- 1Jiangxi Normal University, Nangchang, Jiangxi, China
- 2College of Physical Education, Jinggangshan University, Ji’an, China
- 3College of Physical Education, Beijing Normal University, Beijing, China
Objective: Metabolic syndrome and chronic inflammation significantly impact the quality of life of the elderly. Physical activity and dietary habits are two of the most important modifiable aspects of lifestyle. Thus, this study investigated the effects of physical activity and dietary habits on relevant biomarkers in the elderly.
Methods: A total of 2349 elderly participants aged 60–75 were recruited. Physical activity was measured using the International Physical Activity Questionnaire (IPAQ) short form. Dietary habits and intakes were assessed via the Dietary Quality Questionnaire (DQQ) and 24-h recalls. Fasting blood samples were analyzed for glycolipid metabolism and inflammatory markers, such as C - reactive protein (CRP) and interleukin - 6 (IL - 6).
Results: There was a close association between physical activity and diet. Moderate - intensity physical activity (MPA) was positively associated with the intake of dark green leafy vegetables (β = 0.174) and negatively associated with the intake of unprocessed red meat (β = −0.112) and deep - fried foods (β = −0.117). Both physical activity and diet affected biomarkers. Vigorous physical activity was positively correlated with high - density lipoprotein cholesterol (r = 0.144), while MPA was negatively correlated with blood glucose (r = −0.127) and CRP (r = −0.129). The percentage of protein intake was positively correlated with triglycerides (r = 0.118). Mediation analysis demonstrated the combined effects of physical activity and diet. The results showed that MPA significantly and negatively affected CRP levels, with the intake of dark green leafy vegetables mediating this relationship (P < 0.05). MPA also significantly and negatively affected blood glucose levels, with vitamin B12 intake mediating this relationship (P < 0.05).
Conclusion: The study indicates that physical activity and diet interact with each other and jointly affect blood glucose and inflammation in the elderly. Diet mediates the effect of physical activity on biomarkers. Further longitudinal studies are needed to verify the findings of this study.
1 Introduction
The global population is aging at an unprecedented rate, making the health of older adults a critical public health priority. By 2050, the proportion of individuals aged 60 years and older is projected to rise dramatically, straining healthcare systems, economies, and social services worldwide (1). Aging is associated not only with physical and cognitive decline (2) but also with metabolic disturbances, including dysregulated glucose and lipid metabolism (3). These changes significantly impair quality of life and elevate the risk of chronic conditions such as cardiovascular disease and diabetes (4). Consequently, age-related glycolipid metabolic disorders and low-grade chronic inflammation have emerged as pressing global health challenges (5).
Aging disrupts glycolipid metabolism through mechanisms such as insulin resistance, visceral fat accumulation, and impaired lipid processing (6–8). Older adults–particularly women–exhibit reduced insulin sensitivity, exacerbated by shifts in body fat distribution and loss of skeletal muscle mass, a key site for glucose uptake (9–11). These alterations increase susceptibility to diabetes, metabolic syndrome, and cardiovascular disease, underscoring the need for interventions targeting metabolic health in aging populations.
Simultaneously, aging is characterized by chronic low-grade inflammation (“inflammaging”), marked by elevated levels of inflammatory mediators like C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) (12–14). This state is further amplified in individuals with metabolic disorders (15) and contributes to atherosclerosis, bone density loss, and immune dysfunction (16, 17). Crucially, chronic inflammation and glycolipid dysregulation form a vicious cycle, accelerating age-related health decline (18).
Modifiable lifestyle factors, particularly physical activity and diet, offer promising avenues to mitigate these effects. Regular exercise improves insulin sensitivity, reduces blood glucose and lipid levels, and dampens inflammatory responses (19). Aerobic activities (e.g., walking, swimming) enhance cardiorespiratory fitness and glycemic control (20), while resistance training preserves muscle mass and metabolic rate (21). Similarly, dietary patterns influence metabolic health; high-fiber, low-glycemic diets and anti-inflammatory foods (e.g., Mediterranean diet) ameliorate dyslipidemia and inflammation (22, 23).
Numerous studies have demonstrated the independent and combined effects of physical activity and dietary interventions on metabolic and inflammatory markers in older adults. For instance, a study found that moderate-intensity exercise significantly increased postprandial carbohydrate oxidation rates after high-carbohydrate meals while reducing fat oxidation, suggesting exercise modulates nutrient metabolism in a diet-dependent manner (24). Similarly, research comparing Mediterranean and traditional Chinese diets under isocaloric conditions revealed that both dietary patterns improved glycemic control, though Mediterranean diets were more effective in reducing hypoglycemic episodes, highlighting the role of dietary composition in metabolic regulation (25).
Other investigations have explored the mechanistic pathways linking lifestyle factors to metabolic health. One study demonstrated that high-protein diets counteract post-dieting fat mass rebound by suppressing gut Lactobacillus-mediated lipid absorption, underscoring the interplay between dietary protein and gut microbiota in obesity prevention (26). Additionally, time-restricted feeding, particularly morning-based eating windows, was shown to enhance insulin sensitivity and reduce inflammation in healthy non-obese adults, suggesting meal timing as a modifiable factor in metabolic regulation (27).
Despite these advances, few studies have systematically examined how dietary habits mediate the effects of physical activity on glycolipid metabolism and inflammation in elderly populations. While some research has noted synergistic benefits–such as combined aerobic exercise and high-fiber diets improving insulin sensitivity more than either intervention alone 28–the specific mediating role of nutrients remains underexplored. This gap underscores the originality of the current study, which aims to elucidate these interactions through biomarker analysis and mediation modeling, offering novel insights for targeted lifestyle interventions in aging.
While the independent benefits of physical activity and diet are well-documented (28–30), their combined effects on glycolipid metabolism and inflammation in older adults remain underexplored. This study aims to address this gap by investigating how interactions between physical activity and dietary habits influence these outcomes. We hypothesize that: Higher physical activity levels and healthier dietary patterns will correlate with improved glycolipid profiles (e.g., lower fasting glucose, favorable lipid levels) and reduced inflammatory markers (e.g., CRP, IL-6).
This study is the first to systematically examine the synergistic effects of moderate-to-vigorous physical activity and specific dietary patterns (e.g., dark green leafy vegetable intake) on glycolipid metabolism and inflammatory markers in the elderly. It reveals the mediating role of diet–particularly dark green leafy vegetables and vitamin B12–in the relationship between physical activity and biomarkers. By providing direct evidence for combined lifestyle interventions (exercise and nutrition), this research addresses a critical gap in understanding how dietary habits mediate exercise-induced metabolic benefits. These findings offer a scientific foundation for tailored health strategies in aging populations.
2 Materials and methods
2.1 Participants
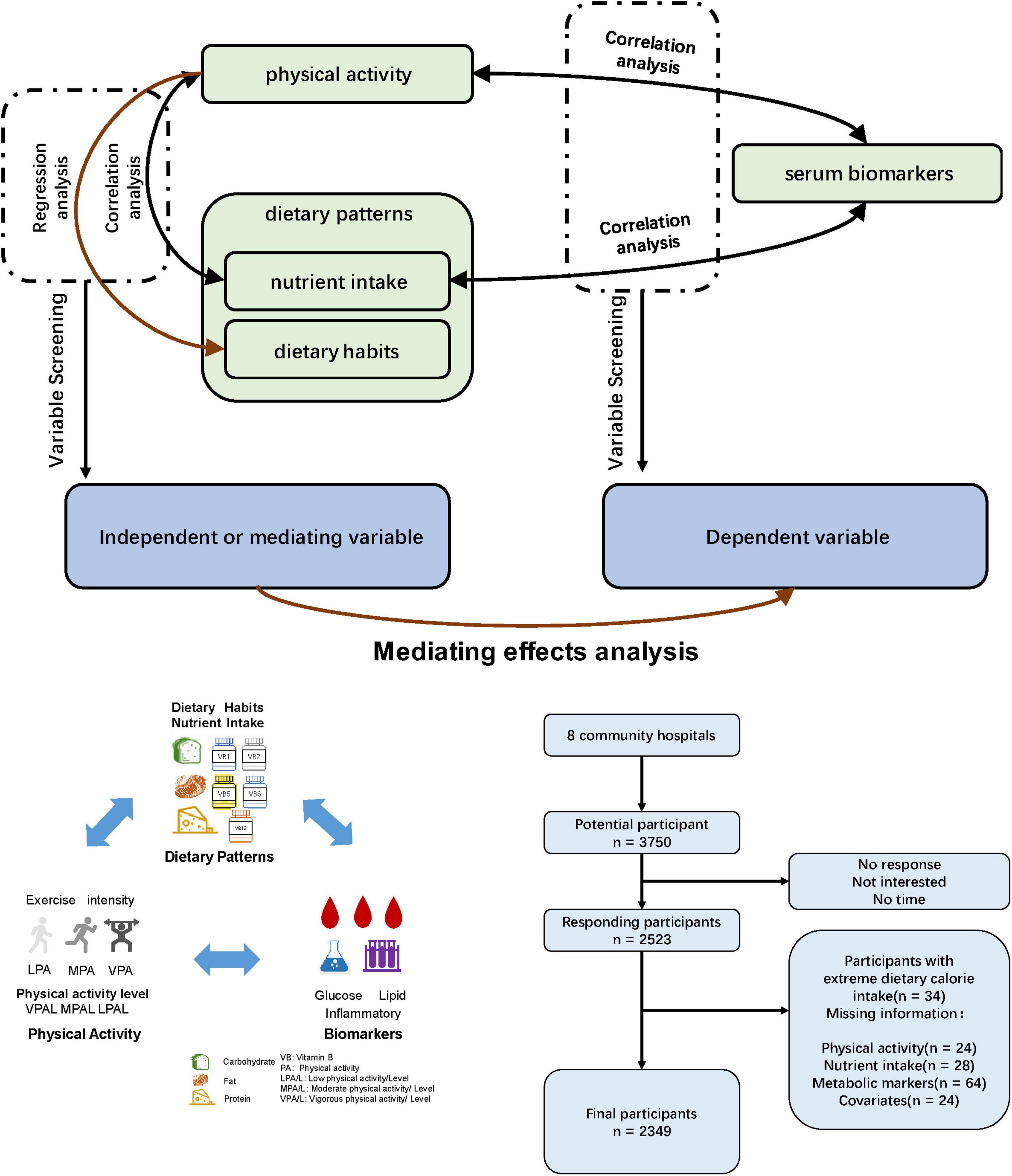
The study was conducted in a northern city in China. Recruitment took place from September to October 2023, targeting 3750 potential participants from various community hospitals across the city. Eight communities were selected from different districts to ensure representation. Questionnaires were distributed via poster advertisements for initial screening. Out of the 2523 responses received, 34 participants with extreme dietary calorie intake and 140 who did not complete the full questionnaire were excluded. This resulted in a final sample of 2349 participants. The study design is presented in Figure 1.
Participants were included in the study if they were aged between 60 and 75 years. They had to be non-smokers and have no history of long-term alcohol consumption, defined as more than 50 g per week. Additionally, only those who were not on any medication were selected. Cognitive function was also assessed, and individuals with no cognitive impairment, as evidenced by a Montreal Cognitive Assessment (MoCA) score of 26 or higher, were included. Participants were excluded from the study if they had major acute or chronic diseases. Those self-reporting any cardiovascular or cerebrovascular diseases were also excluded. Furthermore, individuals using weight-loss medications or with a history of depression, Parkinson’s disease, Alzheimer’s disease, severe mental disorders, stroke, or brain injury were not eligible for the study.
The study protocol was approved by the Ethics Committee of Jiangxi Normal University (RB_0343_56). The research was conducted in accordance with the ethical standards of the 1964 Helsinki Declaration.
2.2 Physical activity assessment
Physical activity levels of participants were quantified using the International Physical Activity Questionnaire (IPAQ) short form, a seven-item instrument that evaluates the frequency and duration of physical activities across different intensities, including vigorous (VPA), moderate (MPA), and low (LPA) physical activity. Administered by trained personnel through one-on-one interviews with elderly participants, the IPAQ short form ensures comprehension and clarity of responses, and is recognized for its high reliability and validity in the Chinese elderly population (31). Trained personnel received training through a combination of online and offline sessions. The training covered the structure and scientific basis of the IPAQ, standardized instructions for administering the questionnaire, and how to explain items clearly to participants. Standardized scripts were developed to maintain consistency. Based on total Metabolic Equivalent of Task (MET) minutes per week, participants were categorized into three activity levels: Low Physical Activity level (LPAL) for scores below 600 MET⋅min⋅wk–1, Moderate Physical Activity level (MPAL) for scores between 600 and 3000 MET⋅min⋅wk–1, and Vigorous Physical Activity level (VPAL) for scores of 3000 MET⋅min⋅wk–1 or higher.
2.3 Dietary data collection
Dietary intake data were collected using the Dietary Quality Questionnaire(DQQ), which comprises yes/no questions regarding the consumption of sentinel foods corresponding to 24 food groups over the past 24 h. The Chinese version of the DQQ has been validated for its ability to capture food group consumption patterns within the Chinese population (32).
2.3.1 Nutrient intake assessment
To assess dietary habits and estimate daily intakes of carbohydrates, fats, proteins, and vitamin B complex from the diet, three 24-h dietary recalls were conducted by trained dietitians over two weekdays and one weekend day. These recalls were collected via validated telephone interviews. The dietitians received structured training to ensure standardized and accurate data collection. This included comprehensive guidance on the 24-h dietary recall method, practice in interviewing techniques (e.g., rapid listing, probing for forgotten foods, estimating portion sizes), and calibration using visual aids. They were also trained to use neutral questioning techniques to minimize bias. Continuous quality control was maintained through supervised mock interviews and calibration meetings.
Participants were first educated on the proper use of visual aids for portion size estimation of common foods. This training featured real plates, glasses, and food models, complemented by hands-on experiential communication to enhance understanding and accuracy.
Food consumption data were collected through multiple 24-h dietary recall interviews, ensuring a comprehensive capture of dietary intake patterns. Macronutrient and micronutrient intakes were calculated using the nutritional software MetaDieta (Meteda s.r.l., Ascoli-Piceno).
Daily total food intake was expressed in grams per day (g/day), with vitamin B1-6 intake reported in milligrams per day (mg/day), vitamin B9-12 reported in micrograms per day (μg/day). Energy intake values falling outside the range of 1000–6000 kcal/day were considered implausible and were excluded from the analysis.
2.4 Measurement of glycolipemic metabolism and inflammatory markers
2.4.1 Blood sample collection
Fasting blood samples were collected in the early morning by nurses at local community hospitals. Participants were instructed to fast for at least 8 h prior to the blood draw to ensure the accuracy of the metabolic measurements.
2.4.2 Metabolic markers
Blood glucose, glycated hemoglobin (HbA1c), and lipid profile, including triglycerides, total cholesterol, high-density lipoprotein (HDL), and low-density lipoprotein (LDL), were measured by the community hospitals and reported accordingly.
2.4.3 Inflammatory markers
C-reactive protein (CRP), a widely recognized marker of systemic inflammation, was also assessed by the community hospitals and reported accordingly. The concentration of IL-6, a key cytokine involved in regulating immune responses and inflammation, was measured using the Human IL-6 ELISA Kit (YEASENELISA). The assay was performed strictly according to the manufacturer’s instructions, with a detection range of 0.16–10.5 pg/mL. The analyses were conducted in the university laboratory. Blood samples were immediately placed in insulated boxes with ice after collection. The samples were transported to the laboratory within 10 min and centrifuged at 1500 rpm for 10 min to separate plasma/serum for analysis. The measurements were conducted using a microplate reader. If analysis could not be conducted immediately, samples were stored at −80 °C until analysis, with strict limits on freeze-thaw cycles (≤1). The overall inter-assay coefficient of variation for IL-6 was 6.7%.
2.5 Covariates
In the analysis, potential confounding variables were controlled for to isolate the effects of physical activity and dietary habits on metabolic outcomes. These covariates encompassed socio-demographic factors such as gender (male or female), age (ranging from 60 to 75 years), marital status (married, widowed, divorced, or single), educational attainment (classified into primary school or below, junior high school, high school, and higher education), and annual household income. Additionally, lifestyle factors were considered, with sleep quality being evaluated using the Pittsburgh Sleep Quality Index (PSQI), a self-rated questionnaire that assesses sleep quality and disturbances over a 1-month period, yielding a score from 0 to 21, where higher scores denote poorer sleep quality (33).
2.6 Statistical analysis
Data analysis was conducted using IBM SPSS version 26 (IBM Corp., Armonk, NY, USA). To assess the normality of the distribution, the Shapiro-Wilk test was applied. Data that deviated from a normal distribution were reported as medians with interquartile ranges (IQR). The Mann-Whitney U test was employed to evaluate significant intergroup differences in physical activity and nutrient intake between genders. Comparisons of rate type data were made using the chi-square test. Spearman’s rank correlation coefficient was utilized to assess the strength and direction of correlations between variables. For the categorical variables derived from the Dietary Quality Questionnaire (DQQ), multiple linear regression analyses were conducted to explore the associations with other variables. To investigate the mediating relationships among physical activity, diet, and various biomarkers, we employed the PROCESS macro for SPSS (34). This tool enabled us to test the mediating effects of physical activity and dietary habits on the relationships between biomarkers. We alternately considered physical activity and diet as independent or mediating variables, with each biomarker serving as the dependent variable. This approach facilitated the identification of significant mediating relationships. Statistical significance was set at P < 0.05.
3 Results
3.1 Sociodemographic characteristics
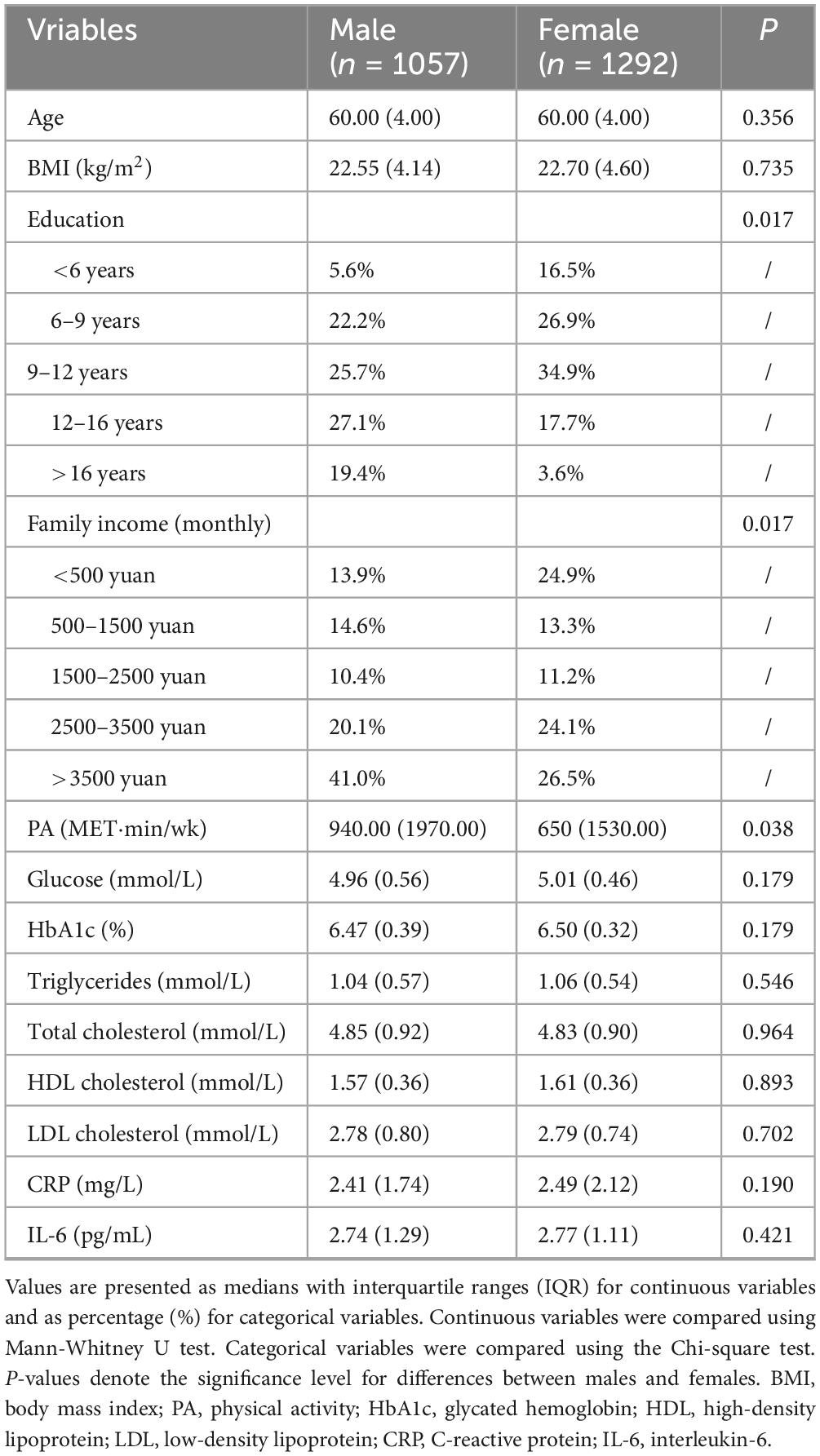
Table 1 presents the sociodemographic characteristics stratified by gender. Comparative analyses revealed statistically significant differences between males and females in terms of education and income (P = 0.017). Males demonstrated significantly higher levels of total physical activity compared to females (P = 0.038). Differences in other indices were not statistically significant.
3.2 Correlation analysis
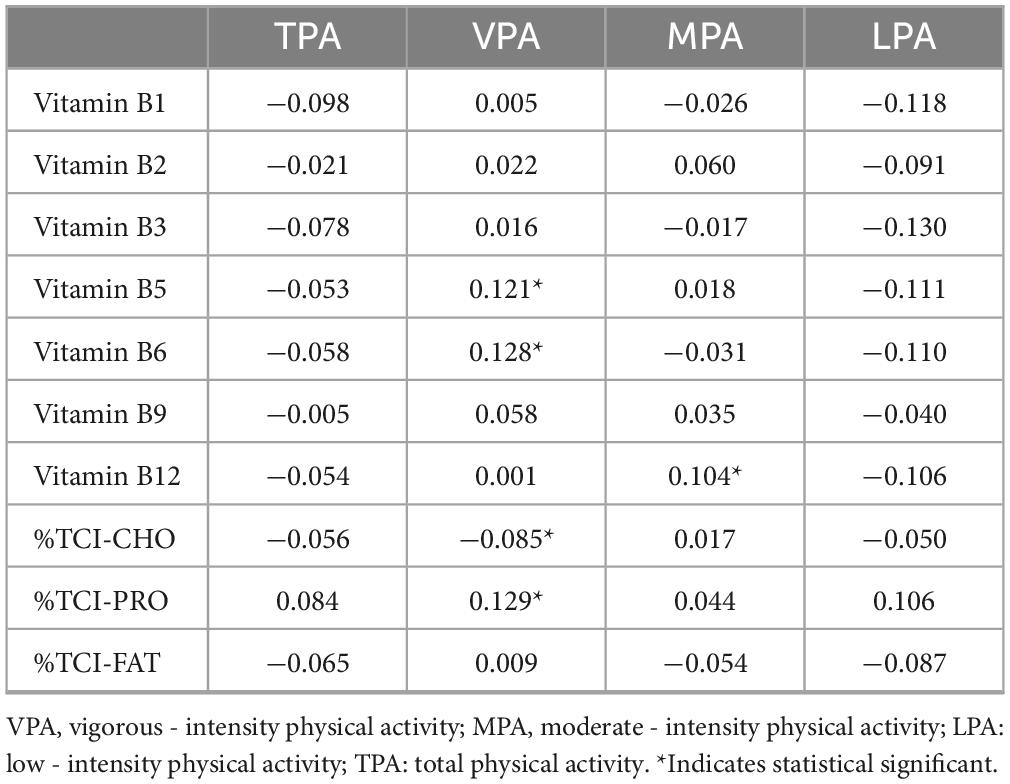
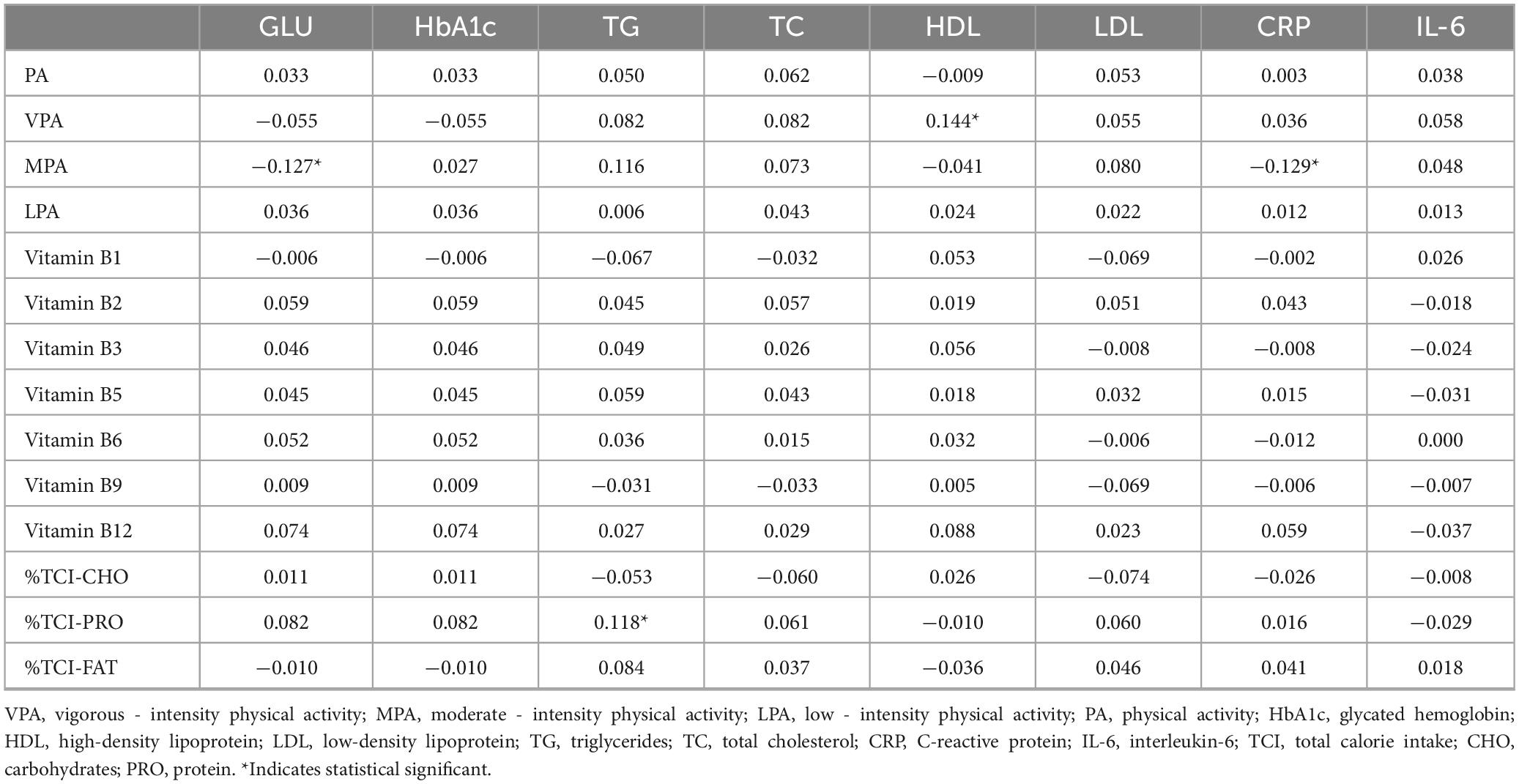
Table 2 results show that vigorous-intensity physical activity was positively correlated with the percentage of protein intake from total caloric intake, vitamins B5, B6, and B12 (r = 0.121; r = 0.128; r = 0.129), and negatively correlated with the percentage of caloric intake from carbohydrates (r = −0.085). Moderate-intensity physical activity was positively correlated with vitamin B12 (r = 0.104).
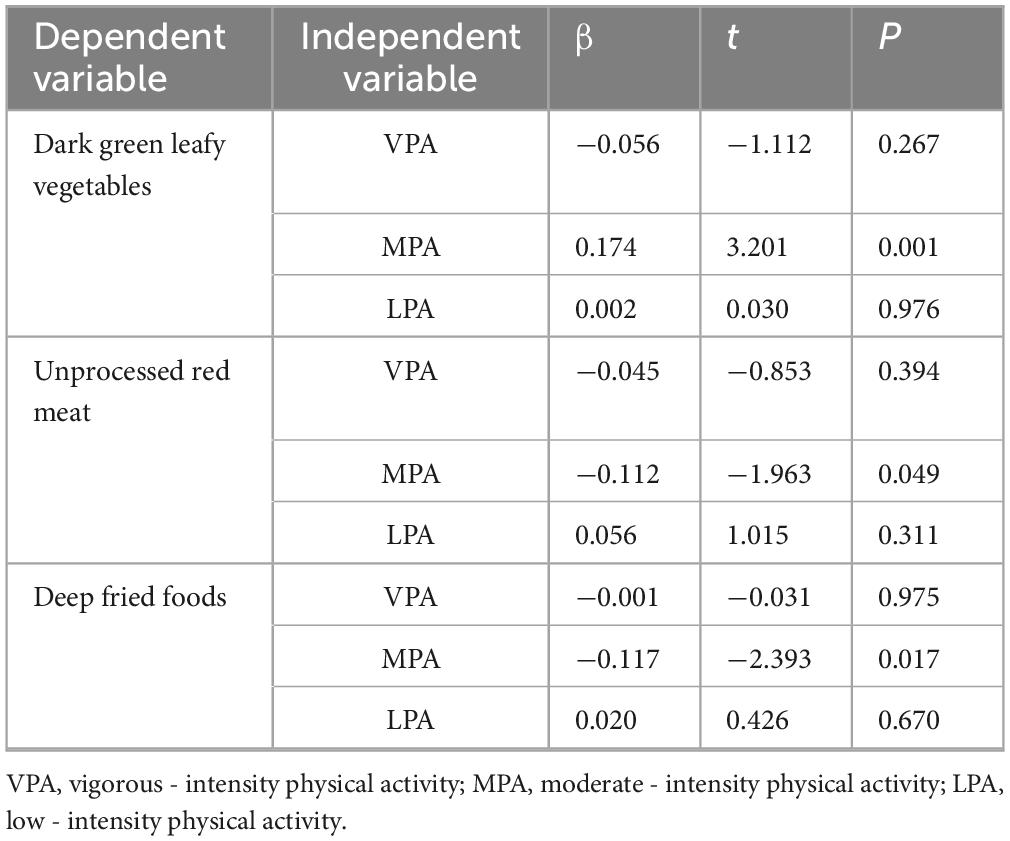
Table 3 results, derived from multivariate linear regression controlling for confounding factors, indicate a positive association between moderate-intensity physical activity and the intake of dark green leafy vegetables (β = 3.201). Conversely, moderate-intensity physical activity was negatively associated with the intake of unprocessed red meat (β = −1.963) and deep-fried foods (β = −2.393).
Table 4 indicates that vigorous levels of physical activity were significantly positively correlated with high-density lipoprotein cholesterol (HDL-C) (r = 0.144). Moderate levels of physical activity were significantly negatively correlated with blood glucose (r = −0.127) and C-reactive protein (CRP) (r = −0.129). Additionally, the percentage of protein intake relative to total caloric intake was significantly positively correlated with triglycerides (r = 0.118).
3.3 Mediating effect analysis
Table 5 results demonstrate that, after controlling for confounding factors, moderate-intensity physical activity has a significant effect on C-reactive protein levels, with the intake of dark green leafy vegetables mediating this relationship, indicating a significant mediating effect. Furthermore, after controlling for confounding factors, moderate-intensity physical activity also significantly affects blood glucose levels, with vitamin B12 intake mediating this relationship, indicating a significant mediating effect Figure 2.
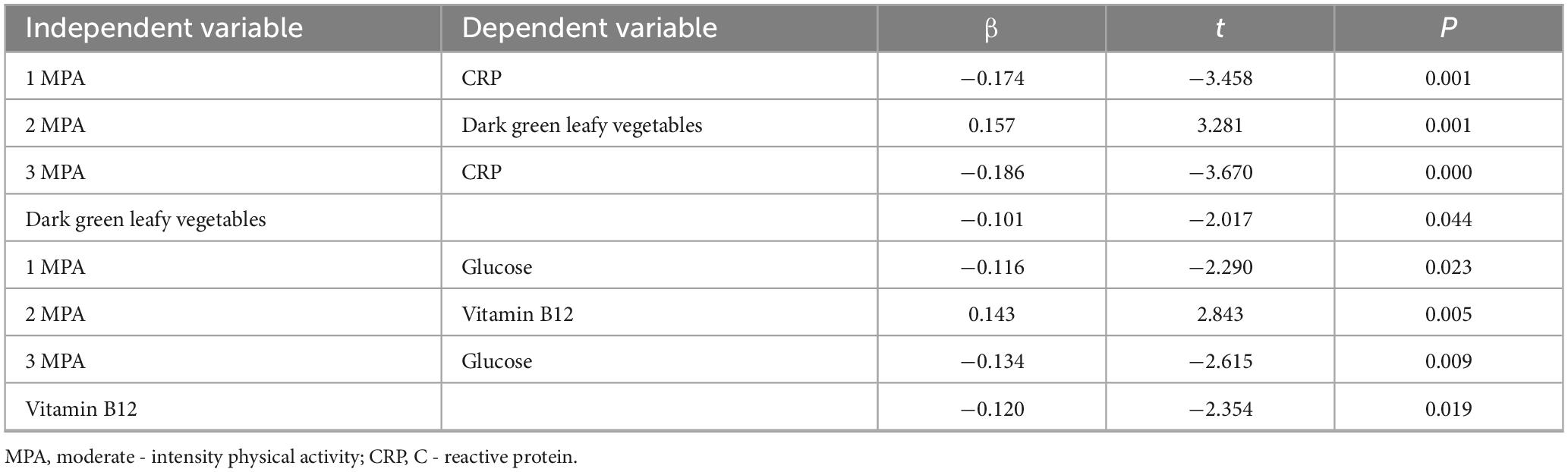
Table 5. Significant mediating effects between physical activity, dietary habits and serum biomarkers.
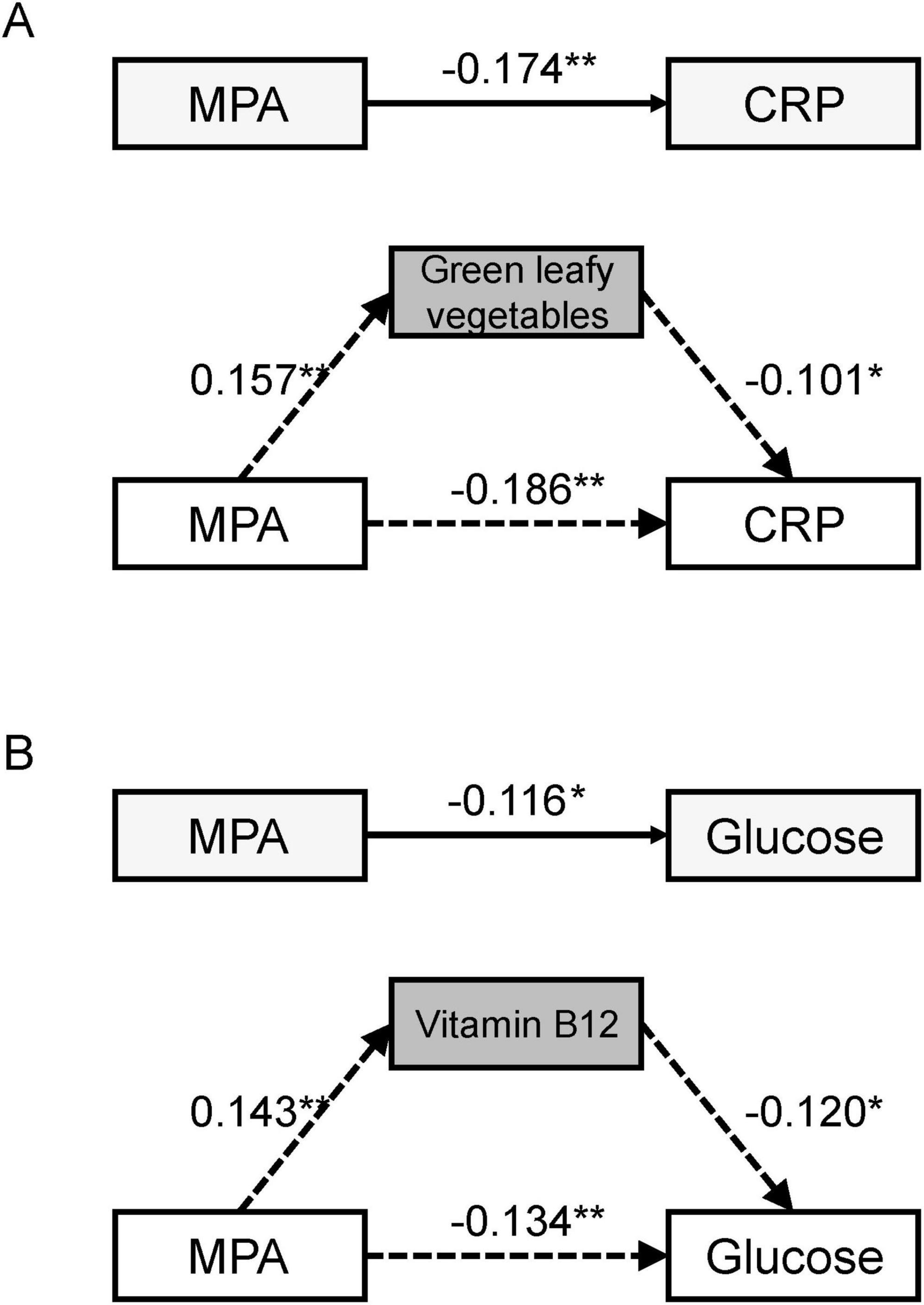
Figure 2. The mediation model of diet mediating the effects of physical activity on biomarkers. (A) Significant mediating effects between MPA, dietary habits and CRP. (B) Significant mediating effects between MPA, nutrient intake and glucose. MPA, moderate - intensity physical activity; CRP, C - reactive protein. *P < 0.05, **P < 0.01.
4 Discussion
4.1 Analysis of correlation results
4.1.1 Correlation analysis of physical activity, nutrient intake and serum biomarkers
This study aimed to examine how physical activity and dietary habits collectively influence glycolipid metabolism and inflammatory markers in elderly individuals. Our findings demonstrate three key outcomes: high-intensity physical activity significantly improves HDL-C levels, moderate-intensity exercise effectively reduces blood glucose and CRP levels, and dietary protein proportion shows a complex relationship with triglyceride levels.
The results reveal that higher intensity physical activity was positively correlated with HDL-C levels. Previous studies have shown that regular high-intensity exercise enhances lipid metabolism by increasing HDL-C concentrations, which may substantially lower atherosclerosis risk (35). Furthermore, research indicates that exercise promotes cardiovascular health through optimized lipid metabolism and decreased visceral fat accumulation (36).
Regarding glucose regulation and inflammation control, moderate-intensity physical activity exhibited significant negative associations with both blood glucose and CRP levels. These observations align with studies demonstrating that moderate exercise improves insulin sensitivity while simultaneously reducing systemic inflammation (37). This dual benefit suggests moderate physical activity may serve as an effective intervention for metabolic disorders in aging populations.
Nutritional analyses uncovered important relationships between dietary patterns and metabolic markers. The positive association between protein intake proportion and triglyceride levels warrants particular attention. While studies confirm that high-protein diets can enhance fat oxidation, research also indicates that excessive protein intake may adversely affect lipid profiles (38). Additionally, the complex interactions observed between macronutrient ratios and metabolic regulation confirm that dietary composition plays a critical role in elderly health outcomes (39).
These findings collectively emphasize the synergistic importance of physical activity and dietary habits in maintaining metabolic health. High-intensity exercise proves particularly beneficial for lipid profiles, while moderate exercise shows advantages for glucose metabolism and inflammation control. Simultaneously, balanced dietary patterns emerge as equally crucial factors. For elderly populations, comprehensive lifestyle interventions incorporating appropriate exercise regimens and nutritional strategies may offer optimal protection against metabolic disorders and support healthy aging.
4.1.2 Correlation analysis between physical activity and nutrient intake
This study aimed to investigate the relationship between physical activity levels and nutritional intake patterns in elderly individuals. The key findings demonstrate that: high-intensity physical activity was positively associated with protein and B vitamin intake while negatively correlated with carbohydrate proportion; moderate-intensity exercise showed a specific positive correlation with vitamin B12 intake.
The results revealed significant positive correlations between high-intensity physical activity and the intake of protein, vitamins B5, B6, and B12. Previous research has demonstrated that high-intensity exercise stimulates greater protein consumption and increases requirements for B vitamins involved in protein metabolism and energy production (40). Studies indicate these nutrients play vital roles in muscle recovery and metabolic processes following intense physical activity (41).
Conversely, high-intensity exercise showed a negative association with carbohydrate intake proportion. Research suggests this may reflect altered energy substrate utilization during high-intensity activity, where the body preferentially utilizes fats and proteins over carbohydrates as primary fuel sources (42). These findings highlight how exercise intensity influences macronutrient selection and metabolic demands.
Regarding moderate-intensity activity, the positive correlation with vitamin B12 intake is particularly noteworthy. Vitamin B12 is known to be essential for neurological function and energy metabolism. Studies have shown that moderate physical activity may increase physiological demands for this vitamin, potentially explaining the observed dietary pattern (43).
These findings collectively suggest that different exercise intensities are associated with distinct nutritional intake patterns in elderly individuals. High-intensity activity appears linked to increased protein and B vitamin consumption with reduced carbohydrate proportion, while moderate exercise shows specific associations with vitamin B12 intake. These exercise-nutrient relationships may have important implications for optimizing dietary recommendations to support physical activity in aging populations.
The interaction between physical activity and nutritional intake observed in this study provides valuable insights for developing targeted lifestyle interventions. Future research should explore whether these associations reflect conscious dietary choices or physiological adaptations to different exercise regimens.
4.1.3 Significant associations between physical activity and dietary habits
This study aimed to examine the relationship between moderate-intensity physical activity (MPA) and dietary patterns in elderly individuals. The key findings demonstrate that: MPA was positively associated with consumption of dark green leafy vegetables, while showing negative associations with intake of unprocessed red meat and fried foods.
The positive correlation between MPA and dark green leafy vegetable intake suggests that regular moderate exercise may promote healthier food choices. Research has shown that dark green leafy vegetables are rich sources of vitamin K, folate, fiber and antioxidants, whose consumption can enhance immune function and lower chronic disease risk (44). These findings support the notion that physical activity may influence dietary preferences toward more nutrient-dense options.
Conversely, the negative associations between MPA and consumption of unprocessed red meat and fried foods indicate that active individuals may naturally reduce their intake of less healthy food options. Studies have demonstrated that regular physical activity tends to decrease consumption of high-fat, high-cholesterol foods like red meat, which are linked to increased cardiovascular and metabolic disease risk (45). Similarly, research indicates that active individuals typically consume fewer fried foods, which are high in trans fats and calories associated with obesity and heart disease (46).
These findings collectively suggest that moderate-intensity physical activity may promote healthier dietary patterns through multiple mechanisms. First, it appears to increase preference for nutrient-rich vegetables. Second, it seems to decrease consumption of potentially harmful food items. The combined effect of these dietary shifts could significantly contribute to better long-term health outcomes in elderly populations.
The observed associations between physical activity and dietary habits provide important insights for developing integrated lifestyle interventions. Future research should investigate whether these relationships reflect conscious behavior changes or more subtle physiological and psychological adaptations to regular exercise. Additionally, longitudinal studies could help determine if these dietary changes mediate the known health benefits of physical activity.
4.2 Significant mediating effects analysis between physical activity, dietary habits and serum biomarkers
This study aimed to investigate the mediating role of dietary habits in the relationship between moderate-intensity physical activity (MPA) and key serum biomarkers in elderly individuals. The findings demonstrate that: MPA showed significant negative associations with CRP and blood glucose levels while positively correlating with vitamin B12 levels, with dietary habits (particularly dark green leafy vegetable intake) serving as important mediators of these relationships.
The observed negative association between MPA and CRP levels aligns with existing research showing that physical activity can modulate inflammatory responses. Studies have demonstrated that regular moderate exercise reduces CRP concentrations, potentially lowering cardiovascular disease risk through anti-inflammatory mechanisms (47). CRP elevation, a well-established marker of chronic inflammation, is consistently reduced through physical activity interventions (48).
The mediating role of dark green leafy vegetable intake in the MPA-CRP relationship represents a key finding. Research indicates that physical activity promotes healthier food choices, particularly increasing consumption of nutrient-dense vegetables (49). These vegetables provide antioxidants and anti-inflammatory compounds that may synergize with exercise to further reduce systemic inflammation.
Regarding glucose metabolism, MPA showed both direct and indirect (diet-mediated) effects on blood glucose regulation. Previous studies confirm that moderate exercise enhances insulin sensitivity through multiple pathways (50). Our results extend this understanding by suggesting that dietary improvements, particularly increased vegetable intake, may amplify these metabolic benefits.
The positive association between MPA and vitamin B12 levels may reflect exercise-induced changes in dietary patterns. As vitamin B12 plays crucial roles in neurological function and erythropoiesis, research suggests that physically active individuals tend to consume more B12-rich foods, potentially explaining this relationship (51).
These findings have important clinical implications. The dual approach of combining moderate physical activity with dietary modifications (particularly increased vegetable consumption) appears particularly effective for improving metabolic and inflammatory markers in elderly populations. This synergistic effect may offer enhanced protection against chronic diseases compared to either intervention alone (52).
Future research should explore whether these mediating relationships vary by demographic factors or baseline health status. Additionally, intervention studies could help determine optimal combinations of physical activity and dietary changes for maximal health benefits in aging populations.
4.3 Limitations
This study has several limitations. The cross-sectional design prevents conclusions about causality. Self-reported physical activity and dietary data may be affected by recall bias. The sample was limited to elderly individuals in specific communities, which may affect generalizability. Future research using longitudinal designs and objective measurements would help confirm these findings.
5 Conclusion
Physical activity levels and dietary habits in older adults play an important role in metabolic health. Specifically, moderate-intensity physical activity was significantly associated with high-density lipoprotein cholesterol levels, whereas lower levels of physical activity were negatively associated with blood glucose and C-reactive protein (CRP) levels. In addition, dietary protein intake was significantly and positively correlated with triglycerides. These results suggest that by promoting moderate physical activity and optimizing dietary structure, glycolipid metabolism and reducing inflammation levels in older adults can be effectively improved. Therefore, health interventions for older adults should emphasize the integrated management of physical activity and nutritional intake to enhance their overall metabolic health.
Data availability statement
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The study protocol was approved by the Ethics Committee of Jiangxi Normal University (Approval No. RB_0343_56). All participants provided written informed consent prior to participation, and the study was conducted in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments.
Author contributions
WZ: Funding acquisition, Writing – original draft, Formal analysis, Writing – review & editing, Software, Visualization, Project administration, Supervision, Methodology, Conceptualization, Investigation. LZ: Funding acquisition, Writing – review & editing, Writing – original draft, Project administration, Resources, Formal analysis, Software, Visualization, Conceptualization, Validation, Investigation, Data curation. JC: Visualization, Writing – original draft, Resources, Formal analysis, Project administration, Funding acquisition, Methodology, Data curation, Supervision, Validation, Investigation, Writing – review & editing, Conceptualization, Software.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the General Project of Jiangxi Provincial Social Science Planning titled “Evaluation and Promotion Path of Public Sports Service Accessibility for the Elderly in Urban Communities of Jiangxi Province” (Project No. 25TY03), the 2023 Planning Fund Project of Humanities and Social Sciences Research of the Ministry of Education titled “Evaluation and Improvement Path of Public Fitness Public Service Accessibility in Rural Areas under the Background of ‘Rural Revitalization”’ (Project No. 23YJA890052), the Planning Project of Humanities and Social Sciences Research in Jiangxi Provincial Colleges and Universities titled “Inheritance and Innovative Development of Village Sports Performance Culture in Jiangxi Province from the Perspective of Rural Revitalization” (Project No. TY23102), and the Humanities and Social Sciences Research Project of Jiangxi Provincial Colleges and Universities (Jiangxi Provincial Department of Education) (Project No. TY20111).
Acknowledgments
We gratefully acknowledge all participants for their time and cooperation. We also thank the community health staff for their assistance in data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author declares that Gen AI was used in the creation of this manuscript. During the preparation of this work, the authors used ChatGPT (OpenAI) for language polishing and grammatical refinement. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dziechciaż M, Filip R. Biological psychological and social determinants of old age: bio-psycho-social aspects of human aging. Ann Agric Environ Med. (2014) 21:835–8.
2. Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, Verdin E. From discoveries in ageing research to therapeutics for healthy ageing. Nature. (2019) 571:183–92. doi: 10.1038/s41586-019-1365-2
3. De Winter G. Aging as disease. Med Health Care Philos. (2015) 18:237–43. doi: 10.1007/s11019-014-9600-y
4. Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, et al. Geroscience: linking aging to chronic disease. Cell. (2014) 159:709–13. doi: 10.1016/j.cell.2014.10.039
5. Uyar B, Palmer D, Kowald A, Murua Escobar H, Barrantes I, Möller S, et al. Single-cell analyses of aging, inflammation and senescence. Ageing Res Rev. (2020) 64:101156. doi: 10.1016/j.arr.2020.101156
6. Hoogwerf BJ. Hypoglycemia in older patients. Clin Geriatr Med. (2020) 36:395–406. doi: 10.1016/j.cger.2020.04.001
8. Yang H, Han L, Sheng T, He Q, Liang J. Effects of replenishing qi, promoting blood circulation and resolving phlegm on vascular endothelial function and blood coagulation system in senile patients with hyperlipemia. J Tradit Chin Med. (2006) 26:120–4.
9. Roberts CK, Hevener AL, Barnard RJ. Metabolic syndrome and insulin resistance: underlying causes and modification by exercise training. Compr Physiol. (2013) 3:1–58. doi: 10.1002/cphy.c110062
10. Zhang F, Li Y, Zhao Y, Zhou X, Ji L. Is visceral abdominal fat area a better indicator for hyperglycemic risk? Results from the pinggu metabolic disease study. J Diabetes Investig. (2020) 11:888–95. doi: 10.1111/jdi.13217
11. Ying L, Wang L, Guo K, Hou Y, Li N, Wang S, et al. Paracrine FGFs target skeletal muscle to exert potent anti-hyperglycemic effects. Nat Commun. (2021) 12:7256. doi: 10.1038/s41467-021-27584-y
12. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol. (2014) 69:S4–9.
13. Thiagarajan S, Tan JW, Zhou S, Tan QX, Hendrikson J, Ng WH, et al. Postoperative inflammatory marker surveillance in colorectal peritoneal carcinomatosis. Ann Surg Oncol. (2021) 28:6625–35.
14. Yihui C, Yanfeng G. Inflammatory markers in patients with hypertension. Br J Hosp Med. (2023) 84:1–8. doi: 10.12968/hmed.2022.0531
15. Bovolini A, Garcia J, Andrade MA, Duarte JA. Metabolic syndrome pathophysiology and predisposing factors. Int j Sports Med. (2021) 42:199–214. doi: 10.1055/a-1263-0898
16. Pawelec G. Aging as an inflammatory disease and possible reversal strategies. J Allergy Clin Immunol. (2020) 145:1355–6. doi: 10.1016/j.jaci.2020.02.022
17. Gomes MJ, Martinez PF, Pagan LU, Damatto RL, Cezar MDM, Lima ARR, et al. Skeletal muscle aging: influence of oxidative stress and physical exercise. Oncotarget. (2017) 8:20428–40. doi: 10.18632/oncotarget.14670
18. Soysal P, Stubbs B, Lucato P, Luchini C, Solmi M, Peluso R, et al. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev. (2016) 31:1–8. doi: 10.1016/j.arr.2016.08.006
19. Battista F, Bettini S, Verde L, Busetto L, Barrea L, Muscogiuri G. Diet and physical exercise in elderly people with obesity: the state of the art. Eur J Intern Med. (2024) 130:9–18. doi: 10.1016/j.ejim.2024.08.007
20. Valenzuela PL, Castillo-García A, Morales JS, Izquierdo M, Serra-Rexach JA, Santos-Lozano A, et al. Physical exercise in the oldest old. Compr Physiol. (2019) 9:1281–304. doi: 10.1002/cphy.c190002
21. Yasuda T. Selected methods of resistance training for prevention and treatment of sarcopenia. Cells. (2022) 11:1389. doi: 10.3390/cells11091389
22. Tolkien K, Bradburn S, Murgatroyd C. An anti-inflammatory diet as a potential intervention for depressive disorders: a systematic review and meta-analysis. Clin Nutr. (2019) 38:2045–52. doi: 10.1016/j.clnu.2018.11.007
23. Mazza E, Ferro Y, Pujia R, Mare R, Maurotti S, Montalcini T, et al. Mediterranean diet in healthy aging. J Nutr Health Aging. (2021) 25:1076–83. doi: 10.1007/s12603-021-1675-6
24. Gao Y, Zhang W, Zeng LQ, Bai H, Li J, Zhou J, et al. Exercise and dietary intervention ameliorate high-fat diet-induced NAFLD and liver aging by inducing lipophagy. Redox Biol. (2020) 36:101635. doi: 10.1016/j.redox.2020.101635
25. Smith HA, Betts JA. Nutrient timing and metabolic regulation. J Physiol. (2022) 600:1299–312. doi: 10.1113/JP280756
26. Rong L, Zou J, Ran W, Qi X, Chen Y, Cui H, et al. Advancements in the treatment of non-alcoholic fatty liver disease (NAFLD). Front Endocrinol. (2023) 13:1087260. doi: 10.3389/fendo.2022.1087260
27. Busetto L, Bettini S, Makaronidis J, Roberts CA, Halford JCG, Batterham RL. Mechanisms of weight regain. Eur J Intern Med. (2021) 93:3–7. doi: 10.1016/j.ejim.2021.01.002
28. Śliwicka E, Popierz-Rydlewska N, Straburzyńska-Lupa A, Nikolov J, Pilaczyńska-Szcześniak Ł, Gogojewicz A. Prevention is better than cure-body composition and glycolipid metabolism after a 24-week physical activity program without nutritional intervention in healthy sedentary women. Nutrients. (2024) 16:2536. doi: 10.3390/nu16152536
29. Amare F, Alemu Y, Enichalew M, Demilie Y, Adamu S. Effects of aerobic, resistance, and combined exercise training on body fat and glucolipid metabolism in inactive middle-aged adults with overweight or obesity: a randomized trial. BMC Sports Sci Med Rehabil. (2024) 16:189. doi: 10.1186/s13102-024-00982-7
30. Roberts CK, Won D, Pruthi S, Kurtovic S, Sindhu RK, Vaziri ND, et al. Effect of a short-term diet and exercise intervention on oxidative stress, inflammation, MMP-9, and monocyte chemotactic activity in men with metabolic syndrome factors. J Appl Physiol. (2006) 100:1657–65. doi: 10.1152/japplphysiol.01292.2005
31. Ma M, Dong FW, Lan JY. Associations between sleep duration, physical activity, and cognitive impairment in older adults-empirical analysis based on CHARLS data. Front Public Health. (2025) 13:1589606. doi: 10.3389/fpubh.2025.1589606
32. Gao M, Wang J, Qiu Y, Chen Y, Cao Q, Pan Y, et al. Association between dietary diversity and subjective cognitive decline in the middle-aged and elderly chinese population: a cross-sectional study. Nutrients. (2024) 16:3603. doi: 10.3390/nu16213603
33. Wang Y, Li Y, Liu X, Liu R, Mao Z, Tu R, et al. Gender-specific prevalence of poor sleep quality and related factors in a Chinese rural population: the Henan Rural Cohort Study. Sleep Med. (2019) 54:134–41. doi: 10.1016/j.sleep.2018.10.031
34. Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instruments Comput. (2004) 36:717–31. doi: 10.3758/bf03206553
35. Ha M-S, Kim J-H, Kim Y-S, Kim D-Y. Effects of aquarobic exercise and burdock intake on serum blood lipids and vascular elasticity in Korean elderly women. Exp Gerontol. (2018) 101:63–8. doi: 10.1016/j.exger.2017.11.005
36. Gröne M, Duse DA, Kramser N, Ophoff N, Schweers H, Voß F, et al. Cocoa flavanols improve peakVO 2 and exercise capacity in a randomized double blinded clinical trial in healthy elderly people. Food Funct. (2023) 14:7562–73. doi: 10.1039/d3fo01737k
37. Koh S, Kim D, Kim M, Kim T. Aerobic exercise effects on systolic blood pressure and endothelial inflammation in obese and non-obese elderly women with isolated systolic hypertension. J Hypertens. (2024) 42:1743–9. doi: 10.1097/HJH.0000000000003794
38. He J, Yu S, Fang A, Shen X, Li K. Association between protein intake and the risk of hypertension among chinese men and women: a longitudinal study. Nutrients. (2022) 14:1276. doi: 10.3390/nu14061276
39. Fart F, Tingö L, Engelheart S, Lindqvist CM, Brummer RJ, Kihlgren A, et al. Gut health and its association with wellbeing and nutrient intake in community-dwelling older adults. Gastroenterol Insights. (2022) 13:349–64. doi: 10.3390/gastroent13040035
40. Liu S, Liu H, Yang L, Wang K, Chen N, Zhang T, et al. A review of rehabilitation benefits of exercise training combined with nutrition supplement for improving protein synthesis and skeletal muscle strength in patients with cerebral stroke. Nutrients. (2022) 14:4995. doi: 10.3390/nu14234995
41. Skalny A, Aschner M, Tsatsakis A, Rocha J, Santamaria A, Spandidos D, et al. Role of vitamins beyond vitamin D 3 in bone health and osteoporosis (Review). Int J Mol Med. (2023) 53:9. doi: 10.3892/ijmm.2023.5333
42. Khademosharie M, Mollanovruzi A. Effects of high-intensity training upon appetite, body mass, aerobic capacity, and metabolic hormones in overweight women. Endocrinol Res Pract. (2023) 27:21–7. doi: 10.5152/erp.2023.22047
43. Alzohily B, AlMenhali A, Gariballa S, Munawar N, Yasin J, Shah I. Unraveling the complex interplay between obesity and vitamin D metabolism. Sci Rep. (2024) 14:7583. doi: 10.1038/s41598-024-58154-z
44. Lin S-C, Wang C-Y, Hou T-H, Chen H-C, Wang C-C. Impact of fruit and vegetable enzyme supplementation on aerobic performance and lactate response in older adults following high-intensity interval exercise through exergaming: randomized experimental matched-pair study. JMIR Ser Games. (2024) 12:e52231. doi: 10.2196/52231
45. Yan LL, Li C, Zou S, Li Y, Gong E, He Z, et al. Healthy eating and all-cause mortality among Chinese aged 80 years or older. Int J Behavi Nutr Phys Activ. (2022) 19:60. doi: 10.1186/s12966-022-01280-6
46. Ho E, Qualls C, Villareal DT. Effect of diet, exercise, or both on biological age and healthy aging in older adults with obesity: secondary analysis of a randomized controlled trial. J Nutr Health Aging. (2022) 26:552–7. doi: 10.1007/s12603-022-1812-x
47. Estébanez B, Visavadiya NP, Vargas JE, Rivera-Viloria M, Khamoui AV, de Paz JA, et al. Resistance training modulates reticulum endoplasmic stress, independent of oxidative and inflammatory responses, in elderly people. Antioxidants. (2022) 11:2242. doi: 10.3390/antiox11112242
48. Maria Gonzalez L. Therapeutic effects of physical exercise and the mesenchymal stem cell secretome by modulating neuroinflammattory response in multiple sclerosis. Curr Stem Cell Res Ther. (2022) 17:621–32. doi: 10.2174/1574888516666211209155333
49. Babaei P, Hoseini R. Exercise training modulates adipokine dysregulations in metabolic syndrome. Sports Med Health Sci. (2022) 4:18–28. doi: 10.1016/j.smhs.2022.01.001
50. Chien Y-J, Tsao J-P, Tsai C-T, Cheng I-S, Hsu C-L. Antifatigue effect of okara protein hydrolysate supplementation during cycling exercise in men: a pre-post uncontrolled pilot study. J Int Soc Sports Nutr. (2024) 21:6479. doi: 10.1080/15502783.2024.2416479
51. Niklewicz A, Smith AD, Smith A, Holzer A, Klein A, McCaddon A, et al. The importance of vitamin B12 for individuals choosing plant-based diets. Eur J Nutr. (2023) 62:1551–9. doi: 10.1007/s00394-022-03025-4
Keywords: physical activity, vitamin B, inflammatory markers, elderly, mediation analysis
Citation: Zhang W, Zou L and Chen J (2025) The impact of physical activity and dietary habits on glycolipemic metabolism and inflammatory markers in the elderly: a cross-sectional study. Front. Nutr. 12:1600038. doi: 10.3389/fnut.2025.1600038
Received: 25 March 2025; Accepted: 03 September 2025;
Published: 22 September 2025.
Edited by:
Xin Zhang, Ningbo University, ChinaReviewed by:
Carlos Farinha, Polytechnic Institute of Castelo Branco, PortugalRola Angga Lardika, Riau University, Indonesia
Copyright © 2025 Zhang, Zou and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Limin Zou, Y2hlbmppYW5nYW5nQHNubnUuZWR1LmNu
 Wentao Zhang1
Wentao Zhang1 Jiangang Chen
Jiangang Chen