- 1Department of Food and Nutrition, College of Human Ecology, Seoul National University, Seoul, Republic of Korea
- 2Division of Population Health Research, Department of Precision Medicine, National Institute of Health, Cheongju, Republic of Korea
- 3Department of Food Science and Nutrition, Soonchunhyang University, Asan-si, Republic of Korea
- 4Department of Public Health, Graduate School of Public Health, Seoul National University, Seoul, Republic of Korea
- 5Research Institute of Human Ecology, Seoul National University, Seoul, Republic of Korea
Background: The association between dietary soy and isoflavone intake and mortality remains inconclusive. This study aimed to examine the relationships of dietary intakes of isoflavones, soy protein, and soy foods with all-cause, cancer, and cardiovascular disease (CVD) mortality in Korean adults.
Methods: This prospective cohort study included 118,450 Korean adults aged 40–79 years from the Health Examinees Study (2004–2013). Dietary intakes of isoflavones, soy protein, and soy foods were assessed using a food frequency questionnaire. Cox proportional hazards models were used to estimate adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for mortality risk according to quartiles of dietary soy and isoflavone intake.
Results: During a median follow-up of 10.1 years (interquartile range: 8.7–11.4 years), 2,614 deaths were documented, including 1,290 from cancer and 389 from CVD. Multivariable analyses showed no significant associations between dietary isoflavone intake and the risk of all-cause and cause-specific mortality. The HRs (95% CIs) comparing the highest vs. the lowest quartile of isoflavone intake were 1.04 (0.93–1.15) for all-cause mortality, 0.98 (0.84–1.14) for cancer mortality, and 1.04 (0.79–1.38) for CVD mortality. Similarly, no significant associations were observed for soy protein or soy food intake in relation to all-cause, cancer, and CVD mortality.
Conclusion: Our study found no significant associations of dietary intakes of isoflavones, soy protein, and soy foods with the risks of all-cause, cancer, and CVD mortality.
1 Introduction
Soy foods are a major dietary source of isoflavones, plant-derived compounds classified as phytoestrogens due to their structural similarity to estrogens (1). Isoflavones are also considered natural selective estrogen receptor modulators (SERMs), exerting tissue-specific estrogenic or anti-estrogenic effects (2). Tamoxifen and raloxifene, for example, are widely used SERMs for the treatment of breast cancer and osteoporosis, respectively. In addition to their hormone-related actions, isoflavones have been reported to possess antioxidant, anti-inflammatory, anti-proliferative effects, and tyrosine kinase-inhibitory properties, which may contribute to a range of health outcomes (3). Soybeans also provide high-quality protein, which has been shown to exert a modest but clinically relevant cholesterol-lowering effect (4). Based on these biological properties, higher intakes of soy and isoflavones have been hypothesized to be associated with a reduced risk of coronary heart disease (CHD), certain cancers, improved bone health, and alleviation of menopausal symptoms such as hot flashes (5). However, findings from clinical and observational studies remain inconclusive across health outcomes, highlighting the need for further research to clarify the potential health effects of soy and isoflavones (5, 6).
Specifically, the relationship between dietary soy and isoflavone intake and mortality risk remains inconsistent (7–10). A recent meta-analysis of prospective cohort studies reported that the highest category of soy food intake, compared with the lowest, was significantly associated with lower risks of all-cause and cardiovascular disease (CVD) mortality, but not cancer mortality (8). In contrast, meta-analyses of isoflavone intake found no significant associations with mortality from CVD (9) or cancer (10). However, evidence on isoflavone intake and mortality risk is limited, particularly in populations with high habitual soy consumption. For example, in the Singapore Chinese Health Study, dietary intakes of soy protein, isoflavones, and tofu equivalents were not significantly associated with CVD mortality in the overall analysis (11). In sex-stratified analyses, higher soy protein intake was associated with a slightly increased risk of CVD mortality among men, but not women. In the Takayama Study, natto intake was inversely associated with CVD mortality among Japanese men and women, whereas no significant associations were found for soy protein or isoflavone intake (12). These limited and inconsistent findings underscore the need for further evidence from prospective studies conducted in populations with high habitual soy consumption.
Therefore, in this study, we aimed to examine the associations of dietary intakes of isoflavones, soy protein, and soy foods with all-cause, cancer, and CVD mortality in Korean adults, using data from a large-scale prospective cohort study.
2 Methods and materials
2.1 Study population
The Health Examinees (HEXA) Study is a prospective cohort study established as part of the Korean Genome and Epidemiology Study (KoGES) Consortium, conducted by the National Institute of Health within the Korea Disease Control and Prevention Agency (KDCA) (13). The HEXA study was designed to investigate risk factors for chronic diseases among Korean adults aged 40 years and older. Between 2004 and 2013, a total of 173,208 participants were recruited from 39 health examination centers, primarily general hospitals in metropolitan areas and major cities across Korea. Participants underwent an interview and health examination at baseline. For those who provided consent and had valid resident registration numbers, baseline data were linked to the Cause of Death Statistics provided by the Statistics Korea (14). Of the 130,230 participants in the linked dataset, individuals with a history of cancer or CVD (n = 8,664) were excluded. Participants who died within 3 years of follow-up (n = 474) were excluded to minimize potential reverse causation. Among the remaining participants, those without dietary data (n = 1,425) or with implausible energy intake (n = 1,217; beyond ± 3 standard deviations [SD] from the mean of log-transformed energy intake) were excluded, leaving 118,450 participants in the current analysis.
The HEXA study was approved by the Institutional Review Boards (IRBs) of the KoGES group collaborators and the KDCA. All participants provided written informed consent. This study received exempt approval from the IRB of Seoul National University (IRB No. E2012/001-002), and permission to use the data was granted by the National Institute of Health in Korea.
2.2 Dietary assessment
A validated semi-quantitative food frequency questionnaire (FFQ) with 106 food items was administered to participants through personal interviews (15). Participants were asked to report their average frequency of consumption for each food item over the past year, using nine frequency categories ranging from “almost never” to “three times per day.” They were also asked to report their usual portion size as small, medium, or large. Daily intake was calculated based on the selected portion size and frequency of consumption. The nutrient database for the FFQ was based on the food composition table of the Korean Nutrition Society (16).
2.3 Estimation of soy and isoflavone intake
Soy food intake (g/day) was calculated as the sum of the intake amounts of soybeans, tofu, soy sprouts, soy milk, and soybean paste (doenjang, cheonggukjang, and ssamjang). Soy protein intake (g/day) was calculated by summing the protein content of these soy foods. Total isoflavone content was calculated as the sum of daidzein, genistein, and glycitein, using the Flavonoid Database of Common Korean Foods (17). Each food item in the FFQ was matched to those in the isoflavone database, and dietary isoflavone intake (mg/day) was calculated by multiplying the amount of food intake by the isoflavone content. The percentage contribution of each food or food group to total isoflavone intake was calculated.
2.4 Ascertainment of mortality
Mortality data were obtained from the Cause of Death Statistics. The cause of death was categorized according to the Korean Standard Classification of Diseases, 6th revision, which is based on the International Classification of Diseases, 10th revision. Cause-specific mortality was classified into death from cancer (C00 to C97) and CVD (I00 to I99). The follow-up time for each participant was calculated from baseline to the date of death or the end of the follow-up period (December 31, 2019).
2.5 Covariate assessment
Data on demographics, lifestyle factors, and medical history were assessed using an interviewer-administered questionnaire (13). Average alcohol drinking over the past year was estimated as ethanol intake in grams per day, and smoking history was assessed in pack-years. Physical activity was measured by self-reported hours per week of regular exercise, defined as activity intense enough to cause sweating. Anthropometric measurements were obtained by trained staff, and body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Diabetes was defined based on a self-reported physician diagnosis. Participants also reported their regular use of multivitamins over the past year. Daily intake of total energy, fruits, vegetables, and red/processed meats was estimated from the FFQ.
2.6 Statistical analyses
Energy-adjusted intakes of isoflavones, soy protein, and soy foods were estimated using the residual method (18) and categorized into quartiles. All reported values for dietary soy and isoflavone intake were adjusted for energy intake. Cox proportional hazards models were applied to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between dietary soy and isoflavone intake and mortality risk. Model 1 was stratified by 5- or 10-year age groups and adjusted for age in years and sex. Model 2 additionally adjusted for education level, current alcohol drinking (none, <5, 5 to <15, 15 to <30, ≥30 g/day), smoking status (none, <10, 10 to <20, 20 to <30, ≥ 30 pack-years), regular exercise [none, ≤ 3.5 (median), >3.5 hours/week], BMI (<18.5, 18.5 to <23, 23 to <25, ≥25 kg/m2), history of diabetes (no, yes), energy intake (kcal/day), and fruit and vegetable intake (g/day). Multivitamin use and red/processed meat intake were not included in the final model as they did not substantially affect the estimates. P-values for trend were calculated by entering the median value of each quartile of soy or isoflavone intake as a continuous variable in the model. The proportional hazards assumption was assessed using time-dependent covariates. Main analyses were repeated for men and women separately, using sex-specific quartiles. Subgroup analyses were conducted to examine the association between dietary soy isoflavone intake and all-cause mortality, stratified by demographic and lifestyle factors. Statistical significance for the interaction was tested using the likelihood ratio test.
A two-tailed P-value of < 0.05 was considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA).
3 Results
3.1 Characteristics of participants
The median values (interquartile range) dietary intakes of isoflavones, soy protein, and soy foods were 10.2 (6.5–15.9) mg/day, 3.1 (2.0–5.1) g/day, and 34.8 (21.3–56.0) g/day, respectively. The major food sources of isoflavones were tofu (49%), soybeans (15%), soy milk (12%), soybean paste (11%), soybean sprouts (10%), with a combined contribution of 97%. Table 1 shows the baseline characteristics of participants according to quartiles of isoflavone intake. Participants with higher isoflavone intake were more likely to be older, women, engage in regular exercise, report a history of diabetes, and consume more fruits and vegetables.
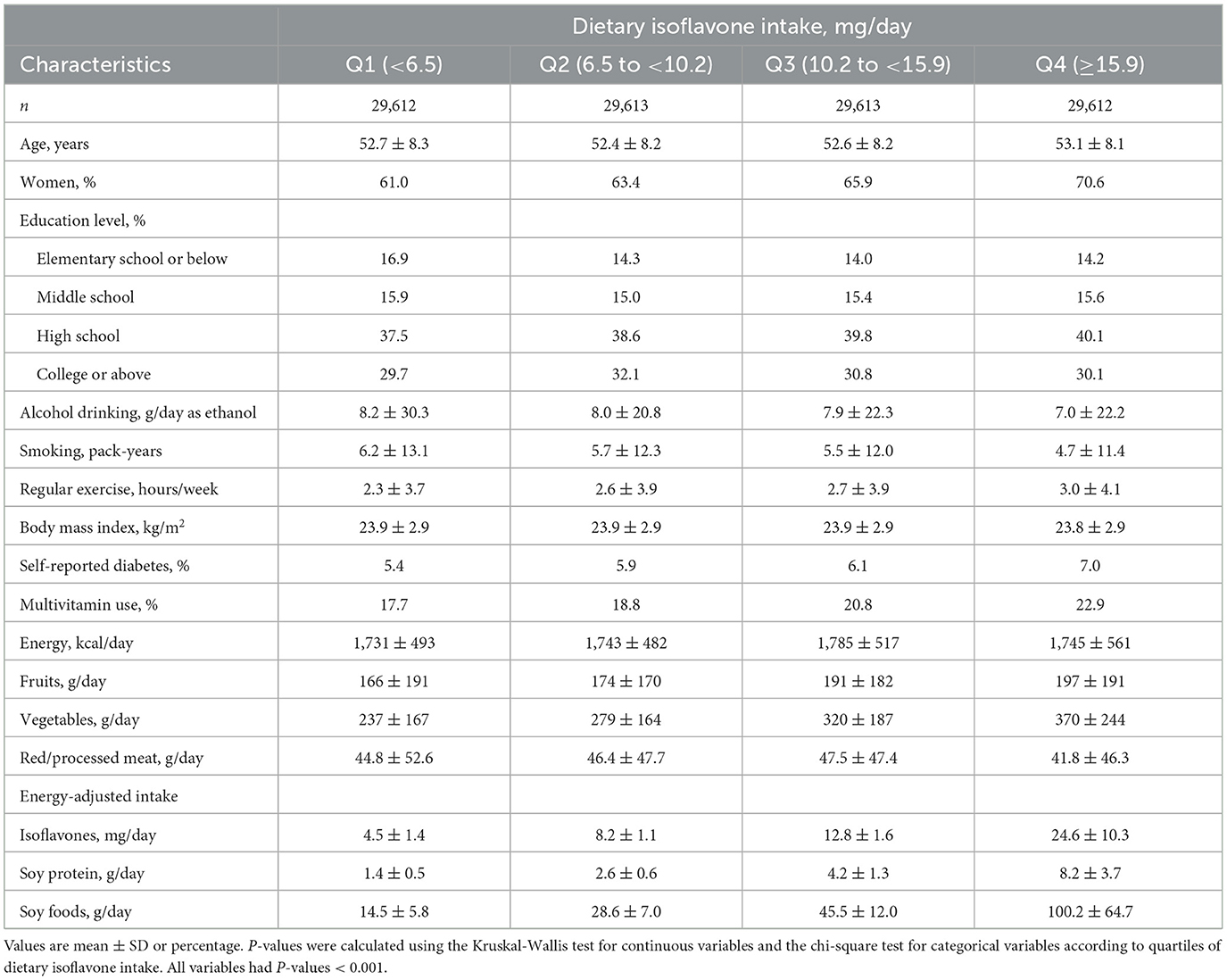
Table 1. Baseline characteristics of Korean adults according to quartiles of dietary isoflavone intake.
3.2 Dietary soy and isoflavone intake and mortality risk
During a median follow-up of 10.1 years (interquartile range: 8.7–11.4), 2,614 deaths were identified, including 1,290 attributed to cancer and 389 to CVD. Associations between dietary soy and isoflavone intake and mortality risk are presented in Table 2. In fully adjusted models, dietary isoflavone intake was not significantly associated with the risks of all-cause or cause-specific mortality. The HRs (95% CIs) for the highest quartile compared to the lowest quartile of isoflavone intake were 1.04 (0.93–1.15) for all-cause mortality, 0.98 (0.84–1.14) for cancer mortality, and 1.04 (0.79–1.38) for CVD mortality. Similarly, soy protein and soy food intake were not significantly associated with the risk of all-cause, cancer, or CVD mortality. When we conducted sex-specific analyses, no apparent association was observed in either sex (Table 3). The HRs (95% CIs) comparing extreme quartiles of isoflavones in men and women were 1.03 (0.90–1.19) and 1.03 (0.87–1.23) for all-cause mortality, 0.94 (0.76–1.16) and 1.06 (0.84–1.34) for cancer mortality, and 0.86 (0.60–1.24) and 1.21 (0.79–1.86) for CVD mortality.
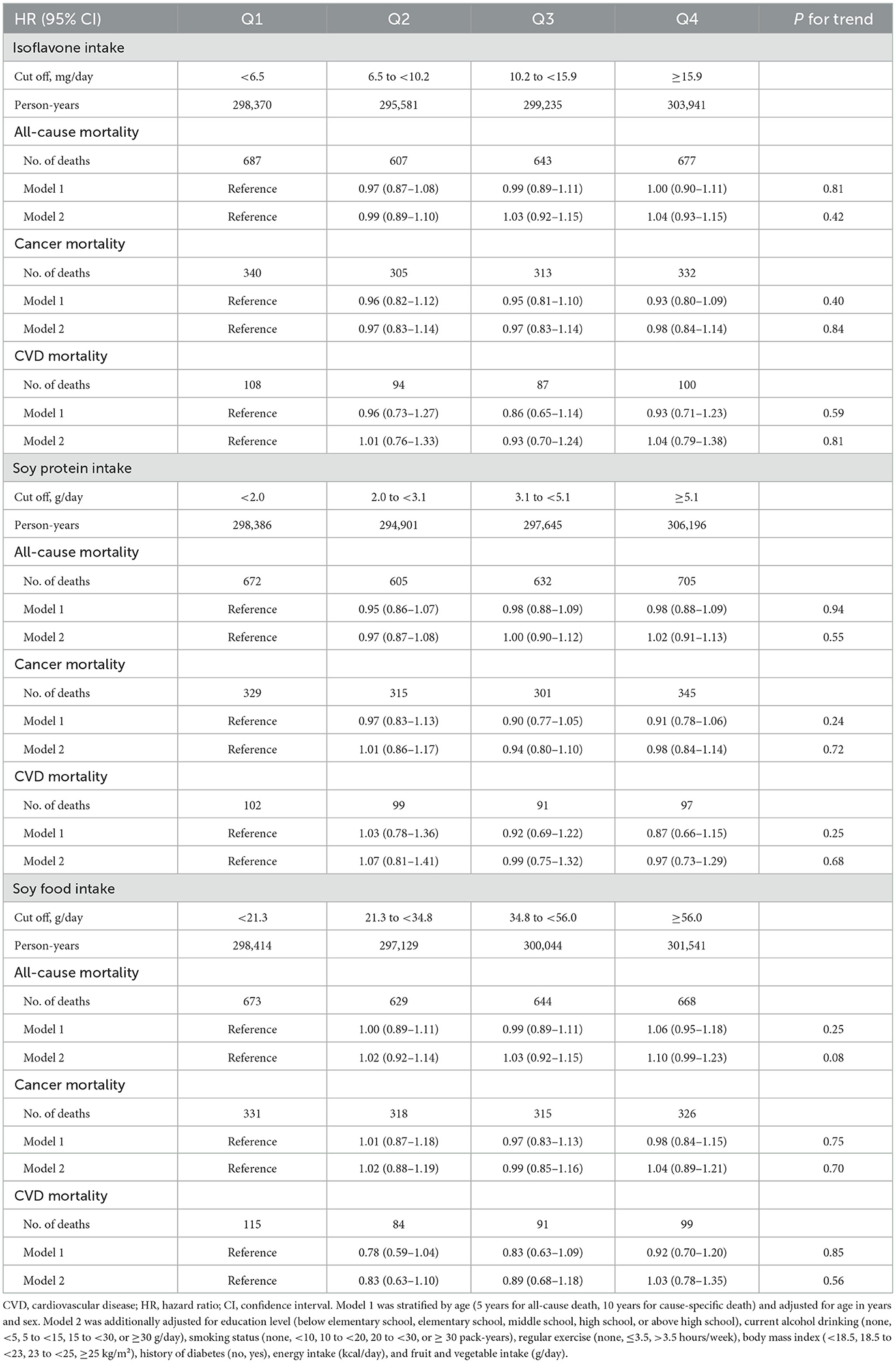
Table 2. Hazard ratios and 95% confidence intervals for all-cause, cancer, and CVD mortality according to quartiles of dietary intakes of isoflavones, soy protein, and soy foods.
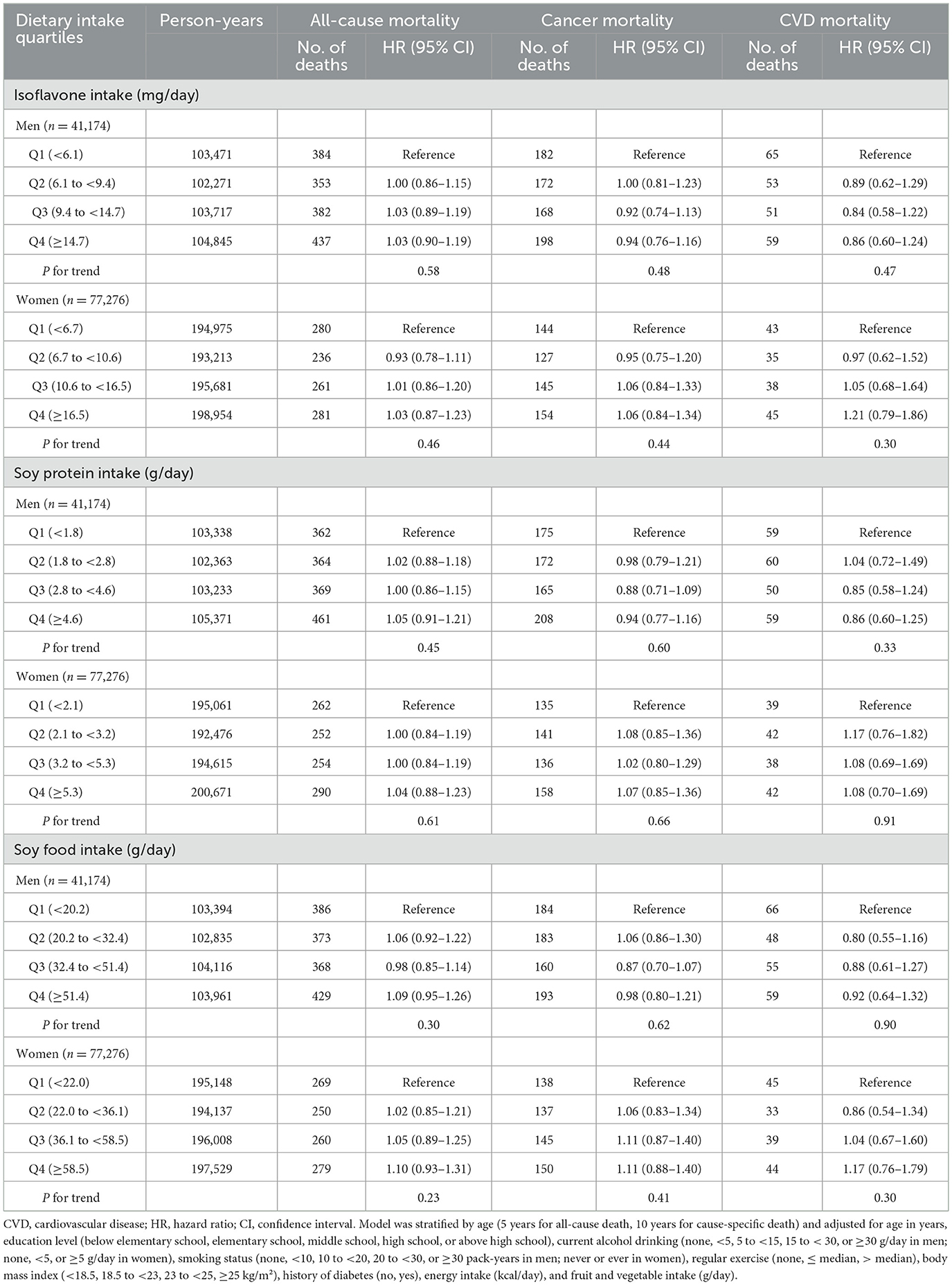
Table 3. Hazard ratios and 95% confidence intervals for all-cause, cancer, and CVD mortality according to sex-specific quartiles of dietary intakes of isoflavones, soy protein, and soy foods.
3.3 Subgroup analyses
When we examined the association between dietary isoflavone intake and all-cause mortality, stratified by demographic and lifestyle factors, no significant interaction was observed (Figure 1). Although the interaction did not reach significance, higher isoflavone intake was associated with a slightly increased risk of all-cause mortality among those with an education level of middle school or below: HR (95% CI) comparing extreme quartiles was 1.18 (1.01–1.39).
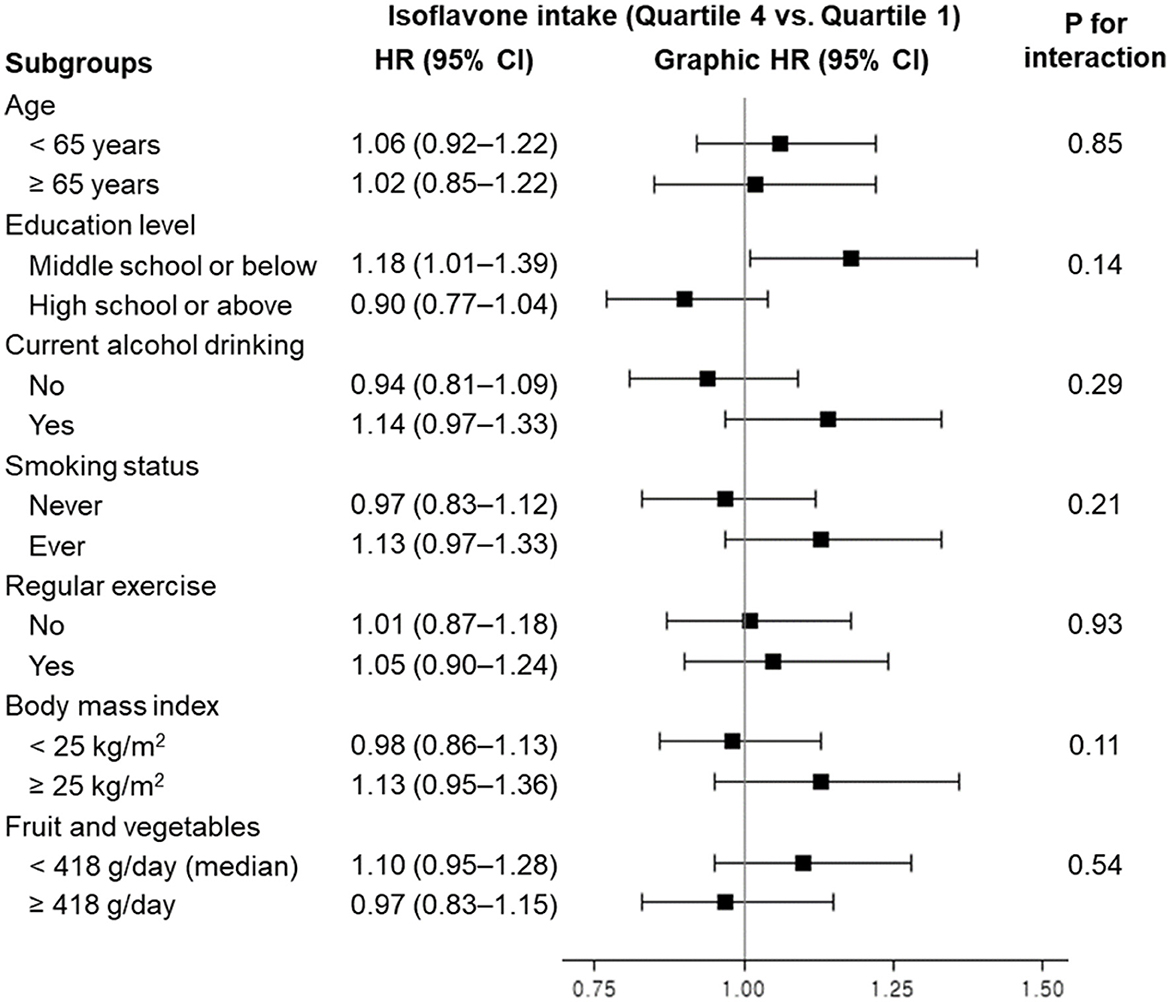
Figure 1. Hazard ratios and 95% confidence intervals for all-cause mortality according to quartiles of dietary isoflavone intake, stratified by demographic and lifestyle factors. HR, hazard ratio; CI, confidence interval. Model was stratified by age group and adjusted for age in years, sex, education level, current alcohol drinking, smoking status, regular exercise, body mass index, history of diabetes, energy intake, and fruit and vegetable intake (as applicable).
4 Discussion
In this prospective cohort study of Korean adults, dietary intakes of isoflavones, soy protein, and soy foods did not appear to reduce the risks of all-cause, cancer, and CVD mortality.
Regarding all-cause mortality, most studies have shown an inverse association with isoflavone and/or soy food intake (19–24), but a few have not, particularly in studies where soy intake was low (25–27). No significant association was observed between dietary isoflavone intake and all-cause mortality risk among US postmenopausal women (25) and Spanish adults (26). Among Italian adults, higher isoflavone intake was associated with an increased risk of all-cause mortality (27). The median isoflavone intake in these studies was <1 mg/day. In US studies reporting significant inverse associations despite low isoflavone intake (median <1 mg/day), dose-response analyses demonstrated a linear association in men and women within the analyzed range (up to ~6 mg/day, excluding the top 2.5% of intake values) (23), and a non-linear pattern in women, with risk reduction plateauing around 6 mg/day and no additional benefit observed at higher levels (24). In our study, the cutoff for the lowest quartile of isoflavone intake was 6.5 mg/day, indicating substantially higher intake levels compared to populations with low soy food consumption. If threshold effects exist at very low intake levels, the habitual intake of soy foods in our population may partly explain the null findings. Additionally, among studies reporting significant associations between soy intake and mortality risk, several studies found no additional benefit at higher intake levels. The Guangzhou Biobank Cohort Study found that Chinese adults who consumed 1 to 6 portions of soy foods per week had a lower risk of all-cause mortality compared with those who consumed none, but no significant association was observed for those who consumed 7 or more portions per week (8). Similarly, a study in Hong Kong found comparable reductions in all-cause mortality risk in the third and fourth quartiles of soy food intake relative to the lowest quartile (21). In the Japan Public Health Centre-based Prospective Study, the reduction in all-cause mortality risk in the highest quintile of fermented soy intake was similar to that in the moderate quintiles, compared to the lowest quintile (22). In contrast, another Japanese study found that men who consumed soy either rarely or almost daily had higher risks of all-cause mortality than those who consumed soy 1–2 times per week, whereas no such association was observed among women (28).
Overall, we found no significant association between soy and isoflavone intake and mortality risk. However, in subgroup analyses, higher isoflavone intake was associated with a slightly increased risk of all-cause mortality among individuals with lower education levels. Given the prevalent consumption of soy foods in the Korean population, higher isoflavone intake in this subgroup might reflect differences in overall dietary patterns or diet quality, and residual confounding cannot be ruled out. Furthermore, inter-individual variability in isoflavone metabolism, particularly the equol producer phenotype, may have influenced the observed associations. Equol, a metabolite of daidzein produced by gut microbiota in only some individuals, is more prevalent in Asian populations (~50%−60%) than in Western populations (29). Considering its higher affinity for estrogen receptor-β compared to daidzein, equol has been hypothesized to enhance the biological activity of soy isoflavones (5, 29). Although the role of metabolic variability remains uncertain, it may have contributed to the null findings and highlights the need for further investigation.
In our overall and sex-specific analyses, no significant associations were found between dietary soy and isoflavone intake and the risk of mortality from cancer or CVD. Some studies have reported that higher intakes of soy and/or isoflavones are associated with a decreased risk of cancer (19, 21) or CVD (22, 23) mortality. Conversely, a few studies have also reported positive associations between isoflavone and soy food intake and prostate cancer mortality (30), and between soy protein intake and CVD mortality among men (11). Consistent with our study, meta-analyses of prospective cohort studies have reported null associations between soy and isoflavone intake and cancer mortality (8, 10), as well as between isoflavone intake and CVD mortality (9). A meta-analysis found that higher soy food intake was associated with a reduced risk of CVD mortality compared to lower intake (8). Moreover, significant inverse associations have been reported between soy and/or isoflavone intake and the risk of cancer (10) and CHD (9). The biological properties of soy and isoflavones, including their potential to reduce oxidative stress, inflammation, and low-density lipoprotein cholesterol levels, may contribute to their protective effects in various diseases (3, 4, 31, 32). The lack of association between soy and isoflavone intake and mortality in observational studies, including ours, may be partially explained by changes in dietary habits following the diagnosis of these diseases. Additionally, in our study, the relatively small number of CVD deaths may have limited the statistical power in sex-specific analyses. Although the associations were not statistically significant, we observed a modest trend toward lower CVD mortality with higher intakes of soy and isoflavones among men. Further investigation with repeated assessments of soy and isoflavone intake, relevant lifestyle factors, and longer follow-up is warranted to clarify their potential role in mortality risk.
Our study has several strengths, including a large sample size, long-term follow-up, and the use of registry-based mortality data. However, there are several limitations to consider. First, dietary intake was assessed only at baseline, and participants' dietary habits may have changed during the follow-up period. Second, measurement errors in dietary assessment, including recall bias, are inevitable. These limitations may have led to non-differential misclassification, which tends to attenuate associations toward the null. Third, the observational nature of the study limits our ability to infer causality, and unmeasured or residual confounding cannot be ruled out. To reduce the potential for reverse causation, participants with cancer or CVD at baseline or early follow-up were excluded. Fourth, the number of events in certain subgroups may have limited the statistical power to detect modest associations. Lastly, because participants were recruited from selected metropolitan areas in Korea, the generalizability of our findings may be somewhat limited.
5 Conclusion
In this prospective cohort study of Korean adults, we observed no significant associations between dietary soy and isoflavone intake and mortality from all causes, cancer, and cardiovascular disease. The potential threshold effects suggested in previous studies may not have been detectable in our population, possibly due to the relatively high habitual intake of soy. These findings highlight the need for further research incorporating repeated assessments of diet and lifestyle factors, along with consideration of individual variability in isoflavone metabolism, to better understand the complex relationship between soy intake and long-term health outcomes.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: the data that support the findings of this study are available with the permission of the National Institute of Health in Korea. Requests to access these datasets should be directed to the Clinical & Omics Data Archive (CODA) [https://coda.nih.go.kr/frt/index.do].
Ethics statement
The studies involving humans were approved by the HEXA study was approved by the Institutional Review Boards (IRBs) of the KoGES group collaborators and the KDCA. This study received exempt approval from the IRB of Seoul National University (IRB No. E2012/001-002), and permission to use the data was granted by the National Institute of Health in Korea. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SS: Formal analysis, Methodology, Writing – original draft. SJ: Data curation, Resources, Writing – review & editing. HJ: Data curation, Resources, Writing – review & editing. JEL: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (Nos. 2021R1F1A1062476 and RS-2025-00560416).
Acknowledgments
Data in this study were from the Korean Genome and Epidemiology Study (KoGES; 6635-302), National Institute of Health, Korea Disease Control and Prevention Agency, and Cause of Death Statistics, Statistics Korea.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Messina MJ. Legumes and soybeans: overview of their nutritional profiles and health effects. Am J Clin Nutr. (1999) 70:439s−50s. doi: 10.1093/ajcn/70.3.439s
2. Oseni T, Patel R, Pyle J, Jordan VC. Selective estrogen receptor modulators and phytoestrogens. Planta Med. (2008) 74:1656–65. doi: 10.1055/s-0028-1088304
3. Setchell KDR. The history and basic science development of soy isoflavones. Menopause. (2017) 24:1338–50. doi: 10.1097/GME.0000000000001018
4. Jenkins DJ, Mirrahimi A, Srichaikul K, Berryman CE, Wang L, Carleton A, et al. Soy protein reduces serum cholesterol by both intrinsic and food displacement mechanisms. J Nutr. (2010) 140:2302S−11S. doi: 10.3945/jn.110.124958
5. Messina M, Duncan A, Messina V, Lynch H, Kiel J, Erdman JW Jr. The health effects of soy: a reference guide for health professionals. Front Nutr. (2022) 9:970364. doi: 10.3389/fnut.2022.970364
6. Messina M. Soy and health update: evaluation of the clinical and epidemiologic literature. Nutrients. (2016) 8:754. doi: 10.3390/nu8120754
7. Namazi N, Saneei P, Larijani B, Esmaillzadeh A. Soy product consumption and the risk of all-cause, cardiovascular and cancer mortality: a systematic review and meta-analysis of cohort studies. Food Funct. (2018) 9:2576–88. doi: 10.1039/C7FO01622K
8. Lu TY, Zhang WS, Jiang CQ, Jin YL, Au Yeung SL, Cheng KK, et al. Associations of soy product intake with all-cause, cardiovascular disease and cancer mortality: Guangzhou Biobank Cohort Study and updated meta-analyses. Eur J Nutr. (2024) 63:1731–45. doi: 10.1007/s00394-024-03363-5
9. Naghshi S, Tutunchi H, Yousefi M, Naeini F, Mobarak S, Asadi M, et al. Soy isoflavone intake and risk of cardiovascular disease in adults: a systematic review and dose-response meta-analysis of prospective cohort studies. Crit Rev Food Sci Nutr. (2024) 64:6087–101. doi: 10.1080/10408398.2022.2163372
10. Fan Y, Wang M, Li Z, Jiang H, Shi J, Shi X, et al. Intake of soy, soy isoflavones and soy protein and risk of cancer incidence and mortality. Front Nutr. (2022) 9:847421. doi: 10.3389/fnut.2022.847421
11. Talaei M, Koh W-P, van Dam RM, Yuan J-M, Pan A. Dietary soy intake is not associated with risk of cardiovascular disease mortality in Singapore Chinese adults. J Nutr. (2014) 144:921–8. doi: 10.3945/jn.114.190454
12. Nagata C, Wada K, Tamura T, Konishi K, Goto Y, Koda S, et al. Dietary soy and natto intake and cardiovascular disease mortality in Japanese adults: the Takayama study. Am J Clin Nutr. (2017) 105:426–31. doi: 10.3945/ajcn.116.137281
13. Kim Y, Han B-G, KoGES Group. Cohort profile: the Korean Genome and Epidemiology Study (KoGES) Consortium. Int J Epidemiol. (2017) 46:e20. doi: 10.1093/ije/dyv316
14. Jung EJ, Lee SH, Na JC, Soo KS. Introduction to data on death causes linked with the Korean Genome and Epidemiology Study (KoGES) epidemiological data. Public Health Wkly Rep. (2018) 11:159–62.
15. Ahn Y, Kwon E, Shim JE, Park MK, Joo Y, Kimm K, et al. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur J Clin Nutr. (2007) 61:1435–41. doi: 10.1038/sj.ejcn.1602657
16. The Korean Nutrition Society. Food composition table. Recommended dietary allowances for Koreans. 7th ed. Seoul: The Korean Nutrition Society (2000).
17. Jun S, Shin S, Joung H. Estimation of dietary flavonoid intake and major food sources of Korean adults. Br J Nutr. (2016) 115:480–9. doi: 10.1017/S0007114515004006
19. Hejazi J, Ghanavati M, Hejazi E, Poustchi H, Sepanlou SG, Khoshnia M, et al. Habitual dietary intake of flavonoids and all-cause and cause-specific mortality: Golestan cohort study. Nutr J. (2020) 19:1–25. doi: 10.1186/s12937-020-00627-8
20. Xue T, Wen J, Wan Q, Qin G, Yan L, Wang G, et al. Association of soy food with cardiovascular outcomes and all-cause mortality in a Chinese population: a nationwide prospective cohort study. Eur J Nutr. (2022) 61:1609–20. doi: 10.1007/s00394-021-02724-8
21. Yang J, Yang A, Yeung S, Woo J, Lo K. Joint associations of food groups with all-cause and cause-specific mortality in the Mr. OS and Ms. OS Study: a prospective cohort. Nutrients. (2022) 14:3915. doi: 10.3390/nu14193915
22. Katagiri R, Sawada N, Goto A, Yamaji T, Iwasaki M, Noda M, et al. Association of soy and fermented soy product intake with total and cause specific mortality: prospective cohort study. BMJ. (2020) 368:m34. doi: 10.1136/bmj.m34
23. Chen Z, Qian F, Hu Y, Voortman T, Li Y, Rimm E, et al. Dietary phytoestrogens and total and cause-specific mortality: results from 2 prospective cohort studies. Am J Clin Nutr. (2023) 117:130–40. doi: 10.1016/j.ajcnut.2022.10.019
24. Yan Y, Qiu M, Li J. Association of dietary isoflavones intake with all-cause mortality and heart disease mortality: a prospective cohort study. BMC Public Health. (2025) 25:1026. doi: 10.1186/s12889-025-21349-8
25. Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong C-P, Nettleton JA, et al. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr. (2007) 85:895–909. doi: 10.1093/ajcn/85.3.895
26. Zamora-Ros R, Jiménez C, Cleries R, Agudo A, Sánchez M-J, Sanchez-Cantalejo E, et al. Dietary flavonoid and lignan intake and mortality in a Spanish cohort. Epidemiology. (2013) 24:726–33. doi: 10.1097/EDE.0b013e31829d5902
27. Ponzo V, Goitre I, Fadda M, Gambino R, De Francesco A, Soldati L, et al. Dietary flavonoid intake and cardiovascular risk: a population-based cohort study. J Transl Med. (2015) 13:218. doi: 10.1186/s12967-015-0573-2
28. Yamasaki K, Kayaba K, Ishikawa S. Soy and soy products intake, all-cause mortality, and cause-specific mortality in Japan: the Jichi Medical School Cohort Study. Asia Pac J Public Health. (2015) 27:531–41. doi: 10.1177/1010539514539545
29. Setchell KD, Clerici C. Equol: history, chemistry, and formation. J Nutr. (2010) 140:1355s−62s. doi: 10.3945/jn.109.119776
30. Sawada N, Iwasaki M, Yamaji T, Shimazu T, Inoue M, Tsugane S, et al. Soy and isoflavone consumption and subsequent risk of prostate cancer mortality: the Japan Public Health Center-based Prospective Study. Int J Epidemiol. (2020) 49:1553–61. doi: 10.1093/ije/dyaa177
31. Sarkar FH, Li Y. Soy isoflavones and cancer prevention. Cancer Invest. (2003) 21:744–57. doi: 10.1081/CNV-120023773
Keywords: isoflavones, soy foods, mortality, cancer, cardiovascular disease
Citation: Song S, Jun S, Joung H and Lee JE (2025) Dietary soy and isoflavone intake and mortality in Korean adults: a prospective cohort study. Front. Nutr. 12:1613685. doi: 10.3389/fnut.2025.1613685
Received: 17 April 2025; Accepted: 13 June 2025;
Published: 09 July 2025.
Edited by:
Jonathan S. Tam, Children's Hospital of Los Angeles, United StatesReviewed by:
Antonella Smeriglio, University of Messina, ItalyMeleksen Akin, Iǧdır Üniversitesi, Türkiye
Xiaojian Yin, East China Normal University, China
Copyright © 2025 Song, Jun, Joung and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jung Eun Lee, anVuZ2VsZWVAc251LmFjLmty
 Sihan Song
Sihan Song Shinyoung Jun3
Shinyoung Jun3 Jung Eun Lee
Jung Eun Lee