- 1Manitoba Interdisciplinary Lactation Centre (MILC), Winnipeg, MB, Canada
- 2Children’s Hospital Research Institute of Manitoba, Winnipeg, MB, Canada
- 3Department of Community Health Sciences, University of Manitoba, Winnipeg, MB, Canada
- 4Manitoba Centre for Health Policy, University of Manitoba, Winnipeg, MB, Canada
- 5Department of Psychology, University of Manitoba, Winnipeg, MB, Canada
- 6Faculty of Kinesiology and Recreation Management, University of Manitoba, Winnipeg, MB, Canada
- 7Department of Pediatrics, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada
- 8Department of Pediatrics, University of British Columbia, Vancouver, BC, Canada
- 9Department of Pediatrics and Child Health, University of Manitoba, Winnipeg, MB, Canada
- 10Department of Pediatrics, Physiology and Dalla Lana School of Public Health, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada
- 11Department of Pediatrics, Larsson-Rosenquist Foundation Mother-Milk-Infant Center of Research Excellence (MOMI CORE), and the Human Milk Institute (HMI), University of California San Diego, San Diego, CA, United States
- 12Department of Pediatrics, University of Alberta, Edmonton, AB, Canada
- 13Department of Agriculture, Food and Nutrition, University of Alberta, Edmonton, AB, Canada
- 14School and Child Psychology Program, University of Alberta, Edmonton, AB, Canada
- 15Faculty of Medicine and Health Sciences, UCSI University, Kuala Lumpur, Malaysia
Introduction: Human milk fatty acids and human milk oligosaccharides (HMOs) are milk components inconsistently associated with neurodevelopment. The objective of this research is to examine the link between fatty acids, HMOs and neurodevelopment.
Methods: This study includes a subset of 240 parent-infant pairs from the Edmonton site of the CHILD Cohort Study. At 3–4 months post-partum, breastfeeding parents provided a milk sample which was analyzed to identify 20 fatty acids and 19 HMOs. Research assistants administered the Bayley Scales of Infant and Toddler Development at 1 and 2 years of age, comprising cognitive, language and motor development scales (standardized to a mean of 100 and a standard deviation of 15; higher scores indicate better development). Adjusted linear regression was used to estimate the relationships between individual milk components or principal components and neurodevelopment, adjusting for maternal and infant factors. Interactions were tested with infant sex and maternal secretor status.
Results: After adjustment, the first fatty acid principal component, characterized by high saturated fat and low n-3 and n-6 fatty acids, was related to higher motor scores (β = 1.59; 95% CI: 0.75, 2.43). Higher concentrations of disialyllacto-N-tetraose were related to lower motor scores (β = −3.91, 95% CI: −5.81, −2.01). Higher concentrations of difucosyllacto-N-hexaose were related to higher language and motor scores for infants of maternal non-secretors, while higher concentrations of 3′-sialyllactose were related to higher scores for infants of maternal secretors.
Conclusion: Both fatty acids and HMOs are related to early neurodevelopment. Maternal secretor status moderates the relationship between select HMOs and neurodevelopment.
Introduction
Decades of research have shown that longer breastfeeding duration and more exclusive breastfeeding are related to better neurodevelopmental outcomes such as performance on intelligence tests and standardized measures of cognitive, language or motor development (1–4). A prominent hypothesis to explain the association between breastfeeding and child neurodevelopment is that human milk contains the optimal source of nutrients (ex. fats, protein, carbohydrates) and other bioactive components (ex. human milk oligosaccharides, brain-derived neurotrophic factor, milk fat globule membrane) to support the developing brain (5, 6). Thus, longer duration, and more exclusive breastfeeding would provide more of these nutritional and bioactive components and confer more benefits to the infant. However, our current understanding of which human milk components contribute to neurodevelopment is limited and requires further study.
Human milk fatty acids and infant neurodevelopment
Fatty acids are the most well-studied milk component related to neurodevelopment (7). It is well known that both n-3 and n-6 long chain polyunsaturated fatty acids (LCPUFAs) have essential roles in human brain development and function (8, 9); however, the available observational data on the association between human milk LCPUFAs and child neurodevelopment has produced mixed results. Some previous studies have shown that higher concentrations of human milk LCPUFAs, measured within the first 4 months post-partum, are related to better neurodevelopment (i.e., infant temperament and psychomotor development) (10, 11); while others have shown no relationship with neurodevelopment (i.e., cognition and intelligence) (12, 13). Further, it is largely unknown if other human milk fatty acids, such as saturated and monounsaturated fats, are related to child neurodevelopment; this relationship needs to be explored further.
Evidence from rodent and human adult studies have shown sex-specific pathways for fatty acid metabolism (14, 15), and emerging research indicates that concentrations of human milk ALA and total n-3 LCPUFAs measured after 8 weeks post-partum are higher in milk made for female infants compared to males (16). In addition, language scores at 13 months, and cognitive scores at 24 months are known to differ based on infant gender, with girls having higher mean scores than boys (17). This evidence provides rational to study the moderating role of child sex on the association between human milk fatty acids and child neurodevelopment to glean more nuanced and specific understandings of these relationships. In general, further research is needed to clarify the associations between human milk n-3 and n-6 fatty acids and child neurodevelopment, investigate novel associations between saturated and monounsaturated fats and child neurodevelopment, and test for sex differences.
Human milk oligosaccharides and infant neurodevelopment
Another human milk component that has received attention for its association with infant neurodevelopment are human milk oligosaccharides (HMOs) (18). HMOs are complex, bioactive, sugar molecules that have multiple functions, one of which is to shape the infant gut microbiome, which can modulate infant brain development through the gut-brain axis (19). In addition, sialic acid, one of the HMO building blocks, is essential for human brain ganglioside development which play an important role in cell signaling and communication (20). A recent narrative review identified only five previous human studies in term infants (four of which n < 100) examining the association between HMOs and infant neurodevelopment up to 2 years of age (18).
There is some evidence that associations between individual HMOs and child outcomes, including neurodevelopment, may vary depending on maternal secretor status, a genetic trait that has a large influence on the type and number of HMOs produced in human milk (21, 22). Additionally, it is largely unknown if the relationships between HMOs and neurodevelopment are moderated by infant sex. This emerging area of research requires more study to understand the associations between HMOs and child neurodevelopment and the potential moderation by maternal secretor status and infant sex.
Objectives
The objectives of these exploratory analyses are to 1), examine the associations between 20 fatty acids and 19 HMOs in relation to child cognitive, language and motor development at 1 and 2 years of age, 2), using dimension reduction techniques, assess the association between fatty acids, HMOs and neurodevelopment, and 3), determine if these associations are moderated by sex, or maternal secretor status (HMOs only).
Methods
Data and sample
This study uses data from the CHILD cohort study, a pan-Canadian longitudinal cohort ongoing since 2009 (n = 3,407) (23). Data were collected in four sites across Canada; Toronto, Manitoba (including Winnipeg, Morden and Winkler), Edmonton and Vancouver, and at several time points (i.e., two pre-natal assessments and annual data collection through a combination of visits and questionnaires). The CHILD study is unique because it combines data from biological samples as well as survey data to better understand relationships between environmental exposures and biological functions. Parent-infant pairs were included if the infant was born at or after 35 weeks gestation, the parent had the ability to read and write English and the pregnant parent was greater than 18 years of age at the time of recruitment. Pairs were excluded if the child was born with major congenital abnormalities, a child of multiple births, a child resulting from in vitro fertilization or a child who would not spend at least 80% of nights in the index home (n = 111). Written informed consent was obtained from all participants at enrollment. The study was approved by the Human Research Ethics Boards at the University of Alberta, the University of British Columbia, McMaster University and the University of Manitoba.
Parents who were breastfeeding at a mean age of 4 months post-partum (range: <1 month to 11 months) were asked to provide a 10 mL breastmilk sample that consisted of a mix of foremilk and hindmilk from multiple feedings during a 24-h period (n = 2,571) (24). Following collection, parents were instructed to place the sample in the refrigerator for up to 24 h until the sample was collected by study staff and placed in a −80 °C freezer. A subset of milk samples was preselected to be analyzed for both fatty acids and HMOs (n = 1,200), however, only 1,181 samples were analyzed for both milk components and had corresponding survey data. This subsample was partly representative of the CHILD study population (about 1/3 of the samples) and partly enriched for maternal and infant health conditions (i.e., asthma, allergies and obesity; about 2/3 of the samples). To improve normality of the dataset, we excluded dyads with ≥ one milk fatty acid or oligosaccharide value that was ± > 6 standard deviations (SD) from the mean (n = 26). Two further dyads were excluded because the infant had parent-reported, physician diagnosed Trisomy 21. The main outcome of this study, the Bayley Scales of Infant and Toddler Development III (Bayley-III), was only measured in the Edmonton cohort, therefore, 913 dyads from the other three sites were excluded because they did not have Bayley-III data. In total 240 parent–child dyads were included in the current analysis (Supplementary Figure S1).
Measures
Milk fatty acids
Milk fatty acids were analyzed by gas chromatography at the University of Alberta and expressed as relative percentages of total identified fatty acids (25). Relative percentages were used instead of absolute concentrations because the sampling protocol did not involve a full breast expression, which is necessary to accurately determine fat content and concentrations. Relative percentages of milk fatty acids are commonly used in human milk research including previously within the CHILD Study (26–28). All values were z-scored with a mean of zero and a standard deviation (SD) of one so the values could be comparable across all fatty acids. Twenty commonly identified fatty acids were included in the present analysis. These included saturated fatty acids [SFA; capric acid (10:0), lauric acid (12:0), myristic acid (14:0), palmitic acid (16:0), margaric acid (17:0) and stearic acid (18:0)], monounsaturated fatty acids [MUFA; palmitoleic acid (16:ln-7), oleic acid (18:ln-9), and vaccenic acid (18:1 c-11)], n-3 polyunsaturated fatty acids [n-3 PUFA; α-linoleic acid (ALA; 18:3n-3), eicosatetraenoic acid (ESA; 20:4n-3), eicosapentaenoic acid (EPA; 20:5n-3), docosapentaenoic acid (DPA; 22:5n-3), and docosahexaenoic acid (DHA; 22:6n-3)] and n-6 polyunsaturated fatty acids [n-6 PUFA; linoleic acid (LA; 18:2n-6), γ-linolenic acid (GLA; 18:3n-6), conjugated linoleic acid (CLA; 18:2c-9, t-11), dihomo-γ-linoleic acid (DGLA; 20:3n-6), arachidonic acid (ARA; 20:4n-6) and adrenic acid (22,4n-6)]. In addition, similar to previous research, five biologically meaningful ratio variables and four summary variables were created (28). The ratios included: ARA: (DHA + EPA); ARA: DHA; total n-6:total n-3; LA: ALA; (EPA + DPA): DHA (all calculated using non-transformed data), and the summary measures included: total n-3; total n-6; total n-3 without ALA and total n-6 without LA.
Human milk oligosaccharides
We identified 19 HMOs using high-throughput solid-phase extraction and analyzed by liquid chromatography at the University of California San Diego. The 19 HMOs identified account for approximately 90% of the total HMO content and were summed to approximate the total HMO content per sample (29). The absolute concentrations of each HMO were log-transformed for normality and then z-score transformed with a mean of zero and a SD of one to be comparable across all HMOs in the sample. The HMOs included fucodisialyllacto-N-hexaose (FDSLNH); 2′-fucosyllactose (2’FL); 3-fucosyllactose (3FL); difucosyllactose (DFLac); lacto-N-fucopentaose-I (LNFP I); lacto-N-fucopentaose-II (LNFP II); lacto-N-fucopentaose-III (LNFP III); difucosyllacto-N-tetrose (DFLNT); fucosyllacto-N-hexaose (FLNH); difucosyllacto-N-hexaose (DFLNH); 3′-sialyllactose (3’SL); 6′-sialyllactose (6’SL); sialyllacto-N-tetraose b (LSTb); sialyllacto-N-tetraose c (LSTc); disialyllacto-N-tetraose (DSLNT); disialyllacto- N-hexaose (DSLNH); lacto-N-neotetraose (LNnT); lacto-N-tetrose (LNT) and lacto-N-hexaose (LNH). In addition, three summary measures were included: total HMO-bound sialic acid, total HMO-bound fucose and total HMOs.
Bayley Scales of Infant and Toddler Development III
The Bayley Scales of Infant and Toddler Development III (Bayley-III) was administered to children at 1 and 2 years of age in the Edmonton cohort by trained research assistants (4, 30). The Bayley-III is an assessment that measures developmental functioning of infants and toddlers between 1 and 42 months of age. For the purposes of these analyses, we included three assessed domains: cognitive, language, and motor development. Composite scores for each domain are standardized to a mean of 100 and a SD of 15, with higher scores indicating better development.
Maternal secretor status
Maternal secretor status is a genetic trait determined by the fucosyltransferase 2 (FUT2) gene, which in about 20% of the population is inactivated by a single nucleotide polymorphism leading to truncation of the gene product (31). Secretor status impacts the number and type of HMOs that the mother produces, particularly 2’FL which is the most abundant HMO among secretors but is virtually absent among non-secretors (29). Maternal secretor status was determined by the presence or near absence of the 2’FL in the mother’s milk.
Confounders
We developed a directed acyclic graph to identify variables to use as confounders in the present analysis (Supplementary Figure S2). These include child sex (male or female), birth mode (cesarian or vaginal), birthweight (continuous in kilograms), gestational age (continuous in weeks), number of older siblings (none, one or two or more), maternal race (White, Asian, or Other), completed maternal post-secondary education (yes/no) and infant age at milk sampling (continuous in weeks). In sex interaction models, child sex was used as a moderator, not as a confounder.
Statistical analyses
Characteristics of the sample were described and compared to those who had Bayley-III scores at 1 year but no milk component data. Then, we ran Pearson correlations between fatty acids, HMOs and cognitive, language and motor scores at 1 year and 2 years of age. Pearson correlations were used because the milk components and Bayley-III scores generally follow a normal distribution. A sensitivity analysis was conducted repeating the analysis among just the exclusively breastfed infants with the intent of reducing statistical variance in the outcome due to the consumption of infant formula or other food. To understand the shape of the relationships and visually assess the potential impact of outliers, scatter plots were generated displaying the linear, quadradic and cubic fit lines for the one-year Bayley-III outcomes.
Next, to assess for potential confounding, we ran linear regression models between milk components and Bayley-III scores at 1 and 2 years, adjusting for sex, birth mode, birthweight, gestational age, number of older siblings, maternal race, maternal post-secondary degree and infant age at milk sampling. We combined the two milk component types into one analysis to assess their potential interdependence in relation to neurodevelopment using principal component analysis as a dimension reduction technique. We determined the first component that explained the largest amount of variance in the sample of fatty acids and HMOs, separately. Then, we entered both first components in a regression model, with Bayley-III scores at 1 and 2 years at the outcomes, to determine if either milk component accounted for variation in the other component. We also limited the regression models to secretors and non-secretors to determine if there were differences in the associations with Bayley-III scores when looking at these specific subgroups. Sex interactions, adjusting for all confounders, were tested for all fatty acids and HMOs in relation to all Bayley-III scores. Adjusted maternal secretor status interactions were tested for HMOs. All p-values throughout the analysis were corrected for multiple comparisons using the Benjamini Hochberg False Discovery Rate (32).
Results
Demographics of the sample
Of the 653 infants who had Bayley-III scores at 1 year, 240 were included in the present analysis because they also had an analyzed milk sample (Table 1). At the time of milk sampling, 45.4% of infants were being exclusively breastfed. The mean infant age at milk sampling was 18.1 (± 4.7) weeks. About 72.0% of the mothers who provided milk samples were secretors. The mean Bayley-III scores at 1 year were 110.9 (±10.1) for the cognitive domain, 109.8 (±11.4) for the language domain and 104.7 (±14.5) for the motor domain. At 2 years, the scores were slightly lower with mean scores of 106.9 (± 15.6) for the cognitive domain, 101.2 (±12.2) for the language domain and 99.1 (± 9.7) for the motor domain. Compared to infants in the CHILD study with a Bayley-III assessment but no milk sample (n = 413), those with milk samples were more likely to be exclusively breastfed at 3 months and have higher Bayley-III language scores at 1 year (p-values ≤0.001 and 0.02, respectively); the other demographics were not statistically different between the subset used for this analysis and all infants with a Bayley-III assessments at 1 year.
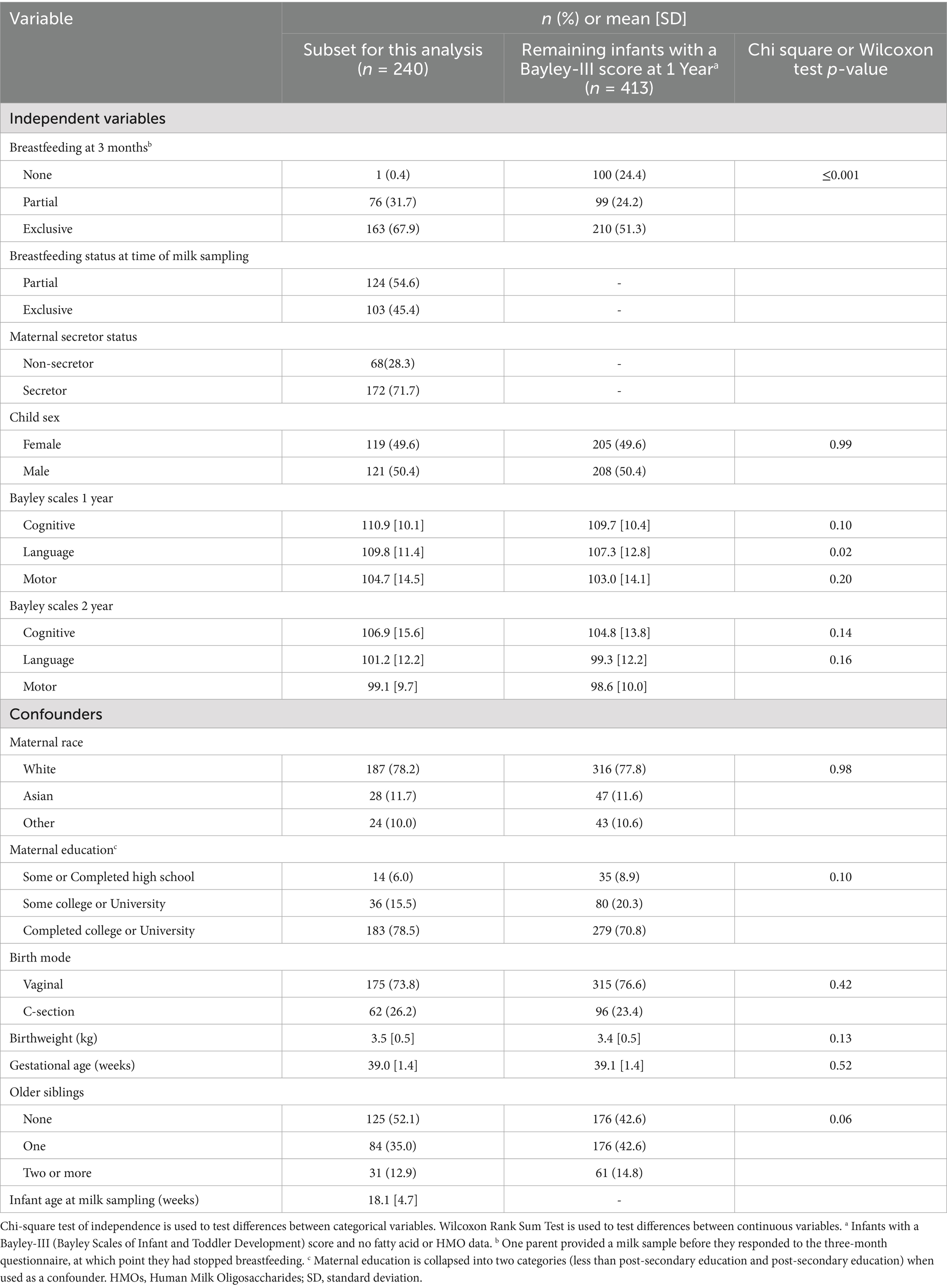
Table 1. Characteristics of CHILD cohort subset included in the current analysis of fatty acid and HMO data.
Select fatty acids and HMOs are related to cognitive, language and motor development at 1 year
There were significant correlations between select fatty acids and cognitive and motor development at 1 year of age after FDR adjustment (Figure 1A; Supplementary Figure S3A). Specifically, higher proportions of n-3 and n-6 PUFAs including, EPA (20:5n-3), DPA (22:5n-3), DHA (22:6n-3), total n-3 without ALA, LA (18:2n-6) and total n-6 fatty acids were related to lower (worse) cognitive scores (rho range from −0.24 to −0.18). These correlations generally became stronger when limiting to those who were exclusively breastfed (rho range from –0.36 to −0.28; Supplementary Figures S4A, S5A). Two saturated fatty acids were related to higher (better) motor development at 1 year: palmitic acid (16:0) and margaric acid (17:0) (rho = 0.19 and 0.20, respectively). In addition, several monounsaturated, n-3 and n-6 PUFAs were related to lower (worse) motor development at 1 year including: oleic acid (18:ln-9), EPA (20:5n-3), DPA (22:5n-3), LA (18:2n-6), ARA (20:4n-6), AA (22:4n-6) and total n-6 (rho range from −0.22 to −0.16). These correlations followed a similar trend when limiting to those who were exclusively breastfed, but none reached statistical significance after FDR correction. After FDR correction, no significant correlations were observed between fatty acids and language development at 1 year or any Bayley-III scales at 2 years and these trends remained when limiting to those who were exclusively breastfed.
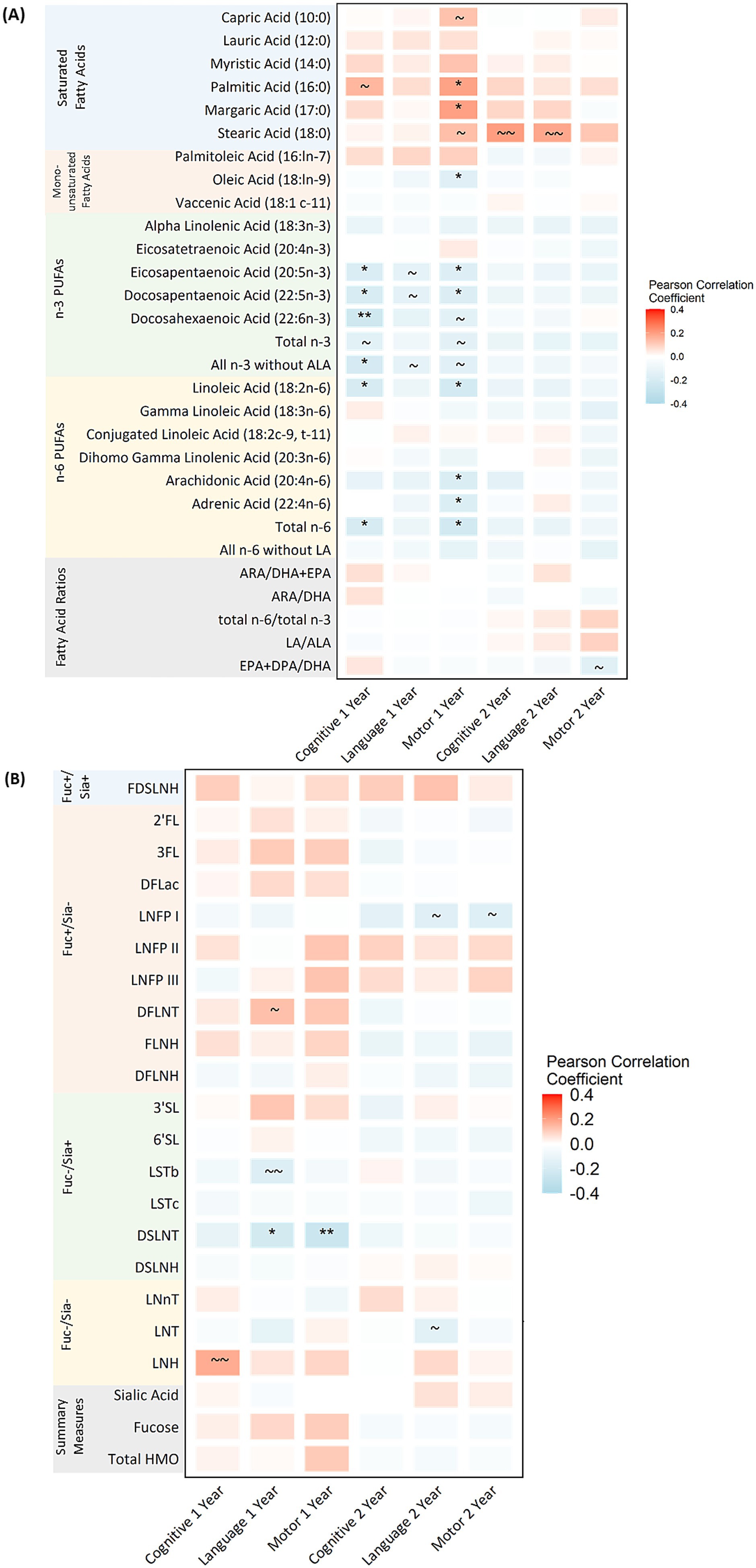
Figure 1. Pearson correlations between human milk fatty acids (A), HMOs (B) and Bayley-III scores at 1 and 2 years of age in the CHILD cohort study. Bayley-III, Bayley Scales of Infant and Toddler Development; DFLac, difucosyllactose; DFLNH, difucosyllacto-N-hexaose; DFLNT, difucosyllacto-N-tetrose; DSLNH, disialyllacto- N-hexaose; DSLNT, disialyllacto-N-tetraose; FLNH, fucosyllacto-N-hexaose; FDSLNH, fucodisialyllacto-N-hexaose; Fuc, Fucosylated HMO; Fucose, human milk oligosaccharide–bound fucose; HMO, human milk oligosaccharide; LNFP I/II/III, lacto-N-fucopentaose-I/II/III; LNH, lacto-N-hexaose; LNnT, lacto-N-neotetraose; LNT, lacto-N-tetrose; LSTb/c, sialyllacto-N-tetraose b/c; Sia, Sialylated HMO; Sialic Acid, HMO-bound Sialic Acid; 2’FL, 2′-fucosyllactose; 3FL, 3-fucosyllactose; 3’SL, 3′-sialyllactose; 6’SL, 6′-sialyllactose; PUFA, Polyunsaturated Fatty Acid; ALA, Alpha Linolenic Acid; EPA, Eicosapentaenoic Acid; DPA, Docosapentaenoic Acid; DHA, Docosahexaenoic Acid; LA, Linoleic Acid; ARA, Arachidonic Acid. All milk components are expressed as z-scores aside from fatty acid ratios which are not z-scored. See Supplementary Figure S3 for exact correlation coefficients. See Supplementary Figures S4, S5 for a sensitivity analysis limited to exclusively breastfed infants and Supplementary Figures S6, S7 for scatter plots indicating linear and polynomial fit lines. *FDR corrected p-value ≤0.05, ** FDR corrected p-value ≤0.01, ~ uncorrected p-value ≤0.05, ~ ~ uncorrected p-values ≤0.01.
Only one HMO was significantly correlated with Bayley-III scores after FDR adjustment; higher DSLNT concentrations were related to lower (worse) language and motor scores at 1 year of age (rho = −0.22 for language and −0.26 for motor; Figure 1B; Supplementary Figure S3B). When limiting to those being exclusively breastfed, there were no significant correlations with one-year outcomes; however, LNFP1 was negatively correlated to motor development at 2 years (rho = −0.39; Supplementary Figures S4B, S5B).
To understand if the relationships between milk components and Bayley-III scores at 1 year followed a linear relationship, we present scatter plots with the linear, quadradic and cubic (i.e., polynomial) fit lines for fatty acids and HMOs. There were no obvious polynomial fit lines for fatty acids or HMOs and Bayley-III scores at 1 year (Supplementary Figures S6A–C, S7A–C), suggesting generally linear relationships.
DSLNT remains significantly associated with motor development after adjusting for confounders
After adjusting for sex, birth mode, birthweight, gestational age, number of older siblings, maternal race, maternal post-secondary degree and infant age at milk sampling, and applying FDR correction, there were no significant associations between human milk fatty acids and Bayley-III scores at 1 or 2 years (Figure 2A). DSLNT remained significantly associated with lower motor scores at 1 year after confounder adjustment and FDR correction (B = −3.91, 95% CI: −5.81, −2.01; i.e. one SD increase in DSLNT is related to a 3.91 point (about ¼ of a SD) decrease in motor scores), however, no other HMOs were significantly related to Bayley-III scores at 1 or 2 years (Figure 2B).
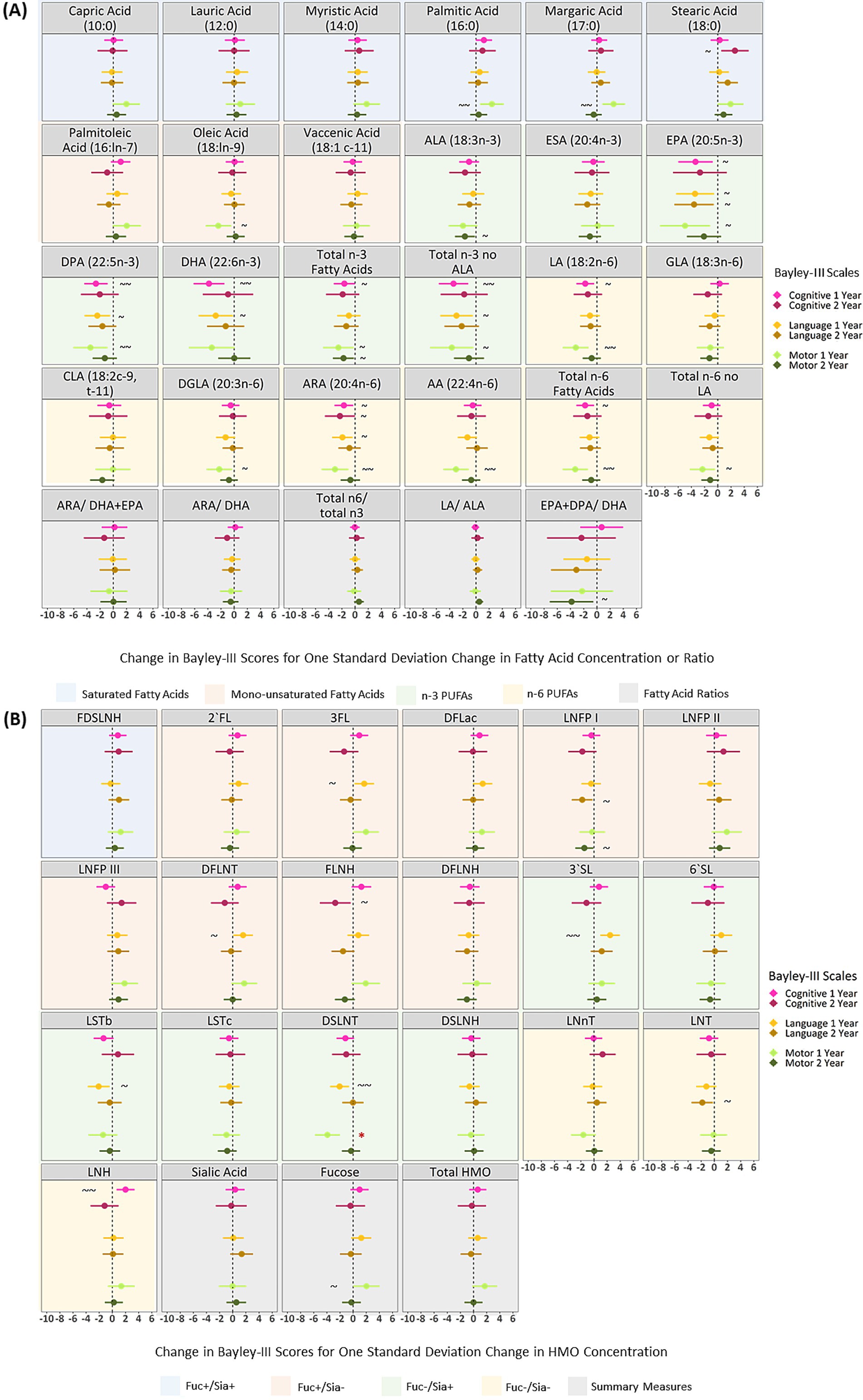
Figure 2. Adjusted linear associations between human milk fatty acids (A), HMOs (B) and Bayley-III scores at 1 and 2 years of age in the CHILD cohort study. All models are adjusted for: infant sex, birthweight, birth mode, number of older siblings, gestational age, maternal race, maternal education, infant age at milk sampling. Bayley-III, Bayley Scales of Infant and Toddler Development; DFLac, difucosyllactose; DFLNH, difucosyllacto-N-hexaose; DFLNT, difucosyllacto-N-tetrose; DSLNH, disialyllacto- N-hexaose; DSLNT, disialyllacto-N-tetraose; FLNH, fucosyllacto-N-hexaose; FDSLNH, fucodisialyllacto-N-hexaose; Fuc, Fucosylated HMO; Fucose, human milk oligosaccharide–bound fucose; HMO, human milk oligosaccharide; LNFP I/II/III, lacto-N-fucopentaose-I/II/III; LNH, lacto-N-hexaose; LNnT, lacto-N-neotetraose; LNT, lacto-N-tetrose; LSTb/c, sialyllacto-N-tetraose b/c; Sia, Sialylated HMO; Sialic Acid, HMO-bound Sialic Acid; 2’FL, 2′-fucosyllactose; 3FL, 3-fucosyllactose; 3’SL, 3′-sialyllactose; 6’SL, 6′-sialyllactose; PUFA, Polyunsaturated Fatty Acid; ALA, Alpha Linolenic Acid; ESA, Eicosatetraenoic Acid; EPA, Eicosapentaenoic Acid; DPA, Docosapentaenoic Acid; DHA, Docosahexaenoic Acid; LA, Linoleic Acid; GLA, Gamma Linoleic Acid; CLA, Conjugated Linoleic Acid; DGLA, Dihomo Gamma Linolenic Acid; ARA, Arachidonic Acid; AA, Adrenic Acid. All milk components are expressed at z-scores aside from fatty acid ratios which are not z-scored. *FDR corrected p-value ≤0.05, ~ uncorrected p-value ≤0.05, ~ ~ uncorrected p-value ≤0.01.
The first fatty acid principal component is related to motor development at 1 year and this association is unchanged after accounting for HMOs
Figure 3A shows the factor loadings and biplots for the first fatty acid and HMO principal components (PC1). PC1 explained 25.2% of the variance in the fatty acids data and 27.2% of the variance in the HMO data, which indicates how much information is preserved from the full dataset. Based on the loadings, the PC1s can be described as high saturated fat and low n-3 and n-6 PUFAs and high secretors (ex. 2’FL, DLFac) and low non-secretors (ex. LNFP11, FDSLNH). After adjusting for all confounders and applying the FDR correction, fatty acid PC1 was associated with higher motor scores at 1 year, and this association was unchanged after adjusting for HMO PC1 (B = 1.59; 95% CI: 0.75, 2.43; Figure 3B). Fatty acid PC1 was not significantly associated with Bayley-III scores at 2 years. HMO PC1 was not associated with Bayley-III scores at 1 or 2 years, and the estimates were relatively unchanged after adjusting for FA PC1. In line with the full sample, when limiting to subgroups of either secretors or non-secretors, there were no significant associations between HMO PC1 and Bayley-III scores at 1 or 2 years.
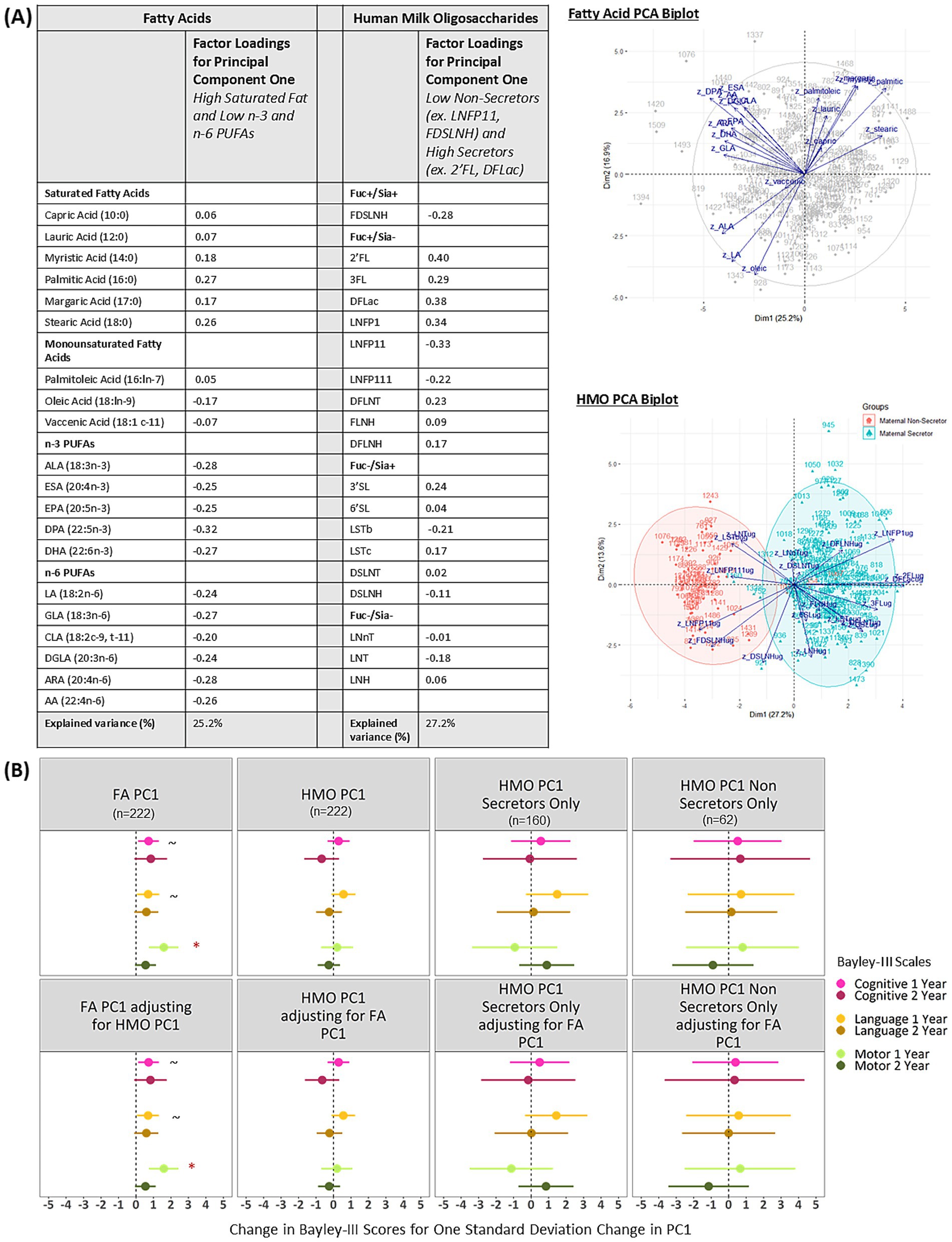
Figure 3. (A) Factor loadings and (B) Adjusted associations between fatty acid and HMO principal components and Bayley-III scores at 1 and 2 years in the CHILD cohort study. FA PC1 is significantly associated with Bayley-III scores and these relationships are not dependent on HMO PC1. HMO PC1 is not significantly associated with Bayley-III scores; estimates are similar among secretors and non-secretors. Regression models are adjusted for: infant sex, birthweight, birth mode, number of older siblings, gestational age, maternal race, maternal education, infant age at milk sampling. PC, Principal Component; Bayley-III, Bayley Scales of Infant and Toddler Development; DFLac, difucosyllactose; DFLNH, difucosyllacto-N-hexaose; DFLNT, difucosyllacto-N-tetrose; DSLNH, disialyllacto- N-hexaose; DSLNT, disialyllacto-N-tetraose; FLNH, fucosyllacto-N-hexaose; FDSLNH, fucodisialyllacto-N-hexaose; Fuc, Fucosylated HMO; Fucose, human milk oligosaccharide–bound fucose; HMO, human milk oligosaccharide; LNFP I/II/III, lacto-N-fucopentaose-I/II/III; LNH, lacto-N-hexaose; LNnT, lacto-N-neotetraose; LNT, lacto-N-tetrose; LSTb/c, sialyllacto-N-tetraose b/c; Sia, Sialylated HMO; Sialic Acid, HMO-bound Sialic Acid; 2’FL, 2′-fucosyllactose; 3FL, 3-fucosyllactose; 3’SL, 3′-sialyllactose; 6’SL, 6′-sialyllactose; PUFA, Polyunsaturated Fatty Acid; ALA, Alpha Linolenic Acid; ESA, Eicosatetraenoic Acid; EPA, Eicosapentaenoic Acid; DPA, Docosapentaenoic Acid; DHA, Docosahexaenoic Acid; LA, Linoleic Acid; GLA, Gamma Linoleic Acid; CLA, Conjugated Linoleic Acid; DGLA, Dihomo Gamma Linolenic Acid; ARA, Arachidonic Acid; AA, Adrenic Acid. See Supplementary Figure S8 for factor loadings for FA and HMO PC2 and PC3. *FDR corrected p-value ≤0.05, ~ uncorrected p-value ≤0.05.
Maternal secretor status moderates the association between select HMOs and language and motor development at 1 year
There were limited adjusted sex interactions in the relationships between milk fatty acids, HMOs and Bayley-III scores at 1 or 2 years of age and none reached statistical significance after confounder adjustment and FDR correction (Supplementary Figures S9, S10). Maternal secretor status interactions showed that DFLNH was significantly related to better language and motor scores at 1 year for infants of maternal non-secretors, while 3’SL was related to better language and motor scores at 1 year for infants of maternal secretors (Figure 4). Significant interactions between 3’SL, 3FL and secretor status also showed better motor scores at 2 years for infants of maternal secretors. These results indicate that maternal secretor status may play an important role in moderating the relationships between DFLNH, 3’SL, 3FL, and language and motor development, however, the direction of the moderation effect is not consistent (i.e., having a positive secretor status is not always better).
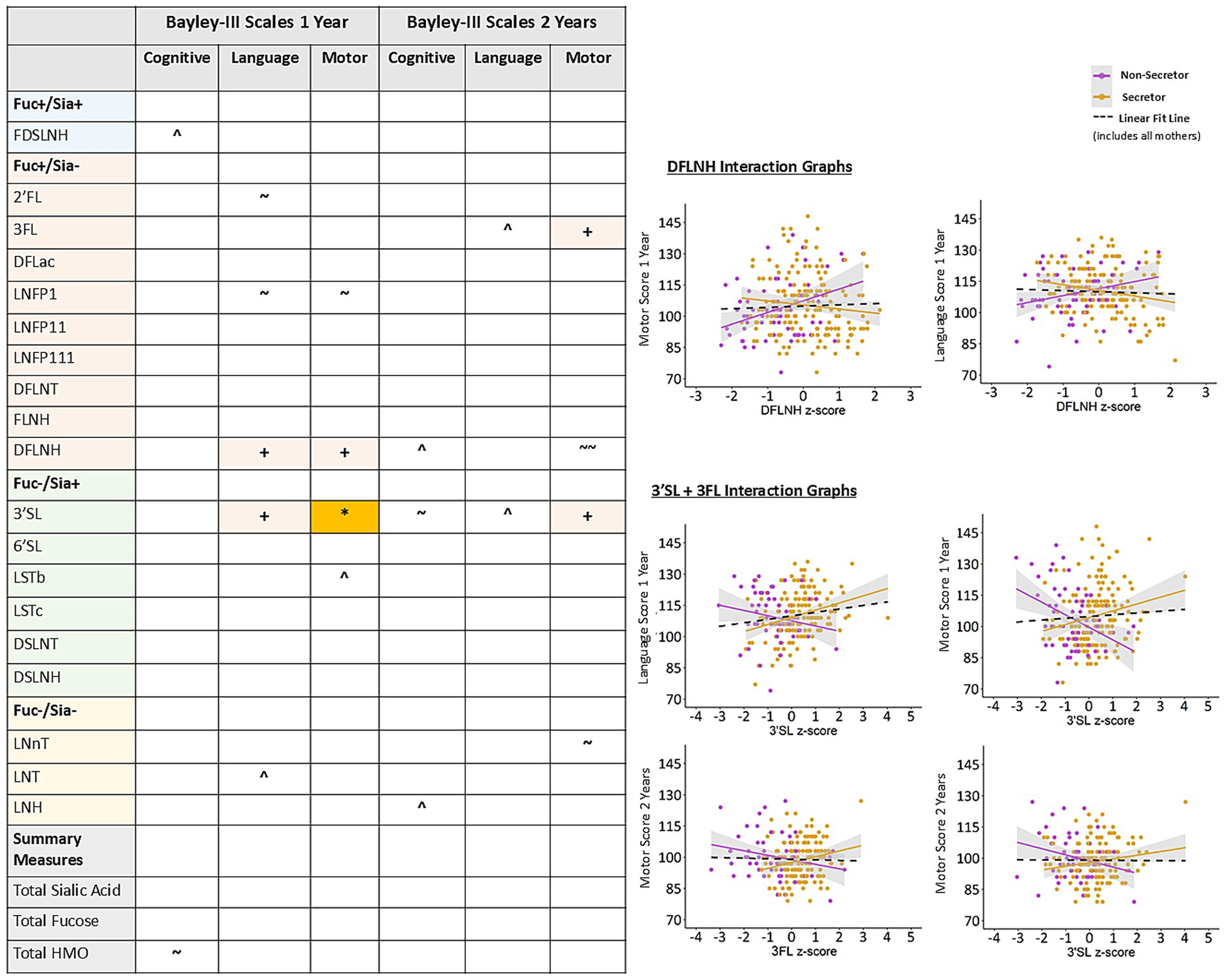
Figure 4. Adjusted interactions between maternal secretor status and HMOs on Bayley-III scores at 1 and 2 years in the CHILD cohort study. Colored cells in the table are represented as scatter plots. All models are adjusted for: infant sex, birthweight, birth mode, number of older siblings, gestational age, maternal race, maternal education, infant age at milk sampling. Bayley-III, Bayley Scales of Infant and Toddler Development DFLac, difucosyllactose; DFLNH, difucosyllacto-N-hexaose; DFLNT, difucosyllacto-N-tetrose; DSLNH, disialyllacto- N-hexaose; DSLNT, disialyllacto-N-tetraose; FLNH, fucosyllacto-N-hexaose; FDSLNH, fucodisialyllacto-N-hexaose; Fuc, Fucosylated HMO; Fucose, human milk oligosaccharide–bound fucose; HMO, human milk oligosaccharide; LNFP I/II/III, lacto-N-fucopentaose-I/II/III; LNH, lacto-N-hexaose; LNnT, lacto-N-neotetraose; LNT, lacto-N-tetrose; LSTb/c, sialyllacto-N-tetraose b/c; Sia, Sialylated HMO; Sialic Acid, HMO-bound Sialic Acid; 2’FL, 2′-fucosyllactose; 3FL, 3-fucosyllactose; 3’SL, 3′-sialyllactose; 6’SL, 6′-sialyllactose. Linear fit line is from the full model that does not include the interaction term in Figure 2B. + FDR corrected p-value ≤0.1, *FDR corrected p-value ≤0.05, ^ uncorrected p-value ≤0.1, ~ uncorrected p-value ≤0.05, ~ ~ uncorrected p-value ≤0.01.
Discussion
This is the largest study to date to examine relationships between both human milk fatty acids and HMOs with child neurodevelopment at ages 1 and 2 years. Our results suggest that select n-3 and n-6 PUFAs were related to lower cognitive and motor scores at 1 year of age, while select saturated fatty acids were related to higher motor scores. Although these individual associations did not withstand correction for multiple comparisons, they were corroborated by results from principal component analyses where fatty acid profiles high in saturated fat and low in n-3 and n-6 PUFAs were significantly related to better motor scores at 1 year. Higher concentrations of DSLNT, a sialylated HMO, were related to lower motor scores at 1 year and this association was robust to confounding and correction for multiple comparisons. However, overall HMO profiles were not associated with neurodevelopment at 1 or 2 years. We found that secretor status moderates associations between DFLNH, 3’SL, 3FL and language and motor scores, however, the direction of the moderation effect was inconsistent between these HMOs.
Select n-3 and n-6 PUFAs are related lower cognitive and motor development scores at 1 year
Contrary to the existing literature (10, 11, 33), in unadjusted models, higher concentrations of n-3 PUFAs were related to worse cognitive and motor scores at 1 year. There is existing research showing no association between n-3 PUFAs and neurodevelopment both in observational studies of human milk n-3 PUFAs and RCTs of n-3 formula supplementation (7, 34); however, to our knowledge, no studies have shown negative associations between n-3 PUFAs and neurodevelopment in term infants. A previous analysis using data from the CHILD cohort found that DHA was negatively associated with head circumference, a proxy for brain development (35), which may also be related to cognitive, language and motor scores (36). While the associations shown in the current study did not persist after adjustment and FDR correction, or extend to the two-year time point, the direction of the associations are consistently negative across most n-3 and n-6 PUFAs. It is important to note that the relative proportion of fatty acids in the milk does not reflect the actual amount of fatty acid that gets absorbed and utilized by the infant body, or how absorption is affected by the presence of other milk components. These cautions may shed light on the reasons for our negative findings. Future research could examine infant serum to determine absorbed fatty acids levels and how this may be associated with Bayley-III scores. Additionally, future work is needed to examine different combinations of human milk components to determine if and how their bioavailability interacts in the infant body.
Saturated fatty acids are related to better motor development at 1 year
This is one of the first studies to examine the relationship between human milk saturated fatty acids and child neurodevelopment. Palmitic and margaric acid were related to better motor scores at 1 year in unadjusted models, although the associations did not remain significant in adjusted models and after FDR correction. However, the fatty acid profile high in saturated fat and low n-3 and n-6 PUFAs (the first principal component) was related to better motor scores at 1 year and this association was robust to confounding and FDR correction. Principal component analysis preserves variance which may translate into increased statistical power to observe a significant effect compared to individual analyses (37).
Saturated fats comprise the largest proportion of milk fat [42.2% in mature milk (38)] and can help to absorb fat-soluble vitamins and assist other biological functions, however, most research on the health effects of saturated fat has been in older children or adults (39, 40). Typically, increased dietary saturated fat is related to poorer health outcomes, including lower cognitive flexibility in children ages 7 to 10 years old (39). The results from the current study suggest that human milk saturated fatty acids may play a positive role in infant neurodevelopment but requires replication in other cohorts.
DSLNT is related to lower motor development scores at 1 year
Two previous human studies have examined the relationship between DSLNT and motor development and results were counter to what was observed in the current analysis. Both Ferrira et al. (41) and Sato et al. (42) showed that higher concentrations of DSLNT were related to better fine motor development, as measured by the Ages and Stages Questionnaire, in infants at 6 months and 1 year of age. However, in alignment with the current study, the association between DSLNT and motor development did not persist to the two-year time point in Sato et al. (42). Differences in the timing of human milk sample collection (i.e., at 1 month in both Ferrira et al. (41) and Sato et al. (42), while mean sample collection was 4 months in the current study), or the neurodevelopment measure, could explain the diverging findings.
It is currently understood that the primary role of HMOs is to act as prebiotics for gut bacteria and that certain HMOs have strong effects on shaping the infant microbiome (43). One study showed that DSLNT was related to multiple different bacterial genera in the infant gut, including a negative association with Bacteroides (44). Previous research using the CHILD study data has linked higher abundance of Bacteroides with improved motor development (4). Therefore, it is possible that higher concentrations of DSLNT could be linked to lower motor development scores through the reduction of Bacteroides in the infant gut.
It is important to note that the main clinical utility of the Bayley-III scale is to assess development and identify children who may not be on a typical developmental trajectory (30). However, the Bayley-III is also used for population-level research, which may result in small effect sizes that can have population-level relevance despite not having individual clinical meaning, as observed in the current study. As explained by Roses Theorem, small effect sizes can still be interpreted as meaningful on a population level because large number of people at a small risk may result in more incidence of the outcome than a small number who are at high risk (45). Infant feeding is a universal exposure, and in Canada, 69% of infants receive some human milk for the first 6 months of life, indicating widespread human milk exposure (46). An alternative method for interpreting the relevance of a novel exposure (e.g., DSLNT in human milk) is to compare the adjusted effect size to that of another variable already known to be associated with that same outcome (i.e., improved neurodevelopment), such as high maternal post-secondary education (47). In our analysis, completing maternal post-secondary education, compared to less than a college or university degree, was related to a 0.33 point increase in Bayley-III motor scores at 1 year (not statistically significant), while a one standard deviation increase in DSLNT was related to a 3.91 point decrease in Bayley-III motor scores (FDR-corrected p ≤ 0.05). This demonstrates that, in our sample, DSLNT has an even stronger association with motor development at 1 year than high maternal education and gives context to our findings.
Fatty acid and HMO principal components are not interdependent
We did not observe interdependent effects of fatty acids and HMO profiles on neurodevelopment. In fact, the significant association between fatty acid PC1 and motor development was unchanged after adjusting for HMO PC1, and similarly, the non-significant associations between HMO PC1 and neurodevelopment were unchanged after adjusting for fatty acid PC1. Previous research has examined combinations of other human milk components (ex. phospholipids, choline and fatty acids) in relation to recognition memory or cognitive development and has found both joint and synergistic associations between milk components (48, 49). To more comprehensively understand relationships between fatty acids and HMOs on neurodevelopment, future work could use more advanced methods such as machine learning or clustering analyses.
Maternal secretor status moderates the relationship between select HMOs and language and motor scores
The current results align with one previous study that examined differences between HMOs and neurodevelopment based on secretor status. Similar to our findings, Cho et al. (21), found that 3’SL was related to better language development in children between the ages of 2 and 25 months who were born to maternal secretors, with no relationship among children of maternal non-secretors. No other studies have shown associations between DFLNH and higher language and motor scores among infants of maternal non-secretors. While the exact mechanisms of these interactions are unknown, it is possible that certain HMOs have stronger biological effects in a microbial environment that is characterized by secretor status. Typically, infants of maternal secretors have higher abundance of gut Bifidobacteria (50). It is possible that the biological effects of DFLNH are more available in an infant gut that is colonized by bacteria informed by non-secretor milk (ex. less Bifidobacteria). More research in this area may discover neurodevelopmental benefits for infants of maternal non-secretors as well as for infants of maternal secretors.
Limitations
The results of this exploratory study should be interpreted in the context of several limitations. First, the sampling protocol for milk collection instructed mothers to collect a combination of fore and hind milk from multiple feeds over 24 h and only one milk sample was collected. Concentrations of human milk components can change throughout lactation and concentrations of fatty acids can vary from day to day, throughout a single day, and from the beginning to the end of a feed. However, concentrations of HMOs appear to be stable over 6 h and 7 day periods (51). The current sampling protocol was not designed to capture temporal changes in milk components, and could obscure relevant associations and/or limit the real world-applicability of the results, especially for fatty acids. The CHILD study also did not collect a full breast expression which means the total fat volume in a feed, and therefore the absolute concentrations of fatty acids, cannot be determined. Second, maternal secretor status was phenotypically determined and there is a small chance that genetically determined secretor status could provide different results. Third, CHILD study participants without milk component data were excluded from the analysis; however, there were minimal differences between those included and excluded from the study sample on most variables aside from language scores at 1 year where included infants had higher scores than excluded infants. Therefore, those with lower language scores are underrepresented in this analysis which may underestimate the association between milk components and language scores at 1 year. Fourth, the CHILD study cohort has a higher income, education, and prevalence of married or cohabiting parents than participants in a general population, representative Canadian sample (52). Therefore, the results of this study are not fully generalizable to the general Canadian population.
Conclusion
In this longitudinal, exploratory study, our results suggest that fatty acid principal component one (comprised of high saturated fat and low n-3 and n-6 PUFAs) is related to higher motor scores at 1 year of age. Additionally, DSLNT, a sialylated HMO, may be related to lower motor scores at 1 year of age. Maternal secretor status may moderate associations between 3FL, 3’SL, DFLNH, and infant language and motor scores; however, positive secretor status did not consistently provide benefits. Results from this work can inform future studies seeking to understand the mechanisms of fatty acids and HMOs on infant neurodevelopment.
Data availability statement
The datasets presented in this article are not readily available because the data requires approval from the CHILD National Coordinating Centre (NCC) before they can be accessed. Requests to access the datasets should be directed to the CHILD NCC to discuss their needs before initiating a formal request. More information about data access for the CHILD Cohort Study can be found at https://childstudy.ca/for-researchers/data-access/. Requests to access these datasets should be directed to Y2hpbGRAbWNtYXN0ZXIuY2E=.
Ethics statement
This study involving Humans was approved by Human Research Ethics Boards at the University of Alberta, the University of British Columbia, McMaster University and the University of Manitoba. The study was conducted in accordance with local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians.
Author contributions
SaT: Methodology, Writing – review & editing, Conceptualization, Writing – original draft, Data curation, Visualization, Formal analysis. LR: Conceptualization, Writing – review & editing, Supervision. NN: Writing – review & editing, Supervision, Conceptualization. TM: Conceptualization, Writing – review & editing. StT: Conceptualization, Writing – review & editing. ES: Conceptualization, Writing – review & editing. PS: Conceptualization, Funding acquisition, Writing – review & editing. BR: Data curation, Writing – review & editing, Methodology. JC: Writing – review & editing, Data curation. SG: Writing – review & editing, Methodology, Data curation. CF: Data curation, Writing – review & editing, Methodology, Conceptualization. LB: Writing – review & editing, Methodology, Conceptualization, Data curation. JP: Methodology, Writing – review & editing, Conceptualization. PM: Conceptualization, Writing – review & editing, Data curation, Methodology. MA: Data curation, Methodology, Supervision, Writing – review & editing, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The Canadian Institutes of Health Research (CIHR) and the Allergy, Genes and Environment Network of Centers of Excellence (AllerGen NCE) provided core support for the CHILD study (grants to founding CHILD director Malcolm Sears and current director PS). Human milk analysis was funded by the Manitoba Medical Services Foundation, Research Manitoba and the Canadian Institutes of Health Research. SaT was funded as a Canada Vanier Scholar during the development of this manuscript.
Acknowledgments
We are grateful to all the families who are participating in the CHILD Study and the entire CHILD Study team, which includes interviewers, nurses, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, and receptionists.
Conflict of interest
LR receives funding from the Canadian Institutes of Health Research (CIHR), the Social Sciences and Humanities Research Council of Canada, the Canada Foundation for Innovation, Research Manitoba, the Children’s Hospital Research Institute of Manitoba. NN holds funding from the CIHR, Health Canada and Public Health Agency of Canada (PHAC). As Director of Manitoba Center for Health Policy he receives funding from the Manitoba provincial government. He has received funding from the Breastfeeding Committee of Canada for quality improvement work focused on the Baby Friendly Initiative. StT holds the Tier 1 Canada Research Chair in Pediatric Precision Health and the Aubrey J. Tingle Professor of Pediatric Immunology. PS holds the Tier 1 Canada Research Chair in Pediatric Asthma and Lung Health. PM receives funding from the Women’s and Children’s Health Research Institute. CF holds a Tier 1 Canada Research Chair in Nutrition and Metabolism. She serves on the scientific advisory board for Dairy Farmers of Canada and By Heart. LB is the UC San Diego Chair of Collaborative Human Milk Research, endowed by the Family Larsson-Rosenquist Foundation, Switzerland. He is also an inventor on several patents related to functional benefits of human milk oligosaccharides related to inflammatory diseases and conditions. MA holds the Tier 2 Canada Research Chair in Developmental Origins of Chronic Disease. She receives research funding from the CIHR, the Canada Foundation for Innovation, the Bill and Melinda Gates Foundation, Research Manitoba, Mitacs, CIFAR, the Garfield Weston Foundation. She has consulted for DSM Nutritional Products and serves on the Malaika Vx and Tiny Health Scientific Advisory Boards.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1606169/full#supplementary-material
References
1. Horta, BL, and Victora, CG. (2013). Long-term effects of breastfeeding: a systematic review. Available online at: https://www.who.int/publications/i/item/9789241505307 (Accessed March 5, 2025).
2. McGowan, C, and Bland, R. The benefits of breastfeeding on child intelligence, behavior, and executive function: a review of recent evidence. Breastfeed Med. (2023) 18:172–87. doi: 10.1089/bfm.2022.0192
3. Horta, B, Bahl, R, Martines, J, and Victora, C. (2007). Evidence on the long-term effects of breastfeeding: systematic reviews and meta-analyses. Available online at: https://iris.who.int/bitstream/handle/10665/43623/9789241595230_eng.pdf (Accessed March 5, 2025).
4. Tamana, SK, Tun, HM, Konya, T, Chari, RS, Field, CJ, Guttman, DS, et al. Bacteroides-dominant gut microbiome of late infancy is associated with enhanced neurodevelopment. Gut Microbes. (2021) 13:1–17. doi: 10.1080/19490976.2021.1930875
5. Kim, SY, and Yi, DY. Components of human breast milk: from macronutrient to microbiome and microRNA. Clin Exp Pediatr. (2020) 63:301–9. doi: 10.3345/cep.2020.00059
6. Peila, C, Riboldi, L, and Coscia, A. Role of the biological active components of human milk on long-term growth and neurodevelopmental outcome. Ital J Pediatr. (2024) 50:201. doi: 10.1186/s13052-024-01773-z
7. Mitguard, S, Doucette, O, and Miklavcic, J. Human milk polyunsaturated fatty acids are related to neurodevelopmental, anthropometric, and allergic outcomes in early life: a systematic review. J Dev Orig Health Dis. (2023) 14:763–72. doi: 10.1017/S2040174423000454
8. Sherzai, D, Moness, R, Sherzai, S, and Sherzai, A. A systematic review of omega-3 fatty acid consumption and cognitive outcomes in neurodevelopment. Am J Lifestyle Med. (2023) 17:649–85. doi: 10.1177/15598276221116052
9. Hadley, KB, Ryan, AS, Forsyth, S, Gautier, S, and Salem, N. The essentiality of arachidonic acid in infant development. Nutrients. (2016) 8:216. doi: 10.3390/nu8040216
10. Hahn-Holbrook, J, Fish, A, and Glynn, LM. Human milk omega-3 fatty acid composition is associated with infant temperament. Nutrients. (2019) 11:1–12. doi: 10.3390/nu11122964
11. Zielinska, M, Hamulka, J, Grabowicz-Chądrzyńska, I, Bryś, J, and Wesolowska, A. Association between breastmilk LC PUFA, carotenoids and psychomotor development of exclusively breastfed infants. Int J Environ Res Public Health. (2019) 16:1144. doi: 10.3390/ijerph16071144
12. Keim, S, Daniels, J, Siega-Riz, AM, Herring, AH, Dole, N, and Scheidt, PC. Breastfeeding and long-chain polyunsaturated fatty acid intake in the first four post-natal months and infant cognitive development: an observational study. Matern Child Nutr. (2012) 8:471–82. doi: 10.1038/jid.2014.371
13. Gustafsson, PA, Duchén, K, Birberg, U, and Karlsson, T. Breastfeeding, very long polyunsaturated fatty acids (PUFA) and IQ at 6/4 years of age. Acta Paediatr. (2004) 93:1280–7. doi: 10.1111/j.1651-2227.2004.tb02924.x
14. Lohner, S, Fekete, K, Marosvölgyi, T, and Decsi, T. Gender differences in the long-chain polyunsaturated fatty acid status: systematic review of 51 publications. Ann Nutr Metab. (2013) 62:98–112. doi: 10.1159/000345599
15. Christiansen, K, Gan, M, and Holman, R. Sex differences in the metabolism of fatty acids in virto. Biochim Biophys Acta. (1969) 187:19–25. doi: 10.1016/0005-2795(69)90136-6
16. Meng, F, Uniacke-lowe, T, Lanfranchi, E, Meehan, G, O'Shea, CA, Dennehy, T, et al. A longitudinal study of fatty acid profiles, macronutrient levels, and plasmin activity in human milk. Front Nutr. (2023) 10:2613. doi: 10.3389/fnut.2023.1172613
17. Krogh, MT, and Væver, MS. Does gender affect Bayley-III scores and test-taking behavior? Infant Behav Dev. (2019) 57:101352. doi: 10.1016/j.infbeh.2019.101352
18. Berger, PK, Ong, ML, Bode, L, and Belfort, MB. Human milk oligosaccharides and infant neurodevelopment: a narrative review. Nutrients. (2023) 15:1–10. doi: 10.3390/nu15030719
19. Carlson, AL, Xia, K, Azcarate-Peril, MA, Goldman, BD, Ahn, M, Styner, MA, et al. Infant gut microbiome associated with cognitive development. Biol Psychiatry. (2018) 83:148–59. doi: 10.1016/j.biopsych.2017.06.021
20. Wang, B. Molecular mechanism underlying sialic acid as an essential nutrient for brain development and cognition. Adv Nutr. (2012) 3:465S–72S. doi: 10.3945/an.112.001875
21. Cho, S, Zhu, Z, Li, T, Baluyot, K, Howell, BR, and Hazlett, HC. Human milk 3′ -sialyllactose is positively associated with language development during infancy. Am J Clin Nutr. (2021) 114:1–10. doi: 10.1093/ajcn/nqab103
22. Jorgensen, JM, Young, R, Ashorn, P, Ashorn, U, Chaima, D, Davis, JCC, et al. Associations of human milk oligosaccharides and bioactive proteins with infant growth and development among Malawian mother-infant dyads. Am J Clin Nutr. 113:209–20. doi: 10.1093/ajcn/nqaa272
23. Subbarao, P, Anand, SS, Becker, AB, Befus, AD, Brauer, M, Brook, JR, et al. The Canadian healthy infant longitudinal development (CHILD) study: examining developmental origins of allergy and asthma. Thorax. (2015) 70:998–1000. doi: 10.1136/thoraxjnl-2015-207246
24. Moraes, TJ, Lefebvre, DL, Chooniedass, R, Becker, AB, Brook, JR, Denburg, J, et al. The Canadian healthy infant longitudinal development birth cohort study: biological samples and biobanking. Paediatr Perinat Epidemiol. (2015) 29:84–92. doi: 10.1111/ppe.12161
25. Ewaschuk, JB, Unger, S, Connor, DLO, Stone, D, Harvey, S, Clandinin, MT, et al. Effect of pasteurization on selected immune components of donated human breast milk. J Perinatol. (2011) 31:593–8. doi: 10.1038/jp.2010.209
26. Jensen, CL, Maude, M, Anderson, RE, and Heird, WC. Effect of docosahexaenoic acid supplementation of lactating women on the fatty acid composition of breast milk lipids and maternal and infant plasma phospholipids 1 – 4. Am J Clin Nutr. (2000) 71:292S–9S. doi: 10.1093/ajcn/71.1.292S
27. Munhoz, J, Wattar, N, Goruk, S, Pakseresht, M, Jarman, M, Forbes, L, et al. Determinants of maternal and infant omega-3 status at 3 months postpartum: findings from the APrON longitudinal cohort study. Am J Clin Nutr. (2025) 121:629–42. doi: 10.1016/j.ajcnut.2025.01.002
28. Miliku, K, Duan, QL, Moraes, TJ, Becker, AB, Mandhane, PJ, Turvey, SE, et al. Human milk fatty acid composition is associated with dietary, genetic, sociodemographic, and environmental factors in the CHILD cohort study. Am J Clin Nutr. (2019) 110:1370–83. doi: 10.1093/ajcn/nqz229
29. Azad, M, Robertson, B, Atakora, F, Azad, MB, Becker, AB, Subbarao, P, et al. Human milk oligosaccharide concentrations are associated with multiple fixed and modifiable maternal characteristics, environmental factors, and feeding practices. J Nutr. (2018) 148:1733–42. doi: 10.1093/jn/nxy175
30. Bayley, N. Bayley scales of infant and toddler development–third edition. J Psychoeduc Assess. (2007) 25:180–90. doi: 10.1177/0734282906297199
31. Bode, L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology. (2012) 22:1147–62. doi: 10.1093/glycob/cws074
32. Hochberg, B. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Biometrika. (1995) 61:1–15. doi: 10.2307/2346101
33. Guxens, M, Mendez, MA, Moltó-Puigmartí, C, Julvez, J, García-Esteban, R, Forns, J, et al. Breastfeeding, long-chain polyunsaturated fatty cids in colostrum, and infant mental development. Pediatrics. (2011) 128:e880–9. doi: 10.1542/peds.2010-1633
34. Beyerlein, A, Hadders-algra, M, Kennedy, K, Fewtrell, M, Singhal, A, Rosenfeld, E, et al. Infant formula supplementation with long-chain polyunsaturated fatty acids has no effect on Bayley developmental scores at 18 months of age-IPD meta-analysis of 4 large clinical trials. J Pediatr Gastroenterol Nutr. (2010) 50:79–84. doi: 10.1097/MPG.0b013e3181acae7d
35. Gale, CR, O’Callaghan, FJ, Bredow, M, and Martyn, CN. The influence of head growth in fetal life, infancy, and childhood on intelligence at the ages of 4 and 8 years. Pediatrics. (2006) 118:1486–92. doi: 10.1542/peds.2005-2629
36. Becker, M, Fehr, K, Goguen, S, Miliku, K, Field, C, Robertson, B, et al. Multimodal machine learning for modeling infant head circumference, mothers’ milk composition, and their shared environment. Sci Rep. (2024) 14:2977–18. doi: 10.1038/s41598-024-52323-w
37. Jollife, IT, and Cadima, J. Principal component analysis: a review and recent developments. Philos Trans R Soc Lond A Math Phys Eng Sci. (2016) 374:1–16. doi: 10.1098/rsta.2015.0202
38. Zhang, Z, Wang, Y, Yang, X, Cheng, Y, Zhang, H, Xu, X, et al. Human milk lipid profiles around the world: a systematic review and meta-analysis. Adv Nutr. (2022) 13:2519–36. doi: 10.1093/advances/nmac097
39. Khan, N, Raine, L, Drollette, E, Scudder, M, and Hillman, C. The relation of saturated fats and dietary cholesterol to childhood cognitive flexibility. Appetitie. (2015) 93:51–6. doi: 10.1016/j.appet.2015.04.012
40. Eskelinen, MH, Ngandu, T, Helkala, EL, Tuomilehto, J, Nissinen, A, Soininen, H, et al. Fat intake at midlife and cognitive impairment later in life: a population-based CAIDE study. Int J Geriatr Psychiatry. (2008) 23:741–7. doi: 10.1002/gps.1969
41. Ferreira, ALL, Alves-Santos, NH, Freitas-Costa, NC, Santos, PPT, Batalha, MA, Figueiredo, ACC, et al. Associations between human milk oligosaccharides at 1 month and infant development throughout the first year of life in a Brazilian cohort. J Nutr. (2021) 151:3543–54. doi: 10.1093/jn/nxab271
42. Sato, K, Nakamura, Y, Fujiyama, K, Ohneda, K, Nobukuni, T, Ogishima, S, et al. Absolute quantification of eight human milk oligosaccharides in breast milk to evaluate their concentration profiles and associations with infants’ neurodevelopmental outcomes. J Food Sci. (2024) 89:10152–70. doi: 10.1111/1750-3841.17597
43. Zhang, S, Li, T, Xie, J, Zhang, D, Pi, C, Zhou, L, et al. Gold standard for nutrition: a review of human milk oligosaccharide and its effects on infant gut microbiota. Microb Cell Factories. (2021) 20:108–16. doi: 10.1186/s12934-021-01599-y
44. Wang, M, Li, M, Wu, S, Lebrilla, CB, Chapkin, RS, Ivanov, I, et al. Fecal microbiota composition of breast-fed infants is correlated with human milk oligosaccharides consumed. J Pediatr Gastroenterol Nutr. (2015) 60:825–33. doi: 10.1097/MPG.0000000000000752
45. Rose, G. Sick individuals and sick populations. Int J Epidemiol. (2001) 30:427–32. doi: 10.1093/ije/30.3.427
46. Government of Canada. (2024). Canada’s breastfeeding dashboard. Available online at: https://health-infobase.canada.ca/breastfeeding/. (Accessed March 5, 2025)
47. Koutra, K, Chatzi, L, Roumeliotaki, T, Vassilaki, M, Giannakopoulou, E, Batsos, C, et al. Socio-demographic determinants of infant neurodevelopment at 18 months of age: mother–child cohort (Rhea study) in Crete, Greece. Infant Behav Dev. (2012) 35:48–59. doi: 10.1016/j.infbeh.2011.09.005
48. Cheatham, CL, and Sheppard, KW. Synergistic effects of human milk nutrients in the support of infant recognition memory: an observational study. Nutrients. (2015) 7:9079–95. doi: 10.3390/nu7115452
49. Li, T, Samuel, TM, Zhu, Z, Howell, B, Cho, S, Baluyot, K, et al. Joint analyses of human milk fatty acids, phospholipids, and choline in association with cognition and temperament traits during the first 6 months of life. Front Nutr. (2022) 9:1–17. doi: 10.3389/fnut.2022.919769
50. Lewis, ZT, Totten, SM, Smilowitz, JT, Popovic, M, Parker, E, Lemay, DG, et al. Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants. Microbiome. (2015) 3:13–7. doi: 10.1186/s40168-015-0071-z
51. Berger, PK, Hampson, HE, Schmidt, KA, Alderete, TL, Furst, A, Yonemitsu, C, et al. Stability of human-milk oligosaccharide concentrations over 1 week of lactation and over 6 hours following a standard meal. J Nutr. (2022) 152:2727–33. doi: 10.1093/jn/nxac214
Keywords: cognitive development, language developement, motor development, breast milk composition, Bayley-III Scales of Infant and Toddler Development, cohort study
Citation: Turner SE, Roos LE, Nickel NC, Moraes TJ, Turvey SE, Simons E, Subbarao P, Robertson B, Chikuma J, Goruk S, Field CJ, Bode L, Pei J, Mandhane PJ and Azad MB (2025) Examining associations between human milk fatty acids, oligosaccharides, and early infant cognitive, language and motor development in the CHILD cohort study. Front. Nutr. 12:1606169. doi: 10.3389/fnut.2025.1606169
Edited by:
Francisco José Pérez-Cano, University of Barcelona, SpainReviewed by:
Brittany Rollins Howell, Virginia Tech Carilion, United StatesLijun Chen, Beijing Sanyuan Foods Co., Ltd., China
Copyright © 2025 Turner, Roos, Nickel, Moraes, Turvey, Simons, Subbarao, Robertson, Chikuma, Goruk, Field, Bode, Pei, Mandhane and Azad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meghan B. Azad, TWVnaGFuLmF6YWRAdW1hbml0b2JhLmNh
 Sarah E. Turner
Sarah E. Turner Leslie E. Roos
Leslie E. Roos Nathan C. Nickel1,2,3,4,6
Nathan C. Nickel1,2,3,4,6 Theo J. Moraes
Theo J. Moraes Stuart E. Turvey
Stuart E. Turvey Elinor Simons
Elinor Simons Padmaja Subbarao
Padmaja Subbarao Susan Goruk
Susan Goruk Catherine J. Field
Catherine J. Field Lars Bode
Lars Bode Jacqueline Pei
Jacqueline Pei Piushkumar J. Mandhane
Piushkumar J. Mandhane Meghan B. Azad
Meghan B. Azad