- 1Gulbali Institute, School of Agricultural, Environmental, and Veterinary Sciences, Charles Sturt University, Wagga Wagga, NSW, Australia
- 2Department of Anatomy and Histology, Faculty of Veterinary Medicine and Animal Science, Gazipur Agricultural University (formerly Bangabandhu Sheikh Mujibur Rahman Agricultural University), Gazipur, Bangladesh
- 3Nutrition Research Institute, Junlebao Dairy Group Co., Ltd, Shijiazhuang, China
Human milk oligosaccharides (HMOs), a diverse group of complex sugars, are increasingly recognized for their health advantages for infants. These bioactive molecules are believed to be critical in shaping gut microbiota, infant immunity, and overall health. Recent clinical studies have focused on supplementation of infant formulas with manufactured HMOs to replicate some of the benefits observed in breastfed infants. This review aims to summarize the latest evidence from human clinical trials on manufactured HMO supplementation, highlighting its associated health benefits and the underlying mechanisms of action. A comprehensive literature search was conducted using PubMed, Medline, and Scopus databases from 1964 to 2024, identifying clinical intervention studies on manufactured HMOs across different populations, ranging from pre-term infants to adults with or without medical conditions. Findings reveal that manufactured HMOs are safe, well-tolerated, and show promising benefits for immune health and gut microbiota composition, closely mirroring the effects of natural HMOs found in breast milk. Although studies have explored the prebiotic role of HMOs in modulating neuroactive metabolites such as short-chain fatty acids (SCFAs) produced by gut microbiota, there is a notable lack of research directly evaluating the cognitive outcomes of HMOs using MRI or standardized developmental assessment tools. Furthermore, this review highlights two novel clinical findings: the potential therapeutic role of HMOs in obesity prevention by promoting fat loss while preserving muscle mass and their beneficial effects in osteoarthritis by reducing pain and enhancing mobility. However, the variability in dosage, participant groups, intervention duration, and outcomes, along with the limited studies on the mechanistic pathways of HMOs, makes it difficult to draw definitive conclusions, underscoring the need for well-designed clinical trials across diverse health conditions to better understand the full potential of HMO supplementation.
Introduction
Human milk oligosaccharides (HMOs) are key complex carbohydrates of breast milk, ranking as the third most abundant solid constituent after lactose and lipids (1, 2). Their concentration ranges from 12.9 g/L in mature milk to 20.9 g/L in early postpartum milk (3). This represents a ~ 100-fold higher concentration than what was found in bovine milk or infant formulas (4, 5). Over 200 distinct types of HMOs have been characterized, with approximately 70–75% being neutral HMOs, which include both fucosylated and non-fucosylated, and 10–30% being sialylated HMOs, distinguished by the presence of a sialic acid molecule at the terminal position (4, 5). HMOs play a crucial role in infant health, although they are not directly digestible by humans. Instead, they serve as prebiotics, fostering the growth of beneficial bacteria, particularly Bifidobacteria, in the infant’s gut, modulating the immune system, and supporting infant growth and cognitive development.
One of the major compositional differences between breast milk and infant formula is the presence of HMOs (6). Recent advancements in biotechnology have made it possible to produce HMOs identical to those found in human milk through bacterial fermentation (7, 8) and enzymatic conversion (9). As a result, specific HMOs such as 2′-fucosyllactose (2’-FL), 3′-fucosyllactose (3’-FL), Lacto-N-neotetraose (LNnT), 3’-Sialyllactose (3’-SL), and 6’-Sialyllactose (6’-SL) are now commercially available and being incorporated into infant formulas to narrow this gap (10, 11). While observational studies have provided valuable insights into the role of HMOs, a growing number of prospective human clinical trials are now focusing on the efficacy, safety, and health benefits of manufactured HMOs in various populations. These trials aim to assess the impact of HMOs in clinical settings, particularly in infant nutrition, gut microbiota composition, immune support, and disease prevention.
This review critically evaluates current evidence from clinical trials that specifically investigate the impact of manufactured HMOs on clinical applications across diverse populations ranging from pre-term infants to adults with or without medical conditions, excluding observational studies. It highlights key findings on the clinical applications of HMOs in infant nutrition, immune support, neurodevelopment, and disease prevention. Additionally, the review explores potential mechanisms of action identified in these clinical studies, addresses significant knowledge gaps, and outlines directions for future research.
Methods
A systematic literature search was performed in the PubMed, Medline, and Scopus electronic databases up to December 2024 without any restriction on the publication date. The search terms included “human milk oligosaccharide” AND “clinical trial”; “human milk oligosaccharide” AND “clinical intervention”; “human milk oligosaccharide” AND “infant formula.” Only original studies published in English were considered. Additionally, reference lists from primary articles and related reviews were examined to identify other studies for potential inclusion. The complete search strategy is shown in detail in Table 1. In brief, a total of 706 articles were initially retrieved. Following the elimination of duplicates (287) and the application of exclusion and inclusion criteria, a total of 38 articles were ultimately selected for this review. A summary of these studies including study design, HMO intervention, population range, doses, and outcomes, are presented in Figure 1, with further details outlined in Tables 2–4.
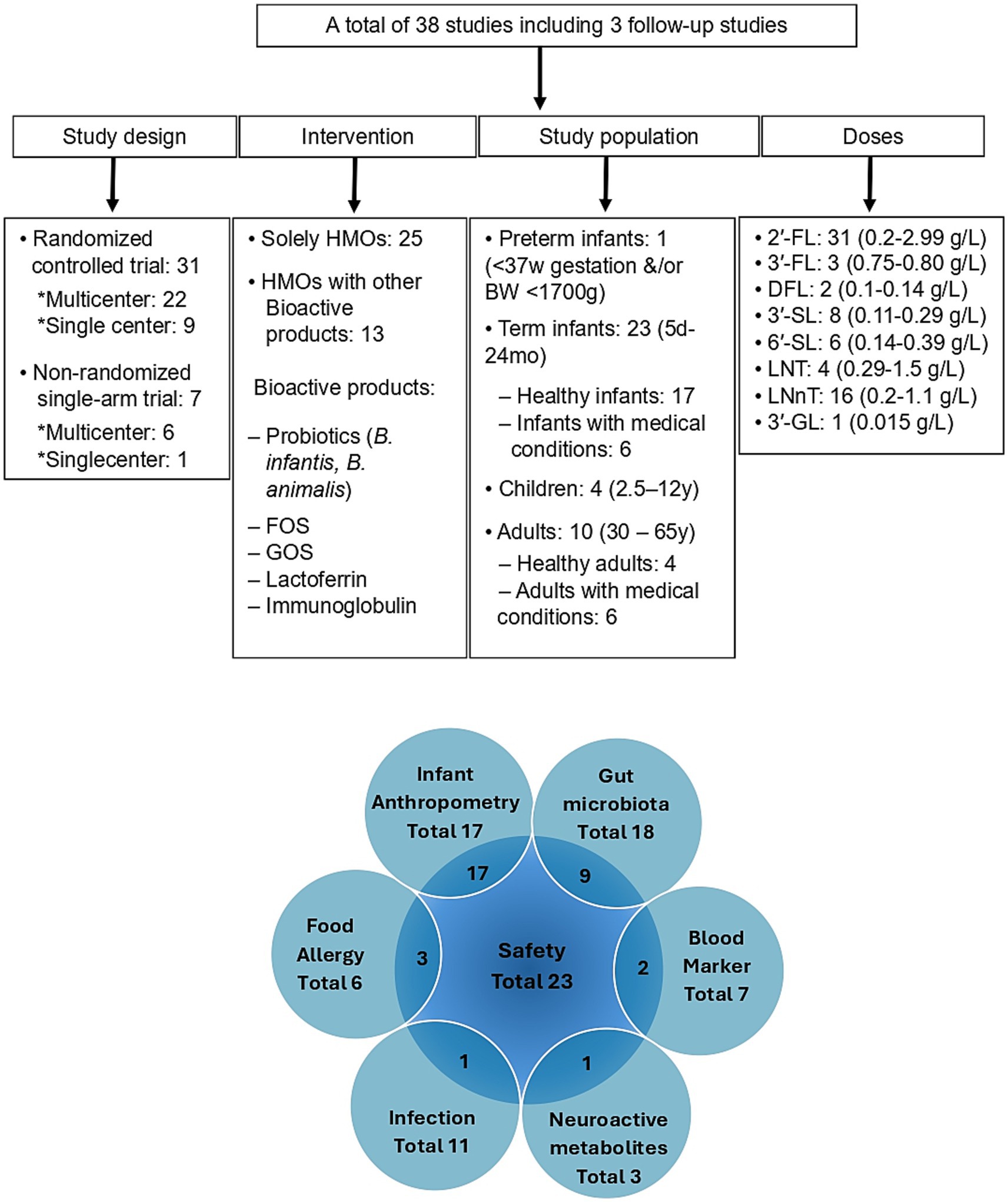
Figure 1. Summary of clinical intervention studies investigating the health effects of manufactured human milk oligosaccharides (HMOs), focusing on study population characteristics, administered dose ranges, and evaluated clinical endpoints. Children’s medical conditions are CMPA, food protein allergy/sensitivity, and feeding intolerance symptoms. Adult medical conditions are Ulcerative colitis, Helicobacter pylori, IBS, diabetes, obesity, and osteoarthritis. HMOs, Human milk oligosaccharides; 2’-FL, 2’-Fucosyllactose; 3’-FL, 3’-Fucosyllactose; DFL, Difucosyllactose; 3’-SL, 3’-Sialyllactose; 6’-SL, 6’-Sialyllactose; LNT, Lacto-N-tetraose; LNnT, Lacto-N-neotetraose; 3′-GL, 3′-Galactosyllactose; FOS, Fructo-oligosaccharides; GOS, Galacto-oligosaccharides; SCFAs, Short chain fatty acids; B, BW, Body weight; CMPA, Cow’s milk protein allergy; W, Week; mo, Month; Y, Year; d, Day.
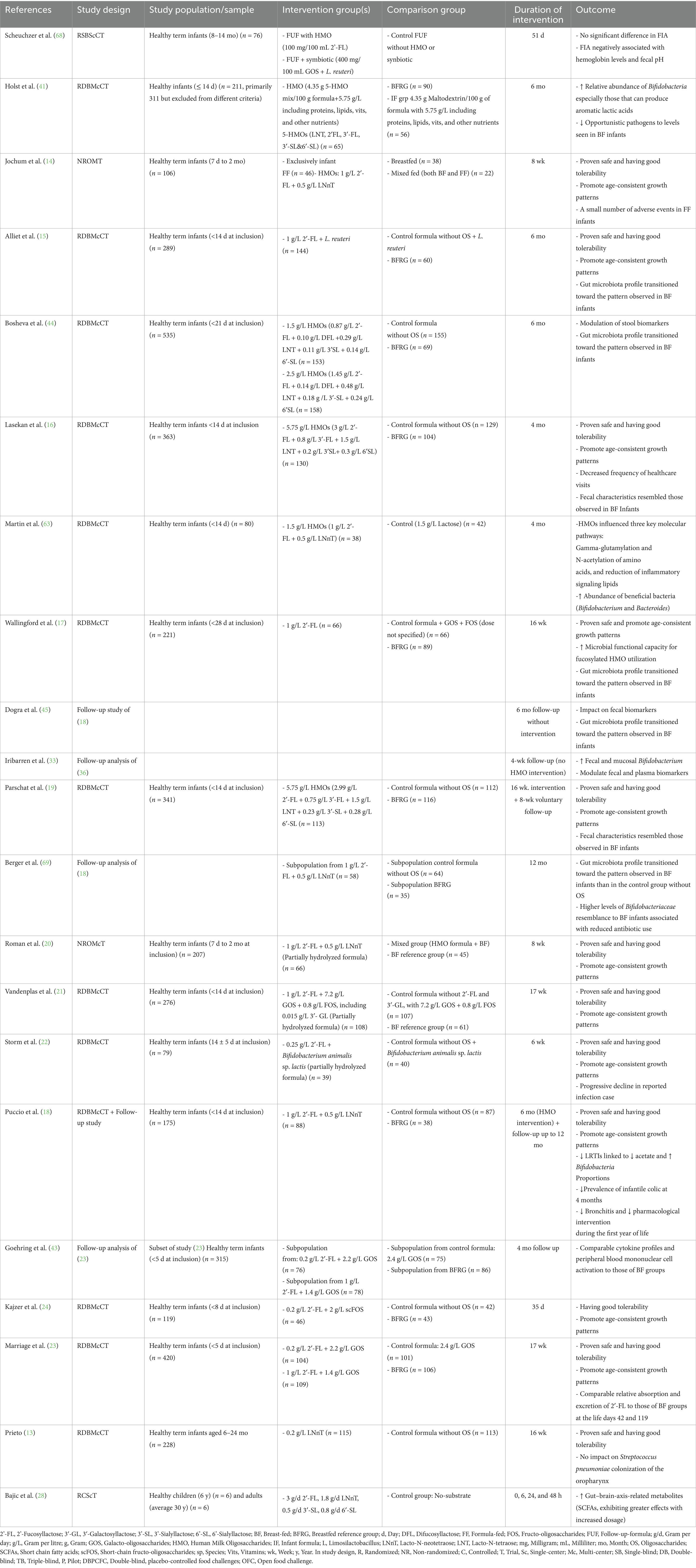
Table 2. Clinical studies investigating the effect of manufactured HMOs on healthy infants and children.
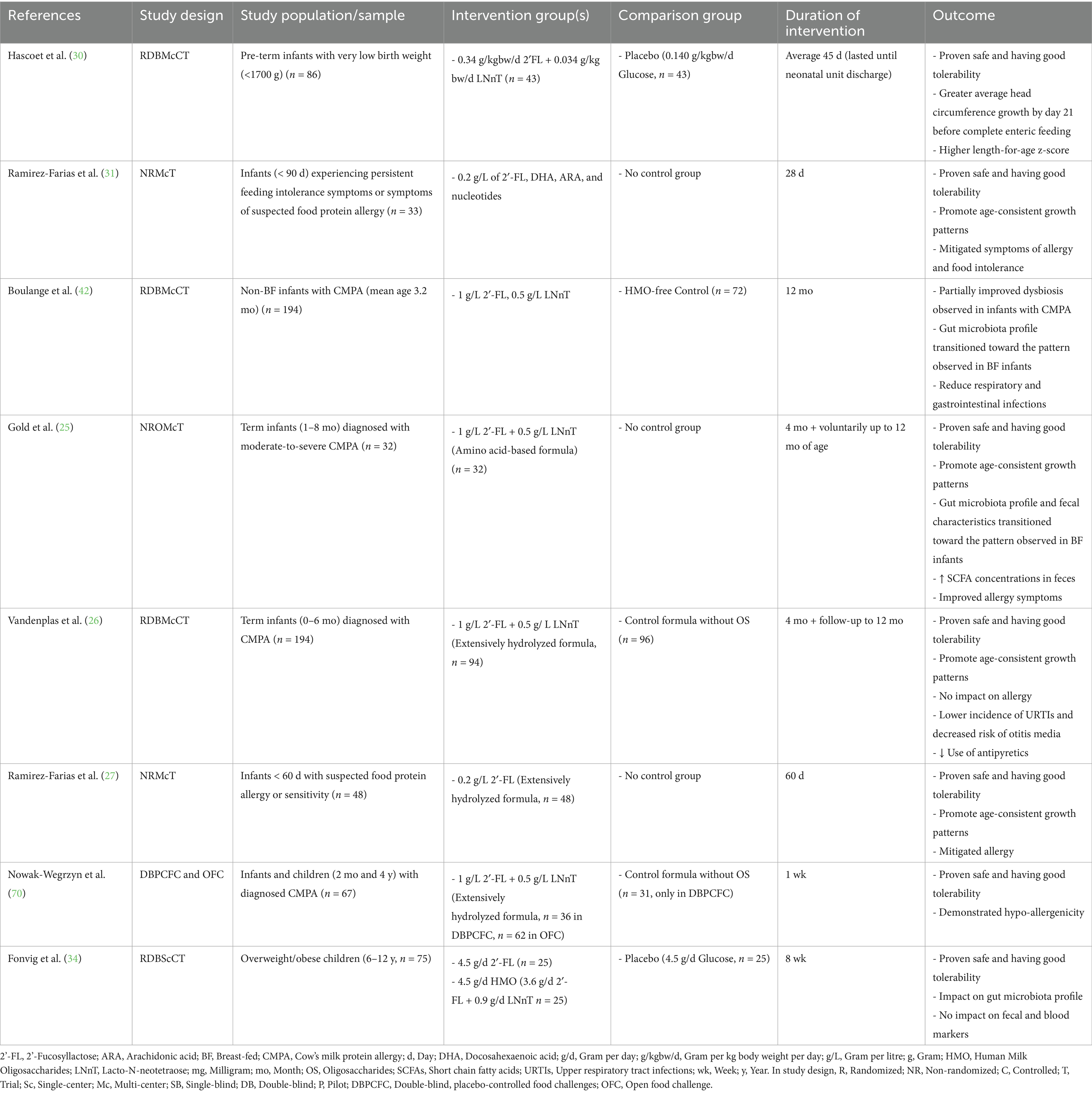
Table 3. Clinical studies investigating the effect of manufactured HMOs on pre-term infants, full-term infants, and children with medical conditions.
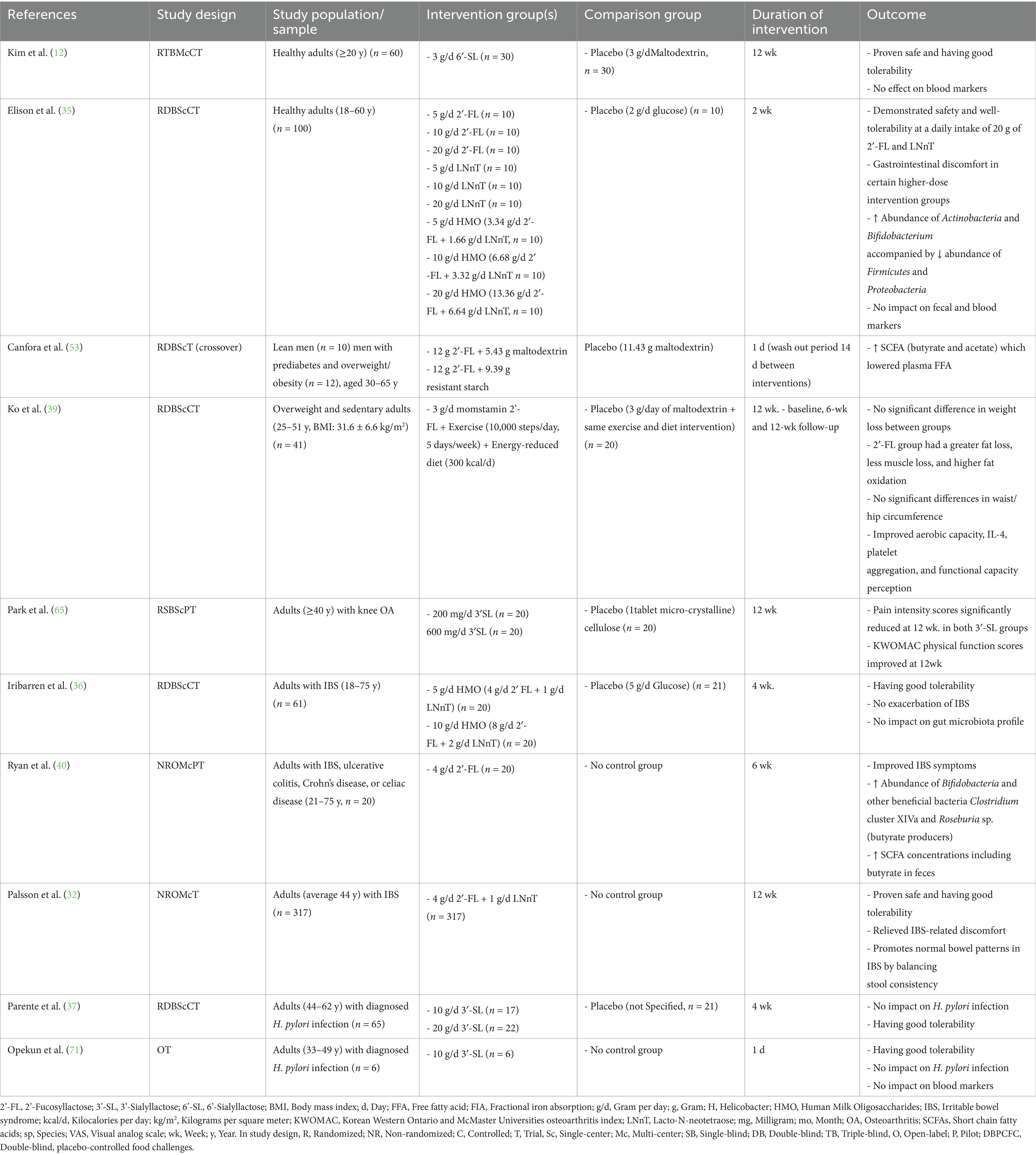
Table 4. Clinical studies investigating the effect of manufactured HMOs on adults with or without medical conditions.
Safety assessment of manufactured HMO intervention
The primary safety endpoints assessed in these clinical trials are as follows:
• Gastrointestinal tolerance: incidence of diarrhea, constipation, bloating, gas, or vomiting.
• Allergic reactions: monitoring hypersensitivity, rashes, or anaphylaxis.
• Metabolic effects: blood glucose levels, lipid profiles, and metabolic markers.
• Immune response: biomarkers of inflammation and immune activation.
• Microbiota composition: changes in gut microbial diversity and balance.
Most of the reviewed studies do not explicitly report structural verification of HMOs (e.g., 2’-FL and 6’-SL) to ensure accurate manufacturing. Additionally, detailed source verification, such as confirming the absence of microbial contaminants and DNA residues for microbial fermentation-derived HMOs or chemical contaminants for enzymatically synthesized HMOs is often missing. Addressing these points is critical for ensuring product safety and consistency. However, a few studies have incorporated these quality control measures. For instance, Kim et al. (12) verified the identity of enzymatically synthesized 6′-SL by confirming a purity of 98.8% and the presence of Sia content. The source was validated as non-pathogenic E. coli, with assurances of safety through the absence of microbial contaminants (12). Similarly, Prieto (13) described rigorous production and purification methods for LNnT, employing mass spectrometry and chromatography to eliminate contaminants. These studies highlight the importance of integrating robust quality control measures into HMO research.
The safety of HMOs has been evaluated across diverse populations, including pre-term and term infants, children, and adults, with or without medical conditions or food allergies. The safety of HMOs has primarily focused on three key aspects: growth measures, adverse events (AEs), and gastrointestinal tolerance. Growth measures were assessed using anthropometric parameters to evaluate the nutritional adequacy of HMOs (14–29). These measures provide insights into whether HMO supplementation supports normal growth and development, particularly in infants. AEs were recorded through validated questionnaires completed by parents or caregivers and medical confirmation provided by physicians (15–17, 19, 21, 25, 29–32). These AEs included infections, such as gastrointestinal, respiratory tract, and ear infections, allergic reactions like skin rashes and hypersensitivity responses, and general medical conditions such as pyrexia and other mild to moderate illnesses. AEs were classified based on severity (mild, moderate, or severe). The details of growth measures and AEs are elaborated separately under the sections “Infant Anthropometric Parameters” and “Infection” in this article.
Gastrointestinal tolerance was assessed by using standardized tools, including the Gastrointestinal Symptom Rating Scale (GSRS) (33, 34), Gastrointestinal Symptom Questionnaire (GISQ) (22), and the Bristol Stool Form Scale (BSFS) (34). Monitored symptoms included diarrhea, vomiting, gastric residual volumes, flatulence, and stool consistency and frequency. Dose–response studies established safe dosage levels for pre-term infants to term infants irrespective of their medical conditions. For pre-term infants, a daily dose of 0.35 g/kg body weight of 2’-FL and LNnT in a 10:1 ratio was established as safe (30). However, in healthy term infants, the combination of 1 g/L 2’-FL and 0.5 g/L LNnT demonstrated safety and tolerability (21–23, 27). In HMO blends, a combination of five HMOs, including neutral, sialylated, and fucosylated oligosaccharides, was found to be safe at 5.75 g/L (19). Furthermore, in children with or without medical conditions such as cow’s milk protein allergy (CMPA) or other food allergies, a 4.5 g/L dose of 2’-FL, in a 4:1 ratio with LNnT, demonstrated excellent tolerability (25, 26, 29, 34).
In healthy adults, higher doses of HMOs have been investigated, revealing nuanced gastrointestinal effects. A daily intake of 20 g of 2’-FL led to increased abdominal rumbling, while the same dose of LNnT resulted in harder stools. However, a mixture of 2’-FL and LNnT showed better tolerability than the individual oligosaccharides at these high doses (35). In patients with irritable bowel syndrome (IBS), daily intake of a 4:1 mix of 2′-FL and LNnT at doses of 5 g or 10 g over 4 weeks significantly increased Bifidobacterium spp. abundance, modulated fecal and plasma metabolite profiles, and improved IBS symptoms—all without inducing adverse immune or mucosal responses—supporting a favorable safety profile (33, 36). Complementing this, a more extensive 12-week open-label study involving 317 IBS patients confirmed that daily supplementation with 5 g of the same 4:1 mix of 2′FL and LNnT improved bowel function, reduced IBS symptom severity, and enhanced quality of life, with only mild gastrointestinal discomforts reported as side effects (32). Additionally, in a placebo-controlled trial among dyspeptic adults, daily administration of 3-′SL at 10 g or 20 g was well tolerated. However, it did not eradicate Helicobacter pylori infection, indicating that even high doses of isolated HMOs can be safely consumed in humans (37). Collectively, these findings support the safety and tolerability of manufactured HMOs across diverse populations and clinical contexts while also underscoring the need for standardized quality control to ensure consistent formulation. Consequently, the optimal HMO dose and composition may need to be tailored to the target population and the intended physiological or clinical outcome.
Functional impacts of HMOs
Anthropometric parameters
The nutritional adequacy of HMOs has been widely evaluated through their impact on growth and development, as measured by weight, length, head circumference, waist/hip circumference, and body mass index (BMI). In pre-term infants, HMO-enriched formulas containing 2′-FL and LNnT have demonstrated a faster transition to full enteral feeding compared to placebo, with no adverse effects reported. Growth outcomes in these infants were comparable to standard feeding practices, with increased length-for-age z-scores, indicating a positive role in postnatal growth and development (30). In healthy-term infants, studies adhering to World Health Organization (WHO) growth standards have compared weight-for-age and length-for-age z-scores, across HMO-enriched formulas, breastfeeding, and standard formula groups (14–29). The results demonstrated that infants consuming HMO-enriched formula exhibited non-inferior growth compared to the breastfed reference group. Furthermore, growth outcomes in the HMO-formula group were superior to those observed in infants fed standard formula. Moreover, in infants with dietary sensitivities—such as persistent feeding intolerance or suspected food protein allergies—a 28-day intervention with hydrolyzed rice infant formula (HRF) enriched with 2′-FL resulted in statistically significant improvements in feeding tolerance (31). Head circumference, a critical neurodevelopmental marker, has also been examined in several clinical studies (14, 17, 19, 31). Parshat et al. (19) demonstrated that an infant formula containing a mix of five HMOs (5HMO-Mix), including sialylated HMOs, supported head circumference growth within the normal range, showing non-inferiority compared to breastfed infants. While this finding does not establish a direct link, it suggests a potential role of sialylated HMOs, such as 3′-SL and 6′-SL, in contributing to early neurodevelopment, possibly through the provision of sialic acid (Sia), a key nutrient for brain growth (38). Additionally, a recent placebo-controlled study investigated the effects of 2′-FL supplementation (3 g/day) combined with a calorie-reduced diet and structured exercise regimen on body composition over 12 weeks in overweight adults (39). While the 2′-FL group showed improvements such as fat mass reduction, better fat oxidation, and loss of lean mass compared to baseline, these effects were not significantly different from those in the placebo group when adjusted for group-by-time interactions. Moreover, an increase in work-related physical activity was observed, which may have contributed to changes in body composition independent of the intervention. Although the results suggest a potential role for 2′-FL in modulating metabolic health markers, these findings should be interpreted cautiously due to the study’s modest sample size, non-placebo-adjusted primary outcomes, and variability in lifestyle behaviors. However, clinical trials assessing the direct effects of HMOs, particularly 2′-FL, on infant body composition and fat regulation remain limited. Furthermore, evidence gaps remain regarding the effects of specific HMOs in low-resource settings, where challenges such as postnatal linear growth faltering, undernutrition, and stunting persist. Clinical studies investigating the effects of manufactured HMOs on healthy infants and children, as well as those with medical conditions, are shown in Tables 2, 3.
Infections
As bioactive constituents of human milk, HMOs provide both direct and indirect protection against infections caused by pathogenic microorganisms. Puccio et al. (18) reported that healthy infants consuming a formula with 1.0 g/L 2′-FL and 0.5 g/L LNnT for 6 months experienced significantly fewer parental reports of bronchitis and lower respiratory tract infections (LRTIs) (18). A marked reduction in the use of antibiotics and antipyretics further suggests potential respiratory health benefits (18). Similarly, Vandenplas et al. (26) demonstrated that infants with CMPA receiving an extensively hydrolyzed formula (EHF) supplemented with these HMOs for 4 months showed a statistically significant reduction in the frequency of upper respiratory tract infections (URTIs) and a lower incidence of ear infections at 12 months. Additionally, the relative risk of LRTIs and gastrointestinal infections was reduced by 30–40%, underscoring the broad protective effects of HMO supplementation in vulnerable populations.
Leung et al. (29), however, observed that 2′-FL supplementations in healthy Chinese children aged 1–2.5 years over 6 months extended the duration of URTIs in some cases, suggesting that HMO effects may vary depending on demographic or health context (29). In contrast, Prieto (13) reported no significant effect of LNnT supplementation on oropharyngeal colonization with Streptococcus pneumoniae in children aged 6–24 months in Chile following a 16-week intervention. Beyond respiratory health, HMOs have been investigated for their potential benefits in gastrointestinal disorders (32, 40). Palsson et al. (32) found that a 5-g intervention with HMOs (2′-FL and LNnT in a 4:1 ratio) over 12 weeks significantly improved bowel movement consistency in patients with IBS, particularly within the first 4 weeks. Similarly, Ryan et al. (40) observed improvements in both intestinal and extra-intestinal symptoms, alongside bifidogenic and butyrogenic effects, in IBS and ulcerative colitis patients consuming 2′-FL-enriched nutritional formulas.
While HMOs appear beneficial across multiple domains, their role in urinary tract infections (UTIs) remains unclear. Irribaren et al. (33) detected 2′-FL in urine, but no clinical findings have confirmed its role in preventing UTIs by inhibiting bacterial adhesion to the urothelial lining. Collectively, these findings have demonstrated that supplementation with specific HMOs can reduce the incidence of infections in infants and adults. However, mechanistic insights remain limited or inconsistent across trials. Proposed pathways include the direct inhibition of pathogen adhesion to epithelial surfaces by acting as decoy receptors (35, 41) as well as the modulation of gut microbiota composition (15, 42)—particularly the enrichment of Bifidobacterium spp. and strengthening of the intestinal epithelial barrier. Additionally, HMOs are thought to influence immune responses through the regulation of cytokine production and enhancement of mucosal immunity (34, 43). Despite these promising leads, most clinical trials have not concurrently measured molecular markers of these mechanisms, limiting the ability to establish a causal relationship. Therefore, future research should integrate mechanistic endpoints with clinical outcomes to better elucidate how HMOs exert their protective effects against infections.
Gut microbiota and benefits
HMOs significantly influence the composition, function, and diversity of the gut microbiota, particularly during early life. Microbiome diversity is assessed by evaluating α-diversity (richness and Shannon index) and β-diversity (weighted UniFrac distances). α-diversity indicates the overall diversity within the gut microbiome of individual infants, while β-diversity highlights differences in microbiome composition between groups. Supplementation of infant formula with five HMOs [2’-FL, 3’-FL, Lacto-N-tetraose (LNT), 3’-SL, and 6’-SL] at a total natural concentration of 5.75 g/L induced distinct shifts in β-diversity, aligning the microbial composition more closely with that of breastfed infants (41). Similarly, Fonvig et al. (34) observed a significant increase in α-diversity after 8 weeks of supplementation with a mix of 2′-FL and LNT in infants. In contrast, no significant changes were observed in the placebo group or those receiving only 2’-FL at a dose of 4.5 g/day (34). While lower doses of 2’-FL or LNnT alone did not influence fecal alpha diversity, higher doses (10 g of 2’-FL /LNnT) modulated fecal microbial β diversity profiles (33).
This clinical evidence supports the role of HMOs as prebiotics, preferentially stimulating the growth and activity of beneficial bacteria such as Bifidobacterium, Bacteroides, and helicon strains in infants and children (15, 17, 19, 25, 42, 44, 45). A diverse and abundant bifidobacterial community during early life is associated with positive extended health outcomes (46). In contrast, reduced abundance and diversity of Bifidobacteria have been linked to medical conditions such as allergies (47), dermatitis (48, 49), and pediatric obesity (50). Fonvig et al. (34) demonstrated that infant formula supplemented with 2′-FL and LNnT significantly increased Bifidobacteria abundance in overweight children after 4 and 8 weeks. In addition, an amino acid-based formula (AAF) supplemented with these two HMOs significantly enriched HMO-utilizing Bifidobacteria and reduced the abundance of fecal Proteobacteria in infants with CMPA. These findings suggest that the HMO-supplemented formula may help to correct gut microbial dysbiosis in CMPA infants (25).
Beyond promoting beneficial microbes, HMOs act as decoy receptors, preventing pathogenic bacteria, such as Escherichia coli and Clostridium difficile, from adhering to the intestinal epithelium (35, 41). Elison et al. (35) further demonstrated that HMO supplementation in adults led to substantial increases in Actinobacteria and Bifidobacterium while reducing Firmicutes and Proteobacteria, reinforcing their bifidogenic effects.
Blood and fecal markers
Blood marker: HMOs play a pivotal role in modulating immune function and gut health. Plasma cytokine concentrations and the composition of major lymphocyte subsets within peripheral blood mononuclear cells (PBMCs) serve as key biomarkers of immune functions. Goehring et al. (51) provided the first direct evidence that 2′-fucosyllactose (2′-FL) is present in the systemic circulation of both breastfed infants and those fed formula supplemented with 2′-FL, with plasma concentrations ranging from 0.1 to 4.0 mg/L, as measured using a validated liquid chromatography–mass spectrometry (LC–MS) method (51). Although human milk oligosaccharides (HMOs) are generally considered non-digestible and are thought to reach the colon intact, these findings suggest that a fraction of HMOs—particularly smaller, neutrally charged structures like 2′-FL—may be absorbed in the small intestine and transported into the bloodstream. The authors propose that this absorption may occur via paracellular pathways, such as through tight junctions between intestinal epithelial cells (51). Similarly, Irribaren et al. (33) identified the presence of 2’-FL in plasma, highlighting its systemic absorption and implying potential roles in immune modulation. However, further studies are needed to elucidate the metabolic fate of 2′-FL and confirm these findings. The presence of 2′-FL in circulation has been associated with reduced plasma concentrations of inflammatory cytokines in both breastfed infants and those receiving 2′-FL-enriched formula. For example, Goehring et al. (43) reported that infants fed 2’-FL-enriched formula exhibited significantly lower plasma levels of IL-1α, IL-1β, IL-6, and TNF-α compared to control groups, reflecting innate cytokine profiles more closely resembling those of breastfed infants. Notably, a higher concentration of 2′-FL (1 g/L) did not demonstrate greater efficacy than the lower dose (0.2 g/L) in altering cytokine profiles. The potential mechanisms underlying these anti-inflammatory effects induced by 2′-FL may involve Bifidobacteria, which utilize HMOs as their sole carbon source and suppress inflammatory gene expression in colonic epithelial cells (52). Furthermore, 2’-FL supplementation has been shown to narrow the differences in adaptive immune parameters, including the proportions of total T lymphocytes (helper and cytotoxic T cells) and the percentages of apoptotic cells, particularly CD8 + T cells, among infants who are breastfed and those who are formula-fed (43). This modulation of apoptosis—a crucial process for cellular homeostasis and immune regulation—emphasizes the multifaceted role of 2’-FL in supporting immune development. However, not all studies demonstrate consistent effects. Fonvig et al. (34) observed no significant changes in blood markers related to inflammation, such as IL-6, IL-8, IL-10, TNF-α, and C-reactive protein in obese children supplemented with 2’-FL and LNnT over 8 weeks. This variability underscores the influence of demographic and health factors on immune responses to HMOs.
Regarding nutritional biomarkers, Kim et al. (12) reported no significant changes in blood glucose, cholesterol, or triglycerides in healthy adults supplemented with 6’-SL for 12 weeks, confirming the metabolic safety of HMOs at clinical doses (3 gm/day). Canfora et al. (53) reported that 2′-FL supplementations significantly increased systemic butyrate levels in lean individuals and those with prediabetes or obesity. This elevation in butyrate was linked to improved metabolic health markers, including reductions in plasma-free fatty acids and inflammation. Interestingly, acetate levels increased only in lean men, highlighting a phenotype-specific response to supplementation. Consistent with these observations, Bajic et al. (28) reported dose-dependent increases in acetate, propionate, and butyrate following HMO supplementation, starting from estimated doses of 0.3–0.5 g/day. Their findings highlighted distinct effects of individual HMOs: 6′-SL had the strongest impact on propionate, 2’-FL and 3′-SL significantly elevated acetate, and LNnT notably increased butyrate, a key molecule associated with neuroactive pathways due to its capacity to traverse the blood–brain barrier and influence neurotransmitter synthesis (54).
Fecal marker: Fecal metabolites serve as indicators of gut microbial function and play a critical role in host health. Iribarren et al. (55) observed distinct modulation of metabolite profiles, including changes in asparagine, an amino acid associated with intestinal barrier integrity, and tryptophan, another essential amino acid linked to the pathogenesis of IBS (56). Tryptophan also serves as a precursor to serotonin, which is vital for mood regulation, cognitive function, and sleep (57). These changes were more pronounced in the intervention group receiving 10 g 2′-FL/LNnT than those receiving 5 g 2′-FL/LNnT or a placebo.
Furthermore, the untargeted metabolomic analysis demonstrated that even the lowest dose of HMOs (0.3 g/day) in children and adults significantly increased immune-associated metabolites, including aromatic lactic acids (indole-3-lactic acid and 3-phenyllactic acid) and 2-hydroxyisocaproic acid, as well as metabolites associated with the gut–brain axis, such as γ-aminobutyric acid, 3-hydroxybutyric acid, and acetylcholine (28). Notably, these aromatic lactic acids have been implicated in immune regulation (58) and neural processes mediated through the aryl hydrocarbon receptor (59–62).
The supplementation of 2′-FL and LNnT in an AAF for up to 12 months resulted in a significant increase in fecal SCFAs (17), mirroring previously observed elevation in plasma SCFA concentrations. This included marked increases in acetate, propionate, and butyrate, with acetate levels remaining consistently high throughout the study. These changes reflect the metabolic activity of Bifidobacteria, contributing to a lowered colonic pH and creating a protective acidic environment against entero-pathogens. The findings align with SCFA profiles commonly observed in breastfed infants, further underscoring the metabolic benefits of HMOs in infant nutrition.
Clinical evidence of mechanistic insights into HMO action
HMOs exert their health benefits through a range of complex and interconnected mechanisms, influencing immune function, metabolic regulation, gut health, microbial composition, and systemic physiological processes. While few clinical studies have comprehensively mapped the specific pathways of HMO activity, emerging research highlights several key mechanisms.
Immunomodulatory effects of HMOs
Among HMOs, 2′-FL, being the most widely studied, plays a significant role in immune development. Studies show that 2′-FL supplementations significantly reduce pro-inflammatory cytokines in the plasma, aligning the innate immune profiles of formula-fed infants more closely with those of breastfed infants (34, 43). This modulation is especially beneficial in managing conditions such as CMPA, which is associated with heightened inflammatory cytokine expression. Beyond innate immunity, 2′-FL supplementation influences adaptive immunity responses by reducing disparities in T lymphocyte proportions and enhancing apoptotic activity, particularly in CD8 + T cells (43). By promoting the clearance of activated T cells, HMOs also help downregulate inflammatory responses, fostering a more regulated immune environment and potentially alleviating or preventing allergic reactions associated with CMPA.
Infection resistance
HMOs contribute to infection-resistance properties and gastrointestinal and metabolic health. Infants receiving HMO-supplemented formulas exhibit low incidences of URTIs and LRTIs and gastrointestinal disorders such as IBS (18, 26, 32, 40). The protective effects of HMOs are attributed to multiple mechanisms:
a. Through gut microbiota modulation: HMOs promote beneficial gut bacteria, such as Bifidobacterium, Bacteroides, and Lactobacillus, thereby enhancing gut homeostasis (15, 42).
b. Pathogen inhibition by acting as decoy receptors: HMOs prevent the adhesion of pathogens such as Escherichia coli and Clostridium difficile, reducing infection risks (35, 41).
c. Through anti-inflammation: HMOs stimulate the synthesis of anti-inflammatory compounds, including N-acetyl amino acids and gamma-glutamyl amino acids, while reducing pro-inflammatory sphingolipids (Figure 2) (63).
d. Innate immune enhancement: In vitro studies suggest that HMOs, such as 2′-FL and LNnT, can reduce respiratory syncytial virus (RSV) and influenza A viral loads by enhancing innate immune defense (64). However, these findings need to be confirmed through additional clinical studies.
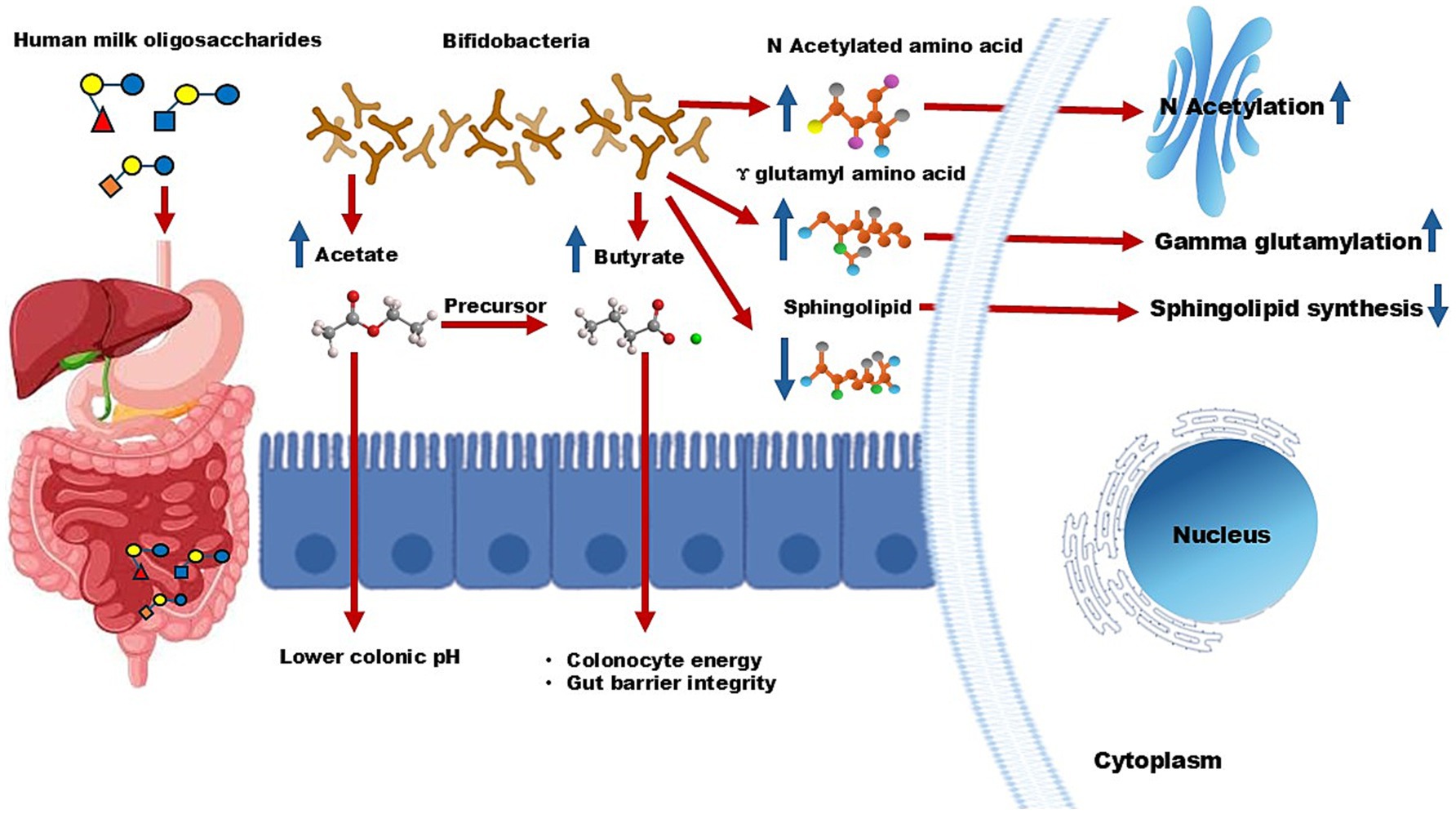
Figure 2. Mechanistic pathway of human milk oligosaccharides. Bifidobacteria in the colon utilize HMOs, leading to the production of acetate. This short-chain fatty acid lowers colonic pH and further works as a precursor for butyrate, which provides energy and maintains intestinal integrity. Additionally, HMOs boost levels of N-acetylated amino acids and gamma-glutamyl amino acids while reducing sphingolipid synthesis. These metabolic changes contribute to lower inflammation and enhance immune protection.
Gut health and barrier integrity
Gastrointestinal disorders such as IBS, typically characterized by gut dysbiosis and compromised gut barrier integrity, are another area where HMOs show promise. Clinical studies reported that supplementation with 2′-FL and LNnT significantly improves both intestinal and systemic IBS symptoms (32, 40). These benefits are attributed, at least in part, to the prebiotic role of HMOs in promoting the growth of Bifidobacteria, which produce SCFAs such as acetate. Acetate lowers colonic pH, creating a favorable gut environment, and serves as a precursor for butyrate synthesis (Figure 2). Butyrate is essential for colonocyte energy metabolism and maintaining gut barrier integrity, further underscoring the role of HMOs in alleviating IBS symptoms.
Metabolic health benefits
Emerging evidence suggests that HMOs contribute to metabolic regulation by modulating gut microbiota and systemic inflammation. In line with this, a recent clinical trial was the first to examine the therapeutic effects of 3′-SL supplementation in patients with osteoarthritis, demonstrating that 200 or 600 mg/day of 3′-SL for 12 weeks led to significant pain relief and enhanced mobility (65). Besides, Canfora et al. (53) reported increased systemic butyrate levels in lean individuals and those with prediabetes or obesity after HMO supplementation. This elevation in butyrate was linked to improved metabolic markers, including reduced plasma-free fatty acids and systemic inflammation. These findings suggest that HMOs may play a beneficial role in preventing osteoarthritis, obesity, and associated metabolic disorders by modulating gut microbiota and promoting anti-inflammatory pathways. Clinical studies investigating the effects of manufactured HMOs on adults, with or without medical conditions, are shown in Table 4.
Gut–brain axis and neurodevelopment
Preclinical studies have demonstrated that SCFAs influence neuroactive pathways by crossing the blood–brain barrier, where they regulate brain energy metabolism, modulate inflammation, and affect neurotransmitter synthesis (66, 67). These mechanisms provide a plausible explanation for clinical findings that associate SCFA activity with indicators of appropriate neurodevelopment, as reflected in anthropometric measures such as head circumference. This link underscores the potential role of SCFAs in supporting early brain development and highlights their significance in shaping neurophysiological outcomes.
Future perspectives
While considerable advancements have been made in understanding the benefits of HMOs, future research must address several limitations. One research gap is how HMOs influence cognitive development, which remains a key area requiring further exploration. Additionally, the metabolic fate of HMOs and the functional roles of their metabolites, particularly short-chain fatty acids (SCFAs), in peripheral organs involved in metabolic homeostasis—such as the liver, adipose tissue, and pancreas—also warrant further investigation. Furthermore, there has been limited focus on the mechanistic pathways in clinical investigations, with most studies emphasizing observation outcomes rather than the specific biological processes involved in reducing infections, limiting the ability to confirm causal relationships. Future clinical research should incorporate mechanistic endpoints to validate these effects. This is particularly important for comprehending how HMOs modulate immune responses, gut microbiota, and cognitive development. The current heterogeneity in study designs—such as variations in dosage, participant characteristics, and duration—further complicates the ability to draw consistent conclusions. To deepen our understanding, more randomized controlled trials (RCTs) are needed, especially those exploring long-term effects on immune modulation/inflammatory regulation, infection resistance/antimicrobial effects, gut microbiota modulation, gastrointestinal health, metabolic health, neurodevelopment, and the gut–brain axis, as well as the specific HMO structures and dosages required for clinical benefits. Addressing these gaps will be essential for developing targeted nutritional strategies to optimize human health outcomes.
Future directions should address broader applications in infant and adult nutrition, therapeutic potential in chronic diseases, and enhanced clinical research, including deeper exploration of the molecular pathways and systemic effects of HMOs in various physiological and pathological contexts. Large-scale, long-term trials evaluate the efficacy and safety of HMOs in diverse populations, including vulnerable groups like pre-term infants and elderly adults, and use of advanced technologies, such as genomics, metabolomics, and proteomics, to map HMO interactions and outcomes comprehensively. HMOs have the potential to redefine nutritional and therapeutic paradigms. Continued interdisciplinary research, technological innovation, and collaboration between academia, industry, and regulatory bodies will be critical in unlocking their full potential.
Author contributions
TA: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. MA: Visualization, Writing – review & editing, Methodology, Data curation. AA: Writing – review & editing, Data curation. XZ: Writing – review & editing. YN: Writing – review & editing. BW: Supervision, Writing – review & editing, Validation, Funding acquisition, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. We also thank the Nutrition Research Institute, Junlebao Dairy Group Co., Ltd. 103878, for partially providing research funding to BW. Australian Government Research Training Program (AGRTP) Scholarships from Gulbali Institute, Charles Sturt University (CSU) to TA, and from CSU to MA and AA. The funder was not involved in the study design, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of interest
YN was employed by the Junlebao Dairy Group Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kim, SY, and Yi, DY. Components of human breast milk: from macronutrient to microbiome and microRNA. Clin Exp Pediatr. (2020) 63:301–9. doi: 10.3345/cep.2020.00059
2. Urashima, T, Asakuma, S, Leo, F, Fukuda, K, Messer, M, and Oftedal, OT. The predominance of type I oligosaccharides is a feature specific to human breast milk. Adv Nutr. (2012) 3:473S–82S. doi: 10.3945/an.111.001412
3. Mazzocchi, A, and Agostoni, C. Human milk oligosaccharides and infant growth: a global health approach. Pediatr Res. (2024) 96:277–8. doi: 10.1038/s41390-024-03037-2
4. Martin-Sosa, S, Martin, MJ, Garcia-Pardo, LA, and Hueso, P. Sialyloligosaccharides in human and bovine milk and in infant formulas: variations with the progression of lactation. J Dairy Sci. (2003) 86:52–9. doi: 10.3168/jds.S0022-0302(03)73583-8
5. Bode, L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology. (2012) 22:1147–62. doi: 10.1093/glycob/cws074
6. Soyyilmaz, B, Miks, MH, Rohrig, CH, Matwiejuk, M, Meszaros-Matwiejuk, A, and Vigsnaes, LK. The mean of Milk: a review of human Milk oligosaccharide concentrations throughout lactation. Nutrients. (2021) 13:737. doi: 10.3390/nu13082737
7. Lu, M, Mosleh, I, and Abbaspourrad, A. Engineered microbial routes for human Milk oligosaccharides synthesis. ACS Synth Biol. (2021) 10:923–38. doi: 10.1021/acssynbio.1c00063
8. Bode, L, Contractor, N, Barile, D, Pohl, N, Prudden, AR, Boons, GJ, et al. Overcoming the limited availability of human milk oligosaccharides: challenges and opportunities for research and application. Nutr Rev. (2016) 74:635–44. doi: 10.1093/nutrit/nuw025
9. Biel-Nielsen, TL, Li, K, Sorensen, SO, Sejberg, JJP, Meyer, AS, and Holck, J. Utilization of industrial citrus pectin side streams for enzymatic production of human milk oligosaccharides. Carbohydr Res. (2022) 519:108627. doi: 10.1016/j.carres.2022.108627
10. Dinleyici, M, Barbieur, J, Dinleyici, EC, and Vandenplas, Y. Functional effects of human milk oligosaccharides (HMOs). Gut Microbes. (2023) 15:2186115. doi: 10.1080/19490976.2023.2186115
11. Sprenger, N, Tytgat, HLP, Binia, A, Austin, S, and Singhal, A. Biology of human milk oligosaccharides: from basic science to clinical evidence. J Hum Nutr Diet. (2022) 35:280–99. doi: 10.1111/jhn.12990
12. Kim, JH, Yong, SY, Kim, SH, Baek, A, Go, TH, and Kang, DR. Randomized, triple-blind, placebo-controlled study to evaluate the safety of 6′-sialyllactose in healthy adults. Regul Toxicol Pharmacol. (2022) 129:105110. doi: 10.1016/j.yrtph.2021.105110
13. Prieto, PA. In vitro and clinical experiences with a human milk oligosaccharide, lacto-N-neotetraose, and fructooligosaccharides. Foods Food Ingredients J Jpn. (2005) 210:1018–30.
14. Jochum, F, Meyer-Krott, M, Hubler, T, Lorenz, M, Bedikian, R, Zakarian, J, et al. Real-world evidence study on tolerance and growth in infants fed an infant formula with two human milk oligosaccharides vs mixed fed and exclusively breastfed infants. Mol Cell Pediatr. (2023) 10:7. doi: 10.1186/s40348-023-00162-6
15. Alliet, P, Vandenplas, Y, Roggero, P, Jespers, SNJ, Peeters, S, Stalens, JP, et al. Safety and efficacy of a probiotic-containing infant formula supplemented with 2′-fucosyllactose: a double-blind randomized controlled trial. Nutr J. (2022) 21:11. doi: 10.1186/s12937-022-00764-2
16. Lasekan, J, Choe, Y, Dvoretskiy, S, Devitt, A, Zhang, S, Mackey, A, et al. Growth and gastrointestinal tolerance in healthy term infants fed milk-based infant formula supplemented with five human milk oligosaccharides (HMOs): a randomized multicenter trial. Nutrients. (2022) 14:625. doi: 10.3390/nu14132625
17. Wallingford, JC, Neve Myers, P, and Barber, CM. Effects of addition of 2-fucosyllactose to infant formula on growth and specific pathways of utilization by Bifidobacterium in healthy term infants. Front Nutr. (2022) 9:961526. doi: 10.3389/fnut.2022.961526
18. Puccio, G, Alliet, P, Cajozzo, C, Janssens, E, Corsello, G, Sprenger, N, et al. Effects of infant formula with human Milk oligosaccharides on growth and morbidity: a randomized multicenter trial. J Pediatr Gastroenterol Nutr. (2017) 64:624–31. doi: 10.1097/MPG.0000000000001520
19. Parschat, K, Melsaether, C, Japelt, KR, and Jennewein, S. Clinical evaluation of 16-week supplementation with 5HMO-mix in healthy-term human infants to determine tolerability, safety, and effect on growth. Nutrients. (2021) 13:871. doi: 10.3390/nu13082871
20. Roman, E, Moreno Villares, JM, Dominguez Ortega, F, Carmona Martinez, A, Pico Sirvent, L, Santana Sandoval, L, et al. Real-world study in infants fed with an infant formula with two human milk oligosaccharides. Nutr Hosp. (2020) 37:698–706. doi: 10.20960/nh.03084
21. Vandenplas, Y, de Halleux, V, Arciszewska, M, Lach, P, Pokhylko, V, Klymenko, V, et al. A partly fermented infant formula with postbiotics including 3′-GL, specific oligosaccharides, 2'-FL, and milk fat supports adequate growth, is safe and well-tolerated in healthy term infants: a double-blind, randomised, controlled, multi-country trial. Nutrients. (2020) 12:3650. doi: 10.3390/nu12113560
22. Storm, HM, Shepard, J, Czerkies, LM, Kineman, B, Cohen, SS, Reichert, H, et al. 2′-fucosyllactose is well tolerated in a 100% whey, partially hydrolyzed infant formula with Bifidobacterium lactis: a randomized controlled trial. Glob Pediatr Health. (2019) 6:2333794X19833995. doi: 10.1177/2333794X19833995
23. Marriage, BJ, Buck, RH, Goehring, KC, Oliver, JS, and Williams, JA. Infants fed a lower calorie formula with 2'FL show growth and 2'FL uptake like breast-fed infants. J Pediatr Gastroenterol Nutr. (2015) 61:649–58. doi: 10.1097/MPG.0000000000000889
24. Kajzer, J, Oliver, J, and Marriage, B. Gastrointestinal tolerance of formula supplemented with oligosaccharides. FASEB J. (2016) 30:671. doi: 10.1096/fasebj.30.1_supplement.671.4
25. Gold, MS, Quinn, PJ, Campbell, DE, Peake, J, Smart, J, Robinson, M, et al. Effects of an amino acid-based formula supplemented with two human Milk oligosaccharides on growth, tolerability, safety, and gut microbiome in infants with cow's Milk protein allergy. Nutrients. (2022) 14:297. doi: 10.3390/nu14112297
26. Vandenplas, Y, Zolnowska, M, Berni Canani, R, Ludman, S, Tengelyi, Z, Moreno-Alvarez, A, et al. Effects of an extensively hydrolyzed formula supplemented with two human Milk oligosaccharides on growth, tolerability, safety and infection risk in infants with cow's Milk protein allergy: a randomized, multi-center trial. Nutrients. (2022) 14:530. doi: 10.3390/nu14030530
27. Ramirez-Farias, C, Baggs, GE, and Marriage, BJ. Growth, tolerance, and compliance of infants fed an extensively hydrolyzed infant formula with added 2'-FL Fucosyllactose (2'-FL) human Milk oligosaccharide. Nutrients. (2021) 13:186. doi: 10.3390/nu13010186
28. Bajic, D, Wiens, F, Wintergerst, E, Deyaert, S, Baudot, A, and Abbeele, PVD. HMOs impact the gut microbiome of children and adults starting from low predicted daily doses. Meta. (2024) 14:239. doi: 10.3390/metabo14040239
29. Leung, TF, Ulfman, LH, Chong, MKC, Hon, KL, Khouw, I, Chan, PKS, et al. A randomized controlled trial of different young child formulas on upper respiratory and gastrointestinal tract infections in Chinese toddlers. Pediatr Allergy Immunol. (2020) 31:745–54. doi: 10.1111/pai.13276
30. Hascoet, JM, Chevallier, M, Gire, C, Brat, R, Roze, JC, Norbert, K, et al. Use of a liquid supplement containing 2 human Milk oligosaccharides: the first double-blind, randomized, controlled trial in pre-term infants. Front Pediatr. (2022) 10:858380. doi: 10.3389/fped.2022.858380
31. Ramirez-Farias, C, Oliver, JS, Schlezinger, J, and Stutts, JT. Tolerance of infants fed a hydrolyzed Rice infant formula with 2'-Fucosyllactose (2'-FL) human Milk oligosaccharide (HMO). Nutrients. (2024) 16:863. doi: 10.3390/nu16121863
32. Palsson, OS, Peery, A, Seitzberg, D, Amundsen, ID, McConnell, B, and Simren, M. Human milk oligosaccharides support normal bowel function and improve symptoms of irritable bowel syndrome: a multicenter, open-label trial. Clin Transl Gastroenterol. (2020) 11:e00276. doi: 10.14309/ctg.0000000000000276
33. Iribarren, C, Magnusson, MK, Vigsnaes, LK, Aziz, I, Amundsen, ID, Suligoj, T, et al. The effects of human Milk oligosaccharides on gut microbiota, metabolite profiles and host mucosal response in patients with irritable bowel syndrome. Nutrients. (2021) 13:836. doi: 10.3390/nu13113836
34. Fonvig, CE, Amundsen, ID, Vigsnaes, LK, Sorensen, N, Frithioff-Bojsoe, C, Christiansen, M, et al. Human Milk oligosaccharides modulate fecal microbiota and are safe for use in children with overweight: a randomized controlled trial. J Pediatr Gastroenterol Nutr. (2021) 73:408–14. doi: 10.1097/MPG.0000000000003205
35. Elison, E, Vigsnaes, LK, Rindom Krogsgaard, L, Rasmussen, J, Sorensen, N, McConnell, B, et al. Oral supplementation of healthy adults with 2'-O-fucosyllactose and lacto-N-neotetraose is well tolerated and shifts the intestinal microbiota. Br J Nutr. (2016) 116:1356–68. doi: 10.1017/S0007114516003354
36. Iribarren, C, Tornblom, H, Aziz, I, Magnusson, MK, Sundin, J, Vigsnaes, LK, et al. Human milk oligosaccharide supplementation in irritable bowel syndrome patients: a parallel, randomized, double-blind, placebo-controlled study. Neurogastroenterol Motil. (2020) 32:e13920. doi: 10.1111/nmo.13920
37. Parente, F, Cucino, C, Anderloni, A, Grandinetti, G, and Bianchi, PG. Treatment of Helicobacter pylori infection using a novel antiadhesion compound (3'sialyllactose sodium salt). A double blind, placebo-controlled clinical study. Helicobacter. (2003) 8:252–6. doi: 10.1046/j.1523-5378.2003.00152.x
38. Wang, B. Sialic acid is an essential nutrient for brain development and cognition. Annu Rev Nutr. (2009) 29:177–222. doi: 10.1146/annurev.nutr.28.061807.155515
39. Ko, J, Yoo, C, Xing, D, Chun, J, Gonzalez, DE, Dickerson, BL, et al. Effects of human Milk oligosaccharide 2'-Fucosyllactose ingestion on weight loss and markers of health. Nutrients. (2024) 16:387. doi: 10.3390/nu16193387
40. Ryan, JJ, Monteagudo-Mera, A, Contractor, N, and Gibson, GR. Impact of 2'-Fucosyllactose on gut microbiota composition in adults with chronic gastrointestinal conditions: batch culture fermentation model and pilot clinical trial findings. Nutrients. (2021) 13:938. doi: 10.3390/nu13030938
41. Holst, AQ, Myers, P, Rodriguez-Garcia, P, Hermes, GDA, Melsaether, C, Baker, A, et al. Infant formula supplemented with five human Milk oligosaccharides shifts the fecal microbiome of formula-fed infants closer to that of breastfed infants. Nutrients. (2023) 15:3087. doi: 10.3390/nu15143087
42. Boulange, CL, Pedersen, HK, Martin, FP, Siegwald, L, Palleja Caro, A, Eklund, AC, et al. An extensively hydrolyzed formula supplemented with two human Milk oligosaccharides modifies the fecal microbiome and metabolome in infants with cow's Milk protein allergy. Int J Mol Sci. (2023) 24:422. doi: 10.3390/ijms241411422
43. Goehring, KC, Marriage, BJ, Oliver, JS, Wilder, JA, Barrett, EG, and Buck, RH. Similar to those who are breastfed, infants fed a formula containing 2′-fucosyllactose have lower inflammatory cytokines in a randomized controlled trial. J Nutr. (2016) 146:2559–66. doi: 10.3945/jn.116.236919
44. Bosheva, M, Tokodi, I, Krasnow, A, Pedersen, HK, Lukjancenko, O, Eklund, AC, et al. Infant formula with a specific blend of five human Milk oligosaccharides drives the gut microbiota development and improves gut maturation markers: a randomized controlled trial. Front Nutr. (2022) 9:920362. doi: 10.3389/fnut.2022.920362
45. Dogra, SK, Martin, FP, Donnicola, D, Julita, M, Berger, B, and Sprenger, N. Human Milk oligosaccharide-stimulated Bifidobacterium species contribute to prevent later respiratory tract infections. Microorganisms. (2021) 9:939. doi: 10.3390/microorganisms9091939
46. Saturio, S, Nogacka, AM, Alvarado-Jasso, GM, Salazar, N, de Los Reyes-Gavilan, CG, Gueimonde, M, et al. Role of Bifidobacteria on infant health. Microorganisms. (2021) 9:415. doi: 10.3390/microorganisms9122415
47. He, F, Ouwehand, AC, Isolauri, E, Hashimoto, H, Benno, Y, and Salminen, S. Comparison of mucosal adhesion and species identification of bifidobacteria isolated from healthy and allergic infants. FEMS Immunol Med Microbiol. (2001) 30:43–7. doi: 10.1111/j.1574-695X.2001.tb01548.x
48. Kalliomaki, M, Kirjavainen, P, Eerola, E, Kero, P, Salminen, S, and Isolauri, E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol. (2001) 107:129–34. doi: 10.1067/mai.2001.111237
49. Rahman, T, Sarwar, PF, Potter, C, Comstock, SS, and Klepac-Ceraj, V. Role of human milk oligosaccharide metabolizing bacteria in the development of atopic dermatitis/eczema. Front Pediatr. (2023) 11:1090048. doi: 10.3389/fped.2023.1090048
50. Kalliomaki, M, Collado, MC, Salminen, S, and Isolauri, E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. (2008) 87:534–8. doi: 10.1093/ajcn/87.3.534
51. Goehring, KC, Kennedy, AD, Prieto, PA, and Buck, RH. Direct evidence for the presence of human milk oligosaccharides in the circulation of breastfed infants. PLoS One. (2014) 9:e101692. doi: 10.1371/journal.pone.0101692
52. Wickramasinghe, S, Pacheco, AR, Lemay, DG, and Mills, DA. Bifidobacteria grown on human milk oligosaccharides downregulate the expression of inflammation-related genes in Caco-2 cells. BMC Microbiol. (2015) 15:172. doi: 10.1186/s12866-015-0508-3
53. Canfora, EE, Vliex, LMM, Wang, T, Nauta, A, Bouwman, FG, Holst, JJ, et al. 2′-fucosyllactose alone or combined with resistant starch increases circulating short-chain fatty acids in lean men and men with prediabetes and obesity. Front Nutr. (2023) 10:1200645. doi: 10.3389/fnut.2023.1200645
55. Zhu, H, Pi, D, Leng, W, Wang, X, Hu, CA, Hou, Y, et al. Asparagine preserves intestinal barrier function from LPS-induced injury and regulates CRF/CRFR signaling pathway. Innate Immun. (2017) 23:546–56. doi: 10.1177/1753425917721631
56. Bosi, A, Banfi, D, Bistoletti, M, Giaroni, C, and Baj, A. Tryptophan metabolites along the microbiota-gut-brain Axis: an Interkingdom communication system influencing the gut in health and disease. Int J Tryptophan Res. (2020) 13:1178646920928984. doi: 10.1177/1178646920928984
57. Jenkins, TA, Nguyen, JC, Polglaze, KE, and Bertrand, PP. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients. (2016) 8:56. doi: 10.3390/nu8010056
58. Laursen, MF, Sakanaka, M, von Burg, N, Morbe, U, Andersen, D, Moll, JM, et al. Bifidobacterium species associated with breastfeeding produce aromatic lactic acids in the infant gut. Nat Microbiol. (2021) 6:1367–82. doi: 10.1038/s41564-021-00970-4
59. Latchney, SE, Hein, AM, O'Banion, MK, DiCicco-Bloom, E, and Opanashuk, LA. Deletion or activation of the aryl hydrocarbon receptor alters adult hippocampal neurogenesis and contextual fear memory. J Neurochem. (2013) 125:430–45. doi: 10.1111/jnc.12130
60. Swann, JR, Spitzer, SO, and Diaz Heijtz, R. Developmental signatures of microbiota-derived metabolites in the mouse brain. Meta. (2020) 10:172. doi: 10.3390/metabo10050172
61. Kimura, E, and Tohyama, C. Embryonic and postnatal expression of aryl hydrocarbon receptor mRNA in mouse brain. Front Neuroanat. (2017) 11:4. doi: 10.3389/fnana.2017.00004
62. Schroeder, JC, Dinatale, BC, Murray, IA, Flaveny, CA, Liu, Q, Laurenzana, EM, et al. The uremic toxin 3-indoxyl sulfate is a potent endogenous agonist for the human aryl hydrocarbon receptor. Biochemistry. (2010) 49:393–400. doi: 10.1021/bi901786x
63. Martin, FP, Tytgat, HLP, Krogh Pedersen, H, Moine, D, Eklund, AC, Berger, B, et al. Host-microbial co-metabolites modulated by human milk oligosaccharides relate to reduced risk of respiratory tract infections. Front Nutr. (2022) 9:935711. doi: 10.3389/fnut.2022.935711
64. Duska-McEwen, G, Senft, AP, Ruetschilling, TL, Barrett, EG, and Buck, RH. Human milk oligosaccharides enhance innate immunity to respiratory syncytial virus and influenza in vitro. Food Nutr Sci. (2014) 5:1387–98. doi: 10.4236/fns.2014.514151
65. Park, EJ, Kim, LL, Go, H, and Kim, SH. Effects of 3'-Sialyllactose on symptom improvement in patients with knee osteoarthritis: a randomized pilot study. Nutrients. (2024) 16:410. doi: 10.3390/nu16193410
66. Silva, YP, Bernardi, A, and Frozza, RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol. (2020) 11:25. doi: 10.3389/fendo.2020.00025
67. Mansuy-Aubert, V, and Ravussin, Y. Short chain fatty acids: the messengers from down below. Front Neurosci. (2023) 17:1197759. doi: 10.3389/fnins.2023.1197759
68. Scheuchzer, P, Sinawat, S, Donze, AS, Zeder, C, Sabatier, M, Garcia-Garcera, M, et al. Iron absorption from an Iron-fortified follow-up formula with and without the addition of a Synbiotic or a human-identical Milk oligosaccharide: a randomized crossover stable isotope study in young Thai children. J Nutr. (2024) 154:2988–98. doi: 10.1016/j.tjnut.2024.08.016
69. Berger, B, Porta, N, Foata, F, Grathwohl, D, Delley, M, Moine, D, et al. Linking human milk oligosaccharides, infant fecal community types, and later risk to require antibiotics. MBio. (2020) 11:3196. doi: 10.1128/mBio.03196-19
70. Nowak-Wegrzyn, A, Czerkies, L, Reyes, K, Collins, B, and Heine, RG. Confirmed Hypoallergenicity of a novel whey-based extensively hydrolyzed infant formula containing two human Milk oligosaccharides. Nutrients. (2019) 11:447. doi: 10.3390/nu11071447
Keywords: human milk oligosaccharides, infant formula, gut-microbiota, infection, mechanistic pathway
Citation: Amin T, Amin MM, Adikari AADI, Zheng X, Ning Y and Wang B (2025) Clinical evidence and mechanistic pathways of human milk oligosaccharide supplementation for health benefits: an updated review. Front. Nutr. 12:1599678. doi: 10.3389/fnut.2025.1599678
Edited by:
Claude Billeaud, Independent Paediatrics Nutrition Expert, Bordeaux, FranceReviewed by:
Aristea Binia, Nestlé Research Center, SwitzerlandJean-Michel Hascoet, Université de Lorraine, France
Copyright © 2025 Amin, Amin, Adikari, Zheng, Ning and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bing Wang, Yml3YW5nQGNzdS5lZHUuYXU=
 Tanjina Amin
Tanjina Amin Md Mahmudul Amin
Md Mahmudul Amin Adikari Arachchige Dilki Indrachapa Adikari1
Adikari Arachchige Dilki Indrachapa Adikari1 Xiaoming Zheng
Xiaoming Zheng Bing Wang
Bing Wang