- 1Department of Geriatrics, The Affiliated Hospital of Qingdao University, Qingdao, China
- 2Department of Geriatrics, Qingdao Medical College, Qingdao University, Qingdao, China
Objectives: Evaluating prognosis in geriatric inpatients presents significant complexity and challenges. The aim of this retrospective study was to investigate the association between phase angle (PhA) and clinical outcomes in hospitalized elderly patients, specifically focusing on prolonged hospital stays, one-year readmission, or all-cause mortality.
Methods: The study enrolled individuals aged ≥65 years hospitalized in the geriatric medical ward of our hospital. PhA was assessed using BIA, and the length of hospital stay (LOS) was documented. Readmission and mortality outcomes were extracted from electronic medical records and supplemented by telephone follow-ups with patients or their relatives within 1 year following discharge. Optimal PhA thresholds for out-come prediction were determined using Receiver Operating Characteristic curve (ROC). Multivariable Cox proportional hazards regression was employed to evaluate the relationship between PhA and the composite endpoint of readmission or mortality, whereas logistic regression examined its association with LOS.
Results: This study enrolled a total of 218 geriatric patients over a median follow-up duration of 1 year. Among these participants, 42 patients (19.3%) experienced composite endpoint events, defined as either one-year readmission or all-cause mortality. Adverse event rates across the PhA tertiles (T1–T3) were 32.9%, 18.6%, and 5.6%, respectively, indicating a significant decrease in event incidence as PhA values increased. Multivariable-adjusted Cox regression analysis revealed that low PhA was significantly associated with a higher risk of one-year composite endpoint events (HR = 3.657, 95% CI: 1.625–8.229). Additionally, patients with low PhA based on the optimal ROC-derived cutoff had 3.243 times higher odds of prolonged LOS (95% CI: 1.146–9.177).
Conclusion: Low PhA is independently associated with prolonged LOS and higher one-year adverse outcomes in elderly medical inpatients. PhA can serve as a valuable indicator for monitoring malnutrition in hospitalized elderly patients and functions as a reliable independent predictor of prognosis.
1 Introduction
The disease burden resulting from population aging has emerged as a major challenge in the fields of biomedical and public health research (1, 2). Older adults aged ≥ 60 years account for 23% of the global disease burden (3). The rising prevalence of chronic diseases and age-related functional decline substantially contributes to higher hospitalization rates and adverse clinical outcomes in the elderly population. A Chinese epidemiological study revealed that the hospitalization rates among adults aged 65 and above have been increasing annually, with a one-year readmission rate as high as 25.27% (4). Poor clinical outcomes among hospitalized elderly patients contribute to increased healthcare expenditures, diminished quality of medical care, and disruptions to the daily lives of both patients and their families (5). Therefore, recognizing risk factors and implementing tailored interventions can potentially enhance quality of life, improve functional capacity, and decrease readmission and mortality rates in this population (6).
Malnutrition is a prevalent geriatric syndrome. Research has demonstrated that over than 50% of hospitalized older adults present with nutritional risk or malnutrition, and this condition may further deteriorate at discharge (7, 8). Nutritional status serves as a crucial role in determining health outcomes, as malnourished individuals face increased hospitalization costs, longer hospital length of stay (LOS), and elevated risks of readmission and mortality (7, 9, 10). Multiple nutritional screening tools are currently available, and body composition analysis has increasingly demonstrated its clinical relevance in the context of nutritional assessment in recent years (11). As a validated, non-invasive tool, bioelectrical impedance analysis (BIA) provides cost-effective body composition analysis with broad clinical applicability across medical specialties (12).
Calculated from BIA-derived resistance and reactance, phase angle (PhA) is a validated measure of cellular health, with lower PhA values indicating impaired cellular integrity or impending cell death (13). This independent parameter serves as a biomarker for evaluating cellular function, hydration status and nutritional condition, exhibiting strong correlations with disease diagnosis and prognostic indicators (14). As evidence has shown, PhA functions as a biomarker for evaluating and monitoring morbidity and mortality in respiratory diseases, and may serve as a critical indicator for predicting the mortality risk of COVID-19 (15, 16). In addition, PhA exhibits negative correlations with muscle mass and function in elderly individuals, potentially serving as a marker for the identification of sarcopenia (17, 18). Lower PhA significantly increases the risk of frailty, disability and adverse outcomes among elderly patients (19–21). Additionally, a separate study suggests that the PhA predicts a poor prognosis in general medical patients (22).
Geriatric patients often present challenges in prognostic evaluation due to complex factors such as multimorbidity and polypharmacy. While existing evidence substantiates the clinical validity of PhA for disease assessment, the predictive capacity of PhA for clinical outcomes among hospitalized elderly adults remains underexplored. To address this knowledge gap, we hypothesized that lower PhA would independently predict the composite endpoint of one-year readmission or all-cause mortality in elderly medical inpatients. Additionally, we examined the influence of PhA on LOS.
2 Materials and methods
2.1 Study design
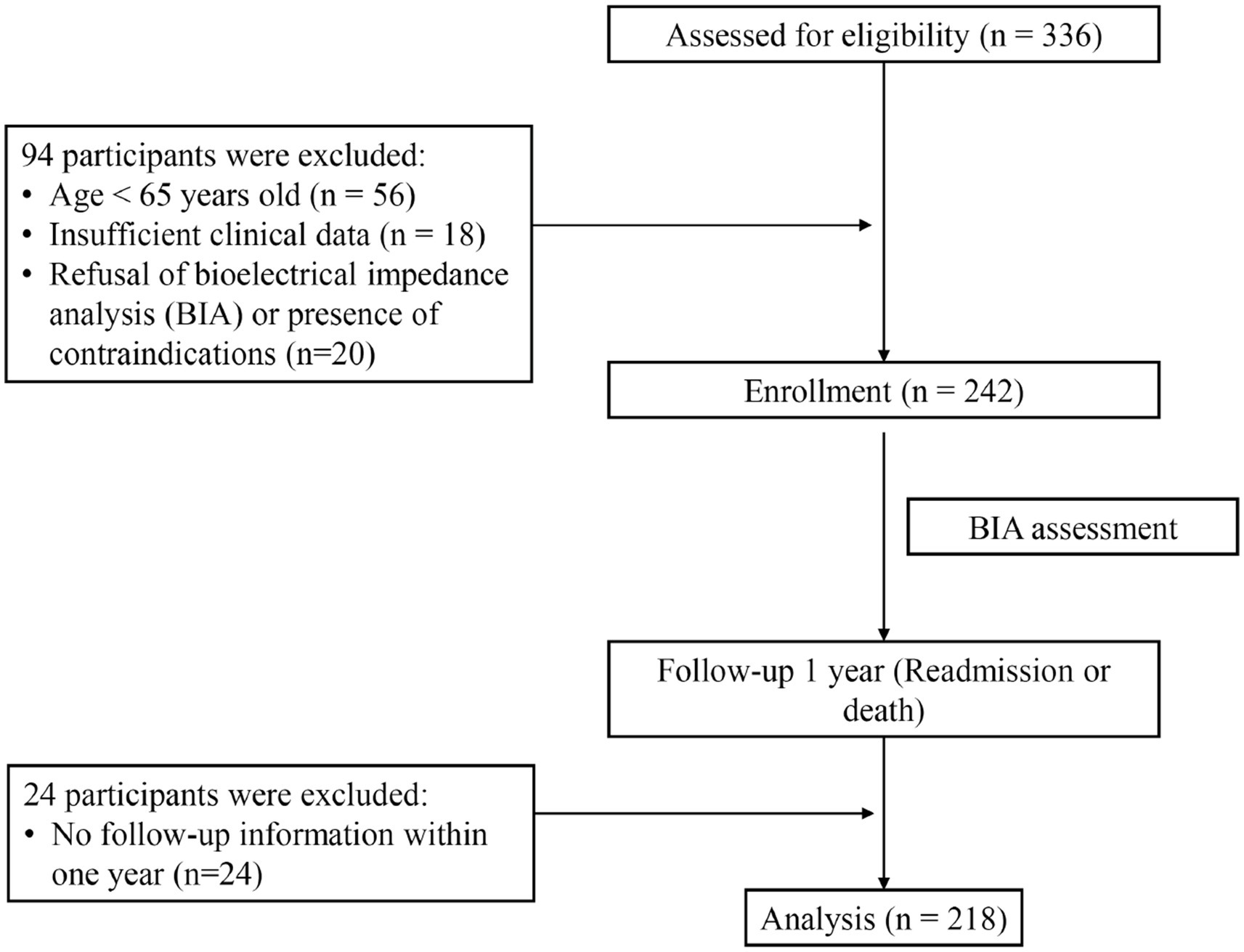
The study enrolled patients aged 65 years or older in the geriatric medical ward of the Affiliated Hospital of Qingdao University between July 2019 and January 2023. A total of 336 individuals were initially screened. We excluded patients younger than 65 years, those with incomplete clinical records, individuals who declined or had contraindicated for BIA examination, and cases lost to follow-up. Patients with severe edema were excluded prior to BIA, as severe fluid retention significantly distorts body composition measurements. Finally, a cohort of 218 patients was included in the analysis, with the research flowchart shown in Figure 1.
2.2 Assessment of PhA by BIA
The PhA was assessed by BIA using the InBody S10® device (InBody, Biospace Co., Ltd., Seoul, Korea), in accordance with the manufacturer’s protocol and a previously published methodological guideline (23). Parameters recorded included PhA, skeletal muscle mass index (SMI), visceral fat area (VFA), and waist circumference (WC).
2.3 Sociodemographic data and laboratory test indicators
Data were collected by well-trained assessors. Sociodemographic data and clinical information were obtained through a systematic review of medical records or structured interviews. Body mass index (BMI) was derived from anthropometrically measured height and weight. All patients underwent blood pressure assessments and routine laboratory examinations. The cardiac and renal functions, as well as the hydration status of the participants, were evaluated through measurements of NT-ProBNP and estimated glomerular filtration rate (eGFR).
2.4 Assessment of geriatric syndrome
The medication history, functional status, multimorbidity, and nutritional status of elderly inpatients were evaluated through structured interview questionnaires. Polypharmacy was assigned to patients receiving ≥5 medications concomitantly. Cognitive function was evaluated by the Mini-Mental State Examination (MMSE). Functional dependence was assessed by the 100-point Activities of Daily Living (ADL) scale with established cutoffs: 100 (normal), 61–99 (mild disability), 41–60 (moderate disability), and ≤40 (severe disability). We categorized functional trajectory as either “stable/improved” or “declined/death” by comparing pre-admission (2-week baseline) and pre-discharge ADL scores. Multimorbidity was quantified based on the presence of seven chronic diseases in older inpatients, including coronary heart disease, hyper-tension, diabetes, chronic obstructive pulmonary disease (COPD), cerebrovascular disease, cancer, and chronic kidney disease, as self-reported by patients and confirmed by physician diagnosis. Nutritional assessment was performed via the Geriatric Nutritional Risk Index (GNRI), with a score of ≤98 indicating malnutrition classification (24). The GNRI formula applied was: Sex-specific ideal weights were calculated as follows: for men, , while for women it was calculated as: (24).
2.5 Endpoints
With a median observation period of 1 year, we examined a composite adverse outcome that included all-cause mortality and unplanned readmission occurring within 12 months after discharge. Follow-up events were determined by reviewing electronic medical records or telephone interview records. During follow-up, the period spanning from initial hospitalization to the occurrence of the first reported adverse event was measured to determine the timing of the composite adverse outcome. For participants who did not experience any adverse events, follow-up duration reflected the period from initial assessment to last available survey date. Elderly patients were categorized based on the interquartile range (IQR) of hospitalization duration into two groups: prolonged stay (upper IQR) and normal stay, in order to evaluate the association between PhA and LOS.
2.6 Statistical analysis
Normality testing was performed using the Shapiro–Wilk test. Continuous variables were presented as mean ± standard deviation (SD) for normally distributed data or median (Q1–Q3) for non-normally distributed data, according to the normality test results. Categorical variables were presented as numbers and percentages [n, (%)], and were compared using chi-square tests or Fisher ‘s exact test, as appropriate. Group comparisons used t-tests/ANOVA for normally distributed data and Mann–Whitney U/Kruskal–Wallis tests for non-normal distributions. Bivariate correlations were assessed using Pearson’s or Spearman’s correlation coefficients.
Kaplan–Meier analysis was performed to evaluate the one-year cumulative incidence of composite adverse outcomes among elderly patients categorized by PhA tertiles. Survival curves were compared using the log-rank test. Receiver operating characteristic curve (ROC) analysis was utilized to establish the optimal PhA threshold for one-year composite outcomes and LOS prediction, with the area under the curve (AUC) serving as a metric for quantifying predictive accuracy. Multivariate Cox regression analysis was utilized to quantify the relationship between PhA and the risk of composite adverse outcome. To assess the proportional hazards assumption, Schoenfeld residuals tests were performed, demonstrating no significant deviations (all p > 0.05; Supplementary Table S1). Multivariate logistic regression models were used to evaluated the relationship between PhA and LOS. Variance inflation factor (VIF) analysis was conducted to evaluate potential multicollinearity and confirmed no significant multicollinearity among the adjusted variables (all VIF < 5; Supplementary Table S2). Additionally, bootstrapping methods were employed in sensitivity analyses to validate the consistency of the results. A p-value < 0.05 was considered statistically significant. Data analysis was performed with SPSS 26.0, R 4.5.1 and Origin 2024 software.
3 Results
3.1 Baseline characteristics
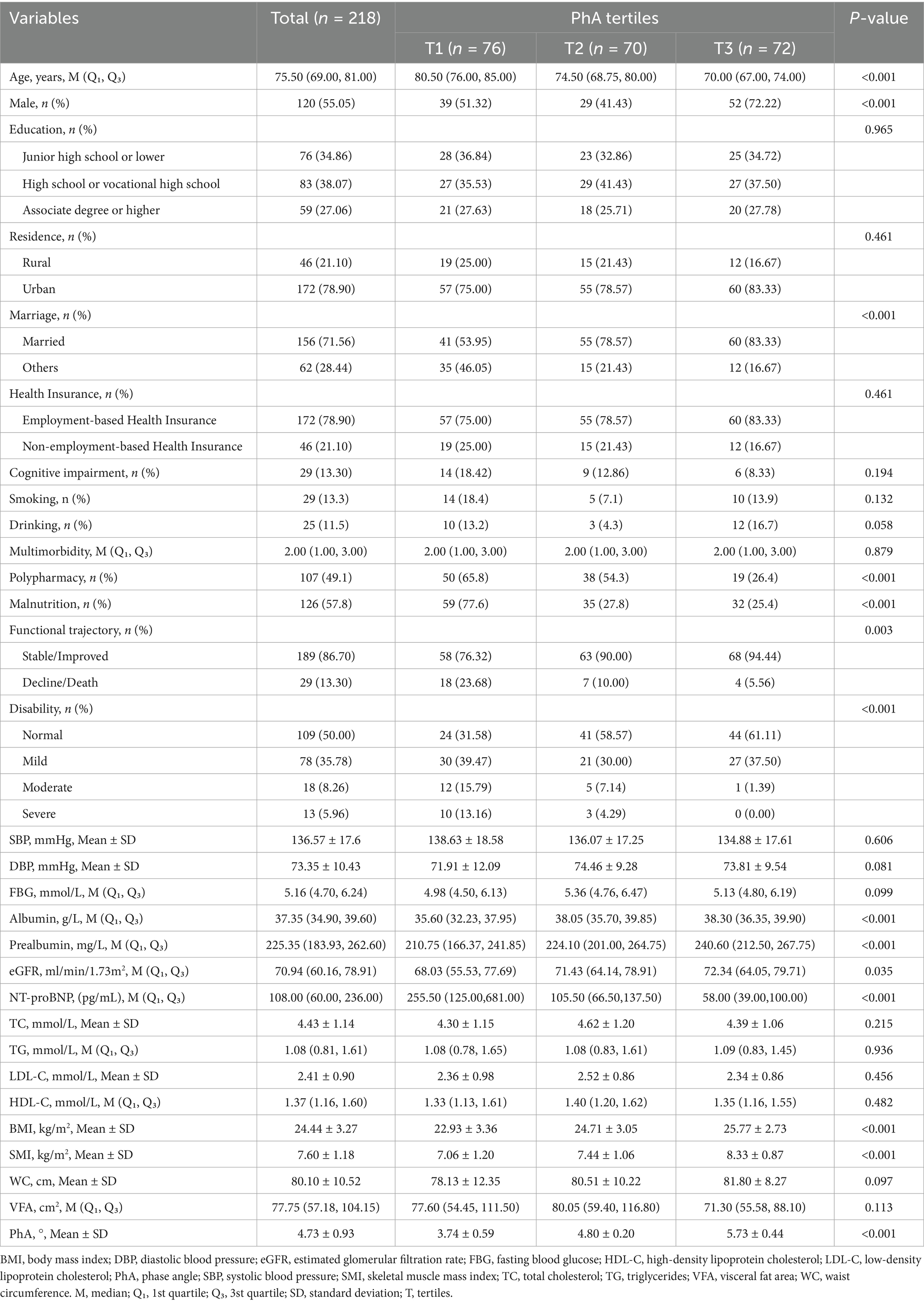
The study enrolled 218 elderly inpatients with a median age of 75.50 (69.00, 81.00), of whom 55.05% were male. The mean PhA was 4.73 ± 0.93°. Participants were stratified into tertiles (T) based on PhA: low PhA (T1, ≤4.4°), medium PhA (T2, 4.4°–5.1°), and high PhA (T3, >5.1°). Baseline characteristics of the patients were presented in Table 1. Participants in the lowest PhA tertile were significantly older and exhibited a higher prevalence of polypharmacy, malnutrition, and disability (all p < 0.001). This group also demonstrated elevated NT-proBNP levels, along with reduced albumin, prealbumin, BMI, and SMI (all p < 0.001). Notably, these participants showed a declining functional trajectory and lower eGFR (all p < 0.05).
3.2 The correlation between PhA and in-hospital prognosis risk factors
Figure 2 illustrated the relationship between PhA and various prognostic risk factors in elderly medical inpatients. The results revealed a significant positive correlation between PhA and albumin (0.448, p < 0.001), prealbumin (0.387, p < 0.001), eGFR (0.156, p < 0.05), BMI (0.388, p < 0.001), and SMI (0.451, p < 0.001). Conversely, it exhibited significant negative correlations with age (−0.585, p < 0.001), polypharmacy (−0.333, p < 0.001), malnutrition (−0.361, p < 0.001), functional trajectory (−0.241, p < 0.001), disability (−0.449, p < 0.001) and NT-proBNP (−0.595, p < 0.001).
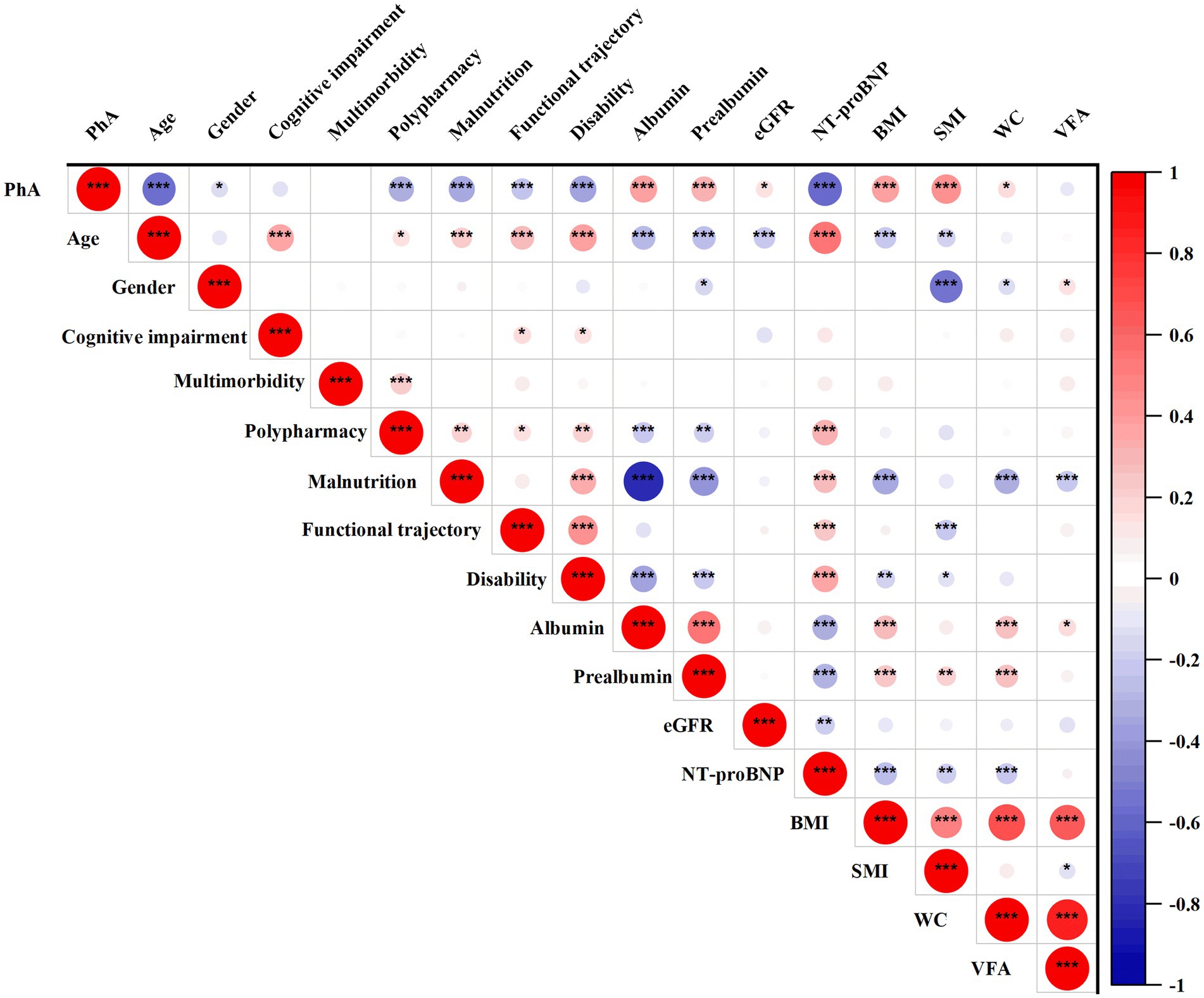
Figure 2. Correlation between PhA and prognostic risk factors in geriatric inpatient. The positive correlation is represented in red, while the negative correlation is depicted in blue. The correlation coefficient ranges from −1 to +1; a higher absolute value of correlation corresponds to a larger circle. BMI, Body Mass Index; eGFR, Estimated Glomerular Filtration Rate; PhA, Phase Angle; SMI, Skeletal Muscle Mass Index; VFA, Visceral Fat Area; WC, Waist Circumference. Significant level: *p < 0.05, **p < 0.01, ***p < 0.001.
3.3 PhA and one-year composite adverse outcomes
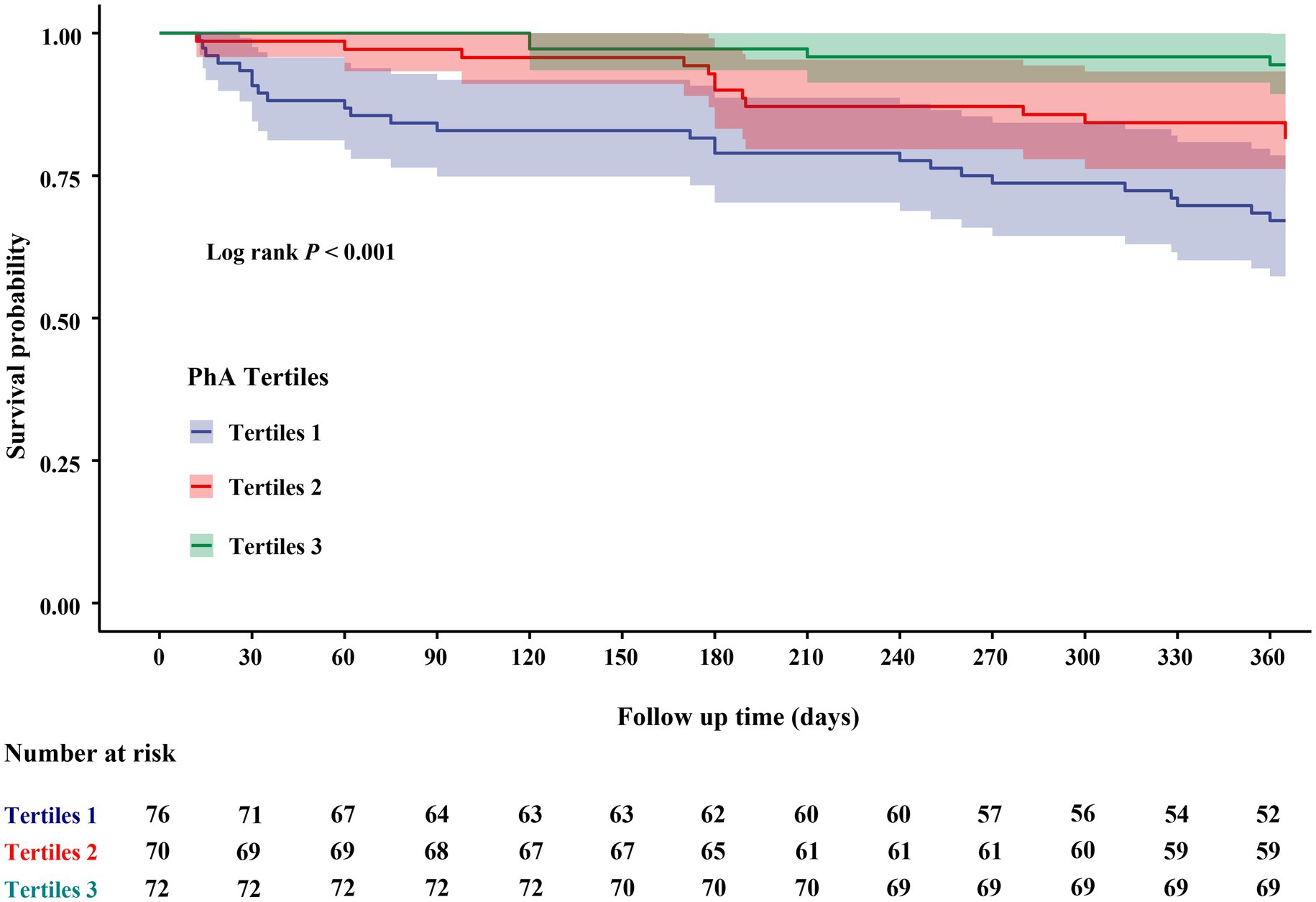
During the one-year follow-up period, 42 individuals (19.3%) experienced composite endpoint events (Supplementary Table S3). Patients who experienced composite adverse outcomes were significantly older, exhibited lower levels of serum albumin and HDL-C (p < 0.05), demonstrated a higher prevalence of disability and polypharmacy, as well as significantly higher NT-proBNP and lower PhA values (p < 0.001), relative to those without adverse outcomes. Kaplan–Meier survival curve was presented in Figure 3. Within one-year, elderly participants in the lowest one-third PhA group exhibited a significantly higher incidence of composite adverse outcomes, with cumulative incidences of 32.9% for T1, 18.6% for T2, and 5.6% for T3 (log-rank p < 0.001). Generally, a lower PhA was associated with an increased risk of composite adverse outcomes during the follow-up period.
3.4 The predictive value of PhA for composite adverse outcomes within 1 year
The ROC analysis assessed the predictive performance of PhA for composite adverse outcomes within 1 year for elderly hospitalized patients. As shown in Figure 4A, PhA exhibited moderate predictive accuracy, with an AUC of 0.730 (95% confidence interval [CI]: 0.650–0.809), indicating clinically meaningful discriminatory capacity. Furthermore, subgroup analyses stratified by gender revealed that PhA remained a significant predictor of one-year composite adverse outcomes for both male (AUC = 0.775) and female (AUC = 0.670) participants (Figure 4B). The optimal cut-off values were identified as 4.55° for males and 4.25° for females, respectively.
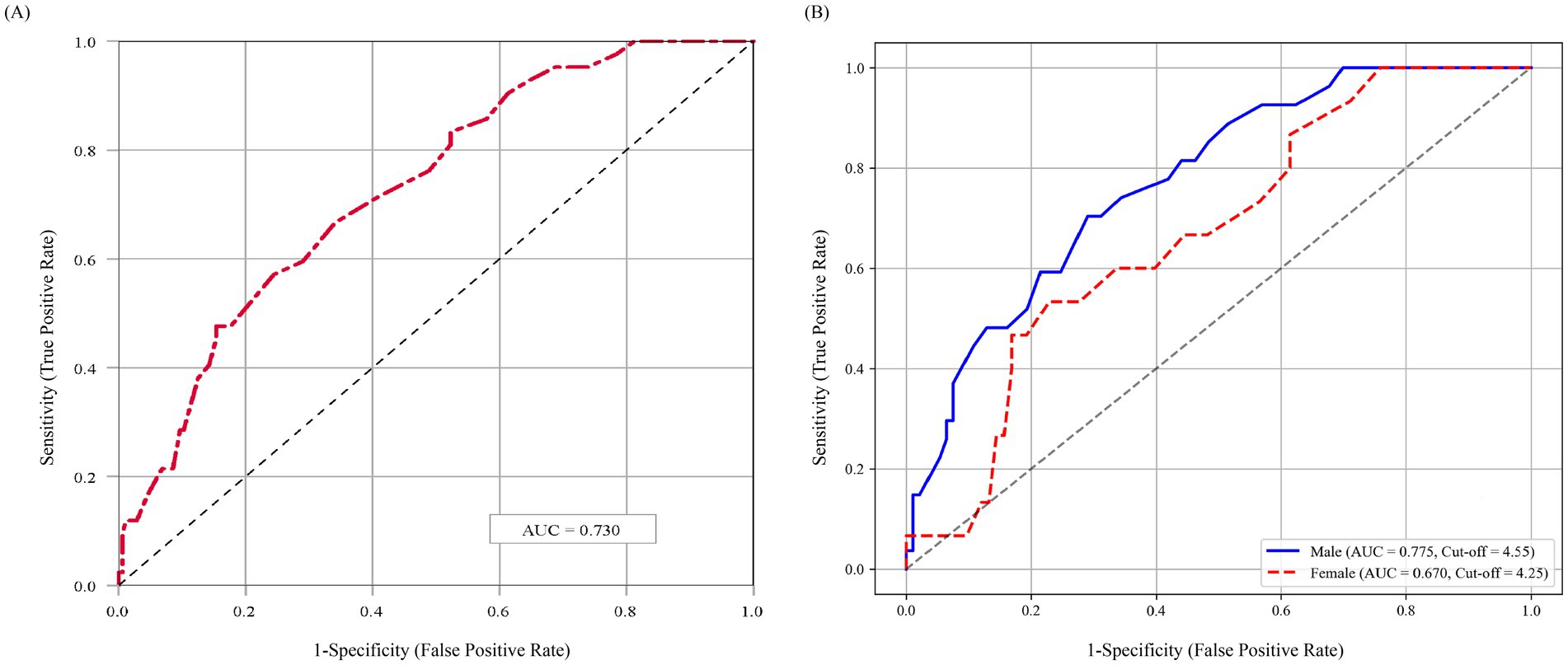
Figure 4. ROC curve of PhA for predicting composite adverse outcomes. (A) ROC analysis based on the overall study population. (B) Sex-specific ROC analyses. AUC, area under the Curve; PhA, phase angle. The PhA cutoff values were 4.55° for men and 4.25° for women.
3.5 The relationship between PhA dichotomized by cutoff value and prognosis of elderly medical inpatients
Based on sex-specific optimal cutoff values of PhA, the study population was categorized into low-PhA and normal-PhA groups. A total of 73 participants (33.5%) were classified into the low-PhA group. Comparative analyses of clinical characteristics between these two groups were presented in Supplementary Table S4.
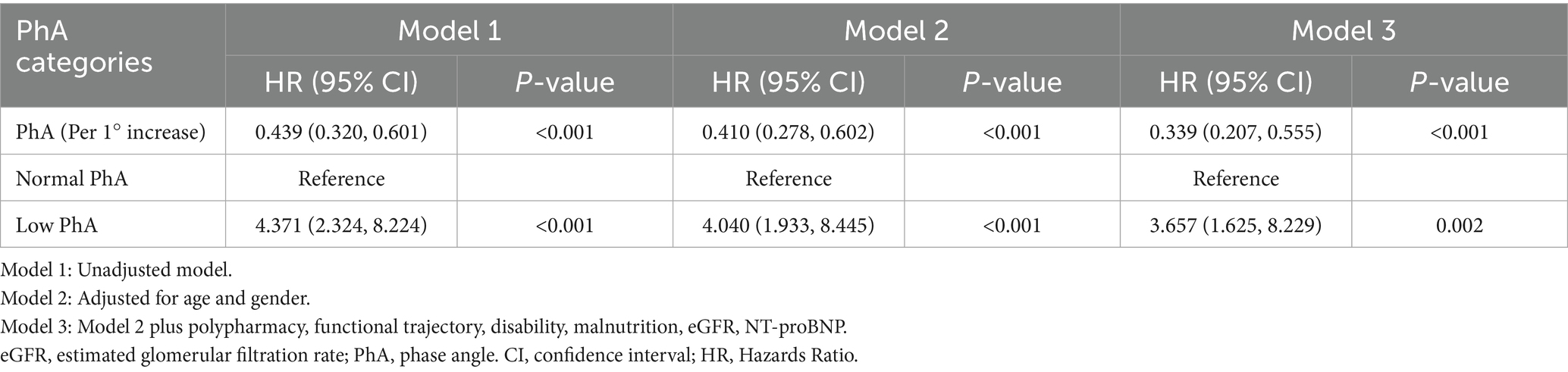
Cox proportional hazards regression was used to evaluate the association between PhA and one-year composite adverse outcomes (Table 2). The unadjusted model showed that the low PhA group had a 4.371-fold increased risk of composite adverse outcomes (hazards ratio [HR] = 4.371, 95% CI: 2.324–8.224). After adjusting for geriatric prognostic factors and fluid status in multivariate models, this association remained significant. The fully adjusted model (Model 3) demonstrated that the low PhA group maintained a 3.657-fold higher risk (adjusted HR = 3.657, 95% CI: 1.625–8.229). When analyzed as a continuous variable, each 1-degree increase in PhA was associated with a 66.1% reduction in adverse outcome risk (adjusted HR = 0.339, 95% CI: 0.207–0.555). Sensitivity analysis using 1,000 bootstrap resamples yielded consistent results, showing an average 56.1% risk reduction per 1° PhA increase (mean HR = 0.439, 95% CI: 0.309–0.578).
3.6 The relationship between PhA and prolonged LOS
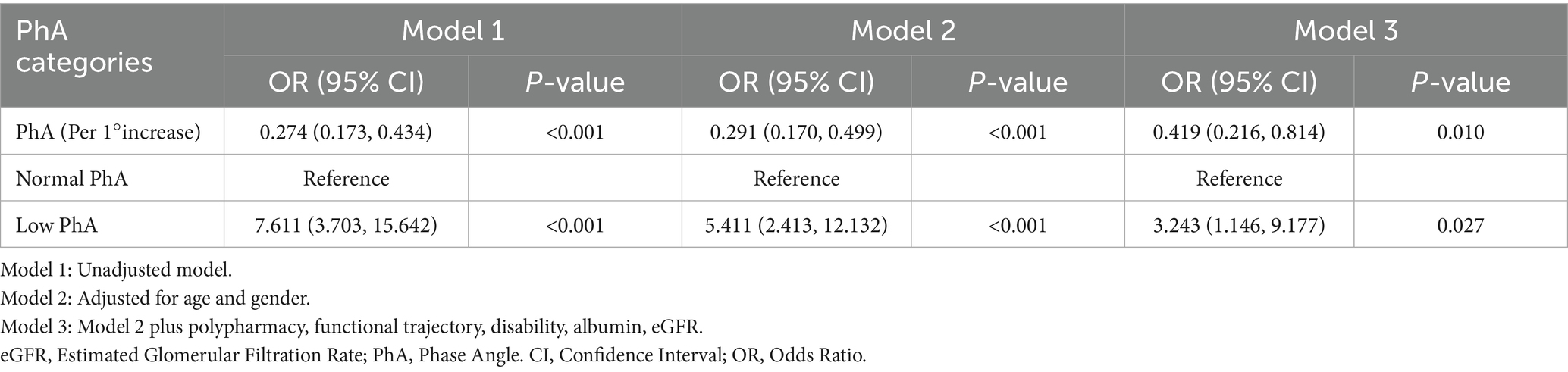
The study examined the association between PhA and LOS. ROC analysis demonstrated that PhA effectively predicted prolonged LOS, with an AUC of 0.774 (95% CI: 0.698–0.850). The optimal PhA cutoffs for identifying prolonged hospitalization were 4.45° for males and 4.25° for females (Supplementary Figure S1). Logistic regression analysis further assessed this association (Table 3). In the unadjusted model, each 1° increase in PhA was associated with a 72.6% decrease in the risk of prolonged LOS (odds ratio [OR] = 0.274, 95% CI: 0.173–0.434). This protective association maintained statistical significance after adjustment for additional risk factors (adjusted OR = 0.419, 95% CI: 0.216–0.814). In contrast, ROC-based stratification revealed that patients with low PhA exhibited a 3.243-fold higher risk of prolonged hospitalization (adjusted OR = 3.243, 95% CI: 1.146–9.177). The bootstrap analysis with 1,000 resamples provided robust validation, indicating approximately a 70% decrease in prolonged LOS risk for every 1° increment in PhA (mean OR = 0.293, 95% CI: 0.187–0.422). These consistent findings underscore PhA’s role as an independent protective factor against extended hospital stays.
4 Discussion
Elderly hospitalized patients often exhibit complex multimorbidity, yet sensitive prognostic markers for this population remain lacking. PhA has recently emerged as a promising prognostic indicator, with growing evidence supporting its clinical significance (14, 25–27). While some studies have explored PhA in clinical populations, its relationship with outcomes in geriatric medical inpatients has not been extensively investigated. Therefore, this study aims to specifically focuses on the predictive value of PhA for adverse outcomes following admission to geriatric medical wards. Our findings demonstrated that lower PhA is an independent predictor for one-year adverse outcomes and is significantly associated with prolonged LOS in geriatric patients.
Substantial evidence indicates that nutritional impairments elevate the risk of adverse clinical outcomes, negatively impact disease progression and recovery, extend hospitalization durations, and lead to functional decline or mortality (28, 29). Researches had confirmed the utility of PhA in the early identification of malnutrition among geriatric patients with multiple chronic conditions (30). In older patients with subacute stroke, a PhA below 4.08° predicted high nutritional risk (31). Additionally, studies have established PhA as an independent predictor of malnutrition and sarcopenia in elderly COPD individuals (32). In this study, we focused on the associations between PhA and nutritional status, the overall prevalence of malnutrition, as defined by the GNRI score, was 57.8%, which was in good agreement with previous reports by Hongyuan et al. (7). Significantly, the prevalence of malnutrition in the lowest tertile of the PhA group reached as high as 77.6%.
Numerous studies have consistently demonstrated the prognostic significance of PhA across a range of clinical outcomes. A prospective study indicated a significant association between reduced PhA and impaired ADL function in hemodialysis patients (33). Another cohort study revealed an independent association between low PhA and all-cause mortality within 1 year in ICU patients (34). In this study, we evaluated the predictive ability of PhA for adverse outcomes in geriatric inpatients using ROC curve analysis. The results demonstrated that PhA had moderate predictive value for one-year composite adverse outcomes (AUC = 0.730). The sex-specific optimal PhA cutoffs were 4.55° for males and 4.25° for females. Currently, no consensus exists on PhA reference values, limiting comparisons with broader populations. Previous studies have reported PhA cutoffs of 5.04° (males) and 4.20° (females) for predicting sarcopenia in older adults (18), while another study identified a PhA < 4.0° as an independent predictor of 90-day readmission or mortality in acute heart failure patients (35). In older hip fracture patients, sex-specific PhA cutoffs for 12-month mortality were 4.05° (females) and 4.65° (males) (36). The PhA cutoff ranges observed in our study align closely with previously reported values, however, further research is still required to establish universally standardized thresholds. To quantify the association between PhA and adverse outcomes, we performed a Cox regression analysis. After adjusting for covariates, each 1° increase in PhA was associated with a 66.1% decrease in the risk of one-year readmission or mortality. Similarly, low PhA based on sex-specific ROC-derived cutoffs significantly increased this risk (adjusted HR = 3.657, 95% CI: 1.625–8.229). This association may be attributed to PhA reflecting malnutrition, decreased skeletal muscle mass, impaired cellular health, and underlying oxidative stress or inflammatory damage (37, 38). Our findings support PhA as a valuable prognostic indicator for readmission and mortality in geriatric inpatients.
The LOS is a reliable indicator of healthcare quality, impacting both medical burden and disease prognosis. Previous studies have consistently shown a significant relationship between malnutrition and prolonged LOS (39, 40). A study focusing on critically ill ICU patients revealed that low PhA accurately discriminated nutritionally high-risk cases and was associated with more than double the ICU stay duration (41). Our findings similarly indicate that, after adjusting for confounding factors, low PhA increased the risk of prolonged LOS in elderly patients. However, it is important to note that prolonged LOS definitions vary widely and may be influenced by non-medical determinants, making it challenging to account for all potential confounding factors in clinical research. Therefore, this association should be interpreted with caution. Nevertheless, PhA remains a relatively effective and convenient tool for evaluating prolonged LOS in elderly hospitalized patients.
Several limitations should be acknowledged. First, the single-center retrospective design and reliance on BIA-measured participants may limit generalizability and introduce selection bias. Second, although sex-specific PhA cutoffs were derived, potential overfitting remains a concern due to the lack of external validation; further analyses would have strengthened causal inferences and enhanced the reliability of the findings. In addition, variations in BIA devices complicate cross-study comparisons. Third, although multivariate adjustments and sensitivity analyses were applied, unmeasured confounders could persist due to the observational nature of this study. Future multicenter prospective studies are needed to validate PhA’s prognostic utility and explore its mechanistic links with clinical outcomes.
In conclusion, this study demonstrated that PhA derived from BIA serves as a valuable predictive biomarker for clinical outcomes in elderly medical inpatients, independently predicting prolonged LOS and the risk of one-year readmission or all-cause death. As a non-invasive measurement, it does not require additional physical effort and serves as an elder-friendly assessment tool that is particularly well-suited for elderly patients and geriatric medical care settings.
5 Conclusion
In summary, elderly hospitalized patients exhibited a high incidence of adverse outcomes within 1 year, and PhA appears to be a promising independent predictor. Actively incorporating PhA into routine evaluations in geriatric medical wards may facilitate the identification of the most vulnerable patients, thereby enabling the provision of enhanced care and support.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Institutional Ethics Committee of the Affiliated Hospital of Qingdao University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because due to the retrospective design, the requirement for written informed consent was waived by the Institutional Ethics Committee of the Affiliated Hospital of Qingdao University.
Author contributions
JL: Methodology, Writing – original draft, Conceptualization. SH: Methodology, Writing – original draft. SW: Investigation, Data curation, Writing – original draft. TL: Data curation, Visualization, Writing – original draft. YD: Writing – original draft, Data curation, Visualization. JZ: Validation, Writing – review & editing. LJ: Writing – review & editing, Resources, Supervision. NA: Supervision, Writing – review & editing, Resources. YM: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Qingdao Science and Technology Plan Project (No. 21–1-2-2-zyyd-nsh).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1623983/full#supplementary-material
References
1. Chang, AY, Skirbekk, VF, Tyrovolas, S, Kassebaum, NJ, and Dieleman, JL. Measuring population ageing: an analysis of the global burden of disease study 2017. Lancet Public Health. (2019) 4:e159–67. doi: 10.1016/S2468-2667(19)30019-2
2. Choi, M, Sempungu, JK, Lee, EH, and Lee, YH. Living longer but in poor health: healthcare system responses to ageing populations in industrialised countries based on the findings from the global burden of disease study 2019. BMC Public Health. (2024) 24:576. doi: 10.1186/s12889-024-18049-0
3. Prince, MJ, Wu, F, Guo, Y, Gutierrez, RLM, O'Donnell, M, Sullivan, R, et al. The burden of disease in older people and implications for health policy and practice. Lancet. (2015) 385:549–62. doi: 10.1016/S0140-6736(14)61347-7
4. Gao, S, Sun, S, Sun, T, Lu, T, Ma, Y, Che, H, et al. Chronic diseases spectrum and multimorbidity in elderly inpatients based on a 12-year epidemiological survey in China. BMC Public Health. (2024) 24:509. doi: 10.1186/s12889-024-18006-x
5. Jencks, SF, Williams, MV, and Coleman, EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. (2009) 360:1418–28. doi: 10.1056/NEJMsa0803563
6. Lo, YT, Liao, JC, Chen, MH, Chang, CM, and Li, CT. Predictive modeling for 14-day unplanned hospital readmission risk by using machine learning algorithms. BMC Med Inform Decis Mak. (2021) 21:288. doi: 10.1186/s12911-021-01639-y
7. Hongyuan, C, Mingwei, Z, Wei, C, Hanping, S, Weixin, C, Birong, D, et al. Nutritional status of elderly inpatients in China: a multicenter survey. Chin J Geriatr. (2021) 40:364–9. doi: 10.3760/cma.j.issn.0254-9026.2021.03.019
8. Chen, L, Liu, C, Cui, H, Yang, X, Ding, L, Chen, Y, et al. Malnutrition dynamics according to GLIM criteria in hospitalized elderly. Asia Pac J Clin Nutr. (2022) 31:543–50. doi: 10.6133/apjcn.202209_31(3).0022
9. Cong, WJ, Liu, ZP, Liang, YX, Ye, SL, Cai, ZM, Chen, HM, et al. Predictive value of nutritional risk for all-cause death and functional outcomes in Chinese elderly patients with acute stroke: a 3-year follow-up study. Clin Interv Aging. (2024) 19:109–18. doi: 10.2147/CIA.S447038
10. Mohammed, HO, Hassan, AM, Mostafa, A, Khater, MS, Aboelfotoh, A, and Abd, EKM. Geriatric nutritional risk index and adverse medical outcomes among Egyptian patients admitted to a geriatric hospital: a prospective cohort study. BMC Geriatr. (2024) 24:62. doi: 10.1186/s12877-024-04671-5
11. Bazzocchi, A, Gazzotti, S, Santarpia, L, Madeddu, C, Petroni, ML, and Aparisi, GMP. Editorial: importance of body composition analysis in clinical nutrition. Front Nutr. (2022) 9:1080636. doi: 10.3389/fnut.2022.1080636
12. Catapano, A, Trinchese, G, Cimmino, F, Petrella, L, D'Angelo, M, Di Maio, G, et al. Impedance analysis to evaluate nutritional status in physiological and pathological conditions. Nutrients. (2023) 15:2264. doi: 10.3390/nu15102264
13. Lukaski, HC, and Talluri, A. Phase angle as an index of physiological status: validating bioelectrical assessments of hydration and cell mass in health and disease. Rev Endocr Metab Disord. (2023) 24:371–9. doi: 10.1007/s11154-022-09764-3
14. Bellido, D, Garcia-Garcia, C, Talluri, A, Lukaski, HC, and Garcia-Almeida, JM. Future lines of research on phase angle: strengths and limitations. Rev Endocr Metab Disord. (2023) 24:563–83. doi: 10.1007/s11154-023-09803-7
15. De Benedetto, F, Marinari, S, and De Blasio, F. Phase angle in assessment and monitoring treatment of individuals with respiratory disease. Rev Endocr Metab Disord. (2023) 24:491–502. doi: 10.1007/s11154-023-09786-5
16. Cornejo-Pareja, I, Vegas-Aguilar, IM, Garcia-Almeida, JM, Bellido-Guerrero, D, Talluri, A, Lukaski, H, et al. Phase angle and standardized phase angle from bioelectrical impedance measurements as a prognostic factor for mortality at 90 days in patients with COVID-19: a longitudinal cohort study. Clin Nutr. (2022) 41:3106–14. doi: 10.1016/j.clnu.2021.02.017
17. Zhang, J, Wang, N, Li, J, Wang, Y, Xiao, Y, and Sha, T. The diagnostic accuracy and cutoff value of phase angle for screening sarcopenia: a systematic review and Meta-analysis. J Am Med Dir Assoc. (2024) 25:105283. doi: 10.1016/j.jamda.2024.105283
18. Akamatsu, Y, Kusakabe, T, Arai, H, Yamamoto, Y, Nakao, K, Ikeue, K, et al. Phase angle from bioelectrical impedance analysis is a useful indicator of muscle quality. J Cachexia Sarcopenia Muscle. (2022) 13:180–9. doi: 10.1002/jcsm.12860
19. Ida, S, Kaneko, R, Imataka, K, Okubo, K, Azuma, K, and Murata, K. A low phase angle is a factor independently associated with falls in elderly diabetic patients. Nihon Ronen Igakkai Zasshi. (2023) 60:261–7. doi: 10.3143/geriatrics.60.261
20. Guner, M, Ceylan, S, Okyar, BA, Kahyaoglu, Z, Coteli, S, Koca, M, et al. Phase angle is associated with frailty in community-dwelling older adults. Nutrition. (2023) 116:112157. doi: 10.1016/j.nut.2023.112157
21. Morisawa, T, Saitoh, M, Takahashi, T, Watanabe, H, Mochizuki, M, Kitahara, E, et al. Association of phase angle with hospital-acquired functional decline in older patients undergoing cardiovascular surgery. Nutrition. (2021) 91-92:111402. doi: 10.1016/j.nut.2021.111402
22. Del Giorno, R, Quarenghi, M, Stefanelli, K, Rigamonti, A, Stanglini, C, De Vecchi, V, et al. Phase angle is associated with length of hospital stay, readmissions, mortality, and falls in patients hospitalized in internal-medicine wards: a retrospective cohort study. Nutrition. (2021) 85:111068. doi: 10.1016/j.nut.2020.111068
23. Martins, AD, Oliveira, R, Brito, JP, Costa, T, Ramalho, F, Pimenta, N, et al. Phase angle cutoff value as a marker of the health status and functional capacity in breast cancer survivors. Physiol Behav. (2021) 235:113400. doi: 10.1016/j.physbeh.2021.113400
24. Kubo, Y, Noritake, K, Nakashima, D, Fujii, K, and Yamada, K. Relationship between nutritional status and phase angle as a noninvasive method to predict malnutrition by sex in older inpatients. Nagoya J Med Sci. (2021) 83:31–40. doi: 10.18999/nagjms.83.1.31
25. Amano, K, Bruera, E, and Hui, D. Diagnostic and prognostic utility of phase angle in patients with cancer. Rev Endocr Metab Disord. (2023) 24:479–89. doi: 10.1007/s11154-022-09776-z
26. Yoshimura, Y, Wakabayashi, H, Nagano, F, Matsumoto, A, Shimazu, S, Shiraishi, A, et al. Phase angle is associated with sarcopenic obesity in post-stroke patients. Clin Nutr. (2023) 42:2051–7. doi: 10.1016/j.clnu.2023.08.018
27. Gheri, CF, Scalfi, L, Luisi, MLE, and Di Vincenzo, O. Bioelectrical impedance analysis (BIA) phase angle in stroke patients: a systematic review. Clin Nutr. (2024) 43:63–72. doi: 10.1016/j.clnu.2024.10.001
28. Bellanti, F, Lo, BA, Quiete, S, and Vendemiale, G. Malnutrition in hospitalized old patients: screening and diagnosis, clinical outcomes, and management. Nutrients. (2022) 14:910. doi: 10.3390/nu14040910
29. Schuetz, P, Seres, D, Lobo, DN, Gomes, F, Kaegi-Braun, N, and Stanga, Z. Management of disease-related malnutrition for patients being treated in hospital. Lancet. (2021) 398:1927–38. doi: 10.1016/S0140-6736(21)01451-3
30. Wang, Y, Liu, T, Zheng, T, Zhang, Y, Li, L, and Gao, M. Phase angle as a predictor of mortality in elderly patients with multimorbidity: a matched case-control study. PeerJ. (2024) 12:e18592. doi: 10.7717/peerj.18592
31. Di Vincenzo, O, Pagano, E, Ballarin, G, Pasanisi, F, and Scalfi, L. Phase angle is associated with nutritional risk in subacute stroke patients at the beginning of rehabilitation. Nutrition. (2025) 131:112637. doi: 10.1016/j.nut.2024.112637
32. Murakami, T, Kobayashi, T, Ono, H, Shibuma, H, Tsuji, K, Nikkuni, E, et al. Phase angle as an indicator of sarcopenia and malnutrition in patients with chronic obstructive pulmonary disease. Respir Investig. (2024) 62:651–6. doi: 10.1016/j.resinv.2024.05.012
33. Li, J, Wang, Z, Zhang, Q, Zhang, H, Shen, Y, Zhang, Q, et al. Association between disability in activities of daily living and phase angle in hemodialysis patients. BMC Nephrol. (2023) 24:350. doi: 10.1186/s12882-023-03400-1
34. Stellingwerf, F, Beumeler, LFE, Rijnhart-de, JH, Boerma, EC, and Buter, H. The predictive value of phase angle on long-term outcome after ICU admission. Clin Nutr. (2022) 41:1256–9. doi: 10.1016/j.clnu.2022.03.029
35. Bernal-Ceballos, MF, Hernandez-Gilsoul, T, Reyes-Paz, Y, Villanueva-Juarez, JL, and Castillo-Martinez, L. Cutoff value of phase angle by bioelectrical impedance analysis at admission as a prognostic factor in patients with acute heart failure. J Vis Exp. (2025) 220:e68203. doi: 10.3791/68203
36. Sanchez-Torralvo, FJ, Perez-Del-Rio, V, Navas, VLI, Garcia-Olivares, M, Porras, N, Abuin, FJ, et al. Phase angle as a predictor of mortality in older patients with hip fracture. Nutrients. (2024) 16:2221. doi: 10.3390/nu16142221
37. Lukaski, HC, and Garcia-Almeida, JM. Phase angle in applications of bioimpedance in health and disease. Rev Endocr Metab Disord. (2023) 24:367–70. doi: 10.1007/s11154-023-09799-0
38. Zheng, WH, Zhao, YH, Yao, Y, and Huang, HB. Prognostic role of bioelectrical impedance phase angle for critically ill patients: a systemic review and meta-analysis. Front Med (Lausanne). (2022) 9:1059747. doi: 10.3389/fmed.2022.1059747
39. Wang, X, Naito, Y, Nakatani, H, Ida, M, and Kawaguchi, M. Prevalence of undernutrition in surgical patients and the effect on length of hospital stay. J Anesth. (2022) 36:89–95. doi: 10.1007/s00540-021-03013-8
40. Song, D, Zhang, L, Zhang, Y, Liu, S, Shen, R, Zhou, W, et al. Risk factors for inpatient malnutrition and length of stay assessed by 'nutritionday' in China. Asia Pac J Clin Nutr. (2022) 31:561–9. doi: 10.6133/apjcn.202209_31(3).0024
41. Razzera, EL, Marcadenti, A, Rovedder, SW, Alves, FD, Fink, JDS, and Silva, FM. Parameters of bioelectrical impedance are good predictors of nutrition risk, length of stay, and mortality in critically ill patients: a prospective cohort study. JPEN J Parenter Enteral Nutr. (2020) 44:849–54. doi: 10.1002/jpen.1694
Glossary
ADL - Activities of Daily Living
AUC - Area Under the Curve
BIA - Bioelectrical Impedance Analysis
BMI - Body Mass Index
COPD - Chronic Obstructive Pulmonary Disease
CI - Confidence Interval
DBP - Diastolic Blood Pressure
eGFR - Estimated Glomerular Filtration Rate
FBG - Fasting Blood Glucose
GNRI - Geriatric Nutritional Risk Index
HDL-C - High-Density Lipoprotein Cholesterol
HR - Hazards Ratio
IQR - Interquartile Range
LOS - Length of Hospital Stay
LDL-C - Low-Density Lipoprotein Cholesterol
MMSE - Mini-Mental State Examination
OR - Odds Ratio
PhA - Phase Angle
ROC - Receiver Operating Characteristic Curve
SD - Standard Deviation
SBP - Systolic Blood Pressure
SMI - Skeletal Muscle Mass Index
TC - Total Cholesterol
TG - Triglycerides
T - Tertiles
VIF - Variance Inflation Factor
VFA - Visceral Fat Area
WC - Waist Circumference
Keywords: elderly, malnutrition, bioelectrical impedance, phase angle, adverse outcomes
Citation: Liu J, Hu S, Wang S, Luan T, Duan Y, Zhou J, Jia L, An N and Mao Y (2025) Phase angle as a predictor of prolonged length of hospital stay and adverse outcomes in elderly medical inpatients: a retrospective cohort study. Front. Nutr. 12:1623983. doi: 10.3389/fnut.2025.1623983
Edited by:
Gerson Ferrari, University of Santiago, ChileReviewed by:
Dina Khodeer, Suez Canal University, EgyptBatoul Khoundabi, Iranian Red Crescent Society, Iran
Copyright © 2025 Liu, Hu, Wang, Luan, Duan, Zhou, Jia, An and Mao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongjun Mao, bWFveW9uZ2p1bkBxZHVob3NwaXRhbC5jbg==
 Jia Liu
Jia Liu Song Hu1
Song Hu1 Shan Wang
Shan Wang Yuting Duan
Yuting Duan