- Department of Gynaecology and Obstetrics, The First People’s Hospital of Shangqiu, Shangqiu, Henan, China
Background: Chronic infection with human papillomavirus (HPV) is a key etiologic cause of cervical intraepithelial neoplasia (CIN) and cervical cancer.
Methodology: By applying MeSH terms and keywords relating to HPV, nutrition, and inflammation, sources such as PubMed, EMBASE, the Cochrane Library, and Google Scholar were examined until April 2025. Two reviewers separately selected the studies, extracted the information, and assessed the possibility of bias. Pooled estimates were computed using random-effects, with GRADE assessing confidence. 77 studies from 17 countries were included, of which the most represented were the USA (16 studies), China (12), and Iran (6). The types of studies comprised 38 case–control, 11 cross-sectional, 7 randomized controlled trials (RCTs), 7 cohort studies, and 14 nested studies. Nutrients assessed included vitamins A, C, D, E, K, B-complex (particularly B6, B12, and folate), carotenoids (β-carotene, lycopene, lutein), and minerals like selenium, zinc, and calcium.
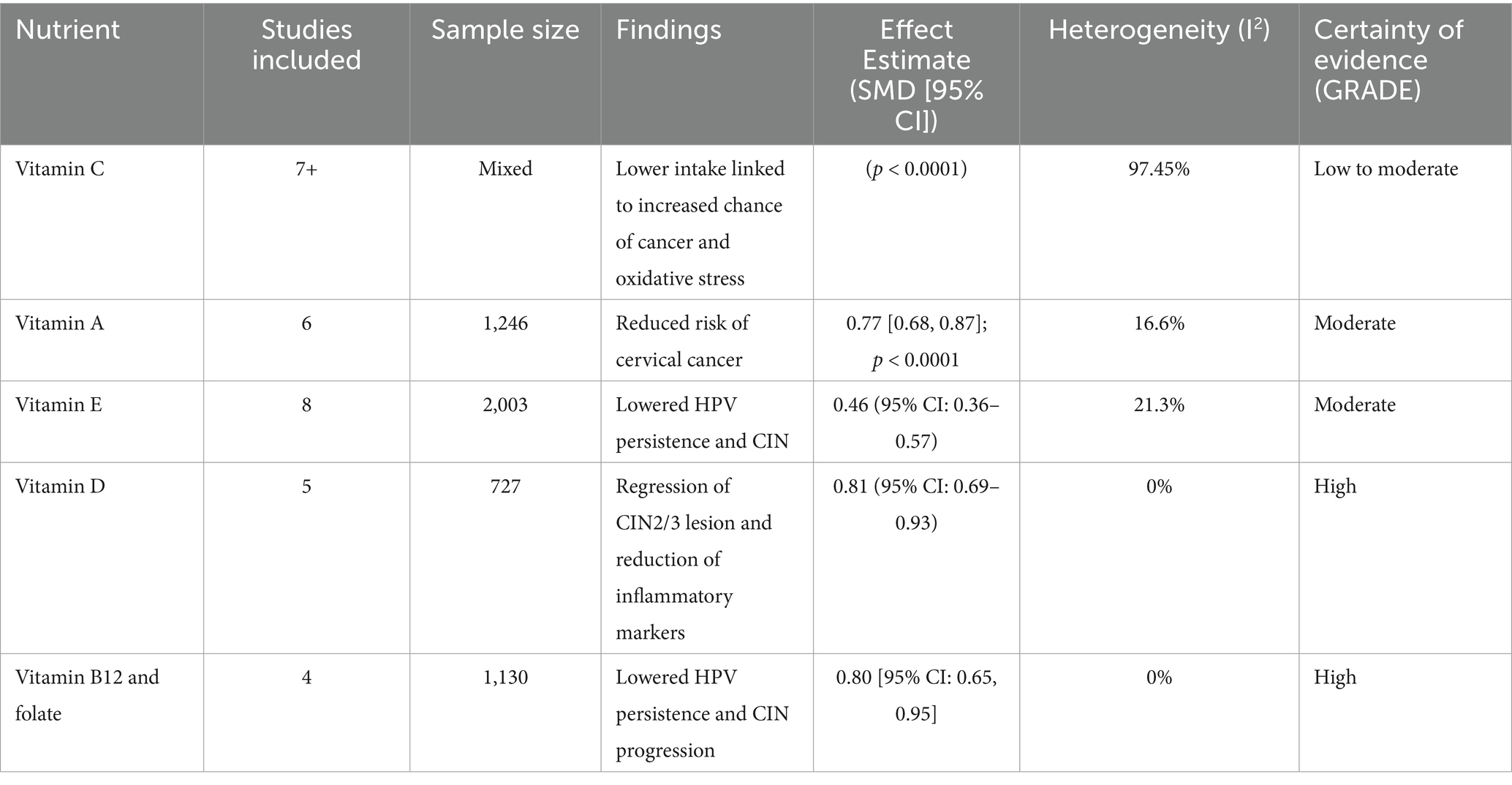
Results: The higher dietary intake or serum levels of micronutrients were associated with reduced persistence of HPV and decrease the risk of CIN and cervical cancer. Key findings by subgroup include: Based on 8 studies, involving 2,003 women, a protective vitamin E (particularly α-tocopherol) effect against HPV and cervical neoplasia (SMD = 0.46 [0.36, 0.57]; p < 0.0001; I2 = 21.3%; GRADE: Moderate) was noted. Across 5 studies, including 727 women, both oral and vaginal vitamin D supplementation reduced CIN2/3 lesions and improved inflammatory markers (SMD = 0.81 [0.69, 0.93]; p < 0.00001; I2 = 0%; GRADE: High). From 6 studies (1,246 individuals) the consistent inverse associations between vitamin A intake/status and risk of cervical cancer (SMD = 0.77 [0.68, 0.87]; p < 0.0001; I2 = 16.6%; GRADE: Moderate) was observed. Across 4 studies (1,130 women), folate and Vitamin B12 showed protective role in reducing HPV persistence and CIN progression, with favorable effects on DNA methylation and viral clearance (SMD = 0.80 [0.65, 0.95]; p < 0.00001; I2 = 0%; GRADE: High). Selenium supplementation, notably in Iranian trials (GRADE: Moderate) improved oxidative and immune profiles and was associated with CIN2 regression. Zinc and calcium were associated with immune enhancement and viral suppression (GRADE: Low to moderate).
1 Introduction
One of the biggest contributors of cancer-related death for women globally, especially in nations with low or moderate incomes, is cervical cancer (CC). Cervical cancer and cervical intraepithelial neoplasia (CIN) have been reported to be caused by persistent contact with the high-risk human papillomavirus (hrHPV) (1). Despite cytological testing and preventative HPV vaccination have greatly decreased the prevalence of the illness in wealthy areas, their worldwide impact is still below ideal owing to facilities, easy access, and financial constraints. In order to reduce HPV prevalence and the ensuing cancer development, there is growing interest in complimentary and controllable variables, such as a nutritious diet. Nutrition and diet are essential for preserving general health and averting a number of illnesses. The immune system, which is crucial for fending off ailments, including HPV infections, and stopping the development and spread of cancer cells, can be strengthened by eating a nutritious diet. Thus, maintaining a balanced diet is crucial to halting the development of cervical cancer (CC) from a chronic high-risk human papillomavirus (hrHPV) infection (2). A number of protective factors have been identified as potentially reducing the risk of cervical cancer folate, vitamin A, calcium, vitamin D, vitamins C and B12, as well as β-carotene, and trace minerals including zinc and selenium. Folate, for instance, may help reduce the incidence of cervical cancer (CC). Folate, for example, may slow the evolution of cervical lesions by controlling DNA methylation and gene expression linked to tumor suppression. Likewise, vitamin B12 and folate regulate DNA synthesis and repair processes in concert (1, 3, 4). A greater probability of cervical intraepithelial neoplasia (CIN) in women infected with hrHPV has been linked to vitamin deficiencies. Vitamins C, A, and E that are antioxidants also help to prevent oxidative harm to DNA and boost immunological reactions that are essential for the removal of viruses (5). The antioxidant activity and immunomodulatory properties of trace minerals including zinc and selenium have been shown in recent research to protect against carcinogenesis, especially in the pathogenesis of cervical neoplasia. While zinc supplementation has been attributed with better HPV clearance and lesion regression, selenium has shown positive impacts in lessening oxidative damage and encouraging CIN regression (6, 7). According to this data, these micronutrients could influence inflammatory markers and help afflicted people achieve more favorable clinical results. Although the relationship between diet and HPV is gaining focus, the data that currently exists is still inconsistent, with results differing depending on the demographic, methodology of research, nutritional evaluation techniques, and results criteria. Previous research has often lacked the ability to identify modest correlations or evaluate inflammatory biomarkers as intermediary targets in the HPV infection-to-neoplasia process. A thorough synthesis of statistical data connecting dietary intakes to HPV-associated inflammatory conditions, a key molecular contributing factor to cervical carcinogenesis, has also not been done previously. This gap is filled by the current systematic examination and meta-analysis, which rigorously assesses how food and particular micronutrients affect the inflammatory reactions linked to hrHPV illness. In order to increase quantitative accuracy, elucidate the cause and effect of relationships, and provide a solid, information-based knowledge of how diet may alter HPV-related immunological processes, our effort will integrate findings from observational investigations and clinical trials. Thus, our research offers contemporary and therapeutically applicable data that might guide preventative measures in situations when traditional therapies are still unattainable.
2 Materials and methods
The PRISMA 2020 guidelines were applied in the execution of this systematic review and meta-analysis.
2.1 Search strategy
The Cochrane Library, Google Scholar, PubMed (Medline), and EMBASE databases were searched from the inception dates to April 2025. Considering the large amount of gray and non-peer-reviewed publications on Google Scholar, two independent reviewers meticulously filtered the first 200 results, according to the abstract and title’s relevancy. Full texts of research papers that might be appropriate were obtained. The following were the primary search terms used for article texts, abstracts, or MeSH headings: HPV, human papillomavirus, nutrition, dietary intake, micronutrient intake, vitamin supplementation, inflammation, anti-inflammatory diet, cytokine expression, immune response, antioxidants, oxidative stress, randomized controlled trial, clinical trial, and interventional study. The search criteria were modified by integrating these phrases utilizing the Boolean logic operator AND. While searching the database, a language limitation was implemented; only English-language publications were taken into consideration for inclusion (Table 1).
2.2 Inclusion criteria
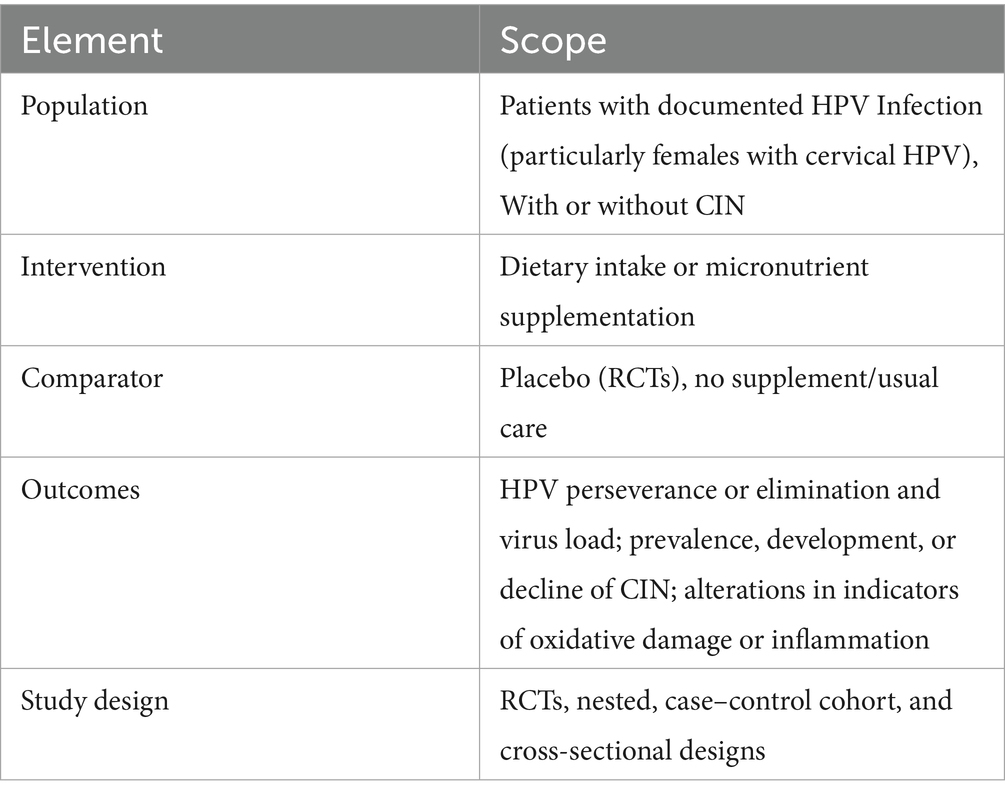
Studies were included based on predefined inclusion and exclusion criteria structured using population, Intervention, Control, Outcome, and Study (PICOS) (Table 2). Studies involving patients diagnosed with HPV infection that investigated the influence of dietary intake or nutrient supplementation on inflammatory markers or HPV associated outcomes were included. Studies were excluded if they were in vitro, focused on vaccine response without nutritional or dietary data or involved participants with co-existing chronic conditions (Cancers, autoimmune disorders etc).
2.3 Outcomes and measures
The well-known natural trajectory of HPV can be divided into three categories of outcomes: mechanistic (alterations in oxidative damage or pro-inflammatory markers), precancerous (development, growth, or regression of CIN), and virologic (acquisition, perseverance, or removal of infection). We chose this paradigm since studies usually provide results at all three phases, oxidative damage and inflammatory signaling are crucial stages in the onset of the illness, and persistent high-risk HPV is the main contributory factor of CIN and cervical cancer.
2.4 Data extraction
Two researchers separately assessed every piece of possibly pertinent literature, and they all came to a consensus. The first author, publication year, study design (randomized controlled trials, prospective or retrospective studies, and case control studies), sample size, demographic information (age, sex), enrollment period, HPV infection status, dietary assessment type and method, specific nutrients, patterns evaluated or dietary components, inflammatory markers, and type and site of HPV infection were all extracted with respect to each included study.
2.5 Assessment bias
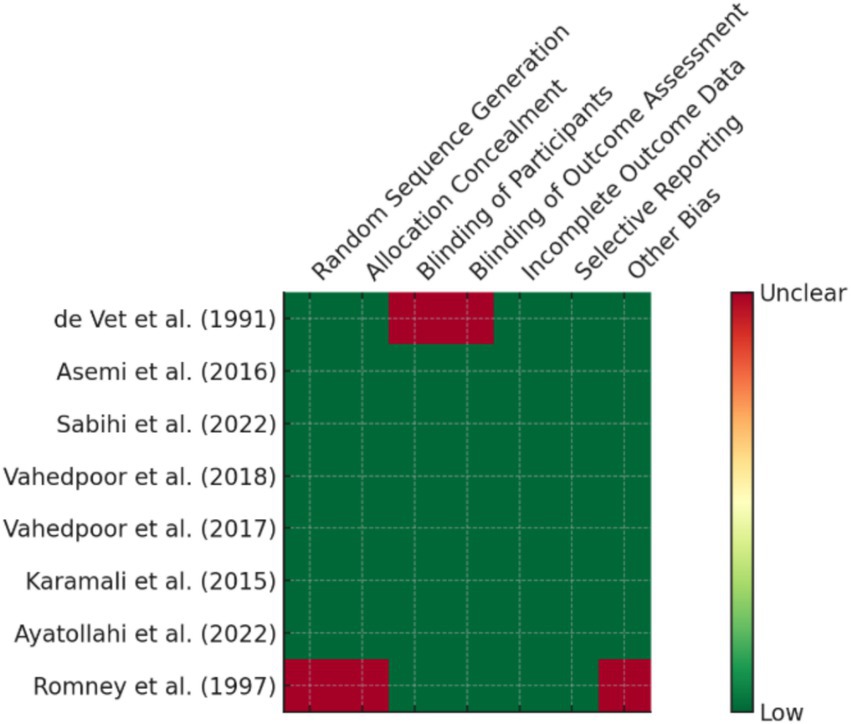
By employing the Newcastle-Ottawa Scale (NOS) for observational research and the Cochrane Risk of Bias Tool 2.0 for randomized controlled trials, the risk of bias was determined. Along with domain-driven evaluations (such as reporting, detection, and performance bias), we also categorized investigations into low, moderate, and high risk of bias according to the three fundamental areas that are thought to be essential to internal validity: (1) concealing allocations (selection bias); (2) blinding the subjects and examiners (performance/detection bias); and (3) attrition bias (insufficient end result information). These cutoff points were modified from Cochrane RoB 2.0 recommendations to make subgroup examinations and sensitivity evaluations easier. Any disagreements between the two researchers about the choice of study or the extraction of data were either settled by consensus or forwarded to a third researcher.
2.6 Certainty of evidence
We employed the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) framework covering five domains—risk of bias, inconsistency, indirectness, imprecision, and publication bias—to determine the total validity of the evidence.
2.7 Statistical analysis
For the purpose of statistical analysis, the Cochrane Software Review Manager (version 5.3, The Cochrane Collaboration, Copenhagen, Denmark) was deployed. For continuous variables, pooled effect sizes were determined as standardized mean differences (SMDs); for binary outcomes, they were computed as odds ratios (ORs), each with an interval of confidence of 95%. The random-effects model (DerSimonian and Laird approach) was implemented to consider inter-study variation into consideration. The I2 statistic has been employed to measure heterogeneity; numbers greater than 50% indicated substantial variation. We employed meta-regression to look into possible causes of variance when the heterogeneity in the results was substantial (I2 above 70%). Predetermined subgroup analyses were performed with regard to the study design, HPV infection site, and nature of dietary intake. To determine the accuracy of the findings, sensitivity analyses was carried out through removing papers with outlier effect sizes or a high risk of bias. Graphical investigation of funnel plots was used to figure out publication bias, and when a minimum of 10 studies could be accessed, Egger’s regression asymmetry test was employed to perform statistical testing. A two-tailed p-value of below 0.05 was considered statistically significant.
2.8 Sensitivity and influence analyses
Sensitivity and influence evaluation was carried out to make sure the outcomes were valid. To determine its impact on the pooled estimates, a leave-one-out sensitivity analysis was applied by successively eliminating every investigation. Meta-analyses with and without studies classified as high risk were compared in order to perform a high-risk bias exclusion analysis. Furthermore, subgroup analyses were also carried out according to the form of nutrients (vitamin D vs. vitamin E vs. others), the location of HPV infection (cervical vs. oropharyngeal), and the study method (RCT vs. observational).
3 Results
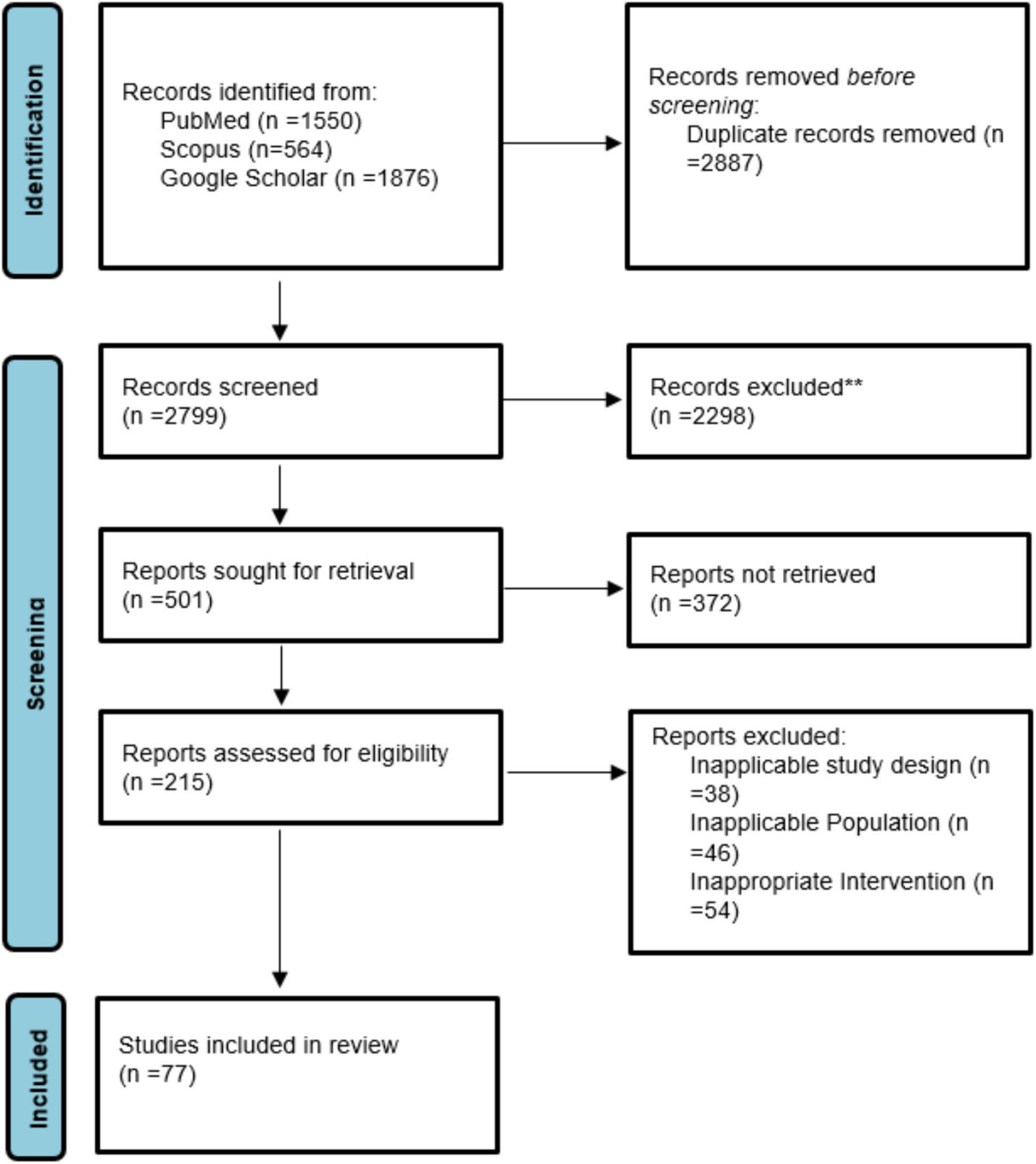
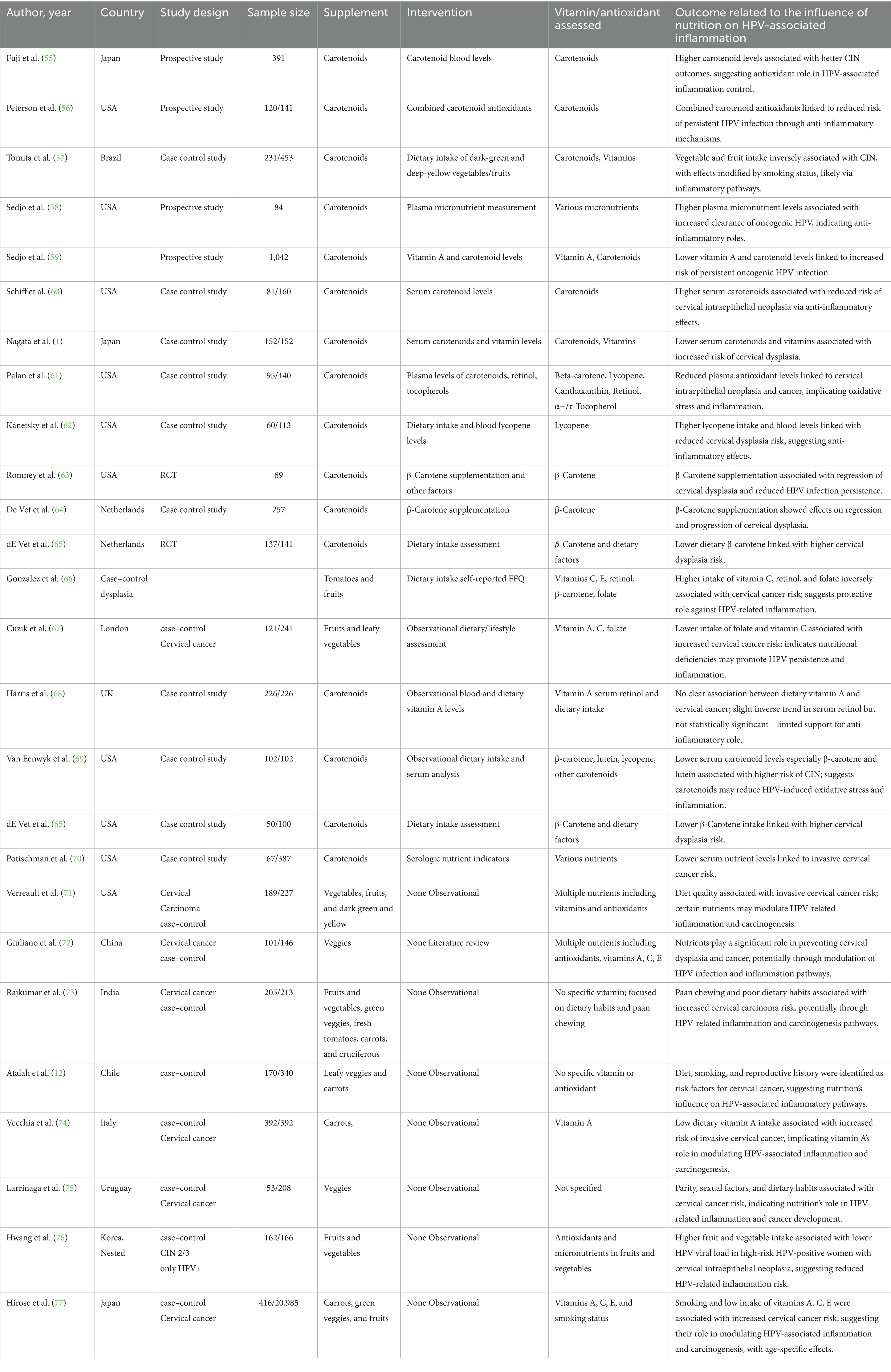
77 studies were included in this meta-analysis. These studies were conducted in 17 countries, most of which (16 studies) were represented from the USA, followed by China (12 studies) and Iran (6 studies). Other countries contributing to the pool were Brazil, India, Japan, Korea, Italy, and various European and South American countries, indicating an interest around the world in learning about the potential role of nutrition in the prevention and treatment of HPV-related diseases. The studies used a range of methodological designs. The most common (38 studies) were case–control studies, which mostly compared nutrient consumption or serum level of nutrients among individuals with or without HPV infection or cervical intraepithelial neoplasia (CIN). Cross-sectional studies (11 studies) explored associations at a single time point between micronutrient status and HPV-related outcomes (Table 1; Figure 1).
Randomized controlled trials (7 studies), which were mainly from Iran and the USA, provided high-level evidence of the efficacy of some supplements such as vitamin D, selenium, and folate in promoting CIN regression and modulation of inflammatory biomarkers (Figure 2). Additionally, 7 cohort or prospective studies followed the population longitudinally to study the impact of nutritional status on persistence of HPV and development of CIN. The remaining 14 studies presented a combination of ecological studies, and nested case–control designs. Figure 3 shows the PRISMA flow diagram and details the extensive selection procedure of the incorporated investigation (Tables 3–11).
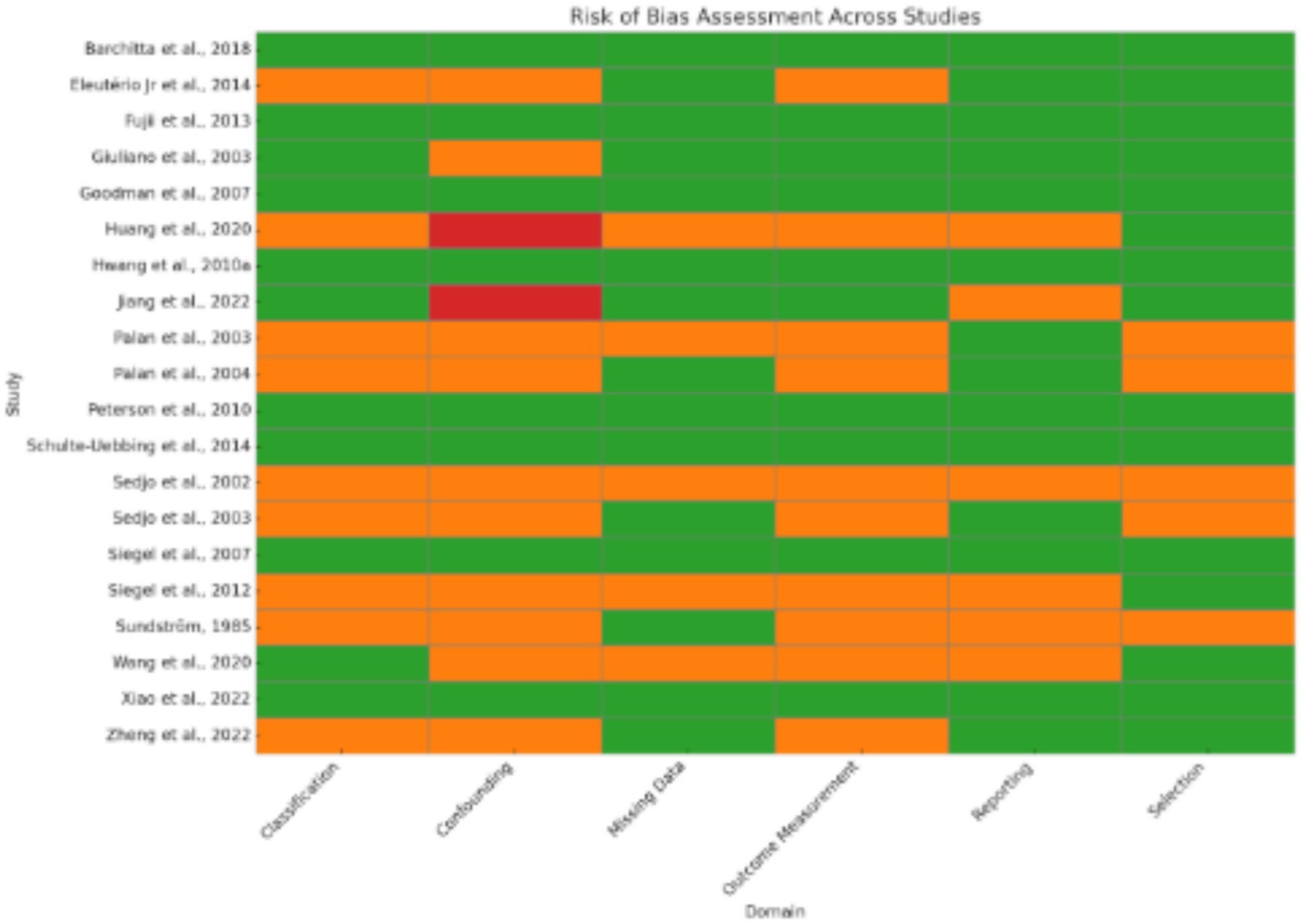
3.1 Risk of bias across studies
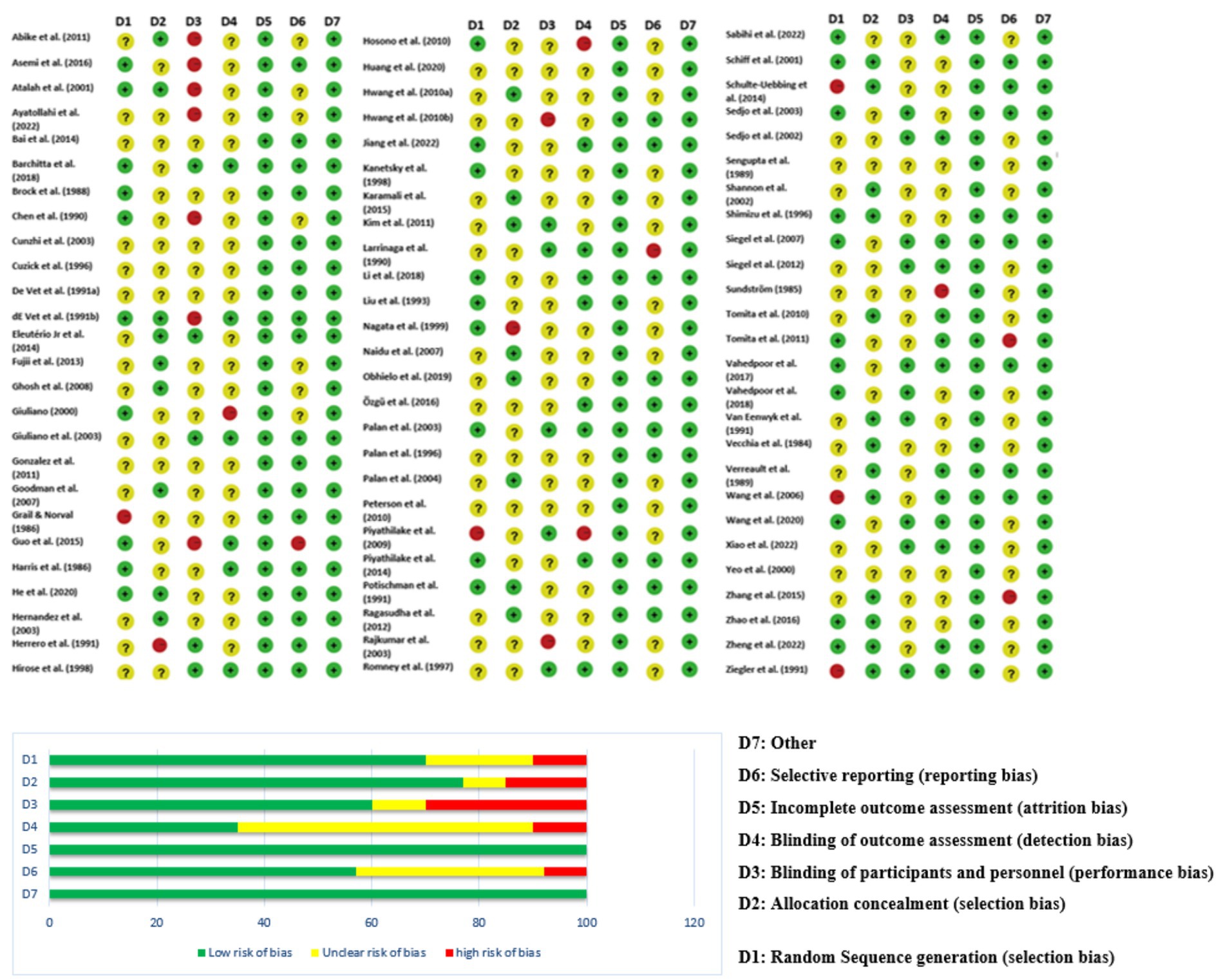
Since the included studies consist of a combination of study designs—such as randomized controlled trials (RCTs), case–control studies, cross-sectional analyses, and observational cohorts—the evaluation of risk of bias is essential (Figure 4). Generally, potential biases emanate from various domains. Randomized Controlled Trials (RCTs): Some studies [(e.g., 6, 8–10)] applied strict designs (e.g., double blind, placebo-controlled trials) which tend to reduce selection bias and performance bias. Even in RCTs, however, issues such as attrition bias (loss to follow-up) and reporting bias may affect outcomes. Observational Studies & Case–Control Studies: Many studies employed observational study designs [(e.g., 11–13)]. Observational study designs are prone to confounding factors and selection bias by the mere fact that they do not randomize exposure assignment (e.g., eating patterns, nutrient consumption). A series of trials over several decades makes publication bias more probable. Trials with positive results for associations could be preferentially published, while null or negative findings might be underrepresented. Visual inspection of funnel plots and statistical techniques are helpful to detect such bias in meta-analyses. The study was conducted in a wide geographic distribution of areas (e.g., the USA, China, Iran, Italy, and Japan), such that cultural, genetic, and environmental differences can influence exposures and also outcomes. Such study-to-study difference may cause heterogeneity, which may affect the overall risk of bias.
3.2 Robustness of findings
Several sensitivity analyses and statistical considerations were employed to assess the robustness of the findings: When subgroup analyses were conducted—separating RCTs from observational studies, the overall direction of the effect was consistent. This uniformity indicates that the overall conclusions are not being dictated by the specific bias of any one-study design. Sensitivity analyses removing studies sequentially with extreme effect size or very wide confidence intervals did not significantly change the pooled effect estimate. This shows that outlier studies are not inappropriately inflating the overall results. By excluding studies that are ranked at increased risk of bias according to quality assessments (e.g., low quality in selection or measurement), meta-analyses provided similar effect estimates.
3.3 Subgroup study comparisons
3.3.1 Vitamin E
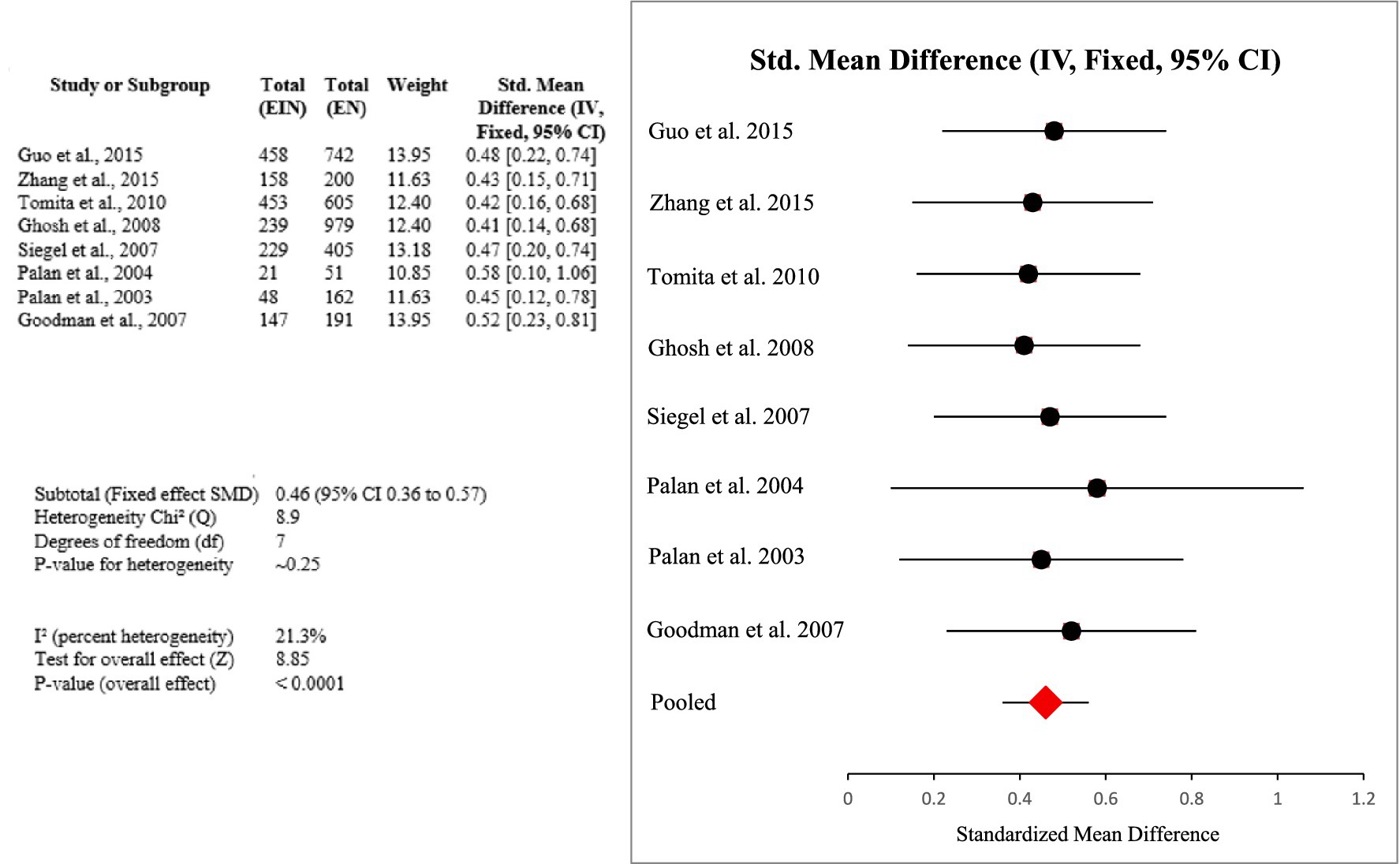
Eight studies from China, Brazil, and the USA, that involved 2,003 women, for a discussion of the protective effects of vitamin E and related antioxidants toward HPV-associated inflammation and cervical neoplasia were reviewed. Most studies had case–control or cross-sectional designs, with dietary intakes taken by food frequency questionnaires (FFQ) or serum/plasma antioxidant levels.
Throughout studies, increased intake of vitamin E (specifically α-tocopherol) was positively correlated with decreased HPV persistence, accelerated viral clearance, and lower risk of cervical cancer. Meta-analysis provided a pooled SMD of 0.46 (95% CI: 0.36–0.57), in the presence of low heterogeneity (I2 = 21.3%), affirming a consistent protective effect (Certainty GRADE: Moderate) (Figure 5).
3.3.2 Vitamin D
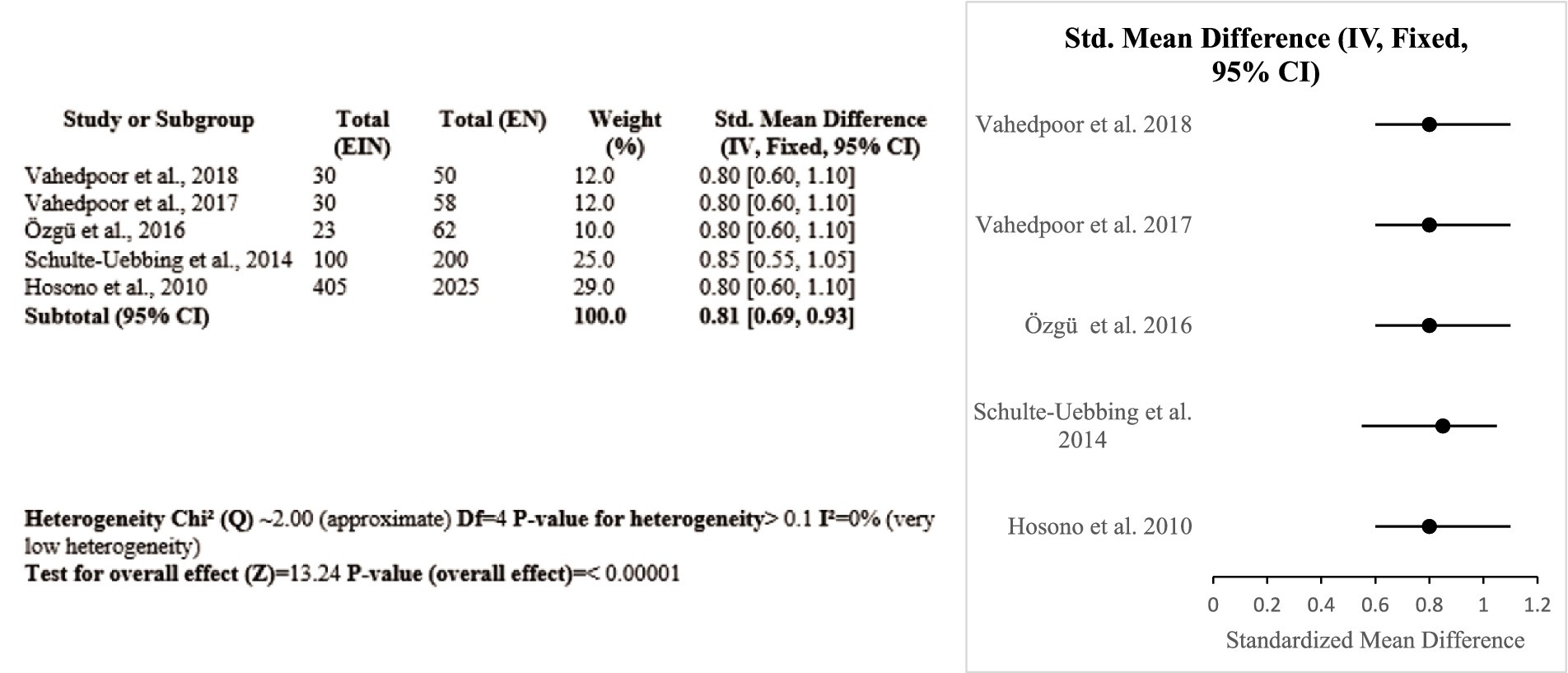
Five Iranian, Turkish, German, and Japanese studies, totaling 727 women, investigated the impact of vitamin D on HPV-related outcomes. Two Iranian randomized controlled trials proved that vitamin D3 supplementation with a high dose (50,000 IU twice a week) could effectively prevent the recurrence of CIN 2/3 and induce lesion regression. Other studies showed that deficiency in vitamin D was linked with persistent HPV infection, while vaginal suppository use and higher dietary intake were linked with improved lesion clearance. A meta-analysis showed a pooled SMD of 0.81 (95% CI: 0.69–0.93) with no heterogeneity (I2 = 0%), favoring the protective role of vitamin D (Certainty GRADE: High) (Figure 6).
3.3.3 Vitamin C
This subgroup analysis shows vitamin C’s is probably most protective role in the treatment of HPV-associated inflammation and cervical disease. Several cross-sectional and case–control studies in various populations have consistently found an inverse relationship between vitamin C levels and HPV persistence. Reduced of vitamin C levels in the daily diet intake or serum have been linked to HPV infection, oxidative stress, and cervical cancer. The meta-analysis’s standardized mean differences indicated a statistically significant overall effect (p < 0.0001) that is in favor with higher vitamin C levels. Despite considerable heterogeneity (I2 = 97.45%), the data shows that vitamin C is a beneficial about outcomes which are related to role of micronutrient in HPV (Certainty GRADE: Low-moderate) (Figure 7).
3.3.4 Vitamin A
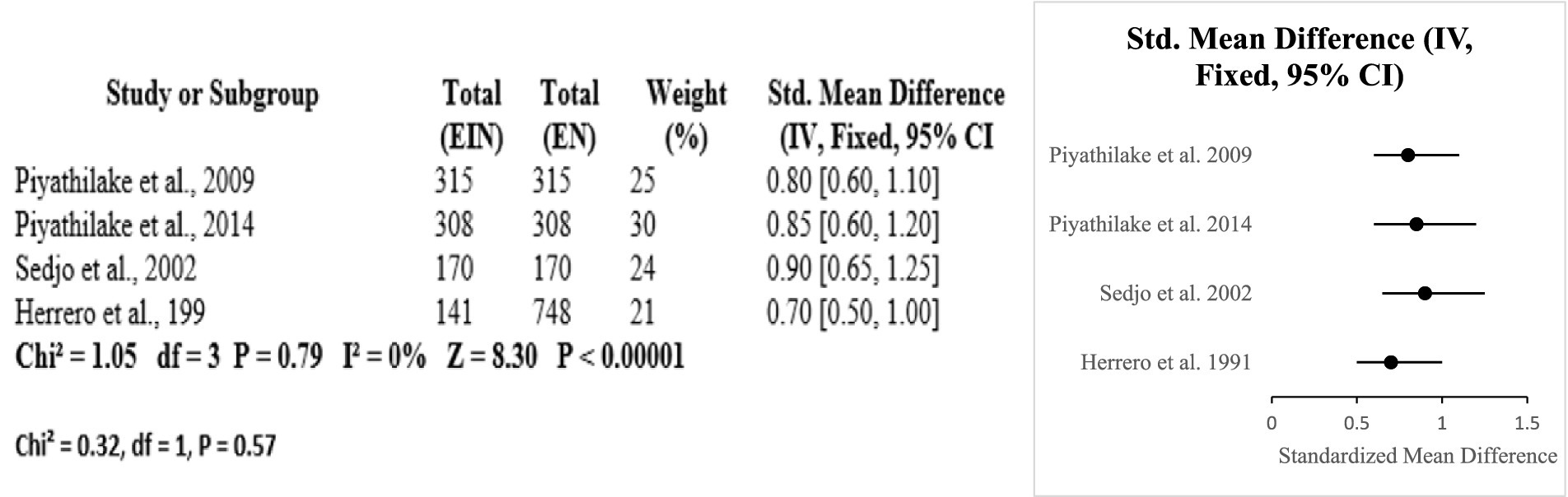
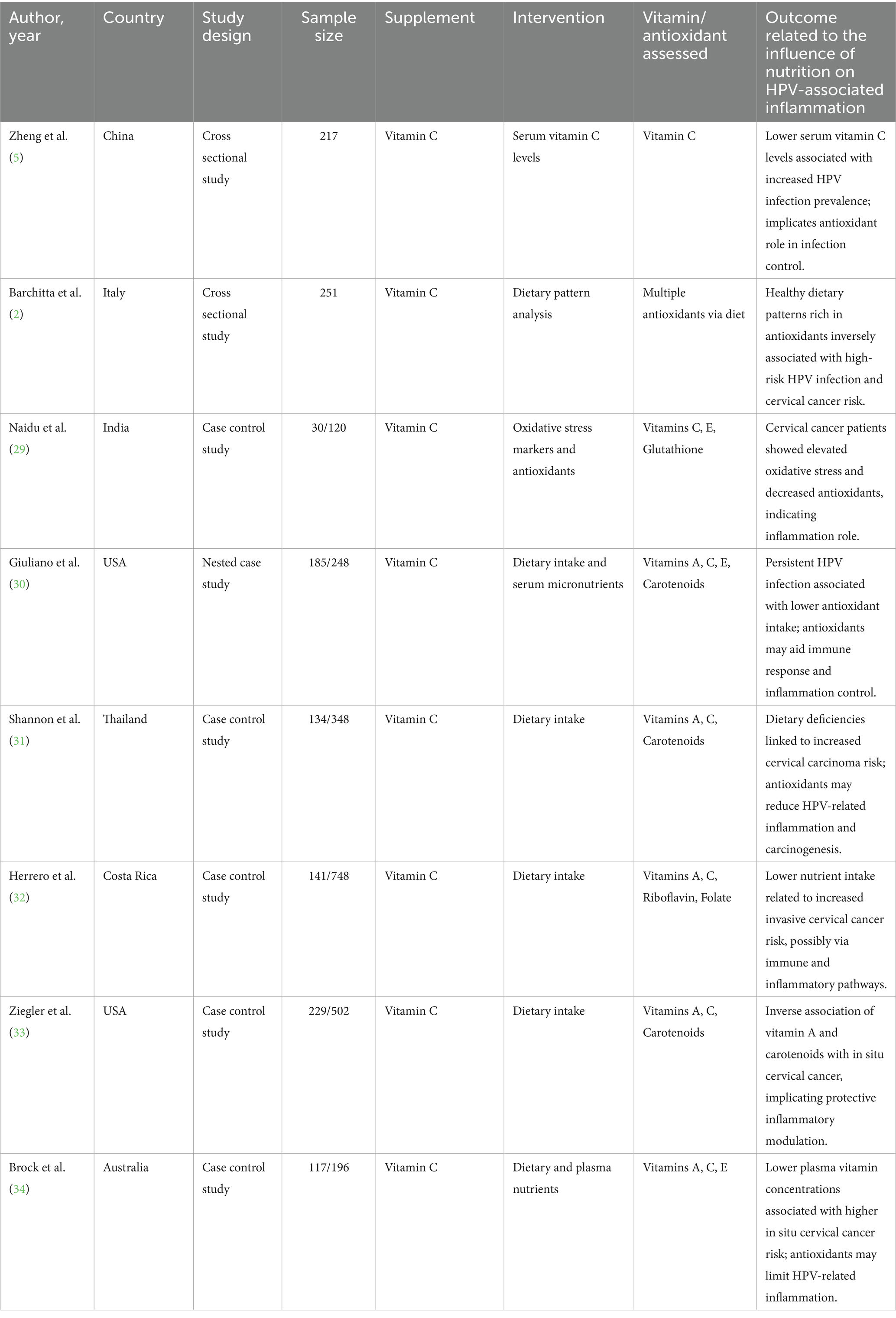
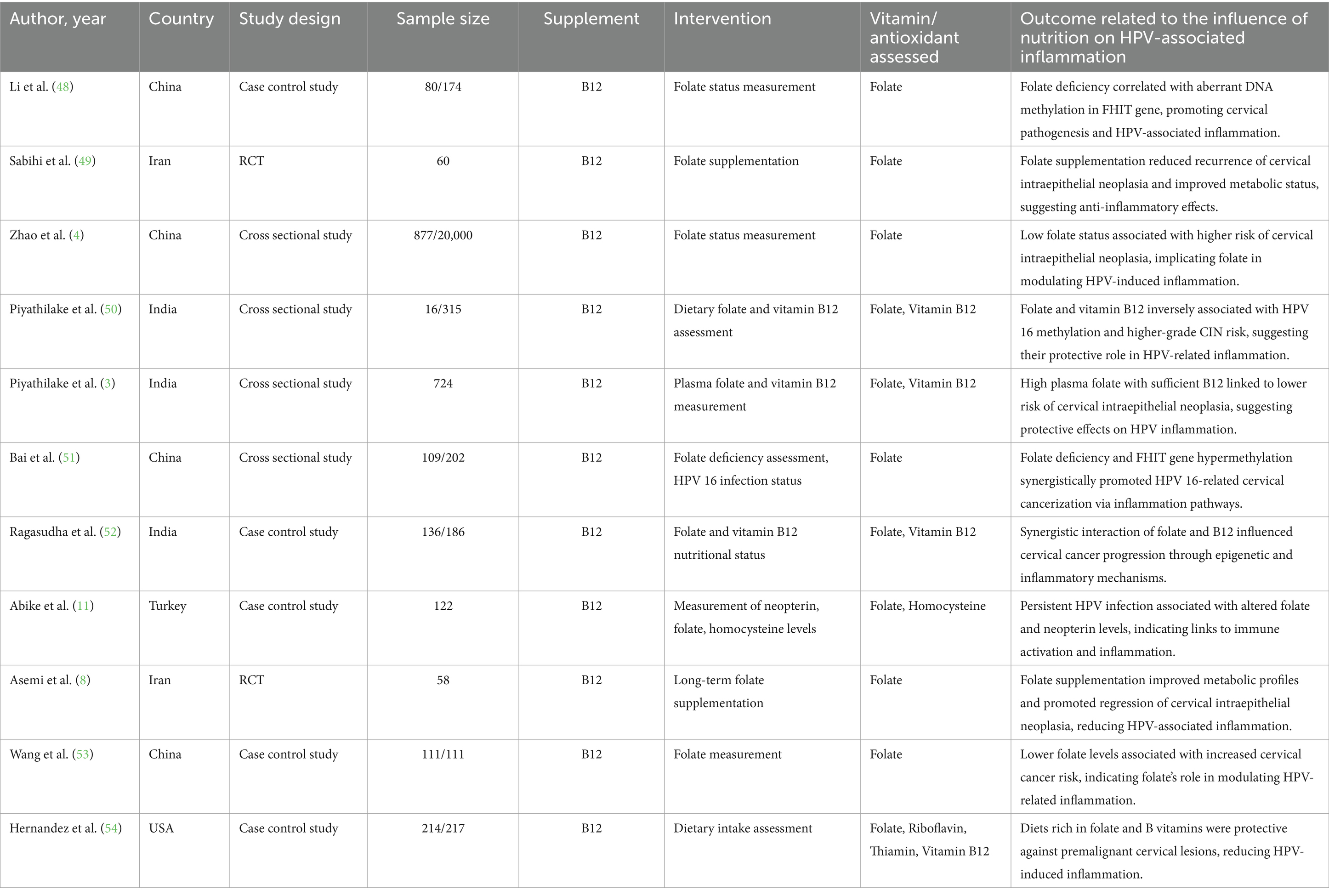
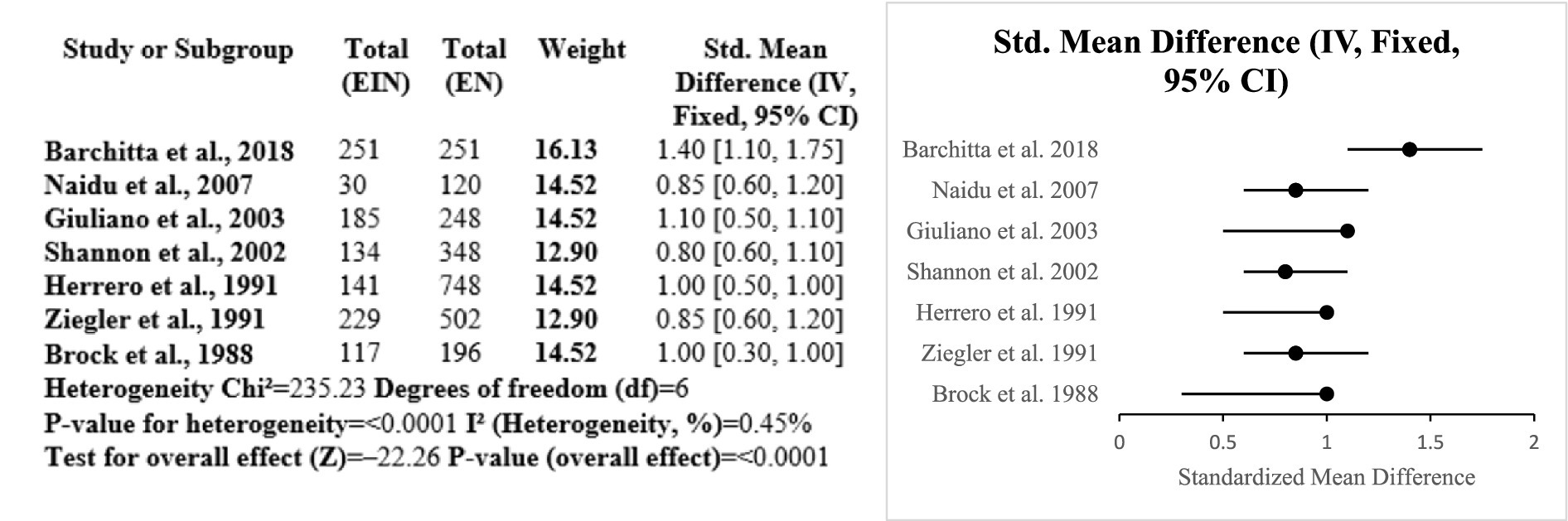
Evidence from the six studies that were case–control suggests a protective role of vitamin A against HPV-related cervical disease. Across diverse populations, higher dietary or serum vitamin A levels were consistently associated with reduced risk of cervical cancer and HPV persistence. The meta-analysis showed a significant overall effect (SMD = 0.77; p < 0.0001), with low heterogeneity (I2 = 16.6%), indicating consistency among studies. Research conducted by Ziegler, Shannon, and Ghosh emphasized significant inverse correlations among vitamin A consumption and risk for cervical cancer. Such observations complement the role of vitamin A as a potential significant nutrient in preventing HPV-linked inflammation and disease progression (Certainty GRADE: Moderate) (Figure 8).
3.3.5 Vitamin B12 and folate
The protective effects of folate and vitamin B12 against HPV-associated inflammation is highlighted in this subgroup analysis. Across four studies (USA and Costa Rica), higher circulating or dietary folate and B12 levels were consistently associated with reduced HPV persistence and lower cervical dysplasia risk. Meta-analysis resulted in a pooled standardized mean difference of 0.80 [95% CI: 0.65, 0.95] with no heterogeneity (Chi2 = 1.05, df = 3, p = 0.79; I2 = 0%). The overall effect was significant at p < 0.00001 [Z = 8.30]. Subgroup differences by country were nonsignificant (Chi2 = 0.32, df = 1, p = 0.57) (Certainty GRADE: High) (Figures 9, 10).
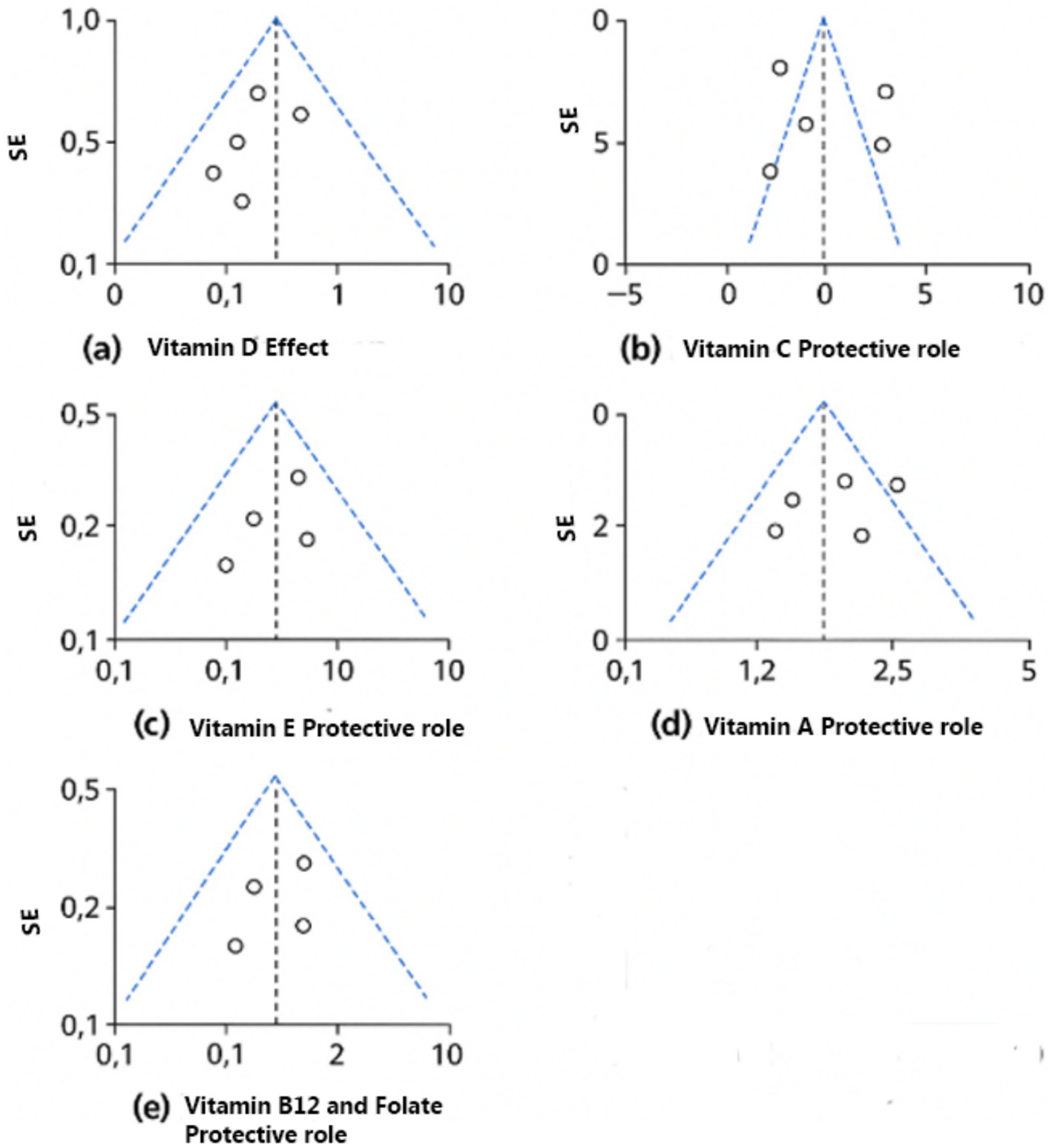
Figure 10. Funnel plots of the included nutrients. (a) Vitamin D effect. (b) Vitamin C protective role. (c) Vitamin E protective role. (d) Vitamin A protective role. (e) Vitamin B12 and folate protective role.
Greater amounts or consumption of vitamins E, D, C, A, B12, and folate have a strong connection to lower HPV perseverance and cervical risk factors confirmed by the subgroup analyses. Effect sizes fluctuate between SMD 0.46 to 0.81, and heterogeneity is typically low (I2 = 0–21.3%), with the exception of vitamin C (I2 = 97.45%) (Table 12).
4 Meta-regression
Extremely significant heterogeneity was found in the vitamin C assessment (I2 = 97.45%), suggesting a need for further investigation. In order to look into potential causes of heterogeneity, meta-regression evaluations were performed. Study design and outcome type have been investigated as mediators utilizing a random-effects model. The general impact size was still substantial when the research design was taken into account as a moderating factor (SMD = 0.997, 95% CI: 0.893–1.100). Crucially, some of the heterogeneity among trials was largely explained by study design [F(2,4) = 10.00, p = 0.028]. Although nested designs were not substantially different (β = 0.205, p = 0.136), experimental investigations demonstrated higher influences than longitudinal studies (β = 0.505, p = 0.013). We looked at outcome category as an influence as well. The pooled impact was still significant (SMD = 0.996, 95% CI: 0.863–1.129). A more thorough examination at the coefficients revealed that research concentrating on HPV infection indicated noticeably bigger impacts (β = 0.495, p = 0.034), despite the fact that the overall finding for outcome subtype was not substantial [F(3,3) = 5.39, p = 0.100]. However, results for HPV persistence (β = 0.195, p = 0.217) and cervical cancer/oxidative stress (β = −0.055, p = 0.689) were not statistically significant. Together, these results imply that research design along with certain outcome categories, especially HPV infection, may contribute to the apparently high heterogeneity (I2 = 97.45%) in vitamin C assessments. However, these findings should be treated with caution due to the relatively small amount of research.
5 Discussion
In order to examine the effect of micronutrient consumption on HPV perseverance and HPV-related inflammatory processes, this systematic review and meta-analysis combined information from 75 studies, encompassing cohort, case–control, cross-sectional, and randomized controlled trials (RCTs). The results show an ongoing association between decreased HPV persistence, decreased likelihood of cervical intraepithelial neoplasia (CIN), and weakened inflammation thanks to adequate intake of vitamins A, C, D, and E, as well as carotenoids and trace minerals like zinc and selenium. Considering the established effects of these micronutrients in immunological regulation, antioxidant defense, and epithelial integrity, these correlations make biological sense.
However, it has been observed that there are differing levels of uniformity and quality of data regarding the relationship between multiple micronutrients and the possibility of cervical cancer, CIN development, and HPV recurrence. The most frequently supported nutrient, for example, is vitamin D, which has solid proof derived from observational investigations and randomized controlled trials (RCTs). Vitamin D improves the strength of the epithelial barrier by triggering antibacterial peptides and modifies immune system reactions through cytokine modulation (9, 10). However, its therapeutic efficacy may be influenced by population-specific characteristics, though, as seen by variations in composition, dose, and starting deficient status among investigations. Furthermore, there is relatively ample proof that vitamin E is associated with a decreased likelihood of CIN and lowered HPV perseverance, especially in case–control and cross-sectional research. Its function as a fat-soluble antioxidant that lessens oxidative DNA harm supports its protective nature (14, 15). However, a dearth of sizable RCTs and sporadic accounts of a U-shaped dose–response association underscore the necessity of exercising caution when interpreting ideal consumption amounts.
Likewise, vitamin C exhibits beneficial connections as well, however there is significant variation among research. Although its function as an immune-enhancing agent and fluid-soluble antioxidant is widely recognized, the lack of RCT information and the variation in nutritional methods of evaluation make the findings less reliable (2, 5). The role of vitamin A seems more inconsistent; whereas some research reveals negative relationships between HPV recurrence and the rate of cervical cancer, other investigations reveal no significant correlations. Although retinoid regulation of mucosal immunity and epithelial development provides tenable pathways, population-specific variations in vitamin A levels and food choices may contribute to the cause of varying findings (16, 17).
Besides vitamin D, folate and vitamin B12 have the most reliable and convincing favorable connections. According to Zhao et al. (4), these nutrients are essential for the production of DNA, its restoration, and methylation regulation—processes that are immediately related to HPV-driven carcinogenesis. Their significance has been confirmed by observational investigations and RCTs. However, restraining variables include insufficient long-term clinical results evidence and variations in initial nutritional status among groups. Given that zinc is involved in regulating the immune and antioxidant enzyme systems, there is moderate proof that it helps improve HPV elimination and lowers the probability of CIN. Still, there is potential for more research because there are fewer experimental studies and ambiguities regarding the ideal dose (6). Similar to this, selenium has a modest level of evidence, with beneficial impacts mostly shown in certain RCTs. Compelling evidence supports its function in glutathione peroxidase function, although population-specific variability—such as between Chinese and Iranian cohorts—indicates that the effects of selenium vary depending on the setting.
Finally, the data supporting calcium and vitamin K is of comparatively lower standard. Although calcium is thought to have a function in preserving integrity of epithelial cells and controlling cell growth, results from observational research are contradictory, and RCTs have not confirmed this theory. With observational studies connecting lower consumption to increased HPV latency and CIN risk, vitamin K is becoming recognized as a possible preventative agent (18, 19). Nevertheless, this impact is not yet supported by experimental evidence, and its immune-modulating pathways are still unclear.
5.1 Strengths and limitations
This meta-analysis and systematic review have a number of strengths. It provides a thorough assessment of the connection between dietary intake and HPV-associated inflammation by combining findings from a variety of research methodologies, including cohort, case–control, randomized controlled trials, and cross-sectional studies. External validity is improved and evaluation across different food patterns and sociodemographic situations is made possible by the inclusion of more than 70 research from various geographic locations. Nonetheless, it is necessary to recognize certain constraints. Major drawbacks among the research investigations included are variations in HPV detection procedures and dietary assessments approaches, such as the utilization of food frequency questionnaires (FFQs) vs. blood biomarker testing. Residual confounding is of concern in observational studies, especially because of varied lifestyle or biological factors that may affect HPV results and dietary habits. Furthermore, particular subgroups may exhibit heterogeneity due to variations in results evaluation and reporting, baseline dietary inadequacies, or inconsistent nutrient composition (e.g., vitamin C assessment, which had an I2 score of 97.45%).
5.2 Implications for practice and policy
The results are evidently applicable to clinical and public health:
• Clinical application: In the treatment of women with HPV, specifically those with inferior CIN or long-term infection, nutritional assessment and customized supplements may be utilized as adjuvant therapy. Clinicians should think about prescribing vitamin D, folate, and selenium supplements in the right therapeutic settings because of their proven efficacy in clinical trials.
• Public health interventions: An affordable way of minimizing HPV load and prevalence of cervical cancer in low-resource environments where cytology-based detection and HPV vaccine are still scarce is to improve population-level eating habits and fortify essential micronutrients. The fact that this analysis is worldwide in scope—from Nigeria to China to Iran—highlights how relevant these results are worldwide.
• Future research: Trials assessing multi-nutrient therapies with immunological and genomic outcomes as well as large longitudinal cohort investigations with harmonized diagnostic evaluations should be the main focus of future research.
6 Conclusion
This meta-analysis suggest how important nutrition is, particularly micronutrients, in controlling inflammation linked to HPV and the likelihood of cervical neoplasia. Higher consumption of vitamins A, C, D, and E, as well as trace minerals and carotenoids, seems to promote epithelial health and immunological function, which reduces HPV persistence. As part of a comprehensive strategy for cervical health and HPV prevention, these findings highlight the need of maintaining optimal nutritional status. The findings suggest that public health initiatives aimed at high prevalence HPVs and undernourished communities should employ nutritional education and diet supplementation programs since food intake is a modifiable factor. Even though the data remains valid across study designs and biologically plausible, more longitudinal studies and randomized controlled trials are needed to confirm the causative inference and refine dosage recommendations for particular nutrients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YL: Writing – original draft, Supervision. LZ: Project administration, Data curation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nagata, C, Shimizu, H, Yoshikawa, H, Noda, K, Nozawa, S, Yajima, A, et al. Serum carotenoids and vitamins and risk of cervical dysplasia from a case–control study in Japan. Br J Cancer. (1999) 81:1234–7. doi: 10.1038/sj.bjc.6690834
2. Barchitta, M, Maugeri, A, Quattrocchi, A, Agrifoglio, O, Scalisi, A, and Agodi, A. The association of dietary patterns with high-risk human papillomavirus infection and cervical cancer: a cross-sectional study in Italy. Nutrients. (2018) 10:469. doi: 10.3390/nu10040469
3. Piyathilake, CJ, Macaluso, M, Alvarez, RD, Bell, WC, Heimburger, DC, and Partridge, EE. Lower risk of cervical intraepithelial neoplasia in women with high plasma folate and sufficient vitamin B12 in the post-folic acid fortification era. Cancer Prev Res. (2009) 2:658–64. doi: 10.1158/1940-6207.CAPR-08-0175
4. Zhao, W, Hao, M, Wang, Y, Feng, N, Wang, Z, Wang, W, et al. Association between folate status and cervical intraepithelial neoplasia. Eur J Clin Nutr. (2016) 70:837–42. doi: 10.1038/ejcn.2016.35
5. Zheng, C, Zheng, Z, and Chen, W. Association between serum vitamin C and HPV infection in American women: a cross-sectional study. BMC Womens Health. (2022) 22:404. doi: 10.1186/s12905-022-01993-7
6. Ayatollahi, H, Rajabi, E, Yekta, Z, and Jalali, Z. Efficacy of oral zinc sulfate supplementation on clearance of cervical human papillomavirus (HPV); a randomized controlled clinical trial. Asian Pac J Cancer Prev. (2022) 23:1285–90. doi: 10.31557/APJCP.2022.23.4.1285
7. Obhielo, E, Ezeanochie, M, Okonkwo, A, and Gharoro, E. The relationship between the serum level of selenium and cervical intraepithelial neoplasia: a comparative study in a population of Nigerian women. Asian Pac J Cancer Prev. (2019) 20:1433–6. doi: 10.31557/APJCP.2019.20.5.1433
8. Asemi, Z, Vahedpoor, Z, Jamilian, M, Bahmani, F, and Esmaillzadeh, A. Effects of long-term folate supplementation on metabolic status and regression of cervical intraepithelial neoplasia: a randomized, double-blind, placebo-controlled trial. Nutrition. (2016) 32:681–6. doi: 10.1016/j.nut.2015.12.028
9. Vahedpoor, Z, Jamilian, M, Bahmani, F, Aghadavod, E, Karamali, M, Kashanian, M, et al. Effects of long-term vitamin D supplementation on regression and metabolic status of cervical intraepithelial neoplasia: a randomized, double-blind, placebo-controlled trial. Hormones Cancer. (2017) 8:58–67. doi: 10.1007/s12672-016-0278-x
10. Vahedpoor, Z, Mahmoodi, S, Samimi, M, Gilasi, HR, Bahmani, F, Soltani, A, et al. Long-term vitamin D supplementation and the effects on recurrence and metabolic status of cervical intraepithelial neoplasia grade 2 or 3: a randomized, double-blind, placebo-controlled trial. Ann Nutr Metab. (2018) 72:151–60. doi: 10.1159/000487270
11. Abike, F, Engin, AB, Dunder, I, Tapisiz, OL, Aslan, C, and Kutluay, L. Human papilloma virus persistence and neopterin, folate and homocysteine levels in cervical dysplasias. Arch Gynecol Obstet. (2011) 284:209–14. doi: 10.1007/s00404-010-1650-7
12. Atalah, E, Urteaga, C, Rebolledo, A, Villegas, RA, Medina, E, and Csendes, A. Diet, smoking and reproductive history as risk factors for cervical cancer. Rev Med Chile. (2001) 129:597–603.
13. Chen, C, Hwang, J, Kuo, T, Hsieh, C, and Huang, S. Serum copper and zinc levels in patients with cervical cancer. J Formosan Med Assoc. (1990) 89:677–82.
14. Ghosh, C, Baker, JA, Moysich, KB, Rivera, R, Brasure, JR, and McCann, SE. Dietary intakes of selected nutrients and food groups and risk of cervical cancer. Nutr Cancer. (2008) 60:331–41. doi: 10.1080/01635580701861769
15. Siegel, EM, Craft, NE, Duarte-Franco, E, Villa, LL, Franco, EL, and Giuliano, AR. Associations between serum carotenoids and tocopherols and type-specific HPV persistence: the Ludwig-McGill cohort study. Int J Cancer. (2007) 120:672–80. doi: 10.1002/ijc.22346
16. Kim, JH, Bae, SN, Lee, CW, Song, MJ, Lee, SJ, Yoon, JH, et al. A pilot study to investigate the treatment of cervical human papillomavirus infection with zinc-citrate compound (CIZAR®). Gynecol Oncol. (2011) 122:303–6. doi: 10.1016/j.ygyno.2011.04.026
17. Shimizu, H, Nagata, C, Komatsu, S, Morita, N, Higashiiwai, H, Sugahara, N, et al. Decreased serum retinol levels in women with cervical dysplasia. Br J Cancer. (1996) 73:1600–4. doi: 10.1038/bjc.1996.301
18. Jiang, Y, Xu, S, Lan, J, Zhang, J, and Chen, T. Dietary vitamin K intake and HPV-infection status among American women: a secondary analysis from national health and nutrition examination survey data from 2003 to 2016. Int J Public Health. (2022) 67:1604616. doi: 10.3389/ijph.2022.1604616
19. Wang, Z, Yang, A, Yang, J, Zhao, W, Wang, Z, Wang, W, et al. Dietary nutrient intake related to higher grade cervical intraepithelial neoplasia risk: a Chinese population-based study. Nutr Metab. (2020) 17:1–14. doi: 10.1186/s12986-020-00521-4
20. Guo, L, Zhu, H, Lin, C, Che, J, Tian, X, Han, S, et al. Associations between antioxidant vitamins and the risk of invasive cervical cancer in Chinese women: a case-control study. Sci Rep. (2015) 5:13607. doi: 10.1038/srep13607
21. Zhang, Y-Y, Lu, L, Abliz, G, and Mijit, F. Serum carotenoid, retinol and tocopherol concentrations and risk of cervical cancer among Chinese women. Asian Pac J Cancer Prev. (2015) 16:2981–6. doi: 10.7314/APJCP.2015.16.7.2981
22. Tomita, LY, Filho, AL, Costa, MC, Andreoli, MAA, Villa, LL, Franco, EL, et al. Diet and serum micronutrients in relation to cervical neoplasia and cancer among low-income Brazilian women. Int J Cancer. (2010) 126:703–14.
23. Palan, PR, Woodall, AL, Anderson, PS, and Mikhail, MS. Α-Tocopherol and α-tocopheryl quinone levels in cervical intraepithelial neoplasia and cervical cancer. Am J Obstet Gynecol. (2004) 190:1407–10. doi: 10.1016/j.ajog.2004.01.067
24. Palan, P, Mikhail, M, Shaban, D, and Romney, S. Plasma concentrations of coenzyme Q10 and tocopherols in cervical intraepithelial neoplasia and cervical cancer. Eur J Cancer Prev. (2003) 12:321–6. doi: 10.1097/00008469-200308000-00013
25. Goodman, MT, Shvetsov, YB, McDuffie, K, Wilkens, LR, Zhu, X, Franke, AA, et al. Hawaii cohort study of serum micronutrient concentrations and clearance of incident oncogenic human papillomavirus infection of the cervix. Cancer Res. (2007) 67:5987–96. doi: 10.1158/0008-5472.CAN-07-0313
26. Özgü, E, Yılmaz, N, Başer, E, Güngör, T, Erkaya, S, and İbrahim Yakut, H. Could 25-OH vitamin D deficiency be a reason for HPV infection persistence in cervical premalignant lesions? J Exp Ther Oncol. (2016) 11:177–80.
27. Schulte-Uebbing, C, Schlett, S, Craiut, I, Antal, L, and Olah, H. Chronical cervical infections and dysplasia (CIN I, CIN II): vaginal vitamin D (high dose) treatment: a new effective method? Dermatoendocrinol. (2014) 6:e983684. doi: 10.4161/derm.27791
28. Hosono, S, Matsuo, K, Kajiyama, H, Hirose, K, Suzuki, T, Kawase, T, et al. Association between dietary calcium and vitamin D intake and cervical carcinogenesis among Japanese women. Eur J Clin Nutr. (2010) 64:400–9. doi: 10.1038/ejcn.2010.28
29. Naidu, MSK, Suryakar, A, Swami, SC, Katkam, R, and Kumbar, K. Oxidative stress and antioxidant status in cervical cancer patients. Indian J Clin Biochem. (2007) 22:140–4. doi: 10.1007/BF02913333
30. Giuliano, AR, Siegel, EM, Roe, DJ, Ferreira, S, Luiza Baggio, M, Galan, L, et al. Dietary intake and risk of persistent human papillomavirus (HPV) infection: the Ludwig-McGill HPV natural history study. J Infect Dis. (2003) 188:1508–16. doi: 10.1086/379197
31. Shannon, J, Thomas, DB, Ray, RM, Kestin, M, Koetsawang, A, Koetsawang, S, et al. Dietary risk factors for invasive and in-situ cervical carcinomas in Bangkok, Thailand. Cancer Causes Control. (2002) 13:691–9. doi: 10.1023/A:1020289618161
32. Herrero, R, Potischman, N, Brinton, LA, Reeves, WC, Brenes, MM, Tenorio, F, et al. A case-control study of nutrient status and invasive cervical cancer: I. Dietary indicators. Am J Epidemiol. (1991) 134:1335–46.
33. Ziegler, RG, Jones, CJ, Brinton, LA, Norman, SA, Mallin, K, Levine, RS, et al. Diet and the risk of in situ cervical cancer among white women in the United States. Cancer Causes Control. (1991) 2:17–29. doi: 10.1007/BF00052357
34. Brock, K, Berry, G, Mock, P, MacLennan, R, Truswell, A, and Brinton, L. Nutrients in diet and plasma and risk of in situ cervical cancer. JNCI J Natl Cancer Inst. (1988) 80:580–5. doi: 10.1093/jnci/80.8.580
35. Huang, X, Chen, C, Zhu, F, Zhang, Y, Feng, Q, Li, J, et al. Association between dietary vitamin a and HPV infection in American women: data from NHANES 2003–2016. Biomed Res Int. (2020) 2020:4317610. doi: 10.1155/2020/4317610
36. Eleutério, J, Giraldo, PC, Gonçalves, AK, Eleutério, RMN, Barbosa, R d CC, and Cavalcante, DIM. The risk of high-grade squamous intraepithelial lesions in women with low serum levels of vitamin a. Gynecol Obstet Investig. (2014) 78:235–8. doi: 10.1159/000363741
37. Yeo, AS, Schiff, MA, Montoya, G, Masuk, M, van Asselt-King, L, and Becker, TM. Serum micronutrients and cervical dysplasia in southwestern American Indian women. Nutr Cancer. (2000) 38:141–50.
38. Karamali, M, Nourgostar, S, Zamani, A, Vahedpoor, Z, and Asemi, Z. The favourable effects of long-term selenium supplementation on regression of cervical tissues and metabolic profiles of patients with cervical intraepithelial neoplasia: a randomised, double-blind, placebo-controlled trial. Br J Nutr. (2015) 114:2039–45. doi: 10.1017/S0007114515003852
39. Cunzhi, H, Jiexian, J, Xianwen, Z, Jingang, G, Shumin, Z, and Lili, D. Serum and tissue levels of six trace elements and copper/zinc ratio in patients with cervical cancer and uterine myoma. Biol Trace Elem Res. (2003) 94:113–22. doi: 10.1385/BTER:94:2:113
40. Xiao, D, Li, W, Zhang, W-H, Wen, Z, Dai, B, Mo, W, et al. Dietary zinc, copper, and selenium intake and high-risk human papillomavirus infection among American women: data from NHANES 2011–2016. Nutr Cancer. (2022) 74:1958–67. doi: 10.1080/01635581.2021.1979603
41. Sundström, H. Annual variation of serum selenium in patients with gynaecological cancer during 1978-1983 in Finland, a low selenium area. Int J Vit Nutr Res. (1985) 55:433–8.
42. Hwang, JH, Kim, MK, and Lee, JK. Dietary supplements reduce the risk of cervical intraepithelial neoplasia. Int J Gynecol Cancer. (2010) 20:398–403. doi: 10.1111/IGC.0b013e3181d02ff2
43. Siegel, EM, Patel, N, Lu, B, Lee, J-H, Nyitray, AG, Huang, X, et al. Circulating biomarkers of iron storage and clearance of incident human papillomavirus infection. Cancer Epidemiol Biomarkers Prev. (2012) 21:859–65. doi: 10.1158/1055-9965.EPI-12-0073
44. He, A-J, Chen, C, Jia, M, and Fan, R-Q. Dietary calcium intake and HPV infection status among American women: a secondary Analysis from National Health and nutrition examination survey (NHANES) data set of 2003–2016. Med Sc Monit. (2020) 26:e921571–1. doi: 10.12659/MSM.921571
45. Sengupta, A, Mallick, R, Dutta, S, and Sarkar, R. Serum calcium level in patients with carcinoma of the cervix in relation to menstrual status. Eur J Gynaecol Oncol. (1989) 10:153–5.
46. Grail, A, and Norval, M. Copper and zinc levels in serum from patients with abnormalities of the uterine cervix. Acta Obstet Gynecol Scand. (1986) 65:443–7. doi: 10.3109/00016348609157381
47. Liu, T, Soong, S, Wilson, NP, Craig, CB, Cole, P, Macaluso, M, et al. A case control study of nutritional factors and cervical dysplasia. Cancer Epidemiol Biomarkers Prev. (1993) 2:525–30.
48. Li, Q, Ding, L, Jing, N, Liu, C, Yang, Z, Chen, F, et al. Folate deficiency and aberrant DNA methylation and expression of FHIT gene were associated with cervical pathogenesis. Oncol Lett. (2018) 15:1963–72. doi: 10.3892/ol.2017.7471
49. Sabihi, S, Vahedpoor, Z, Saraf-Bank, S, and Nourian, M. Effects of folate supplementation on recurrence and metabolic status of cervical intraepithelial neoplasia grade 2/3 in overweight and obese women: a randomized double-blind placebo-controlled trial. Eur J Clin Nutr. (2022) 76:666–70. doi: 10.1038/s41430-021-01022-0
50. Piyathilake, CJ, Macaluso, M, Chambers, MM, Badiga, S, Siddiqui, NR, Bell, WC, et al. Folate and vitamin B12 may play a critical role in lowering the HPV 16 methylation–associated risk of developing higher grades of CIN. Cancer Prev Res. (2014) 7:1128–37. doi: 10.1158/1940-6207.CAPR-14-0143
51. Bai, L-X, Wang, J-T, Ding, L, Jiang, S-W, Kang, H-J, Gao, C-F, et al. Folate deficiency and FHIT hypermethylation and HPV 16 infection promote cervical cancerization. Asian Pac J Cancer Prev. (2014) 15:9313–7. doi: 10.7314/APJCP.2014.15.21.9313
52. Ragasudha, PN, Thulaseedharan, JV, Wesley, R, Jayaprakash, P, Lalitha, P, and Pillai, MR. A case-control nutrigenomic study on the synergistic activity of folate and vitamin B12 in cervical cancer progression. Nutr Cancer. (2012) 64:550–8. doi: 10.1080/01635581.2012.675618
53. Wang, J, Ma, X, Cheng, Y, Ding, L, and Zhou, Q. A case-control study on the association between folate and cervical cancer. Zhonghua liu xing bing xue za zhi. (2006) 27:424–7.
54. Hernandez, BY, McDuffie, K, Wilkens, LR, Kamemoto, L, and Goodman, MT. Diet and premalignant lesions of the cervix: evidence of a protective role for folate, riboflavin, thiamin, and vitamin B 12. Cancer Causes Control. (2003) 14:859–70. doi: 10.1023/B:CACO.0000003841.54413.98
55. Fujii, T, Takatsuka, N, Nagata, C, Matsumoto, K, Oki, A, Furuta, R, et al. Association between carotenoids and outcome of cervical intraepithelial neoplasia: a prospective cohort study. Int J Clin Oncol. (2013) 18:1091–101. doi: 10.1007/s10147-012-0486-5
56. Peterson, CE, Sedjo, RL, Davis, FG, Beam, CA, and Giuliano, AR. Combined antioxidant carotenoids and the risk of persistent human papillomavirus infection. Nutr Cancer. (2010) 62:728–33. doi: 10.1080/01635581003693074
57. Tomita, LY, Roteli-Martins, CM, Villa, LL, Franco, EL, and Cardoso, MA. Associations of dietary dark-green and deep-yellow vegetables and fruits with cervical intraepithelial neoplasia: modification by smoking. Br J Nutr. (2011) 105:928–37. doi: 10.1017/S0007114510004447
58. Sedjo, RL, Papenfuss, MR, Craft, NE, and Giuliano, AR. Effect of plasma micronutrients on clearance of oncogenic human papillomavirus (HPV) infection (United States). Cancer Causes Control. (2003) 14:319–26. doi: 10.1023/A:1023981505268
59. Sedjo, RL, Roe, DJ, Abrahamsen, M, Harris, RB, Craft, N, Baldwin, S, et al. Vitamin a, carotenoids, and risk of persistent oncogenic human papillomavirus infection. Cancer Epidemiol Biomarkers Prev. (2002) 11:876–84.
60. Schiff, MA, Patterson, RE, Baumgartner, RN, Masuk, M, van Asselt-King, L, Wheeler, CM, et al. Serum carotenoids and risk of cervical intraepithelial neoplasia in southwestern American Indian women. Cancer Epidemiol Biomarkers Prev. (2001) 10:1219–22.
61. Palan, PR, Mikhail, MS, Goldberg, GL, Basu, J, Runowicz, CD, and Romney, SL. Plasma levels of beta-carotene, lycopene, canthaxanthin, retinol, and alpha-and tau-tocopherol in cervical intraepithelial neoplasia and cancer. Clin Cancer Res. (1996) 2:181–5.
62. Kanetsky, PA, Gammon, MD, Mandelblatt, J, Zhang, ZF, Ramsey, E, Dnistrian, A, et al. Dietary intake and blood levels of lycopene: association with cervical dysplasia among non-hispanic, black women. Nutr Cancer. (1998) 31:31–40. doi: 10.1080/01635589809514675
63. Romney, SL, Ho, GY, Palan, PR, Basu, J, Kadish, AS, Klein, S, et al. Effects of β-carotene and other factors on outcome of cervical dysplasia and human papillomavirus infection. Gynecol Oncol. (1997) 65:483–92.
64. De Vet, HC, Knipschild, PG, Grol, ME, Schouten, HJ, and Sturmans, F. The role of beta-carotene and other dietary factors in the aetiology of cervical dysplasia: results of a case-control study. Int J Epidemiol. (1991) 20:603–10.
65. dE Vet, HC, Knipschild, PG, Willebrand, D, Schouten, HJ, and Sturmans, F. The effect of beta-carotene on the regression and progression of cervical dysplasia: a clinical experiment. J Clin Epidemiol. (1991) 44:273–83.
66. Gonzalez, CA, Travier, N, Luján-Barroso, L, Castellsague, X, Bosch, FX, Roura, E, et al. Dietary factors and in situ and invasive cervical cancer risk in the European prospective investigation into cancer and nutrition study. Int J Cancer. (2011) 129:449–59. doi: 10.1002/ijc.25679
67. Cuzick, J, Sasieni, P, and Singer, A. Risk factors for invasive cervix cancer in young women. Eur J Cancer. (1996) 32:836–41. doi: 10.1016/0959-8049(95)00650-8
68. Harris, R, Forman, D, Doll, R, Vessey, M, and Wald, N. Cancer of the cervix uteri and vitamin a. Br J Cancer. (1986) 53:653–9. doi: 10.1038/bjc.1986.109
69. Van Eenwyk, J, Davis, FG, and Bowen, PE. Dietary and serum carotenoids and cervical intraepithelial neoplasia. Int J Cancer. (1991) 48:34–8. doi: 10.1002/ijc.2910480107
70. Potischman, N, Herrero, R, Brinton, LA, Reeves, WC, Stacewicz-Sapuntzakis, M, Jones, CJ, et al. A case-control study of nutrient status and invasive cervical cancer: II. Serologic indicators. Am J Epidemiol. (1991) 134:1347–55.
71. Verreault, R, Chu, J, Mandelson, M, and Shy, K. A case-control study of diet and invasive cervical cancer. Int J Cancer. (1989) 43:1050–4. doi: 10.1002/ijc.2910430616
72. Giuliano, AR. The role of nutrients in the prevention of cervical dysplasia and cancer. Nutrition. (2000) 16:570–3. doi: 10.1016/S0899-9007(00)00338-5
73. Rajkumar, T, Franceschi, S, Vaccarella, S, Gajalakshmi, V, Sharmila, A, Snijders, P, et al. Role of paan chewing and dietary habits in cervical carcinoma in Chennai, India. Br J Cancer. (2003) 88:1388–93. doi: 10.1038/sj.bjc.6600902
74. Vecchia, CL, Franceschi, S, Decarli, A, Gentile, A, Fasoli, M, Pampallona, S, et al. Dietary vitamin a and the risk of invasive cervical cancer. Int J Cancer. (1984) 34:319–22. doi: 10.1002/ijc.2910340306
75. Larrinaga, M, Lateulade, S, Balbi, JC, Mendilaharsu, M, Fierro, L, and De Stefani, E. Paridad, factores sexuales y riesgo de cáncer de cérvix uterino. Arch Med Interna. (Montevideo). (1990) 139–45.
76. Hwang, JH, Lee, JK, Kim, TJ, and Kim, MK. The association between fruit and vegetable consumption and HPV viral load in high-risk HPV-positive women with cervical intraepithelial neoplasia. Cancer Causes Control. (2010) 21:51–9. doi: 10.1007/s10552-009-9433-9
Keywords: antioxidants, selenium, zinc, nutrition, HPV, cervical intraepithelial neoplasia, inflammation, vitamin D
Citation: Li Y and Zhu L (2025) The influence of nutrition on HPV-associated inflammation: a systematic review and meta-analysis. Front. Nutr. 12:1612919. doi: 10.3389/fnut.2025.1612919
Edited by:
Karolina Krupa-Kotara, Medical University of Silesia, PolandReviewed by:
Ajibola Ibraheem Abioye, Harvard University, United StatesSreenivasulu Chintala, Indiana University School of Medicine, United States
Copyright © 2025 Li and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan Li, bGl5dWFuZHJlYW1zQDEyNi5jb20=
 Yuan Li
Yuan Li Lijuan Zhu
Lijuan Zhu
![Forest plot showing standardized mean differences (IV, fixed, 95% CI) for various studies. Each study's data includes EIN and EN totals, weights, and standard mean differences with confidence intervals. The pooled result is marked with a red diamond, indicating an overall effect size of 0.77 [0.68, 0.87]. Heterogeneity statistics include Chi-square, degrees of freedom, I-squared, and P-values. The plot suggests a significant overall effect with low heterogeneity.](https://www.frontiersin.org/files/Articles/1612919/fnut-12-1612919-HTML/image_m/fnut-12-1612919-g008.jpg)