- 1The Second Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, China
- 2Wuming Hospital of Guangxi Medical University, Nanning, Guangxi, China
Background: Sepsis patients often have immune dysfunction and malnutrition, which is a high-risk disease for death in critically ill patients. Although various biomarkers can predict the prognosis of sepsis patients, they are cumbersome to implement clinically. This study evaluates the prognostic potential of the Prognostic Nutritional Index (PNI) to fill this gap.
Methods: We conducted a retrospective analysis of data from patients admitted to the Intensive Care Unit (ICU) of Beth Israel Deaconess Medical Center with sepsis between 2008 and 2022. The Prognostic Nutritional Index (PNI) was calculated using the first measurement within 24 h of admission. Kaplan–Meier analysis was used to compare mortality risks among three groups, and a multivariable Cox proportional hazards regression model assessed the link between PNI and mortality risk in sepsis patients. Restricted cubic splines (RCS) explored the potential dose—response relationship between PNI and mortality, and threshold analysis determined the critical threshold of PNI. Receiver operating characteristic (ROC) analysis evaluated the predictive ability, sensitivity, and specificity of LAR for all—cause mortality in patients with liver cirrhosis and sepsis, and calculated the area under the curve (AUC). Finally, subgroup analyses were performed to evaluate the relationship between PNI and prognosis in different populations.
Results: A total of 6,234 patients were included Kaplan—Meier analysis showed that patients with high PNI had lower 14, 28, and 90-day all—cause mortality risks (all log—rank P < 0.001). The multivariable Cox proportional hazards model indicated that high PNI was independently associated with 14, 28, and 90-day all—cause mortality, with HRs of 0.62, 0.56, and 0.59 (all P < 0.0001), before and after adjusting for confounders RCS analysis revealed a non-linear link between PNI and short—and medium—term all—cause mortality in sepsis patients. A two—segment Cox proportional hazards model identified inflection points at 11.6 for 14-day, 11.2 for 28-day, and 11.2 for 90-day all-cause mortality ROC analysis showed PNI has lower predictive value for sepsis prognosis than sequential organ failure assessment and acute physiology and chronic health evaluation, yet it can enhance their predictive power Subgroup analyses found no significant interaction between PNI and specific subgroups.
Conclusion: There is a significant association between short-term and medium—term all—cause mortality in sepsis patients and PNI, indicating that PNI can be a valuable indicator for predicting in—hospital and ICU mortality risk.
1 Introduction
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. It is one of the common reasons for sending critically ill patients to the intensive care unit and a major cause of death in such patients (1). This dysregulated response to infection can lead to cellular dysfunction and ultimately organ dysfunction. According to relevant studies, the mortality rate of sepsis can be as high as 40% (2, 3).
Albumin is a good indicator of nutritional status and is closely related to the prognosis of sepsis patients (4). However, baseline nutritional status or chronic inflammatory diseases can affect albumin levels, limiting its use as a single prognostic indicator. Peripheral blood lymphocyte levels are key immune indicators in patients with infection, and changes in their number and function can reflect the body’s immune status (5, 6). For example, in sepsis, patients experience increased lymphocyte apoptosis, leading to reduced immune cell numbers and decreased function. However, severe infections and certain medications can cause abnormal lymphocyte levels, limiting the prediction of patient prognosis based solely on lymphocyte levels (7). Therefore, identifying indicators to assess the prognosis of sepsis patients is crucial for timely recognition and intervention.
Recently, the Prognostic Nutritional Index (PNI), calculated from albumin and lymphocyte levels, has shown potential as a predictor of mortality in various conditions, including cancer, cardiovascular disease, liver disease, and chronic kidney disease (8–12). However, the mid-term prognostic value of PNI in sepsis patients has not been reported.
In this study, we aim to retrospectively analyze the clinical data of critically ill sepsis patients to explore the potential of PNI as a mid-term prognostic tool, with the goal of aiding early clinical recognition and improving outcomes.
2 Materials and methods
2.1 Data source
We retrospectively analyzed clinical data of sepsis patients extracted from the Medical Information Mart for Intensive Care IV (MIMIC-IV) database (version 3.1) between 2008 and 2022 (13). The MIMIC-IV database, developed by the Massachusetts Institute of Technology’s Computational Physiology Laboratory, includes records of patients admitted to Beth Israel Deaconess Medical Center. Our research team completed the Collaborative Institutional Training Initiative (CITI) course, passed the “Conflict of Interest” and “Research Data or Specimens Only” exams, and obtained access to the MIMIC-IV database (version 3.1). The study aimed to explore the potential of PNI as a prognostic tool to aid early clinical recognition and improve outcomes.
2.2 Study population
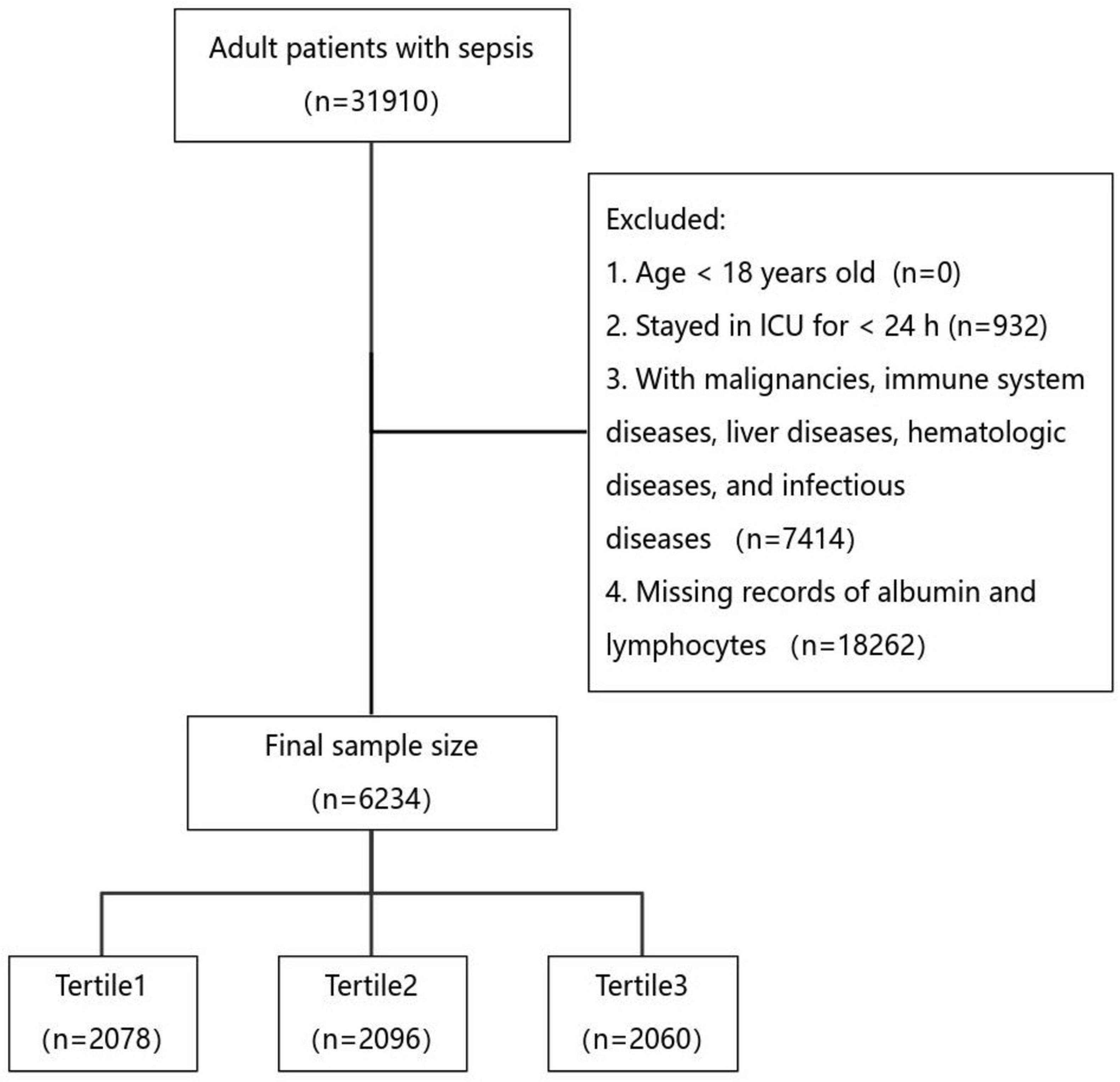
Patients were classified as having sepsis according to the Sepsis 3.0 definition if they had suspected infection and a Sequential Organ Failure Assessment (SOFA) score ≥ 2 (1). Strict exclusion criteria were applied to ensure robust results, including patients under 18 years of age at first admission, patients with ICU stays less than 24 h, patients with conditions affecting albumin and lymphocyte levels, and patients without albumin and lymphocyte records within the first 24 h of admission. Only the first admission data were included for patients with multiple ICU stays. Ultimately, 6,234 patients were included in the study (Figure 1).
2.3 Data extraction
Data extraction was performed using PostgreSQL software (version 13.7.2) and Navicat Premium software (version 16) with the aid of Structured Query Language (SQL). Information on demographic characteristics, vital signs, comorbidities, laboratory data, microbiological data, clinical treatments, survival status, and severity scores was extracted.
2.4 Handling of outliers and missing values
Outliers were handled using the winsor2 command in STATA with 1 and 99% cutoff points. Missing values were imputed using multiple imputation, excluding variables with over 10% missing data and imputing the remaining using this method.
2.5 Grouping and clinical outcomes
PNI was calculated as: PNI = albumin (g/dL) + 5 × absolute lymphocyte count (× 109/L) (14). Patients were divided into three groups by tertiles. The primary endpoint was 90-day all—cause mortality, with secondary endpoints at 14 and 28 days.
2.6 Ethical statement
This study adhered to the Declaration of Helsinki. As it used de-identified data from MIMIC-IV (version 3.1), the Beth Israel Deaconess Medical Center’s ethics review committee waived the requirement for informed consent. No formal ethical approval or patient consent was needed due to the use of de-identified data.
2.7 Statistical analysis
Continuous variables are presented as median (IQR). The Mann-Whitney U or Kruskal-Wallis test was used for group comparisons. Categorical variables are summarized as frequency (percentage), with group comparisons done via chi—square or Fisher’s exact tests.
Patients were divided into three groups based on PNI tertiles. Kaplan–Meier curves were plotted to analyze survival trajectories for primary and secondary endpoints, with log—rank tests assessing group differences. Univariate and multivariate Cox proportional hazards models evaluated the association between PNI (as a categorical variable) and 14, 28, and 90-day all—cause mortality. The multivariate models were adjusted for potential confounding factors such as age, gender, race, SOFA score, hematocrit, platelets, anion gap, calcium, sodium, lactate, acute kidney injury, chronic kidney disease, myocardial infarction, continuous renal replacement therapy, mechanical ventilation, vasoactive drugs, albumin, immunosuppressants, and corticosteroids.
Restrictive cubic spline (RCS) analysis based on Cox regression models was performed to explore the potential non-linear relationship between PNI levels and mortality. A two—segment Cox proportional hazards model was used to determine threshold effects and identify inflection points.
Receiver operating characteristic (ROC) analysis was conducted to evaluate the predictive ability of PNI, SOFA, APACHE II, and their combinations for all—cause mortality at different time points, and the area under the curve (AUC) was calculated.
Subgroup analyses were performed to assess the consistency of the association between PNI levels and mortality across predefined subgroups, including age, sex, acute kidney injury, chronic kidney disease, myocardial infarction, albumin treatment, immunosuppressive treatment, and corticosteroid therapy.
All analyses were performed using the Decision Linnc (Decision Linnc Core Team 2023) analytical platform, a comprehensive platform that combines various programming environments and enables data processing and analysis. Hang Zhou, CHN. Retrieved from https://www.statsape.com/. All the statistical tests were two-tailed, with a significance threshold of P < 0.05.
3 Results
3.1 Baseline characteristics comparison of sepsis patients in three groups
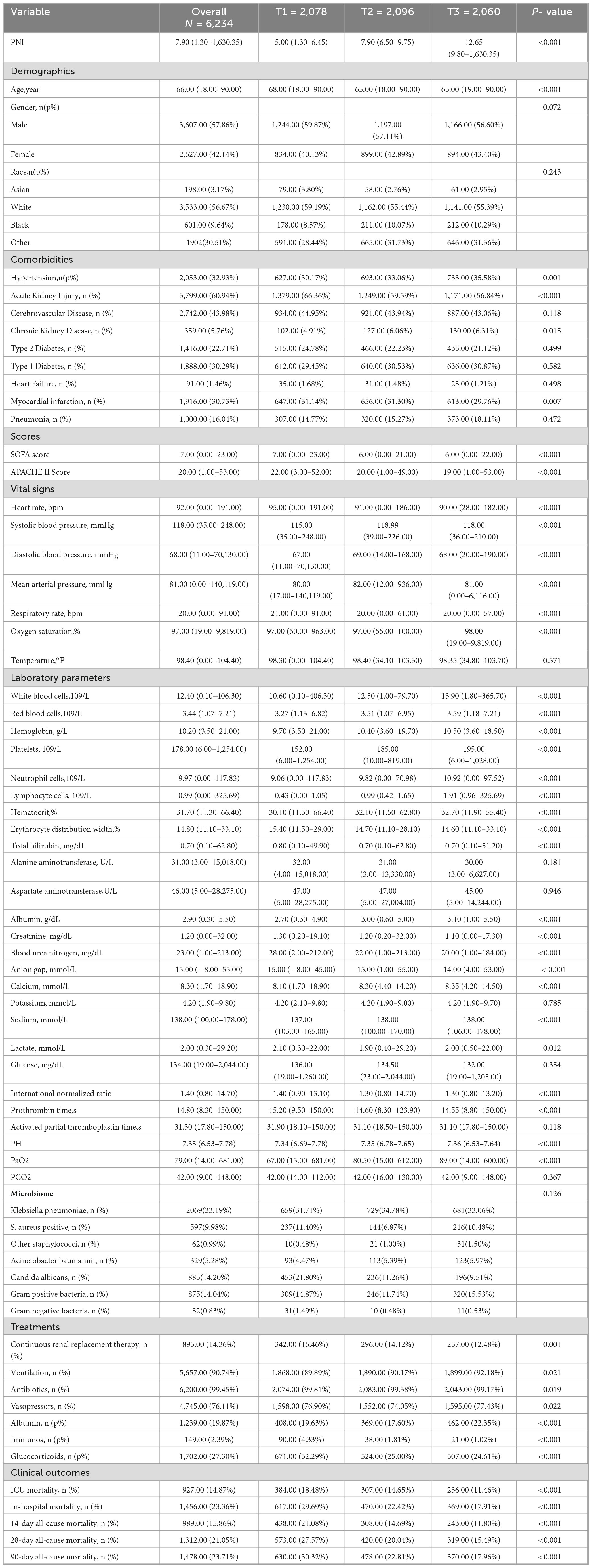
As shown in Table 1, this study included 6,234 patients, with a mean age of 66.00 (18.00–90.00) years, and 2,627 (42.14%) were female. ICU mortality was 14.87%, in—hospital mortality 23.36%, 14-day all—cause mortality 15.86%, 28-day 21.05%, and 90-day 23.71%. Patients were divided into three groups by PNI tertiles: T1 (low), T2 (medium), and T3 (high). Baseline characteristics differed significantly across groups. The high—PNI group had higher rates of hypertension, chronic kidney disease, and pneumonia, with a higher likelihood of Acinetobacter baumannii and gram—positive bacterial infections. The low—PNI group had more heart failure and myocardial infarction cases and was more prone to Candida albicans and gram—negative bacterial infections. Additionally, the high—PNI group had lower SOFA and APACHE II scores, received more albumin therapy, and less immunosuppressive therapy. Notably, the high—PNI group had lower mortality rates across all time endpoints compared to other groups (Table 1).
3.2 Kaplan–Meier survival curves
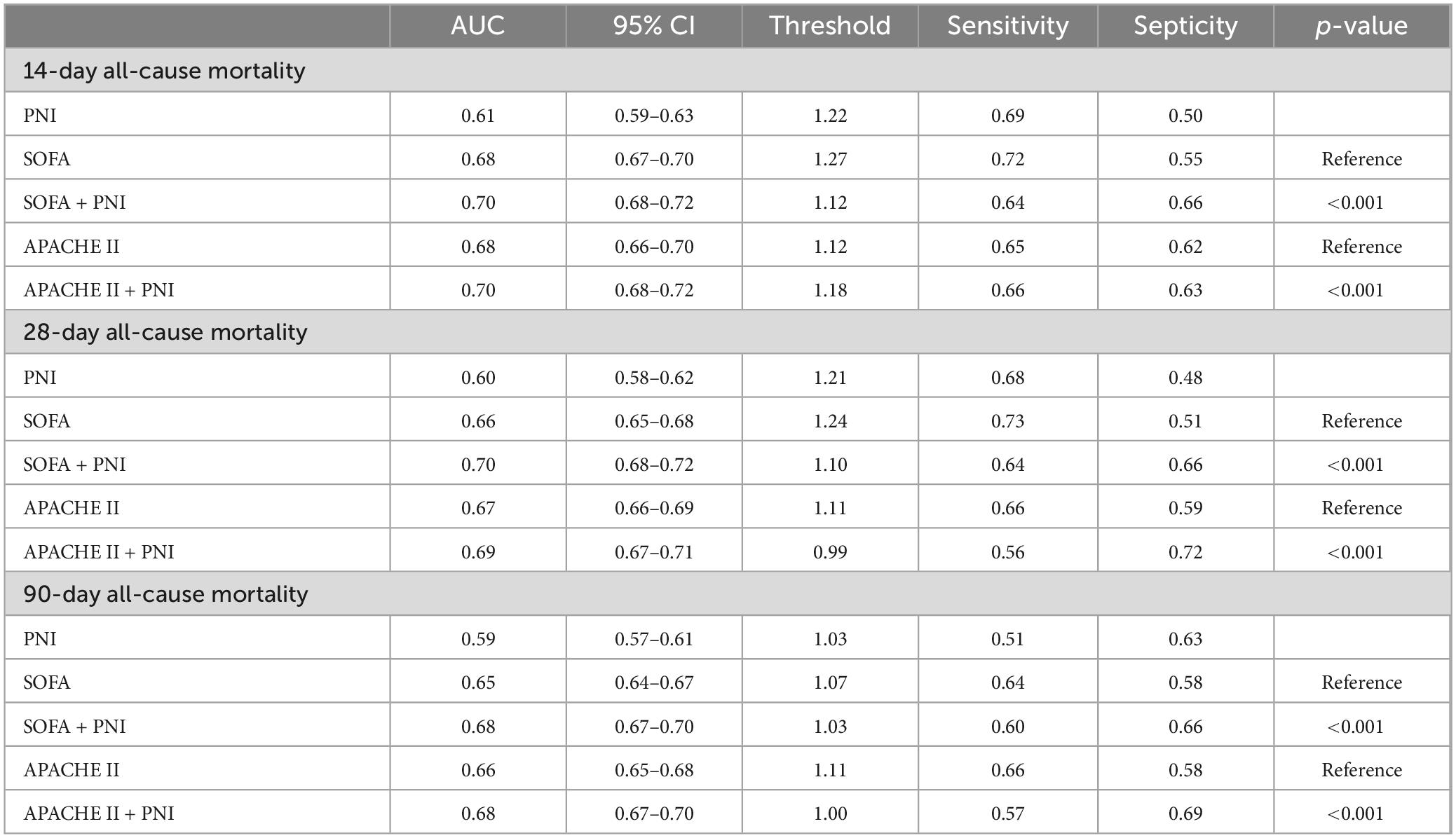
A total of 989 patients died within 14 days, 1,312 within 28 days, and 1,478 within 90 days. Kaplan–Meier survival curves showed that patients with higher PNI had lower 14, 28, and 90-day all—cause mortality (Figure 2, all log-rank P < 0.001). [Kaplan—Meier curves and cumulative incidence of 14-day (A), 28-day (B), and 90-day (C) all-cause mortality stratified by PNI groups].
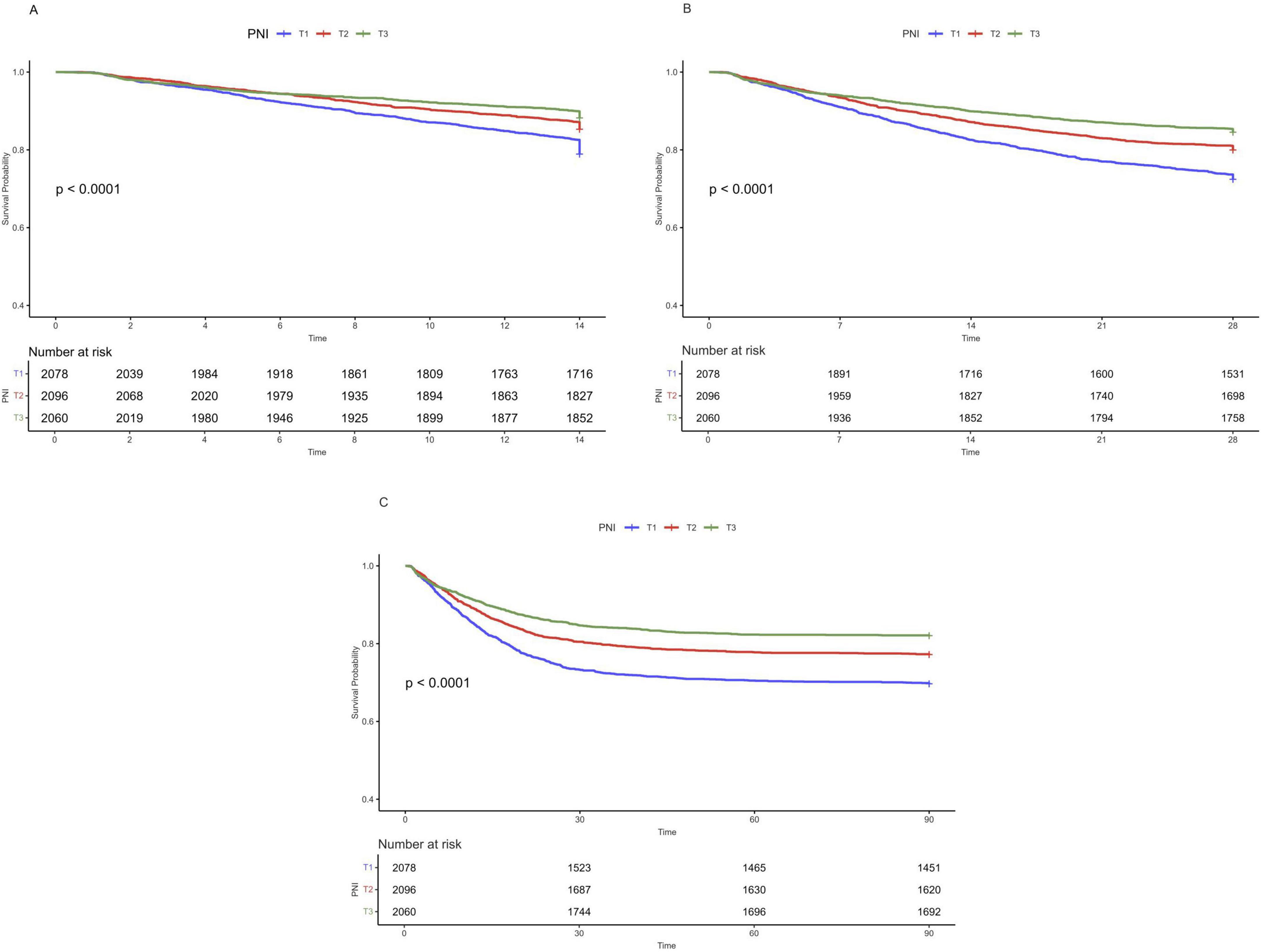
Figure 2. Kaplan-Meier curves and cumulative incidence of 14-day (A), 28-day (B), and 90-day (C) all-cause mortality stratified by PNI groups.
3.3 Relationship between PNI levels and clinical outcomes
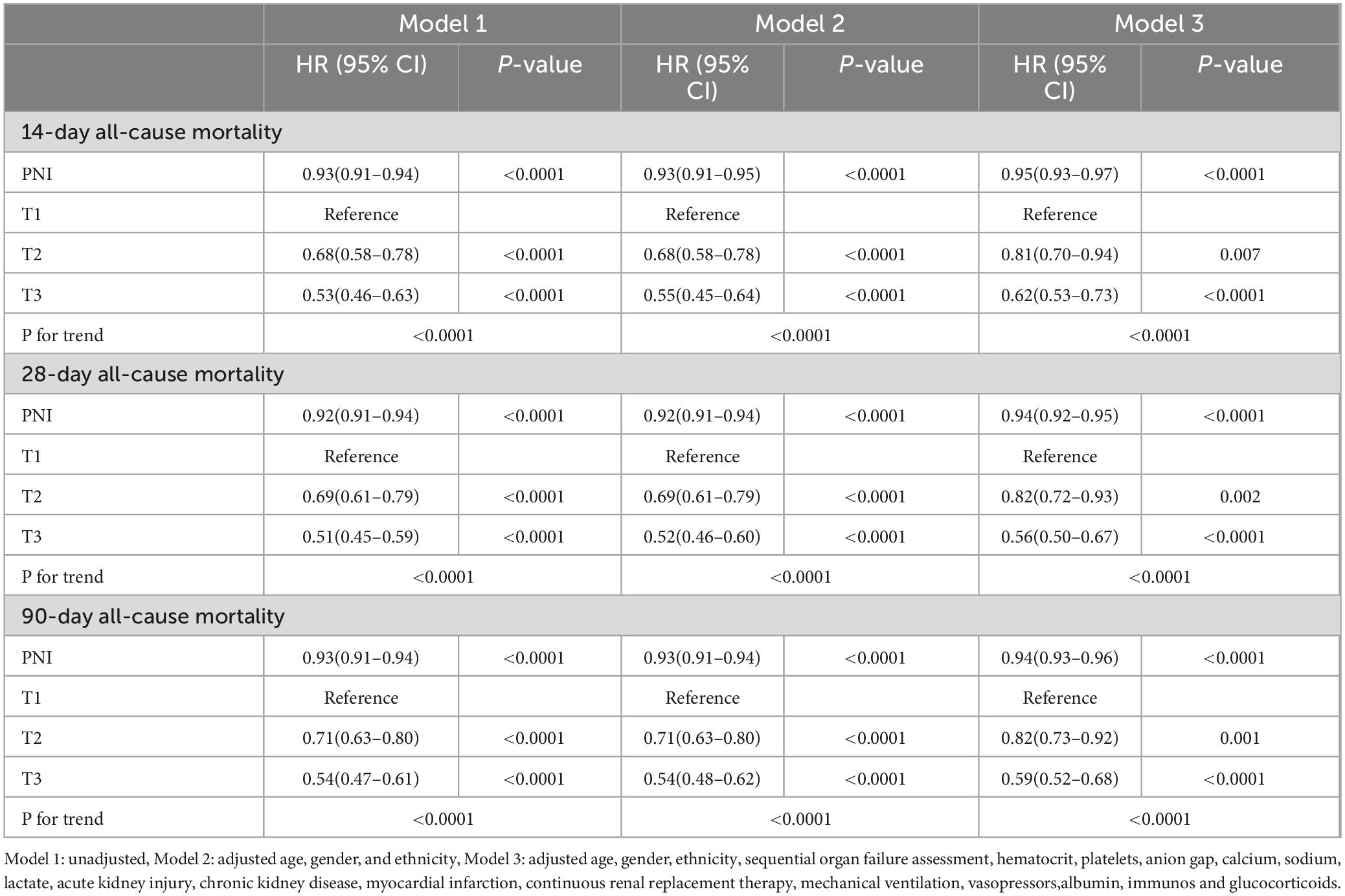
A multivariable Cox regression model was constructed to analyze the association between PNI levels and 14, 28, and 90-day all—cause mortality in sepsis patients. In the unadjusted model (Model 1), higher PNI was significantly associated with lower mortality risk: HR = 0.53 (95% CI:0.46–0.63, P < 0.0001) for 14-day, HR = 0.51 (95% CI:0.45–0.59, P < 0.0001) for 28-day, and HR = 0.54 (95% CI:0.47–0.61, P < 0.0001) for 90-day mortality. Model 2 adjusted for age, sex, and race, and also showed lower mortality risk with higher PNI: HR = 0.55 (95% CI:0.47–0.64, P < 0.0001) for 14-day, HR = 0.52 (95% CI:0.45–0.60, P < 0.0001) for 28-day, and HR = 0.54 (95% CI:0.48–0.62, P < 0.0001) for 90-day mortality. Model 3 further adjusted for potential confounders, including SOFA score, hematocrit, platelets, anion gap, calcium, sodium, lactate, acute kidney injury, chronic kidney disease, myocardial infarction, renal replacement therapy, mechanical ventilation, vasoactive drugs, albumin, immunosuppressants, and corticosteroids. Higher PNI levels remained significantly associated with lower all—cause mortality (Table 2).
3.4 Detection of non-linear relationships
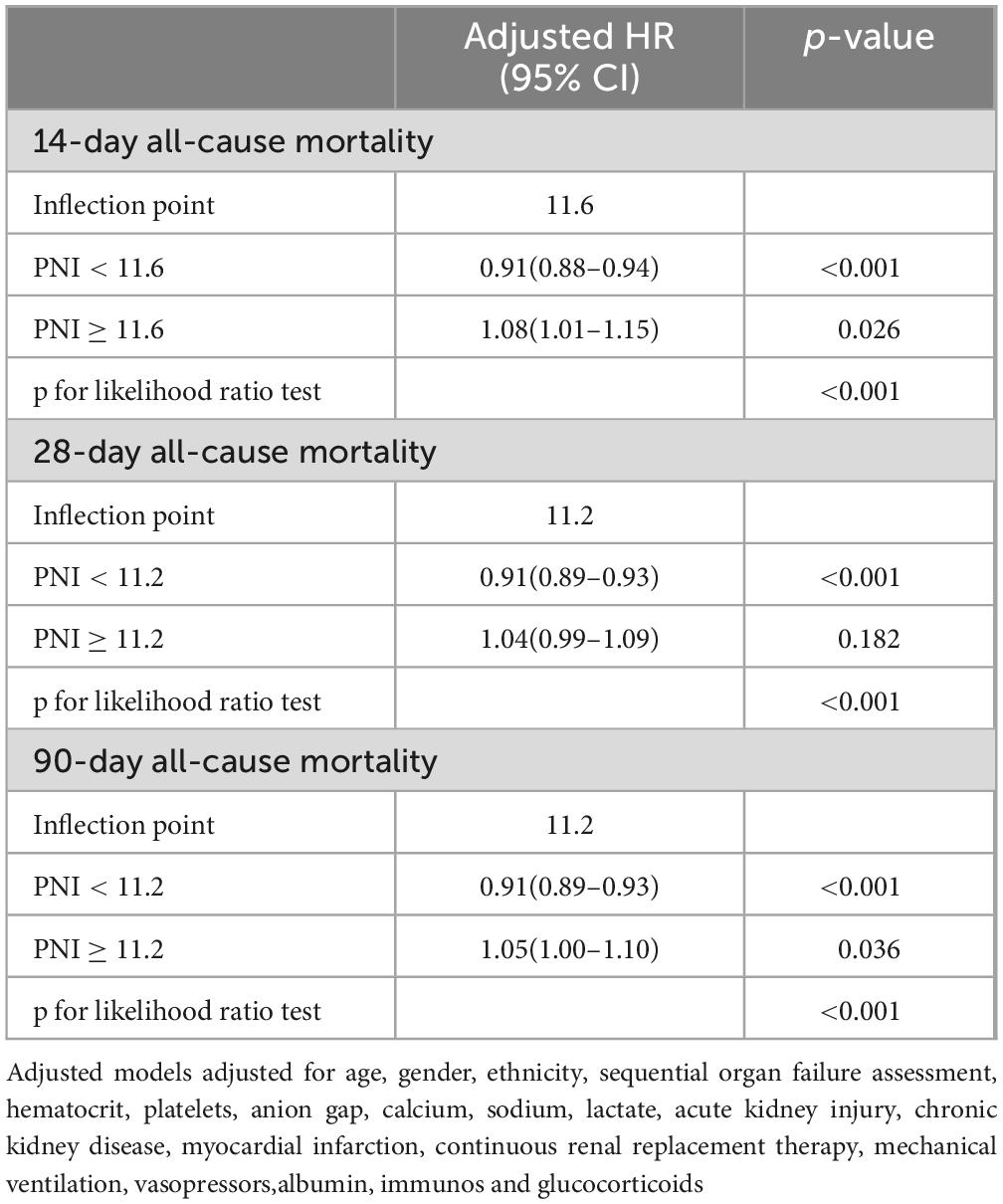
RCS analysis showed a non-linear relationship between PNI and 14, 28, and 90-day all—cause mortality (Figure 3, all P for non-linearity < 0.01). A two-segment Cox proportional hazards model identified inflection points at 14 days (11.6), 28 days (11.2), and 90 days (11.2). Below these thresholds, each 1-unit PNI increase was linked to a 9% drop in mortality risk, with HRs of 0.91 (95% CI: 0.88–0.94) for 14-day, 0.91 (95% CI: 0.89–0.93) for 28-day, and 0.91 (95% CI: 0.89–0.93) for 90-day mortality, indicating a negative PNI-mortality risk correlation. Conversely, when PNI was above these thresholds, the mortality risk rose, with HRs of 1.08 (95% CI: 1.01–1.15) for 14-day and 1.05 (95% CI: 1.00–1.10) for 90-day mortality. All associations were statistically significant (p < 0.001 by likelihood ratio test) (Table 3).
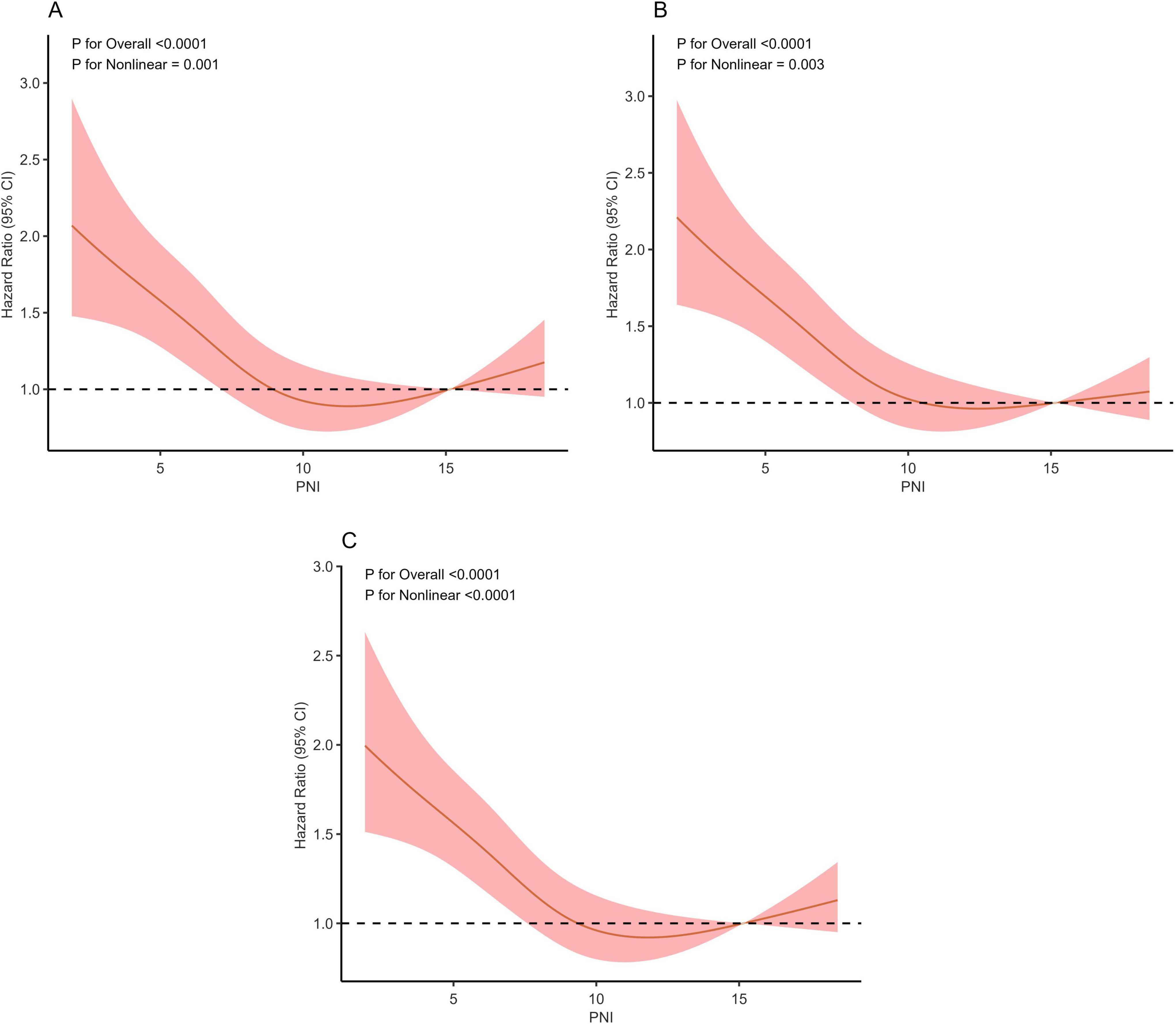
Figure 3. Restricted cubic spline regression analysis of PNI with 14-day (A), 28-day (B), and 90-day (C) all-cause mortality.
3.5 Predictive efficacy of PNI for all-cause mortality in in sepsis patients
The predictive value of PNI for all—cause mortality in sepsis patients was assessed by plotting ROC curves for PNI, SOFA, APACHE II, and their combinations, analyzing their predictive power for 14, 28, and 90-day all-cause mortality (Figures 4A–C). For 90-day mortality, the AUC for PNI was 0.59 (95% CI: 0.57–0.61), for SOFA 0.65 (95% CI: 0.64–0.67), for SOFA plus PNI 0.68 (95% CI: 0.67–0.70), for APACHE II 0.66 (95% CI: 0.65–0.68), and for APACHE II plus PNI 0.68 (95% CI: 0.67–0.70). While PNI alone had a lower predictive value than SOFA and APACHE II, it could enhance their predictive power when used in combination. Detailed results are presented in Figure 4 and Table 4.
Figure 4. Receiver operating characteristic curves assesses the predictive capability of the PNI for 14-day (A), 28-day (B), and 90-day (C) all-cause mortality.
3.6 Subgroup analysis
We further explored the link between PNI and the risk of 14, 28, and 90-day all—cause mortality across different populations. Forest-plot analysis showed no significant PNI-subgroup interactions when stratifying by age, sex, acute kidney injury, chronic kidney disease, myocardial infarction, albumin, immunosuppressant, and corticosteroid use (all P-values for interaction > 0.05) (Figure 5).
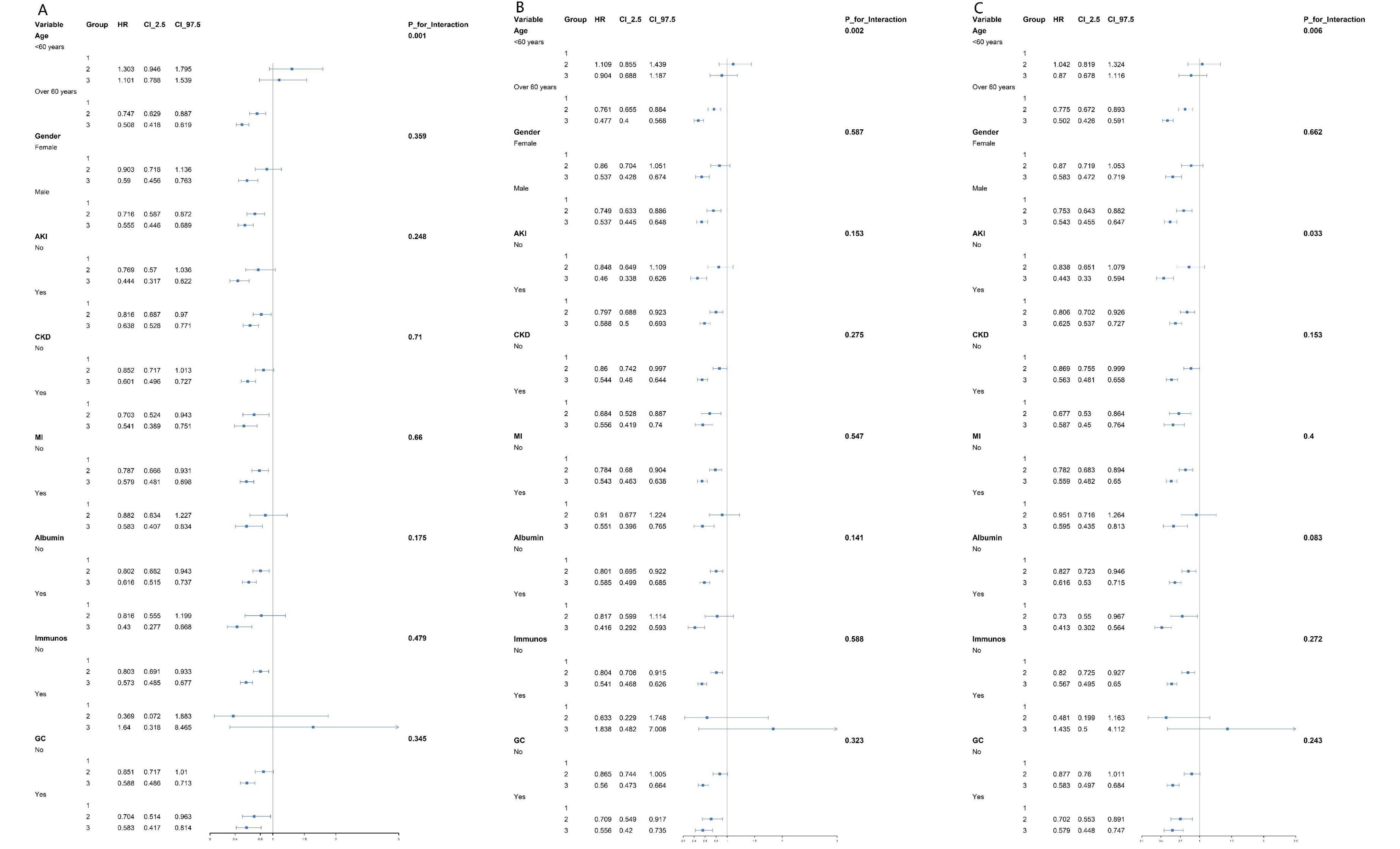
Figure 5. Forest plots of stratified analyses of PNI and 14-day (A), 28-day (B), and 90-day (C) all-cause mortality in patients.
4 Discussion
To our knowledge, this is the first study to evaluate the relationship between PNI and mid-term prognosis in sepsis patients. Our results indicate a significant association between PNI levels and 14, 28, and 90-day all—cause mortality in this population, even after adjusting for potential confounders.
Sepsis is a systemic inflammatory response syndrome caused by infection, characterized by an excessive host response leading to systemic inflammation and immune dysfunction (1). This excessive response triggers systemic inflammation and immune dysfunction, and is often accompanied by malnutrition and immune disorders. During the early stages of sepsis, the immune system is activated, releasing a large number of pro-inflammatory cytokines. These cytokines can cause tissue damage and organ dysfunction (7), leading to high mortality and readmission rates among sepsis patients (8–10). Therefore, identifying a clinical biomarker that is easily obtainable and has high predictive value for early intervention in sepsis patients is crucial for improving their prognosis.
Albumin is a key indicator of nutritional status and also reflects the severity of inflammation (4). However, albumin levels can be influenced by various factors such as liver dysfunction and chronic inflammation, which limit the predictive value of albumin alone (15–18). Peripheral blood lymphocytes are important indicators of immune function and serve as a key component of the indirect markers of immune dysfunction in sepsis (5, 19). Nevertheless, in sepsis patients with concurrent infections or abnormal immune system activation, peripheral blood lymphocytes may increase (20–22). This reduces the reliability of using lymphocytes alone to predict patient outcomes.
The PNI, calculated based on serum albumin and lymphocyte counts, helps to reduce the confounding effects of nutrition and inflammation on albumin interpretation. It comprehensively evaluates a patient’s nutritional and immune status, thereby predicting their prognosis. Generally, a higher PNI value indicates better nutritional status and immune function, and a lower risk of death.
Previous studies have confirmed the prognostic value of PNI in various diseases, such as liver cirrhosis, heart failure, stroke-related pneumonia, and tumors (23–26). In critically ill patients (27), PNI can effectively identify patients at high nutritional risk, who often have poor clinical outcomes, including higher mortality, longer mechanical ventilation time, and longer ICU stay. Moon Seong Baek et al. (28) further pointed out that elderly sepsis patients with low PNI levels have higher mortality rates.
4.1 This study further confirms the prognostic significance of PNI in sepsis patients
We collected data from 6,234 sepsis patients and compared the baseline information of patients in three PNI groups. Multivariable Cox regression analysis of mortality outcomes showed that PNI was an independent risk factor for this patient population. Our results are consistent with previous studies, indicating that patients with lower PNI may have inadequate nutrition, poor infection control, and immune imbalance, which can lead to reduced albumin and lymphocyte levels. Conversely, patients in the high PNI group have improved nutritional status and organ function due to controlled infections and nutritional support. This study also reveals a non-linear relationship between PNI and mortality risk in sepsis patients, consistent with existing evidence. This further emphasizes the prognostic importance of PNI in this population and identifies specific risk thresholds.
Scoring systems like SOFA and APACHE II have long been key tools for assessing disease severity and organ dysfunction in critically ill patients (29–31). Our study shows that PNI has relatively good predictive power and can enhance the ability of these two scoring systems to predict the prognosis of sepsis patients.
Clinically, based on PNI assessment, early nutritional intervention should be implemented for high-risk critically ill patients. Appropriate early nutritional support improves status, reduces complications, and enhances survival. Individualized nutritional management plans, based on PNI and other assessments, are necessary due to varying baseline nutrition, disease types, and severities. Dynamic monitoring of PNI and adjusting support ensures optimal outcomes.
A major strength of this study is establishing PNI as an independent predictor of short-term and long-term all-cause mortality in sepsis patients. The diverse population data in the MIMIC-IV database (version 3.1) allowed robust statistical adjustments to mitigate confounding effects.
Despite these strengths, our study has limitations. As a single-center retrospective study, our findings may lack generalizability and require prospective validation. We only assessed initial PNI levels at admission and did not evaluate dynamic changes over time. Future studies on dynamic PNI measurements are needed to further clarify its clinical utility. Additionally, while we used measurements within the first 24 h to minimize the impact of interventions, we could not confirm if patients received nutritional or immune interventions before albumin and lymphocyte measurements due to MIMIC-IV limitations. Future research should explore how such interventions affect albumin, lymphocyte levels, and PNI predictive ability. Lastly, despite multivariable adjustments and subgroup analyses, other unmeasured confounders may influence results.
5 Conclusion
This study shows a significant association between short-term and medium-term all-cause mortality in sepsis patients and PNI, indicating PNI can be a valuable indicator for predicting in-hospital and ICU mortality risk. Low PNI levels correlate with higher mortality risk and poorer prognosis, while increasing PNI may improve clinical outcomes. Clinically, monitoring PNI in sepsis patients is essential, with timely nutritional support and other interventions to optimize prognosis.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: mimic.physionet.org/.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
MP: Software, Writing – review & editing, Writing – original draft, Formal Analysis, Visualization, Methodology. ZL: Resources, Project administration, Writing – review & editing, Supervision, Funding acquisition. SS: Writing – original draft, Formal Analysis, Supervision, Data curation, Methodology. XT: Writing – original draft, Formal Analysis, Visualization, Methodology. YL: Supervision, Writing – original draft, Methodology.
Funding
The author declare that financial support was received for the research and/or publication of this article. This work was supported by the Guangxi Health Care Technology Development and Promotion Project (S2023100).
Acknowledgments
Thanks for Zheng Li help and support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Singer M, Deutschman C, Seymour C, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). Jama. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
2. Xie J, Wang H, Kang Y, Zhou L, Liu Z, Qin B, et al. The epidemiology of sepsis in Chinese ICUs. A National Cross-Sectional Survey. Crit Care Med. (2020) 48:e209–18. doi: 10.1097/CCM.0000000000004155
3. Berg D, Gerlach H. Recent advances in understanding and managing sepsis. F1000Res. (2018) 7:F1000 Faculty Rev-1570. doi: 10.12688/f1000research.15758.1
4. Shin J, Hwang S, Jo I, Kim W, Ryoo S, Kang G, et al. Prognostic value of the lactate/albumin ratio for predicting 28-Day mortality in critically ILL sepsis patients. Shock. (2018) 50:545–50. doi: 10.1097/SHK.0000000000001128
5. Sheu T, Chiang B. Lymphopenia, lymphopenia-induced proliferation, and autoimmunity. Int J Mol Sci. (2021) 22:4152. doi: 10.3390/ijms22084152
6. Li D, Zhang J, Cheng W, Zhao G, Lei X, Xie Y, et al. Dynamic changes in peripheral blood lymphocyte trajectory predict the clinical outcomes of sepsis. Front Immunol. (2025) 16:1431066. doi: 10.3389/fimmu.2025.1431066
7. Wu Y, Wang L, Li Y, Cao Y, Wang M, Deng Z, et al. Immunotherapy in the context of sepsis-induced immunological dysregulation. Front Immunol. (2024) 15:1391395. doi: 10.3389/fimmu.2024.1391395
8. Ge J, Lei Y, Wen Q, Zhang Y, Kong X, Wang W, et al. The prognostic nutritional index, an independent predictor of overall survival for newly diagnosed follicular lymphoma in China. Front Nutr. (2022) 9:981338. doi: 10.3389/fnut.2022.981338
9. Li B, Lu Z, Wang S, Hou J, Xia G, Li H, et al. Pretreatment elevated prognostic nutritional index predicts a favorable prognosis in patients with prostate cancer. BMC Cancer. (2020) 20:361. doi: 10.1186/s12885-020-06879-1
10. Hu Y, Cai Y, Ma W, Hu H, Gu H, Jin Y, et al. The prognostic nutritional index and tumor pathological characteristics predict the prognosis of elderly patients with early-stage hepatocellular carcinoma after surgery. Biosci Trends. (2023) 17:369–80. doi: 10.5582/bst.2023.01212
11. Ning Y, Pan D, Guo J, Su Z, Wang J, Wu S, et al. Association of prognostic nutritional index with the risk of all-cause mortality and cardiovascular mortality in patients with type 2 diabetes: Nhanes 1999-2018. BMJ Open Diabetes Res Care. (2023) 11:e003564. doi: 10.1136/bmjdrc-2023-003564
12. Liu C, Liu P, Chen H, Chen J, Lee C, Cheng W, et al. Association of preoperative prognostic nutritional index with risk of postoperative acute kidney injury: A meta-analysis of observational studies. Nutrients. (2023) 15:2929. doi: 10.3390/nu15132929
13. Johnson A, Bulgarelli L, Shen L, Gayles A, Shammout A, Horng S, et al. MIMIC-IV, a freely accessible electronic health record dataset. Sci Data. (2023) 10:1. doi: 10.1038/s41597-022-01899-x
14. Zeng D, Wen N, Wang Y, Cheng N, Li B. Prognostic roles nutritional index in patients with resectable and advanced biliary tract cancers. World J Gastroenterol. (2025) 31:97697. doi: 10.3748/wjg.v31.i6.97697
15. Gray A, Oatey K, Grahamslaw J, Irvine S, Cafferkey J, Kennel T, et al. Albumin versus balanced crystalloid for the early resuscitation of sepsis: An open parallel-group randomized feasibility trial- the ABC-sepsis trial. Crit Care Med. (2024) 52:1520–32. doi: 10.1097/CCM.0000000000006348
16. Tsao F, Li Z, Amessoudji A, Jawdat D, Sadat M, Arabi Y, et al. The role of serum albumin and secretory phospholipase A2 in sepsis. Int J Mol Sci. (2024) 25:9413. doi: 10.3390/ijms25179413
17. Arnau-Barrés I, Güerri-Fernández R, Luque S, Sorli L, Vázquez O, Miralles R. Serum albumin is a strong predictor of sepsis outcome in elderly patients. Eur J Clin Microbiol Infect Dis. (2019) 38:743–6. doi: 10.1007/s10096-019-03478-2
18. Liu Q, Zheng H, Wu M, Wang Q, Yan S, Wang M, et al. Association between lactate-to-albumin ratio and 28-days all-cause mortality in patients with acute pancreatitis: A retrospective analysis of the MIMIC-IV database. Front Immunol. (2022) 13:1076121. doi: 10.3389/fimmu.2022.1076121
19. Letessier W, Demaret J, Gossez M, Allam C, Venet F, Rimmelé T, et al. Decreased intra-lymphocyte cytokines measurement in septic shock patients: A proof of concept study in whole blood. Cytokine. (2018) 104:78–84. doi: 10.1016/j.cyto.2017.09.029
20. Nates J, Pène F, Darmon M, Mokart D, Castro P, David S, et al. Septic shock in the immunocompromised cancer patient: A narrative review. Crit Care. (2024) 28:285. doi: 10.1186/s13054-024-05073-0
21. Yang J, Zhu X, Feng J. The changes in the quantity of lymphocyte subpopulations during the process of sepsis. Int J Mol Sci. (2024) 25:1902. doi: 10.3390/ijms25031902
22. Gustave C, Gossez M, Demaret J, Rimmelé T, Lepape A, Malcus C, et al. Septic shock shapes B cell response toward an exhausted-like/immunoregulatory profile in patients. J Immunol. (2018) 200:2418–25. doi: 10.4049/jimmunol.1700929
23. Xie Y, He C, Wang W. Prognostic nutritional index: A potential biomarker for predicting the prognosis of decompensated liver cirrhosis. Front Nutr. (2023) 9:1092059. doi: 10.3389/fnut.2022.1092059
24. Chen M, Wen J, Lu M, Jian X, Wan X, Xu Z, et al. Association between prognostic nutritional index and prognosis in patients with heart failure: A meta-analysis. Front Cardiovasc Med. (2022) 9:918566. doi: 10.3389/fcvm.2022.918566
25. Cai Q, Chen K, Rao H, Jiang W, Huang H, Lin T, et al. Novel and significant predictor in extranodal natural killer/T-Cell lymphoma, nasal type: The prognostic nutritional index (PNI). Blood. (2014) 124:1650–1650. doi: 10.1182/blood.V124.21.1650.1650
26. Wang T, Qi L, Zhao Y, Ma X, Li T. Inflammatory biomarker correlations and prognosis in high-risk gastrointestinal stromal tumor patients: A multicenter retrospective analysis. BMC Gastroenterol. (2025) 25:119. doi: 10.1186/s12876-025-03710-8
27. Shimoyama Y, Umegaki O, Kadono N, Minami T. Presepsin values and prognostic nutritional index predict mortality in intensive care unit patients with sepsis: A pilot study. BMC Res Notes. (2021) 14:245. doi: 10.1186/s13104-021-05659-9
28. Baek M, Kwon Y, Kang S, Shim D, Yoon Y, Kim J. Association of malnutrition status with 30-day mortality in patients with sepsis using objective nutritional indices: A multicenter retrospective study in South Korea. Acute Crit Care. (2024) 39:127–37. doi: 10.4266/acc.2023.01613
29. Lambden S, Laterre P, Levy M, Francois B. The SOFA score-development, utility and challenges of accurate assessment in clinical trials. Crit Care. (2019) 23:374. doi: 10.1186/s13054-019-2663-7
30. Mumtaz H, Ejaz M, Tayyab M, Vohra L, Sapkota S, Hasan M, et al. APACHE scoring as an indicator of mortality rate in ICU patients: A cohort study. Ann Med Surg. (2023) 85:416–21. doi: 10.1097/MS9.0000000000000264
Keywords: mortality, prognostic nutritional index, sepsis, prognosis, lymphocyte, albumin
Citation: Pan M, Li Z, Sheng S, Teng X and Li Y (2025) Prognostic nutritional index as a potential predictor of prognosis in patients with sepsis: a retrospective cohort study. Front. Nutr. 12:1600943. doi: 10.3389/fnut.2025.1600943
Received: 27 March 2025; Accepted: 12 June 2025;
Published: 01 July 2025.
Edited by:
Yuetian Yu, Shanghai Jiao Tong University, ChinaReviewed by:
Zhaowei Xu, Fujian Medical University, ChinaTing Zhou, Huazhong University of Science and Technology, China
Saleh Al Omar, Al-Balqa Applied University, Jordan
Copyright © 2025 Pan, Li, Sheng, Teng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zheng Li, bGl6aGVuZzE3MTdAMTI2LmNvbQ==
 Mingyuan Pan
Mingyuan Pan Zheng Li
Zheng Li Shanfeng Sheng
Shanfeng Sheng Xiao Teng2
Xiao Teng2