- 1Department of Nutrition, School of Public Health, Anhui Medical University, Hefei, Anhui, China
- 2Key Laboratory of Population Health Across Life Cycle (Anhui Medical University), Ministry of Education of the People’s Republic of China, Hefei, Anhui, China
- 3NHC Key Laboratory of Study on Abnormal Gametes and Reproductive Tract, Hefei, Anhui, China
- 4Anhui Provincial Key Laboratory of Population Health and Aristogenics/Key Laboratory of Environmental Toxicology of Anhui Higher Education Institutes, Anhui Medical University, Hefei, Anhui, China
- 5Department of Laboratory, The Second Affiliated Hospital of Anhui Medical University Economic and Technological Development Zone, Hefei, Anhui, China
Background: The association between enterolignans (the bioavailable metabolites of dietary lignans) including enterolactone (ENL) and enterodiol (END) and long-term risk of mortality remains limited and inconclusive. Involvement of human serum albumin (HSA) could be a possible reason behind the inconsistency. We prospectively examined the associations between urinary enterolignans and the risk of overall and cause-specific mortality among US adults and evaluated the impact of adjusting for HSA by comparing the results before and after its inclusion as a covariate.
Methods: The data was obtained from the US National Health and Nutrition Examination Survey. Urinary END and ENL concentrations were measured using high-performance liquid chromatography with tandem mass spectrometric detection. Deaths from baseline until December 31, 2015 were identified through linkage to the National Death Index. Cox regression was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) with and without HSA adjustment. Joint analysis and stratified analysis were used to evaluated the impact of HSA on the associations of enterolignans with mortality risk.
Results: We documented 1,578 deaths among 10,664 participants after a median follow-up of 9.8 years. Higher concentrations of ENL were associated with lower all-cause mortality risk (comparing extreme tertiles, HR = 0.86, 95% CI: 0.74–1.00, Ptrend = 0.031). However, the inverse association between urinary ENL and all-cause mortality risk became non-significant when further adjusting for HSA. Compared to individuals with low levels of both ENL and HSA, those with high levels of both ENL and HSA had the lowest mortality risk (HR = 0.71, 95% CI: 0.60–0.84). Meanwhile, urinary ENL concentrations were associated with decreased all-cause mortality risk (HR = 0.74, 95% CI: 0.59–0.93, Ptrend = 0.020) only in the group with higher HSA levels.
Conclusion: Adjustment of HSA attenuated the inverse association between urinary ENL and all-cause death risk to non-significance. HSA can be considered as an important covariate in the future epidemiological studies on enterolignans.
1 Introduction
Lignans are a large group of non-flavonoid phenolic compounds widely distributed in edible plants such as seed oils, whole-grain cereals and beans (1). Dietary lignans have recently gained considerable attention for their potential health benefits extensively studied in many in vitro and in vivo studies (2–6). There is an essential need for more epidemiological evidence to confirm the protective benefits of lignans in human populations. Most previous studies used a food frequency questionnaire (FFQ) to estimate habitual intake of plant foods, which may have been subject to measurement errors (7–9). In addition, the incomplete coverage of lignans in food composition databases may have compromised the accuracy of dietary lignan intake estimations (10). Lignans, which show individual variation in metabolism across populations, can be converted into more bioactive enterolignans including enterolactone (ENL) and enterodiol (END) by the host gut microbiota in the colon (11, 12). Therefore, some epidemiological studies used circulating enterolignans as biomarkers to reflect lignan intakes (11).
Circulating ENL and END are the most commonly used biomarkers. Dietary lignans are metabolized to ENL and END, which can passively diffuse across the enterocyte membrane (13, 14), with enhanced bioavailability and activity compared to their precursors (15). However, the association of serum or urinary ENL and END with long-term death have been less examined, with inconsistent results (16–19). Moreover, consideration of human serum albumin (HSA) could be a possible reason behind the inconsistency. Absorbed polyphenols can be stored and transported by HSA as it is the most abundant protein in plasma (20–23). The binding of lignans and other polyphenols to HSA is an important factor in determining their pharmacokinetics, pharmacodynamics and biological activities (20, 24, 25). Moreover, findings from in vitro studies confirm the antioxidant activity of enterolignans (15). Albumin can serve as a trap for reactive oxygen and nitrogen species due to the free thiol group of Cys34 (26). It has been reported that enterolignan binding to HSA also leads to an increase in the antioxidant activity of HSA in vitro (24, 27). Hence, HSA may play a role in the currently discovered health benefit of lignans. Besides, HSA levels have been reported to be correlated with circulating ENL and END (28), as well as with mortality risk (29, 30), rendering it to be a potential confounder for the relationship between circulating ENL and END with mortality. While to the best of our knowledge, few epidemiological research on lignans has considered albumin as a covariate (16). Hence, determining if HSA levels affect the association between lignans and health outcomes, thereby requiring adjustment in correlation analyses, is an important issue to address.
We hypothesized that further adjustment of HSA would influence the associations between urinary enterolignans and mortality risk. In the present study, we prospectively examined the associations between urinary enterolignans and mortality risk with or without serum albumin adjustment. Furthermore, joint analysis and stratified analysis were used to evaluated the impact of HSA on the associations of enterolignans with mortality risk.
2 Methods
2.1 Study population
Participants in our study were selected from the National Health and Nutrition Examination Survey (NHANES). NHANES consists of a series of continuous cross-sectional surveys of the civilian, noninstitutionalized US population since 1999, with a complex, stratified, multistage probability sampling design. NHANES incorporates personal interviews, physical examinations, and laboratory tests conducted by trained staff to collect nationally representative data. Further details can be found at https://www.cdc.gov/nchs/nhanes/about_nhanes.htm. For the present study, participants of the NHANES survey cycles from 1999–2000 to 2008–2010 were included. We excluded the participants who were younger than 18 years old (n = 26,781), had missing urinary enterolignans data (n = 24,641) and did not have linked mortality data (n = 14). Therefore, a total of 10,664 participants were included in the final analysis (Supplementary Figure 1). All participants provided the written informed consent, and the NHANES study protocol was approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board.
2.2 Urinary enterolignans and creatinine measurement
Spot urine samples of the participants were collected at NHANES mobile examination centers (MEC) in collection cups, transferred to specimen vials and stored frozen in borosilicate glass or polypropylene vials or specimen cups. Vials were plugged by Teflon coated stoppers and sealed with an aluminum seal. Spot urine specimens were then labeled, immediately frozen to −20°C, and then shipped to the Division of Environmental Health Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention for analysis. The methods for the analysis of urine samples for END and ENL concentrations have been described in detail elsewhere.1 Briefly, urine samples were spiked with stable isotope-labeled internal standards to enhance method accuracy and precision. The samples were then subjected to solid-phase extraction to eliminate interferences and improve sensitivity. Finally, the samples were analyzed using negative ion mode electrospray ionization high-performance liquid chromatography–tandem mass spectrometry (HPLC-MS/MS). To adjust for the impact of glomerular filtration rate on urinary biomarker levels, urinary creatinine was determined using the Jaffe reaction on the Beckman CX3 (1999–2006) or an enzymatic (creatinase) method on the Roche ModP (2007 onwards). Creatinine-adjusted urinary concentration (μg/g creatinine) was calculated by dividing the enterolignans levels (μg/L) by the creatinine level (g/L) (11).
2.3 Serum albumin measurement
Serum specimens are stored under the conditions of 2–8°C, and shipped to Collaborative Laboratory Services for testing and analysis. Serum albumin concentrations were measured using the DcX800 method, a bichromatic digital endpoint method. In the reaction, serum albumin formed a complex with the Bromcresol Purple reagent. Then the change in absorbance at 600 nm was detected by the system. The content of albumin in the sample was directly proportional to the change in absorbance. More details of the serum albumin measurement process were described on the official website of NHANES (see text footnote 1).
2.4 Ascertainment of deaths
We ascertained mortality status via record linkage to the National Death Index (NDI) through 31 December 2015. In our analysis, cardiovascular disease (CVD) mortality was defined using the 10th revision of the International Classification of Diseases (ICD-10), including deaths from diseases of the heart (ICD-10 codes I00-I09, I11, I13, I20-I51) and cerebrovascular diseases (I60–I69). Cancer mortality was defined as code C00-C97. The NDI has been proven to be a reliable and efficient utility for ascertainment of deaths in large epidemiological studies, and over 98% of deaths can be identified using this approach (31, 32).
2.5 Assessment of covariates
Information on covariates was collected through questionnaires, administrated during the household interview, including demographic and lifestyle factors (i.e., age, sex, race/ethnicity, educational level, physical activity, and smoking status). Information on body weight, height, alcohol drinking status, menopausal status, use of female hormones was obtained during the MEC visit. The body mass index (BMI) was calculated as weight (kg) divided by the square of height (m2). Histories of diabetes and hypertension were defined according to self-reported medical diagnoses of these diseases or use of prescribed medications due to these diseases. The participants with a fasting glucose of 126 mg/dL or greater were also defined as diabetic patients. Hypertension (a systolic blood pressure ≥140 mmHg or a diastolic blood pressure ≥90 mmHg) was also identified through physical examination in the MEC. Abnormal liver function is defined by elevated levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) that exceed the upper limits of the established normal range (ALT and AST: 0–40 U/L) (33). Estimate glomerular filtration rate (eGFR) was calculated according to the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation (34). Declined renal function was defined as eGFR <60 mL/min per 1.73 m2 (35).
2.6 Statistical analysis
All analyses incorporated appropriate sampling weights, stratification, and clustering of the complex sampling design to ensure nationally representative estimates. Cox regression models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) of death according to tertiles of enterolignan concentrations. HRs of death risk for each 1-standard deviation (SD) increase in concentrations of END and ENL were also calculated. Since dietary lignan intake and circulating enterolignan levels may differ between men and women (36, 37), urinary enterolignan concentrations were divided into sex-specific tertiles. Sex, age, race/ethnicity, education, marital status, ratio of family income to poverty, physical activity, smoking status, alcohol drinking status, body mass index, diabetes, hypertension, abnormal liver function, declined renal function, menopausal status, use of female hormones, urinary creatinine, and total energy intake were adjusted in the model 1. HSA was further adjusted in the model 2. We removed continuous covariates with missing values. For each categorical covariate in the models, we created a missing value indicator. We compared the distribution characteristics of the above-mentioned covariates between study population and overall population. Linear trend was tested by treating each exposure as a continuous variable in the models. We used restricted cubic splines to test the potential non-linear relationships between enterolignan concentrations and death risk. We also examined joint associations of enterolignan levels and HSA on mortality risk. According to the median values of enterolignan and HSA concentrations, participants were divided into four groups: participants with low enterolignans and low HSA levels, with low enterolignan and high HSA levels, with high enterolignan and low HSA levels, and with high enterolignan and high HSA levels. Using the first group as the reference group, and the Cox proportional hazards model was employed to calculate HRs for the other three groups. In stratified analysis, participants were stratified into two groups based on median HSA concentrations: participants with lower HSA levels and those with higher HSA levels. We examined the association of enterolignan levels and death risk in each subgroup. Interaction was tested using Wald test by evaluating whether the cross-product term between albumin and enterolignan levels was statistically significant. All statistical analyses were performed using R version 4.2.0 and statistical significance was defined as a two-tailed p value < 0.05.
3 Results
3.1 Baseline characteristics of participants
Among 10,664 participants aged 18–85 years (mean age, 46.6 years; SD, 19.6 years), we documented 1,568 deaths including 343 CVD-specific deaths and 349 cancer -specific deaths during a median follow-up of 9.8 years. Participants with higher urinary ENL levels were more likely to be married and non-Hispanic White, were older, were better educated, had a higher ratio of family income to poverty and energy intake, were more physically active, were more likely to be current drinkers, were less likely to be current smokers, had lower BMI. Similar trends were observed among participants with higher urinary END levels (Table 1). The median concentrations of ENL and END were 341.9 μg/g creatinine (interquartile range, IQR: 99.8–836.7 μg/g creatinine) and 38.5 μg/g creatinine (IQR: 14.7–91.6 μg/g creatinine), respectively. After comparing the covariate distributions of the overall population, we found no significant difference, indicating the representativeness of our sample (Supplementary Table 1).
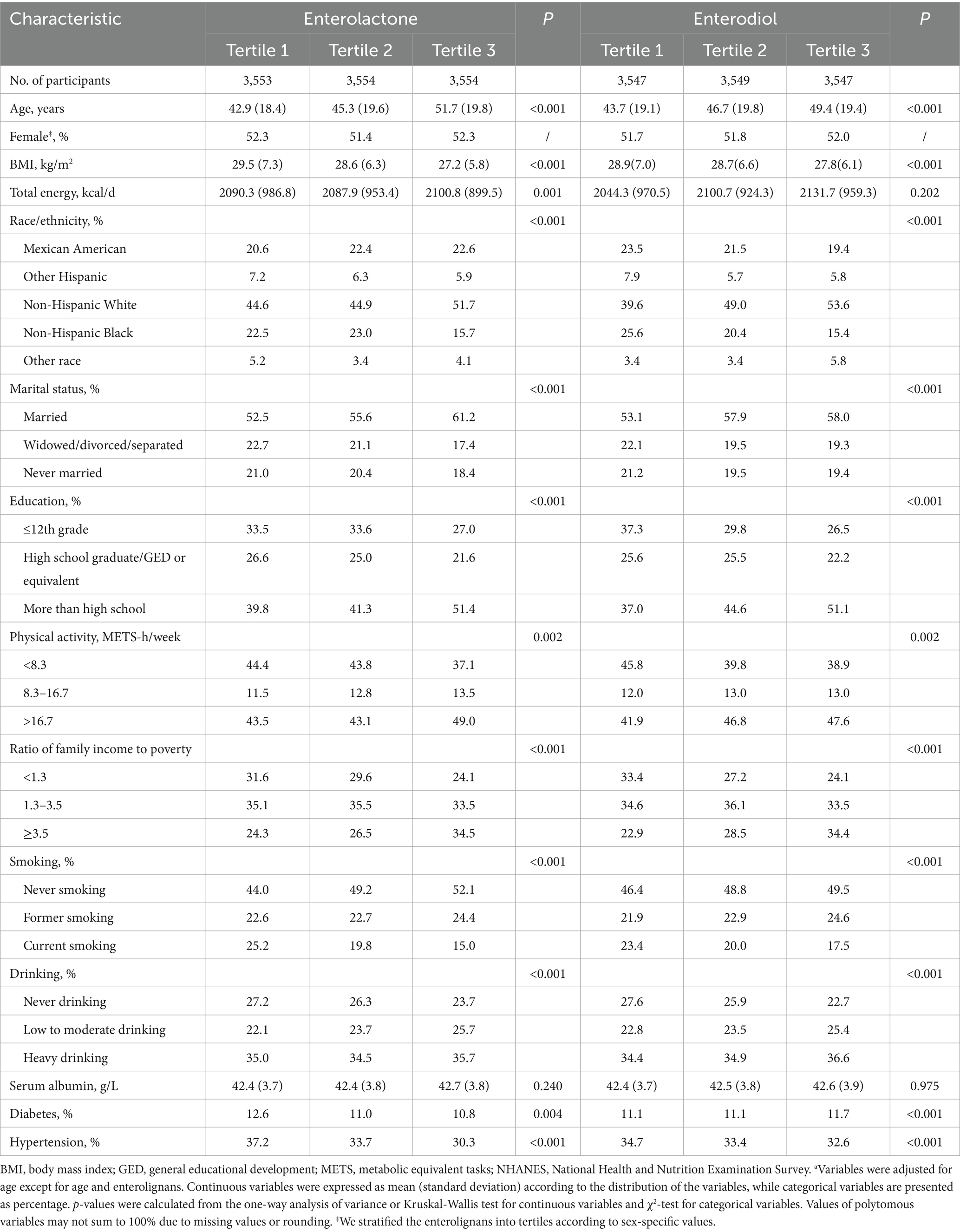
Table 1. Age-adjusted characteristics of participants according to urinary enterolignans concentrations in NHANES (1999–2010)a.
3.2 Association between urinary enterolignans and mortality
As shown in Table 2, all-cause death risk of participants with the highest tertile of urinary ENL concentrations decreased by 14% (HR = 0.86, 95% CI: 0.74–1.00, Ptrend = 0.031) compared with those in the first tertile. When HSA was further adjusted, the inverse association between urinary ENL and all-cause mortality became non-significant. In the restricted cubic spline analyses (Supplementary Figure 2), we found a non-linear association between ENL and all-cause mortality (Pnonlinearity = 0.543) and ENL lost its association with all-cause mortality after 4.7 μg/g creatinine. Additionally, ENL showed non-linear associations with CVD and cancer mortality (All Pnonlinearity > 0.05). Similarly, there was non-linear correlation between END and all-cause, CVD, and cancer mortality (All Pnonlinearity > 0.05). Urinary ENL levels did not present to be associated with CVD or cancer specific mortality risk. No significant association was observed between urinary END and decreasing all-cause, CVD and cancer mortality risk, regardless of whether HSA was adjusted for in the model.
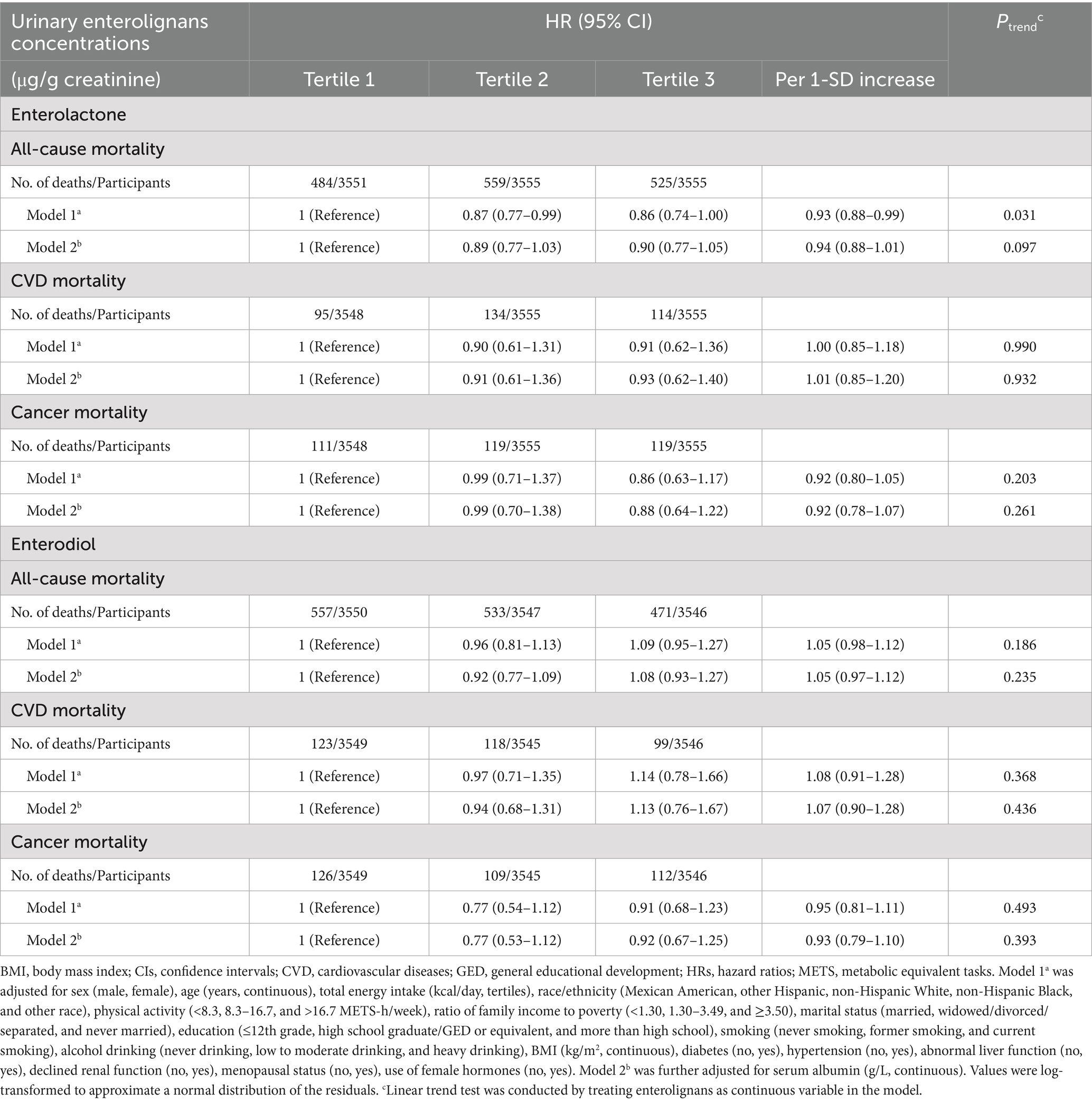
Table 2. HRs (95% CIs) for mortality risk according to urinary enterolignans concentrations in NHANES (1999–2010).
In order to further investigate the interaction of enterolignans and HSA on death risk, we performed joint analysis. The results were shown in Figure 1. Compared to individuals with low levels of both ENL and HSA (low/low group), those in all three other groups had decreased all-cause mortality risk. Participants with high levels of both ENL and HSA (high/high group) had the lowest mortality risk (HR = 0.71, 95% CI: 0.60–0.84). The all-cause mortality risk of individuals with low ENL and high HSA levels (low/high group) reduced by 24% (HR = 0.76, 95% CI: 0.62–0.93), that of participants with high ENL and low HSA levels (high/low group) reduced by 14% (HR = 0.86, 95% CI: 0.74–1.00).
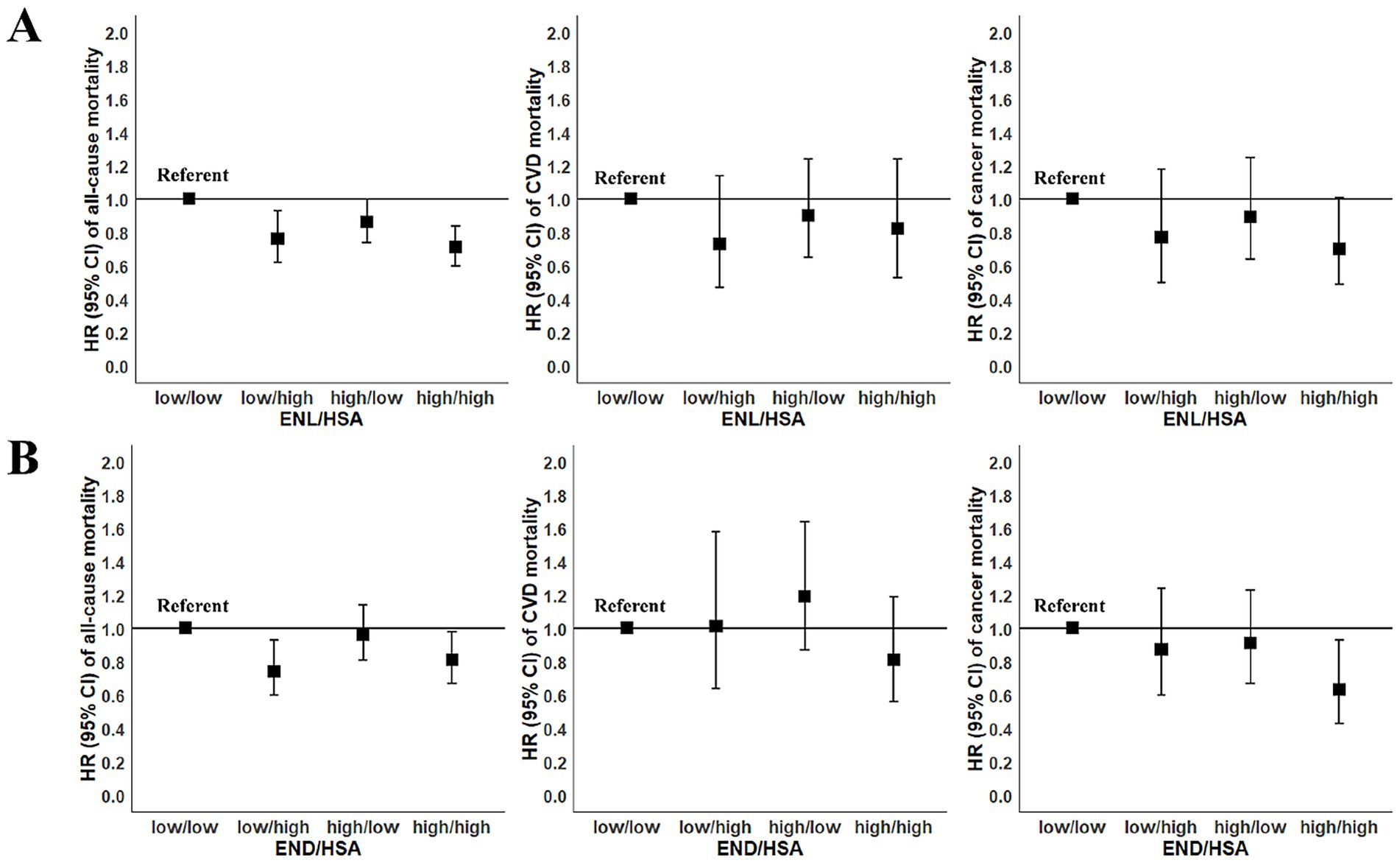
Figure 1. Joint association of enterolignans and human serum albumin (HSA) with risk of mortality among participants in the National Health and Nutrition Examination Survey (NHANES 1999–2010). According to the median values of enterolignans and HSA concentrations, participants were divided into four groups: participants with low enterolignans and low HSA levels (low/low), with low enterolignans and high HSA levels (low/high), with high enterolignans and low HSA levels (high/low), and with high enterolignans and high HSA levels (high/high). Using the first group as the reference group, and the Cox proportional hazards model was employed to calculate HRs for the other three groups. All models were adjusted for sex (male, female), age (years, continuous), total energy intake (kcal/day, tertiles), race/ethnicity (Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, and other race), physical activity (<8.3, 8.3–16.7, and >16.7 METS-h/week), ratio of family income to poverty (<1.30, 1.30–3.49, and ≥3.50), marital status (married, widowed/divorced/separated, and never married), education (≤12th grade, high school graduate/GED or equivalent, and more than high school), smoking (never smoking, former smoking, and current smoking), alcohol drinking (never drinking, low to moderate drinking, and heavy drinking), BMI (kg/m2, continuous), diabetes (no, yes), hypertension (no, yes), abnormal liver function (no, yes), declined renal function (no, yes), menopausal status (no, yes), use of female hormones (no, yes). (A) The joint association of ENL and HSA with mortality risk; (B) the joint association of END and HSA with mortality risk. BMI, body mass index; CI, confidence interval; CVD, cardiovascular diseases; ENL, enterolactone; END, enterodiol; GED, general educational development; HR, hazard ratio; METS, metabolic equivalent tasks.
In stratified analysis (Figure 2), we found that urinary ENL levels were only correlated with a lower all-cause mortality risk among participants with higher HSA. In this subgroup, all-cause death risk of participants with the highest tertile of urinary ENL concentrations decreased by 10% (HR = 0.90, 95% CI: 0.83–0.98, Pinteraction < 0.001). In the subgroup of participants with comparatively lower HSA levels, this association was not observed. Urinary ENL levels did not present to be associated with CVD or cancer specific mortality risk in both subgroups. Urinary END levels did not correlate with mortality in both subgroups.
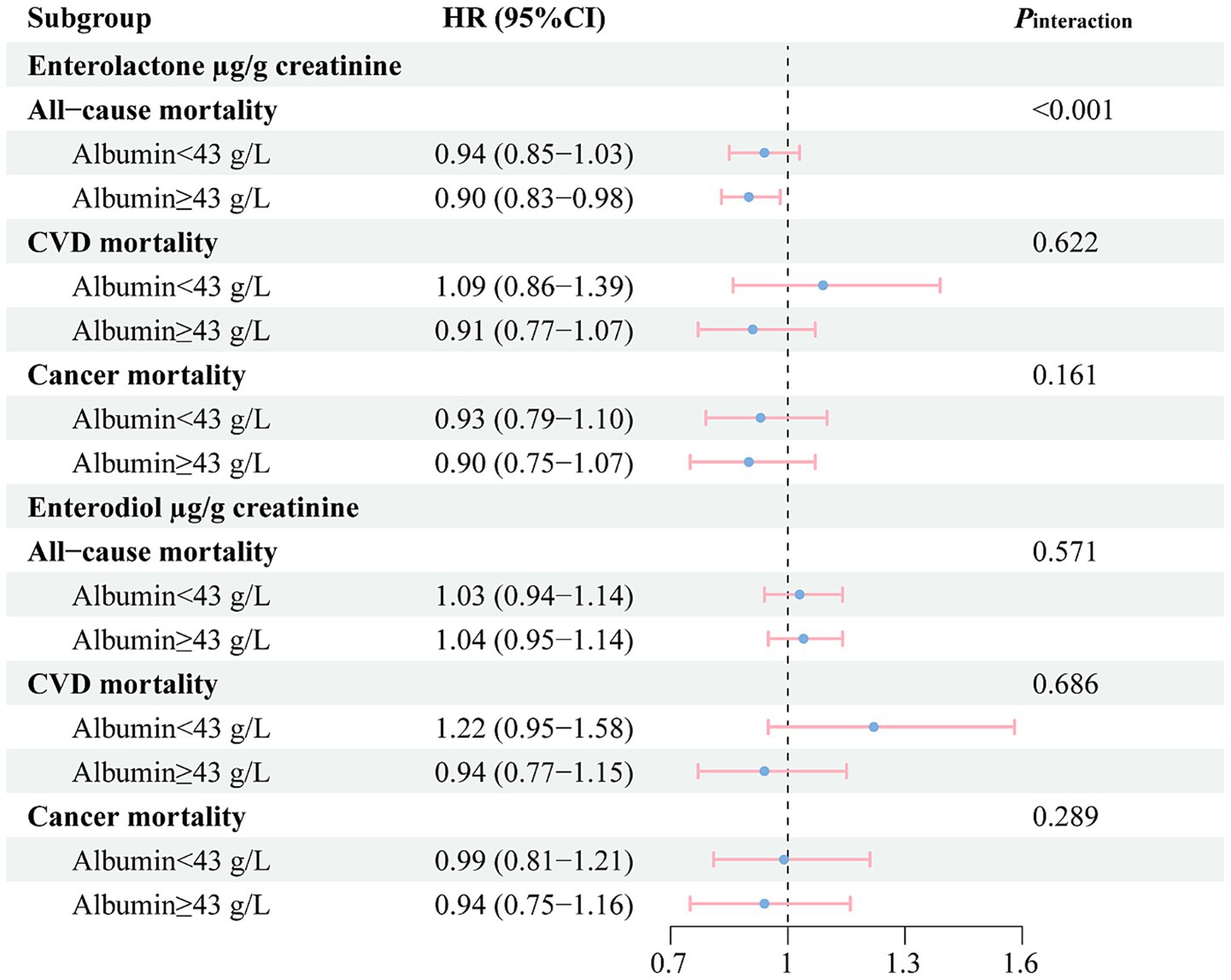
Figure 2. Stratified analysis for the associations between the concentrations of enterolignans (per 1-SD increase) and risk of mortality among participants in the National Health and Nutrition Examination Survey (NHANES 1999–2010). According to the median values of human serum albumin (HSA) concentrations, participants were divided into two groups: low HSA levels (Albumin <43 g/L) and high HSA levels (Albumin ≥43 g/L). All models were adjusted for sex (male, female), age (years, continuous), total energy intake (kcal/day, tertiles), race/ethnicity (Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, and other race), physical activity (<8.3, 8.3–16.7, and >16.7 METS-h/week), ratio of family income to poverty (<1.30, 1.30–3.49, and ≥3.50), marital status (married, widowed/divorced/separated, and never married), education (≤12th grade, high school graduate/GED or equivalent, and more than high school), smoking (never smoking, former smoking, and current smoking), alcohol drinking (never drinking, low to moderate drinking, and heavy drinking), BMI (kg/m2, continuous), diabetes (no, yes), hypertension (no, yes), abnormal liver function (no, yes), declined renal function (no, yes), menopausal status (no, yes), use of female hormones (no, yes). BMI, body mass index; CVD, cardiovascular diseases; GED, general educational development; CI, confidence interval; HR, hazard ratio; METS, metabolic equivalent tasks. SD, Standard Deviation.
4 Discussion
In this large nationally representative cohort study, our findings confirmed that further adjustment of HSA rendered the inverse association between urinary ENL and all-cause mortality marginal and non-significant. The joint effect and stratified analysis suggested that the protective role of ENL was more pronounced in individuals with higher HSA levels.
As the bioavailable metabolites of dietary lignans, the health-protective role of enterolignans have been extensively investigated by in vitro and in vivo studies (38–43). Enterolignans has been demonstrated to possess antioxidant activity in a variety of test media systems (15, 39, 40). It has been found that enterolignans could also suppress the inflammatory responses in female Alzheimer’s disease mice (41), atopic dermatitis mice (42), and an in vitro intestinal epithelium model (43). But the epidemiological studies on circulating enterolignans and mortality risk, an indicator of long-term health, are scarce with inconsistent results. A study using NHANES data (1999–2004) reported that higher urinary ENL concentrations were associated with a reduced all-cause mortality, but not with cardiovascular disease-related mortality or cancer mortality. Urinary END did not manifest any association (17). The difference in the results between ENL and END may lie in the fact that ENL is the main lignan metabolite in both urine and blood (44), as is supported by the higher urinary ENL levels than that of END in this study. Our present study expanded the sample size from 5,179 to 10,664 with consideration of more potential confounders, yielding similar results before adjustment for HSA. While another study conducted on 1,889 middle age Finnish men found an inverse but non-significant association of serum ENL with reduced all-cause mortality risk. The authors also revealed that cardiovascular death risk decreased with elevated serum ENL levels (18). Such associations were not observed in our present study and another case-cohort study of 6,065 Finnish male smokers (19). Both of these two studies were conducted in Finnish men. Differences in race, gender, dietary habits and lifestyle factors may partly explain such inconsistency (45). Additionally, results of the present study indicated that HSA levels should also be taken into consideration when assessing the associations between enterolignans and health outcomes.
In this study, we found that urinary ENL was inversely associated with all-cause mortality, while the inverse association became non-significant when HSA was further adjusted. One possible reason is that the binding of ENL to HSA may affect their health benefits. Enterolignans and other metabolites of polyphenols are known to non-covalently interact with HSA in blood through hydrophobic or hydrophilic interactions (25). Biological properties of polyphenols depend on their bioavailability. The interaction between HSA and polyphenols may influence the bioavailability of polyphenols by modulating their transport, biological activity, delivery to tissues and organs, and ultimate clearance (25, 46). This may potentially account for the potential influence of HSA on the health-beneficial properties of ENL observed in the present study. Another possibility is that, due to residual confounding, HSA may simply act as a confounder in the statistical model. HSA levels have also been proven to be negatively related with death risk among healthy participants (29, 30) and patients (47, 48). Although one cross-sectional study has reported a negative correlation between enterolignans and HSA levels among Chinese pregnant women (28), ENL concentrations were not associated with HSA levels in our study (data not shown). Hence, it is possible that the alteration in the relationship between ENL and death before and after HSA adjustment may be a consequence of the interaction of HSA with the biological activities of ENL, rather than HSA being a confounder in the model. Additionally, we cannot rule out that this attenuation reflects confounding or a statistical artifact. Further studies are needed to confirm our conclusions and above hypotheses. Moreover, RCS analysis suggested a linear dose–response relationship between ENL and all-cause mortality. The protective association for ENL became no significant at approximately 4.7 μg/g creatinine, which may suggest that ENL levels require more attention.
Our findings suggested that the association between ENL and mortality was more pronounced in participants with higher HSA levels. A potential explanation for the result could be improved antioxidant activity. Enterolactone and albumin have both been found to exhibit antioxidant properties (15, 26). Some in vitro studies suggested that polyphenol-albumin interaction could enhance the antioxidant activity of the complex. Binding of ENL and END to HSA could increase the reactivity of the HSA Cys34 thiol group, which plays the most important role for the antioxidant activity of HSA (24). HSA-bound quercetin was found to repair the phenoxy radical of LDL-bound tocopherol as well as the tryptophan radical of HSA (46). Almajano et al. found albumin caused a synergistic increasing antioxidant activity of green tea catechins in oil-in-water emulsions. The probable mechanism is that albumin binds with catechins and transports it to the oil–water interface, where it is highly effective at weakening the oxidation (49). This potential synergistic interaction between albumin and ENL may partially account for our observations. Albumin is an indicator of nutritional status and can be associated with function and health status (47), thus individuals with lower HSA levels might have other underlying factors affecting health, which could mask the potential inverse relationship between ENL and death. In the present study, we also observed the lowest mortality risk among participants with high levels of both ENL and HSA, suggesting that these findings are unlikely to be due to chance. However, given some potential factors, more research is necessary.
The current study has several strengths, including the use of a nationally representative sample of US adults, large sample size and reliable urinary enterolignan measurements for lignan exposure assessment. However, several limitations should be noted. Firstly, END and ENL concentrations were determined in spot urine rather than 24-h urine samples, which might introduce additional random and systematic errors because of potential circadian rhythm. However, enterolignan levels in spot urine were proven to be in good accordance with serum concentrations (50–52). Additionally, most studies utilize single-void urine enterolignans, as a biomarker of exposure. However, urinary enterolignans, measured only once, might not accurately represent habitual dietary intake, thus necessitating repeated measurements. Yet, such repeats are unavailable in NHANES due to feasibility constraints. Secondly, although we have adjusted for a wide range of potential confounders, some residual confounders due to unmeasured or inaccurately measured covariates, such as genetic susceptibility, gut microbiota, or dietary structures, cannot be entirely ruled out, and these factors may influence the associations in the study. Thirdly, since this is an observational study, we cannot determine if there is a causal relationship between urinary enterolignans and mortality risk.
5 Conclusion
In summary, our findings revealed that adjustment of HSA could influence the association between urinary enterolignans and mortality risk. Urinary ENL concentrations were inversely correlated with all-cause mortality only among individuals with higher HSA levels. Despite the need for more research, our work offers preliminary insights that HSA might be an important covariate to be considered in future epidemiological studies on enterolignans.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: The datasets generated and/or analyzed for this study can be found in the NHANES repository (https://www.cdc.gov/nchs/nhanes/.irba98.htm).
Ethics statement
The studies involving humans were approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ZS: Writing – original draft, Investigation, Formal analysis. QW: Validation, Investigation, Writing – review & editing. TS: Supervision, Writing – review & editing, Resources. JZ: Investigation, Writing – review & editing. HW: Investigation, Writing – review & editing. WY: Supervision, Project administration, Funding acquisition, Writing – review & editing. ZZ: Methodology, Funding acquisition, Supervision, Conceptualization, Writing – review & editing, Project administration. QZ: Writing – review & editing, Methodology, Supervision, Conceptualization, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82373673, 82103796), Scientific Research Projects of Universities in Anhui Province (2024AH040104). The funding agency had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
The authors thank all the participants and researchers who contributed to the data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1600857/full#supplementary-material
Abbreviations
BMI, body mass index; CVD, cardiovascular disease; CIs, confidence intervals; CDC, Centers for Disease Control and Prevention; ENL, enterolactone; END, enterodiol; GED, general educational development; HSA, human serum albumin; HRs, hazard ratios; HPLC-MS/MS, high-performance liquid chromatography–tandem mass spectrometry; IQR, interquartile range; MEC, mobile examination centers; METS, metabolic equivalent tasks; NHANES, National Health and Nutrition Examination Survey; NCHS, National Center for Health Statistics; NDI, National Death Index; SD, standard deviation.
Footnotes
References
1. Peterson, J, Dwyer, J, Adlercreutz, H, Scalbert, A, Jacques, P, and McCullough, ML. Dietary lignans: physiology and potential for cardiovascular disease risk reduction. Nutr Rev. (2010) 68:571–603. doi: 10.1111/j.1753-4887.2010.00319.x
2. Jang, WY, Kim, MY, and Cho, JY. Antioxidant, anti-inflammatory, anti-menopausal, and anti-cancer effects of lignans and their metabolites. Int J Mol Sci. (2022) 23:15482. doi: 10.3390/ijms232415482
3. Wang, LX, Wang, HL, Huang, J, Chu, TZ, Peng, C, Zhang, H, et al. Review of lignans from 2019 to 2021: newly reported compounds, diverse activities, structure-activity relationships and clinical applications. Phytochemistry. (2022) 202:113326. doi: 10.1016/j.phytochem.2022.113326
4. Chhillar, H, Chopra, P, and Ashfaq, MA. Lignans from linseed (Linum usitatissimum L.) and its allied species: retrospect, introspect and prospect. Crit Rev Food Sci Nutr. (2021) 61:2719–41. doi: 10.1080/10408398.2020.1784840
5. Chen, ZL, Qian, F, Hu, Y, Voortman, T, Li, YP, Rimm, EB, et al. Dietary phytoestrogens and total and cause-specific mortality: results from 2 prospective cohort studies. Am J Clin Nutr. (2023) 117:130–40. doi: 10.1016/j.ajcnut.2022.10.019
6. Lin, XC, Zhao, J, Ge, S, Lu, HD, Xiong, QQ, Guo, XL, et al. Dietary polyphenol intake and risk of hypertension: an 18-y Nationwide cohort study in China. Am J Clin Nutr. (2023) 118:264–72. doi: 10.1016/j.ajcnut.2023.05.001
7. Morisset, AS, Lemieux, S, Veilleux, A, Bergeron, J, Weisnagel, SJ, and Tchernof, A. Impact of a lignan-rich diet on adiposity and insulin sensitivity in post-menopausal women. Br J Nutr. (2009) 102:195–200. doi: 10.1017/S0007114508162092
8. Peñalvo, JL, Moreno-Franco, B, Ribas-Barba, L, and Serra-Majem, L. Determinants of dietary lignan intake in a representative sample of young Spaniards: association with lower obesity prevalence among boys but not girls. Eur J Clin Nutr. (2012) 66:795–8. doi: 10.1038/ejcn.2012.45
9. Li, YP, Wang, FL, Li, J, Ivey, KL, Wilkinson, JE, Wang, DD, et al. Dietary lignans, plasma enterolactone levels, and metabolic risk in men: exploring the role of the gut microbiome. BMC Microbiol. (2022) 22:82. doi: 10.1186/s12866-022-02495-0
10. Rizzolo-Brime, L, Caro-Garcia, EM, Alegre-Miranda, CA, Felez-Nobrega, M, and Zamora-Ros, R. Lignan exposure: a worldwide perspective. Eur J Nutr. (2022) 61:1143–65. doi: 10.1007/s00394-021-02736-4
11. Mo, YF, Li, YM, Liang, SX, Wang, WQ, Zhang, HH, Zhao, JJ, et al. Urinary enterolignans and enterolignan-predicting microbial species are favourably associated with liver fat and other obesity markers. Food Funct. (2024) 15:7305–13. doi: 10.1039/d3fo05632e
12. Rowland, IR, Wiseman, H, Sanders, TA, Adlercreutz, H, and Bowey, EA. Interindividual variation in metabolism of soy isoflavones and lignans: influence of habitual diet on equol production by the gut microflora. Nutr Cancer. (2000) 36:27–32. doi: 10.1207/s15327914nc3601_5
13. Adlercreutz, H. Lignans and human health. Crit Rev Clin Lab Sci. (2007) 44:483–525. doi: 10.1080/10408360701612942
14. Landete, JM, Arqués, J, Medina, M, Gaya, P, de Las Rivas, B, and Muñoz, R. Bioactivation of phytoestrogens: intestinal bacteria and health. Crit Rev Food Sci Nutr. (2016) 56:1826–43. doi: 10.1080/10408398.2013.789823
15. Laveriano-Santos, EP, Luque-Corredera, C, Trius-Soler, M, Lozano-Castellón, J, Dominguez-López, I, Castro-Barquero, S, et al. Enterolignans: from natural origins to cardiometabolic significance, including chemistry, dietary sources, bioavailability, and activity. Crit Rev Food Sci Nutr. (2024) 65:3764–84. doi: 10.1080/10408398.2024.2371939
16. Xuan, C, Zhao, C, Zhou, TT, Guo, JJ, Pan, D, Wang, ZB, et al. Associations of urinary phytoestrogens with all-cause and cardiovascular mortality in adults: a population-based cohort study. Front Endocrinol. (2024) 15:1400182. doi: 10.3389/fendo.2024.1400182
17. Reger, MK, Zollinger, TW, Liu, ZY, Jones, J, and Zhang, JJ. Urinary phytoestrogens and cancer, cardiovascular, and all-cause mortality in the continuous National Health and nutrition examination survey. Eur J Nutr. (2016) 55:1029–40. doi: 10.1007/s00394-015-0917-y
18. Vanharanta, M, Voutilainen, S, Rissanen, TH, Adlercreutz, H, and Salonen, JT. Risk of cardiovascular disease-related and all-cause death according to serum concentrations of enterolactone: Kuopio ischaemic heart disease risk factor study. Arch Intern Med. (2003) 163:1099–104. doi: 10.1001/archinte.163.9.1099
19. Kilkkinen, A, Erlund, I, Virtanen, MJ, Alfthan, G, Ariniemi, K, and Virtamo, J. Serum enterolactone concentration and the risk of coronary heart disease in a case-cohort study of Finnish male smokers. Am J Epidemiol. (2006) 163:687–93. doi: 10.1093/aje/kwj080
20. Xiao, JB, and Kai, GY. A review of dietary polyphenol-plasma protein interactions: characterization, influence on the bioactivity, and structure-affinity relationship. Crit Rev Food Sci Nutr. (2012) 52:85–101. doi: 10.1080/10408398.2010.499017
21. López-Yerena, A, Perez, M, Vallverdú-Queralt, A, and Escribano-Ferrer, E. Insights into the binding of dietary phenolic compounds to human serum albumin and food-drug interactions. Pharmaceutics. (2020) 12:1123. doi: 10.3390/pharmaceutics12111123
22. Manach, C, Scalbert, A, Morand, C, Rémésy, C, and Jiménez, L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. (2004) 79:727–47. doi: 10.1093/ajcn/79.5.727
23. Xiao, J, Cao, H, Wang, Y, Yamamoto, K, and Wei, X. Structure-affinity relationship of flavones on binding to serum albumins: effect of hydroxyl groups on ring a. Mol Nutr Food Res. (2010) 54:S253–60. doi: 10.1002/mnfr.200900454
24. Takic, MM, Jovanovic, VB, Pavicevic, ID, Uzelac, TN, Acimovic, JM, Ristic-Medic, DK, et al. Binding of enterolactone and enterodiol to human serum albumin: increase of cysteine-34 thiol group reactivity. Food Funct. (2016) 7:1217–26. doi: 10.1039/c5fo01346a
25. Zou, DF, and Xie, AZ. Influence of polyphenol-plasma protein interaction on the antioxidant properties of polyphenols. Curr Drug Metab. (2013) 14:451–5. doi: 10.2174/1389200211314040008
26. Belinskaia, DA, Voronina, PA, Shmurak, VI, Jenkins, RO, and Goncharov, NV. Serum albumin in health and disease: esterase, antioxidant, transporting and signaling properties. Int J Mol Sci. (2021) 22:10318. doi: 10.3390/ijms221910318
27. Zhu, Y, Kawaguchi, K, and Kiyama, R. Differential and directional estrogenic signaling pathways induced by enterolignans and their precursors. PLoS One. (2017) 12:e0171390. doi: 10.1371/journal.pone.0171390
28. Wang, XG, Lei, L, Wang, LJ, Huang, DD, Huang, JC, Guo, EN, et al. Associations between maternal serum phytoestrogens and liver function markers: a cross-sectional study from China. Environ Sci Pollut R. (2023) 30:122038–50. doi: 10.1007/s11356-023-30761-9
29. Goldwasser, P, and Feldman, J. Association of serum albumin and mortality risk. J Clin Epidemiol. (1997) 50:693–703. doi: 10.1016/s0895-4356(97)00015-2
30. Phillips, A, Shaper, AG, and Whincup, PH. Association between serum albumin and mortality from cardiovascular disease, cancer, and other causes. Lancet. (1989) 2:1434–6. doi: 10.1016/s0140-6736(89)92042-4
31. Stampfer, MJ, Willett, WC, Speizer, FE, Dysert, DC, Lipnick, R, Rosner, B, et al. Test of the National Death Index. Am J Epidemiol. (1984) 119:837–9. doi: 10.1093/oxfordjournals.aje.a113804
32. Rich-Edwards, JW, Corsano, KA, and Stampfer, MJ. Test of the National Death Index and Equifax Nationwide death search. Am J Epidemiol. (1994) 140:1016–9. doi: 10.1093/oxfordjournals.aje.a117191
33. Gawrieh, S, Wilson, LA, Cummings, OW, Clark, JM, Loomba, R, Hameed, B, et al. Histologic findings of advanced fibrosis and cirrhosis in patients with nonalcoholic fatty liver disease who have Normal aminotransferase levels. Am J Gastroenterol. (2019) 114:1626–35. doi: 10.14309/ajg.0000000000000388
34. Levey, AS, Stevens, LA, Schmid, CH, Zhang, YL, Castro, AF 3rd, Feldman, HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
35. Zhang, K, Li, Q, Chen, Y, Wang, NJ, and Lu, YL. Visceral adiposity and renal function: an observational study from SPECT-China. Lipids Health Dis. (2017) 16:205. doi: 10.1186/s12944-017-0597-0
36. Kuijsten, A, Arts, JCW, Vree, TB, and Hollman, PCH. Pharmacokinetics of enterolignans in healthy men and women consuming a single dose of secoisolariciresinol diglucoside. J Nutr. (2005) 135:795–801. doi: 10.1093/jn/135.4.795
37. Kuhnle, GGC, Ward, HA, Vogiatzoglou, A, Luben, RN, Mulligan, A, Wareham, NJ, et al. Association between dietary phyto-oestrogens and bone density in men and postmenopausal women. Br J Nutr. (2011) 106:1063–9. doi: 10.1017/S0007114511001309
38. Hu, Y, Li, YP, Sampson, L, Wang, ML, Manson, JE, Rimm, E, et al. Lignan intake and risk of coronary heart disease. J Am Coll Cardiol. (2021) 78:666–78. doi: 10.1016/j.jacc.2021.05.049
39. Kitts, DD, Yuan, YV, Wijewickreme, AN, and Thompson, LU. Antioxidant activity of the flaxseed lignan secoisolariciresinol diglycoside and its mammalian lignan metabolites enterodiol and enterolactone. Mol Cell Biochem. (1999) 202:91–100. doi: 10.1023/a:1007022329660
40. Hu, C, Yuan, YV, and Kitts, DD. Antioxidant activities of the flaxseed lignan secoisolariciresinol diglucoside, its aglycone secoisolariciresinol and the mammalian lignans enterodiol and enterolactone. Food Chem Toxicol. (2007) 45:2219–27. doi: 10.1016/j.fct.2007.05.017
41. Jia, MZ, Ning, FJ, Wen, JQ, Wang, XR, Chen, J, Hu, J, et al. Secoisolariciresinol diglucoside attenuates neuroinflammation and cognitive impairment in female Alzheimer's disease mice via modulating gut microbiota metabolism and GPER/CREB/BDNF pathway. J Neuroinflamm. (2024) 21:201. doi: 10.1186/s12974-024-03195-4
42. Yu, L, Xu, QS, Wang, P, Luo, JL, Zheng, ZJ, Zhou, J, et al. Secoisolariciresinol diglucoside-derived metabolite, enterolactone, attenuates atopic dermatitis by suppressing Th2 immune response. Int Immunopharmacol. (2022) 111:109039. doi: 10.1016/j.intimp.2022.109039
43. Almousa, AA, Meurens, F, Krol, ES, and Alcorn, J. Linoorbitides and enterolactone mitigate inflammation-induced oxidative stress and loss of intestinal epithelial barrier integrity. Int Immunopharmacol. (2018) 64:42–51. doi: 10.1016/j.intimp.2018.08.012
44. Lampe, JW. Isoflavonoid and lignan phytoestrogens as dietary biomarkers. J Nutr. (2003) 133:956s–64s. doi: 10.1093/jn/133.3.956S
45. Halldin, E, Eriksen, AK, Brunius, C, da Silva, AB, Bronze, M, Hanhineva, K, et al. Factors explaining interpersonal variation in plasma enterolactone concentrations in humans. Mol Nutr Food Res. (2019) 63:e1801159. doi: 10.1002/mnfr.201801159
46. Poloni, DM, Dangles, O, and Vinson, JA. Binding of plant polyphenols to serum albumin and LDL: healthy implications for heart disease. J Agric Food Chem. (2019) 67:9139–47. doi: 10.1021/acs.jafc.8b06674
47. Cabrerizo, S, Cuadras, D, Gomez-Busto, F, Artaza-Artabe, I, Ciancas, FM, and Malafarina, V. Serum albumin and health in older people: review and meta analysis. Maturitas. (2015) 81:17–27. doi: 10.1016/j.maturitas.2015.02.009
48. Sun, J, Su, HT, Lou, YH, and Wang, MJ. Association between serum albumin level and all-cause mortality in patients with chronic kidney disease: a retrospective cohort study. Am J Med Sci. (2021) 361:451–60. doi: 10.1016/j.amjms.2020.07.020
49. Almajano, MP, Delgado, ME, and Gordon, MH. Albumin causes a synergistic increase in the antioxidant activity of green tea catechins in oil-in-water emulsions. Food Chem. (2007) 102:1375–82. doi: 10.1016/j.foodchem.2006.06.067
50. Knust, U, Spiegelhalder, B, Strowitzki, T, and Owen, RW. Contribution of linseed intake to urine and serum enterolignan levels in German females: a randomised controlled intervention trial. Food Chem Toxicol. (2006) 44:1057–64. doi: 10.1016/j.fct.2005.12.009
51. Nesbitt, PD, Lam, Y, and Thompson, LU. Human metabolism of mammalian lignan precursors in raw and processed flaxseed. Am J Clin Nutr. (1999) 69:549–55. doi: 10.1093/ajcn/69.3.549
Keywords: enterolignans, enterodiol, enterolactone, mortality risk, serum albumin
Citation: Sun Z, Wang Q, Shen T, Zhu J, Wang H, Yang W, Zhang Z and Zhou Q (2025) Associations of urinary enterolignans and risk of overall and cause-specific mortality with or without serum albumin adjustment: a prospective cohort study. Front. Nutr. 12:1600857. doi: 10.3389/fnut.2025.1600857
Edited by:
Weimin Ye, Karolinska Institutet (KI), SwedenReviewed by:
Raffaella Comitato, Council for Agricultural Research and Agricultural Economy Analysis|CREA, ItalyMeng Zhang, Inner Mongolia Agricultural University, China
Copyright © 2025 Sun, Wang, Shen, Zhu, Wang, Yang, Zhang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Zhou, emhvdXFpYW5nMTk3M0AxNjMuY29t; Zhuang Zhang, emh6aF9haG11QG91dGxvb2suY29t
†These authors share first authorship
‡These authors share last authorship
 Zisuo Sun1,2,3,4†
Zisuo Sun1,2,3,4† Tianyi Shen
Tianyi Shen Wanshui Yang
Wanshui Yang Zhuang Zhang
Zhuang Zhang