- 1Heilongjiang University of Chinese Medicine, Harbin, China
- 2First Affiliated Hospital of Heilongjiang University of Chinese Medicine, Harbin, China
Background: Stroke ranks among the main diseases resulting in death and disability, imposing a heavy burden on both the country and individuals. Healthy foods can effectively prevent the occurrence of stroke, and fermented dairy products are among them. However, in previous studies, the correlation between stroke and fermented dairy products remains controversial.
Methods: The intake of fermented dairy products and the identification of stroke both originated from the data of the NHANES database from 2007 to 2018. This study used a weighted regression model to analyze the association between the total intake of fermented dairy products and the intake of various types of fermented dairy products (yogurt, cheese and buttermilk) and stroke, and conducted subgroup analyses and interaction tests.
Results: This study included 27,487 American adults, of whom 2.9% had suffered from a stroke. The results of regression analysis indicated that total intake of fermented dairy products and yogurt intake were negatively correlated with stroke. For total intake, after adjusting for all confounding variables, the results revealed that every 50 g rise in intake led to a 7% decline in the stroke risk (OR = 0.93, 95% CI: 0.88–1.00). Meanwhile, when compared to participants having no consumption of fermented dairy products, those with a low intake had a 21% lower probability of stroke (OR = 0.79, 95% CI: 0.66–0.95). Subgroup analysis showed that smoking interacted with stroke and fermented dairy products (p = 0.047). For yogurt, after adjusting for all confounding variables, the results indicated that for every 50 g rise in intake, the probability of stroke declined by 7% (OR = 0.93, 95% CI: 0.86–1.00). However, only high intake of yogurt was associated with a protective effect against stroke, and this relationship remained stable across three models (Model I: OR = 0.49, 95% CI: 0.33–0.75; Model II: OR = 0.50, 95% CI: 0.33–0.75; Model III: OR = 0.62, 95% CI: 0.40–0.96). In contrast, no significant associations were found between cheese and buttermilk intake and stroke risk.
Conclusion: This study discovered that, among American adults, the total quantity of fermented dairy products as well as yogurt had an inverse correlation with the risk of stroke, while this correlation did not exist for cheese or buttermilk.
1 Introduction
Stroke is a disease primarily characterized by symptoms of ischemic or hemorrhagic brain injury, posing a severe threat to human health and ranking as the third leading cause of death after ischemic heart disease and COVID-19 (1). According to the latest data, the incidence and prevalence of stroke have declined globally between 1990 and 2021 (2). However, the incidence among younger populations is on the rise (3). Meanwhile, stroke-attributable deaths and disabilities have increased by 43 and 32% (2), respectively. Due to its elevated mortality and disability levels, stroke persists in placing a substantial economic strain on both society and families. Studies predict that by 2050, the burden of stroke will increase by 81%, with a more significant rise in upper-middle-income countries (4). Therefore, preventing stroke is of utmost importance.
To prevent the occurrence of stroke, it is essential to identify the inducing factors of stroke. Although there are numerous contributing factors, in recent years, the theory that explains the occurrence of stroke from the perspective of the brain-gut microbiota mechanism has received widespread attention (5). There exists a bidirectional communication between the brain and the gut. When the gut microbiota is dysbiotic, it can trigger the abnormal emission of bacterial metabolites or neurotransmitters in the intestine. These substances can be transmitted via the vagus nerve or directly enter the bloodstream and cross the blood–brain barrier, thereby affecting the neurovascular system and triggering a stroke (6). Furthermore, the gut microbiota can also indirectly have an effect on the happening of stroke by exerting an influence on pathogenic factors including body weight, blood pressure, and blood glucose levels (7). Food is a direct factor influencing the gut microbiota. As an effective carrier of probiotics, fermented dairy products have a significant impact on the gut microenvironment, making them an indispensable part of the diet. As a traditional food, fermented dairy products not only appear in the food guidelines of many countries (8) but also in the recommended dietary patterns for stroke prevention, such as the Mediterranean diet (9). Compared with milk, fermented dairy products not only contain all the nutrients of milk, but some components, such as proteins and minerals, are present in higher concentrations than in milk (10). Moreover, the probiotics generated during the fermentation process can make the raw materials more easily absorbed by transforming their chemical compositions (11). Meanwhile, the fermentation process breaks down the lactose in dairy products, making them suitable for consumption by people with lactose intolerance (12), thus expanding the group of beneficiaries of dairy products. A study from Sweden (13) found that an appropriate intake of fermented dairy products (400–600 g/day) can lower the probability of death (HR = 0.93, 95% CI: 0.87–0.99), while a high-dose intake of non-fermented dairy products may increase the risk of death (HR = 1.34, 95% CI: 1.14–1.59). Evidently, fermented dairy products are beneficial to our health.
The link between fermented dairy products and stroke has been documented in previous reports. As early as in the 1980s, a study on adult women found that consuming yogurt could reduce the risk of stroke, and the same relationship also existed for hard cheese (14). A cohort study involving 74,138 participants found that the intake of a small amount of fermented dairy products could reduce the incidence of stroke (RR = 0.89, 95% CI: 0.81–0.98), but there was no association between non-fermented dairy products and the risk of stroke (15). A meta-analysis of prospective cohort studies showed that fermented milk (RR = 0.80, 95% CI: 0.71–0.89) and cheese (RR = 0.94, 95% CI: 0.89–0.995) were both tied to a lower incidence of stroke, but this relationship did not exist for non-fermented milk (RR = 1.02, 95% CI: 0.89–1.17) (16). Thus, it is evident that fermented dairy products are negatively correlated with the occurrence of stroke and can be considered a healthy dietary option for stroke prevention.
However, a study targeting individuals aged 55 and above found no significant correlation between stroke and fermented dairy products (17). Similarly, Susanna et al. (18) conducted a 10-year follow-up study involving 74,961 individuals from Sweden. The results showed that after adjusting for all variables, the negative correlation between yogurt or cheese and stroke disappeared. Another study from Sweden reached a similar conclusion (19). In contrast to the aforementioned studies, a study including 26,566 men found that yogurt consumption may increase the risk of subarachnoid hemorrhage (RR = 1.83, 95% CI: 1.20–2.0) (20). Additionally, a meta-analysis pointed out that most of the existing research into the connection of yogurt or cheese with stroke risk centers around comparing low and high intake amounts, but a clear dose–response relationship has not yet been established (21). It is evident that the relationship between fermented dairy products and stroke remains controversial. The association between different types of fermented dairy products and stroke is not well-defined, and most relevant studies have been conducted in European countries. Only 0.1% of Americans have healthy eating habits according to data (22), while dietary modifications are of vital significance in the primary prevention of stroke (23). Therefore, it is particularly urgent to clarify the association between fermented dairy products and stroke among American adults.
This study analyzed the National Health and Nutrition Examination Survey (NHANES) data in order to clarify the association between total fermented dairy product intake and individual fermented dairy product intake (yogurt, cheese and buttermilk) with stroke among adult Americans.
2 Methods
2.1 Study population
NHANES is a cross-sectional survey organized and carried out biennially by the Centers for Disease Control and Prevention (CDC) of the United States. This database adopts a sophisticated sampling design and contains comprehensive information, enabling it to representatively mirror the health and nutritional conditions of Americans.
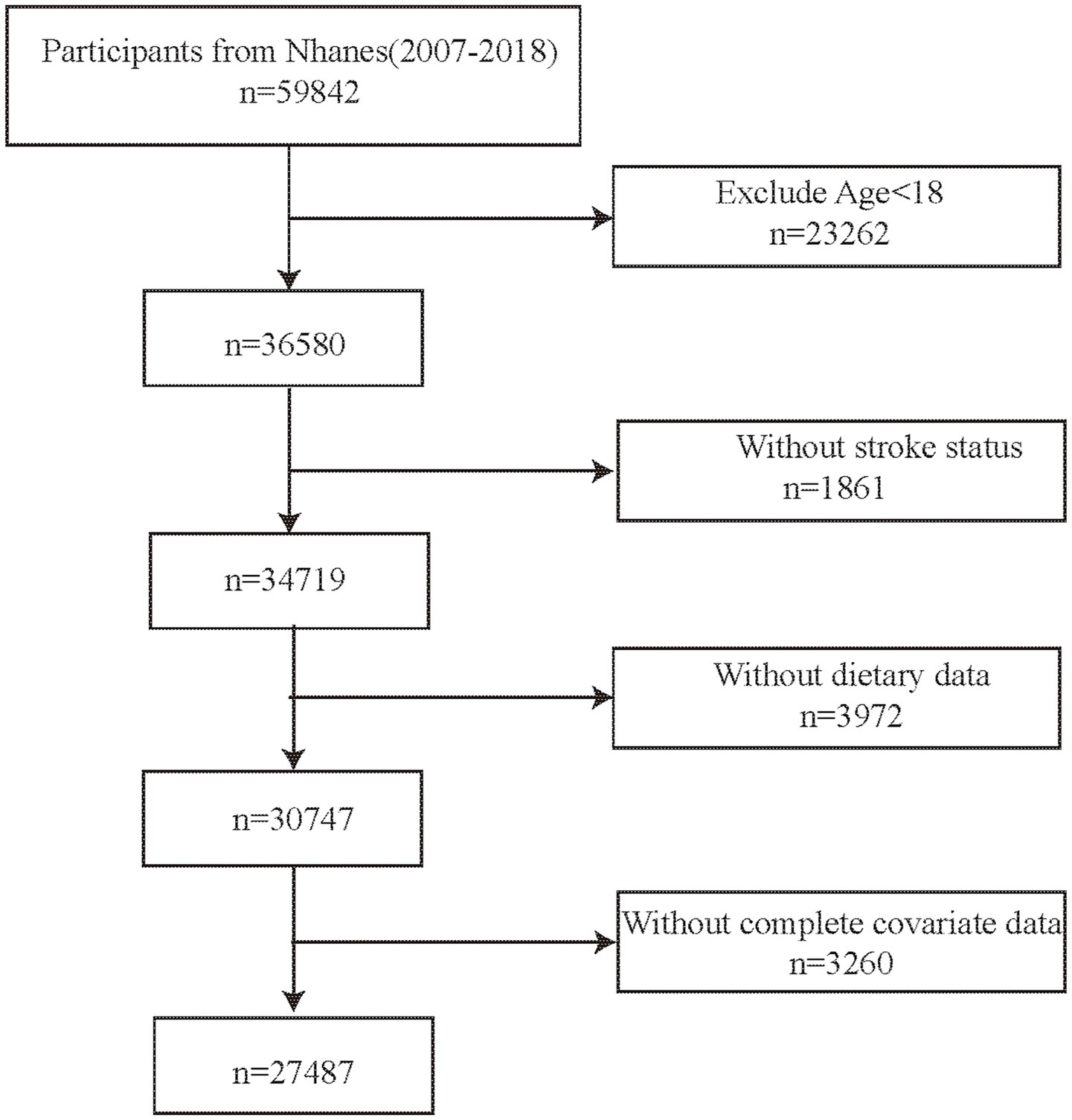
In the study, six NHANES cycles from 2007 to 2018 were selected. The inclusion criteria were as follows: (1) age ≥18 years, (2) participants with complete stroke status, (3) participants with complete dietary data (4) participants with complete information on other confounding factors. Finally, this survey included 27,487 participants (Figure 1).
2.2 Definition of stroke
The primary outcome was ascertained using the Medical Conditions Questionnaire administered via face-to-face interviews. During these interviews, participants were specifically asked whether they had ever been diagnosed with a stroke. Those who answered affirmatively were categorized as having defined with stroke.
2.3 Fermented dairy products intake
The intake amount of fermented dairy products was obtained from the 24-h dietary recall interview. Each participant provided information on the types, weights, times, occasions, and other relevant details of the foods or beverages they consumed. Different foods and beverages were assigned corresponding codes. Querying the codes provided on the website revealed that the database only included three types of fermented dairy products: yogurt, cheese, and buttermilk. It should be noted that buttermilk products included in the analysis were specifically cultured buttermilk, as defined by their fermentation-based production process. Therefore, this study separately calculated the weights of these three fermented dairy products consumed by participants and summed them up to derive the total intake. Ultimately, the mean consumption quantity of different fermented dairy products obtained from two 24-h dietary recall surveys served as the foundation for analysis. Those participants without records of these codes were defined as non-consumers. After the statistics were compiled, the participants were separated into three groups based on their median intake of each fermented dairy product: (1) Non-consumers; (2) low-intake group (0—median value); (3) high-intake group (> median value).
2.4 Covariates
Referring to relevant articles (24–26), we selected 15 confounding variables from the database. Among them, gender, race, age, educational attainment, drinking (having at least 12 alcohol drinks peer year or not), smoking (smoking cigarettes now or not), physical activity (having work activity or recreational activity), hypertension, diabetes, cardiovascular disease (CVD) and medication use were all obtained from face-to-face interviews. CVDs included conditions such as coronary heart disease, congestive heart failure, heart attack, and angina. It should be noted that CVD mentioned in this study did not include stroke, as stroke was the outcome measure. Medication use included participants’ current use of anti-hypertensive drug, anti-lipemic drug, and anti-diabetic medication (insulin or diabetic pills). Body mass index (BMI) was from physical examination and categorized into three groups: (1) normal weight (BMI = 18.5–24.9) (2) underweight (BMI < 18.5), (3) overweight (BMI ≥ 25.0). For the diagnosis of hyperlipidemia, in addition to the information obtained from the questionnaire survey, it also includes those who meet any one of the following blood test results (27): (1) total cholesterol ≥200 mg/dL, (2) triglycerides ≥150 mg/dL, (3) high-density lipoprotein ≤50 mg/dL, (4) low-density lipoprotein ≥130 mg/dL. Additionally, participants’ cow milk consumption was included as a covariate, which was derived from 24-h dietary recalls.
2.5 Statistical analysis
To guarantee that the results accurately represented the American civilian population, each participant was allocated a sample weight when they accepted the survey. Based on the official website descriptions and analytical standards, weight was also taken into account during data analysis. The data was organized and analyzed using R software (version 4.4.2).1 The study used a two-tailed p-value of <0.05 for statistical significance.
In baseline data analysis, the median and quartiles were used to describe continuous data, and non-parametric test was used to analyze differences; in contrast, percentages were used to describe categorical data, and chi-square tests were used to evaluate differences. Multivariate regression analysis was employed across three distinct models to evaluate the association between fermented dairy intake and stroke risk. Model I was unadjusted for any covariates. Model II was adjusted for sex, age, and race. Model III was fully adjusted for all potential confounding variables included in the study. Then, fermented dairy products were treated as categorical variables in the regression analysis. These analyses were carried out four times, with the categories of total amount, yogurt, cheese and buttermilk.
Subsequently, subgroup analyses were conducted based on sex, race, education attainment, smoking, drinking, BMI, physical activity, diabetes, hypertension, hyperlipidemia, CVD and medication use to evaluate the association between fermented dairy product consumption and stroke risk in specific population subgroups. Statistical interaction tests were also performed to assess potential effect modification by these demographic factors.
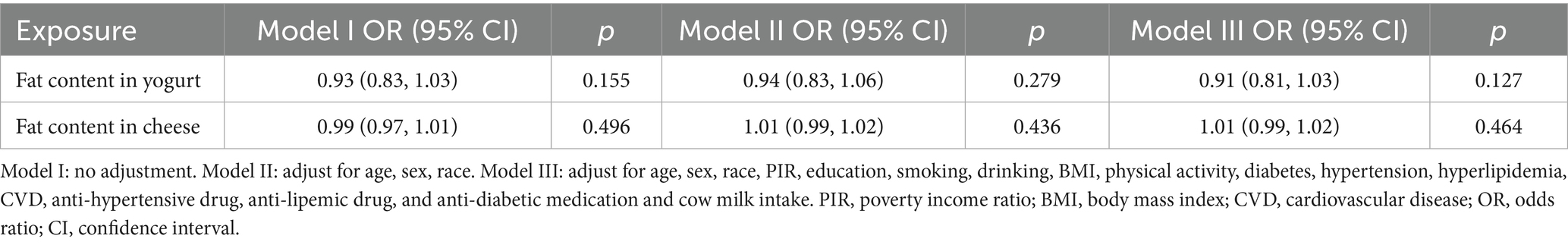
Additionally, to explore whether fat content in fermented dairy products affects stroke occurrence, this study also conducted regression analysis between fat content and stroke. This analysis was performed separately in subgroups of individuals consuming yogurt, buttermilk, and cheese.
3 Results
3.1 Baseline characteristics
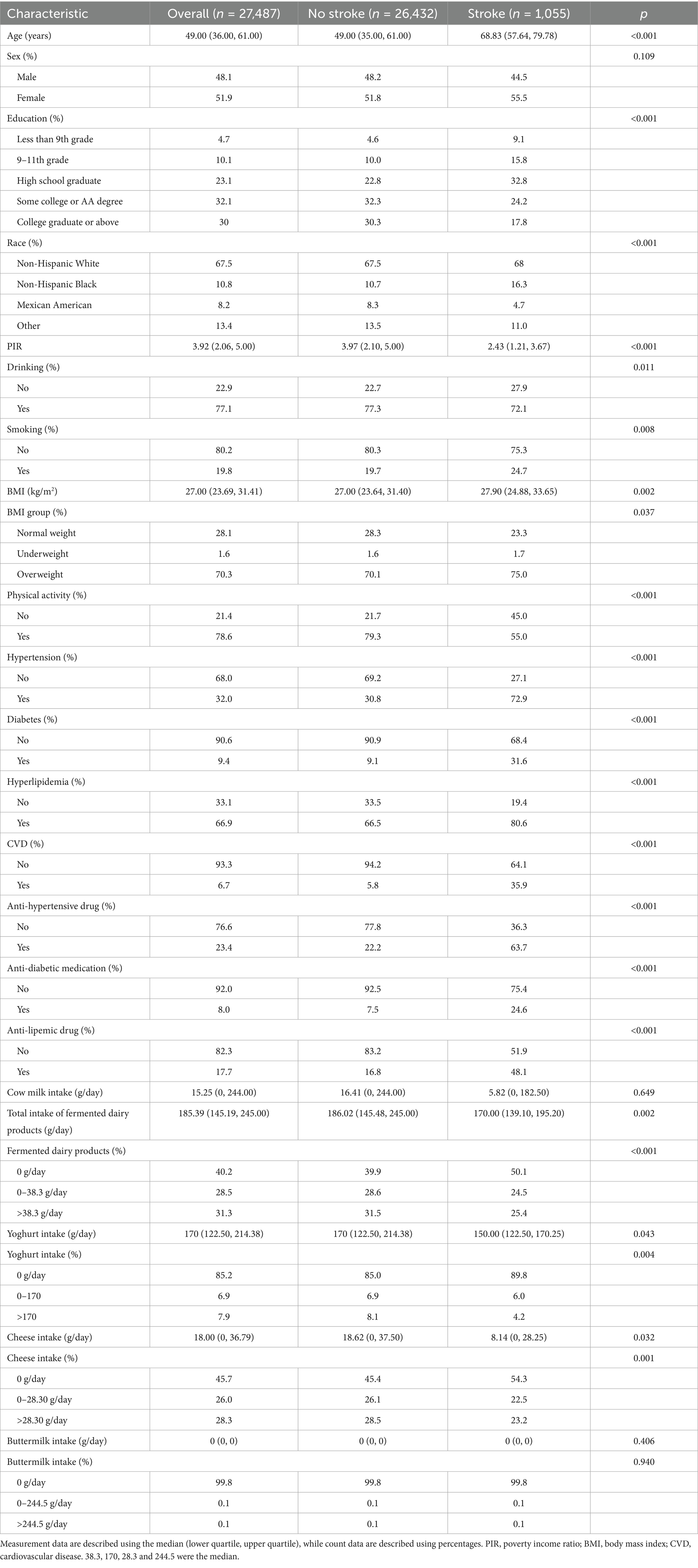
The study ultimately included 27,487 participants, among whom 1,055 had experienced a stroke. Participants with a habit of consuming fermented dairy products accounted for 59.8% of the total population. Yogurt consumers made up 14.8%, cheese consumers accounted for 54.3%, and only 0.2% of the participants consumed buttermilk. Due to the low prevalence of buttermilk consumption, the median (intake) of buttermilk was zero. Overall, as shown in Table 1, the risk of stroke was significantly associated with age (p < 0.001), education (p < 0.001), race (p < 0.001), PIR (p < 0.001), drinking (p = 0.011), smoking (p = 0.008), BMI (p = 0.002), Physical activity (p < 0.001), hypertension (p < 0.001), diabetes (p < 0.001), hyperlipidemia (p = 0.001), CVD (p < 0.001), anti-hypertensive drug (p < 0.001), anti-diabetic medication (p < 0.001) and anti-lipemic drug (p < 0.001). Compared with participants without stroke, those who had a stroke were older, had lower educational attainment, had a higher prevalence of smoking, had a higher proportion of Black people, had a lower proportion of Mexican Americans, had lower income levels and had a lower prevalence of physical activity. Additionally, these individuals had a higher likelihood of being overweight, along with a more widespread prevalence of hypertension, diabetes, hyperlipidemia, and CVD. Similarly, a higher proportion of individuals with stroke were on medications. However, the study found that participants without stroke were more likely to have a habit of alcohol consumption. This study also found no association between stroke and sex.
3.2 Stroke prevalence according to kinds of fermented dairy products
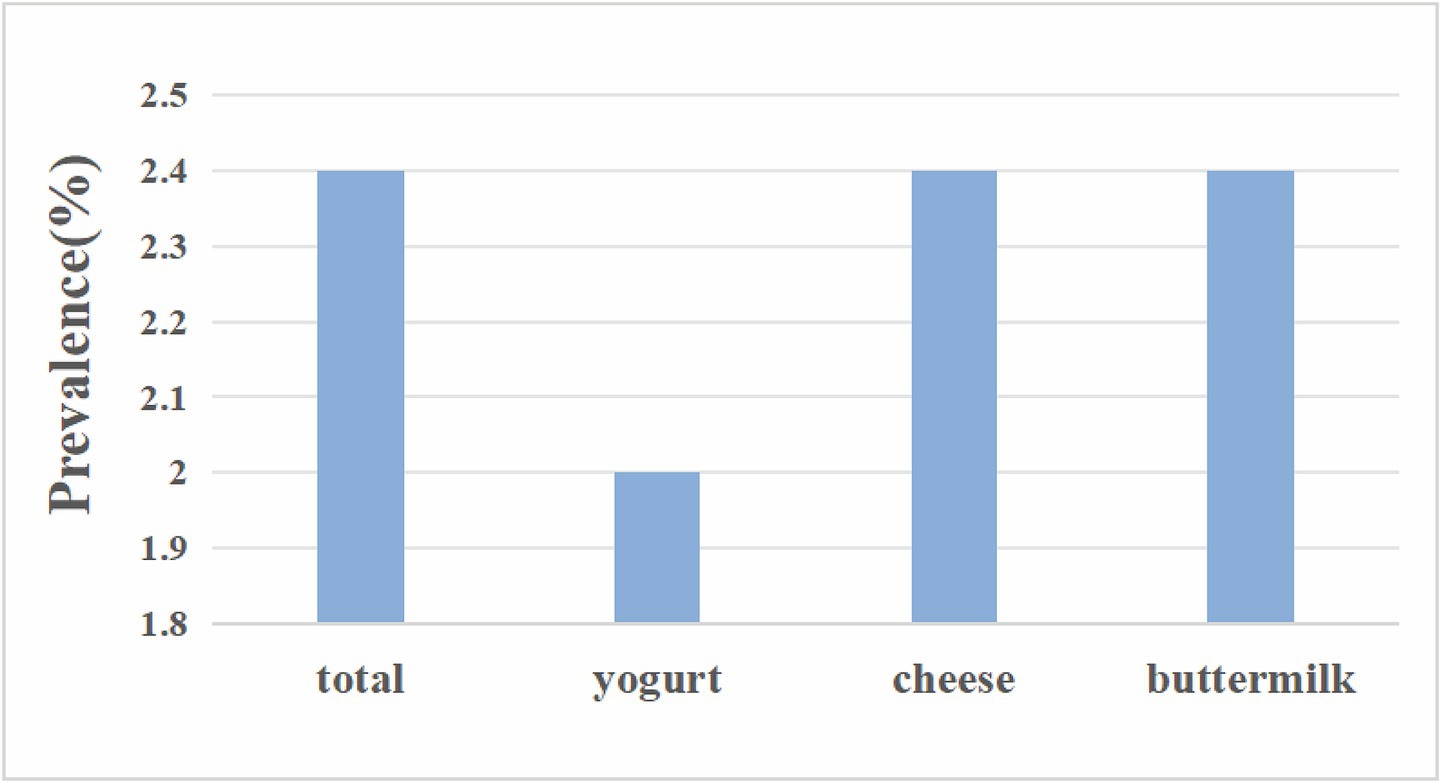
This study calculated the stroke incidence rates among populations consuming different types of fermented dairy products, as shown in Figure 2. The incidence rate of stroke was 2.40% (95% CI: 2.11–2.74) totally, 2% (95% CI: 1.56–2.54) for participants who had yogurt, 2.40% (95% CI: 2.11–2.77) for who ate cheese, and 2.40% (95% CI: 0.49–10.63) for who drank buttermilk.
3.3 The association between fermented dairy intake and stroke
This study explored the associations among the overall intake of fermented dairy products, as well as the intakes of yogurt, cheese, and buttermilk, and the incidence of stroke. Initially, the intake of fermented dairy products (total, yogurt and buttermilk) was analyzed as a continuous variable and its unit was changed to 50 g/day. According to the Dietary Guidelines for Americans (28), 1 cup of dairy equivalent is approximately 240 ml, and 50 g of fermented milk is roughly to 1/5 cup. When analyzing the association between cheese and stroke, the unit was 20 g/day. Then they were divided into three groups: no intake, low intake and high intake for regression analysis.
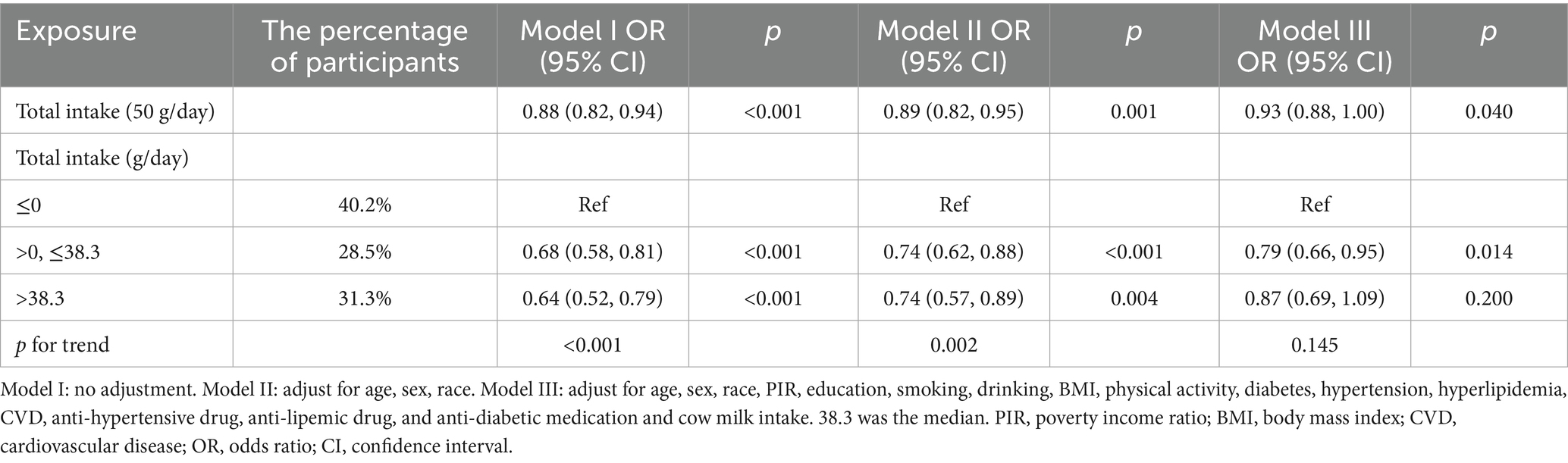
For total intake (Table 2), when analyzed as a continuous variable, the results from all three models demonstrated a negative association between intake and stroke risk (Model I: OR = 0.88, 95% CI: 0.82–0.94; Model II: OR = 0.89, 95% CI: 0.82–0.95; Model III: OR = 0.93, 95% CI: 0.88–1.00). When treated as a categorical variable, compared with participants who did not consume fermented dairy products, those with a daily intake of 0–38.3 g (38.3 was the median) had a lower stroke risk, and this relationship was consistent across all three models (Model I: OR = 0.68, 95% CI: 0.58–0.81; Model II: OR = 0.74, 95% CI: 0.62–0.88; Model III: OR = 0.79, 95% CI: 0.66–0.95). For participants with high intake, the association with reduced stroke risk was only observed in Model I (OR = 0.64, 95% CI: 0.58–0.81) and Model II (OR = 0.74, 95% CI: 0.57–0.89), but disappeared after full adjustment in Model III. In both Model I and Model II, we also confirmed that there was a relationship between stroke and the amount of fermented dairy intake shown in the trend test (Model I p for trend <0.001, Model II p for trend = 0.002).
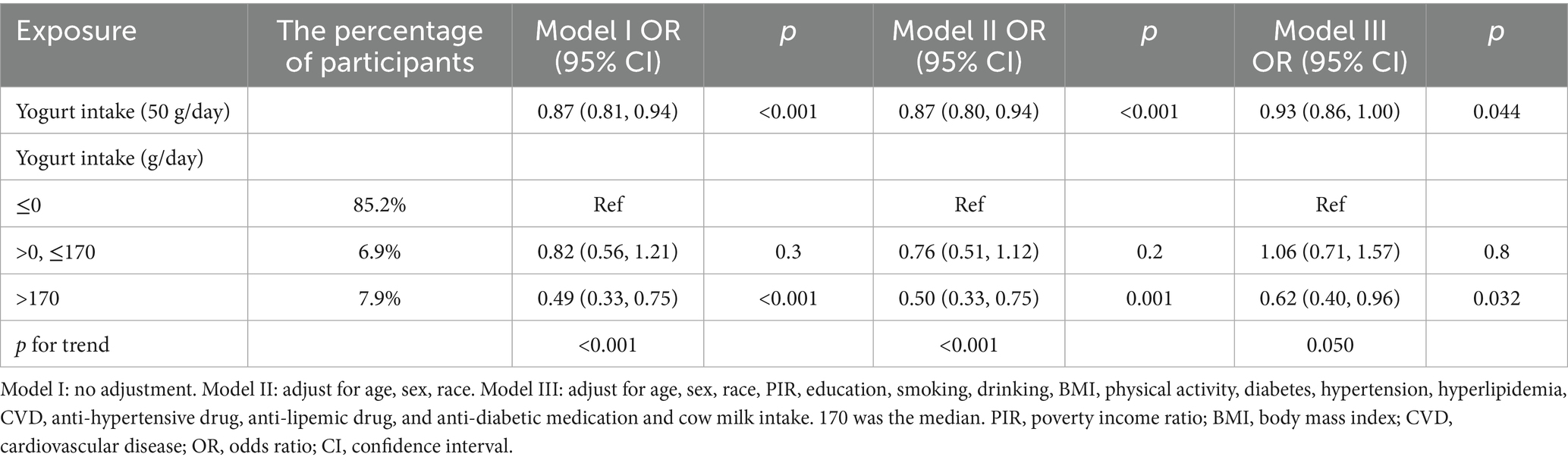
Then, this study analyzed the link between the consumption of yogurt and stroke (Table 3). Similarly, the unit for yogurt intake was also converted to 50 g/day. For continuous variables, the results showed that consuming yogurt could reduce the risk of stroke, and this relationship was consistent across all three models (Model I: OR = 0.87, 95% CI: 0.81–0.94; Model II: OR = 0.87, 95% CI: 0.80–0.94; Model III: OR = 0.93, 95% CI: 0.86–1.00). For categorical variables, only those who consumed more than 170 g (170 was the median) of yogurt per day had a reduced stroke risk (Model I: OR = 0.49, 95% CI: 0.33–0.75; Model II: OR = 0.50, 95% CI: 0.33–0.75; Model III: OR = 0.62, 95% CI: 0.40–0.96). Finally, we also found that there was a relationship between the amount of yogurt intake and the risk of stroke (Model I p for tend <0.001, Model II p for tend <0.001, Model III p for tend = 0.050).
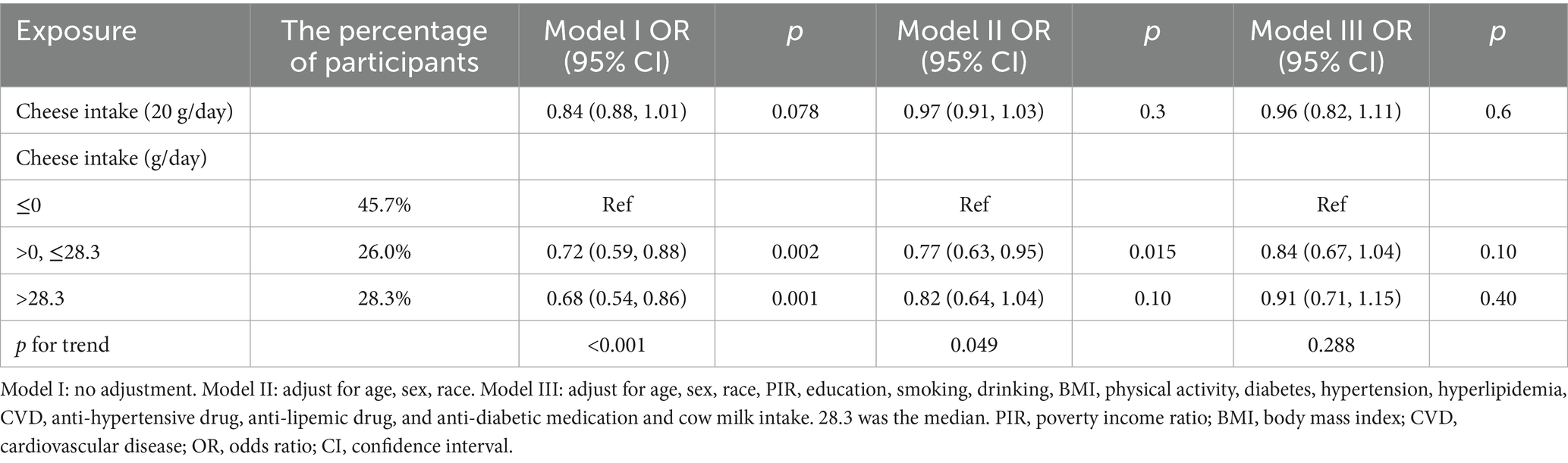
Next, we analyzed the relationship between cheese intake and stroke (Table 4). The results showed that when cheese intake was analyzed as a continuous variable, there was no association between the two (p > 0.05). For categorical variables, the negative correlation between stroke and cheese intake was only statistically significant in Model I (participants (0–28.3 g/day): OR = 0.72, 95% CI: 0.59–0.88; participants (>28.3 g/day): OR = 0.68, 95% CI: 0.54–0.86), but this relationship lost statistical significance after adjusting for variables (28.3 was the median). In the trend test, we only found statistical significance in Model I and Model II (Model I p for trend <0.001, Model II p for trend = 0.049).
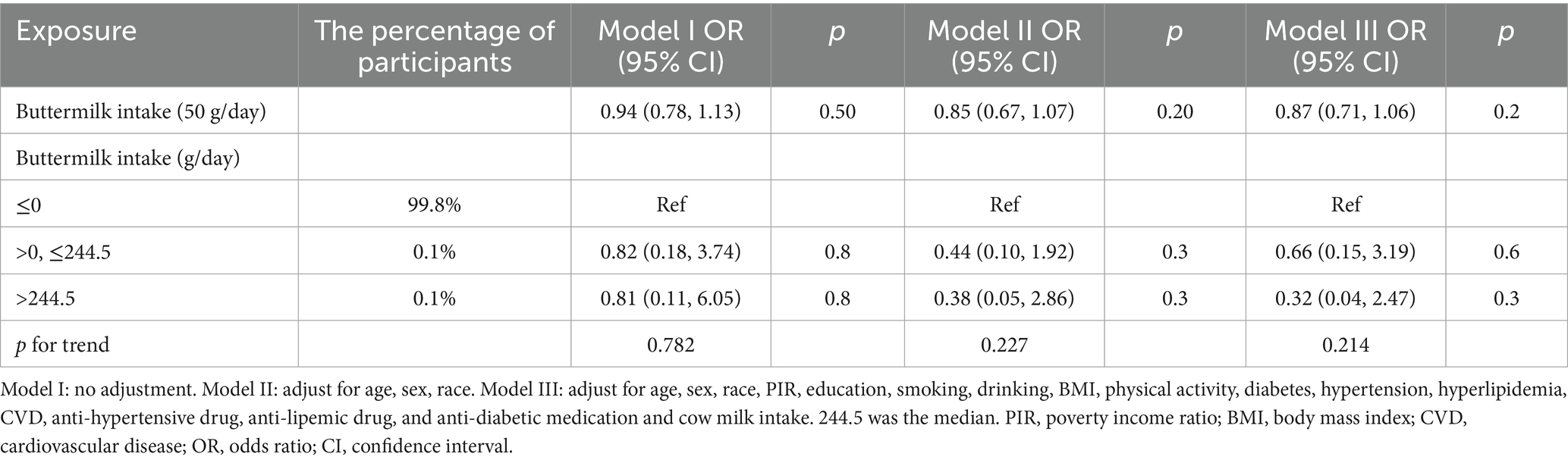
As shown in Table 5, the association between buttermilk intake and stroke was not statistically significant.
3.4 Subgroup analysis
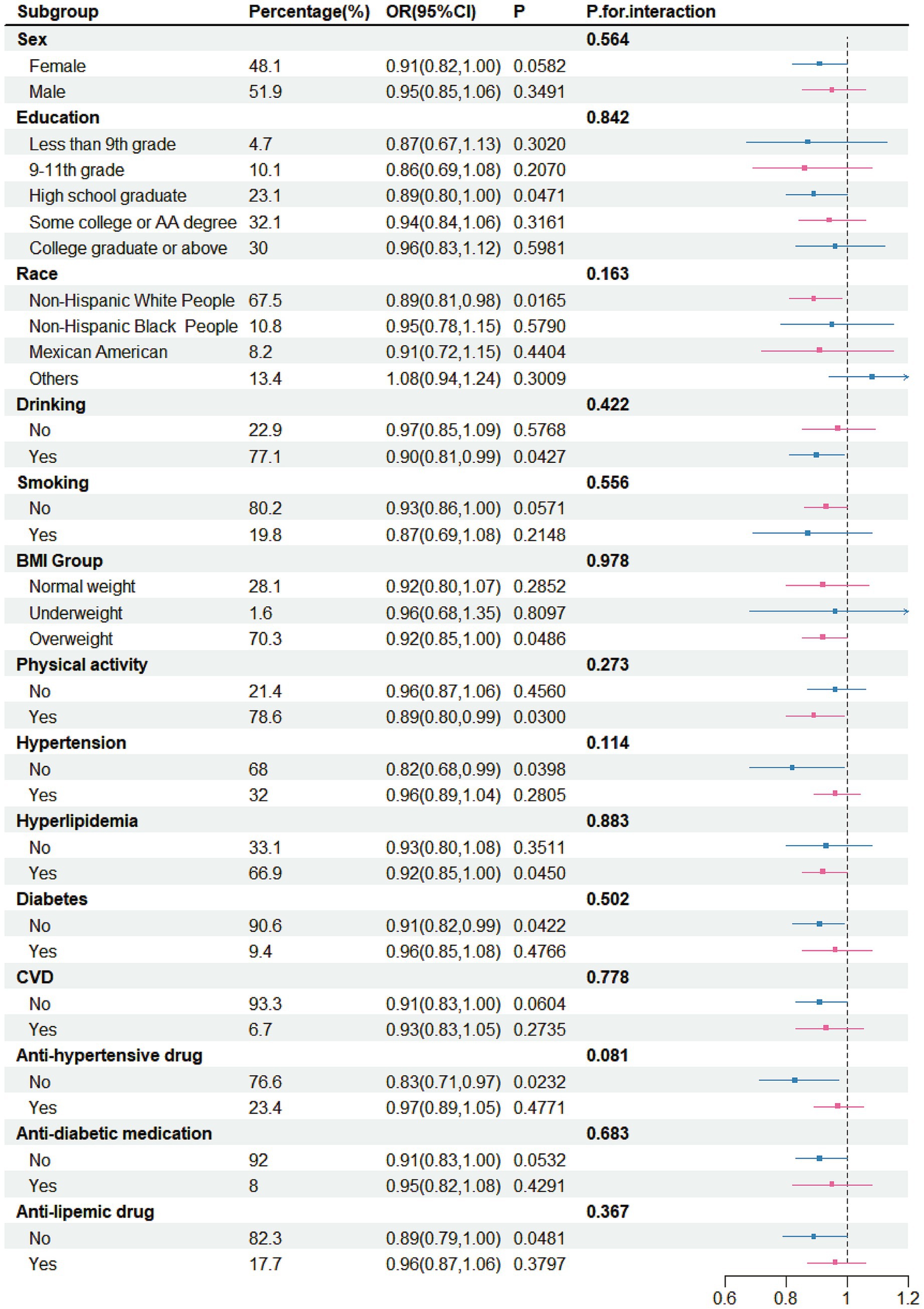
The above results showed that both total intake and yogurt intake were negatively correlated with stroke risk. Therefore, detailed subgroup analyses conducted. In these analyses, both total fermented dairy intake (50 g/day) and yogurt intake (50 g/day) were treated as continuous variables. For the relationship between total intake and stroke risk (Figure 3), no significant differences were observed across different sex groups, education groups, race groups, BMI groups, drinker groups, CVD groups, hypertension groups, diabetes groups, and hyperlipidemia groups. However, in certain specific populations, the ingestion of fermented dairy products was related to a lowered risk of stroke, and this relationship was statistically significant. These populations included White people (OR = 0.91, 95% CI: 0.83–0.99), drinkers (OR = 0.90, 95% CI: 0.82–0.99), smokers (OR = 0.78, 95% CI: 0.63–0.96), overweight individuals (OR = 0.92, 95% CI: 0.86–0.99), those with physical activity (OR = 0.90, 95% CI: 0.82–0.98), those without CVD (OR = 0.92, 95% CI: 0.85–1.00), those without diabetes (OR = 0.90, 95% CI: 0.83–0.98), those without anti-diabetic medication (OR = 0.93, 95% CI: 0.86–1.00) and those without anti-lipemic drug (OR = 0.91, 95% CI: 0.84–0.99). However, we found ferment milk may benefit the smoker more (p for interaction = 0.047).
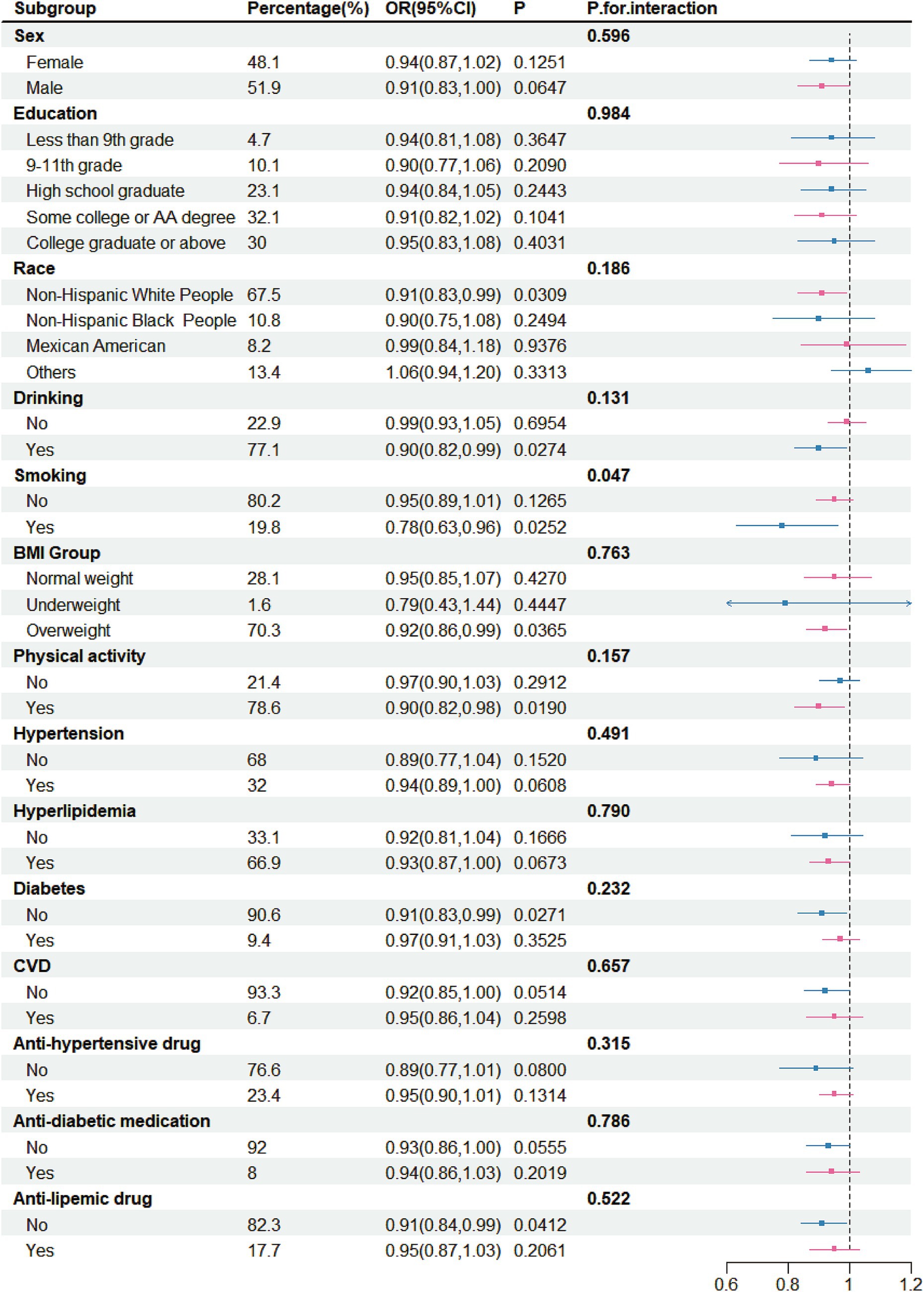
Figure 3. Subgroup analysis for the association between intake of fermented dairy products and stroke, weighted.
There was no significant correlation between yogurt intake and stroke risk across different subgroups (Figure 4). However, a negative correlation was observed in certain specific groups, including female (OR = 0.91, 95% CI 0.82–1.00), participants with high school education (OR = 0.89, 95% CI 0.80–1.00), White people (OR = 0.89, 95% CI: 0.81–0.98), drinkers (OR = 0.90, 95% CI: 0.81–0.99), non-smokers (OR = 0.93, 95% CI: 0.86–1.00), individuals who were overweight (OR = 0.92, 95% CI: 0.85–1.00), those with physical activity (OR = 0.89, 95% CI: 0.80–0.99), those without hypertension (OR = 0.82, 95% CI: 0.68–0.99), individuals with hyperlipidemia (OR = 0.92, 95% CI: 0.85–1.00), those without diabetes (OR = 0.91, 95% CI: 0.82–0.99), those without anti-hypertensive drug (OR = 0.83, 95% CI: 0.71–0.97), those without anti-diabetic medication (OR = 0.91, 95% CI: 0.83–1.00) and those without anti-lipemic drug (OR = 0.89, 95% CI: 0.79–1.00).
3.5 The association between fat content in fermented dairy products and stroke
This analysis was performed separately in three consumption subgroups across three distinct models: yogurt consumers, buttermilk consumers, and cheese consumers (Table 6). Due to the insufficient number of individuals consuming buttermilk, no statistical results were obtained, thus the data were not presented in the table. Results showed that the fat content in both yogurt and cheese was not associated with stroke risk (p > 0.05).
4 Discussion
This research, based on an in-depth analysis of data from the NHANES database (2007–2018), revealed that fermented dairy products may potentially mitigate stroke risk. Notably, it was yogurt, as opposed to cheese and buttermilk, that exhibited a remarkably negative correlation with stroke incidence. Specifically, among the people who consumed yogurt, the incidence of stroke was the lowest. The findings demonstrated that for each incremental 50-gram increase in the daily intake of fermented dairy products, the risk of stroke was diminished by 7%. Moreover, the optimal range for minimizing the stroke risk lay within the daily consumption of 0–38.3 grams of fermented dairy products. Similarly, in the case of yogurt consumption, a 50-gram increment in daily intake corresponded to a 7% reduction in stroke risk. However, consuming more than 170 g of yogurt daily could reduce the risk of stroke. Conversely, the results of this study did not indicate any significant correlation between cheese, buttermilk, and the risk of stroke. In conclusion, having a sufficient quantity of fermented dairy products, particularly yogurt, every day can help American people avoid stroke by promoting a balanced diet.
Diet is a modifiable factor in stroke prevention, and dairy products are an essential component of a wholesome diet. Previous studies have yielded results similar to ours. A prospective cohort study (29) reported a trend toward an inverse association between fermented dairy consumption and the risk of stroke (p = 0.07). An investigation of a cohort in the Netherlands showed that the ingestion of yogurt was notably linked to a lower risk of stroke in both genders (30). Another Dutch study (31) involving 36,886 participants showed that full-fat yogurt, compared with other dairy products, was related to a decreased likelihood of stroke (HR = 0.34–0.37). Similarly, observations from nine European countries indicated that a daily ingestion of fermented dairy products showed an inverse relationship to the risk of ischemic stroke (yogurt: 100 g/day, HR = 0.91, 95% CI: 0.85–0.97; cheese: 30 g/day, HR = 0.88, 95% CI: 0.81–0.97) (32). Additionally, a Danish cohort study including 55,211 participants found that replacing other dairy products with full-fat fermented dairy was associated with a lower incidence of ischemic stroke (33). However, these studies do not appear to elucidate the causal relationship between fermented dairy products and stroke risk. In summary, both previous studies and this research provide a common suggestion that the intake of fermented dairy products, particularly yogurt, may reduce the risk of stroke. Moreover, while moderate consumption is generally appropriate for total fermented dairy intake, a daily yogurt intake of more than 170 g is required to achieve a protective effect.
However, in these results, the relationship between cheese and stroke was not statistically significant. Moreover, when compared with those who did not consume cheese, the negative correlation disappeared after adjusting for covariates. This result may be related to the insufficiently detailed classification of cheese in the database. Due to the diversity of food types nowadays, perhaps not all the consumed cheese was included in the statistics. Meanwhile, cheese, with its characteristics of high fat and high sodium, is considered a food that is not conducive to cardiovascular and cerebrovascular health (34, 35). The results of some studies also show that the correlation between cheese and stroke is not statistically significant (RR = 0.97, 95% CI: 0.94–1.01) (36). Regarding buttermilk, only 74 people (0.27%) in the population covered by this study consumed it. The extremely small sample size makes the relationship between buttermilk and stroke not particularly clear. However, as a by-product of the butter manufacturing process, buttermilk is characterized by its low fat and low salt content. The milk fat globule membrane (MFGM), which is abundant in buttermilk, has been verified to have anti-inflammatory properties and the potential to reduce cholesterol levels (37), and can restore the normal flora structure of mice with dysbacteriosis (38). MFGM, a trilayer structure composed of proteins and polar lipids (phospholipids and sphingolipids), tightly encapsulates the triglyceride core (fat globule) as a natural protective layer. Sphingolipids and bovine phospholipids within MFGM can reduce serum cholesterol levels and alleviate hepatic lipid accumulation by decreasing intestinal cholesterol absorption and regulating hepatic gene expression related to lipid metabolism (39, 40). Moreover, buttermilk processed by certain special techniques may even substitute for milk (41). Evidently, it is also a potentially healthy beverage.
In the analysis of baseline data, there was no difference in the gender distribution between stroke and non-stroke participants. This may be related to the relatively small sample size. However, some studies have also shown that the gender-specific differences in the prevalence of stroke have been decreasing year by year (42). It is widely recognized that alcohol consumption is a factor in stroke risk. However, the results of this study show that a significant number of participants who did not suffer from stroke had a habit of drinking alcohol. Similarly, some studies have shown that moderate alcohol consumption and the intake of certain types of alcoholic beverages could be related to a reduced risk of stroke (43, 44). Therefore, the relationship between alcohol consumption and stroke risk remains a topic of debate.
In the subgroup analysis, correlations are not universal because the sample sizes of each subgroup are relatively small. When the total intake was selected for analysis, a negative correlation between stroke and it was observed among White people, drinkers, smoker, overweight individuals, those with physical activity, those without CVD, those without diabetes, those without anti-diabetic medication and those without anti-lipemic drug. After that, subgroup analyses were also conducted on the relationship between yogurt and stroke. The negative correlation was not present in any of the subgroups. However, there were some populations that might benefit, such as participants with a high—school education, White people, drinkers, non-smokers, overweight individuals, those with physical activity, those without hypertension, individuals with hyperlipidemia, those without diabetes, those without anti-hypertensive drug, those without anti-diabetic medication and those without anti-lipemic drug. This suggests that healthcare workers should provide targeted prevention strategies for specific populations when formulating preventive policies. The above-mentioned population should pay more attention to the intake of fermented dairy products.
Fermented dairy product, as a traditional food, has its health benefits well-documented by extensive research, providing an important research entry point for exploring the potential mechanisms underlying the association between fermented milk and stroke risk. The gut-brain axis is a complex bidirectional signaling system connecting the gastrointestinal tract and the brain (45). This connection is mediated through the synergistic actions of the Central Nervous System (CNS), the Enteric Nervous System (ENS), and the gut microbiota. The ENS is an intricate system composed of neurons and glial cells embedded within the intestinal wall, regulating most gastrointestinal functions. Furthermore, the ENS establishes bidirectional communication with the CNS via autonomic nerves and primary afferent nerves originating from the gastrointestinal tract (46). In this process, it is the gut microbiota that consistently plays a crucial role. Probiotics are collectively defined as live microorganisms that, when ingested in appropriate amounts, exhibit biological activity, promote ecological balance of the intestinal microbiota, and confer health benefits to the host (47). Fermented dairy products are primary food carriers for probiotics (48), and their stroke-preventive function is inseparable from the role of these beneficial microorganisms. Under normal physiological conditions, the gut microbiota sustains a dynamic equilibrium, forming a gut barrier that supports host health (49). When the abundance and diversity of gut microbiota are reduced, it may directly trigger a stroke. A review of 14 clinical studies found that stroke patients had lower gut microbiota diversity compared to healthy individuals (50). However, this effect is relatively weak, and the impact of gut microbiota dysbiosis on stroke occurrence is more likely mediated through influencing risk factors (7, 51). Current research indicates that obesity (52), diabetes (53), and hypertension (54) are risk factors directly associated with gut microbiota imbalance. Studies have shown that one serving of yogurt contains approximately 10 million CFUs (colony-forming units) of bacteria (11),with high diversity (55), including Bifidobacterium lactis, Lactobacillus rhamnosus, and Streptococcus thermophilus. And most of these bacteria are beneficial to human health. For instance, Lactobacillus rhamnosus can inhibit inflammation (56), and Bifidobacterium lactis reduces BMI (57). The intake of fermented dairy products can increase the number of probiotics (58), improve the gut microenvironment, and produce a series of beneficial effects on the human body, thereby influencing risk factors (59) and ultimately reducing the incidence of stroke.
In addition to affecting the homeostasis of the gut microbiota, probiotics can also influence the metabolites or intermediate products of the gut microbiota, which may be another mechanism by which fermented dairy products prevent stroke. Certainly, this preventive effect is also achieved by influencing risk factors. These substances include short-chain fatty acids (SCFAs) and conjugated linoleic acid. Conjugated linoleic acid is widely present in dairy products, but the lactic acid bacteria and bifidobacterium in yogurt can increase its content (60), thereby further enhancing its effects of reducing blood pressure, blood lipids, and body weight (61). Similarly, these bacteria can ferment some dietary fibers, thus increasing the content of metabolites, and this is how SCFAs are increased (62). A literature review found that fermented milk can significantly reduce cholesterol compared with non-fermented milk, and this has been verified both in humans and animals (63). This may be related to the role of short-chain fatty acids in inhibiting cholesterol synthesis in the liver. In addition, experiments have found that SCFAs can promote the secretion of peptide YY by intestinal endothelial cells, thereby increasing insulin secretion and achieving the effect of lowering blood glucose (64). Moreover, an animal experiment has found that SCFAs can directly act on the nerve endings in the lamina propria of intestinal mucosal epithelial cells, thereby activating the afferent nerve fibers of the vagus nerve and affecting blood pressure regulation (65). Another factor related to stroke is the inhibitory neurotransmitter—GABA. Lactobacillus rhamnosus and Bifidobacterium have been proven to produce this neurotransmitter in the intestine (66), and yogurt and cheese themselves are foods rich in GABA (67, 68). Different from other factors, GABA can also regulate emotions, and the influence of emotional issues on stroke occurrence has garnered significant attention nowadays. Multiple meta-analyses and systematic reviews (69, 70) have shown that negative emotions (depression) are positively correlated with stroke. GABA can relieve depression (71), thereby reducing the impact of emotional problems on stroke risk. At the same time, GABA has also been reported to have the effect of lowering blood pressure (72). Certainly, existing studies have indeed confirmed that fermented dairy products can prevent or improve these risk factors (73–77). In conclusion, probiotics and their derivatives significantly contribute to stroke prevention through fermented dairy products.
In addition, we hypothesize that the high calcium content in fermented dairy products is another important factor in stroke prevention. As early as 1931, it was discovered that yogurt contains higher levels of calcium than milk, and the presence of probiotics facilitates calcium absorption. Studies have shown that calcium intake is associated with a lower prevalence of stroke. Zhu et al. analyzed the dietary intake of 6,411 Chinese individuals and found that calcium intake appeared to correlate with a reduced likelihood of stroke (HR = 0.53, 95% CI: 0.29–0.96) (78). A 14-year follow-up study (14) revealed that low calcium intake increased the stroke risk in adult women, while those with high calcium intake had a relative stroke risk of 0.69 (95% CI: 0.50–0.95). This inverse correlation was more pronounced for calcium obtained from dairy sources (dairy: RR = 0.68, 95% CI: 0.50–0.94; non-dairy: RR = 0.82, 95% CI: 0.58–1.16). Tian et al. also obtained similar results through a meta-analysis of several prospective cohort studies (79). Similarly, a study focused on men also found that dietary calcium intake can lower stroke incidence (80). In summary, dietary calcium can reduce stroke risk to some extent, which may be related to its ability to influence stroke risk factors. For example, calcium can directly affect the contraction and relaxation of vascular smooth muscle, thereby regulating blood pressure (81). Additionally, calcium can lower cholesterol levels in the blood (82). This explains why calcium-rich fermented dairy products can help prevent stroke.
Finally, the preventive effect of fermented milk against stroke may be related to its anti-inflammatory properties. Chronic inflammation is recognized as a key factor in stroke occurrence (83). Inflammatory factors (such as interleukins and tumor necrosis factor) produced by chronic inflammation can alter the state of vascular endothelium, activate platelets, inhibit the decomposition of fibrinogen, promote a hypercoagulable state, form blood clots, and increase the risk of stroke (84). Fortunately, diet can modulate the systemic inflammatory response. Moreover, an analysis of the NHANES database reveals a positive correlation between the dietary inflammatory index and stroke risk, indicating that an anti-inflammatory diet can reduce the risk of stroke (25). Notably, fermented dairy products have demonstrated anti-inflammatory effects (10). For instance, a 9-week randomized controlled trial on healthy premenopausal women showed that compared with women who consumed soy pudding daily, women who consumed 226 grams of low-fat yogurt daily for 9 weeks had a decrease in the marker of chronic inflammation, IL-6, in their bodies (85). Additionally, some studies have also shown that Bifidobacterium can significantly reduce the levels of tumor necrosis factor-α and IL-6 in patients (57). Collectively, these findings suggest that the preventive effect of fermented dairy products against stroke is likely mediated by their ability to alleviate the pro-inflammatory response.
This research elucidated the connection between overall consumption of fermented dairy products and stroke risk, alongside the links between specific fermented dairy items (yogurt, cheese, and buttermilk) and stroke incidence. Additionally, we established the dose–response relationships for these associations: stroke risk decreased by 7% for every 50 g increase in total fermented dairy intake. Notably, yogurt intake was associated with an even greater reduction in stroke risk, with each 50 g increase lowering it by 7%. No such associations were observed for cheese or buttermilk. However, our study has certain limitations. First, stroke and confounding variables were determined based on participants recall, which may introduce recall bias. The survey questionnaire also did not specify the type of stroke. Second, as this was a cross-sectional study, we could not establish causality between fermented dairy intake and stroke. Third, the intake of dairy products was based on participants’ recall, which may be inaccurate. Furthermore, due to the diversification of modern food products, some emerging fermented dairy products may not have been included in the database. Participants may have consumed fermented dairy products in other foods that were not accounted for, potentially affecting the results. Additionally, the dietary intake data represented only a two-day average for participants, which might not accurately represent their long-term dietary patterns. Nevertheless, studies have shown that this method of data collection is representative (86). Furthermore, the NHANES database did not analyze statistically the participants’ background of daily dairy consumption or their dietary patterns, which may have influenced the results. Fourth, non-dairy fermented foods might affect stroke occurrence through similar pathways. However, due to challenges in accurately quantifying their intake, they were not included as covariates, potentially introducing errors. Finally, given the complexity of stroke occurrence, there may still be unconsidered confounding factors in the NHANES database, such as family history of stroke.
5 Conclusion
This research revealed a negative correlation between fermented dairy products and stroke among adult Americans, particularly with yogurt. Consuming fermented dairy products in appropriate amounts daily may lower stroke risk. However, no significant correlation was observed between cheese, buttermilk, and stroke. These findings can provide recommendations for adult Americans to prevent stroke. Nonetheless, numerous prospective cohort studies remain necessary to confirm these findings and elucidate the causal relationship.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: https://www.cdc.gov/nchs/nhanes/.
Ethics statement
The studies involving humans were approved by National Center for Health Statistics Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
SM: Writing – review & editing, Writing – original draft, Methodology. YM: Writing – review & editing, Formal analysis, Data curation. XW: Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would thank the NHANES for providing such detailed data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. GBD 2021 Causes of Death Collaborators. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet. (2024) 403:2100–32. doi: 10.1016/S0140-6736(24)00367-2
2. GBD 2021 Stroke Risk Factor Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet Neurol. (2024) 23:973–1003. doi: 10.1016/S1474-4422(24)00369-7
3. Ma, Z, He, W, Zhou, Y, Mai, L, Xu, L, Li, C, et al. Global burden of stroke in adolescents and young adults (aged 15-39 years) from 1990 to 2019: a comprehensive trend analysis based on the global burden of disease study 2019. BMC Public Health. (2024) 24:2042. doi: 10.1186/s12889-024-19551-1
4. Cheng, Y, Lin, Y, Shi, H, Cheng, M, Zhang, B, Liu, X, et al. Projections of the stroke burden at the global, regional, and National Levels up to 2050 based on the global burden of disease study 2021. J Am Heart Assoc. (2024) 13:e036142. doi: 10.1161/JAHA.124.036142
5. Raghani, N, Postwala, H, Shah, Y, Chorawala, M, and Parekh, P. From gut to brain: unraveling the intricate link between microbiome and stroke. Probiotics Antimicrob Proteins. (2024) 16:2039–53. doi: 10.1007/s12602-024-10295-3
6. Wu, Y, He, H, Cheng, Z, Bai, Y, and Ma, X. The role of neuropeptide Y and peptide YY in the development of obesity via gut-brain Axis. Curr Protein Pept Sci. (2019) 20:750–8. doi: 10.2174/1389203720666190125105401
7. Chidambaram, SB, Rathipriya, AG, Mahalakshmi, AM, Sharma, S, Hediyal, TA, Ray, B, et al. The influence of gut Dysbiosis in the pathogenesis and Management of Ischemic Stroke. Cells. (2022) 11:1239. doi: 10.3390/cells11071239
8. Smug, LN, Salminen, S, Sanders, ME, and Ebner, S. Yoghurt and probiotic bacteria in dietary guidelines of the member states of the European Union. Benefic Microbes. (2014) 5:61–6. doi: 10.3920/BM2013.0050
9. Román, GC, Jackson, RE, Gadhia, R, Román, AN, and Reis, J. Mediterranean diet: the role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev Neurol. (2019) 175:724–41. doi: 10.1016/j.neurol.2019.08.005
10. Yerlikaya, O. A review of fermented milks: potential beneficial effects on human nutrition and health. Afr Health Sci. (2023) 23:498–507. doi: 10.4314/ahs.v23i4.54
11. Rizzoli, R, and Biver, E. Role of fermented dairy products in the health benefits of a mediterranean diet. Aging Clin Exp Res. (2024) 36:75. doi: 10.1007/s40520-024-02721-x
12. Masoumi, SJ, Mehrabani, D, Saberifiroozi, M, Fattahi, MR, Moradi, F, and Najafi, M. The effect of yogurt fortified with lactobacillus acidophilus and Bifidobacterium sp. probiotic in patients with lactose intolerance. Food Sci Nutr. (2021) 9:1704–11. doi: 10.1002/fsn3.2145
13. Sonestedt, E, Borné, Y, Wirfält, E, and Ericson, U. Dairy consumption, lactase persistence, and mortality risk in a cohort from southern Sweden. Front Nutr. (2021) 8:779034. doi: 10.3389/fnut.2021.779034
14. Iso, H, Stampfer, MJ, Manson, JE, Rexrode, K, Hennekens, CH, Colditz, GA, et al. Prospective study of calcium, potassium, and magnesium intake and risk of stroke in women. Stroke. (1999) 30:1772–9. doi: 10.1161/01.STR.30.9.1772
15. Dukuzimana, J, Janzi, S, Habberstad, C, Zhang, S, Borné, Y, and Sonestedt, E. High consumption of dairy products and risk of major adverse coronary events and stroke in a Swedish population. Br J Nutr. (2024) 131:500–11. doi: 10.1017/S0007114523001939
16. Hu, D, Huang, J, Wang, Y, Zhang, D, and Qu, Y. Dairy foods and risk of stroke: a meta-analysis of prospective cohort studies. Nutr Metab Cardiovasc Dis. (2014) 24:460–9. doi: 10.1016/j.numecd.2013.12.006
17. Praagman, J, Franco, OH, Ikram, MA, Soedamah-Muthu, SS, Engberink, MF, van Rooij, FJ, et al. Dairy products and the risk of stroke and coronary heart disease: the Rotterdam study. Eur J Nutr. (2015) 54:981–90. doi: 10.1007/s00394-014-0774-0
18. Larsson, SC, Virtamo, J, and Wolk, A. Dairy consumption and risk of stroke in Swedish women and men. Stroke. (2012) 43:1775–80. doi: 10.1161/STROKEAHA.111.641944
19. Olsson, E, Larsson, SC, Höijer, J, Kilander, L, and Byberg, L. Milk and fermented Milk consumption and risk of stroke: longitudinal study. Nutrients. (2022) 14:1070. doi: 10.3390/nu14051070
20. Larsson, SC, Männistö, S, Virtanen, MJ, Kontto, J, Albanes, D, and Virtamo, J. Dairy foods and risk of stroke. Epidemiology. (2009) 20:355–60. doi: 10.1097/EDE.0b013e3181935dd5
21. Chen, Z, Ahmed, M, Ha, V, Jefferson, K, Malik, V, Ribeiro, PAB, et al. Dairy product consumption and cardiovascular health: a systematic review and Meta-analysis of prospective cohort studies. Adv Nutr (Bethesda, Md). (2022) 13:439–54. doi: 10.1093/advances/nmab118
23. Iacoviello, L, Bonaccio, M, Cairella, G, Catani, MV, Costanzo, S, D'Elia, L, et al. Diet and primary prevention of stroke: systematic review and dietary recommendations by the ad hoc working Group of the Italian Society of human nutrition. Nutr Metab Cardiovasc Dis. (2018) 28:309–34. doi: 10.1016/j.numecd.2017.12.010
24. Lee, M, Lakshminarayan, K, Sedaghat, S, Sabayan, B, Chen, LY, Johansen, MC, et al. Population attributable fraction of total stroke associated with modifiable risk factors in the United States. Am J Epidemiol. (2024) 193:1712–9. doi: 10.1093/aje/kwae132
25. Mao, Y, Weng, J, Xie, Q, Wu, L, Xuan, Y, Zhang, J, et al. Association between dietary inflammatory index and stroke in the US population: evidence from NHANES 1999-2018. BMC Public Health. (2024) 24:50. doi: 10.1186/s12889-023-17556-w
26. Miao, Y, Ma, S, and Wu, X. Association between tea consumption and stroke in the American adult females: analyses of NHANES 2011-2018 data. Front Nutr. (2024) 11:1452137. doi: 10.3389/fnut.2024.1452137
27. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. (2002) 106:3143–421. doi: 10.1161/circ.106.25.3143
28. U.S. USDoAa. Department of Health and Human Services. Dietary guidelines for Americans, 2020–2025. Washington, DC: USDA (2020).
29. Dalmeijer, GW, Struijk, EA, van der Schouw, YT, Soedamah-Muthu, SS, Verschuren, WM, Boer, JM, et al. Dairy intake and coronary heart disease or stroke--a population-based cohort study. Int J Cardiol. (2013) 167:925–9. doi: 10.1016/j.ijcard.2012.03.094
30. Goldbohm, RA, Chorus, AM, Galindo Garre, F, Schouten, LJ, and van den Brandt, PA. Dairy consumption and 10-y total and cardiovascular mortality: a prospective cohort study in the Netherlands. Am J Clin Nutr. (2011) 93:615–27. doi: 10.3945/ajcn.110.000430
31. Laursen, ASD, Sluijs, I, Boer, JMA, Verschuren, WMM, van der Schouw, YT, and Jakobsen, MU. Substitutions between dairy products and risk of stroke: results from the European investigation into Cancer and nutrition-Netherlands (EPIC-NL) cohort. Br J Nutr. (2019) 121:1398–404. doi: 10.1017/S0007114519000564
32. Tong, TYN, Appleby, PN, Key, TJ, Dahm, CC, Overvad, K, Olsen, A, et al. The associations of major foods and fibre with risks of ischaemic and haemorrhagic stroke: a prospective study of 418 329 participants in the EPIC cohort across nine European countries. Eur Heart J. (2020) 41:2632–40. doi: 10.1093/eurheartj/ehaa007
33. Laursen, ASD, Dahm, CC, Johnsen, SP, Tjønneland, A, Overvad, K, and Jakobsen, MU. Substitutions of dairy product intake and risk of stroke: a Danish cohort study. Eur J Epidemiol. (2018) 33:201–12. doi: 10.1007/s10654-017-0271-x
34. Givens, DI. Saturated fats, dairy foods and cardiovascular health: no longer a curious paradox? Nutr Bull. (2022) 47:407–22. doi: 10.1111/nbu.12585
35. Zhang, M, Dong, X, Huang, Z, Li, X, Zhao, Y, Wang, Y, et al. Cheese consumption and multiple health outcomes: an umbrella review and updated meta-analysis of prospective studies. Adv Nutr (Bethesda, Md). (2023) 14:1170–86. doi: 10.1016/j.advnut.2023.06.007
36. de Goede, J, Soedamah-Muthu, SS, Pan, A, Gijsbers, L, and Geleijnse, JM. Dairy consumption and risk of stroke: a systematic review and updated dose-response Meta-analysis of prospective cohort studies. J Am Heart Assoc. (2016) 5:2787. doi: 10.1161/JAHA.115.002787
37. Barukčić, I, Lisak Jakopović, K, and Božanić, R. Valorisation of whey and buttermilk for production of functional beverages – an overview of current possibilities. Food Technol Biotechnol. (2019) 57:448–60. doi: 10.17113/ftb.57.04.19.6460
38. Bellés, A, Abad, I, Sánchez, L, and Grasa, L. Whey and buttermilk-based formulas modulate gut microbiota in mice with antibiotic-induced dysbiosis. Mol Nutr Food Res. (2023) 67:e2300248. doi: 10.1002/mnfr.202300248
39. Mozaffarian, D, and Wu, JHY. Flavonoids, dairy foods, and cardiovascular and metabolic health: a review of emerging biologic pathways. Circ Res. (2018) 122:369–84. doi: 10.1161/CIRCRESAHA.117.309008
40. Pan, J, Chen, M, Li, N, Han, R, Yang, Y, Zheng, N, et al. Bioactive functions of lipids in the milk fat globule membrane: a comprehensive review. Food Secur. (2023) 12:3755. doi: 10.3390/foods12203755
41. Szkolnicka, K, Dmytrów, I, and Mituniewicz-Małek, A. The characteristics of quark cheese made from buttermilk during refrigerated storage. Foods (Basel). (2021) 10:1783. doi: 10.3390/foods10081783
42. Madsen, TE, Khoury, JC, Leppert, M, Alwell, K, Moomaw, CJ, Sucharew, H, et al. Temporal trends in stroke incidence over time by sex and age in the GCNKSS. Stroke. (2020) 51:1070–6. doi: 10.1161/STROKEAHA.120.028910
43. Patel, A, and Figueredo, VM. Alcohol and cardiovascular disease: helpful or hurtful. Rev Cardiovasc Med. (2023) 24:121. doi: 10.31083/j.rcm2404121
44. Chiva-Blanch, G, and Badimon, L. Benefits and risks of moderate alcohol consumption on cardiovascular disease: current findings and controversies. Nutrients. (2019) 12:108. doi: 10.3390/nu12010108
45. Cryan, JF, O'Riordan, KJ, Cowan, CSM, Sandhu, KV, Bastiaanssen, TFS, Boehme, M, et al. The microbiota-gut-brain Axis. Physiol Rev. (2019) 99:1877–2013. doi: 10.1152/physrev.00018.2018
46. Sharkey, KA, and Mawe, GM. The enteric nervous system. Physiol Rev. (2023) 103:1487–564. doi: 10.1152/physrev.00018.2022
47. Vallianou, N, Stratigou, T, Christodoulatos, GS, Tsigalou, C, and Dalamaga, M. Probiotics, prebiotics, Synbiotics, Postbiotics, and obesity: current evidence, controversies, and perspectives. Curr Obes Rep. (2020) 9:179–92. doi: 10.1007/s13679-020-00379-w
48. López-Yerena, A, de Santisteban Villaplana, V, Badimon, L, Vilahur, G, and Padro, T. Probiotics: a potential strategy for preventing and managing cardiovascular disease. Nutrients. (2024) 17:52. doi: 10.3390/nu17010052
49. Blaut, M, and Clavel, T. Metabolic diversity of the intestinal microbiota: implications for health and disease. J Nutr. (2007) 137:751s–5s. doi: 10.1093/jn/137.3.751S
50. Peh, A, O'Donnell, JA, Broughton, BRS, and Marques, FZ. Gut microbiota and their metabolites in stroke: a double-edged sword. Stroke. (2022) 53:1788–801. doi: 10.1161/STROKEAHA.121.036800
51. Pluta, R, Januszewski, S, and Czuczwar, SJ. The role of gut microbiota in an ischemic stroke. Int J Mol Sci. (2021) 22:915. doi: 10.3390/ijms22020915
52. Enache, RM, Profir, M, Roşu, OA, Creţoiu, SM, and Gaspar, BS. The role of gut microbiota in the onset and progression of obesity and associated comorbidities. Int J Mol Sci. (2024) 25:2321. doi: 10.3390/ijms252212321
53. Zhang, L, Wang, P, Huang, J, Xing, Y, Wong, FS, Suo, J, et al. Gut microbiota and therapy for obesity and type 2 diabetes. Front Endocrinol. (2024) 15:1333778. doi: 10.3389/fendo.2024.1333778
54. Li, J, Zhao, F, Wang, Y, Chen, J, Tao, J, Tian, G, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. (2017) 5:14. doi: 10.1186/s40168-016-0222-x
55. Aryana, KJ, and Olson, DW. A 100-year review: yogurt and other cultured dairy products. J Dairy Sci. (2017) 100:9987–10013. doi: 10.3168/jds.2017-12981
56. Feng, J, Cen, Q, Cui, Y, Hu, X, Li, M, Wang, L, et al. Lactobacillus rhamnosus: an emerging probiotic with therapeutic potential for depression. Pharmacol Res. (2025) 211:107541. doi: 10.1016/j.phrs.2024.107541
57. Bernini, LJ, Simão, AN, Alfieri, DF, Lozovoy, MA, Mari, NL, de Souza, CH, et al. Beneficial effects of Bifidobacterium lactis on lipid profile and cytokines in patients with metabolic syndrome: a randomized trial. Effects of probiotics on metabolic syndrome. Nutrition. (2016) 32:716–9. doi: 10.1016/j.nut.2015.11.001
58. Toscano, M, De Grandi, R, Pastorelli, L, Vecchi, M, and Drago, L. A consumer's guide for probiotics: 10 golden rules for a correct use. Dig Liver Dis. (2017) 49:1177–84. doi: 10.1016/j.dld.2017.07.011
59. Huang, Q, Cai, G, Liu, T, and Liu, Z. Relationships among gut microbiota, ischemic stroke and its risk factors: based on research evidence. Int J Gen Med. (2022) 15:2003–23. doi: 10.2147/IJGM.S353276
60. Gebereyowhans, S. Potential strategies to enhance conjugated linoleic acid content of milk and dairy products: a review. Heliyon. (2024) 10:e38844. doi: 10.1016/j.heliyon.2024.e38844
61. Dilzer, A, and Park, Y. Implication of conjugated linoleic acid (CLA) in human health. Crit Rev Food Sci Nutr. (2012) 52:488–513. doi: 10.1080/10408398.2010.501409
62. De Vuyst, L, Moens, F, Selak, M, Rivière, A, and Leroy, F. Summer meeting 2013: growth and physiology of bifidobacteria. J Appl Microbiol. (2014) 116:477–91. doi: 10.1111/jam.12415
63. St-Onge, MP, Farnworth, ER, and Jones, PJ. Consumption of fermented and nonfermented dairy products: effects on cholesterol concentrations and metabolism. Am J Clin Nutr. (2000) 71:674–81. doi: 10.1093/ajcn/71.3.674
64. Larraufie, P, Martin-Gallausiaux, C, Lapaque, N, Dore, J, Gribble, FM, Reimann, F, et al. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci Rep. (2018) 8:74. doi: 10.1038/s41598-017-18259-0
65. Lal, S, Kirkup, AJ, Brunsden, AM, Thompson, DG, and Grundy, D. Vagal afferent responses to fatty acids of different chain length in the rat. Am J Physiol Gastrointest Liver Physiol. (2001) 281:G907–15. doi: 10.1152/ajpgi.2001.281.4.G907
66. Strandwitz, P, Kim, KH, Terekhova, D, Liu, JK, Sharma, A, Levering, J, et al. GABA-modulating bacteria of the human gut microbiota. Nat Microbiol. (2019) 4:396–403. doi: 10.1038/s41564-018-0307-3
67. Nomura, M, Kimoto, H, Someya, Y, Furukawa, S, and Suzuki, I. Production of gamma-aminobutyric acid by cheese starters during cheese ripening. J Dairy Sci. (1998) 81:1486–91. doi: 10.3168/jds.S0022-0302(98)75714-5
68. Park, KB, and Oh, SH. Production of yogurt with enhanced levels of gamma-aminobutyric acid and valuable nutrients using lactic acid bacteria and germinated soybean extract. Bioresour Technol. (2007) 98:1675–9. doi: 10.1016/j.biortech.2006.06.006
69. Pan, A, Sun, Q, Okereke, OI, Rexrode, KM, and Hu, FB. Depression and risk of stroke morbidity and mortality: a meta-analysis and systematic review. JAMA. (2011) 306:1241–9. doi: 10.1001/jama.2011.1282
70. Pérez-Piñar, M, Ayerbe, L, González, E, Mathur, R, Foguet-Boreu, Q, and Ayis, S. Anxiety disorders and risk of stroke: a systematic review and meta-analysis. Eur Psychiatry. (2017) 41:102–8. doi: 10.1016/j.eurpsy.2016.11.004
71. Chuang, CY, Shi, YC, You, HP, Lo, YH, and Pan, TM. Antidepressant effect of GABA-rich monascus-fermented product on forced swimming rat model. J Agric Food Chem. (2011) 59:3027–34. doi: 10.1021/jf104239m
72. Oketch-Rabah, HA, Madden, EF, Roe, AL, and Betz, JM. United States Pharmacopeia (USP) safety review of gamma-aminobutyric acid (GABA). Nutrients. (2021) 13:2742. doi: 10.3390/nu13082742
73. Pei, R, Martin, DA, DiMarco, DM, and Bolling, BW. Evidence for the effects of yogurt on gut health and obesity. Crit Rev Food Sci Nutr. (2017) 57:1569–83. doi: 10.1080/10408398.2014.883356
74. Baraquet, ML, Rivarola, E, and Perovic, NR. Dairy product consumption and type 2 diabetes in an Argentinian population: is there an association? Nutr Hospitalaria. (2024) 41:186–93. doi: 10.20960/nh.04700
75. Ansari, S, Mohammadifard, N, Hajihashemi, P, Haghighatdoost, F, Zarepur, E, Mahmoudi, S, et al. The relationship between fermented and nonfermented dairy products consumption and hypertension among premature coronary artery disease patients: Iran premature coronary artery disease study. Food Sci Nutr. (2024) 12:3322–35. doi: 10.1002/fsn3.3998
76. Saleem, GN, Gu, R, Qu, H, Bahar Khaskheli, G, Rashid Rajput, I, Qasim, M, et al. Therapeutic potential of popular fermented dairy products and its benefits on human health. Front Nutr. (2024) 11:1328620. doi: 10.3389/fnut.2024.1328620
77. Ayala-Bribiesca, E, Turgeon, SL, and Britten, M. Effect of calcium on fatty acid bioaccessibility during in vitro digestion of Cheddar-type cheeses prepared with different milk fat fractions. J Dairy Sci. (2017) 100:2454–70. doi: 10.3168/jds.2016-11902
78. Zhu, HL, Liu, Y, Zhang, J, Wang, MX, Jiang, H, Guo, F, et al. Dietary calcium, magnesium, and phosphorus intakes and risk of stroke in Chinese adults. Sci Rep. (2021) 11:11270. doi: 10.1038/s41598-021-90388-z
79. Tian, DY, Tian, J, Shi, CH, Song, B, Wu, J, Ji, Y, et al. Calcium intake and the risk of stroke: an up-dated meta-analysis of prospective studies. Asia Pac J Clin Nutr. (2015) 24:245–52. doi: 10.6133/apjcn.2015.24.2.22
80. Abbott, RD, Curb, JD, Rodriguez, BL, Sharp, DS, Burchfiel, CM, and Yano, K. Effect of dietary calcium and milk consumption on risk of thromboembolic stroke in older middle-aged men. The Honolulu heart program. Stroke. (1996) 27:813–8. doi: 10.1161/01.str.27.5.813
81. Gibson, R, Aljuraiban, GS, Oude Griep, LM, Vu, TH, Steffen, LM, Appel, LJ, et al. Relationship of calcium and magnesium intakes with the dietary approaches to stop hypertension score and blood pressure: the international study of macro/micronutrients and blood pressure. J Hypertens. (2024) 42:789–800. doi: 10.1097/HJH.0000000000003648
82. Jolma, P, Kööbi, P, Kalliovalkama, J, Kähönen, M, Fan, M, Saha, H, et al. Increased calcium intake reduces plasma cholesterol and improves vasorelaxation in experimental renal failure. Am J Physiol Heart Circ Physiol. (2003) 285:H1882–9. doi: 10.1152/ajpheart.01148.2002
83. Parikh, NS, Merkler, AE, and Iadecola, C. Inflammation, autoimmunity, infection, and stroke: epidemiology and lessons from therapeutic intervention. Stroke. (2020) 51:711–8. doi: 10.1161/STROKEAHA.119.024157
84. Chu, AJ. Role of tissue factor in thrombosis. Coagulation-inflammation-thrombosis circuit. Front Biosci. (2006) 11:256–71. doi: 10.2741/1796
85. Pei, R, DiMarco, DM, Putt, KK, Martin, DA, Chitchumroonchokchai, C, Bruno, RS, et al. Premeal low-fat yogurt consumption reduces postprandial inflammation and markers of endotoxin exposure in healthy premenopausal women in a randomized controlled trial. J Nutr. (2018) 148:910–6. doi: 10.1093/jn/nxy046
Keywords: NHANES, stroke, fermented dairy products, yogurt, adult
Citation: Ma S, Miao Y and Wu X (2025) Fermented dairy products intake and stroke risk: analyses of NHANES 2007–2018 data. Front. Nutr. 12:1593174. doi: 10.3389/fnut.2025.1593174
Edited by:
Rachel Anderson, AgResearch, New ZealandReviewed by:
Ardy Ardiansyah, Bakrie University, IndonesiaYasuaki Wada, Morinaga Milk Industry Co Ltd., Japan
Alexander P. Kanon, University College Cork, Ireland
Copyright © 2025 Ma, Miao and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xian Wu, SFVDTVdYQDE2My5jb20=
 Sijia Ma
Sijia Ma Yongyue Miao
Yongyue Miao Xian Wu2*
Xian Wu2*