- 1Department of Anesthesiology, Chi Mei Medical Center, Tainan City, Taiwan
- 2School of Medicine, College of Medicine, National Sun Yat-sen University, Kaohsiung City, Taiwan
- 3Department of Anesthesiology, E-Da Hospital, I-Shou University, Kaohsiung City, Taiwan
- 4Center of General Education, Chia Nan University of Pharmacy and Science, Tainan City, Taiwan
- 5Department of Anesthesiology, Chi Mei Medical Center, Liouying, Tainan City, Taiwan
Background: To assess the impact of preoperative vitamin D deficiency (VDD) on the risk of postoperative delirium (POD) in patients undergoing musculoskeletal surgery.
Methods: This cohort study utilized the TriNetX Healthcare Commercial Organizations database. We included patients aged 50 years or older who underwent musculoskeletal surgery requiring hospital admission. Patients were categorized according to their preoperative vitamin D levels into three groups: deficient (≤20 ng/mL), insufficient (21–29 ng/mL), and sufficient (≥30 ng/mL). The primary outcome was POD within 30 days of surgery. Secondary outcomes included risks of surgical site infections, emergency department (ED) visits, and intensive care unit (ICU) admissions. Risk factors for POD were assessed using multivariate logistic regression analysis.
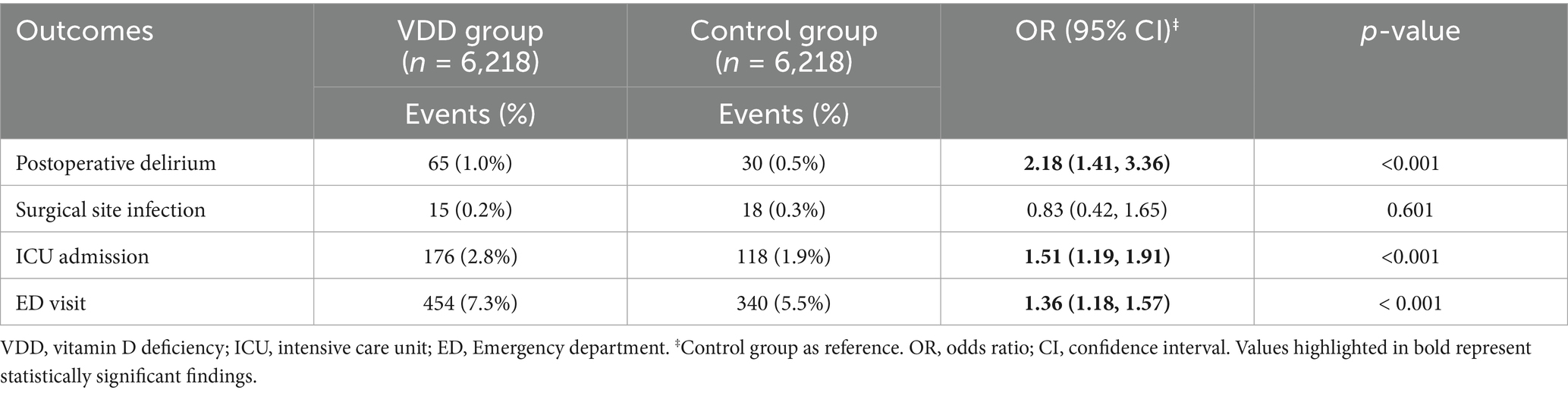
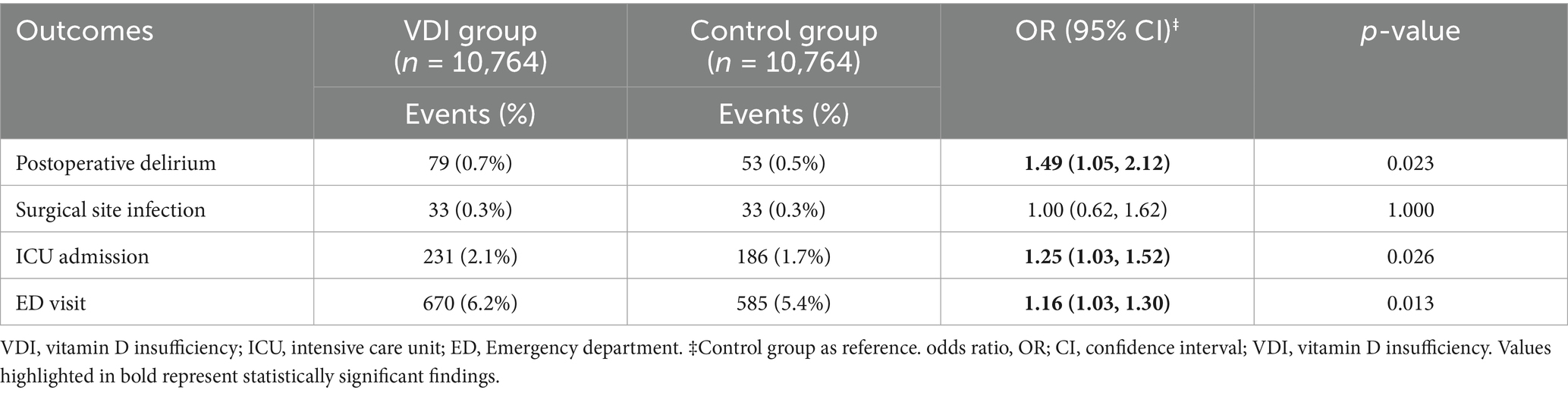
Results: After matching, 6,218 pairs of vitamin D-deficient and sufficient patients were compared. VDD was related to a significantly higher risk of POD [1.0% vs. 0.5%; odds ratio (OR): 2.18, 95% confidence interval (CI): 1.41–3.36, p < 0.001]. Vitamin D-deficient patients also had higher rates of ED visits (OR: 1.36, 95% CI: 1.18–1.57, p < 0.001) and ICU admissions (OR: 1.51, 95% CI: 1.19–1.91, p < 0.001). Similarly, vitamin D insufficiency (10,764 matched pairs) was associated a smaller but significant increase in delirium risk (OR: 1.49, 95%CI: 1.05–2.12, p = 0.023), along with increased ED visits (OR: 1.16, 95% CI: 1.03–1.30, p = 0.013) and ICU admissions (OR: 1.25, 95% CI: 1.03–1.52, p = 0.0261), suggesting a dose-dependent relationship. Risk factor analysis revealed that advanced age, male sex, chronic kidney disease, and malnutrition were significant predictors of POD in patients with VDD.
Conclusion: Individuals with VDD experienced a higher risk of POD, suggesting the potential benefits of preoperative vitamin D screening and supplementation as a strategy to improve outcomes in surgical patients. While our findings highlight the potential benefit of vitamin D assessment and optimization before surgery, the retrospective design limits the ability to draw causal inferences. Prospective interventional studies are warranted to determine whether treating VDD can meaningfully lower the risk of POD.
1 Introduction
Postoperative delirium (POD) involves fluctuating attention, impaired thinking, and changes in awareness. In lower-risk elective procedures, such as joint replacement, POD occurs in approximately 1–22.8% of cases (1, 2). However, in high-risk settings—such as cardiac procedures—the incidence rises sharply to 4.1–54.9% (3). This acute change in mental status is associated not only with prolonged hospital stays and higher healthcare costs (4) but also with increased mortality and long-term cognitive decline (5–7), potentially leading to reduced quality of life, loss of independence, and heightened caregiver strain. Notably, research indicates that up to 54% of POD cases may be preventable through targeted interventions (8, 9), highlighting the critical need to identify and address modifiable risk factors. Although numerous risk factors for POD have been established, including advanced age, pre-existing cognitive impairment, depth of anesthesia, and specific surgical procedures (10–12), the role of modifiable nutritional factors remains incompletely understood. As the surgical population continues to age, recognizing risk factors and implementing effective prevention strategies has become increasingly essential.
Vitamin D deficiency (VDD) is gaining recognition as a possible driver of numerous negative health outcomes. Beyond its well-established role in bone metabolism, vitamin D exhibits significant neurosteroid properties and influences numerous neurological processes (13, 14). Recent evidence indicates that vitamin D receptors are broadly expressed throughout the central nervous system, especially in regions involved in cognitive function and behavioral regulation (15). Furthermore, VDD has been linked to various neurological conditions, including cognitive decline and dementia (16, 17). Despite the biological plausibility of the association between vitamin D status and postoperative neurological complications, the association of preoperative VDD with POD are poorly characterized. Previous studies examining this association have been limited by small sample sizes, single-institution designs, and inadequate control of confounding factors (18–20). Additionally, the potential dose-dependent effects of vitamin D levels on POD have not been adequately evaluated. In an effort to better characterize this relationship, we performed a retrospective analysis across multiple institutions using data from the TriNetX Healthcare Commercial Organizations database. We proposed that preoperative VDD independently contributes to a higher risk of POD and other complications.
2 Methods
2.1 Data sources
This study utilized data from the TriNetX database, which containing de-identified patient data from over 140 healthcare institutions. Through the TriNetX platform, researchers had access to real-time de-identified electronic health records, including demographic information, ICD-10-coded diagnoses, procedural data (CPT/ICD-10-PCS codes), medication records, laboratory test results, and clinical observations. All data were fully de-identified in accordance with the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule using standardized, certified processes before researcher access. The TriNetX platform also maintains robust data governance through secure access protocols, institutional agreements, and continuous audit trials to protect patient privacy. In accordance with the Declaration of Helsinki, this study was approved by the Institutional Review Board of Chi Mei Medical Center (approval no. 11310-E04), which also waived the requirement for informed consent.
2.2 Inclusion and exclusion criteria
We included patients aged 50 years or older who underwent musculoskeletal surgery (e.g., arthroscopy, femur and knee joint surgery, and spine surgery) requiring hospital admission. According to preoperative vitamin D status within 4 weeks before surgery, patients were classified into a VDD group (≤20 ng/mL) and a control group (≥30 ng/mL). Individuals with any vitamin D measurement >20 ng/mL in the VDD group or <30 ng/mL in the control group within 1 month before surgery were excluded. Additional exclusion criteria included pre-existing neurological or psychiatric diseases documented within 1 year before surgery (including dementia, Alzheimer’s disease, Parkinson’s disease, schizophrenia, bipolar disorders, substance use disorders, and any documented cognitive impairment), history of cerebrovascular events or brain disorders within 3 months before surgery (including stroke, intracranial hemorrhage, and other brain disorders), and recent critical illness within 1 month before surgery [including sepsis and intensive care unit (ICU) admission] (21).
2.3 Propensity score matching
The propensity score was calculated by incorporating demographic characteristics [e.g., age at index, body mass index (BMI), race, sex], preoperative comorbidities (e.g., hypertension and diabetes mellitus), and laboratory data (e.g., hemoglobin). To minimize potential confounding due to protein-bound vitamin D levels (22), serum albumin also was included in the propensity score matching process. In TriNetX, data on vitamin D supplementation, such as prescription records, dosing, and patient compliance, are often unavailable or inconsistently documented. Consequently, we did not use vitamin D supplementation status as a matching variable. Instead, we used serum 25-hydroxyvitamin D [25(OH)D] levels as the primary exposure variable, based on the rationale that measured serum concentrations more accurately reflect patients’ physiological vitamin D status than prescription data alone. In addition, while preoperative cognitive status is an important risk factor for delirium, standardized cognitive assessments are not routinely performed before surgery. Thus, we were unable to match or adjust for this variable because of low availability. A 1:1 nearest neighbor propensity score matching was conducted without replacement, applying a caliper of 0.1 standard deviations of the logit-transformed score.
2.4 Study outcomes
We defined the primary outcome as the occurrence of POD within 30 days after surgery, as indicated by ICD-10 codes F05 or R41.0. Secondary outcomes included postoperative complications, such as emergency department (ED) visits, ICU admission, and surgical site infections (ICD-10: T81.41 or ICD-10: T81.42). All outcomes were assessed from the time of surgery to the 30-day postoperative period.
2.5 Risk factors assessment
A multivariate logistic regression analysis was performed to evaluate risk factors for POD, incorporating demographic variables (age, sex, race) and preoperative comorbidities such as hypertension and diabetes mellitus. For continuous variables, such as age, odds ratios were calculated per unit increase. We performed separate analyses for the vitamin D-deficient and sufficient groups to identify potentially different risk patterns between these populations.
2.6 Additional analysis
An additional analysis was conducted on patients with vitamin D insufficiency (20–30 ng/mL) to explore a potential dose-dependent relationship. Using identical inclusion and exclusion criteria, we compared vitamin D-insufficient patients with matched controls with sufficient levels. This analysis evaluated whether the relationship between VDD and POD follows a dose-dependent pattern across deficient, insufficient, and sufficient levels.
In addition to our primary matching model, we conducted two sensitivity analyses to further validate our findings. In Model I, we restricted the analysis to patients aged ≥65 years to account for age-related vulnerability to delirium. In Model II, we included C-reactive protein (CRP), a commonly used inflammatory marker, as an additional covariate in the matching process to evaluate the role of systemic inflammation.
2.7 Statistical analysis
Mean ± standard deviation was used to summarize continuous variables; categorical variables were presented as numbers and percentages. To compare propensity score-matched groups, we calculated odds ratios (OR) with 95% confidence intervals (CI). For risk factor assessment, we conducted separate multivariate logistic regression analyses for the vitamin D-deficient and vitamin D-sufficient groups. In these analyses, we estimated the ORs for each independent variable, including continuous variables such as age, for which the OR was calculated per unit increase. We performed 1:1 nearest-neighbor matching without replacement. This approach is widely accepted as a conservative threshold to reduce bias while retaining an adequate sample size. Matching without replacement was chosen to maintain the integrity of individual matches and avoid repeated controls. To assess the adequacy of matching, we calculated standardized mean differences (SMDs) for all covariates, with values below 0.1 indicating acceptable balance. A plot of SMDs before and after matching was used to visually confirm covariate balance. Statistical analyses were carried out utilizing the TriNetX Analytics platform. A two-sided p-value of less than 0.05 was considered indicative of statistical significance across all analyses.
3 Results
3.1 Characteristics of patients
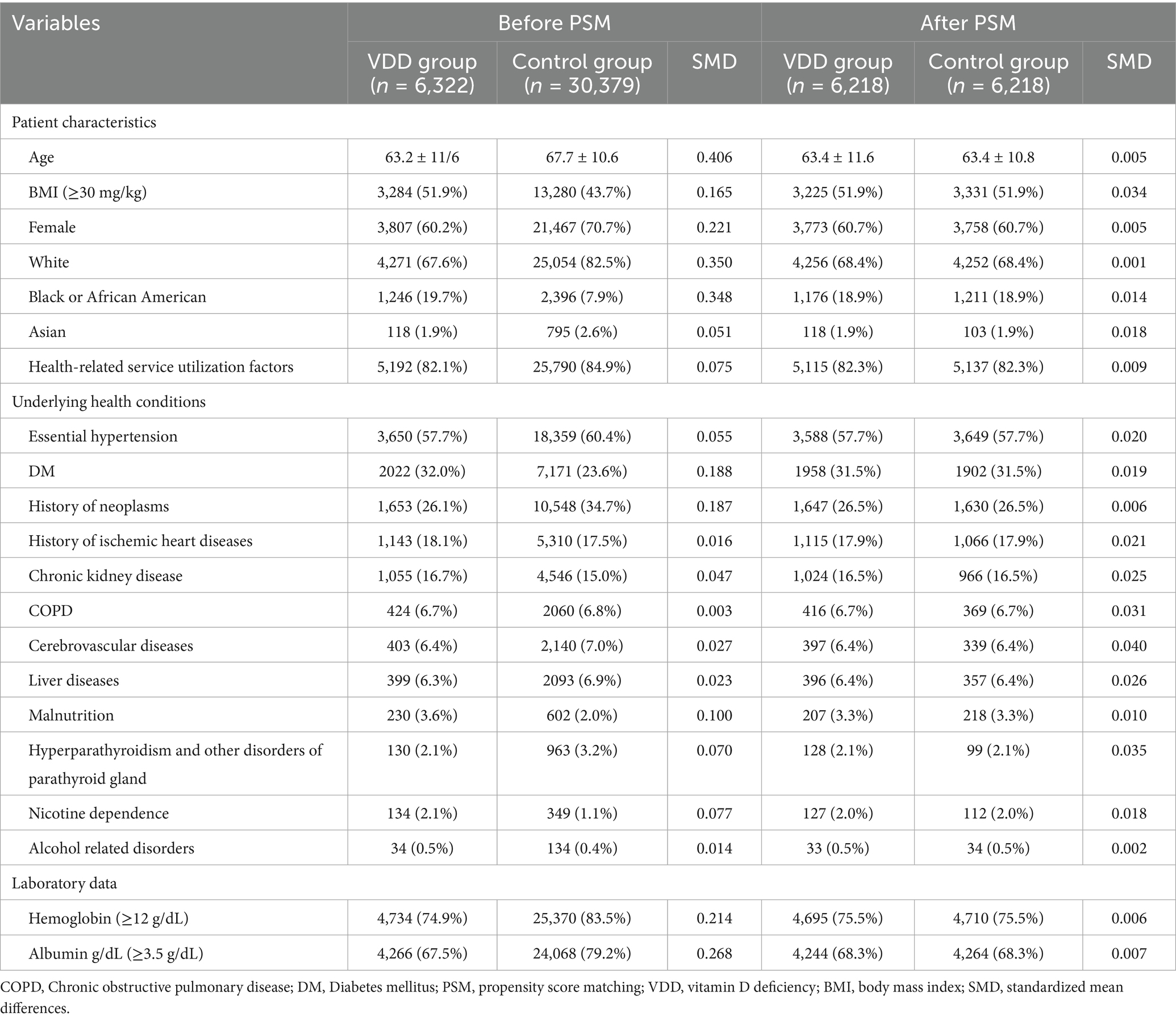
In this study, 6,322 patients with VDD and 30,379 patients without VDD were identified before matching (Figure 1). After matching, 6,218 pairs were analyzed in the study (Table 1). The balance between cohorts before and after matching was assessed using SMDs for all baseline covariates and visualized using propensity score density plots (Figure 2). The density plots demonstrate substantial improvement in the overlap between groups post-matching, indicating a good balance and appropriate use of the matching algorithm. The mean age was 63.4 years in both groups. Both groups had similar proportions of females (60.7%), and racial distribution was comparable with that of predominantly White patients (68.4%). BMI distributions were similar between the groups (51.9% vs. 51.9% with BMI ≥ 30 mg/kg). Regarding comorbidities, the matched groups showed a similar prevalence of essential hypertension (57.7%), diabetes mellitus (31.5%), neoplasms (26.5%), and ischemic heart disease (17.9%). Other clinical characteristics, including cerebrovascular diseases (6.4%), liver diseases (6.4%), and chronic obstructive pulmonary disease (6.7%), were also well balanced between groups.
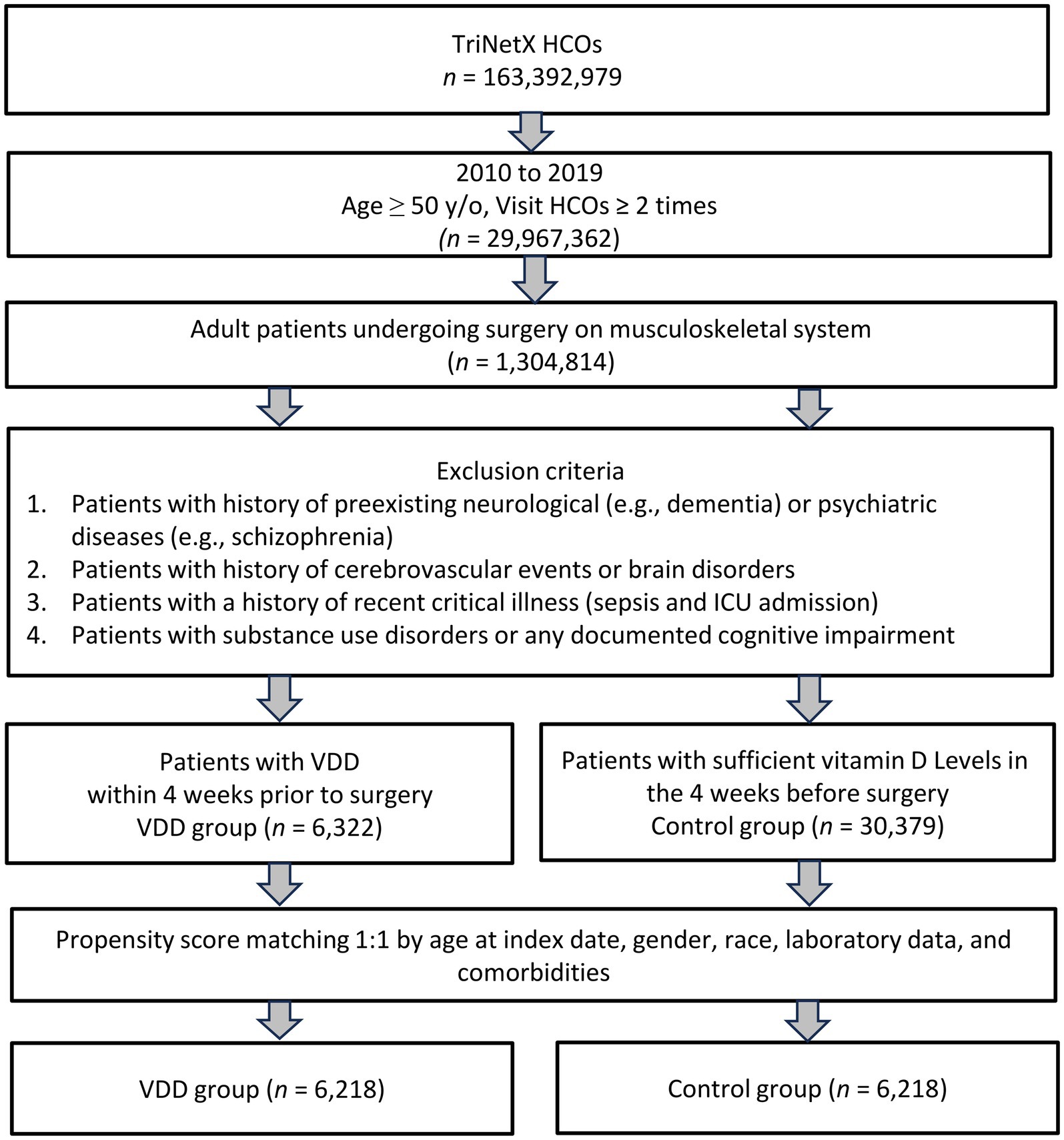
Figure 1. This flowchart illustrates the selection process for the study cohort. HCOs, Healthcare Commercial Organizations; ICU, Intensive Care Unit; PSM, Propensity Score Matching; VDD, Vitamin D Deficiency.
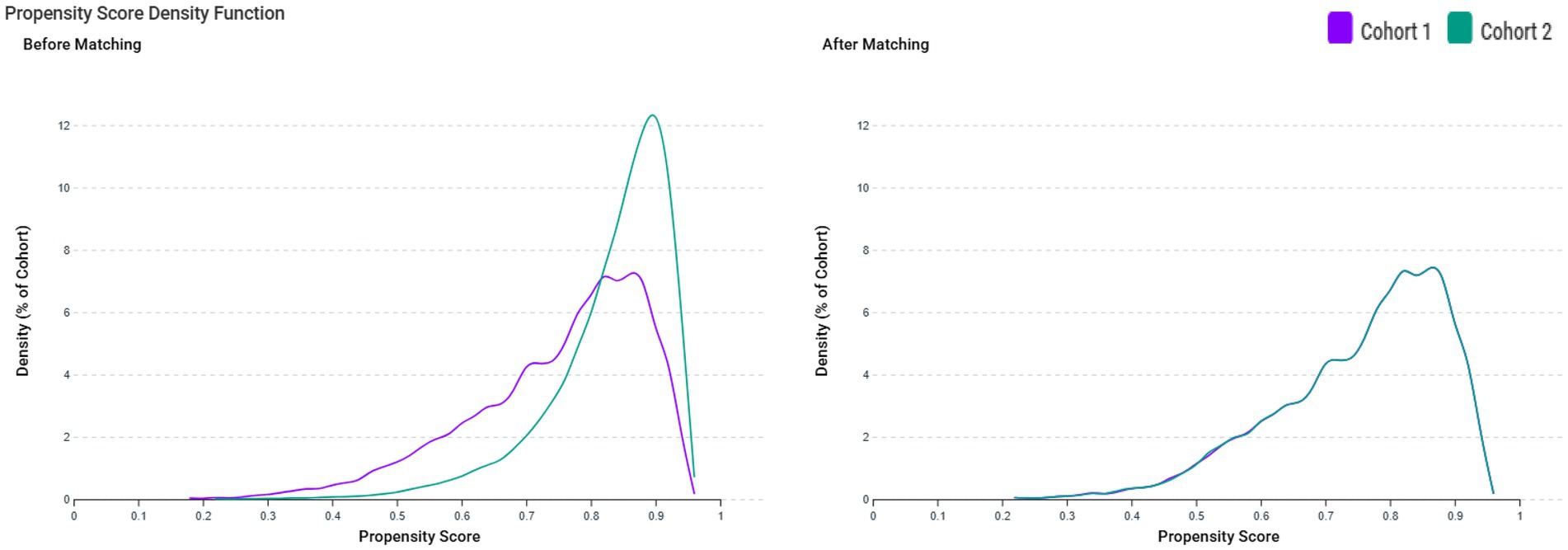
Figure 2. Propensity score density function before and after matching between patients with vitamin D deficiency (VDD) (Cohort 1, purple line) and those without VDD (Cohort 2, green line). The left panel displays the distribution of propensity scores before matching, showing a lack of overlap between the groups. The right panel shows the distribution after matching, demonstrating improved balance between the cohorts.
3.2 Postoperative clinical outcomes in individuals with VDD
The incidence of POD was significantly higher in patients with VDD (1.0%) than in patients without VDD (0.5%) (OR: 2.18, 95%CI: 1.41–3.36, p < 0.001) (Table 2). This finding indicates that VDD is related to more than double the odds of POD development. Among the secondary outcomes, we observed significant differences in healthcare utilization between the two groups. ED visits within 30 days after surgery were more frequent in vitamin D-deficient patients (7.3% vs. 5.5%, OR 1.36, 95%CI: 1.18–1.57, p < 0.001). Similarly, ICU admission risk was significantly higher in vitamin D-deficient patients (OR: 1.51, 95%CI 1.19–1.91, p < 0.001). Regarding postoperative complications, risk of surgical site infection was comparable between the two groups (OR: 0.83, 95%CI 0.42–1.65, p = 0.601).
3.3 Association of vitamin D insufficiency with postoperative outcomes
In our matched cohort analysis of 10, 764 pairs of individuals with vitamin D insufficiency, we observed several significant associations between vitamin D insufficiency and postoperative outcomes. The risk of POD was higher in patients with vitamin D insufficiency than in those with sufficient levels (OR: 1.49, 95% CI 1.05–2.12, p = 0.023) (Table 3). While this association was notable, the magnitude of the effect was less pronounced than that observed in the vitamin D-deficient group, suggesting a possible dose-dependent relationship. The analysis of healthcare utilization revealed consistent patterns in vitamin D-sufficient patients. ED visits were also more frequent among patients with vitamin D insufficiency (OR: 1.16, 95%CI:1.03–1.30, p = 0.013), as were ICU admissions (OR 1.25, 95%CI: 1.03–1.52, p = 0.026). The magnitude of the effect was less pronounced than that observed in the vitamin D-deficient group. Similarly, surgical site infection rates were identical between the two groups (OR: 1.00, 95%CI: 0.62–1.62, p = 1.000).
3.4 Sensitivity analysis
In Model I (n = 4,049 per group) (Table 4), VDD continued to show a significant association with elevated POD risk (OR: 2.09, 95%CI: 1.32–3.31, p = 0.001) and ED visits (OR: 1.26, 95%CI: 1.05–1.50, p = 0.012). The associations between ICU admission and surgical site infection were not statistically significant in this older subgroup.
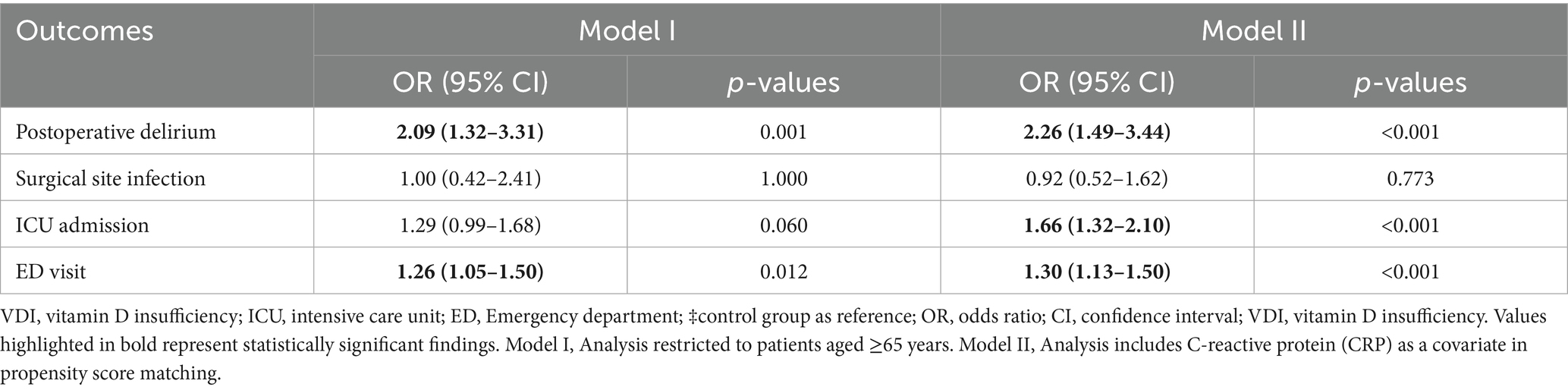
Table 4. Sensitivity analyses of 30-day postoperative outcomes in patients with vitamin D deficiency.
In Model II, which incorporated C-reactive protein (CRP) as a covariate in the matching process, VDD significantly elevated the risk of POD (OR: 2.26, 95%CI: 1.49–3.44, p < 0.001), ICU admission (OR: 1.66, 95%CI: 1.32–2.10, p < 0.001), and ED visits (OR: 1.30, 95%CI: 1.13–1.50, p < 0.001) (Table 4). Surgical site infection was not significantly associated with the studied variable (OR: 0.92, 95%CI: 0.52–1.62, p = 0.773). These findings support the robustness of our results and indicate that the observed associations persist even after accounting for advanced age and systemic inflammation.
3.5 Risk factors for POD in patients with VDD
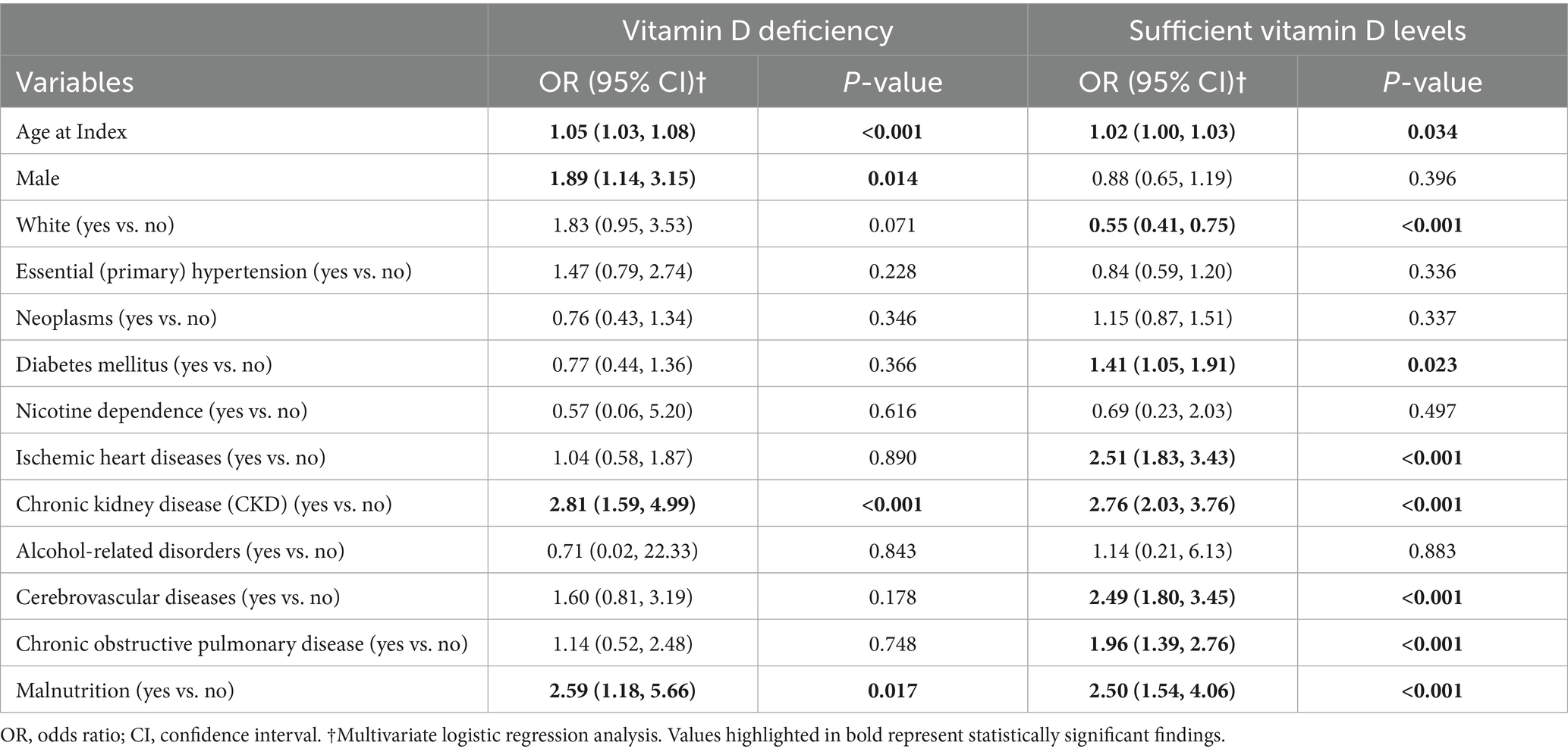
Multivariate analysis of vitamin D-deficient patients revealed several risk predictors (Table 5). Age (OR: 1.05, p < 0.001) and male sex (OR: 1.89, p = 0.014) were associated with higher risks. Among comorbidities, chronic kidney disease emerged as a strong risk factor (OR: 2.81, p < 0.001), along with malnutrition (OR: 2.59, p = 0.017).
3.6 Risk factors for POD in patients without VDD
Multivariate analysis among patients without VDD highlighted multiple independent predictors of POD (Table 5). Advanced age also revealed a significant relationship (OR: 1.02, p = 0.034), but White patients demonstrated a protective effect (OR: 0.55, p < 0.001). Among comorbidities, chronic kidney disease exhibited the strongest association (OR: 2.76, p < 0.001), followed by ischemic heart disease (OR: 2.51, p < 0.001), malnutrition (OR: 2.50, p < 0.001), and cerebrovascular disease (OR: 2.49, p < 0.001). Additional significant risk factors included chronic obstructive pulmonary disease (OR: 1.96, p < 0.001) and diabetes mellitus (OR: 1.41, p = 0.023).
4 Discussion
This large-scale, multi-institutional study revealed that preoperative VDD doubled the risk of POD compared with vitamin D-sufficient patients (1.0% vs. 0.5%). This study also demonstrated a dose-dependent relationship, as vitamin D insufficiency showed a smaller but significant increase in delirium risk (0.7% vs. 0.5%). Secondary outcomes indicated higher rates of ED visits and ICU admissions in both the vitamin D deficient and insufficient groups than in the controls, although the effect was more pronounced in the deficient group. Risk factor analysis highlighted distinct patterns across groups. Among vitamin D-deficient patients, significant predictors of POD included increasing age, male sex, chronic kidney disease, and malnutrition. In contrast, while age, CKD, and malnutrition remained risk factors in vitamin D-sufficient patients, additional predictors emerged, including ischemic heart disease, cerebrovascular disease, COPD, and diabetes mellitus.
Our findings revealed a strong link between preoperative VDD and POD, supported by both biological mechanisms and clinical evidence. Vitamin D, functioning as a neurosteroid, influences the central nervous system by helping regulate neurotransmitter activity, reduce oxidative stress, and modulate neuroinflammatory processes (23, 24). Delirium can be triggered by oxidative stress, neuroinflammation, and neurotransmitter imbalances, particularly acetylcholine deficiency and excess dopamine (25). In animal models, vitamin D reduced neuroinflammation by modulating cytokines (26) and helped restore neurotransmitter balance by boosting acetylcholine synthesis (27) and regulating dopamine levels (28). However, much of the mechanistic evidence stems from experimental or preclinical studies, and the direct relevance of these pathways in perioperative settings remains uncertain. Therefore, while these mechanisms provide a possible explanatory framework, they should be interpreted with caution and considered hypothesis generating rather than conclusive. The perioperative period is particularly vulnerable to vitamin D metabolism. Studies have shown that serum 25(OH)D levels can decrease substantially following surgery, potentially due to increased cellular uptake during tissue regeneration and impaired postoperative generation of the active form 1,25(OH)2D (29, 30). This decline in vitamin D levels, combined with the metabolic stress of surgery, may contribute to an increased risk of delirium. The dose-dependent relationship observed between vitamin D status and delirium outcomes aligns with previous findings, which suggest a dose-dependent neuroprotective effect of vitamin D (31, 32). Our findings suggest that vitamin D supplementation is a potentially modifiable factor for delirium prevention. Further research is needed to establish precise preoperative target levels and develop evidence-based supplementation protocols.
In our previous meta-analysis of 2,673 patients (33), we found that preoperative VDD significantly increased the risk of POD and cognitive dysfunction (OR = 1.54). However, the limited sample size (33) prompted us to conduct this large-scale, multi-institutional, retrospective study to further evaluate the association between vitamin D status and POD. Our current findings were largely consistent with the meta-analysis (33), demonstrating that patients with VDD experienced increased risk of POD, though with a stronger association (OR 2.18 vs. 1.54). This stronger effect size in our current study might be attributed to our larger sample size and better control for confounding factors. Interestingly, our previous meta-analysis (33) did not identify a significant association between vitamin D insufficiency and POD. This discrepancy is likely due to the limited sample size in the meta-analysis (33), which included only four studies and 1,410 patients for vitamin D insufficiency analysis, whereas our current study examined a significantly larger cohort.
The increased rates of ED visits and ICU admissions among vitamin D-deficient patients suggest that vitamin D status may have broader implications for postoperative recovery beyond cognitive outcomes. Our findings may partially align with those of a previous study of 872 patients undergoing hip fracture surgery, which reported that low vitamin D levels were associated with an increased risk of medical readmissions within 30 days (34). A notable finding was the dose-dependent relationship; while both vitamin D deficient and insufficient patients showed higher rates of ED visits and ICU admissions compared to those with sufficient levels, the magnitude of the effect was substantially larger in the deficient group. These findings suggest that low vitamin D levels may compromise postoperative recovery and increase the need for higher levels of care. Interestingly, we found no significant difference in surgical site infection rates between the groups. This contrasts with previous studies suggesting a role for vitamin D in immune function and wound healing (35, 36). The absence of a significant association may reflect the low overall rate of surgical site infections in our cohort, which likely reduced the statistical power to identify meaningful differences.
Among vitamin D-deficient patients, significant predictors of POD included increasing age, male sex, chronic kidney disease, and malnutrition. This age-related vulnerability aligns with a previous review article that consistently identified advanced age as a risk factor for POD (37). Male sex was identified as a significant risk factor for POD among vitamin D-deficient patients in the current study. This result aligns with earlier research identifying male sex as a contributing risk factor for POD (38, 39). However, in patients with sufficient vitamin D levels, male sex did not appear to be associated with an increased risk of POD in our study. This novel observation suggests that adequate vitamin D levels might help mitigate certain risk factors associated with male sex. Chronic kidney disease and malnutrition exhibited a notably strong association with delirium in both groups, indicating that these predictors were independent of vitamin D status. While previous studies have established links between these factors and POD (40, 41), our study, with its large sample size, provides robust evidence that vitamin D levels exert minimal influence on these associations. Additional risk factors, such as ischemic heart disease, cerebrovascular disease, chronic obstructive pulmonary disease, and diabetes mellitus, were identified exclusively in vitamin D-sufficient patients. This implies that VDD may alter the impact of traditional risk factors on the development of delirium.
The interpretation of these findings should take into account several notable limitations of the study. First, as a retrospective observational study, our analysis can only demonstrate associations and cannot establish causality between preoperative VDD and POD. Second, although propensity score matching was applied, key variables were unavailable in the TriNetX database, including preoperative cognitive status, perioperative vitamin D supplementation, seasonal variation, duration of surgery, and intraoperative factors, such as anesthesia depth, pain management, and medication use (e.g., anticholinergics and benzodiazepines). These unmeasured factors may have contributed to the residual confounding. Third, an additional limitation of this study is the reliance on ICD-10 diagnostic codes (F05, R41.0) to identify POD cases. Although administrative coding allows for large-scale analysis, it is subject to variability in clinical recognition and documentation practices, which may lead to underreporting or misclassification, particularly in cases of hypoactive or transient delirium. This limitation may result in the underestimation of the true incidence of POD. Validation against standardized clinical tools, such as the Confusion Assessment Method (CAM), would improve diagnostic accuracy, but such information was not available in the TriNetX database. Furthermore, the database structure prevented us from examining the relationship between specific vitamin D levels and delirium severity. Fourth, the database lacks granular information about critical perioperative factors, such as surgical duration and depth of anesthesia, which could significantly impact delirium risk. Fifth, patients with vitamin D insufficiency were excluded from the main analysis to ensure a clear comparison between well-defined deficient and sufficient groups. However, we acknowledge that excluding this intermediate group may introduce selection bias and limit generalizability. Additionally, because the data were retrospectively retrieved, information on vitamin D supplementation during the perioperative period was not available. These factors may affect the accuracy of group classification and outcome interpretation. Finally, we focused exclusively on patients undergoing musculoskeletal surgery to reduce heterogeneity and enhance internal validity. However, this narrow focus limits the generalizability of our findings to other surgical populations, such as those undergoing cardiovascular, thoracic, or abdominal procedures, which may involve different perioperative risk profiles and delirium mechanisms. Future studies should investigate whether the observed association between preoperative VDD and POD holds across diverse surgical specialties.
5 Conclusion
Our findings demonstrate that preoperative VDD is independently associated with an increased risk of POD and adverse healthcare utilization outcomes following musculoskeletal surgery. However, because this was a retrospective observational study, causal relationships could not be inferred. The dose-dependent link between vitamin D levels and postoperative complications highlights the possible value of assessing and correcting vitamin D status before surgery. The distinct risk factor profiles in patients with or without VDD highlight the need for tailored preventive strategies. Future trials should incorporate standardized delirium assessments, detailed perioperative data, and longitudinal vitamin D measurements to evaluate whether preoperative optimization of vitamin D status (e.g., vitamin D supplementation) can reduce delirium risk and improve postoperative outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of the Chi Mei Medical Center (approval no. 11310-E04), which granted a waiver for the informed consent requirement. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin in accordance with the local legislation and institutional requirements. Only de-identified data were used in the current study.
Author contributions
K-CH: Software, Investigation, Writing – original draft, Writing – review & editing, Visualization, Conceptualization, Formal analysis. T-SY: Supervision, Methodology, Writing – review & editing, Writing – original draft, Data curation. S-WL: Writing – review & editing, Writing – original draft, Software, Supervision, Validation. Y-CL: Methodology, Writing – original draft, Data curation, Resources, Writing – review & editing. P-HFu: Conceptualization, Writing – original draft, Writing – review & editing, Formal analysis, Data curation. P-HFe: Investigation, Software, Conceptualization, Writing – original draft, Writing – review & editing. I-WC: Conceptualization, Writing – original draft, Resources, Investigation, Writing – review & editing, Methodology, Data curation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yang, Q, Wang, J, Chen, Y, Lian, Q, Shi, Z, and Zhang, Y. Incidence and risk factors of postoperative delirium following total knee arthroplasty: a retrospective Nationwide inpatient sample database study. Knee. (2022) 35:61–70. doi: 10.1016/j.knee.2022.02.006
2. Memtsoudis, S, Cozowicz, C, Zubizarreta, N, Weinstein, SM, Liu, J, Kim, DH, et al. Risk factors for postoperative delirium in patients undergoing lower extremity joint arthroplasty: a retrospective population-based cohort study. Reg Anesth Pain Med. (2019) 44:934–43. doi: 10.1136/rapm-2019-100700
3. Chen, H, Mo, L, Hu, H, Ou, Y, and Luo, J. Risk factors of postoperative delirium after cardiac surgery: a meta-analysis. J Cardiothorac Surg. (2021) 16:1–11. doi: 10.1186/s13019-021-01496-w
4. Kirfel, A, Guttenthaler, V, Mayr, A, Coburn, M, Menzenbach, J, and Wittmann, M. Postoperative delirium is an independent factor influencing the length of stay of elderly patients in the intensive care unit and in hospital. J Anesth. (2022) 36:341–8. doi: 10.1007/s00540-022-03049-4
5. Huang, H, Li, H, Zhang, X, Shi, G, Xu, M, Ru, X, et al. Association of postoperative delirium with cognitive outcomes: a meta-analysis. J Clin Anesth. (2021) 75:110496. doi: 10.1016/j.jclinane.2021.110496
6. Deiner, S, and Silverstein, J. Postoperative delirium and cognitive dysfunction. Br J Anaesth. (2009) 103 Suppl 1(Suppl 1):i41–6. doi: 10.1093/bja/aep291
7. Bai, J, Liang, Y, Zhang, P, Liang, X, He, J, Wang, J, et al. Association between postoperative delirium and mortality in elderly patients undergoing hip fractures surgery: a meta-analysis. Osteoporos Int. (2020) 31:317–26. doi: 10.1007/s00198-019-05172-7
8. Inouye, SK, Bogardus, ST Jr, Charpentier, PA, Leo-Summers, L, Acampora, D, Holford, TR, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. (1999) 340:669–76. doi: 10.1056/NEJM199903043400901
9. Zhao, Q, Liu, S, Zhao, H, Dong, L, Zhu, X, and Liu, J. Non-pharmacological interventions to prevent and treat delirium in older people: an overview of systematic reviews. Int J Nurs Stud. (2023) 148:104584. doi: 10.1016/j.ijnurstu.2023.104584
10. Albanese, AM, Ramazani, N, Greene, N, and Bruse, L. Review of postoperative delirium in geriatric patients after hip fracture treatment. Geriatr Orthopaed Surg Rehabil. (2022) 13:21514593211058947. doi: 10.1177/21514593211058947
11. Park, SK, Han, DW, Chang, CH, Jung, H, Kang, H, and Song, Y. Association between intraoperative electroencephalogram burst suppression and postoperative delirium: a systematic review and meta-analysis. Anesthesiology. (2025) 142:107–20. doi: 10.1097/ALN.0000000000005255
12. Varpaei, HA, Robbins, LB, Farhadi, K, and Bender, CM. Preoperative cognitive function as a risk factor of postoperative delirium in cancer surgeries: a systematic review and meta-analysis. J Surg Oncol. (2024) 130:222–40. doi: 10.1002/jso.27730
13. Rihal, V, Kaur, A, Singh, TG, and Abdel-Daim, MM. Therapeutic and mechanistic intervention of vitamin D in neuropsychiatric disorders. Psychiatry Res. (2022) 317:114782. doi: 10.1016/j.psychres.2022.114782
14. Cui, X, and Eyles, DW. Vitamin D and the central nervous system: causative and preventative mechanisms in brain disorders. Nutrients. (2022) 14:4353. doi: 10.3390/nu14204353
15. Gáll, Z, and Székely, O. Role of vitamin D in cognitive dysfunction: new molecular concepts and discrepancies between animal and human findings. Nutrients. (2021) 13:3672. doi: 10.3390/nu13113672
16. Zhang, X-X, Wang, H-R, Meng-Wei,, Hu, Y-Z, Sun, H-M, Feng, Y-X, et al. Association of vitamin D levels with risk of cognitive impairment and dementia: a systematic review and meta-analysis of prospective studies. J Alzheimers Dis. (2024) 98:373–85. doi: 10.3233/JAD-231381
17. Chen, H, Xue, W, Li, J, Fu, K, Shi, H, Zhang, B, et al. 25-Hydroxyvitamin D levels and the risk of dementia and Alzheimer's disease: a dose–response meta-analysis. Front Aging Neurosci. (2018) 10:368. doi: 10.3389/fnagi.2018.00368
18. Velayati, A, Vahdat Shariatpanahi, M, Dehghan, S, Zayeri, F, and Vahdat Shariatpanahi, Z. Vitamin D and postoperative delirium after coronary artery bypass grafting: a prospective cohort study. J Cardiothorac Vasc Anesth. (2020) 34:1774–9. doi: 10.1053/j.jvca.2020.02.008
19. Qiu, Y, Sessler, DI, Chen, L, Halvorson, S, Cohen, B, Bravo, M, et al. Preoperative vitamin D deficiency is associated with postoperative delirium in critically ill patients. J Intensive Care Med. (2022) 37:655–62. doi: 10.1177/08850666211021330
20. Tumer, NB, Tekeli Kunt, A, Gunaydin, S, and Ozisik, K. Preoperative vitamin D level is associated with postoperative delirium after cardiac surgery in patients over 65 years of age. Heart Surg Forum. (2020) 23:E264–9. doi: 10.1532/hsf.2961
21. Zhou, Q, Zhou, X, Zhang, Y, Hou, M, Tian, X, Yang, H, et al. Predictors of postoperative delirium in elderly patients following total hip and knee arthroplasty: a systematic review and meta-analysis. BMC Musculoskelet Disord. (2021) 22:1–13. doi: 10.1186/s12891-021-04825-1
22. Ghosh, A, Prabhu, M, Prabhu, K, Changani, M, Patel, N, and Belle, VS. Vitamin D as a biomarker in predicting sepsis outcome at a tertiary care hospital. Asian J Med Sci. (2023) 14:89–94.
23. Menéndez, SG, and Manucha, W. Vitamin D as a modulator of neuroinflammation: implications for brain health. Curr Pharm Des. (2024) 30:323–32. doi: 10.2174/0113816128281314231219113942
24. Ali, A, Shah, SA, Zaman, N, Uddin, MN, Khan, W, Ali, A, et al. Vitamin D exerts neuroprotection via SIRT1/nrf-2/ NF-kB signaling pathways against D-galactose-induced memory impairment in adult mice. Neurochem Int. (2021) 142:104893. doi: 10.1016/j.neuint.2020.104893
25. Maldonado, JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. (2013) 21:1190–222. doi: 10.1016/j.jagp.2013.09.005
26. de Oliveira, LRC, Mimura, LAN, Fraga-Silva, TFC, Ishikawa, LLW, Fernandes, AAH, Zorzella-Pezavento, SFG, et al. Calcitriol prevents neuroinflammation and reduces blood-brain barrier disruption and local macrophage/microglia activation. Front Pharmacol. (2020) 11:161. doi: 10.3389/fphar.2020.00161
27. Rastegar-Moghaddam, SH, Hosseini, M, Alipour, F, Rajabian, A, and Ebrahimzadeh Bideskan, A. The effects of vitamin D on learning and memory of hypothyroid juvenile rats and brain tissue acetylcholinesterase activity and oxidative stress indicators. Naunyn Schmiedeberg Arch Pharmacol. (2022) 395:337–51. doi: 10.1007/s00210-021-02195-y
28. Bayo-Olugbami, A, Nafiu, AB, Amin, A, Ogundele, OM, Lee, CC, and Owoyele, BV. Vitamin D attenuated 6-OHDA-induced behavioural deficits, dopamine dysmetabolism, oxidative stress, and neuro-inflammation in mice. Nutr Neurosci. (2022) 25:823–34. doi: 10.1080/1028415X.2020.1815331
29. Binkley, N, Coursin, D, Krueger, D, Iglar, P, Heiner, J, Illgen, R, et al. Surgery alters parameters of vitamin D status and other laboratory results. Osteoporos Int. (2017) 28:1013–20. doi: 10.1007/s00198-016-3819-9
30. Blomberg Jensen, M, Husted, H, Bjerrum, PJ, Juul, A, and Kehlet, H. Compromised activation of vitamin D after elective surgery: a prospective pilot study. JBMR Plus. (2018) 2:281–8. doi: 10.1002/jbm4.10053
31. AlJohri, R, AlOkail, M, and Haq, SH. Neuroprotective role of vitamin D in primary neuronal cortical culture. eNeurol Sci. (2019) 14:43–8. doi: 10.1016/j.ensci.2018.12.004
32. Farghali, M, Ruga, S, Morsanuto, V, and Uberti, F. Can brain health be supported by vitamin D-based supplements? A critical review. Brain Sci. (2020) 10:660. doi: 10.3390/brainsci10090660
33. Hung, K-C, Wang, L-K, Lin, Y-T, Yu, C-H, Chang, C-Y, Sun, C-K, et al. Association of preoperative vitamin D deficiency with the risk of postoperative delirium and cognitive dysfunction: a meta-analysis. J Clin Anesth. (2022) 79:110681. doi: 10.1016/j.jclinane.2022.110681
34. Ingstad, F, Solberg, LB, Nordsletten, L, Thorsby, PM, Hestnes, I, and Frihagen, F. Vitamin D status and complications, readmissions, and mortality after hip fracture. Osteoporos Int. (2021) 32:873–81. doi: 10.1007/s00198-020-05739-9
35. Ismailova, A, and White, JH. Vitamin D, infections and immunity. Rev Endocr Metab Disord. (2022) 23:1. doi: 10.1007/s11154-021-09679-5
36. Lin, J, Mo, X, Yang, Y, Tang, C, and Chen, J. Association between vitamin D deficiency and diabetic foot ulcer wound in diabetic subjects: a meta-analysis. Int Wound J. (2023) 20:55–62. doi: 10.1111/iwj.13836
37. Bramley, P, McArthur, K, Blayney, A, and McCullagh, I. Risk factors for postoperative delirium: an umbrella review of systematic reviews. Int J Surg. (2021) 93:106063. doi: 10.1016/j.ijsu.2021.106063
38. Wang, H, Guo, X, Zhu, X, Li, Y, Jia, Y, Zhang, Z, et al. Gender differences and postoperative delirium in adult patients undergoing cardiac valve surgery. Front Cardiovasc Med. (2021) 8:751421. doi: 10.3389/fcvm.2021.751421
39. Sadeghirad, B, Dodsworth, BT, Gelsomino, NS, Goettel, N, Spence, J, Buchan, TA, et al. Perioperative factors associated with postoperative delirium in patients undergoing noncardiac surgery: an individual patient data meta-analysis. JAMA Netw Open. (2023) 6:e2337239-e. doi: 10.1001/jamanetworkopen.2023.37239
40. Adogwa, O, Elsamadicy, AA, Sergesketter, A, Oyeyemi, D, Galan, D, Vuong, VD, et al. The impact of chronic kidney disease on postoperative outcomes in patients undergoing lumbar decompression and fusion. World Neurosurg. (2018) 110:e266–70. doi: 10.1016/j.wneu.2017.10.147
Keywords: postoperative delirium, vitamin D deficiency, musculoskeletal surgery, propensity score matching, cognitive dysfunction, perioperative risk factors
Citation: Hung K-C, Yu T-S, Liao S-W, Lai Y-C, Fu P-H, Feng P-H and Chen I-W (2025) Preoperative vitamin D deficiency and postoperative delirium risk: multicenter retrospective study. Front. Nutr. 12:1617670. doi: 10.3389/fnut.2025.1617670
Edited by:
Song Qiao, Zhejiang Hospital, ChinaReviewed by:
Silvio Pires Gomes, University of São Paulo, BrazilHongjian Pu, University of Pittsburgh, United States
Anirban Ghosh, AIIMS, India
Copyright © 2025 Hung, Yu, Liao, Lai, Fu, Feng and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: I-Wen Chen, Y2hlbml3ZW5hNjA5MTJAZ21haWwuY29t
 Kuo-Chuan Hung
Kuo-Chuan Hung Ting-Sian Yu3
Ting-Sian Yu3 Yi-Chen Lai
Yi-Chen Lai