- 1Clinical Nutrition Division, The Second Affiliated Hospital of Soochow University, Suzhou, China
- 2School of Public Health, Suzhou Medical College of Soochow University, Suzhou, China
Background: We aimed to investigate the associations of 25-hydroxyvitamin D (25OHD) with lipid, inflammatory, and injury biomarkers, and their potential mediating roles in vitamin D-related mortality.
Methods: We analyzed data from 4,144 participants in the 2015–2018 National Health and Nutrition Examination Survey (NHANES). Glycemic status was classified using fasting plasma glucose and hemoglobin A1c levels. Key biomarkers were assessed, including visceral adiposity index (VAI) and systemic inflammation response index (SIRI). Multivariate linear regression and restricted cubic spline models examined associations.
Results: In females, 25OHD was positively associated with HDL-cholesterol across all glycemic subgroups, and with triglycerides and total cholesterol in the normal glycemic subgroup (all p < 0.05). In males, inverse associations of 25OHD with triglycerides and VAI were most pronounced in the prediabetes group (p < 0.05). 25OHD was inversely associated with SIRI in normoglycemic individuals and prediabetic males. Mediation analyses revealed that SIRI partially mediated the inverse association between 25OHD and mortality only in prediabetic males, while lipid and injury biomarkers showed no significant mediation effects in any subgroup.
Conclusion: Gender and glycemic status influence the associations between vitamin D and biomarkers, with inflammation potentially mediating the relationship between vitamin D and mortality in prediabetic males.
1 Introduction
As a classic fat-soluble secosteroid hormone, vitamin D plays a crucial role in calcium homeostasis and bone metabolism. Nevertheless, growing evidence over the past decades indicates that vitamin D’s influence extends far beyond skeletal health, encompassing a wide array of physiological processes, including glucose-lipid metabolism, inflammation response, and immune function (1, 2). Observational studies and meta-analyses have linked vitamin D deficiency to an increased risk of various non-communicable chronic diseases, including cardiovascular disease (3), type 2 diabetes (4), certain cancers (5), and even all-cause mortality (6). Consequently, maintaining optimal vitamin D levels has been proposed as a potential strategy for improving overall health and preventing chronic diseases (7).
Despite growing recognition of its multiple effects, the precise mechanisms by which vitamin D influences metabolic health remain incompletely understood. In particular, the extent to which vitamin D modulates distinct biological processes such as lipid metabolism, systemic inflammation, and cellular injury, as well as whether these factors mediate its effects on long-term health and mortality, remains an area of active investigation. A substantial body of evidence indicates that the effects of vitamin D on metabolic health are significantly modulated by both gender and glycemic status (8, 9). Therefore, a central, a priori hypothesis of our study was that these factors would act as critical effect modifiers, making a stratified analysis a necessary, hypothesis-driven component of our research design. A nuanced understanding of these specific interactions is particularly important, as the progression from normoglycemia to diabetes is tightly linked to disturbances in adiposity and systemic inflammation—two core components of overall metabolic health.
To better characterize these complex relationships, researchers are increasingly moving beyond single-marker analysis to gain a more holistic view of biological processes. This trend is evident across diverse fields, employing strategies that range from reference-free transcriptomic analyses in oncology (10) to the use of composite indices in metabolic research, such as the visceral adiposity index (VAI). The VAI, which integrates waist circumference (WC), body mass index (BMI), triglycerides (TG), and high-density lipoprotein cholesterol (HDL-C), provides a robust measure of metabolic dysfunction (11). Similarly, the systemic inflammation response index (SIRI) and systemic immune-inflammation index (SII), derived from complete blood counts, offer valuable insights into an individual’s systemic inflammatory burden (12). However, the extent to which vitamin D is associated with these indices—and whether these associations vary by gender and glycemic status—remains largely unexplored.
Therefore, in this study, we aimed to examine the associations between vitamin D status and lipid, inflammation, and cellular injury biomarkers, including VAI, SII, and SIRI. Additionally, we sought to determine whether these factors mediate the relationship between vitamin D and mortality risk, with a particular focus on gender- and glycemia-specific differences.
2 Materials and methods
2.1 Study design and participant selection
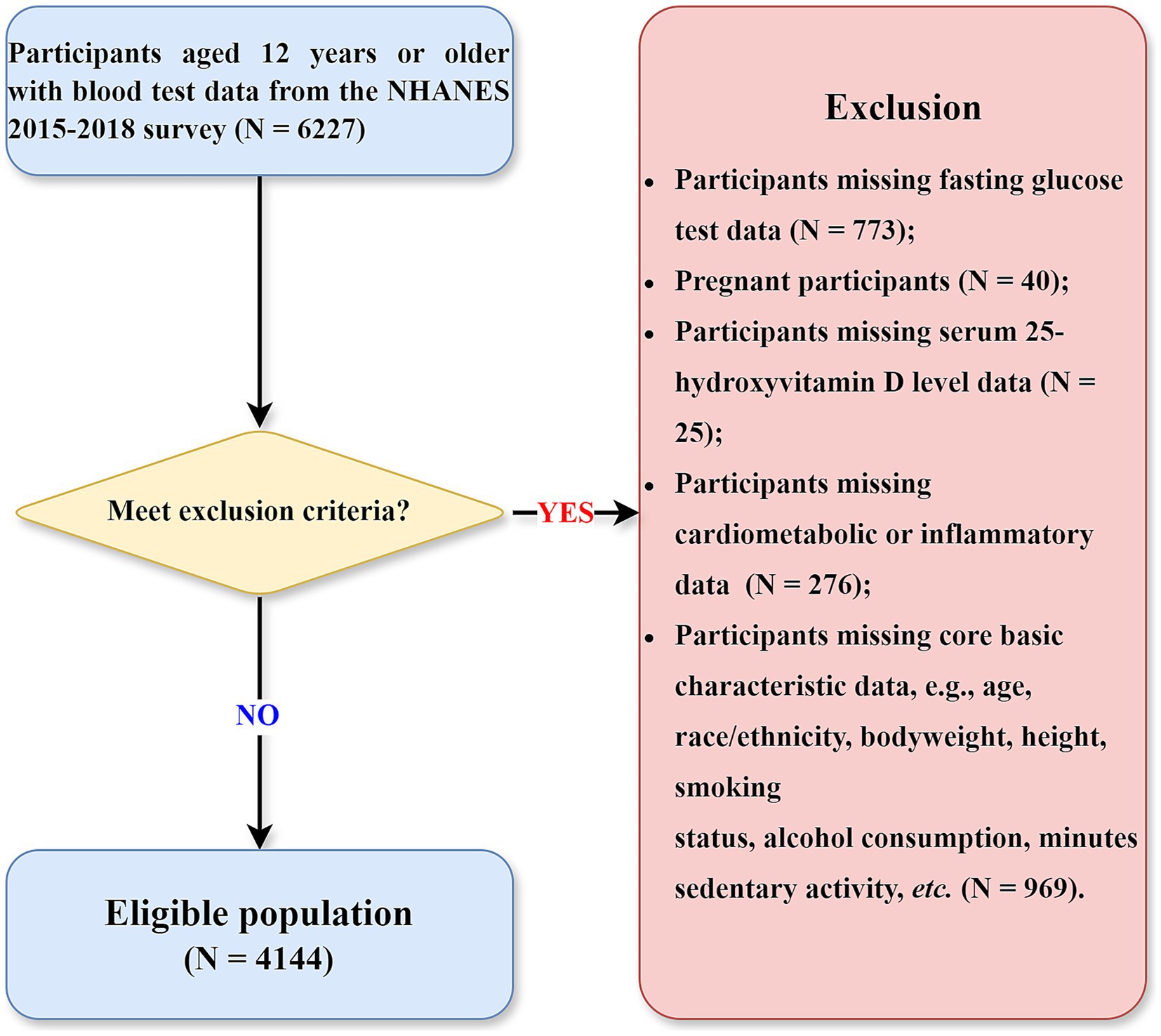
This study utilized baseline data from the National Health and Nutrition Examination Survey (NHANES) 2015–2018 cycles, with mortality outcomes ascertained prospectively through linkage to National Death Index (NDI) mortality files. This design allows for a temporal relationship to be established between baseline exposures and subsequent mortality. NHANES is a nationally representative program of studies designed to assess the health and nutritional status of adults and children in the United States, employing a complex, multistage probability sampling design (13). Data collection encompasses detailed questionnaires, standardized physical examinations, and extensive laboratory analyses. The flow chart of this study is presented in Figure 1. Initially, 6,227 participants aged over 12 years who provided blood samples were considered. Participants were excluded according to the following exclusion criteria: (1) absence of valid fasting glucose measurements (number = 773); (2) pregnancy status (number = 40); (3) missing serum 25-hydroxyvitamin D (25OHD) measurements (number = 25); (4) incomplete cardiometabolic or inflammatory biomarker data (number = 276); and (5) missing critical demographic or lifestyle information (age, race/ethnicity, anthropometric measurements, smoking status, alcohol consumption, or sedentary behavior; number = 969). Although the initial inclusion criterion allowed participants aged ≥ 12 years with available laboratory data, all individuals under 18 years of age were ultimately excluded due to missing data on key variables required for this analysis—such as fasting glucose, lipid and inflammatory markers, lifestyle factors, and mortality follow-up. As a result, the final analytic sample consisted of 4,144 adults aged 18 years and older.
2.2 Anthropometric and laboratory assessments
Body weight and height were measured by trained health technicians using standardized protocols. BMI was calculated as weight in kilograms divided by height in meters squared. WC was measured at the level just above the iliac crest using a flexible tape, with participants standing and breathing normally. Blood samples were collected following a standardized protocol and analyzed at laboratories certified by the Centers for Disease Control and Prevention. All assays were performed using validated enzymatic or hematological methods under rigorous quality control, as detailed in the NHANES Laboratory Procedures Manuals. Serum triglycerides and HDL-C were measured enzymatically using the Roche Modular P chemistry analyzer. The same platform was also used to measure injury markers including creatine phosphokinase (CPK), lactate dehydrogenase (LDH), alanine aminotransferase (ALT), aspartate aminotransferase (AST). Complete blood counts, including neutrophils, lymphocytes, monocytes, and platelets, were assessed using the Beckman Coulter DxH 800 hematology analyzer. All assays followed rigorous quality control and quality assurance procedures as outlined in the NHANES Laboratory Procedures Manuals. Full details of these examination and laboratory procedures are publicly available at https://www.cdc.gov/nchs/nhanes/ (Accessed on 29 August 2025).
2.3 Outcome assessment
Mortality status was determined by linkage of NHANES participants to the NDI through probabilistic matching using a combination of personal identifiers (e.g., Social Security number, name, date of birth). The publicly available Linked Mortality File (LMF) used in this study, which provides data through December 31, 2019, can be accessed at http://www.cdc.gov/nchs/linked-data/mortality-files/index.html (Accessed on 29 August 2025). From this file, we identified all-cause mortality as the primary outcome. The follow-up time was calculated from the date of the NHANES examination to the date of death or the end of the follow-up period (December 31, 2019), whichever came first.
2.4 Definition of diabetes status
Diabetes status was determined according to the American Diabetes Association criteria (14): diabetes was defined as a fasting plasma glucose (FPG) level ≥ 7.0 mmol/L or hemoglobin A1C (HbA1c) ≥ 6.5%; prediabetes was defined as 5.6 mmol/L ≤ FPG < 7.0 mmol/L or 5.7% ≤ HbA1c < 6.5%; and normoglycemic status was defined as FPG < 5.6 mmol/L or HbA1c < 5.7%.
2.5 Calculation of Indices
The following composite indices were calculated based on previously established formulas. SII, SIRI, and VAI are calculated as follows: SII = (platelet count × neutrophil count)/lymphocyte count (15); SIRI = (neutrophil count × monocyte count)/lymphocyte count (16); VAI (male) = (WC/[39.68 + 1.88 × BMI]) × (TG/1.03) × (1.31/HDL-C); VAI (female) = (WC/[36.58 + 1.89 × BMI]) × (TG/0.81) × (1.52/HDL-C) (17).
2.6 Statistical analysis
Baseline participant characteristics were summarized using descriptive statistics. Data are presented as numbers (percentages) for categorical variables or medians (interquartile ranges) for continuous variables. Categorical variables were compared using the chi-square (χ2) test, while continuous variables were compared using the Kruskal–Wallis H test.
2.6.1 Multivariate linear regression
Associations between serum 25OHD levels and lipid, inflammatory, and cellular injury biomarkers were evaluated using multivariate linear regression models. The general form of the model is:
where Y denotes the dependent biomarker, X25OHD is the serum 25OHD level, Xi (i = 2, …, p) are adjustment covariates, βᵢ are the corresponding regression coefficients, p is the total number of predictors in the model, and ϵ is the random error term. Model 1 was adjusted for age, race/ethnicity, and BMI, and model 2 additionally adjusted for alcohol consumption, smoking status, and sedentary activity.
2.6.2 Restricted cubic splines (RCS)
To assess the potential nonlinear association between serum 25OHD and mortality, we fitted RCS within a Cox proportional hazards model:
where s(X) denotes the spline function of serum 25OHD, Zj is the j-th covariate, γj its coefficient, and q is the number of covariates. This corresponds to the proportional hazards form h(t∣X, Z) = h0 (t) exp.{s(X) + ∑γj Zj}, where h0 (t) is the baseline hazard. We used three knots placed at the 10th, 50th, and 90th percentiles of the 25OHD distribution. Nonlinearity was tested using likelihood ratio tests, and the proportional hazards assumption was verified using Schoenfeld residuals.
2.6.3 Mediation analyses
To investigate whether biomarkers mediated the effect of 25OHD (X) on mortality (Y), we applied a counterfactual-based mediation framework. The mediator (M) model was:
and the outcome model for time-to-event mortality was:
where T is the survival time, Wⱼ denotes the j-th covariate (indexed from 1 to r, the total number of covariates); a quantifies the effect of X on M; b reflects the effect of M on the log-survival time given X; and c’ represents the direct effect of X on the log-survival time given M. The term σεY represents the error, where σ is the scale parameter of the survival distribution. Based on these models, which were fitted using a Weibull distribution in the survreg function, the average causal mediation effect (ACME) and average direct effect (ADE) were estimated using the mediation package in R with quasi-Bayesian simulations (1,000 iterations). The analysis allowed for an exposure-mediator interaction.
All statistical analyses were performed using R software (version 4.3.3). A two-sided p-value < 0.05 was considered statistically significant.
3 Results
3.1 Baseline characteristics of participants
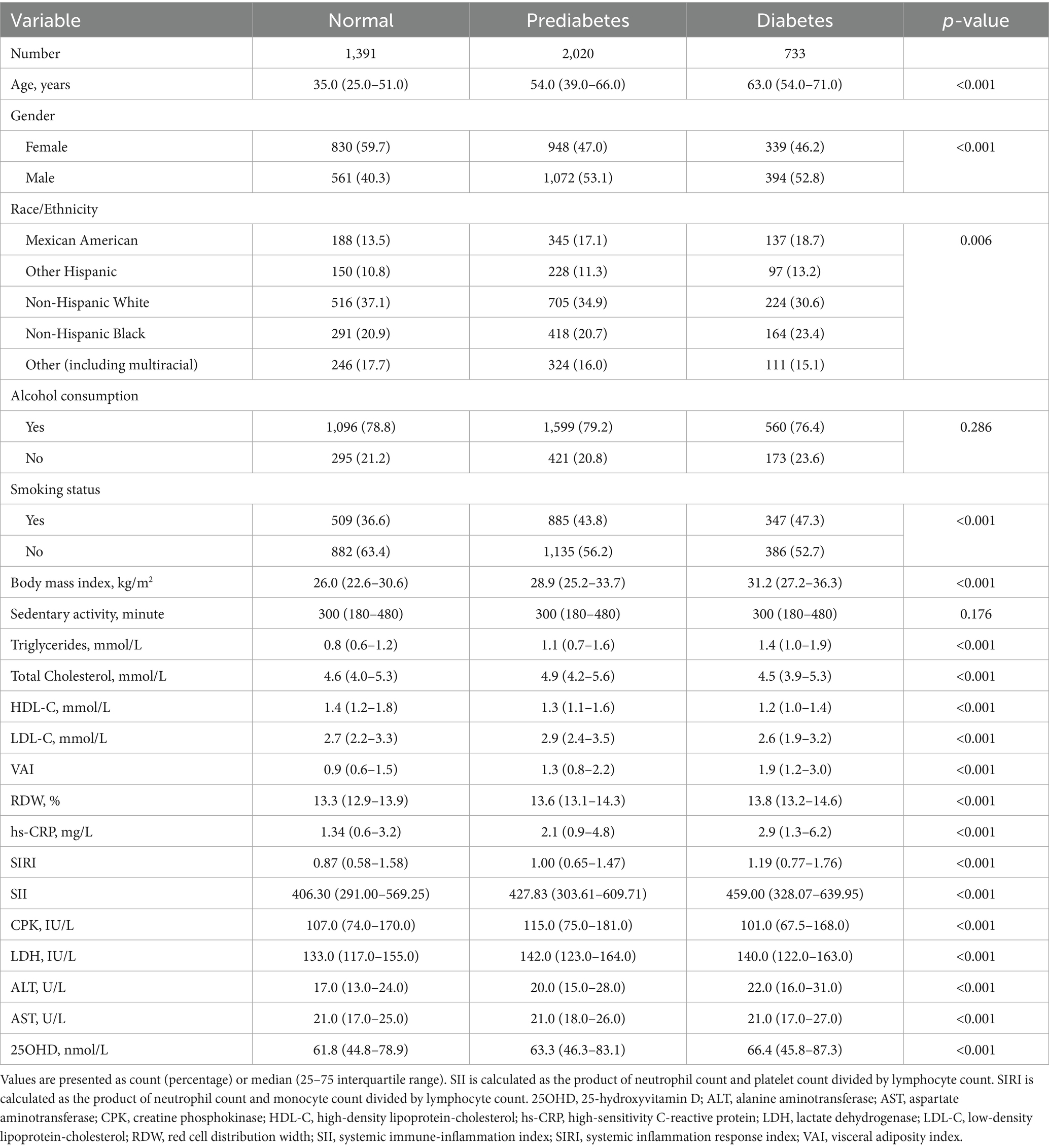
As shown in Table 1, the study population comprised 1,391 normoglycemic, 2020 prediabetic, and 733 diabetic individuals. The median age increased significantly from 35.0 years in the normoglycemic group to 63.0 years in the diabetic group (p < 0.001). The proportion of females was significantly higher in the normoglycemic group (59.7%) compared with the diabetic group (46.2%; p < 0.001). Racial/ethnic composition also differed significantly, with a higher prevalence of non-Hispanic Whites among normoglycemic and prediabetic participants. Additionally, smoking prevalence was greater among diabetic participants (47.3%) compared with normoglycemic individuals (36.6%; p < 0.001), and BMI increased progressively across groups. Triglyceride was highest, while HDL-C was lowest in the diabetic group. Other laboratory indices—including SIRI, LDH, and ALT—were significantly elevated, whereas serum 25(OH)D levels were highest in the diabetic group.
3.2 Associations of 25OHD with lipid, inflammation, and injury biomarkers
The associations between vitamin D and various lipid parameters were modified by both diabetes status and gender (Table 2). In normoglycemic females, 25OHD was positively associated with triglycerides, total cholesterol, and HDL-C (all p < 0.05). In prediabetes, 25OHD was positively associated with HDL-C in both genders, while inverse associations with triglycerides and VAI were evident only in males. Among diabetic participants, a consistent positive association between 25OHD and HDL-C was observed in females, with no significant associations in other lipid measures for either gender.
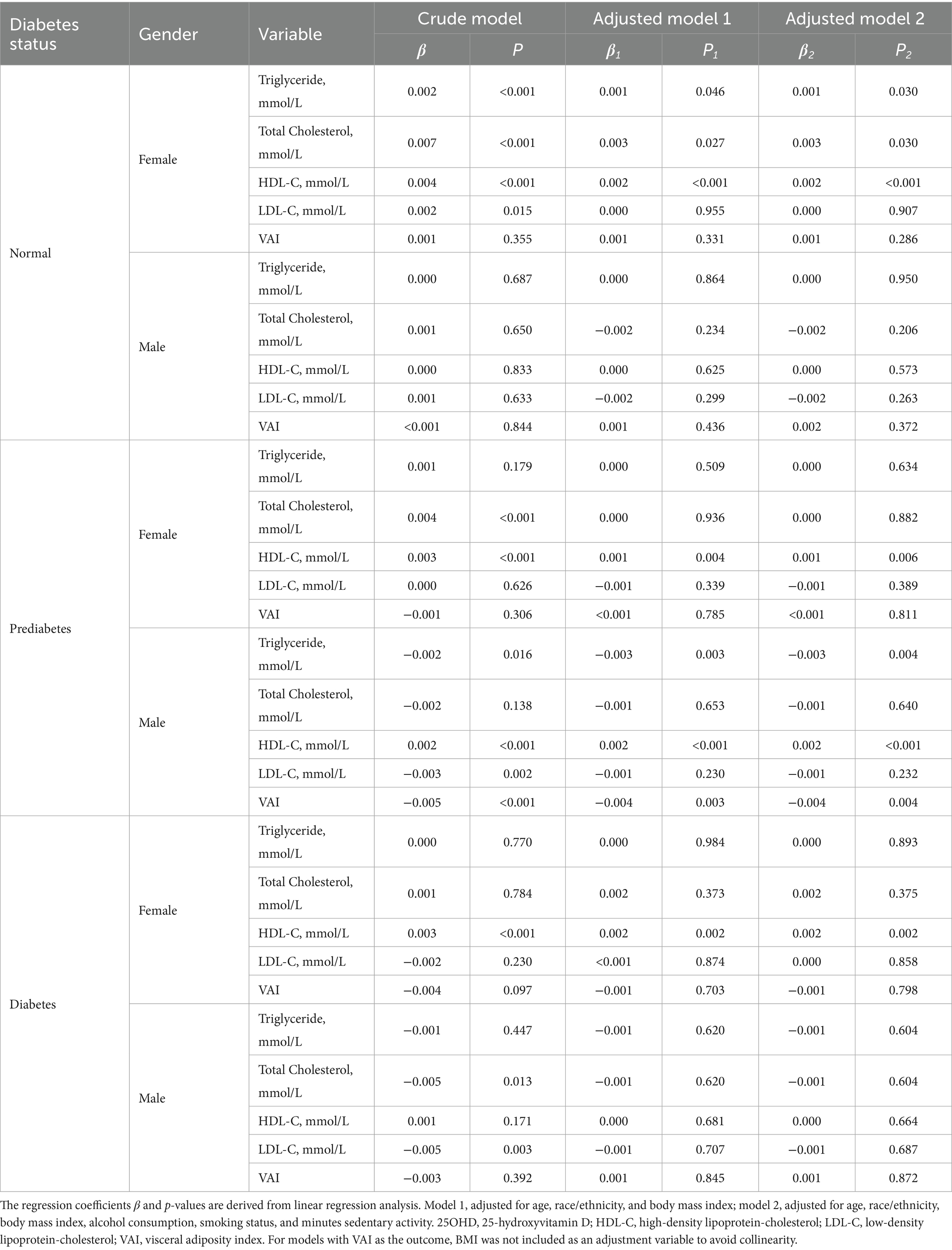
Table 2. The association of serum 25OHD levels with lipid biomarkers in NHANES 2015–2018 participants.
As presented in Table 3, in normoglycemic individuals, 25OHD was inversely associated with SIRI in females, and with both SII and SIRI in males after adjustment (all p < 0.05), while no significant association was found with high-sensitivity C-reactive protein (hs-CRP) or red cell distribution width (RDW) in certain subgroups. Similarly, in males, the serum 25OHD level was significantly and inversely associated with SII and SIRI after adjustment. In prediabetic males, the inverse association with SIRI remained significant (p = 0.032), whereas such associations were not observed in females. In the diabetic group, initial positive associations in males were attenuated after adjustment.
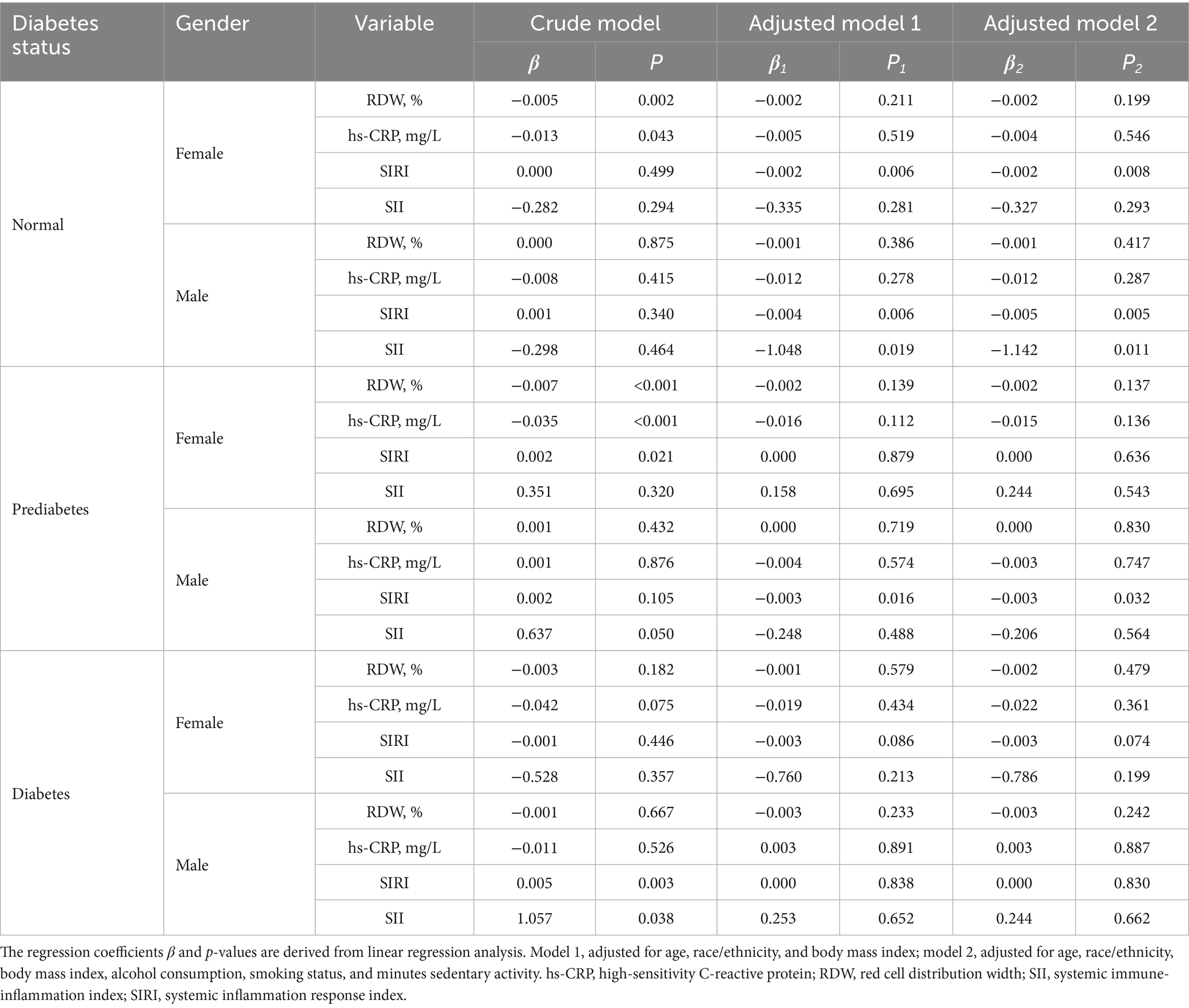
Table 3. The association of serum 25OHD levels with inflammation biomarkers in NHANES 2015–2018 participants.
The association between 25OHD and cellular injury is demonstrated in Table 4. Normoglycemic females exhibited a positive association between 25OHD and AST (p = 0.005). Normoglycemic males showed an inverse association between 25OHD and ALT in the adjusted model 1 (p = 0.042), however, this association lost significance upon further adjustment in the adjusted model 2 (p = 0.052). In prediabetic males and diabetic individuals, 25OHD was inversely associated with CPK in crude analyses, but these associations were not maintained after adjustment.
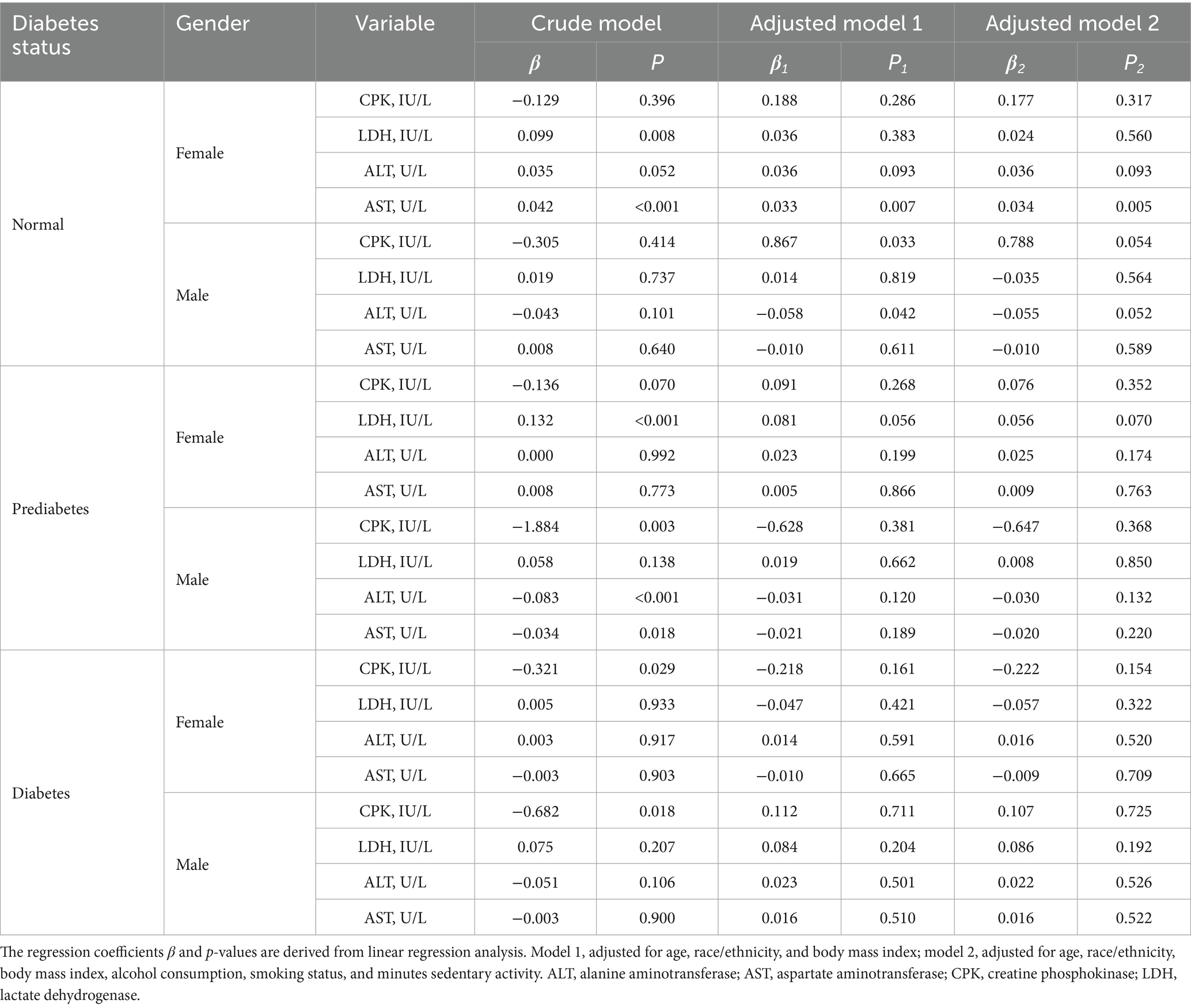
Table 4. The association of serum 25OHD levels with cellular injury biomarkers in NHANES 2015–2018 participants.
3.3 Vitamin D and total mortality
Figure 2 presents the non-linear associations between serum 25OHD levels and total mortality across different diabetes statuses and genders. Among normoglycemic, prediabetic, and female diabetic participants, mortality risk declined with increasing serum 25OHD levels up to a certain threshold, after which the association plateaued. Notably, a statistically significant inverse association was observed only in male prediabetic participants. Although an inverted U-shaped relationship was observed in male diabetic participants, this association did not reach statistical significance.
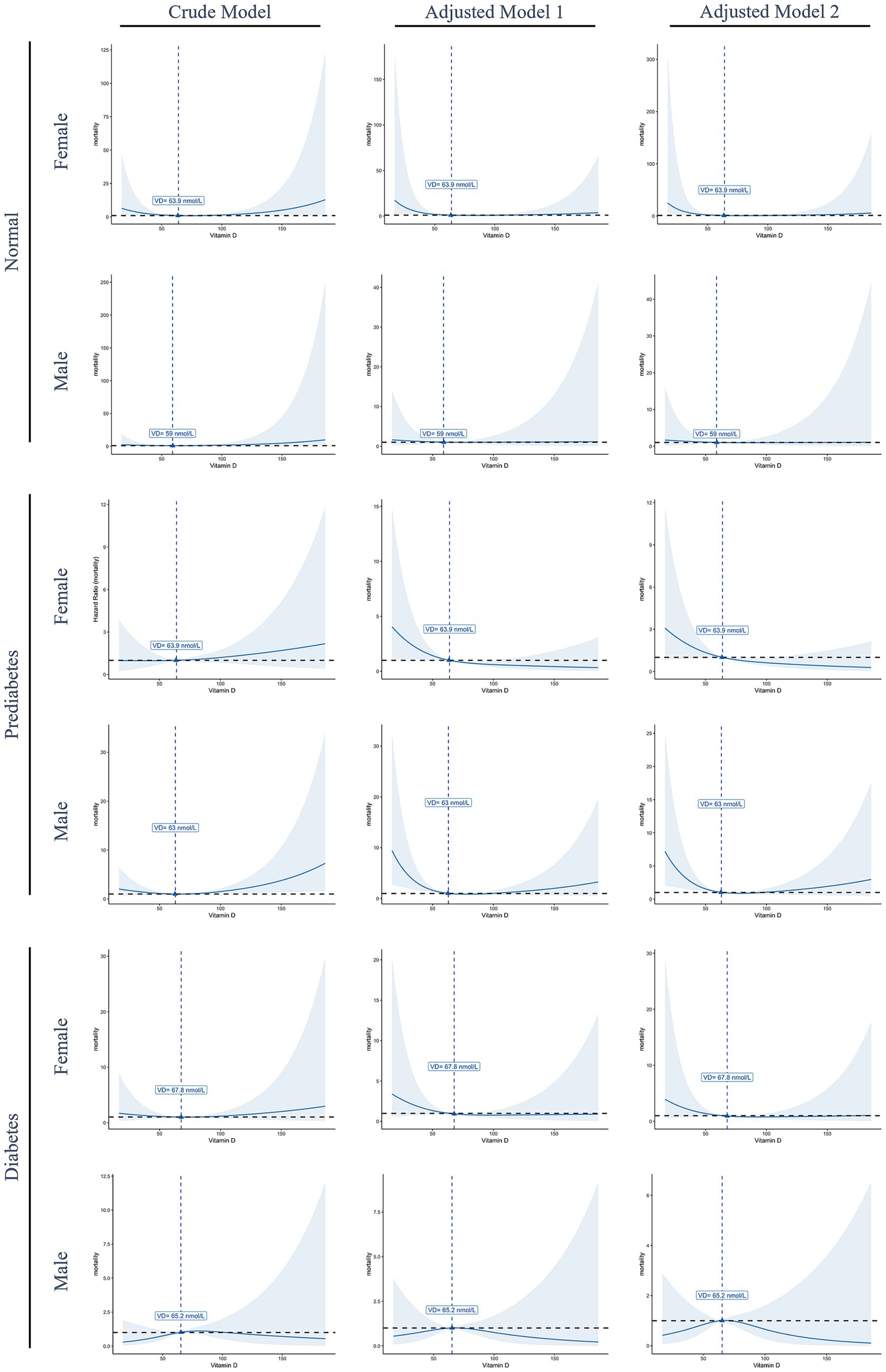
Figure 2. Restricted cubic spline curves illustrate the non-linear association between serum 25OHD levels and total mortality, stratified by gender and diabetes status. Solid blue lines represent the estimated hazard ratios (HRs), and shaded areas indicate 95% confidence intervals. The dashed horizontal black line denotes the reference value (HR = 1), while dashed vertical blue lines indicate the estimated threshold points where the slope of the association changes. Model 1, Adjusted for age, race/ethnicity, and body mass index; model 2, adjusted for age, race/ethnicity, body mass index, alcohol consumption, smoking status, and minutes sedentary activity.
3.4 Mediation analyses of lipids, inflammation, and injury
The results of mediation analyses are summarized in Tables 5–7. In normoglycemic and diabetic participants, lipid biomarkers did not significantly mediate the relationship between 25OHD and mortality in either gender. Among prediabetics, significant ADEs for total cholesterol, HDL-C, and low-density lipoprotein-cholesterol (LDL-C) were observed in females. For inflammatory markers, only prediabetic males exhibited a significant ACME for SIRI (p = 0.042), with no significant mediation observed in other groups. Similarly, mediation analyses for cellular injury markers revealed no significant mediation effects in normoglycemic or diabetic participants, with only prediabetic females showing significant ADEs in LDH, ALT, and AST.
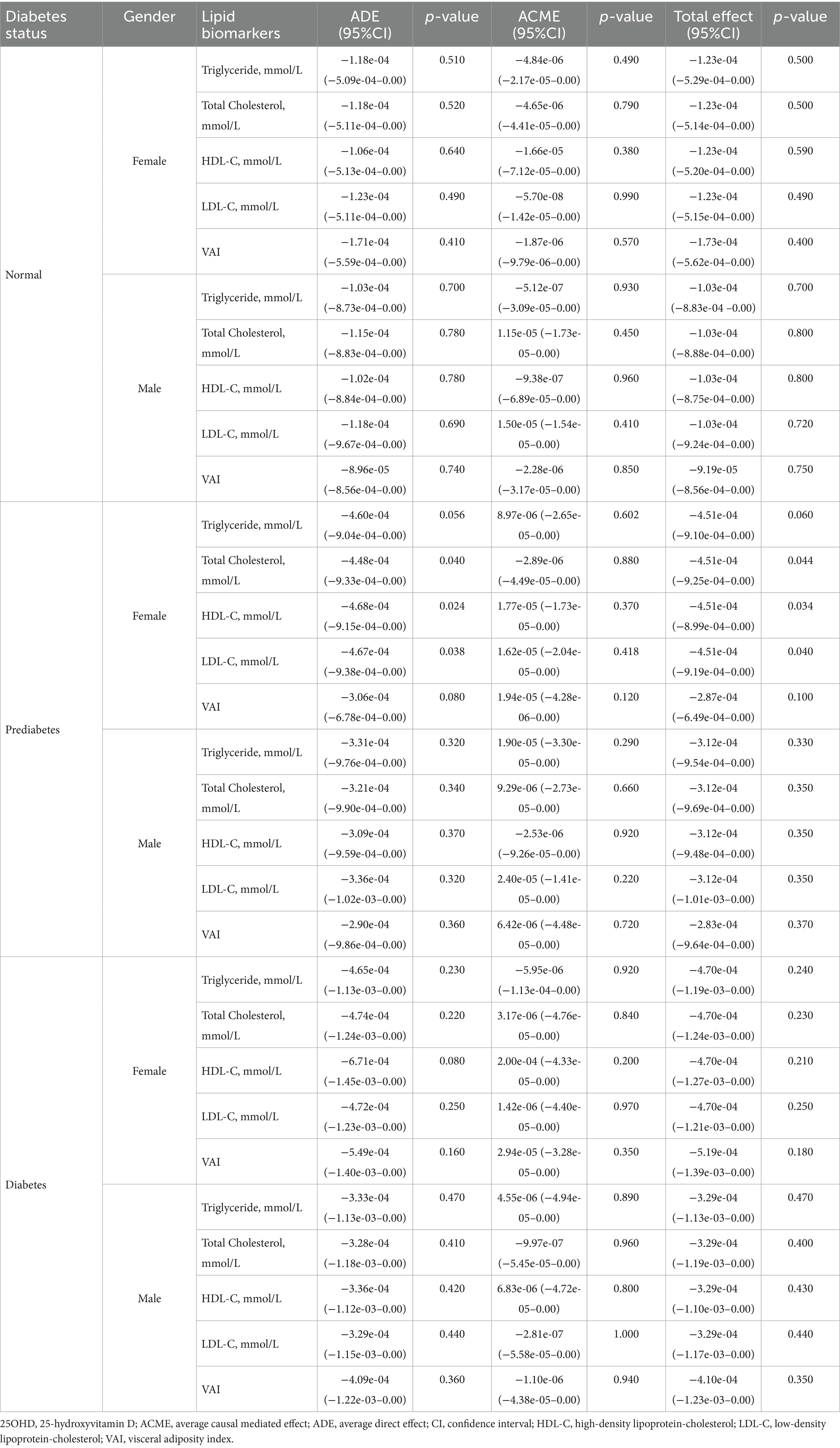
Table 5. The mediation effect of lipid biomarkers in the association between 25OHD and mortality in NHANES 2015–2018 participants.
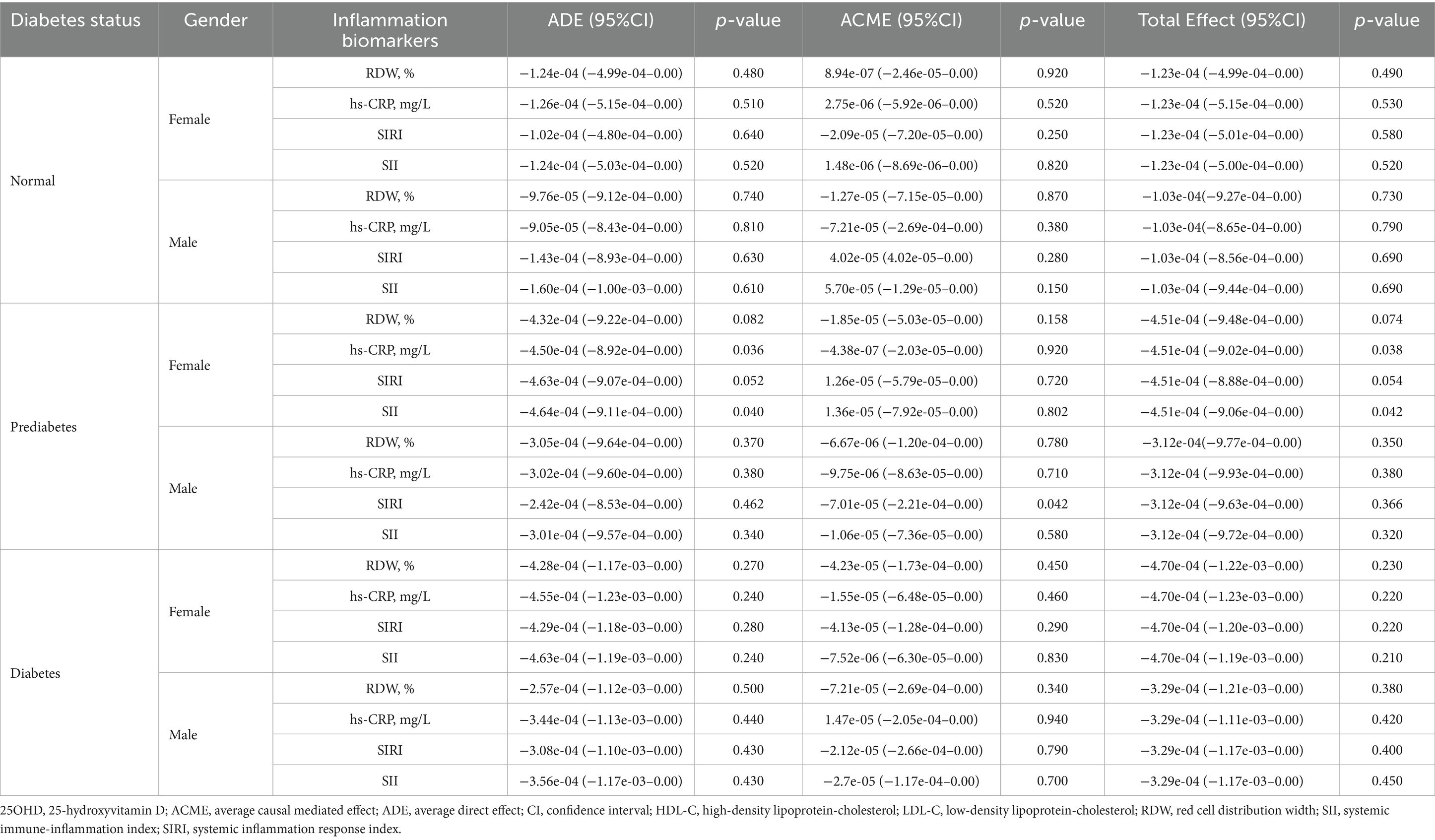
Table 6. The mediation effect of inflammation biomarkers in the association between 25OHD and mortality in NHANES 2015–2018 participants.
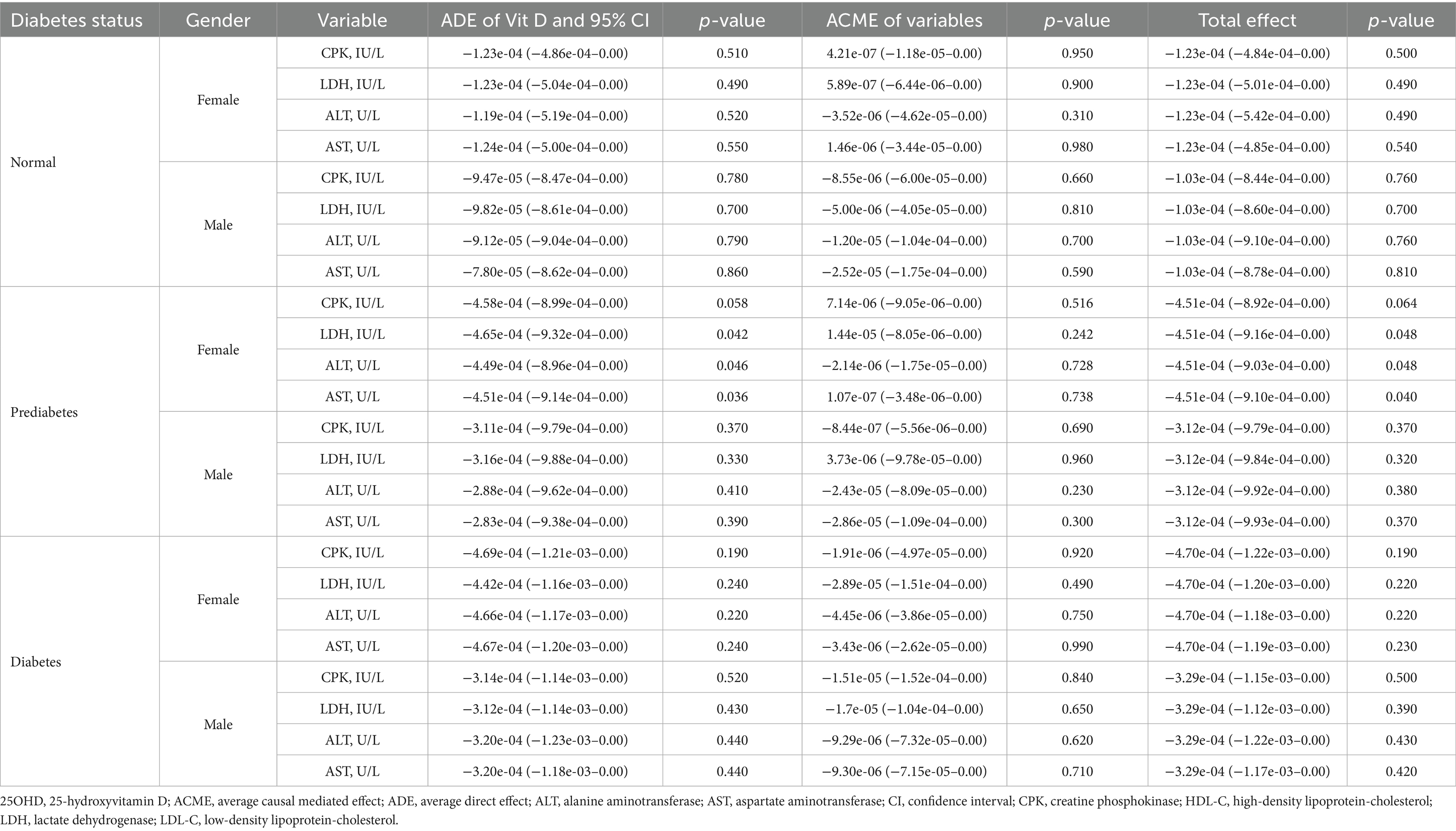
Table 7. The mediation effect of injury biomarkers in the association between 25OHD and mortality in NHANES 2015–2018 participants.
4 Discussion
This nationwide cohort study provides a novel and comprehensive exploration of the complex associations between vitamin D status, lipid metabolism, inflammation, cellular injury, and mortality, with a key focus on identifying gender-specific variations across different stages of glucose dysregulation. This nuanced approach allows for the identification of subgroups that may benefit most from targeted interventions in contrast to a one-size-fits-all approach.
The pronounced gender-specific associations we observed between vitamin D, lipid parameters, and inflammatory biomarkers are consistent with previous research, although specific findings across studies vary (18–20). For instance, Li et al. (20) reported that 25OHD levels were inversely correlated with LDL-C and total cholesterol (TC) in men aged over 50, but positively correlated with LDL-C and total cholesterol in women before age 50. In contrast, our study found that serum 25OHD was not associated with LDL-C in the overall population and was positively associated with total cholesterol only in normoglycemic females. These gender differences may be attributed to several factors. Firstly, sex hormones such as estrogen and testosterone play critical roles in lipid metabolism and immune regulation, which may influence how vitamin D exerts its biological effects. An animal study has shown that estrogen enhances vitamin D signaling by modulating hepatic estrogen receptor expression and vitamin D metabolic enzymes (21). In ovariectomized mice, concurrent deficiency of estrogen and vitamin D led to exacerbated hepatic steatosis, increased expression of lipogenesis-related genes [e.g., sterol regulatory element-binding protein 1c (SREBP1c), fatty acid synthase (FASn)], and elevated inflammatory cytokines [e.g., interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α)], suggesting a synergistic role of estrogen and vitamin D in regulating lipid homeostasis and inflammation. Secondly, genetic factors may contribute to the observed disparities, as genes involved in vitamin D metabolism, vitamin D receptor (VDR) expression, and downstream signaling pathways might exhibit sex-specific polymorphisms or expression patterns. For instance, studies in rats have shown that the hepatic expression of Vdr (the gene encoding VDR) and Cyp2r1 (a key 25-hydroxylase involved in vitamin D activation) is significantly higher in females compared with males (22). Such genetically determined differences in VDR levels and the activity of vitamin D metabolizing enzymes can logically lead to distinct intracellular vitamin D signaling capacities and quantitatively different physiological responses to vitamin D between sexes. Thirdly, differences in body composition, particularly body fat distribution, may affect vitamin D bioavailability since vitamin D is fat-soluble and can be sequestered in adipose tissue. Research by Cominacini et al. (23) indicates that visceral adipose tissue (VAT) particularly can trap vitamin D, thereby diminishing its metabolic availability. Their study also observed that distinct body fat distribution patterns in males and females correlated differently with vitamin D levels, suggesting these sex-specific adiposity characteristics contribute to differential vitamin D storage and bioavailability between men and women.
The association of vitamin D status with mortality risk among populations has been evaluated for several years; however, conclusions remain uncertain due to contradictory findings (24). In our study, mortality risk slightly declined with increasing serum 25OHD levels up to a threshold among normoglycemic, prediabetic, and female diabetic participants—aligning with several studies reporting inverse associations between vitamin D levels and mortality to a certain degree (6, 25–28). However, some studies have not found a significant association between blood 25OHD levels and all-cause mortality (29, 30). These discrepancies extend to intervention studies on vitamin D supplementation, where some studies reported beneficial effects on mortality (31, 32), while others did not (33, 34). Variations in study design, population characteristics, vitamin D dosage and form, as well as follow-up duration, may all contribute to these inconsistent findings (24, 34).
Vitamin D appears to exert its protective effects primarily by modulating immune pathways, such as regulating pro-inflammatory cytokines like TNF-α, IL-6, and interleukin-1β (IL-1β) (35). This regulation is often mediated through the VDR, which can interfere with pro-inflammatory signaling cascades, such as the nuclear factor-κB (NF-κB) pathway, a central regulator of cytokine gene expression (36). The potential links between vitamin D and lipid parameters, inflammation, cellular damage, and mortality prompted us to investigate whether these factors mediate the association between vitamin D and mortality. To mitigate the limitations of single biomarkers, we incorporated composite indices including VAI, SII, and SIRI for a more comprehensive assessment. Regarding the significant mediation by SIRI in prediabetic males, this finding should be interpreted with caution. Although the ACME was statistically significant, the effect size was modest, suggesting that SIRI may play only a limited role in the observed association. Therefore, the result should be viewed as a potential direction for future research rather than conclusive evidence of a clinically meaningful pathway. Larger-scale epidemiological research and well-designed experimental studies are warranted to further elucidate the underlying mechanisms and validate these findings across diverse populations.
Contrary to expectations, our mediation analyses revealed that neither lipid-related nor injury-related biomarkers significantly mediated the association between vitamin D and mortality across subgroups. This absence of mediation is particularly noteworthy, suggesting that inflammation may represent a more dominant and statistically detectable pathway than lipid modulation in the vitamin D–mortality relationship. Nevertheless, the lack of significant findings does not rule out potential involvement of these pathways, as previous studies have reported inconsistent results. For example, Huang et al. (37) found no association between plasma 25OHD and lipid metabolism markers, and reported no significant causal relationship. In contrast, Lhilali et al. (38) observed that low 25OHD levels were associated with elevated lipid profiles and atherogenic indices. Similar inconsistencies were found in studies exploring injury-related biomarkers. Bello et al.’s (39) meta-analysis concluded that vitamin D supplementation had no significant effect on LDH or creatine kinase, whereas Reichrath et al. (40) reported that average serum 25OHD levels ≥ 10 ng/mL were associated with significantly lower LDH levels, with a 3.86 U/L decrease per 1 ng/mL increase in 25OHD. Taken together, these findings highlight the heterogeneous and context-dependent nature of vitamin D’s biological effects, underscoring the need for further mechanistic investigations in diverse populations and clinical settings.
This study benefits from a large, nationally representative sample, allowing for robust analysis and generalizability to the broader United States population. By further stratifying our analysis by gender and glycemic status, we were able to identify important group-specific associations and different mediation pathways that would likely be missed in an analysis of the whole population. Taken together, our findings highlight why a ‘one-size-fits-all’ approach to vitamin D may be insufficient. Instead, our results support the need for a more personalized approach, where vitamin D interventions could be tailored to an individual’s specific characteristics, such as their gender and glycemic state, to achieve better health outcomes. Nevertheless, several limitations in our study still need to be considered. First and foremost, while mortality outcomes were ascertained prospectively, baseline vitamin D levels and various biomarkers—including potential mediators—were measured cross-sectionally. Therefore, the temporal order required for causal inference is lacking. As such, the observed associations and estimated mediation effects should be interpreted with caution. Future longitudinal studies assessing changes in both vitamin D status and biomarker profiles over time are warranted to further elucidate and confirm these potentially mediated relationships; additionally, the reliance on single time-point 25OHD measurements—common in many observational studies including NHANES—may not capture long-term vitamin D status. This introduces potential exposure misclassification due to seasonal fluctuations, supplement use, and lifestyle variation, which may not only bias internal estimates but also complicate comparison across studies. These limitations should be considered when interpreting the observed associations; furthermore, despite adjusting for key confounders, the potential for residual confounding remains. Our models could not fully account for several unmeasured variables, such as detailed dietary habits, specific physical activity patterns, or the precise dosage and duration of vitamin D supplementation. These lifestyle factors could correlate with both vitamin D status and cardiometabolic health, thereby potentially confounding the observed associations. Therefore, while our findings are robust to the included covariates, we cannot entirely exclude the possibility that these unmeasured lifestyle factors may have contributed to the observed associations; and finally, as all participants were from the United States, caution is warranted when generalizing the findings to other populations.
In conclusion, our study demonstrates that the associations of vitamin D with lipid metabolism, inflammation, and cellular injury are strongly influenced by gender and glycemic status. Systemic inflammation partially mediates the relationship between vitamin D and mortality in prediabetic males. Although further research is needed to confirm these findings, our study suggests that vitamin D intervention strategies may need to be tailored based on an individual’s gender and glycemic status.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://wwwn.cdc.gov/nchs/nhanes/; www.cdc.gov/nchs/linked-data/mortality-files/index.html.
Ethics statement
The studies involving humans were approved by National Center for Health Statistics (NCHS) Ethics Review Board (ERB). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MC: Project administration, Writing – review & editing, Funding acquisition, Conceptualization, Methodology. XJ: Visualization, Formal analysis, Software, Methodology, Writing – original draft. XX: Writing – original draft, Data curation, Funding acquisition, Validation, Formal analysis. SZ: Writing – original draft, Validation. YS: Writing – original draft, Data curation. YY: Writing – original draft, Software, Visualization. YG: Funding acquisition, Writing – original draft, Data curation, Validation. YH: Supervision, Conceptualization, Writing – review & editing, Project administration, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Suzhou Science and Education Strengthening Health Youth Project, grant numbers KJXW2021011 and KJXW2022014; the Suzhou Medical Innovation and Application Research Project, grant number SKY2023119; the Extracurricular Academic Research Fund for Undergraduates, grant number KY2024266B; the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Acknowledgments
The authors appreciate the efforts of the NHANES staff, researchers, and participants whose contributions have made this valuable dataset accessible for public health research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ao, T, Kikuta, J, and Ishii, M. The effects of vitamin D on immune system and inflammatory diseases. Biomolecules. (2021) 11:1624. doi: 10.3390/biom11111624
2. Surdu, AM, Pînzariu, O, Ciobanu, DM, Negru, AG, Cainap, SS, Lazea, C, et al. vitamin d and its role in the lipid metabolism and the development of atherosclerosis. Biomedicine. (2021) 9:172. doi: 10.3390/biomedicines9020172
3. Kamimura, D, Yimer, WK, Shah, AM, Mentz, RJ, Oshunbade, A, Hamid, A, et al. Vitamin D Levels in black americans and the association with left ventricular remodeling and incident heart failure with preserved ejectin fraction: the Jackson Heart Study. J Card Fail. (2023) 29:150–7. doi: 10.1016/j.cardfail.2022.07.049
4. Berridge, MJ. Vitamin D deficiency and diabetes. Biochem J. (2017) 474:1321–32. doi: 10.1042/BCJ20170042
5. Zhu, K, Knuiman, M, Divitini, M, Hung, J, Lim, EM, Cooke, BR, et al. Lower serum 25-hydroxyvitamin D is associated with colorectal and breast cancer, but not overall cancer risk: a 20-year cohort study. Nutr Res. (2019) 67:100–7. doi: 10.1016/j.nutres.2019.03.010
6. Park, KY, Han, KYD, Hwang, HS, Park, HK, and Park, K. Serum 25-hydroxyvitamin D concentrations are inversely associated with all-cause mortality among Koreans: a nationwide cohort study. Nutr Res. (2023) 113:49–58. doi: 10.1016/j.nutres.2023.02.008
7. Holick, MF. revisiting vitamin d guidelines: a critical appraisal of the literature. Endocr Pract. (2024) 30:1227–41. doi: 10.1016/j.eprac.2024.10.011
8. Wierzbicka, A, and Oczkowicz, M. Sex differences in vitamin D metabolism, serum levels and action. Br J Nutr. (2022) 128:2115–30. doi: 10.1017/S0007114522000149
9. Alaidarous, TA, Alkahtani, NM, Aljuraiban, GS, and Abulmeaty, MMA. Impact of the glycemic control and duration of type 2 diabetes on vitamin D level and cardiovascular disease risk. J Diabetes Res. (2020) 2020:1–10. doi: 10.1155/2020/8431976
10. Eralp, B, and Sefer, E. Reference-free inferring of transcriptomic events in cancer cells on single-cell data. BMC Cancer. (2024) 24:607. doi: 10.1186/s12885-024-12331-5
11. Bijari, M, Jangjoo, S, Emami, N, Raji, S, Mottaghi, M, Moallem, R, et al. The accuracy of visceral adiposity index for the screening of metabolic syndrome: a systematic review and meta-analysis. Int J Endocrinol. (2021) 2021:1–14. doi: 10.1155/2021/6684627
12. Li, J, He, D, Yu, JZ, Chen, SH, Wu, Q, Cheng, ZX, et al. dynamic status of sii and siri alters the risk of cardiovascular diseases: evidence from Kailuan Cohort Study. J Inflamm Res. (2022) 15:5945–57. doi: 10.2147/JIR.S378309
13. Seligman, HK, Levi, R, Adebiyi, VO, Coleman-Jensen, A, Guthrie, JF, and Frongillo, EA. Assessing and monitoring nutrition security to promote healthy dietary intake and outcomes in the United States. Annu Rev Nutr. (2023) 43:409–29. doi: 10.1146/annurev-nutr-062222-023359
14. Elsayed, NA, Comm, PP, Mccoy, RG, Aleppo, G, Balapattabi, K, Beverly, EA, et al. 2. Diagnosis and classification of diabetes: standards of care in diabetes-2025. Diabetes Care. (2025) 48:S27–49. doi: 10.2337/dc25-S002
15. Candemir, M, Kiziltunç, E, Nurkoç, S, and Sahinarslan, A. Relationship between systemic immune-inflammation index (SII) and the severity of stable coronary artery disease. Angiology. (2021) 72:575–81. doi: 10.1177/0003319720987743
16. Li, XW, Cui, L, and Xu, HY. Association between systemic inflammation response index and chronic kidney disease: a population-based study. Front Endocrinol. (2024) 15:1329256. doi: 10.3389/fendo.2024.1329256
17. Parra-Soto, S, Boonpor, J, Lynskey, N, Araya, C, Ho, F, Pell, JP, et al. Association between visceral adiposity index and cancer risk in the UK Biobank cohort. Cancer. (2025) 131:e35576. doi: 10.1002/cncr.35576
18. Almesri, N, Das, NS, Ali, ME, Gumaa, K, and Giha, HA. Gender-dependent association of vitamin D deficiency with obesity and hypercholesterolemia (LDLC) in adults. Endoc Metab Immune Disord. (2020) 20:425–36. doi: 10.2174/1871530319666191009154528
19. Abudawood, M, Tabassum, H, Ansar, S, Almosa, K, Sobki, S, Ali, MN, et al. Assessment of gender-related differences in vitamin D levels and cardiovascular risk factors in Saudi patients with type 2 diabetes mellitus. Saudi J Biol Sci. (2018) 25:31–6. doi: 10.1016/j.sjbs.2017.04.001
20. Li, XT, Liu, YN, Wang, JY, Chen, X, Reichetzeder, C, Elitok, S, et al. Vitamin D Is associated with lipid metabolism: a sex- and age-dependent analysis of a large outpatient cohort. Nutrients. (2024) 16:3936. doi: 10.3390/nu16223936
21. Borges, CC, Bringhenti, I, Mandarim-de-Lacerda, CA, and Aguila, MB. Vitamin D deficiency aggravates the liver metabolism and inflammation in ovariectomized mice. Biomed Pharmacother. (2018) 107:878–88. doi: 10.1016/j.biopha.2018.08.075
22. Oczkowicz, M, Szymczyk, B, Swiatkiewicz, M, Furgal-Dzierzuk, I, Koseniuk, A, Wierzbicka, A, et al. Analysis of the effect of vitamin D supplementation and sex on and gene expression in Wistar rats’ tissues. J Steroid Biochem Mol Biol. (2021) 212:105918. doi: 10.1016/j.jsbmb.2021.105918
23. Cominacini, M, Fumaneri, A, Ballerini, L, Braggio, M, Valenti, MT, and Carbonare, LD. Unraveling the connection: visceral adipose tissue and vitamin D levels in obesity. Nutrients. (2023) 15:4259. doi: 10.3390/nu15194259
24. Heath, AK, Kim, IY, Hodge, AM, English, DR, and Muller, DC. Vitamin D status and mortality: a systematic review of observational studies. Int J Environ Res Public Health. (2019) 16:383. doi: 10.3390/ijerph16030383
25. Luo, W, Xu, D, Zhang, J, Zhou, Y, Yang, Q, Lv, Q, et al. Low serum 25-hydroxyvitamin D levels are associated with increased cardiovascular morbidity and mortality. Postgrad Med. (2023) 135:93–101. doi: 10.1080/00325481.2022.2161250
26. Wan, ZZ, Guo, JY, Pan, A, Chen, C, Liu, LG, and Liu, G. Association of serum 25-hydroxyvitamin D concentrations with all-cause and cause-specific mortality among individuals with diabetes. Diabetes Care. (2021) 44:350–7. doi: 10.2337/dc20-1485
27. Jin, XR, Xiong, SZ, Ju, SY, Zeng, Y, Yan, LJL, and Yao, Y. Serum 25-hydroxyvitamin D, albumin, and mortality among Chinese older adults: a population-based longitudinal study. J Clin Endocrinol Metab. (2020) 105:2762–70. doi: 10.1210/clinem/dgaa349
28. Ye, HW, Li, YX, Liu, SM, Zhang, XF, Liang, HZ, Wang, Y, et al. Association between serum 25-hydroxyvitamin D and vitamin D dietary supplementation and risk of all-cause and cardiovascular mortality among adults with hypertension. Nutr J. (2024) 23:33. doi: 10.1186/s12937-024-00914-8
29. Lin, SW, Chen, W, Fan, JH, Dawsey, SM, Taylor, PR, Qiao, YL, et al. Prospective study of serum 25-hydroxyvitamin D concentration and mortality in a Chinese population. Am J Epidemiol. (2012) 176:1043–50. doi: 10.1093/aje/kws285
30. Jevalikar, G, Mithal, A, Singh, A, Sharma, R, Farooqui, KJ, Mahendru, S, et al. Lack of association of baseline 25-hydroxyvitamin D levels with disease severity and mortality in Indian patients hospitalized for COVID-19. Sci Rep. (2021) 11:6258. doi: 10.1038/s41598-021-85809-y
31. Sha, S, Nguyen, TMN, Kuznia, S, Niedermaier, T, Zhu, A, Brenner, H, et al. Real-world evidence for the effectiveness of vitamin D supplementation in reduction of total and cause-specific mortality. J Intern Med. (2022) 293:384–97. doi: 10.1111/joim.13578
32. Acharya, P, Dalia, T, Ranka, S, Sethi, P, Oni, OA, Safarova, MS, et al. The effects of vitamin D supplementation and 25-hydroxyvitamin D levels on the risk of myocardial infarction and mortality. J Endocr Soc. (2021) 5:bvab124. doi: 10.1210/jendso/bvab124
33. Manson, JE, Cook, NR, Lee, IM, Christen, W, Bassuk, SS, Mora, S, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. (2019) 380:33–44. doi: 10.1056/NEJMoa1809944
34. Zhang, Y, Fang, F, Tang, JJ, Jia, L, Feng, YN, Xu, P, et al. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. (2019) 366:l4673. doi: 10.1136/bmj.l4673
35. Xiaohua, G, Dongdong, L, Xiaoting, N, Shuoping, C, Feixia, S, Huajun, Y, et al. Severe Vitamin D deficiency is associated with increased expression of inflammatory cytokines in painful diabetic peripheral neuropathy. Front Nutr. (2021) 8:612068. doi: 10.3389/fnut.2021.612068
36. Argano, C, Mirarchi, L, Amodeo, S, Orlando, V, Torres, A, and Corrao, S. The role of vitamin D and its molecular bases in insulin resistance, diabetes, metabolic syndrome, and cardiovascular disease: state of the art. Int J Mol Sci. (2023) 24:15485. doi: 10.3390/ijms242015485
37. Huang, T, Afzal, S, Yu, CQ, Guo, Y, Bian, Z, Yang, L, et al. Vitamin D and cause-specific vascular disease and mortality: a Mendelian randomisation study involving 99,012 Chinese and 106,911 European adults. BMC Med. (2019) 17:160. doi: 10.1186/s12916-019-1401-y
38. Lhilali, I, Zouine, N, Godderis, L, El Midaoui, A, El Jaafari, S, and Filali-Zegzouti, Y. Relationship between Vitamin D insufficiency, lipid profile and atherogenic indices in healthy women aged 18–50 years. Eur J Invest Health Psychol Educ. (2024) 14:2337–57. doi: 10.3390/ejihpe14080155
39. Bello, HJ, Caballero-García, A, Pérez-Valdecantos, D, Roche, E, Noriega, DC, and Córdova-Martínez, A. Effects of vitamin D in post-exercise muscle recovery. A systematic review and meta-analysis. Nutrients. (2021) 13:4013. doi: 10.3390/nu13114013
40. Reichrath, J, Biersack, F, Wagenpfeil, S, Schoepe, J, Pfoehler, C, Saternus, R, et al. Low vitamin D status predicts poor clinical outcome in advanced melanoma treated with immune checkpoint or BRAF/MEK inhibitors: a prospective non-interventional side-by-side analysis. Front Oncol. (2022) 12:839816. doi: 10.3389/fonc.2022.839816
Glossary
25OHD - 25-hydroxyvitamin D
ALT - alanine aminotransferase
AST - aspartate aminotransferase
BMI - body mass index
CPK - creatine phosphokinase
FASn - fatty acid synthase
FPG - fasting plasma glucose
HbA1c - hemoglobin A1c
HDL-C - high-density lipoprotein cholesterol
hs-CRP - high-sensitivity C-reactive protein
IL-1β - interleukin-1β
IL-6 - interleukin-6
LDH - lactate dehydrogenase
LDL-C - low-density lipoprotein cholesterol
NF-κB - nuclear factor-κB
NDI - national death index
NHANES - National Health and Nutrition Examination Survey
RDW - red cell distribution width
SII - systemic immune-inflammation index
SIRI - systemic inflammation response index
SREBP1c - sterol regulatory element-binding protein 1c
TC - total cholesterol
TG - triglycerides
TNF-α - tumor necrosis factor-α
VAI - visceral adiposity index
WC - waist circumference
Keywords: vitamin D, dyslipidemia, systemic inflammation, oxidative stress, cardiometabolic risk, gender differences, NHANES, glycemic control
Citation: Cai M, Jiang X, Xu X, Zhao S, Sun Y, Yang Y, Gu Y and Huang Y (2025) Associations of vitamin D with lipid metabolism, inflammation, and mortality vary by glycemic status and gender: a nationwide prospective study. Front. Nutr. 12:1597527. doi: 10.3389/fnut.2025.1597527
Edited by:
Kathryn Hart, University of Surrey, United KingdomReviewed by:
Alessandro De Oliveira, Universidade Federal de São João del-Rei, BrazilEmre Sefer, Özyeğin University, Türkiye
Yuan Tian, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, China
Muhammad Mazhar, Shenzhen University, China
Copyright © 2025 Cai, Jiang, Xu, Zhao, Sun, Yang, Gu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meizhi Cai, Y2FpbWVpemhpMTk4N0Bob3RtYWlsLmNvbQ==; Yifan Huang, eXl6aHlmQGhvdG1haWwuY29t
†These authors have contributed equally to this work
 Meizhi Cai
Meizhi Cai Xuan Jiang
Xuan Jiang Xinyi Xu
Xinyi Xu Sidi Zhao1
Sidi Zhao1 Yue Sun
Yue Sun Yushuo Yang
Yushuo Yang Yifan Huang
Yifan Huang