- 1Department of Biomedical Sciences, School of Biosciences and Technology, Vellore Institute of Technology, Vellore, India
- 2Faculty of Engineering, Department of Science and Humanities, Karpagam Academy of Higher Education, Coimbatore, India
The gut microbiome plays a fundamental role in regulating host immunity, metabolism, and overall health. Disruptions to this microbial ecosystem, known as dysbiosis, have been implicated in various conditions such as colorectal cancer, inflammatory bowel diseases, and metabolic syndromes. Although probiotics are widely used to restore microbial balance, their efficacy is often inconsistent due to variable colonization and concerns over antimicrobial resistance gene transfer. This review explores the growing body of literature surrounding postbiotics—bioactive metabolites produced by probiotics—as a promising alternative to live microbial therapy. We focus on key classes of postbiotics including exopolysaccharides, cell-free supernatants, short-chain fatty acids, and bacteriocins, summarizing their reported immunomodulatory, antimicrobial, antioxidant, and anti-cancer properties. We also highlight recent developments in formulation techniques, such as encapsulation, which enhance their stability and bioavailability. While current findings are promising, limitations persist, including variability in postbiotic composition and a lack of standardized clinical evaluations. Future research should aim to clarify their mechanisms of action, define optimal delivery strategies, and assess long-term safety. Overall, postbiotics present a sustainable, non-viable, and functionally rich alternative to probiotics, aligning with global health goals—Sustainable Development Goal 3 (Good Health and Well-being) and Sustainable Development Goal 9 (Industry, Innovation, and Infrastructure)—by offering safer, scalable, and effective therapeutic solutions.
1 Introduction
The gut microbiome is one of the most extensively studied microbiomes, housing a vast number of microorganisms that is 100 times greater than any other bacterial community in the human body, that are pivotal in shaping the host’s immune system and overall health (1, 2). Gut dysbiosis is characterized by disruptions in the composition and function of gut microbial communities—has been associated with a range of conditions, including colorectal cancer (CRC), inflammatory bowel diseases (IBDs), irritable bowel syndrome (IBS), and coeliac disease, among others (2, 3). Alterations in microbial composition—such as reduced abundance of beneficial Firmicutes and Bacteroidetes, and increased levels of pathogenic species like Fusobacterium and Proteobacteria have been observed in IBD and CRC patients. In individuals affected by IBD, a decreased prevalence of anti-inflammatory microbes such as Firmicutes and Bacteroidetes and an increased prevalence of pro-inflammatory taxa like Fusobacterium spp. and Proteobacteria have been documented by Sokol et al. (4) and Feizi et al. (5). Similar microbial imbalances have been observed in CRC, where specific bacterial drivers such as Clostridium septicum, Streptococcus bovis, and Enterococcus faecalis are implicated in promoting hyperproliferative and genotoxic processes within the gut epithelium (6).
Several factors can lead to gut dysbiosis that include use of antibiotics, stress, poor diet, and infections leading to the loss of beneficial bacteria and dominance of pathogenic bacteria. To treat dysbiosis, a healthy gut microbiome can be restored using fecal microbiota transplantation (FMT), prebiotics, and probiotics (7). Specifically, antibiotic-induced dysbiosis can be alleviated through the introduction of probiotic-rich foods or supplements containing well-characterized strains. Qualified Presumption of Safety (QPS) strains, including Lactobacillus rhamnosus GG, Bifidobacterium longum, Lactobacillus plantarum, and Saccharomyces boulardii, have demonstrated effectiveness in restoring gut homeostasis, enhancing mucosal immunity, and reducing inflammation (8). For example, L. rhamnosus GG has been shown to prevent antibiotic-associated diarrhea and modulate gut barrier function (9), while S. boulardii effectively shortens the duration of acute infectious diarrhea and reduces recurrence rates of Clostridioides difficile infections (10). Moreover, probiotic interventions can modulate the gut-brain axis and inflammatory signaling pathways. Bifidobacterium and Lactobacillus strains have been reported to regulate neurotransmitter metabolism, lower pro-inflammatory cytokines, and improve stress-related symptoms (11).
However, clinical outcomes depend on strain specificity, dosing regimen, duration, and host factors such as baseline microbiota composition and antibiotic exposure (12). Thus, probiotic supplementation with clinically validated QPS strains offers a targeted strategy to mitigate antibiotic-induced dysbiosis, improve gut health, and support systemic immune function under specific conditions where microbial diversity and resilience have been compromised.
This growing recognition of probiotic benefits has encouraged individuals to incorporate probiotic-containing dietary supplements into their routines. These products are valued for their ability to manage gastrointestinal tract infections, inhibit the growth of pathogenic microbes, and alleviate symptoms associated with lactose intolerance.
However, certain probiotic strains commonly found in dietary supplements, including Enterococcus faecalis, Lactobacillus rhamnosus GG, and Bifidobacterium animalis subsp. lactis, have been reported to carry antimicrobial resistance (AMR) genes such as tet(M), erm(B), and vanA. These genes confer resistance to tetracyclines, macrolides, and vancomycin, respectively (13). Horizontal gene transfer, particularly via plasmids and transposons, facilitates the transmission of these resistance genes from probiotics to resident gut microbiota under selective pressure from antibiotic use (14). This mechanism poses a significant risk for the enrichment of multidrug-resistant bacterial populations within the intestinal environment (15). Hence, although probiotics offer health benefits, the potential for AMR gene dissemination warrants careful strain selection and genomic screening prior to probiotic application in clinical and dietary contexts (13).
Despite probiotic use, conclusive evidence of their colonization in the gut mucosa is lacking. The effectiveness of probiotics is highly variable due to individual microbiome differences influenced by factors like diet and medication. Furthermore, stool analysis is not a reliable indicator of probiotic impact on the gut lining. While in vitro and mouse studies show some promise, a single human study during colonoscopy failed to demonstrate significant probiotic colonization (12). Recent findings suggest that probiotics’ benefits might come more from the production of metabolites (postbiotics) than from the live bacteria themselves (probiotics) (16).
Recent literature suggests that the beneficial effects attributed to probiotics may arise primarily from their metabolic byproducts, collectively termed postbiotics. Postbiotics, also referred to as cell-free supernatants (CFS), metabolites, or biogenics, are bioactive compounds produced by live probiotic bacteria or released during bacterial lysis. These include diverse substances such as microbial fractions, functional proteins, extracellular polysaccharides, cell wall components (e.g., teichoic acids and peptidoglycans), and short-chain fatty acids (SCFAs) (17, 18). Functionally, postbiotics exert a range of health-promoting effects. They possess immunomodulatory, antimicrobial, antioxidant, and anti-inflammatory properties, which contribute to host defense and intestinal homeostasis. Additionally, certain postbiotic metabolites have been reported to support glucose metabolism, regulate lipid accumulation and obesity, and inhibit pathogenic microorganisms (19). Therefore, postbiotics have the potential to serve as safe next-generation therapeutics without the need to administer live bacteria.
Additionally, postbiotics, including short-chain fatty acids (SCFAs) and bacteriocins, play a vital role in maintaining gut microbial balance and preventing the overgrowth of pathogenic organisms. These metabolites support the integrity of the intestinal barrier and contribute to gut health by promoting the growth of beneficial commensals (17).
Unlike probiotics, postbiotics do not involve the administration of live microorganisms, thereby eliminating the risks associated with AMR gene transfer and the variability of microbial colonization outcomes in different hosts (18). Their inherent stability and targeted effects make them attractive candidates for therapeutic development. This makes them a safer therapeutic option, particularly in immunocompromised or critically ill individuals. Functionally, postbiotics exhibit a diverse range of biological activities, including immunomodulatory, anti-inflammatory, and antimicrobial effects. These properties enable them to be valuable adjuncts in the management of gut dysbiosis, metabolic syndromes, and infectious diseases (20). Furthermore, postbiotics offer substantial industrial potential as stable, non-viable bioactive compounds suitable for incorporation into functional foods, pharmaceuticals, and nutraceuticals. Their production is scalable and sustainable, aligning with global initiatives targeting antibiotic resistance mitigation and supporting innovations in biotechnology and public health (21).
Their stability and efficacy compared to probiotics make them ideal for large-scale industrial applications, fostering advancements in precision medicine, nutraceuticals, and antimicrobial alternatives. Additionally, postbiotic-based products can reduce reliance on antibiotics, aligning with global efforts to combat AMR while promoting sustainable health solutions.
This perspective aims to consolidate recent findings on the functional roles and mechanisms of postbiotics derived from probiotic bacteria with emphasis on their emerging value as next-generation therapeutics. We discuss their biological activities, clinical relevance, formulation strategies, and potential for industrial translation. By bridging health and innovation, postbiotics support both sustainable development goal 3 (Good Health and Well-Being) and SDG 9 (Industry, Innovation, and Infrastructure), paving the way for safer, more effective, and accessible healthcare solutions (Figure 1).
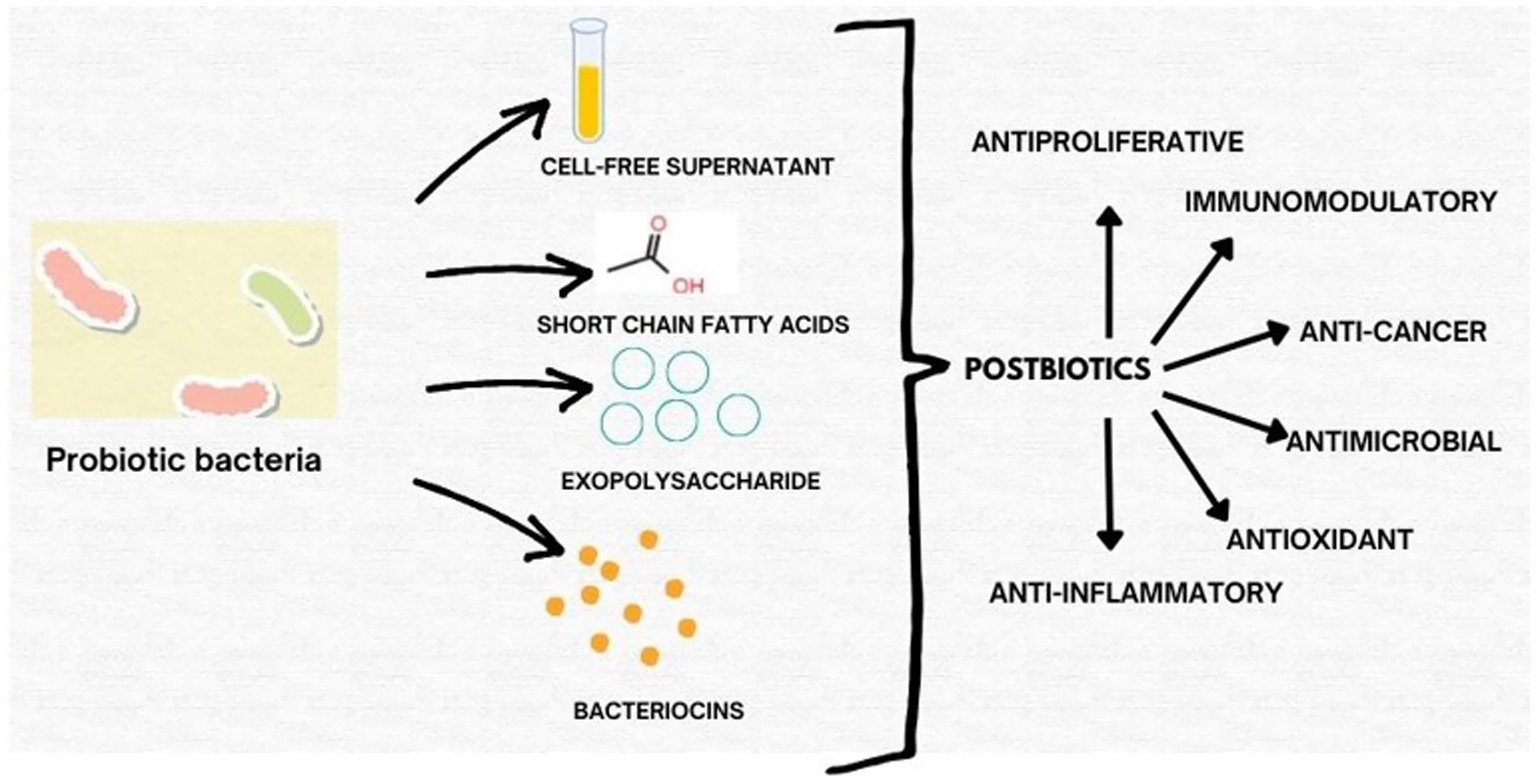
Figure 1. Schematic representation of key classes of postbiotics derived from probiotic bacteria, including exopolysaccharides (EPS), short-chain fatty acids (SCFAs), cell-free supernatants (CFS), and bacteriocins, highlighting their bioactivities.
2 Materials and methods
This narrative review was conducted to synthesize current scientific literature on the therapeutic potential of postbiotics derived from probiotic organisms. Relevant articles were identified through comprehensive electronic searches of PubMed, Scopus, ScienceDirect, and Google Scholar databases. The search strategy included combinations of the following keywords: “postbiotics,” “probiotic metabolites,” “cell-free supernatant,” “bacteriocins,” “short-chain fatty acids,” “exopolysaccharides,” “gut microbiome,” “next-generation therapeutics,” and “antimicrobial resistance.”
Over 120 peer-reviewed publications were initially screened. After removing duplicates and applying inclusion criteria, a total of 67 key studies were reviewed in-depth and synthesized to support this article’s discussion. Where appropriate, data from animal models and clinical studies were also included to highlight translational relevance. References were managed using Mendeley and verified against journal citation formats. This perspective follows a narrative approach, emphasizing mechanistic insights, therapeutic relevance, and gaps in current knowledge for future research directions.
3 Exopolysaccharides from probiotic bacteria as therapeutic agents
Exopolysaccharides (EPSs) are carbohydrate polymers produced by probiotic bacteria, typically composed of repeating monosaccharide units with molecular weights ranging from 10 to 2000 kDa. These bioactive compounds have demonstrated diverse therapeutic properties, including antioxidant, immunomodulatory, antithrombotic, anticoagulant, anticancer, and wound healing activities (22).
3.1 Antioxidant activity
EPSs derived from Lactobacillus plantarum RJF4 demonstrated antioxidant potential, achieving 23.63% DPPH radical scavenging activity and 32% total antioxidant capacity (23). Similarly, Lactobacillus paracasei EPS isolated from homemade sauerkraut exhibited robust antioxidant effects, with 76.34% total antioxidant capacity, 71.15% reducing power, 68.65% hydrogen peroxide scavenging efficiency, and 60.31% DPPH scavenging activity (24). Bacillus albus DM-15, isolated from the Ayurvedic formulation Dasamoolarishta, also displayed DPPH radical scavenging activity across concentrations of 0.5–30 mg/mL (25). EPSs are extracellular carbohydrate polymers secreted by many lactic acid bacteria. Their structure, molecular weight, and monosaccharide composition significantly influence their bioactivity. EPSs derived from Lactobacillus plantarum RJF4, for example, demonstrate free radical scavenging capacity and α-amylase inhibition, suggesting potential applications in oxidative stress-related diseases and diabetes (23).
3.2 Anticancer properties
Several EPSs exhibit antiproliferative effects against cancer cell lines. Lactobacillus plantarum RJF4 EPS demonstrated cytotoxicity toward the MiaPaCa2 pancreatic cancer cell line (26). Bacillus albus DM-15 EPS showed notable cytotoxic effects against the lung cancer cell line A549, with an IC50 value of 20 ± 0.97 μg/mL (25). Additionally, Lactobacillus pantheris TCP102 produced three EPSs (EPS1–EPS3) that inhibited the proliferation of gastric (BCG-803), colon (HCT-116), and ovarian (A-2780) cancer cells (26). EPSs produced by Lactobacillus pantheris TCP102 have been shown to stimulate IL-6 and TNF-α production in macrophages, while also suppressing proliferation of multiple cancer cell lines (26). Their antitumor potential may be attributed to activation of the NF-κB and MAPK signaling pathways, although further mechanistic studies are warranted.
3.3 Immunomodulatory activity
EPSs from Lactobacillus pantheris TCP102 stimulated pro-inflammatory cytokines such as IL-6 and TNF-α, as well as nitric oxide production in macrophages and Ana-1 cells (26), indicating potential for immune regulation and adjunctive therapy in immunodeficiency disorders.
3.4 Wound healing effects
An EPS derived from Lactiplantibacillus plantarum EI6 exhibited significant wound-healing capacity. In a scratch assay, EPS-treated cells achieved 50% wound closure, compared to 41% in untreated controls (27). EPSs from probiotic bacteria exhibit multifaceted therapeutic potential and present a viable alternative to the administration of live probiotics for functional food, pharmaceutical, and biomedical applications.
4 Cell-free supernatants from probiotic Bacteria as therapeutic agents
Cell-free supernatants (CFS) produced by probiotic bacteria contain secreted bioactive compounds with potent biological activities. These supernatants demonstrate antimicrobial, anticancer, and immunomodulatory properties, making them promising alternatives to conventional probiotics for therapeutic use (28). The Figure 2 provides a schematic representation of postbiotics derived from different probiotic bacteria, including exopolysaccharides, bacteriocins, cell-free supernatants, and short-chain fatty acids. The figure highlights their respective microbial sources such as Lactobacillus plantarum, Bifidobacterium breve, and Saccharomyces boulardii, and their associated bioactivities like antioxidant, anti-inflammatory, antibacterial, cytotoxic, and immunomodulatory effects. This integrative visual framework complements the discussion by summarizing the diverse therapeutic roles of postbiotics.
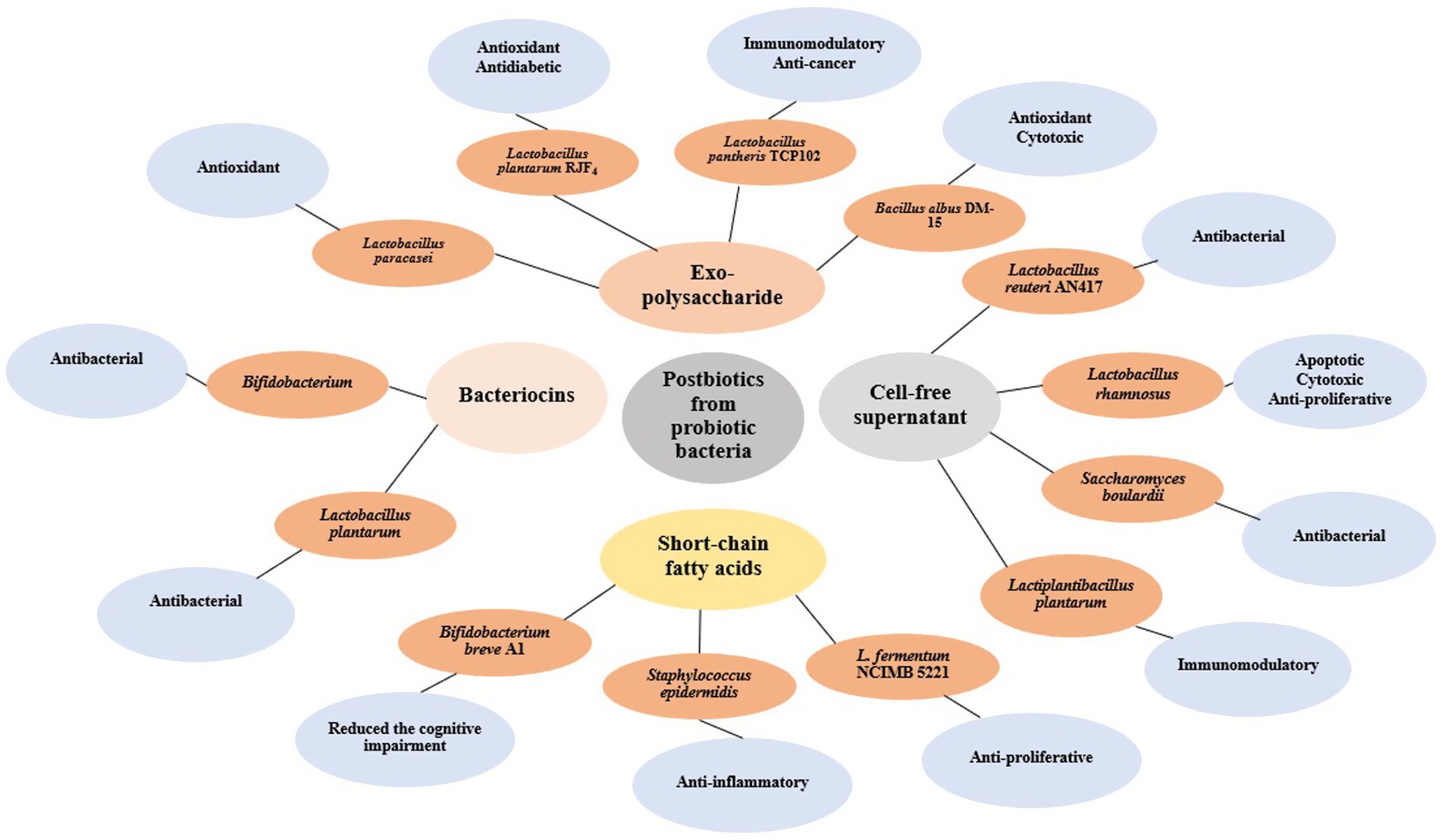
Figure 2. Shematic representation of major classes of postbiotics derived from various probiotic bacteria. The illustration includes exopolysaccharides (EPS) produced by Lactobacillus plantarum, Lactobacillus rhamnosus, and Bifidobacterium breve; bacteriocins produced by Lactobacillus acidophilus and Lactococcus lactis; cell-free supernatants (CFS) from Lactobacillus casei, Streptococcus thermophilus, and Saccharomyces boulardii; and short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate commonly released by Bifidobacterium and Faecalibacterium species. These postbiotics are associated with diverse bioactivities including antioxidant, antibacterial, anti-inflammatory, cytotoxic, and immunomodulatory effects.
4.1 Anticancer effects
CFS from Lactobacillus rhamnosus has shown apoptotic, cytotoxic, and anti-proliferative effects on HT-29 colon cancer cells in a dose- and time-dependent manner. Gene expression analysis revealed increased Bax, caspase-3, and caspase-9 levels, indicating induction of apoptosis and suggesting its potential as a biological anticancer agent (29). Similarly, both live Lactobacillus casei and its CFS demonstrated anti-proliferative effects against CT26 and HT29 colon cancer cells (30).
4.2 Antimicrobial activity
Several studies have reported the antimicrobial potential of probiotic-derived CFS. The purified CFS from Lactobacillus rhamnosus L60 and Lactobacillus fermentum L23 produced inhibition zones of 19.01 ± 2.69 mm and 18.33 ± 2.48 mm, respectively, against Neisseria gonorrhoeae, with all tested strains displaying complete susceptibility (31). Similarly, the cell-free spent medium (CFSM) from four lactic acid bacteria (LAB) strains—Lactobacillus acidophilus, Lactobacillus delbrueckii, Lactobacillus johnsonii, and Lactiplantibacillus plantarum—exhibited strong antimicrobial effects against Pseudomonas aeruginosa strains 27853™ and 9027™ (32). In addition, the CFS from Saccharomyces boulardii metabolites demonstrated significant antibacterial activity, particularly against Escherichia coli (15.43 ± 0.26 mm), with efficacy persisting at pH ≤ 4 and reduced at higher pH values (33). Protease treatment partially diminished activity, indicating the presence of both proteinaceous and non-protein antimicrobial compounds (34).
4.3 Immunomodulatory properties
CFS can also modulate immune responses. Lactiplantibacillus plantarum CFS was shown to regulate cytokine activity in human macrophage cultures by increasing TNF-α production, suppressing pro-inflammatory IL-8 and TNF-α in response to inflammatory stimuli, and enhancing anti-inflammatory IL-10 levels (34). Additionally, Lactobacillus reuteri AN417 CFS demonstrated antimicrobial and anti-biofilm effects against oral pathogens, reducing biofilm integrity, downregulating virulence gene expression, and lowering ATP levels. Minimal inhibitory volumes varied by pathogen: 30% for Streptococcus mutans, 20% for Fusobacterium nucleatum, and 10% for Porphyromonas gingivalis (35). These findings collectively underscore the broad-spectrum biological activities of probiotic-derived CFS, including antimicrobial, anticancer, and immune-regulatory properties. By eliminating the need for live bacterial administration, CFS offers a safer, more controlled, and potentially scalable option for functional food and pharmaceutical applications. Continued research is warranted to isolate, characterize, and optimize these bioactive metabolites for clinical use.
5 Short-chain fatty acids from probiotic bacteria as therapeutic agents
Short-chain fatty acids (SCFAs) such as butyrate, propionate, and acetate are primary metabolic end-products generated by the gut microbiome through the fermentation of complex dietary carbohydrates, particularly dietary fibers. This process involves carbohydrate-active enzymes absent in mammals and also produces organic acids and gasses as secondary metabolites (36). SCFAs exert diverse physiological effects, playing essential roles in gut barrier integrity, immunomodulation, and energy metabolism.
5.1 Neuroprotective effects
Emerging evidence suggests that SCFAs may reduce the frequency of seizures and alleviate psychiatric comorbidities in individuals with epilepsy. By modulating oxidative stress, balancing neurotransmitter levels, and influencing psychosocial parameters, SCFAs help address depression, anxiety, and seizure susceptibility. Dietary strategies aimed at enhancing SCFA production have been proposed as potential therapeutic interventions for neurological disorders (37). Probiotic-derived SCFAs have emerged as important mediators in the microbiota–gut–brain axis, exerting neuroprotective effects by modulating immune and inflammatory pathways. Recent studies suggest that butyrate and propionate can influence neurological health through G-protein-coupled receptor (GPR109A)-dependent anti-inflammatory mechanisms and by promoting M2 macrophage polarization, which contributes to neuroprotection in neurodegenerative disorders (38). Additionally, SCFA-producing probiotic strains such as Lactobacillus, Bifidobacterium, and Clostridium species have demonstrated potential psychobiotic effects, reducing anxiety, depression, and cognitive impairment via modulation of the microbiota–gut–brain axis (39). A recent comprehensive review in Signal Transduction and Targeted Therapy (40) further emphasizes the role of microbial metabolites, including SCFAs, in regulating neuroinflammation and glial cell function, proposing them as actionable targets for neurodegenerative disease management.
5.2 Anticancer properties
Probiotic-derived SCFAs have demonstrated selective anticancer activity, particularly in colorectal cancer (CRC) models. In a comparative study, Lactobacillus fermentum NCIMB 5221 exhibited stronger anti-proliferative effects against CRC cells than Lactobacillus rhamnosus and Lactobacillus acidophilus. This effect was attributed to elevated levels of SCFAs, including butyrate, propionate, and acetate, which induced apoptosis in malignant cells while preserving normal colonocyte viability (41).
5.3 Dermatological applications
Within the skin microbiome, the probiotic Staphylococcus epidermidis ferments glycerol, generating butyric acid. In murine models, topical application of butyric acid or S. epidermidis with glycerol effectively suppressed UVB-induced inflammation by lowering IL-6 expression. Notably, this anti-inflammatory effect was abolished upon blocking the free fatty acid receptor 2 (FFAR2), confirming its role as a mediator of SCFA-driven dermatological benefits (42). SCFAs also contribute to skin health through their role in the gut–skin axis. They have been shown to modulate skin inflammation and may alleviate conditions such as atopic dermatitis, psoriasis, urticaria, and acne. Staphylococcus epidermidis, a commensal skin probiotic, produces SCFAs like butyrate and propionate that inhibit Cutibacterium acnes-induced inflammation by activating GPR41 and suppressing histone deacetylase (HDAC) activity (43, 44). Moreover, recent findings highlight the broader regulatory roles of gut-derived SCFAs on systemic and cutaneous immunity, offering novel therapeutic avenues for inflammatory skin diseases (45). These insights reinforce the potential of probiotic-derived SCFAs in dermatological and cosmetic formulations targeting inflammatory skin conditions.
5.4 Cognitive health benefits
SCFAs also appear to benefit cognitive function. In an Alzheimer’s disease mouse model, acetate derived from Bifidobacterium breve strain A1 attenuated cognitive decline. The observed neuroprotective effects were linked to the modulation of inflammatory and neurochemical pathways associated with memory and learning processes (11). In summary, probiotic-derived SCFAs possess broad-spectrum therapeutic potential, contributing to neurological, oncological, dermatological, and cognitive health through diverse bioactive mechanisms.
6 Bacteriocins from probiotic bacteria as therapeutic agents
Bacteriocins are ribosomally synthesized antimicrobial peptides produced by probiotic and other bacteria, known for their selective bactericidal or bacteriostatic activity against closely related or pathogenic species (46). Produced primarily by food-grade lactic acid bacteria (LAB), bacteriocins have attracted attention for their potential applications in food preservation, human health, and as alternatives to conventional antibiotics amidst rising antimicrobial resistance concerns (47).
6.1 Antimicrobial properties in clinical pathogens
Bacteriocin-producing probiotic strains, particularly those within LAB and Bifidobacterium genera, have demonstrated significant antimicrobial activity against clinically relevant pathogens. For example, Bifidobacterium species isolated from the human gut inhibited Staphylococcus aureus ATCC 25923, Escherichia coli B-6954, Salmonella enterica ATCC 14028, and Clostridium tyrobutyricum LMG, effects attributed to bacteriocin production (48). This pathogen suppression suggests their utility in both gastrointestinal and extraintestinal infections.
6.2 Applications in biofilm-associated infections
Notably, Lactobacillus plantarum produces plantaricin A, a well-characterized bacteriocin exhibiting potent antimicrobial activity against Staphylococcus aureus MTCC 96, particularly strains adhering to extracellular matrix components such as mucin and collagen (49). This activity highlights the potential of plantaricin A in combating biofilm-associated infections, including those occurring on medical implants, where biofilms contribute to treatment resistance and chronicity.
6.3 Therapeutic potential summary
Together, SCFAs and bacteriocins derived from probiotics offer promising avenues for next-generation therapeutics. SCFAs contribute to neurological health, inflammation control, and cancer chemoprevention, while bacteriocins provide effective antimicrobial defense against pathogenic bacteria, including biofilm-forming strains. Their diverse mechanisms of action, high safety profile, and demonstrated efficacy position these postbiotic products as valuable candidates for addressing a range of health challenges in clinical, food safety, and biotechnological contexts (Table 1).
7 Conclusion
Postbiotics derived from probiotics represent an emerging frontier in next-generation therapeutics. By harnessing non-viable microbial components such as exopolysaccharides, cell-free supernatants, short-chain fatty acids, and bacteriocins, they have demonstrated diverse bioactivities including antimicrobial, anti-inflammatory, immunomodulatory, and anticancer effects. Due to their stability and targeted action, postbiotics may help overcome key limitations associated with live probiotics, such as inconsistent colonization and the risk of antimicrobial resistance gene transfer. Unlike probiotics that rely on active colonization, postbiotics act through their metabolites, offering more predictable outcomes. However, their transient effects may require enhancement through formulation technologies. Approaches such as nanoemulsions, hydrogels, liposomes, and polymer-based delivery systems are being explored to improve their stability, controlled release, and bioavailability in the gut. As research progresses, postbiotics are being positioned as a reliable adjunct or alternative to traditional probiotic therapy. Their development aligns with broader global health goals—specifically SDG 3 (Good Health and Well-being) by contributing to alternatives to antibiotics, and SDG 9 (Industry, Innovation, and Infrastructure) through their role in advancing biotechnological innovation. Overall, postbiotics hold promising potential for application in functional foods, pharmaceuticals, and precision medicine, supporting a sustainable and accessible future in healthcare.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
VP: Methodology, Validation, Formal analysis, Writing – original draft, Conceptualization. SR: Investigation, Project administration, Validation, Writing – original draft. BK: Investigation, Formal analysis, Writing – original draft, Data curation. JC: Validation, Investigation, Writing – review & editing, Data curation, Methodology, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dahiya, D, and Nigam, PS. Antibiotic-therapy-induced gut dysbiosis affecting gut microbiota—brain axis and cognition: restoration by intake of probiotics and synbiotics. Int J Mol Sci. (2023) 24:3074. doi: 10.3390/ijms24043074
2. Winter, SE, and Bäumler, AJ. Gut dysbiosis: ecological causes and causative effects on human disease. Proc Natl Acad Sci USA. (2023) 120:e2316579120. doi: 10.1073/pnas.2316579120
3. De Vos, WM, Tilg, H, Van Hul, M, and Cani, PD. Gut microbiome and health: mechanistic insights. Gut. (2022) 71:1020–32. doi: 10.1136/gutjnl-2021-326789
4. Sokol, H, Leducq, V, Aschard, H, Pham, HP, Jegou, S, Landman, C, et al. Fungal microbiota dysbiosis in IBD. Gut. (2017) 66:1039–48. doi: 10.1136/GUTJNL-2015-310746
5. Feizi, H, Rezaee, MA, Ghotaslou, R, Sadrkabir, M, Jadidi-Niaragh, F, Gholizadeh, P, et al. Gut microbiota and colorectal Cancer risk factors. Curr Pharm Biotechnol. (2022) 24:1018–34. doi: 10.2174/1389201023666221005103340
6. Lau, AWY, Tan, LTH, Ab Mutalib, NS, Wong, SH, Letchumanan, V, and Lee, LH. The chemistry of gut microbiome in health and diseases. Prog Microb Mol Biol. (2021) 4:a0000175. doi: 10.36877/pmmb.a0000175
7. Acevedo-Román, A, Pagán-Zayas, N, Velázquez-Rivera, LI, Torres-Ventura, AC, and Godoy-Vitorino, F. Insights into gut dysbiosis: inflammatory diseases, obesity, and restoration approaches. Int J Mol Sci. (2024) 25:9715. doi: 10.3390/ijms25179715
8. Hill, C, Guarner, F, Reid, G, Gibson, GR, Merenstein, DJ, Pot, B, et al. Expert consensus document: the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. (2014) 11:506–14. doi: 10.1038/NRGASTRO.2014.66
9. Szajewska, H, Canani, RB, Guarino, A, Hojsak, I, Indrio, F, Kolacek, S, et al. Probiotics for the prevention of antibiotic-associated diarrhea in children. J Pediatr Gastroenterol Nutr. (2016) 62:495–506. doi: 10.1097/MPG.0000000000001081
10. McFarland, LV. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J Gastroenterol. (2010) 16:2202–22. doi: 10.3748/WJG.V16.I18.2202
11. Kobayashi, Y, Sugahara, H, Shimada, K, Mitsuyama, E, Kuhara, T, Yasuoka, A, et al. Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s disease. Sci Rep. (2017) 7:1–10. doi: 10.1038/s41598-017-13368-2
12. Zmora, N, Zilberman-Schapira, G, Suez, J, Mor, U, Dori-Bachash, M, Bashiardes, S, et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell. (2018) 174:1388–1405.e21. doi: 10.1016/J.CELL.2018.08.041
13. Zheng, M, Zhang, R, Tian, X, Zhou, X, Pan, X, and Wong, A. Assessing the risk of probiotic dietary supplements in the context of antibiotic resistance. Front Microbiol. (2017) 8:264345. doi: 10.3389/FMICB.2017.00908
14. Huddleston, JR. Horizontal gene transfer in the human gastrointestinal tract: potential spread of antibiotic resistance genes. Infect Drug Resist. (2014) 7:167–76. doi: 10.2147/IDR.S48820
15. Tan, R, Jin, M, Li, J, and Yang, D. The dissemination, health risks, and mitigation approaches of antibiotic resistance genes in the gut microbiome. J Hazard Mater Adv. (2025) 17:100634. doi: 10.1016/J.HAZADV.2025.100634
16. Prajapati, N, Patel, J, Singh, S, Yadav, VK, Joshi, C, Patani, A, et al. Postbiotic production: harnessing the power of microbial metabolites for health applications. Front Microbiol. (2023) 14:1306192. doi: 10.3389/fmicb.2023.1306192
17. Aguilar-Toalá, JE, Garcia-Varela, R, Garcia, HS, Mata-Haro, V, González-Córdova, AF, Vallejo-Cordoba, B, et al. Postbiotics: an evolving term within the functional foods field. Trends Food Sci Technol. (2018) 75:105–14. doi: 10.1016/J.TIFS.2018.03.009
18. Tsilingiri, K, and Rescigno, M. Postbiotics: what else? Benef Microbes. (2013) 4:101–7. doi: 10.3920/BM2012.0046
19. Liang, B, and Xing, D. The current and future perspectives of postbiotics. Probiot Antimicrob Proteins. (2023) 15:1626–43. doi: 10.1007/s12602-023-10045-x
20. Wegh, CAM, Geerlings, SY, Knol, J, Roeselers, G, and Belzer, C. Postbiotics and their potential applications in early life nutrition and beyond. Int J Mol Sci. (2019) 20:4673. doi: 10.3390/IJMS20194673
21. Taverniti, V, and Guglielmetti, S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept). Genes Nutr. (2011) 6:261–74. doi: 10.1007/S12263-011-0218-X
22. Birch, J, Van Calsteren, MR, Pérez, S, and Svensson, B. The exopolysaccharide properties and structures database: EPS-DB. Application to bacterial exopolysaccharides. Carbohydr Polym. (2019) 205:565–70. doi: 10.1016/j.carbpol.2018.10.063
23. Dilna, SV, Surya, H, Aswathy, RG, Varsha, KK, Sakthikumar, DN, Pandey, A, et al. Characterization of an exopolysaccharide with potential health-benefit properties from a probiotic Lactobacillus plantarum RJF4. LWT. (2015) 64:1179–86. doi: 10.1016/J.LWT.2015.07.040
24. Shankar, T, Palpperumal, S, Kathiresan, D, Sankaralingam, S, Balachandran, C, Baskar, K, et al. Biomedical and therapeutic potential of exopolysaccharides by Lactobacillus paracasei isolated from sauerkraut: screening and characterization. Saudi J Biol Sci. (2021) 28:2943–50. doi: 10.1016/j.sjbs.2021.02.030
25. Vinothkanna, A, Sathiyanarayanan, G, Rai, AK, Mathivanan, K, Saravanan, K, Sudharsan, K, et al. Exopolysaccharide produced by probiotic Bacillus albus DM-15 isolated from Ayurvedic fermented Dasamoolarishta: characterization, antioxidant, and anticancer activities. Front Microbiol. (2022) 13:832109. doi: 10.3389/FMICB.2022.832109
26. Sheng, S, Fu, Y, Pan, N, Zhang, H, Xiu, L, Liang, Y, et al. Novel exopolysaccharide derived from probiotic Lactobacillus pantheris TCP102 strain with immune-enhancing and anticancer activities. Front Microbiol. (2022) 13:1015270. doi: 10.3389/FMICB.2022.1015270
27. Zaghloul, EH, and Ibrahim, MIA. Production and characterization of exopolysaccharide from newly isolated marine probiotic Lactiplantibacillus plantarum EI6 with in vitro wound healing activity. Front Microbiol. (2022) 13:903363. doi: 10.3389/FMICB.2022.903363
28. Pelyuntha, W, Chaiyasut, C, Kantachote, D, and Sirilun, S. Cell-free supernatants from cultures of lactic acid bacteria isolated from fermented grape as biocontrol against salmonella Typhi and Salmonella Typhimurium virulence via autoinducer-2 and biofilm interference. PeerJ. (2019) 7:e7555. doi: 10.7717/PEERJ.7555
29. Dehghani, N, Tafvizi, F, and Jafari, P. Cell cycle arrest and anti-cancer potential of probiotic Lactobacillus rhamnosus against HT-29 cancer cells. Bioimpacts. (2021) 11:245–52. doi: 10.34172/BI.2021.32
30. Tiptiri-Kourpeti, A, Spyridopoulou, K, Santarmaki, V, Aindelis, G, Tompoulidou, E, Lamprianidou, EE, et al. Lactobacillus casei exerts anti-proliferative effects accompanied by apoptotic cell death and up-regulation of TRAIL in colon carcinoma cells. PLoS One. (2016) 11:e0147960. doi: 10.1371/JOURNAL.PONE.0147960
31. Ruíz, FO, Pascual, L, Giordano, W, and Barberis, L. Bacteriocins and other bioactive substances of probiotic lactobacilli as biological weapons against Neisseria gonorrhoeae. Pathog Dis. (2015) 73:ftv013. doi: 10.1093/FEMSPD/FTV013
32. Drumond, MM, Tapia-Costa, AP, Neumann, E, Nunes, ÁC, Barbosa, JW, Kassuha, DE, et al. Cell-free supernatant of probiotic bacteria exerted antibiofilm and antibacterial activities against Pseudomonas aeruginosa: a novel biotic therapy. Front Pharmacol. (2023) 14:1152588. doi: 10.3389/FPHAR.2023.1152588
33. Fu, JJ, Liu, J, Wen, XP, Zhang, G, Cai, J, Qiao, Z, et al. Unique probiotic properties and bioactive metabolites of Saccharomyces boulardii. Probiot Antimicrob Proteins. (2023) 15:967–82. doi: 10.1007/S12602-022-09953-1
34. Rocchetti, MT, Russo, P, De Simone, N, Capozzi, V, Spano, G, and Fiocco, D. Immunomodulatory activity on human macrophages by cell-free supernatants to explore the probiotic and Postbiotic potential of Lactiplantibacillus plantarum strains of plant origin. Probiot Antimicrob Proteins. (2024) 16:911–26. doi: 10.1007/S12602-023-10084-4
35. Yang, KM, Kim, JS, Kim, HS, Kim, YY, Oh, JK, Jung, HW, et al. Lactobacillus reuteri AN417 cell-free culture supernatant as a novel antibacterial agent targeting oral pathogenic bacteria. Sci Rep. (2021) 11:1–16. doi: 10.1038/S41598-020-80921-X
36. Martin-Gallausiaux, C, Marinelli, L, Blottière, HM, Larraufie, P, and Lapaque, N. Conference on diet and digestive disease symposium 2: sensing and signalling of the gut environment: SCFA: mechanisms and functional importance in the gut. Proc Nutr Soc. (2021) 80:37–49. doi: 10.1017/S0029665120006916
37. Kim, S, Park, S, Choi, TG, and Kim, SS. Role of short chain fatty acids in epilepsy and potential benefits of probiotics and prebiotics: targeting “health” of epileptic patients. Nutrients. (2022) 14:2982. doi: 10.3390/NU14142982
38. Kalyanaraman, B, Cheng, G, and Hardy, M. Gut microbiome, short-chain fatty acids, alpha-synuclein, neuroinflammation, and ROS/RNS: relevance to Parkinson’s disease and therapeutic implications. Redox Biol. (2024) 71:103092. doi: 10.1016/J.REDOX.2024.103092
39. Cheng, Y, Liu, J, and Ling, Z. Short-chain fatty acids-producing probiotics: a novel source of psychobiotics. Crit Rev Food Sci Nutr. (2022) 62:7929–59. doi: 10.1080/10408398.2021.1920884
40. Loh, JS, Mak, WQ, Tan, LKS, Ng, CX, Chan, HH, Yeow, SH, et al. Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct Target Ther. (2024) 9:1–53. doi: 10.1038/s41392-024-01743-1
41. Kahouli, I, Malhotra, M, Alaoui-Jamali, M, and Prakash, S. In-vitro characterization of the anti-cancer activity of the probiotic bacterium Lactobacillus fermentum NCIMB 5221 and potential against colorectal cancer. J Cancer Sci Ther. (2015) 7:7. doi: 10.4172/1948-5956.1000354
42. Keshari, S, Balasubramaniam, A, Myagmardoloonjin, B, Herr, DR, Negari, IP, and Huang, CM. Butyric acid from probiotic staphylococcus epidermidis in the skin microbiome down-regulates the ultraviolet-induced pro-inflammatory IL-6 cytokine via short-chain fatty acid receptor. Int J Mol Sci. (2019) 20:4477. doi: 10.3390/IJMS20184477
43. Xiao, X, Hu, X, Yao, J, Cao, W, Zou, Z, Wang, L, et al. The role of short-chain fatty acids in inflammatory skin diseases. Front Microbiol. (2023) 13:1083432. doi: 10.3389/FMICB.2022.1083432
44. Tegegne, BA, and Kebede, B. Probiotics, their prophylactic and therapeutic applications in human health development: a review of the literature. Heliyon. (2022) 8:e09725. doi: 10.1016/J.HELIYON.2022.E09725
45. Gao, T, Wang, X, Li, Y, and Ren, F. The role of probiotics in skin health and related gut–skin axis: a review. Nutrients. (2023) 15:3123. doi: 10.3390/NU15143123
46. Ozma, MA, Abbasi, A, Akrami, S, Lahouty, M, Shahbazi, N, Ganbarov, K, et al. Postbiotics as the key mediators of the gut microbiota-host interactions. Inf Med. (2022) 30:180–93. doi: 10.53854/LIIM-3002-3
47. Sugrue, I, Ross, RP, and Hill, C. Bacteriocin diversity, function, discovery and application as antimicrobials. Nat Rev Microbiol. (2024) 22:556–71. doi: 10.1038/S41579-024-01045-X
48. Prosekov, AY, Dyshlyuk, LS, Milentyeva, IS, Sykhikh, SA, Babich, OO, Ivanova, SA, et al. Antioxidant and antimicrobial activity of bacteriocin-producing strains of lactic acid bacteria isolated from the human gastrointestinal tract. Prog Nutr. (2017) 19:67–80. doi: 10.23751/PN.V19I1.5147
49. Mukherjee, S, and Ramesh, A. Bacteriocin-producing strains of Lactobacillus plantarum inhibit adhesion of Staphylococcus aureus to extracellular matrix: quantitative insight and implications in antibacterial therapy. J Med Microbiol. (2015) 64:1514–26. doi: 10.1099/JMM.0.000181
50. Zhang, Q, Wang, J, Sun, Q, Zhang, SM, Sun, XY, Li, CY, et al. Characterization and antioxidant activity of released exopolysaccharide from potential probiotic Leuconostoc mesenteroides LM187. J Microbiol Biotechnol. (2021) 31:1144–53. doi: 10.4014/JMB.2103.03055
51. Qian, X, Si, Q, Lin, G, Zhu, M, Lu, J, Zhang, H, et al. Bifidobacterium adolescentis is effective in relieving type 2 diabetes and may be related to its dominant core genome and gut microbiota modulation capacity. Nutrients. (2022) 14:2479. doi: 10.3390/NU14122479/S1
52. Hao, R, Liu, Q, Wang, L, Jian, W, Cheng, Y, Zhang, Q, et al. Anti-inflammatory effect of Lactiplantibacillus plantarum T1 cell-free supernatants through suppression of oxidative stress and NF-κB- and MAPK-signaling pathways. Appl Environ Microbiol. (2023) 89:e00608-23. doi: 10.1128/AEM.00608-23
Keywords: gut microbiome, postbiotics, dysbiosis, immunomodulation, next-generation therapeutics
Citation: Pattapulavar V, Ramanujam S, Kini B and Christopher JG (2025) Probiotic-derived postbiotics: a perspective on next-generation therapeutics. Front. Nutr. 12:1624539. doi: 10.3389/fnut.2025.1624539
Edited by:
Andrey Santos, State University of Campinas, BrazilReviewed by:
Sónia Norberto, University of Campinas (UNICAMP), BrazilMaftei Nicoleta, Dunarea de Jos University, Romania
Copyright © 2025 Pattapulavar, Ramanujam, Kini and Christopher. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John Godwin Christopher, Z29kd2luakB2aXQuYWMuaW4=
 Veilumuthu Pattapulavar
Veilumuthu Pattapulavar Sathiyabama Ramanujam
Sathiyabama Ramanujam Bhagyashree Kini
Bhagyashree Kini John Godwin Christopher
John Godwin Christopher