- 1Weill Cornell Medicine Qatar, Doha, Qatar
- 2Cancer, Infection and Therapeutics Research Group, School of Applied and Health Sciences, College of Health and Life Sciences, London South Bank University, London, United Kingdom
Obesity has been implicated as the driving force of many diseases including cancer through multiple biological mechanisms, including gut microbial imbalances, compromised intestinal barrier integrity, persistent low-grade inflammation, and alterations in energy uptake. As lifestyle factors such as diet, physical activity, and sleep are known to influence disease susceptibility, understanding the role of the gut microbiome in these interactions is critical. A deeper understanding of the intricate connections between gut microbiota, obesity, and various cancers could be used to better inform effective strategies for disease prevention and treatment. Investigating the microbiome’s influence on tumor progression and systemic metabolic health may be the way forward for novel diagnostic and therapeutic approaches. It is essential to investigate how lifestyle factors are linked to both obesity and cancer, and what role the microbiome is playing. This review synthesizes current research on the mechanistic role of the gut microbiome in obesity and cancer, highlighting its potential role in early detection, prognosis, and its use as a targeted intervention to restore gut eubiosis.
1 Introduction
Obesity, as defined as those with a BMI > 30 represents a complex chronic disease that is one of the most important public health challenges of the 21st century. The WHO (World Health Organization) reported that obesity rates have seen a vast growth, almost tripling since 1975, with over 650 million adults classified as obese in 2022 (1). Characterized by excessive adipose tissue accumulation, the health significance of obesity proceeds beyond the immediate and obvious impact on body weight, physical immobility and skeletal issues that comes with carrying an excess of weight. It serves as a risk factor for several complex health conditions, one of which is cancer. Obesity is known to induce chronic, low-level inflammation in the body which creates a pro-tumour environment. Obesity is linked to numerous types of cancers including breast, colorectal, esophageal, kidney, gallbladder, uterine, pancreatic, and liver (2).
In 2022 there were approximately 20 million new cases of cancer diagnosed globally and 9.7 million deaths from cancer (3) Female breast cancer is now the most diagnosed cancer, closely followed by lung, colorectal (CRC) and prostate cancer (4). Cancer represents a complex disease characterized by the uncontrolled growth of abnormal cells that have the ability to change their surrounding environment to make it more favorable, destroy surrounding tissues and spread to distant parts of the body through metastasis. At the most basic level, cancer is caused by changes to the genes that disrupt normal cellular mechanisms of growth, division, and apoptosis. This change is often brought about by environmental determinants and lifestyle factors including obesity.
The human gut microbiome is a complex ecosystem composed of trillions of microorganisms, including bacteria, viruses, fungi, and protozoa, that reside in the gastrointestinal tract. This microbial community contains over 100 trillion cells and plays a far bigger role than previously thought. The gut microbiome is now known to influence host metabolism, immune function, neurological processes, and overall health (5). Scientific advances have revealed that the gut microbiome plays an active part in a number of physiological processes, with the composition and diversity linked to a wide range of health conditions, from metabolic disorders and inflammatory diseases to cancer and neurological pathologies (6, 7). This review examines the nexus between lifestyle factors – obesity – cancer and proposes a mechanism involving gut microbes to explain how lifestyle can relate to obesity and cancer. Mechanistic relationships between the gut microbiome and both obesity and cancer, with particular emphasis on how lifestyle factors, specifically diet, physical activity, and sleep patterns influence microbial communities. While previous reviews have explored these topics separately, our analysis uniquely integrates these interconnected elements to address a critical gap in the literature: the comprehensive understanding of how lifestyle modifications alter the gut microbiome to potentially mitigate disease progression.
2 Methods
Before the initial search, key terms and concepts were decided to ensure a concise and accurate search of the available literature. Keywords were chosen based on our search questions: 1/ causes of obesity; 2/ link between diet and microbiome; 3/ link between gut microbes, metabolites and cancer/obesity/epigenetics; 4/ association between obesity and physical activity; 5/ gut metabolites and sleep/hunger/psychiatric disorders; 6/ link between physical activity/sleep/psychiatric disorders and diet.; 7/ probiotics and fecal transplant and microbiome. Using the pre-decided key terms and Boolean operators PubMed was searched to identify relevant studies. Specific filters were applied to narrow the results (e.g., publication date, language). A manual search of the reference lists from key articles was performed to identify additional studies not captured in the initial database search.
3 Obesity and cancer
Obesity is one of the most significant modifiable risk factors for cancer development, second only to tobacco use in preventable cancer causes. Excess adipose tissue increases the risk for at least 13 different types of cancers, including endometrial, esophageal adenocarcinoma, colorectal, postmenopausal breast, prostate, and renal cell cancers (2, 8). The biological mechanisms underlying this relationship are multifaceted. As global obesity rates continue to rise, understanding these connections has become increasingly urgent.
The microbiome was reported to play an important role in some cancers including CRC. CRC is the most common cancer for both sexes in the US and the third most prevalent cancer worldwide: previously a cancer of high economic countries, an increase in cases from low-income countries is thought to be due to the rapid change in diet from traditional foods to one that more closely resembles the Western Diet (WD) (9, 10). The sharp rise in CRC, particularly early-onset CRC, is linked to obesity and a diet high in saturated fats and low in fibre (11, 12). Multiple studies have highlighted the change in bacterial species in cases of CRC as compared to healthy individuals, with the most noted differences being a loss of diversity, and the presence in the colon of bacteria normally associated with the oral cavity in healthy individuals, notably genotoxin-producing Fusobacterium nucleatum (13). Indeed, it has even been suggested (14) that the gut microbiota could be used as a tool to identify colonic lesions, as CRC patients contained higher levels of bacterial taxa traditionally thought of as oral pathogens, notably Fusobacterium, Porphyromonas, Peptostreptococus, Gemella, Parvimonas, and Prevotella. This increase in pathogenic bacterial species and decrease in butyrate-producing bacteria leads to a change in the local immune response and a shift in mucosal state to one that supports tumor progression (12). The relationship between dysbiosis of the gut microbiome and cancer is best studied for CRC (15, 16), but there is some evidence for the role of the microbiome in other cancers. Breast cancer is the most common cancer of women worldwide. It has been shown that women with breast cancer have a different gut microbiota than women without breast cancer (17).
Fusobacterium nucleatum has also been implicated in promoting tumor progression in pancreatic ductal adenocarcinoma, the most prevalent form of pancreatic cancer through triggering chronic inflammation and the increased release of cytokines (18, 19). Additionally, gut microbiota-derived bile acids have been shown to suppress immune surveillance, and antibiotic intervention in mouse models of liver cancer has demonstrated the potential to inhibit tumor growth. The lung microbiome, influenced by microbial communities from the oral, nasal, and gastrointestinal tracts, has also emerged as a contributing factor in lung cancer (20–22). Notably, individuals with lung cancer often exhibit reduced microbial diversity within lung tissue (23).
4 Factors affecting the gut microbiome
The composition of diet plays a role in obesity. Highly processed carbohydrates can cause rapid glucose absorption, triggering insulin spikes and promoting adipose storage; added sugars are associated with visceral adiposity; and a reduced fibre intake can lead to constipation and affect gut microbiome health (5). Excessive consumption of saturated and trans fats is linked to inflammation and metabolic dysregulation (24), while the quality of protein consumed in a diet can affect satiety, muscle protein synthesis, and body composition (25). One dietary factor now recognized as playing a major role is the high consumption of ultra-processed foods that are designed to be hyperpalatable but have a poor nutrient profile (Figure 1). Lane, Gamage (26) found that energy from ultra-processed foods ranges from between 42 to 58% in countries such as Australia and the US. Other factors that play a role in diet and obesity are hormonal regulation, chrononutrition, physical activity levels, and sleep (27). The quality of the food consumed can impact the signalling of the hunger and satiety hormones leptin and ghrelin, as well as the stress hormone cortisol (28). Irregular eating patterns, fasting and late-night snacking have also been associated with an increased risk of obesity due to the effect of meal timing on cell cycle regulation (29).
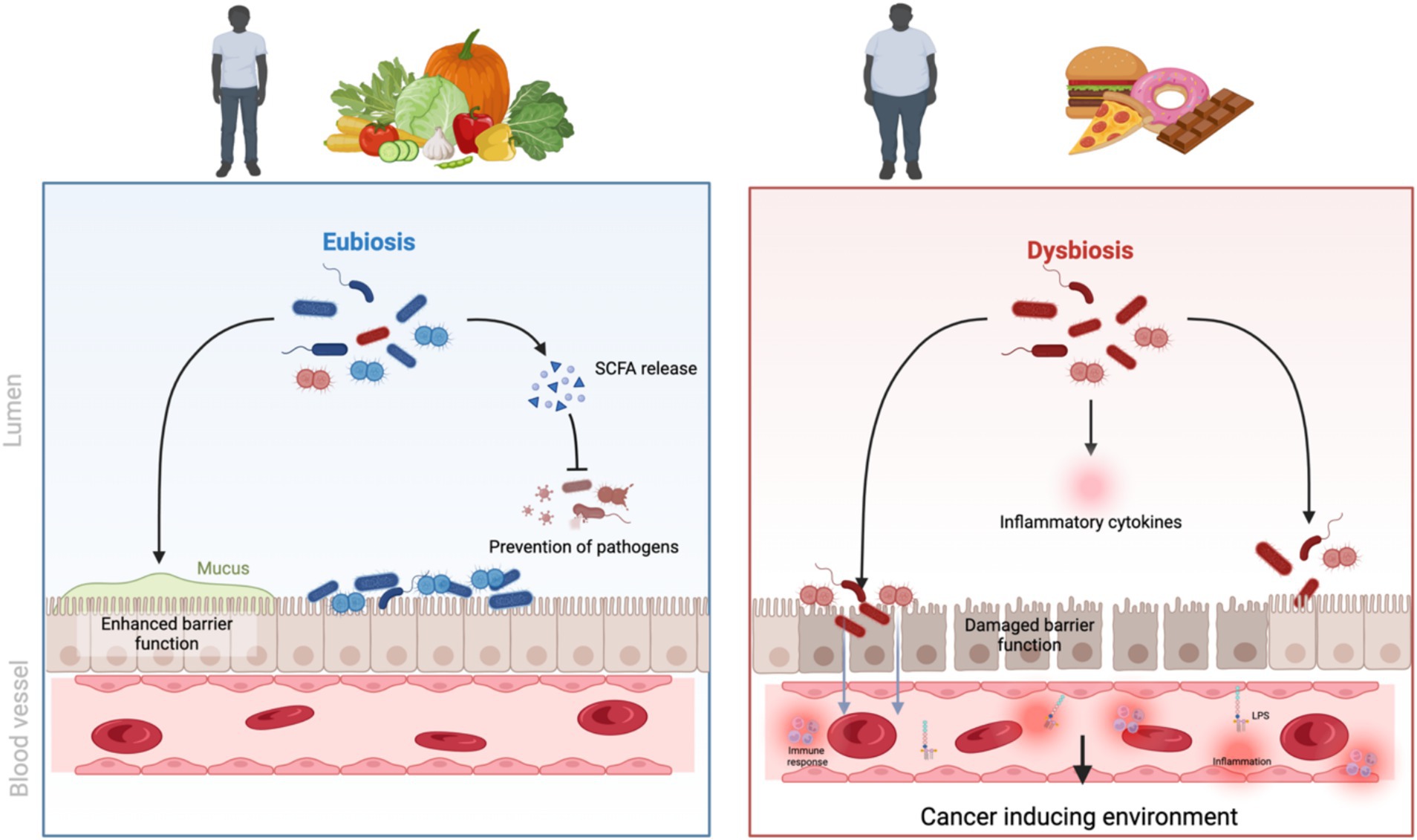
Figure 1. The effect of an unhealthy diet and obesity on gut microbiome and cancer initiation. Created in BioRender.
While diet is a major contributing factor in obesity, it is crucial to recognize that other environmental factors, such as antibiotic exposure, medications, and pollutants, also play significant roles. Accumulating evidence suggests that these factors may contribute to the development of obesity by influencing the gut microbiota (7). Early-life antibiotic exposure has been associated with an increased risk of obesity due to its effects on the intestinal microbial community (30). This may be due to dramatic changes in the intestinal microbiota in response to oral antibiotic treatments: a study of Finnish children (31) found those who had consumed antibiotics belonging to the macrolide group had a long-lasting shift in the microbial composition that included a depletion of Actinobacteria and increases in Bacteroidetes and Proteobacteria. Similarly, environmental pollutants and certain medications can disrupt the delicate balance of the gut microbiome, promoting dysbiosis and potentially impacting energy metabolism and weight regulation (32). The role of hormones in obesity has also gathered interest recently, due to the changing environment and the inclusion of Endocrine Disrupting Chemicals (EDC) in everyday products such as food packaging. These EDC are thought to alter lipid metabolism and alter the hormonal pathway for satiety leading to weight gain and alteration in the gut microbiome (33, 34).
Studies have shown that the gut microbiomes of lean and obese individuals differ significantly in their composition (6, 35–37). Obese individuals tend to have a higher abundance of Firmicutes and a lower abundance of Bacteroidetes, a microbial profile that has been associated with increased energy harvesting and storage (38). This shift in the gut microbiome composition is thought to be driven, in part, by the WD, which is typically high in fat and low in fibre. While earlier research suggested a consistent pattern of higher Firmicutes and lower Bacteroidetes in obese individuals, more recent studies reveal a more complex picture (39). The ratio of Firmicutes to Bacteroidetes may not be as consistent as initially thought, and specific changes at the species level seem more relevant. Alterations to the ratio of Firmicutes to Bacteroidetes is affected by diet and weight loss can impact the diversity found in an individual’s gut. Obese individuals often exhibit reduced microbial diversity and an altered abundance of specific bacterial groups (40). For instance, some studies have reported a depletion of Blautia species in obese children, which correlates with intestinal inflammation and worsened metabolic phenotype (41). Akkermansia muciniphila, a bacterium known for its mucin-degrading properties, has gained attention for its potential role in modulating metabolism and improving gut health (42). In addition, although most studies focus on bacteria, some studies also suggest obesity changes the human gut mycobiome (43). It’s important to consider that the relationship between specific microbial changes and obesity can be influenced by various factors, including diet, genetics, and even geographical location (44).
5 The microbiome -metabolome-obesity-cancer link
The gut microbiome’s influence goes beyond that of energy homeostasis, shaping the host’s metabolic landscape through interactions with various physiological pathways, influencing cancer risk. Obesity-related shifts in the gut microbiome led to altered production of key metabolites, including short-chain fatty acids (SCFAs), which play a crucial role in modulating inflammation, immune responses, and metabolic regulation. These microbial changes influence cytokine production, promoting a pro-inflammatory environment that can contribute to tumorigenesis (Table 1). Additionally, disruptions in gut-derived hormones and neurotransmitters affect metabolic signaling, further exacerbating obesity and increasing cancer susceptibility. Epigenetic modifications induced by microbial metabolites further regulate gene expression, reinforcing the complex interplay between the gut microbiome, obesity, and cancer.
5.1 Short chain fatty acids
A number of metabolomic changes observed in obesity have been directly linked to the gut microbiome composition. Microbial fermentation of dietary fibre found in foods such as vegetables produce short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate (55). In particular, Bacteroidetes in the Firmicutes are responsible for producing butyrate and propionate. Firmicutes have both a harmful effect, through pro-inflammatory response and a beneficial response depending on the bacteria species. Butyrate in particular may play a number of roles in reducing inflammation or reducing cancer risk. It serves as the main energy source for the cells of the colon (56) and plays a crucial role in maintaining intestinal barrier integrity by facilitating tight junction assembly (57) as well as being an anti-inflammatory agent, inhibiting pro-inflammatory cytokines, and supporting regulatory T-cell function (58). Butyrate produced by gut bacteria also regulates gut mucus barrier repair, potentially by polarizing macrophages into a M2 state (59). SCFAs also act to down-regulate fatty acids synthesis and lipolysis, leading to a decrease in body weight and reduced likelihood of obesity (56, 60). Butyrate has been observed in vitro to inhibit proliferation and promote apoptosis in some cancer cell lines (61).
5.2 Cytokines
A dysbiosis in the gut microbiome as the result of obesity leads to a change in metabolites such as SCFA that cause a pro-inflammatory state characterized by increased levels of pro-inflammatory cytokines such as IL-1, IL-6, and TNF-α, the so-called “inflammatory triad” (62). This systemic inflammatory state may be initiated or enhanced through multiple adipose tissue-dependent mechanisms. Adipose tissue, particularly in the context of obesity, undergoes pathological expansion characterized by adipocyte hypertrophy, hypoxia, and stress responses that trigger the production of pro-inflammatory cytokines including TNF-α, IL-6, and IL-1β. These mechanisms are described further in the following sections (62, 63).
There are two forms of adipose tissue in the human body, white adipose tissue (WAT) and brown adipose tissue (BAT) (64). It is now known that WAT is an active endocrine organ, responsible for the production and secretion of various adipokines, with leptin and adiponectin being among the most physiologically significant. Leptin regulates appetite and energy balance by influencing the hypothalamus, while adiponectin is responsible for enhancing insulin sensitivity and is known to have anti-inflammatory properties, with lower levels associated with obesity, diabetes, and increased cancer risk (65–67). In contrast, resistin, primarily produced by circulating monocytes in human adipose tissue, plays a significant role in metabolic dysfunction by impairing insulin signaling and promoting systemic inflammation. By interfering with insulin action, resistin contributes to the development of insulin resistance, a key factor in metabolic disorders such as type 2 diabetes, and cancers such as breast, colorectal, pancreatic and endometrial (68–70). Additionally, it enhances the release of pro-inflammatory cytokines from mononuclear cells, further exacerbating chronic low-grade inflammation. This inflammatory response not only disrupts glucose homeostasis but also contributes to the progression of obesity-related complications, cardiovascular diseases, certain cancers and other metabolic syndromes. Visfatin is also involved in glucose metabolism and immune response, functioning both as a cytokine and an adipokine (67). Visfatin exhibits insulin-like properties by binding to insulin receptors at a site distinct from insulin itself, thereby promoting glucose uptake in peripheral tissues. Furthermore, visfatin plays a crucial role in inflammation, with elevated levels observed in various inflammatory conditions and obesity (65). Its expression increases in response to proinflammatory cytokines, and it subsequently stimulates the production of inflammatory mediators such as IL-6, TNF-α, and IL-1β, creating a potential feedback loop that may exacerbate chronic low-grade inflammation (65, 71, 72).
Whilst both lean and obese individuals have WAT present, the quantity and composition differ with obese individuals having a dysregulated configuration leading to an increased pro-inflammatory state and a reduction in adiponectin (64). Adiponectin, a 247-amino acid peptide has been shown in multiple studies to enhance insulin sensitivity in muscle and adipocytes, it improves endothelial health through local nitric oxide production and promotes weight loss through increased oxidation of lipids. Adiponectin’s ability to suppress tumor growth and angiogenesis while triggering apoptosis indicates that lower circulating levels of adiponectin as found in obese individuals could be a mechanism linking excess weight to tumor development (65).
Within the WAT, fat-storing adipocytes are responsible for a host of responses within the body such as metabolism of lipids and glucose, inflammation, blood pressure and angiogenesis (67). An increase in body fat that causes an increase in the volume of the adipocyte is referred to as hypertrophy (73); hypertrophy can lead to adipocyte hypoxia when blood vessels do not grow quickly enough to match the expansion of the adipocytes. This leads to a low-oxygen environment causing the cells to produce cytokines and chemokines that attract circulating monocytes (Figure 2).
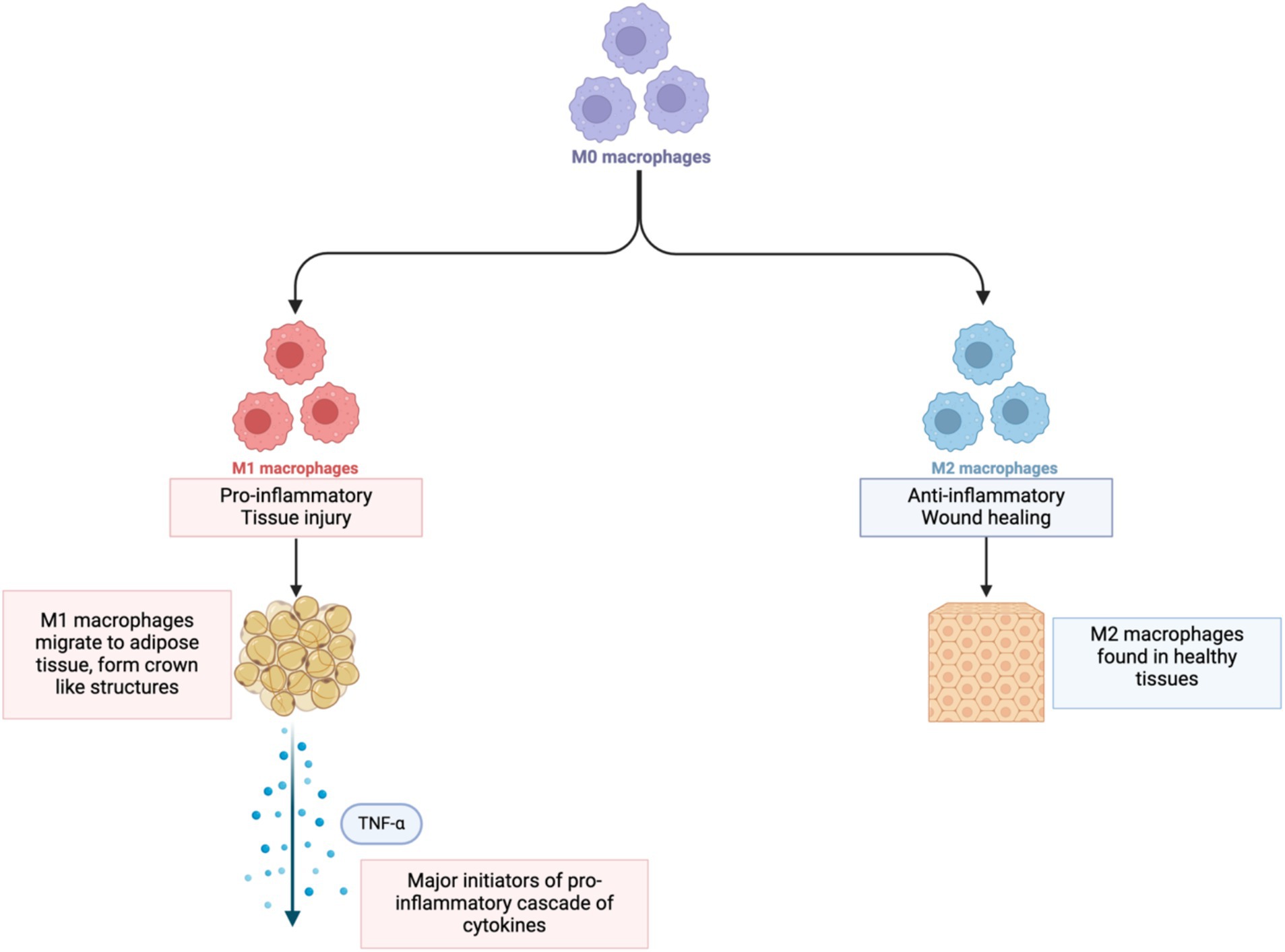
Figure 2. Change in monocytes to M1 and M2 macrophages. Created in BioRender.
Upon infiltration of the hypoxic adipose tissue, M1 macrophages become major initiators of a pro-inflammatory cascade of cytokines, particularly TNF-α, which is that is responsible for disrupting insulin signaling, reducing adiponectin, and increasing free fatty acids. It also stimulates other inflammatory molecules (74) which work in parallel with TNF-α to promote inflammation by affecting insulin sensitivity, fat metabolism, and appetite regulation, and deregulating expression of genes involved in tumor growth via JAK/STAT pathways (64).
While TNF-α is produced as a result of tissue stress in WAT, it has also been reported that one third of total circulating IL-6 originates from adipose tissue (73). Maqoud, Calabrese (75) also found that in individuals placed into three groups (group 1 – overweight; group 2 – obese; group 3 – morbidly obese) there was a significant increase in IL-6 levels between group 1 and group 3 (but no change in levels between the groups for TNF-α).
Together, increasing levels of these cytokines create a harmful feedback loop, with TNF-α and IL-6 recruiting more M1 macrophages, whilst also causing adipocyte dysregulation. The malfunctioning adipocytes produce more inflammatory signals that attract even more M1 macrophages, and the cycle continues.
A study by Xue et al. (76) investigated the association between gut microbiota and inflammatory cytokines, finding seven significant associations between bacteria phyla such as Euryarchaeota with IL-2 and IL-8. This is of particular interest in the case of gastric cancer in which Helicobacter pylori is known to induce the production of IL-8, driving inflammation with the potential to lead to cancer (77).
5.3 Hormones
Gut dysbiosis can directly affect hormone production and signaling, including that of hormones involved in appetite regulation, satiety, and stress response. Altered gut hormone levels can lead to increased food intake, cravings for unhealthy foods, and decreased energy expenditure, contributing to weight gain and obesity.
The relationship between dysbiosis of the gut microbiome and cancer is best studied for CRC (15, 16), but there is some evidence for the role of the microbiome in other cancers. Breast cancer is the most common cancer of women worldwide. It has been shown that women with breast cancer have a different gut microbiota than women without breast cancer (17).
As highlighted in the previous section, adipose tissue is an active endocrine organ that influences hormone metabolism. Excess adipose tissue is a core attribute of obesity and can significantly affect the levels and balance of various sex hormones, including estrogen, androgens, and progesterone, all of which play critical roles in the development and progression of hormone-sensitive cancers such as breast, endometrial, and prostate cancer. Additionally, adipose tissue is a significant source of aromatase, an enzyme that catalyzes the conversion of androgens into estrogens, further contributing to the elevated estrogen levels associated with obesity (78). This positive relationship between fat mass, aromatase expression, and higher estrogen levels is particularly prominent in the post-menopausal state (79). The complex interplay between obesity, metabolic dysregulation, and altered sex hormone levels creates an inflammatory state that can promote a tumorigenic environment, thereby increasing the risk of hormone-sensitive cancers (80–83). If gut microbiome alterations play a role in breast cancer, the mechanism is still not fully understood, and Baker, Al-Nakkash (84) note that estrogen receptor-positive (ER+) breast cancer has been linked to a hyperactive estrobolome – a collection of genes in commensal bacteria responsible for estrogen metabolism that causes increased intestinal absorption of free estrogen leading to an elevated risk of developing breast cancer. The gut microbiota is also responsible for modulating the estrogen-metabolizing enzyme beta-glucuronidase which influences estrogen metabolism (85).
The discussion around obesity and breast cancer in women considers two distinct groups: postmenopausal and premenopausal. This is due to the differences in circulating hormone profiles in the different stages of a woman’s life (86). In postmenopausal women, an increased BMI is linked to an increased incidence of breast cancer; however, in premenopausal women, obesity has been shown to have the opposite effect and play a protective role (82, 87, 88). Adipose tissue produces excess estrogen, which is implicated in hormone-sensitive cancers such as breast and endometrial cancer. Adipose tissue becomes the main source of estrogen production in postmenopausal women with obesity. This is due to androgens that are released from the adrenal glands being converted into estrogens by adipocytes, in a process called aromatization. As a result, the greater the levels of adipose tissue, the more androgens are converted, and the greater the circulating levels of estrogen. Adipose tissue also secretes cytokines, growth factors, and inflammatory molecules that further impact aromatization, leading to increased estrogen levels. Elevated estrogen levels interact with hormone-sensitive breast tissue, increasing the risk of estrogen receptor-positive (ER+) breast cancer (65) via an increased cell proliferation and reduction in apoptosis (89). In contrast, estrogen production in premenopausal women occurs mostly in the ovaries: this results in obesity having a lesser impact on estrogen production as estrogen levels in premenopausal women tend to be lower because of significant absorption of estradiol into fat tissue and an increased rate of estrogen metabolism and clearance by the liver (86). Loh et al. (90) found that obesity in premenopausal women had a protective effect for ER + breast cancer. Choi, Choi (87) also found that obesity and/or higher waist circumference was linked to an increased incidence of gastric cancer in postmenopausal women, although no association was found between obesity and or waist circumference and gastric cancer in premenopausal women. Changes to the microbiome diversity involved in estrogen and hormone metabolism can also lead to an increased level of circulating estrogen, increasing the risk of breast cancer. Additionally, a decreased abundance of Methylobacterium in breast cancer patients has been associated with more invasive tumors (91).
In the case of endometrial cancer, there are interactions between hormonal fluctuations, disturbances in gut microbial balance, and chronic inflammation. Post-menopausal women exhibit a heightened cancer risk, potentially linked to shifts in gut microbial composition and intestinal environment that accompany hormonal changes (92). The menstrual cycle also plays a role in the cyclical timing of the proliferation on the endometrium. Estrogen increases cell proliferation as a normal stage in the cycle and is counter-balanced by progesterone (81). Obese women have a lower circulating level of progesterone which leads to continued cell proliferation. Once menopause has occurred, ovarian estrogen production starts to decline rapidly and is replaced as previously described by production in adipose tissue (83). Sex Hormone-Binding Globulin (SHBG) levels are lowered in those with obesity, meaning more estrogen and testosterone are circulating. Lowering of SHBG occurs due to hyperinsulinemia increasing insulin growth factor-1 (IGF-1) levels, creating a potentially cancer inducing environment (78, 93). There are alterations in the diversity and structure of the microbial community in endometrial cancer compared to healthy controls. Patients with endometrial cancer had a reduction in alpha diversity, with a shift from anti-inflammatory Firmicutes and Clostridia to a pro-inflammatory Proteobacteria-dominated microbiome (94). This dysbiosis appears connected to estrogen metabolism disruption, suggesting the gut microbiome as a critical mediator of endometrial cancer risk (95).
Geographical variations in endometrial cancer show that Europe and North America have the highest rates which may be due to lifestyle factors that impact gut microbial health. Unhealthy dietary patterns, sedentary behaviors, and extensive antibiotic use contribute to microbial imbalances (95). The microbiome’s role extends beyond passive observation, actively participating in hormonal regulation, inflammation modulation, and potentially cancer progression.
Prostate cancer is linked to an imbalance between estrogen and androgens. As men age their testosterone levels decline but estrogen levels normally increase (96): this increase is brought about by an increase in the activity of aromatase, which converts testosterone to estrogen. The subsequent shift in hormone levels can lead to inflammation, cell proliferation and a decrease in apoptosis causing a tumor-creating environment. However, research findings are contradictory regarding the association of obesity and prostate cancer. Several studies have found that BMI did not have a significant effect on prostate cancer total risk (90, 97–99). Although it has been noted by Agalliu et al. (97) that cases with aggressive prostate cancer had a lower BMI, they postulated it to be a result of weight loss due to the effects of the cancer. In contrast, a randomized trial by Chau, Till (98) found that increased BMI was associated with high-grade prostate cancer. Vidal, Oyekunle (100) also found obesity to be linked to high-grade prostate cancer, and those with obesity to be at a younger age for surgical treatment of prostate cancer. An explanation for the differences observed in the studies is the availability of prostate cancer screening: Agalliu et al. (97) involved participants who had received a positive histological diagnosis of prostate cancer up to 6 months before the date of enrollment, whereas participants in Hurwitz, Dogbe (99) were required to undergo screening for prostate cancer every six years, influencing detection rates, with earlier detection having a better prognosis. A pilot study by Golombos, Ayangbesan (101) investigated the gut microbiome of 20 men with high-risk prostate cancer, the study revealed a higher abundance of Bacteroides massiliensis in prostate cancer patients compared to the controls and a higher level of Faecalibacterium prausnitzii and Eubacterium rectalie in the control group.
5.4 Neurotransmitters
The gut microbiome acts as a neurochemical factory, directly producing or modulating the production of key neurotransmitters responsible for appetite and mood (102). These include serotonin, gamma-aminobutyric acid (GABA), dopamine and norepinephrine. Almost all of the body’s serotonin is produced within the gut (103), and it acts on the hypothalamus to regulate hunger and satiety, with deficiencies in serotonin being linked to cravings for high calorie foods and overeating (104). Specific spore-forming bacteria found in both mouse and human gut microbiota stimulate serotonin production from enterochromaffin cells (ECs) in the colon, influencing the gut mucosa and the platelets in the circulating blood effect gastrointestinal motility as well as platelet function (105). This, plus the further influence of serotonin on sleep (see Section 5.3), highlights the fact that the effects of the gut microbiome are not only local but systemic.
An imbalance in GABA, which is an inhibitory neurotransmitter, has been linked to a number of neurological disorders that include stress, anxiety, and Parkinsons disease. A number of gastrointestinal bacteria such as Bifidobacterium, Lactobacillus, and Bacteroides have the genetic code for glutamic acid decarboxylase (GAD), the enzyme responsible for synthesizing (GABA). In fact, Bacteroides are thought to be the driving force in the influence of gut microbiota on mental health by regulating GABA production (106). GABA helps regulate dopamine and serotonin, and disruptions in its production may lead to increased cravings for high-calorie foods, further promoting weight gain (107). Ma, Yan (108) tested white-to-beige adipocyte conversion using GABA supplementation as a potential treatment for obesity. They found GABA supplementation successful in not only reducing body weight but also adipose inflammation. Analysis of gut microbiota composition revealed that GABA supplementation increased beneficial bacteria such as Bacteroidetes and Akkermansia, while decreasing the levels of Firmicutes levels that are linked to obesity and inflammation. This highlights a potential gut-brain axis mechanism in obesity management.
In individuals with obesity, dopamine signaling is often dysregulated, leading to reduced dopamine receptor availability (especially D2 receptors) in the brain. This can result in overeating to compensate for reduced reward sensitivity, similar to addiction mechanisms. Additionally, dopamine influences energy expenditure and physical activity, with lower dopamine levels being linked to reduced motivation for exercise (109). Norepinephrine’s role in obesity is through the regulation of metabolism, appetite, and energy expenditure. It influences the sympathetic nervous system, which controls thermogenesis and lipolysis (110). High norepinephrine activity stimulates BAT to burn calories, promoting weight loss. Norepinephrine also affects appetite regulation by acting on the hypothalamus, contributing to increased hunger and food intake (111). Chronic stress has been known to elevate norepinephrine levels, perhaps leading to stress-induced overeating and weight gain (112).
The dysbiosis brought about by obesity disrupts not only the production of these neurotransmitters but also their regulation due to the reduction of the diversity in the gut microbiome. Obesity-associated neurotransmitter dysfunction has the potential to lead to metabolic disturbances that create a cancer inducing environment, that may act in concert with the effect of altered hormone levels (see Section 4.3). A neurotransmitter imbalance can directly influence cancer cell behavior as seen in the imbalance of serotonin which stimulates cell proliferation in colorectal and breast cancers (113). An imbalance in norepinephrine leads to activation of β-adrenergic signaling promoting tumor growth as well as inducing anti-apoptotic activity (114).
Emerging data suggest that cancer cells take advantage of the neurotransmitters-initiated signaling pathway to activate uncontrolled proliferation and dissemination. The gut microbiome influences neurotransmitter levels, including serotonin, dopamine, gamma-aminobutyric acid (GABA), and norepinephrine, which not only regulate mood and cognition but also affect immune function, inflammation, and tumor growth (115). Norepinephrine and dopamine have been linked to stress-related tumor growth, with chronic stress activating beta-adrenergic receptors, leading to increased inflammation, immune suppression, and enhanced tumor cell survival (116). Gut dysbiosis may alter the balance of these neurotransmitters, creating a tumor-promoting microenvironment, particularly in cancers such as colorectal, breast, and prostate cancer (117).
5.5 Epigenetics, the tumor microenvironment and the microbiome
Metabolomic alterations can impact gene expression and contribute to both obesity and cancer through several mechanisms which involve either changing the availability of molecules used for DNA modifications or directly blocking the function of enzymes that regulate these genetic control mechanisms (118). Gut microbial metabolites influence epigenetic modifications such as DNA methylation, by silencing tumor suppressor genes, activating oncogenic pathways, and/or modifying gene expression related to metabolism. Bacterial-derived metabolites can also cause alterations to histone acetylation and methylation, creating changes in the chromatin structure that impacts gene expression. Additionally, the modulation of non-coding RNA populations can influence gene regulation and cellular signaling pathways (119).
The altered metabolic environment in obesity can influence the tumor microenvironment, promoting cancer development and progression (120). This is seen in the elevated levels of free fatty acids that activate inflammatory pathways and increase cancer risk (121). Adipokines, originating from body fat and within the tumor capsule, can exert both proinflammatory and anti-inflammatory effects, impacting tumor growth (122). The tumor microenvironment has been compared to that of a wound healing site, with the production of proinflammatory molecules and growth factors. It is also affected by the gut microbiota through the previously highlighted epigenetic modifications (120).
5.6 Microbial influence of tumors
The relationship between microbiota and cancer goes beyond the intestinal environment, with studies showing associations between microbial dysbiosis and the onset and progression of multiple cancer types such as pancreatic, prostate, endometrial, and brain cancers (123–126).
There are two routes of microbial influence on tumors. The first is microbial presence at the tumor site, where specific bacteria interact with tumor and immune cells directly within the tumor microenvironment. For example, Fusobacterium nucleatum, commonly found in colorectal tumors and increasingly identified in pancreatic cancer tissue, has been shown to promote tumor growth and resistance to therapy through enhancement of inflammatory signaling (127, 128).
The second route involves the gut-derived microbial metabolites short-chain fatty acids, bile acids, and lipopolysaccharides. These metabolites circulate through the bloodstream and lymphatics, affecting the immune responses, influencing systemic inflammation, and endocrine pathways. In prostate and endometrial cancers, metabolites are thought to contribute to a pro-tumorigenic systemic environment by impacting hormone regulation and immune function (123, 129).
The microbial influence is highlighted in glioblastoma multiforme (GBM), where the gut-brain axis has emerged as a key area of interest. Gut microbiota-derived metabolites are believed to traverse the blood–brain barrier, potentially supporting the immunosuppressive microenvironment characteristic of GBM and modulating the phenotype of tumor-associated macrophages, which play a crucial role in tumor progression (130–132).
These interconnected findings across various cancer types point toward a shared underlying concept: both local and distant microbial communities can shape cancer development and progression through metabolic and immune-related mechanisms.
6 The gut-health nexus: how diet, sleep, and physical activity modulate dysbiosis, obesity, and cancer risk
Lifestyle factors significantly influence the composition and function of the gut microbiome, and the resulting dysbiosis can initiate a cycle that promotes obesity and, ultimately, increases cancer risk. This cycle is fueled by the interplay between dysbiosis, obesity, and lifestyle factors, further exacerbating dysbiosis. We will analyze how these factors interact.
6.1 Diet
There is a growing body of research examining the connection between diet, gut microbiota composition/diversity, and their impact on obesity and cancer development. There are three gut microbiome enterotypes according to high abundances of specific bacterial groups in healthy individuals: Bacteroides (Enterotype 1); Prevotella (Enterotype 2); and Ruminococcus (Enterotype 3). It is possible these broad profiles are associated with different dietary patterns: for instance, individuals with a diet high in protein and animal fat show a higher abundance of Bacteroides, compared to individuals who consume diets high in carbohydrates, who have a higher ratio of Prevotella (133).
Several diets have been investigated with regards to their association with nutrient intake and cancer. One of the most studied diets in relation to health and disease is the Mediterranean diet (MD), although it is now accepted that the MD is not so much a dietary pattern as a way of life (134). The MD is high in fruit, vegetables, olive oil (with anacidity rate lower than 0.8%), wholegrains, moderate consumption of fish, red wine and dairy, and low intake of red meats (135). The high consumption of fruit and vegetables means the diet is high in micronutrients and antioxidants with anti-inflammatory properties providing protection to the cell membranes from free radicals, reduction in the proliferation of cancer cells, the prevention of damage to DNA, and reducing pro-inflammatory signaling (136). With one third of cancer mortality being linked to diet and the associated inflammation caused by certain foods, the MD with its naturally anti-inflammatory properties shows promise in reducing the risk of certain cancers. A large study by (137), for instance, found a weak association between anti-inflammatory diet risk of cancer, although oddly, no evidence for protection from CRC. Ricceri, Giraudo (138) found that women who followed the MD had a 50% less risk of developing endometrial cancer than those who either did not follow the MD or had a low adherence to it. Shively, Register (139) found that monkeys who consumed an MD over a prolonged period of time (31 months) had a shift in the microbiome in their mammary glands tenfold in comparison to those consuming a Western-style diet.
In contrast to the MD, the WD is associated with increased risk of a number of non-communicable diseases (NCD) including cancer, and a sharp increase in obesity. The WD is characterized by a high intake of processed foods, meats, refined sugars, sweets and caloric drinks (140). A diet that is high in processed foods and refined sugars is associated with high levels of inflammation and an increased risk of CRC (11), and the low intake of dietary fibre and the increased intake of sugar and fats in the WD is hypothesized as being the leading cause of dysbiosis, notably an increased number of Bacterioides spp. (141). The WD has also been found to have an impact on the diversity of the gut microbiota, with a study on immigrants in the US showing a replacement of Prevotella, which is responsible for breaking down plant fibre, by dominant strains from the Bacteroides genus (142). This shift characterizes the first enterotype described by Güven Gülhan, Nikerel (143), which is marked by high Bacteroides levels and is commonly observed in individuals following a long-term WD. Similarly, levels of Fusobacterium nucleatum, a bacterium linked with CRC, increased following a 2-week WD intervention that consisted of high-fat and low fibre (144). The dysbiosis brought about by the WD also causes the mucosal lining to become thinner and a low-grade persistent inflammation to occur (145).
The documented benefits of fruits and vegetables would suggest adopting a plant-based diet pattern such as a vegan/vegetarian diet would be the best option for a healthy gut microbiome in relation to health and disease. However, a meta-analysis (146) found there was no evidence to support a vegetarian diet in the prevention of cancer mortality compared to a non-vegetarian diet, despite the anti-inflammatory and anti-oxidative effects provided by fruits and vegetables against the development and progression of cancer. In contradiction to this, one study (147) found that Chinese people who consume four to five servings of fruit, vegetables and legumes daily had a reduced risk of cancer mortality, although this could be different for hormone dependent cancers. This is supported by a finding that the consumption of legumes and lentils was linked to a 49% reduction in the risk of cancer mortality (148).
Based on studies such as those above, some attempts have been made to change the gut microbiota with targeted dietary interventions. Wastyk, Fragiadakis (149) assessed the influence of two such dietary interventions in healthy adults, one a plant-based fibre diet, and the second a diet based on fermented foods. The plant-based fibre diet showed no change in alpha diversity but an increase in microbial proteins per gram of stool, possibly showing a change in microbial density due to the increased fibre consumption. Surprisingly, there was no change in the levels of SCFA such as butyrate, which had been reported in other studies. The participants who consumed the fermented food diet showed an increase in alpha diversity as well as a decrease in inflammatory proteins. In another study (6), the “Microbiome Enhancer Diet” (MBD) aimed to ensure more nutritional components reached the colon in order to influence the gut microbiome. The diet was centered around four key components: increased consumption of dietary fibre; increased resistant starch; larger food particle sizes; and as little processed food ingredients as possible. The findings reveled that when compared to the WD, participants on the MBD excreted significantly more calories in their feces meaning they absorbed a smaller percentage of the consumed energy on the MBD compared to the statistically significant (p < 0.0001) higher absorption rate seen in the participants on the WD. Notably, this difference was seen to occur in energy absorption without any changes in the individual’s energy expenditure, feelings of hunger or the amount of food consumed. This study suggests there is a possibility the MBD creates a gut environment where more calories pass through the digestive system unabsorbed, providing metabolic benefits without the need to eat less or experience increased hunger. The reduction in the calories absorbed appears to be as a result of how the gut microbiome interacts with food rather than through behavioral modifications. No change was observed in alpha-diversity, but beta-diversity highlighted a significant difference.
Attempts to modify the gut microbiome through dietary changes face several significant challenges due to the nature of existing microbial communities. The gut microbiome is usually well established in adults and is resilient to temporary changes, actively resisting modification (150). Long-term dietary patterns/ lifestyles of an individual create metabolic pathways and selective pressures that favor the growth of certain bacterial populations. Established microbial colonies are known to occupy niches in the gut. Thus, new beneficial microbes introduced through diet face competitive disadvantage against well-established populations that have optimized their environment over time. Research indicates that creating a change in the gut microbiome with diet requires a sustained intervention lasting a minimum of 6 months (151).
6.2 Physical activity
Dysbiosis and obesity can indirectly affect physical activity levels when obesity-related inflammation, fatigue, or discomfort reduce the individual’s motivation or ability to engage in physical activity. A sedentary lifestyle can further disrupt the gut microbiome, creating a vicious cycle of dysbiosis, reduced physical activity, and weight gain.
Physical activity is widely recognized as a key factor in promoting overall health, reducing the risk of chronic diseases such as obesity and cancer, and influencing gut microbiome composition. Research has demonstrated the connection between physical activity and increased diversity of the gut microbiota: athletes and individuals who partake in regular vigorous exercise exhibit a more diverse gut microbial population. The difference in diversity is not only seen in the number of species but in the types of species, with shifts in bacterial composition (152). Several studies (35, 36, 153) show that exercise has been associated with an increase in microbial richness and the proliferation of beneficial bacteria such as Bifidobacterium, Akkermansia muciniphila, and Faecalibacterium prausnitzii, which are known to have anti-inflammatory effects.
Physical activity has also been shown to increase levels of SCFAs, particularly butyrate, which supports gut barrier integrity (see Section 3.1). Exercise helps prevent the translocation of LPS – a key driver of chronic inflammation and metabolic dysregulation – from the gut into the bloodstream (154). The anti-inflammatory effects of physical activity have been shown to alter the composition of the gut microbiota, reducing circulating LPS, and decreasing the levels of IL-6 and TNF-α. However, not all exercise has the same outcomes with effects depending on intensity, duration, and type of physical activity. Aerobic exercises such as running and cycling have been shown to enhance microbial diversity more than resistance training. Further, a review by Clauss, Gérard (155) has shown that too much exercise can actually cause harm, and excessively high-intensity exercise can lead to an increased gut permeability, a decrease in gut mucus thickness, and dysbiosis. Physical activity is associated with a reduced risk of several types of cancer, including CRC, breast, and prostate cancer (156). There are a number of pathways by which this may come about, including reduction of inflammation and regulation of insulin and glucose metabolism. The cycle of diet-induced dysbiosis, hormonal and sleep disruption, and reduced physical activity creates a self-reinforcing loop that promotes obesity. Obesity, in turn, further exacerbates dysbiosis and the risk of certain cancers.
Physical activity and exercise have been shown to influences gut transit time and motility, which in turn affects microbial composition and function. Physical activity and exercise accelerates the transit time in the gastrointestinal tract, reducing the opportunity for pathogenic bacterial colonization and promoting the growth of beneficial bacteria that can adapt to this environment Studies have shown that moderate-intensity exercise enhances colonic motility and reduces transit time (157, 158). This altered motility affects substrate availability for different microbial populations, favoring the growth of specific bacterial communities that can thrive under these conditions. The enhanced gut motility also aids in the mechanical removal of potential pathogens, reducing their residence time in the gut.
Physical activity and exercise influence the stress response through effects on the hypothalamic–pituitary–adrenal (HPA) axis and the sympathetic nervous system. These neuroendocrine pathways influence gut physiology and microbial composition. Regular moderate exercise reduces chronic stress and cortisol levels (159), which have been associated with increased intestinal permeability and dysbiosis. In contrast, excessive or high-intensity exercise may induce acute stress responses that temporarily affect gut barrier function and microbial composition (160). The integrity of the intestinal barrier is crucial for preventing translocation of bacteria and bacterial products from the gut lumen into systemic circulation, and physical activity has been shown to enhance intestinal barrier function through multiple mechanisms. Moderate exercise upregulates the expression of tight junction proteins that maintain epithelial barrier integrity (161). Exercise also promotes the production of heat shock proteins (HSPs) and intestinal alkaline phosphatase (IAP), which protect against stress-induced damage to the intestinal epithelium and detoxify bacterial endotoxins (162). Improved barrier function prevents bacterial translocation and the subsequent inflammatory response, creating a more favorable environment for beneficial microbes.
While the focus has been on how exercise affects the gut microbiome, it’s important to acknowledge the bidirectional nature of this relationship. Evidence suggests that the gut microbiome may influence exercise performance and adaptations to training. For instance, microbially derived metabolites, particularly SCFAs, enhance energy harvesting, muscle function, and endurance capacity (88).
6.3 Sleep
Emerging research is revealing a complex relationship between gut microbiome composition and sleep patterns. Far from being independent biological processes as previously thought, gut dysbiosis and sleep demonstrate a bidirectional interaction that significantly impacts human health and metabolic function.
The gut microbiome plays an important role in the production of neurotransmitters (see Section 4.4), particularly serotonin and melatonin, which are fundamental to sleep regulation. Approximately 95% of the body’s serotonin and a significant portion of melatonin are produced in the gut, highlighting the microbiome’s direct neurochemical influence (103). Disruptions in microbial composition can alter these critical neurotransmitter pathways, potentially compromising sleep quality and circadian rhythms. An imbalance in the microbial population can trigger increased production of pro-inflammatory cytokines, which disrupt normal sleep architecture. These inflammatory markers activate neural pathways that interfere with sleep onset, maintenance, and overall quality (163). The circadian rhythm governs not only sleep–wake cycles but also microbial populations, with disrupted sleep leading to shifts in microbial diversity, potentially reducing beneficial bacterial populations and promoting inflammatory microorganisms (164). Poor sleep has been shown to alter the gut microbiome composition: a decrease in sleep duration and quality associated with gut dysbiosis creates a negative feedback loop with sleep deprivation causing an increase in appetite hormones, cravings for calorie-dense foods, reduced metabolic efficiency and decreased physical activity (165). These factors contribute to weight gain and obesity, further exacerbating gut microbiome imbalances. Disrupted sleep patterns have been linked to cancer risk, with growing evidence suggesting that the gut microbiome may also play a role in this relationship. Sleep deprivation and disruptions to the circadian rhythm, such as those seen in shift can lead to gut dysbiosis with a reduction in abundance of species such as Bifidobacterium leading to inflammation (166). Furthermore, a lack of quality sleep is linked to increased levels of pro-inflammatory which create a pro-tumorigenic environment and accelerates cancer progression (167).
7 Stress, anxiety, and the gut: bidirectional interactions
The connection between psychological states and gut function is a strong example of mind–body interaction. The gut-brain axis, a well-established network linking the central and enteric nervous systems, acts as the key pathway through which mental states impact digestive processes and vice versa. Understanding these interactions offers insights for developing integrated approaches to managing both psychological and gastrointestinal disorders. The gut-brain axis encompasses multiple pathways that enable bidirectional communication between the central nervous system and the gastrointestinal tract. For example, the vagus nerve is the primary component of the parasympathetic nervous system innervating the gut (the “second brain”), and 80–90% of its component nerve fibres are afferent carriers of information from the gut to the brain. The hypothalamic–pituitary–adrenal axis, a neuroendocrine system that controls stress responses and influences gut function through the release of corticotropin-releasing factor (CRF), adrenocorticotropic hormone (ACTH), and cortisol, and through microbial signaling that can affect brain function, including neurotransmitters such as serotonin, gamma-aminobutyric acid (GABA), and SCFAs.
Acute stress speeds up the transit time in the colon whilst delaying gastric emptying, a pattern mediated primarily by CRF. In animal models, CRF administration mimics stress-induced alterations in gut motility, while CRF antagonists block these effects (168). In humans, these changes to motility are thought to be an evolutionarily adaptation, preparing the organism for “fight or flight” by diverting resources away from digestion. However, chronic activation of this system can lead to motility issues. Stress and anxiety contribute to “leaky gut,” through alterations to the tight junction proteins and disruption of the intestinal mucus layer (169). Transferring fecal microbiota from depressed patients to microbiota-depleted rats showed an increase in intestinal permeability and depressive-like behaviors (170). These stress and anxiety-induced changes in turn induce significant alterations in gut microbial composition, with a reduction in microbial diversity, a decrease in beneficial species, an increase in pathogenic species, and an alteration in metabolite production. These changes have been noted as taking place within a relatively short period of time after the stressor has occurred in mice (171). Diets that have a particularly high intake of refined sugar, significantly influence the gut microbiome and, consequently, anxiety levels. This has been demonstrated in Western-style diets, with their high refined sugars and associated selective promotion of pathogenic bacteria (e.g., certain Clostridia). High-sugar diets decrease the abundance of bacteria that produce butyrate and other SCFAs, which are crucial for maintaining intestinal barrier integrity and have anti-inflammatory properties. Lower SCFA levels are associated with increased gut permeability and systemic inflammation. a reduction in beneficial SCFA-producing bacteria, decreased microbial diversity, and increased intestinal permeability (172, 173). Magnusson, Hauck (174) demonstrated that high-sugar diets promoted the growth of Proteobacteria while reducing beneficial Bacteroidetes, leading to intestinal dysbiosis. This dysbiosis was correlated with increased anxiety-like behaviors in rodent models (175). Stress and depression have been seen to reduce physical activity through pathways such low energy levels, reduced pleasure in activities, and poor sleep pattern (27, 176). Studies have consistently shown that individuals with depression engage in significantly less physical activity than non-depressed controls (177). One meta-analysis (178) found that people with depression are 50–60% more likely to be physically inactive compared to the general population.
The interplay between depression, physical activity, and gut health appears to create a self-perpetuating cycle in which depression reduces physical activity, leading to alterations in gut microbiome composition and function. This gut dysbiosis contributes to intestinal inflammation and increased permeability, allowing inflammatory mediators and bacterial translocation to influence brain function. The resulting neuroinflammation exacerbates depressive symptoms, further deepening the cycle by reducing physical activity even more. Cancer diagnosis has a huge psychological impact on an individual. Lee, Nam (179) found that psychiatric disorders were common in patients with cancer and patients with cancer and a newly diagnosed psychiatric disorder had a higher mortality rate. This supports (180) who highlighted that between 30 and 60% of cancer patients had a psychiatric disorder such as extreme stress, depression, anxiety and insomnia. As of yet there are no studies showing how the mechanistic role of the gut microbiome influences stress, depression and anxiety in relation to cancer.
8 The promising potential of microbial reprogramming
The reprogramming of the gut microbiome through therapeutic interventions has recently been shown as a promising tool to address a number of diseases. This includes the use of probiotics to restore microbial balance and fecal microbiota transplant (FMT) which has been used to completely replace a recipient’s gut microbiome.
8.1 Microbial reprogramming via probiotics
Probiotics – live microorganisms that, when administered in adequate amounts, confer a health benefit on the host – are another avenue for potentially reprogramming the gut microbiome (181). The idea is that introducing beneficial bacteria can help to restore balance to the gut, although the effects can be variable and depend on the specific strains used, the individual’s existing microbiome, and other factors such as diet. Probiotics can be used to modulate the gut microbiota by releasing SCFA such as butyric or acetic acid, which can help restore balance to a microbiome in dysbiosis and help to improve intestinal permeability and gut barrier function (182). Probiotics such as Lactobacillus and Bifidobacterium can control obesity through regulating the functions of the hosts own gut microbiome (183). It has been shown in animal models that probiotics have the potential to produce anticancer effects through the regulation of the gut microbiota and thus achieve immune modulation to reduce chronic inflammation by modulating both Toll-like receptors (TLRs) and G-protein coupled receptors (GPRs), lowering intestinal pH, and inhibiting enzymes that produce carcinogens (184, 185). However, it is worth noting that the effects and results from the use of probiotics are strain-specific and so may vary from individual to individual (186).
8.2 Microbial reprogramming via fecal microbiota transplant
FMT is being explored as a strategy to reprogram the gut microbiome in the context of obesity and related metabolic disorders. It represents one of the most direct interventions for microbiome reprogramming currently available (187). While traditional dietary and probiotic approaches offer incremental changes to the gut ecosystem, FMT provides a complete microbial community transfer, potentially offering more rapid and comprehensive microbiome reprogramming. Although the use of fecal matter is not new, with ancient Chinese medicine using ‘yellow soup’ as a treatment for diarrhea, it was not until the early 21st century that FMT has gained recognition as a viable tool for treating gut dysbiosis and its associated diseases. A start has been made to use FMT to treat a number of diseases such as inflammatory bowel disease (IBD). A systematic review by Paramsothy, Paramsothy (188) found 41 studies with overall clinical remission rates of 36% for ulcerative colitis, 50.5% for Crohn’s disease, and 21.5% for pouchitis following FMT. Research investigating the gut-brain axis has also prompted exploration of FMT for neuropsychiatric conditions. Preliminary studies in autism spectrum disorder (ASD) have shown promising results: Kang, Adams (189) reported improved gastrointestinal and behavioral symptoms in children with ASD following microbiota transfer therapy, with benefits persisting two years post-treatment. In relation to obesity, a meta-analysis looking at 10 studies for a total of 334 participants showed that individuals who received FMT showed a negative association with calorie intake, fasting glucose levels, and total cholesterol (190). Zhang, Zuo (191) showed minor weight loss in obese patients who received FMT and an increase in Bacteroides in the mucosal microbiome in the colon, but FMT appeared to have less influence over changing the composition of the microbiota of the small intestine.
While FMT may be a direct and potentially powerful intervention, there are still subtilties in its use. For instance, two studies (192, 193) showed no difference in fat mass, lean mass, or metabolic parameters in individuals who had received FMT after 12 weeks. In addition, a study comparing young mice (3 months) vs. older mice (24 months) found that key species were transferred between the mice despite the differences in age, with the older mice developing an increased intestinal barrier when receiving FMT from the young mice (194). However, when the young mice received FMT from the older mice the inflammatory cytokine levels of IL- and TNF- became elevated to match that of the older mice donors. This study shows the influence of the FMT on the recipient and why the screening and history of the donor is vital before considering any transplant. Despite these caveats, FMT is firmly established for treating recurrent C. difficile infection (195), and its potential extends to numerous conditions with emerging evidence supporting applications in inflammatory, metabolic, and neuropsychiatric disorders. In the context of cancer, FMT has been seen as a way to improve the efficacy of immunotherapy and a reduction in associated toxic events. Species such as Bifidobacterium fragilis were found to have anti-cancer properties (196). As the interplay between gut microbiota, health and disease becomes more understood, FMT will likely play an increasingly important role in microbiome reprogramming across a number of diseases.
9 Conclusion
Lifestyle plays a role in the development of both obesity and cancer, with factors such as diet, physical activity, and sleep known to influence disease risk and progression. This review proposes a mechanistic model that places the gut microbiome at the intersection of these lifestyle factors and disease processes. Dysbiosis of the gut microbiota has been strongly associated not only with obesity but also with the development and progression of cancers such as colorectal and breast cancers where mechanistic pathways are best characterized, and there is emerging but compelling evidence for pancreatic and other malignancies (15, 17, 128). Microbial species have been implicated in promoting obesity through mechanisms such as enhanced energy harvest from the diet, increased fat storage, modulation of appetite regulation, disruption of circadian rhythms, and the promotion of low-grade chronic inflammation (5, 38, 51, 53).
The review demonstrates a cycle of lifestyle-induced dysbiosis that promotes obesity, which further disrupts the microbial balance. These pathways offer promising targets for intervention, as shown by initiatives like the BE GONE trial, which targeted microbiome modulation in obese patients with history of colorectal neoplasia to mitigate cancer risk. At the end of the 16 weeks trial the study reported an increased alpha diversity in the participants who consumed the prebiotics foods (197).
Studies have highlighted how physical activity can be an influential microbiome modulator with implications for cancer prevention. Beyond its established benefits for energy balance and systemic inflammation, exercise creates distinct alterations in gut microbial ecosystems through physiological mechanisms (198, 199). Regular physical activity enhances microbiome diversity, a key indicator of gut health whilst simultaneously enriching beneficial bacterial species associated with improved metabolic health and immune function (200, 201). Exercise-induced changes in transit time, mucosal immunity, and bile acid metabolism collectively help to shape the intestinal environment, promoting microbial profiles linked to a reduced cancer risk (202, 203).
While many studies report associations between physical activity, diet, and microbial diversity, only a few interventional trials have demonstrated causative links of lifestyle factors to cancer outcomes. One such trial was conducted by Wastyk, Fragiadakis (149), who found that diet significantly influenced the gut microbiome, which in turn affects immune function. In a 17-week randomized study involving healthy adults, the researchers compared the effects of two dietary interventions, a high-fibre diet, and a diet focused on fermented foods. The fermented food diet led to a steady increase in microbiota diversity and a reduction in inflammation. These findings suggest that fermented foods may be particularly effective in improving microbiome health and lowering inflammation. A recent meta-analysis showed consumption of fermented dairy products such as yogurt was significantly linked with a decreased risk of cancers such as bladder, CRC and esophageal. Kefir, a fermented milk, has also been shown to have potential in the prevention and treatment of cancer through its anti-bacterial and anti-inflammatory properties (149, 204, 205).
Significant gaps remain in understanding the precise mechanisms and how they interact with modifiable lifestyle factors. Future research would benefit from studies that can establish causality rather than correlation, examining how physical activity, dietary patterns, and sleep quality can modulate microbiome composition over time. Additional to this is the importance of addressing individual variability by exploring how genetics, sex, age, and the individual’s environment can influence the microbiome in relation to lifestyle interventions (Figure 3).
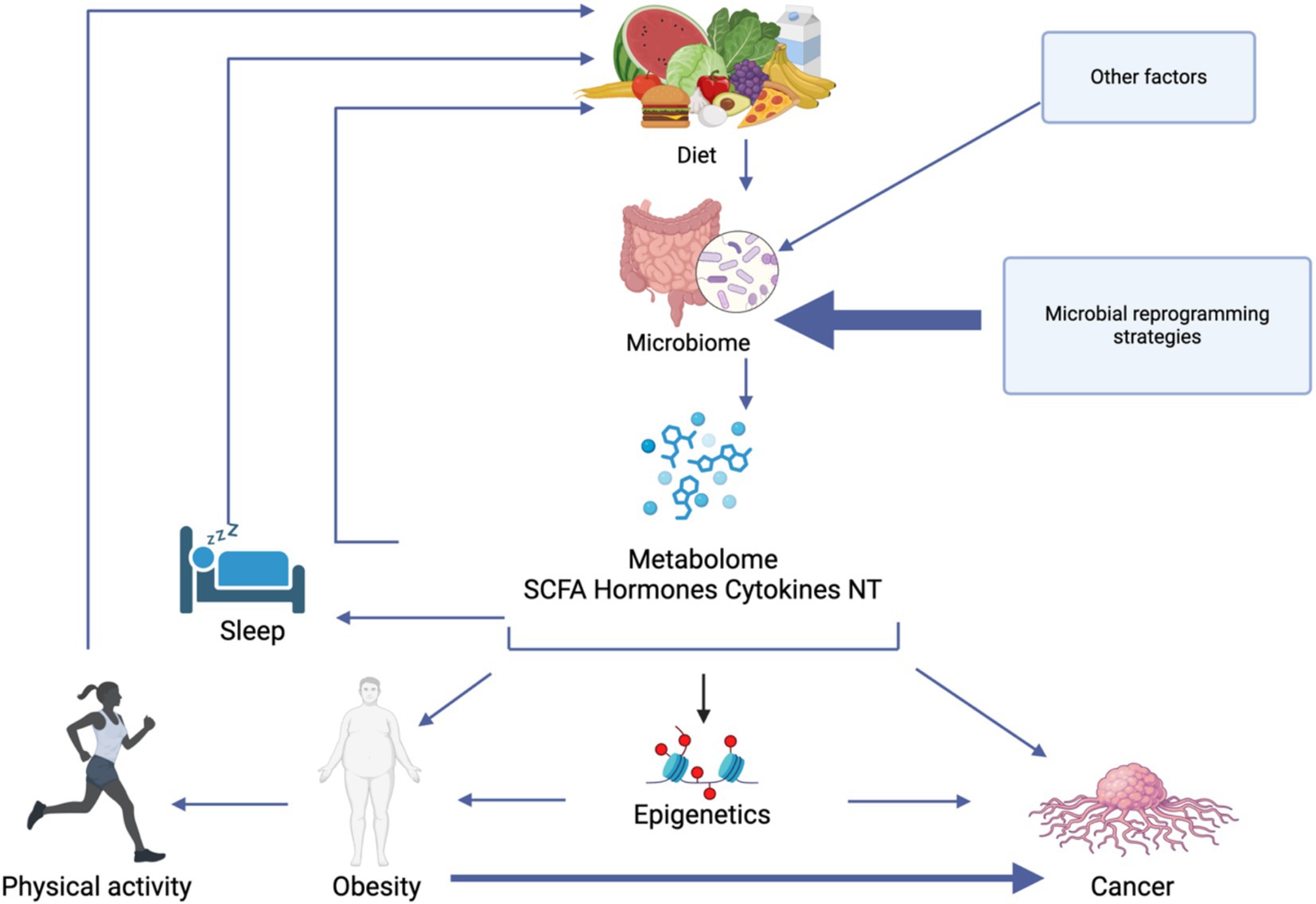
Figure 3. A model of the complex interplay between lifestyle factors, gut microbiome composition, and metabolic outcomes that influence obesity and cancer development. Diet directly modulates the gut microbiome, which in turn produces various metabolites including short-chain fatty acids (SCFAs), hormones, cytokines, and neurotransmitters (NT). These metabolites mediate numerous physiological effects, including epigenetic modifications that can influence both obesity and cancer pathways. Sleep quality and physical activity both influence and are influenced by the microbiome-metabolite axis. Created in BioRender.
Author contributions
CG: Methodology, Visualization, Writing – original draft. RM: Writing – original draft. EA: Supervision, Writing – review & editing. GB: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The publication of this work was funded by the Weill Cornell Medicine-Qatar Health Sciences Library and the Premedical Education Department.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Heath Organization. Obesity and overweight (2024). Available online at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed March 15, 2025).
2. Gaskell, C, Lutimba, S, Bendriss, G, and Aleem, E. Obesity, physical activity, and cancer incidence in two geographically distinct populations; the Gulf cooperation council countries and the United Kingdom-a systematic review and meta-analysis. Cancers. (2024) 16:4206. doi: 10.3390/cancers16244205
3. Bray, F, Laversanne, M, Sung, H, Ferlay, J, Siegel, RL, Soerjomataram, I, et al. Global Cancer statistics 2022: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
4. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global Cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
5. Cronin, P, Joyce, SA, O'Toole, PW, and O'Connor, EM. Dietary fibre modulates the gut microbiota. Nutrients. (2021) 13:1655. doi: 10.3390/nu13051655
6. Corbin, KD, Carnero, EA, Dirks, B, Igudesman, D, Yi, F, Marcus, A, et al. Host-diet-gut microbiome interactions influence human energy balance: a randomized clinical trial. Nat Commun. (2023) 14:3161. doi: 10.1038/s41467-023-38778-x
7. Leong, KSW, Derraik, JGB, Hofman, PL, and Cutfield, WS. Antibiotics, gut microbiome and obesity. Clin Endocrinol. (2018) 88:185–200. doi: 10.1111/cen.13495
8. Kyrgiou, M, Kalliala, I, Markozannes, G, Gunter, MJ, Paraskevaidis, E, Gabra, H, et al. Adiposity and Cancer at major anatomical sites: umbrella review of the literature. BMJ. (2017) 356:j477. doi: 10.1136/bmj.j477
9. Alsheridah, N, and Akhtar, S. Diet, obesity and colorectal carcinoma risk: results from a National Cancer Registry-Based Middle-Eastern Study. BMC Cancer. (2018) 18:1227. doi: 10.1186/s12885-018-5132-9
10. Bradbury, KE, Murphy, N, and Key, TJ. Diet and colorectal Cancer in Uk biobank: a prospective study. Int J Epidemiol. (2020) 49:246–58. doi: 10.1093/ije/dyz064
11. Song, M, Garrett, WS, and Chan, AT. Nutrients, foods, and colorectal Cancer prevention. Gastroenterology. (2015) 148:1244–60.e16. doi: 10.1053/j.gastro.2014.12.035
12. Valciukiene, J, Lastauskiene, E, Laurinaviciene, A, Jakubauskas, M, Kryzauskas, M, Valkiuniene, RB, et al. Interaction of human gut microbiota and local immune system in progression of colorectal adenoma (Mimica-1): a protocol for a prospective, observational cohort study. Front Oncol. (2024) 14:1495635. doi: 10.3389/fonc.2024.1495635
13. Zepeda-Rivera, M, Minot, SS, Bouzek, H, Wu, H, Blanco-Míguez, A, Manghi, P, et al. A distinct Fusobacterium Nucleatum clade dominates the colorectal Cancer niche. Nature. (2024) 628:424–32. doi: 10.1038/s41586-024-07182-w
14. Baxter, NT, Ruffin, MT, Rogers, MA, and Schloss, PD. Microbiota-based model improves the sensitivity of fecal immunochemical test for detecting colonic lesions. Genome Med. (2016) 8:37. doi: 10.1186/s13073-016-0290-3
15. Song, M, Chan, AT, and Sun, J. Influence of the gut microbiome, diet, and environment on risk of colorectal Cancer. Gastroenterology. (2020) 158:322–40. doi: 10.1053/j.gastro.2019.06.048
16. Wang, Z, Dan, W, Zhang, N, Fang, J, and Yang, Y. Colorectal Cancer and gut microbiota studies in China. Gut Microbes. (2023) 15:2236364. doi: 10.1080/19490976.2023.2236364
17. Fernández, MF, Reina-Pérez, I, Astorga, JM, Rodríguez-Carrillo, A, Plaza-Díaz, J, and Fontana, L. Breast Cancer and its relationship with the microbiota. Int J Environ Res Public Health. (2018) 15:1747. doi: 10.3390/ijerph15081747
18. Del Castillo, E, Meier, R, Chung, M, Koestler, DC, Chen, T, Paster, BJ, et al. The microbiomes of pancreatic and duodenum tissue overlap and are highly subject specific but differ between pancreatic Cancer and noncancer subjects. Cancer Epidemiol Biomarkers Prev. (2019) 28:370–83. doi: 10.1158/1055-9965.Epi-18-0542
19. Kandalai, S, Li, H, Zhang, N, Peng, H, and Zheng, Q. The human microbiome and Cancer: a diagnostic and therapeutic perspective. Cancer Biol Ther. (2023) 24:2240084. doi: 10.1080/15384047.2023.2240084
20. Greathouse, KL, White, JR, Vargas, AJ, Bliskovsky, VV, Beck, JA, von Muhlinen, N, et al. Interaction between the microbiome and Tp53 in human lung Cancer. Genome Biol. (2018) 19:123. doi: 10.1186/s13059-018-1501-6
21. Souza, VGP, Forder, A, Pewarchuk, ME, Telkar, N, de Araujo, RP, Stewart, GL, et al. The complex role of the microbiome in non-small cell lung Cancer development and progression. Cells. (2023) 12:2801. doi: 10.3390/cells12242801
22. Lucaciu, SR, Domokos, B, Puiu, R, Ruta, V, Motoc, SN, Rajnoveanu, R, et al. Lung microbiome in lung Cancer: a systematic review. Microorganisms. (2024) 12:2439. doi: 10.3390/microorganisms12122439
23. Yang, K, Wang, S, Ding, Z, Zhang, K, Zhu, W, Wang, H, et al. Unveiling microbial dynamics in lung adenocarcinoma and adjacent nontumor tissues: insights from nicotine exposure and diverse clinical stages via Nanopore sequencing technology. Front Cell Infect Microbiol. (2024) 14:1397989. doi: 10.3389/fcimb.2024.1397989
24. Okamura, T, Hashimoto, Y, Majima, S, Senmaru, T, Ushigome, E, Nakanishi, N, et al. Trans fatty acid intake induces intestinal inflammation and impaired glucose tolerance. Front Immunol. (2021) 12:669672. doi: 10.3389/fimmu.2021.669672
25. Morell, P, and Fiszman, S. Revisiting the role of protein-induced satiation and satiety. Food Hydrocoll. (2017) 68:199–210. doi: 10.1016/j.foodhyd.2016.08.003
26. Lane, MM, Gamage, E, Du, S, Ashtree, DN, McGuinness, AJ, Gauci, S, et al. Ultra-processed food exposure and adverse health outcomes: umbrella review of epidemiological Meta-analyses. BMJ. (2024) 384:e077310. doi: 10.1136/bmj-2023-077310
27. Gaskell, C, Sarada, P, Aleem, E, and Bendriss, G. Identifying lifestyle factors associated to co-morbidity of obesity and psychiatric disorders, a pilot study. Front Public Health. (2023) 11:1132994. doi: 10.3389/fpubh.2023.1132994
28. Wittekind, DA, Kratzsch, J, Mergl, R, Baber, R, Wirkner, K, Schroeter, ML, et al. Leptin, but not ghrelin, is associated with food addiction scores in a population-based subject sample. Front Psych. (2023) 14:1200021. doi: 10.3389/fpsyt.2023.1200021
29. de Oliveira Melo, NC, Cuevas-Sierra, A, Souto, VF, and Martínez, JA. Biological rhythms, Chrono-nutrition, and gut microbiota: Epigenomics insights for precision nutrition and metabolic health. Biomol Ther. (2024) 14:559. doi: 10.3390/biom14050559
30. Turta, O, and Rautava, S. Antibiotics, obesity and the link to microbes - what are we doing to our children? BMC Med. (2016) 14:57. doi: 10.1186/s12916-016-0605-7
31. Korpela, K, Salonen, A, Virta, LJ, Kekkonen, RA, Forslund, K, Bork, P, et al. Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nat Commun. (2016) 7:10410. doi: 10.1038/ncomms10410
32. Peivasteh-Roudsari, L, Barzegar-Bafrouei, R, Sharifi, KA, Azimisalim, S, Karami, M, Abedinzadeh, S, et al. Origin, dietary exposure, and toxicity of endocrine-disrupting food chemical contaminants: a comprehensive review. Heliyon. (2023) 9:e18140. doi: 10.1016/j.heliyon.2023.e18140
33. de Paula, LCP, and Alves, C. Food packaging and endocrine disruptors. J Pediatr. (2024) 100:S40–7. doi: 10.1016/j.jped.2023.09.010
34. Gupta, R, Kumar, P, Fahmi, N, Garg, B, Dutta, S, Sachar, S, et al. Endocrine disruption and obesity: a current review on environmental obesogens. Curr Res Green Sustain Chem. (2020) 3:100009. doi: 10.1016/j.crgsc.2020.06.002
35. Allen, JM, Mailing, LJ, Niemiro, GM, Moore, R, Cook, MD, White, BA, et al. Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc. (2018) 50:747–57. doi: 10.1249/mss.0000000000001495
36. Bressa, C, Bailén-Andrino, M, Pérez-Santiago, J, González-Soltero, R, Pérez, M, Montalvo-Lominchar, MG, et al. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS One. (2017) 12:e0171352. doi: 10.1371/journal.pone.0171352
37. Rosenbaum, M, Knight, R, and Leibel, RL. The gut microbiota in human energy homeostasis and obesity. Trends Endocrinol Metab. (2015) 26:493–501. doi: 10.1016/j.tem.2015.07.002
38. Gomes, AC, Hoffmann, C, and Mota, JF. The human gut microbiota: metabolism and perspective in obesity. Gut Microbes. (2018) 9:308–25. doi: 10.1080/19490976.2018.1465157
39. Castaner, O, Goday, A, Park, YM, Lee, SH, Magkos, F, Shiow, STE, et al. The gut microbiome profile in obesity: a systematic review. Int J Endocrinol. (2018) 2018:4095789. doi: 10.1155/2018/4095789
40. Enache, RM, Profir, M, Roşu, OA, Creţoiu, SM, and Gaspar, BS. The role of gut microbiota in the onset and progression of obesity and associated comorbidities. Int J Mol Sci. (2024) 25:12321. doi: 10.3390/ijms252212321
41. Benítez-Páez, A, Gómez Del Pugar, EM, López-Almela, I, Moya-Pérez, Á, Codoñer-Franch, P, and Sanz, Y. Depletion of Blautia species in the microbiota of obese children relates to intestinal inflammation and metabolic phenotype worsening. mSystems. (2020) 5:e00857-19. doi: 10.1128/mSystems.00857-19
42. Xu, Y, Wang, N, Tan, HY, Li, S, Zhang, C, and Feng, Y. Function of Akkermansia Muciniphila in obesity: interactions with lipid metabolism, immune response and gut systems. Front Microbiol. (2020) 11:219. doi: 10.3389/fmicb.2020.00219
43. Mar Rodríguez, M, Pérez, D, Javier Chaves, F, Esteve, E, Marin-Garcia, P, Xifra, G, et al. Obesity changes the human gut Mycobiome. Sci Rep. (2015) 5:14600. doi: 10.1038/srep14600
44. Tandon, D, Haque, MM, R, S, Shaikh, S, P, S, Dubey, AK, et al. A snapshot of gut microbiota of an adult urban population from western region of India. PLoS One. (2018) 13:e0195643. doi: 10.1371/journal.pone.0195643
45. Song, Y, and Yang, JM. Role of interleukin (Il)-17 and t-helper (Th)17 cells in cancer. Biochem Biophys Res Commun. (2017) 493:1–8. doi: 10.1016/j.bbrc.2017.08.109
46. Abuqwider, JN, Mauriello, G, and Altamimi, M. Akkermansia Muciniphila, a new generation of beneficial microbiota in modulating obesity: a systematic review. Microorganisms. (2021) 9:1089. doi: 10.3390/microorganisms9051098
47. Zhou, K. Strategies to promote abundance of Akkermansia Muciniphila, an emerging probiotics in the gut, evidence from dietary intervention studies. J Funct Foods. (2017) 33:194–201. doi: 10.1016/j.jff.2017.03.045
48. Ju, T, Bourrie, BCT, Forgie, AJ, Pepin, DM, Tollenaar, S, Sergi, CM, et al. The gut commensal Escherichia Coli aggravates high-fat-diet-induced obesity and insulin resistance in mice. Appl Environ Microbiol. (2023) 89:e0162822. doi: 10.1128/aem.01628-22
49. Gong, H, Gao, H, Ren, Q, and He, J. The abundance of Bifidobacterium in relation to visceral obesity and serum uric acid. Sci Rep. (2022) 12:13073. doi: 10.1038/s41598-022-17417-3
50. Sharma, D, Gajjar, D, and Seshadri, S. Understanding the role of gut microfloral Bifidobacterium in Cancer and its potential therapeutic applications. Microbiome Res Rep. (2024) 3:3. doi: 10.20517/mrr.2023.51
51. Maioli, TU, Borras-Nogues, E, Torres, L, Barbosa, SC, Martins, VD, Langella, P, et al. Possible benefits of Faecalibacterium Prausnitzii for obesity-associated gut disorders. Front Pharmacol. (2021) 12:740636. doi: 10.3389/fphar.2021.740636
52. Formiga, R, and Sokol, H. Faecalibacterium Prausnitzii: one species with multiple potential implications in Cancer research. Gut. (2024):gutjnl-2024-334338. doi: 10.1136/gutjnl-2024-334338
53. Odunitan, TT, Apanisile, BT, Akinboade, MW, Abdulazeez, WO, Oyaronbi, AO, Ajayi, TM, et al. Microbial mysteries: staphylococcus aureus and the enigma of carcinogenesis. Microb Pathog. (2024) 194:106831. doi: 10.1016/j.micpath.2024.106831
54. Sánchez-Alcoholado, L, Ordóñez, R, Otero, A, Plaza-Andrade, I, Laborda-Illanes, A, Medina, JA, et al. Gut microbiota-mediated inflammation and gut permeability in patients with obesity and colorectal Cancer. Int J Mol Sci. (2020) 21:6782. doi: 10.3390/ijms21186782
55. Facchin, S, Bertin, L, Bonazzi, E, Lorenzon, G, De Barba, C, Barberio, B, et al. Short-chain fatty acids and human health: from metabolic pathways to current therapeutic implications. Life. (2024) 14:559. doi: 10.3390/life14050559
56. den Besten, G, van Eunen, K, Groen, AK, Venema, K, Reijngoud, DJ, and Bakker, BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. (2013) 54:2325–40. doi: 10.1194/jlr.R036012
57. Peng, L, Li, ZR, Green, RS, Holzman, IR, and Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of amp-activated protein kinase in Caco-2 cell monolayers. J Nutr. (2009) 139:1619–25. doi: 10.3945/jn.109.104638
58. Hodgkinson, K, El Abbar, F, Dobranowski, P, Manoogian, J, Butcher, J, Figeys, D, et al. Butyrate's role in human health and the current Progress towards its clinical application to treat gastrointestinal disease. Clin Nutr. (2023) 42:61–75. doi: 10.1016/j.clnu.2022.10.024
59. Liang, L, Liu, L, Zhou, W, Yang, C, Mai, G, Li, H, et al. Gut microbiota-derived butyrate regulates gut mucus barrier repair by activating the macrophage/Wnt/Erk signaling pathway. Clin Sci. (2022) 136:291–307. doi: 10.1042/cs20210778
60. Lin, HV, Frassetto, A, Kowalik, EJ Jr, Nawrocki, AR, Lu, MM, Kosinski, JR, et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. (2012) 7:e35240. doi: 10.1371/journal.pone.0035240
61. Canani, RB, Costanzo, MD, Leone, L, Pedata, M, Meli, R, and Calignano, A. Potential beneficial effects of butyrate in intestinal and Extraintestinal diseases. World J Gastroenterol. (2011) 17:1519–28. doi: 10.3748/wjg.v17.i12.1519
62. Divella, R, De Luca, R, Abbate, I, Naglieri, E, and Daniele, A. Obesity and Cancer: the role of adipose tissue and Adipo-cytokines-induced chronic inflammation. J Cancer. (2016) 7:2346–59. doi: 10.7150/jca.16884
63. Arias, C, Álvarez-Indo, J, Cifuentes, M, Morselli, E, Kerr, B, and Burgos, PV. Enhancing adipose tissue functionality in obesity: Senotherapeutics, autophagy and cellular senescence as a target. Biol Res. (2024) 57:51. doi: 10.1186/s40659-024-00531-z
64. Kern, L, Mittenbühler, MJ, Vesting, AJ, Ostermann, AL, Wunderlich, CM, and Wunderlich, FT. Obesity-induced Tnfα and Il-6 signaling: the missing link between obesity and inflammation-driven liver and colorectal cancers. Cancers (Basel). (2018) 11:24. doi: 10.3390/cancers11010024
65. Avgerinos, KI, Spyrou, N, Mantzoros, CS, and Dalamaga, M. Obesity and Cancer risk: emerging biological mechanisms and perspectives. Metabolism. (2019) 92:121–35. doi: 10.1016/j.metabol.2018.11.001
66. Clemente-Suárez, VJ, Redondo-Flórez, L, Beltrán-Velasco, AI, Martín-Rodríguez, A, Martínez-Guardado, I, Navarro-Jiménez, E, et al. The role of Adipokines in health and disease. Biomedicines. (2023) 11:1290. doi: 10.3390/biomedicines11051290
67. Savulescu-Fiedler, I, Mihalcea, R, Dragosloveanu, S, Scheau, C, Baz, RO, Caruntu, A, et al. The interplay between obesity and inflammation. Life (Basel). (2024) 14:856. doi: 10.3390/life14070856
68. Aleem, E, Nehrbass, D, Klimek, F, Mayer, D, and Bannasch, P. Upregulation of the insulin receptor and type I insulin-like growth factor receptor are early events in Hepatocarcinogenesis. Toxicol Pathol. (2011) 39:524–43. doi: 10.1177/0192623310396905
69. Ray, A, Alalem, M, and Ray, BK. Insulin signaling network in cancer. Indian J Biochem Biophys. (2014) 51:493–8.
70. Deb, A, Deshmukh, B, Ramteke, P, Bhati, FK, and Bhat, MK. Resistin: a journey from metabolism to Cancer. Transl Oncol. (2021) 14:101178. doi: 10.1016/j.tranon.2021.101178
71. Khanna, D, Khanna, S, Khanna, P, Kahar, P, and Patel, BM. Obesity: a chronic low-grade inflammation and its markers. Cureus. (2022) 14:e22711. doi: 10.7759/cureus.22711
72. Yang, HM, Kim, J, Kim, BK, Seo, HJ, Kim, JY, Lee, JE, et al. Resistin regulates inflammation and insulin resistance in humans via the endocannabinoid system. Research. (2024) 7:0326. doi: 10.34133/research.0326
73. Ellulu, MS, Patimah, I, Khaza'ai, H, Rahmat, A, and Abed, Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. (2017) 13:851–63. doi: 10.5114/aoms.2016.58928
74. Brown, KA. Metabolic pathways in obesity-related breast Cancer. Nat Rev Endocrinol. (2021) 17:350–63. doi: 10.1038/s41574-021-00487-0
75. Maqoud, F, Calabrese, FM, Celano, G, Mallardi, D, Goscilo, F, D'Attoma, B, et al. Role of increasing body mass index in gut barrier dysfunction, systemic inflammation, and metabolic dysregulation in obesity. Nutrients. (2024) 17:72. doi: 10.3390/nu17010072
76. Xue, F, He, Z, Zhuang, DZ, and Lin, F. The influence of gut microbiota on circulating inflammatory cytokines and host: a Mendelian randomization study with Meta-analysis. Life Sci. (2023) 332:122105. doi: 10.1016/j.lfs.2023.122105
77. Duan, Y, Xu, Y, Dou, Y, and Xu, D. Helicobacter pylori and gastric Cancer: mechanisms and new perspectives. J Hematol Oncol. (2025) 18:10. doi: 10.1186/s13045-024-01654-2
78. Naaman, SC, Shen, S, Zeytinoglu, M, and Iyengar, NM. Obesity and breast Cancer risk: the oncogenic implications of metabolic dysregulation. J Clin Endocrinol Metab. (2022) 107:2154–66. doi: 10.1210/clinem/dgac241
79. Chen, Y, Liu, L, Zhou, Q, Imam, MU, Cai, J, Wang, Y, et al. Body mass index had different effects on premenopausal and postmenopausal breast Cancer risks: a dose-response Meta-analysis with 3,318,796 subjects from 31 cohort studies. BMC Public Health. (2017) 17:936. doi: 10.1186/s12889-017-4953-9
80. Harvey, SV, Wentzensen, N, Bertrand, K, Black, A, Brinton, LA, Chen, C, et al. Associations of life course obesity with endometrial Cancer in the epidemiology of endometrial Cancer consortium (E2c2). Int J Epidemiol. (2023) 52:1086–99. doi: 10.1093/ije/dyad046
81. Kitson, SJ, and Crosbie, EJ. Endometrial cancer and obesity. Obstet Gynaecol. (2019) 21:237–45. doi: 10.1111/tog.12601
82. Lee, KR, Hwang, IC, Han, KD, Jung, J, and Seo, MH. Waist circumference and risk of breast Cancer in Korean women: a Nationwide cohort study. Int J Cancer. (2018) 142:1554–9. doi: 10.1002/ijc.31180
83. Onstad, MA, Schmandt, RE, and Lu, KH. Addressing the role of obesity in endometrial Cancer risk, prevention, and treatment. J Clin Oncol. (2016) 34:4225–30. doi: 10.1200/jco.2016.69.4638
84. Baker, JM, Al-Nakkash, L, and Herbst-Kralovetz, MM. Estrogen-gut microbiome Axis: physiological and clinical implications. Maturitas. (2017) 103:45–53. doi: 10.1016/j.maturitas.2017.06.025
85. Hu, S, Ding, Q, Zhang, W, Kang, M, Ma, J, and Zhao, L. Gut Microbial Beta-Glucuronidase: A Vital Regulator in Female Estrogen Metabolism. Gut Microbes. (2023) 15:2236749. doi: 10.1080/19490976.2023.2236749
86. Park, JW, Han, K, Shin, DW, Yeo, Y, Chang, JW, Yoo, JE, et al. Obesity and breast Cancer risk for pre- and postmenopausal women among over 6 million Korean women. Breast Cancer Res Treat. (2021) 185:495–506. doi: 10.1007/s10549-020-05952-4
87. Choi, IY, Choi, YJ, Shin, DW, Han, KD, Jeon, KH, Jeong, SM, et al. Association between obesity and the risk of gastric cancer in premenopausal and postmenopausal women: a nationwide cohort study. J Gastroenterol Hepatol. (2021) 36:2834–40. doi: 10.1111/jgh.15558
88. Patel, BK, Patel, KH, Lee, CN, and Moochhala, S. Intestinal microbiota interventions to enhance athletic performance-a review. Int J Mol Sci. (2024) 25:10076. doi: 10.3390/ijms251810076
89. Chimento, A, De Luca, A, Avena, P, De Amicis, F, Casaburi, I, Sirianni, R, et al. Estrogen receptors-mediated apoptosis in hormone-dependent cancers. Int J Mol Sci. (2022) 23:1242. doi: 10.3390/ijms23031242
90. Loh, NY, Wang, W, Noordam, R, and Christodoulides, C. Obesity, fat distribution and risk of cancer in women and men: a Mendelian randomisation study. Nutrients. (2022) 14:5259. doi: 10.3390/nu14245259
91. Plaza-Díaz, J, Álvarez-Mercado, AI, Ruiz-Marín, CM, Reina-Pérez, I, Pérez-Alonso, AJ, Sánchez-Andujar, MB, et al. Association of Breast and gut Microbiota Dysbiosis and the risk of breast Cancer: a case-control clinical study. BMC Cancer. (2019) 19:495. doi: 10.1186/s12885-019-5660-y
92. Han, M, Wang, N, Han, W, Ban, M, Sun, T, and Xu, J. Gut microbes in gynecologic cancers: causes or biomarkers and therapeutic potential. Front Oncol. (2022) 12:902695. doi: 10.3389/fonc.2022.902695
93. Zhao, S, Gu, J, Tian, Y, Wang, R, and Li, W. Low levels of sex hormone-binding globulin predict an increased breast Cancer risk and its underlying molecular mechanisms. Open Life Sci. (2024) 19:20220822. doi: 10.1515/biol-2022-0822
94. Li, Y, Liu, G, Gong, R, and Xi, Y. Gut microbiome dysbiosis in patients with endometrial cancer vs. healthy controls based on 16s Rrna gene sequencing. Curr Microbiol. (2023) 80:239. doi: 10.1007/s00284-023-03361-6
95. Sobstyl, M, Brecht, P, Sobstyl, A, Mertowska, P, and Grywalska, E. The role of microbiota in the Immunopathogenesis of endometrial Cancer. Int J Mol Sci. (2022) 23:5756. doi: 10.3390/ijms23105756
96. Daniels, JP, Freedland, SJ, and Gresham, G. The growing implications of obesity for prostate Cancer risk and mortality: where do we go from Here? J Natl Cancer Inst. (2023) 115:1448–50. doi: 10.1093/jnci/djad140
97. Agalliu, I, Lin, WJ, Zhang, JS, Jacobson, JS, Rohan, TE, Adusei, B, et al. Overall and central obesity and prostate cancer risk in African men. Cancer Causes Control. (2022) 33:223–39. doi: 10.1007/s10552-021-01515-0
98. Chau, CH, Till, C, Price, DK, Goodman, PJ, Neuhouser, ML, Pollak, MN, et al. Serum markers, obesity and prostate Cancer risk: results from the prostate Cancer prevention trial. Endocr Relat Cancer. (2022) 29:99–109. doi: 10.1530/erc-21-0107
99. Hurwitz, LM, Dogbe, N, Barry, KH, Koutros, S, and Berndt, SI. Obesity and prostate Cancer screening, incidence, and mortality in the prostate, lung, colorectal, and ovarian Cancer screening trial. J Natl Cancer Inst. (2023) 115:1506–14. doi: 10.1093/jnci/djad113
100. Vidal, AC, Oyekunle, T, Howard, LE, De Hoedt, AM, Kane, CJ, Terris, MK, et al. Obesity, race, and Long-term prostate Cancer outcomes. Cancer. (2020) 126:3733–41. doi: 10.1002/cncr.32906
101. Golombos, DM, Ayangbesan, A, O'Malley, P, Lewicki, P, Barlow, L, Barbieri, CE, et al. The role of gut microbiome in the pathogenesis of prostate cancer: a prospective, pilot study. Urology. (2018) 111:122–8. doi: 10.1016/j.urology.2017.08.039
102. Bendriss, G, MacDonald, R, and McVeigh, C. Microbial reprogramming in obsessive-compulsive disorders: a review of gut-brain communication and emerging evidence. Int J Mol Sci. (2023) 24:11978. doi: 10.3390/ijms241511978
103. Appleton, J. The gut-brain axis: influence of microbiota on mood and mental health. Integr Med. (2018) 17:28–32.
104. van Galen, KA, Ter Horst, KW, and Serlie, MJ. Serotonin, food intake, and obesity. Obes Rev. (2021) 22:e13210. doi: 10.1111/obr.13210
105. Yano, JM, Yu, K, Donaldson, GP, Shastri, GG, Ann, P, Ma, L, et al. Indigenous Bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. (2015) 161:264–76. doi: 10.1016/j.cell.2015.02.047
106. Otaru, N, Ye, K, Mujezinovic, D, Berchtold, L, Constancias, F, Cornejo, FA, et al. Gaba production by human intestinal Bacteroides Spp.: prevalence, regulation, and role in acid stress tolerance. Front Microbiol. (2021) 12:656895. doi: 10.3389/fmicb.2021.656895
107. Nagao, T, Braga, JD, Chen, S, Thongngam, M, Chartkul, M, Yanaka, N, et al. Synergistic effects of peripheral Gaba and Gaba-transaminase inhibitory drugs on food intake control and weight loss in high-fat diet-induced obese mice. Front Pharmacol. (2024) 15:1487585. doi: 10.3389/fphar.2024.1487585
108. Ma, X, Yan, H, Hong, S, Yu, S, Gong, Y, Wu, D, et al. Gamma-aminobutyric acid promotes beige adipocyte reconstruction by modulating the gut microbiota in obese mice. Nutrients. (2023) 15:456. doi: 10.3390/nu15020456
109. Kravitz, AV, O'Neal, TJ, and Friend, DM. Do dopaminergic impairments underlie physical inactivity in people with obesity? Front Hum Neurosci. (2016) 10:514. doi: 10.3389/fnhum.2016.00514
110. Ryu, V, and Buettner, C. Fat cells gobbling up norepinephrine? PLoS Biol. (2019) 17:e3000138. doi: 10.1371/journal.pbio.3000138
111. Miller, GD. Appetite regulation: hormones, peptides, and neurotransmitters and their role in obesity. Am J Lifestyle Med. (2019) 13:586–601. doi: 10.1177/1559827617716376
112. Kumar, R, Rizvi, MR, and Saraswat, S. Obesity and stress: a contingent paralysis. Int J Prev Med. (2022) 13:95. doi: 10.4103/ijpvm.IJPVM_427_20
113. Balakrishna, P, George, S, Hatoum, H, and Mukherjee, S. Serotonin pathway in Cancer. Int J Mol Sci. (2021) 22:1268. doi: 10.3390/ijms22031268
114. Wang, X, Wang, Y, Xie, F, Song, ZT, Zhang, ZQ, Zhao, Y, et al. Norepinephrine promotes glioma cell migration through up-regulating the expression of Twist1. BMC Cancer. (2022) 22:213. doi: 10.1186/s12885-022-09330-9
115. Dinan, TG, and Cryan, JF. The microbiome-gut-brain axis in health and disease. Gastroenterol Clin N Am. (2017) 46:77–89. doi: 10.1016/j.gtc.2016.09.007
116. Cole, SW, Nagaraja, AS, Lutgendorf, SK, Green, PA, and Sood, AK. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer. (2015) 15:563–72. doi: 10.1038/nrc3978
117. Jiang, SH, Hu, LP, Wang, X, Li, J, and Zhang, ZG. Neurotransmitters: emerging targets in cancer. Oncogene. (2020) 39:503–15. doi: 10.1038/s41388-019-1006-0
118. Hullar, MA, and Fu, BC. Diet, the gut microbiome, and epigenetics. Cancer J. (2014) 20:170–5. doi: 10.1097/ppo.0000000000000053
119. Tourancheau, A, Mead, EA, Zhang, XS, and Fang, G. Discovering multiple types of DNA methylation from Bacteria and microbiome using Nanopore sequencing. Nat Methods. (2021) 18:491–8. doi: 10.1038/s41592-021-01109-3
120. Iyengar, NM, Gucalp, A, Dannenberg, AJ, and Hudis, CA. Obesity and Cancer mechanisms: tumor microenvironment and inflammation. J Clin Oncol. (2016) 34:4270–6. doi: 10.1200/jco.2016.67.4283
121. Blücher, C, and Stadler, SC. Obesity and breast Cancer: current insights on the role of fatty acids and lipid metabolism in promoting breast Cancer growth and progression. Front Endocrinol. (2017) 8:293. doi: 10.3389/fendo.2017.00293
122. Kim, JW, Kim, JH, and Lee, YJ. The role of Adipokines in tumor progression and its association with obesity. Biomedicines. (2024) 12:97. doi: 10.3390/biomedicines12010097
123. Feng, Y, Ramnarine, VR, Bell, R, Volik, S, Davicioni, E, Hayes, VM, et al. Metagenomic and Metatranscriptomic analysis of human prostate microbiota from patients with prostate Cancer. BMC Genomics. (2019) 20:146. doi: 10.1186/s12864-019-5457-z
124. Haque, S, Raina, R, Afroze, N, Hussain, A, Alsulimani, A, Singh, V, et al. Microbial Dysbiosis and epigenetics modulation in Cancer development - a Chemopreventive approach. Semin Cancer Biol. (2022) 86:666–81. doi: 10.1016/j.semcancer.2021.06.024
125. Mei, S, Deng, Z, Chen, Y, Ning, D, Guo, Y, Fan, X, et al. Dysbiosis: the first hit for digestive system Cancer. Front Physiol. (2022) 13:1040991. doi: 10.3389/fphys.2022.1040991
126. Wong-Rolle, A, Wei, HK, Zhao, C, and Jin, C. Unexpected guests in the tumor microenvironment: microbiome in Cancer. Protein Cell. (2021) 12:426–35. doi: 10.1007/s13238-020-00813-8
127. Nejman, D, Livyatan, I, Fuks, G, Gavert, N, Zwang, Y, Geller, LT, et al. The human tumor microbiome is composed of tumor type-specific intracellular Bacteria. Science. (2020) 368:973–80. doi: 10.1126/science.aay9189
128. Pollini, T, Adsay, V, Capurso, G, Dal Molin, M, Esposito, I, Hruban, R, et al. The tumour immune microenvironment and microbiome of pancreatic Intraductal papillary mucinous neoplasms. Lancet Gastroenterol Hepatol. (2022) 7:1141–50. doi: 10.1016/s2468-1253(22)00235-7
129. Katongole, P, Sande, OJ, Joloba, M, Reynolds, SJ, and Niyonzima, N. The human microbiome and its link in prostate Cancer risk and pathogenesis. Infect Agent Cancer. (2020) 15:53. doi: 10.1186/s13027-020-00319-2
130. Liang, J, Li, T, Zhao, J, Wang, C, and Sun, H. Current understanding of the human microbiome in glioma. Front Oncol. (2022) 12:781741. doi: 10.3389/fonc.2022.781741
131. Mohamed, ZS, Wu, Q, Jacome, MA, Chen, J, and Etame, AB. The role of gut microbiome on glioblastoma Oncogenesis and malignant evolution. Int J Mol Sci. (2025) 26:2935. doi: 10.3390/ijms26072935
132. Zhang, H, Hong, Y, Wu, T, Ben, E, Li, S, Hu, L, et al. Role of gut microbiota in regulating immune checkpoint inhibitor therapy for glioblastoma. Front Immunol. (2024) 15:1401967. doi: 10.3389/fimmu.2024.1401967
133. Amabebe, E, Robert, FO, Agbalalah, T, and Orubu, ESF. Microbial Dysbiosis-induced obesity: role of gut microbiota in homoeostasis of energy metabolism. Br J Nutr. (2020) 123:1127–37. doi: 10.1017/s0007114520000380
134. Serra-Majem, L, Román-Viñas, B, Sanchez-Villegas, A, Guasch-Ferré, M, Corella, D, and La Vecchia, C. Benefits of the mediterranean diet: epidemiological and molecular aspects. Mol Asp Med. (2019) 67:1–55. doi: 10.1016/j.mam.2019.06.001
135. Zheng, Y, Meng, L, Liu, H, Sun, L, Nie, Y, Wu, Q, et al. Let food be thy medicine: the role of diet in colorectal Cancer: a narrative review. J Gastrointest Oncol. (2022) 13:2020–32. doi: 10.21037/jgo-22-32
136. Klement, RJ, and Pazienza, V. Impact of different types of diet on gut microbiota profiles and cancer prevention and treatment. Medicina (Kaunas). (2019) 55:84. doi: 10.3390/medicina55040084
137. Bodén, S, Myte, R, Wennberg, M, Harlid, S, Johansson, I, Shivappa, N, et al. The inflammatory potential of diet in determining Cancer risk; a prospective investigation of two dietary pattern scores. PLoS One. (2019) 14:e0214551. doi: 10.1371/journal.pone.0214551
138. Ricceri, F, Giraudo, MT, Fasanelli, F, Milanese, D, Sciannameo, V, Fiorini, L, et al. Diet and endometrial Cancer: a focus on the role of fruit and vegetable intake, Mediterranean diet and dietary inflammatory index in the endometrial Cancer risk. BMC Cancer. (2017) 17:757. doi: 10.1186/s12885-017-3754-y
139. Shively, CA, Register, TC, Appt, SE, Clarkson, TB, Uberseder, B, Clear, KYJ, et al. Consumption of Mediterranean versus Western diet leads to distinct mammary gland microbiome populations. Cell Rep. (2018) 25:47–56.e3. doi: 10.1016/j.celrep.2018.08.078
140. Solans, M, Castelló, A, Benavente, Y, Marcos-Gragera, R, Amiano, P, Gracia-Lavedan, E, et al. Adherence to the Western, prudent, and Mediterranean dietary patterns and chronic lymphocytic leukemia in the mcc-Spain study. Haematologica. (2018) 103:1881–8. doi: 10.3324/haematol.2018.192526
141. Tomasello, G, Mazzola, M, Leone, A, Sinagra, E, Zummo, G, Farina, F, et al. Nutrition, oxidative stress and intestinal Dysbiosis: influence of diet on gut microbiota in inflammatory bowel diseases. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. (2016) 160:461–6. doi: 10.5507/bp.2016.052
142. Vangay, P, Johnson, AJ, Ward, TL, Al-Ghalith, GA, Shields-Cutler, RR, Hillmann, BM, et al. Us immigration westernizes the human gut microbiome. Cell. (2018) 175:962–72.e10. doi: 10.1016/j.cell.2018.10.029
143. Güven Gülhan, Ü, Nikerel, E, Çakır, T, Erdoğan Sevilgen, F, and Durmuş, S. Species-level identification of Enterotype-specific microbial markers for colorectal Cancer and adenoma. Mol Omics. (2024) 20:397–416. doi: 10.1039/d4mo00016a
144. O'Keefe, SJ, Li, JV, Lahti, L, Ou, J, Carbonero, F, Mohammed, K, et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun. (2015) 6:6342. doi: 10.1038/ncomms7342
145. Malesza, IJ, Malesza, M, Walkowiak, J, Mussin, N, Walkowiak, D, Aringazina, R, et al. High-fat, Western-style diet, systemic inflammation, and gut microbiota: a narrative review. Cells. (2021) 10:3164. doi: 10.3390/cells10113164
146. Molina-Montes, E, Salamanca-Fernández, E, Garcia-Villanova, B, and Sánchez, MJ. The impact of plant-based dietary patterns on Cancer-related outcomes: a rapid review and Meta-analysis. Nutrients. (2020) 12:2010. doi: 10.3390/nu12072010
147. Liu, BN, Liu, XT, Liang, ZH, and Wang, JH. Gut microbiota in obesity. World J Gastroenterol. (2021) 27:3837–50. doi: 10.3748/wjg.v27.i25.3837
148. Papandreou, C, Becerra-Tomás, N, Bulló, M, Martínez-González, M, Corella, D, Estruch, R, et al. Legume consumption and risk of all-cause, cardiovascular, and cancer mortality in the Predimed study. Clin Nutr. (2019) 38:348–56. doi: 10.1016/j.clnu.2017.12.019
149. Wastyk, HC, Fragiadakis, GK, Perelman, D, Dahan, D, Merrill, BD, Yu, FB, et al. Gut-microbiota-targeted diets modulate human immune status. Cell. (2021) 184:4137–53.e14. doi: 10.1016/j.cell.2021.06.019
150. Van Hul, M, Cani, PD, Petitfils, C, De Vos, WM, Tilg, H, and El-Omar, EM. What defines a healthy gut microbiome? Gut. (2024) 73:1893–908. doi: 10.1136/gutjnl-2024-333378
151. Leeming, ER, Johnson, AJ, Spector, TD, and Le Roy, CI. Effect of diet on the gut microbiota: rethinking intervention duration. Nutrients. (2019) 11:2862. doi: 10.3390/nu11122862
152. Aya, V, Jimenez, P, Muñoz, E, and Ramírez, JD. Effects of exercise and physical activity on gut microbiota composition and function in older adults: a systematic review. BMC Geriatr. (2023) 23:364. doi: 10.1186/s12877-023-04066-y
153. Monda, V, Villano, I, Messina, A, Valenzano, A, Esposito, T, Moscatelli, F, et al. Exercise modifies the gut microbiota with positive health effects. Oxidative Med Cell Longev. (2017) 2017:3831972. doi: 10.1155/2017/3831972
154. Varghese, S, Rao, S, Khattak, A, Zamir, F, and Chaari, A. Physical exercise and the gut microbiome: a bidirectional relationship influencing health and performance. Nutrients. (2024) 16:3663. doi: 10.3390/nu16213663
155. Clauss, M, Gérard, P, Mosca, A, and Leclerc, M. Interplay between exercise and gut microbiome in the context of human health and performance. Front Nutr. (2021) 8:637010. doi: 10.3389/fnut.2021.637010
156. Matthews, CE, Moore, SC, Arem, H, Cook, MB, Trabert, B, Håkansson, N, et al. Amount and intensity of leisure-time physical activity and lower Cancer risk. J Clin Oncol. (2020) 38:686–97. doi: 10.1200/jco.19.02407
157. Jensen, MM, Pedersen, HE, Clemmensen, KKB, Ekblond, TS, Ried-Larsen, M, Færch, K, et al. Associations between physical activity and gastrointestinal transit times in people with Normal weight, overweight, and obesity. J Nutr. (2024) 154:41–8. doi: 10.1016/j.tjnut.2023.06.005
158. Stanich, PP, Peck, J, Murphy, C, Porter, KM, and Meyer, MM. Physical activity during video capsule endoscopy correlates with shorter bowel transit time. Endosc Int Open. (2017) 5:E856–60. doi: 10.1055/s-0043-115385
159. De Nys, L, Anderson, K, Ofosu, EF, Ryde, GC, Connelly, J, and Whittaker, AC. The effects of physical activity on cortisol and sleep: a systematic review and Meta-analysis. Psychoneuroendocrinology. (2022) 143:105843. doi: 10.1016/j.psyneuen.2022.105843
160. Clark, A, and Mach, N. Exercise-induced stress behavior, gut-microbiota-brain Axis and diet: a systematic review for athletes. J Int Soc Sports Nutr. (2016) 13:43. doi: 10.1186/s12970-016-0155-6
161. Shin, HE, Kwak, SE, Zhang, DD, Lee, J, Yoon, KJ, Cho, HS, et al. Effects of treadmill exercise on the regulation of tight junction proteins in aged mice. Exp Gerontol. (2020) 141:111077. doi: 10.1016/j.exger.2020.111077
162. Lian, P, Kovynev, A, Wang, L, Pronk, ACM, Verhoeven, A, Giera, M, et al. Exercise training at different intensities induces heat stress, disrupts barrier function and alters microbiota in the gut of mice. eLife. (2024) 13:RP100630. doi: 10.7554/elife.100630.1
163. Irwin, MR. Sleep disruption induces activation of inflammation and heightens risk for infectious disease: role of impairments in thermoregulation and elevated ambient temperature. Temperature. (2023) 10:198–234. doi: 10.1080/23328940.2022.2109932
164. Wankhede, NL, Kale, MB, Kyada, A, M, RM, Chaudhary, K, Naidu, KS, et al. Sleep deprivation-induced shifts in gut microbiota: implications for neurological disorders. Neuroscience. (2025) 565:99–116. doi: 10.1016/j.neuroscience.2024.11.070
165. Papatriantafyllou, E, Efthymiou, D, Zoumbaneas, E, Popescu, CA, and Vassilopoulou, E. Sleep deprivation: effects on weight loss and weight loss maintenance. Nutrients. (2022) 14:1549. doi: 10.3390/nu14081549
166. Matenchuk, BA, Mandhane, PJ, and Kozyrskyj, AL. Sleep, circadian rhythm, and gut microbiota. Sleep Med Rev. (2020) 53:101340. doi: 10.1016/j.smrv.2020.101340
167. Walker, WH 2nd, and Borniger, JC. Molecular mechanisms of cancer-induced sleep disruption. Int J Mol Sci. (2019) 20:2890. doi: 10.3390/ijms20112780
168. Lobo, B, Tramullas, M, Finger, BC, Lomasney, KW, Beltran, C, Clarke, G, et al. The stressed gut: region-specific immune and neuroplasticity changes in response to chronic psychosocial stress. J Neurogastroenterol Motil. (2023) 29:72–84. doi: 10.5056/jnm22009
169. Leigh, SJ, Uhlig, F, Wilmes, L, Sanchez-Diaz, P, Gheorghe, CE, Goodson, MS, et al. The impact of acute and chronic stress on gastrointestinal physiology and function: a microbiota-gut-brain Axis perspective. J Physiol. (2023) 601:4491–538. doi: 10.1113/jp281951
170. Kelly, JR, Kennedy, PJ, Cryan, JF, Dinan, TG, Clarke, G, and Hyland, NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. (2015) 9:392. doi: 10.3389/fncel.2015.00392
171. Kelly, LS, Apple, CG, Gharaibeh, R, Pons, EE, Thompson, CW, Kannan, KB, et al. Stress-related changes in the gut microbiome after trauma. J Trauma Acute Care Surg. (2021) 91:192–9. doi: 10.1097/ta.0000000000003209
172. Di Rienzi, SC, and Britton, RA. Adaptation of the gut microbiota to modern dietary sugars and sweeteners. Adv Nutr. (2020) 11:616–29. doi: 10.1093/advances/nmz118
173. Satokari, R. High intake of sugar and the balance between pro- and anti-inflammatory gut Bacteria. Nutrients. (2020) 12:1348. doi: 10.3390/nu12051348
174. Magnusson, KR, Hauck, L, Jeffrey, BM, Elias, V, Humphrey, A, Nath, R, et al. Relationships between diet-related changes in the gut microbiome and cognitive flexibility. Neuroscience. (2015) 300:128–40. doi: 10.1016/j.neuroscience.2015.05.016
175. Luo, X, Yang, X, Tan, S, Zhang, Y, Liu, Y, Tian, X, et al. Gut microbiota mediates anxiety-like behaviors induced by chronic infection of toxoplasma Gondii in mice. Gut Microbes. (2024) 16:2391535. doi: 10.1080/19490976.2024.2391535
176. Wanjau, MN, Möller, H, Haigh, F, Milat, A, Hayek, R, Lucas, P, et al. Physical activity and depression and anxiety disorders: a systematic review of reviews and assessment of causality. AJPM Focus. (2023) 2:100074. doi: 10.1016/j.focus.2023.100074
177. Rutherford, ER, Vandelanotte, C, Chapman, J, and To, QG. Associations between depression, domain-specific physical activity, and BMI among US adults: Nhanes 2011-2014 cross-sectional data. BMC Public Health. (2022) 22:1618. doi: 10.1186/s12889-022-14037-4
178. Schuch, F, Vancampfort, D, Firth, J, Rosenbaum, S, Ward, P, Reichert, T, et al. Physical activity and sedentary behavior in people with major depressive disorder: a systematic review and Meta-analysis. J Affect Disord. (2017) 210:139–50. doi: 10.1016/j.jad.2016.10.050
179. Lee, SA, Nam, CM, Kim, YH, Kim, TH, Jang, SI, and Park, EC. Impact of onset of psychiatric disorders and psychiatric treatment on mortality among patients with Cancer. Oncologist. (2020) 25:e733–42. doi: 10.1634/theoncologist.2019-0396
180. Anuk, D, Özkan, M, Kizir, A, and Özkan, S. The characteristics and risk factors for common psychiatric disorders in patients with Cancer seeking help for mental health. BMC Psychiatry. (2019) 19:269. doi: 10.1186/s12888-019-2251-z
181. Maftei, NM, Raileanu, CR, Balta, AA, Ambrose, L, Boev, M, Marin, DB, et al. The potential impact of probiotics on human health: an update on their health-promoting properties. Microorganisms. (2024) 12:234. doi: 10.3390/microorganisms12020234
182. Bell, V, Ferrão, J, Pimentel, L, Pintado, M, and Fernandes, T. One health, fermented foods, and gut microbiota. Food Secur. (2018) 7:195. doi: 10.3390/foods7120195
183. Torres, B, Sánchez, MC, Virto, L, Llama-Palacios, A, Ciudad, MJ, and Collado, L. Use of probiotics in preventing and treating excess weight and obesity. A systematic review. Obes Sci Pract. (2024) 10:e759. doi: 10.1002/osp4.759
184. Green, M, Arora, K, and Prakash, S. Microbial medicine: prebiotic and probiotic functional foods to target obesity and metabolic syndrome. Int J Mol Sci. (2020) 21:2890. doi: 10.3390/ijms21082890
185. Lu, K, Dong, S, Wu, X, Jin, R, and Chen, H. Probiotics in cancer. Front Oncol. (2021) 11:638148. doi: 10.3389/fonc.2021.638148
186. Śliżewska, K, Markowiak-Kopeć, P, and Śliżewska, W. The role of probiotics in cancer prevention. Cancers (Basel). (2020) 13:20. doi: 10.3390/cancers13010020
187. Al-Ali, D, Ahmed, A, Shafiq, A, McVeigh, C, Chaari, A, Zakaria, D, et al. Fecal microbiota transplants: a review of emerging clinical data on applications, efficacy, and risks (2015–2020). Qatar Med J. (2021) 2021:5. doi: 10.5339/qmj.2021.5
188. Paramsothy, S, Paramsothy, R, Rubin, DT, Kamm, MA, Kaakoush, NO, Mitchell, HM, et al. Faecal microbiota transplantation for inflammatory bowel disease: a systematic review and Meta-analysis. J Crohns Colitis. (2017) 11:1180–99. doi: 10.1093/ecco-jcc/jjx063
189. Kang, DW, Adams, JB, Gregory, AC, Borody, T, Chittick, L, Fasano, A, et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome. (2017) 5:10. doi: 10.1186/s40168-016-0225-7
190. Zecheng, L, Donghai, L, Runchuan, G, Yuan, Q, Qi, J, Yijia, Z, et al. Fecal microbiota transplantation in obesity metabolism: a Meta analysis and systematic review. Diabetes Res Clin Pract. (2023) 202:110803. doi: 10.1016/j.diabres.2023.110803
191. Zhang, F, Zuo, T, Wan, Y, Xu, Z, Cheung, C, Li, AY, et al. Multi-Omic analyses identify mucosa Bacteria and fecal metabolites associated with weight loss after fecal microbiota transplantation. Innovation. (2022) 3:100304. doi: 10.1016/j.xinn.2022.100304
192. Allegretti, JR, Kassam, Z, Mullish, BH, Chiang, A, Carrellas, M, Hurtado, J, et al. Effects of fecal microbiota transplantation with oral capsules in obese patients. Clin Gastroenterol Hepatol. (2020) 18:e2:855–63. doi: 10.1016/j.cgh.2019.07.006
193. Yu, EW, Gao, L, Stastka, P, Cheney, MC, Mahabamunuge, J, Torres Soto, M, et al. Fecal microbiota transplantation for the improvement of metabolism in obesity: the Fmt-trim double-blind placebo-controlled pilot trial. PLoS Med. (2020) 17:e1003051. doi: 10.1371/journal.pmed.1003051
194. Parker, A, Romano, S, Ansorge, R, Aboelnour, A, Le Gall, G, Savva, GM, et al. Fecal microbiota transfer between young and aged mice reverses hallmarks of the aging gut, eye, and brain. Microbiome. (2022) 10:68. doi: 10.1186/s40168-022-01243-w
195. Bednárik, DS, Földvári-Nagy, KC, Simon, V, Rancz, A, Gede, N, Veres, DS, et al. Comparative effectiveness of different therapies for Clostridioides difficile infection in adults: a systematic review and network Meta-analysis of randomized controlled trials. Lancet Reg Health Eur. (2025) 49:101151. doi: 10.1016/j.lanepe.2024.101151
196. Xu, H, Cao, C, Ren, Y, Weng, S, Liu, L, Guo, C, et al. Antitumor effects of fecal microbiota transplantation: implications for microbiome modulation in Cancer treatment. Front Immunol. (2022) 13:949490. doi: 10.3389/fimmu.2022.949490
197. Zhang, X, Irajizad, E, Hoffman, KL, Fahrmann, JF, Li, F, Seo, YD, et al. Modulating a prebiotic food source influences inflammation and immune-regulating gut microbes and metabolites: insights from the be Gone trial. EBioMedicine. (2023) 98:104873. doi: 10.1016/j.ebiom.2023.104873
198. Allen, JM, Mailing, LJ, Cohrs, J, Salmonson, C, Fryer, JD, Nehra, V, et al. Exercise training-induced modification of the gut microbiota persists after microbiota colonization and attenuates the response to chemically-induced colitis in Gnotobiotic mice. Gut Microbes. (2018) 9:115–30. doi: 10.1080/19490976.2017.1372077
199. Kern, T, Blond, MB, Hansen, TH, Rosenkilde, M, Quist, JS, Gram, AS, et al. Structured exercise alters the gut microbiota in humans with overweight and obesity-a randomized controlled trial. Int J Obes. (2020) 44:125–35. doi: 10.1038/s41366-019-0440-y
200. Bycura, D, Santos, AC, Shiffer, A, Kyman, S, Winfree, K, Sutliffe, J, et al. Impact of different exercise modalities on the human gut microbiome. Sports. (2021) 9:14. doi: 10.3390/sports9020014
201. Denou, E, Marcinko, K, Surette, MG, Steinberg, GR, and Schertzer, JD. High-intensity exercise training increases the diversity and metabolic capacity of the mouse distal gut microbiota during diet-induced obesity. Am J Physiol Endocrinol Metab. (2016) 310:E982–93. doi: 10.1152/ajpendo.00537.2015
202. Erlandson, KM, Liu, J, Johnson, R, Dillon, S, Jankowski, CM, Kroehl, M, et al. An exercise intervention alters stool microbiota and metabolites among older, sedentary adults. Ther Adv Infect Dis. (2021) 8:20499361211027067. doi: 10.1177/20499361211027067
203. Mailing, LJ, Allen, JM, Buford, TW, Fields, CJ, and Woods, JA. Exercise and the gut microbiome: a review of the evidence, potential mechanisms, and implications for human health. Exerc Sport Sci Rev. (2019) 47:75–85. doi: 10.1249/jes.0000000000000183
204. Sharifi, M, Moridnia, A, Mortazavi, D, Salehi, M, Bagheri, M, and Sheikhi, A. Kefir: a powerful probiotics with anticancer properties. Med Oncol. (2017) 34:183. doi: 10.1007/s12032-017-1044-9
Keywords: bidirectional, cancer, gut microbiota, lifestyle, obesity, physical activity
Citation: Gaskell C, MacDonald R, Aleem E and Bendriss G (2025) Obesity and cancer: unravelling the microbiome’s hidden role. Front. Nutr. 12:1602603. doi: 10.3389/fnut.2025.1602603
Edited by:
Falak Zeb, University of Sharjah, United Arab EmiratesReviewed by:
Florinda Jimenez Vega, Universidad Autónoma de Ciudad Juárez, MexicoXutong Xue, Harvard Medical School, United States
Copyright © 2025 Gaskell, MacDonald, Aleem and Bendriss. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christine Gaskell, Z2Fza2VsYzJAbHNidS5hYy51aw==
 Christine Gaskell
Christine Gaskell Ross MacDonald
Ross MacDonald Eiman Aleem
Eiman Aleem Ghizlane Bendriss
Ghizlane Bendriss