- 1Department of Chronic Disease Prevention and Control, Zhejiang Provincial Center for Disease Control and Prevention, Hangzhou, China
- 2Department of Epidemiology, School of Public Health, Southeast University, Nanjing, China
- 3Key Laboratory of Systems Health Science of Zhejiang Province, School of Life Science, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, Hangzhou, China
Background: Serum vitamins A and B12, as essential micronutrients, play pivotal roles in maintaining physiological homeostasis; however, the association between these vitamins and aging remains unclear. Therefore, this study aims to investigate potential threshold effects of these nutrients on accelerated biological aging using multidimensional DNA methylation biomarkers.
Methods: This study included 2,530 participants with DNA methylation data from the National Health and Nutrition Examination Survey 1999–2000 and 2001–2002. Two age acceleration metrics, derived from epigenetic clocks (PhenoAge and GrimAge), were calculated as the residuals obtained by regressing the epigenetic clock estimates on chronological age. Multivariable logistic regression models were used to analyze the association of vitamins A and B12 with epigenetic clocks. Additionally, generalized additive models and two-piecewise logistic regression were used to explore the non-linear relationships between vitamins A and B12 and epigenetic clocks.
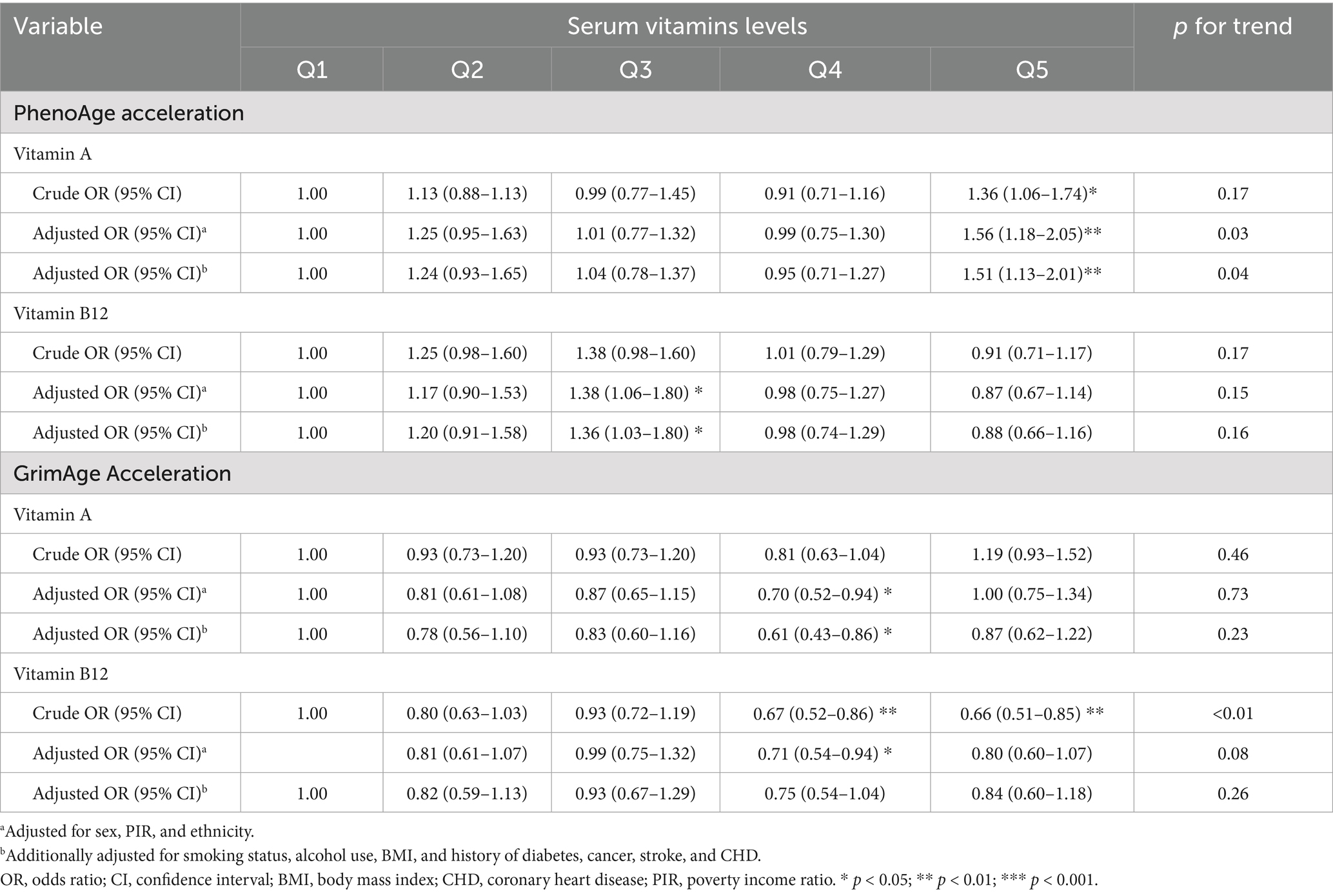
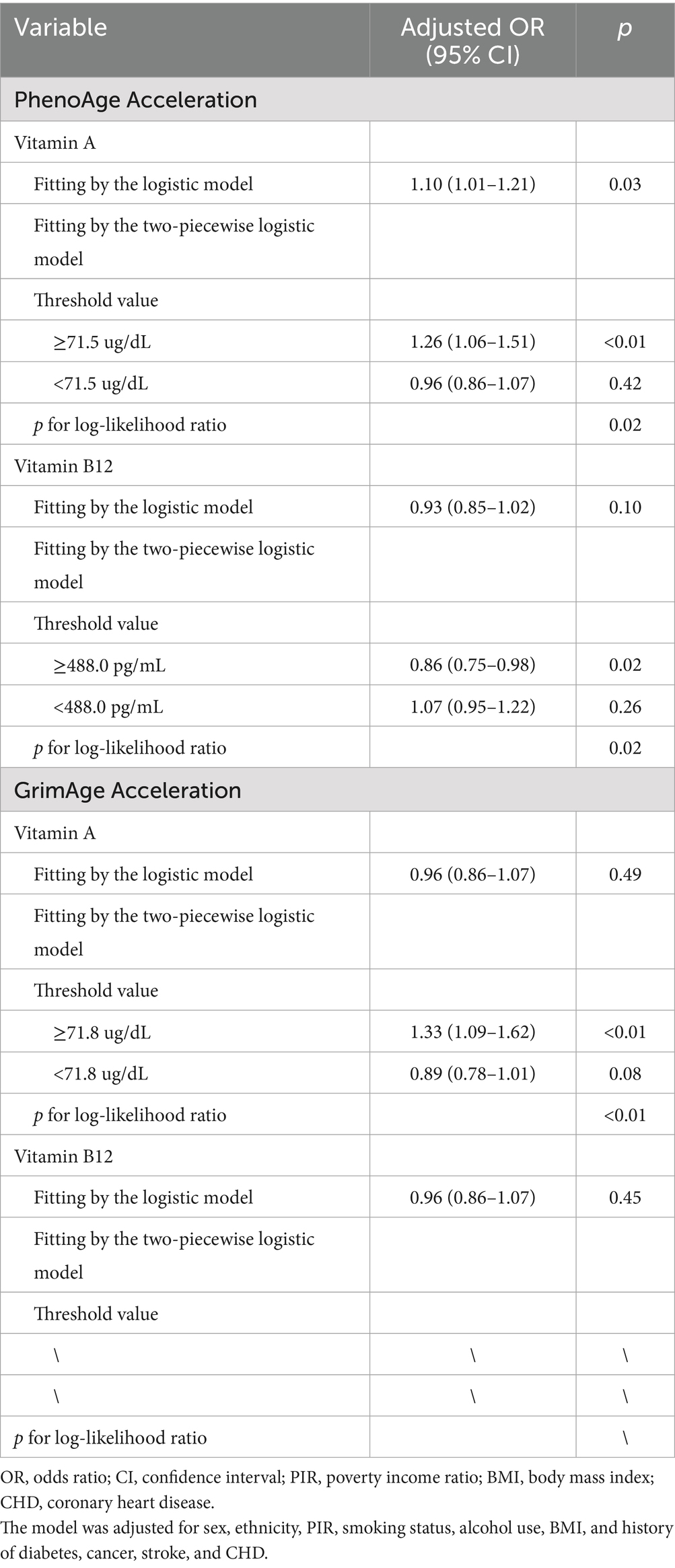
Results: Compared to the first quintile of vitamin A, the odds ratios (ORs) for PhenoAge acceleration in the next four quintiles were 1.24 (0.93–1.65), 1.04 (0.78–1.37), 0.95 (0.71–1.27), and 1.51 (1.13–2.01), respectively. No linear associations were found between vitamin B12 and PhenoAge acceleration, nor between vitamins A and B12 and GrimAge acceleration. However, the generalized additive model showed significant non-linear associations between serum levels of vitamins A and B12 and PhenoAge acceleration, with inflection points at 71.5 and 488.0 pg/mL, respectively. In addition, a non-linear association was observed between serum levels of vitamin A with GrimAge acceleration, with an inflection point at 71.8 μg/dL. Two-piecewise logistic regression also indicated that higher vitamin B12 delayed aging, while higher vitamin A accelerated aging. Sensitivity analyses showed a similar non-linear association between vitamins A and B12 and HannumAge acceleration.
Conclusion: This study suggests that higher vitamin A concentrations may be related to an increased risk of aging, while adequate vitamin B12 intake may offer protective benefits against epigenetic changes associated with aging.
1 Introduction
According to the report of the World Health Organization (WHO), the population aged 60 years and above currently exceeds 1 billion. By 2050, this number is expected to double, reaching 2.1 billion globally (1). As the challenges associated with an aging population intensify, the importance of healthy aging has become increasingly critical. Aging is a natural physiological process in most living organisms, characterized by a time-dependent decline in body function. Traditionally, chronological age has been used as the primary measure of aging. However, recent studies showed that biological age could more accurately reflect a person’s aging status compared to chronological age, as the latter did not consider an individual’s health status (2–4). Biological aging could raise the accumulation of molecular changes or “hallmarks” that deteriorate the function and resilience of tissues and organs, eventually leading to death and age-related diseases such as cardiovascular disease and cancer (3, 4).
Recent evidence from human and mouse studies demonstrates that DNA methylation-based (DNAm) biomarkers satisfy the formerly elusive criteria of a molecular biomarker of aging and thus serve as vital inputs for constructing epigenetic clocks (5). These clocks can accurately predict biological aging by accounting for both genetic predispositions and environmental factors (6). HannumAge and HorvathAge were the “first-generation” clocks, which are primarily based on chronological age. In contrast, “second-generation” clocks, such as GrimAge and PhenoAge, emphasize health and mortality outcomes (7). Among these, PhenoAge and GrimAge have stronger evidence of association with morbidity and mortality and with risk factors for shorter and less healthy lives (8). When the epigenetic clock exceeds chronological age, individuals are considered to be in the state of accelerated aging, which is linked to an increased risk of diseases (6, 9). However, few studies have explored the factors that affect accelerated epigenetic aging, especially the association of nutrients with aging (7).
Vitamins are essential nutrients that are significantly associated with aging-related diseases. Vitamin A, a crucial fat-soluble vitamin, is an indispensable dietary micronutrient essential for human health and development, supporting physiological processes, including immune function, cellular growth, and metabolic regulation (10, 11). Previous studies have shown that adults in high-income countries often use dietary supplements containing vitamin A, which increases the risk of intake above tolerable upper intake levels (12). However, vitamin A deficiency has been linked to obesity and carcinogenesis in the oral cavity (13, 14). In addition, vitamin B12, a water-soluble vitamin, plays a critical role in physiological functions such as DNA synthesis and methylation, inflammatory response, and neurological function. Previous studies have shown that vitamin B12 deficiency could elevate homocysteine levels, thereby increasing the risk of cardiovascular disease (15). Moreover, vitamin B12 deficiency was associated with neurological sequelae such as peripheral neuropathy, subacute combined degeneration of the spinal cord, cognitive decline, and psychiatric disturbances (16, 17). However, few studies explored the association between vitamins and epigenetic aging.
Previous studies have shown that serum levels of vitamin D are negatively associated with aging (18–22). Limited evidence exists regarding the associations between serum levels of vitamins A and B12 and aging. While most prior investigations examined linear associations of vitamins with health outcomes (e.g., mortality or disease risk), the optimal concentration ranges for these vitamins remain unknown. For example, previous studies showed that serum levels of vitamin A <30 μg/dL or >80 μg/dL may indicate a high risk of subsequent mortality (23). Additionally, both low (<369.1 pg/mL) and high (≥506.1 pg/mL) serum vitamin B12 levels were associated with a higher risk of cardiovascular disease (CVD) mortality in diabetes patients (24). To fill these knowledge gaps, we examined the non-linear associations between serum vitamins A and B12 concentrations and GrimAge and PhenoAge acceleration, based on the National Health and Nutrition Examination Survey (NHANES). Furthermore, the inflection points of these associations were also determined.
2 Materials and methods
2.1 Study design and sample
Participants of this cross-sectional study came from the NHANES, a nationally representative sample of the US population, with methodological details reported previously (25). Briefly, the NHANES was conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention. All participants were recruited from the civilian, non-institutionalized household population of the United States between 1988 and 2018. Baseline information was collected using standardized questionnaires and home interviews, and blood samples were collected and tested following standard procedures.
The data for our study were obtained from the two-cycle data of NHANES (1999–2000 and 2001–2002). After excluding the participants with missing DNAm PhenoAge (N = 18,472) and serum levels of vitamin A (N = 5) and vitamin B12 (N = 2) at baseline, 2,527 and 2,530 participants were used to calculate the association of serum levels of vitamins A and vitamin B12 with epigenetic clocks, respectively (Supplementary Figure 1).
2.2 Serum levels of vitamins A and B12 assessment
The serum levels of vitamins A and B12 were measured using high-performance liquid chromatography with photodiode array detection in the NHANES 1999–2000 and 2001–2002. The NHANES quality assurance and quality control protocols complied with the 1988 Clinical Laboratory Improvement Act Mandates.
2.3 Accelerated epigenetic aging assessment
This study evaluated accelerated epigenetic aging through DNAm-based epigenetic clocks. DNA methylation profiling was performed on purified whole blood samples obtained from participants in NHANES during the 1999–2000 and 2001–2002 cycles. Genome-wide DNAm levels were quantified using the Illumina Infinium MethylationEPIC BeadChip v1.0 (Illumina, San Diego, CA, United States). Data preprocessing, normalization, and quality control procedures followed established protocols, as detailed in NHANES (26). Two widely validated epigenetic clocks, including PhenoAge (27) and GrimAge (28) were computed to capture distinct dimensions of biological aging. PhenoAge and GrimAge incorporate clinical biomarkers and mortality-related predictors to quantify morbidity- and mortality-associated aging trajectories (29). Age acceleration, a measure of epigenetic aging discordance, was derived for each clock by regressing chronological age against its corresponding epigenetic clock estimate. Accelerated epigenetic aging was defined as the positive age acceleration, indicating that the epigenetic clock was older than chronological age.
2.4 Covariate assessment
As both vitamins (A and B12) and accelerated epigenetic aging could be influenced by sex, age, ethnicity, socioeconomic factors [education and poverty income ratio (PIR)], lifestyle factors [smoking and alcohol use], body mass index (BMI), and history of disease [diabetes, cancer, coronary heart disease (CHD) and stroke]. The history of disease was defined based on self-reported diagnoses by doctors or the use of drugs associated with those diseases.
2.5 Statistical analyses
To reduce the effect of false positives, our study used winsorization to adjust for outliers prior to log-transformation (30). The outliers were capped by the 5th percentile (Q5) or 95th percentile (Q95) of vitamin levels. The chi-squared test was used to compare baseline categorical variables by quintiles of serum levels of vitamins A and B12, and analysis of variance (ANOVA) or Kruskal–Wallis rank sum test for continuous variables. Logistic regression was used to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for the association of serum levels of vitamins A and B12 with accelerated epigenetic aging. Model 1 was the crude model without adjustment. In multivariable analyses, model 2 was adjusted for sex, age, ethnicity, and PIR, and model 3 additionally adjusted for lifestyle factors (smoking and alcohol use), BMI, and comorbidities (history of diabetes, cancer, stroke, and CHD).
Furthermore, potential non-linear associations between serum levels of vitamins A and B12 and accelerated epigenetic aging were explored using logistic regression with generalized additive model and smoothed curve fitting (penalized spline method). Vitamins A and B12 were included in the models as continuous, log2-transformed, and scaled values. A recursive algorithm was then employed to estimate the inflection points from these non-linear associations, and a two-segment logistic regression model based on inflection points was used.
2.6 Sensitivity analysis
To assess potential effect modification, a log-likelihood ratio test was used to calculate the model fit by comparing models with and without interaction terms of vitamins A and B12 with selected potential effect modifiers. When significant interactions were found, we conducted subgroup analyses by the possible effect modifiers. To assess the robustness of our findings, we used HorvathAge and HannumAge acceleration, which have been extensively validated in previous studies. In addition, we analyzed the association between serum vitamin quantiles and epigenetic age acceleration, with model fit assessed using Akaike Information Criterion (AIC) values. Two DNAm ages were trained on chronological age and reflect mitotic and tissue-specific aging processes (31, 32). Statistical analysis was performed using R (version 4.4.1; R Core Team, Vienna, Austria). Two-sided p < 0.05 were considered statistically significant.
3 Results
3.1 Participant characteristics
Table 1 shows that at baseline compared with those in the lowest quintile (Q1) of vitamin A level, participants in the highest quintile (Q5) were more likely to be men, older and non-Hispanic White, had higher family income, more smoking and alcohol use, were less likely to be obese (all p < 0.05). They had a higher prevalence of diabetes, cancer, and CVD (all p < 0.05), but similar prevalence of stroke (p = 0.28). In contrast, participants in the highest quintile (Q5) of vitamin B12 were more likely to be women and non-Hispanic Black, with lower smoking and alcohol use. At the same time, age, income, and prevalence of cancer and CHD were similar.
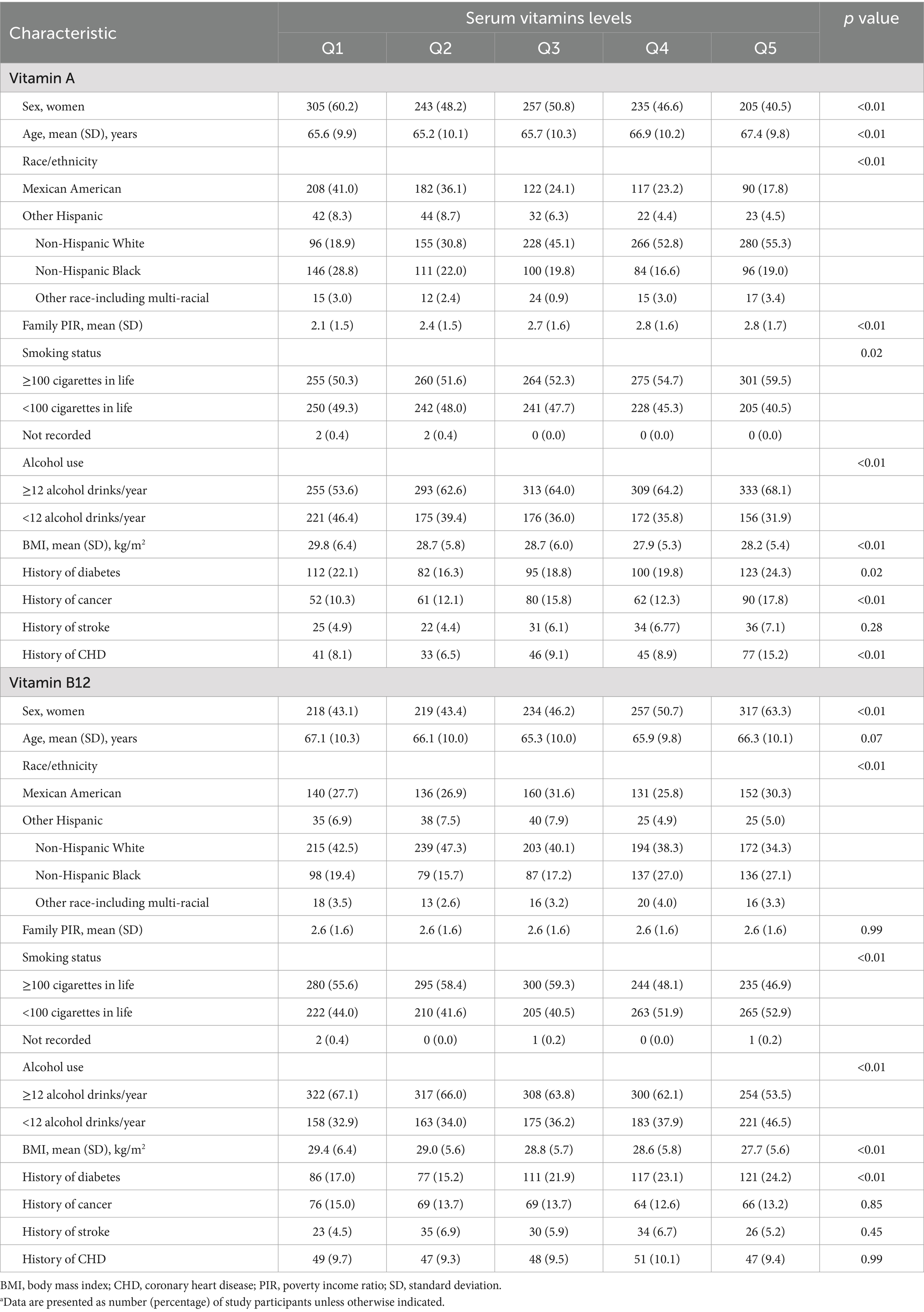
Table 1. Baseline characteristics of participants according to serum levels of vitamins A and vitamin B12a.
3.2 Association of vitamins A and B12 with accelerated epigenetic aging
Table 2 shows that compared to the first quintile of vitamin A, the fully adjusted OR (95% CI) of PhenoAge acceleration for the second to fifth quintiles were 1.24 (95% CI: 0.93–1.65), 1.04 (95% CI: 0.78–1.37), 0.95 (95% CI: 0.71–1.27), and 1.51 (95% CI: 1.13–2.01), respectively. Although we found no significant linear association of serum levels of vitamin B12 with PhenoAge acceleration [Q1: reference; Q2: OR = 1.20, 95% CI: 0.91–1.58; Q3: OR = 1.36, 95% CI: 1.03–1.80; Q4: OR = 0.98, 95% CI: 0.74–1.29; and Q5: OR = 0.88, 95% CI: 0.66–1.16; p for trend = 0.16], the trend of the OR values suggested a potential non-linear dose–response association. Similarly, we found no significant linear association of serum levels of vitamins A and B12 with GrimAge acceleration (p for trend = 0.23 and 0.26, respectively). However, nonmonotonic trends in ORs across quintiles suggested possible threshold or biphasic effects.
3.3 The detection of non-linear association
Figure 1 shows that the generalized additive model revealed a non-linear association of serum levels of vitamin A (p for non-linear = 0.02 for PhenoAge acceleration and p for non-linear = 0.01 for GrimAge acceleration) and serum levels of vitamin B12 (p for non-linear = 0.03 for PhenoAge acceleration) with accelerated epigenetic aging. The inflection points of serum levels of vitamins A and B12 with PhenoAge acceleration were 71.5 μg/dL and 488.0 pg/mL, respectively. In addition, the points at which the curve crossed the horizontal vector were similar for the PhenoAge acceleration and GrimAge acceleration (71.8 μg/dL for vitamin A). However, no significant non-linear association was found between vitamin B12 and GrimAge acceleration (p for non-linear = 0.30).
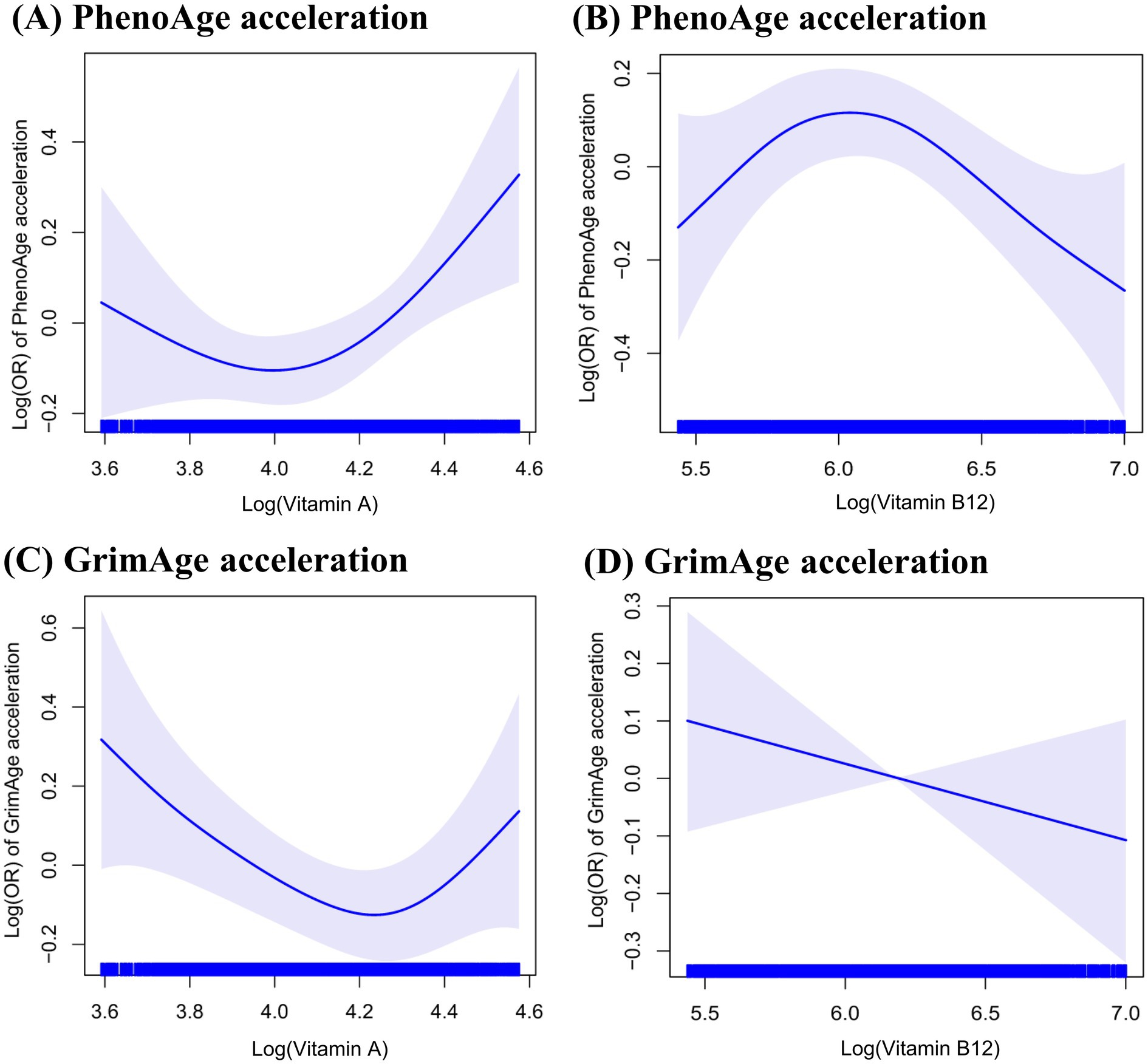
Figure 1. The non-linear association of serum levels of vitamins A and B12 concentrations with accelerated epigenetic aging.
Furthermore, two-piecewise logistic regression shows similar results (all p for log-likelihood ratio < 0.05, Table 3). Meanwhile, if the serum vitamin A concentration is ≥71.5 μg/dL, each standard deviation (SD) raises the risk of PhenoAge acceleration by 26% (OR = 1.26, 95% CI: 1.06–1.51). However, per 1 SD increase in serum levels of vitamin B12 was associated with a 14% lower risk of PhenoAge acceleration when the concentration was ≥488.0 pg/mL (OR = 0.86, 95% CI: 0.75–0.98). When serum levels of vitamins A and B12 were <71.5 μg/dL and 488.0 pg/mL, respectively, no associations were observed between those vitamins and PhenoAge acceleration (OR = 0.96, 95% CI: 0.86–1.07 and OR = 1.07, 95% CI: 0.95–1.22, respectively). If the serum vitamin A concentration is ≥71.8 μg/dL, each SD raises the risk of GrimAge acceleration by 33% (OR = 1.33, 95% CI: 1.09–1.62). However, no associations were observed between vitamin A and GrimAge acceleration (OR = 0.89, 95% CI: 0.78–1.01).
3.4 Subgroup analysis and sensitivity analysis
Figure 2 shows that serum levels of vitamins A and B12 were categorized into low and high groups using a cut-off value of 71.5 μg/dL and 488.0 pg/mL, respectively. The association of high vs. low serum vitamins A and B12 concentrations on PhenoAge acceleration was consistent across various subgroups by age, sex, ethnicity, BMI, and history of diabetes, stroke, and CHD. No significant interactions were observed between serum levels of vitamins (A and B12) and subgroup variables related to epigenetic acceleration of aging (PhenoAge acceleration and GrimAge acceleration), except in cancer subgroup analyses. The positive association between serum levels of vitamin A and PhenoAge Acceleration was stronger in participants with cancer than in participants without cancer (p for interaction <0.01), with the OR being 2.46 (1.49–4.05) and 1.12 (0.91–1.39), respectively. Figure 3 shows that the direction of the association between vitamins A and B12 and GrimAge was almost consistent with the aforementioned association, although it was not statistically significant.
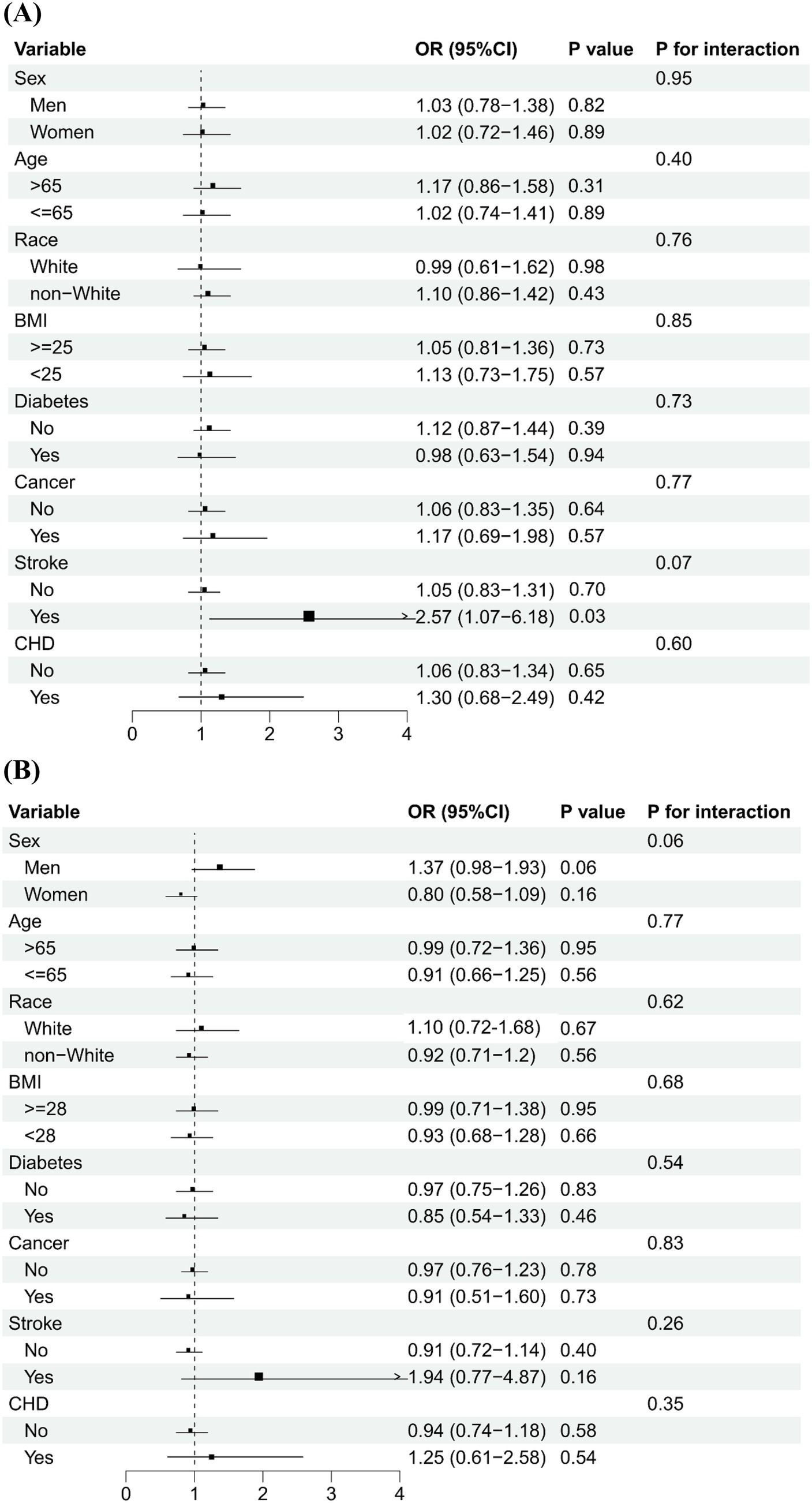
Figure 2. Subgroup analysis of the association of higher levels of serum vitamins A and B12 with PhenoAge acceleration.
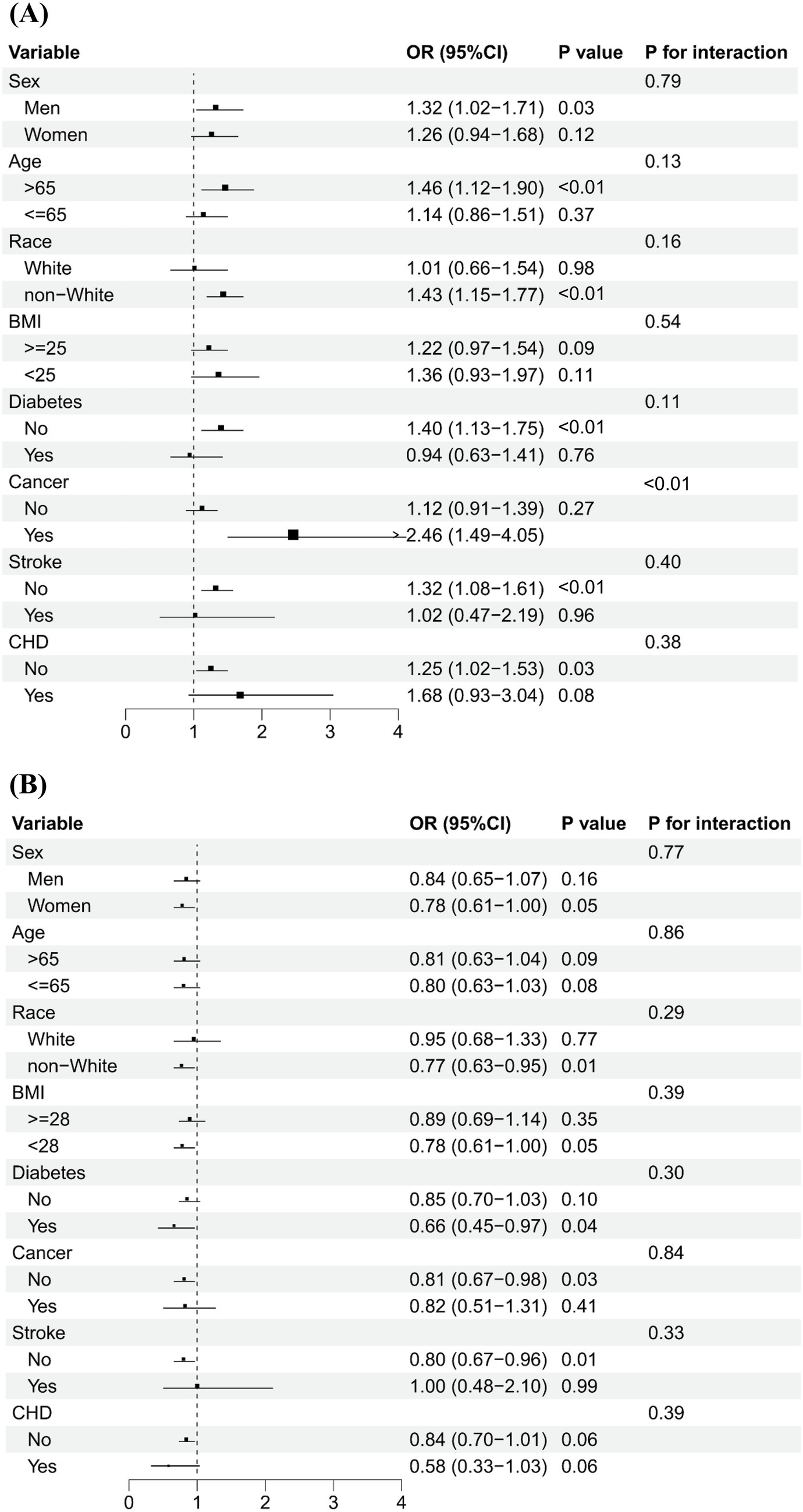
Figure 3. Subgroup analysis of the association of high levels of serum vitamins A and B12 with GrimAge acceleration.
In addition, the associations between serum levels of vitamins A and B12 and HorvathAge acceleration or HannumAge acceleration show similar results. Supplementary Table 1 shows that compared to the first quintile of vitamin A, the highest quintile was associated with higher risks of HorvathAge acceleration (OR = 1.44, 95% CI: 1.07–1.92) and HannumAge acceleration (OR = 1.36, 95% CI: 1.01–1.83). This study also showed no associations between vitamin B12 and HorvathAge acceleration or HannumAge acceleration. Supplementary Table 2 shows consistent associations between serum vitamin quartiles and epigenetic age acceleration in the quartile-based model. Compared to the quartile-based model, the quintile-based model yielded higher AIC values for the association between vitamin A and epigenetic age acceleration (PhenoAge Acceleration: 2,826.80 vs. 2,829.43; GrimAge Acceleration: 2,368.77 vs. 2,369.52), as well as for vitamin B12 (PhenoAge Acceleration: 2,835.98 vs. 2,831.86; GrimAge Acceleration: 2,366.98 vs. 2,366.00). Supplementary Figure 2 shows similar non-linear associations of vitamins A and B12 with HannumAge acceleration (p for non-linear = 0.04 and 0.02). Although no significant non-linear associations of those vitamins with HorvathAge acceleration (p for non-linear = 0.09 and 0.21 for vitamins A and B12, respectively), high vitamin A and low vitamin B12 concentrations may be associated with HorvathAge acceleration according to the plot of the generalized additive model.
4 Discussion
This study found significant non-linear associations between serum levels of vitamins A and B12 and accelerated epigenetic aging. Specifically, higher serum vitamin B12 concentrations (≥488.0 pg/mL) were associated with delayed PhenoAge acceleration. Conversely, higher serum vitamin A concentrations (≥71.5 μg/dL) were linked to accelerated PhenoAge, GrimAge, and HannumAge. Compared with the results of logistic regression between vitamins and biological aging, these results could inform recommendations for optimal serum vitamin concentrations to delay biological aging and have substantial public health implications.
4.1 Comparison with previous studies
Previous studies have primarily focused on the association of serum vitamins and mortality risk, as well as aging-related diseases. For example, Chen et al. (33) demonstrated that serum vitamin D deficiency was associated with an increased risk of dementia, while Xiao et al. (34) revealed an L-shaped relationship between vitamin D levels and mortality. However, few studies explored the association between serum vitamins and aging. We searched the PubMed database using the keywords “serum vitamin” and “aging” or “biological age” up to 29 May 2025 and found seven studies exploring the association between vitamins and aging (18–22, 35, 36). Nevertheless, only one study has reported the association of serum B12 levels with phenotypic aging measured by chemistry biomarkers. Although similar results were found, this study did not further investigate the non-linear association of serum B12 with phenotypic aging (35). Additionally, some studies have found an association between serum levels of vitamins A and B12 and age-related diseases, which partially supports our findings (37, 38). For example, one cohort study showed that serum levels of vitamin A were also positively associated with the development of CVD (38). Conversely, low serum vitamin B12 concentrations were associated with higher risks of age-related diseases, such as Alzheimer’s disease and metabolic syndrome (39, 40). Accelerated epigenetic aging provides a more comprehensive assessment of an individual’s overall health status than age-related diseases alone (41). This holistic evaluation allows for timely interventions and the potential to delay the onset of diseases (42).
Furthermore, the clinical reference ranges for vitamins A and B12 are typically above 24.08 μg/dL and 270.8 pg/mL, respectively. These references are disease-defined thresholds. However, optimal biological ranges and the prevention level for aging and age-related disease remain undefined. Therefore, this study further identified a non-linear association of serum levels of vitamin A with PhenoAge, GrimAge, and HannumAge acceleration, and of serum levels of vitamin B12 with PhenoAge acceleration. These results are partially consistent with previous studies (23, 43, 44). For instance, Min et al. (23) observed that serum levels of vitamin A < 30 μg/dL or A > 80 μg/dL indicate a higher risk of subsequent mortality. Liu et al. (24) reported that both low (<369.1 pg/mL) and high (≥506.1 pg/mL) serum levels of vitamin B12 were associated with increased CVD mortality risk in diabetes patients. A cross-sectional study showed that the relationship between circulating vitamin B12 and α-Klotho in American adults was inverted U-shaped, which is consistent with the present results (36). While previous studies indicate that serum levels of vitamins A and B12 may reduce premature mortality, they failed to identify protective concentration ranges for health outcomes. In contrast, the present study suggested that the potential optimal concentrations of serum levels of vitamins A and B12 to delay accelerated epigenetic aging were <71.5 μg/dL and ≥488.0 pg/mL, respectively.
4.2 Possible explanations
A previous study has shown that vitamin A induces epigenetic changes in monocytes (45). Moreover, excess serum levels of vitamin A could also increase the transport of retinol-binding protein 4 (RBP4), subsequently increasing the risk of CHD, stroke, metabolic syndrome, and cardiovascular risk factors, including triglycerides and hypertension (46–51). Conversely, previous studies found that vitamin B12 deficiency is positively associated with inflammatory factors [such as C-reactive protein (CRP) and interleukin 6 (IL-6)] (52, 53), and leads to metabolic syndrome onset and an increase in cardiovascular risk factors (40, 54). Furthermore, the α-Klotho (known for inhibiting cellular senescence) is a hallmark of aging (3), and it can decelerate the aging process in both animal and human studies (55). One study reported a positive relationship between serum levels of vitamin B12 (<1,020 pg/mL) and α-Klotho concentration (36). Thus, maintaining appropriate serum vitamins A and B12 concentrations may help mitigate accelerated epigenetic aging.
5 Study limitations
The present study had some limitations. First, the results of the association analysis between vitamins and the methylation clock are not entirely consistent, and there is no gold standard for measuring biological aging. Recent studies have demonstrated that PhenoAge can effectively predict age-related diseases and mortality in large populations (56). Second, despite adjusting for 11 potential confounders, the lack of adjustment for dietary and total energy intake may have attenuated the observed association toward the null. Third, the sample size may have been insufficient to detect subtle associations, and recall bias could have led to underestimation of the association. Fourth, because of the cross-sectional design, causal relationships cannot be established based on our findings. Further prospective studies are warranted to confirm and clarify this relationship. Fifth, due to a lack of genetic and multiomics data, further studies are warranted to explore the potential mechanism of vitamins A and B12 with epigenetic aging. Finally, although no significant interaction was observed between the vitamins and ethnicity on accelerated epigenetic aging, the generalizability of our findings to ethnic populations should be approached with caution due to potential representativeness limitations.
6 Conclusion
This study identified biphasic effects of serum levels of vitamins A and B12 on accelerated epigenetic aging. The findings suggest that higher vitamin A levels may be associated with an increased aging risk, whereas adequate vitamin B12 may offer protective benefits against epigenetic changes associated with aging. This study offers promising insights for developing preventive public health strategies and dietary recommendations to delay accelerated epigenetic aging; however, validation in large-scale clinical trials is necessary.
Data availability statement
The datasets presented in this article are not readily available because this study analyzed datasets that are publicly accessible. Requests to access the datasets should be directed to the NHANES repository. This data can be found at: https://www.cdc.gov/nchs/nhanes/.
Ethics statement
The studies involving humans were approved by National Center for Health Statistics Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ZM: Writing – original draft, Formal analysis. MA: Formal analysis, Methodology, Writing – review & editing. WG: Writing – review & editing, Methodology. XC: Methodology, Writing – review & editing, Validation. ML: Validation, Writing – review & editing, Methodology. JZ: Writing – review & editing. XD: Writing – review & editing. FL: Writing – review & editing, Validation, Methodology. QH: Methodology, Supervision, Writing – review & editing. MW: Supervision, Writing – review & editing. JMZ: Supervision, Writing – review & editing. CS: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Healthy Zhejiang One Million People Cohort (K-20230085).
Acknowledgments
We extend our gratitude to the NHANES administration for providing access to publicly available data, which has been instrumental in preparing this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1599205/full#supplementary-material
References
1. World Health Organization. Ageing and health. (2024). Available at: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (Accessed October 1, 2024).
2. Yoo, J, Kim, Y, Cho, ER, and Jee, SH. Biological age as a useful index to predict seventeen-year survival and mortality in koreans. BMC Geriatr. (2017) 17:7. doi: 10.1186/s12877-016-0407-y
3. López-Otín, C, Blasco, MA, Partridge, L, Serrano, M, and Kroemer, G. Hallmarks of aging: an expanding universe. Cell. (2023) 186:243–78. doi: 10.1016/j.cell.2022.11.001
4. Thomas, A, Belsky, DW, and Gu, Y. Healthy lifestyle behaviors and biological aging in the u. S. National health and nutrition examination surveys 1999-2018. J Gerontol A Biol Sci Med Sci. (2023) 78:1535–42. doi: 10.1093/gerona/glad082
5. Horvath, S, and Raj, K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. (2018) 19:371–84. doi: 10.1038/s41576-018-0004-3
6. Suárez-Pérez, A, Macias-Gómez, A, Fernández-Pérez, I, Vallverdú-Prats, M, Cuadrado-Godia, E, Giralt-Steinhauer, E, et al. Epigenetic age and long-term cancer risk following a stroke. Genome Med. (2024) 16:135. doi: 10.1186/s13073-024-01408-2
7. Boyer, K, Domingo-Relloso, A, Jiang, E, Haack, K, Goessler, W, Zhang, Y, et al. Metal mixtures and DNA methylation measures of biological aging in american indian populations. Environ Int. (2023) 178:108064. doi: 10.1016/j.envint.2023.108064
8. Belsky, DW, Caspi, A, Corcoran, DL, Sugden, K, Poulton, R, Arseneault, L, et al. Dunedinpace, a DNA methylation biomarker of the pace of aging. eLife. (2022) 11:e73420. doi: 10.7554/eLife.73420
9. Guo, Y, Ma, G, Wang, Y, Lin, T, Hu, Y, and Zang, T. Causal associations and shared genetic etiology of neurodegenerative diseases with epigenetic aging and human longevity. Aging Cell. (2024) 23:e14271. doi: 10.1111/acel.14271
10. Tanumihardjo, SA. Vitamin a: biomarkers of nutrition for development. Am J Clin Nutr. (2011) 94:658s–65s. doi: 10.3945/ajcn.110.005777
11. Larange, A, and Cheroutre, H. Retinoic acid and retinoic acid receptors as pleiotropic modulators of the immune system. Annu Rev Immunol. (2016) 34:369–94. doi: 10.1146/annurev-immunol-041015-055427
12. Bailey, RL, Fulgoni, VL 3rd, Keast, DR, and Dwyer, JT. Examination of vitamin intakes among us adults by dietary supplement use. J Acad Nutr Diet. (2012) 112:657–63. doi: 10.1016/j.jand.2012.01.026
13. Geisen, C, Denk, C, Gremm, B, Baust, C, Karger, A, Bollag, W, et al. High-level expression of the retinoic acid receptor beta gene in normal cells of the uterine cervix is regulated by the retinoic acid receptor alpha and is abnormally down-regulated in cervical carcinoma cells. Cancer Res. (1997) 57:1460–7. doi: 10.1006/viro.2000.0282
14. Osman, AA, Chin, SF, Teh, LK, Abdullah, N, Abdul Murad, NA, and Jamal, R. Lipids as key biomarkers in unravelling the pathophysiology of obesity-related metabolic dysregulation. Heliyon. (2025) 11:e42197. doi: 10.1016/j.heliyon.2025.e42197
15. Stabler, SP. Clinical practice. Vitamin B12 deficiency. N Engl J Med. (2013) 368:149–60. doi: 10.1056/NEJMcp1113996
16. Allen, LH. How common is vitamin B-12 deficiency? Am J Clin Nutr. (2009) 89:693s–6s. doi: 10.3945/ajcn.2008.26947A
17. Carmel, R. Subclinical cobalamin deficiency. Curr Opin Gastroenterol. (2012) 28:151–8. doi: 10.1097/MOG.0b013e3283505852
18. Liu, C, Hua, L, and Xin, Z. Synergistic impact of 25-hydroxyvitamin d concentrations and physical activity on delaying aging. Redox Biol. (2024) 73:103188. doi: 10.1016/j.redox.2024.103188
19. Zhou, J, Chen, H, Wang, Q, Chen, S, Wang, R, Wang, Z, et al. Sirt1 overexpression improves senescence-associated pulmonary fibrosis induced by vitamin d deficiency through downregulating il-11 transcription. Aging Cell. (2022) 21:e13680. doi: 10.1111/acel.13680
20. Dawoud, NM, Bakry, OA, Shoeib, MA, and Ismael, HN. Serum vitamin d and facial aging: is there a link? Skin Pharmacol Physiol. (2016) 29:76–82. doi: 10.1159/000443839
21. Strath, LJ, Meng, L, Rani, A, Sinha, P, Johnson, AJ, Huo, Z, et al. Accelerated epigenetic aging mediates the association between vitamin d levels and knee pain in community-dwelling individuals. J Nutr Health Aging. (2022) 26:318–23. doi: 10.1007/s12603-022-1758-z
22. Vetter, VM, Spira, D, Banszerus, VL, and Demuth, I. Epigenetic clock and leukocyte telomere length are associated with vitamin d status but not with functional assessments and frailty in the berlin aging study ii. J Gerontol A Biol Sci Med Sci. (2020) 75:2056–63. doi: 10.1093/gerona/glaa101
23. Min, KB, and Min, JY. Relation of serum vitamin a levels to all-cause and cause-specific mortality among older adults in the nhanes iii population. Nutr Metab cardiovasc Dis. (2014) 24:1197–203. doi: 10.1016/j.numecd.2014.06.004
24. Liu, Y, Geng, T, Wan, Z, Lu, Q, Zhang, X, Qiu, Z, et al. Associations of serum folate and vitamin b12 levels with cardiovascular disease mortality among patients with type 2 diabetes. JAMA Netw Open. (2022) 5:e2146124. doi: 10.1001/jamanetworkopen.2021.46124
25. Zipf, G, Chiappa, M, Porter, KS, Ostchega, Y, Lewis, BG, and Dostal, J. National health and nutrition examination survey: plan and operations, 1999–2010. Vital Health Stat. (2013) 1:1–37.
26. National Health and Nutrition Examination Survey (2024). NHANES 1999–2002 DNA methylation array and epigenetic biomarkers. Available at: https://wwwn.cdc.gov/nchs/nhanes/dnam (Accessed July 31, 2024).
27. Levine, ME, Lu, AT, Quach, A, Chen, BH, Assimes, TL, Bandinelli, S, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging. (2018) 10:573–91. doi: 10.18632/aging.101414
28. Lu, AT, Quach, A, Wilson, JG, Reiner, AP, Aviv, A, Raj, K, et al. DNA methylation grimage strongly predicts lifespan and healthspan. Aging. (2019) 11:303–27. doi: 10.18632/aging.101684
29. Li, A, Koch, Z, and Ideker, T. Epigenetic aging: biological age prediction and informing a mechanistic theory of aging. J Intern Med. (2022) 292:733–44. doi: 10.1111/joim.13533
30. Yang, L, Zhang, X, and Chen, J. Winsorization greatly reduces false positives by popular differential expression methods when analyzing human population samples. Genome Biol. (2024) 25:282. doi: 10.1186/s13059-024-03230-w
31. Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. (2013) 14:R115. doi: 10.1186/gb-2013-14-10-r115
32. Hannum, G, Guinney, J, Zhao, L, Zhang, L, Hughes, G, Sadda, S, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. (2013) 49:359–67. doi: 10.1016/j.molcel.2012.10.016
33. Chen, LJ, Sha, S, Stocker, H, Brenner, H, and Schöttker, B. The associations of serum vitamin d status and vitamin d supplements use with all-cause dementia, alzheimer's disease, and vascular dementia: a Uk biobank based prospective cohort study. Am J Clin Nutr. (2024) 119:1052–64. doi: 10.1016/j.ajcnut.2024.01.020
34. Xiao, Q, Cai, B, Yin, A, Huo, H, Lan, K, Zhou, G, et al. L-shaped association of serum 25-hydroxyvitamin d concentrations with cardiovascular and all-cause mortality in individuals with osteoarthritis: results from the nhanes database prospective cohort study. BMC Med. (2022) 20:308. doi: 10.1186/s12916-022-02510-1
35. Tang, F, Qiu, H, Liu, Y, Guo, J, Huang, Z, Fang, S, et al. Decreased cobalamin sensitivity and biological aging acceleration in the general population. J Nutr Health Aging. (2024) 28:100262. doi: 10.1016/j.jnha.2024.100262
36. Li, YS, Gong, XJ, Du, WJ, Li, Y, He, DY, Yao, J, et al. Inverted u-shaped relationship between serum vitamin b12 and α-klotho levels in us adults: a cross-sectional study. Front Nutr. (2024) 11:1473196. doi: 10.3389/fnut.2024.1473196
37. Chen, D, Wan, L, Chen, Z, Yuan, X, Liu, M, Tang, Z, et al. Serum vitamin levels in multiple system atrophy: a case-control study. Front Aging Neurosci. (2022) 14:1105019. doi: 10.3389/fnagi.2022.1105019
38. Olsen, T, and Blomhoff, R. Retinol, retinoic acid, and retinol-binding protein 4 are differentially associated with cardiovascular disease, type 2 diabetes, and obesity: an overview of human studies. Adv Nutr. (2020) 11:644–66. doi: 10.1093/advances/nmz131
39. Liu, XX, Wu, PF, Liu, YZ, Jiang, YL, Wan, MD, Xiao, XW, et al. Association between serum vitamins and the risk of alzheimer's disease in chinese population. J Alzheimers Dis. (2022) 85:829–36. doi: 10.3233/JAD-215104
40. Zhu, J, Chen, C, Lu, L, Shikany, JM, D'Alton, ME, and Kahe, K. Folate, vitamin B6, and vitamin B12 status in association with metabolic syndrome incidence. JAMA Netw Open. (2023) 6:e2250621. doi: 10.1001/jamanetworkopen.2022.50621
41. You, Y, Chen, Y, Wang, X, Wei, M, Zhang, Q, and Cao, Q. Accelerometer-measured physical activity patterns are associated with phenotypic age: Isotemporal substitution effects. Heliyon. (2023) 9:e19158. doi: 10.1016/j.heliyon.2023.e19158
42. Chen, L, Wu, B, Mo, L, Chen, H, Zhao, Y, Tan, T, et al. Associations between biological ageing and the risk of, genetic susceptibility to, and life expectancy associated with rheumatoid arthritis: a secondary analysis of two observational studies. Lancet Healthy Longevity. (2024) 5:e45–55. doi: 10.1016/S2666-7568(23)00220-9
43. Xu, K, Liu, X, Liu, J, Zhang, Y, Ding, X, Li, L, et al. Association between serum vitamin b12 and risk of all-cause mortality in elderly adults: a prospective cohort study. BMC Geriatr. (2021) 21:497. doi: 10.1186/s12877-021-02443-z
44. Wolffenbuttel, BHR, Heiner-Fokkema, MR, Green, R, and Gans, ROB. Relationship between serum B12 concentrations and mortality: experience in NHANES. BMC Med. (2020) 18:307. doi: 10.1186/s12916-020-01771-y
45. Arts, RJ, Blok, BA, van Crevel, R, Joosten, LA, Aaby, P, Benn, CS, et al. Vitamin a induces inhibitory histone methylation modifications and down-regulates trained immunity in human monocytes. J Leukoc Biol. (2015) 98:129–36. doi: 10.1189/jlb.6AB0914-416R
46. Newcomer, ME, and Ong, DE. Plasma retinol binding protein: structure and function of the prototypic lipocalin. Biochim Biophys Acta. (2000) 1482:57–64. doi: 10.1016/S0167-4838(00)00150-3
47. Ingelsson, E, Sundström, J, Melhus, H, Michaëlsson, K, Berne, C, Vasan, RS, et al. Circulating retinol-binding protein 4, cardiovascular risk factors and prevalent cardiovascular disease in elderly. Atherosclerosis. (2009) 206:239–44. doi: 10.1016/j.atherosclerosis.2009.02.029
48. Mallat, Z, Simon, T, Benessiano, J, Clément, K, Taleb, S, Wareham, NJ, et al. Retinol-binding protein 4 and prediction of incident coronary events in healthy men and women. J Clin Endocrinol Metab. (2009) 94:255–60. doi: 10.1210/jc.2008-0253
49. Cabré, A, Lázaro, I, Girona, J, Manzanares, J, Marimón, F, Plana, N, et al. Retinol-binding protein 4 as a plasma biomarker of renal dysfunction and cardiovascular disease in type 2 diabetes. J Intern Med. (2007) 262:496–503. doi: 10.1111/j.1365-2796.2007.01849.x
50. Bobbert, T, Raila, J, Schwarz, F, Mai, K, Henze, A, Pfeiffer, AF, et al. Relation between retinol, retinol-binding protein 4, transthyretin and carotid intima media thickness. Atherosclerosis. (2010) 213:549–51. doi: 10.1016/j.atherosclerosis.2010.07.063
51. Alkharfy, KM, Al-Daghri, NM, Vanhoutte, PM, Krishnaswamy, S, and Xu, A. Serum retinol-binding protein 4 as a marker for cardiovascular disease in women. PLoS One. (2012) 7:e48612. doi: 10.1371/journal.pone.0048612
52. Domínguez-López, I, Kovatcheva, M, Casas, R, Toledo, E, Fitó, M, Ros, E, et al. Higher circulating vitamin b12 is associated with lower levels of inflammatory markers in individuals at high cardiovascular risk and in naturally aged mice. J Sci Food Agric. (2024) 104:875–82. doi: 10.1002/jsfa.12976
53. Mahalle, N, Kulkarni, MV, Garg, MK, and Naik, SS. Vitamin b12 deficiency and hyperhomocysteinemia as correlates of cardiovascular risk factors in indian subjects with coronary artery disease. J Cardiol. (2013) 61:289–94. doi: 10.1016/j.jjcc.2012.11.009
54. Guarnizo-Poma, M, Urrunaga-Pastor, D, Montero-Suyo, C, Lazaro-Alcantara, H, Paico-Palacios, S, Pantoja-Torres, B, et al. Association between serum vitamin b12 levels and metabolic syndrome in a euthyroid population. Diabetes Met Syndr. (2018) 12:943–8. doi: 10.1016/j.dsx.2018.05.022
55. Xu, Y, and Sun, Z. Molecular basis of klotho: from gene to function in aging. Endocr Rev. (2015) 36:174–93. doi: 10.1210/er.2013-1079
Keywords: accelerated epigenetic aging, biological aging, vitamin A, vitamin B12, non-linear association
Citation: Ma Z, An M, Gong W, Chen X, Liang M, Zhang J, Du X, Lu F, He Q, Wang M, Zhong J and Sun C (2025) Non-linear association between serum levels of vitamins A and B12 and accelerated epigenetic aging. Front. Nutr. 12:1599205. doi: 10.3389/fnut.2025.1599205
Edited by:
Laura Bordoni, University of Camerino, ItalyReviewed by:
Shooka Mohammadi, University of Malaya, MalaysiaDaniel Austin, Lake Erie College of Osteopathic Medicine, United States
Radhika Kumar, University of Southern California, United States
Copyright © 2025 Ma, An, Gong, Chen, Liang, Zhang, Du, Lu, He, Wang, Zhong and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meng Wang, bXdhbmdAY2RjLnpqLmNu; Jieming Zhong, am16aG9uZ0BjZGMuemouY24=; Ce Sun, c3VuYzVAbWFpbDIuc3lzdS5lZHUuY24=
 Zhimin Ma
Zhimin Ma Mingxing An2
Mingxing An2 Weiwei Gong
Weiwei Gong Xiangyu Chen
Xiangyu Chen Qingfang He
Qingfang He Meng Wang
Meng Wang Ce Sun
Ce Sun