- 1School of Medicine, Jiangsu University, Zhenjiang, Jiangsu, China
- 2Department of ICU, Xinghua People's Hospital Affiliated to Yangzhou University, Xinghua, Jiangsu, China
- 3Center for Intravenous Infusion Therapy and Nursing, Affiliated People's Hospital of Jiangsu University, Zhenjiang, Jiangsu, China
- 4Department of Internal Medicine, Xinghua People's Hospital Affiliated to Yangzhou University, Xinghua, Jiangsu, China
Oral cavity cancer exhibits high mortality rates with conventional therapies often causing nutritional complications. Emerging evidence highlights the critical role of micronutrients in modulating oxidative stress, a key driver of carcinogenesis in precancerous lesions including oral lichen planus, leukoplakia and submucous fibrosis. Zinc deficiency impairs antioxidant defenses while copper excess promotes angiogenesis. Selenium maintains redox balance through selenoproteins and vitamins A, E and C exhibit chemopreventive effects through reactive oxygen species scavenging and immunomodulation. Immunonutrition strategies incorporating omega-3 fatty acids and arginine demonstrate benefits in postoperative outcomes. This review summarizes the mechanistic roles of antioxidant micronutrients including zinc, copper, selenium and vitamins A, D, E, C and B complex in oral squamous cell carcinoma pathogenesis and explores personalized nutritional interventions to enhance treatment tolerance and quality of life. Optimizing micronutrient status represents a promising adjuvant approach in comprehensive oral cancer management.
1 Introduction
Oral cavity cancer is associated with significant mortality, with fatality rates approaching 50% of diagnosed cases (1, 2). Surgery, radiotherapy, and chemotherapy frequently induce adverse effects such as dysphagia, taste alterations, and oral mucositis (3–5). These treatment-related complications, if not properly managed, can lead to nutritional deterioration, potentially establishing a detrimental cycle that may compromise clinical outcomes (6). Evidence suggests that appropriate nutritional interventions may provide multiple benefits, including enhancing the treatment tolerance, reducing treatment interruptions, improving therapeutic efficacy, supporting disease control, and facilitating post-treatment recovery (7).
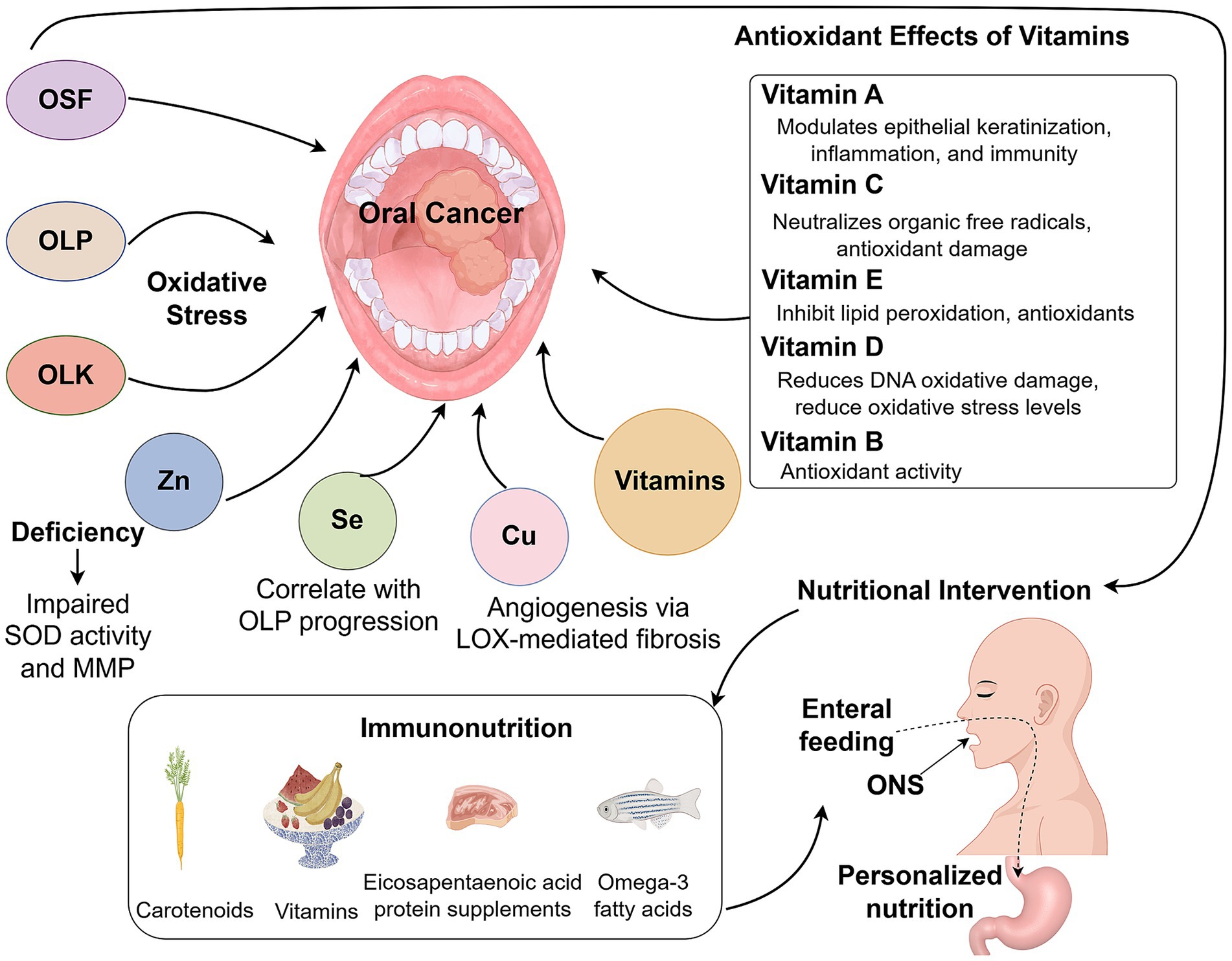
In recent years, with the continuous advancement of micronutrient research techniques, an increasing body of evidence suggests a link between the occurrence of oral cancer and antioxidant micronutrients (8). Oxidative stress refers to a state in which the balance between the formation of reactive oxygen species (ROS) and antioxidant defenses is disrupted, and it plays a role in the development of precancerous lesions such as oral lichen planus (OLP), oral leukoplakia (OLK), and oral submucous fibrosis (OSF) (9–12). The body’s defense against oxidative stress can be achieved through the antioxidant activity of micronutrients (minerals and vitamins) and the interconnected systems of enzymes, which can eliminate or inhibit the formation of free radicals or repair damage caused by free radicals, thereby protecting the body from the harmful effects of oxidative stress and potentially preventing disease onset (13, 14). Therefore, nutritional management, as part of the comprehensive treatment plan for oral cancer, holds significant importance in improving patient prognosis and enhancing quality of life (15). This review explores the relationship between the antioxidant mechanisms of micronutrients and the development of oral cavity cancer, as well as their potential therapeutic applications.
2 Antioxidant role of minerals in oral cancer development
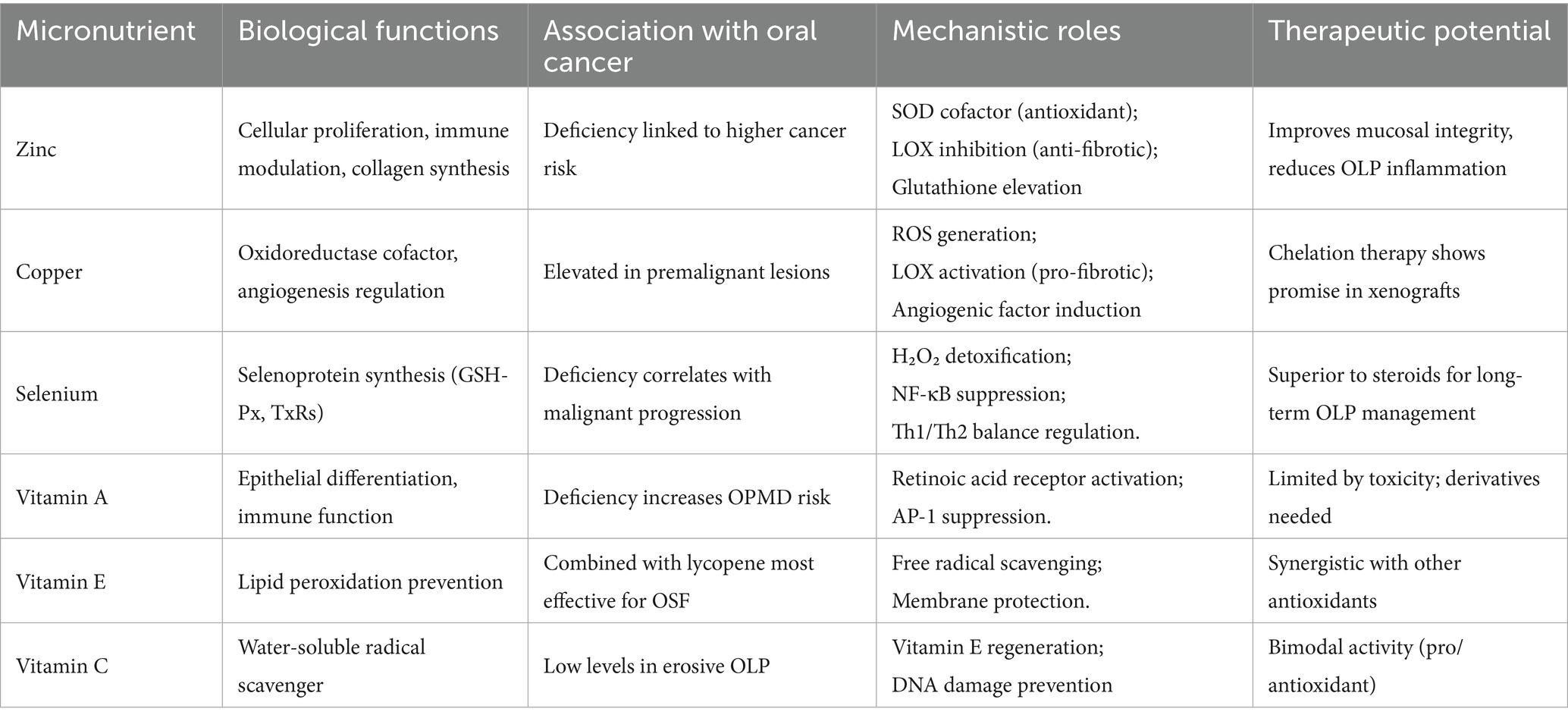
2.1 Zinc
Zinc, an essential trace element, is widely distributed throughout oral ecosystems, including dental plaque, saliva, dental structures, and mucosal tissues, where it serves as a critical biological reservoir (16). This micronutrient plays a pivotal role in multiple physiological processes, including cellular proliferation, immune regulation, collagen synthesis, and tissue repair (17). Notably, clinical studies have established a significant association between zinc deficiency and increased susceptibility to oral cancer (18), with tumor patients frequently exhibiting depleted zinc levels, likely due to its consumption during fibrotic processes, inflammatory responses, and free radical-scavenging activities (19). The therapeutic and prophylactic effects of zinc are mediated through several distinct mechanisms: First, as a cofactor for copper-zinc superoxide dismutase (SOD), zinc exhibits anticancer activity, particularly in OSF (20). Second, it modulates collagen metabolism by suppressing lysyl oxidase (LOX)-mediated collagen deposition while simultaneously promoting matrix metalloproteinase (MMP)-dependent degradation, thereby enhancing mucosal flexibility and alleviating clinical symptoms (21–24). Third, by upregulating glutathione levels, zinc synergizes with vitamins A and C to maintain epithelial integrity and reduce mucosal discomfort (25, 26). Furthermore, emerging evidence suggests that zinc reinforces mucosal barrier function through immunomodulatory effects and oxidative stress mitigation (27, 28). Research indicates that zinc supplementation physiologically modulates immune responses by suppressing excessive activation, while its depletion during severe infections leads to widespread upregulation of NF-κB signaling. In vitro experiments reveal that zinc downregulates NF-κB-mediated pathways along with associated pro-inflammatory cytokines, including TNF-α and IL-1β. Concurrently, it enhances transcriptional activity of A20 and PPAR-α, both of which are zinc-dependent regulators exhibiting anti-inflammatory functions (27).
In the pathogenesis of OLP, a condition marked by cytotoxic T-cell activation, MMP dysregulation, cyclooxygenase-2 (COX-2) overexpression, and redox imbalance, zinc demonstrates significant protective effects (29). As MMPs depend on zinc for their activation, zinc exerts precise control over inflammatory responses by inhibiting MMP-1-mediated lymphocyte infiltration and preventing MMP-9-induced basement membrane disruption (22, 23). Additionally, zinc functions as a potent reactive oxygen species (ROS) scavenger, suppressing COX-2/prostaglandin E2 (PGE2) signaling and thereby attenuating oxidative stress-driven inflammation in OLP patients (30). Collectively, zinc exerts its anti-carcinogenic and anti-inflammatory effects through multifaceted mechanisms, including modulation of the SOD/MMP axis, inhibition of COX-2–PGE2 signaling, and maintenance of redox homeostasis and epithelial integrity. These mechanistic pathways are strongly correlated with enhanced mucosal healing and clinical symptom relief in patients with oral cancer and OLP.
2.2 Copper (cu)
Copper ions serve as a critical micronutrient in numerous oxidoreductases including cytochrome oxidase and tyrosinase, essential for cellular homeostasis and biological functions (31, 32). While physiologically important, dysregulated copper metabolism exhibits dual roles in oral pathogenesis. On one hand, copper deficiency impairs SOD1 activity, compromising antioxidant defenses and leading to oxidative stress accumulation (33). On the other hand, elevated copper levels in biological fluids have been consistently associated with premalignant conditions and squamous cell carcinomas, particularly in OSF, a condition with high malignant transformation potential (34, 35). The oncogenic properties of copper involve multiple interconnected mechanisms: (1) activation of LOX-mediated collagen deposition, driving OSF progression (36); (2) induction of ROS-dependent growth genes (c-fos, c-jun); (3) stimulation of angiogenic factors (VEGF, b-FGF); and (4) promotion of proliferative signaling pathways (37–39). Notably, the copper-CER-SOD1 axis forms a critical regulatory network, where ceruloplasmin facilitates copper delivery for SOD1 biosynthesis, while SOD1 upregulation conversely depletes circulating copper pools (20). This delicate balance is further evidenced by studies showing that copper deficiency reduces antioxidant enzyme activities (GSH-Px, SOD1) while increasing oxidative markers (LPO, MDA), effects reversible upon copper supplementation (40, 41). The therapeutic potential of copper modulation is underscored by preclinical studies demonstrating that chelation therapy can simultaneously target multiple oncogenic pathways, including ROS reduction, SOD1 inhibition, and suppression of angiogenic factors (VEGF, MMP-9) (41, 42). However, current evidence remains limited to xenograft models, highlighting the need for clinical investigations in oral cavity cancers (43, 44). Importantly, dose–response studies reveal a narrow therapeutic window, with micromolar copper iron concentrations stimulating keratinocyte proliferation by ROS accumulation (45), emphasizing the necessity to precisely define physiological ranges that maintain redox homeostasis without inducing either deficiency or toxicity.
2.3 Selenium (se)
Selenium (Se), an essential trace element, functions through selenoproteins including glutathione peroxidase (GSH-Px) and thioredoxin reductase (TxRs), which serve as pivotal antioxidants (46). These proteins exhibit organelle-specific localization and tissue-dependent expression patterns, with activity sensitive to Se availability (47). Epidemiological evidence associates Se deficiency with elevated cancer risk due to impaired selenoprotein function (48). In lichen planus (LP), serum Se levels inversely correlate with lesion severity and chronicity (49). Notably, OLP demonstrates reduced Se levels in malignant progression, suggesting its chemopreventive role (50). Mechanistically, Se insufficiency diminishes GPX-1 activity, compromising H₂O₂ detoxification post-SOD2 reaction, thereby accelerating neoplastic transformation in OLP (51). The selenium-GPX axis restores H₂O₂ detoxification and suppresses pro-inflammatory cytokines via inhibition of NF-κB transcriptional activity (52). These pathways correlate with reduced OLP recurrence, improved epithelial repair, and reduced mucosal pain, demonstrating both molecular and clinical relevance. Beyond antioxidant effects, Se modulates immune responses and oxidative stress (53, 54). At the molecular level, Se suppresses NF-κB binding to cytokine gene promoters to mitigate inflammation, including TNF-α, IL-1, and IL-6 (55, 56). It also normalizes CD3+/CD4+ and CD4+/CD8+ ratios and Th1/Th2 balance, reducing OLP recurrence (57, 58). OLP pathogenesis involves ROS amplification, where ROS overproduction by CD4+ T cells perpetuates keratinocyte lipid membrane damage and localized inflammation. Se interrupts this cycle by neutralizing H₂O₂ and organic peroxides, preserving mucosal integrity (55).
2.4 Vitamin A and vitamin E
Vitamin A encompasses fat-soluble compounds, including retinol, retinoic acid, retinal, and carotenoids, that play crucial roles in modulating epithelial keratinization, inflammatory responses, and immune function (59–61). As a key antioxidant within the glutathione peroxidase system, vitamin E (α-tocopherol) effectively mitigates oxidative membrane damage by neutralizing ROS (62). While both vitamins exhibit lipid peroxidation inhibitory effects, emerging evidence suggests potential antagonistic interactions when administered concurrently (63). Clinical studies have established a strong correlation between deficiencies in these antioxidants and an elevated risk of oral cavity carcinogenesis, particularly in OLK and OLP progression (64–66). The pathogenesis involves tobacco-and betel nut-derived carcinogens that generate excessive ROS and malondialdehyde (MDA), leading to cytotoxic and genotoxic effects that promote mucosal malignant transformation (67). Antioxidant supplementation demonstrates therapeutic potential, with carotenoids showing particular efficacy in precancerous lesion regression (68). Notably, vitamin A or β-carotene supplementation reduces OLK lesion size and nuclear abnormalities, even with persistent carcinogen exposure (69). For refractory OLP cases, isotretinoin (9-cis retinoic acid) exhibits clinical efficacy, potentially through retinoic acid receptor activation or AP-1 pathway suppression, though the mechanisms underlying treatment resistance require further investigation (70, 71). α-Tocopherol demonstrates significant regulatory effects on free radicals and lipid peroxides in precancerous conditions (72). A recent network meta-analysis identified lycopene combined with vitamin E as the most effective intervention for OSF (73). However, the clinical application of systemic vitamin A therapy remains limited by its transient efficacy and notable adverse effects, including cheilitis, mucosal pigmentation, and impaired wound healing (74). These limitations underscore the need for developing safer vitamin A derivatives to enable sustained chemoprevention strategies in oral potentially malignant disorders.
2.5 Vitamin C
Vitamin C (L-ascorbic acid) is a potent water-soluble antioxidant that plays a crucial role in neutralizing organic free radicals and protecting biological membranes from oxidative damage (75). Its antioxidant mechanism involves two key aspects: direct radical scavenging and synergistic interaction with other antioxidants. Notably, vitamin C regenerates α-tocopherol from oxidized vitamin E, thereby restoring the antioxidant capacity of vitamin E (76, 77). In the context of carcinogenesis, where reactive oxygen/nitrogen species (ROS/RNS) induce significant DNA damage, vitamin C demonstrates diagnostic potential when combined with other biomarkers. Studies show that the combination of vitamins C/E significantly improves diagnostic sensitivity for oral precancerous lesions compared to using single biomarkers alone (78). The unique solubility properties of vitamin C enable its antioxidant action in both intracellular and extracellular compartments, effectively mitigating oxidative stress induced by infections (79). Furthermore, vitamin C exhibits a bimodal activity pattern through dose-dependent modulation of redox-sensitive signaling pathways, including NF-κB and MAPK cascades. These molecular interactions can lead to either DNA repair activation or cytotoxic effects, depending on the concentration of vitamin C, highlighting its complex role in cellular redox regulation. Nicolae et al. observed reduced urinary vitamin C in infected lichen planus (LP) patients, correlating with disease severity (79). Animal studies reveal elevated ascorbate in immune cells, bolstering infection resistance (79). Abdolsamadi et al. reported higher salivary MDA and lower antioxidants, such as vitamins A/E, in erosive OLP patients, linking OS to lesion susceptibility (63). Vitamin C also modulates OS-driven metabolic pathways. Depletion elevates ROS, oxidizing DNA (8-hydroxydeoxyguanosine), proteins (carbonyls), and lipids (8-iso-PGF2α), while altering glucose/cholesterol metabolism, enhancing cancer invasiveness (80). Importantly, Vitamin C exhibits concentration-dependent “bimodal” behavior. At physiological concentrations, it functions as an antioxidant, quenching ROS and stabilizing cell membranes (81). However, at pharmacologic or supraphysiological doses, it reduces transition metal ions such as Fe3+ to Fe2+ or Cu2+ to Cu+, facilitating Fenton-like reactions that produce hydrogen peroxide (H₂O₂) and hydroxyl radicals in situ (82, 83). This pro-oxidant effect selectively induces oxidative stress in cancer cells, which often have impaired catalase activity and a weakened antioxidant defense system, leading to DNA strand breaks, mitochondrial dysfunction, and apoptosis. This mechanism underpins the cytotoxic activity of high-dose Vitamin C in tumor settings (80).
2.6 Antioxidant effects of other vitamins
2.6.1 Vitamin B complex
The Vitamin B complex consists of eight water-soluble vitamins: thiamine (VB1), riboflavin (VB2), niacin (VB3), pantothenic acid (VB5), pyridoxine (VB6), biotin (VB7), folate (VB9), and cobalamin (VB12). These vitamins are interconnected in their roles in protein, lipid, and nucleic acid synthesis, metabolism, and immune defense (84). Each B vitamin has demonstrated considerable antioxidant activity (85). Chen et al. (85) observed a significant association between anemia due to hemoglobin, iron, or vitamin B12 deficiencies and elevated homocysteine levels, with an increased prevalence of erosive OLP. Vitamin B12 and iron deficiencies, which lead to anemia, reduce the oxygen supply to the oral mucosal tissues, causing atrophy. Elevated homocysteine levels in erosive OLP patients contribute to OS, promoting thrombosis in small arteries supplying the oral epithelium, thereby compromising the epithelial barrier and increasing the frequency of OLP lesions. Studies indicate that elevated homocysteine levels in OLP patients correlate with deficiencies in vitamin B6, B12, and folate, and this increase has become a key marker of the Vitamin B complex’s involvement in antioxidant stress responses (86). Although empirical supplementation of B vitamins has alleviated subjective symptoms in some cases, studies show that deficiencies in B1, B6, C, folate, and carotenoids are not primary contributors to OLP pathogenesis. Furthermore, no complete recovery was observed in any patients after two months of intensive B vitamin supplementation (87).
2.6.2 Vitamin D
Vitamin D is a fat-soluble vitamin that, through its metabolites such as 7-dehydrocholesterol, calcidiol, vitamin D2, and calcitriol, exhibits antioxidant properties by reducing lipid peroxidation (88). Existing research has established a close relationship between vitamin D deficiency and an increased risk of OLP (89). The active form of vitamin D, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), exerts its biological effects primarily through the vitamin D receptor (VDR), a nuclear receptor expressed in various epithelial cells (89). In vitro studies using HaCat cell models demonstrated that 1,25(OH)2D3, via VDR, can attenuate lipopolysaccharide-induced inflammatory cytokine expression by modulating the NF-κB signaling pathway, thereby reducing inflammation associated with OLP (90). Additionally, vitamin D plays a crucial role in mitigating DNA oxidative damage in mucosal tissues. Supplementation with exogenous vitamin D has been shown to significantly improve oxidative stress markers in patients with ulcerative colitis, including oxidized low-density lipoproteins, lipid peroxides, MDA, and superoxide dismutase, contributing to the repair of intestinal mucosal oxidative damage. Given that the oral cavity is part of the digestive tract and expresses VDR in keratinocytes, the binding of vitamin D to VDR in these cells can reduce oxidative stress levels and clear ROS to facilitate the repair of damaged oral mucosal barriers and promoting lesion healing (91). The 25(OH)2D3-VDR signaling pathway plays a protective role in maintaining the integrity of oral mucosal tissues, suggesting that vitamin D supplementation may serve as a potential strategy for managing OLP lesions (Table 1).
3 Nutritional intervention strategies for oral cancer patients
For oral cancer patients with preserved swallowing function, modifying food texture and increasing nutrient density can meet nutritional needs (92). Oral nutritional supplements (ONS) have been shown to improve nutrient intake and quality of life, although they do not significantly affect mortality rates (93). When combined with dietary counseling, ONS enhances micronutrient intake and helps maintain body weight. In patients with impaired oral intake, enteral feeding via nasogastric or gastrostomy tubes significantly improves immune function, clinical recovery, nutritional status, and reduces hospital stay duration (94). Personalized nutrition assessments offer dynamic, tailored interventions, leading to greater improvements in serum albumin levels, handgrip strength, and lower rates of gastrointestinal complications compared to standard approaches (15). Immunonutrition, involving targeted supplementation with amino acids, fatty acids, nucleotides, and vitamins, modulates immune cell activity, particularly natural killer (NK) cell function, and improves clinical outcomes. Nutrients such as carotenoids, vitamin E, selenium, n-6/n-3 fatty acids, and eicosapentaenoic acid protein supplements have shown potential in preventing oral cancer (95, 96). Specifically, omega-3 fatty acids and arginine have been associated with enhanced progression-free survival, improved serum protein levels, and higher lymphocyte counts (97). Moreover, immune-modulating formulas have been reported to reduce postoperative inflammation in oral cancer patients (Figure 1) (98).
4 Comparative efficacy and limitations of micronutrient and Immunonutrition interventions
While multiple micronutrients and immunonutritional components have demonstrated potential in the management of oral cancer, their relative efficacy, safety, and mechanistic strengths require further critical evaluation (99). Zinc supplementation is associated with enhanced mucosal repair and oxidative stress reduction, particularly in OSF and OLP (100); however, prolonged high-dose use may disrupt copper metabolism, leading to hypocupremia-induced anemia and immunosuppression (101). Selenium, especially in its organic forms such as selenomethionine, exhibits potent anti-inflammatory and redox-stabilizing effects, with clinical evidence supporting its comparability to corticosteroids in the short term and superiority in long-term symptom control in OLP (50). Nevertheless, its narrow therapeutic index limits broader applicability due to risks of selenosis. Vitamin A is effective in reversing precancerous lesions like OLK but poses significant toxicity risks during long-term use, including mucosal dryness and hepatotoxicity (102). In contrast, vitamin E shows a more favorable safety profile and may synergize with lycopene in OSF management (103); however, antagonistic interactions with vitamin A have been reported, indicating the need for empirical testing of combined regimens. Vitamin C displays a bimodal redox activity, acting as an antioxidant at physiological levels and a pro-oxidant at pharmacological concentrations, raising interest in its therapeutic role in selectively inducing cancer cell death, although its clinical application requires definition of safe dosing windows (104, 105). Vitamin D, through the VDR signaling pathway, offers consistent antioxidant and immunomodulatory benefits in oral mucosal disorders, with minimal adverse effects, making it a promising adjunct in managing inflammatory and neoplastic oral lesions (89, 90). Among broader nutritional strategies, immunonutrition, incorporating omega-3 fatty acids, arginine, and nucleotides have shown superior outcomes in reducing inflammation, preserving lean mass, and improving treatment tolerance relative to standard nutritional support (106, 107). While combined immunonutrition regimens appear more effective than isolated micronutrient supplementation, variability in formulations and dosages complicates cross-study comparisons, highlighting the need for standardized, multicenter trials to validate their clinical utility (104, 108).
5 Conclusion
The critical role of micronutrients in oral carcinogenesis and therapy is underscored by their dual capacity to modulate oxidative stress and inflammatory pathways. Deficiencies in zinc and selenium disrupt redox homeostasis, impairing SOD and GPX activity, while copper excess promotes fibrosis and angiogenesis in OSF. Vitamins A, C, and E demonstrate chemopreventive potential but require precise dosing to avoid antagonistic or pro-oxidant effects. Immunonutrition strategies, particularly those incorporating omega-3 fatty acids and arginine, show promise in enhancing treatment tolerance and immune function. However, the therapeutic window for many micronutrients remains narrow, necessitating further research to optimize dosing regimens.
Future studies should focus on biomarker-guided supplementation to enable personalized nutrition interventions. Mechanistic investigations into vitamin D’s role in mucosal repair via VDR signaling may offer novel therapeutic avenues. Additionally, standardized protocols for combined antioxidant therapies are needed to maximize efficacy while minimizing adverse effects. Integrating these nutritional approaches with conventional treatments could improve clinical outcomes and quality of life for oral cancer patients, bridging a critical gap in comprehensive cancer care.
Author contributions
YFa: Writing – original draft. YFe: Writing – original draft. WL: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cracchiolo, JR, Starc, MT, Wong, RJ, Ho, A, Ganly, I, Pfister, DG, et al. Defining response-adapted surgery after neoadjuvant therapy in oral cavity cancer. Oral Oncol. (2025) 165:107349. doi: 10.1016/j.oraloncology.2025.107349
2. Zhang, Z, Sun, D, Tang, H, Ren, J, Yin, S, and Yang, K. PER2 binding to HSP90 enhances immune response against oral squamous cell carcinoma by inhibiting IKK/NF-κB pathway and PD-L1 expression. J Immunother Cancer. (2023) 11:e007627. doi: 10.1136/jitc-2023-007627
3. Gallant, JN, Vivek, N, McKeon, MG, Sharma, RK, Kim, YJ, Rosenthal, EL, et al. Establishing a role for the oral microbiome in infectious complications following major oral cavity cancer surgery. Oral Oncol. (2024) 156:106926. doi: 10.1016/j.oraloncology.2024.106926
4. Draghini, L, Lancellotta, V, Fionda, B, De Angeli, M, Cornacchione, P, Massaccesi, M, et al. Can interventional radiotherapy (brachytherapy) be an alternative to surgery in early-stage oral cavity cancer? A systematic review. Strahlenther Onkol. (2024) 200:367–76. doi: 10.1007/s00066-023-02184-5
5. Yaniv, D, Seiwert, TY, Margalit, DN, Williams, MD, Barbon, CEA, Largo, RD, et al. Neoadjuvant chemotherapy for advanced oral cavity cancer. CA Cancer J Clin. (2024) 74:213–23. doi: 10.3322/caac.21829
6. Hiraoka, SI, Shimada, Y, Kawasaki, Y, Akutagawa, M, and Tanaka, S. Preoperative nutritional evaluation, surgical site infection, and prognosis in patients with oral cancer. Oral Surg Oral Med Oral Pathol Oral Radiol. (2022) 134:168–75. doi: 10.1016/j.oooo.2022.01.009
7. Matsuda, Y, Okui, T, Tatsumi, H, Okuma, S, Kato, A, Morioka, R, et al. Oral dysfunction in patients with Oral Cancer could occur before treatment and require early nutritional improvement: a cross-sectional study. Dysphagia. (2023) 38:1096–105. doi: 10.1007/s00455-022-10531-4
8. Reiter, RJ, De Almeida Chuffa, LG, Simão, VA, Martín Giménez, VM, De Las Heras, N, Spandidos, DA, et al. Melatonin and vitamin D as potential synergistic adjuvants for cancer therapy (review). Int J Oncol. (2024) 65:114. doi: 10.3892/ijo.2024.5702
9. Li, W, Zeng, Q, Wang, B, Lv, C, He, H, Yang, X, et al. Oxidative stress promotes oral carcinogenesis via Thbs 1-mediated M1-like tumor-associated macrophages polarization. Redox Biol. (2024) 76:103335. doi: 10.1016/j.redox.2024.103335
10. Gonzaga, AKG, Vasconcelos, RC, Lopes, M, Medeiros, MRS, de Araújo, AA, da Silveira, ÉJD, et al. Oxidative stress markers in the saliva of patients with oral lichen planus. Pathol Res Pract. (2023) 248:154569. doi: 10.1016/j.prp.2023.154569
11. Yang, M, Chen, X, Cheng, C, Yan, W, Guo, R, Wang, Y, et al. Cucurbitacin B induces ferroptosis in oral leukoplakia via the SLC7A11/mitochondrial oxidative stress pathway. Phytomedicine. (2024) 129:155548. doi: 10.1016/j.phymed.2024.155548
12. Lu, MY, Hsieh, PL, Chao, SC, Fang, CY, Ohiro, Y, Liao, YW, et al. Targeting Meta Lnc 9/mi R-143/FSCN1 axis inhibits oxidative stress and myofibroblast transdifferentiation in oral submucous fibrosis. J Dent Sci. (2024) 19:1416–25. doi: 10.1016/j.jds.2024.04.008
13. Renke, G, Starling-Soares, B, Baesso, T, Petronio, R, Aguiar, D, and Paes, R. Effects of vitamin D on cardiovascular risk and oxidative stress. Nutrients. (2023) 15:769. doi: 10.3390/nu15030769
14. Kapper, C, Oppelt, P, Ganhör, C, Gyunesh, AA, Arbeithuber, B, Stelzl, P, et al. Minerals and the menstrual cycle: impacts on ovulation and endometrial health. Nutrients. (2024) 16:1008. doi: 10.3390/nu16071008
15. Ding, C, Chen, Q, Zhang, F, Xu, B, and Zhang, H. Effect of a personalized enteral nutrition protocol on the postoperative nutritional status in patients who underwent Oral Cancer surgery. Nutr Cancer. (2023) 75:815–24. doi: 10.1080/01635581.2022.2157449
16. Agare, GI, Chidike Ezeorba, TP, Michael, DC, Agbamu, E, Aghoja, OC, and Alalor, CA. Zinc supplementation for mitigating Oral mucositis in head and neck Cancer patients undergoing radiotherapy and Chemoradiotherapy - a systematic review. Clin Nutr ESPEN. (2025) 67:8–24. doi: 10.1016/j.clnesp.2025.02.011
17. Duan, M, Li, T, Liu, B, Yin, S, Zang, J, Lv, C, et al. Zinc nutrition and dietary zinc supplements. Crit Rev Food Sci Nutr. (2023) 63:1277–92. doi: 10.1080/10408398.2021.1963664
18. Gholizadeh, N, and Sheykhbahaei, N. Micronutrients profile in Oral lichen planus: a review literature. Biol Trace Elem Res. (2021) 199:912–24. doi: 10.1007/s12011-020-02221-9
19. Roselletti, E, Pericolini, E, Nore, A, Takacs, P, Kozma, B, Sala, A, et al. Zinc prevents vaginal candidiasis by inhibiting expression of an inflammatory fungal protein. Sci Transl Med. (2023) 15:eadi3363. doi: 10.1126/scitranslmed.adi3363
20. Sachdev, PK, Freeland-Graves, J, Beretvas, SN, and Sanjeevi, N. Zinc, copper, and Iron in Oral submucous fibrosis: a Meta-analysis. Int J Dent. (2018) 2018:1–14. doi: 10.1155/2018/3472087
21. Bhattacharya, PT, Misra, SR, and Hussain, M. Nutritional aspects of essential trace elements in Oral health and disease: an extensive review. Scientifica. (2016) 2016:5464373. doi: 10.1155/2016/5464373
22. Kim, DY, Kim, JH, Lee, JC, Won, MH, Yang, SR, Kim, HC, et al. Zinc oxide nanoparticles exhibit both cyclooxygenase-and lipoxygenase-mediated apoptosis in human bone marrow-derived mesenchymal stem cells. Toxicol Res. (2019) 35:83–91. doi: 10.5487/TR.2019.35.1.083
23. Rashid, ZA, and Bardaweel, SK. Novel matrix Metalloproteinase-9 (MMP-9) inhibitors in Cancer treatment. Int J Mol Sci. (2023) 24:12133. doi: 10.3390/ijms241512133
24. Kim, MK, Kim, HW, Jang, M, Oh, SS, Yong, SJ, Jeong, Y, et al. LOX family and ZFPM2 as novel diagnostic biomarkers for malignant pleural mesothelioma. Biomark Res. (2020) 8:1. doi: 10.1186/s40364-019-0180-0
25. Bhandarkar, GP, Shetty, KV, and Kulkarni, A. Thioctic acid in oral submucous fibrosis (India's disease) - a better tomorrow. J Stomatol Oral Maxillofac Surg. (2018) 119:129–34. doi: 10.1016/j.jormas.2017.12.006
26. Sharif, N, Opu, RR, Khan, A, Alzahrani, KJ, Banjer, HJ, Alzahrani, FM, et al. Impact of zinc, vitamins C and D on disease prognosis among patients with COVID-19 in Bangladesh: a cross-sectional study. Nutrients. (2022) 14:5029. doi: 10.3390/nu14235029
27. Jarosz, M, Olbert, M, Wyszogrodzka, G, Młyniec, K, and Librowski, T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology. (2017) 25:11–24. doi: 10.1007/s10787-017-0309-4
28. Bederska-Łojewska, D, Szczepanik, K, Turek, J, Machaczka, A, Gąsior, Ł, Pochwat, B, et al. Dietary zinc restriction and chronic restraint stress affect mice physiology, immune organ morphology, and liver function. Nutrients. (2024) 16:3934. doi: 10.3390/nu16223934
29. Yang, JY, Zhang, J, and Zhou, G. Black pepper and its bioactive constituent piperine: promising therapeutic strategies for oral lichen planus. Inflammopharmacology. (2019) 27:5–13. doi: 10.1007/s10787-018-0540-7
30. Zhai, J, Li, S, Cheng, X, Chen, ZJ, Li, W, and Du, Y. A candidate pathogenic gene, zinc finger gene 217 (ZNF217), may contribute to polycystic ovary syndrome through prostaglandin E2. Acta Obstet Gynecol Scand. (2020) 99:119–26. doi: 10.1111/aogs.13719
31. Song, Y, Li, J, Tian, H, Xiang, H, Chen, S, Li, L, et al. Copper chelating peptides derived from tilapia (Oreochromis niloticus) skin as tyrosinase inhibitor: biological evaluation, in silico investigation and in vivo effects. Food Res Int. (2023) 163:112307. doi: 10.1016/j.foodres.2022.112307
32. Xue, Q, Kang, R, Klionsky, DJ, Tang, D, Liu, J, and Chen, X. Copper metabolism in cell death and autophagy. Autophagy. (2023) 19:2175–95. doi: 10.1080/15548627.2023.2200554
33. Lu, Y, Chan, YT, Wu, J, Feng, Z, Yuan, H, Li, Q, et al. CRISPR/Cas 9 screens unravel mi R-3689a-3p regulating sorafenib resistance in hepatocellular carcinoma via suppressing CCS/SOD1-dependent mitochondrial oxidative stress. Drug Resist Updat. (2023) 71:101015. doi: 10.1016/j.drup.2023.101015
34. Srivastava, R, Singh, DK, Thomas, PA, Raza, H, Sangamesh, NC, and Bagde, HS. Comparative analysis of tissue copper levels in Oral submucous fibrosis (OSMF) patients. J Pharm Bioallied Sci. (2024) 16:S629–30. doi: 10.4103/jpbs.jpbs_904_23
35. Gupta, R, Jayanti, I, Das, A, Patel, A, Achalli, S, and Reddy, MSR. Estimation of the salivary copper levels in Oral submucous fibrosis condition: an in vivo study. J Contemp Dent Pract. (2024) 25:458–502. doi: 10.5005/jp-journals-10024-3661
36. Shah, R, Khidri, FF, Waryah, YM, Nigar, R, Mahmood, A, Shaikh, H, et al. Serum and salivary cu/Zn ratio as a diagnostic biomarker for oral submucosal fibrosis: an analysis of trace metals and LOX gene variants. Biometals. (2024) 37:447–59. doi: 10.1007/s10534-023-00561-2
37. Das, A, Ash, D, Fouda, AY, Sudhahar, V, Kim, YM, Hou, Y, et al. Cysteine oxidation of copper transporter CTR1 drives VEGFR2 signalling and angiogenesis. Nat Cell Biol. (2022) 24:35–50. doi: 10.1038/s41556-021-00822-7
38. Han, J, Hassani Besheli, N, Deng, D, van Oirschot, B, Leeuwenburgh, SCG, and Yang, F. Tailoring copper-doped bioactive glass/chitosan coatings with angiogenic and antibacterial properties. Tissue Eng Part C Methods. (2022) 28:314–24. doi: 10.1089/ten.TEC.2022.0014
39. Noori, A, Hoseinpour, M, Kolivand, S, Lotfibakhshaiesh, N, Azami, M, Ai, J, et al. Synergy effects of copper and L-arginine on osteogenic, angiogenic, and antibacterial activities. Tissue Cell. (2022) 77:101849. doi: 10.1016/j.tice.2022.101849
40. Cfg Dem,, Soares, GR, Ribeiro, FAP, Silva, MJD, Vilegas, W, Santamarina, AB, et al. Evaluation of the chemopreventive activity of grape skin extract using medium-term oral carcinogenesis assay induced by 4-nitroquinoline 1-oxide. Anticancer Res. (2019) 39:177–82. doi: 10.21873/anticanres.13095
41. Wang, XX, Chen, WZ, Li, C, and Xu, RS. Current potential pathogenic mechanisms of copper-zinc superoxide dismutase 1 (SOD1) in amyotrophic lateral sclerosis. Rev Neurosci. (2024) 35:549–63. doi: 10.1515/revneuro-2024-0010
42. Wakisaka, Y, Chu, Y, Miller, JD, Rosenberg, GA, and Heistad, DD. Critical role for copper/zinc-superoxide dismutase in preventing spontaneous intracerebral hemorrhage during acute and chronic hypertension in mice. Stroke. (2010) 41:790–7. doi: 10.1161/STROKEAHA.109.569616
43. Torabinia, N, Aghakouchakzadeh, A, Kargahi, N, and Motamedi, A. Evaluation of copper salivary level in oral squamous cell carcinoma, occupationally copper exposed, and its normal population and its association with cytomorphologic changes of oral mucosa. Dent Res J. (2023) 20:80. doi: 10.4103/1735-3327.382133
44. Lee, HM, Patel, V, Shyur, LF, and Lee, WL. Copper supplementation amplifies the anti-tumor effect of curcumin in oral cancer cells. Phytomedicine. (2016) 23:1535–44. doi: 10.1016/j.phymed.2016.09.005
45. Coger, V, Million, N, Rehbock, C, Sures, B, Nachev, M, Barcikowski, S, et al. Tissue concentrations of zinc, Iron, copper, and magnesium during the phases of full thickness wound healing in a rodent model. Biol Trace Elem Res. (2019) 191:167–76. doi: 10.1007/s12011-018-1600-y
46. Diyabalanage, S, Dangolla, A, Mallawa, C, Rajapakse, S, and Chandrajith, R. Bioavailability of selenium (se) in cattle population in Sri Lanka based on qualitative determination of glutathione peroxidase (GSH-Px) activities. Environ Geochem Health. (2020) 42:617–24. doi: 10.1007/s10653-019-00395-3
47. Guillin, OM, Vindry, C, Ohlmann, T, and Chavatte, L. Selenium, selenoproteins and viral infection. Nutrients. (2019) 11:2101. doi: 10.3390/nu11092101
48. Hughes, DJ, Schomburg, L, Jenab, M, Biessy, C, Méplan, C, Moskal, A, et al. Prediagnostic selenium status, selenoprotein gene variants and association with breast cancer risk in a European cohort study. Free Radic Biol Med. (2023) 209:381–93. doi: 10.1016/j.freeradbiomed.2023.10.401
49. Belal, MH. Management of symptomatic erosive-ulcerative lesions of oral lichen planus in an adult Egyptian population using selenium-ACE combined with topical corticosteroids plus antifungal agent. Contemp Clin Dent. (2015) 6:454–60. doi: 10.4103/0976-237X.169837
50. Qataya, PO, Elsayed, NM, Elguindy, NM, Ahmed Hafiz, M, and Samy, WM. Selenium: a sole treatment for erosive oral lichen planus (randomized controlled clinical trial). Oral Dis. (2020) 26:789–804. doi: 10.1111/odi.13285
51. Ekoue, DN, He, C, Diamond, AM, and Bonini, MG. Manganese superoxide dismutase and glutathione peroxidase-1 contribute to the rise and fall of mitochondrial reactive oxygen species which drive oncogenesis. Biochim Biophys Acta Bioenerg. (2017) 1858:628–32. doi: 10.1016/j.bbabio.2017.01.006
52. Jia, B, Yang, W, Li, H, Chang, G, Zhang, X, Zhang, N, et al. Ophiopogonis Radix fructan-selenium nanoparticles for dual amelioration of ulcerative colitis and anti-colon cancer. Int J Biol Macromol. (2025) 307:142327. doi: 10.1016/j.ijbiomac.2025.142327
53. Vaivode, I, Zake, T, Strele, I, Upmale-Engela, S, Gogins, D, Gersone, G, et al. Stress-related immune response and selenium status in autoimmune thyroid disease patients. Int J Mol Sci. (2023) 24:2440. doi: 10.3390/ijms24032440
54. Lei, L, Jing, M, Yingce, Z, Pei, Z, and Yun, L. Selenium deficiency causes oxidative stress and activates inflammation, apoptosis, and necroptosis in the intestine of weaned calves. Metallomics. (2023) 15:mfad028. doi: 10.1093/mtomcs/mfad028
55. Lei, L, Zhang, F, Huang, J, Yang, X, Zhou, X, Yan, H, et al. Selenium deficiency causes hypertension by increasing renal AT (1) receptor expression via GPx1/H (2) O (2)/NF-κB pathway. Free Radic Biol Med. (2023) 200:59–72. doi: 10.1016/j.freeradbiomed.2023.02.021
56. Yin, K, Sun, X, Zheng, Y, Zhang, W, and Lin, H. Bisphenol a exacerbates selenium deficiency-induced pyroptosis via the NF-κB/NLRP3/Caspase-1 pathway in chicken trachea. Comp Biochem Physiol C Toxicol Pharmacol. (2023) 263:109488. doi: 10.1016/j.cbpc.2022.109488
57. Xu, J, Liu, Z, Zhang, S, Xiang, J, Lan, H, and Bao, Y. Anti-hepatoma immunotherapy of Pholiota adiposa polysaccharide-coated selenium nanoparticles by reversing M2-like tumor-associated macrophage polarization. Int J Biol Macromol. (2024) 277:133667. doi: 10.1016/j.ijbiomac.2024.133667
58. Wang, F, Zuo, Z, Chen, K, Peng, X, Fang, J, Cui, H, et al. Selenium rescues aflatoxin B (1)-inhibited T cell subsets and cytokine levels in cecal tonsil of chickens. Biol Trace Elem Res. (2019) 188:461–7. doi: 10.1007/s12011-018-1412-0
59. Lin, B, Shah, VS, Chernoff, C, Sun, J, Shipkovenska, GG, Vinarsky, V, et al. Airway hillocks are injury-resistant reservoirs of unique plastic stem cells. Nature. (2024) 629:869–77. doi: 10.1038/s41586-024-07377-1
60. Amimo, JO, Michael, H, Chepngeno, J, Jung, K, Raev, SA, Paim, FC, et al. Maternal immunization and vitamin a sufficiency impact sow primary adaptive immunity and passive protection to nursing piglets against porcine epidemic diarrhea virus infection. Front Immunol. (2024) 15:1397118. doi: 10.3389/fimmu.2024.1397118
61. Rossholt, ME, Wendel, K, Bratlie, M, Aas, MF, Gunnarsdottir, G, Fugelseth, D, et al. Vitamin a status in preterm infants is associated with inflammation and dexamethasone exposure. Nutrients. (2023) 15:441. doi: 10.3390/nu15020441
62. Pallavi, M, and Rajashekaraiah, V. Synergistic activity of vitamin-C and vitamin-E to ameliorate the efficacy of stored erythrocytes. Transfus Clin Biol. (2023) 30:87–95. doi: 10.1016/j.tracli.2022.09.002
63. Abdolsamadi, H, Rafieian, N, Goodarzi, MT, Feradmal, J, Davoodi, P, Jazayeri, M, et al. Levels of salivary antioxidant vitamins and lipid peroxidation in patients with oral lichen planus and healthy individuals. Chonnam Med J. (2014) 50:58–62. doi: 10.4068/cmj.2014.50.2.58
64. See, JKL, Liu, X, Canfora, F, Moore, C, McCullough, M, Yap, T, et al. The role of vitamins in oral potentially malignant disorders and oral cancer: a systematic review. J Pers Med. (2023) 13:1520. doi: 10.3390/jpm13101520
65. Iqubal, MA, Khan, M, Kumar, P, Kumar, A, and Ajai, K. Role of vitamin e in prevention of oral cancer: -a review. J Clin Diagn Res. (2014) 8:Ze05-07. doi: 10.7860/JCDR/2014/9166.4958
66. Divyambika, CV, Sathasivasubramanian, S, Vani, G, Vanishree, AJ, and Malathi, N. Correlation of clinical and histopathological grades in Oral submucous fibrosis patients with oxidative stress markers in saliva. Indian J Clin Biochem. (2018) 33:348–55. doi: 10.1007/s12291-017-0689-7
67. Mohideen, K, Sudhakar, U, Balakrishnan, T, Almasri, MA, Al-Ahmari, MM, Al Dira, HS, et al. Malondialdehyde, an oxidative stress marker in Oral squamous cell carcinoma-a systematic review and Meta-analysis. Curr Issues Mol Biol. (2021) 43:1019–35. doi: 10.3390/cimb43020072
68. Tschuck, J, Padmanabhan Nair, V, Galhoz, A, Zaratiegui, C, Tai, HM, Ciceri, G, et al. Suppression of ferroptosis by vitamin a or radical-trapping antioxidants is essential for neuronal development. Nat Commun. (2024) 15:7611. doi: 10.1038/s41467-024-51996-1
69. Stich, HF, Mathew, B, Sankaranarayanan, R, and Nair, MK. Remission of precancerous lesions in the oral cavity of tobacco chewers and maintenance of the protective effect of beta-carotene or vitamin a. Am J Clin Nutr. (1991) 53:298s–304s.
70. Kunz, M, Urosevic-Maiwald, M, Goldinger, SM, Frauchiger, AL, Dreier, J, Belloni, B, et al. Efficacy and safety of oral alitretinoin in severe oral lichen planus--results of a prospective pilot study. J Eur Acad Dermatol Venereol. (2016) 30:293–8. doi: 10.1111/jdv.13444
71. Klaassen, I, and Braakhuis, BJ. Anticancer activity and mechanism of action of retinoids in oral and pharyngeal cancer. Oral Oncol. (2002) 38:532–42. doi: 10.1016/s1368-8375(01)00118-x
72. Zulkapli, R, Abdul Razak, F, and Zain, RB. Vitamin E (α-tocopherol) exhibits antitumour activity on Oral squamous carcinoma cells ORL-48. Integr Cancer Ther. (2017) 16:414–25. doi: 10.1177/1534735416675950
73. Rajesh Kashyap, R, and Shanker Kashyap, R. Herbal derivatives in the management of mouth opening in oral submucous fibrosis-a network meta-analysis. Oral Dis. (2021) 27:1606–15. doi: 10.1111/odi.13544
74. Barnish, M, Sheikh, M, and Scholey, A. Nutrient therapy for the improvement of fatigue symptoms. Nutrients. (2023) 15:2154. doi: 10.3390/nu15092154
75. Zhou, J, Chen, C, Chen, X, Fei, Y, Jiang, L, and Wang, G. Vitamin C promotes apoptosis and cell cycle arrest in Oral squamous cell carcinoma. Front Oncol. (2020) 10:976. doi: 10.3389/fonc.2020.00976
76. Li, XY, and Zhang, ZC. Assessment of serum malondialdehyde, uric acid, and vitamins C and E levels in patients with recurrent aphthous stomatitis. J Dent Sci. (2016) 11:401–4. doi: 10.1016/j.jds.2016.06.002
77. Hondal, RJ. Selenium vitaminology: the connection between selenium, vitamin C, vitamin E, and ergothioneine. Curr Opin Chem Biol. (2023) 75:102328. doi: 10.1016/j.cbpa.2023.102328
78. Kaur, J, Politis, C, and Jacobs, R. Salivary 8-hydroxy-2-deoxyguanosine, malondialdehyde, vitamin C, and vitamin E in oral pre-cancer and cancer: diagnostic value and free radical mechanism of action. Clin Oral Investig. (2016) 20:315–9. doi: 10.1007/s00784-015-1506-4
79. Nicolae, I, Mitran, CI, Mitran, MI, Ene, CD, Tampa, M, and Georgescu, SR. Ascorbic acid deficiency in patients with lichen planus. J Immunoassay Immunochem. (2017) 38:430–7. doi: 10.1080/15321819.2017.1319863
80. Kaźmierczak-Barańska, J, Boguszewska, K, Adamus-Grabicka, A, and Karwowski, BT. Two faces of vitamin C-antioxidative and pro-oxidative agent. Nutrients. (2020) 12:1501. doi: 10.3390/nu12051501
81. Li, Y, and Schellhorn, HE. New developments and novel therapeutic perspectives for vitamin C. J Nutr. (2007) 137:2171–84. doi: 10.1093/jn/137.10.2171
82. Timoshnikov, VA, Kobzeva, TV, Polyakov, NE, and Kontoghiorghes, GJ. Redox interactions of vitamin C and Iron: inhibition of the pro-oxidant activity by Deferiprone. Int J Mol Sci. (2020) 21:3967. doi: 10.3390/ijms21113967
83. Nowak, M, Tryniszewski, W, Sarniak, A, Wlodarczyk, A, Nowak, PJ, and Nowak, D. Effect of physiological concentrations of vitamin C on the Inhibitation of hydroxyl radical induced light emission from Fe (2+)-EGTA-H (2)O (2) and Fe (3+)-EGTA-H(2)O(2) systems in vitro. Molecules. (2021) 26:1993. doi: 10.3390/molecules26071993
84. Lindschinger, M, Tatzber, F, Schimetta, W, Schmid, I, Lindschinger, B, Cvirn, G, et al. A randomized pilot trial to evaluate the bioavailability of natural versus synthetic vitamin B complexes in healthy humans and their effects on homocysteine, oxidative stress, and antioxidant levels. Oxidative Med Cell Longev. (2019) 2019:1–14. doi: 10.1155/2019/6082613
85. Chen, HM, Wang, YP, Chang, JY, Wu, YC, Cheng, SJ, and Sun, A. Significant association of deficiencies of hemoglobin, iron, folic acid, and vitamin B12 and high homocysteine level with oral lichen planus. J Formos Med Assoc. (2015) 114:124–9. doi: 10.1016/j.jfma.2014.10.004
86. Gupta, P, Chandra, S, Jha, AK, Khaitan, T, Shukla, AK, and Naik, SR. Increased vitamin B12 levels in patients with oral cancer. Indian J Dent Res. (2023) 34:164–8. doi: 10.4103/ijdr.ijdr_1124_21
87. Vitamin B shown to reduce mouth cancer risk. Br Dent J. (2010) 209:548. doi: 10.1038/sj.bdj.2010.1099
88. Cakici, C, Yigitbasi, T, Ayla, S, Karimkhani, H, Bayramoglu, F, Yigit, P, et al. Dose-dependent effects of vitamin 1, 25(OH)2D3 on oxidative stress and apoptosis. J Basic Clin Physiol Pharmacol. (2018) 29:271–9. doi: 10.1515/jbcpp-2017-0121
89. Zhao, B, Li, R, Yang, F, Yu, F, Xu, N, Zhang, F, et al. LPS-induced vitamin D receptor decrease in Oral keratinocytes is associated with Oral lichen planus. Sci Rep. (2018) 8:763. doi: 10.1038/s41598-018-19234-z
90. Du, J, Li, R, Yu, F, Yang, F, Wang, J, Chen, Q, et al. Experimental study on 1, 25(OH)(2) D (3) amelioration of oral lichen planus through regulating NF-κB signaling pathway. Oral Dis. (2017) 23:770–8. doi: 10.1111/odi.12659
91. Nuszkiewicz, J, Czuczejko, J, Maruszak, M, Pawłowska, M, Woźniak, A, Małkowski, B, et al. Parameters of oxidative stress, vitamin D, Osteopontin, and melatonin in patients with lip, Oral cavity, and pharyngeal Cancer. Oxidative Med Cell Longev. (2021) 2021:2364931. doi: 10.1155/2021/2364931
92. Chen, WF, Tsai, SC, Zhang, YH, Chang, HM, Wu, WJ, Su, JH, et al. Rhopaloic acid a triggers mitochondria damage-induced apoptosis in oral cancer by JNK/BNIP3/nix-mediated mitophagy. Phytomedicine. (2024) 132:155855. doi: 10.1016/j.phymed.2024.155855
93. Ferreira, IB, Lima, E, Canto, PPL, Gontijo, CA, Maia, YCP, and Pena, GDG. Oral nutritional supplementation affects the dietary intake and body weight of head and neck cancer patients during (chemo) radiotherapy. Nutrients. (2020) 12:2516. doi: 10.3390/nu12092516
94. Nett, H, Steegmann, J, Tollkühn-Prott, B, Hölzle, F, and Modabber, A. A prospective randomized comparative trial evaluating postoperative nutritional intervention in patients with oral cancer. Sci Rep. (2022) 12:14213. doi: 10.1038/s41598-022-18292-8
95. Hegde, SK, Rao, S, D'Souza, RK, and Baliga, MS. Efficacy of eicosapentaenoic acid (EPA) containing protein supplement in preventing weight loss in head and neck cancer patients undergoing curative radiotherapy: retrospective observations with historical controls. Indian J Otolaryngol Head Neck Surg. (2024) 76:587–95. doi: 10.1007/s12070-023-04217-y
96. Edefonti, V, Bravi, F, La Vecchia, C, Randi, G, Ferraroni, M, Garavello, W, et al. Nutrient-based dietary patterns and the risk of oral and pharyngeal cancer. Oral Oncol. (2010) 46:343–8. doi: 10.1016/j.oraloncology.2009.11.017
97. Lessa, RC, Alves, FA, Fortunati, E, and Lu, J. Oral mucositis in cancer and potential use of Omega-3 free fatty acids in its management: a review. Biomedicines. (2021) 9:1531. doi: 10.3390/biomedicines9111531
98. Cereda, E, Veronese, N, and Caccialanza, R. Nutritional therapy in chronic wound management for older adults. Curr Opin Clin Nutr Metab Care. (2024) 27:3–8. doi: 10.1097/MCO.0000000000000990
99. Fang, Y, Xu, Y, Zhang, Y, Ren, F, and Baker, JS. Mixed treatments comparison of oral nutrition interventions for blood immune cell parameters in cancer patients: systematic review and network meta-analysis. Meta. (2022) 12:868. doi: 10.3390/metabo12090868
100. Aboushousha, A, Kamal, Y, and Ali, S. Supplementary zinc and vitamin D in management of symptomatic oral lichen planus: a three-arm randomized clinical trial. BMC Oral Health. (2025) 25:872. doi: 10.1186/s12903-025-06173-1
101. Qin, Y, Liu, Y, Xiang, X, Long, X, Chen, Z, Huang, X, et al. Cuproptosis correlates with immunosuppressive tumor microenvironment based on pan-cancer multiomics and single-cell sequencing analysis. Mol Cancer. (2023) 22:59. doi: 10.1186/s12943-023-01752-8
102. Sankaranarayanan, R, Mathew, B, Varghese, C, Sudhakaran, PR, Menon, V, Jayadeep, A, et al. Chemoprevention of oral leukoplakia with vitamin a and beta carotene: an assessment. Oral Oncol. (1997) 33:231–6. doi: 10.1016/s0964-1955(97)00010-9
103. Garewal, H. Chemoprevention of oral cancer: beta-carotene and vitamin E in leukoplakia. Eur J Cancer Prev. (1994) 3:101–7.
104. Kouakanou, L, Peters, C, Brown, CE, Kabelitz, D, and Wang, LD. Vitamin C, from supplement to treatment: a re-emerging adjunct for Cancer immunotherapy? Front Immunol. (2021) 12:765906. doi: 10.3389/fimmu.2021.765906
105. Roa, FJ, Peña, E, Gatica, M, Escobar-Acuña, K, Saavedra, P, Maldonado, M, et al. Therapeutic use of vitamin C in Cancer: physiological considerations. Front Pharmacol. (2020) 11:211. doi: 10.3389/fphar.2020.00211
106. Prieto, I, Montemuiño, S, Luna, J, de Torres, MV, and Amaya, E. The role of immunonutritional support in cancer treatment: current evidence. Clin Nutr. (2017) 36:1457–64. doi: 10.1016/j.clnu.2016.11.015
107. Motallebi, M, Bhia, M, Rajani, HF, Bhia, I, Tabarraei, H, Mohammadkhani, N, et al. Naringenin: a potential flavonoid phytochemical for cancer therapy. Life Sci. (2022) 305:120752. doi: 10.1016/j.lfs.2022.120752
Keywords: oral cavity cancer, micronutrients, inflammation, immunity, nutritional intervention, oxidative stress
Citation: Fan Y, Feng Y and Liu W (2025) Role of micronutrition in patients with oral cancer and nutritional intervention strategies. Front. Nutr. 12:1616344. doi: 10.3389/fnut.2025.1616344
Edited by:
Biao Zhang, Dalian Medical University, ChinaReviewed by:
Binggang Liu, The Central Hospital of Yongzhou, ChinaCopyright © 2025 Fan, Feng and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenxin Liu, NDUwOTMwMTczQHFxLmNvbQ==
 Yunwei Fan
Yunwei Fan Yuling Feng3
Yuling Feng3