- 1Department of Gastrointestinal Surgery, Northern Jiangsu People’s Hospital Affliated to Yangzhou University/Clinical Medical College, Yangzhou University, Yangzhou, China
- 2Department of Nursing, Northern Jiangsu People’s Hospital Affliated to Yangzhou University/Clinical Medical College, Yangzhou University, Yangzhou, China
Background: Bariatric surgery has become a widely utilized therapeutic approach for obesity management and glycemic regulation in individuals with type 2 diabetes mellitus (T2DM). This meta-analysis examines the effects of bariatric surgery on key glycemic and metabolic parameters.
Methods: A systematic literature search was performed across PubMed, Scopus, Embase, and Web of Science to identify relevant studies assessing alterations in outcomes following bariatric surgery compared to baseline measurements. Eligible studies were analyzed using a random-effects model to compute weighted mean differences (WMD) and their corresponding 95% confidence intervals (CIs).
Results: Bariatric surgery resulted in 39 with 3,855 participants in significant reductions in fasting blood glucose (FBG) (WMD: −0.82 mg/dL; 95%CI: −0.92 to −0.72), postprandial glucose (PPG) (WMD: −4.15 mg/dL; 95%CI: −5.38 to −2.92), Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) levels (WMD: -2.81; 95% CI: −3.06 to −2.56), C-peptide (WMD: -0.38; 95%CI: −0.73 to −0.03) and fasting insulin (WMD: -0.62; 95% CI: −0.88 to −0.36). No significant reduction in glycated hemoglobin (HbA1c) levels was observed (WMD: -0.17; 95%CI: −0.39 to 0.04). Follow-up periods ranging from 2.3 to 120 months.
Conclusion: It was concluded that the bariatric surgery may have improved the glycemic and metabolic outcomes. Therefore, the results underscore the value of incorporating bariatric surgery into diabetes care strategies, highlighting its potential to enhance long-term diabetes management and mitigate the risk of complications.
Introduction
Globally, obesity and type 2 diabetes mellitus (T2DM) have reached epidemic proportions. According to recent WHO data, one in eight people worldwide was living with obesity in 2022, and nearly half of all adults (43%) are overweight.1 Concurrently, the prevalence of diabetes has surged: by 2022 an estimated 830 million people had diabetes worldwide.2 Obesity and T2DM are tightly linked through complex metabolic and inflammatory pathways. Expanded adipose tissue in obesity secretes dysregulated adipokines and cytokines, leading to chronic low-grade inflammation. Adipocyte hypertrophy and hypoxia provoke macrophage infiltration into adipose tissue and release of factors such as pro-inflammatory cytokines. These pro-inflammatory signals impair insulin receptor signaling in muscle and liver, reducing glucose uptake and increasing hepatic gluconeogenesis, while also promoting pancreatic β-cell dysfunction (1).
Bariatric surgery has emerged as the most effective treatment for severe obesity, consistently producing greater and more durable weight loss than non-surgical interventions. In addition to weight loss, bariatric procedures confer profound metabolic benefits. Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG) in particular achieve sustained weight reduction beyond 10 years while dramatically improving obesity-related comorbidities (2). These weight-independent effects implicate altered gut physiology as key mechanisms. Postoperatively, patients typically show exaggerated postprandial incretin responses: elevations in glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) that enhance insulin secretion and satiety (3, 4). RYGB, by bypassing the proximal intestine, acutely delivers nutrients to the distal gut and evokes strong GLP-1 and bile acid signals, whereas SG reduces ghrelin (a hunger hormone) and similarly boosts GLP-1/PYY responses (5). In parallel, adipose-derived inflammation is attenuated after surgery (6).
Despite clear overall benefits, debate persists regarding which bariatric procedure optimally treats T2DM. Observational and trial evidence often favors RYGB for glycemic remission. For example, in a pooled analysis of randomized trials, RYGB produced a higher diabetes remission rate than SG at 1 year (7). However, differences tend to attenuate over time: in studies with 2–5-year follow-up, remission rates with SG approached those of RYGB (5, 7). Adjustable gastric banding (AGB) is generally less effective. Comparative data show much lower remission rates after AGB than after RYGB or SG (8). For instance, a landmark U. K. cohort found 2-year remission in only ~7% of AGB patients versus ~41% after RYGB and 26% after SG (8). Biliopancreatic diversion (BPD) or duodenal switch, a more complex procedure, yields the highest remission (often >90%) (8). A recent study of insulin-treated patients confirmed that BPD-DS conferred superior long-term remission compared to other procedures (9). The literature contains some conflicting signals: some reviews assert RYGB’s superiority, while others find only transient differences (5, 7). Variability among patients (age, diabetes duration, baseline β-cell function, etc.) also affects outcomes. For example, the one study found that patients with lower preoperative hemoglobin A1c (HbA1c) and fewer diabetes medications were far more likely to maintain remission at 10 years (10).
Given these gaps, a comprehensive synthesis of the most recent evidence is needed. Our systematic review and meta-analysis evaluates all major bariatric procedures (RYGB, SG, AGB, and BPD) and their effects on a wide range of glycemic (HbA1c, fasting blood glucose (FBG), postprandial glucose (PPG)) and metabolic markers (fasting insulin, homeostatic model assessment of insulin resistance (HOMA-IR), C-peptide) in people with T2DM. This broad comparison offers a more complete picture of their relative efficacy. Importantly, we formally assess the certainty of the evidence using GRADE methodology to provide clinicians with clearer guidance.
Methods
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (11). It was registered in PROSPERO with an ID: CRD420251063420.
Search strategy
A thorough literature search was conducted across several electronic databases, including PubMed, EMBASE, Scopus, and Web of Science, from their inception until November 2024, without language restrictions. Clinical trial registries such as ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP)—were also screened for ongoing or unpublished studies to minimize publication bias. The search strategy incorporated MeSH terms and keywords related to bariatric surgery, type 2 diabetes mellitus, and metabolic outcomes.
Eligibility criteria
All identified citations were imported into EndNote X9 (Thomson Reuters, New York) for reference management, with duplicate records subsequently eliminated. Two independent investigators screened the titles and abstracts of the remaining publications to determine eligibility. Eligible studies underwent full-text review, during which they were assessed for compliance with the predefined inclusion and exclusion criteria. The inclusion criteria were: Population: Adults (≥18 years) with a confirmed diagnosis of T2DM and obesity (BMI ≥ 30 kg/m2). Exposure and Comparator: Any form of bariatric surgery, including RYGB, SG, AGB, or BPD compared with pre-surgery baseline data. Outcomes: Changes in HbA1c, FBG, postprandial glucose, C-peptide levels, HOMA-IR, and fasting insulin levels. Study Design: Randomized controlled trials (RCTs), cohort studies, or quasi-experimental studies with sufficient data for quantitative synthesis. Exclusion criteria included studies involving patients with type 1 diabetes (T1D); those without pre- and post-operative blood glucose data; Case reports, conference abstracts, and non-English-language publications.
Research selection
Studies were independently screened and selected by two authors based on inclusion criteria, with agreement reached after consultation with the third reviewer. These authors were also responsible for extracting relevant data, including the first author’s name, year of publication, sample size, study design, Mean age, sex distribution, BMI, diabetes duration, and baseline glycemic/metabolic values and outcome data.
Study risk of bias assessment and meta-evidence
Methodological quality assessment was performed using the Cochrane Risk of Bias 2.0 (RoB 2) tool for RCTs (12), evaluating key domains including randomization processes, deviations from intended interventions, and outcome reporting. For non-randomized trials, the Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) tool was applied to assess potential biases arising from confounding, participant selection, and outcome measurement (13). Two independent reviewers conducted the assessments, with any discrepancies resolved through consensus discussion. The overall certainty of evidence was subsequently appraised using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) framework (14).
Statistical analysis
The data were analyzed using the STATA version 17 software. The weighted mean difference (WMD) or standardized mean difference (SMD) with 95% confidence intervals (CI) was described for continuous outcomes. Since all included trials were heterogeneous by design, the data were analyzed using a random effects model. Heterogeneity across RCTs was assessed using prediction intervals instead of the traditional I2 statistic (15). Between-study heterogeneity was quantified using I2 statistics and τ2 estimates. A leave-one-out sensitivity analysis was carried out to examine whether any individual study influenced the overall effect size estimates (16). Publication bias was assessed via funnel plot symmetry and Egger’s regression test (17).
Results
Search results
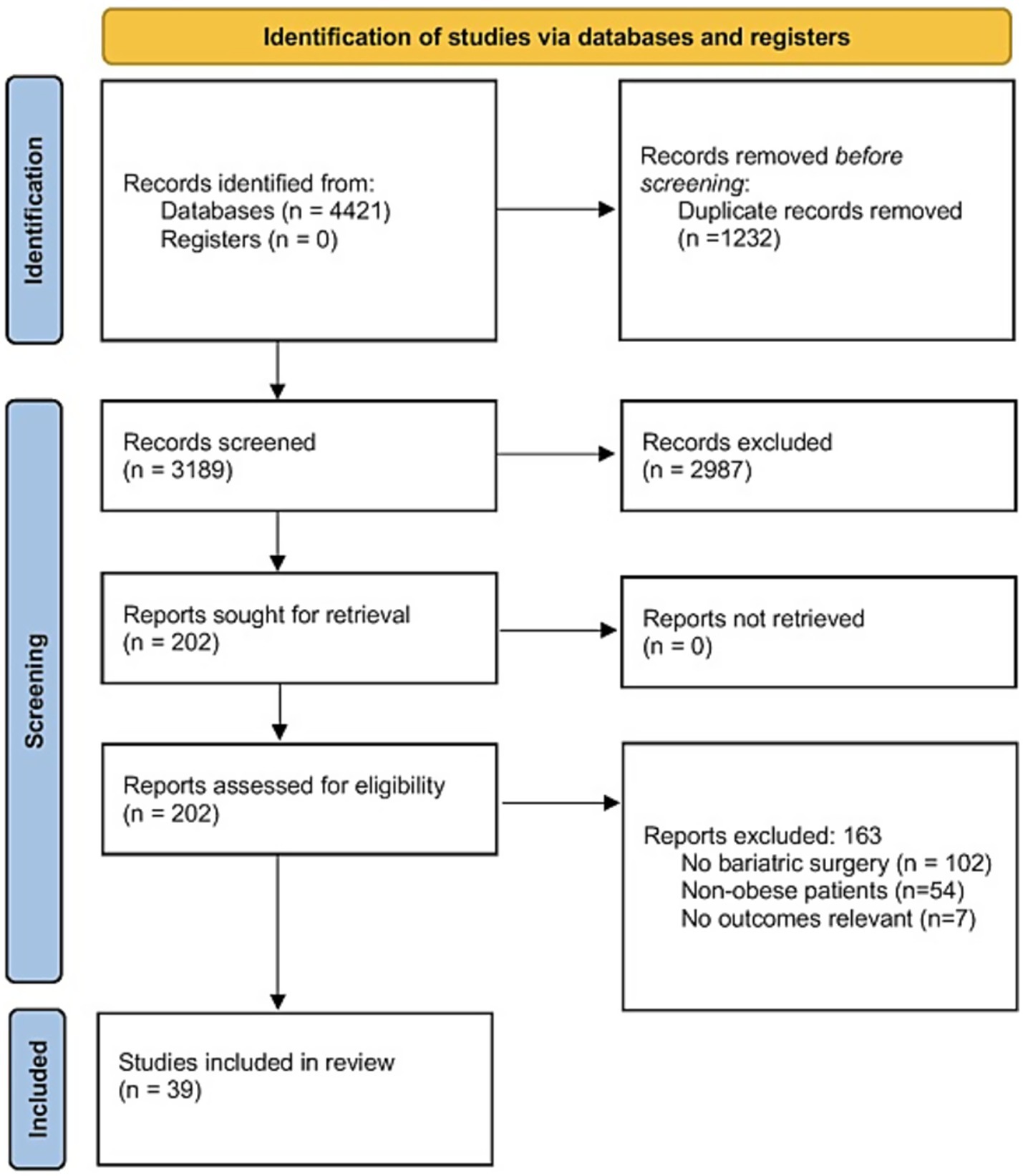
A total of 4,421 studies were identified through the literature search and subsequently screened. After removing duplicates, 3,189 records were screened based on titles and abstracts, resulting in 202 full-text articles for further evaluation. 163 full-text articles were then excluded, due to the following reasons: no bariatric surgery (n = 102), non-obese patients (n = 54), and non-relevant outcomes (n = 7). Finally, a total of 39 eligible studies met all inclusion criteria (18–56). The PRISMA flow diagram is depicted in Figure 1.
Characteristics of the included studies
A total of 39 studies were included in this meta-analysis, spanning a diverse range of global settings. The majority of studies originated from Asia, the Americas, and Europe, with China, Korea, Brazil, and the United States being the most frequently represented countries. This reflects the broad geographic distribution of bariatric surgical research. Sample sizes across studies varied from 6 to 479 participants, with mean ages ranging between 41.3 and 63.8 years. The studies evaluated various surgical interventions, including RYGB (Roux-en-Y Gastric Bypass), LAGB (Laparoscopic Adjustable Gastric Banding), and DJB (Duodenal-Jejunal Bypass), with follow-up periods ranging from 2.3 to 120 months. Risk of bias assessments varied across studies, with 15 classified as “High,” 15 as “Moderate” or having “Some concerns,” and 9 as “Low” (Table 1). Considering the GRADE quality of evidence, FBG, PPG, C-peptide levels, HOMA-IR, and fasting insulin levels had high GRADE quality. Low quality was noted for HbA1c.
FBG levels
Data from 30 studies involving 3,494 participants showed that FBG levels decreased significantly after bariatric surgery compared to pre-surgery values (WMD = −0.82 mg/dL, 95% CI: −0.92, −0.72; p < 0.001, I2 = 0.0%; tau2 value 0.001; Figure 2). Further analysis based on sample size and mean age showed more reductions in studies with more than 50 individuals, and age less than 50 years, with age and sample size identified as a source of heterogeneity. Sensitivity analysis confirmed the robustness of the overall findings with WMD. Funnel plot was asymmetrical (Supplementary Figure S1) with highly significant Egger’s test (p = 0.02). Then, trim and fill analysis was performed with 45 studies (15 imputed studies, WMD = −0.81, 95% CI, −0.96, −0.65; p < 0.05; Supplementary Figure S2).
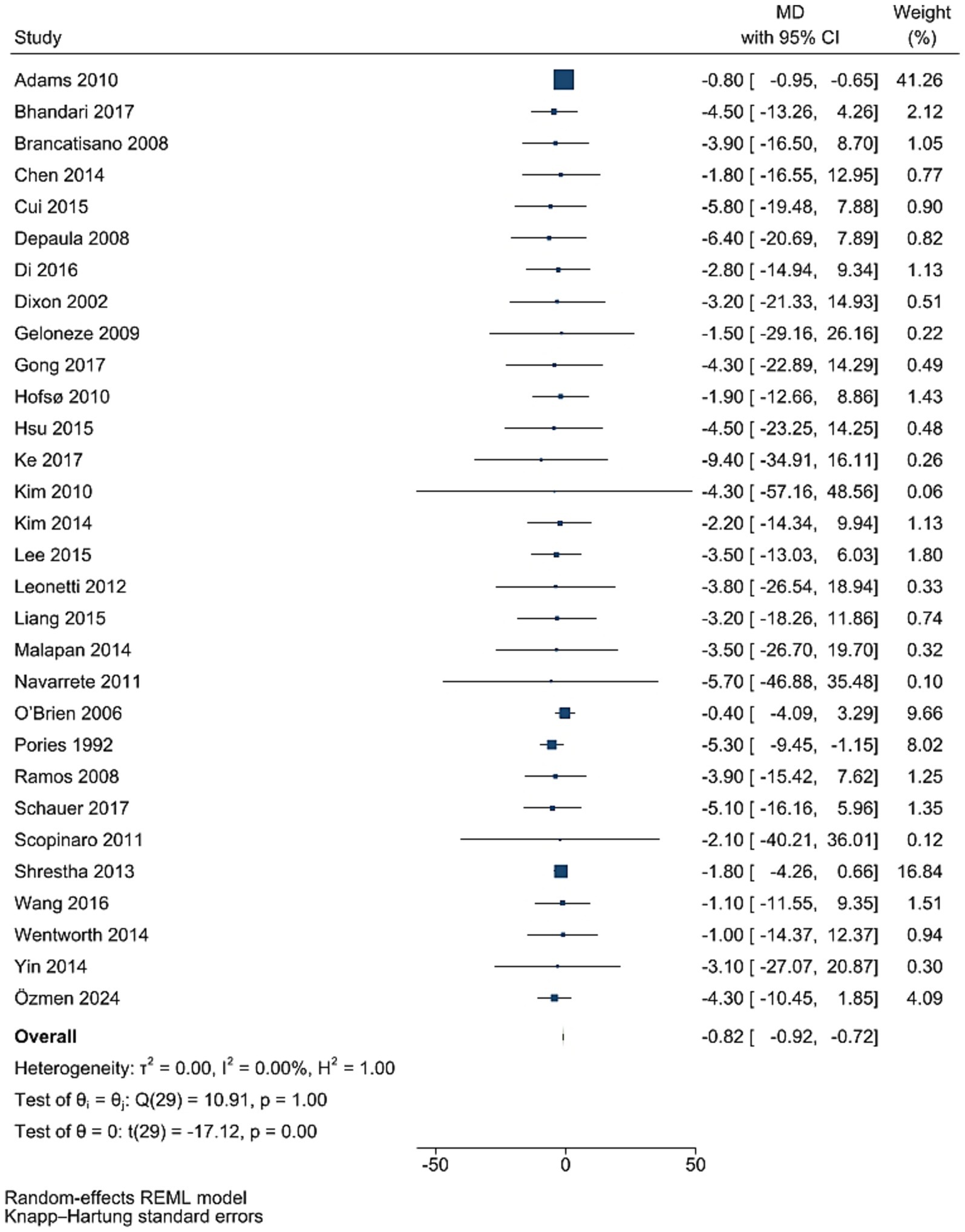
Figure 2. Forest plot detailing mean difference and 95% confidence intervals (CIs), the effects of bariatric surgery supplementation on FPG.
HbA1C levels
Data from 37 studies involving 3,855 participants showed that HbA1c levels did not significantly decrease after bariatric surgery compared to pre-surgery values (WMD = −0.17, 95% CI: −0.39, 0.04; p = 0.115, I2 = 0.0%; tau2 value 0.001; Figure 3). Further analysis based on sample size and mean age showed more reductions in studies with less than 50 individuals, and age less than 50 years, with age and sample size identified as a source of heterogeneity. Sensitivity analysis revealed no single RCT effect on the overall estimates. Funnel plot was asymmetrical (Supplementary Figure S3) with highly significant Egger’s test (p < 0.001). Then, trim and fill analysis was performed with 54 studies, and led to significant effect on HbA1c (17 imputed studies, WMD = −3.99, 95% CI: −5.21, −2.77; p < 0.05; Supplementary Figure S4).
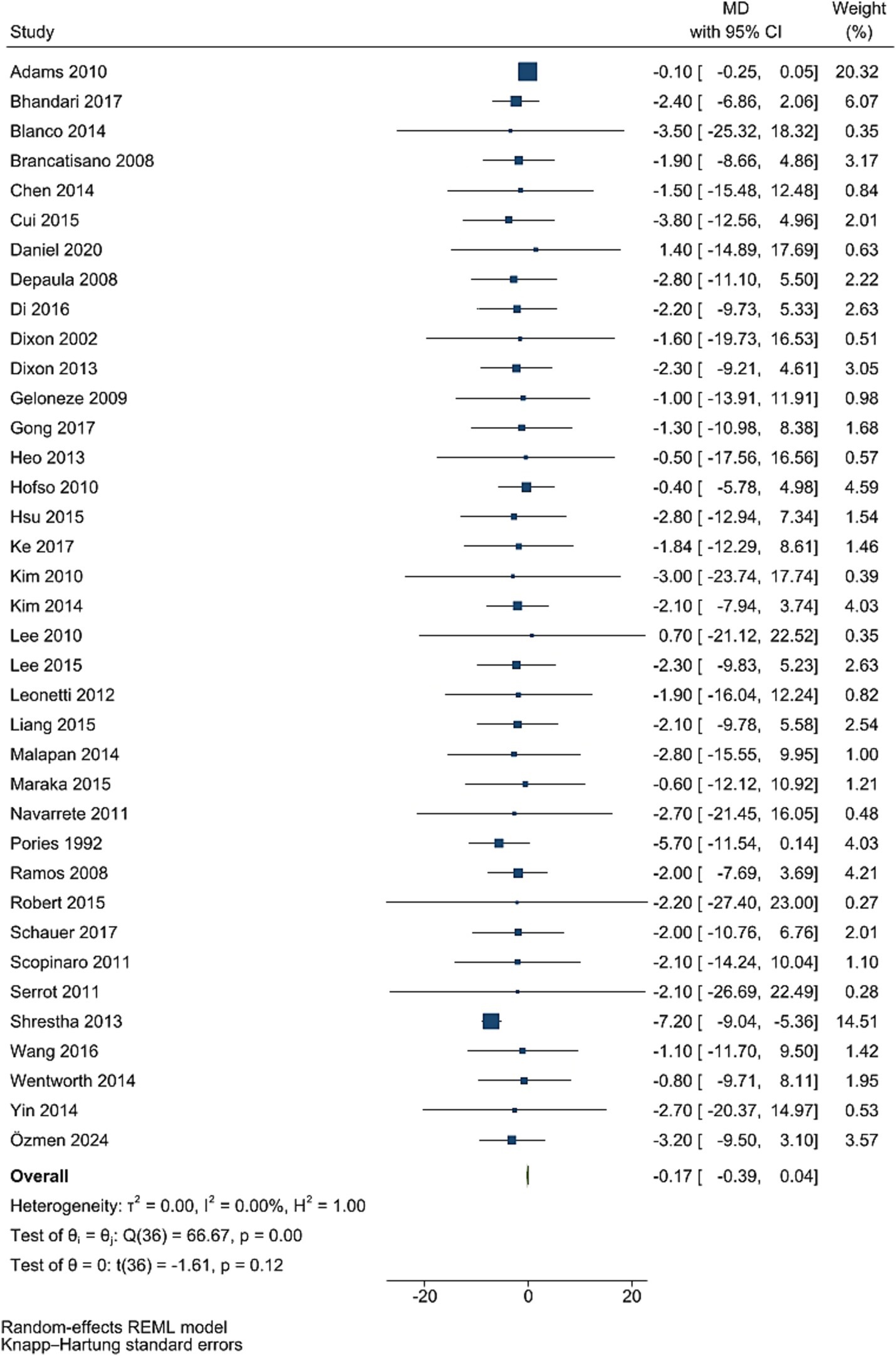
Figure 3. Forest plot detailing mean difference and 95% confidence intervals (CIs), the effects of bariatric surgery supplementation on HbA1C.
PPG levels
Post bariatric surgery, compared to pre-surgery values from nine studies involving 1,154 participants, led to a significant reduction in PPG levels (WMD = −4.15 mg/dL, 95% CI: −5.38, −2.92; p < 0.001, I2 = 0.0%; tau2 value 0.001; Figure 4). Sensitivity analyses revealed robust pooled estimates, with no single study significantly affecting the pooled effect size. Funnel plot was asymmetrical (Supplementary Figure S5) with highly significant Egger’s test (p = 0.532). Then, trim and fill analysis was performed with 13 studies (four imputed studies, WMD = −3.74, 95% CI: −7.22, −0.26; p < 0.05; Supplementary Figure S6).
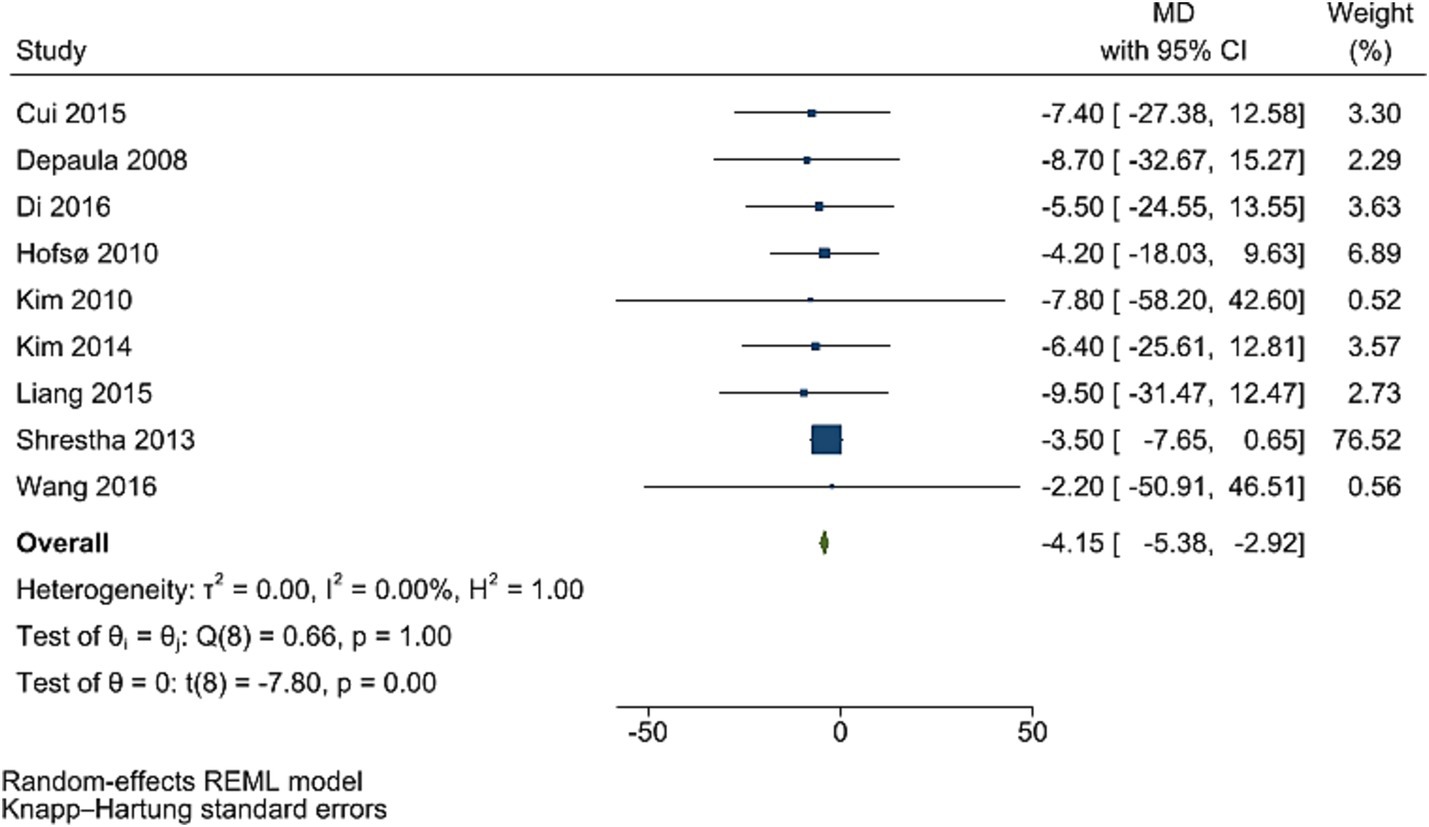
Figure 4. Forest plot detailing mean difference and 95% confidence intervals (CIs), the effects of bariatric surgery supplementation on PPG.
C-peptide levels
The improving effect of post-bariatric surgery compared to pre-surgery values on C peptide levels was significant (WMD = −0.38, 95% CI: −0.73, −0.03, p = 0.037, I2 = 0.0%; tau2 value 0.001, 12 studies involving 1,216 participants; Figure 5). Sensitivity analyses revealed robust pooled estimates. Egger’s test showed no evidence of publication bias (p = 0.988).
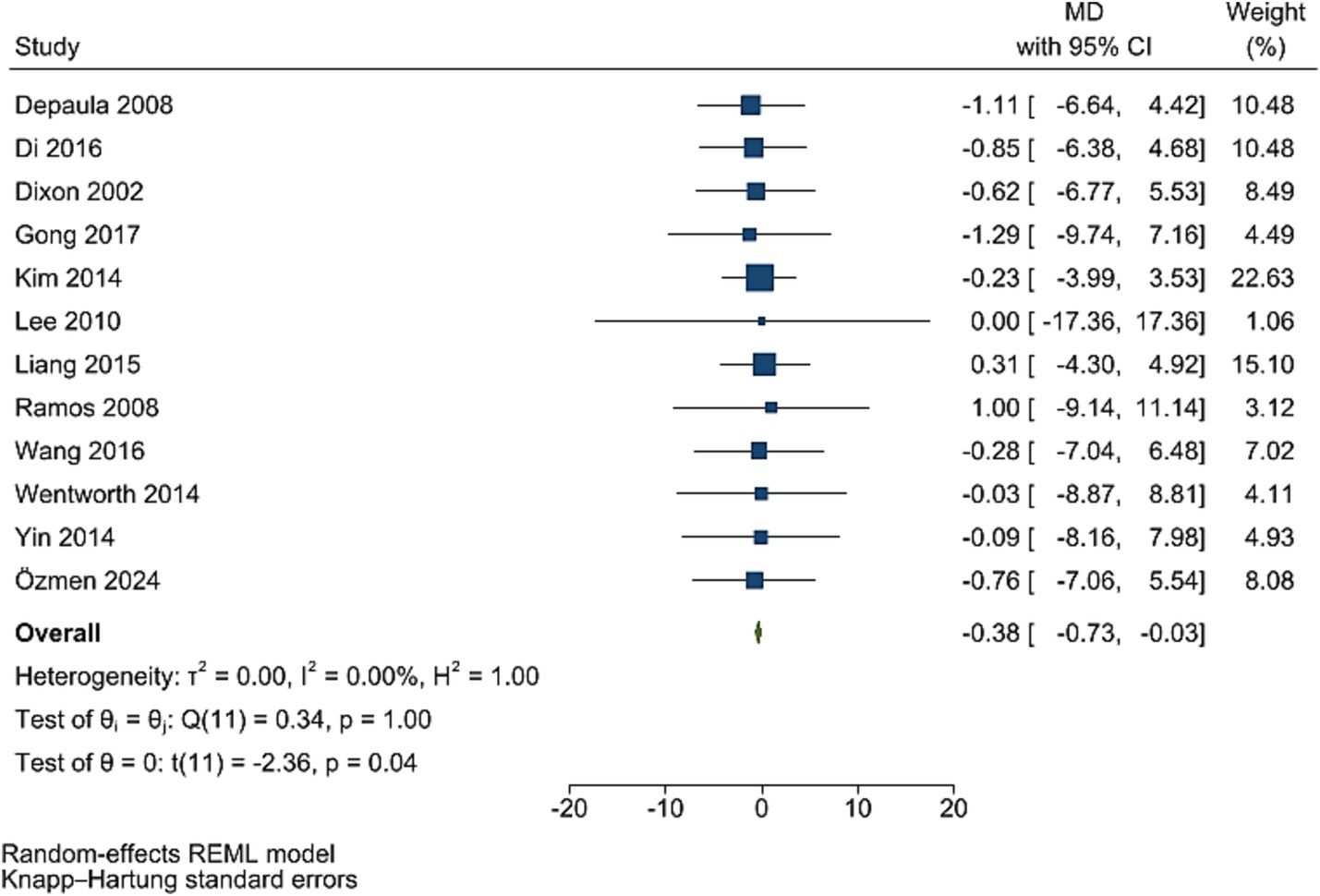
Figure 5. Forest plot detailing mean difference and 95% confidence intervals (CIs), the effects of bariatric surgery supplementation on c-peptide.
HOMA-IR
The improving effect of post-bariatric surgery compared to pre-surgery values on HOMA-IR levels was significant (WMD = −2.81, 95% CI: −3.06, −2.56, p < 0.001, I2 = 0.0%; tau2 value 0.001, five studies involving 1,361 participants; Figure 6). Sensitivity analyses revealed robust overall effect size estimates.
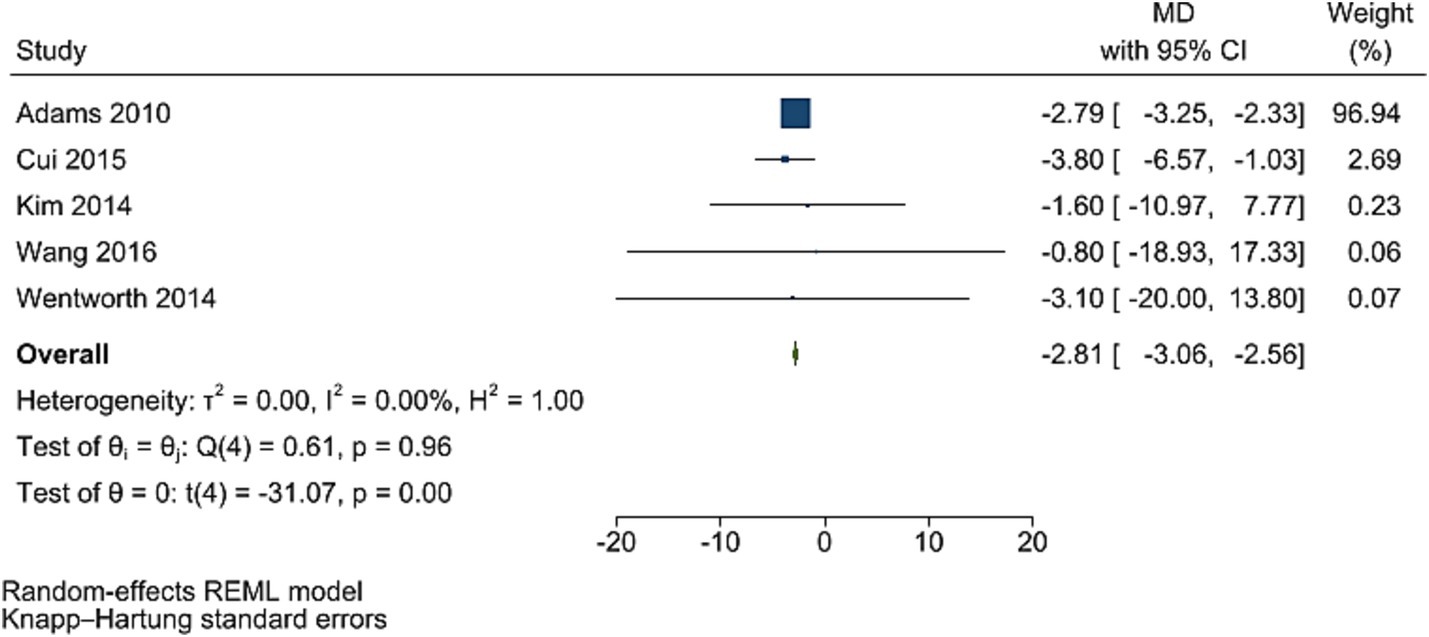
Figure 6. Forest plot detailing mean difference and 95% confidence intervals (CIs), the effects of bariatric surgery supplementation on HOMA-IR.
Fasting insulin levels
Post bariatric surgery, compared to pre-surgery values from 11 studies involving 1,831 participants, led to a significant reduction in insulin levels (WMD = −0.62, 95% CI: −0.88, −0.36, p < 0.001, I2 = 0.0%; tau2 value 0.001; Figure 7). A sensitivity analysis showed no significant change in effect overall estimates after the removal of single studies. Funnel plot was symmetrical (Supplementary Figure S7) with no significant Egger’s test (p = 0.922).
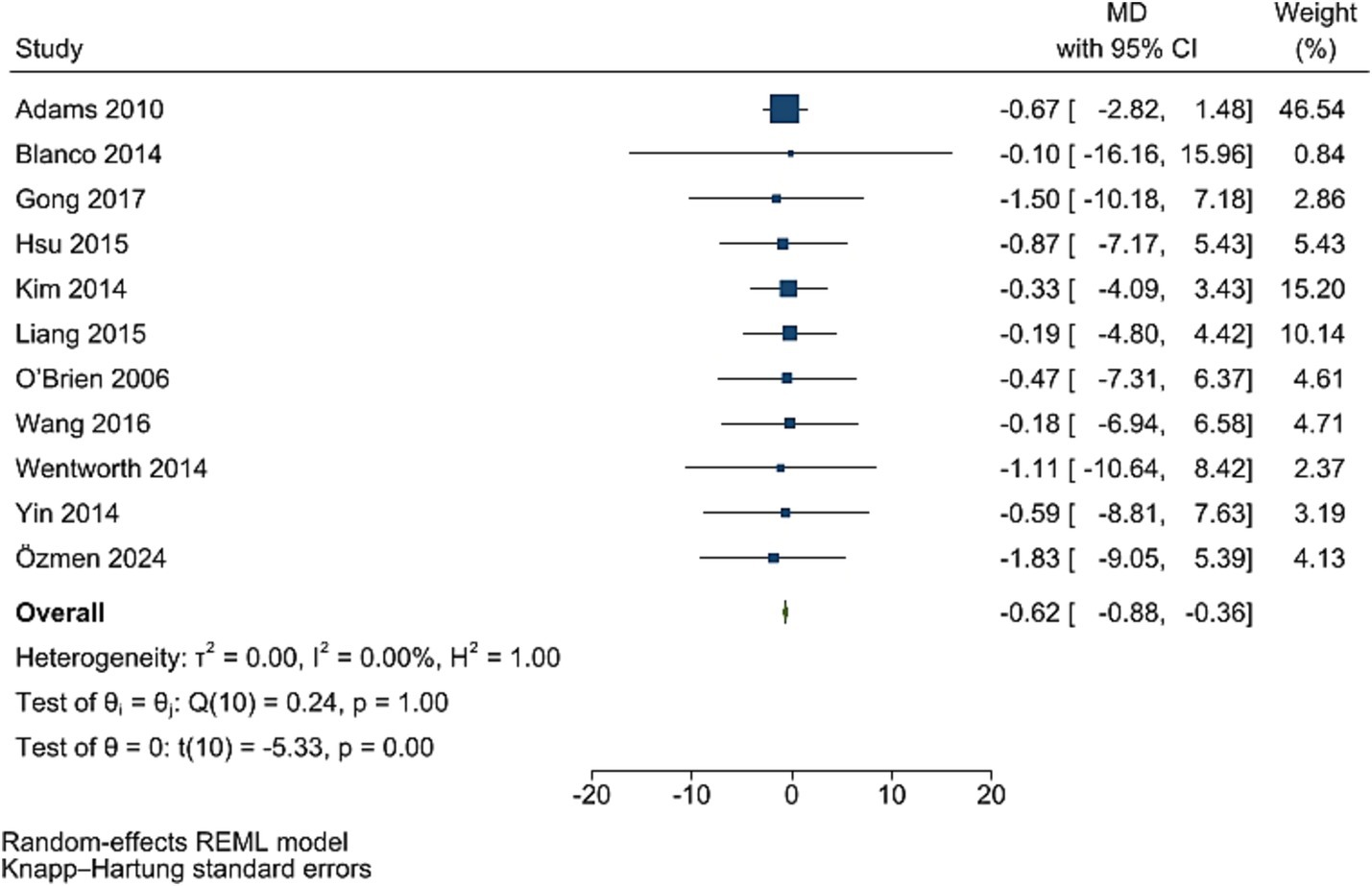
Figure 7. Forest plot detailing mean difference and 95% confidence intervals (CIs), the effects of bariatric surgery supplementation on insulin.
Discussion
The findings from this meta-analysis underscore the profound impact of bariatric surgery on improving glycemic and metabolic outcomes in obese individuals with T2DM. Despite the overall improvements in glycemic parameters, the effect of bariatric surgery on HbA1c levels was not statistically significant in the primary analysis. This finding may reflect the complex interplay of factors influencing long-term glycemic control, including patient adherence, variability in surgical procedures, and baseline glycemic status. Notably, the GRADE quality for HbA1c was rated as low, indicating limited confidence in the reliability of this outcome. This suggests that future high-quality, long-term RCTs are needed to better clarify the true effect of bariatric surgery on HbA1c. Interestingly, after performing the trim and fill analysis to account for potential publication bias, the effect on HbA1c became statistically significant, highlighting how methodological limitations and potential reporting biases may obscure true effects. Significant reductions were observed in FBG, PPG, and HOMA-IR, along with modest improvements in C-peptide and fasting insulin levels. Evidence on FBG, PPG, C-peptide levels, HOMA-IR, and fasting insulin levels had high GRADE quality. These results align with the growing body of evidence demonstrating that bariatric surgery offers not only weight loss but also effective glycemic control and metabolic regulation, often resulting in partial or complete diabetes remission.
Multiple meta-analyses of RCTs have shown dramatic improvements in glycemic control and diabetes remission with bariatric surgery. For example, Wu et al. pooled 8 RCTs and found bariatric surgery gave a ~ 5.8-fold higher remission rate and much larger drops in HbA1c and fasting glucose than medical therapy (57). Kim et al. similarly reported T2DM remission rates of ~47% after RYGB, 42% after sleeve, and 25% after banding, versus only ~5% with medical care; mean HbA1c fell by ~0.96–0.97% after RYGB/SG but not with medical treatment (58). Meta-analyses comparing surgical procedures found RYGB tends to induce higher short-term remission than SG. Borgeraas et al. analyzed 10 RCTs and observed 1-year remission of 57% after RYGB vs. 47% after SG (RR ≈ 1.20), though by 2–5 years the rates were similar (7). Large pooled analyses (n ≈ 22,000) have also reported ~83.8% diabetes resolution after RYGB vs. ~ 47.8% after gastric banding (59). Overall, prior work consistently showed that more aggressive procedures (RYGB, BPD) yielded higher remission and glycemic improvements than restrictive ones. Few meta-reviews focused on insulin/HOMA. Rao et al. (2012) documented dramatic insulin-sensitivity gains after surgery – e.g. HOMA-IR fell by ~40–60% or more by 6–12 months, with particularly rapid drops after RYGB and BPD (60). However, most earlier meta-analyses did not report insulin, C-peptide, or HOMA-IR changes. These earlier analyses generally pooled relatively small numbers of trials and focused on a limited set of outcomes (mostly weight, HbA1c, fasting glucose, and remission). Many did not stratify results by procedure type or include newer RCTs, and none had fully comprehensive quality/GRADE appraisals of the evidence.
The significant reduction in FBG levels, as highlighted by the pooled WMD of −0.82 mg/dL, was consistent with previous studies, further emphasizing the effectiveness of surgical intervention over conventional medical management (61). The high heterogeneity observed in this analysis is consistent with variability in baseline characteristics, surgical techniques, and follow-up durations among the included studies. Following the trim-and-fill analysis, the pooled estimate for HbA1c demonstrated a WMD of −3.99%, confirming a substantial and clinically meaningful impact of bariatric surgery on long-term glycemic control compared to non-surgical interventions. This adjusted estimate suggests that the initial non-significant findings may have been influenced by publication bias. Our results align with prior long-term studies reporting sustained reductions in HbA1c levels up to 5 years postoperatively in patients undergoing bariatric surgery (61). While heterogeneity remains a concern, likely due to differences in patient populations and pre-surgical glycemic status, the findings strongly support the role of bariatric surgery as a metabolic intervention for diabetes management. The results for PPG reduction were consistent with prior studies that demonstrated improved incretin responses post-surgery, which contributes to better PPG control (62). The modest improvement in C-peptide levels, reflected by the pooled WMD of 0.38 ng/mL, suggests partial restoration of beta-cell function following bariatric surgery. This finding corroborates earlier research indicating that bariatric surgery improves pancreatic function and preserves residual beta-cell activity (63). However, the variability in results across studies points to heterogeneity in baseline beta-cell function and the degree of insulin resistance among patients. The significant reduction in HOMA-IR levels, with pooled WMD of −2.81, highlights the role of bariatric surgery in reducing insulin resistance, a hallmark of T2DM. Prior studies have consistently demonstrated that weight loss and improved adipokine profiles post-surgery contribute to enhanced insulin sensitivity (63). Lastly, the improvement in fasting insulin levels (pooled WMD of −0.62 μU/mL) aligns with prior evidence indicating that bariatric surgery reduces compensatory hyperinsulinemia. This outcome is likely driven by a combination of reduced insulin resistance and improved glucose metabolism post-surgery (64). The variability observed in the magnitude of fasting insulin changes across studies may reflect differences in baseline insulin levels, surgical methods, and follow-up durations. Overall, the results strongly support bariatric surgery as an effective intervention for improving glycemic control and metabolic outcomes in obese patients with T2DM.
The observed reductions in fasting blood glucose, postprandial glucose, and HbA1c levels post-bariatric surgery can be attributed to several physiological mechanisms (65). One of the most significant is the improvement in insulin sensitivity due to weight loss and changes in adipokine secretion. Bariatric surgery reduces visceral adiposity, which is strongly associated with insulin resistance, and leads to decreased secretion of pro-inflammatory cytokines like tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6). These changes reduce systemic inflammation and improve insulin signaling (66). Additionally, the alterations in gut hormone dynamics play a critical role. Increased levels of GLP-1 following surgery enhance glucose dependent insulin secretion, suppress glucagon release, and slow gastric emptying, all of which contribute to better glycemic control (67). The improvement in C-peptide levels and reductions in HOMA-IR suggest partial restoration of beta-cell function and reduced insulin resistance (68). The “foregut hypothesis” proposes that exclusion of the proximal small intestine enhances insulin sensitivity, while the “hindgut hypothesis” highlights the role of accelerated nutrient delivery to the distal intestine in stimulating GLP-1 secretion. Furthermore, bariatric surgery leads to a reduction in hepatic glucose output, likely through decreased lipotoxicity and improved liver insulin sensitivity. These metabolic changes collectively contribute to the observed reductions in fasting insulin levels and better overall glycemic control (69).
This meta-analysis is comprehensive, synthesizing data from 39 studies with a large sample size of participants, which enhances the robustness of the findings. The inclusion of multiple glycemic and metabolic outcomes provides a nuanced understanding of the impact of bariatric surgery on T2DM. Rigorous statistical methods, including sensitivity analyses and assessments of publication bias, lend credibility to the results. The adherence to PRISMA guidelines ensures transparency and reproducibility, making the findings reliable for clinical and research purposes. Despite its strengths, this review has several limitations. It is important to acknowledge that many included studies were rated as having a high risk of bias, primarily due to variability in laboratory follow-up protocols and inconsistent reporting standards. These factors may reduce the overall confidence in the pooled results and should be considered when interpreting the findings. The substantial heterogeneity observed across studies for outcomes like FBG, and PPG levels limits the generalizability of the findings. The included studies exhibited considerable variability in sample sizes and follow-up durations, ranging from as few as 6 to nearly 480 participants and from just over 2 months to 10 years. Such heterogeneity may influence the precision and generalizability of the pooled estimates. Smaller studies are more prone to random error, and shorter follow-up periods may not capture the durability of metabolic improvements, particularly for longer-term markers like HbA1c. This variation highlights the need for more standardized, long-term trials with adequate sample sizes to confirm and extend these findings. Additionally, the lack of uniform reporting on comorbid conditions and medication use makes it challenging to isolate the effects of bariatric surgery. Finally, factors such as the presence of cardiovascular disease, hypertension, or dyslipidemia, as well as the type and duration of antidiabetic medication use can significantly affect metabolic outcomes and glycemic response following bariatric surgery. Due to inconsistent or missing data, we were unable to perform adjusted analyses or stratify results based on these clinical variables. Future studies should prioritize standardized reporting of comorbidity profiles and medication regimens to allow for more precise assessments of surgical efficacy and its interaction with medical management.
The findings of this meta-analysis have significant implications for clinical practice. Bariatric surgery should be considered a key therapeutic option for obese patients with T2DM, particularly those with poor glycemic control despite optimal medical therapy. The observed reductions in fasting blood glucose, and postprandial blood glucose levels suggest that surgery can achieve better glycemic outcomes compared to conventional treatments. These benefits are likely to translate into reduced risks of diabetes-related complications, such as retinopathy, nephropathy, and cardiovascular disease. Additionally, the improvements in insulin sensitivity and beta-cell function may delay or prevent disease progression, further enhancing patient quality of life. Clinicians should weigh the risks and benefits of surgery and provide individualized recommendations based on patient preferences, comorbidities, and surgical eligibility criteria.
Future research should focus on understanding the long-term durability of glycemic and metabolic improvements following bariatric surgery. Studies with extended follow-up durations are needed to assess the sustainability of outcomes such as HbA1c and HOMA-IR reductions. Randomized controlled trials comparing different bariatric procedures in diverse populations could provide valuable insights into procedure-specific effects. Additionally, research should explore the role of genetic and epigenetic factors in modulating responses to surgery. Mechanistic studies investigating changes in gut microbiota and their relationship with glycemic outcomes could shed light on novel pathways of metabolic regulation. Finally, economic evaluations assessing cost-effectiveness in different healthcare settings could guide policy decisions.
Conclusion
This meta-analysis demonstrates that bariatric surgery significantly improves glycemic and metabolic outcomes in obese individuals with T2DM. The substantial reductions in FBG, and PPG, coupled with improvements in insulin sensitivity and beta-cell function, highlight the metabolic benefits of surgical intervention beyond weight loss. These findings underscore the need for greater integration of bariatric surgery into diabetes care pathways, offering hope for better disease management and improved patient outcomes.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
XW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. HY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. DW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. FF: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1603670/full#supplementary-material
Footnotes
1. ^ https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
2. ^ https://www.who.int/news-room/fact-sheets/detail/diabetes
References
1. Chandrasekaran, P, and Weiskirchen, R. The role of obesity in type 2 diabetes mellitus—an overview. Int J Mol Sci. (2024) 25:1882. doi: 10.3390/ijms25031882
2. Brzozowska, MM, Isaacs, M, Bliuc, D, Baldock, PA, Eisman, JA, White, CP, et al. Effects of bariatric surgery and dietary intervention on insulin resistance and appetite hormones over a 3 year period. Sci Rep. (2023) 13:6032. doi: 10.1038/s41598-023-33317-6
3. Mokadem, M, Zechner, JF, Margolskee, RF, Drucker, DJ, and Aguirre, V. Effects of roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency. Mol Metab. (2014) 3:191–201. doi: 10.1016/j.molmet.2013.11.010
4. Ramracheya, RD, McCulloch, LJ, Clark, A, Wiggins, D, Johannessen, H, Olsen, MK, et al. PYY-dependent restoration of impaired insulin and glucagon secretion in type 2 diabetes following roux-En-Y gastric bypass surgery. Cell Rep. (2016) 15:944–50. doi: 10.1016/j.celrep.2016.03.091
5. Wilmington, R, Ardavani, A, Hasan, N, Alhindi, Y, Ramzan, I, Anyiam, O, et al. Mechanism of diabetes remission or improvement in glucose control following roux-en-Y gastric bypass versus sleeve gastrectomy: a systematic review and meta-analysis. Obesities. (2025) 5:14. doi: 10.3390/obesities5010014
6. Ferraz-Bannitz, R, Welendorf, CR, Coelho, PO, Salgado, W Jr, Nonino, CB, Beraldo, RA, et al. Bariatric surgery can acutely modulate ER-stress and inflammation on subcutaneous adipose tissue in non-diabetic patients with obesity. Diabetol Metab Syndr. (2021) 13:19. doi: 10.1186/s13098-021-00623-w
7. Borgeraas, H, Hofsø, D, Hertel, JK, and Hjelmesæth, J. Comparison of the effect of roux-en-Y gastric bypass and sleeve gastrectomy on remission of type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. (2020) 21:e13011. doi: 10.1111/obr.13011
8. Vetter, ML, Ritter, S, Wadden, TA, and Sarwer, DB. Comparison of bariatric surgical procedures for diabetes remission: efficacy and mechanisms. Diabetes Spectr. (2012) 25:200–10. doi: 10.2337/diaspect.25.4.200
9. Kapeluto, JE, Tchernof, A, Masckauchan, D, Biron, S, Marceau, S, Hould, FS, et al. Ten-year remission rates in insulin-treated type 2 diabetes after biliopancreatic diversion with duodenal switch. Surg Obes Relat Dis. (2020) 16:1701–12. doi: 10.1016/j.soard.2020.06.052
10. Meira, I, Menino, J, Ferreira, P, Leite, AR, Gonçalves, J, Ferreira, HU, et al. Diabetes remission after bariatric surgery: a 10-year follow-up study. Obes Surg. (2025) 35:161–9. doi: 10.1007/s11695-024-07592-9
11. Page, MJ, JE, MK, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
12. Eldridge, S, Campbell, M, Campbell, M, Dahota, Amy, Giraudeau, B, Higgins, J, et al. Revised Cochrane risk of bias tool for randomized trials (RoB 2.0): additional considerations for cluster-randomized trials. Cochrane Methods Cochrane Database Syst Rev. (2016) 10:11.
13. Seo, H-J, Kim, SY, Lee, YJ, and Park, JE. RoBANS 2: a revised risk of bias assessment tool for nonrandomized studies of interventions. Korean J Family Med. (2023) 44:249–60. doi: 10.4082/kjfm.23.0034
14. Guyatt, GH, Oxman, AD, Vist, GE, Kunz, R, Falck-Ytter, Y, Alonso-Coello, P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
15. Huedo-Medina, TB, Sánchez-Meca, J, Marín-Martínez, F, and Botella, J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. (2006) 11:193. doi: 10.1037/1082-989X.11.2.193
17. Egger, M, Smith, GD, Schneider, M, and Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
18. Ramos, AC, Galvão Neto, MP, de Souza, YM, Galvão, M, Murakami, AH, Silva, AC, et al. Laparoscopic duodenal–jejunal exclusion in the treatment of type 2 diabetes mellitus in patients with BMI< 30 kg/m 2 (LBMI). Obes Surg. (2009) 19:307–12. doi: 10.1007/s11695-008-9759-5
19. DePaula, A, DePaula, AL, Macedo, ALV, Mota, BR, and Schraibman, V. Laparoscopic ileal interposition associated to a diverted sleeve gastrectomy is an effective operation for the treatment of type 2 diabetes mellitus patients with BMI 21–29. Surg Endosc. (2009) 23:1313–20. doi: 10.1007/s00464-008-0156-x
20. Geloneze, B, Geloneze, SR, Fiori, C, Stabe, C, Tambascia, MA, Chaim, EA, et al. Surgery for nonobese type 2 diabetic patients: an interventional study with duodenal–jejunal exclusion. Obes Surg. (2009) 19:1077–83. doi: 10.1007/s11695-009-9844-4
21. Kim, Z, and Hur, KY. Laparoscopic mini-gastric bypass for type 2 diabetes: the preliminary report. World J Surg. (2011) 35:631–6. doi: 10.1007/s00268-010-0909-2
22. Lee, HC, Kim, MK, Kwon, HS, Kim, E, and Song, KH. Early changes in incretin secretion after laparoscopic duodenal–jejunal bypass surgery in type 2 diabetic patients. Obes Surg. (2010) 20:1530–5. doi: 10.1007/s11695-010-0248-2
23. Navarrete, SA, Leyba, JL, and Llopis, SN. Laparoscopic sleeve gastrectomy with duodenojejunal bypass for the treatment of type 2 diabetes in non-obese patients: technique and preliminary results. Obes Surg. (2011) 21:663–7. doi: 10.1007/s11695-011-0371-8
24. Scopinaro, N, Adami, GF, Papadia, FS, Camerini, G, Carlini, F, Briatore, L, et al. The effects of biliopancreatic diversion on type 2 diabetes mellitus in patients with mild obesity (BMI 30–35 kg/m 2) and simple overweight (BMI 25–30 kg/m 2): a prospective controlled study. Obes Surg. (2011) 21:880–8. doi: 10.1007/s11695-011-0407-0
25. Dixon, J, Hur, KY, Lee, WJ, Kim, MJ, Chong, K, Chen, SC, et al. Gastric bypass in type 2 diabetes with BMI< 30: weight and weight loss have a major influence on outcomes. Diabet Med. (2013) 30:e127–34. doi: 10.1111/dme.12107
26. García-Caballero, M, Valle, M, Martínez-Moreno, JM, Miralles, F, Toval, JA, Mata, JM, et al. Resolución de la diabetes mellitus y del síndrome metabólico. Nutr Hosp. (2012) 27:623–31. doi: 10.3305/nh.2012.27.2.5674
27. Shrestha, C, He, H, Liu, Y, Zhu, S, Xiong, J, and Mo, Z. Changes in Adipokines following laparoscopic roux-en-Y gastric bypass surgery in Chinese individuals with type 2 diabetes mellitus and BMI of 22–30 kg· m− 2. Int J Endocrinol. (2013) 2013:240971. doi: 10.1155/2013/240971
28. Blanco, J, Jiménez, A, Casamitjana, R, Flores, L, Lacy, A, Conget, I, et al. Relevance of beta-cell function for improved glycemic control after gastric bypass surgery. Surg Obes Relat Dis. (2014) 10:9–13. doi: 10.1016/j.soard.2013.07.020
29. Maraka, S, Kudva, YC, Kellogg, TA, Collazo-Clavell, ML, and Mundi, MS. Bariatric surgery and diabetes: implications of type 1 versus insulin-requiring type 2. Obesity. (2015) 23:552–7. doi: 10.1002/oby.20992
30. Robert, M, Belanger, P, Hould, FS, Marceau, S, Tchernof, A, and Biertho, L. Should metabolic surgery be offered in morbidly obese patients with type I diabetes? Surg Obes Relat Dis. (2015) 11:798–805. doi: 10.1016/j.soard.2014.12.016
31. Chen, W, Yan, Z, Liu, S, Zhang, G, Sun, D, and Hu, S. The better effect of roux-en-Y gastrointestinal reconstruction on blood glucose of nonobese type 2 diabetes mellitus patients. Am J Surg. (2014) 207:877–81. doi: 10.1016/j.amjsurg.2013.06.011
32. Cui, J-F, Chen, T, Shi, L, Yan, HT, and Tang, LJ. Gastric bypass surgery in non-obese patients with type 2 diabetes mellitus: a 1-year follow-up of 58 cases in Chinese. Int J Clin Exp Med. (2015) 8:4393.
33. Di, J, Zhang, H, Yu, H, Zhang, P, Wang, Z, and Jia, W. Effect of roux-en-Y gastric bypass on the remission of type 2 diabetes: a 3-year study in Chinese patients with a BMI< 30 kg/m2. Surg Obes Relat Dis. (2016) 12:1357–63. doi: 10.1016/j.soard.2016.02.007
34. Gong, K, Li, K, Zhang, N, Zhu, B, du, D, Zhang, D, et al. Gastric bypass procedure for type 2 diabetes patients with BMI< 28 kg/m 2. Surg Endosc. (2017) 31:1172–9. doi: 10.1007/s00464-016-5087-3
35. Heo, Y, Ahn, JH, Shin, SH, and Lee, YJ. The effect of duodenojejunal bypass for type 2 diabetes mellitus patients below body mass index 25 kg/m2: one year follow-up. J Korean Surg Soc. (2013) 85:109–15. doi: 10.4174/jkss.2013.85.3.109
36. Ke, Z, Li, F, Chen, J, Gao, Y, Zhou, X, Sun, F, et al. Effects of laparoscopic Roux-en-Y gastric bypass for type 2 diabetes mellitus: comparison of BMI> 30 and< 30 kg/m 2. Obes Surg. (2017) 27:3040–7. doi: 10.1007/s11695-017-2926-9
37. Kim, MJ, and Hur, KY. Short-term outcomes of laparoscopic single anastomosis gastric bypass (LSAGB) for the treatment of type 2 diabetes in lower BMI (<30 kg/m(2)) patients. Obes Surg. (2014) 24:1044–51. doi: 10.1007/s11695-014-1202-5
38. Lee, WJ, Almulaifi, A, Chong, K, Chen, SC, Tsou, JJ, Ser, KH, et al. The effect and predictive score of gastric bypass and sleeve gastrectomy on type 2 diabetes mellitus patients with BMI < 30 kg/m(2). Obes Surg. (2015) 25:1772–8. doi: 10.1007/s11695-015-1603-0
39. Liang, H, Guan, W, Yang, Y, Mao, Z, Mei, Y, Liu, H, et al. Roux-en-Y gastric bypass for Chinese type 2 diabetes mellitus patients with a BMI < 28 kg/m(2): a multi-institutional study. J Biomed Res. (2015) 29:112–7. doi: 10.7555/JBR.29.20140109
40. Malapan, K, Goel, R, Tai, CM, Kao, YH, Chang, PC, and Huang, CK. Laparoscopic roux-en-Y gastric bypass for nonobese type II diabetes mellitus in Asian patients. Surg Obes Relat Dis. (2014) 10:834–40. doi: 10.1016/j.soard.2014.01.018
41. Wang, G, Zhu, L, Li, W, Yang, X, Li, P, and Zhu, S. Can low BMI Chinese patients with type 2 diabetes benefit from laparoscopic roux-en-Y gastric bypass surgery? Surg Obes Relat Dis. (2016) 12:1890–5. doi: 10.1016/j.soard.2016.06.005
42. Yin, J, Xu, L, Mao, Z, Zhou, X, Zhu, Z, Chen, X, et al. Laparoscopic roux-en-Y gastric bypass for type 2 diabetes mellitus in nonobese Chinese patients. Surg Laparosc Endosc Percutan Tech. (2014) 24:e200–6. doi: 10.1097/SLE.0000000000000068
43. Horwitz, D, Padron, C, Kelly, T, Saunders, JK, Ude-Welcome, A, Schmidt, AM, et al. Long-term outcomes comparing metabolic surgery to no surgery in patients with type 2 diabetes and body mass index 30-35. Surg Obes Relat Dis. (2020) 16:503–8. doi: 10.1016/j.soard.2020.01.016
44. Hsu, CC, Almulaifi, A, Chen, JC, Ser, KH, Chen, SC, Hsu, KC, et al. Effect of bariatric surgery vs medical treatment on type 2 diabetes in patients with body mass index lower than 35: five-year outcomes. JAMA Surg. (2015) 150:1117–24. doi: 10.1001/jamasurg.2015.2602
45. Bhandari, M, Mathur, W, Kumar, R, and Mishra, A. Surgical and advanced medical therapy for the treatment of type 2 diabetes in class I obese patients: a short-term outcome. Obes Surg. (2017) 27:3267–72. doi: 10.1007/s11695-017-2770-y
46. Wentworth, JM, Playfair, J, Laurie, C, Ritchie, ME, Brown, WA, Burton, P, et al. Multidisciplinary diabetes care with and without bariatric surgery in overweight people: a randomised controlled trial. Lancet Diabetes Endocrinol. (2014) 2:545–52. doi: 10.1016/S2213-8587(14)70066-X
47. Özmen, MM . Metabolic effects of bariatric surgery on type 2 diabetes mellitus. Laparoscopic Endoscopic Surg Sci. (2016) 23:147–54.
48. Adams, TD, Pendleton, RC, Strong, MB, Kolotkin, RL, Walker, JM, Litwin, ES, et al. Health outcomes of gastric bypass patients compared to nonsurgical, nonintervened severely obese. Obesity (Silver Spring). (2010) 18:121–30. doi: 10.1038/oby.2009.178
49. Serrot, FJ, Dorman, RB, Miller, CJ, Slusarek, B, Sampson, B, Sick, BT, et al. Comparative effectiveness of bariatric surgery and nonsurgical therapy in adults with type 2 diabetes mellitus and body mass index <35 kg/m2. Surgery. (2011) 150:684–91. doi: 10.1016/j.surg.2011.07.069
50. Leonetti, F, Capoccia, D, Coccia, F, Casella, G, Baglio, G, Paradiso, F, et al. Obesity, type 2 diabetes mellitus, and other comorbidities: a prospective cohort study of laparoscopic sleeve gastrectomy vs medical treatment. Arch Surg. (2012) 147:694–700. doi: 10.1001/archsurg.2012.222
51. Pories, WJ, MacDonald, KG, Morgan, EJ, Sinha, MK, Dohm, GL, Swanson, MS, et al. Surgical treatment of obesity and its effect on diabetes: 10-y follow-up. Am J Clin Nutr. (1992) 55:582s–5s. doi: 10.1093/ajcn/55.2.582s
52. Dixon, JB, and O'Brien, PE. Health outcomes of severely obese type 2 diabetic subjects 1 year after laparoscopic adjustable gastric banding. Diabetes Care. (2002) 25:358–63. doi: 10.2337/diacare.25.2.358
53. Brancatisano, A, Wahlroos, S, Matthews, S, and Brancatisano, R. Gastric banding for the treatment of type 2 diabetes mellitus in morbidly obese. Surg Obes Relat Dis. (2008) 4:423–9. doi: 10.1016/j.soard.2007.10.011
54. Schauer, PR, Bhatt, DL, Kirwan, JP, Wolski, K, Aminian, A, Brethauer, SA, et al. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. (2017) 376:641–51. doi: 10.1056/NEJMoa1600869
55. O'Brien, PE, Dixon, JB, Laurie, C, Skinner, S, Proietto, J, McNeil, J, et al. Treatment of mild to moderate obesity with laparoscopic adjustable gastric banding or an intensive medical program: a randomized trial. Ann Intern Med. (2006) 144:625–33. doi: 10.7326/0003-4819-144-9-200605020-00005
56. Hofsø, D, Nordstrand, N, Johnson, LK, Karlsen, TI, Hager, H, Jenssen, T, et al. Obesity-related cardiovascular risk factors after weight loss: a clinical trial comparing gastric bypass surgery and intensive lifestyle intervention. Eur J Endocrinol. (2010) 163:735–45. doi: 10.1530/EJE-10-0514
57. Wu, G-z, Cai, B, Yu, F, Fang, Z, Fu, XL, Zhou, HS, et al. Meta-analysis of bariatric surgery versus non-surgical treatment for type 2 diabetes mellitus. Oncotarget. (2016) 7:961. doi: 10.18632/oncotarget.11961
58. Kim, JC, Kim, MG, Park, JK, Lee, S, Kim, J, Cho, YS, et al. Outcomes and adverse events after bariatric surgery: an updated systematic review and meta-analysis, 2013-2023. J Metab Bariatr Surg. (2023) 12:76–88. doi: 10.17476/jmbs.2023.12.2.76
59. Buchwald, H, Avidor, Y, Braunwald, E, Jensen, MD, Pories, W, Fahrbach, K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. (2004) 292:1724–37. doi: 10.1001/jama.292.14.1724
60. Rao, RS, Yanagisawa, R, and Kini, S. Insulin resistance and bariatric surgery. Obes Rev. (2012) 13:316–28. doi: 10.1111/j.1467-789X.2011.00955.x
61. Baskota, A, Li, S, Dhakal, N, Liu, G, and Tian, H. Bariatric surgery for type 2 diabetes mellitus in patients with BMI <30 kg/m2: a systematic review and Meta-analysis. PLoS One. (2015) 10:e0132335. doi: 10.1371/journal.pone.0132335
62. Zhou, X, and Zeng, C. Diabetes remission of bariatric surgery and nonsurgical treatments in type 2 diabetes patients who failure to meet the criteria for surgery: a systematic review and meta-analysis. BMC Endocr Disord. (2023) 23:46. doi: 10.1186/s12902-023-01283-9
63. Ji, G, Li, P, Li, W, Sun, X, Yu, Z, Li, R, et al. The effect of bariatric surgery on Asian patients with type 2 diabetes mellitus and body mass index < 30 kg/m(2): a systematic review and Meta-analysis. Obes Surg. (2019) 29:2492–502. doi: 10.1007/s11695-019-03861-0
64. Chow, A, Switzer, NJ, Dang, J, Shi, X, de Gara, C, Birch, DW, et al. A systematic review and Meta-analysis of outcomes for type 1 diabetes after bariatric surgery. J Obes. (2016) 2016:6170719. doi: 10.1155/2016/6170719
65. Sandoval, DA, and Patti, ME. Glucose metabolism after bariatric surgery: implications for T2DM remission and hypoglycaemia. Nat Rev Endocrinol. (2023) 19:164–76. doi: 10.1038/s41574-022-00757-5
66. Goktas, Z, Moustaid-Moussa, N, Shen, C-L, Boylan, M, Mo, H, and Wang, S. Effects of bariatric surgery on adipokine-induced inflammation and insulin resistance. Front Endocrinol (Lausanne). (2013) 4:69. doi: 10.3389/fendo.2013.00069
67. Müller, TD, Finan, B, Bloom, SR, D'Alessio, D, Drucker, DJ, Flatt, PR, et al. Glucagon-like peptide 1 (GLP-1). Mol Metab. (2019) 30:72–130. doi: 10.1016/j.molmet.2019.09.010
68. Batterham, RL, and Cummings, DE. Mechanisms of diabetes improvement following bariatric/metabolic surgery. Diabetes Care. (2016) 39:893–901. doi: 10.2337/dc16-0145
Keywords: bariatric surgery, diabetes mellitus, meta-analysis, obesity, systematic review
Citation: Wei X, Yuan H, Wang D, Zhao J and Fang F (2025) Effect of bariatric surgery on glycemic and metabolic outcomes in people with obesity and type 2 diabetes mellitus: a systematic review, meta-analysis, and meta-evidence of 39 studies. Front. Nutr. 12:1603670. doi: 10.3389/fnut.2025.1603670
Edited by:
Mona Vintilă, West University of Timișoara, RomaniaReviewed by:
Keerthana Kesavarapu, Rutgers, The State University of New Jersey, United StatesMable Pereira, Lincoln American University, Guyana
Copyright © 2025 Wei, Yuan, Wang, Zhao and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Fang, ZmZ6eXp4bm9rbEAxNjMuY29t
 Xiao Wei1
Xiao Wei1
 Fang Fang
Fang Fang