- 1Key Lab of Health Technology Assessment of Ministry of Health, School of Public Health, Fudan University, Shanghai, China
- 2Department of Obstetrics, The People's Hospital of Pingyang, Wenzhou, Zhejiang, China
- 3Joseph L. Mailman School of Public Health, Columbia University, New York, NY, United States
- 4Wenzhou Center for Disease Control and Prevention, Wenzhou, Zhejiang, China
- 5School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, ON, Canada
Introduction: Gestational diabetes mellitus (GDM) poses significant health risks for both the mother and fetus, and it also increases the mother’s risk of developing type 2 diabetes later in life. Mineral elements may play a crucial role in the development of GDM by influencing insulin metabolism. However, comprehensive studies on serum mineral levels during pregnancy remain limited. This study aims to evaluate the relationship between serum mineral levels in pregnant women during the second and third trimesters and the risk of developing GDM.
Methods: This retrospective cohort study included 17,224 singleton pregnancies delivered between 2016 and 2022 at a tertiary hospital in China. Maternal demographic data and serum mineral concentration information from the mid and late stages of pregnancy were collected through the hospital information system. Analyses were conducted using restricted cubic spline models and multivariate logistic regression models.
Results: The prevalence of GDM in this study was 15.07%. Chloride [P for overall = 0.01; P for non-linear = 0.373; OR (95% CI) = 1.03 (1.01, 1.05)] showed a significant linear positive association with GDM. Additionally, serum levels of calcium (P for non-linear < 0.001), potassium (P for nonlinear = 0.036), and magnesium (P for nonlinear < 0.001) were found to have non-linear relationships with the risk of GDM. The interactions between calcium and magnesium [OR (95% CI) = 0.05 (0.01, 0.27), P for interaction < 0.001], potassium and magnesium [OR (95% CI) = 0.11 (0.03, 0.37), P for interaction < 0.001], and potassium and chloride [OR (95% CI) = 1.06 (1.01, 1.11), P for interaction < 0.001] were significant.
Discussion: The study indicates that specific serum mineral levels in pregnant women are closely associated with the risk of gestational diabetes mellitus. A deeper understanding of the mechanisms and interactions of these minerals could aid in developing effective prevention and treatment strategies, thereby reducing the incidence of GDM and improving pregnancy outcomes.
1 Introduction
Gestational diabetes mellitus (GDM) is a type of diabetes that first appears or is diagnosed during pregnancy due to impaired glucose tolerance and is one of the most common metabolic disorders in pregnant women (1). GDM can lead to severe adverse health outcomes for both the mother and fetus, including macrosomia, low birth weight, and congenital abnormalities (2). Additionally, women with GDM have a higher risk of developing type 2 diabetes (T2DM) later in life (3). In 2021, the International Diabetes Federation (IDF) released a report titled “Global Diabetes Map,” which indicated that the global prevalence of GDM is approximately 16.7% (4). In China, the overall prevalence of GDM is about 14.8% (5). However, with the recent implementation of the two-child and even three-child policies, the increasing number of elder pregnant women, and improvements in economic development, living standards, and lifestyle changes, the number of GDM patients in China continues to rise (6).
In addition to traditional risk factors such as overweight and obesity, advanced maternal age, ethnicity, history of GDM, and a family history of T2DM, mineral elements may also play a significant role in the occurrence of GDM (7–9). Minerals may influence the occurrence of GDM through their effects on the secretion, synthesis, storage, and metabolism of insulin (10). Trace elements are closely related to the incidence of GDM. Insufficient intake of trace elements can impair cellular and tissue functions, leading to GDM and pregnancy-related hypertension (11). Some studies have shown that the plasma level of zinc (Zn) is negatively correlated with GDM risk, with Zn promoting the development of GDM by disrupting insulin metabolism and glucose homeostasis (12, 13). Other research suggests that high levels of iron (Fe) and copper (Cu) increase the incidence of GDM (14). Elevated Fe levels can induce oxidative stress, β-cell toxicity, and insulin resistance, potentially leading to an increased risk of GDM (15).
It is noteworthy that macroelements also play an important role in glucose metabolism and insulin function. Evidence indicates that deficiencies in calcium and magnesium may increase the risk of GDM by impairing insulin secretion and promoting insulin resistance (16, 17). In addition, elevated potassium levels and imbalances between sodium and potassium have been associated with a higher risk of GDM (18, 19). Abnormal phosphate levels and disturbances in chloride balance may also indirectly influence insulin action by affecting energy metabolism and acid–base homeostasis (20, 21). Therefore, specific abnormalities in both trace and macroelements levels may contribute to the development of GDM through their effects on insulin function and glucose metabolism.
Although the impact of trace elements on GDM has been extensively studied, there is a lack of research on the relationship and interactions between macroelements in minerals and GDM. Therefore, investigating this relationship is of considerable significance. Hence, we conducted a large-scale retrospective epidemiological study to examine the association between serum mineral levels in the second and third trimesters of pregnancy and the occurrence of GDM.
2 Materials and methods
2.1 Study population
This study was a retrospective cohort study involving 17,224 pregnant women at the People’s Hospital of Pingyang, Wenzhou City, Zhejiang Province, China, conducted between January 2016 and December 2022. The hospital is the primary provider of medical services in Pingyang County and operates as the only tertiary hospital in the area. Most pregnant women in the region choose this hospital for delivery. The hospital has an effective information system that archives demographic and clinical data for each patient. In this study, all participants underwent pregnancy registration and examination, with all cases involving singleton deliveries.
Inclusion Criteria: (1) Met the diagnostic criteria for GDM (22); (2) Singleton pregnancies; (3) Natural conception or in vitro fertilization; (4) Received routine prenatal care.
Exclusion Criteria: (1) Diabetes complicating pregnancy (pre-existing diabetes); (2) Pregnancy-related hypertensive disorders, including chronic hypertension (pre-existing), gestational hypertension, preeclampsia, and eclampsia; (3) Missing data and other reasons.
The study was approved by the Medical Research Ethics Committee of the School of Public Health, Fudan University (The international registry no. IRB00002408 and FWA00002399). This retrospective study utilized existing data with all personally identifiable information removed. Informed consent was not required, which was also approved by the Medical Research Ethics Committee of the School of Public Health, Fudan University.
2.2 Data collection and measurement
The demographic and clinical data were obtained from the hospital information system.
The diagnostic criteria for GDM, as recommended by the Diabetes and Pregnancy Study Group (IADPSG), are based on the oral glucose tolerance test (OGTT) conducted between 24 and 28 weeks of gestation. GDM was diagnosed if any of the following criteria were met: fasting plasma glucose ≥ 5.1 mmol/L (≥92 mg/dL), 1-h plasma glucose ≥ 10.0 mmol/L (≥180 mg/dL), or 2-h plasma glucose ≥ 8.5 mmol/L (≥153 mg/dL) (23).
Mineral information was obtained from the prenatal check-up records before hospitalization for delivery, covering both the mid and late stages of pregnancy. Blood samples were collected from the antecubital vein of fasting pregnant women (fasting for more than 8 h) using 5 mL BD vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ, United States). After allowing the blood samples to clot, they were centrifuged at 3,500 rpm for 10 min. The serum samples were then processed to remove any interference from hemolysis, jaundice, and lipid turbidity. Serum sodium (Na), potassium (K), chloride (Cl), calcium (Ca), magnesium (Mg), and phosphate (P) were measured on the ARCHITECT c16000 clinical chemistry analyzer (Abbott Diagnostics, Abbott Park, United States). Na, K, and Cl levels were measured using ion-selective electrode methods; Ca levels were determined using the Arsenazo III method; Mg levels were assessed using enzymatic methods; and P were measured using the molybdenum blue method; According to published literature and Abbott manufacturer data, using three levels of commercial quality control sera (low, medium, and high), within-run CVs for these analytes ranged from 0.5–1.5% and between-run CVs from 1.0–2.0% (24, 25), indicating that the system exhibits good analytical precision across different concentration levels.
2.3 Covariates
Covariates included age, parity (previous live births), in vitro fertilization (IVF), and neonatal sex. Information on age and parity was recorded by the physician during the first pregnancy registration visit.
2.4 Statistical analysis
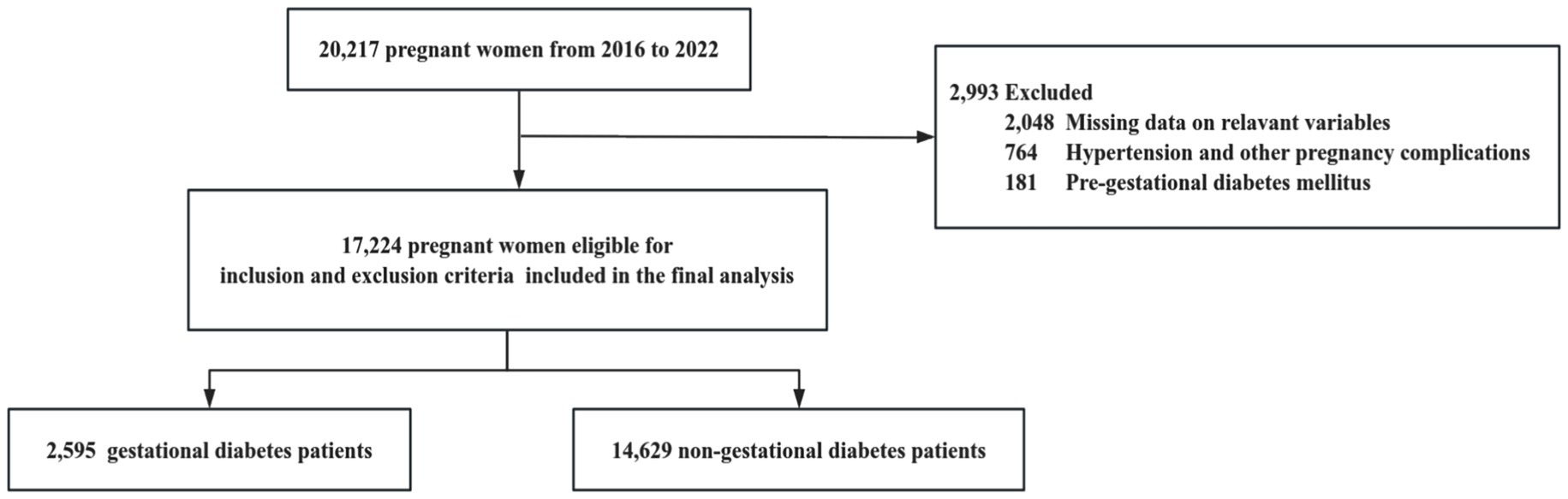
Statistical analyses were conducted using R 4.2.31. Continuous variables were reported as means with standard deviations (SD) or medians with interquartile ranges (IQR). Categorical variables were presented as frequencies and percentages. We investigated the dose–response relationship between maternal mineral levels and GDM risk using restricted cubic splines with four knots. The number of knots used in the restricted cubic spline analysis was selected based on the minimum Akaike Information Criterion (AIC). Restricted cubic splines were chosen because they can flexibly capture nonlinear relationships while maintaining linearity beyond the data boundaries, thereby avoiding unreasonable extrapolation and overfitting. This approach allows for more accurate and interpretable models, better reflecting the complex relationship between maternal mineral levels and GDM risk. Given the non-direct interpretability of the regression parameters from the spline regression (26), we used binary logistic regression models to derive interpretable parameters for linear relationships. For nonlinear relationships, we constructed piecewise logistic regression models to estimate inflection points and analyze threshold effects. In logistic regression models, we computed odds ratios (ORs) and 95% confidence intervals (CIs) associated with a one standard deviation change in mineral levels. Restricted cubic spline analysis and logistic regression analysis adjusted for age, parity (previous live births), IVF, and neonatal sex. A p-value less than 0.05 was considered statistically significant (Figure 1).
3 Results
3.1 Characteristics of study participants
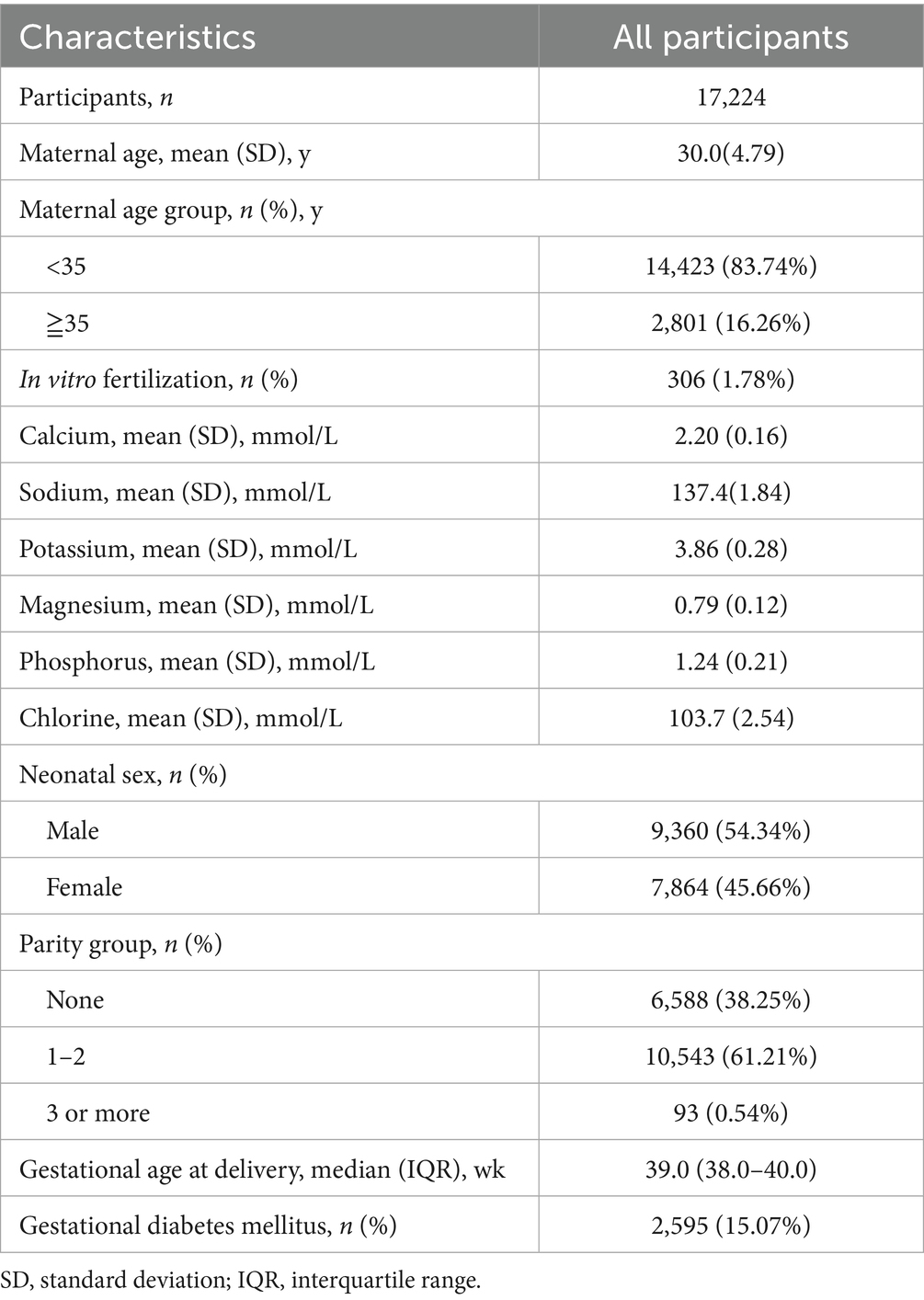
This study included a total of 17,224 singleton mothers, with an average delivery age of 30.0 ± 4.79 years, of whom 16.26% were of advanced maternal age (≥35 years). Among the participants, 38.25% were primiparous, and 1.78% had undergone in vitro fertilization (IVF). The average serum levels of minerals during mid or late pregnancy were as follows: Ca 2.20 ± 0.16 mmol/L, Na 137.4 ± 1.84 mmol/L, K 3.86 ± 0.28 mmol/L, Mg 0.79 ± 0.12 mmol/L, P 1.24 ± 0.21 mmol/L, and Cl 103.7 ± 2.54 mmol/L. The median gestational age at delivery was 39.0 weeks (range: 38.0–40.0 weeks), and 15.07% of the participants were diagnosed with GDM (see Table 1).
3.2 Relationship between mineral levels and GDM
Restricted cubic spline regression analysis showed nonlinear relationships between serum levels of Ca (P for non-linear < 0.001; Figure 2A) and GDM risk. The inflection point for Ca was 2.10 mmol/L. Ca levels below the inflection point were associated with a protective effect against GDM (OR: 0.61; 95% CI: 0.37, 0.99) (Table 2), while levels above the inflection point were associated with an increased risk of GDM (OR: 7.19; 95% CI: 4.49, 11.5) (Table 2).
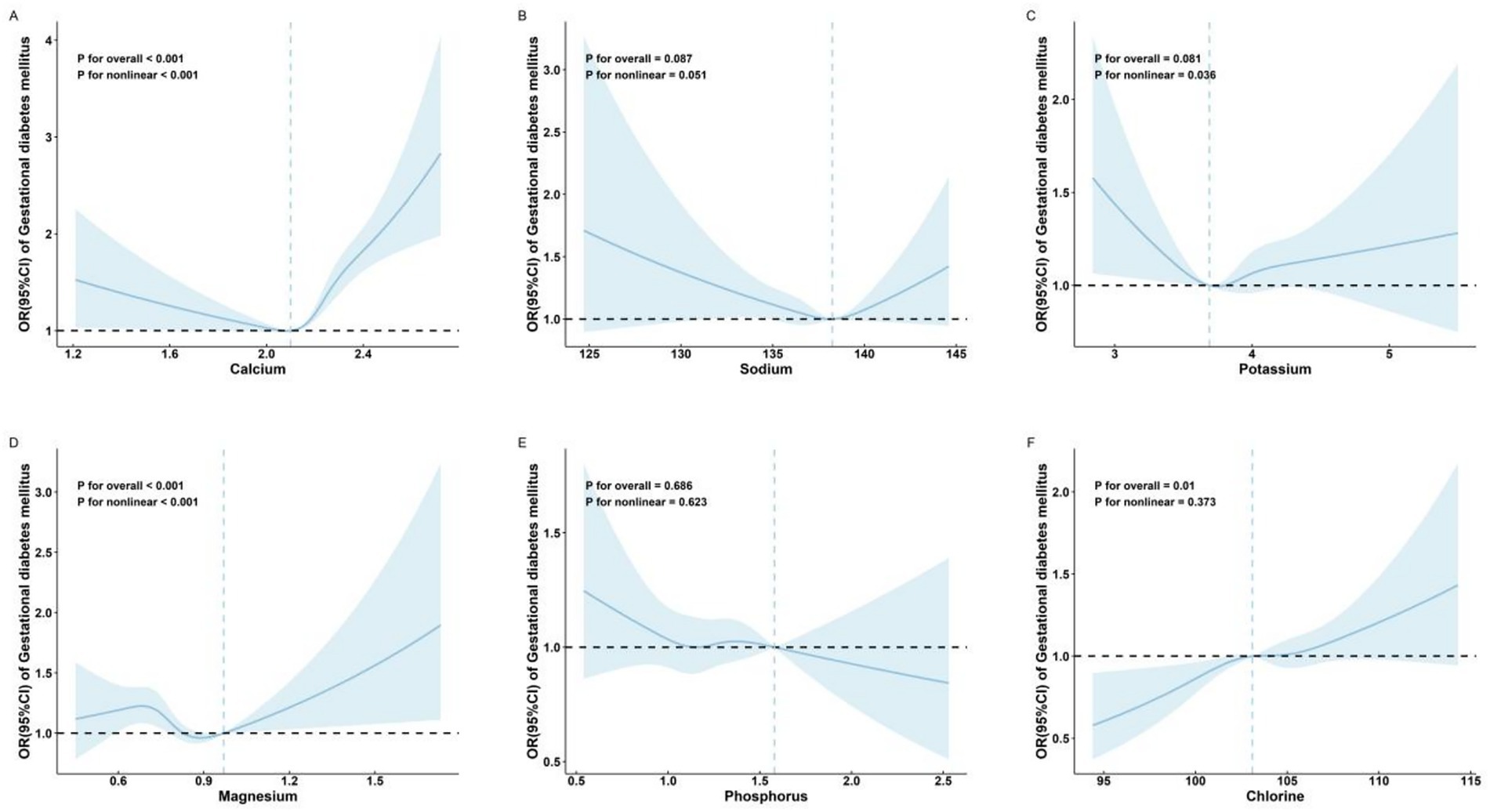
Figure 2. Adjusted* odds ratios and 95% confidence intervals for GDM in association with maternal serum mineral levels in restricted cubic spline regression. (A) calcium; (B) sodium; (C) potassium; (D) magnesium; (E) phosphorus; and (F) chlorine. The x-axis represents the full range of measured serum mineral levels to comprehensively display the data distribution captured by the restricted cubic spline model. *Adjusted for age (continuous), parity, in vitro fertilization, and neonatal sex.
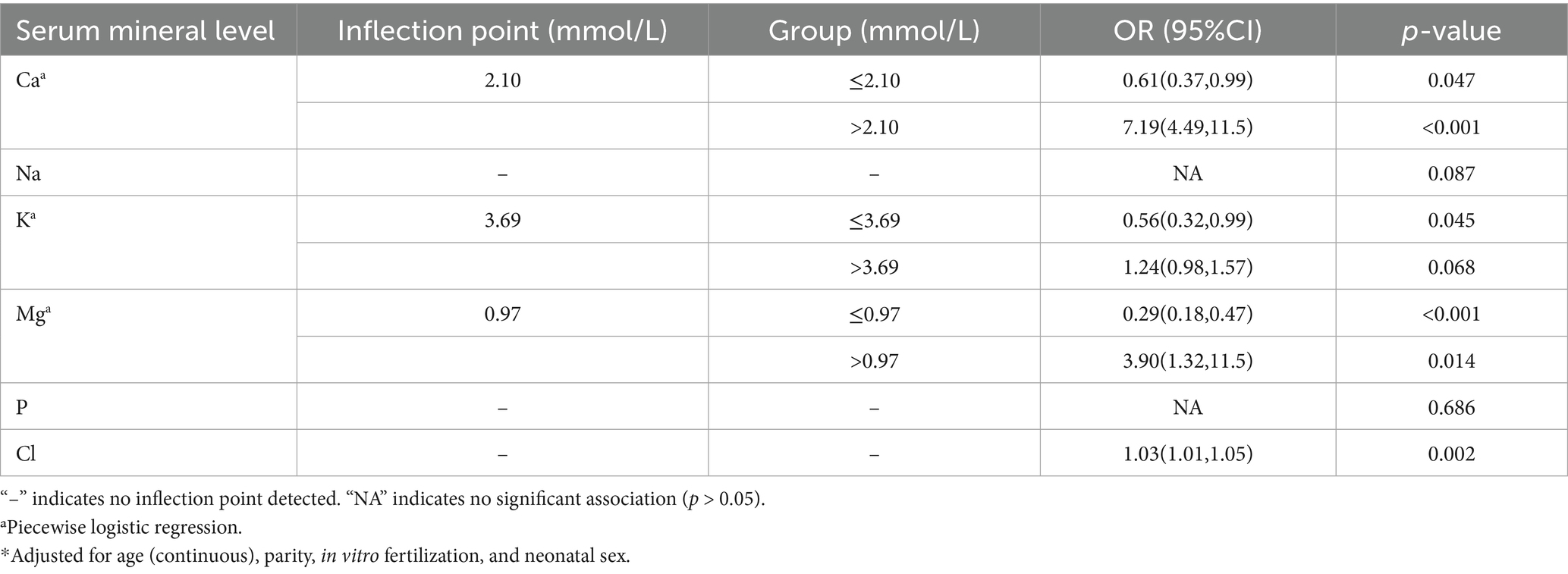
Table 2. Adjusted* odds ratios (ORs) and 95% confidence intervals (95% CIs) for GDM in association with maternal serum mineral levels in multivariable logistic regression.
No significant association was observed between Na (P for overall = 0.087; Figure 2B) and GDM risk.
Restricted cubic spline regression analysis also showed a nonlinear relationship between serum levels of K (P for non-linear = 0.036; Figure 2C) and Mg (P for non-linear < 0.001; Figure 2D) and GDM risk.
No significant association was observed between P (P for overall = 0.686; Figure 2E) and GDM risk.
The Cl was significantly linearly positively associated with GDM risk [P for overall = 0.01; P for non-linear = 0.373; OR (95% CI) = 1.03 (1.01, 1.05); Table 2 and Figure 2F].
3.3 Interaction between maternal serum mineral levels and risk of GDM
To further explore the associations between maternal mineral levels and GDM, we focused on minerals (Ca, K, Mg, and Cl) that demonstrated significant independent associations with GDM in our primary analyses and incorporated their interaction terms as independent variables in multivariable logistic regression models. After controlling for confounding factors such as age (continuous), parity, in vitro fertilization, and newborn sex, we found significant interactions between maternal serum Ca and Mg (OR: 0.05; 95% CI: 0.01, 0.27; P for interaction < 0.001) (Table 3). This indicates that when both Ca and Mg levels are elevated, their combined effect on GDM is negative, meaning that the joint effect of Ca and Mg significantly reduces the risk of GDM after adjusting for other variables. Similarly, the interaction between K and Mg was also significant (OR: 0.11; 95% CI: 0.03, 0.37; P for interaction < 0.001) (Table 3), suggesting that the combined effect of K and Mg significantly lowers the risk of GDM after controlling for other variables. The interaction between K and Cl was significant (OR: 1.06; 95% CI: 1.01, 1.11; P for interaction < 0.001) (Table 3), but the combined effect of K and Cl significantly increased the risk of GDM.
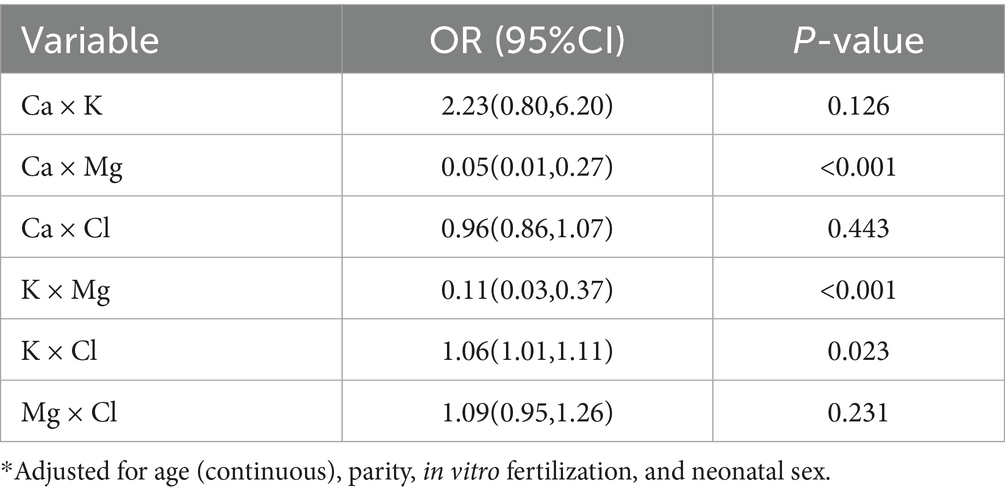
Table 3. Adjusted* the relationship between the risk of GDM and the multiplicative interaction of maternal serum mineral levels with odds ratio (ORs) and 95% confidence interval (95%CI).
4 Discussion
We conducted a large-scale retrospective epidemiological study involving 17,224 singleton pregnancies. After adjusting for various confounding variables, our study revealed significant associations between maternal serum levels of Ca, K, Mg, and Cl with GDM. Notably, interactions between Ca and Mg, as well as K and Mg, were negatively associated with GDM risk, while the interaction between K and Cl was positively associated with GDM risk.
Our findings indicated that elevated maternal serum Cl levels are associated with an increased risk of GDM. Cl, primarily present in the body as chloride ions, plays essential roles in maintaining fluid balance, contributing to gastric acid formation, and helping to regulate acid–base balance. While some studies have reported increased Cl levels due to exposure to organic pollutants in pregnant women (27, 28), direct research on the relationship between Cl and GDM is limited. Electrolyte imbalances can lead to metabolic disturbances, potentially impacting insulin sensitivity and secretion (29). However, Cl’s role in overall electrolyte balance suggests it may indirectly affect insulin function and glucose regulation.
Ca, the most abundant mineral in the body, is crucial for insulin secretion and intracellular signaling, helping to stabilize blood glucose levels (30). Our study found a U-shaped relationship between maternal serum Ca levels and GDM risk. Specifically, when maternal serum Ca levels fall below a certain threshold, the risk of GDM increases. During the second and third trimesters of pregnancy, Ca absorption in pregnant women significantly increases, particularly when dietary intake is low (31). Ca metabolism is influenced by both active (1,25(OH)₂D) and inactive (25(OH)D) forms of vitamin D. During pregnancy, although serum 25(OH)D levels remain unchanged, increased conversion to 1,25(OH)₂D due to elevated 1-α-hydroxylase activity in the placenta doubles serum 1,25(OH)₂D levels, enhancing Ca absorption (32, 33). Consequently, Ca deficiency could disrupt these processes, increasing insulin resistance and GDM risk. Conversely, elevated Ca levels above a critical threshold may also increase GDM risk, potentially due to hypercalcemia, kidney stones, alkalosis, and renal failure (34). While there is no strong evidence or large-scale clinical studies supporting the link between excessive Ca intake and increased GDM risk, some studies suggest that Ca supplementation may impact metabolic health, including insulin sensitivity and glucose metabolism (35), and could potentially affect the risk of GDM under certain conditions. The risk of GDM is lowest within a moderate range of serum Ca levels. A randomized, placebo-controlled study found that Ca plus vitamin D supplementation had beneficial effects on the metabolic status of women with GDM, which indicated that maintaining Ca levels within an optimal range is crucial for lowering GDM risk (36, 37).
Mg is an essential mineral involved in regulating body temperature, synthesizing proteins and nucleic acids, and maintaining cellular electrical potentials (38). Our study showed that, up to a Mg level of 0.97 mmol/L, an increase in Mg levels is associated with a transient rise in the risk of GDM followed by a decrease. However, beyond this threshold, higher Mg levels are linked to an increased risk of GDM. Mg participates in numerous enzymatic reactions, including those related to glucose metabolism and insulin secretion, which is vital for maintaining normal blood glucose levels and insulin function (39). Low Mg levels have been associated with insulin resistance and metabolic syndrome, as Mg regulates ion channels, cellular processes, and physiological functions and is involved in insulin signaling pathways (40). Reduced intracellular Mg2+ can impair insulin receptor (IR) activity, damage post-receptor effects, and increase insulin resistance, all of which are risk factors for GDM (17). Mg supplementation may help improve insulin sensitivity and reduce GDM risk. Recent meta-analyses have shown that Mg supplementation positively affects glucose homeostasis in GDM patients and may reduce preeclampsia and increase neonatal birth weight (41–43).
K helps maintain fluid balance inside and outside cells and is involved in insulin secretion and function. It also plays a crucial role in regulating blood pressure and glucose levels (44). Our study found that low K levels are protective against GDM. Low K levels at the start of pregnancy are associated with a significantly reduced risk of GDM. Early pregnancy K levels can predict the risk of severe pregnancy complications such as GDM and preeclampsia (18). However, some studies suggest that low K levels in non-pregnant individuals are linked to an increased risk of type 2 diabetes and hypertension (45, 46). Ensuring adequate K intake may help mitigate these risks, but further research is needed to confirm the role of serum K levels as predictors of GDM.
Our study found that maternal serum levels of Ca, Mg, K, and Cl significantly affect GDM risk both independently and through their interactions. A randomized, double-blind, placebo-controlled trial indicated that Mg-Zn-Ca-vitamin D supplementation has beneficial effects on glucose control in GDM patients (47). It has been suggested that Ca and Mg, or K and Mg, may enhance insulin sensitivity, regulate IR and glucose transporter (GLUT4) activity, and exhibit anti-inflammatory and antioxidant effects (42, 48). These combined effects may reduce insulin resistance caused by inflammation and oxidative stress, protect pancreatic β-cell function, and stabilize blood glucose levels, thereby lowering GDM risk. Additionally, Mg facilitates the absorption and utilization of K within cells, helping to maintain electrolyte balance and the stability of cellular functions (49). In contrast, the interaction between K and chloride positively correlates with GDM risk, possibly due to their combined impact on intracellular electrolyte balance. Excessive chloride may interfere with K function, leading to IR dysfunction and impaired insulin signaling, thus increasing insulin resistance (50). Chloride may also promote inflammatory responses, exacerbating insulin resistance (51). Traditionally, healthcare providers have primarily focused on the supplementation and monitoring of trace elements like folic acid, iron, and vitamins during pregnancy. However, our results suggest that the levels of these major minerals should also be given equal attention. Additionally, rather than focusing solely on individual mineral levels, it is important to consider the interactions between different minerals and their impact on GDM risk.
5 Limitations
There are several limitations of our study. First, as a retrospective cohort study, we were unable to collect data on key confounding factors such as pre-pregnancy body mass index (BMI), polycystic ovary syndrome (PCOS), and socioeconomic status. The lack of detailed information on vitamin and nutritional supplement use, including specific dosages of calcium, iron, and vitamin D, also limited our ability to fully assess their impact on GDM development. Second, our analysis was based solely on serum mineral concentrations, which may not fully reflect the true mineral status throughout the body, as these elements are differentially distributed across organs and tissues. Finally, our findings are subject to selection bias because we excluded pregnant women with complications who were likely to have had additional mineral tests. Given that these high-risk individuals often have more pronounced electrolyte or mineral abnormalities and are more prone to GDM, their exclusion may have underestimated the true association between mineral levels and GDM risk (52–54). Consequently, the generalizability of our results is limited to a general population of pregnant women without severe complications. Additionally, as mineral levels were measured only in a small proportion of individuals during early pregnancy, our analysis was largely confined to data from the second and third trimesters. Future studies should therefore explore the dynamic relationship between mineral status and GDM risk across different stages of gestation.
6 Conclusion
Our study found significant associations between certain maternal mineral levels and the risk of GDM. Specifically, the balance of minerals such as Ca, K, Mg, and Cl plays a crucial role in the prevention and management of GDM. Therefore, healthcare providers should closely monitor these mineral levels during pregnancy to ensure that expectant mothers receive adequate and balanced intake of minerals to reduce the risk of GDM. Furthermore, larger and higher-quality studies are needed to confirm these associations and explore the underlying mechanisms.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
YH: Data curation, Funding acquisition, Methodology, Visualization, Writing – original draft, Writing – review & editing. NW: Conceptualization, Supervision, Visualization, Writing – review & editing. SH: Conceptualization, Data curation, Formal analysis, Resources, Writing – original draft, Writing – review & editing. YL: Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. GL: Conceptualization, Data curation, Software, Validation, Writing – original draft, Writing – review & editing. XX: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. YZ: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. XW: Conceptualization, Data curation, Methodology, Validation, Writing – original draft, Writing – review & editing. BS: Conceptualization, Data curation, Investigation, Project administration, Software, Writing – original draft, Writing – review & editing. HW: Conceptualization, Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing. MH: Formal analysis, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing. JW: Conceptualization, Investigation, Supervision, Validation, Writing – original draft, Writing – review & editing. YC: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. QJ: Conceptualization, Data curation, Investigation, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We are grateful to the staff of the People’s Hospital of Pingyang for their assistance in data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. You, H, Hu, J, Liu, Y, Luo, B, and Lei, A. Risk of type 2 diabetes mellitus after gestational diabetes mellitus: a systematic review & meta-analysis. Indian J Med Res. (2021) 154:62–77. doi: 10.4103/ijmr.IJMR_852_18
2. Srichumchit, S, Luewan, S, and Tongsong, T. Outcomes of pregnancy with gestational diabetes mellitus. Int J Gynecol Obstet. (2015) 131:251–4. doi: 10.1016/j.ijgo.2015.05.033
3. Pinho-Gomes, AC, Morelli, G, Jones, A, and Woodward, M. Association of lactation with maternal risk of type 2 diabetes: a systematic review and meta-analysis of observational studies. Diabetes Obes Metab. (2021) 23:1902–16. doi: 10.1111/dom.14417
4. Wang, H, Li, N, Chivese, T, Werfalli, M, Sun, H, Yuen, L, et al. IDF diabetes atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by International Association of Diabetes in pregnancy study group’s criteria. Diabetes Res Clin Pract. (2022) 183:109050. doi: 10.1016/j.diabres.2021.109050
5. X-w, Lan, Wu, Z-h, J-x, Zhao, Zhang, H, Yang, B, and Chen, W. Research progress of dietary components in helping alleviate gestational diabetes mellitus. Food Ferment. Ind. (2023) 49:311–9. doi: 10.13995/j.cnki.11-1802/ts.033439
6. Juan, J, and Yang, H. Prevalence, prevention, and lifestyle intervention of gestational diabetes mellitus in China. Int J Environ Res Public Health. (2020) 17:9517. doi: 10.3390/ijerph17249517
7. Dubey, P, Thakur, V, and Chattopadhyay, M. Role of minerals and trace elements in diabetes and insulin resistance. Nutrients. (2020) 12:1864. doi: 10.3390/nu12061864
8. Khan, R, Ali, K, and Khan, Z. Socio-demographic risk factors of gestational diabetes mellitus. Pak J Med Sci. (2013) 29:843. doi: 10.12669/pjms.293.3629
9. McIntyre, HD, Catalano, P, Zhang, C, Desoye, G, Mathiesen, ER, and Damm, P. Gestational diabetes mellitus. Nat Rev Dis Primers. (2019) 5:47. doi: 10.1038/s41572-019-0098-8
10. Catalano, PM, Kirwan, JP, Haugel-de Mouzon, S, and King, J. Gestational diabetes and insulin resistance: role in short-and long-term implications for mother and fetus. J Nutr. (2003) 133:1674S–83S. doi: 10.1093/jn/133.5.1674S
11. Derakhshanian, H, Javanbakht, M, Zarei, M, Djalali, E, and Djalali, M. Vitamin D increases IGF-I and insulin levels in experimental diabetic rats. Growth Hormon IGF Res. (2017) 36:57–9. doi: 10.1016/j.ghir.2017.09.002
12. Foster, M, and Samman, S. Zinc and regulation of inflammatory cytokines: implications for cardiometabolic disease. Nutrients. (2012) 4:676–94. doi: 10.3390/nu4070676
13. Hassan, S, Elsheikh, W, Rahman, N, and ElBagir, NM. Serum calcium levels in correlation with glycated hemoglobin in type 2 diabetic Sudanese patients. Adv Diabet Metabol. (2016) 4:59–64. doi: 10.13189/adm.2016.040401
14. Yang, K, Yang, Y, Pan, B, Fu, S, Cheng, J, and Liu, J. Relationship between iron metabolism and gestational diabetes mellitus: a systemic review and meta analysis. Asia Pac J Clin Nutr. (2022) 31:242–54. doi: 10.3316/informit.543499471066754
15. Zhu, G, Zheng, T, Xia, C, Qi, L, Papandonatos, GD, Ming, Y, et al. Plasma levels of trace element status in early pregnancy and the risk of gestational diabetes mellitus: a nested case-control study. J Trace Elem Med Biol. (2021) 68:126829. doi: 10.1016/j.jtemb.2021.126829
16. Osorio-Yáñez, C, Qiu, C, Gelaye, B, Enquobahrie, DA, and Williams, MA. Risk of gestational diabetes mellitus in relation to maternal dietary calcium intake. Public Health Nutr. (2017) 20:1082–9. doi: 10.1017/S1368980016002974
17. Ren, Q, Wang, H, Zeng, Y, Tan, X, Cheng, X, Zhou, T, et al. The association between serum magnesium levels and gestational diabetes mellitus: a systematic review and meta-analysis. Biol Trace Elem Res. (2023) 201:5115–25. doi: 10.1007/s12011-023-03591-6
18. Wolak, T, Sergienko, R, Wiznitzer, A, Ben Shlush, L, Paran, E, and Sheiner, E. Low potassium level during the first half of pregnancy is associated with lower risk for the development of gestational diabetes mellitus and severe pre-eclampsia. J Matern Fetal Neonatal Med. (2010) 23:994–8. doi: 10.3109/14767050903544736
19. Wolak, T, Shoham-Vardi, I, Sergienko, R, and Sheiner, E. High potassium level during pregnancy is associated with future cardiovascular morbidity. J Matern Fetal Neonatal Med. (2016) 29:1021–4. doi: 10.3109/14767058.2015.1032238
20. Keshvari Delavar, M, Mozaffari-Khosravi, H, Nadjarzadeh, A, Farhadian, Z, Khazaei, S, and Rezaeian, S. Comparison of growth parameters, Apgar scores, the blood zinc, magnesium, calcium and phosphorus between gestational diabetic and non-diabetic pregnant women. Int J Pediatr. (2016) 4:1767–75. doi: 10.22038/IJP.2016.6704
21. Berend, K, Van Hulsteijn, LH, and Gans, RO. Chloride: the queen of electrolytes? Eur J Intern Med. (2012) 23:203–11. doi: 10.1016/j.ejim.2011.11.013
22. Gao, C, Sun, X, Lu, L, Liu, F, and Yuan, J. Prevalence of gestational diabetes mellitus in mainland China: a systematic review and meta-analysis. J Diabet Invest. (2019) 10:154–62. doi: 10.1111/jdi.12854
23. Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. (2010) 33:S62–9. doi: 10.2337/dc10-S062
24. Bailey, D, Martens, P, Mah, W, and Yip, P. A next generation enzymatic magnesium assay on the Abbott ARCHITECT chemistry system meets performance goals based on biological variation. Clin Biochem. (2014) 47:142–4. doi: 10.1016/j.clinbiochem.2013.09.018
25. Food and Drug Administration. 510(k) substantial equivalence determination decision summary: Abbott ISE electrolyte module (K170320). Silver Spring, MD: U.S. Food and Drug Administration (2017).
26. Muggeo, VM. Estimating regression models with unknown break-points. Stat Med. (2003) 22:3055–71. doi: 10.1002/sim.1545
27. Rahman, ML, Zhang, C, Smarr, MM, Lee, S, Honda, M, Kannan, K, et al. Persistent organic pollutants and gestational diabetes: a multi-center prospective cohort study of healthy US women. Environ Int. (2019) 124:249–58. doi: 10.1016/j.envint.2019.01.027
28. Yan, D, Jiao, Y, Yan, H, Liu, T, Yan, H, and Yuan, J. Endocrine-disrupting chemicals and the risk of gestational diabetes mellitus: a systematic review and meta-analysis. Environ Health. (2022) 21:53. doi: 10.1186/s12940-022-00858-8
29. Attama, CT. Electrolyte imbalance in type II diabetes. J Clin Diag Res. (2018). Available online at: http://eprints.gouni.edu.ng/id/eprint/806
30. Domínguez, M, Martínez, M, Méndez, J, and Rodríguez, M. Supplements in pregnant women: controversies, evidence and recommendations. Nat Health Syst. (2010) 34:1–12. Available online at: https://www.msssi.gob.es/biblioPublic/publicaciones/recursos_propios/infMedic/docs/vol34n4_Suplementos.pdf
31. Ritchie, LD, Fung, EB, Halloran, BP, Turnlund, JR, Van Loan, MD, Cann, CE, et al. A longitudinal study of calcium homeostasis during human pregnancy and lactation and after resumption of menses. Am J Clin Nutr. (1998) 67:693–701. doi: 10.1093/ajcn/67.4.693
32. Bouillon, R, Van Assche, FA, Van Baelen, H, Heyns, W, and De Moor, P. Influence of the vitamin D-binding protein on the serum concentration of 1, 25-dihydroxyvitamin D 3: significance of the free 1, 25-dihydroxyvitamin D 3 concentration. J Clin Invest. (1981) 67:589–96. doi: 10.1172/JCI110072
33. Hacker, AN, Fung, EB, and King, JC. Role of calcium during pregnancy: maternal and fetal needs. Nutr Rev. (2012) 70:397–409. doi: 10.1111/j.1753-4887.2012.00491.x
34. Tinawi, M. Disorders of calcium metabolism: hypocalcemia and hypercalcemia. Cureus. (2021) 13:e12420. doi: 10.7759/cureus.12420
35. Asemi, Z, Karamali, M, and Esmaillzadeh, A. Effects of calcium–vitamin D co-supplementation on glycaemic control, inflammation and oxidative stress in gestational diabetes: a randomised placebo-controlled trial. Diabetologia. (2014) 57:1798–806. doi: 10.1007/s00125-014-3293-x
36. Karamali, M, Asemi, Z, Ahmadi-Dastjerdi, M, and Esmaillzadeh, A. Calcium plus vitamin D supplementation affects pregnancy outcomes in gestational diabetes: randomized, double-blind, placebo-controlled trial. Public Health Nutr. (2016) 19:156–63. doi: 10.1017/S1368980015000609
37. Safary, K, Ahmed, H, and Moghaddam-Banaem, L. Maternal calcium status in first half of pregnancy and gestational diabetes mellitus. Int J Womens Health Wellness. (2016) 2:5. doi: 10.23937/2474-1353/1510012
38. Jahnen-Dechent, W, and Ketteler, M. Magnesium basics. Clin Kidney J. (2012) 5:i3–i14. doi: 10.1093/ndtplus/sfr163
39. Paolisso, G, Scheen, A, d'Onofrio, F, and Lefebvre, P. Magnesium and glucose homeostasis. Diabetologia. (1990) 33:511–4. doi: 10.1007/BF00404136
40. Kostov, K. Effects of magnesium deficiency on mechanisms of insulin resistance in type 2 diabetes: focusing on the processes of insulin secretion and signaling. Int J Mol Sci. (2019) 20:1351. doi: 10.3390/ijms20061351
41. Jin, S, Sha, L, Dong, J, Yi, J, Liu, Y, Guo, Z, et al. Effects of nutritional strategies on glucose homeostasis in gestational diabetes mellitus: a systematic review and network meta-analysis. J Diabetes Res. (2020) 2020:6062478. doi: 10.1155/2020/6062478
42. Li, D, Cai, Z, Pan, Z, Yang, Y, and Zhang, J. The effects of vitamin and mineral supplementation on women with gestational diabetes mellitus. BMC Endocr Disord. (2021) 21:1–15. doi: 10.1186/s12902-021-00712-x
43. Santander Ballestín, S, Giménez Campos, MI, Ballestin Ballestin, J, and Luesma Bartolomé, MJ. Is supplementation with micronutrients still necessary during pregnancy? A review. Nutrients. (2021) 13:3134. doi: 10.3390/nu13093134
44. Palmer, BF, and Clegg, DJ. Physiology and pathophysiology of potassium homeostasis. Adv Physiol Educ. (2016) 40:480–90. doi: 10.1152/advan.00121.2016
45. Krishna, GG. Effect of potassium intake on blood pressure. J Am Soc Nephrol. (1990) 1:43–52. doi: 10.1681/ASN.V1143
46. Zillich, AJ, Garg, J, Basu, S, Bakris, GL, and Carter, BL. Thiazide diuretics, potassium, and the development of diabetes: a quantitative review. Hypertension. (2006) 48:219–24. doi: 10.1161/01.HYP.0000231552.10054.aa
47. Karamali, M, Bahramimoghadam, S, Sharifzadeh, F, and Asemi, Z. Magnesium–zinc–calcium–vitamin D co-supplementation improves glycemic control and markers of cardiometabolic risk in gestational diabetes: a randomized, double-blind, placebo-controlled trial. Appl Physiol Nutr Metab. (2018) 43:565–70. doi: 10.1139/apnm-2017-0521
48. Omu, AE. Pro-inflammatory cytokines, lipid metabolism and inflammation in gestational diabetes mellitus as cause of insulin resistance. Gestational diabetes–causes, diagnosis and treatment, InTech, Rijeka (Croatia); (2013). 79–102.
49. Al Alawi, AM, Majoni, SW, and Falhammar, H. Magnesium and human health: perspectives and research directions. Int J Endocrinol. (2018) 2018:9041694. doi: 10.1155/2018/9041694
50. Das, UN. Molecular biochemical aspects of salt (sodium chloride) in inflammation and immune response with reference to hypertension and type 2 diabetes mellitus. Lipids Health Dis. (2021) 20:83. doi: 10.1186/s12944-021-01507-8
51. Yu, H, Wang, M, Wang, D, Kalogeris, TJ, McHowat, J, Ford, DA, et al. Chlorinated lipids elicit inflammatory responses in vitro and in vivo. Shock. (2019) 51:114–22. doi: 10.1097/SHK.0000000000001112
52. Eslamzadeh, A, Amin Kashani, SM, Asadi, N, Bazmi, S, Rezaei, S, Karimimoghadam, Z, et al. Serum calcium and magnesium levels in women presenting with pre-eclampsia: a systematic review and Meta-analysis based on observational studies. Galen Med J. (2023) 12:e3151. doi: 10.31661/gmj.v12i.3151
53. Al-Jameil, N, Tabassum, H, Al-Mayouf, H, Aljohar, HI, Alenzi, ND, Hijazy, SM, et al. Analysis of serum trace elements-copper, manganese and zinc in preeclamptic pregnant women by inductively coupled plasma optical emission spectrometry: a prospective case controlled study in Riyadh, Saudi Arabia. Int J Clin Exp Pathol. (2014) 7:1900. doi: 10.7314/APJCP.2014.15.11.4733
Keywords: mineral, gestational diabetes mellitus, micronutrients, gestational age, retrospective study
Citation: Hao Y, Wang N, Hong S, Liu Y, Lin G, Xu X, Zhou Y, Wen X, Sun B, Wang H, Huang M, Wang J, Chen Y and Jiang Q (2025) Relationship between serum mineral levels in the second and third trimester of pregnancy and the risk of gestational diabetes mellitus: a retrospective cohort study. Front. Nutr. 12:1634419. doi: 10.3389/fnut.2025.1634419
Edited by:
Javier Diaz-Castro, University of Granada, SpainReviewed by:
Jim Philip, NMC Specialty Hospital, Al Ain, United Arab EmiratesConstanza Méndez, Pontificia Universidad Católica de Chile, Chile
Copyright © 2025 Hao, Wang, Hong, Liu, Lin, Xu, Zhou, Wen, Sun, Wang, Huang, Wang, Chen and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Na Wang, V2FuZ25hNzYwMzIwQDE2My5jb20=; Jiwei Wang, aml3ZWl3YW5nQGZ1ZGFuLmVkdS5jbg==
‡ORCID: Yuxin Hao, orcid.org/0009-0002-6871-0749
†These authors have contributed equally to this work and share first authorship
 Yuxin Hao
Yuxin Hao Na Wang2*†
Na Wang2*† Baochang Sun
Baochang Sun Hexing Wang
Hexing Wang Jiwei Wang
Jiwei Wang