- 1Department of Gastrointestinal Surgery, Yongchuan Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Respiratory Medicine, The Affiliated Yongchuan District Traditional Chinese Medicine Hospital of Chongqing Medical University, Chongqing, China
- 3Central Laboratory, Yongchuan Hospital of Chongqing Medical University, Chongqing, China
Purpose: To investigate the combined effects of oral nutritional supplement (ONS) and intestinal microecology on postoperative nutrition status, inflammatory response and intestinal flora regulation colorectal cancer (CRC) patients.
Methods: This prospective single-center randomized controlled trial (RCT) was conducted at Chongqing Yongchuan Hospital between December 2023 and December 2024. CRC patients were randomly assigned to one of two groups: a control group receiving ONS (55.8 g per dose, three times daily) or a test group receiving ONS (55.8 g per dose, three times daily) combined with bifidobacteria (1.5 g per dose, three times daily).
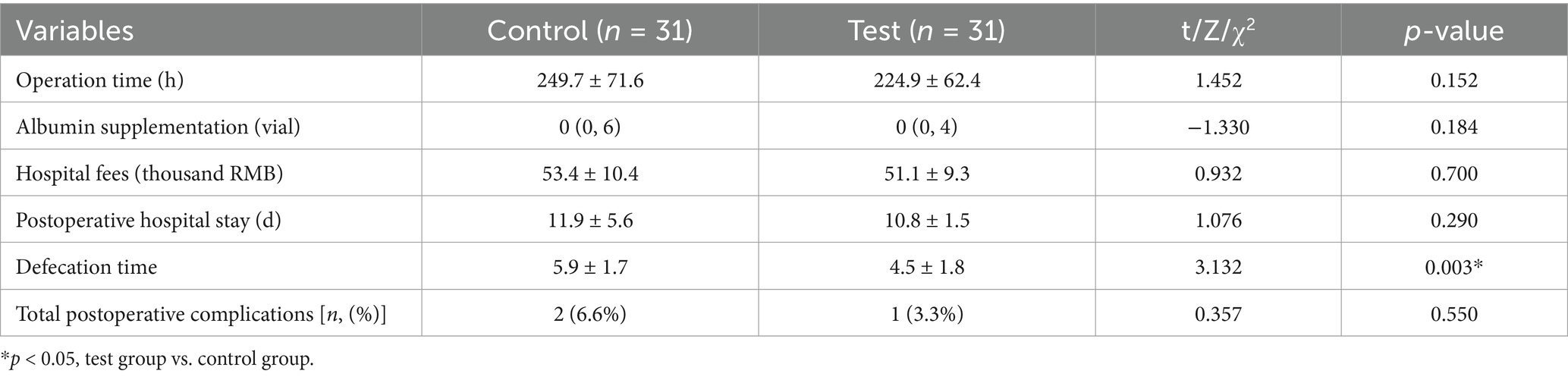
Results: A total of 62 patients who undergoing radical colorectal cancer resection were enrolled. Participants were equally randomized into control and test groups (n = 31 each). At baseline, no significant differences in demographic characteristics, nutritional status, or inflammatory markers were observed between groups (p > 0.05). Prealbumin (PA; 174.0 ± 38.0 g/L vs. 149.7 ± 42.9 g/L, t = −2.358, p = 0.022), albumin (ALB; 36.6 ± 3.3 g/L vs. 33.1 ± 4.0 g/L, t = −3.745, p < 0.000), total protein (TP; 65.8 ± 5.1 g/L vs. 62.5 ± 6.3 g/L, t = −2.266, p = 0.027), and the changes in ΔT3–T2 in PA (32.9 ± 36.1 g/L vs. 13.3 ± 34.9 g/L, t = −2.180, p = 0.033), ALB (4.0 ± 4.5 g/L vs. 1.0 ± 3.7 g/L, t = −2.862, p = 0.006), and TP (7.5 ± 5.9 g/L vs. 4.0 ± 5.9 g/L, t = −2.333, p = 0.023) were significantly greater in the test group than in the control group. The reduction in C-reactive protein (CRP) from T2 to T3 (42.1 (27.1, 62.9) mg/L vs. 26.8 (10.7, 46.4) mg/L, Z = −2.752, p = 0.006) was significantly greater in the test group. Fecal DNA fingerprint analysis revealed that, compared with the control group, the test group presented significantly greater intestinal flora species richness and abundance. The time to first defecation was significantly shorter in the test group (4.5 ± 1.8 vs. 5.9 ± 1.7 days, t = 3.132, p = 0.003).
Conclusion: Perioperative ONS combined with intestinal microbiota interventions improves postoperative nutritional status, modulates inflammatory dynamics, and accelerates intestinal function recovery. However, these interventions show limited impact on hospitalization duration and complication rates.
1 Introduction
Colorectal cancer (CRC), one of the most common global malignant tumors, exhibits an annually increasing incidence rate and ranks among the leading digestive system causes of death (1). While laparoscopic surgery combined with synchronous chemoradiotherapy is commonly used in CRC clinical treatment, preoperative tumor growth often induces intestinal mucosa ischemia and hypoxia. This disrupts intestinal microecological balance, damages the mucosal barrier, and causes malnutrition (2, 3). Furthermore, preoperative bowel preparation and postoperative fasting exacerbate nutritional deficiencies, leading to reduced immunity, worsening nutritional status, and impaired postoperative recovery. Consequently, CRC treatment strategies have shifted from single-surgery approaches to multidisciplinary, comprehensive perioperative models. The integration of oral nutritional supplements (ONS) and intestinal microecology has emerged as a key research focus.
For patients undergoing radical CRC surgery, malnutrition serves as an independent risk factor for postoperative complications and is closely linked to mortality, prolonged hospitalization, and readmission rates (4). Correcting perioperative malnutrition improves surgical tolerance, reduces postoperative complications, and enhances patient prognosis (5). ONS is the preferred method for delivering supplemental energy and nutrients for special medical purposes beyond regular food (6). Clinical studies and systematic reviews consistently show that ONS improves nutritional status and clinical outcomes in hospitalized patients while also reducing overall costs, underscoring its cost-effectiveness (7, 8). However, ONS alone often fails to address intestinal dysfunction and impaired nutrient absorption in CRC patients, potentially limiting its efficacy.
Intestinal microecosystems have gained significant attention in CRC adjuvant therapy due to their beneficial properties. Studies show that probiotics like Bifidobacterium enhance gastrointestinal motility and may slow CRC progression via specific protein and metabolite secretion (9). Recent clinical research indicates that intestinal ecosystem modulation improves perioperative immune indices and postoperative gastrointestinal symptoms in CRC patients while enriching intestinal flora diversity. These interventions effectively correct dysbiosis, accelerate postoperative recovery, and play key roles in CRC prevention and adjuvant treatment (10, 11).
Many existing studies have used single-intervention approaches, limiting CRC clinical management. This study explored the perioperative combination of ONS and intestinal microbial agents. By leveraging intestinal microecology to regulate flora balance and using ONS to supply essential nutrients, the study investigated their combined effects on postoperative recovery, including intestinal flora composition, inflammation, and nutritional status. The goal was to develop more precise and effective CRC treatment strategies.
2 Information and methods
2.1 Study design
This prospective, single-center, randomized controlled trial enrolled subjects who met the inclusion criteria during the study period. The participants were randomly assigned to either the control or test group via a computer-generated randomization sequence. All the subjects provided written informed consent.
The study was conducted in strict accordance with relevant guidelines and regulations and was approved by the Medical Ethics Committee of Yongchuan Hospital, Chongqing Medical University (Approval No. 2023LLS040).
The inclusion criteria were as follows: (1) patients who underwent elective surgery for CRC and were aged ≥18 years (with no sex restrictions); (2) patients who underwent preoperative colonoscopy biopsy confirming CRC, without multiple primary tumors; (3) patients with no distant metastasis and no preoperative neoadjuvant therapy; (4) patients with no preoperative infections or immunodeficiency diseases and no recent use of nutritional or intestinal microbial preparations; (5) patients with no acute complications, such as intestinal obstruction, intestinal perforation, or gastrointestinal bleeding; and (6) patients who underwent their first laparoscopic radical resection for CRC, with a preoperative nutritional risk screening (NRS2002) score ≥3; (7) patients with normal mental status, with intact acceptance, cognition, judgment, and language communication abilities; and voluntary consent to participate in the study after being informed of its details.
The exclusion criteria were as follows: (1) patients with multiple primary tumors; (2) patients with advanced preoperative or intraoperative tumors or those who received preoperative neoadjuvant therapy; (3) patients with preoperative infections, immunodeficiency diseases, nutritional support therapy, or recent antibiotic use; (4) patients with acute complications, such as intestinal obstruction, perforation, or bleeding; (5) patients with severe immune, cardiovascular, or respiratory diseases; and (6) patients who underwent repeat surgery or who had a preoperative NRS2002 score <3; (7) patients who were allergic to the study drug or were unable to complete the study for other reasons.
2.2 Methods
2.2.1 Intervention drugs and ONS
The intestinal microbial agents used in this study were bifidobacterium tetrad live tablets (0.5 g/tablet; Hangzhou Yuanda Biopharmaceutical Co., Ltd.). These agents are composed of the following strains: Bifidobacterium infantis, Lactobacillus acidophilus, Enterococcus faecalis, and Bacillus cereus.
The ONS product used was ENSURE TP (400 g/tub; Abbott Laboratories B.V.), a balanced nutritional supplement providing approximately 450 kcal of energy, 15.9 g of protein, 15.9 g of fat, 60.7 g of carbohydrates, vitamins, and minerals per 100 g.
2.3 Patient management
Both groups were fed a liquid diet beginning on the day of enrollment. The control group received oral enteral nutrition powder (55.8 g per dose, three times per day) until 1 day before surgery (average duration: 4 days). The test group received the same regimen as the control group, with an additional 1.5 g of Bifidobacterium per dose, three times per day.
On the night before surgery, all enrolled patients received polyethylene glycol electrolyte powder (3,000 mL). Prophylactic antibiotics were administered 30 min before surgery, with additional doses given over the next three hours. Surgical procedures followed the China Code for Diagnosis and Treatment of Colorectal Cancer (2023 edition) (12) and were performed by the same surgical team.
In the postoperative period, both groups received routine intravenous nutritional support on days 1 and 2. Patients were allowed to drink small amounts of water on day 2, provided that there was no discomfort (nausea, vomiting, abdominal distension, or abdominal pain). On day 3, patients began taking a small amount of oral enteral nutrition powder (55.8 g per day). On day 4, the dose was increased to 55.8 g twice daily, and on day 5, the regimen was adjusted to 55.8 g three times daily until discharge. For the control group, bifidobacterium (1.5 g per dose, three times daily) was added starting on day 3.
The daily energy requirements for all patients were 25 to 30 kcal/kg/day, and the protein requirements were 1.0 to 2.0 g/kg/day. ONS and parenteral nutrition (PN) were initiated on postoperative day 3. The PN energy supply gradually decreased as the ONS energy supply increased. Intravenous fluid intake was maintained at 2000 to 2,500 mL for the first 2 days and then gradually decreased on the basis of the patient’s ONS intake from day 3 onward.
2.4 Study endpoints
2.4.1 Primary study endpoints
The primary endpoints of the study were postoperative nutritional measures, including prealbumin (PA), albumin (ALB), total protein (TP), and hemoglobin (HGB). These parameters were measured on the enrollment day (T1), postoperative day 1 (T2), and postoperative day 8 (T3).
2.4.2 Secondary study endpoints
The secondary endpoints of the study were postoperative inflammatory markers and fecal bacterial DNA fingerprints.
2.4.2.1 Inflammatory indicators
Blood samples were collected at T1, T2, and T3. The inflammatory markers measured included white blood cell count (WBC), lymphocyte count (Lym), neutrophil count (Neu), and C-reactive protein (CRP).
2.4.2.2 Fecal bacterial DNA fingerprint analysis
In this study, 10 fecal samples (3 g each) were randomly selected from the first postoperative stools of both groups and stored at −80°C. For analysis, the fecal samples were processed as follows:
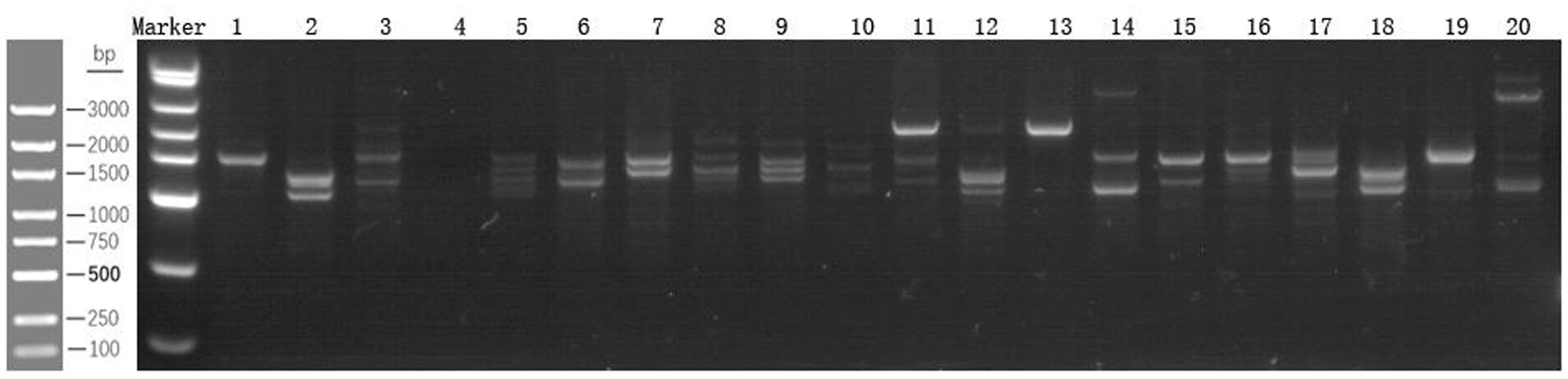
For sample preparation, 100–200 mg of each stool sample was weighed, Buffer SLA, Proteinase K, and grinding beads were added. The mixture was incubated at 70°C for 10 min, followed by centrifugation at 13,000 rpm for 1 min. DNA Extraction: RNase A, Buffer SLB, and Buffer SGB were added sequentially. Multiple centrifugation and washing steps were performed. Finally, the genomic DNA was eluted with TE buffer. PCR amplification: The primers PS2 (5′-TG(C/T)ACACACCGCCCGT-3′) and PL2 (5′-GGGT(G/C/T)CCCCCATTC(A/G)G-3′) were used for specific amplification. The PCR protocol included predenaturation at 98°C for 2 min; 30 cycles of denaturation at 98°C for 20 s, annealing at 55°C for 20 s, and extension at 72°C for 10 s; a final extension at 72°C for 5 min; and cooling at 16°C for 2 min. Product analysis: The amplified products were analyzed via electrophoresis on a 2% agarose gel, visualized under UV light, and photographed to preserve the results.
2.4.3 Clinical observation indicators
The number and incidence of operation time and postoperative complications (such as lung infection, urinary tract infection, and abdominal infection), postoperative bowel function recovery (measured by time to first postoperative defecation), blood albumin injection utilization, postoperative hospitalization duration, and associated hospitalization costs were recorded.
2.5 Sample size calculation
The primary outcome measures in this study were postoperative nutritional indicators, specifically prealbumin levels. In the current literature, the mean prealbumin levels in the test group and control group were reported to be 288.4 ± 58.6 and 239.8 ± 71.8, respectively (13). With a significance level (α) of 0.05 and a power (1−β) of 0.8, the required sample size for each group was calculated to be 30 cases via PASS software. Considering the short follow-up period and low risk of loss to follow-up in this study, a total of 60 cases (30 per group) were deemed sufficient. This study is a single-center, randomized controlled trial with a minimum of 30 patients in each group (test and control).
2.6 Statistical analysis
Statistical analyses were performed via SPSS 26.0 software. A two-sided p value of less than 0.05 was considered statistically significant. Normally distributed continuous data are presented as the means ± standard deviations (Means ± SD), whereas nonnormally distributed data are reported as medians and interquartile ranges (M [P25, P75]). Repeated measures analysis of variance (ANOVA), independent samples t tests, or Mann–Whitney U tests were used as appropriate. Categorical data are expressed as counts and proportions [n, (%)] and were analyzed via the χ2 test or Fisher’s exact test, depending on the sample size.
3 Results
3.1 Comparison of the baseline data between the two patient groups
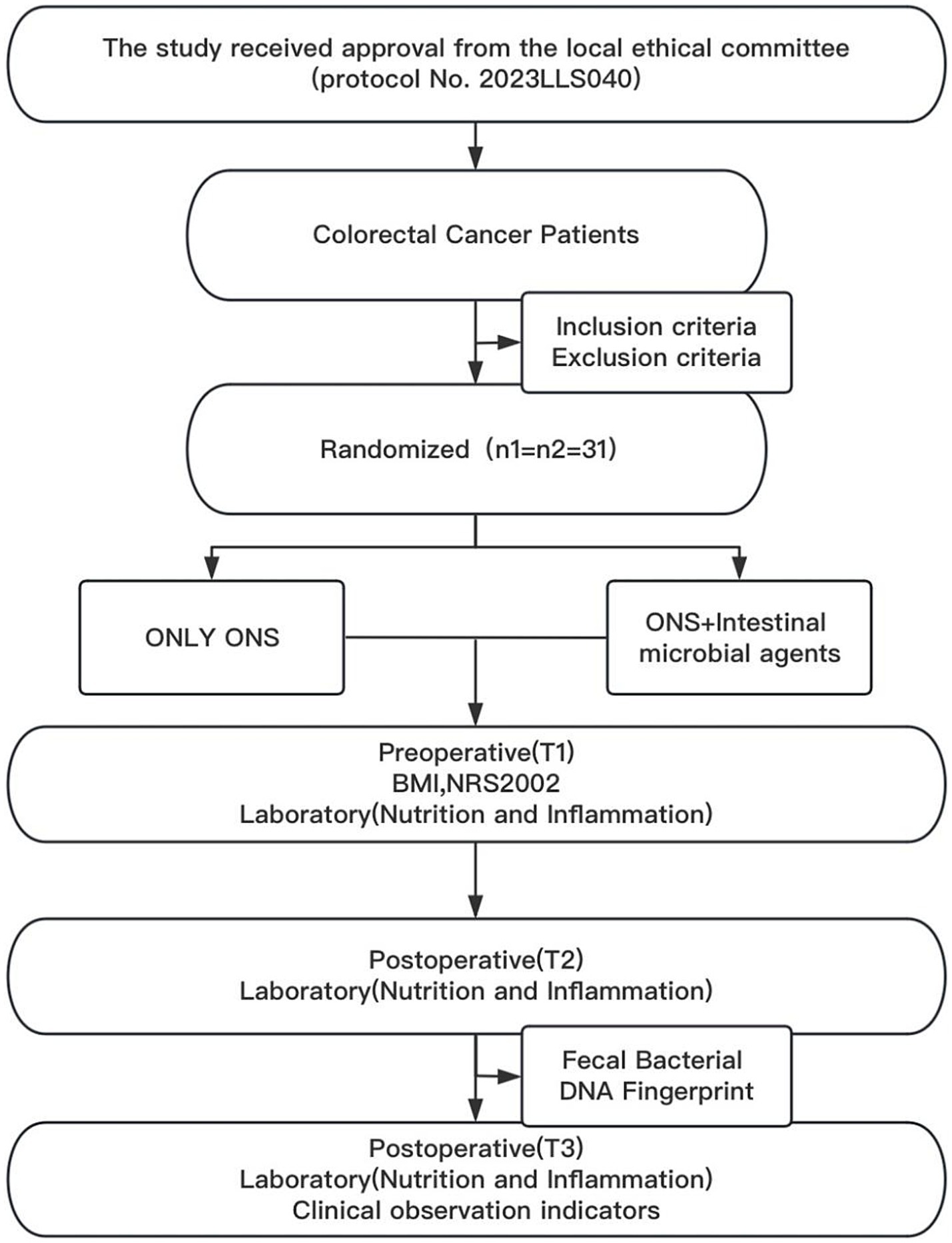
A total of 62 patients who underwent radical CRC surgery were included in the study and randomized into control and trial groups (n = 31 per group) (Figure 1). Demographic analysis revealed no significant differences between the groups in terms of sex composition (male: 14 vs. 17; female: 17 vs. 14), age (66.8 ± 13.0 years vs. 64.7 ± 10.3 years; t = 0.703, p = 0.485), or BMI (22.98 ± 3.15 kg/m2 vs. 23.06 ± 2.91 kg/m2; t = −0.106, p = 0.916).
In terms of oncological characteristics, tumor anatomical location distribution (right colon: 9 vs. 7; left colon: 4 vs. 3; sigmoid colon: 3 vs. 5; rectum: 15 vs. 16 cases; χ2 = 0.925, p = 0.859), maximum tumor diameter (4.7 ± 1.7 cm vs. 4.0 ± 1.7 cm; t = 1.571, p = 0.121), and AJCC 8th Edition TNM stage (stage I: 5 vs. 8 cases; stage II: 14 vs. 11 cases; stage III: 12 vs. 12 cases; χ2 = 1.052, p = 0.647) were all balanced between groups (all p > 0.05). These results confirmed the validity of the randomization and the matching of the baseline characteristics (Table 1).
3.2 Comparison of nutritional and inflammatory indicators between the two groups
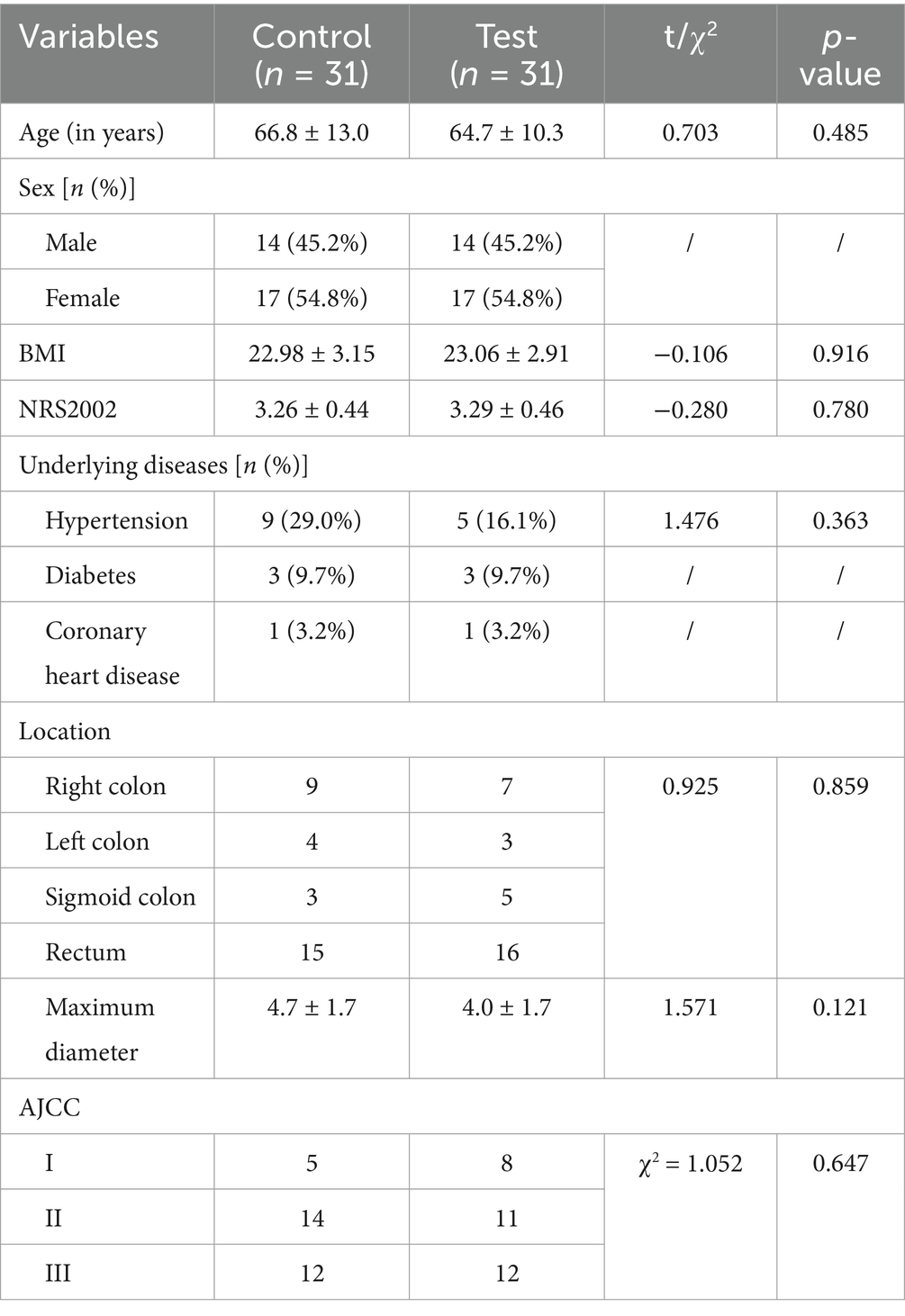
A repeated-measures two-factor analysis of variance (group × time) was conducted. The time main effect revealed that all nutritional indicators (PA, ALB, TP, HGB) and inflammatory indicators (CRP, WBC, etc.) exhibited statistically significant changes over time (p < 0.05). These findings suggest substantial dynamic fluctuations in nutritional and inflammatory status during postoperative recovery. The interaction effect showed that the time × group interaction for CRP was significant (F = 3.298, p = 0.04), indicating that the intervention differentially regulated the dynamic trajectory of CRP. No significant interaction effects were detected for the other indicators (p > 0.05). The main effect between groups was not significant for CRP (F = 0.363, p = 0.549) or other indicators (p > 0.05), indicating that the groups were comparable at baseline (Table 2).
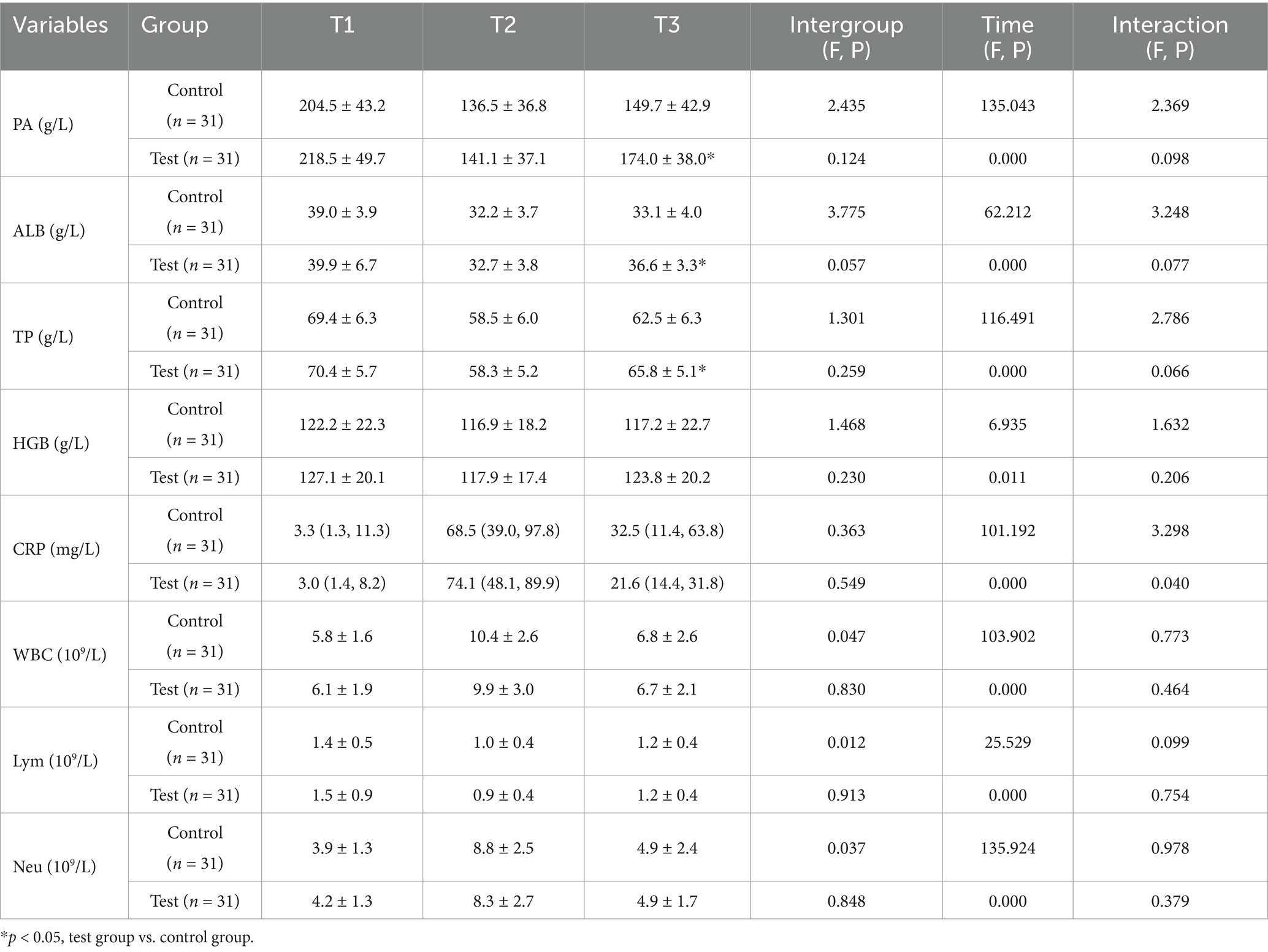
Post hoc analyses of nutritional and inflammatory indicators at different time points and change values revealed no significant differences between the two groups (p > 0.05), confirming good baseline balance and comparability. At T2, the levels of nutritional indicators (PA, ALB, TP, HGB) and Lym significantly decreased compared with those at T1, whereas the levels of inflammatory markers (CRP, WBC) increased with stress. However, these differences were not statistically significant between the groups (p > 0.05). At T3, the test group exhibited superior nutritional recovery compared with the control group, with significant increases in PA (174.0 ± 38.0 vs. 149.7 ± 42.9 g/L; p = 0.022), ALB (36.6 ± 3.3 vs. 33.1 ± 4.0 g/L; p < 0.001), and TP (65.8 ± 5.1 g/L vs. 62.5 ± 6.3 g/L; p = 0.027) (Table 2; Figure 2). Analysis of the change values revealed that, compared with the control group, the test group presented a significantly greater rebound in the ALB concentration (4.0 ± 4.5 vs. 1.0 ± 3.7 g/L; p = 0.006). Additionally, the rate of CRP decline was faster in the test group (42.1 vs. 26.8 mg/L; p = 0.006), indicating that the combined intervention accelerated postoperative inflammation resolution and nutritional recovery (Table 3). ΔT2–T1 and ΔT3–T1: No significant differences were observed between groups during the surgical stress period (T1–T2) or over the full recovery period (T1–T3) (p > 0.05).
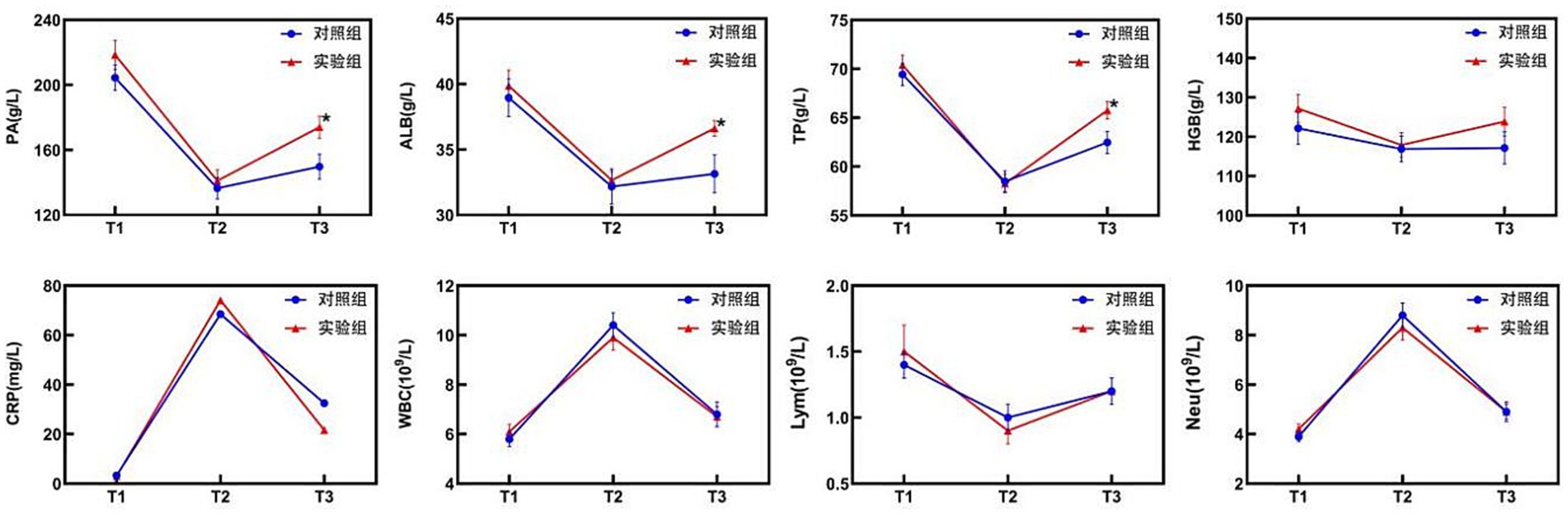
Figure 2. Changes in nutritional and inflammatory indicators in the two groups. CRP levels are presented as the median (upper/lower limit); * T3, test group vs. control group, p < 0.05.
3.3 Comparison of fecal bacterial DNA fingerprint analysis between the two groups
According to the map analysis, the test group presented an increase in band intensity compared with the control group, indicating a greater number of intestinal bacteria. This suggests an increase in both species richness and abundance, as well as a significant change in the diversity of the intestinal flora (Figure 3).
3.4 Clinical observation indicators in both groups
Clinical observation indicators included operation time, albumin supplementation, hospital fees, postoperative hospital stay, time to first postoperative defecation, and incidence of postoperative complications. The defecation time was significantly shorter in the test group than in the control group (p < 0.05). However, no significant differences were observed in other indicators, including human ALB injection use, hospitalization cost, and incidence of postoperative complications (Table 4).
4 Discussion
The development of CRC is associated with imbalances in nutritional metabolism and the intestinal flora. This association presents dual challenges for perioperative management: addressing insufficient nutrient supply and mitigating flora-mediated immunosuppression. Compared with normal cells, tumor cells exploit the host’s glucose metabolism via the Warburg effect, increasing their glycolysis rate 20- to 30-fold. This results in a high catabolic state (14). However, surgical trauma exacerbates metabolic disorders, and intestinal ischemia–reperfusion injury increases intestinal epithelial cell apoptosis. This triggers intestinal barrier dysfunction and enhances the translocation of gram-negative bacteria, leading to a vicious cycle of endotoxemia and systemic inflammation (3, 15, 16). In this context, traditional single-intervention models, such as simple enteral nutrition or antibiotics, often fail to address these interconnected issues comprehensively.
ONS are specialized nutritional formulations designed to provide patients with energy, protein, and micronutrients. They are widely used in pre- and postoperative care, for patients with chronic wasting diseases, and among elderly populations at nutritional risk. The primary advantages of ONS include rapid energy replenishment, the ability to mitigate the negative nitrogen balance, and reduced muscle catabolism. These effects help maintain organ function and immune status, thereby improving the overall nutritional health of patients (17). In 2020, the European Society for Parenteral and Enteral Nutrition (ESPEN) recommended preoperative ONS use regardless of the patient’s nutritional status, as the clinical benefits outweigh potential surgical risks (18). However, ONS focuses primarily on the macronutrient supply and lacks targeted regulation of the intestinal microecology. This limitation makes it difficult to interrupt the inflammatory cascade triggered by intestinal dysbiosis, which can persist postoperatively or during disease states.
In recent years, increasing evidence has highlighted a causal link between gut microbial dysbiosis and cancer development. Specific pathogens, such as Fusobacterium nucleatum, anaerobic Streptococcus, and enterotoxin-producing Bacteroides fragilis, have been shown to promote CRC development through mechanisms including tumor proliferation, inflammation, DNA damage, and immune evasion (19, 20). Consequently, modulating the gut microbiota to restore microbial homeostasis has emerged as a promising strategy for CRC prevention and treatment. Gut microbial agents are biological preparations that enhance host health by regulating the composition or function of the intestinal flora. These agents primarily include probiotics, prebiotics, and synbiotics. The mechanisms of different probiotic strains vary on the basis of their specific activities. Its clinical value lies in restoring the intestinal microecological balance by restoring dominant bacterial populations and inhibiting inflammatory pathways (21). However, colonization efficiency is significantly reduced when the host lacks sufficient metabolic substrates.
In the present study, we innovatively applied a combination of bifidobacterium tetrad live bacterial tablets (Bifidobacterium infantis, Lactobacillus acidophilus, Enterococcus faecalis, and Bacillus cereus) and ENSURE TP (containing 15.9 g protein per 100 g) in the perioperative management of CRC. The results revealed that the serum ALB levels on postoperative day 8 (36.6 ± 3.3 vs. 33.1 ± 4.0 g/L; p < 0.001), total protein levels (65.8 ± 5.1 vs. 62.5 ± 6.3 g/L; p = 0.027), and prealbumin levels (174.0 ± 38.0 vs. 149.7 ± 42.9 g/L; p = 0.022) were significantly greater in the test group than in the control group. These findings suggest that the combined intervention overcomes the limitations of traditional single interventions, thereby improving patients’ nutritional status and promoting postoperative recovery.
Moreover, fecal DNA fingerprint analysis of the ITS region revealed that, compared with the control group, the test group presented increased bacterial richness and abundance. Specifically, the numbers of intestinal Bifidobacterium (p = 0.017) and Enterococcus (p = 0.02) increased, as did the abundances of the four commensal microbiota genera previously reported by Xie et al. (10). This effect may be attributed to the dual mechanism of short-chain fatty acids (SCFAs) provided by ENSURE TP (22, 23): (1) SCFAs serve as the primary energy substrate for colonic epithelial cells, enhancing amino acid and micronutrient transport efficiency, and (2) Bifidobacterium upregulates the expression of the tight junction proteins ZO-1 and Occludin, thereby strengthening the structural integrity of the tight junction complex between intestinal epithelial cells and reducing intestinal mucosal permeability. Collectively, these mechanisms establish a positive cycle of “microbiota metabolism–intestinal barrier repair–nutrient absorption”. Although Feijo et al. (24) reported no improvement in ALB levels with ω-3 fatty acid-enriched ONS in gastric cancer patients undergoing chemotherapy, and a meta-analysis by Rinninella et al. (25) found no significant differences in the serum ALB concentration (p > 0.05). Our study demonstrated that the dual-axis strategy of nutrient supply–microbiota metabolic regulation reversed the deterioration of nutritional indicators and confirmed the synergistic benefits of the combined intervention. Future research should employ metagenomic sequencing and metabolomics to further elucidate the interaction mechanisms between the functional microbiota and host metabolism.
Perioperative stress induces proinflammatory stimuli in patients, increasing intestinal mucosal permeability. The intestinal microecology can mitigate intestinal inflammation and repair the intestinal epithelial barrier by reducing cytokine release (26). In this study, although the temporal trends of WBC, Neu, and CRP initially decreased (with Lym decreasing before increasing), the differences in WBC, Neu, Lym, and CRP were not significant (p > 0.05). This aligns with Feijo et al.’s (24) neutral conclusion on the effect of ω-3-enriched ONS on the CRP but contradicts Niu et al. (27). A meta-analysis revealed that perioperative immunonutrition significantly reduced WBC and CRP levels in gastrointestinal cancer patients.
Notably, in the analysis of dynamic changes in inflammatory markers, the decrease in CRP from T2 to T3 was 42.1 mg/L in the test group, which was significantly greater than the 26.8 mg/L observed in the control group (p = 0.006). Similarly, a randomized controlled study by Park et al. (28), which included 73 CRC patients who received oral prebiotics 7 days before surgery, revealed that the inflammatory markers IL-6 and CRP were significantly higher in the test group than in the control group. Mechanistic studies suggest that live bifidobacterium tetrad tablets may coregulate the inflammatory response through multiple pathways. Lactobacillus acidophilus can competitively inhibit pathogenic bacteria (e.g., Escherichia coli), reducing endotoxin release and inhibiting the activation of the MAPK and NF-κB signaling pathways (29). Indole-3-lactate, produced by Bifidobacterium infantis via tryptophan metabolism, activates AhR receptors, thereby inhibiting NF-κB signaling pathway activity (30, 31). A moderate amount of linoleic acid in ENSURE TP can accelerate the inflammatory phase of wound healing, reduce the inflammatory response, and promote wound proliferation and remodeling (32). Thus, the combined application of ONS and microbial agents can create a dual-coordination strategy of “nutrient supply–microflora regulation”, overcoming the metabolic and microecological limitations of single interventions.
Despite positive results in terms of nutritional and inflammatory measures, no significant differences were observed between the two groups in terms of hospital length of stay (10.8 ± 1.5 vs. 11.9 ± 1.9 days) or complication rates (12.9 vs. 16.1%). Similar to the findings of Lee SY et al. (33), patients receiving oral arginine-rich ONS and ω-3 fatty acids for 7 days showed no significant differences in morbidity (31.6 vs. 29.3%; p = 0.743) or length of stay (7.6 ± 2.5 vs. 7.4 ± 2.3 days; p = 0.635). However, a meta-analysis of multiple randomized controlled trials (RCTs) confirmed the value of prebiotics and probiotics in reducing postoperative complications, particularly infectious complications (34). This discrepancy may be related to insufficient statistical power due to the small sample size in our study. Additionally, we observed accelerated recovery of intestinal function in the test group, with a shorter time to first postoperative defecation than in the control group (4.5 ± 1.8 vs. 5.9 ± 1.7 days; p = 0.003). Similar findings were reported by Wang et al. (35), who demonstrated that early enteral nutrition can shorten the time to first defecation in CRC patients (p < 0.001) and promote postoperative intestinal recovery.
In conclusion, our study demonstrated that perioperative ONS combined with intestinal microecological interventions in CRC patients significantly improved nutritional status (e.g., increased levels of PA, ALB, and TP), regulated the inflammatory process (as evidenced by reduced CRP levels), and accelerated gastrointestinal recovery (shorter time to first postoperative defecation). However, several limitations should be acknowledged. First, the single-center study design may introduce selection bias, and the small sample size limits statistical power. Second, the mechanistic insights are insufficient, with a lack of key functional biomarkers such as SCFAs, ZO-1, and Occludin. Third, the short observation period (30 days) precludes a comprehensive evaluation of long-term outcomes, including 90-day postoperative complications and long-term prognosis indicators (e.g., 3-year disease-free survival rate, overall survival rate, and tumor recurrence and metastasis rates). Future research should focus on the following directions: utilizing metagenomics technology to analyze the functional genomic characteristics of key microbiota, such as Bifidobacterium infantis and Lactobacillus acidophilus, precisely. Multicenter randomized controlled trials (RCTs) should be conducted to comprehensively evaluate the cost-effectiveness of the intervention program and its impact on optimizing medical resource utilization.
5 Conclusion
Perioperative ONS combined with intestinal microecological interventions can effectively increase serum PA, ALB, and TP levels in patients who have undergone CRC surgery. This combination therapy has synergistic effects on improving postoperative nutritional status, alleviating inflammatory responses, and promoting the recovery of gastrointestinal function.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of Yongchuan Hospital, Chongqing Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XL: Funding acquisition, Project administration, Writing – original draft, Writing – review & editing. WM: Data curation, Writing – original draft. GZ: Data curation, Writing – original draft. JL: Data curation, Writing – original draft. QL: Data curation, Funding acquisition, Writing – original draft, Writing – review & editing. GH: Data curation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The present study was sponsored by the Natural Science Fund Project of Chongqing Yongchuan District Science and Technology Bureau (2023yc-jckx20042) and the Graduate Student Innovation Fund of the Fifth Clinical College of Chongqing Medical University (YJSCX202307). No external funding was received for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel, RL, Giaquinto, AN, and Jemal, A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
2. Thanikachalam, K, and Khan, G. Colorectal Cancer and nutrition. Nutrients. (2019) 11:1. doi: 10.3390/nu11010164
3. Deng, F, Lin, ZB, Sun, QS, Min, Y, Zhang, Y, Chen, Y, et al. The role of intestinal microbiota and its metabolites in intestinal and extraintestinal organ injury induced by intestinal ischemia–reperfusion injury. Int J Biol Sci. (2022) 18:3981–92. doi: 10.7150/ijbs.71491
4. Martínez-Escribano, C, Arteaga Moreno, F, Pérez-López, M, Cunha-Pérez, C, Belenguer-Varea, Á, Cuesta Peredo, D, et al. Malnutrition and increased risk of adverse outcomes in elderly patients undergoing elective colorectal Cancer surgery: a case-control study nested in a cohort. Nutrients. (2022) 14:207. doi: 10.3390/nu14010207
5. Achilli, P, Mazzola, M, Bertoglio, CL, Magistro, C, Origi, M, Carnevali, P, et al. Preoperative immunonutrition in frail patients with colorectal cancer: an intervention to improve postoperative outcomes. Int J Color Dis. (2020) 35:19–27. doi: 10.1007/s00384-019-03438-4
6. Chinese Society of Parenteral and Enteral Nutrition (CSPEN). Guideline for clinical application of parenteral and enteral nutrition in adults patients in China (2023 edition). Zhonghua Yi Xue Za Zhi. (2023) 103:946–74. doi: 10.3760/cma.j.cn112137-20221116-02407
7. Zhang, B, Najarali, Z, Ruo, L, Alhusaini, A, Solis, N, Valencia, M, et al. Effect of perioperative nutritional supplementation on postoperative complications-systematic review and Meta-analysis. J Gastrointest Surg. (2019) 23:1682–93. doi: 10.1007/s11605-019-04173-5
8. Pimiento, JM, Evans, DC, Tyler, R, Barrocas, A, Hernandez, B, Araujo-Torres, K, et al. Value of nutrition support therapy in patients with gastrointestinal malignancies: a narrative review and health economic analysis of impact on clinical outcomes in the United States. J Gastrointest Oncol. (2021) 12:864–73. doi: 10.21037/jgo-20-326
9. Sun, W, and Zhang, L. Antioxidant indexes and immune function of the intestinal Flora of compound microecological preparations. Oxidative Med Cell Longev. (2022) 2022:1–13. doi: 10.1155/2022/5498514
10. Xie, X, He, Y, Li, H, Yu, D, Na, L, Sun, T, et al. Effects of prebiotics on immunologic indicators and intestinal microbiota structure in perioperative colorectal cancer patients. Nutrition. (2019) 61:132–42. doi: 10.1016/j.nut.2018.10.038
11. Yingli, ZH, Qianqian, CH, and Ying, LI. Effect of microecological agents on early colorectal cancer after radical resection. Chin J Microecol. (2020) 32:1167–72. doi: 10.13381/j.cnki.cjm.202010012
12. National Health Commission of the People’s Republic of China; Chinese Society of Oncology. Chinese Protocol of Diagnosis and Treatment of Colorectal Cancer (2023 edition). (2023) 14:617–644. doi: 10.3760/cma.j.cn112139-20230603-00222
13. Longbo, G, and Chunhui, L. Clinical observation of oral nutrition supplement during perioperative of laparoscopic colorectal surgery. Chin J Colorectal Dis. (2020) 9:460–3. doi: 10.3877/cma.j.issn.2095-3224.2020.05.005
14. Hamanaka, RB, and Chandel, NS. Cell biology. Warburg effect and redox balance. Science. (2011) 334:1219–20. doi: 10.1126/science.1215637
15. Trone, K, Rahman, S, Green, CH, Venegas, C, Martindale, R, and Stroud, A. Synbiotics and surgery: can prebiotics and probiotics affect inflammatory surgical outcomes? Curr Nutr Rep. (2023) 12:238–46. doi: 10.1007/s13668-023-00464-1
16. Si, H, Yang, Q, Hu, H, Ding, C, Wang, H, and Lin, X. Colorectal cancer occurrence and treatment based on changes in intestinal flora. Semin Cancer Biol. (2021) 70:3–10. doi: 10.1016/j.semcancer.2020.05.004
17. Wobith, M, and Weimann, A. Oral nutritional supplements and enteral nutrition in patients with gastrointestinal surgery. Nutrients. (2021) 13:2655. doi: 10.3390/nu13082655
18. Lobo, DN, Gianotti, L, Adiamah, A, Barazzoni, R, Deutz, NEP, Dhatariya, K, et al. Perioperative nutrition: recommendations from the ESPEN expert group. Clin Nutr. (2020) 39:3211–27. doi: 10.1016/j.clnu.2020.03.038
19. Long, X, Wong, CC, Tong, L, Chu, ESH, Ho Szeto, C, Go, MYY, et al. Peptostreptococcus anaerobius promotes colorectal carcinogenesis and modulates tumor immunity. Nat Microbiol. (2019) 4:2319–30. doi: 10.1038/s41564-019-0541-3
20. Chung, L, Thiele Orberg, E, Geis, AL, Chan, JL, Fu, K, DeStefano Shields, CE, et al. Bacteroides fragilis toxin coordinates a pro-carcinogenic inflammatory Cascade via targeting of colonic epithelial cells. Cell Host Microbe. (2018) 23:203–14.e5. doi: 10.1016/j.chom.2018.01.007
21. Martín, R, Chamignon, C, Mhedbi-Hajri, N, Chain, F, Derrien, M, Escribano-Vázquez, U, et al. The potential probiotic Lactobacillus rhamnosus CNCM I-3690 strain protects the intestinal barrier by stimulating both mucus production and cytoprotective response. Sci Rep. (2019) 9:5398. doi: 10.1038/s41598-019-41738-5
22. Monteagudo-Mera, A, Rastall, RA, Gibson, GR, Charalampopoulos, D, and Chatzifragkou, A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl Microbiol Biotechnol. (2019) 103:6463–72. doi: 10.1007/s00253-019-09978-7
23. Abdulqadir, R, Engers, J, and Al-Sadi, R. Role of Bifidobacterium in modulating the intestinal epithelial tight junction barrier: current knowledge and perspectives. Curr Dev Nutr. (2023) 7:102026. doi: 10.1016/j.cdnut.2023.102026
24. Feijó, PM, Rodrigues, VD, Viana, MS, Dos Santos, MP, Abdelhay, E, Viola, JP, et al. Effects of ω-3 supplementation on the nutritional status, immune, and inflammatory profiles of gastric cancer patients: a randomized controlled trial. Nutrition. (2019) 61:125–31. doi: 10.1016/j.nut.2018.11.014
25. Rinninella, E, Cintoni, M, Raoul, P, Pozzo, C, Strippoli, A, Bria, E, et al. Effects of nutritional interventions on nutritional status in patients with gastric cancer: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr ESPEN. (2020) 38:28–42. doi: 10.1016/j.clnesp.2020.05.007
26. Gou, HZ, Zhang, YL, Ren, LF, Li, ZJ, and Zhang, L. How do intestinal probiotics restore the intestinal barrier? Front Microbiol. (2022) 13:929346. doi: 10.3389/fmicb.2022.929346
27. Niu, JW, Zhou, L, Liu, ZZ, Pei, DP, Fan, WQ, and Ning, W. A systematic review and Meta-analysis of the effects of perioperative Immunonutrition in gastrointestinal Cancer patients. Nutr Cancer. (2021) 73:252–61. doi: 10.1080/01635581.2020.1749291
28. Park, IJ, Lee, JH, Kye, BH, Oh, HK, Cho, YB, Kim, YT, et al. Effects of PrObiotics on the symptoms and surgical ouTComes after anterior REsection of Colon Cancer (POSTCARE): a randomized, double-blind, placebo-controlled trial. J Clin Med. (2020) 9:2181. doi: 10.3390/jcm9072181
29. Lu, Q, Guo, Y, Yang, G, Cui, L, Wu, Z, Zeng, X, et al. Structure and anti-inflammation potential of Lipoteichoic acids isolated from Lactobacillus strains. Foods. (2022) 11:1610. doi: 10.3390/foods11111610
30. Meng, D, Sommella, E, Salviati, E, Campiglia, P, Ganguli, K, Djebali, K, et al. Indole-3-lactic acid, a metabolite of tryptophan, secreted by Bifidobacterium longum subspecies infantis is anti-inflammatory in the immature intestine. Pediatr Res. (2020) 88:209–17. doi: 10.1038/s41390-019-0740-x
31. Yu, K, Li, Q, Sun, X, Peng, X, Tang, Q, Chu, H, et al. Bacterial indole-3-lactic acid affects epithelium-macrophage crosstalk to regulate intestinal homeostasis. Proc Natl Acad Sci USA. (2023) 120:e2309032120. doi: 10.1073/pnas.2309032120
32. Silva, JR, Burger, B, Kühl, CMC, Candreva, T, Dos Anjos, MBP, and Rodrigues, HG. Wound healing and Omega-6 fatty acids: from inflammation to repair. Mediat Inflamm. (2018) 2018:1–17. doi: 10.1155/2018/2503950
33. Lee, SY, Lee, J, Park, HM, Kim, CH, and Kim, HR. Impact of preoperative Immunonutrition on the outcomes of Colon Cancer surgery: results from a randomized controlled trial. Ann Surg. (2023) 277:381–6. doi: 10.1097/SLA.0000000000005140
34. Veziant, J, Bonnet, M, Occean, BV, Dziri, C, Pereira, B, and Slim, K. Probiotics/Synbiotics to reduce infectious complications after colorectal surgery: a systematic review and Meta-analysis of randomized controlled trials. Nutrients. (2022) 14:3066. doi: 10.3390/nu14153066
Keywords: colorectal cancer, perioperative period, oral nutritional supplements, intestinal microecology, postoperative recovery
Citation: Liu X, Mao W, Zhao G, Liao J, Li Q and He G (2025) Effect of perioperative ONS combined with intestinal microecology in patients with colorectal cancer: a randomized clinical trial. Front. Nutr. 12:1588399. doi: 10.3389/fnut.2025.1588399
Edited by:
Falak Zeb, University of Sharjah, United Arab EmiratesReviewed by:
Maha Gasmi, University of Manouba, TunisiaHuma Naqeeb, Women University Mardan, Pakistan
Copyright © 2025 Liu, Mao, Zhao, Liao, Li and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qigang Li, eWNsaXFpZ2FuZ0AxNjMuY29t; Gan He, ODM5NjIwNTE0QHFxLmNvbQ==
†These authors have contributed equally to this work
 Xuanjun Liu
Xuanjun Liu Weixu Mao
Weixu Mao Guowei Zhao1
Guowei Zhao1 Juan Liao
Juan Liao Gan He
Gan He