- Department of Endoscopy, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Background: Mediterranean diet (MD) adherence is linked to improved health outcomes, yet evidence among cancer survivors remains limited. This study investigated the association between Mediterranean Diet Score (MDS) adherence and all-cause mortality among cancer survivors.
Methods: We analyzed data from 2,669 cancer survivors participating in the National Health and Nutrition Examination Survey (2005–2018). Dietary adherence was assessed using the MDS based on 2-day dietary recalls. Multivariable Cox regression, mediation, subgroup, and sensitivity analyses were conducted.
Results: Higher MDS adherence was significantly associated with reduced all-cause mortality (hazard ratio (HR) = 0.845, 95% confidence interval (CI): 0.779–0.917, p < 0.001), with a linear dose–response trend. Mediation analysis showed that red blood cell distribution width and neutrophils explained 18.5 and 7.8% of the association, respectively. Subgroup analyses revealed stronger protective effects in females, older adults, individuals with lower BMI or higher socioeconomic status, smokers, drinkers, and survivors of digestive, urinary, and skin cancers. Sensitivity analyses confirmed the robustness of the findings.
Conclusion: Greater adherence to the Mediterranean diet is associated with lower all-cause mortality in cancer survivors, partly mediated by inflammatory biomarkers. Integrating Mediterranean dietary counseling into survivorship care may help improve long-term outcomes.
Introduction
Cancer survivorship has become a critical focus in public health worldwide (1–3). Advances in diagnosis and treatment have improved survival rates, yet long-term mortality and quality of life remain significant concerns. Among modifiable post-diagnosis factors, dietary patterns have garnered increasing attention for their potential role in improving prognosis and reducing the risk of recurrence and comorbidities (4–8).
The Mediterranean diet (MD), characterized by high consumption of vegetables, fruits, legumes, whole grains, fish, nuts, and olive oil, along with a low intake of red meat and saturated fats, has been extensively studied for its cardiometabolic and anti-inflammatory benefits (9–14). The Mediterranean Diet Score (MDS), a composite score reflecting adherence to the MD, has been linked to a lower risk of chronic diseases and all-cause mortality in general populations (15, 16). However, its prognostic implications for cancer survivors remain less well established.
However, most existing studies have focused primarily on breast, colorectal, or prostate cancer survivors, leaving other cancer types underrepresented in the literature (17–22). Survivors of cancers such as lung, digestive system, reproductive system, and hematologic malignancies, which often carry worse prognoses and a greater systemic burden, have been largely excluded or insufficiently powered in prior cohort studies. This limits the generalizability of findings and impedes the development of broad dietary recommendations for the cancer survivor population as a whole. Some prospective studies have reported improved survival and reduced recurrence with higher MDS adherence, while others have found null associations (23). These discrepancies may arise from differences in cancer types, dietary assessment methods, or follow-up durations. Furthermore, most studies have not addressed potential biological mechanisms linking MDS to outcomes. Inflammation and oxidative stress are known to play central roles in cancer progression and survivorship health, yet few studies have explored these pathways as potential mediators (24, 25).
Therefore, we aimed to investigate the association between MDS adherence and all-cause mortality in a nationally representative cohort of U.S. cancer survivors from the NHANES 2005–2018 cycles. Leveraging a robust analytical framework, we used Cox proportional hazards models, restricted cubic splines, and stratified subgroup analyses to assess the dose–response and effect modification. Additionally, we conducted mediation analyses to explore potential inflammatory pathways, focusing on biomarkers such as red cell distribution width (RDW) and neutrophil count (NEU), which may partially explain the observed associations.
Methods
Participant selection
The present study utilized data from the National Health and Nutrition Examination Survey (NHANES), administered by the National Center for Health Statistics (NCHS). NHANES cycles prior to 2005 were excluded due to inconsistencies in dietary data collection protocols and the limited availability of key variables required for constructing the MDS and covariate adjustments. Initially, 70,190 individuals from the 2005–2018 cycles were considered. Participants completed a standardized questionnaire that included the item: “Has a medical practitioner or healthcare provider ever informed you that you had cancer or a malignancy of any kind?” Individuals who answered affirmatively were categorized as cancer survivors. Following a rigorous screening process, 2,669 participants were included in the final analysis. Details of the participant selection process are illustrated in Figure 1.
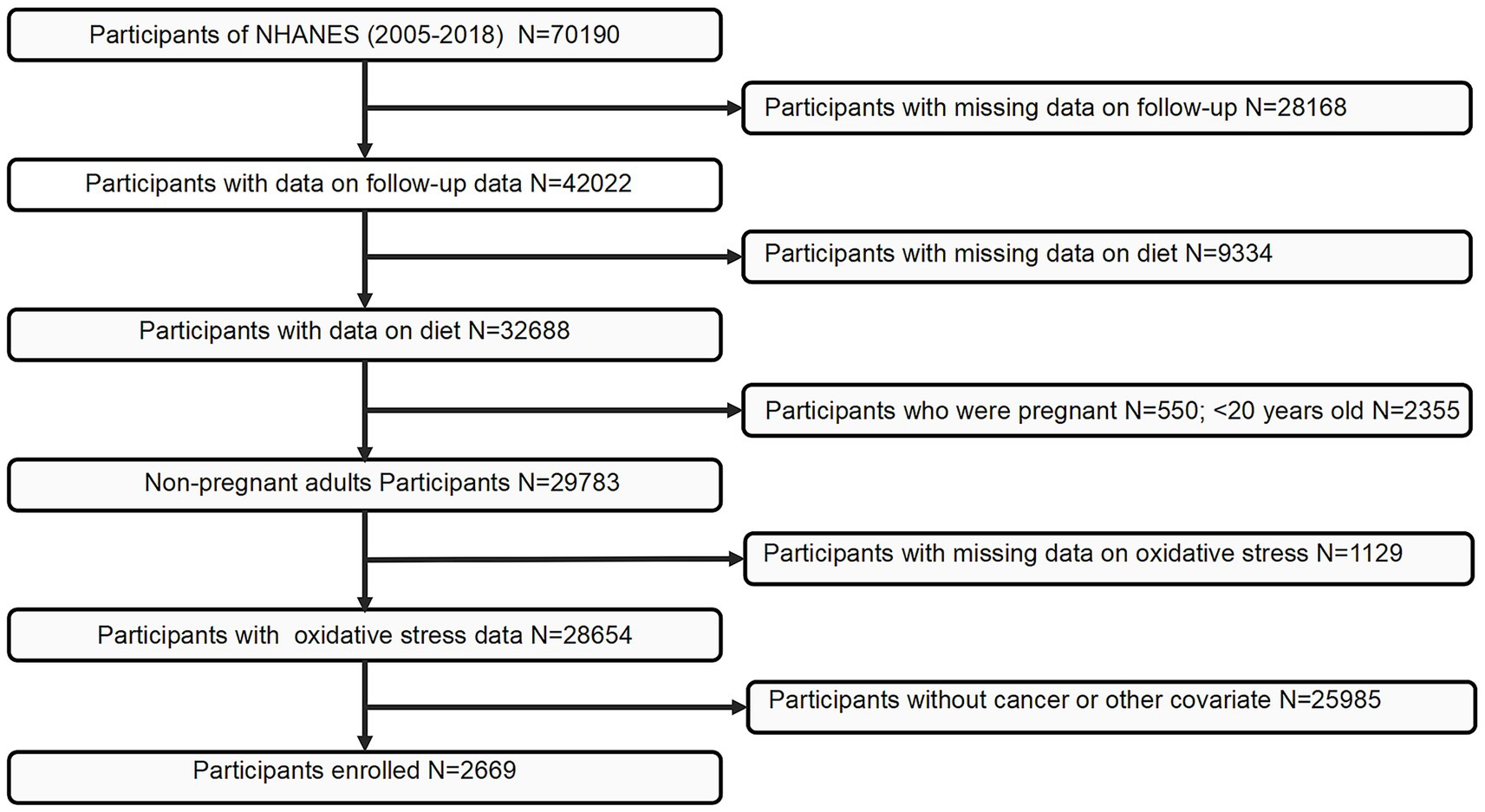
Figure 1. Flowchart of the study population. NHANES, National Health and Nutrition Examination Survey.
Assessment of the Mediterranean diet
Following interviews conducted by specially trained personnel, dietary data were collected using computer-assisted 24-h dietary recall interviews. Dedicated team members were responsible for data review and processing. The United States Department of Agriculture has repeatedly simulated and refined this process to ensure stringent quality control. In this study, dietary data from the first and second days were utilized. While 24-h recalls are subject to recall bias, NHANES uses standardized protocols, trained interviewers, and repeated measures to improve data reliability. This method has been validated for assessing dietary patterns in large populations. Although cancer stage and treatment data were unavailable, our analyses were adjusted for multiple health-related and sociodemographic variables, aligning with the study’s population-level objectives.
The Mediterranean Diet Score (MDS) is an index designed to assess adherence to the Mediterranean diet (26). We used a modified MDS adapted for the dataset in the study (27). The score comprises 10 components: one point is assigned for intakes equal to or above the median for non-refined cereals, legumes, fruits and nuts, vegetables, and fish and for a favorable ratio of monounsaturated to saturated fatty acids. The median cutoffs for each MDS component were derived from the study sample of cancer survivors. Since MDS is a relative index, absolute intake values are not used for scoring. To facilitate interpretability and examine dose–response patterns, participants were categorized into quartiles (Q1–Q4) based on their MDS distribution within the analytic sample. Although group sizes were not perfectly equal due to score discreteness, quartile-based classification is a standard method in nutritional epidemiology.
Ascertainment of mortality
All-cause mortality rate was determined through probabilistic matching of unique identifiers with the National Death Index. Participants lacking a corresponding record were assumed to be alive as of the specified cutoff date. Follow-up duration was measured in person-years from the baseline assessment until death or the conclusion of the follow-up period on 31 December 2019. In accordance with the guidelines of the International Classification of Diseases, 10th Revision (ICD-10), deaths due to all causes were identified. All-cause mortality encompassed deaths attributable to cardiovascular diseases, malignant neoplasms, and other causes.
Covariates
This study incorporated several covariate factors, as identified by previous research, for comprehensive analysis and discussion. The covariates included age, sex, ethnicity, education, poverty-to-income ratio (PIR), body mass index (BMI), smoking status, alcohol consumption, diabetes, and hypertension. Ethnicity was categorized as Mexican American, other Hispanic, non-Hispanic white, non-Hispanic Black, and other ethnicities. Education levels were stratified into less than 9th grade, 9th–11th grade, high school, college, and graduate-level or higher. Smoking status was defined as having smoked more than 100 cigarettes over a lifetime, while alcohol consumption was determined by the annual intake equivalent to 12 ounces of beer, 5 ounces of wine, or 1.5 ounces of liquor. Diabetes was diagnosed based on medical history and fasting blood glucose levels, and hypertension was ascertained through a combination of medical history and multiple blood pressure measurements. NHANES does not collect data on cancer site, diagnosis date, stage, or treatment modalities in a systematic manner. As such, these cancer-specific covariates could not be included and are acknowledged as a limitation.
Statistical analyses
Participants were divided into four groups based on their MDS, and baseline characteristics were summarized using frequencies (percentages) for categorical variables and means (standard errors) for continuous variables.
To evaluate the association between MDS and all-cause mortality in cancer survivors, multivariable Cox regression models were used. Hazard ratios (HRs) along with their corresponding 95% confidence intervals (CIs) were calculated to quantify these associations. Three progressively adjusted models were constructed: Model 1 was adjusted solely for age and sex; Model 2 further incorporated ethnicity, education, PIR, and BMI; and Model 3 additionally controlled for mortality risk factors, including diabetes, hypertension, alcohol consumption, and smoking status. Furthermore, a restricted cubic spline (RCS) analysis was performed to explore the relationship between MDS and prognosis. Consistent with common epidemiologic practice, the node of RCS was set to four (28, 29).
Previous studies have demonstrated that adherence to the Mediterranean diet is closely associated with markers of inflammation and oxidative stress, which are critical contributors to the pathogenesis of cancer prognosis (30–33). In line with these findings, a mediation analysis was conducted to investigate the potential mediating roles of neutrophils (NEU), lymphocytes (LYM), red blood cell distribution width (RDW), alkaline phosphatase (ALP), γ-glutamyltransferase (GGT), total bilirubin (TB), and uric acid (UA) in the relationship between MDS and prognosis. This analysis aimed to elucidate the underlying biological mechanisms and identify potential targets for intervention by quantifying the average causal mediation effects (ACMEs), average direct effects (ADEs), total effect, and the proportion of the mediating effect relative to the total effect.
To further explore the relationships, subgroup analyses were performed by stratifying participants according to age (20–65 years vs. ≥65 years), sex (male vs. female), smoking status (smoking vs. non-smoking), alcohol consumption (drinking vs. non-drinking), BMI (<median vs. ≥median), poverty income ratio (PIR; <median vs. ≥median), and type of malignancy (urinary system, digestive system, skin and soft tissues, lungs and mediastinal organs, breast, and reproductive system, or others). Multivariable Cox regression analyses were conducted within each subgroup. Interaction terms were tested for each subgroup to evaluate effect modification.
To assess the robustness of our findings, we performed three sensitivity analyses: (1) excluding participants who died within 1 year of the interview, (2) sequentially removing data from each NHANES cycle (each spanning 2 years) to evaluate the stability of the results, and (3) utilizing the first-day dietary data to validate the association, ensuring that the observed relationship was not affected by the averaging method of the 2-day data. Analyses were conducted using R (Revision 4.4.1), with statistical significance defined as a p-value of < 0.05.
Result
Baseline characteristics
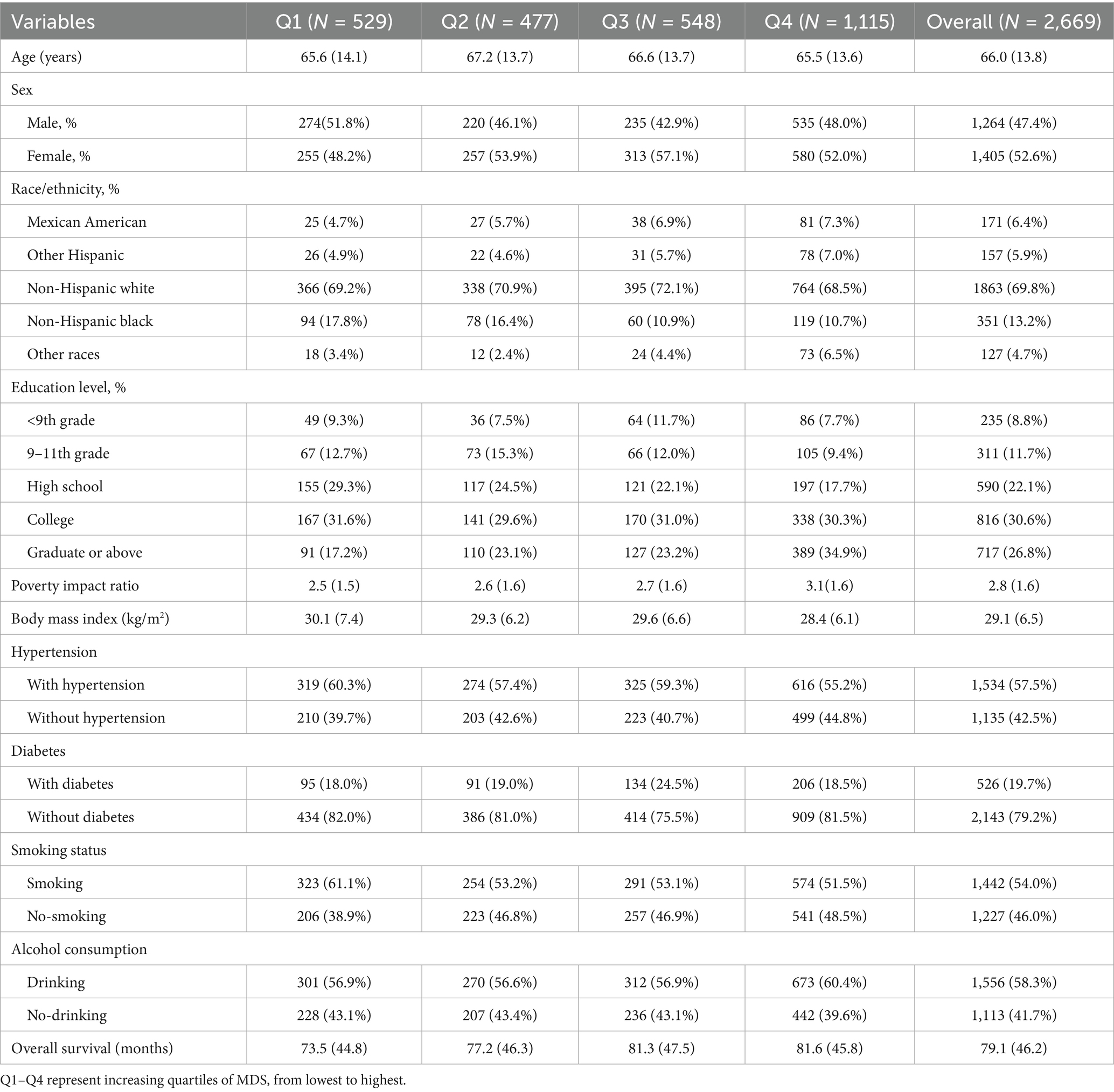
The baseline characteristics of the study population across MDS quartiles are presented in Table 1. The mean age of the participants was 66.0 years (SD: 13.8), with slight variations across quartiles. Females accounted for 52.6% of the overall cohort, with a higher proportion observed in Q2 and Q3. The majority of participants were non-Hispanic white (69.8%), although Q4 showed a modest increase in the representation of Hispanic individuals. Educational attainment varied substantially across groups, with the highest proportion of participants holding graduate-level education in Q4 (34.9%) and the lowest in Q1 (17.2%).
A decreasing trend in BMI was observed across MDS quartiles, from 30.1 kg/m2 in Q1 to 28.4 kg/m2 in Q4. Similarly, the prevalence of hypertension decreased with increasing MDS adherence, from 60.3% in Q1 to 55.2% in Q4. Smoking prevalence was highest in Q1 (61.1%) and gradually decreased across higher quartiles. Alcohol consumption was relatively consistent across all groups, although it was slightly higher in Q4 (60.4%). Mean overall survival increased progressively with higher MDS, from 73.5 months in Q1 to 81.6 months in Q4. Details are shown in Table 1.
Survival analysis
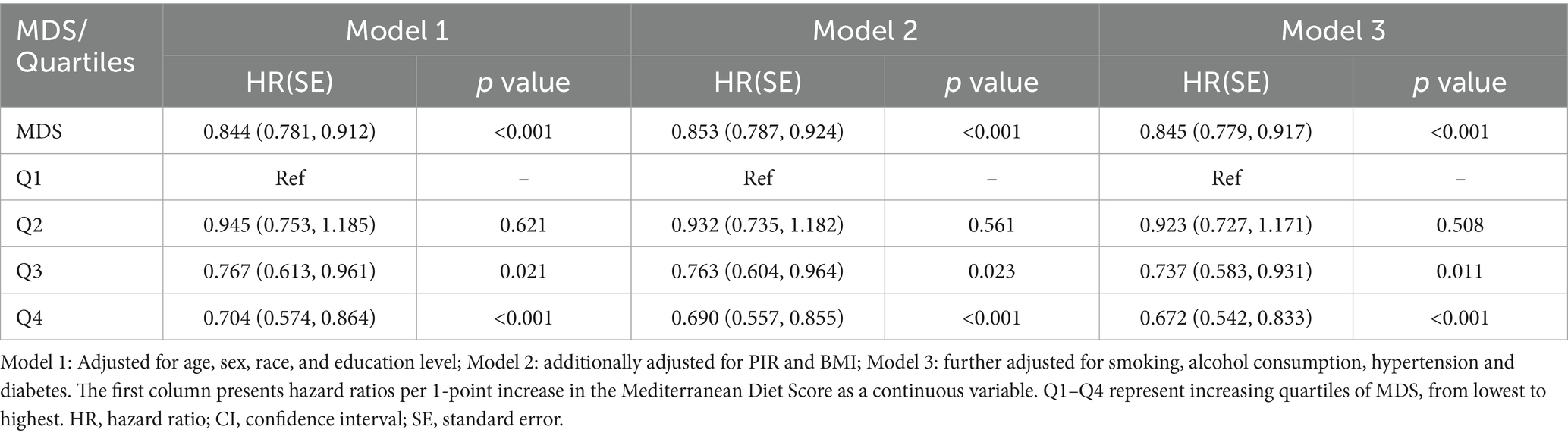
As shown in Table 2 and Figure 2, higher MDS was significantly associated with improved survival in cancer survivors. In the minimally adjusted model (Model 1), individuals in Q4 had a 29.6% lower risk of mortality compared to those in Q1 (HR = 0.704, 95% CI: 0.574–0.864, p < 0.001). This inverse association remained robust after further adjustment for socioeconomic factors in Model 2 (HR = 0.690, 95% CI: 0.557–0.855, p < 0.001), and lifestyle-related risk factors in Model 3 (HR = 0.672, 95% CI: 0.542–0.833, p < 0.001).
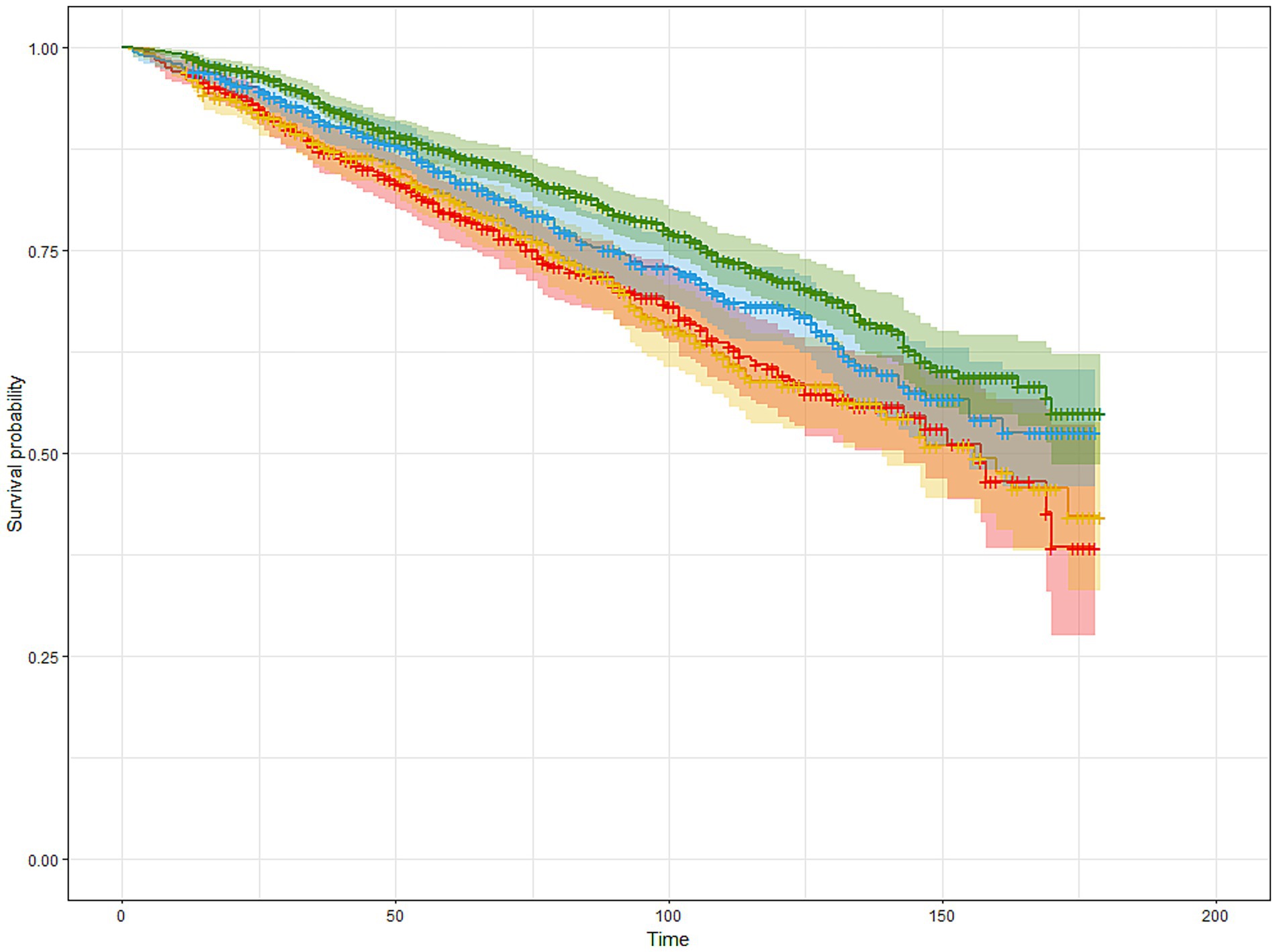
Figure 2. The Kaplan–Meier estimated survival curves of participants with cancer based on MDS quartiles. Participants were stratified into four groups (Q1–Q4) based on sex-specific quartiles of MDS. The horizontal axis represents follow-up time (months, 0–200), and the vertical axis shows survival probability. Curves were adjusted for age, sex, ethnicity, education level, PIR, BMI, smoking status, alcohol consumption, hypertension, and diabetes. Red, blue, yellow, and green lines correspond to Q1, Q2, Q3, and Q4 of MDS, respectively.
While Q2 and Q3 showed a trend toward lower mortality risk compared to Q1, only Q3 reached statistical significance in Model 3 (HR = 0.737, 95% CI: 0.583–0.931, p = 0.011), suggesting a dose–response relationship primarily driven by higher adherence to MDS.
In addition, RCS analysis indicated a significant linear association between MDS and all-cause mortality (p for overall association < 0.001), without evidence of non-linearity (p for non-linearity = 0.29), as shown in Figure 3. These findings suggest that increasing adherence to the MDS is linearly associated with reduced mortality risk among cancer survivors.

Figure 3. The restricted cubic spline estimated survival curves of participants with cancer based on MDS quartiles. The horizontal axis represents the MDS, and the vertical axis displays the estimated HR for all-cause mortality. The blue line indicates the adjusted HR values, while the shaded area represents the 95% CI. The reference value (HR = 1.0) corresponds to the median MDS. Estimates were derived from Cox proportional hazards models adjusted for age, sex, ethnicity, education level, PIR, BMI, smoking status, alcohol consumption, hypertension, and diabetes.
Mediation analysis
Mediation analysis was performed to explore the potential biological mechanisms linking MDS to prognosis, with inflammatory and oxidative stress-related biomarkers considered as mediators. As shown in Table 3, the total effect of MDS on prognosis was estimated at −0.024. Among the biomarkers assessed, RDW exhibited the strongest mediating effect, with an ACME of −0.004, accounting for 18.5% of the total effect. NEU also showed a modest mediation proportion of 7.82% (ACME = −0.002). TB and UA contributed to 9.01 and 4.13% of the total effect, respectively. Conversely, LYM, ALP, and GGT showed minimal mediation, with ACME values near zero and proportion mediated below 3.5%. These results suggest that certain inflammatory markers, particularly RDW and NEU, may partially mediate the protective association between MDS and mortality risk among cancer survivors.
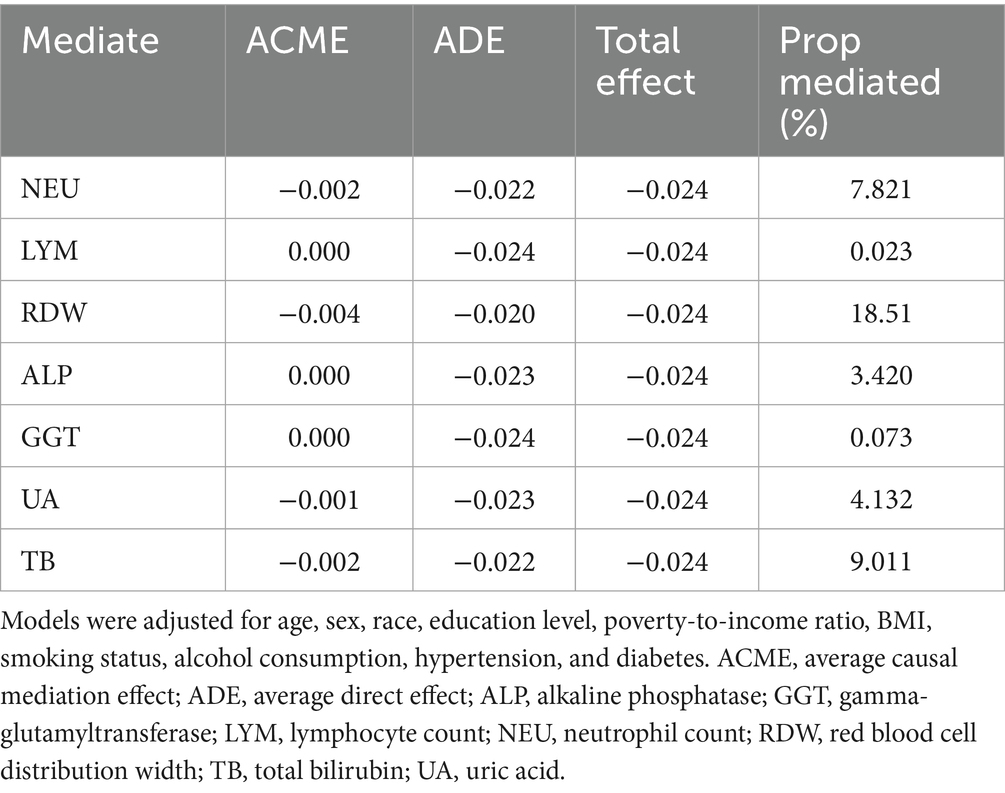
Table 3. Mediation effects of inflammatory markers on the association between mds and prognosis in cancer survivors.
Subgroup analysis
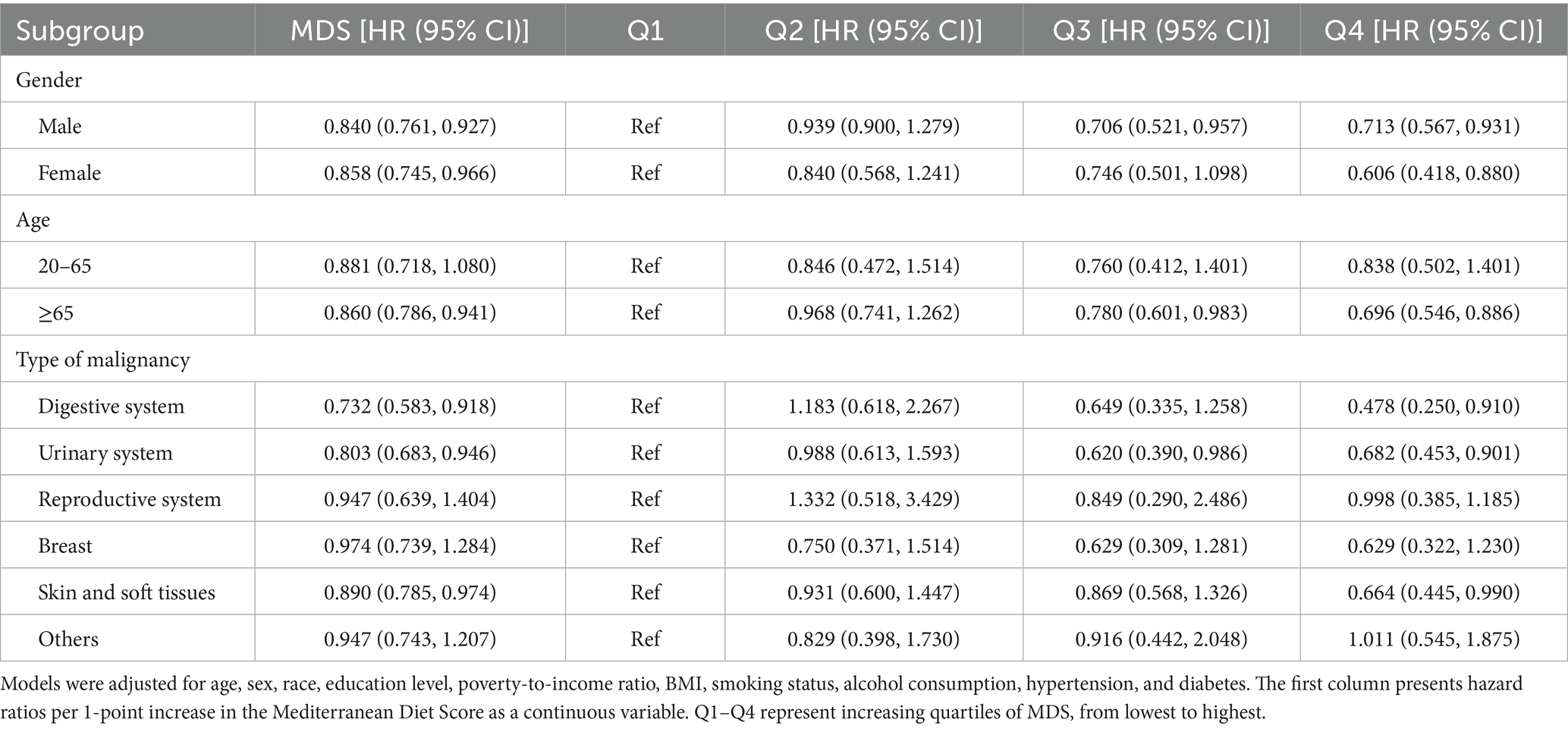
Subgroup analyses demonstrated that the inverse association between MDS and all-cause mortality was largely consistent across the demographic and clinical strata, with several notable differences in effect magnitude. Both male and female participants exhibited significant protective associations, with a stronger effect observed in female participants (HR = 0.606, 95% CI: 0.418–0.880) than in male participants (HR = 0.713, 95% CI: 0.567–0.931). The association was more pronounced among participants aged ≥65 years (HR = 0.696, 95% CI: 0.546–0.886) compared to their younger counterparts, where the trend did not reach statistical significance.
Greater benefits were also observed in current smokers and drinkers, suggesting that adherence to the Mediterranean diet may mitigate some of the adverse effects associated with these behaviors. Individuals with lower BMI and higher socioeconomic status derived more pronounced survival benefits.
Stratification by cancer type revealed heterogeneity in effect size. The strongest inverse associations were observed among survivors of digestive system, urinary system, and skin/soft tissue cancers, whereas associations were weaker or non-significant in other cancer types. These patterns are summarized in Table 4 and Supplement Table 1.
Sensitivity analysis
To evaluate the robustness of the findings, multiple sensitivity analyses were conducted. First, participants who died within 1 year of the interview were excluded, and the analysis was repeated. Second, data from each NHANES cycle were sequentially removed to assess the stability of the effect estimates. Across all scenarios, the inverse association between MDS and all-cause mortality remained consistent. Additionally, the results obtained from different model specifications and methodological approaches were largely similar, further supporting the reliability of the main findings.
Discussion
In this nationally representative cohort of cancer survivors from the NHANES dataset, we found that greater adherence to the MDS was significantly associated with reduced all-cause mortality. This association remained robust across progressively adjusted Cox regression models, was consistent in multiple subgroup and sensitivity analyses, and exhibited a linear dose–response pattern in RCS modeling. Furthermore, the mediation analysis suggested that specific inflammatory and oxidative stress-related biomarkers—particularly RDW and NEU—may partially mediate this relationship. While several prior NHANES-based studies have linked dietary patterns to mortality in the general population or patients with cardiometabolic diseases, few have focused specifically on cancer survivors. Moreover, most existing analyses have not explored the underlying biological mechanisms. By applying mediation analysis and incorporating inflammatory biomarkers such as RDW and NEU, our study offers mechanistic insights into how dietary adherence may affect survival in this high-risk population.
Our results align with previous findings that link MDS to improved survival in the general populations and among individuals with chronic diseases such as cardiovascular disease, diabetes, and metabolic syndrome (34–37). However, studies specifically focusing on cancer survivors remain limited. The current analysis highlights that, even among individuals who have undergone the physiologic burden of malignancy and its treatment, dietary quality may serve as an important prognostic indicator. This finding underscores the potential of dietary interventions as a modifiable post-diagnosis factor that may influence long-term outcomes in cancer survivorship.
The biological plausibility of these findings is supported by several mechanisms. The Mediterranean diet is rich in anti-inflammatory and antioxidant nutrients, such as monounsaturated fats, polyphenols, dietary fiber, and omega-3 fatty acids, all of which have been shown to modulate key pathways involved in carcinogenesis, immune regulation, and systemic inflammation (13, 38, 39). In the mediation analysis, RDW accounted for 18.5% of the total effect of MDS on mortality, followed by NEU (7.8%) and TB (9.0%). These biomarkers have previously been associated with poor prognosis in various cancers and may reflect systemic inflammation, oxidative stress, or bone marrow dysfunction (40–45). Although RDW, NEU, and TB were found to partially mediate the association between MDS and mortality, the overall mediation proportion was modest. This finding suggests that other unmeasured pathways may play a more prominent role in this relationship.
The consistency of the association across subgroups further strengthens the robustness of the results. Notably, the inverse relationship between MDS and mortality was more pronounced among older adults, females, individuals with lower BMI, and those with higher socioeconomic status, as reflected by PIR. These interactions may reflect differential susceptibility to the benefits of dietary quality, possibly due to underlying metabolic or immunologic differences. The stronger protective effect observed in smokers and drinkers is particularly noteworthy, suggesting that dietary modification may mitigate some of the adverse health risks associated with these behaviors (46, 47). These findings provide compelling evidence that, even among high-risk populations, dietary quality remains an actionable target. Biologically, this may be explained by the antioxidant and anti-inflammatory properties of the Mediterranean diet, which could counteract oxidative stress and systemic inflammation induced by smoking or alcohol consumption. Previous research has shown that high adherence to anti-inflammatory diets significantly lowers inflammatory biomarkers in high-risk populations, including smokers, lending support to this hypothesis. Nonetheless, residual confounding cannot be ruled out, as individuals with healthy dietary habits within these high-risk groups may also differ in other unmeasured lifestyle or health-related factors. Thus, these findings should be interpreted with caution and warrant further investigation in stratified or intervention-based studies.
Stratified analyses by cancer type revealed that the association between MDS and survival was strongest among patients with digestive system, urinary system, and skin and soft tissue malignancies. This may be due to the direct interaction of diet with gastrointestinal mucosal integrity, microbiota composition, and systemic inflammation, which are known to influence both local and systemic carcinogenic processes (48–50). The less consistent findings among reproductive, breast, and other cancer types may reflect smaller sample sizes or the influence of hormonal and genetic factors that attenuate the impact of dietary patterns. Additionally, the wide confidence intervals observed in several of these subgroups suggest limited statistical power, and the findings should therefore be interpreted as exploratory.
While the robustness of our findings was supported by sensitivity analyses and consistent subgroup patterns, the potential for reverse causality cannot be entirely excluded. It is possible that cancer survivors with better baseline health or greater health awareness were more likely to adhere to a Mediterranean-style diet, leading to an overestimation of its protective effect. To address this concern, we performed a sensitivity analysis excluding participants who died within 1 year of follow-up to reduce the likelihood of bias from pre-existing illness. Nonetheless, prospective or interventional studies are needed to establish temporality and causality with greater confidence.
Nonetheless, this study has several limitations. Residual confounding cannot be entirely ruled out despite adjustment for a wide range of covariates. Dietary data were based on 24-h recall interviews, which may not accurately capture long-term dietary patterns and are subject to recall and reporting bias. Moreover, cancer stage, treatment modalities, and time since diagnosis were not available in the NHANES dataset, limiting the ability to stratify analyses by disease severity or treatment status. Finally, although mediation analysis provides insights into potential biological pathways, it cannot fully disentangle complex interactions, and the proportion of mediation was relatively modest. Cancer-specific mortality outcomes, which could have provided more targeted insights, were not analyzed due to limited ICD-10 resolution and small event counts for specific cancer types.
In conclusion, higher adherence to the Mediterranean diet is independently associated with improved survival among cancer survivors in the United States. These findings highlight the potential of dietary quality as a modifiable prognostic factor in cancer survivorship and support the incorporation of Mediterranean dietary principles into post-diagnosis lifestyle recommendations. Specifically, dietary counseling interventions that emphasize Mediterranean components could be integrated into routine survivorship care, particularly targeting high-risk groups such as smokers, drinkers, or individuals with digestive or urinary system malignancies. Future prospective and interventional studies—including randomized controlled trials—are needed to confirm causality, evaluate dietary responsiveness across different cancer types, and determine the long-term efficacy of tailored nutritional interventions in improving survivorship outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by NCHS Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
BZ: Project administration, Writing – original draft, Formal analysis, Resources, Methodology, Validation, Conceptualization, Supervision, Visualization, Data curation, Software, Investigation. LD: Funding acquisition, Software, Conceptualization, Writing – original draft, Supervision, Data curation, Visualization, Investigation, Resources, Formal analysis, Validation. CZ: Investigation, Writing – original draft, Validation, Conceptualization, Project administration, Supervision. SH: Resources, Funding acquisition, Project administration, Writing – review & editing, Formal analysis, Software, Validation, Writing – original draft, Methodology, Supervision, Conceptualization, Visualization, Investigation, Data curation. GW: Conceptualization, Writing – review & editing, Methodology, Funding acquisition, Software, Validation, Investigation, Project administration, Formal analysis, Resources, Supervision, Data curation, Visualization, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by grants from (1) CAMS Innovation Fund for Medical Sciences (CIFMS) (grant no. 2021-I2M-1-015, 2021-I2M-1-061, 2021-I2M-1-013, 2022-I2M-C&T-B-054), (2) the Sanming Project of Medicine in Shenzhen (no. SZSM201911008), (3) Capital’s Funds for Health Improvement and Research (grant no. CRF2020-2-4025), (4) Beijing Hope Run Special Fund of Cancer Foundation of China (grant no. LC2021A03). (5) Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2024-JKCS-30), (6) Beijing Hope Run Special Fund of Cancer Foundation of China (LC2022B05), and (7)National High Level Hospital Clinical Research Funding.
Acknowledgments
The authors are grateful to all the participants and staff members involved in the NHANES.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1607522/full#supplementary-material
References
1. Gallicchio, L, Burnett-Hartman, AN, Filipski, KK, Shelburne, N, and Freedman, AN. National cancer institute-funded cancer epidemiology survivor cohorts: overview, progress, and opportunities. Cancer Epidemiol Biomarkers Prev. (2025) 34:627–40. doi: 10.1158/1055-9965.EPI-24-1750
2. Hudson, SV, Mollica, MA, Crystal, R, Hahn, EE, O'Malley, DM, Radhakrishnan, A, et al. Enhancing capacity for primary care research in cancer survivorship: National Cancer Institute Meeting Report. J Natl Cancer Inst. (2024) 117:827–32. doi: 10.1093/jnci/djae276
3. Singh, A, Jain, AG, Hamilton, BK, and Adjei, A. Care models for cancer survivors. Annu Rev Med. (2025) 76:225–41. doi: 10.1146/annurev-med-042423-044004
4. Fretwell, A, Dobson, C, Orange, ST, and Corfe, BM. Diet and physical activity advice for colorectal cancer survivors: critical synthesis of public-facing guidance. Support Care Cancer. (2024) 32:609. doi: 10.1007/s00520-024-08797-5
5. Joseph, R, Hart, NH, Bradford, N, Wallen, MP, Han, CY, Pinkham, EP, et al. Essential elements of optimal dietary and exercise referral practices for cancer survivors: expert consensus for medical and nursing health professionals. Support Care Cancer. (2022) 31:46. doi: 10.1007/s00520-022-07509-1
6. Lan, T, Wang, M, Ehrhardt, MJ, Jiang, S, Lanctot, JQ, Armstrong, GT, et al. Adherence to healthy diet and risk of cardiovascular disease in adult survivors of childhood cancer in the St. Jude lifetime cohort: a cross-sectional study. BMC Med. (2023) 21:242. doi: 10.1186/s12916-023-02956-x
7. Lan, T, Wang, M, Williams, AM, Ehrhardt, MJ, Lanctot, JQ, Jiang, S, et al. Plant-based, fast-food, Western-contemporary, and animal-based dietary patterns and risk of premature aging in adult survivors of childhood cancer: a cross-sectional study. BMC Med. (2025) 23:120. doi: 10.1186/s12916-025-03940-3
8. Maino Vieytes, CA, Zhu, R, Gany, F, Koester, BD, and Arthur, AE. Dietary patterns among U.S. food insecure cancer survivors and the risk of mortality: NHANES 1999-2018. Cancer Causes Control. (2024) 35:1075–88. doi: 10.1007/s10552-024-01868-2
9. Abene, J, and Deng, J. Evaluating the role of dietary interventions in reducing chemotherapy toxicities in cancer patients: a systematic review. J Cancer Surviv. (2025). doi: 10.1007/s11764-025-01777-6
10. Abreu, MT, Devkota, S, and Issokson, K. A Mediterranean diet for Crohn's disease: embracing colorful diversity to improve the microbiome. Gastroenterology. (2025) 168:872–4. doi: 10.1053/j.gastro.2025.02.003
11. Aguilera-Buenosvinos, I, Morales Berstein, F, González-Gil, EM, Dossus, L, Gunter, MJ, Biessy, C, et al. Adherence to the Mediterranean diet and obesity-linked Cancer risk in EPIC. JAMA Netw Open. (2025) 8:e2461031. doi: 10.1001/jamanetworkopen.2024.61031
12. Li, SY, Lu, ZH, Leung, J, Su, Y, Yu, B, and Kwok, T. Dietary patterns modify the association between body mass index and mortality in older adults. Clin Nutr. (2025) 46:20–9. doi: 10.1016/j.clnu.2025.01.016
13. Reyneke, GL, Lambert, K, and Beck, EJ. Food-based indexes and their association with dietary inflammation. Adv Nutr. (2025) 16:100400. doi: 10.1016/j.advnut.2025.100400
14. Marino, P, Mininni, M, Deiana, G, Marino, G, Divella, R, Bochicchio, I, et al. Healthy lifestyle and cancer risk: modifiable risk factors to prevent cancer. Nutrients. (2024) 16:800. doi: 10.3390/nu16060800
15. Reytor-González, C, Zambrano, AK, Montalvan, M, Frias-Toral, E, Simancas-Racines, A, and Simancas-Racines, D. Adherence to the Mediterranean diet and its association with gastric cancer: health benefits from a Planeterranean perspective. J Transl Med. (2024) 22:483. doi: 10.1186/s12967-024-05176-w
16. Panagiotakos, DB, Pitsavos, C, Arvaniti, F, and Stefanadis, C. Adherence to the Mediterranean food pattern predicts the prevalence of hypertension, hypercholesterolemia, diabetes and obesity, among healthy adults; the accuracy of the MedDietScore. Prev Med. (2007) 44:335–40. doi: 10.1016/j.ypmed.2006.12.009
17. Di Maso, M, Augustin, LSA, Toffolutti, F, Stocco, C, Dal Maso, L, Jenkins, DJA, et al. Adherence to Mediterranean diet, physical activity and survival after prostate cancer diagnosis. Nutrients. (2021) 13:243. doi: 10.3390/nu13010243
18. Di Maso, M, Dal Maso, L, Augustin, LSA, Puppo, A, Falcini, F, Stocco, C, et al. Adherence to the Mediterranean diet and mortality after breast cancer. Nutrients. (2020) 12:3649. doi: 10.3390/nu12123649
19. El Kinany, K, Hatime, Z, El Asri, A, Benslimane, A, Mint Sidi Deoula, M, Zarrouq, B, et al. Adherence to the Mediterranean diet and colorectal cancer risk: a large case control study in the Moroccan population. Public Health Nutr. (2025) 28:e48. doi: 10.1017/S1368980025000199
20. Gregg, JR, Zhang, X, Chapin, BF, Ward, JF, Kim, J, Davis, JW, et al. Adherence to the Mediterranean diet and grade group progression in localized prostate cancer: an active surveillance cohort. Cancer. (2021) 127:720–8. doi: 10.1002/cncr.33182
21. De Matteis, C, Crudele, L, Gadaleta, RM, Di Buduo, E, Novielli, F, Petruzzelli, S, et al. Low adherence to Mediterranean diet characterizes metabolic patients with gastrointestinal Cancer. Nutrients. (2024) 16:630. doi: 10.3390/nu16050630
22. De Matteis, C, Crudele, L, Battaglia, S, Loconte, T, Rotondo, A, Ferrulli, R, et al. Identification of a novel score for adherence to the Mediterranean diet that is inversely associated with visceral adiposity and cardiovascular risk: the Chrono med diet score (CMDS). Nutrients. (2023) 15:1910. doi: 10.3390/nu15081910
23. Moazzen, S, van der Sloot, KWJ, Bock, GH, and Alizadeh, BZ. Systematic review and meta-analysis of diet quality and colorectal cancer risk: is the evidence of sufficient quality to develop recommendations? Crit Rev Food Sci Nutr. (2021) 61:2773–82. doi: 10.1080/10408398.2020.1786353
24. Negrati, M, Razza, C, Biasini, C, Di Nunzio, C, Vancini, A, Dall'Asta, M, et al. Mediterranean diet affects blood circulating lipid-soluble micronutrients and inflammatory biomarkers in a cohort of breast cancer survivors: results from the SETA study. Nutrients. (2021) 13:3482. doi: 10.3390/nu13103482
25. Panagiotakos, DB, Pitsavos, C, and Stefanadis, C. Dietary patterns: a Mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. Nutr Metab Cardiovasc Dis. (2006) 16:559–68. doi: 10.1016/j.numecd.2005.08.006
26. Jayanama, K, Theou, O, Godin, J, Cahill, L, Shivappa, N, Hébert, JR, et al. Relationship between diet quality scores and the risk of frailty and mortality in adults across a wide age spectrum. BMC Med. (2021) 19:64. doi: 10.1186/s12916-021-01918-5
27. Estruch, R, Ros, E, Salas-Salvadó, J, Covas, MI, Corella, D, Arós, F, et al. Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. (2018) 378:e34. doi: 10.1056/NEJMoa1800389
28. Zha, B, Liu, Y, and Xu, H. Associations of mixed urinary metals exposure with metabolic syndrome in the US adult population. Chemosphere. (2023) 344:140330. doi: 10.1016/j.chemosphere.2023.140330
29. Zhuang, T, Zhang, Y, Ren, X, Pan, Q, and Sun, J. Non-linear association between interpregnancy interval after vaginal delivery and singleton preterm birth: a retrospective cohort study. BMC Pregnancy Childbirth. (2025) 25:275. doi: 10.1186/s12884-025-07373-x
30. Kang, M, Song, S, Cho, HJ, Kim, Z, Youn, HJ, Cho, J, et al. Adherence to the American Cancer Society guidelines on nutrition and physical activity for cancer survivors and biomarkers of inflammation among breast cancer survivors. Epidemiol Health. (2024) 46:e2024026. doi: 10.4178/epih.e2024026
31. Bekar, C, Armagan, B, Sari, A, and Ayaz, A. Evaluation of serum total antioxidant level, nutritional status and Mediterranean diet adherence of adult women with rheumatoid arthritis: a case-control study. Br J Nutr. (2025) 133:239–45. doi: 10.1017/S0007114524003386
32. Demirer, B, Yardımcı, H, and Erem Basmaz, S. Inflammation level in type 2 diabetes is associated with dietary advanced glycation end products, Mediterranean diet adherence and oxidative balance score: a pathway analysis. J Diabetes Complicat. (2023) 37:108354. doi: 10.1016/j.jdiacomp.2022.108354
33. Haskey, N, Letef, C, Sousa, JA, Yousuf, M, Taylor, LM, McKay, DM, et al. Exploring the connection between erythrocyte membrane fatty acid composition and oxidative stress in patients undergoing the Crohn's disease therapeutic diet intervention (CD-TDI). Ther Adv Gastroenterol. (2025) 18:17562848251314827. doi: 10.1177/17562848251314827
34. Guasch-Ferré, M, and Willett, WC. The Mediterranean diet and health: a comprehensive overview. J Intern Med. (2021) 290:549–66. doi: 10.1111/joim.13333
35. Oliveira, JS, da Silva, JA, de Freitas, BVM, Alfenas, RCG, and Bressan, J. A Mediterranean diet improves glycation markers in healthy people and in those with chronic diseases: a systematic review of clinical trials. Nutr Rev. (2025) 83:e317–31. doi: 10.1093/nutrit/nuae045
36. Xiao, Y, Xiao, X, Zhang, X, Yi, D, Li, T, Hao, Q, et al. Mediterranean diet in the targeted prevention and personalized treatment of chronic diseases: evidence, potential mechanisms, and prospects. EPMA J. (2024) 15:207–20. doi: 10.1007/s13167-024-00360-w
37. Xiong, Y, Shi, X, Xiong, X, Li, S, Zhao, H, Song, H, et al. A systematic review and meta-analysis of randomized controlled trials: effects of Mediterranean diet and low-fat diet on liver enzymes and liver fat content of NAFLD. Food Funct. (2024) 15:8248–57. doi: 10.1039/d4fo01461h
38. Frye, BM, Negrey, JD, Johnson, CSC, Kim, J, Barcus, RA, Lockhart, SN, et al. Mediterranean diet protects against a neuroinflammatory cortical transcriptome: associations with brain volumetrics, peripheral inflammation, social isolation, and anxiety in nonhuman primates (Macaca fascicularis). Brain Behav Immun. (2024) 119:681–92. doi: 10.1016/j.bbi.2024.04.016
39. Wang, Z, Yuan, C, Zhang, Y, Abdelaty, NS, Chen, C, Shen, J, et al. Food inflammation index reveals the key inflammatory components in foods and heterogeneity within food groups: how do we choose food? J Adv Res. (2024) 74:87–98. doi: 10.1016/j.jare.2024.10.010
40. Wang, P, Moses, AS, Li, C, Chen, S, Qi, X, Xu, K, et al. Prognosis factors of predicting survival in spontaneously ruptured hepatocellular carcinoma. Hepatol Int. (2022) 16:1330–8. doi: 10.1007/s12072-022-10403-x
41. Wang, X, He, S, Gong, X, Lei, S, Zhang, Q, Xiong, J, et al. Neutrophils in colorectal cancer: mechanisms, prognostic value, and therapeutic implications. Front Immunol. (2025) 16:1538635. doi: 10.3389/fimmu.2025.1538635
42. Peng, D, Li, ZW, Liu, F, Liu, XR, and Wang, CY. Predictive value of red blood cell distribution width and hematocrit for short-term outcomes and prognosis in colorectal cancer patients undergoing radical surgery. World J Gastroenterol. (2024) 30:1714–26. doi: 10.3748/wjg.v30.i12.1714
43. Chen, GP, Huang, Y, Yang, X, and Feng, JF. A nomogram to predict prognostic value of red cell distribution width in patients with esophageal cancer. Mediat Inflamm. (2015) 2015:854670. doi: 10.1155/2015/854670
44. Cheng, J, Wang, S, Jia, J, Chen, Q, Song, Y, and Li, J. Association between pre-treatment and post-treatment 3-month red cell distribution width with three-year prognosis of prostate cancer. J Inflamm Res. (2021) 14:6115–27. doi: 10.2147/JIR.S342272
45. Hiraoka, A, Kumada, T, Michitaka, K, and Kudo, M. Newly proposed ALBI grade and ALBI-T score as tools for assessment of hepatic function and prognosis in hepatocellular carcinoma patients. Liver Cancer. (2019) 8:312–25. doi: 10.1159/000494844
46. Lin, J, Yang, R, Zhang, S, Li, H, Li, S, Yang, H, et al. Associations of the inflammatory diet index and smoking status with the risk of chronic obstructive pulmonary disease and lung cancer. Food Funct. (2023) 14:6083–92. doi: 10.1039/D2FO03429H
47. Wang, X, Peng, Y, Liu, F, Wang, P, Si, C, Gong, J, et al. Joint association of biological aging and lifestyle with risks of cancer incidence and mortality: a cohort study in the UK biobank. Prev Med. (2024) 182:107928. doi: 10.1016/j.ypmed.2024.107928
48. Chen, J, Liu, X, Zou, Y, Gong, J, Ge, Z, Lin, X, et al. A high-fat diet promotes cancer progression by inducing gut microbiota-mediated leucine production and PMN-MDSC differentiation. Proc Natl Acad Sci USA. (2024) 121:e2306776121. doi: 10.1073/pnas.2306776121
49. John Kenneth, M, Tsai, HC, Fang, CY, Hussain, B, Chiu, YC, and Hsu, BM. Diet-mediated gut microbial community modulation and signature metabolites as potential biomarkers for early diagnosis, prognosis, prevention and stage-specific treatment of colorectal cancer. J Adv Res. (2023) 52:45–57. doi: 10.1016/j.jare.2022.12.015
Keywords: Mediterranean diet, cancer survivors, all-cause mortality, NHANES, cox regression, mediation analysis
Citation: Zha B, Dou L, Zhang C, He S and Wang G (2025) Adherence to the Mediterranean diet and mortality in cancer survivors: a Nationwide study with mediation and subgroup analyses. Front. Nutr. 12:1607522. doi: 10.3389/fnut.2025.1607522
Edited by:
Paula Ravasco, Catholic University of Portugal, PortugalReviewed by:
Carlo De Matteis, University of Bari Aldo Moro, ItalyMehmet Emin Arayici, Dokuz Eylül University, Türkiye
Copyright © 2025 Zha, Dou, Zhang, He and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guiqi Wang, d2FuZ2d1aXFAMTI2LmNvbQ==; Shun He, aGVzaHVuQGNpY2Ftcy5hYy5jbg==
†These authors have contributed equally to this work
 Bowen Zha
Bowen Zha Lizhou Dou†
Lizhou Dou†