- College of Life Sciences, 237 SFH, Brigham Young University, Provo, UT, United States
Background: This investigation was conducted to determine the relationship between fruit intake and abdominal adiposity in 1,707 U.S. children.
Methods: The children were randomly selected as part of the National Health and Nutrition Examination Survey (NHANES), so the sample represented U.S. children 8–11 years old. A cross-sectional design was employed. Fruit consumption was measured using the average of two 24-h dietary recalls. Fruit intake was expressed as the percent of total energy derived from fruit, not including fruit juices. Abdominal adiposity was indexed using two methods: waist circumference and the sagittal abdominal diameter (SAD). Covariates were age, sex, race, household size, year of assessment, recreational computer time, physical activity, total energy consumption, and intake of carbohydrate, protein, fat, fiber, sugar, and saturated fat. The outcome measures were waist circumference and sagittal abdominal diameter.
Results: According to the findings, mean fruit consumption was 10.1% of total energy intake. With fruit intake and abdominal adiposity both treated as continuous variables, after controlling for all the covariates, there were significant inverse linear relationships between the log10 of fruit intake and waist circumference (F = 6.5, p = 0.0143) and SAD (F = 7.0, p = 0.0112). Similarly, with fruit consumption divided into 3 categories (None, Low, and Moderate/High), means of the abdominal adiposity variables differed across the fruit categories in a dose–response pattern (SAD: F = 3.4, p = 0.0407; Waist: F = 2.9, p = 0.0657), after adjusting for all the covariates.
Conclusion: In this nationally representative sample of U.S. children, fruit consumption was low. Higher levels of fruit consumption were predictive of lower levels of abdominal adiposity. These findings support the recommendation of the U.S. Dietary Guidelines for Americans that encourage children to eat more fruit. Given the results, physicians, teachers, and parents should educate and encourage children about the importance of fruit consumption.
1 Introduction
In the United States, over 40% of children have overweight or obesity (1). In the past 50 years, the prevalence of overweight and obesity in children and adolescents has increased almost five-fold (2). Unfortunately, the risk of serious health conditions is much higher among youth who carry excess weight and body fat. Specifically, these individuals are more likely to have insulin resistance, high cholesterol, and high blood pressure, to name a few (1). They are also at increased risk of type 2 diabetes and cardiovascular disease early in their lives. Emotional issues such as depression and anxiety, as well as bullying and other social troubles, are also more likely in children with overweight or obesity (1).
The fact that overweight and obesity often lead to a multitude of health problems in children and adults is well-established. However, research indicates that the location of body fat is also a critical factor affecting disease risk. In their recent 2025 publication, the Lancet Diabetes & Endocrinology Commission stated that the commonly used body mass index (BMI) does not differentiate between fat and lean mass, and it does not account for differences in the distribution of body fat. The Commission declared that alternative measures, such as the waist circumference, might be more accurate for the detection of excess body fat and obesity-related health risks (2).
In a sample of 174 children from the United Kingdom, abdominal adiposity was a significant predictor of artery stiffness (3). In an investigation of 948 Saudi children, there were significant relationships between abdominal adiposity and numerous metabolic parameters (4). Labyak et al. (5) found that abdominal obesity in 145 youth was predictive of elevated triglycerides, HbA1c, systolic blood pressure, and a total risk score. Other investigations have also shown that abdominal adiposity is a good predictor of metabolic issues in children (6, 7).
The causes of childhood obesity and abdominal adiposity are multidimensional and complex. However, it is clear that diet plays a substantial role. Although there are many dietary components that contribute to excess weight and body fat, numerous investigations indicate that the energy density of food is an important factor (1, 8–12). Food energy density is the amount of energy in a food divided by its weight (kilocalories/grams). In short, the number of kilocalories (kcal) per gram of food. Some foods have very low energy densities, such as watermelon [0.30], strawberries [0.32], and peaches [0.39], whereas other foods have high energy densities, such as many candy bars [~4.8], bacon [5.3], and potato chips [5.4] (13).
The energy density of food is a function of the water content, the amount of fiber in the food, and the fat level (14). Foods that contain little water and fiber and have large amounts of dietary fat are energy dense. These items contain high amounts of energy but provide relatively little food to eat (14). Dietary patterns that include large amounts of energy dense foods often lead to excess body weight, whereas regular consumption of low energy dense foods tends to result in less obesity and abdominal adiposity (8, 9, 11, 12). Among the many foods available in the U.S., fruits tend to be among the lowest in energy density.
The relationship between fruit intake and obesity has been studied extensively in adults. For example, in a meta-analysis of prospective cohort studies, higher fruit intake was inversely and favorably associated with weight change in 17 of 20 investigations (15). In the classic Women’s Health Study, those in the highest quintile of baseline fruit intake had 13% less risk of having overweight or obesity compared to those in the lowest quintile (16). In a recent cross-sectional investigation, over 10,000 Korean adults were studied. Frequency of whole fruit intake was associated significantly and inversely with the prevalence of abdominal obesity (17). Similarly, in a large cross-sectional study of Peruvian adults, servings of fruit were negatively associated with central fat distribution (18).
Although the fruit consumption and adiposity relationship has been investigated many times in adults, research in children is less common. Moreover, few investigations have focused on the link between fruit intake and central adiposity in children, and even fewer studies have employed a large sample of children representative of U.S. youth. Therefore, the present investigation was conducted to evaluate the association between fruit intake and abdominal adiposity in a large random sample of U.S. children.
2 Methods
2.1 Study design and sample
The present cross-sectional investigation was conducted using data supplied by the U.S. National Health and Nutrition Examination Survey, NHANES. The survey is a government-sponsored project administered by the U.S. National Center for Health Statistics (NCHS) and the Centers for Disease Control and Prevention (CDC).
Written informed consent was obtained before data were collected by NHANES. The data gathering process and the data files were approved by the Ethics Review Board of the NCHS (19). The NHANES data files included no confidential information that could be connected to an individual. The data that support the findings of this study are available in NHANES Questionnaires, Datasets, and Related Documentation at https://wwwn.cdc.gov/nchs/nhanes/Default.aspx (20).
The data associated with the current investigation were gathered during a 6-year period, from 2011–2016. Other years were not included because the sagittal abdominal diameter variable was only measured during this time frame. The ethical approval code associated with these 6 years of data collection was Protocol #2011–17 (19).
NHANES employs a 4-step sampling procedure to randomly select participants using census data. Specifically, U.S. counties, then blocks or roads, then dwelling units, and lastly individuals were randomly selected. A total of 1,814 children, 8–11 years of age, were randomly selected. Children who reported extremely low energy intakes (< 400 kcal) on either of the two 24-h recall days were not included in the sample (n = 10). Children who were classified as underweight based on the age and sex-specific results of the CDC 2000 Growth Charts were not included in the sample due to concerns of an eating disorder or significant illness (n = 56). Also, children with missing data for one or more variables used in the present investigation were not included in the sample (n = 41). Hence, the final sample included 1,707 participants.
2.2 Measurement methods
Fruit intake was the exposure variable in this investigation. Abdominal adiposity, measured using two methods, was the outcome measure. Age, sex, race, household size, year of assessment, physical activity, recreational computer use, total energy intake, and consumption (grams per 1,000 kcal) of carbohydrate, protein, fat, sugar, saturated fat, and dietary fiber were the covariates.
2.2.1 Fruit intake and other dietary variables
Fruit consumption and intake of 7 additional dietary variables were measured using the average of two 24-h dietary recall assessments. When appropriate, a parent or guardian assisted with the dietary assessment. The initial dietary evaluation was in-person. A telephone interview was employed to gather dietary information 3 to 10 days later for the second recall assessment. Mean values from the two recall assessments were used. Both 24-h recall assessments collected in-depth information about all foods and beverages consumed during the 24-h period before the interview (12:00 AM to 12:00 AM) (21). A computer provided a standardized interview outline. Scripts were employed to guide the interviewer (21). The Automated Multiple Pass Method (AMPM) guided the interviewer (22).
The in-person dietary interviews included several examples to help gauge the amount of food consumed, such as different sized plates, bowls, glasses, cups, spoons, etc. Following completion of the initial 24-h recall assessment, participants were furnished with sample glasses, cups, plates, etc. and a food model booklet to help during the subsequent telephone diet interview.
The focus of the present study was on fruit consumption, defined according to the U.S. Department of Agriculture (USDA) (13). Fruit juice was not included. Because larger individuals typically consume more total food and more food energy than smaller individuals, rather than focusing on the grams of fruit consumed, the present study concentrated on the percentage of total energy derived from fruit. Specifically, based on the weight of each fruit eaten during the two 24-h recall assessments, the energy value of each fruit was calculated. Then, the total energy intake from all fruits consumed was divided by the average total energy intake of the individual over the two dietary assessments, resulting in the percentage of total energy derived from fruit.
2.2.2 Abdominal adiposity
Abdominal adiposity was indexed using two methods: waist circumference (waist) and sagittal abdominal diameter (SAD). Both are excellent measures of abdominal obesity and metabolic risk in adults (23–28) and children (4, 6, 29).
For the waist and SAD measurements, health technicians participated in 2 days of extensive training and periodic follow-up evaluations. The individual performing the measurements was assisted by a recorder. For the waist measurement, a wall mirror was also used to check the level of the measuring tape. The waist measurement was calculated to the nearest 0.1 cm after the individual exhaled a normal breath. Detailed measurement methods are available online (30).
The sagittal abdominal diameter (SAD) is an index of abdominal height with the participant in the supine position on an examination table. SAD was assessed by using a sliding-beam, abdominal caliper (Holtain, Ltd., Wales, UK). A second SAD measurement was always taken, and the mean was calculated. If the SAD measurements differed by more than 0.5 cm, then a third and possibly a fourth measurement was taken, and the mean was calculated (30). SAD intra-tester precision is excellent (31). The measurement method for assessing SAD is explained in detail online (30).
2.2.3 Covariates
NHANES recorded sex as male or female based on the sex recorded at birth. Race was recorded as non-Hispanic Black, non-Hispanic White, Mexican American, Other race/multiracial, or Other Hispanic. Household size was the number of individuals currently living in the dwelling unit, truncated at 7. Year of assessment was based on the NHANES 2-year data collection cycles. SAD data were only collected by NHANES from 2011 to 2016. Physical activity was the number of days during the past week the child participated in physical activity for an hour or more. Time spent in recreational computer use was the average number of hours spent on a computer each day over the past 30 days, outside of work or school. Information about the dietary covariates was collected as part of the two 24-h dietary recall assessments.
2.3 Data analysis
Because NHANES utilizes a unique, multi-stage random sampling procedure to select participants, statistical procedures involving NHANES data require special procedures. Specifically, each statistical analysis of NHANES data must be weighted so that the results can be generalized to the U.S. population. Additionally, even though the number of children in this investigation was large (N = 1,707), the unique sampling procedure employed by NHANES resulted in a significant reduction in the degrees of freedom (df) available. Specifically, df equaled the number of clusters [91] minus the number of strata [44], resulting in 47 df, instead of approximately 1,707 df. Therefore, statistical power was much lower than the sample size would typically dictate.
There were two outcome variables, SAD and waist circumference, both measures of abdominal adiposity. The exposure variable was the percentage of total energy derived from fruit. Five demographic covariates were controlled, age, sex, race, year of assessment, and household size. Additionally, physical activity, recreational computer use, and numerous dietary measures were the covariates.
To assess the associations between the exposure variable, fruit intake, and the outcome variables (waist and SAD), two different analyses were performed. First, both fruit intake and the outcome variables were treated as continuous variables. This allowed the linear association to be evaluated using the SAS SurveyReg procedure. Because the fruit intake variable was skewed and more than 1 in 4 participants reported no energy derived from fruit, the log10 of the fruit intake variable was used as the exposure variable. Because the log10 of 0 cannot be calculated, 0.1 was used instead of 0, resulting in a substantial reduction in the skewness of the distribution.
Second, fruit intake was treated as a categorical variable and mean differences in abdominal adiposity (SAD and waist) were compared across the fruit consumption categories using SAS SurveyReg. There were three fruit intake categories: None, Low, and Moderate/High. Children in the “None” category had zero fruit intake. Youth in the Low category consumed some fruit but less than 9.2% of their total energy intake was derived from fruit. Children in the Moderate/High category consumed at least 9.2% of their energy from fruit. The Low and Moderate/High fruit groups were constructed to be equal in size. Partial correlation and the LSmeans procedure were used to control differences in the covariates and to compare the adjusted means to determine if they differed significantly.
Effect modification was tested to determine the extent that the fruit intake and the abdominal adiposity relationships were consistent within subgroups. SAS version 9.4 (SAS Institute, Inc., Cary, NC) was utilized to examine relationships of interest. Alpha was set at < 0.05 to establish significance. Marginal significance was p > 0.05 and p < 0.10. The statistical tests were all two-sided.
3 Results
From 2011 to 2016, there were 44 primary sampling units (PSU) selected randomly by NHANES in the U.S. From these PSU, 91 clusters were randomly selected. A total of 1707 randomly selected children were included in the sample. They represented the population of 8–11-year-old children in the U.S. Mean (±SE) age, SAD, and waist of the sample were 9.5 ± 0.04 years, 15.4 ± 0.12 (cm), and 68.5 ± 0.44 (cm), respectively. Average (±SE) number of days per week that the children engaged in at least 1 h of physical activity was 5.7 ± 0.07 and mean (±SE) recreational computer use per day was 1.2 ± 0.05 h. Average (±SE) fruit intake per day was 10.1 ± 0.6% of total energy intake. Table 1 shows the percentile distributions associated with each of the continuous measures included in the investigation.
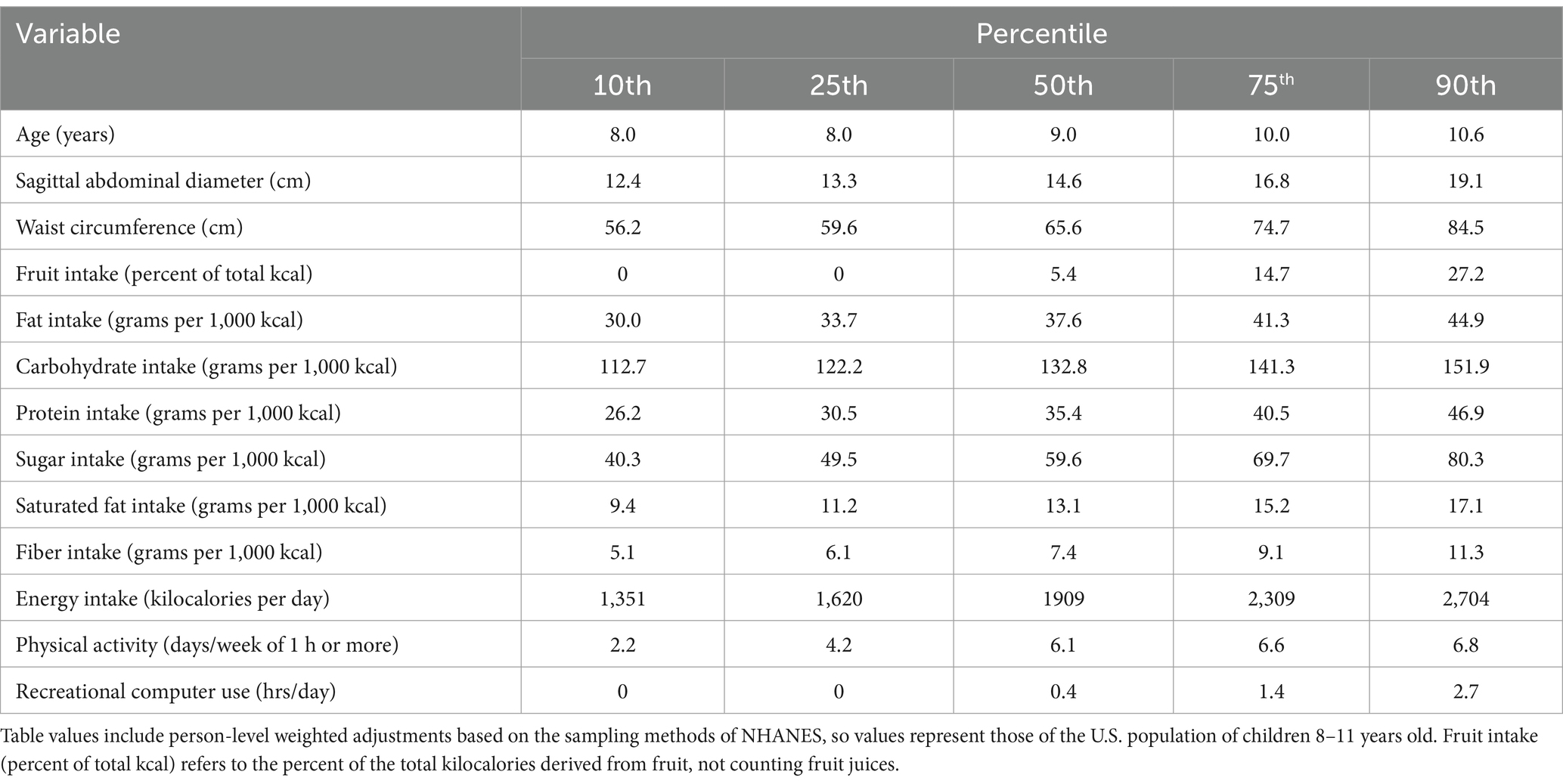
Table 1. Percentile distributions of the continuous variables representing U.S. children, (n = 1,707).
For the categorical variables, which are shown in Table 2, there was almost an even split between girls and boys. The race distribution differed slightly from the U.S. population because NHANES intentionally oversampled Mexican Americans. One-third of the sample had households with four individuals and about one-fourth of the sample included households with a total of five individuals. Almost 27% of the sample reported no fruit intake, and the remaining 73% were intentionally divided evenly into two categories.
The exposure variable was fruit intake, expressed as a percentage of the total energy consumed. As shown in Table 3, there was a significant linear relationship between fruit intake (normalized using log10) and SAD (df = 47), after adjusting for the demographic covariates (Model 1: F = 6.4, p = 0.0148), after adjusting for the demographic and lifestyle covariates together (Model 2: F = 7.0, p = 0.0110), and after controlling for the demographic, lifestyle, and additional dietary covariates, including intake (grams per 1,000 kcal) of carbohydrate, protein, fat, sugar, saturated fat, and dietary fiber (Model 3: F = 7.0, p = 0.0112). The association between fruit intake and waist circumference (df = 47) was also inverse, linear, and significant across all three statistical models (Table 3).
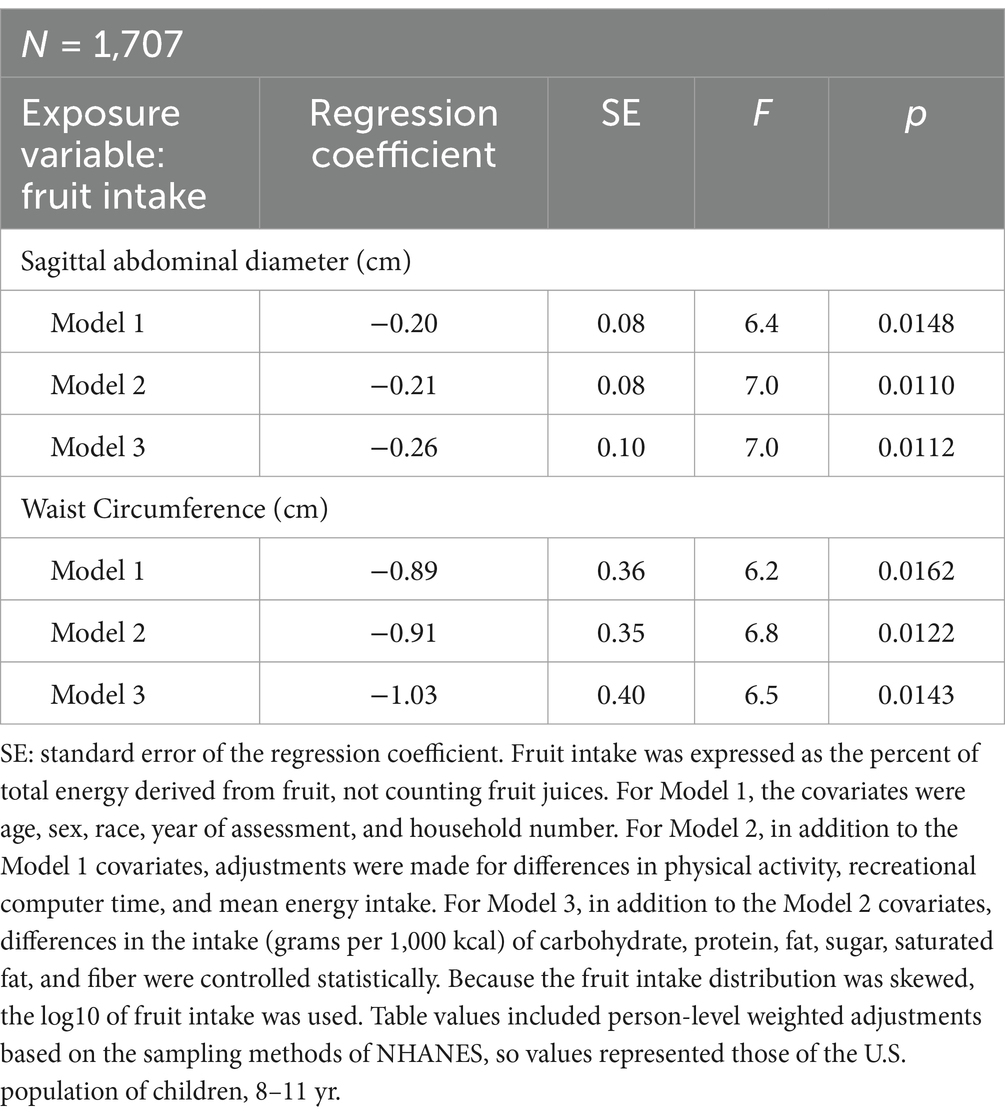
Table 3. Regression analysis showing the linear relationship between fruit intake and abdominal adiposity after adjusting for different covariates.
In Table 3, both fruit consumption and abdominal adiposity (SAD and waist) were treated as continuous variables. However, in Table 4, abdominal adiposity was expressed as a continuous variable and fruit intake was treated as a categorical variable. In Table 4, with fruit intake divided into 3 categories, again there was a dose–response relationship. With all the covariates controlled, SAD (abdominal height) was significantly smaller for the children who consumed a moderate to high percentage of their energy from fruit compared to children who ate no fruit. Likewise, waist circumferences tended to be smaller for youth who ate a moderate to high percentage of energy from fruit compared to children who ate no fruit. In each case, the associations were inverse and dose–response.
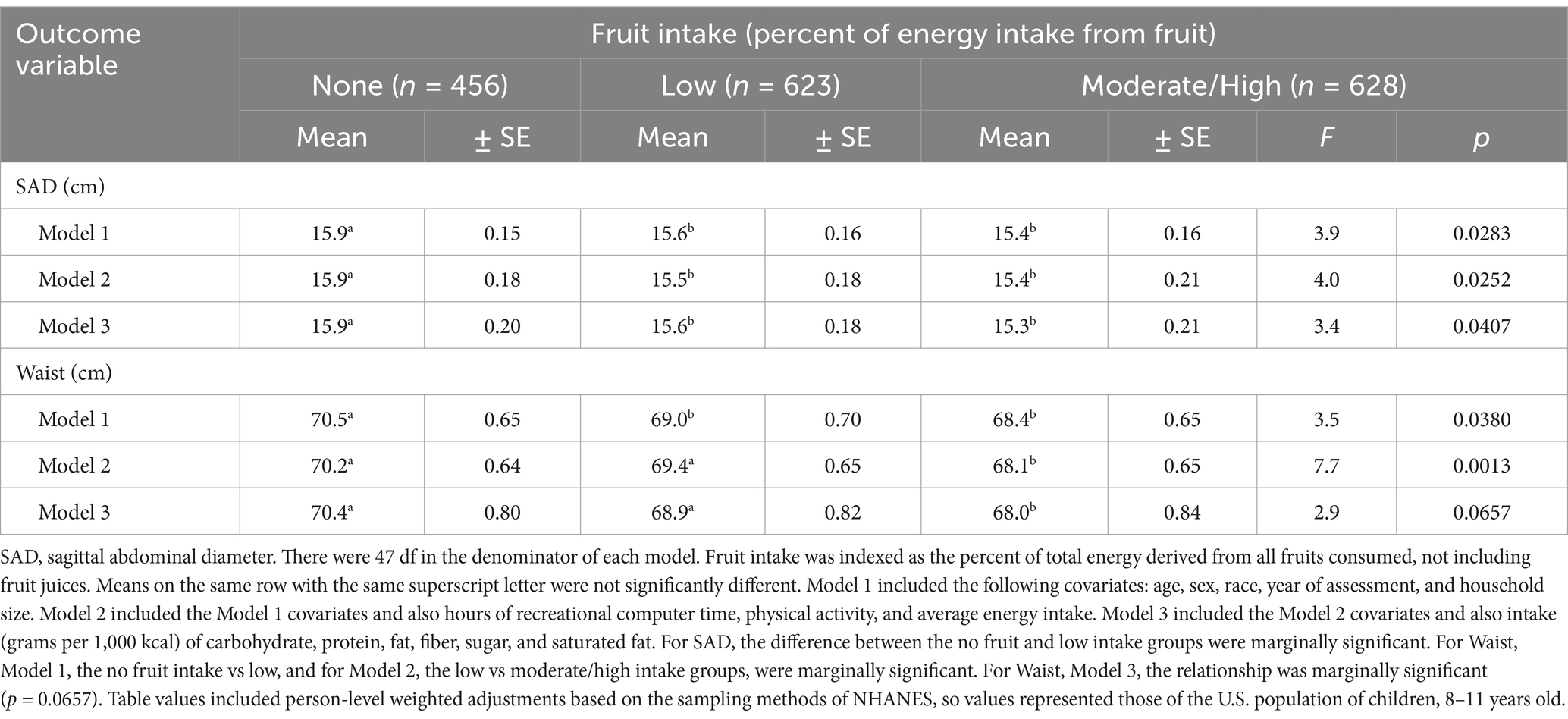
Table 4. Mean differences in abdominal adiposity, indexed using waist circumference and SAD, across categories of fruit intake in U.S. children, after adjusting for the covariates.
To test the extent that fruit intake was related to SAD and waist circumference within specific subgroups, gender, race and ethnicity, recreational computer use, and physical activity were divided into meaningful categories. The results are shown in Table 5.
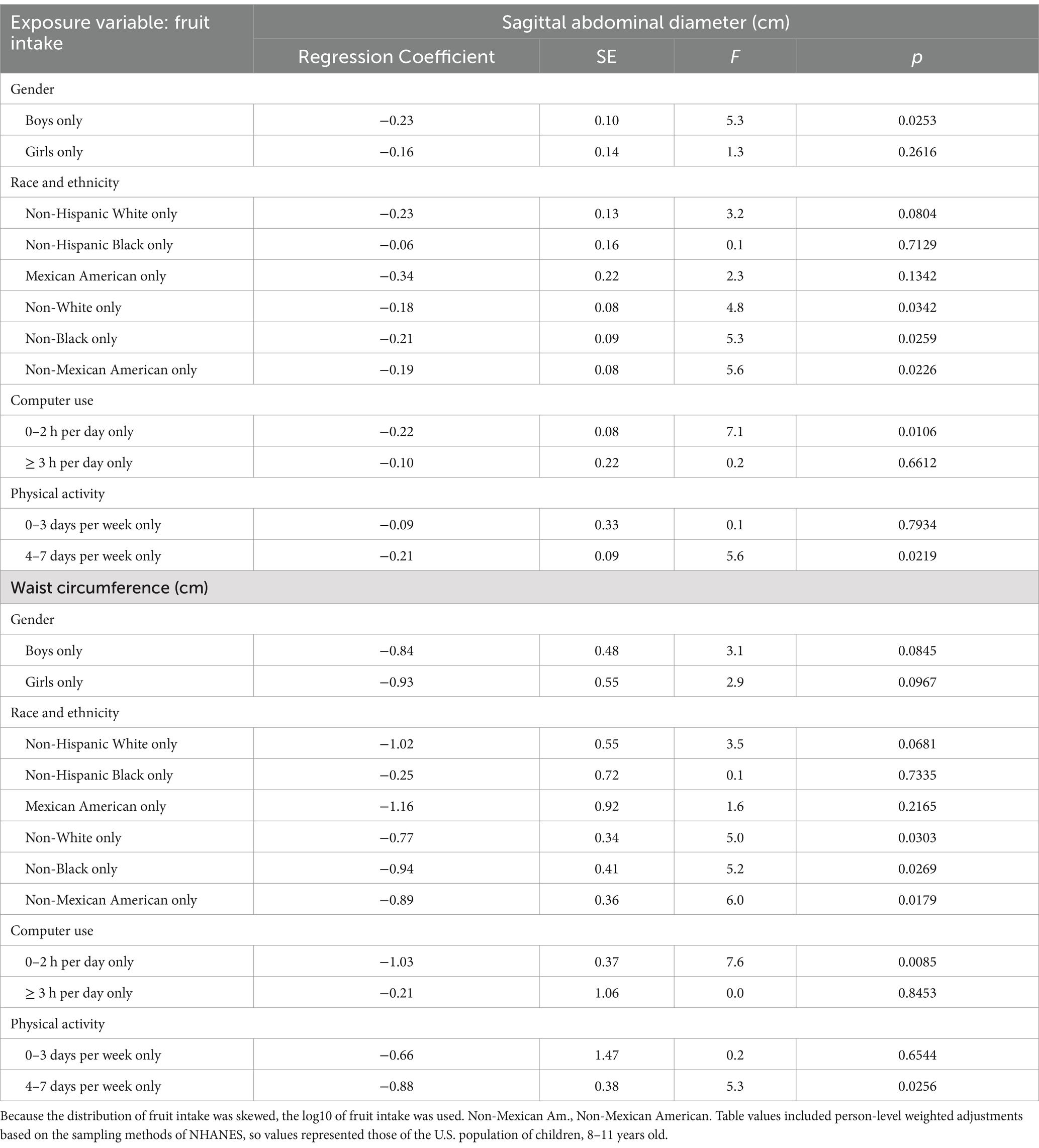
Table 5. The relationship between fruit intake, sagittal abdominal diameter, and waist circumference within key subgroups.
According to Table 5, the relationship between fruit intake and SAD was significant for boys but not for girls. For waist size, the association was marginally significant for both girls and boys considered separately. For subgroups based on race and ethnicity, the abdominal adiposity measures were significant or marginally significant for all the subgroups except Non-Hispanic Black and Mexican Americans. For subgroups based on recreational computer use, the fruit intake and abdominal obesity associations were inverse and significant for youth who kept their recreational computer use to 2 h or less per day. However, the relationship was not significant for youth who spent 3 h per day or more in recreational computer use. Similarly, based on subgroups of physical activity, the association between fruit intake and abdominal adiposity was inverse and significant in youth who engaged in at least 1 h of physical activity 4 days per week or more, but the relationship was not significant in youth who engaged in less than 4 days per week of physical activity.
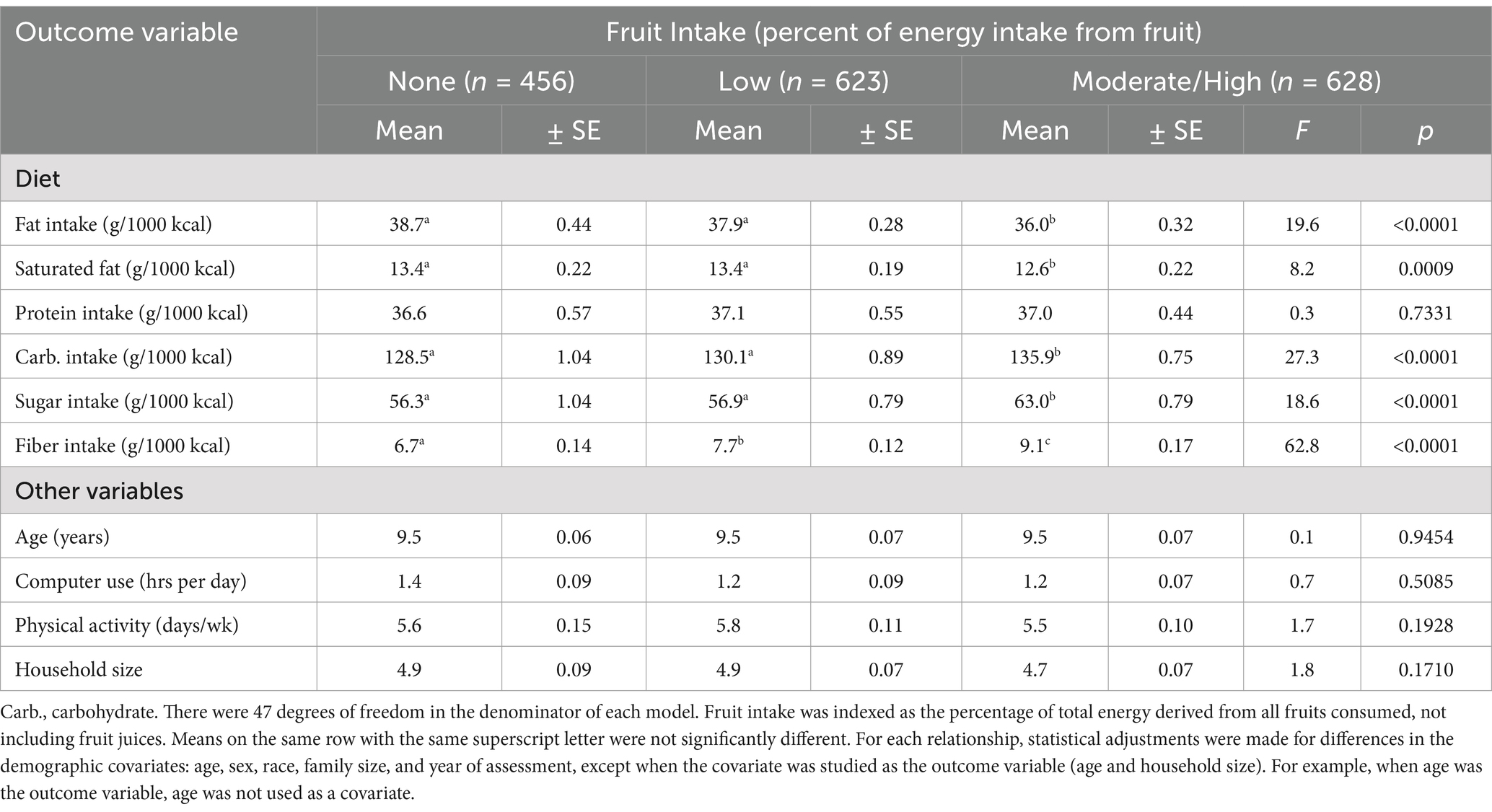
Table 6 shows the extent that the dietary and other covariates differed across the 3 fruit intake categories. Fiber intake had the strongest relationship with fruit intake (F = 62.8, p < 0.0001). The association was dose–response. Those in the highest fruit intake category consumed 37% more fiber than those in the no fruit intake category.
Carbohydrate and sugar consumption were also higher in the children reporting the most fruit intake compared to children with lower intakes of fruit. The association between dietary fat consumption and fruit intake was also strong (F = 19.6, p < 0.0001). The relationship was dose–response. Children with the highest intakes of fruit also had the lowest intakes of saturated fat (F = 8.2, p = 0.0009). The only dietary variable that was not related to fruit intake was protein consumptioin (F = 0.3, p = 0.7331).
Other covariates and their associations across the fruit intake categories included age, recreational computer use, physical activity, and the number of individuals in the household. Mean ages were identical across the 3 fruit categories. Moreover, there were no mean differences in the other covariates across the fruit intake categories.
4 Discussion
The main focus of this investigation was to determine the relationship between fruit intake and abdominal adiposity, indexed using SAD and waist circumference, in 1707 randomly selected U.S. children 8–11 years old. There were four key findings resulting from this study: (1) children consumed only 5–6% of their total energy from fruit (median), not including fruit drinks. (2) With fruit intake and abdominal adiposity both treated as continuous variables, the associations were linear, inverse, and significant. (3) With fruit consumption divided into 3 categories (None, Low, and Moderate/High), children with moderate to high fruit intake had less abdominal adiposity (both SAD and waist circumference) than children who did not consume fruit. (4) The association between fruit intake and abdominal adiposity was stronger and more consistent in U.S. boys than in girls.
The relationship between fruit intake and adiposity has been evaluated many times in adults. Hebden et al. (32) published a review that included 17 studies. The findings showed that fruit intake (not including juice) reduced the risk of long-term weight gain. The authors concluded that their review affirmed the U.S. Dietary Guidelines, which encourage Americans to consume more fruit. Although the review showed a consistent, inverse relationship between fruit intake and body weight in adults and adolescents, it did not include children as participants.
The relationship between fruit intake and adiposity has been studied infrequently in children compared to adults, and findings in children have been mixed. In a large prospective study of approximately 15,000 children and adolescents, Field et al. determined that fruit consumption was not predictive of changes in BMI (33). Similarly, in a prospective study by Bayer et al. (34), over 1,200 children who were about 6 years old at baseline were followed for 4 years. Gains in BMI were lower in children who increased fruit consumption over the 4 years, but the reduced gains were not statistically significant.
On the other hand, in a study by Wall et al. (35) of almost 200,000 adolescents who completed a food frequency questionnaire, those who reported eating fruit once or twice per week and those who reported consuming fruit three of more times per week had significantly lower BMIs than those who never ate fruit. Finally, Wang et al. (36) conducted a meta-analysis using only RCT. They found that fruit intake had no effect on BMI-z scores in children. However, the interventions decreased waist circumference in three of the four investigations that assessed waist circumference. None of the studies included a measure of sagittal abdominal diameter. It appears that abdominal adiposity may be a more sensitive measure of overweight and obesity than BMI or BMI-z in children.
In the present study of U.S. children, as fruit intake increased, abdominal adiposity decreased. The potential mechanisms accounting for this relationship are many and varied. However, one likely mechanism is the low energy density of most fruits because fruits typically contain large amounts of water, some fiber, and little or no dietary fat. Consequently, fruits tend to provide little food energy for their weight (14). Therefore, individuals can eat relatively large amounts of fruit without consuming a large amount of energy (14). Additionally, fruits are good sources of many vitamins, minerals, and bioactive compounds, such as carotenoids and phytochemicals.
Numerous investigations indicate that dietary patterns that include a large number of low energy dense foods, such as fruits, discourage over-consumption of energy and can assist with the management of body weight. For example, in a 2023 study by Diktas et al. (37), at snack time, 52 children consumed a larger proportion of self-served strawberries than pretzels, but because of the energy density difference between the foods, the children derived 55 ± 4 kcal more from the pretzels than the strawberries (p < 0.0001). In short, although the children ate more of the lower-energy dense strawberries, they consumed more energy from the higher-energy dense pretzels, emphasizing the impact of energy density in children’s energy intake.
In another study, Smethers et al. (38) employed a crossover design to determine the effect of varying the energy density of three main dishes and 1 snack per day for 5 days on 49 children. At baseline, the children’s energy intakes were consistent with their daily needs. However, serving higher energy dense foods resulted in higher energy intakes by 84 kcal per day, and serving lower energy dense foods resulted in lower energy intakes by 72 kcal per day (both p < 0.0001) over the 5 days. Adjusting the energy density of the foods had a meaningful and consistent impact on the children’s daily energy intakes (38).
In 2023, Rolls et al. (39) performed a secondary analysis based on the weighed intakes of 6,355 meals served to 94 children. The energy consumed at the meals was significantly related to the energy density of the consumed foods (p < 0.0001). Additionally, Leahy et al. employed a crossover design and evaluated a test lunch once per week for 6 weeks in children (40). Reducing the energy density of the entrée by 30% decreased the kcal consumption from the entrée by 25%. Although the children consumed more of the lower energy dense entrée, the children ate fewer kcal (40).
Guyenet (41) reported that randomized controlled trials (RCT), particularly those of high quality, show that fruit intake promotes weight maintenance or weight loss over time, and that high intake of fruit promotes weight loss. The review also suggested that single-meal RCTs show that fruit consumption tends to decrease kcal intake.
Another potential mechanism that could account for some of the association between fruit intake and abdominal adiposity in U.S. children is dietary fiber consumption. As shown in Table 6, in the current investigation, there was a very strong association between fruit and fiber intake. Children in the highest category of fruit consumption ate about 36% more fiber when compared to youth who reported no fruit consumption. Many studies in the literature have reported strong relationships between dietary fiber intake and reduced weight gain, lower body fat, and other body composition outcomes (42–45).
Numerous investigations over the past few decades have studied the impact of gut bacteria on obesity, body weight, and health (46–49). Given consumption of fruit and dietary fiber tend to have positive effects on the gut microbiome (46), it is reasonable that participants in the present study who consumed the most fruit and fiber had healthier gut microbiomes. This could be a mechanism accounting for the lower abdominal adiposity in those with higher fruit and fiber intakes. In short, increased fruit and fiber consumption may have resulted in less abdominal adiposity in the children because of their healthier gut microbiomes (46–49).
Differences in dietary fat intake could be another mechanism accounting for the inverse relationship between fruit intake and abdominal obesity. As displayed in Table 6, there was a substantial, inverse relationship between fruit intake and fat consumption (F = 19.6, p < 0.0001). Children who reported not eating fruit ate about 8% more dietary fat than youth with moderate to high intakes of fruit. Numerous studies indicate that as dietary fat increases in the diet of individuals eating ad libitum, body weight and obesity tend to increase (44, 50, 51). Moreover, according to the results of 32 randomized controlled trials reviewed by Hooper et al. (52), almost all the studies showed that eating less dietary fat results in greater weight loss than higher fat intakes. The increased dietary fat intake among youth eating less fruit could account for some of the differences in abdominal adiposity found in the present investigation.
Another mechanism that could help explain the inverse association between fruit intake and abdominal adiposity in children is the substitution effect. When children eat fruit, they are less likely to consume high calorie snacks, such as chips, cookies, and soft drinks. Over time, this could account for reduced energy consumption and less abdominal obesity. For example, a longitudinal study of 1,339 children in Norway found that 15 schools participating in a free fruit program saw a significant decrease in children’s intake of unhealthy snacks compared to children in 12 schools that did not participate. Specifically, the frequency of unhealthy snack consumption decreased from 6.9 to 4.6 times per week in the schools that participated in the free fruit program compared to the schools that did not participate (53).
Lastly, fruits are rich in vitamins, antioxidants, flavonoids, and phytochemicals that tend to reduce inflammation, as shown in a review and meta-analysis of 83 studies by Hosseini et al. (54). Overall, the meta-analysis revealed that circulating C-reactive protein and tumor necrosis factor alpha were improved by fruit and also vegetable consumption. Similarly, in a dietary study of adolescents, higher levels of fruit intake were associated with lower levels of C-reactive protein, interleukin-6, and urinary F2-isoprostanes, indicative of decreased inflammation and oxidative stress (55). Decreasing inflammation tends to increase insulin sensitivity and normalize hunger hormones, such as leptin and ghrelin, potentially improving weight management (56–58).
According to the 2020–2025 Dietary Guidelines for Americans, about 80% of the U.S. population do not meet the fruit consumption recommendations (1). The Guidelines indicate that most individuals would benefit from adding more fruit to their diet, particularly whole fruits in nutrient-dense forms. The U.S. Guidelines indicate that individuals should eat more whole fruits as snacks and also consume fruit as part of meals. Given the U.S. Guideline recommendations and the findings of this study, healthcare professionals, educators, and parents should encourage children to consume more fruit.
The current study was not without flaws. The most significant weakness was that a cross-sectional design was employed. Hence, conclusions pointing toward causation are not justified. However, it should be noted that randomized controlled trials have a significant weakness in that participants often do not eat the diet they are prescribed. In the present study, no diet was prescribed, so compliance, or lack of compliance, was not an issue. Participants simply reported what they ate, so the study focused on typical, unmanipulated intake. Another weakness could be that children who ate large amounts of fruit may be unique and have other characteristics that encourage less abdominal adiposity. Age, sex, race, year of assessment, household size, physical activity, recreational computer time, average energy intake, and consumption of carbohydrate, protein, fat, sugar, fiber, and saturated fat were controlled statistically to minimize this issue, but there are always unmeasured factors that could influence the results. Moving forward, scientists should focus on conducting prospective cohort studies and intervention studies to further clarity the causal link between fruit consumption and abdominal obesity.
The study also had multiple strengths. Participants were randomly selected from the U.S. population. Consequently, the results can be generalized to the U.S. population of children 8–11 years old. Additionally, the sample included nearly an equal number of girls and boys and all major races and ethnic groups within the U.S., so the findings fit a diverse population. Furthermore, the variables were measured using high-quality methods, and highly trained technicians collected the data, independent of the present investigation, so there were no experimental biases influencing the measurements. Also, a total of 14 potentially confounding variables were measured and controlled statistically. Finally, the sample was large (n = 1,707), providing stable results.
5 Conclusion
U.S. children tend to consume significantly less fruit than the U.S. Dietary Guidelines for Americans (2020–2025) recommend. In this investigation, as fruit consumption increased, abdominal adiposity, specifically the sagittal abdominal diameter and the waist circumference, decreased in U.S. children. The associations were inverse and linear, even after adjusting for differences in many potential confounding variables. Low energy dense fruits allow children to consume more food without consuming more energy. Fruits are also rich in vitamins, antioxidants, flavonoids, and phytochemicals, which may increase insulin sensitivity, and potentially help to normalize hunger-related hormones and promote other health benefits. Although causal conclusions are not warranted, it appears that abdominal adiposity in U.S. children is partly accounted for by different levels of fruit intake. Results from the present investigation align with the U.S. Dietary Guidelines which recommend that American children should eat more fruit. Consequently, healthcare professionals, teachers, and parents should encourage children to consume multiple servings of fruit each day.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: The data that support the findings of this study are openly available in NHANES Questionnaires, Datasets, and Related Documentation at: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx.
Ethics statement
The studies involving humans was performed according to the guidelines of the Declaration of Helsinki. The Ethics Review Board (ERB) of the National Center for Health Statistics (USA) approved the NHANES data collection protocol. The ethical approval code for NHANES data collection from 2011-2016 was: Protocol #2011–17. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
LT: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Gratitude and appreciation are expressed to all those who participated in and helped with the study.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author declares that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. U.S. Department of Agriculture and the U.S. Department of Health and Human Services (2020) Dietary Guidelines for Americans, 2020-2025. 9th. Available online at: https://www.dietaryguidelines.gov/ (Accessed May 22, 2025)
2. Rubino, F, Cummings, DE, Eckel, RH, Cohen, RV, Wilding, JPH, Brown, WA, et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. (2025) 13:221–62. doi: 10.1016/S2213-8587(24)00316-4
3. Hudson, L, Kinra, S, Wong, I, Cole, TJ, Deanfield, J, and Viner, R. Is arterial stiffening associated with adiposity, severity of obesity and other contemporary cardiometabolic markers in a community sample of adolescents with obesity in the UK? BMJ Paediatr Open. (2017) 1:e000061. doi: 10.1136/bmjpo-2017-000061
4. Al-Attas, OS, Al-Daghri, NM, Alokail, MS, Alkharfy, KM, Draz, H, Yakout, S, et al. Association of body mass index, sagittal abdominal diameter and waist-hip ratio with cardiometabolic risk factors and adipocytokines in Arab children and adolescents. BMC Pediatr. (2012) 12:119. doi: 10.1186/1471-2431-12-119
5. Labyak, CA, Janicke, DM, Lim, CS, Colee, J, and Mathews, AE. Anthropometrics to identify overweight children at Most risk for the development of Cardiometabolic disease. Infant Child Adolesc Nutr. (2013) 5:341–6. doi: 10.1177/1941406413501379
6. da Silva, CC, Vasques, ACJ, Zambon, MP, Camilo, DF, De Bernardi Rodrigues, AM, Antonio, M, et al. Sagittal abdominal diameter resembles waist circumference as a surrogate marker of insulin resistance in adolescents-Brazilian metabolic syndrome study. Pediatr Diabetes. (2018) 19:882–91. doi: 10.1111/pedi.12664
7. Summer, S, Jenkins, T, Inge, T, Deka, R, and Khoury, J. The association of sagittal abdominal diameter with metabolic syndrome risk before and after weight-loss surgery in adolescents. Surg Obes Relat Dis. (2023) 19:350–5. doi: 10.1016/j.soard.2022.10.015
8. Vernarelli, JA, Mitchell, DC, Rolls, BJ, and Hartman, TJ. Dietary energy density is associated with obesity and other biomarkers of chronic disease in US adults. Eur J Nutr. (2015) 54:59–65. doi: 10.1007/s00394-014-0685-0
9. Ledikwe, JH, Rolls, BJ, Smiciklas-Wright, H, Mitchell, DC, Ard, JD, Champagne, C, et al. Reductions in dietary energy density are associated with weight loss in overweight and obese participants in the PREMIER trial. Am J Clin Nutr. (2007) 85:1212–21. doi: 10.1093/ajcn/85.5.1212
10. Rolls, BJ. The relationship between dietary energy density and energy intake. Physiol Behav. (2009) 97:609–15. doi: 10.1016/j.physbeh.2009.03.011
11. Rolls, BJ, Drewnowski, A, and Ledikwe, JH. Changing the energy density of the diet as a strategy for weight management. J Am Diet Assoc. (2005) 105:S98–S103. doi: 10.1016/j.jada.2005.02.033
12. Ello-Martin, JA, Roe, LS, Ledikwe, JH, Beach, AM, and Rolls, BJ. Dietary energy density in the treatment of obesity: a year-long trial comparing 2 weight-loss diets. Am J Clin Nutr. (2007) 85:1465–77. doi: 10.1093/ajcn/85.6.1465
13. U.S. Department of Agriculture (2023) Find a food. Available online at: https://fdc.nal.usda.gov/food-search (Accessed May 22, 2025).
14. Rolls, BJ. The volumetrics eating plan: Techniques and recipes for feeling full on fewer calories. 1st ed. New York: Harper (2007). 316 p.
15. Schwingshackl, L, Hoffmann, G, Kalle-Uhlmann, T, Arregui, M, Buijsse, B, and Boeing, H. Fruit and vegetable consumption and changes in anthropometric variables in adult populations: a systematic review and Meta-analysis of prospective cohort studies. PLoS One. (2015) 10:e0140846. doi: 10.1371/journal.pone.0140846
16. Rautiainen, S, Wang, L, Lee, IM, Manson, JE, Buring, JE, and Sesso, HD. Higher intake of fruit, but not vegetables or Fiber, at baseline is associated with lower risk of becoming overweight or obese in middle-aged and older women of Normal BMI at baseline. J Nutr. (2015) 145:960–8. doi: 10.3945/jn.114.199158
17. Choi, A, Ha, K, Joung, H, and Song, Y. Frequency of consumption of whole fruit, not fruit juice, is associated with reduced prevalence of obesity in Korean adults. Journal of the academy of. Nutr Diet. (2019) 119:1842–1851.e2. doi: 10.1016/j.jand.2019.04.015
18. Guerra Valencia, J, Ramos, W, Cruz-Ausejo, L, Torres-Malca, JR, Loayza-Castro, JA, Zenas-Trujillo, GZ, et al. The fruit intake-adiposity paradox: findings from a Peruvian cross-sectional study. Nutrients. (2023) 15:1183. doi: 10.3390/nu15051183
19. NHANES. National Center of Health Statistics Research Ethics Review Board (ERB) Approval: Centers for Disease Control and Prevention; (2025) Available online at: https://www.cdc.gov/nchs/nhanes/about/erb.html?CDC_AAref_Val=https://www.cdc.gov/nchs/nhanes/irba98.htm (Accessed May 22, 2025).
20. NHANES. Data Files: Questionnaires, Datasets, and Related Documentation: Centers for Disease Control and Prevention; (1999-2016) Available online at: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx (Accessed May 22, 2025).
21. NHANES. Dietary interview, individual foods, first day (2011-2012) Available online at: https://wwwn.cdc.gov/Nchs/Data/Nhanes/Public/2011/DataFiles/DR1IFF_G.htm (Accessed May 22, 2025).
22. U.S. Department of Agriculture. Food surveys research group: AMPM, USDA automated multiple-pass method Beltsvillem MD: Agricultural Research Service; (2021) Available online at: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/ampm-usda-automated-multiple-pass-method/ (Accessed May 22, 2025).
23. Ross, R, Leger, L, Morris, D, de Guise, J, and Guardo, R. Quantification of adipose tissue by MRI: relationship with anthropometric variables. J Appl Physiol. (1992) 72:787–95. doi: 10.1152/jappl.1992.72.2.787
24. Gletsu-Miller, N, Kahn, HS, Gasevic, D, Liang, Z, Frediani, JK, Torres, WE, et al. Sagittal abdominal diameter and visceral adiposity: correlates of beta-cell function and dysglycemia in severely obese women. Obes Surg. (2013) 23:874–81. doi: 10.1007/s11695-013-0874-6
25. Kahn, HS, and Bullard, KM. Beyond body mass index: advantages of abdominal measurements for recognizing Cardiometabolic disorders. Am J Med. (2016) 129:74–81.e2. doi: 10.1016/j.amjmed.2015.08.010
26. Kahn, HS, and Cheng, YJ. Comparison of adiposity indicators associated with fasting-state insulinemia, triglyceridemia, and related risk biomarkers in a nationally representative, adult population. Diabetes Res Clin Pract. (2018) 136:7–15. doi: 10.1016/j.diabres.2017.11.019
27. Zamboni, M, Turcato, E, Armellini, F, Kahn, HS, Zivelonghi, A, Santana, H, et al. Sagittal abdominal diameter as a practical predictor of visceral fat. Int J Obes Relat Metab Disord. (1998) 22:655–60. doi: 10.1038/sj.ijo.0800643
28. Firouzi, SA, Tucker, LA, LeCheminant, JD, and Bailey, BW. Sagittal abdominal diameter, waist circumference, and BMI as predictors of multiple measures of glucose metabolism: an NHANES investigation of US adults. J Diabetes Res. (2018) 2018:1–14. doi: 10.1155/2018/3604108
29. Krishnappa, SK, Yashoda, HT, Boraiah, G, and Vishwa, S. Sagittal abdominal diameter to measure visceral adipose tissue in overweight or obese adolescent children and its role as a marker of insulin resistance. J Clin Diagn Res. (2015) 9:SC09-12. doi: 10.7860/JCDR/2015/15971.6742
30. NHANES. Anthropometry Procedures Manual Hyattsville, MD: Centers for Disease Control and Prevention; (2016) Available online at: https://www.cdc.gov/nchs/data/nhanes/nhanes_15_16/2016_anthropometry_procedures_manual.pdf (Accessed May 22, 2025).
31. Williamson, DF, Kahn, HS, Worthman, CM, Burnette, JC, and Russell, CM. Precision of recumbent anthropometry. Am J Hum Biol. (1993) 5:159–67. doi: 10.1002/ajhb.1310050205
32. Hebden, L, O'Leary, F, Rangan, A, Singgih Lie, E, Hirani, V, and Allman-Farinelli, M. Fruit consumption and adiposity status in adults: a systematic review of current evidence. Crit Rev Food Sci Nutr. (2017) 57:2526–40. doi: 10.1080/10408398.2015.1012290
33. Field, AE, Gillman, MW, Rosner, B, Rockett, HR, and Colditz, GA. Association between fruit and vegetable intake and change in body mass index among a large sample of children and adolescents in the United States. Int J Obes Relat Metab Disord. (2003) 27:821–6. doi: 10.1038/sj.ijo.0802297
34. Bayer, O, Nehring, I, Bolte, G, and von Kries, R. Fruit and vegetable consumption and BMI change in primary school-age children: a cohort study. Eur J Clin Nutr. (2014) 68:265–70. doi: 10.1038/ejcn.2013.139
35. Wall, CR, Stewart, AW, Hancox, RJ, Murphy, R, Braithwaite, I, Beasley, R, et al. Association between frequency of consumption of fruit, vegetables, nuts and pulses and BMI: analyses of the international study of asthma and allergies in childhood (ISAAC). Nutrients. (2018) 10:316. doi: 10.3390/nu10030316
36. Wang, F, Zhang, P, Ren, Y, Huang, D, Xu, F, Ma, J, et al. The estimated effect of increasing fruit interventions on controlling body weight in children and adolescents: a meta-analysis. Prev Med. (2023) 179:107785. doi: 10.1016/j.ypmed.2023.107785
37. Diktas, HE, Roe, LS, Keller, KL, and Rolls, BJ. The effects of snack foods of different energy density on self-served portions and consumption in preschool children. Appetite. (2023) 185:106527. doi: 10.1016/j.appet.2023.106527
38. Smethers, AD, Roe, LS, Sanchez, CE, Zuraikat, FM, Keller, KL, and Rolls, BJ. Both increases and decreases in energy density lead to sustained changes in preschool children's energy intake over 5 days. Physiol Behav. (2019) 204:210–8. doi: 10.1016/j.physbeh.2019.02.042
39. Rolls, BJ, Roe, LS, and Keller, KL. Children's energy intake generally increases in response to the energy density of meals but varies with the amounts and types of foods served. Am J Clin Nutr. (2023) 119:185–95. doi: 10.1016/j.ajcnut.2023.10.019
40. Leahy, KE, Birch, LL, and Rolls, BJ. Reducing the energy density of an entree decreases children's energy intake at lunch. J Am Diet Assoc. (2008) 108:41–8. doi: 10.1016/j.jada.2007.10.015
41. Guyenet, SJ. Impact of whole, fresh fruit consumption on energy intake and adiposity: a systematic review. Front Nutr. (2019) 6:66. doi: 10.3389/fnut.2019.00066
42. Tucker, LA. Legume intake, body weight, and abdominal adiposity: 10-year weight change and cross-sectional results in 15, 185 U.S. Adults. Nutrients. (2023) 15. doi: 10.3390/nu15020460
43. Tucker, LA, and Thomas, KS. Increasing total fiber intake reduces risk of weight and fat gains in women. J Nutr. (2009) 139:576–81. doi: 10.3945/jn.108.096685
44. Nelson, LH, and Tucker, LA. Diet composition related to body fat in a multivariate study of 203 men. J Am Diet Assoc. (1996) 96:771–7. doi: 10.1016/S0002-8223(96)00215-5
45. Zhang, L, Pagoto, S, Olendzki, B, Persuitte, G, Churchill, L, Oleski, J, et al. A nonrestrictive, weight loss diet focused on fiber and lean protein increase. Nutrition. (2018) 54:12–8. doi: 10.1016/j.nut.2018.02.006
46. Dreher, ML. Whole fruits and fruit Fiber emerging health effects. Nutrients. (2018) 10. doi: 10.3390/nu10121833
47. Ortega-Santos, CP, and Whisner, CM. The key to successful weight loss on a high-Fiber diet may be in gut microbiome Prevotella abundance. J Nutr. (2019) 149:2083–4. doi: 10.1093/jn/nxz248
48. Hjorth, MF, and Astrup, A. The role of viscous fiber for weight loss: food for thought and gut bacteria. Am J Clin Nutr. (2020) 111:242–3. doi: 10.1093/ajcn/nqz334
49. Yang, Z, Yang, M, Deehan, EC, Cai, C, Madsen, KL, Wine, E, et al. Dietary fiber for the prevention of childhood obesity: a focus on the involvement of the gut microbiota. Gut Microbes. (2024) 16:2387796. doi: 10.1080/19490976.2024.2387796
50. Tucker, LA, Seljaas, GT, and Hager, RL. Body fat percentage of children varies according to their diet composition. J Am Diet Assoc. (1997) 97:981–6. doi: 10.1016/S0002-8223(97)00237-X
51. Tucker, LA, and Kano, MJ. Dietary fat and body fat: a multivariate study of 205 adult females. Am J Clin Nutr. (1992) 56:616–22. doi: 10.1093/ajcn/56.4.616
52. Hooper, L, Abdelhamid, A, Bunn, D, Brown, T, Summerbell, CD, and Skeaff, CM. Effects of total fat intake on body weight. Cochrane Database Syst Rev. (2015) 2016:CD011834. doi: 10.1002/14651858.CD011834
53. Overby, NC, Klepp, KI, and Bere, E. Introduction of a school fruit program is associated with reduced frequency of consumption of unhealthy snacks. Am J Clin Nutr. (2012) 96:1100–3. doi: 10.3945/ajcn.111.033399
54. Hosseini, B, Berthon, BS, Saedisomeolia, A, Starkey, MR, Collison, A, Wark, PAB, et al. Effects of fruit and vegetable consumption on inflammatory biomarkers and immune cell populations: a systematic literature review and meta-analysis. Am J Clin Nutr. (2018) 108:136–55. doi: 10.1093/ajcn/nqy082
55. Holt, EM, Steffen, LM, Moran, A, Basu, S, Steinberger, J, Ross, JA, et al. Fruit and vegetable consumption and its relation to markers of inflammation and oxidative stress in adolescents. J Am Diet Assoc. (2009) 109:414–21. doi: 10.1016/j.jada.2008.11.036
56. Wu, Y, Yu, Y, Szabo, A, Han, M, and Huang, XF. Central inflammation and leptin resistance are attenuated by ginsenoside Rb1 treatment in obese mice fed a high-fat diet. PLoS One. (2014) 9:e92618. doi: 10.1371/journal.pone.0092618
57. de Git, KC, and Adan, RA. Leptin resistance in diet-induced obesity: the role of hypothalamic inflammation. Obes Rev. (2015) 16:207–24. doi: 10.1111/obr.12243
Keywords: epidemiology, energy density, dietetics, public health, abdominal obesity, waist
Citation: Tucker LA (2025) Association between fruit intake and abdominal adiposity in 1,707 randomly selected U.S. children. Front. Nutr. 12:1592654. doi: 10.3389/fnut.2025.1592654
Edited by:
Fei Xu, Nanjing Municipal Center for Disease Control and Prevention, ChinaReviewed by:
Xiongfeng Huang, Fuzhou Medical College of Nanchang University, ChinaBiriz Çakır, Kırıkkale University, Türkiye
Copyright © 2025 Tucker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Larry A. Tucker, dHVja2VyQGJ5dS5lZHU=
 Larry A. Tucker
Larry A. Tucker