- 1Department of Medical Oncology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2School of Clinical Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3School of Acupuncture and Tuina, Chengdu University of Traditional Chinese Medicine, Chengdu, China
Objective: To evaluate the effects and underlying mechanisms of resveratrol—a plant-derived polyphenol abundantly found in natural dietary sources such as grapes and blueberries—on the amelioration of liver fibrosis.
Methods: Data were obtained from a systematic review of 46 animal studies identified across seven databases. Study quality was assessed using the SYRCLE tool for risk of bias. Meta-analysis was performed with Stata 17.0. Outcome measures included collagen deposition, hydroxyproline content, extracellular matrix components (HA, LN, CIV, PIIINP), key fibrogenic mediators (TGF-β, α-SMA, Col1α1), liver function markers (albumin, ALT, AST, ALP), as well as inflammatory and oxidative stress indicators.
Results: Resveratrol markedly attenuated collagen deposition and reduced hydroxyproline levels, a central marker of fibrotic progression. It significantly inhibited the accumulation of extracellular matrix components and modulated profibrotic mediators. Improvement in liver function was indicated by elevated albumin levels and decreased activities of ALT, AST, and ALP. Mechanistically, resveratrol exerted dual modulation through the following pathways: Inflammatory pathways: downregulation of IL-6 and TNF-α; Oxidative stress responses: enhancement of SOD and GSH activities, accompanied by reduction in MDA levels.
Conclusion: Resveratrol significantly alleviates liver fibrosis in animal models via anti-inflammatory and antioxidant mechanisms. However, translation to clinical practice requires further validation owing to interspecies differences and notable heterogeneity across included studies. Standardized preclinical study designs and cross-species mechanistic investigations are warranted to support future clinical applications.
Systematic review registration: The registered website: https://www.crd.york.ac.uk/PROSPERO/view/CRD42025633941.
1 Introduction
Liver fibrosis (LF) represents a pathological state characterized by excessive extracellular matrix deposition, primarily collagen, secondary to chronic hepatic injury. This process disrupts hepatic architecture, progressively impairing function and potentially advancing to cirrhosis, liver failure, or hepatocellular carcinoma (1). Affecting 2–19% of the global population (2, 3), chronic liver diseases impact approximately 1.5 billion individuals (4). LF exacerbates complications including ascites, portal hypertension, hepatic encephalopathy, liver failure, and elevates the risk of carcinogenesis, imposes substantial burdens on healthcare systems and societies (5, 6). Primary etiologies encompass chronic viral hepatitis (hepatitis B/C), alcohol-related liver damage, non-alcoholic fatty liver disease (NAFLD), and autoimmune hepatic disorders (7, 8).
The pathogenesis of LF involves a complex interplay among inflammatory responses, activation of hepatic stellate cells (HSC), and dysregulated extracellular matrix (ECM) turnover, driven primarily by key signaling pathways including transforming growth factor-β (TGF-β)/Smad and Wnt/β-catenin (9, 10). Despite advances in understanding LF’s molecular mechanisms, current therapeutic options remain limited. Antifibrotic drugs, such as pirfenidone and nintedanib, demonstrate limited antifibrotic efficacy, significant side effects, and variable effectiveness in heterogeneous disease presentations (11, 12). Liver transplantation, provides a curative approach, is constrained by donor shortages, high costs, and post-transplant complications (13). Therefore, there is an urgent need to identify novel therapeutic agents with improved safety and efficacy profiles that can prevent or reverse LF.
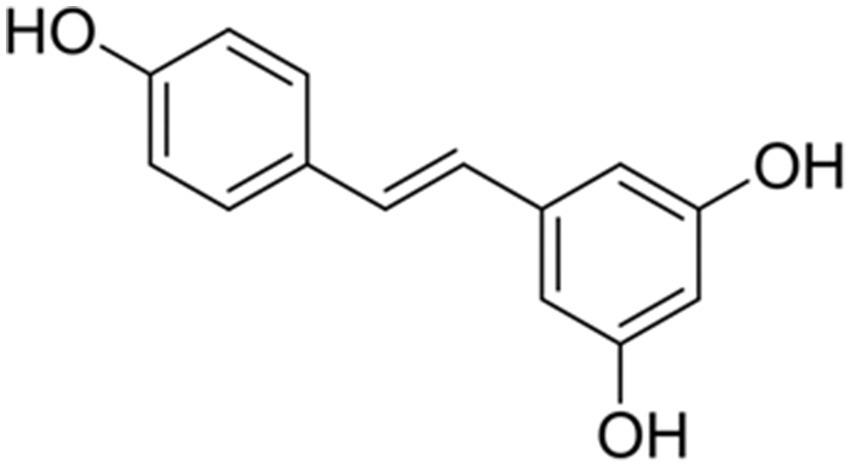
Resveratrol (3,5,4′-trihydroxystilbene, Figure 1), a natural polyphenolic compound derived from botanical sources including grapes, berries, and peanuts with particularly high concentrations in red wine (14). It has been consumed as part of the human diet for centuries and is generally recognized as safe (GRAS) by regulatory agencies, making it an attractive candidate for therapeutic applications (15). Beyond nutritional functions, resveratrol exhibits therapeutic promise for cardiovascular, metabolic, and oncological disorders via its antioxidant, anti-inflammatory, and anti-fibrotic actions (16, 17). In recent years, preclinical studies have highlighted the potential of resveratrol in attenuating LF in animal models. For instance, resveratrol has been shown to HSC activation, reduce oxidative stress, and modulate key fibrogenic pathways such as TGF-β/Smad and nuclear factor kappa-B (NF-Κb) (18, 19). Despite these promising findings, clinical translation faces significant bottlenecks. First, the bioavailability of resveratrol is extremely low (<1% following oral administration). Although nano-delivery systems (e.g., liposomes) or structural modifications (e.g., resveratrol derivatives) can enhance stability, the long-term toxicity and industrial-scale production feasibility still require verification (20, 21). Second, substantial heterogeneity exists in preclinical studies. Differences in modeling methods, dosage regimens, and efficacy evaluation criteria among various animal models compromise the comparability of results. Integrating data through meta-analysis is urgently needed to clarify the dose–response relationship. Finally, the discrepancy between animal models and human pathology limits predictive value. Existing models are primarily based on single causes, whereas human LF is often driven by the interaction of multiple factors. Interspecies differences may also overestimate resveratrol’s in vivo effects. Although anatomical and physiological differences exist between animal models and humans, animal research remains crucial for exploring the pathophysiology of human diseases.
2 Methods
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (22). The protocol was registered in the PROSPERO International Prospective Register of Systematic Reviews (registration number: CRD42025633941).
2.1 Search strategy
A comprehensive literature search was performed to identify all relevant preclinical animal studies investigating the efficacy of resveratrol in preventing LF. The following electronic databases were searched: Web of Science, Embase, PubMed, China Biology Medicine (CBM), China National Knowledge Infrastructure (CNKI), Wanfang Database (WF), and China Science Journal Database (VIP). The search was limited to studies published before December 31, 2024, to ensure the inclusion of the most recent evidence. Additionally, manual searches of reference lists from included studies and relevant reviews were conducted to identify potentially eligible studies that might have been missed in the electronic database searches. The search strategy employed a combination of Medical Subject Headings (MeSH) terms and free-text words to maximize the sensitivity and specificity of the search (Supplementary Table 1).
2.2 Eligibility criteria
The inclusion criteria for this study were defined based on the PICO framework to ensure the selection of relevant and high-quality preclinical animal studies. Animal: Studies utilizing animal models of LF were included, regardless of the species, sex, age, or weight of the animals. Both induced (e.g., chemically induced, diet-induced) and genetic models of LF were considered eligible. Intervention: Studies in which animals were treated with resveratrol, regardless of the dose, duration, frequency, or route of administration, were included. Comparison: Studies must have included a control group receiving either an equivalent vehicle, physiological saline, or no treatment. Outcomes: Studies reporting outcomes related to the protective effects of resveratrol on LF were included. Primary outcomes of interest included histopathological changes in LF (e.g., collagen deposition, degree of tissue fibrosis), LF progression markers [e.g., hydroxyproline (HYP), α-smooth muscle actin (α-SMA)], TGF-β, and inflammatory cytokine levels [e.g., interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α)]. Secondary outcomes such as liver function biomarkers (e.g., ALT, AST) and oxidative stress markers [e.g., malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione (GSH)] were also considered.
Exclude the following studies: (1) Study Type: Clinical studies, in vitro experiments, and computer simulation studies were excluded. (2) Intervention: Studies without a control group or those in which the treatment group received a combination of resveratrol and other therapeutic interventions were excluded. (3) Duplicate Publications: In cases of duplicate publications, the most recent or comprehensive study was retained, and earlier versions were excluded.
2.3 Data extraction
Data extraction was conducted independently by two researchers (QH and JK) to ensure accuracy and minimize bias. The process involved the following steps: (1) Initial Screening: Titles and abstracts of all retrieved studies were screened to exclude irrelevant publications. Studies that did not meet the inclusion criteria were removed at this stage. (2) Full-Text Review: The remaining studies were subjected to a full-text review to assess their eligibility based on the predefined inclusion and exclusion criteria. (3) Data Collection: For studies meeting the inclusion criteria, the following information was extracted: Publication Details: Authors and year of publication. Animal Characteristics: Species, sex, age, weight, and sample size. LF Model: Method used to induce LF (e.g., chemical induction, diet-induced, genetic models). Intervention Details: Resveratrol administration parameters, including dose, duration, frequency, route of administration, and control group treatment. Outcome Measures: Data on histopathological changes, LF progression markers, inflammatory cytokine levels, liver function biomarkers, and oxidative stress markers. If outcome data were presented only in graphical form, attempts were made to contact the corresponding authors to obtain raw data. If raw data were unavailable, graphical data were digitized using WebPlotDigitizer 4.5,1 a validated tool for extracting numerical data from graphs. For studies reporting multiple data points due to varying doses or time points, data from the group receiving the maximum effective dose or the latest effective time point were extracted for meta-analysis. Any disagreements between the two researchers during data extraction were resolved through discussion and, if necessary, consultation with a third researcher (SX) to reach a consensus.
2.4 Quality assessment
The methodological quality and risk of bias of the included studies were independently assessed by two reviewers (ZS and QX) using the Systematic Review Center for Laboratory Animal Experimentation (SYRCLE) risk of bias tool (23). This tool is specifically designed to evaluate the risk of bias in animal studies and includes the following domains: sequence generation, baseline characteristics, allocation concealment, random housing, blinding of experimentalists, random outcome assessment, blinding of outcome assessors, incomplete outcome data, selective outcome reporting, other sources of bias. Each domain was assessed and categorized as “yes” (low risk of bias), “no” (high risk of bias), or “unclear” (uncertain risk of bias) based on the information provided in the studies. Discrepancies between the two reviewers were resolved through discussion, and if consensus could not be reached, a third reviewer (SX) was consulted to make the final decision.
2.5 Statistical analysis
Statistical analyses were performed using STATA software (version 17.0). For continuous outcome measures, the overall effect size was expressed as the standardized mean difference (SMD) with 95% confidence intervals (CIs). A p-value of < 0.05 was considered statistically significant. Heterogeneity among studies was assessed using the I2 statistic, which quantifies the proportion of total variability in effect estimates attributable to heterogeneity rather than chance. The following thresholds were used to interpret the I2 values: I2 ≤ 50%: Low to moderate heterogeneity, indicating that a fixed-effects model was appropriate for meta-analysis. I2 > 50%: Substantial heterogeneity, prompting further investigation through sensitivity analysis and subgroup analysis to identify potential sources of heterogeneity. If significant heterogeneity persisted and could not be resolved, a random-effects model was applied to account for between-study variability. Sensitivity analysis was conducted by sequentially excluding individual studies to evaluate their impact on the overall effect size and heterogeneity. Subgroup analyses were performed based on predefined factors, such as animal species, LF induction method, resveratrol dosage, and treatment duration, to explore potential sources of heterogeneity. Publication bias was assessed using Egger’s linear regression test and Begg’s rank correlation test. If evidence of publication bias was detected (p < 0.05), the trim-and-fill method was employed to adjust for potential bias and estimate the corrected effect size.
3 Results
3.1 Study selection
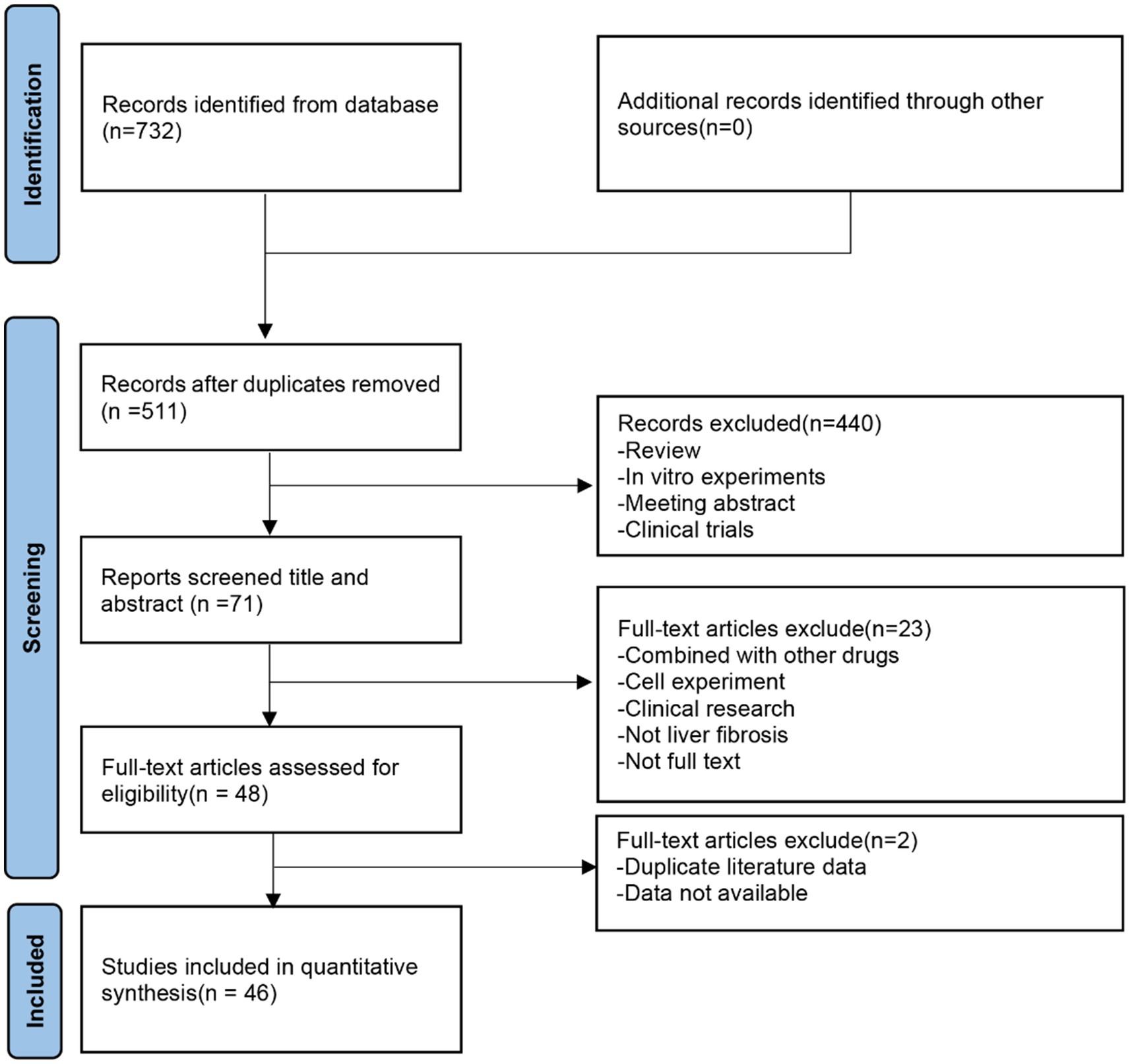
A total of 732 potentially relevant articles were retrieved from seven online databases, including PubMed (24), Embase (295), Web of Science (233), CNKI (25), CBM (26), Wanfang (27), and VIP (19). After removing duplicates, 511 articles remained. Subsequently, 440 articles were excluded based on the screening of titles and abstracts. Following a full-text review, an additional 25 articles were excluded, resulting in the final inclusion of 46 studies. The flow diagram of the study selection process is presented in Figure 2.
3.2 Characteristics of included studies
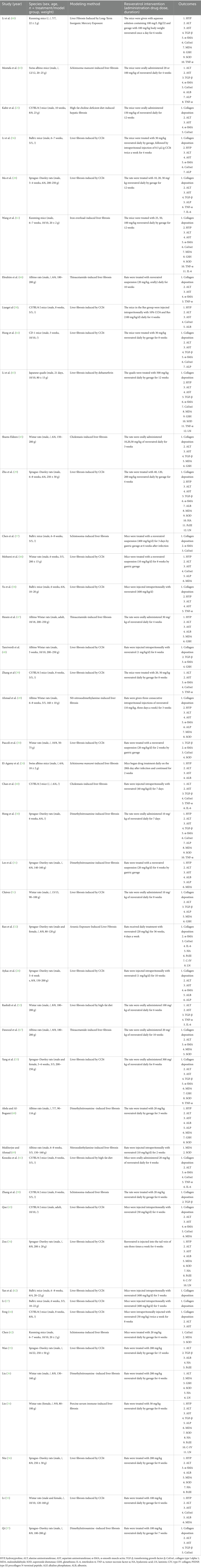
The 46 included studies involved a total of 751 animals, with 375 in the treatment groups and 376 in the control groups. Among these, 11 studies used 172 Sprague–Dawley (SD) rats (26, 28–37), 8 studies used 124 C57BL/6 J mice (25, 38–44), 11 studies used 229 Wistar rats (45–55), 5 studies used 46 Balb/c mice (27, 56–59), 3 study used 40 Kunming mice (60–62), 2 studies used 32 Swiss mice (24, 63), 1 study used CD-1 mice (64), and 1 study used 20 Japanese quails (65). Additionally, 4 studies involved 86 rats of unspecified strains (66–69). Regarding the sex of the animals, 39 studies used male animals, 1 study used female animals, 4 studies used both male and female animals, and 2 studies did not specify the sex. The age of the animals was reported in 26 studies, and the weight was described in 36 studies. In terms of resveratrol administration, 34 studies utilized oral gavage or intragastric administration, 11 studies employed intraperitoneal injection, and 1 study used tail vein injection. For LF assessment, 24 studies reported histopathological changes in LF, 18 studies measured TGF-β levels, 21 studies evaluated α-SMA expression, 19 studies assessed type I collagen, 18 studies measured HYP, 6 studies reported hyaluronic acid (HA), 5 studies evaluated type III procollagen N-terminal propeptide (PIINP), 3 studies measured collagen type IV (COL-IV), and 6 studies reported laminin (LN). Regarding liver function, 37 studies reported ALT levels, 31 studies reported AST levels, 11 studies measured albumin (ALB), and 12 studies evaluated alkaline phosphatase (ALP). For oxidative stress markers, 21 studies reported MDA levels, 10 studies measured GSH, and 15 studies evaluated SOD. In terms of inflammatory cytokine levels, 13 studies reported TNF-α, 6 studies measured IL-6, 5 studies assessed IL-1β, and 6 studies evaluated NF-κB. Detailed characteristics of the included studies are presented in Table 1.
3.3 Study quality
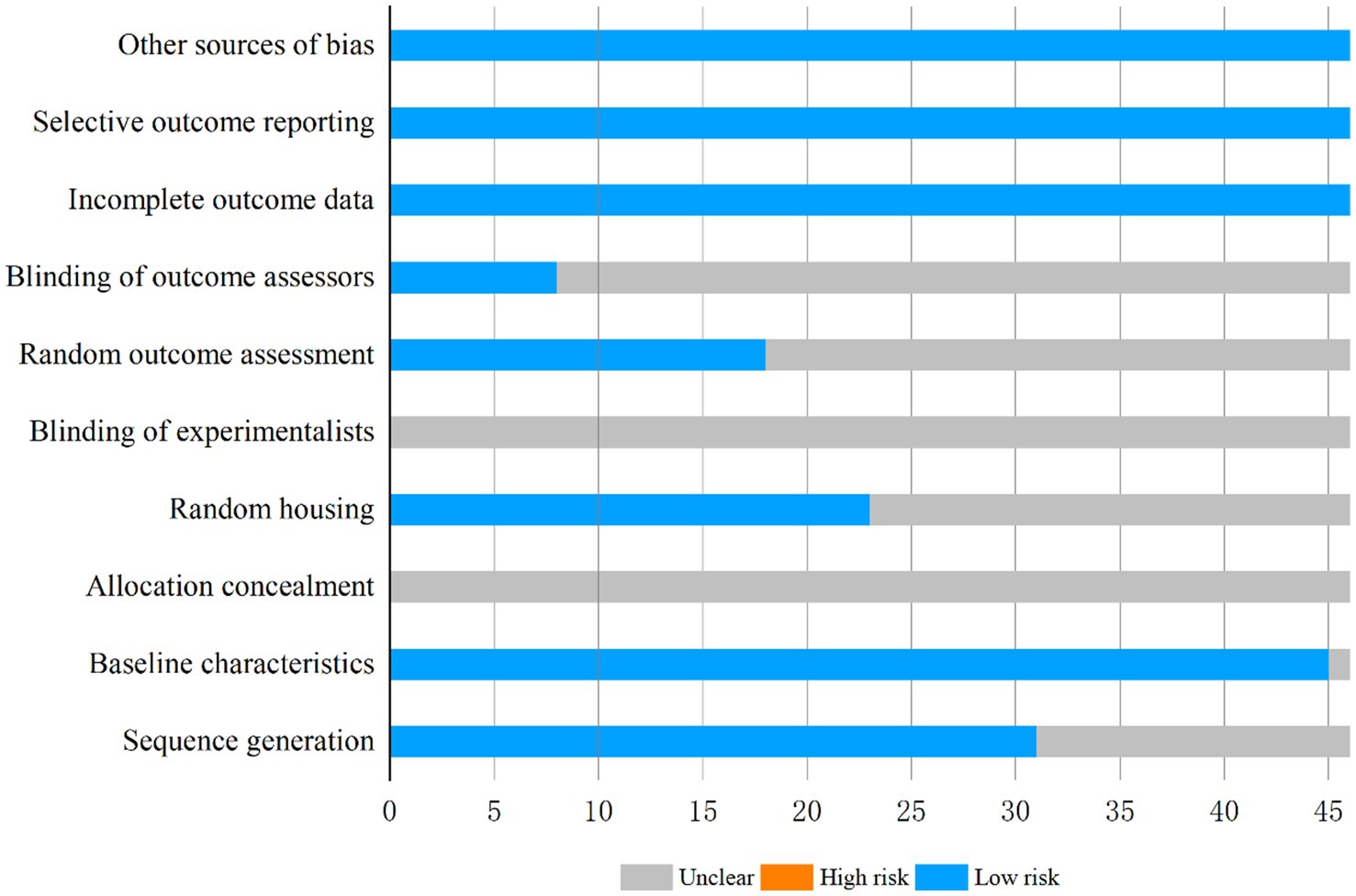
Quality assessment of the 46 included studies using the SYRCLE risk-of-bias tool revealed the following score distribution: 4 studies scored 4, 7, and 8 points each, with 14 and 17 studies attaining 5 and 6 points, respectively. Methodological quality analysis (Figure 3) demonstrated that baseline characteristics of experimental subjects were documented in 45 studies, among which 31 explicitly implemented animal randomization, 23 reported randomized housing, and 18 utilized randomized outcome assessment. Notably, while 8 studies declared blinded outcome evaluation, none provided specific details regarding allocation concealment or operator blinding. All included studies met low-risk criteria in three critical domains: data completeness, avoidance of selective reporting, and control of additional biases. Comprehensive evaluation data are compiled in Supplementary Table 2.
3.4 Effectiveness
3.4.1 Primary outcomes
3.4.1.1 The condition of liver fibrosis
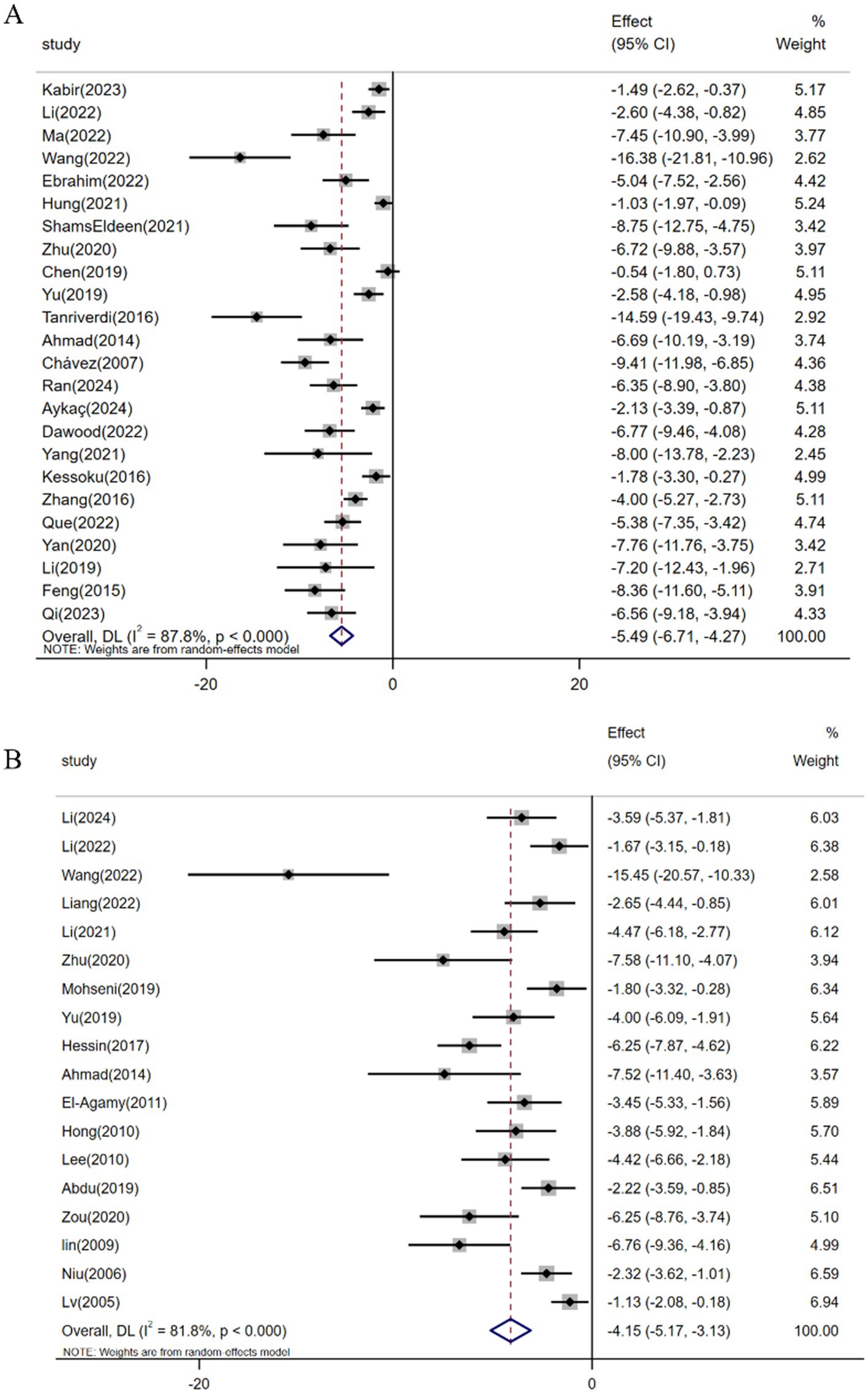
Among the 37 studies evaluating resveratrol’s antifibrotic effects, a meta-analysis of 24 investigations (n = 358) demonstrated a significant reduction in hepatic collagen deposition under pathological conditions [SMD: -5.49 (95% CI: −6.71, −4.27), p < 0.001; heterogeneity: I2 = 87.8%, p < 0.001; Figure 4A]. Hyp, a non-essential amino acid serving as a collagen-specific biomarker, reflects hepatic collagen synthesis status (70). Pooled analysis of 18 studies (n = 273) revealed resveratrol’s efficacy in reducing Hyp levels and ameliorating fibrotic progression in animal models [SMD: -4.15 (95% CI: −5.17, −3.13), p < 0.001; heterogeneity: I2 = 81.8%, p < 0.001; Figure 4B].
3.4.2 Secondary outcomes
3.4.2.1 Liver fibrosis-related biomarkers
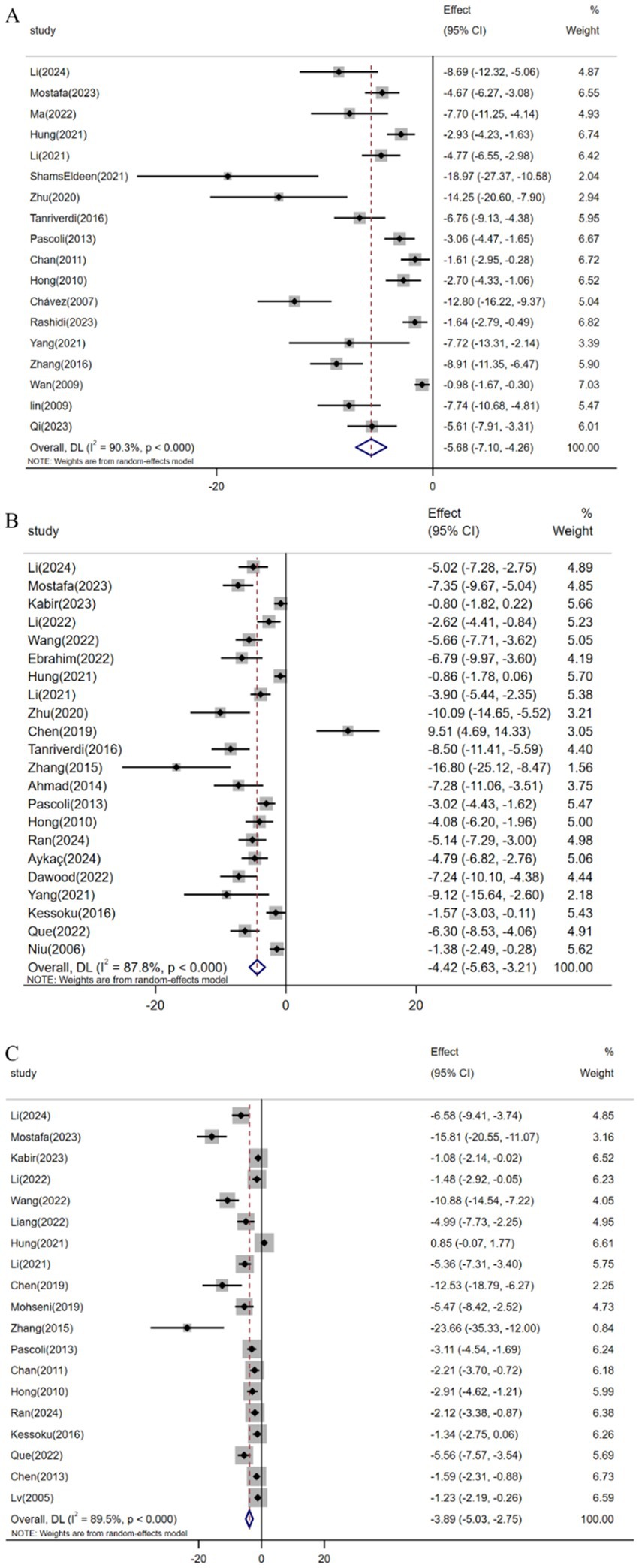
Pooled analysis of 18 studies (n = 329) demonstrated resveratrol significantly suppressed TGF-β expression versus controls [SMD: -5.68 (95% CI: −7.10, −4.26), p < 0.001; I2 = 90.3%, p < 0.001; Figure 5A]. Similarly, meta-analysis of 22 studies (n = 328) revealed reduced α-SMA levels in resveratrol-treated groups [SMD: -4.42 (95% CI: −5.63, −3.21), p < 0.001; I2 = 87.8%, p < 0.001; Figure 5B]. Furthermore, analysis of 19 trials (n = 312) confirmed attenuated Col1α1 expression following resveratrol intervention [SMD: -3.89 (95% CI: −5.03, −2.75), p < 0.001; I2 = 89.5%, p < 0.001; Figure 5C].
3.4.2.2 Liver function
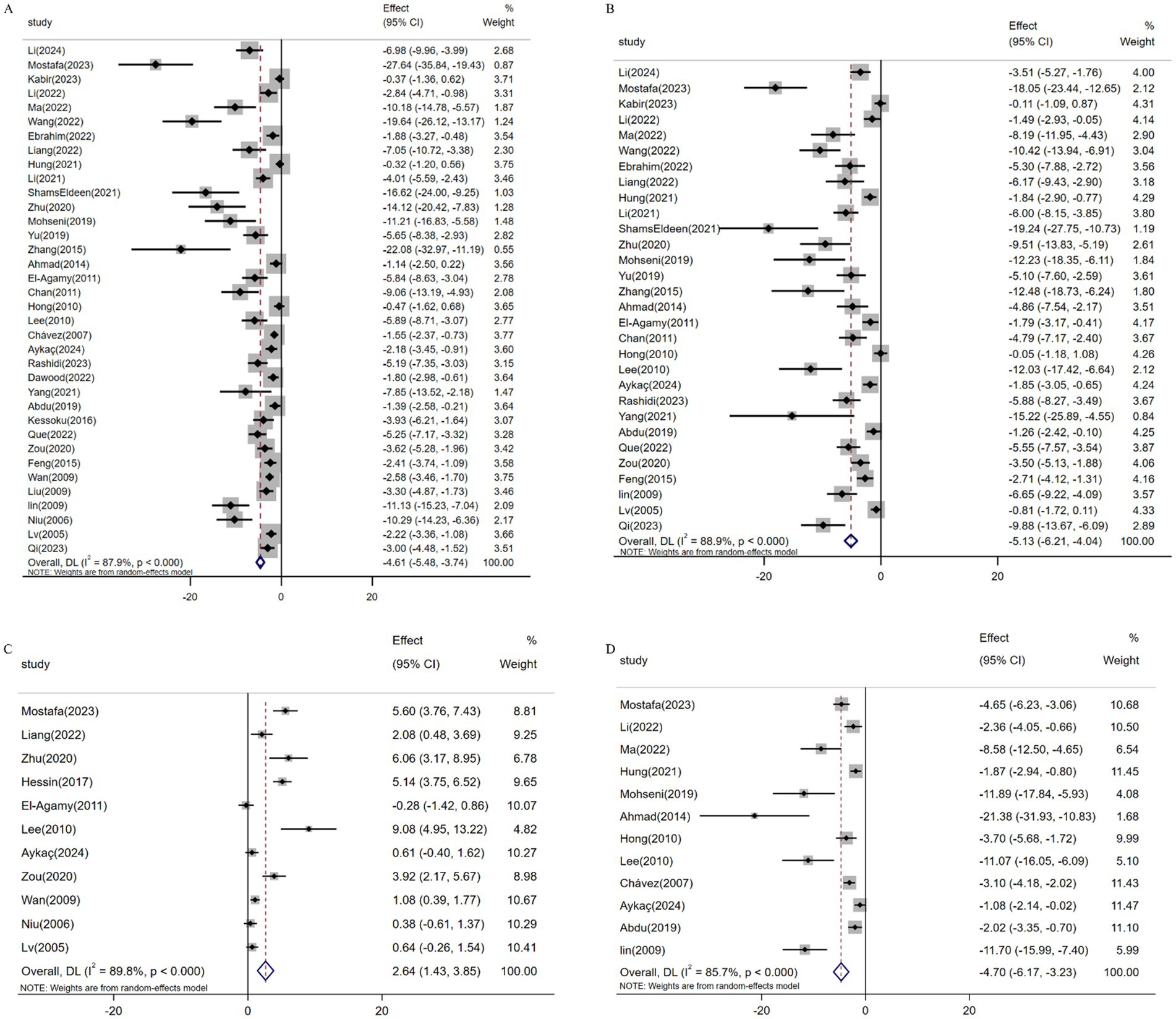
Meta-analysis of 36 studies (n = 555) demonstrated resveratrol significantly reduced ALT levels versus controls [SMD: -4.61 (95% CI: −5.48, −3.74), p < 0.001; I2 = 87.9%; Figure 6A]. Similarly, pooled data from 30 studies (n = 429) revealed suppressed AST expression [SMD: -5.13 (95% CI: −6.21, −4.04; Figure 6B), p < 0.001; I2 = 88.9%]. Conversely, analysis of 11 studies (n = 212) confirmed elevated ALB levels following resveratrol treatment [SMD: 2.64 (95% CI: 1.43, 3.85), p < 0.001; I2 = 89.8%; Figure 6C]. Additionally, 12 studies (n = 187) showed reduced ALP activity [SMD: -4.70 (95% CI: −6.17, −3.23), p < 0.001; I2 = 85.7%; Figure 6D].
3.4.2.3 Oxidative stress
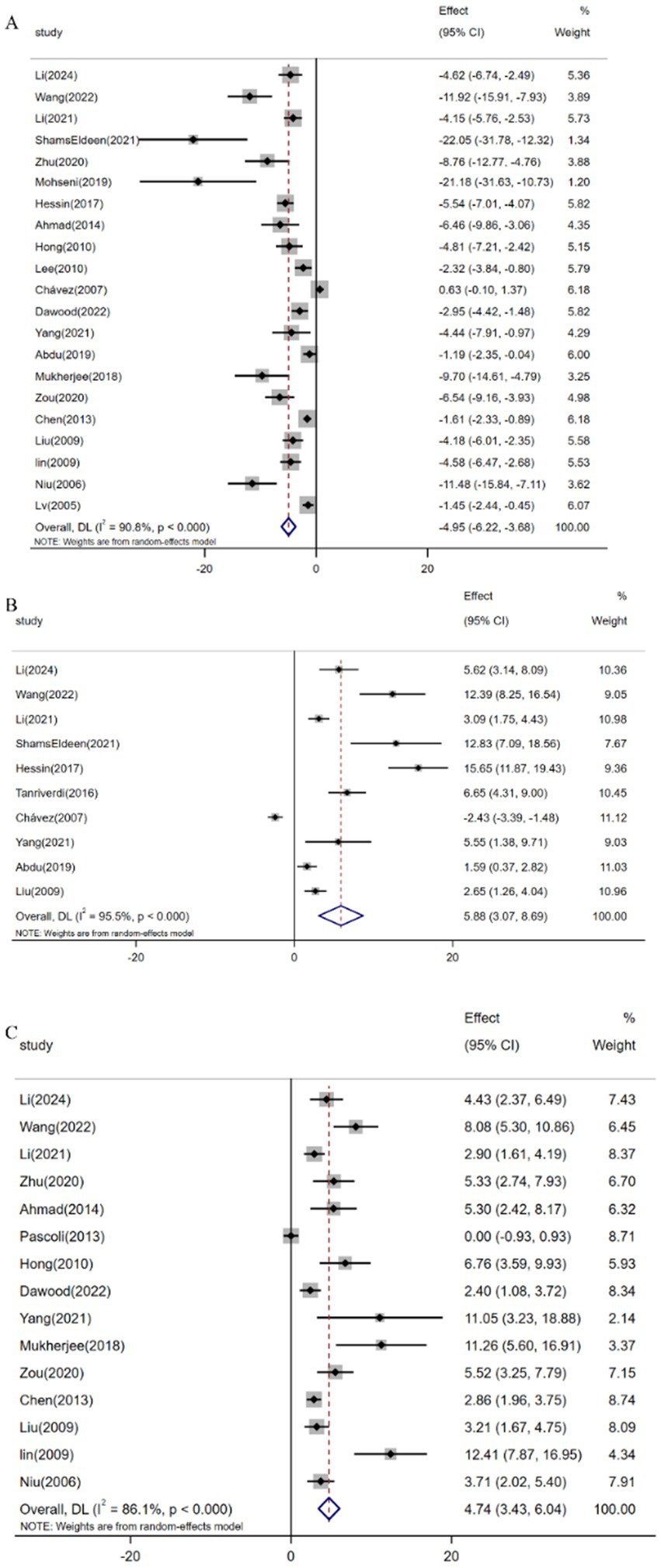
Pooled analysis of 21 studies (n = 359) demonstrated resveratrol significantly reduced MDA levels versus controls [SMD: -4.95 (95% CI: −6.22, −3.68), p < 0.001; I2 = 90.8%; Figure 7A]. Similarly, meta-analysis of 10 studies (n = 188) revealed elevated GSH expression following resveratrol intervention [SMD: 5.88 (95% CI: 3.07, 8.69), p < 0.001; I2 = 95.5%; Figure 7B]. Furthermore, analysis of 15 trials (n = 243) confirmed increased SOD activity [SMD: 4.74 (95% CI: 3.43, 6.04), p < 0.001; I2 = 86.1%; Figure 7C].
3.4.2.4 Inflammation
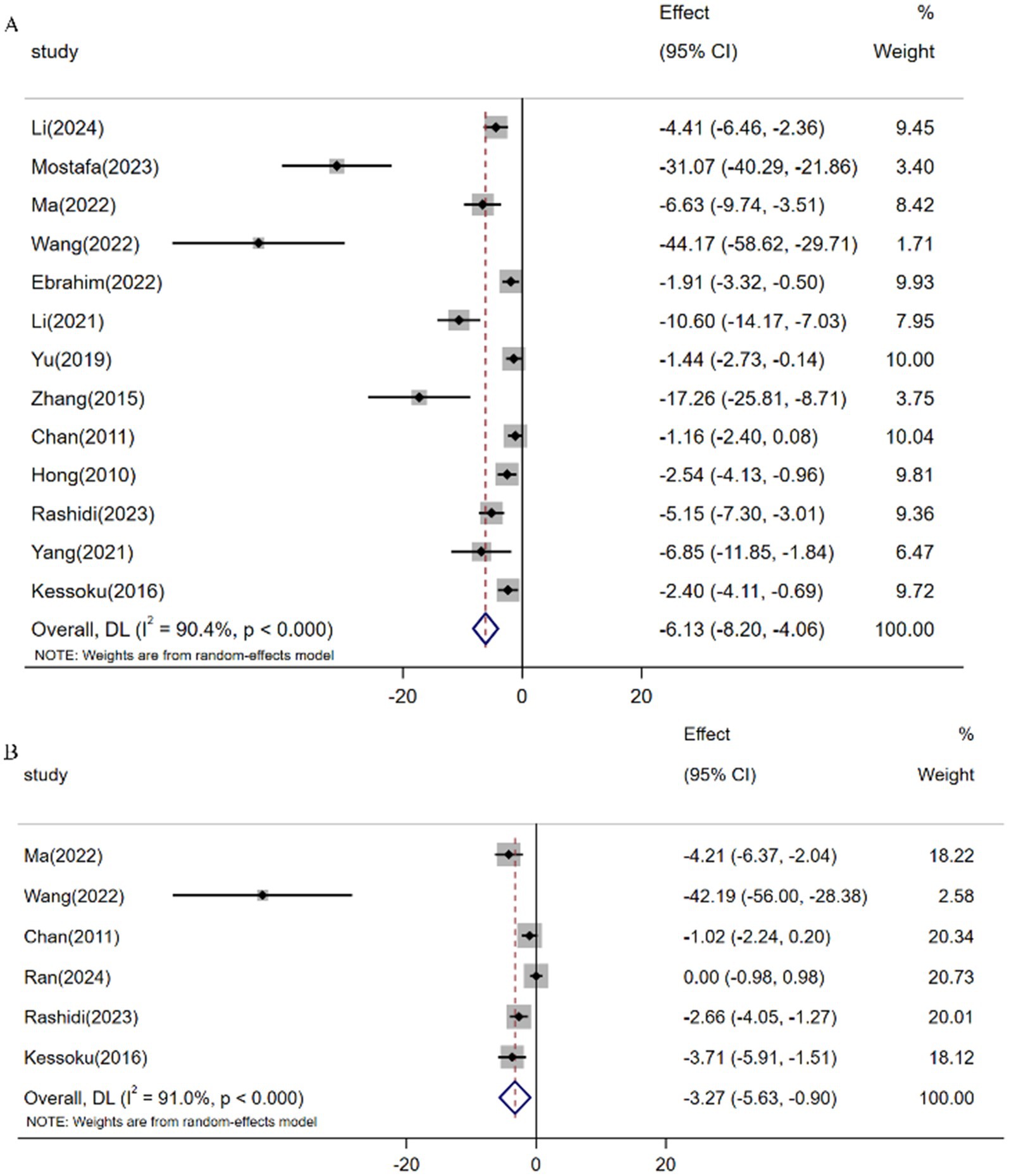
Meta-analysis of 13 studies (n = 180) demonstrated resveratrol significantly reduced TNF-α levels versus controls [SMD: -6.13 (95% CI: −8.20, −4.07), p < 0.001; I2 = 90.4%; Figure 8A]. Similarly, pooled analysis of 6 trials (n = 86) revealed attenuated IL-6 expression following resveratrol intervention [SMD: -3.27 (95% CI: −5.63, −0.90), p < 0.001; I2 = 91.0%; Figure 8B].
3.4.2.5 Extracellular matrix
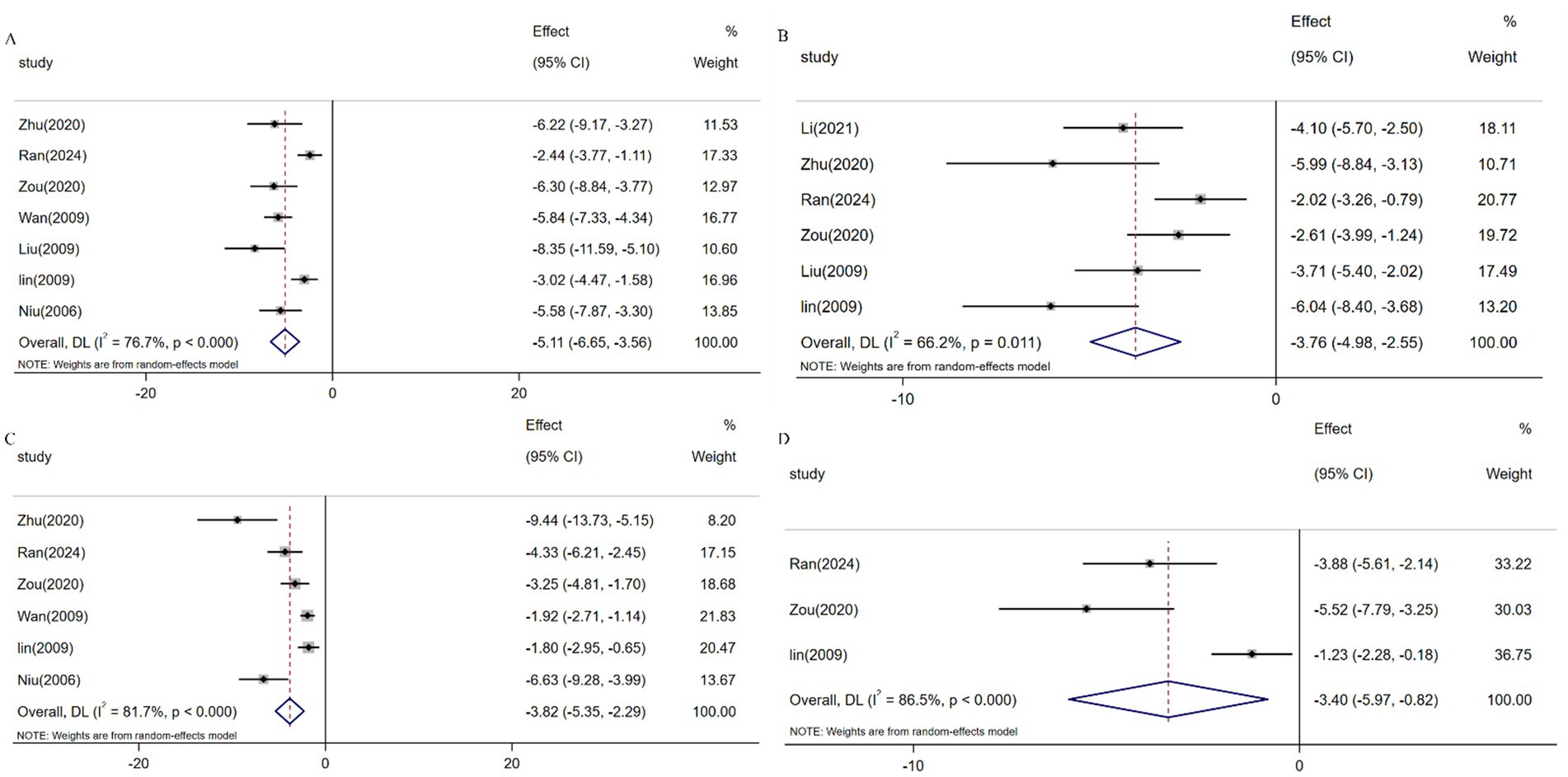
Serum biomarkers including HA, LN, PIINP, and COL-IV serve as critical indicators for assessing hepatic inflammatory activity and fibrotic progression, with elevated levels correlating with ECM deposition and hepatocyte injury (19782946). Meta-analysis of 7 studies (n = 131) demonstrated resveratrol significantly reduced HA expression versus controls [SMD: -5.11 (95% CI: −6.65, −3.56), p < 0.001; I2 = 76.7%; Figure 9A]. Similarly, pooled analysis of 6 trials (n = 97) revealed attenuated LN levels [SMD: -3.77 (95% CI: −4.98, −2.55), p < 0.001; I2 = 66.2%; Figure 9B], while 6 studies (n = 115) showed suppressed PIINP expression [SMD: -3.82 (95% CI: −5.35, −2.29), p < 0.001; I2 = 81.7%; Figure 9C]. Additionally, 3 studies (n = 49) confirmed reduced COL-IV levels following resveratrol intervention [SMD: -3.40 (95% CI: −5.97, −0.82), p < 0.001; I2 = 86.5%; Figure 9D].
3.5 Sensitivity analysis
Sensitivity analysis excluded through sequential research indicates that despite high heterogeneity, the estimated effects of all outcome measures were stable. The exclusion of Chávez (51) and Hung et al. (64) yielded marginal variations in collagen deposition effect sizes (minimum: −3.04, 95% CI: −3.43 to −2.65; maximum: −3.64, 95% CI: −4.07 to −3.21). Similarly, removing Hessin et al. (47) and Lv (2005) revealed comparable HYP effect size ranges (minimum: −2.93, 95% CI: −3.36 to −2.51; maximum: −3.61, 95% CI: −4.07 to −3.16). The results of secondary outcomes still have robustness (Supplementary Table 4).
3.6 Subgroup analysis
To address substantial between-study heterogeneity, stratified subgroup analyses of collagen deposition and HYP levels were performed across four covariates: disease induction methods, animal species, administration routes, and dosing regimens. The analysis revealed disease modeling approaches, animal species selection, and treatment duration as key contributors to collagen deposition heterogeneity. For HYP variability, primary sources included disease induction protocols, administration methods, and dosage parameters. Comprehensive stratification data are presented in the attached Supplementary Table 3.
3.7 Publication bias
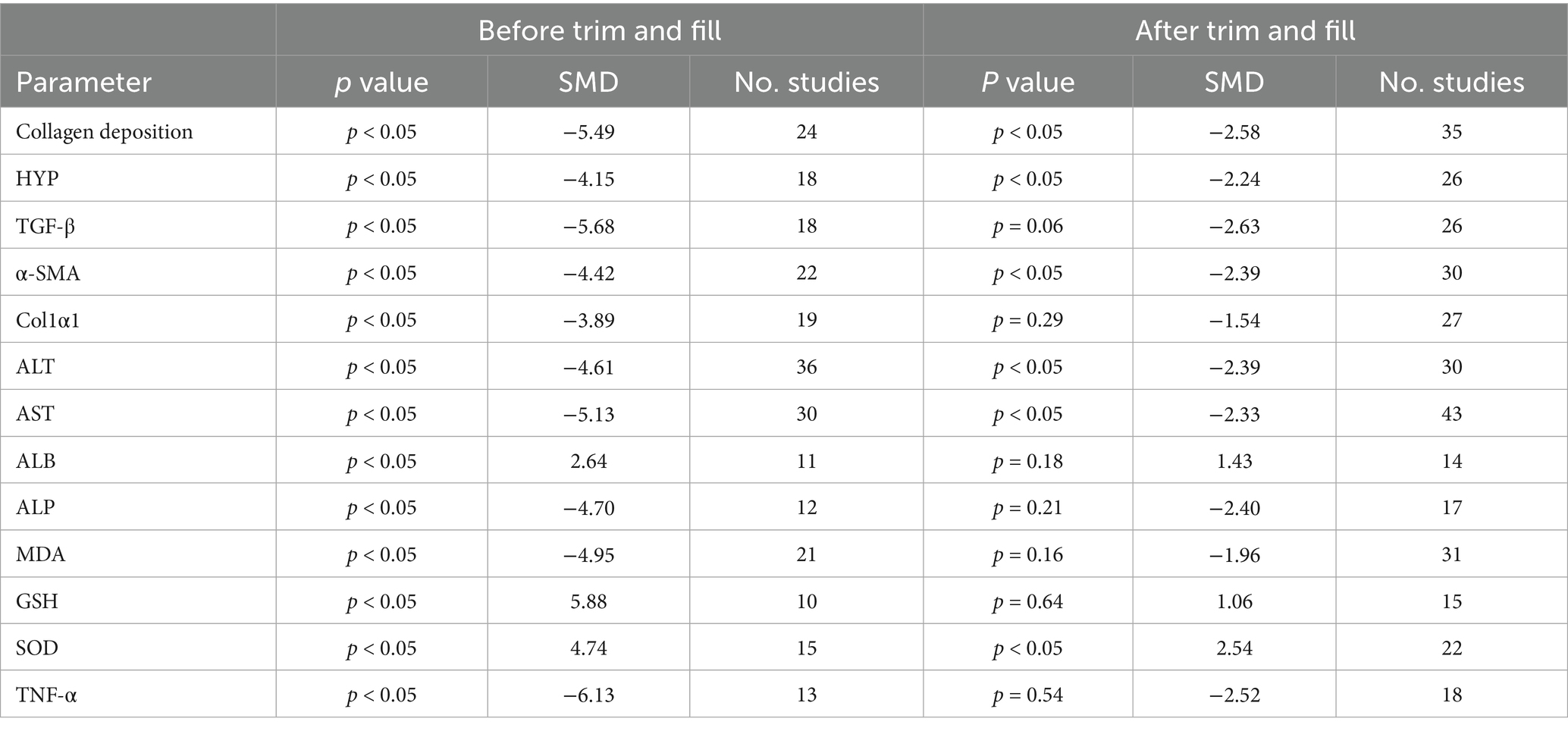
In the presence of sufficient data, Egger and Begg were used to assess publication bias, and the results showed that all outcome measures included in the analysis were at risk of publication bias (Supplementary Figure 1). To address potential missing research effects, sensitivity analysis conducted through the pruning and filling methods showed that unpublished data had no significant impact on the combined effect estimation of degree of liver fibrosis, HYP, α-SMA, ALT, AST, and SOD, and the research results were robust (Table 2; Figure 10). However, unpublished data had a significant impact on the combined effect estimation of TGF -β, Col1α1, ALB, ALP, MDA, GSH, and TNF-α, and the results obtained from the study were not robust.
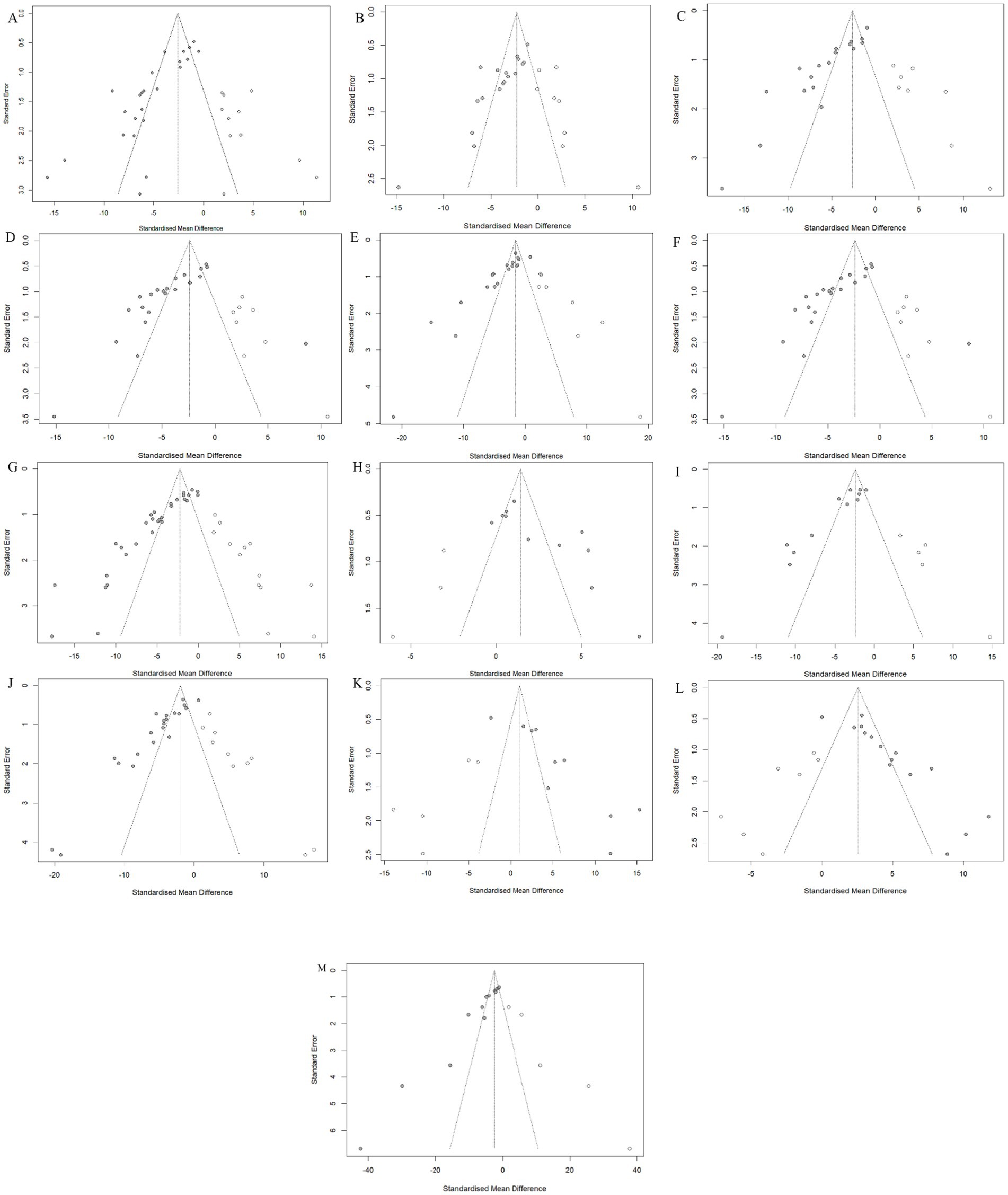
Figure 10. Trim-and-fill analysis for (A) Hepatic collagen deposition, (B) HYP, (C) TGF-β, (D) α-SMA, (E) Col1α1, (F) ALT, (G) AST, (H) ALB, (I) ALP, (J) MDA, (K) GSH, (L) SOD and (M) TNF-α.
4 Discussion
4.1 Effectiveness and evidence summary
This meta-analysis synthesizing 46 preclinical studies demonstrates resveratrol’s therapeutic potential against hepatic fibrosis by attenuating collagen deposition and HYP accumulation, suppressing fibrogenic markers (TGF-β, α-SMA, Col1α1), and modulating ECM components (HA, LN, PIIINP, COL-IV). Hepatoprotective effects were evidenced by reduced ALT/AST/ALP levels and elevated albumin expression. Notwithstanding substantial heterogeneity observed in primary outcomes (collagen deposition: I2 = 87.8%; HYP: I2 = 81.8%), sensitivity analyses confirmed result stability. Stratified subgroup analyses identified disease induction methods, animal species, administration routes, and dosage regimens as key heterogeneity contributors. Potential publication bias was identified in all results, the robustness of secondary outcome measures decreased after adjusting for pruning and padding, indicating that the bias may have been exaggerated.
4.2 Potential mechanism
The activation of HSC constitutes the central pathological mechanism underlying LF (71, 72). Activated HSCs generate excessive reactive oxygen species (ROS) (73), inducing persistent oxidative stress that compromises cellular membrane integrity and organelle architecture, ultimately driving hepatocyte injury, necrosis, and apoptosis (74). This cyclical process perpetuates HSC activation and fibrotic progression. Mechanistically, MDA, a terminal lipid peroxidation byproduct, serves as a dual biomarker of oxidative damage severity and hepatocyte injury (75, 76). SOD, the primary oxygen radical scavenger, becomes depleted under oxidative assault, while its restoration inhibits MDA-mediated free radical generation (77). GSH depletion exacerbates mitochondrial ROS leakage, triggering apoptotic cascades and profibrotic factor release (78). Resveratrol shows anti-fibrotic potential in preclinical studies via MDA reduction and enhanced SOD/GSH defenses, indicating oxidative stress mitigation.
Fibrogenesis critically depends on inflammatory dysregulation, where early repair-to-pathology transition follows pro−/anti-inflammatory imbalance (79). Chronic inflammation, whether driven by steatosis-induced lipotoxicity or ROS-mediated oxidative stress (80), activates proapoptotic pathways and inflammatory cascades, perpetuating HSC activation via phagocytosis of cellular debris (81–83). Interleukin is a key link in immune regulation. Among them, IL-6 plays a role in promoting fibrosis, and IL-22, IL-24 exerts anti-fibrotic effects (10). TNF-α can regulate the expression of matrix metalloproteinase (MMP) -9, thereby promoting LF (84). Oxidative stress can also activate NF-κB, promote the release of inflammatory factors such as TNF-α and IL-6, exacerbate inflammatory reactions, and lead to fibrosis (85). In addition, inflammation can induce epithelial-mesenchymal transition in liver cells which was associated with reduced TGF-β expression. TGF-β is the initial signal for activation and transformation of quiescent HSC and plays an important role in the occurrence and development of LF (86–88). Studies have shown that inhibiting the TGF-β/SMAD signaling pathway can alleviate the progression of LF (89). Resveratrol can downregulate the expression of TNF-α, IL-6, and TGF-β. Therefore, we speculate that resveratrol may delay the progression of LF by inhibiting inflammatory responses and the TGF-β/SMAD signaling pathway.
4.3 Heterogeneity of methodology and animal models
The substantial heterogeneity observed in this study primarily stems from differences in experimental design and limitations of animal models. Chemically induced models (e.g., CCl₄/TAA) induce rapid fibrosis through acute liver injury, whereas metabolic models (high-fat diet/choline deficiency) replicate the progressive mechanism of NAFLD. Consequently, resveratrol’s inhibitory effect on TGF-β is markedly weaker in metabolic models compared to chemical models. Meanwhile, the essential differences between the CYP1B1 metabolism of resveratrol in rodents and the human CYP1A2 pathway, as well as the lack of core comorbidity features of human liver fibrosis in existing models (such as insulin resistance and gut microbiota disorders), further weaken the clinical extrapolation of the results.
4.4 Clinical translational disorders
The 46 preclinical studies included in this meta-analysis lacked dose-ranging toxicity evaluations and standardized documentation of organ-specific adverse reactions (e.g., weight loss, multi-organ injury, or abnormal mortality). Although doses varied significantly (10–500 mg/kg), only 17.4% (8/46) monitored biochemical parameters beyond baseline liver function (ALT/AST), and none provided histopathological assessment of extrahepatic organs (e.g., kidneys, heart). This precludes assessment of whether resveratrol’s known pharmacological risks (e.g., CYP450 enzyme inhibition or estrogen receptor modulation) manifest at anti-fibrotic doses. Furthermore, the longest-duration study (36 weeks) evaluated efficacy endpoints exclusively, neglecting chronic exposure cumulative toxicity assessment. Consequently, neither the No Observed Adverse Effect Level (NOAEL) nor the safety margin required for regulatory dose translation could be established.
4.5 Optimization path for future research
Future research must mandate tiered dosing designs (encompassing 20–50 mg/kg human-equivalent and 2–5 therapeutic doses), pathological screening of core organs (liver/kidney/heart), and dynamic CYP450 activity monitoring to address critical gaps in systematic safety reporting. In the preclinical phase, humanized liver models (e.g., FRG mice) or organoid co-culture systems should integrate metabolic-inflammatory interactions, alongside establishing multi-etiological sequential injury models (e.g., HCV infection combined with high-fat diet). In addition, future research on the mechanism of action can focus on microRNAs to comprehensively elucidate the role of resveratrol in preventing and treating LF. Early clinical trials should prioritize liposomal formulations like ResVida®, conducting Phase I maximum tolerated dose studies focused on CYP450/hormonal disturbances while quantifying target engagement (e.g., p-Smad2/3 inhibition rates) via Phase IIa liver biopsies. Ultimately, a precision treatment framework should be developed using validated biomarkers (plasma miR-29a, CK-18) for cohort stratification, coupled with antioxidant-synergistic combination regimens such as obeticholic acid or selonsertib.
5 Conclusion
Preclinical evidence demonstrates resveratrol’s capacity to attenuate hepatic fibrogenesis and restore hepatic functional markers in animal models. Mechanistically, the observed therapeutic effects coincide with concurrent improvements in both inflammatory markers and oxidative stress parameters. Despite resveratrol’s anti-fibrotic potential in preclinical studies, clinical validation remains essential for therapeutic translation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
DL: Visualization, Writing – original draft, Resources, Methodology, Investigation, Conceptualization, Validation, Writing – review & editing. ZS: Resources, Methodology, Writing – original draft, Visualization, Writing – review & editing, Investigation, Conceptualization, Validation. QH: Writing – original draft, Methodology, Investigation, Validation. JK: Validation, Writing – original draft, Methodology, Investigation. QX: Validation, Methodology, Writing – original draft, Investigation. SD: Investigation, Methodology, Validation, Writing – review & editing. SS: Validation, Writing – review & editing, Supervision, Formal analysis, Software, Methodology, Data curation, Project administration, Visualization. SX: Writing – original draft, Conceptualization, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1606603/full#supplementary-material
Footnotes
References
1. Friedman, SL. Liver fibrosis -- from bench to bedside. J Hepatol. (2003) 38:S38–53. doi: 10.1016/s0168-8278(02)00429-4
2. He, Z, Yang, D, Fan, X, Zhang, M, Li, Y, Gu, X, et al. The roles and mechanisms of lnc RNAs in liver fibrosis. Int J Mol Sci. (2020) 21:1482. doi: 10.3390/ijms21041482
3. Huang, DQ, Mathurin, P, Cortez-Pinto, H, and Loomba, R. Global epidemiology of alcohol-associated cirrhosis and HCC: trends, projections and risk factors. Nat Rev Gastroenterol Hepatol. (2023) 20:37–49. doi: 10.1038/s41575-022-00688-6
4. Asrani, SK, Devarbhavi, H, Eaton, J, and Kamath, PS. Burden of liver diseases in the world. J Hepatol. (2019) 70:151–71. doi: 10.1016/j.jhep.2018.09.014
5. Xie, R, Xiao, M, Li, L, Ma, N, Liu, M, Huang, X, et al. Association between SII and hepatic steatosis and liver fibrosis: a population-based study. Front Immunol. (2022) 13:925690. doi: 10.3389/fimmu.2022.925690
6. Atta, HM. Reversibility and heritability of liver fibrosis: implications for research and therapy. World J Gastroenterol. (2015) 21:5138–48. doi: 10.3748/wjg.v21.i17.5138
7. Bataller, R, and Brenner, DA. Liver fibrosis. J Clin Invest. (2005) 115:209–18. doi: 10.1172/JCI24282
8. Lee, YA, Wallace, MC, and Friedman, SL. Pathobiology of liver fibrosis: a translational success story. Gut. (2015) 64:830–41. doi: 10.1136/gutjnl-2014-306842
9. Higashi, T, Friedman, SL, and Hoshida, Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev. (2017) 121:27–42. doi: 10.1016/j.addr.2017.05.007
10. Kisseleva, T, and Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat Rev Gastroenterol Hepatol. (2021) 18:151–66. doi: 10.1038/s41575-020-00372-7
11. Roehlen, N, Crouchet, E, and Baumert, TF. Liver fibrosis: mechanistic concepts and therapeutic perspectives. Cells. (2020) 9:875. doi: 10.3390/cells9040875
12. Tsochatzis, EA, Bosch, J, and Burroughs, AK. Liver cirrhosis. Lancet. (2014) 383:1749–61. doi: 10.1016/S0140-6736(14)60121-5
13. Kwong, AJ, Kim, WR, Lake, JR, Schladt, DP, Schnellinger, EM, Gauntt, K, et al. OPTN/SRTR 2022 annual data report: liver. Am J Transplant. (2024) 24:S176–265. doi: 10.1016/j.ajt.2024.01.014
14. Baur, JA, and Sinclair, DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. (2006) 5:493–506. doi: 10.1038/nrd2060
15. Smoliga, JM, Baur, JA, and Hausenblas, HA. Resveratrol and health--a comprehensive review of human clinical trials. Mol Nutr Food Res. (2011) 55:1129–41. doi: 10.1002/mnfr.201100143
16. Szkudelski, T, and Szkudelska, K. Resveratrol and diabetes: from animal to human studies. Biochim Biophys Acta. (2015) 1852:1145–54. doi: 10.1016/j.bbadis.2014.10.013
17. Chen, Q, Wang, T, Li, J, Wang, S, Qiu, F, Yu, H, et al. Effects of natural products on fructose-induced nonalcoholic fatty liver disease (NAFLD). Nutrients. (2017) 9:96. doi: 10.3390/nu9020096
18. Faghihzadeh, F, Adibi, P, Rafiei, R, and Hekmatdoost, A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr Res. (2014) 34:837–43. doi: 10.1016/j.nutres.2014.09.005
19. Chang, C, Chang, C, Lin, P, Chang, CC, Chang, CY, Lin, PC, et al. Administration of low-dose resveratrol attenuated hepatic inflammation and lipid accumulation in high cholesterol-fructose diet-induced rat model of nonalcoholic fatty liver disease. Chin J Physiol. (2020) 63:149–55. doi: 10.4103/CJP.CJP_43_20
20. Farhan, M, and Rizvi, A. The pharmacological properties of red grape polyphenol resveratrol: clinical trials and obstacles in drug development. Nutrients. (2023) 15:486. doi: 10.3390/nu15204486
21. Mohammadi, S, Moghadam, MD, Nasiriasl, M, Akhzari, M, and Barazesh, M. Insights into the therapeutic and pharmacological properties of resveratrol as a nutraceutical antioxidant polyphenol in health promotion and disease prevention. Curr Rev Clin Exp Pharmacol. (2024) 19:327–54. doi: 10.2174/0127724328268507231218051058
22. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
23. Hooijmans, CR, Rovers, MM, de Vries, RBM, Vries, RB, Leenaars, M, Ritskes-Hoitinga, M, et al. Syrcle's risk of bias tool for animal studies. BMC Med Res Methodol. (2014) 14:43. doi: 10.1186/1471-2288-14-43
24. El-Agamy, DS, Shebl, AM, and Said, SA. Prevention and treatment of Schistosoma mansoni-induced liver fibrosis in mice. Inflammopharmacology. (2011) 19:307–16. doi: 10.1007/s10787-011-0092-6
25. Kabir, T, Yoshiba, H, Agista, AZ, Sultana, H, Ohsaki, Y, Yeh, CL, et al. Protective effects of Gnetin C from Melinjo seed extract against high-fat diet-induced hepatic steatosis and liver fibrosis in NAFLD mice model. Nutrients. (2023) 15:388. doi: 10.3390/nu15183888
26. Aykac, M, Balkan, E, Gedi Kli, S, and Ozturk, N. Resveratrol treatment ameliorates hepatic damage via the TGF-beta/SMAD signaling pathway in a phenobarbital/CCl (4)-induced hepatic fibrosis model. Iran J basic. Med Sci. (2024) 27:1124–33. doi: 10.22038/IJBMS.2024.75737.16398
27. L, F. Study on Resveratrol Regulation of NLRP3 Inflammasome and Its Role in Liver Fibrosis in Mice [Master's thesis]. Guilin Medical University. (2019).
28. Ma, Z, Sheng, L, Li, J, Qian, J, Wu, G, Wang, Z, et al. Resveratrol alleviates hepatic fibrosis in associated with decreased endoplasmic reticulum stress-mediated apoptosis and inflammation. Inflammation. (2022) 45:812–23. doi: 10.1007/s10753-021-01586-w
29. Zhu, L, Mou, Q, Wang, Y, Zhu, Z, and Cheng, M. Resveratrol contributes to the inhibition of liver fibrosis by inducing autophagy via the micro RNA-20a-mediated activation of the PTEN/PI3K/AKT signaling pathway. Int J Mol Med. (2020) 46:2035–46. doi: 10.3892/ijmm.2020.4748
30. Hong, S, Jung, KH, Zheng, H, Hong, SW, Zheng, HM, Lee, HS, et al. The protective effect of resveratrol on dimethylnitrosamine-induced liver fibrosis in rats. Arch Pharm Res. (2010) 33:601–9. doi: 10.1007/s12272-010-0415-y
31. Lee, E, Shin, M, Yoon, S, and Moon, J. Resveratrol inhibits dimethylnitrosamine-induced hepatic fibrosis in rats. Arch Pharm Res. (2010) 33:925–32. doi: 10.1007/s12272-010-0616-4
32. Ran, Q, Song, D, Wang, Q, Wang, D, Chen, X, Zhang, A, et al. Resveratrol alleviates arsenic exposure-induced liver fibrosis in rats by inhibiting hepatocyte senescence. Biol Trace Elem Res. (2025) 203:1528–38. doi: 10.1007/s12011-024-04255-9
33. Yang, GL, Zhan, JF, Yang, YW, Yang, G, Zhan, J, Yang, Y, et al. Inhibitory effects of oxyresveratrol on ERK and Smad 1/2 phosphorylation and HSC activation in preventing carbon tetrachloride-induced rat liver fibrosis. Food Sci Human Wellness. (2021) 10:6–12. doi: 10.1016/j.fshw.2020.08.007
34. Z, R. Preparation of Resveratrol Liver-Targeted Liposomes and Study on Their Preventive Effect Against Liver Fibrosis in Rats [Master's thesis]. Hubei University of Chinese Medicine. (2020).
35. Rong, W, Jianye, W, Jun, L, and E, A. Inhibition of resveratrol, a herbal medicine, on expression of E-cadherin in hepatic tissues of rats with CCl4 induced fibrosis. J Clin Hepatol. (2009) 12:335–8.
36. Pq, N. Experimental Study on the Anti-fibrotic Effects of Resveratrol in Rat Liver Fibrosis [Master's thesis]. Tongji University (2006).
37. Xr, Q. Mechanism of Resveratrol Inhibition of DEN-Induced Pre-cancerous Liver Lesions in Rats Based on Metabolomics [Master's thesis]. Anhui University of Chinese Medicine (2023).
38. Liang, F, Xu, X, and Tu, Y. Resveratrol inhibited hepatocyte apoptosis and alleviated liver fibrosis through mi R-190a-5p/HGF axis. Bioorg Med Chem. (2022) 57:116593. doi: 10.1016/j.bmc.2021.116593
39. Zhang, H, Sun, Q, Xu, T, Hong, L, Fu, R, Wu, J, et al. Resveratrol attenuates the progress of liver fibrosis via the Akt/nuclear factor-kappa B pathways. Mol Med Rep. (2016) 13:224–30. doi: 10.3892/mmr.2015.4497
40. Chan, C, Cheng, L, Lin, C, Chan, CC, Cheng, LY, Lin, CL, et al. The protective role of natural phytoalexin resveratrol on inflammation, fibrosis and regeneration in cholestatic liver injury. Mol Nutr Food Res. (2011) 55:1841–9. doi: 10.1002/mnfr.201100374
41. Kessoku, T, Imajo, K, Honda, Y, Kato, T, Ogawa, Y, Tomeno, W, et al. Resveratrol ameliorates fibrosis and inflammation in a mouse model of nonalcoholic steatohepatitis. Sci Rep. (2016) 6:22251. doi: 10.1038/srep22251
42. Wei-Wei, Z, Ji-Feng, Z, Ren, W, Yanan, G, Junfeng, Z, and Shujuan, T. Resveratrol inhibited hepatic fibrosis in mice with schistosomiasis japonica by modulating Th1 and Th2 responses. Chin Pharmacol Bull. (2016) 32:1091–7.
43. Renye, Q, Yancheng, DA, Yong, L, and Yi, Z. Resveratrol improves CCl4induced liver fibrosis in mice by regulating liver circadian clock. Tianjin J Tradit Chin Med. (2022) 39:1604–10.
44. Fq, W. The Role of SIRT1 in Mouse Liver Fibrosis and Related Molecular Omics Study [Doctoral dissertation]. Fourth Military Medical University (2015).
45. Shams Eldeen, AM, Al-Ani, B, Ebrahim, HA, Rashed, L, Badr, AM, Attia, A, et al. Resveratrol suppresses cholestasis-induced liver injury and fibrosis in rats associated with the inhibition of TGFbeta1-Smad 3-mi R21 axis and profibrogenic and hepatic injury biomarkers. Clin Exp Pharmacol Physiol. (2021) 48:1402–11. doi: 10.1111/1440-1681.13546
46. Mohseni, R, Arab Sadeghabadi, Z, Goodarzi, MT, and Karimi, J. Co-administration of resveratrol and beta-aminopropionitrile attenuates liver fibrosis development via targeting lysyl oxidase in CCl (4)-induced liver fibrosis in rats. Immunopharmacol Immunotoxicol. (2019) 41:644–51. doi: 10.1080/08923973.2019.1688829
47. Hessin, AF, Hegazy, RR, Hassan, AA, Yassin, NZ, and Kenawy, SA. Resveratrol prevents liver fibrosis via two possible pathways: modulation of alpha fetoprotein transcriptional levels and normalization of protein kinase C responses. Indian J Pharmacol. (2017) 49:282–9. doi: 10.4103/ijp.IJP_299_16
48. Tanriverdi, G, Kaya-Dagistanli, F, Ayla, S, Demirci, S, Eser, M, Unal, ZS, et al. Resveratrol can prevent CCl (4)-induced liver injury by inhibiting notch signaling pathway. Histol Histopathol. (2016) 31:769–84. doi: 10.14670/HH-11-720
49. Ahmad, A, and Ahmad, R. Resveratrol mitigate structural changes and hepatic stellate cell activation in N'-nitrosodimethylamine-induced liver fibrosis via restraining oxidative damage. Chem Biol Interact. (2014) 221:1–12. doi: 10.1016/j.cbi.2014.07.007
50. Di Pascoli, M, Divi, M, Rodriguez-Vilarrupla, A, Rosado, E, Gracia-Sancho, J, Vilaseca, M, et al. Resveratrol improves intrahepatic endothelial dysfunction and reduces hepatic fibrosis and portal pressure in cirrhotic rats. J Hepatol. (2013) 58:904–10. doi: 10.1016/j.jhep.2012.12.012
51. Chavez, E, Reyes-Gordillo, K, Segovia, J, Shibayama, M, Tsutsumi, V, Vergara, P, et al. Resveratrol prevents fibrosis, NF-kappa B activation and TGF-beta increases induced by chronic CCl4 treatment in rats. J Appl Toxicol. (2008) 28:35–43. doi: 10.1002/jat.1249
52. Rashidi, M, Afarin, R, Kouchak, M, Kabizadeh, B, Shamsi, M, Hatami, M, et al. Resveratrol and saroglitazar: a promising combination for targeting TGF-β/Smad3 signaling and attenuating inflammatory response in nonalcoholic steatohepatitis in rats. Hepat Mon. (2023) 23:237. doi: 10.5812/hepatmon-138237
53. Liu, YZ, Liu, YG, and Ye, FQ. Effects of resveratrol on DMN-induced hepatic fibrosis in rats. Zhong Yao Cai. (2009) 32:1429–31.
54. L, JH. Prevention and Treatment of Liver Fibrosis in Rats by Resveratrol and Related Mechanism Research [Master's thesis]. Guangzhou University of Chinese Medicine (2009).
55. L, QJ, X, JQ, W, LQ, C, YY, and Z, M. Effects of resveratrol on chronic hepatic fibrosis in rats. Chin J New Drugs. (2005) 1:855–8.
56. Li, Z, Dong, J, Wang, M, Yan, J, Hu, Y, Liu, Y, et al. Resveratrol ameliorates liver fibrosis induced by nonpathogenic Staphylococcus in BALB/c mice through inhibiting its growth. Mol Med. (2022) 28:52. doi: 10.1186/s10020-022-00463-y
57. Chen, TT, Peng, S, Wang, Y, Hu, Y, Shen, Y, Xu, Y, et al. Improvement of mitochondrial activity and fibrosis by resveratrol treatment in mice with Schistosoma japonicum infection. Biomolecules. (2019) 9:658. doi: 10.3390/biom9110658
58. Yu, B, Qin, S, Hu, B, Qin, QY, Jiang, HX, and Luo, WL. Resveratrol improves CCL4-induced liver fibrosis in mouse by upregulating endogenous IL-10 to reprogramme macrophages phenotype from M (LPS) to M(IL-4). Biomed Pharmacother. (2019) 117:109110. doi: 10.1016/j.biopha.2019.109110
59. Yudong, Y, Haixing, J, Wei, L, Bangli, H, Bing, Y, Fan, L, et al. Resveratrol can improve liver fibrosis by inhibiting the NF-κB pathway in liver ma crophages. Chin J Gastroenterol Hepatol. (2020) 29:576–80.
60. Li, S, Han, B, Li, J, Lv, Z, Jiang, H, Liu, Y, et al. Resveratrol alleviates liver fibrosis induced by long-term inorganic mercury exposure through activating the Sirt1/PGC-1alpha signaling pathway. J Agric Food Chem. (2024) 72:15985–97. doi: 10.1021/acs.jafc.4c02349
61. Wang, H, Jiang, C, Yang, Y, Li, J, Wang, Y, Wang, C, et al. Resveratrol ameliorates iron overload induced liver fibrosis in mice by regulating iron homeostasis. PeerJ. (2022) 10:e13592. doi: 10.7717/peerj.13592
62. Yan, C, and Zheng, X. Therapeutic effect of resveratrol as well as resveratrol combined with Praziquantel on the liver fibrosis due to Schistosoma japonicum. Infection in mice. Chin J Parasitol Parasit Dis. (2013) 31:337–41.
63. Mostafa, DK, Eissa, MM, Ghareeb, DA, Abdulmalek, S, and Hewedy, WA. Resveratrol protects against Schistosoma mansoni-induced liver fibrosis by targeting the Sirt-1/NF-kappaB axis. Inflammopharmacology. (2024) 32:763–75. doi: 10.1007/s10787-023-01382-y
64. Hung, W, Hsiao, Y, Chiou, Y, Nagabhushanam, K, Ho, CT, and Pan, MH. Correction: Hepatoprotective effect of piceatannol against carbon tetrachloride-induced liver fibrosis in mice. Food Funct. (2021) 12:12159–60. doi: 10.1039/D1FO90103F
65. Li, S, Zheng, X, Zhang, X, Yu, H, Han, B, Lv, Y, et al. Exploring the liver fibrosis induced by deltamethrin exposure in quails and elucidating the protective mechanism of resveratrol. Ecotoxicol Environ Saf. (2021) 207:111501. doi: 10.1016/j.ecoenv.2020.111501
66. Ebrahim, HA, Kamar, SS, Haidara, MA, Latif, NSA, Ellatif, MA, ShamsEldeen, AM, et al. Association of resveratrol with the suppression of TNF-alpha/NF-kB/iNOS/HIF-1alpha axis-mediated fibrosis and systemic hypertension in thioacetamide-induced liver injury. Naunyn Schmiedeberg's Arch Pharmacol. (2022) 395:1087–95.
67. Dawood, AF, Al Humayed, S, Momenah, MA, El-Sherbiny, M, Ashour, H, Kamar, SS, et al. MiR-155 dysregulation is associated with the augmentation of ROS/p 53 Axis of fibrosis in Thioacetamide-induced hepatotoxicity and is protected by resveratrol. Diagnostics (Basel). (2022) 12:1762. doi: 10.3390/diagnostics12071762
68. Abdu, SB, and Al-Bogami, FM. Influence of resveratrol on liver fibrosis induced by dimethylnitrosamine in male rats. Saudi J Biol Sci. (2019) 26:201–9. doi: 10.1016/j.sjbs.2017.09.003
69. Mukherjee, D, and Ahmad, R. Resveratrol attenuates nitrosodiethylamine-induced liver injury in anti-inflammatory manner via regulating cyclooxygenase-2. J Food Biochem. (2018) 42:594. doi: 10.1111/jfbc.12594
70. Attallah, AM, Toson, EA, Shiha, GE, Omran, MM, Abdel-Aziz, MM, and el-Dosoky, I. Evaluation of serum procollagen aminoterminal propeptide III, laminin, and hydroxyproline as predictors of severe fibrosis in patients with chronic hepatitis C. J Immunoassay Immunochem. (2007) 28:199–211. doi: 10.1080/15321810701454649
71. Chen, Y, Li, D, Wu, J, Chen, Y, and Lu, H. Tetrandrine inhibits activation of rat hepatic stellate cells stimulated by transforming growth factor-beta in vitro via up-regulation of Smad 7. J Ethnopharmacol. (2005) 100:299–305. doi: 10.1016/j.jep.2005.03.027
72. Gabele, E, Brenner, DA, and Rippe, RA. Liver fibrosis: signals leading to the amplification of the fibrogenic hepatic stellate cell. Front Biosci. (2003) 8:d69–77. doi: 10.2741/887
73. Trivedi, P, Wang, S, and Friedman, SL. The power of plasticity-metabolic regulation of hepatic stellate cells. Cell Metab. (2021) 33:242–57. doi: 10.1016/j.cmet.2020.10.026
74. Arroyave-Ospina, JC, Wu, Z, Geng, Y, and Moshage, H. Role of oxidative stress in the pathogenesis of non-alcoholic fatty liver disease: implications for prevention and therapy. Antioxidants (Basel). (2021) 10:174. doi: 10.3390/antiox10020174
75. Janero, DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. (1990) 9:515–40. doi: 10.1016/0891-5849(90)90131-2
76. Li, S, Tan, H, Wang, N, Zhang, ZJ, Lao, L, Wong, CW, et al. The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci. (2015) 16:26087–124. doi: 10.3390/ijms161125942
77. Allameh, A, Niayesh-Mehr, R, Aliarab, A, Sebastiani, G, and Pantopoulos, K. Oxidative stress in liver pathophysiology and disease. Antioxidants (Basel). (2023) 12:1653. doi: 10.3390/antiox12091653
78. Sies, H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. (2015) 4:180–3. doi: 10.1016/j.redox.2015.01.002
79. Peiseler, M, Schwabe, R, Hampe, J, Kubes, P, Heikenwälder, M, and Tacke, F. Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease - novel insights into cellular communication circuits. J Hepatol. (2022) 77:1136–60. doi: 10.1016/j.jhep.2022.06.012
80. Kumar, S, Duan, Q, Wu, R, Harris, EN, and Su, Q. Pathophysiological communication between hepatocytes and non-parenchymal cells in liver injury from NAFLD to liver fibrosis. Adv Drug Deliv Rev. (2021) 176:113869. doi: 10.1016/j.addr.2021.113869
81. Tsuchida, T, and Friedman, SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. (2017) 14:397–411. doi: 10.1038/nrgastro.2017.38
82. Shu, Y, Liu, X, Huang, H, Wen, Q, and Shu, J. Research progress of natural compounds in anti-liver fibrosis by affecting autophagy of hepatic stellate cells. Mol Biol Rep. (2021) 48:1915–24. doi: 10.1007/s11033-021-06171-w
83. Campana, L, and Iredale, JP. Regression of liver fibrosis. Semin Liver Dis. (2017) 37:1–10. doi: 10.1055/s-0036-1597816
84. Beringer, A, and Miossec, P. IL-17 and TNF-alpha co-operation contributes to the proinflammatory response of hepatic stellate cells. Clin Exp Immunol. (2019) 198:111–20. doi: 10.1111/cei.13316
85. Khadrawy, SM, Mohamed, HM, and Mahmoud, AM. Mesenchymal stem cells ameliorate oxidative stress, inflammation, and hepatic fibrosis via Nrf 2/HO-1 signaling pathway in rats. Environ Sci Pollut Res Int. (2021) 28:2019–30. doi: 10.1007/s11356-020-10637-y
86. Runyan, CE, Hayashida, T, Hubchak, S, Curley, JF, and Schnaper, HW. Role of SARA (SMAD anchor for receptor activation) in maintenance of epithelial cell phenotype. J Biol Chem. (2009) 284:25181–9. doi: 10.1074/jbc.M109.032847
87. Yu, K, Li, Q, Shi, G, and Li, N. Involvement of epithelial-mesenchymal transition in liver fibrosis. Saudi J Gastroenterol. (2018) 24:5–11. doi: 10.4103/sjg.SJG_297_17
88. Fabregat, I, Moreno-Caceres, J, Sanchez, A, Dooley, S, Dewidar, B, Giannelli, G, et al. TGF-beta signalling and liver disease. FEBS J. (2016) 283:2219–32. doi: 10.1111/febs.13665
89. Song, Y, Wei, J, Li, R, Fu, R, Han, P, Wang, H, et al. Tyrosine kinase receptor B attenuates liver fibrosis by inhibiting TGF-beta/SMAD signaling. Hepatology. (2023) 78:1433–47.
Glossary
HYP - hydroxyproline
ALT - alanine aminotransferase
AST - aspartate aminotransferase
α-SMA - α-smooth muscle actin
TGF-β - transforming growth factor-β
Col1α1 - collagen type I alpha 1
MDA - malondialdehyde
SOD - superoxide dismutase
GSH - glutathione
IL-6 - interleukin-6
TNF-α - tumor necrosis factor-α
HA - hyaluronic acid
LN - laminin
CIV - type IV collagen
PIIINP - type III procollagen N-terminal peptide
ALP - alkaline phosphatase
ALB - albumin
NF-Κb - nuclear factor kappa-B
ECM - extracellular matrix
ROS - reactive oxygen species
LF - Liver fibrosis
Keywords: resveratrol, liver fibrosis, preclinical studies, meta–analysis, systematic review
Citation: Luo D, Shang Z, He Q, Ke J, Xian Q, Dai S, Sun S and Xiong S (2025) The efficacy of resveratrol in the treatment of liver fibrosis: a systematic review and meta-analysis of preclinical studies. Front. Nutr. 12:1606603. doi: 10.3389/fnut.2025.1606603
Edited by:
Thomas Brzozowski, Jagiellonian University Medical College, PolandReviewed by:
Sara Taha Elazab, Mansoura University, EgyptZheng Xu, Guangzhou University of Chinese Medicine, China
Hataichanok Chuljerm, Chiang Mai University, Thailand
Lingjie Meng, Zunyi Medical University, China
Copyright © 2025 Luo, Shang, He, Ke, Xian, Dai, Sun and Xiong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaoquan Xiong, eHNxdWFuXzEwNkAxNjMuY29t
 Dehua Luo
Dehua Luo Zhoubiao Shang1
Zhoubiao Shang1 Jianlong Ke
Jianlong Ke Shaoquan Xiong
Shaoquan Xiong