- 1College of Physical Education, Shanghai University, Shanghai, China
- 2College of Physical Education, Shanghai Normal University, Shanghai, China
- 3Exercise Biological Center, China Institute of Sport Science, Beijing, China
Anthocyanins, a class of polyphenol flavonoids widely present in various fruits and vegetables, have attracted significant attention due to their potent anti-inflammatory, antioxidant, and anti-aging properties. Recent studies indicate that anthocyanins may play important roles in extending life and preventing or treating age-related diseases. This review systematically summarizes the chemical characteristics of anthocyanins and their potential roles in age-related diseases, including lifespan extension, neurodegenerative diseases, skeletal diseases, cardiovascular diseases, cancer, and metabolic syndrome. Furthermore, we explore the effects of anthocyanins on age-related diseases and their potential mechanisms of action to establish a theoretical foundation for future clinical applications.
1 Introduction
Aging is a natural process where the ability of organisms to adapt both physically and mentally to their environment gradually decreases, ultimately resulting in death. Aging drives the development of various diseases related to old age. Common age-related diseases include cardiovascular disease (CVD) (1), neurodegenerative disease (2), cancer (3), metabolic syndrome (4) and bone diseases (5). These conditions can diminish the quality of life for older adults and impose a significant economic burden on families and society. Thus, creating new and effective anti-aging strategies to reduce or delay age-related diseases and improve the quality of life for older adults is a crucial public health challenge that must be addressed. Strong evidence from both animal and human studies shows a clear but complex link between nutrition and aging (6–8). In recent years, numerous studies have investigated the anti-aging effects of nutritional strategies, such as antioxidant nutrient supplementation, which helps reduce health risks and promote healthy aging.
Vegetables and fruits, abundant in polyphenolic compounds, have been shown to effectively extend lifespan and reduce the risk of age-related diseases (9, 10). Anthocyanins (ACNs), a class of water-soluble plant pigments classified as flavonoids, are abundantly found in numerous fruits and vegetables such as blueberries, blackberries, red grapes, and purple cabbage. ACNs provide vibrant coloration to plants and exhibit various biological properties, such as anti-inflammatory, antioxidant, and antitumor activities. Moreover, the molecular structure of anthocyanins, which includes conjugated cyclic systems and hydroxyl substituents-especially catechol moieties-confers potent antioxidant capacity (11). Recent clinical and experimental studies show that anthocyanins can extend lifespan and help prevent or alleviate various age-related diseases, including neurodegenerative diseases, cardiovascular disorders, metabolic syndrome, bone diseases, and cancer. Therefore, understanding the therapeutic effects and underlying mechanisms of anthocyanins in age-related diseases has significant scientific and clinical implications.
2 Chemical properties of anthocyanins
Anthocyanins are important natural pigments found in plants, classified as flavonoids. They have a unique structure known as a benzopyran skeleton, which consists of a benzene ring attached to a pyran ring. The structure of anthocyanins greatly affects their stability, solubility, and bioavailability, which in turn influences their use in food, pharmaceuticals, and nutraceuticals. Currently, over 650 different anthocyanin compounds have been identified in plants. These compounds can be categorized into six main aglycone variants based on their substituent patterns: Pelargonidin (Pg), Cyanidin (Cy), Delphinidin (Dp), Peonidin (Pn), Malvidin (Mv), and Petunidin (Pt) (12). Anthocyanins are mainly found in a variety of fruits, vegetables, and some flowers, such as blueberries, blackberries, red grapes, purple cabbage, and purple sweet potatoes. The chemical structure of anthocyanins contains multiple hydroxyl and carboxyl groups, functional moieties that confer potent antioxidant capacity and bioactivity. Studies, both in vivo and in vitro, have shown that anthocyanins have various biological functions, including antioxidant, anti-inflammatory, anti-aging, antimicrobial, anti-tumor, hypoglycemic, vision-protective, and immunomodulatory effects (13–15). In addition, more and more studies have shown that anthocyanins have important roles in prolonging life span and the prevention or treatment of aging-related diseases, including cardiovascular diseases (16), neurodegenerative diseases (17), metabolic diseases (18), skeletal diseases (19), cancer (20) and eye-related diseases (21).
3 Anthocyanins and lifespan extension
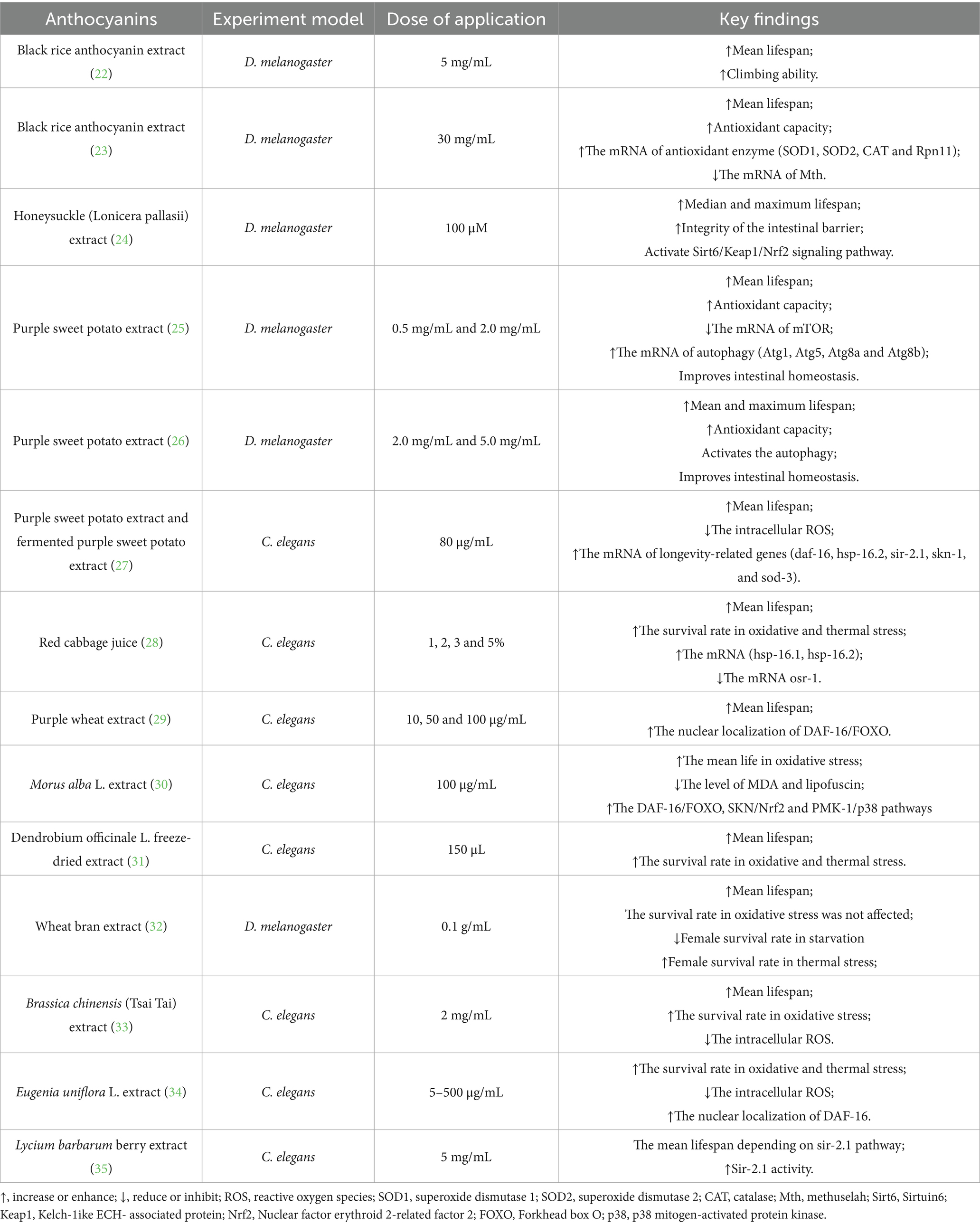
Extending lifespan is a key objective of anti-aging research and serves as a crucial indicator of its effectiveness. One of the mechanisms of aging is the excessive accumulation of oxygen radicals, which leads to oxidative damage. Studies have shown that anthocyanins have antioxidant biological activity and can prolong the life span of Drosophila and Caenorhabditis elegans (C. elegans). For example, studies indicate that black rice anthocyanins extract (BRAE) can extend Drosophila lifespan by 20% while also delaying the loss of motor function (22). Additionally, Zuo et al. reported an increase in Drosophila lifespan of 14% due to BRAE (23). The proposed mechanism indicates that BRAE may enhance the mRNA levels of CuZnSOD (SOD1), MnSOD (SOD2), catalase (CAT), and Rpn11 in fruit flies, while simultaneously downregulating the mRNA level of methuselah (Mth). This modulation strengthens the antioxidant system and contributes to lifespan extension in fruit flies (23). Honeysuckle (Lonicera pallasii) extract is an excellent source of anthocyanins. Studies have shown that 100 μM honeysuckle extract, by activating the silent information regulator 6 (Sirt 6)/Lelch-like ECH-associated protein 1 (Keap 1)/nuclear factor-erythroid 2-related factor 2 (Nrf2) signaling pathway, can increase the lifespan of Drosophila melanogaster by 8%. The integrity of the intestinal barrier increased by 4%; inhibition of Sirt-6 expression blocked the effect of honeysuckle extract on lifespan extension in Drosophila melanogaster (24). Furthermore, purple sweet potato extract (PSPE) is not only rich in anthocyanins but also exhibits greater stability compared to anthocyanins found in other plants, such as blueberries and cranberries. Studies have shown that PSPE activates the autophagy pathway by increasing the activity of antioxidant enzymes and inhibiting the mammalian target of rapamycin (mTOR) pathway, improving intestinal homeostasis and mitigating intestinal barrier dysfunction, thus extending the lifespan of Drosophila (25, 26).
Moreover, anthocyanins can also effectively improve the lifespan of C. elegans. Studies show that PSPE improves the antioxidant enzyme activity in C. elegans and reduces malondialdehyde, reactive oxygen species (ROS), and lipofuscin accumulation. This leads to a 26.7% increase in their lifespan. In contrast, fermented PSPA extends their lifespan by 37.5% (27). The primary component of red cabbage anthocyanins, cyanidin-3-diglycoside-5-glucoside (CY3D5G), exhibits antioxidant activity. The study demonstrated that the derivatives of red cabbage CY3D5G (RCJ) significantly increased the survival rate and average lifespan of C. elegans under oxidative and heat stress, with improvements of 171.63, 31.64, and 28.16%, respectively. The life-prolonging effect of RCJ may be related to the heat shock transcription factor pathway, deacetylase signaling pathway and calmodulin kinase II pathway (28). Alternatively, Chen et al. showed that anthocyanin-rich purple wheat has a lifespan-extending effect, partially dependent on the activation of DAF-16/FOXO transcription factors (29). Similarly, the nutrients from mulberry anthocyanin extract (MAE) can effectively prolong the longevity of paraquat-damaged C. elegans by inhibiting mitogen-activated protein kinase (MAPK)/Nrf2 signaling in vitro (30). Moreover, other natural compounds such as wheat bran, Dendrobium officinale flower, extracts of Tsai Tai, purple pitanga fruit, lycium barbarum extracts have been shown to effectively extend the life span, but the specific mechanism needs to be further explored (31–35). In summary, these studies underscore the crucial role of anthocyanins in promoting healthy aging. Anthocyanins play a key role in delaying aging and improving lifespan by activating autophagy, inhibiting oxidative stress, and improving intestinal homeostasis, providing new perspectives for future aging research (Table 1).
4 Role of anthocyanin in age-related diseases
4.1 Anthocyanins and neurodegenerative diseases
Aging is the primary risk factor for neurodegenerative diseases, particularly Alzheimer’s Disease (AD), Parkinson’s Disease (PD), and Amyotrophic Lateral Sclerosis (ALS), all of which become more prevalent with age (36). Recent studies have shown that anthocyanins and anthocyanin-rich extracts can alleviate the cognitive deficits associated with PD, AD, and ALS.
4.1.1 Anthocyanins and Alzheimer’s disease
Alzheimer’s disease (AD) is a common and severe neurodegenerative disorder related to aging, marked by cognitive decline and synaptic dysfunction. Currently, about 50 million people aged 65 and older have Alzheimer’s disease (AD) worldwide, and this number is expected to triple by 2050 (37). Human studies have shown that consuming 200 milliliters of cherry juice daily for 12 weeks significantly enhances language fluency, short-term memory, and long-term memory in older adults aged 70 and above with mild to moderate dementia (38). Supplementation with anthocyanin-rich blueberry concentrate (30 mL/day for 12 weeks) may not only improve brain perfusion and activation in brain areas associated with cognitive function in healthy older adults (39), but also enhance neural activation in patients with mild cognitive impairment and strength neural responses during working memory challenges in older adults with cognitive decline (40). Animal studies have also shown that anthocyanin-rich blackcurrant extract (3% anthocyanin for 9 weeks) also improves long-term recognition memory and normalized anxiety levels in senescence-accelerated mouse prone 8 (SAMP 8) mice (41). Additionally, mulberry extract (0.18 and 0.9% mulberry extract for 12 weeks) reduces brain β-amyloid levels and improves learning and memory in SAMP 8 mice (42). These studies indicate that anthocyanins can effectively address age-related cognitive decline and may serve as a promising compound for preventing and treating Alzheimer’s disease.
The brain is particularly vulnerable to oxidative stress, as previous studies indicate that reactive oxygen species (ROS) levels are significantly elevated in the brains of Alzheimer’s disease (AD) patients and animal models (43). Mechanistic studies on the neuroprotective effects of anthocyanins indicate that Korean black bean anthocyanin (12 mg/kg/day for 30 days) regulates the phosphorylated phosphatidylinositol 3-kinase (p-PI3K)/protein kinase B (Akt)/glycogen synthase kinase 3β (GSK3β) pathway, thereby reducing ROS levels and oxidative stress in the brains of APP/PS1 transgenic mice, which improves cognitive function in these AD models. In vitro experiments have also shown that anthocyanins mitigate neurotoxicity induced by amyloid β oligomers (AβO) through the PI3K/Akt/Nrf2 signaling pathway (44). In addition, anthocyanins-containing PEG-AuNPs (12 μg/g/day for 14 days) also modulated the p-PI3K/p-Akt/p-GSK3β pathway, thereby inhibiting the hyperphosphorylation of tau at serine 413 and 404 and apoptosis of neurons in the brains of mice injected with Aβ1-42 (45). Excessive neuroinflammation is directly related to the development of AD, and microglia are the main effectors of neuroinflammation (46). Supplementing with bilberry anthocyanins (20 mg/kg/day for 3 months) can activate astrocytes and microglia, and improve their phagocytic function of beta amyloid plaques in APP/PSEN1 mice (47). Activation of c-Jun N-terminal kinase (JNK) in the brain can stimulate microglia and increase the expression of proinflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and monocyte chemoattractant protein-1 (MCP-1) (48), anthocyanin supplementation in LPS treated mice inhibited JNK activation and reduced the expression of nuclear factor kappa-B (NF-κB), TNF-α and interleukin-1beta (IL-1β). In addition, anthocyanins also reduced neuroinflammatory markers in Aβ1-42-induced mouse model by inhibiting the p-JNK/NF-κB/p-GSK3β pathway (49). High-fat diet is an important risk factor for inducing neurodegenerative diseases (50). Anthocyanin supplementation (4% blueberry diet for 5 months) was able to reverse some of the behavioral deficits in high-fat diet-induced mice, particularly object recognition memory (51). The neuroprotective effects of anthocyanins may be related to attenuated microglial activation and increased neuroplasticity (52). Anthocyanin supplementation (100 mg/kg/day for 20 weeks) could also further block oxidative stress by improving AMPK-mediated autophagy, restore brain-derived neurotrophic factor protein levels in the hippocampus of mice on a high-fat diet, and ultimately inhibit hippocampal cell apoptosis and ameliorate cognitive deficits (53). Anthocyanins (700 mg/kg/day for 20 weeks) can also alleviate high-fat diet-induced neuroinflammation by inhibiting extracellular signal-regulated kinases, JNK, p38, and NF-κB activation (54). In summary, both animal studies and randomized clinical trials demonstrate that anthocyanins enhance cognition and neuroprotection. The mechanisms underlying these neuroprotective benefits are linked to anthocyanins’ ability to reduce oxidative stress, inflammation, and apoptosis in the brain. To fully realize the neuroprotective effects, further research should determine the best dose and frequency of anthocyanins for human use (Table 2).
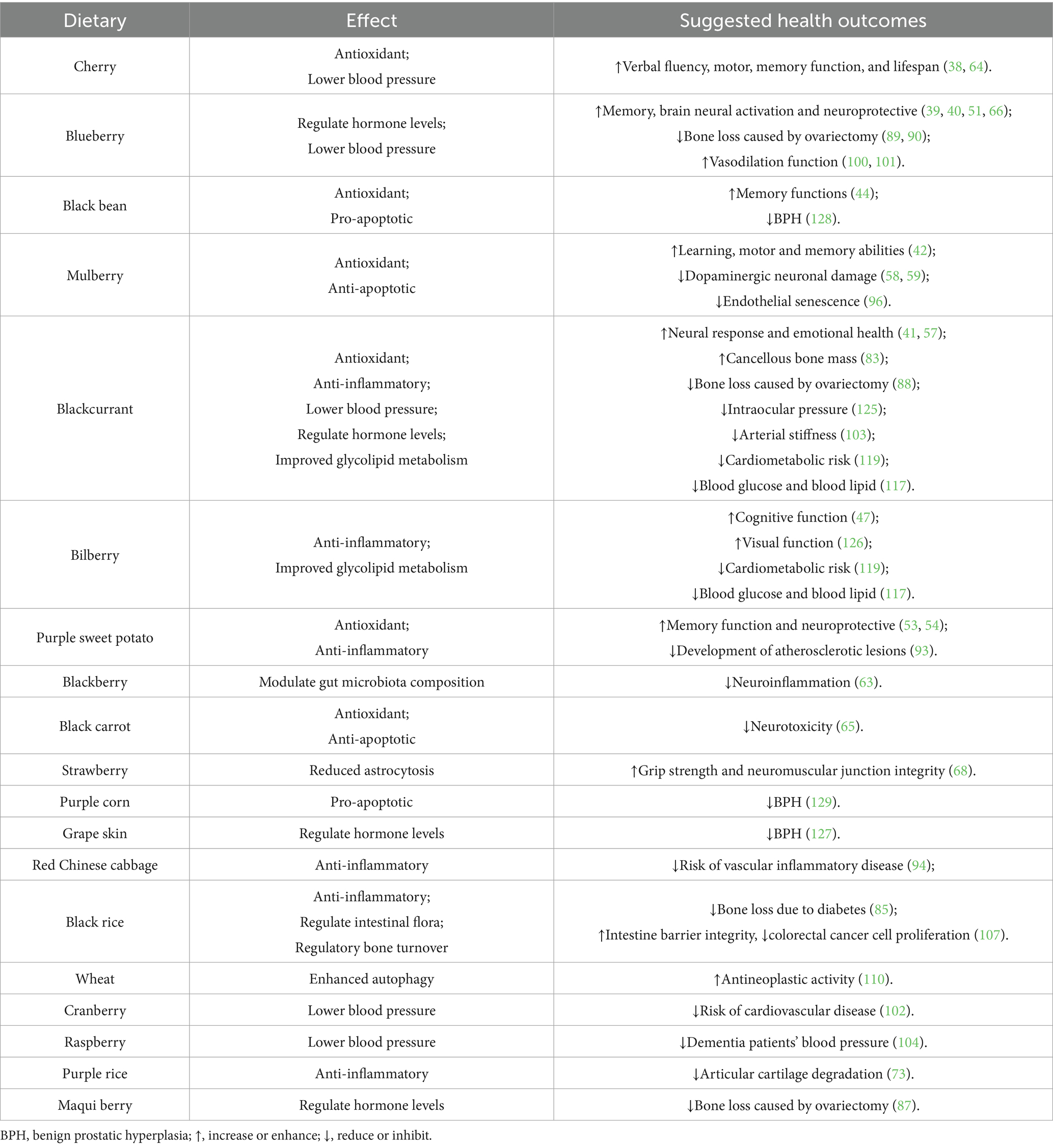
Table 2. Common dietary sources of anthocyanins and health outcomes associated with aging-related diseases.
4.1.2 Anthocyanins and Parkinson’s disease
Aging significantly increases the risk of developing Parkinson’s disease (PD), with prevalence rising from age 50 to 80. The pathogenesis of PD is diverse, including α-synuclein misfolding and aggregation, oxidative stress, mitochondrial dysfunction, and neuroinflammation (55). Current treatments for PD are limited; common medications provide only symptom relief and often come with significant side effects. Human studies have shown that dietary anthocyanins can effectively reduce mortality risk and have a positive impact on the mood of patients with PD (56, 57). The main lesions in Parkinson’s disease are the midbrain substantia nigra (SN) and the striatum, accompanied by degeneration and death of dopaminergic neurons. 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induces the death of specific dopaminergic neurons. Mulberry (Morus alba L.) extract (500 mg/kg/day for 15 days) can mitigate this cell death, reduce pro-apoptotic protein levels, and alleviate symptoms of Parkinson’s disease (58). A similar study also suggests that the mulberry (Morus alba L.) extract (250 mg/kg/day for 38 days) significantly inhibited the expression of Lewy body α-synuclein and ubiquitin, which are induced by MPTP (59). The injection of 6-hydroxydopamine (6-OHDA) leads to oxidative damage in neurons, which is associated with the death of neurons in Parkinson’s disease (60). Pelargonidin supplementation (20 mg/kg 1 day before and on the day of surgery) significantly increased the number of dopaminergic neurons in the substantia nigra, reduced lipid peroxidation levels, and improved motor function in rats that were injected with 6-OHDA (61). Some studies indicate that neurodegeneration in Parkinson’s disease (PD) is linked to gastrointestinal dysregulation. Additionally, anthocyanin supplementation exhibits neuroprotective effects in PD mice (10, 20, 40 mg/kg/day for 4 weeks) (62) and high-fat diet-induced obese rats (25 mg/kg/day for 17 weeks) (63) by modulation the composition and metabolism of gut microbiota. Moreover, other foods rich in anthocyanins, including sweet cherries (64), black carrot (65), blueberries and grape seed (66), may alleviate PD symptoms by providing antioxidant benefits, preventing cell death, and improving mitochondrial function. These studies indicate that anthocyanins could be a promising new element of treatment strategy for PD, requiring further investigation in clinical trials (Table 2).
4.1.3 Anthocyanins and amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is a severe neurodegenerative disease characterized by the progressive degeneration and death of motor neurons. While current research on anthocyanins in ALS remains limited, preliminary evidence from animal models suggests potential protective effects on ALS. For instance, anthocyanin-derived metabolites such as protocatechuic acid (100 mg/kg after onset until death, 1 time/day, 5 times/week) (67) and anthocyanin-enriched strawberry extract (2 mg/kg/day after 60 days of age until death) (68), were shown to attenuate spinal cord astrogliosis, inhibit motor neuron apoptosis, and preserve neuromuscular junction integrity in SOD1 mutant mice—a widely used ALS model. These interventions reportedly delayed disease progression, improved motor performance, and extended survival in preclinical settings. However, no recent studies have further explored anthocyanins’ therapeutic mechanisms or translational potential in ALS, nor have clinical trials investigated their efficacy in human patients. The scarcity of research highlights a critical gap in understanding how dietary polyphenols might intersect with ALS pathophysiology. Future studies should prioritize (1) validating these findings in additional ALS models (e.g., TDP-43 or C9orf72-related models), (2) elucidating gut-brain axis interactions, and (3) assessing bioavailability and dosing regimens for clinical translation (Table 2).
4.2 Anthocyanins and bone diseases
4.2.1 Anthocyanins and osteoarthritis
Osteoarthritis (OA) is a chronic disease primarily affecting the elderly. A global study showed that there were about 300 million cases of hip and knee OA in 2017 (69). As the elderly population increases, osteoarthritis has emerged as a serious disease that affects their quality of life. The main pathological mechanism of OA is the degradation of the articular cartilage matrix, whose formation is related to cellular senescence, aging-related mitochondrial dysfunction, and oxidative stress (70). Additionally, both malvidin and pelargonidin can alleviate inflammation, cartilage degradation, and pain in OA by inhibiting the NF-κB pathway (71). Drugs for osteoarthritis (OA) can have several side effects, while nutritional health products are seen as an effective alternative for protecting and enhancing bone health (72). Studies have shown that anthocyanins improve OA symptoms by inhibiting inflammatory responses and the degradation of articular cartilage. For example, the anthocyanin in purple rice (6.25–50 μg/mL for 35 days) can reduce articular chondrocyte damage by inhibiting IL-1β-induced matrix metalloproteinase expression, which is closely related to the NF-κB and ERK/MAPK signaling pathways (73). Additionally, malvidin (74) and pelargonidin (75) can enhance the inflammatory response, reduce cartilage degradation, and alleviate pain in OA by inhibiting the NF-κB pathway. Research indicates that Sirtuin 6 improves chondrocyte aging and slows the progression of osteoarthritis (OA) (76). Cyanidin administration (50 mg/kg/day for 8 weeks), both in vivo and in vitro, enhances Sirt6 activity and inhibits the NF-κB signaling pathway. It also prevents IL-1β-induced degradation of the extracellular matrix (ECM) and reduces the inflammatory response in human OA chondrocytes. Additionally, it mitigates proteoglycan loss and cartilage damage caused by destabilization of the medial meniscus (DMM) in OA mice (77). Clinical studies indicate that consuming foods rich in anthocyanins can help balance immune markers in patients with osteoarthritis, thereby reinforcing the potential of anthocyanins as an additional therapeutic strategy (78). Therefore, anthocyanins can reduce OA symptoms and improve patients’ quality of life (Table 2).
4.2.2 Anthocyanins and osteoporosis
Osteoporosis is a disease marked by low bone mass and changes in bone microstructure, leading to increased fragility and susceptibility to fractures, which adversely impacts patients’ quality of life (79). Advanced age is a major risk factor for chronic diseases. Hormonal imbalances that occur with age lead to dysfunction of osteoclasts and osteoblasts, oxidative stress, and chronic inflammation, all of which significantly contribute to the development of osteoporosis (80, 81). Anthocyanins are known for their anti-inflammatory, anti-oxidative, and anti-apoptotic effects. Studies suggest that anthocyanin-rich foods can improve bone remodeling biomarkers in middle-aged and elderly people, indicating their potential role in osteoporosis management (82). Sakaki et al. found that blackcurrant diet (a standard chow diet with 1% (w/w) anthocyanin for 4 months) improved cancellous bone mass loss in young mice by increasing glutathione peroxidase (GPX) activity in the humerus. However, this diet only modestly reduced TNF-α expression in older mice, with no significant effect on cancellous bone mass. This suggests that early administration of anthocyanins may help prevent age-related bone loss (83). Osteoporosis involves a gradual decline in osteoblasts and increased bone resorption by osteoclasts. Cyandin-3-glucoside plays a role in regulating osteoblast differentiation via the ERK1/2 signaling pathway (84). Osteoporosis related to diabetes is a systemic endocrine metabolic bone disease characterized by reduced bone density and destruction of bone microstructure. Studies show that anthocyanins in black rice extract (0.5, 1.0 and 2.0 g/kg/day for 8 weeks) can improve bone loss in diabetes rats by inhibiting bone turnover and bone marrow fat production, and up regulating the ratio of RUNX2 and OPG/RANKL in bone tissue of diabetes rats (85). Decreased estrogen levels are the primary cause of bone loss in postmenopausal women, with more than 30% of them affected by osteoporosis (86). Studies have demonstrated that supplements containing anthocyanins from blueberries, blackcurrants, or maqui berries can reduce bone loss induced by ovariectomy (87–90). This finding suggests that anthocyanins may help alleviate osteoporosis in postmenopausal women; however, the exact mechanism of action remains unclear and requires further investigation. Thus, anthocyanins, as natural bioactive compounds, may offer innovative strategies for preventing and treating osteoporosis (Table 2).
4.3 Anthocyanins and cardiovascular diseases
4.3.1 Effects of anthocyanins on endothelial function
Aging is a complex biological process, and epidemiological studies prove that aging is an independent risk factor leading to the occurrence of cardiovascular diseases. As people age, the heart transitions from compensatory adaptation to maladaptation, resulting in cardiac hypertrophy, changes in left ventricular diastolic function and contractile reserve, increased arterial stiffness, and impaired endothelial function (1). Cardiac dysfunction due to aging can lead to various cardiovascular diseases, including atherosclerosis, hypertension, and dyslipidemia. Atherosclerosis is a chronic and progressive vascular disease that is a precursor of an ischemic heart attack. The initial stage of atherosclerotic lesion development involves the activation of endothelial cells. Activated endothelial cells release the inflammatory mediator MCP-1 into the bloodstream and express adhesion molecules (ICAM-1 and VCAM-1) to attract circulating monocytes and other immune cells to the site of oxidized low-density lipoprotein accumulation (91, 92). Oral administration of anthocyanins has been recognized as a therapeutic option for managing cardiovascular disease. Research indicates that purple sweet potato, red Chinese cabbage (93, 94), and protocatechuic acid (95) can reduce plasma VCAM-1 levels and inhibit the expression of adhesion molecules on arterial endothelial surfaces. Furthermore, daily intake of an extract high in Chinese cabbage anthocyanins (150 and 300 mg/kg/day for 12 weeks) can lower inflammatory cytokines and adhesion molecule levels, thus preventing plaque buildup in the arteries of hyperlipidemic mice (94). This suggests that anthocyanins suppress inflammation and alleviate the progression of atherosclerosis. Furthermore, Cyanidin-3-O-β-glucoside (100, 200 and 300 mg/kg for 8 weeks) enhances endothelial nitric oxide synthase phosphorylation and preserves nitric oxide availability, promoting endothelial cell migration and survival (96, 97). Cyanidin-3-O-β-glucoside (0.2% C3G for 6 weeks) also enhances the function of endothelial progenitor cells and promotes endothelial repair, thereby slowing atherosclerosis in apolipoprotein E-deficient mice (98). More importantly, anthocyanin metabolites enhance endothelial function by influencing the gut microbiota (99). These studies suggest that anthocyanins slow atherosclerosis progression by regulating vascular endothelial function. In conclusion, anthocyanins are crucial for cardiovascular health due to their antioxidant and anti-inflammatory properties, as well as their role in regulating endothelial cell function (Table 2).
4.3.2 Anthocyanins and hypertension
Hypertension is a significant risk factor for cardiovascular diseases. Chronic hypertension increases the heart’s workload, requiring it to pump blood more forcefully. Over time, this can lead to cardiac hypertrophy and ultimately result in heart failure. In addition, hypertension damages vascular endothelial cells and promotes the formation of atherosclerosis, which can lead to coronary heart disease, strokes, and other serious conditions. Clinical pilot studies indicate that healthy elderly individuals aged 65 to 80, who consume 26 grams of freeze-dried wild blueberry powder (containing 302 mg of anthocyanins) daily for 12 weeks, experience significant increases in blood flow-mediated vasodilation and decreases in 24 h dynamic systolic blood pressure compared to the placebo group (100). Studies have also found that 5-week low-dose wild blueberry extract (222 mg of anthocyanins) significantly reduced systolic blood pressure in healthy elderly people (68–75 years old) (101). Moreover, a 6-week regimen of 85 mg of cranberry extract (25% anthocyanins) per day significantly lowered both systolic and diastolic blood pressure in patients with myocardial infarction (102). Short-term (28 days) ingestion of 300 mg New Zealand blackcurrant extract capsules (35% blackcurrant extract) reduced arterial stiffness and blood pressure in elderly individuals with an average age of 73.3 years (103). These studies indicate that anthocyanins can lower blood pressure. Growing evidence suggests that anthocyanin’s anti-hypertensive effects primarily stem from its antioxidant, anti-inflammatory, and ACE inhibitory properties, along with its ability to inhibit the growth of vascular endothelial cells. However, the intake of anthocyanin-rich blood orange juice (50 mg/500 mL) for 4 weeks also had no effect on blood pressure in healthy people (25–84 years old) (104), but it could significantly reduce blood pressure in patients with dementia (104). Therefore, the antihypertensive effects of anthocyanins depend on both the dosage and the duration of administration, and additional clinical trials are needed to determine the ideal nutritional intake and specific mechanisms, which will help create a stronger scientific basis for the prevention and treatment of cardiovascular diseases (Table 2).
4.4 Anthocyanins and cancer
Aging is a key risk factor for both the onset and progression of cancer, which is a leading cause of the rising mortality rate globally (105). Several studies have confirmed that anthocyanins possess anti-cancer properties. For example, it has been shown that anthocyanins (200 mg/kg) are able to significantly inhibit the growth of colorectal cancer cells, and to promote the apoptosis of cancer cells by regulating the PI3K/AKT signaling pathway (106). Anthocyanin can also further activate the aryl hydrocarbon receptor pathway by regulating intestinal flora, improve the intestinal barrier function, reduce inflammatory, and inhibit the proliferation and cell cycle of colorectal cancer cells (107). In addition, anthocyanins can slow tumor development by inhibiting tumor-associated inflammatory responses and reducing pro-inflammatory factors in the tumor microenvironment (20). In breast cancer and prostate cancer studies, anthocyanins have inhibited the growth of cancer cells by regulating the cell cycle and inducing apoptosis, thus showing a good preventive effect (108, 109). The latest studies show that anthocyanin-rich cereal diets (anthocyanin content 140 mM/g for 4.5 months) enhance autophagy by reducing M1 macrophage markers in tumors and promoting the expression of M2 macrophage markers, thereby exerting antitumor effects in Lewis lung cancer mice (110). Anthocyanins diet (0.5% CAN for 15 weeks) can also reduce lipid deposition in cancer cells by regulating the AMPK/mTOR signaling pathway, thereby inhibiting the development of urethane-induced lung cancer in C57BL/6 J mice (111). While multiple studies have confirmed the anti-cancer effects of anthocyanins, further research is needed to determine their effective dosage and long-term clinical effects. Furthermore, the current study has focused on the relationship between anthocyanins intake and cancer risk, and some epidemiological studies showing that a diet rich in anthocyanins may be associated with reduced risk of some cancers (112). However, more randomized controlled trials are still needed to validate the specific mechanism of action of anthocyanins and its potential use in cancer prevention (Table 2).
4.5 Anthocyanins and metabolic syndrome
Aging is a major risk factor for developing metabolic syndrome, a complex condition characterized by symptoms like obesity, glucose intolerance, insulin resistance, dyslipidemia, and hypertension (113). These symptoms significantly increase the risk of cardiovascular disease and diabetes mellitus. Compared with healthy individuals, the proliferation of harmful bacterial flora in the gut of patients with metabolic syndrome is increased, and the beneficial bacterial flora is inhibited (114). Research shows that anthocyanin metabolites promote the growth of beneficial gut flora, improving intestinal health and metabolic function (115). Chronic inflammation is a hallmark of metabolic syndrome, and anthocyanins (320 mg/day for 4 weeks) can lower systemic inflammation by inhibiting proinflammatory factors like TNF-α and IL-6, thus alleviating metabolic syndrome symptoms (116, 117). Anthocyanins can also ameliorate the development of metabolic syndrome by improving the hypertrophy and inflammatory status of adipose tissue by regulating the leptin signaling pathway (118). These studies suggest that anthocyanins alleviate key features of metabolic syndrome by regulating gut microbiota, reducing chronic inflammation, and modulating leptin signaling pathways. In addition, anthocyanins play an important role in regulating lipid metabolism. Studies have shown that anthocyanins (640 mg/day for 4 weeks) can reduce the levels of low-density lipoprotein cholesterol (LDL-C) and triglyceride (TG) levels in serum, while increasing the level of high-density lipoprotein cholesterol (HDL-C), thus improving abnormal lipid metabolism (119, 120). Anthocyanins (320 mg/day for 4 weeks) effectively improve insulin resistance by activating AMPK and PPAR-γ signaling pathways, enhancing cell sensitivity to insulin (99, 117). Oxidative stress is considered a key factor in the development of metabolic syndrome, and anthocyanins effectively reduce oxidative damage by scavenging free radicals and boosting the activity of antioxidant enzymes (116, 121). These researches indicate that anthocyanins mitigate metabolic disturbances associated with metabolic syndrome by regulating lipid metabolism, insulin sensitivity, and antioxidative stress. Although these studies provide a rationale for anthocyanins as a natural drug for potential antimetabolic syndrome, future studies should investigate the potential benefits and optimal dosing of anthocyanins in clinical applications (Table 2).
4.6 Anthocyanins and other diseases
Glaucoma is a chronic, progressive optic nerve disease that is a leading cause of irreversible blindness worldwide (122). The likelihood of developing glaucoma and other common eye diseases, like cataracts and macular degeneration, rises with age (123, 124). Studies have shown that black currant anthocyanins (50 mg/day for 4 weeks) are effective in lowering intraocular pressure in both healthy individuals and glaucoma patients (125). Additionally, bilberry anthocyanins (120 mg/day for 24.32 ± 10.34 months) can improve visual function in patients with normal-tension glaucoma (126). However, the specific mechanism of action has not been reported in the literature and requires further investigation. Benign prostatic hyperplasia (BPH) is a common chronic disease of the urinary system among elderly men. An imbalance of androgens in older men is one of the main causes of BPH. Dihydrotestosterone (DHT) and converted testosterone by 5-α reductase type 2 (5AR2), binding with androgen receptor (AR), affect prostate proliferation and growth. In BPH, androgen signaling boosts the levels of prostate-specific antigen (PSA) and certain cytokines, like proliferating cell nuclear antigen (PCNA) and cyclin D1. Research has demonstrated that polymerized anthocyanin (PA) reduces the expression of proteins related to androgen signaling, including 5AR2, AR, and PSA in LNCaP cell lines. Oral administration of PA (100 mg/kg/day for 4 weeks) can reduce the expression levels of AR, 5ar2, PSA, PCNA, cyclin D1, Bcl-2 in prostate tissue and serum DHT level, ultimately improving prostate weight in rats with BPH (127). Similarly, Jang et al. demonstrated that a 4-week black soybean (40, 80, and 160 mg/kg for 4 weeks) anthocyanin intervention effectively reduced prostate volume in benign prostatic hyperplasia (BPH) rats (128). Meanwhile, purple corn extract enhanced (10, and 50 mg/kg/day for 4 weeks) pro-apoptotic gene expression by inhibiting androgen and AR signaling markers and regulating the PI3K/AKT signaling cascade, resulting in reduced prostate hypertrophy weight (129). These findings suggest that anthocyanin may be a promising natural treatment for BPH (Table 2).
5 Conclusion
Anthocyanins, as plant-derived bioactive compounds, hold significant potential to extend lifespan and combat age-related diseases through pleiotropic mechanisms such as autophagy activation, oxidative stress reduction, and promoting intestinal health. Preclinical evidence supports their therapeutic benefits in neurodegenerative disorders, osteoporosis, cancer, cardiovascular diseases, and other aging-associated conditions, mediated by antioxidant, anti-inflammatory, and metabolic regulatory properties (Figure 1). However, critical knowledge gaps persist in translating these findings to elderly populations: (1) Existing studies predominantly rely on animal models or young/middle-aged cohorts, with minimal data on long-term efficacy and safety in frail older adults (≥75 years) exhibiting multimorbidity or polypharmacy. (2) Age-related declines in gastrointestinal absorption, hepatic metabolism, and renal excretion may alter anthocyanin pharmacokinetics, yet no studies have systematically addressed this. (3) The impact of genetic polymorphisms (e.g., GST enzymes), sex hormones, and baseline microbiota diversity on anthocyanin effects remains unexplored in aging contexts.
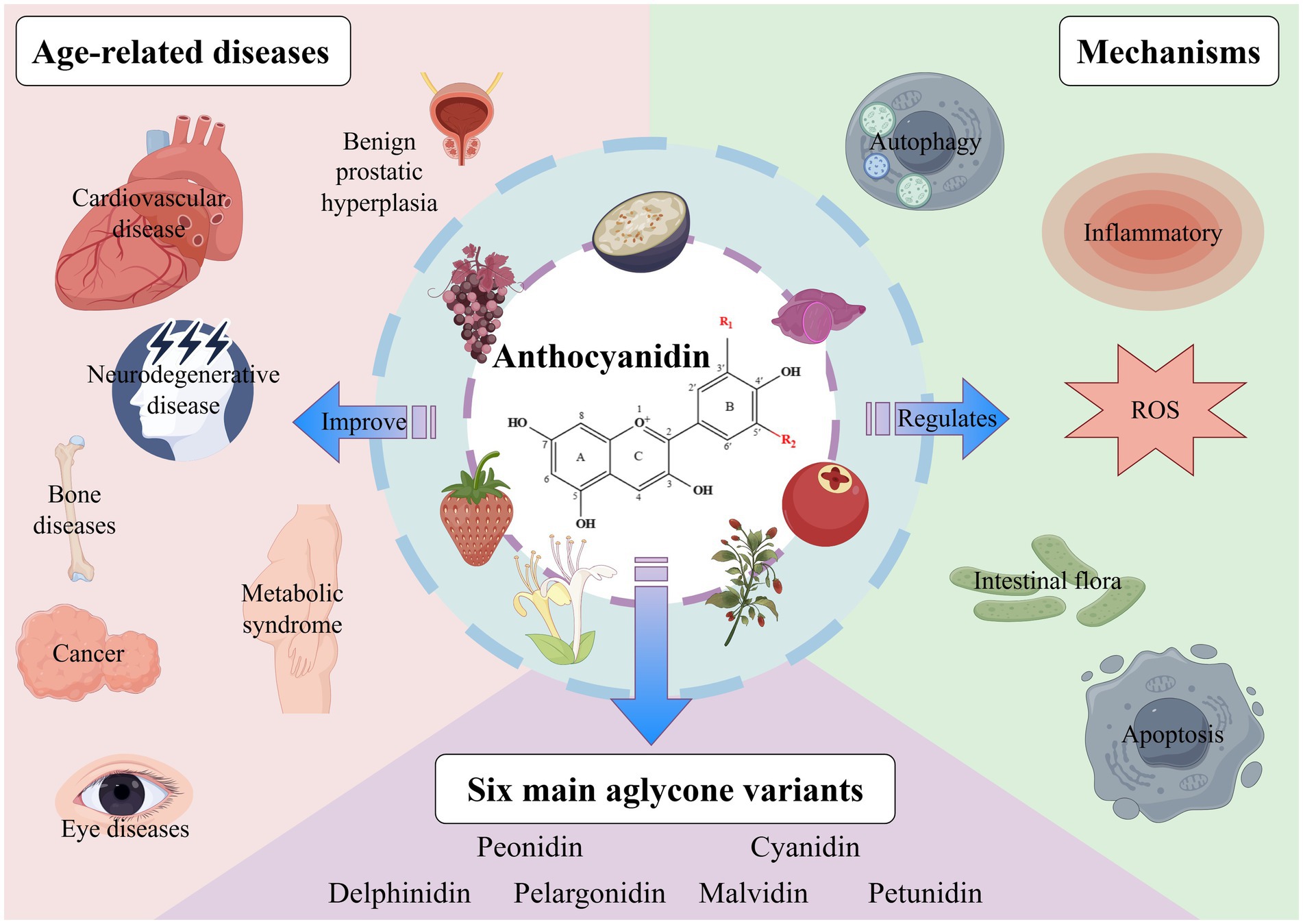
Figure 1. The chemical properties, biological functions, and ability to improve age-related diseases of anthocyanins. ROS, reactive oxygen species. Created with Figdraw.com.
Author contributions
XM: Investigation, Methodology, Writing – original draft. ZJ: Investigation, Methodology, Writing – original draft. ZR: Funding acquisition, Supervision, Writing – review & editing. LZ: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was sponsored by the National Natural Science Foundation of China (32400956); the Shanghai University Young Talents Sailing Plan (N.13-G21022367); and China Postdoctoral Science Foundation (2023M740870).
Acknowledgments
Figure 1 were created using Figdraw with the publication licenses (YPYSO57fa8).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. North, BJ, and Sinclair, DA. The intersection between aging and cardiovascular disease. Circ Res. (2012) 110:1097–108. doi: 10.1161/circresaha.111.246876
2. Hou, Y, Dan, X, Babbar, M, Wei, Y, Hasselbalch, SG, Croteau, DL, et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. (2019) 15:565–81. doi: 10.1038/s41582-019-0244-7
3. Montégut, L, López-Otín, C, and Kroemer, G. Aging and cancer. Mol Cancer. (2024) 23:106. doi: 10.1186/s12943-024-02020-z
4. Dominguez, LJ, and Barbagallo, M. The biology of the metabolic syndrome and aging. Curr Opin Clin Nutr Metab Care. (2016) 19:5–11. doi: 10.1097/mco.0000000000000243
5. Zhang, L, Guan, Q, Wang, Z, Feng, J, Zou, J, and Gao, B. Consequences of aging on bone. Aging Dis. (2023) 15:2417–52. doi: 10.14336/ad.2023.1115
6. Fontana, L. The science of nutritional modulation of aging. Ageing Res Rev. (2017) 39:1–2. doi: 10.1016/j.arr.2017.08.002
7. Fontana, L, and Partridge, L. Promoting health and longevity through diet: from model organisms to humans. Cell. (2015) 161:106–18. doi: 10.1016/j.cell.2015.02.020
8. Longo, VD, and Anderson, RM. Nutrition, longevity and disease: from molecular mechanisms to interventions. Cell. (2022) 185:1455–70. doi: 10.1016/j.cell.2022.04.002
9. Li, R, Tao, M, Xu, T, Pan, S, Xu, X, and Wu, T. Small berries as health-promoting ingredients: a review on anti-aging effects and mechanisms in Caenorhabditis elegans. Food Funct. (2022) 13:478–500. doi: 10.1039/d1fo02184b
10. Luo, J, Si, H, Jia, Z, and Liu, D. Dietary anti-aging polyphenols and potential mechanisms. Antioxidants. (2021) 10:283. doi: 10.3390/antiox10020283
11. Gonçalves, AC, Nunes, AR, Falcão, A, Alves, G, and Silva, LR. Dietary effects of anthocyanins in human health: a comprehensive review. Pharmaceuticals (Basel). (2021) 14:690. doi: 10.3390/ph14070690
12. Kumkum, R, Aston-Mourney, K, McNeill, BA, Hernández, D, and Rivera, LR. Bioavailability of anthocyanins: whole foods versus extracts. Nutrients. (2024) 16:1403. doi: 10.3390/nu16101403
13. Xue, H, Zha, M, Tang, Y, Zhao, J, Du, X, and Wang, Y. Research Progress on the extraction and purification of anthocyanins and their interactions with proteins. Molecules. (2024) 29:2815. doi: 10.3390/molecules29122815
14. Smeriglio, A, Barreca, D, Bellocco, E, and Trombetta, D. Chemistry, pharmacology and health benefits of anthocyanins. Phytother Res. (2016) 30:1265–86. doi: 10.1002/ptr.5642
15. He, J, and Giusti, MM. Anthocyanins: natural colorants with health-promoting properties. Annu Rev Food Sci Technol. (2010) 1:163–87. doi: 10.1146/annurev.food.080708.100754
16. Wallace, TC, Slavin, M, and Frankenfeld, CL. Systematic review of anthocyanins and markers of cardiovascular disease. Nutrients. (2016) 8:32. doi: 10.3390/nu8010032
17. Winter, AN, and Bickford, PC. Anthocyanins and their metabolites as therapeutic agents for neurodegenerative disease. Antioxidants (Basel). (2019) 8:333. doi: 10.3390/antiox8090333
18. Godyla-Jabłoński, M, Raczkowska, E, Jodkowska, A, Kucharska, AZ, Sozański, T, and Bronkowska, M. Effects of anthocyanins on components of metabolic syndrome-a review. Nutrients. (2024) 16:1103. doi: 10.3390/nu16081103
19. Hubert, PA, Lee, SG, Lee, SK, and Chun, OK. Dietary polyphenols, berries, and age-related bone loss: a review based on human, animal, and cell studies. Antioxidants (Basel). (2014) 3:144. doi: 10.3390/antiox3010144
20. Lin, BW, Gong, CC, Song, HF, and Cui, YY. Effects of anthocyanins on the prevention and treatment of cancer. Br J Pharmacol. (2017) 174:1226–43. doi: 10.1111/bph.13627
21. Bungau, S, Abdel-Daim, MM, Tit, DM, Ghanem, E, Sato, S, Maruyama-Inoue, M, et al. Health benefits of polyphenols and carotenoids in age-related eye diseases. Oxidative Med Cell Longev. (2019) 2019:1–22. doi: 10.1155/2019/9783429
22. Li, X, Zhang, Z, Zhang, X, Cheng, J, Liu, D, Yan, Y, et al. Transcriptomic analysis of the life-extending effect exerted by black rice anthocyanin extract in D. melanogaster through regulation of aging pathways. Exp Gerontol. (2019) 119:33–9. doi: 10.1016/j.exger.2019.01.015
23. Zuo, Y, Peng, C, Liang, Y, Ma, KY, Yu, H, Edwin Chan, HY, et al. Black rice extract extends the lifespan of fruit flies. Food Funct. (2012) 3:1271–9. doi: 10.1039/c2fo30135k
24. Golubev, D, Zemskaya, N, Shevchenko, O, Shaposhnikov, M, Kukuman, D, Patov, S, et al. Honeysuckle extract (Lonicera pallasii L.) exerts antioxidant properties and extends the lifespan and healthspan of Drosophila melanogaster. Biogerontology. (2022) 23:215. doi: 10.1007/s10522-022-09954-1
25. Han, Y, Guo, Y, Cui, SW, Li, H, Shan, Y, and Wang, H. Purple sweet potato extract extends lifespan by activating autophagy pathway in male Drosophila melanogaster. Exp Gerontol. (2021) 144:111190. doi: 10.1016/j.exger.2020.111190
26. Zhang, X, Wang, H, Han, Y, Pei, Y, Guo, Y, and Cui, SW. Purple sweet potato extract maintains intestinal homeostasis and extend lifespan through increasing autophagy in female Drosophila melanogaster. J Food Biochem. (2021) 45:e13861. doi: 10.1111/jfbc.13861
27. Zhao, J, Yu, J, Zhi, Q, Yuan, T, Lei, X, Zeng, K, et al. Anti-aging effects of the fermented anthocyanin extracts of purple sweet potato on Caenorhabditis elegans. Food Funct. (2021) 12:12647–58. doi: 10.1039/d1fo02671b
28. Zhang, N, Jiao, S, and Jing, P. Red cabbage rather than green cabbage increases stress resistance and extends the lifespan of Caenorhabditis elegans. Antioxidants (Basel). (2021) 10:930. doi: 10.3390/antiox10060930
29. Chen, W, Müller, D, Richling, E, and Wink, M. Anthocyanin-rich purple wheat prolongs the life span of Caenorhabditis elegans probably by activating the DAF-16/FOXO transcription factor. J Agric Food Chem. (2013) 61:3047–53. doi: 10.1021/jf3054643
30. Yan, F, Chen, Y, Azat, R, and Zheng, X. Mulberry anthocyanin extract ameliorates oxidative damage in HepG2 cells and prolongs the lifespan of Caenorhabditis elegans through MAPK and Nrf2 pathways. Oxidative Med Cell Longev. (2017) 2017:7956158. doi: 10.1155/2017/7956158
31. Li, S, Wang, J, Zhang, L, Zheng, Y, Ma, G, Sun, X, et al. Preparation of Dendrobium officinale flower anthocyanin and extended lifespan in Caenorhabditis elegans. Molecules. (2022) 27:8608. doi: 10.3390/molecules27238608
32. Mikhailova, DV, Shevchenko, OG, Golubev, DA, Platonova, EY, Zemskaya, NV, Shoeva, OY, et al. Antioxidant properties and Geroprotective potential of wheat bran extracts with increased content of anthocyanins. Antioxidants (Basel). (2023) 12:2010. doi: 10.3390/antiox12112010
33. Chen, J, Zhang, J, Xiang, Y, Xiang, L, Liu, Y, He, X, et al. Extracts of Tsai tai (Brassica chinensis): enhanced antioxidant activity and anti-aging effects both in vitro and in Caenorhabditis elegans. Food Funct. (2016) 7:943–52. doi: 10.1039/c5fo01241d
34. Tambara, AL, de Los Santos Moraes, L, Dal Forno, AH, Boldori, JR, Gonçalves Soares, AT, de Freitas Rodrigues, C, et al. Purple Pitanga fruit (Eugenia uniflora L.) protects against oxidative stress and increase the lifespan in Caenorhabditis elegans via the DAF-16/FOXO pathway. Food Chem Toxicol. (2018) 120:639–50. doi: 10.1016/j.fct.2018.07.057
35. Zhou, H, Ding, S, Sun, C, Fu, J, Yang, D, Wang, X, et al. Lycium barbarum extracts extend lifespan and alleviate proteotoxicity in Caenorhabditis elegans. Front Nutr. (2021) 8:815947. doi: 10.3389/fnut.2021.815947
36. Trevisan, K, Cristina-Pereira, R, Silva-Amaral, D, and Aversi-Ferreira, TA. Theories of aging and the prevalence of Alzheimer's disease. Biomed Res Int. (2019) 2019:1–9. doi: 10.1155/2019/9171424
37. Scheltens, P, De Strooper, B, Kivipelto, M, Holstege, H, Chételat, G, Teunissen, CE, et al. Alzheimer's disease. Lancet. (2021) 397:1577–90. doi: 10.1016/s0140-6736(20)32205-4
38. Kent, K, Charlton, K, Roodenrys, S, Batterham, M, Potter, J, Traynor, V, et al. Consumption of anthocyanin-rich cherry juice for 12 weeks improves memory and cognition in older adults with mild-to-moderate dementia. Eur J Nutr. (2017) 56:333–41. doi: 10.1007/s00394-015-1083-y
39. Bowtell, JL, Aboo-Bakkar, Z, Conway, ME, Adlam, AR, and Fulford, J. Enhanced task-related brain activation and resting perfusion in healthy older adults after chronic blueberry supplementation. Appl Physiol Nutr Metab. (2017) 42:773. doi: 10.1139/apnm-2016-0550
40. Boespflug, EL, Eliassen, JC, Dudley, JA, Shidler, MD, Kalt, W, Summer, SS, et al. Enhanced neural activation with blueberry supplementation in mild cognitive impairment. Nutr Neurosci. (2018) 21:297–305. doi: 10.1080/1028415x.2017.1287833
41. Shimada, M, Maeda, H, Nanashima, N, Yamada, K, and Nakajima, A. Anthocyanin-rich blackcurrant extract improves long-term memory impairment and emotional abnormality in senescence-accelerated mice. J Food Biochem. (2022) 46:e14295. doi: 10.1111/jfbc.14295
42. Shih, PH, Chan, YC, Liao, JW, Wang, MF, and Yen, GC. Antioxidant and cognitive promotion effects of anthocyanin-rich mulberry (Morus atropurpurea L.) on senescence-accelerated mice and prevention of Alzheimer's disease. J Nutr Biochem. (2010) 21:598–605. doi: 10.1016/j.jnutbio.2009.03.008
43. Bhatt, S, Puli, L, and Patil, CR. Role of reactive oxygen species in the progression of Alzheimer's disease. Drug Discov Today. (2021) 26:794–803. doi: 10.1016/j.drudis.2020.12.004
44. Ali, T, Kim, T, Rehman, SU, Khan, MS, Amin, FU, Khan, M, et al. Natural dietary supplementation of anthocyanins via PI3K/Akt/Nrf2/HO-1 pathways mitigate oxidative stress, neurodegeneration, and memory impairment in a mouse model of Alzheimer's disease. Mol Neurobiol. (2018) 55:6076–93. doi: 10.1007/s12035-017-0798-6
45. Ali, T, Kim, MJ, Rehman, SU, Ahmad, A, and Kim, MO. Anthocyanin-loaded PEG-gold nanoparticles enhanced the neuroprotection of anthocyanins in an aβ(1-42) mouse model of Alzheimer's disease. Mol Neurobiol. (2017) 54:6490–506. doi: 10.1007/s12035-016-0136-4
46. Zhang, B, Gaiteri, C, Bodea, LG, Wang, Z, McElwee, J, Podtelezhnikov, AA, et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer's disease. Cell. (2013) 153:707–20. doi: 10.1016/j.cell.2013.03.030
47. Li, J, Zhao, R, Jiang, Y, Xu, Y, Zhao, H, Lyu, X, et al. Bilberry anthocyanins improve neuroinflammation and cognitive dysfunction in APP/PSEN1 mice via the CD33/TREM2/TYROBP signaling pathway in microglia. Food Funct. (2020) 11:1572–84. doi: 10.1039/c9fo02103e
48. Mehan, S, Meena, H, Sharma, D, and Sankhla, R. JNK: a stress-activated protein kinase therapeutic strategies and involvement in Alzheimer's and various neurodegenerative abnormalities. J Mol Neurosci. (2011) 43:376–90. doi: 10.1007/s12031-010-9454-6
49. Kim, MJ, Rehman, SU, Amin, FU, and Kim, MO. Enhanced neuroprotection of anthocyanin-loaded PEG-gold nanoparticles against aβ(1-42)-induced neuroinflammation and neurodegeneration via the NF-(K)B /JNK/GSK3β signaling pathway. Nanomedicine. (2017) 13:2533–44. doi: 10.1016/j.nano.2017.06.022
50. Kalmijn, S. Fatty acid intake and the risk of dementia and cognitive decline: a review of clinical and epidemiological studies. J Nutr Health Aging. (2000) 4:202–7.
51. Carey, AN, Gomes, SM, and Shukitt-Hale, B. Blueberry supplementation improves memory in middle-aged mice fed a high-fat diet. J Agric Food Chem. (2014) 62:3972–8. doi: 10.1021/jf404565s
52. Carey, AN, Gildawie, KR, Rovnak, A, Thangthaeng, N, Fisher, DR, and Shukitt-Hale, B. Blueberry supplementation attenuates microglia activation and increases neuroplasticity in mice consuming a high-fat diet. Nutr Neurosci. (2019) 22:253–63. doi: 10.1080/1028415x.2017.1376472
53. Zhuang, J, Lu, J, Wang, X, Wang, X, Hu, W, Hong, F, et al. Purple sweet potato color protects against high-fat diet-induced cognitive deficits through AMPK-mediated autophagy in mouse hippocampus. J Nutr Biochem. (2019) 65:35–45. doi: 10.1016/j.jnutbio.2018.10.015
54. Li, J, Shi, Z, and Mi, Y. Purple sweet potato color attenuates high fat-induced neuroinflammation in mouse brain by inhibiting MAPK and NF-κB activation. Mol Med Rep. (2018) 17:4823–31. doi: 10.3892/mmr.2018.8440
55. Jankovic, J, and Tan, EK. Parkinson's disease: etiopathogenesis and treatment. J Neurol Neurosurg Psychiatry. (2020) 91:795–808. doi: 10.1136/jnnp-2019-322338
56. Zhang, X, Molsberry, SA, Yeh, TS, Cassidy, A, Schwarzschild, MA, Ascherio, A, et al. Intake of flavonoids and flavonoid-rich foods and mortality risk among individuals with Parkinson disease: a prospective cohort study. Neurology. (2022) 98:e1064–76. doi: 10.1212/wnl.0000000000013275
57. Alamri, Y. The use of dietary supplements and perceived quality of life in patients with Parkinson's disease. J Clin Neurosci. (2018) 56:137–8. doi: 10.1016/j.jocn.2018.06.029
58. Kim, HG, Ju, MS, Shim, JS, Kim, MC, Lee, SH, Huh, Y, et al. Mulberry fruit protects dopaminergic neurons in toxin-induced Parkinson's disease models. Br J Nutr. (2010) 104:8–16. doi: 10.1017/s0007114510000218
59. Gu, PS, Moon, M, Choi, JG, and Oh, MS. Mulberry fruit ameliorates Parkinson's-disease-related pathology by reducing α-synuclein and ubiquitin levels in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid model. J Nutr Biochem. (2017) 39:15–21. doi: 10.1016/j.jnutbio.2016.08.014
60. Simola, N, Morelli, M, and Carta, AR. The 6-hydroxydopamine model of Parkinson's disease. Neurotox Res. (2007) 11:151–67. doi: 10.1007/bf03033565
61. Roghani, M, Niknam, A, Jalali-Nadoushan, MR, Kiasalari, Z, Khalili, M, and Baluchnejadmojarad, T. Oral pelargonidin exerts dose-dependent neuroprotection in 6-hydroxydopamine rat model of hemi-parkinsonism. Brain Res Bull. (2010) 82:279–83. doi: 10.1016/j.brainresbull.2010.06.004
62. Wang, W, Zhu, G, Wang, Y, Li, W, Yi, S, Wang, K, et al. Multi-omics integration in mice with Parkinson's disease and the intervention effect of Cyanidin-3-O-glucoside. Front Aging Neurosci. (2022) 14:877078. doi: 10.3389/fnagi.2022.877078
63. Marques, C, Fernandes, I, Meireles, M, Faria, A, Spencer, JPE, Mateus, N, et al. Gut microbiota modulation accounts for the neuroprotective properties of anthocyanins. Sci Rep. (2018) 8:11341. doi: 10.1038/s41598-018-29744-5
64. Filaferro, M, Codeluppi, A, Brighenti, V, Cimurri, F, González-Paramás, AM, Santos-Buelga, C, et al. Disclosing the antioxidant and neuroprotective activity of an anthocyanin-rich extract from sweet cherry (Prunus avium L.) using in vitro and in vivo models. Antioxidants. (2022) 11:211. doi: 10.3390/antiox11020211
65. Zaim, M, Kara, I, and Muduroglu, A. Black carrot anthocyanins exhibit neuroprotective effects against MPP+ induced cell death and cytotoxicity via inhibition of oxidative stress mediated apoptosis. Cytotechnology. (2021) 73:827–40. doi: 10.1007/s10616-021-00500-4
66. Strathearn, KE, Yousef, GG, Grace, MH, Roy, SL, Tambe, MA, Ferruzzi, MG, et al. Neuroprotective effects of anthocyanin- and proanthocyanidin-rich extracts in cellular models of Parkinson′s disease. Brain Res. (2014) 1555:60–77. doi: 10.1016/j.brainres.2014.01.047
67. Koza, LA, Winter, AN, Holsopple, J, Baybayon-Grandgeorge, AN, Pena, C, Olson, JR, et al. Protocatechuic acid extends survival, improves motor function, diminishes gliosis, and sustains neuromuscular junctions in the hSOD1(G93A) mouse model of amyotrophic lateral sclerosis. Nutrients. (2020) 12:1824. doi: 10.3390/nu12061824
68. Winter, AN, Ross, EK, Wilkins, HM, Stankiewicz, TR, Wallace, T, Miller, K, et al. An anthocyanin-enriched extract from strawberries delays disease onset and extends survival in the hSOD1(G93A) mouse model of amyotrophic lateral sclerosis. Nutr Neurosci. (2018) 21:414–26. doi: 10.1080/1028415x.2017.1297023
69. Safiri, S, Kolahi, AA, Smith, E, Hill, C, Bettampadi, D, Mansournia, MA, et al. Global, regional and national burden of osteoarthritis 1990-2017: a systematic analysis of the global burden of disease study 2017. Ann Rheum Dis. (2020) 79:819–28. doi: 10.1136/annrheumdis-2019-216515
70. Loeser, RF, Collins, JA, and Diekman, BO. Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. (2016) 12:412–20. doi: 10.1038/nrrheum.2016.65
71. Haseeb, A, Chen, D, and Haqqi, TM. Delphinidin inhibits IL-1β-induced activation of NF-κB by modulating the phosphorylation of IRAK-1(Ser376) in human articular chondrocytes. Rheumatology (Oxford). (2013) 52:998–1008. doi: 10.1093/rheumatology/kes363
72. Mével, E, Monfoulet, LE, Merceron, C, Coxam, V, Wittrant, Y, Beck, L, et al. Nutraceuticals in joint health: animal models as instrumental tools. Drug Discov Today. (2014) 19:1649–58. doi: 10.1016/j.drudis.2014.06.012
73. Wongwichai, T, Teeyakasem, P, Pruksakorn, D, Kongtawelert, P, and Pothacharoen, P. Anthocyanins and metabolites from purple rice inhibit IL-1β-induced matrix metalloproteinases expression in human articular chondrocytes through the NF-κB and ERK/MAPK pathway. Biomed Pharmacother. (2019) 112:108610. doi: 10.1016/j.biopha.2019.108610
74. Dai, T, Shi, K, Chen, G, Shen, Y, and Pan, T. Malvidin attenuates pain and inflammation in rats with osteoarthritis by suppressing NF-κB signaling pathway. Inflamm Res. (2017) 66:1075–84. doi: 10.1007/s00011-017-1087-6
75. Zeng, Z, Li, H, Luo, C, Hu, W, Weng, TJ, and Shuang, F. Pelargonidin ameliorates inflammatory response and cartilage degeneration in osteoarthritis via suppressing the NF-κB pathway. Arch Biochem Biophys. (2023) 743:109668. doi: 10.1016/j.abb.2023.109668
76. Ji, ML, Jiang, H, Li, Z, Geng, R, Hu, JZ, Lin, YC, et al. Sirt6 attenuates chondrocyte senescence and osteoarthritis progression. Nat Commun. (2022) 13:7658. doi: 10.1038/s41467-022-35424-w
77. Jiang, C, Sun, ZM, Hu, JN, Jin, Y, Guo, Q, Xu, JJ, et al. Cyanidin ameliorates the progression of osteoarthritis via the Sirt6/NF-κB axis in vitro and in vivo. Food Funct. (2019) 10:5873–85. doi: 10.1039/c9fo00742c
78. Pomilio, AB, Szewczuk, NA, and Duchowicz, PR. Dietary anthocyanins balance immune signs in osteoarthritis and obesity – update of human in vitro studies and clinical trials. Crit Rev Food Sci Nutr. (2024) 64:2634–72. doi: 10.1080/10408398.2022.2124948
79. Rachner, TD, Khosla, S, and Hofbauer, LC. Osteoporosis: now and the future. Lancet. (2011) 377:1276–87. doi: 10.1016/s0140-6736(10)62349-5
80. Clarke, BL, and Khosla, S. Physiology of bone loss. Radiol Clin North Am. (2010) 48:483–95. doi: 10.1016/j.rcl.2010.02.014
81. Manolagas, SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. (2010) 31:266–300. doi: 10.1210/er.2009-0024
82. Quek, YY, Cheng, LJ, Ng, YX, Hey, HWD, and Wu, XV. Effectiveness of anthocyanin-rich foods on bone remodeling biomarkers of middle-aged and older adults at risk of osteoporosis: a systematic review, meta-analysis, and meta-regression. Nutr Rev. (2024) 82:1187–207. doi: 10.1093/nutrit/nuad121
83. Sakaki, J, Melough, M, Lee, SG, Kalinowski, J, Koo, SI, Lee, SK, et al. Blackcurrant supplementation improves trabecular bone mass in young but not aged mice. Nutrients. (2018) 10:1671. doi: 10.3390/nu10111671
84. Hu, B, Chen, L, Chen, Y, Zhang, Z, Wang, X, and Zhou, B. Cyanidin-3-glucoside regulates osteoblast differentiation via the ERK1/2 signaling pathway. ACS Omega. (2021) 6:4759–66. doi: 10.1021/acsomega.0c05603
85. Qi, S, He, J, Han, H, Zheng, H, Jiang, H, Hu, CY, et al. Anthocyanin-rich extract from black rice (Oryza sativa L. japonica) ameliorates diabetic osteoporosis in rats. Food Funct. (2019) 10:5350–60. doi: 10.1039/c9fo00681h
86. Lane, NE. Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obstet Gynecol. (2006) 194:S3–S11. doi: 10.1016/j.ajog.2005.08.047
87. Nagaoka, M, Maeda, T, Chatani, M, Handa, K, Yamakawa, T, Kiyohara, S, et al. A delphinidin-enriched maqui berry extract improves bone metabolism and protects against bone loss in osteopenic mouse models. Antioxidants. (2019) 8:386. doi: 10.3390/antiox8090386
88. Zheng, X, Mun, S, Lee, SG, Vance, TM, Hubert, P, Koo, SI, et al. Anthocyanin-rich blackcurrant extract attenuates Ovariectomy-induced bone loss in mice. J Med Food. (2016) 19:390–7. doi: 10.1089/jmf.2015.0148
89. Li, T, Wu, SM, Xu, ZY, and Ou-Yang, S. Rabbiteye blueberry prevents osteoporosis in ovariectomized rats. J Orthop Surg Res. (2014) 9:56. doi: 10.1186/s13018-014-0056-9
90. Devareddy, L, Hooshmand, S, Collins, JK, Lucas, EA, Chai, SC, and Arjmandi, BH. Blueberry prevents bone loss in ovariectomized rat model of postmenopausal osteoporosis. J Nutr Biochem. (2008) 19:694–9. doi: 10.1016/j.jnutbio.2007.09.004
91. Falk, E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. (2006) 47:C7–C12. doi: 10.1016/j.jacc.2005.09.068
92. Zhu, Y, Xian, X, Wang, Z, Bi, Y, Chen, Q, Han, X, et al. Research Progress on the relationship between atherosclerosis and inflammation. Biomol Ther. (2018) 8:80. doi: 10.3390/biom8030080
93. Miyazaki, K, Makino, K, Iwadate, E, Deguchi, Y, and Ishikawa, F. Anthocyanins from purple sweet potato Ipomoea batatas cultivar Ayamurasaki suppress the development of atherosclerotic lesions and both enhancements of oxidative stress and soluble vascular cell adhesion molecule-1 in apolipoprotein E-deficient mice. J Agric Food Chem. (2008) 56:11485–92. doi: 10.1021/jf801876n
94. Joo, HK, Choi, S, Lee, YR, Lee, EO, Park, MS, Park, KB, et al. Anthocyanin-rich extract from red Chinese cabbage alleviates vascular inflammation in endothelial cells and Apo E(−/−) mice. Int J Mol Sci. (2018) 19:816. doi: 10.3390/ijms19030816
95. Wang, D, Wei, X, Yan, X, Jin, T, and Ling, W. Protocatechuic acid, a metabolite of anthocyanins, inhibits monocyte adhesion and reduces atherosclerosis in apolipoprotein E-deficient mice. J Agric Food Chem. (2010) 58:12722–8. doi: 10.1021/jf103427j
96. Lee, GH, Hoang, TH, Jung, ES, Jung, SJ, Han, SK, Chung, MJ, et al. Anthocyanins attenuate endothelial dysfunction through regulation of uncoupling of nitric oxide synthase in aged rats. Aging Cell. (2020) 19:e13279. doi: 10.1111/acel.13279
97. Wautier, JL, and Wautier, MP. Vascular permeability in diseases. Int J Mol Sci. (2022) 23:3645. doi: 10.3390/ijms23073645
98. Zhang, Y, Wang, X, Wang, Y, Liu, Y, and Xia, M. Supplementation of cyanidin-3-O-β-glucoside promotes endothelial repair and prevents enhanced atherogenesis in diabetic apolipoprotein E-deficient mice. J Nutr. (2013) 143:1248–53. doi: 10.3945/jn.113.177451
99. Saini, RK, Khan, MI, Shang, X, Kumar, V, Kumari, V, Kesarwani, A, et al. Dietary sources, stabilization, health benefits, and industrial application of anthocyanins-a review. Food Secur. (2024) 13:1227. doi: 10.3390/foods13081227
100. Wood, E, Hein, S, Mesnage, R, Fernandes, F, Abhayaratne, N, Xu, Y, et al. Wild blueberry (poly)phenols can improve vascular function and cognitive performance in healthy older individuals: a double-blind randomized controlled trial. Am J Clin Nutr. (2023) 117:1306–19. doi: 10.1016/j.ajcnut.2023.03.017
101. Cheng, N, Barfoot, KL, Le Cozannet, R, Fança-Berthon, P, Lamport, DJ, and Williams, CM. Wild blueberry extract intervention in healthy older adults: a multi-study, randomised, controlled investigation of acute cognitive and cardiovascular effects. Nutrients. (2024) 16:1180. doi: 10.3390/nu16081180
102. Naruszewicz, M, Laniewska, I, Millo, B, and Dłuzniewski, M. Combination therapy of statin with flavonoids rich extract from chokeberry fruits enhanced reduction in cardiovascular risk markers in patients after myocardial infraction (MI). Atherosclerosis. (2007) 194:e179–84. doi: 10.1016/j.atherosclerosis.2006.12.032
103. Okamoto, T, Hashimoto, Y, Kobayashi, R, Nakazato, K, and Willems, MET. Effects of blackcurrant extract on arterial functions in older adults: a randomized, double-blind, placebo-controlled, crossover trial. Clin Exp Hypertens. (2020) 42:640–7. doi: 10.1080/10641963.2020.1764015
104. Igwe, EO, Roodenrys, S, Probst, YC, do Rosario, V, Netzel, ME, Hong, HT, et al. Low anthocyanin plum nectar does not impact cognition, blood pressure and gut microbiota in healthy older adults: a randomized crossover trial. Nutr Res. (2020) 82:74–87. doi: 10.1016/j.nutres.2020.08.003
105. Aunan, JR, Cho, WC, and Søreide, K. The biology of aging and Cancer: a brief overview of shared and divergent molecular hallmarks. Aging Dis. (2017) 8:628–42. doi: 10.14336/ad.2017.0103
106. Zhao, X, Feng, P, He, W, Du, X, Chen, C, Suo, L, et al. The prevention and inhibition effect of anthocyanins on colorectal cancer. Curr Pharm Des. (2019) 25:4919–27. doi: 10.2174/1381612825666191212105145
107. Wang, L, Tu, YX, Chen, L, Yu, KC, Wang, HK, Yang, SQ, et al. Black rice diet alleviates colorectal cancer development through modulating tryptophan metabolism and activating AHR pathway. iMeta. (2024) 3:e165. doi: 10.1002/imt2.165
108. Bars-Cortina, D, Sakhawat, A, Piñol-Felis, C, and Motilva, MJ. Chemopreventive effects of anthocyanins on colorectal and breast cancer: a review. Semin Cancer Biol. (2022) 81:241–58. doi: 10.1016/j.semcancer.2020.12.013
109. Mottaghipisheh, J, Doustimotlagh, AH, Irajie, C, Tanideh, N, Barzegar, A, and Iraji, A. The promising therapeutic and preventive properties of Anthocyanidins/anthocyanins on prostate Cancer. Cells. (2022) 11:1070. doi: 10.3390/cells11071070
110. Tikhonova, MA, Shoeva, OY, Tenditnik, MV, Akopyan, AA, Litvinova, EA, Popova, NA, et al. Antitumor effects of an anthocyanin-rich grain diet in a mouse model of Lewis lung carcinoma. Int J Mol Sci. (2024) 25:5727. doi: 10.3390/ijms25115727
111. Luo, H, Gao, M, Lu, H, Chen, Q, and Lian, X. Anthocyanins prevent the development and progression of urethane-induced lung cancer by regulating energy metabolism in mice. Food Nutr Res. (2024) 68:10-29219. doi: 10.29219/fnr.v68.10434
112. Wang, LS, and Stoner, GD. Anthocyanins and their role in cancer prevention. Cancer Lett. (2008) 269:281–90. doi: 10.1016/j.canlet.2008.05.020
113. Alberti, KG, Zimmet, P, and Shaw, J. The metabolic syndrome--a new worldwide definition. Lancet. (2005) 366:1059–62. doi: 10.1016/s0140-6736(05)67402-8
114. Wang, PX, Deng, XR, Zhang, CH, and Yuan, HJ. Gut microbiota and metabolic syndrome. Chin Med J. (2020) 133:808–16. doi: 10.1097/cm9.0000000000000696
115. Du, L, Ding, X, Tian, Y, Chen, J, and Li, W. Effect of anthocyanins on metabolic syndrome through interacting with gut microbiota. Pharmacol Res. (2024) 210:107511. doi: 10.1016/j.phrs.2024.107511
116. Wang, S, Du, Q, Meng, X, and Zhang, Y. Natural polyphenols: a potential prevention and treatment strategy for metabolic syndrome. Food Funct. (2022) 13:9734–53. doi: 10.1039/d2fo01552h
117. Aboonabi, A, and Aboonabi, A. Anthocyanins reduce inflammation and improve glucose and lipid metabolism associated with inhibiting nuclear factor-kappaB activation and increasing PPAR-γ gene expression in metabolic syndrome subjects. Free Radic Biol Med. (2020) 150:30–9. doi: 10.1016/j.freeradbiomed.2020.02.004
118. Liu, M, Li, S, Guan, M, Bai, S, Bai, W, and Jiang, X. Leptin pathway is a crucial target for anthocyanins to protect against metabolic syndrome. Crit Rev Food Sci Nutr. (2024) 65:2046–61. doi: 10.1080/10408398.2024.2323093
119. Aboonabi, A, Meyer, RR, Gaiz, A, and Singh, I. Anthocyanins in berries exhibited anti-atherogenicity and antiplatelet activities in a metabolic syndrome population. Nutr Res. (2020) 76:82–93. doi: 10.1016/j.nutres.2020.02.011
120. Guo, J, Li, K, Lin, Y, and Liu, Y. Protective effects and molecular mechanisms of tea polyphenols on cardiovascular diseases. Front Nutr. (2023) 10:10. doi: 10.3389/fnut.2023.1202378
121. Duarte-Casar, R, González-Jaramillo, N, Bailon-Moscoso, N, Rojas-Le-Fort, M, and Romero-Benavides, JC. Five underutilized Ecuadorian fruits and their bioactive potential as functional foods and in metabolic syndrome: a review. Molecules. (2024) 29:2904. doi: 10.3390/molecules29122904
122. Tham, YC, Li, X, Wong, TY, Quigley, HA, Aung, T, and Cheng, CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. (2014) 121:2081–90. doi: 10.1016/j.ophtha.2014.05.013
123. Baskaran, M, Foo, RC, Cheng, CY, Narayanaswamy, AK, Zheng, YF, Wu, R, et al. The prevalence and types of glaucoma in an urban Chinese population: the Singapore Chinese eye study. JAMA Ophthalmol. (2015) 133:874. doi: 10.1001/jamaophthalmol.2015.1110
124. Klein, R, and Klein, BE. The prevalence of age-related eye diseases and visual impairment in aging: current estimates. Invest Ophthalmol Vis Sci. (2013) 54:ORSF5–ORSF13. doi: 10.1167/iovs.13-12789
125. Ohguro, H, Ohguro, I, and Yagi, S. Effects of black currant anthocyanins on intraocular pressure in healthy volunteers and patients with glaucoma. J Ocul Pharmacol Ther. (2013) 29:61–7. doi: 10.1089/jop.2012.0071
126. Shim, SH, Kim, JM, Choi, CY, Kim, CY, and Park, KH. Ginkgo biloba extract and bilberry anthocyanins improve visual function in patients with normal tension glaucoma. J Med Food. (2012) 15:818–23. doi: 10.1089/jmf.2012.2241
127. Choi, YJ, Fan, M, Tang, Y, Yang, HP, Hwang, JY, and Kim, EK. In vivo effects of polymerized anthocyanin from grape skin on benign prostatic hyperplasia. Nutrients. (2019) 11:2444. doi: 10.3390/nu11102444
128. Jang, H, Ha, US, Kim, SJ, Yoon, BI, Han, DS, Yuk, SM, et al. Anthocyanin extracted from black soybean reduces prostate weight and promotes apoptosis in the prostatic hyperplasia-induced rat model. J Agric Food Chem. (2010) 58:12686–91. doi: 10.1021/jf102688g
Keywords: anthocyanidins, age-related diseases, neurodegenerative disease, bone diseases, cardiovascular disease, cancer
Citation: Ma X, Jin Z, Rao Z and Zheng L (2025) Health benefits of anthocyanins against age-related diseases. Front. Nutr. 12:1618072. doi: 10.3389/fnut.2025.1618072
Edited by:
Dongmin Liu, Virginia Tech, United StatesReviewed by:
Satheesh Babu Adhini Kuppuswamy, The University of Utah, United StatesMichaela Godyla-Jabłoński, Wroclaw University of Environmental and Life Sciences, Poland
Copyright © 2025 Ma, Jin, Rao and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lifang Zheng, emhlbmdsZjIxQHNodS5lZHUuY24=
†These authors have contributed equally to this work
 Xiaojie Ma
Xiaojie Ma Zhihai Jin
Zhihai Jin Zhijian Rao
Zhijian Rao Lifang Zheng
Lifang Zheng