- 1School of Public Health, Guangxi Medical University, Nanning, China
- 2School of Nursing, Shandong First Medical University and Shandong Academy of Medical Sciences, Taian, China
Background: Cardiovascular-kidney-metabolic (CKM) syndrome is a complex condition that encompasses cardiovascular, renal, and metabolic disorders. Dietary metal intake plays a crucial role in maintaining normal physiological functions. This study aims to examine the relationship between dietary intake of multiple metals and CKM syndrome.
Methods: We analyzed data from 15,233 participants aged 20–79 years in the National Health and Nutrition Examination Survey (NHANES) 2003–2018. Dietary metal intake included nine metals: potassium (K), calcium (Ca), magnesium (Mg), phosphorus (P), iron (Fe), copper (Cu), zinc (Zn), and selenium (Se). CKM syndrome was classified into non-advanced (stages 0–2) and advanced (stages 3–4) stages. We employed weighted logistic regression, restricted cubic splines (RCS) regression, weighted quantile sum (WQS) regression, and quantile-based g computation (qgcomp) models to evaluate the associations between individual metal intake and metal intake mixtures with CKM stages. Subgroup analysis was used to explore potential interaction effect between metal intake and other variables.
Results: Weighted logistic regression models showed that Q2 (≤0.80–1.12 mg/d) (OR = 0.74, 95% CI = 0.60, 0.92), Q3 (≤1.12–1.53 mg/d) (OR = 0.74, 95% CI = 0.58, 0.93) and Q4 (>1.53 mg/d) (OR = 0.73, 95% CI = 0.55, 0.95) groups of Cu intake were significantly associated with a reduced incidence of advanced CKM stages compared with Q1 (≤0.80 mg/d) group. The RCS regression models indicated that higher Cu intake was significantly associated with a lower risk of advanced CKM stages (p for overall < 0.05). WQS regression and qgcomp models did not reveal significant effect of the mixture. Subgroup analysis found that the effect of Cu was robust in various subgroups.
Conclusion: In conclusion, higher dietary intake Cu was linked to a reduced prevalence of advanced CKM stages in the U. S. adult population.
1 Introduction
The Cardiovascular-Kidney-Metabolic (CKM) syndrome, a recently recognized multi-systemic affliction as delineated by the American Heart Association (AHA), involves the complex interactions between obesity, diabetes mellitus, chronic renal conditions, and cardiovascular illnesses (1, 2). This syndrome significantly influences patient outcomes and is associated with elevated mortality rates that surpass the aggregate risks of each individual condition (3). In the United States, the incidence of CKM is escalating. Information gleaned from the National Health and Nutrition Examination Survey (NHANES) reveals that nearly 90% of the adult population in the US fulfill the criteria for CKM stage 1 or beyond, with roughly 15% being categorized under the advanced stages of CKM (stages 3 or 4) (4). Therefore, it is particularly important to find effective measures to prevent CKM syndrome.
Metal elements, including constant and trace metals, are key nutrients for maintaining normal physiological functions of the body (5, 6). Previous studies have shown that metals are closely related to cardiovascular, renal, and metabolic processes. Calcium exerts its influence on the susceptibility to cardiovascular disease (CVD) via a variety of pathways, encompassing modulation of serum cholesterol levels, insulin release, and insulin sensitivity, as well as impacting vasodilation, adiposity, and vascular calcification. A deficiency in zinc and copper may elevate the risk of developing coronary artery disease (7). In addition, dietary zinc and selenium intake can significantly reduce the risk of chronic kidney disease (CKD) in adults (8, 9). Magnesium, phosphorus and selenium may have beneficial effects on lipid metabolism (10, 11). CKM syndrome involves complex interactions between metabolism, CKD, and cardiovascular disease, and it is unclear what role metals play in CKM syndrome. Importantly, previous studies have mostly focused on single metals, and the interactions between metals may affect each other’s physiological functions (12, 13).
To address this gap, we conducted a large-scale cross-sectional study using the National Health and Nutrition Examination Survey (NHANES) data to investigate the association between multiple metal intakes in American adults and CKM syndrome. We hope to reveal the association between dietary metal mixtures and CKM and provide clues from a dietary perspective for the prevention and treatment of CKM syndrome.
2 Methods
2.1 The study design and population
The NHANES is an extensive research initiative conducted by the Centers for Disease Control and Prevention (CDC) in the United States to evaluate the health condition and associated health determinants among the U. S. population. Furthermore, the programs of NHANES have been sanctioned by the ethical review board of the National Center for Health Statistics (NCHS) (14).
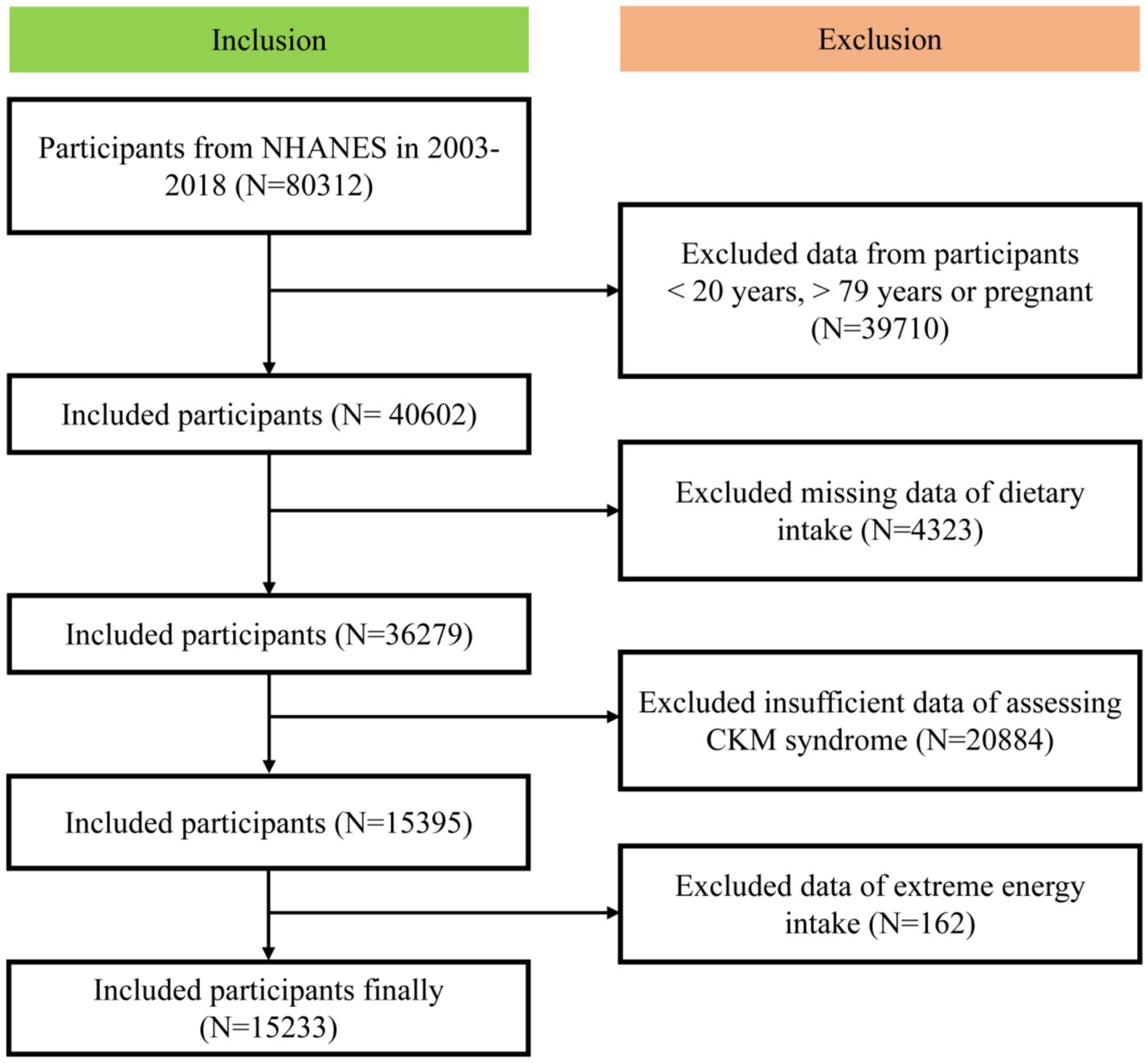
In this study, we included participants from the NHANES between 2003 and 2018. The exclusion criteria were: under 20 years old, over 79 years old, pregnant, missing dietary intake data, with insufficient data for assessing CKM syndrome and with extreme energy intake data. Extreme energy intake was total energy intake < 500 kcal/day or > 5,000 kcal/day for females or <500 kcal/day or > 8,000 kcal/day males (15). Ultimately, our analysis included a final cohort of 15,233 participants (Figure 1).
2.2 Assessment of CKM syndrome
The AHA has released diagnostic criteria for CKM syndrome, but the indicators involved in this criterion cannot be fully found in the NHANES database. Therefore, we referred to the diagnostic criteria applicable to the NHANES database mentioned in previous literature, which are very similar to those proposed by the AHA (4). Overall, CKM syndrome consists of 5 stages (stages 0 to 4), divided according to each individual’s obesity, cardiovascular, renal, and metabolic status. Please refer to Supplementary Table 1 for detailed diagnostic criteria.
Complex calculated indicators included the estimated glomerular filtration rate (eGFR) and 10-year cardiovascular disease risk. The eGFR was calculated using the 2021 race-and ethnicity-free Chronic Kidney Disease Epidemiology Collaboration creatinine equation which included serum creatinine and sex (16). And the 10-year CVD risk was assessed using prediction equations for absolute risk of total CVD, which included factors such as sex, age, blood lipids, blood pressure, diabetes, smoking status, eGFR, and the use of lipid-lowering and antihypertensive medications (17). High CVD risk was operationally defined as a ≥ 20% 10-year CVD risk. Please refer to Supplementary Table 2 for detailed calculation process. CKD stage was determined by eGFR and (Urine Albumin-to-Creatinine Ratio) UACR (18). We defined the CKM stage as a binary variable, with 0, 1, or 2 being nonadvanced CKM stages and stages 3 or 4 being advanced CKM stages (19).
2.3 Dietary metal intake
Dietary intakes were from a 24-h dietary review survey. Trained investigators conduct interviews with respondents, with the first dietary survey conducted in person and the second conducted through telephone interviews 3 to 10 days after the first survey. Considering the accuracy and authenticity of face-to-face interview data, we only included data from the first dietary survey (20). In this study, we included 9 types of dietary metal intake, including potassium (K), calcium (Ca), magnesium (Mg), phosphorus (P), iron (Fe), copper (Cu), zinc (Zn), and selenium (Se).
2.4 Covariates
Covariates encompassed a spectrum of demographic, lifestyle factors and energy intake. Demographic factors comprised gender (male, female), age (20–39, 40–59, and 60 years and above), ethnicity (Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, and other race including multi-racial), educational attainment (below high school, high school graduate/GED or equivalent, and above high school), household poverty-to-income ratio (PIR) (≤1.3, 1.3–3.5, and above 3.5), and marital status (married/living with partner, widowed/divorced/separated, and unmarried). Lifestyle factors included smoking status, drinking status, and physical exercise. Participants were also classified into nonsmokers, former smokers, and current smokers based on their smoking habits. The status of alcohol intake was ascertained by the quantity of alcohol consumed last year, including nondrinkers (no alcohol consume), moderate drinkers (≤ 2 drinks/day for male, ≤ 1 drinks/day for female), heavy drinkers (> 2 drinks/day for male, > 1 drinks/day for female) (21). Low physical activity was defined as no moderate or vigorous physical activity in a typical week from self-reporting (1). The daily energy intake came from the dietary survey on the first day.
2.5 Statistical analysis
All statistical models were completed using R (version 4.4.1). Two-tailed p values < 0.05 were considered statistically significant. Given the multi-stage sampling methodology employed in NHANES, we utilized the dietary survey weight “WTDRD1” to stand for the U. S. population (22). In the descriptive statistical analysis, continuous variables, encompassing energy intake and 9 dietary metal intakes, were depicted through weighted medians (25th, 75th), while categorical variables were exhibited as counts (weighted percentages). Participants were grouped into two categories: non-advanced stages and advanced stages. We contrasted the distribution differences of the variables between these two groups, employing weighted Kruskal-Wallis tests for continuous variables and weighted chi-square tests for categorical variables (23). Spearman rank correlation analysis was implemented to appraise the correlation of dietary metal intake (24). To address the missing values of covariates, aiming to optimize the sample size to the fullest extent, we applied multiple imputation techniques in the “MICE” package (25).
To investigate the relationship between individual dietary metal intake and advanced CKM stages, we applied weighted logistic regression. Each dietary metal intake was stratified into four groups based on its quartiles (Q1-Q4), with the Q1 group serving as a reference point (26). Three models were constructed in this segment: Model 1, the unadjusted model; Model 2, adjusted for demographic variables; Model 3, adjusted for all covariates. Restricted cubic splines (RCS) regression model according to the quantity of knots that meet the minimal Akaike information criterion (AIC) each dietary metal intake was applied to evaluate the potential nonlinear relationship between dietary metal intake and advanced CKM stages (27).
Weighted Quantile Sum (WQS) regression model is extensively utilized in environmental epidemiology to explore the impacts of pollutant mixtures on health outcomes. We substituted environmental pollutants with dietary metal intake and assessed the impact of a mixture of nine dietary metal intakes on advanced CKM stages. In essence, WQS regression categorized various dietary metal intakes into quantiles and computed a weighted index represents the combined effects of the mixture of 9 dietary metal intakes. Additionally, WQS regression established two models by hypothesizing a positive or negative correlation between the mixture and the outcome (28). Specifically, each dietary metal intake will possess a corresponding weight denoting its contribution to advanced CKM stages in each model. If the weight of a dietary metal intake surpasses 0.11 (1/9), it is deemed to play a predominant role in the mixture. The dataset was randomly divided into a training set (50%) and a validation set (50%), and was sampled 1,000 times to achieve more precise results. We also established a Quantile-based computation (gqcomp) model to substantiate the outcomes of WQS regression. This method integrates the straightforward inference of WQS regression with the adaptability of g-computation (29). Unlike WQS regression, the qgcomp model does not presuppose a positive or negative correlation between the mixture and the outcome separately. It also generates a weight for each component to articulate the significance of each component within the mixture. The WQS regression and qgcomp models are not yet suitable for weighted analysis of NHANES (30).
Ultimately, we established subgroup analyses to assess the potential interaction between the dietary metal mixture and subgroup variables including age, sex, smoking status, drinking status, physical activity and energy intake (Classified by median calorie count).
3 Results
3.1 Study population and dietary metal intake
The basic characteristics of 15,233 participants were displayed in Table 1. 12,931 (approximately 88.2%) were in nonadvanced stages of CKM and 2,302 participants (approximately 11.8%) were in advanced stages of CKM. Moreover, about half of the participants (50.2%) were female. By comparing the differences in the distribution of different demography and lifestyle variables between the two groups, we found that there were significant differences between the two groups in all the variables. In terms of metal intake, participants in the advanced stage might have lower levels of all metal intakes (All p < 0.05).
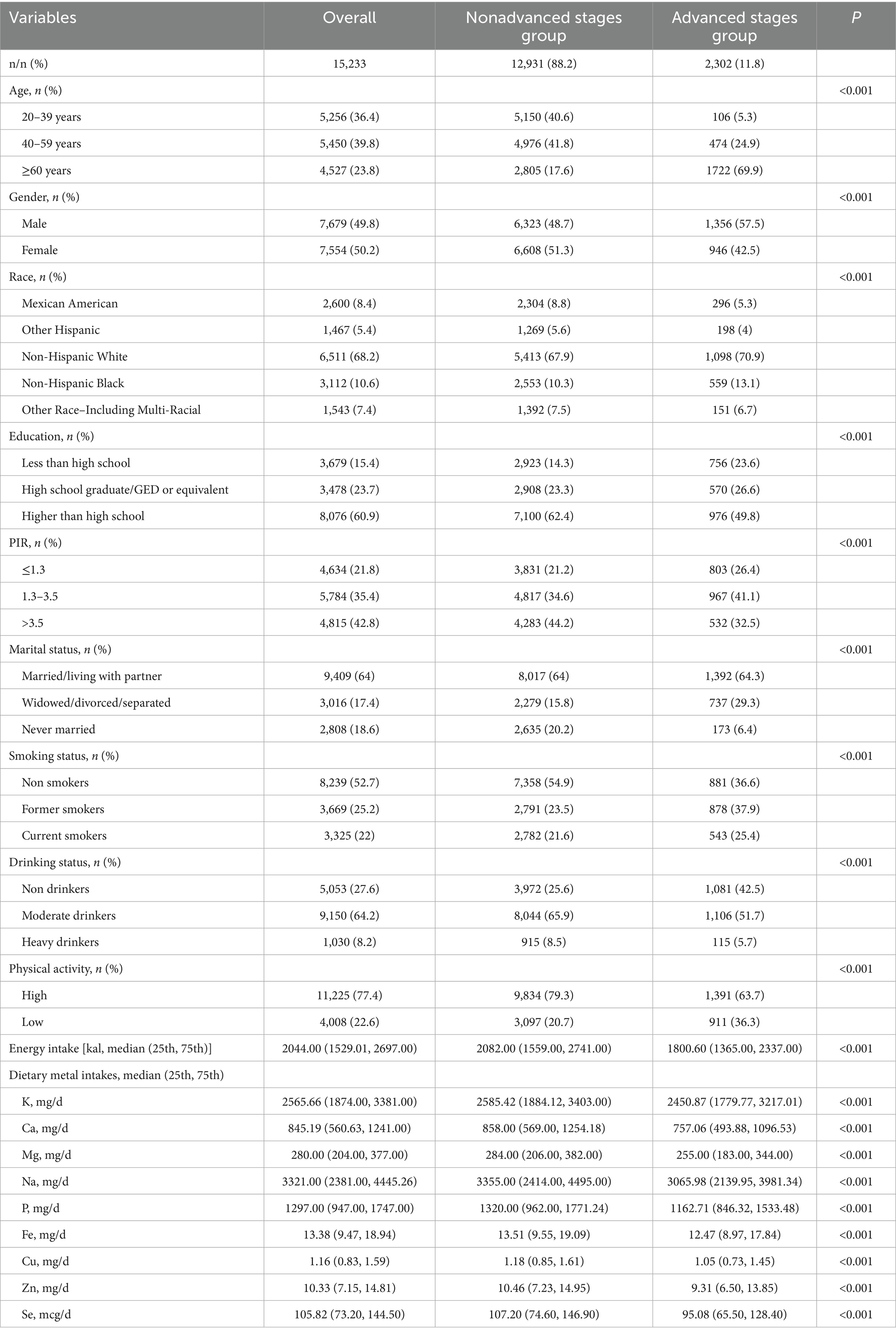
Table 1. Characteristics and dietary metal intakes of participants grouped by CKM stages in NHANES 2003–2018.
Supplementary Figure 1 showed the correlation heatmap of 9 metal intakes. All the metal intakes are positively correlated. The highest correlation was observed between Mg and Cu (Correlation coefficient = 0.85).
3.2 Associations between single metal intake and advanced CKM stages
Table 2 showed the associations between single metal intake and advanced CKM stages in weighted logistic regression models. The population was grouped into Q1-Q4 according to each metal intake. In the unadjusted model, K (Q4), Ca (Q3, Q4), Mg (Q2, Q3, Q4), Na (Q2, Q3, Q4), P (Q3, Q4), Fe (Q3, Q4), Cu (Q2, Q3, Q4), Zn (Q3, Q4) and Se (Q2, Q3, Q4) were negative with advanced CKM stages compared with Q1. After adjusting for demography variables, we found that K (Q4), Ca (Q4), Mg (Q2, Q4), Na (Q2, Q3, Q4), P (Q3, Q4), Fe (Q4), Cu (Q2, Q3, Q4), Zn (Q3, Q4) and Se (Q2, Q3, Q4) were still negative associations with advanced CKM stages compared with Q1. In the fully-adjusted model, only Q2 (OR = 0.74, 95% CI = 0.60, 0.92), Q3 (OR = 0.74, 95% CI = 0.58, 0.93) and Q4 (OR = 0.73, 95% CI = 0.55, 0.95) groups of Cu intake remain negative associations with advanced CKM stages compared with Q1 group (All p < 0.05).
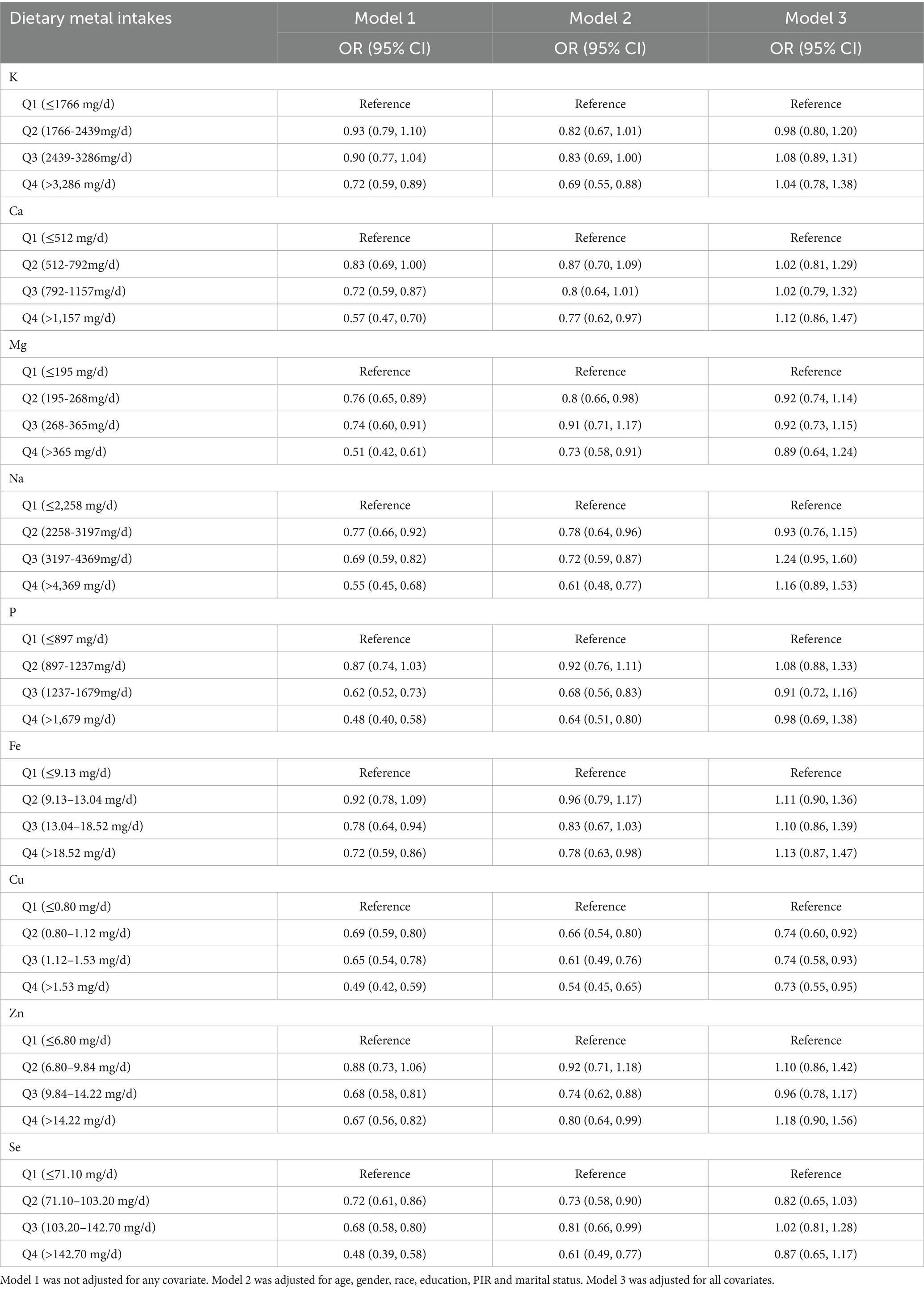
Table 2. The associations of single metal intake with advanced CKM stages in weighted logistic regression models.
The results of RCS regression models were displayed in Figure 2. After adjusting for all covariates, higher intake of Cu was linearly associated with lower risk of advanced CKM stages (p for overall < 0.05 and P for non-linear > 0.05). However, higher intake of Na was linearly associated with higher risk of advanced CKM stages (p for overall < 0.05 and P for non-linear > 0.05). No significant association was found between the intake of other metals and advanced CKM stages (All p for overall > 0.05).
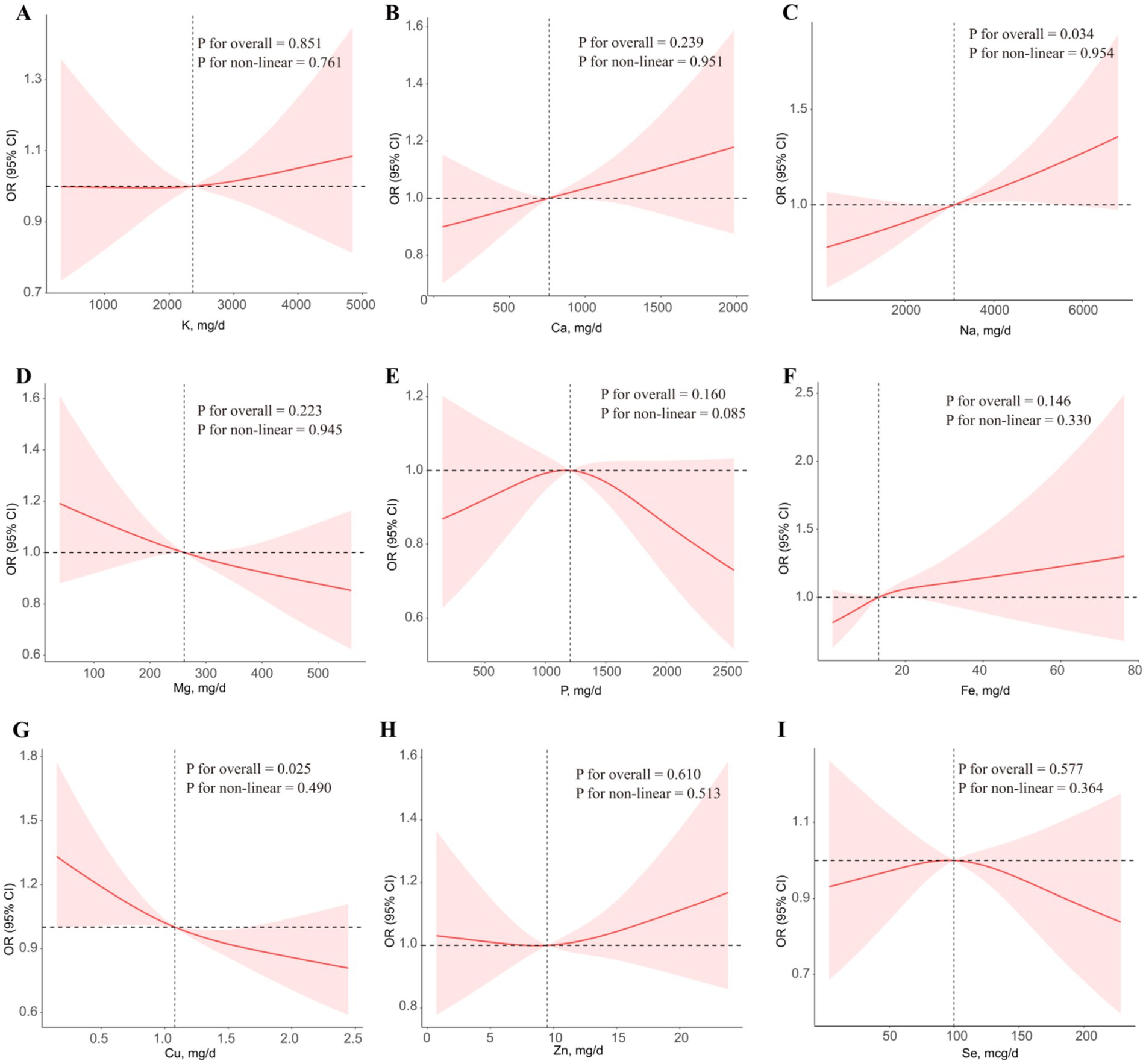
Figure 2. The associations of single metal intake with advanced CKM stages in RCS regression models. (A) K, (B) Ca, (C) Mg, (D) Na, (E) P, (F) Fe, (G) Cu, (H) Zn, (I) Se. All the models were adjusted for all covariates.
3.3 Associations between vitamin intake mixture and advanced CKM stages
Subsequently, the associations between the metal intake mixture and advanced CKM stages were examined using WQS regression and qgcomp models (Figure 3). Due to the single metal intake model indicating a significant negative correlation between copper intake and advanced CKM stages, we firstly hypothesized a negative relationship between metal intake mixture and advanced CKM stages. However, we did not find a significant effect of metal ingestion mixtures (OR: 1.10, 95% CI: 0.95, 1.26). Conversely, when we hypothesized a positive relationship between metal intake mixture and advanced CKM stages, no significant effect was observed either (OR: 1.13, 95% CI: 0.98, 1.29; Supplementary Figure 2). The qgcomp model yielded similar results to the WQS regression, corroborating an insignificant effect of metal ingestion mixtures (OR: 1.00, 95% CI: 0.94, 1.08; Figure 3B). Although the overall effect of multiple metal intake was not significant, copper intake seemed to be associated with a low risk of advanced CKM stages.
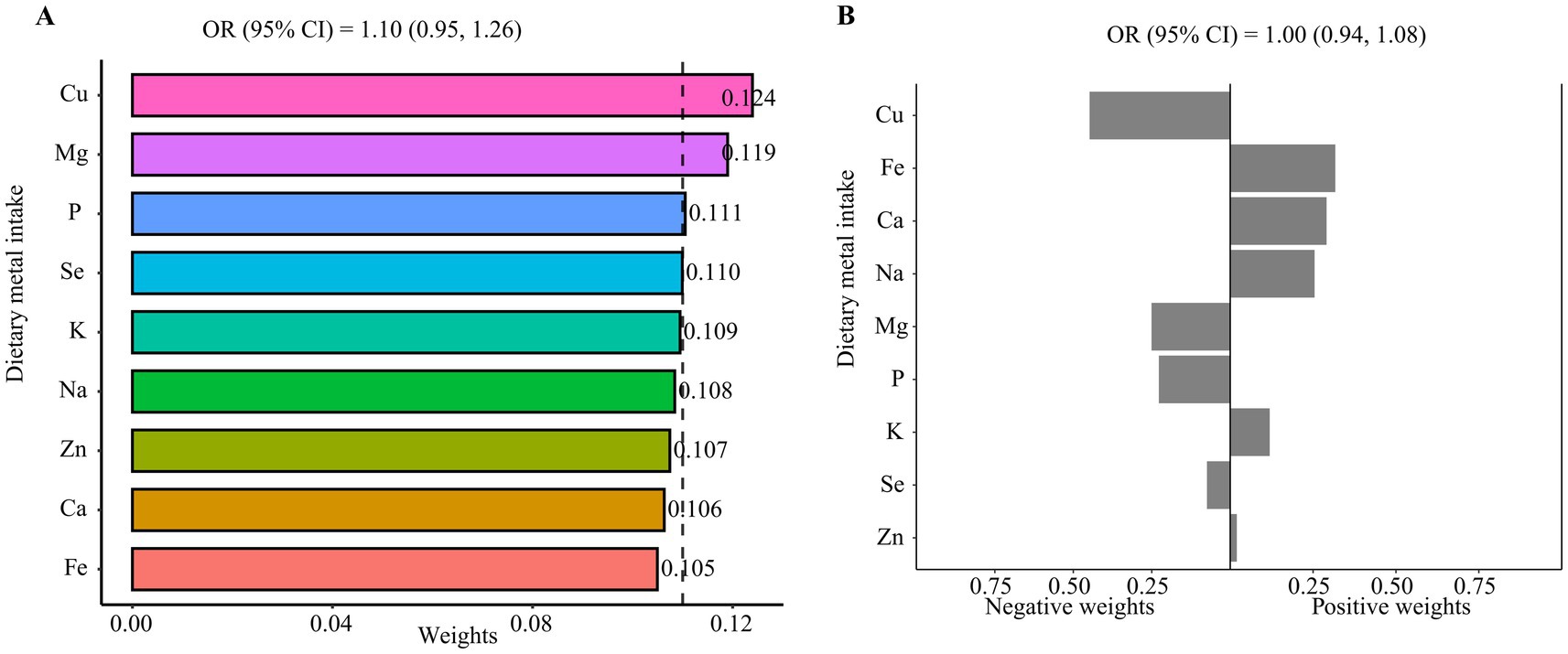
Figure 3. The associations of metal intake mixture with advanced CKM stages. (A) WQS regression model, (B) qgcomp model. All the models were adjusted for all covariates.
3.4 Subgroup analysis of dietary cu intake in relation to advanced CKM stages
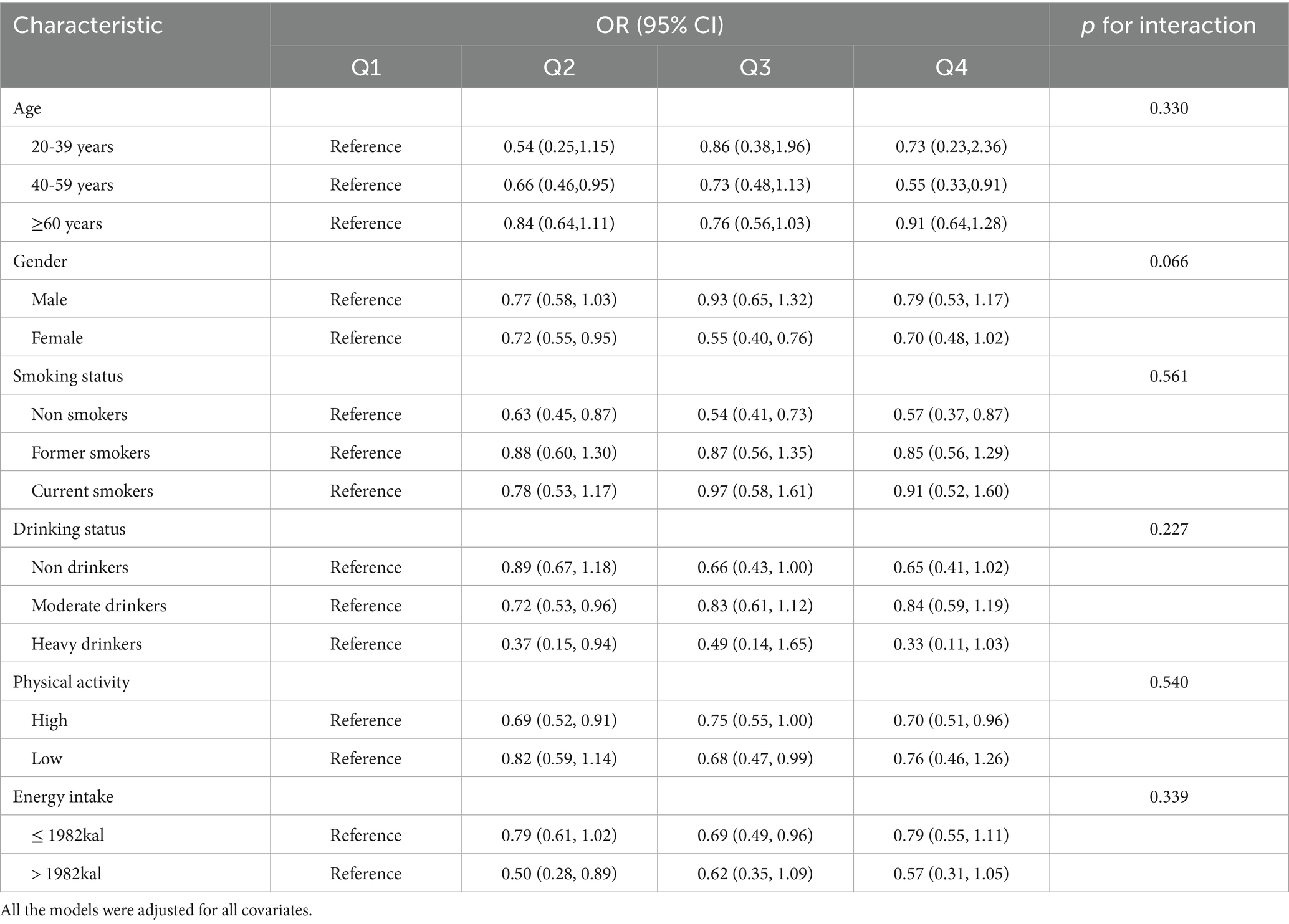
We further evaluated the potential interaction between the Cu intake and subgroup variables, including age, sex, smoking status, drinking status, physical activity and energy intake (Table 3). The association between Cu intake and advanced CKM stages was robust across subgroups. In some subgroups, we still found a correlation between groups of high copper intake and lower risk of advanced CKM stages compared with the lowest Cu intake group.
4 Discussion
In a word, we utilized the data of the NHANES 2003–2018 and established a large-scale cross-sectional study and examine the association between dietary metal intake and CKM syndrome among American adults. We used weighted logistic regression model, RCS regression model, WQS regression model, and qgcomp model to assess individual and combined effects of metal intake comprehensively. Weighted logistic regression models showed that Q2 (≤0.80–1.12 mg/d), Q3 (≤1.12–1.53 mg/d) and Q4 (>1.53 mg/d) groups of Cu intake were significantly associated with a reduced incidence of advanced CKM stages compared with Q1 (≤0.80 mg/d) group. The RCS regression models indicated that higher Cu intake was significantly associated with a lower risk of advanced CKM stages. The two mixture models did not reveal a significant effect of metal ingestion mixtures. Subgroup analysis revealed the robust association between copper intake and advanced CKM stages across subgroups. In summary, the intake of multiple metal mixture by American adults may not be associated with the risk of advanced CKM stages.
CKM syndrome is an emerging disease that encompasses complex associations between the cardiovascular, renal, and metabolic systems. In this study, we identified the correlation of higher Cu intake and lower risk of CKM syndrome risk. A study from the NHANES showed that dietary copper intake was significantly negatively correlated with the incidence rate of CVD (31). Copper is closely related to the metabolic system. A randomized controlled trial showed that participants’ fasting blood glucose levels and insulin resistance improved with an increase in copper intake (32). In addition, high copper exposure in early pregnancy can significantly increase the level of high-density lipoprotein cholesterol (HDL-C) in Chinese pregnant women (33). Conversely, Urinary copper may lead to the development of abnormal blood lipids (34). Abnormal copper metabolism plays a key role in the progression of CKD (35). In a cross-sectional survey of 2,210 adults in China, high concentration of serum Cu was significantly associated with high-risk CKD (36). However, a large prospective cohort study showed a U-shaped association between dietary copper intake and CKD incidence 30 years later (37). In summary, a large amount of literature has confirmed the correlation between copper and multiple systems in the human body, although research conclusions are inconsistent. Firstly, there may be differences in dietary copper intake and copper concentration among different biological samples. Secondly, differences in conclusions are caused by research populations from different regions or ethnicities. Thirdly, research design, statistical methods, and sample size may all lead to statistical differences in the results. Most importantly, CKM syndrome is a syndrome that combines three systems, not just one of the cardiovascular, metabolic, or renal systems and. We have discovered for the first time the association between dietary copper intake and this complex disease, and this conclusion requires further research to confirm.
Although we did not find significant associations between other metals and CKM syndrome, previous literature has reported their relationships with cardiovascular, renal, and metabolic systems. Maintaining normal levels of P intake may be beneficial for CVD and CKD (38). A meta-analysis showed elevated levels of dietary Mg or serum Mg were linearly and negatively correlated with the risk of total CVD events (39). Kuria et al. found that compared with the low Se state in the body, the incidence of CVD was lower under physiological high Se state (40). High Se exposure may also lower total cholesterol (TC) and increase HDL-C (41). K was the most abundant cations in intracellular fluids and play a decisive role in maintaining normal cellular function (42). Higher K levels may lower the risk of CVD by lowering blood pressure. The main way for the human body to ingest Na is through table salt (43). Due to insufficient human control over salt intake, the current research results indicate that strict Na intake is crucial for human health. However, the importance of low Na levels in maintaining human health cannot be ignored (44). In addition, Ca and Zn have also shown beneficial effects on the cardiovascular and renal systems (45, 46). Similarly, the inconsistency between the above conclusions and our research findings may be attributed to the complexity of CKM syndrome compared to a single systemic disease, study design, study population, and statistical methods.
After Cu enters the human body, it is mainly absorbed by the duodenum and small intestine, and then secreted into the bloodstream to combine with various soluble substances. Then Cu was transported to different organs to exert its effects (47). The mechanism by which Cu affect the process of CKM syndrome is still unclear. We speculate that inflammation and oxidative stress are common influencing factors of the cardiovascular, renal, and metabolic systems. The antioxidant and anti-inflammatory effects of Cu seem to have become a consensus. Experimental studies have shown that dietary Reduce NF-κB and IRF3 activation in macrophages stimulated by LPS (48). Moreover, Cu is a cofactor of superoxide dismutase (SOD) and can regulate the activity of SOD (49). On the other hand, the close relationship between aging and chronic diseases has been reported in literature. An epidemiological study reveals that dietary Cu intake obviously reduced aging (50). Further research is needed to explore the potential mechanisms underlying the beneficial effects of copper on CKM syndrome.
We applied advanced mixed exposure models to investigate the comprehensive effects of multiple metal ingestion on CKM syndrome. It is basically impossible to consume only one metal under a normal dietary pattern, as the nutrients in food are diverse (51). Our design conforms to the real dietary patterns of humans and fills the gap in research on single metal intake. In addition, there are interactions between different metal elements or other nutrients, and relying solely on a single variable model may not accurately identify their effects on the human body. Therefore, we call on researchers to pay more attention to the intake of multiple nutrients in future studies. In the mixture model used in this study, we did not determine whether dietary metal mixtures have a significant positive or negative effect on CKM syndrome. This may be due to the cancelation of positive or negative effects caused by the intake of different metals, as demonstrated by the RCS model.
Our research has several advantages. Firstly, this is the first study to explore the association between dietary metal intake mixtures and an emerging disease called CKM syndrome. Our research provided dietary recommendations for the prevention and treatment of CKM syndrome. Secondly, we utilized reliable mixed exposure models to identify the dominant metal ingestion. Thirdly, the large sample size of this study ensured the authenticity of the results. The shortcomings of this study cannot be ignored. Firstly, short-term dietary survey may not fully reflect the long-term dietary habits of participants. However, numerous studies have confirmed the reliability of short-term dietary surveys (52). Secondly, the staging criteria for CKM syndrome in NHANES have not been unified yet, which may have a certain impact on the results. We compared other literature and found that the difference in staging criteria is negligible. Finally, cross-sectional studies have less ability to validate causal associations compared to longitudinal studies. Another key point is that we did not account for the potential influence of other dietary nutrients, foods, or dietary patterns because of potential collinearity. In the future, new statistical methods such as machine learning may be reused to comprehensively explore the role of nutrients in CKM syndrome. We believe that more research will confirm our conclusions in the future.
5 Conclusion
Our findings indicated that higher dietary Cu (>1.53 mg/d) was associated with a lower prevalence of advanced CKM stages compared lower dietary Cu in the U. S. adult population. However, multiple dietary metal intakes did not show a significant effect on advanced CKM stages. Further experimental and longitudinal cohort studies are warranted to corroborate these observations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
SH: Formal analysis, Data curation, Methodology, Writing – review & editing, Conceptualization, Software, Investigation, Writing – original draft. BW: Writing – review & editing, Project administration, Visualization, Resources, Supervision, Validation, Funding acquisition. AZ: Resources, Funding acquisition, Project administration, Visualization, Writing – review & editing, Validation, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We are grateful to the National Health and Nutrition Examination Survey (NHANES) staff and participants for their valuable contributions, particularly for providing data licenses. We extend special thanks to Professor AZ for her invaluable contributions to the research ideas for this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1612458/full#supplementary-material
References
1. Li, J, Lei, L, Wang, W, Ding, W, Yu, Y, Pu, B, et al. Social risk profile and cardiovascular-kidney-metabolic syndrome in US adults. J Am Heart Assoc. (2024) 13:e034996. doi: 10.1161/JAHA.124.034996
2. Ndumele, CE, Neeland, IJ, Tuttle, KR, Chow, SL, Mathew, RO, Khan, SS, et al. A synopsis of the evidence for the science and clinical Management of Cardiovascular-Kidney-Metabolic (CKM) syndrome: a scientific statement from the American Heart Association. Circulation. (2023) 148:1636–64. doi: 10.1161/CIR.0000000000001186
3. Chen, Y, Wu, S, Liu, H, Zhong, Z, Bucci, T, Wang, Y, et al. Role of oxidative balance score in staging and mortality risk of cardiovascular-kidney-metabolic syndrome: insights from traditional and machine learning approaches. Redox Biol. (2025) 81:103588. doi: 10.1016/j.redox.2025.103588
4. Zhou, X, Tao, XL, Zhang, L, Yang, QK, Li, ZJ, Dai, L, et al. Association between cardiometabolic index and depression: National Health and nutrition examination survey (NHANES) 2011-2014. J Affect Disord. (2024) 351:939–47. doi: 10.1016/j.jad.2024.02.024
5. Iqbal, S, and Ali, I. Dietary trace element intake and risk of breast Cancer: a Mini review. Biol Trace Elem Res. (2022) 200:4936–48. doi: 10.1007/s12011-021-03089-z
6. Cui, A, Yan, J, Li, H, Fan, Z, Wei, X, Wang, H, et al. Association between dietary copper intake and bone mineral density in children and adolescents aged 8-19 years: a cross-sectional study. PLoS One. (2024) 19:e0310911. doi: 10.1371/journal.pone.0310911
7. Malekahmadi, M, Firouzi, S, Rezayi, M, Ghazizadeh, H, Ranjbar, G, Ferns, GA, et al. Association of Zinc and Copper Status with cardiovascular diseases and their assessment methods: a review study. Mini Rev Med Chem. (2020) 20:2067–78. doi: 10.2174/1389557520666200729160416
8. Xie, C, Zeng, M, Shi, Z, Li, S, Jiang, K, and Zhao, Y. Association between selenium status and chronic kidney disease in middle-aged and older Chinese based on CHNS data. Nutrients. (2022) 14:2695. doi: 10.3390/nu14132695
9. Sun, Y, Wang, Y, Wang, D, and Zhou, Q. Dietary zinc intake, supplemental zinc intake and serum zinc levels and the prevalence of kidney stones in adults. J Trace Elem Med Biol. (2020) 57:126410. doi: 10.1016/j.jtemb.2019.126410
10. Zhang, Q, Qian, ZY, Zhou, PH, Zhou, XL, Zhang, DL, He, N, et al. Effects of oral selenium and magnesium co-supplementation on lipid metabolism, antioxidative status, histopathological lesions, and related gene expression in rats fed a high-fat diet. Lipids Health Dis. (2018) 17:165. doi: 10.1186/s12944-018-0815-4
11. Liu, X, Zhao, T, Wei, X, Zhang, D, Lv, W, and Luo, Z. Dietary phosphorus reduced hepatic lipid deposition by activating Ampk pathway and Beclin1 phosphorylation levels to activate Lipophagy in Tilapia Oreochromis niloticus. Front Nutr. (2022) 9:841187. doi: 10.3389/fnut.2022.841187
12. Wang, G, Fang, L, Chen, Y, Ma, Y, Zhao, H, Wu, Y, et al. Association between exposure to mixture of heavy metals and hyperlipidemia risk among U.S. adults: a cross-sectional study. Chemosphere. (2023) 344:140334. doi: 10.1016/j.chemosphere.2023.140334
13. Wang, PP, Lei, JY, Wang, Y, Wang, HL, Sun, L, Hu, B, et al. The association between the essential metal mixture and fasting plasma glucose in Chinese community-dwelling elderly people. Ecotoxicol Environ Saf. (2023) 263:115289. doi: 10.1016/j.ecoenv.2023.115289
14. Pan, J, Hu, Y, Pang, N, and Yang, L. Association between dietary niacin intake and nonalcoholic fatty liver disease: NHANES 2003-2018. Nutrients. (2023) 15:4128. doi: 10.3390/nu15194128
15. Liu, Y, Han, Y, Gao, Y, Yao, N, Wang, Y, Wang, F, et al. The association between oxidative balance score and frailty in adults across a wide age spectrum: NHANES 2007-2018. Food Funct. (2024) 15:5041–9. doi: 10.1039/D4FO00870G
16. Inker, LA, Eneanya, ND, Coresh, J, Tighiouart, H, Wang, D, Sang, Y, et al. New creatinine-and cystatin C-based equations to estimate GFR without race. N Engl J Med. (2021) 385:1737–49. doi: 10.1056/NEJMoa2102953
17. Khan, SS, Coresh, J, Pencina, MJ, Ndumele, CE, Rangaswami, J, Chow, SL, et al. Novel prediction equations for absolute risk assessment of Total cardiovascular disease incorporating cardiovascular-kidney-metabolic health: a scientific statement from the American Heart Association. Circulation. (2023) 148:1982–2004. doi: 10.1161/CIR.0000000000001191
18. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. 2024 clinical practice guideline for the evaluation and Management of Chronic Kidney Disease. Kidney Int. (2024) 105:S117–314. doi: 10.1016/j.kint.2023.10.018
19. Zhu, R, Wang, R, He, J, Wang, L, Chen, H, Niu, X, et al. Prevalence of cardiovascular-kidney-metabolic syndrome stages by social determinants of health. JAMA Netw Open. (2024) 7:e2445309. doi: 10.1001/jamanetworkopen.2024.45309
20. Peeri, NC, Egan, KM, Chai, W, and Tao, MH. Association of magnesium intake and vitamin D status with cognitive function in older adults: an analysis of US National Health and nutrition examination survey (NHANES) 2011 to 2014. Eur J Nutr. (2021) 60:465–74. doi: 10.1007/s00394-020-02267-4
21. Wang, J, Zhu, R, Fang, H, Xing, X, Ge, L, and Cai, G. Association of prognostic nutritional index with the presence and all-cause mortality of rheumatoid arthritis: the National Health and nutrition examination survey 2003-2018. BMC Public Health. (2024) 24:3281. doi: 10.1186/s12889-024-20795-0
22. You, Y, Chen, Y, Fang, W, Li, X, Wang, R, Liu, J, et al. The association between sedentary behavior, exercise, and sleep disturbance: a mediation analysis of inflammatory biomarkers. Front Immunol. (2022) 13:1080782. doi: 10.3389/fimmu.2022.1080782
23. Huang, Y, Wang, Y, Su, H, Wang, H, Xu, H, Xu, C, et al. Association between polyunsaturated fatty acid intake and the prevalence of erectile dysfunction: a cross-sectional analysis of the NHANES 2001-2004. Lipids Health Dis. (2023) 22:182. doi: 10.1186/s12944-023-01950-9
24. Balogun, M, and Obeng-Gyasi, E. Association of Combined PFOA, PFOS, metals and allostatic load on hepatic disease risk. J Xenobiot. (2024) 14:516–36. doi: 10.3390/jox14020031
25. White, IR, Royston, P, and Wood, AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. (2011) 30:377–99. doi: 10.1002/sim.4067
26. Ye, J, Hu, Y, Chen, X, Yin, Z, Yuan, X, Huang, L, et al. Association between the weight-adjusted waist index and stroke: a cross-sectional study. BMC Public Health. (2023) 23:1689. doi: 10.1186/s12889-023-16621-8
27. Dang, K, Wang, X, Hu, J, Zhang, Y, Cheng, L, Qi, X, et al. The association between triglyceride-glucose index and its combination with obesity indicators and cardiovascular disease: NHANES 2003-2018. Cardiovasc Diabetol. (2024) 23:8. doi: 10.1186/s12933-023-02115-9
28. Cheng, X, Wei, Y, Wang, R, Jia, C, Zhang, Z, An, J, et al. Associations of essential trace elements with epigenetic aging indicators and the potential mediating role of inflammation. Redox Biol. (2023) 67:102910. doi: 10.1016/j.redox.2023.102910
29. Guo, JY, Wang, SN, Zhang, ZL, and Luan, M. Associations between organophosphate esters and bone mineral density in adults in the United States: 2011-2018 NHANES. Ecotoxicol Environ Saf. (2024) 278:116414. doi: 10.1016/j.ecoenv.2024.116414
30. Tan, Y, Fu, Y, Huang, F, Wen, L, Weng, X, Yao, H, et al. Association between blood metal exposures and hyperuricemia in the U.S. general adult: a subgroup analysis from NHANES. Chemosphere. (2023) 318:137873. doi: 10.1016/j.chemosphere.2023.137873
31. Yin, T, Zhu, X, Xu, D, Lin, H, Lu, X, Tang, Y, et al. The association between dietary antioxidant micronutrients and cardiovascular disease in adults in the United States: a cross-sectional study. Front Nutr. (2021) 8:799095. doi: 10.3389/fnut.2021.799095
32. Eljazzar, S, Abu-Hijleh, H, Alkhatib, D, Sokary, S, Ismail, S, Al-Jayyousi, GF, et al. The role of copper intake in the development and Management of Type 2 diabetes: a systematic review. Nutrients. (2023) 15:1655. doi: 10.3390/nu15071655
33. Yang, R, Sun, F, Pan, XF, Su, Y, Wu, P, Yuan, J, et al. Metal exposure and blood lipid biomarkers in early pregnancy: a cross-sectional study. Environ Pollut. (2024) 355:124238. doi: 10.1016/j.envpol.2024.124238
34. Ma, J, Xie, Y, Zhou, Y, Wang, D, Cao, L, Zhou, M, et al. Urinary copper, systemic inflammation, and blood lipid profiles: Wuhan-Zhuhai cohort study. Environ Pollut. (2020) 267:115647. doi: 10.1016/j.envpol.2020.115647
35. Jiayi, H, Ziyuan, T, Tianhua, X, Mingyu, Z, Yutong, M, Jingyu, W, et al. Copper homeostasis in chronic kidney disease and its crosstalk with ferroptosis. Pharmacol Res. (2024) 202:107139. doi: 10.1016/j.phrs.2024.107139
36. Yu, Y, Meng, W, Kuang, H, Chen, X, Zhu, X, Wang, L, et al. Association of urinary exposure to multiple metal(loid)s with kidney function from a national cross-sectional study. Sci Total Environ. (2023) 882:163100. doi: 10.1016/j.scitotenv.2023.163100
37. Zhang, Y, Gan, X, Xiang, H, Zhang, Y, Yang, S, Ye, Z, et al. U-shaped association between dietary copper intake and new-onset chronic kidney disease: a 30-year follow-up study from young adulthood to midlife. Mol Nutr Food Res. (2025) 69:e202400761. doi: 10.1002/mnfr.202400761
38. Uribarri, J, and Calvo, MS. Dietary phosphorus excess: a risk factor in chronic bone, kidney, and cardiovascular disease? Adv Nutr. (2013) 4:542–4. doi: 10.3945/an.113.004234
39. Zhao, L, Hu, M, Yang, L, Xu, H, Song, W, Qian, Y, et al. Quantitative association between serum/dietary magnesium and cardiovascular disease/coronary heart disease risk: a dose-response Meta-analysis of prospective cohort studies. J Cardiovasc Pharmacol. (2019) 74:516–27. doi: 10.1097/FJC.0000000000000739
40. Kuria, A, Tian, H, Li, M, Wang, Y, Aaseth, JO, Zang, J, et al. Selenium status in the body and cardiovascular disease: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. (2021) 61:3616–25. doi: 10.1080/10408398.2020.1803200
41. Mojadadi, A, Au, A, Salah, W, Witting, P, and Ahmad, G. Role for selenium in metabolic homeostasis and human reproduction. Nutrients. (2021) 13:3256. doi: 10.3390/nu13093256
42. Stone, MS, Martyn, L, and Weaver, CM. Potassium intake, bioavailability, hypertension, and glucose control. Nutrients. (2016) 8:444. doi: 10.3390/nu8070444
43. O'Donnell, M, Mente, A, and Yusuf, S. Evidence relating sodium intake to blood pressure and CVD. Curr Cardiol Rep. (2014) 16:529. doi: 10.1007/s11886-014-0529-9
44. Wilck, N, Balogh, A, Markó, L, Bartolomaeus, H, and Müller, DN. The role of sodium in modulating immune cell function. Nat Rev Nephrol. (2019) 15:546–58. doi: 10.1038/s41581-019-0167-y
45. Shi, C, Cao, H, Zeng, G, Wu, H, and Wang, Y. Mendelian randomization analyses explore the effects of micronutrients on different kidney diseases. Front Nutr. (2024) 11:1440800. doi: 10.3389/fnut.2024.1440800
46. Narayanam, H, Chinni, SV, and Samuggam, S. The impact of micronutrients-calcium, vitamin D, selenium, zinc in cardiovascular health: a Mini review. Front Physiol. (2021) 12:742425. doi: 10.3389/fphys.2021.742425
47. Chen, L, Min, J, and Wang, F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct Target Ther. (2022) 7:378. doi: 10.1002/mco2.724
48. Zangiabadi, S, Chamoun, KP, Nguyen, K, Tang, Y, Sweeney, G, and Abdul-Sater, AA. Copper infused fabric attenuates inflammation in macrophages. PLoS One. (2023) 18:e0287741. doi: 10.1371/journal.pone.0287741
49. Jomova, K, Alomar, SY, Nepovimova, E, Kuca, K, and Valko, M. Heavy metals: toxicity and human health effects. Arch Toxicol. (2025) 99:153–209. doi: 10.1007/s00204-024-03903-2
50. Ma, J, Li, P, Jiang, Y, Yang, X, Luo, Y, Tao, L, et al. The association between dietary nutrient intake and acceleration of aging: evidence from NHANES. Nutrients. (2024) 16:1635. doi: 10.3390/nu16111635
51. Chiavaroli, L, Wang, YF, Ahmed, M, Ng, AP, DiAngelo, C, Marsden, S, et al. Intakes of nutrients and food categories in Canadian children and adolescents across levels of sugars intake: cross-sectional analyses of the Canadian community health survey 2015 public use microdata file. Appl Physiol Nutr Metab. (2022) 47:415–28. doi: 10.1139/apnm-2021-0517
Keywords: multiple metal intake, CKM syndrome, NHANES, American, cross-section study
Citation: Hu S, Wei B and Zhang A (2025) Association of multiple dietary metal intake with cardiovascular-kidney-metabolic syndrome: a cross-sectional study based on NHANES 2003–2018. Front. Nutr. 12:1612458. doi: 10.3389/fnut.2025.1612458
Edited by:
Sandra Wagner, INSERM CIC1433 Centre d’Investigation Clinique Nancy, FranceReviewed by:
Francesco Di Giacomo Barbagallo, University of Catania, ItalyHaoxian Tang, First Affiliated Hospital of Shantou University Medical College, China
Copyright © 2025 Hu, Wei and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baojian Wei, Ymp3ZWlAc2RmbXUuZWR1LmNu; Aihua Zhang, YWh6aGFuZ0BzZGZtdS5lZHUuY24=
 Sihan Hu
Sihan Hu Baojian Wei2*
Baojian Wei2*