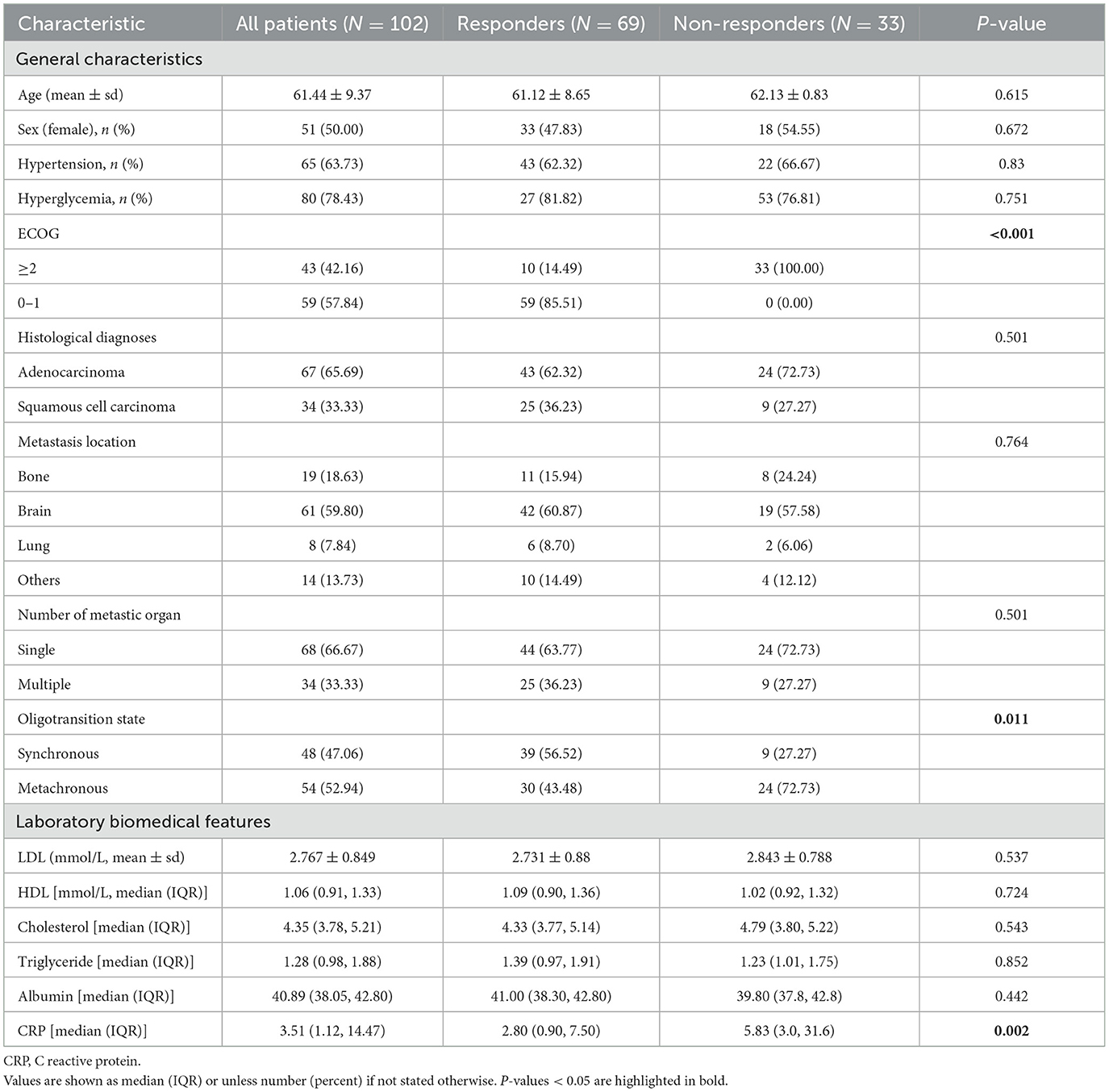
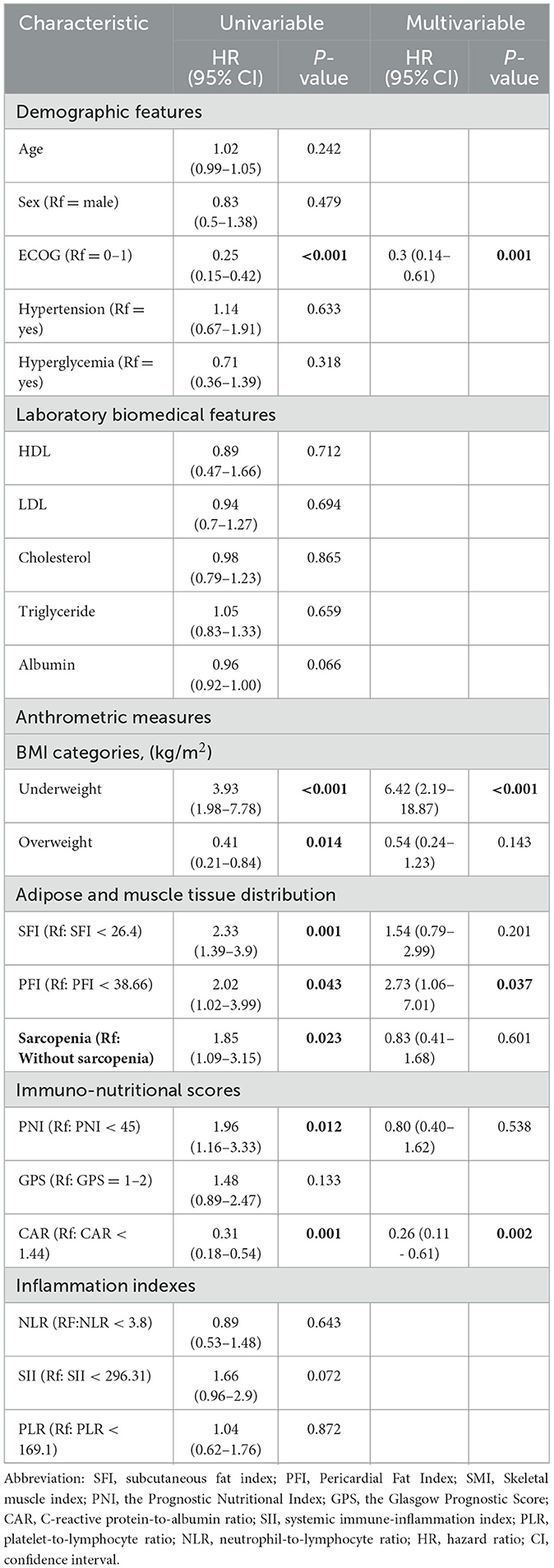
After conducting cutoff analyses, we delineated high and low-risk groups according to body composition markers (PFI, SFI, SMI) and immuno-nutritional scores (CAR, PNI, SII, GPS, NLR, PLR). In the univariate Cox regression analysis, ECOG performance status, BMI, PFI, SFI, sarcopenia, PNI, and CAR were significantly linked to OS (P < 0.05). ECOG performance status, underweight status, CAR, and PFI emerged as independent prognostic markers (P < 0.05), following inclusion in multivariate Cox regression analysis (Table 3). For PFS, ECOG performance status, albumin levels, BMI, SFI, sarcopenia, PNI, and CAR demonstrated significant correlations (P < 0.05). In the multivariate Cox regression analysis, ECOG performance status, underweight status, SFI, and CAR were identified as significant predictors of PFS (P < 0.05) (Table 4). K-M analyses revealed significant differences in OS between high and low-risk groups stratified by SFI, and PFI (P < 0.05). Notably, significant differences in PFS were observed only between high and low-risk groups based on SFI (P < 0.05). SFI, and PFI were associated with comparatively better prognoses (Figure 2). Among patients without sarcopenia, both OS and PFS were markedly improved (P < 0.05) (Figure 2). Furthermore, significant differences in OS and PFS were noted between groups stratified by PNI and CAR (P < 0.05), with higher PNI and lower CAR indicating relatively better prognostic outcomes (Figure 3).
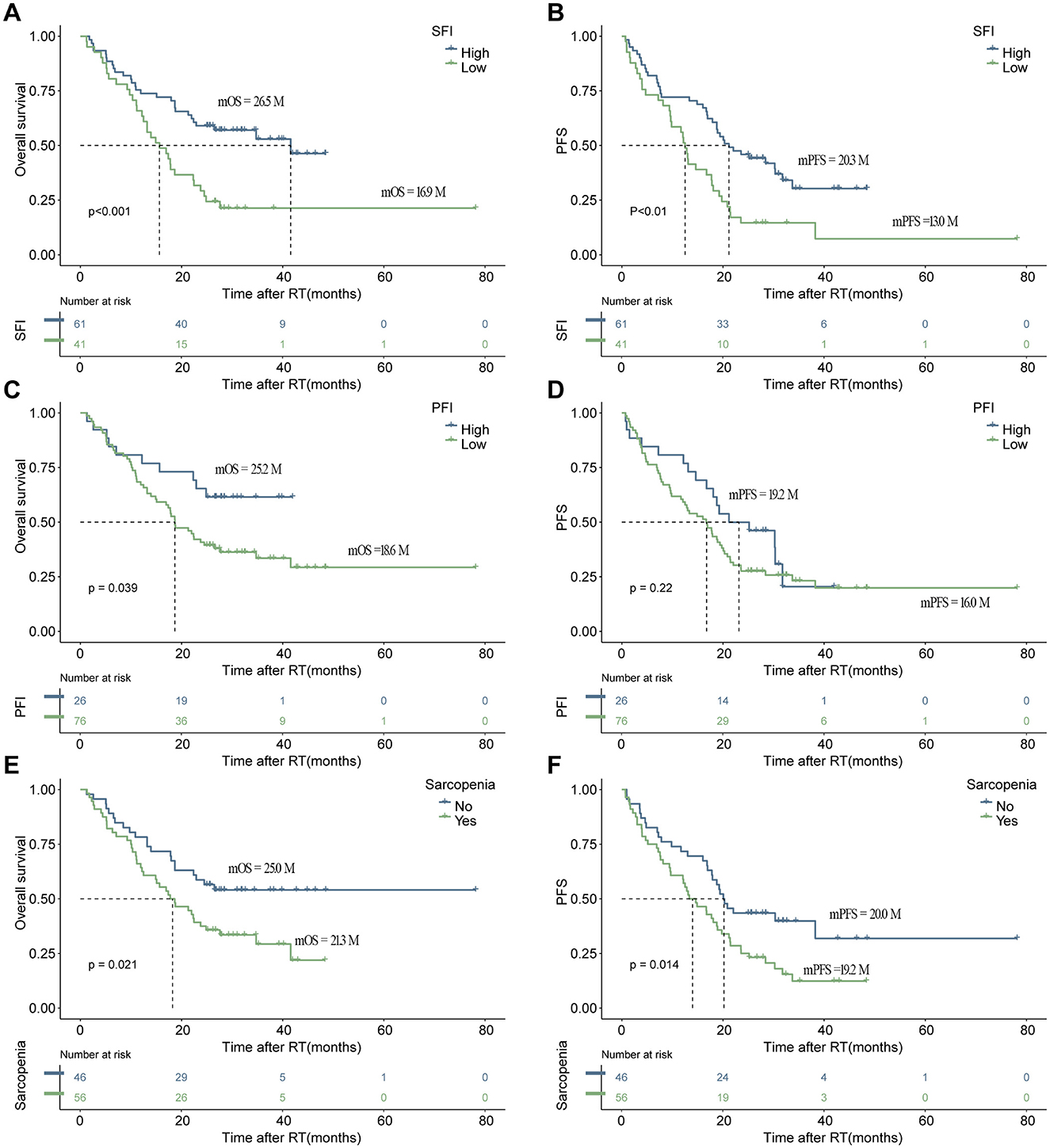
Figure 2. The depicts the association of increased body composition with superior progression-free survival (PFS) and overall survival (OS). Kaplan–Meier estimates show median OS (left) and PFS (right) stratified by SFI (A, B), and PFI (C, D), as well as sarcopenia (E, F). The respective cutoff for each parameter and median survival in months are indicated above each graph. The P-value from the Mantel–Cox log-rank test is provided in the graph inset. SFI, subcutaneous fat index; PFI, Pericardial Fat Index.
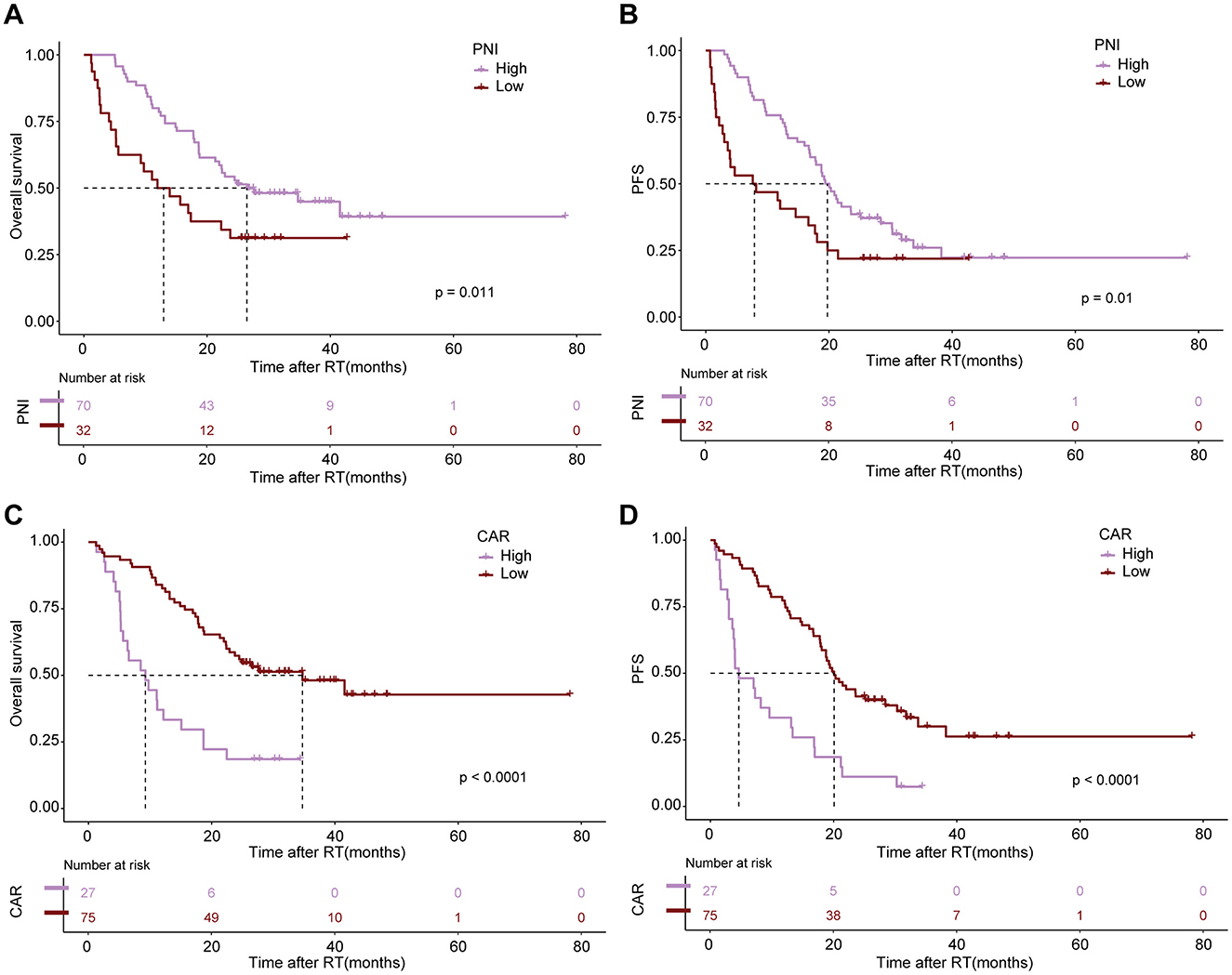
Figure 3. The impact of altered immuno-nutritional scores as negative prognostic markers for survival outcomes. Kaplan–Meier estimates display median OS (left) and PFS (right) stratified by Prognostic Nutritional Index [PNI; (A, B)] and CRP-to-Albumin ratio [CAR; (C, D)]. The respective cutoff for each parameter and median survival in months are depicted above each graph. The P-value from the Mantel–Cox log-rank test is indicated in the graph inset.
3.4 Systemic inflammation parameters with body composition
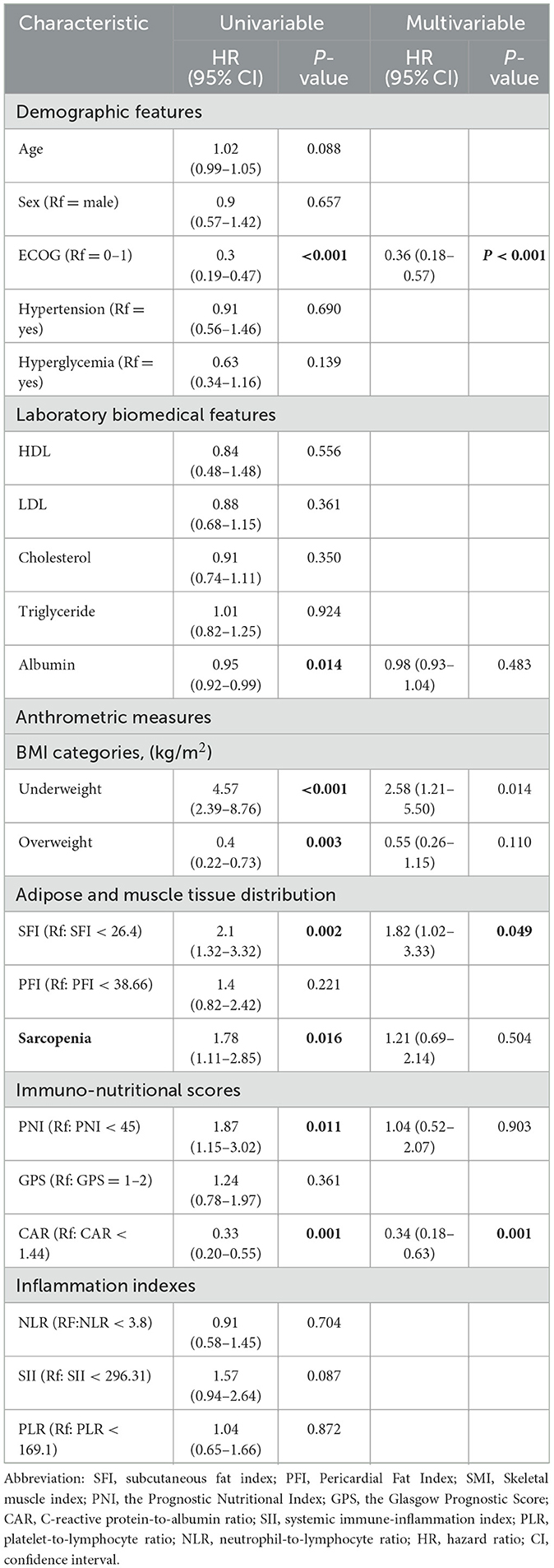
Through logistic regression analyses, we investigated the relationship between body composition markers (PFI, SFI, SMI) and systemic immune-nutritional scores (CAR, PNI, SII, GPS, NLR, PLR), while adjusting for age, sex, PNI, SII, PLR, NLR, CAR, GPS, tumor histology, ECOG performance status, smoking status, radiation treatment sites, BMI, hypertension, hyperglycemia, albumin levels, triglycerides, cholesterol, HDL, and LDL. Detailed results are presented in Table 5, Supplementary Tables S2, S3. The results indicated that among all systemic immune-nutritional scores, only PNI was associated with body composition biomarkers, emerging as an independent risk factor for all three markers, including SFI [odds ratio (OR) = 2.77, 95% CI 1.01–7.99, P = 0.042] (Table 5), SMI (OR = 1.98, 95% CI 1.66–6.17, P = 0.026) (Supplementary Table S2), and PFI (OR = 1.41, 95% CI 1.28–4.37, P = 0.030) (Supplementary Table S3).
3.5 Body composition and prognosis nutritional index are predictors for PFS and OS in oligometastatic NSCLC receiving radiotherapy
To further elucidate the impact of these parameters on survival outcomes, we conducted K-M analyses by combining PNI (high vs. low) with SFI (high vs. low) and sarcopenia (sarcopenia vs. non-sarcopenia). The results indicated significant prognostic differences among the various groups (Figure 4). The group with high PNI and high SFI exhibited markedly improved OS and PFS, as well as the one with high PNI and without sarcopenia (P < 0.001). Therefore, the combination of elevated subcutaneous fat or muscle mass with high PNI emerges as a favorable prognostic indicator for patients undergoing radiotherapy.
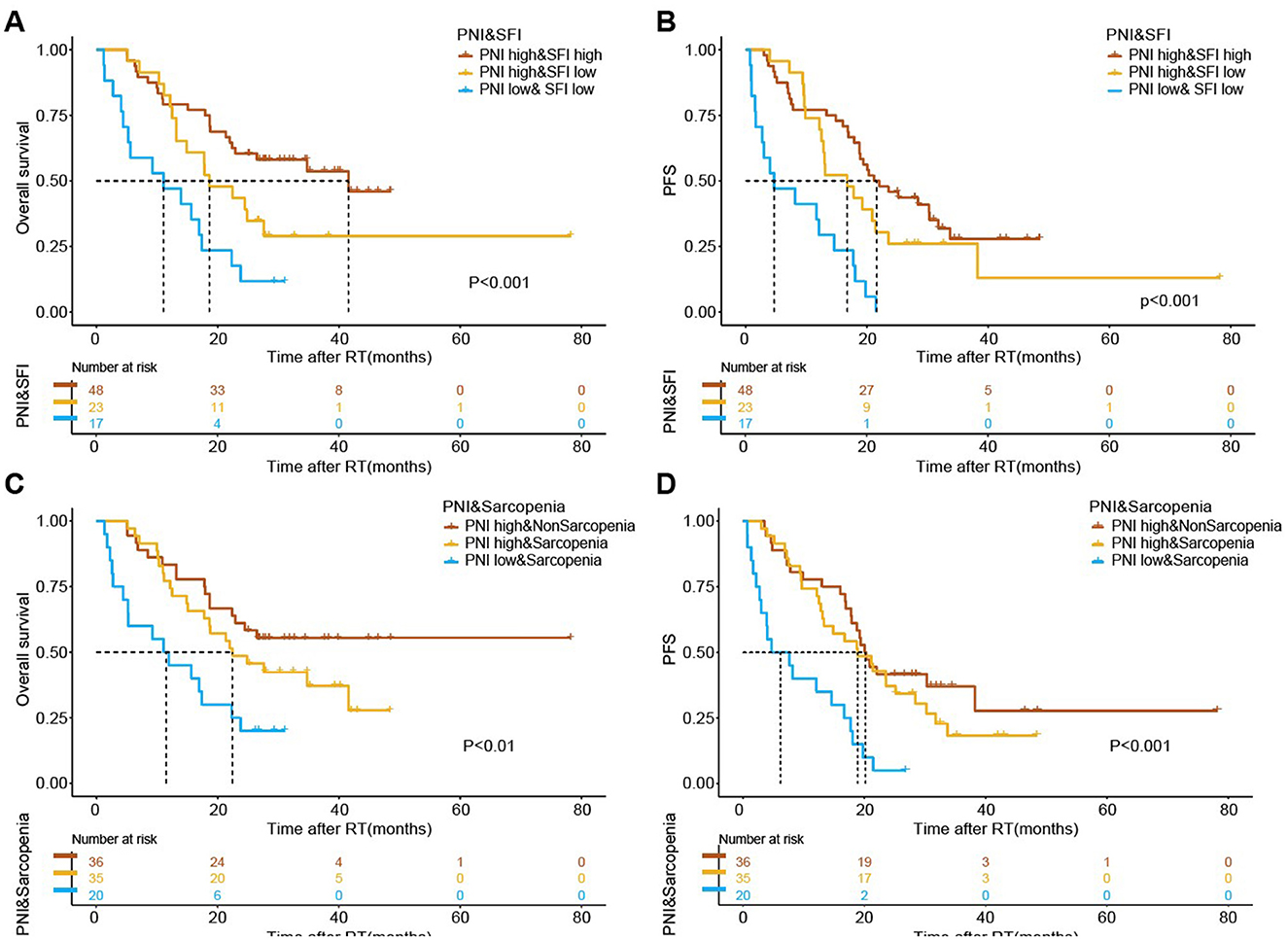
Figure 4. The association of combined body composition and immuno-nutritional scores with survival outcomes following radiotherapy. Kaplan-Meier estimates for median overall survival (OS) and progression-free survival (PFS) stratified by the combination of Prognostic Nutritional Index (PNI) and Subcutaneous Fat Index (SFI) (A, B), and PNI and sarcopenia (C, D). The respective cutoff for each parameter and median survival in months are depicted above each graph. The P-value from the Mantel–Cox log-rank test is indicated in the graph inset.
4 Discussion
This study represented the first investigation into the independent and combined impact of body composition and immune-nutritional status on the prognosis of oligometastatic NSCLC patients undergoing radiotherapy. Our findings underscored the significant associations among sarcopenia, adiposity indices, and various inflammatory markers. BMI, ECOG performance status, PFI, and CAR emerged as critical prognostic factors for OS, and BMI, ECOG performance status, SFI, and CAR were identified as key predictors for PFS. These results highlighted the pivotal role of body composition in influencing treatment response and underscored the necessity for future translational research to elucidate potential immune-metabolic mechanisms.
Inflammation has been recognized as a crucial prognostic determinant in cancers receiving radiotherapy, such as melanoma and renal cell carcinoma (28, 29). The inflammatory status discussed here needs to be distinguished from the concept of tumor inflammatory microenvironment. Our results indicated that the PNI, which reflects nutritional status and immune balance, serves as a robust predictive indicator of survival in these patients, with higher PNI values correlating with improved outcomes. This study provided oncologists with compelling evidence that comprehensive nutritional assessment is vital for optimizing the care of patients with oligometastatic NSCLC.
Our analyses also revealed the significant prognostic value of evaluating muscle-related body composition. Elevated muscle mass indicators, such as SMI, correlated positively with favorable survival outcomes. In the context of cancer, systemic inflammatory responses markedly contribute to muscle depletion through mechanisms involving increased oxidative stress and mitochondrial dysfunction, which adversely affect skeletal muscle cells within the tumor microenvironment (30). Consequently, the decline in muscle mass disrupts the secretion of antitumor factors (myokines), ultimately leading to poorer prognoses. These physiological alterations are expected in malnourished cancer patients, with SMI acting as a crucial prognostic marker (31).
Adipose tissue, encompassing subcutaneous and visceral fat, also plays a vital role in patients with NSCLC (27). Our results indicated that more subcutaneous adipose tissue is beneficial for PFS in these individuals. Similarly, an increase in pericardial fat content correlates with improved prognosis, showing significant associations with visceral fat. We quantified skeletal muscle and fat, establishing critical thresholds for sarcopenia, SFI and PFI reductions. Obesity and elevated PFI were found to be independently associated with OS, after adjusting for body composition variables. In contrast, sarcopenia did not independently impact OS, potentially due to the heightened energy requirements of cancer patients, with fat serving as a key energy source (32). In cancer patients, fat depletion often precedes muscle wasting, a pattern also observed in gastrointestinal cancers (33). This phenomenon is characterized by enhanced lipolysis and reduced adipocyte volume, leading to depleted energy reserves and disrupted homeostasis, which correlates with diminished survival times (34). Therefore, we attribute the obesity paradox in patients with oligometastatic NSCLC more to adipose tissue than to skeletal muscle.
However, relying solely on these parameters may not comprehensively determine treatment responses or prognosis. Other disease-specific and host intrinsic factors must also be considered. While developing a universal tool to guide treatment for all patients remains challenging, thorough assessment, combined with early palliative interventions, may constitute the most effective strategy prior to initiating treatment. Consequently, exploring a multimodal approach that integrates nutritional, exercise, and anti-inflammatory interventions with cancer therapies may enhance therapeutic efficacy (Figure 5).
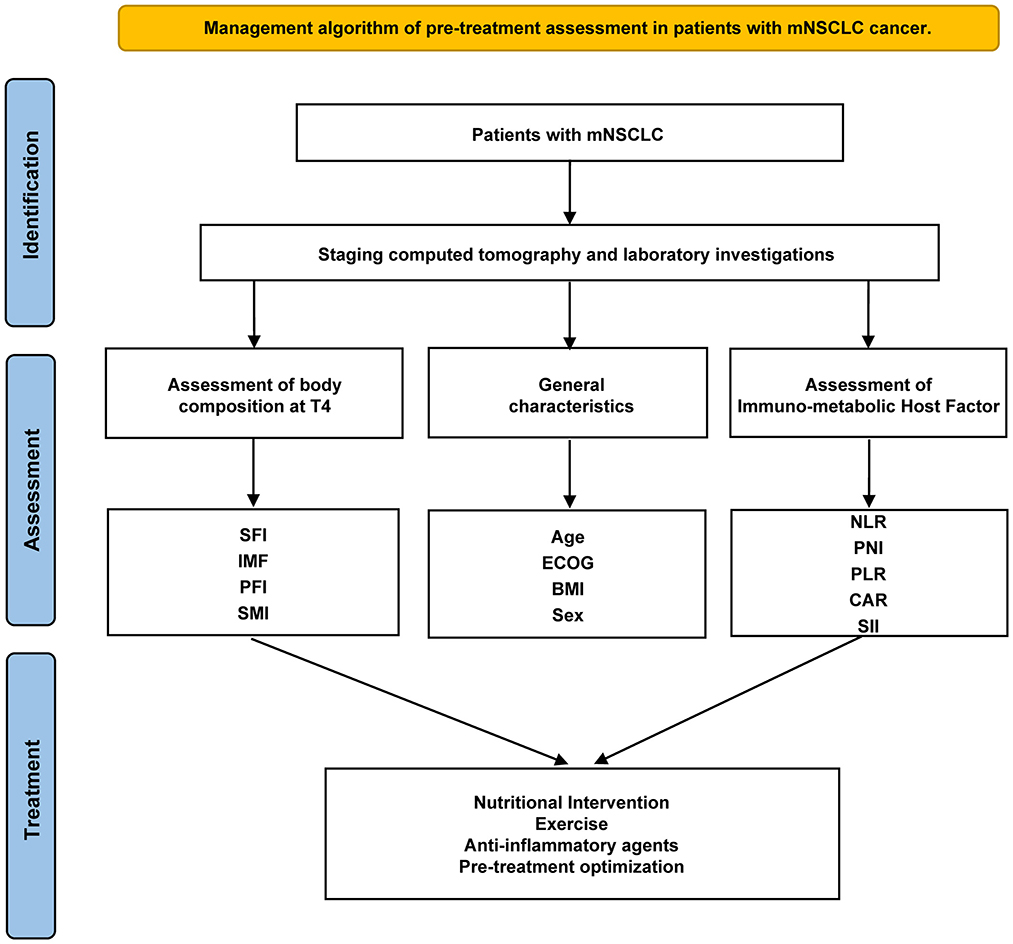
Figure 5. Management algorithm of pre-treatment assessment in patients with mNSCLC. SFI, subcutaneous fat index; PFI, Pericardial Fat Index; SMI, Skeletal muscle index; PNI, the Prognostic Nutritional Index; GPS, the Glasgow Prognostic Score; CAR, C-reactive protein-to-albumin ratio; SII, systemic immune-inflammation index; PLR, platelet-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio.
There are several limitations to our study. First, selection bias is unavoidable due to the retrospective and single-center design. Second, we did not assess the impact of changes in body composition post-radiotherapy or the effects of neoadjuvant and adjuvant chemotherapy. Third, considering clinical feasibility, we measured body composition at the Th4 level rather than the standard L3 level, which may affect comparability. Future directions include prospective data collection to further validate our findings and the necessity for multicenter studies to confirm these results. If the preliminary findings of this study are substantiated in future prospective investigations, they will provide significant guidance for clinical management. Additionally, forthcoming research should investigate the impact of physical activity and diet on outcomes. In particular, a better understanding is needed regarding the impact of diet in modulating gut microbiome composition and its potential effects on immune responses is warranted. Increasing evidence indicates the role of the gut microbiome in immune modulation, and elucidating the interplay between diet and the gut microbiome holds clinical significance for enhancing the efficacy of radiotherapy and other cancer treatments (35). Therefore, future studies must also focus on the relationship between diet, the gut microbiome, and cancer treatment responses to develop more personalized and effective therapeutic strategies.
5 Conclusion
In summary, our study underscores the significance of immune-nutritional scores, and body composition assessed via baseline CT as vital prognostic indicators for oligometastatic NSCLC patients. The findings imply that implementing multimodal clinical management strategies aimed at mitigating the loss of subcutaneous adiposity could enhance the life quality and prognosis of patients with advanced NSCLC. The underlying mechanisms and validation of these results warrant further investigation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the First Affiliated Hospital of Bengbu Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin because our retrospective study did not expose patient privacy and did not affect the treatment strategy, so we did not provide or request the informed consent form.
Author contributions
ZHu: Investigation, Methodology, Writing – original draft. ZG: Investigation, Methodology, Writing – review & editing. BG: Investigation, Writing – review & editing. SL: Writing – review & editing, Software. YY: Writing – review & editing, Methodology. SZ: Writing – review & editing, Investigation. ZHe: Investigation, Writing – review & editing, Methodology, Supervision. HC: Methodology, Supervision, Writing – review & editing. HJ: Methodology, Supervision, Writing – review & editing, Funding acquisition.
Funding
The author (s) declare that financial support was received for the research and/or publication of this article. This work was supported in part by Research Funds of Joint Research Center for Regional Diseases of IHM (2023bydjk003); Anhui Provincial Key R&D Programmes (202304295107020078).
Acknowledgments
We appreciate the polishing services and database technical support was provided by LEVIN (Wuhan) Institute of Biomedical Research (http://www.laierwen.com).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1588391/full#supplementary-material
Abbreviations
SII, systemic immune-inflammation index; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; CAR, C reactive protein-to-albumin ratio; PNI, the Prognostic Nutritional Index; CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; OS, overall survival; PFS, progression free survival; GPS, the Glasgow Prognostic Score; CRP, C reactive protein; SFI, subcutaneous fat index; PFI, pericardial Fat Index; SMI, skeletal muscle index; HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; Oligo-NSCLC, oligometastatic non-small cell lung cancer.
References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
2. Xie S, Wu Z, Qi Y, Wu B, Zhu X. The metastasizing mechanisms of lung cancer: Recent advances and therapeutic challenges. Biomed Pharmacother. (2021) 138:111450. doi: 10.1016/j.biopha.2021.111450
3. Iyengar P, All S, Berry MF, Boike TP, Bradfield L, Dingemans AC, et al. Treatment of oligometastatic non-small cell lung cancer: an ASTRO/ESTRO clinical practice guideline. Pract Radiat Oncol. (2023) 13:393–412. doi: 10.1016/j.prro.2023.04.004
4. Gomez DR, Tang C, Zhang J, Blumenschein GR Jr, Hernandez M, Lee JJ, et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. (2019) 37:1558–65. doi: 10.1200/JCO.19.00201
5. Caan BJ, Cespedes Feliciano EM, Prado CM, Alexeeff S, Kroenke CH, Bradshaw P, et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol. (2018) 4:798–804. doi: 10.1001/jamaoncol.2018.0137
6. Kosumi K, Baba Y, Hara Y, Wang H, Nomoto D, Toihata T, et al. Body composition and clinical outcomes in esophageal cancer patients treated with immune checkpoint inhibitors. Ann Surg Oncol. (2024) 31:3839–49. doi: 10.1245/s10434-024-15093-3
7. Vedire Y, Nitsche L, Tiadjeri M, McCutcheon V, Hall J, Barbi J, et al. Skeletal muscle index is associated with long term outcomes after lobectomy for non-small cell lung cancer. BMC Cancer. (2023) 23:778. doi: 10.1186/s12885-023-11210-9
8. Baastrup NN, Christensen JK, Jensen KK, Jørgensen LN. Visceral obesity and short-term outcomes after laparoscopic rectal cancer resection. Surg Endosc. (2020) 34:177–85. doi: 10.1007/s00464-019-06748-4
9. Donkers H, Fasmer KE, Mcgrane J, Pijnenborg JMA, Bekkers R, Haldorsen IS, et al. Obesity and visceral fat: survival impact in high-grade endometrial cancer. Eur J Obstet Gynecol Reprod Biol. (2021) 256:425–32. doi: 10.1016/j.ejogrb.2020.11.050
10. Xu FQ, Xu QY, Zhu ZJ, Jin L, Ye TW, Du CF, et al. Visceral and ectopic fat are more predictively associated with primary liver cancer than overall obesity from genetic sights: a Mendelian randomization study. Int J Cancer. (2024) 154:530–7. doi: 10.1002/ijc.34751
11. Xu J, Wan CS, Ktoris K, Reijnierse EM, Maier AB. Sarcopenia is associated with mortality in adults: a systematic review and meta-analysis. Gerontology. (2022) 68:361–76. doi: 10.1159/000517099
12. Bano G, Trevisan C, Carraro S, Solmi M, Luchini C, Stubbs B, et al. Inflammation and sarcopenia: a systematic review and meta-analysis. Maturitas. (2017) 96:10–5. doi: 10.1016/j.maturitas.2016.11.006
13. Pan L, Xie W, Fu X, Lu W, Jin H, Lai J, et al. Inflammation and sarcopenia: a focus on circulating inflammatory cytokines. Exp Gerontol. (2021) 154:111544. doi: 10.1016/j.exger.2021.111544
14. Shinohara S, Otsuki R, Kobayashi K, Matsuo M, Harada K, Sugaya M, et al. The prognostic impact of pericardial fat volumes in resected non-small cell lung cancer. Ann Surg Oncol. (2020) 27:481–9. doi: 10.1245/s10434-019-07703-2
15. Jimenez-Gutierrez GE, Martínez-Gómez LE, Martínez-Armenta C, Pineda C, Martínez-Nava GA, Lopez-Reyes A. Molecular mechanisms of inflammation in sarcopenia: diagnosis and therapeutic update. Cells. (2022) 11:52359. doi: 10.3390/cells11152359
16. Yamanouchi K, Murakami S, Sato A, Ogawa S, Shinagawa H, Kamohara Y. Integrated evaluation of inflammatory, nutritional, and sarcopenia markers to predict survival in metastatic breast cancer patients. In Vivo. (2023) 37:811–7. doi: 10.21873/invivo.13146
17. Zhao M, Duan X, Han X, Wang J, Han G, Mi L, et al. Sarcopenia and systemic inflammation response index predict response to systemic therapy for hepatocellular carcinoma and are associated with immune cells. Front Oncol. (2022) 12:854096. doi: 10.3389/fonc.2022.854096
18. Zhou E, Wu J, Zhou X, Yin Y. Systemic inflammatory biomarkers are novel predictors of all-cause and cardiovascular mortality in individuals with osteoarthritis: a prospective cohort study using data from the NHANES. BMC Public Health. (2024) 24:1586. doi: 10.1186/s12889-024-19105-5
19. Laird BJA, Skipworth RJE. The obesity paradox in cancer: is bigger better? J Cachexia Sarcopenia Muscle. (2022) 13:1440–1. doi: 10.1002/jcsm.13007
20. Ohliger MA. Body composition at CT and risk of future disease. Radiology. (2023) 306:e222162. doi: 10.1148/radiol.222162
21. Wu TH, Tsai YT, Chen KY, Yap WK, Luan CW. Utility of high-sensitivity modified glasgow prognostic score in cancer prognosis: a systemic review and meta-analysis. Int J Mol Sci. (2023) 24:1318. doi: 10.3390/ijms24021318
22. Wang S, Xu S, Wang J, Ye H, Zhang K, Fan X, et al. Preoperative C-reactive protein to albumin ratio may be a good prognostic marker in patients undergoing hepatectomy for hepatocellular carcinoma: a meta-analysis. Front Nutr. (2024) 11:1444352. doi: 10.3389/fnut.2024.1444352
23. Liu J, Li S, Zhang S, Liu Y, Ma L, Zhu J, et al. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J Clin Lab Anal. (2019) 33:e22964. doi: 10.1002/jcla.22964
24. Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. (2017) 111:176–81. doi: 10.1016/j.lungcan.2017.07.024
25. Xia H, Zhang W, Zheng Q, Zhang Y, Mu X, Wei C, et al. Predictive value of the prognostic nutritional index in advanced non-small cell lung cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Heliyon. (2023) 9:e17400. doi: 10.1016/j.heliyon.2023.e17400
26. Sun C, Anraku M, Kawahara T, Karasaki T, Kitano K, Nagayama K, et al. Prognostic significance of low pectoralis muscle mass on preoperative chest computed tomography in localized non-small cell lung cancer after curative-intent surgery. Lung Cancer. (2020) 147:71–6. doi: 10.1016/j.lungcan.2020.07.008
27. Tao J, Fang J, Chen L, Liang C, Chen B, Wang Z, et al. Increased adipose tissue is associated with improved overall survival, independent of skeletal muscle mass in non-small cell lung cancer. J Cachexia Sarcopenia Muscle. (2023) 14:2591–601. doi: 10.1002/jcsm.13333
28. De Wolf K, Vermaelen K, De Meerleer G, Lambrecht BN, Ost P. The potential of radiotherapy to enhance the efficacy of renal cell carcinoma therapy. Oncoimmunology. (2015) 4:e1042198. doi: 10.1080/2162402X.2015.1042198
29. Newton JM, Hanoteau A, Liu HC, Gaspero A, Parikh F, Gartrell-Corrado RD, et al. Immune microenvironment modulation unmasks therapeutic benefit of radiotherapy and checkpoint inhibition. J Immunother Cancer. (2019) 7:216. doi: 10.1186/s40425-019-0698-6
30. Bilen MA, Martini DJ, Liu Y, Shabto JM, Brown JT, Williams M, et al. Combined effect of sarcopenia and systemic inflammation on survival in patients with advanced stage cancer treated with immunotherapy. Oncologist. (2020) 25:e528–35. doi: 10.1634/theoncologist.2019-0751
31. Salavatizadeh M, Soltanieh S, Radkhah N, Ataei Kachouei AH, Bahrami A, Khalesi S, et al. The association between skeletal muscle mass index (SMI) and survival after gastrectomy: a systematic review and meta-analysis of cohort studies. Eur J Surg Oncol. (2023) 49:106980. doi: 10.1016/j.ejso.2023.07.006
32. Li Z, Ji BW, Dixit PD, Tchourine K, Lien EC, Hosios AM, et al. Cancer cells depend on environmental lipids for proliferation when electron acceptors are limited. Nat Metab. (2022) 4:711–23. doi: 10.1038/s42255-022-00588-8
33. Ebadi M, Mazurak VC. Evidence and mechanisms of fat depletion in cancer. Nutrients. (2014) 6:5280–97. doi: 10.3390/nu6115280
34. Setiawan T, Sari IN, Wijaya YT, Julianto NM, Muhammad JA, Lee H, et al. Cancer cachexia: molecular mechanisms and treatment strategies. J Hematol Oncol. (2023) 16:54. doi: 10.1186/s13045-023-01454-0
Keywords: body composition, immune-nutritional scores, oligometastasis, NSCLC, radiotherapy
Citation: Huang Z, Guo Z, Gao B, Li S, Yu Y, Zhu S, He Z, Chen H and Jiang H (2025) Prognostic impact of body composition and immune-nutritional status in oligometastatic NSCLC patients receiving radiotherapy. Front. Nutr. 12:1588391. doi: 10.3389/fnut.2025.1588391
Received: 05 March 2025; Accepted: 30 May 2025;
Published: 01 July 2025.
Edited by:
Artur Delgado, Hospital das Clínicas, Sao Paulo University, BrazilReviewed by:
Hesong Wang, Fourth Hospital of Hebei Medical University, ChinaJieun Kim, University of Texas Health Science Center at Houston, United States
Copyright © 2025 Huang, Guo, Gao, Li, Yu, Zhu, He, Chen and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Jiang, Ynl5ZnlqaEBiYm11LmVkdS5jbg==; Haiyan Chen, Y2hlbmhhaXlhbkB6anUuZWR1LmNu
†These authors have contributed equally to this work
 Zhifei Huang1,2,3,4†
Zhifei Huang1,2,3,4† Yaner Yu
Yaner Yu Zelai He
Zelai He Haiyan Chen
Haiyan Chen