- 1Department of Pharmacy, Shaoxing People’s Hospital, Shaoxing, China
- 2School of Medicine, Shaoxing University, Shaoxing, Zhejiang, China
- 3State Key Laboratory of Genetic Engineering, Department of Pharmaceutical Sciences, School of Life Sciences, Fudan University, Shanghai, China
- 4MOE Engineering Research Center of Gene Technology, School of Life Sciences, Fudan University, Shanghai, China
Numerous studies have demonstrated an association between high-sodium diet and the incidence of ischemic heart disease (IHD). This study aims to assess the global impact of IHD associated with diet high in sodium from 1990 to 2021. We retrieved relevant data from the Global Burden of Disease Study 2021 (GBD 2021). The data encompassed the number of IHD-related deaths due to high-sodium intake, age-standardized mortality rates (ASMR), disability-adjusted life years (DALYs), and age-standardized DALYs rates (ASDR), all of which were estimated using GBD’s statistical model. Subsequently, we calculated the burden of IHD associated with high-sodium intake for each country and territory, stratified by age, sex, and socio-demographic index (SDI). The Bayesian age-period-cohort (BAPC) model was used to predict future trends of the IHD burden up to 2035. We discovered that excessive dietary sodium intake is associated with an elevated global burden of IHD. Although ASMR and ASDR have declined over time, this situation continues to impede global socioeconomic progress, and this trend might be associated with population growth and aging. These paradoxical trends underscore the urgent necessity for public health policymakers to prioritize the development of sodium reduction strategies customized to regional epidemiological patterns and gender-specific risk profiles.
Introduction
Ischemic heart disease (IHD) is one of the leading causes of high global incidence and mortality rates and represents the most burdensome type of cardiovascular disease (CVD) (1). It is a complex disease characterized by coronary artery stenosis, which results in myocardial ischemia, hypoxia, and even necrosis. In 2019 alone, IHD caused 9.14 million deaths (2, 3). The burden of IHD on sustainable development in the 21st century is significant. Despite advancements in treatment strategies and preventive measures, IHD remains a major public health challenge for countries and regions (4). The 2019 Global Burden of Disease (GBD) study estimated that there were approximately 197 million cases of IHD worldwide in 2019 (5). It is noteworthy that the attributable burden data reported in the GBD are derived from a statistical synthesis of surveys, hospital records, death certificates, and literature-based relative risks, rather than direct counts.
The influence of dietary risk factors on CVD has received attention, and their importance as a major factor was widely acknowledged (6). High-sodium diet has emerged as a significant risk factor associated with various health complications, and mounting evidence indicates a significant association between excessive sodium intake and the onset and progression of CVD (7, 8). As per the recommendations of the World Health Organization (WHO), the daily dietary sodium intake of healthy adults should be limited to less than 2 g [WHO Sodium Reduction Fact Sheet (2025)] (9). Existing studies have demonstrated that diet high in sodium (DHIS) is associated with elevated blood pressure and may be a significant contributing factor to the increased risk of CVD-related mortality in adults (10). Therefore, the high-sodium diet may substantially elevate the risk of developing IHD.
In this study, we conducted a comprehensive analysis of the GBD database to assess the global burden of IHD associated with diet high in sodium from 1990 to 2021. This study aims to establish an evidence-based framework for policymakers and public health stakeholders to formulate targeted interventions, offering novel perspectives for optimizing prevention strategies and therapeutic approaches in IHD management.
Materials and methods
Study data
This study utilized data on ischemic heart disease mortality and DALYs associated with diet high in sodium from 1990 to 2021, obtained from the Global Health Data Exchange (GHDx) query tool,1 based on gender, age, region, and country. This study classified 204 countries and territories into five distinct levels according to their Socio-Demographic Index (SDI) as defined by GBD: high, high-middle, middle, low-middle, and low SDI. We calculated the SDl through several social factors, with the fertility rate of the population aged <25 years, the education level of the population aged >15 years, and per capita income. Based on geographic continuity, the world was divided geographically into 21 distinct GBD regions, such as Western Europe and East Asia. Utilizing the latest epidemiological data and refined standardized methods, the GBD 2021 study provides a comprehensive assessment of health loss associated with 371 diseases, injuries, and impairments, as well as 87 risk factors in 204 nations and territories (11).
This study is based on data from the GBD 2021 study and employs a comparative risk assessment (CRA) framework to estimate the attributable burden of ischemic heart disease (IHD) due to diet high in sodium. The definition of IHD employed in this study adheres to the International Classification of Diseases (ICD). It encompassed corresponding diagnostic codes within this classification: I20-I21.6, I21.9- I25.9 (11). Diet high in sodium is defined as a daily sodium intake exceeding the theoretical minimum risk exposure level (TMREL), as an average 24-h urinary sodium excretion of 1–5 g/day. This study is from multiple data sources, including dietary surveys, urinary sodium monitoring, and relevant epidemiological studies, were integrated to estimate sodium intake exposure distributions across regions, ages, and genders. A spatio-temporal gaussian process regression (ST-GPR) model is used for spatial and temporal estimation. The population attributable fraction (PAF) is used to calculate the burden of IHD associated with diet high in sodium exposure above the TMREL.
Statistical analysis
The burden of ischemic heart disease associated with diet high in sodium was assessed using metrics including numbers of mortality and DALYs, the age-standardized mortality rate (ASMR), age-standardized DALYs rate (ASDR). DALYs were computed as the sum of the years lived with disability and the years of life lost. According to the age group construction of the standard population, the ASR (per 100, 000 population) were calculated using the following formula:
(ai refers to the incidence of the ith age group. wi denotes the number of persons (or weight) in the same age subgroup i of the assigned reference standard population).
Estimated annual percentage change (EAPC) is used to estimate the trends of the ASRs, and it quantitatively calculates the average annual rate of change of ASR for a specified period (12), which follows this formula, i.e., y = α + βx + ε, where y = ln (ASR), and x = the calendar year. The EAPC calculation formula, 100 × (exp(β) −1), and its 95% confidence intervals (CI) can also be calculated from the linear regression model.
In this study, we employed a Bayesian age–period–cohort (BAPC) modeling framework to estimate and project temporal trends in disease burden. Consistent with the classical APC approach, temporal variation was decomposed into additive contributions from age, period, and birth cohort effects. To ensure smoothness of the estimated effects and enhance predictive performance, we specified second-order random walk (RW2) priors for each temporal dimension, under the assumption that changes between adjacent points are gradual and temporally correlated. Parameter estimation was conducted using integrated nested Laplace approximation (INLA). The model rests on the following key assumptions: (1) age, period, and cohort effects evolve smoothly over time; (2) the effects are additive and independent; and (3) observed historical patterns can be extrapolated into the projection period in the absence of major unforeseen shocks (13, 14).
All data analyses were performed using the open-source software R (version 4.3.3). Statistical significance was set at P < 0.05.
Results
The burden of ischemic heart disease associated with diet high in sodium at global level
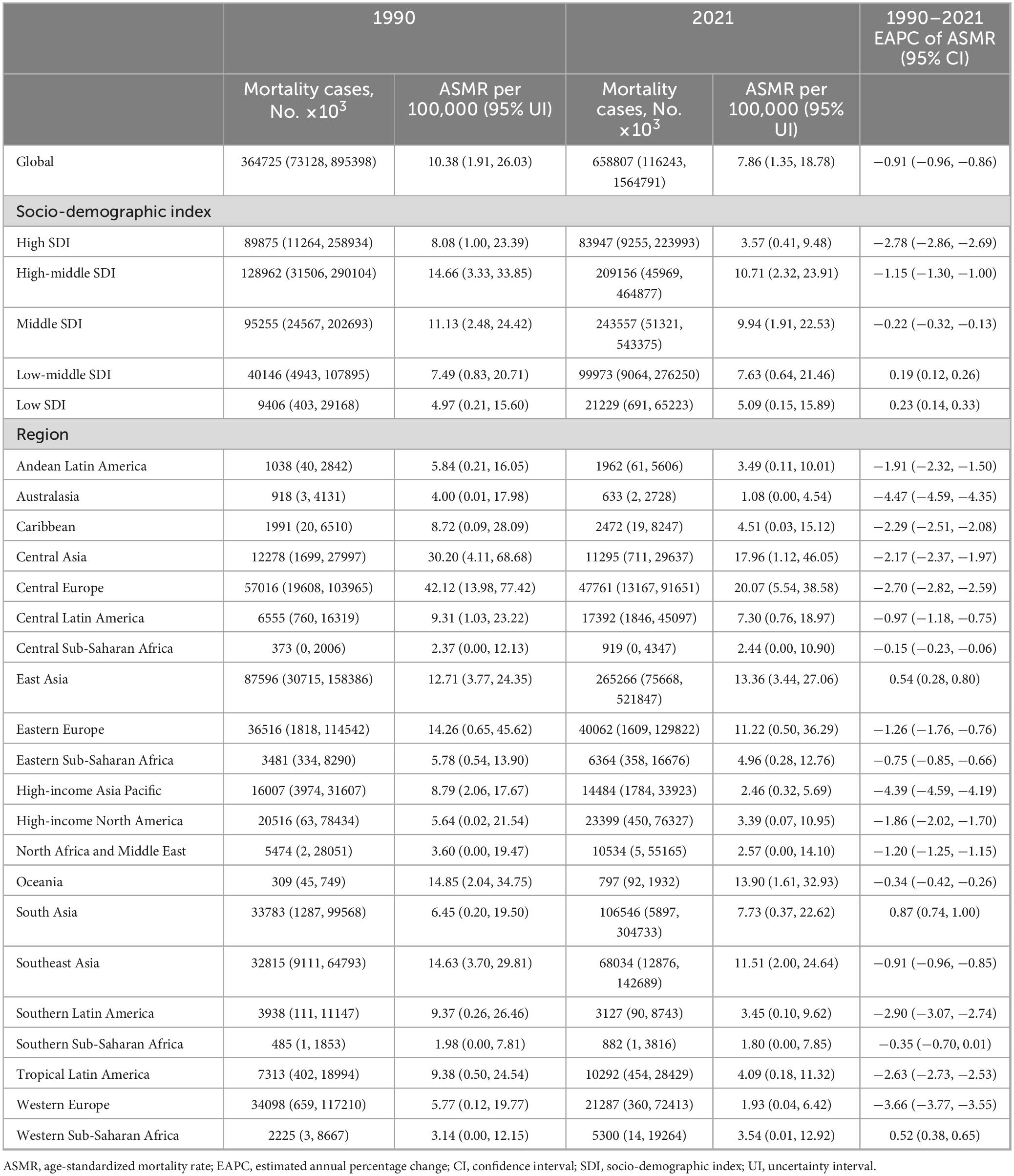
Between 1990 and 2021, the global mortality cases of ischemic heart disease associated with diet high in sodium increased by 81%, rising from 364725 (95% uncertainty interval (UI): 73128–895398) to 658807 (95% UI: 116243–1564791). The age-standardized mortality rate (ASMR) of IHD associated with diet high in sodium decreased by 24%, dropping from 10.38 per 100 000 population (95% UI: 1.91–26.03) in 1990 to 7.86 per 100 000 population (95% UI: 1.35–18.78) in 2021, with an estimated annual percentage change (EAPC) of −0.91 (95% confidence interval (CI): −0.96 to −0.86) (Table 1).
Similarly, disability-adjusted life years (DALYs) associated with diet high in sodium for IHD increased by 70% between 1990 and 2021, rising from 8296172 (95% UI: 1864218–19526026) to 14079694 (95% UI: 2844791–32526250). The age-standardized DALYs rate (ASDR) decreased by 24%, dropping from 213.88 per 100 000 population (95% UI: 46.15–513.06) in 1990 to 163.37 per 100 000 population (95% UI: 32.53–379.07) in 2021, with an EAPC of −0.90 (95% CI: −0.96 to −0.84) (Table 2).
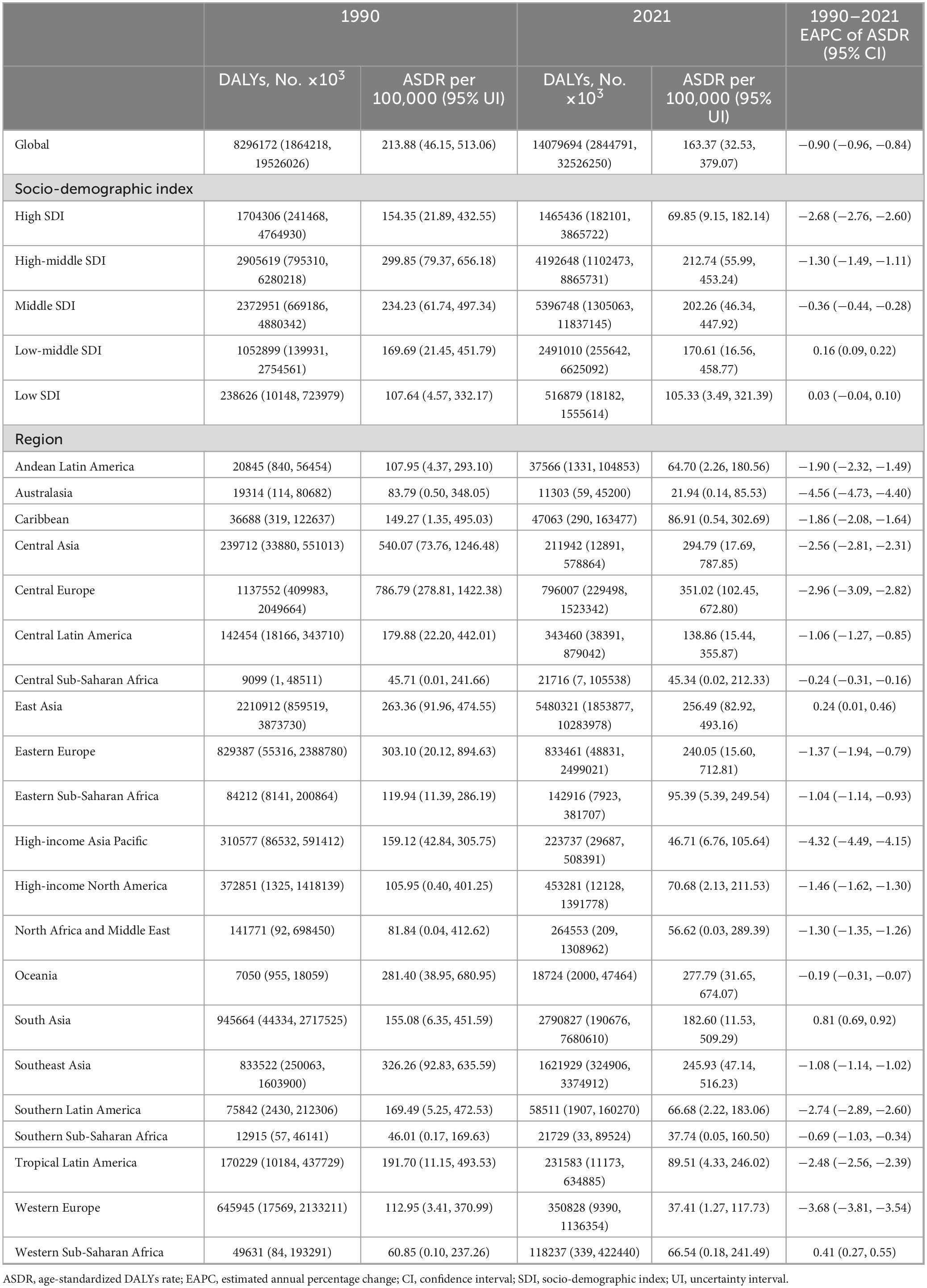
Table 2. Disability-adjusted life years (DALYs) and ASDR of IHD in 1990 and 2021, and temporal trends.
The burden of ischemic heart disease associated with diet high in sodium at national level
In 2021, at the national level, Nauru had the highest ASMR (35.60 per 100 000 population; 95% UI: 3.66–286.87), followed by Montenegro (32.63 per 100 000 population; 95% UI: 9.19–61.84) and Bulgaria (30.76 per 100 000 population; 95% UI: 8.67–58.33). Spain had the lowest ASMR (0.78 per 100 000 population; 95% UI: 0.01–3.21), closely followed by Australia (0.89 per 100 000 population; 95% UI: 0.002–4.02) and San Marino (0.92 per 100 000 population; 95% UI: 0.007–3.21). In 2021, Nauru and Bulgaria had the highest ASDR (18.29 per 100 000 population, 95% UI: 0.08–75.28; 18.41 per 100 000 population, 95% UI: 0.21–59.99) (Figures 1A, B).
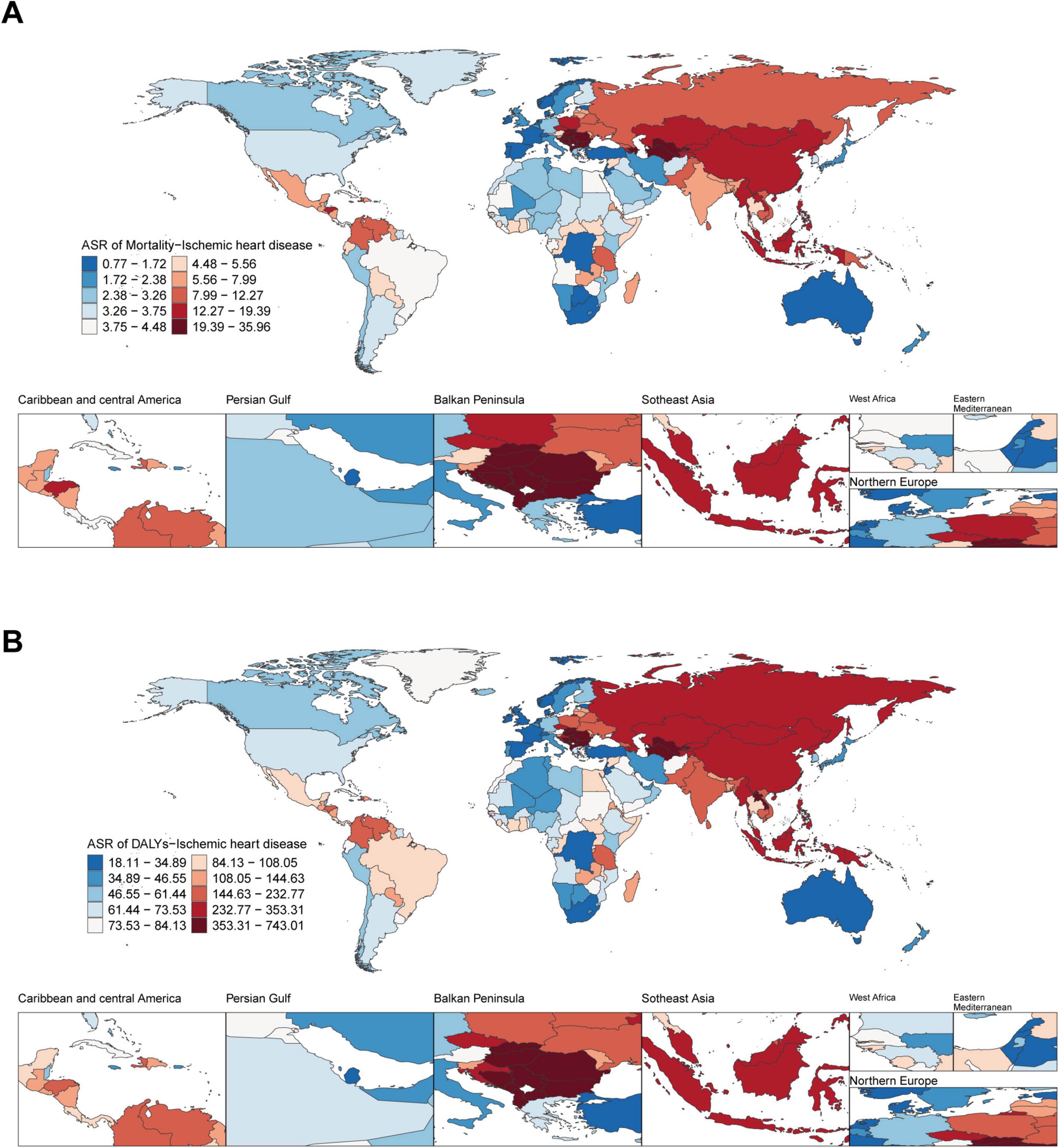
Figure 1. Global disease burden of ischemic heart disease associated with diet high in sodium in 204 countries and territories. (A) ASMR of ischemic heart disease in 2021; (B) ASDR of ischemic heart disease in 2021.
From 1990 to 2021, at the national level, ASMR and ASDR showed a downward trend in most countries and territories. Pakistan had the highest growth rate of ASMR (EAPC: 2.56; 95% CI: 2.35–2.78), closely followed by Lesotho (EAPC: 2.36; 95% CI: 1.87–2.86) and Nepal (EAPC: 2.24; 95% CI: 1.93–2.54). Denmark experienced the fastest decline in ASMR (EAPC: −5.59; 95% CI: −5.79 to −5.40), followed by Israel (EAPC: −5.42; 95% CI: −5.64 to −5.19). From 1990 to 2021, Pakistan also had the highest growth rate of ASDR (EAPC: 2.58; 95% CI: 2.36–2.81), closely followed by Lesotho (EAPC: 2.56; 95% CI: 2.05–3.07). The most rapid decline in ASDR was observed in Denmark (EAPC: −5.56; 95% CI: −5.78 to −5.33), Norway (EAPC: −5.40; 95% CI: −5.50 to −5.30), and Israel (EAPC: −5.36; 95% CI: −5.62 to −5.11) (Figures 2A, B).
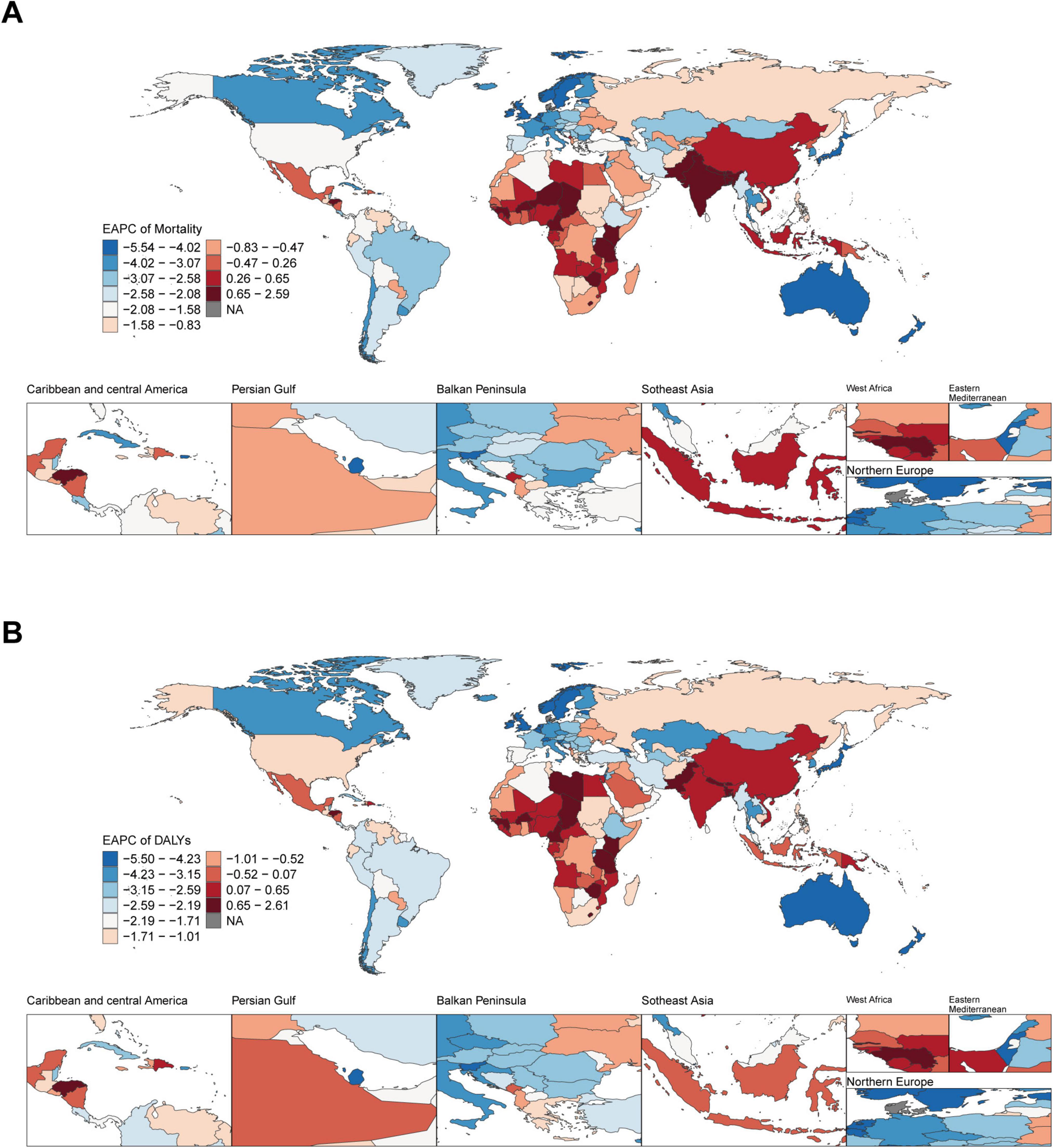
Figure 2. Global temporal trend of ischemic heart disease burden associated with diet high in sodium in 204 countries and territories from 1990 to 2021. (A) EPAC of the ASMR in ischemic heart disease; (B) EPAC of the ASDR in ischemic heart disease. EAPC, estimated annual percentage change.
The burden of ischemic heart disease associated with diet high in sodium at regional level
From 1990 to 2021, high-middle SDI region had the highest ASMR and ASDR. In contrast, in 2021, high SDI region had the lowest ASMR and ASDR (Figures 3A, B and Tables 1, 2). The most rapid decline in both ASMR and ASDR was observed in high SDI region, followed by high-middle SDI region. In other regions, ASMR and ASDR remained largely unchanged (Figures 3A, B and Tables 1, 2).
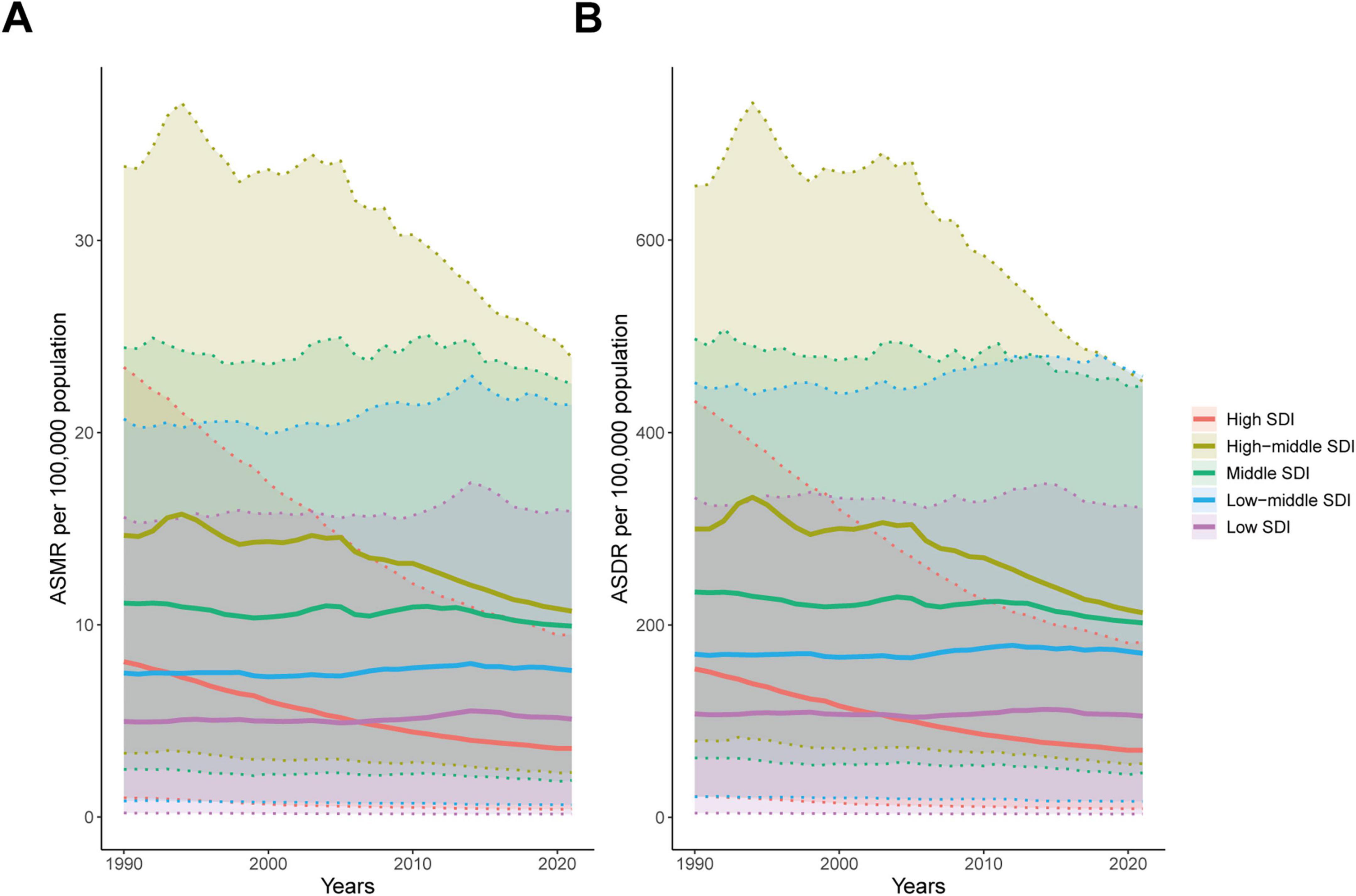
Figure 3. Age-standardized rates of ischemic heart disease associated with diet high in sodium at different SDI levels from 1990 to 2021. (A) ASMR; (B) ASDR.
Subsequently, we examined the relationship between SDI and the corresponding ASMR and ASDR in 21 GBD regions from 1990 to 2021. At the regional level, ASMR and ASDR presented an inverted U-shaped relationship with SDI (Figures 4A, B). When the SDI was less than 0.7, ASMR and ASDR gradually increased as the SDI increased, and then decreased significantly. From 1990 to 2021, ASMR and ASDR showed a decreasing trend in most regions except South Asia, East Asia, and Western Sub-Saharan Africa (Figures 4A, B and Tables 1, 2). ASMR and ASDR in Central Europe and Central Asia were the highest (Tables 1, 2), exceeding the expected levels based on SDI from 1990 to 2021, but also showing significant declines (Figures 4A, B). Australia, the high-income Asia-Pacific region, and Western Europe had the fastest decline rates in ASMR and ASDR (Tables 1, 2).
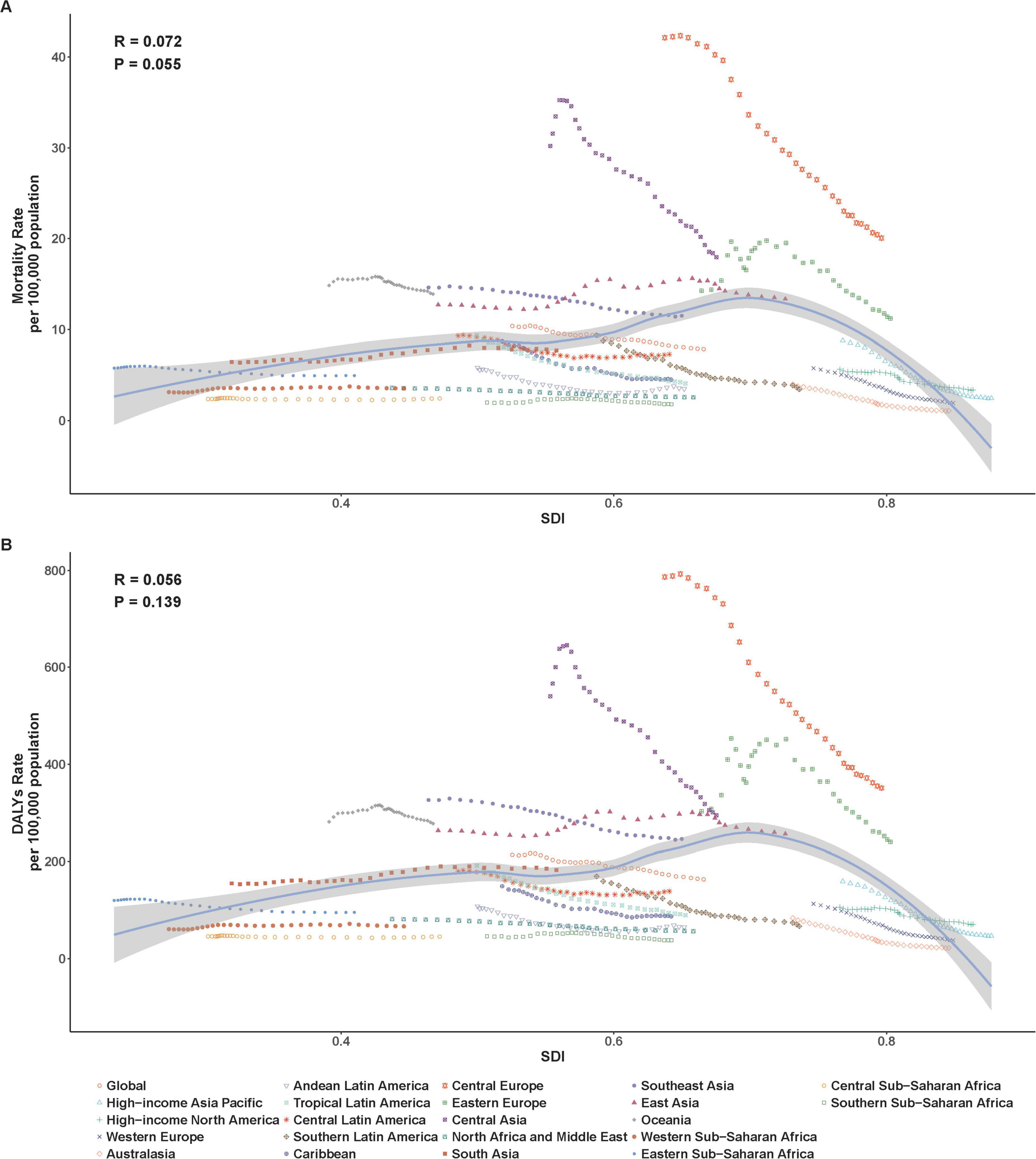
Figure 4. Age-standardized rates of ischemic heart disease associated with diet high in sodium among regions based on SDI levels from 1990 to 2021. (A) ASMR; (B) ASDR.
Age and sex patterns
In 2021, the number of mortality cases and DALYs of IHD associated with diet high in sodium were higher in males than in females before the age of 85–89. In males, the number of mortality cases and DALYs increased with age, reaching their peaks at 65–69 and 70–74 years, and then gradually declined with age. In females, the number of mortality cases attributed increased with age, reaching its peak between 80 and 84 years, and then gradually declined with age. ASMR and ASDR of males and females gradually increased with advancing age (Figures 5A, B). From 1990 to 2021, the number of mortality cases and DALYs for both males and females showed a continuous increasing trend, with males having a higher number than females (Figures 6A, C). Meanwhile, both ASMR and ASDR decreased, with males having significantly higher rates than females (Figures 6B, D).
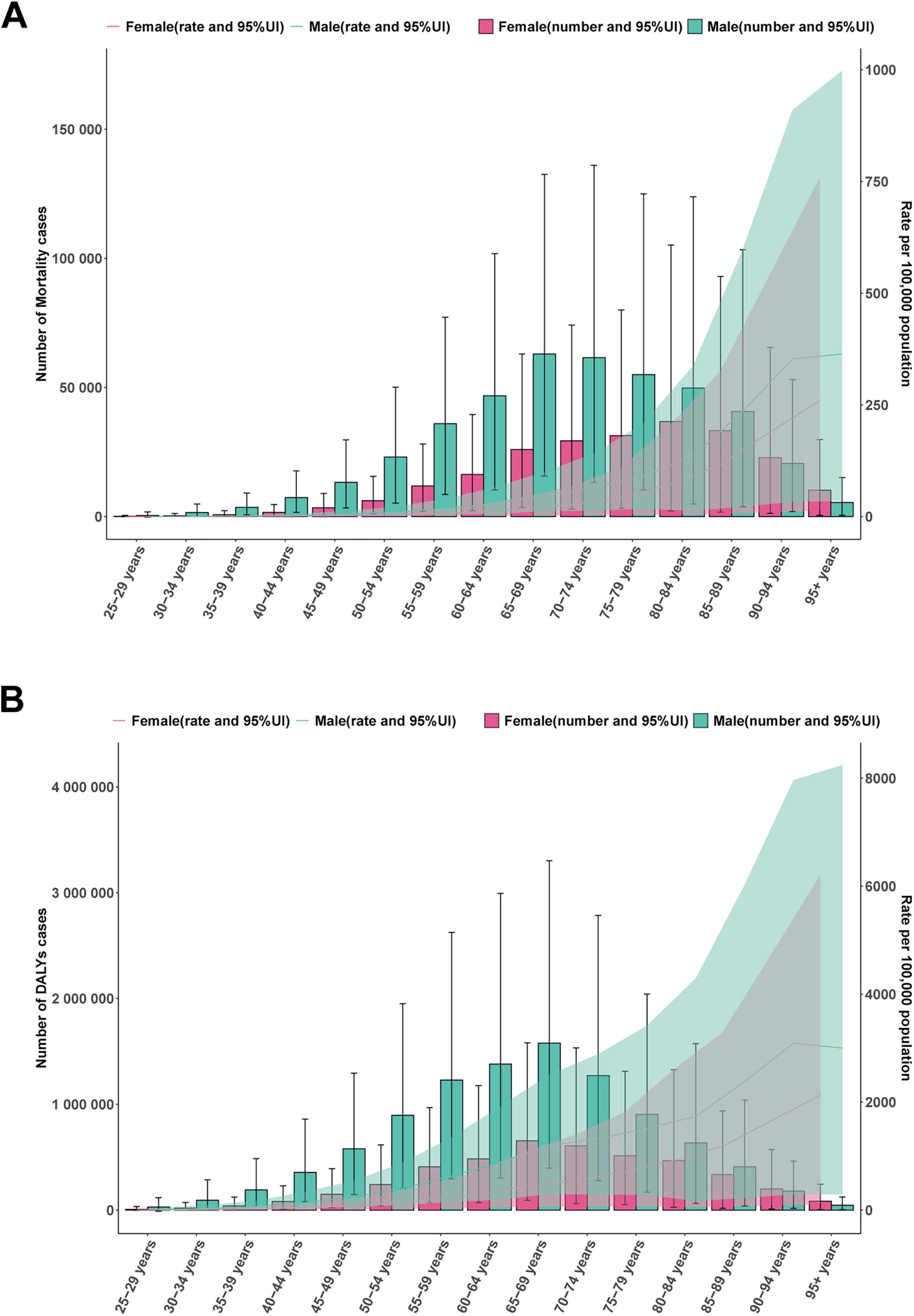
Figure 5. Global disease burden of ischemic heart disease associated with diet high in sodium in males and females by different age group in 2021. (A) Mortality cases and ASMR; (B) DALYs and ASDR.
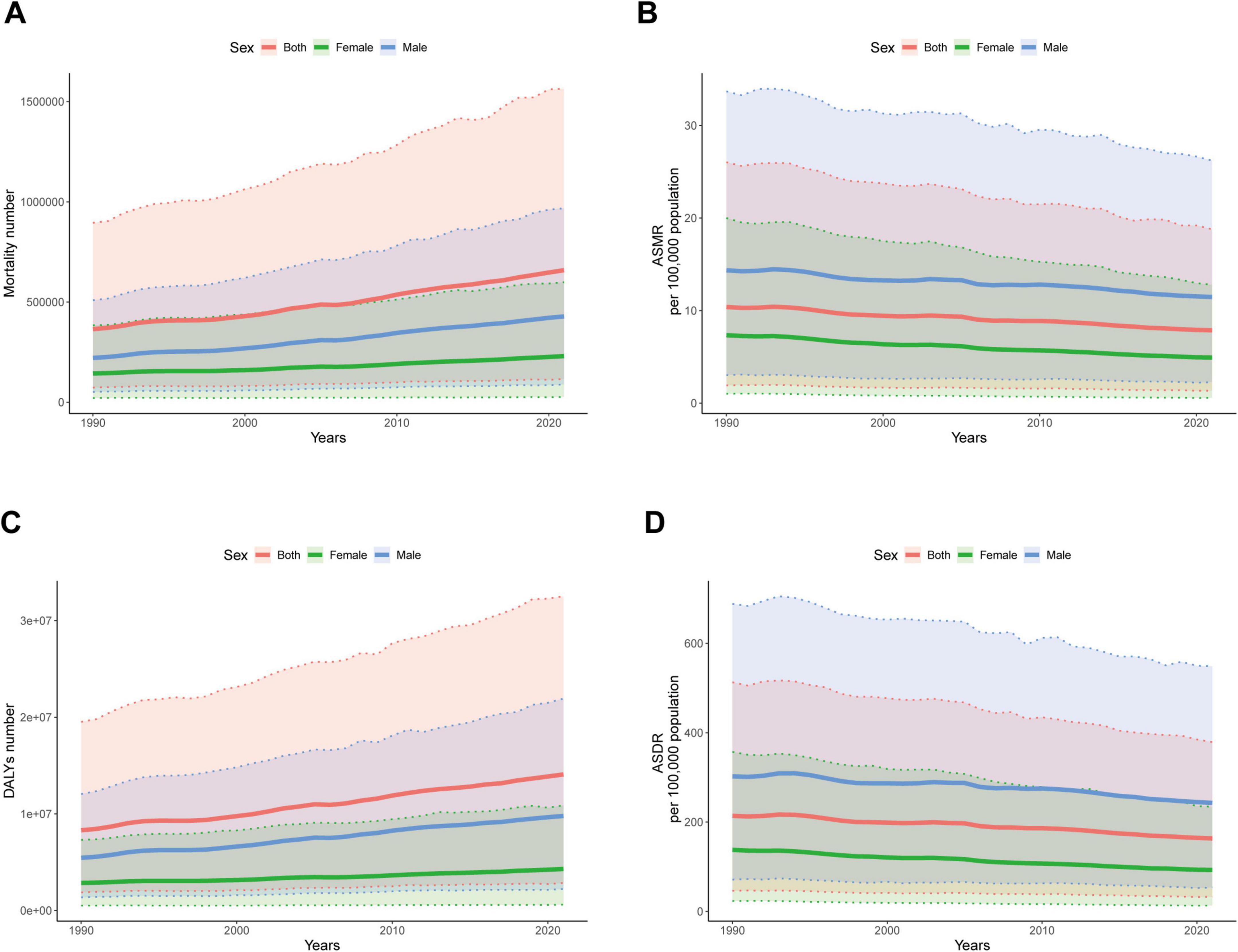
Figure 6. Global disease burden of ischemic heart disease associated with diet high in sodium with different sexes from 1990 to 2021. (A) Mortality number; (B) ASMR; (C) DALYs number; (D) ASDR.
From 1990 to 2021, the number of mortality cases and DALYs in all age groups showed an overall increasing trend (Figure 7A). In contrast, ASMR and ASDR showed a decreasing trend in all age groups. The older the age, the higher the ASDR and ASMR (Figure 7B).
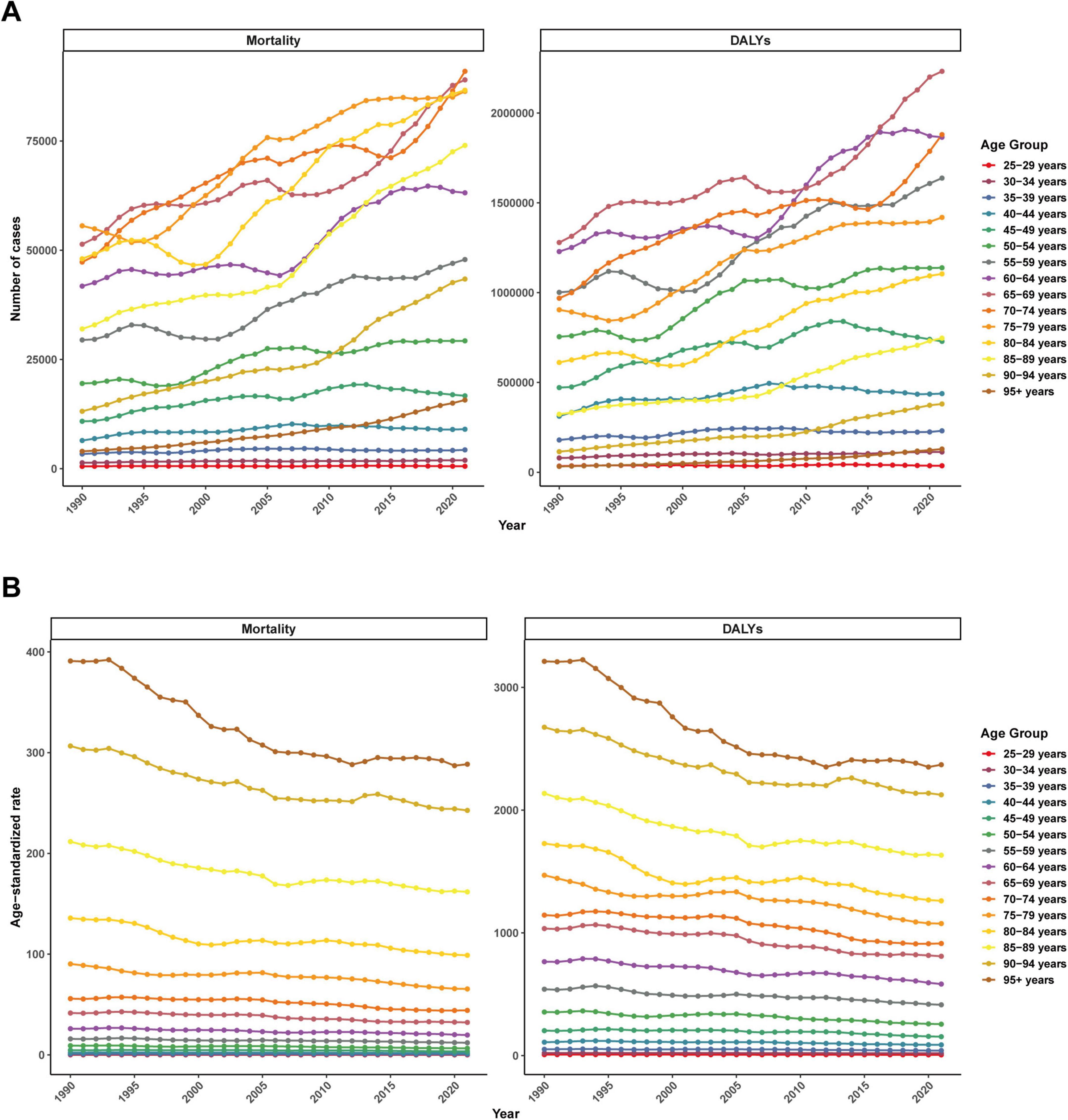
Figure 7. Global disease burden of ischemic heart disease associated with diet high in sodium by different age group from 1990 to 2021. (A) Mortality number and DALYs number; (B) ASMR and ASDR.
Projection of the burden of ischemic heart disease associated with diet high in sodium
To clarify the development trend of IHD associated with diet high in sodium after 2021, we used the Bayesian age-period-cohort (BAPC) model to predict ASMR and ASDR from 2021 to 2035 (Figure 8). The BAPC model uses Bayesian methods to decompose the disease development trend into age, period, and cohort effects to quantify their independent impacts. The model projects the future disease burden (e.g., incidence) by extrapolating historical parameters, relying on stable age/cohort effects and continued period trends. The projection results indicate that from 2021 to 2035, ASDR will continue to decline, while changes in ASMR are not significant (Figures 8A, B).
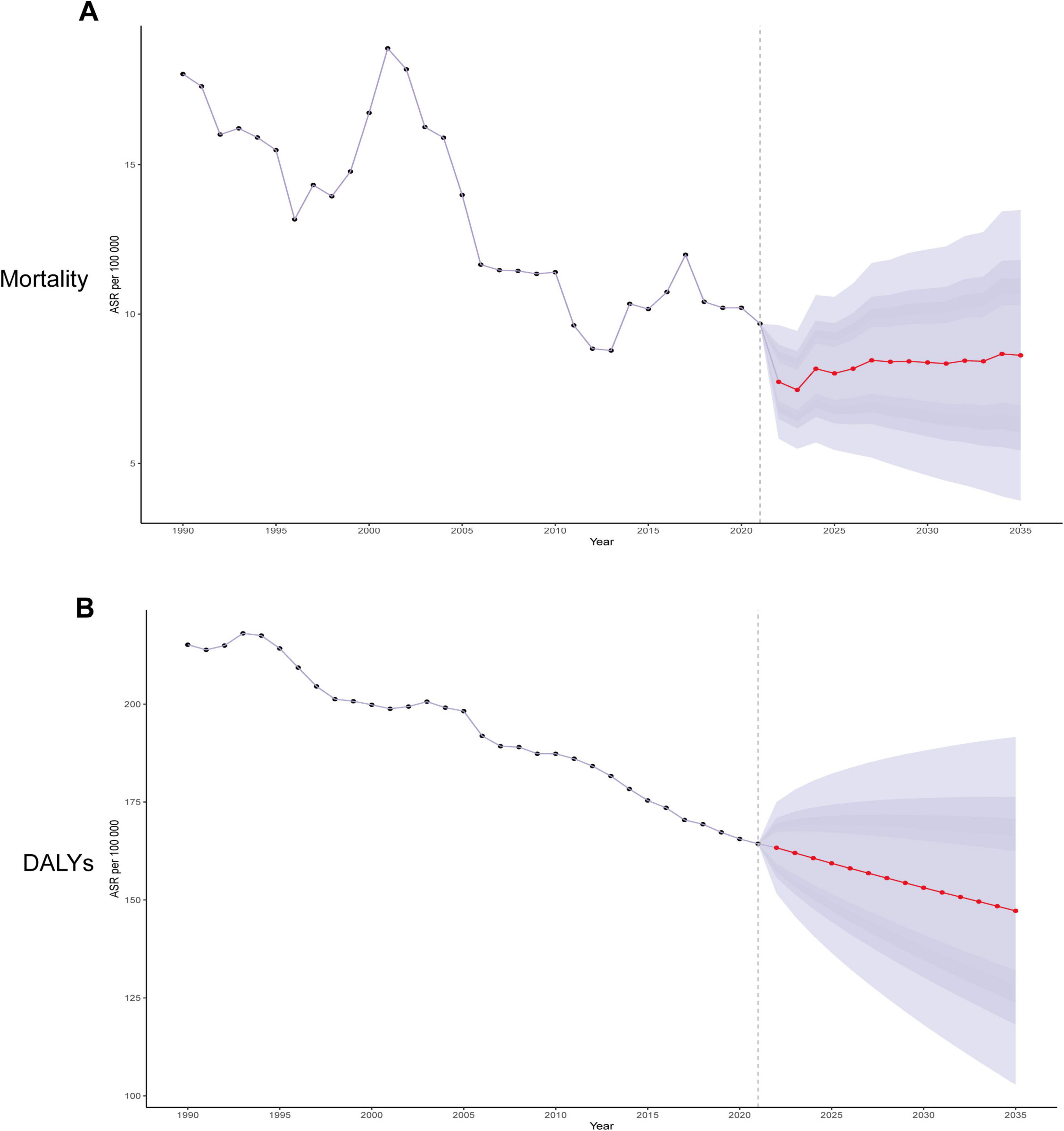
Figure 8. Trends in ischemic heart disease attributable to diet high in sodium with ASMR and ASDR in the global from 1990 to 2035 (BAPC models). (A) Trends in ASMR; (B) Trends in ASDR.
Discussion
This study conducted a comprehensive analysis of the global, national, and regional burdens of IHD associated with diet high in sodium from 1990 to 2021 and forecasted the development trend from 2021 to 2035. The results indicated that between 1990 and 2021, the number of mortality cases and DALYs associated with high-sodium diet as a risk factor for IHD increased globally, whereas the ASMR and ASDR exhibited a decreasing trend. However, the trends of ASMR and ASDR in SDI regions differed, and there were disparities in disease burden across different regions and countries. Comparisons based on age and sex revealed that the disease burden was higher among males than females, and the elderly population had the heaviest burden. The recent implementation guidelines of the WHO stress that population-level intervention measures, such as mandatory sodium content targets for processed foods, front-of-pack labeling, and mass media campaigns, are effective strategies for reducing sodium intake and preventing CVD (WHO ELENA Sodium-CVD Intervention, 2023). Consequently, controlling high-sodium intake is critical for mitigating mortality and DALY burden associated with IHD.
Sodium is an essential dietary mineral and electrolyte in human physiology, playing an indispensable role in sustaining life processes. It plays a pivotal role in supporting cellular homeostasis, facilitating neurotransmission, regulating fluid and acid-base equilibrium, and preserving plasma volume (15). However, high-sodium diet has emerged as a significant trigger for numerous non-communicable diseases. Diseases such as IHD, hypertension, and stroke are strongly associated with excessive salt intake. In fact, it has become the leading risk factor for diet-related deaths globally (16). Multiple studies have demonstrated a positive correlation between high dietary sodium intake and the onset or progression of IHD (17, 18). Studies indicate that each 1 g/day increase in sodium intake will elevate the risk of CVD by 6%, whereas reducing sodium intake may decrease the relative risk of atherosclerotic cardiovascular disease by approximately 9% (19, 20). In a high-sodium environment, the equilibrium of vascular endothelial cells is disrupted. This disruption causes abnormal vasoconstriction and accelerates atherosclerotic plaque formation (21). High-sodium diet can stimulate the production of reactive oxygen species (ROS) in vascular walls. Accumulated ROS trigger oxidative damage to endothelial cells, impairing the endothelial layer of blood vessels. Simultaneously, this process activates immune pathways (e.g., nuclear factor kappa B signaling), stimulates cytokine production (including IL-6), and aggravates vascular wall inflammation (21, 22). Excessive sodium intake results in an increase in extracellular fluid volume. When kidney function is compromised, this may give rise to the development of hypertension. Meanwhile, a decline in the reactivity of the renin-angiotensin-aldosterone system may enhance the salt-sensitivity of blood pressure (23, 24). Our study validated that high-sodium intake is a risk factor for IHD, which is further corroborated by earlier studies. A stratified analysis of disease burden statistics across different age groups, sexes, and geographic regions offers more direct and comprehensive evidence for clarifying the risk of IHD associated with elevated sodium intake and the necessity of dietary sodium reduction strategies.
This study revealed the persistent global burden of high-sodium-related IHD from 1990 to 2021. Despite a decreasing trend in ASMR and ASDR, there was a significant increase in absolute deaths and DALYs, which indicated that public health challenges remained unaddressed. This contradictory phenomenon can be attributed to population aging, the uneven distribution of medical resources, partial improvements in dietary sodium intake patterns, and regional differences in the implementation of sodium-reduction interventions. Our analysis revealed significant differences in the burden of IHD across different countries and territories. The disparities among these countries may stem from changes in dietary structure and the influence of public health policies. Countries with a high burden of IHD, such as Nauru and Bulgaria, often rely on high-sodium processed foods (such as cured meats and canned goods) or traditional high-salt diets. Coupled with inadequate regulation of sodium content in the food processing industry, limited coverage of health education, and scarce medical resources, these factors collectively exacerbate the harm of excessive sodium intake to the heart (15). Furthermore, middle-to-high SDI regions bore a heavier burden compared to high-SDI regions, as ASMR and ASDR exhibited an inverse U-shaped relationship with the SDI. These findings indicate a strong association among the level of socioeconomic development, the prevention and control of IHD, and salt-reduction strategies. High-SDI regions managed to significantly reduce the burden of sodium-related IHD burden through integrated systems of social welfare, universal healthcare, health literacy improvement programs, and proactive preventive measures. By 2021, these efforts led to the lowest regional ASMR and ASDR. This suggests that global sodium-reduction measures may have yielded relatively favorable outcomes. In 2019, reports indicated that 96 countries had implemented national salt-reduction initiatives, and an additional 16 countries were in the planning phase (25). The primary implementation strategies center on intervention measures in specific settings, food reformulation, consumer education, front-of-pack labeling, and salt taxes. Continuous monitoring and reporting of the progress of each intervention measure can assist in evaluating the effectiveness of policies, identifying gaps, and formulating follow-up measures required to achieve the goal of reducing salt intake by 30% (26). Conversely, middle-to-high SDI regions such as Central Europe and Central Asia, despite showing declining trends, still reported the highest SDI-related rate globally, which might be attributed to policy lag during economic transitions. The most concerning issue is that there was no significant decline in the ASMR and ASDR in South Asia, East Asia, and Sub-Saharan West Africa, suggesting synergistic effects of metabolic comorbidities (particularly elevated fasting blood glucose) and excessive sodium intake. Overall, these findings recommend the adoption of tailored prevention strategies, fully taking into account regional socioeconomic development trajectories, cultural dietary habits, and healthcare capacities. Such precision approaches are essential to disrupt the persistent global burden of sodium-related CVD.
Global epidemiological analyses demonstrate significant sex disparities in sodium-related cardiovascular pathology. In comparison to females, males shoulder a disproportionately higher burden of IHD associated with excessive sodium intake. From 1990 to 2021, age-adjusted mortality rates and DALYs were consistently higher among males than females, a trend corroborated by multiple longitudinal studies (27). Another crucial factor is that females can more effectively regulate the sodium balance in their bodies, rendering them less susceptible to the adverse effects of excessive sodium intake (28). Additionally, compared with females, males generally exhibit poorer dietary patterns and lifestyle habits, especially in aspects such as smoking and alcohol abuse (29, 30). Notably, it is reported that estrogen can offer vascular protection to females and impede the process of atherosclerosis (31). Therefore, this clinically significant sex difference demands greater attention to implement sex-specific dietary salt control measures. We also observed that the ASMR and ASDR for both males and females gradually increased with age, suggesting that older adults confronted a heavier burden of IHD due to high-sodium intake compared to younger populations. This phenomenon may be attributed to the following factors. Firstly, aging is associated with reduced vascular compliance and diminished renal filtration function, which may render older adults more vulnerable to the adverse effects of excessive sodium intake. Secondly, most elderly individuals already have other cardiovascular risk factors, such as arteriosclerosis and diabetes. High-sodium intake can exacerbate these pre-existing diseases, leading to more severe IHD (32). Additionally, due to diminished taste perception or lack of awareness of healthy eating habits, elderly individuals may increase their salt intake, resulting in long-term high-sodium intake issues (33). To enhance the quality of life of the elderly and alleviate the global disease burden, attention should be paid to their dietary habits while implementing strict sodium restriction strategies.
Nevertheless, this study still has certain limitations. Firstly, dependence on secondary GBD data rather than primary sources may limit reliability to some extent, which is a common limitation in previous GBD studies (34, 35). Secondly, inadequate understanding of asymptomatic IHD cases may lead to an underestimation of the burden of IHD associated with high-sodium intake. Underestimating the disease burden can influence policy-making, potentially leading to insufficient resource allocation and unnoticed financial burdens. Finally, when evaluating the impact of high-sodium intake on IHD using ASMR and ASDR, this analysis did not fully account for the severity of the disease.
Conclusion
Our study demonstrated that high-sodium diet exerted a significant influence on the global burden of IHD. Meanwhile, it revealed substantial disparities in SDI regions, countries, age groups, and sexes. Utilizing the BAPC model, we analyzed the development trend of IHD associated with diet high in sodium from age, period, and cohort perspectives. Additionally, we were capable of predicting future changes and the disease burden of IHD. The prediction results of the BAPC model suggested that by 2035, the ASDR will continue to decline, whereas the change in the ASMR will be relatively minor. This implies that although current preventive strategies sustain the reduction of IHD mortality, control of disease burden has reached a plateau requiring breakthroughs through targeted interventions and integrated management. Therefore, priority should be placed on strengthening sodium-reduction interventions, particularly through targeted low-sodium diet education and evidence-based salt restriction policies, to mitigate the escalating burden of IHD globally.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: Global Health Data Exchange query tool, http://ghdx.healthdata.org/gbd-results-tool.
Author contributions
YM: Writing – review & editing, Conceptualization, Writing – original draft. JW: Writing – review & editing, Writing – original draft. SX: Writing – review & editing, Writing – original draft. MW: Writing – review & editing. SY: Writing – review & editing. QW: Writing – review & editing. ZT: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Shanghai Medical Innovation and Development Foundation, the Yangtze River Delta pharmaceutical high-quality development research promotion program (SMIDF-142-3 to MW).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
IHD, ischemic heart disease; GBD, global burden of disease; ASR, age-standardized rate; ASMR, age-standardized mortality rate; ASDR, age-standardized DALYs rate; DALYs, disability-adjusted life years; EAPC, estimated annual percentage change; SDI, socio-demographic index; UI, uncertainty interval; CI, confidence interval; BAPC, Bayesian age-period-cohort; CVD, cardiovascular disease; WHO, World Health Organization; DHIS, diet high in sodium.
Footnotes
References
1. Wang W, Hu M, Liu H, Zhang X, Li H, Zhou F, et al. Global burden of disease study 2019 suggests that metabolic risk factors are the leading drivers of the burden of ischemic heart disease. Cell Metab. (2021) 33:1943–56 e2. doi: 10.1016/j.cmet.2021.08.005.
2. Roth G, Mensah G, Johnson C, Addolorato G, Ammirati E, Baddour L, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the Gbd 2019 Study. J Am Coll Cardiol. (2020) 76:2982–3021. doi: 10.1016/j.jacc.2020.11.010
3. Shu T, Tang M, He B, Liu X, Han Y, Liu C, et al. Assessing global, regional, and national time trends and associated risk factors of the mortality in ischemic heart disease through global burden of disease 2019 study: population-based study. JMIR Public Health Surveill. (2024) 10:e46821. doi: 10.2196/46821
4. Malakar A, Choudhury D, Halder B, Paul P, Uddin A, Chakraborty SA. Review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol. (2019) 234:16812–23. doi: 10.1002/jcp.28350
5. Yan D, Liu K, Li F, Shi D, Wei L, Zhang J, et al. Global burden of ischemic heart disease associated with high red and processed meat consumption: an analysis of 204 Countries and Territories between 1990 and 2019. BMC Public Health. (2023) 23:2267. doi: 10.1186/s12889-023-16954-4
6. Bechthold A, Boeing H, Schwedhelm C, Hoffmann G, Knuppel S, Iqbal K, et al. Food groups and risk of coronary heart disease, stroke and heart failure: a systematic review and dose-response meta-analysis of prospective studies. Crit Rev Food Sci Nutr. (2019) 59:1071–90. doi: 10.1080/10408398.2017.1392288
7. Li H, Song X, Liang Y, Bai X, Liu-Huo W, Tang C, et al. Global, Regional, and National burden of disease study of atrial fibrillation/flutter, 1990-2019: results from a global burden of disease study, 2019. BMC Public Health. (2022) 22:2015. doi: 10.1186/s12889-022-14403-2
8. He F, MacGregor G. Reducing population salt intake worldwide: from evidence to implementation. Prog Cardiovasc Dis. (2010) 52:363–82. doi: 10.1016/j.pcad.2009.12.006
9. World Health Organization [WHO]. Guideline: Sodium Intake for Adults and Children. Who Guidelines Approved by the Guidelines Review Committee. Geneva: World Health Organization (2012).
10. Wang K, Jin Y, Wang M, Liu J, Bu X, Mu J, et al. Global cardiovascular diseases burden attributable to high sodium intake from 1990 to 2019. J Clin Hypertens. (2023) 25:868–79. doi: 10.1111/jch.14717
11. GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (Ylds), disability-adjusted life-years (Dalys), and healthy life expectancy (Hale) for 371 Diseases and Injuries in 204 Countries and Territories and 811 Subnational Locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. (2024) 403:2133–61. doi: 10.1016/S0140-6736(24)00757-8
12. Qian X, Wan J, Xu J, Liu C, Zhong M, Zhang J, et al. Epidemiological trends of Urolithiasis at the Global, Regional, and National Levels: a population-based study. Int J Clin Pract. (2022) 2022:6807203. doi: 10.1155/2022/6807203
13. Liu Z, Xu K, Jiang Y, Cai N, Fan J, Mao X, et al. Global trend of aetiology-based primary liver cancer incidence from 1990 to 2030: a modelling study. Int J Epidemiol. (2021) 50:128–42. doi: 10.1093/ije/dyaa196
14. Riebler A, Held L. Projecting the future burden of cancer: Bayesian age-period-cohort analysis with integrated nested laplace approximations. Biom J. (2017) 59:531–49. doi: 10.1002/bimj.201500263
15. Liu W, Zhou L, Yin W, Wang J, Zuo X. Global, Regional, and National Burden of chronic kidney disease attributable to high sodium intake from 1990 to 2019. Front Nutr. (2023) 10:1078371. doi: 10.3389/fnut.2023.1078371
16. Durazzo A, Lombardi-Boccia G, Santini A, Lucarini M. Sodium intake and related diseases 2.0. Int J Mol Sci. (2021) 23:170. doi: 10.3390/ijms23010170
17. Heaney R. Sodium: How and how not to set a nutrient intake recommendation. Am J Hypertens. (2013) 26:1194–7. doi: 10.1093/ajh/hpt130
18. Heaney R. The nutrient problem, as seen through the lens of calcium. J Clin Endocrinol Metab. (2011) 96:2035–7. doi: 10.1210/jc.2011-1545
19. Zhao D, Li H, Li C, Zhou B. 24-hour urinary sodium excretion association with cardiovascular events: a systematic review and dose-response meta-analysis. Biomed Environ Sci. (2022) 35:921–30. doi: 10.3967/bes2022.119
20. Knauss H, Kovell L, Miller E III, Appel L, Mukamal K, Plante T, et al. Dietary sodium reduction lowers 10-year atherosclerotic cardiovascular disease risk score: results from the dash-sodium trial. Am J Prev Cardiol. (2025) 22:100980. doi: 10.1016/j.ajpc.2025.100980
21. Patik J, Lennon S, Farquhar W, Edwards D. Mechanisms of dietary sodium-induced impairments in endothelial function and potential countermeasures. Nutrients. (2021) 13:270. doi: 10.3390/nu13010270
22. Jaques D, Wuerzner G, Ponte B. Sodium intake as a cardiovascular risk factor: a narrative review. Nutrients. (2021) 13:3177. doi: 10.3390/nu13093177
23. Farquhar W, Edwards D, Jurkovitz C, Weintraub W. Dietary sodium and health: more than just blood pressure. J Am Coll Cardiol. (2015) 65:1042–50. doi: 10.1016/j.jacc.2014.12.039
24. Koomans H, Roos J, Boer P, Geyskes G, Mees E. Salt sensitivity of blood pressure in chronic renal failure. evidence for renal control of body fluid distribution in man. Hypertension. (1982) 4:190–7. doi: 10.1161/01.hyp.4.2.190
25. Santos J, Tekle D, Rosewarne E, Flexner N, Cobb L, Al-Jawaldeh A, et al. A systematic review of salt reduction initiatives around the world: a midterm evaluation of progress towards the 2025 global non-communicable diseases salt reduction target. Adv Nutr. (2021) 12:1768–80. doi: 10.1093/advances/nmab008
26. NCD Countdown 2030 collaborators. NCD Countdown 2030: efficient pathways and strategic investments to accelerate progress towards the sustainable development goal target 3.4 in low-income and middle-income Countries. Lancet. (2022) 399:1266–78. doi: 10.1016/S0140-6736(21)02347-3
27. Chen X, Du J, Wu X, Cao W, Sun S. Global burden attributable to high sodium intake from 1990 to 2019. Nutr Metab Cardiovasc Dis. (2021) 31:3314–21. doi: 10.1016/j.numecd.2021.08.033
28. Gohar E, De Miguel C, Obi I, Daugherty E, Hyndman K, Becker B, et al. Acclimation to a high-salt diet is sex dependent. J Am Heart Assoc. (2022) 11:e020450. doi: 10.1161/JAHA.120.020450
29. Safiri S, Nejadghaderi S, Abdollahi M, Carson-Chahhoud K, Kaufman J, Bragazzi N, et al. Global, Regional, and National Burden of cancers attributable to tobacco smoking in 204 Countries and Territories, 1990-2019. Cancer Med. (2022) 11:2662–78. doi: 10.1002/cam4.4647
30. Hernandez-Vasquez A, Chacon-Torrico H, Vargas-Fernandez R, Grendas L, Bendezu-Quispe G. Gender differences in the factors associated with alcohol binge drinking: a population-based analysis in a Latin American Country. Int J Environ Res Public Health. (2022) 19:4931. doi: 10.3390/ijerph19094931
31. Wang Z, Zhang G, Hu S, Fu M, Zhang P, Zhang K, et al. Research progress on the protective effect of hormones and hormone drugs in myocardial ischemia-reperfusion injury. Biomed Pharmacother. (2024) 176:116764. doi: 10.1016/j.biopha.2024.116764
32. Zhang L, Tong Z, Han R, Guo R, Zang S, Zhang X, et al. Global, Regional, and National burdens of ischemic heart disease attributable to smoking from 1990 to 2019. J Am Heart Assoc. (2023) 12:e028193. doi: 10.1161/JAHA.122.028193
33. Zeanandin G, Molato O, Le Duff F, Guerin O, Hebuterne X, Schneider S. Impact of restrictive diets on the risk of undernutrition in a free-living elderly population. Clin Nutr. (2012) 31:69–73. doi: 10.1016/j.clnu.2011.08.007
34. GBD 2019 Diseases and Injuries Collaborators. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
Keywords: ischemic heart disease, global burden of disease, diet high in sodium, risk factors, health projections
Citation: Mao Y, Wang J, Xuan S, Wang M, Yang S, Wu Q and Tang Z (2025) Global, regional, and national burden of ischemic heart disease associated with diet high in sodium from 1990 to 2021, and its projections to 2035: a systematic analysis of the global burden of disease study 2021. Front. Nutr. 12:1630331. doi: 10.3389/fnut.2025.1630331
Received: 17 May 2025; Accepted: 26 August 2025;
Published: 16 September 2025.
Edited by:
Yang Yang, The First Affiliated Hospital of Zhengzhou University, ChinaReviewed by:
Tri Siswati, Health Polytechnic Ministry of Health, Yogyakarta, IndonesiaNereida Spahia, University Hospital Center “Mother Teresa” (QSUT), Albania
Copyright © 2025 Mao, Wang, Xuan, Wang, Yang, Wu and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhihua Tang, c3h0emhAMTYzLmNvbQ==; Qiuji Wu, cWp3dTIyQG0uZnVkYW4uZWR1LmNu; Yuqin Mao, bXlxMTk5NTAwMUAxNjMuY29t
†These authors have contributed equally to this work
 Yuqin Mao
Yuqin Mao Jiong Wang1,2†
Jiong Wang1,2† Minxiu Wang
Minxiu Wang