- 1Department of Internal Medicine, Diabetes, Endocrinology and Metabolism, Mansoura University, Mansoura, Egypt
- 2Department of Medical Analysis, Medical Laboratory Technique College, The Islamic University of Al Diwaniyah, Al Diwaniyah, Iraq
- 3Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Khalid University, Abha, Saudi Arabia
- 4Department of Medical Laboratory, College of Applied Medical Sciences, Prince Sattam Bin Abdulaziz University, Al-Kharj, Saudi Arabia
Background: Colorectal cancer (CRC) remains one of the leading causes of cancer-related morbidity and mortality worldwide. Emerging evidence suggests that dietary patterns can significantly influence CRC risk, with beverages playing a critical role. The Healthy Beverage Index (HBI) is a tool to assess the healthfulness of beverage consumption, yet its relationship with colorectal cancer risk has not been extensively studied.
Methods: A total of 250 participants diagnosed with colorectal cancer and 250 age- and sex-matched control subjects were recruited for the study. Beverage intake was assessed using a validated dietary questionnaire, and HBI scores were calculated to reflect the quality of beverage consumption. Logistic regression analyses were performed to examine the association between HBI scores and colorectal cancer risk, controlling for potential confounders such as energy intake, physical activity, family history of cancer, and other lifestyle factors were assessed.
Results: The case group had an average age of 48.91 years and BMI of 29.61, while the control group averaged 47.13 years and 29.07 BMI. CRC patients had a higher waist circumference (p < 0.05) and lower vitamin D intake and HBI scores than controls (p < 0.05). Those in the highest HBI quartile consumed more nutrients compared to the lowest (p < 0.05). Higher HBI scores correlated with increased physical activity. The highest HBI quartile significantly reduced CRC odds (OR: 0.28; 95% CI: 0.19–0.51), remaining significant after adjustments (OR: 0.43; 95% CI: 0.25–0.76).
Conclusion: The HBI is inversely associated with the risk of colorectal cancer, suggesting that improvements in beverage choices may serve as an effective dietary strategy for CRC prevention. These results underscore the critical role of beverage consumption in dietary assessments and cancer risk management, warranting further examination in prospective studies.
Introduction
Colorectal cancer (CRC) is a major global health problem, with a high incidence and the second highest rate of cancer-related deaths (1). In 2020, the World Health Organization estimated 1.93 million new CRC cases and 935,000 deaths (2). Rising trends in low-income countries are compounded by aging populations and the increased adoption of Western lifestyles, creating an urgent need for effective prevention and management strategies. Diet, obesity, physical inactivity, smoking, and excessive alcohol intake are modifiable risk factors for CRC. High consumption of red and processed meats, coupled with a low-fiber diet and a sedentary lifestyle, significantly increases the risk (3). Preventative strategies refer to the lifestyle modifications to prevent these risks and hence reduce overall incidence (4). Dietary interventions have great potential for CRC prevention. Increasing fiber intake, reducing red and processed meat consumption, and increasing the intake of fruits and vegetables can significantly reduce risk. However, these dietary shifts, combined with regular physical activity, form the cornerstone of effective CRC prevention strategies (5).
A novel assessment tool for evaluating the quality of beverage intake in relation to chronic disease prevention is the Healthy Beverage Index (HBI). This index measures consumption patterns based on criteria such as sugar content, the presence of beneficial nutrients, and overall caloric contribution (6). Selecting water, unsweetened teas, and nutrient-dense drinks, such as certain juices, can help optimize HBI scores, improve overall dietary quality, and decrease disease risk (7).
A high intake of sugary beverages appears to increase inflammation and insulin resistance, which may raise colorectal cancer risk, according to studies (8). Regular consumption of coffee, which is rich in antioxidants and polyphenols, has been linked to a lower risk, most likely due to its anti-inflammatory properties (9). The antiangiogenic and antiproliferative mechanisms of green tea catechins also protect against colorectal cancer. A link between alcohol and colorectal cancer has been established, particularly with excessive consumption, possibly through the production of acetaldehyde and the impairment of folate metabolism (10).
Despite growing evidence on the impact of beverage consumption on colorectal cancer risk, few studies have comprehensively evaluated this relationship using standardized measures such as the HBI. This case-control study aims to investigate the association between HBI and the risk of colorectal cancer.
Method
Study population
We conducted a comprehensive hospital-based case-control study at King Khalid Hospital. Cases included patients aged 30–79 years with pathologically confirmed colorectal cancer (CRC), diagnosed within 3 months prior to the interview. Patients with a history of other cancers or previous adenomatous polyps were excluded. Additionally, patients diagnosed with adenomatous colorectal polyps via colonoscopy and histological confirmation were included as a separate group.
Control subjects were randomly selected from individuals admitted to the same hospital during the same period. Controls were frequency-matched to cases by age (±10 years) and sex. Inclusion criteria for controls were admission for acute or minor non-neoplastic conditions unrelated to diet-related chronic illnesses (such as fractures, minor infections, or elective surgeries). Controls with any history of cancer, adenomatous polyps, or chronic diseases related to diet (e.g., diabetes, cardiovascular disease, chronic kidney disease) were excluded to minimize confounding.
Out of 516 participants initially recruited (259 controls, 257 CRC cases), 16 individuals (seven controls and nine cases) were excluded due to incomplete food frequency questionnaires or total energy intake values outside ±3 standard deviations from the mean. The final sample included 250 cases and 250 controls.
The research protocol was reviewed and approved by the Ethics Committee of King Khalid University. All participants provided written informed consent prior to their inclusion in the study. This study was approved by the Ethics Committee of King Khalid University (approval code: ECM#2023-11-137).
Dietary intake assessment
The dietary intake of the participants was assessed through the utilization of a validated semi-quantitative food frequency questionnaire, which comprised a total of 152 distinct food items (11). Participants were instructed to report their average consumption of various food items over the course of the previous year by selecting one of several predefined options. These options included: never or less than once a month, three to four times a month, once a week, two to four times a week, five to six times a week, once a day, two to three times a day, four to five times a day, or six times or more each day. Finally, the calculated daily intake was input into Nutritionist IV software to determine total energy and nutrient intake.
Assessment of anthropometric variables and physical activity
Standardized methodologies were employed to systematically gather anthropometric data from the participants involved in the study. The weights of the patients were accurately assessed while they were attired in comfortable clothing and without any footwear, utilizing a Seca digital scale, which is a product manufactured in Germany and is known for its precision, capable of measuring weight with an accuracy of 100 g. In addition, the measurement of standing height was conducted using a tape measure, ensuring that the participants were barefoot during this process. To calculate the body mass index (BMI), the weight of the individual in kilograms was divided by the square of their height measured in meters squared (m2). Furthermore, waist circumference (WC) was measured at its narrowest point using a non-elastic tape, ensuring that no pressure was exerted on the surface of the body during the measurement process.
We employed a reliable abbreviated version of the International Physical Activity Questionnaire (short IPAQ) to assess the physical activity levels of participants. The calculation of physical activity in MET-minutes per week was performed as follows: Walking MET-minutes/week = 3.3 × Walking minutes × Walking days. Moderate MET-minutes/week = 4.0 × Minutes of moderate-intensity activity × Days of moderate activity. Vigorous MET-minutes/week = 8.0 × Minutes of vigorous-intensity activity × Days of vigorous activity. The total physical activity in MET-minutes/week was determined by summing the scores of Walking, Moderate, and Vigorous MET-minutes/week. Additionally, we secured informed written consent from all participants.
Calculation of Healthy Beverage Index (HBI)
The HBI was developed by Duffey and Davy (12), as referenced in their work. This index serves a purpose similar to that of the Healthy Eating Index, as it can be utilized to assess the overall quality of beverage consumption and to detect any alterations in drinking habits over time. It is important to note that health-related changes are often associated with specific consumption patterns. In the framework of the beverage guidance system, all items that were classified as beverages were categorized into eight distinct types. These categories include 100% fruit juice, plain water, unsweetened varieties of coffee and tea, low-fat milk, diet beverages—which encompass caffeine-free coffee and tea as well as other drinks that are artificially sweetened—alcoholic beverages like beer, wine, and spirits, and finally, full-fat milk. Eight distinct categories of beverages were consumed by individuals, which included fruit juices, sweetened coffee and tea, as well as carbonated soft drinks. A higher score indicates greater adherence to drinking standards and healthier drinking practices, with the final HBI score ranging from 0 to 100 (12). Since our study’s target demographic did not consume diet beverages (rated between 0 and 5) or alcoholic drinks (scoring between 0 and 5), the highest possible final HBI score was 90. The focus of this research was to examine compliance with healthy beverage consumption guidelines rather than total fluid intake; therefore, liquids consumed during meals (like soup) were excluded.
Statistical analysis
Statistical analyses were conducted using SPSS software (version 26.0; SPSS Inc., Chicago IL). The Shapiro–Wilk tests evaluated the normal distribution of the variables. Baseline characteristics and dietary intakes for quantitative factors were expressed as mean ± standard deviation (SD), while qualitative variables were reported as counts and percentages. We compared the data between two groups using independent sample T-Tests or, when appropriate, the non-parametric alternative (Mann–Whitney test) for continuous variables, and chi-squared tests for categorical variables. A conditional logistic regression model was employed to calculate odds ratios (ORs) and 95% confidence intervals (CIs), adjusting for various covariates in a separate model. In all analyses, a significance level of p < 0.05 was established.
Results
The average (SD) age and BMI of the case groups were 48.91 (10.46) years and 29.61 (4.55) kg/m2, respectively, whereas the average age and BMI of the control groups were 47.13 (10.08) years and 29.07 (4.39) kg/m2, respectively. Table 1 presents the demographic information, lifestyle factors, and medical history of the study participants in both the case and control groups. Patients diagnosed with colorectal cancer (CRC) exhibited a significantly greater waist circumference (WC) in comparison to the control group (p < 0.05). Furthermore, the average consumption of vitamin D supplements and the HBI score among the case group was found to be considerably lower than that observed in the control group (p-value < 0.05). In contrast, for other characteristics and factors examined in the study, there were no significant differences identified between the patients in the case group and those in the control group.
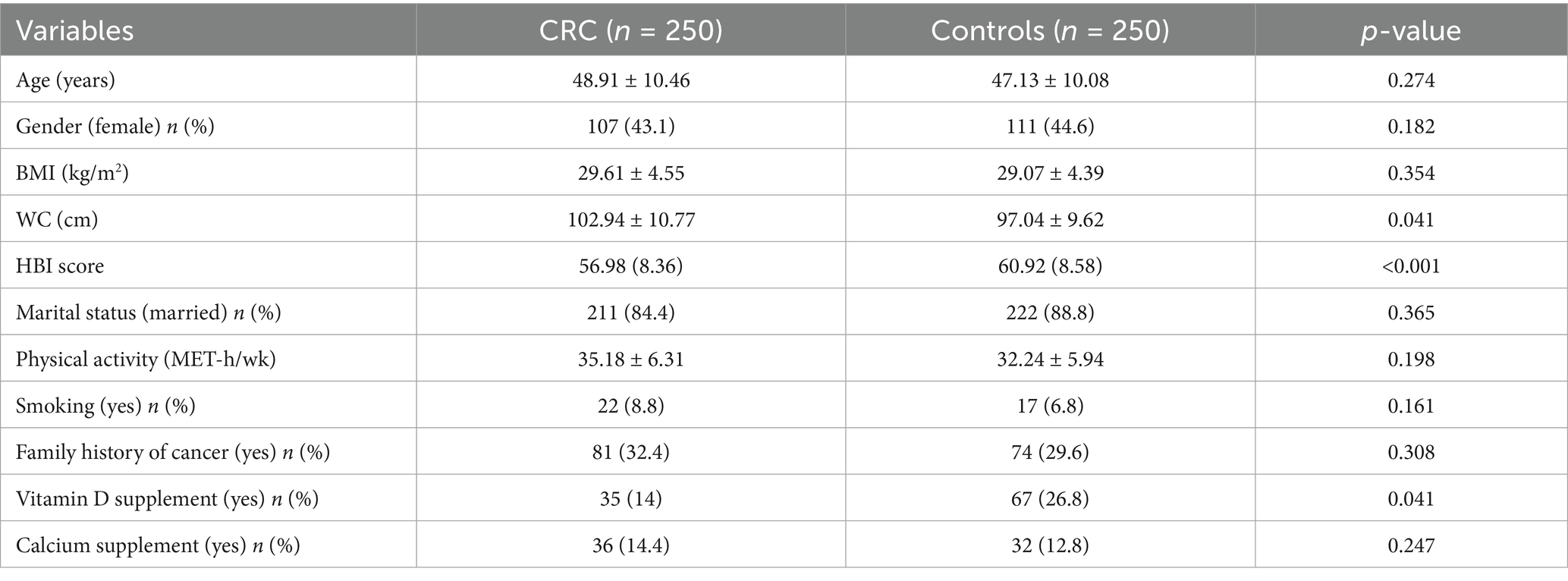
Table 1. The demographic information, lifestyle factors, and medical history of the study participants in both the case and control groups.
Table 2 presents a detailed overview of the mean food consumption patterns observed among the research participants, categorized into case and control groups. When making a comparison with the control group, it is evident that individuals diagnosed with CRC exhibited a higher intake of various macronutrients, including energy, carbohydrates, and fats. Furthermore, these subjects also consumed greater amounts of saturated fatty acids (SFA), cholesterol, additional carbohydrates, sodium, folate, iron, sugar-sweetened beverages, grains, and starches. Conversely, their consumption of protein, potassium, phosphorus, calcium, vitamin B12, and several micronutrients, including antioxidants such as zinc, magnesium, and vitamins E, C, and D, was notably lower. This difference in dietary intake was statistically significant, with a p-value of less than 0.05.
The dietary intakes, anthropometric measurements, and various lifestyle characteristics of the participants involved in the study, categorized according to their HBI quartiles, are presented in detail in Table 3. Notably, individuals who fell within the highest quartile of the HBI exhibited significantly greater consumption levels of calories, carbohydrates, proteins, fats, fibers, salts, potassium, calcium, magnesium, zinc, as well as vitamins C, E, and B9, when compared to those situated in the lowest quartile. This observation was statistically significant, with a p-value of less than 0.05. Furthermore, there was a marked increase in both the HBI score and the level of physical activity among participants as they progressed through the index quartiles.
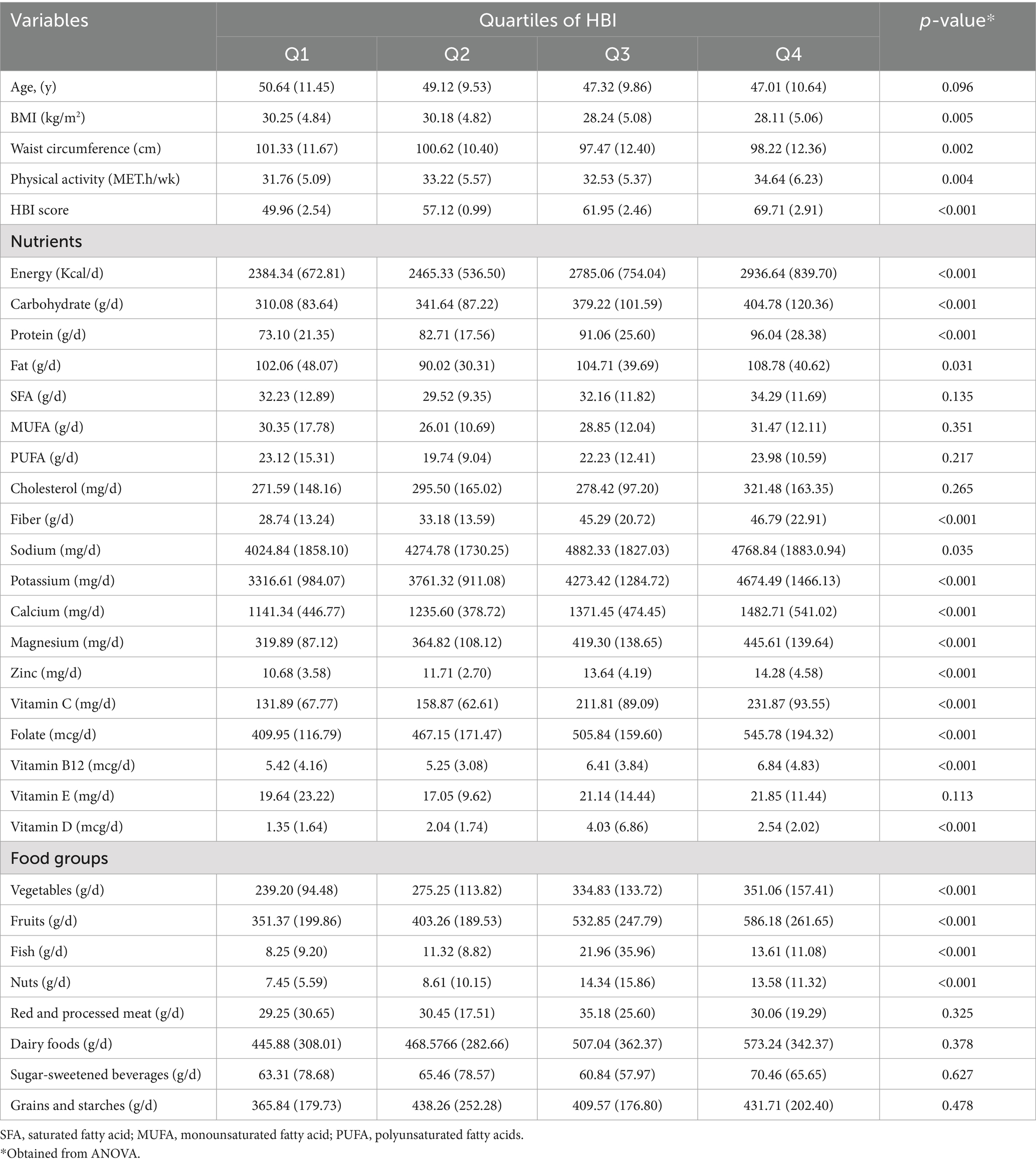
Table 3. Dietary intakes, anthropometric, and lifestyle characteristics of study participants across quartiles of HBI.
Table 4 displays the odds ratios (ORs) and 95% confidence intervals (CIs) for colorectal cancer (CRC) patients categorized by HBI. In the crude model, the highest quartile of HBI scores, when compared to the lowest quartile, showed a reduction in CRC odds for the population (OR: 0.28; 95% CI: 0.19–0.51). Additionally, after further adjustments for potential confounders in the final adjusted model, the decrease in CRC odds remained statistically significant (OR: 0.43; 95% CI: 0.25–0.76 for the population).
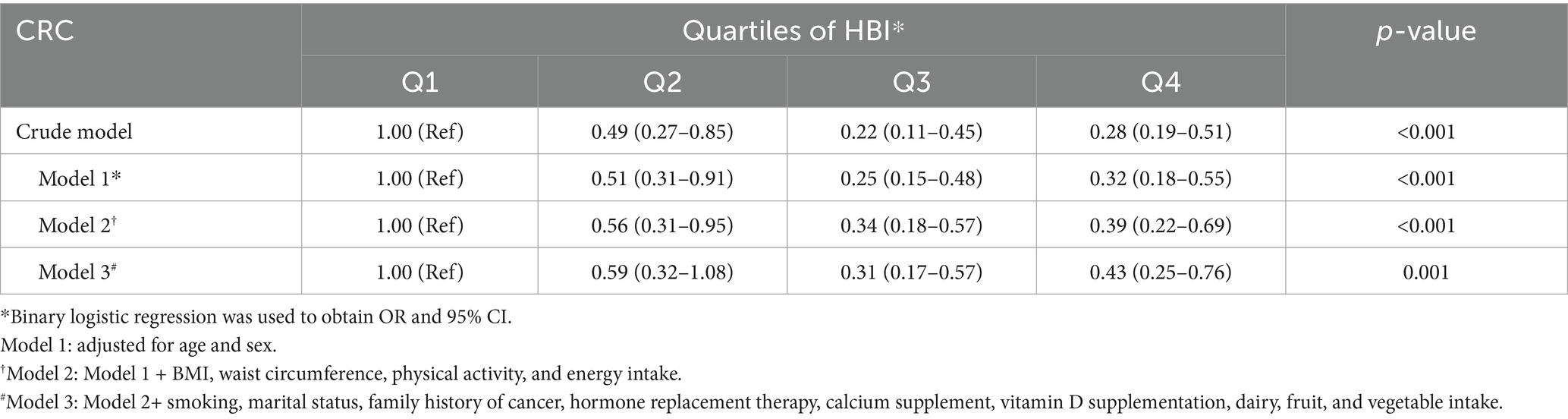
Table 4. The odds ratios (ORs) and 95% confidence intervals (CIs) for colorectal cancer (CRC) patients categorized by HBI.
Discussion
The aim of this hospital-based study was to investigate the relationship between consumption of healthy beverages and risk of colorectal cancer (CRC). The study included patients with CRC and control participants chosen from individuals with similar demographic characteristics. An anthropometric measurement and physical activity level measurement was performed according to standardized methods, and dietary intake was assessed using a food frequency questionnaire. The HBI was used to evaluate the quality of beverage consumption and the possibility that healthier beverage choices may reduce CRC risk. Those with higher adherence to healthy beverage consumption patterns were less likely to develop colorectal cancer than those with less healthy drinking habits, the results showed. The role of diet and lifestyle factors in preventing disease, in particular colorectal cancer, is stressed in this study.
To gain insight into the long-term effects of healthy beverage consumption on colorectal cancer (CRC) risk and whether behavioral interventions can change beverage consumption patterns, researchers leverage both longitudinal studies and randomized controlled trials (RCTs) (13). Evidence for such an effect is obtained from long-term observational studies that demonstrate that consumption of beverages containing green tea, coffee, and some fruit juices is associated with reduced CRC risk because of their anti-inflammatory and antioxidant properties (14). For example, the Nurses’ Health Study and the Health Professionals Follow-up Study, as these have over the decades tracked health behaviors, indicate that there is a slight but real connection between regular coffee consumption and some reduction in CRC risk (15). These studies point to the influence of long-term dietary habits on cancer risk.
Conversely, beverages low in nutrients and high in sugar, such as sugar-sweetened sodas and certain fruit-flavored drinks, have been associated with an increased risk of CRC. High consumption of these beverages can contribute to systemic inflammation, insulin resistance, and obesity, all of which are established risk factors for CRC (8, 16). For instance, a longitudinal study by Hur et al. (16) found that sugar-sweetened beverage intake during adulthood and adolescence was linked to an elevated risk of early-onset CRC in women, potentially due to the disruption of glucose metabolism and promotion of pro-inflammatory pathways. Similarly, Llaha et al. (17) reported that sugary beverages, including sodas and sweetened fruit juices, are associated with increased cancer risk, likely through mechanisms such as increased visceral adiposity and oxidative stress. These findings suggest that replacing nutrient-poor, high-sugar beverages with healthier alternatives, such as water or unsweetened teas, could mitigate CRC risk by reducing metabolic and inflammatory stressors.
The RCTs and behavioral interventions evaluate the effectiveness of particular change strategies for inducing change in beverage consumption patterns. For instance, some success has been achieved in changing long-term consumption habits through interventions that include educational components about the benefits of certain beverages and personalized feedback (18). Follow-ups are often used in these studies to find out if changes in beverage consumption have a lasting impact on health outcomes (e.g., CRC risk). Validation of the protective effects of beverage consumption against CRC and the development of effective public health strategies to promote long-term healthy beverage choices are these research efforts (19).
Dietary and lifestyle factors which influence colorectal cancer (CRC) risk have been extensively researched. Bahrami et al. (20) reported that diets rich in calcium, vitamin C, riboflavin, folate, and fiber are protective against CRC and adenoma, while Fereidooni et al. (21) found that a healthy dietary pattern is associated with decreased CRC risk and a Western dietary pattern increases it. Aleksandrova et al. (22) showed that a lifestyle index composed of five modifiable factors decreases risk of CRC, Reedy et al. (23), as well as Steck et al. (24), linked adherence to dietary guidelines and anti-inflammatory diets to lower CRC risk, Tabung et al. (25), as well as Magalhães et al. (26), confirmed the protective role of fruit and vegetable rich diets and Mediterranean dietary patterns.
On the other hand, Vieira et al. (27) found that red/processed meats and alcohol increase CRC risk, but milk and whole grains are protective. In women, sugar-sweetened beverages in adulthood and adolescence raise the risk of early-onset CRC, Hur et al. (16) found. Huxley et al. (28) outlined the complete picture of dietary and lifestyle risk factors for CRC outcomes. Other studies include Jafari Nasab et al. (29) who found that higher Healthy Eating Index scores are associated with a reduced CRC risk; Hang et al. (30) who showed the combined effect of lifestyle factors and comorbidities on CRC risk; and Tabung et al. (25) who found that proinflammatory diets increase CRC risk, especially in postmenopausal women.
While Duffey and Davy (12) and Jahanbazi et al. (31) found that the HBI was associated with lower cardiometabolic risk, there was no direct association with CRC. Mahmoodi et al. (32) and Rasaei et al. (33) examined the HBI association with sarcopenia and obesity, rather than CRC. HBI’s inverse relationship with non-alcoholic fatty liver disease was investigated by Sadafi et al. (34).
There are many studies that have shown that dietary patterns and some beverages are associated with colorectal cancer risk. Some examples are that of Llaha et al. (17) who found that sugary beverage consumption (such as sodas or fruit juices) is related to an increased risk for some cancers. Vieira et al. (27) also reviewed that high intake of red and processed meats and alcohol increases the risk of colorectal cancer, but milk and whole grains have a protective effect. Like Huang et al. (35) found no significant association between tea consumption and colorectal cancer risk. According to Je et al. (36), high coffee consumption has no significant effects on colorectal cancer incidence, and according to a meta-analysis by Giovannucci (37), coffee consumption might reduce risk for colorectal cancer, but the results are not definitive. An independent study conducted by Akter et al. (38) has shown that a higher or reduced coffee consumption is not associated with increased, nor decreased colorectal cancer risk in the Japanese population. There, research by Schwingshackl et al. (39) showed that a high intake of whole grains, vegetables, fruits, and dairy is associated with a lower risk of colorectal cancer and high intake of red and processed meats with a higher risk.
Several biological mechanisms have been identified as being associated with a reduced risk of colorectal cancer (CRC) through consumption of healthy beverages, such as green tea, coffee, and certain fruit juices. Importantly, these beverages are rich in antioxidants, polyphenols, and other bioactive compounds, which are well known to play important roles in preventing cancer (40). An example is green tea with rich content in catechins, especially epigallocatechin-3 gallate (EGCG). It is shown that EGCG can induce apoptosis (programmed cell death) and arrest cells in the cell cycle, which research indicates inhibits the growth of colorectal cancer cells. In addition, it also has anti-inflammatory properties that may lower the chronic inflammation associated with increased cancer risk (41). By reducing oxidation, caffeine and other antioxidants present in coffee may protect the colon from digestion of carcinogens and the development of oxidative stress as seen in the colonic mucosa (42). Coffee also contains anti-carcinogenic chlorogenic acids that modulate the expression of genes related to tumor necrosis and cell proliferation (43). Besides, some fruit juices such as those from berries and citrus fruits are high in vitamin C, flavonoids, and other phytochemicals. They have been shown to inhibit the formation of cancer cells by means of their antioxidant activity and their ability to modulate detoxification enzyme functions (44). In contrast, high-sugar, low-nutrient beverages may exacerbate CRC risk by promoting chronic inflammation and insulin resistance. For example, excessive sugar intake can lead to elevated blood glucose levels, which may stimulate insulin-like growth factor (IGF-1) signaling pathways, promoting cell proliferation and inhibiting apoptosis in colorectal tissue (8). Additionally, high-sugar beverages contribute to weight gain and visceral fat accumulation, which are linked to increased CRC risk through inflammatory cytokine production and altered gut microbiota (16, 17). The consumption of these healthy beverages provides a potential dietary strategy for the reduction of the risk of colorectal cancer by multiple protective mechanisms: antioxidant activity, anti-inflammatory effects, and stimulation of cell growth and apoptosis, while avoiding high-sugar beverages can prevent adverse metabolic effects (45).
This study concludes that there appears to be an association between good health beverage consumption (as measured by the HBI) and reduced risk of CRC. This suggests that beverage consumption might be modified as a preventive strategy. Reducing risk may also be promoted by encouraging healthier beverage choices, such as water, unsweetened beverages, and low-fat milk, while discouraging the consumption of sugar-sweetened beverages. Nevertheless, this study has idiosyncratic limitations, and additional research is needed to verify these findings and reveal the mechanistic basis for them. These results can inform public health professionals and clinicians on how to provide dietary advice to reduce CRC risk. CRC prevention requires important public education about the importance of healthy beverage choices, as well as the development and implementation of interventional programs to modify beverage consumption patterns.
The hospital-based case-control study increases the risk for selection bias. Assessment of beverage intake using a food frequency questionnaire is subject to recall bias. Limitations to generalizability of the findings exist due to study focus on a Saudi population. Although some were controlled for confounders, others may not have been. Additionally, the inability to fully assess the maximum HBI score was also present because some beverage types were not present in the study population. Limitations to these studies could be overcome in future studies with more precise measures of beverage intake, such as 24-h dietary recalls. To increase generalizability, longitudinal studies need to include larger, more diverse populations. One limitation of our study is that beverage intake was assessed based on consumption over the past year. Given the long latency period of colorectal cancer, this timeframe may not fully capture long-term dietary exposures that contribute to disease development. However, adult dietary patterns are generally stable over time, and recent intake may still play a role in cancer progression or influence risk in the late stages of carcinogenesis. Another limitation of the present study is the modification of the original HBI to better fit the beverage consumption patterns and available data in our population. Although this adaptation increases the contextual relevance, it may reduce the comparability of our findings with studies using the original HBI scoring system, potentially limiting the generalizability of our results across different populations.
Conclusion
The findings suggest that adherence to a healthier beverage consumption pattern, as measured by the HBI, is significantly associated with a reduced risk of colorectal cancer (CRC). These findings suggest that there is the potential to alter beverage choices, for example by increasing water and unsweetened tea intake and nutrient dense beverages intake and reducing sugary and alcoholic beverage intake, as a potent CRC prevention strategy. While we consistently observed protective effects of the HBI, even after adjusting for confounding factors, further research with more diverse populations and more sophisticated assessment tools is needed to confirm these findings and to elucidate the underlying biological mechanisms. These insights are important to be used to develop dietary strategies targeted at reducing CRC risk and healthier lifestyles, and for designing public health policies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of King Khalid University (Approval No. ECM#2023-11-137). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. All procedures performed in studies involving human participants adhered to the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Author contributions
AA: Data curation, Methodology, Writing – original draft, Visualization, Investigation, Conceptualization, Resources, Validation, Software, Project administration, Supervision, Writing – review & editing, Formal analysis. MK: Data curation, Resources, Validation, Methodology, Formal analysis, Investigation, Writing – review & editing, Software, Writing – original draft. IA: Writing – original draft, Investigation, Conceptualization, Visualization, Resources, Software, Formal analysis, Validation, Project administration, Supervision, Writing – review & editing, Data curation, Methodology. AH: Data curation, Writing – review & editing, Methodology, Writing – original draft, Investigation, Software, Validation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors are thankful to the Deanship of Research and Graduate Studies, King Khalid University, Abha, Saudi Arabia, for financially supporting this work through the Large Research Group Project under Grant no. R.G.P.2/409/46.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tazinkeng, NN, Pearlstein, EF, Manda-Mapalo, M, Adekunle, AD, Monteiro, JFG, Sawyer, K, et al. Incidence and risk factors for colorectal cancer in Africa: a systematic review and meta-analysis. BMC Gastroenterol. (2024) 24:303. doi: 10.1186/s12876-024-03385-7
2. Morgan, E, Arnold, M, Gini, A, Lorenzoni, V, Cabasag, C, Laversanne, M, et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. (2023) 72:338–44. doi: 10.1136/gutjnl-2022-327736
3. Saha, B, R, AT, Adhikary, S, Banerjee, A, Radhakrishnan, AK, Duttaroy, AK, et al. Exploring the relationship between diet, lifestyle and gut microbiome in colorectal cancer development: a recent update. Nutr Cancer. (2024) 76:1–26. doi: 10.1080/01635581.2024.2367266
4. Desai, N, Federico, L, and Baker, JF. Lifestyle, hormonal, and metabolic environmental risks for rheumatoid arthritis. Rheum Dis Clin N Am. (2022) 48:799–811. doi: 10.1016/j.rdc.2022.06.003
5. Tammi, R, Kaartinen, NE, Harald, K, Maukonen, M, Tapanainen, H, Smith-Warner, SA, et al. Partial substitution of red meat or processed meat with plant-based foods and the risk of colorectal cancer. Eur J Epidemiol. (2024) 39:419–28. doi: 10.1007/s10654-024-01096-7
6. Parker, MK, Davy, BM, and Hedrick, VE. Preliminary assessment of the healthy beverage index for US children and adolescents: a tool to quantify the overall beverage intake quality of 2-to 19-year olds. J Acad Nutr Diet. (2022) 122:371–383.e6. doi: 10.1016/j.jand.2021.07.007
7. Jacobo Cejudo, MG, Ochoa-Rosales, C, Ahmadizar, F, Kavousi, M, Geleijnse, JM, and Voortman, T. The healthy beverage index is not associated with insulin resistance, prediabetes and type 2 diabetes risk in the Rotterdam study. Eur J Nutr. (2023) 62:3021–31. doi: 10.1007/s00394-023-03209-6
8. Makarem, N, Bandera, EV, Nicholson, JM, and Parekh, N. Consumption of sugars, sugary foods, and sugary beverages in relation to cancer risk: a systematic review of longitudinal studies. Annu Rev Nutr. (2018) 38:17–39. doi: 10.1146/annurev-nutr-082117-051805
9. Rai, SP, Ansari, AH, Singh, D, and Singh, S. Coffee, antioxidants, and brain inflammation. Prog Brain Res. (2024) 289:123–50. doi: 10.1016/bs.pbr.2024.06.005
10. Siegel, RL, Wagle, NS, Cercek, A, Smith, RA, and Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J Clin. (2023) 73:233–54. doi: 10.3322/caac.21772
11. Aljohani, N. (2017). Development and validation of a semi-quantitative food frequency questionnaire to measure macro-micro nutrients intake for Saudi population in the western region of Saudi Arabia. United States: DRUM - Digital Repository at the University of Maryland.
12. Duffey, KJ, and Davy, BM. The healthy beverage index is associated with reduced Cardiometabolic risk in US adults: a preliminary analysis. J Acad Nutr Diet. (2015) 115:1682–9.e2. doi: 10.1016/j.jand.2015.05.005
13. Liu, VN, Zuniga, KB, Paciorek, A, Zhang, L, Chan, JM, Carroll, PR, et al. Barriers and confidence among colorectal and prostate cancer survivors participating in two behavioral intervention studies. Support Care Cancer. (2023) 31:453. doi: 10.1007/s00520-023-07901-5
14. Sonestedt, E, and Lukic, M. Beverages–a scoping review for Nordic nutrition recommendations 2023. Food Nutr Res. (2024) 68:68. doi: 10.29219/fnr.v68.10458
15. Kantor, ED, Zhang, X, Wu, K, Signorello, LB, Chan, AT, Fuchs, CS, et al. Use of glucosamine and chondroitin supplements in relation to risk of colorectal cancer: results from the nurses’ health study and health professionals follow-up study. Int J Cancer. (2016) 139:1949–57. doi: 10.1002/ijc.30250
16. Hur, J, Otegbeye, E, Joh, H-K, Nimptsch, K, Ng, K, Ogino, S, et al. Sugar-sweetened beverage intake in adulthood and adolescence and risk of early-onset colorectal cancer among women. Gut. (2021) 70:2330–6. doi: 10.1136/gutjnl-2020-323450
17. Llaha, F, Gil-Lespinard, M, Unal, P, de Villasante, I, Castañeda, J, and Zamora-Ros, R. Consumption of sweet beverages and cancer risk. A systematic review and meta-analysis of observational studies. Nutrients. (2021) 13:516. doi: 10.3390/nu13020516
18. Eckel-Mahan, K. Treat time matters: untimely sugar consumption implicated in long-term energy imbalance. Acta Physiol. (2023) 239:e14051. doi: 10.1111/apha.14051
19. Brown, AD, Bolton, KA, Clarke, B, Fraser, P, Lowe, J, Kays, J, et al. System dynamics modelling to engage community stakeholders in addressing water and sugar sweetened beverage consumption. Int J Behav Nutr Phys Act. (2022) 19:118. doi: 10.1186/s12966-022-01363-4
20. Bahrami, A, Rafiee, P, Nasab, SJ, Hekmatdoost, A, Sohrab, G, Sadeghi, A, et al. The relationship between the index of nutritional quality and the risk of colorectal cancer and adenoma: a case-control study. Eur J Cancer Prev. (2020) 29:222–8. doi: 10.1097/CEJ.0000000000000550
21. Fereidooni, F, Rashidkhani, B, Kandiah, M, Shariff, ZM, and Safari, A. Dietary patterns and risk of colorectal cancer in Tehran Province: a case-control study. BMC Public Health. (2013) 13:222. doi: 10.1186/1471-2458-13-222
22. Aleksandrova, K, Pischon, T, Jenab, M, Bueno-de-Mesquita, HB, Fedirko, V, Norat, T, et al. Combined impact of healthy lifestyle factors on colorectal cancer: a large European cohort study. BMC Med. (2014) 12:1–15. doi: 10.1186/s12916-014-0168-4
23. Reedy, J, Mitrou, P, Krebs-Smith, S, Wirfält, E, Flood, A, Kipnis, V, et al. Index-based dietary patterns and risk of colorectal cancer: the NIH-AARP diet and health study. Am J Epidemiol. (2008) 168:38–48. doi: 10.1093/aje/kwn097
24. Steck, SE, Guinter, M, Zheng, J, and Thomson, CA. Index-based dietary patterns and colorectal cancer risk: a systematic review. Adv Nutr. (2015) 6:763–73. doi: 10.3945/an.115.009746
25. Tabung, FK, Brown, LS, and Fung, TT. Dietary patterns and colorectal cancer risk: a review of 17 years of evidence (2000–2016). Curr Colorectal Cancer Rep. (2017) 13:440–54. doi: 10.1007/s11888-017-0390-5
26. Magalhães, B, Peleteiro, B, and Lunet, N. Dietary patterns and colorectal cancer: systematic review and meta-analysis. Eur J Cancer Prev. (2012) 21:15–23. doi: 10.1097/CEJ.0b013e3283472241
27. Vieira, A, Abar, L, Chan, D, Vingeliene, S, Polemiti, E, Stevens, C, et al. Foods and beverages and colorectal cancer risk: a systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR continuous update project. Ann Oncol. (2017) 28:1788–802. doi: 10.1093/annonc/mdx171
28. Huxley, RR, Ansary-Moghaddam, A, Clifton, P, Czernichow, S, Parr, CL, and Woodward, M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer. (2009) 125:171–80. doi: 10.1002/ijc.24343
29. Jafari Nasab, S, Bahrami, A, Rafiee, P, Hekmatdoust, A, Ghanavati, M, Rashidkhani, B, et al. Healthy eating Index-2010 and Mediterranean-style dietary pattern score and the risk of colorectal cancer and adenoma: a case-control study. Nutr Cancer. (2020) 72:1326–35. doi: 10.1080/01635581.2019.1683212
30. Hang, J, Cai, B, Xue, P, Wang, L, Hu, H, Zhou, Y, et al. The joint effects of lifestyle factors and comorbidities on the risk of colorectal cancer: a large Chinese retrospective case-control study. PLoS One. (2015) 10:e0143696. doi: 10.1371/journal.pone.0143696
31. Jahanbazi, L, Farhangi, MA, Tousi, AZ, and Nikrad, N. The association between healthy beverage index (HBI) with metabolic risk factors among apparently metabolically healthy overweight and obese individuals. Clin Nutr Res. (2023) 12:218–28. doi: 10.7762/cnr.2023.12.3.218
32. Mahmoodi, M, Shateri, Z, Nouri, M, Vali, M, Nasimi, N, Sohrabi, Z, et al. The association between healthy beverage index and sarcopenia in Iranian older adults: a case-control study. BMC Geriatr. (2024) 24:244. doi: 10.1186/s12877-024-04790-z
33. Rasaei, N, Ghaffarian-Ensaf, R, Gholami, F, Shiraseb, F, Khadem, A, Fatemi, SF, et al. The association between healthy beverage index and sarcopenic obesity among women with overweight and obesity: a cross-sectional study. BMC Endocr Disord. (2023) 23:25. doi: 10.1186/s12902-023-01274-w
34. Sadafi, S, Azizi, A, Rezaeian, S, and Pasdar, Y. Association between healthy beverage index and nonalcoholic fatty liver disease in the Ravansar noncommunicable disease cohort study. Sci Rep. (2024) 14:3622. doi: 10.1038/s41598-024-54288-2
35. Huang, Y, Chen, Q, Liu, Y, Tian, R, Yin, X, Hao, Y, et al. Association between tea consumption and colorectal cancer: a systematic review and meta-analysis of a population-based study. BMC Gastroenterol. (2023) 23:294. doi: 10.1186/s12876-023-02928-8
36. Je, Y, Liu, W, and Giovannucci, E. Coffee consumption and risk of colorectal cancer: a systematic review and meta-analysis of prospective cohort studies. Int J Cancer. (2009) 124:1662–8. doi: 10.1002/ijc.24124
37. Giovannucci, E. Meta-analysis of coffee consumption and risk of colorectal cancer. Am J Epidemiol. (1998) 147:1043–52. doi: 10.1093/oxfordjournals.aje.a009398
38. Akter, S, Kashino, I, Mizoue, T, Matsuo, K, Ito, H, Wakai, K, et al. Coffee drinking and colorectal cancer risk: an evaluation based on a systematic review and meta-analysis among the Japanese population. Jpn J Clin Oncol. (2016) 46:781–7. doi: 10.1093/jjco/hyw059
39. Schwingshackl, L, Schwedhelm, C, Hoffmann, G, Knüppel, S, Laure Preterre, A, Iqbal, K, et al. Food groups and risk of colorectal cancer. Int J Cancer. (2018) 142:1748–58. doi: 10.1002/ijc.31198
40. Na, H, Lee, J, Cho, S, Shin, W-K, Choi, J-Y, Kang, D, et al. Consumption of coffee and green tea and the risk of colorectal cancer in Korea: the health examinees study. J Cancer Prev. (2022) 27:229–38. doi: 10.15430/JCP.2022.27.4.229
41. Kouzu, K, Tsujimoto, H, Kishi, Y, Ueno, H, and Shinomiya, N. Role of microbial infection-induced inflammation in the development of gastrointestinal cancers. Medicine. (2021) 8:45. doi: 10.3390/medicines8080045
42. Soares, PV, Kannen, V, Jordao Junior, AA, and Garcia, SB. Coffee, but neither decaffeinated coffee nor caffeine, elicits chemoprotection against a direct carcinogen in the colon of Wistar rats. Nutr Cancer. (2019) 71:615–23. doi: 10.1080/01635581.2018.1506489
43. Machado, F, Coimbra, MA, Castillo, MD, and Coreta-Gomes, F. Mechanisms of action of coffee bioactive compounds–a key to unveil the coffee paradox. Crit Rev Food Sci Nutr. (2024) 64:10164–86. doi: 10.1080/10408398.2023.2221734
44. Drewnowski, A, and Burton-Freeman, B. A new category-specific nutrient rich food (NRF9f.3) score adds flavonoids to assess nutrient density of fruit. Food Funct. (2020) 11:123–30. doi: 10.1039/C9FO02344E
Keywords: Healthy Beverage Index, HBI, colorectal cancer, dietary, case-control
Citation: Abdelgawwad El-Sehrawy AAM, Kadem M, Ahmad I and Hjazi A (2025) Associations of the Healthy Beverage Index and the risk of colorectal cancer: a case-control study. Front. Nutr. 12:1595246. doi: 10.3389/fnut.2025.1595246
Edited by:
Sylwia Dziegielewska-Gesiak, Medical University of Silesia, PolandReviewed by:
Daniela Caetano Gonçalves, Federal University of São Paulo, BrazilMohammad Ali Mohsenpour, Shiraz University of Medical Sciences, Iran
Copyright © 2025 Abdelgawwad El-Sehrawy, Kadem, Ahmad and Hjazi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Irfan Ahmad, SXJmYW4uYWhtYWQucmVzZWFyY2hAZ21haWwuY29t
 Amr Ali Mohamed Abdelgawwad El-Sehrawy
Amr Ali Mohamed Abdelgawwad El-Sehrawy Mundher Kadem2
Mundher Kadem2 Irfan Ahmad
Irfan Ahmad