- 1Department of Nephrology, The Second Hospital of Longyan, Longyan, Fujian, China
- 2Department of Neurosurgery, The Second Affiliated Hospital of Fujian Medical University, Quanzhou, Fujian, China
- 3Department of Pediatrics, The Second Hospital of Longyan, Longyan, Fujian, China
Background: Omega-3 fatty acids are known for their anti-inflammatory and antioxidant properties. However, the relationship between Omega-3 intake and the systemic inflammatory response index (SIRI) remains unclear. This study aimed to examine the potential association between Omega-3 fatty acid intake and SIRI.
Methods: A cross-sectional study was conducted using comprehensive data from the National Health and Nutrition Examination Survey (NHANES) for 2005–2018, assessing total Omega-3 fatty acid intake and SIRI among adults. SIRI was calculated using the formula monocyte × neutrophil count/lymphocyte count. The total dietary intake of Omega-3 fatty acids was calculated by summing the intakes of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). Subgroup analysis, smoothed curve fitting, and segmented linear regression were employed to investigate the relationship between SIRI and Omega-3 fatty acid consumption across genders.
Results: A total of 26,416 participants were included in the study. Participants were classified into quartiles of Omega-3 fatty acid intake: 0–0.014, 0.015–0.037, 0.037–0.093, and 0.093–5.215. The participants’ SIRI ranged from 1.242 ± 0.916, with levels decreasing as Omega-3 fatty acid intake quartiles increased (Q1: 1.27 ± 0.88; Q2: 1.27 ± 1.01; Q3: 1.25 ± 0.91; Q4: 1.18 ± 0.87, P for trend < 0.001). In the fully adjusted model, total Omega-3 fatty acid consumption was negatively correlated with SIRI (β: −0.05; 95% CI: −0.09, −0.01). Subgroup analysis and interaction tests indicated no significant correlation between this negative association and age, sex, BMI, hypertension, diabetes mellitus, or coronary heart disease (p > 0.05 for all interactions). A “J”-shaped curve was observed in male participants, with an inflection point at 2.7 g Omega-3 fatty acid intake. On the left side of the inflection point, a negative correlation was observed (β: −0.07; 95% CI: −0.14, −0.00), whereas a positive and statistically significant correlation was found on the right side (β: 0.43; 95% CI: 0.05, 0.80; Logarithmic likelihood ratio test P = 0.014.
Conclusion: A negative association may exist between SIRI and the consumption of omega-3 fatty. Further extensive studies are still needed to analyze their interaction.
Introduction
The inflammatory response is a protective mechanism that the body employs in response to pathogen invasion and external environmental damage, involving processes such as the proliferation of inflammatory cells, the release of inflammatory mediators, and mobilization of immune responses. However, while protecting the organism, the inflammatory response can also lead to damage to the host organ, particularly under certain conditions (1–3). The primary goal of medical treatment is to enhance the host’s defense mechanisms and facilitate recovery, while also promoting the elimination of pathogens and mitigating external damage during the inflammatory response. This can be achieved through measures such as the appropriate use of antibiotics with minimal side effects and enhancing the body’s immune functions to eliminate foreign substances. In many cases, it is necessary to suppress the inflammatory response to prevent excessive damage to the organism (4).
Docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), the two principal components of omega-3 polyunsaturated fatty acids, are naturally occurring anti-inflammatory agents predominantly found in fish and other human food sources. This study focuses on the relationship between omega-3 polyunsaturated fatty acids and the systemic inflammatory response. Most research on omega-3 fatty acids and inflammatory responses has focused on therapeutic studies related to various diseases. For instance, Al Biltagi et al. (5) found that omega-3 polyunsaturated fatty acids significantly improved lung function and controlled asthma, while Amer et al. (1), Tao et al. (3) reported that omega-3 fatty acid supplementation reduced all-cause mortality in cardiovascular diseases (6, 7). In the context of chronic kidney disease, Hu et al. (8) demonstrated the beneficial effects of omega-3 fatty acid supplementation. However, these studies do not explore the connection between omega-3 polyunsaturated fatty acids and the systemic inflammatory response at the organism level. The systemic inflammatory response index (SIRI), a novel index, reflects the overall level of systemic inflammation (9, 10). To date, no studies have reported the association between increased intake of omega-3 polyunsaturated fatty acids and decreased SIRI. However, the present study, using data from the NHANES database (2005–2018), demonstrates, for the first time, a strong association between these two variables.
Subjects and methods
Data and sample sources
This study utilizes data from the National Health and Nutrition Examination Surveys (NHANES), a research program administered by the National Center for Health Statistics (NCHS), a division of the Centers for Disease Control and Prevention (CDC). The objective of the program is to assess the nutritional and general health of the United States population through laboratory testing, physical examinations, and interviews. NHANES data is updated biennially. This study use stratified multistage random sample, ensuring representativeness. The publicly accessible website1 allows users to explore all the NHANES data used in this study.
This study utilized data from the NHANES surveys conducted between 2005 and 2018, encompassing seven survey cycles. Initially, 70,190 participants were enrolled in the study and screened to exclude individuals with dietary deficiencies in omega-3 fatty acids (n = 17,654), absent SIRI data (n = 6,022), and missing data on other covariates (n = 20,098). Ultimately, data from 26,416 participants were analyzed (Figure 1).
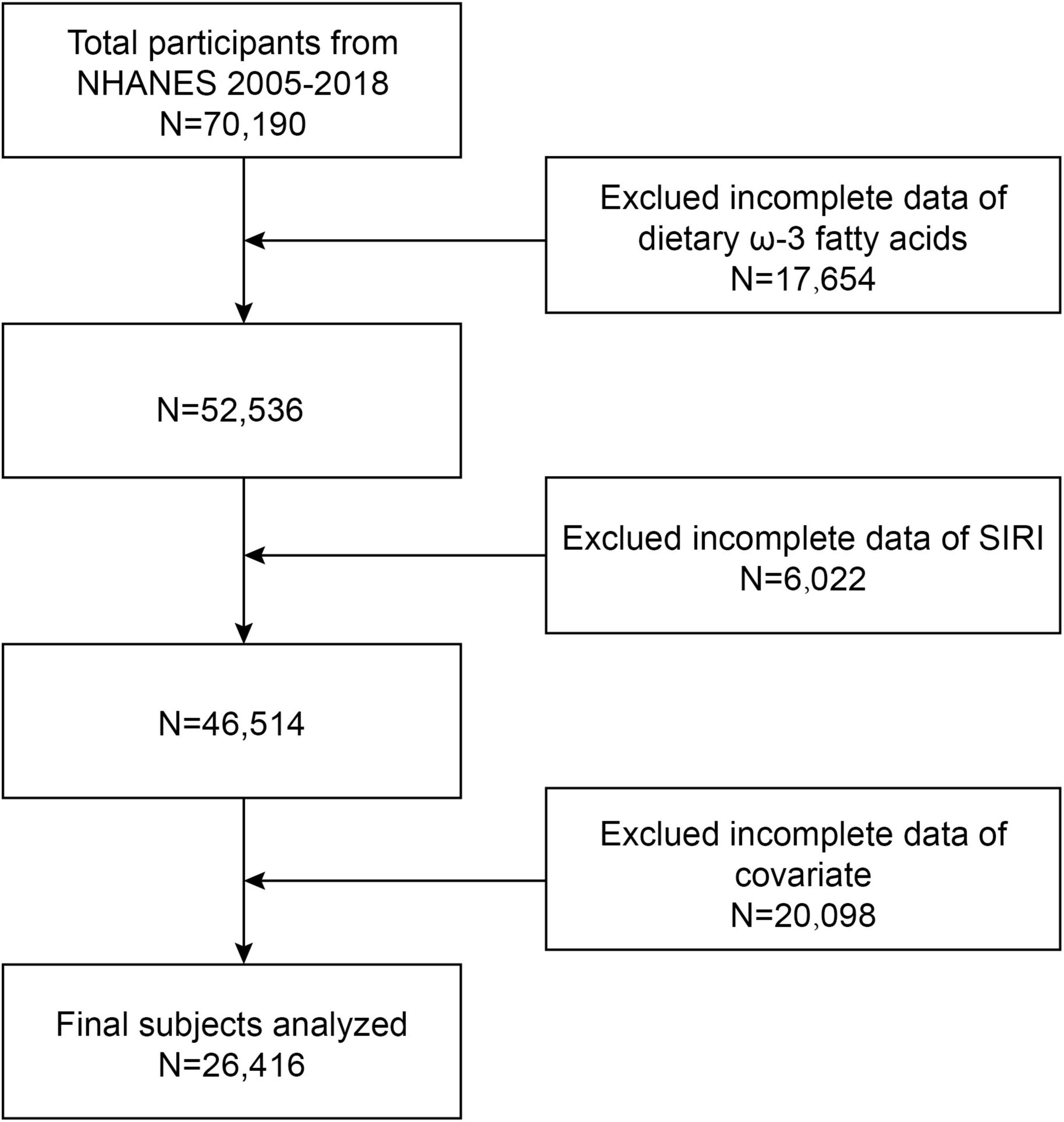
Figure 1. Flow chart. Exclued incomplete data of covariates included: hypertension (n = 13,209), diabetes (n = 14), coronary heart disease (n = 722), smoking (n = 3,036), education (n = 24), alcohol consumption (n = 20), marriage (n = 9), poverty ratio (n = 2,311), body mass index (BMI) (n = 259), high-density lipoprotein cholesterol (HDL-C) (n = 315), aspartate aminotransferase (AST) (n = 158), lanine aminotransferase (ALT) (n = 14), cholesterol (n = 4), and triglycerides (n = 3).
Exposure and outcome definitions
In this study, the total dietary intake of omega-3 fatty acids was calculated by summing the intake of EPA and DHA (11). Data on omega-3 component intake were collected using a food frequency questionnaire (FFQ) and a 24 h dietary recall method. The FFQ employed an automated multiple-pass method (AMPM) to ensure that the generated nutrient intake data did not deviate by more than 10% from actual intake (12). Participants recalled food and drink intake from the previous 24 h. Trained interviewers conducted the first dietary assessment, interviewing participants and collecting detailed dietary information for the preceding 24 h. A follow-up interview was scheduled by telephone 3–10 days later. The average of the two 24 h dietary recall surveys was used to calculate dietary omega-3 intake. The primary exposure variable in this study was omega-3 fatty acid intake.
Blood samples are given a complete blood cell count using the Beckman Coulter DxH 800 device in the NHANES Mobile Examination Center (MEC), which also distributes blood cells to each patient. According to a previous study, SIRI was calculated using the formula monocyte × neutrophil count/lymphocyte count (13). In this study, SIRI was used as an outcome variable.
Covariates
In accordance with previous studies, the following covariates were considered in our analysis: age, gender, race, education level, household income-to-poverty ratio, marital status, body mass index (BMI), aspartate aminotransferase (AST), alanine aminotransferase (ALT), high-density lipoprotein cholesterol (HDL-C), blood cholesterol levels, blood triglyceride levels, alcohol consumption, smoking status, hypertension, diabetes, and coronary heart disease. Alcohol consumption was categorized as none, moderate (1–2 drinks/day), or excessive (three or more drinks/day) (14). Smoking status was defined as non-smoking or having smoked at least 100 cigarettes in a lifetime. Hypertension and coronary heart disease were categorized as “yes/no,” while diabetes mellitus was classified as “yes/no/preclinical diabetes” based on the questionnaire.
Statistical analysis
The Student’s t-test was used for continuous data (reported as mean ± standard deviation), while the chi-square test was employed for categorical variables (reported as percentages). Multifactorial logistic regression analysis was utilized to investigate the relationship between SIRI and the dietary consumption of omega-3 fatty acids. Model 1 did not adjust for any covariates. Model 2 was partially adjusted for age, sex, and race. Model 3 was a fully adjusted model, accounting for age, sex, race, education level, marital status, household income-to-poverty ratio, body mass index (BMI), aspartate aminotransferase (AST), alanine aminotransferase (ALT), high-density lipoprotein cholesterol (HDL-C), cholesterol, triglycerides, alcohol consumption, smoking, hypertension, diabetes mellitus, and coronary heart disease. To further explore the relationship between omega-3 fatty acid intake and SIRI, smoothed curves were fitted, and two-stage linear regression was used to identify curve thresholds. Subgroup analyses based on gender, age, BMI, hypertension, coronary heart disease, and diabetes were also performed to assess the association between omega-3 fatty acid intake and SIRI in various subgroups. An interaction test was conducted to examine potential heterogeneity in the associations across subgroups. All analyses were performed using R software (version 4.1) and EmpowerStats (version 4.0).
Results
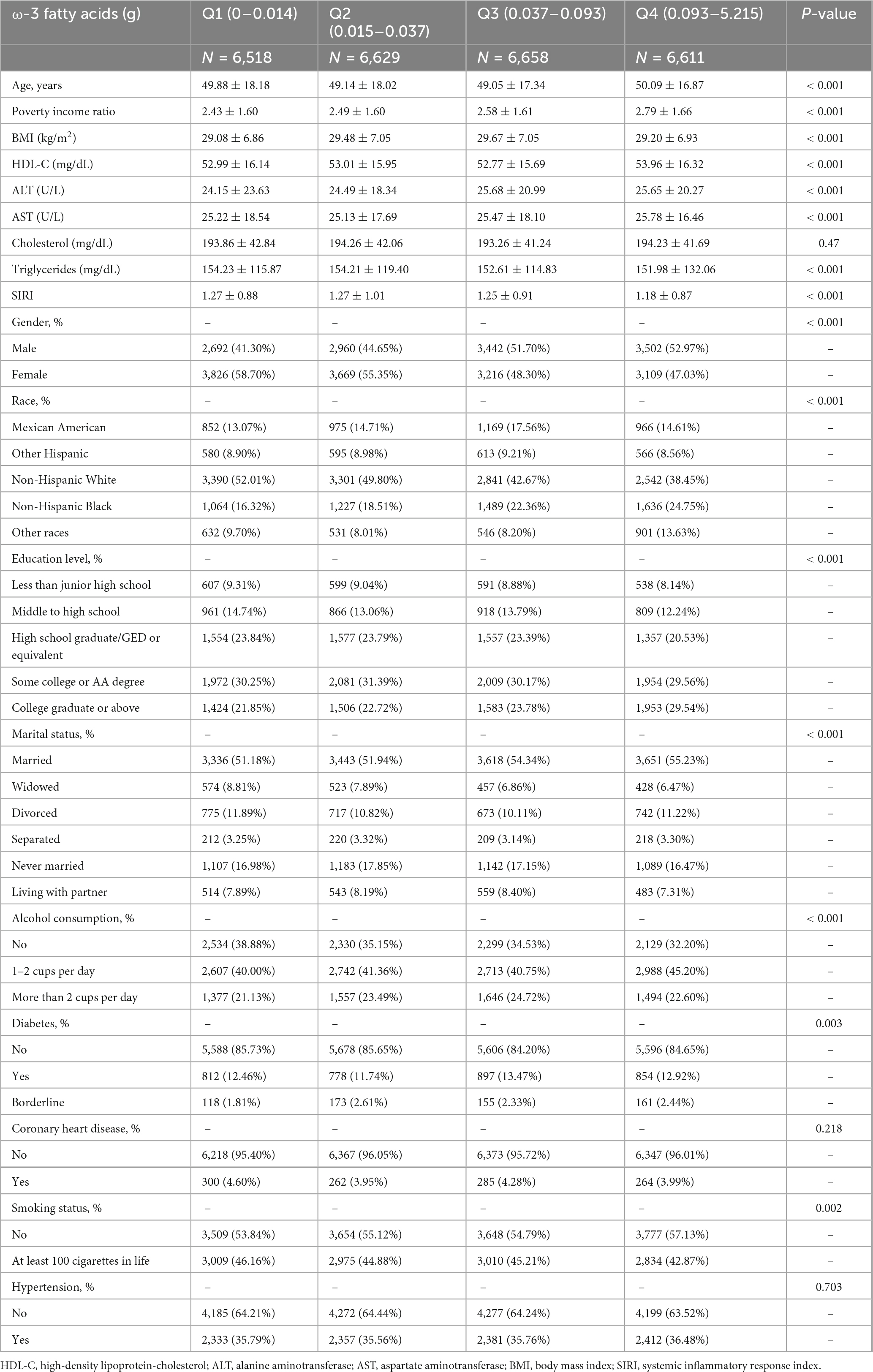
In this study, a total of 26,416 participants were included, with 47.68% males and 52.32% females. The participants had a mean age of 49.54 ± 17.61 years. The omega-3 fatty acid intake was divided into four interquartile ranges: 0–0.014, 0.015–0.037, 0.037–0.093, and 0.093–5.215. The participants’ SIRI ranged from 1.242 ± 0.916. Moreover, as dietary omega-3 fatty acid intake quartiles increased, SIRI levels progressively decreased (Q1: 1.27 ± 0.88; Q2: 1.27 ± 1.01; Q3: 1.25 ± 0.91; Q4: 1.18 ± 0.87, p < 0.001). Based on the Omega-3 fatty acid intake quartiles, the main characteristics of the population with higher Omega-3 fatty acid intake were older married men, moderate alcohol consumption, non-smokers, income and education level, higher HDL cholesterol, aspartate aminotransferase levels, and low triglyceride levels (Table 1).
Connection of Omega-3 fatty acid intake with SIRI
In the unadjusted model (Model 1), we found a negative association between overall omega-3 intake and SIRI (β: −0.10; 95% CI: −0.14, −0.05) (Table 2). Dietary Omega-3 intake remained negatively associated with SIRI when partially adjusted for potential confounders (Model 2: β: −0.08; 95% CI: −0.12, −0.04), and the association persisted in the fully adjusted model (Model 3: β: −0.05; 95% CI: −0.09, −0.01). In the fully adjusted model, SIRI levels decreased by 0.05 for each 1 g increase in omega-3 intake. To further validate the findings, dietary omega-3 consumption was converted from a continuous to a categorical variable. In the fully adjusted model, SIRI levels were significantly reduced by 0.041 (β: −0.041; 95% CI: −0.071, −0.010) in participants in the highest quartile (Q4) compared with those in the lowest quartile (Q1) of omega-3 intake, and this reduction was statistically significant (P for trend < 0.00058).
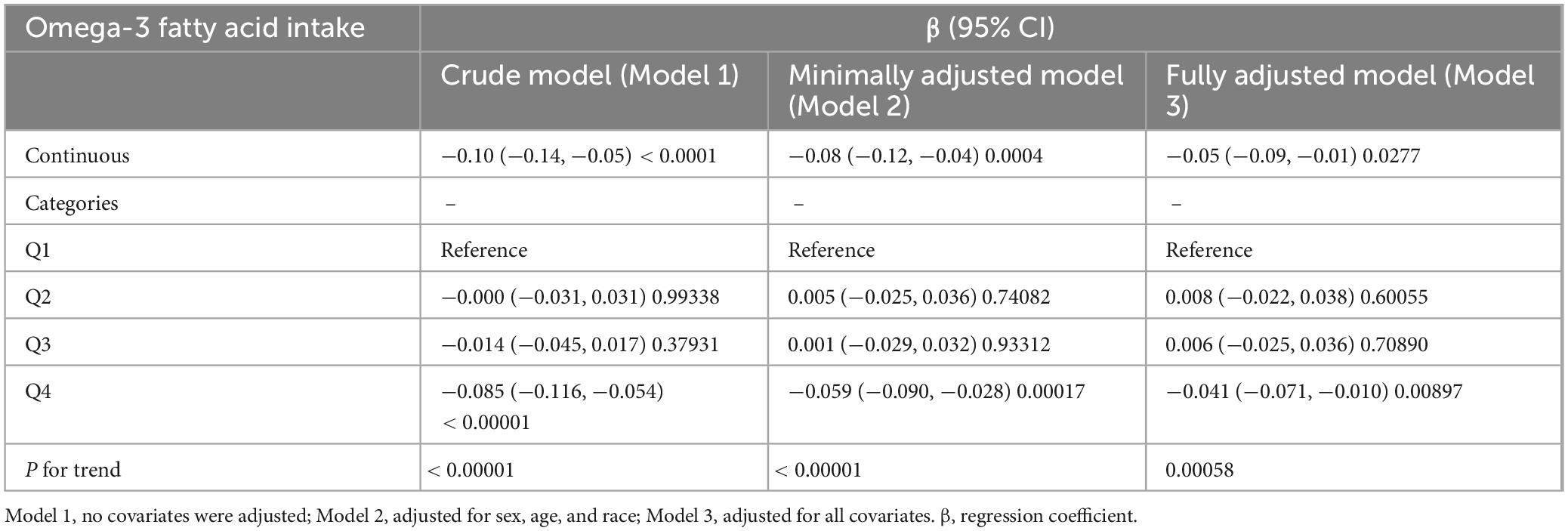
Table 2. Correlation between Omega-3 fatty acid intake and systemic inflammatory response index (SIRI).
Non-linear relationship between Omega-3 fatty acid intake and SIRI
To explore the potential non-linear relationship between dietary omega-3 fatty acid intake and SIRI, smoothed curve fitting was performed. The results in Figure 2A show a significant non-linear relationship between dietary omega-3 fatty acid intake and SIRI. Further smoothed curve fitting, stratified by gender (Figure 2B), revealed a “J”-shaped curve between dietary omega-3 fatty acid intake and SIRI in male participants, while a linear relationship was observed in female participants. Segmented linear regression was then applied to further validate this non-linear relationship. In the male population, the inflection point of the “J”-shaped curve occurred at 2.7 g. On the left side of the inflection point, a negative correlation between SIRI and omega-3 fatty acid intake was observed (β: −0.07; 95% CI: −0.14, −0.00), whereas a positive correlation was found on the right side (β: 0.43; 95% CI: 0.05, 0.80) (Logarithmic likelihood ratio test P = 0.014). In contrast, no non-linear relationship was observed in female participants (Logarithmic likelihood ratio test P = 0.365) (Table 3).
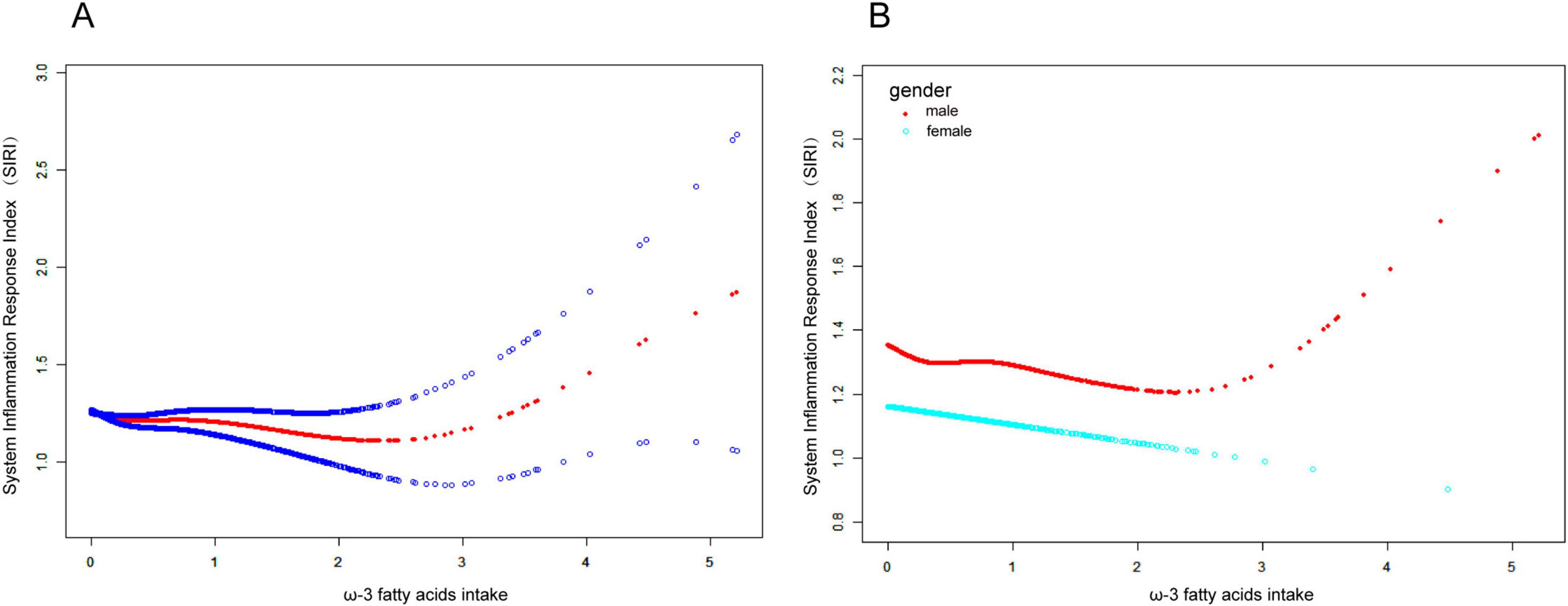
Figure 2. Connection between Omega-3 fatty acid intake and SIRI. (A) The smoothing of the fit is indicated by the red curve, and the 95% confidence interval is indicated by the blue curve. There is a non-linear correlation between Omega-3 fatty acid intake and SIRI. (B) Association between Omega-3 fatty acid intake and SIRI stratified by gender. Omega-3 fatty acid intake showed a “J”-shaped curve relationship with SIRI in male participants, but a linear positive relationship in female patients.
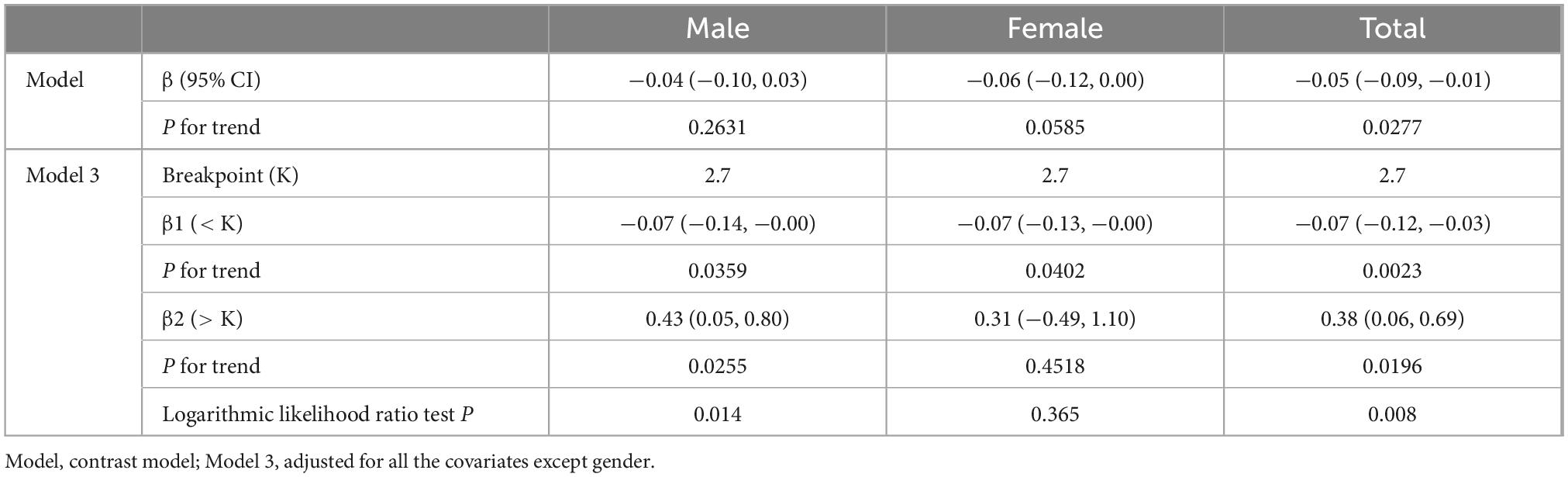
Table 3. Threshold effect analysis of Omega-3 fatty acid intake and systemic inflammatory response index (SIRI).
Subgroup analysis
The correlation between omega-3 fatty acid intake and SIRI levels varied across the subgroups analyzed (Figure 3). In the subgroups based on age, sex, BMI, coronary heart disease, hypertension, and diabetes mellitus, a stronger correlation between dietary omega-3 fatty acid intake and SIRI was observed in participants aged 40–59 years, females, those with obesity, individuals without coronary heart disease, those with preclinical diabetes, and participants with hypertension. To assess whether this relationship held across the entire population, an interaction test was conducted. The results indicated that the negative association between omega-3 fatty acid intake and SIRI levels was independent of subgroups based on age, sex, BMI, coronary heart disease, hypertension, and diabetes mellitus (all P for interaction > 0.05).
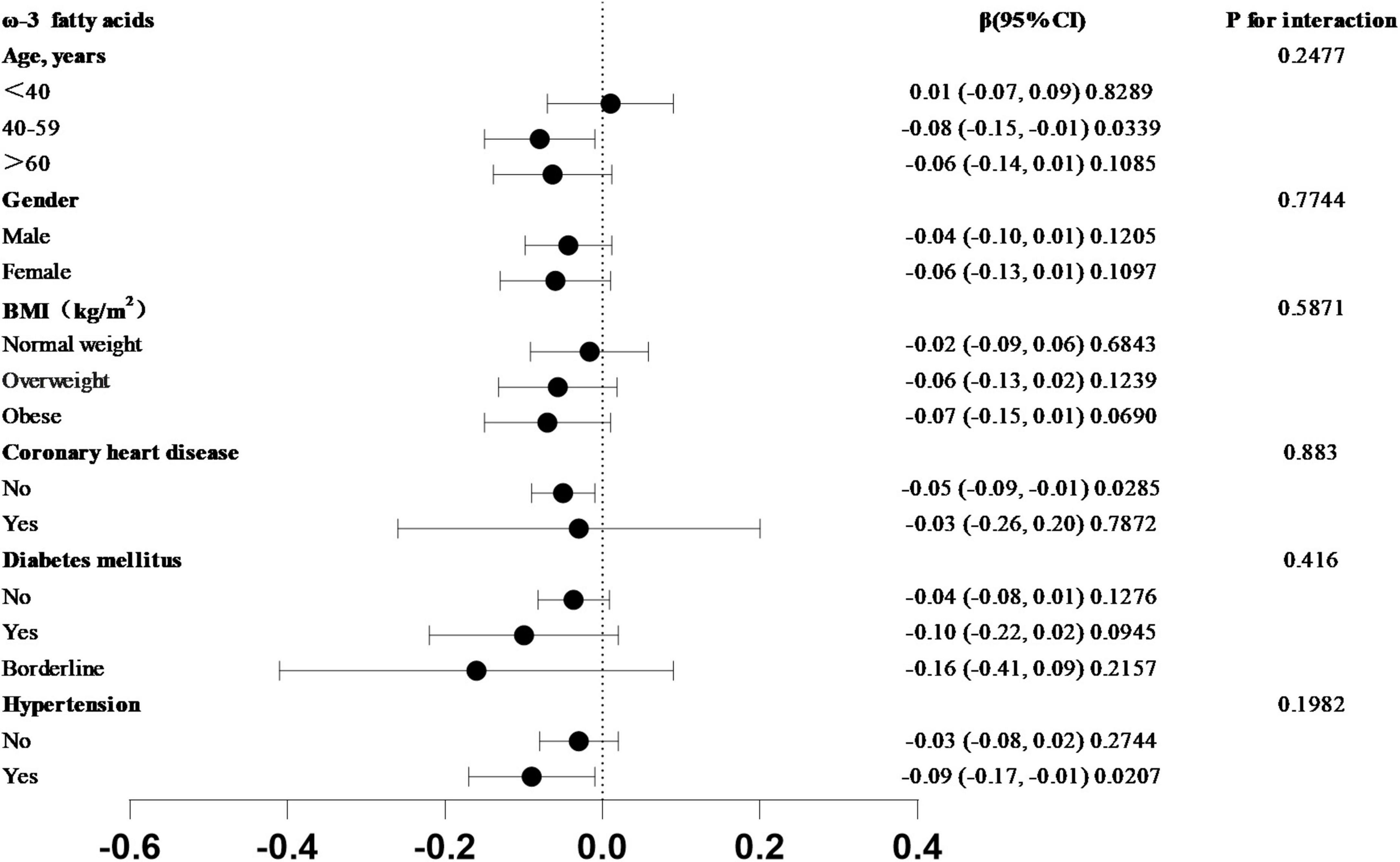
Figure 3. Subgroup analysis of the association between Omega-3 fatty acid intake and SIRI in forest plot. In the subgroups of age, sex, BMI, coronary heart disease, hypertension, and diabetes mellitus, which trend test p-value greater than 0.05.
Discussion
This study examined data from seven cycles of the NHANES survey (2005–2018) in a cross-sectional analysis. To date, this study is the first to report an association between increased dietary intake of omega-3 polyunsaturated fatty acids and lower SIRI levels. To control for confounding factors, age, gender, and race were included as covariates in Model 2, while Model 3 was fully adjusted, incorporating all relevant factors, and yielded similar results. Omega-3 polyunsaturated fatty acid intake was divided into quartiles, with Q1 as the reference group. A test for trend was performed, and the P-values for trend in all three models were less than 0.05. A strong negative correlation was observed between omega-3 polyunsaturated fatty acid intake and SIRI.
Systemic inflammatory response index is a systemic inflammatory response index that reflects the overall severity of inflammation in the body (15). Inflammation serves as a fundamental defense mechanism against infection and injury, involving the activation of immune cells and release of cytokines such as TNF-α and IL-6 (16). While normally self-limiting, inflammation can become chronic due to metabolic dysregulation, contributing to tissue damage in conditions such as atherosclerosis and obesity (17, 18). In these cases, patients often show immune cell infiltration in tissues and elevated systemic inflammatory mediators, though anti-inflammatory medications are not routinely used. Instead, dietary strategies, particularly those involving anti-inflammatory nutrients, are gaining attention. Omega-3 polyunsaturated fatty acids (PUFAs), notably DHA and EPA, have been widely studied for their role in modulating inflammatory pathways. Recent research suggests that higher omega-3 intake may help reduce systemic inflammation by limiting leukocyte activation, decreasing pro-inflammatory cytokine release, and stabilizing cell membranes (19). In diseases such as cardiovascular disorders, rheumatoid arthritis, and asthma, omega-3 PUFAs have shown beneficial adjunctive effects. Given these findings, dietary omega-3 intake may represent a feasible approach to mitigating chronic inflammation in the general population.
The total dietary intake of omega-3 fatty acids was calculated by summing the intake of DHA and EPA (20). Numerous recent studies have confirmed the anti-inflammatory effects of omega-3 polyunsaturated fatty acids. A study by Zhang et al. (21) supplemented the diet of patients with rheumatoid arthritis with omega-3 polyunsaturated fatty acids, significantly relieving symptoms. Zeyda et al. (22) demonstrated that omega-3 polyunsaturated fatty acids can inhibit inflammatory responses and exert anti-inflammatory effects in rats by regulating prostatic transmembrane protein-2 (STAMP2) in animal models. In a cross-sectional study of chronic kidney disease, the addition of omega-3 polyunsaturated fatty acid-rich fish oil to the diet was shown to complement medical therapy, alleviating symptoms and prolonging survival by reducing the degree of inflammatory response (23). Several recent studies on the anti-inflammatory mechanisms of omega-3 polyunsaturated fatty acids suggest the following possible mechanisms: First, omega-3 polyunsaturated fatty acids inhibit the proliferation of leukocytes and reduce their accumulation at the site of inflammation by altering the secretion of leukocyte adhesion factors in the vascular wall, thereby decreasing the local inflammatory response. Second, the chemotaxis of leukocytes reduces their migration from the lesion site. Leukocytes respond to the release of chemicals at the site of inflammation by migrating toward them through a mechanism called chemotaxis. These substances, including the eicosanoids LTB4 produced from arachidonic acid, are known as chemoattractants. Studies in healthy human subjects supplemented with fish oil rich in omega-3 polyunsaturated fatty acids have shown that reduced leukocyte chemotaxis to various chemoattractants leads to a decrease in the production of inflammatory factors such as IL-6, IL-1β, and TNF-α. Third, the anti-inflammatory effect of omega-3 polyunsaturated fatty acids is also attributed to their protection of cell membranes from damage at the site of inflammation. Omega-3 fatty acids can reduce the secretion of arachidonic acid through competitive binding, stabilizing the phospholipid structure of the cell membrane and protecting cells from inflammation (24). In conclusion, omega-3 polyunsaturated fatty acids are associated with lower SIRI levels, suggesting potential anti-inflammatory properties, and the incorporation of omega-3 fatty acid-rich foods into the diet can improve the level of inflammation in the organism’s internal environment, thereby protecting the organism.
Subgroup analyses were conducted across different populations to assess the stability of the model associating omega-3 polyunsaturated fatty acids with SIRI. The results revealed that the negative association between omega-3 polyunsaturated fatty acid intake and SIRI levels was independent of age, sex, BMI, coronary heart disease, hypertension, and diabetes mellitus subgroups (P for interaction > 0.05). No significant differences were observed in the intake of adequate fish-based diets across age and gender. In diabetic patients, increasing the intake of fish rich in omega-3 polyunsaturated fatty acids reduces complications and improves prognosis, particularly the incidence of diabetic nephropathy (23). Several recent studies on cardiovascular disease treatment have demonstrated that omega-3 polyunsaturated fatty acids improve prognosis and quality of life (25–27). According to dietary guidelines for individuals with obesity, foods high in omega-3 polyunsaturated fatty acids from fish should be consumed regularly over an extended period. These findings align with the subgroup analysis of the present study, where the negative correlation between omega-3 polyunsaturated fatty acid intake and SIRI levels remained stable across different populations.
Our study has several strengths. First, the large, nationally representative sample enhanced the statistical robustness and reliability of our results. Second, this study is the first to evaluate the dose-response relationship between dietary omega-3 fatty acid intake and SIRI. Third, we adjusted for several potential confounders in our exploration of the association between omega-3 fatty acid intake and SIRI. However, our study has several limitations. First, establishing causality is challenging due to the cross-sectional design of this study. Additionally, despite adjusting for several variables, confounding bias from unmeasured factors cannot be entirely ruled out. Third, nutritional data were collected through two 24 h dietary recall interviews; however, these interviews may not fully capture an individual’s average intake. Finally, the conclusions of this study apply to the United States adult population for which data on omega-3 fatty acid intake are available, and the results should not be generalized to individuals who lack relevant dietary data or to populations in other countries and regions.
Conclusion
In conclusion, the results of this study indicate a negative correlation between omega-3 polyunsaturated fatty acid intake and SIRI levels. This negative association remains significant even after adjusting for potential confounders, including age, sex, and BMI. Our findings support the hypothesis that omega-3 fatty acids are anti-inflammatory effects and may contribute to reducing systemic inflammation. Further longitudinal studies are needed to clarify the mechanisms linking omega-3 intake and systemic inflammation. Additionally, further research is necessary to explore the optimal dosage and long-term effects of omega-3 fatty acid supplementation in diverse populations.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of The Second Hospital of Longyan, Fujian. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from as the study utilized publicly available, de-identified data from the National Health and Nutrition Examination Survey (NHANES), written informed consent was waived due to the retrospective nature of the study. All participant data were anonymized to ensure confidentiality and to uphold ethical standards in research. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
XL: Data curation, Writing – original draft. LL: Formal Analysis, Writing – review and editing, Funding acquisition, Investigation. JL: Writing – review and editing, Software, Validation, Methodology. XX: Methodology, Project administration, Visualization, Investigation, Writing – review and editing, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Science and technology plan Project of Quanzhou City, Fujian Province, China (CQZ2023N001S0077).
Acknowledgments
We would like to express our sincere gratitude to the National Health and Nutrition Examination Survey (NHANES) program for providing the publicly available data used in this study. We also thank the participants of the NHANES for their contributions to the data, which made this research possible. Additionally, we would like to acknowledge the support of The Second Hospital of Longyan, Fujian, for their institutional resources and encouragement throughout this project. Finally, we would like to extend our appreciation to our colleagues and research staff for their valuable assistance in data analysis and manuscript revision.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Amer O, Dkhil M, Hikal W, Al-Quraishy S. Antioxidant and anti-inflammatory activities of pomegranate (punica granatum) on eimeria papillata -induced infection in mice. BioMed Res Int. (2015) 2015:1–7. doi: 10.1155/2015/219670
2. Jia Y, Wang G, Ye Y, Kang E, Chen H, Guo Z, et al. Niche cells crosstalk in neuroinflammation after traumatic brain injury. Int J Biol Sci. (2021) 17:368–78. doi: 10.7150/ijbs.52169
3. Tao F, Zhu J, Duan L, Wu J, Zhang J, Yao K, et al. Anti-inflammatory effects of doxepin hydrochloride against LPS-induced C6-glioma cell inflammatory reaction by PI3K-mediated akt signaling. J Biochem Mol Toxicol. (2020) 34:e22424. doi: 10.1002/jbt.22424
4. Divella R, Mazzocca A, Daniele A, Sabbà C, Paradiso A. Obesity, nonalcoholic fatty liver disease and adipocytokines network in promotion of cancer. Int J Biol Sci. (2019) 15:610–6. doi: 10.7150/ijbs.29599
5. Saadeh D, Salameh P, Baldi I, Raherison C. Diet and allergic diseases among population aged 0 to 18 years: Myth or reality? Nutrients. (2013) 5:3399–423. doi: 10.3390/nu5093399
6. Buonomo A, Scotto R, Zappulo E, Nerilli M, Pinchera B, Perruolo G, et al. Severe vitamin D deficiency increases mortality among patients with liver cirrhosis regardless of the presence of HCC. In Vivo. (2019) 33:177–82. doi: 10.21873/invivo.11456
7. Farías J, Carrasco-Pozo C, Carrasco Loza R, Sepúlveda N, Álvarez P, Quezada M, et al. Polyunsaturated fatty acid induces cardioprotection against ischemia-reperfusion through the inhibition of NF-kappaB and induction of Nrf2. Exp Biol Med. (2017) 242:1104–14. doi: 10.1177/1535370216649263
8. Bi J, Chen C, Sun P, Tan H, Feng F, Shen J. Neuroprotective effect of omega-3 fatty acids on spinal cord injury induced rats. Brain Behav. (2019) 9:e01339. doi: 10.1002/brb3.1339
9. Chen J, Zhai E, Yuan Y, Wu K, Xu J, Peng J, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. (2017) 23:6261. doi: 10.3748/wjg.v23.i34.6261
10. Huang H, Liu Q, Zhu L, Zhang Y, Lu X, Wu Y, et al. Prognostic value of preoperative systemic immune-inflammation index in patients with cervical cancer. Sci Rep. (2019) 9:3284. doi: 10.1038/s41598-019-39150-0
11. Zhao M, Xiao M, Tan Q, Ji J, Lu F. Association between dietary omega-3 intake and coronary heart disease among american adults: The NHANES, 1999–2018. PLoS One. (2023) 18:e0294861. doi: 10.1371/journal.pone.0294861
12. Thompson F, Dixit-Joshi S, Potischman N, Dodd K, Kirkpatrick S, Kushi L, et al. Comparison of interviewer-administered and automated self-administered 24-hour dietary recalls in 3 diverse integrated health systems. Am J Epidemiol. (2015) 181:970–8. doi: 10.1093/aje/kwu467
13. Xia Y, Xia C, Wu L, Li Z, Li H, Zhang J. Systemic immune inflammation index (SII), system inflammation response index (SIRI) and risk of all-cause mortality and cardiovascular mortality: A 20-year follow-up cohort study of 42,875 US adults. J Clin Med. (2023) 12:1128. doi: 10.3390/jcm12031128
14. Di X, Liu S, Xiang L, Jin X. Association between the systemic immune-inflammation index and kidney stone: A cross-sectional study of NHANES 2007-2018. Front Immunol. (2023) 14:1116224. doi: 10.3389/fimmu.2023.1116224
15. Fu H, Zheng J, Cai J, Zeng K, Yao J, Chen L, et al. Systemic immune-inflammation index (SII) is useful to predict survival outcomes in patients after liver transplantation for hepatocellular carcinoma within hangzhou criteria. Cell Physiol Biochem. (2018) 47:293–301. doi: 10.1159/000489807
16. Wen W, Chen J, Zhou Y, Li G, Zhang Y. Loss of Ripk3 attenuated neutrophil accumulation in a lipopolysaccharide-induced zebrafish inflammatory model. Cell Death Discov. (2022) 8:88. doi: 10.1038/s41420-022-00891-z
17. Li J, Shang C, Rong Y, Sun J, Cheng Y, He B, et al. Review on laser technology in intravascular imaging and treatment. Aging Dis. (2022) 13:246. doi: 10.14336/AD.2021.0711
18. Chen D, Zhang M, Shi Z, Li C, Wang Q, Song J, et al. Anti-inflammatory and anti-infectious dietary paradigms may be crucial for visceral weight reduction. Front Immunol. (2019) 10:422. doi: 10.3389/fimmu.2019.00422
19. Wang R, Feng Y, Chen J, Chen Y, Ma F. Association between polyunsaturated fatty acid intake and infertility among american women aged 20–44 years. Front Public Health. (2022) 10:938343. doi: 10.3389/fpubh.2022.938343
20. Rosendahl-Riise H, Sulo G, Karlsson T, Drevon C, Dierkes J, Tell G. Limited benefit of fish consumption on risk of hip fracture among men in the community-based hordaland health study. Nutrients. (2018) 10:873. doi: 10.3390/nu10070873
21. Zhang A, Downie L. Preliminary validation of a food frequency questionnaire to assess long-chain omega-3 fatty acid intake in eye care practice. Nutrients. (2019) 11:817. doi: 10.3390/nu11040817
22. Zeyda M, Säemann M, Stuhlmeier K, Mascher D, Nowotny P, Zlabinger G, et al. Polyunsaturated fatty acids block dendritic cell activation and function independently of NF-κB activation. J Biol Chem. (2005) 280:14293–301. doi: 10.1074/jbc.M410000200
23. Li W, Zhang N, Ge S, Xu G. Dietary omega-3 fatty acid intake and mortality in CKD population: A 1999–2014 NHANES analysis. Am J Nephrol. (2021) 52:909–18. doi: 10.1159/000520027
24. Oppedisano F, Macrì R, Gliozzi M, Musolino V, Carresi C, Maiuolo J, et al. The anti-inflammatory and antioxidant properties of n-3 PUFAs: Their role in cardiovascular protection. Biomedicines. (2020) 8:306. doi: 10.3390/biomedicines8090306
25. Ellulu M, Khaza’ai H, Patimah I, Rahmat A, Abed Y. Effect of long chain omega-3 polyunsaturated fatty acids on inflammation and metabolic markers in hypertensive and/or diabetic obese adults: A randomized controlled trial. Food Nutr Res. (2016) 60:29268. doi: 10.3402/fnr.v60.29268
26. Krantz M, Havranek E, Pereira R, Beaty B, Mehler P, Long C. Effects of omega-3 fatty acids on arterial stiffness in patients with hypertension: A randomized pilot study. J Negat Results BioMed. (2015) 14:21. doi: 10.1186/s12952-015-0040-x
Keywords: Omega-3 fatty acids, systemic inflammatory response index, NHANES, multifactorial logistic regression analysis, subgroup analysis
Citation: Liang X, Luo L, Lu J and Xie X (2025) Dietary Omega-3 fatty acids inversely associated with systemic inflammatory response index (SIRI): a population-based analysis of NHANES 2005–2018. Front. Nutr. 12:1614427. doi: 10.3389/fnut.2025.1614427
Received: 18 April 2025; Accepted: 09 June 2025;
Published: 25 June 2025.
Edited by:
Everson Araujo Nunes, McMaster University, CanadaReviewed by:
Yujia Zhang, Centers for Disease Control and Prevention (CDC), United StatesViviana Paz Sandoval Sandoval, San Sebastián University, Chile
Copyright © 2025 Liang, Luo, Lu and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoying Xie, MTM4NTk1NzkwODFAMTYzLmNvbQ==
†These authors share first authorship
 Xinglan Liang
Xinglan Liang Liangqin Luo2†
Liangqin Luo2†