- 1Department of Respiratory and Critical Care Medicine, The Affiliated Changsha Central Hospital, Hengyang Medical School, University of South China, Changsha, China
- 2Department of Cardiovascular Medicine, The Affiliated Changsha Central Hospital, Hengyang Medical School, University of South China, Changsha, China
Background: Research indicates that Life’s Essential 8 (LE8) has health-promoting effects for many diseases, yet few studies have explored its association with asthma patients. This research aimed to investigate the relationships between LE8 and all-cause mortality in asthma patients.
Methods: We conducted a retrospective cohort analysis of seven cycles of data from the 2005–2018 National Health and Nutrition Examination Survey (NHANES). The impact of LE8, which includes four health behaviors (diet, physical activity, smoking, and sleep) and four health factors (BMI, lipids, blood glucose, and blood pressure), on asthma mortality was analyzed using multivariate Cox proportional hazards models. Dose–response relationships between these indicators and mortality were examined using restricted cubic spline (RCS) analysis. Subgroup analyses and interaction tests were performed to verify the stability of the results.
Results: The study included 3,321 asthma patients aged 20 or older, with a median follow-up of 91.03 months, during which 331 patients died. Each one-unit increase in LE8 score was associated with a 1.4% reduction in all-cause mortality risk (HR = 0.986, 95% CI: 0.974–0.998; p < 0.001). Patients with scores ≥80 had a 58.8% lower mortality risk than those with scores <50 (HR = 0.412, 95% CI: 0.203–0.837, p = 0.014). Each one-point increase in health behavior score was linked to a 1.3% decrease in mortality risk (HR = 0.987, 95% CI: 0.982–0.992; p < 0.001). Participants with optimal health behaviors (scores ≥80) had a 53.8% lower mortality risk than those with poor scores (<50; HR = 0.462, 95% CI: 0.275–0.777; p = 0.004). RCS analysis revealed linear associations of LE8 and health behavior scores with mortality, while the relationship between health factor scores and mortality was non-linear, with mortality risk decreasing as scores increased above 80. Subgroup analyses showed stable associations between exposure variables and mortality, particularly strong protective associations in high-income groups.
Conclusion: Optimized LE8, health behavior scores, and health factor scores above 80 are associated with reduced all-cause mortality risk in asthma patients, supporting ideal cardiovascular health as an intervention strategy to lower asthma mortality.
Introduction
Asthma is a heterogeneous chronic airway inflammatory disease that imposes a significant burden on global public health (1). In 2019, approximately 300 million people worldwide were affected by asthma, resulting in about 461,070 deaths every year (2). While the age-standardized prevalence of asthma has decreased globally, its incidence and mortality rates are still on the rise in many regions (3–5). Asthma’s burden in terms of premature death or reduced quality of life remains a big concern (2).
In recent years, there has been a rising concern over the impact of lifestyle factors on the occurrence and prognosis of asthma. Research revealed that unhealthy lifestyle factors are linked to an increased risk of asthma in adults (6), whereas healthy lifestyles can significantly affect the mortality risk of asthma patients. The Oxidative Balance Score (OBS) evaluates both dietary antioxidants (e.g., vitamins C, E, and polyunsaturated fatty acids) and pro-oxidants (e.g., smoking and alcohol). Each quartile increase in OBS is associated with a 63% reduction in all-cause mortality risk among asthma patients (HR = 0.37) (7, 8), indicating that healthy eating habits may lower the risk of asthma death. A 20-year Danish cohort study showed that self-reported leisure-time physical activity is related to a nearly 50% decrease in all-cause mortality for asthma patients (9). Moreover, avoiding tobacco smoke exposure can lead to a 41% decrease in hospitalizations for severe asthma attacks, indirectly reducing deaths associated with acute attacks (10). Identifying multiple modifiable risk factors offers ways to reduce asthma exacerbations and improve prognosis. Obesity and metabolic abnormalities can further worsen asthma prognosis and increase mortality (11, 12). Comprehensive management of comorbidities like diabetes and hypertension can increase the five-year survival rate of elderly asthma patients by 18% (13). Currently, comprehensive assessment studies on the impact of these factors on the prognosis of asthma patients are still limited.
Cardiovascular health (CVH) is closely linked to modifiable behavioral and environmental factors, including tobacco use, passive smoke inhalation, sedentary lifestyles, and pollutant exposure (14). To promote CVH, the American Heart Association (AHA) introduced the ‘Life’s Essential 8’ (LE8) framework in 2022, which integrates eight core metrics targeting both health behaviors and clinical indicators (15). Lifestyle-related practices focused on diet, physical activity, tobacco avoidance, and sleep; and biomarker-based parameters involving body mass index (BMI), lipid metabolism, glycemic control, and vascular pressure regulation. Higher LE8 levels are associated with a lower risk of all causes and cardiovascular-related mortality in US adults, regardless of Cardiovascular disease (CVD) (16, 17). This association extends to a reduced risk of multiple chronic diseases, including chronic obstructive pulmonary disease (COPD), gallstones, metabolic syndrome, non-alcoholic fatty liver disease, and metabolic-associated steatohepatitis (18–23).
Emerging evidence suggests an inverse relationship between LE8 composite scores and asthma incidence. Analyses of National Health and Nutrition Examination Survey (NHANES) datasets further demonstrated that individuals with optimized LE8 profiles exhibit a statistically significant reduction in asthma risk (24). Moreover, United Kingdom Biobank research found that for every standard deviation increase in LE8, the risk of new-onset asthma in adults decreases by 17% (HR = 0.83) (25). Research indicates that changing health-related behaviors, such as quitting smoking, exercising regularly, and controlling weight can prevent CVD and lower asthma incidence by improving immune regulation and airway health.
Despite growing interest in LE8’s clinical relevance, its potential role in predicting mortality outcomes among individuals with asthma remains unexplored. To address this knowledge gap, we leverage NHANES data to comprehensively evaluate associations between LE8 composite metrics (including health behavior and health factor scores) and all-cause mortality risk in this population.
Methods
Research design
Administered by the United States (US) Centers for Disease Control and Prevention, the NHANES serves as a critical epidemiological resource for evaluating population-level health metrics and disease determinants. It systematically gathers nationally representative datasets spanning nutrition, clinical health parameters, and epidemiological trends through a multi-phase stratified probabilistic sampling framework. By enrolling participants from diverse age groups, genders, racial/ethnic categories, and socioeconomic strata across the US, the survey ensures robust generalizability of its findings to the broader population.
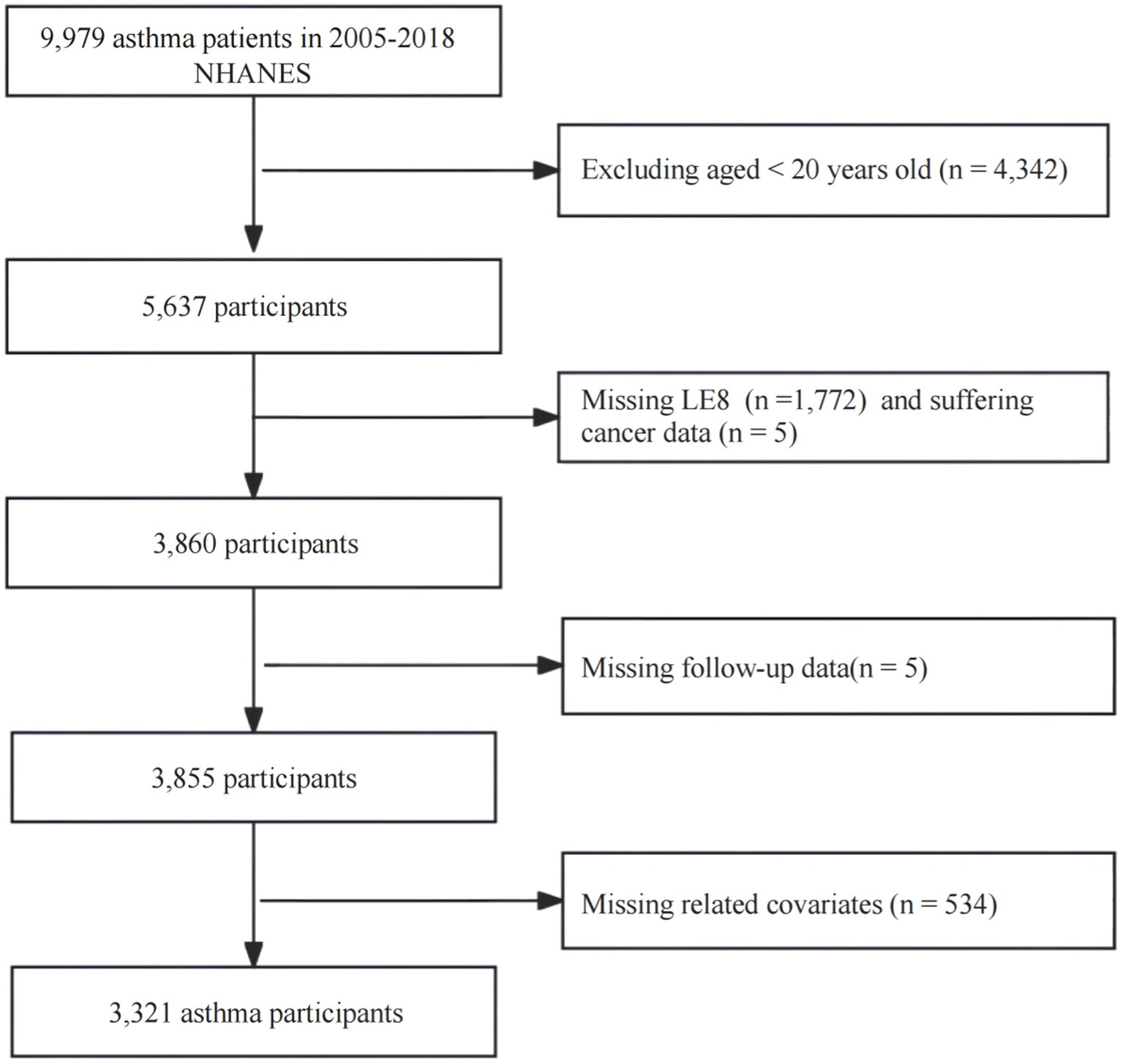
This study adopted a retrospective cohort study design. The study subjects were asthma patients (n = 9,979) from the 2005–2018 NHANES. We excluded the following related data: (1) < 20 years old (n = 4,342); (2) missing LE8 score (n = 1,772); (3) suffering cancer (n = 5); (4) missing follow-up data (n = 5); (5) missing values of other related variables (n = 534). Ultimately, 3,321 participants were included in the analysis.
Ascertainment of deaths
Data on all-cause mortality during the follow-up period were collected, up to December 31, 2019.
The concept of LE8
LE8 is a comprehensive CVH assessment tool developed by the AHA, aimed at measuring an individual’s health level through quantifying health behaviors and biological markers (16, 26, 27). Its core includes the following eight dimensions:
Dietary Quality: Emphasizes balanced nutrition. 2. Physical Activity: Assesses exercise frequency and intensity. 3. Nicotine Exposure: Includes smoking or secondhand smoke exposure. 4. Sleep Health: Focuses on sleep duration and quality. 5. Body Mass Index (BMI): Measures the ratio of weight to height. 6. Lipid Level: Assesses the level of non-high-density lipoprotein (HDL) cholesterol in the blood. 7. Blood Glucose Level: Measured by fasting blood glucose or hemoglobin A1c (HbA1c). 8. Blood Pressure: Assesses the state of vascular health. Each dimension is quantified through standardized scores (0–100 points), with the total score reflecting the overall CVD status. A higher score indicates better health levels (16, 27).
The AHA has documented the detailed scoring methods for each indicator (15). The LE8 scoring framework stratifies cardiovascular health status into three tiers: high CVH (80–100), moderate CVH (50–79), and low CVH (0–49), based on cumulative adherence to the eight defined health metrics.
Dietary patterns were quantitatively evaluated through the Healthy Eating Index (HEI)-2015 framework, which assigns scores based on adherence to dietary guidelines for nutrient adequacy and food group balance. Relevant studies have been published on how to calculate the HEI-2015 from NHANES data (18, 28). Supplementary Table S1 summarizes the components of the HEI-2015 and their scoring criteria. Information on physical activity, smoking, and sleep was collected via self-reported questionnaires. During the physical examination, trained professionals measured participants’ blood pressure, height, and weight. Body mass index (BMI) was derived using the standard formula of body weight (kg) divided by the square of standing height (m2). Venous blood specimens collected from participants underwent centralized laboratory processing to quantify metabolic biomarkers, including fasting lipid profiles, plasma glucose concentrations, and HbA1c levels.
The definition of asthma
The diagnostic criteria for asthma was based on a standardized questionnaire: ‘Have you ever been diagnosed with asthma by a healthcare professional?’ (mcq010).
Data collection
We collected baseline data, including demographic characteristics (gender, age, race), socioeconomic factors (education level, marital status, poverty income ratio (PIR)), comorbid conditions (chronic bronchitis, emphysema, cancer, and CVD), and laboratory indicators (white blood cell count (WBC), glomerular filtration rate (GFR)).
Age was divided into three stages: 20 to 39 years, 40 to 59 years, and 60 years and above (18). Race classification included non-Hispanic (NH) white, NH black, Mexican Americans, Hispanic, and other races. PIR was calculated by the ratio of family monthly income to the poverty line level and was divided into three groups: less than 1.3 (low income), 1.3 to 3.5 (middle income), and 3.5 and above (high income) (18).
Educational attainment was stratified into three tiers: (1) incomplete secondary education, (2) high school diploma or equivalent qualification, (3) undergraduate degree or higher academic achievement (23). Marital status was divided into never married, married or cohabiting, and widowed/divorced/separated. Alcohol use status was operationalized through the validated survey item: ‘Within the past 12 months, have you consumed 12 or more standard alcoholic drinks, regardless of beverage type’. Drinking status was divided into never drank, drank before, and currently drinks.
Data on cancer (has a doctor or other health professional ever told you that you had cancer or malignancy), chronic bronchitis (ever told you that you have chronic bronchitis), emphysema (ever told you that you have emphysema), and CVD (ever been told you had coronary heart disease, congestive heart failure, a heart attack (myocardial infarction), angina, or a stroke) were collected through questionnaires. Venous blood specimens were obtained through standardized phlebotomy protocols and subsequently transferred to certified laboratories for WBC enumeration. GFR was estimated via the Modification of Diet in Renal Disease formula (29).
Statistical analysis
To address the complex stratified probabilistic sampling framework inherent to NHANES, all analytical models incorporated the designated survey weights (wtmec2yr), thereby preserving the dataset’s national-level generalizability. Descriptive statistics for categorical variables were expressed as frequency distributions with adjusted proportions, whereas continuous measures were reported as weighted arithmetic means accompanied by their standard errors. To explore the relationship between LE8 and its components with all-cause mortality in asthma patients, we employed multivariate Cox regression models. Three models were established: Model 1 with no covariate adjustment; Model 2 adjusted for age, gender, and race; and Model 3 further adjusted for education level, marital status, PIR, drinking history, chronic bronchitis, emphysema, cancer, CVD, WBC and GFR. The relationship between LE8 score, healthy behavior score, healthy factor score, and all-cause mortality in asthma was described using Kaplan–Meier Survival Analysis. The dose–response associations between LE8 composite scores (including healthy behavior and healthy factor scores) and all-cause mortality in asthma were modeled via restricted cubic spline (RCS). For non-monotonic relationships, segmented Cox proportional hazards models were applied to estimate differential hazard ratio (HR) above and below identified inflection thresholds. To validate result stability, we performed sensitivity analyses excluding patients with chronic bronchitis and emphysema and also carried out subgroup analyses and interaction tests. The analytical framework was executed within the R software (v4.2.2), with a statistical significance threshold of p < 0.05.
Results
Baseline characteristics
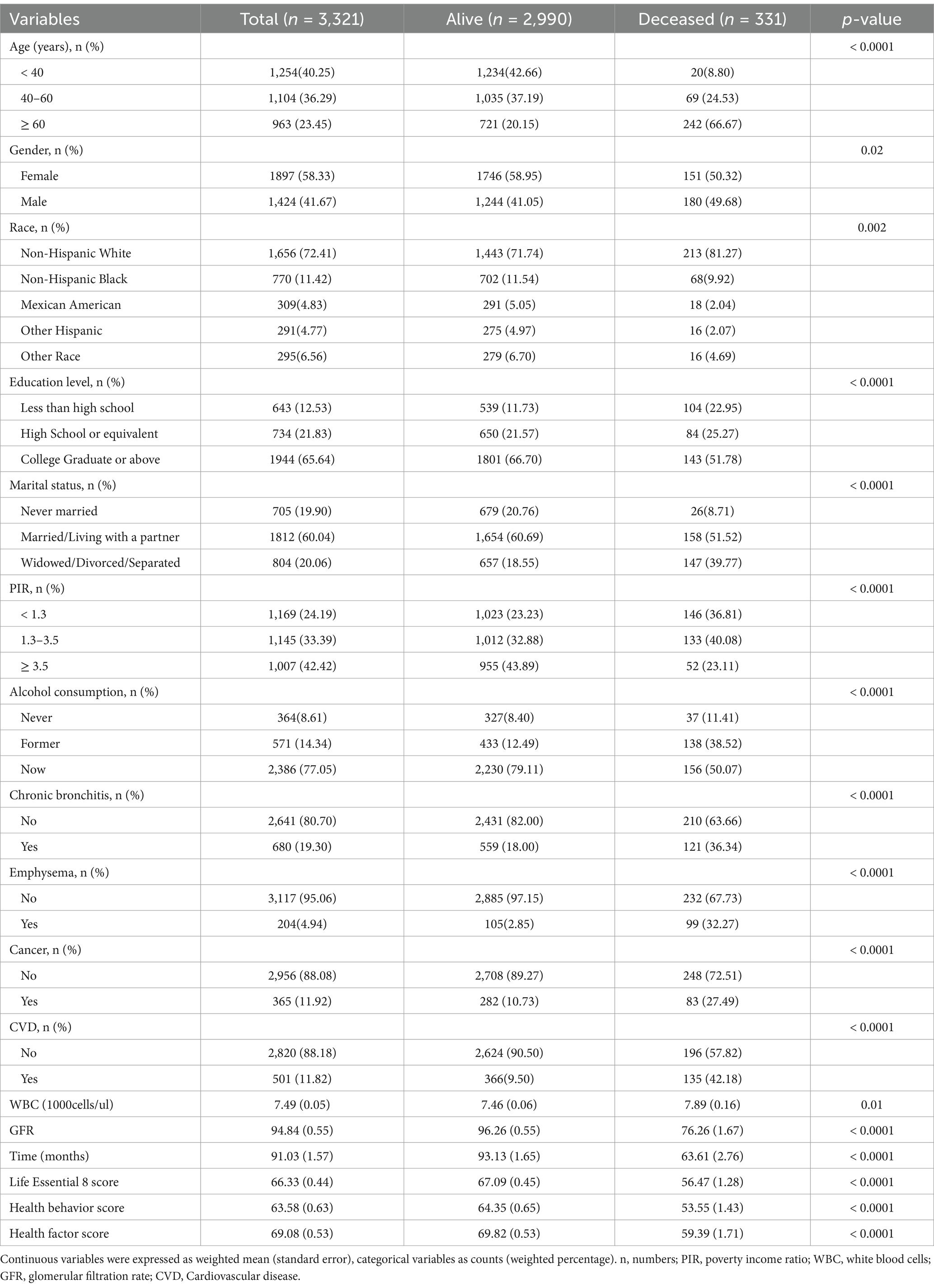
A total of 3,321 adult asthma patients were included in the study. Participants were followed for an average of 91.03 months. The proportion of individuals aged ≥ 60 years was significantly higher in the mortality group (66.67%) compared to the survival group (20.15%; p < 0.0001). The mortality rate was significantly lower among those with a college degree or higher, with 51.78% in the mortality group versus 66.70% in the survival group (p < 0.0001). A higher mortality rate was observed among males, individuals who were widowed or divorced or separated, those with a low income (PIR < 1.3), and those with comorbid chronic diseases (chronic bronchitis, emphysema, cancer, and CVD). The survival group had significantly better scores in LE8, health behavior, and health factors compared to the mortality group (p < 0.0001). Detailed data are presented in Figure 1 and Table 1.
LE8 score and asthma
Analyses presented in Table 2 revealed an inverse association between LE8 score and mortality risk. For each unit increase in LE8 score, the adjusted hazard of death decreased by 4.0% (HR = 0.960, 95% CI: 0.951–0.970; p < 0.001). This protective association persisted in fully adjusted models (Model II), with LE8 scores independently predicting a 1.4% reduction in mortality risk per unit increment (HR = 0.986, 95% CI: 0.974–0.998; p < 0.001). In terms of categorical comparison, the group with a score of ≥ 80 had a 58.8% reduction in the risk of death compared to the group with a score of < 50 (Model 2, p = 0.014), indicating a significant reduction in the risk of death among those with higher scores.
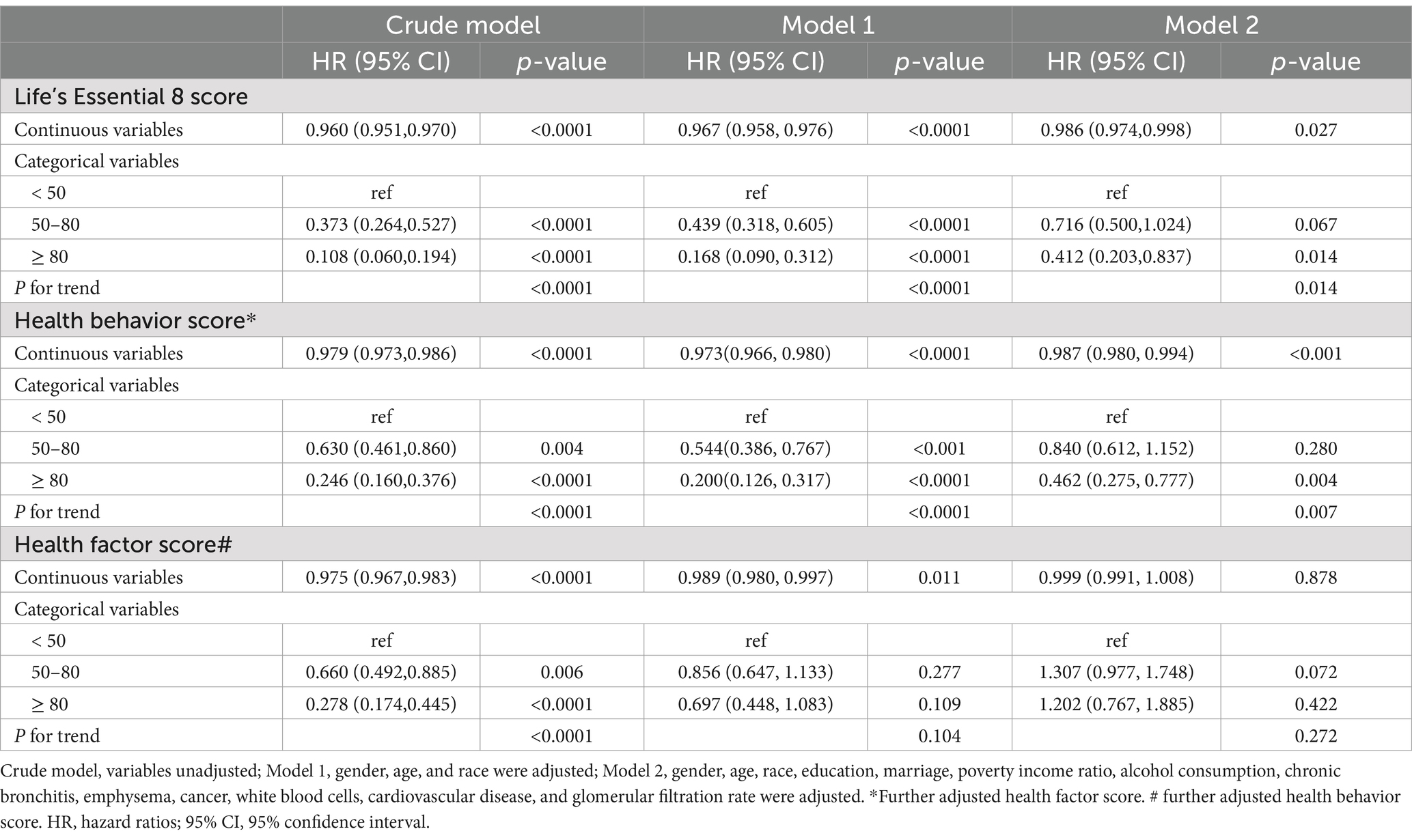
Table 2. Associations of Life’s Essential 8 score, health behavior score, and health factor score with all-cause mortality in patients of asthma.
Kaplan–Meier survival analyses further illustrated that elevated LE8 composite score, health behavior score, and health factor score collectively corresponded to progressive declines in cumulative mortality incidence (Figure 2). Following full adjustment for covariates, RCS modeling confirmed a monotonic inverse relationship between LE8 scores and mortality risk in asthma cohorts (P-nonlinear = 0.820; Figure 3A).
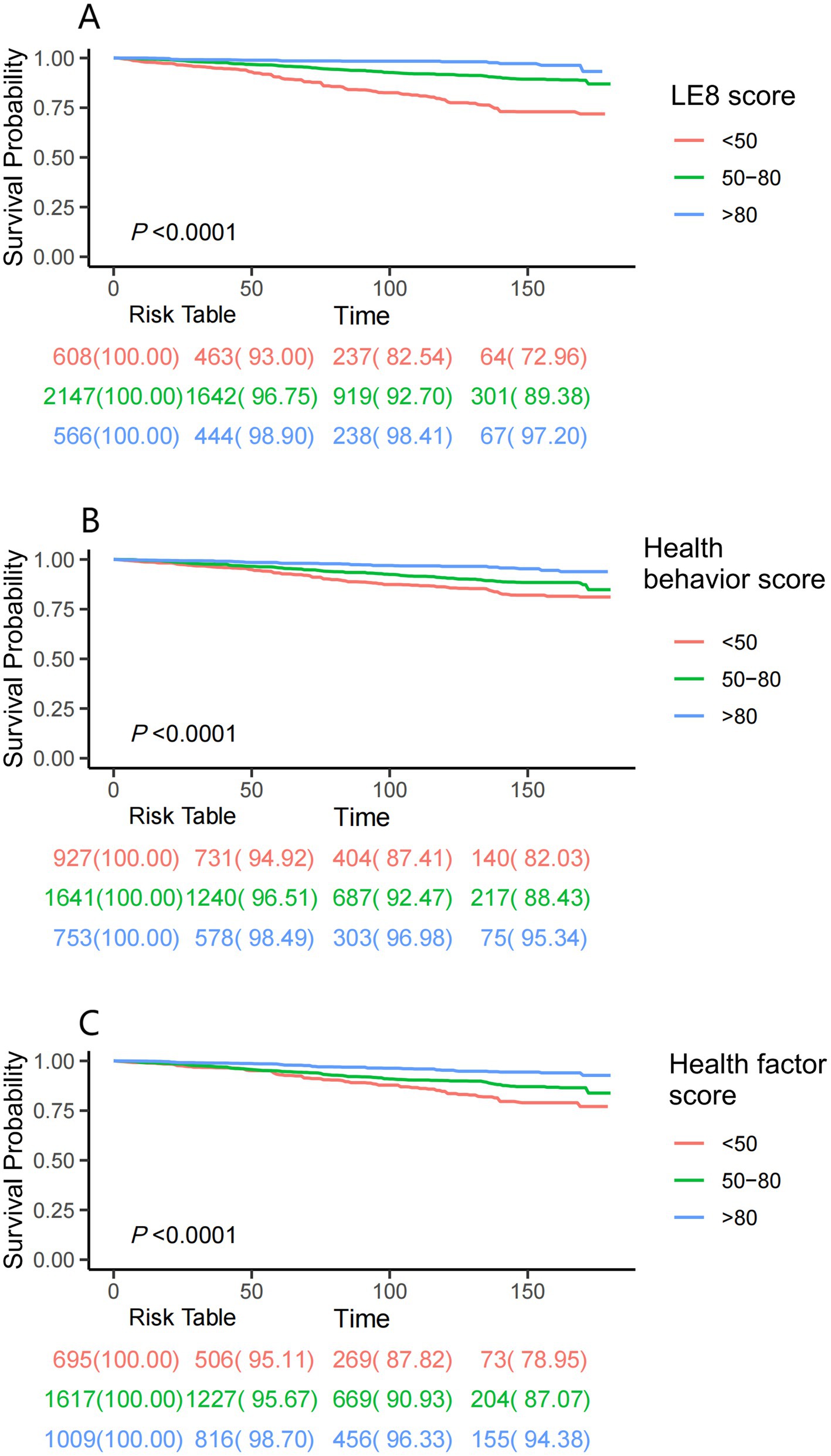
Figure 2. Kaplan–Meier survival analyses were used to assess the associations between LE8 score (A), health behavior score (B), health factor score (C), and all-cause mortality in asthma patients. LE8: Life’s Essential 8.
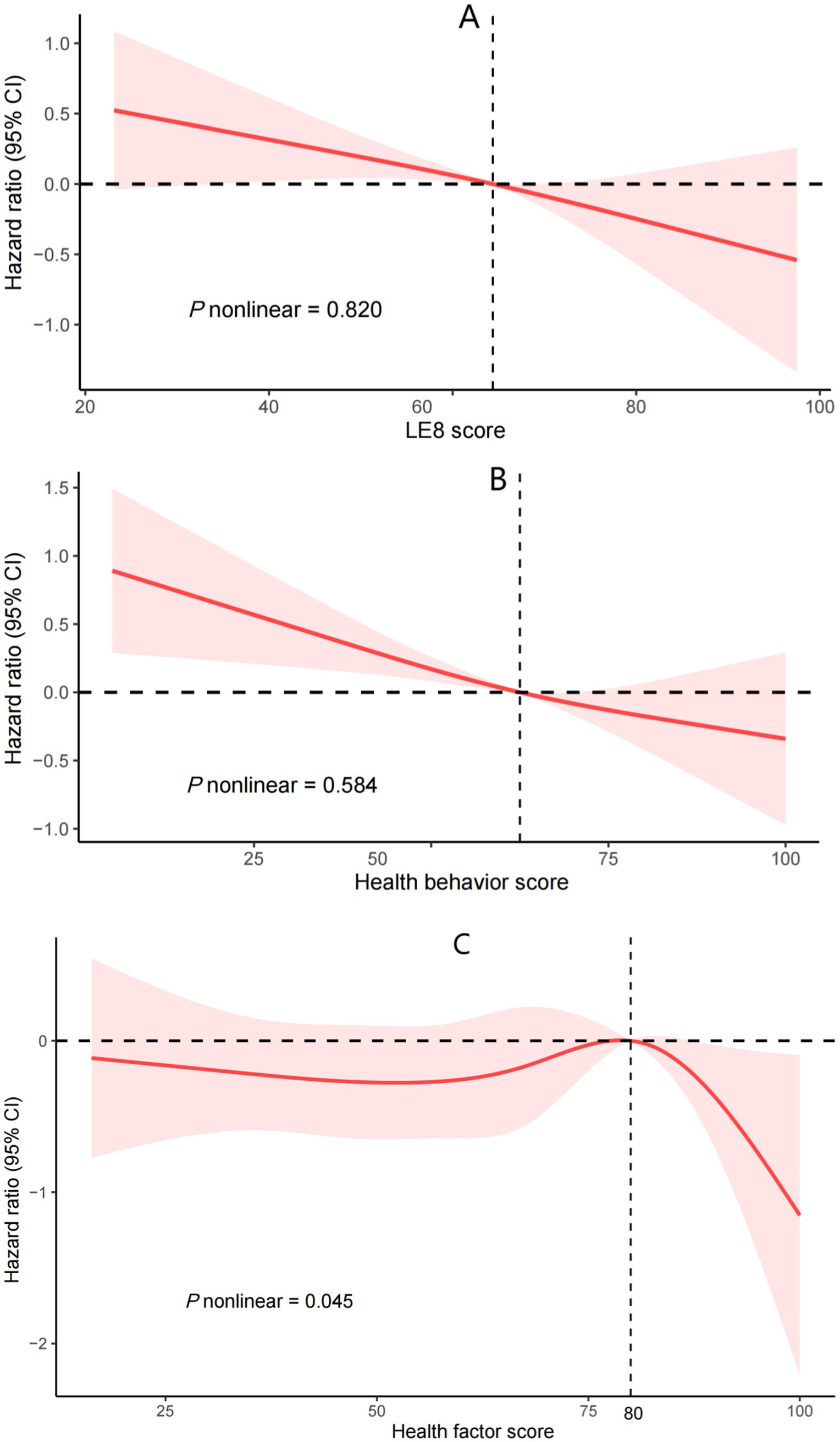
Figure 3. Cox regression using restricted cubic spline regression of LE8 score (A), health behavior score (B), health factor score (C), with all-cause mortality. LE8: Life’s Essential 8. (A) was adjusted for gender, age, race, education, marriage, poverty income ratio, alcohol consumption, chronic bronchitis, emphysema, cancer, white blood cells, cardiovascular disease, and glomerular filtration rate. (B) was further adjusted for health factor score. (C) was further adjusted for health behavior score.
Health behavior score and asthma
In multivariable-adjusted analyses (Model 2), each incremental point in the health behavior score conferred a 1.3% reduced risk of death. (HR = 0.987, 95% CI: 0.982–0.992; p < 0.001). Categorical stratification further revealed a 53.8% mortality risk reduction among participants with optimal behavioral profiles (score ≥ 80) relative to those with suboptimal scores (< 50; p = 0.004). RCS analyses corroborated a monotonic dose–response relationship between health behavior scores and survival outcomes (P-nonlinear = 0.584; Figure 3B).
Health factor score and asthma
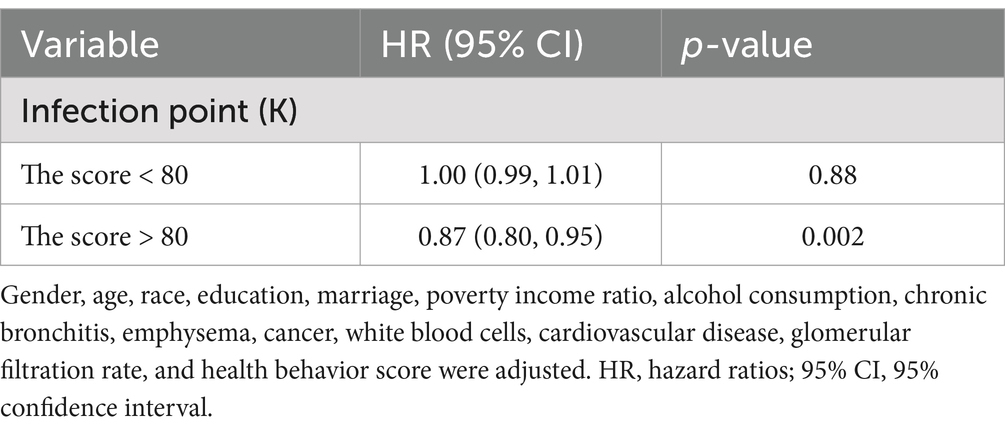
Initial analyses (Crude and Model 1) demonstrated a statistically significant linkage between health factor metrics and mortality risk (p < 0.05). However, this relationship attenuated to non-significance upon full covariate adjustment in Model 2 (HR = 0.999, 95% CI: 0.988–1.010; p = 0.878). Restricted cubic spline modeling revealed a nonlinear dose–response pattern (P-nonlinear = 0.045; Figure 3C), with threshold analysis identifying 80 as the critical inflection point for mortality risk stratification. Below 80, it was unrelated to all - cause mortality (p = 0.88). Above 80, for every unit increase in the score, the risk of all-cause death increased by 13% (HR = 0.87;95% CI: 0.80, 0.95; p = 0.002; Table 3).
Stratified analysis and interaction tests
Subgroup analyses consistently demonstrated homogeneity in the inverse association between LE8 score and mortality risk across most demographic strata (P for interaction > 0.05). Notably, the mortality risk reduction attributable to LE8 optimization was most substantial among high-income participants (PIR ≥ 3.5; HR = 0.96, 95% CI: 0.93–0.99; P for interaction = 0.004). The impact of exposed variables on all-cause mortality was more pronounced in the non-emphysema or non-CVD subgroups (P for interaction < 0.05; Figure 4).
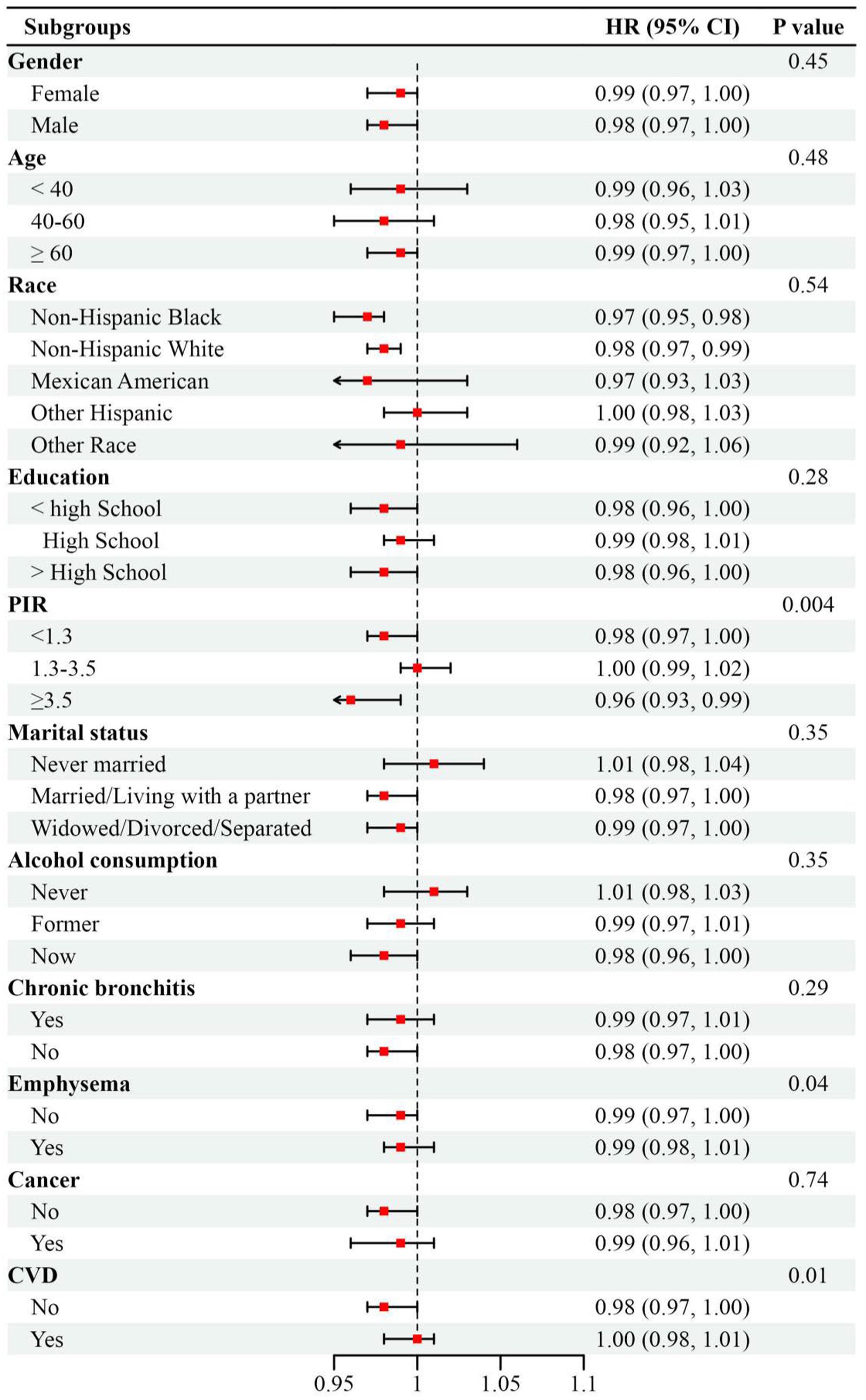
Figure 4. Subgroup analysis between Life’s Essential 8 score with all-cause mortality in patients of asthma, weighted. Adjusted by gender, age, race, education, marriage, poverty income ratio, alcohol consumption, chronic bronchitis, emphysema, cancer, white blood cells, cardiovascular disease, and glomerular filtration rate except for the stratification variable. Abbreviations: HR, hazard ratios; 95% CI, 95% confidence interval; PIR, poverty income ratio; CVD, Cardiovascular disease.
Sensitivity analyses
We further excluded participants with chronic bronchitis (n = 680) and emphysema (n = 91). Sensitivity analyses showed similar inverse associations between LE8 score, health behavior score, health factor score, and all-cause mortality in asthma patients (Supplementary Tables 1, 2; Supplementary Figure 1). Stratified analyses also confirmed that the association between LE8 score and reduced all-cause mortality risk was more pronounced in high - income participants (Supplementary Table 3).
Discussion
This study analyzed NHANES data and for the first time assessed the relationship between the LE8 comprehensive score, its components of health behaviors and health factors, and the all-cause mortality rate of asthma patients. Results showed that higher LE8 and health behavior score were significantly associated with reduced mortality risk. Specifically, compared to scores <50, higher LE8 scores, particularly those ≥80, were linked to a nearly 60% reduction in mortality risk. Subgroup analyses confirmed the robustness of these findings, revealing a stronger protective effect of LE8 scores in high-income individuals (P for interaction = 0.004). This suggests that limited access to healthcare resources and higher environmental exposure risks in economically disadvantaged groups may diminish the potential benefits of healthy behaviors.
Growing evidence suggests that the association between LE8 metrics and asthma pathogenesis may predominantly arise from shared etiological pathways, including chronic inflammatory states, systemic oxidative imbalance, and adiposity-related metabolic dysregulation, alongside modifiable behavioral and environmental determinants such as active/passive tobacco exposure, sedentary lifestyles, and exposure to environmental pollutants (14). Asthma is linked to higher levels of inflammation and oxidative stress (24, 30). Dietary patterns may influence inflammation, oxidative stress, mucus hypersecretion, and airway remodeling, with healthier eating habits associated with fewer asthma symptoms and better asthma control (31, 32). Tobacco smoke boosts asthma risk as its reactive oxygen species can trigger oxidative stress, worsening airway hyper-responsiveness in asthma (33–35). Smoking cessation can curb inflammation and oxidative stress (36). Higher physical activity levels are linked to better asthma control and reduced systemic inflammation markers like IL-6 and TNF-α (37–39). Conversely, unhealthy lifestyle factors such as poor diet, sedentary behavior, and obesity are associated with worse asthma control and reduced quality of life (40). The core features of metabolic syndrome, including obesity, insulin resistance, hypertension, and dyslipidemia, are closely related to chronic low-grade inflammation and oxidative stress. These conditions can worsen airway inflammation, leading to deteriorated asthma control and increased risks of exacerbations and mortality (14). These are potential health behavior and health factor indicators within LE8.
Previous studies have examined individual behavioral indicators’ links to asthma, whereas LE8 assesses multiple health behavior and factor indicators. Evidence showed LE8, health behavior score, and health factor score were negatively associated with asthma risk, with inflammation and oxidative stress partly mediating this link (24). This study revealed a linear inverse association between LE8, health factor score, and asthma mortality. However, health factor score exhibited a non-linear link to asthma mortality. Our findings align with Jiao Xu et al., who reported a nonlinear association between health factor scores and asthma prevalence, and a linear association with health behavior scores (41). Compared to previous studies on LE8-related mortality, in individuals with COPD, a 10-point increase in the LE8 score is associated with a 4% reduction in all-cause mortality (23). In patients with metabolic syndrome, LE8 and health behavior score shows linear associations with all-cause and CVD mortality, whereas health factor score exhibits a nonlinear relationship (22). This is consistent with the results of this study. Possible reasons include survivor bias, reverse causality, or overadjustment in participants. These results suggest that different LE8 levels might have diverse impacts on asthma. When the score exceeds 80, higher scores are linked to a lower risk of asthma-related death. The results highlight the importance of optimizing cardiovascular health metrics, enhancing healthy behaviors, and achieving high health factor score for better asthma prognosis. Subgroup analyses revealed that higher LE8 scores offer the strongest protective effect in the PIR > 3.5 group. This aligns with past research indicating that disadvantaged socioeconomic groups are more adversely affected by unhealthy lifestyles, and that low socioeconomic status plays a significant role in adult asthma progression (6, 42). These findings provide a theoretical basis for developing public health strategies to improve asthma patient prognoses. It is necessary to promote low-cost lifestyle interventions, such as community fitness programs, free smoking cessation services, and education on healthy eating and proper sleep. Moreover, poverty-reduction social policies are essential and must be implemented.
This study has several limitations. Residual confounding factors, such as unmeasured environmental, genetic factors, or the absence of asthma-specific variables, such as medication adherence, lung function, or exacerbation history, which may influence both LE8 components and mortality risk. The reliance on self-reported behaviors may introduce bias. The cross-sectional nature of the LE8 score fails to capture the dynamic changes in health behaviors. Nonetheless, this study offers new evidence on the all-cause mortality risk in asthma patients through a large sample size, multi-dimensional adjustments, and subgroup analyses. Future research could explore intervention trials testing LE8-guided lifestyle programs across diverse socioeconomic settings and further examine the health impacts of each LE8 component in asthma patients. Additionally, more research is needed to uncover the mechanisms behind the observed associations and develop targeted interventions based on these findings.
Conclusion
Higher LE8, health behavior score, and health factor score are linked to lower all-cause mortality risk in asthma patients. Optimizing cardiovascular health, especially through modifiable behaviors, is an intervenable strategy to reduce mortality in adults with asthma.
Data availability statement
The datasets presented in this article are not readily available because authors can provide raw data if reasonably requested. Requests to access the datasets should be directed to Sue Zhao, MjAxODA1MDc4NUB1c2MuZWR1LmNu.
Ethics statement
This study was approved by the National Center for Health Statistics (NCHS) Ethics Review Board. All methods were carried out in accordance with the Declaration of Helsinki. Written informed consent from the patients/participants or patients/participants legal guardian/next of kin was required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
JZ: Writing – review & editing, Conceptualization. RT: Methodology, Writing – review & editing, Conceptualization. SZ: Writing – original draft, Formal analysis, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by Hunan Provincial Natural Science Foundation of China (2023JJ60407).
Acknowledgments
We would like to express our gratitude to both the study participants and the NHANES researchers. Thanks to Zhang Jing (Second Department of Infectious Disease, Shanghai Fifth People’s Hospital, Fudan University) for his work on the NHANES database. His outstanding work, nhanesR package and webpage, makes it easier for us to explore NHANES database.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1603875/full#supplementary-material
References
1. Bakakos, P, Kostikas, K, Loukides, S, Makris, M, Papadopoulos, NG, Steiropoulos, P, et al. Reducing tolerance for SABA and OCS towards the extreme ends of asthma severity. J Personalized Med. (2022) 12:504. doi: 10.3390/jpm12030504
2. Wang, Z, Li, Y, Gao, Y, Fu, Y, Lin, J, Lei, X, et al. Global, regional, and national burden of asthma and its attributable risk factors from 1990 to 2019: a systematic analysis for the global burden of disease study 2019. Respir Res. (2023) 24:169. doi: 10.1186/s12931-023-02475-6
3. Rajvanshi, N, Kumar, P, and Goyal, JP. Global initiative for asthma guidelines 2024: an update. Indian Pediatr. (2024) 61:781–6. doi: 10.1007/s13312-024-3260-7
4. Davitte, J, DeBarmore, B, Hinds, D, Zhang, S, Chao, J, and Sansbury, L. Asthma control among treated US asthma patients in practice fusion's electronic medical record research database. NPJ Primary Care Respiratory Med. (2023) 33:17. doi: 10.1038/s41533-023-00338-7
5. Huang, K, Wang, W, Wang, Y, Li, Y, Feng, X, Shen, H, et al. Evaluation of a global initiative for asthma education and implementation program to improve asthma CARE quality (CARE4ALL): protocol for a multicenter, single-arm study. JMIR Res Protoc. (2025) 14:e65197. doi: 10.2196/65197
6. Fan, Z, Xu, M, Chen, S, Wang, J, Gong, Y, Feng, X, et al. Association of Socioeconomic Status and a broad combination of lifestyle factors with adult-onset asthma: a cohort study. J Allergy Clin Immunol Pract. (2024) 12:2066–73. doi: 10.1016/j.jaip.2024.04.009
7. Zhao, W, Sun, Y, and Zhu, B. Association of oxidative balance scores with cardiovascular and all cause mortality in patients with asthma. Sci Rep. (2025) 15:3901. doi: 10.1038/s41598-025-87996-4
8. Liu, C, Liang, D, Xiang, G, Zhao, X, Xiao, K, and Xie, L. Association between oxidative balance score and all-cause, CVD and respiratory-related mortality in the US older adults of asthma patients with diabetes. Front Nutr. (2024) 11:1519570. doi: 10.3389/fnut.2024.1519570
9. Tupper, OD, Andersen, ZJ, and Ulrik, CS. Demographic, lifestyle and comorbid risk factors for all-cause mortality in a Danish cohort of middle-aged adults with incident asthma. BMJ Open. (2021) 11:e049243. doi: 10.1136/bmjopen-2021-049243
10. Liu, T, Woodruff, PG, and Zhou, X. Advances in non-type 2 severe asthma: from molecular insights to novel treatment strategies. Eur Respir J. (2024) 64:2300826. doi: 10.1183/13993003.00826-2023
11. Wang, S, Li, D, and Sun, L. Weight-adjusted waist index is an independent predictor of all-cause and cause-specific mortality in patients with asthma. Heart Lung: J Critical Care. (2024) 68:166–74. doi: 10.1016/j.hrtlng.2024.07.002
12. Lin, P, Zhao, Y, Shi, Y, and Liang, Z. Associations of asthma control with hypertension, cardiovascular disease, and mortality in obese individuals. Intern Emerg Med. (2024) 19:951–7. doi: 10.1007/s11739-024-03554-2
13. Listyoko, AS, Okazaki, R, Harada, T, Inui, G, and Yamasaki, A. Exploring the association between asthma and chronic comorbidities: impact on clinical outcomes. Front Med. (2024) 11:1305638. doi: 10.3389/fmed.2024.1305638
14. Aggarwal, K, Bansal, V, Mahmood, R, Kanagala, SG, and Jain, R. Asthma and cardiovascular diseases: uncovering common ground in risk factors and pathogenesis. Cardiol Rev. (2023) 33:219–26. doi: 10.1097/CRD.0000000000000600
15. Lloyd-Jones, DM, Allen, NB, Anderson, CAM, Black, T, Brewer, LC, Foraker, RE, et al. Life's essential 8: updating and enhancing the American Heart Association's construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. (2022) 146:e18–43. doi: 10.1161/CIR.0000000000001078
16. Nguyen, XT, Li, Y, Gong, Y, Houghton, S, Ho, YL, Pyatt, M, et al. Cardiovascular health score and atherosclerotic cardiovascular disease in the million veteran program. JAMA Netw Open. (2024) 7:e2447902. doi: 10.1001/jamanetworkopen.2024.47902
17. Yan, Z, Pu, X, Cai, Y, Chang, X, Liu, Z, and Liu, R. Biological aging traits mediate the association between cardiovascular health levels and all-cause and cardiovascular mortality among adults in the U.S. without cardiovascular disease. Biogerontology. (2025) 26:40. doi: 10.1007/s10522-025-10185-3
18. Liu, Y, Li, W, Tang, J, and Gao, S. Association of life's essential 8 with chronic obstructive pulmonary disease: a population-based analysis of NHANES 2007-2018. BMC Public Health. (2024) 24:3144. doi: 10.1186/s12889-024-20534-5
19. Xiao, Y, Zhao, W, Zhao, Q, Pang, K, Gao, Q, Yang, X, et al. Exploring the association between life's essential 8 and gallstone disease in the US adult population: a population-based study utilizing NHANES data from 2017-2018. BMC Gastroenterol. (2024) 24:453. doi: 10.1186/s12876-024-03545-9
20. Li, X, Zhang, Y, Gong, J, Ni, X, Yin, J, and Lv, Z. Association of life's essential 8 with all-cause and cause-specific mortality in metabolic dysfunction-associated steatotic liver disease. Sci Rep. (2024) 14:30624. doi: 10.1038/s41598-024-82875-w
21. Xu, N, Lu, X, Luo, C, and Chen, J. Race/ethnicity-specific association between the American Heart Association's new life's essential 8 and stroke in US adults with nonalcoholic fatty liver disease: evidence from NHANES 2005-2018. J Clin Neurosci: Official J Neurosurg Society of Australasia. (2025) 132:111005. doi: 10.1016/j.jocn.2024.111005
22. Zhou, DC, Liang, JL, Hu, XY, Fang, HC, Liu, DL, Zhao, HX, et al. Adherence to higher life's essential 8 scores is linearly associated with reduced all-cause and cardiovascular mortality among US adults with metabolic syndrome: results from NHANES 2005-2018. PLoS One. (2024) 19:e0314152. doi: 10.1371/journal.pone.0314152
23. Sun, C, Zhang, XX, and Cao, J. Low life essential 8 score as a risk factor for long-term mortality in chronic obstructive pulmonary disease: a study based on the analysis of NHANES data from 2007 to 2012. Int J Chron Obstruct Pulmon Dis. (2024) 19:2545–57. doi: 10.2147/COPD.S469584
24. Shen, G, Yang, Y, Wang, N, Shi, S, Chen, Y, Qiao, Y, et al. Association of life's essential 8 and asthma: mediating effect of inflammation and oxidative stress. J Asthma. (2025) 62:328–35. doi: 10.1080/02770903.2024.2400613
25. Zhang, H, Chang, Q, Yang, H, Yu, H, Chen, L, Zhao, Y, et al. Life's essential 8, genetic predisposition, and risk of incident adult-onset asthma: a prospective cohort study. Am J Clin Nutr. (2024) 119:100–7. doi: 10.1016/j.ajcnut.2023.11.009
26. Zhang, W, Yuan, Z, Wang, Y, Jin, Z, Luo, Z, and Wang, X. The association between life's essential 8 and psoriasis in American adults: a cross-sectional NHANES study. Clin Cosmet Investig Dermatol. (2024) 17:2555–63. doi: 10.2147/CCID.S476594
27. Liang, Q, Zou, M, and Peng, Z. Associations between life's essential 8 and liver function: a cross-sectional study. Front Nutr. (2024) 11:1515883. doi: 10.3389/fnut.2024.1515883
28. Krebs-Smith, SM, Pannucci, TE, Subar, AF, Kirkpatrick, SI, Lerman, JL, Tooze, JA, et al. Update of the healthy eating index: HEI-2015. J Acad Nutr Diet. (2018) 118:1591–602. doi: 10.1016/j.jand.2018.05.021
29. Shchekochikhin, D, Nikiforova, T, Shilova, A, Nesterov, A, Baturina, O, Gognieva, D, et al. Evaluation of discriminative capacity of two formulas of CKD-EPI to predict complications after the first episode of heart failure with preserved ejection fraction. Int J Nephrol Renov Dis. (2019) 12:113–8. doi: 10.2147/IJNRD.S196976
30. Michaeloudes, C, Abubakar-Waziri, H, Lakhdar, R, Raby, K, Dixey, P, Adcock, IM, et al. Molecular mechanisms of oxidative stress in asthma. Mol Asp Med. (2022) 85:101026. doi: 10.1016/j.mam.2021.101026
31. Ignacio Carlotto, C, Bernardes, S, Zanella, P, and Silva, FM. Dietary patterns and risk of chronic obstructive pulmonary disease (COPD) and clinical outcomes in diagnosed patients: a scoping review. Respir Med. (2024) 233:107773. doi: 10.1016/j.rmed.2024.107773
32. Andrianasolo, RM, Kesse-Guyot, E, Adjibade, M, Hercberg, S, Galan, P, and Varraso, R. Associations between dietary scores with asthma symptoms and asthma control in adults. Eur Respir J. (2018) 52:1702572. doi: 10.1183/13993003.02572-2017
33. Pietinalho, A, Pelkonen, A, and Rytilä, P. Linkage between smoking and asthma. Allergy. (2009) 64:1722–7. doi: 10.1111/j.1398-9995.2009.02208.x
34. Del Giacco, SR, Firinu, D, Bjermer, L, and Carlsen, KH. Exercise and asthma: an overview. European Clin Respiratory J. (2015) 2:27984. doi: 10.3402/ecrj.v2.27984
35. Ding, S, and Zhong, C. Exercise and asthma. Adv Exp Med Biol. (2020) 1228:369–80. doi: 10.1007/978-981-15-1792-1_25
36. King, CC, Piper, ME, Gepner, AD, Fiore, MC, Baker, TB, and Stein, JH. Longitudinal impact of smoking and smoking cessation on inflammatory markers of cardiovascular disease risk. Arterioscler Thromb Vasc Biol. (2017) 37:374–9. doi: 10.1161/ATVBAHA.116.308728
37. Cordova-Rivera, L, Gibson, PG, Gardiner, PA, Powell, H, and McDonald, VM. Physical activity and exercise capacity in severe asthma: key clinical associations. J Allergy Clin Immunol Pract. (2018) 6:814–22. doi: 10.1016/j.jaip.2017.09.022
38. Loponen, J, Vähätalo, I, Tuomisto, LE, Niemelä, O, Lehtimäki, L, Hämäläinen, M, et al. Physical exercise, systemic inflammation and adult-onset asthma: a 12-year follow-up study. J Asthma. (2024) 62:714–24. doi: 10.1080/02770903.2024.2438096
39. Heikkinen, SAM, Mäkikyrö, EMS, Hugg, TT, Jaakkola, MS, and Jaakkola, JJK. Effects of regular exercise on asthma control in young adults. J Asthma. (2018) 55:726–33. doi: 10.1080/02770903.2017.1366510
40. Nyenhuis, SM, Dixon, AE, and Ma, J. Impact of lifestyle interventions targeting healthy diet, physical activity, and weight loss on asthma in adults: what is the evidence? J Allergy Clin Immunol Pract. (2018) 6:751–63. doi: 10.1016/j.jaip.2017.10.026
41. Xu, J, and Tang, J. Associations between asthma and life's essential 8: a cross-sectional study. Front Med. (2025) 12:1446900. doi: 10.3389/fmed.2025.1446900
Keywords: asthma, Life’s Essential 8, health behaviors, cardiovascular health, mortality
Citation: Zhu J, Tao R and Zhao S (2025) Association of Life’s Essential 8 with all-cause mortality in asthma patients: evidence from NHANES 2005–2018. Front. Nutr. 12:1603875. doi: 10.3389/fnut.2025.1603875
Edited by:
Alexandr Ceasovschih, Grigore T. Popa University of Medicine and Pharmacy, RomaniaReviewed by:
Cristina Toma, Nicolae Testemiţanu State University of Medicine and Pharmacy, MoldovaIrina Starodubtseva, Voronezh State Medical Academy named after N.N. Burdenko, Russia
Anastasia Balta, Emergency County Hospital Saint Spiridon, Romania
Copyright © 2025 Zhu, Tao and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sue Zhao, MjAxODA1MDc4NUB1c2MuZWR1LmNu
†These authors have contributed equally to this work
‡ORCID: Sue Zhao, http://orcid.org/0000-0001-7472-5272
 Jinqi Zhu1†
Jinqi Zhu1† Sue Zhao
Sue Zhao