- 1Biotech Research Institute, Grape King Bio Ltd., Taoyuan, Taiwan
- 2Department of Food Science, Nutrition, and Nutraceutical Biotechnology, Shih Chien University, Taipei, Taiwan
- 3Institute of Food Science and Technology, National Taiwan University, Taipei, Taiwan
- 4Graduate Institute of Sports Science, National Taiwan Sport University, Taoyuan, Taiwan
- 5Tajen University, Pingtung, Taiwan
Background: Exercise-induced muscle fatigue is a major challenge for athletes. Our previous study indicated that Levilactobacillus brevis GKEX improved endurance and reduced fatigue in mice. This study aimed to further evaluate the effects of different preparations of L. brevis GKEX on exercise performance and fatigue resistance.
Methods: Eight-week-old male ICR mice were divided into six groups (n = 8): vehicle, BCAA, low-dose GKEX (0.0615 mg/day), high-dose GKEX (0.615 mg/day), heat-killed GKEX (0.615 mg/day), and freeze-killed GKEX (0.615 mg/day). Treatments lasted for four consecutive weeks. Exercise performance, fatigue-related biochemical markers, and gut microbiota composition were assessed.
Results: Compared with BCAA, L. brevis GKEX significantly improved aerobic performance, including forelimb grip strength and running-exhaustion time. It enhanced lactate clearance and glycogen storage in the liver and muscles while reducing lactate production and blood urea nitrogen levels after exercise. L. brevis GKEX supplementation also increased key short-chain fatty-acid–producing bacteria in the intestines.
Conclusion: Oral administration of different doses of live and dead L. brevis GKEX promoted exercise performance and ameliorated fatigue, especially live GKEX. These findings suggest that L. brevis GKEX may serve as an ergogenic aid for athletes and support broader applications across various product forms.
1 Introduction
When the body experiences energy depletion or tissue damage, it may result in a sensation of fatigue primarily caused by exercise, inadequate sleep, illness, or other factors. Exercise-induced muscle fatigue is one of the most prevalent and easily identifiable types of fatigue. During exercise, the body utilizes various energy sources, including liver glycogen and glucose. When energy expenditure, coupled with the accumulation of lactate, can lead to feelings of weakness or muscle pain, it ultimately affects exercise performance (1). Prolonged muscle fatigue may even lead to neuromuscular disorders, metabolic diseases, and other adverse health conditions (2). Athletes and other individuals typically use various supplements, such as vitamins, traditional herbs, and minerals, to alleviate fatigue and improve their athletic performance. However, consuming supplements without scientific validation of their efficacy can potentially increase the burden on the body, leading to side effects, such as allergic reactions or digestive problems.
Probiotics are defined as non-pathogenic microorganisms that, when ingested, exert a positive influence on the host’s health or physiology (3). Among the most common probiotics are lactic acid bacteria, with Lactobacillus being the most prevalent genus. Studies indicate that Lactobacilli have proven functions, including reducing allergic reactions (4) and exerting antioxidant and anti-aging effects (5). They also have applications in intestinal inflammatory and infectious diseases (6), in addition to being used as an adjuvant for cancer prevention (7). Thus, it is evident that Lactobacilli have a beneficial impact on the overall health maintenance and promotion of bodily functions.
In recent years, there has been a growing interest in Lactobacillus supplements for reducing post-exercise fatigue while enhancing exercise performance. For instance, supplementation with Limosilactobacillus reuteri (formerly Lactobacillus reuteri) ID-D01 enhanced exercise endurance and muscle growth in C57BL/6 mice (8). Additionally, supplementation with heat-killed Lactiplantibacillus plantarum (formerly Lactobacillus plantarum) TWK10 has been found to significantly reduce physical fatigue, improve exercise endurance capacity, and increase overall muscle weight in healthy humans (9). Specifically, supplementation with Lactobacillus salivarius SA-03 increases muscle strength, decreases fatigue levels, and also affects the distribution of intestinal microbiota (10).
Levilactobacillus brevis (L. brevis) GKEX is a heterofermentative, Gram-positive lactic acid bacterium isolated from douchi (fermented black soybeans). The strain was identified and selected by Grape King Bio Ltd. (Taoyuan, Taiwan) based on its potential probiotic properties, including acid and bile salt tolerance. Our previous study demonstrated that supplementation with L. brevis GKEX significantly enhanced exercise endurance in a murine model, reduced fatigue-related biomarkers such as lactate and blood urea nitrogen levels, and increased glycogen content in the liver and muscles (11). Additionally, in our recent human trials, a six-week resistance exercise training program combined with L. brevis GKEX supplementation resulted in increased muscle mass and improved strength performance (12). These findings highlight the strain’s potential as a novel probiotic in exercise supplementation. Furthermore, non-viable probiotics offer several practical advantages, such as greater safety, stability, and shelf life, especially in immunocompromised populations (13). Therefore, this study aims to further investigate the fatigue-relieving and performance-enhancing effects of both live and non-viable forms of L. brevis GKEX, as well as its dose-dependent efficacy for broader applications. This will be evaluated through endurance testing, the regulation of fatigue-related biomarkers, and gut microbiota analysis.
2 Materials and methods
2.1 Sample preparation
The experimental strain of L. brevis GKEX provided by Grape King Bio Ltd. (Taoyuan, Taiwan) was originally isolated from douchi (fermented black soybeans) and preserved at the National Institute of Technology and Evaluation, Biological Resource Center (NBRC, Chiba, Japan) under accession number NITE BP-03696. A single colony was selected from an MRS agar plate and cultured in MRS broth (Difco, BD, United States) at 32 °C for 16 h with shaking at 100 rpm. The activated culture was then scaled up in 1 L of MRS medium with 0.1% inoculation and incubated under the same conditions. Afterward, the fermented broth was centrifuged at 25 °C at 5,000 rpm (Heraeus Megafuge 40R, Thermo Fisher Scientific Inc., United States) for 10 min to collect the bacterial pellet. The pellet was washed with reverse osmosis (RO) water, mixed with 20%(w/v) skim milk powder, and freeze-dried (FD24, Kingmech Scientific, Taoyuan, Taiwan) to obtain a live bacterial powder containing approximately 4 × 1011 colony-forming units (CFU) per gram, as determined by the plate counting method. For the preparation of the heat-killed GKEX sample (HK-GKEX), the washed bacterial pellet was resuspended in an equal volume of RO water, autoclaved at 121 °C for 15 min, and then freeze-dried. To prepare the freeze-killed GKEX sample (FK-GKEX), the washed bacterial pellet was resuspended in RO water, frozen at −20 °C for 3 days, and thawed at room temperature. This freeze–thaw cycle was repeated three times before processing into the powder by freeze-drying. All the bacterial powders were stored at 4 °C until use in subsequent animal experiments.
2.2 Animals and experimental designs
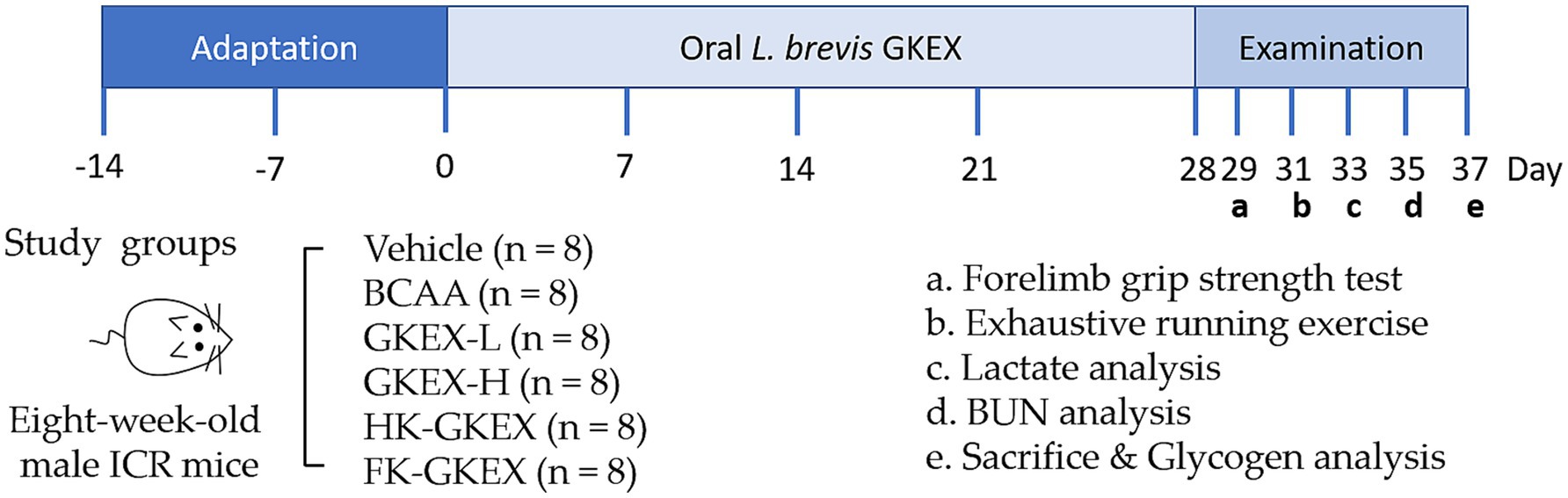
Eight-week-old male ICR mice with an average weight of approximately 30 g were sourced from BioLASCO Taiwan (Yi-Lan Breeding Center, Yi-Lan, Taiwan). The Institutional Animal Care and Use Committee of the National Taiwan Sport University (IACUC of NTSU) approved (IACUC-11108) all animal experiments conducted. All animals were provided LabDiet® 5,001 (PMI Nutrition International, Purina Mills, MO, United States) and distilled water ad libitum. The room temperature was maintained at 24 ± 2 °C with a humidity of 65 ± 5% and a 12-h light–dark cycle. A 2-week acclimation period was provided during which the mice were subjected to adaptive training to minimize stress and familiarize them with treadmill use. The adaptive training protocol consisted of placing the mice on a stationary treadmill on days 1–3, followed by light running at a speed of 5–10 m/min for 10 min/day from days 4 to 7, with gradual increases in duration and intensity over the second week to prepare them for later endurance testing. A total of 48 mice were randomly divided into 6 groups, with each group containing 8 mice: vehicle (water only) and branched-chain amino acids (BCAA, positive control, 1,500 mg/day (14)). Feeding doses of different preparations of L. brevis GKEX groups in mice were based on the human-to-mouse conversion factor of 12.3 (15): low-dosage L. brevis GKEX (GKEX-L) (0.0615 mg/day, equivalent to 10 mg/day in a human with 60 kg), high-dosage L. brevis GKEX (GKEX-H) (0.615 mg/day, equivalent to 100 mg/day in a human with 60 kg), HK-GKEX (0.615 mg/day, equivalent to 100 mg/day in a human with 60 kg), and FK-GKEX (0.615 mg/day, equivalent to 100 mg/day in a human with 60 kg). During the animal experiment, L. brevis GKEX was administered daily at 9 a.m. via oral gavage for four consecutive weeks. Throughout this period, the body weight, food intake, and water consumption were recorded. The intensity of exercise was gradually increased from low to high in consideration of animal welfare. Endurance fatigue analysis was conducted on days 29 to 37 of the experiment. This included analysis of forelimb grip strength, an exhaustive running test, post-exercise blood lactate and blood urea nitrogen (BUN) concentrations, as well as liver and muscle glycogen content. The experimental procedure is shown in Figure 1.
2.3 Forelimb grip strength
The forelimbs of the mice were placed on a tension rod (diameter 2 mm, length 7.5 cm) using a grip strength meter (Model-RX-5, Aikoh Engineering, Nagoya, Japan). During this process, the tail of the mouse was repeatedly pulled back to record the maximum grip force (peak power) as previously described (16).
2.4 Exhaustive running exercise
The mouse was placed on a mouse treadmill (MK680C, Muromachi Kikai, Chuo, Tokyo, Japan), set at a 15-degree incline with an initial speed of 12 m/min, increasing by 3 m/min every 2 min until the mouse falls into the shock zone (electric current of 2 Hz, 1.22 mA) for 5 s, showing no willingness to continue running forward, thereby indicating exhaustion. The time from the start to exhaustion was recorded as a measure of endurance performance (17).
2.5 Post-exercise blood biochemistry analysis
Unloaded swimming tests were conducted according to a previous study (18, 19). Mice swam for 10 min and rested for 20 min for lactate analysis, whereas mice swam for 90 min and rested for 60 min for BUN analysis. The swimming test was conducted in water at 28 ± 1 °C, and blood samples were immediately collected after swimming. The blood samples were centrifuged at 4 °C, 1,500 × g for 10 min to collect blood for analysis. Post-exercise fatigue markers, including lactate and BUN, were measured using an automatic blood biochemical analyzer (Hitachi 7,060, Hitachi, Tokyo).
2.6 Hematological profiles
At the end of the experiment, the mice were euthanized by 95% CO2 asphyxiation. Their blood was collected and then centrifuged at 4 °C, 1,500 × g for 15 min. The serum was aliquoted and analyzed for general clinical blood biochemistry, including glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT), creatine kinase (CK), BUN, creatinine (CREA), uric acid (UA), total cholesterol (TC), triglycerides (TG), glucose (GLU), and albumin (ALB), using a blood biochemical automatic analyzer (Hitachi 7,080, Hitachi, Tokyo, Japan).
2.7 Determination of tissue glycogen levels
This analytical method directly quantifies glycogen according to Chamberland and Rioux (20). Mice were sacrificed 30 min after the last feeding, and the liver and hind limb muscles were collected. After washing with physiological saline and drying, the tissues were homogenized with 5 times (w/v) tissue homogenization buffer using a Bullet Blender tissue homogenizer (Next Advance, Cambridge, MA, United States). The homogenates were then centrifuged at 4 °C, 12,000 × g for 15 min, and the supernatant was used for hepatic glycogen content analysis (21). Additionally, a calibration curve was prepared using a commercially available glycogen standard (Glycogen Sigma) to calculate changes in glycogen storage in the liver and muscle tissues among the different groups.
2.8 Analysis of gut microbiota composition
Fresh samples from the cecum of mice were collected after sacrifice and stored at −80 °C for DNA extraction using the Qiagen DNA Mini Kit (Qiagen, MD, United States). DNA was quantified using a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Wilmington, DE, United States) and maintained at −80 °C. Furthermore, the V3–V4 region of the 16S rRNA gene was amplified using the forward primer 341F (5’-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG-3′) and reverse primer 805R (5’-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC-3′). Subsequently, the PCR product was subjected to a second PCR amplification using the Nextera XT Index Kit (Illumina, United States) and quantified by real-time quantitative polymerase using a KAPA library quantification kit (KAPA Biosystems, United States). The Illumina HiSeq platform was applied to the V3 and V4 regions of 16S rRNA genes for taxonomic profiling of microbiota in cecums from kingdom to species, according to the Greengenes database1. Linear discriminant analysis effect size (LEfSe) analysis was performed to identify the differences between the abundance of taxonomic biomarkers of the gut microbiota in each group, and the results were visualized using a heatmap. Alpha diversity was assessed using the Shannon diversity index to evaluate the species richness and evenness within each group. The results were presented as boxplots to visualize differences in microbial diversity. Beta diversity using Bray–Curtis dissimilarity, unweighted UniFrac, and weighted UniFrac distances, which were visualized via constrained principal coordinates analysis (CPCoA).
2.9 Statistical analysis
Values are presented as mean ± SD, with each group consisting of eight mice. To assess group differences, a one-way analysis of variance (ANOVA) using Duncan’s post hoc test was conducted. The analysis was performed using SAS 9.0 (SAS Institute, Cary, NC, United States). Statistical differences in alpha diversity among groups were analyzed using the Kruskal–Wallis test, and for pairwise comparisons between groups, post hoc Dunn’s tests were performed. PERMANOVA accompanies CPCoA to determine whether the observed separation is statistically significant. A p-value of < 0.05 was considered statistically significant.
3 Results
3.1 Characteristics of mice following 4 weeks of Levilactobacillus brevis GKEX supplementation
During the experimental period, the body weight of mice in all six groups showed a stable increase over time, indicating no adverse effects associated with L. brevis GKEX supplementation. Additionally, there were no significant changes in the average body weight among the groups during the 4-week experimental period (Figure 2). As shown in Supplementary Table S1, no significant differences were observed in the average daily water and chow intake among the groups. Therefore, supplementation of different treatment methods of L. brevis GKEX did not significantly affect the drinking or feeding behavior of the mice. Furthermore, there were no significant differences observed in the absolute or relative weight of the tissues and organs among the groups (Supplementary Table S4), suggesting that no abnormal damage and changes were observed.
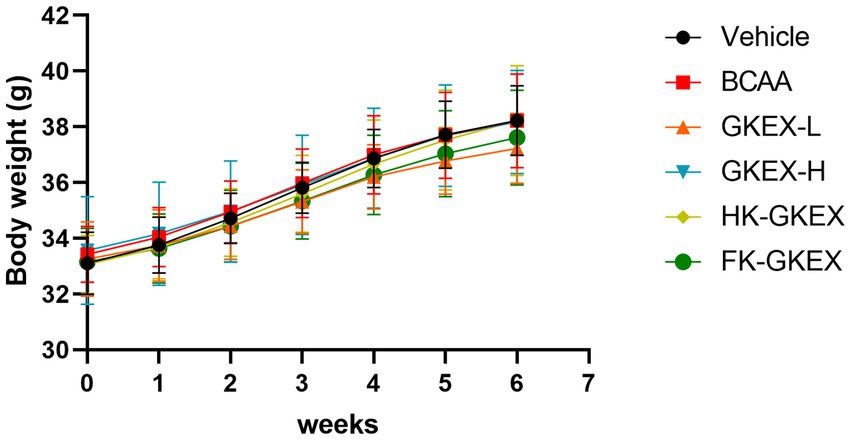
Figure 2. Body weights of the mice in each group during the experimental period. Data are expressed as mean ± SD (n = 8 mice per group). GKEX-L: low-dosage Levilactobacillus brevis GKEX, GKEX-H: high-dosage L. brevis GKEX, HK-GKEX: heat-killed L. brevis GKEX, and FK-GKEX: freeze-killed L. brevis GKEX.
3.2 Levilactobacillus brevis GKEX supplementation enhances forelimb grip strength in ICR mice
In order to determine whether L. brevis GKEX supplementation can enhance mouse muscle strength, the mice were fed GKEX for 4 weeks, and the forelimb grip strength of the L. brevis GKEX groups was compared with that of the control group. Forelimb grip strength performance may be affected by individual body weight. To adjust for this, the relative grip strength normalized to body weight (%) was calculated, as shown in Figure 3. The relative forelimb grip strength for the Vehicle, BCAA, GKEX-L, GKEX-H, HK-GKEX, and FK-GKEX groups was 347.02 ± 17.68, 385.02 ± 25.23, 415.55 ± 28.74, 426.50 ± 15.13, 407.79 ± 28.11, and 418.07 ± 20.45, respectively. Compared to the Vehicle group, the L. brevis GKEX group significantly increased the forelimb grip strength. Furthermore, compared to the BCAA group, the GKEX-L, GKEX-H, and FK-GKEX groups significantly increased by 1.08-fold (p = 0.0117), 1.11-fold (p = 0.0009), and 1.09-fold (p = 0.0069), respectively. The absolute values of grip strength are presented in Supplementary Table S2.
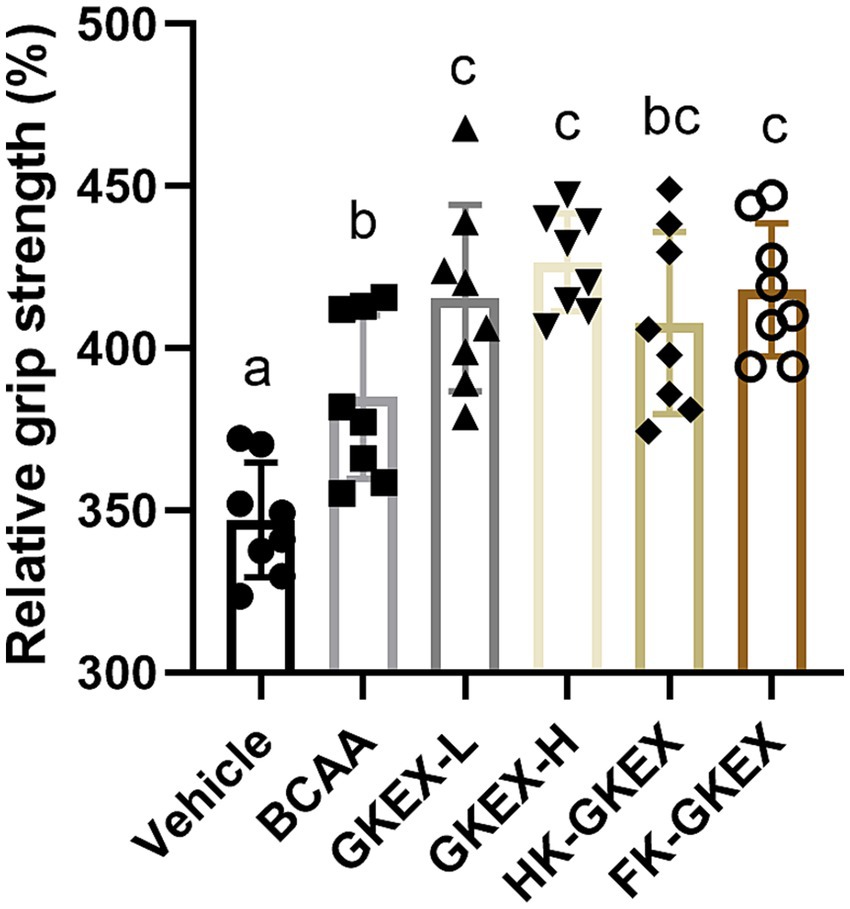
Figure 3. Effect of L. brevis GKEX supplementation on relative grip strength. Relative grip strength is calculated as forelimb grip strength divided by body weight. Data are expressed as mean ± SD (n = 8 mice per group). Different letters (a, b, c, d) indicate significant differences at p < 0.05, as determined by one-way ANOVA. GKEX-L: low-dosage L. brevis GKEX, GKEX-H: high-dosage L. brevis GKEX, HK-GKEX: heat-killed L. brevis GKEX, and FK-GKEX: freeze-killed L. brevis GKEX.
3.3 Levilactobacillus brevis supplementation increased exhaustive running time in ICR mice
The running exhaustion times for the Vehicle, BCAA, GKEX-L, GKEX-H, HK-GKEX, and FK-GKEX groups were 7.59 ± 1.52, 10.23 ± 0.97, 13.45 ± 1.11, 16.28 ± 0.72, 13.00 ± 1.06, and 14.96 ± 0.80 min, respectively (Figure 4). Compared to the Vehicle group, the BCAA, GKEX-L, GKEX-H, HK-GKEX, and FK-GKEX groups showed significantly improved running exhaustion performance. Additionally, compared to the BCAA group, the GKEX-L, GKEX-H, HK-GKEX, and FK-GKEX groups showed significantly enhanced running exhaustion performance by 1.31-fold, 1.59-fold, 1.27-fold, and 1.46-fold, respectively (p < 0.0001). Thus, continuous supplementation with L. brevis GKEX effectively increased the running exhaustion performance.
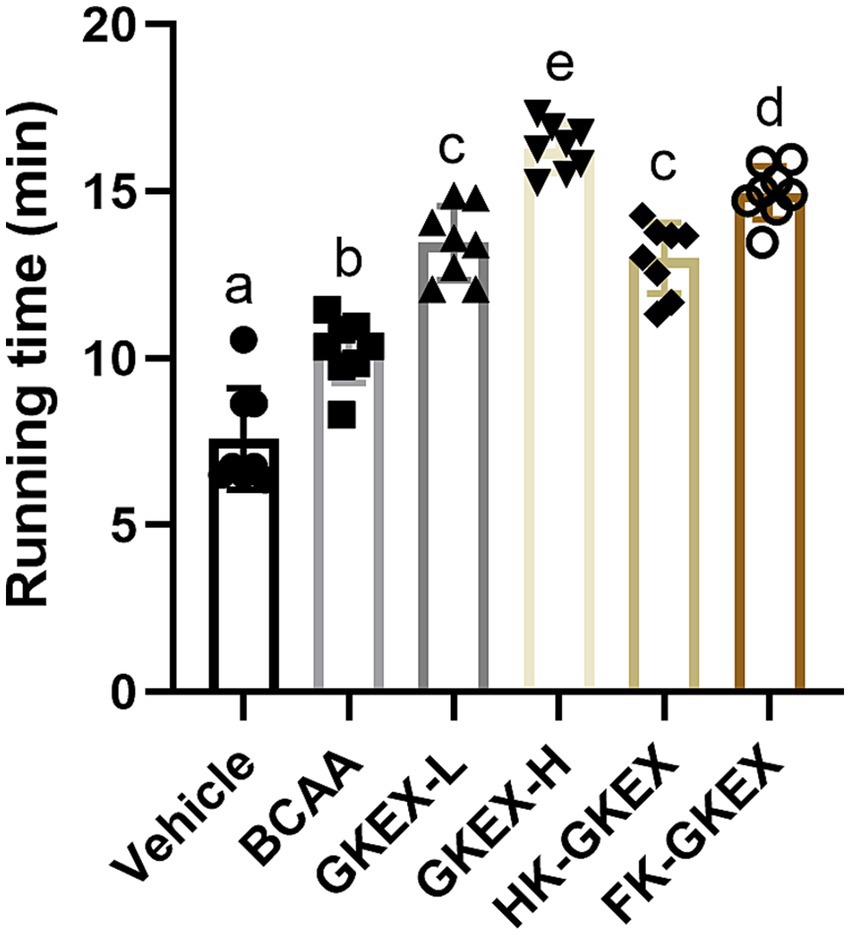
Figure 4. Effect of L. brevis GKEX supplementation on exhaustive running test. Data are expressed as mean ± SD (n = 8 mice per group). Different letters (a, b, c, d) indicate significant differences at p < 0.05, as determined by one-way ANOVA. GKEX-L: low-dosage L. brevis GKEX, GKEX-H: high-dosage L. brevis GKEX, HK-GKEX: heat-killed L. brevis GKEX, and FK-GKEX: freeze-killed L. brevis GKEX.
3.4 Effect of Levilactobacillus brevis GKEX supplementation on serum lactate levels in post 10-min swimming test and after 20-min rest
The serum lactate levels were measured before swimming, after 10 min of swimming, and after 20 min of rest, followed by swimming. The absolute values of lactate were recorded at different timepoints in Supplementary Table S3. Before swimming, there were no significant differences in serum lactate levels between the Vehicle, BCAA, GKEX-L, GKEX-H, HK-GKEX, and FK-GKEX groups. After 10 min of swimming, the lactate increase ratio was calculated based on the lactate levels before and 10 min after the exercise. Compared to the Vehicle group, the GKEX-H group showed a significant decrease of 1.2-fold (p = 0.0213) in the lactate increase rate (Figure 5A).
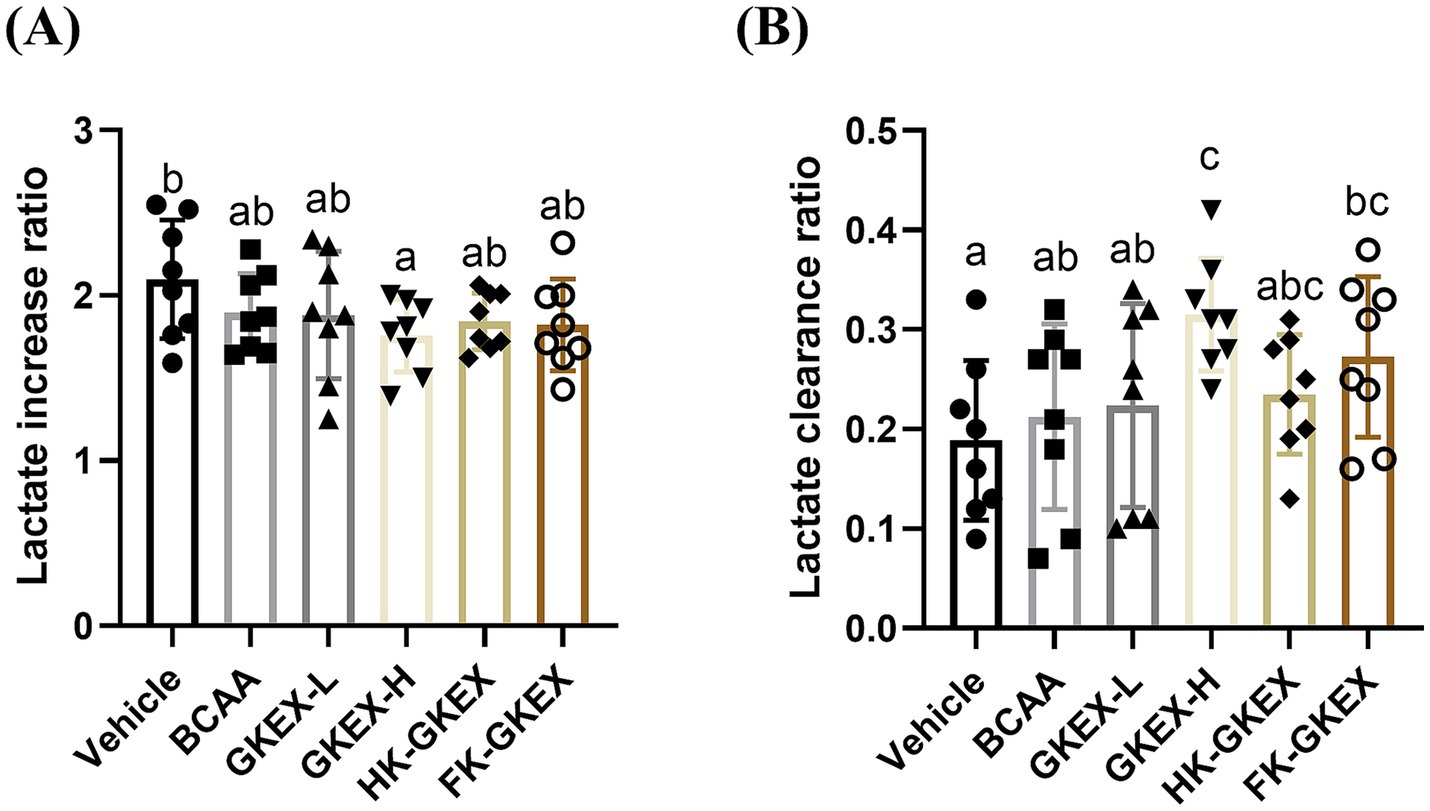
Figure 5. Effect of L. brevis GKEX supplementation on serum (A) lactate increase and (B) clearance ratio in post-10-min swimming test. The lactate increase ratio represents the ratio of the lactate level after exercise to that before exercise. The clearance ratio is calculated as the difference between the lactate level after swimming and that after 20 min of rest, divided by the lactate level after swimming. Data are expressed as mean ± SD (n = 8 mice per group). Different letters (a, b, c, d) indicate significant differences at p < 0.05, as determined by one-way ANOVA. GKEX-L: low-dosage L. brevis GKEX, GKEX-H: high-dosage L. brevis GKEX, HK-GKEX: heat-killed L. brevis GKEX, and FK-GKEX: freeze-killed L. brevis GKEX.
The lactate clearance ratio, determined following a 20-min rest period after exercise, was used to assess lactate recovery. Compared to the Vehicle group, GKEX-H and FK-GKEX groups significantly increased the lactate clearance ratio. Furthermore, compared to the BCAA group, the supplementation of the GKEX-H significantly increased the lactate clearance ratio by 1.48-fold (p = 0.0145) (Figure 5B).
3.5 Effect of Levilactobacillus brevis supplementation on blood urea nitrogen levels after a 90-min swimming test followed by a 60-min rest
As shown in Figure 6, BUN concentrations following a 90-min swimming test were measured in each group of mice. The BUN concentrations in the Vehicle, BCAA, GKEX-L, GKEX-H, HK-GKEX, and FK-GKEX groups showed no significant differences among the six groups.
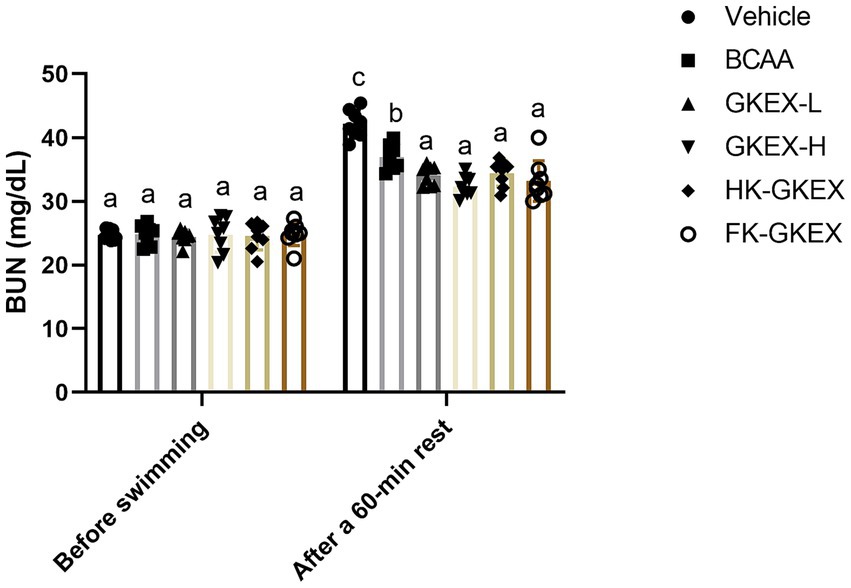
Figure 6. Effect of L. brevis GKEX supplementation on blood urea nitrogen levels after a 90-min exercise followed by a 60-min rest. Data are expressed as mean ± SD (n = 8 mice per group). Different letters (a, b, c, d) indicate significant differences at p < 0.05, as determined by one-way ANOVA. GKEX-L: low-dosage L. brevis GKEX, GKEX-H: high-dosage L. brevis GKEX, HK-GKEX: heat-killed L. brevis GKEX, and FK-GKEX: freeze-killed L. brevis GKEX.
Additionally, BUN concentrations after a 90-min swimming test followed by a 60-min rest were tested for each group of mice. Compared to the Vehicle group, supplementation with L. brevis GKEX significantly reduced BUN concentration. Furthermore, compared to the BCAA group, supplementation with GKEX-L, GKEX-H, HK-GKEX, and FK-GKEX significantly decreased BUN concentrations by 1.09-fold (p = 0.0094), 1.14-fold (p < 0.0001), 1.07-fold (p = 0.0217), and 1.11-fold (p = 0.0011), respectively.
3.6 Levilactobacillus brevis supplementation increases hepatic and muscular glycogen contents
Hepatic and muscular glycogen contents are shown in Figure 7. The hepatic glycogen content in the Vehicle, BCAA, GKEX-L, GKEX-H, HK-GKEX, and FK-GKEX groups was 1.45 ± 0.26, 1.66 ± 0.31, 2.05 ± 0.28, 2.54 ± 0.36, 1.99 ± 0.24, and 2.08 ± 0.19 (mg/g), respectively. Compared to the Vehicle group, the L. brevis GKEX group showed significantly increased hepatic glycogen content. In addition, compared to the BCAA group, the GKEX-L, GKEX-H, HK-GKEX, and FK-GKEX groups significantly increased hepatic glycogen content by 1.23-fold (p = 0.0074), 1.53-fold (p < 0.0001), 1.20-fold (p = 0.0227), and 1.25-fold (p = 0.0044), respectively. On the other hand, the muscular glycogen content in the Vehicle, BCAA, GKEX-L, GKEX-H, HK-GKEX, and FK-GKEX groups was 0.57 ± 0.07, 0.79 ± 0.06, 1.13 ± 0.87, 1.37 ± 0.08, 1.08 ± 0.10, and 1.25 ± 0.08 (mg/g), respectively. Compared to the Vehicle group, the GKEX-H and HK-GKEX groups showed significantly increased muscular glycogen content. Compared to the BCAA group, the GKEX-H group significantly increased muscular glycogen content by 1.38-fold (p = 0.0444).
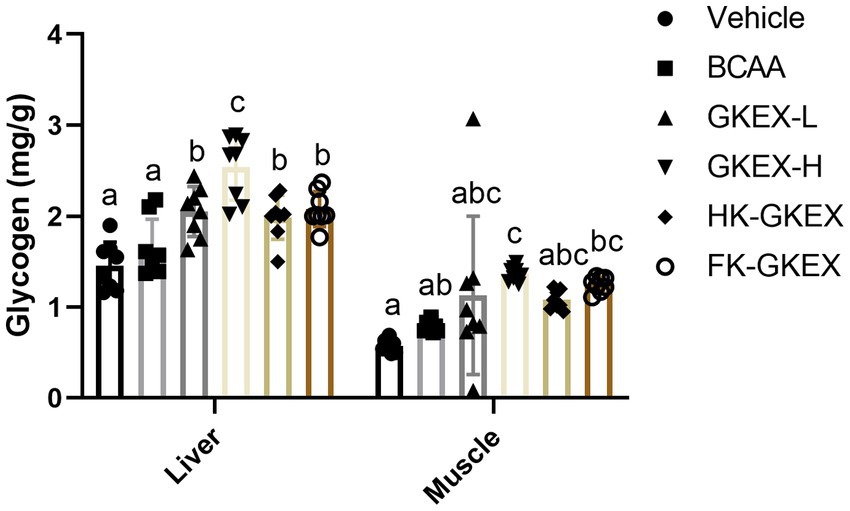
Figure 7. Effect of L. brevis GKEX supplementation on liver and muscular glycogen content. Mice were pretreated with vehicle, BCAA, GKEX-L, GKEX-H, HK-GKEX, and FK-GKEX for 28 days. Data are expressed as mean ± SD (n = 8 mice per group). Different letters (a, b, c, d) indicate significant differences at p < 0.05, as determined by one-way ANOVA. GKEX-L: low-dosage L. brevis GKEX, GKEX-H: high-dosage L. brevis GKEX, HK-GKEX: heat-killed L. brevis GKEX, and FK-GKEX: freeze-killed L. brevis GKEX.
3.7 Effect of Levilactobacillus brevis GKEX supplementation on gut microbiota
The heatmap resulting from LEfSe analysis shows the taxonomic groups in the intestines and their abundance distribution in each group. As shown in Figure 8A, the GKEX-L group showed higher proportions of Mollicutes (Class), Anaeroplasmatales (Order), Anaeroplasmataceae (Family), and Anaeroplasma (Genus). Furthermore, an LDA score above 2 is widely considered a threshold for biologically meaningful differences in microbiota analysis, assuming that it passes the statistical significance test (Supplementary Figure S1). The abundances of Roseburia, Blautia (Family Lachnospiraceae), and Eubacterium at the genus level and that of Christensenella minuta (Phylum Firmicutes) significantly increased in the GKEX-H group compared with the vehicle group. In addition, the genus Peptococcus in the HK-GKEX group was significantly increased, whereas the phylum Proteobacteria was significantly increased in the FK-GKEX group. Supplementation with different doses of live and dead bacteria, particularly high doses of live L. brevis GKEX, increased the taxa of the microbiome.
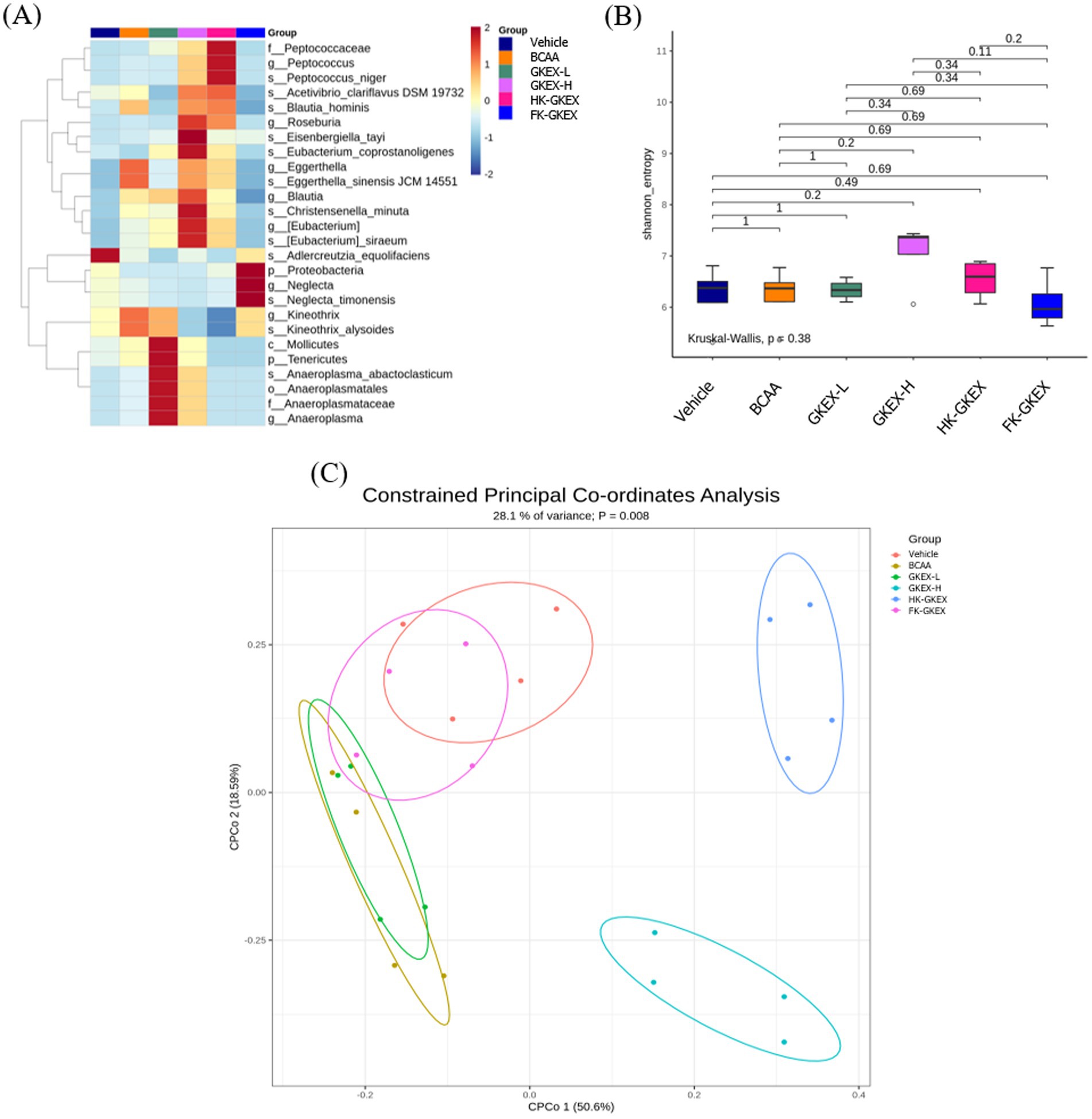
Figure 8. Effect of L. brevis supplementation on gut microbiota. (A) Linear discriminant analysis effect size (LEfSe) analysis of the abundance of the gut microbiota along grazing gradients. The X- and Y-axes of the thermal graph represent the experimental groups and species, respectively. p: phylum; c: class; f: family; o: order; g: genus; s: species. (B) The boxplot of alpha diversity using the Shannon diversity index. (C) Constrained principal coordinates analysis (CPCoA) based on Bray–Curtis distances of the gut microbiota profiles. Each point represents the microbial community of a sample, and the colors indicate the treatment groups. GKEX-L: low-dosage L. brevis GKEX, GKEX-H: high-dosage L. brevis GKEX, HK-GKEX: heat-killed L. brevis GKEX, and FK-GKEX: freeze-killed L. brevis GKEX.
Alpha diversity refers to the diversity of the microbial species within the experimental groups. The α-diversity of the gut microbiota, as measured by the Shannon index, showed no significant differences among the groups (p = 0.38). Pairwise comparisons also revealed no statistically significant differences (all p > 0.05), although a trend of increased diversity was observed in the GKEX-H and HK-GKEX groups (Figure 8B). Beta diversity was visualized by CPCoA (Figure 8C). Beta diversity measures the differences in microbial community composition between each group. BCAA and GKEX-L groups showed a high degree of overlap, indicating that their microbiota effects were similar and not markedly distinct. The FK-GKEX group overlapped partly with the Vehicle group and partly with the BCAA and GKEX-L groups, suggesting that its microbiota composition shared similarities with all three. In contrast, the GKEX-H and HK-GKEX groups were clearly separated from the others, indicating that their microbiota profiles were substantially different from both the Vehicle and other treatment groups. A clear separation between groups was observed, and PERMANOVA indicated a significant difference (p = 0.008) in community structure between groups.
4 Discussion
Probiotics can potentially promote health, exercise adaptation, and performance in athletes (22). A recent study has also indicated the potential of Lactobacilli in enhancing athletic performance (9). In our previous study, supplementation with L. brevis GKEX significantly improved forelimb grip strength and exercise endurance time in mice and maintained lower levels of blood lactate and blood urea nitrogen after exercise (11). In this study, Levilactobacillus brevis GKEX supplementation not only significantly improved forelimb grip strength and endurance running time but also demonstrated superior performance compared to both the vehicle and BCAA groups, particularly in lactate clearance, BUN reduction, and glycogen preservation. Moreover, unlike many studies that focus solely on live probiotics, this study highlights the beneficial effects of both live and inactivated GKEX formulations (HK-GKEX and FK-GKEX). The FK-GKEX group, in particular, revealed distinct microbiota profiles and performance outcomes, potentially due to preserved intracellular metabolites. These findings underscore the unique versatility and efficacy of GKEX across different preparation forms, which is rarely reported in previous probiotic-exercise studies. Most importantly, with the efficacy of L. brevis GKEX supplementation with the probiotic did not result in any abnormal changes in the weights and pathological sections of tissues and organs (Supplementary Table S4 and Supplementary Figure S2), liver function, kidney function, or blood lipid-related indices (Supplementary Table S5).
First, we evaluated the exercise performance-related tests. Forelimb grip strength was used to detect changes in motor function and muscle strength, while the exhaustive running test is a common way to measure fatigue-like behavior (17). BCAAs, as a positive control, might play a role in the central fatigue hypothesis by reducing the synthesis of brain serotonin (5-HT), which is associated with fatigue during prolonged exercise, thereby delaying the onset of fatigue symptoms (23, 24). Moreover, BCAAs may influence glycogen metabolism during prolonged exercise, potentially preserving liver and muscle glycogen levels (25). Surprisingly, the results revealed that ingestion of different types of L. brevis GKEX significantly enhanced both forelimb grip strength and exhaustive running time. This was evident not only when compared to the vehicle group but also when comparing the live L. brevis GKEX and FK-GKEX groups to the BCAA group (Figures 3, 4). This finding correlates with our prior human trials, where L. brevis GKEX supplementation combined with resistance exercise training resulted in increased muscle mass and grip strength (12); however, the underlying mechanism requires further investigation. According to reports, L. plantarum TWK10 (LP10) has also been shown to increase muscle mass and grip strength, enhance athletic performance, and further reduce physical fatigue (26). It is evident that Lactobacillus shows potential in enhancing athletic performance and alleviating exercise fatigue.
Lactate production plays a significant role in understanding metabolic responses to exercise and is frequently used as a fatigue marker in athletes (27, 28). During higher-intensity exercise, a significant increase occurs in lactate and hydrogen ion concentrations, leading to muscle acidification and subsequent fatigue (29). Another explanation is that the addition of lactate to muscles, both in vivo and in vitro, is associated with increased extracellular osmolarity. This causes water to exit the muscle fibers, resulting in increased intracellular ionic strength and the subsequent inhibition of force production (30). In our study, the ingestion of live L. brevis GKEX significantly reduced the lactate increase ratio. Furthermore, there was a significant increase in the lactate clearance ratio in the GKEX-H and FK-GKEX groups (Figure 5). Recent studies suggest that probiotic supplementation has the potential to remove and utilize blood lactate after exercise (22). The mechanism may involve lactate converting into acetyl-CoA, which is then transformed into butyryl-CoA by lactate-utilizing bacteria (31). This process is followed by the conversion of butyryl-CoA to butyrate and coenzyme A by the enzymes phosphotransbutyrylase and butyrate kinase, simultaneously generating adenosine triphosphate (ATP), thereby maintaining the host’s physiological performance during exercise (22). This suggests that live L. brevis GKEX has the potential to enhance metabolic capacity, delaying muscle cell inhibition and improving exercise endurance. In addition, a previous study has found that lactate from muscle enters the intestinal lumen through the bloodstream, and lactate serves as a carbon source for specific microbes, resulting in increased microbiome-derived short-chain fatty acids (SCFAs). These SCFAs, upon absorption by the host, enhance athletic performance (32). This occurrence could also explain the higher lactate clearance ratio observed after supplementation with L. brevis GKEX.
The elevated BUN levels indicate protein and amino acid decomposition, negatively impacting muscle contraction strength and contributing to fatigue. Thus, BUN serves as a critical biochemical marker of fatigue (33). As the exercise workload increases, BUN levels rise more significantly, leading to a slower recovery time. In our study, supplementation with different strains of L. brevis GKEX resulted in significantly lower BUN levels compared to the vehicle group as well as the BCAA group after prolonged exercise followed by an equivalent rest period (Figure 6). This indicates that supplementation with L. brevis GKEX can enhance the efficiency of physiological energy supply, thereby alleviating fatigue.
Glycogen, stored primarily in the liver and muscle tissues, serves as the main source of energy during exercise (16). Liver glycogen directly contributes to the release of glucose into the bloodstream, providing energy for the entire body, while muscle glycogen primarily serves as an energy substrate, converting it into ATP during exercise (34). We found that supplementation with different types of L. brevis GKEX, particularly the live GKEX group, significantly increased liver and muscular glycogen content (Figure 7). The mechanism may be related to the production of SCFAs such as propionate and butyrate by gut bacteria, which can serve as energy sources for liver and muscle cells, maintaining stable blood glucose levels to improve endurance performance during exercise (32, 35). Additionally, numerous scientific studies support the efficacy of probiotics in regulating the intestinal microbiome and influencing the levels of SCFAs in the colon (36). Specifically, lactate-utilizing bacteria can convert lactate into butyrate (37). Therefore, this process can regulate the storage and utilization of bodily energy, thereby enhancing physical endurance and exercise performance.
In this study, microbial diversity (alpha diversity) of the GKEX-H and HK-GKEX groups was elevated, and differences in species composition among groups were significant, indicating that different L. brevis GKEX processing methods influence the diversity of the measured community. Multiple studies have noted that an increase in fecal SCFAs produced by the gut microbiota facilitates the metabolism of carbohydrates and proteins, which further enhances skeletal muscle glucose uptake and lean muscle mass, contributing to improvements in endurance exercise capacity and muscular strength (38–40). However, we did not include the direct measurement of SCFAs in the current study, which could not elucidate the role of microbial metabolites in mediating the physiological effects of GKEX supplementation. Nevertheless, the common SCFA-producing bacteria were upregulated, including the phylum Firmicutes, in particular Roseburia, Blautia spp., and Eisenbergiella tayi of the family Lachnospiraceae, Christensenella minuta of the family Christensenellaceae, and Peptococcus of the family Peptococcaceae (41–45) after different doses and formulations of L. brevis GKEX were used. The GKEX-H and HK-GKEX groups demonstrated similar microbiota patterns, with increased levels of Peptococcus, Roseburia, Blautia, Eisenbergiella tayi, and Eubacterium (Figure 8). The diversity of key SCFA-producing bacteria in the live L. brevis GKEX-H group was higher than that in the dead L. brevis HK-GKEX group, which possibly resulted in better exercise performance and fatigue resistance in the GKEX-H group. Furthermore, the freeze–thaw process in the FK-GKEX group may cause the secretion of intracellular metabolites, such as enzymes, after cell membrane rupture, which does not appear in other groups. Additionally, because there is no heat treatment, some substances that are different from those in dead bacteria may be preserved. This resulted in a different microbiota pattern compared to that of the HK-GKEX and FK-GKEX groups, with an enhanced abundance of the phylum Proteobacteria, a potential butyrate producer (46, 47).
Overall, regardless of the type of L. brevis GKEX, live cells, cell debris, and cell lysates had beneficial effects on exercise performance. Compared to strains like L. plantarum TWK10 and L. casei Shirota, whose effects are primarily limited to muscle strength or immune modulation, L. brevis GKEX demonstrates a more comprehensive impact, from energy metabolism to microbiota modulation and systemic fatigue markers. Furthermore, SCFA levels should be measured to clarify the relationship between gut microbiota and exercise duration. Although L. brevis GKEX demonstrated positive effects on fatigue alleviation and exercise performance in animal models, the underlying mechanisms remain to be elucidated. In particular, genomic and metabolomic profiling of this strain has not yet been performed. Future studies should include omics-based approaches to clarify the metabolic pathways and functional genes involved in GKEX’s strain-specific ergogenic effects.
5 Conclusion
Our study evaluated the effects of supplementing with L. brevis GKEX on enhancing exercise performance and reducing fatigue. We found that after continuous supplementation with live or dead L. brevis GKEX for 4 weeks, mice showed improved endurance and aerobic performance during exercise, accompanied by a reduction in lactate production, even better than that in the BCAA group. Additionally, there was an enhanced lactate clearance ratio during the rest periods post-exercise and decreased BUN levels. Furthermore, there was increased glycogen content in the liver and muscles, thereby improving the physiological energy supply efficiency. Finally, supplementation promoted the growth of key SCFA-producing bacteria in the gut. In summary, we hypothesize that during exercise, the lactate produced enters the intestine via the bloodstream. The increased SCFA-producing bacteria, enhanced by L. brevis GKEX supplementation, can convert the lactate into SCFAs, thereby reducing exercise fatigue in the host and improving exercise performance. In this study, the modulation of fatigue-related biomarkers and energy metabolism, along with alterations in gut microbiota composition, implies an indirect influence via the gut-organ axis. Further investigations, such as targeted metabolomics and receptor pathway analysis, are needed to determine whether GKEX-derived compounds directly engage the host tissues.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here in the NCBI Sequence Read Archive (SRA) under BioProject accession number PRJNA1338346.
Ethics statement
The animal study was approved by the Institutional Animal Care and Use Committee of National Taiwan Sport University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
T-CL: Software, Writing – original draft, Formal analysis, Visualization, Data curation. C-CC: Writing – review & editing, Supervision, Project administration, Conceptualization, Resources. Y-ST: Writing – review & editing, Validation. S-WL: Validation, Writing – review & editing. C-CH: Supervision, Writing – review & editing, Conceptualization, Resources, Project administration. Y-JH: Software, Supervision, Investigation, Writing – review & editing, Resources, Formal analysis, Methodology, Conceptualization, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the University–Industry Cooperation Fund, National Taiwan Sport University, Taoyuan, Taiwan (NTSU No. 1111032).
Conflict of interest
T-CL, C-CC, Y-ST, and S-WL was employed by Biotech Research Institute, Grape King Bio Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2025.1625645/full#supplementary-material
Footnotes
References
1. González-Izal, M, Malanda, A, Gorostiaga, E, and Izquierdo, M. Electromyographic models to assess muscle fatigue. J Electromyogr Kinesiol. (2012) 22:501–12. doi: 10.1016/j.jelekin.2012.02.019
2. Torri, F, Lopriore, P, Montano, V, Siciliano, G, Mancuso, M, and Ricci, G. Pathophysiology and Management of Fatigue in neuromuscular diseases. Int J Mol Sci. (2023) 24:24055005. doi: 10.3390/ijms24055005
3. Pandey, KR, Naik, SR, and Vakil, BV. Probiotics, prebiotics and synbiotics-a review. J Food Sci Technol. (2015) 52:7577–87. doi: 10.1007/s13197-015-1921-1
4. Velez, EM, Galdeano, CM, Carmuega, E, Weill, R, Bonet, MEB, and Perdigón, G. Probiotic fermented milk consumption modulates the allergic process induced by ovoalbumin in mice. Br J Nutr. (2015) 114:566. doi: 10.1017/S0007114515001981
5. Ge, Q, Yang, B, Liu, R, Jiang, D, Yu, H, Wu, M, et al. Antioxidant activity of Lactobacillus plantarum NJAU-01 in an animal model of aging. BMC Microbiol. (2021) 21:182. doi: 10.1186/s12866-021-02248-5
6. Huang, R, Wu, F, Zhou, Q, Wei, W, Yue, J, Xiao, B, et al. Lactobacillus and intestinal diseases: mechanisms of action and clinical applications. Microbiol Res. (2022) 260:127019. doi: 10.1016/j.micres.2022.127019
7. Escamilla, J, Lane, MA, and Maitin, V. Cell-free supernatants from probiotic Lactobacillus casei and Lactobacillus rhamnosus GG decrease colon cancer cell invasion in vitro. Nutr Cancer. (2012) 64:871. doi: 10.1080/01635581.2012.700758
8. Choi, HS, Jang, Y-J, Oh, I, Chung, JH, and Moon, JS. Limosilactobacillus reuteri ID-D01 improves exercise performance and reduces muscle fatigue in C57BL/6 mice through regulation of oxidative capacity. J Funct Foods. (2024) 116:106125. doi: 10.1016/j.jff.2024.106125
9. Cheng, YC, Lee, CC, Lee, MC, Hsu, HY, Lin, JS, Huang, CC, et al. Effects of heat-killed Lactiplantibacillus plantarum TWK10 on exercise performance, fatigue, and muscle growth in healthy male adults. Physiol Rep. (2023) 11:e15835. doi: 10.14814/phy2.15835
10. Lee, M-C, Hsu, Y-J, Ho, H-H, Hsieh, S-H, Kuo, Y-W, Sung, H-C, et al. Lactobacillus salivarius subspecies salicinius SA-03 is a new probiotic capable of enhancing exercise performance and decreasing fatigue. Microorganisms. (2020) 8:545. doi: 10.3390/microorganisms8040545
11. Chen, Y, Tsai, Y, Lin, S, and Wu, W. Evaluation of lactic acid Bacteria with anti-fatigue effects. Hans Journal of Food and Nutrition Science. (2022) 11:322–31. doi: 10.12677/hjfns.2022.114037
12. Lee, M-C, Hsu, Y-J, Ho, C-S, Tsai, Y-S, Chen, C-C, and Huang, C-C. Supplementation with Lactiplantibacillus brevis GKEX combined with resistance exercise training improves muscle mass, strength performance, and body fat condition in healthy humans. Foods. (2024) 13:1030. doi: 10.3390/foods13071030
13. Cuevas-González, P, Liceaga, A, and Aguilar-Toalá, J. Postbiotics and paraprobiotics: from concepts to applications. Food Res Int. (2020) 136:109502. doi: 10.1016/j.foodres.2020.109502
14. Fernstrom, JD. Branched-chain amino acids and brain function. J Nutr. (2005) 135:1539S–46S. doi: 10.1093/jn/135.6.1539S
15. Nair, AB, and Jacob, S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. (2016) 7:27. doi: 10.4103/0976-0105.177703
16. Huang, C-C, Hsu, M-C, Huang, W-C, Yang, H-R, and Hou, C-C. Triterpenoid-rich extract from Antrodia camphorata improves physical fatigue and exercise performance in mice. Evid Based Complement Altern Med. (2012) 2012:364741. doi: 10.1155/2012/364741
17. Dougherty, JP, Springer, DA, and Gershengorn, MC. The treadmill fatigue test: a simple, high-throughput assay of fatigue-like behavior for the mouse. J Visual Exper. (2016) 111:54052. doi: 10.3791/54052PMC4927751
18. Hsu, Y-J, Huang, W-C, Chiu, C-C, Liu, Y-L, Chiu, W-C, Chiu, C-H, et al. Capsaicin supplementation reduces physical fatigue and improves exercise performance in mice. Forum Nutr. (2016) 8:648. doi: 10.3390/nu8100648
19. Wen-Ching, H, Wan-Chun, C, Hsiao-Li, C, Deh-Wei, T, Zon-Min, L, Li, W, et al. Effect of curcumin supplementation on physiological fatigue and physical performance in mice. Forum Nutr. (2015) 7:905–21. doi: 10.3390/nu7020905
20. Chamberland, V, and Rioux, P. Not only students can express alcohol dehydrogenase: goldfish can too! Adv Physiol Educ. (2010) 34:222–7. doi: 10.1152/advan.00088.2009
21. Chun-Ching, H, Chia-Chen, L, Jung-Piao, T, Chin-Lin, H, and I-Shiung, C. Effects of oral resveratrol supplementation on glycogen replenishment and mitochondria biogenesis in exercised human skeletal muscle. Forum Nutr. (2020) 12:3721. doi: 10.3390/nu12123721
22. Jäger, R, Mohr, AE, Carpenter, KC, Kerksick, CM, Purpura, M, Moussa, A, et al. International society of sports nutrition position stand: probiotics. J Int Soc Sports Nutr. (2019) 16:1–44. doi: 10.1186/s12970-019-0329-0
23. Davis, JM. Carbohydrates, branched-chain amino acids, and endurances: the central fatigue hypothesis. Int J Sport Nutr Exerc Metab. (1995) 5:S29–38. doi: 10.1123/ijsn.5.s1.s29
24. Falavigna, G, Alves,, Rogero, MM, Pires, IS, Pedrosa, RG, Martins,, et al. Effects of diets supplemented with branched-chain amino acids on the performance and fatigue mechanisms of rats submitted to prolonged physical exercise. Forum Nutr. (2012) 4:1767. doi: 10.3390/nu4111767
25. Shimomura, Y, Murakami, T, Nakai, N, Nagasaki, M, Obayashi, M, Li, Z, et al. Suppression of glycogen consumption during acute exercise by dietary branched-chain amino acids in rats. J Nutr Sci Vitaminol. (2000) 46:71–7. doi: 10.3177/jnsv.46.71
26. Chen, Y-M, Wei, L, Chiu, Y-S, Hsu, Y-J, Tsai, T-Y, Wang, M-F, et al. Lactobacillus plantarum TWK10 supplementation improves exercise performance and increases muscle mass in mice. Forum Nutr. (2016) 8:205. doi: 10.3390/nu8040205
27. Goodwin, ML, Harris, JE, Hernández, A, and Gladden, LB. Blood lactate measurements and analysis during exercise: a guide for clinicians. J Diabetes Sci Technol. (2007) 1:558–69. doi: 10.1177/193229680700100414
28. Takeda, R, Nonaka, Y, Kakinoki, K, Miura, S, Kano, Y, and Hoshino, D. Effect of endurance training and PGC-1α overexpression on calculated lactate production volume during exercise based on blood lactate concentration. Sci Rep. (2022) 12:1635. doi: 10.1038/s41598-022-05593-1
29. Hobson, RM, Saunders, B, Ball, G, Harris, R, and Sale, C. Effects of β-alanine supplementation on exercise performance: a meta-analysis. Amino Acids. (2012) 43:25–37. doi: 10.1007/s00726-011-1200-z
30. Allen, DG, Lamb, GD, and Westerblad, H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. (2008) 88:287–332. doi: 10.1152/physrev.00015.2007
31. Duncan, SH, Louis, P, and Flint, HJ. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl Environ Microbiol. (2004) 70:5810–7. doi: 10.1128/AEM.70.10.5810-5817.2004
32. Scheiman, J, Luber, JM, Chavkin, TA, MacDonald, T, Tung, A, Pham, L-D, et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat Med. (2019) 25:1104–9. doi: 10.1038/s41591-019-0485-4
33. Hsu, Y-J, Huang, W-C, Lin, J-S, Chen, Y-M, Ho, S-T, Huang, C-C, et al. Kefir supplementation modifies gut microbiota composition, reduces physical fatigue, and improves exercise performance in mice. Forum Nutr. (2018) 10:862. doi: 10.3390/nu10070862
34. Jensen, J, Rustad, PI, Kolnes, AJ, and Lai, Y-C. The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise. Front Physiol. (2011) 2:112. doi: 10.3389/fphys.2011.00112
35. Zhang, W-Q, Zhao, T-T, Gui, D-K, Gao, C-L, Gu, J-L, Gan, W-J, et al. Sodium butyrate improves liver glycogen metabolism in type 2 diabetes mellitus. J Agric Food Chem. (2019) 67:7694–705. doi: 10.1021/acs.jafc.9b02083
36. Markowiak-Kopeć, P, and Śliżewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Forum Nutr. (2020) 12:1107. doi: 10.3390/nu12041107
37. Tao, Y, Hu, X, Zhu, X, Jin, H, Xu, Z, Tang, Q, et al. Production of butyrate from lactate by a newly isolated Clostridium sp. BPY5. Appl Biochem Biotechnol. (2016) 179:361–74. doi: 10.1007/s12010-016-1999-6
38. Frampton, J, Murphy, KG, Frost, G, and Chambers, ES. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat Metab. (2020) 2:840–8. doi: 10.1038/s42255-020-0188-7
39. Han, J-H, Kim, I-S, Jung, S-H, Lee, S-G, Son, H-Y, and Myung, C-S. The effects of propionate and valerate on insulin responsiveness for glucose uptake in 3T3-L1 adipocytes and C2C12 myotubes via G protein-coupled receptor 41. PLoS One. (2014) 9:9. e95268. doi: 10.1371/journal.pone.0095268
40. Lahiri, S, Kim, H, Garcia-Perez, I, Reza, MM, Martin, KA, Kundu, P, et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci Transl Med. (2019) 11:11. eaan5662. doi: 10.1126/scitranslmed.aan5662
41. Amir, I, Bouvet, P, Legeay, C, Gophna, U, and Weinberger, A. Eisenbergiella tayi gen. Nov., sp. nov., isolated from human blood. Int J Syst Evol Microbiol. (2014) 64:907. doi: 10.1099/ijs.0.057331-0
42. Estaki, M, Pither, J, Baumeister, P, Little, JP, Gill, SK, Ghosh, S, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome. (2016) 4:42. doi: 10.1186/s40168-016-0189-7
43. Ignatyeva, O, Tolyneva, D, Kovalyov, A, Matkava, L, Terekhov, M, Kashtanova, D, et al. Christensenella minuta, a new candidate next-generation probiotic: current evidence and future trajectories. Front Microbiol. (2024) 14:1241259. doi: 10.3389/fmicb.2023.1241259
44. Rodríguez-García, A, Arroyo, A, García-Vicente, R, Morales, ML, Gómez-Gordo, R, Justo, P, et al. Short-chain fatty acid production by gut microbiota predicts treatment response in multiple myeloma. Clin Cancer Res. (2024) 30:904–17. doi: 10.1158/1078-0432.CCR-23-0195
45. Wang, Z, and Qi, Q. Gut microbial metabolites associated with HIV infection. Future Virol. (2019) 14:335–47. doi: 10.2217/fvl-2019-0002
46. Vital, M, Howe, AC, and Tiedje, JM. Revealing the bacterial butyrate synthesis pathways by analyzing (meta) genomic data. MBio. (2014) 5:2e00889. doi: 10.1128/mbio.00889-14
47. Wang, G, Xia, Y, Cui, J, Gu, Z, Song, Y, Chen, YQ, et al. The roles of moonlighting proteins in bacteria. Curr Issues Mol Biol. (2014) 16:15–22. doi: 10.21775/cimb.016.015
Glossary
L. brevis - Levilactobacillus brevis
CFU - colony-forming units
HK-GKEX - Heat-killed GKEX
FK-GKEX - Freeze-killed GKEX
IACUC of NTSU - Institutional Animal Care and Use Committee of National Taiwan Sport University
BCAA - Branched-chain amino acids
GKEX-L - Low-dosage L. brevis GKEX
GKEX-H - High-dosage L. brevis GKEX
BUN - Blood urea nitrogen
GOT - Glutamic oxaloacetic transaminase
GPT - Glutamic pyruvic transaminase
CK - Creatine kinase
CREA - Creatinine
UA - Uric Acid
TC - Total Cholesterol
TG - Triglyceride
GLU - Glucose
ALB - Albumin
LEfSe - Linear discriminant analysis effect size
ANOVA - Analysis of variance
SCFAs - short-chain fatty acids
Keywords: Levilactobacillus brevis GKEX, exercise performance, fatigue resistance, lactate, blood urea nitrogen, short-chain fatty-acid-producing bacteria
Citation: Lin T-C, Chen C-C, Tsai Y-S, Lin S-W, Huang C-C and Hsu Y-J (2025) Effects of Levilactobacillus brevis GKEX supplementation on exercise performance and fatigue resistance in mice. Front. Nutr. 12:1625645. doi: 10.3389/fnut.2025.1625645
Edited by:
David Christopher Nieman, Appalachian State University, United StatesReviewed by:
Thiruppathi Govindhan, Bharathiar University, IndiaFu Jian, Yangzhou University, China
Copyright © 2025 Lin, Chen, Tsai, Lin, Huang and Hsu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi-Ju Hsu, cnVieTc4MDIwMkBudHN1LmVkdS50dw==
 Tzu-Chun Lin
Tzu-Chun Lin Chin-Chu Chen
Chin-Chu Chen You-Shan Tsai1
You-Shan Tsai1 Chi-Chang Huang
Chi-Chang Huang Yi-Ju Hsu
Yi-Ju Hsu