- 1Department of Pediatrics, The First Hospital of China Medical University, Shenyang, Liaoning, China
- 2Department of Dermatology, The First Hospital of China Medical University, Shenyang, Liaoning, China
- 3NHC Key Laboratory of Immunodermatology, National Joint Engineering Research Center for Theranostics of Immunological Skin Diseases, China Medical University, Shenyang, Liaoning, China
Acrodermatitis enteropathica (AE) primarily affects children and is characterized by periorificial and acral dermatitis, alopecia, and diarrhea. Currently, zinc remains an effective treatment for pediatric AE. However, there is a lack of consensus regarding the optimal dosage for zinc therapy. Furthermore, emerging evidence suggests that basic zinc supplementation may be ineffective in certain cases. Therefore, we performed a literature search using PubMed and Web of Science in July 2024, focusing on pediatric AE patients, and reviewed 190 articles involving 231 patients (aged < 18 years) to address the gaps in knowledge about hereditary and acquired zinc deficiencies in these patients and to evaluate cases occurring during metabolic disease decompensation. In summary, zinc deficiency was observed in 75.9% of the patients. Among the 174 AE patients who received zinc supplementation at various dosages, 159 (91.4%) demonstrated therapeutic efficacy, with 1–3 mg/kg/day as the most commonly used effective dosage. Additionally, zinc supplementation was frequently shown to be ineffective in patients with AE associated with metabolic disorders. It is imperative to address the underlying metabolic perturbations to achieve optimal management of this condition. Therefore, a comprehensive evaluation of pediatric patients is crucial when addressing cases of AE.
Introduction
Acrodermatitis enteropathica (AE) is a rare condition characterized by periorificial and acral dermatitis, alopecia, and diarrhea; and was first described by Danbolt and Closs in 1942 (1). Historically, AE was considered an autosomal recessive disorder (2). With the discovery of zinc supplementation as a treatment for AE in approximately 1974 (3), acquired zinc deficiency was incorporated into the etiology of AE (4, 5). Since 2002, researchers believed that AE was linked to an inherited genetic defect in the SLC39A4 gene affecting the zinc transporter Zip4 (6). However, recent findings suggest that the link between metabolic disorders and AE may be more complex: several metabolic disorders have been reported to manifest as AE-like syndromes (7), some of which are unrelated to zinc deficiency (8, 9). This broader spectrum of underlying causes indicates that AE may not solely be attributed to zinc transport defects, highlighting the importance of considering metabolic disorders in the diagnosis and management of AE.
Currently, there are no systematic reviews that encompass all published cases of pediatric AE, including those related to hereditary and acquired zinc deficiencies, as well as cases arising from metabolic disorders. Moreover, although zinc supplementation remains the cornerstone of therapy for AE, there is currently a lack of consensus regarding the appropriate dosage for zinc treatment. While lifelong supplementation of elemental zinc at a dosage of 3 mg/kg/day is recommended, variations in practice persist (10). Therefore, we conducted a systematic review of the literature to gain a deeper understanding of all subtypes of pediatric AE, with a specific emphasis on their therapeutic approaches.
Methods
We adhered to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines for conducting and reporting this systematic review. We aimed to improve the diagnosis and treatment of AE in clinical practice.
Search strategy and selection criteria
We conducted PubMed and Web of Science database searches in July 2024 for all the literature on pediatric patients with AE using the following key words: “acrodermatitis enteropathica” AND “pediatric” OR “infant” OR “toddler” OR “child” OR “adolescent.” The review protocol was registered in the Open Science Framework (Registration DOI: https://doi.org/10.17605/OSF.IO/BN3TD). Studies from all years were considered. A flowchart summarizing the process of selecting studies on AE in the pediatric population is presented in Figure 1.
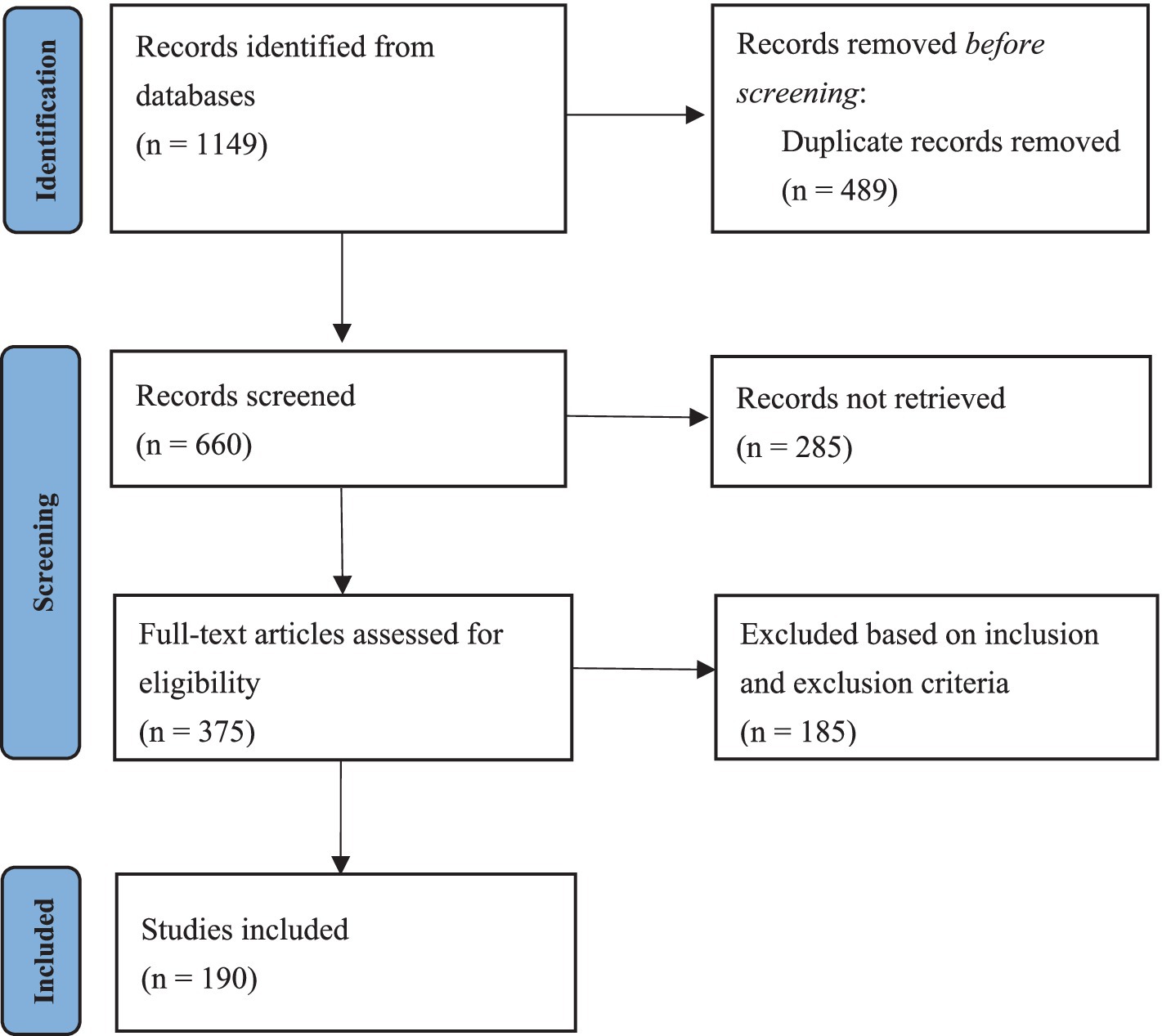
Figure 1. A flowchart summarizing the process of selecting patients with AE in the pediatric population based on the PRISMA guidelines.
Exclusion criteria
Studies were excluded according to the following criteria: age of onset over 18 years, review articles, or nondetailed cases.
Data collection
The patient data recorded included gender, age of onset and diagnosis, cutaneous characteristics, lesion location, and initial lesion site. Other documented clinical manifestations included diarrhea, alopecia, ocular involvement, growth retardation, and neurological complications. Laboratory and dermatological tests were used to assess blood zinc levels, alkaline phosphatase (ALP) levels, albumin levels, hemoglobin levels, SLC39A4 mutations, skin swab cultures, and biopsies. Additionally, medical history, treatment, and prognosis were recorded. Not all studies provided data on every measure, and the total number of patients included in each data analysis reflects the available records. Qualitative outcomes were analyzed descriptively, while quantitative outcomes were combined and analyzed using descriptive statistics. We performed the statistical analysis using SPSS 24.0 (IBM-SPSS, Chicago, USA). The chi-square test was applied in Tables 1, 2, with χ2 and p values calculated. A p value of less than 0.05 was considered statistically significant.
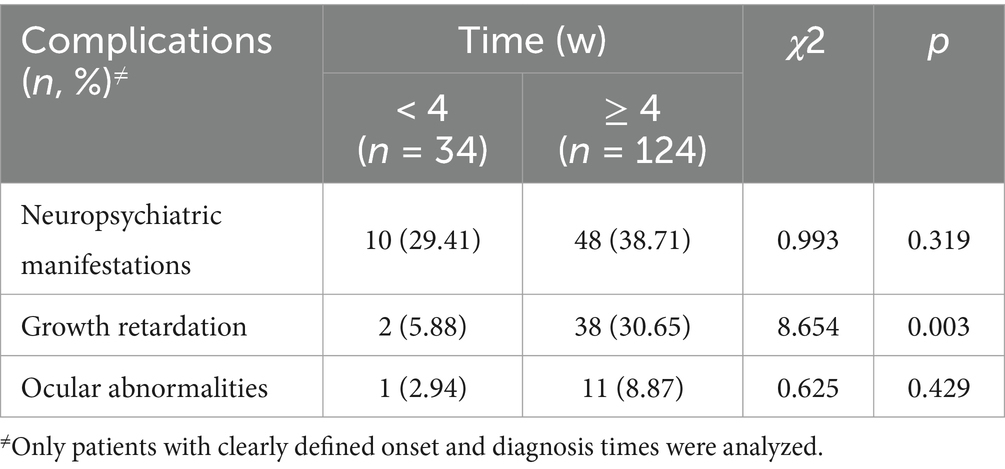
Table 1. Cross (Chi-square) analysis results of complications at different durations from onset to diagnosis.
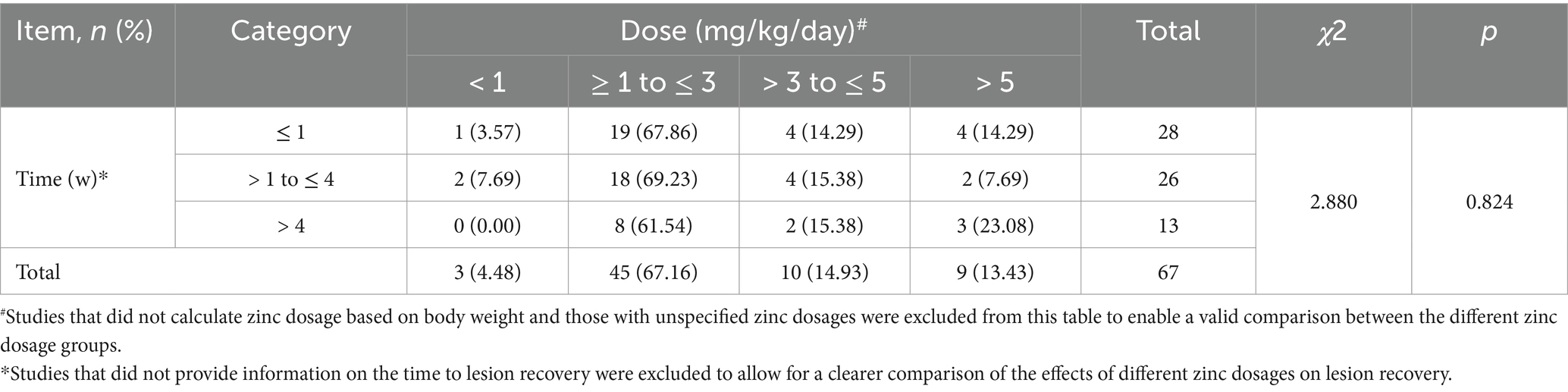
Table 2. Cross (Chi-square) analysis results of the effects of zinc supplementation on lesion recovery.
Results
The initial literature search yielded 660 results after removing duplicates. Manual screening resulted in 190 studies, including a total of 231 patients (129 boys, 55.8%) (7, 8, 11–198). Among these studies, 189 were isolated case reports, and 1 was a retrospective study. The age at onset was recorded for 179 patients, with a mean age of 10.5 months (range: 0.2–188 months). Among the 216 patients, 189 (87.5%) experienced their first episode of AE, whereas 27 (12.5%) experienced recurrence. In 164 patients with defined onset and diagnosis times, the average duration from onset to diagnosis was 40 weeks (range: 0.3–1,008 weeks). Table 3 summarizes the detailed information.
Clinical characteristics
Among the 231 pediatric patients included, all exhibited dermatitis, with 209 having additional clinical symptoms recorded. Diarrhea was present in 98 patients (46.9%), and alopecia was present in 88 patients (42.1%). Only 44 patients (21.1%) exhibited the triad of dermatitis, diarrhea, and alopecia simultaneously. Other symptoms included neuropsychiatric manifestations in 70 patients (33.5%), irritability in 62 patients (29.7%), and apathy or depression in 8 patients (3.8%). Growth retardation was noted in 49 patients (23.4%), fever in 35 patients (16.8%), and ocular abnormalities in 17 patients (8.1%). Ocular issues included photophobia in 7 patients (3.4%), blepharitis in 6 patients (2.9%), keratitis or conjunctivitis in 5 patients (2.4%), and decreased vision with macular atrophy in 1 patient (0.5%). Delayed puberty was observed in 2 patients (1.0%). Notably, 28 patients (13.4%) had dermatitis as the sole manifestation of AE. The incidence of clinical manifestations—neuropsychiatric manifestations, growth retardation, and ocular abnormalities—was compared between patients whose time from onset to diagnosis was ≥ 4 weeks and patients whose time from onset to diagnosis was < 4 weeks. Table 1 shows that a delayed diagnosis was associated with an increased risk of growth retardation (χ2 = 8.654, p = 0.003).
Dermatologic manifestations
Dermatitis can present in various forms among patients. A detailed analysis of the lesion morphology in 200 patients revealed that the most common lesion morphology was erythema, which was observed in 160 patients (80.0%). This was followed by desquamation in 101 patients (50.5%), blisters or vesicles in 85 patients (42.5%), erosion or ulceration in 65 patients (32.5%), pustules in 22 patients (11.0%), scaly macules in 16 patients (8.0%), exudation in 11 patients (5.5%), eczematous plaques in 3 patients (1.5%), pigmentation in 2 patients (1.0%) and depigmentation in 2 patients (1.0%).
In 225 patients whose skin lesion locations were identified, the lesions were most commonly present on the hands and feet and limbs (187 patients, 83.1%), followed by the perioral area (171 patients, 76.0%) and the perianal area (163 patients, 72.4%). Other locations included the perineum (133 patients, 59.1%), cheeks (91 patients, 40.4%), eye corners (54 patients, 24.0%), trunk (50 patients, 22.2%), auricles (29 patients, 12.9%), and occipital region (27 patients, 12.0%). Among the 80 patients for whom the sequence of lesion appearance was recorded, the lesions initially appeared in the perianal area in 28 patients (35.0%), the hands and feet and limbs in 19 patients (23.8%), the perioral area in 12 patients (15.0%), the cheeks in 9 patients (11.3%), the perineum in 7 patients (8.8%), the occipital region in 2 patients (2.5%), the trunk in 2 patients (2.5%), and the auricles in 1 patient (1.3%).
Dermatologic tests
Skin swab culture was performed in 54 out of 231 patients (23.4%), with 35 patients (64.8%) showing the growth of various bacterial and/or fungal organisms. Among these, single bacterial infection was the most common, observed in 17 patients (48.6%), followed by mixed bacterial infections in 8 patients (22.9%), bacterial and fungal coinfections in 6 patients (17.1%), and single fungal infection in 4 patients (11.4%). Among the 35 patients, Staphylococcus aureus was the most common pathogen, identified in 18 patients (51.4%), followed by Candida albicans in 8 patients (22.9%).
Skin biopsy was performed in 61 (26.4%) patients. A histopathological examination of biopsy samples supported the diagnosis of AE in 55 patients (90.2%); this examination typically revealed necrolysis in the upper part of the epidermis.
Laboratory abnormalities
Among 191 patients, 145 (75.9%) had zinc deficiency, with the lowest zinc level in the blood reported as 0.8 μg/dL (124) and the average level reported as 31.2 μg/dL. Among the 63 patients with reported causes of zinc deficiency, 37 cases (58.7%) were attributed to insufficient zinc intake, which included 24 cases (64.9%) related to low breast milk zinc levels. Additionally, 38 cases (60.3%) were attributed to impaired absorption, and 10 cases (15.9%) were attributed to increased demand. Furthermore, 13 patients (20.6%) presented with two or more contributing factors. Among the 87 patients whose ALP levels were recorded, 40 patients (46.0%) exhibited decreased ALP levels, with a minimum level of 15 U/L (94) and an average level of 74.3 U/L. Among the 55 patients with albumin measurements, 33 patients (60.0%) had reduced albumin levels, with a minimum level of 1.2 g/dL (70, 182, 184) and an average level of 2.0 g/dL. Hemoglobin levels were recorded in 63 patients, of whom 39 (61.9%) had decreased hemoglobin levels, with a minimum level of 5.5 g/dL (117) and an average level of 9.2 g/dL. Genetic information was identified in only 34 out of 231 cases (of which 156 were reported after the contribution of the SLC39A4 gene was established), and mutations in the SLC39A4 gene were confirmed in 32 of these individuals.
Associated conditions
Fifty-seven patients (24.7%) (8, 18, 19, 21, 23, 26, 35, 37, 44, 47, 56, 57, 70, 87, 91, 101, 102, 104, 106–108, 112, 117, 125, 134, 137, 138, 145, 148, 151, 165, 167, 169, 172, 179, 181, 182, 184, 186, 193) were diagnosed with metabolic diseases: cystic fibrosis (CF) was present in 18 patients (31.6%), followed by maple syrup urine disease (MSUD) in 15 patients (26.3%), methylmalonic acidemia (MMA) in 7 patients (12.3%), ornithine transcarbamylase deficiency (OTC) in 4 patients (7.0%), and propionic acidemia (PA) in 4 patients (7.0%). Additionally, phenylketonuria (PKU) was diagnosed in 3 patients (5.3%), whereas biotinidase deficiency, Hartnup disease, d < 1.063 lipoprotein deficiency, nonketotic hyperglycinemia, aminoacidemia, and type 1 glutaric acidemia were each diagnosed in 1 patient (1.8%). Among those with metabolic diseases, 11 patients (19.3%) had zinc deficiency, and 7 of these patients (63.6%) had CF. Additionally, 29 patients had normal zinc levels, while 17 patients did not have their zinc levels tested.
Twenty-eight patients (12.1%) (11, 27, 33, 36, 43, 49, 53, 66, 80, 86, 96, 100, 107, 109, 121, 123, 129, 152, 158, 159, 163, 168–171, 192, 195, 198) were documented as preterm, of whom 26 (92.9%) had zinc deficiency.
Twenty patients (8.7%) (39, 45–47, 50, 79, 90, 92, 140, 153, 166, 169, 175, 181, 184, 185, 191, 198) were diagnosed with gastrointestinal diseases, among whom 7 (35.0%) had received total parenteral nutrition. Zinc levels were not recorded in 1 patient (169), but zinc deficiency was observed in all of the remaining 19 patients (95.0%). No patients were found to have liver or kidney diseases.
Furthermore, malnutrition was identified in 9 patients (3.9%) (11, 32, 39, 65, 84, 87, 102, 127, 128), including 7 patients (77.8%) presenting with diarrhea, 1 patient (11.1%) who was diagnosed with MSUD (87), and 1 patient (11.1%) who was a preterm infant born at 31 weeks gestation (11). Zinc deficiency was observed in 6 of the malnourished patients (66.7%).
Treatment and prognosis
Among the 231 patients, 174 (75.3%) received zinc supplementation, including 137 patients (78.7%) with zinc deficiency, 25 patients (14.4%) with normal zinc levels, and 12 patients (6.9%) with unrecorded zinc levels; of the patients receiving zinc supplementation, 159 (91.4%) demonstrated therapeutic efficacy. Among the 25 patients with normal zinc levels who received supplementation, 21 patients (84.0%) responded effectively, whereas 4 patients (16.0%) had an ineffective response, all of whom also had concomitant metabolic disorders (35, 37, 44, 145).
Among the 174 patients who received zinc supplementation, 4 patients (2.3%) were treated with a zinc dose of < 1 mg/kg/day, 55 patients (31.6%) received a dose of ≥ 1 to ≤ 3 mg/kg/day, 20 patients (11.5%) received a dose of > 3 to ≤ 5 mg/kg/day, and 10 patients (5.8%) were treated with a dose of > 5 mg/kg/day (Table 3). In 85 patients (48.9%), either the zinc dose was not weight-adjusted or the dosage was not recorded. With respect to rash recovery time, 46 patients (26.4%) experienced resolution of the rash within 1 week, 50 patients (28.7%) recovered within 1–4 weeks, and 22 patients (12.6%) experienced resolution of the rash after more than 4 weeks. In 41 patients (23.6%), zinc supplementation was effective, but the time to rash resolution was not specified. For 5 patients (2.9%), the outcome was unknown. Zinc supplementation was ineffective in 10 patients (5.8%), all of whom had metabolic disorders, including CF, PA, MMA, MUSD, nonketotic hyperglycinemia and aminoacidemia. We compared the effects of different doses of zinc supplementation on lesion recovery time, as shown in the chi-square analysis in Table 2, and found that different dosages of zinc had no significant effect on lesion recovery time (χ2 = 2.880, p = 0.824).
Thirty out of 32 (93.8%) patients with SLC39A4 gene mutations received zinc supplementation, with 29 patients (96.7%) showing improvement (12, 15, 25, 29, 61, 65, 68, 71, 73, 75, 77, 80, 95, 98, 110, 118, 120, 130, 135, 143, 156, 173, 187–189, 194). Only one patient did not respond to zinc treatment and subsequently died (164). Among these patients, 12 (40.0%) were treated with a zinc dose of ≥ 1 to ≤ 3 mg/kg/day, whereas 6 patients (20.0%) received a dose of > 3 mg/kg/day. In 12 patients (40.0%), the zinc dosage was either not weight-adjusted or was not recorded. Among the 29 patients who recovered, 12 (41.4%) achieved resolution within 1–4 weeks, 5 patients (17.2%) experienced resolution after more than 4 weeks, and the resolution time was unspecified for 12 patients (41.4%).
Among the 57 patients with metabolic diseases, 53 received treatment, 21 of whom received zinc supplementation. Among these patients, only 2 patients (9.5%) improved (47, 169), 5 patients (23.8%) experienced ineffective zinc supplementation and subsequently died from sepsis or metabolic decompensation (37, 104, 112, 125), 5 patients (23.8%) recovered after correcting metabolic disorders following ineffective zinc treatment (35, 44, 117, 134, 145), and 9 patients (42.9%) recovered with a combination of zinc supplementation and correction of metabolic imbalances (8, 18, 47, 57, 107, 108, 167, 181, 186). In contrast, 32 patients were solely treated for metabolic imbalances, and all (100%) recovered (19, 21, 23, 26, 37, 56, 70, 87, 91, 104, 106, 137, 138, 148, 151, 172, 179, 182).
All 26 (100%) premature infants with zinc deficiency improved after zinc supplementation. Among patients with gastrointestinal diseases, 18 (90%) received zinc supplementation, and all (100%) demonstrated improvement (39, 45, 47, 50, 79, 90, 92, 140, 153, 166, 169, 175, 181, 185, 191, 198). Three malnourished patients who received zinc supplementation (100.0%) showed improvement. Similarly, all patients (100%) with zinc deficiency due to low breast milk zinc levels improved after zinc supplementation (27, 28, 33, 42, 49, 96, 98, 103, 110, 118, 121, 126, 129, 133, 154, 158, 160, 170, 176, 178, 183, 198).
In summary, there are significant differences in treatment methods, efficacy, and prognosis for AE based on their various etiologies. Figure 2 provides a brief summary of this information.
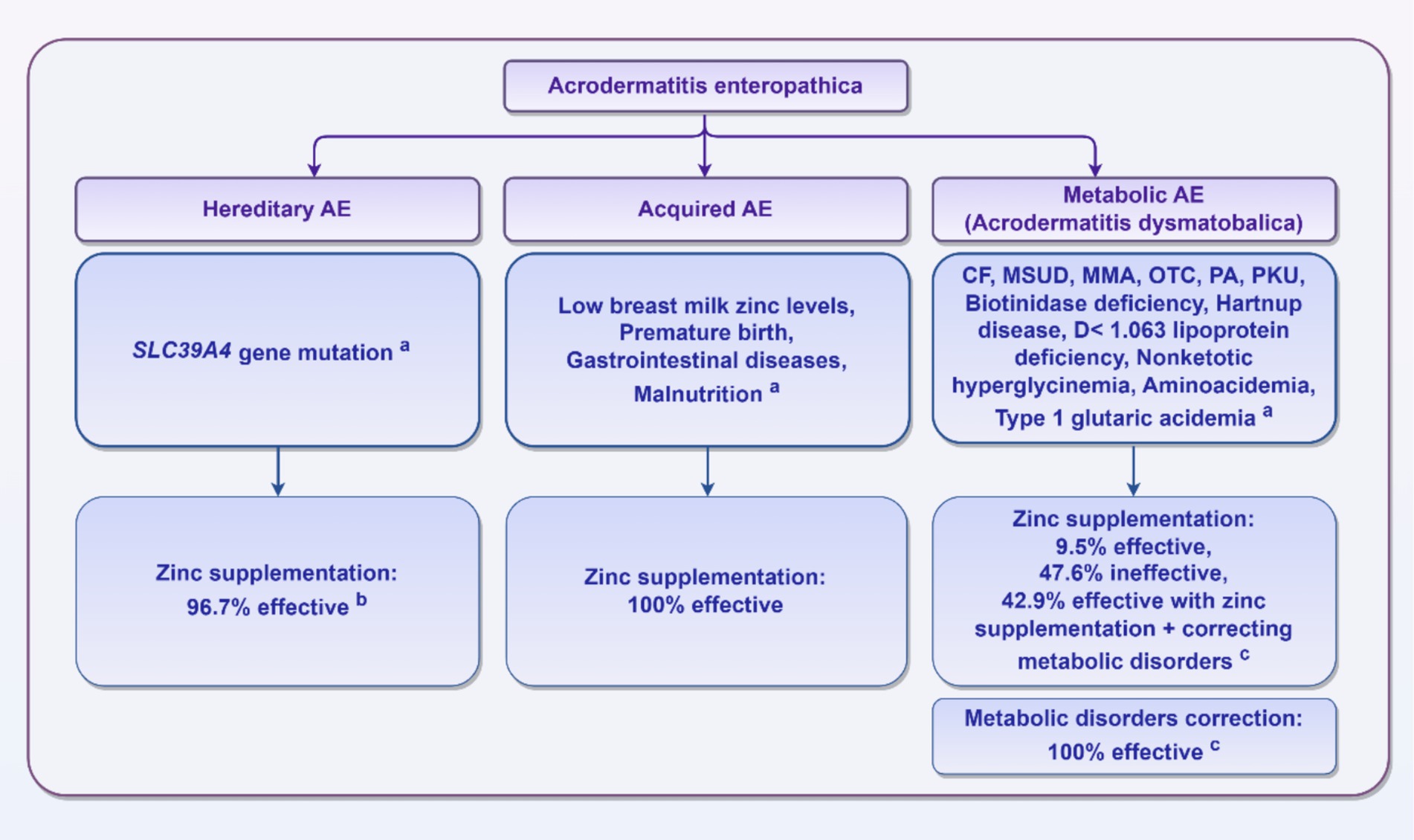
Figure 2. AE classification and treatment effects. aThese reasons are limited to the specific reasons mentioned in the published literature summarized in this review and do not include unreported cases. bThirty patients with SLC39A4 gene mutations received zinc supplementation, with 29 patients (96.7%) showing improvement; one patient did not respond to zinc treatment and subsequently died. cAmong the 57 patients with metabolic diseases, 53 patients received treatment, including 21 patients who received zinc supplementation. Among these, only 2 patients (9.5%) showed improvement, 10 patients (47.6%) had an ineffective treatment response, and 9 patients (42.9%) recovered with a combination of zinc supplementation and correction of metabolic imbalances. In contrast, 32 patients were solely treated for metabolic imbalances, and all (100%) recovered.
Discussion
The diagnosis of AE relies on clinical dermatological characteristics, with the presence of zinc deficiency often supporting the clinical diagnosis (10). According to our analysis, zinc deficiency was observed in only 75.9% of cases, which is consistent with previously reported findings (199). Our results indicate that the two major causes of zinc deficiency are insufficient intake and impaired absorption. Zinc deficiency occurs when the zinc supply fails to meet the physiological needs of children. Among the various causes, low zinc levels in breast milk are particularly common, which may be partly attributed to mutations in the ZNT2 gene, which is responsible for supplying human milk with zinc (200–202). Furthermore, zinc absorption disorders are prevalent in genetic AE and various gastrointestinal diseases, where damage to intestinal epithelial cells and their barrier leads to impaired zinc absorption. The patients in our review included those with gastrointestinal conditions such as intestinal immaturity in preterm infants, celiac disease, infantile refractory diarrhea, neonatal necrotizing enterocolitis (NEC), food allergies, and postintestinal resection (25, 27, 29, 36, 39, 46, 47, 65, 68, 71, 100, 159, 166).
Consistent with previous reports (203), the triad of dermatitis, diarrhea, and alopecia simultaneously appeared in 21.1% of patients. In addition, neuropsychiatric manifestations (33.5%) were the most prominent complications of AE, with growth restrictions observed in more than 20% of patients. Remarkably, 13.4% of patients presented with dermatitis as the only symptom of AE. After analyzing the characteristics of these 28 patients, we found that patients with only skin symptoms were all first-episode AE patients (8, 12, 25, 30, 31, 37, 59, 86, 89, 97, 103, 104, 126, 129, 134, 137, 138, 159, 163, 165, 175–177, 198), and the time from the onset of symptoms to diagnosis was relatively short (3.8 weeks, range: 0.7–8 weeks). These findings suggest that AE should be considered even in the absence of the full triad, particularly in first-onset cases. Early diagnosis and treatment can help avoid adverse complications and improve patient prognosis, emphasizing the need for clinician vigilance.
Currently, zinc supplementation remains the primary treatment approach for AE. Based on our data, 174 AE patients received zinc supplementation at varying dosages, with 159 (91.4%) demonstrating therapeutic efficacy. Interestingly, this group also included 21 patients (13.2%) who had normal zinc levels, suggesting that zinc levels in the blood should not be the sole indicator of zinc deficiency. Patients with normal zinc blood levels may still benefit from zinc supplementation, and a comprehensive assessment should consider the patient’s response to zinc supplementation as well. In terms of dosage distribution, when cases with unclear zinc supplementation doses were excluded, the most commonly used dosage for AE, including hereditary AE, was 1–3 mg/kg/day, which was administered in 61.8% (55/89) of the patients receiving supplementation. Table 2 further confirms the efficacy and appropriateness of the 1–3 mg/kg/day dosage in AE treatment. With respect to lesion recovery time, after excluding patients with unrecorded recovery times and unrecorded therapeutic efficacy, the majority of patients (81.4%, 96/118) recovered within 4 weeks, with nearly half of the patients (47.9%, 46/96) recovering within 1 week. However, no patients with hereditary AE were observed to recover within 1 week after zinc supplementation, suggesting that it may take more time to replenish the zinc transport pool. In summary, zinc supplementation has shown significant efficacy, with 1–3 mg/kg/day reported as the most common dosage; however, individualized dose adjustment is necessary, particularly for the long-term management of patients with hereditary AE.
As mentioned earlier, reports have indicated that metabolic disorders can mimic the manifestations of AE, a condition that Tabanhoglu and colleagues referred to as acrodermatitis dysmetabolica (19). Consistent with these findings, our analysis of 57 cases of AE associated with metabolic disorders revealed that the majority of cases occurring during the decompensation of metabolic diseases exhibited improvement following targeted nutrient supplementation. These metabolic diseases include, but are not limited to, CF, MSUD, MMA, OTC, PA, and PKU. In fact, the most important aspect of managing these metabolic diseases is dietary control, particularly the use of specific formulas during early life. However, if monitoring is insufficient, it can easily result in deficiencies in amino acids or lead to malnutrition. Some factors such as pancreatic enzymes (204) and albumin (205, 206) have been reported to affect zinc absorption and bioavailability, and zinc ionophores such as amino acids and peptides have been documented to enhance zinc absorption (207). Furthermore, some essential amino acids, such as isoleucine and valine, are essential for keratinocyte metabolism (208). As shown in the results, most patients with AE and metabolic disorders recovered by solely addressing their metabolic imbalances, which included supplementation with essential amino acids or pancreatic enzymes. Additionally, a few patients only showed improvement after supplementing with pancreatic enzymes or amino acids when zinc supplementation was ineffective. Therefore, it is reasonable to hypothesize that certain amino acid metabolic abnormalities that occur during the inadequate management of metabolic diseases contribute directly to AE. However, at present, the molecular mechanisms of nonhereditary AE have not been sufficiently studied, particularly AE secondary to metabolic disorders. In the future, research can further explore the relationship between metabolic disorders, zinc metabolism, and AE manifestations.
In summary, AE is classified into three types: hereditary, acquired, and metabolic AE (Acrodermatitis dysmetabolica). Hereditary AE results from inherited zinc malabsorption, often due to mutations in the SLC39A4 gene. It responds well to zinc supplementation, which usually requires lifelong treatment. Acquired AE is caused by low zinc intake, which may result from factors like low zinc levels in breast milk, prematurity, gastrointestinal diseases, or malnutrition. It also responds to zinc supplementation. Metabolic AE occurs alongside metabolic comorbidities, treatment may focus solely on correcting metabolic imbalances, or it may include zinc supplementation. Clinicians should be vigilant to identify AE subtype early and provide proper treatment to avoid adverse complications and improve patient prognosis. During the clinical diagnosis and treatment process, physicians should develop a reasonable therapeutic strategy by integrating a comprehensive patient history and a methodological collaborative approach, including laboratory tests, screening for genetic metabolic disorders, and genetic analysis.
Limitations
This literature review has several limitations, including publication bias and the predominance of case reports. Additionally, there is limited information regarding SLC39A4 mutations, making it difficult to analyze the incidence of hereditary AE. Furthermore, there is insufficient quantitative data for statistical analysis, particularly concerning different dosages and recovery times. Another limitation is that given the increased understanding of metabolic diseases in recent years, several metabolic disorders related to AE symptoms have been identified. While this review aims to include such patients as comprehensively as possible, it is possible that some cases may not have been captured.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
WC: Conceptualization, Data curation, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. NW: Data curation, Investigation, Methodology, Validation, Visualization, Writing – original draft. MS: Data curation, Investigation, Methodology, Validation, Visualization, Writing – original draft. XX: Supervision, Validation, Visualization, Writing – review & editing. HJ: Conceptualization, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by National Natural Science Foundation of China (No. 81300130), Basic Research Projects of Liaoning Province (JYTMS20230073) and Technology Research and Development Program Project of Liaoning Province (2024JH2/102600318).
Acknowledgments
We would like to express our heartfelt gratitude to Xinghua Gao, Chair at China Medical University (PRC) and head of the NHC Key Laboratory of Immunodermatology, as well as the National Joint Engineering Research Center for Theranostics of Immunological Skin Diseases, for his valuable insights and for proofreading the article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Dillaha, CJ, Lorincz, AL, and Aavik, OR. Acrodermatitis enteropathica; review of the literature and report of a case successfully treated with diodoquin. JAMA J Am Med Assoc. (1953) 152:509–12. doi: 10.1001/jama.1953.03690060025009
3. Moynahan, EJ. Letter: Acrodermatitis enteropathica: a lethal inherited human zinc-deficiency disorder. Lancet. (1974) 2:399–400. doi: 10.1016/s0140-6736(74)91772-3
4. Arakawa, T, Tamura, T, Igalesioni, Y, Suzuki, H, and Sandstead, HH. Zinc deficiency in two infants during total parenteral alimentation for diarrhea. Am J Clin Nutr. (1976) 29:197–204. doi: 10.1093/ajcn/29.2.197
5. Tucker, SB, Schroeter, AL, Brown, PW Jr, and McCall, JT. Acquired zinc deficiency. Cutaneous manifestations typical of acrodermatitis enteropathica. JAMA. (1976) 235:2399–402. doi: 10.1001/jama.235.22.2399
6. Wang, K, Zhou, B, Kuo, YM, Zemansky, J, and Gitschier, J. A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. Am J Hum Genet. (2002) 71:66–73. doi: 10.1086/341125
7. Hansen, RC, Lemen, R, and Revsin, B. Cystic fibrosis manifesting with acrodermatitis enteropathica-like eruption. Association with essential fatty acid and zinc deficiencies. Arch Dermatol. (1983) 119:51–5. doi: 10.1001/archderm.1983.01650250055016
8. Giacoia, GP, and Berry, GT. Acrodermatitis enteropathica-like syndrome secondary to isoleucine deficiency during treatment of maple syrup urine disease. Am J Dis Child. (1993) 147:954–6. doi: 10.1001/archpedi.1993.02160330044015
9. Bosch, AM, Sillevis Smitt, JH, Van Gennip, AH, Abeling, NG, Schutgens, RB, Bakker, HD, et al. Iatrogenic isolated isoleucine deficiency as the cause of an acrodermatitis enteropathica-like syndrome. Br J Dermatol. (1998) 139:488–91. doi: 10.1046/j.1365-2133.1998.02415.x
10. Jagadeesan, S, and Kaliyadan, F. Acrodermatitis Enteropathica [Updated 2023 Apr 3]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing. (2025).
11. Gu, L, He, XH, and Zhu, P. Analysis of similarities and differences between transient symptomatic zinc deficiency and acrodermatitis enteropathica in children: a case report of a Chinese Yi-ethnic infant. BMC Pediatr. (2024) 24:338. doi: 10.1186/s12887-024-04830-y
12. Cleminson, K, Hull, PR, Price, E, and Green, PJ. Acute onset of blisters in an infant with acrodermatitis enteropathica: a case report. SAGE Open Med Case Rep. (2021) 9:2050313X20984119. doi: 10.1177/2050313X20984119
13. Portnoy, B, and Marsden, CW. Acrodermatitis enteropathica without diarrhea. Arch Dermatol. (1961) 83:420–4. doi: 10.1001/archderm.1961.01580090070008
14. Cash, R, and Berger, CK. Acrodermatitis enteropathica: defective metabolism of unsaturated fatty acids. J Pediatr. (1969) 74:717–29. doi: 10.1016/s0022-3476(69)80134-4
15. Vardi, A, Anikster, Y, Eisenkraft, A, Shohat, M, Abu-Much, J, Eisenkraft, S, et al. A new genetic isolate of acrodermatitis enteropathica with a novel mutation. Br J Dermatol. (2009) 160:1346–8. doi: 10.1111/j.1365-2133.2009.09128.x
16. Romaine, RA, and Lachman, AB. Letter: Acrodermatitis enteropathica-like clinical findings in two children. Arch Dermatol. (1974) 109:96–7. doi: 10.1001/archderm.1974.01630010068022
17. Bartosinska, J, Chodorowska, G, Jazienicka, I, Pucuła, J, Prystupa, A, Wawrzycki, B, et al. Skin lesions in a 16-month-old toddler with impaired zinc absorption. Adv Med Sci. (2011) 56:369–72. doi: 10.2478/v10039-011-0021-3
18. Muñiz, AE, Bartle, S, and Foster, R. Edema, anemia, hypoproteinemia, and acrodermatitis enteropathica: an uncommon initial presentation of cystic fibrosis. Pediatr Emerg Care. (2004) 20:112–4. doi: 10.1097/01.pec.0000113881.10140.50
19. Tabanlıoğlu, D, Ersoy-Evans, S, and Karaduman, A. Acrodermatitis enteropathica-like eruption in metabolic disorders: acrodermatitis dysmetabolica is proposed as a better term. Pediatr Dermatol. (2009) 26:150–4. doi: 10.1111/j.1525-1470.2008.00803.x
20. Ciampo, IRLD, Sawamura, R, Ciampo, LAD, and Fernandes, MIM. Acrodermatitis Enteropathica: clinical manifestations and pediatric diagnosis. Rev Paul Pediatr. (2018) 36:238–41. doi: 10.1590/1984-0462/;2018;36;2;00010
21. Puzenat, E, Durbise, E, Fromentin, C, Humbert, P, and Aubin, F. Iatrogenic acrodermatitis enteropathica-like syndrome in leucinosis. Ann Dermatol Venereol. (2004) 131:801–4. doi: 10.1016/s0151-9638(04)93764-7
22. Bronson, DM, Barsky, R, and Barsky, S. Acrodermatitis enteropathica. J Am Acad Dermatol. (1983) 9:140–4. doi: 10.1016/s0190-9622(83)70120-9
23. Patra, S, Senthilnathan, G, and Bhari, N. Acrodermatitis enteropathica-like skin eruption with neonatal seizures in a child with biotinidase deficiency. Clin Exp Dermatol. (2020) 45:266–7. doi: 10.1111/ced.14053
24. Mack, D, Koletzko, B, Cunnane, S, Cutz, E, and Griffiths, A. Acrodermatitis enteropathica with normal serum zinc levels: diagnostic value of small bowel biopsy and essential fatty acid determination. Gut. (1989) 30:1426–9. doi: 10.1136/gut.30.10.1426
25. Nakano, A, Nakano, H, Nomura, K, Toyomaki, Y, and Hanada, K. Novel SLC39A4 mutations in acrodermatitis enteropathica. J Invest Dermatol. (2003) 120:963–6. doi: 10.1046/j.1523-1747.2003.12243.x
26. Lemke, J, Gaertner, M, Kemen, C, and Höger, PH. Clinical diagnosis of mucoviscidosis in false-negative mucoviscidosis neonatal screening. Monatsschr Kinderheilkd. (2018) 166:889–93. doi: 10.1007/s00112-017-0344-7
27. Benedix, F, Hermann, U, Brod, C, Metzler, G, Sönnichsen, C, Röcken, M, et al. Transient zinc deficiency in preterm infants. Hautarzt. (2008) 59:563–6. doi: 10.1007/s00105-007-1409-7
28. Kaur, S, Thami, GP, and Kanwar, AJ. Acrodermatitis enteropathica in a full-term breast-fed infant. Indian J Pediatr. (2002) 69:631–3. doi: 10.1007/BF02722694
29. Coromilas, A, Brandling-Bennett, HA, Morel, KD, and Chung, WK. Novel SLC39A4 mutation in acrodermatitis enteropathica. Pediatr Dermatol. (2011) 28:697–700. doi: 10.1111/j.1525-1470.2011.01637.x
30. Kelly, R, Davidson, GP, Townley, RR, and Campbell, PE. Reversible intestinal mucosal abnormality in acrodermatitis enteropathica. Arch Dis Child. (1976) 51:219–22. doi: 10.1136/adc.51.3.219
31. Wiznia, LE, Bhansali, S, Brinster, N, Al-Qaqaa, YM, Orlow, SJ, and Oza, V. Acquired acrodermatitis enteropathica due to zinc-depleted parenteral nutrition. Pediatr Dermatol. (2019) 36:520–3. doi: 10.1111/pde.13865
32. Koletzko, B, Bretschneider, A, and Bremer, HJ. Fatty acid composition of plasma lipids in acrodermatitis enteropathica before and after zinc supplementation. Eur J Pediatr. (1985) 143:310–4. doi: 10.1007/BF00442310
33. Stapleton, KM, O'Loughlin, E, and Relic, JP. Transient zinc deficiency in a breast-fed premature infant. Australas J Dermatol. (1995) 36:157–9. doi: 10.1111/j.1440-0960.1995.tb00959.x
34. Satria, B, Chen, W, Soebono, H, Radiono, S, and Danarti, R. Concurrence of Acrodermatitis Enteropathica and eczema Herpeticum in a child with atopic dermatitis. Case Rep Dermatol. (2019) 11:240–8. doi: 10.1159/000502509
35. Page, SS, and Foster, R. Acrodermatitis dysmetabolica in a child with cystic fibrosis. Pediatr Dermatol. (2016) 33:e93–4. doi: 10.1111/pde.12789
36. Blom, I, Jameson, S, Krook, F, Larsson-Stymne, B, and Wranne, L. Zinc deficiency with transitory acrodermatitis enteropathica in a boy of low birth weight. Br J Dermatol. (1981) 104:459–64. doi: 10.1111/j.1365-2133.1981.tb15318.x
37. De Raeve, L, De Meirleir, L, Ramet, J, Vandenplas, Y, and Gerlo, E. Acrodermatitis enteropathica-like cutaneous lesions in organic aciduria. J Pediatr. (1994) 124:416–20. doi: 10.1016/s0022-3476(94)70364-7
38. Whitmore, CW. Probable acrodermatitis enteropathica treated with nystatin (mycostain). AMA Arch Derm. (1959) 79:594. doi: 10.1001/archderm.1959.01560170092020
39. Mishra, P, Sirka, CS, Das, RR, and Nanda, D. Secondary acrodermatitis enteropathica-like lesions in a child with newly diagnosed coeliac disease. Paediatr Int Child Health. (2016) 36:72–5. doi: 10.1179/2046905514Y.0000000168
40. Cameron, JD, and McClain, CJ. Ocular histopathology of acrodermatitis enteropathica. Br J Ophthalmol. (1986) 70:662–7. doi: 10.1136/bjo.70.9.662
41. Alvares, M, Kao, L, Mittal, V, Wuu, A, Clark, A, and Bird, JA. Misdiagnosed food allergy resulting in severe malnutrition in an infant. Pediatrics. (2013) 132:e229–32. doi: 10.1542/peds.2012-2362
42. Ohlsson, A. Acrodermatitis enteropathica reversibility of cerebral atrophy with zinc therapy. Acta Paediatr Scand. (1981) 70:269–73. doi: 10.1111/j.1651-2227.1981.tb05556.x
43. Nistor, N, Ciontu, L, Frasinariu, OE, Lupu, VV, Ignat, A, and Streanga, V. Acrodermatitis Enteropathica: a case report. Medicine (Baltimore). (2016) 95:e3553. doi: 10.1097/MD.0000000000003553
44. Flores, K, Chikowski, R, and Morrell, DS. Acrodermatitis dysmetabolica in an infant with maple syrup urine disease. Clin Exp Dermatol. (2016) 41:651–4. doi: 10.1111/ced.12876
45. Proli, F, Margiotta, G, Ferretti, S, Drosi, A, Valentini, P, Buonsenso, D, et al. Acrodermatitis enteropathica during parenteral nutrition: a pediatric case report. Acta Biomed. (2023) 94:e2023180. doi: 10.23750/abm.v94iS1.14489
46. Morishima, T, Yagi, S, Kuwabara, K, Endo, M, and Takemura, T. An acquired form of acrodermatitis enteropathica due to longterm lactose-free milk alimentation. J Dermatol. (1980) 7:121–5. doi: 10.1111/j.1346-8138.1980.tb01954.x
47. Martin, DP, Tangsinmankong, N, Sleasman, JW, Day-Good, NK, and Wongchantara, DR. Acrodermatitis enteropathica-like eruption and food allergy. Ann Allergy Asthma Immunol. (2005) 94:398–401. doi: 10.1016/S1081-1206(10)60994-5
48. Mohammed, J, Mehrotra, S, Schulz, H, and Lim, R. Severe infant lesion resistant to therapy due to zinc deficiency. Pediatr Emerg Care. (2017) 33:582–4. doi: 10.1097/PEC.0000000000001218
49. Stevens, J, and Lubitz, L. Symptomatic zinc deficiency in breast-fed term and premature infants. J Paediatr Child Health. (1998) 34:97–100. doi: 10.1046/j.1440-1754.1998.00164.x
50. Jat, KR, Marwaha, RK, Panigrahi, I, and Kaur, S. Fulminant candida infection in an infant with acrodermatitis enteropathica. Indian J Pediatr. (2009) 76:941–2. doi: 10.1007/s12098-009-0145-7
51. Chen, MY, James, K, Hsu, LL, Chang, SW, Huang, LH, and Wang, EK. Health-related behavior and adolescent mothers. Public Health Nurs. (2005) 22:280–8. doi: 10.1111/j.0737-1209.2005.220403.x
52. Braun, OH, Heilmann, K, Pauli, W, Rossner, JA, and Bergmann, KE. Acrodermatitis enteropathica: recent findings concerning clinical features, pathogenesis, diagnosis and therapy. Eur J Pediatr. (1976) 121:247–61. doi: 10.1007/BF00443018
53. Niemi, KM, Anttila, PH, Kanerva, L, and Johansson, E. Histopathological study of transient acrodermatitis enteropathica due to decreased zinc in breast milk. J Cutan Pathol. (1989) 16:382–7. doi: 10.1111/j.1600-0560.1989.tb00590.x
54. Krieger, I, and Evans, GW. Acrodermatitis enteropathica without hypozincemia: therapeutic effect of a pancreatic enzyme preparation due to a zinc-binding ligand. J Pediatr. (1980) 96:32–5. doi: 10.1016/s0022-3476(80)80319-2
55. Rogers, M. Acrodermatitis enteropathica. Australas J Dermatol. (1980) 21:71–5. doi: 10.1111/j.1440-0960.1980.tb00145.x
56. Rosa, J, Fraga, AB, de Carvalho, R, Maia, AL, Rodrigues, AL, and Gomes, MF. Acrodermatitis dysmetabolica as a sign of methylmalonic aciduria decompensation. Clin Case Rep. (2018) 6:1048–50. doi: 10.1002/ccr3.1509
57. Seyhan, ME, Selimoglu, MA, Ertekin, V, Fidanoğlu, O, and Altinkaynak, S. Acrodermatitis enteropathica-like eruptions in a child with hartnup disease. Pediatr Dermatol. (2006) 23:262–5. doi: 10.1111/j.1525-1470.2006.00231.x
58. Mortimer, PS, Gough, P, Newbold, PC, Dawber, RP, and Ryan, TJ. Acrodermatitis enteropathica. J R Soc Med. (1984) 77:67–8. doi: 10.1177/014107688407700117
59. Karadag, AS, Bilgili, SG, and Calka, O. Acrodermatitis enteropathica in three siblings. Indian J Dermatol Venereol Leprol. (2013) 79:268. doi: 10.4103/0378-6323.107667
60. Ruiz-Maldonado, R, and Tamayo, L. Zinc therapy of acrodermatitis enteropathica. Int J Dermatol. (1978) 17:423–6. doi: 10.1111/ijd.1978.17.5.423
61. Alwadany, MM, Al Wadani, AF, Almarri, FH, Alyami, HS, and Al-Subaie, MA. Acrodermatitis Enteropathica: a rare case with lifelong implications. Cureus. (2023) 15:e37783. doi: 10.7759/cureus.37783
62. Bock, DE, Prabhakaran, V, and Filler, G. Picture of the month: severe zinc deficiency in infancy (acrodermatitis enteropathica-like picture). Arch Pediatr Adolesc Med. (2009) 163:765–6. doi: 10.1001/archpediatrics.2009.126-a
63. Dev, T, and Sethuraman, G. Diagnosis of acrodermatitis enteropathica in resource limited settings. BMJ Case Rep. (2017) 2017:bcr2017220928. doi: 10.1136/bcr-2017-220928
64. Der Kaloustian, VM, Musallam, SS, Sanjad, SA, Murib, A, Hammad, WD, and Idriss, ZH. Oral treatment of acrodermatitis enteropathica with zinc sulfate. Am J Dis Child. (1976) 130:421–3. doi: 10.1001/archpedi.1976.02120050079015
65. S,, Taskesen, M, Coskun, T, Gürakan, F, Tokatli, A, and Sivri, HS. A zinc Sulphate-resistant Acrodermatitis Enteropathica patient with a novel mutation in SLC39A4 gene. JIMD Rep. (2012) 2:25–8. doi: 10.1007/8904_2011_38
66. Borroni, G, Brazzelli, V, Vignati, G, accone, C, Vignoli, GP, and Rabbiosi, G. Bullous lesions in acrodermatitis enteropathica. Histopathologic findings regarding two patients. Am J Dermatopathol. (1992) 14:304–9. doi: 10.1097/00000372-199208000-00003
67. Hussain, T, Kumar, J, Aslam, T, Khan, S, Ishaq, M, and Kumar, D. A rare case of severe Acrodermatitis Enteropathica during Covid-19 lockdown. J Ayub Med Coll Abbottabad. (2022) 34:880–2. doi: 10.55519/JAMC-04-10371
68. Hammersen, J, Has, C, Galiano, M, Lindner, M, Rossi, R, Kohlhase, J, et al. Sustained need for high-dose zinc supplementation in children with Acrodermatitis Enteropathica. Clin Pediatr (Phila). (2018) 57:99–102. doi: 10.1177/0009922816685820
69. Inamadar, AC, and Palit, A. Acrodermatitis enteropathica with depigmented skin lesions simulating vitiligo. Pediatr Dermatol. (2007) 24:668–9. doi: 10.1111/j.1525-1470.2007.00567.x
70. Lovett, A, Kokta, V, and Maari, C. Diffuse dermatitis: an unexpected initial presentation of cystic fibrosis. J Am Acad Dermatol. (2008) 58:S1–4. doi: 10.1016/j.jaad.2006.04.012
71. Hua, W, Zou, J, Zhuang, Y, and Zhou, T. Case report: Acrodermatitis enteropathica result from a novel SLC39A4 gene mutation. Front Pediatr. (2022) 10:972030. doi: 10.3389/fped.2022.972030
72. Khanna, U, Dhali, TK, and D'Souza, P. Scaly erythematous perineal dermatitis in twins. Clin Pediatr (Phila). (2015) 54:192–3. doi: 10.1177/0009922814543326
73. Zhou, XY, Chen, XJ, Wang, S, Xue, J, Liu, W, Wang, Q, et al. One recurrent homozygous mutation of SLC39A4 in a girl with acrodermatitis enteropathica from southwestern China. Int J Dermatol. (2016) 55:223–5. doi: 10.1111/ijd.12442
74. al, A, al, M, and Kaliyadan, F. Acrodermatitis enteropathica in a pair of twins. J Dermatol Case Rep. (2016) 10:65–7. doi: 10.3315/jdcr.2016.1238
75. Garza-Rodriguez, V, de la Fuente-Garcia, A, Liy-Wong, C, Küry, S, Schmitt, S, Jamall, IS, et al. Acrodermatitis Enteropathica: a novel SLC39A4 gene mutation in a patient with Normal zinc levels. Pediatr Dermatol. (2015) 32:e124–5. doi: 10.1111/pde.12555
76. Garretts, M, and Molokhia, M. Acrodermatitis enteropathica without hypozincemia. J Pediatr. (1977) 91:492–4. doi: 10.1016/s0022-3476(77)81333-4
77. Meftah, SP, Kuivaniemi, H, Tromp, G, Kerkeni, A, Sfar, MT, Ayadi, A, et al. A new mutation in exon 3 of the SCL39A4 gene in a Tunisian family with severe acrodermatitis enteropathica. Nutrition. (2006) 22:1067–70. doi: 10.1016/j.nut.2006.05.008
78. Portnoy, B, and Molokhia, M. Acrodermatitis enteropathica treated by zinc. Br J Dermatol. (1974) 91:701–3. doi: 10.1111/j.1365-2133.1974.tb12458.x
79. Sawai, T, Sugiura, H, Danno, K, Uchiyama, M, and Ohta, S. Acquired acrodermatitis enteropathica during chemotherapy for acute lymphocytic leukaemia in a child with down syndrome. Br J Dermatol. (1996) 135:659–60. doi: 10.1111/j.1365-2133.1996.tb03862.x
80. Li, KY, Tang, JP, Shu, Y, Yue, SZ, Wang, YW, Wen, R, et al. Recurrent systemic sporadic lesion for 10 years in a girl aged 11 years. Chin J Contemp Pediatr. (2022) 24:1047–52. doi: 10.7499/j.issn.1008-8830.2204123
81. Paul, SP, Vamvakiti, E, Atherton, DJ, and Candy, DC. Acrodermatitis enteropathica variant with borderline plasma zinc concentrations. J Pediatr Gastroenterol Nutr. (2011) 52:630. doi: 10.1097/MPG.0b013e3182034cd6
82. Garcia-Perez, A, Castro, C, Franco, A, and Escribano, R. A case of optic atrophy possibly induced by quinoline in acrodermatitis enteropathica. Br J Dermatol. (1974) 90:453–5. doi: 10.1111/j.1365-2133.1974.tb06433.x
83. Oh, KI, Kim, JH, Lee, JE, Lim, DH, and Son, BK. A case of acquired acrodermatitis enteropathica with a normal serum zinc level but a low level in the hair. Korean J Pediatr. (2007) 50:209–12. doi: 10.3345/kjp.2007.50.2.209
84. Rodin, AE, and Goldman, AS. Autopsy findings in acrodermatitis enteropathica. Am J Clin Pathol. (1969) 51:315–22. doi: 10.1093/ajcp/51.3.315
85. Mostafa, WZ, and al-Zayer, AA. Acrodermatitis enteropathica in Saudi Arabia. Int J Dermatol. (1990) 29:134–8. doi: 10.1111/j.1365-4362.1990.tb04086.x
86. Sepehr, A, and Burnett, M. Bullous acrodermatitis enteropathica. J Cutan Pathol. (2008) 35:120. doi: 10.1111/j.1600-0560.2007.00929.x
87. Oeztuerk, Y. Acrodermatitis enteropathica-like syndrome secondary to branched-chain amino acid deficiency in inborn errors of metabolism. Pediatr Dermatol. (2008) 25:415. doi: 10.1111/j.1525-1470.2008.00707.x
88. Aggett, PJ, Delves, HT, Thorn, JM, Atherton, DJ, Harris, JT, and Bangham, AD. The therapeutic effect of amphotericin in acrodermatitis enteropathica: hypothesis and implications. Eur J Pediatr. (1981) 137:23–5. doi: 10.1007/BF00441164
89. Dinulos, JG, and Zembowicz, A. Case 32-2008. N Engl J Med. (2008) 359:1718–24. doi: 10.1056/NEJMcpc0805312
90. Yazbeck, N, Muwakkit, S, Abboud, M, and Saab, R. Zinc and biotin deficiencies after pancreaticoduodenectomy. Acta Gastroenterol Belg. (2010) 73:283–6.
91. Hou, J-W. Maple syrup urine disease complicated with kyphoscoliosis and myelopathy. Pediatr Neonatol. (2016) 57:431–5. doi: 10.1016/j.pedneo.2013.10.013
92. Stern, M, Gruttner, R, and Krumbach, J. Protracted diarrhoea: secondary monosaccharide malabsorption and zinc deficiency with cutaneous manifestations during total parenteral nutrition. Eur J Pediatr. (1980) 135:175–80. doi: 10.1007/BF00441638
93. Gözdasoglu, S, Taçyildiz, N, Günlemez, A, Bayhan, H, Sencer, H, Ünal, E, et al. Acrodermatitis enteropathica: case report analyses of zinc metabolism electron microscopic examination and immune function. J Trace Elem Exp Med. (2000) 13:317–25. doi: 10.1002/1520-670X(2000)13:3<317::AID-JTRA9>3.0.CO;2-7
94. Kumar, S, Thakur, V, Choudhary, R, and Vinay, K. Acrodermatitis Enteropathica. J Pediatr. (2020) 220:258–9. doi: 10.1016/j.jpeds.2020.01.017
95. Ashkenazi-Hoffnung, L, Bilavsky, E, and Amir, J. Acrodermatitis enteropathica in a 9 month old infant. Isr Med Assoc J. (2011) 13:258.
96. D'Amico, G, De Laet, C, Smits, G, Salik, D, Deprez, G, Vilain, C, et al. Acquired zinc deficiency mimicking acrodermatitis enteropathica in a breast-fed premature infant. Pediatr Rep. (2021) 13:444–9. doi: 10.3390/pediatric13030051
97. Edle Von Jaschke, AK, and Kruse, R. Facial and acral dermatitis in a breast-fed infant. Diagnosis: Acrodermatitis enteropathica. Dtsch Med Wochenschr. (2010) 135:1427–8. doi: 10.1055/s-0030-1262428
98. El Fekih, N, Monia, K, Schmitt, S, Dorbani, I, Küry, S, and Kamoun, MR. Transient symptomatic zinc deficiency in a breast-fed infant: relevance of a genetic study. Nutrition. (2011) 27:1087–9. doi: 10.1016/j.nut.2011.06.002
99. van Gool, JD, Went, K, and Zegers, BJ. Letter: Acrodermatitis enteropathica and cellular immune deficiency. Lancet. (1976) 1:1085. doi: 10.1016/s0140-6736(76)92271-6
100. Young, HS, Khan, ASA, Power, S, Ehrhardt, P, and Coulson, IH. Case 4. Transient symptomatic zinc deficiency in a breast-fed premature infant: an acrodermatitis enteropathica-like eruption. Clin Exp Dermatol. (2003) 28:109–10. doi: 10.1046/j.1365-2230.2003.01182.x
101. Ghali, FE, Steinberg, JB, and Tunnessen, WW Jr. Picture of the month. Acrodermatitis enteropathica-like lesion in cystic fibrosis. Arch Pediatr Adolesc Med. (1996) 150:99–100. doi: 10.1001/archpedi.1996.02170260103018
102. Wallis, K, Azizi, E, Kook, AJ, Herczeg, E, Julsary, A, Szeinberg, A, et al. Acrodermatitis enteropathica associated with low density lipoproteins deficiency. Clin Pediatr (Phila). (1974) 13:749–54. doi: 10.1177/000992287401300911
103. Tatlican, S, Yamangokturk, B, Eren, C, Gulbahar, O, and Eskioglu, F. A diagnostic challenge: a case of Acrodermatitis Enteropathica without Hypozincemia and with maternal Milk of low zinc level. Pediatr Dermatol. (2010) 27:534–5. doi: 10.1111/j.1525-1470.2010.01268.x
104. Gupta, N, Jain, P, Kabra, M, Gulati, S, and Sethuraman, G. Acrodermatitis Dysmetabolica - report of two cases. Indian J Pediatr. (2015) 82:869–70. doi: 10.1007/s12098-015-1715-5
105. Robertson, AF, and Putz, J. Serum and erythrocyte fatty acids in a case of acrodermatitis enteropathica. J Pediatr. (1967) 70:279–81. doi: 10.1016/s0022-3476(67)80425-6
106. Pekcan, S, Kose, M, Dogru, D, Sekerel, B, Atakan, N, Ozcelik, U, et al. A 4-month-old boy with acrodermatitis enteropathica-like symptoms. Eur J Pediatr. (2009) 168:119–21. doi: 10.1007/s00431-008-0825-8
107. Starbek Zorko, M, and Brecelj, J. Severe dermatitis with anemia and edema in an infant. Pediatr Dermatol. (2020) 37:939–40. doi: 10.1111/pde.14268
108. Madhusudan, M, Raman, R, and Sathyasekaran, M. Acrodermatitis Enteropathica as a presentation of cystic fibrosis in an infant. Indian Pediatr. (2020) 57:573. doi: 10.1007/s13312-020-1860-4
109. Chew, AL, Chan, I, McGrath, JA, and Atherton, DJ. Infantile acquired zinc deficiency resembling acrodermatitis enteropathica. Clin Exp Dermatol. (2005) 30:594–5. doi: 10.1111/j.1365-2230.2005.01833.x
110. Kharfi, M, Zaraa, I, Kury, S, Moisan, JP, and Kamoun, MR. Acrodermatitis enteropathica in full-term breast-fed infant. Ann Dermatol Venereol. (2005) 132:246–8. doi: 10.1016/s0151-9638(05)79254-1
111. Cheng, HC, Wang, JD, Chen, CH, and Yang, CS. A Young infant with Periorificial and Acral dermatitis. J Pediatr. (2014) 165:408–408.e1. doi: 10.1016/j.jpeds.2014.04.056
112. Samady, JA, Schwartz, RA, Shih, LY, Piela, Z, Lambert, WC, and Janniger, CK. Acrodermatitis enteropathica-like eruption in an infant with nonketotic hyperglycinemia. J Dermatol. (2000) 27:604–8. doi: 10.1111/j.1346-8138.2000.tb02236.x
113. Leung, AKC, Leong, KF, and Lam, JM. Acrodermatitis enteropathica in a 3-month-old boy. Can Med Assoc J. (2021) 193:e243. doi: 10.1503/cmaj.201181
114. Sheddon, I. Letter: acrodermatitis enteropathica. Br J Dermatol. (1974) 91:716. doi: 10.1111/j.1365-2133.1974.tb12465.x
115. Gutierrez-Gonzalez, E, Alvarez-Perez, A, Loureiro, M, Sánchez-Aguilar, D, and Toribio, J. Acrodermatitis enteropathica in a breast-fed infant. Actas Dermosifiliogr. (2012) 103:170–2. doi: 10.1016/j.ad.2011.04.019
116. Bhat, MR, Dandekeri, S, Kambil, SM, and Pinto, M. Acrodermatitis enteropathica in an infant with normal zinc levels. Indian J Dermatol Venereol Leprol. (2015) 81:70–1. doi: 10.4103/0378-6323.148583
117. Peroni, DG, Banzato, C, Guerresi, S, Schena, D, Deganello, M, Boner, AL, et al. Severe refractory dermatitis and cystic fibrosis. Arch Dis Child. (2012) 97:205. doi: 10.1136/archdischild-2011-300731
118. Santiago, F, Matos, J, Moreno, A, Schmitt, S, Bézieau, S, and Tellechea, O. Acrodermatitis enteropathica: a novel SLC39A4 gene mutation found in a patient with an early-onset. Pediatr Dermatol. (2011) 28:735–6. doi: 10.1111/j.1525-1470.2011.01487.x
119. Sheu, J, and Huang, JT. Erythematous scaly plaques and papules in a 9-month-old infant. J Pediatr. (2013) 163:1222. doi: 10.1016/j.jpeds.2013.05.031
120. Shah, A, Veitch, D, and Harman, KE. Alopecia universalis-like hair loss in acrodermatitis enteropathica. Clin Exp Dermatol. (2020) 45:929–31. doi: 10.1111/ced.14232
121. Leverkus, M, Kütt, S, Bröcker, EB, Frank, J, and Hamm, H. Nutritional zinc deficiency mimicking acrodermatitis enteropathica in a fully breast-fed infant. J Eur Acad Dermatol Venereol. (2006) 20:1380–1. doi: 10.1111/j.1468-3083.2006.01734.x
122. Vogel, CA, Baron, J, and Hansen, RC. A scaly rash. Clin Pediatr (Phila). (2003) 42:187–90. doi: 10.1177/000992280304200215
123. Al Naamani, A, and Al Lawati, T. Acrodermatitis enteropathica: a case report. Oman Med J. (2020) 35:e201. doi: 10.5001/omj.2020.97
124. Vashist, S, Rana, A, and Mahajan, VK. Transient symptomatic zinc deficiency in a breastfed infant associated with low zinc levels in maternal serum and breast Milk improving after zinc supplementation: an uncommon phenotype? Indian Dermatol Online J. (2020) 11:623–6. doi: 10.4103/idoj.IDOJ_386_19
125. Zedek, D, Morrell, DS, Graham, M, Goodman, D, and Groben, P. Acrodermatitis enteropathica-like eruption and failure to thrive as presenting signs of cystic fibrosis. J Am Acad Dermatol. (2008) 58:S5–8. doi: 10.1016/j.jaad.2006.04.038
126. Brar, BK, Pall, A, and Gupta, RR. Acrodermatitis enteropathica-like lesion in an exclusively breast fed infant with zinc deficiency. J Dermatol. (2003) 30:259–60. doi: 10.1111/j.1346-8138.2003.tb00386.x
127. da Matta Ain, AC, Valente, ES, Mallozi, MC, Sarni, RO, Furquim, M, and Solé, D. Acrodermatitis enteropathica-like simulating severe atopic dermatitis: a case report. Allergol Immunopathol (Madr). (2008) 36:176–9. doi: 10.1016/S0301-0546(08)72543-6
128. Freier, S, Faber, J, Goldstein, R, and Mayer, M. Treatment of acrodermatitis enteropathica by intravenous amino acid hydrolysate. J Pediatr. (1973) 82:109–12. doi: 10.1016/s0022-3476(73)80026-5
129. Chue, CD, Rajpar, SF, and Bhat, J. An acrodermatitis enteropathica-like eruption secondary to acquired zinc deficiency in an exclusively breast-fed premature infant. Int J Dermatol. (2008) 47:372–3. doi: 10.1111/j.1365-4632.2008.03492.x
130. Jahnke, I, Vogt, A, Stieler, KM, Hennermann, J, and Blume-Peytavi, U. Symmetrical inflammatory erosive plaques and blisters in an infant diagnosis: Acrodermatitis. J Dtsch Dermatol Ges. (2017) 15:956–9. doi: 10.1111/ddg.12985
131. Oztürkcan, S, Içağasioğlu, D, Akyol, M, and Cevit, O. A case of acrodermatitis enteropathica. J Dermatol. (2000) 27:475–7. doi: 10.1111/j.1346-8138.2000.tb02210.x
132. Mancini, AJ, and Tunnessen, WW Jr. Picture of the month. Acrodermatitis enteropathica-like rash in a breast-fed, full-term infant with zinc deficiency. Arch Pediatr Adolesc Med. (1998) 152:1239–40. doi: 10.1001/archpedi.152.12.1239
133. Roberts, LJ, Shadwick, CF, and Bergstresser, PR. Zinc deficiency in two full-term breast-fed infants. J Am Acad Dermatol. (1987) 16:301–4. doi: 10.1016/s0190-9622(87)70039-5
134. Kim, SK, Hwang, JS, Kang, HY, and Kang, HY. Acrodermatitis enteropathica-like dermatosis associated with combined deficiency of zinc and amino acids. Int J Dermatol. (2009) 48:909–10. doi: 10.1111/j.1365-4632.2009.04165.x
135. Kaya Erdogan, H, Bulur, I, Saracoglu, ZN, Aslan, H, Aydogdu, SD, and Yildiz, B. Acrodermatitis enteropathica: a novel mutation of the SLC39A4 gene in a Turkish boy. J Dermatol. (2016) 43:966–8. doi: 10.1111/1346-8138.13313
136. Milla, PJ, and Moynahan, EJ. Acrodermatitis enteropathica with lactose intolerance. Proc R Soc Med. (1972) 65:600–1. doi: 10.1177/003591577206500712
137. Patrizi, A, Bianchi, F, Neri, I, and Specchia, F. Acrodermatitis enteropathica-like eruption: a sign of malabsorption in cystic fibrosis. Pediatr Dermatol. (2003) 20:187–8. doi: 10.1046/j.1525-1470.2003.20221_6.x
138. Lee, JY, Chang, SE, Suh, CW, Choi, JH, Sung, KJ, Moon, KC, et al. A case of acrodermatitis enteropathica-like dermatosis caused by ornithine transcarbamylase deficiency. J Am Acad Dermatol. (2002) 46:965–7. doi: 10.1067/mjd.2002.120595
139. White, HB Jr, and Montalvo, JM. Serum fatty acids before and after recovery from acrodermatitis enteropathica: comparison of an infant with her family. J Pediatr. (1973) 83:999–1006. doi: 10.1016/s0022-3476(73)80535-9
140. Smith, SZ, Scheen, SR, and Wine, LJ. Acrodermatitis enteropathica induced by iatrogenic zinc deficiency. South Med J. (1978) 71:1582–3. doi: 10.1097/00007611-197812000-00043
141. Parra, CA, and Smálik, AV. Percutaneous absorption of zinc in acrodermatitis enteropathica. Dermatologica. (1981) 163:413–6. doi: 10.1159/000250194
142. Ozkan, S, Ozkan, H, Fetil, E, Corapçioglu, F, Yilmaz, S, and Ozer, E. Acrodermatitis enteropathica with Pseudomonas aeruginosa sepsis. Pediatr Dermatol. (1999) 16:444–7. doi: 10.1046/j.1525-1470.1999.00114.x
143. Panzer, R, Küry, S, Schmitt, S, and Fölster-Holst, R. Identification of a novel mutation in the SLC39A4 gene in a case of Acrodermatitis Enteropathica. Acta Derm Venereol. (2016) 96:424–5. doi: 10.2340/00015555-2240
144. Webber, NK, Pope, MF, Powell, AM, and Mellerio, J. Recalcitrant generalized eruption and low alkaline phosphatase: think zinc. Clin Exp Dermatol. (2011) 36:225–6. doi: 10.1111/j.1365-2230.2010.03902.x
145. Arora, K, Das, RR, Panda, SS, and Kabra, M. Acrodermatitis enteropathica-like skin lesions in a neonate. BMJ Case Rep. (2013) 2013:bcr2013200190. doi: 10.1136/bcr-2013-200190
146. Cvancara, KG, and Cvancara, JL. Irritable baby with weight loss and a periorificial and truncal lesion acrodermatitis enteropathica. Cutis. (2020) 106:E7–9. doi: 10.12788/cutis.0102
147. Vinay, K, Yadav, S, and Handa, S. Zinc deficiency and Canities: an unusual manifestation. JAMA Dermatol. (2014) 150:1116–7. doi: 10.1001/jamadermatol.2014.368
148. Templier, I, Reymond, JL, Nguyen, MA, Boujet, C, Lantuejoul, S, Beani, JC, et al. Acrodermatitis enteropathica-like syndrome secondary to branched-chain amino acid deficiency during treatment of maple syrup urine disease. Ann Dermatol Venereol. (2006) 133:375–9. doi: 10.1016/s0151-9638(06)70919-x
149. Vu, M, Gillooly, Z, Becker, E, and Osswald, S. Acquired acrodermatitis enteropathica in an infant. Cutis. (2022) 110:281–3. doi: 10.12788/cutis.0642
150. Ranugha, P, Sethi, P, and Shastry, V. Acrodermatitis enteropathica: the need for sustained high dose zinc supplementation. Dermatol Online J. (2018) 24:13030/qt1w9002sr. doi: 10.5070/D32412042450
151. Dalgic, B, and Egritas, O. Gray hair and acrodermatitis enteropathica-like dermatitis: an unexpected presentation of cystic fibrosis. Eur J Pediatr. (2011) 170:1305–8. doi: 10.1007/s00431-011-1447-0
152. Kiechl-Kohlendorfer, U, Fink, F-M, and Steichen-Gersdorf, E. Transient symptomatic zinc deficiency in a breast-fed preterm infant. Pediatr Dermatol. (2007) 24:536–40. doi: 10.1111/j.1525-1470.2007.00512.x
153. Martínez-Bustamante, ME, Peña-Vélez, R, Almanza-Miranda, E, Aceves-Barrios, CA, Vargas-Pastrana, T, and Morayta-Ramírez Corona, ARR. Acrodermatitis enteropathica. Bol Med Hosp Infant Mex. (2017) 74:295–300. doi: 10.1016/j.bmhimx.2017.05.002
154. Zeriouh, L, Hali, F, Khadir, K, and Benchikhi, H. Acrodermatitis enteropathica-like disease in a full-term exclusively breast-fed infant. Arch Pediatr. (2013) 20:1129–32. doi: 10.1016/j.arcped.2013.07.007
155. Sandström, B, Cederblad, A, Lindblad, BS, and Lönnerdal, B. Acrodermatitis enteropathica, zinc metabolism, copper status, and immune function. Arch Pediatr Adolesc Med. (1994) 148:980–5. doi: 10.1001/archpedi.1994.02170090094017
156. Kilic, SS, Giraud, M, Schmitt, S, Bézieau, S, and Küry, S. A novel mutation of the SLC39A4 gene causing acrodermatitis enteropathica. Br J Dermatol. (2007) 157:386–7. doi: 10.1111/j.1365-2133.2007.08000.x
157. Zhou, Z, Liu, TH, and Zhang, ZK. Zinc-responsive exfoliative dermatitis in a 17-year-old girl with delayed puberty. Clin Exp Dermatol. (2018) 43:746–8. doi: 10.1111/ced.13564
158. Heinen, F, Matern, D, Pringsheim, W, Leititis, JU, and Brandis, M. Zinc deficiency in an exclusively breast-fed preterm infant. Eur J Pediatr. (1995) 154:71–5. doi: 10.1007/BF01972977
159. Buehning, LJ, and Goltz, RW. Acquired zinc deficiency in a premature breast-fed infant. J Am Acad Dermatol. (1993) 28:499–501. doi: 10.1016/s0190-9622(08)81766-5
160. Lee, MG, Hong, KT, and Kim, JJ. Transient symptomatic zinc deficiency in a full-term breast-fed infant. J Am Acad Dermatol. (1990) 23:375–9. doi: 10.1016/0190-9622(90)70226-8
161. Traupe, H, Happle, R, Gröbe, H, and Bertram, HP. Polarization microscopy of hair in acrodermatitis enteropathica. Pediatr Dermatol. (1986) 3:300–3. doi: 10.1111/j.1525-1470.1986.tb00529.x
162. Businco, L, Menghi, AM, Rossi, P, D'Amelio, R, and Galli, E. Zinc-dependent chemotactic defect in an infant with acrodermatitis. Arch Dis Child. (1980) 55:966–8. doi: 10.1136/adc.55.12.966
163. Jensen, SL, McCuaig, C, Zembowicz, A, and Hurt, MA. Bullous lesions in acrodermatitis enteropathica delaying diagnosis of zinc deficiency: a report of two cases and review of the literature. J Cutan Pathol. (2008) 35:1–13. doi: 10.1111/j.1600-0560.2008.00981.x
164. Park, CH, Lee, MJ, Kim, HJ, Lee, G, Park, JW, and Cinn, YW. Congenital zinc deficiency from mutations of the SLC39A4 gene as the genetic background of Acrodermatitis Enteropathica. J Korean Med Sci. (2010) 25:1818–20. doi: 10.3346/jkms.2010.25.12.1818
165. Pascual, JC, Matarredona, J, and Mut, J. Acrodermatitis enteropathica-like dermatosis associated with ornithine Transcarbamylase deficiency. Pediatr Dermatol. (2007) 24:394–6. doi: 10.1111/j.1525-1470.2007.00457.x
166. Barbarot, S, Chantier, E, Kuster, A, Hello, M, Roze, JC, Blouin, E, et al. Symptomatic acquired zinc deficiency in at-risk premature infants: high dose preventive supplementation is necessary. Pediatr Dermatol. (2010) 27:380–3. doi: 10.1111/j.1525-1470.2010.01174.x
167. Sahoo, B, Jain, MK, Mishra, R, and Patnaik, S. Atypical presentation of cystic fibrosis in an infant. Indian J Dermatol. (2022) 67:287–9. doi: 10.4103/ijd.IJD_243_17
168. Aggett, PJ, Atherton, DJ, More, J, Davey, J, Delves, HT, and Harries, JT. Symptomatic zinc deficiency in a breast-fed preterm infant. Arch Dis Child. (1980) 55:547–50. doi: 10.1136/adc.55.7.547
169. Mazzocchi, C, Michel, JL, Chalencon, V, Teyssier, G, Rayet, I, and Cambazard, F. Carence en zinc au cours de la mucoviscidose. Arch Pediatr. (2000) 7:1081–4.
170. Weymouth, RD, Kelly, R, and Lansdell, BJ. Symptomatic zinc deficiency in a premature infant. Aust Paediatr J. (1982) 18:208–10. doi: 10.1111/j.1440-1754.1982.tb02031.x
171. Hon, KL, Chow, CM, and Hung, EC. Periorificial and acral dermatitis in a newborn having milk intolerance. Indian J Pediatr. (2010) 77:805–6. doi: 10.1007/s12098-010-0101-6
172. Alkhayal, FA, Al Haddad, S, Bakraa, RM, and Alqahtani, A. Acrodermatitis dysmetabolica secondary to isoleucine deficiency in infant with maple syrup urine disease. Dermatol Rep. (2023) 15:9750. doi: 10.4081/dr.2023.9750
173. He, Y, Yang, Q, Pradhan, S, Ran, Y, and Wang, S. Transient symptomatic zinc deficiency resembling Acrodermatitis Enteropathica in a full-term breastfed infant. Indian J Pediatr. (2021) 88:292–3. doi: 10.1007/s12098-020-03518-2
174. Julius, R, Schulkind, M, Sprinkle, T, and Rennert, O. Acrodermatitis enteropathica with immune deficiency. J Pediatr. (1973) 83:1007–11. doi: 10.1016/s0022-3476(73)80536-0
175. Couvreur, Y, Quarre, JP, Bailly, A, and Cornut, P. Zinc deficiency and lymphocyte subpopulations. A study by flow cytometry. JPEN J Parenter Enteral Nutr. (1986) 10:239–41. doi: 10.1177/0148607186010002239
176. Lova Navarro, M, Vera Casaño, A, Benito López, C, Fernández Ballesteros, MD, Godoy Díaz, DJ, Crespo Erchiga, A, et al. Transient neonatal zinc deficiency due to a new autosomal dominant mutation in gene SLC30A2 (ZnT-2). Pediatr Dermatol. (2014) 31:251–2. doi: 10.1111/pde.12257
177. Olholm-Larsen, P. Serum zinc levels in heterozygous carriers of the gene for acrodermatitis enteropathica: identification of a carrier state is not possible. Hum Genet. (1979) 46:65–74. doi: 10.1007/BF00278903
178. Crisóstomo, M, Santos, MC, Tavares, E, and Cunha, F. Transient symptomatic zinc deficiency in an exclusively breastfed infant. BMJ Case Rep. (2021) 14:e241754. doi: 10.1136/bcr-2021-241754
179. Ross, B, Kumar, M, Srinivasan, H, and Ekbote, AV. Isoleucine deficiency in a neonate treated for maple syrup urine disease masquerading as Acrodermatitis Enteropathica. Indian Pediatr. (2016) 53:738–40. doi: 10.1007/s13312-016-0922-0
180. Harms, FL, Nampoothiri, S, Kortüm, F, Thomas, J, Panicker, VV, Alawi, M, et al. Coinheritance of biallelic SLURP1 and SLC39A4 mutations cause a severe genodermatosis with skin peeling and hair loss all over the body. Br J Dermatol. (2018) 179:1192–4. doi: 10.1111/bjd.16912
181. Ma, L, Savory, S, and Agim, NG. Acquired protein energy malnutrition in Glutaric Acidemia. Pediatr Dermatol. (2013) 30:502–4. doi: 10.1111/pde.12067
182. O'Regan, GM, Canny, G, and Irvine, AD. 'Peeling paint' dermatitis as a presenting sign of cystic fibrosis. J Cyst Fibros. (2006) 5:257–9. doi: 10.1016/j.jcf.2006.05.003
183. Li, Z, Wang, J, Yang, Y, and Wang, S. A novel homozygous mutation p.E88K in maternal SLC30A2 gene as a cause of transient neonatal zinc deficiency. Exp Dermatol. (2020) 29:556–61. doi: 10.1111/exd.14099
184. Wenk, KS, Higgins, KB, and Greer, KE. Cystic fibrosis presenting with dermatitis. Arch Dermatol. (2010) 146:171–4. doi: 10.1001/archdermatol.2009.361
185. Strobel, CT, Byrne, WJ, Abramovits, W, Newcomer, VJ, Bleich, R, and Ament, ME. A zinc-deficiency dermatitis in patients on total parenteral nutrition. Int J Dermatol. (1978) 17:575–81. doi: 10.1111/j.1365-4362.1978.tb06003.x
186. Pode-Shakked, B, Shemer-Meiri, L, Harmelin, A, Stettner, N, Brenner, O, Abraham, S, et al. Man made disease: clinical manifestations of low phenylalanine levels in an inadequately treated phenylketonuria patient and mouse study. Mol Genet Metab. (2013) 110:S66. doi: 10.1016/j.ymgme.2013.10.006
187. Zhao, M, and Shen, C. Acrodermatitis Enteropathica. N Engl J Med. (2023) 389:1803. doi: 10.1056/NEJMicm2304003
188. Zhong, W, Yang, C, Zhu, L, Huang, YQ, and Chen, YF. Analysis of the relationship between the mutation site of the SLC39A4 gene and acrodermatitis enteropathica by reporting a rare Chinese twin: a case report and review of the literature. BMC Pediatr. (2020) 20:34. doi: 10.1186/s12887-020-1942-4
189. Higuchi, S, Yorifuji, T, Nishida, M, Fukai, K, and Nakano, H. Acrodermatitis enteropathica: a hereditary form of zinc deficiency. QJM. (2021) 114:270–1. doi: 10.1093/qjmed/hcaa176
190. Moynahan, EJ. Acrodermatitis enteropathica. Proc R Soc Med. (1962) 55:240. doi: 10.1177/003591576205500321
191. Osmani, S, Smidt, AC, Phan, CM, and Johnson, DW. Acquired acrodermatitis enteropathica from a ketogenic diet. JAAD Case Rep. (2021) 9:75–7. doi: 10.1016/j.jdcr.2021.01.016
192. Kunzler, E, Cernik, C, and Weaver, J. Psoriasiform lesion in a toddler. J Pediatr. (2016) 170:332–e1. doi: 10.1016/j.jpeds.2015.11.032
193. Crone, J, Huber, WD, Eichler, I, and Granditsch, G. Acrodermatitis enteropathica-like eruption as the presenting sign of cystic fibrosis--case report and review of the literature. Eur J Pediatr. (2002) 161:475–8. doi: 10.1007/s00431-002-0982-0
194. Jung, AG, Mathony, UA, Behre, B, Küry, S, Schmitt, S, Zouboulis, CC, et al. Acrodermatitis enteropathica: an uncommon differential diagnosis in childhood - first description of a new sequence variant. J Dtsch Dermatol Ges. (2011) 9:999–1002. doi: 10.1111/j.1610-0387.2011.07742.x
195. Sahuquillo-Torralba, A, Calle-Andrino, A, Ferrer-Lorente, B, and Évole-Buselli, M. Erosions and blisters in a premature infant with exclusive breastfeeding. Med Clin (Barc). (2017) 149:e49. doi: 10.1016/j.medcli.2016.12.040
196. Azevedo, PM, Gavazzoni-Dias, MF, Avelleira, JC, Lerer, C, De Sousa, AS, and Azulay, DR. Acrodermatitis enteropathica in a full-term breast-fed infant: case report and literature review. Int J Dermatol. (2008) 47:1056–7. doi: 10.1111/j.1365-4632.2008.03626.x
197. Perafán-Riveros, C, França, LF, Alves, AC, and Sanches, JA Jr. Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. (2002) 19:426–31. doi: 10.1046/j.1525-1470.2002.00200.x
198. Guillot, I, Roth, B, Causeret, AS, Jullien, D, Claris, O, Faure, M, et al. Acquired zinc deficiency in a breast-fed premature infant. Arch Pediatr. (2003) 10:442–4. doi: 10.1016/s0929-693x(03)00094-0
199. Lim, Y-S, Lee, M-W, Choi, J-H, and Sung, K-J. The clinical study of zinc deficiency presented as a skin manifestation of acrodermatitis enteropathica. Korean J Dermatol. (2000) 38:155–62.
200. Kasana, S, Din, J, and Maret, W. Genetic causes and gene–nutrient interactions in mammalian zinc deficiencies: acrodermatitis enteropathica and transient neonatal zinc deficiency as examples. J Trace Elem Med Biol. (2015) 29:47–62. doi: 10.1016/j.jtemb.2014.10.003
201. Ogawa, Y, Kinoshita, M, Shimada, S, and Kawamura, T. Zinc and skin disorders. Nutrients. (2018) 10:199. doi: 10.3390/nu10020199
202. Golan, Y, Kambe, T, and Assaraf, YG. The role of the zinc transporter SLC30A2/ZnT2 in transient neonatal zinc deficiency. Metallomics. (2017) 9:1352–66. doi: 10.1039/c7mt00162b
203. Sehgal, VN, and Jain, S. Acrodermatitis enteropathica. Clin Dermatol. (2000) 18:745–8. doi: 10.1016/s0738-081x(00)00150-4
204. Easley, D, Krebs, N, Jefferson, M, Miller, L, Erskine, J, Accurso, F, et al. Effect of pancreatic enzymes on zinc absorption in cystic fibrosis. J Pediatr Gastroenterol Nutr. (1998) 26:136–9. doi: 10.1097/00005176-199802000-00003
205. Maares, M, and Haase, H. A guide to human zinc absorption: general overview and recent advances of in vitro intestinal models. Nutrients. (2020) 12:762. doi: 10.3390/nu12030762
206. Reyes, JG. Zinc transport in mammalian cells. Am J Phys. (1996) 270:C401–10. doi: 10.1152/ajpcell.1996.270.2.C401
207. Hall, AG, and King, JC. The molecular basis for zinc bioavailability. Int J Mol Sci. (2023) 24:6561. doi: 10.3390/ijms24076561
Keywords: acrodermatitis enteropathica, zinc deficiency, SLC39A4, metabolic disorder, pediatric dermatology
Citation: Cui W, Wang N, Shi M, Xu X and Jiang H (2025) Acrodermatitis enteropathica in the pediatric population: a literature review of real-world studies. Front. Nutr. 12:1590075. doi: 10.3389/fnut.2025.1590075
Edited by:
Devinder Mohan Thappa, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), IndiaReviewed by:
Andrew Hall, University of California, Davis, United StatesSai Divija Kamma, Godavari Skin Hospital, India
Copyright © 2025 Cui, Wang, Shi, Xu and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongkun Jiang, aGtqaWFuZ0BjbXUuZWR1LmNu; Xuegang Xu, eGd4dUBjbXUuZWR1LmNu
†These authors have contributed equally to this work
 Wanlin Cui
Wanlin Cui Ningning Wang1†
Ningning Wang1† Mingyue Shi
Mingyue Shi Hongkun Jiang
Hongkun Jiang