- 1Department of Nutrition, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
- 2Student Research Committee, Mashhad University of Medical Sciences, Mashhad, Iran
- 3Metabolic Syndrome Research Center, Mashhad University of Medical Sciences, Mashhad, Iran
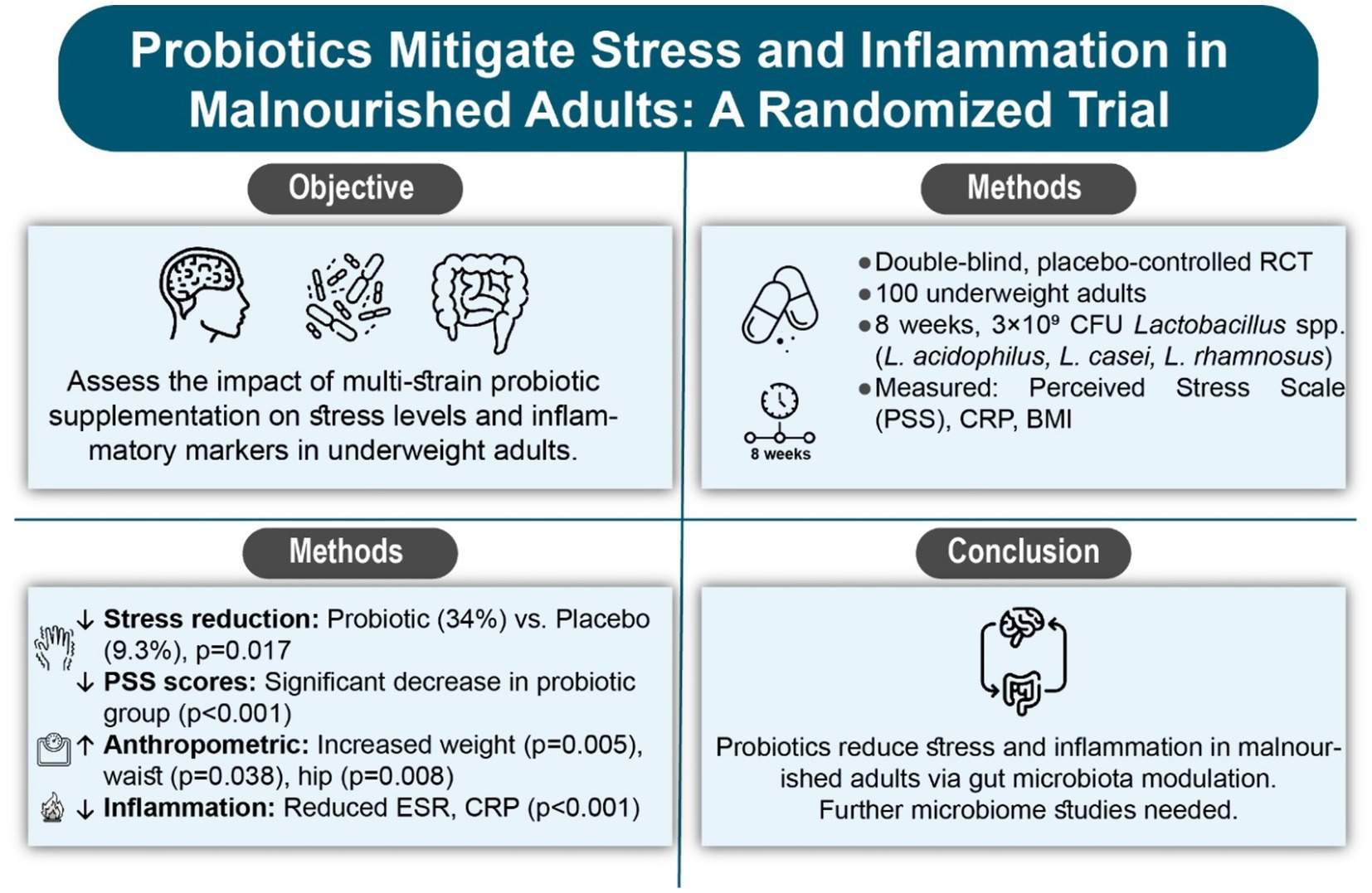
Objective: Malnutrition negatively affects mental health by altering neurotransmitter function and increasing stress responses. The gut-brain axis is pivotal in this process, and probiotics may mitigate stress. The current study examined the effects of multi-strain probiotic supplementation on stress levels in underweight individuals using the Perceived Stress Scale (PSS).
Methods: A double-blind, randomized, placebo-controlled trial involved 100 underweight participants were randomized to receive either a probiotic supplement (Lactobacillus acidophilus, L. casei, L. rhamnosus; 3 × 109 CFU) or placebo for 8 weeks. Stress levels, anthropometric measures, and inflammatory markers (ESR, CRP) evaluated at baseline and post-intervention.
Results: Ninety participants (mean age: 26.22 ± 7.42 years) completed the study (probiotic: n = 47; placebo: n = 43). Baseline age (p = 0.051) and gender (p = 0.101) showed no significant differences. Post-intervention, the probiotic group exhibited significant weight increases (p = 0.005), waist circumference (p = 0.038), and hip circumference (p = 0.008), and a significant reduction in Perceived Stress Scale (PSS) scores (p < 0.001) in comparison to the placebo. Inflammatory markers (ESR, CRP) also decreased significantly in the probiotic group (p < 0.001). Within-group analysis revealed improvements in anthropometric measures and inflammatory markers in both groups (p < 0.05), but stress reduction was more pronounced in the probiotic group (34% vs. 9.3%, p = 0.017). A significant time-group interaction was observed for stress scores (p < 0.001).
Discussion: The findings suggest that probiotic supplementation reduces stress levels in underweight individuals, possibly through gut microbiota modulation and inflammation reduction. Further research with larger samples and microbiome analysis is warranted.
Conclusion: In conclusion, administering probiotics to underweight patients positively impacts their mental health and exhibits anti-inflammatory effects.
Clinical trial registration: https://irct.behdasht.gov.ir/trial/69130, identifier IRCT20230310057667N1.
Introduction
Malnutrition, defined as nutrient deficiencies, excesses, or imbalances, adversely affects body composition, physiological function, and clinical outcomes (1). Undernutrition, specifically underweight conditions, is marked by body weight below healthy standards across age groups (2). In 2022, approximately 183 million women (95% CI: 169–197 million) and 164 million men (95% CI: 148–180 million) were underweight globally, down by 44.9 million women and 47.6 million men since 1990 (3).
Undernutrition disrupts neurotransmitter synthesis (e.g., serotonin, dopamine, GABA), impairing mood, sleep, and stress regulation, and increasing risks of depression and anxiety (4). Chronic malnutrition elevates cortisol, amplifies stress, and, through oxidative stress and inflammation, impairs cognitive functions like memory and attention. This creates a cycle where poor nutrition exacerbates psychological stress, further reducing appetite and worsening health (5, 6).
The gut-brain axis is critical in this interplay. Poor nutrition and gut dysbiosis, mediated by neural, metabolic, and immune pathways, contribute to stress and depression (7). Probiotics, or “psychobiotics,” restore microbiome balance, modulate hormones (e.g., cortisol, serotonin), and reduce pro-inflammatory cytokines (e.g., IFN-γ, TNF-α), alleviating stress and enhancing mental well-being (7).
Probiotics also increase short-chain fatty acid production, which reduces inflammation in conditions like autoimmune disorders and inflammatory bowel disease (8).
While the gut-brain axis provides a promising framework for understanding the interplay between nutrition and mental health, the application of probiotic interventions in underweight individuals is less understood due to limited baseline data on their gut microbiota. While the gut-brain axis and probiotic interventions have been extensively studied in healthy and obese populations, data on gut microbiota alterations in underweight individuals remain limited (9). Undernutrition is associated with reduced microbial diversity, lower SCFA production, and compromised gut barrier integrity, which may uniquely influence the efficacy of probiotics in this population (10). Evidence from healthy or obese cohorts may not fully apply to underweight individuals due to these distinct microbial and physiological profiles. Consequently, this study cautiously interprets the effects of multi-strain probiotic supplementation, recognizing the need for baseline microbiota data specific to underweight individuals to enhance result validity and generalizability.
Recent studies demonstrate mental health benefits: Lactobacillus casei Shirota reduced anxiety by 16% and stress by 20% in athletes over 6 weeks (11), while Lacticaseibacillus rhamnosus HN001 improved happiness and lowered stress in adults after 28 days (12). Synbiotics reduced stress and depression in adults with obesity over 8 weeks (13). Anti-inflammatory effects include lowered hs-CRP in type 2 diabetes and rheumatoid arthritis with Bacillus coagulans and Lactobacillus casei supplementation (14, 15).
However, the effects of specific multi-strain probiotics (Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus rhamnosus) on psychological stress and inflammation in underweight individuals remain unexplored. This study evaluates the impact of eight-week multi-strain probiotic supplementation on psychological stress, measured via the Perceived Stress Scale, and inflammatory biomarkers in underweight adults, advancing nutritional strategies for mental and physiological health.
Methods
Study design and participants
This double-blind, randomized, placebo-controlled trial was conducted at the Specialized Nutrition Clinic of Imam Reza Hospital, Mashhad, Iran, between October 1, 2024, and February 28, 2025. The trial was registered with the Iranian Registry of Clinical Trials (IRCT Identifier: IRCT20230310057667N1). Participants were recruited through advertisements and screened for eligibility. Participants were one hundred adults (18–65 years) with undernutrition, defined by BMI <18.5 kg/m2 and low FFMI (<17 kg/m2 for men, <15 kg/m2 for women), assessed to confirm reduced muscle mass. Exclusion criteria included a history of chronic diseases or gastrointestinal disorders, pregnancy, lactation, smoking, and use of antibiotics, probiotics, or foods containing probiotics within 3 months before the study. At baseline, participants completed a detailed questionnaire capturing demographic details (e.g., age, occupation, education), socio-economic status (e.g., household size, housing conditions), medical history, and current medication or supplement use. The sample size was determined based on prior research by Pan et al. (16), which investigated BMI changes following multi-species probiotic supplementation (Bifidobacterium longum, Lactobacillus bulgaricus, Streptococcus thermophilus, 1 × 109 CFU/day) in adults undergoing peritoneal dialysis, a population with nutritional challenges. With an alpha of 0.01, power of 90% (beta = 0.10), and an effect size of 0.4, a minimum of 45 participants per group was required. Allowing for a 10% dropout, 50 participants per group were enrolled, totaling 100 participants (Figure 1).
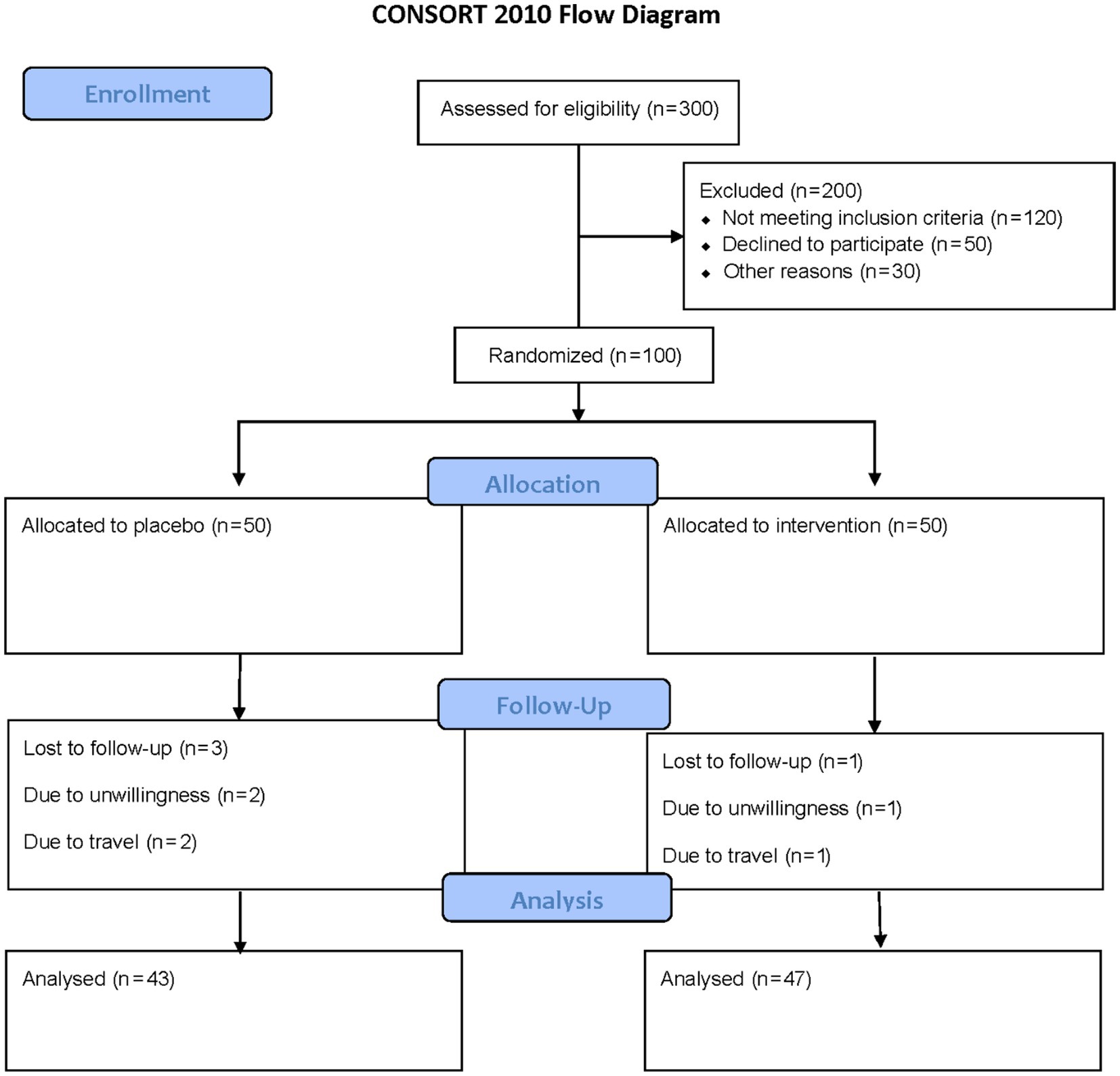
Figure 1. The flowchart of the study. Including patient screening, enrollment, randomization, and follow-up assessments at week 8 for outcome measurements.
Randomization and blinding
Participants were randomized into the probiotic or placebo group using permuted block randomization with a fixed block size of four, ensuring a 1:1 allocation ratio across 25 blocks for 100 participants (50 per group). The randomization sequence was generated by an independent statistician using a web-based platform.1 Research assistants enrolled participants after screening, and a study coordinator assigned interventions using numbered, opaque, sealed envelopes to ensure allocation concealment. Both participants and study personnel, including those administering interventions and analyzing data, remained blind to group assignments throughout the study.
Intervention
Probiotic and placebo capsules, supplied in identical coded containers, contained 3 × 109 CFU of Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus rhamnosus, and maltodextrin filler (probiotic) or maltodextrin alone (placebo), manufactured by ParsiLact Company. Participants were instructed to consume two capsules daily, one after lunch and one after dinner, for 8 weeks. Adherence was monitored via participant self-reported daily logs and capsule counts at week 8, with compliance defined as consuming ≥80% of prescribed capsules. Dietary intake was assessed at baseline and week 8 using 24-h dietary recalls to monitor potential changes. Weekly phone calls and text message reminders were used to reinforce adherence to the probiotic or placebo capsule regimen and to record any reported dietary changes.
Measurements
Perceived Stress Scale (PSS) questionnaire, blood sampling, and anthropometric indices were measured at the baseline and after 8 weeks.
Perceived stress scale
The PSS is one of the most commonly used tools for assessing the perception of stress (17, 18). The PSS-10 comprises ten items designed to evaluate the extent to which individuals perceive their life situations as stressful. Each item is scored on a scale from 0 to 4 (0 = never, 4 = very often). Scoring for the PSS-10 was conducted following the guidelines established by Cohen et al. (17) and Cohen (18). Total scores (0–40) categorize stress as none (0), low (0–13), moderate (14–26), or high (27–40).
Anthropometric indices
Height was measured to 0.5 cm using a stadiometer, weight to 0.1 kg with a digital scale (participants barefoot, lightly clothed), waist circumference at the midpoint between the lowest rib and iliac crest, and hip circumference at the widest point (19).
Blood sampling
Blood samples (8 mL) were collected at baseline and week 8 from a forearm vein by a trained technician at Navid Laboratory, Mashhad, Iran, between 8:00 and 9:30 AM. Venipuncture used 5-mL EDTA anticoagulant tubes. Samples were centrifuged at 3,000×g for 10 min at 4°C within 30 min of collection to separate serum and analyzed immediately. Complete blood count (CBC) was measured using Sysmex KX21, C-reactive protein (CRP) via Roche Cobas 6000 (immunoturbidimetric assay, detection limit 0.1 mg/L, intra-assay CV <5%), and erythrocyte sedimentation rate (ESR) via the Westergren method (ICSH standardized). Derived indices [neutrophil-to-lymphocyte (NLR), platelet-to-lymphocyte (PLR), monocyte-to-lymphocyte (MLR), and neutrophil-lymphocyte-platelet (NLPR) ratios] were calculated. Changes in these markers were analyzed as continuous outcomes using repeated measures ANOVA, with no predefined cut-off points applied.
Statistical analysis
Data were analyzed using SPSS v24 (IBM Corp, USA). Normality was tested with the Kolmogorov–Smirnov test. Normally distributed variables were reported as mean ± standard deviation (SD), compared within and between groups using paired and independent t-tests, respectively. Non-normal data were presented as median (IQR), analyzed with Wilcoxon and Mann–Whitney tests. Categorical variables were compared using chi-square tests. Repeated measures ANOVA was used to evaluate stress scores over time, with baseline variables (age, sex, socio-economic status, BMI) included as covariates to control for confounding effects. All tests were two-sided, with p < 0.05 considered significant.
Results
Ninety participants (mean age: 26.22 ± 7.42 years) completed the study (probiotic: n = 47; placebo: n = 43). The median age (interquartile range) was 25 (22–32) years for the probiotic group and 23 (20–27) years for the placebo group, with no significant difference (p = 0.051, Mann–Whitney test). Gender distribution was similar (male: female ratio was 2.92:1 in the intervention and 1.39:1 in the control group, p = 0.101, chi-square test).
Baseline anthropometric measures showed no significant differences (p > 0.05). Post-intervention, the probiotic group had significant increases in weight (p = 0.005), waist circumference (p = 0.038), and hip circumference (p = 0.008) compared to placebo. Within the probiotic group, significant improvements occurred in weight (p < 0.001), BMI (p < 0.001), waist circumference (p < 0.001), and hip circumference (p < 0.001). The placebo group showed improvements in weight (p = 0.003) and BMI (p = 0.001). Inflammatory markers differed significantly at baseline for ESR (1 and 2 h, p < 0.001 each). Post-intervention, ESR (2 h, p < 0.001) and CRP (p < 0.001) were significantly lower in the probiotic group. Within-group changes showed significant reductions in ESR (1 h: p < 0.001; 2 h: p = 0.002) and CRP (p = 0.036) in the probiotic group, and ESR (1 and 2 h: plinha <0.001 each) and CRP (p = 0.017) in the placebo group (Table 1).
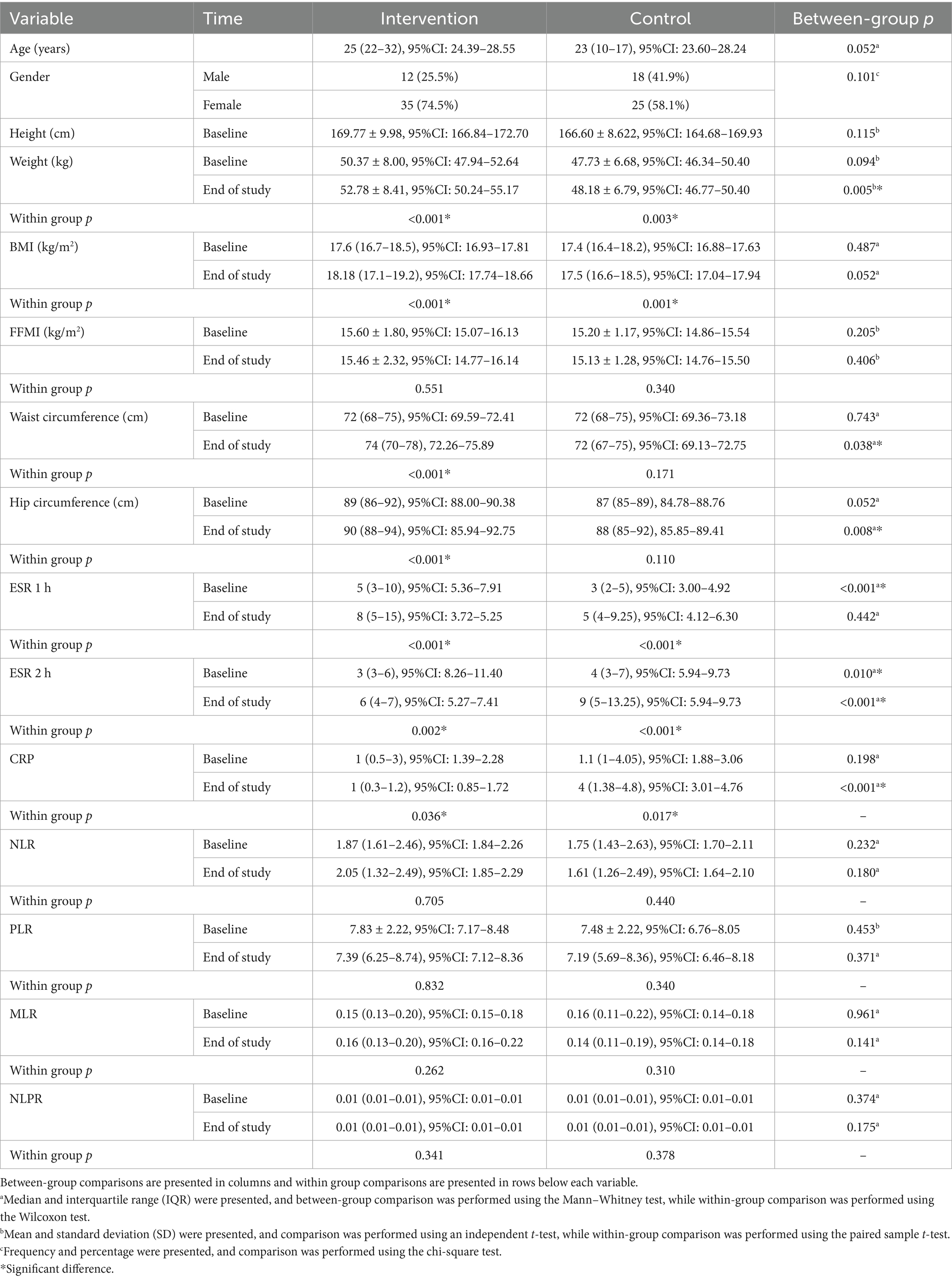
Table 1. Comparison of the demographic, anthropometric and laboratory variables between the intervention and control groups.
A comparison of anthropometric and laboratory variables changes between the intervention and control groups is presented in Table 1.
Changes in stress scores between the intervention and control groups are presented and compared in Table 2 and Figure 2. A significant time effect (p < 0.001) and time-group interaction (p < 0.001) were observed for stress scores in the probiotic group. At the end of the study, stress scores differed significantly between groups (p = 0.032). Within the probiotic group, stress scores decreased significantly from baseline to week eight (p < 0.001), whereas no significant change was observed in the placebo group (Table 3).
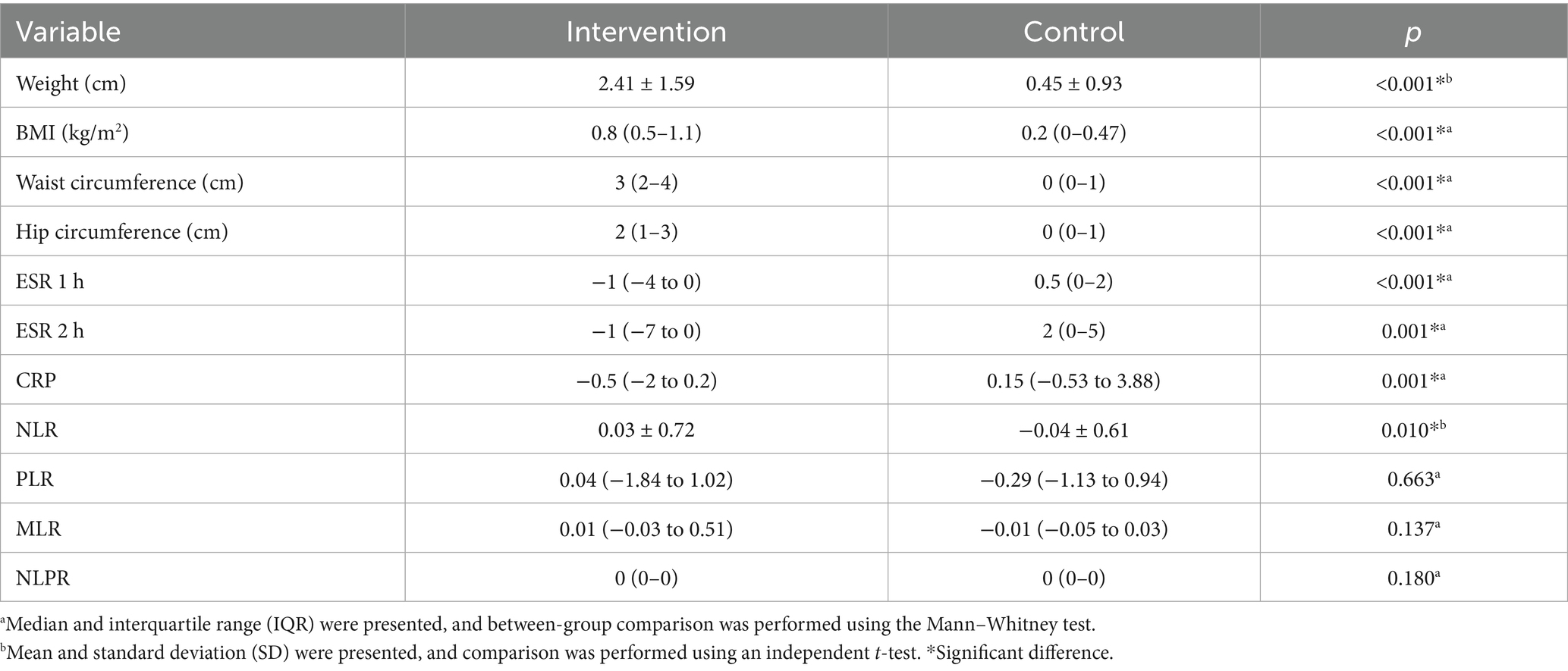
Table 2. Comparison of the changes in the anthropometric and laboratory variables between the intervention and control groups.
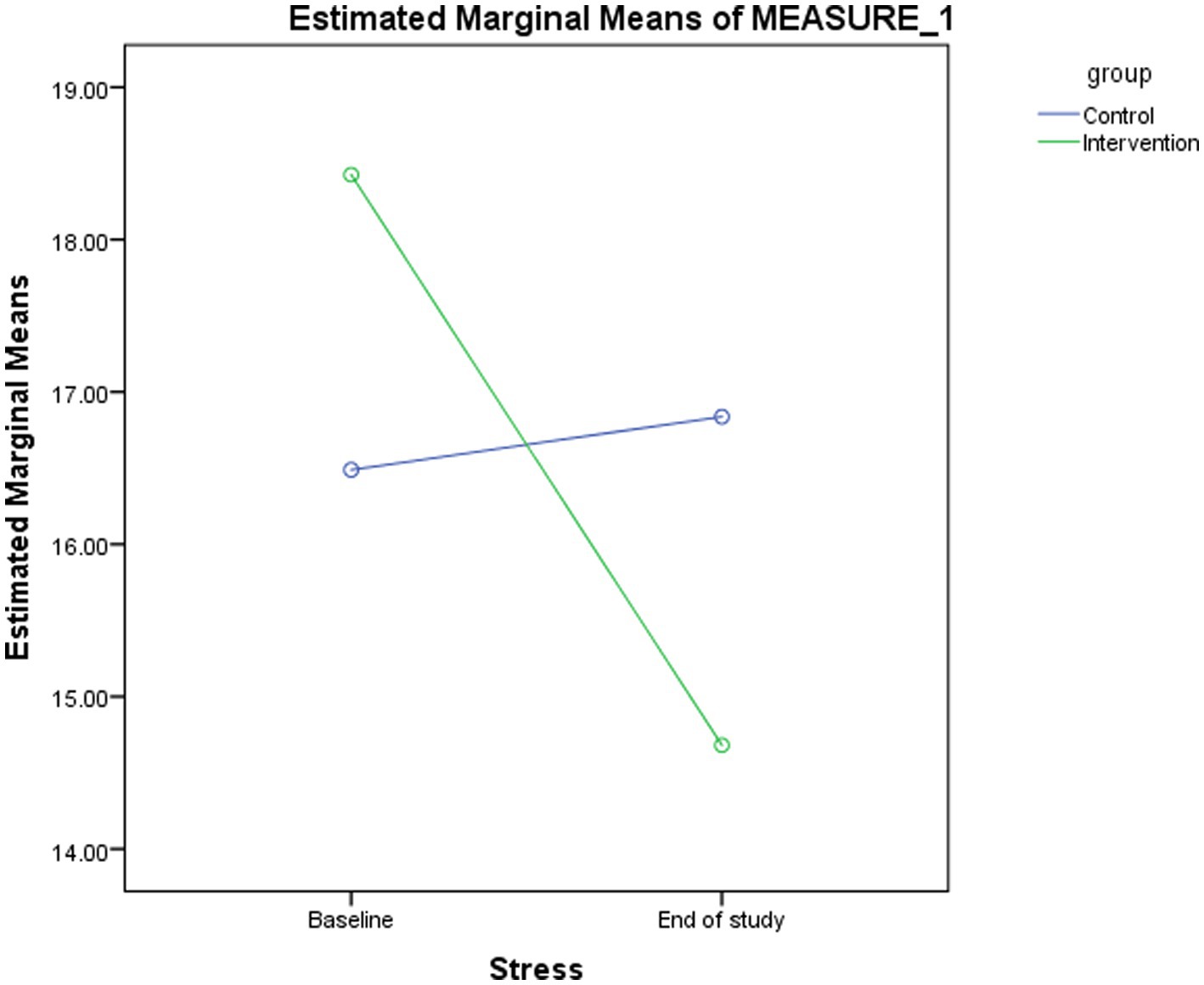
Figure 2. Changes in the estimated marginal means for stress score between baseline and the end of study in the intervention and control groups.
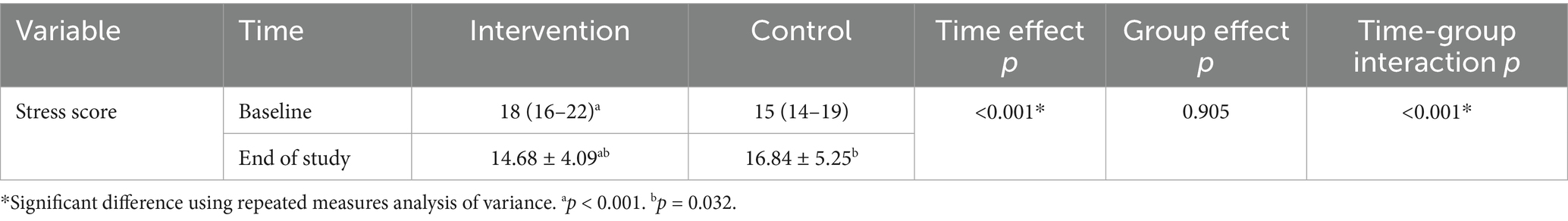
Table 3. Comparison of stress score between the intervention and control groups at baseline and the end of the study.
Comparison of the stress levels between the intervention and control groups at baseline and the end of the study, and changes in stress category over time between groups are presented in Table 4. There was no significant difference in the distribution pattern of stress levels between groups at baseline (p = 0.801) or at the end of the study (p = 0.108). Of the participants in the control group, the stress level was reduced in four (9.3%), did not change in 37 (86%), and increased in two (4.7%) over the study duration. In contrast, in the intervention group, the stress level was reduced in 16 (34%), did not change in 30 (63.8%), and increased in one (2.2%) participant. A significant difference in stress level changes was observed between groups (p = 0.017, chi-square test).
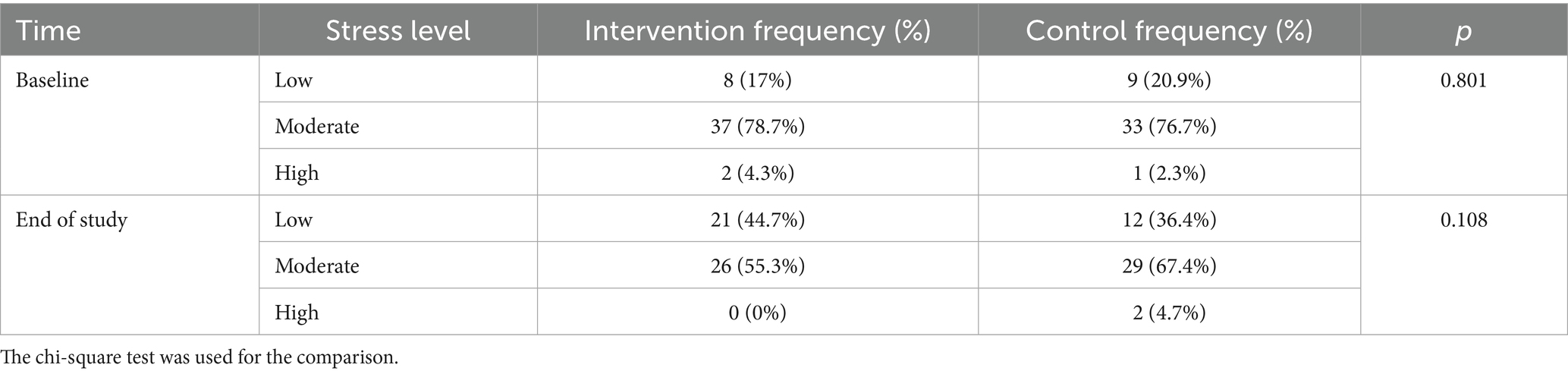
Table 4. Comparison of the stress levels between the intervention and control groups at baseline and the end of the study and changes in stress category over time between groups.
Discussion
This study is the first to investigate the effects of multi-strain probiotic supplementation (Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus rhamnosus) on psychological stress and inflammatory markers in underweight adults. Our findings demonstrate significant improvements in PSS scores and reductions in CRP and ESR levels in the intervention group, highlighting probiotics’ potential in addressing stress and inflammation in this population.
The gut-brain axis, a dynamic network of neural, immune, hormonal, and metabolic pathways, significantly influences mental health (20). Gut microbes regulate brain function by controlling inflammatory markers like interleukin-1, which can trigger cortisol release through the hypothalamic–pituitary–adrenal axis (21). Additionally, short-chain fatty acids (SCFAs) produced by gut microbiota contribute to mental health by modulating the immune system and neurotransmitter production. Probiotics enhance gut microbiome composition, reinforcing the intestinal barrier and producing antimicrobial substances that support mental well-being (22). To further elucidate the mechanisms underlying these effects, the potential direct and indirect actions of the probiotic strains used in this study warrant exploration. The observed improvements in PSS scores and reductions in CRP and ESR levels may result from both direct and indirect effects of the multi-strain probiotic supplementation. Directly, Lactobacillus strains may produce bioactive metabolites, such as short-chain fatty acids (SCFAs), which modulate immune responses and neurotransmitter synthesis, enhancing mental well-being (20–22). These strains may also directly interact with the hypothalamic–pituitary–adrenal (HPA) axis, potentially reducing cortisol levels by downregulating pro-inflammatory cytokines (e.g., IL-1, TNF-α) that stimulate cortisol release (21). Indirectly, probiotics may alter gut microbiota composition, strengthening the intestinal barrier and reducing systemic inflammation, although some studies suggest probiotic supplementation does not always significantly change microbiota composition (23). The absence of microbiome analysis in this study limits our ability to confirm these mechanisms. Future research should include microbial profiling to elucidate whether the observed effects are primarily driven by direct probiotic actions or microbiota-mediated changes.
SCFAs, such as acetate, propionate, and butyrate, produced by Lactobacillus strains, likely contribute to the observed reductions in PSS scores and inflammatory markers by modulating the gut-brain-immune axis. SCFAs enhance GABAergic activity by upregulating GABA receptor expression in the brain, potentially reducing stress and improving emotional regulation (24). Additionally, SCFAs inhibit pro-inflammatory cytokines (e.g., IL-6, TNF-α) by suppressing NF-κB signaling, which may explain the reductions in CRP and ESR levels (25). Although IL-6 and TNF-α were not measured in this study, their involvement in SCFA-mediated immune modulation suggests a mechanistic pathway for future investigation. These effects underscore the role of SCFAs in linking gut microbiota to brain function and systemic inflammation.
Research into natural alternatives for cognitive and mental health improvement has expanded, leading to the concept of ‘psychobiotics,’ probiotics that confer mental health benefits (26). Probiotics influence the gut-brain axis and provide a natural approach to managing stress and enhancing mental health outcomes (27). Several clinical trials have examined probiotics’ effects on psychological health, with varying results depending on the population and probiotic strain used (12).
Studies on Lactobacillus rhamnosus have reported both positive and neutral results. For example, supplementation with Lactobacillus rhamnosus improved depressive symptoms and quality of life in post-myocardial infarction patients (28), and reduced postnatal depression and anxiety in pregnant women (29). However, no significant benefits were observed in university students or healthcare workers during the COVID-19 pandemic (30, 31). These discrepancies suggest that probiotic efficacy may depend on population characteristics and external factors. Lactobacillus casei supplementation has been shown to improve sleep quality and reduce stress-related symptoms in medical students during exams and athletes under competitive pressure (11, 32). Furthermore, synbiotic supplementation combining Lactobacillus acidophilus with other strains has demonstrated reductions in stress, anxiety, and depression in individuals with various conditions (13, 33, 34).
Regarding inflammatory markers, ESR and CRP levels decreased in the intervention group, but the between-group differences were not statistically significant, possibly due to the small sample size or low baseline inflammation levels. The use of more sensitive biomarkers, such as IL-6 or TNF-α, may better capture subtle inflammatory changes in future studies (35). This aligns with previous studies that reported reductions in hs-CRP levels following probiotic supplementation in patients with rheumatoid arthritis, coronary artery disease, and type-2 diabetes (14, 15, 36). The reason for the no significant difference observed in NLR, PLR, MLR and NLPR was hypothesized to be due to the changes in these parameters being within the normal range. Limitations include the absence of microbiome analysis to clarify mechanisms and a modest sample size that limits generalizability. Future research should involve larger samples and microbial profiling to optimize probiotics interventions. This study did not evaluate gut permeability, absorption efficacy, psychological effects and other possible factors that might affect the outcomes. Therefore, it I suggested that further studies evaluate these factors and the mechanism of the observed effects.
Conclusion
In conclusion, probiotic supplementation in underweight patients benefits mental health and reduces inflammation in underweight adults, offering a complementary approach to stress management. Further studies are needed to validate these findings and explore probiotics as a primary stress intervention.
Data availability statement
Data described in the manuscript, will be made available upon written request to the corresponding author and approval by the Vice Chancellor of Research and technology of the Mashhad University of Medical Sciences.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Research Vice-Chancellor Mashhad University of Medical Sciences, Mashhad, Iran. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MA-K: Investigation, Writing – review & editing, Data curation, Conceptualization, Writing – original draft, Project administration, Supervision. AH: Investigation, Writing – review & editing, Resources, Visualization, Writing – original draft, Data curation, Supervision, Conceptualization, Project administration. PA: Writing – review & editing, Writing – original draft. AJ: Software, Formal analysis, Methodology, Writing – review & editing. AA: Resources, Supervision, Methodology, Writing – review & editing. FJ: Writing – review & editing, Data curation. MN: Conceptualization, Resources, Funding acquisition, Project administration, Validation, Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was financially supported by a grant from Mashhad University of Medical Sciences (MUMS), Mashhad, Iran (award/grant numbers: 4012306).
Acknowledgments
The authors would like to express their thanks to the patients enrolled in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BMI, Body Mass Index; CAD, Coronary Artery Disease; CFU, Colony Forming Units; CRP, C-reactive protein; ESR, Erythrocyte Sedimentation Rate; HPA, Hypothalamic–Pituitary–Adrenal (axis); IBD, Inflammatory Bowel Disease; IBS, Irritable Bowel Syndrome; IL, Interleukin; MDD, Major Depressive Disorder; NLR, Neutrophil-to-Lymphocyte Ratio; PLR, Platelet-to-Lymphocyte Ratio; PSS-10, 10-item Perceived Stress Scale; QOL, Quality of Life; SD, Standard Deviation; SPSS, Statistical Package for the Social Sciences; SCFAs, Short-Chain Fatty Acids; TNF-α, Tumor Necrosis Factor alpha.
Footnotes
References
1. Saunders, J, and Smith, T. Malnutrition: causes and consequences. Clin Med (Lond). (2010) 10:624–7. doi: 10.7861/clinmedicine.10-6-624
2. Uzogara, SG. Underweight, the less discussed type of unhealthy weight and its implications: a review. Am J Food Sci Nutr Res. (2016) 3:126–42.
3. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet. (2024) 403:1027–50. doi: 10.1016/s0140-6736(23)02750-2
4. Kotur-Stevuljevic, J, Simic-Ogrizovic, S, Dopsaj, V, Stefanovic, A, Vujovic, A, Ivanic-Corlomanovic, T, et al. A hazardous link between malnutrition, inflammation and oxidative stress in renal patients. Clin Biochem. (2012) 45:1202–5. doi: 10.1016/j.clinbiochem.2012.04.021
6. Alam, SM. Nutrient uptake by plants under stress conditions In: N Kosaric and JCT Kwak, editors. Handbook of plant and crop stress, vol. 19990540. Marcel Dekker Inc. Publishers (1999). 285–313.
7. Zhang, N, Zhang, Y, Li, M, Wang, W, Liu, Z, Xi, C, et al. Efficacy of probiotics on stress in healthy volunteers: a systematic review and meta-analysis based on randomized controlled trials. Brain Behav. (2020) 10:e01699. doi: 10.1002/brb3.1699
8. Milajerdi, A, Mousavi, SM, Sadeghi, A, Salari-Moghaddam, A, Parohan, M, Larijani, B, et al. The effect of probiotics on inflammatory biomarkers: a meta-analysis of randomized clinical trials. Eur J Nutr. (2020) 59:633–49. doi: 10.1007/s00394-019-01931-8
9. Million, M, Diallo, A, and Raoult, D. Gut microbiota and malnutrition. Microb Pathog. (2017) 106:127–38. doi: 10.1016/j.micpath.2016.02.003
10. Gentile, CL, and Weir, TL. The gut microbiota at the intersection of diet and human health. Science. (2018) 362:776–80. doi: 10.1126/science.aau5812
11. Salleh, RM, Kuan, G, Aziz, MNA, Rahim, MRA, Rahayu, T, Sulaiman, S, et al. Effects of probiotics on anxiety, stress, mood and fitness of badminton players. Nutrients. (2021) 13:1783. doi: 10.3390/nu13061783
12. Al Kassaa, I, and Fuad, M. Effects of Lacticaseibacillus rhamnosus HN001 on happiness and mental well-being: findings from a randomized controlled trial. Nutrients. (2024) 16:2936. doi: 10.3390/nu16172936
13. Hadi, A, Sepandi, M, Marx, W, Moradi, S, and Parastouei, K. Clinical and psychological responses to synbiotic supplementation in obese or overweight adults: a randomized clinical trial. Complement Ther Med. (2019) 47:102216. doi: 10.1016/j.ctim.2019.102216
14. Velayati, A, Kareem, I, Sedaghat, M, Sohrab, G, Nikpayam, O, Hedayati, M, et al. Does symbiotic supplementation which contains Bacillus Coagulans Lactobacillus rhamnosus, Lactobacillus acidophilus and fructooligosaccharide has favourite effects in patients with type-2 diabetes? A randomised, double-blind, placebo-controlled trial. Arch Physiol Biochem. (2023) 129:1211–8. doi: 10.1080/13813455.2021.1928225
15. Alipour, B, Homayouni-Rad, A, Vaghef-Mehrabany, E, Sharif, SK, Vaghef-Mehrabany, L, Asghari-Jafarabadi, M, et al. Effects of Lactobacillus casei supplementation on disease activity and inflammatory cytokines in rheumatoid arthritis patients: a randomized double-blind clinical trial. Int J Rheum Dis. (2014) 17:519–27. doi: 10.1111/1756-185x.12333
16. Pan, Y, Yang, L, Dai, B, Lin, B, Lin, S, and Lin, E. Effects of probiotics on malnutrition and health-related quality of life in patients undergoing peritoneal dialysis: a randomized controlled trial. J Ren Nutr. (2021) 31:199–205. doi: 10.1053/j.jrn.2020.04.008
17. Cohen, S, Kamarck, T, and Mermelstein, R. A global measure of perceived stress. J Health Soc Behav. (1983) 24:385–96. doi: 10.2307/2136404
18. Cohen, S. Perceived stress in a probability sample of the United States In: The social psychology of health. Newbury Park, California: Sage Publications (1988)
19. Anuurad, E, Shiwaku, K, Nogi, A, Kitajima, K, Enkhmaa, B, Shimono, K, et al. The new BMI criteria for Asians by the regional office for the western pacific region of WHO are suitable for screening of overweight to prevent metabolic syndrome in elder Japanese workers. J Occup Health. (2003) 45:335–43. doi: 10.1539/joh.45.335
20. Chen, P, Zhang, L, Feng, Y, Liu, YF, Si, TL, Su, Z, et al. Brain-gut axis and psychiatric disorders: a perspective from bibliometric and visual analysis. Front Immunol. (2022) 13:1047007. doi: 10.3389/fimmu.2022.1047007
21. Kasarello, K, Cudnoch-Jedrzejewska, A, and Czarzasta, K. Communication of gut microbiota and brain via immune and neuroendocrine signaling. Front Microbiol. (2023) 14:1118529. doi: 10.3389/fmicb.2023.1118529
22. Merkouris, E, Mavroudi, T, Miliotas, D, Tsiptsios, D, Serdari, A, Christidi, F, et al. Probiotics' effects in the treatment of anxiety and depression: a comprehensive review of 2014-2023 clinical trials. Microorganisms. (2024) 12:411. doi: 10.3390/microorganisms12020411
23. Kristensen, NB, Bryrup, T, Allin, KH, Nielsen, T, Hansen, TH, and Pedersen, O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med. (2016) 8:52. doi: 10.1186/s13073-016-0300-5
24. Bravo, JA, Forsythe, P, Chew, MV, Escaravage, E, Savignac, HM, Dinan, TG, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. (2011) 108:16050–5. doi: 10.1073/pnas.1102999108
25. Tedelind, S, Westberg, F, Kjerrulf, M, and Vidal, A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World J Gastroenterol. (2007) 13:2826–32. doi: 10.3748/wjg.v13.i20.2826
26. Vasiliu, O. The current state of research for psychobiotics use in the management of psychiatric disorders–a systematic literature review. Front Psych. (2023) 14:1074736. doi: 10.3389/fpsyt.2023.1074736
27. Lou, H, Liu, X, and Liu, P. Mechanism and implications of pro-nature physical activity in antagonizing psychological stress: the key role of microbial-gut-brain axis. Front Psychol. (2023) 14:1143827. doi: 10.3389/fpsyg.2023.1143827
28. Moludi, J, Alizadeh, M, Mohammadzad, MHS, and Davari, M. The effect of probiotic supplementation on depressive symptoms and quality of life in patients after myocardial infarction: results of a preliminary double-blind clinical trial. Psychosom Med. (2019) 81:770–7. doi: 10.1097/psy.0000000000000749
29. Slykerman, RF, Hood, F, Wickens, K, Thompson, JMD, Barthow, C, Murphy, R, et al. Effect of Lactobacillus rhamnosus HN001 in pregnancy on postpartum symptoms of depression and anxiety: a randomised double-blind placebo-controlled trial. EBioMedicine. (2017) 24:159–65. doi: 10.1016/j.ebiom.2017.09.013
30. Slykerman, RF, Li, E, and Mitchell, EA. Probiotics for reduction of examination stress in students (PRESS) study: a randomized, double-blind, placebo-controlled trial of the probiotic Lacticaseibacillus rhamnosus HN001. PLoS One. (2022) 17:e0267778. doi: 10.1371/journal.pone.0267778
31. Slykerman, RF, and Li, E. A randomized trial of probiotic supplementation in nurses to reduce stress and viral illness. Sci Rep. (2022) 12:14742. doi: 10.1038/s41598-022-19104-9
32. Takada, M, Nishida, K, Gondo, Y, Kikuchi-Hayakawa, H, Ishikawa, H, Suda, K, et al. Beneficial effects of Lactobacillus casei strain Shirota on academic stress-induced sleep disturbance in healthy adults: a double-blind, randomised, placebo-controlled trial. Benef Microbes. (2017) 8:153–62. doi: 10.3920/bm2016.0150
33. Akkasheh, G, Kashani-Poor, Z, Tajabadi-Ebrahimi, M, Jafari, P, Akbari, H, Taghizadeh, M, et al. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition. (2016) 32:315–20. doi: 10.1016/j.nut.2015.09.003
34. Sarkawi, M, Raja Ali, RA, Abdul Wahab, N, Abdul Rathi, ND, and Mokhtar, NM. A randomized, double-blinded, placebo-controlled clinical trial on Lactobacillus-containing cultured milk drink as adjuvant therapy for depression in irritable bowel syndrome. Sci Rep. (2024) 14:9478. doi: 10.1038/s41598-024-60029-2
35. Dinarello, CA. Anti-inflammatory agents: present and future. Cell. (2010) 140:935–50. doi: 10.1016/j.cell.2010.02.043
36. Moludi, J, Khedmatgozar, H, Nachvak, SM, Abdollahzad, H, Moradinazar, M, and Sadeghpour tabaei, A. The effects of co-administration of probiotics and prebiotics on chronic inflammation, and depression symptoms in patients with coronary artery diseases: a randomized clinical trial. Nutr Neurosci. (2022) 25:1659–68. doi: 10.1080/1028415x.2021.1889451
Keywords: malnutrition, probiotics, perceived stress score, inflammation, stress
Citation: Ahmadi-Khorram M, Hatami A, Asghari P, Jafarzadeh Esfehani A, Afshari A, Javdan F and Nematy M (2025) Probiotics mitigate stress and inflammation in malnourished adults via gut microbiota modulation: a randomized controlled trial. Front. Nutr. 12:1615607. doi: 10.3389/fnut.2025.1615607
Edited by:
Bowen Li, Southwest University, ChinaReviewed by:
Pugazhendhi Srinivasan, University of Kansas Medical Center, United StatesJurairat Khongrum, Chiang Mai University, Thailand
Copyright © 2025 Ahmadi-Khorram, Hatami, Asghari, Jafarzadeh Esfehani, Afshari, Javdan and Nematy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohsen Nematy, TmVtYXR5TUBtdW1zLmFjLmly
†ORCID: Mohsen Nematy, https://orcid.org/0000-0003-3202-2709
 Maryam Ahmadi-Khorram1,2
Maryam Ahmadi-Khorram1,2 Alireza Hatami
Alireza Hatami