- 1University of Lausanne, Lausanne, Switzerland
- 2Nestlé Institute of Health Sciences, Nestlé Research, Lausanne, Switzerland
- 3Department of Epidemiology and Health Systems, Unisanté, University Center for Primary Care and Public Health, Lausanne, Switzerland
Background: Plant-based diets with reduced animal protein intake are increasingly recommended for health and sustainability reasons that have potential implications for nutrient intake, including protein quality.
Objective: To develop a non-linear optimization model to determine the optimal ratio needed from plant and animal protein foods to obtain a high protein and nutrient quality in primarily plant-based meals.
Methods: Sixty-two protein foods were grouped by their limiting amino acid: “lysine-limiting” foods were mainly “grains, nuts and seeds,” “sulfur amino acids” mainly consisted of “beans, peas and lentils” and “non-limiting” included “soy-foods” for vegan and vegetarian meals and/or “animal protein foods” for pesco/semi-vegetarian meals.” A non-linear optimization approach was used to maximize protein quality using the Protein Digestibility Corrected Amino Acid Score (PDCAAS 1) while considering essential nutrients such as energy, protein, dietary fiber, iron, calcium, and zinc in three plant-based meal models. The three models considered all contained protein foods from the first two groups, “grains, nuts and seeds” and “beans, peas, lentils, and others” and some variations of the third group being either “soy-foods only” (vegan), “soy-foods, dairy and egg” (vegetarian), or “soy-foods and/or animal-based foods” (pesco/semi-vegetarian).
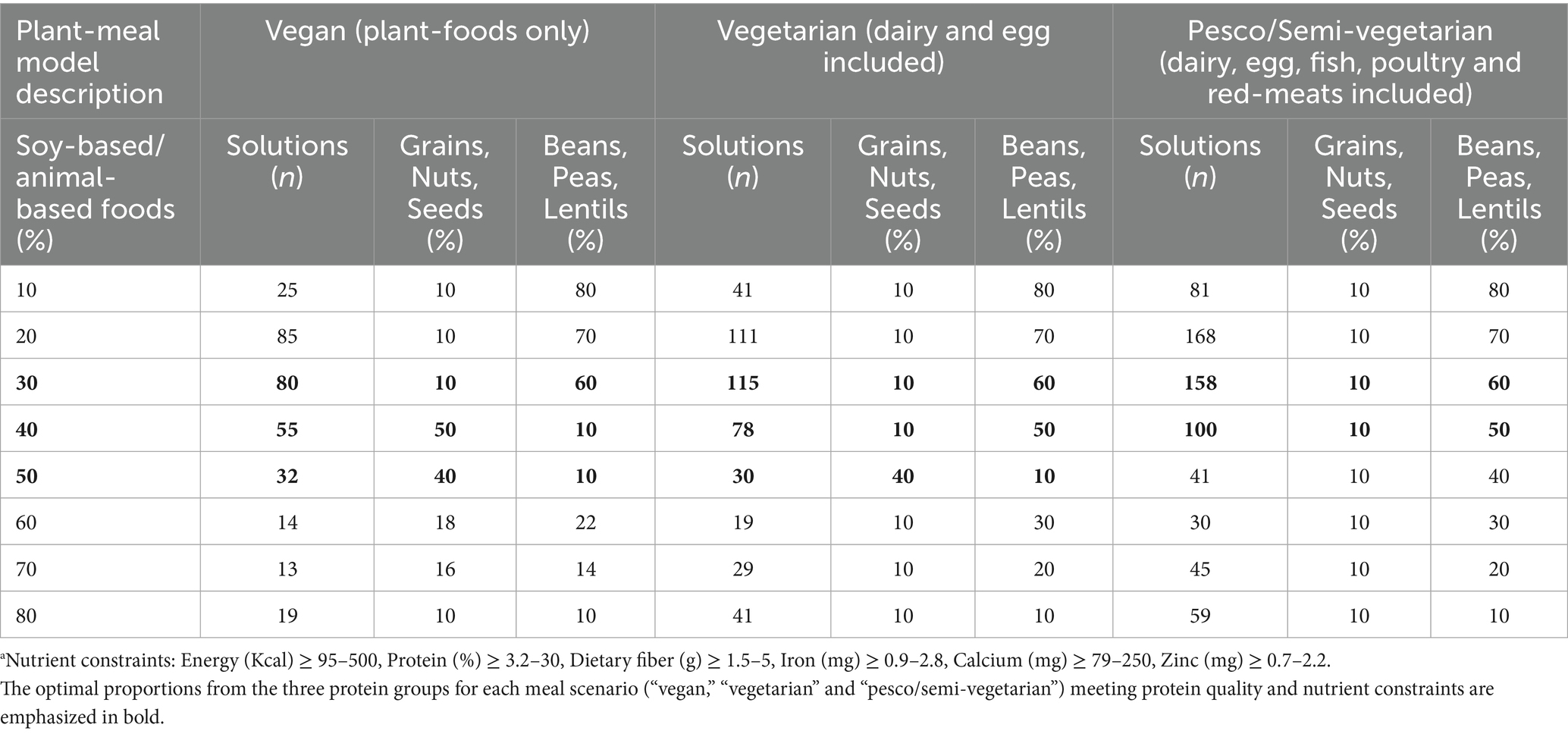
Results: To achieve optimal protein quality, calcium, iron, and zinc levels in a vegan and vegetarian meal, the optimal protein ratio based on total protein intake was at least 10% grains, nuts, and seeds; 10–60% beans, peas, and lentils; and 30–50% soy-based foods only and/or dairy and eggs. The optimal pesco/semi-vegetarian meal had at least 10% grains, nuts, and seeds, 50–60% beans, peas, and lentils, and 30–40% from soy-foods and/or animal-based foods. The vegan meal had more variety than models including animal protein foods.
Conclusion: The optimal ratios of protein foods determined could be used to define easy-to-follow guidance for selecting protein foods that deliver high protein quality while also contributing to nutrient quality in primarily plant-based meals.
1 Introduction
Plant-based meals and diets with reduced intake from animal-based foods, especially in high-income countries, are increasingly recommended for health and sustainability reasons and may have potential implications for overall diet quality and nutrient intakes, including protein quality (1, 2). Foods contain proteins that consist of two main types of amino acids: (i) dispensable amino acids, which the human body can synthesize on its own, and (ii) indispensable amino acids (IAA), which the human body cannot synthesize and must obtain from the diet (3). Apart from consuming sufficient protein, the quality of protein consumed in the human diet is also important as it considers how well protein is digested and absorbed for use by the body. Protein quality consists of amino acid composition, protein content, individual amino acid digestibility, and the requirement of IAA in the target population (3). The protein digestibility amino acid score (PDCAAS) is the most widely used protein quality metric and is based on the first limiting amino acid relative to a reference pattern (3, 4). High-quality protein foods such as meat, poultry, fish, eggs, certain soy-based foods, and dairy foods (milk, cheese, and yogurt) provide all the 9 IAA and are also carriers of several key nutrients such as iron, zinc, calcium, vitamin B12, and phosphorus (3, 4). Although plant protein foods such as legumes, grains, nuts, seeds, and vegetables can be deficient in one or more IAA, these foods contribute highly to dietary fiber, vitamin E, and magnesium intakes (5). Therefore, for individuals mainly relying on plant protein foods, high-quality protein, and other nutrient intakes can only be achieved by specific combinations of plant and animal protein foods, allowing them to compensate for the various limiting amino acids and key nutrients (6). For example, a study that simulated flexitarian dietary patterns, which involved reducing animal protein intake from 75 to 25%, revealed higher Healthy Eating Index-2015 scores with reduced animal protein intake as compared to an omnivorous diet that consisted of over 75% of animal protein (7).
Existing dietary recommendations from several Western and Asian populations emphasize consuming more plant proteins (50–75%) without specifying proportions from the different types of plant-protein foods (8–11). Our previous work has shown that individuals following a semi-vegetarian or flexitarian diet reducing animal protein foods tended to consume mainly plant protein foods such as grains, nuts, and seeds and least from beans, peas, lentils, and soy-based foods (12). Diet modeling analyses of national dietary surveys in the United States and Canada found that replacing amino acids from grain protein with those from legumes, lentils, or non-grain plant proteins increased overall protein quality as well as levels of fiber, folate, iron, and zinc in the diet (13, 14). Mainly relying on cereal-based foods as a plant protein food instead of incorporating a variety from other high-quality plant-protein foods such as beans, peas, lentils, and soy-based foods could affect protein quality and other key nutrient intakes (12).
Currently, most dietary guidelines are clear on including a variety of protein foods in meals and diets for optimal protein and overall diet quality (8–11). However, it remains uncertain which types of plant protein foods should be consumed and in which proportions. Consequently, it becomes important to define easy-to-follow dietary guidance for the general population aiming to reduce animal-based foods by selecting foods delivering high protein quality while contributing to nutrient quality to help reach a balanced primarily plant-based meal. The main aim of this study is to develop a non-linear optimization method to determine optimal ratios of plant and animal protein foods to obtain a high protein and nutrient quality in primarily plant-based meals (vegan, vegetarian, and pesco, or semi-vegetarian).
2 Methods
2.1 Data origin
All cooked and ready-to-eat plant protein foods (N = 51) and animal protein foods (N = 11) had their IAA, macronutrients, and micronutrients information extracted from the US Department of Agriculture (USDA) national nutrient database for standard reference release 28, considering that the preparation method could influence nutrient availability and also to reflect the typical consumption of foods; for example, almonds are usually consumed raw, while rice is cooked (15) (Table 1; Supplementary Table 1). The protein digestibility values, and processing method of each food item were obtained from various sources of the literature as indicated in Table 1 and Supplementary Table 1. There was an exception with three ingredients (soy milk, seitan, and carrots), where the total protein content, IAA information, and protein digestibility values were obtained from the literature alone (16, 17). The selection process for the 62 foods has been described in Supplementary Figure 1.
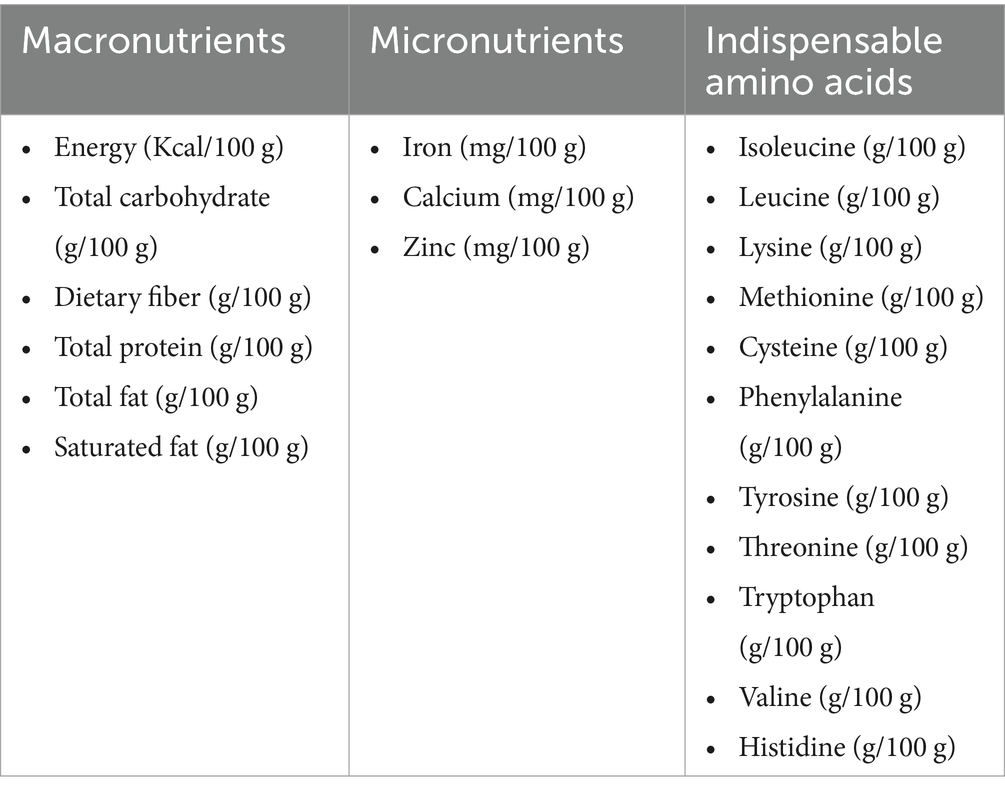
Table 1. Information extracted on protein foods (N = 62) from the USDA national database standard reference release 28 (15).
As shown in Table 2, the limiting amino acid was identified as the lowest amount of IAA present in the protein food. The amino acid score assesses how effectively the absorbed dietary nitrogen can fulfill the IAA requirements at a safe level of protein consumption and was calculated by comparing the limiting amino acid content (mg per g) in each protein food with the amino acid requirement pattern (mg per g) (18). The protein digestibility of protein foods was measured by how efficiently a protein food is digested and absorbed in the body while also considering protein that would be lost through the feces. The protein digestibility values of the included plant and animal protein foods in this study were extracted from the literature (Table 2), prioritizing human ileal digestibility where available, followed by digestibility from studies in pigs and rats, and finally values from in vitro models if needed (4). The PDCAAS value for each protein food was calculated by multiplying the amino acid score with the protein digestibility value.
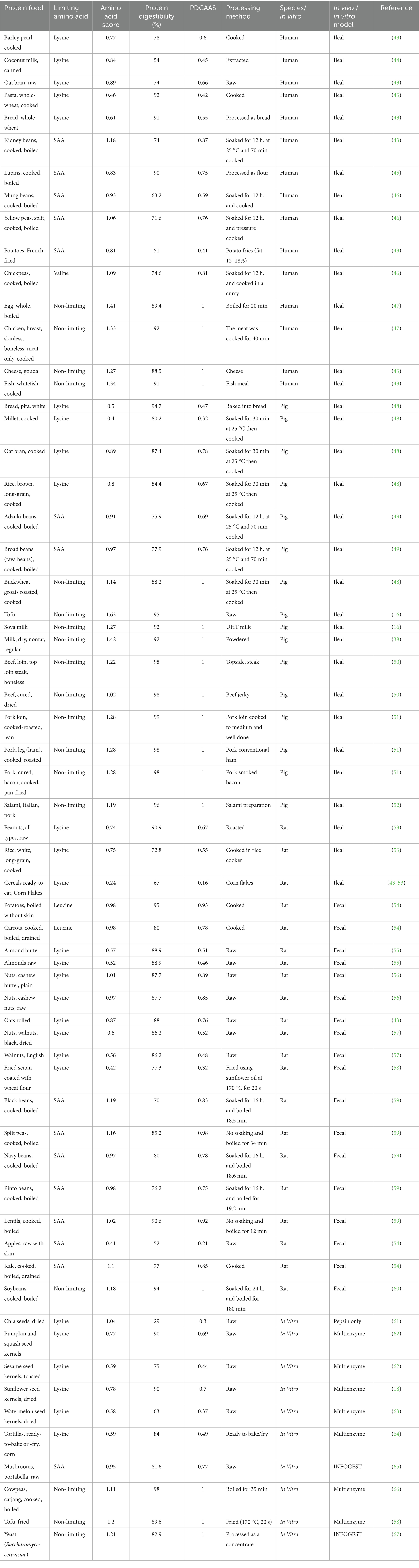
Table 2. Plant protein foods with amino acid score, protein digestibility %, food processing method used, species protein digestibility tested in vivo or in vitro method and calculated PDCAAS.
2.2 Protein foods categorization approach
In step 1 of Figure 1, the fifty-one plant protein foods and eleven animal protein foods were grouped by protein food groups according to their limiting amino acid profile. Based on the protein foods and their corresponding limiting amino acid as shown in Table 2. The protein food groups were defined as “lysine-limiting,” “sulfur amino acids (SAA)-limiting” and “non-limiting” which mainly consisted of “grains, nuts and seeds,” “beans, peas, lentils and others” and “soy-based foods only and/or animal foods and others,” respectively (Table 3). A few protein foods fell into groups that would differ from the corresponding food group. Examples include peanut and coconut (botanically classified as legumes and fruit, respectively), which are categorized as “lysine limiting.” Similarly, apples, carrots, kale, and potatoes (i.e., classified as fruit and vegetables) were grouped under “SAA-limiting,” whereas buckwheat, cowpeas, and yeast (Saccharomyces cerevisiae) had “non-limiting” amino acid profiles, thus grouped as a “non-limiting” protein food. Foods such as boiled potatoes and carrots were “leucine-limiting” and chickpeas “valine-limiting”; they grouped under “SAA-limiting” as the methionine and cysteine levels were reported to be the second lowest amino acids present, with carrots reflecting levels below the reference intake for methionine and cysteine in adults.
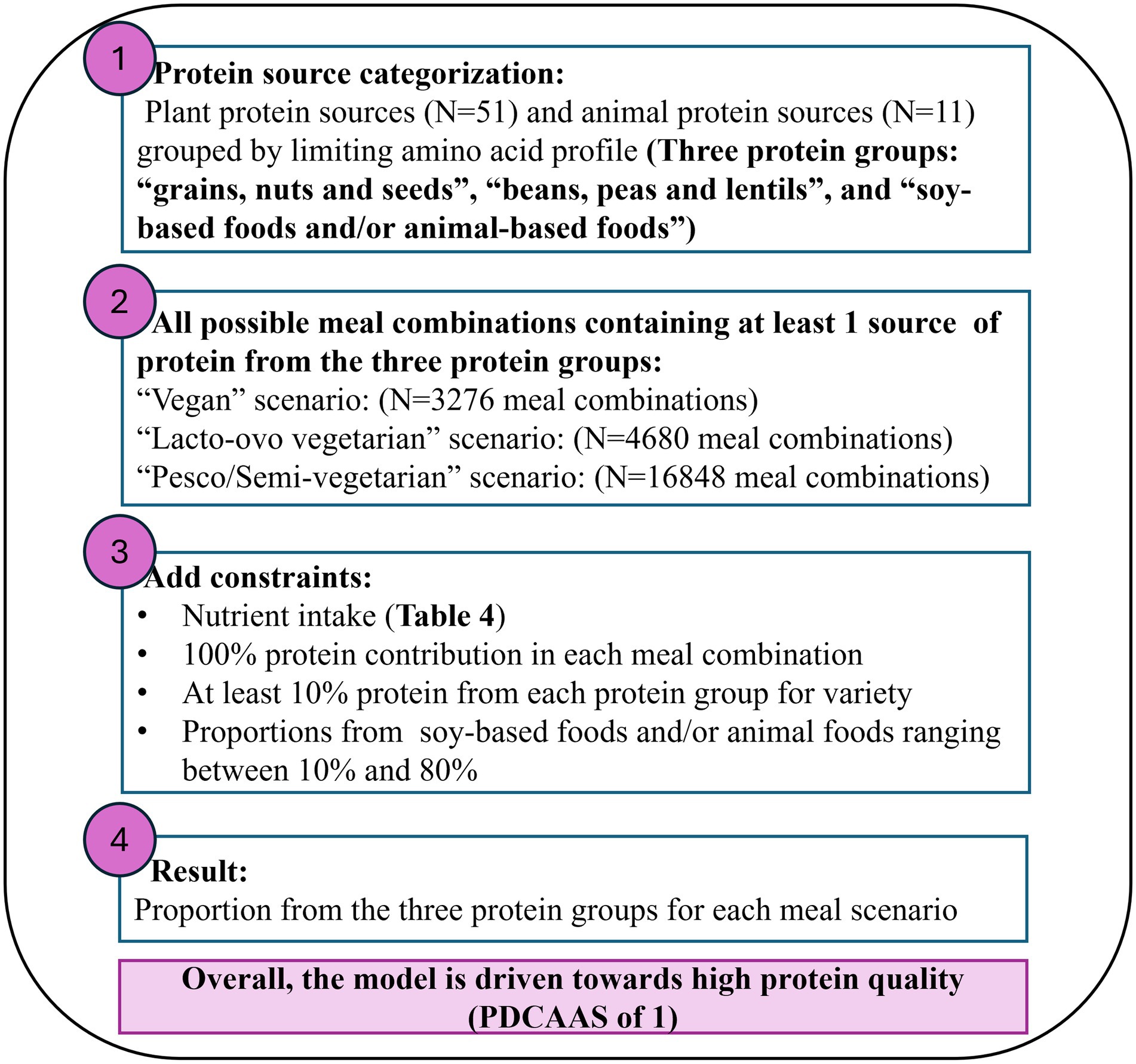
Figure 1. Overview of the non-linear model process to obtain the optimal protein ratios from the three protein food groups based on limiting amino acid profile.
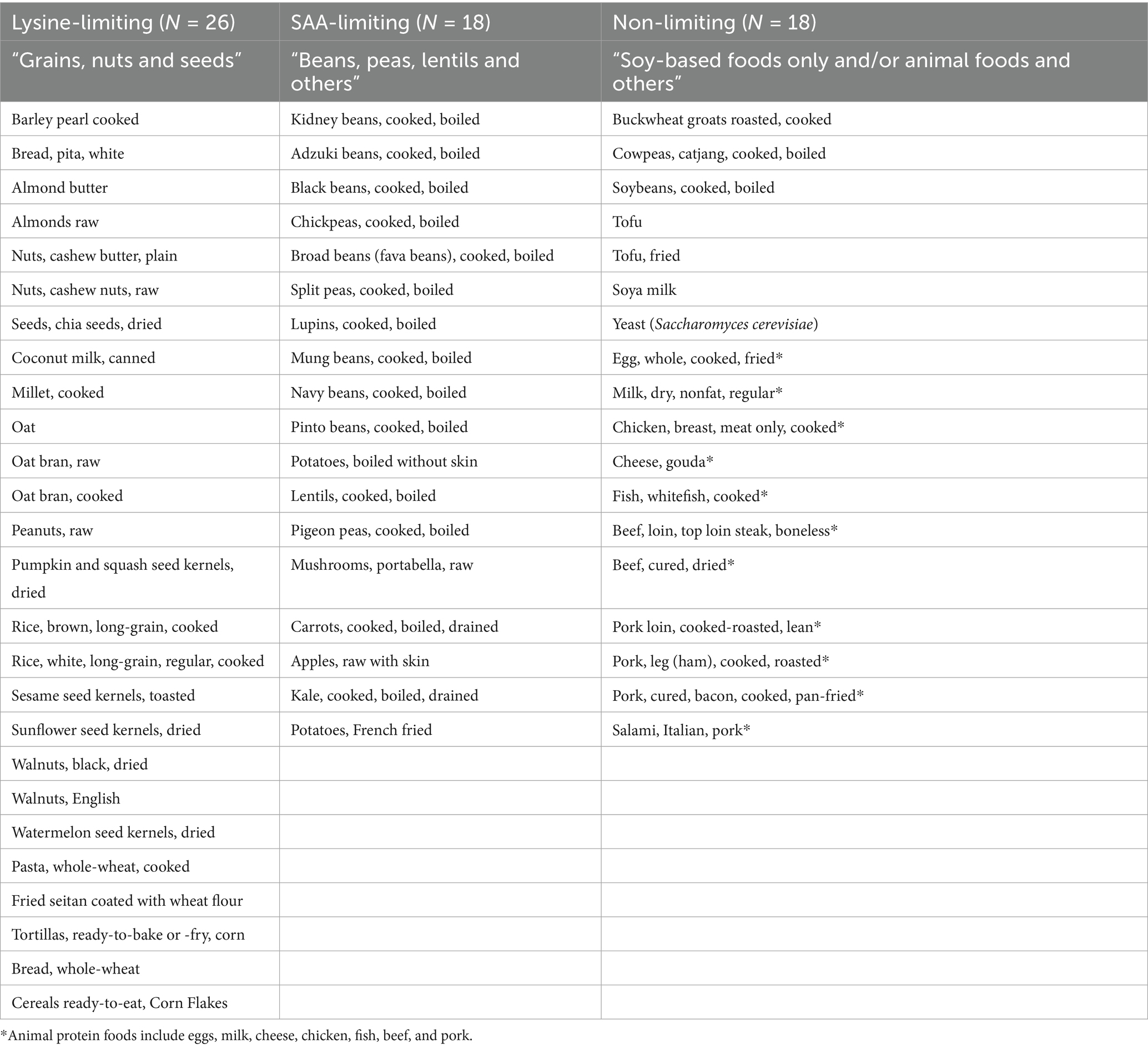
Table 3. Plant protein foods are grouped according to their limiting amino acid profile based on Table 2.
2.3 Non-linear optimization programming approach
The non-linear optimization approach is a mathematical methodology used to find the best solution from a set of possible solutions where the objective function or the defined constraints are non-linear (19). Although the Digestible Indispensable Amino Acid Score (DIAAS) is a more accurate measurement of protein quality, the data available for a variety of foods remains limited. Thus, in this study. PDCAAS was considered as the most appropriate measurement for protein quality. The key aspects covered in a non-linear optimization model include an objective function, which in this study aims to maximize the PDCAAS to enhance protein quality in a meal. The decision variables in this model represent the proportions of different foods from the respective food groups included in the meal. Additionally, the model includes constraints that define a feasible region within which the optimal solution for protein quality and nutrient content in a meal can be identified. In this study, these constraints relate to the determined nutrient contributions of the meal. As shown in Figure 1, a non-linear optimization model using R package NlcOptim was built to maximize the objective function [PDCAAS = (amino acid score × protein digestibility %)] between all meal combinations of the three protein food groups (Table 3). In step 2 of Figure 1, all meal combinations were determined in three primarily plant-based meal scenarios across the three protein food groups (Table 3). The first meal category was defined as a typical “vegan” meal using foods from (i) “grains, nuts and seeds,” (ii) “beans, peas, lentils and others” and (iii) “soy-based foods and others” creating 3,276 unique meal combinations. The second meal category was defined as a typical “vegetarian” meal using foods from (i) “grains, nuts, and seeds,” (ii) “beans, peas, lentils, and others” and (iii) “soy-based foods and inclusion of dairy and/or eggs” creating 4,680 unique meal combinations. Finally, the last meal category was defined as a typical “pesco/semi-vegetarian” meal using foods from combinations of (i) “grains, nuts, and seeds,” (ii) “beans, peas, lentils, and others” and (iii) “soy-based foods and inclusion of dairy, eggs, fish, poultry and red meats” creating 16,848 unique meal combinations. The detailed calculations to obtain a PDCAAS for each meal combination from the three protein food groups are shown in Supplementary Table 2.
2.4 Constraints added to non-linear optimization model
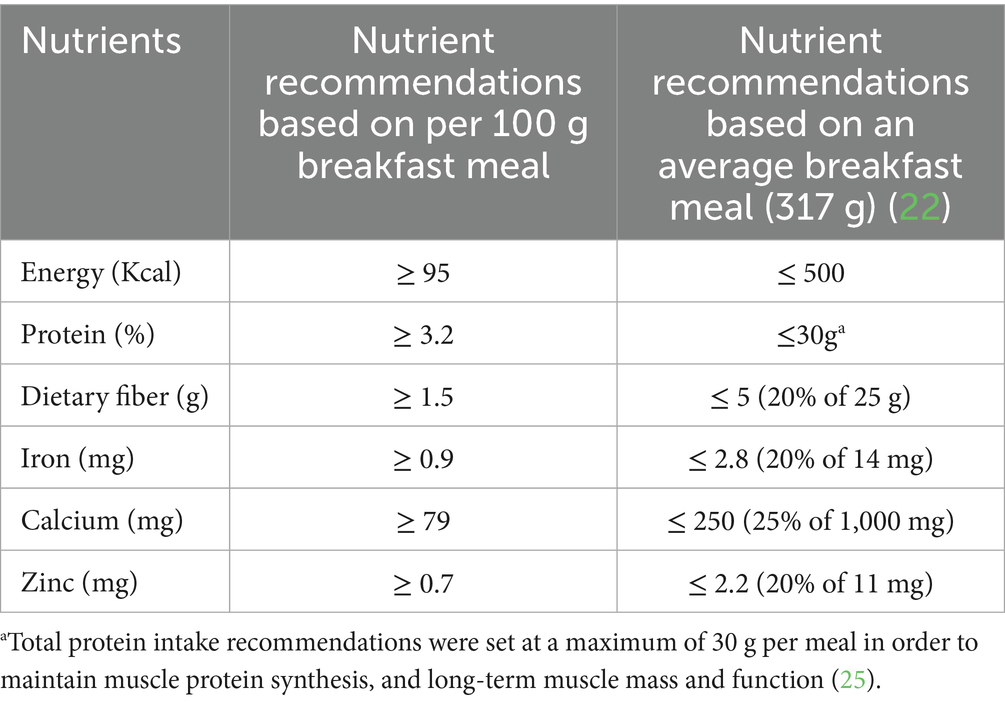
In step 3 of Figure 1, the model was restricted to providing solutions that resulted in a total contribution of 100% protein intake from all protein food groups for each meal combination (Table 3). A constraint of at least 10% was determined to come from the “grains, nuts and seeds” and “beans, peas, lentils, and others” groups to allow for a variety of consumption from all protein food groups (1, 20). Finally, constraints ranging from 10 to 80% were determined on the “soy-based foods and others” or “soy, animal-based foods, and others” protein food group to prevent the model from favoring a solution that consisted entirely of soy or animal protein foods as all foods within these groups had a PDCAAS of 1. The nutrient constraints were selected based on critical nutrients found to be low in plant-based diets (21). Vitamin B12 and vitamin D are essential nutrients for plant-based meals and diets. However, they were not considered due to plant protein foods having no vitamin B12 present, and there was a lack of data available for vitamin D in selected foods.
Dietary intake values were determined using the International Breakfast Initiative nutrient proposal for a breakfast meal in Western Europe and North America to determine an ideal contribution of nutrients from plant protein foods in a plant-based meal (22). Breakfast was used as an example meal for several reasons, including the availability of nutrient guidance for this meal and the daily energy intake recommendations being generally lower for breakfast, allocating 20–25% of total intake thus allowing for adaptation based on individual snacking habits (23). The average-sized breakfast of 317 g was determined by assessing the 20 breakfast recipes listed under the International Breakfast Initiative (24). As shown in Table 4, the range for each nutrient constraint was determined based on nutrient recommendations per 100 g breakfast to an average-sized breakfast of 317 g. As protein intake is a driving factor for optimal protein quality, the total protein intake recommendations were set at a maximum of 30 g per meal to maintain muscle protein synthesis and long-term muscle mass and function (25).
Plant-based foods naturally contain high levels of antinutrients such as phytates which impede the bioavailability of key nutrients such as iron and zinc as well as protein digestibility (26). Therefore, the phytate for each protein food was sourced from the FAO/INFOODS/IZiNCG Global food composition database (27). The phytate-to-iron and phytate-to-zinc molar ratio were calculated for each plant-based meal combination to assess the potential bioavailability of iron and zinc (28).
Higher ratios indicate a greater presence of more phytate to mineral intake. The WHO and FAO guidance on phytate ratios states if there are no iron absorption enhancers like vitamin C consumed in a meal, the phytate-to-iron molar ratio should be aimed to be < 1:1 (low impact on iron absorption) or phytate-to-zinc molar ratio < 5 to 15 (low to moderate impact on zinc absorption) (29). Furthermore, FAO/IZiNCG states a molar ratio >18 would adversely affect zinc absorption (27).
The optimal solution would be defined as high protein quality (PDCAAS 1) and meet the defined target nutrient quality. In step 4 of Figure 1, the non-linear optimization model aimed to maximize the PDCAAS for every combination of a plant-based meal, thereby producing solutions that represented the percentage contribution of total protein intake from each protein group and considered the quality of selected macro and micronutrients. The algorithm did not converge for some combinations of a plant-based meal, thus making these meal combinations “non-applicable.” From all the potential optimal solutions for each meal combination, we selected the most frequent output from the three protein groups in combination with most mixes having a PDCAAS of 1. The distribution of PDCAAS, nutrient intake, and phytate to iron and zinc ratios across all protein ratio solutions comparing vegan, vegetarian, and pesco/semi-vegetarian meals were presented as boxplots. The boxplots display the minimum, interquartile range, median, and maximum for each plant protein food, and any outliers were represented as black dots. The R script used is available here: Mrolands123/GitHub.
3 Results
As presented in Figure 2, the protein quality (PDCAAS) distribution to achieve optimal protein quality (PDCAAS 1) indicates that most meal combinations must have contributions of at least 30 to 50 and 80% from soy-based foods for vegan meals. The results were similar when including dairy and/or eggs for vegetarian meals, and animal protein foods for pesco/semi-vegetarian meals.
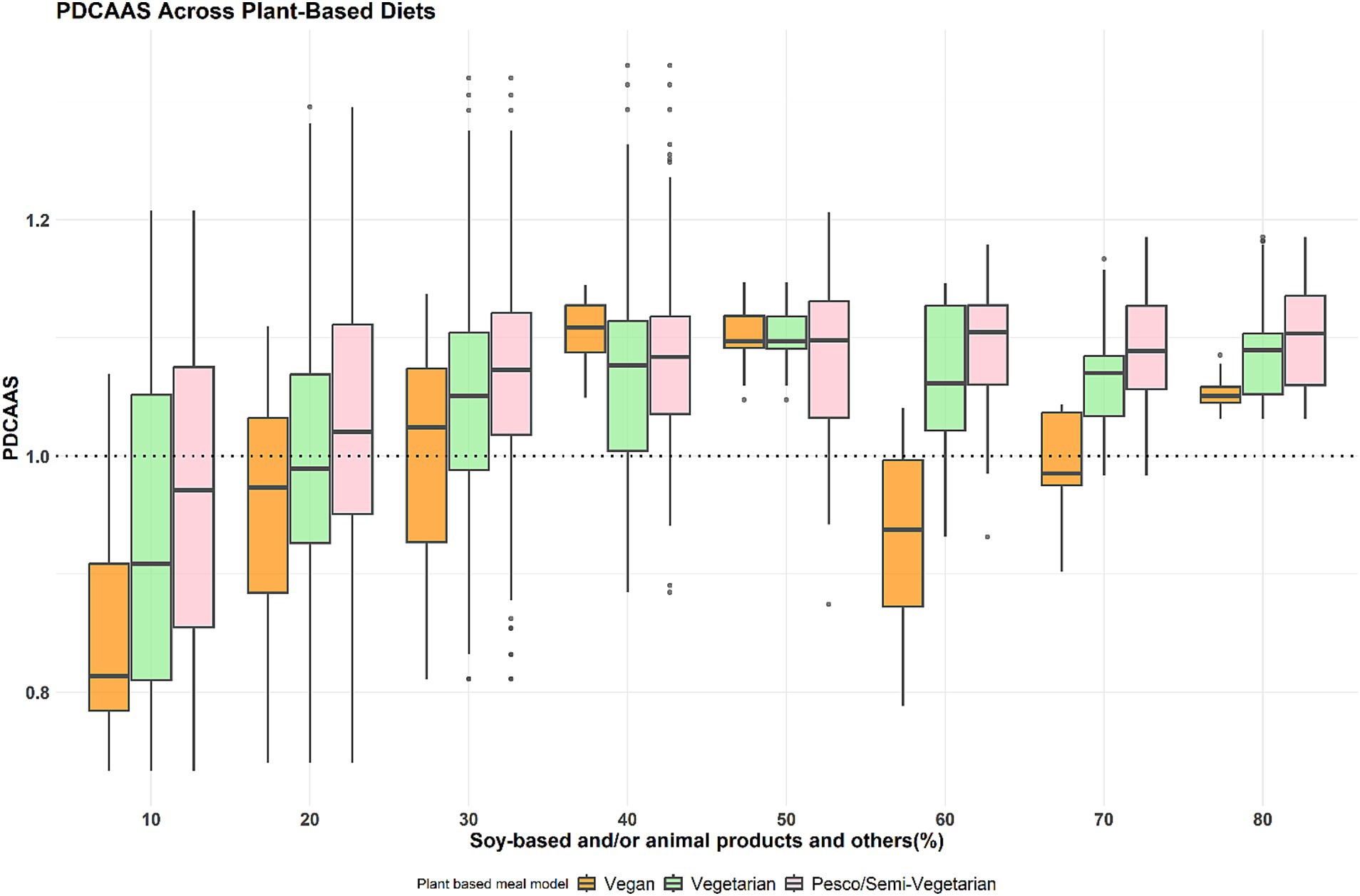
Figure 2. PDCAAS distribution across the three plant-based meal models at different protein food contributions. These boxplots display the minimum, interquartile range, median, and maximum PDCAAS for the percentage contribution of soy-based only and/or animal products to total protein intake across three primarily plant-based meals: vegan, vegetarian, and pesco/semi-vegetarian. The left whisker indicates the minimum contribution, while the upper and lower whiskers represent the lower and upper 25% of intake values, respectively. The box shows the interquartile range, encompassing the middle 50% of intakes (25th to 75th percentile), with the median marked by a line within the box. The right whisker denotes the maximum contribution, and any outliers are displayed as black dots outside the whiskers. The dotted line represents PDCAAS 1 (optimal protein quality). The green box highlights the protein ratios with a median PDCAAS 1 across all three primarily plant-based meals.
At this optimal ratio for protein quality, the macronutrient distribution indicated that, on average, per 100 g, the energy content ranged from 158 to 195 kcals. The percentage of energy from carbohydrates varied between 26 and 41%, while dietary fiber content ranged from 2.8 to 3.7 g. The proportion of energy derived from protein was between 21 and 24%, and total fat contributed 43 to 57%, with saturated fat accounting for 7 to 8% of energy (Supplementary Figure 2). With 80% of protein foods from soy-based foods only and/or animal protein, the macronutrient distribution across the different plant-based diets showed that, on average, per 100 g, the energy content ranged from 100 to 185 kcals. The percentage of energy from carbohydrates varied between 15.5 and 30.9%, while dietary fiber content ranged from 4.1 to 4.2 g. Protein contributed 27.4 to 32% of energy, and total fat accounted for 40 to 56% of energy. Notably, saturated fat at 80% soy-based foods and/or animal protein contributed 4.9 to 13.7% of energy.
At optimal ratios for protein quality (PDCAAS 1), with 30 to 50% protein from soy-based foods only and/or animal protein, the micronutrient distribution in the vegan and vegetarian models showed that, on average, per 100 g, the iron content ranged from 1.85 to 2.71 mg, calcium content ranged from 90.7 to 191.5 mg, and zinc content ranged from 1.03 to 1.3 mg (Figure 3). Whereas at 50% soy and/or animal protein foods contribution, the pesco/semi-vegetarian meal model had the lowest contribution from iron (1.48 mg), calcium (88.5 mg), and zinc (1.19 mg). Therefore, regarding iron and calcium distribution, the optimal soy and/or animal protein contribution in a pesco/semi-vegetarian meal would be 30 to 40%. The distribution of iron, calcium, and zinc intake across the plant-based meals was lowest at 80% contribution from soy and/or animal protein. In addition to iron, zinc and calcium, vitamin B12 was also observed to increase when animal protein foods are included as a solution in either a vegetarian or pesco/semi-vegetarian meal.
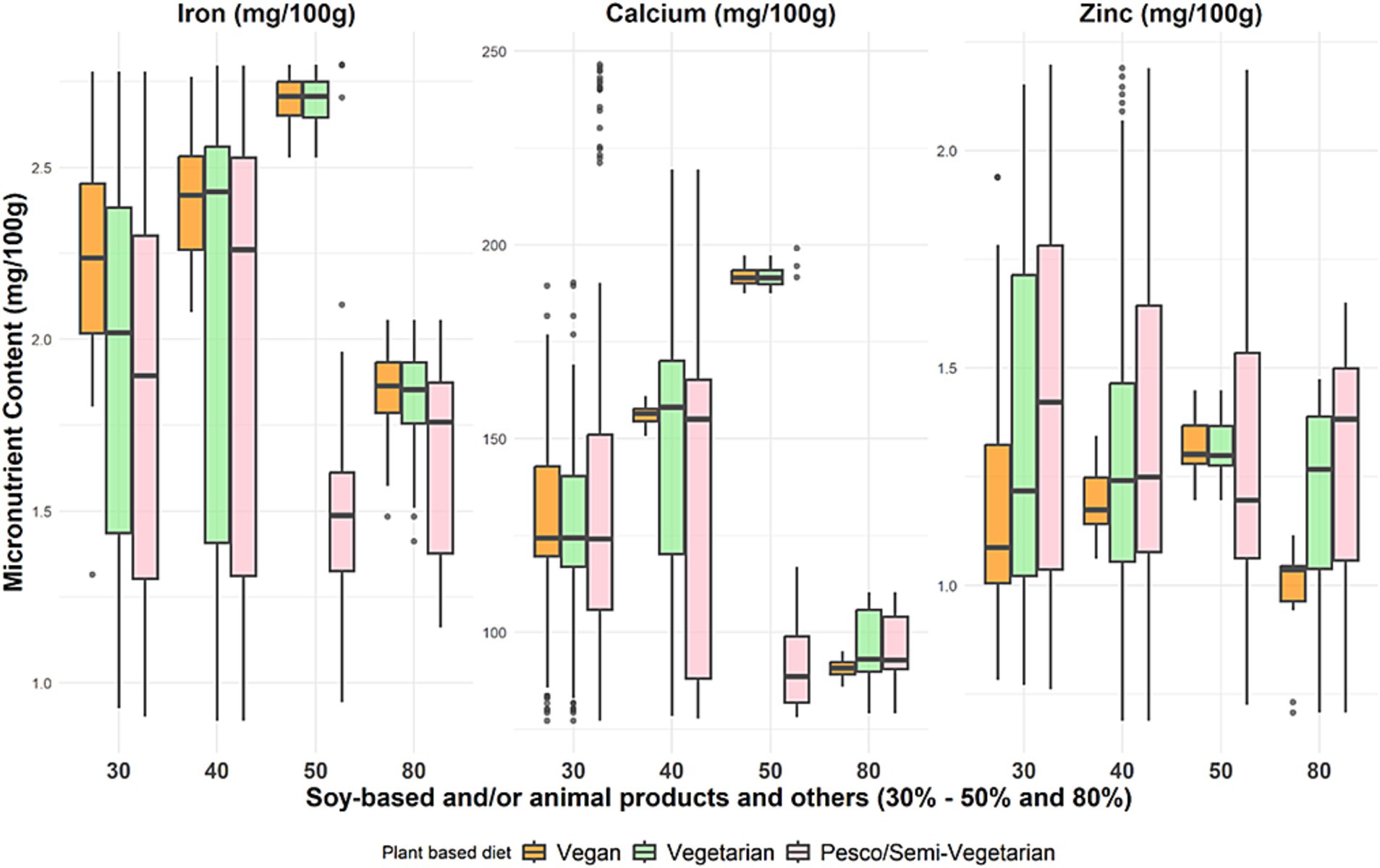
Figure 3. Distribution of selected micronutrient content across the three plant-based meal models at different protein food contributions. These boxplots display the minimum, interquartile range, median, and maximum iron, zinc, and calcium levels for the percentage contribution of soy-based only and/or animal products to total protein intake across three primarily plant-based meals: vegan, vegetarian, and pesco/semi-vegetarian. The left whisker indicates the minimum contribution, while the upper and lower whiskers represent the lower and upper 25% of intake values, respectively. The box shows the interquartile range, encompassing the middle 50% of intakes (25th to 75th percentile), with the median marked by a line within the box. The right whisker denotes the maximum contribution, and any outliers are displayed as black dots outside the whiskers.
The highest phytate-to-iron and phytate-to-zinc ratios resulted from the vegan meal model, with a 40% contribution from soy-based foods, indicating higher levels of phytate present in comparison to iron and zinc (Figure 4). Notably, all three plant-based meal models had a consistently lower phytate-to-iron and phytate-to-zinc ratio when there was a 30% contribution from soy-based foods alone or the inclusion of animal protein foods. However, none of the plant-based meals met the phytate-to-iron molar ratio threshold (<1:1) for optimal iron absorption. The phytate-to-zinc molar ratio across all plant-based meals was below the threshold for adverse zinc absorption (1, 18), except for the vegan meals with 40 and 80% soy food contribution.
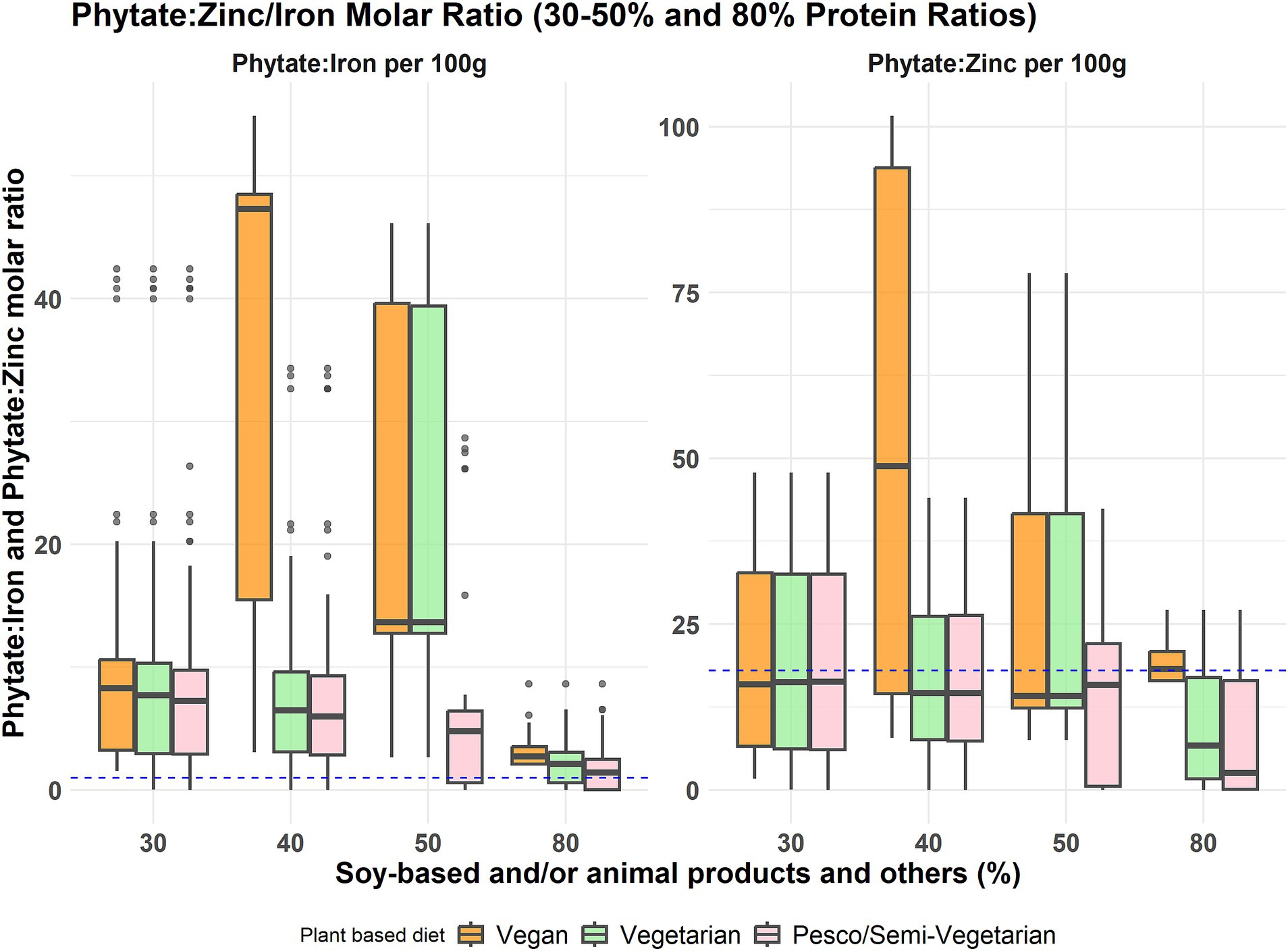
Figure 4. Phytate to iron and phytate to zinc molar ratio distribution across the three plant-based meal models at different protein food contributions. These boxplots display the minimum, interquartile range, median, and maximum phytate to iron molar ratio and phytate to zinc molar ratio levels for the percentage contribution of soy-based and/or animal products to total protein intake across three primarily plant-based meals: vegan, vegetarian, and pesco/semi-vegetarian. The left whisker indicates the minimum contribution, while the upper and lower whiskers represent the lower and upper 25% of intake values, respectively. The box shows the interquartile range, encompassing the middle 50% of intakes (25th to 75th percentile), with the median marked by a line within the box. The right whisker denotes the maximum contribution, and any outliers are displayed as black dots outside the whiskers. The blue dashed line indicates the threshold for phytate-to-iron (<1:1) and phytate-to-zinc ratio (<18:1).
Results from the non-optimization model showed that when a meal was modeled to be vegan or vegetarian, the optimal ratio guidance from protein foods could be at least 10% coming from grains, nuts, and seeds, 10 to 60% from beans, peas, and lentils, and 30 to 50% from soy-based foods only and/or dairy and eggs. These protein ratios met all nutrient constraints while providing the highest protein quality for a plant-based meal (Figure 5; Table 5). However, given the low levels of iron and calcium at a 50% contribution from soy and/or animal protein in pesco/semi-vegetarian meals, the optimal protein ratio should consist of at least 10% from grains, nuts, and seeds, 50 to 60% from beans, peas, and lentils, and a minimum of 30 to 40% from soy-based foods, dairy, eggs, fish, poultry, and red meats (Figure 5; Table 5). Notably, with at least 30% contribution from soy-based foods or the inclusion of any animal protein, the protein ratio contributions across all three plant-based meal models were able to meet optimal protein quality and nutrient quality.
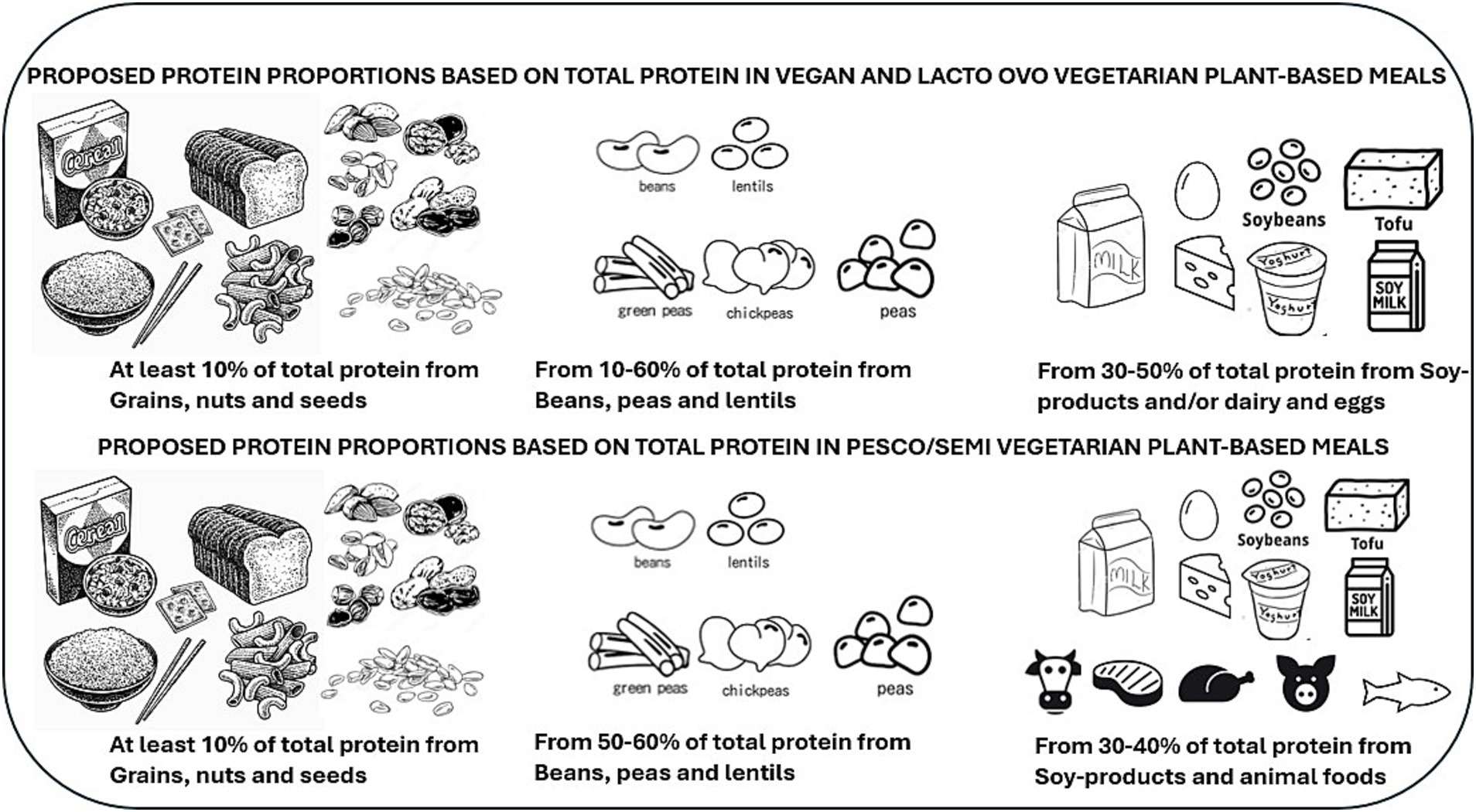
Figure 5. An overview of protein food recommendations that incorporates a diversity of protein food sources needed across various plant-based meals to achieve optimal protein as well as nutrient quality.
Table 6 illustrates examples of protein foods with a proportion contribution to total protein intake meeting optimal protein quality and nutrient quality. Each row in Table 6 represents one plant-based meal solution; for example, 50% of the total protein intake should come from whole wheat bread, 10% from carrots, and 40% from tofu. The vegan meal model had more variety coming from grains, nuts and seeds, beans, peas, and lentils than meals including animal protein foods. With the vegetarian meal, no dairy and eggs were observed in the optimal 50% “soy-based foods, dairy products, eggs and others” solution. Similarly, the model included all animal protein foods with no beef in the 40% “soy-based foods, dairy products, eggs, fish, poultry, red-meats and others” solution. It is important to note that the model did not identify beef as an optimal ingredient for semi-vegetarian meals, as its high levels of iron and zinc per 100 grams exceeded the set nutrient constraints for protein and micronutrient quality. Therefore, beef could still be a viable option for inclusion in a semi-vegetarian meal.
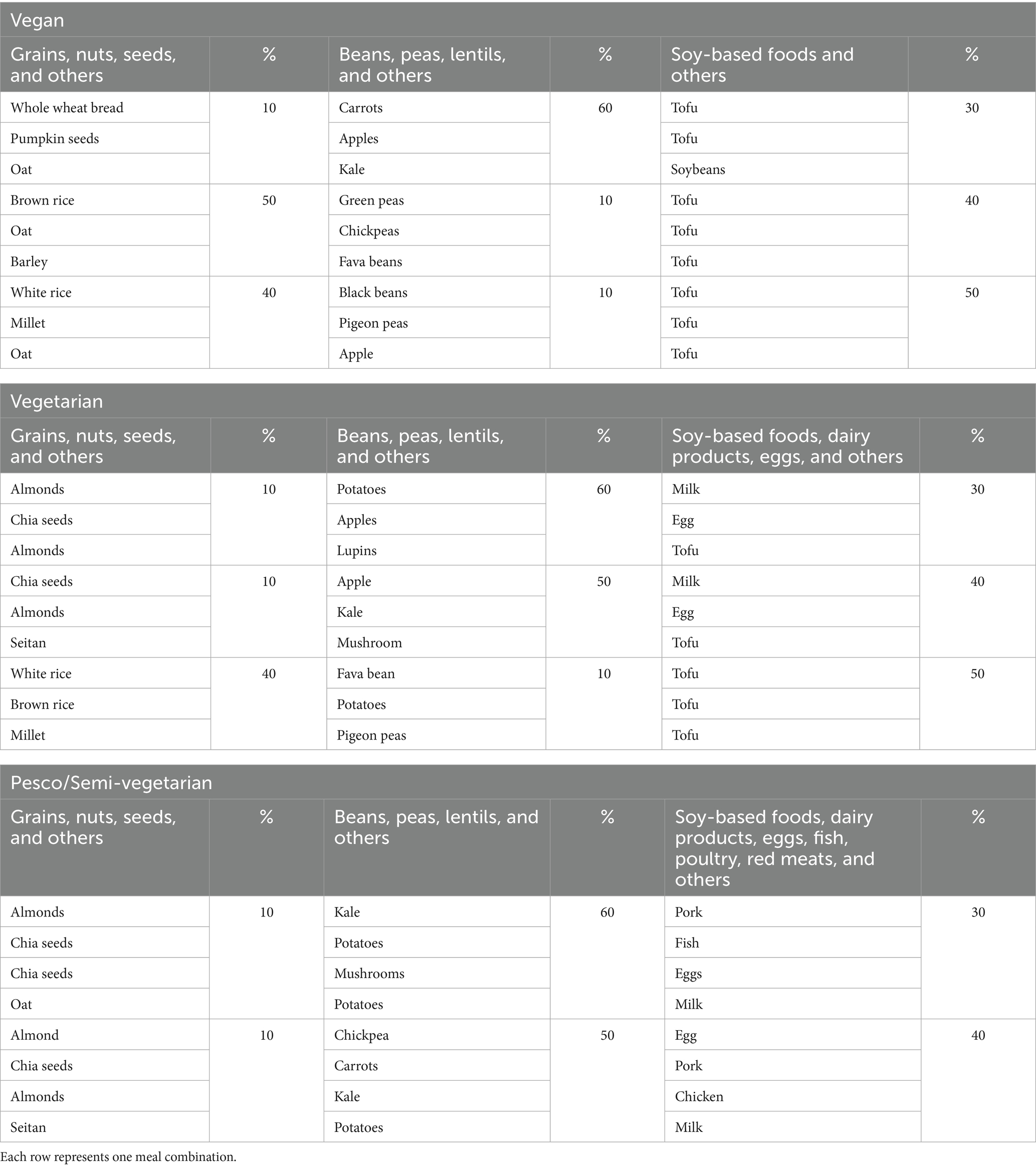
Table 6. Example of types of protein food contributions to total protein meeting all nutrient constraints.
4 Discussion
4.1 The protein quality and nutritional composition across the protein ratios
Current dietary recommendations from various populations guide the increased variety of plant-protein foods without specifying proportions from each type of plant protein (8–11). Our study provides insights into the diversity of protein foods needed across different plant-based meals to achieve optimal protein as well as nutrient quality. Our findings indicate that a contribution of 30 to 50% from soy-based foods for vegan meals and/or the inclusion of dairy foods and eggs for vegetarian meals presented optimal protein quality (PDCAAS 1) and had the highest levels of calcium and iron (Figures 2, 3). In the case of pesco/semi-vegetarian meals, a contribution of 50% from soy and/or animal protein foods contained the lowest levels of these nutrients, consequently, this proportion was not considered optimal for meals including more animal protein foods. This guidance on protein proportions in plant-based meals could support future dietary guidelines by helping plant-based eaters meet their protein needs, including essential nutrients such as calcium, zinc, and iron (Figure 5).
Overall, at least 30% of either plant-only foods or the inclusion of animal protein foods was required to reach optimal protein and nutrient quality across the different plant-based meals (Figures 2–4). The protein ratio guidance of consuming at least 30% high-quality protein foods such as soy-based foods only and/or animal-based foods is in line with another study’s finding on healthier diets providing adequate nutrients and minimal health risk at a plant protein percentage range from 25 to 70% [53]. Another study in Canada had similar findings and found a meal to be nutritionally balanced when plant protein foods contributed 25–74.9% of the total protein intake [54]. Despite these findings, it is essential to emphasize that the optimized vegan and vegetarian meals could still be low in eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), iodine, vitamin B12, and vitamin D as unfortified plant protein foods have minimal to none of these nutrients present (30–33). Therefore, including some animal protein in dietary recommendations for vegetarian or pesco/semi-vegetarian meals could aid in increasing the intake of these nutrients found low in plant-based meals, leading to a nutritionally balanced meal. Although several studies have found the vegan diet to have the highest diet quality in comparison to other plant-based diets (34, 35), this was mostly attributed to higher scores from an increased intake of fruits, vegetables, whole grains, plant-protein, fatty acid profile, and lower saturated fat. The diet quality indices used in these studies did not consider the diversity of protein foods consumed or assess whether the intake of any nutrients was low or missing. Therefore, future work should include protein food diversity and consider key nutrient intakes such as iron, calcium, zinc, EPA, DHA, iodine, vitamin B12, and vitamin D when assessing diet quality in plant-based meals.
Other optimization studies for protein quality have been conducted to define optimal plant ingredient combinations that achieve a desirable amino acid profile or high-quality protein meals. For instance, a study based in the Netherlands developed a meal protein scoring system to create high-protein quantity and quality of strict plant-based meals based on indispensable amino acids and digestibility of foods aimed specifically for older adults (36). In contrast, our study findings are applicable for the general population plant-based dieters who are looking to reduce their consumption of animal protein whilst maintaining nutrient quality in a meal, which is a novelty of this study. Another study conducted in France uses a linear programming technique to determine the optimal plant-based ingredient combinations for potential plant-based substitutes. This study results in a combination of plant ingredients meeting three distinct amino acid profiles: first, a ‘balanced amino acid profile’ based on the amino acid requirements for adults; second, ‘animal profiles’ reflecting the compositions of typical animal proteins; and third, a ‘cardioprotective profile’ linked to a reduced risk of cardiovascular disease (37). However, our study takes a different approach by using the PDCAAS as the primary measure of protein quality. Additionally, our study considered key nutrient contributions and examined how phytate may affect the absorption of key minerals such as iron and zinc in plant-based meals.
This study’s vegan and vegetarian meal solutions included a variety of plant protein foods within grains, nuts, seeds, beans, peas, and lentils, unlike the pesco/semi-vegetarian meals, including fish, poultry, and red meats. The increased variety could be due to the need for various plant protein foods to address the limiting amino acids in different plant proteins (38, 39). In contrast, when animal protein foods such as fish, poultry, and red meats were added to the pesco/semi-vegetarian meal model, the optimal ratios differed, with a higher percentage coming from beans, peas, and lentils. Such differences could be due to the constraints on nutrients such as dietary fiber, iron, and calcium in the optimization model. To meet the nutrient constraints included in the optimization model for proposing protein ratios in meals containing animal protein foods such as dairy, eggs, fish, poultry, and red meats, which are high in iron and calcium but lack dietary fiber, the solution was increasing the proportion of beans, peas, and lentils. This solution aims to compensate for the absence of dietary fiber in animal foods while maintaining high protein quality in a meal.
4.2 The presence of phytate and selecting the ideal protein ratio
Apart from being important sources of nutrients, plant protein foods such as whole grains, nuts, beans, legumes, and some vegetables contain high amounts of antinutrients, mainly phytates, tannins, lectins, and oxalates (26). Antinutrients like phytates could inhibit protein and mineral absorption. Thus, it is primarily a concern in plant-based meals or diets, which are mainly made up of plant-protein foods (35). The protein foods assessed in this study, such as walnuts, oats, peanuts, and buckwheat, contributed the most phytate from 1,385–4,029 mg per 100 g food (Supplementary Table 1). Our study considered one potential aspect affecting the bioavailability of protein and micronutrients by estimating the phytate levels present in the identified ideal protein ratios. To mitigate excessive amounts of phytates the meal should contain only limited amounts of grains, nuts, and seeds (at least 10% of total protein intake). In the vegan meal model, the molar ratios of phytate to iron and zinc were highest at 50% grains, nuts, and seeds, 10% beans, peas, and lentils, and 40% soy-based foods contribution, indicating a potential decreased bioavailability of iron and zinc at this ratio (Figure 4). Across all plant-based meals, the ratios of phytate to iron and zinc were consistently lowest at 30% soy-based foods only and/or animal protein foods contribution in comparison to all proportions from soy/animal foods, suggesting that this ratio may represent the most effective solution for addressing the presence of antinutrients in plant protein foods. However, it is important to note that across all protein food proportions in all plant-based meals, the phytate-to-iron ratio did not meet the threshold for optimal iron absorption. Therefore, iron absorption enhancers such as vitamin C rich foods, known to counteract the effect of iron absorption inhibitors, should be encouraged when incorporating these proportions into a plant-based meal (29). In our model, enhancers of iron absorption were not considered. However, reasonable consumption of animal protein foods such as meats and fish in a pesco/semi-vegetarian diet could help mitigate the effects of antinutrient factors (40). Additionally, in line with these recommendations from our findings, recent reports indicate that a 30% inclusion of soy in the diet does not significantly impair total iron absorption (41). Further research is needed to evaluate this aspect on mineral absorption, particularly concerning zinc.
4.3 The application of an ideal ratio of sources of proteins into dietary guidance
Across all plant-based meals (vegan, vegetarian, and pesco/semi-vegetarian), a consistent finding for achieving optimal protein and nutrient quality was the recommended protein ratio: a minimum of 10% from grains, nuts, and seeds; 60% from beans, peas, and lentils; and 30% from soy-based foods and/or animal protein foods. These ratios also have the most consistent phytate-to-iron and phytate-to-zinc ratios, and if coupled with consuming vitamin C rich foods, this meal proportion could be the most optimal across all plant-based meals. The vegan meal model exhibited the highest diversity among food groups, featuring a broader selection of grains, nuts, seeds, beans, peas, and lentils compared to models that included animal protein foods. Furthermore, a recent review suggests that greater diversity within food groups may enhance nutritional quality and health outcomes (42). With the availability of more data on protein digestibility of foods in the future, this aspect of dietary diversity, both within and between food groups utilizing these ideal protein ratios, will need further investigation to assess its significance for diet quality and health in different study populations.
4.4 Strengths and limitations
To our knowledge, this is the first study to use this unique approach to group protein foods by their limiting acids followed by adding nutrient constraints to determine optimal protein and nutrient quality across the different primarily plant-based meals. Furthermore, cooked foods were included in the analysis whenever data was available to account for potential differences between raw and cooked protein foods. The use of secondary data to compile information on amino acid profiles, macronutrients, and micronutrients of the foods included in this study leverages existing USDA datasets that provide complete information on each food item related to protein, amino acids, and nutrients. Another strength to this study was the amount of phytate estimated in the meals to determine if the bioavailability of iron and zinc would be affected across the different primarily plant-based meals. The key findings from this study adds to current knowledge on plant-based dietary guidelines by providing understanding into the types and proportions of protein foods to consume in a plant-based meal.
As the USDA dataset provided comprehensive information on amino acids but lacked data on the protein digestibility of foods, the literature was used to obtain the necessary information for calculating the PDCAAS of each food. The use of different data sources could lead to inconsistencies in the data and the final PDCAAS values. However, due to the absence of a complete data set for protein digestibility of foods, this approach represents the most optimal method for determining the PDCAAS of the foods included in this study. Other limitations of this study include the paucity of in vivo data on protein digestibility of protein foods. The PDCAAS is less precise in assessing protein quality than the Digestible Indispensable Amino Acid Score (DIAAS) method. However, as the data on DIAAS for a variety of foods remains limited, the PDCAAS is the most appropriate measurement for protein quality. Future studies could implement a similar model using DIAAS as the objective function instead, leading to an increased accuracy on protein quality measurement. Additionally, this study used the available nutrient guidelines proposed by the International Breakfast Initiative to address the nutrients needed for a plant-based meal. Future studies could adapt to potential developed thresholds for what an ideal protein proportion would be for lunches or dinners, particularly across cultural contexts. This study could not assess vitamin D intakes due to the lack of available data on vitamin D in selected foods, which may have overlooked key differences across the different primarily plant-based meals. Additionally, we included an estimation of phytate levels in foods based on the FAO/INFOODS/IZiNCG Global Food Composition Database for phytate; however, this may not accurately reflect the actual phytate levels in the foods. Finally, enhancers of mineral absorption would also need to be considered in future modeling approaches.
5 Conclusion
The main findings from this study provide comprehensive easy-to-follow guidance for selecting a variety of protein foods providing high protein quality while also contributing to nutrient quality in a balanced plant-based meal. These protein food proportions could inform future dietary guidelines by enhancing current recommendations, which primarily emphasize variety in protein sources. The optimal protein ratio from protein foods in vegan meals should be at least 10% grains, nuts, and seeds, 10–60% beans, peas and lentils and 30–50% soy-based foods with the inclusion of dairy and/or eggs in vegetarian meals. As for a pesco/semi-vegetarian meal, the optimal protein ratio should be at least 10% grains, nuts, seeds, 50–60% beans, peas, and lentils, and 30–40% soy-based foods and/or the inclusion of animal protein foods. To reduce animal protein intake in meals, the guidance could be at least 30% of protein foods coming from animal protein foods to be sufficient to meet optimal protein and nutrient quality in a plant-based meal.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MR: Conceptualization, Writing – review & editing, Formal analysis, Methodology, Writing – original draft. FM: Supervision, Methodology, Writing – review & editing, Writing – original draft, Conceptualization. MB: Writing – review & editing, Writing – original draft, Conceptualization, Supervision. K-AL: Conceptualization, Methodology, Supervision, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank Laura Hackl and Magalie Sabatier for their constructive input and support of the manuscript. All authors and those acknowledged have granted their permission to be mentioned in this manuscript.
Conflict of interest
MR, FM, K-AL are employees of Société des Produits Produits Nestlé S.A.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at:https://www.frontiersin.org/articles/10.3389/fnut.2025.1624633/full#supplementary-material
References
1. World Health Organisation. Plant-based diets and their impact on health, sustainability and the environment: a review of the evidence. WHO European Office for the Prevention and Control of Noncommunicable Diseases. Copenhagen. (2021).
2. Marinangeli, CPF. Chasing the transition to plant-based diets: a need for more focus and guidance to facilitate effective dietary changes. J Nutr. (2025) 155:4–6. doi: 10.1016/j.tjnut.2024.10.045
3. Food and Agriculture Organization. Dietary protein quality evaluation in human nutrition. Report of an FAQ expert consultation. Rome, Italy: FAO food and nutrition paper. (2013) 92:1–66.
4. Adhikari, S, Schop, M, de Boer, IJM, and Huppertz, T. Protein quality in perspective: a review of protein quality metrics and their applications. Nutrients. (2022) 14. doi: 10.3390/nu14050947
5. Phillips, SM, Fulgoni, VL, Heaney, RP, Nicklas, TA, Slavin, JL, and Weaver, CM. Commonly consumed protein foods contribute to nutrient intake, diet quality, and nutrient adequacy23456. Am J Clin Nutr. (2015) 101:1346S–52S. doi: 10.3945/ajcn.114.084079
6. Zhubi-Bakija, F, Bajraktari, G, Bytyci, I, Mikhailidis, DP, Henein, MY, Latkovskis, G, et al. The impact of type of dietary protein, animal versus vegetable, in modifying cardiometabolic risk factors: a position paper from the international lipid expert panel (ILEP). Clin Nutr. (2021) 40:255–76. doi: 10.1016/j.clnu.2020.05.017
7. Vasiloglou, MF, Elortegui Pascual, P, Scuccimarra, EA, Plestina, R, Mainardi, F, Mak, TN, et al. Assessing the quality of simulated food patterns with reduced animal protein using meal data from NHANES 2017-2018. Nutrients. (2023) 15. doi: 10.3390/nu15112572
8. Blomhoff, R, Andersen, R, Arnesen, EK, Christensen, JJ, Eneroth, H, Erkkola, M, et al. Nordic nutrition recommendations. Copenhagen: Nordic Council of Ministers (2023).
9. German Society for Nutrition e.V. DGE nutrition circle (2024). Available online at: https://www.dge.de/gesunde-ernaehrung/gut-essen-und-trinken/dge-ernaehrungskreis/.
10. Brink, E, van Rossum, C, Postma-Smeets, A, Stafleu, A, Wolvers, D, van Dooren, C, et al. Development of healthy and sustainable food-based dietary guidelines for the Netherlands. Public Health Nutr. (2019) 22:2419–35. doi: 10.1017/S1368980019001435
11. National Institute of Nutrition. Dietary guidelines for Indian - a manual. Hyderabad: National Institute of Nutrition. (2011).
12. Rolands, MR, Hackl, LS, Bochud, M, and Lê, KA. Protein adequacy, plant protein proportion, and Main plant protein sources consumed across vegan, vegetarian, Pescovegetarian, and Semivegetarian diets: a systematic review. J Nutr. (2025) 155:153–67. doi: 10.1016/j.tjnut.2024.07.033
13. Marinangeli, CPF, Fabek, H, Ahmed, M, Sanchez-Hernandez, D, Foisy, S, and House, JD. The effect of increasing intakes of plant protein on the protein quality of Canadian diets. Appl Physiol Nutr Metab. (2021) 46:771–80. doi: 10.1139/apnm-2020-1027
14. Marinangeli, CPF, Miller, K, and Fulgoni, VL 3rd. Effect of increasing plant protein intake on protein quality and nutrient intake of US adults. Appl Physiol Nutr Metab. (2023) 48:49–61. doi: 10.1139/apnm-2022-0054
15. US Department of Agriculture ARS. USDA National Nutrient Database for standard reference, release 28 (slightly revised) (2016). Available online at: http://www.ars.usda.gov/nea/bhnrc/mafcl.
16. Reynaud, Y, Buffière, C, Cohade, B, Vauris, M, Liebermann, K, Hafnaoui, N, et al. True ileal amino acid digestibility and digestible indispensable amino acid scores (DIAASs) of plant-based protein foods. Food Chem. (2021) 338:128020. doi: 10.1016/j.foodchem.2020.128020
17. Anwar, D, and El-Chaghaby, G. Nutritional quality, AMINO ACID profiles, protein digestibility corrected AMINO ACID scores and antioxidant properties of fried tofu and seitan. Suceava, Romania: Food Engineering Faculty, Ştefan cel Mare University (2019).
18. WHO/FAO/UNU Expert Consultation. Protein and amino acid requirements in human nutrition. World Health Organ Tech Rep Ser. (2007) 935:1–265.
19. Dewangan, SK, Choubey, S, Patra, J, and Choubey, A. Nonlinear optimization methods—overview and future scope In: Modern optimization methods for science, engineering and technology [internet]. GR Sinha editor. Bristol, England: IOP Publishing. (2019).
20. British Nutrition Foundation. Plant based diets (2019) Available online at: https://www.nutrition.org.uk/putting-it-into-practice/plant-based-diets/plant-based-diets/.
21. Neufingerl, N, and Eilander, A. Nutrient intake and status in adults consuming plant-based diets compared to meat-eaters: a systematic review. Nutrients. (2021) 14. doi: 10.3390/nu14010029
22. Gibney, MJ, Barr, SI, Bellisle, F, Drewnowski, A, Fagt, S, Hopkins, S, et al. Towards an evidence-based recommendation for a balanced breakfast-a proposal from the international breakfast research initiative. Nutrients. (2018) 10. doi: 10.3390/nu10101540
23. O'Neil, CE, Byrd-Bredbenner, C, Hayes, D, Jana, L, Klinger, SE, and Stephenson-Martin, S. The role of breakfast in health: definition and criteria for a quality breakfast. J Acad Nutr Diet. (2014) 114:S8–S26. doi: 10.1016/j.jand.2014.08.022
24. Dietitians TEFotAo. (2024). Balanced breakfast recipes. Available online at: https://www.efad.org/wp-content/uploads/2023/11/Balanced-Breakfast-Recipes-Booklet.pdf].
25. Moore, DR, Churchward-Venne, TA, Witard, OC, Breen, L, Burd, NA, Tipton, KD, et al. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontology. (2014) 70:57–62. doi: 10.1093/gerona/glu103
26. Popova, A, and Mihaylova, D. Antinutrients in plant-based foods: a review. Open Biotechnol J. (2019) 13:68–76. doi: 10.2174/1874070701913010068
27. Dahdouh, S, Grande, F, Espinosa, SN, Vincent, A, Gibson, R, Bailey, K, et al. Development of the FAO/INFOODS/IZINCG global food composition database for phytate. J Food Compos Anal. (2019) 78:42–8. doi: 10.1016/j.jfca.2019.01.023
28. Hurrell, RF. Phytic acid degradation as a means of improving iron absorption. Int J Vitam Nutr Res. (2004) 74:445–52. doi: 10.1024/0300-9831.74.6.445
29. Allen, L, Dary, O, and Hurrell, R. Guidelines on food fortification with micronutrients. Geneva: World Health Organization; (2006).
30. Americans DGf. Food sources of vitamin D (2019). Available online at: https://www.dietaryguidelines.gov/resources/2020-2025-dietary-guidelines-online-materials/food-sources-select-nutrients/food-sources-vitamin-d.
31. Health NIo. Omega-3 fatty acids fact sheet for health professionals (2024) Available online at: https://ods.od.nih.gov/factsheets/Omega3FattyAcids-HealthProfessional/].
32. Health NIo. Iodine (2024) Available online at: https://ods.od.nih.gov/factsheets/Iodine-HealthProfessional/].
33. Health NIo. Vitamin B12 (2024) Available online at: https://ods.od.nih.gov/factsheets/VitaminB12-HealthProfessional/].
34. Clarys, P, Deliens, T, Huybrechts, I, Deriemaeker, P, Vanaelst, B, De Keyzer, W, et al. Comparison of nutritional quality of the vegan, vegetarian, semi-vegetarian, pesco-vegetarian and omnivorous diet. Nutrients. (2014) 6:1318–32. doi: 10.3390/nu6031318
35. Peruvemba, S, Gieng, J, Chen, S, and Pereira Pignotti, GA. Diet quality, diet motives, and nutrition literacy of vegans, vegetarians, and semi-vegetarians. Public Health Nutr. (2024) 27:1–19. doi: 10.1017/S1368980024001241
36. Grootswagers, P, Højlund Christensen, S, Timmer, M, Riley, W, de Groot, L, and Tetens, I. Meal protein quality score: a novel tool to evaluate protein quantity and quality of meals. Curr Develop Nut. (2024) 8:104439. doi: 10.1016/j.cdnut.2024.104439
37. Dimina, L, Remond, D, Huneau, JF, and Mariotti, F. Combining plant proteins to achieve amino acid profiles adapted to various nutritional objectives-an exploratory analysis using linear programming. Front Nutr. (2021) 8:809685. doi: 10.3389/fnut.2021.809685
38. Mathai, JK, Liu, Y, and Stein, HH. Values for digestible indispensable amino acid scores (DIAAS) for some dairy and plant proteins may better describe protein quality than values calculated using the concept for protein digestibility-corrected amino acid scores (PDCAAS). Br J Nutr. (2017) 117:490–9. doi: 10.1017/S0007114517000125
39. Hertzler, SR, Lieblein-Boff, JC, Weiler, M, and Allgeier, C. Plant proteins: assessing their nutritional quality and effects on health and physical function. Nutrients. (2020) 12. doi: 10.3390/nu12123704
40. Hurrell, R, and Egli, I. Iron bioavailability and dietary reference values. Am J Clin Nutr. (2010) 91:1461s–7s. doi: 10.3945/ajcn.2010.28674F
41. Hackl, LS, Moretti, D, and Sabatier, M. Absorption of Iron naturally present in soy. Adv Nutr. (2025) 16:100396. doi: 10.1016/j.advnut.2025.100396
42. Bolo, A, Verger, E, Fouillet, H, and Mariotti, F. Exploring multidimensional and within-food group diversity for diet quality and long-term health in high-income countries. Adv Nutr. (2024) 15:100278. doi: 10.1016/j.advnut.2024.100278
43. Sub-Committee of the 2011 FAO Consultation. The assessment of amino acid digestibility in foods for humans and including a collation of published ileal amino acid digestibility data for human foods. Rome, Italy: Food and Agriculture Organization of the United Nations. (2011).
44. Sauvant, D, Perez, J-M, and Tran, G. Tables of composition and nutritional value of feed materials: Pigs, poultry, cattle, sheep, goats, rabbits, horses and fish. Wageningen, Netherlands: Wageningen Academic (2023).
45. Mariotti, F, Pueyo, ME, Tomé, D, and Mahé, S. The bioavailability and postprandial utilisation of sweet lupin (Lupinus albus)-flour protein is similar to that of purified soyabean protein in human subjects: a study using intrinsically 15N-labelled proteins. Br J Nutr. (2002) 87:315–23. doi: 10.1079/BJN2002526
46. Kashyap, S, Varkey, A, Shivakumar, N, Devi, S, Reddy, BHR, Thomas, T, et al. True ileal digestibility of legumes determined by dual-isotope tracer method in Indian adults. Am J Clin Nutr. (2019) 110:873–82. doi: 10.1093/ajcn/nqz159
47. Kashyap, S, Shivakumar, N, Varkey, A, Duraisamy, R, Thomas, T, Preston, T, et al. Ileal digestibility of intrinsically labeled hen's egg and meat protein determined with the dual stable isotope tracer method in Indian adults. Am J Clin Nutr. (2018) 108:980–7. doi: 10.1093/ajcn/nqy178
48. Han, F, Han, F, Wang, Y, Fan, L, Song, G, Chen, X, et al. Digestible indispensable amino acid scores of nine cooked cereal grains. Br J Nutr. (2019) 121:30–41. doi: 10.1017/S0007114518003033
49. Han, F, Moughan, PJ, Li, J, and Pang, S. Digestible indispensable Amino Acid scores (DIAAS) of six cooked Chinese pulses. Nutrients. (2020) 12. doi: 10.3390/nu12123831
50. Hodgkinson, SM, Montoya, CA, Scholten, PT, Rutherfurd, SM, and Moughan, PJ. Cooking conditions affect the true ileal digestible Amino Acid content and digestible indispensable Amino Acid score (DIAAS) of bovine meat as determined in pigs. J Nutr. (2018) 148:1564–9. doi: 10.1093/jn/nxy153
51. Bailey, HM, Mathai, JK, Berg, EP, and Stein, HH. Pork products have digestible indispensable Amino Acid scores (DIAAS) that are greater than 100 when determined in pigs, but processing does not always increase DIAAS. J Nutr. (2020) 150:475–82. doi: 10.1093/jn/nxz284
52. Bailey, HM, Mathai, JK, Berg, EP, and Stein, HH. Most meat products have digestible indispensable amino acid scores that are greater than 100, but processing may increase or reduce protein quality. Br J Nutr. (2020) 124:14–22. doi: 10.1017/S0007114520000641
53. Rutherfurd, SM, Fanning, AC, Miller, BJ, and Moughan, PJ. Protein digestibility-corrected Amino Acid scores and digestible indispensable Amino Acid scores differentially describe protein quality in growing male Rats1, 2, 3. J Nutr. (2015) 145:372–9. doi: 10.3945/jn.114.195438
54. Jørgensen, H, and Lauridsen, C, editors. Nutrient composition and bioavailability of protein and energy in common fruits and vegetables prepared for human consumption. Søborg, Denmark: GSB-tryk. (2004).
55. House, JD, Hill, K, Neufeld, J, Franczyk, A, and Nosworthy, MG. Determination of the protein quality of almonds (Prunus dulcis L.) as assessed by in vitro and in vivo methodologies. Food Sci Nutr. (2019) 7:2932–8. doi: 10.1002/fsn3.1146
56. Freitas, JB, Fernandes, DC, Czeder, LP, Lima, JCR, Sousa, AGO, and Naves, MMV. Edible seeds and nuts grown in Brazil as sources of protein for human nutrition. Food Nutr Sci. (2012) 3:6. doi: 10.4236/fns.2012.36114
57. Lackey, KA, and Fleming, SA. Brief research report: estimation of the protein digestibility-corrected amino acid score of defatted walnuts. Front Nutr. (2021) 8. doi: 10.3389/fnut.2021.702857
58. Dina, A, and El-Chaghaby, G. Nutritional quality, AMINO ACID profiles, protein digestibility corrected AMINO ACID scores and antioxidant properties of fried tofu and seitan. Suceava, Romania: Food Engineering Faculty, Ştefan cel Mare University. (2019) 176–190.
59. Nosworthy, MG, Neufeld, J, Frohlich, P, Young, G, Malcolmson, L, and House, JD. Determination of the protein quality of cooked Canadian pulses. Food Sci Nutr. (2017) 5:896–903. doi: 10.1002/fsn3.473
60. Agengo, F, Serrem, C, and Wamunga, F. Evaluation of protein nutritional quality of four soybean varieties grown in Western Kenya. J Food Res. (2018) 7:69. doi: 10.5539/jfr.v7n5p69
61. Monroy -Torres, R, Mancilla-Escobar, M, Gallaga-Solórzano, J, Medina, S, and Santiago-García, E. Protein digestibility of chia seed Salvia hispanica L. Revista Salud Publicay Nutricion. (2008) 9. doi: 10.1002/mnfr.201900021
62. Sá, AGA, Moreno, YMF, and Carciofi, BAM. Food processing for the improvement of plant proteins digestibility. Crit Rev Food Sci Nutr. (2020) 60:3367–86. doi: 10.1080/10408398.2019.1688249
63. Jyothi lakshmi, A, and Kaul, P. Nutritional potential, bioaccessibility of minerals and functionality of watermelon (Citrullus vulgaris) seeds. LWT Food Sci Technol. (2011) 44:1821–6. doi: 10.1016/j.lwt.2011.04.001
64. Martínez-Velasco, A, Alvarez-Ramirez, J, Rodríguez-Huezo, E, Meraz-Rodríguez, M, Vernon-Carter, EJ, and Lobato-Calleros, C. Effect of the preparation method and storage time on the in vitro protein digestibility of maize tortillas. J Cereal Sci. (2018) 84:7–12. doi: 10.1016/j.jcs.2018.09.016
65. Colunga, A, Cruz-Hernández, M, Losoya, C, Nobre Gonçalves, C, Treviño, A, Rodriguez-Jasso, R, et al. Edible mushrooms as a novel protein source for functional foods. Food Funct. (2020) 11. doi: 10.1039/d0fo01746a
66. Khattab, RY, Arntfield, SD, and Nyachoti, CM. Nutritional quality of legume seeds as affected by some physical treatments, part 1: protein quality evaluation. LWT Food Sci Technol. (2009) 42:1107–12. doi: 10.1016/j.lwt.2009.02.008
Keywords: plant-based diet, vegetarian, vegan, semi-vegetarian, protein source, plant protein, animal protein, protein quality
Citation: Rolands MR, Mainardi F, Bochud M and Lê K-A (2025) Identifying the optimal ratio from protein foods for protein and nutrient quality in plant-based meals using a non-linear optimization approach. Front. Nutr. 12:1624633. doi: 10.3389/fnut.2025.1624633
Edited by:
Benjamin K. Mintah, CSIR-Food Research Institute, GhanaReviewed by:
Omar A. Obeid, American University of Beirut, LebanonGengjun Chen, South Dakota State University, United States
Seydi Yıkmış, Namik Kemal University, Türkiye
Copyright © 2025 Rolands, Mainardi, Bochud and Lê. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maryann R. Rolands, bWFyeWFubi5yb2xhbmRzQHJkLm5lc3RsZS5jb20=
 Maryann R. Rolands
Maryann R. Rolands Fabio Mainardi
Fabio Mainardi Murielle Bochud
Murielle Bochud Kim-Anne Lê
Kim-Anne Lê