Abstract
The Composite Dietary Antioxidant Index (CDAI), a comprehensive measure of dietary antioxidant intake, quantifies the combined effects of key micronutrients, including vitamins A, C, and E, zinc (Zn), and selenium (Se), to evaluate overall antioxidant capacity. Existing evidence suggests that CDAI is inversely associated with cardiovascular diseases, including myocardial infarction and stroke. This study aims to investigate the relationship between CDAI and peripheral artery disease (PAD), which remains unclear in the current literature. In this study, we analyzed data from 2,332 participants with available ankle-brachial index (ABI) measurements from the NHANES database. Multivariable logistic regression and smooth curve fitting were employed to evaluate the association between CDAI and PAD. Additionally, subgroup analyses and interaction tests were conducted to assess the generalizability and stability of these relationships. Our findings revealed a significant inverse association between CDAI and PAD. In the fully adjusted model, each one-unit increase in CDAI was associated with a 12% reduction in PAD prevalence (OR = 0.88, 95% CI: 0.81–0.95). Moreover, participants in the highest quartile of CDAI had a 53% lower likelihood of developing PAD (OR = 0.47, 95% CI: 0.24–0.93) compared with those in the lowest quartile. These results demonstrate a strong correlation between CDAI and PAD risk, suggesting that diets rich in antioxidants (reflected by higher CDAI scores) may play a role in PAD prevention. However, further comprehensive research and prospective cohort studies are needed to explore causal relationships and validate these findings.
1 Introduction
PAD, the manifestation of atherosclerosis in lower extremity arteries, is a common cardiovascular condition linked to increased risks of amputation, myocardial infarction, stroke, and death (1). PAD is an established predictor of all-cause mortality and cardiovascular death. Current epidemiological evidence demonstrates that PAD significantly raises the risk of all-cause mortality, cardiovascular death, coronary artery disease, and cerebrovascular disease (2). The global burden of PAD affects more than 200 million people, corresponding to over 3% of the world’s population (3).
The CDAI developed by Wright et al. (4) is an integrative scoring system that measures cumulative antioxidant intake (including vitamins A, C, E, selenium, zinc, and carotenoids) from dietary sources. A higher CDAI has been associated with a reduced risk of multiple chronic conditions, including diabetes, chronic obstructive pulmonary disease (COPD), low back pain, and sleep disorders (5–8). Two additional studies have, respectively, identified inverse relationships between the CDAI and the probability of cardiovascular disease in general adult populations and postmenopausal women (9, 10).
However, no studies have investigated the association between CDAI and the prevalence of PAD. Therefore, we initiated cross-sectional research to explore the relationship between CDAI and PAD using data from the 1999–2004 NHANES.
2 Materials and methods
2.1 Study population
NHANES is an extensive health assessment initiative conducted by the Centers for Disease Control and Prevention (CDC). The research design and associated materials obtained formal authorization from the designated regulatory authorities and were approved through ethical review. Because NHANES data on the ABI questionnaire were only available from 1999 through 2004, our study primarily focused on this period, and 31,125 people were included in this research. The exclusion criteria were as follows: (a) no ABI data (23,555); (b) no CDAI data (5,236); and (c) CDAI outliers (2). Based on the above criteria, 2,332 participants were enrolled. The baseline information of the participants is illustrated in Figure 1.
Figure 1
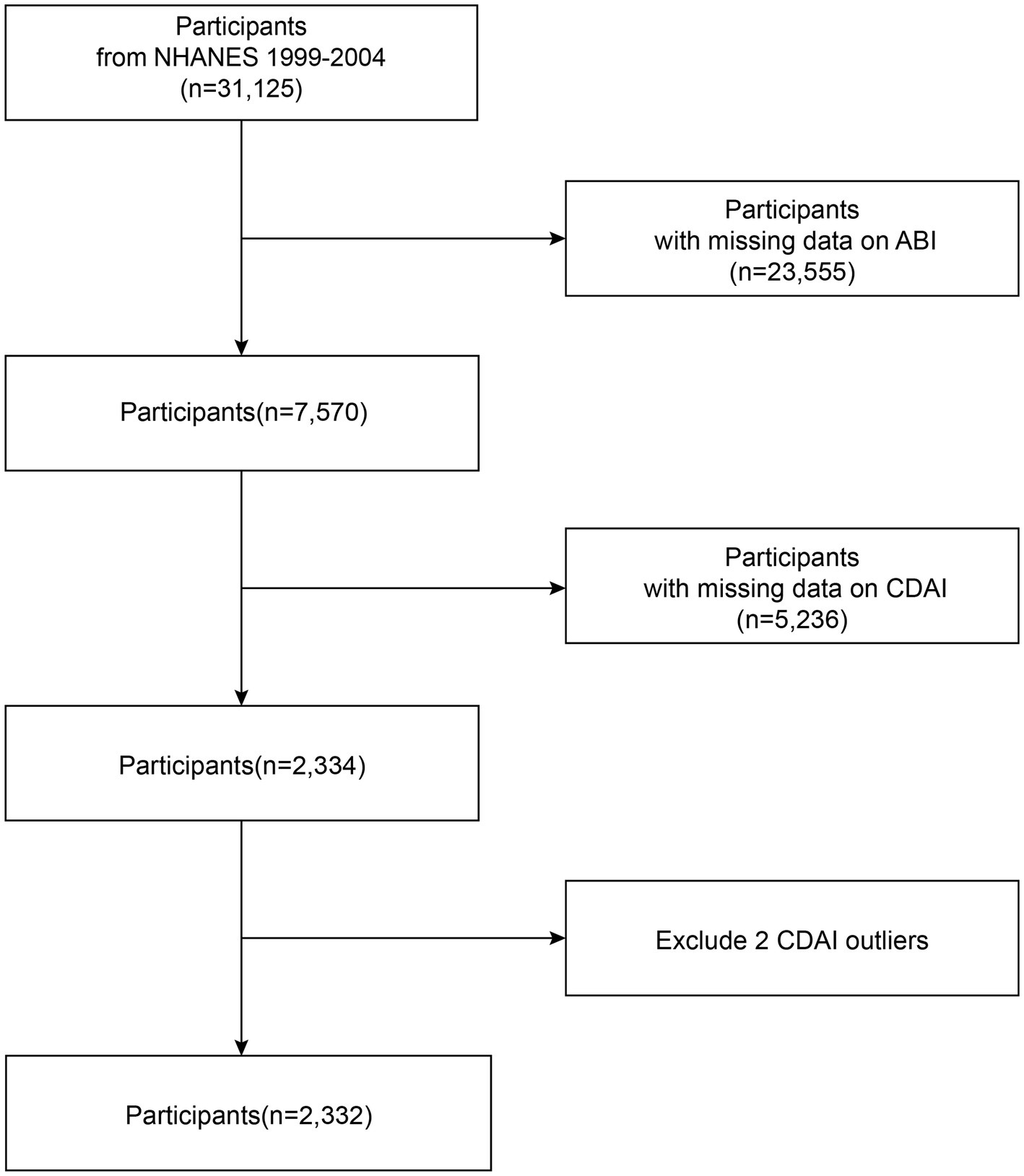
A detailed flow chart of participant recruitment.
2.2 Composite dietary antioxidant index
CDAI served as the primary exposure factor in this study. It was derived from the NHANES Dietary Interview Questionnaire and calculated using a specific formula. Existing literature has validated the reliability of this methodological approach (11–13). The questionnaire collects dietary intake data over any two consecutive days within a calendar year, and calculates the intake of various nutrients and trace elements. The data were collected using two different methods: the first time at the Mobile Examination Center (MEC), a specially designed bus, and the second time via telephone follow-up. The specific dietary intake collection items and the proportion of nutritional components are defined by the USDA’s (U. S. Department of Agriculture) Dietary Research Food and Nutrition Database (FNDDS). To calculate CDAI, we selected the daily intake of six dietary antioxidants (vitamins A, C, E, selenium, zinc, and carotenoids) obtained from the Dietary Interview Questionnaire (Days 1 and 2). The CDAI is computed as the sum of standardized values for each antioxidant using the formula:
2.3 Peripheral artery disease
We evaluated the ankle-brachial blood pressure index (ABI) as an indicator for assessing lower extremity disease using the NHANES examination data component. During the measurement, participants were required to lie on the examination table in the supine position. A cuff tonometer was placed on the right arm and both ankles, and blood pressure was measured at each site. If the subject’s right upper extremity had skin lesions, amputation, or contraindications to compression, the left arm was used. According to the protocol, measurements were taken twice at each site, while for participants older than 65 years, only one measurement was taken. Finally, the ABI was calculated by the ratio of ankle systolic blood pressure to brachial systolic blood pressure. Based on clinical guidelines, participants with an ABI ≤ 0.9 in either leg were defined as suffering PAD (14).
2.4 Covariables
In this study, covariates were rigorously selected based on two principles: (1) documented associations with PAD in previous research, or (2) recognition as established clinical risk factors supported by epidemiological studies or consensus guidelines. The included covariates encompassed demographic characteristics (age, gender, race), socioeconomic factors (education level, marital status, income-to-poverty ratio), anthropometric and lifestyle measures—including body mass index, physical activity levels, dietary factors (excluding antioxidants such as total energy, calcium, and iron intake), and adverse health behaviors (smoking status and alcohol consumption). Additionally, comorbid conditions (diabetes mellitus, hypertension, and hypercholesterolemia) were incorporated due to their well-established role in PAD pathogenesis, pro-inflammatory and pro-oxidative effects, and potential confounding influence on the relationship between CDAI and PAD outcomes. Adjustment for these covariates enhances the robustness of our analyses by minimizing residual confounding, thereby improving the validity and interpretability of the observed associations.
2.5 Statistical analysis
The baseline characteristics of the participants were evaluated using the chi-square test and t-test. The effect sizes of exposure factors on outcomes were examined using logistic regression analyses. In this study, three distinct multivariate logistic regression models were employed to investigate the relationship between CDAI and PAD, while progressively adjusting for and controlling potential confounders to more accurately assess the independent associations between the two variables. These models differed in their covariate adjustments: Model 1 was unadjusted for any covariates, while Model 2 included age, gender, race, and educational status. In contrast, Model 3 combined covariates including gender, age, race, marital status, education level, household poverty rate, BMI, physical activity, energy intake, calcium intake, iron intake, drinking status, smoking status, diabetes, hypertension, and high cholesterol. Additionally, CDAI was divided into quartiles of continuous measurements to allow for trend analysis to determine potential correlations with PAD, with values categorized as follows: quartile 1 (Q1): −5.62 to −2.26; quartile 2 (Q2): −2.25 to −0.75; quartile 3 (Q3): −0.74 to 1.18; and quartile 4 (Q4): 1.19 to 29.17. Smoothed curve fitting methods were used to evaluate nonlinear correlations. Stratified multiple regression analysis with interaction terms was conducted to assess heterogeneity and stability across subgroups, including sex, age, race, education, PIR, BMI, drinking, smoking, diabetes, hypertension, and hypercholesterolemia. All statistical analyses were conducted utilizing R software (version 3.4.3) in conjunction with EmpowerStats (version 2.0); statistical significance was defined as p < 0.05.
3 Results
3.1 Sociodemographic and clinical characteristics
Baseline sociodemographic and clinical characteristics of the participants are presented in Table 1. A total of 2,332 participants aged over 40 years were included in this analysis. The mean age was 60.27 years (SD = 12.74). Among the participants, 50.21% were male, and 47.60% identified as non-Hispanic White. The mean CDAI score for the overall cohort was 0.02 (SD = 3.46). Participants with PAD had significantly lower CDAI values than those without PAD (−1.08 vs. 0.06, p < 0.001). 7.12% of participants were diagnosed with PAD. Participants with PAD were generally older and more likely to be non-Hispanic Black, smokers, or have hypertension, diabetes mellitus, or hypercholesterolemia. They were also more likely to have family PIR ≤ 100% and lower energy intake. In addition, patients with PAD had lower educational levels. Significant differences appeared in marital status, with higher proportions of widowed/divorced/separated PAD participants and lower proportions of married/living with a partner. Individuals with PAD tended to have a BMI in the normal or overweight range.
Table 1
| Variables | Overall | ABI >0.9 | ABI ≤0.9 | p-value |
|---|---|---|---|---|
| N, % | 2,332(100%) | 2,166(92.88%) | 166(7.12%) | <0.001 |
| Age (years) | 60.27 ± 12.74 | 59.49 ± 12.50 | 70.49 ± 11.29 | <0.001 |
| Gender, % | 0.670 | |||
| Male | 1,171 (50.21%) | 1,085 (50.09%) | 86 (51.81%) | |
| Female | 1,161 (49.79%) | 1,081 (49.91%) | 80 (48.19%) | |
| Race/Ethnicity, % | 0.008 | |||
| Mexican American | 628 (26.93%) | 596 (27.52%) | 32 (19.28%) | |
| Other Hispanic | 125 (5.36%) | 118 (5.45%) | 7 (4.22%) | |
| Non-Hispanic White | 1,110 (47.60%) | 1,026 (47.37%) | 84 (50.60%) | |
| Non-Hispanic Black | 408 (17.50%) | 366 (16.90%) | 42 (25.30%) | |
| Other | 61 (2.62%) | 60 (2.77%) | 1 (0.60%) | |
| Education level, % | 0.002 | |||
| <9th grade | 541 (23.20%) | 492 (22.71%) | 49 (29.52%) | |
| 9–11th grade | 445 (19.08%) | 407 (18.79%) | 38 (22.89%) | |
| High school graduate | 513 (22.00%) | 469 (21.65%) | 44 (26.51%) | |
| Some college or AA degree | 461 (19.77%) | 440 (20.31%) | 21 (12.65%) | |
| College graduate or above | 372 (15.95%) | 358 (16.53%) | 14 (8.43%) | |
| Family PIR, % | 0.029 | |||
| ≤100 | 367 (15.74%) | 331 (15.28%) | 36 (21.69%) | |
| >100 | 1965 (84.26%) | 1835 (84.72%) | 130 (78.31%) | |
| BMI (kg/m2), % | 0.004 | |||
| <18.5 | 25 (1.07%) | 23 (1.06%) | 2 (1.20%) | |
| 18.5–24.9 | 624 (26.76%) | 565 (26.08%) | 59 (35.54%) | |
| 25.0–29.9 | 916 (39.28%) | 846 (39.06%) | 70 (42.17%) | |
| ≥30.0 | 767 (32.89%) | 732 (33.80%) | 35 (21.08%) | |
| Physical activity, % | 0.714 | |||
| Active | 1962 (84.13%) | 1824 (84.21%) | 138 (83.13%) | |
| Moderate | 370 (15.87%) | 342 (15.79%) | 28 (16.87%) | |
| Smoking, % | <0.001 | |||
| Yes | 1,238 (53.09%) | 1,122 (51.80%) | 116 (69.88%) | |
| No | 1,094 (46.91%) | 1,044 (48.20%) | 50 (30.12%) | |
| Drinking, % | 0.410 | |||
| Yes | 1,543 (66.17%) | 1,438 (66.39%) | 105 (63.25%) | |
| No | 789 (33.83%) | 728 (33.61%) | 61 (36.75%) | |
| Diabetes, % | <0.001 | |||
| Yes | 298 (12.78%) | 260 (12.00%) | 38 (22.89%) | |
| No | 2034 (87.22%) | 1906 (88.00%) | 128 (77.11%) | |
| Hypertension, % | <0.001 | |||
| Yes | 930 (39.88%) | 837 (38.64%) | 93 (56.02%) | |
| No | 1,402 (60.12%) | 1,329 (61.36%) | 73 (43.98%) | |
| Hypercholesterolemia, % | 0.009 | |||
| Yes | 796 (34.13%) | 724 (33.43%) | 72 (43.37%) | |
| No | 1,536 (65.87%) | 1,442 (66.57%) | 94 (56.63%) | |
| CDAI | 0.02 ± 3.46 | 0.06 ± 3.50 | −1.08 ± 2.67 | <0.001 |
| Energy Intake (kcal) | 1923.99 ± 888.95 | 1944.31 ± 901.60 | 1658.80 ± 649.33 | <0.001 |
| Calcium Intake (mg) | 748.20 ± 519.36 | 753.80 ± 522.61 | 675.16 ± 470.26 | 0.060 |
| Iron Intake (mg) | 14.62 ± 9.24 | 14.73 ± 9.21 | 13.32 ± 9.52 | 0.059 |
Clinical characteristics of study population.
Continuous data were presented as the mean and 95% confidence interval, category data were presented as the proportion and 95% confidence interval, CDAI, composite dietary antioxidant index, BMI, body mass index, PIR, poverty income ratio. Bold values indicate statistical significance (p < 0.05).
3.2 Association between CDAI and PAD
The association between CDAI and PAD was assessed using logistic regression models. The findings from logistic regression on PAD are summarized in Table 2. After adjusting for all covariates, a one-unit increment in CDAI was linked to a 14% reduction in PAD prevalence [OR = 0.86 (95% CI: 0.78–0.95)]. After categorizing the CDAI into four groups (Q1-4) based on its values, the inverse association remained statistically significant (p < 0.05). Compared to participants in Q1 (reference group), those in Q4 had a 53% reduction in PAD risk [OR = 0.47 (95% CI: 0.24–0.93)]. The smoothed curve fitting visualized the relationship between CDAI and PAD, confirming a negative correlation between the two variates (Figure 2). The P for trend indicated a significant dose–response relationship (unadjusted model: p = 0.001; model I: p = 0.005; model II: p = 0.025). Threshold effect analysis also revealed a significant nonlinear relationship between CDAI and PAD prevalence (p for nonlinearity = 0.008). Using a two-piecewise linear regression model, an inflection point was identified at CDAI = −1.32. This result demonstrated distinct dose–response patterns on either side of this threshold. Below this value, each unit increase in CDAI corresponded to a 35% reduction in PAD probability (adjusted OR = 0.65, 95% CI: 0.51–0.82, p < 0.001). However, beyond this threshold, the protective association plateaued (adjusted OR = 0.92, 95% CI: 0.83–1.02, p = 0.097). No further clinical benefit was seen for increasing CDAI levels above this point (Table 3).
Table 2
| Result | Non-adjusted model | Model I | Model II | |||
|---|---|---|---|---|---|---|
| OR [95% CI] | p value | OR [95% CI] | P value | OR [95% CI] | P value | |
| Continuous CDAI | 0.87 (0.82, 0.93) | <0.0001 | 0.89 (0.83, 0.95) | 0.0011 | 0.86 (0.78, 0.95) | 0.0030 |
| CDAI-Q1 (−5.62 to −2.26) | 1 (ref) | — | 1 (ref) | — | 1 (ref) | — |
| CDAI-Q2 (−2.25 to −0.75) | 0.69 (0.46, 1.05) | 0.0806 | 0.63 (0.41, 0.98) | 0.0411 | 0.57 (0.35, 0.92) | 0.0203 |
| CDAI-Q3 (−0.74 to 1.18) | 0.57 (0.37, 0.88) | 0.0114 | 0.55 (0.35, 0.87) | 0.0110 | 0.52 (0.30, 0.91) | 0.0212 |
| CDAI-Q4 (1.19 to 29.17) | 0.42 (0.26, 0.68) | 0.0003 | 0.51 (0.31, 0.84) | 0.0090 | 0.47 (0.24, 0.93) | 0.0303 |
| P for trend | <0.001 | 0.005 | 0.0025 | |||
Results from logistic regression analysis on PAD.
Model 1: no covariates were adjusted. Model 2: adjusted for age, sex, race/ethnicity, and education levels. Model 3: adjusted for age, sex, race/ethnicity, education levels, PIR, BMI, physical activity, energy intake, calcium intake, iron intake, smoking, drinking, hypertension, diabetes and hypercholesterolemia.
Figure 2
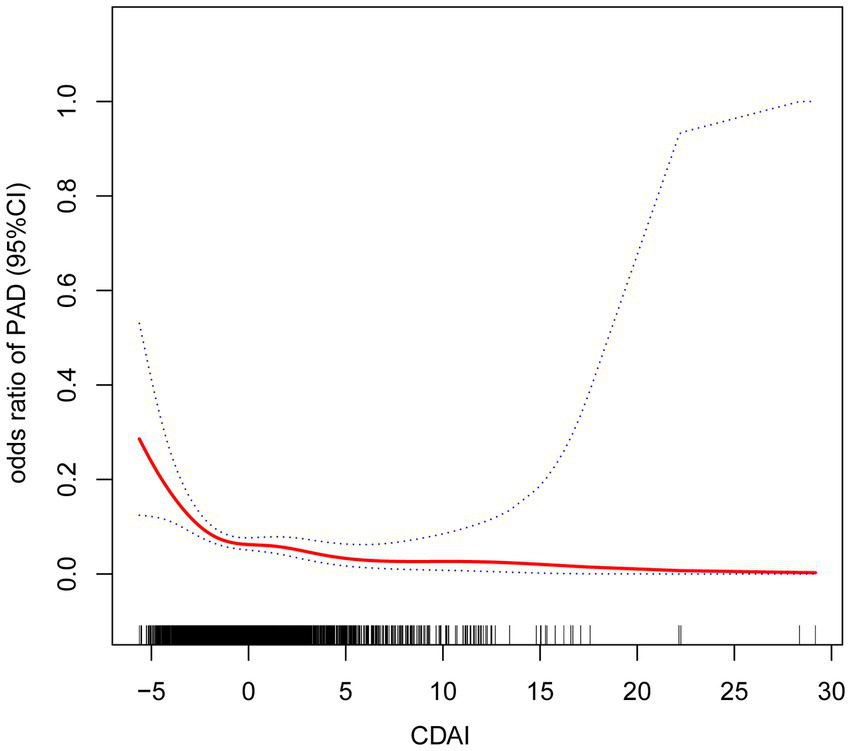
Results from smooth curve fitting.
Table 3
| Inflection point | Adjusted OR (95%CI) | P-value |
|---|---|---|
| Model I | ||
| One line effect | 0.86 (0.78, 0.95) | 0.0032 |
| Model II | ||
| Turning point (k) | −1.32 | |
| < K effect 1 | 0.65 (0.51, 0.82) | 0.0002 |
| > K effect 2 | 0.92 (0.83, 1.02) | 0.0968 |
| Effect 2–1 | 1.42 (1.10, 1.83) | 0.0073 |
| Model fit value at K | −2.60 (−2.86, −2.35) | |
| Log-likelihood ratio | 0.0080 | |
Threshold effect analysis of CDAI on PAD using a two-piecewise linear regression model.
3.3 Subgroup analysis
Analyses were stratified by sex, age, race, education, PIR, BMI, energy intake, calcium intake, iron intake, smoking, drinking, hypertension, diabetes, and hypercholesterolemia. The forest plot (Figure 3) shows a consistent and statistically significant inverse association between CDAI and PAD across all genders, age ≥65 years, Non-Hispanic Black, participants with family PIR above 100, 9th grade to high school graduates, widowed/divorced/separated participants, BMI 25.0–29.9, smokers, non-smokers, drinkers, non-drinkers, participants with hypertension, participants without diabetes, and participants with hypercholesterolemia. In all subgroup analyses, the interaction tests were statistically insignificant (P for interaction > 0.05), indicating the negative association has good robustness and generalizability. Smoothing curves (Figure 4) suggested steeper negative slopes in females and participants aged <65 years. However, formal interaction tests remained non-significant. The underlying mechanisms warrant further investigation.
Figure 3
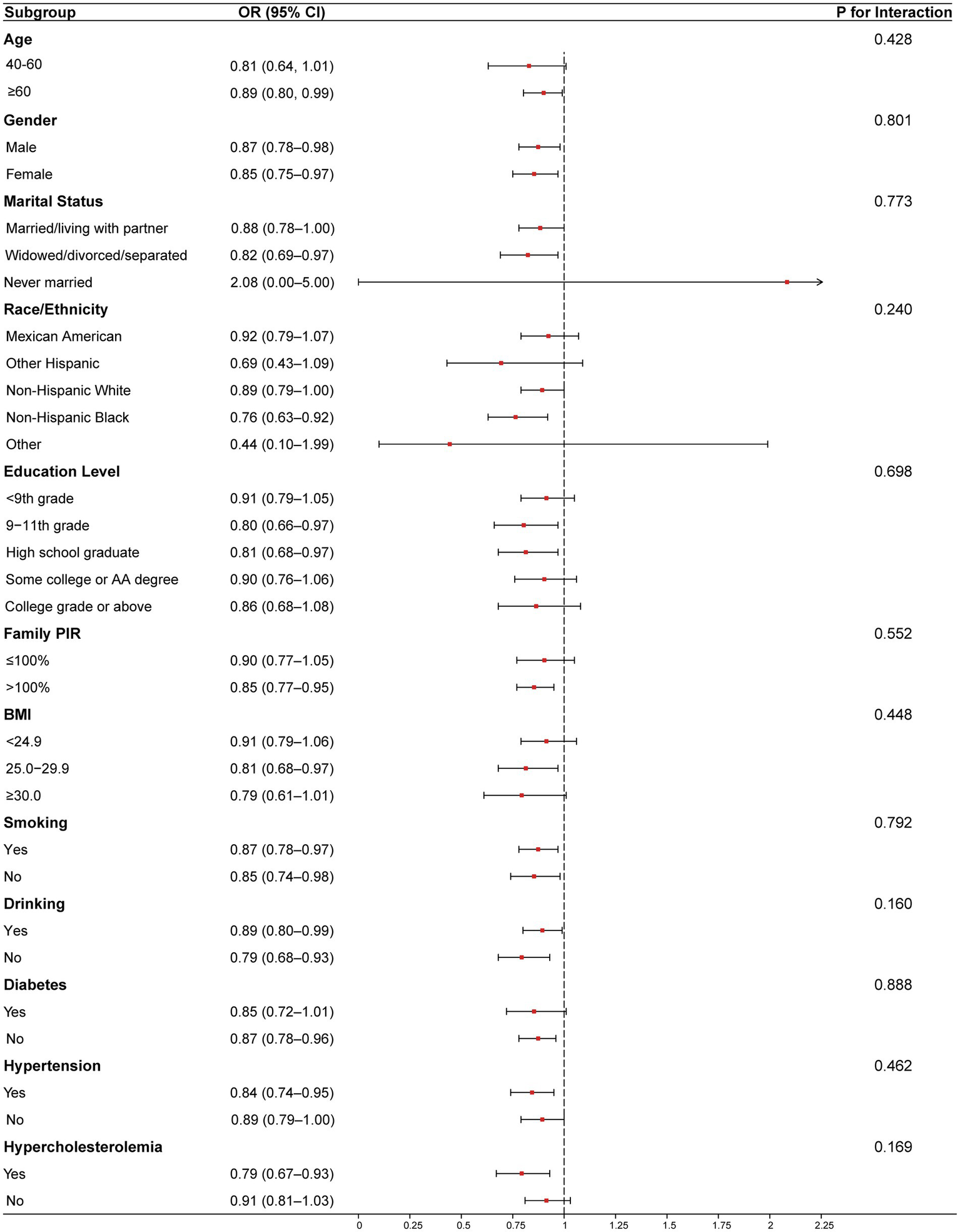
Results from subgroup analyses. Age, sex, race/ethnicity, education levels, PIR, BMI, physical activity, energy intake, calcium intake, iron intake, smoking, drinking, hypertension, diabetes and hypercholesterolemia were adjusted. Abbreviation: CDAI, composite dietary antioxidant index, BMI, body mass index, PIR, poverty Income Ratio.
Figure 4
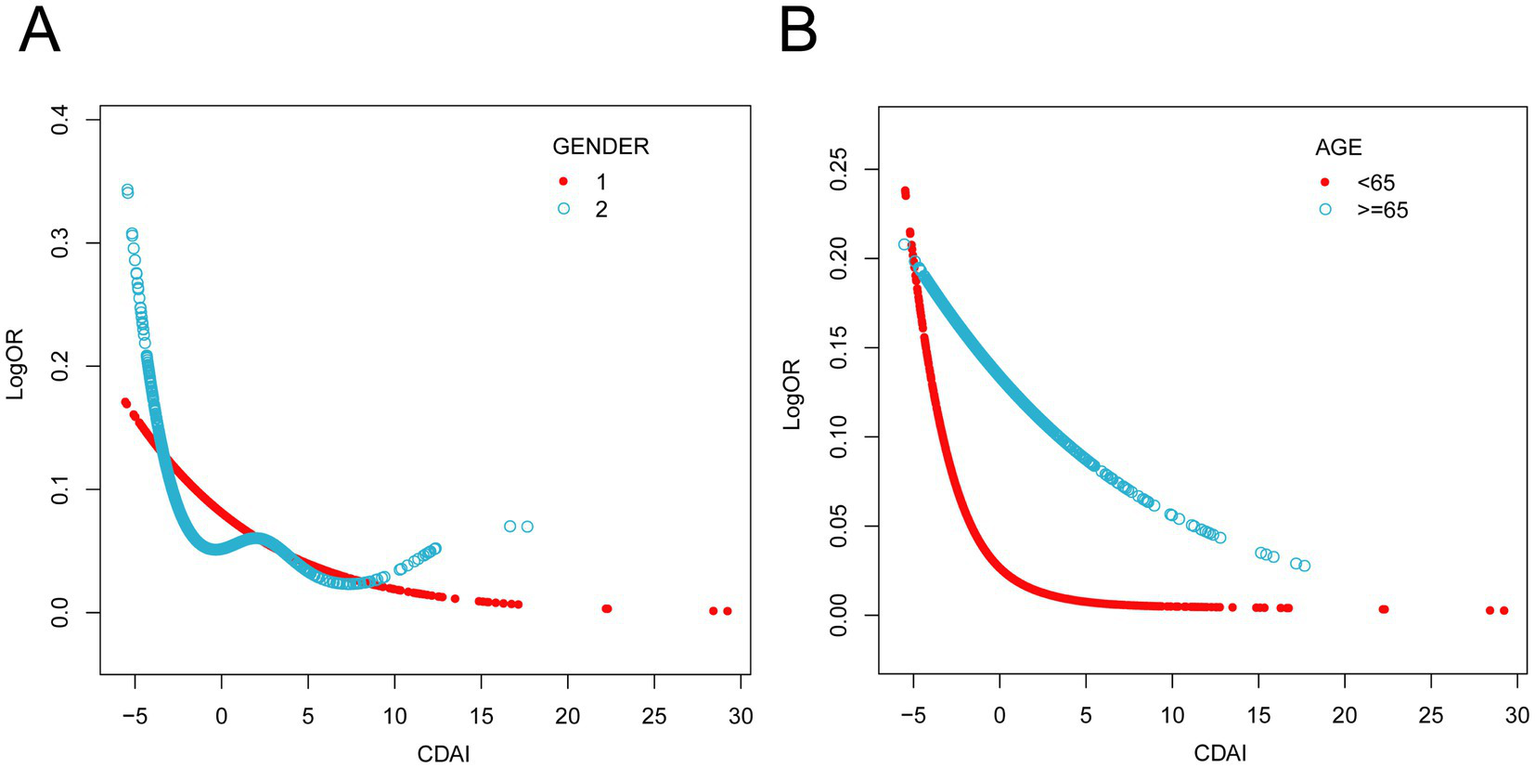
Results from subgroups smooth curve fitting analyses. (A) sex (male and female), (B) age (< 65 years, ≥ 65 years). Age, sex, race/ethnicity, education levels, PIR, BMI, physical activity, energy intake, calcium intake, iron intake, smoking, drinking, hypertension, diabetes and hypercholesterolemia were adjusted.
4 Discussion
In the cross-sectional study, we enrolled 2,332 representative participants and observed the negative relationship between CDAI and PAD. This suggests that higher levels of dietary antioxidants may be linked to a lower risk of developing PAD, particularly in high-risk populations with antioxidant deficiency.
Our results suggest that CDAI may have potential clinical value in the prevention of PAD. To our knowledge, this is the first population-based study to evaluate the association between CDAI and PAD, independent of traditional cardiovascular risk factors. By analyzing the NHANES database, Teng et al. (11) demonstrated a negative association between CDAI and stroke, with the antioxidant-based nomogram model showing strong predictive capability for stroke risk. Analogously, Ma et al. (12) demonstrated that the CDAI exhibited an L-shaped inverse association with the risk of coronary heart disease (CHD), reducing it by up to 65%. A study has also found that CDAI improves Atherosclerosis, Maugeri et al. (15) using the Kardiovize Brno 2030 study found that CDAI reduced carotid intima-media thickness (cIMT) in women. Consistent with the results of the previous study, CDAI is a protective factor against cardiovascular diseases. In this study, we found a negative relationship between the CDAI and PAD, indicating that individuals with high levels of dietary antioxidants may have a lower risk of developing PAD.
Atherosclerosis is a major cause of stenosis or occlusion of the arteries lumen in the lower extremities, affecting the blood supply to the lower extremities. Oxidative stress, inflammatory response, and lipid infiltration are the primary causes of atherosclerosis formation (16). Of these, reactive oxygen species (ROS), the primary byproducts of oxidative stress, initiate atherosclerosis by inducing endothelial dysfunction, chronic inflammation, and dysregulated lipid metabolism (17). Moreover, oxidative stress critically influences PAD progression by promoting vascular inflammation and endothelial dysfunction (18, 19). Oxidative stress results from the overproduction of reactive oxygen species (ROS) combined with impaired or inadequate antioxidant defense systems (20, 21). Animal and clinical studies indicate that oxidative stress drives the initiation and progression of PAD via diverse mechanisms. In animal models, experimental hindlimb ischemia (HLI) studies have shown that the generation of ROS is closely associated with angiogenesis, endothelial dysfunction, and inflammatory responses (22). Increased muscle ROS formation was found after ligation of unilateral iliac and femoral arteries (23). Moreover, ROS interferes with vascular endothelial growth factor (VEGF) mediated angiogenesis by decreasing nitric oxide (NO) bioavailability, thereby affecting the recovery of ischemic tissues (24). In addition, animal studies of cigarette smoke exposure have demonstrated that major risk factors for PAD, such as smoking and diabetes, further exacerbate inflammation by enhancing oxidative stress and decreasing NO bioactivity (25, 26). In terms of clinical studies, a cross-sectional analysis comparing blood markers in patients with type 2 diabetes with or without PAD found higher levels of AGEs and MDA (two biomarkers closely linked to oxidative stress), and lower levels of vitamin E and total reactive antioxidant potentials (TRAPs) in patients with PAD compared to those without PAD (27). A small clinical study (n = 74,12 months duration) noted that propionyl L-carnitine improved ABI, maximum walking distance, pre-morbid walking distance, and quality of life in patients (28).
The key antioxidants included in the CDAI—such as carotenoids, vitamins A, C, E, and essential trace metals (e.g., Zn, Se)—exert protective effects against oxidative stress through diverse mechanisms. Vitamin C is a water-soluble compound generated through glucose metabolism (29, 30). It synthesizes the reducing agents needed for collagen fibers, and it also protects the body from free radicals. Moreover, Vitamin C has been demonstrated to ameliorate oxidative stress through the modulation of key signaling pathways (31). Carotenoids are structurally similar to vitamin A (32). They may be involved in antioxidant protection against oxidative free radical attack (33). Some studies indicate that vitamin A and carotenoids are potent antioxidants that may inhibit the development of cardiovascular diseases and enhance visual health (34, 35). They can impact both free radicals and peroxyl radicals. Vitamin E, as an essential nutrient that can only be ingested exogenously, is a fat-soluble vitamin group present in high concentrations throughout the body, including cell membranes and cytoplasmic proteins, and is involved in regulating the body’s redox balance (36). Numerous studies have demonstrated that Vitamin E exhibits anti-atherosclerotic and anti-cardiovascular effects (37, 38). The trace element selenium (Se) is regarded as a beneficial dietary supplement due to its significant antioxidant capabilities and function of enhancing health (39). Amino acids, peptides, and enzymes are selenium-containing compounds, also playing crucial biological roles in the human body (40). Selenium and its derivatives, especially selenoproteins containing selenocysteine form selenium, have been shown to have antioxidant properties (41). Zn(II), Cu(I), and iron, the metal thiolate groups, also function as redox switches (42), serving as crucial and irreplaceable elements in the reaction pathway.
However, the US Preventive Services Task Force (USPSTF) found through a systematic review of RCTs that current evidence is insufficient to evaluate the balance of benefits and risks of using single or combined nutritional supplements for the prevention of certain diseases (43). Findings from retrospective research also support similar conclusions (44, 45).
Although the effects of single nutrients are not significant or insufficiently supported by evidence, some studies have found that the Mediterranean diet, rich in antioxidant components, is linked to a lower risk of cardiovascular disease and PAD (46). The Mediterranean Diet is predominantly plant-based, particularly in fruits, vegetables, legumes, and nuts, which are abundant in antioxidants such as vitamin A, C, E, polyphenols and others (47). The Mediterranean diet has been used for the prevention of cardiovascular diseases, including peripheral arterial disease and ischemic stroke. The Mediterranean diet was found to be superior to a low-fat diet in the prevention of cardiovascular events, including peripheral arterial disease, myocardial infarction, and ischemic stroke, in a 1,000-person RCT, supporting the secondary prevention of cardiovascular disease through the Mediterranean diet (48). Additionally, an exploratory, non-prespecified analysis of a large-scale randomized controlled trial revealed that the Mediterranean diet, characterized by an unrestricted energy intake and rich in antioxidants, significantly reduces the prevalence of PAD, underscoring its value in cardiovascular disease prevention (49, 50).
Interestingly, as depicted in Figure 4, our study revealed a potential differential benefit of antioxidant dietary intake across sex and age groups. Specifically, female participants and individuals under 65 years of age exhibited a more pronounced reduction in PAD risk with increasing CDAI levels. Accumulating evidence indicates that female sex hormones, particularly estrogen, exert multifaceted atheroprotective effects (51). A prospective study on oxidative balance scores and hypertension also observed similar sex-specific differences and demonstrated that it may be mediated by oxidative stress mechanisms (52). In elderly individuals, advanced age drives vascular aging process. It includes oxidative stress, endothelial dysfunction, and progressive arterial stiffening, which collectively may compromise the efficacy of antioxidant interventions (53). Therefore, this may raise different guidelines for the prevention of PAD in people of different genders and ages. For instance, premenopausal women or middle-aged people at risk of PAD might benefit from a diet plan that emphasizes foods high in antioxidants, while older populations may require adjunct therapies to overcome age-related declines in antioxidant absorption or utilization.
This study has several advantages. Firstly, this study is grounded in data extracted from the NHANES, which employs a randomized and scientifically rigorous sampling methodology. This approach ensures that the selected cohort reflects the demographic diversity of the US population, providing a robust representation of the US population’s health and nutritional status. Additionally, this study benefits from a substantial sample size, enhancing statistical precision and reliability. Additionally, we also adjusted for a large number of covariates to make the results more independent and more credible. Nevertheless, the limitations of this study must be acknowledged. Firstly, due to the cross-sectional study design, the authors were unable to establish clear causal relationships. Secondly, dietary information was self-reported, which may introduce inaccuracies due to recall bias. Moreover, due to the NHANES study protocol, which excluded individuals aged below 40 years from screening for these variables, the association between CDAI and PAD could not be comprehensively evaluated across a broader demographic spectrum.
5 Conclusion
In conclusion, our study demonstrates that CDAI is inversely associated with PAD in U. S. adults aged ≥40 years. Moreover, this association remained consistent across all subgroups, supporting the potential role of antioxidant-rich diets (reflected by higher CDAI scores) in PAD prevention in the general population. However, further comprehensive research and prospective cohort studies are needed to explore causal relationships and validate these findings.
Statements
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: wwwn.cdc.gov/nchs/nhanes/.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the [patients/ participants OR patients/participants legal guardian/next of kin] was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
ZF: Conceptualization, Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. RZ: Data curation, Supervision, Writing – review & editing. SG: Data curation, Writing – review & editing, Writing – original draft. QW: Data curation, Writing – review & editing. JL: Data curation, Writing – original draft. SL: Data curation, Writing – review & editing. ZS: Data curation, Writing – review & editing. WW: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was funded by the Beijing Tsinghua Changgung Hospital Fund (Grant No.12022C6020).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Gornik HL Aronow HD Goodney PP Arya S Brewster LP Byrd L et al . 2024 ACC/AHA/AACVPR/APMA/ABC/SCAI/SVM/SVN/SVS/SIR/VESS guideline for the Management of Lower Extremity Peripheral Artery Disease: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2024) 83:2497–604. doi: 10.1016/j.jacc.2024.02.013
2.
Heald CL Fowkes FG Murray GD Price JF . Risk of mortality and cardiovascular disease associated with the ankle-brachial index: systematic review. Atherosclerosis. (2006) 189:61–9. doi: 10.1016/j.atherosclerosis.2006.03.011
3.
Song P Rudan D Zhu Y Fowkes FJI Rahimi K Fowkes FGR et al . Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health. (2019) 7:e1020–30. doi: 10.1016/S2214-109X(19)30255-4
4.
Wright ME Mayne ST Stolzenberg-Solomon RZ Li Z Pietinen P Taylor PR et al . Development of a comprehensive dietary antioxidant index and application to lung cancer risk in a cohort of male smokers. Am J Epidemiol. (2004) 160:68–76. doi: 10.1093/aje/kwh173
5.
Liu Z Li J Chen T Zhao X Chen Q Xiao L et al . Association between dietary antioxidant levels and chronic obstructive pulmonary disease: a mediation analysis of inflammatory factors. Front Immunol. (2023) 14:1310399. doi: 10.3389/fimmu.2023.1310399
6.
Feng C Yao J Xie Y Yang F Fan X . Association between different composite dietary antioxidant indexes and low back pain in American women adults: a cross-sectional study from NHANES. BMC Public Health. (2024) 24:147. doi: 10.1186/s12889-024-17649-0
7.
Xiong B Wang J He R Qu G . Composite dietary antioxidant index and sleep health: a new insight from cross-sectional study. BMC Public Health. (2024) 24:609. doi: 10.1186/s12889-024-18047-2
8.
Chen X Lu H Chen Y Sang H Tang Y Zhao Y . Composite dietary antioxidant index was negatively associated with the prevalence of diabetes independent of cardiovascular diseases. Diabetol Metab Syndr. (2023) 15:183. doi: 10.1186/s13098-023-01150-6
9.
Lin Z Xie Y Lin Y Chen X . Association between composite dietary antioxidant index and atherosclerosis cardiovascular disease in adults: a cross-sectional study. Nutr Metabol Cardiovascul Dis. (2024) 34:2165–72. doi: 10.1016/j.numecd.2024.06.002
10.
Liu C Lai W Zhao M Zhang Y Hu Y . Association between the composite dietary antioxidant index and atherosclerotic cardiovascular disease in postmenopausal women: a cross-sectional study of NHANES data, 2013-2018. Antioxidants. (2023) 12:1740. doi: 10.3390/antiox12091740
11.
Teng TQ Liu J Hu FF Li QQ Hu ZZ Shi Y . Association of composite dietary antioxidant index with prevalence of stroke: insights from NHANES 1999-2018. Front Immunol. (2024) 15:1306059. doi: 10.3389/fimmu.2024.1306059
12.
Ma R Zhou X Zhang G Wu H Lu Y Liu F et al . Association between composite dietary antioxidant index and coronary heart disease among US adults: a cross-sectional analysis. BMC Public Health. (2023) 23:2426. doi: 10.1186/s12889-023-17373-1
13.
Li W Bai J Ge Y Fan Y Huang Q Deng Z . Association between compound dietary antioxidant index and all-cause and cancer mortality in patients with chronic obstructive pulmonary disease: results from NHANES 1999-2018. Front Med. (2025) 12:1544841. doi: 10.3389/fmed.2025.1544841
14.
Gornik HL Aronow HD Goodney PP Arya S Brewster LP Byrd L et al . 2024 ACC/AHA/AACVPR/APMA/ABC/SCAI/SVM/SVN/SVS/SIR/VESS guideline for the Management of Lower Extremity Peripheral Artery Disease: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. (2024) 149:e1313–410. doi: 10.1161/CIR.0000000000001251
15.
Maugeri A Hruskova J Jakubik J Kunzova S Sochor O Barchitta M et al . Dietary antioxidant intake decreases carotid intima media thickness in women but not in men: a cross-sectional assessment in the Kardiovize study. Free Radic Biol Med. (2019) 131:274–81. doi: 10.1016/j.freeradbiomed.2018.12.018
16.
Marchio P Guerra-Ojeda S Vila JM Aldasoro M Victor VM Mauricio MD . Targeting early atherosclerosis: a focus on oxidative stress and inflammation. Oxidative Med Cell Longev. (2019) 2019:1–32. doi: 10.1155/2019/8563845
17.
Batty M Bennett MR Yu E . The role of oxidative stress in atherosclerosis. Cells. (2022) 11:3843. doi: 10.3390/cells11233843
18.
Higashi Y Noma K Yoshizumi M Kihara Y . Endothelial function and oxidative stress in cardiovascular diseases. Circ J. (2009) 73:411–8. doi: 10.1253/circj.cj-08-1102
19.
Shaito A Aramouni K Assaf R Parenti A Orekhov A Yazbi AE et al . Oxidative stress-induced endothelial dysfunction in cardiovascular diseases. Front Biosci. (2022) 27:105. doi: 10.31083/j.fbl2703105
20.
Sies H . Oxidative stress: a concept in redox biology and medicine. Redox Biol. (2015) 4:180–3. doi: 10.1016/j.redox.2015.01.002
21.
Sies H . Oxidative eustress: on constant alert for redox homeostasis. Redox Biol. (2021) 41:101867. doi: 10.1016/j.redox.2021.101867
22.
Steven S Daiber A Dopheide JF Münzel T Espinola-Klein C . Peripheral artery disease, redox signaling, oxidative stress - basic and clinical aspects. Redox Biol. (2017) 12:787–97. doi: 10.1016/j.redox.2017.04.017
23.
Lejay A Choquet P Thaveau F Singh F Schlagowski A Charles AL et al . A new murine model of sustainable and durable chronic critical limb ischemia fairly mimicking human pathology. Eur J Vascul Endovascu Surg. (2015) 49:205–12. doi: 10.1016/j.ejvs.2014.12.010
24.
Neufeld G Cohen T Gengrinovitch S Poltorak Z . Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. (1999) 13:9–22. doi: 10.1096/fasebj.13.1.9
25.
Michaud SE Dussault S Groleau J Haddad P Rivard A . Cigarette smoke exposure impairs VEGF-induced endothelial cell migration: role of NO and reactive oxygen species. J Mol Cell Cardiol. (2006) 41:275–84. doi: 10.1016/j.yjmcc.2006.05.004
26.
Brevetti G Giugliano G Brevetti L Hiatt WR . Inflammation in peripheral artery disease. Circulation. (2010) 122:1862–75. doi: 10.1161/CIRCULATIONAHA.109.918417
27.
Lapolla A Piarulli F Sartore G Ceriello A Ragazzi E Reitano R et al . Advanced glycation end products and antioxidant status in type 2 diabetic patients with and without peripheral artery disease. Diabetes Care. (2007) 30:670–6. doi: 10.2337/dc06-1508
28.
Mingorance C Rodriguez-Rodriguez R Justo ML Herrera MD de Sotomayor MA . Pharmacological effects and clinical applications of propionyl-L-carnitine. Nutr Rev. (2011) 69:279–90. doi: 10.1111/j.1753-4887.2011.00387.x
29.
Valdés F . Vitamin C. Actas Dermosifiliogr. (2006) 97:557–68. doi: 10.1016/s0001-7310(06)73466-4
30.
Doseděl M Jirkovský E Macáková K Krčmová LK Javorská L Pourová J et al . Vitamin C-sources, physiological role, kinetics, deficiency, use, toxicity, and determination. Nutrients. (2021) 13:615. doi: 10.3390/nu13020615
31.
Fatima S Alrashoudi RH Alqarni SS Alshehri S Alsaigh SM Malik A et al . Vitamin C ameliorates potassium dichromate-induced oxidative stress and mitochondrial dysfunction via PGC-1α/Nrf-2/TFAM pathway. J Biochem Mol Toxicol. (2025) 39:e70061. doi: 10.1002/jbt.70061
32.
Carazo A Macáková K Matoušová K Krčmová LK Protti M Mladěnka P . Vitamin a update: forms, sources, kinetics, detection, function, deficiency, therapeutic use and toxicity. Nutrients. (2021) 13:1703. doi: 10.3390/nu13051703
33.
Bates CJ . Vitamin A. Lancet. (1995) 345:31–5. doi: 10.1016/S0140-6736(95)91157-X
34.
Palace VP Khaper N Qin Q Singal PK . Antioxidant potentials of vitamin a and carotenoids and their relevance to heart disease. Free Radic Biol Med. (1999) 26:746–61. doi: 10.1016/S0891-5849(98)00266-4
35.
Terao J . Revisiting carotenoids as dietary antioxidants for human health and disease prevention. Food Funct. (2023) 14:7799–824. doi: 10.1039/D3FO02330C
36.
Miyazawa T Burdeos GC Itaya M Nakagawa K Miyazawa T . Vitamin E: regulatory redox interactions. IUBMB Life. (2019) 71:430–41. doi: 10.1002/iub.2008
37.
Shibata A Kobayashi T Asai A Eitsuka T Oikawa S Miyazawa T et al . High purity tocotrienols attenuate atherosclerotic lesion formation in apoE-KO mice. J Nutr Biochem. (2017) 48:44–50. doi: 10.1016/j.jnutbio.2017.06.009
38.
Ramanathan N Tan E Loh LJ Soh BS Yap WN . Tocotrienol is a cardioprotective agent against ageing-associated cardiovascular disease and its associated morbidities. Nutrition metabolism. (2018) 15:6. doi: 10.1186/s12986-018-0244-4
39.
Bjørklund G Shanaida M Lysiuk R Antonyak H Klishch I Shanaida V et al . Selenium: an antioxidant with a critical role in anti-aging. Molecules. (2022) 27:6613. doi: 10.3390/molecules27196613
40.
Mocchegiani E Malavolta M Muti E Costarelli L Cipriano C Piacenza F et al . Zinc, metallothioneins and longevity: interrelationships with niacin and selenium. Curr Pharm Des. (2008) 14:2719–32. doi: 10.2174/138161208786264188
41.
Zhang F Li X Wei Y . Selenium and selenoproteins in health. Biomolecules. (2023) 13:799. doi: 10.3390/biom13050799
42.
Palumaa P . Biological redox switches. Antioxid Redox Signal. (2009) 11:981–3. doi: 10.1089/ars.2009.2468
43.
Mangione CM Barry MJ Nicholson WK Cabana M Chelmow D Coker TR et al . Vitamin, mineral, and multivitamin supplementation to prevent cardiovascular disease and cancer: US preventive services task force recommendation statement. JAMA. (2022) 327:2326–33. doi: 10.1001/jama.2022.8970
44.
Sunkara A Raizner A . Supplemental vitamins and minerals for cardiovascular disease prevention and treatment. Methodist Debakey Cardiovasc J. (2019) 15:179–84. doi: 10.14797/mdcj-15-3-179
45.
Fortmann SP Burda BU Senger CA Lin JS Whitlock EP . Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the U.S. preventive services task force. Ann Intern Med. (2013) 159:824–34. doi: 10.7326/0003-4819-159-12-201312170-00729
46.
Mentella MC Scaldaferri F Ricci C Gasbarrini A Miggiano GAD . Cancer and Mediterranean diet: a review. Nutrients. (2019) 11:2059. doi: 10.3390/nu11092059
47.
Davis C Bryan J Hodgson J Murphy K . Definition of the Mediterranean diet; a literature review. Nutrients. (2015) 7:9139–53. doi: 10.3390/nu7115459
48.
Delgado-Lista J Alcala-Diaz JF Torres-Peña JD Quintana-Navarro GM Fuentes F Garcia-Rios A et al . Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): a randomised controlled trial. Lancet. (2022) 399:1876–85. doi: 10.1016/S0140-6736(22)00122-2
49.
Wan D Li V Banfield L Azab S de Souza RJ Anand SS . Diet and nutrition in peripheral artery disease: a systematic review. Can J Cardiol. (2022) 38:672–80. doi: 10.1016/j.cjca.2022.01.021
50.
Ros E Martínez-González MA Estruch R Salas-Salvadó J Fitó M Martínez JA et al . Mediterranean diet and cardiovascular health: teachings of the PREDIMED study. Adv Nutr. (2014) 5:330s–6s. doi: 10.3945/an.113.005389
51.
Kamon T Kaneko H Itoh H Kiriyama H Mizuno Y Morita H et al . Possible gender difference in the association between abdominal obesity, chronic inflammation, and preclinical atherosclerosis in the general population. Int Heart J. (2021) 62:837–42. doi: 10.1536/ihj.20-654
52.
Lee JH Son DH Kwon YJ . Association between oxidative balance score and new-onset hypertension in adults: a community-based prospective cohort study. Front Nutr. (2022) 9:1066159. doi: 10.3389/fnut.2022.1066159
53.
Donato AJ Machin DR Lesniewski LA . Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ Res. (2018) 123:825–48. doi: 10.1161/CIRCRESAHA.118.312563
Summary
Keywords
oxidative stress, antioxidant, CDAI, PAD, NHANES
Citation
Fan Z, Zhu R, Guo S, Wu Q, Li J, Liu S, Su Z and Wu W (2025) Association of composite dietary antioxidant index and peripheral artery disease: a national cross-sectional study. Front. Nutr. 12:1606769. doi: 10.3389/fnut.2025.1606769
Received
06 April 2025
Accepted
26 August 2025
Published
10 September 2025
Volume
12 - 2025
Edited by
Gentaro Ikeda, Stanford University, United States
Reviewed by
Xin Chen, Tongji University, China
Yazareni José Mercadante Urquía, Universidade Federal do Espírito Santo, Brazil
Updates
Copyright
© 2025 Fan, Zhu, Guo, Wu, Li, Liu, Su and Wu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiwei Wu, wwwa00906@btch.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.