- 1Chengdu Pidu District Hospital of Traditional Chinese Medicine, Chengdu, Sichuan, China
- 2Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, China
Background: Significant correlations exist between gut microbiota, dietary habits, and cognitive function; the objective of this research was to evaluate the correlation between the dietary index for gut microbiota (DI-GM) and cognitive performance. The primary objective of this study was to evaluate the strength and direction of the association between Dietary Index for Gut Microbiota (DI-GM) scores and cognitive performance among older adults, and to further explore whether a dose-response relationship exists, thereby informing potential dietary strategies for cognitive risk stratification.
Methods: Complete DI-GM and cognitive function evaluation data for older adults were taken from the 2011–2014 National Health and Nutrition Examination Survey (NHANES) database. Cognitive function was assessed by standardized test scales. The weighted linear regression models were used to examine the association between DI-GM and cognitive function. Restricted cubic spline and threshold analysis evaluated the existence of non-linear correlations among variables. Subgroup studies were conducted to evaluate the consistency of the connection across different demographics.
Results: The outcome analysis showed that among the 2,207 participants, there was a positive and statistically significant relationship between higher DI-GM scores and scores of beneficial gut microbiota and total scores of cognitive functions (β = 0.03, 95% CI: 0.01–0.05, P = 0.034). Both RCS and threshold analyses confirmed the linear correlation between DI-GM and beneficial gut flora and total scores of cognitive functions (P for non-linear > 0.05). Additionally, our study demonstrated that the correlation between DI-GM and total scores of cognitive functions was maintained in subgroup analyses (P for interaction > 0.05).
Conclusion: The findings of the study indicated that DI-GM profoundly impacts cognitive performance, which suggests that dietary modifications based on DI-GM may help lower the level of cognitive impairment in the elderly, but further high-caliber research is required to elucidate the precise processes and application modalities, and to provide more effective strategies for improving cognitive function in the elderly.
1 Introduction
With the increasing trend of global population aging, cognitive dysfunction has become an important issue affecting public health. Cognitive decline elevates the probability of disability among older adults and amplifies the caregiving burden on families and society (1). Although traditional pharmacological treatments can delay cognitive decline to a certain extent, their effects are limited and side effects cannot be ignored (2, 3). Therefore, more and more researchers are turning their attention to safe and intervenable lifestyle factors, especially the role of diet in cognitive health maintenance (4).
The significance of gut bacteria as an intermediary between nutrition and cognition has recently attracted considerable focus (5). Studies have shown that gut microbiota modulate neuroinflammation, neurotransmitter levels, and thus brain structure and function through the gut-brain axis by a variety of immune, endocrine, neural, and metabolic mechanisms (6, 7). Furthermore, a research examining the impact of probiotics on cognition and mood in elderly adults revealed that probiotics exert broad impacts on the gut-brain axis in healthy older individuals, enhancing cognitive and psychological well-being while modifying gut bacteria composition (8). Conversely, diet is a pivotal element in modulating the makeup and functionality of the gut microbiota. Foods rich in dietary fiber, polyphenols, and prebiotics may enhance the proliferation of probiotics, yielding beneficial neuroprotective benefits.
Nevertheless, the majority of research have only investigated the connection between nutrition and cognitive performance, as well as flora and cognitive function separately, lacking an integrative perspective; and there is a lack of standardized metrics that can quantitatively measure the potential impact of diet on gut flora, especially in the context of the US dietary structure; furthermore, existing dietary assessment tools (e.g., Mediterranean Dietary Score, DASH Dietary Score), while available to some degree, are not designed specifically to be intestinal flora friendliness, and the mechanistic link with microecology still needs to be clarified (9, 10).
To systematically examine the effect of food on the gut microbiota, several researchers have created the Dietary Index for Gut Microbiota (DI-GM). The development of this index was informed by a comprehensive review of 106 studies, from which 14 food groups or nutrients closely associated with microbial diversity and function were identified. This encompasses advantageous elements including fermented dairy products and whole grains, with harmful elements like red meat and processed grains. The index was formally introduced by Kase et al. in 2024 (11), with the aim of providing a standardized tool for dietary assessment in microbiome-related health research. The DI-GM index incorporates several key dietary components—such as fermented dairy products, whole grains, and red or processed meats—that have been shown to influence gut microbiota composition and, through the gut-brain axis, may impact cognitive function. For example, fermented dairy products among other fiber-rich components—has been associated with increased gut microbial diversity and predicted short-chain fatty acid production, as well as improvements in frailty and health status in older adults (12). Whole grains and dietary fiber are known to promote the growth of butyrate I-producing bacteria, reduce systemic inflammation—factors linked to better cognitive outcomes (13). In contrast, high intake of red and processed meats has been correlated with pro-inflammatory microbial profiles, which may contribute to neuroinflammation and cognitive decline (14–16). While DI-GM has not yet been widely applied in clinical dietary planning, its structure suggests potential utility for microbiota I-informed dietary guidance in aging populations. This highlights the importance of exploring its association with cognitive performance and its possible integration into personalized nutrition strategies or population-level dietary models for older adults.
The objective of our work is to statistically evaluate the interaction between food and intestinal microecology, informed by the dietary features of the US population, and to further investigate the correlation between this index and cognitive performance. This is anticipated to yield novel concepts for dietary therapies targeting cognitive impairments and a theoretical framework for public health intervention techniques.
2 Methods
2.1 Data source
The National Health and Nutrition Examination Survey (NHANES) was utilized to analyze an adult population with complete dietary recall and cognitive function test results. The CDC's NHANES is a national, stratified multistage probability sample survey that is representative of the population. All subjects gave written informed permission for NHANES, which followed the Declaration of Helsinki and the National Center for Health Statistics' Research Ethics Review Board's guidelines. National Center for Health Statistics Research Ethics Review Board accepted the procedure.
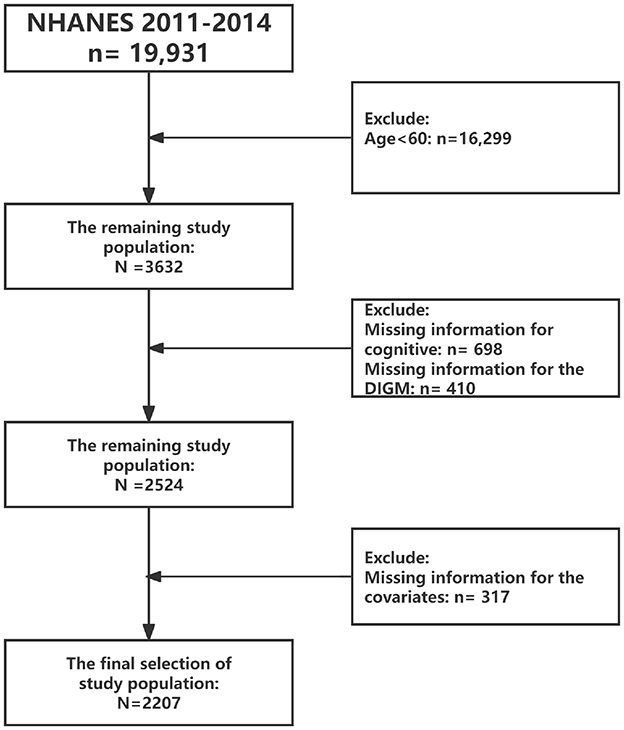
The survey period included in this study was 2011–2014, as that period included cognitive functioning assessment modules. Inclusion criteria included: (1) we excluded participants aged < 60 years (n = 16,299); (2) excluded missing data on cognitive function (n = 698); (3) excluded missing data on DIGM (n = 410); and (4) excluded missing data on covariates (317). As shown in Figure 1. Ultimately, a total of 2,207 eligible respondents were included and weighed variables provided by NHANES.
2.2 Cognitive function
Cognitive function was assessed using four standardized neuropsychological tests included in NHANES 2011–2014: (1) the Consortium to Establish a Registry for Alzheimer's Disease Word Learning subtest (CERAD-WL) for immediate recall; (2) CERAD Delayed Recall (CERAD-DR) for delayed memory; (3) the Animal Fluency Test (AFT) for verbal fluency and semantic memory; and (4) the Digit Symbol Substitution Test (DSST) for attention, processing speed, and executive function. Composite cognitive scores were calculated by standardizing and summing all individual test scores. Test-specific Z-scores (including DSST, CERAD-WL, CERAD-WL, AFT) were created using the SD of the sample mean and test scores. Standardized overall cognitive Z-scores were then generated by dividing the test-specific Z-scores by the SD mean (17).
2.3 DIGM
DI-GM was calculated based on participants' dietary intake data. The DI-GM scoring system was developed using dietary data collected by the self-reported 24-h dietary recall method in NHANES (11). The index covers food groups related to gut flora diversity and health, such as dietary fiber, prebiotics, fermented foods, etc. Higher DI-GM scores indicate greater beneficial effects of dietary structure on the gut microbiota. Please refer to Supplementary Table S1 for specific definitions.
2.4 Covariates
In addition, we include age gender, ethnicity, marriage status, education level, PIR, BMI, smoking status, alcohol consumption, hypertension, diabetes, depression, sleep disorders, hyperlipidemia as covariates. These covariates were obtained from self-reported questionnaires (Supplementary Table S2).
2.5 Statistical analysis
We used sample weights officially provided by NHANES to guarantee that the findings accurately represent the whole United States. All analyses considered NHANES' complex sampling design, including stratification, clustering, and weighting factors.
Initially, we computed descriptive statistics pertaining to participant characteristics. Secondly, multivariate linear regression models were employed to investigate the relationships between varying levels of DIGM and its components with specific assessments and overall cognitive Z-scores. The independent variables were categorized into four subgroups to determine the presence of a trend effect in these associations. Model 1 was an unadjusted model, Model 2 was adjusted just for age, gender, and ethnicity; Model 3 was adjusted for all factors. Subgroup studies were performed to examine the stability of the association. Moreover, restricted cubic spline and threshold analysis evaluated the existence of non-linear correlations among variables. All statistical analyses in this study were conducted using R software (version 4.4.3).
3 Results
3.1 Baseline characteristics
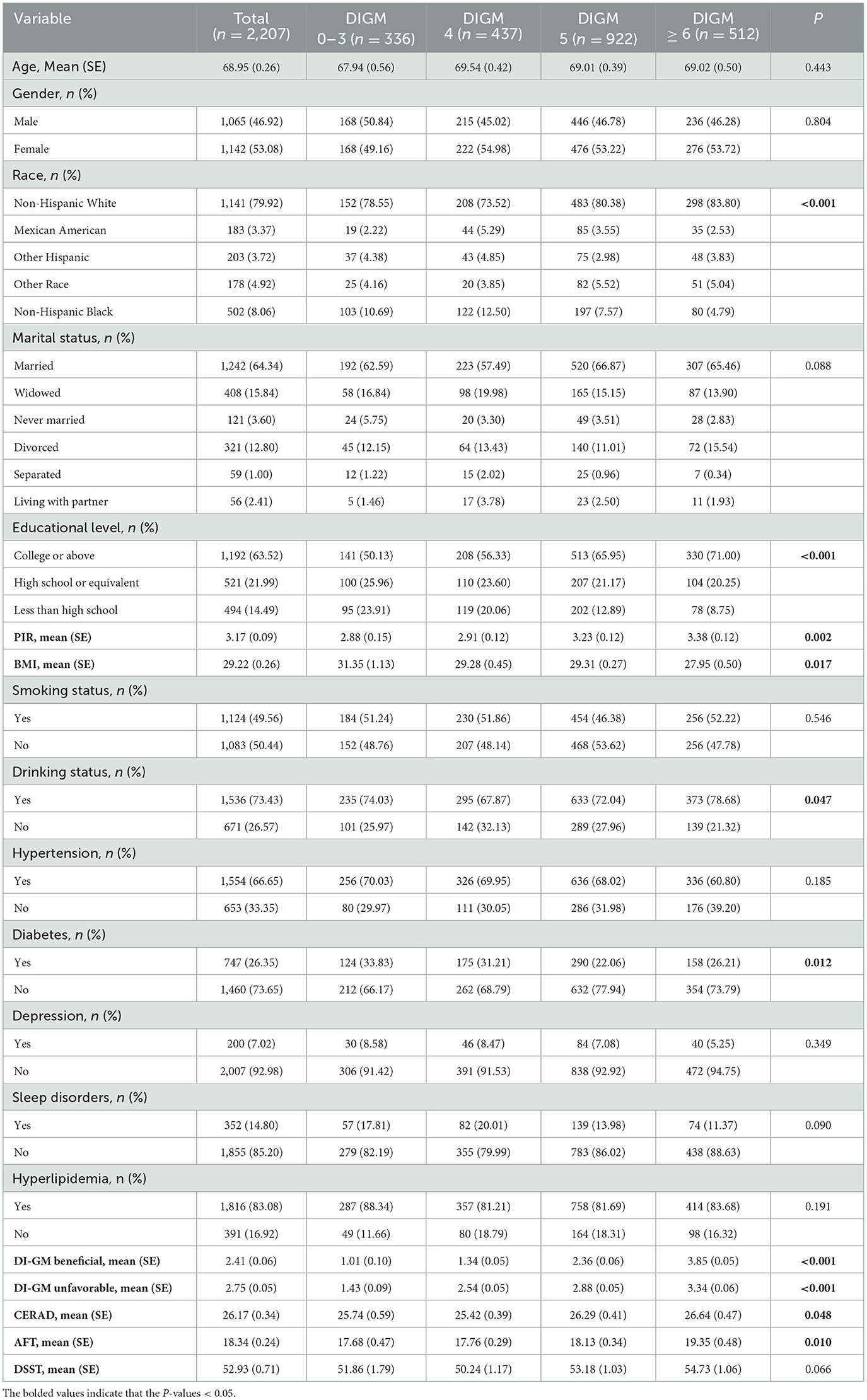
Table 1 displayed the sample's characteristics, including a mean age of 68.95 years (SE, 0.26) and a higher proportion of females than men. Of note, those with a DIGM score of 6 or higher tended to be more likely to present as non-Hispanic White, higher PIR and lower BMI, more educated, drinker, non-diabetic, higher DI-GM Beneficial scores, higher DI-GM Unfavorable scores, and higher CERAD and AFT scores, with statistically significant differences (P < 0.05).
3.2 Multivariable linear regression
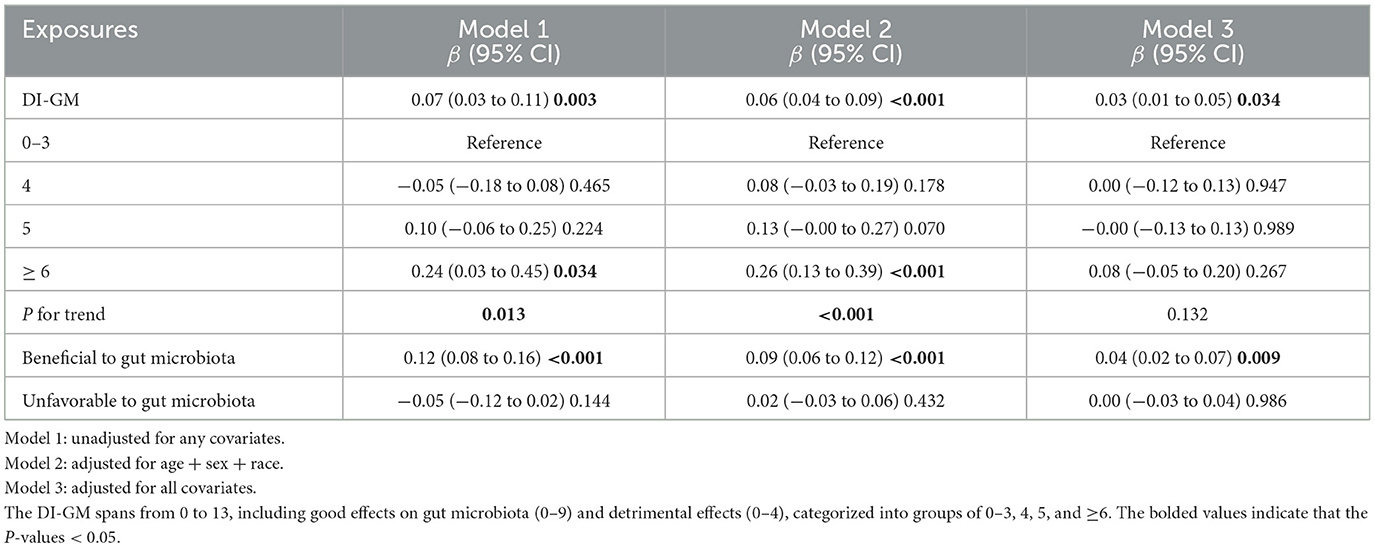
Table 2 demonstrated that the original model's overall cognitive function score rose 0.07 units for each 1-point DI-GM increase. (β = 0.07, 95% CI: 0.03–0.11, P = 0.003). After controlling all confounders, DI-GM score boosted cognitive function by 0.03 units every 1-point rise (β = 0.03, 95% CI: 0.01–0.05, P = 0.034). In addition, although the results were not significant in model 3, the association with cognitive function scores was significantly increased for the group with DI-GM ≥ 6 compared to those with 0–3 scores in model 1 and model 2 (model 1: P for trend = 0.013; model 2: P for trend < 0.001), and for each unit of elevation of the DIGM, the cognitive function scores increased by 0.24 and 0.26 units higher, respectively (Model 1: β = 0.24, 95% CI: 0.03–0.45, P = 0.034; model 2: β = 0.26, 95% CI: 0.13–0.39, P < 0.001). Additionally, cognitive function scores significantly increased with increasing benefit to gut flora, (β = 0.04, 95% CI: 0.02–0.07, P = 0.009). Whereas the correlation between adverse gut flora and cognitive function scores was not substantial (β = 0.00, 95% CI: −0.03–0.04, P = 0.986).
3.3 Dose-response relationship between DI-GM and cognitive function
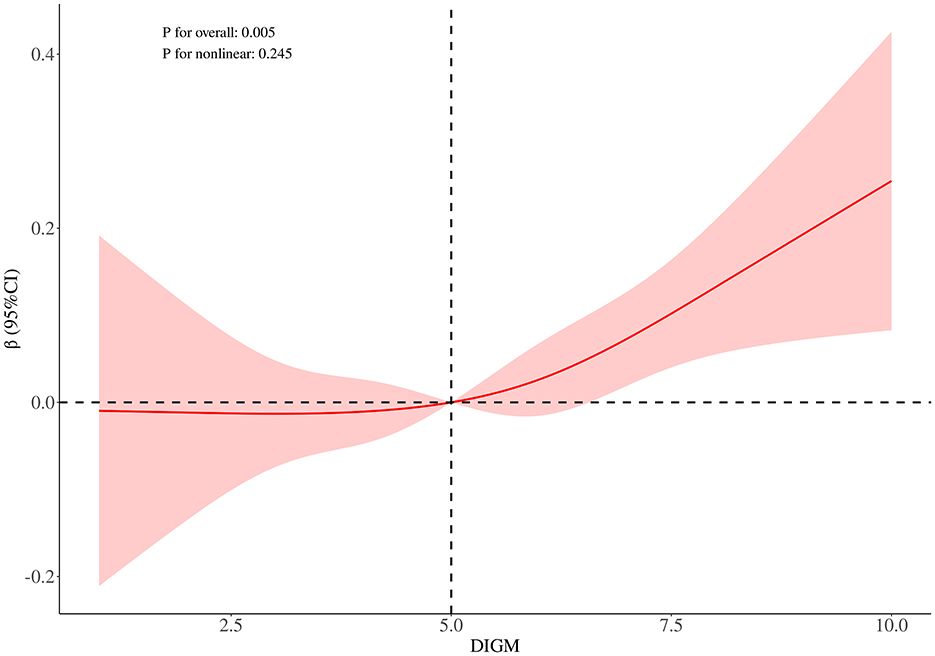
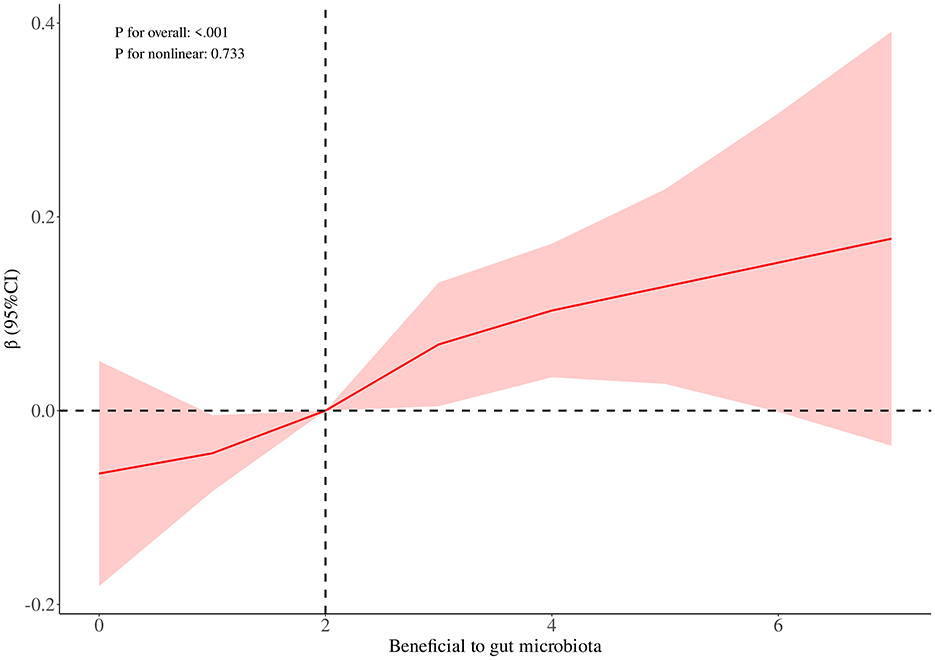
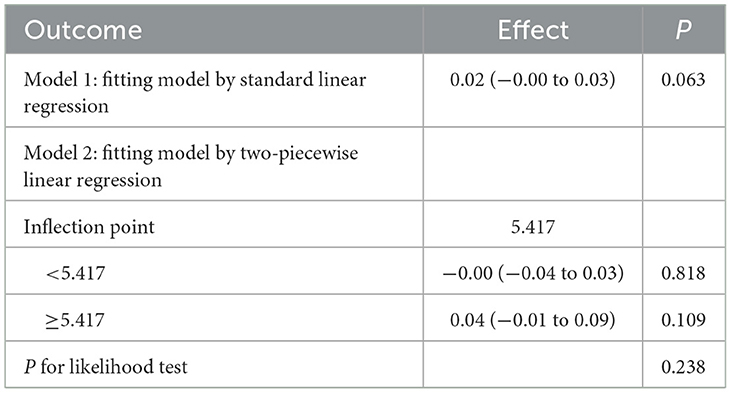
RCS curve analysis revealed a significant positive association between DIGM and the outcome variables (P for overall = 0.005), with no significant non-linear trend (P for nonlinear = 0.245), but the effect only increased after DIGM exceeded 5, suggesting that it had a significant impact on health outcomes only at higher levels (Figure 2). On the other hand, the good for gut microbes factor showed a sustained positive effect from low levels (P for overall < 0.001) without a significant non-linear trend (P for nonlinear = 0.733), suggesting that its benefits on health outcomes are broadly applicable with a linear cumulative effect (Figure 3). This was also demonstrated through threshold analysis, as shown in Tables 3, 4.
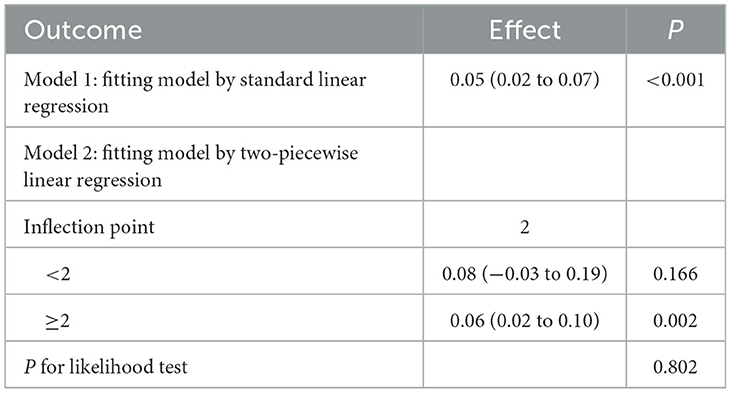
Table 4. Validation of the linear relationship of beneficial to gut microbiota with cognitive function.
3.4 Subgroup analyses between DI-GM and cognitive function
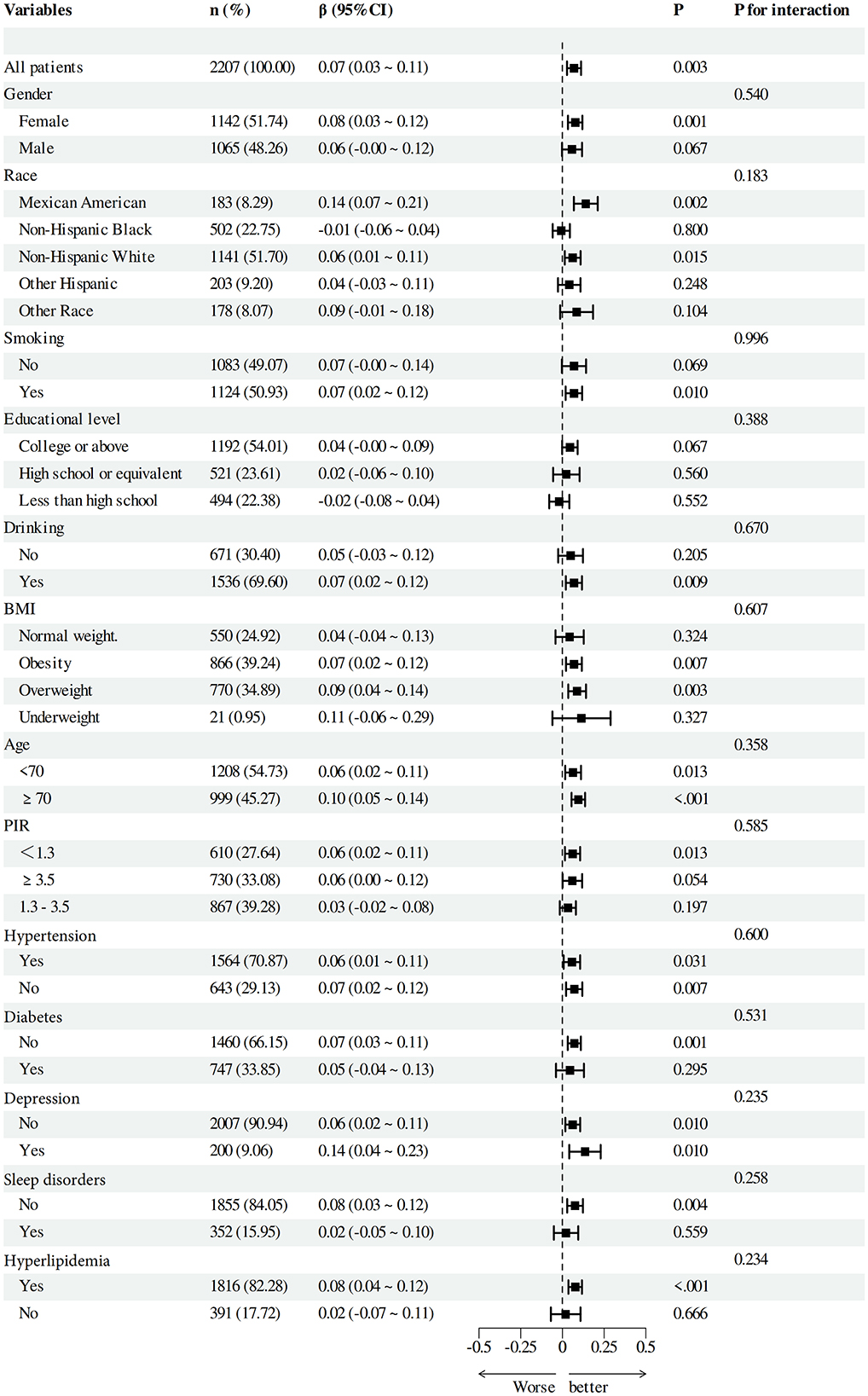
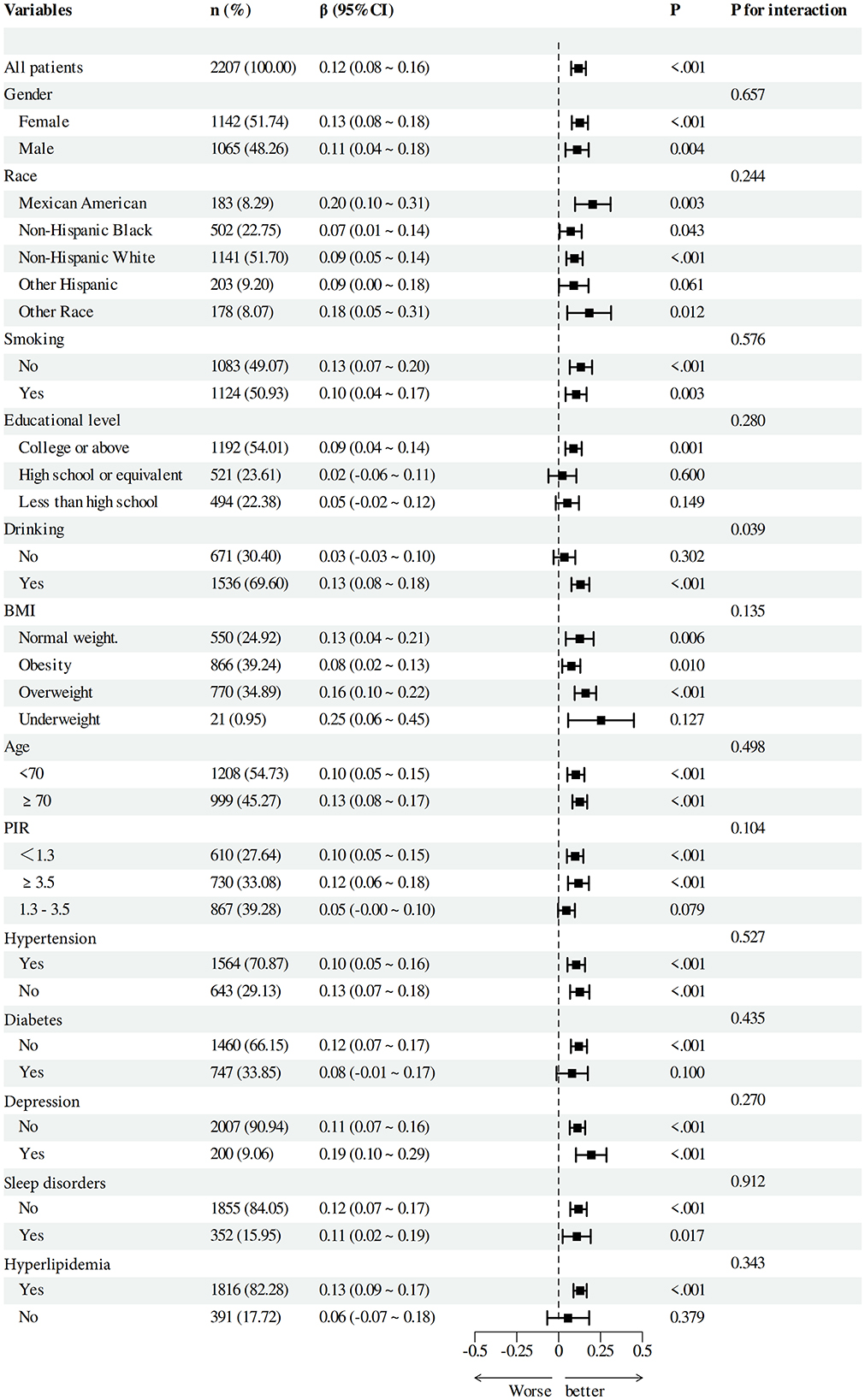
To verify the solidity of the correlation between DI-GM and Beneficial to gut microbiota and cognitive function scores, we performed subgroup analyses based on categorical factors (Figures 4, 5). The findings indicated that the positive correlation between DI-GM and cognitive function scores remained stable across all subgroups, including age, gender, ethnicity, smoking, education, alcohol consumption, BMI, PIR, hypertension, diabetes, depression, and hyperlipidemia, and no significant differences were found between subgroups (P > 0.05).
4 Discussion
The results of our investigation revealed a strong positive linear correlation between increased DI-GM scores—especially those elements deemed beneficial to the gut microbiota—and overall cognitive performance scores. Furthermore, the identified correlation between the dietary index and cognitive function persisted throughout many subgroup analyses, demonstrating the strength of the link regardless of stratification variables.
There has been an increasing amount of research that has concentrated on the systemic health implications of nutrition on intestinal microbiota. In this context, dietary indices of gut flora have been proposed as an integrative dietary assessment tool to quantify the potential modulatory effects of diet on gut microecology. Latest studies have shown that these dietary indicators are inversely correlated with chronic conditions, including the constipation index (18), diabetes mellitus (19), sleep disorders (20), and stroke (21), which preliminarily validates the value of the application of DIGM in health prediction and intervention assessment.
Although the observed effect size in our study was modest (e.g., β = 0.03), its clinical relevance should not be overlooked. Prior studies have demonstrated that even small changes in cognitive test performance can reflect meaningful shifts in cognitive function, particularly in older adults. For instance, Jehu et al. (22) reported that a change of 3–5 points on the DSST may represent the minimal clinically important difference (MCID) in community-dwelling older adults. Similarly, for the Montreal Cognitive Assessment (MoCA), an MCID of 1–2 points has been established in populations with neurological conditions such as stroke and subarachnoid hemorrhage (23).
Given that the Dietary Index for Gut Microbiota (DI-GM) represents a modifiable lifestyle factor, even slight improvements in cognitive performance associated with higher DI-GM scores may translate into meaningful benefits at the population level, particularly when scaled across aging societies. Moreover, from a public health perspective, small individual-level improvements in cognitive function—if maintained over time—could help delay the onset of cognitive impairment and reduce dementia incidence. Therefore, while the effect sizes were numerically small, they may still carry substantial clinical and epidemiological significance.
The gut-brain axis is a complex network facilitating reciprocal interaction between the gastrointestinal system and the brain, significantly influencing cognitive function. This interaction is mediated through multiple interconnected pathways, including neural, hormonal, and immunological mechanisms. Among these, the neural route—particularly the vagus nerve—serves as a principal conduit, facilitating the transmission of signals from the gut to the brain and vice versa. The vagus nerve enables the central nervous system to receive and respond to physiological information originating from the intestinal environment, thereby supporting the dynamic regulation of brain function (24). For example, glutamate stimulation in the gut can cause activation of specific areas of the brain (e.g., insular cortex, limbic system, and hypothalamus) via the vague nerve and induce conditioned taste preferences (25). Our study provides further evidence that microecological improvements led by a healthy diet (e.g., high dietary fiber, increased intake of prebiotics) may enhance cognitive performance by elevating the DI-GM index.
In terms of immune pathways, the gut microbiota regulates the function of immune cells in the gut, systemically and in the central nervous system. Imbalances in gut flora can trigger inflammatory responses that affect neural function in the brain through the release of cytokines. For example, in sepsis-associated encephalopathy, an imbalance in gut flora activates inflammatory signaling pathways that lead to neuronal damage and cognitive deficits through the neuroimmune pathway of the gut-brain axis (26). Furthermore, Intestinal metabolites such short-chain fatty acids, bile acids, and tryptophan alter the gut-brain axis or directly act on microglia to modulate central nervous system activity, leading to neurodegenerative and neurodevelopmental diseases (27).
Emerging evidence indicates that individual DI-GM components engage the gut-brain axis through distinct, nutrient-specific pathways. Fermented dairy products deliver viable lactic-acid bacteria and bifidobacteria that transiently colonize the colon and generate metabolites such as lactate and acetate. These species, together with their short-chain fatty acid (SCFA) by-products, have been shown to reinforce epithelial tight-junction expression, attenuate microglial activation, and ultimately support memory performance (28–30).
By contrast, the prebiotic fraction of whole grains—principally β-glucans—selectively enriches butyrate-producing taxa, including Faecalibacterium prausnitzii and Roseburia spp. Randomized trials demonstrate that the resulting rise in colonic butyrate not only enhances gut barrier integrity but also modulates histone-deacetylase activity and synaptic plasticity, thereby providing a mechanistic link to slower cognitive decline observed in prospective cohorts (29, 31, 32).
In red and processed meats, high concentrations of heme iron and lipid peroxidation products stimulate bile secretion and favor the proliferation of bile-tolerant, lipopolysaccharide (LPS). Animal-based diets have been shown to raise circulating LPS levels and systemic cytokine concentrations, setting in motion neuroinflammatory cascades that compromise hippocampal long-term potentiation (16, 33).
Several studies have shown that specific dietary components and patterns can positively affect cognitive function. For example, a systematic review and meta-analysis of cognitively healthy adults found that interventions involving key dietary components improved global cognition, executive function and processing speed. An analysis, which included 15 trials (with a total of 6,480 participants), showed that interventions led to improvements in global cognition, executive function and processing speed (34). Furthermore, two mouse models used in neurodegenerative research—the APPswe/PS1De9 model and the senescence-accelerated mice-prone-8—show that supplementation with MCTs and DHA affects gut microbiota, inflammation, and cognitive performance (35). Moreover, research involving maintenance haemodialysis patients indicates that Roseburia within the gut microbiota may significantly influence cognitive performance, with some bacterial genera exhibiting favorable correlations with cognitive abilities or domains (36).
Our cognitive battery included CERAD for verbal learning and delayed recall, AFT for semantic fluency, and DSST for processing speed and executive control. While sensitive to early change, these tools do not cover domains such as visuospatial perception (e.g., Rey–Osterrieth Figure) or pure working-memory span (e.g., digit span tasks). Each test also imposes distinct cognitive demands—CERAD on encoding, AFT on rapid retrieval, and DSST on psychomotor speed—so our results primarily reflect these functions. Future work should add measures like the Trail Making Test B and N-back tasks to capture unmeasured domains.
Our findings provide a foundation for developing actionable dietary guidelines to support cognitive health via gut microbiota modulation. Practically, the DI-GM results can be translated into microbiota-supportive dietary patterns that align closely with established Mediterranean dietary frameworks. Specifically, increasing consumption of fermented dairy products (such as yogurt and kefir), whole grains rich in fermentable fiber, and polyphenol-rich fruits and vegetables, coupled with limiting intake of red and processed meats, could effectively enhance gut microbiota health and cognitive resilience. At the personalized level, DI-GM scores can serve as simple screening tools to identify suboptimal dietary habits linked to microbiota-related cognitive risk. Dietitians and clinicians could utilize this index to provide targeted dietary counseling tailored to individual needs. At the population level, integrating DI-GM-informed dietary recommendations into public health guidelines can offer cost-effective, evidence-based preventive strategies to mitigate cognitive decline through improved dietary quality and microbiota diversity.
We acknowledge several limitations. First, NHANES relies on 24 h dietary recall without direct microbiome sequencing, so we used the DI-GM as a proxy—justified by studies linking long-term diet to microbiota (37, 38)—but future work should include sequencing data and more comprehensive dietary assessments (e.g., repeated recalls or FFQs). Second, DI-GM equally weights all items, which may oversimplify differential effects [e.g., fermented dairy or fiber-rich whole grains likely have greater microbial and neurocognitive impact than coffee or tea (39–41)]; data-driven weighting should be explored. Third, the index covers individual foods rather than broader groups and omits items unavailable in NHANES (e.g., green tea) or unrepresented components (e.g., non-fermented dairy), and it does not account for cooking methods—factors that may affect comparability and predictive utility. Fourth, our cognitive battery (CERAD, AFT, DSST) captures key but not all domains. Future work should add measures like the Trail Making Test B and N-back tasks to capture unmeasured domains. Finally, the cross-sectional design precludes causal inference, and selection bias from excluded participants (older, less educated, poorer health) may yield conservative estimates. Despite these constraints, the rigorous dietary and cognitive assessments in a nationally representative sample enhance the study's validity and generalizability.
To strengthen the robustness and generalizability of the DI-GM, future research should prioritize validating this dietary index in diverse populations of older adults across various geographic and ethnic backgrounds. Additionally, the cross-sectional nature of the current study precludes causal interpretations. Thus, well-designed longitudinal studies, including prospective cohort studies or randomized controlled dietary intervention trials, are necessary to determine whether dietary changes aligned with higher DI-GM scores effectively enhance gut microbiota health and cognitive function over time. Such studies would provide stronger evidence of causality, clarify temporal relationships, and inform targeted dietary guidelines aimed at promoting cognitive resilience through microbiota modulation.
In addition to validating the DI-GM in diverse populations and conducting longitudinal analyses, future research should further explore the role of specific dietary components—particularly macronutrients (e.g., fiber, saturated fat, protein types) and micronutrients (42, 43) (e.g., B vitamins, polyphenols, magnesium)—in shaping gut microbiota and cognitive function. While DI-GM offers a food-based, microbiota-oriented dietary measure, it does not capture the full complexity of nutrient interactions. Detailed nutrient-level analyses may help clarify the mechanisms through which diet influences the gut-brain axis and identify specific bioactive compounds that mediate cognitive benefits. Future studies leveraging NHANES nutrient intake data and biomarker panels could yield important insights into these pathways and complement the findings derived from food-based indices like DI-GM.
5 Conclusions
A favorable linear connection between cognitive performance and diet-affected DI-GM has been demonstrated in this investigation. This study supports the concept of “cognitive intervention targeting gut microecology” and provides a theoretical basis for dietary intervention strategies for cognitive impairment. Nonetheless, due to the observational nature of this investigation, foreseeable cohort studies and randomized controlled trials are essential to further substantiate the direct effect of DI-GM on cognitive function and to explore its molecular processes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
CS: Conceptualization, Data curation, Methodology, Software, Writing – original draft, Writing – review & editing. TT: Conceptualization, Supervision, Writing – original draft. ZC: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
All authors would like to acknowledge the NHANES participants who contributed to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1618220/full#supplementary-material
References
1. GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the global burden of disease study 2019. Lancet Public Health. (2022) 7:e105–25.
2. Do D, Schnittker J. Utilization of medications with cognitive impairment side effects and the implications for older adults' cognitive function. J Aging Health. (2020) 32:1165–77. doi: 10.1177/0898264319895842
3. López-Álvarez J, Zea Sevilla MA, Agüera Ortiz L, Fernández Blázquez MÁ, Valentí Soler M, Martínez-Martín P. Effect of anticholinergic drugs on cognitive impairment in the elderly. Revista de Psiquiatría y Salud Mental (English Edition). (2015) 8:35–43. doi: 10.1016/j.rpsmen.2015.03.001
4. Petersson SD, Philippou E. Mediterranean diet, cognitive function, and dementia: a systematic review of the evidence. Adv Nutr. (2016) 7:889–904. doi: 10.3945/an.116.012138
5. Liu Z, Dai X, Zhang H, Shi R, Hui Y, Jin X, et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat Commun. (2020) 11:855. doi: 10.1038/s41467-020-14676-4
6. Chen Y, Xu J, Chen Y. Regulation of Neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients. (2021) 13:2099. doi: 10.3390/nu13062099
7. Chen C, Liao J, Xia Y, Liu X, Jones R, Haran J, et al. Gut microbiota regulate Alzheimer's disease pathologies and cognitive disorders via PUFA-associated neuroinflammation. Gut. (2022) 71:2233–52. doi: 10.1136/gutjnl-2021-326269
8. Kim C-S, Cha L, Sim M, Jung S, Chun WY, Baik HW, et al. Probiotic supplementation improves cognitive function and mood with changes in gut microbiota in community-dwelling older adults: a randomized, double-blind, placebo-controlled, multicenter trial. J Gerontol A Biol Sci Med Sci. (2020) 76:32–40. doi: 10.1093/gerona/glaa090
9. Ribeiro G, Ferri A, Clarke G, Cryan JF. Diet and the microbiota - gut - brain-axis: a primer for clinical nutrition. Curr Opin Clin Nutr Metab Care. (2022) 25:443–50. doi: 10.1097/MCO.0000000000000874
10. Barber TM, Kabisch S, Pfeiffer AFH, Weickert MO. The effects of the mediterranean diet on health and gut microbiota. Nutrients. (2023) 15:2150. doi: 10.3390/nu15092150
11. Kase BE, Liese AD, Zhang J, Murphy EA, Zhao L, Steck SE. The development and evaluation of a literature-based dietary index for gut microbiota. Nutrients. (2024) 16:1045. doi: 10.3390/nu16071045
12. Ghosh TS, Rampelli S, Jeffery IB, Santoro A, Neto M, Capri M, et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut. (2020) 69:1218–28. doi: 10.1136/gutjnl-2019-319654
13. Berding K, Carbia C, Cryan JF. Going with the grain: fiber, cognition, and the microbiota-gut-brain-axis. Exp Biol Med. (2021) 246:796–811. doi: 10.1177/1535370221995785
14. Wang Y, Uffelman CN, Bergia RE, Clark CM, Reed JB, Cross T-WL, et al. Meat consumption and gut microbiota: a scoping review of literature and systematic review of randomized controlled trials in adults. Adv Nutr. (2023) 14:215–37. doi: 10.1016/j.advnut.2022.10.005
15. Gao J, Guo X, Wei W, Li R, Hu K, Liu X, et al. The association of fried meat consumption with the gut microbiota and fecal metabolites and its impact on glucose homoeostasis, intestinal endotoxin levels, and systemic inflammation: a randomized controlled-feeding trial. Diabetes Care. (2021) 44:1970–9. doi: 10.2337/dc21-0099
16. Engler-Chiurazzi EB, Russell AE, Povroznik JM, McDonald KO, Porter KN, Wang DS, et al. Intermittent systemic exposure to lipopolysaccharide-induced inflammation disrupts hippocampal long-term potentiation and impairs cognition in aging male mice. Brain Behav Immun. (2023) 108:279–91. doi: 10.1016/j.bbi.2022.12.013
17. Wei J, Wang L, Kulshreshtha A, Xu H. Adherence to life's simple 7 and cognitive function among older adults: the national health and nutrition examination survey 2011 to 2014. J Am Heart Assoc. (2022) 11:e022959–e022959. doi: 10.1161/JAHA.121.022959
18. Zhang Z, Bi C, Wu R, Qu M. Association of the newly proposed dietary index for gut microbiota and constipation: a cross-sectional study from NHANES. Front Nutr. (2025) 12:1529373. doi: 10.3389/fnut.2025.1529373
19. Huang Y, Liu X, Lin C, Chen X, Li Y, Huang Y, et al. Association between the dietary index for gut microbiota and diabetes: the mediating role of phenotypic age and body mass index. Front Nutr. (2025) 12:1519346. doi: 10.3389/fnut.2025.1519346
20. Li Y, Pan F, Shen X. Association of the dietary index for gut microbiota with sleep disorder among US adults: the mediation effect of dietary inflammation index. Front Nutr. (2025) 12:1528677. doi: 10.3389/fnut.2025.1528677
21. Liu J, Huang S. Dietary index for gut microbiota is associated with stroke among US adults. Food Funct. (2025) 16:1458–68. doi: 10.1039/D4FO04649H
22. Jehu DA, Davis JC, Madden K, Parmar N, Liu-Ambrose T. Minimal clinically important difference of executive function performance in older adults who fall: a secondary analysis of a randomized controlled trial. Gerontology. (2022) 68:771–9. doi: 10.1159/000518939
23. Wong GKC, Mak JSY, Wong A, Zheng VZY, Poon WS, Abrigo J, et al. Minimum clinically important difference of montreal cognitive assessment in aneurysmal subarachnoid hemorrhage patients. J Clin Neurosci. (2017) 46:41–4. doi: 10.1016/j.jocn.2017.08.039
24. Arneth BM. Gut-brain axis biochemical signalling from the gastrointestinal tract to the central nervous system: gut dysbiosis and altered brain function. Postgrad Med J. (2018) 94:446–52. doi: 10.1136/postgradmedj-2017-135424
25. Nakamura E, Uneyama H, Torii K. Gastrointestinal nutrient chemosensing and the gut-brain axis: significance of glutamate signaling for normal digestion. J Gastroenterol Hepatol. (2013) 28:2–8. doi: 10.1111/jgh.12408
26. Wang X, Wen X, Yuan S, Zhang J. Gut-brain axis in the pathogenesis of sepsis-associated encephalopathy. Neurobiol Dis. (2024) 195:106499. doi: 10.1016/j.nbd.2024.106499
27. Deng W, Yi P, Xiong Y, Ying J, Lin Y, Dong Y, et al. Gut metabolites acting on the gut-brain axis: regulating the functional state of microglia. Aging Dis. (2024) 15:480. doi: 10.14336/AD.2023.0727
28. Marco ML, Heeney D, Binda S, Cifelli CJ, Cotter PD, Foligné B, et al. Health benefits of fermented foods: Microbiota and beyond. Curr Opin Biotechnol. (2017) 44:94–102. doi: 10.1016/j.copbio.2016.11.010
29. Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. (2019) 16:461–78. doi: 10.1038/s41575-019-0157-3
30. Fock E, Parnova R. Mechanisms of blood-brain barrier protection by microbiota-derived short-chain fatty acids. Cells. (2023) 12:657. doi: 10.3390/cells12040657
31. Martínez I, Lattimer JM, Hubach KL, Case JA, Yang J, Weber CG, et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. ISME J. (2012) 7:269–80. doi: 10.1038/ismej.2012.104
32. Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: the bread and butter of the microbiota-gut-brain axis? Neurochem Int. (2016) 99:110–32. doi: 10.1016/j.neuint.2016.06.011
33. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. (2013) 505:559–63. doi: 10.1038/nature12820
34. McEvoy CT, Leng Y, Peeters GM, Kaup AR, Allen IE, Yaffe K. Interventions involving a major dietary component improve cognitive function in cognitively healthy adults: a systematic review and meta-analysis. Nutr Res. (2019) 66:1–12. doi: 10.1016/j.nutres.2019.02.008
35. Wang Z, Sun Y, Zhang D, Wang Y, Zhou D, Li W, et al. Gut microbiota and inflammation analyses reveal the protective effect of medium-chain triglycerides combined with docosahexaenoic acid on cognitive function in APP/PS1 and SAMP8 mice. Nutr Res. (2024) 132:27–39. doi: 10.1016/j.nutres.2024.09.015
36. Gao Q, Li D, Wang Y, Zhao C, Li M, Xiao J, et al. Analysis of intestinal flora and cognitive function in maintenance hemodialysis patients using combined 16S ribosome DNA and shotgun metagenome sequencing. Aging Clin Exp Res. (2024) 36:28. doi: 10.1007/s40520-023-02645-y
37. Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y-Y, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. (2011) 334:105–8. doi: 10.1126/science.1208344
38. De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. (2010) 107:14691–6. doi: 10.1073/pnas.1005963107
39. Saygili S, Hegde S, Shi X-Z. Effects of coffee on gut microbiota and bowel functions in health and diseases: a literature review. Nutrients. (2024) 16:3155. doi: 10.3390/nu16183155
40. Ross AB, Shertukde SP, Livingston Staffier K, Chung M, Jacques PF, McKeown NM. The relationship between whole-grain intake and measures of cognitive decline, mood, and anxiety-a systematic review. Adv Nutr. (2023) 14:652–70. doi: 10.1016/j.advnut.2023.04.003
41. Schneider E, Balasubramanian R, Ferri A, Cotter PD, Clarke G, Cryan JF. Fibre & fermented foods: differential effects on the microbiota-gut-brain axis. Proc Nutr Soc. (2024) 1–16. doi: 10.1017/S0029665124004907
42. Rosa A, Pujia AM, Arcuri C. The protective role antioxidant of vitamin C in the prevention of oral disease: a scoping review of current literature. Eur J Dent. (2024) 18:965–70. doi: 10.1055/s-0044-1786845
Keywords: dietary index for gut microbiota, cognitive function, cross-sectional study, NHANES, older people
Citation: Sun C, Tan T and Chen Z (2025) Exploring the relationship between dietary index for gut microbiota and cognitive function. Front. Nutr. 12:1618220. doi: 10.3389/fnut.2025.1618220
Received: 25 April 2025; Accepted: 21 July 2025;
Published: 07 August 2025.
Edited by:
Patrick Noël Pallier, Queen Mary University of London, United KingdomReviewed by:
Alessio Rosa, University of Rome Tor Vergata, ItalyÖzge Cemali, Trakya University, Türkiye
Copyright © 2025 Sun, Tan and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zeping Chen, emVwaW5nNDYxNEAxNjMuY29t
 Changhu Sun1
Changhu Sun1 Zeping Chen
Zeping Chen