- 1Institute for Applied Research in Public Health, School of Public Health, Nantong University, Nantong, Jiangsu, China
- 2Department of Chronic Non-Communicable Diseases Prevention and Control, Jiangsu Provincial Center for Disease Control and Prevention, Nanjing, Jiangsu, China
- 3Medical Department of Xinglin College, Nantong University, Nantong, Jiangsu, China
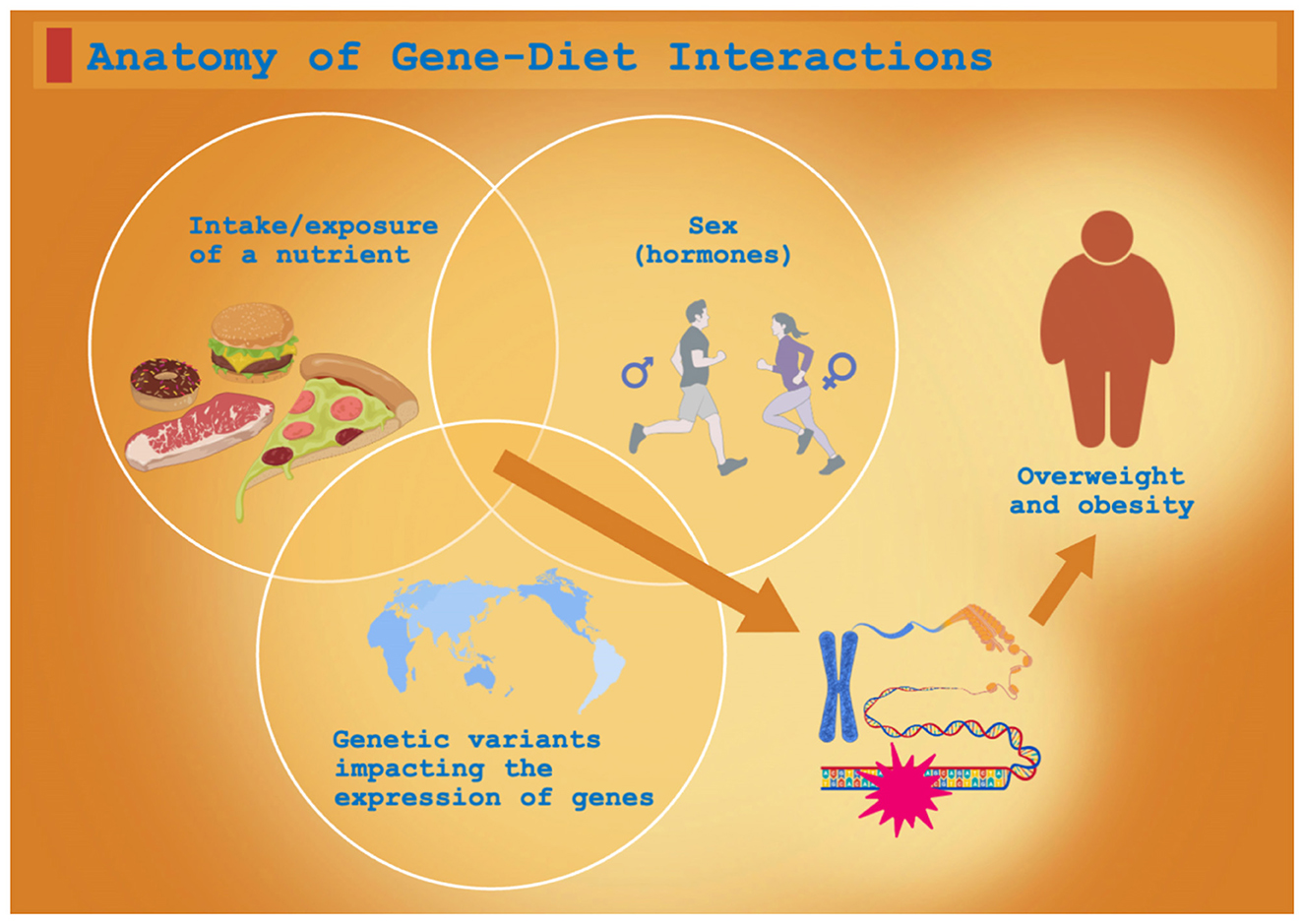
Obesity is a major global public health challenge caused by a complex interplay of genetic predisposition, environmental factors (notably dietary transitions), and their interactions. In 2025, China's National Health Commission (NHC) launched the “Year of Weight Management” initiative during its key policy-setting sessions, generating substantial public and scientific attention. Despite extensive research on obesity, a comprehensive analysis integrating gene–diet interactions with sex differences specifically in Chinese adults remains lacking. This review synthesizes recent advances in understanding obesity-related gene polymorphisms, dietary influences, and gene–diet interactions in Chinese adults, with particular emphasis on multigene synergistic effects. Our analysis demonstrates that such genetic synergism shows potential for predicting obesity intervention outcomes, while gene–diet interactions substantially contribute to obesity heterogeneity. Furthermore, maintaining a balanced dietary structure is particularly crucial for specific genotypes, with sex differences exerting distinct moderating effects. By transcending conventional single-factor analytical frameworks and incorporating population-specific genetic and dietary backgrounds, this study proposes that future research should prioritize developing a three-dimensional gene–diet–metabolic phenotype model (an integrative framework considering genotype, dietary intake, and dynamic metabolic outcomes) to identify critical windows and mechanisms of metabolic dysregulation. This approach holds substantial promise for informing personalized precision strategies for obesity prevention and control.
1 Introduction
Obesity is a chronic and progressive disease that elevates the risk for numerous comorbidities, such as cardiovascular disease, diabetes, other metabolic disorders, depression, and breast cancer (1–3). As such, it represents one of the most pressing public health challenges globally (4). This global concern is mirrored in China, where national survey data reveal that over half of the adult population is now overweight or obese (5), signaling a rapid rise in obesity prevalence. Against this backdrop, the topic of “weight loss for all” gained prominence as a key agenda item during the two sessions of the Healthy China Initiative, highlighting the government's focused commitment to advancing weight management and chronic disease prevention.
The shift from a traditional lifestyle to a modern, obesogenic environment—characterized by pervasive sedentary behaviors, reduced physical activity, and excessive energy intake—is widely recognized as a principal driver of the escalating obesity epidemic in China (6). Within this context, dietary transitions toward increased consumption of cooking oils, animal-source foods, sugar-sweetened products, and energy-dense snacks and fried items have been especially influential (7, 8). It is important to note, however, that while these dietary shifts have collectively driven population-level obesity trends, individual susceptibility to weight gain in response to such an environment varies substantially (9, 10), likely due to genetic susceptibility. Genome-wide association studies (GWAS) have successfully identified multiple obesity susceptibility loci while also elucidating their potential biological mechanisms (11–13). Nevertheless, the collective contribution of these identified single-nucleotide polymorphisms (SNPs) explains less than 5% of the variation in body mass index (BMI)—a figure strikingly lower than the estimated heritability of 40%−70% (14–16). This substantial gap underscores the value of consolidating existing findings to facilitate the discovery of additional loci, potentially through the investigation of gene–gene associations. Recent evidence indicates that gene–environment interactions (GxE) represent a key factor accounting for obesity heterogeneity, providing novel insights into obesity development that cannot be fully explained by genetic susceptibility or dietary intake independently (11, 17).
This review synthesizes recent advances in understanding how dietary factors, specific SNPs, and their interactions influence obesity development, with the aim of informing the design of comprehensive interventions for overweight and obesity in Chinese adults (Figure 1).
2 Methods
This narrative review was conducted to synthesize and critically evaluate recent evidence on the interactions between SNPs, dietary patterns, and overweight/obesity in Chinese adults. The synthesis was structured around several key themes: the influence of dietary factors, the role of specific obesity-related SNPs (with attention to sex and ethnic differences), multigene synergistic effects, and the interplay between genetics and diet.
To elucidate this discussion, a literature search was performed in PubMed, China National Knowledge Infrastructure (CNKI), and Wanfang Data for studies published between January 2018 and April 2025. The literature search and study selection followed a structured process, as detailed in the Supplementary material. Given the substantial heterogeneity in study designs, exposures, and outcomes identified through this process, the evidence was integrated through a narrative synthesis, organizing the findings thematically to provide a coherent overview of the current research landscape.
3 Recent research on the influence of dietary factors on overweight and obesity in Chinese adults
Obesity is a major global public health challenge strongly influenced by multiple factors—with dietary macronutrients playing a key role. The overconsumption of dietary fat, specifically long-chain saturated fats, potently stimulates leptin secretion from expanding adipose tissue. Subsequently, circulating leptin crosses the blood–brain barrier to bind hypothalamic leptin receptors (LepR), primarily activating the JAK2-STAT3 signaling pathway in POMC neurons. This activation ultimately promotes satiety and elevates energy expenditure, thereby facilitating the restoration of energy homeostasis (18, 19). However, chronic overnutrition induces a state of leptin resistance, which disrupts these regulatory pathways and leads to uncontrolled fat deposition (20). Longitudinal evidence shows a positive correlation between dietary fat intake and BMI (21). In contrast, findings from a prospective cohort study suggest that a dietary pattern with moderately high-fat content (30%−40% of energy) was associated with a lower risk of obesity (22). These apparent inconsistencies in macronutrient research highlight the constraints of adopting an isolated, nutrient-centric perspective. The metabolic response to dietary intake is significantly influenced by an individual's genetic makeup—a concept that will be further explored in the following sections. For example, polymorphisms in genes such as the fatty acid desaturase gene cluster 1 (FADS1) can modify fatty acid metabolism efficiency, making health outcomes dependent on both the specific nutrient and the individual's genotype (23–25). Similarly, variants in appetite-regulating genes including, the melanocortin 4 receptor (MC4R) and fat mass and obesity-associated gene (FTO), may enhance susceptibility to weight gain under high-energy diets (26, 27), whereas sex-specific genetic effects [e.g., in ANK4 or ankyrin 1 (ANK1)] can lead to divergent dietary responses between males and females (28). Thus, the heterogeneity commonly observed in nutritional studies likely stems from the intricate interplay between dietary composition and genetic susceptibility, highlighting a critical dimension for future research. Although carbohydrates serve as the body's primary energy source, their relationship with obesity exhibits considerable complexity. Evidence indicates that low-carbohydrate diets may support weight management and enhance metabolic function (29). Notably, research reveals significant gender disparities in this association: higher carbohydrate intake is linked to a reduced risk of overweight and obesity among Chinese women, whereas this relationship remains less defined in men (30). Protein represents a pivotal component in macronutrient balance, with its effects being closely intertwined with the intake levels of carbohydrates and fats (22). Specifically, under free-living conditions, habitual high protein intake has been independently associated with elevated obesity risk (31). In contrast, within the context of specific weight-loss interventions such as ketogenic diets, increased protein consumption may support weight loss by enhancing satiety and promoting energy-expending processes including gluconeogenesis, which can incur an additional energy cost of 400–600 kcal per day (32). Similarly, the relationship between dietary fiber and obesity in Chinese adults exhibits a paradoxical complexity. Longitudinal studies demonstrate that neither total dietary fiber intake nor its intake from various food sources shows a significant association with obesity risk (33). In contrast, circulating levels of short-chain fatty acids (SCFAs)—such as butyrate derived from microbial fermentation of dietary fiber—are positively correlated with both BMI and central obesity (34). This paradox finds a critical explanation in intervention trials, which elucidate that dietary fiber (e.g., from fruits and vegetables) exerts significant benefits on weight reduction and metabolic health when it acts in concert with synbiotics that optimize gut microbiota composition (35).
The seemingly contradictory findings from single-nutrient studies collectively underscore the challenges in formulating consistent conclusions and emphasize the necessity of shifting research attention toward overall dietary patterns. Evidence suggests that specific macronutrient intake ratios (e.g., high-fat, low-carbohydrate patterns) are associated with a significantly elevated risk of obesity, whereas balanced energy distributions correlate with reduced risks of both obesity and related chronic diseases (36). Consequently, adopting a balanced dietary structure emerges as a fundamental strategy for effective obesity management and public health promotion.
The traditional Chinese diet, characterized by its plant-based composition, is increasingly being displaced by Western dietary patterns high in fat, sugar, and processed foods—a transition correlated with elevated obesity risk (37). Indeed, obesity incidence is twice as high among individuals consuming Western diets compared to those following traditional Chinese eating patterns (8). Regionally, traditional southern Chinese diets demonstrate protective effects against obesity (38–40), whereas northern diets—typically richer in refined carbohydrates and sodium—correlate with increased overweight risk, showing a 2.04-fold higher obesity prevalence than southern regions (38). Similarly, studies of Tibetan populations reveal that pastoral diets protect against central obesity (particularly in men), while urban diets high in red meat and refined carbohydrates elevate risks of metabolic syndrome and central adiposity (41, 42).
Dietary behaviors represent another critical dimension in obesity development. The practice of healthy eating habits—such as consuming light diets, selecting moderately textured foods, and eating slowly—has been shown to aid in the control and prevention of metabolic dysfunction (43). Notably, women and adults aged 45–60 years old demonstrate heightened susceptibility to unhealthy dietary practices (43). Beyond diet, other modifiable lifestyle factors, including smoking and alcohol consumption, constitute significant risk factors for abnormal BMI. Evidence indicates that smoking exhibits a dual association between underweight and overweight/obesity, whereas alcohol intake specifically elevates the risk of overweight and obesity. Collectively, these findings emphasize the multifactorial nature of lifestyle influences on BMI and underscore the importance of implementing integrated interventions that concurrently address these interrelated risk factors (44).
4 Recent progress on SNPs related to overweight and obesity in Chinese adults
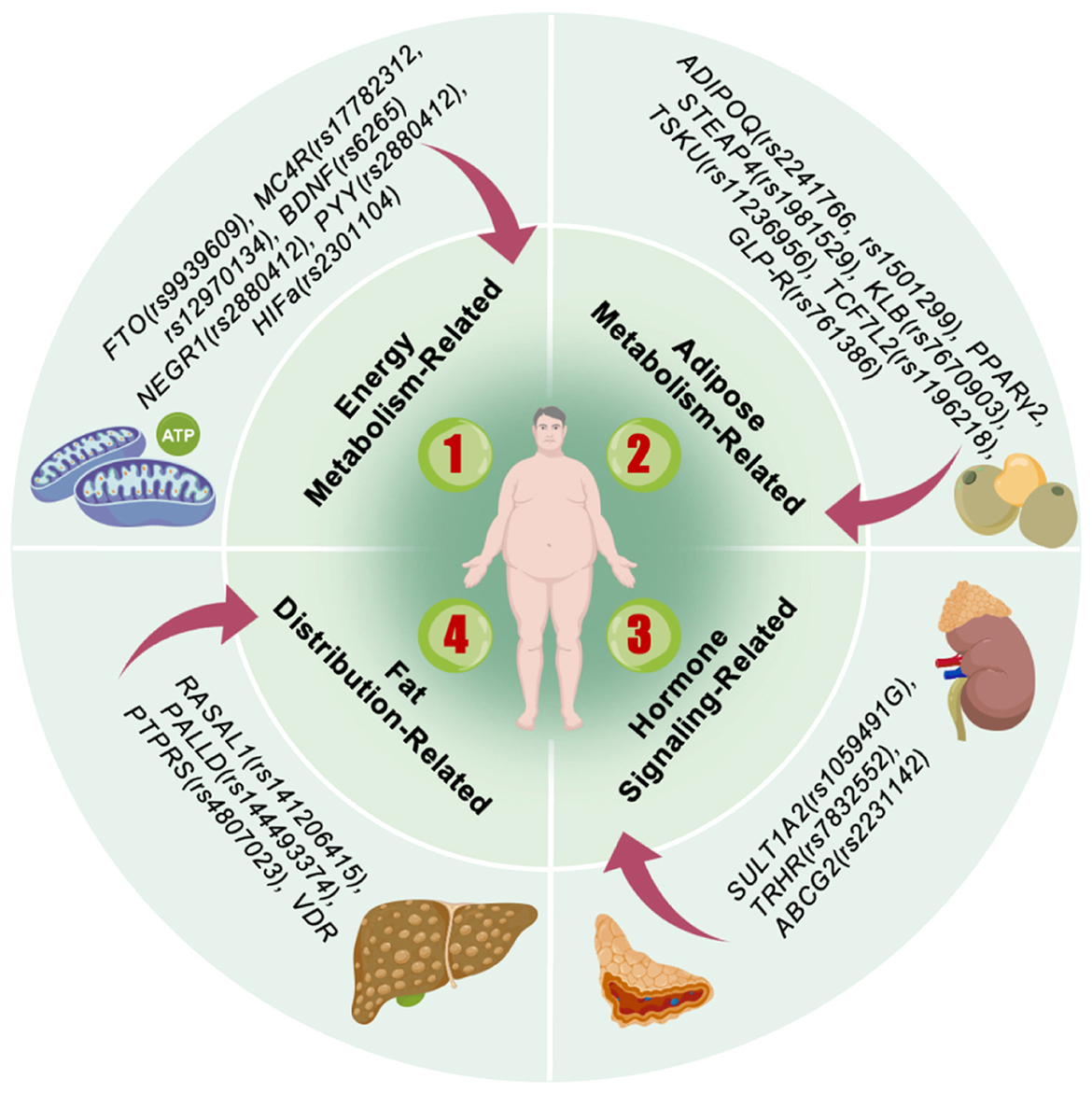
Obesity, commonly termed polygenic obesity, represents a quantitative trait arising from variations at multiple SNP loci. Collectively, these modest-effect variants are estimated to account for only approximately 5% of the observed heritability in obesity (14). Nevertheless, recent genome-wide investigations have revealed that obesity-associated SNP loci consistently demonstrate specific biological mechanisms. These include modulation of appetite regulatory pathways—exemplified by genes encoding brain-derived neurotrophic factor (BDNF) and MC4R—as well as genes participating in insulin secretion, energy metabolism, and lipogenesis, such as FTO, transcription factor 7-like 2 (TCF7L2), insulin receptor substrate 1 (IRS1), and forkhead box O3 (FOXO3) (13). This section systematically examines the relationship between SNPs (Figure 2) and overweight/obesity in Chinese adults, with particular emphasis on underlying functional abnormalities, sex- and ethnicity-specific regulation, and the synergistic effects exerted by multiple genes at key loci.
4.1 Function and regulation of core SNP loci
4.1.1 Energy metabolism-related genes
The FTO gene, a well-established locus in obesity research, harbors SNPs (e.g., rs9939609) that elevate obesity susceptibility by modulating hunger and appetite pathways or by disrupting inter-locus balance (26, 45). This gene is associated with severe obesity in the Han Chinese population (46). Notably, the rs1121980 and rs17817449 loci showed no significant association with obesity in Tibetan cohorts, indicating ethnic-specific genetic effects (47).
The MC4R gene encodes a receptor pivotal in appetite and energy balance regulation. Its polymorphisms (e.g., rs17782313 and rs12970134) are linked to obesity susceptibility across diverse ethnic groups, including Han Chinese and Tibetan populations, demonstrating cross-ethnic relevance (26, 27, 47–49).
Gong et al. demonstrated that the BDNF polymorphism (rs6265) was correlated with BMI and waist circumference (WC) in sedentary individuals, likely mediated through its indirect role in energy balance via neuroplasticity (26). Liu et al. reported that the peptide YY (PYY) gene polymorphism (rs2880412) displayed a dual effect on obesity susceptibility: the CC genotype elevated obesity risk, whereas the AA genotype conferred protection through the modulation of lipoprotein metabolism [triglycerides (TG)↑ and low-density lipoprotein cholesterol (LDL-C)↓] (50, 51).
Hypoxia-inducible factor 1 alpha (HIF1α), a key transcription factor mediating hypoxic responses and metabolic reprogramming in tumor cells (52), plays a critical role in lipid metabolism—promoting fatty acid synthesis, inhibiting lipolysis and fatty acid oxidation, and enhancing cholesterol metabolism—beyond its established functions in glucose metabolism and angiogenesis. Metabolic reprogramming enables cellular adaptation to physiological or pathological stressors, including hypoxia, nutrient overload, and inflammation (53). HIF1α activation disrupts the balance between glycolysis and fatty acid oxidation, promoting a “compensation-inhibitory model” wherein depleted core metabolic functions are compensated by other genes while non-essential pathways are suppressed (54), thereby exacerbating obesity. In contrast, evidence suggests that the rs2301104 CC/CG genotype may serve as a protective factor against obesity in Chinese adults by reducing pro-inflammatory mediators (55), indicating that the C allele may counteract obesity through modulations in lipid metabolism, energy expenditure, and inflammatory responses. Additionally, ferroptosis signaling has been proposed to limit HIF1α availability, thereby restraining adipose tissue expansion during obesity and alleviating associated metabolic dysregulation (56).
4.1.2 Adipose metabolism-related genes
SNPs in the adiponectin, C1q, and collagen domain-containing (ADIPOQ) gene (rs2241766, rs1501299) are associated with reduced susceptibility to multiple obesity phenotypes, underscoring the importance of their encoded lipocalins in insulin sensitivity and inflammatory regulation (57). The Pro/Ala polymorphism in the peroxisome proliferator-activated receptor gamma 2 (PPARγ2) gene shows geographical stratification, with carriers in southern China displaying elevated obesity risk (58). This pattern may reflect temperature-mediated selection pressure influencing gene expression (59). In the Xinjiang Uyghur population, Zhu et al. (60) reported a significant protective effect for the STEAP4 rs1981529 CC genotype, although its expression decreased during adipocyte differentiation and maturation, indicating a primary role in early-stage obesity development. The klotho beta (KLB) gene encodes Klotho β, an essential co-receptor in the FGF21–KLB–FGFR signaling pathway. Its rs7670903 polymorphism is associated with increased BMI and participates in a cross-regulatory network with non-alcoholic fatty liver disease (NAFLD) pathological processes (61). Emerging genetic targets include the TSKU gene rs11236956-G allele, which has been linked to obesity-related metabolic disorders. Carriers of this allele exhibit elevated serum Tsukushi protein levels, suggesting its potential involvement in obesity development (62). The TCF7L2 and glucagon-like peptide-1 receptor (GLP1-R) genes encode proteins involved in glucose homeostasis and glucose-stimulated insulin secretion. Studies have demonstrated that individuals with the AA/AG genotype of TCF7L2 rs11196218 have a 2.54-fold higher obesity risk compared to GG genotype carriers. Similarly, the GLP1-R rs761386 T allele is recognized as a risk factor for obesity (63).
4.1.3 Hormone signaling-related genes
Carriers of the rs1059491G allele in the sulfotransferase family 1A member 2 (SULT1A2) gene exhibited a 54% reduction in overweight/obesity risk compared to non-carriers, a phenomenon potentially mediated through its location within the PPARγ2 and RXRA transcription factor binding sites (64). Although these associations did not survive multiple testing corrections, they provide novel insights into the potential involvement of metabolic detoxification pathways in obesity regulation (65). Gong et al. demonstrated that the rs7832552 polymorphism in the thyrotropin-releasing hormone receptor (TRHR) gene independently influenced waist circumference variation through the thyroid hormone signaling axis, suggesting the existence of specific endocrine regulatory nodes for central obesity (26). Additionally, the Q141K missense mutation (rs2231142) in the ATP-binding cassette subfamily G member 2 (ABCG2) gene shows association with overweight/obesity independent of FTO effects. The underlying mechanism, possibly involving cross-regulation between uric acid (UA) metabolism and energy homeostasis, requires further investigation (66).
4.1.4 Fat distribution-related genes
A genome-wide association study (GWAS) conducted in a Chinese Han population identified significant associations between the RAS protein activator-like 1 (RASAL1) gene (rs141206415) and the palladin (PALLD) gene (rs144493374) with trunk fat mass, whereas the protein tyrosine phosphatase receptor type S (PTPRS) gene (rs4807023) was significantly correlated with leg fat mass (64). Additionally, Shen et al. reported that the C allele at the Fok1 locus and the T allele at the Apal locus of the vitamin D receptor (VDR) gene were associated with elevated body fat percentage (BFP). Furthermore, the rs2239179 G allele and the Apal locus T allele were linked to increased triceps skinfold thickness, independently of BMI and waist circumference (67). These findings collectively suggest that genetic regulatory pathways for distinct adipose tissue depots may operate through independent mechanisms.
4.2 Sex differences in SNP associations between overweight and obesity in Chinese adults
SNPs associated with overweight and obesity in Chinese adults exhibit pronounced sex differences. For instance, the LGR4 rs11029986 polymorphism significantly influences BMI through the regulation of adipose transformation in females while exerting a more limited effect in males (68, 69). Both BMAL1 rs6486121 and CYP19A1 rs752760 contribute to sexually dimorphic obesity phenotypes, potentially mediated by sex hormone-regulated variations in fat distribution (70–72). Moreover, SNPs in mitochondrial genes also demonstrate sex-specific associations: the MT-ND6 14431T>C mutation correlates with obesity in males, whereas the MT-TF 152T>C mutation elevates obesity risk in females (73). Similarly, the MFN2 rs2295281 T allele and CT genotype are associated with increased overweight/obesity risk in males, while the TT genotype confers elevated risk in females (68). Furthermore, SH2B1—which regulates energy balance through insulin sensitivity and adipocyte function—shows male-specific obesity association at the rs7359397 locus (49).
4.3 The influence of multi-gene synergistic effects on overweight and obesity in Chinese adults
4.3.1 Gene interactions and metabolic imbalance
Specific genotype combinations of MC4R rs17782313 and SH2B1 rs7359397 (TC-CC and TC-CT) confer a substantially elevated obesity risk−15.58-fold higher than other genotypes—through synergistic regulation of the leptin–melanocortin pathway (49). Separately, polymorphisms in the estrogen receptor 1 (ESR1) gene (rs712221 TT) and peroxisome proliferator-activated receptor delta (PPARD) gene (rs2016520 TC/TT) impair estrogen receptor activity and fatty acid oxidation capacity, thereby reducing overall lipid metabolic efficiency. Consequently, even under controlled energy intake, obesity risk increases substantially, indicating that impairments in metabolic efficiency—mediated through disrupted energy balance—represent a central mechanism in obesity pathogenesis (69).
4.3.2 Multigene risk assessment
Polygenic risk score (PRS) enhances obesity risk prediction and facilitates personalized weight management strategies by integrating genome-wide SNPs associated with obesity phenotypes through weighted summation based on GWAS effect sizes (74). This approach demonstrates superior predictive capability compared to single-SNP analyses. For instance, studies integrating FTO variants (rs9930501, rs9930506, and rs9932754) and ADRB2 polymorphisms (rs1042713 and rs1042714) through PRS have successfully predicted obesity susceptibility in Malay–Chinese populations. Notably, when combined with randomized controlled trial (RCT) data, these studies revealed more substantial improvements in inflammatory markers following dietary interventions in high-PRS subgroups (75). However, as a statistical model, PRS cannot elucidate the biological mechanisms through which SNPs influence obesity or intervention responses, and its predictive accuracy may diminish when applied to populations of different genetic backgrounds.
The weighted gene score (WGS) model quantifies genetic risk by calculating the weighted sum of risk alleles across multiple SNP loci, normalized by the total weight. This approach accounts for the differential contribution of variants at distinct loci to disease susceptibility (76). For instance, studies investigating olfactory pathway genes—including olfactory receptor 2A2 (OR2AK2) and olfactory receptor 2L8 (OR2L8)—have revealed their roles in modulating food cue perception, energy allocation, and adipose storage. While individual SNPs within this pathway exhibited both positive and negative correlations with obesity risk, the aggregate WGS of all olfactory-related SNPs demonstrated a significant inverse association with obesity susceptibility (77). It should be noted, however, that the WGS in this study was computed under an additive genetic model, which may underestimate the contribution of SNPs operating through dominance or epistatic effects, potentially introducing bias if key functional variants are overlooked. In a related direction, WGS analysis of G protein-coupled receptor (GPCR) signaling pathway genes has identified specific genotypes linked to obesity risk, providing novel perspectives on the neuroendocrine regulation of energy homeostasis (78).
The generalized multifactor dimensionality reduction (GMDR) method, which accommodates discrete and quantitative covariates, is suitable for analyzing both dichotomous and continuous phenotypes across diverse population-based study designs (79). This approach demonstrates superior cross-validation consistency (CVC) and balanced testing accuracy. For instance, studies using GMDR have identified specific locus combinations—FokI, rs2239179, and Apal—associated with elevated body fat percentage (BFP) susceptibility, while a four-locus combination (FokI, rs2239179, Apal, and rs218980) has been linked to increased triceps skinfold thickness risk (67). However, as GMDR primarily focuses on detecting interactions, it may overlook the main effects of individual SNPs. Therefore, combining GMDR with single-locus analyses (e.g., linear regression) is recommended to obtain more comprehensive insights. Additionally, the computational burden increases exponentially with the number of interaction sites analyzed, which may compromise analysis efficiency, particularly when handling large-scale genomic datasets.
This study transcends conventional single-gene analytical frameworks by underscoring the crucial role of gene–gene interactions in obesity pathogenesis and establishing a predictive model through integration of multi-genomic data, thereby informing the development of precision nutrition strategies.
In summary, investigation of polygenic synergistic effects and their corresponding statistical approaches (e.g., PRS, WGS, and GMDR) has substantially enhanced our understanding of obesity's genetic architecture, overcoming limitations inherent in single-locus analyses. To systematically summarize the core obesity-associated genes addressed in this section and their functional attributes, Table 1 presents key information on these major loci—including biological functions, risk alleles, and related phenotypes. This table organizes principal genetic factors modulating energy homeostasis, adipocyte metabolism, hormonal signaling, and fat distribution in Chinese adults, thereby providing a structured genetic framework for subsequent exploration of interactions between these genetic backgrounds and dietary patterns.
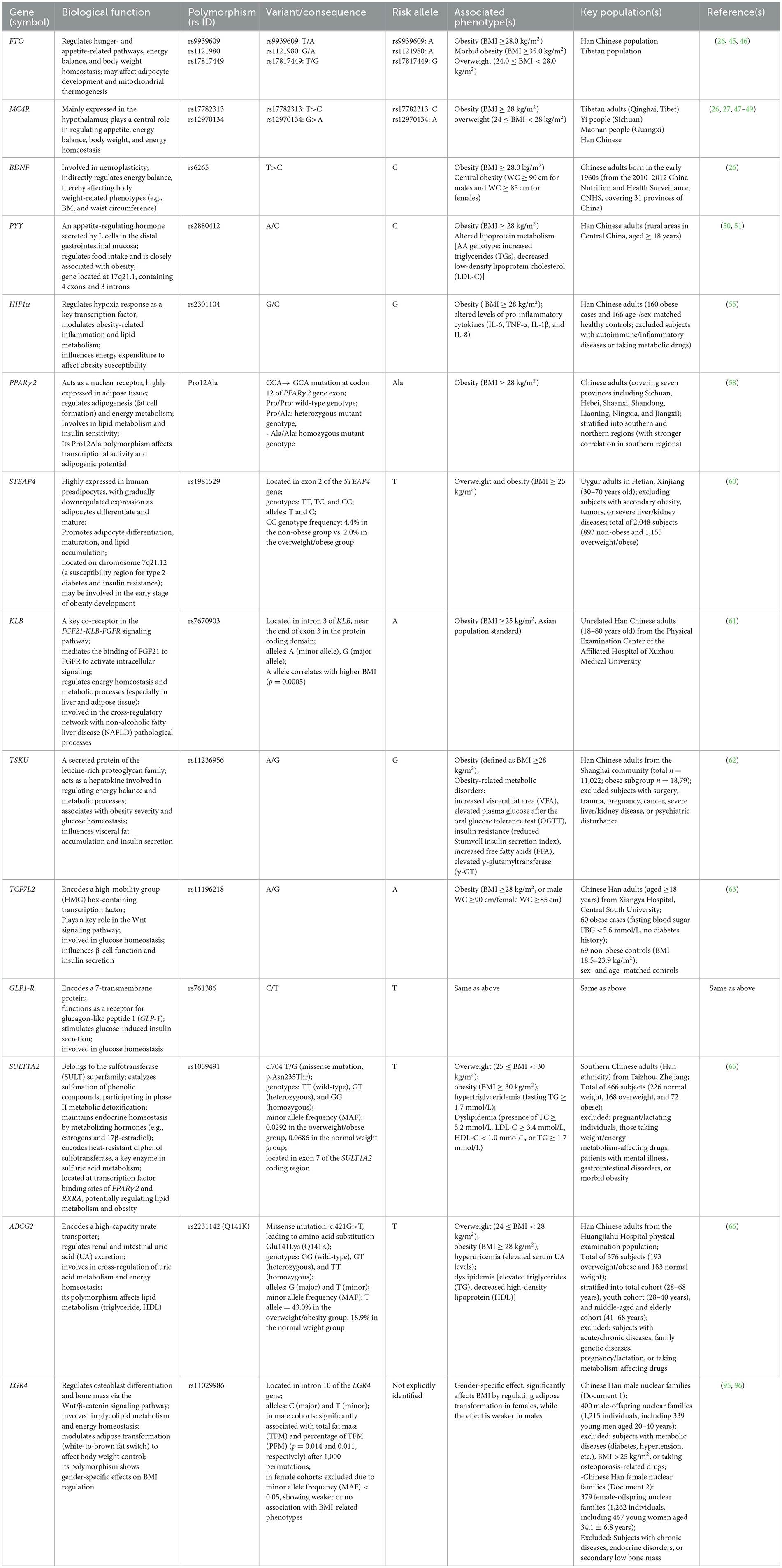
Table 1. Summary of gene polymorphisms associated with obesity and metabolic phenotypes in Chinese populations.
5 Recent progress of interactions between SNPs and diet on overweight and obesity in Chinese adults
While each gene locus exerts a modest effect on obesity, its interaction with dietary factors leads to complex phenotypic outcomes, thereby accounting for the observed variations in nutritional intake behaviors and energy metabolism among individuals with different genetic backgrounds (80).
5.1 The effects of specific dietary interventions in populations with different genotypes are modulated by multiple factors
High-energy diets are prevalent worldwide, and the interaction of MC4R—a key gene regulating appetite and energy balance—plays a critical role. Gong et al. conducted a 2-year prospective cohort study of 2,216 Chinese adults from the 2010–2012 China Nutrition and Health Survey. The researchers found that carriers of the MC4R rs12970134 risk allele experienced an average BMI increase of 0.140 kg/m2 (p = 0.049) when consuming a high-energy diet (defined as total energy intake above the median), compared to wild-type individuals (26). This gene–diet interaction was similarly observed in a Yi migrant population, where carriers of the rs17782313 C/C genotype exhibited a tenfold higher obesity risk following rural-to-urban migration (27), potentially linked to greater exposure to high-fat and high-sugar foods in urban diets. This mechanism further explains the persistent rise in obesity rates in modern Chinese society against a stable genetic background (81).
Further investigating gene–diet interactions, Gong et al. (26) reported that carriers of the FTO rs8050136 risk allele exhibited a 77% increased obesity risk when consuming a high-energy diet. The researchers also observed that FTO was positively correlated with dietary fat intake proportion and inversely correlated with carbohydrate energy share (82), suggesting that this gene influences obesity susceptibility partly through modulating dietary behaviors. This principle—that genetic background shapes dietary response—is further demonstrated in intervention trials. In an 8-week RCT involving Chinese overweight/obese adults (mean baseline BMI ~27 kg/m2), Kuang et al. (83) showed that the weight reduction efficacy of defatted flaxseed flour (13.5 g fiber/100 g) was strictly genotype-dependent. Significant body weight reductions occurred exclusively in carriers of specific alleles: the A allele of FTO rs11076023 (−2.48 kg), the T allele of PCSK1 rs155971 (−2.81 kg), and the A allele of BDNF rs6265 (−2.34 kg), with no significant effect in non-carriers (p for interaction < 0.05 for all). These findings underscore that nutritional interventions can produce divergent effects—even promoting weight gain in certain genetic subgroups—thus challenging the universal application of dietary guidelines and emphasizing the crucial role of nutrigenetics in identifying potential responders (84).
As described previously, PRS further expands the scope of personalized nutrition. In a 6-month RCT involving 128 overweight/obese Malaysian adults (mean baseline BMI ~29.5 kg/m2), Tan and Mitra (75) examined interactions between a PRS—integrating FTO (rs9930501, rs9930506, and rs9932754) and ADRB2 (rs1042713, rs1042714) variants—and a high-protein, energy-restricted, high-vitamin E, high-fiber (Hipcref) diet. Notably, participants in the highest PRS tertile (range: 3.60–8.18) achieved substantially greater weight loss (−4.6 kg) following the Hipcref intervention than those in the lowest tertile (−2.5 kg). Waist circumference reduction was also more pronounced in the high-PRS group (−7.2 vs. −5.2 cm). Moreover, the high-PRS group exhibited a significantly larger decrease in high-sensitivity C-reactive protein (hsCRP) under the Hipcref diet relative to the control diet, indicating an enhanced anti-inflammatory response among genetically predisposed individuals (75).
Nevertheless, the limitations of SNPs in predicting dietary intervention efficacy warrant consideration. Despite adherence to a high-fiber, low-energy diet, carriers of the ESR1 rs712221 TT and PPARD rs2016520 TC/TT genotypes exhibited significantly greater increases in BMI and waist circumference than non-carriers (69). This effect may be explained by the PPARD rs2016520 TC/TT genotype impairing fatty acid oxidation, combined with the ESR1 rs712221 TT genotype disrupting fat distribution regulation. This specific genotypic combination appears to strongly shape obesity predisposition by reprogramming metabolic priorities—an effect that appears resistant to dietary intervention. These findings challenge the generalizability of universal dietary recommendations and highlight the necessity of precision nutrition to identify genotype-imposed constraints on metabolic plasticity. Future research should aim to develop a three-dimensional response map integrating genotype, dietary composition, and metabolic phenotype to facilitate dynamic correction of compensatory metabolic defects.
5.2 Genetic polymorphisms in nutrient metabolism genes modulate dietary responses
In addition to direct regulation, SNPs may also indirectly influence metabolic networks, leading to differences in dietary responses associated with polymorphisms in nutrient metabolic pathways. Metabolic flux redistribution—a process wherein metabolite flow is altered in metabolic networks due to genetic variation, environmental changes, or intervention—can significantly impact cellular physiology. Enzymes encoded by FADS1/2 serve as central catalysts in n-3/n-6 polyunsaturated fatty acid (PUFA) metabolism. Rs174547 TT genotype carriers displayed reduced high-density lipoprotein cholesterol (HDL-C) levels [(1.1 ± 0.3) mmol/L vs. (1.4 ± 0.3) mmol/L] under moderate-to-high linoleic acid (LA) intake (23). This likely stems from the TT genotype impairing FADS1 catalytic efficiency in converting alpha-linolenic acid (ALA) to eicosapentaenoic acid/ docosahexaenoic acid (EPA/DHA; conversion rate only 5%−10%) (24), while high LA further competitively inhibits the n-3 pathway, creating a metabolic bottleneck. Consequently, ALA supplementation failed to reverse the TT phenotype [HDL-c: (1.2 ± 0.3) mmol/L vs. (1.3 ± 0.5) mmol/L, p > 0.05] (25). These findings underscore the need to integrate multi-omics data in future studies to elucidate how key SNPs modulate metabolic flux redistribution and influence obesity, thereby establishing a cross-scale theoretical framework for precision nutrition.
Notably, although n-3 PUFAs are generally considered beneficial for weight management and the rs174570 T allele has been identified as an adaptive marker linked to high-fat fish and n-3 PUFA intake, carriers of this allele showed greater BMI increases following high fish consumption (≥1 time/day) than non-carriers [BMI change: (1.55 ± 0.25) kg/m2 vs. (1.15 ± 0.13) kg/m2] (85). This paradoxical observation may result from linkage disequilibrium between rs174547 and rs174570 (86), which jointly alter desaturase activity and thereby transform conventionally beneficial dietary components into metabolic liabilities in specific genetic backgrounds.
The potent antioxidant properties of haptoglobin (Hp) offer additional insight into the metabolic benefits of dietary interventions (87). Under balanced dietary regimens, Hp 1-1 carriers with elevated free hemoglobin-binding capacity exhibited more substantial reductions in abdominal adiposity (waist circumference: −7.91 cm vs. −5.19 cm) and greater improvement in the homeostasis model assessment of insulin resistance (HOMA-IR) (88). These findings underscore the critical role of antioxidant activity in mitigating oxidative stress induced by high-fat diets. Nevertheless, the influence of the Hp phenotype is dually modulated by pro-inflammatory factors such as IL-6 and TNF-α, as well as nutritional status (89, 90), indicating that future interventions should integrate anti-inflammatory dietary strategies to enhance efficacy.
Research on angiotensin-related genes (e.g., AGTR1/2) further demonstrates that healthy dietary patterns can partially counteract genetically driven adverse lipid profiles through genotype-specific mechanisms. Specifically, only carriers of the AGTR rs1403543 AA genotype exhibited U-shaped distributions of total cholesterol (TC), LDL-C, and HDL-C under the vegetable–fruit–soybean (VFSD) dietary pattern (91), with no significant associations observed for other genotypes or dietary patterns such as REFD. In a similar vein, the small ubiquitin-like modifier 4 (SUMO4) gene rs237025 polymorphism causes an M55V substitution that enhances nuclear factor kappa B (NF-κB) expression, promoting insulin resistance and lipid accumulation in adipocytes. Conversely, Shandong participants carrying the GG genotype showed improved lipid metabolic profiles when adhering to weight management strategies involving dietary modification and salt restriction (92).
5.3 Sex-related gene–diet interaction
Sex differences not only influence SNPs, as discussed in the previous section, but also play a significant role in gene polymorphism–diet interactions.
Notably, the rs516946 T allele of the ankyrin 1 (ANK1) gene significantly counteracted adverse dietary iron effects in men, but not in women (28). This sex-specific effect may be attributed to the absence of cyclic blood loss in males, which enables the ANK1 genotype to mitigate oxidative damage-related metabolic disorders by enhancing iron metabolic efficiency. Furthermore, Zhao et al. demonstrated that rs429358 exerts stronger effects in males by regulating apolipoprotein E (APOE) production and influencing lipid metabolic pathways, representing a key determinant of lipid profiles (93). Their findings further indicated that maintaining ideal body composition in men requires emphasis on high-quality protein sources, with eggs being preferable to red meat (93).
Multiple SNPs are associated with female body shape regulation (Table 2) (93), revealing a complex network of gene–nutrition interactions. For instance, rs4994 regulates ADRB3 protein expression—a key mediator of lipolysis and energy reserve modulation—thereby contributing to individual variations in energy expenditure, with more pronounced effects in females (93). Substantial heterogeneity exists in the metabolic responses to nutrient sources among women with different genotypes. Carriers of either the ANKK1 rs1800497 GG/GA or FABP2 rs3751812 GG genotype, for example, exhibit synergistic benefits for body size maintenance when consuming nuts and other high-quality fat sources. Carbohydrate responsiveness also varies genotypically: the rs1800497 AA and rs1800544 GG genotypes show particularly distinct responses (93). The AA genotype reduces dopamine receptor D2 (DRD2) density, promoting increased sugar consumption for neural reward stimulation and thereby elevating obesity risk (93). In contrast, the G allele elevates fasting glucose levels in Chinese individuals, predisposing them to central obesity. Macronutrient source specificity is further evidenced by the rs662799 polymorphism, wherein AG and AA genotypes respond differentially to plant vs. animal proteins (93). Interestingly, fried food consumption does not appear to adversely affect body shape among AG-genotype women, a subgroup well-represented in the Chinese Han population (93).
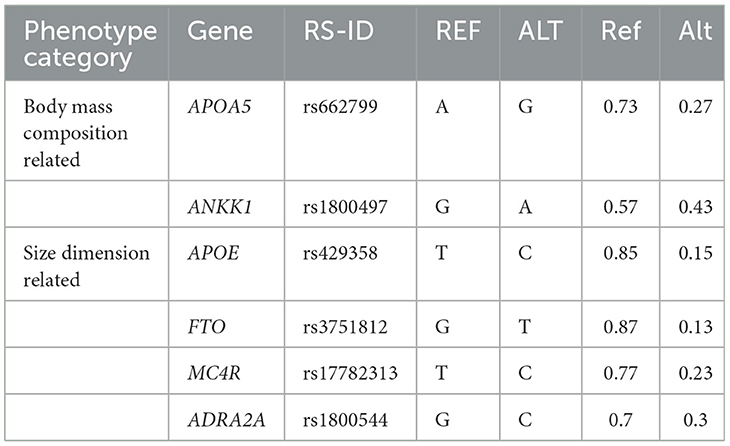
Table 2. Information on important SNPs with high impact on Chinese women (93).
6 Conclusion and future perspective
Obesity, as a complex polygenic disease, arises from the interplay of genetic susceptibility, environmental factors (particularly dietary patterns), and their interactions. This review has synthesized recent advances in obesity-related genetic SNPs and gene–diet interactions, highlighting the crucial roles of multigene synergism, metabolic network regulation, and sex differences in obesity pathogenesis.
Despite notable progress, current evidence remains largely static, failing to elucidate the critical timing and mechanisms behind the “triggering event” in the “genes load the gun, environment pulls the trigger” paradigm. Furthermore, the majority of studies are constrained by limited sample sizes and brief intervention durations, hindering the assessment of long-term exposure cumulative effects and metabolic compensation sustainability. The scarcity of cross-regional validation also restricts the generalizability of reported associations.
Looking forward, it is imperative to construct a three-dimensional gene–diet–metabolic phenotype model via long-term, large-scale multi-ethnic cohort studies to identify critical windows and reversible mechanisms of metabolic dysregulation. This approach will accelerate the transition from universal dietary recommendations to precision nutrition. Furthermore, integrating artificial intelligence and machine learning can enable dynamic tools to quantify non-linear gene–diet interactions and metabolic flux redistribution patterns. For instance, a recent BMI prediction model leveraging gut microbiome SNP density and random forest algorithms demonstrates enhanced efficacy (94). Future studies should also integrate genomic, metabolomic, and immunomic data to elucidate how multi-level regulatory networks shape obesity phenotypes. Particular attention should be paid to the unique characteristics of Chinese populations, with more localized research initiatives needed to inform region-specific prevention and control strategies.
Author contributions
WY: Conceptualization, Writing – original draft, Supervision. HX: Writing – original draft, Formal analysis, Investigation. CX: Writing – review & editing, Data curation, Validation. KC: Writing – review & editing, Visualization, Methodology. YP: Writing – original draft, Writing – review & editing, Formal analysis. RG: Writing – original draft, Visualization, Software. QZ: Conceptualization, Supervision, Writing – review & editing. JX: Supervision, Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Nantong Municipal Science and Technology Project (JC2024101).
Acknowledgments
We would like to express our sincere gratitude to our mentors, Jing Xiao and Qi Zhu, for their valuable guidance and support throughout this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1603038/full#supplementary-material
References
1. Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. (2003) 289:187–93. doi: 10.1001/jama.289.2.187
2. Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, et al. Body-mass index and mortality among 146 million white adults. N Engl J Med. (2010) 363:2211–9. doi: 10.1056/NEJMoa1000367
3. Prospective Studies Collaboration, Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet Lond Engl. (2009) 373:1083–96. doi: 10.1016/S0140-6736(09)60318-4
4. Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol. (2013) 9:13–27. doi: 10.1038/nrendo.2012.199
6. Bouchard C. Gene-environment interactions in the etiology of obesity: defining the fundamentals. Obes Silver Spring Md. (2008) 16:S5–10. doi: 10.1038/oby.2008.528
7. Popkin BM, Du S, Zhai F, Zhang B. Cohort profile: the China health and nutrition survey–monitoring and understanding socio-economic and health change in China, 1989-2011. Int J Epidemiol. (2010) 39:1435–40. doi: 10.1093/ije/dyp322
8. Zhen S, Ma Y, Zhao Z, Yang X, Wen D. Dietary pattern is associated with obesity in Chinese children and adolescents: data from China health and nutrition survey (CHNS). Nutr J. (2018) 17:68. doi: 10.1186/s12937-018-0372-8
9. Makris A, Foster GD. Dietary approaches to the treatment of obesity. Psychiatr Clin North Am. (2011) 34:813–27. doi: 10.1016/j.psc.2011.08.004
10. Malik VS, Hu FB. Popular weight-loss diets: from evidence to practice. Nat Clin Pract Cardiovasc Med. (2007) 4:34–41. doi: 10.1038/ncpcardio0726
11. Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. (2010) 42:937–48.
12. Fall T, Ingelsson E. Genome-wide association studies of obesity and metabolic syndrome. Mol Cell Endocrinol. (2014) 382:740–57. doi: 10.1016/j.mce.2012.08.018
13. Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. (2015) 518:197–206.
14. Yengo L, Sidorenko J, Kemper KE, Zheng Z, Wood AR, Weedon MN, et al. Meta-analysis of genome-wide association studies for height and body mass index in ~700000 individuals of European ancestry. Hum Mol Genet. (2018) 27:3641–9. doi: 10.1093/hmg/ddy271
15. Rose KM, Newman B, Mayer-Davis EJ, Selby JV. Genetic and behavioral determinants of waist-hip ratio and waist circumference in women twins. Obes Res. (1998) 6:383–92. doi: 10.1002/j.1550-8528.1998.tb00369.x
16. Sandholt CH, Hansen T, Pedersen O. Beyond the fourth wave of genome-wide obesity association studies. Nutr Diabetes. (2012) 2:e37. doi: 10.1038/nutd.2012.9
17. Qi L, Cho YA. Gene-environment interaction and obesity. Nutr Rev. (2008) 66:684–94. doi: 10.1111/j.1753-4887.2008.00128.x
18. Lackey DE, Lazaro RG Li P, Johnson A, Hernandez-Carretero A, Weber N, et al. The role of dietary fat in obesity-induced insulin resistance. Am J Physiol Endocrinol Metab. (2016) 311:E989–97. doi: 10.1152/ajpendo.00323.2016
19. Percopo CM, McCullough M, Limkar AR, Druey KM, Rosenberg HF. Impact of controlled high-sucrose and high-fat diets on eosinophil recruitment and cytokine content in allergen-challenged mice. PLoS ONE. (2021) 16:e0255997. doi: 10.1371/journal.pone.0255997
20. Jiang P, Ma D, Wang X, Wang Y, Bi Y, Yang J, et al. Astragaloside IV prevents obesity-associated hypertension by improving pro-inflammatory reaction and leptin resistance. Mol Cells. (2018) 41:244–55. doi: 10.14348/molcells.2018.2156
21. Huang J, Rong R, Ma ZF, Chen Y. Trajectory patterns of macronutrient intake and their associations with obesity, diabetes, and all-cause mortality: a longitudinal analysis over 25 years. Nutrients. (2024) 16:2567. doi: 10.3390/nu16152567
22. Zhang X, Zhang J, Du W, Su C, Ouyang Y, Huang F, et al. Multi-trajectories of macronutrient intake and their associations with obesity among Chinese adults from 1991 to 2018: a prospective study. Nutrients. (2021) 14:13. doi: 10.3390/nu14010013
23. Pledger SL, Ahmadizar F. Gene-environment interactions and the effect on obesity risk in low and middle-income countries: a scoping review. Front Endocrinol. (2023) 14:1230445. doi: 10.3389/fendo.2023.1230445
24. Dumont J, Goumidi L, Grenier-Boley B, Cottel D, Marécaux N, Montaye M, et al. Dietary linoleic acid interacts with FADS1 genetic variability to modulate HDL-cholesterol and obesity-related traits. Clin Nutr Edinb Scotl. (2018) 37:1683–9. doi: 10.1016/j.clnu.2017.07.012
25. Lopez-Huertas E. The effect of EPA and DHA on metabolic syndrome patients: a systematic review of randomised controlled trials. Br J Nutr. (2012) 107:S185–194. doi: 10.1017/S0007114512001572
26. Gong W, Li H, Song C, Yuan F, Ma Y, Chen Z, et al. Effects of gene-environment interaction on obesity among Chinese adults born in the early 1960s. Genes. (2021) 12:270. doi: 10.3390/genes12020270
27. Wang Y, Pan L, Wan S, Yihuo W, Yang F, He H, et al. MC4R gene polymorphisms interact with the urbanized living environment on obesity: results from the Yi Migrant Study. Front Genet. (2022) 13:849138. doi: 10.3389/fgene.2022.849138
28. Zhu Z, Wang Z, Zang J, Lu Y, Xiao Z, Zheng G, et al. The SNP rs516946 interacted in the association of MetS with dietary iron among Chinese males but not females. Nutrients. (2022) 14:2024. doi: 10.3390/nu14102024
29. Sun S, Kong Z, Shi Q, Hu M, Zhang H, Zhang D, et al. Non-energy-restricted low-carbohydrate diet combined with exercise intervention improved cardiometabolic health in overweight Chinese females. Nutrients. (2019) 11:3051. doi: 10.3390/nu11123051
30. Cao YJ, Wang HJ, Zhang B, Qi SF, Mi YJ, Pan XB, et al. Associations of fat and carbohydrate intake with becoming overweight and obese: an 11-year longitudinal cohort study. Br J Nutr. (2020) 124:715–28. doi: 10.1017/S0007114520001579
31. Liu X, Gan W, Gao C, Qi X, Liao X, Lin J, et al. The independent associations of protein consumption with body fat and glycaemic control in adult Chinese. Eur J Nutr. (2019) 58:1981–90. doi: 10.1007/s00394-018-1751-9
32. Kong Z, Sun S, Shi Q, Zhang H, Tong TK, Nie J. Short-term ketogenic diet improves abdominal obesity in overweight/obese Chinese young females. Front Physiol. (2020) 11:856. doi: 10.3389/fphys.2020.00856
33. Zhang Z, Chen B, Zeng J, Fan M, Xu W, Li X, et al. Associations between consumption of dietary fibers and the risk of type 2 diabetes, hypertension, obesity, cardiovascular diseases, and mortality in Chinese adults: longitudinal analyses from the China health and nutrition survey. Nutrients. (2022) 14:2650. doi: 10.3390/nu14132650
34. Wang Y, Wang H, Howard AG, Meyer KA, Tsilimigras MCB, Avery CL, et al. Circulating short-chain fatty acids are positively associated with adiposity measures in Chinese adults. Nutrients. (2020) 12:2127. doi: 10.3390/nu12072127
35. Lauw S, Kei N, Chan PL, Yau TK, Ma KL, Szeto CYY, et al. Effects of synbiotic supplementation on metabolic syndrome traits and gut microbial profile among overweight and obese Hong Kong Chinese individuals: a randomized trial. Nutrients. (2023) 15:4248. doi: 10.3390/nu15194248
36. Yuan X, Wei Y, Jiang H, Wang H, Wang Z, Dong M, et al. Longitudinal relationship between the percentage of energy intake from macronutrients and overweight/obesity among Chinese adults from 1991 to 2018. Nutrients. (2024) 16:666. doi: 10.3390/nu16050666
37. Popkin BM. Synthesis and implications: China's nutrition transition in the context of changes across other low- and middle-income countries. Obes Rev. (2014) 15:60–7. doi: 10.1111/obr.12120
38. Tang D, Bu T, Feng Q, Liu Y, Dong X. Differences in overweight and obesity between the north and south of China. Am J Health Behav. (2020) 44:780–93. doi: 10.5993/AJHB.44.6.4
39. Li T, Xie J, Shuai P, Huang J, He B. Dietary patterns, skeletal muscle mass loss, and cardiovascular risk among elderly men: a preliminary cross-sectional study in Sichuan Province. Environ Res. (2022) 208:112719. doi: 10.1016/j.envres.2022.112719
40. Song P, Zhang X, Li Y, Man Q, Jia S, Zhang J, et al. MetS prevalence and its association with dietary patterns among Chinese middle-aged and elderly population: results from a national cross-sectional study. Nutrients. (2022) 14:5301. doi: 10.3390/nu14245301
41. Wang H, Wang Y, Shi Z, Zhao L, Jian W, Li K, et al. Association between dietary patterns and metabolic syndrome and modification effect of altitude: a cohort study of Tibetan adults in China. Nutrients. (2023) 15:2226. doi: 10.3390/nu15092226
42. Peng W, Liu Y, Malowany M, Chen H, Su X, Liu Y. Metabolic syndrome and its relation to dietary patterns among a selected urbanised and semi-urbanised Tibetan population in transition from nomadic to settled living environment. Public Health Nutr. (2021) 24:984–92. doi: 10.1017/S1368980019004798
43. Pu F, Lin J, Wei Y, Li J, Liao X, Shi L, et al. Association of dietary behavior patterns of middle-aged and older adults with their obesity metabolic phenotype: a cross-sectional study. BMC Public Health. (2024) 24:2311. doi: 10.1186/s12889-024-19781-3
44. Zhang M, Guan Q, Mai J, Li S, Liu C, Zhou L, et al. How exercise frequency affects BMI: a nationwide cross-sectional study exploring key influencing factors, including dietary behavior. Front Public Health. (2024) 12:1514805. doi: 10.3389/fpubh.2024.1514805
45. Peters U, North KE, Sethupathy P, Buyske S, Haessler J, Jiao S, et al. A systematic mapping approach of 16q122/FTO and BMI in more than 20,000 African Americans narrows in on the underlying functional variation: results from the population architecture using genomics and epidemiology (PAGE) study. PLoS Genet. (2013) 9:e1003171. doi: 10.1371/journal.pgen.1003171
46. Chiang KM, Chang HC, Yang HC, Chen CH, Chen HH, Lee WJ, et al. Genome-wide association study of morbid obesity in Han Chinese. BMC Genet. (2019) 20:97. doi: 10.1186/s12863-019-0797-x
47. Wang Y, Pan L, He H, Li Z, Cui S, Yang A, et al. Prevalence, associated factors, and gene polymorphisms of obesity in Tibetan adults in Qinghai, China. BMC Public Health. (2024) 24:305. doi: 10.1186/s12889-023-17181-7
48. Wei BL, Yin RX, Liu CX, Deng GX, Guan YZ, Zheng PF. The MC4R SNPs, their haplotypes and gene-environment interactions on the risk of obesity. Mol Med Camb Mass. (2020) 26:77. doi: 10.1186/s10020-020-00202-1
49. 李强. 西藏藏族mc4r、sH2b1、negr1基因多态性与肥胖的关系 [Internet] [硕士学位论文]. 锦州医科大学. (2021). doi: 10.27812/d.cnki.glnyx.2021.000059 (Accessed March 24, 2025).
50. 刘卫刚, 刘志军, 张龙玉, 王琳, 刘贵京, 陆云, et al. 中国农村地区汉族人群rs2880412、rs2880416和rs1047214基因多态性与肥胖的相关性研究. 现代预防医学(2019) 46:3082–6, 3115.
51. 刘卫刚, 许忠新, 张洪亮, 王琳, 刘少军, 史文宗. Rs2880412位点多态性与肥胖易感性的病例对照研究. 现代预防医学. (2020) 47:164–7. doi: 10.26549/jxffcxysj.v3i2.3527
52. Wu H, Zhao X, Hochrein SM, Eckstein M, Gubert GF, Knöpper K, et al. Mitochondrial dysfunction promotes the transition of precursor to terminally exhausted T cells through HIF-1α-mediated glycolytic reprogramming. Nat Commun. (2023) 14:6858. doi: 10.1038/s41467-023-42634-3
53. Bouhamida E, Morciano G, Perrone M, Kahsay AE, Della Sala M, Wieckowski MR, et al. The interplay of hypoxia signaling on mitochondrial dysfunction and inflammation in cardiovascular diseases and cancer: from molecular mechanisms to therapeutic approaches. Biology. (2022) 11:300. doi: 10.3390/biology11020300
54. Li X, Zhang H, Hodder T, Wang W, Myers CL, Yilmaz LS, et al. Systems-level design principles of metabolic rewiring in an animal. Nature. (2025) 640:203–11. doi: 10.1038/s41586-025-08636-5
55. Zheng X, Ma J, Long J, Wei Q, Ren W. The HIF1α polymorphism rs2301104 is associated with obesity and obesity-related cytokines in Han Chinese population. Acta Diabetol. (2023) 60:285–92. doi: 10.1007/s00592-022-02008-5
56. Wang X, Wu Q, Zhong M, Chen Y, Wang Y, Li X, et al. Adipocyte-derived ferroptotic signaling mitigates obesity. Cell Metab. (2025) 37:673–91.e7. doi: 10.1016/j.cmet.2024.11.010
57. Zhu X, Hu J, Yang M, Guo H, Ji D, Li Y, et al. A genetic analysis identifies haplotype at adiponectin locus: association with the metabolic health and obesity phenotypes. Gene. (2021) 784:145593. doi: 10.1016/j.gene.2021.145593
58. 李美艳, 王永治, 阿依古丽•阿力木, 海且木汗•阿布杜热曼, 刘早玲. 中国七省人群PPARγ2基因Pro12Ala多态性与肥胖相关性的Meta分析. 卫生研究. (2021) 50:315–9. doi: 10.19813/j.cnki.weishengyanjiu.2021.02.026
59. Ji L, Wu D, Xie H, Yao B, Chen Y, Irwin DM, et al. Ambient temperature is a strong selective factor influencing human development and immunity. Genomics Proteomics Bioinformatics. (2020) 18:489–500. doi: 10.1016/j.gpb.2019.11.009
61. Ji F, Liu Y, Hao JG, Wang LP Dai MJ, Shen GF, et al. KLB gene polymorphism is associated with obesity and non-alcoholic fatty liver disease in the Han Chinese. Aging. (2019) 11:7847–58. doi: 10.18632/aging.102293
62. Li Y, Jin L, Yan J, Huang Y, Zhang H, Zhang R, et al. Tsukushi and TSKU genotype in obesity and related metabolic disorders. J Endocrinol Invest. (2021) 44:2645–54. doi: 10.1007/s40618-021-01572-x
63. Xu T, Liu M, Liu Q, Wang B, Wang M, Qu M, et al. Associations of TCF7L2 rs11196218 (a/G) and GLP-1R rs761386 (C/T) gene polymorphisms with obesity in Chinese population. Diabetes Metab Syndr Obes Targets Ther. (2021) 14:2465–72. doi: 10.2147/DMSO.S310069
64. Qi L, Zhang Z, Hu W. Identification of gene polymorphisms associated with fat mass by genome-wide association study in Chinese people. Arch Med Sci AMS. (2023) 19:1541–4. doi: 10.5114/aoms/170851
65. Lv HY, Shi G, Li C, Ye YF, Chen YH, Chen LH, et al. Association of SULT1A2 rs1059491 with obesity and dyslipidaemia in southern Chinese adults. Sci Rep. (2023) 13:7256. doi: 10.1038/s41598-023-35762-9
66. 宁美微. Fto和abcg2基因多态性与超重/肥胖和代谢参数的相关性研究 [Internet] [硕士学位论文]. 湖北中医药大学 (2023). Available online at: https://doi.org/10.27134/d.cnki.ghbzc.2022.000061 (Accessed August 30, 2025).
67. Shen F, Wang Y, Sun H, Zhang D, Yu F, Yu S, et al. Vitamin D receptor gene polymorphisms are associated with triceps skin fold thickness and body fat percentage but not with body mass index or waist circumference in Han Chinese. Lipids Health Dis. (2019) 18:97. doi: 10.1186/s12944-019-1027-2
69. Huang YM, Wang W, Hsieh PP, Chen HH. Haplotype of ESR1 and PPARD genes is associated with higher anthropometric changes in Han Chinese obesity by adjusting dietary factors-an 18-month follow-up. Nutrients. (2022) 14:4425. doi: 10.3390/nu14204425
70. Fang Z, Zhu L, Zhang T, Jin Y, Chen Y, Yao Y. Sex-specific genetic association of brain and muscle arnt-like protein-1 (BMAL1) and obesity in Chinese youth. Obes Res Clin Pract. (2022) 16:464–9. doi: 10.1016/j.orcp.2022.10.008
71. Sebo ZL, Rodeheffer MS. Testosterone metabolites differentially regulate obesogenesis and fat distribution. Mol Metab. (2021) 44:101141. doi: 10.1016/j.molmet.2020.101141
72. Lu Y, Qin L, Wei Y, Mo X, Tang X, Liu Q, et al. Association between barium exposed, CYP19A1 and central obesity: a cross-sectional study in rural China. J Trace Elem Med Biol Organ Soc Miner Trace Elem GMS. (2023) 78:127170. doi: 10.1016/j.jtemb.2023.127170
73. 于婷. 西藏藏族线粒体基因多态性与肥胖的相关性研究 [Internet] [硕士学位论文]. 锦州医科大学 (2021). doi: 10.27812/d.cnki.glnyx.2021.000162 (Accessed March 24, 2025).
74. Jansen PR, Vos N, van Uhm J, Dekkers IA, van der Meer R, Mannens MMAM, et al. The utility of obesity polygenic risk scores from research to clinical practice: a review. Obes Rev. (2024) 25:e13810. doi: 10.1111/obr.13810
75. Tan PY, Mitra SR. The combined effect of polygenic risk from FTO and ADRB2 gene variants, odds of obesity, and post-hipcref diet differences. Lifestyle Genomics. (2020) 13:84–98. doi: 10.1159/000505662
76. Young AI, Wauthier F, Donnelly P. Multiple novel gene-by-environment interactions modify the effect of FTO variants on body mass index. Nat Commun. (2016) 7:12724. doi: 10.1038/ncomms12724
77. Sun M, Li S, Ning F, Zhang L, Wang W, Duan H, et al. Association between olfactory pathway gene variants and obesity in Chinese Han population: a case-control study based on genetic score. Gene. (2022) 825:146442. doi: 10.1016/j.gene.2022.146442
78. 张丽. Gpcr信号通路基因遗传变异与肥胖的关联研究 [Internet] [硕士学位论文]. 青岛大学 (2023). Available online at: doi: 10.27262/d.cnki.gqdau.2022.001145
79. Lou XY, Chen GB, Yan L, Ma JZ, Zhu J, Elston RC, et al. A generalized combinatorial approach for detecting gene-by-gene and gene-by-environment interactions with application to nicotine dependence. Am J Hum Genet. (2007) 80:1125–37. doi: 10.1086/518312
80. Pigeyre M, Yazdi FT, Kaur Y, Meyre D. Recent progress in genetics, epigenetics and metagenomics unveils the pathophysiology of human obesity. Clin Sci. (2016) 130:943–86. doi: 10.1042/CS20160136
81. Elks CE, den Hoed M, Zhao JH, Sharp SJ, Wareham NJ, Loos RJF, et al. Variability in the heritability of body mass index: a systematic review and meta-regression. Front Endocrinol. (2012) 3:29. doi: 10.3389/fendo.2012.00029
82. Park SL, Cheng I, Pendergrass SA, Kucharska-Newton AM, Lim U, Ambite JL, et al. Association of the FTO obesity risk variant rs8050136 with percentage of energy intake from fat in multiple racial/ethnic populations: the PAGE study. Am J Epidemiol. (2013) 178:780–90. doi: 10.1093/aje/kwt028
83. Kuang X, Li K, Shi Y, Shao X, Li H, Li D. Gene-diet interaction in response to defatted flaxseed flour supplementation on obesity-related traits in Chinese overweight and obese adults: a randomized controlled trial. Nutr Burbank Los Angel Cty Calif. (2023) 105:111870. doi: 10.1016/j.nut.2022.111870
84. Hellstrand S, Sonestedt E, Ericson U, Gullberg B, Wirfält E, Hedblad B, et al. Intake levels of dietary long-chain PUFAs modify the association between genetic variation in FADS and LDL-C. J Lipid Res. (2012) 53:1183–9. doi: 10.1194/jlr.P023721
85. Obregón AM, Valladares M, Goldfield G. Association of the dopamine D2 receptor rs1800497 polymorphism and eating behavior in Chilean children. Nutr Burbank Los Angel Cty Calif. (2017) 35:139–45. doi: 10.1016/j.nut.2016.11.005
86. 田烨. 多囊卵巢综合征和卵巢过度刺激综合征易感基因相关研究 [Internet] [博士学位论文]. 上海交通大学 (2020). doi: 10.27307/d.cnki.gsjtu.2016.003412
87. Langlois MR, Delanghe JR. Biological and clinical significance of haptoglobin polymorphism in humans. Clin Chem. (1996) 42:1589–600. doi: 10.1093/clinchem/42.10.1589
88. Tang KY, Huang SY, Cheng TM, Bai CH, Chang JS. Haptoglobin phenotype influences the effectiveness of diet-induced weight loss in middle-age abdominally obese women with metabolic abnormalities. Clin Nutr Edinb Scotl. (2020) 39:225–33. doi: 10.1016/j.clnu.2019.01.019
89. Oliviero S, Cortese R. The human haptoglobin gene promoter: interleukin-6-responsive elements interact with a DNA-binding protein induced by interleukin-6. EMBO J. (1989) 8:1145–51. doi: 10.1002/j.1460-2075.1989.tb03485.x
90. Maffei M, Funicello M, Vottari T, Gamucci O, Costa M, Lisi S, et al. The obesity and inflammatory marker haptoglobin attracts monocytes via interaction with chemokine (C-C motif) receptor 2 (CCR2). BMC Biol. (2009) 7:87. doi: 10.1186/1741-7007-7-87
91. Yap RWK, Shidoji Y, Yap WS, Masaki M. Association and interaction effect of AGTR1 and AGTR2 gene polymorphisms with dietary pattern on metabolic risk factors of cardiovascular disease in Malaysian adults. Nutrients. (2017) 9:853. doi: 10.3390/nu9080853
92. Tong Z, Qi J, Ma W, Wang D, Hu B, Li Y, et al. SUMO4 gene SNP rs237025 and the synergistic effect with weight management: a study of risk factors and interventions for MetS. Front Genet. (2021) 12:786393. doi: 10.3389/fgene.2021.786393
93. 赵婧雅, 李辉. 遗传背景和饮食习惯协同影响肥胖进程. 解剖学杂志. (2018) 41:446–51. Available online at: https://kns.cnki.net/kcms2/article/abstract?v=ZHE1803t14sUg59R93t5n-ktJtqGQZQuacLoRcXWMw2jrYcIqA8dpigdZ4qewfGU1Tt5W2DxVGIhSB53p0cKCyeliLuApUeJyYwHiAjunQ-Cup7p-KGjHVVObvYlHSdiOw0NpqYVYLvOJyz1hH5zzHCga7zfoYjfafMgZlaC4hFaMe5oQOfMig==&uniplatform=NZKPT&language=CHS
94. Liang Y, Dou S, Zhao G, Shen J, Fu G, Fu L, et al. Prediction of BMI traits in the Chinese population based on the gut metagenome. Microb Cell Factories. (2023) 22:250. doi: 10.1186/s12934-023-02255-3
95. Shi SQ, Li SS, Zhang XY, Wei Z, Fu WZ, He JW, et al. LGR4 gene polymorphisms are associated with bone and obesity phenotypes in Chinese female nuclear families. Front Endocrinol. (2021) 12:656077. doi: 10.3389/fendo.2021.656077
Keywords: SNPs, nutrition, obesity, Chinese adults, gene-diet interactions
Citation: Yang W, Xu H, Xu C, Cao K, Pan Y, Gu R, Zhu Q and Xiao J (2025) Research progress on the mechanistic impact of single-nucleotide polymorphisms and dietary pattern interactions on overweight and obesity in Chinese adults: a narrative review. Front. Nutr. 12:1603038. doi: 10.3389/fnut.2025.1603038
Received: 31 March 2025; Accepted: 28 October 2025;
Published: 18 November 2025.
Edited by:
Claudia Ojeda-Granados, University of Catania, ItalyReviewed by:
Jerzy Beltowski, Medical University of Lublin, PolandIngrid Rivera-Iñiguez, MedicaUniversity of California, San Diego, United States
Akingbolabo Daniel Ogunlakin, Bowen University, Nigeria
Copyright © 2025 Yang, Xu, Xu, Cao, Pan, Gu, Zhu and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Xiao, anhpYW9udEBudHUuZWR1LmNu; Qi Zhu, emdqc250enFAbnR1LmVkdS5jbg==
†These authors have contributed equally to this work
 Weiling Yang
Weiling Yang Hong Xu1,2†
Hong Xu1,2† Renjie Gu
Renjie Gu