- Eye Hospital, China Academy of Chinese Medical Sciences, Beijing, China
Background: Although many studies have pointed to the role of manganese in various diseases. However, there are surprisingly few studies on the potential relationship between manganese and diabetic retinopathy (DR). The available literature fails to provide definitive conclusions regarding the directionality and strength of this particular association.
Methods: The analytical cohort comprised 2,558 adults from NHANES 2011–2020 cycles. We employed binary logistic regression to evaluate manganese-DR associations, supplemented by subgroup analyses, nonparametric smoothing techniques, and propensity score weighting to address potential confounding.
Results: Our multivariate model failed to find a significant linear relationship between manganese concentration and the likelihood of DR (p > 0.05). However, we found that manganese levels above 7.66 μg/L DR were less severe (adjusted OR = 0.84, p = 0.0007). This suggests a nonlinear dose–response relationship.
Conclusion: The manganese and DR relationship followed a U-shaped dose–response pattern. The least severe condition was observed at 7.66 μg/L, while the disease was aggravated at both insufficient and excessive concentrations.
1 Introduction
DR is a major cause of adult vision loss (1). It involves damage to small blood vessels and nerve cells as well as neovascularization due to chronic hyperglycemia in the body (2). Globally, diabetes affects approximately 537 million people (3). About 103 million adults with diabetes have DR (4). The burden of DR continues to grow. And also from high-income countries to low- and middle-income regions (5). Consequently, combating DR is critical. The pathogenesis of DR primarily stems from long-term diabetes, making glycemic control the cornerstone of its management. Yet it remains highly challenging (6). Growing evidence suggests that trace elements, particularly those involved in antioxidant defense, may modulate DR progression (7–9). Exploring the relationship between micronutrients and DR may help control DR.
Manganese is an important trace element that is widely found in plant foods. It is also effective in preventing inflammation, vascular disease and cancer (10). Manganese acts as a key component of Mn-SOD, the main mitochondrial enzyme responsible for neutralizing superoxide radicals. Therefore, manganese helps relieve oxidative stress and chronic inflammation (11).
It is well known that oxidative Stress as a Major Cause of DR Progression. Experimental studies have shown that Mn-SOD activity attenuates oxidative damage in retinal cells (12). Manganese plays an important role in glucose metabolism (13). Manganese significantly inhibits pro-inflammatory signaling such as TNF-α and IL-11 at moderate concentrations (14). However, while manganese is crucial for health, excessive intake can be harmful (15). Accumulation of excess Manganese may exacerbate oxidative stress via Fenton chemical reaction and and destroys the retinal structure (16). Both manganese deficiency and overload may aggravate oxidative stress and inflammation, worsening DR. While mechanistic understanding has advanced, population-based evidence regarding manganese and DR associations remains limited. Our analysis of NHANES 2011–2020 data seeks to clarify this relationship. Our findings clarify manganese’s dual role in DR pathogenesis, and reveal its potential in diabetes prevention and management.
2 Materials and methods
2.1 Study participants
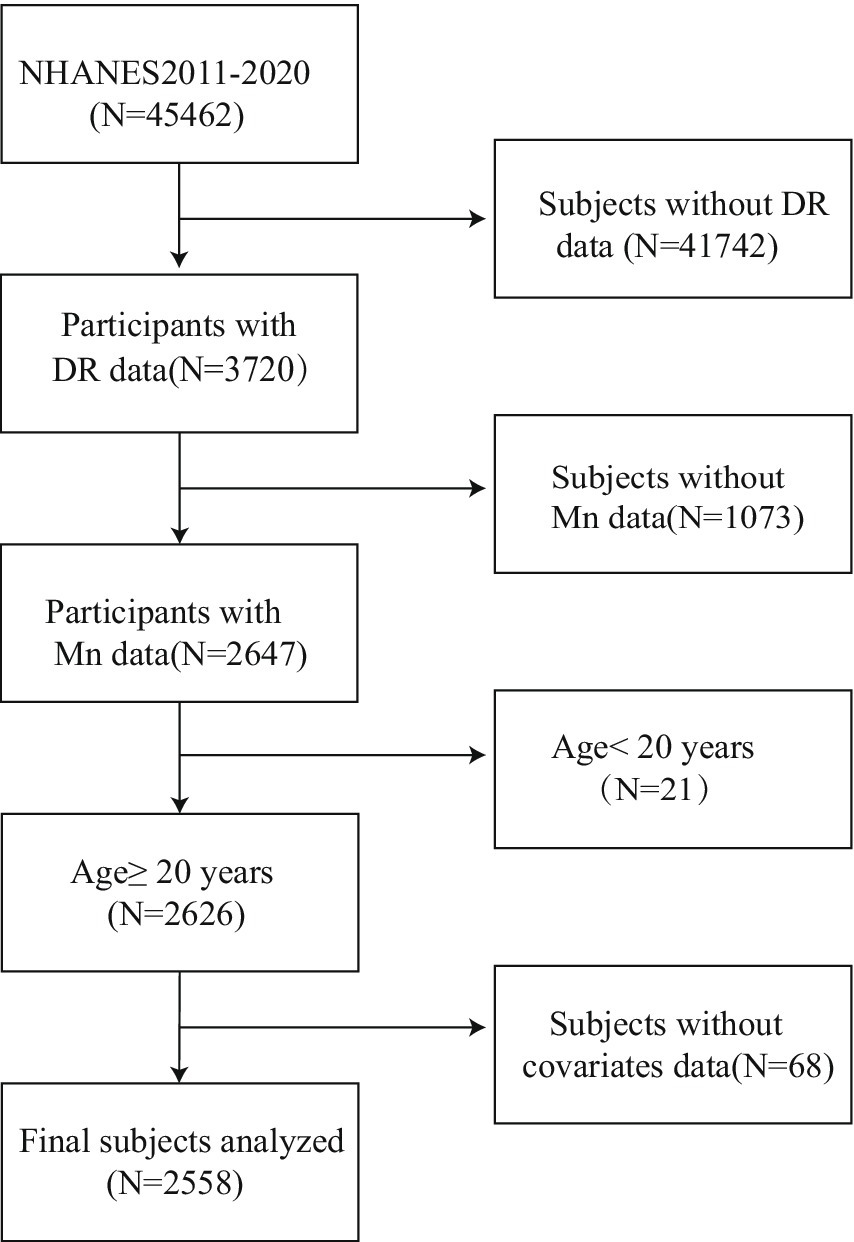
TheNHANES implements a complex, multistage sampling design to generate nationally representative estimates. Our analysis utilized 2011–2020 survey cycles, incorporating both physical examinations and standardized interviews from participating adults. During this period, there were 45,462 participants. We excluded participants with missing DR data (N = 41,742) and missing blood manganese data (N = 1,073). The final analytical cohort comprised 2,558 eligible adults after excluding minors (n = 21) and participants with incomplete covariate data (n = 68). The complete participant selection flowchart is presented in Figure 1.
2.2 Diabetic retinopathy assessment
Diabetes case identification required meeting at least one of five criteria: (1) HbA1c ≥ 6.5%; (2) Fasting glucose ≥7.0 mmol/L; (3) 2-h OGTT glucose ≥11.1 mmol/L; (4) Self-reported physician-diagnosed diabetes; (5) Current use of glucose-lowering therapy. Among identified diabetics, retinopathy status was determined through standardized self-report (DIQ080), with affirmative responses (“yes”) classifying participants as DR cases.
2.3 Determination of blood manganese
Whole blood samples were processed to measure hemorrhagic manganese levels. Measured blood manganese levels are cross-sectional in nature. All blood samples were placed in test tubes containing anticoagulant reagents. They were frozen at −30°C and sent to CDC labs in Atlanta for testing. Before analysis, we mixed samples well to avoid clotting. Whole blood manganese (Mn) concentrations were determined using inductively coupled plasma mass spectrometry (ICP-MS). We diluted them (1:1:48 with water and diluent) before ICP-MS analysis. This method enables high-sensitivity multi-element analysis, ensuring accurate quantification of trace elements. All results met minimum detection limits.
2.4 Covariates assessment
Demographic variables comprised sex, age, racial/ethnic background, marital situation, and educational attainment. BMI classifications followed standard cutoffs: under 25 (normal), 25–29.9 (overweight), and 30 + kg/m2 (obese). Alcohol consumption was categorized as whether or not they drank more than 4–5 drinks per day based on self-reports. Smoking status was dichotomized into “never smokers” (lifetime smoking <100 cigarettes) and “ever smokers.” Economic status was grouped as “below poverty” (income-to-poverty ratio <1) or “poverty or above” (≥1). Sleep disorders were recorded as present or absent based on self-reports. Participants were considered hypertensive if they met any of these criteria: (1) physician-diagnosed hypertension; (2) current antihypertensive drug use; (3) blood pressure measurements exceeding normal limits.
2.5 Statistical analysis
We used weighted statistical methods to improve accuracy. Analyses were performed using weighted multivariate logistic regression models. Independent samples t-test was used for continuous variables. Categorical variables were analyzed using chi-square tests. Logistic regression was examined for manganese and DR relationship. Subgroup analyses were conducted using covariates and further stratified multiple regression analyses were conducted to investigate the differences and potential interaction effects between the different subgroups. All analyses were performed using R (version 4.1.1) and EmpowerStats (version 4.2). A p-value ≤ 0.05 was considered statistically significant.
3 Results
3.1 Baseline population profiles
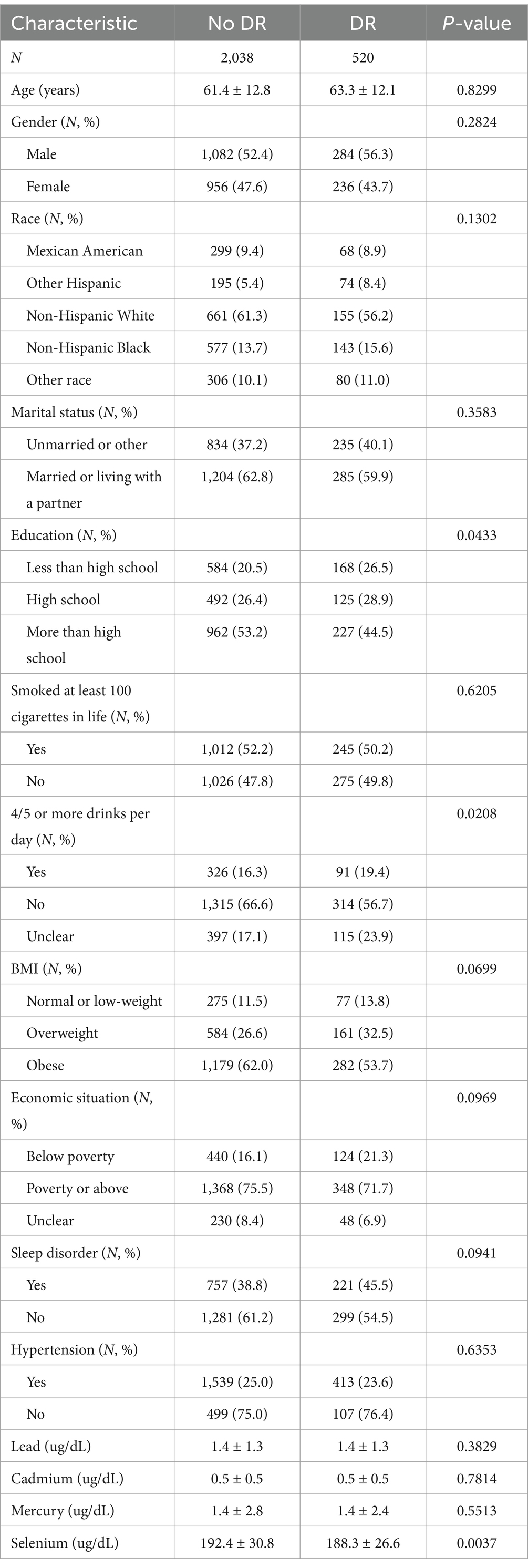
Table 1 presents the study population stratified by DR status. The analysis of DR-related factors revealed that participants with DR had significantly lower educational attainment (p = 0.0433). Heavy alcohol consumption (≥4–5 drinks/day) was more prevalent in the DR group (p = 0.0208). The prevalence of low educational level and alcohol abuse was higher in the DR group. Highlights the potential role of socioeconomic differences in DR risk. Suggests possible confounders of lifestyle factors. No intergroup variations were detected in lead, cadmium, or mercury levels (p > 0.05). However, a significant difference in selenium levels (p = 0.0037) was noted, potentially reflecting its antioxidant role in mitigating retinal oxidative damage. No other measured variables demonstrated statistically significant intergroup variations (all p > 0.05).
3.2 Manganese and DR association analysis
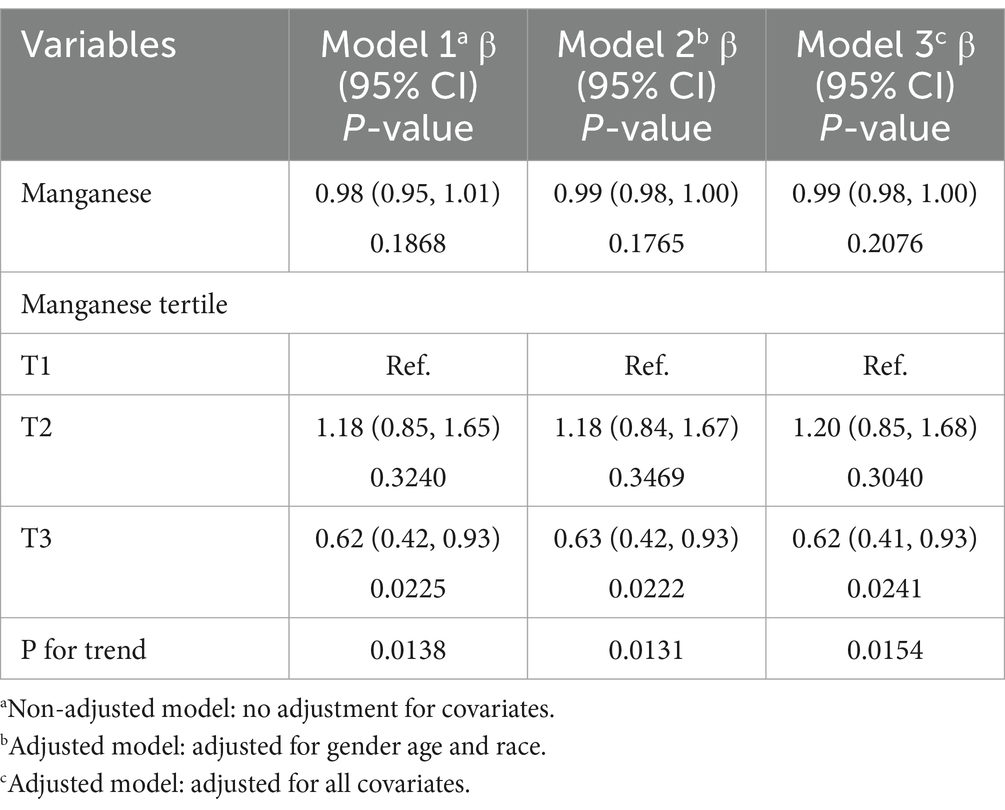
The nonlinear relationship between Manganese and DR was shown by regression analysis (Table 2). Although the linear model showed no association (p > 0.05). However, the tertile analysis revealed a U-shaped dose–response. The risk of DR was significantly lower in T3 participants compared to T1 participants (OR = 0.62, p = 0.022). In contrast, the middle tertile (T2) had no significant effect (OR = 1.18, p > 0.05). Significant trend tests (p-trend = 0.013–0.015) for all models further confirmed this nonlinear relationship. This protective effect remained stable after multivariable adjustment, suggesting a potential U-shaped relationship between manganese and DR. This suggests that manganese may have a threshold effect and that only optimal levels can be protective against DR.
3.3 Identifcation of nonlinear relationship
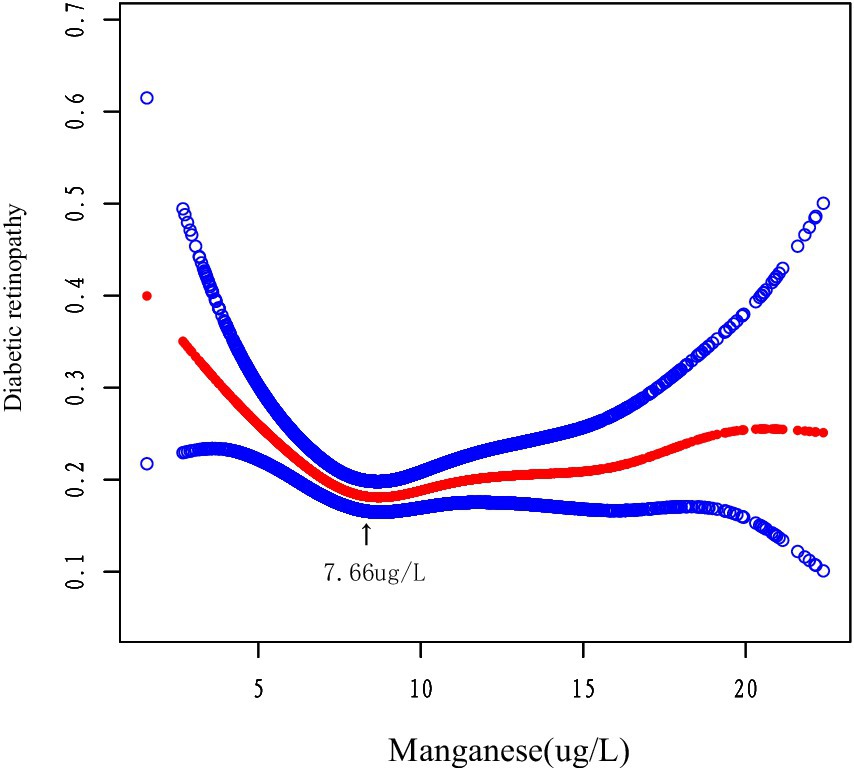
We using smoothed curve fitting (Figure 2) and segmented regression analysis (Table 3). And nonlinear modeling revealed a U-shaped manganese and DR association. Below 7.66 μg/L, each 1-unit manganese increase conferred 16% lower DR risk (OR = 0.84, p < 0.001), while higher concentrations showed null effects (OR = 1.01, p = 0.608). The piecewise model significantly outperformed linear regression (p = 0.002), indicating concentration-dependent protection.
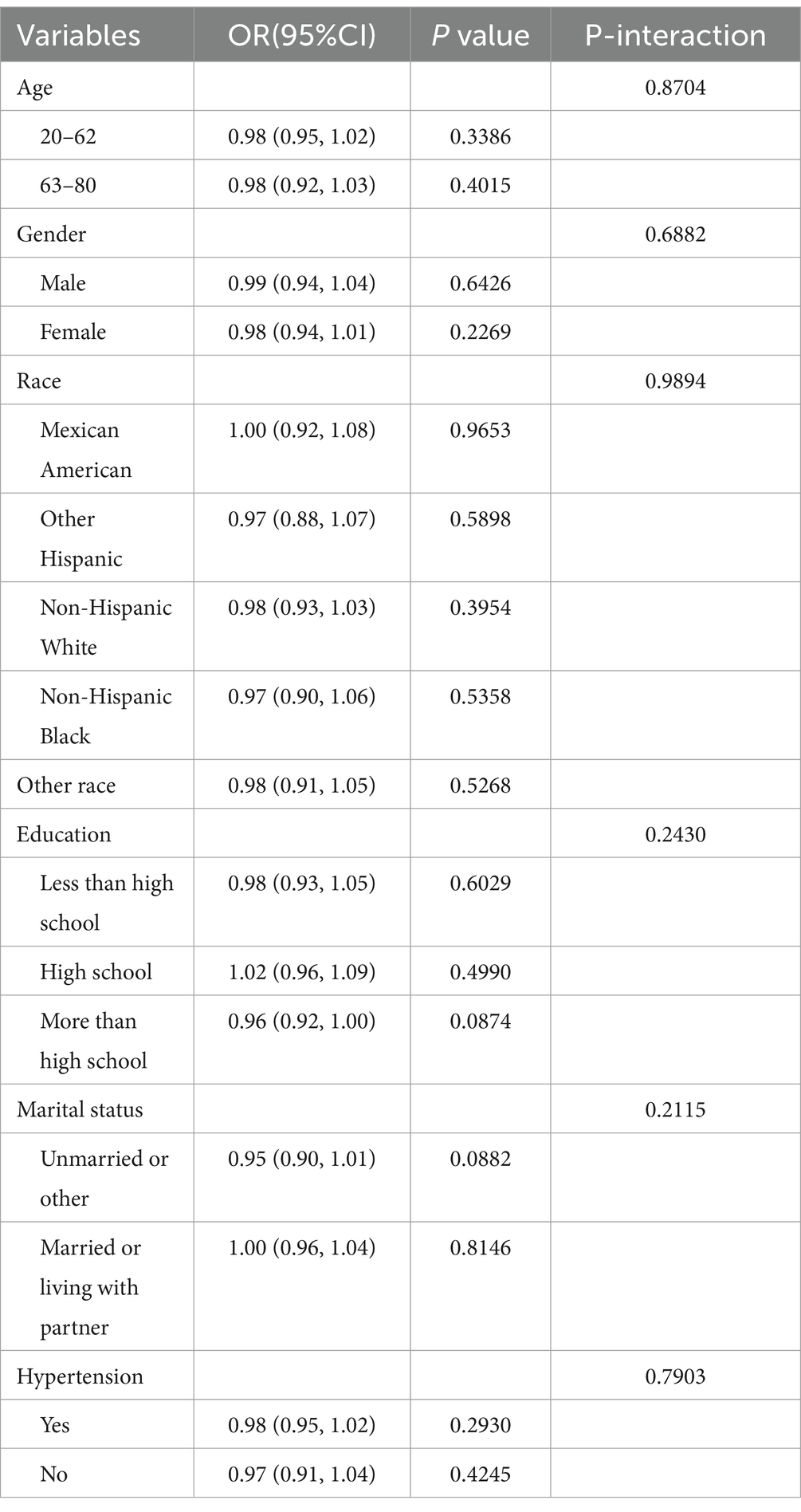
3.4 Subgroup analyses
As shown in Table 4, subgroup analyses confirmed the main null association, with no significant Mn and DR relationship observed in any population. This consistency was maintained regardless of age, biological sex, racial/ethnic background, education, marital status, or hypertension (all p values > 0.05). Notably, none of the interaction terms reached statistical significance (all p-values >0.05), indicating that the manganese and DR relationship was not modified by these demographic or clinical characteristics.
4 Discussion
This study demonstrated for the first time a U-shaped relationship between manganese levels and the risk of DR. The association remained robust after controlling for potential confounders. Our results showed that the likelihood of developing DR was significantly lower when manganese levels reached 7.66 μg/L (OR = 0.84, p = 0.0007). These results suggest that maintaining manganese levels around 7.66 μg/L may confer protective benefits. The U-shaped pattern was further confirmed through smoothed curve fitting analysis. There is no globally standardized clinical criteria for a healthy range of blood manganese. The United States Agency for Toxic Substances and Disease Registry (ATSDR) suggests that a blood manganese level of 4–15 μg/L is a common physiologic level. Notably, our data indicate that both excessively high and low manganese levels are associated with increased DR risk. This aligns with existing research demonstrating that elevated manganese concentrations may indeed elevate DR risk (17, 18). Therefore, it may be more appropriate to control blood manganese levels close to 7.66 ug/L. Additionally, the DR group showed significantly lower selenium levels compared to controls (p = 0.0037), further supporting the hypothesis that trace nutrient status may influence DR development.
Previous studies have examined associations between serum heavy metals and DR (19). Their findings indicated a significant inverse correlation between manganese and DR, while no significant associations were observed for other heavy metals including selenium. In contrast, our study identified a strong U-shaped relationship between blood manganese levels and DR risk, as well as a significant association between blood selenium levels and DR risk. The reason for this discrepancy may be that they were looking at serum heavy metals from NHANES 2011–2020. However, the NHANES database lacks data on serum selenium and manganese for 2017–2020. By selecting participants with complete blood manganese and selenium data, our results may be more reliable. Although the two studies differ in analytical methods and focus, both highlight the potential role of trace elements in DR pathogenesis. Other research has linked heavy metals to DR risk (20), though manganese was not investigated. Our study specifically addressed this gap by evaluating blood manganese and DR associations. Another study demonstrated that manganese mitigates high-glucose-induced oxidative stress in the retina (21), underscoring its therapeutic relevance. However, the protective effects at specific manganese concentrations remain unclear. Our findings reveal a U-shaped manganese and DR relationship, with levels below the threshold associated with significantly lower DR risk. Additionally, selenium deficiency emerged as a potential risk factor for DR, consistent with existing evidence (22, 23). However, the precise mechanisms by which selenium and manganese influences DR remain unknown. Future studies should further elucidate the roles of these elements in DR prevention and treatment.
Manganese may influence DR through multiple protective mechanisms. First, as an essential cofactor of manganese superoxide dismutase (Mn-SOD). Low concentration of manganese converts superoxide anions (O₂−) into H₂O₂ in mitochondria, thereby alleviating hyperglycemia-induced oxidative stress (24, 25). Second, Low concentration of manganese suppresses chronic low-grade inflammation in diabetic retina by reducing NF-κB nuclear translocation and IL-8 release (26–28). Finally, it stabilizes HIF-1α degradation, indirectly inhibiting pathological VEGF overexpression and reducing vascular leakage, thus protecting retinal vasculature (29). However, excessively high blood manganese levels may exacerbate DR. First, High concentration of manganese overload intensifies oxidative stress by accumulating in mitochondria and generating hydroxyl radicals through Fenton reaction, directly damaging retinal pigment epithelial cells (30). Second, High concentration of manganese inhibits tyrosine hydroxylase (TH) activity, reducing retinal dopamine synthesis and impairing microvascular autoregulation (31, 32). Lastly, High concentration of manganese competitively antagonizes selenium/zinc, suppressing selenium-dependent GPx and zinc metalloenzyme activities, thereby weakening coordinated antioxidant defenses (33, 34). In summary, manganese exhibits multifaceted mechanisms affecting DR pathogenesis.
This study has several limitations. First, the cross-sectional design does not provide causal inference of the relationship between Manganese and DR. The directionality of the relationship between Mn exposure and DR is unclear. We can only conclude that there is an association between the two. Thus longitudinal validation is needed. Secondly, determining DR by self-report may introduce bias. Third, because NHANES performs only a single test for diabetes-related indicators. There may be false positives in the diagnosis of diabetes. And it also fails to differentiate the typology of diabetic patients. Finally, although we have tried to be as thorough as possible in analyzing confounding factors that may affect the results of the study. However, due to the presence of unmeasured confounding variables in the database, such as diabetes duration. There are still unmeasured variables that may have influenced. Future studies should incorporate prospective designs, clinical DR assessment, and more extensive covariate collection.
5 Conclusion
In this study, the U-shaped relationship between manganese and DR was determined. It was indicating that manganese levels were associated with milder degrees of DR. In particular, moderate concentrations (7.66–12.5 μg/L) of manganese were associated with the mildest degree of DR. In addition, selenium may also reduce DR. It is hoped that future mechanistic studies will elucidate the temporal dynamics of the effects of these micronutrients in order to realize a precision nutritional approach to diabetes prevention.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://wwwn.cdc.gov/nchs/nhanes/default.aspx.
Ethics statement
The studies involving humans were approved by National Center for Health Statistics Ethics Review Board (ERB) Approval. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XC: Writing – review & editing, Investigation, Data curation, Visualization, Software, Methodology, Conceptualization, Validation, Writing – original draft, Formal analysis. ZG: Writing – review & editing, Writing – original draft. YQ: Writing – original draft, Writing – review & editing. XH: Writing – review & editing, Project administration, Writing – original draft, Supervision, Methodology. LX: Writing – original draft, Validation, Writing – review & editing, Funding acquisition, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the project to establish a High-Level Chinese Medicine Hospital at the Eye Hospital of the China Academy of Traditional Chinese Medicine (Grant No. GSP4-02-2, Grant No. GSP4-02-3), the Capital Health Development Scientific Research Specialised Key Projects (Grant No. 2020-1-4181).
Acknowledgments
We sincerely thank our research team members for their expertise and collaborative efforts. This work was made possible through access to the NHANES datasets, for which we express our profound appreciation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Heng, L, Comyn, O, Pető, T, Tadros, C, Ng, E, Sivaprasad, S, et al. Diabetic retinopathy: pathogenesis, clinical grading, management and future developments. Diabet Med. (2013) 30:640–50. doi: 10.1111/dme.12089
2. Evans, MA, Saint-Aubin, J, and Landry, N. Letter names and alphabet book reading by senior kindergarteners: an eye movement study. Child Dev. (2009) 80:1824–41. doi: 10.1111/j.1467-8624.2009.01370.x
3. Ogurtsova, K, da Rocha Fernandes, JD, Huang, Y, Linnenkamp, U, Guariguata, L, Cho, NH, et al. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. (2017) 128:40–50. doi: 10.1016/j.diabres.2017.03.024
4. Teo, ZL, Tham, YC, Yu, M, Chee, ML, Rim, TH, Cheung, N, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and Meta-analysis. Ophthalmology. (2021) 128:1580–91. doi: 10.1016/j.ophtha.2021.04.027
5. Tan, TE, and Wong, TY. Diabetic retinopathy: looking forward to 2030. Front Endocrinol (Lausanne). (2023) 13:1077669. doi: 10.3389/fendo.2022.1077669
6. Le, NT, Kroeger, ZA, Lin, WV, Khanani, AM, and Weng, CY. Novel treatments for diabetic macular edema and proliferative diabetic retinopathy. Curr Diab Rep. (2021) 21:43. doi: 10.1007/s11892-021-01412-5
7. Heidari, Z, Sepehri, Z, and Doostdar, A. Serum selenium status in patients with type 2 diabetes and control group. Glob J Health Sci. (2016) 9:234. doi: 10.5539/gjhs.v9n5p234
8. Li, YQ, Zhang, ST, Ke, NY, Fang, YC, Hu, WL, Li, GA, et al. The impact of multiple metals exposure on the risk of developing proliferative diabetic retinopathy in Anhui, China: a case-control study. Environ Sci Pollut Res Int. (2023) 30:112132–43. doi: 10.1007/s11356-023-30294-1
9. Mehta, N, Akram, M, and Singh, YP. The impact of zinc supplementation on hyperglycemia and complications of type 2 diabetes mellitus. Cureus. (2024) 16:e73473. doi: 10.7759/cureus.73473
10. Jomova, K, Makova, M, Alomar, SY, Alwasel, SH, Nepovimova, E, Kuca, K, et al. Essential metals in health and disease. Chem Biol Interact. (2022) 367:110173. doi: 10.1016/j.cbi.2022.110173
11. Liu, M, Sun, X, Chen, B, Dai, R, Xi, Z, and Xu, H. Insights into manganese superoxide dismutase and human diseases. Int J Mol Sci. (2022) 23:15893. doi: 10.3390/ijms232415893
12. Yoshinaga, A, Kajihara, N, Kukidome, D, Motoshima, H, Matsumura, T, Nishikawa, T, et al. Hypoglycemia induces mitochondrial reactive oxygen species production through increased fatty acid oxidation and promotes retinal vascular permeability in diabetic mice. Antioxid Redox Signal. (2021) 34:1245–59. doi: 10.1089/ars.2019.8008
13. Szentmihályi, K, Klébert, S, and Somogyi, A. Diabetes and trace elements. Orv Hetil. (2022) 163:1303–10. doi: 10.1556/650.2022.32550
14. Mapuskar, KA, Pulliam, CF, Tomanek-Chalkley, A, Rastogi, P, Wen, H, Dayal, S, et al. The antioxidant and anti-inflammatory activities of avasopasem manganese in age-associated, cisplatin-induced renal injury. Redox Biol. (2024) 70:103022. doi: 10.1016/j.redox.2023.103022
15. Sun, Y, and Zhang, Y. Blood manganese level and gestational diabetes mellitus: a systematic review and meta-analysis. J Obstet Gynaecol. (2023) 43:2266646. doi: 10.1080/01443615.2023.2266646
16. Aranda-Rivera, AK, Cruz-Gregorio, A, Aparicio-Trejo, OE, Ortega-Lozano, AJ, and Pedraza-Chaverri, J. Redox signaling pathways in unilateral ureteral obstruction (UUO)-induced renal fibrosis. Free Radic Biol Med. (2021) 172:65–81. doi: 10.1016/j.freeradbiomed.2021.05.034
17. Wang, X, Zhang, M, Lui, G, Chang, H, Zhang, M, Liu, W, et al. Associations of serum manganese levels with prediabetes and diabetes among >/=60-year-old Chinese adults: a population-based cross-sectional analysis. Nutrients. (2016) 8:497. doi: 10.3390/nu8080497
18. Chen, H, Cui, Z, Lu, W, Wang, P, Wang, J, Zhou, Z, et al. Association between serum manganese levels and diabetes in Chinese adults with hypertension. J Clin Hypertens. (2022) 24:918–27. doi: 10.1111/jch.14520
19. Zhang, Y, Liu, X, Zhang, X, Li, L, Li, Q, Geng, H, et al. Association between serum heavy metal levels and diabetic retinopathy in NHANES 2011-2020. Sci Rep. (2024) 14:1268. doi: 10.1038/s41598-024-51749-6
20. Gui, Y, Gui, S, Wang, X, Li, Y, Xu, Y, and Zhang, J. Exploring the relationship between heavy metals and diabetic retinopathy: a machine learning modeling approach. Sci Rep. (2024) 14:13049. doi: 10.1038/s41598-024-63916-w
21. Bryl, A, Mrugacz, M, Falkowski, M, and Zorena, K. The effect of diet and lifestyle on the course of diabetic retinopathy-a review of the literature. Nutrients. (2022) 14:1252. doi: 10.3390/nu14061252
22. Wróblewska, J, Nuszkiewicz, J, Wróblewski, M, Wróblewska, W, and Woźniak, A. Selected trace elements and their impact on redox homeostasis in eye health. Biomol Ther. (2024) 14:1356 2024. doi: 10.3390/biom14111356
23. Öztürk Kurt, HP, Karagöz Özen, DS, Genç, İ, Erdem, M, and Demirdağ, MD. Comparison of selenium levels between diabetic patients with and without retinopathy: selenium levels and diabetic retinopathy. J Surg Med. (2023) 7:58–62. doi: 10.28982/josam.7673
24. Costa, RD, Thomaz Neto, FJ, Moustafa, MT, Atilano, SR, Chwa, M, Cáceres-del-Carpi, J, et al. The role of mitochondrial genes on nuclear gene expression in neovascular age related macular degeneration: analysis of nuclear VEGF gene expression after ranibizumab treatment in cytoplasmic hybrid retinal pigment epithelial cell lines correlated with clinical evolution. Int J Retina Vitreous. (2023) 9:44 2023 Jul 25. doi: 10.1186/s40942-023-00476-7
25. Angelova, PR, and Abramov, AY. Functional role of mitochondrial reactive oxygen species in physiology. Free Radic Biol Med. (2016) 100:81–5. doi: 10.1016/j.freeradbiomed.2016.06.005
26. Dhar, SK, and St Clair, DK. Manganese superoxide dismutase regulation and cancer. Free Radic Biol Med. (2012) 52:2209–22. doi: 10.1016/j.freeradbiomed.2012.03.009
27. Miao, L, and St Clair, DK. Regulation of superoxide dismutase genes: implications in disease. Free Radic Biol Med. (2009) 47:344–56. doi: 10.1016/j.freeradbiomed.2009.05.018
28. Joseph, A, Li, Y, Koo, HC, Davis, JM, Pollack, S, and Kazzaz, JA. Superoxide dismutase attenuates hyperoxia-induced interleukin-8 induction via AP-1. Free Radic Biol Med. (2008) 45:1143–9. doi: 10.1016/j.freeradbiomed.2008.07.006
29. Dong, JW, Xu, MJ, Zhang, WY, and Che, XM. Effects of sevoflurane pretreatment on myocardial ischemia-reperfusion injury through the Akt/hypoxia-inducible factor 1-alpha (HIF-1 alpha)/vascular endothelial growth factor (VEGF) signaling pathway. Med Sci Monit. (2019) 25:3100–7. doi: 10.12659/MSM.914265
30. Jomova, K, Alomar, SY, Nepovimova, E, Kuca, K, and Valko, M. Heavy metals: toxicity and human health effects. Arch Toxicol. (2025) 99:153–209. doi: 10.1007/s00204-024-03903-2
31. Pajarillo, E, Rizor, A, Son, DS, Aschner, M, and Lee, E. The transcription factor REST up-regulates tyrosine hydroxylase and antiapoptotic genes and protects dopaminergic neurons against manganese toxicity. J Biol Chem. (2020) 295:3040–54. doi: 10.1074/jbc.RA119.011446
32. Ponzoni, S. Manganese tissue accumulation and tyrosine hydroxylase immunostaining response in the Neotropical freshwater crab, Dilocarcinus pagei, exposed to manganese. Invertebr Neurosci. (2017) 17:5. doi: 10.1007/s10158-017-0198-7
33. Baj, J, Flieger, W, Barbachowska, A, Kowalska, B, Flieger, M, Forma, A, et al. Consequences of disturbing manganese homeostasis. Int J Mol Sci. (2023) 24:14959. doi: 10.3390/ijms241914959
Keywords: manganese, heavy metal, diabetic retinopathy, NHANES, epidemiology
Citation: Chen X, Gu Z, Qi Y, Hao X and Xie L (2025) Manganese exposure and its U-shaped relationship with diabetic retinopathy: analysis of NHANES 2011–2020. Front. Nutr. 12:1619751. doi: 10.3389/fnut.2025.1619751
Edited by:
Martina Tomić, Merkur University Hospital, CroatiaReviewed by:
Melek Ozdemir, Pamukkale University, TürkiyeIkram Kenfaoui, Ibn Tofail University, Morocco
Qingqing Li, First Affiliated Hospital of Fujian Medical University, China
Copyright © 2025 Chen, Gu, Qi, Hao and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaofeng Hao, Zm1tdWhhb0AxNjMuY29t; Like Xie, Ymp4aWVsaWtlQHNpbmEuY29t
 Xi Chen
Xi Chen Zhenzhen Gu
Zhenzhen Gu Yixin Qi
Yixin Qi Like Xie
Like Xie