- 1University Institute of Diet and Nutritional Sciences UIDNS, The University of Lahore UOL, Lahore, Pakistan
- 2Faculty of Medicine, Rabigh-King Abdulaziz University, Rabigh, Saudi Arabia
- 3Preventive Medicine Executive Directory Makkah Health Cluster, Makkah, Saudi Arabia
- 4Department of Pharmacology and Toxicology, Faculty of Medicine, Umm Al-Qura University, Makkah, Saudi Arabia
Background: Metabolic syndrome (MetS) is a growing global health concern and a major risk factor for conditions such as diabetic nephropathy and atherosclerosis. It is marked by chronic inflammation, insulin resistance, and dyslipidemia. Dietary interventions, including carbohydrate-controlled diets, have shown potential in improving metabolic outcomes. Cuminum cyminum (cumin), containing the bioactive compound cuminaldehyde, is known for its hypoglycemic and antioxidant properties.
Aim: This study aimed to evaluate the combined effect of a carbohydrate-controlled diet and cumin herbal infusion on metabolic and biochemical parameters in patients with metabolic syndrome.
Methods: A pre- and post-interventional study was conducted on 132 patients (aged 18–60 years) diagnosed with MetS based on the ATP III criteria. Participants were recruited from the University of Lahore Teaching Hospital (ULTH) through purposive sampling and were randomly assigned to control and intervention groups (n = 66 each). The intervention group (INEG) received a carbohydrate-controlled diet and cumin herbal infusion for 8 weeks. Anthropometric, biochemical, and physiological parameters were assessed at baseline and post-intervention. A two-way repeated measures ANOVA was used for statistical analysis.
Results: Significant improvements were observed in body mass index (BMI), body weight, and lipid profile parameters (p < 0.005). The intervention group showed notable reductions in triglycerides, low-density lipoprotein (LDL), and fasting blood glucose levels compared to the control group (CG).
Conclusion: A carbohydrate-controlled diet combined with cumin herbal infusion may support glycemic control and improve lipid metabolism in individuals with metabolic syndrome. This combined approach shows potential as an adjunct dietary strategy for managing cardiometabolic risk factors.
1 Introduction
Metabolic syndrome (MetS) is a multi-component condition characterized by multiple physiological abnormalities, often resulting from a disparity between energy intake and expenditure, and is further influenced by an individual’s genetic and epigenetic factors (1). MetS has emerged as a public health concern in both developed and developing countries. It is characterized by abnormalities such as impaired fasting glucose, central obesity, low high-density lipoprotein (HDL) cholesterol (HDL-C), elevated triglyceride (TG) levels, and hypertension, which cluster together to create a morbid state (2). Metabolic syndrome is linked to a higher likelihood of developing type 2 diabetes mellitus and cardiovascular diseases among 25% of the world’s population. The underlying contributing factors are abdominal adiposity and insulin resistance (3). MetS has been found to have a higher prevalence in men than in women with a heightened likelihood of developing cardiovascular disease, type 2 diabetes, and overall mortality (4). Over the past decade, the gut microbiota has been identified as a major predictor of metabolic conditions such as type 2 diabetes and obesity. Dysbiosis is caused by an obesogenic diet that activates pro-inflammatory processes and causes metabolic endotoxemia, which can promote insulin resistance and cardiometabolic disorders in the population (5). Sedentary lifestyle and excessive calorie intake from a diet high in fats and carbohydrates contribute to vascular alterations and hypercholesterolemia (6). A high-fat diet (HFD) contributes to metabolic disturbances by disrupting redox balance, which weakens the body’s natural antioxidant defense mechanisms against oxidative stress. Additionally, it impairs glucose and lipid regulation and promotes the accumulation of visceral fat (7). Excessive salt consumption has been linked to metabolic diseases, such as insulin resistance, which often coexist with hypertension (8). A high intake of oral sodium in obese individuals is linked to increased urinary sodium excretion, which may contribute to the formation of renal calculi. The kidneys play a key role in regulating electrolyte balance, and excessive sodium intake can lead to hypercalciuria, thereby promoting the formation of calcium stones. In obesity, altered dietary habits and higher metabolic load further disrupt urinary composition. In addition, an elevated body mass index (BMI) itself is an independent risk factor for nephrolithiasis (9). Effective dietary strategies to address metabolic syndrome-associated comorbidities should include lifestyle modifications, such as following a balanced, energy-restricted diet, incorporating functional foods and bioactive nutrients, achieving weight loss, and increasing physical activity (10). Healthy eating and lifestyle changes are the mainstays of metabolic syndrome (MetS) treatment (11). To combat chronic diseases linked to obesity that can jeopardize overall health, strict adherence to healthy eating habits combined with calorie restriction may be beneficial. The Mediterranean diet is considered the best paradigm for evaluating individuals who are overweight or obese (12). Recent studies have shown that cuminaldehyde, a compound found in cumin, exhibits significant α-glucosidase and aldose reductase inhibitory activity (13). Cuminum cyminum, a member of the Apiaceous family, is extensively utilized in Ayurvedic medicine for managing various ailments (14). Cuminaldehyde (4-isopropylbenzaldehyde) is an organic compound classified as a natural aldehyde, with the molecular formula C10H12O. It is a bioactive component of Cuminum cyminum, Carum caraway, and Cinnamomum verum, among others. Studies have shown that cuminaldehyde exhibits potent antidiabetic effects in streptozotocin-induced diabetic rats and functions as an effective lipoxygenase inhibitor (13, 15). Zujko et al. (11) investigated the effects of individualized nutrition intervention on reducing the components of metabolic syndrome (MetS) in 90 participants diagnosed with MetS. The participants were assigned to an intervention group (INEG) and a control group (CG) for 3 months. The results showed that individual nutrition education led to improvement in dietary habits, knowledge, and physical activity. The diet modification, which included a higher intake of polyphenols, fiber, PUFA, and PUFA n-3 and a lower intake of SFA, significantly improved the risk factors associated with MetS, such as waist circumference, fasting glucose levels, and HDL cholesterol (11). A 12-month lifestyle program for managing metabolic syndrome (MetS), involving a healthcare team, successfully reversed the participants’ metabolic abnormalities. The study assessed nutrient intake and diet quality over the year, revealing reduced total energy intake, increased consumption of fruits, vegetables, and nuts, and decreased intake of processed and commercially baked foods. Notably, these dietary improvements were observed within the first 3 months and were sustained throughout the 12-month period (16). MetS can be managed through dietary modification. The DASH diet emphasizes the inclusion of fruits, vegetables, and low-fat dairy products while restricting total fat, saturated fat, and sodium intake. This dietary approach helps reduce body weight, lower total and low-density lipoprotein cholesterol (LDL-C), and improve insulin and glucose regulation, as evidenced by previous studies (17). Adherence to the Mediterranean diet has been associated with reductions in body weight and homeostatic model assessment of insulin resistance (HOMA-IR) and improvements in lipid profiles among metabolic syndrome patients, as evidenced by recent studies (18).
Pakistan ranks ninth among the world’s most obese nations, and obesity is associated with a multitude of risk factors, including dyslipidemia. Metabolic syndrome affects 18 to 46% of the Pakistani population. Lifestyle-related risk factors are associated with metabolic syndrome; therefore, addressing these factors is considered a primary focus target for preventive therapies. The researcher aimed to investigate the synergistic effect of a carbohydrate-controlled diet and cumin seed herbal infusion on ameliorating metabolic syndrome, which is a major cause of mortality. Consuming cumin tea regularly, along with diet therapy, leads to improvements in hematological biomarkers and anthropometric measurements.
2 Materials and methods
2.1 Study design
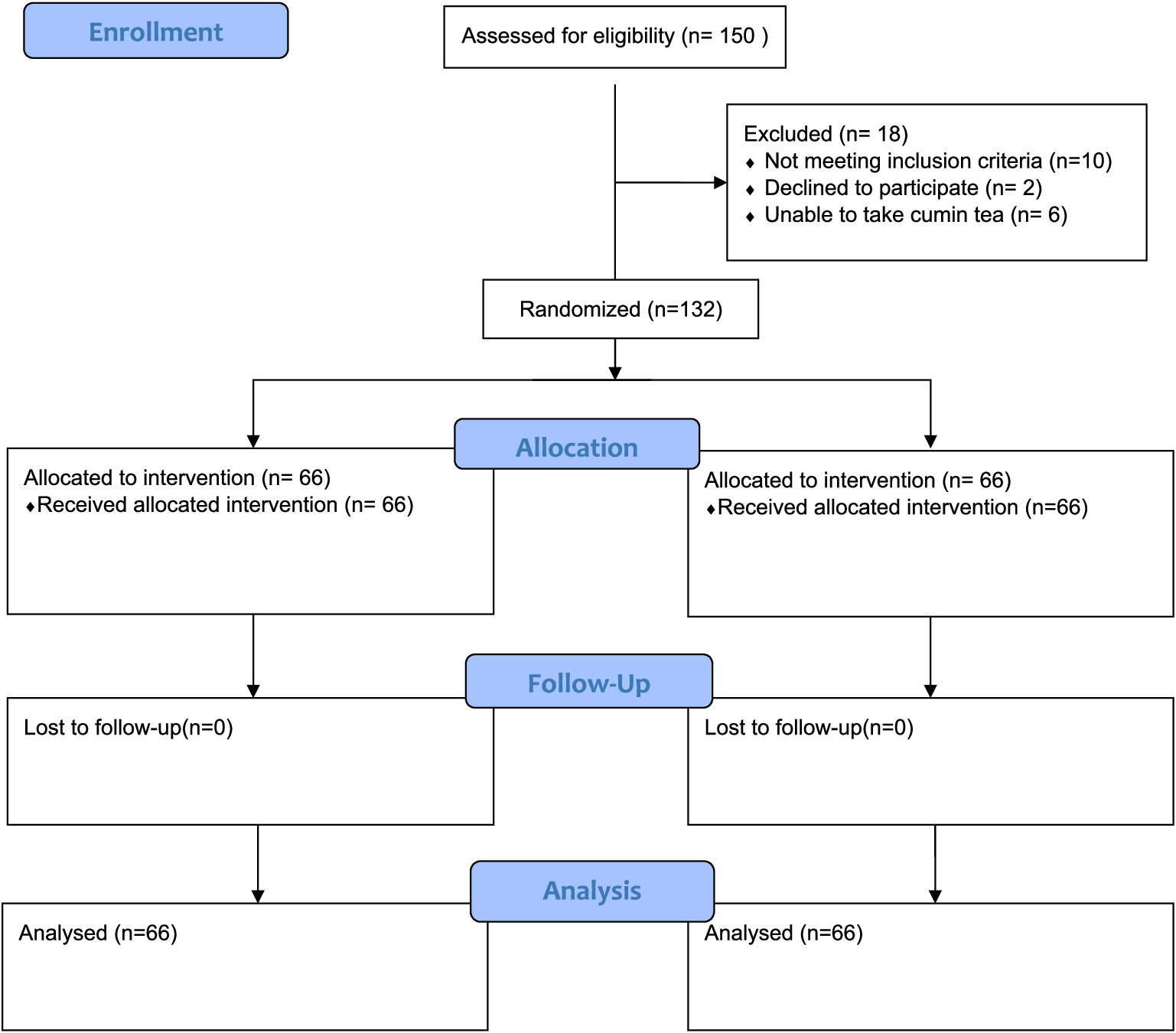
A pre- and post-interventional trial was carried out to elucidate the synergistic effect of a carbohydrate-controlled diet and Cuminum cyminum herbal infusion in metabolic syndrome patients. The study aimed to assess the combined effect of the diet and cumin seed herbal infusion on clinical outcomes in these patients (see Figure 1).
2.2 Sampling technique
Patients of either gender, aged 18 to 60 years, and diagnosed with MetS based on the ATP III criteria were recruited through a purposive sampling technique, followed by simple random assignment to control and intervention groups.
2.3 Sample selection
2.3.1 Inclusion criteria
Patients of any body mass index with a confirmed diagnosis of MetS who had not used any dietary supplements or adhered to special diets at least 1 month before baseline were included. In addition, patients with at least three symptoms of metabolic syndrome who visited the University of Lahore Teaching Hospital (ULTH) were included and assigned through simple random sampling to control and intervention groups.
2.3.2 Exclusion criteria
Genetically obese individuals, patients already on lipid-lowering drugs according to the recommendation of physicians, those taking any nutritional supplements, individuals allergic to any drugs or specific foods, patients with a medical history of renal diseases, infections, liver disorders, or cardiovascular diseases, patients receiving weight loss drugs or supplements, those undergoing any changes in diet or daily exercise programs, breastfeeding mothers, and pregnant women were excluded from the study.
2.4 Sample/cohort size
The sample/cohort size was calculated with a study power of 80% and a significance level of 5%. A total of 132 patients with at least three symptoms of metabolic syndrome who visited the University of Lahore Teaching Hospital (ULTH), Lahore, Pakistan, participated in this research. The Institutional Review Board of the University of Lahore (Ethical code: IRB-UOL-FAHS/808/2021) approved this research. Furthermore, the trial is registered on clinicaltrials.gov (https://clinicaltrials.gov/study/NCT06728449).
2.5 Data collection procedure
The selected participants were assigned through simple random sampling to either the control or intervention group. Anthropometric measurements and biochemical tests, such as fasting blood sugar level, lipid profiles (including triglycerides, total cholesterol, LDL, and HDL), liver function tests (LFTs), and renal function tests (RFTs), were taken at the start of the study (0 day) and after 60 days.
2.6 Intervention
Following enrollment, the 132 eligible participants diagnosed with metabolic syndrome were randomly assigned to two groups using simple random sampling (n = 66 per group). The intervention period lasted for 8 weeks (60 days).
T0: Control group receiving a carbohydrate-controlled diet along with conventional therapy.
T1: Intervention group receiving the same carbohydrate-controlled diet and conventional therapy, along with cumin seed herbal infusion.
2.7 Carbohydrate-controlled diet
Both groups were prescribed individualized meal plans that provided 45–50% of total daily energy from carbohydrates, with emphasis on low-glycemic index foods such as whole grains, legumes, and non-starchy vegetables. Refined sugars, white bread, sweetened beverages, and high-GI foods were limited. Meal distribution and portion sizes were adjusted to maintain glycemic balance throughout the day. Total energy requirements were calculated using the Mifflin-St Jeor equation, and the diet plans were reviewed weekly by a clinical nutritionist to monitor adherence.
2.8 Cumin herbal infusion
2.8.1 Formulation
The average weight of one teabag was 2 grams (19). The filter paper was used for packaging the tea bags. Cuminum cyminum (cumin seeds) was procured from local markets in Lahore. The cumin seeds were sifted to remove impurities and then coarsely ground for use in the preparation of the tea bags.
The herbal infusion was prepared by placing a cumin tea bag in boiling water and allowing it to steep for 5–7 min before consumption. The patients were instructed to consume the herbal infusion twice daily—once in the mid-morning and once in the evening—using a standard cup size of 200 mL, without adding sugar.
2.9 Conventional therapy
All participants continued to receive standard medical care for metabolic syndrome, which included lifestyle counseling and prescribed medications for blood pressure, blood glucose, or cholesterol, as needed. The type and dosage of any medications were not altered by the research team.
2.10 Compliance monitoring
Dietary adherence was monitored using weekly food diaries, 24-h dietary recalls, and follow-up visits. Cumin intake compliance was tracked through daily checklists provided to the intervention group. The participants were regularly reminded and motivated through weekly calls or in-person sessions.
2.11 Follow-up
During data collection, the patients were informed about weekly follow-up calls using their provided contact numbers. Follow-up calls were conducted every 2 weeks (15 days) to monitor adherence, identify any side effects, and track follow-up or dropout rates. The patients were advised to visit the hospital every 15 days so that compliance could be monitored and cumin seed tea bags could be given for the corresponding days or months. If any issues or side effects were reported, they were treated, adjusted, or eliminated accordingly. After 8 weeks, anthropometric and biochemical tests were conducted to assess the effects of the intervention.
2.12 Data analysis
Data were analyzed using SPSS version 24. Two-way repeated measures ANOVA was performed to assess the interaction between time (pre- and post-intervention) and group (control vs. intervention), evaluating differences across the intervention period. The significance level was fixed at a p-value of 0.005.
3 Results
According to the result, 56% of the patients had three disorders, whereas 44% of the patients had four to five disorders. The mean age of the patients with MetS was 37.54 ± 7.59 years. The age range of the participants was 21–60 years.
3.1 Anthropometric measurements
Table 1 shows the changes in the mean scores of BMI and waist circumference in both the control and intervention groups. According to the result, the mean BMI in the control group (T0) was 31.44 ± 1.42 before the intervention and 28.48 ± 1.78 after the intervention. In the intervention group (T1), the mean BMI was 30.93 ± 1.73 before the intervention and 27.32 ± 1.64 after the intervention. A significant time × group interaction was observed for BMI, with F (1, 65) = 5.41 and a p-value = 0.023. The mean waist circumference in the control group (T0) was 107.83 ± 3.49 before the intervention and 97.87 ± 3.40 after the intervention, while, in the intervention group (T1), the mean waist circumference was 108.46 ± 3.77 before the intervention and 97.87 ± 3.40 after the intervention. A significant time × group interaction was observed for waist circumference, with F (1, 65) = 9.77 and a p-value = 0.003, suggesting that the intervention group had greater improvement compared to the control group.
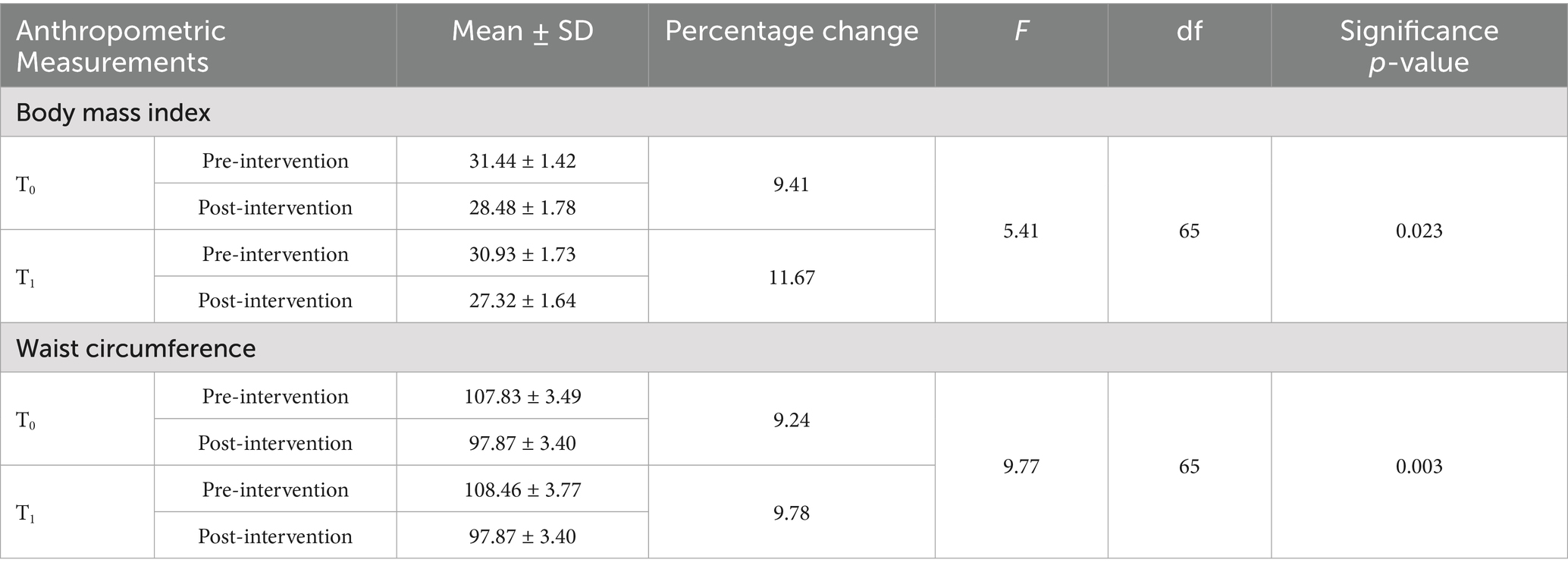
Table 1. Distribution of the participants according to the changes observed in body mass index and waist circumference.
3.2 Glucose and uric acid levels
Table 2 shows the changes in the mean scores of fasting blood glucose and uric acid levels in both the control and intervention groups. The mean fasting blood glucose level in the control group (T0) was 106.27 ± 4.84 before the intervention and 96.39 ± 5.03 after the intervention. In the intervention group (T1), the mean fasting blood glucose level was 108.09 ± 4.90 before the intervention and 95.25 ± 4.20 after the intervention. A significant time × group interaction was observed for fasting blood glucose levels, with F (1, 65) = 21.10 and a p-value < 0.001. The mean uric acid value in the control group (T0) was 7.88 ± 0.57 before the intervention and was 7.01 ± 0.47 after the intervention. In the intervention group (T1), the mean uric acid value was 8.19 ± 0.55 before the intervention and was 6.99 ± 0.49 after the intervention. A statistically significant interaction was found between time and group for uric acid levels, with F (1, 65) = 25.77 and a p-value < 0.001.
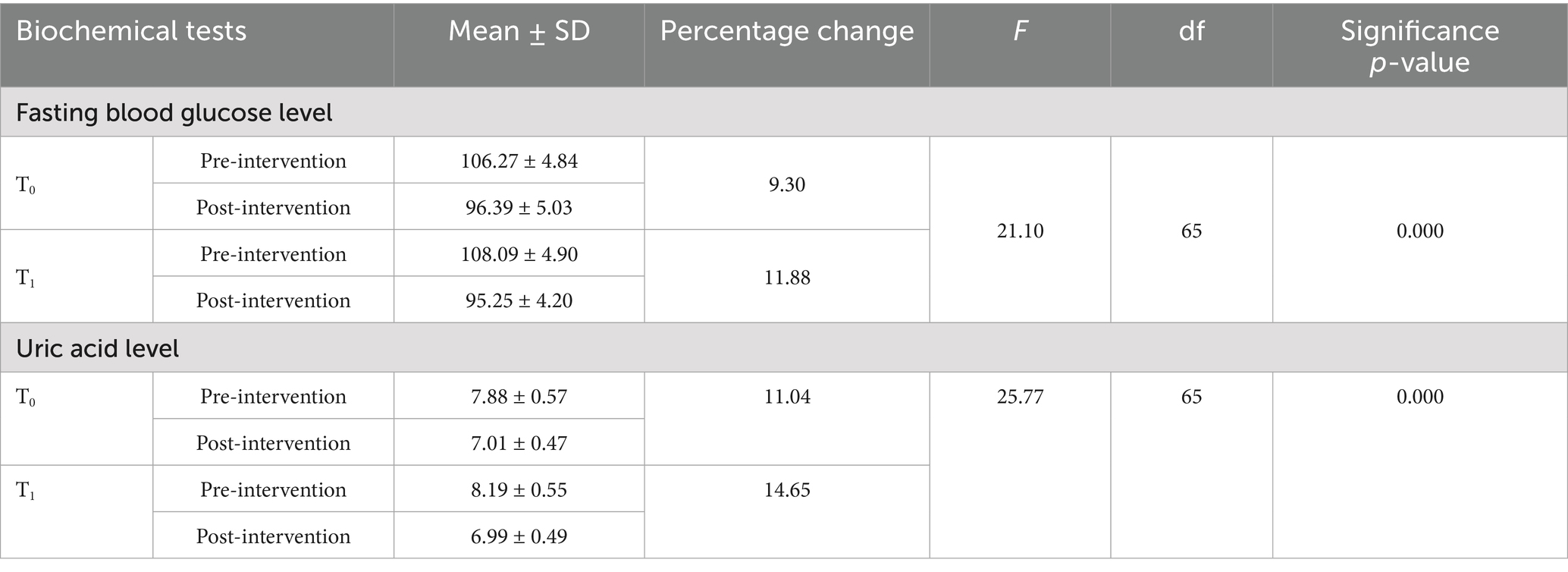
Table 2. Distribution of the participants according to the changes observed in fasting blood glucose and uric acid levels.
3.3 Lipid profile
Table 3 shows the changes in the mean scores of low- and high-density lipoprotein (LDL/HDL), triglyceride, and total cholesterol levels in both the control and intervention groups. According to the result, the mean LDL level in the control group (T0) was 134.09 ± 11.48 before the intervention and 112.43 ± 11.09 after the intervention. In the intervention group (T1), the mean LDL level was 131.34 ± 12.89 before the intervention and 107.98 ± 10.76 after the intervention. A significant time × group interaction was observed for LDL levels, with F (1, 65) = 11.43 and a p-value< 0.001.
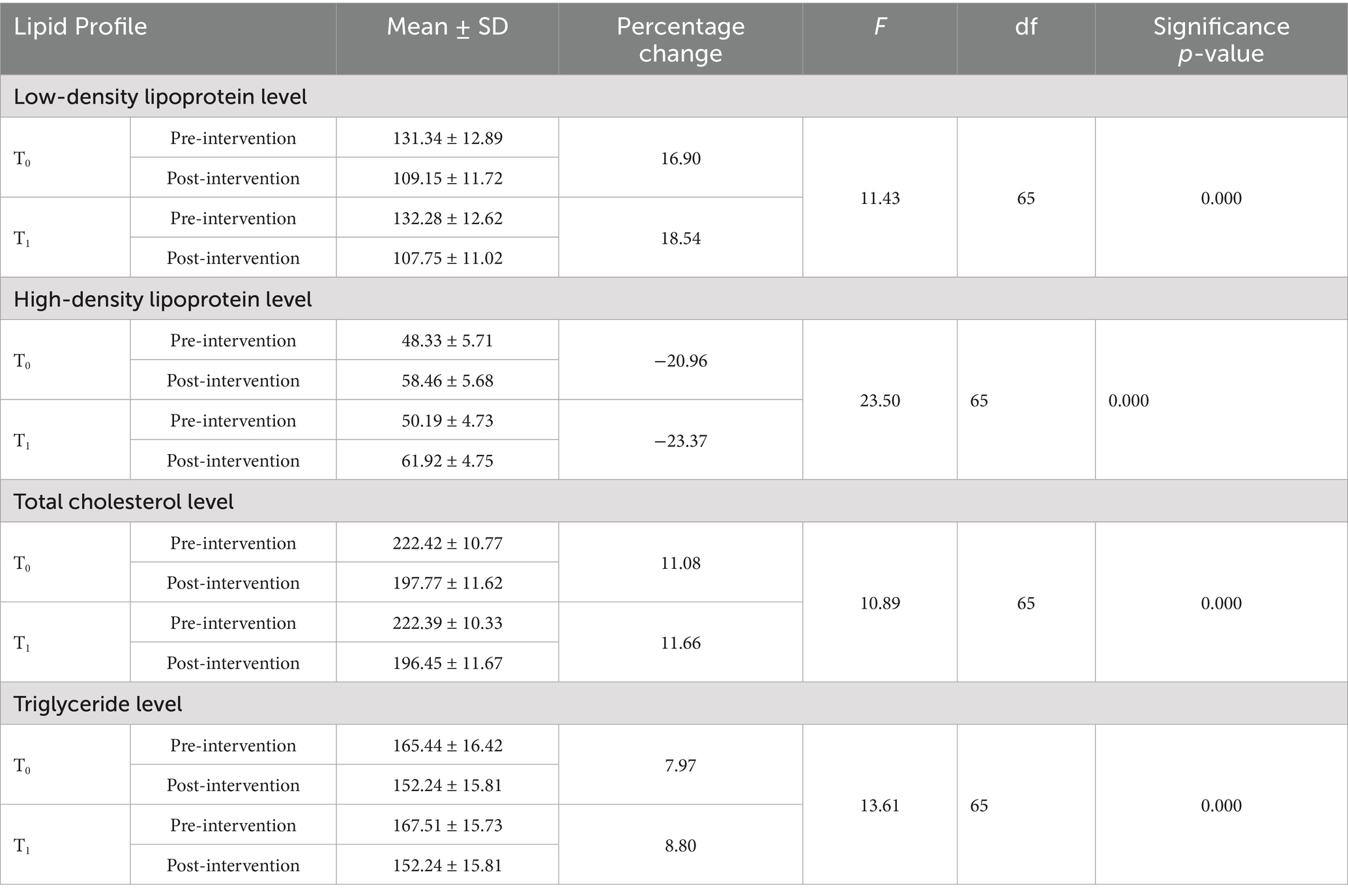
Table 3. Distribution of the participants according to the changes observed in low- and high-density lipoprotein, cholesterol, and triglyceride levels.
The mean HDL level in the control group (T0) was 48.33 ± 5.71 before the intervention and 58.46 ± 5.68 after the intervention. In the intervention group (T1), the HDL level was 50.19 ± 4.73 before the intervention and 61.92 ± 4.75 after the intervention. A significant time × group interaction was observed for HDL levels, with F (1, 65) = 23.50 and a p-value < 0.001.
The mean triglyceride level in the control group (T0) was 167.51 ± 15.73 before the intervention and 152.77 ± 15.07 after the intervention. In the intervention group (T1), the mean triglyceride level was 165.43 ± 16.42 before the intervention and 152.24 ± 15.81 after the intervention. A significant time × group interaction was observed for triglyceride levels, with F (1, 65) = 13.61 and a p-value < 0.001.
The mean cholesterol level in the control group (T0) was 222.42 ± 10.77 before the intervention and 197.77 ± 11.62 after the intervention, with T0: t (65) = 55.34 and a p-value< 0.001. In the intervention group (T1), the mean cholesterol level was 222.39 ± 10.33 before the intervention and 196.45 ± 11.67 after the intervention, with F (1, 65) = 10.89 and a p-value < 0.001.
4 Blood pressure
Table 4 shows the changes in the mean scores of blood pressure readings in both the control and intervention groups. In the control group, the mean systolic blood pressure was 146.34 ± 5.25 before the intervention and 142.00 ± 4.88 after the intervention. In the intervention group (T1), the mean systolic blood pressure was 144.18 ± 3.29 before the intervention and 136.84 ± 3.98 after the intervention. A statistically significant interaction was found between time and group for systolic blood pressure, with F (1, 65) = 86.28 and a p-value < 0.001.
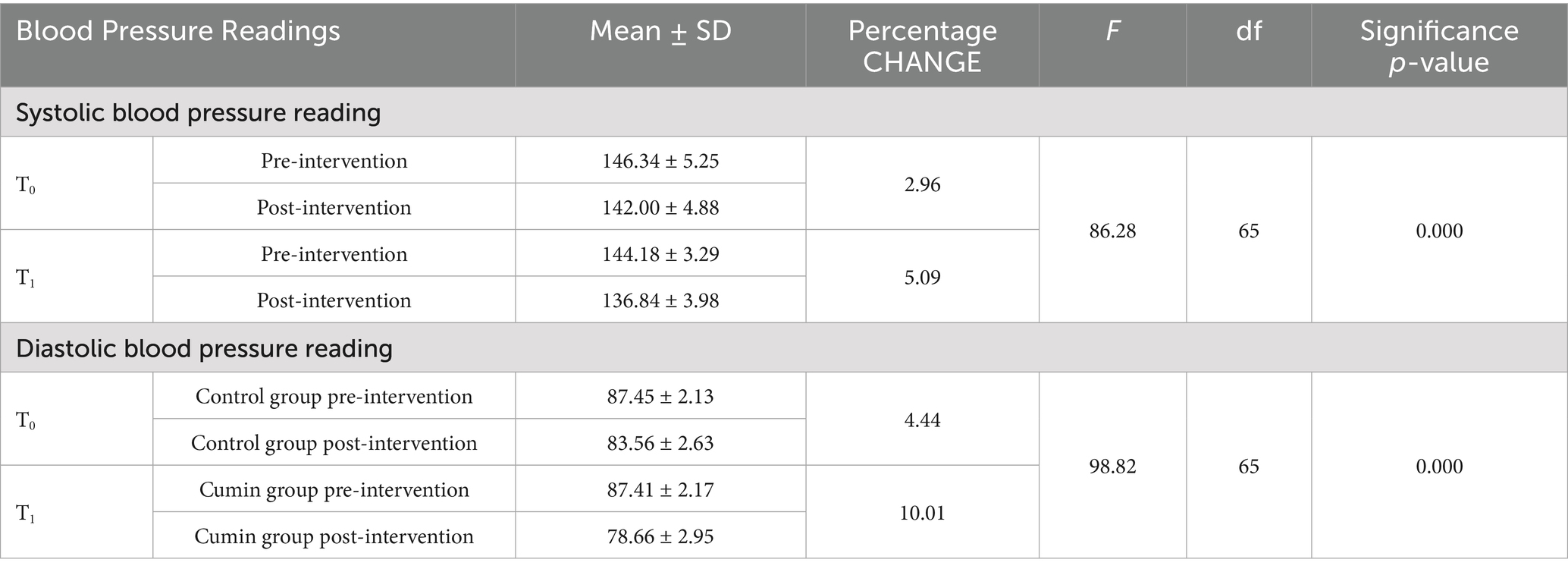
Table 4. Distribution of the participants according to the changes observed in blood pressure readings.
In the control group, the mean diastolic blood pressure was 87.45 ± 2.13 before the intervention and 83.56 ± 2.63 after the intervention. In the intervention group (T1), the mean diastolic blood pressure was 87.41 ± 2.17 before the intervention and 78.66 ± 2.99 after the intervention. A statistically significant interaction was found between time and group for diastolic blood pressure, with F (1, 65) = 98.82 and a p-value < 0.001, indicating a substantial difference in the pre- to post-intervention changes between the intervention and control groups.
4.1 Liver and renal function tests
Table 5 shows the changes in the mean scores of liver enzyme levels in both the control and intervention groups. In the alkaline phosphatase (ALP) test, the mean level in the control group (T0) was 128.30 ± 4.75 before the intervention and 120.13 ± 4.22 after the intervention. In the intervention group (T1), the mean level was 128.30 ± 4.75 before the intervention and 118.03 ± 3.49 after the intervention. A statistically significant interaction was found between time and group for ALP levels, with F (1, 65) = 14.45 and a p-value < 0.001. In the aspartate aminotransferase (AST) test, the pre-intervention mean score in the control group(T0) was 58.13 ± 6.08 and the post-intervention mean score was 50.19 ± 4.73. In the intervention group (T1), the mean level was 58.54 ± 6.21 before the intervention and 49.53 ± 4.96 after the intervention. There was no significant interaction between time and group for AST levels, with F (1, 65) = 7.25 and a p-value > 0.005.
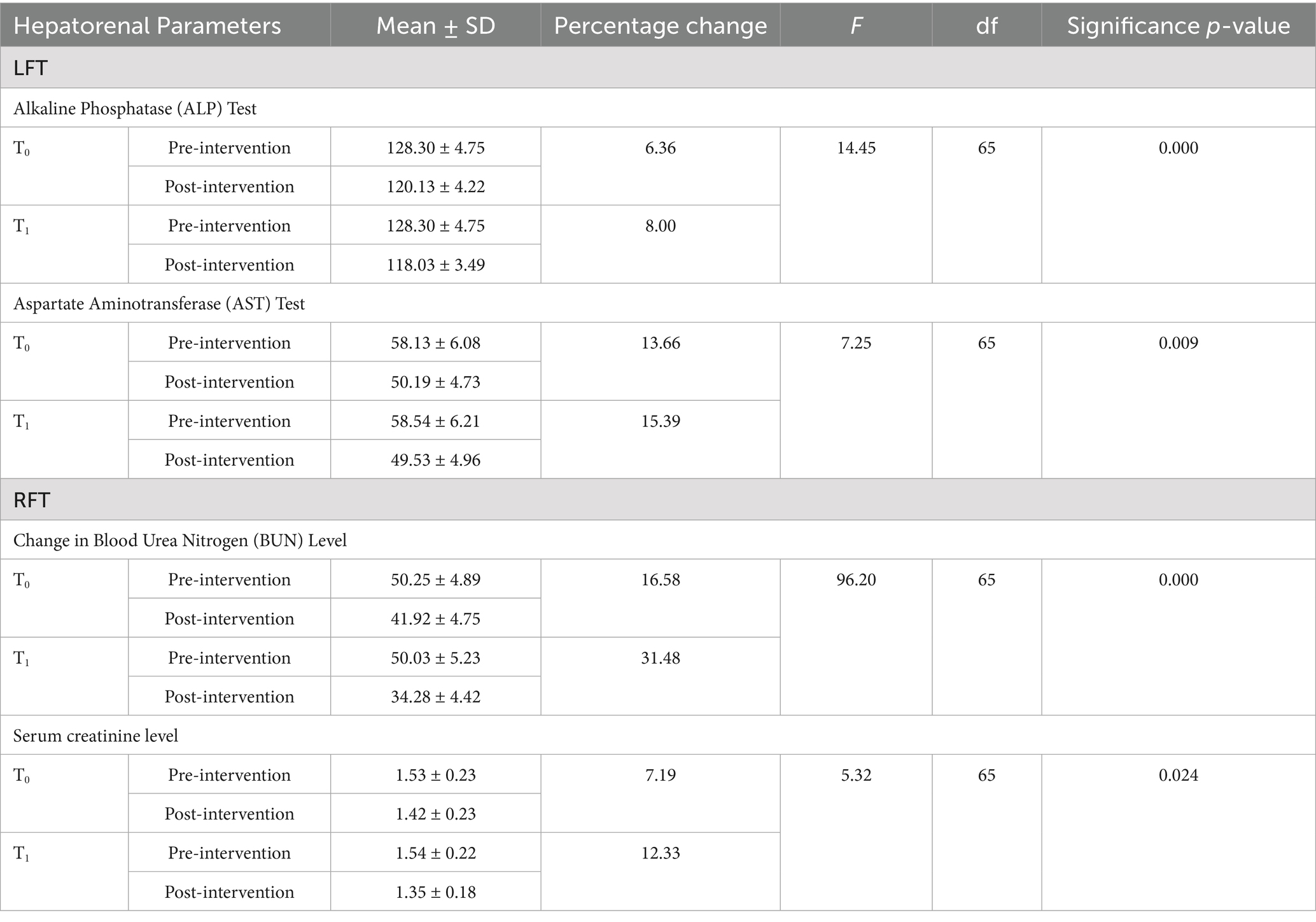
Table 5. Distribution of the participants according to the changes observed in liver enzyme levels and renal functioning.
In the control group (T0), the mean blood urea nitrogen (BUN) level was 50.25 ± 4.89 before the intervention and 41.92 ± 4.75 after the intervention. In the intervention group (T1), the mean BUN level was 50.03 ± 5.23 before the intervention and 34.28 ± 4.42 after the intervention. A significant time × group interaction was observed for BUN levels, with F (1, 65) = 96.20 and a p-value < 0.001, suggesting that the intervention group had greater improvement compared to the control group.
According to the result, the mean serum creatinine level in the control group (T0) was 1.53 ± 0.23 before the intervention and 1.42 ± 0.23 after the intervention. In the intervention group (T1), the mean serum creatinine level was 1.54 ± 0.22 before the intervention and 1.35 ± 0.18 after the intervention. No significant time × group interaction was observed for serum creatinine levels, with F (1, 65) = 5.32 and a p-value > 0.005, suggesting that the intervention group had greater improvement compared to the control group.
5 Discussion
The current study aimed to determine the combined effect of diet and cumin herbal tea on the clinical outcomes of metabolic syndrome, including abdominal obesity, dyslipidemia, hypertension, and insulin resistance. The findings demonstrated that integrating cumin tea with a carbohydrate-controlled dietary approach significantly enhanced metabolic outcomes compared to either intervention alone. Metabolic syndrome is a chronic disease with lifestyle interventions serving as a foundation of treatment. Nutritional interventions, focused primarily on weight loss, are ineffective in addressing all risk factors. Diet quality is also a major contributor to reducing the risk of metabolic syndrome (20).
The participants in the combined intervention group exhibited substantial improvements in fasting blood glucose levels, as evidenced by the results (F (1, 65) = 21.10, p < 0.001). The results are based on the hypoglycemic effects of cumin, attributed to its bioactive compound cuminaldehyde, which improves glucose metabolism and reduces oxidative stress. The carbohydrate-controlled diet complements this by reducing postprandial glucose levels and promoting a stable insulin response through the inclusion of low glycemic index foods. The combined intervention also led to significant reductions in triglycerides and increases in HDL cholesterol. These lipid improvements likely resulted from the synergistic effects of cumin’s lipid-lowering properties and the metabolic benefits of carbohydrate restriction. A study reported by Zare et al. found that the administration of cumin (3gm /day) to overweight and obese women resulted in reductions in serum fasting cholesterol levels, triglycerides, weight, body mass index, waist circumference, and body fat percentage. It also enhanced the concentration of high-density lipoprotein and improved anthropometric parameters (21).
Taghizadeh et al. (22) conducted a study on 72 overweight participants to investigate the combined impact of Cuminum cyminum L. and lime, administered in doses of 75 mg and 25 mg, respectively, over 8 weeks. The higher dose of the nutraceutical (cumin and lime) resulted in declines in fasting plasma glucose levels (p < 0.001), serum triglycerides (p = 0.03), total cholesterol levels (p = 0.004), and LDL cholesterol levels (p = 0.01) (22).
Aktas et al. conducted a study on 80 obese participants to investigate the impact of a low-calorie diet and physical activity on body weight over 12 weeks. Turkish Muslims residing in Germany are prone to developing obesity, type 2 diabetes mellitus, and metabolic syndrome. The results revealed that tailored lifestyle interventions led to a significant reduction of 6% in body weight and improvements in the levels of glucose, glycosylated hemoglobin, and cholesterol (23).
Abdominal obesity, measured by waist circumference and BMI, improved significantly in the combined intervention group compared to the control group. While carbohydrate restriction promoted fat loss through reduced calorie intake and enhanced satiety, cumin’s thermogenic properties and ability to regulate fat metabolism amplified these results (p < 0.001). Karpagam and Priya (24) conducted a human trial to evaluate the impact of Cuminum cyminum combined with lime water on obesity. Participants consumed the preparation every morning on an empty stomach for 3 weeks. The preparation involved boiling 200 mL of water with 2 grams of cumin seeds, allowing it to steep overnight, and then adding the juice of half a lime before consumption. The findings demonstrated that the Cuminum cyminum and lime water combination effectively reduced overweight and obesity (p < 0.005) (24). Cumin supplementation has also been linked to a decrease in body mass index (BMI) (25).
Cumin (Cuminum cyminum L.) supplementation significantly reduces total cholesterol and low-density lipoprotein cholesterol while increasing high-density lipoprotein cholesterol. It shows no significant effect on triglyceride levels, except in individuals without hypertriglyceridemia, indicating its efficacy in managing lipid profiles. Cumin supplementation resulted in a significant reduction in TC, with studies reporting mean differences of −3.96 mg/dL and −10.90 mg/dL. Cumin was effective in lowering LDL-C, with reductions of −6.94 mg/dL noted. An increase in HDL-C levels was observed, with a mean difference of 3.35 mg/dL (26). Cumin supplementation has been associated with a significant decrease in total cholesterol levels, with studies reporting reductions ranging from approximately 3.96 mg/dL to 10.90 mg/dL (25, 26).
Cuminum cyminum supplementation has been shown to lower total cholesterol and BMI. It also improves waist circumference, high-density lipoprotein, and triglyceride levels, although its effect on low-density lipoprotein is inconclusive without longer supplementation periods. Cumin supplementation resulted in a significant reduction in TC, with studies reporting a mean difference of −3.96 mg/dL (25).
Notable improvements in blood pressure were observed, with greater reductions in systolic and diastolic blood pressure observed in the combined intervention group. The antihypertensive effects of cumin, attributed to its antioxidant properties and enhancement of nitric oxide-mediated vasodilation, likely complimented the weight loss and metabolic improvements induced by the carbohydrate-controlled diet. Together, these interventions may improve endothelial function and reduce arterial stiffness over time. Although cumin exhibits promising nephroprotective properties, a study conducted by Alfahdawi et al. (27) investigated the effect of an aqueous extract of cumin seeds on kidney function in albino rats. The results showed improvements in kidney function, with a p-value of <0.05 (27). Similar results were reported by Kumar et al. (28), where the administration of Cuminum cyminum aqueous extract significantly restored elevated levels of urea, uric acid, and creatinine in mice (28).
In conjunction with exercise, cumin extract has been shown to improve insulin resistance, further supporting metabolic health. Cumin extract consumption, when combined with pilates training, significantly improved lipid profiles in overweight and obese women. The intervention led to decreased serum triglyceride, cholesterol, and LDL levels, while increasing HDL levels, as observed in a previous study (29).
Cuminum cyminum exhibits a hepatoprotective effect, as evidenced by significant reductions in liver enzyme levels. This is demonstrated by the results for ALP: F (1, 65) = 14.45, p < 0.001. Cuminum cyminum seeds powder exhibits hepatoprotective properties, suggesting it may aid in liver function recovery following acetaminophen-induced injury. This indicates potential therapeutic benefits for liver health, particularly in mitigating damage caused by certain pharmaceutical agents (30). Previous research indicates that cumin seed powder supplementation can prevent non-alcoholic fatty liver disease and reduce oxidative stress in rats that are fed a high-fat diet, suggesting a potential protective effect on liver function related to dietary fat intake (31).
This study’s key strength lies in the novel exploration of the combined effects of two complementary interventions, providing a comprehensive approach to managing metabolic syndrome-associated comorbidities. The findings of the study underscore the potential of integrating functional foods such as cumin into tailored dietary strategies for metabolic syndrome management. This discussion highlights the potential synergistic effects of cumin and a carbohydrate-controlled diet, emphasizing their complementary roles in improving metabolic health.
6 Conclusion
The findings of this study suggest that combining a carbohydrate-controlled diet with Cuminum cyminum (cumin) infusion may support improvements in metabolic parameters among individuals with metabolic syndrome. Notable reductions were observed in lipid profile markers, including total cholesterol, LDL, and triglycerides, along with modest improvements in renal function indicators. These outcomes may be attributed in part to the bioactive compounds in cumin, such as cuminaldehyde, which are known for their antioxidant and anti-inflammatory properties. While the results are promising, the intervention should be considered a complementary approach alongside standard dietary and lifestyle modifications. Additional long-term, blinded, and standardized studies are needed to confirm these findings and clarify the role of cumin as an adjunct therapy in managing metabolic syndrome.
6.1 Limitations
This study has several limitations. The purposive sampling method may limit the generalizability of the results. The open-label design without blinding might have introduced bias. Cumin infusion was not standardized for active compounds, restricting the ability to attribute observed effects to specific phytochemicals. The short intervention period (8 weeks) limits insights into long-term outcomes. Compliance was based on self-reported dietary records, which might not have been fully accurate. Additionally, variations in participants’ medications were not controlled and might have influenced the findings.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by University of Lahore ethics review board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MA: Writing – original draft, Methodology, Project administration. H-u-R: Writing – review & editing, Investigation, Project administration. TT: Methodology, Writing – review & editing, Investigation. YA: Writing – review & editing, Resources, Funding acquisition, Formal analysis. WA: Data curation, Writing – review & editing, Formal analysis, Validation, Funding acquisition. AA: Writing – original draft, Software, Data curation, Investigation, Resources, Validation, Funding acquisition. IS: Methodology, Writing – original draft, Formal analysis, Conceptualization, Funding acquisition, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research work was funded by Umm Al-Qura University, Saudi Arabia under grant number: 25UQU4330924GSSR07.
Acknowledgments
The authors extend their appreciation to Umm Al-Qura University, Saudi Arabia for funding this research work through grant number:25UQU4330924GSSR07.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Hajihashemi, P, Hassannejad, R, Haghighatdoost, F, Mohammadifard, N, Sadeghi, M, Roohafza, H, et al. The long-term association of different dietary protein sources with metabolic syndrome. Sci Rep. (2021) 11:19394. doi: 10.1038/s41598-021-98688-0
3. Prasad, H, Ryan, DA, Celzo, MF, and Stapleton, D. Metabolic syndrome: definition and therapeutic implications. Postgrad Med. (2012) 124:21–30. doi: 10.3810/pgm.2012.01.2514
4. He, J, Lai, Y, Yang, J, Yao, Y, Li, Y, Teng, W, et al. The relationship between thyroid function and metabolic syndrome and its components: a cross-sectional study in a chinese population. Front Endocrinol. (2021) 12:661160. doi: 10.3389/fendo.2021.661160
5. Le Barz, M, Anhê, FF, Varin, TV, Desjardins, Y, Levy, E, Roy, D, et al. Probiotics as complementary treatment for metabolic disorders. Diabetes Metab J. (2015) 39:291–303. doi: 10.4093/dmj.2015.39.4.291
6. Lozano, WM, Arias-Mutis, OJ, Calvo, CJ, Chorro, FJ, and Zarzoso, MJA. Diet-induced rabbit models for the study of metabolic syndrome. Animals. (2019) 9:463. doi: 10.3390/ani9070463
7. Di Majo, D, Sardo, P, Giglia, G, Di Liberto, V, Zummo, FP, Zizzo, MG, et al. Correlation of metabolic syndrome with redox homeostasis biomarkers: evidence from high-fat diet model in Wistar rats. Antioxidants. (2023) 12:89. doi: 10.3390/antiox12010089
8. Takase, H, Hayashi, K, Kin, F, Nakano, S, Machii, M, Takayama, S, et al. Dietary salt intake predicts future development of metabolic syndrome in the general population. Hypertens Res. (2023) 46:236–43. doi: 10.1038/s41440-022-01035-7
9. Atar, FA, and Verep, S. Dietary advices for patients with metabolic syndrome and obesity. World J Urol. (2023) 41:1. doi: 10.1007/s00345-022-04250-6
10. Andersen, CJ, and Fernandez, ML. Dietary strategies to reduce metabolic syndrome. Rev Endocr Metab Disord. (2013) 14:241–54. doi: 10.1007/s11154-013-9251-y
11. Zujko, ME, Rożniata, M, and Zujko, KJN. Individual diet modification reduces the metabolic syndrome in patients before pharmacological treatment. Nutrients. (2021) 13:2102. doi: 10.3390/nu13062102
12. Sayón-Orea, C, Razquin, C, Bulló, M, Corella, D, Fitó, M, Romaguera, D, et al. Effect of a nutritional and behavioral intervention on energy-reduced Mediterranean diet adherence among patients with metabolic syndrome: interim analysis of the PREDIMED-plus randomized clinical trial. JAMA. (2019) 322:1486–99. doi: 10.1001/jama.2019.14630
13. Haldar, S, Gan, L, Tay, SL, Ponnalagu, S, and Henry, CJ. Postprandial glycemic and insulinemic effects of the addition of aqueous extracts of dried corn silk, cumin seed powder or tamarind pulp, in two forms, consumed with high Glycemic index Rice. Food Secur. (2019) 8:437. doi: 10.3390/foods8100437
14. Dhandapani, S, Subramanian, VR, Rajagopal, S, and Namasivayam, N. Hypolipidemic effect of Cuminum cyminum L. on alloxan-induced diabetic rats. Pharmacol Res. (2002) 46:251–5. doi: 10.1016/S1043-6618(02)00131-7
15. Haque, M, and Ansari, S. Aromatic aldehyde compound cuminaldehyde protects nonalcoholic fatty liver disease in rats feeding high fat diet. Hum Exp Toxicol. (2019) 38:823–32. doi: 10.1177/0960327119842248
16. Brauer, P, Royall, D, Li, A, Rodrigues, A, Green, J, Macklin, S, et al. Nutrient intake and dietary quality changes within a personalized lifestyle intervention program for metabolic syndrome in primary care. Appl Physiol Nutr Metab. (2019) 44:1297–304. doi: 10.1139/apnm-2019-0070
17. Akhlaghi, MJ. Dietary approaches to stop hypertension (DASH): potential mechanisms of action against risk factors of the metabolic syndrome. Nutr Res Rev. (2020) 33:1–18. doi: 10.1017/S0954422419000155
18. Koopen, AM, Almeida, EL, Attaye, I, Witjes, JJ, Rampanelli, E, Majait, S, et al. Effect of fecal microbiota transplantation combined with Mediterranean diet on insulin sensitivity in subjects with metabolic syndrome. Front Microbiol. (2021) 12:662159. doi: 10.3389/fmicb.2021.662159
19. Oduro, I, Twumasi, P, Tandoh, M, Ankar-Brewoo, G, and De-Heer, N. Formulation and sensory evaluation of herb tea from Moringa oleifera, Hibiscus sabdariffa and Cymbopogon citratus. J Ghana Sci Assoc. (2013) 15:53–62.
20. Chauhan, H, Belski, R, Bryant, E, and Cooke, MJN. Dietary assessment tools and metabolic syndrome: Is it time to change the focus? Nutrients. (2022) 14:1557. doi: 10.3390/nu14081557
21. Zare, R, Heshmati, F, Fallahzadeh, H, and Nadjarzadeh, A. Effect of cumin powder on body composition and lipid profile in overweight and obese women. Complement Ther Clin Pract. (2014) 20:297–301. doi: 10.1016/j.ctcp.2014.10.001
22. Taghizadeh, M, Memarzadeh, MR, Abedi, F, Sharifi, N, Karamali, F, Kashan, ZF, et al. The effect of cumin cyminum L. plus lime administration on weight loss and metabolic status in overweight subjects: a randomized double-blind placebo-controlled clinical trial. Iran Red Crescent Med J. (2016) 18:e34212. doi: 10.5812/ircmj.34212
23. Aktas, MF, Mähler, A, Hamm, M, Perger, G, Simon, F, Westenhöfer, J, et al. Lifestyle interventions in Muslim patients with metabolic syndrome—a feasibility study. Eur J Clin Nutr. (2019) 73:805. doi: 10.1038/s41430-018-0371-z
24. Karpagam, K, and Priya, U. Effectiveness of cumin cyminum plus lime administration on obesity among womens. Int J Res Pharm Sci. (2019) 10:2965–9.
25. Tavakoli-Rouzbehani, OM, Faghfouri, AH, Anbari, M, Nikpayam, O, PourMirzaei Olyaei, H, and Alizadeh, M. Efficacy of Cuminum Cyminum supplementation on lipid profile and anthropometric parameters: A systematic review and meta-analysis of randomized controlled clinical trials. Phytother Res. (2022) 36:380–94. doi: 10.1002/ptr.7327
26. Hadi, A, Mohammadi, H, Hadi, Z, Roshanravan, N, and Kafeshani, M. Cumin (Cuminum cyminum L.) is a safe approach for management of lipid parameters: a systematic review and meta-analysis of randomized controlled trials. Phytother Res. (2018) 32:2146–54. doi: 10.1002/ptr.6162
27. Alfahdawi, OAS, Alfahdawi, AAS, and Mohammed, IM. Effects of aqueous extract of cumin seed (Cuminum cyminum) on the structure and function of albino rat kidneys treated with dibutyl phthalate. Iraqi J Sci. (2023):2168–77.
28. Kumar, R, Ali, M, and Kumar, A. Nephroprotective effect of Cuminum cyminum on chloropyrifos induced kidney of mice. Adv J Pharm Life sci Res. (2014) 2:46–53.
29. Salem, L, Abedi, B, and Khansooz, MJH. The effect of six-weeks Pilates exercise and cumin extract consumption on lipid profile and insulin resistance index in obese and overweight women. Health Dev J. (2019) 7:295–304.
30. Mozaffarinia, A, Gol, A, and Mohammadzadeh, A. Hepatoprotective properties of Cuminum cyminum seeds powder as post-treatment for acetaminophen-induced injury. J Adv Med Biomed Res. (2023) 31:170–6. doi: 10.30699/jambs.31.145.170
Keywords: carbohydrate diet, Cuminum cyminum, metabolic syndrome, BMI, cumin aldehyde, glucose
Citation: Aslam M, Habib-ur-Rehman, Tufail T, Almehmadi Y, Abualamah WA, Alzahrani AR and Shahid I (2025) Synergistic effects of a carbohydrate-controlled diet and Cuminum cyminum herbal infusion on metabolic syndrome. Front. Nutr. 12:1623478. doi: 10.3389/fnut.2025.1623478
Edited by:
Imran Khan, Abdul Wali Khan University Mardan, PakistanReviewed by:
Mustapha Umar Imam, Zhengzhou University, ChinaMohammed Faris Abdulghani, University of Nineveh, Iraq
Copyright © 2025 Aslam, Habib-ur-Rehman, Tufail, Almehmadi, Abualamah, Alzahrani and Shahid. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Aslam, bW5hcnouYXNsYW1AZ21haWwuY29t; bWFyaWEuYXNsYW1AYWhzLnVvbC5lZHUucGs=
 Maria Aslam
Maria Aslam Habib-ur-Rehman1
Habib-ur-Rehman1 Tabussam Tufail
Tabussam Tufail Imran Shahid
Imran Shahid