- 1Department of Gynecology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2Chengdu Women's and Children's Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
This review examines the neurobiological mechanisms by which plant-derived compounds influence women's reproductive health through the neuroendocrine-reproductive axis. Gynecological disorders frequently present with neurological manifestations, including cognitive decline in perimenopause, anxiety and depression in polycystic ovary syndrome (PCOS), and central sensitization in endometriosis. Bioactive compounds from medicinal plants, including polyphenols and phytoestrogens, demonstrate therapeutic potential through their anti-inflammatory, antioxidant, and neuromodulatory properties. These multi-target compounds offer advantages over conventional single-target therapies by simultaneously regulating multiple physiological processes. The review explores applications in specific gynecological conditions and discusses the development of dietary supplements and functional foods incorporating these plant-derived ingredients. The growing market for these products presents opportunities for innovative formulations with enhanced bioavailability and personalized approaches. Future research directions include integrating neuroimaging with herbal research, improving clinical translation, and establishing regulatory frameworks for the global application of these plant-derived interventions to enhance female neuroendocrine-reproductive health.
1 Introduction
The neuroendocrine-reproductive axis represents a complex and precisely regulated physiological network connecting the central nervous system, endocrine system, and female reproductive system. Proper functioning of this axis is essential for maintaining female reproductive health, emotional stability, and cognitive function (1, 2). With modern lifestyle changes and growing environmental pressures, an increasing number of women experience health issues related to neuroendocrine-reproductive axis dysfunction, including premenstrual syndrome (PMS), polycystic ovary syndrome (PCOS), endometriosis, and menopausal symptoms (3–6).
Epidemiological studies suggest that these gynecological disorders not only impair reproductive function but are also frequently associated with significant neurological symptoms. Studies report that 44% to 62% of perimenopausal women experience cognitive decline, more than 60% of PCOS patients present with anxiety or depression, and 42% of endometriosis patients suffer from chronic pain and central sensitization (7–9). These findings underscore the intricate interplay between neurological and reproductive dysfunctions, emphasizing the need for holistic interventions targeting the neuroendocrine-reproductive axis.
Traditional dietary practices across various cultures have long recognized the unique health benefits of certain plant-based foods for women. These empirical observations have been substantiated by modern nutritional research, demonstrating that bioactive compounds in plant-based foods can modulate neurological and reproductive functions through multiple mechanisms (10–12). Unlike conventional single-target drug therapies, plant-derived compounds typically exhibit multi-target effects, simultaneously regulating multiple physiological processes, making them particularly suitable for addressing complex disorders associated with the neuroendocrine-reproductive axis (13, 14).
This review systematically examines the neurobiological mechanisms through which plant-derived compounds influence women's reproductive health via the neuroendocrine-reproductive axis. We analyze the physiological foundations of this axis, explore the mechanisms and biological activities of various plant-derived compounds, evaluate their therapeutic potential in specific gynecological disorders, and propose strategies for their clinical translation. By integrating traditional knowledge with contemporary scientific evidence, we provide comprehensive insights for understanding and utilizing plant-derived therapeutics to enhance female neuroendocrine-reproductive health. The conceptual framework of this review is illustrated in Figure 1, which summarizes the interconnections between plant-derived compounds, their neurobiological mechanisms, and their effects on women's reproductive health.
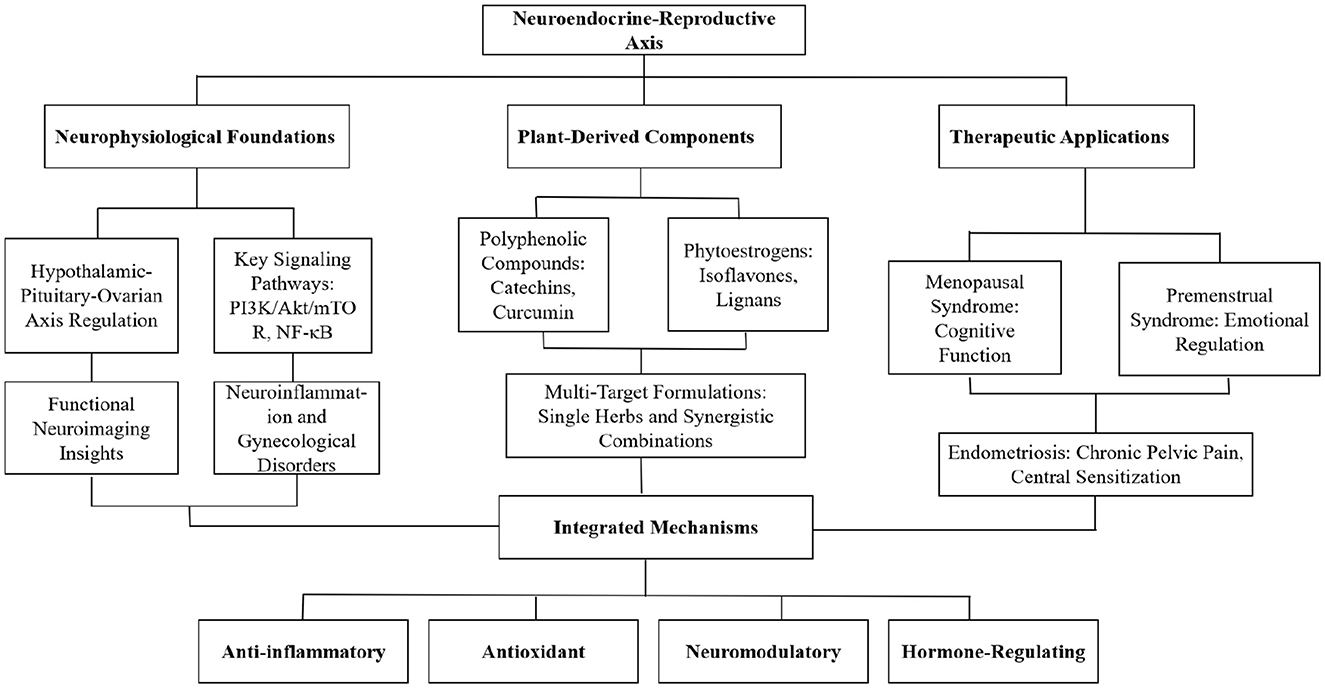
Figure 1. Conceptual framework of the neurobiology of plant-based therapeutics in women's reproductive health.
2 Neurophysiological foundations of the neuroendocrine-reproductive axis
2.1 Hypothalamic-pituitary-ovarian axis regulation
The hypothalamic-pituitary-ovarian (HPO) axis constitutes the central regulatory network controlling reproductive function throughout a woman's lifespan. This intricate system precisely coordinates the menstrual cycle, follicular development, ovulation, and reproductive aging via tightly regulated hormonal secretion and feedback mechanisms (15). The hypothalamus, serving as the neural command center, secretes gonadotropin-releasing hormone (GnRH) in a pulsatile manner, stimulating the secretion of gonadotropins from the anterior pituitary (16). The subsequently released follicle-stimulating hormone (FSH) and luteinizing hormone (LH) act upon the ovaries to promote follicular growth, ovulation, and the synthesis of estradiol and progesterone (17). Multiple neurotransmitter systems play crucial roles in regulating HPO axis function, particularly the kisspeptin signaling networks in the arcuate nucleus and anteroventral periventricular nucleus (18, 19). These neuronal populations integrate various central and peripheral signals to modulate GnRH pulse release. This neural regulation extends beyond kisspeptin to include dopaminergic, serotonergic, noradrenergic, GABAergic, and glutamatergic inputs, which collectively participate in coordinating reproductive function (20). Evidence from animal and human studies suggests that plant-derived compounds can modulate these neurotransmitter systems, providing a mechanistic basis for their effects on reproductive function (21, 22).
2.2 Key signaling pathways in the neuro-endocrine-reproductive axis
As shown in Figure 2, the neuroendocrine-reproductive axis involves several key signaling pathways, including the kisspeptin-GPR54 signaling system, GABA/glutamate balance system, PI3K/Akt/mTOR pathway, inflammation-related pathways, and BDNF-TrkB neurotrophic pathway. These pathways collectively form the molecular foundation for the actions of plant-derived compounds. Regulation of the HPO axis involves the precise coordination of multiple signaling pathways, which form the molecular foundation for the actions of plant-derived compounds. The kisspeptin-GPR54 signaling system is particularly critical, functioning as an upstream regulator of the GnRH pulse generator and integrating various endogenous and exogenous signals (23). Kisspeptin neurons activate GnRH neurons through the GPR54 receptor, enhancing their pulsatile release, and research indicates that certain plant flavonoids can influence this process by modulating kisspeptin expression (24, 25).
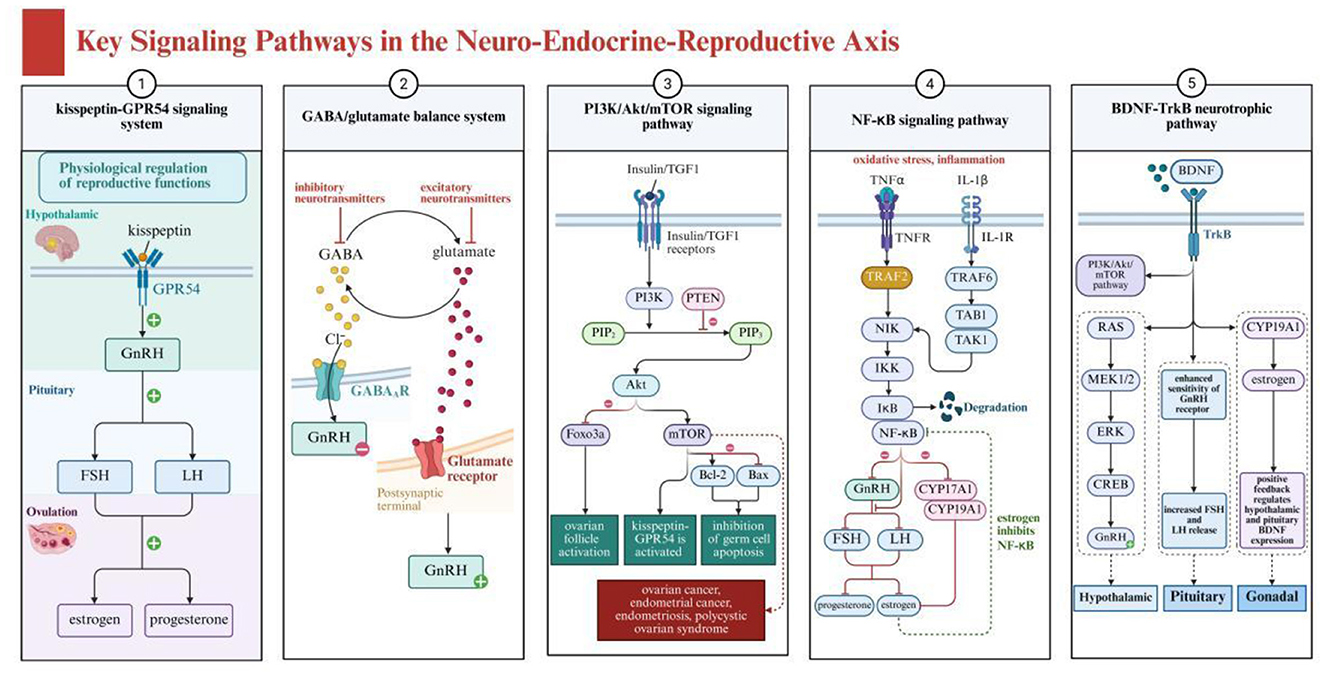
Figure 2. Key signaling pathways in the neuro-endocrine-reproductive axis showing interconnected neural and reproductive regulation systems. Created with BioRender.com.
The GABA/glutamate balance system plays a dual role in HPO axis function regulation. GABA primarily inhibits GnRH neuronal activity through GABA-A receptors, while glutamate promotes activity via N-methyl-D-aspartate (NMDA) and non-NMDA receptors (26). Various active components from traditional Chinese medicine, such as saikosaponins and paeoniflorin, have been found to regulate this balance, indirectly affecting GnRH release patterns (27, 28).
The PI3K/Akt/mTOR signaling pathway plays a crucial role in ovarian function and follicular development, while also being closely related to energy sensing and stress responses in the brain. This pathway is regulated by multiple factors, including insulin, IGF-1, and oxidative stress levels (29). Studies show that various plant polyphenolic compounds such as resveratrol and curcumin can modulate this pathway, simultaneously affecting neuroprotection and ovarian function, achieving multi-level regulation of the neuroendocrine-reproductive axis (30, 31).
These plant compounds, by inhibiting the PI3K/Akt/mTOR pathway, can reduce cell proliferation and promote apoptosis, thus playing a role in cancer treatment (30, 31). Additionally, their antioxidant properties help alleviate oxidative stress, protecting the nervous system and supporting reproductive health. Inflammation-related pathways, particularly the NF-κB signaling pathway, constitute an important bridge connecting the immune system, nervous system, and reproductive system. Chronic inflammation can affect hypothalamic GnRH secretion, pituitary gonadotropin release, and ovarian responsiveness to these hormones through this pathway (32–34). Various anti-inflammatory compounds in traditional Chinese medicine, such as tanshinone and tetramethylpyrazine, can effectively inhibit NF-κB activation, reducing inflammatory interference with the neuroendocrine-reproductive axis (35–37).
The BDNF-TrkB neurotrophic pathway not only plays a role in neuroplasticity and emotional regulation but also participates in local ovarian function regulation (38, 39). Plant active ingredients such as ginkgolide B and ginsenoside Rg1 can enhance BDNF expression and TrkB activation, potentially simultaneously improving neural function and promoting ovarian health, providing a theoretical basis for the application of traditional Chinese medicine components in neuroendocrine-reproductive axis disorders (40, 41).
2.3 Functional neuroimaging insights into neuro-reproductive connections
While traditional physiological and molecular studies have established the foundational mechanisms of the neuroendocrine-reproductive axis, modern neuroimaging techniques now provide critical visual evidence of the dynamic interactions between neural and reproductive systems (42, 43). These non-invasive methods are essential for understanding the neurophysiological foundations of the axis, as they reveal real-time functional connections that cannot be observed through conventional research approaches (43, 44).
The development of functional magnetic resonance imaging (fMRI) and related technologies has revolutionized our ability to visualize the neural correlates of reproductive hormone fluctuations (45). These advanced techniques provide unprecedented insights into how the central nervous system and reproductive organs communicate, offering objective visualization of the neuro-reproductive interface that strengthens our understanding of the neurophysiological mechanisms underlying women's reproductive health.
During the menstrual cycle, fMRI studies reveal significant fluctuations in brain activity corresponding to hormonal changes. During the follicular phase, when estrogen levels rise, there is enhanced activation of the prefrontal cortex, improving cognitive control and emotional regulation (46). In contrast, during the luteal phase when progesterone levels peak, there is increased activity in the amygdala and anterior cingulate cortex, areas associated with emotional processing and pain perception (46). These cyclic changes in neural activity patterns demonstrate the profound influence of reproductive hormones on brain function, helping to explain many neuropsychological symptoms experienced by women at specific points in their menstrual cycle.
In women with gynecological conditions, fMRI studies have identified characteristic neural signatures that differ from healthy controls. Women with primary dysmenorrhea show altered resting-state functional connectivity between the anterior cingulate cortex and periaqueductal gray matter, suggesting central sensitization of pain pathways (47). Similarly, endometriosis patients with chronic pelvic pain show increased gray matter volume in pain processing regions and enhanced functional connectivity in pain networks, even during pain-free periods (48). These neuroimaging findings provide objective markers for central nervous system changes in gynecological conditions and suggest potential neural targets for nutritional interventions.
2.4 Neuroinflammation and gynecological disorders
Following our understanding of the neuroanatomical pathways and functional connections of the neuroendocrine-reproductive axis, neuroinflammation emerges as another fundamental mechanism that shapes the physiological foundations of this system (32, 49). Inflammatory processes represent not merely secondary consequences but core regulatory elements within the axis, influencing both normal reproductive function and pathological states (50–52). The complex interplay between inflammatory mediators and neuroendocrine signaling constitutes an essential component of the axis's basic neurophysiology, providing critical insights into how the brain-reproductive system communication can be disrupted in various gynecological conditions (51, 52).
Neuroinflammation represents a key pathophysiological mechanism connecting the nervous system and gynecological health. The female reproductive system and central nervous system share common inflammatory signaling pathways, establishing a bidirectional relationship where inflammation in one system can significantly affect the other. Inflammatory factors, including interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α), can cross the blood-brain barrier or activate neural afferent pathways, thereby modulating central nervous system function (53).
In endometriosis, systemic and local inflammation appears to promote central sensitization of pain pathways. Multiple studies have documented elevated levels of inflammatory cytokines in the peritoneal fluid and circulation of women with endometriosis (54, 55). These inflammatory mediators can induce neuroplastic changes in central pain processing networks, evidenced by altered brain activation in pain perception and regulation-related areas shown in fMRI studies. This central sensitization may explain why many women with endometriosis continue to experience pain even after surgical removal of lesions (48).
3 Plant-derived nutritional components and their effects on the neuro-endocrine-reproductive axis
As shown in Table 1, Figures 3, 4, different categories of plant-derived compounds, including polyphenols and phytoestrogens, interact with distinct yet overlapping signaling pathways to produce their effects on the neuroendocrine-reproductive axis. Figure 3 illustrates the molecular mechanisms of polyphenolic compounds, while Figure 4 depicts the action mechanisms of phytoestrogens.

Figure 3. Molecular mechanisms of polyphenolic compounds (EGCG, curcumin, and resveratrol) in neuroendocrine-reproductive health regulation. Created with BioRender.com.
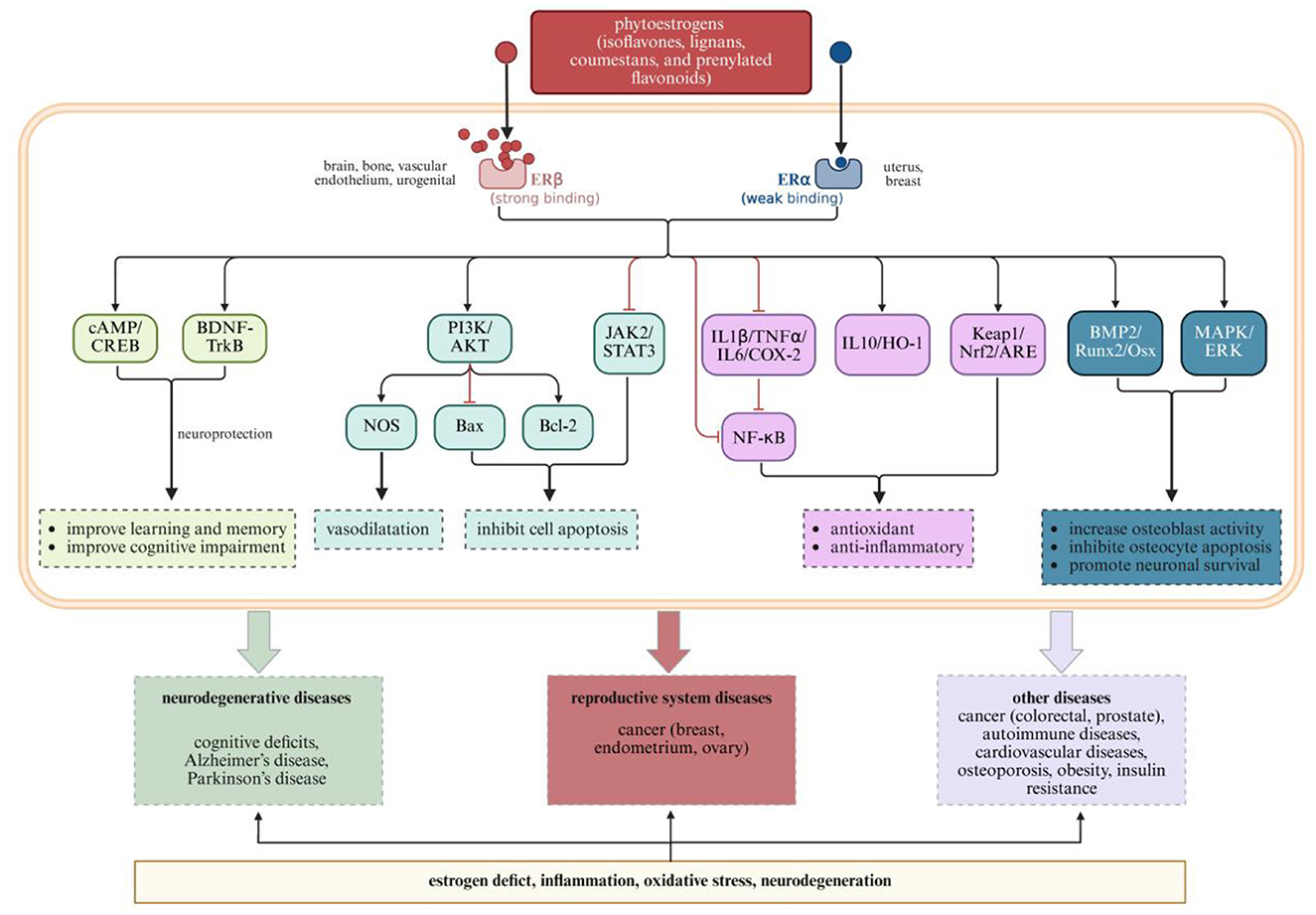
Figure 4. Phytoestrogen action mechanisms and their effects on neurological and reproductive health outcomes. Created with BioRender.com.
3.1 Polyphenolic compounds
3.1.1 Catechins from green tea
Green tea (Camellia sinensis) contains several catechins, with epigallocatechin gallate (EGCG) being the most abundant and biologically active. EGCG exhibits powerful antioxidant properties through multiple mechanisms: it directly neutralizes free radicals through its hydroxyl groups, demonstrates iron chelating activity, and inhibits lipid oxidation (56–58). These antioxidant actions provide protection against oxidative damage for both neural and reproductive tissues.
EGCG modulates multiple signaling pathways critical to neuroendocrine-reproductive function (59, 60). In particular, EGCG activates the PI3K/Akt pathway, which plays a dual role in neuroprotection and reproductive cell function (60, 61). Within the central nervous system, this pathway activation enhances neuronal survival and reduces apoptosis, contributing to EGCG's cognitive benefits (62, 63). EGCG affects protein kinase C (PKC), MAPK, JNK, and ASK-1 pathways, creating a comprehensive network of neuroprotective mechanisms.
A key mechanism of EGCG's action is its inhibition of the NF-κB pathway, which explains its potent anti-inflammatory effects across both neural and reproductive tissues (64, 65). By suppressing this pathway, EGCG reduces the production of pro-inflammatory cytokines that can disrupt normal hypothalamic-pituitary-ovarian axis function (50, 66). This anti-inflammatory action is particularly relevant for conditions like endometriosis and PCOS, where inflammation contributes significantly to pathophysiology (66, 67).
For gynecological health, EGCG exhibits estrogen-modulating properties through interactions with estrogen receptors and inhibition of aromatase activity (68–71). In PCOS models, these mechanisms help reduce androgen levels, improve insulin sensitivity, and normalize ovarian morphology (66, 72–74). These molecular interactions and their physiological outcomes are illustrated in Figure 3. EGCG's actions culminate in several beneficial outcomes for women's health, including improved insulin sensitivity, reduced androgen levels, and inhibition of endometriotic cell growth and invasion.
3.1.2 Curcumin from turmeric
Curcumin, the principal curcuminoid in turmeric (Curcuma longa), exerts multi-target effects on the neuroendocrine-reproductive axis through several key signaling pathways. Curcumin modulates PI3K/Akt, JAK/STAT, and NF-κB pathways, creating an integrated network of anti-inflammatory and antioxidant actions.
Curcumin's inhibition of the NF-κB pathway is a central mechanism underlying its anti-inflammatory effect (75, 76). By suppressing this pathway, curcumin reduces the production of pro-inflammatory cytokines including TNF-α, IL-1β, and IL-6, which otherwise could disrupt neuroendocrine signaling between the brain and reproductive organs (75, 77). This anti-inflammatory action is particularly important for conditions like endometriosis, where inflammation drives disease progression (77–79).
Curcumin's modulation of the PI3K/Akt pathway contributes to its effects on both neural and reproductive tissues. In neural cells, this modulation enhances survival mechanisms and reduces oxidative stress-induced damage (80, 81). In reproductive tissues like the endometrium, the same pathway modulation helps regulate cell proliferation and angiogenesis, explaining curcumin's beneficial effects in endometriosis (78, 79, 82).
Regarding gynecological health, curcumin demonstrates significant effects on endometriosis through multiple mechanisms illustrated in Figure 3. It inhibits the proliferation, migration, and invasion of endometriotic cells, reduces the expression of inflammatory mediators, and inhibits angiogenesis by downregulating vascular endothelial growth factor (VEGF) (79, 83). These multifaceted actions explain clinical observations where curcumin supplementation significantly reduced the severity of primary dysmenorrhea symptoms compared to placebo (84–86).
3.1.3 Resveratrol from grapes and berries
Resveratrol (3,5,4′-trihydroxy-trans-stilbene) exhibits complex molecular interactions with the neuroendocrine-reproductive axis. It modulates multiple signaling pathways, including PI3K/Akt, SIRT1/STAT3, NF-κB, and VEGF pathways, creating an integrated network of beneficial effects.
A key mechanism of resveratrol's action is its activation of SIRT1, an NAD+-dependent deacetylase that regulates various cellular processes including energy metabolism, stress resistance, and longevity (87–90). SIRT1 activation enhances mitochondrial biogenesis through PGC-1α, improving energy production while reducing oxidative stress (88, 89). This mechanism is particularly important for energy-intensive tissues in the neuroendocrine-reproductive axis, including the hypothalamus and ovaries (90, 91).
Resveratrol's inhibition of the NF-κB pathway explains its anti-inflammatory effects in both neural and reproductive tissues (92, 93). By suppressing microglial activation and reducing pro-inflammatory cytokine production, resveratrol creates a favorable environment for proper neuroendocrine signaling. This anti-inflammatory action may benefit conditions characterized by neuroinflammatory processes, such as the mood and cognitive symptoms associated with PCOS and menopause (94, 95).
For gynecological health, resveratrol's multiple actions converge to protect ovarian function and address conditions like endometriosis. Resveratrol protects ovarian follicles from oxidative damage, enhances oocyte quality, and may delay ovarian aging through SIRT1 activation and inhibition of mTOR signaling (96, 97). In endometriosis models, resveratrol inhibits the proliferation and invasion of endometriotic cells, reduces inflammation, and inhibits angiogenesis, thereby limiting lesion growth and development (98–100).
3.2 Phytoestrogens
Phytoestrogens are plant-derived compounds with structural similarities to 17β-estradiol, enabling them to bind to estrogen receptors and produce estrogen-like or estrogen-modulating effects (101). These compounds interact with the neuroendocrine-reproductive axis through multiple signaling pathways, creating tissue-specific effects relevant to women's health.
3.2.1 Isoflavones from soy and red clover
Isoflavones, primarily daidzein, genistein, and glycitein, interact with the neuroendocrine-reproductive axis through their preferential binding to estrogen receptor β (ERβ) over estrogen receptor α (ERα), with binding affinities for ERβ ~17–324 times higher than for ERα (102). This selective receptor binding property explains their tissue-specific effects, as ERβ is predominantly expressed in the brain, bone, and vascular endothelium, while ERα is more abundant in reproductive tissues like the uterus and breast.
This selective estrogen receptor modulator (SERM) activity allows isoflavones to exert beneficial estrogenic effects in certain tissues while minimizing unwanted stimulation in others. In the brain, ERβ activation by isoflavones triggers neuroprotective pathways including BDNF-TrkB and cAMP/CREB signaling, which enhance neuroplasticity and support cognitive function (103, 104). This activation improves learning, memory, and cognitive function—effects particularly relevant for menopausal women experiencing cognitive changes.
Isoflavones also modulate PI3K/AKT signaling, which influences both neuronal survival and reproductive cell function (105–107). In neural tissue, this pathway activation enhances cell survival mechanisms and promotes vasodilation through nitric oxide synthase (NOS) activation (108). These effects may explain the observed benefits of isoflavone supplementation on cognitive function and cerebrovascular health in menopausal women.
3.2.2 Lignans from flaxseed and whole grains
Lignans are diphenolic compounds found in various plant foods, with flaxseed being the richest dietary source (109). Lignans interact with the neuroendocrine-reproductive axis through multiple pathways following their conversion to bioactive enterolignans by intestinal bacteria (101, 110–112). These compounds, like isoflavones, bind to estrogen receptors but with different binding characteristics, producing distinct biological effects.
Lignan metabolites modulate multiple signaling cascades beyond direct estrogen receptor binding. They activate antioxidant pathways including Keap1/Nrf2/ARE signaling, which enhances cellular defense against oxidative stress in both neural and reproductive tissues (113). Additionally, they have an inhibitory effect on inflammatory pathways including IL1β/TNFα/IL6/COX-2 and NF-κB signaling, creating an anti-inflammatory environment conducive to proper neuroendocrine function.
These molecular actions culminate in prevention of various conditions affecting women's health, from cognitive deficits to hormone-dependent cancers. For the nervous system, lignans help prevent cognitive deficits and neurodegenerative conditions through their combined antioxidant and anti-inflammatory actions. For the reproductive system, these same mechanisms help address hormone-dependent conditions including reproductive system cancers. This multi-system protective effect exemplifies the integrative approach of phytoestrogens in supporting the neuroendocrine-reproductive axis.
3.3 Formulation approaches and multi-target mechanisms
3.3.1 Single plant extracts with multi-target properties
Many single Chinese medicinal plants exhibit multi-target therapeutic effects, showing significant advantages particularly in neurological health (114). The complex chemical composition and multiple bioactivities of Chinese herbal components enable them to target various disease pathways, producing synergistic effects (115). For example, flavonoid compounds in Scutellaria baicalensis possess significant antioxidant and anti-inflammatory actions, improving cognitive function and slowing neurodegeneration progression by inhibiting neuroinflammation and improving blood-brain barrier permeability (116, 117). Polysaccharides, carotenoids, and flavonoids in Lycium barbarum have antioxidant and anti-aging properties, protecting neurons and improving memory and cognitive function by regulating the immune system and enhancing neurotrophic factors (118). Active components in Salvia miltiorrhiza, such as salvianolic acids, demonstrate anti-inflammatory, antioxidant, and circulation-promoting effects, helping improve cerebral blood flow and oxygen supply, reducing neurodegeneration, and showing extensive application potential in treating Alzheimer's disease (119).
3.3.2 Synergistic effects in complex herbal formulations
Chinese herbal formulations embody the principle of synergy, whereby combinations of herbal components work collectively to enhance therapeutic efficacy, minimize adverse effects, and target multiple pathological pathways simultaneously (115, 120). Traditional Chinese herbal formulation follows the “Jun-Chen-Zuo-Shi” (monarch-minister-assistant-courier) principle, where primary herbs address the main pathology (Jun), supporting herbs enhance the primary action (Chen), adjuvant herbs reduce side effects or target secondary symptoms (Zuo), and courier herbs direct the therapeutic action to specific organs or tissues (Shi). This structured approach ensures systematic synergy throughout the formulation process, maximizing therapeutic outcomes while minimizing adverse effects.
Research demonstrates that herbal ingredients within a formulation can potentiate each other's bioactivity through several synergistic mechanisms, including pharmacokinetic synergy where one herb enhances the absorption or bioavailability of another (121, 122). For instance, piperine from black pepper has been shown to increase the bioavailability of curcumin by up to 2,000%, inhibiting hepatic and intestinal glucuronidation and slowing intestinal transit time. This pharmacokinetic enhancement significantly improves the therapeutic potential of herbal combinations compared to individual components (121, 122).
For example, combining tetramethylpyrazine from Ligusticum chuanxiong with astragaloside IV from Astragalus membranaceus significantly enhances neuroprotective outcomes in spinal cord injury by modulating astrocyte polarization. Tetramethylpyrazine exerts anti-inflammatory and neuroprotective effects via NF-κB inhibition, while astragaloside IV potentiates these effects by activating SIRT1 pathways, together creating a robust neuroinflammatory control mechanism superior to either component alone (123–128).
Similarly, the combination of salvianolic acid IIA (from Salvia miltiorrhiza) and tetramethylpyrazine nanoemulsion exhibits synergistic neuroprotective effects in Alzheimer's disease models. Salvianolic acid IIA independently possesses strong antioxidant and anti-apoptotic effects by targeting MAPK/ERK/CREB pathways, while tetramethylpyrazine enhances blood circulation and reduces neuronal inflammation (129). Their co-delivery in nanoemulsion form significantly enhances bioavailability, improving therapeutic outcomes related to cognitive impairment and neuronal survival beyond what single ingredients achieve independently.
The Yangyin Tongnao Granules (YYTN), a complex formulation including formononetin, calycosin, ligustrazine, puerarin, and ferulic acid, exemplify multi-component synergy. This formulation simultaneously targets inflammatory pathways (reducing TNF-α), oxidative stress (elevating T-SOD levels), and apoptosis (lowering Cyt-C levels) in cerebral ischemia-reperfusion injury models. Individually, each herbal component has specific biological activities, but their coordinated interaction within YYTN achieves enhanced neuroprotective efficacy through combined modulation of inflammatory, oxidative, and apoptotic pathways (130).
In women's reproductive health specifically, the traditional formula Gui Zhi Fu Ling Wan demonstrates remarkable synergistic effects in treating endometriosis and dysmenorrhea (131, 132). This formula combines Cinnamomi Ramulus (Gui Zhi) as the monarch herb providing analgesic effects, Poria (Fu Ling) and Moutan Cortex (Mu Dan Pi) as minister herbs enhancing blood circulation and resolving blood stasis, with Persicae Semen (Tao Ren) and Paeoniae Radix (Chi Shao) as assistant herbs that further promote blood circulation while modulating inflammation (133, 134). The combined action of these herbs creates a comprehensive therapeutic effect that simultaneously addresses pain, inflammation, and tissue remodeling in endometriotic lesions, with clinical studies showing superior efficacy compared to single-herb treatments (132, 133, 135).
Additionally, Kai Xin San (KXS), a traditional herbal formula, demonstrates how herbal synergy operates through a multi-pathway approach in Alzheimer's disease. Its combination of herbs collectively enhances cognitive and memory functions by activating the Wnt/β-catenin signaling pathway, facilitating mitochondrial autophagy, and suppressing neuroinflammation via NLRP3 inflammasome inhibition. These coordinated actions provide comprehensive therapeutic effects against neuropathological processes, illustrating the unique strength of synergistic formulations in complex neuroendocrine-reproductive conditions (136, 137).
Therefore, understanding and leveraging the synergistic interactions between herbal ingredients within traditional formulations can significantly optimize their therapeutic potential, providing enhanced efficacy and broader clinical applications for managing complex women's reproductive health disorders.
4 Applications of plant-derived nutritional components in specific gynecological conditions
4.1 Menopausal syndrome and cognitive function
The menopausal transition marks a significant phase in a woman's life, characterized by declining ovarian function and hormonal fluctuations, ultimately leading to cessation of menstrual cycles. Beyond reproductive changes, menopause is frequently accompanied by a range of symptoms affecting multiple body systems, including vasomotor symptoms (hot flashes, night sweats), psychological disturbances (mood fluctuations, anxiety, depression), cognitive changes (“brain fog,” memory decline), and urogenital symptoms (vaginal dryness, urinary issues) (138). The neurological dimension of menopausal syndrome is increasingly recognized as a significant contributor to declining quality of life and functional capacity. Menopausal estrogen decline affects various neurotransmitter systems, cerebral blood flow, and neuroplasticity, potentially leading to cognitive changes and psychological symptoms (139). Additionally, sleep disruption due to vasomotor symptoms may exacerbate cognitive difficulties and mood disorders, creating a cycle of symptoms that significantly impacts daily functioning (140).
4.1.1 Research on traditional Chinese herbal applications in menopausal cognitive impairment
Various plant-derived compounds found in traditional Chinese herbs demonstrate potential value in improving cognitive function during menopause, particularly those with phytoestrogenic activity. Literature reviews indicate that multiple Chinese herbal extracts can influence cognitive function through multiple mechanisms, including antioxidant, anti-inflammatory, and neuroprotective effects, which are closely related to cognitive changes resulting from declining estrogen levels during menopause (141).
Studies show that flavonoid compounds from Scutellaria baicalensis provide protection to neural cells by regulating oxidative stress and neuroinflammatory processes, mitigating cognitive decline in menopausal women. These compounds not only reduce free radical production but also enhance neuroplasticity through regulation of specific signaling pathways, thereby supporting cognitive functions such as memory and attention (117). Additionally, since Scutellaria extracts modulate activity in the prefrontal cortex, an area that commonly shows decreased activity in menopausal women, they may have specific beneficial effects on executive function (141–143).
Soy isoflavones, as a major source of phytoestrogens, have been extensively studied for their role in improving menopausal cognitive function. Systematic reviews suggest that isoflavones may provide cognitive protection through selective binding to estrogen receptor β (ERβ), which is highly expressed in cognitive-related brain regions such as the hippocampus and prefrontal cortex (144–146). Research has observed that long-term supplementation with soy isoflavones may positively affect verbal memory and executive function, particularly in late-stage postmenopausal women (144, 147, 148). Some research findings indicate that isoflavone intervention may slow the trend of hippocampal volume atrophy in menopausal women and enhance functional connectivity between the hippocampus and prefrontal cortex, with these structural changes correlating with cognitive improvements (149, 150). However, it is worth noting that heterogeneity exists between different studies, suggesting that individual factors, dosage, and intervention duration may influence intervention effects (144).
Traditional Chinese herbal formulations, such as the Ningshen mixture, have demonstrated synergistic advantages in treating menopausal cognitive function (151). Observational studies suggest that these formulations not only alleviate vasomotor symptoms such as hot flashes but also improve cognitive test performance, particularly in memory and attention domains. Mechanistically, these formulations may function by regulating the expression of neurotrophic factors and reducing neuroinflammation (152–154). Compared to single-component interventions, Chinese herbal formulations can simultaneously act on multiple targets, potentially providing more comprehensive regulation for menopausal syndrome symptoms.
4.1.2 Advantages of multi-target mechanisms of Chinese herbal medicine in menopausal symptom management
Unlike single-target Western medications, active components from traditional Chinese medicine exhibit multi-target regulatory characteristics for menopausal syndrome. For example, tanshinone IIA not only selectively activates ERβ and demonstrates estrogen-like effects but simultaneously inhibits inflammatory factor (IL-1β, TNF-α) release, activates the Nrf2 antioxidant pathway, and enhances BDNF expression (155–157). This multi-target characteristic enables a single Chinese herbal component to simultaneously improve vasomotor symptoms, mood fluctuations, and cognitive decline, providing a comprehensive solution for menopausal women.
Modern neuroimaging research has revealed the regulatory mechanisms of Chinese herbal components on menopausal brain function. A study applying electroencephalography (EEG) and functional near-infrared spectroscopy (fNIRS) demonstrated that honeysuckle extract could restore alpha wave activity in the prefrontal cortex of menopausal women and enhance blood oxygen levels in this region, changes significantly correlated with improved cognitive test scores (158). Another study using resting-state fMRI found that ginsenoside Rg1 could enhance functional connectivity in the default mode network of menopausal women, closely related to emotional regulation and self-referential processing, potentially explaining its antidepressant effect (159–161).
In clinical practice, menopausal symptoms often present with individualized differences, requiring personalized intervention strategies (162). Traditional Chinese medicine emphasizes syndrome differentiation and treatment, selecting appropriate herbal formulations based on women's specific symptom combinations, an approach highly consistent with modern precision medicine concepts. Recent research confirms that personalized Chinese herbal interventions based on traditional Chinese medicine constitution typing are superior to standardized prescriptions in improving menopausal symptoms, especially for complex patients with concurrent cognitive and emotional symptoms (162–165).
4.2 Premenstrual syndrome and emotional regulation
Premenstrual syndrome (PMS) encompasses a range of physical, emotional, and behavioral symptoms occurring during the luteal phase of the menstrual cycle and disappearing shortly after menstruation begins (166). A more severe form, premenstrual dysphoric disorder (PMDD), is characterized by significant mood disturbances that substantially impact functioning. The pathophysiology of PMS/PMDD involves complex interactions between ovarian hormones, neurotransmitter systems, and neuroendocrine pathways, providing multiple targets for herbal nutritional interventions.
4.2.1 Chinese herbal regulation of serotonergic pathways to improve PMS emotional symptoms
Serotonergic dysfunction occupies a central position in PMS/PMDD pathology, with affected women showing altered serotonin function and increased sensitivity to normal hormonal fluctuations (167). Various active components from Chinese herbs can influence serotonergic neurotransmission, potentially alleviating mood disorders associated with PMS/PMDD (168).
St. John's wort (Hypericum perforatum) contains multiple bioactive compounds, including hypericin and hyperforin, which inhibit serotonin reuptake, potentially enhancing serotonergic neurotransmission (169, 170). A randomized controlled trial found that St. John's wort extract (900 mg daily) significantly reduced physical and behavioral symptoms of PMS, with improvements particularly notable for mood disturbances (171). These effects were comparable to selective serotonin reuptake inhibitors (SSRIs), the first-line pharmacological treatment for PMS/PMDD (172).
Molecular mechanism studies indicate that Chinese flavonoid compounds such as saikosaponins from Bupleurum and apigenin from celery can regulate serotonergic function through multiple pathways. These compounds inhibit monoamine oxidase (MAO) activity, modulate serotonin receptor sensitivity, and influence neurotransmitter reuptake. For example, in PMS animal models, saikosaponin treatment significantly increased serotonin levels in the hypothalamus and hippocampus while upregulating 5-HT1A receptor expression, closely related to antidepressant and anxiolytic effects (173). Functional magnetic resonance imaging (fMRI) studies provide insights into how Chinese herbal compounds affect emotional regulation neural circuits in women with PMS (174).
4.2.2 Anti-inflammatory Chinese herbal components and PMS symptom reduction
Inflammatory processes appear to be important contributing factors to PMS symptoms, with affected women showing elevated inflammatory markers during symptomatic luteal phases (175). Various plant-derived anti-inflammatory compounds may help alleviate these inflammatory aspects of PMS/PMDD.
Alpha-linolenic acid (ALA) from flaxseed and other plant-derived omega-3 fatty acids demonstrate significant anti-inflammatory properties by reducing pro-inflammatory eicosanoid production and inducing specialized pro-resolving mediators (176, 177). A randomized controlled trial found that omega-3 supplementation significantly reduced physical and psychological symptoms of PMS, with particular improvements in depression, anxiety, and bloating (178). These benefits were associated with reductions in inflammatory markers, suggesting a mechanistic link between anti-inflammatory actions and symptom improvement.
Curcumin from turmeric is an effective anti-inflammatory compound that inhibits NF-κB signaling and reduces pro-inflammatory cytokine production (179). Clinical research shows that curcumin supplementation (100 mg every 12 h from 7 days before menstruation to 3 days after) significantly reduces PMS symptoms, with improvements in both physical and behavioral symptoms. These effects were comparable to non-steroidal anti-inflammatory drugs (NSAIDs), suggesting that curcumin may benefit the inflammatory aspects of PMS (180).
4.3 Endometriosis and chronic pelvic pain
Endometriosis is characterized by the presence of endometrial-like tissue outside the uterus, affecting ~5–10% of reproductive-age women and is a leading cause of chronic pelvic pain and infertility (181). Its pathophysiology involves complex interactions between inflammatory processes, hormonal factors, and neural sensitization (181–183). Beyond local pelvic manifestations, endometriosis is increasingly recognized as having significant systemic dimensions, with affected women more susceptible to comorbid fibromyalgia, chronic fatigue syndrome, and mood disorders (183).
4.3.1 Chinese herbal anti-inflammatory components' regulation of peripheral pain mechanisms
Chronic inflammation is a core feature of endometriosis pathophysiology, with endometriotic lesions producing various inflammatory mediators that promote pain, fibrosis, adhesion formation, and infertility (184). Local inflammation also sensitizes peripheral nociceptors, lowering pain thresholds and enhancing pain perception. Various Chinese herbal anti-inflammatory components may help address these peripheral aspects of endometriosis-associated pain (185, 186).
Resveratrol, a stilbenoid polyphenol found in Chinese herbs such as Polygonum cuspidatum, demonstrates significant anti-inflammatory and antioxidant properties relevant to endometriosis management (98). The anti-inflammatory actions of resveratrol in endometriosis involve multiple pathways, including inhibition of NF-κB signaling, reduction of pro-inflammatory cytokine production (particularly IL-1β, IL-6, and TNF-α), and inhibition of inflammatory enzyme activity (98, 187, 188). Additionally, resveratrol inhibits aromatase activity in endometriotic tissue, potentially reducing local estrogen production that drives lesion growth and inflammation (189, 190).
Beyond anti-inflammatory properties, resveratrol exhibits significant anti-angiogenic effects that may inhibit the development and progression of endometriotic lesions (98). Angiogenesis is a key process in endometriosis pathogenesis, as new blood vessel formation is necessary for lesion survival and growth. Functional imaging studies indicate that resveratrol treatment significantly reduces microvessel density and vascular endothelial growth factor (VEGF) expression in endometriotic lesions, resulting in reduced lesion size and pain improvement (189).
4.3.2 Chinese herbal neuromodulation of central pain processing
Beyond peripheral inflammation, central sensitization of pain pathways is a key component of endometriosis-associated chronic pelvic pain (191). This process involves neuroplastic changes in the central nervous system, resulting in increased responsiveness of nociceptive neurons to normal and subthreshold stimuli (192). Various plant-derived compounds possess neuromodulatory properties that may help address these central aspects of endometriosis-associated pain, providing a more comprehensive approach to pain management (193–195). Beyond traditional anti-inflammatory and analgesic Chinese herbs, recent research has identified various Chinese herbal components that directly modulate central pain pathways. Tetramethylpyrazine, a key component in pain-relieving formulas, acts by activating descending inhibitory pain pathways, particularly through enhancing noradrenergic and serotonergic projections from the brainstem to the spinal cord (196, 197). Gastrodin from Gastrodia elata demonstrates significant central nervous system modulatory effects that may be particularly applicable to centrally sensitized pain in endometriosis (198–200). Gastrodin inhibits microglial activation, reduces catecholaminergic system hyperactivity in the spinal cord and brain, and normalizes NMDA receptor expression (198, 200).
5 Dietary supplements and functional foods: development and practical applications
5.1 Global development status of dietary supplements and functional foods
The market for dietary supplements and functional foods targeting women's reproductive health has experienced unprecedented growth over the past decade. In just 30 years, the U.S. dietary supplement market has evolved from several hundred products primarily consisting of vitamins, minerals, and select herbal extracts to >75,000 products today. The United States remains the world's largest dietary supplement market, having shown remarkable stability even during economic downturns. Historical data from the 2007–2010 National Health and Nutrition Examination Survey (NHANES) indicated that 49% of US adults reported using supplements (201, 202). The growth in this sector parallels rising healthcare costs and increasing prevalence of conditions affecting the female neuroendocrine-reproductive axis. Functional foods and supplements offer an accessible means for women to take proactive approaches to health maintenance, particularly in addressing symptoms associated with hormonal fluctuations throughout the reproductive lifespan. Importantly, these products often align with patient preferences for natural approaches and preventative strategies rather than solely relying on pharmaceutical interventions after symptoms develop (203, 204).
5.2 Current research and application of plant-based ingredients for women's reproductive health
The application of plant-derived compounds in women's health supplements has evolved considerably, with formulations becoming increasingly sophisticated and targeted. For menopausal health, products containing standardized extracts of soy isoflavones, red clover, and black cohosh dominate the market, with clinical evidence supporting their efficacy in managing vasomotor symptoms and potentially offering protective effects for cognitive health. These ingredients are commonly formulated as capsules, tablets, or incorporated into functional beverages and food products (205–208).
For premenstrual syndrome management, formulations containing chasteberry (Vitex agnus-castus), evening primrose oil, and St. John's wort have gained popularity, with varying levels of clinical evidence supporting their efficacy (209–213). Delivery systems for these ingredients range from traditional capsules to innovative formats such as functional teas, chocolates, and gummies, which may enhance compliance and address consumer preferences. Products targeting fertility and reproductive health often contain combinations of antioxidant-rich botanicals, including green tea extracts, resveratrol, and various adaptogens (66, 214, 215). These formulations aim to reduce oxidative stress, modulate inflammatory pathways, and optimize hormonal balance—all factors that contribute to reproductive health and fertility outcomes. The application of these ingredients spans multiple delivery formats, including specialized prenatal supplements, fertility-supporting beverage powders, and condition-specific formulations.
6 Future research directions and opportunities
6.1 Integrating advanced neuroimaging with Chinese herbal research
Functional magnetic resonance imaging (fMRI) and other advanced neuroimaging techniques provide unprecedented opportunities for studying the effects of Chinese herbal components on the neuroendocrine-reproductive axis (1, 216). These non-invasive methods track changes in brain activity and connectivity patterns, providing direct visualization of mechanisms underlying herbal effects (217, 218).
Each neuroimaging modality offers distinct insights into how plant compounds affect neural function: functional MRI reveals real-time brain activation and network connectivity changes (219, 220); diffusion tensor imaging (DTI) visualizes white matter structural integrity modifications that may reflect neuroplastic responses to herbal interventions (221, 222); and arterial spin labeling (ASL) measures cerebral blood flow changes that may underlie vascular mechanisms of herbal actions (223–225).
Multimodal imaging approaches are particularly valuable for herbal research because they can capture the multifaceted effects of plant compounds. For example, resveratrol supplementation not only enhances functional connectivity between the left posterior hippocampus and medial prefrontal cortex in older adults, but these connectivity improvements also correlate significantly with enhanced memory performance and decreased glycated hemoglobin (HbA1c) levels. These findings suggest a potential mechanism whereby resveratrol may support cognitive function in older adults by improving communication between key brain regions and optimizing metabolic function (226).
A promising application is tracking changes in prefrontal-hippocampal connectivity before and after phytoestrogen interventions in menopausal women, correlating these changes with cognitive improvements and hormone regulation (227–229). Advanced neuroimaging analytical methods could further elucidate how plant compounds affect broader neural networks, potentially revealing mechanisms beyond isolated regional effects and providing more comprehensive insights into their neuromodulatory actions (229, 230).
For endometriosis-related chronic pain, neuroimaging can identify predictive markers for herbal treatment response (231, 232). Neuroimaging could help visualize how traditional plant compounds affect central pain processing pathways in the brain (233, 234). Future research utilizing functional MRI could examine how herbal interventions modulate activity in key pain-related regions such as the anterior cingulate cortex and insula (235–238). Such studies may eventually reveal whether baseline functional connectivity patterns in pain processing networks could predict individual responses to herbal formulations, potentially enabling more personalized treatment approaches (239).
Future research should standardize neuroimaging protocols for herbal studies and incorporate advanced analytical approaches to capture the complex neural effects of multi-component herbal formulations (240, 241). The integration of neuroimaging with pharmacokinetic and genetic assessments could further elucidate individual variations in neural responses to plant-derived compounds, advancing personalized botanical interventions for women's neuroendocrine-reproductive health (242).
6.2 Clinical translation challenges and opportunities
Laboratory and preclinical studies on Chinese herbal active components in neuroendocrine-reproductive health require clinical translation. This process faces significant challenges, primarily the limited number of high-quality randomized controlled trials (RCTs) that incorporate objective biomarkers and neuroimaging endpoints (243, 244). Future clinical studies should adopt more rigorous methodologies with appropriate randomization, blinding, adequate sample sizes, and objective outcome assessments. Adaptive trial designs may be particularly suitable for herbal formulation research, allowing modifications based on preliminary results while maintaining scientific rigor. For example, studies evaluating menopausal cognitive interventions could adjust formulations based on participant responses, mirroring the personalized approach of traditional Chinese medicine pattern differentiation while maintaining methodological standards (245). Additionally, long-term studies examining the effects of Chinese herbs across different female life stages are needed. Longitudinal research tracking herbal interventions from puberty through post-menopause could assess preventive strategies for neuroendocrine-reproductive axis dysregulation, aligning with traditional Chinese medicine's preventive philosophy while generating valuable evidence for broader women's health strategies.
6.3 Innovative approaches in herbal formulation development
The application of Chinese herbal active components in functional foods represents a rapidly evolving field with significant market potential. Combining traditional wisdom with modern food technology can yield innovative products targeting neuroendocrine-reproductive health (114, 246). A key challenge is the limited bioavailability of many active components like curcumin and resveratrol, which often show promising results in vitro but fail to achieve therapeutic concentrations in vivo due to poor absorption, rapid metabolism, or limited solubility (247, 248).
6.3.1 Advanced delivery systems for enhanced bioavailability
Nanotechnology offers promising solutions for overcoming bioavailability limitations of plant compounds. Nanoparticle-based delivery systems significantly enhance the solubility, stability, and cellular uptake of poorly bioavailable compounds (249). For instance, curcumin-loaded nanoparticles demonstrate substantially greater bioavailability compared to unformulated curcumin, with correspondingly enhanced therapeutic effects in both neurological and reproductive tissues (250–252). Polymeric nanoparticles, utilizing biodegradable materials like PLGA (poly lactic-co-glycolic acid), provide controlled release profiles particularly beneficial for compounds targeting the neuroendocrine-reproductive axis, where sustained therapeutic concentrations are often required for optimal efficacy (253, 254).
Liposomal encapsulation represents another effective strategy for enhancing bioavailability of plant compounds. These phospholipid vesicles can encapsulate both hydrophilic and hydrophobic compounds, protecting them from degradation while facilitating cellular uptake through membrane fusion or endocytosis (255). Liposomal formulations of resveratrol have demonstrated markedly increased bioavailability and significantly enhanced neuroprotective effects in cognitive studies (256). For women's reproductive health applications, liposomal delivery systems can be designed with specific surface modifications to target reproductive tissues, enhancing local concentration of therapeutic compounds while minimizing systemic exposure.
Micelle-based delivery systems utilize amphiphilic molecules that spontaneously form nano-sized core-shell structures in aqueous environments, encapsulating hydrophobic compounds within their cores (257). These systems dramatically increase the apparent water solubility of compounds like EGCG and curcumin (258–260). Mixed polymeric micelles combining different polymers can be particularly effective for delivering multiple plant compounds simultaneously, supporting the synergistic effects characteristic of traditional herbal formulations while improving their bioavailability profiles.
6.3.2 Bioenhancement strategies for plant compounds
Beyond delivery systems, specific bioenhancement strategies can significantly improve the absorption and effectiveness of plant compounds. Co-administration with natural absorption enhancers represents a traditional approach now validated by modern science. Piperine from black pepper inhibits UDP-glucuronosyltransferase and hepatic aryl-hydrocarbon hydroxylase, significantly reducing the metabolism of compounds like curcumin and resveratrol (261–264). Studies demonstrate that co-administration of piperine substantially increases curcumin bioavailability, potentially enhancing its therapeutic effects across various applications (262, 263).
Phytosomal technology, combining plant compounds with phospholipids to form molecular complexes with improved lipophilicity, offers another effective enhancement strategy. Phytosomes demonstrate superior bioavailability compared to simple herbal extracts while maintaining the natural origin preferred by many consumers (265–267).
Self-emulsifying drug delivery systems (SEDDS) spontaneously form fine oil-in-water emulsions upon mild agitation in the gastrointestinal tract, significantly enhancing the solubility and absorption of lipophilic compounds (268–270). These systems are particularly valuable for enhancing the bioavailability of fat-soluble plant compounds like phytoestrogens and certain polyphenols, which otherwise show limited absorption. SEDDS formulations can be easily incorporated into soft gel capsules or functional food formats, supporting patient compliance while significantly enhancing therapeutic efficacy (269, 271).
Future research should optimize these technologies for women's health applications, such as developing phytoestrogen delivery systems capable of crossing the blood-brain barrier to enhance cognitive protection. Functional food formulations should also consider life-cycle approaches, targeting specific stages and transitions in female physiology. For example, formulations based on classical Chinese herbal prescriptions could be adapted for different age groups—adding menstruation-regulating herbs for young women, antioxidant components for middle-aged women, and cognitive support components for older women, each with optimized bioavailability enhancement strategies appropriate for their target compounds and tissues.
6.4 Interdisciplinary integration for future research
The fusion of Chinese herbal research with neuroendocrinology, gynecology, and nutritional science represents a particularly promising direction. This interdisciplinary integration can generate new therapeutic paradigms combining traditional wisdom with cutting-edge science (272, 273). To advance research in this field, international collaborative platforms integrating diverse expertise should be established. These platforms should bring together traditional Chinese medicine practitioners, neuroscientists, endocrinologists, gynecologists, and nutritionists to design and execute comprehensive research projects. Standardized methods are essential for generating comparable and reproducible results, including standardized analytical protocols for herbal extracts and active components, comprehensive assessment frameworks for neuroendocrine-reproductive axis function, approaches that integrate traditional diagnostics with modern biomarkers, and quality control standards for herbal formulations (274). Natural language processing and machine learning technologies offer new opportunities to analyze ancient Chinese medical texts, potentially identifying herbal patterns for specific gynecological conditions that may have been overlooked by modern research. These data-driven discoveries can guide the development of novel therapeutic strategies, bridging traditional knowledge with contemporary science (272).
6.5 Regulatory considerations and global application
The global application of Chinese herbal products faces regulatory challenges, with different regions having varying classifications and requirements. Developing harmonized regulatory frameworks that recognize traditional evidence while ensuring safety and efficacy is essential for promoting the legitimate use of Chinese herbal products internationally (275). Future efforts should include developing international quality standards for Chinese herbal products, establishing innovative clinical trial designs for evaluating herbal formulations, creating methodological frameworks that integrate traditional evidence with modern clinical data, and training healthcare professionals to incorporate Chinese herbs into women's healthcare (272, 276, 277). These regulatory and educational advances will accelerate the translation of Chinese herbal research into clinical practice and consumer products, expanding access to these potential therapeutic approaches.
7 Conclusion
Plant-derived bioactive compounds from Chinese medicinal plants demonstrate significant advantages in anti-inflammatory, antioxidant, neuroregenerative, and mitochondrial protective functions relevant to neuroendocrine-reproductive health. This review systematically examined how these compounds simultaneously modulate neurological and reproductive systems through multiple pathways, providing evidence for their applications in menopausal cognitive function, premenstrual syndrome, polycystic ovary syndrome, and endometriosis-related pain management. Future research should focus on developing optimized clinical trial designs specifically adapted for complex herbal formulations, such as adaptive designs that allow for personalization while maintaining scientific rigor. Establishing multi-center collaborative studies with standardized protocols will generate more robust evidence across diverse populations, while investigating integrative therapeutic strategies that combine plant-based compounds with conventional pharmaceuticals could enhance efficacy and reduce side effects in conditions resistant to single-modality treatments. Additionally, applying personalized medicine approaches using advanced -omics technologies to identify biomarkers that predict individual responses would allow for more precise herbal interventions. Advanced functional neuroimaging and molecular research increasingly validate the regulatory mechanisms of these compounds on the female neuroendocrine-reproductive axis. As core components of dietary supplements and functional foods, Chinese herbal ingredients offer substantial market potential while addressing contemporary neurological health concerns, providing evidence-based options for improving women's neuroendocrine-reproductive health globally.
Author contributions
XL: Writing – original draft, Writing – review & editing. CB: Writing – original draft. ZZ: Writing – original draft. TZ: Resources, Writing – original draft. KW: Data curation, Writing – original draft. YL: Data curation, Resources, Writing – original draft. QL: Formal analysis, Writing – original draft. SW: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The work was supported by the grants of the National Natural Science Foundation of China (No. 82174431) and the National Celebrated Traditional Chinese Medicine Expert Inheritance Studio of SW (Project No: CJJ2023062).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Gen AI was used in the creation of this manuscript. Generative AI technology was used to generate the abstract and to refine certain sections of the manuscript, which has been thoroughly audited for plagiarism and false content, and the author(s) take full responsibility for the statement. AI details: Name: Claude; Version: 3.7; Model: Claude 3.7 Sonnet; Source: Anthropic.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Phumsatitpong C, Wagenmaker ER, Moenter SM. Neuroendocrine interactions of the stress and reproductive axes. Front Neuroendocrinol. (2021) 63:100928. doi: 10.1016/j.yfrne.2021.100928
2. Cutia CA, Christian-Hinman CA. Mechanisms linking neurological disorders with reproductive endocrine dysfunction: Insights from epilepsy research. Front Neuroendocrinol. (2023) 71:101084. doi: 10.1016/j.yfrne.2023.101084
3. Hantsoo L, Jagodnik KM, Novick AM, Baweja R, di Scalea TL, Ozerdem A, et al. The role of the hypothalamic-pituitary-adrenal axis in depression across the female reproductive lifecycle: current knowledge and future directions. Front Endocrinol. (2023) 14:1295261. doi: 10.3389/fendo.2023.1295261
4. Liao B, Qiao J, Pang Y. Central Regulation of PCOS: Abnormal Neuronal-Reproductive-Metabolic Circuits in PCOS Pathophysiology. Front Endocrinol. (2021) 12:667422. doi: 10.3389/fendo.2021.667422
5. Neal-Perry G, Nejat E, Dicken C. The neuroendocrine physiology of female reproductive aging: an update. Maturitas. (2010) 67:34–8. doi: 10.1016/j.maturitas.2010.04.016
6. Bonavina G, Taylor HS. Endometriosis-associated infertility: From pathophysiology to tailored treatment. Front Endocrinol. (2022) 13:1020827. doi: 10.3389/fendo.2022.1020827
7. Conde DM, Verdade RC, Valadares ALR, Mella LFB, Pedro AO, Costa-Paiva L. Menopause and cognitive impairment: a narrative review of current knowledge. World J Psychiatry. (2021) 11:412–28. doi: 10.5498/wjp.v11.i8.412
8. Barry JA, Kuczmierczyk AR, Hardiman PJ. Reporting the rates of depression in polycystic ovary syndrome (PCOS). J Sex Med. (2014) 11:1882–3. doi: 10.1111/jsm.12503
9. Raimondo D, Raffone A, Renzulli F, Sanna G, Raspollini A, Bertoldo L, et al. Prevalence and risk factors of central sensitization in women with endometriosis. J Minim Invasive Gynecol. (2023) 30:73–80.e1. doi: 10.1016/j.jmig.2022.10.007
10. Chavez GN, Jaworsky K, Basu A. The effects of plant-derived phytochemical compounds and phytochemical-rich diets on females with polycystic ovarian syndrome: a scoping review of clinical trials. Int J Environ Res Public Health. (2023) 20:15. doi: 10.3390/ijerph20156534
11. Clemente-Suárez VJ, Redondo-Flórez L, Martín-Rodríguez A, Curiel-Regueros A, Rubio-Zarapuz A, Tornero-Aguilera JF. Impact of vegan and vegetarian diets on neurological health: a critical review. Nutrients. (2025) 17:884. doi: 10.3390/nu17050884
12. Dietz BM, Hajirahimkhan A, Dunlap TL, Bolton JL. Botanicals and their bioactive phytochemicals for women's health. Pharmacol Rev. (2016) 68:1026–73. doi: 10.1124/pr.115.010843
13. Makhoba XH, Viegas C, Mosa RA, Viegas FPD, Pooe OJ. Potential impact of the multi-target drug approach in the treatment of some complex diseases. Drug Des Devel Ther. (2020) 14:3235–49. doi: 10.2147/DDDT.S257494
14. Huo X, Gu Y, Zhang Y. The discovery of multi-target compounds with anti-inflammation activity from traditional Chinese medicine by TCM-target effects relationship spectrum. J Ethnopharmacol. (2022) 293:115289. doi: 10.1016/j.jep.2022.115289
15. Downs JL, Wise PM. The role of the brain in female reproductive aging. Mol Cell Endocrinol. (2009) 299:32–8. doi: 10.1016/j.mce.2008.11.012
16. Blair JA, McGee H, Bhatta S, Palm R, Casadesus G. Hypothalamic-pituitary-gonadal axis involvement in learning and memory and Alzheimer's disease: more than “just” estrogen. Front Endocrinol. (2015) 6:45. doi: 10.3389/fendo.2015.00045
17. Ferin M. The hypothalamic-hypophyseal-ovarian axis and the menstrual cycle. Glob Libr Women's Med, (2008) 10:10283. doi: 10.3843/GLOWM.10283
18. Tng EL. Kisspeptin signalling and its roles in humans. Singapore Med J. (2015) 56:649–56. doi: 10.11622/smedj.2015183
19. Xie Q, Kang Y, Zhang C, Xie Y, Wang C, Liu J, et al. The role of kisspeptin in the control of the hypothalamic-pituitary-gonadal axis and reproduction. Front Endocrinol. (2022) 13:925206. doi: 10.3389/fendo.2022.925206
20. Zheng JY, Zhu J, Wang Y, Tian ZZ. Effects of acupuncture on hypothalamic-pituitary-adrenal axis: current status and future perspectives. J Integr Med. (2024) 22:445–58. doi: 10.1016/j.joim.2024.06.004
21. Patisaul HB. Reproductive toxicology: endocrine disruption and reproductive disorders: impacts on sexually dimorphic neuroendocrine pathways. Reproduction. (2021) 162:F111–f30. doi: 10.1530/REP-20-0596
22. Minich DM. The phytoneuroendocrine system: connecting plants to human systems biology. Integr Med. (2024) 23:28–31.
23. Mattam U, Talari NK, Thiriveedi VR, Fareed M, Velmurugan S, Mahadev K, et al. Aging reduces kisspeptin receptor (GPR54) expression levels in the hypothalamus and extra-hypothalamic brain regions. Exp Ther Med. (2021) 22:1019. doi: 10.3892/etm.2021.10451
24. Liu X, Herbison AE. Kisspeptin regulation of neuronal activity throughout the central nervous system. Endocrinol Metab. (2016) 31:193–205. doi: 10.3803/EnM.2016.31.2.193
25. Niama W, Ben Said S, Rame C, Froment P, Mahouachi M, Dupont J. Selected plant extracts and female fertility: role in the regulation of the hypothalamo-pituitary-ovarian axis in normal and pathological conditions. Reprod Fertil Dev. (2025) 37:24120. doi: 10.1071/RD24120
26. Camille Melón L, Maguire J. GABAergic regulation of the HPA and HPG axes and the impact of stress on reproductive function. J Steroid Biochem Mol Biol. (2016) 160:196–203. doi: 10.1016/j.jsbmb.2015.11.019
27. Hong H, Lu X, Wu C, Chen J, Chen C, Zhang J, et al. A review for the pharmacological effects of paeoniflorin in the nervous system. Front Pharmacol. (2022) 13:898955. doi: 10.3389/fphar.2022.898955
28. Zhou XD, Yang XJ, Zheng Y, Qin ZS, Sha W, Chen G, et al. A proprietary herbal medicine, ameliorates mood disorder-like behavior and cognitive impairment in estrogen-deprived mice exposed to chronic unpredictable mild stress: implication for a potential therapy of menopause syndrome. Front Psychiatry. (2020) 11:579995. doi: 10.3389/fpsyt.2020.579995
29. Makker A, Goel MM, Mahdi AA. PI3K/PTEN/Akt and TSC/mTOR signaling pathways, ovarian dysfunction, and infertility: an update. J Mol Endocrinol. (2014) 53:R103–18. doi: 10.1530/JME-14-0220
30. Zughaibi TA, Suhail M, Tarique M, Tabrez S. Targeting PI3K/Akt/mTOR pathway by different flavonoids: a cancer chemopreventive approach. Int J Mol Sci. (2021) 22:12455. doi: 10.3390/ijms222212455
31. Muhanmode Y, Wen MK, Maitinuri A, Shen G. Curcumin and resveratrol inhibit chemoresistance in cisplatin-resistant epithelial ovarian cancer cells via targeting P13K pathway. Hum Exp Toxicol. (2022) 41:9603271221095929. doi: 10.1177/09603271221095929
32. Barabás K, Szabó-Meleg E, Ábrahám IM. Effect of inflammation on female gonadotropin-releasing hormone (GnRH) neurons: mechanisms and consequences. Int J Mol Sci. (2020) 21:529. doi: 10.3390/ijms21020529
33. Ameho S, Klutstein M. The effect of chronic inflammation on female fertility. Reproduction. (2025) 169:0197. doi: 10.1530/REP-24-0197
34. Garcia C, Velez LM, Ujagar N, Del Mundo Z, Nguyen T, Fox C, et al. Lipopolysaccharide-induced chronic inflammation increases female serum gonadotropins and shifts the pituitary transcriptomic landscape. Front Endocrinol. (2023) 14:1279878. doi: 10.3389/fendo.2023.1279878
35. Liu YW, Huang YT. Inhibitory effect of tanshinone IIA on rat hepatic stellate cells. PLoS ONE. (2014) 9:e103229. doi: 10.1371/journal.pone.0103229
36. Jang SI, Kim HJ, Kim YJ, Jeong SI, You YO. Tanshinone IIA inhibits LPS-induced NF-kappaB activation in RAW 2647 cells: possible involvement of the NIK-IKK, ERK1/2, p38 and JNK pathways. Eur J Pharmacol. (2006) 542:1–7. doi: 10.1016/j.ejphar.2006.04.044
37. Yu L, She T, Li M, Shi C, Han L, Cheng M. Tetramethylpyrazine inhibits angiotensin II-induced cardiomyocyte hypertrophy and tumor necrosis factor-α secretion through an NF-κB-dependent mechanism. Int J Mol Med. (2013) 32:717–22. doi: 10.3892/ijmm.2013.1436
38. Kawamura K, Kawamura N, Mulders SM, Sollewijn Gelpke MD, Hsueh AJ. Ovarian brain-derived neurotrophic factor (BDNF) promotes the development of oocytes into preimplantation embryos. Proc Natl Acad Sci U S A. (2005) 102:9206–11. doi: 10.1073/pnas.0502442102
39. Liu B, Liu Y, Li S, Chen P, Zhang J, Feng L. BDNF promotes mouse follicular development and reverses ovarian aging by promoting cell proliferation. J Ovarian Res. (2023) 16:83. doi: 10.1186/s13048-023-01163-9
40. Feng H, Xue M, Deng H, Cheng S, Hu Y, Zhou C. Ginsenoside and its therapeutic potential for cognitive impairment. Biomolecules. (2022) 12:1310. doi: 10.3390/biom12091310
41. Yang P, Fan M, Chen Y, Yang D, Zhai L, Fu B, et al. A novel strategy for the protective effect of ginsenoside Rg1 against ovarian reserve decline by the PINK1 pathway. Pharm Biol. (2025) 63:68–81. doi: 10.1080/13880209.2025.2453699
42. Pritschet L, Taylor CM, Santander T, Jacobs EG. Applying dense-sampling methods to reveal dynamic endocrine modulation of the nervous system. Curr Opin Behav Sci. (2021) 40:72–8. doi: 10.1016/j.cobeha.2021.01.012
43. Cieri F, Cera N, Griffa A, Mantini D, Esposito R. Editorial: Dynamic functioning of resting state networks in physiological and pathological conditions. Front Neurosci. (2020) 14:624401. doi: 10.3389/fnins.2020.624401
44. Aguirre GK. Functional neuroimaging: technical, logical, and social perspectives. Hastings Cent Rep. (2014) 44:S8-18. doi: 10.1002/hast.294
45. Avila-Varela DS, Hidalgo-Lopez E, Dagnino PC, Acero-Pousa I, Agua Ed, Deco G, et al. Whole-brain dynamics across the menstrual cycle: the role of hormonal fluctuations and age in healthy women. NPJ Women's Health. (2024) 2:8. doi: 10.1038/s44294-024-00012-4
46. Pletzer B, Harris TA, Scheuringer A, Hidalgo-Lopez E. The cycling brain: menstrual cycle related fluctuations in hippocampal and fronto-striatal activation and connectivity during cognitive tasks. Neuropsychopharmacology. (2019) 44:1867–75. doi: 10.1038/s41386-019-0435-3
47. Zhang Y, Huang Y, Liu N, Wang Z, Wu J, Li W, et al. Abnormal interhemispheric functional connectivity in patients with primary dysmenorrhea: a resting-state functional MRI study. Quant Imaging Med Surg. (2022) 12:1958–67. doi: 10.21037/qims-21-731
48. As-Sanie S, Kim J, Schmidt-Wilcke T, Sundgren PC, Clauw DJ, Napadow V, et al. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain. (2016) 17:1–13. doi: 10.1016/j.jpain.2015.09.008
49. Lainez NM, Coss D. Obesity, neuroinflammation, and reproductive function. Endocrinology. (2019) 160:2719–36. doi: 10.1210/en.2019-00487
50. Lonardo MS, Cacciapuoti N, Guida B, Di Lorenzo M, Chiurazzi M, Damiano S, et al. Hypothalamic-ovarian axis and adiposity relationship in polycystic ovary syndrome: physiopathology and therapeutic options for the management of metabolic and inflammatory aspects. Curr Obes Rep. (2024) 13:51–70. doi: 10.1007/s13679-023-00531-2
51. Zheng CY, Yu YX, Cao SY, Bai X. Epigenetics of inflammation in hypothalamus pituitary gonadal and neuroendocrine disorders. Semin Cell Dev Biol. (2024) 154:340–5. doi: 10.1016/j.semcdb.2023.04.001
52. Jabbour HN, Sales KJ, Catalano RD, Norman JE. Inflammatory pathways in female reproductive health and disease. Reproduction. (2009) 138:903–19. doi: 10.1530/REP-09-0247
53. Goshi N, Lam D, Bogguri C, George VK, Sebastian A, Cadena J, et al. Direct effects of prolonged TNF-α and IL-6 exposure on neural activity in human iPSC-derived neuron-astrocyte co-cultures. Front Cell Neurosci. (2025) 19:1512591. doi: 10.3389/fncel.2025.1512591
54. Wei Y, Liang Y, Lin H, Dai Y, Yao S. Autonomic nervous system and inflammation interaction in endometriosis-associated pain. J Neuroinflammation. (2020) 17:80. doi: 10.1186/s12974-020-01752-1
55. Maddern J, Grundy L, Castro J, Brierley SM. Pain in endometriosis. Front Cell Neurosci. (2020) 14:590823. doi: 10.3389/fncel.2020.590823
56. Weinreb O, Amit T, Mandel S, Youdim MB. Neuroprotective molecular mechanisms of (-)-epigallocatechin-3-gallate: a reflective outcome of its antioxidant, iron chelating and neuritogenic properties. Genes Nutr. (2009) 4:283–96. doi: 10.1007/s12263-009-0143-4
57. Singh NA, Mandal AK, Khan ZA. Potential neuroprotective properties of epigallocatechin-3-gallate (EGCG). Nutr J. (2016) 15:60. doi: 10.1186/s12937-016-0179-4
58. Li S, Wang Z, Liu G, Chen M. Neurodegenerative diseases and catechins: (–)-epigallocatechin-3-gallate is a modulator of chronic neuroinflammation and oxidative stress. Front Nutri. (2024) 11:1425839. doi: 10.3389/fnut.2024.1425839
59. Gu Q, Xia L, Du Q, Shao Y, He J, Wu P, et al. The therapeutic role and potential mechanism of EGCG in obesity-related precocious puberty as determined by integrated metabolomics and network pharmacology. Front Endocrinol. (2023) 14:1159657. doi: 10.3389/fendo.2023.1159657
60. Hung SW, Li Y, Chen X, Chu KO, Zhao Y, Liu Y, et al. Green tea epigallocatechin-3-gallate regulates autophagy in male and female reproductive cancer. Front Pharmacol. (2022) 13:906746. doi: 10.3389/fphar.2022.906746
61. Deng Y, Zhang X, Chen F, Huang J, Zhang D, Luo J. HO-1 mediated by PI3K/Akt/Nrf2 signaling pathway is involved in (-)-epigallocatechin-3-gallate-rescueing impaired cognitive function induced by chronic cerebral hypoperfusion in rat model. Exp Aging Res. (2022) 48:428–43. doi: 10.1080/0361073X.2021.2011689
62. Nan W, Zhonghang X, Keyan C, Tongtong L, Wanshu G, Zhongxin X. Epigallocatechin-3-gallate reduces neuronal apoptosis in rats after middle cerebral artery occlusion injury via PI3K/AKT/eNOS signaling pathway. Biomed Res Int. (2018) 2018:6473580. doi: 10.1155/2018/6473580
63. Ding ML, Ma H, Man YG, Lv HY. Protective effects of a green tea polyphenol, epigallocatechin-3-gallate, against sevoflurane-induced neuronal apoptosis involve regulation of CREB/BDNF/TrkB and PI3K/Akt/mTOR signalling pathways in neonatal mice. Can J Physiol Pharmacol. (2017) 95:1396–405. doi: 10.1139/cjpp-2016-0333
64. Suhail M, Rehan M, Tarique M, Tabrez S, Husain A, Zughaibi TA. Targeting a transcription factor NF-κB by green tea catechins using in silico and in vitro studies in pancreatic cancer. Front Nutr. (2022) 9:1078642. doi: 10.3389/fnut.2022.1078642
65. Joo SY, Song YA, Park YL, Myung E, Chung CY, Park KJ, et al. Epigallocatechin-3-gallate inhibits LPS-induced NF-κB and MAPK signaling pathways in bone marrow-derived macrophages. Gut Liver. (2012) 6:188–96. doi: 10.5009/gnl.2012.6.2.188
66. Kamal DAM, Salamt N, Zaid SSM, Mokhtar MH. Beneficial effects of green tea catechins on female reproductive disorders: a review. Molecules. (2021) 26:2675. doi: 10.3390/molecules26092675
67. Chen X, Man GCW, Hung SW, Zhang T, Fung LWY, Cheung CW, et al. Therapeutic effects of green tea on endometriosis. Crit Rev Food Sci Nutr. (2023) 63:3222–35. doi: 10.1080/10408398.2021.1986465
68. Hallman K, Aleck K, Quigley M, Dwyer B, Lloyd V, Szmyd M, et al. The regulation of steroid receptors by epigallocatechin-3-gallate in breast cancer cells. Breast Cancer (Dove Med Press). (2017) 9:365–73. doi: 10.2147/BCTT.S131334
69. Baker KM, Bauer AC. Green Tea Catechin, EGCG, Suppresses PCB 102-induced proliferation in estrogen-sensitive breast cancer cells. Int J Breast Cancer. (2015) 2015:163591. doi: 10.1155/2015/163591
70. Balunas MJ, Kinghorn AD. Natural compounds with aromatase inhibitory activity: an update. Planta Med. (2010) 76:1087–93. doi: 10.1055/s-0030-1250169
71. Satoh K, Sakamoto Y, Ogata A, Nagai F, Mikuriya H, Numazawa M, et al. Inhibition of aromatase activity by green tea extract catechins and their endocrinological effects of oral administration in rats. Food Chem Toxicol. (2002) 40:925–33. doi: 10.1016/S0278-6915(02)00066-2
72. Maleki V, Taheri E, Varshosaz P, Tabrizi FPF, Moludi J, Jafari-Vayghan H, et al. A comprehensive insight into effects of green tea extract in polycystic ovary syndrome: a systematic review. Reprod Biol Endocrinol. (2021) 19:147. doi: 10.1186/s12958-021-00831-z
73. Wahid S, Ramli MDC, Fazleen NE, Naim RM, Mokhtar MH. Exploring the therapeutic potential of natural products in polycystic ovarian syndrome (PCOS): a mini-review of lipid profile, blood glucose, and ovarian histological improvements. Life. (2024) 14:150. doi: 10.3390/life14010150
74. Hazimeh D, Massoud G, Parish M, Singh B, Segars J, Islam MS. Green tea and benign gynecologic disorders: a new trick for an old beverage? Nutrients. (2023) 15:1439. doi: 10.3390/nu15061439
75. Buhrmann C, Mobasheri A, Busch F, Aldinger C, Stahlmann R, Montaseri A, et al. Curcumin modulates nuclear factor kappaB (NF-kappaB)-mediated inflammation in human tenocytes in vitro: role of the phosphatidylinositol 3-kinase/Akt pathway. J Biol Chem. (2011) 286:28556–66. doi: 10.1074/jbc.M111.256180
76. Jobin C, Bradham CA, Russo MP, Brenner DA, Sartor RB, Juma B, et al. Curcumin blocks cytokine-mediated NF-κB activation and proinflammatory gene expression by inhibiting inhibitory factor I-κB kinase activity. J Immunol. (1999) 163:3474–83. doi: 10.4049/jimmunol.163.6.3474
77. Cho JW, Lee KS, Kim CW. Curcumin attenuates the expression of IL-1beta, IL-6, and TNF-alpha as well as cyclin E in TNF-alpha-treated HaCaT cells; NF-kappaB and MAPKs as potential upstream targets. Int J Mol Med. (2007) 19:469–74. doi: 10.3892/ijmm.19.3.469
78. Chowdhury I, Banerjee S, Driss A, Xu W, Mehrabi S, Nezhat C, et al. Curcumin attenuates proangiogenic and proinflammatory factors in human eutopic endometrial stromal cells through the NF-κB signaling pathway. J Cell Physiol. (2019) 234:6298–312. doi: 10.1002/jcp.27360
79. Vallée A, Lecarpentier Y. Curcumin and endometriosis. Int J Mol Sci. (2020) 21:2440. doi: 10.3390/ijms21072440
80. Cai B, Wang Q, Zhong L, Liu F, Wang X, Chen T, et al. Integrating network pharmacology, transcriptomics to reveal neuroprotective of curcumin activate PI3k/AKT pathway in Parkinson's disease. Drug Design Dev Ther. (2024) 18:2869–81. doi: 10.2147/DDDT.S462333
81. Cui Q, Li X, Zhu H. Curcumin ameliorates dopaminergic neuronal oxidative damage via activation of the Akt/Nrf2 pathway. Mol Med Rep. (2016) 13:1381–8. doi: 10.3892/mmr.2015.4657
82. Zhang Y, Cao H, Hu YY, Wang H, Zhang CJ. Inhibitory effect of curcumin on angiogenesis in ectopic endometrium of rats with experimental endometriosis. Int J Mol Med. (2011) 27:87–94. doi: 10.3892/ijmm.2010.552
83. Cao H, Wei YX, Zhou Q, Zhang Y, Guo XP, Zhang J. Inhibitory effect of curcumin in human endometriosis endometrial cells via downregulation of vascular endothelial growth factor. Mol Med Rep. (2017) 16:5611–7. doi: 10.3892/mmr.2017.7250
84. Talebpour A, Mohammadifard M, Zare Feyzabadi R, Mahmoudzadeh S, Rezapour H, Saharkhiz M, et al. Effect of curcumin on inflammatory biomarkers and iron profile in patients with premenstrual syndrome and dysmenorrhea: a randomized controlled trial. Physiol Rep. (2023) 11:e15763. doi: 10.14814/phy2.15763
85. Bahrami A, Zarban A, Rezapour H, Agha Amini Fashami A, Ferns GA. Effects of curcumin on menstrual pattern, premenstrual syndrome, and dysmenorrhea: a triple-blind, placebo-controlled clinical trial. Phytothera Res. (2021) 35:6954–62. doi: 10.1002/ptr.7314
86. Sharifipour F, Siahkal SF, Qaderi K, Mohaghegh Z, Zahedian M, Azizi F. Effect of curcumin on dysmenorrhea and symptoms of premenstrual syndrome: a systematic review and meta-analysis. Korean J Fam Med. (2024) 45:96–104. doi: 10.4082/kjfm.23.0184
87. Zhang W, Qian S, Tang B, Kang P, Zhang H, Shi C. Resveratrol inhibits ferroptosis and decelerates heart failure progression via Sirt1/p53 pathway activation. J Cell Mol Med. (2023) 27:3075–89. doi: 10.1111/jcmm.17874
88. Zhao M, Li J, Li Z, Yang D, Wang D, Sun Z, et al. SIRT1 regulates mitochondrial damage in N2a cells treated with the prion protein fragment 106-126 via PGC-1α-TFAM-mediated mitochondrial biogenesis. Int J Mol Sci. (2024) 25:9707. doi: 10.3390/ijms25179707
89. Wu SK, Wang L, Wang F, Zhang J. Resveratrol improved mitochondrial biogenesis by activating SIRT1/PGC-1α signal pathway in SAP. Sci Rep. (2024) 14:26216. doi: 10.1038/s41598-024-76825-9
90. Yamamoto M, Takahashi Y. The essential role of SIRT1 in hypothalamic-pituitary axis. Front Endocrinol. (2018) 9:605. doi: 10.3389/fendo.2018.00605
91. Estienne A, Bongrani A, Ramé C, Kurowska P, Błaszczyk K, Rak A, et al. Energy sensors and reproductive hypothalamo-pituitary ovarian axis (HPO) in female mammals: Role of mTOR (mammalian target of rapamycin), AMPK (AMP-activated protein kinase) and SIRT1 (Sirtuin 1). Mol Cell Endocrinol. (2021) 521:111113. doi: 10.1016/j.mce.2020.111113
92. Iervolino M, Lepore E, Forte G, Laganà AS, Buzzaccarini G, Unfer V. Natural molecules in the management of polycystic ovary syndrome (PCOS): an analytical review. Nutrients. (2021) 13:1677. doi: 10.3390/nu13051677
93. Xu L, Botchway BOA, Zhang S, Zhou J, Liu X. Inhibition of NF-κB signaling pathway by resveratrol improves spinal cord injury. Front Neurosci. (2018) 12:690. doi: 10.3389/fnins.2018.00690
94. Karimi A, Tutunchi H, Naeini F, Vajdi M, Mobasseri M, Najafipour F. The therapeutic effects and mechanisms of action of resveratrol on polycystic ovary syndrome: a comprehensive systematic review of clinical, animal and in vitro studies. Clin Exp Pharmacol Physiol. (2022) 49:935–49. doi: 10.1111/1440-1681.13698
95. Evans HM, Howe PR, Wong RH. Effects of resveratrol on cognitive performance, mood and cerebrovascular function in post-menopausal women; a 14-week randomised placebo-controlled intervention trial. Nutrients. (2017) 9:27. doi: 10.3390/nu9010027
96. Wu H, Xue J, Di H, Lv C, Hao Y, Nie Z. Resveratrol improves ovarian function in aged rat by inhibiting oxidative stress and activating the Sirt1. Gen Physiol Biophys. (2022) 41:53–61. doi: 10.4149/gpb_2021040
97. Nishigaki A, Tsubokura H, Tsuzuki-Nakao T, Okada H. Hypoxia: role of SIRT1 and the protective effect of resveratrol in ovarian function. Reprod Med Biol. (2022) 21:e12428. doi: 10.1002/rmb2.12428
98. Dull AM, Moga MA, Dimienescu OG, Sechel G, Burtea V, Anastasiu CV. Therapeutic approaches of resveratrol on endometriosis via anti-inflammatory and anti-angiogenic pathways. Molecules. (2019) 24:667. doi: 10.3390/molecules24040667
99. Wang C, Chen Z, Zhao X, Lin C, Hong S, Lou Y, et al. Transcriptome-based analysis reveals therapeutic effects of resveratrol on endometriosis in arat model. Drug Des Devel Ther. (2021) 15:4141–55. doi: 10.2147/DDDT.S323790
100. Wang J, Jia R, Celi P, Zhuo Y, Ding X, Zeng Q, et al. Resveratrol alleviating the ovarian function under oxidative stress by alternating microbiota related tryptophan-kynurenine pathway. Front Immunol. (2022) 13:911381. doi: 10.3389/fimmu.2022.911381
101. Desmawati D, Sulastri D. Phytoestrogens and their health effect. Open Access Maced J Med Sci. (2019) 7:495–9. doi: 10.3889/oamjms.2019.086
102. Jiang Y, Gong P, Madak-Erdogan Z, Martin T, Jeyakumar M, Carlson K, et al. Mechanisms enforcing the estrogen receptor β selectivity of botanical estrogens. FASEB J. (2013) 27:4406–18. doi: 10.1096/fj.13-234617
103. Jiang T, Wang XQ, Ding C, Du XL. Genistein attenuates isoflurane-induced neurotoxicity and improves impaired spatial learning and memory by regulating cAMP/CREB and BDNF-TrkB-PI3K/Akt signaling. Korean J Physiol Pharmacol. (2017) 21:579–89. doi: 10.4196/kjpp.2017.21.6.579
104. Ariyani W, Koibuchi N. The effect of soy isoflavones in brain development: the emerging role of multiple signaling pathways and future perspectives. Endocr J. (2024) 71:317–33. doi: 10.1507/endocrj.EJ23-0314
105. Yoo DY, Jung S, Kang JS, Baek JH, Park KH, Lee DH, et al. Isoflavone-enriched soybean leaves (glycine max) alleviate cognitive impairment induced by ovariectomy and modulate PI3K/Akt signaling in the hippocampus of C57BL6 mice. Nutrients. (2022) 14:4753. doi: 10.3390/nu14224753
106. Ariyani W, Miyazaki W, Amano I, Hanamura K, Shirao T, Koibuchi N. Soy isoflavones accelerate glial cell migration via GPER-mediated signal transduction pathway. Front Endocrinol. (2020) 11:554941. doi: 10.3389/fendo.2020.554941
107. Acosta-Martinez M. PI3K: an attractive candidate for the central integration of metabolism and reproduction. Front Endocrinol. (2011) 2:110. doi: 10.3389/fendo.2011.00110
108. Gu C, Zhang Q, Li Y, Li R, Feng J, Chen W, et al. The PI3K/AKT Pathway-the potential key mechanisms of traditional chinese medicine for stroke. Front Med. (2022) 9:900809. doi: 10.3389/fmed.2022.900809
109. Mueed A, Shibli S, Korma SA, Madjirebaye P, Esatbeyoglu T, Deng Z. Flaxseed bioactive compounds: chemical composition, functional properties, food applications and health benefits-related gut microbes. Foods. (2022) 11:3307. doi: 10.3390/foods11203307
110. Baldi S, Tristán Asensi M, Pallecchi M, Sofi F, Bartolucci G, Amedei A. Interplay between Lignans and gut microbiota: nutritional, functional and methodological aspects. Molecules. (2023) 28:343. doi: 10.3390/molecules28010343
111. Senizza A, Rocchetti G, Mosele JI, Patrone V, Callegari ML, Morelli L, et al. Lignans and gut microbiota: an interplay revealing potential health implications. Molecules. (2020) 25:5709. doi: 10.3390/molecules25235709
112. Jang WY, Kim MY, Cho JY. Antioxidant, anti-inflammatory, anti-menopausal, and anti-cancer effects of lignans and their metabolites. Int J Mol Sci. (2022) 23:15482. doi: 10.3390/ijms232415482
113. Hassanein EHM, Althagafy HS, Baraka MA, Abd-Alhameed EK, Ibrahim IM, Abd El-Maksoud MS, et al. The promising antioxidant effects of lignans: Nrf2 activation comes into view. Naunyn Schmiedebergs Arch Pharmacol. (2024) 397:6439–58. doi: 10.1007/s00210-024-03102-x
114. Meng W, Chao W, Kaiwei Z, Sijia M, Jiajia S, Shijie X. Bioactive compounds from Chinese herbal plants for neurological health: mechanisms, pathways, and functional food applications. Front Nutr. (2025) 12:1537363. doi: 10.3389/fnut.2025.1537363
115. Zhou X, Seto SW, Chang D, Kiat H, Razmovski-Naumovski V, Chan K, et al. Synergistic effects of Chinese herbal medicine: a comprehensive review of methodology and current research. Front Pharmacol. (2016) 7:201. doi: 10.3389/fphar.2016.00201
116. Liu Y, Gao Z, Zhao Y, Kong L, Ji X, Wu J, et al. Exploring bioactive constituents and pharmacological effects of Scutellaria baicalensis georgi: a review. Nat Prod Commun. (2024) 19:1934578X241266692. doi: 10.1177/1934578X241266692
117. Sowndhararajan K, Deepa P, Kim M, Park SJ, Kim S. Neuroprotective and cognitive enhancement potentials of baicalin: a review. Brain Sci. (2018) 8:104. doi: 10.3390/brainsci8060104
118. Gao Y, Wei Y, Wang Y, Gao F, Chen Z. Lycium Barbarum: a traditional chinese herb and a promising anti-aging agent. Aging Dis. (2017) 8:778–91. doi: 10.14336/AD.2017.0725
119. Uță G, Manolescu D, Avram S. Therapeutic properties of several chemical compounds of Salvia officinalis L. in Alzheimer's disease. Mini Rev Med Chem. (2021) 21:1421–30. doi: 10.2174/1389557521999201230200209
120. Su X, Yao Z, Li S, Sun H. Synergism of Chinese herbal medicine: illustrated by Danshen compound. Evid Based Complement Alternat Med. (2016) 2016:7279361. doi: 10.1155/2016/7279361
121. Patil VM, Das S, Balasubramanian K. Quantum chemical and docking insights into bioavailability enhancement of curcumin by piperine in pepper. J Phys Chem A. (2016) 120:3643–53. doi: 10.1021/acs.jpca.6b01434
122. Pratti VL, Thomas M, Bhoite R, Satyavrat V. Investigating bioavailability of curcumin and piperine combination in comparison to turmeric rhizomes: an in vitro study. J Exp Pharmacol. (2024) 16:37–47. doi: 10.2147/JEP.S427818
123. Li H, Liu Y, Sun Y, Guo H, Lv S, Guo W, et al. Targeting astrocytes polarization after spinal cord injury: a promising direction. Front Cellular Neurosci. (2024) 18:1478741. doi: 10.3389/fncel.2024.1478741
124. Rao Y, Li J, Qiao R, Luo J, Liu Y. Synergistic effects of tetramethylpyrazine and astragaloside IV on spinal cord injury via alteration of astrocyte A1/A2 polarization through the Sirt1-NF-κB pathway. Int Immunopharmacol. (2024) 131:111686. doi: 10.1016/j.intimp.2024.111686
125. Rao Y, Li J, Qiao R, Luo J, Liu Y. Tetramethylpyrazine and Astragaloside IV have synergistic effects against spinal cord injury-induced neuropathic pain via the OIP5-AS1/miR-34a/Sirt1/NF-κB axis. Int Immunopharmacol. (2023) 115:109546. doi: 10.1016/j.intimp.2022.109546
126. Chen Y, Peng F, Yang C, Hou H, Xing Z, Chen J, et al. SIRT1 activation by 2,3,5,6-tetramethylpyrazine alleviates neuroinflammation via inhibiting M1 microglia polarization. Front Immunol. (2023) 14:1206513. doi: 10.3389/fimmu.2023.1206513
127. Kuang G, Zhao Y, Wang L, Wen T, Liu P, Ma B, et al. Astragaloside IV alleviates acute hepatic injury by regulating macrophage polarization and pyroptosis via activation of the AMPK/SIRT1 signaling pathway. Phytother Res. (2025) 39:733–46. doi: 10.1002/ptr.8403
128. Li L, Zou J, Zhou M, Li H, Zhou T, Liu X, et al. Phenylsulfate-induced oxidative stress and mitochondrial dysfunction in podocytes are ameliorated by Astragaloside IV activation of the SIRT1/PGC1α/Nrf1 signaling pathway. Biomed Pharmacother. (2024) 177:117008. doi: 10.1016/j.biopha.2024.117008
129. Fang L, Cheng H, Chen W, Peng C, Liu Y, Zhang C. Therapeutic effects of Tanshinone IIA and Tetramethylpyrazine nanoemulsions on cognitive impairment and neuronal damage in Alzheimer's disease rat models. J Pharm Pharmacol. (2024) 76:1169–77. doi: 10.1093/jpp/rgae069
130. Chen J, Chen Q, Xiao P, Jin W, Yu L. A novel framework for uncovering the coordinative spectrum-effect correlation of the effective components of Yangyin Tongnao Granules on cerebral ischemia-reperfusion injury in rats. J Ethnopharmacol. (2025) 337:118844. doi: 10.1016/j.jep.2024.118844
131. Fang RC, Tsai YT, Lai JN, Yeh CH, Wu CT. The traditional Chinese medicine prescription pattern of endometriosis patients in Taiwan: a population-based study. Evid Based Complement Alternat Med. (2012) 2012:591391. doi: 10.1155/2012/591391
132. Meng W, Ta N, Wang F. Add-on effect of Guizhi Fuling formula to mifepristone for endometriosis: a meta-analysis of randomized controlled trials. Medicine. (2019) 98:e16878. doi: 10.1097/MD.0000000000016878
133. Zhang S, Lai X, Wang X, Liu G, Wang Z, Cao L, et al. Deciphering the pharmacological mechanisms of guizhi-fuling capsule on primary dysmenorrhea through network pharmacology. Front Pharmacol. (2021). 12:613104. doi: 10.3389/fphar.2021.613104
134. Wang X, Shi Y, Xu L, Wang Z, Wang Y, Shi W, et al. Traditional Chinese medicine prescription Guizhi Fuling Pills in the treatment of endometriosis. Int J Med Sci. (2021) 18:2401–8. doi: 10.7150/ijms.55789
135. Zheng W, Li M, Wang Y, Lv B, Zhang X, Chen L, et al. Guizhi fuling capsule exhibits antidysmenorrhea activity by inhibition of cyclooxygenase activity. Evid Based Complement Alternat Med. (2020) 2020:8607931. doi: 10.1155/2020/8607931
136. Xu YM, Lu FM, Xu HC, Zhang J, Hei SY, Qiu YH, et al. Kai-Xin-San improves cognitive impairment via Wnt/β-catenin and IRE1/XBP1s signalings in APP/PS1 mice. Rejuvenation Res. (2023) 26:105–15. doi: 10.1089/rej.2022.0063
137. Shan X, Lv S, Huang P, Zhang W, Jin C, Liu Y, et al. Classic famous prescription kai-xin-san ameliorates alzheimer's disease via the wnt/β-catenin signaling pathway. Mol Neurobiol. (2024) 61:2297–312. doi: 10.1007/s12035-023-03707-y
138. Arar MA, Erbil N. The effect of menopausal symptoms on women's daily life activities. Prz Menopauzalny. (2023) 22:6–15. doi: 10.5114/pm.2023.126436
139. Gava G, Orsili I, Alvisi S, Mancini I, Seracchioli R, Meriggiola MC. Cognition, mood and sleep in menopausal transition: the role of menopause hormone therapy. Medicina. (2019) 55:668. doi: 10.3390/medicina55100668
140. Shieu MM, Braley TJ, Becker J, Dunietz GL. The interplay among natural menopause, insomnia, and cognitive health: a population-based study. Nat Sci Sleep. (2023) 15:39–48. doi: 10.2147/NSS.S398019
141. Echeverria V, Echeverria F, Barreto GE, Echeverría J, Mendoza C. Estrogenic plants: to prevent neurodegeneration and memory loss and other symptoms in women after menopause. Front Pharmacol. (2021) 12:644103. doi: 10.3389/fphar.2021.644103
142. Gaire B, Song J, Lee S, Kim H. Neuroprotective effect of four flavonoids in the root of Scutellaria baicalensis Georgi. Planta Med. (2012) 78:PF71. doi: 10.1055/s-0032-1320618
143. Han Y, Gu S, Li Y, Qian X, Wang F, Huang JH. Neuroendocrine pathogenesis of perimenopausal depression. Front Psychiatry. (2023) 14:1162501. doi: 10.3389/fpsyt.2023.1162501
144. Cheng PF, Chen JJ, Zhou XY, Ren YF, Huang W, Zhou JJ, et al. Do soy isoflavones improve cognitive function in postmenopausal women? A meta-analysis. Menopause. (2015) 22:198–206. doi: 10.1097/GME.0000000000000290
145. Cui C, Birru RL, Snitz BE, Ihara M, Kakuta C, Lopresti BJ, et al. Effects of soy isoflavones on cognitive function: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev. (2020) 78:134–44. doi: 10.1093/nutrit/nuz050
146. Ronchetti S, Labombarda F, Del Core J, Roig P, De Nicola AF, Pietranera L. The phytoestrogen genistein improves hippocampal neurogenesis and cognitive impairment and decreases neuroinflammation in an animal model of metabolic syndrome. J Neuroendocrinol. (2025) 37:e13480. doi: 10.1111/jne.13480
147. Henderson VW, St John JA, Hodis HN, Kono N, McCleary CA, Franke AA, et al. Long-term soy isoflavone supplementation and cognition in women: a randomized, controlled trial. Neurology. (2012) 78:1841–8. doi: 10.1212/WNL.0b013e318258f822
148. Chen LR, Ko NY, Chen KH. Isoflavone supplements for menopausal women: a systematic review. Nutrients. (2019) 11: 2649. doi: 10.3390/nu11112649
149. Alhazmi MA, Khalifa FK, Zeyadi M. A Effect of Soy Isoflavones on the Performance of Cerebral Neurotransmitter Systems in Adult and Aged Male Rats. Jeddah: King Abdulaziz University Jeddah. (2021).
150. Zhang S, Fan W, Hu H, Wen L, Gong M, Liu B, et al. Subcortical volume changes in early menopausal women and correlation with neuropsychological tests. Front Aging Neurosci. (2021) 13:738679. doi: 10.3389/fnagi.2021.738679
151. Yang W, Ye Y, Cai Y, Wang G, Wang M, Zhang X. Exploration on the improvement of cognitive function and inflammatory response in perimenopausal patients with mild cognitive impairment by self-prepared Ningshen prescription. Evid Based Complement Alternat Med. (2022) 2022:4311031. doi: 10.1155/2022/4311031
152. Cao C, Xiao J, Liu M, Ge Z, Huang R, Qi M, et al. Active components, derived from Kai-xin-san, a herbal formula, increase the expressions of neurotrophic factor NGF and BDNF on mouse astrocyte primary cultures via cAMP-dependent signaling pathway. J Ethnopharmacol. (2018) 224:554–62. doi: 10.1016/j.jep.2018.06.007
153. Wei S. Potential therapeutic action of natural products from traditional Chinese medicine on Alzheimer's disease animal models targeting neurotrophic factors. Fundam Clin Pharmacol. (2016) 30:490–501. doi: 10.1111/fcp.12222
154. Li C, Huang B, Zhang YW. Chinese herbal medicine for the treatment of depression: effects on the neuroendocrine-immune network. Pharmaceuticals. (2021) 14:65. doi: 10.3390/ph14010065
155. Zhang X, Li S, Chen Z, Liang W, Pei S, Gou F, et al. Tanshinone IIA participates in the treatment of endometriosis by regulating adhesion, invasion, angiogenesis and inhibition of PI3K/Akt/mTOR signaling pathway. Mol Med Rep. (2023) 28:13108. doi: 10.3892/mmr.2023.13108
156. Wang Y, Gao L, Chen J, Li Q, Huo L, Wang Y, et al. Pharmacological modulation of Nrf2/HO-1 signaling pathway as a therapeutic target of Parkinson's disease. Front Pharmacol. (2021) 12:757161. doi: 10.3389/fphar.2021.757161
157. Zhao P, Soukup ST, Hegevoss J, Ngueu S, Kulling SE, Diel P. Anabolic effect of the traditional Chinese medicine compound tanshinone IIA on myotube hypertrophy is mediated by estrogen receptor. Planta Med. (2015) 81:578–85. doi: 10.1055/s-0035-1545883
158. Gorniak SL, Wagner VE, Vaughn K, Perry J, Cox LG, Hibino H, et al. Functional near infrared spectroscopy detects cortical activation changes concurrent with memory loss in postmenopausal women with Type II Diabetes. Exp Brain Res. (2023) 241:1555–67. doi: 10.1007/s00221-023-06581-1
159. Ficek-Tani B, Horien C, Ju S, Xu W, Li N, Lacadie C, et al. Sex differences in default mode network connectivity in healthy aging adults. Cereb Cortex. (2023) 33:6139–51. doi: 10.1093/cercor/bhac491
160. Frey BN, Hall GB, Attard S, Yucel K, Skelin I, Steiner M, et al. Shift in the brain network of emotional regulation in midlife women: is the menopausal transition the turning point? Menopause. (2010) 17:840–5. doi: 10.1097/gme.0b013e3181df840f
161. Carmichael OT, Pillai S, Shankapal P, McLellan A, Kay DG, Gold BT, et al. A combination of essential fatty acids, panax ginseng extract, and green tea catechins modifies brain fMRI signals in healthy older adults. J Nutr Health Aging. (2018) 22:837–46. doi: 10.1007/s12603-018-1028-2
162. Xu LW, Jia M, Salchow R, Kentsch M, Cui XJ, Deng HY, et al. Efficacy and side effects of chinese herbal medicine for menopausal symptoms: a critical review. Evid Based Complement Alternat Med. (2012) 2012:568106. doi: 10.1155/2012/568106
163. Zhu X, Liew Y, Liu ZL. Chinese herbal medicine for menopausal symptoms. Cochrane Database Syst Rev. (2016) 3:Cd009023. doi: 10.1002/14651858.CD009023.pub2
164. Nedeljkovic M, Tian L, Ji P, Déglon-Fischer A, Stute P, Ocon E, et al. Effects of acupuncture and Chinese herbal medicine (Zhi Mu 14) on hot flushes and quality of life in postmenopausal women: results of a four-arm randomized controlled pilot trial. Menopause. (2014) 21:15–24. doi: 10.1097/GME.0b013e31829374e8
165. Wang YP, Yu Q. The treatment of menopausal symptoms by traditional Chinese medicine in Asian countries. Climacteric. (2021) 24:64–7. doi: 10.1080/13697137.2020.1832461
166. Ayhan I, Altuntaş I, Üzümcü I, Erbaş O. Premenstrual syndrome mechanism in the brain. Demiroglu Sci Univers Florence Nightingale J Med. (2021) 7:213–24. doi: 10.5606/fng.btd.2021.25069
167. Parry BL. The role of central serotonergic dysfunction in the aetiology of premenstrual dysphoric disorder: therapeutic implications. CNS Drugs. (2001) 15:277–85. doi: 10.2165/00023210-200115040-00003
168. Shorter E, Segesser K. Traditional Chinese medicine and Western psychopharmacology: building bridges. Phytother Res. (2013) 27:1739–44. doi: 10.1002/ptr.4940
169. Rychlewski P, Kamgar E, Zembrzuska J, Mildner-Szkudlarz S, Kowalczewski PŁ. Determination of the contents of bioactive compounds in St. John's wort (Hypericum perforatum): Comparison of commercial and wild samples. Open Chemistry. (2023) 21:20220347. doi: 10.1515/chem-2022-0347
170. Fornal CA, Metzler CW, Mirescu C, Stein SK, Jacobs BL. Effects of standardized extracts of St. John's wort on the single-unit activity of serotonergic dorsal raphe neurons in awake cats: Comparisons with fluoxetine and sertraline. Neuropsychopharmacology. (2001) 25:858–70. doi: 10.1016/S0893-133X(01)00297-4
171. Canning S, Waterman M, Orsi N, Ayres J, Simpson N, Dye L. The efficacy of Hypericum perforatum (St John's wort) for the treatment of premenstrual syndrome: a randomized, double-blind, placebo-controlled trial. CNS Drugs. (2010) 24:207–25. doi: 10.2165/11530120-000000000-00000
172. Cui YH, Zheng Y. A meta-analysis on the efficacy and safety of St John's wort extract in depression therapy in comparison with selective serotonin reuptake inhibitors in adults. Neuropsychiatr Dis Treat. (2016) 12:1715–23. doi: 10.2147/NDT.S106752
173. Sun X, Li X, Pan R, Xu Y, Wang Q, Song M. Total Saikosaponins of Bupleurum yinchowense reduces depressive, anxiety-like behavior and increases synaptic proteins expression in chronic corticosterine-treated mice. BMC Complement Altern Med. (2018) 18:117. doi: 10.1186/s12906-018-2186-9
174. Gao M, An L, Yu Y, Wang J, Hou Y, Xu Q, et al. Brain activation during processing of depression emotion in college students with premenstrual syndrome in China: preliminary findings. Front Psychiatry. (2022) 13:856443. doi: 10.3389/fpsyt.2022.856443
175. Heidari H, Amani R, Feizi A, Askari G, Kohan S, Tavasoli P. Vitamin D Supplementation for Premenstrual Syndrome-Related inflammation and antioxidant markers in students with vitamin D deficient: a randomized clinical trial. Sci Rep. (2019) 9:14939. doi: 10.1038/s41598-019-51498-x
176. Kumar N, Gupta G, Anilkumar K, Fatima N, Karnati R, Reddy GV, et al. 15-Lipoxygenase metabolites of α-linolenic acid, [13-(S)-HPOTrE and 13-(S)-HOTrE], mediate anti-inflammatory effects by inactivating NLRP3 inflammasome. Sci Rep. (2016) 6:31649. doi: 10.1038/srep31649
177. Zhao G, Etherton TD, Martin KR, Gillies PJ, West SG, Kris-Etherton PM. Dietary alpha-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. Am J Clin Nutr. (2007) 85:385–91. doi: 10.1093/ajcn/85.2.385
178. Mohammadi MM, Dehghan Nayeri N, Mashhadi M, Varaei S. Effect of omega-3 fatty acids on premenstrual syndrome: a systematic review and meta-analysis. J Obstet Gynaecol Res. (2022) 48:1293–305. doi: 10.1111/jog.15217
179. Olivera A, Moore TW, Hu F, Brown AP, Sun A, Liotta DC, et al. Inhibition of the NF-κB signaling pathway by the curcumin analog, 3,5-Bis(2-pyridinylmethylidene)-4-piperidone (EF31): anti-inflammatory and anti-cancer properties. Int Immunopharmacol. (2012) 12:368–77. doi: 10.1016/j.intimp.2011.12.009
180. Khayat S, Fanaei H, Kheirkhah M, Moghadam ZB, Kasaeian A, Javadimehr M. Curcumin attenuates severity of premenstrual syndrome symptoms: a randomized, double-blind, placebo-controlled trial. Complement Ther Med. (2015) 23:318–24. doi: 10.1016/j.ctim.2015.04.001
181. Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet. (2021) 397:839–52. doi: 10.1016/S0140-6736(21)00389-5
182. Chen Y, LI T. Unveiling the mechanisms of pain in endometriosis: comprehensive analysis of inflammatory sensitization and therapeutic potential. Int J Mol Sci. (2025) 26:1770. doi: 10.3390/ijms26041770
183. McNamara HC, Frawley HC, Donoghue JF, Readman E, Healey M, Ellett L, et al. Peripheral, central, and cross sensitization in endometriosis-associated pain and comorbid pain syndromes. Front Reprod Health. (2021) 3:729642. doi: 10.3389/frph.2021.729642
184. Rathod S, Shanoo A, Acharya N. Endometriosis: a comprehensive exploration of inflammatory mechanisms and fertility implications. Cureus. (2024) 16:e66128. doi: 10.7759/cureus.66128
185. Lin Y, Hou R, Zhang T, Chung JPW, Wang CC, Zhao R. Efficacy and safety of Chinese herbal medicine for endometriosis associated pain. Am J Chinese Med. (2022) 50:1095–111. doi: 10.1142/S0192415X22500446
186. Burks-Wicks C, Cohen M, Fallbacher J, N Taylor R, Wieser F. A western primer of chinese herbal therapy in endometriosis and infertility. Women's Health. (2005) 1:447–63. doi: 10.2217/17455057.1.3.447
187. Kolahdouz-Mohammadi R, Shidfar F, Khodaverdi S, Arablou T, Heidari S, Rashidi N, et al. Resveratrol treatment reduces expression of MCP-1, IL-6, IL-8 and RANTES in endometriotic stromal cells. J Cell Mol Med. (2021) 25:1116–27. doi: 10.1111/jcmm.16178
188. Gołabek-Grenda A, Juzwa W, Kaczmarek M, Olejnik A. Resveratrol and its natural analogs mitigate immune dysregulation and oxidative imbalance in the endometriosis niche simulated in a co-culture system of endometriotic cells and macrophages. Nutrients. (2024) 16:3483. doi: 10.3390/nu16203483
189. Arablou T, Aryaeian N, Khodaverdi S, Kolahdouz-Mohammadi R, Moradi Z, Rashidi N, et al. The effects of resveratrol on the expression of VEGF, TGF-β, and MMP-9 in endometrial stromal cells of women with endometriosis. Sci Rep. (2021) 11:6054. doi: 10.1038/s41598-021-85512-y
190. Mendes da Silva D, Gross LA, Neto EPG, Lessey BA, Savaris RF. The use of resveratrol as an adjuvant treatment of pain in endometriosis: a randomized clinical trial. J Endocr Soc. (2017) 1:359–69. doi: 10.1210/js.2017-00053
191. Bashir ST, Redden CR, Raj K, Arcanjo RB, Stasiak S, Li Q, et al. Endometriosis leads to central nervous system-wide glial activation in a mouse model of endometriosis. J Neuroinflammation. (2023) 20:59. doi: 10.1186/s12974-023-02713-0
192. Orr NL, Huang AJ, Liu YD, Noga H, Bedaiwy MA, Williams C, et al. Association of central sensitization inventory scores with pain outcomes after endometriosis surgery. JAMA Network Open. (2023) 6:e230780-e. doi: 10.1001/jamanetworkopen.2023.0780
193. Forouzanfar F, Hosseinzadeh H. Medicinal herbs in the treatment of neuropathic pain: a review. Iran J Basic Med Sci. (2018) 21:347–58. doi: 10.22038/IJBMS.2018.24026.6021
194. Bina F, Soleymani S, Toliat T, Hajimahmoodi M, Tabarrai M, Abdollahi M, et al. Plant-derived medicines for treatment of endometriosis: a comprehensive review of molecular mechanisms. Pharmacol Res. (2019) 139:76–90. doi: 10.1016/j.phrs.2018.11.008
195. Meresman GF, Götte M, Laschke MW. Plants as source of new therapies for endometriosis: a review of preclinical and clinical studies. Hum Reprod Update. (2021) 27:367–92. doi: 10.1093/humupd/dmaa039
196. Liu C, Liu R, Tang M, Yang X, Gong X. Role and mechanism of nursing cooperation and tetramethylpyrazine application in post-operative pain in patients undergoing total knee arthroplasty. Exp Ther Med. (2019) 17:2366–72. doi: 10.3892/etm.2019.7203
197. Leng YF, Gao XM, Wang SX, Xing YH. Effects of tetramethylpyrazine on neuronal apoptosis in the superficial dorsal horn in a rat model of neuropathic pain. Am J Chin Med. (2012) 40:1229–39. doi: 10.1142/S0192415X12500917
198. Liu Y, Gao J, Peng M, Meng H, Ma H, Cai P, et al. A review on central nervous system effects of gastrodin. Front Pharmacol. (2018) 9:24. doi: 10.3389/fphar.2018.00024
199. Dai Y, Ban W, Yang Z. Gastrodin, a promising natural small molecule for the treatment of central nervous system disorders, and its recent progress in synthesis, pharmacology and pharmacokinetics. Int J Mol Sci. (2024) 25:9540. doi: 10.3390/ijms25179540
200. Yao YY, Bian LG, Yang P, Sui Y, Li R, Chen YL, et al. Gastrodin attenuates proliferation and inflammatory responses in activated microglia through Wnt/β-catenin signaling pathway. Brain Res. (2019) 1717:190–203. doi: 10.1016/j.brainres.2019.04.025
201. Phillips MM, Rimmer CA. Functional foods and dietary supplements. Anal Bioanal Chem. (2013) 405:4323–4. doi: 10.1007/s00216-013-6846-9
202. Stepanova A, Plutnitskiy A, Gameeva E. Biologically active supplements: safety, efficacy, market review. Farmakoekonomika Modern Pharmacoeconomics and Pharmacoepidemiology. (2025) 17:558–71. doi: 10.17749/2070-4909/farmakoekonomika.2024.269
203. Mackinnon M. The Hormone Type Cookbook: Nourishing Recipes for balAncing the 7 Different Hormone Types-Recipes for Healthy Cycles, PMS, PCOS, Fertility, and Menopause. Beverly: Fair Winds Press. (2023).
204. Thornfeldt C. Cosmeceuticals containing herbs: fact, fiction, and future. Dermatol Surg. (2005) (7 Pt 2):873–80. doi: 10.1111/j.1524-4725.2005.31734
205. Geller SE, Studee L. Botanical and dietary supplements for menopausal symptoms: what works, what does not. J Womens Health (Larchmt). (2005) 14:634–49. doi: 10.1089/jwh.2005.14.634
206. Carroll DG. Nonhormonal therapies for hot flashes in menopause. Am Fam Physician. (2006) 73:457–64. doi: 10.2165/00128413-200615370-00005
207. Franco OH, Chowdhury R, Troup J, Voortman T, Kunutsor S, Kavousi M, et al. Use of plant-based therapies and menopausal symptoms: a systematic review and meta-analysis. JAMA. (2016) 315:2554–63. doi: 10.1001/jama.2016.8012
208. Geller SE, Shulman LP, van Breemen RB, Banuvar S, Zhou Y, Epstein G, et al. Safety and efficacy of black cohosh and red clover for the management of vasomotor symptoms: a randomized controlled trial. Menopause. (2009) 16:1156–66. doi: 10.1097/gme.0b013e3181ace49b
209. Loch EG, Selle H, Boblitz N. Treatment of premenstrual syndrome with a phytopharmaceutical formulation containing Vitex agnus castus. J Womens Health Gend Based Med. (2000) 9:315–20. doi: 10.1089/152460900318515
210. van Die MD, Burger HG, Teede HJ, Bone KM. Vitex agnus-castus extracts for female reproductive disorders: a systematic review of clinical trials. Planta Med. (2013) 79:562–75. doi: 10.1055/s-0032-1327831
211. Mahboubi M. Evening primrose (Oenothera biennis) oil in management of female ailments. J Menopausal Med. (2019) 25:74–82. doi: 10.6118/jmm.18190
212. Csupor D, Lantos T, Hegyi P, Benkő R, Viola R, Gyöngyi Z, et al. Vitex agnus-castus in premenstrual syndrome: A meta-analysis of double-blind randomised controlled trials. Complement Ther Med. (2019) 47:102190. doi: 10.1016/j.ctim.2019.08.024
213. Canning SE, Waterman MG, Dye L. Dietary supplements and herbal remedies for premenstrual syndrome (PMS): a systematic research review of the evidence for their efficacy. J Reprod Infant Psychol. (2006) 24:363–78. doi: 10.1080/02646830600974170
214. Ochiai A, Kuroda K. Preconception resveratrol intake against infertility: friend or foe? Reprod Med Biol. (2020) 19:107–13. doi: 10.1002/rmb2.12303
215. Rahman SU, Huang Y, Zhu L, Feng S, Khan IM, Wu J, et al. Therapeutic role of green tea polyphenols in improving fertility: a review. Nutrients. (2018) 10:834. doi: 10.3390/nu10070834
216. Ottowitz WE, Dougherty DD, Fischman AJ, Hall JE. [18F]2-fluoro-2-deoxy-D-glucose positron emission tomography demonstration of estrogen negative and positive feedback on luteinizing hormone secretion in women. J Clin Endocrinol Metab. (2008) 93:3208–14. doi: 10.1210/jc.2008-0203
217. Chan AS, Cheung MC, Sze SL, Leung WW, Shi D. An herbal nasal drop enhanced frontal and anterior cingulate cortex activity. Evid Based Complement Alternat Med. (2011) 2011:543648. doi: 10.1093/ecam/nep198
218. Yang H, Li X, Guo XL, Zhou J, Shen ZF, Liu LY, et al. Moxibustion for primary dysmenorrhea: A resting-state functional magnetic resonance imaging study exploring the alteration of functional connectivity strength and functional connectivity. Front Neurosci. (2022) 16:969064. doi: 10.3389/fnins.2022.969064
219. Carhart-Harris RL, Erritzoe D, Williams T, Stone JM, Reed LJ, Colasanti A, et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci U S A. (2012) 109:2138–43. doi: 10.1073/pnas.1119598109
220. Gao H, Zhang H, Wang L, Zhang C, Feng Z, Li Z, et al. Altered amygdala functional connectivity after real-time functional MRI emotion self-regulation training. Neuroreport. (2023) 34:537–45. doi: 10.1097/WNR.0000000000001921
221. Xie Y, Cai K, Dai J, Wei G. Enhanced integrity of white matter microstructure in mind-body practitioners: a whole-brain diffusion tensor imaging study. Brain Sci. (2023) 13:691. doi: 10.3390/brainsci13040691
222. Keong NC, Lock C, Soon S, Hernowo AT, Czosnyka Z, Czosnyka M, et al. Diffusion tensor imaging profiles can distinguish diffusivity and neural properties of white matter injury in hydrocephalus vs. non-hydrocephalus using a strategy of a periodic table of DTI elements. Front Neurol. (2022) 13:868026. doi: 10.3389/fneur.2022.868026
223. Haller S, Zaharchuk G, Thomas DL, Lovblad KO, Barkhof F, Golay X. Arterial spin labeling perfusion of the brain: emerging clinical applications. Radiology. (2016) 281:337–56. doi: 10.1148/radiol.2016150789
224. Li Q, Shen S, Lei M. Sensitivity of functional arterial spin labelling in detecting cerebral blood flow changes. Br J Hosp Med (Lond). (2024) 85:1–21. doi: 10.12968/hmed.2024.0433
225. Qiu M, Zhou D, Zhu H, Shao Y, Li Y, Wang Y, et al. Alterations of cerebral blood flow and its connectivity patterns measured with arterial spin labeling in mild cognitive impairment. Curr Alzheimer Res. (2023) 20:567–76. doi: 10.2174/0115672050241163231017073139
226. Witte AV, Kerti L, Margulies DS, Flöel A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J Neurosci. (2014) 34:7862–70. doi: 10.1523/JNEUROSCI.0385-14.2014
227. Girard R, Météreau E, Thomas J, Pugeat M, Qu C, Dreher JC. Hormone therapy at early post-menopause increases cognitive control-related prefrontal activity. Sci Rep. (2017) 7:44917. doi: 10.1038/srep44917
228. Shanmugan S, Epperson CN. Estrogen and the prefrontal cortex: towards a new understanding of estrogen's effects on executive functions in the menopause transition. Hum Brain Mapp. (2014) 35:847–65. doi: 10.1002/hbm.22218
229. Marchant IC, Chabert S, Martínez-Pinto J, Sotomayor-Zárate R, Ramírez-Barrantes R, Acevedo L, et al. Estrogen, cognitive performance, and functional imaging studies: what are we missing about neuroprotection? Front Cellular Neurosci. (2022) 16:866122. doi: 10.3389/fncel.2022.866122
230. Avberšek LK, Repovš G. Deep learning in neuroimaging data analysis: Applications, challenges, and solutions. Front Neuroimag. (2022) 1:981642. doi: 10.3389/fnimg.2022.981642
231. Szabo E, Timmers I, Borsook D, Simons LE, Sieberg CB. Altered anterior insula functional connectivity in adolescent and young women with endometriosis-associated pain: Pilot resting-state fMRI study. Eur J Paediatr Neurol. (2022) 41:80–90. doi: 10.1016/j.ejpn.2022.10.004
232. Zheng P, Jia S, Guo D, Chen S, Zhang W, Cheng A, et al. Central sensitization-related changes in brain function activity in a rat endometriosis-associated pain model. J Pain Res. (2020) 13:95–107. doi: 10.2147/JPR.S232313
233. Zeb A, Ahmad S, Ullah F, Ayaz M, Sadiq A. Anti-nociceptive activity of ethnomedicinally important analgesic plant isodon rugosus wall ex benth: mechanistic study and identifications of bioactive compounds. Front Pharmacol. (2016) 7:200. doi: 10.3389/fphar.2016.00200
234. Sic A, Manzar A, Knezevic NN. The role of phytochemicals in managing neuropathic pain: how much progress have we made? Nutrients. (2024) 16:4342. doi: 10.3390/nu16244342
235. Monti DA, Vedaei F, Tobia A, Navarreto E, Hriso C, Ross R, et al. Brain functional connectivity changes on fMRI in patients with chronic pelvic pain treated with the Neuro Emotional Technique: a randomised controlled trial. J Obstet Gynaecol. (2025) 45:2472767. doi: 10.1080/01443615.2025.2472767
236. Sankarasubramanian V, Cunningham DA, Potter-Baker KA, Beall EB, Roelle SM, Varnerin NM, et al. Transcranial direct current stimulation targeting primary motor versus dorsolateral prefrontal cortices: proof-of-concept study investigating functional connectivity of thalamocortical networks specific to sensory-affective information processing. Brain Connect. (2017) 7:182–96. doi: 10.1089/brain.2016.0440
237. Letzen JE, Craggs JG, Perlstein WM, Price DD, Robinson ME. Functional connectivity of the default mode network and its association with pain networks in irritable bowel patients assessed via lidocaine treatment. J Pain. (2013) 14:1077–87. doi: 10.1016/j.jpain.2013.04.003
238. Weizman L, Dayan L, Brill S, Nahman-Averbuch H, Hendler T, Jacob G, et al. Cannabis analgesia in chronic neuropathic pain is associated with altered brain connectivity. Neurology. (2018) 91:e1285–e94. doi: 10.1212/WNL.0000000000006293
239. Yu S, Xie M, Liu S, Guo X, Tian J, Wei W, et al. Resting-state functional connectivity patterns predict acupuncture treatment response in primary dysmenorrhea. Front Neurosci. (2020) 14:559191. doi: 10.3389/fnins.2020.559191
240. Park SM, Lee SH, Zhao H, Kim J, Jang JY, Choi Y, et al. Literature review on the interdisciplinary biomarkers of multi-target and multi-time herbal medicine therapy to modulate peripheral systems in cognitive impairment. Front Neurosci. (2023) 17:1108371. doi: 10.3389/fnins.2023.1108371
241. Dalenberg JR, Peretti DE, Marapin LR, van der Stouwe AMM, Renken RJ, Tijssen MAJ. Next move in movement disorders: neuroimaging protocols for hyperkinetic movement disorders. Front Hum Neurosci. (2024) 18:1406786. doi: 10.3389/fnhum.2024.1406786
242. Gerretsen P, Müller DJ, Tiwari A, Mamo D, Pollock BG. The intersection of pharmacology, imaging, and genetics in the development of personalized medicine. Dialogues Clin Neurosci. (2009) 11:363–76. doi: 10.31887/DCNS.2009.11.4/pgerretsen
243. Zhou X, Li CG, Chang D, Bensoussan A. Current status and major challenges to the safety and efficacy presented by Chinese herbal medicine. Medicines. (2019) 6:14. doi: 10.3390/medicines6010014
244. Zhang B, Zhang J, Hu J. Thinking about translational medicine and traditional Chinese medicine. J Transl Med. (2012) 10:A33. doi: 10.1186/1479-5876-10-S2-A33
245. Zhou Y, Yang J, He Y, Lv Y, Wang C, Deng H, et al. Characteristic analysis of clinical trials for new traditional Chinese medicines in mainland China from 2013 to 2021. Front Med. (2022) 9:1008683. doi: 10.3389/fmed.2022.1008683
246. Deng C, Chen H, Meng Z, Meng S. Roles of traditional chinese medicine regulating neuroendocrinology on AD treatment. Front Endocrinol. (2022) 13:955618. doi: 10.3389/fendo.2022.955618
247. Shindikar A, Singh A, Nobre M, Kirolikar S. Curcumin and resveratrol as promising natural remedies with nanomedicine approach for the effective treatment of triple negative breast cancer. J Oncol. (2016) 2016:9750785. doi: 10.1155/2016/9750785
248. Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. (2007) 4:807–18. doi: 10.1021/mp700113r
249. Manzari-Tavakoli A, Babajani A, Tavakoli MM, Safaeinejad F, Jafari A. Integrating natural compounds and nanoparticle-based drug delivery systems: A novel strategy for enhanced efficacy and selectivity in cancer therapy. Cancer Med. (2024) 13:e7010. doi: 10.1002/cam4.7010
250. Hamilton AE, Gilbert RJ. Curcumin release from biomaterials for enhanced tissue regeneration following injury or disease. Bioengineering. (2023) 10:262. doi: 10.3390/bioengineering10020262
251. Gera M, Sharma N, Ghosh M, Huynh DL, Lee SJ, Min T, et al. Nanoformulations of curcumin: an emerging paradigm for improved remedial application. Oncotarget. (2017) 8:66680–98. doi: 10.18632/oncotarget.19164
252. Saghari Y, Movahedi M, Tebianian M, Entezari M. The neuroprotective effects of curcumin nanoparticles on the cerebral ischemia-reperfusion injury in the rats-the roles of the protein kinase RNA-like ER kinase/extracellular signal-regulated kinase and transcription factor EB proteins. Cell J. (2024) 26:62–9. doi: 10.22074/cellj.2023.1995696.1257
253. Makadia HK, Siegel SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers (Basel). (2011) 3:1377–97. doi: 10.3390/polym3031377
254. Han FY, Thurecht KJ, Whittaker AK, Smith MT. Bioerodable PLGA-based microparticles for producing sustained-release drug formulations and strategies for improving drug loading. Front Pharmacol. (2016) 7:185. doi: 10.3389/fphar.2016.00185
255. Hua S, Vaughan B. In vitro comparison of liposomal drug delivery systems targeting the oxytocin receptor: a potential novel treatment for obstetric complications. Int J Nanomedicine. (2019) 14:2191–206. doi: 10.2147/IJN.S198116
256. Khishvand MA, Yeganeh EM, Zarei M, Soleimani M, Mohammadi M, Mahjub R. Development, statistical optimization, and characterization of resveratrol-containing solid lipid nanoparticles (SLNs) and determination of the efficacy in reducing neurodegenerative symptoms related to Alzheimer's disease: in vitro and in vivo study. Biomed Res Int. (2024) 2024:7877265. doi: 10.1155/2024/7877265
257. Negut I, Bita B. Polymeric micellar systems-a special emphasis on “smart” drug delivery. Pharmaceutics. (2023) 15:976. doi: 10.3390/pharmaceutics15030976
258. Chen Q, Jiang Y, Yuan L, Liu L, Zhu X, Chen R, et al. Preparation, characterization, and antioxidant properties of self-assembled nanomicelles of curcumin-loaded amphiphilic modified chitosan. Molecules. (2024) 29:2693. doi: 10.3390/molecules29112693
259. Yang X, Li Z, Wang N, Li L, Song L, He T, et al. Curcumin-encapsulated polymeric micelles suppress the development of colon cancer in vitro and in vivo. Sci Rep. (2015) 5:10322. doi: 10.1038/srep10322
260. Liu C, Wu H, Duan H, Hou Y, Wang S, Liu Y, et al. An EGCG-mediated self-assembled micellar complex acts as a bioactive drug carrier. Food Chem. (2023) 418:135939. doi: 10.1016/j.foodchem.2023.135939
261. Akter T, Zahan MS, Nawal N, Rahman MH, Tanjum TN, Arafat KI, et al. Potentials of curcumin against polycystic ovary syndrome: Pharmacological insights and therapeutic promises. Heliyon. (2023) 9:e16957. doi: 10.1016/j.heliyon.2023.e16957
262. Volak LP, Ghirmai S, Cashman JR, Court MH. Curcuminoids inhibit multiple human cytochromes P450, UDP-glucuronosyltransferase, and sulfotransferase enzymes, whereas piperine is a relatively selective CYP3A4 inhibitor. Drug Metab Dispos. (2008) 36:1594–605. doi: 10.1124/dmd.108.020552
263. Atal CK, Dubey RK, Singh J. Biochemical basis of enhanced drug bioavailability by piperine: evidence that piperine is a potent inhibitor of drug metabolism. J Pharmacol Exp Ther. (1985) 232:258–62. doi: 10.1016/S0022-3565(25)20081-7
264. Singh J, Dubey RK, Atal CK. Piperine-mediated inhibition of glucuronidation activity in isolated epithelial cells of the guinea-pig small intestine: evidence that piperine lowers the endogeneous UDP-glucuronic acid content. J Pharmacol Exp Ther. (1986) 236:488–93. doi: 10.1016/S0022-3565(25)38910-X
265. Kanojiya D, Parmar G, Chauhan B, Gondalia S, Rakholiya M. Phytosomes: a contemporary method for delivering novel herbal drugs. J Nat Remed. (2024). doi: 10.18311/jnr/2024/34470
266. Lu M, Qiu Q, Luo X, Liu X, Sun J, Wang C, et al. Phyto-phospholipid complexes (phytosomes): A novel strategy to improve the bioavailability of active constituents. Asian J Pharm Sci. (2019) 14:265–74. doi: 10.1016/j.ajps.2018.05.011
267. Gnananath K, Sri Nataraj K, Ganga Rao B. Phospholipid complex technique for superior bioavailability of phytoconstituents. Adv Pharm Bull. (2017) 7:35–42. doi: 10.15171/apb.2017.005
268. Baghel P, Roy A, Verma S, Satapathy T, Bahadur S. Amelioration of lipophilic compounds in regards to bioavailability as self-emulsifying drug delivery system (SEDDS). Future J Pharmaceut Sci. (2020) 6:42. doi: 10.1186/s43094-020-00042-0
269. Salawi A. Self-emulsifying drug delivery systems: a novel approach to deliver drugs. Drug Deliv. (2022) 29:1811–23. doi: 10.1080/10717544.2022.2083724
270. Araya H, Nagao S, Tomita M, Hayashi M. The novel formulation design of self-emulsifying drug delivery systems (SEDDS) type O/W microemulsion I: enhancing effects on oral bioavailability of poorly water soluble compounds in rats and beagle dogs. Drug Metab Pharmacokinet. (2005) 20:244–56. doi: 10.2133/dmpk.20.244
271. Nigade PM, Patil SL, Tiwari SS. Self emulsifying drug delivery system (SEDDS): a review. Int J Pharm Biol Sci. (2012) 2:42–52.
272. Huang N, Huang W, Wu J, Long S, Luo Y, Huang J. Possible opportunities and challenges for traditional Chinese medicine research in 2035. Front Pharmacol. (2024) 15:1426300. doi: 10.3389/fphar.2024.1426300
273. Zhou J, Qu F. Treating gynaecological disorders with traditional Chinese medicine: a review. Afr J Tradit Complement Altern Med. (2009) 6:494–517. doi: 10.4314/ajtcam.v6i4.57181
274. Wang H, Chen Y, Wang L, Liu Q, Yang S, Wang C. Advancing herbal medicine: enhancing product quality and safety through robust quality control practices. Front Pharmacol. (2023) 14:1265178. doi: 10.3389/fphar.2023.1265178
275. Dubale S, Usure RE, Mekasha YT, Hasen G, Hafiz F, Kebebe D, et al. Traditional herbal medicine legislative and regulatory framework: a cross-sectional quantitative study and archival review perspectives. Front Pharmacol. (2025) 16:1475297. doi: 10.3389/fphar.2025.1475297
276. Mück F, Scotti F, Mauvisseau Q, Raclariu-Manolică AC, Schrøder-Nielsen A, Wangensteen H, et al. Complementary authentication of Chinese herbal products to treat endometriosis using DNA metabarcoding and HPTLC shows a high level of variability. Front Pharmacol. (2023) 14:1305410. doi: 10.3389/fphar.2023.1305410
277. Mück F, Scotti F, Mauvisseau Q, Thorbek BLG, Wangensteen H, de Boer HJ. Three-tiered authentication of herbal traditional Chinese medicine ingredients used in women's health provides progressive qualitative and quantitative insight. Front Pharmacol. (2024) 15:1353434. doi: 10.3389/fphar.2024.1353434
Keywords: neuroendocrine-reproductive axis, plant-derived compounds, women's health, functional foods, phytoestrogens
Citation: Liu X, Bin C, Zhou Z, Zeng T, Wu K, Luo Y, Liu Q and Wei S (2025) The neurobiology of plant-based therapeutics in women's reproductive health: mechanisms, efficacy, and clinical translation. Front. Nutr. 12:1591534. doi: 10.3389/fnut.2025.1591534
Received: 11 March 2025; Accepted: 24 April 2025;
Published: 20 May 2025.
Edited by:
Chuanfeng Tang, Nanjing University of Chinese Medicine, ChinaReviewed by:
Shaoxing Guan, Sun Yat-sen University, ChinaKaihui Zhang, Guangzhou University of Chinese Medicine, China
Ruitong Li, Sun Yat-sen University, China
Copyright © 2025 Liu, Bin, Zhou, Zeng, Wu, Luo, Liu and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaobin Wei, d2Vpc2hhb2JpbjU2MjBAMTYzLmNvbQ==
†These authors have contributed equally to this work
‡These authors share first authorship
 Xue Liu
Xue Liu Chengli Bin
Chengli Bin Zehui Zhou1†‡
Zehui Zhou1†‡ Tongtong Zeng
Tongtong Zeng Shaobin Wei
Shaobin Wei