- 1Anesthesia Department, Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou, China
- 2Anesthesia Department, Suining Central Hospital, Suining, Sichuan, China
- 3School of Pharmacy, Key Laboratory of Basic Pharmacology of Ministry of Education and Joint International Research Laboratory of Ethnomedicine of Ministry of Education, Zunyi Medical University, Zunyi, Guizhou, China
- 4Anesthesia Department, Affiliated Hospital of Chengdu University, Chengdu, Sichuan, China
- 5Anesthesia Department, The First People's Hospital of Zunyi, Zunyi, Guizhou, China
- 6Early Clinical Research Ward, Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou, China
This literature review examines the relationship between malnutrition and perioperative neurocognitive disorders (PND), which encompass cognitive impairments occurring throughout the perioperative period, including pre-existing cognitive impairments, postoperative delirium, delayed neurocognitive recovery, and postoperative cognitive dysfunction. Malnutrition is associated with an increased incidence of PND, affecting patient recovery and quality of life. Studies suggest that preoperative malnutrition may heighten the risk of PND, and that preoperative nutritional diagnosis and perioperative nutritional interventions could reduce the occurrence of PND. The review discusses the definition, diagnosis, and indicators of malnutrition, as well as the mechanisms by which malnutrition leads to PND, including direct pathways such as psychological factors, abnormal neurotransmitter synthesis, and changes in brain structure and function, and indirect pathways like impaired immune function, neuroinflammation, mitochondrial dysfunction, intestinal barrier damage, disruption of the gut-brain axis, lymphatic system dysfunction, and endocrine disruption. Finally, this paper summarizes the existing nutritional intervention strategies for improving PND, explores the research directions of malnutrition and PND, and emphasizes that future research needs to clarify the role of nutritional intervention in specific populations and conduct in-depth studies on the molecular mechanisms of nutritional intervention and PND prevention.
1 Introduction
Perioperative neurocognitive disorders (PND) encompasses cognitive impairment or decline that occurs throughout the perioperative period, manifesting as deficits in orientation, attention, memory, and reaction time (1). This includes pre-existing cognitive impairments diagnosed preoperatively, delirium occurring during the postoperative hospital stay postoperative delirium (POD), delayed neurocognitive recovery diagnosed within 30 days postoperatively delayed neurocognitive recovery (dNCR), and persistent or diagnosed cognitive decline within 12 months postoperatively, classified as severe or mild neurocognitive dysfunction postoperative cognitive dysfunction (POCD) (Figure 1) (2).
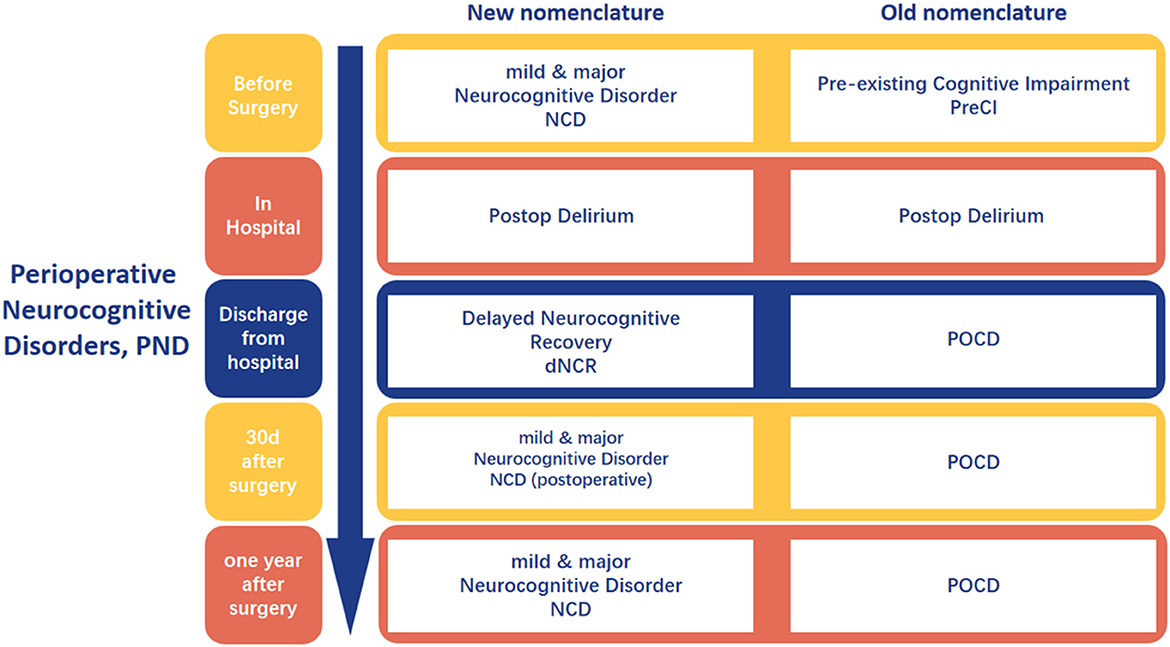
Figure 1. The nomenclature about the perioperative neurocognitive disorder. Created with FigDraw.com.
PND is a widely recognized public health issue that may affect millions of patients annually (1). It is associated with increased postoperative mortality, increased complications, prolonged hospital stays, and higher medical costs (3). Furthermore, long-term follow-up studies suggest that patients with PND are at an increased risk of dementia, reduced quality of life, and diminished work capacity (4).
Studies indicate that various perioperative risk factors may be associated with the development of PND, including advanced age, surgical site, type of surgery, duration of surgery, intraoperative blood loss, hypotension and higher ASA physical status classes (5); preoperative comorbidities such as hypertension, diabetes, obesity, sleep disorders, presence of depressive, anxiety states, and dementia (6); history of delirium, alcohol abuse, and substance use, postoperative sleep disturbances, inadequate pain management, postoperative infections, repeated use of anesthetics (7).
In recent years, the nutritional status has gained increasing research attention, with up to one-third of patients presenting with malnutrition or being at risk of malnutrition upon admission (8). Causes include disease-related anorexia, medication-related side effects, fasting orders related to diagnostic procedures, diseases that impair normal gastrointestinal function, suboptimal overall nutritional management in hospitalized patients, and metabolic consumption caused by diseases (9, 10). These conditions can lead to decreased appetite or impaired nutrient absorption.
Recent research on the relationship between malnutrition and PND has highlighted the significant association between the two (11). Numerous studies have demonstrated that preoperative malnutrition may increase the risk of PND, and that preoperative nutritional diagnosis and perioperative nutritional interventions may be beneficial in reducing the incidence of PND (12). Early identification and intervention of malnutrition are expected to effectively reduce the incidence of PND, improve the quality of patient recovery, and enhance postoperative quality of life (13). This review of the literature on malnutrition leading to perioperative neurocognitive disorders aims to provide a multifaceted perspective for the mechanistic study of PND.
To conduct this narrative review, we searched the following databases: PubMed, Web of Science, and Scopus. The search was performed using the keywords (“Malnutrition”[Mesh] OR “Nutrition Disorders”[Mesh] OR “Nutritional Status”[Mesh] OR “Nutrition Assessment”[Mesh] OR “Nutritional Support”[Mesh] OR “Nutrition Therapy”[Mesh] OR “Nutritional Deficiency”[Mesh] OR “Protein-Energy Malnutrition”[Mesh] OR “Undernutrition” OR “Nutrition Risk” OR “Nutritional Risk” OR “Nutritional Screening” OR “Malnourished” OR “Nutritional Inadequacy” OR “Nutritional Depletion”) AND (“Perioperative Period”[Mesh] OR “Perioperative Care”[Mesh] OR “Surgical Procedures, Operative”[Mesh] OR “Anesthesia”[Mesh] OR “Anesthesia Recovery Period”[Mesh] OR “Postoperative Period”[Mesh] OR “Preoperative Period”[Mesh] OR “Intraoperative Period”[Mesh] OR “Surgery” OR “Surgical” OR “Operative” OR “Anesthesia” OR “Anesthetic” OR “Preoperative” OR “Intraoperative” OR “Postoperative” OR “Post-surgery” OR “Post-surgical” OR “Perioperative”) AND (“Cognition Disorders”[Mesh] OR “Cognitive Dysfunction”[Mesh] OR “Postoperative Cognitive Complications”[Mesh] OR “Delirium”[Mesh] OR “Confusion”[Mesh] OR “Neurocognitive Disorders”[Mesh] OR “Postoperative Cognitive Dysfunction” OR “POCD” OR “Postoperative Cognitive Decline” OR “Postoperative Delirium” OR “Cognitive Impairment” OR “Cognitive Decline” OR “Neurocognitive Decline” OR “Cognitive Deficit” OR “Mental Status Change”)and was limited to articles published in English between January 2010 and January 2025. Foundational studies published prior to 2010 were included where critical to contextualize key concepts. We included original research articles, reviews, and case studies that focused on “perioperative cognitive dysfunction caused by malnutrition” and excluded articles that were not relevant to the main topic, duplicates, and those with insufficient data. The articles were screened based on their titles and abstracts, and full texts were retrieved for those that met the inclusion criteria. The key findings and conclusions were extracted and synthesized into major themes for discussion in this review.
2 Malnutrition definition, diagnosis, and indicators
In 2015, the European Society for Clinical Nutrition and Metabolism (ESPEN) released a consensus statement on malnutrition, defining it as: “Malnutrition is a condition resulting from a lack of food intake or absorption, leading to changes in body composition that affect body and mental function and impact clinical outcomes of disease” (14). The statement introduced the concept of nutrition disorder and its diagnostic framework, categorizing nutrition disorders into three types: Malnutrition, Micronutrients abnormalities, and Overnutrition. Malnutrition was further divided into four subtypes: Starvation-related underweight, Cachexia/Disease-related malnutrition, Sarcopenia, and Frailty (Figure 2). In 2018, the Global Leadership Initiative on Malnutrition (GLIM), a group of global nutrition leaders, launched the latest criteria for malnutrition assessment, simply referred to as the GLIM criteria (15). The GLIM criteria clearly distinguish between two steps: “nutrition screening” and “diagnostic assessment” (16). The first step is nutrition screening, which emphasizes the use of clinically validated nutrition screening tools for patient assessment. The criteria list the Nutritional Risk Screening (NRS 2002) (17), Malnutrition Universal Screening Tool (MUST) (18), and Mini Nutritional Assessment-Short Form (MNA-SF) (19) as the primary screening tools. The second step, following a positive screen, is to assess and grade the severity of malnutrition. The malnutrition assessment criteria include five items: involuntary weight loss, low BMI, and reduced muscle mass, which are phenotypic criteria; and reduced food intake or absorption, and disease burden/inflammation, which are etiologic criteria (15). To make a diagnosis of malnutrition, at least one phenotypic diagnostic criterion and one etiologic diagnostic criterion must be met. Based on the degree of weight loss, low BMI, and muscle wasting, malnutrition is classified as moderate malnutrition (Phase 1) and severe malnutrition (Phase 2) (Table 1).
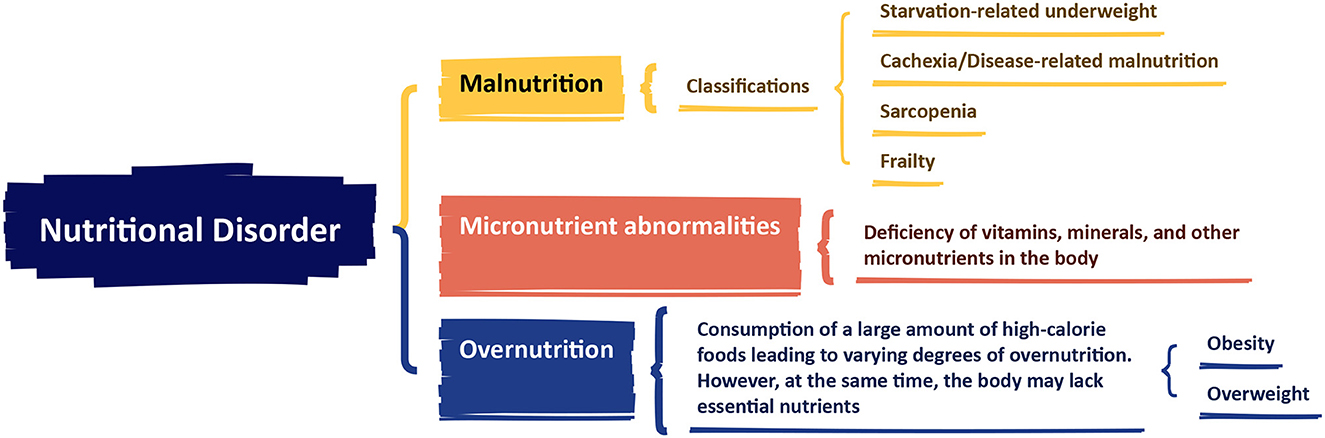
Figure 2. The European Society for Clinical Nutrition and Metabolism (ESPEN) consensus statement on malnutrition. Created with FigDraw.com.
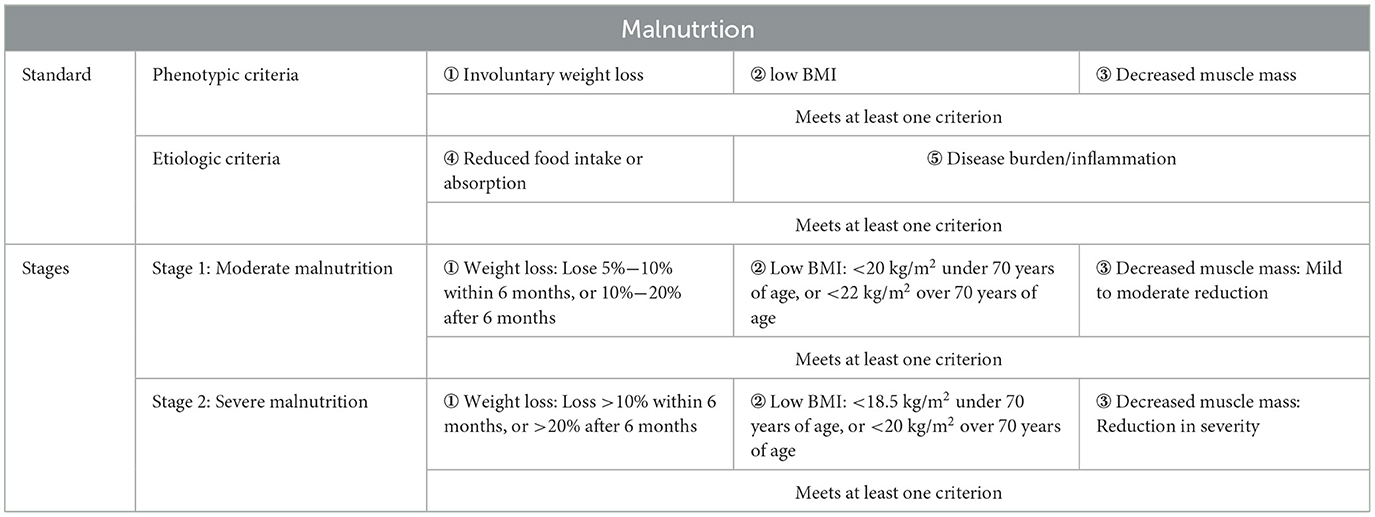
Table 1. The criteria for malnutrition assessment from Global Leadership Initiative on Malnutrition (GLIM).
Currently, commonly used nutritional indicators in clinical and research settings include Albumin (ALB), Prognostic Nutritional Index (PNI), and Geriatric Nutritional Risk Index (GNRI).
Plasma ALB is influenced by various factors (such as infection, blood loss, severe hepatic or renal dysfunction, etc.), and its ability to assess nutritional risk is controversial (20). Nevertheless, plasma ALB at admission or before surgery is still used as an indicator to assess patient nutritional risk in clinical practice. PNI is a comprehensive indicator for assessing the nutritional status of surgical patients, predicting surgical risks, and making prognostic judgments. It reflects the immune-nutritional condition by calculating the function of peripheral blood lymphocyte counts and serum albumin (21). In a study related to spinal deformity correction surgery, it was found (22). The significant risk factors for POD were age (OR 1.11, 95%CI 1.03–1.19) and PNI (OR 0.87, 95%CI 0.79–0.96). The risk factors for POD after spinal deformity correction are PNI <49.7 and age > 68.5 years old. GNRI is a simple and objective indicator for assessing the nutritional status of elderly patients and can be used to predict the risk of nutrition-related complications and death (23). The calculation of GNRI involves weight, height, and serum albumin levels, with the value range reflecting the patient's nutritional status, such as 97.5–100 indicating mild malnutrition, 83.5–97.5 indicating moderate malnutrition, and <83.5 indicating severe malnutrition (24).
3 Malnutrition leading to the occurrence of PND
3.1 The influence of different types of malnutrition on PND
3.1.1 Energy-related malnutrition (ERM) and PND
A pathological condition specifically caused by prolonged energy-protein intake deficiency, characterized by energy imbalance (chronic intake below basal metabolic requirements) and protein deficiency (<0.8 g/kg/day). ERM is commonly seen in cases of insufficient intake, absorption disorders, or a hypermetabolic state of the body caused by disease-related reasons. ERM increases PND risk through three mechanisms: ① Inflammatory cascade: Hypoproteinemia triggers IL-6 and TNF-α release, disrupting the blood-brain barrier and directly damaging neurons (25); ② Neurotransmitter imbalance: Protein deficiency leads to tryptophan metabolism disorder and accumulation of neurotoxic quinolinic acid (26); ③ Cerebral energy crisis: Insufficient glucose supply forces neurons to rely on ketones, exacerbating oxidative damage through mitochondrial dysfunction (27). Relevant studies demonstrate: 79.3% of elderly hip fracture patients exhibit ERM, with a 2.3-fold increased risk of postoperative delirium (OR = 3.4, 95%CI 1.9–6.1) (28); Cardiac surgery patients with GNRI <92 show a 3.5-fold increased PND incidence (23); Intervention studies confirm that 7-day preoperative high-protein supplementation (≥1.5 g/kg/day) combined with vitamin D (2,000 IU/day) reduces PND risk by 48% (29). In conclusion, ERM represents a modifiable key risk factor for PND, with early nutritional screening and targeted protein supplementation potentially improving neurocognitive outcomes.
3.1.2 Sarcopenia and PND
It is manifested as muscle strength reduction and muscle mass decline, and may be accompanied by a decline in physical function. Increasing the intake of protein and vitamins can prevent and alleviate sarcopenia. Relevant studies have shown (30) that the prevalence of sarcopenia ranges from 18% in diabetic patients to 66% in patients with inoperable esophageal cancer. Sarcopenia is associated with a high risk of various adverse health outcomes, including poor overall survival and disease-free progression survival rates, postoperative complications, prolonged hospital stays for patients with different medical conditions, as well as falls and fractures, metabolic disorders, cognitive impairment, and mortality in the general population. In a prospective cohort study on the impact of sarcopenia on cognitive function in 2015 (31), a total of 5,715 participants over 60 years old (43.8% were female; average age 67.3 ± 6.0 years) participated in a cross-sectional association study, and 2,982 elderly people were followed up in 2018. Logistic regression models showed that compared with the non-sarcopenia group, the OR value of the possible sarcopenia group was 1.43 (95% CI: 1.06–1.91, P = 0.017), and the OR value of the sarcopenia group was 1.72 (95% CI: 1.04–2.85, P = 0.035). Sarcopenia is associated with more severe cognitive impairment, providing new evidence for a strong association.
3.1.3 Frailty and PND
Frailty is a non-specific state characterized by a decline in the physiological reserves of the nervous, muscular, metabolic and immune systems, increased vulnerability of the body, and reduced stress resistance. Some people, although they do not have specific diseases, are prone to fatigue, weakness and weight loss. The imbalance between energy supply and demand related to nutrition and the disorder of the internal environment may be one of the reasons. In existing studies, there are many reports on the association between frailty and PND. Cheng et al. (32) found in a recent cohort study of 2,080 elderly people that the incidence of delirium was higher in the frailty group (29.2% vs. 16.4%, P < 0.05). After adjusting for related variable factors, multivariate logistic regression showed that the risk of delirium in frailty patients was significantly increased (adjusted OR: 1.61, 95% CI: 1.23–2.10, P < 0.001, E value: 1.85). In addition, in a prospective observational study in Taiwan (33), frailty was found to be an independent risk factor for POD in elderly cancer patients after elective abdominal surgery. In another meta-analysis of cohort studies (34), a total of 15 cohort studies were included, involving 3,250 adult patients who underwent surgery. The preoperative prevalence of frailty was 27.1% (880/3,250). The pooled results showed that frailty was significantly associated with a higher risk of POD (adjusted OR: 3.23, 95% CI: 2.56–4.07, P < 0.001).
3.1.4 Micronutrients abnormalities and PND
Malnutrition caused by a deficiency of micronutrients such as vitamins and minerals. In a systematic review on micronutrient deficiency and POD (35), it was found that micronutrient deficiency (i.e., cobalamin, thiamine, and vitamin D) was associated with an increased incidence of delirium, with a higher prevalence among hospitalized patients. In a prospective cohort study on vitamin D and the occurrence of delirium after coronary artery bypass grafting (36), multivariate logistic regression analysis indicated that severe vitamin D deficiency at admission was associated with the occurrence of delirium (OR: 3.18; 95% CI: 1.29–7.78; P = 0.01). A 2022 meta-analysis on preoperative vitamin deficiency and POD and cognitive dysfunction (37) also reached the same conclusion, that preoperative vitamin D deficiency was associated with postoperative cognitive impairment. The results of a systematic review showed (38) that low levels of B vitamins (folic acid and vitamin B12), vitamin D, vitamin A, vitamin E, omega-3 fatty acids, and albumin, as well as high levels of homocysteine in the blood, were significantly associated with an increased risk of mild cognitive impairment in the elderly.
Beyond classical micronutrients, dietary and non-dietary antioxidants play pivotal roles in mitigating oxidative stress-induced neuronal damage, a key pathway in PND pathogenesis. Evidence suggests that nutritional antioxidants (e.g., vitamin C, selenium) enhance endogenous defense systems by scavenging reactive oxygen species (ROS) and supporting glutathione peroxidase activity (39, 40). Phenolic compounds (e.g., resveratrol, curcumin) exert neuroprotection via anti-inflammatory signaling (e.g., NF-κB suppression) and mitochondrial biogenesis regulation (e.g., PGC-1α activation) (41, 42). Clinical studies report that perioperative supplementation with these antioxidants correlates with reduced PND incidence, likely through preserving blood-brain barrier integrity and synaptic function (43).
3.1.5 Overnutrition and PND
Obesity represents a distinct form of malnutrition characterized by chronic oxidative stress and micronutrient deficiencies, even amidst caloric excess. Although obese people consume excessive calories, they often have insufficient intake or absorption of nutrients such as vitamins (like B vitamins, D, and E) and minerals (such as iron, zinc, and magnesium), presenting a state of “energy surplus but nutritional imbalance”. At the same time, a preference for high-sugar, high-fat and high-oil foods leads to excessive calorie intake, while lacking key nutrients such as dietary fiber and high-quality protein, which affects metabolism. In addition, obese individuals are prone to insulin resistance, abnormal leptin secretion, and other conditions, which lead to fat accumulation and nutrient utilization disorders, creating a vicious cycle. High intake of processed foods rich in saturated fats (e.g., palmitic acid) and refined sugars—hallmarks of the Western Diet—induces mitochondrial dysfunction and ROS overproduction in the brain (44). This persistent oxidative state exacerbates neuroinflammation and blood-brain barrier disruption, significantly elevating POD risk beyond isolated micronutrient deficiencies (45). Clinical evidence confirms this linkage: Feinkohl et al. (46) reported a 1.85-fold higher POD risk (95% CI: 1.26–2.70) in obese patients with metabolic syndrome, where Western Diet patterns drive systemic oxidation. In overweight hip fracture patients, malnutrition (often coexisting with obesogenic diets) increased POD incidence (OR = 3.64) (47). Crucially, the Western Diet's impact on POD is amplified by: ① Deficiencies in antioxidant cofactors (zinc, selenium) required for ROS-detoxifying enzymes (e.g., SOD). ② Low intake of phenolic compounds (e.g., flavonoids in berries), which synergistically regulate Nrf2/NF-κB pathways (48). This is consistent with our emphasis in the previous section on dietary antioxidants as a response to PND.
3.2 The influence of malnutrition on PND in different surgical types
Although the degree of trauma and stress response varies among different types of surgery, the correlation between malnutrition and the occurrence of PND after surgery appears to be well-established. A study from West China Hospital of Sichuan University assessed preoperative nutritional status using GNRI and MNA-SF and followed up with patients for the occurrence of POD and length of hospital stay (LOS), finding that preoperative malnutrition is significantly associated with POD, and that low/high nutritional risk of preoperative GNRI and malnutrition of MNA-SF are independent predictive factors for prolonged LOS (49). Wang et al. (50) found in a retrospective study that among 1,440 patients who underwent hip fracture surgery, the incidence of POD was 19.1%, and the risk of POD in patients with hypoalbuminemia increased by 2.99 times, with a dose-response relationship between low albumin levels and POD risk. Hung et al.'s (51) meta-analysis found a negative correlation between PNI and POD, with the risk of POD nearly doubling. Chen et al. (23) explored the predictive value of GNRI for POD in elderly cardiac surgery patients and found that malnourished patients (GNRI ≤ 98) had a significantly higher risk of POD compared to non-malnourished patients (GNRI > 98), with a negative correlation between preoperative GNRI and POD. Another study found that a lower GNRI increases the risk of delirium and dementia and is a risk factor for death within 1 year after hip surgery (24). In Mazzola's study, elderly patients who underwent hip fracture surgery and were malnourished or severely malnourished were more likely to experience POD (52). A prospective observational study found that 44.5% of patients experienced POD, with advanced age, hypoalbuminemia, malnutrition, and uncontrolled diabetes being strong predictive factors for POD in elderly hip fracture patients (53). A prospective cohort study using the National Hip Fracture Database (NHFD) also found that preoperative cognitive deficits and malnutrition, among other factors, were associated with POD (54).
4 Mechanisms of malnutrition leading to PND
4.1 Direct pathways
4.1.1 Psychological factors and preoperative cognitive function
Patients with malnutrition often have poor physical conditions, which can lead to adverse emotions such as anxiety and depression, affecting their sleep quality and psychological state, thereby increasing their susceptibility to PND. A survey study based on the NHANES database analyzed the association between the GNRI and the prevalence and scores of depression, indicating that the GNRI in the depressed group was significantly lower than in the non-depressed group (55). Multivariate logistic regression showed that GNRI is an important predictor of depression. Depression and anxiety are correlated with cognitive decline that depression is a major risk factor for dementia and mild cognitive impairment (56). If patients already have preoperative cognitive decline, their brain function may be further challenged after surgery and anesthesia, potentially exacerbating cognitive dysfunction and leading to POD.
The pathophysiological links between malnutrition and mood disorders involve specificmicronutrient deficiencies. Notably, deficiencies in B vitamins (folate/folic acid, B6, B12) impair one-carbon metabolism, elevating homocysteine while reducing S-adenosyl methionine (SAM) (57). As SAM is the methyl donor for synthesizing serotonin, dopamine, and norepinephrine (58), its depletion in malnourished patients directly disrupts neurotransmitter homeostasis—a mechanism corroborated by lower SAM levels in depressed individuals (59). Concurrently, excess homocysteine promotes neurotoxicity via overactivation of NMDA receptors (60), further exacerbating depressive pathophysiology.
Simultaneously, broader nutritional factors modulate anxiety pathways. Key nutrients (B vitamins, vitamin C, magnesium, zinc) regulate stress responses through neurotransmitter synthesis/metabolism and neuronal membrane stability (61). Chronic stress impairs these processes, creating a vicious cycle that heightens anxiety risk. Furthermore, these nutrients facilitate conversion of α-linolenic acid (ALA) to neuroprotective n-3 fatty acids (62), which are independently associated with reduced anxiety. Collectively, these mechanisms elucidate how malnutrition propagates emotional dysregulation, ultimately predisposing to PND.
4.1.2 Abnormal synthesis of neurotransmitters
Neurotransmitters, as key messengers in the transmission of nervous system information, rely on various nutrients for their synthesis. Tryptophan and tyrosine, as precursors of serotonin and dopamine, respectively, are insufficiently ingested during malnutrition, particularly under conditions of prolonged hunger or energy deficit. Crucially, in such states, the body may prioritize these amino acids for energy production or gluconeogenesis over neurotransmitter synthesis, further limiting their availability for neural signaling. This leads to a significant decrease in the activity of enzymes involved in the synthesis of these neurotransmitters, resulting in the obstruction of neurotransmitter synthesis, an imbalance in neurotransmitter levels, and subsequently affects the brain's emotional regulation and cognitive functions, increasing the risk of POD (63). Importantly, this neurotransmitter imbalance, especially involving serotonin and dopamine which are key regulators of appetite, mood, and motivation, can disrupt hypothalamic feeding centers and reward pathways. This disruption may manifest as altered appetite perception, reduced motivation to seek food, or changes in metabolic set points, thereby exacerbating the initial malnutrition and perpetuating a detrimental cycle of nutritional deficiency and neurological dysfunction. Neurotrophic B vitamins also play a crucial role in neurotransmitter metabolism and the maintenance of a healthy nervous system, particularly vitamins B1, B6, and B12. Studies have shown that vitamin B12 can assist in the synthesis of methylmalonyl-CoA, and a deficiency in this coenzyme can lead to impaired myelin formation, thereby disrupting the conduction of nerve impulses (64).
4.1.3 Changes in brain structure and function
Long-term malnutrition can lead to significant changes in brain structure and function. Neuroimaging examinations suggest that in children with malnutrition, MRI results mainly show brain atrophy, which tends to be reversible after appropriate nutritional intervention (65). Elderly patients with malnutrition have a higher incidence of white matter hyperintensities compared to those in good nutritional status, and the study also indicates that low levels of vitamins B1 and B12 increase the risk of white matter hyperintensities (66). Another study shows that vitamin D deficiency in the elderly is associated with increased white matter hyperintensities in periventricular, cortical, and juxtacortical regions, as well as atrophy of the hippocampus, anterior cingulate cortex, and left calcarine sulcus gray matter (67). Animal studies suggest that in mice deficient in vitamin A, there are changes in the proteome of the cerebral cortex and hippocampus (68). These structural and functional abnormalities affect the brain's information transmission and integration, greatly increasing the risk of postoperative cognitive dysfunction and delirium symptoms in patients.
4.2 Indirect pathways
4.2.1 Impaired immune function and neuroinflammation
Malnutrition, encompassing both undernutrition (insufficient intake) and overnutrition (excess intake leading to overweight/obesity), can lead to significant immune dysfunction, albeit through distinct mechanisms. In the context of undernutrition, there is a comprehensive impairment of immune function, characterized by significant reductions in the function of immune cells such as macrophages and T-cells, and an imbalance in their proportions (69). Perioperative patients with undernutrition have a weakened ability to resist external pathogens, resulting in a significantly increased incidence of infections (70). Conversely, in individuals with overnutrition, such as those who are overweight or obese, and particularly those with associated comorbidities like metabolic syndrome, diabetes, and hypertension, a state of chronic low-grade inflammation (metaflammation) is prevalent even in the absence of overt infection. This state is characterized by persistently elevated levels of pro-inflammatory cytokines, including interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α).
Regardless of the underlying cause (infection in undernutrition or chronic metaflammation in overnutrition), elevated levels of circulating inflammatory factors pose a risk to the central nervous system. These cytokines can partially enter the brain through the weak areas of the blood-brain barrier, and another part achieves transmembrane transport by activating transport proteins on endothelial cells (71, 72). Noah et al. (73), in a meta-analysis, explored the association between preoperative blood levels of inflammatory mediators and POD by assessing these levels before surgery. The results indicated that participants who developed POD had significantly higher preoperative IL-6 levels compared to those who did not, suggesting that a pre-existing inflammatory state (whether from infection, chronic disease, or nutritional imbalance) may increase the risk of POD.
After infiltrating the brain, inflammatory factors rapidly activate microglia, the innate immune cells of the central nervous system, transforming them from a resting state to an activated state with phagocytic and antigen-presenting functions, releasing more inflammatory mediators and neurotoxic substances, such as reactive oxygen species (ROS) and nitric oxide (NO) (74). The activation of microglia is often considered a key contributor to POCD, as it involves the interaction between neuroinflammation and neurofunctional abnormalities, neuronal apoptosis, and synaptic damage, leading to neurologic dysfunction (75, 76). In addition to the excessive production of inflammatory cytokines and reactive oxygen species, other perioperative injury factors, such as brain iron metabolism imbalance and downregulation of type 2 cannabinoid receptors, can also induce microglial activation (77, 78). Activated microglia form a positive feedback loop with injury factors, exacerbating the damage process and ultimately leading to postoperative neurological sequelae.
4.2.2 Mitochondrial dysfunction
The brain is one of the most metabolically active organs in the human body, primarily dependent on the oxidation of glucose and fatty acids for energy supply. Mitochondria, as cellular organelles of energy metabolism, are important sites for the complete oxidation of glucose or its transformation into substances like fatty acids. In states of malnutrition, deficiencies in vitamins or trace elements affect the activity of key cofactors like Mg2+ in glycolytic enzymes such as hexokinase and pyruvate kinase, leading to reduced efficiency in glucose uptake and utilization (79). In addition to energy supply, mitochondria play a significant role in the generation of reactive oxygen species, cell death, and Ca2+ buffering. Under normal physiological conditions, mitochondria produce a small amount of reactive oxygen to participate in intracellular signal transduction, while mitochondrial dysfunction can lead to the production of large amounts of reactive oxygen, triggering oxidative stress damage and contributing to neurological diseases (80). Mitochondria are also involved in various forms of cell death, including apoptosis, pyroptosis, ferroptosis, and cuproptosis (81, 82). Furthermore, mitochondria play a crucial role in maintaining intracellular calcium ion homeostasis, which is particularly important for excitable cells like neurons (83). A substantial body of evidence suggests that mitochondrial dysfunction can lead to hippocampal neuron damage and is a key factor in triggering cognitive impairment in PND (84, 85). Improving mitochondrial dysfunction in elderly mice with abdominal exploration can inhibit oxidative stress, insufficient energy supply, and mitochondrial ultrastructural abnormalities (86). In PND mice, glucose metabolism disruption mediated by glucose transporter 1 and reduced ATP production due to mitochondrial dysfunction have been observed (87). Isoflurane anesthesia in mice leads to cognitive impairment, opening of the mitochondrial permeability transition pore in hippocampal tissue, and a decrease in ATP levels and mitochondrial membrane potential. In the isoflurane/surgery-induced cognitive impairment model, a decrease in basal oxygen consumption, ATP levels, and maximum mitochondrial respiratory capacity in the hippocampus of mice has been noted (88). Isoflurane can cause energy supply deficiency in the brain, leading to a reduction in postsynaptic density protein 95 in hippocampal tissue, inhibition of the brain-derived neurotrophic factor/tyrosine kinase receptor B pathway, synaptic dysfunction, and ultimately learning and memory impairments (89). It is evident that mitochondrial energy metabolism disorders leading to insufficient brain energy supply are one of the mechanisms promoting the occurrence of PND.
4.2.3 Damage to the intestinal barrier and disruption of the gut-brain axis
In recent years, the gut microbiome-gut-brain axis has become an important field in biological research. The gut, as the largest immune and nutrient-absorbing organ in the human body, sees its mucosal barrier integrity compromised and intestinal permeability increased under conditions of malnutrition, allowing the translocation of bacterial endotoxins and pathogens into the bloodstream (90). At the same time, malnutrition causes an imbalance in the gut microbiota, with a reduction in beneficial bacteria (lactobacilli, bifidobacteria) increasing the risk of postoperative cognitive impairment, and surgical anesthesia exacerbates the ecological imbalance of the gut microbiota, shifting it toward a more toxic phenotype (91). Other perioperative factors also affect the gut microbiota, including antibiotics, opioids, or acidifying drugs (92).
A critical aspect of gut-brain communication involves serotonin (5-hydroxytryptamine, 5-HT). Notably, approximately 90% of the body's serotonin is produced in the gut, primarily by enterochromaffin (EC) cells lining the intestinal epithelium (93). This peripheral serotonin plays a vital role not only in regulating gut motility and secretion (94), but also, crucially, in modulating mood, cognition, and stress responses via the gut-brain axis (95). Gut microbiota composition significantly influences intestinal serotonin production. Specific commensal bacteria, particularly those producing short-chain fatty acids (SCFAs) like butyrate, stimulate EC cells to synthesize and release serotonin (96, 97). Malnutrition-induced dysbiosis, characterized by a reduction in these beneficial SCFA-producing bacteria, can therefore lead to diminished serotonin synthesis in the gut (98).
Studies have found a strong link between abnormal gut microbiota composition and the onset of autism, depression, schizophrenia, and Alzheimer's disease (99, 100). An increasing body of evidence suggests that gut microbiota communicates with the central nervous system and can influence brain function and behavior through neural, endocrine, and immune pathways (101). For example, gut microbiota dysbiosis can lead to abnormal synthesis and release of neurotransmitters such as gamma-aminobutyric acid (GABA), and, as discussed, impair gut serotonin production. Dysbiosis can also induce neuroinflammation, disrupt the balance of brain neurotransmitters (including central serotonin levels, which may be influenced by peripheral precursor availability and signaling), and ultimately promote the occurrence of PND (102). The reduction in gut-derived serotonin and its downstream effects on central serotonergic signaling and neuroinflammation represent a significant pathway linking malnutrition, microbiota disruption, and impaired mental health/PND risk.
Animal studies suggest that surgical anesthesia can change the gut microbiome and impair cognitive function in elderly mice, and probiotic pretreatment can prevent the negative impact of surgical anesthesia on postoperative memory (103). Probiotics may exert part of their beneficial effect by restoring microbial communities that support healthy serotonin production and gut-brain signaling.
4.2.4 Lymphatic system dysfunction
In the central nervous system, lymphatic drainage relies on the meningeal lymphatics in the dura mater and the glymphatic system, which interact to ensure the clearance of neurotoxic substances and the stability of the brain's microenvironment. Malnutrition impairs the lymphatic system's drainage function (104), leading to the accumulation of neuroinflammatory factors and metabolic products, thereby affecting neurocognitive function. Additionally, under conditions such as anesthetic drugs, surgical trauma, and changes in the patient's internal environment, immune imbalance in the brain can also lead to postoperative cognitive dysfunction. Lymphatic clearance is closely related to the sleep-wake cycle, with sleep promoting faster clearance of metabolites (105). Melatonin, a hormone that regulates the circadian rhythm and sleep-wake cycle and enhances sleep, is found in fruits and nuts, and its intake may be reduced in patients with malnutrition (106). Meanwhile, Mg2+, as one of the key elements in the human body, ensures sleep quality and regulates the body's circadian rhythm (107), and its deficiency may indirectly affect lymphatic clearance efficiency through sleep quality. Furthermore, sleep disruption increases neuronal activity and produces more waste, including lactate, which is then output through the lymphatic fluid (108).
4.2.5 Endocrine disruption
Malnutrition can trigger functional disorders in the hypothalamic-pituitary-target gland axis, with the thyroid and adrenal axes being the most typical. Thyroid hormones play a crucial role in brain development, neuronal excitation, and metabolic rate. Malnutrition affects the synthesis and function of thyroid hormones due to insufficient dietary and trace element intake, thereby affecting cognitive function (109, 110). Additionally, patients with malnutrition have a disorder in the secretion of adrenocortical hormones under stress, leading to oxidative stress responses, neuronal damage, and promoting the occurrence of PND (Figure 3).
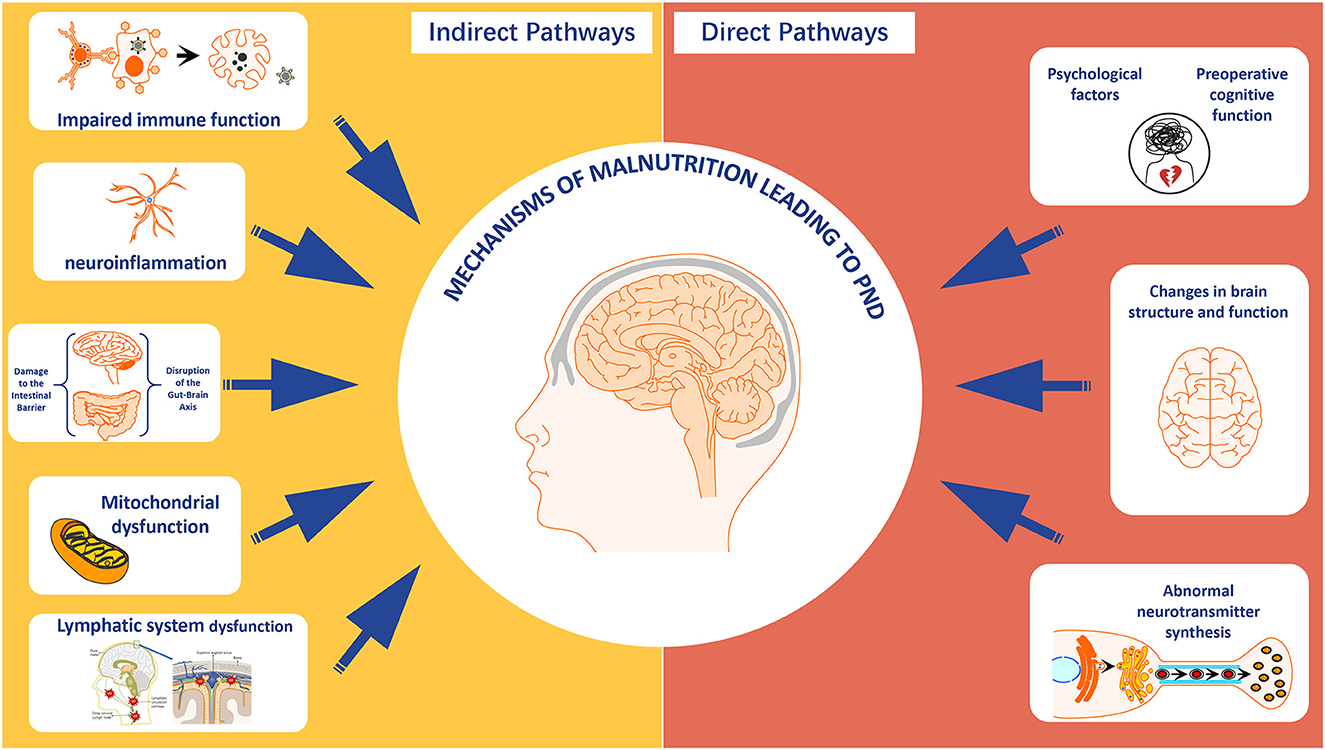
Figure 3. The direct and indirect mechanisms of malnutrition leading to perioperative neurocognitive disorder. Created with FigDraw.com.
5 Nutritional intervention and PND
5.1 Immunonutrition
Current evidence supports the clinical benefits of immunonutrition formulas (containing arginine, nucleotides, and ω-3 fatty acids) in gastrointestinal and head/neck cancer surgeries. Two meta-analyse (111, 112) demonstrated that preoperative immunonutrition administration for 5–7 days significantly reduces the risk of postoperative infectious complications (RRs 0.71–0.74) and shortens hospital length of stay (MDs −1.22 to −2.12 days). These effects may be attributed to the mitigation of systemic inflammatory responses. Although direct evidence linking immunonutrition to delirium prevention remains sparse, the reduction in infectious complications could indirectly ameliorate neuroinflammation via decreased pro-inflammatory cytokine release (e.g., IL-6 and TNF-α). Emerging research highlights the gut microbiota-brain axis as a potential mechanistic pathway, where immunonutrition-derived short-chain fatty acids modulate blood-brain barrier permeability and neuroinflammatory microenvironments (113). Future studies should investigate longitudinal gut microbial dynamics in relation to neuroinflammatory biomarkers (e.g., CSF GFAP, neurofilament light chain) and optimize immunonutrition protocols within Enhanced Recovery After Surgery (ERAS) frameworks. Key considerations include dose-response relationships, timing of administration, and potential synergies/antagonisms with anti-inflammatory therapies.
5.2 Oral nutritional supplements (ONS)
For malnourished patients (NRS-2002 ≥3), preoperative ONS improves nutritional parameters (e.g., albumin and prealbumin levels) and reduces postoperative morbidity. High-biological-value protein formulations (e.g., whey protein) enhance nitrogen retention and hepatic protein synthesis (114, 115), with a meta-analysis revealing a 12.5% increase in prealbumin (95% CI: 5.3–19.7%; P < 0.01) following 7-day preoperative supplementation. Arginine/ω-3-enriched formulations further suppress NF-κB-mediated pro-inflammatory cascades (IL-6↓34%, TNF-α↓28%) while enhancing lymphocyte proliferation (113). A Cochrane review (116) confirmed ONS efficacy in reducing infectious complications in malnourished subgroups (RR = 0.58), though no mortality benefit was observed. Notably, evidence regarding cognitive outcomes remains inconclusive, warranting exploration of neuroprotective formulations incorporating choline, antioxidants (e.g., vitamin E, selenium), or omega-3 derivatives. Clinical implementation requires vigilant metabolic monitoring to avoid hyperglycemia and refeeding syndrome, ideally coordinated by multidisciplinary nutrition support teams (NSTs) for dynamic protocol adjustments (117).
5.3 Micronutrient optimization
Observational studies consistently associate vitamin D and B-complex deficiencies with POD risk. A retrospective cohort study in arthroplasty patients (118) demonstrated that post-operative vitamin D surveillance and supplementation improved bone turnover markers, though cognitive implications require validation. Iron homeostasis dysregulation (common post-gastrectomy) may exacerbate delirium via cerebral hypoxia and mitochondrial dysfunction (115), necessitating targeted repletion strategies. Prospective trials should evaluate multi-micronutrient cocktails modulating neuroinflammatory pathways (e.g., NLRP3 inflammasome inhibition) while addressing surgical subtype-specific deficiencies (e.g., vitamin B12 malabsorption after gastric resection).
5.4 Combined nutritional intervention and exercise training
Researchers have conducted several intervention studies to evaluate the effectiveness of combining nutritional improvement with exercise training in POD. A single-center study in the Netherlands investigated the use of multimodal prehabilitation to reduce the incidence of POD after elective abdominal surgery. The 5-week prehabilitation program, which included nutritional counseling and home-based exercise, reduced the incidence of POD by one-third (119). Another retrospective cohort study involving over 160,151 patients undergoing hip/femur surgery examined the use of early nutritional intervention (initiated on postoperative day 1) in malnourished patients. Although nutritional intervention was underutilized, its implementation was associated with reduced LOS without increasing hospital costs. However, the intervention did not improve secondary outcomes such as infection rates, in-hospital mortality, or ICU admission rates (120). While some randomized controlled trials (RCTs) suggest that appropriate nutrition can help reverse frailty and increase muscle mass index (121), a meta-analysis by Oktaviana et al. (122) of eight studies found that protein supplementation alone did not significantly improve muscle mass or frailty indices in frail populations. However, more promising results emerged when protein supplementation was combined with exercise intervention. A meta-analysis of 22 RCTs demonstrated significant improvements in frailty status, lean mass, muscle strength, and physical performance among frail older adults (123). Further research is needed to establish effective interventions for frail populations. Specifically, studies should investigate whether multimodal approaches combining exercise modalities—such as aerobic exercise, resistance training, balance training, and functional training—can enhance cardiopulmonary function, muscle strength, and muscle mass, thereby positively influencing perioperative cognitive function.
6 Research direction
In summary, as awareness of the impact of malnutrition continues to grow, interventions such as enteral nutrition (124), parenteral nutrition (125), and oral nutritional supplements (126) have been evaluated to explore the effect of nutritional improvement on preventing perioperative neurocognitive disorders. Moving forward, more targeted interventions tailored to different types of malnutrition are needed. For patients with metabolic obesity, the primary goal should be to alleviate chronic inflammation rather than achieving short-term weight loss (rapid weight loss before surgery may accelerate catabolism). Therefore, the future research direction should focus on how to improve the dietary structure and supplement antioxidant substances to reduce oxidative stress, regulate the activity of inflammasomes, inhibit the NF-κB pathway, and alleviate neuroinflammation. For patients with frail sarcopenia, the core goal is to reverse protein synthesis resistance and protect neuromuscular function. A single nutritional intervention strategy may be limited. Therefore, in the future, large-sample clinical trial evidence is needed to prove that a multi-dimensional collaborative approach integrating nutrition, exercise, metabolic regulation, and cognitive intervention may be beneficial for this population.
In addition, future research must prioritize elucidating how distinct malnutrition subtypes (undernutrition, specific micronutrient deficiencies and general malnutrition) contribute differentially to PND through discrete pathophysiological mechanisms. Current knowledge gaps center on two critical areas: (1) the cellular-level effects of nutrients on neuronal metabolism and neuroimmune crosstalk, and (2) subtype-specific pathways linking nutritional deficits to PND pathogenesis. To address these gaps, investigations should focus on subtype-targeted mechanisms:
For undernutrition, determine whether amino acid supplementation (e.g., tryptophan) restores serotonin synthesis in energy-deprived neurons to prevent delirium, and elucidate how protein-calorie deficits impair hippocampal neurogenesis.
For micronutrient deficiencies, test if vitamin B12 repletion mitigates neuroinflammation by preventing myelin degradation (contrasted with folate's role in dopamine methylation), and assess how zinc deficiency disrupts blood-brain barrier integrity via matrix metalloproteinase activation.
For general malnutrition, map synergistic effects of combined macro/micronutrient deficits on microglial activation, and characterize gut-brain axis dysregulation in patients with concurrent weight loss and vitamin depletion.
Collectively, these mechanistic insights will establish a foundation for precision nutritional interventions tailored to specific malnutrition phenotypes in clinical practice.
7 Conclusion
The review highlights a significant association between malnutrition and PND, with malnutrition increasing PND incidence and impacting postoperative recovery and quality of life. Mechanisms linking malnutrition to PND include psychological impacts, neurotransmitter synthesis abnormalities, brain structural and functional changes, immune dysfunction, neuroinflammation, mitochondrial issues, intestinal barrier damage, gut-brain axis disruption, lymphatic system dysfunction, and endocrine imbalances. Future research should delve into the efficacy of nutritional interventions in specific populations and the molecular links between nutrition and PND prevention, with studies on gene expression, neurotransmitter metabolism, and inflammatory modulation potentially informing more targeted and potent clinical nutrition strategies.
Author contributions
LL: Writing – original draft, Writing – review & editing. QG: Writing – review & editing. MH: Investigation, Writing – review & editing. YZ: Investigation, Writing – review & editing. WY: Investigation, Writing – review & editing. TG: Investigation, Writing – review & editing. PZ: Investigation, Writing – review & editing. ZZ: Investigation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by National Natural Science Foundation of China (No. 82160223), Science and Technology Research Project of Sichuan Administration of Traditional Chinese Medicine (No. 2024MS458), and Youth Innovation Project of Sichuan Medical Association (No. Q2024021).
Acknowledgments
The authors appreciate the support from the Translational Neurology Laboratory and Wang TingHua Translation Institute, Affiliated Hospital of ZunYi Medical University. Thanks are due to MH and YZ for designing this manuscript. We also thank Figdraw for the assistance in creating figures.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cheng C, Wan H, Cong P, Huang X, Wu T, He M, et al. Targeting neuroinflammation as a preventive and therapeutic approach for perioperative neurocognitive disorders. J Neuroinflammation. (2022) 19:297. doi: 10.1186/s12974-022-02656-y
2. Evered L, Silbert B, Knopman DS, Scott DA, DeKosky ST, Rasmussen LS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br J Anaesth. (2018) 121:1005–12. doi: 10.1097/ALN.0000000000002334
3. Boone MD, Sites B, von Recklinghausen FM, Mueller A, Taenzer AH, Shaefi S. Economic burden of postoperative neurocognitive disorders among us medicare patients. JAMA Netw Open. (2020) 3:e208931. doi: 10.1001/jamanetworkopen.2020.8931
4. Jia S, Yang H, Huang F, Fan W. Systemic inflammation, neuroinflammation and perioperative neurocognitive disorders. Inflamm Res. (2023) 72:1895–907. doi: 10.1007/s00011-023-01792-2
5. Dilmen OK, Meco BC, Evered LA, Radtke FM. Postoperative neurocognitive disorders: a clinical guide. J Clin Anesth. (2024) 92:111320. doi: 10.1016/j.jclinane.2023.111320
6. Varpaei HA, Farhadi K, Mohammadi M, Khafaee Pour Khamseh A, Mokhtari T. Postoperative cognitive dysfunction: a concept analysis. Aging Clin Exp Res. (2024) 36:133. doi: 10.1007/s40520-024-02779-7
7. Ron D, Deiner S. Postoperative delirium and neurocognitive disorders: updates for providers caring for cancer patients. Curr Oncol Rep. (2024) 26:1176–87. doi: 10.1007/s11912-024-01584-9
8. Dent E, Wright ORL, Woo J, Hoogendijk EO. Malnutrition in older adults. Lancet Lond Engl. (2023) 401:951–66. doi: 10.1016/S0140-6736(22)02612-5
9. Moellmann HL, Alhammadi E, Boulghoudan S, Kuhlmann J, Mevissen A, Olbrich P. Risk of sarcopenia, frailty and malnutrition as predictors of postoperative delirium in surgery. BMC Geriatr. (2024) 24:971. doi: 10.1186/s12877-024-05566-1
10. Han D, Wang P, Wang SK, Cui P, Lu SB. Frailty and malnutrition as predictors of major complications following posterior thoracolumbar fusion in elderly patients: a retrospective cohort study. Spine J. (2024) 25:679–87. doi: 10.1016/j.spinee.2024.10.004
11. Hou TY, Lin YH, Liu YW, Liu YY, Li WF, Kuo MC, et al. The impact of preoperative nutritional status on postoperative outcomes: an insight from Geriatric Nutritional Risk Index in elderly pancreaticoduodenectomy patients. BMC Surg. (2024) 24:100. doi: 10.1186/s12893-024-02397-0
12. Zhang B, Najarali Z, Ruo L, Alhusaini A, Solis N, Valencia M, et al. Effect of perioperative nutritional supplementation on postoperative complications-systematic review and meta-analysis. J Gastrointest Surg. (2019) 23:168–293. doi: 10.1007/s11605-019-04173-5
13. Lobo DN, Gianotti L, Adiamah A, Barazzoni R, Deutz NEP, Dhatariya K, et al. Perioperative nutrition: recommendations from the ESPEN expert group. Clin Nutr Edinb Scotl. (2020) 39:3211–27. doi: 10.1016/j.clnu.2020.03.038
14. Cederholm T, Bosaeus I, Barazzoni R, Bauer J, Van Gossum A, Klek S, et al. Diagnostic criteria for malnutrition—an ESPEN Consensus Statement. Clin Nutr Edinb Scotl. (2015) 34:335–40. doi: 10.1016/j.clnu.2015.03.001
15. Jensen GL, Cederholm T, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition—a consensus report from the global clinical nutrition community. Clin Nutr Edinb Scotl. (2019) 38:1–9. doi: 10.1016/j.clnu.2019.02.033
16. Rothenberg E, Tsagari A, Erickson N, Katsagoni CN, Malone A, de van der Schueren M, et al. Global Leadership Initiative on Malnutrition (GLIM) for the diagnosis of malnutrition - a framework for consistent dietetic practice. Clin Nutr ESPEN. (2024) 60:261–5. doi: 10.1016/j.clnesp.2024.02.009
17. Kondrup J, Rasmussen HH, Hamberg O, Stanga Z, Ad Hoc ESPEN Working Group. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr Edinb Scotl. (2003) 22:321–36. doi: 10.1016/S0261-5614(02)00214-5
18. Kruizenga H, van Keeken S, Weijs P, Bastiaanse L, Beijer S, Huisman-de Waal G, et al. Undernutrition screening survey in 564,063 patients: patients with a positive undernutrition screening score stay in hospital 14 d longer. Am J Clin Nutr. (2016) 103:1026–32. doi: 10.3945/ajcn.115.126615
19. Guigoz Y, Vellas B. Nutritional assessment in older adults: MNA® 25 years of a screening tool and a reference standard for care and research; what next? J Nutr Health Aging. (2021) 25:528–83. doi: 10.1007/s12603-021-1601-y
20. Gremese E, Bruno D, Varriano V, Perniola S, Petricca L, Ferraccioli G. Serum albumin levels: a biomarker to be repurposed in different disease settings in clinical practice. J Clin Med. (2023) 12:6017. doi: 10.3390/jcm12186017
21. Chen Y, Bei M, Liu G, Zhang J, Ge Y, Tan Z, et al. Prognostic nutritional index (PNI) is an independent predictor for functional outcome after hip fracture in the elderly: a prospective cohort study. Arch Osteoporos. (2024) 19:107. doi: 10.1007/s11657-024-01469-1
22. Oe S, Togawa D, Yamato Y, Hasegawa T, Yoshida G, Kobayashi S, et al. Preoperative age and prognostic nutritional index are useful factors for evaluating postoperative delirium among patients with adult spinal deformity. Spine. (2019) 44:472–78. doi: 10.1097/BRS.0000000000002872
23. Chen Z, Hao Q, Sun R, Zhang Y, Fu H, Liu S, et al. Predictive value of the geriatric nutrition risk index for postoperative delirium in elderly patients undergoing cardiac surgery. CNS Neurosci Ther. (2024) 30:e14343. doi: 10.1111/cns.14343
24. Chiavarini M, Ricciotti GM, Genga A, Faggi MI, Rinaldi A, Toscano OD, et al. Malnutrition-related health outcomes in older adults with hip fractures: a systematic review and meta-analysis. Nutrients. (2024) 16:1069. doi: 10.3390/nu16071069
25. Chen Y, Chen H, Zhuang Y, Wang Y, Dai Z. Association between the geriatric nutritional risk index and postoperative delirium in gastric surgery patients: an analysis of the MIMIC-IV database. BMC Anesthesiology. (2024) 24:477. doi: 10.1186/s12871-024-02874-2
26. Zhao G, Deng J, Shen Y, Zhang P, Dong H, Xie Z, et al. Hyperhomocysteinemia is key for increased susceptibility to PND in aged mice. Ann Clin Transl Neurol. (2019) 6:1435–44. doi: 10.1002/acn3.50838
27. Velayati A, Vahdat Shariatpanahi M, Shahbazi E, Vahdat Shariatpanahi Z. Association between preoperative nutritional status and postoperative delirium in individuals with coronary artery bypass graft surgery: a prospective cohort study. Nutrition. (2019) 66:227–32. doi: 10.1016/j.nut.2019.06.006
28. Drevet S, Bioteau C, Mazière S, Couturier P, Merloz P, Tonetti J, et al. Prevalence of protein-energy malnutrition in hospital patients over 75 years of age admitted for hip fracture. Orthop Traumatol Surg Res. (2014) 100:669–74. doi: 10.1016/j.otsr.2014.05.003
29. Zhang Y, Shan GJ, Zhang YX, Cao SJ, Zhu SN, Li HJ, et al. Preoperative vitamin D deficiency increases the risk of postoperative cognitive dysfunction: a predefined exploratory sub-analysis. Acta Anaesthesiol Scand. (2018) 62:924–35. doi: 10.1111/aas.13116
30. Yuan, S S.C. Larsson, Epidemiology of sarcopenia: prevalence, risk factors, and consequences. Metabolism. (2023) 144:155533. doi: 10.1016/j.metabol.2023.155533
31. Hu Y, Peng W, Ren R, Wang Y, Wang G. Sarcopenia and mild cognitive impairment among elderly adults: the first longitudinal evidence from CHARLS. J Cachexia Sarcopenia Muscle. (2022) 13:2944–52. doi: 10.1002/jcsm.13081
32. Cheng H, Ling Y, Li Q, Li X, Tang Y, Guo J, et al. Association between modified frailty index and postoperative delirium in patients after cardiac surgery: a cohort study of 2080 older adults. CNS Neurosci Ther. (2024) 30: e14762. doi: 10.1111/cns.14762
33. Tsai CY, Liu KH, Lai CC, Hsu JT, Hsueh SW, Hung CY, et al. Association of preoperative frailty and postoperative delirium in older cancer patients undergoing elective abdominal surgery: a prospective observational study in Taiwan. Biomed J. (2023) 46:100557. doi: 10.1016/j.bj.2022.08.003
34. Fu D, Tan X, Zhang M, Chen L, Yang J. Association between frailty and postoperative delirium: a meta-analysis of cohort study. Aging Clin Exp Res. (2022) 34:25–37. doi: 10.1007/s40520-021-01828-9
35. Ceolin C, Papa MV, De Rui M, Devita M, Sergi G, Coin A. Micronutrient deficiency and its potential role in delirium onset in older adults: a systematic review. J Nutr Health Aging. (2023) 27:785–90. doi: 10.1007/s12603-023-1976-z
36. Velayati A, Vahdat Shariatpanahi M, Dehghan S, Zayeri F, Vahdat Shariatpanahi Z. Vitamin D and postoperative delirium after coronary artery bypass grafting: a prospective cohort study. J Cardiothorac Vasc Anesth. (2020) 34:1774–9. doi: 10.1053/j.jvca.2020.02.008
37. Hung KC, Wang LK, Lin YT, Yu CH, Chang CY, Sun CK, et al. Association of preoperative vitamin D deficiency with the risk of postoperative delirium and cognitive dysfunction: a meta-analysis. J Clin Anesth. (2022) 79:110681. doi: 10.1016/j.jclinane.2022.110681
38. Mustafa Khalid N, Haron H, Shahar S, Fenech M. Current evidence on the association of micronutrient malnutrition with mild cognitive impairment, frailty, and cognitive frailty among older adults: a scoping review. Int J Environ Res Public Health (2022) 19:15722. doi: 10.3390/ijerph192315722
39. Song X, Zhang L, Hui X, Sun X, Yang J, Wang J, et al. Selenium-containing protein from selenium-enriched Spirulina platensis antagonizes oxygen glucose deprivation-induced neurotoxicity by inhibiting ROS-mediated oxidative damage through regulating MPTP opening. Pharm Biol. (2021) 59:629–38. doi: 10.1080/13880209.2021.1928715
40. Naeini AM, Elmadfa I, Djazayery A, Barekatain M, Ghazvini MR, Djalali M, et al. The effect of antioxidant vitamins E and C on cognitive performance of the elderly with mild cognitive impairment in Isfahan, Iran: a double-blind, randomized, placebo-controlled trial. Eur J Nutr. (2014) 53:1255–62. doi: 10.1007/s00394-013-0628-1
41. Galiniak S, Aebisher D, Bartusik-Aebisher D. Health benefits of resveratrol administration. Acta Biochim Pol. (2019) 66:13–21. doi: 10.18388/abp.2018_2749
42. Askarizadeh A, Barreto GE, Henney NC, Majeed M, Sahebkar A. Neuroprotection by curcumin: a review on brain delivery strategies. Int J Pharm. (2020) 585:119476. doi: 10.1016/j.ijpharm.2020.119476
43. Kelsey NA, Wilkins HM, Linseman DA. Nutraceutical antioxidants as novel neuroprotective agents. Molecules. (2010) 15:7792–814. doi: 10.3390/molecules15117792
44. Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, et al. Saturated fatty acids produce an inflammatory response predominantly through TLR4 signaling in hypothalamus. J Neurosci. (2009) 29:359–70. doi: 10.1523/JNEUROSCI.2760-08.2009
45. Agrawal R, Gómez-Pinilla F. Metabolic syndrome in the brain: deficiency in omega-3 fatty acid exacerbates dysfunctions in insulin receptor signalling and cognition. J Physiol. (2012) 590:2485–99. doi: 10.1113/jphysiol.2012.230078
46. Feinkohl I, Janke J, Slooter AJC, Winterer G, Spies C, Pischon T, et al. Metabolic syndrome and the risk of postoperative delirium and postoperative cognitive dysfunction: a multi-centre cohort study. Br J Anaesth. (2023) 131:338–47. doi: 10.1016/j.bja.2023.04.031
47. Bell JJ, Pulle RC, Lee HB, Ferrier R, Crouch A, Whitehouse SL. Diagnosis of overweight or obese malnutrition spells DOOM for hip fracture patients: a prospective audit. Clin Nutr. (2021) 40:1905–10. doi: 10.1016/j.clnu.2020.09.003
48. Abdelsalam SA, Renu K, Zahra HA, Abdallah BM, Ali EM, Veeraraghavan VP, et al. Polyphenols mediate neuroprotection in cerebral ischemic stroke-an update. Nutrients. (2023) 15:1107. doi: 10.3390/nu15051107
49. Zhao Y, Ge N, Xie D, Gao L, Wang Y, Liao Y, et al. The geriatric nutrition risk index versus the mini-nutritional assessment short form in predicting postoperative delirium and hospital length of stay among older non-cardiac surgical patients: a prospective cohort study. BMC Geriatr. (2020) 20:107. doi: 10.1186/s12877-020-1501-8
50. Wang W, Yao W, Tang W, Li Y, Lv Q, Ding W. Association between preoperative albumin levels and postoperative delirium in geriatric hip fracture patients. Front Med. (2024) 11:1344904. doi: 10.3389/fmed.2024.1344904
51. Hung KC, Chiu CC, Hsu CW, Ho CN, Ko CC, Chen IW, et al. Association of preoperative prognostic nutritional index with risk of postoperative delirium: a systematic review and meta-analysis. Front Med. (2023) 9:1017000. doi: 10.3389/fmed.2022.1017000
52. Mazzola P, Ward L, Zazzetta S, Broggini V, Anzuini A, Valcarcel B, et al. Association between preoperative malnutrition and postoperative delirium after hip fracture surgery in older adults. J Am Geriatr Soc. (2017) 65:1222–8. doi: 10.1111/jgs.14764
53. Venkatakrishnaiah NK, Anandkumar UM, Wooly S, Rajkamal G, Gadiyar HB, Janakiraman P. Identification of factors contributing to the development of postoperative delirium in geriatric patients with hip fractures—a prospective study. J Fam Med Prim Care. (2022) 11:4785–90. doi: 10.4103/jfmpc.jfmpc_238_22
54. Hawley S, Inman D, Gregson CL, Whitehouse M, Johansen A, Judge A. Risk factors and 120-day functional outcomes of delirium after hip fracture surgery: a prospective cohort study using the UK National Hip Fracture Database (NHFD). J Am Med Dir Assoc. (2023) 24:694–701.e7. doi: 10.1016/j.jamda.2023.02.008
55. Li Z, Zhang L, Yang Q, Zhou X, Yang M, Zhang Y, et al. Association between geriatric nutritional risk index and depression prevalence in the elderly population in NHANES. BMC Public Health. (2024) 24:469. doi: 10.1186/s12889-024-17925-z
56. Hu L, Chen J, Li X, Zhang H, Zhang J, Lu Y, et al. Disruptive and complementary effects of depression symptoms on spontaneous brain activity in the subcortical vascular mild cognitive impairment. Front Aging Neurosci. (2024) 16:1338179. doi: 10.3389/fnagi.2024.1338179
57. Almeida OP, Ford AH, Flicker L. Systematic review and meta-analysis of random-ized placebo-controlled trials of folate and vitamin B12 for depression. Int Psychogeriatr. (2015) 27:727–37. doi: 10.1017/S1041610215000046
58. Sugden C. One-carbon metabolism in psychiatric illness. Nutr Res Rev. (2006) 19:117–36. doi: 10.1079/NRR2006119
59. Bottiglieri T, Godfrey P, Flynn T, Carney MW, Toone BK, Reynolds EH. Cerebrospinal fluid S-adenosylmethionine in depression and dementia: effects of treatment with parenteral and oral S- adenosylmethionine. J Neurol Neurosurg Psychiatry. (1990) 53:1096–8. doi: 10.1136/jnnp.53.12.1096
60. Iadarola ND, Niciu MJ, Richards EM, Vande Voort JL, Ballard ED, Lundin NB, et al. Ketamine and other N-methyl-D-aspar- ate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis. (2015) 6:97–114. doi: 10.1177/2040622315579059
61. McCabe D, Colbeck M. The effectiveness of essential fatty acid, B vitamin, Vitamin C, magnesium and zinc supplementation for managing stress in women: asystematic review protocol. JBI Database System Rev Implement Rep. (2015) 13:104–18. doi: 10.11124/jbisrir-2015-2298
62. Horrobin D, Bennett C. “Phospholipid metabolism and the pathophysiology of psychiatric and neurological disorders”. In:Peet M, Glen I, Horrobin D, , editors. Phospholipid Spectrum Disorders in Psychiatry and Neurology. 2nd ed. Carnforth, UK: Maius Press (2003). p. 3–47.
63. Nimgampalle M, Chakravarthy H, Sharma S, Shree S, Bhat AR, Pradeepkiran JA, et al. Neurotransmitter systems in the etiology of major neurological disorders: emerging insights and therapeutic implications. Ageing Res Rev. (2023) 89:101994. doi: 10.1016/j.arr.2023.101994
64. Calderón-Ospina CA, Nava-Mesa MO, B. Vitamins in the nervous system: current knowledge of the biochemical modes of action and synergies of thiamine, pyridoxine, and cobalamin. CNS Neurosci Ther. (2020) 26:5–13. doi: 10.1111/cns.13207
65. Ayaz A, Nisar I, Muhammad A, Ahmed K, Chand P, Jehan F. Structural changes in the brain on magnetic resonance imaging in malnourished children: a scoping review of the literature. Pediatr Neurol. (2023) 149:151–8. doi: 10.1016/j.pediatrneurol.2023.08.020
66. de van der Schueren MA, Lonterman-Monasch S, van der Flier WM, Kramer MH, Maier AB, Muller M. Malnutrition and risk of structural brain changes seen on magnetic resonance imaging in older adults. J Am Geriatr Soc. (2016) 64:2457–63. doi: 10.1111/jgs.14385
67. Sultan S. Neuroimaging changes associated with vitamin D deficiency—a narrative review. Nutr Neurosci. (2022) 25:1650–8. doi: 10.1080/1028415X.2021.1888206
68. Zhang M, Huang K, Zhang Z, Ji B, Zhu H, Zhou K, et al. Proteome alterations of cortex and hippocampus tissues in mice subjected to vitamin A depletion. J Nutr Biochem. (2011) 22:1003–8. doi: 10.1016/j.jnutbio.2010.08.012
69. Bourke CD, Berkley JA, Prendergast AJ. Immune dysfunction as a cause and consequence of malnutrition. Trends Immunol. (2016) 37:386–98. doi: 10.1016/j.it.2016.04.003
70. Schaible UE, Kaufmann SHE. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med. (2007) 4:e115. doi: 10.1371/journal.pmed.0040115
71. Bhol NK, Bhanjadeo MM, Singh AK, Dash UC, Ojha RR, Majhi S, et al. The interplay between cytokines, inflammation, and antioxidants: mechanistic insights and therapeutic potentials of various antioxidants and anti-cytokine compounds. Biomed Pharmacother. (2024) 178:117177. doi: 10.1016/j.biopha.2024.117177
72. Banks WA. Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Curr Pharm Des. (2005) 11:973–84. doi: 10.2174/1381612053381684
73. Noah AM, Almghairbi D, Evley R, Moppett IK. Preoperative inflammatory mediators and postoperative delirium: systematic review and meta-analysis. Br J Anaesth. (2021) 127:424–34. doi: 10.1016/j.bja.2021.04.033
74. Borst K, Dumas AA, Prinz M. Microglia: immune and non-immune functions. Immunity. (2021) 54:2194–208. doi: 10.1016/j.immuni.2021.09.014
75. Skvarc DR, Berk M, Byrne LK, Dean OM, Dodd S, Lewis M, et al. Post-operative cognitive dysfunction: an exploration of the inflammatory hypothesis and novel therapies. Neurosci Biobehav Rev. (2018) 84:116–33. doi: 10.1016/j.neubiorev.2017.11.011
76. Hovens IB, van Leeuwen BL, Nyakas C, Heineman E, van der Zee EA, Schoemaker RG. Postoperative cognitive dysfunction and microglial activation in associated brain regions in old rats. Neurobiol Learn Mem. (2015) 118:74–9. doi: 10.1016/j.nlm.2014.11.009
77. Li Y, Pan K, Chen L, Ning JL, Li X, Yang T, et al. Deferoxamine regulates neuroinflammation and iron homeostasis in a mouse model of postoperative cognitive dysfunction. J Neuroinflammation. (2016) 13:268. doi: 10.1186/s12974-016-0740-2
78. Sun L, Dong R, Xu X, Yang X, Peng M. Activation of cannabinoid receptor type 2 attenuates surgery-induced cognitive impairment in mice through anti-inflammatory activity. J Neuroinflammation. (2017) 14:138. doi: 10.1186/s12974-017-0913-7
79. Pilchova I, Klacanova K, Tatarkova Z, Kaplan P, Racay P. The involvement of Mg2+ in regulation of cellular and mitochondrial functions. Oxid Med Cell Longev. (2017) 2017:6797460. doi: 10.1155/2017/6797460
80. Dan Dunn J, Alvarez LA, Zhang X, Soldati T. Reactive oxygen species and mitochondria: a nexus of cellular homeostasis. Redox Biol. (2015) 6:472–85. doi: 10.1016/j.redox.2015.09.005
81. Bock FJ, Tait SWG. Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol. (2020) 21:85–100. doi: 10.1038/s41580-019-0173-8
82. Cobine PA, Brady DC. Cuproptosis: cellular and molecular mechanisms underlying copper-induced cell death. Mol Cell. (2022) 82:1786–7. doi: 10.1016/j.molcel.2022.05.001
83. Garbincius JF, Elrod JW. Mitochondrial calcium exchange in physiology and disease. Physiol Rev. (2022) 102:893–992. doi: 10.1152/physrev.00041.2020
84. Li J, Zhu X, Yang S, Xu H, Guo M, Yao Y, et al. Lidocaine attenuates cognitive impairment after isoflurane anesthesia by reducing mitochondrial damage. Neurochem Res. (2019) 44:1703–14. doi: 10.1007/s11064-019-02799-0
85. Zhang L, Wang X, Yu W, Ying J, Fang P, Zheng Q, et al. CB2R activation regulates TFEB-mediated autophagy and affects lipid metabolism and inflammation of astrocytes in POCD. Front Immunol. (2022) 13:836494. doi: 10.3389/fimmu.2022.836494
86. Zhang X, Wu W, Luo Y, Wang Z. Transcranial photobiomodulation therapy ameliorates perioperative neurocognitive disorder through modulation of mitochondrial function in aged mice. Neuroscience. (2022) 490:236–49. doi: 10.1016/j.neuroscience.2021.12.033
87. Ge X, Zuo Y, Xie J, Li X, Li Y, Thirupathi A, et al. A new mechanism of POCD caused by sevoflurane in mice: cognitive impairment induced by cross-dysfunction of iron and glucose metabolism. Aging. (2021) 13:22375–89. doi: 10.18632/aging.203544
88. Miao HH, Wang M, Wang HX, Tian M, Xue FS. Ginsenoside Rg1 attenuates isoflurane/surgery-induced cognitive disorders and sirtuin 3 dysfunction. Biosci Rep. (2019) 39:BSR20190069. doi: 10.1042/BSR20190069
89. Wu J, Zhang M, Li H, Sun X, Hao S, Ji M, et al. BDNF pathway is involved in the protective effects of SS-31 on isoflurane-induced cognitive deficits in aging mice. Behav Brain Res. (2016) 305:115–21. doi: 10.1016/j.bbr.2016.02.036
90. Chelakkot C, Ghim J, Ryu SH. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med. (2018) 50:1–9. doi: 10.1038/s12276-018-0126-x
91. Guyton K, Alverdy JC. The gut microbiota and gastrointestinal surgery. Nat Rev Gastroenterol Hepatol. (2017) 14:43–54. doi: 10.1038/nrgastro.2016.139
92. Zheng Z, Hu Y, Tang J, Xu W, Zhu W, Zhang W. The implication of gut microbiota in recovery from gastrointestinal surgery. Front Cell Infect Microbiol. (2023) 13:1110787. doi: 10.3389/fcimb.2023.1110787
93. Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes. (2013) 20:14–21. doi: 10.1097/MED.0b013e32835bc703
94. Fukumoto S, Tatewaki M, Yamada T, Fujimiya M, Mantyh C, Voss M, et al. Short-chain fatty acids stimulate colonic motility via intestinal serotonin release. Am J Physiol Regul Integr Comp Physiol. (2003) 285:R126–33. doi: 10.1152/ajpregu.00442.2002
95. O'Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. (2015) 277:32–48. doi: 10.1016/j.bbr.2014.07.027
96. Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. (2015) 161:264–76. doi: 10.1016/j.cell.2015.02.047
97. Reigstad CS, Salmonson CE, Rainey JF 3rd, Szurszewski JH, Linden DR, Sonnenburg JL, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. (2015) 29:1395–403. doi: 10.1096/fj.14-259598
98. Magnúsdóttir S, Ravcheev D, de Crécy-Lagard V, Thiele I. Systematic genome assessment of B-vitamin biosynthesis reveals co-operation between gut microbes and host. Nat Biotechnol. (2015) 33:115–20. doi: 10.3389/fgene.2015.00148
99. Zhan G, Yang N, Li S, Huang N, Fang X, Zhang J, et al. Abnormal gut microbiota composition contributes to cognitive dysfunction in SAMP8 mice. Aging. (2018) 10:1257–67. doi: 10.18632/aging.101464
100. Yang C, Fujita Y, Ren Q, Ma M, Dong C, Hashimoto K. Bifidobacterium in the gut microbiota confer resilience to chronic social defeat stress in mice. Sci Rep. (2017) 7:45942. doi: 10.1038/srep45942
101. Lynch JB, Hsiao EY. Microbiomes as sources of emergent host phenotypes. Science. (2019) 365:1405–9. doi: 10.1126/science.aay0240
102. Chidambaram SB, Essa MM, Rathipriya AG, Bishir M, Ray B, Mahalakshmi AM, et al. Gut dysbiosis, defective autophagy and altered immune responses in neurodegenerative diseases: tales of a vicious cycle. Pharmacol Ther. (2022) 231:107988. doi: 10.1016/j.pharmthera.2021.107988
103. Jiang XL, Gu XY, Zhou XX, Chen XM, Zhang X, Yang YT, et al. Intestinal dysbacteriosis mediates the reference memory deficit induced by anaesthesia/surgery in aged mice. Brain Behav Immun. (2019) 80:605–15. doi: 10.1016/j.bbi.2019.05.006
104. Gonzales GB, Njunge JM, Gichuki BM, Wen B, Ngari M, Potani I, et al. The role of albumin and the extracellular matrix on the pathophysiology of oedema formation in severe malnutrition. EBioMedicine. (2022) 79:103991. doi: 10.1016/j.ebiom.2022.103991
105. Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. (2013) 342:373–7. doi: 10.1126/science.1241224
106. Mogavero MP, DelRosso LM, Fanfulla F, Bruni O, Ferri R. Sleep disorders and cancer: state of the art and future perspectives. Sleep Med Rev. (2021) 56:101409. doi: 10.1016/j.smrv.2020.101409
107. Arslan N, Bozkir E, Koçak T, Akin M, Yilmaz B. From garden to pillow: understanding the relationship between plant-based nutrition and quality of sleep. Nutrients. (2024) 16:2683. doi: 10.3390/nu16162683
108. Lundgaard I, Lu ML, Yang E, Peng W, Mestre H, Hitomi E, et al. Glymphatic clearance controls state-dependent changes in brain lactate concentration. J Cereb Blood Flow Metab. (2017) 37:2112–24. doi: 10.1177/0271678X16661202
109. Knezevic J, Starchl C, Tmava Berisha A, Amrein K. Thyroid-gut-axis: how does the microbiota influence thyroid function? Nutrients. (2020) 12:1769. doi: 10.3390/nu12061769
110. Street ME, Shulhai AM, Petraroli M, Patianna V, Donini V, Giudice A, et al. The impact of environmental factors and contaminants on thyroid function and disease from fetal to adult life: current evidence and future directions. Front Endocrinol. (2024) 15:1429884. doi: 10.3389/fendo.2024.1429884
111. Matsui R, Sagawa M, Sano A, Sakai M, Hiraoka SI, Tabei I, et al. Impact of perioperative immunonutrition on postoperative outcomes for patients undergoing head and neck or gastrointestinal cancer surgeries: a systematic review and meta-analysis of randomized controlled trials. Ann Surg. (2024) 279:419–28. doi: 10.1097/SLA.0000000000006116
112. Yu K, Zheng X, Wang G, Liu M, Li Y, Yu P, et al. Immunonutrition vs Standard nutrition for cancer patients: a systematic review and meta-analysis (part 1). JPEN J Parenter Enteral Nutr. (2020) 44:742–67. doi: 10.1002/jpen.1736
113. McGrattan AM, McGuinness B, McKinley MC, Kee F, Passmore P, Woodside JV, et al. Diet and inflammation in cognitive ageing and Alzheimer's disease. Curr Nutr Rep. (2019) 8:53–65. doi: 10.1007/s13668-019-0271-4
114. Xu R, Chen XD, Ding Z. Perioperative nutrition management for gastric cancer. Nutrition. (2022) 93:111492. doi: 10.1016/j.nut.2021.111492
115. Triantafillidis JK, Papakontantinou J, Antonakis P, Konstadoulakis MM, Papalois AE. Enteral nutrition in operated-on gastric cancer patients: an update. Nutrients. (2024) 16:1639. doi: 10.3390/nu16111639
116. Sowerbutts AM, Burden S, Sremanakova J, French C, Knight SR, Harrison EM. Preoperative nutrition therapy in people undergoing gastrointestinal surgery. Cochrane Database Syst Rev. (2024) 4:CD008879. doi: 10.1002/14651858.CD008879.pub3
117. Martínez-Ortega AJ, Piñar-Gutiérrez A, Serrano-Aguayo P, González-Navarro I, Remón-Ruíz PJ, Pereira-Cunill JL, et al. Perioperative nutritional support: a review of current literature. Nutrients. (2022) 14:1601. doi: 10.3390/nu14081601
118. Duggan JL, Fitz W, Lange JK, Shah VM, Olsen A, Iorio R, et al. Postoperative Vitamin D surveillance and supplementation in revision total knee arthroplasty patients: a retrospective cohort analysis. Orthop Clin North Am. (2024) 55:323–32. doi: 10.1016/j.ocl.2024.02.002
119. Janssen TL, Steyerberg EW, Langenberg JCM, de Lepper CCHAVH, Wielders D, Seerden TCJ, et al. Multimodal prehabilitation to reduce the incidence of delirium and other adverse events in elderly patients undergoing elective major abdominal surgery: an uncontrolled before-and-after study. PLoS ONE. (2019) 14:e0218152. doi: 10.1371/journal.pone.0218152
120. Williams DGA, Ohnuma T, Haines KL, Krishnamoorthy V, Raghunathan K, Sulo S, et al. Association between early postoperative nutritional supplement utilisation and length of stay in malnourished hip fracture patients. Br J Anaesth. (2021) 126:730–7. doi: 10.1016/j.bja.2020.12.026
121. Ng TP, Feng L, Nyunt MS, Feng L, Niti M, Tan BY, et al. Nutritional, physical, cognitive, and combination interventions and frailty reversal among older adults: a randomized controlled trial. Am J Med. (2015) 128:1225–36.e1. doi: 10.1016/j.amjmed.2015.06.017
122. Oktaviana J, Zanker J, Vogrin S, Duque G. The effect of protein supplements on functional frailty in older persons: a systematic review and meta-analysis. Arch Gerontol Geriatr. (2020) 86:103938. doi: 10.1016/j.archger.2019.103938
123. Liao CD, Wu YT, Tsauo JY, Chen PR, Tu YK, Chen HC, et al. Effects of protein supplementation combined with exercise training on muscle mass and function in older adults with lower-extremity osteoarthritis: a systematic review and meta-analysis of randomized trials. Nutrients. (2020) 12:2422. doi: 10.3390/nu12082422
124. Ma Y, Cheng J, Liu L, Chen K, Fang Y, Wang G, et al. Intermittent versus continuous enteral nutrition on feeding intolerance in critically ill adults: a meta-analysis of randomized controlled trials. Int J Nurs Stud. (2021) 113:103783. doi: 10.1016/j.ijnurstu.2020.103783
Keywords: perioperative neurocognitive disorders (PND), malnutrition, cognitive dysfunction, nutritional intervention, gut-brain axis
Citation: Luo L, Gong Q, He M, Zhu Y, Yu W, Gong T, Zhao P and Zhu Z (2025) Bridging nutrition and neurology: malnutrition's role in perioperative neurocognitive disorders. Front. Nutr. 12:1601021. doi: 10.3389/fnut.2025.1601021
Received: 27 March 2025; Accepted: 21 July 2025;
Published: 13 August 2025.
Edited by:
Lenin Pavón, National Institute of Psychiatry Ramon de la Fuente Muñiz (INPRFM), MexicoReviewed by:
Yvonne Flores Medina, National Institute of Psychiatry Ramon de la Fuente Muñiz (INPRFM), MexicoDenisse Castro-Eguiluz, National Council of Science and Technology (CONACYT), Mexico
Lucrecia Carrera, University of Guadalajara, Mexico
Copyright © 2025 Luo, Gong, He, Zhu, Yu, Gong, Zhao and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaoqiong Zhu, emh1emhhb3Fpb25nQHptdS5lZHUuY24=
 Li Luo
Li Luo Qihai Gong3
Qihai Gong3 Miao He
Miao He Taowu Gong
Taowu Gong