- 1Shandong Institute of Pomology, Tai'an, Shandong, China
- 2College of Food Science and Engineering, Shandong Agricultural University, Tai'an, Shandong, China
To investigate the characteristic flavor compounds and their differences in commercially available cherry wines, this study employed headspace solid-phase microextraction combined with gas chromatography–mass spectrometry (HS-SPME-GC-MS), headspace gas chromatography–ion mobility spectrometry (HS-GC-IMS), and sensory evaluation techniques to comprehensively analyze the volatile flavor compounds and sensory characteristics of 11 commercial cherry wines. The results showed that HS-SPME-GC-MS and HS-GC-IMS identified 74 and 49 volatile compounds, respectively. The combined use of both techniques detected a total of 101 compounds, identifying 15 additional key compounds compared to a single method, significantly improving the comprehensiveness of flavor characterization. Through odor activity value (OAV), relative odor activity value (ROAV), and variable importance in projection (VIP), 28 key flavor compounds were screened. Among them, esters (such as ethyl acetate, ethyl hexanoate, and ethyl octanoate) and alcohols (such as phenethyl alcohol) were the main contributors to the fruity and floral aroma of cherry wines, while benzaldehyde and 3-furfural were significantly associated with woody characteristics. Sensory evaluation indicated that sample C10 received the highest overall score, exhibiting prominent floral and fruity notes. Partial least squares regression (PLSR) analysis further confirmed that the floral and fruity attributes were strongly positively correlated with compounds such as linalool and α-terpineol, while the woody attribute was closely associated with benzaldehyde. Based on volatile compound fingerprinting and principal component analysis (PCA), samples C5, C9, and C10 exhibited similar flavor profiles with notable floral and fruity characteristics, whereas C11 displayed a unique woody character due to its high benzaldehyde content. This study provides a theoretical basis and data support for improving the flavor quality and differentiated quality control of cherry wine.
1 Introduction
Cherries are highly favored by consumers for their bright colors and rich nutritional components (1). Notably, cherries are rich in bioactive compounds, such as flavonoids, polyphenols, and anthocyanins. These substances possess excellent antioxidant capabilities (2). However, freshly harvested cherries have a relatively short shelf life due to their high water and sugar content. To extend their shelf life and enhance economic viability, cherries are frequently processed into juice or fruit wine.
The flavor of fruit wines is of utmost importance in determining their sensory quality and is a key element that impacts consumer preference (40). Extensive studies have been conducted on the flavor compounds in fruit wines. Wattanakul et al. (3) discovered that ethyl esters and acetate constituted the key flavor groups in the process of mango wine fermentation, while fatty acid methyl esters showed positive correlations with volatile acids, sugars, aldehydes, higher alcohols, and ketones. Li et al. (4) found that isoamyl acetate, β-damascenone, 2,3-butanediol, and phenyl ethanol were the main contributors to sweetness in dry red wine, while berry flavor characteristics were formed by geranylacetone, ethyl 2-methylbutyrate, and ethyl isobutyrate.
In cherry wine, volatile flavor compounds including alcohols, aldehydes, ketones, esters, and monoterpenes engage in intricate interactions with one another. These interactions are responsible for generating the unique and characteristic flavors that define cherry wine. Through their interactions, these diverse compounds combine to create a complex flavor profile, with each component contributing to the overall sensory experience, making the flavor of cherry wine distinct and recognizable (5). Nevertheless, most cherry wines on the market have less distinct characteristic aromas, weaker fruit and wine aromas, and suboptimal taste profiles (6).
A diverse range of chromatographic techniques, including gas chromatography (GC), gas chromatography-olfactory determination (GC-O) (7), and gas chromatography-mass spectrometry (GC-MS) (8) have been utilized for detecting the aroma compounds in fruit wines. Currently, numerous investigations have been conducted on analyzing the aroma compounds in cherry wines. Solid phase microextraction (SPME) and headspace sampler (HS) combined with gas chromatography (GC/FID) were utilized to determine the flavor compounds of Marasca cherry wine produced under varying fermentation conditions. It was found that ethyl butyrate, butyl acetate, and hexyl acetate had the highest content and imparted a fruit aroma to cherry wine (9). Xiao et al. (10) analyzed the aroma composition and aroma active ingredients of three different-priced kiraschins with HS-SPME-GC-MS and noticed that kiraschins with different prices exhibited diverse characteristics in aroma combination and aroma properties. Niu et al. (11) employed GC-O and GC-MS to conduct an in-depth study on the ester aroma compounds in cherry wine. Their research findings indicate that several esters, including ethyl butyrate, ethyl acetate, ethyl caprylate, ethyl isovalerate, ethyl decanoate, methyl salicylate and hexyl acetate, play a crucial role in endowing cherry wines with their characteristic sweetness and fruity aroma. However, there is a paucity of reports on determining the characteristic flavor compounds in cherry wines through the integration of various coupled chromatographic techniques and sensory analysis.
Although researchers have analyzed the aroma components of cherry wine using instruments such as GC-MS and conducted preliminary evaluations of its quality through sensory assessments, current studies still exhibit significant limitations: the detection methods are relatively singular, there is a lack of systematic analysis of differences in characteristic flavor compounds among different products, and the relationship between flavor substances and sensory attributes has not yet been established. To further reveal the diversity and characteristics of the flavor of commercially available cherry wines, this study selected 11 cherry wines from different brands as samples and employed a combination of HS-SPME-GC-MS and HS-GC-IMS techniques to comprehensively capture their volatile flavor compounds. By integrating chemical analysis with sensory evaluation and applying multivariate statistical methods, this research aims to differentiate the flavor profiles of cherry wines from various brands, identify key discriminatory compounds, and establish correlations between flavor components and sensory properties. The study seeks to elucidate the product characteristics of commercially available cherry wines from a flavoromics perspective, providing data support and theoretical reference for understanding their flavor composition and market differentiation.
2 Materials and methods
2.1 Material information
Ten different types of cherry wines (C1-C6,C8-C11) with high consumer ratings and market share were purchased from the Chinese e-commerce platform JD.com. C7 sample was obtained through homemade production. The main process is as follows: Cherries were rinsed, stemmed, and crushed. The sugar content was adjusted to 20%, followed by yeast inoculation. Fermentation was carried out at 20°C. After completion, the fermented mixture was filtered and stored in a sealed container at 4°C, with details provided in Table 1.
2.2 Physiochemical parameters analysis
In accordance with the Chinese national standard GB/T 15038-2006, the reducing sugars, total sugars, total soluble solids, total acids, alcohol content, and pH in cherry wines were measured.
2.3 HS-GC-IMS analysis
The HS-GC–IMS analysis method was referenced from the approach of Xie et al. (12) with modifications. Briefly, 2.0 g of cherry wine was transferred into a 20-mL headspace vial, and duplicate samples were obtained. An MXT-5 chromatographic column (15 m × 0.53 mm, 1 μm) was utilized to desorb the volatile compounds at 60 °C. The MXT-5 column excels at separating compounds based on their volatility (boiling point). This provides sufficient separation for the subsequent IMS dimension while enabling faster analysis speeds and improved robustness. An automatic headspace injection technique was used, with an injection volume of 100 μL. The cherry wine was placed at 65 °C for 10 min while rotating at 500 rpm. The temperature of injection needle was maintained at 85 °C. Nitrogen served as the carrier gas, with a total analysis time period of 40 min. Initially, the gas flow rate was set at 2 mL/min and held constant for 2 min, then increased to 10 mL/min over the following 8 min, and further raised to 90 mL/min over the following 30 min. Additionally, nitrogen at a flow rate of 150 mL/min was utilized as the drift gas. Each group of samples was analyzed in duplicate.
2.4 HS-SPME-GC-MS analysis
The procedure was referenced from the method of Niu et al. (11) with modifications for analyzing the volatile compounds in cherry wine using HS–SPME–GC–MS. The specific operational steps are as follows: a 20 mL sample of cherry wine was transferred to a container with a volume of 50 mL, followed by adding 4 g of NaCl and 80 μL of 2-octanol (0.411 g/L). The container was then sealed with a Teflon lid. The SPME fiber (DVB/C-WR/PDMS) head was inserted into the container and allowed to adsorb volatile compounds at 50 °C for 45 min, with continuous stirring using a magnetic stirrer bar. The analysis was conducted with GC-MS (GCMS-TQ8040 NX). A VF-WAX capillary column (30 m × 0.25 mm × 0.55 μm) was employed, with helium as the carrier gas at a flow rate of 1.2 mL/min. The primary reason for using a polar column (VF-Wax) in HS-SPME-GC-MS is that the flavor compounds analyzed by GC-MS are typically polar, thereby maximizing the resolution and sensitivity of the analysis. Additionally, the retention indices and mass spectra obtained from the detection can be directly compared with data from large commercial databases (e.g., NIST), significantly enhancing the accuracy and reliability of compound identification. The specific operational procedure is as follows: the injection port maintained at 260°C and the injection was conducted in splitless mode. The temperature program was as follows: the initial temperature was set at 45°C and held for 1 min, then increased at a rate of 4 °C/min to 80 °C and maintained for 2 min. It was then raised at a rate of 5 °C/min to 230°C and held for 10 min. The ion source temperature remained constant at 230 °C. The ionization was performed in EI mode, with an electron energy of 70 eV and a mass scanning range of 30–450 m/z. Volatile compounds were identified by matching their mass spectra against the NIST 20.0 mass spectral library. 2-Octanol is typically absent in cherry wine. It does not belong to the group of major or common alcohols, such as ethanol, isoamyl alcohol, or isobutanol, produced during the fermentation of cherry wine. 2-Octanol exhibits a well-defined peak shape and stable retention time in GC analysis, making it suitable for using as an internal standard. Quantitative analysis of volatile components was performed by comparing the peak area with that of the internal standard (2-octanol). Each group of samples was analyzed in triplicate.
2.5 Calculation of odor activity value and relative odor activity value
The OAV value represents the ratio of the mass concentration to the odor threshold of a volatile compound and is primarily used to assess its contribution to the cherry wine aroma (13). Typically, volatile compounds with an OAV ≥ 1 remarkably affect the flavor profile of cherry wine (14). The following formula is used for the calculation of OAV:
Where Ci represents the compound concentration, Ti denotes the threshold level of compound, and OAVi indicates the value of odor activity associated with the compound.
The formula for calculating ROAV is presented as follows:
ROAVi is the relative odor activity value of a compound, while OAVmax refers to the maximum OAV value among all compounds in the sample.
2.6 Sensory analysis
The sensory evaluation panel consists of 14 trained wine tasters, comprising 6 males and 8 females, with the ages from 23 to 45 years old. All tasters panelists had prior experience in wine tasting. Additionally, to enhance the accuracy of cherry wine evaluation, the 14 panelists received a 1-week training session specifically on cherry wine tasting. The entire sensory analysis adhered to sensory ethical standards. Before the experiment commenced, every participant was comprehensively briefed on the study's objective. Subsequently, they all furnished written consent to take part in the experiment. All panelists were non-smokers and had no known medical conditions, particularly those associated with the oral and olfactory organs. The sensory evaluation of cherry wine was conducted according to the requirements of GB/T15038-2006. Fourteen panelists evaluated the samples, describing the wine based on six aroma attributes (floral, fruity, fermented, burnt, sour, and woody) and scoring each wine sample accordingly.
2.7 Data analysis
In this study, SPSS 26.0 software is used for one-way analysis of variance. SIMCA software is employed to conduct OPLS-DA and VIP analysis on the volatile compounds of the samples. PLSR analysis was performed with Unscramble X. For visualizing HS-GC-IMS data, interpolation software such as Reporter, Gallery Plot, and Dynamic PCA was utilized. This combination of analytical and visualization tools enabled a comprehensive and in-depth exploration of the data, providing deeper insights into the relationships and characteristics within the dataset.
3 Results and discussion
3.1 Physicochemical indicators of cherry wines
Table 2 summarizes the basic physical and chemical indices of 11 kinds of cherry wines. The cherry wine with the lowest total sugar content is C2 (34.93 g/L), while the one with the highest is C11 (318.53 g/L). The reason why the total sugar content of C11 is greater than that of other wines may be the addition of some sugar to enhance the taste. The initial sugar level directly determines the potential alcohol content, which in turn influences the aroma profile via the alcohol degree (15). Ethanol is an excellent organic solvent that enhances the solubility of many aroma compounds—such as higher alcohols and esters (e.g., ethyl acetate, ethyl hexanoate)—in the wine, allowing them to remain more stable within the matrix rather than volatilizing. This contributes to a richer and more complex aromatic profile (16). Acidity determines the pH environment of cherry wine, and pH acts as a critical condition for many chemical reactions (17). A lower pH value (i.e., higher acidity) favors the progression of esterification, thereby promoting the formation and accumulation of fruit-aroma ester compounds—such as ethyl acetate and ethyl hexanoate (18). Excessively high acidity may bring an off—flavor to cherry wine, while insufficient acidity may lead to a lack of flavor. The total acid content of C1 is the highest (6.95 g/L), and the significantly lower total acid content of C11 (1.92 g/L) compared to other wines may be related to its production process. The 11 kinds of cherry wines show significant differences in various physical and chemical indices.
3.2 HS-GC-IMS results of the cherry wines
The volatile flavor compounds characterized through HS-GC-IMS are listed in Table 3. A total of 49 signal peaks were characterized in 11 cherry wine samples, such as 1 organic acid, 3 aldehydes, 4 ketones, 8 alcohols, and 14 esters. Additionally, due to database limitations, 15 compounds remained unidentified. Additionally, 15 compounds remained unidentified due to database limitations. Certain flavor compounds are present as proton-bound dimers and monomers, likely resulting from variations in volatile compound concentrations, which can generate multiple signals, including dimers and even trimers (19).
In order to assess the differences among samples, the sample C1 spectrum was utilized as a benchmark. Subsequently, the remaining samples' spectra were withdrawn from that of sample C1. This approach allowed for a clear visualization and quantification of the variances between the samples, facilitating a more in—depth analysis of their unique characteristics. A white subtracted background indicates consistent flavor compounds; red means a greater concentration than the reference, and blue represents a lower one. As presented in Figure 1a, which illustrates the comparison of volatile compound content in different cherry wine samples with C1 as the reference, the main compound content in C4, C5, C6, C9, and C10 samples were similar to those in C1.
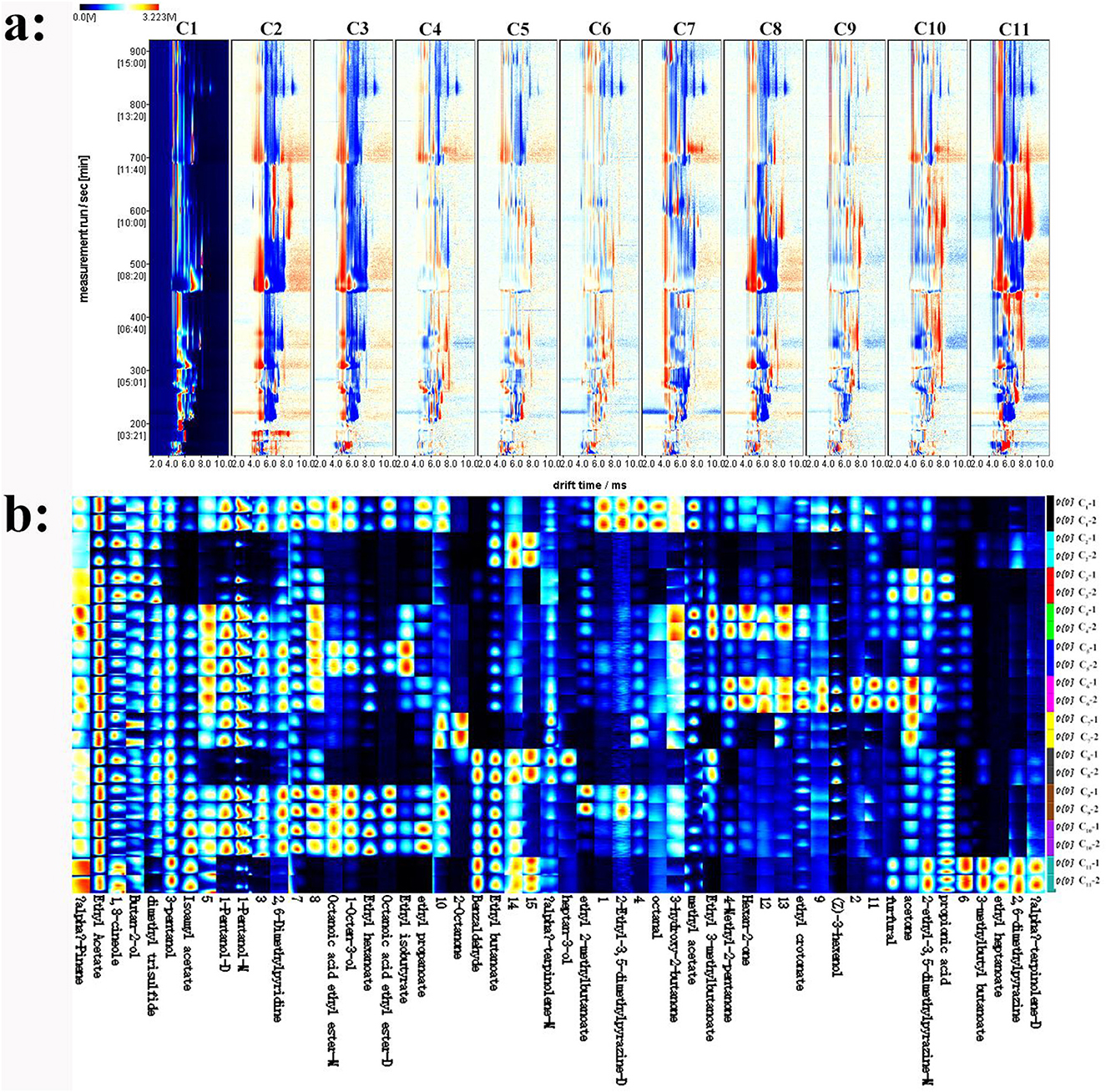
Figure 1. Analyzing volatile compounds in cherry wines with HS-GC-IMS. (a) Comparative difference map of volatile compounds. (b) Fingerprint of volatile compounds.
To clearly highlight the specific differences in compounds among cherry wines, each peak was chosen for fingerprint comparison (Figure 1b). The volatile compounds in different cherry wines were qualitatively characterized through fingerprint analysis. In Figure 1b, each row represents all the signal peaks selected for a specific sample, while each column corresponds to the signal peaks of the same volatile organic compounds (VOCs) across different cherry wines. The figure employs color coding to indicate the relative content of VOCs, providing a clear and intuitive visualization of their distribution and abundance. Brighter colors signify higher concentrations (20). This figure offers a comprehensive overview of the volatile compound profiles in each sample, highlighting the differences in VOC composition among them. It enables a detailed examination of the unique volatile compound profiles of individual samples and emphasizes the variations between them. Notably, α-pinene, ethyl acetate, 1,8-cineole, and 2-butanol are present in relatively high concentrations in the cherry wines. α-Pinene and 1,8-cineole contribute woody and resinous aromas, while ethyl acetate and 2-butanol impart a fruity aroma. These compounds may serve as key characteristic volatiles in cherry wine, significantly influencing its overall flavor profile. Among the samples, C1 contains the highest levels of octanal, 3-hydroxy-2-butanone, and methyl acetate. C2 exhibites the highest concentration of ethyl butyrate, which provides a pronounced fruity aroma. However, apart from ethyl butyrate and two unidentified compounds (designated as 14 and 15), the types and numbers of the remaining volatile compounds in C2 were substantially lower than in other samples. Additionally, 2-octanone was abundant in C7, imparting a distinct natural woody and herbal aroma.
3.3 Results from HS-SPME-GC-MS analysis of the cherry wines
To obtain a deeper awareness of the volatile organic compounds (VOCs) in cherry wines, the HS-SPME-GC-MS technique was employed. This analysis successfully identified 74 VOCs, such as 16 alcohols, 25 esters, 8 ketones, 6 phenols, 8 acids, 7 aldehydes, and 4 monoterpenoids (as detailed in Table 4). Marked differences were observed in the types and concentrations of volatile compounds among the 11 cherry wine varieties. In terms of total volatile compound concentration, C1 and C10 exhibited the highest levels, measuring 396.46 mg/L and 819.05 mg/L, respectively. In contrast, the total concentration in the remaining cherry wines ranged between 60 and 200 mg/L, with most samples clustering around 100 mg/L. Variations in volatile compound composition were also evident among the samples. Notably, C7 had the greatest number of volatile compounds (42 types) but had a relatively lower total concentration. In contrast, C10 had the fewest volatile compounds (27 types) but exhibited a significantly higher total concentration.
Among all the volatile compounds detected, esters were the most predominant in both quantity and content. These compounds are produced via the esterification of fatty acids with alcohols (21). Studies have shown that esters play a critical role in defining the aroma of fruit wines and are key contributors to their fruity taste and sweetness (22). Ethyl esters, in particular, are essential aroma components in fruit wines, with their concentrations influenced by factors including yeast strains, sugar content, and fermentation temperature. These compounds significantly enhance the overall quality of fruit wines, as most exhibit ripe and fruity aromas, contributing to the “floral” and “fruity” sensory characteristics (23). Ethyl acetate, ethyl caproate, ethyl lactate, and ethyl caprylate were detected in all 11 cherry wines, with their average concentrations exceeding their respective threshold levels. Previous studies have similarly concluded that ester compounds, including ethyl butyrate, ethyl acetate, ethyl caprylate, and ethyl lactate, impart a distinctive fruit aroma and play an essential role in the volatile flavor profile of fruit wines (24). Each ester contributes specific sensory attributes: ethyl acetate typically imparts apple and banana aromas, ethyl caproate provides pineapple and strawberry aroma, ethyl lactate is associated with fruity and creamy aromas, and ethyl caprylate exhibits fruity and floral characteristics.
Higher alcohols are essential secondary metabolites generated during the fermentation process of Saccharomyces cerevisiae. At appropriate levels, they result in the smoothness and balance of cherry wines (25). However, the types and content of alcohols varied significantly among the 11 cherry wine samples. C2, C3, and C11 contained the fewest detected alcohols. Isoamyl alcohol, phenyl ethanol, and benzyl alcohol were present in all samples except C11. Phenylethanol and isoamyl alcohol, known for their floral and grassy aromas, are likely generated via yeast metabolism through the Ehrlich pathway (26). Additionally, certain alcohols were exclusive to specific wine samples. For instance, 1-butanol and n-decanol were detected only in C9, while 2-propyl-1-amyl alcohol, 1-cetanol, and 2-methyl-1-amyl alcohol were unique to C4. Diisobutylcarbinol was found only in C8, whereas 1-nonyl alcohol and 2-nonyl alcohol were exclusive to C7. Current studies have demonstrated that differences in raw materials for winemaking (such as cherry varieties) (27) and variations in fermentation process parameters (e.g., yeast strains, fermentation temperature, pH, etc.) (28) can significantly influence the yeast metabolic pathways, thereby altering the composition and content of higher alcohols. The differences observed among samples in this study may stem from the application of the aforementioned factors in the production processes of different commercial wines.
Acid compounds in cherry wines primarily originate during fermentation. Their concentrations are influenced by two key factors: Acid compounds in cherry wines primarily originate from cherry juice and yeast fermentation. Cherry juice contains citric acid, malic acid, and other organic acids, and yeast produces a large quantity of acids through glycolysis (the EMP pathway) during the fermentation. Furthermore, yeast may generate a small amount of acetic acid via the acetaldehyde pathway) under oxygen-deficient or stressful conditions (such as excessively high temperature or nutrient deficiency). Because of their high odor thresholds, acids are conducive minimally to the overall flavor profile of cherry wines. In this study, nine acids were characterized, with acetic acid, caprylic acid, and capric acid detected in all 11 samples. This aligns with previous research, confirming the consistent presence of these acids in cherry wines (10). Additionally, the corresponding ethyl esters of these acids were also detected, indicating active esterification of ethanol with free fatty acids. The presence of these fatty acids suggests that a robust esterification reaction occurred during fermentation, playing a key role in the chemical transformation of the wine—making process. This reaction also significantly influences the flavor and aroma characteristics of cherry wines. It is noteworthy that benzoic acid was detected in samples C4 and C6 of this study. Although cherry raw materials may inherently contain benzoic acid and its precursors (29), in alcoholic products, the detection of free benzoic acid is generally regarded as an indicator of the addition of preservatives such as sodium benzoate. This may be a process measure adopted by producers to ensure product stability.
Seven aldehydes were identified in cherry wines, with benzaldehyde being present in all samples. Notably, C8 and C11 demonstrated markedly higher benzaldehyde concentrations in comparison to the other samples, surpassing its odor threshold. This suggests that benzaldehyde could have a more pronounced influence on the aroma profile of C8 and C11. At appropriate concentrations, benzaldehyde imparts fruity, sweet, nutty, and caramel-like notes (30). Although aldehydes generally have low OAV values, they can enhance woody aroma intensity while reducing fruity aroma perception (31). The sensory analysis of C11 revealed it has a strong oak aroma, further supporting the role of benzaldehyde and other aldehydes in shaping the peculiar flavor profile of cherry wines.
Apart from the previously mentioned volatile compounds, 9 types of ketones and 6 types of phenols were also detected in the cherry wines. Although these compounds present in relatively low concentrations, their low sensory thresholds allow them to significantly influence the overall flavor profile. Research findings indicate that ketones have the ability to augment the flavor complexity in cherry wines. They play a pivotal role in orchestrating the release of various aromas (32).
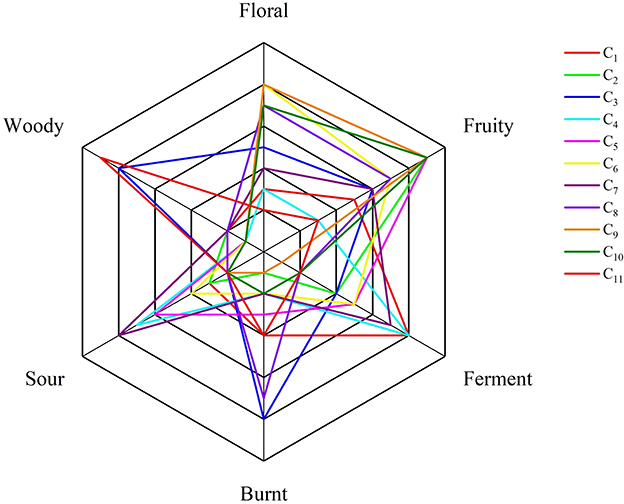
3.4 Sensory evaluation
The sensory evaluation and aroma scoring results for cherry wine, assessed by 14 trained panelists, are illustrated in Figure 2. The sensory profiles of the cherry wines encompass a diverse range of aroma attributes, including fruity, sour, woody, fermented, roasted, and floral notes. Notably, most cherry wines exhibited dominant floral and fruity characteristics, suggesting that these aroma components significantly enhance the overall aromatic appeal of cherry wine. This observation aligns with the volatile compound analysis, where esters and alcohols key contributors to floral and fruity aromas were observed in high concentrations.
3.5 Comparison of analysiss between HS-GC-IMS and HS-SPME-GC-MS
3.5.1 Comparison of PCA analysis results
PCA is an unsupervised multivariate statistical analysis technique that effectively captures variations in volatile components among samples. By applying PCA, researchers can assess the consistency and variability of different samples, facilitating more accurate comparisons and interpretations of similarities and differences. In PCA plots, samples with similar flavor characteristics cluster together, whereas those with distinct flavor profiles are positioned farther apart. As exhibited in Figure 3, PCA results of HS-SPME-GC-MS and HS-GC-IMS revealed certain variations. Notably, C1, C4, C5, C6, and C7 were closely positioned in both analytical methods, suggesting minimal differences in their volatile compound profiles. This finding indicates that these cherry wines likely share similar aroma characteristics. In contrast, C11 was distinctly separated from the other samples, highlighting significant differences in its volatile compound composition. This separation suggests that C11 possesses a unique aromatic profile, potentially contributing to distinct sensory attributes compared to the other cherry wine samples.
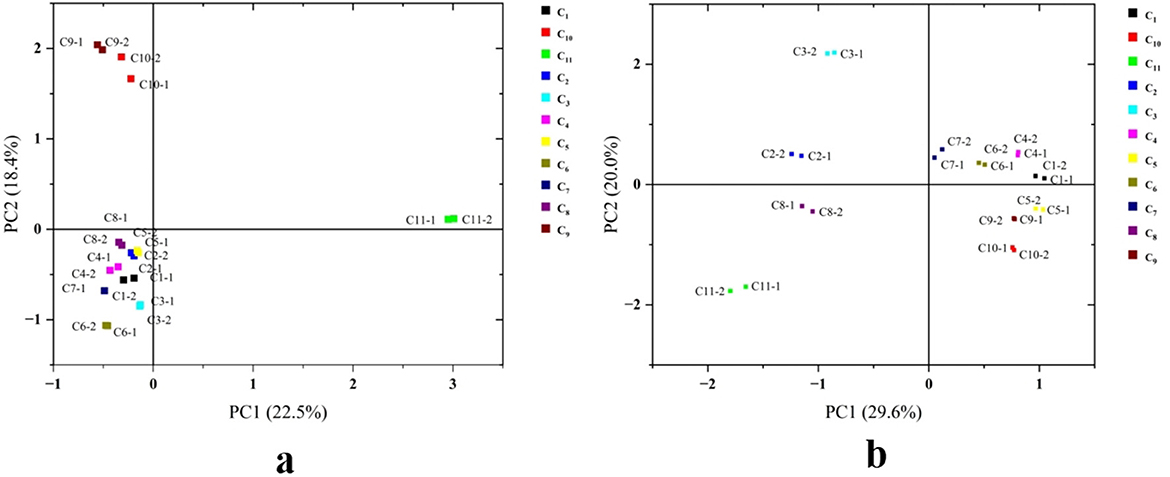
Figure 3. PCA analysis of cherry wines. (a) PCA score plot for HS-SPME-GC-MS. (b) PCA score plot for HS-GC-IMS.
3.5.2 A comparison of the detection results from HS-GC-IMS and HS-SPME-GC-MS
The flavor compounds identified using the HS-SPME-GC-MS and HS-GC-IMS techniques were predominantly esters and alcohols, reinforcing their key role in determining the flavor profile of cherry wines. This result aligns with previous research, confirming the substantial contribution of flavor compounds to the overall sensory characteristics of cherry wines. However, notable differences were observed between the two analytical methods. Compared to GC-MS, GC-IMS demonstrated superior sensitivity in detecting dimer volatiles, terpenes, and heterocyclic compounds. This advantage stems from the distinct detection principles of IMS and MS (33). Despite its strengths, HS-GC-IMS is an effective method in food flavor analysis, but its compound identification capability is constrained by the NIST database, making it unable to identify some compounds. Some of these unidentified compounds may have a significant impact on flavor. As a result, certain compounds remain unidentified, some of which could significantly influence the flavor profile (34). For example, in the HS-GC-IMS results (Figure 1b), unidentified compounds 3, 7, 8, 10, and 15 were present at high levels in the cherry wines, suggesting their potential importance in defining the wine's aroma. Regarding the identification of volatile components, HS-SPME-GC-MS identified 75 compounds, whereas HS-GC-IMS identified only 49 compounds. Some compounds detected by HS-GC-IMS were absent in HS-SPME-GC-MS, including ethyl propionate, ethyl isovalerate, 1-octene-3-ol, (Z)-3-hexanol, 1-pentanol-M, 1-pentanol-D, 1,8-cineole, 3-pentanol, and tertiary amyl alcohol. These differences highlight the complementary nature of the two techniques. To achieve a comprehensive characterization of volatile flavor compounds in cherry wines, a combined approach with both HS-SPME-GC-MS and HS-GC-IMS is necessary. Together, these methods provide a more complete and nuanced understanding of the wine's flavor composition.
3.6 The key flavor compounds of cherry wines
3.6.1 OPLS-DA analysis
To assess each VOC's contribution to the flavor profile of cherry wines, the VIP values within the OPLS-DA model were analyzed. VOCs with VIP values over 1 were considered remarkable contributors to the overall aroma and taste characteristics (35). Figures 4a, b display the VIP values of VOCs identified by HS-SPME-GC-MS and HS-GC-IMS, respectively, according to the OPLS-DA model. From the HS-SPME-GC-MS analysis, five VOCs including benzaldehyde, 1-butanol, 2-methyl, 3-furaldehyde, 3-methyl, 1-propanol, and ethyl acetate exhibited VIP values exceeding 1, indicating their major influence on the flavor profile. These compounds contribute distinct sensory attributes, with benzaldehyde imparting sweet, nutty, and fruity notes, while ethyl acetate enhances fruity and floral characteristics. In contrast, HS-GC-IMS identified 14 VOCs with VIP values over 1, emphasizing the broader range of impactful compounds detected by this method. These key contributors included (Z)-3-hexenol, 1-pentanol-M, ethyl hexanoate, ethyl butanoate, isoamyl acetate, benzaldehyde, ethyl acetate, 3-methylbutyl butanoate, 1,8-cineole, methyl acetate, alpha-terpinolene-M, dimethyl trisulfide, 3-pentanol, and 2-octanone. The presence of additional esters, alcohols, and terpenes in this list highlights the enhanced sensitivity of HS-GC-IMS in detecting flavor-relevant compounds. These findings confirm that benzaldehyde and ethyl acetate were consistently identified as significant contributors across both analytical methods, further reinforcing their importance in determining the characteristic aroma of cherry wines. The differences in detected key VOCs also underscore the necessity of combining HS-SPME-GC-MS and HS-GC-IMS to achieve a comprehensive understanding of cherry wine flavor composition.
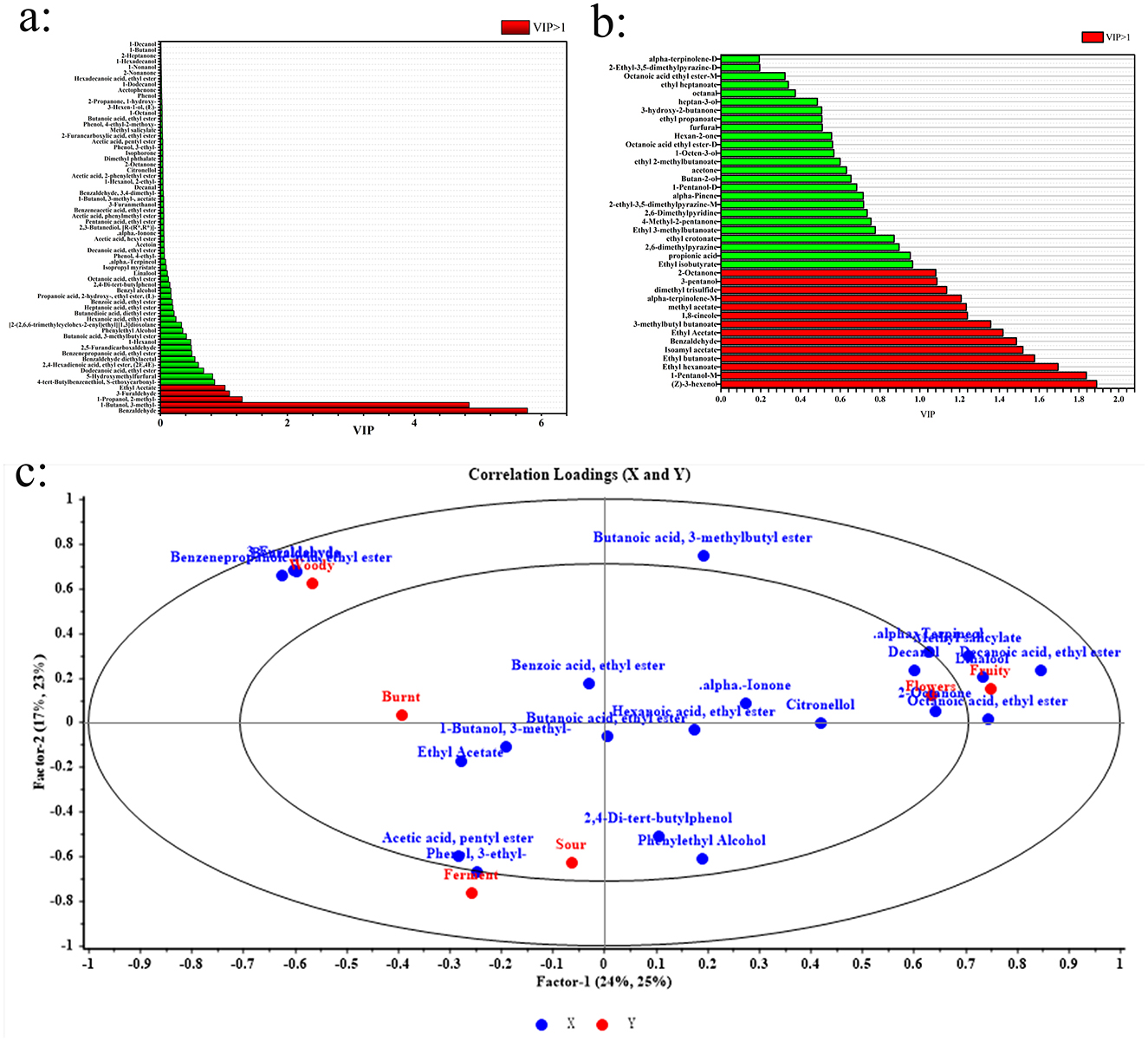
Figure 4. Analysis of key flavor compounds in cherry wines. (a) VIP rankings of HS-SPME-GC-MS. (b) VIP rankings of HS-GC-IMS. (c) PLSR analysis of characteristic flavor compounds and sensory properties of cherry wines.
3.6.2 The determination of the OAV by HS-SPME-GC-MS and the ROAV by HS-GC-IMS
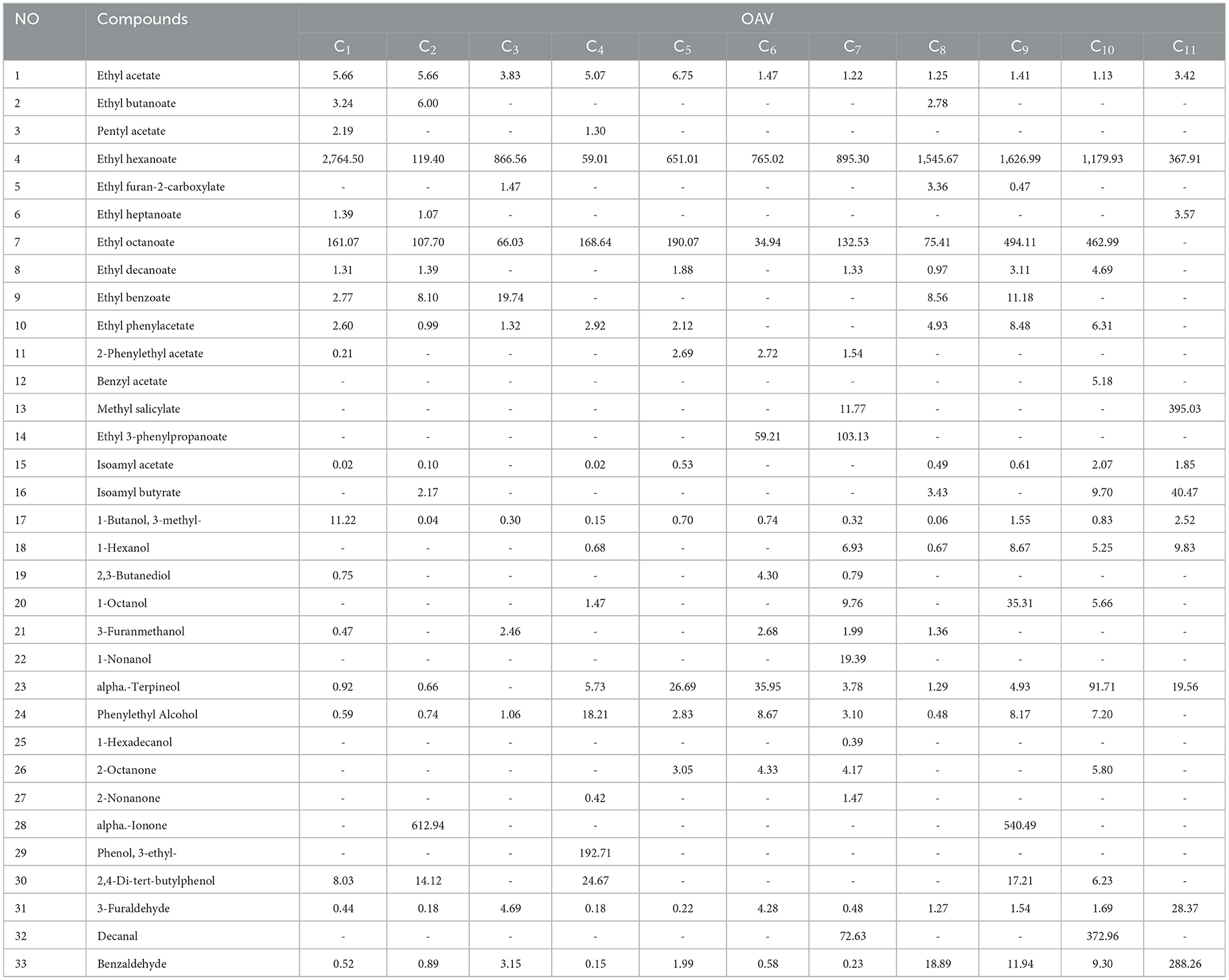
To better understand the volatile flavor compounds' contribution to the overall composition of cherry wines, the OAV of VOCs was calculated. The OAV is determined by dividing the concentration of a compound by its corresponding odor threshold. A higher OAV indicates a stronger influence of the compound on the wine's overall flavor profile. Combining HS-SPME-GC-MS with OAV analysis provides an effective method for identifying the key flavor compounds in cherry wines. As shown in Table 5, compounds with an OAV >1 included 12 esters, 6 alcohols, 4 ketones, 2 phenols, and 3 aldehydes. Specifically, the esters detected were ethyl acetate, ethyl butyrate, isoamyl acetate, ethyl heptanoate, ethyl hexanoate, ethyl octanoate, ethyl benzoate, ethyl phenylacetate, methyl salicylate, ethyl phenylpropionate, and isoamyl butyrate. Among the alcohols, n-hexanol was identified. The terpenoid compounds included linalool, α-terpineol, and citronellol, while the ketones comprised 2-octanone, α-ionone, and isophorone. The phenolic compounds detected were 3-ethylphenol and 2,4-di-tert-butylphenol, and the aldehydes included decanal and benzaldehyde. The key flavor compounds identified were consistent with previous studies (11). Isoamyl acetate and ethyl hexanoate contributed a banana-like aroma, while ethyl octanoate imparted a pineapple aroma. Ethyl acetate and ethyl butyrate enhanced the fruity and floral scent. 2-Octanone and α-ionone provided fruity characteristics, whereas n-hexanol reinforced the floral aroma. Benzaldehyde was a major contributor to the woody aroma, and monoterpenes such as citronellol, geraniol, and α-terpineol contributed floral, fruity, and citrusy nuances.
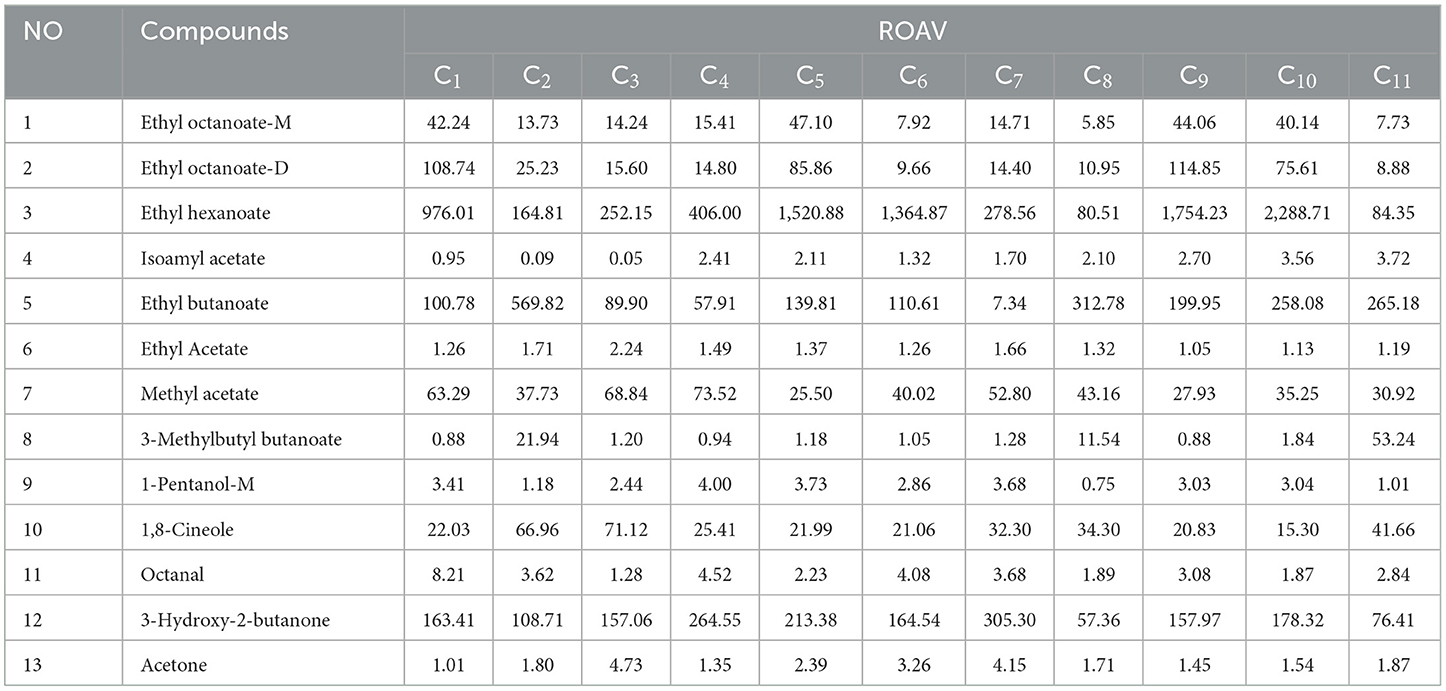
Compared to OAV analysis, ROAV analysis only requires data of relative concentration. Hence, ROAV analysis was performed using the volatile compounds identified through HS-GC-IMS to determine the key volatile flavor compounds in cherry wine. As exhibited in Table 6, 13 volatile organic compounds with ROAV > 1 were identified, such as ethyl hexanoate, ethyl octanoate-M, ethyl octanoate-D, ethyl butyrate, ethyl isovalerate, ethyl acetate, ethyl formate, butyl 3-methylbutyrate, 1-pentanol-M, 1,8-cineole, octyl aldehyde, 3-hydroxy-2-butanone, and acetone. In the OAV analysis, ethyl acetate, ethyl hexanoate, ethyl octanoate, and ethyl butyrate all showed OAV values over 1, confirming their significant contribution to the overall aroma. To further refine the identification of key flavor compounds, the OAV, ROAV, and VIP values were integrated. Among the esters, ethyl butyrate, ethyl acetate, amyl acetate, ethyl hexanoate, ethyl benzoate, ethyl octanoate, methyl salicylate, ethyl phenylpropionate, 3-methylbutyl butyrate, ethyl 3-methylbutyrate, and methyl acetate were included; among the alcohols, 3-methyl-1-butanol and phenylethyl alcohol and were included; among the ketone compounds, 2-octanone, α-nonanone, 3-hydroxy-2-butanone, acetone, phenol, 3-ethylphenol, and 2,4-di-tert-butylphenol were included; among the aldehyde compounds, decanal, benzaldehyde, and 3-furaldehyde were important components of the flavor of cherry wine.
3.6.3 Characteristic flavor compounds correlated with sensory properties
To further investigate the rrelationship between characteristic flavor compounds and sensory characteristics, PLSR analysis was performed. In this analysis, VOCs with OAV > 1 were designated as the X variables, while sensory characteristics were set as the Y variables. The ellipses in the PLSR plot represent explained variances of 50% and 100%, respectively. If most variables fall within these ellipses, it indicates that the PLSR model effectively explains their relationships. As shown in Figure 4c, the majority of key flavor compounds exhibited a positive correlation with floral and fruity aromas. Specifically, “fruity” and “floral” characteristics were strongly associated with linalool, α-terpinol, isophorone, and ethyl caprylate. The “woody” aroma showed a strong correlation with 3-furfural and benzaldehyde, while the “scorched” aroma was closely linked to 1,8-cineole. Although these individual compounds may not independently produce a distinct sensory aroma, their interactions with other volatile compounds in cherry wine likely contribute to the formation of its characteristic flavor profile. Overall, PLSR analysis further validated the relationship between key flavor compounds and sensory properties, providing deeper insights into the flavor composition of cherry wine.
4 Conclusion
This work comprehensively identified the volatile flavor compounds in cherry wine using HS-SPME-GC-MS and HS-GC-IMS techniques. A total of 101 volatile compounds were identified, with esters and alcohols being the predominant contributors to the overall flavor profile. Among them, ethyl acetate, ethyl caprylate, and hexyl acetate were critical compounds accountable for the fruity aroma, while 3-furfural and benzaldehyde contributed to the woody notes. The integration of OAV and ROAV analyses enabled the identification of 28 primary flavor compounds, further validated through sensory evaluation. Notably, cherry wine samples C5, C9, and C10 exhibited the most pronounced floral and fruity characteristics. The results of this work are of great significance for clarifying the characteristic flavor substances of cherry wines, promoting the improvement of cherry wine quality, and accelerating product innovation.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JQ: Data curation, Formal analysis, Investigation, Software, Writing – original draft, Writing – review & editing. JL: Investigation, Methodology, Software, Writing – review & editing. ZL: Conceptualization, Methodology, Writing – review & editing. XY: Software, Visualization, Writing – review & editing. TQ: Investigation, Methodology, Validation, Writing – review & editing. GW: Methodology, Software, Writing – review & editing. JY: Data curation, Validation, Writing – review & editing. CL: Conceptualization, Funding acquisition, Methodology, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by Key Research and Development Program of Shandong Province (2023TZXD059) and the Young Scientists' Research Fund of Shandong Institute of Pomology (GSS2022QN03).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Liu B, Yang Y, Ren L, Su Z, Bian X, Fan J, et al. HS-GC-IMS and PCA to characterize the volatile flavor compounds in three sweet cherry cultivars and their wines in China. Molecules. (2022) 27:9056. doi: 10.3390/molecules27249056
2. Fonseca LRS, Silva GR, Luís Â, Cardoso HJ, Correia S, Vaz CV, et al. Sweet cherries as anti-cancer agents: from bioactive compounds to function. Molecules. (2021) 26:2941. doi: 10.3390/molecules26102941
3. Wattanakul N, Morakul S, Lorjaroenphon Y, Jom KN. Integrative metabolomics-flavoromics to monitor dynamic changes of ‘Nam Dok Mai' mango (Mangifera indica Linn) wine during fermentation and storage. Food Biosci. (2020) 35:100549. doi: 10.1016/j.fbio.2020.100549
4. Li N, Li G, Guan X, Li A, Tao Y. Volatile aroma compound-based decoding and prediction of sweet berry aromas in dry red wine. Food Chem. (2025) 463:141248. doi: 10.1016/j.foodchem.2024.141248
5. Su Z, Liu B, Ma C. Analyses of the volatile compounds in cherry wine during fermentation and aging in bottle using HS-GC-IMS: note. Food Sci Technol Res. (2021) 27:599–607. doi: 10.3136/fstr.27.599
6. Niu Y, Zhang X, Xiao Z, Song S, Jia C, Yu H, et al. Characterization of taste-active compounds of various cherry wines and their correlation with sensory attributes. J Chromatogr. (2012) 902:55–60. doi: 10.1016/j.jchromb.2012.06.015
7. Wang S, Chen H, Sun B. Recent progress in food flavor analysis using gas chromatography-ion mobility spectrometry (GC-IMS). Food Chem. (2020) 315:126158. doi: 10.1016/j.foodchem.2019.126158
8. Zhao X, Wang Z, Tang F, Cai W, Peng B, Shan C. Exploring jujube wine flavor and fermentation mechanisms by HS-SPME-GC–MS and UHPLC-MS metabolomics. Food Chem X. (2024) 21:101115. doi: 10.1016/j.fochx.2024.101115
9. Pantelic M, Dabic D, Matijasevic S, Davidovic S, Dojcinovic B, Milojkovic-Opsenica D, et al. Chemical characterization of fruit wine made from Oblačinska sour cherry. Sci World J. (2014) 09:454797. doi: 10.1155/2014/454797
10. Xiao Z, Liu S, Gu Y, Xu N, Shang Y, Zhu J. Discrimination of cherry wines based on their sensory properties and aromatic fingerprinting using HS-SPME-GC-MS and multivariate analysis. J Food Sci. (2014) 79:C284–94. doi: 10.1111/1750-3841.12362
11. Niu Y, Wang P, Xiao Z, Zhu J, Sun X, Wang R. Evaluation of the perceptual interaction among ester aroma compounds in cherry wines by GC-MS, GC-O, odor threshold and sensory analysis: an insight at the molecular level. Food Chem. (2019) 275:143–53. doi: 10.1016/j.foodchem.2018.09.102
12. Xie X, Wang Y, Wen B, Tian J, Cheng Z, Tang S, et al. Characterization and metabolism pathway of volatile compounds in blueberries of different varieties and origins analyzed via HS-GC-IMS and HS-SPME-GC–MS. Food Chem. (2025) 480:143813. doi: 10.1016/j.foodchem.2025.143813
13. Albertin W, Setati M, Miot-Sertier C, Mostert T, Colonna-Ceccaldi B, Coulon J, et al. Hanseniaspora uvarum from winemaking environments show spatial and temporal genetic clustering. Front Microbiol. (2016) 6:1569. doi: 10.3389/fmicb.2015.01569
14. Wang C, Mas A, Esteve-Zarzoso B. Interaction between Hanseniaspora uvarum and Saccharomyces cerevisiae during alcoholic fermentation. Int J Food Microbiol. (2015) 206:67–74. doi: 10.1016/j.ijfoodmicro.2015.04.022
15. Zhang BQ, Luan Y, Duan CQ, Yan GL. Use of Torulaspora delbrueckii co-fermentation with two Saccharomyces cerevisiae strains with different aromatic characteristic to improve the diversity of red wine aroma profile. Front Microbiol. (2018) 9:606. doi: 10.3389/fmicb.2018.00606
16. Ao H, Tang C, Lu Y, Zhang Y, He L, Qiu S, et al. Characterization of physicochemical properties, sensory characteristics, and volatile compounds with a special focus on the terpene profile of commercial Chinese kiwifruit wines. J Food Compos Anal. (2025) 140:107187. doi: 10.1016/j.jfca.2025.107187
17. Zhou Y, Fei G, Faridul Hasan KM, Kang Y, Wu Y, Li H, et al. Cultivar difference characterization of kiwifruit wines on phenolic profiles, volatiles and antioxidant activity. Food Chem X. (2023) 18:100691. doi: 10.1016/j.fochx.2023.100691
18. Zhang Y, Chang SKC, Stringer SJ, Zhang Y. Characterization of titratable acids, phenolic compounds, and antioxidant activities of wines made from eight mississippi-grown muscadine varieties during fermentation. LWT. (2017) 86:302–11. doi: 10.1016/j.lwt.2017.07.038
19. Xiong Y, Zheng X, Tian X, Wang C, Chen J, Zhou L, et al. Comparative study of volatile organic compound profiles in aromatic and non-aromatic rice cultivars using HS-GC-IMS and their correlation with sensory evaluation. LWT. (2024) 203:116321. doi: 10.1016/j.lwt.2024.116321
20. Zheng A, Wei C, Wang M, Ju N, Fan M. Characterization of the key flavor compounds in cream cheese by GC-MS, GC-IMS, sensory analysis and multivariable statistics. Curr Res Food Sci. (2024) 8:100772. doi: 10.1016/j.crfs.2024.100772
21. Seung J, Boml A. Comparison of volatile components in fermented soybean pastes using simultaneous distillation and extraction (SDE) with sensory characterization. Food Chem. (2009) 114:600–8. doi: 10.1016/j.foodchem.2008.09.091
22. Qin Z, Mikael A, Wender L. Flavor profiling of apple ciders from the UK and Scandinavian region. Food Res Int. (2018) 105:713–23. doi: 10.1016/j.foodres.2017.12.003
23. Perestrelo A, Fernandes F, Albuquerque J, Marques J. Camara analytical characterization of the aroma of tinta negra mole red wine: identification of the main odorants compounds. Anal Chim Acta. (2006) 563:154–64. doi: 10.1016/j.aca.2005.10.023
24. Li X, Xing Y, Cao L, Xu Q, Li S, Wang R, et al. Effects of six commercial Saccharomyces cerevisiae strains on phenolic attributes. Antioxidant activity, and aroma of kiwifruit (Actinidia deliciosa cv) wine. Biomed Res Int. (2017) 10:2934743. doi: 10.1155/2017/2934743
25. Zhang W, Zhang L, Xu C. Chemical and volatile composition of jujube wines fermented by Saccharomyces cerevisiae with and without pulp contact and protease treatment. Food Sci Technol. (2016) 36:204–9. doi: 10.1590/1678-457X.0011
26. El-Dalatony MM, Saha SP, Govindwar S, Abou-Shanab RAI, Jeon BH. Biological conversion of amino acids to higher alcohols. Trends Biotechnol. (2019) 37:855–69. doi: 10.1016/j.tibtech.2019.01.011
27. Chen H, Bai S, Yang B, Ren R, Tang Z, Zhang Z, et al. Inter- and intra-varietal clonal differences influence the aroma compound profiles of wines analyzed by GC–MS and GC-IMS. Food Chem X. (2025) 25:102136. doi: 10.1016/j.fochx.2024.102136
28. Sun SY, Jiang WG, Zhao YP. Evaluation of different Saccharomyces cerevisiae strains on the profile of volatile compounds and polyphenols in cherry wines. Food Chem. (2011) 127:547–55. doi: 10.1016/j.foodchem.2011.01.039
29. Zhang W, Zhu D, Mao J, Du H, Qin H, Wang J, et al. (2024). Insight into flavor difference of cherry (Prunus avium L.) grown in facility environment and outdoors through metabolomics and correlation analysis. Food Chem X. 24:101802. doi: 10.1016/j.fochx.2024.101802
30. Zea L, Moyano L, Moreno J, Cortes MB, Medina M. Discrimination of the aroma fraction of sherry wines obtained by oxidative and biological ageing. Food Chem. (2001) 75:79–84. doi: 10.1016/S0308-8146(01)00190-X
31. Andrei P, Pascal C. Impact of oak-derived compounds on the olfactory perception of barrel-aged wines. Am J Enol Vitic. (2010) 61:408–13. doi: 10.5344/ajev.2010.61.3.408
32. Wang Z, Wang Y, Zhu T, Wang J, Huang M, Wei J, et al. Characterization of the key odorants and their content variation in Niulanshan Baijiu with different storage years using flavor sensory omics analysis. Food Chem. (2022) 376:131851. doi: 10.1016/j.foodchem.2021.131851
33. Sorribes-Soriano A, De la Guardia M, Esteve-Turrillas FA, Armenta S. Trace analysis by ion mobility spectrometry: From conventional to smart sample preconcentration methods. A review. Analytica Chimica Acta. (2018) 1026:37–50. doi: 10.1016/j.aca.2018.03.059
34. Yao W, Ma S, Wu H, Liu D, Liu J, Zhang M. Flavor profile analysis of grilled lamb seasoned with classic salt, chili pepper, and cumin (Cuminum cyminum) through HS-SPME-GC-MS, HS-GC-IMS, E-Nose techniques, and sensory evaluation on sonit sheep. Food Chem. (2024) 454:139514. doi: 10.1016/j.foodchem.2024.139514
35. Liu N, Shen S, Huang L, Deng G, Wei Y, Ning J, et al. Revelation of volatile contributions in green teas with different aroma types by GC–MS and GC–IMS. Food Res Int. (2023) 169:112845. doi: 10.1016/j.foodres.2023.112845
36. Xiao Q, Zhou X, Xiao Z, Niu Y. Characterization of the differences in the aroma of cherry wines from different price segments using gas chromatography–mass spectrometry, odor activity values, sensory analysis, and aroma reconstitution. Food Sci Biotechnol. (2017) 26:331–8. doi: 10.1007/s10068-017-0045-y
37. Cao W, Shu N, Wen J, Yang Y, Jin Y, Lu W. Characterization of the key aroma volatile compounds in nine different grape varieties wine by headspace gas chromatography–ion mobility spectrometry (HS-GC-IMS), odor activity values (OAV) and sensory analysis. Foods. (2022) 11:2767. doi: 10.3390/foods11182767
38. Naranjo A, Martínez-Lapuente L, Ayestarán B, Guadalupe Z, Pérez I, Canals C, et al. Aromatic and sensory characterization of maturana blanca wines made with different technologies. Beverages. (2021) 7:10. doi: 10.3390/beverages7010010
39. Pérez-Navarro J, Izquierdo-Cañas P, Mena-Morales A, Chacón-Vozmediano J, Martínez-Gascueña J, García-Romero E, et al. Comprehensive chemical and sensory assessment of wines made from white grapes of vitis vinifera cultivars albillo dorado and montonera del casar: a comparative study with airén. Foods. (2020) 9:1282. doi: 10.3390/foods9091282
Keywords: cherry wine, volatile flavor compounds, sensory evaluation, characteristic flavor compounds, HS-GC-IMS, HS-SPME-GC-MS
Citation: Qi J, Li J, Liu Z, Yang X, Qi T, Wei G, Yang J and Liu C (2025) Analysis of key flavor compounds in commercial cherry wines by HS-SPME-GC-MS, HS-GC-IMS, and sensory evaluation. Front. Nutr. 12:1631912. doi: 10.3389/fnut.2025.1631912
Received: 20 May 2025; Accepted: 15 September 2025;
Published: 03 October 2025.
Edited by:
Susana Casal, University of Porto, PortugalReviewed by:
Eduardo Dellacassa, Universidad de la República, UruguayAntónio Manuel Jordão, Instituto Politecnico de Viseu, Portugal
Copyright © 2025 Qi, Li, Liu, Yang, Qi, Wei, Yang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheng Liu, dG9uZ2RvdTFAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Jiawei Qi
Jiawei Qi Jie Li2†
Jie Li2† Guoqin Wei
Guoqin Wei Cheng Liu
Cheng Liu