- 1Key Laboratory of Medical Science and Laboratory Medicine of Jiangsu Province, School of Medicine, Jiangsu University, Zhenjiang, Jiangsu, China
- 2Department of Laboratory Medicine, The Affiliated People’s Hospital, Jiangsu University, Zhenjiang, Jiangsu, China
- 3The People’s Hospital of Danyang, Affiliated Danyang Hospital of Nantong University, Zhenjiang, Jiangsu, China
IBD, which includes Crohn’s and ulcerative colitis, is associated with gut microbiota dysbiosis. The dysbiotic environment results in an elevation of harmful microbiota and a diminution of advantageous microbiota, leading to IBD. Interestingly, plant-based dietary compounds consisting of dietary fibers and polyphenols have demonstrated promise to be safe and successful in IBD treatment, with studies revealing that they can improve dysbiosis, increase anti-inflammatory cytokines, decrease pro-inflammatory cytokines, lower oxidative stress, and improve barrier function. Plant-based dietary compounds have shown potential to reduce IBD by regulating signaling pathways such as TGF-β/Smad, TRL-4/NF-κB/MAPK, TLR2-NF-κB, autophagy, pyroptosis, glycolysis/gluconeogenesis and amino acid metabolism, Nrf-2/HO-1, microbiota-macrophage-arginine metabolism, and bile acid metabolism. Additionally, they assist in forming short-chain fatty acids and other metabolites, which help regulate immune cells to alleviate IBD. Recent research indicates that dietary compounds, either as nanoparticles or encapsulated in nanoparticles, have shown potential in effectively treating IBD. Despite the beneficial role of plant-based dietary compounds, other studies have shown detrimental effects such as cancer promotion and exacerbation of immune responses. Therefore, this will help clinicians/individuals to plan their nutrition to prevent IBD exacerbation. This review highlights the microbiota signatures linked to IBD and examines the impact of gut dysbiosis on IBD. It also provides a comprehensive discussion of how plant-based dietary compounds can influence the modulation of dysbiotic gut microbiota in IBD. Plant-based dietary compounds hold potential for treating IBD.
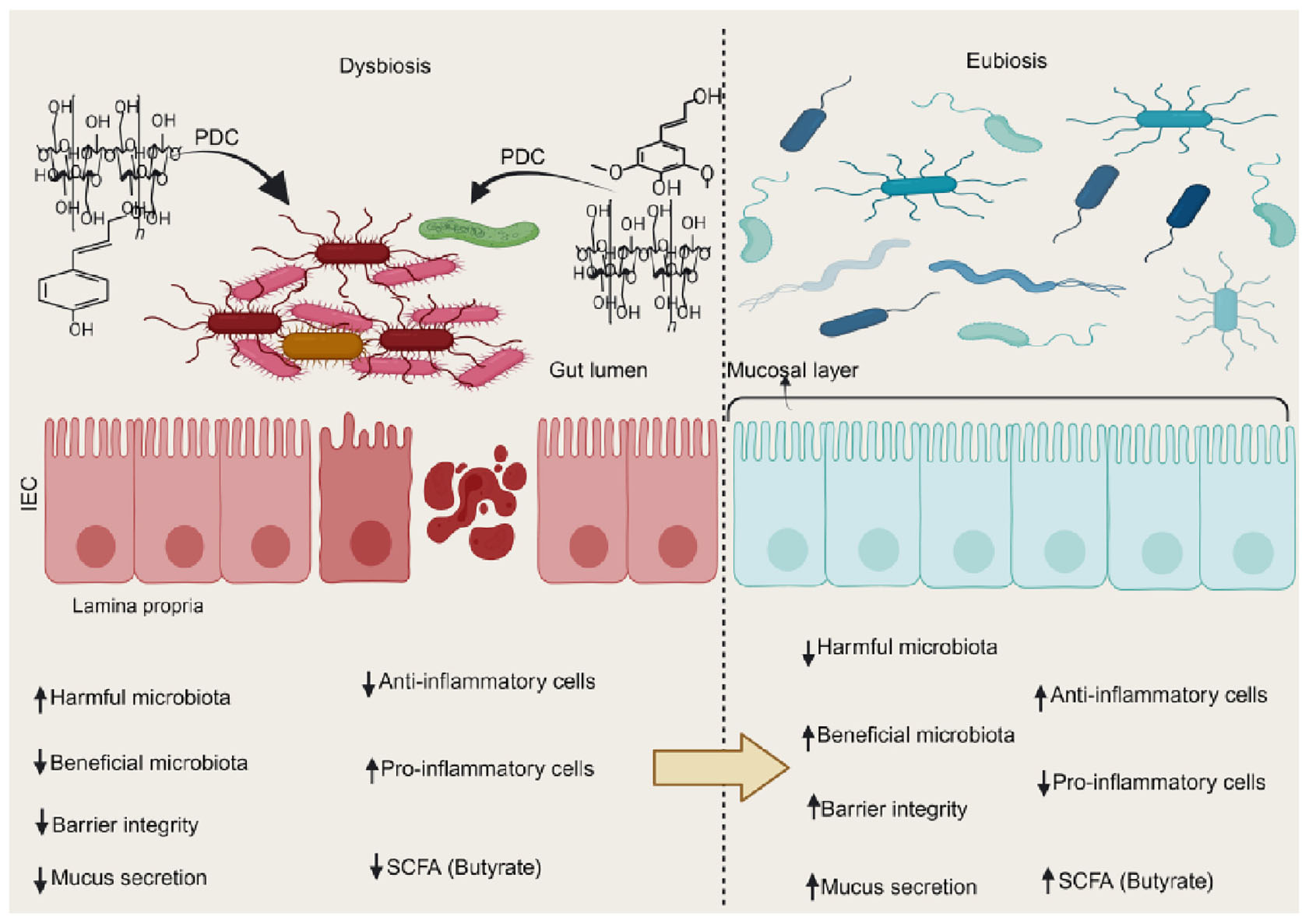
Graphical Abstract. Plant-based dietary compounds help restore a eubiotic environment in dysbiotic settings. This improves gut barrier repair and lowers inflammatory markers, easing IBD symptoms. PDC, plant-based dietary compounds; SCFA, short chain fatty acid.
1 Introduction
Inflammatory bowel diseases (IBD), which include Crohn’s disease (CD) and ulcerative colitis (UC), are characterized by long-term chronic inflammation of the digestive system (1) and have a complex etiology that involves genetic susceptibility, environmental factors, and the intricate interactions between the host’s immune system and gut microbiota (2). IBD is a common condition in Europe and America, and because of changes in dietary habits, its incidence rate is rising in Asia (3). CD and UC patients often experience weight loss, diarrhea, fatigue, bloody stool, fecal urgency, mucoid stool, and abdominal pain (4).
The digestive tract contains numerous microbes that have evolved alongside the host immune system (5). It is now commonly known that a healthy gut flora significantly influences the host’s general health (6). The normal gut microbiota helps protect the gut mucosal barrier and control the immune system (Figure 1). It also helps break down nutrients and drugs and fight off pathogens (6). However, emerging research highlights that intestinal dysbiosis plays a critical role in both the onset and progression of IBD (7). Antibiotics, steroids, immune modulators, and 5-aminosalicylates have all been used to lessen symptoms and keep remission going in IBD (8). Nevertheless, prolonged use of these substances has been shown to cause serious toxicities, which discourage consumers (8). Thus, finding an effective treatment to restore gut microbiota to a state of eubiosis and reduce drug toxicities is essential for addressing the increasing prevalence of IBD. One potential strategy for nutritionally treating IBD with little adverse effects is the use of food bioactive substances (9).
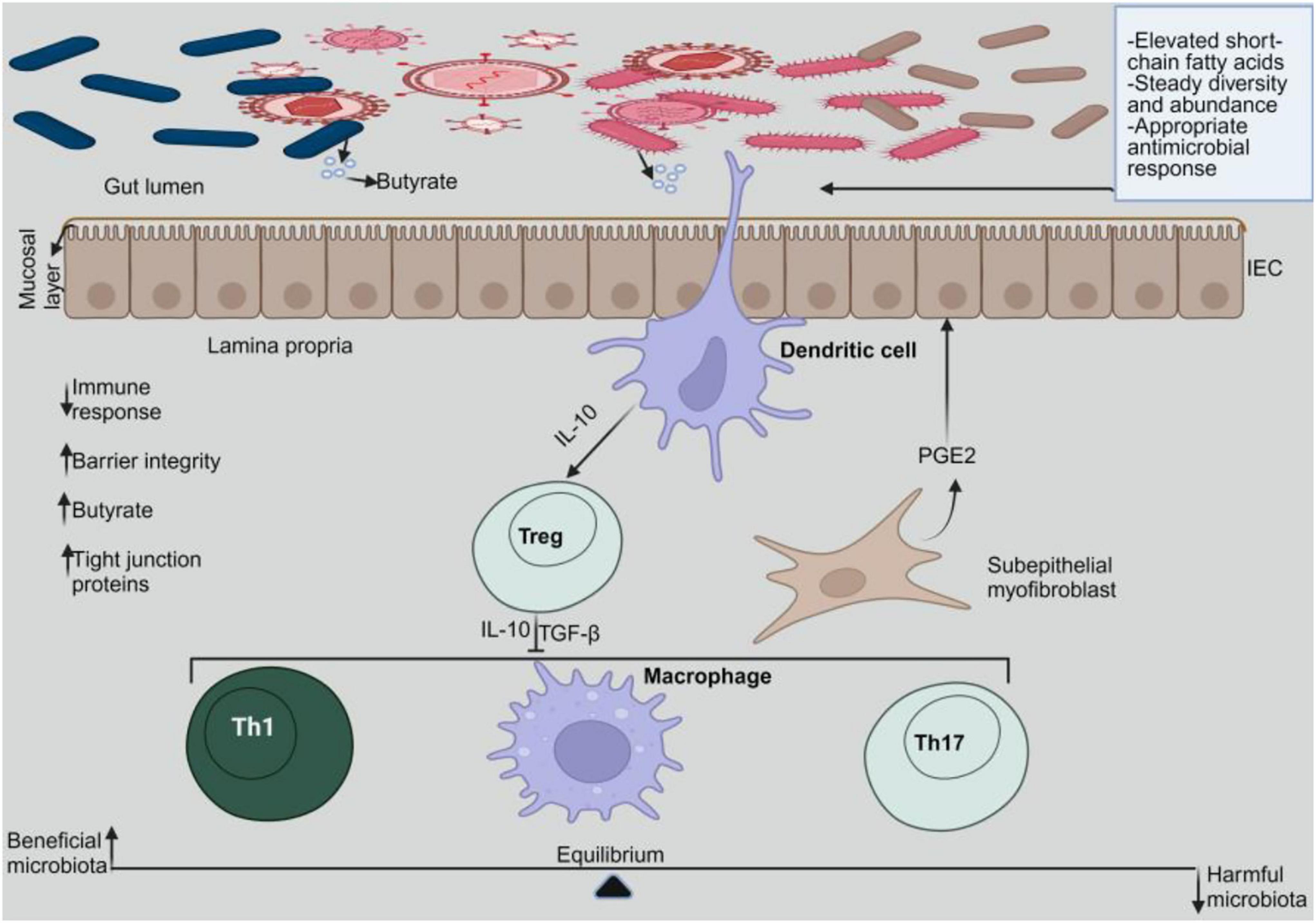
Figure 1. Gut microbiome homeostasis. In a homeostatic environment, the helpful microbes multiply while the toxic ones diminish. Dendritic cells interact with the beneficial microbes to emit IL-10, which stimulates Tregs. Tregs release IL-10 and TGF-β to prevent pro-inflammatory cells from activating.
Plant-based diets have become increasingly popular for enhancing animal welfare, improving human health, and benefiting the environment (10); consuming them offers several advantages, including improved digestive and immune system function (11). The plants’ dietary components have shown the potential to reduce colitis in mouse models by modifying the gut microbiota, lowering immune responses, and minimizing barrier damage. These compounds enhance beneficial intestinal microbes and reduce harmful ones (12–15). Diets based on plants are safe and beneficial for all phases of life, including childhood, old age, pregnancy, and breastfeeding (16). Understanding the role of gut dysbiosis in IBD is crucial for exploring additional plant-based therapies aimed at gut modulation. We therefore examine the critical function of dysbiotic gut microbiota in IBD and demonstrate how plant-based dietary compounds can successfully alter the dysbiotic gut microbiome. This will open up new paths for treating IBD symptoms.
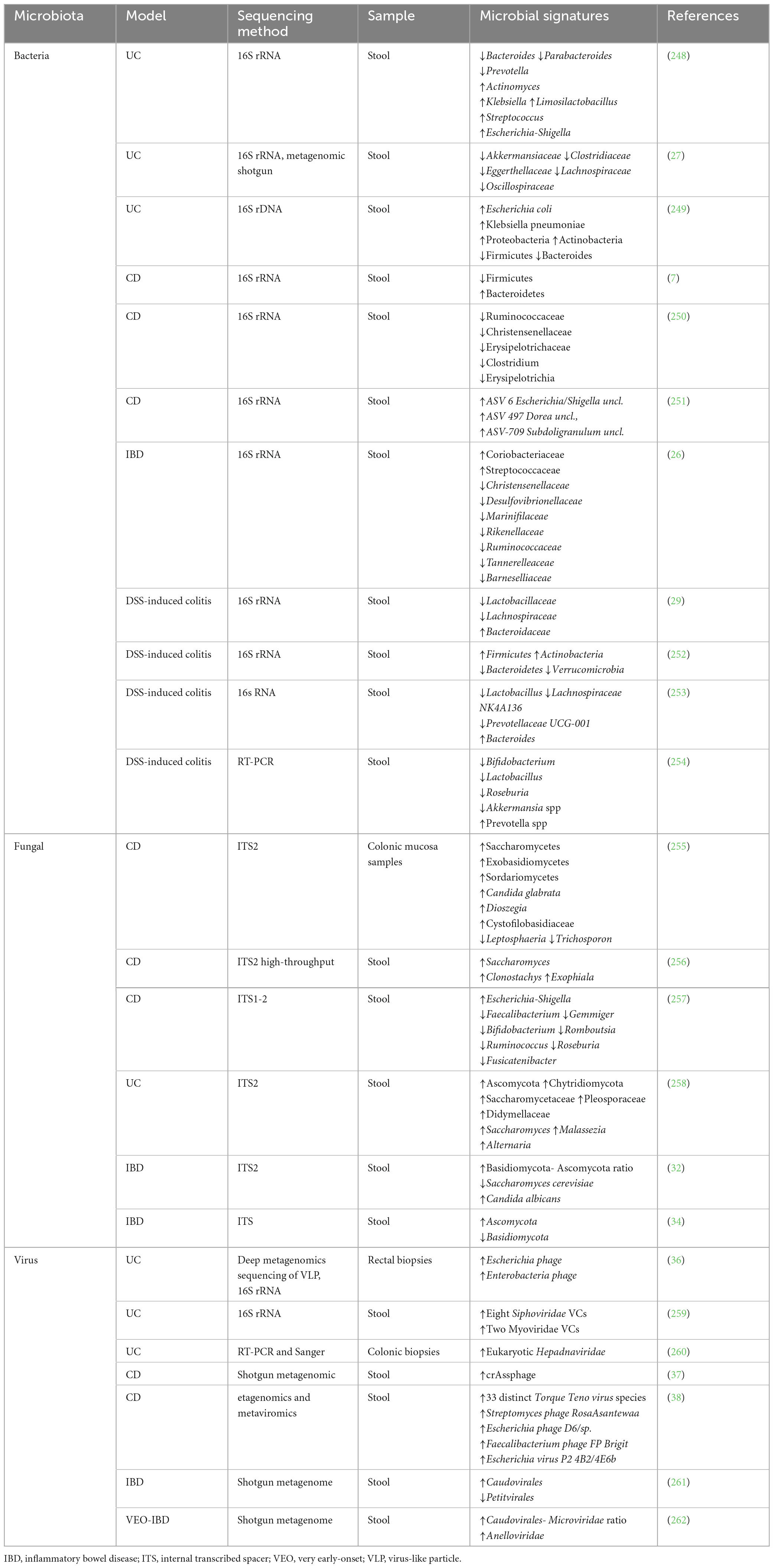
2 Key microbial signatures that are involved in the dysbiosis of IBD
More than 1 trillion microorganisms live in the human body, and the gastrointestinal tract alone is home to various commensal microbes (17). The gut microbiota is an essential component of the human metaorganism that shapes physiologic host immunological responses, including host defense against infections (18). An important factor in IBD and its chronicity is the interplay among the intestinal mucosal barrier, the mucosal immune system, and a disrupted microbial makeup (19). Dysbiosis is any perturbation to the makeup or function of the microbiome (20). Microbial dysbiosis is characterized by reduced biodiversity, changed geographic distribution, abnormal gut microbiota composition, and interactions between microbiota, strains, and the host (21). IBD onset and development are associated with alterations in gut microbiota and metabolites, but the specific microbial communities affected and their potential contribution to the disease remain unclear (22, 23). Several studies have revealed gut microbiota dysbiosis, including viruses, bacteria, and fungi in IBD. The helpful microbiota decreases while the pathogenic microbiome increases. As a result, plant-based dietary components’ potential to reverse these alterations by increasing helpful microorganisms while decreasing pathogenic microbes may aid in the prevention of IBD. These will be covered in the discussion section. Table 1 summarizes the alterations in the gut microbial signatures in IBD.
3 Gut microbiota dysbiosis and its impact on IBD
3.1 Gut microbiota diversities
Dysregulation during infancy can result in diseases later in life since the human gut flora develops and matures throughout this time. The microbiota of infants differs from that of adults in several metabolite groups, including short- and branched-chain fatty acids associated with changes in bacterial populations (24). In particular, during illness and early growth, the gut microbiota can change over time and is incredibly varied (25). Evidence from clinical (26–28) and preclinical (29–31) studies shows that the gut bacteria’s diversity is lower in people with IBD and DSS compared to controls. Reduced biodiversity of the gut fungal (32–35) and the gut virome (36–38) has also been observed in IBD both clinically and preclinically.
3.2 Barrier integrity disruption and influence on gut’s immunological system
The intestinal epithelium facilitates the movement of nutrients, water, and waste products while acting as a barrier to restrict interactions between luminal contents such as the gut microbiota, the immune system, and the body (39). Tight junction proteins like Zonula occludens, occludin, and claudins are essential for maintaining the integrity of the epithelial barrier (40). Tight junctions are crucial for maintaining barrier integrity by regulating the movement of antigens through the intestinal epithelial barrier (41). Multiple studies indicate that gut microbiota comprising bacteria (42, 43), fungi (44), and viruses (45–47) can compromise intestinal barrier integrity, leading to the pathogenesis of IBD. Additionally, the gut bacteria (43, 48–50), virus (51, 52), and fungi (53–55) have shown potential to cause immunological responses in IBD. These findings imply that the gut microbiota may participate in IBD pathogenesis. Figure 2 illustrates how dysbiosis of the gut microbiome leads to compromised intestinal epithelium and heightened immune responses, exacerbating IBD. Dysbiosis increases detrimental gut microbiota, which then induces the generation of proinflammatory cytokines and disrupts the barrier function.
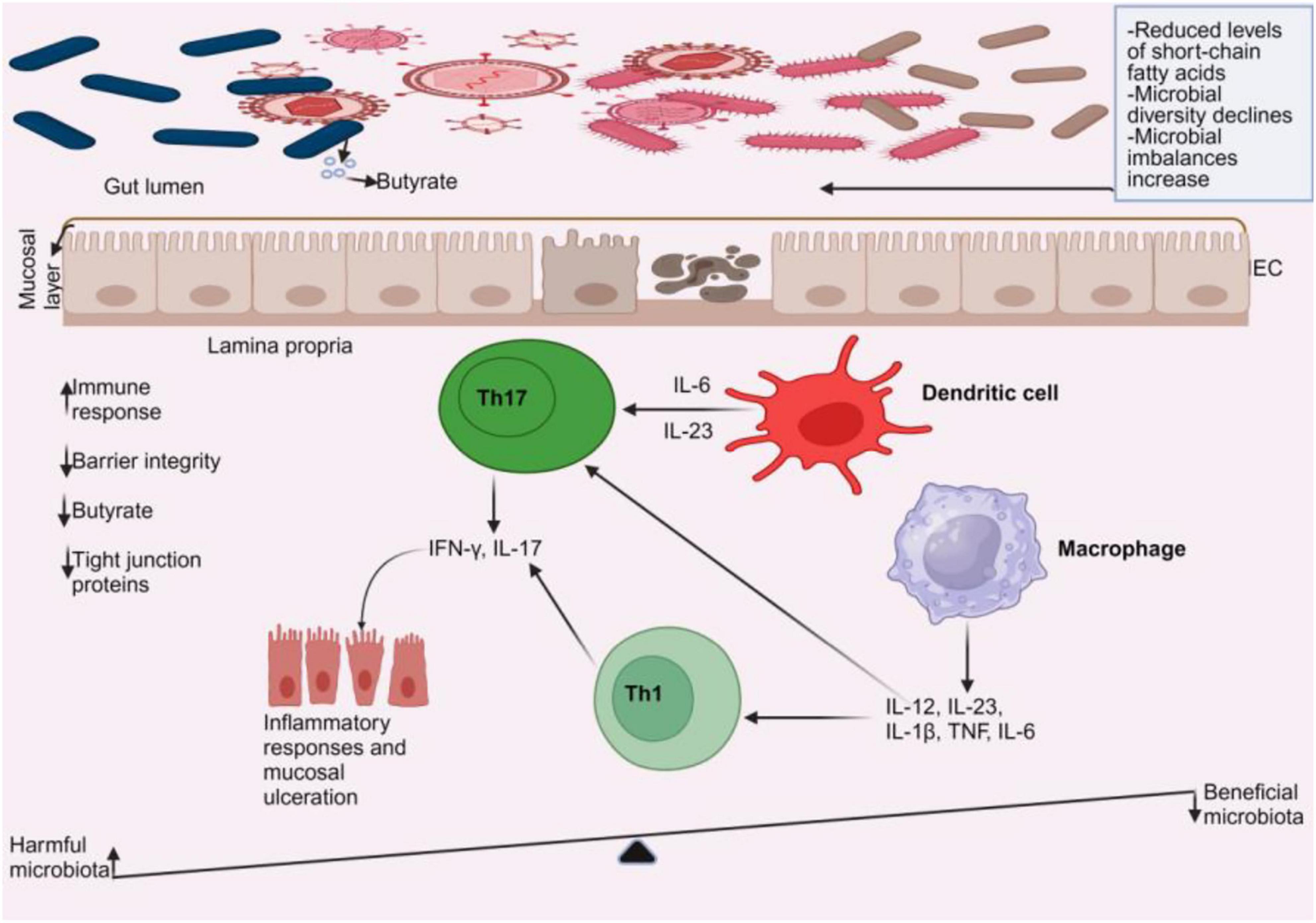
Figure 2. Gut microbiome dysbiosis in IBD. In a dysbiotic environment, short-chain fatty acids (such as butyrate) and gut microbial diversity decrease, accompanied by a rise in microbial imbalances. An increase in harmful microbiota leads to the activation of dendritic cells and macrophages, prompting them to produce cytokines. These cytokines stimulate T helper cells, specifically Th1 and Th17, to release more cytokines that cause mucosal ulcerations. As a result, there is a decrease in tight junction proteins and barrier integrity, along with a reduction in anti-inflammatory cells, while pro-inflammatory cells increase.
4 Plant-based dietary compounds
The American College of Lifestyle Medicine advises consuming a diet high in plant-based foods, including whole grains, legumes, nuts, seeds, fruits, and minimally processed vegetables (56). The benefits of plant-based diets for human and environmental health have made them more popular in recent years (57). Plant-based diets often provide numerous health benefits, including increased intake of essential vitamins and minerals, reduced saturated fat consumption, and increased fiber intake (56). Additionally, research has proven that plant-based diets can reduce the risk of chronic renal disease (58), improve cardiovascular health (59), combat multidrug-resistant bacteria-induced enteric disorders (60), and prevent nonalcoholic fatty liver disease (61). Polyphenols, dietary fibers, and prebiotics are the main components of plant-based diets. These are described below.
4.1 Types of plant-based dietary compounds
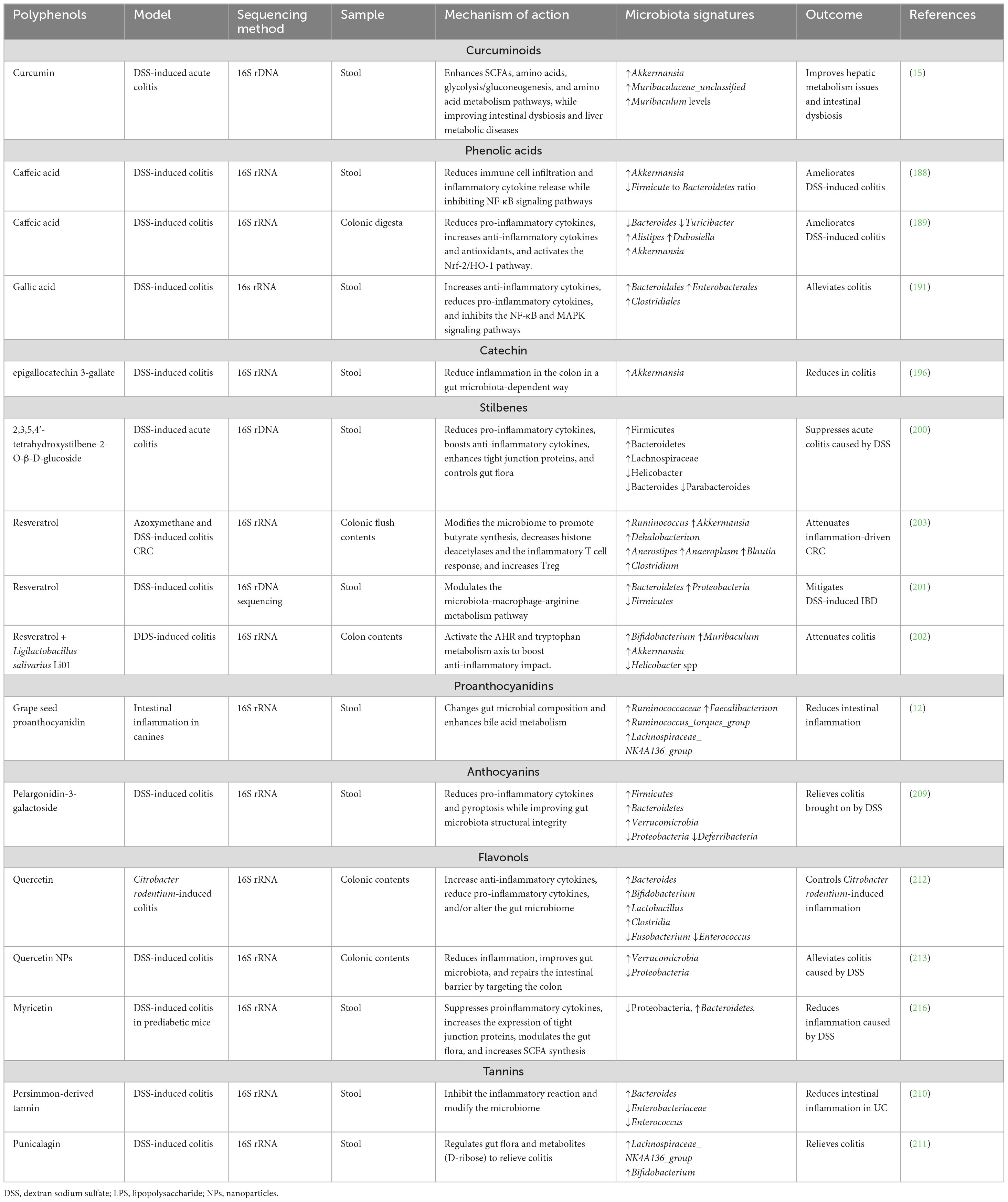
4.1.1 Polyphenols
Nutritionists and food scientists are increasingly interested in the nutraceutical properties of dietary plant polyphenols, which are naturally occurring bioactive substances (62). Phenolic chemicals are significant components of plant-derived diets, as their existence correlates with health-protective properties (63). Phenolic chemicals must be liberated from the matrix during digestion in an absorbable form (bioaccessible), then absorbed and transported to the bloodstream (bioavailable) to exert their biological action (63).
4.1.1.1 Sources
Plant-based foods high in polyphenols, such as fruits, vegetables, tea, coffee, wine, and chocolate, provide several health advantages (64). The gut microbiota may mediate the beneficial effects of polyphenols on host metabolism (65). Renowned for their strong antioxidant qualities, polyphenols neutralize free radicals, therefore addressing oxidative stress and helping to avoid chronic non-communicable disorders like cancer, cardiovascular problems, obesity, and diabetes (64).
4.1.1.2 Types
Dietary polyphenols, such as phenolic acids, flavonoids, catechins, tannins, lignans, stilbenes, and anthocyanidins, are prevalent in grains, cereals, legumes, vegetables, spices, fruits, chocolates, and drinks including fruit juices, tea, coffee, and wine (66). Additionally, curcuminoids are phenolic chemicals frequently added to food as a spice, color, and additive. They serve as a medicinal agent (67).
4.1.1.2.1 Phenolic acids
One important type of dietary polyphenols that are naturally occurring antioxidants is phenolic acids (68). Plant metabolites called phenolic acids are present in various parts of the plant kingdom (69). Mohammed and his team used phytochemical analysis to find phenolic acids in Ephedra alata Decne. These acids include p-coumaric acid, ferulic acid, ellagic acid, caffeic acid, vanillic acid, rosmarinic acid, and chlorogenic acid (70). Researchers have demonstrated that phenolic acids slow down the progression of osteoarthritis by reducing the expression of catabolic factors, mitigate alcohol-induced liver disease by altering the hepatic circadian rhythm signaling pathway through the gut microbiota-NPAS2 axis, prevent inflammation and ferroptosis by controlling the AMPK/mTOR/HIF-1 signaling pathway, alleviate S. aureus-induced endometritis in mice, shield testicular injuries caused by cyclophosphamide, and reduce vascular endothelial growth factor-induced angiogenesis and endothelial permeability. Phenolic acids have also been found to effectively treat neurotoxicity brought on by exposure to neuroendocrine disruptors, reduce splenic tissue inflammation, balance oxidative stress in carp via the nuclear factor erythroid 2-related factor 2 (Nrf2)/NQO-1 pathway, and enhance spleen apoptosis (71–77).
4.1.1.2.2 Flavonoids
Flavonoids are substances that are found in nature and have a variety of health benefits (78). Numerous plants, fruits, vegetables, and leaves contain phytochemicals called flavonoids, which may have uses in medical chemistry (79). Proanthocyanidins are byproducts of flavonoid biosynthesis, consisting of oligo- or polymers made from monomeric flavan-3-ols (80). The degree of unsaturation and oxidation of the C ring and the carbon to which the B ring is linked divide flavonoids into subgroups. Isoflavones are flavonoids with a B ring connected to a C ring at position 3, and neoflavonoids have a B ring connected at position 4. Flavonoids with a B ring connected at position 2 can be further classified into subgroups based on the C ring’s structural characteristics. These subcategories include anthocyanins, chalcones, flavones, flavonols, flavanones, flavanonols, and flavanols or catechins (81). Researchers have proven several advantages from these substances. For instance, increased anthocyanin intake has been associated with a decrease in cardiovascular disease mortality (82). Chalcone T4 also inhibits inflammation in periodontal tissues and the loss of alveolar bone (83). Isoorientin (a natural flavone) therapy in mice with excisional wounds improved tissue healing (84). There is evidence that the natural flavonol kaempferol and flavanonol dietary dihydromyricetin can help treat rheumatoid arthritis and protect against growth retardation and intestinal damage caused by enterotoxigenic Escherichia coli, respectively (85, 86). Other types of flavonoids have also shown promise in slowing the progression of nonalcoholic fatty liver disease, lowering signs of neuroinflammation and cell death in the hippocampus in people with Gulf War Illness, and possibly being used as a treatment for the Omicron version of SARS-CoV-2 (61, 87, 88).
4.1.1.2.3 Curcuminoids
Curcuminoids, commonly used as pigment spices, are phenolic chemicals with antiviral, antitumor, anti-HIV, anti-inflammatory, antiparasitic, anticancer, and antifungal properties. The main active and consistently bioactive components are curcumin, bisdemethoxycurcumin, and demethoxycurcumin (89). Curcumin’s strong anti-inflammatory qualities and regulatory impact on the gut flora make it a research hotspot for IBD treatment (90). Also, bisdemethoxycurcumin holds enormous promise for creating powerful inhibitors that reduce the likelihood of deadly amyotrophic lateral sclerosis (91). Demethoxycurcumin has been shown to upregulate peroxisome proliferator activated receptor γ (PPARγ), which inhibits the growth of cervical cancer cells (92).
4.1.1.2.4 Tannins, lignans, stilbenes
These chemicals, recognized as dietary polyphenols from various sources, exhibit beneficial effects in disease management. For instance, Pterostilbene, a naturally occurring stilbene, has anticancer properties in head and neck cancer cells and prevents liver damage from alcohol consumption, both acute and chronic (93, 94). Schisandra chinensis (Turcz.) Baill’s lignan-enriched extract offers protection against Parkinson’s disease (95). After mandibular molar extraction, green tea tannin has been shown to stop bleeding much better than aqueous and methanolic extracts (96).
4.1.2 Dietary fibers
Fiber consumption improves metabolic homeostasis in both humans and rodents, which in turn leads to changes in the gut microbiota (97). Due to the absence of the digestive enzyme necessary for fiber digestion, dietary fiber is a nondigestible form of carbohydrates in humans (98). Dietary fibers’ structure dictates the metabolic variations and alterations in the gut microbiota brought about by fermentation, which in turn influence the health impacts of gut microbes (99). Consuming dietary fiber, especially insoluble fiber from fruits, vegetables, and other foods, may lower the risk of breast cancer, particularly in premenopausal women (100). Zheng et al. (101) found that total, insoluble, or soluble dietary fibers taken from highland barley can help mice on a high-fat diet (HFD) lose weight, change their blood lipid profiles, and heal damaged tissues (101).
4.1.2.1 Classification of dietary fibers
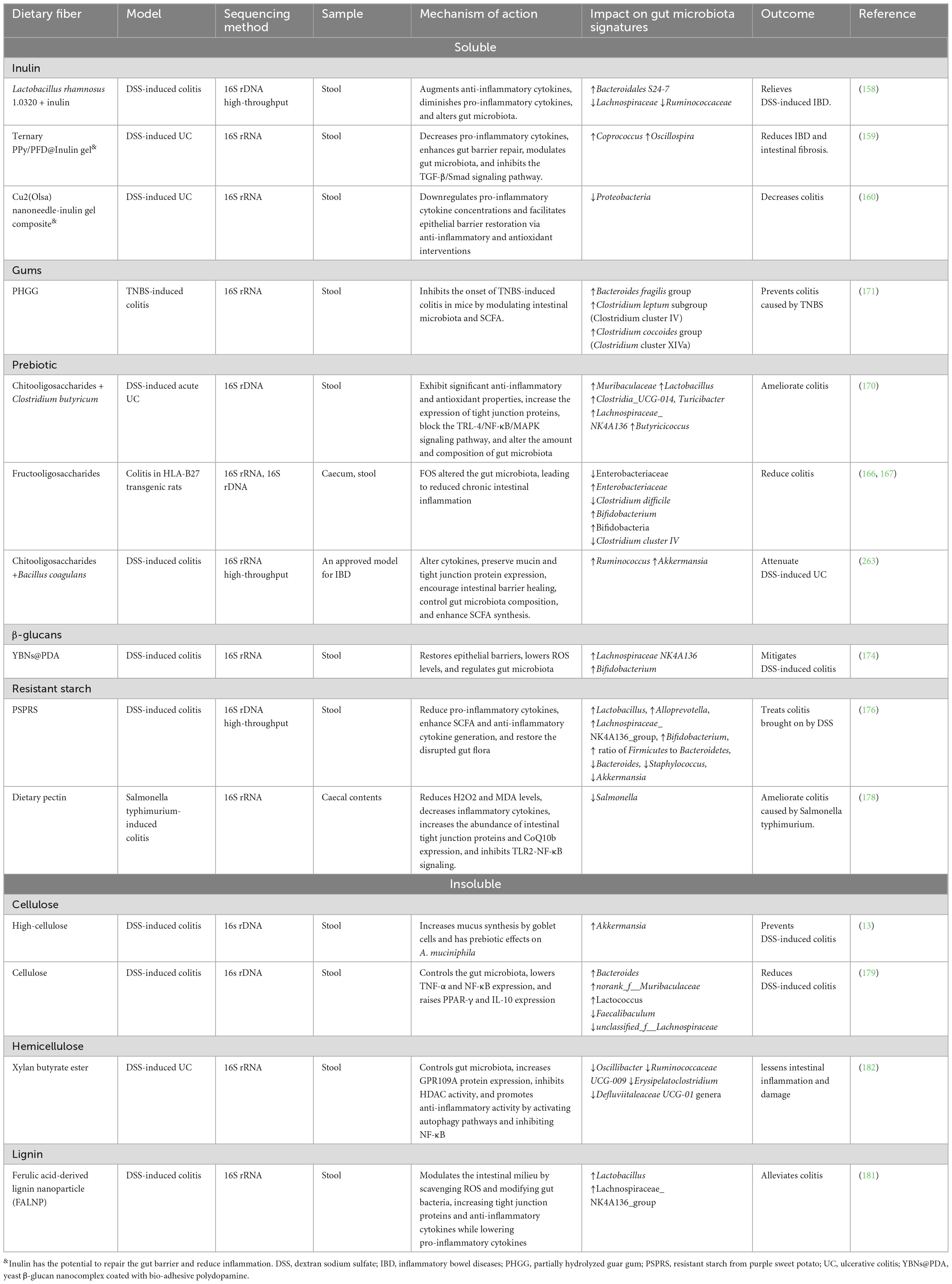
Dietary fiber falls into the soluble or insoluble category based on its water solubility characteristics (98). Soluble fibers comprise pectin, inulin, resistant starch, β-glucan, gums, and mucilages (102–104), while insoluble fibers comprise cellulose, hemicellulose, and lignin (103, 104).
4.1.2.1.1 Soluble fibers
4.1.2.1.1.1 Pectin
Apple and citrus peels are currently the main ingredients used in commercial pectin manufacturing (105). Pectin is a collection of intricate polysaccharides naturally occurring in diverse plants and linked to numerous advantageous health benefits (106). Pectins are dietary fibers recognized for their various positive immunomodulatory effects and their role in managing and preventing different inflammatory disorders (107, 108). Pectins can be classified as high methoxyl pectin or low methoxyl pectin based on the degree of esterification (107). A study has demonstrated that pectin prevents ileitis (109). Liu and colleagues found that pectin from comfrey roots could lessen colon damage from DSS in rats and repair the intestinal barrier (110). In a pilot study, supplementing healthy volunteers with citrus low-methoxy pectin lowers inflammation and anxiety levels (111).
4.1.2.1.1.2 Inulin
As a reserve polysaccharide, inulin, a soluble dietary fiber, is present in over 36,000 plant species (112). Jerusalem artichokes, chicory, onions, garlic, barley, and dahlia are the main sources of inulin (112, 113). The food industry commonly uses chicory roots and Jerusalem artichoke tubers as raw materials for inulin manufacturing (112). A well-known prebiotic component that has been shown to alter the gut flora and its metabolic processes is inulin (114). This suggests that inulin may possess prebiotic properties. Researchers have demonstrated that inulin prevents atherosclerosis by boosting the intestinal barrier and gut microbiota, decreasing inflammation, and increasing lipid metabolism (115). Research suggests that inulin may have antidiabetic effects by enhancing insulin resistance and insulitis, and reducing obesity progression by modulating gene expression in the prefrontal cortex via endocannabinoids (116, 117).
Small interfering RNA (siRNA)-mediated therapy has shown potential in treating various illnesses by inhibiting gene expression, including those involved in cancer initiation and spread, making it a promising treatment option (118). Interestingly, researchers have applied nanoparticles to inulin to treat diseases. For instance, researchers developed systemically administered nanoparticles using inulin modified with α-cyclam-p-toluic acid (CPTA) (IC) and siRNA against p53, which preferentially concentrate in damaged kidneys and significantly decrease p53 expression (119). Mice with cisplatin-induced acute kidney injury showed improved renal function overall and decreased tubular cell death, renal injury, and inflammation due to selective p53 knockdown (119).
4.1.2.1.1.3 β-glucan
β-glucans are a class of β-D-glucose polysaccharides (glucans) found naturally in grain, fungal, and bacterial cell walls (120). A non-starch-soluble polysaccharide, β-glucan, is found in many foods, including barley, oats, yeast, mushrooms, bacteria, and algae (121). Observations show that the structural features of β-glucan, such as specific glycosidic connections, monosaccharide compositions, molecular weight, and chain conformation, influence its physiochemical and biological properties (121). Enzymes called β-glucanases break down β-glucan into glucose and cello-oligosaccharides (120). β-glucans have demonstrated the ability to elevate reactive oxygen species and induce apoptosis in melanoma cells, alter local innate responses in ewes by preserving the integrity of the mammary epithelial barrier, and exhibit unique immune-modulating properties, rendering them compelling adjuvants for prospective allergy therapies (122–124).
4.1.2.1.1.4 Gums
Gums are carbohydrate-based biomolecules that bind water and form gels. Types include exudate, mucilage, and seed gums. Plant gums, due to their bioavailability, are significant and have been used by humans since prehistoric times for various purposes (125). Plant-derived gums and mucilages are suitable pharmaceutical excipients due to their non-toxicity, stability, availability, regulatory compliance, cost-effectiveness, and adaptability to specific requirements (126). According to a phytochemical analysis, Prunus armeniaca and Prunus domestica gum include proteins, carbohydrates, and saponins (127). These gums exhibit potential in pharmaceutical compositions (127, 128). Furthermore, gum can serve as a medium for the oral administration of protein pharmaceuticals (129). Hydrogel made of chicha gum has demonstrated promise as a wound-healing agent (130).
4.1.2.1.1.5 Mucilages
The food industry is interested in mucilage, a hydrophilic biopolymeric substance in high concentrations in agricultural by-products such as the peel of cactus fruits, due to its high dietary fiber content, antioxidant activity, and gelling and thickening properties (131). Plant parts like seeds, rhizomes, and roots can yield mucilage (132). In nature, it is a polysaccharide made up of large sugar molecules and uronic acid components (132). Okra’s mucilage and flesh may be potential remedies for preventing metabolic dysfunction (133). In mice with alloxan-induced diabetes, Abelmoschus esculentus mucilage has good antioxidant potential and hypoglycemic and hypolipidemic effects (134).
4.1.2.1.1.6 Resistant starch
Humans have long used resistant starch (RS) as a food source, and almost all starchy foods contain it (135). RS is a type of starch that can pass through the small intestine and reach the colon, despite being indigestible by human pancreatic amylase (135). Five different RS types modulate gut microbiota to respond differently to chronic illness (136). Short-chain fatty acids (SCFAs) bridge the gut microbiota and RS, and RS has the potential to improve the metabolism of the gut microbiota and increase the population of beneficial bacteria in the gut (136). RS may enhance body weight and carbohydrate and lipid metabolism (137). The RS diet has shown positive effects on renal function indicators and uremic toxin levels in patients with chronic kidney disease (138).
4.1.2.1.1.7 Prebiotics
Prebiotics are indigestible food fibers that have undergone selective fermentation. They specifically encourage the growth of one or more bacterial genera in the gut, which benefits the host’s health (139). Prebiotics that are helpful to human health fall into two major categories: galactooligosaccharides and fructooligosaccharides (140). Prebiotics can feed the gut microbiota, and their breakdown releases SCFAs into the bloodstream that affect the gut and other external organs (140). Consuming prebiotics such as arabinoxylan oligosaccharides and inulin-type fructans can boost the quantity of bifidobacteria in the colon (141). Bifidobacteria play various roles, including breaking down indigestible carbohydrates, protecting against infections, synthesizing vitamin B, antioxidants, and conjugated linoleic acids, and activating the immune system (141). Supplementing male rats with oligofructose prebiotic fiber has been shown to lessen the effects of a diet heavy in fat and sugar and to prevent knee joint deterioration (142). Notably, the majority of prebiotics are soluble fibers.
4.1.2.1.2 Insoluble fibers
4.1.2.1.2.1 Cellulose
Cellulose is the most prevalent polysaccharide on Earth. It can be found in a wide variety of places, including the cell walls of plants and wood, certain types of bacteria, algae, and tunicates—the only known animals that possess cellulose (143). Cellulose can be categorized into fiber, microfibril/nanofibril, or micro/nanocrystalline cellulose based on the selection of physical characteristics, sizes, and shapes (143). SCFAs produced by cellulolytic bacteria from dietary fiber are vital for maintaining gut health and optimizing fiber use (144). The gut microbiota is essential for digesting cellulose, the primary component of plant fiber, in humans and all other mammals. The human gut microbiota contains ruminococcal species that form multi-enzymatic cellulosome structures, which are functional and capable of breaking down plant cell wall polysaccharides. A species closely related to humans likely originated in ruminants’ guts and spread to humans during domestication. It acquired genes from other gut microbes and underwent diversification and diet-related adaptation. These species are common among hunter-gatherers, ancient people, and rural groups but are uncommon in populations from industrialized countries, suggesting they may go extinct due to Westernized lifestyles (145). Microparticles made of highly crystalline seaweed cellulose may help control gut microbial dysbiosis and reduce obesity and metabolic syndromes linked to a high-fat, high-sugar diet in mice (146). Also, cellulose nanocrystals have demonstrated the potential to enhance intestinal retention and assist in body weight management (147).
4.1.2.1.2.2 Hemicellulose
Plant cell walls contain polysaccharides called hemicelluloses, which have beta-(1– > 4)-linked backbones (148). Around one-third of wall biomass comprises hemicelluloses, including heteromannans, xyloglucan, heteroxylans, and mixed-linkage glucan (149). Research suggests finger millet-derived arabinoxylan and Delonix regia galactomannan can be used as nutraceuticals to control high-fat diet-induced obesity and enhance wound healing in murine cutaneous wounds by increasing transforming growth factor-beta (TGF-β) levels (150, 151). Lemieszek and colleagues have also demonstrated Cantharellus cibarius branched mannans as a novel treatment option for colon cancer (152).
4.1.2.1.2.3 Lignin
Lignin, a dietary fiber derived from plant cell walls, has several biological anti-inflammatory and antioxidant properties (153). Lignin is a complex polymer of phenylpropane units that exhibits extensive cross-linking (154). Common lignin structures found in the insoluble dietary fiber of fruits and vegetables comprise glycerols, spirodienones, dibenzodioxocins, and lignin precursors in the gut known as resinols (155). The nature of the cell wall, particularly the amount of lignin present, significantly influences the gut microbiota and fermentation results (156). Lignin prevents ferroptosis in UC by interacting with GPR37 and triggering the extracellular signal-regulated kinase-Nrf2-glutathione peroxidase 4 (GPX4) signaling pathway, providing new clinical intervention concepts for UC treatment (153). Additionally, lignin-carbohydrate complexes reduce the neurotoxicity of bisphenol A in zebrafish by reducing oxidative stress (157).
5 Role of plant-based dietary compounds in modulating the gut microbiome to repair IBD
5.1 Dietary fibers
5.1.1 Soluble dietary fibers
Numerous studies have demonstrated that inulin can avert various disorders, including IBD. Researchers have recognized additional inulins for their prebiotic characteristics. Inulin enhances beneficial microbiota and diminishes detrimental microbiota. These strategies mitigate inflammation and preserve the integrity of the gut mucosal barrier. For instance, Lactobacillus rhamnosus 1.0320 combined with inulin can alleviate colitis caused by DSS, reduce the disease activity index score of colon tissue damage, and increase IL-10 expression while downregulating IL-1β, IL-6, TNF-α, and myeloperoxidase (MPO) (158). Additionally, the combination dramatically increases the abundance of Bacteroidales S24-7 in enteritis-affected mice while decreasing the abundance of Lachnospiraceae and Ruminococcaceae (158). Cao and team also found that the inulin gel matrix can prolong the residence time of polypyrrole (PPy) nanozymes and pirfenidone (PFD) in the gastrointestinal tract, reducing pro-inflammatory cytokine levels, repairing the intestinal epithelial barrier, and suppressing intestinal fibrosis through sustained reactive oxygen and nitrogen species scavenging and attenuation of the TGF-β/Smad signaling pathway. The gut microbiota was altered, enhancing the presence of beneficial genera like Coprococcus and Oscillospira, which aid in butyrate production, a crucial fatty acid for intestinal barrier restoration (159). Zhang and his team also found that in three animal models of IBD, the Cu2(Olsa) nanoneedle-inulin gel composite significantly reduced colitis by promoting the repair of the epithelial barrier through anti-inflammatory and antioxidant therapies while downregulating levels of pro-inflammatory cytokines (Figure 3). The inulin gel composite containing Cu2(Olsa) nanoneedles reduced the number of harmful microorganisms, including Proteobacteria (160). Various studies have demonstrated the potential of inulin in alleviating different ailments. For instance, inulin may improve metabolic diseases by altering the gut microbiota and enhancing the generation of SCFAs, potentially mediated by the angiopoietin-like protein 4-related signaling pathway. Dietary inulin improved gut microbiota dysbiosis, reduced the loss of Bacteroidetes, inhibited the growth of Firmicutes, and enhanced the ratio of Firmicutes to Bacteroidetes (161). Also, inulin improves anxiety- and depression-like behaviors in alcohol-dependent withdrawal mice by increasing the number of Faecalibacterium and Roseburia, boosting the generation of SCFAs, and regulating serotonin metabolism (162). Additionally, inulin27 has significantly decreased rats’ systemic glucose levels and weight gain from an HFD. Inulin7 reduced levels of Lachnospiraceae linked to metabolic disorders while promoting beneficial Bifidobacteriaceae taxa (163). This indicates that inulin enhances gut microbiota in several disorders, including IBD. However, researchers have found that inulin may induce carcinogenesis in IBD. Tian and team found that following DSS treatment, mice fed inulin showed significant colitis, while mice who completed the research showed substantial colon cancer. Inulin caused a shift in gut flora, which supported the increase of succinate in the gut lumen. Interestingly, inulin-fed mice showed a rise in Bacteroidota cecal abundance (164). In a different study, Hoving and the team found that although inulin has prebiotic effects, it did not reduce hypercholesterolemia or atherosclerosis in E3L.CETP mice. However, it did lead to hepatic inflammation when combined with a high cholesterol intake. Inulin significantly increased the abundance of Coprococcus and Allobaculum in mice, while decreasing the abundance of Bacteroides, Parabacteroides, Prevotella, Micispirillum, Clostridium, and Coprobacillus compared to control mice (165). These findings indicate that inulin may exhibit diverse roles in several disorders, including IBD.
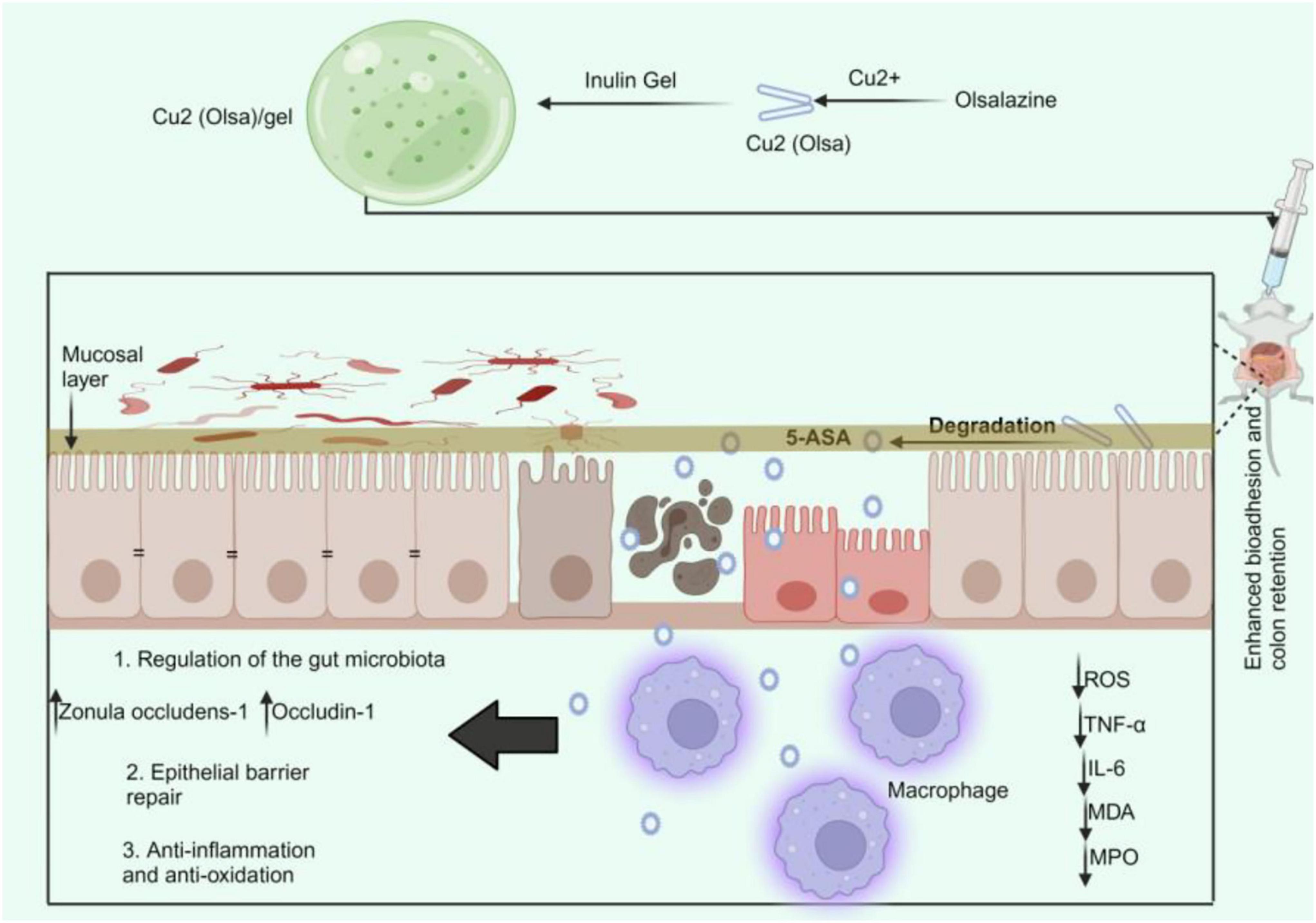
Figure 3. Cu2(Olsa)/Gel treatment of IBD. The oral delivery of Cu2(Olsa)/Gel improves bio-adhesion and colon retention. This makes it easier for 5-ASA to penetrate the inflammatory tissue slowly. It lowers proinflammatory cytokines and speeds up the repair of the epithelial barrier by fighting free radicals and inflammation. This leads to colitis alleviation. 5-ASA, 5-aminosalicylic acid; IL-interleukin; MDA, malondialdehyde; MPO; myeloperoxidase; Olsa, olsalazine; ROS, reactive oxygen species; TNF-α, tumor necrosis factor-alpha.
As previously mentioned, the two primary prebiotics are galactooligosaccharides and fructooligosaccharides. Therefore, Koleva and her team discovered that fructo-oligosaccharides markedly alleviated colitis in rats. While FOS increased the number of Bifidobacterium spp., both fructans (FOS and inulin) decreased the expression of the genes for Clostridium difficile toxin and Clostridium cluster XI, which was associated with a decrease in chronic intestinal inflammation (166). Another study found that FOS lowers colitis and levels of the pro-inflammatory cytokine IL-1β in rats given rat chow. FOS increased the number of copies of Bifidobacteria, Enterobacteriaceae, and butyryl-CoA transferase genes while decreasing those of Clostridium cluster IV. In rats given FOS, the relative content of acetate was noticeably higher (167). In a different study, treatment with fructooligosaccharides improved the changes in pathology in transgenic mice and cognitive impairments. Fructooligosaccharides therapy enhanced the abundance of Lactobacillus while decreasing the abundance of Helicobacter in the transgenic group (168). Conversely, in mice with stress-induced IBS, the injection of FOS increases gut inflammation and visceral hypersensitivity (169). Another study also found that chitooligosaccharides (COS) and Clostridium butyricum decreased clinical symptoms, enhanced colonic morphology, controlled cytokine levels linked to inflammation, prevented the activation of the TLR-4/NF-κB/MAPK signaling pathway, preserved intestinal barrier function, and increased intestinal homeostasis by adjusting the diversity and composition of the gut microbiota (170). These findings indicate that prebiotics may influence the gut microbiota in several disorders, including IBD.
The gums, categorized as soluble dietary fibers, modulate the gut microbiota to prevent IBD. Partially hydrolyzed guar gum (PHGG) lowers increases in myeloperoxidase activity, TNF-α protein, and mRNA expression in the colonic mucosa and repairs damage in the colon caused by TNBS. Mice treated with PHGG exhibited markedly higher caecal proliferation of the Bacteroides fragilis (B. fragilis) group, the Clostridium leptum subgroup (Clostridium cluster IV), and the Clostridium coccoides group (Clostridium cluster XIVa), along with increased SCFAs such as propionic acid and butyric acid (171). The study focused on gut microbiota, excluding significant bacteria like Bifidobacteria, and assessed microbiota and SCFA after TNBS-induced colitis, suggesting further research is needed to address these issues (171). Nevertheless, a study by Paudel and team found that following DSS intervention, mice given a guar gum-containing diet showed more severe colitis than the control group. Primarily, guar gum enhances Actinobacteriota, particularly Bifidobacterium. In the guar gum diet-fed mice, this change in the makeup of the gut microbiota promoted the luminal accumulation of intermediary metabolites lactate and succinate (172). These changes may result from the type of gum diet (refined and partially hydrolyzed guar gum). In a different study, PHGG partially inhibited the development of non-alcoholic fatty liver disease in mice through the gut-liver axis by modifying the microbiota and the resulting SCFA profiles. PHGG dramatically raised the prevalence of Clostridium subcluster XIVa and Bacteroides in the cecum. Furthermore, PHGG therapy significantly raised SCFA levels in the cecal samples, specifically butyric acid, acetic acid, propionic acid, and formic acid (173). Therefore, PHGG may similarly prevent IBD via regulating the gut microbiota and producing SCFAs.
As previously mentioned, β-glucans, which are soluble dietary fibers, have shown the ability to reduce IBD by influencing gut flora. Additionally, certain β-glucans can be encapsulated with nanoparticles to alleviate IBD. For instance, in an acute colitis mouse model, oral administration of a yeast β-glucan nanocomplex coated with bio-adhesive polydopamine (YBNs@PDA) has been demonstrated to improve therapeutic efficacy while restoring epithelial barriers, lowering ROS levels, and minimizing systemic drug exposure (Figure 4). YBNs@PDA significantly increased the number of Bifidobacterium and Lachnospiraceae NK4A136, two probiotics that are essential for reducing colitis by maintaining gut homeostasis (174). In a different study, oat β-glucan (ObG) supplementation changed the gut microbiota profile, enhancing the generation of butyrate in the intestines of both 4-week-old pups and their dams, and increased spatial memory and cognition at week 8 (pups). The Firmicutes phylum was significantly more prevalent in the ObG group’s dams and pups than in the control group (175).
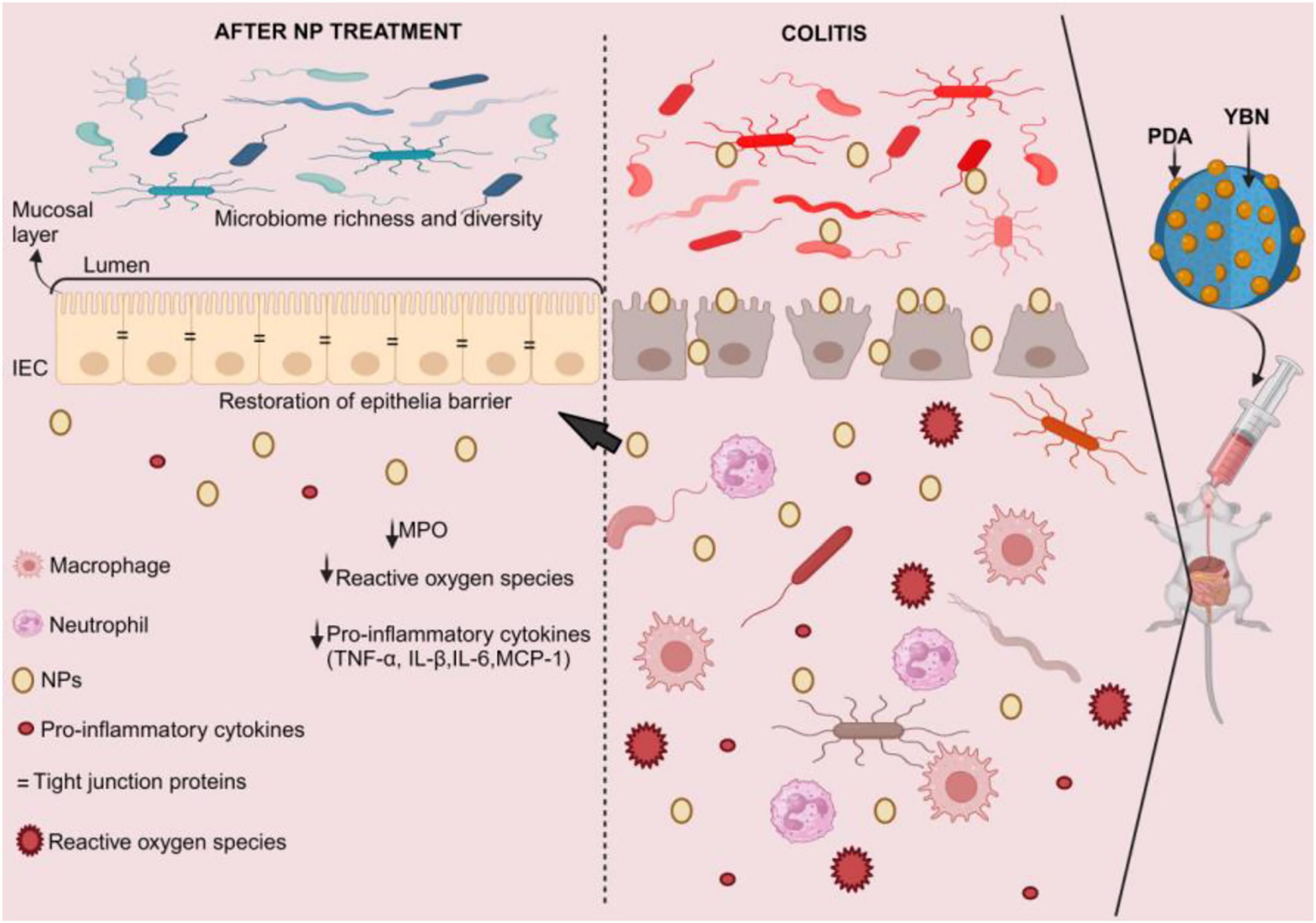
Figure 4. YBNs@PDA intervention in IBD. Due to the mucoadhesive PDA layer that enables YBNs to adhere to inflammatory colon mucosa, these nano-agents extended the time of retention in the intestine, allowing them to optimize the bioavailability of the medicine. This coating also enhances the core YBNs’ potential for beneficial gut flora regulation, enhancing their effectiveness in treating inflammatory colon conditions. This restores the epithelial barrier, lowers the levels of reactive oxygen species and pro-inflammatory cytokines, and raises the diversity and richness of the gut microbiota. MPO, myeloperoxidase; NPs, nanoparticles; PDA, polydopamine; YBNs, yeast β-glucan nanocomplex.
The treatment of resistant starch from purple sweet potato (PSPRS) significantly improved colon inflammation and pathological characteristics in a dose-dependent manner compared to DSS-induced colitis in mice. The PSPRS therapy group had significantly higher levels of putative probiotic bacteria, including Lactobacillus, Alloprevotella, Lachnospiraceae_NK4A136_group, and Bifidobacterium, as well as a higher ratio of Firmicutes to Bacteroidetes (176). High PSPRS dosages, meanwhile, markedly raised butyrate, propionate, and acetate production (176). Nonetheless, the relationship between microbiota and PSPRS structure is unclear, necessitating further research. Furthermore, in DSS-induced colitis mice, a single dose of PSPRS was applied to the gut microbiota and SCFAs; additional doses ought to be selected in subsequent studies to elucidate PSPRS’s anti-inflammatory action (176). The type of RS significantly impacts the gut microbiota’s fermentation of SCFAs, with different RS types possessing unique structural traits that ultimately lead to SCFA generation (177).
Li et al. (178) found that the reduction of Salmonella abundance and the inactivation of TLR2-NF-κB signaling may explain why dietary pectin improved tight junctions, oxidative stress, and colitis caused by Salmonella typhimurium. Interestingly, the study also discovered that Salmonella typhimurium markedly increased the colon’s p-NF-κB/NF-κB ratio and TLR2 protein expressions (178). These findings imply that dietary pectin may ameliorate Salmonella typhimurium-induced colitis by inhibiting the TLR2/NF-κB signaling pathway and oxidative stress.
5.1.2 Insoluble dietary fiber
Insoluble dietary fibers, including cellulose and xylans, have shown promise in modulating gut microbiota to help prevent IBD. In this case, Kim and colleagues discovered that high-cellulose diets (HCD) protect mice from DSS-induced colitis, while low-cellulose diets (LCD) increase intestinal inflammation. Compared to mice fed LCD, mice fed HCD had a higher relative abundance of the genus Akkermansia. Akkermansia muciniphila, given orally to LCD-fed mice, improved colitis, lengthened crypts, and expanded goblet cells. Dietary cellulose reduces inflammation in the gut by regulating gut microbiota and lipid metabolism (13). Additionally, mice fed HCD had greater levels of 13(S)-HODE (hydroxyoctadecadienoic acid), while the precursor of 9(S)-HODE (hydroxyoctadecadienoic acid) was higher in LCD-fed mice than in HCD-fed mice (13). Moreover, cellulose, when used at an optimal dosage of 1.5 g/kg, has been proven to reverse the pathological process of colitis by preventing colon damage, balancing oxidative stress, controlling inflammation, and preventing weight loss. Cellulose primarily targets and regulates the number of unclassified Lactobacilli, Bacteroides, Faecalibaculum, and norank Lactobacilli. Additionally, cellulose raised the levels of SCFAs, including total SCFAs, butyric acid, propionic acid, and valeric acid (179). In another study, treating citric acid-crosslinked carboxymethyl cellulose nanofibers shields mice against diet-induced obesity and metabolic dysfunction by increasing energy expenditure, decreasing food intake, and enriching probiotics like Bifidobacterium (180).
Zhao et al. (181) found that ferulic acid-derived lignin nanoparticles (FALNP) significantly reduced pathogenic symptoms by controlling the gut microbiota and lowering oxidative stress in a mouse model of acute colitis. The mice treated with FALNP showed a considerable rise in Lactobacillus and Lachnospiraceae_NK4A136_group. Through intestinal microenvironment regulation, FALNP can tolerate the gastric acid environment and significantly alleviate pathological symptoms in colitic mice (181). The FALNP-based delivery system, despite its versatility, is not selective for inflammatory areas, necessitating further biological or chemical modification for improved oral delivery therapy of intestinal illnesses (181).
Xylan butyrate ester (XylB) treatments can balance pro- and anti-inflammatory cytokines, lessen damage to mice’s guts, and restore gut microbiota that was harmed by DSS treatment. This lowers the number of Oscillibacter, Ruminococcaceae UCG-009, Erysipelatoclostridium, and Defluviitaleaceae UCG-01 genera. XylB increased colon butyrate concentration, decreased histone deacetylase (HDAC) activity, increased G-protein-coupled receptor 109A protein expression, and activated autophagy and NF-κB, resulting in anti-inflammatory effects (182). In a different study, supplementing with specific xylans, such as arabinoxylan derived from rice bran (RAX), dramatically reduces obesity from a high-fat diet. RAX decreases the relative abundance of pro-inflammatory bacteria such as Anaerotruncus, Helicobacter, Coprococcus, and Desulfovibrio while increasing the relative abundance of anti-inflammatory bacteria like Bifidobacterium and Akkermansia (183). Table 2 summarizes the role of dietary fibers in regulating the gut microbiota to prevent IBD.
5.2 Polyphenols
5.2.1 Curcuminoids
Polyphenols, plant-based substances found naturally or as semi-synthetic or synthetic derivatives, have demonstrated positive health impacts and therapeutic uses in several chronic diseases (184). Healthy, sub-healthy, and sick people use over-the-counter natural products to treat and prevent chronic illnesses, with biomedical researchers and medicine developers focusing on dietary polyphenols like curcumin, a curry component, for treatment and prevention (185). The turmeric plant, Curcuma longa, produces curcumin, a polyphenol belonging to the ginger family. It has long been used in Ayurvedic treatments to treat a variety of illnesses, including anorexia, asthma, coughing, hepatic diseases, diabetes, heart ailments, wound healing, and Alzheimer’s (186). A recent study has shown that curcumin inhibits further body weight and colon length reduction in IBD mice while improving the disease activity index, colonic mucosal damage, and inflammatory infiltration. Curcumin changes the gut microbiota by raising Akkermansia, Muribaculaceae_unclassified, and Muribaculum levels and markedly increasing intestinal concentrations of propionate, butyrate, glycine, tryptophan, and betaine (15). Furthermore, curcumin’s amelioration of intestinal dysbiosis impacted hepatic metabolic performance and enhanced the pathways linked to butanoates, bile acids, glucagon, amino acids, and biotin metabolism (15). These modifications might make it easier to establish the gut-liver axis’s equilibrium. However, curcumin’s impact on the gut-liver axis remains unclear, necessitating further confirmation of genes and proteins linked to gut and hepatic metabolite changes (15). Moreover, the extensive metabolomics data may have overlooked some significant targets due to the limited screening of intrahepatic metabolites with major intestinal genera (15). In another study, curcumin therapy dramatically reduced tumor growth in AOM/DSS-induced CRC model mice while restoring colon length and structural morphology. In the CRC model mice, curcumin decreased the number of harmful bacteria such as Ileibacterium, Monoglobus, and Desulfovibrio while increasing the number of good bacteria such as Clostridia_UCG-014, Bifidobacterium, and Lactobacillus (187).
5.2.2 Phenolics
Polyphenols, like phenolic acids, have shown much promise in helping people with IBD by changing the gut microbiota. For instance, a study revealed that dietary caffeic acid (CA) prevents the rise in the Firmicute to Bacteroidetes ratio and promotes Akkermansia in mice with DSS colitis (188). Nevertheless, the authors did not prove a direct link between the improvement in colitis and the rise in the fraction of Akkermansia populations (188). Therefore, a causal association must be studied in the future. Additionally, CA can significantly decrease the release of IL-6, TNFα, and IFNγ, as well as the colonic infiltration of CD3+T cells, CD177+ neutrophils, and F4/80+ macrophages by blocking the signaling of NF-κB (188). Another study also found that CA supplementation altered the gut microbiome composition, increasing the abundance of Akkermansia, Alistipes, and Dubosiella while decreasing Turicibacter and Bacteroides’ abundance (189). Additionally, the study discovered that while Dubosiella abundance rose after CA injection, the mechanism is not specified (189). Thus, additional research is required to investigate the mechanism of Dubosiella and whether it could impact colitis (189). In a different study, caffeic acid phenethyl ester (CAPE) has shown potential in reducing nonalcoholic fatty liver disease in obese mice by manipulating gut flora. Treatment with CAPE primarily boosted the genera Helicobacter, Bilophila, Enterococcus, and Bacteroides (190). CAPE partially alleviates obesity-related steatosis by inhibiting bacterial bile salt hydrolase activity through the gut microbiota-bile acid-FXR axis (190).
Gallic acid (GA) is another phenolic acid shown to alleviate colitis and improve gut microbial dysbiosis. Treatment with GA restored the number of Bacteroidales, Enterobacterales, and Clostridiales. High-dose GA treatments inhibited the activation of NF-κB and MAPK signaling pathways, which were seen in mRNA levels following DSS treatment (191). In an alternative study, GA alleviates synovial inflammation and fibrosis in knee osteoarthritis by affecting the populations of Bacteroidia and Muribaculaceae, as well as through the metabolic pathways related to arginine biology, glycerophospholipid metabolism, and sphingolipid metabolism (192). However, concurrent use of cytochrome P450 family two subfamily D member 6 substrate medications and supplements containing gallic acid may result in harmful herbal-drug interactions (193). Additionally, gallic acid compounds are also known to have negative effects, including cytotoxicity and mutagenicity (194).
5.2.3 Catechin
Green tea is rich in catechins and polyphenol flavonoids, with the most effective catechin being epigallocatechin 3-gallate (EGCG) (195). Oral EGCG, a key bioactive ingredient in green tea, strengthens the intestinal barrier and reduces inflammation in mice with DSS-induced murine colitis. EGCG alters the gut microbiota by boosting the quantity of Akkermansia and butyrate production (196). In another study, diets rich in green tea polyphenols (GTP) worsened intestinal inflammation and carcinogenesis brought on by DSS. Furthermore, colitis mice fed 1% GTP showed signs of nephrotoxicity, as evidenced by a substantial increase in serum creatinine levels (197). Additionally, green tea catechins have been suggested to decrease intestinal medication absorption by blocking OATP uptake, increasing P-gp export activity, or reducing drug solubility (198). These imply that catechins may have a dual role in disease management.
5.2.4 Stilbenes
Stilbenes, classified as a type of polyphenol, have demonstrated the ability to prevent IBD via modulating gut microbiota. One of the active ingredients in the Chinese medicinal herb Polygonum multiflorum Thunb is tetrahydroxystilbene-2-O-β-D-glucoside (THSG) (199). THSG treatments elicited a beneficial pharmacological response in mice with DSS-induced acute colitis by reinstating epithelial barrier integrity and diminishing the synthesis of pro-inflammatory cytokines. THSG treatments significantly increased Firmicutes and Bacteroidetes abundances, restoring gut microbiota composition disrupted by DSS by increasing the genus Lachnospiraceae (NK4A136) and decreasing the genera Helicobacter, Bacteroides, and Parabacteroides (200). Nonetheless, this study did not investigate the gut metabolites; thus, future studies are needed to explore the metabolites and their association with the gut microbiota.
Resveratrol (RSV), a stilbene, has shown promise in mitigating IBD through its interaction with gut flora, thereby alleviating DSS-induced IBD symptoms. In mice, RSV reduces metabolite dysregulation, improves microbiota variety and composition, increases tight junction molecules, and alleviates colitis’s clinical symptoms. Additionally, RSV reversed the DSS group’s decrease in Bacteroidetes and Proteobacteria and rise in Firmicutes (201). Although the authors concentrated on RSV as a possible treatment for colitis, other substances with similar qualities might provide comparable effects. Consequently, this creates a gap that needs to be filled in future research (201). Co-administration of RSV with the probiotic strain Ligilactobacillus salivarius Li01 (RSV+Li01) has also shown a positive anti-inflammatory impact in DSS-induced colitis mice, promoting the healing of different inflammatory lesions and gut microbiota composition. Mice fed RSV+Li01 had larger relative abundances of Bifidobacterium, Akkermansia, and Muribaculum, but Helicobacter spp. were reduced (202). In another study, RSV therapy inhibited CRC growth in azoxymethane and DSS mice, boosting anti-inflammatory CD4+ FOXP3+ (Tregs) and CD4+ IL10+ cells, decreasing proinflammatory Th1 and Th17 cell growth, and modifying the gut flora (203). Other studies have demonstrated that RSV and its derivatives can reduce liver fibrosis caused by inorganic mercury and alleviate hypertension generated by a high-fat diet by modulating the gut microbiota (204, 205). RSV has been shown to inhibit carcinogenesis; nonetheless, due to the inhibition or activation of specific cytochrome P450s, pharmacological quantities of RSV may exacerbate adverse drug responses or change the efficacy of medications (206). Furthermore, RSV (5 mg/kg) has been shown to extend platelet plug development in mice (207), implying there is a tendency to bleed when using it. RSV may prevent IBD by modifying the gut microbiota, but it can potentially have negative consequences; therefore, caution should be exercised while utilizing specific amounts.
5.2.5 Proanthocyanidins
Grape seed extract, which primarily consists of polymeric and oligomeric proanthocyanidins, epicatechin and monomeric catechin, and gallic acid, is rich in proanthocyanidins (208). Interestingly, research has found that grape seed proanthocyanidin (GSP), a plant-derived polyphenol, enhances inflammatory indices and decreases intestinal permeability, consequently reducing chronic inflammation in dogs. GSP therapy enhanced the number of bacteria, such as Ruminococcaceae, Faecalibacterium, Ruminococcus_torques_group, and Lachnospiraceae_NK4A136_group, that can reduce inflammation and stimulate bile acid metabolism (12). Unfortunately, this study did not evaluate the activities of 7α-dehydroxylase or bile salt hydrolase. The exact mechanism by which GSP regulates bile acids through the gut flora is still unknown (12). Future research may be required to investigate the role of GSP in controlling bile acids through the gut flora in colitis mitigation.
5.2.6 Anthocyanins
It has been demonstrated that anthocyanins, such as pelargonidin-3-galactoside (Pg3gal), which are derived from purple sweet potatoes, considerably reduce DSS-induced UC in mice by preventing intestinal epithelial cell pyroptosis and preserving the structural integrity of the gut microbiota. Pg3gal changed the gut microbiota’s dysbiosis caused by DSS by increasing Firmicutes, Bacteroidetes, and Verrucomicrobia and decreasing Proteobacteria and Deferribacteria (209).
5.2.7 Tannins
Kitabatake et al. (210) discovered that persimmon-derived tannin reduces colon inflammation in UC by changing the immune system and microbiota makeup. Supplementing with tannins considerably enhanced the relative abundance of Bacteroides while decreasing that of Enterobacteriaceae and Enterococcus. Also, mice with colitis fed a tannin diet had higher levels of Bifidobacteria. Thus, future research should evaluate the function of Bifidobacterium since it is unclear how the increased bacteria in mice given tannin supplements contribute to the improvement of colitis (210). Furthermore, more research on how tannin supplementation increases Bifidobacterium during colitis is anticipated to shed light on how probiotics preserve gut homeostasis (210). Additionally, Liu and the team discovered that punicalagin, administered orally to mice, alleviated DSS-induced colitis and elevated Lachnospiraceae_NK4A136_group and Bifidobacterium abundance (211). Punicalagin significantly increased the level of D-ribose. In vitro experiments showed that D-ribose has anti-inflammatory and antioxidant properties (211).
5.2.8 Flavonols
Flavonols such as quercetin have also shown potential to ameliorate IBD. Due to quercetin’s capacity to inhibit pro-inflammatory cytokines and alter gut microbiota, dietary quercetin supplementation has therapeutic effects on colitis caused by Citrobacter rodentium. In colon tissues, quercetin increased the synthesis of IL-10 while inhibiting the production of pro-inflammatory cytokines like IL-17, TNF-α, and IL-6. Quercetin administration greatly decreased the populations of Fusobacterium and Enterococcus while increasing those of Bacteroides, Bifidobacterium, Lactobacillus, and Clostridia (212). One of the study’s noted limitations is the lack of human studies. Therefore, Lin et al. (212) stated that future research involving human participants is needed to validate the effects of quercetin on inflammatory markers and provide a more comprehensive understanding of the changes in human gut flora caused by quercetin. Interestingly, quercetin has been coated with nanoparticles (NPs) for better results. Compared to quercetin, quercetin NPs are better at controlling gut microbiota and SCFAs to help mice with colitis caused by DSS. Quercetin NPs enhance mucus protein and goblet cell density, reduce colon inflammatory infiltration, improve TNF-α, IL-1β, and IL-6, raise IL-10 levels, decrease MPO levels, and restore intestinal barrier integrity. Butyric acid, propanoic acid, and acetic acid concentrations were all raised by quercetin NPs (213). Treatment with quercetin NPs lowered the amounts of Proteobacteria in mice with colitis caused by DSS while increasing the amounts of Verrucomicrobia (213). However, the low pH of the colon may adversely affect the bioavailability of quercetin NPs in patients with UC, especially during the active phase. Therefore, patients in remission or with minor disease should be given more consideration for quercetin delivery methods, and a combination of medications should be used to treat colonic pH imbalance (213). In a different study, researchers have found quercetin to have a hypoglycemic effect, reduce insulin resistance, alter the metabolites of db/db mice, repair the intestinal barrier, and rebuild the intestinal microbiota. Quercetin reduced the number of Escherichia coli, Bacteroides, Proteobacteria, and Escherichia-Shigella (214). Conversely, taking quercetin together with warfarin has been shown to increase blood-thinning effects (215), implying there is a tendency for bleeding when used together.
Another flavonol that has been shown to reduce IBD is myricetin. Yang and colleagues discovered that myricetin controlled the gut microbiota composition in prediabetic mice, preventing DSS-induced colitis. The relative abundance of Bacteroidetes increased while the relative abundance of Proteobacteria declined dramatically after myricetin treatment (216). Furthermore, myricetin therapy elevated SCFAs like acetic, propionic, and butyric acids (216). Although altering the gut microbiota may positively impact the prevention of colitis, this investigation lacked clinical proof. Thus, Yang et al. (216) have proposed future clinical trials to determine the safety and efficacy of myricetin therapy over a lengthy duration. In a different investigation, myricetin modulated the gut-liver axis to have an anti-atherosclerotic effect (217). Myricetin decreased the abundance of genera linked to obesity, such as Rikenellaceae_RC9_gut_group and Alistipes, while increasing that of probiotics g_Lachnospiraceae_NK4A136 (217). Table 3 summarizes the role of polyphenols in regulating the gut microbiota to prevent IBD. Overall, studies have shown that plant-based dietary components reduce experimental colitis by altering the gut microbiome, increasing beneficial bacteria, and decreasing harmful ones. This process maintains the homeostatic equilibrium of gut microorganisms (Figure 5).
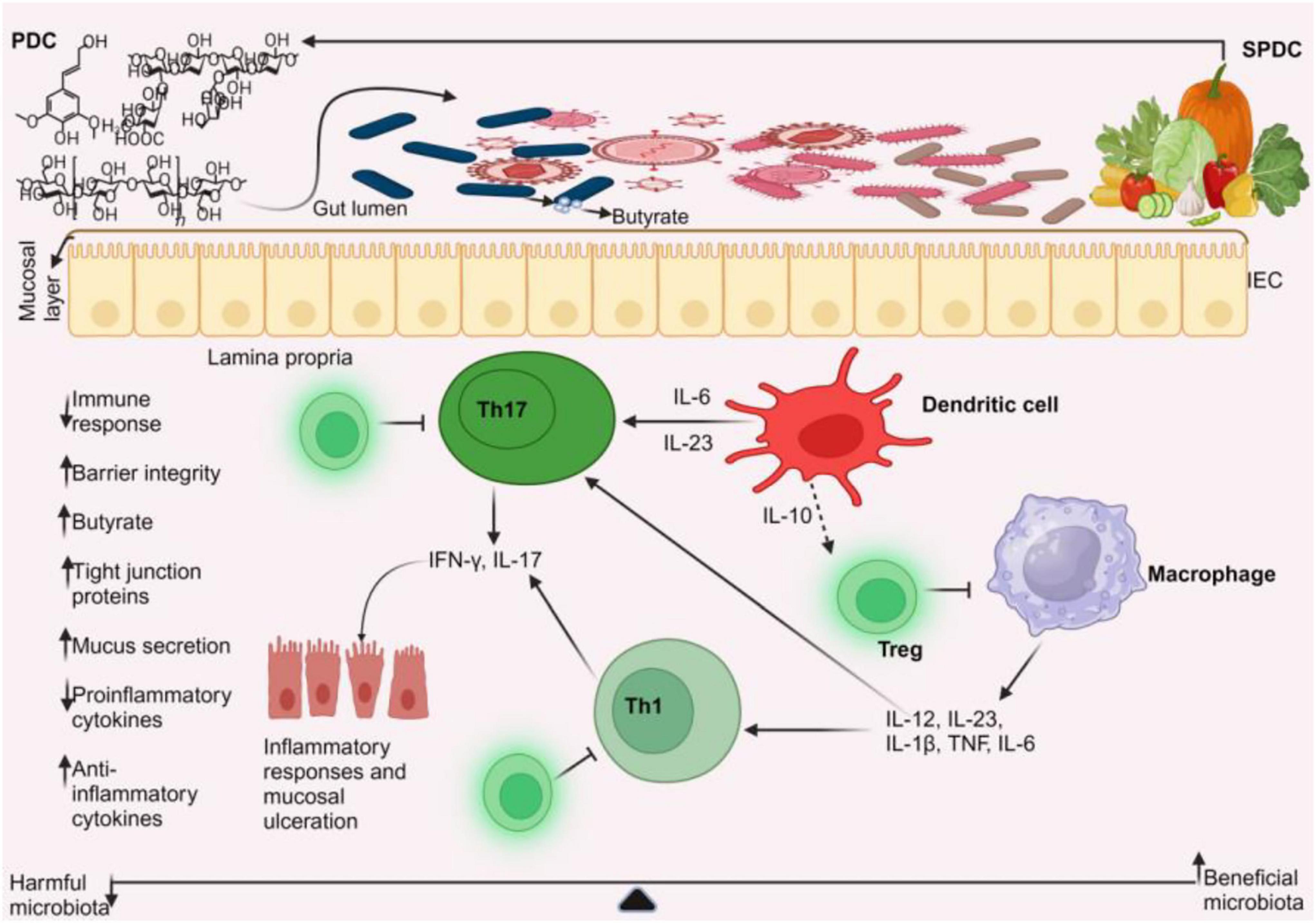
Figure 5. Plant-based management of IBD. Plant-based dietary compounds can help treat IBD by altering the gut microbiota. The beneficial microbiota stimulates dendritic cells to release anti-inflammatory cytokines, such as interleukin-10 (IL-10). This release of IL-10, in turn, promotes the development of regulatory T cells. These regulatory T cells prevent the activation of Th1, Th17 cells, and macrophages from producing pro-inflammatory cytokines, thereby helping to control IBD. This leads to decreased immune response, increased butyrate production, improved barrier integrity, and mucus secretion. PDC, plant-based dietary compounds; SPDC, sources of PDC.
6 Clinical evidence of plant-based dietary compounds
For patients with quiescent UC, curcumin appears to be a safe and promising drug for sustaining remission. Curcumin reduced the morbidity associated with UC by enhancing the endoscopic and clinical activity indices. For 6 months of treatment, 2 out of 43 patients who took curcumin experienced a relapse, while 8 out of 39 patients in the placebo group did the same (218). Another clinical trial found that curcumin supplementation in mesalamine medication was more effective than a placebo combination in causing clinical and endoscopic remission in mild-to-moderate active UC patients. There were no noticeable negative effects (219). This shows that combination therapy with plant-based dietary compounds and IBD medications is safe and effective. A recent study found a novel curcumin derivative called Theracurmin®, with a 27-fold higher absorption rate than natural curcumin powder, clinical and endoscopic effectiveness, and a positive safety profile in patients with active CD (220). A different study also showed that curcumin, a safe and effective adjuvant drug, lowers anti-ds DNA and IL-6 levels, thereby reducing inflammation and autoimmune activity in people with systemic lupus erythematosus (221). Nevertheless, some prebiotics used in a clinical setting showed no improvements. Benjamin et al. (222) found that fructooligosaccharides, despite affecting dendritic cell function, did not have a therapeutic effect in patients with active CD (223).
Despite the limited clinical studies on the role of plant-based dietary compounds in mitigating IBD, other clinical trials and investigations have demonstrated the potential for plant-based diets to prevent various disorders, including IBD. Additionally, researchers have integrated plant-based diets with other drugs to achieve optimal results. Chiba et al. (224) discovered that patients undergoing infliximab medication combined with a lacto-ovo-semivegetarian diet exhibit a reduced mean CD disease activity index score at week 6 post-admission. The average level of C-reactive protein upon admission dropped. Of the cases, 46% (19/41) had mucosal healing. A similar study found that for over half of CD patients, infliximab with a plant-based diet as first-line treatment produced an unparalleled relapse-free course. Three regular infliximab infusions combined with a plant-based diet produced remission in 24 consecutive newly diagnosed adult patients with CD while they were in the hospital (225). Another study also found that relapse rates in UC significantly decreased following lacto-ovo-semivegetarian diet induction therapy compared to standard therapeutics. The relapse rate for initial episode cases of UC after therapy with a lacto-ovo-semivegetarian diet was lower than traditional treatment, with rates of 14% at 1 year and 27% at 5 years. None of the patients reported any significant side effects that a lacto-ovo-semivegetarian diet might have caused (226). Also, 77% of patients with mild or remission of UC who were given nutritional advice and placed on a plant-based diet during a 2-week hospital stay reported improvement, including bloody stool elimination or reduction. At 1, 2, 3, 4, and 5 years of follow-up, the cumulative recurrence rates were 2, 4, 7, 19, and 19%, respectively. None of the patients adhering to plant-based diets encountered any harmful effects (227). These suggest that plant-based diets could be beneficial for use in clinical settings.
7 Role of personalized nutrition in IBD
Nutrition plays a crucial role in the development and progression of disease, making it a potential therapeutic approach to suppress inflammation and symptoms. Given that IBD is a diverse condition clinically and molecularly, tailoring dietary recommendations may be essential to bringing about long-lasting dietary behavior changes that enhance nutritional status and address gut inflammation and abdominal symptoms on a personal basis (228). With the help of data on individual traits like age, insulin sensitivity, or gut flora, personalized nutrition creates individualized dietary recommendations to help patients make positive, long-lasting diet changes (229).
7.1 Individualized triggers
It is crucial to remember that some foods might cause IBD to worsen or subside. For instance, UC risk was linked to a diet imbalance that included low vegetable and high sugar and soft drink intake (230). Opstelten and the team also found that although a distinct dose-response association was not demonstrated, milk consumption may be linked to a lower risk of getting CD (231). Recently, there has been insufficient proof to conclude that dairy products and milk affect the occurrence and progression of IBD (232). Increased consumption of highly processed foods was strongly correlated with an increased incidence of IBD (233, 234). In the case of plant-based diets, some plant-based dietary compounds have been demonstrated to worsen and exacerbate IBD and cause carcinogenesis (164, 172, 197). Therefore, this will help clinicians/individuals to plan their nutrition to prevent IBD exacerbation.
7.2 Nutrient deficiencies and quality of life
Malnutrition is believed to affect 20–85% of people with IBD. Among the many causes of malnutrition in IBD patients are decreased oral meal intake, intestinal bacterial overgrowth, chronic blood and protein loss, and malabsorption. Clinical outcomes, response to treatment, and quality of life are all negatively correlated with poor nutritional status, selective malnutrition, and sarcopenia. Radiological evaluation, functional capacity measurement, and dietetic evaluation involving daily caloric intake and energy expenditure should all be part of the nutritional assessment (235). This reduces IBD exacerbations and improves the quality of life (QoL) for IBD patients. Along with malnutrition brought on by the inflammatory nature of the illness, a significant number of IBD patients exhibit restricted dietary practices (41–93%) and food avoidance (28–89%), which have a negative influence on their QoL when it comes to eating (236, 237). This may lead to nutritional deficiencies, as reported in studies (238, 239), where patients with IBD (active or in clinical remission) developed nutrient constraints including vitamin C, copper, niacin, and zinc, among others. Similarly, individuals who consume fewer or no animal products may be susceptible to dietary deficiencies in protein, calcium, iron, iodine, zinc, vitamin B12, vitamin D, and omega-3 fatty acids. This deficit can result in immediate and long-term health issues (240). Research conducted in Switzerland, Spain, and Germany identified deficiencies in vitamins and minerals, including vitamins B6, B12, and niacin, among plant-based groups (241–243). Therefore, inadequate consumption of vital nutrients highlights the necessity for more effective public health initiatives and enhanced nutrition education, irrespective of eating habits (59).
8 Limitations of plant-based dietary treatments
Although fibers offer many health benefits, not all are equal, and some patients with IBD report intolerance to specific types of fiber (244). Armstrong et al. (244) found that in a subgroup of IBD intestinal biopsies cultivated ex vivo, unfermented dietary β-fructan fibers produced proinflammatory cytokines and immune cells. The NLRP3 and TLR2 pathways had to be activated to release the proinflammatory response to intact β-fructan. Patients without fermentative microbe activity experienced negative effects. Other studies (164, 172, 197, 245) have also shown the negative impacts of plant-based diets on IBD by way of exacerbating the condition and causing carcinogenesis. This may lead to the avoidance of such dietary compounds. Another study (246) demonstrated that plant dietary compound combinations may affect the efficacy of others in reducing colitis. Additionally, studies (218, 224, 225, 227) used smaller sample sizes to determine the effectiveness of plant-based diets in IBD. These studies have indicated the necessity for greater sample sizes in subsequent research.
Regarding the preclinical studies, this review revealed several shortcomings. One significant limitation, for example, was the absence of clinical studies or trials on the plant-based dietary components (except for curcumin). Additional limitations included the use of a single dose in gut microbiome studies, the non-selectivity of nanoparticles in inflammatory areas, the exclusion of important microbiota from studies, and the incomplete and ambiguous mechanisms underlying the effects of some dietary components on metabolites and the gut microbiota. There were no studies on plant-based chemicals and intestinal fungus or virome.
9 Future directions
Due to nutrient deficiencies that may arise from plant-based dietary compound consumption, it is necessary to design a plan to help prevent such issues. Therefore, in this situation, the ability to create a customized meal plan for each patient will likely improve disease treatment, boost adherence since patients are more receptive to individualized approaches, and be more flexible (228). More clinical trials on nanoparticles in IBD patients should be explored. However, when exploring plant-based dietary components as nanoparticles for potential clinical applications, IBD patients’ pH should be considered, as these compounds act best at high pH (preclinical studies), whereas IBD patients typically have low pH (247). As a result, combining these nanoparticles with another treatment regimen to aid in colonic pH regulation may enhance the nanoparticles’ therapeutic effects. Additionally, more intestinal microbiota should be used to evaluate important microbiota. Future studies should include more human studies with larger sample sizes to confirm the role of these dietary compounds in IBD. Most studies centered on gut bacteria; thus, more research is needed on how plant dietary compounds regulate gut fungi and virome. Uncertain and incomplete mechanisms involving the research of plant-based components on the gut microbiota should be elucidated, and more dosages, rather than a single dose, should be investigated in gut microbiome studies.
10 Conclusion
Plant-based dietary components have been demonstrated to reduce IBD symptoms by increasing anti-inflammatory cytokines, decreasing pro-inflammatory cytokines, lowering oxidative stress, and improving barrier function. These compounds prevent IBD by activating/inhibiting multiple signaling pathways, including TGF-β/Smad, TRL-4/NF-κB/MAPK, TLR2-NF-κB, autophagy, pyroptosis, glycolysis/gluconeogenesis and amino acid metabolism, Nrf-2/HO-1, microbiota-macrophage-arginine metabolism, and bile acid metabolism. Furthermore, these dietary components aid in the formation of SCFAs, which promote the development of Tregs, thereby alleviating IBD. While many plant-based nutritional components have been demonstrated to reduce the severity of IBD, others have been shown to increase it or cause cancer. This will aid clinicians when planning diets for their patients. However, the favorable effects make plant-based dietary components a promising alternative for IBD treatment in clinical settings. Increased beneficial microbiota has also been linked to butyrate and anti-inflammatory marker production, whereas bad microbiota leads to inflammatory marker production. Emerging evidence has shown the promising role of dietary compounds used as nanoparticles or dietary compounds encapsulated in nanoparticles for effective treatment of IBD. These nanoparticles are safe and non-toxic in preclinical studies, warranting further studies in clinical settings for IBD patients.
Author contributions
FA: Conceptualization, Writing – original draft. C’eH: Funding acquisition, Writing – review and editing. XW: Software, Writing – review and editing. BW: Visualization, Writing – review and editing. FM: Conceptualization, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Zhenjiang Key Research and Development Plan (Social Development) (Grant No. SH2024047), the Key Research and Development (Social Development) projects of the Innovation Special Fund of Danyang (Grant No. SSF202410), the Henan Province 2024 Science and Technology Development Plan (Grant No. 242102310081), the Open Topic at the University Level of Shangqiu Medical College in 2023 (Grant No. KFKT23005), and the Jiangsu Provincial Medical Key Discipline Cultivation Unit (Grant No. JSDW202241).
Acknowledgments
The figures were drawn using Biorender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fathima A, Jamma T. UDCA ameliorates inflammation driven EMT by inducing TGR5 dependent SOCS1 expression in mouse macrophages. Sci Rep. (2024) 14:24285. doi: 10.1038/s41598-024-75516-9
2. Ning S, Zhang Z, Zhou C, Wang B, Liu Z, Feng B. Cross-talk between macrophages and gut microbiota in inflammatory bowel disease: A dynamic interplay influencing pathogenesis and therapy. Front Med (Lausanne). (2024) 11:1457218. doi: 10.3389/fmed.2024.1457218
3. Cai M, Mao Y, Gao W, Wang Z, Mao J, Sha R. Insights into diosgenin against inflammatory bowel disease as functional food based on network pharmacology and molecular docking. Heliyon. (2024) 10:e37937. doi: 10.1016/j.heliyon.2024.e37937
4. Nóbrega V, Silva I, Brito B, Silva J, Silva M, Santana GO. THE ONSET OF CLINICAL MANIFESTATIONS IN INFLAMMATORY BOWEL DISEASE PATIENTS. Arq Gastroenterol. (2018) 55:290–5. doi: 10.1590/S0004-2803.201800000-73
5. Kogut M, Lee A, Santin E. Microbiome and pathogen interaction with the immune system. Poult Sci. (2020) 99:1906–13. doi: 10.1016/j.psj.2019.12.011
6. Jandhyala S, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. (2015) 21:8787–8783. doi: 10.3748/wjg.v21.i29.8787
7. Tsai Y, Tai W, Liang C, Wu C, Tsai M, Hu W, et al. Alternations of the gut microbiota and the Firmicutes/Bacteroidetes ratio after biologic treatment in inflammatory bowel disease. J Microbiol Immunol Infect. (2024) 58:62–9. doi: 10.1016/j.jmii.2024.09.006
8. Saxena A, Kaur K, Hegde S, Kalekhan F, Baliga M, Fayad R. Dietary agents and phytochemicals in the prevention and treatment of experimental ulcerative colitis. J Tradit Complement Med. (2014) 4:203–17. doi: 10.4103/2225-4110.139111
9. Tie S, Chen Y, Tan M. An evaluation of animal models for using bioactive compounds in the treatment of inflammatory bowel disease. Food Front. (2024) 5:474–93. doi: 10.1002/fft2.360
10. Alcorta A, Porta A, Tárrega A, Alvarez M, Vaquero M. Foods for plant-based diets: Challenges and innovations. Foods. (2021) 10:293. doi: 10.3390/foods10020293
11. Guillamón E, Andreo-Martínez P, Mut-Salud N, Fonollá J, Baños A. Beneficial effects of organosulfur compounds from allium cepa on gut health: A systematic review. Foods. (2021) 10:1680. doi: 10.3390/foods10081680
12. Zhang M, Mo R, Wang H, Liu T, Zhang G, Wu Y. Grape seed proanthocyanidin improves intestinal inflammation in canine through regulating gut microbiota and bile acid compositions. FASEB J. (2023) 37:e23285. doi: 10.1096/fj.202300819RR
13. Kim Y, Hwang S, Kim S, Lee Y, Kim T, Lee S, et al. Dietary cellulose prevents gut inflammation by modulating lipid metabolism and gut microbiota. Gut Microbes. (2020) 11:944–61. doi: 10.1080/19490976.2020.1730149
14. Wen X, Wan F, Wu Y, Liu Y, Zhong R, Chen L, et al. Caffeic acid modulates intestinal microbiota, alleviates inflammatory response, and enhances barrier function in a piglet model challenged with lipopolysaccharide. J Anim Sci. (2024) 102:skae233. doi: 10.1093/jas/skae233
15. Zhou F, Mai T, Wang Z, Zeng Z, Shi J, Zhang F, et al. The improvement of intestinal dysbiosis and hepatic metabolic dysfunction in dextran sulfate sodium-induced colitis mice: Effects of curcumin. J Gastroenterol Hepatol. (2023) 38:1333–45. doi: 10.1111/jgh.16205
16. Craig W, Mangels A, Fresán U, Marsh K, Miles F, Saunders A, et al. The safe and effective use of plant-based diets with guidelines for health professionals. Nutrients. (2021) 13:4144. doi: 10.3390/nu13114144
17. Johnson C, Versalovic J. The human microbiome and its potential importance to pediatrics. Pediatrics. (2012) 129:950–60. doi: 10.1542/peds.2011-2736
18. Bishu S, Kao JY. A step closer to understanding how a diet high in simple carbohydrates may cause dysbiosis. J Clin Invest. (2024) 134:e180001. doi: 10.1172/JCI180001
19. Hartog A, Belle F, Bastiaans J, de Graaff P, Garssen J, Harthoorn L, et al. A potential role for regulatory T-cells in the amelioration of DSS induced colitis by dietary non-digestible polysaccharides. J Nutr Biochem. (2015) 26:227–33. doi: 10.1016/j.jnutbio.2014.10.011
20. Alagiakrishnan K, Morgadinho J, Halverson T. Approach to the diagnosis and management of dysbiosis. Front Nutr. (2024) 11:1330903. doi: 10.3389/fnut.2024.1330903
21. Guo X, Liu X, Hao J. Gut microbiota in ulcerative colitis: Insights on pathogenesis and treatment. J Dig Dis. (2020) 21:147–59. doi: 10.1111/1751-2980.12849
22. Lee M, Chang E. Inflammatory Bowel diseases (IBD) and the microbiome-searching the crime scene for clues. Gastroenterology. (2021) 160:524–37. doi: 10.1053/j.gastro.2020.09.056
23. Ning L, Zhou Y, Sun H, Zhang Y, Shen C, Wang Z, et al. Microbiome and metabolome features in inflammatory bowel disease via multi-omics integration analyses across cohorts. Nat Commun. (2023) 14:7135. doi: 10.1038/s41467-023-42788-0
24. Barker-Tejeda T, Zubeldia-Varela E, Macías-Camero A, Alonso L, Martín-Antoniano I, Rey-Stolle M, et al. Comparative characterization of the infant gut microbiome and their maternal lineage by a multi-omics approach. Nat Commun. (2024) 15:3004. doi: 10.1038/s41467-024-47182-y
25. Lozupone C, Stombaugh J, Gordon J, Jansson J, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. (2012) 489:220–30. doi: 10.1038/nature11550
26. Teofani A, Marafini I, Laudisi F, Pietrucci D, Salvatori S, Unida V, et al. Intestinal taxa abundance and diversity in inflammatory Bowel disease patients: An analysis including covariates and confounders. Nutrients. (2022) 14:260. doi: 10.3390/nu14020260
27. Zuo W, Wang B, Bai X, Luan Y, Fan Y, Michail S, et al. 16S rRNA and metagenomic shotgun sequencing data revealed consistent patterns of gut microbiome signature in pediatric ulcerative colitis. Sci Rep. (2022) 12:6421. doi: 10.1038/s41598-022-07995-7
28. Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. (2006) 55:205–11. doi: 10.1136/gut.2005.073817
29. Yang Y, Chen C, Zheng Y, Wu Z, Zhou M, Liu X, et al. Fucoxanthin alleviates dextran sulfate sodium-induced colitis and gut microbiota dysbiosis in mice. J Agric Food Chem. (2024) 72:4142–54. doi: 10.1021/acs.jafc.3c08811
30. Hu Q, Yuan B, Wu X, Du H, Gu M, Han Y, et al. Dietary intake of Pleurotus eryngii ameliorated dextran-sodium-sulfate-induced colitis in mice. Mol Nutr Food Res. (2019) 63:e1801265. doi: 10.1002/mnfr.201801265
31. Clooney A, Eckenberger J, Laserna-Mendieta E, Sexton K, Bernstein M, Vagianos K, et al. Ranking microbiome variance in inflammatory bowel disease: A large longitudinal intercontinental study. Gut. (2021) 70:499–510. doi: 10.1136/gutjnl-2020-321106
32. Sokol H, Leducq V, Aschard H, Pham H, Jegou S, Landman C, et al. Fungal microbiota dysbiosis in IBD. Gut. (2017) 66:1039–48. doi: 10.1136/gutjnl-2015-310746
33. Chehoud C, Albenberg L, Judge C, Hoffmann C, Grunberg S, Bittinger K, et al. Fungal signature in the gut microbiota of pediatric patients with inflammatory Bowel disease. Inflamm Bowel Dis. (2015) 21:1948–56. doi: 10.1097/MIB.0000000000000454
34. Imai T, Inoue R, Kawada Y, Morita Y, Inatomi O, Nishida A, et al. Characterization of fungal dysbiosis in Japanese patients with inflammatory bowel disease. J Gastroenterol. (2019) 54:149–59. doi: 10.1007/s00535-018-1530-7
35. Catalán-Serra I, Thorsvik S, Beisvag V, Bruland T, Underhill D, Sandvik A, et al. Fungal microbiota composition in inflammatory bowel disease patients: Characterization in different phenotypes and correlation with clinical activity and disease course. Inflamm Bowel Dis. (2024) 30:1164–77. doi: 10.1093/ibd/izad289
36. Zuo T, Lu X, Zhang Y, Cheung C, Lam S, Zhang F, et al. Gut mucosal virome alterations in ulcerative colitis. Gut. (2019) 68:1169–79. doi: 10.1136/gutjnl-2018-318131
37. Imai T, Inoue R, Nishida A, Yokota Y, Morishima S, Kawahara M, et al. Features of the gut prokaryotic virome of Japanese patients with Crohn’s disease. J Gastroenterol. (2022) 57:559–70. doi: 10.1007/s00535-022-01882-8
38. Kong C, Liu G, Kalady M, Jin T, Ma Y. Dysbiosis of the stool DNA and RNA virome in Crohn’s disease. J Med Virol. (2023) 95:e28573. doi: 10.1002/jmv.28573
39. Odenwald M, Turner J. The intestinal epithelial barrier: A therapeutic target? Nat Rev Gastroenterol Hepatol. (2017) 14:9–21. doi: 10.1038/nrgastro.2016.169
40. Chelakkot C, Ghim J, Ryu S. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp Mol Med. (2018) 50:1–9. doi: 10.1038/s12276-018-0126-x
41. Barbara G, Barbaro M, Fuschi D, Palombo M, Falangone F, Cremon C, et al. Inflammatory and microbiota-related regulation of the intestinal epithelial barrier. Front Nutr. (2021) 8:718356. doi: 10.3389/fnut.2021.718356
42. Jakobsson H, Rodríguez-Piñeiro A, Schütte A, Ermund A, Boysen P, Bemark M, et al. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. (2015) 16:164–77. doi: 10.15252/embr.201439263
43. Yuan Y, Wu D, Chen H, Ma Z, Peng X, Li X, et al. Farnesol ameliorates DSS-induced IBD by regulating inflammatory cytokines, repairing the intestinal barrier, reversing the gut microbiota imbalance, and influencing fecal metabolome in C57BL/6 mice. Biomed Pharmacother. (2024) 180:117518. doi: 10.1016/j.biopha.2024.117518
44. Jin J, Tang Y, Cao L, Wang X, Chen Y, An G, et al. Microsporidia persistence in host impairs epithelial barriers and increases chances of inflammatory bowel disease. Microbiol Spectr. (2024) 12:e0361023. doi: 10.1128/spectrum.03610-23
45. Massimino L, Palmieri O, Facoetti A, Fuggetta D, Spanò S, Lamparelli L, et al. Gut virome-colonising Orthohepadnavirus genus is associated with ulcerative colitis pathogenesis and induces intestinal inflammation in vivo. Gut. (2023) 72:1838–47. doi: 10.1136/gutjnl-2022-328375
46. Le-Trilling V, Ebel J, Baier F, Wohlgemuth K, Pfeifer K, Mookhoek A, et al. Acute cytomegalovirus infection modulates the intestinal microbiota and targets intestinal epithelial cells. Eur J Immunol. (2023) 53:e2249940. doi: 10.1002/eji.202249940
47. Seth R, Maqsood R, Mondal A, Bose D, Kimono D, Holland L, et al. Gut DNA virome diversity and its association with host bacteria regulate inflammatory phenotype and neuronal immunotoxicity in experimental gulf war illness. Viruses. (2019) 11:968. doi: 10.3390/v11100968
48. Viladomiu M, Metz M, Lima S, Jin W, Chou L. Adherent-invasive E. coli metabolism of propanediol in Crohn’s disease regulates phagocytes to drive intestinal inflammation. Cell Host Microbe. (2021) 29:607–619.e8. doi: 10.1016/j.chom.2021.01.002
49. Guo Z, Cai X, Guo X, Xu Y, Gong J, Li Y, et al. Let-7b ameliorates Crohn’s disease-associated adherent-invasive E coli induced intestinal inflammation via modulating Toll-Like Receptor 4 expression in intestinal epithelial cells. Biochem Pharmacol. (2018) 156:196–203. doi: 10.1016/j.bcp.2018.08.029
50. Wu M, Li P, An Y, Ren J, Yan D, Cui J, et al. Phloretin ameliorates dextran sulfate sodium-induced ulcerative colitis in mice by regulating the gut microbiota. Pharmacol Res. (2019) 150:104489. doi: 10.1016/j.phrs.2019.104489
51. Hsu C, Paik J, Treuting P, Seamons A, Meeker S, Brabb T, et al. Infection with murine norovirus 4 does not alter Helicobacter-induced inflammatory bowel disease in Il10(-/-) mice. Comp Med. (2014) 64:256–63.
52. Seamons A, Treuting P, Meeker S, Hsu C, Paik J, Brabb T, et al. Obstructive lymphangitis precedes colitis in murine norovirus-infected stat1-deficient mice. Am J Pathol. (2018) 188:1536–54. doi: 10.1016/j.ajpath.2018.03.019
53. Limon J, Tang J, Li D, Wolf A, Michelsen K, Funari V, et al. Malassezia is associated with Crohn’s disease and exacerbates colitis in mouse models. Cell Host Microbe. (2019) 25:377–388.e6. doi: 10.1016/j.chom.2019.01.007
54. Martini G, Tikhonova E, Rosati E, DeCelie M, Sievers L, Tran F, et al. Selection of cross-reactive T cells by commensal and food-derived yeasts drives cytotoxic TH1 cell responses in Crohn’s disease. Nat Med. (2023) 29:2602–14. doi: 10.1038/s41591-023-02556-5
55. Yu M, Ding H, Gong S, Luo Y, Lin H, Mu Y, et al. Fungal dysbiosis facilitates inflammatory bowel disease by enhancing CD4+ T cell glutaminolysis. Front Cell Infect Microbiol. (2023) 13:1140757. doi: 10.3389/fcimb.2023.1140757
56. Landry M, Ward C. Health benefits of a plant-based dietary pattern and implementation in healthcare and clinical practice. Am J Lifestyle Med. (2024) 18:657–65. doi: 10.1177/15598276241237766
57. Metoudi M, Bauer A, Haffner T, Kassam S. A cross-sectional survey exploring knowledge, beliefs and barriers to whole food plant-based diets amongst registered dietitians in the United Kingdom and Ireland. J Hum Nutr Diet. (2024) 38:e13386. doi: 10.1111/jhn.13386
58. Thompson A, Tresserra-Rimbau A, Jennings A, Bondonno N, Candussi C, O’Neill J, et al. Adherence to a healthful plant-based diet and risk of chronic kidney disease among individuals with diabetes. J Am Nutr Assoc. (2024) 44:212–22. doi: 10.1080/27697061.2024.2415917
59. Grygorczuk O, Mrozik M, Lipert A, Kamińska S, Białas A, Drygas W, et al. Cardiovascular health and diet quality among vegetarians, vegans and omnivores: Insights from a large urban population in Poland. Nutrients. (2024) 16:3438. doi: 10.3390/nu16203438
60. Xu W, Ding W, Jia L, Zhu K, Luo Q. Esculetin combats multidrug-resistant Salmonella infection and ameliorates intestinal dysfunction via the Nrf2 pathway. Antioxidants (Basel). (2024) 13:1170. doi: 10.3390/antiox13101170
61. Chen S, Lu H, Yin G, Zhang X, Meng D, Yu W, et al. Hesperitin prevents non-alcoholic steatohepatitis by modulating mitochondrial dynamics and mitophagy via the AMPKα-Drp1/PINK1-Parkin signaling pathway. Biochim Biophys Acta Mol Cell Biol Lipids. (2024) 1870:159570. doi: 10.1016/j.bbalip.2024.159570
62. Arfaoui L. Dietary plant polyphenols: Effects of food processing on their content and bioavailability. Molecules. (2021) 26:2959. doi: 10.3390/molecules26102959
63. Ribas-Agustí A, Martín-Belloso O, Soliva-Fortuny R, Elez-Martínez P. Food processing strategies to enhance phenolic compounds bioaccessibility and bioavailability in plant-based foods. Crit Rev Food Sci Nutr. (2018) 58:2531–48. doi: 10.1080/10408398.2017.1331200
64. Shanmugam G. Polyphenols: Potent protectors against chronic diseases. Nat Prod Res. (2024) 5:1–3. doi: 10.1080/14786419.2024.2386402
65. Jardon K, Goossens G, Most J, Galazzo G, Venema K, Penders J, et al. Examination of sex-specific interactions between gut microbiota and host metabolism after 12-week combined polyphenol supplementation in individuals with overweight or obesity. Gut Microbes. (2024) 16:2392875. doi: 10.1080/19490976.2024.2392875
66. Rudrapal M, Khairnar S, Khan J, Dukhyil A, Ansari M, Alomary M, et al. Dietary polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism(s) of action. Front Pharmacol. (2022) 13:806470. doi: 10.3389/fphar.2022.806470
67. Amalraj A, Pius A, Gopi S, Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives - A review. J Tradit Complement Med. (2017) 7:205–33. doi: 10.1016/j.jtcme.2016.05.005
68. Kumar N, Goel N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol Rep (Amst). (2019) 24:e00370. doi: 10.1016/j.btre.2019.e00370
69. Goleniowski M, Bonfill M, Cusido R, Palazón J. Phenolic acids. In: K Ramawat, J Mérillon editors. Natural products: Phytochemistry, botany and metabolism of alkaloids, phenolics and terpenes. Berlin, Heidelberg: Springer Berlin Heidelberg (2013). p. 1951–73.
70. Mohammed H, Said R, Abbas M, Al-Najjar B, Abd-Elmoniem E, Khan R, et al. Phytochemical, biological, and computational investigations of Ephedra alata Decne. Growing in salinity conditions of Arabian Peninsula. Sci Rep. (2024) 14:21987. doi: 10.1038/s41598-024-69607-w
71. Wang H, Feng S, Pan E, Ji X, Zhou M, Zhang S, et al. Ferulic acid alleviates long-term avermectin-induced damage to the spleen of carp and restores its inflammatory response and oxidative balance. J Environ Sci (China). (2025) 151:616–26. doi: 10.1016/j.jes.2024.03.029
72. Kim D, Hong S, Cho J, Lee S, Cho H. Inhibitory effect of phenolic compounds on vascular endothelial growth factor-induced retinal endothelial permeability and angiogenesis. Prev Nutr Food Sci. (2024) 29:321–31. doi: 10.3746/pnf.2024.29.3.321
73. Eteng O, Ugwor E, James A, Moses C, Ogbonna C, Iwara I, et al. Vanillic acid ameliorates diethyl phthalate and bisphenol S-induced oxidative stress and neuroinflammation in the hippocampus of experimental rats. J Biochem Mol Toxicol. (2024) 38:e70017. doi: 10.1002/jbt.70017
74. Zheng H, Xu Y, Fan S, Qi S, Jia F, Wu W, et al. Potential protective role of chlorogenic acid against cyclophosphamide-induced reproductive damage in male mice. Toxicol Res (Camb). (2024) 13:tfae176. doi: 10.1093/toxres/tfae176
75. Zhang H, Zhou W, Gao P, Li Z, Li C, Li J, et al. Ellagic acid protects against alcohol-related liver disease by modulating the hepatic circadian rhythm signaling through the gut microbiota-NPAS2 axis. J Agric Food Chem. (2024) 72:25103–17. doi: 10.1021/acs.jafc.4c06992
76. Cao L, Liu J, Ye C, Hu Y, Qin R. Caffeic acid inhibits Staphylococcus aureus-induced endometritis through regulating AMPKα/mTOR/HIF-1α signalling pathway. J Cell Mol Med. (2024) 28:e70175. doi: 10.1111/jcmm.70175
77. Sim Y, Kim C, Kim D, Hong J, Lee I, Kwak J, et al. Rosmarinic acid promotes cartilage regeneration through Sox9 induction via NF-κB pathway inhibition in mouse osteoarthritis progression. Heliyon. (2024) 10:e38936. doi: 10.1016/j.heliyon.2024.e38936
78. Li J, Chen G, Xie Z, Lin J, Luo S, Xu S. Association between dietary flavonoid intake and cardiovascular health in cancer survivors: A cross-sectional study. J Multidiscip Healthc. (2024) 17:4815–27. doi: 10.2147/JMDH.S482310
79. Ullah A, Munir S, Badshah S, Khan N, Ghani L, Poulson B, et al. Important flavonoids and their role as a therapeutic agent. Molecules. (2020) 25:5243. doi: 10.3390/molecules25225243
80. Rauf A, Imran M, Abu-Izneid T, Patel S, Pan X. Proanthocyanidins: A comprehensive review. Biomed Pharmacother. (2019) 116:108999. doi: 10.1016/j.biopha.2019.108999
81. Panche A, Diwan A, Chandra S. Flavonoids: An overview. J Nutr Sci. (2016) 5:e47. doi: 10.1017/jns.2016.41
82. Yan Y, Li J. Association of dietary anthocyanidins intake with all-cause mortality and cardiovascular diseases mortality in USA adults: A prospective cohort study. Sci Rep. (2024) 14:26595. doi: 10.1038/s41598-024-76805-z
83. Camilli A, de Godoi M, Costa V, Fernandes N, Cirelli G, da Silva L, et al. Local application of a new chalconic derivative (chalcone T4) reduces inflammation and oxidative stress in a periodontitis model in rats. Antioxidants (Basel). (2024) 13:1192. doi: 10.3390/antiox13101192
84. Hora A, Biano L, Nascimento A, Camargo Z, Heiden G, Albulquerque-Júnior R, et al. Isoorientin improves excisional skin wound healing in mice. Pharmaceuticals (Basel). (2024) 17:1368. doi: 10.3390/ph17101368
85. He X, Wu T, He H, Chen L, Han K, Zheng J, et al. Study of kaempferol in the treatment of rheumatoid arthritis through modulation of the NLRP3/CASP1/GSDMD axis and T-cell activation: Based on network pharmacology, single-cell analysis, and experimental validation. Int Immunopharmacol. (2024) 143:113357. doi: 10.1016/j.intimp.2024.113357
86. Xie K, Qi J, Deng L, Yu B, Luo Y, Huang Z, et al. Dihydromyricetin improves growth performance, immunity, and intestinal functions in weaned pigs challenged by enterotoxigenic Escherichia coli. Front Vet Sci. (2024) 11:1421871. doi: 10.3389/fvets.2024.1421871
87. Ramirez-Sanchez I, Navarrete-Yañez V, Espinosa-Raya J, Rubio-Gayosso I, Palma-Flores C, Mendoza-Lorenzo P, et al. Neurological restorative effects of (-)-epicatechin in a model of gulf war illness. J Med Food. (2024) 27:1070–9. doi: 10.1089/jmf.2023.0200
88. Gyawali K, Maharjan R, Acharya A, Khanal M, Ghimire M, Lamichhane T. Identification of catechin as main protease inhibitor of SARS-CoV-2 Omicron variant using molecular docking, molecular dynamics, PCA, DCCM, MM/GBSA and ADMET profiling. Nat Prod Res. (2024) 84:1–8. doi: 10.1080/14786419.2024.2421907
89. Shahrajabian M, Sun W. The golden spice for life: Turmeric with the pharmacological benefits of curcuminoids components, including curcumin, bisdemethoxycurcumin, and demethoxycurcumins. Curr Org Synth. (2024) 21:665–83. doi: 10.2174/1570179420666230607124949
90. Xie Y, Xu W, Jin Z, Zhao K. Chondroitin sulfate functionalized palmitic acid and cysteine cografted-quaternized chitosan for CD44 and gut microbiota dual-targeted delivery of curcumin. Mater Today Bio. (2023) 20:100617. doi: 10.1016/j.mtbio.2023.100617
91. Kouhi Z, Seyedalipour B, Hosseinkhani S, Chaichi M. Bisdemethoxycurcumin, a novel potent polyphenolic compound, effectively inhibits the formation of amyloid aggregates in ALS-associated hSOD1 mutant (L38R). Int J Biol Macromol. (2024) 282:136701. doi: 10.1016/j.ijbiomac.2024.136701
92. Tang J, Peng H, Xu F, Luo P, Liu D, Chen L. Demethoxycurcumin represses cervical cancer growth through PPARγ-regulated proliferation and apoptosis. Acta Biochim Biophys Sin (Shanghai). (2023) 55:1331–3. doi: 10.3724/abbs.2023077
93. Özdaş T, Özdaş S, Canatar I, Kaypak E. Pterostilbene suppresses head and neck cancer cell proliferation via induction of apoptosis. Turk J Biol. (2024) 48:319–37. doi: 10.55730/1300-0152.2708
94. Dou J, Liu S, Guo J, Wang C, Dai X, Lian L, et al. Dietary supplementation of pterostilbene, a major component in small berries, prevents alcohol-induced liver injury associated with lipid accumulation and inflammation. Food Funct. (2024) 15:11206–19. doi: 10.1039/d4fo03898c
95. Wang S, Li M, Wu J, Sun Y, Pan J, Guan W, et al. Lignans of Schisandra chinensis (Turcz.) Baill inhibits Parkinson’s disease progression through mediated neuroinflammation-TRPV1 expression in microglia. Phytomedicine. (2024) 135:156146. doi: 10.1016/j.phymed.2024.156146
96. Hamid M, Al-Amer S, Alahmari J, Assiri B, Majrashi T, Khan A. Clinical effectiveness of green tea extracts as a local haemostatic agent following mandibular molar extraction. J Coll Physicians Surg Pak. (2024) 34:1281–6. doi: 10.29271/jcpsp.2024.11.1281
97. Howard E, Meyer R, Weninger S, Martinez T, Wachsmuth H, Pignitter M, et al. Impact of plant-based dietary fibers on metabolic homeostasis in high-fat diet mice via alterations in the gut microbiota and metabolites. J Nutr. (2024) 154:2014–28. doi: 10.1016/j.tjnut.2024.05.003
98. Bakr A, Farag M. Soluble dietary fibers as antihyperlipidemic agents: A comprehensive review to maximize their health benefits. ACS Omega. (2023) 8:24680–94. doi: 10.1021/acsomega.3c01121
99. Gao Y, Yu L, Ye Z, Zhang C, Gong Y, Zhang Q, et al. In vitro batch fermentation demonstrates variations in the regulation of gut microbiota and metabolic functions by β-glucans of differing structures. Food Res Int. (2024) 186:114287. doi: 10.1016/j.foodres.2024.114287
100. Zademohammadi F, Sasanfar B, Toorang F, Mozafarinia M, Salehi-Abargouei A, Zendehdel K. Dietary soluble, insoluble, and total fiber intake and their dietary sources in association with breast cancer. BMC Public Health. (2024) 24:2560. doi: 10.1186/s12889-024-19861-4
101. Zheng B, Ao T, Zhao X, Chen Y, Xie J, Gao X, et al. Comprehensive assessment of the anti-obesity effects of highland barley total, insoluble, and soluble dietary fiber through multi-omics analysis. Food Res Int. (2024) 189:114535. doi: 10.1016/j.foodres.2024.114535
102. Scarcella J, Lopes M, Silva E, Andrade G. Valorization of okara by-product for obtaining soluble dietary fibers and their use in biodegradable carboxymethyl cellulose-based film. Int J Biol Macromol. (2024) 280:136032. doi: 10.1016/j.ijbiomac.2024.136032
103. Dhingra D, Michael M, Rajput H, Patil R. Dietary fibre in foods: A review. J Food Sci Technol. (2012) 49:255–66. doi: 10.1007/s13197-011-0365-5
104. Khalid W, Arshad M, Jabeen A, Muhammad Anjum F, Qaisrani T, Suleria H. Fiber-enriched botanicals: A therapeutic tool against certain metabolic ailments. Food Sci Nutr. (2022) 10:3203–18. doi: 10.1002/fsn3.2920
105. Yi L, Cheng L, Yang Q, Shi K, Han F, Luo W, et al. Source, extraction, properties, and multifunctional applications of pectin: A short review. Polymers (Basel). (2024) 16:2883. doi: 10.3390/polym16202883
106. Weber A, Pascale N, Gu F, Ryan E, Respondek F. Nutrition and health effects of pectin: A systematic scoping review of human intervention studies. Nutr Res Rev. (2024) 38:306–23. doi: 10.1017/S0954422424000180
107. Steigerwald H, Blanco-Pérez F, Macías-Camero A, Albrecht M, Huch M, Bender C, et al. Effects of pectin methyl-esterification on intestinal microbiota and its immunomodulatory properties in naive mice. Carbohydr Polym. (2024) 334:122007. doi: 10.1016/j.carbpol.2024.122007
108. Beukema M, Faas M, de Vos P. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: Impact via gut microbiota and direct effects on immune cells. Exp Mol Med. (2020) 52:1364–76. doi: 10.1038/s12276-020-0449-2
109. Sahasrabudhe N, Beukema M, Tian L, Troost B, Scholte J, Bruininx E, et al. Dietary fiber pectin directly blocks toll-like receptor 2-1 and prevents doxorubicin-induced ileitis. Front Immunol. (2018) 9:383. doi: 10.3389/fimmu.2018.00383
110. Liu M, Fu J, Liu Y, Gou W, Yuan W, Shang H. Pectin from comfrey roots alleviate DSS-induced ulcerative colitis in mice through modulating the intestinal barrier. Int J Biol Macromol. (2024) 282:137016. doi: 10.1016/j.ijbiomac.2024.137016
111. Vijay A, Kelly A, Miller S, Marshall M, Alonso A, Kouraki A, et al. Supplementation with citrus low-methoxy pectin reduces levels of inflammation and anxiety in healthy volunteers: A pilot controlled dietary intervention study. Nutrients. (2024) 16:3326. doi: 10.3390/nu16193326
112. Qin Y, Wang L, Yang X, Xu Y, Fan G, Fan Y, et al. Inulin: Properties and health benefits. Food Funct. (2023) 14:2948–68. doi: 10.1039/d2fo01096h
113. Wan X, Guo H, Liang Y, Zhou C, Liu Z, Li K, et al. The physiological functions and pharmaceutical applications of inulin: A review. Carbohydr Polym. (2020) 246:116589. doi: 10.1016/j.carbpol.2020.116589
114. Zhao X, He W, Jakobsen L, Panah F, Barbosa Correia B, Nielsen D, et al. Influence of dairy matrix on the prebiotic effects of inulin related to gut metabolic activity and bone health. Food Funct. (2024) 15:11129–40. doi: 10.1039/d4fo01635a
115. Zhang K, Zeng Y, Li J, Huang Y, Zhang N, Gong Y, et al. Inulin alleviates atherosclerosis through improving lipid metabolism, inflammation, and gut microbiota in ApoE-knockout mice: The short-chain is more efficacious. Front Pharmacol. (2024) 15:1445528. doi: 10.3389/fphar.2024.1445528
116. Ismawati, Saryono, Mukhyarjon, Romus I, Putri VD, Yanti S, et al. Effect of inulin from dahlia tubers (Dahlia variabilis) extract on insulitis severity and insulin expression in diabetic rats. Biomedicine (Taipei). (2024) 14:31–9. doi: 10.37796/2211-8039.1460
117. Alptekin ÝM, Çakıroðlu FP, Reçber T, Nemutlu E. Inulin may prevent the high-fat diet induced-obesity via suppressing endocannabinoid system in the prefrontal cortex in Wistar rats. Int J Food Sci Nutr. (2024) 75:800–11. doi: 10.1080/09637486.2024.2408545
118. Cavallaro G, Sardo C, Scialabba C, Licciardi M, Giammona G. Smart inulin-based polycationic nanodevices for siRNA delivery. Curr Drug Deliv. (2017) 14:224–30. doi: 10.2174/1567201813666160811145855
119. Jogdeo C, Panja S, Kumari N, Tang W, Kapoor E, Siddhanta K, et al. Inulin-based nanoparticles for targeted siRNA delivery in acute kidney injury. J Control Release. (2024) 376:577–92. doi: 10.1016/j.jconrel.2024.10.027
120. Contato A, Vici A, Pinheiro V, de Oliveira T, Ortolan G, de Freitas E, et al. Thermothelomyces thermophilus cultivated with residues from the fruit pulp industry: Enzyme immobilization on ionic supports of a crude cocktail with enhanced production of lichenase. Folia Microbiol (Praha). (2024) 1–11. doi: 10.1007/s12223-024-01208-6
121. Du B, Meenu M, Liu H, Xu B. A concise review on the molecular structure and function relationship of β-glucan. Int J Mol Sci. (2019) 20:4032. doi: 10.3390/ijms20164032
122. Rainer H, Goretzki A, Lin Y, Schiller H, Krause M, Döring S, et al. Characterization of the immune-modulating properties of different β-glucans on myeloid dendritic cells. Int J Mol Sci. (2024) 25:9914. doi: 10.3390/ijms25189914
123. Guamán S, Elhadi A, Salama A, Manuelian C, Caja G, Albanell E. Beta-glucans improve the mammary innate immune response to endotoxin challenge in dairy ewes. Animals (Basel). (2024) 14:3023. doi: 10.3390/ani14203023
124. Arast Y, Esfandiari H, Kamranfar F, Mousavi Z, Ameri Shah Reza M, Pourahmad J. Evaluating the concentration dependent dual effects of β-Glucan on cancerous skin cells and mitochondria isolated from melanoma-induced animal model. Cutan Ocul Toxicol. (2024) 43:347–55. doi: 10.1080/15569527.2024.2410355
125. Amiri M, Mohammadzadeh V, Yazdi M, Barani M, Rahdar A, Kyzas G. Plant-based gums and mucilages applications in pharmacology and nanomedicine: A review. Molecules. (2021) 26:1770. doi: 10.3390/molecules26061770
126. Avachat A, Dash R, Shrotriya S. Recent investigations of plant based natural gums, mucilages and resins in novel drug delivery systems. Ind J Pharm Edu Res. (2011) 45:86–99.
127. Rahim H, Sadiq A, Khan S, Khan M, Amin F, Jan N, et al. Prunus armeniaca and Prunus domestica gums: Exploring their synergistic binding potential in tablets. Lat Am J Pharm. (2018) 37:1672–83.
128. Rahim H, Khan M, Sadiq A, Khan S, Chishti K, Rahman I. Comparative studies of binding potential of Prunus armeniaca and Prunus domestica gums in tablets formulations. Pak J Pharm Sci. (2015) 28:909–14.
129. Freitas A, Ribeiro A, Santos A, Veiga F, Nunes L, Silva D, et al. Sterculia striata gum as a potential oral delivery system for protein drugs. Int J Biol Macromol. (2020) 164:1683–92. doi: 10.1016/j.ijbiomac.2020.07.276
130. Lima I, Ferreira M, Barros E, Rizzo M, Santos J, Ribeiro A, et al. Antibacterial and healing effect of Chicha gum hydrogel (Sterculia striata) with Nerolidol. Int J Mol Sci. (2023) 24:2210. doi: 10.3390/ijms24032210
131. Otálora M, Wilches-Torres A, Gómez Castaño J. Mucilage from yellow Pitahaya (Selenicereus megalanthus) fruit peel: Extraction, proximal analysis, and molecular characterization. Molecules. (2023) 28:786. doi: 10.3390/molecules28020786
132. Kassem I, Joshua Ashaolu T, Kamel R, Elkasabgy N, Afifi S, Farag M. Mucilage as a functional food hydrocolloid: Ongoing and potential applications in prebiotics and nutraceuticals. Food Funct. (2021) 12:4738–48. doi: 10.1039/d1fo00438g
133. Fouda K, Mohamed R. Molecular docking and in vivo protective effects of okra (Abelmoschus esculentus) against metabolic dysfunction in high-fat, high-sodium diet-fed rats. Food Funct. (2024) 15:3566–82. doi: 10.1039/d3fo04407f
134. Uddin Zim A, Khatun J, Khan M, Hossain M, Haque M. Evaluation of in vitro antioxidant activity of okra mucilage and its antidiabetic and antihyperlipidemic effect in alloxan-induced diabetic mice. Food Sci Nutr. (2021) 9:6854–65. doi: 10.1002/fsn3.2641
135. Sánchez-Zapata E, Sendra E, Sayas E, Navarro C, Fernández-López J, Pérez-Alvarez JA. Resistant starch as prebiotic: A review. Starch-Stärke. (2011) 7:406–15. doi: 10.1002/star.201000099
136. Wen J, Li M, Hu J, Tan H, Nie S. Resistant starches and gut microbiota. Food Chem. (2022) 387:132895. doi: 10.1016/j.foodchem.2022.132895
137. Bojarczuk A, Ska̧pska S, Khaneghah A, Marszałek K. Health benefits of resistant starch: A review of the literature. J Funct Foods. (2022) 93:105094. doi: 10.1016/j.jff.2022.105094
138. Zhang Y, Hu X, Yang S, Hu Y, Duan K. Effects of resistant starch supplementation on renal function and inflammatory markers in patients with chronic kidney disease: A meta-analysis of randomized controlled trials. Ren Fail. (2024) 46:2416609. doi: 10.1080/0886022X.2024.2416609
139. Wilson B, Whelan K. Prebiotic inulin-type fructans and galacto-oligosaccharides: Definition, specificity, function, and application in gastrointestinal disorders. J Gastroenterol Hepatol. (2017) 32:64–8. doi: 10.1111/jgh.13700
140. Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi S, et al. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods. (2019) 8:92. doi: 10.3390/foods8030092
141. Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and butyrate-producing colon bacteria: Importance and strategies for their stimulation in the human gut. Front Microbiol. (2016) 7:979. doi: 10.3389/fmicb.2016.00979
142. Abughazaleh N, Smith H, Seerattan R, Hart D, Reimer R, Herzog W. Development of shoulder osteoarthritis and bone lesions in female and male rats subjected to a high fat/sucrose diet. Sci Rep. (2024) 14:25871. doi: 10.1038/s41598-024-76703-4
143. Seddiqi H, Oliaei E, Honarkar H, Jin J, Geonzon L, Bacabac R, et al. Cellulose and its derivatives: Towards biomedical applications. Cellulose. (2021) 28:1893–931. doi: 10.1007/s10570-020-03674-w
144. Liao Y, Wu S, Zhou G, Mei S, Yang Z, Li S, et al. Cellulolytic Bacillus cereus produces a variety of short-chain fatty acids and has potential as a probiotic. Microbiol Spectr. (2024) 12:e0326723. doi: 10.1128/spectrum.03267-23
145. Moraïs S, Winkler S, Zorea A, Levin L, Nagies F, Kapust N, et al. Cryptic diversity of cellulose-degrading gut bacteria in industrialized humans. Science. (2024) 383:eadj9223. doi: 10.1126/science.adj9223
146. Tang S, Dong X, Ma Y, Zhou H, He Y, Ren D, et al. Highly crystalline cellulose microparticles from dealginated seaweed waste ameliorate high fat-sugar diet-induced hyperlipidemia in mice by modulating gut microbiota. Int J Biol Macromol. (2024) 263:130485. doi: 10.1016/j.ijbiomac.2024.130485
147. Feng J, Qin Z, Farmanfarmaee A, Kong F. Comparing gastric emptying of cellulose nanocrystals with sodium alginate and pectin using a dynamic in vitro stomach model. Int J Biol Macromol. (2024) 280:135892. doi: 10.1016/j.ijbiomac.2024.135892
148. Scheller H, Ulvskov P. Hemicelluloses. Annu Rev Plant Biol. (2010) 61:263–89. doi: 10.1146/annurev-arplant-042809-112315
149. Pauly M, Gille S, Liu L, Mansoori N, de Souza A, Schultink A, et al. Hemicellulose biosynthesis. Planta. (2013) 238:627–42. doi: 10.1007/s00425-013-1921-1
150. Sarma S, Singh D, Singh P, Khare P, Mangal P, Singh S, et al. Finger millet arabinoxylan protects mice from high-fat diet induced lipid derangements, inflammation, endotoxemia and gut bacterial dysbiosis. Int J Biol Macromol. (2018) 106:994–993. doi: 10.1016/j.ijbiomac.2017.08.100
151. Lima I, Castro R, Adjafre B, Sousa S, de Paula D, Alves A, et al. Galactomannan of Delonix regia seeds modulates cytokine expression and oxidative stress eliciting anti-inflammatory and healing effects in mice cutaneous wound. Int J Biol Macromol. (2022) 203:342–9. doi: 10.1016/j.ijbiomac.2022.01.144
152. Lemieszek M, Nunes F, Marques G, Rzeski W. Cantharellus cibarius branched mannans inhibits colon cancer cells growth by interfering with signals transduction in NF-κB pathway. Int J Biol Macromol. (2019) 134:770–80. doi: 10.1016/j.ijbiomac.2019.05.072
153. Xie H, Yang J, Liu T, Zhang X, Zuo S, Gao Y, et al. Molecular mechanism study of dietary fiber lignin intervention in ulcerative colitis through GPR37. J Agric Food Chem. (2024) 72:13684–99. doi: 10.1021/acs.jafc.4c01452
154. Asp N. Dietary fibre–definition, chemistry and analytical determination. Mol Aspects Med. (1987) 9:17–29. doi: 10.1016/0098-299790014-8
155. Bunzel M, Ralph J. NMR characterization of lignins isolated from fruit and vegetable insoluble dietary fiber. J Agric Food Chem. (2006) 54:8352–61. doi: 10.1021/jf061525z
156. Xiong W, Devkota L, Flanagan B, Gu Z, Zhang B, Dhital S. Plant cell wall composition modulates the gut microbiota and metabolites in in-vitro fermentation. Carbohydr Polym. (2023) 316:121074. doi: 10.1016/j.carbpol.2023.121074
157. Gu J, Guo M, Zheng L, Yin X, Zhou L, Fan D, et al. Protective effects of lignin-carbohydrate complexes from wheat stalk against bisphenol a neurotoxicity in zebrafish via oxidative stress. Antioxidants (Basel). (2021) 10:1640. doi: 10.3390/antiox10101640
158. Liu Z, Liu F, Wang W, Sun C, Gao D, Ma J, et al. Study of the alleviation effects of a combination of Lactobacillus rhamnosus and inulin on mice with colitis. Food Funct. (2020) 11:3823–37. doi: 10.1039/c9fo02992c
159. Cao X, Tao S, Wang W, Wu S, Hong Y, Wang X, et al. Ternary inulin hydrogel with long-term intestinal retention for simultaneously reversing IBD and its fibrotic complication. Nat Commun. (2024) 15:8428. doi: 10.1038/s41467-024-52722-7
160. Zhang Z, Pan Y, Guo Z, Fan X, Pan Q, Gao W, et al. An olsalazine nanoneedle-embedded inulin hydrogel reshapes intestinal homeostasis in inflammatory bowel disease. Bioact Mater. (2024) 33:71–84. doi: 10.1016/j.bioactmat.2023.10.028
161. Guo J, Zhang M, Wang H, Li N, Lu Z, Li L, et al. Gut microbiota and short chain fatty acids partially mediate the beneficial effects of inulin on metabolic disorders in obese ob/ob mice. J Food Biochem. (2022) 46:e14063. doi: 10.1111/jfbc.14063
162. Li K, Wei W, Xu C, Lian X, Bao J, Yang S, et al. Prebiotic inulin alleviates anxiety and depression-like behavior in alcohol withdrawal mice by modulating the gut microbiota and 5-HT metabolism. Phytomedicine. (2024) 135:156181. doi: 10.1016/j.phymed.2024.156181
163. Ariaee A, Wardill H, Wignall A, Prestidge C, Joyce P. The degree of inulin polymerization is important for short-term amelioration of high-fat diet (HFD)-induced metabolic dysfunction and gut microbiota dysbiosis in rats. Foods. (2024) 13:1039. doi: 10.3390/foods13071039
164. Tian S, Paudel D, Hao F, Neupane R, Castro R, Patterson A, et al. Refined fiber inulin promotes inflammation-associated colon tumorigenesis by modulating microbial succinate production. Cancer Rep (Hoboken). (2023) 6:e1863. doi: 10.1002/cnr2.1863
165. Hoving L, Katiraei S, Pronk A, Heijink M, Vonk K, Amghar-El Bouazzaoui F, et al. The prebiotic inulin modulates gut microbiota but does not ameliorate atherosclerosis in hypercholesterolemic APOE*3-Leiden.CETP mice. Sci Rep. (2018) 8:16515. doi: 10.1038/s41598-018-34970-y
166. Koleva P, Valcheva R, Sun X, Gänzle M, Dieleman L. Inulin and fructo-oligosaccharides have divergent effects on colitis and commensal microbiota in HLA-B27 transgenic rats. Br J Nutr. (2012) 108:1633–43. doi: 10.1017/S0007114511007203
167. Koleva P, Ketabi A, Valcheva R, Gänzle M, Dieleman L. Chemically defined diet alters the protective properties of fructo-oligosaccharides and isomalto-oligosaccharides in HLA-B27 transgenic rats. PLoS One. (2014) 9:e111717. doi: 10.1371/journal.pone.0111717
168. Sun J, Liu S, Ling Z, Wang F, Ling Y, Gong T, et al. Fructooligosaccharides ameliorating cognitive deficits and neurodegeneration in APP/PS1 transgenic mice through modulating gut microbiota. J Agric Food Chem. (2019) 67:3006–17. doi: 10.1021/acs.jafc.8b07313
169. Chen B, Du L, He H, Kim J, Zhao Y, Zhang Y, et al. Fructo-oligosaccharide intensifies visceral hypersensitivity and intestinal inflammation in a stress-induced irritable bowel syndrome mouse model. World J Gastroenterol. (2017) 23:8321–33. doi: 10.3748/wjg.v23.i47.8321
170. Huang X, Hu J, Zhang H, Li J, Zhu X, Liu Y, et al. Clostridium butyricum and chitooligosaccharides in synbiotic combination ameliorate symptoms in a DSS-induced ulcerative colitis mouse model by modulating gut microbiota and enhancing intestinal barrier function. Microbiol Spectr. (2023) 11:e0437022. doi: 10.1128/spectrum.04370-22
171. Takagi T, Naito Y, Higashimura Y, Ushiroda C, Mizushima K, Ohashi Y, et al. Partially hydrolysed guar gum ameliorates murine intestinal inflammation in association with modulating luminal microbiota and SCFA. Br J Nutr. (2016) 116:1199–205. doi: 10.1017/S0007114516003068
172. Paudel D, Nair D, Tian S, Hao F, Goand U, Joseph G, et al. Dietary fiber guar gum-induced shift in gut microbiota metabolism and intestinal immune activity enhances susceptibility to colonic inflammation. Gut Microbes. (2024) 16:2341457. doi: 10.1080/19490976.2024.2341457
173. Takayama S, Katada K, Takagi T, Iida T, Ueda T, Mizushima K, et al. Partially hydrolyzed guar gum attenuates non-alcoholic fatty liver disease in mice through the gut-liver axis. World J Gastroenterol. (2021) 27:2160–76. doi: 10.3748/wjg.v27.i18.2160
174. Yang F, Su Y, Yan C, Chen T, Cheung P. Attenuation of inflammatory bowel disease by oral administration of mucoadhesive polydopamine-coated yeast β-glucan via ROS scavenging and gut microbiota regulation. J Nanobiotechnology. (2024) 22:166. doi: 10.1186/s12951-024-02434-3
175. Katimbwa D, Kim Y, Kim M, Jeong M, Lim J. Solubilized β-glucan supplementation in C57BL/6J mice dams augments neurodevelopment and cognition in the offspring driven by gut microbiome remodeling. Foods. (2024) 13:3102. doi: 10.3390/foods13193102
176. Wang Z, Gao M, Kan J, Cheng Q, Chen X, Tang C, et al. Resistant starch from purple sweet potatoes alleviates dextran sulfate sodium-induced colitis through modulating the homeostasis of the gut microbiota. Foods. (2024) 13:1028. doi: 10.3390/foods13071028
177. Qian Y, Li G, Zhu K, Sun P, Feng X, Zhao X. Effect of resistant starch on HCl/ethanol-induced gastric injury in rats. J Korean Soc Appl Biol Chem. (2013) 56:613–9. doi: 10.1007/s13765-013-3143-4
178. Li J, Bai J, Song Z, Ji Y, Chen Z, Yang Y, et al. Dietary pectin attenuates Salmonella typhimurium-induced colitis by modulating the TLR2-NF-κB pathway and intestinal microbiota in mice. Food Chem Toxicol. (2023) 182:114100. doi: 10.1016/j.fct.2023.114100
179. Cao J, Qin L, Zhang L, Wang K, Yao M, Qu C, et al. Protective effect of cellulose and soluble dietary fiber from Saccharina japonica by-products on regulating inflammatory responses, gut microbiota, and SCFAs production in colitis mice. Int J Biol Macromol. (2024) 267:131214. doi: 10.1016/j.ijbiomac.2024.131214
180. Yu J, Gao M, Wang L, Guo X, Liu X, Sheng M, et al. An insoluble cellulose nanofiber with robust expansion capacity protects against obesity. Int J Biol Macromol. (2024) 277:134401. doi: 10.1016/j.ijbiomac.2024.134401
181. Zhao C, Yang J, Chen M, Chen W, Yang X, Ye H, et al. Synthetic lignin-derived therapeutic nano reagent as intestinal pH-sensitive drug carriers capable of bypassing the gastric acid environment for colitis treatment. ACS Nano. (2023) 17:811–24. doi: 10.1021/acsnano.2c11188
182. Zha Z, Lv Y, Tang H, Li T, Miao Y, Cheng J, et al. An orally administered butyrate-releasing xylan derivative reduces inflammation in dextran sulphate sodium-induced murine colitis. Int J Biol Macromol. (2020) 156:1217–33. doi: 10.1016/j.ijbiomac.2019.11.159
183. Luo S, He L, Zhang H, Li Z, Liu C, Chen T. Arabinoxylan from rice bran protects mice against high-fat diet-induced obesity and metabolic inflammation by modulating gut microbiota and short-chain fatty acids. Food Funct. (2022) 13:7707–19. doi: 10.1039/d2fo00569g
184. Boaru D, Fraile-Martinez O, De Leon-Oliva D, Garcia-Montero C, De Castro-Martinez P, Miranda-Gonzalez A, et al. Harnessing the anti-inflammatory properties of polyphenols in the treatment of inflammatory Bowel disease. Int J Biol Sci. (2024) 20:5608–72. doi: 10.7150/ijbs.98107
185. Jin T. Curcumin and dietary polyphenol research: Beyond drug discovery. Acta Pharmacol Sin. (2018) 39:779–86. doi: 10.1038/aps.2017.179
186. Qadir M, Naqvi S, Muhammad S. Curcumin: A polyphenol with molecular targets for cancer control. Asian Pac J Cancer Prev. (2016) 17:2735–9.
187. Deng W, Xiong X, Lu M, Huang S, Luo Y, Wang Y, et al. Curcumin suppresses colorectal tumorigenesis through restoring the gut microbiota and metabolites. BMC Cancer. (2024) 24:1141. doi: 10.1186/s12885-024-12898-z
188. Zhang Z, Wu X, Cao S, Wang L, Wang D, Yang H, et al. Caffeic acid ameliorates colitis in association with increased Akkermansia population in the gut microbiota of mice. Oncotarget. (2016) 7:31790–9. doi: 10.18632/oncotarget.9306
189. Wan F, Zhong R, Wang M, Zhou Y, Chen Y, Yi B, et al. Caffeic acid supplement alleviates colonic inflammation and oxidative stress potentially through improved gut microbiota community in mice. Front Microbiol. (2021) 12:784211. doi: 10.3389/fmicb.2021.784211
190. Zhong X, Liu Y, Gao X, Krausz K, Niu B, Gonzalez F, et al. Caffeic acid phenethyl ester suppresses intestinal FXR signaling and ameliorates nonalcoholic fatty liver disease by inhibiting bacterial bile salt hydrolase activity. Acta Pharmacol Sin. (2023) 44:145–56. doi: 10.1038/s41401-022-00921-7
191. Peng J, Liu T, Meng P, Luo Y, Zhu S, Wang Y, et al. Gallic acid ameliorates colitis by trapping deleterious metabolite ammonia and improving gut microbiota dysbiosis. mBio. (2024) 15:e0275223. doi: 10.1128/mbio.02752-23
192. Kang J, Jie L, Lu G, Fu H, Liao T, Liu D, et al. Gallic acid ameliorates synovial inflammation and fibrosis by regulating the intestinal flora and its metabolites. Toxicol Appl Pharmacol. (2024) 490:117033. doi: 10.1016/j.taap.2024.117033
193. Athukuri B, Neerati P. Enhanced oral bioavailability of metoprolol with gallic acid and ellagic acid in male Wistar rats: Involvement of CYP2D6 inhibition. Drug Metab Pers Ther. (2016) 31:229–34. doi: 10.1515/dmpt-2016-0029
194. Silva I, Polaquini C, Regasini L, Ferreira H, Pavan F. Evaluation of cytotoxic, apoptotic, mutagenic, and chemopreventive activities of semi-synthetic esters of gallic acid. Food Chem Toxicol. (2017) 105:300–7. doi: 10.1016/j.fct.2017.04.033
195. Kim H, Quon M, Kim J. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. (2014) 2:187–95. doi: 10.1016/j.redox.2013.12.022
196. Wu Z, Huang S, Li T, Li N, Han D, Zhang B, et al. Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis. Microbiome. (2021) 9:184. doi: 10.1186/s40168-021-01115-9
197. Murakami A. Dose-dependent functionality and toxicity of green tea polyphenols in experimental rodents. Arch Biochem Biophys. (2014) 557:3–10. doi: 10.1016/j.abb.2014.04.018
198. Kyriacou N, Gross A, McLachlan A. Green tea catechins as perpetrators of drug pharmacokinetic interactions. Clin Pharmacol Ther. (2025): doi: 10.1002/cpt.3547
199. Ling S, Xu J. Biological activities of 2,3,5,4’-tetrahydroxystilbene-2-O-β-D-glucoside in antiaging and antiaging-related disease treatments. Oxid Med Cell Longev. (2016) 2016:4973239. doi: 10.1155/2016/4973239
200. He X, Liu J, Long G, Xia X, Liu M. 2,3,5,4’-tetrahydroxystilbene-2-O-β-D-glucoside, a major bioactive component from Polygoni multiflori Radix (Heshouwu) suppresses DSS induced acute colitis in BALb/c mice by modulating gut microbiota. Biomed Pharmacother. (2021) 137:111420. doi: 10.1016/j.biopha.2021.111420
201. Xu X, Ocansey D, Pei B, Zhang Y, Wang N, Wang Z, et al. Resveratrol alleviates DSS-induced IBD in mice by regulating the intestinal microbiota-macrophage-arginine metabolism axis. Eur J Med Res. (2023) 28:319. doi: 10.1186/s40001-023-01257-6
202. Fei Y, Zhang S, Han S, Qiu B, Lu Y, Huang W, et al. The role of dihydroresveratrol in enhancing the synergistic effect of ligilactobacillus salivarius LI01 and resveratrol in ameliorating colitis in mice. Research. (2022) 2022:9863845. doi: 10.34133/2022/9863845
203. Alrafas H, Busbee P, Chitrala K, Nagarkatti M, Nagarkatti P. Alterations in the gut microbiome and suppression of histone deacetylases by resveratrol are associated with attenuation of colonic inflammation and protection against colorectal cancer. J Clin Med. (2020) 9:1796. doi: 10.3390/jcm9061796
204. Li S, Han B, Li J, Lv Z, Jiang H, Liu Y, et al. Resveratrol alleviates liver fibrosis induced by long-term inorganic mercury exposure through activating the Sirt 1/ PGC-1α signaling pathway. J Agric Food Chem. (2024) 72:15985–97. doi: 10.1021/acs.jafc.4c02349
205. Tain Y, Hou C, Tzeng H, Lin S, Chang-Chien G, Lee W, et al. Effect of purified resveratrol butyrate ester monomers against hypertension after maternal high-fructose intake in adult offspring. Nutrients. (2024) 16:3132. doi: 10.3390/nu16183132
206. Chow H, Garland L, Hsu C, Vining D, Chew W, Miller J, et al. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev Res (Phila). (2010) 3:1168–75. doi: 10.1158/1940-6207.CAPR-09-0155
207. Shen M, Hsiao G, Liu C, Fong T, Lin K, Chou D, et al. Inhibitory mechanisms of resveratrol in platelet activation: Pivotal roles of p38 MAPK and NO/cyclic GMP. Br J Haematol. (2007) 139:475–85. doi: 10.1111/j.1365-2141.2007.06788.x
208. Rodríguez-Pérez C, García-Villanova B, Guerra-Hernández E, Verardo V. Grape seeds proanthocyanidins: An overview of in vivo bioactivity in animal models. Nutrients. (2019) 11:2435. doi: 10.3390/nu11102435
209. Chen J, Jiang F, Xu N, Dong G, Jiang J, Wang M, et al. Anthocyanin extracted from purple sweet potato alleviates dextran sulfate sodium-induced colitis in mice by suppressing pyroptosis and altering intestinal flora structure. J Med Food. (2024) 27:110–22. doi: 10.1089/jmf.2023.K.0247
210. Kitabatake M, Matsumura Y, Ouji-Sageshima N, Nishioka T, Hara A, Kayano S, et al. Persimmon-derived tannin ameliorates the pathogenesis of ulcerative colitis in a murine model through inhibition of the inflammatory response and alteration of microbiota. Sci Rep. (2021) 11:7286. doi: 10.1038/s41598-021-86608-1
211. Liu H, Yan C, Teng Y, Guo J, Liang C, Xia X. Gut microbiota and D-ribose mediate the anti-colitic effect of punicalagin in DSS-treated mice. Food Funct. (2024) 15:7108–23. doi: 10.1039/d4fo00741g
212. Lin R, Piao M, Song Y. Dietary quercetin increases colonic microbial diversity and attenuates colitis severity in Citrobacter rodentium-infected mice. Front Microbiol. (2019) 10:1092. doi: 10.3389/fmicb.2019.01092
213. Wang L, Fu R, Meng Y, Liang J, Xue W, Hu H, et al. pH sensitive quercetin nanoparticles ameliorate DSS-induced colitis in mice by colon-specific delivery. Mol Nutr Food Res. (2024) 68:e2300051. doi: 10.1002/mnfr.202300051
214. Yuan M, Sun T, Zhang Y, Guo C, Wang F, Yao Z, et al. Quercetin alleviates insulin resistance and repairs intestinal barrier in db/ db mice by modulating gut microbiota. Nutrients. (2024) 16:1870. doi: 10.3390/nu16121870
215. Patel R, Stine A, Zitko K. Enhanced anticoagulant effect of warfarin when co-administered with quercetin. J Pharm Technol. (2022) 38:374–5. doi: 10.1177/87551225221125667
216. Yang L, Gao Y, Wang H, Zhong W, Gong J, Farag M, et al. Myricetin attenuates the inflammatory bowel disease in prediabetic mice via inflammation inhibition and gut microbiota modulation. Food Safety and Health. (2024) 2:303–17. doi: 10.1002/fsh3.12041
217. Liu Y, Wang R, Zhou J, Lyu Q, Zhao X, Yang X, et al. Myricetin alleviates high-fat diet-induced atherosclerosis in ApoE-/- mice by regulating bile acid metabolism involved in gut microbiota remodeling. Food Funct. (2025) 16:2737–49. doi: 10.1039/d5fo00374a
218. Hanai H, Iida T, Takeuchi K, Watanabe F, Maruyama Y, Andoh A, et al. Curcumin maintenance therapy for ulcerative colitis: Randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. (2006) 4:1502–6. doi: 10.1016/j.cgh.2006.08.008
219. Lang A, Salomon N, Wu J, Kopylov U, Lahat A, Har-Noy O, et al. Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Clin Gastroenterol Hepatol. (2015) 13:1444–9.e1. doi: 10.1016/j.cgh.2015.02.019
220. Sugimoto K, Ikeya K, Bamba S, Andoh A, Yamasaki H, Mitsuyama K, et al. Highly bioavailable curcumin derivative ameliorates Crohn’s disease symptoms: A randomized, double-blind, multicenter study. J Crohns Colitis. (2020) 14:1693–701. doi: 10.1093/ecco-jcc/jjaa097
221. Sedighi S, Faramarzipalangar Z, Mohammadi E, Aghamohammadi V, Bahnemiri M, Mohammadi K. The effects of curcumin supplementation on inflammatory markers in systemic lupus erythematosus patients: A randomized placebo-controlled trial. Eur J Nutr. (2024) 64:8. doi: 10.1007/s00394-024-03515-7
222. Benjamin J, Hedin C, Koutsoumpas A, Ng S, McCarthy N, Hart A, et al. Randomised, double-blind, placebo-controlled trial of fructo-oligosaccharides in active Crohn’s disease. Gut. (2011) 60:923–9. doi: 10.1136/gut.2010.232025
223. Valcheva R, Armstrong H, Kovic O, Bording-Jorgensen M, Veniamin S, Pérez-Muñoz M, et al. Double blind placebo-controlled trial for the prevention of ulcerative colitis relapses by β-fructan prebiotics: Efficacy and metabolomic analysis. Medrxiv [Preprint]. (2022). doi: 10.1101/2022.01.16.22269376
224. Chiba M, Tsuji T, Nakane K, Tsuda S, Ishii H, Ohno H, et al. Induction with infliximab and a plant-based diet as first-line (IPF) Therapy for Crohn disease: A single-group trial. Perm J. (2017) 21:17–9. doi: 10.7812/TPP/17-009
225. Chiba M, Tsuji T, Nakane K, Tsuda S, Ohno H, Sugawara K, et al. Relapse-free course in nearly half of Crohn’s disease patients with infliximab and plant-based diet as first-line therapy: A single-group trial. Perm J. (2022) 26:40–53. doi: 10.7812/TPP/21.073
226. Chiba M, Nakane K, Tsuji T, Tsuda S, Ishii H, Ohno H, et al. Relapse prevention by plant-based diet incorporated into induction therapy for ulcerative colitis: A single-group trial. Perm J. (2019) 23:18–20. doi: 10.7812/TPP/18-220
227. Chiba M, Nakane K, Tsuji T, Tsuda S, Ishii H, Ohno H, et al. Relapse prevention in ulcerative colitis by plant-based diet through educational hospitalization: A single-group trial. Perm J. (2018) 22:17–167. doi: 10.7812/TPP/17-167
228. Wellens J, Vissers E, Matthys C, Vermeire S, Sabino J. Personalized dietary regimens for inflammatory Bowel disease: Current knowledge and future perspectives. Pharmgenomics Pers Med. (2023) 16:15–27. doi: 10.2147/PGPM.S359365
229. Ordovas J, Ferguson L, Tai E, Mathers J. Personalised nutrition and health. BMJ. (2018) 361:2173. doi: 10.1136/bmj.k2173
230. Racine A, Carbonnel F, Chan S, Hart A, Bueno-de-Mesquita H, Oldenburg B, et al. Dietary patterns and risk of inflammatory bowel disease in Europe: Results from the EPIC study. Inflamm Bowel Dis. (2016) 22:345–54. doi: 10.1097/MIB.0000000000000638
231. Opstelten J, Leenders M, Dik V, Chan S, van Schaik F, Khaw K, et al. Dairy products, dietary calcium, and risk of inflammatory Bowel disease: Results from a european prospective cohort investigation. Inflamm Bowel Dis. (2016) 22:1403–11. doi: 10.1097/MIB.0000000000000798
232. Kempinski R, Arabasz D, Neubauer K. Effects of milk and dairy on the risk and course of inflammatory Bowel disease versus patients’ dietary beliefs and practices: A systematic review. Nutrients. (2024) 16:2555. doi: 10.3390/nu16152555
233. Narula N, Wong E, Dehghan M, Mente A, Rangarajan S, Lanas F, et al. Association of ultra-processed food intake with risk of inflammatory bowel disease: Prospective cohort study. BMJ. (2021) 374:n1554. doi: 10.1136/bmj.n1554
234. Chen J, Wellens J, Kalla R, Fu T, Deng M, Zhang H, et al. Intake of ultra-processed foods is associated with an increased risk of Crohn’s disease: A cross-sectional and prospective analysis of 187 154 participants in the UK Biobank. J Crohns Colitis. (2023) 17:535–52. doi: 10.1093/ecco-jcc/jjac167
235. Balestrieri P, Ribolsi M, Guarino M, Emerenziani S, Altomare A, Cicala M. Nutritional aspects in inflammatory Bowel diseases. Nutrients. (2020) 12:372. doi: 10.3390/nu12020372
236. Day A, Yao C, Costello S, Andrews J, Bryant R. Food avoidance, restrictive eating behaviour and association with quality of life in adults with inflammatory bowel disease: A systematic scoping review. Appetite. (2021) 167:105650. doi: 10.1016/j.appet.2021.105650
237. Czuber-Dochan W, Morgan M, Hughes L, Lomer M, Lindsay J, Whelan K. Perceptions and psychosocial impact of food, nutrition, eating and drinking in people with inflammatory bowel disease: A qualitative investigation of food-related quality of life. J Hum Nutr Diet. (2020) 33:115–27. doi: 10.1111/jhn.12668
238. Filippi J, Al-Jaouni R, Wiroth J, Hébuterne X, Schneider S. Nutritional deficiencies in patients with Crohn’s disease in remission. Inflamm Bowel Dis. (2006) 12:185–91. doi: 10.1097/01.MIB.0000206541.15963.c3
239. Dunleavy K, Ungaro R, Manning L, Gold S, Novak J, Colombel J. Vitamin C deficiency in inflammatory Bowel disease: The forgotten micronutrient. Crohns Colitis 360. (2021) 3:otab009. doi: 10.1093/crocol/otab009
240. Leonetti M, Kolodinsky J, Trubek A, Belarmino EH. A qualitative study of rural plant-based eaters’ knowledge and practices for nutritional adequacy. Nutrients. (2024) 16:3504. doi: 10.3390/nu16203504
241. Schüpbach R, Wegmüller R, Berguerand C, Bui M, Herter-Aeberli I. Micronutrient status and intake in omnivores, vegetarians and vegans in Switzerland. Eur J Nutr. (2017) 56:283–93. doi: 10.1007/s00394-015-1079-7
242. Gallego-Narbón A, Zapatera B, Barrios L, Vaquero M. Vitamin B12 and folate status in Spanish lacto-ovo vegetarians and vegans. J Nutr Sci. (2019) 8:e7. doi: 10.1017/jns.2019.2
243. Storz M, Müller A, Niederreiter L, Zimmermann-Klemd A, Suarez-Alvarez M, Kowarschik S, et al. A cross-sectional study of nutritional status in healthy, young, physically-active German omnivores, vegetarians and vegans reveals adequate vitamin B12 status in supplemented vegans. Ann Med. (2023) 55:2269969. doi: 10.1080/07853890.2023.2269969
244. Armstrong H, Bording-Jorgensen M, Santer D, Zhang Z, Valcheva R, Rieger A, et al. Unfermented β-fructan fibers fuel inflammation in select inflammatory Bowel disease patients. Gastroenterology. (2023) 164:228–40. doi: 10.1053/j.gastro.2022.09.034
245. Arifuzzaman M, Won T, Yano H, Uddin J, Emanuel E, Hu E, et al. Dietary fiber is a critical determinant of pathologic ILC2 responses and intestinal inflammation. J Exp Med. (2024) 221:e20232148. doi: 10.1084/jem.20232148
246. Wang H, Huang X, Xia S, Chen C, Chen X, Zhang Y, et al. Celery soluble dietary fiber antagonizes flavonoids ameliorative effect on dextran-sodium-sulfate-induced colitis in mice. J Adv Res. (2023) 52:73–88. doi: 10.1016/j.jare.2023.01.013
247. Press A, Hauptmann I, Hauptmann L, Fuchs B, Fuchs M, Ewe K, et al. Gastrointestinal pH profiles in patients with inflammatory Bowel disease. Aliment Pharmacol Ther. (1998) 12:673–8. doi: 10.1046/j.1365-2036.1998.00358.x
248. Laryushina Y, Samoilova-Bedych N, Turgunova L, Kozhakhmetov S, Alina A, Suieubayev M, et al. Alterations of the gut microbiome and TMAO levels in patients with ulcerative colitis. J Clin Med. (2024) 13:5794. doi: 10.3390/jcm13195794
249. Xu X, Ocansey D, Hang S, Wang B, Amoah S, Yi C, et al. The gut metagenomics and metabolomics signature in patients with inflammatory bowel disease. Gut Pathog. (2022) 14:26. doi: 10.1186/s13099-022-00499-9
250. Alam M, Amos G, Murphy A, Murch S, Wellington E, Arasaradnam R. Microbial imbalance in inflammatory bowel disease patients at different taxonomic levels. Gut Pathog. (2020) 12:1. doi: 10.1186/s13099-019-0341-6
251. Pisani A, Rausch P, Bang C, Ellul S, Tabone T, Marantidis Cordina C, et al. Dysbiosis in the gut microbiota in patients with inflammatory Bowel disease during remission. Microbiol Spectr. (2022) 10:e0061622. doi: 10.1128/spectrum.00616-22
252. Liu Y, Bai X, Wu H, Duan Z, Zhu C, Fu R, et al. Ginsenoside CK alleviates DSS-induced IBD in mice by regulating tryptophan metabolism and activating aryl hydrocarbon receptor via gut microbiota modulation. J Agric Food Chem. (2024) 72:9867–79. doi: 10.1021/acs.jafc.4c00245
253. Ullah H, Deng T, Ali M, Farooqui N, Alsholi D, Siddiqui N, et al. Sea conch peptides hydrolysate alleviates DSS-induced colitis in mice through immune modulation and gut microbiota restoration. Molecules. (2023) 28:6849. doi: 10.3390/molecules28196849
254. Sharma S, Bhatia R, Devi K, Rawat A, Singh S, Bhadada S, et al. A synbiotic combination of Bifidobacterium longum Bif10 and Bifidobacterium breve Bif11, isomaltooligosaccharides and finger millet arabinoxylan prevents dextran sodium sulphate induced ulcerative colitis in mice. Int J Biol Macromol. (2023) 231:123326. doi: 10.1016/j.ijbiomac.2023.123326
255. Liguori G, Lamas B, Richard M, Brandi G, da Costa G, Hoffmann T, et al. Fungal dysbiosis in mucosa-associated microbiota of Crohn’s disease patients. J Crohns Colitis. (2016) 10:296–305. doi: 10.1093/ecco-jcc/jjv209
256. Zeng L, Feng Z, Zhuo M, Wen Z, Zhu C, Tang C, et al. Fecal fungal microbiota alterations associated with clinical phenotypes in Crohn’s disease in southwest China. PeerJ. (2022) 10:e14260. doi: 10.7717/peerj.14260
257. Qiu X, Zhao X, Cui X, Mao X, Tang N, Jiao C, et al. Characterization of fungal and bacterial dysbiosis in young adult Chinese patients with Crohn’s disease. Therap Adv Gastroenterol. (2020) 13:1756284820971202. doi: 10.1177/1756284820971202
258. Scanu M, Toto F, Petito V, Masi L, Fidaleo M, Puca P, et al. An integrative multi-omic analysis defines gut microbiota, mycobiota, and metabolic fingerprints in ulcerative colitis patients. Front Cell Infect Microbiol. (2024) 14:1366192. doi: 10.3389/fcimb.2024.1366192
259. Clooney A, Sutton T, Shkoporov A, Holohan R, Daly K, O’Regan O, et al. Whole-virome analysis sheds light on viral dark matter in inflammatory Bowel disease. Cell Host Microbe. (2019) 26:764–778.e5. doi: 10.1016/j.chom.2019.10.009
260. Ungaro F, Massimino L, Furfaro F, Rimoldi V, Peyrin-Biroulet L, D’Alessio S, et al. Metagenomic analysis of intestinal mucosa revealed a specific eukaryotic gut virome signature in early-diagnosed inflammatory Bowel disease. Gut Microbes. (2019) 10:149–58. doi: 10.1080/19490976.2018.1511664
261. Tian X, Li S, Wang C, Zhang Y, Feng X, Yan Q, et al. Gut virome-wide association analysis identifies cross-population viral signatures for inflammatory bowel disease. Microbiome. (2024) 12:130. doi: 10.1186/s40168-024-01832-x
262. Liang G, Conrad M, Kelsen J, Kessler L, Breton J, Albenberg L, et al. Dynamics of the stool virome in very early-onset inflammatory Bowel disease. J Crohns Colitis. (2020) 14:1600–10. doi: 10.1093/ecco-jcc/jjaa094
Keywords: dysbiosis, gut microbiota, IBD, modulation, plant-based dietary compounds
Citation: Akanyibah FA, He C, Wang X, Wang B and Mao F (2025) The role of plant-based dietary compounds in gut microbiota modulation in inflammatory bowel disease. Front. Nutr. 12:1606289. doi: 10.3389/fnut.2025.1606289
Received: 05 April 2025; Accepted: 12 May 2025;
Published: 30 May 2025.
Edited by:
Charalampia Amerikanou, Harokopio University, GreeceReviewed by:
Yan Liu, Southwest University, ChinaFederica Rubbino, Humanitas Research Hospital, Italy
Copyright © 2025 Akanyibah, He, Wang, Wang and Mao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei Mao, bWFvZmVpMjAwM0B1anMuZWR1LmNu
†These authors have contributed equally to this work
 Francis Atim Akanyibah
Francis Atim Akanyibah Chang’e He3†
Chang’e He3† Fei Mao
Fei Mao