- 1Yichang Central Department of Critical Care Medicine, The First College of Clinical Medical Science, China Three Gorges University, Yichang Central People’s Hospital, Yichang, Hubei, China
- 2Yichang Central People’s Hospital, Yichang, Hubei, China
Background: Vitamin C, a water-soluble essential micronutrient, exhibits multifaceted physiological roles including immune modulation and enhanced resistance to infectious pathogens. Evidence suggests that hypovitaminosis C is associated with adverse clinical outcomes in critically ill populations, with notably high prevalence observed in acute kidney injury patients. This retrospective study aimed to evaluate the potential association between vitamin C supplementation during intensive care unit admission and improved clinical outcomes, specifically in sepsis-associated acute kidney injury (SA-AKI).
Methods: Utilizing data from the Medical Information Mart for Intensive Care IV (MIMIC-IV), a repository of ICU patient records from Beth Israel Deaconess Medical Center (United States), we identified patients diagnosed with SA-AKI. Participants were stratified into two cohorts: those receiving intravenous vitamin C supplementation during ICU stay (vitamin C group) and those without supplementation (non-vitamin C group). Primary outcomes, including in-hospital mortality, were evaluated using Kaplan–Meier survival curves, Cox proportional hazards regression models, and subgroup analyses. Propensity score matching (PSM) was employed to mitigate potential confounding. Secondary outcomes encompassed 28-day mortality.
Results: Among 16,140 patients diagnosed with SA-AKI, 589 received vitamin C supplementation, while 15,551 did not. Kaplan–Meier analysis revealed a significant divergence in survival probabilities between cohorts (log-rank p < 0.001). After adjusting for confounders via Cox regression, the vitamin C group demonstrated a 17% reduction in in-hospital mortality risk (adjusted hazard ratio [aHR] 0.67, 95% CI: 0.57–0.79; p < 0.001). Consistency was maintained across PSM, paired algorithm, and overlap weighting analyses, with all p < 0.001.
Conclusion: Vitamin C supplementation during ICU admission may be associated with reduced in-hospital mortality in SA-AKI patients. These findings underscore the need for prospective randomized trials to validate causality.
1 Introduction
Sepsis is clinically defined as a potentially fatal multiorgan dysfunction resulting from a dysregulated host response to infection, which contributes to substantial morbidity and mortality burdens in critically ill populations (1). Global epidemiological data indicate sepsis affects nearly 50 million cases annually, accounting for approximately 11 million fatalities worldwide (2). Renal dysfunction frequently manifests as the earliest and most severe organ involvement in septic patients. Acute kidney injury (AKI), a clinically heterogeneous syndrome characterized by rapid deterioration of glomerular filtration rate (GFR), demonstrates phenotypic variability extending beyond traditional acute renal failure paradigms (3). Importantly, sepsis-associated AKI (SA-AKI) is independently associated with increased hazards for in-hospital mortality, accelerated progression to chronic kidney disease, and persistent renal replacement therapy (RRT) dependency (4, 5).
Emerging evidence has delineated the pleiotropic mechanisms underlying vitamin C’s therapeutic potential in sepsis management, encompassing antioxidant, anti-inflammatory, microvascular stabilizing, and cytoprotective properties (6–9). Functioning as a potent electron donor, this micronutrient effectively neutralizes reactive oxygen species (ROS), thereby reducing lipid peroxidation cascade, preventing DNA strand breaks, and maintaining podocyte cytoskeletal architecture through glutathione regeneration pathways (10, 11). Preclinical studies substantiate its nephroprotective efficacy, particularly in attenuating vancomycin-induced acute tubular necrosis via Nrf2-mediated oxidative stress mitigation (12). Mechanistically, vitamin C exerts immunomodulatory effects through suppression of NF-κB nuclear translocation, subsequent downregulation of proinflammatory mediators (TNF-α, IL-6, HMGB1), induction of M2 macrophage polarization via STAT6 activation, and regulation of neutrophil extracellular trap (NET) formation (11–13). The dual hemodynamic benefits are achieved through enhanced nitric oxide synthase (NOS) coupling for endothelial-dependent vasodilation, concomitantly facilitating catecholamine biosynthesis by serving as an essential cofactor for dopamine β-hydroxylase, which potentiates α1-adrenergic receptor responsiveness to vasoactive agents.
Despite robust preclinical evidence supporting vitamin C’s antioxidant, immunomodulatory, and organ-protective effects in sepsis, its clinical efficacy remains debated. While a review suggests potential mortality reduction in sepsis, concerns have emerged regarding increased risks of death or organ dysfunction in adults receiving vasopressors and intravenous vitamin C therapy (14). Shao et al. (15) reported dose-dependent mortality risks with vitamin C, and meta-analyses reveal conflicting conclusions on combination therapies due to heterogeneous patient populations and variable treatment protocols (16). The C-EASIE trial by Vandervelden et al. (17) found no significant improvement in 28-day mortality or organ function with early vitamin C use. Existing studies predominantly focus on general sepsis populations, overlooking sepsis-associated acute kidney injury (SA-AKI) patients—a high-risk subgroup with distinct pathophysiology. Our study utilizes a real-world ICU database to investigate vitamin C’s association with outcomes in SA-AKI patients through propensity score analyses, aiming to inform targeted interventions and clinical trial design.
2 Methods
2.1 Data source
This population-based cohort study utilized the Medical Information Mart for Intensive Care IV (MIMIC-IV v3.0), an expanded critical care database containing 76,540 ICU admissions from 2008 to 2019. Data access was authorized (Certification ID: 13278787), with ethical approvals granted by the MIT Institutional Review Board (No. 0403000206) and Beth Israel Deaconess Medical Center (2001-P-001699/14). All data were de-identified prior to analysis (18).
2.2 Study population
From 65,366 patients with initial ICU admissions, we included adults (≥18 years) diagnosed with SA-AKI [Sepsis-3 criteria (19)] and ICU stays ≥48 h. AKI was defined per KDIGO 2012 guidelines (20): serum creatinine (sCr) increase ≥0.3 mg/dL (26.5 μmol/L) within 48 h, sCr ≥ 1.5 × baseline within 7 days, or urine output <0.5 mL/kg/h over 6 h. Baseline sCr was derived from pre-ICU records or the first admission measurement if unavailable.
2.3 Exposure and covariates
The primary exposure in this study was defined as the administration of vitamin C supplementation via any route (intravenous or oral) during the ICU stay. This inclusive definition was adopted to comprehensively capture all patients who received vitamin C as part of their clinical management. Patients were categorized into two groups based on their exposure status: those who received vitamin C supplementation during their ICU admission (exposed group), and those who did not receive vitamin C supplementation (non-exposed group). Covariates included in the baseline table comprised demographic characteristics (age, sex, race, BMI), comorbidities (including hypertension, diabetes, congestive heart failure, chronic pulmonary disease, liver disease, and malignancy), lifestyle factors (smoking status), and disease severity scores (SOFA, LODS, SAPS II, Charlson Comorbidity Index, and AKI stage) to adjust for baseline differences and chronic disease burden. In addition, clinical interventions (such as invasive mechanical ventilation, continuous renal replacement therapy, vasopressors, immunosuppressants, glucocorticoids, and antihypertensive agents), vital signs (temperature, heart rate, blood pressure, and oxygenation indices), and laboratory parameters (including lactate, blood cell counts, albumin, creatinine, blood urea nitrogen, coagulation indices, liver function tests, glucose, and urine output) were incorporated to account for acute physiological status and organ function.
2.4 Outcomes
Primary outcome: In-hospital mortality.
Secondary outcomes: 28-day mortality.
2.5 Statistical analysis
Categorical variables were reported as frequencies (%), continuous variables as mean ± SD or median (IQR). Group comparisons employed chi-square, t-, or Kruskal-Wallis tests. Survival analysis used Kaplan–Meier curves with log-rank tests.
To address confounding, 1:1 propensity score matching (PSM, caliper = 0.05) incorporated age, sex, race, vitals, labs, comorbidities, and SOFA scores. Covariate balance was verified via standardized mean differences (SMD < 0.1). Multivariable Cox regression adjusted for propensity score-weighted covariates. Sensitivity analyses included paired algorithm (PA) (21) and overlap weighting (OW) models (22). Subgroup analyses stratified by age, sex, SOFA, interventions, and comorbidities. Analyses utilized R v4.3.3.
3 Results
3.1 Participant selection
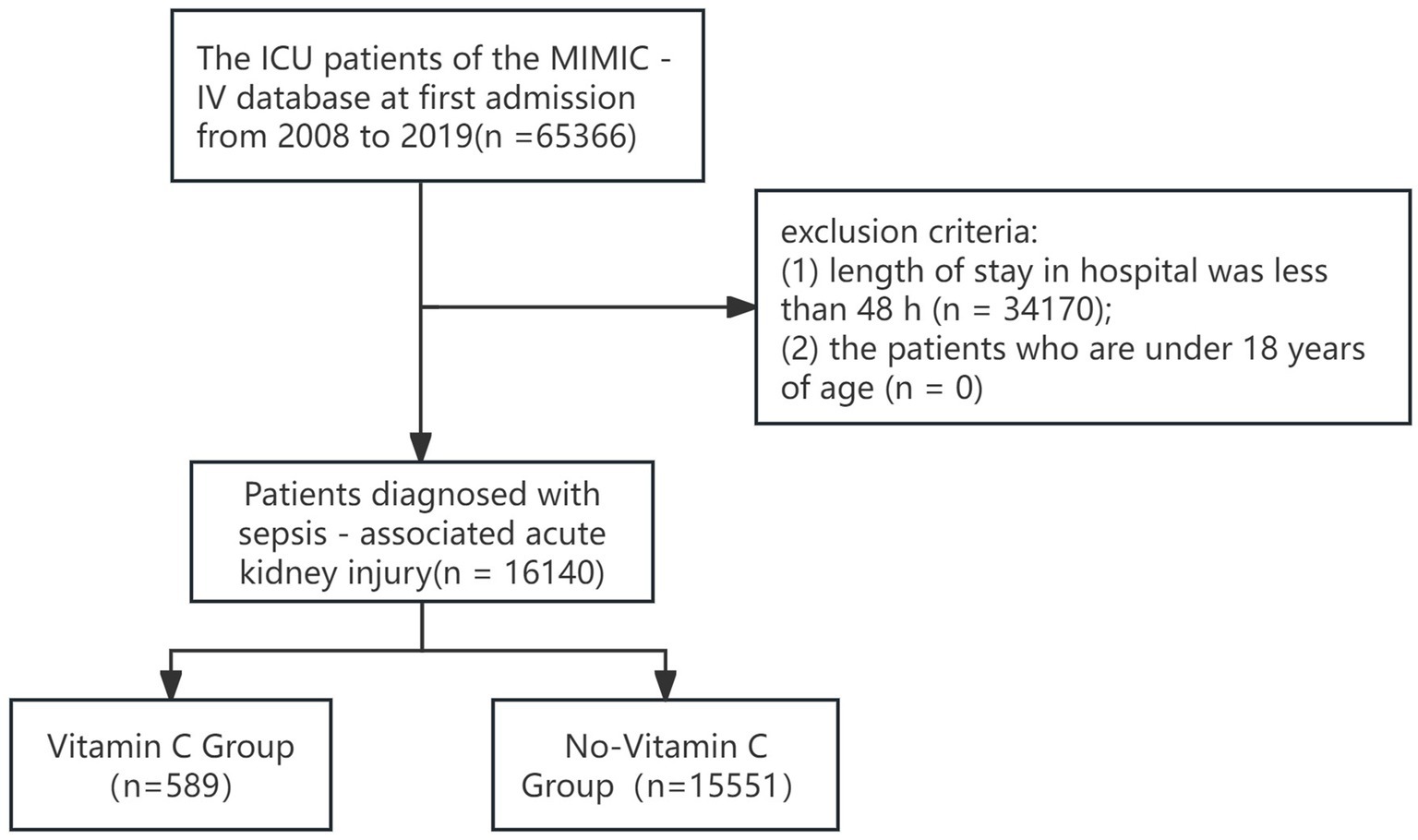
From 65,366 initial ICU admissions, 16,140 SA-AKI patients were enrolled (Figure 1), including 589 vitamin C recipients and 15,551 non-recipients.
3.2 Baseline characteristics
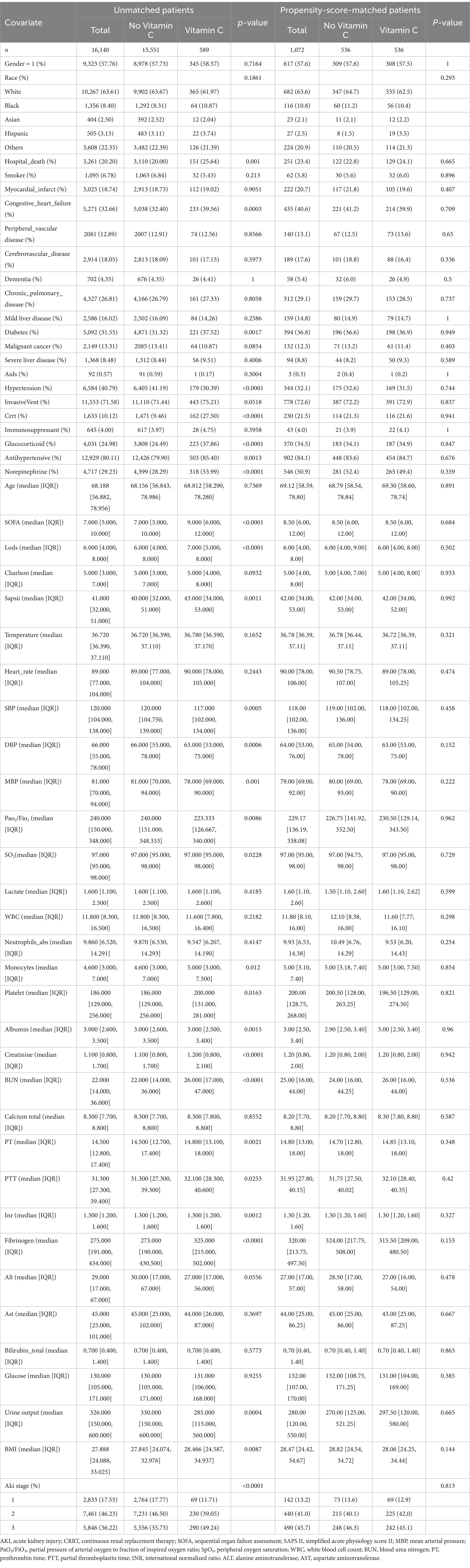
Pre-PSM, the vitamin C group exhibited higher BUN levels, comorbidities (congestive heart failure, chronic lung disease, severe liver disease, diabetes), Charlson indices, and intervention requirements (CRRT, mechanical ventilation). Post-PSM, covariates were balanced across groups (Table 1).
3.3 Primary outcomes
The overall in-hospital mortality rate was 18.8%. Subgroup analysis revealed distinct mortality patterns: the vitamin C supplementation group exhibited significantly lower in-hospital mortality (20.0%, 151/589) compared to the non-supplementation group (25.6%, 3,110/15,551). Kaplan–Meier survival curves demonstrated a pronounced divergence in 28-day mortality favoring the vitamin C cohort (Figure 2).
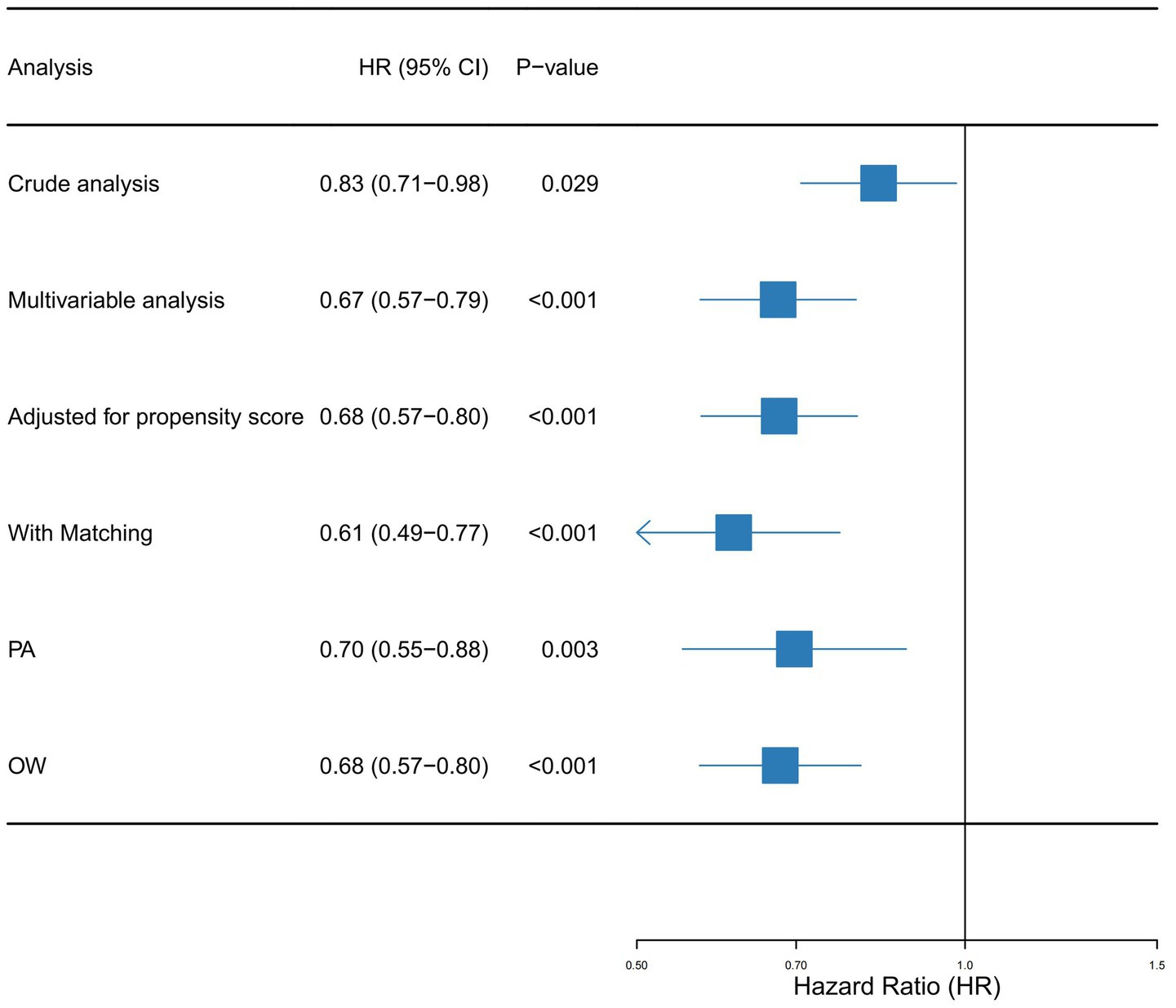
Univariate Cox regression identified vitamin C supplementation as a protective factor against mortality in SA-AKI patients, yielding a hazard ratio (HR) of 0.83 (95% CI: 0.71–0.98). This corresponds to an estimated 17% reduction in mortality risk among supplementation recipients. Multivariable Cox regression incorporating all covariates from Table 1 strengthened this association (adjusted HR: 0.67, 95% CI: 0.57–0.79). Propensity score-matched analyses further validated these findings: PSM-adjusted HR: 0.68 (95% CI: 0.57–0.80); Overlap weighting model HR: 0.61 (95% CI: 0.49–0.77).
Sensitivity analyses using paired algorithm (PA) and overlap weighting (OW) methodologies consistently reinforced the stability of this protective effect (Figure 3). The concordance across multiple statistical approaches underscores the reliability of the observed mortality reduction.
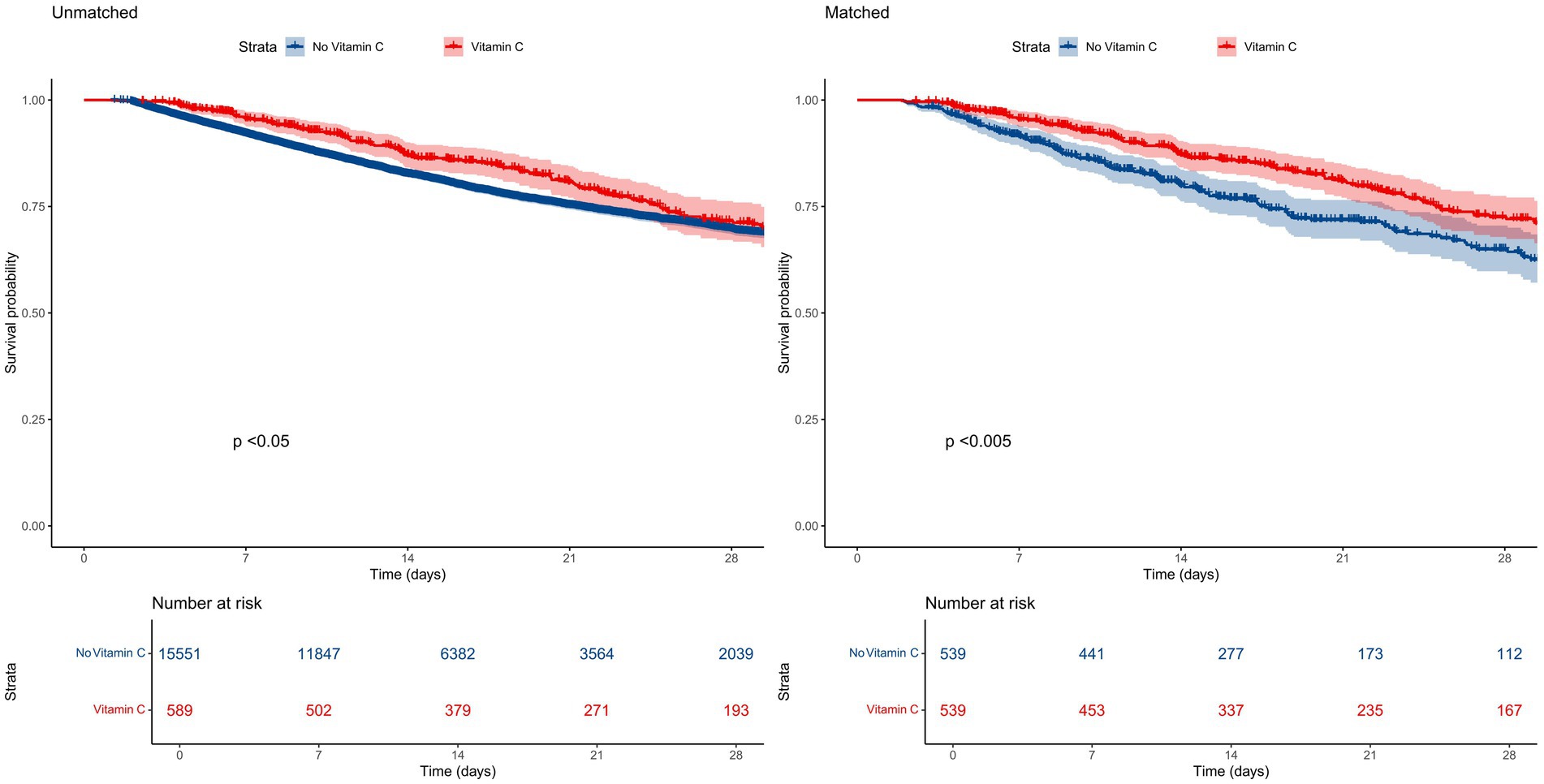
Figure 3. Forest plot shows HRs of in-hospital mortality in vitamin D group using a variety of models.
3.4 Subgroup analysis
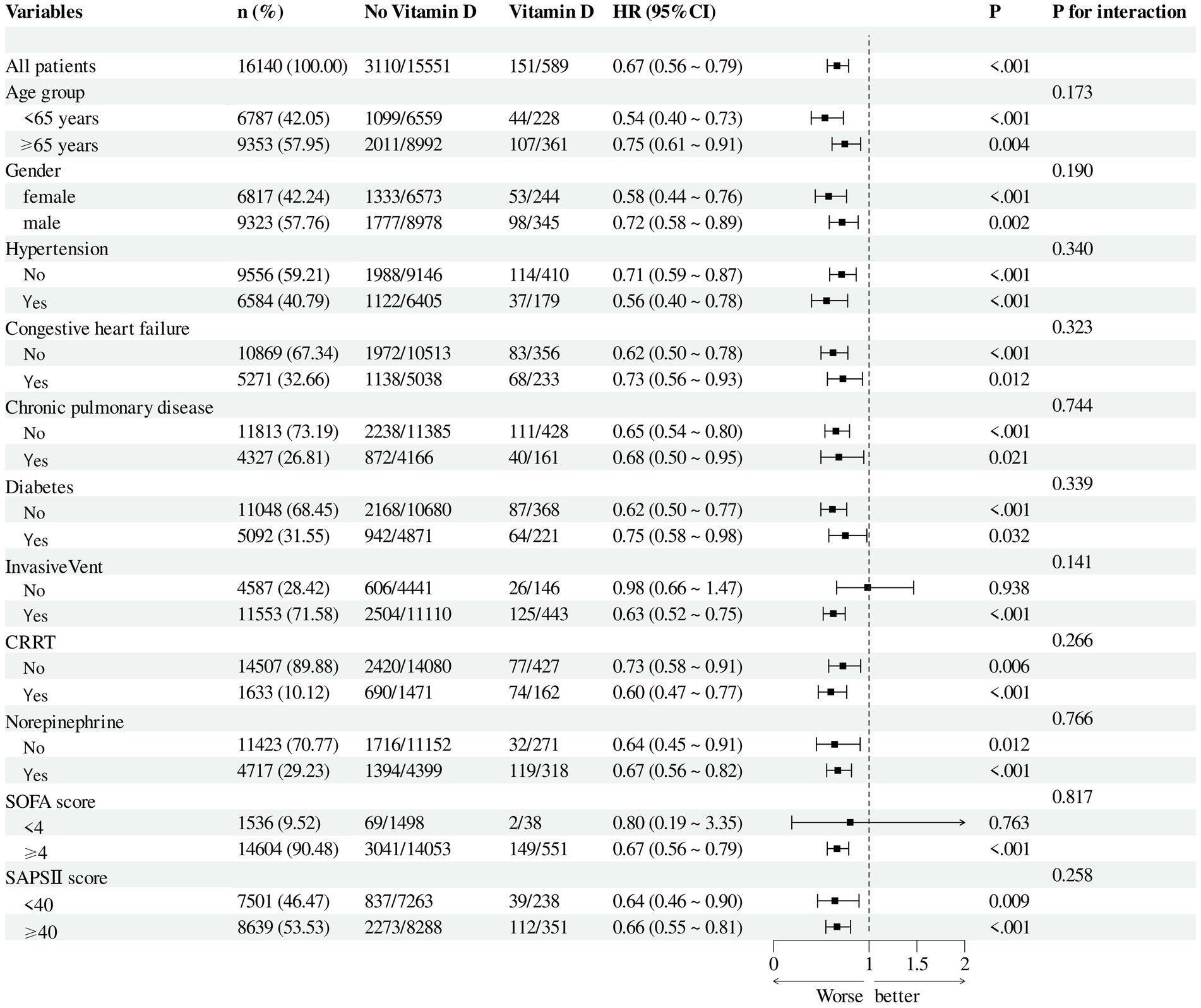
After adjusting for all covariates in Table 1, subgroup analyses were performed based on age, sex, Sequential Organ Failure Assessment (SOFA) score, Simplified Acute Physiology Score II (SAPS II), vasopressor use, presence of invasive mechanical ventilation, and comorbidities including hypertension, diabetes mellitus, chronic kidney disease, chronic pulmonary disease, and congestive heart failure. The results remained consistent across all subgroups (Figure 4).
4 Discussion
Our study demonstrates that in-hospital vitamin C supplementation is associated with a reduced short-term mortality risk in SA-AKI patients, consistent with previous observational studies. The multifaceted renoprotective mechanisms may involve several canonical and recently elucidated pathways. Recent single-cell transcriptomic studies have revealed that vitamin C can restore electron transport chain complex assembly in proximal tubular cells through the upregulation of mitochondrial chaperones (HSP60/70) and prohibitin-1 (PHB1), which are crucial for cristae morphogenesis during sepsis-induced bioenergetic crisis (23). In addition, vitamin C enhances TET2-mediated 5hmC modification at GPX4 promoter regions, counteracting sepsis-induced DNA hypermethylation (mean methylation level decreased by 37.2%, p = 0.009), thereby preserving glutathione peroxidase 4 (GPX4) activity and significantly reducing renal lipid peroxidation markers such as 4-HNE by 54% compared to controls (23). Dynamic contrast-enhanced ultrasonography provides further evidence that vitamin C treatment increases the renal cortical microvascular flow index (MFI) by 1.8-fold (p < 0.01), an effect mechanistically linked to the suppression of PAD4-dependent histone citrullination (62% reduction) and subsequent limitation of neutrophil extracellular trap (NET) formation, thus improving microvascular perfusion heterogeneity (24). Moreover, recent animal model data indicate that vitamin C reverses sepsis-induced gut dysbiosis, increasing the abundance of short-chain fatty acid–producing bacteria such as Roseburia spp. by 3.2-fold, which, through GPR43 receptor activation, enhances tubular autophagic flux (LC3-II/I ratio increased by 2.1-fold) (25, 26).
These novel insights complement established mechanisms whereby vitamin C activates the Nrf2/ARE pathway, enhancing the expression of phase II detoxifying enzymes (HO-1, NQO1), particularly in proximal tubular epithelial cells where oxidative damage is most severe (27). Vitamin C also promotes mitochondrial biogenesis through PGC-1α upregulation, restores ATP production during ischemic injury (28), and inhibits ferroptosis via preservation of GPX4 activity, effectively reducing lipid peroxidation markers like 4-HNE in renal tissue (29). Notably, the immunomodulatory effects of vitamin C exhibit temporal specificity—early enhancement of M1 macrophage bactericidal capacity (evidenced by IL-12 elevation within 24 h) is followed by M2 polarization promoting tissue repair (increased IL-10 at 72 h) (30). This biphasic regulation may explain the reduced vasopressor duration observed in our cohort. Furthermore, vitamin C’s ability to suppress NETosis through PAD4 inhibition could mitigate microvascular thrombosis, a critical pathomechanism in SA-AKI (31).
Vitamin C, which exists in multiple biological forms and is primarily obtained through dietary intake, undergoes hepatic metabolism as its central regulatory pathway. Its deficiency has been mechanistically linked to heightened infection susceptibility, particularly during bacterial and viral challenges (32). Beyond its canonical antioxidant properties, vitamin C exerts pleiotropic effects through enzymatic cofactor roles in tissue repair (33) and endothelial function modulation via oxidative stress mitigation (34). Clinical evidence suggests that intravenous vitamin C administration in critical infections (e.g., sepsis) reduces organ dysfunction and improves survival (35), while enhancing immune cell-mediated pathogen clearance (36). Paradoxically, vitamin C deficiency may exacerbate cardiovascular risks through dyslipidemia and vascular dysregulation (37), underscoring its systemic importance in critical illness (38).
Vitamin C exerts critical immunoregulatory effects in sepsis pathophysiology, mediating the balance between initial pathogen clearance and subsequent hyperinflammation-induced organ failure (30). Experimental models confirm its dual capacity to attenuate cytokine storms while maintaining antimicrobial defenses through three mechanisms: enhanced neutrophil phagocytosis, endothelial barrier stabilization, and NLRP3 inflammasome suppression (39–41). Clinical meta-analyses reveal geographical heterogeneity in mortality outcomes (42), with developing regions showing 28% risk reduction (43) contrasting with neutral effects in multicenter RCTs (44). Clinical evidence from septic shock patients reveals profound vitamin C depletion with preferential accumulation in immune cells (monocytes: 80 × plasma concentration; granulocytes: 25×). Intravenous supplementation achieves rapid plasma concentration elevation followed by swift decline, indicating active cellular uptake (45). A randomized controlled trial demonstrate combination therapy (vitamin C + hydrocortisone + thiamine) significantly reduces SOFA scores, shortens vasopressor dependence duration, and improves 28-day survival rates, particularly in developing regions (46). Notably, early high-dose regimens (3–4 days) show survival benefits in predefined subgroups (47).
Sepsis-associated AKI (SA-AKI), a prevalent complication with mortality exceeding 40% (48), arises from synergistic hemodynamic, inflammatory, and immunometabolic insults (49). Our findings align with emerging paradigms of SA-AKI as a distinct entity involving mitochondrial dysfunction and immunothrombosis (50). Vitamin C deficiency may exacerbate SA-AKI through impaired redox homeostasis and neutrophil extracellular trap dysregulation (51), compounded by micronutrient interactions in malnourished critically ill patients (52). Early intervention bundles—including antimicrobial stewardship, goal-directed resuscitation, and micronutrient repletion—remain cornerstone strategies (53).
4.1 Limitations and future directions
Our study has several limitations requiring cautious interpretation, The MIMIC-IV database lacks detailed records on vitamin C administration patterns (bolus vs. continuous infusion) and concomitant antioxidants use, preventing analysis of dose–response relationships. Previous pharmacokinetic studies suggest plasma concentrations >100 μmol/L require >3 g/d continuous infusion (54), which our data cannot verify. Particularly, the predominance of Caucasian participants (63.6%) raises concerns about generalizability, given ethnic differences in CYP450-mediated vitamin C metabolism (55). Future RCTs should stratify by AKI stage and infection type, while incorporating biomarkers like urinary 8-OHdG to quantify oxidative stress modulation.
5 Conclusion
This large-scale retrospective cohort study establishes vitamin C supplementation as an independent predictor of reduced 28-day mortality in ICU-admitted SA-AKI patients, with robust validation through advanced causal inference methods. The intervention’s cost-effectiveness and safety profile support consideration of protocolized vitamin C status monitoring and supplementation in critical care settings. However, therapeutic optimization requires prospective evaluation of dose–response relationships, administration timing, and combination therapies. Large-scale randomized controlled trials are urgently needed to confirm these observational findings and establish evidence-based clinical guidelines.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
YH: Writing – original draft, Writing – review & editing. JL: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We wish to thank the team of the Laboratory for Computational Physiology from the Massachusetts Institute of Technology (LCP-MIT) for keeping the MIMIC databases available.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cecconi, M, Evans, L, Levy, M, and Rhodes, A. Sepsis and septic shock. Lancet. (2018) 392:75–87. doi: 10.1016/S0140-6736(18)30696-2
2. Rudd, KE, Johnson, SC, Agesa, KM, Shackelford, KA, Tsoi, D, Kievlan, DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of disease study. Lancet. (2020) 395:200–11. doi: 10.1016/S0140-6736(19)32989-7
3. Kellum, JA, Romagnani, P, Ashuntantang, G, Ronco, C, Zarbock, A, and Anders, H-J. Acute kidney injury. Nat Rev Dis Primers. (2021) 7:52. doi: 10.1038/s41572-021-00284-z
4. Manrique-Caballero, CL, Del Rio-Pertuz, G, and Gomez, H. Sepsis-associated acute kidney injury. Crit Care Clin. (2021) 37:279–301. doi: 10.1016/j.ccc.2020.11.010
5. Zarbock, A, Nadim, MK, Pickkers, P, Gomez, H, Bell, S, Joannidis, M, et al. Sepsis-associated acute kidney injury: consensus report of the 28th acute disease quality initiative workgroup. Nat Rev Nephrol. (2023) 19:401–17. doi: 10.1038/s41581-023-00683-3
6. Sahoo, DK, Wong, D, Patani, A, Paital, B, Yadav, VK, Patel, A, et al. Exploring the role of antioxidants in sepsis-associated oxidative stress: a comprehensive review. Front Cell Infect Microbiol. (2024) 14:1348713. doi: 10.3389/fcimb.2024.1348713
7. Vandervelden, S, Cortens, B, Fieuws, S, Eegdeman, W, Malinverni, S, Vanhove, P, et al. Early administration of vitamin C in patients with sepsis or septic shock in emergency departments: a multicenter, double-blind, randomized controlled trial: the C-EASIE trial. Crit Care. (2025) 29:160. doi: 10.1186/s13054-025-05383-x
8. Wei, XB, Wang, ZH, Liao, XL, Guo, WX, Wen, JY, Qin, TH, et al. Efficacy of vitamin C in patients with sepsis: an updated meta-analysis. Eur J Pharmacol. (2020) 868:172889. doi: 10.1016/j.ejphar.2019.172889
9. Shrestha, DB, Budhathoki, P, Sedhai, YR, Mandal, SK, Shikhrakar, S, Karki, S, et al. Vitamin C in critically ill patients: an updated systematic review and meta-analysis. Nutrients. (2021) 13:3564. doi: 10.3390/nu13103564
10. Lin, S, and Ma, J. Effects of vitamin C supplementation on mortality in patients with sepsis and septic shock: a meta-analysis. Chin J Respir Crit Care Med. (2023) 22:182–8. doi: 10.7507/1671-6205.202203019
11. Zhao, G-M, Bian, W-S, Zhen, J, and Chen, W. Effect of vitamin C intravenous injection on prognosis of patients with sepsis or septic shock: a meta-analysis of randomized controlled trial. Chin J Infect Control. (2024) 23:32–41. doi: 10.12138/j.issn.1671-9638.20244248
12. Xu, W, Mao, Z, Zhao, B, Ni, T, Deng, S, Yu, P, et al. Vitamin C attenuates vancomycin induced nephrotoxicity through the reduction of oxidative stress and inflammation in HK-2 cells. Ann Palliat Med. (2021) 10:694. doi: 10.21037/apm-20-694
13. Lauer, A, Burkard, M, Niessner, H, Leischner, C, Renner, O, Vollbracht, C, et al. Ex vivo evaluation of the sepsis triple therapy high-dose vitamin C in combination with vitamin B1 and hydrocortisone in a human peripheral blood mononuclear cells (PBMCs) model. Nutrients. (2021) 13:2366. doi: 10.3390/nu13072366
14. Alberts, A, Moldoveanu, ET, Niculescu, AG, and Grumezescu, AM. Vitamin C: a comprehensive review of its role in health, disease prevention, and therapeutic potential. Molecules. (2025) 30:748. doi: 10.3390/molecules30030748
15. Shao, Q, and Liu, F. Vitamin C in sepsis treatment: not necessarily beneficial. J Trans Crit Care Med. (2025) 7:e24-00020. doi: 10.1097/JTCCM-D-24-00020
16. Khan, A, and Tidman, M. Systematic review on impacts of vitamin C, thiamin, and hydrocortisone in sepsis. J Health Med Sci. (2025) 8:7–23. doi: 10.31014/aior.1994.08.01.335
17. Vandervelden, S, Wauters, L, Breuls, J, Fieuws, S, Vanhove, P, Hubloue, I, et al. Early administration of vitamin C in patients with sepsis or septic shock in emergency departments: a multicenter, double blinded, randomized controlled trial: the C-EASIE trial protocol. PLoS One. (2021) 16:e0259699. doi: 10.1371/journal.pone.0259699
18. Johnson, A, Bulgarelli, L, Pollard, T, Gow, B, Moody, B, Horng, S, et al. MIMIC-IV (version 3.0). PhysioNet. (2024). doi: 10.13026/hxp0-hg59
19. Singer, M, Deutschman, CS, Seymour, CW, Shankar-Hari, M, Annane, D, Bauer, M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
20. Gomez, H, Ince, C, De Backer, D, Pickkers, P, Payen, D, Hotchkiss, J, et al. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. (2014) 41:3–11. doi: 10.1097/SHK.0000000000000052
21. Li, L, and Greene, T. A weighting analogue to pair matching in propensity score analysis. Int J Biostat. (2013) 9:215–34. doi: 10.1515/ijb-2012-0030
22. Li, F, Morgan, KL, and Zaslavsky, AM. Balancing covariates via propensity score weighting. J Am Stat Assoc. (2018) 113:390–400. doi: 10.1080/01621459.2016.1260466
23. He, J, Chen, Y, Li, Y, and Feng, Y. Molecular mechanisms and therapeutic interventions in acute kidney injury: a literature review. BMC Nephrol. (2025) 26:144. doi: 10.1186/s12882-025-04077-4
24. Morrell, ED, Kellum, JA, Pastor-Soler, NM, and Hallows, KR. Septic acute kidney injury: molecular mechanisms and the importance of stratification and targeting therapy. Crit Care. (2014) 18:501. doi: 10.1186/s13054-014-0501-5
25. Li, X, Wang, Q, Wang, F, Jin, Q, Deng, B, Yang, R, et al. Rosa roxburghii Tratt (Cili) has a more effective capacity in alleviating DSS-induced colitis compared to vitamin C through B cell receptor pathway. Food Res Int. (2024) 195:114950. doi: 10.1016/j.foodres.2024.114950
26. Nie, K, Ma, K, Luo, W, Shen, Z, Yang, Z, Xiao, M, et al. Roseburia intestinalis: a beneficial gut organism from the discoveries in genus and species. Front Cell Infect Microbiol. (2021) 11:757718. doi: 10.3389/fcimb.2021.757718
27. Suzuki, T, Takahashi, J, and Yamamoto, M. Molecular basis of the KEAP1-NRF2 signaling pathway. Mol Cells. (2023) 46:133–41. doi: 10.14348/molcells.2023.0028
28. Ni, Y, Wu, GH, Cai, JJ, Zhang, R, Zheng, Y, Liu, JQ, et al. Tubule-mitophagic secretion of SerpinG1 reprograms macrophages to instruct anti-septic acute kidney injury efficacy of high-dose ascorbate mediated by NRF2 transactivation. Int J Biol Sci. (2022) 18:5168–84. doi: 10.7150/ijbs.74430
29. Linkermann, A, Chen, G, Dong, G, Kunzendorf, U, Krautwald, S, and Dong, Z. Regulated cell death in AKI. J Am Soc Nephrol. (2014) 25:2689–701. doi: 10.1681/ASN.2014030262
30. van der Poll, T, Shankar-Hari, M, and Wiersinga, WJ. The immunology of sepsis. Immunity. (2021) 54:2450–64. doi: 10.1016/j.immuni.2021.10.012
31. Zou, S, Jie, H, Han, X, and Wang, J. The role of neutrophil extracellular traps in sepsis and sepsis-related acute lung injury. Int Immunopharmacol. (2023) 124:110436. doi: 10.1016/j.intimp.2023.110436
32. Muñoz-Montesino, C, Peña, E, Roa, FJ, Sotomayor, K, Escobar, E, and Rivas, CI. Transport of vitamin C in cancer. Antioxid Redox Signal. (2021) 35:61–74. doi: 10.1089/ars.2020.8166
33. de Spoelstra-Man, AME, Elbers, PWG, and Van Oudemans-Straaten, HM. Vitamin C: should we supplement? Curr Opin Crit Care. (2018) 24:248–55. doi: 10.1097/MCC.0000000000000510
34. Gordon, DS, Rudinsky, AJ, Guillaumin, J, Parker, VJ, and Creighton, KJ. Vitamin C in health and disease: a companion animal focus. Top Companion Anim Med. (2020) 39:100432. doi: 10.1016/j.tcam.2020.100432
35. Marik, PE. Vitamin C for the treatment of sepsis: the scientific rationale. Pharmacol Ther. (2018) 189:63–70. doi: 10.1016/j.pharmthera.2018.04.007
36. Batra, SD, Nandi, M, Sikri, K, and Tyagi, JS. Genome-wide expression profiling establishes novel modulatory roles of vitamin C in THP-1 human monocytic cell line. BMC Genomics. (2017) 18:252. doi: 10.1186/s12864-017-3635-4
37. Moser, MA, and Chun, OK. Vitamin C and heart health: a review based on findings from epidemiologic studies. Int J Mol Sci. (2016) 17:1328. doi: 10.3390/ijms17081328
38. Ismailova, A, and White, JH. Vitamin D, infections and immunity. Rev Endocr Metab Disord. (2022) 23:265–77. doi: 10.1007/s11154-021-09679-5
39. Lankadeva, YR, Peiris, RM, Okazaki, N, Birchall, IE, Trask-Marino, A, Dornom, A, et al. Reversal of the pathophysiological responses to gram-negative sepsis by megadose vitamin C. Crit Care Med. (2021) 49:e179–90. doi: 10.1097/CCM.0000000000004770
40. May, CN, Bellomo, R, and Lankadeva, YR. Therapeutic potential of megadose vitamin C to reverse organ dysfunction in sepsis and COVID-19. Br J Pharmacol. (2021) 178:3864–8. doi: 10.1111/bph.15579
41. Kashiouris, MG, L'Heureux, M, Cable, CA, Fisher, BJ, Leichtle, SW, and Fowler, AA. The emerging role of vitamin C as a treatment for sepsis. Nutrients. (2020) 12:292. doi: 10.3390/nu12020292
42. Sato, R, Hasegawa, D, Prasitlumkum, N, Ueoka, M, Nishida, K, Takahashi, K, et al. Effect of IV high-dose vitamin C on mortality in patients with sepsis: a systematic review and meta-analysis of randomized controlled trials. Crit Care Med. (2021) 49:2121–30. doi: 10.1097/CCM.0000000000005263
43. Luo, X, Zhu, Y, Zhang, R, Zhu, JQ, Kuang, H, Shao, Y, et al. The effect of vitamin C in adults with sepsis: a meta-analysis of randomized controlled trials. Front Med. (2023) 10:1244484. doi: 10.3389/fmed.2023.1244484
44. Chen, CY, Chiu, CT, Lee, HS, and Lai, CC. The impact of vitamin C-containing treatment on the mortality of patients with sepsis: a systematic review and meta-analysis of randomized controlled trials. J Infect Public Health. (2022) 15:1514–20. doi: 10.1016/j.jiph.2022.11.015
45. Kalil, AC. Lack of benefit of high-dose vitamin C, thiamine, and hydrocortisone combination for patients with Sepsis. JAMA. (2020) 323:419–20. doi: 10.1001/jama.2019.22438
46. Iglesias, J, Vassallo, AV, Patel, VV, Sullivan, JB, Cavanaugh, J, and Elbaga, Y. Outcomes of metabolic resuscitation using ascorbic acid, thiamine, and glucocorticoids in the early treatment of Sepsis: the ORANGES trial. Chest. (2020) 158:164–73. doi: 10.1016/j.chest.2020.02.049
47. Scholz, SS, Borgstedt, R, Ebeling, N, Menzel, LC, Jansen, G, and Rehberg, S. Mortality in septic patients treated with vitamin C: a systematic meta-analysis. Crit Care. (2021) 25:17. doi: 10.1186/s13054-020-03438-9
48. Alobaidi, R, Basu, RK, Goldstein, SL, and Bagshaw, SM. Sepsis-associated acute kidney injury. Semin Nephrol. (2015) 35:2–11. doi: 10.1016/j.semnephrol.2015.01.002
49. Kuwabara, S, Goggins, E, and Okusa, MD. The pathophysiology of sepsis-associated AKI. Clin J Am Soc Nephrol. (2022) 17:1050–69. doi: 10.2215/CJN.00850122
50. Emlet, DR, Shaw, AD, and Kellum, JA. Sepsis-associated AKI: epithelial cell dysfunction. Semin Nephrol. (2015) 35:85–95. doi: 10.1016/j.semnephrol.2015.01.009
51. Zhang, X, Yang, K, Chen, L, Liao, X, Deng, L, Chen, S, et al. Vitamin a deficiency in critically ill children with sepsis. Crit Care. (2019) 23:267. doi: 10.1186/s13054-019-2548-9
52. Ponnarmeni, S, Kumar Angurana, S, Singhi, S, Bansal, A, Dayal, D, Kaur, R, et al. Vitamin D deficiency in critically ill children with sepsis. Paediatr Int Child Health. (2016) 36:15–21. doi: 10.1179/2046905515Y.0000000042
53. Pais, T, Jorge, S, and Lopes, JA. Acute kidney injury in sepsis. Int J Mol Sci. (2024) 25:5924. doi: 10.3390/ijms25115924
54. Fowler, AA, Truwit, JD, Hite, RD, Morris, PE, DeWilde, C, Priday, A, et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA. (2019) 322:1261–70. doi: 10.1001/jama.2019.11825
Keywords: vitamin C supplementation, sepsis-associated acute kidney injury (SA-AKI), 28-day mortality, MIMIC-IV database, ICU
Citation: He Y and Liu J (2025) Vitamin C improves 28-day survival in patients with sepsis-associated acute kidney injury in the intensive care unit: a retrospective study. Front. Nutr. 12:1600224. doi: 10.3389/fnut.2025.1600224
Edited by:
Sree Bhushan Raju, Nizam’s Institute of Medical Sciences, IndiaReviewed by:
Yin Li, Tianjin University, ChinaGawel Solowski, Bingöl University, Türkiye
Khin Phyu Pyar, Ministry of Health Myanmar, Myanmar
Copyright © 2025 He and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinglan Liu, MTEwMzY3OTgzMEBxcS5jb20=
 Yang He
Yang He Jinglan Liu
Jinglan Liu