- 1Department of Gastrointestinal Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2First Clinical Department, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 3Second Clinical Department, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 4The Center for Biomedical Research, Department of Respiratory and Critical Care Medicine, NHC Key Laboratory of Respiratory Diseases, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Depression is a significant public health issue which exerts profound psychological and social impacts on both individuals and society. However, existing therapeutic strategies often exhibit limited efficacy. Accumulating evidence underscores the vital role of gut microbiota in the pathophysiology of depression through the microbiota-gut-brain (MGB) axis. This involves multiple mechanisms, including short-chain fatty acid (SCFA) metabolism, communication via the vagal nerve, regulation of the hypothalamic-pituitary-adrenal (HPA) axis, and immune-inflammatory interactions. This review provides a comprehensive review of the mechanisms through which gut microbiota influences depression via the MGB axis. It synthesizes recent achievements in this field and evaluates the potential of microbiome-targeted therapies for depression treatment. Furthermore, it outlines future research directions to establish a theoretical framework for novel therapeutic approaches and to foster the development of this area.
1 Introduction
Depression is the most prevalent and debilitating mental disorder, ranking as the third leading cause of global disease burden (1). The etiology of depression is multifactorial. For instance, the COVID-19 pandemic can increase the global incidence of depression by 25% (2). Additionally, adolescents with >3 h of daily screen time have a 34% increased risk of developing depression (3). Nowadays, the primary therapy for depression relies heavily on antidepressant medicines, with selective 5-hydroxytryptamine (5-HT) reuptake inhibitors being the most commonly used. However, approximately 30% of patients exhibit treatment resistance or achieve suboptimal therapeutic outcomes (4). Recently, the role of gut microbiota in depression has garnered increasing attention. Emerging studies have uncovered novel pathways through which gut bacteria can influence depression via the MGB axis. The results of this research lay the foundation for the development of novel therapeutic strategies, thereby overcoming the shortcomings of traditional antidepressant therapies.
The human gastrointestinal system hosts a symbiotic microbiota community, which consists of bacteria, fungi, and viruses. Its biomass exceeds that of human cells by over 1.5 times (5–7). The gut bacteria, which make up more than 90% of the microbiota, are primarily composed of Firmicutes, Bacteroidetes, and Actinobacteria, though it varies significantly among individuals (8). The gut microbiota exerts significant effects on human health through various mechanisms: (1) maintenance of intestinal barrier integrity through the production of short-chain fatty acids (SCFAs), B/K vitamins, amino acids, and bile acids (BAs) (9–12); (2) eliminating invasive intestinal pathogens and reducing their colonization (13, 14); (3) regulating inflammatory cytokines and promoting immune system maturation (15). Patients with depression often exhibit reduced gut microbiota diversity and dysbiosis, while restoring the microbiome is linked to improvements in depressive symptoms (16, 17). Substantial evidence highlights the essential role of gut microbiota in influencing depression via the MGB axis.
The MGB axis refers to the bidirectional communication system linking the brain and the gut. It forms a homeostatic network that encompasses neurological, endocrine, and immunological pathways (18). Various mechanisms are implicated, including SCFA metabolism, the monoamine neurotransmitter system, the vagal nerve pathway, the HPA axis, and immune signaling. However, the specific mechanisms underlying these processes are still not fully understood. This review aims to explore how the gut microbiota influences depression via the MGB axis, providing valuable insights for depression research.
Relevant studies were searched in the PubMed, Web of Science, and CNKI databases, including original research articles, observational studies, and reviews. The search strategy terms included gut microbiota, MGB axis, major depressive disorder and depression. The search was restricted to articles published in English and included studies on all species without restrictions on publication date. From an initial pool of 412 publications, we applied rigorous selection criteria focusing on: Mechanistic studies of gut-brain interactions in depression, clinical/preclinical intervention studies with robust methodologies. High-quality reviews and meta-analyses. After thorough screening of abstracts and full texts, 206 studies were ultimately selected based on their scientific quality, relevance to our review objectives, and publication impact.
2 Gut microbiota and depression
Recent studies have increasingly demonstrated a significant association between alterations in gut microbiota and depression (19). Individuals with depression exhibit marked gut microbiota dysbiosis (Figure 1) (20–22), characterized by elevated levels of Enterobacteriaceae, Alistipes, Bacteroidetes, Proteobacteria, and Actinobacteria, while Faecalibacterium and Firmicutes are notably reduced (23). These gut microbiome changes are evident across different age groups in individuals with depression (24–27).
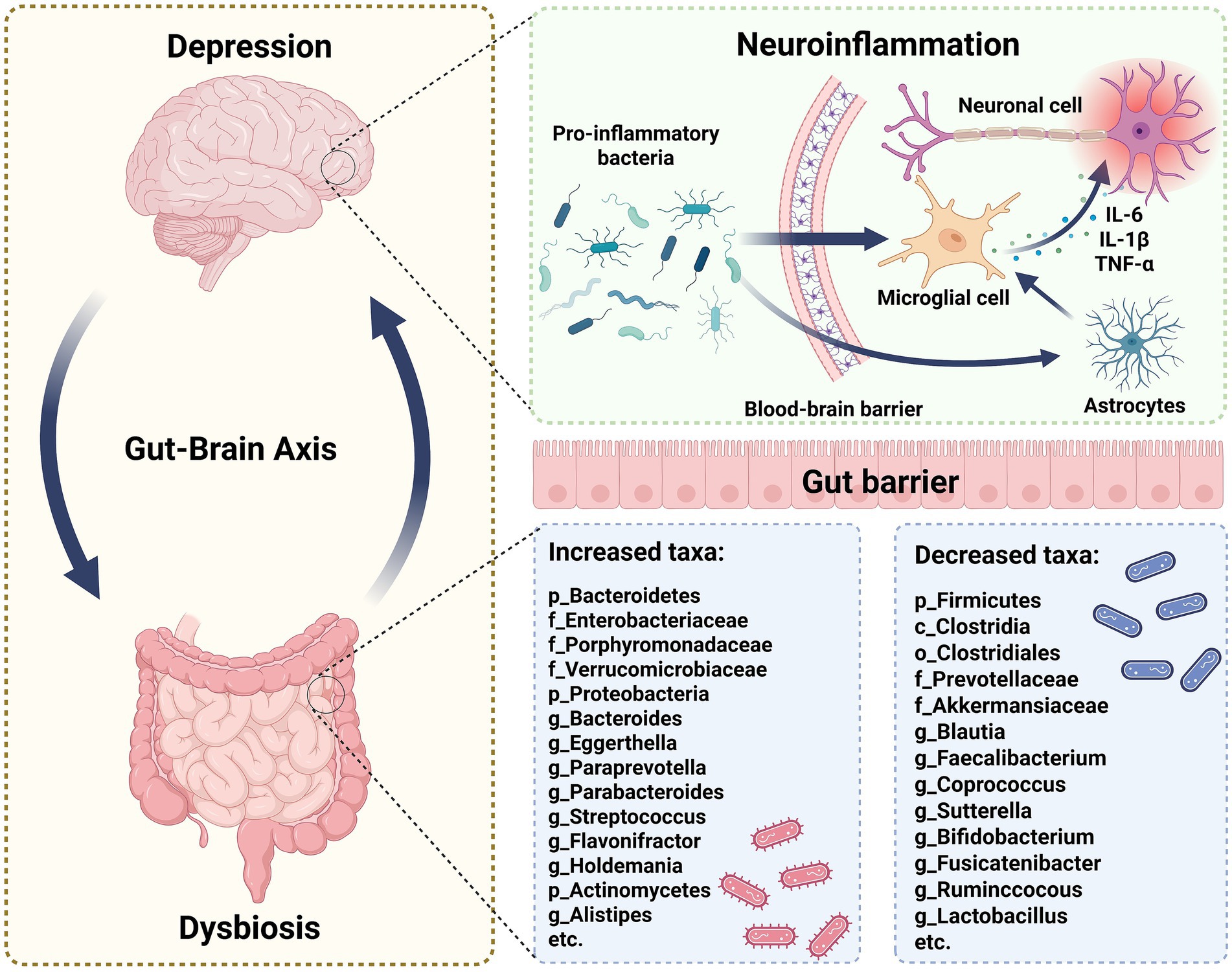
Figure 1. The specific alterations in gut microbiota associated with depression. Depression and gut microbiota dysbiosis share a robust bidirectional relationship. Depression may exacerbate gut dysbiosis, and conversely, dysbiosis can negatively affect mental health. Specifically, certain pro-inflammatory bacteria could penetrate the blood-brain barrier, activating microglia. This activation increases the level of cytokines, such as IL-6, IL-1β, and TNF-α, which can induce neuroinflammation. Moreover, in the gastrointestinal tract of individuals with depression, significant changes in microbiota composition occur, characterized by increased relative abundance of certain microbes and decreased beneficial microbes. These gut microbiota alterations may influence depression development through gut-brain axis mechanisms.
With advancements in macro-genomics and metabolomics, more research has focused on precisely characterizing the gut microbiota alterations related to depression. For instance, the genera Alistipes, Anaerostipes, and Dialister are significantly reduced in individuals with active depression, while Haemophilus is increased in those with mild depression (28, 29). Bacteroidetes levels are markedly elevated in people with mild depression, accompanied by reductions in Faecalibacterium and Escherichia; however, severe depression patients show increased Bacteroidetes but decreased Ruminococcus and Eubacterium (29). The composition of gut microbiota not only varies across different depressive states but also exhibits distinct changes across different depressive species. For instance, a decrease in the Alistipes has been observed in mouse depressive models (30). Therefore, how to accurately and precisely determine the composition of gut microbiota under depressive states remains a critical issue that needs to be addressed.
Some evidence suggests that gut microbiota can directly translocate to the brain parenchyma when the intestinal barrier and the blood–brain barrier (BBB) are compromised, in addition to indirectly influencing depression via the MGB axis (16, 31). This translocation of gut microbiota into the brain may lead to distinct inflammatory changes in different brain regions over time. Research indicates that during the acute phase of depression models, pro-inflammatory gut microbiota primarily infiltrate the brain and induce inflammation, while in the chronic phase, microbiota that penetrate the brain mainly activate neurodegenerative pathways (31). However, it remains unclear whether similar time-dependent changes in gut microbiota occur in the brains of depressed patients. If such changes exist, they could provide a basis for targeted treatments at different stages of depression. Moreover, the effects of gut microbiota invasion vary across different brain regions (31). Given that different depression symptoms may be linked to changes in specific brain regions, future studies could utilize spatial transcriptomics to analyze inflammatory differences across brain regions in mouse models of depression and correlate these with symptomatic changes. This approach could help determine whether gut microbiota induce different depressive symptoms by mediating inflammatory responses in distinct brain regions. The finding that gut microbiota can directly enter the brain and trigger neuroinflammation is interesting and novel, providing a new mechanism and potential new therapies for the onset of depression. However, the mechanism by which gut microbiota can directly enter the brain through damaged intestinal barriers and blood–brain barriers only exists under the specific condition of “intracortical microelectrode implantation,” which is a very special case. Whether this mechanism exists in general patients remains to be explored. If it does exist, it may be possible to alleviate depression by repairing the intestinal barriers and blood–brain barriers. Besides, current studies are limited to animals, with a lack of research in humans. In the future, more research can be conducted in this area.
3 Gut microbiota affects depression through the MGB axis
MGB axis is important in the etiology of depression (Figure 2). The gut microbiota influences the onset of depression through many pathways mediated by the MGB axis, including metabolism, neurotransmitters, neurological pathways, and endocrine systems. Figure 2 illustrates a prevalent MGB axis pathway associated with depression.
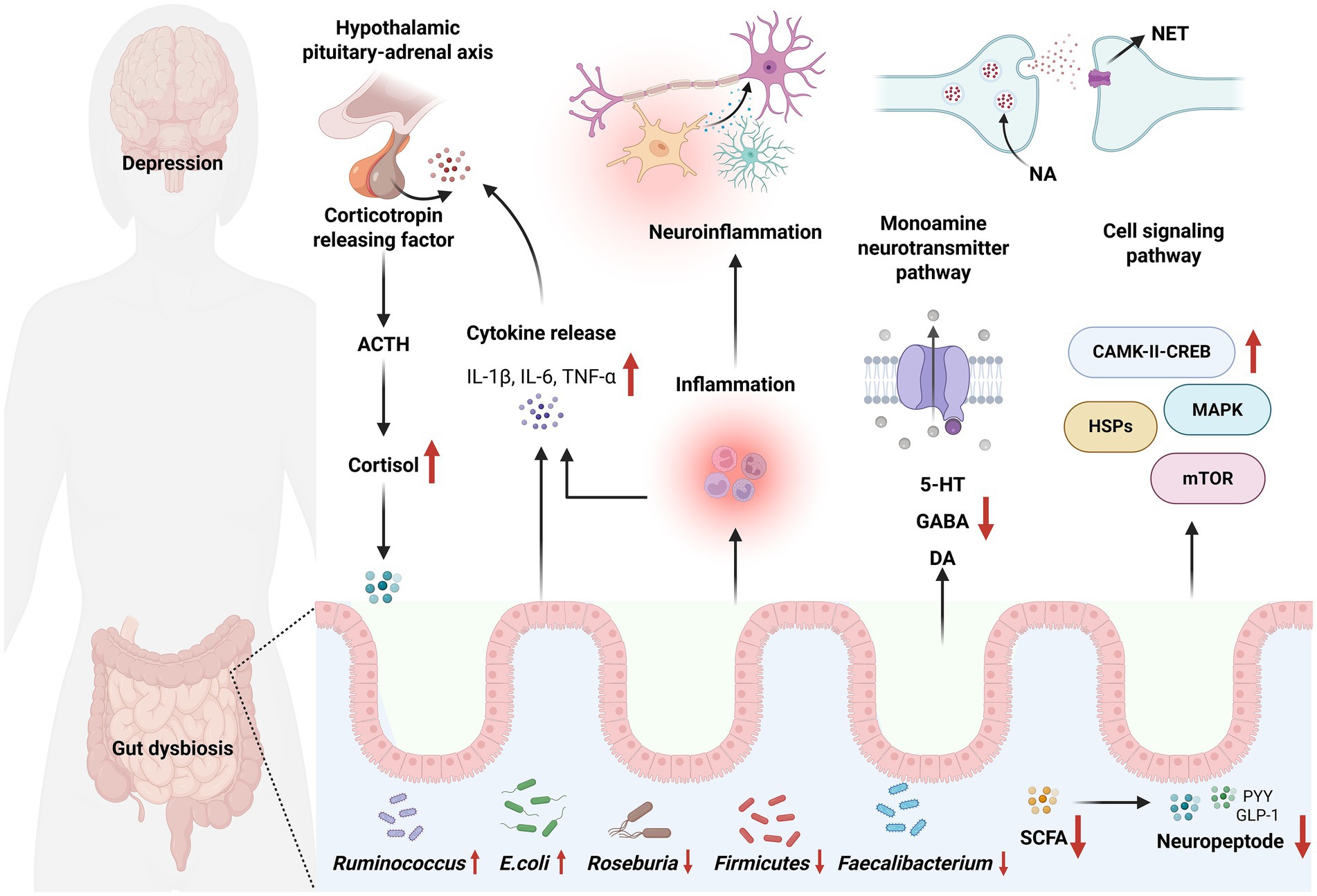
Figure 2. Gut microbiota affects depression through the MGB axis. The HPA axis regulates cortisol levels via corticotropin-releasing factor and adrenocorticotropic hormone. Concurrently, the release of IL-1β, IL-6, and TNF-α can induce neuroinflammation and modulate the HPA axis. Moreover, gut dysbiosis can impact monoamine neurotransmitter pathways and cell signaling pathways, including those involving 5-HT, γ-aminobutyric acid (GABA), dopamine (DA), and mammalian target of rapamycin (mTOR). Alterations in gut microbiota, particularly the reduction of beneficial bacteria and the increase of harmful bacteria, may influence neuropeptide expression. Additional mechanisms by which gut microbiota influence depression via MGB axis are listed in Table 1.
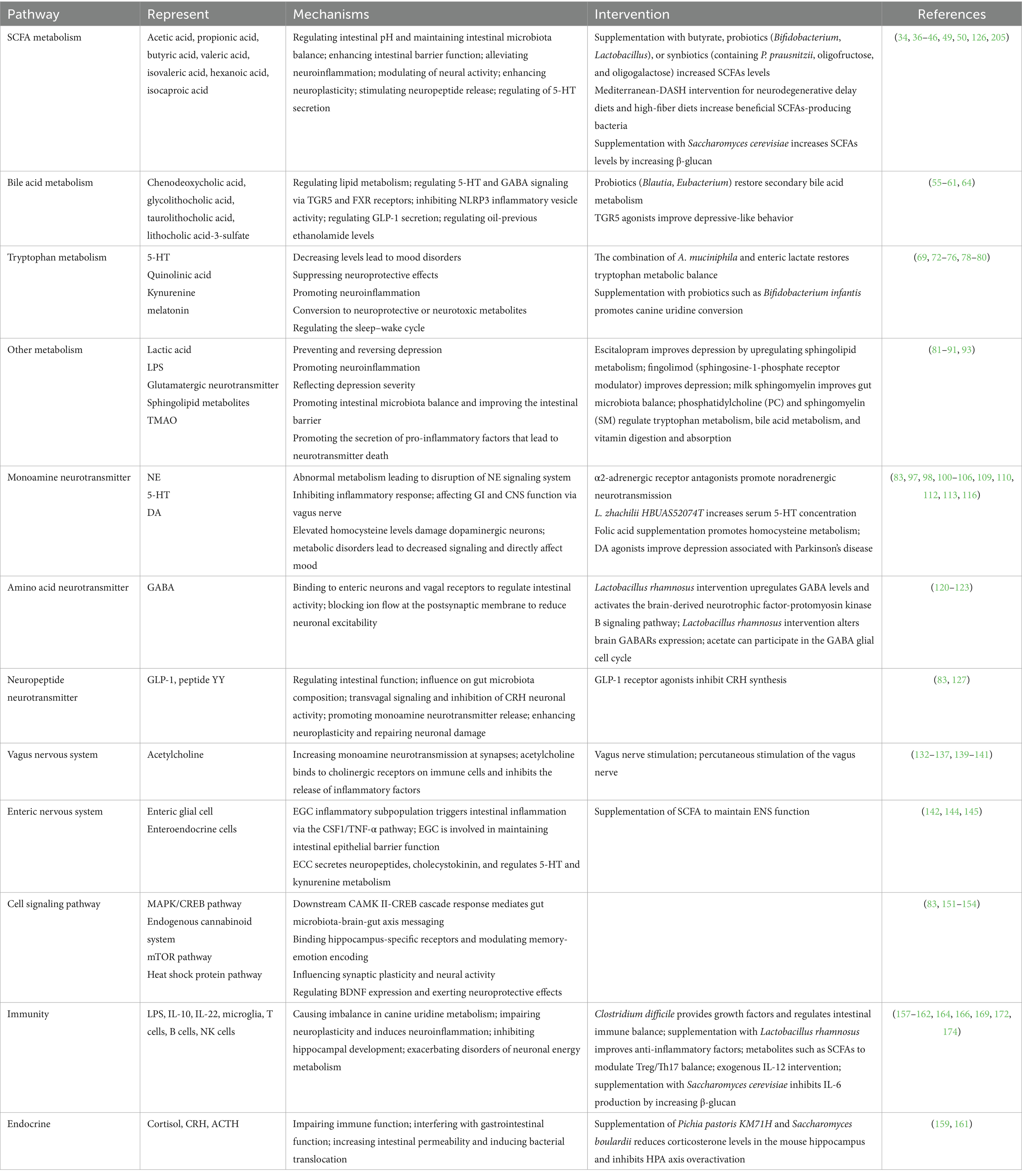
Table 1. Mechanisms of the effect of gut microbes on depression through the microbiota-gut-brain axis.
3.1 Metabolism
3.1.1 SCFAs
SCFAs, including acetic, propionic, and butyric acids along with their salts and esters, are key metabolites produced by gut microbiota (32). SCFA production is influenced by diet and gut microbiota composition, with Bacteroidetes, Firmicutes, and Actinobacteria being notable producers (33).
SCFAs are often deficient in individuals with depression, and their supplementation can improve depressive symptoms (34, 35). The mechanisms involved include: (1) Regulating intestinal pH: SCFAs, mainly present as anions in the gut, lower intestinal pH. This favors the growth of beneficial bacteria like Lactobacillus (optimal pH 3.0–4.5) over pathogenic bacteria such as Salmonella and Staphylococcus (optimal pH 6.0–7.0) (36). (2) Improving intestinal barrier function: SCFAs have demonstrated the potential to mitigate chronic stress-induced elevations in intestinal barrier permeability and facilitate the development of tight junctions within the intestinal barrier (37, 38). Moreover, SCFAs can maintain the integrity of the intestinal mucosa by regulating the transcription and expression of related genes such as specific protein 1 (39). Low concentrations of butyrate enhance barrier function, whereas high concentrations can be detrimental (40). (3) Exerting anti-inflammatory effects: SCFAs bind to the free fatty acid receptor 3, inhibiting microglia M1 polarization, reducing pro-inflammatory cytokines such as IL-6, TNF-α, and increasing anti-inflammatory cytokines such as IL-10 to mitigate neuroinflammation (41, 42). (4) Stimulating of neuropeptide release: SCFAs enhance the release of peptides while reducing hunger hormone secretion (39). (5) Epigenetic mechanisms: SCFAs inhibit histone deacetylase (HDAC), promoting brain-derived neurotrophic factor (BDNF) gene transcription and upregulating hippocampal BDNF expression to enhance neuroplasticity (43). Butyrate also reduces DNA methylation by activating methylcytosine dioxygenase 1, rescuing gene silencing and restoring BDNF expression (44). (6) Regulating neurotransmitters: SCFAs can stimulate 5-HT secretion, thereby influencing mood (45). (7) Crossing the BBB: SCFAs can traverse the BBB directly, modulate neurotransmission, and influence neuronal excitability (34).
Beyond acetic, propionic, and butyric acids, SCFAs also include valeric, isovaleric, hexanoic, and isocaproic acids. Though less studied, these SCFAs may also play roles in depression. For instance, valproate (a valeric acid derivative) affects neuronal function, reduces neuroinflammation, alters gut microbiota, and maintains intestinal barrier integrity (46, 47). Valeric acid diminishes TNF-α levels and regulates the immunological response (48). Isovaleric acid can cross the BBB and disrupt neurotransmitter release, potentially worsening depression (49). Caproic acid is recognized for its roles in metabolic regulation, antibacterial activity, and anti-inflammatory actions (50). The production and function of SCFAs in depression remain incompletely understood, particularly regarding dose-dependent effects as exemplified by butyrate’s dual roles at different concentrations. The transport dynamics of various SCFAs across the blood–brain barrier require further elucidation, while data on minor SCFAs in human depression remain limited. Future studies should establish optimal therapeutic windows for SCFA interventions, develop targeted delivery systems to enhance brain bioavailability, and investigate potential synergistic effects of SCFA combinations as novel antidepressant strategies. The differential effects of various SCFA formulations (salts vs. esters) and their long-term safety profiles also warrant systematic evaluation in clinical populations.
3.1.2 BAs
Gut microbiota dysregulation can influence depression by altering BA concentrations (51). Studies have shown that Blautia and Eubacterium, which are crucial for converting secondary bile acids, are reduced in individuals with depression (52, 53). Depressed patients exhibit significantly lower levels of chenodeoxycholic acid, lithocholylglycine, taurolithocholic acid, and lithocholic acid-3-sulfate, while levels of 23-demethoxycholic acid are significantly higher compared to healthy controls (54). BAs affect lipid metabolism and mediate physiological effects, including immune regulation, by binding to the farnesoid X receptor (FXR) and Takeda G-protein-coupled receptor 5 (TGR5). Mice lacking FXR show reduced depressive behavior, whereas those deficient in TGR5 exhibit increased depression-like behavior (55, 56). Thus, BA metabolism regulated by gut microbiota may influence the MGB axis via TGR5 and FXR, thereby affecting depression development. The specific mechanisms are as follows: (1) Affecting neurotransmitter levels: TGR5 deficiency significantly reduces 5-HT levels in serum and decreases 5-HT1A receptor expression in the hippocampal region of mice, indicating that TGR5 is vital in regulating the 5-HT nervous system (57). Notably, TGR5 expression is reduced in GABA neurons in the lateral hypothalamic area (LHA) of depression model mice. Mechanistically, TGR5 bidirectionally modulates GABA neuronal excitability in the LHA via extracellular regulation of protein kinase-dependent Kv4.2 potassium ion channels. Its antidepressant-like effect stems from the inhibition of GABAergic neuronal inhibitory output in the LHA by TGR5 (58). FXR deficiency reduces the level of the GABA synthase glutamic acid decarboxylase 65 in the hippocampus and upregulates the level of the GABA transporter protein GABA transporter 1, leading to abnormal GABA metabolism. Notably, GABA, 5-hydroxyindole inhibitory acid, and GABA/glutamic acid ratios are elevated in FXR-deficient mice, suggesting that BAs are involved in the pathogenesis of depression through the modulation of neurotransmitter homeostasis (56). (2) Modulating the immune response: FXR and TGR5 can inhibit pro-inflammatory gene expression by regulating endogenous inflammatory vesicles (59). Chenodeoxycholic acid treatment can downregulate NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammatory vesicle activity via FXR (60). (3) Regulating glucagon-like peptide-1 (GLP-1) secretion: BAs are capable of indirectly enhancing GLP-1 signaling through the activation of TGR5 (61). GLP-1 receptor agonists improve depression-like behavior in LPS-treated and obese rodents, possibly by affecting DA synthesis and metabolism or promoting mitochondrial autophagy to inhibit microglial cell focal death (62, 63). (4) Modulating oil-precursor ethanolamide (OEA) levels: Although the exact relationship between BAs and OEA levels has not been fully clarified, BAs increase OEA levels in the jejunum of mice (64). OEA treatment increases Norepinephrine (NE) and 5-HT in the brain and activates peroxisome proliferator-activated receptor α, which, in turn, activates hindbrain neurons and substantia nigra striatal dopaminergic neurons, thereby improving depressive-like behavior in mice (65–67). While bile acids (BAs) show promise in modulating depression via TGR5/FXR pathways, key gaps remain in understanding their precise neuroactive mechanisms, human-specific effects, and clinical applicability. Future work should focus on: human BA metabolomics to identify depression biomarkers, developing CNS-targeted BA receptor modulators, and exploring dietary interventions to optimize BA profiles. Integrating BA research with other gut-brain signals may yield novel therapeutic strategies.
3.1.3 Tryptophan
Tryptophan, a vital amino acid, has metabolic pathways that are closely linked to depression development (68) (Figure 3). It is metabolized through four main pathways: (1) Tryptophan is converted to 5-HT (69). In the human body, 5-HT is distributed unevenly, with approximately 10% in the central nervous system and 90% in the gastrointestinal tract. The BBB prevents gastrointestinal 5-HT from entering the brain, limiting its role as a neurotransmitter there (70). Individuals with depression often have lower serum 5-HT levels (71). 5-HT production is highly dependent on tryptophan availability and tryptophan hydroxylase activity. Low plasma tryptophan levels are associated with impaired immune function, suggesting that such individuals may have inadequate 5-HT synthesis, making them more prone to depression-like symptoms (72). Additionally, the combination of Akkermansia muciniphila and intestinal lactate has been shown to restore tryptophan metabolism balance in people with anxiety disorders and enhance 5-HT effects, thus alleviating anxiety (73). (2) Quinolinic acid pathway: Tryptophan is enzymatically converted to quinolinic acid (QUIN) by tryptophan-2,3-dioxygenase or tryptophan-2,3-monooxygenase. This pathway reduces the conversion of tryptophan to 5-HT, diminishing its neuroprotective effects. Moreover, QUIN and its derivatives may promote neuroinflammation and depressive states (74). Clinically, a combined antidepressant strategy using indoleamine 2,3-dioxygenase 1 inhibitors and probiotics has been proposed and shown to be effective (75). (3) Kynurenine pathway: Over 95% of peripheral tryptophan is oxidized to kynurenine to eliminate excess tryptophan (76). Kynurenine can be further metabolized into kynurenic acid (KYNA), a neuroprotective agent, and QUIN, a neurotoxin (77). Evidence suggests that probiotics like Bifidobacterium infantis can promote the conversion of kynurenine to KYNA, thereby reducing stress-induced depression (78, 79). (4) Melatonin, a significant tryptophan metabolite primarily produced by the pineal gland, is crucial for regulating the sleep–wake cycle. Sleep disturbances in individuals with depression may be related to abnormal melatonin metabolism (80). Current understanding of tryptophan metabolism in depression lacks comprehensive human data on pathway crosstalk and individual variations. Future research should prioritize: (1) clinical validation of microbiota interventions (e.g., Akkermansia, Bifidobacterium) for tryptophan pathway modulation, (2) development of dual-acting therapies targeting both 5-HT synthesis and neuroinflammation (e.g., IDO inhibitors with probiotics), and (3) personalized approaches based on tryptophan metabolic profiling to optimize treatment outcomes.
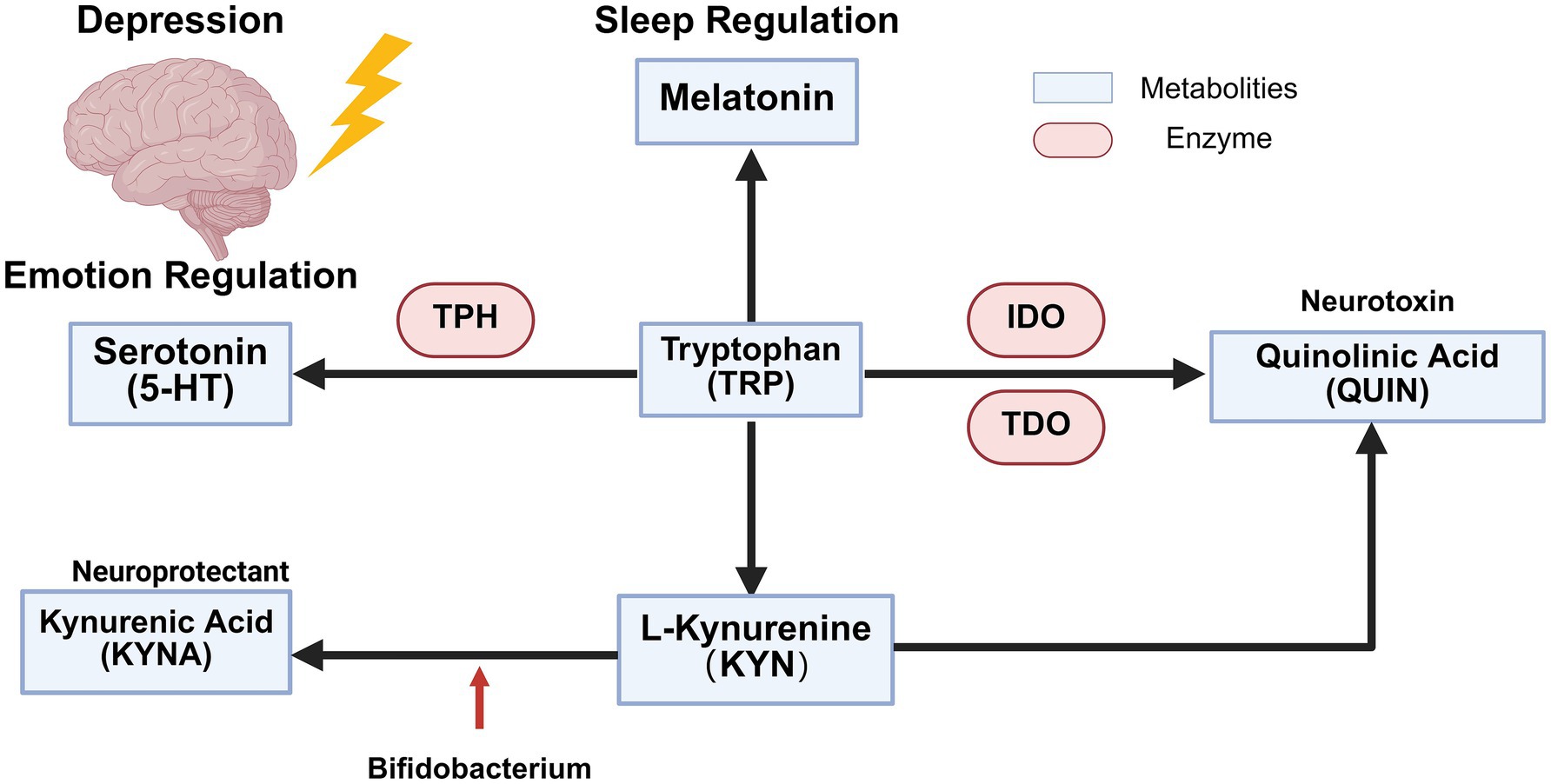
Figure 3. Tryptophan metabolism in depressed patients. Tryptophan is metabolized by tryptophan hydroxylase into 5-HT, a neurotransmitter involved in mood regulation, and into melatonin, which plays a role in sleep regulation. Additionally, enzymes such as indoleamine-2,3-dioxygenase and tryptophan 2,3-dioxygenase convert tryptophan into the neurotoxic metabolite QUIN. Tryptophan can also be converted into KYN, which is subsequently transformed into KYNA by bifidobacteria, exerting neuroprotective effects.
3.1.4 Other metabolic pathways
The gut microbiota can influence the development of depression through various metabolic pathways, such as those involving lactate, sphingolipids, and trimethylamine oxide (TMAO). Lactate has been demonstrated to inhibit and reverse depression (81). Lipopolysaccharides (LPS) produced by gut microbiota can exacerbate the inflammatory response associated with depression (82). Imbalances in gut microbiota can affect protein phosphorylation pathways in the hippocampus, contributing to the onset of depression (83). Additionally, disturbances in carbon and amino acid metabolism have been observed in the hippocampus of rats with depression (84). Elevated glutamatergic neurotransmission is significantly linked to depression, as evidenced by increased glutamate levels in serum, plasma, and brain tissue of individuals with depression. Moreover, plasma glutamate levels have been identified as indicators of depression severity (85–87).
Recent studies indicate that the stress-induced depression model in Wistar rats is associated with alterations in sphingolipid metabolism of phosphatidylcholine and sphingomyelin. Furthermore, the application of milk sphingomyelin, as opposed to egg sphingomyelin, fosters intestinal microbiota homeostasis and enhances the intestinal barrier in mice, potentially ameliorating depression (88–90). Escitalopram was found to enhance depressive symptoms by upregulating critical molecules involved in sphingolipid metabolism (sphingomyelin, sphingosine-1-phosphate). For the first time, an aberration in sphingolipid metabolism was linked to dysbiosis and neuroinflammation, providing a new viewpoint on the connection between sphingolipid metabolism and depression, as well as the MGB axis (91). It has been observed that serum TMAO levels are elevated in methamphetamine (METH)-exposed mice. Increased TMAO levels are associated with higher levels of pro-inflammatory factors, neuronal cell apoptosis, and depression-like behavior, with serum TMAO levels positively correlating with depressive symptoms (92). However, research on TMAO and depression remains limited. The mechanism by which intestinal bacterial metabolites cross the intestinal barrier and the BBB to induce neuroinflammation is still not fully understood. The newly established iPSC model has elucidated how butyric acid can traverse the BBB via the monocarboxylic acid transporter protein (MCT) to stimulate BDNF expression in the hippocampus (93). Further investigation is needed to explore the mechanisms associated with other metabolites.
3.2 Neurotransmitters
3.2.1 Monoamine neurotransmitters
Monoamine neurotransmitters, including catecholamines such as DA, NE, and epinephrine, as well as indoleamines like 5-HT, play a crucial role in mood regulation and are closely associated with depression (94). Current clinical antidepressants primarily rely on the “amine depletion hypothesis,” which involves inhibiting enzymes related to monoamine metabolism to increase synaptic levels of 5-HT, NE, and DA, thereby achieving therapeutic effects (95). The gut microbiota is essential in modulating monoamine neurotransmitter levels, which may influence the onset of depression (83, 96).
3.2.1.1 NE
NE and the norepinephrine transporter (NET) are vital in the pathophysiology of depression. NE binds to NET to recycle excess NE in the synaptic cleft and maintain NE metabolic homeostasis. In the thalamus, increased NET availability accelerates NE recycling in the synaptic cleft, reducing NE signaling and leading to attention deficits (97). The locus coeruleus (LC) is the main source of NE in the brain (98). In depressed patients, reduced NET concentration in the LC results in NE accumulation in the synaptic cleft, disrupting NE signaling and impairing emotional regulation and cognitive function (99).
α2-Adrenergic receptors function as auto-receptors for NE neurons, regulating NE release through negative feedback. In depression patients, increased binding of α2-adrenergic receptors to agonist ligands in NE neuron cytoplasm enhances NE autoreceptor activity, diminishing noradrenergic neurotransmission (100). α-Adrenergic receptors are significant in depression, and antagonizing α2-adrenergic receptors may be a potential therapeutic approach, although direct evidence is limited (101).
Moreover, research indicates that β-adrenergic receptors may enhance stress resistance, offering new insights for depression treatment (102). Chronic antidepressant use affects NE system function. Antidepressants may reduce both the quantity and functionality of β-adrenergic receptors by lowering the sensitivity of NE-responsive adenylate cyclase enzymes, with 5-HT also playing a role. Studies show that 5-HT, which inhibits NE release, interacts with NE, which in turn reduces serotonergic neuron activity (103–105). This interaction is crucial for addressing treatment-resistant depression. Current depression therapies rely on NE reuptake inhibitors and 5-HT reuptake inhibitors, with the interplay between 5-HT and NE potentially impacting therapeutic efficacy and guiding clinical therapy selection (106). Clinically, combining SSRIs with agonists that enhance NE release has shown efficacy in treatment-resistant cases (107). While NE system dysregulation is established in depression, key gaps remain in understanding receptor subtype-specific contributions and optimal therapeutic modulation. The development of CNS-penetrant adrenergic receptor modulators with improved selectivity could address current limitations in targeting noradrenergic pathways. Further elucidation of 5-HT/NE interactions may reveal novel strategies for treatment-resistant cases, particularly through combined receptor modulation approaches. Clinical translation would benefit from advanced NET imaging techniques to guide personalized treatment selection and monitor therapeutic response.
3.2.1.2 5-HT
The primary site of 5-HT production is the intestinal enterochromaffin (EC) cells. These cells absorb tryptophan from the diet via the bloodstream and then convert it into 5-HT through the action of tryptophan hydroxylase (108). This process is governed by gut microbiota, including Lactobacillus and Bifidobacterium, which enhance 5-HT synthesis by modulating tryptophan metabolism and augmenting tryptophan availability in the gut (109, 110). 5-HT plays a crucial role in modulating macrophage polarization by upregulating the expression of genes associated with M2-type macrophages, such as SERPINB2 and THBS1, while simultaneously downregulating the expression of genes characteristic of M1-type macrophages. This dual action effectively reduces the inflammatory response (111). Furthermore, 5-HT regulates immunological responses and systemic signaling through the vagus nerve, consequently influencing gastrointestinal and central nervous system activities. In patients with depression, the concentration of 5-HT and the activity of the 5-HT transporter are reduced, particularly in brain areas such as the amygdala and striatum. This reduction leads to impaired 5-HT signaling, which in turn increases the likelihood of developing depression (112, 113).
3.2.1.3 DA
Roughly 90% of human DA is produced in the gut by Bacillus and Serratia marcescens (114, 115). Most of the L-DOPA synthesized in the gut is transported across the BBB to the brain, where it is subsequently converted into DA. The DA function is influenced by multiple factors. Folate deficiency disrupts homocysteine metabolism, while increased homocysteine levels cause neurotoxicity and damage dopaminergic neurons (83). T-2 toxin, an environmental contaminant generated from fungi, can elicit depressive-like behaviors in mice by increasing DA transporter levels in the nucleus, thereby impairing DA metabolism (116). Furthermore, age-related pro-inflammatory mechanisms correlate with a reduction in DA signaling efficacy, indicating that older adults may represent a lager proportion of individuals with depression (117). Dopaminergic signaling directly influences mood, and its dysfunction is closely associated with depression. Specifically, symptoms of depression such as anhedonia are related to the dysregulation of dopamine signaling (118). Research indicates that DA agonists are useful in alleviating depression linked to Parkinson’s disease; however, there is less empirical data about their efficacy in treating depression more broadly (119).
3.2.2 GABA
Gut microbiota, including Corynebacterium glutamicum, Lactobacillus plantarum, and Lactococcus lactis, produce GABA through a metabolic pathway. This process involves the isomerization of L-glutamic acid to D-glutamic acid, which is then decarboxylated to form GABA (120). Due to the limited dietary GABA content, the current emphasis is on enhancing in vivo levels by manipulating the population of GABA-producing microorganisms (121). GABA crosses the intestinal barrier and binds to receptors on enteric neurons and the vagus nerve, thereby regulating intestinal function. It penetrates the central nervous system through specific transporters at the BBB, binding to GABA receptors on post-synaptic neurons to inhibit the influx of Na+, K+, Ca2+, and Cl−, consequently diminishing neuronal excitability and facilitating various physiological functions, including the promotion of sleep and the alleviation of anxiety (122–124). Subsequent research indicates that chronic stress suppresses the GABAergic neural network, resulting in depression-like behavior, which can be ameliorated with GABA supplementation or probiotic intervention (121, 125). Specific processes involve (1) Administration of Lactobacillus rhamnosus JB-1 modulates the activity of GABA receptors (GABARs) in the brain, thereby resulting in decreased anxiety and depression (23). (2) Gut microbiota metabolites, including SCFAs, are able to permeate the BBB and participate in the GABA-glial cell cycle to mitigate depression-like symptoms (126). Nonetheless, current intervention techniques exhibit dose-effect variability and mechanistic intricacy, requiring additional elucidation of strain specificity and action targets.
3.2.3 Neuropeptides
Neuropeptides are produced by neuroglial cells inside the enteric nervous system and can convey messages to remote organs (83). Alterations in neuropeptide concentrations have been noted in neurological illnesses linked to intestinal inflammation, indicating that immune-neurotransmitter interactions play a role in neurogenesis. Neuropeptide levels have been correlated with depression (83). The precise mechanisms of neuropeptide antidepressants are as follows: Stimulation of the GLP-1 receptor inhibits the synthesis and secretion of corticotropin-releasing hormone (CRH). This mechanism effectively diminishes the overactivation of the HPA axis and attenuates brain damage resulting from chronic stress. Additionally, the engagement of peptide YY and GLP-1 with receptors in the vagal nerve terminals, such as the GLP-1 receptor and neuropeptide Y receptor Y2, transmits signals via vagal afferent fibers to the nucleus tractus solitarius (NTS) in the brainstem. Subsequently, the NTS transmits these impulses to the hypothalamic paraventricular nucleus, suppressing CRH neuronal activity. This interaction facilitates monoamine neurotransmitters’ release, thereby enhancing mood. The signal may also be conveyed to the prefrontal cortex, hippocampus, and other areas to augment neuroplasticity (e.g., BDNF expression) and ameliorate neuronal damage linked to depression (16, 127). Recent studies have found that the tetrapeptide N-acetyl-serine-aspartyl-lysyl-proline (Ac-SDKP) can inhibit neuroinflammatory signaling and alleviate depressive-like behavior in mice (128). Auxin-releasing peptides are closely related to mental disorders and can alleviate depression by activating the HPA axis and promoting DA release (129, 130). Measuring growth hormone-releasing peptide expression levels can also distinguish between depression and bipolar disorder, with the latter having higher growth hormone-releasing peptide levels (119). Targeted growth hormone-releasing peptide therapy for neuropsychiatric disorders is a highly promising and rapidly evolving approach. Recent clinical studies have reported a significant association between elevated growth hormone-releasing peptide levels and reduced depressive symptoms in patients with depression treated with probiotics. However, the exact efficacy of this therapy for depression remains uncertain. Therefore, further clinical trials should be conducted to investigate the efficacy of this growth hormone-releasing peptide (131).
3.3 Nervous pathways
3.3.1 Vagus nerve
The vagus nerve is integral to the MGB axis and functions as the principal conduit for neuronal communication between the gut and the brain (132). The vagus nerve is crucial in neurological and mental disorders (133). The gut microbiota affects intestinal luminal metabolites, which stimulate chemosensory vagal afferent fibers to relay signals to the brain (134). In 2005, vagus nerve stimulation (VNS) has been approved for use in treating treatment-resistant depression, yet its invasiveness has rendered it difficult for many patients to accept. Researchers have been investigating noninvasive alternative therapies (135). Recently, transcutaneous vagus nerve stimulation (tVNS) has enabled noninvasive therapeutic approaches (136). tVNS activates the cortex through electrically modulating the vagus nerve’s auricular branch, thereby stimulating the subcortical nuclei. tVNS is effective in improving depressive symptoms (137, 138). The specific mechanisms include: tVNS augmenting the passage of monoamine neurotransmitters across synapses, rectifying neurochemical and physiological irregularities linked to depression, and improving cognitive performance (139). It facilitates the production of acetylcholine, which attaches to cholinergic receptors on immune cells, suppresses the release of inflammatory mediators, and produces an antidepressant effect (140). tVNS, as a crucial adjunctive treatment for patients with refractory depression, markedly alleviates depressed symptoms when administered with traditional antidepressants, and is anticipated to emerge as a vital supplementary instrument in future depression management (141).
3.3.2 Enteric nervous system
Enteric nervous system (ENS) is an autonomous neuronal plexus within the digestive tract, commonly termed “second brain.” The development initiates with the proliferation and migration of embryonic enteric neural crest cells, governed by the glial cell-derived neurotrophic factor/growth factor receptor signaling pathway, and advances in reaction to gut microbiota colonization (142, 143). ENS is composed of the myenteric plexus, which modulates gut motility, and the submucosal plexus, which controls intestinal secretion.
ENS has a dual purpose. It can autonomously modulate intestinal physiological functions and also lead to depression’s etiology. Enteric glial cells (EGCs) and enteroendocrine cells (EECs) are integral to these processes and are significantly linked to the onset of depression. Research clarifies the interaction between other MGB axis pathways and the enteric nervous system in mediating depression. Germ-free mice demonstrate diminished synaptic growth and a lower density of intermuscular neurons, suggesting that gut microbiota is important in the formation of enteric neurons (143); SCFAs maintain ENS function by restoring neuronal loss (144); reelin, an intrinsic extracellular matrix protein, amalgamates immunomodulatory and neuroplasticity roles within the ENS while upholding intestinal barrier integrity, potentially affecting depression (145, 146); chronic stress activates the HPA axis, leading to an inflammatory subset of EGCs that initiate intestinal inflammation through the CSF1/TNF-α pathway, hence leading to depression (147, 148). EGC is essential for preserving the intestinal epithelial barrier. The ECC releases neuropeptides, including cholecystokinin, transmitting signs from epithelium to submucosal plexus and central nervous system, therefore modulating metabolism (e.g., 5-HT and kynurenine) within the MGB axis (149, 150). Moreover, LPS stimulation in mice enhances gut microbiota growth and repairs damaged neurons without generating new neurons, further emphasizing the interconnectedness and inseparability of MGB axis metabolic pathways (144).
3.4 Cell signaling pathways
Depression is intricately linked to disrupted cellular signaling networks (83). The gut microbiota modulates interactions along the MGB axis via the following fundamental pathways: (1) MAPK/CREB pathway: Gut microbiota activates MAPK pathway via regulation of post-translational changes, resulting in the induction of depression-like behaviors. The downstream CAMK II-CREB cascade is a crucial modulator of signaling within the gut microbiota-MGB axis (151–153). (2) Endogenous cannabinoid system: The endogenous cannabinoid system regulates memory-emotion encoding by the binding of endogenous cannabinoids to hippocampus-specific receptors, which is directly associated with the depressive phenotype (83, 154). (3) mTOR pathway: The deregulation of the mTOR pathway results in compromised synaptic plasticity and neurobehavioral anomalies, which are strongly linked to mood disorders (155). (4) Heat shock protein pathway: Heat shock proteins contribute to the etiology of depression by modulating BDNF expression, preserving neuroprotection and cognitive function, and affecting the HT22 hippocampal cell line and hippocampus tissue (156).
3.5 Immunity
The immune response serves as a crucial pathway through which the gut microbiome affects depression. (1) Centralized migration and modulation of immune cell subsets. Initially, gut microbiota-activated natural killer (NK) cells traverse to the central nervous system (CNS), promoting anti-inflammatory astrocytes’ formation and mitigating neuroinflammation by triggering T-cell death through the TRAIL-DR5 signaling pathway, thus providing a protective effect against depression (157). Secondly, IgA+ plasma cells originating from the gastrointestinal tract traverse to the meninges through the lymphatic system, and their deficiency leads to diminished central resistance to infection in germ-free animals (158). The gut microbiota affects intrinsic lymphocytes to modulate antigen presentation mechanisms, creating a robust connection with the host’s immune system (159). Fourth, Treg cells are diminished in individuals with mood disorders, but Clostridium difficile can supply growth factors like TGF-β to intestinal Treg cells, thus modulating intestinal immune homeostasis and aiding in mood preservation. Fifth, T cell activation in depressed individuals and the downregulation of genes associated with humoral immunity in chronic unpredictable mild stress-induced sad mice indicate that both innate and adaptive immunity lead to depression’s etiology. Interaction between gut bacteria and immune cells can both facilitate immunological tolerance and induce inflammation, thereby serving a dual role in regulating immunity (160–162). (2) Inflammatory factor levels are heightened, while anti-inflammatory factor levels are diminished in individuals with depression (163). Initially, concentrations of the anti-inflammatory cytokine IL-22 are markedly diminished in individuals with depression, and the administration of exogenous IL-22 ameliorates stress-induced depressive behaviors (164, 165). Secondly, IL-10 was markedly enhanced in the plasma of patients treated with Lactobacillus rhamnosus, indicating a widespread neuroimmune influence, although no behavioral effects have been documented (166). Third, the breakdown of the intestinal barrier activates the LPS/TLR4 pathway, resulting in endotoxin translocation and markedly increased serum anti-LPS IgM/IgA concentrations in patients (167, 168). Fourth, pro-inflammatory variables cause an imbalance in kynurenine metabolism, hence enhancing the formation of the neurotoxic compound quinolinic acid and inducing neurotoxicity. (3) Immunometabolism of glial cells. Inflammatory responses may impair neuroplasticity and provoke neuroinflammation. Microglia activation results in the excessive secretion of pro-inflammatory chemicals, which disrupt neuronal connectivity (169, 170). Furthermore, microglial dysfunction impedes hippocampus formation and intensifies depression symptoms (171). Astrocytes modulate microglial activity by preserving the neurochemical milieu and overseeing the BBB, whereas systemic inflammatory reactions induce astrocytic atrophy, worsen microglial dysfunction, and intensify neuroinflammation (172). Dysbiosis additionally prompts excessive generation of reactive oxygen species and impaired mitochondrial autophagy, worsening the crisis in neuronal energy metabolism (173). Recent investigations have demonstrated that the immune system engages with additional channels of the MGB axis. The iPSC-immune cell co-culture paradigm demonstrates that colony metabolites, such as SCFAs, modulate Treg/Th17 homeostasis via epigenetic reprogramming through HDAC inhibition, thereby presenting a novel approach for understanding immune-neurological interactions (174). The gut microbiome influences depression through immune pathways, but key questions remain about how specific microbes drive neuroprotective versus inflammatory responses in humans. Future research should clarify these mechanisms using human cell models and identify immune biomarkers to guide personalized probiotic therapies for depression.
3.6 HPA axis
As a crucial neuroendocrine regulatory axis in the human endocrine system, the HPA axis is the core system regulating the stress response (175). When the body perceives a stress stimulus, the hypothalamus secretes CRH, thereby activating the HPA axis, which subsequently stimulates the pituitary gland to secrete adrenocorticotropic hormone (ACTH) and ultimately leads to the release of cortisol by the adrenal glands (176). The gut microbiota can influence HPA axis activation by regulating stress hormone levels, including cortisol (177). Studies have demonstrated that ACTH levels are significantly elevated in germ-free mice but return to normal levels following the transplantation of normal feces (177). Furthermore, studies have found that the gut microbiota can regulate the circadian secretion of cortisol, and depletion of the gut microbiota can lead to disruption of the glucocorticoid circadian rhythm (178). This finding was further confirmed by gut microbiota transplantation, with Lactobacillus reuteri identified as the key bacterial species involved in this process (178).
It has been demonstrated that the HPA axis is dysregulated in patients with depression, with its overactivation leading to chronic stress responses (179). Overactivation of the HPA axis leads to the release of large amounts of glucocorticoids. In germ-free mice, glucocorticoid receptors are overexpressed and functionally enhanced, leading to impaired immune function, which may be associated with the onset of depression (159). However, a study has shown that HPA activation can induce depression-like behavior by disrupting glucocorticoid receptor expression in the hippocampus (180). Therefore, the specific role of glucocorticoid receptors in HPA axis-mediated depression onset requires further investigation. In addition to its involvement in depression onset through glucocorticoids and their receptors, HPA axis activation can increase intestinal permeability, leading to bacterial translocation and exacerbating inflammatory responses (162). Inflammatory responses are closely associated with the onset and progression of depression. Additionally, in rodent models, vagus nerve stimulation increases CRF mRNA expression in the hypothalamus and elevates plasma ACTH and corticosterone levels (161). This suggests a close interaction between the vagus nerve and the HPA axis, thereby influencing the onset of depression.
Current research indicates that the HPA axis not only serves as a biomarker for the onset of depression but that changes in related hormone levels, such as elevated cortisol levels in the morning and at night, are considered risk factors for the onset of depression (181, 182). For example, fluctuations in ovarian hormones and neurosteroids during perimenopause can alter GABA’s regulation of the HPA axis, leading to HPA axis dysfunction and increased vulnerability to depression (183). Additionally, genetic variations and activity of the HPA axis are considered important predictors of depression and cognitive function in patients. For example, glucocorticoid receptor genetic variations are associated with attention and working memory, while mineralocorticoid receptor is associated with language memory (184).
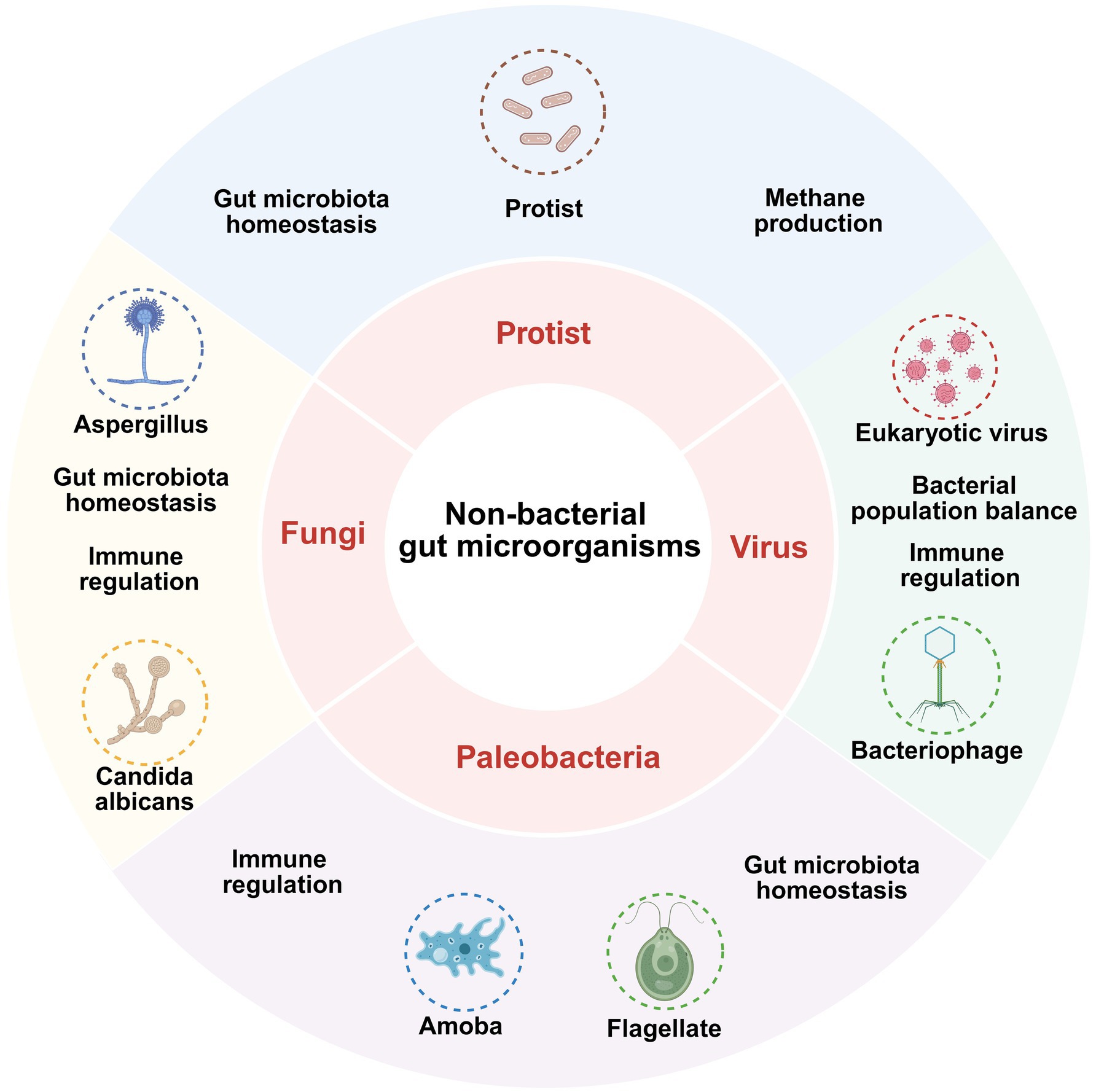
3.7 Non-bacterial gut microbes influence depression through the MGB axis
Although fungi, viruses, and phages form a relatively small part of the gut microbiota, their significance cannot be overlooked and is now receiving more attention (Figure 4).
Fungi such as Aspergillus and Candida albicans contribute to the maintenance of immune regulation and gut microbiota homeostasis; protists are involved in methanogenesis and gut microbiota homeostasis; viruses such as eukaryotic virus affect gut health by regulating bacterial population balance and immune responses; paleobacteria such as Amoeba and Flagellate are also involved in immune regulation and maintenance of gut homeostasis.
Pichia pastoris KM71H and Saccharomyces boulardii demonstrate antidepressant effects via immunomodulation, restoration of the intestinal and BBB, antioxidative stress mitigation, modulation of intestinal microbiota composition, reduction of corticosterone levels in the hippocampus of mice, and inhibition of HPA axis hyperactivation; they are classified as intestinal probiotic fungi (160, 166, 174).
β-glucan (BG) produced by Saccharomyces cerevisiae may influence depression via twin mechanisms: (1) gut microecological regulation: BG enhances the prevalence of advantageous gut bacteria and elevates the concentration of SCFAs, while also restoring the intestinal barrier. (2) Control of neuroinflammation: reducing microglial activation within the hippocampal region and blocking the IL-6-driven pathway of astrocyte apoptosis (185, 186). BG has demonstrated the potential to alleviate symptoms of Alzheimer’s disease, while its direct effectiveness in treating depression necessitates additional elucidation (186).
The known association between human immunodeficiency virus and hepatitis C virus and the risk of depression is acknowledged; nevertheless, the mechanism by which enteroviruses (EV) influence depression via the MGB axis remains contentious (187, 188). Research involving animals has shown that Coxsackievirus B-3 infection inhibits both humoral and cellular immunity, and Coxsackievirus infection in children correlates with heightened anxiety symptoms, although enterovirus infection in children does not elevate the risk of depression (189–191).
4 The impact of gut microbiota on bipolar disorder via the gut-brain axis
Bipolar disorder (BD), also known as manic-depressive disorder, is a common mental disorder characterized by both manic episodes and depressive episodes (a typical feature), differing from depression (192). Gut microbiota is significantly associated with bipolar disorder. Patients with BD exhibit significantly reduced α diversity in gut microbiota, increased abundance of the genera Atopobium, Bacteroidetes, Escherichia-Shigella, and Flavonifractor, and reduced abundance of the phylum Firmicutes (21, 193, 194). Faecalibacterium is negatively correlated with depression severity (195). The mechanism by which the gut-brain axis functions in bipolar disorder is broadly similar to its role in depression. The genus Oscillospira can inhibit the progression of bipolar disorder by producing anti-inflammatory metabolites such as butyrate and participating in fiber fermentation mechanisms, and it is negatively correlated with bipolar disorder (33, 196, 197). B vitamins also play a role in the pathogenesis of BD (198). In the tryptophan metabolic pathway, reduced abundance of KO0837 (aromatic amino acid transferase) and K01667 (tryptophanase) may lead to reduced tryptophan synthesis, while increased abundance of genes such as K00626 (acetyl-CoA transferase) may affect mitochondrial function and neurotransmitter balance. Tryptophan is a precursor to serotonin and kynurenine, and its metabolic pathway undergoes significant changes in BD patients (199). Additionally, studies have found that brain structure acts as an intermediary factor in the influence of gut microbiota on mental disorders, with 13 complete mediation effects identified in BD, including left ventral hypothalamic volume and cerebellar peduncle pathways (196). Bipolar disorder severely impacts patients’ mental health, daily functioning, and quality of life, and is a chronic, recurrent condition. The therapeutic potential of the gut microbiota in mental and psychological disorders is increasingly attracting attention from scientists. Currently, most studies on the gut microbiota and bipolar disorder are conducted in conjunction with other mental disorders, necessitating further independent research on this topic. In the future, combining multi-omics data (such as metabolomics and proteomics) with longitudinal studies could further elucidate the specific mechanisms of the gut-brain axis, providing new insights for the treatment of bipolar disorder.
5 Discussion
Depression, a critical worldwide health issue with profound effects on human well-being and economic status, necessitates heightened scientific focus (200). Recently, the significance of gut microbiota concerning depression has emerged as a prominent area of research. The MGB axis functions as a two-way communication pathway between brain and gut, significantly advancing the research of its involvement in depression (16). This paper comprehensively investigates the mechanisms by which gut microbiota influence depression through MGB axis, systematically summarizes the roles of SCFAs metabolism, tryptophan metabolism, and monoamine neurotransmitters in depression’s pathogenesis, and emphasizes recent research advancements, including the potential roles of sphingolipid metabolism, cellular signaling pathways, and neuropeptides in the etiology of depression, to propose novel strategies for its diagnosis and treatment.
Nonetheless, it is crucial to acknowledge that current investigations possess limits and numerous unresolved enquiries persist, necessitating additional experimental confirmation. Initially, variations in the same microbiota species may differ among depressed individuals of diverse ages and genders, hence precluding the utilization of specific alterations in microbiota as a singular predictor of the onset or advancement of depression (27). Furthermore, despite the acknowledged heterogeneity in individual gut microbiota composition, this variability must be taken into account when targeting gut microorganisms for depression’s treatment. Precise assessment of individual gut microbiota composition has recently been suggested via the integrated study of metagenomics and metabolomics; nevertheless, further studies are required to validate its efficacy. The intricate nature of human gut microbiota, which cannot be entirely replicated by cellular and animal models, renders the translation of fundamental research findings into clinical applications difficult. Current clinical investigations are predominantly observational and are unable to determine a causal association between gut microbiota dysbiosis and depression. Future intervention studies, including probiotic therapy or fecal microbiota transplantation, are necessary to assess enhancements in depressive symptoms and so validate the involvement of gut microorganisms in depression (201, 202). Concurrently, longitudinal cohort studies employing Mendelian randomization analyses are essential to eliminate the influence of environmental factors, including nutrition and stress.
Besides the indirect role of gut microbes in depression through the MGB axis, damage to the BBB in depressed individuals facilitates gut microbiota and their metabolites’ translocation to the brain, resulting in spatially and temporally specific neuroinflammation (31); however, this phenomenon has not been conclusively validated by experimental evidence. Future research may figure out the potential impairment of BBB integrity in individuals with depression, the role of gut microbiota in exacerbating neuroinflammation through this mechanism, and strategies for intervening in the course of depression by focusing on BBB protection. The process via which gut bacterial metabolites penetrate the brain through the intestinal barrier as opposed to crossing the BBB to induce neuroinflammation remains inadequately elucidated. Recent investigations utilizing an iPSC model have elucidated the metabolite cross-barrier process. A co-culture system of induced pluripotent stem cell-derived intestinal epithelial cells (iIECs) and intestinal brain microvascular endothelial cells (iBMECs) demonstrated that butyric acid traverses the BBB through the MCT and stimulates hippocampal BDNF production (93). Additional research is required to validate the processes of other metabolites. Moreover, while SCFAs are known to enhance intestinal barrier integrity, current research indicates that elevated levels of SCFAs may compromise the intestinal barrier (40). Additional validation of the effects of varying SCFA concentrations on intestinal barrier integrity and their influence on the efficacy of depression treatment is required. Simultaneously, the incidence of depression rises among postmenopausal women, potentially linked to estrogen levels, resulting in the suggestion of the “hormone-gut microbe-depression axis” (203). Additional investigation is required to examine this concept. Moreover, the temporal aspects of inflammation in the brains of individuals with depression remain inadequately substantiated. If this hypothesis holds, might the identification of inflammatory markers in the brain or imaging findings establish a foundation for temporal depression interventions, such as anti-inflammatory therapies during the acute phase and anti-neurodegenerative treatments in the chronic phase (31)? METH-exposed mice demonstrated depressive-like behavior; nevertheless, their gut microbiota composition remained mostly unchanged (92). Nevertheless, antibiotic therapy mitigated increased serum TMAO concentrations and depressive-like behaviors (92). The correlation between TMAO and depression, as well as its underlying mechanisms, warrants thorough examination. Although the role of α-adrenergic receptors in depression have been described, direct evidence is still limited. Antagonizing α2-adrenergic receptors may be a promising strategy for treating depression, which requires further experimental verification (101). The role of gut microorganisms in the MGB axis, which frequently leads to comorbidity between depression and other illnesses like anorexia nervosa, necessitates additional investigation (204, 206). Currently, in addition to emerging advances in the treatment of depression based on gut microbiota and the gut-brain axis, treatment methods and approaches for depression are also constantly evolving. Ketamine, dextromethorphan, brain-computer interface technology, and photobiomodulation methods have all shown potential therapeutic effects for depression. In the future, the treatment of depression will undoubtedly become more diversified, with the use of multiple drugs in combination to enhance efficacy. In summary, while substantial advancements have been achieved in examining the correlation between gut bacteria and depression, multiple foundational investigations have elucidated the gut microbes-MGB axis-depression route. Beneficial gut bacteria have been utilized in clinical research to modulate depression, demonstrating modest benefit. Nevertheless, other topics require additional investigation. Future research should prioritize experimental validation and individual variability to enhance strategies for the diagnosis and treatment of depression.
Author contributions
XZ: Software, Investigation, Writing – original draft, Funding acquisition, Resources, Supervision, Writing – review & editing. SW: Investigation, Writing – review & editing, Writing – original draft, Conceptualization, Software. XW: Writing – original draft, Writing – review & editing, Visualization. XC: Writing – original draft, Writing – review & editing, Visualization, Conceptualization. PZho: Software, Writing – review & editing, Investigation, Conceptualization. KM: Conceptualization, Writing – review & editing, Investigation, Software. PZha: Writing – review & editing, Software, Investigation, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the National Science Foundation (NSF) under Grant No. 82102299.
Acknowledgments
All figures have been generated using BioRender (laboratory license is given).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Marx, W, Penninx, BWJH, Solmi, M, Furukawa, TA, Firth, J, Carvalho, AF, et al. Major depressive disorder. Nat Rev Dis Primers. (2023) 9:44. doi: 10.1038/s41572-023-00454-1
2. World Health Organization. (2022). COVID-19 pandemic triggers 25% increase in prevalence of anxiety and depression worldwide. Available online at: https://www.who.int/news/item/02-03-2022-covid-19-pandemic-triggers-25-increase-in-prevalence-of-anxiety-and-depression-worldwide. (Accessed July 12, 2025)
3. Boers, E, Afzali, MH, Newton, N, and Conrod, P. Association of screen time and depression in adolescence. JAMA Pediatr. (2019) 173:853–9. doi: 10.1001/jamapediatrics.2019.1759
4. Kim, H-D, Wei, J, Call, T, Ma, X, Quintus, NT, Summers, AJ, et al. SIRT1 coordinates transcriptional regulation of neural activity and modulates depression-like behaviors in the nucleus accumbens. Biol Psychiatry. (2024) 96:495–505. doi: 10.1016/j.biopsych.2024.03.017
5. Stojanov, S, Berlec, A, and Štrukelj, B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. (2020) 8:1715. doi: 10.3390/microorganisms8111715
6. Sender, R, Fuchs, S, and Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. (2016) 14:e1002533. doi: 10.1371/journal.pbio.1002533
7. Ursell, LK, Metcalf, JL, Parfrey, LW, and Knight, R. Defining the human microbiome. Nutr Rev. (2012) 70:S38–44. doi: 10.1111/j.1753-4887.2012.00493.x
8. Yatsunenko, T, Rey, FE, Manary, MJ, Trehan, I, Dominguez-Bello, MG, Contreras, M, et al. Human gut microbiome viewed across age and geography. Nature. (2012) 486:222–7. doi: 10.1038/nature11053
9. Flint, HJ, Scott, KP, Duncan, SH, Louis, P, and Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. (2012) 3:289–306. doi: 10.4161/gmic.19897
10. Bird, AR, Conlon, MA, Christophersen, CT, and Topping, DL. Resistant starch, large bowel fermentation and a broader perspective of prebiotics and probiotics. Benef Microbes. (2010) 1:423–31. doi: 10.3920/BM2010.0041
11. LeBlanc, JG, Milani, C, de Giori, GS, Sesma, F, van Sinderen, D, and Ventura, M. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol. (2013) 24:160–8. doi: 10.1016/j.copbio.2012.08.005
12. Staley, C, Weingarden, AR, Khoruts, A, and Sadowsky, MJ. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Appl Microbiol Biotechnol. (2017) 101:47–64. doi: 10.1007/s00253-016-8006-6
13. Bäumler, AJ, and Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature. (2016) 535:85–93. doi: 10.1038/nature18849
14. Bernet, MF, Brassart, D, Neeser, JR, and Servin, AL. Lactobacillus acidophilus LA 1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut. (1994) 35:483–9. doi: 10.1136/gut.35.4.483
15. Round, JL, and Mazmanian, SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. (2009) 9:313–23. doi: 10.1038/nri2515
16. Chang, L, Wei, Y, and Hashimoto, K. Brain-gut-microbiota axis in depression: a historical overview and future directions. Brain Res Bull. (2022) 182:44–56. doi: 10.1016/j.brainresbull.2022.02.004
17. Zhang, R, Ding, N, Feng, X, and Liao, W. The gut microbiome, immune modulation, and cognitive decline: insights on the gut-brain axis. Front Immunol. (2025) 16:1529958. doi: 10.3389/fimmu.2025.1529958
18. Mayer, EA, Nance, K, and Chen, S. The gut-brain axis. Annu Rev Med. (2022) 73:439–53. doi: 10.1146/annurev-med-042320-014032
19. Xu, Q, Xiang, Q, Tan, Z, and Yang, Q. Global research trends in the intestinal microflora and depression: bibliometrics and visual analysis. Front Cell Infect Microbiol. (2025) 15:1507667. doi: 10.3389/fcimb.2025.1507667
20. Liu, L, Wang, H, Zhang, H, Chen, X, Zhang, Y, Wu, J, et al. Toward a deeper understanding of gut microbiome in depression: the promise of clinical applicability. Adv Sci. (2022) 9:e2203707. doi: 10.1002/advs.202203707
21. Nikolova, VL, Smith, MRB, Hall, LJ, Cleare, AJ, Stone, JM, and Young, AH. Perturbations in gut microbiota composition in psychiatric disorders: a review and meta-analysis. JAMA Psychiatry. (2021) 78:1343–54. doi: 10.1001/jamapsychiatry.2021.2573
22. Simpson, CA, Diaz-Arteche, C, Eliby, D, Schwartz, OS, Simmons, JG, and Cowan, CSM. The gut microbiota in anxiety and depression—a systematic review. Clin Psychol Rev. (2021) 83:101943. doi: 10.1016/j.cpr.2020.101943
23. Jiang, H, Ling, Z, Zhang, Y, Mao, H, Ma, Z, Yin, Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. (2015) 48:186–94. doi: 10.1016/j.bbi.2015.03.016
24. Chen, J-J, He, S, Fang, L, Wang, B, Bai, S-J, Xie, J, et al. Age-specific differential changes on gut microbiota composition in patients with major depressive disorder. Aging. (2020) 12:2764–76. doi: 10.18632/aging.102775
25. Martino, C, Dilmore, AH, Burcham, ZM, Metcalf, JL, Jeste, D, and Knight, R. Microbiota succession throughout life from the cradle to the grave. Nat Rev Microbiol. (2022) 20:707–20. doi: 10.1038/s41579-022-00768-z
26. Ling, Z, Cheng, Y, Chen, F, Yan, X, Liu, X, Shao, L, et al. Changes in fecal microbiota composition and the cytokine expression profile in school-aged children with depression: a case-control study. Front Immunol. (2022) 13:964910. doi: 10.3389/fimmu.2022.964910
27. Knuesel, T, and Mohajeri, MH. The role of the gut microbiota in the development and progression of major depressive and bipolar disorder. Nutrients. (2021) 14:37. doi: 10.3390/nu14010037
28. Caso, JR, MacDowell, KS, González-Pinto, A, García, S, de Diego-Adeliño, J, Carceller-Sindreu, M, et al. Gut microbiota, innate immune pathways, and inflammatory control mechanisms in patients with major depressive disorder. Transl Psychiatry. (2021) 11:645. doi: 10.1038/s41398-021-01755-3
29. Hu, X, Li, Y, Wu, J, Zhang, H, Huang, Y, Tan, X, et al. Changes of gut microbiota reflect the severity of major depressive disorder: a cross sectional study. Transl Psychiatry. (2023) 13:137. doi: 10.1038/s41398-023-02436-z
30. Zheng, K-Y, Gao, B, Wang, H-J, He, J-G, Chen, H-S, Hu, Z-L, et al. Melatonin ameliorates depressive-like behaviors in ovariectomized mice by improving tryptophan metabolism via inhibition of gut microbe alistipes inops. Adv Sci. (2024) 11:e2309473. doi: 10.1002/advs.202309473
31. Hoeferlin, GF, Grabinski, SE, Druschel, LN, Duncan, JL, Burkhart, G, Weagraff, GR, et al. Bacteria invade the brain following intracortical microelectrode implantation, inducing gut-brain axis disruption and contributing to reduced microelectrode performance. Nat Commun. (2025) 16:1829. doi: 10.1038/s41467-025-56979-4
32. Akhtar, M, Chen, Y, Ma, Z, Zhang, X, Shi, D, Khan, JA, et al. Gut microbiota-derived short chain fatty acids are potential mediators in gut inflammation. Anim Nutr. (2022) 8:350–60. doi: 10.1016/j.aninu.2021.11.005
33. Morrison, DJ, and Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. (2016) 7:189–200. doi: 10.1080/19490976.2015.1134082
34. Tang, C-F, Wang, C-Y, Wang, J-H, Wang, Q-N, Li, S-J, Wang, H-O, et al. Short-chain fatty acids ameliorate depressive-like behaviors of high fructose-fed mice by rescuing hippocampal neurogenesis decline and blood-brain barrier damage. Nutrients. (2022) 14:1882. doi: 10.3390/nu14091882
35. Xiao, W, Li, J, Gao, X, Yang, H, Su, J, Weng, R, et al. Involvement of the gut-brain axis in vascular depression via tryptophan metabolism: a benefit of short chain fatty acids. Exp Neurol. (2022) 358:114225. doi: 10.1016/j.expneurol.2022.114225
36. Fukuda, S, Toh, H, Hase, K, Oshima, K, Nakanishi, Y, Yoshimura, K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. (2011) 469:543–7. doi: 10.1038/nature09646
37. Tong, L-C, Wang, Y, Wang, Z-B, Liu, W-Y, Sun, S, Li, L, et al. Propionate ameliorates dextran sodium sulfate-induced colitis by improving intestinal barrier function and reducing inflammation and oxidative stress. Front Pharmacol. (2016) 7:253. doi: 10.3389/fphar.2016.00253
38. Wang, H-B, Wang, P-Y, Wang, X, Wan, Y-L, and Liu, Y-C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein claudin-1 transcription. Dig Dis Sci. (2012) 57:3126–35. doi: 10.1007/s10620-012-2259-4
39. Parada Venegas, D, De la Fuente, MK, Landskron, G, González, MJ, Quera, R, Dijkstra, G, et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. (2019) 10:277. doi: 10.3389/fimmu.2019.00277
40. Peng, L, He, Z, Chen, W, Holzman, IR, and Lin, J. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatr Res. (2007) 61:37–41. doi: 10.1203/01.pdr.0000250014.92242.f3
41. Wang, W, Dernst, A, Martin, B, Lorenzi, L, Cadefau-Fabregat, M, Phulphagar, K, et al. Butyrate and propionate are microbial danger signals that activate the NLRP3 inflammasome in human macrophages upon TLR stimulation. Cell Rep. (2024) 43:114736. doi: 10.1016/j.celrep.2024.114736
42. Kim, S-J, Lee, H, Lee, G, Oh, S-J, Shin, M-K, Shim, I, et al. CD4+ CD25+ regulatory T cell depletion modulates anxiety and depression-like behaviors in mice. PLoS One. (2012) 7:e42054. doi: 10.1371/journal.pone.0042054
43. Almutawaa, W, Kang, NH, Pan, Y, and Niles, LP. Induction of neurotrophic and differentiation factors in neural stem cells by valproic acid. Basic Clin Pharmacol Toxicol. (2014) 115:216–21. doi: 10.1111/bcpt.12201
44. Xu, L, Cheng, Z, Zhao, J, Liu, Y, Zhao, Y, and Yang, X. Advances in epigenetic regulation of the dioxygenase TET1. Sheng Wu Gong Cheng Xue Bao. (2024) 40:4351–64. doi: 10.13345/j.cjb.240191
45. Fukumoto, S, Tatewaki, M, Yamada, T, Fujimiya, M, Mantyh, C, Voss, M, et al. Short-chain fatty acids stimulate colonic transit via intraluminal 5-HT release in rats. Am J Physiol Regul Integr Comp Physiol. (2003) 284:R1269–76. doi: 10.1152/ajpregu.00442.2002
46. Liu, M, Zhang, Y, Liu, J, Xiang, C, Lu, Q, Lu, H, et al. Revisiting the role of valeric acid in manipulating ulcerative colitis. Inflamm Bowel Dis. (2024) 30:617–28. doi: 10.1093/ibd/izad187
47. Liu, P, Liu, Z, Wang, J, Wang, J, Gao, M, Zhang, Y, et al. Immunoregulatory role of the gut microbiota in inflammatory depression. Nat Commun. (2024) 15:3003. doi: 10.1038/s41467-024-47273-w
48. Kovanda, L, Park, J, Park, S, Kim, K, Li, X, and Liu, Y. Dietary butyrate and valerate glycerides impact diarrhea severity and immune response of weaned piglets under ETEC F4-ETEC F18 coinfection conditions. J Anim Sci. (2023) 101:skad401. doi: 10.1093/jas/skad401
49. Szczesniak, O, Hestad, KA, Hanssen, JF, and Rudi, K. Isovaleric acid in stool correlates with human depression. Nutr Neurosci. (2016) 19:279–83. doi: 10.1179/1476830515Y.0000000007
50. McDonald, JAK, Mullish, BH, Pechlivanis, A, Liu, Z, Brignardello, J, Kao, D, et al. Inhibiting growth of Clostridioides difficile by restoring valerate, produced by the intestinal microbiota. Gastroenterology. (2018) 155:1495–1507.e15. doi: 10.1053/j.gastro.2018.07.014
51. Jia, M, Fan, Y, Ma, Q, Yang, D, Wang, Y, He, X, et al. Gut microbiota dysbiosis promotes cognitive impairment via bile acid metabolism in major depressive disorder. Transl Psychiatry. (2024) 14:503. doi: 10.1038/s41398-024-03211-4
52. Yang, J, Zheng, P, Li, Y, Wu, J, Tan, X, Zhou, J, et al. Landscapes of bacterial and metabolic signatures and their interaction in major depressive disorders. Sci Adv. (2020) 6:eaba8555. doi: 10.1126/sciadv.aba8555
53. Yu, J, Zhang, H, Chen, L, Ruan, Y, Chen, Y, and Liu, Q. Disease-associated gut microbiota reduces the profile of secondary bile acids in pediatric nonalcoholic fatty liver disease. Front Cell Infect Microbiol. (2021) 11:698852. doi: 10.3389/fcimb.2021.698852
54. Sun, N, Zhang, J, Wang, J, Liu, Z, Wang, X, Kang, P, et al. Abnormal gut microbiota and bile acids in patients with first-episode major depressive disorder and correlation analysis. Psychiatry Clin Neurosci. (2022) 76:321–8. doi: 10.1111/pcn.13368
55. Guo, C, Chen, W-D, and Wang, Y-D. TGR5, not only a metabolic regulator. Front Physiol. (2016) 7:646. doi: 10.3389/fphys.2016.00646
56. Huang, F, Wang, T, Lan, Y, Yang, L, Pan, W, Zhu, Y, et al. Deletion of mouse FXR gene disturbs multiple neurotransmitter systems and alters neurobehavior. Front Behav Neurosci. (2015) 9:70. doi: 10.3389/fnbeh.2015.00070
57. Tao, Y, Zhou, H, Li, Z, Wu, H, Wu, F, Miao, Z, et al. TGR5 deficiency-induced anxiety and depression-like behaviors: the role of gut microbiota dysbiosis. J Affect Disord. (2024) 344:219–32. doi: 10.1016/j.jad.2023.10.072
58. Li, X-Y, Zhang, S-Y, Hong, Y-Z, Chen, Z-G, Long, Y, Yuan, D-H, et al. TGR5-mediated lateral hypothalamus-dCA3-dorsolateral septum circuit regulates depressive-like behavior in male mice. Neuron. (2024) 112:1795–1814.e10. doi: 10.1016/j.neuron.2024.02.019
59. Hao, H, Cao, L, Jiang, C, Che, Y, Zhang, S, Takahashi, S, et al. Farnesoid X receptor regulation of the NLRP3 inflammasome underlies cholestasis-associated sepsis. Cell Metab. (2017) 25:856–867.e5. doi: 10.1016/j.cmet.2017.03.007
60. Li, H, Zhu, X, Xu, J, Li, L, Kan, W, Bao, H, et al. The FXR mediated anti-depression effect of CDCA underpinned its therapeutic potentiation for MDD. Int Immunopharmacol. (2023) 115:109626. doi: 10.1016/j.intimp.2022.109626
61. Lasalle, M, Hoguet, V, Hennuyer, N, Leroux, F, Piveteau, C, Belloy, L, et al. Topical intestinal aminoimidazole agonists of G-protein-coupled bile acid receptor 1 promote glucagon like peptide-1 secretion and improve glucose tolerance. J Med Chem. (2017) 60:4185–211. doi: 10.1021/acs.jmedchem.6b01873
62. Ventorp, F, Bay-Richter, C, Nagendra, AS, Janelidze, S, Matsson, VS, Lipton, J, et al. Exendin-4 treatment improves LPS-induced depressive-like behavior without affecting pro-inflammatory cytokines. J Parkinsons Dis. (2017) 7:263–73. doi: 10.3233/JPD-171068
63. Yang, F, Wang, X, Qi, J, Zhang, K, Jiang, Y, Feng, B, et al. Glucagon-like peptide 1 receptor activation inhibits microglial pyroptosis via promoting mitophagy to alleviate depression-like behaviors in diabetic mice. Nutrients. (2022) 15:38. doi: 10.3390/nu15010038
64. Higuchi, S, Wood, C, Nasiri, RH, Giddla, LJ, Molina, V, Diarra, R, et al. The 16α-hydroxylated bile acid, pythocholic acid decreases food intake and increases oleoylethanolamide in male mice. Endocrinology. (2023) 164:bqad116. doi: 10.1210/endocr/bqad116
65. Yu, H-L, Sun, L-P, Li, M-M, and Quan, Z-S. Involvement of norepinephrine and serotonin system in antidepressant-like effects of oleoylethanolamide in the mice models of behavior despair. Neurosci Lett. (2015) 593:24–8. doi: 10.1016/j.neulet.2015.03.019
66. Hankir, MK, Seyfried, F, Hintschich, CA, Diep, T-A, Kleberg, K, Kranz, M, et al. Gastric bypass surgery recruits a gut PPAR-α-striatal D1R pathway to reduce fat appetite in obese rats. Cell Metab. (2017) 25:335–44. doi: 10.1016/j.cmet.2016.12.006
67. Costa, A, Cristiano, C, Cassano, T, Gallelli, CA, Gaetani, S, Ghelardini, C, et al. Histamine-deficient mice do not respond to the antidepressant-like effects of oleoylethanolamide. Neuropharmacology. (2018) 135:234–41. doi: 10.1016/j.neuropharm.2018.03.033
68. Gao, K, Mu, C-L, Farzi, A, and Zhu, W-Y. Tryptophan metabolism: a link between the gut microbiota and brain. Adv Nutr. (2020) 11:709–23. doi: 10.1093/advances/nmz127
69. Yohn, CN, Gergues, MM, and Samuels, BA. The role of 5-HT receptors in depression. Mol Brain. (2017) 10:28. doi: 10.1186/s13041-017-0306-y
70. Cryan, JF, and Dinan, TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. (2012) 13:701–12. doi: 10.1038/nrn3346
71. Tahiri, J, Mian, M, Aftan, F, Habbal, S, Salehi, F, Reddy, PH, et al. Serotonin in depression and Alzheimer’s disease: focus on SSRI’S beneficial effects. Ageing Res Rev. (2024) 101:102537. doi: 10.1016/j.arr.2024.102537
72. Schröcksnadel, K, Wirleitner, B, Winkler, C, and Fuchs, D. Monitoring tryptophan metabolism in chronic immune activation. Clin Chim Acta. (2006) 364:82–90. doi: 10.1016/j.cca.2005.06.013
73. Pan, M, Qian, C, Huo, S, Wu, Y, Zhao, X, Ying, Y, et al. Gut-derived lactic acid enhances tryptophan to 5-hydroxytryptamine in regulation of anxiety via Akkermansia muciniphila. Gut Microbes. (2025) 17:2447834. doi: 10.1080/19490976.2024.2447834
74. Zhou, M, Fan, Y, Xu, L, Yu, Z, Wang, S, Xu, H, et al. Microbiome and tryptophan metabolomics analysis in adolescent depression: roles of the gut microbiota in the regulation of tryptophan-derived neurotransmitters and behaviors in human and mice. Microbiome. (2023) 11:145. doi: 10.1186/s40168-023-01589-9
75. Tian, P, Zou, R, Wang, L, Chen, Y, Qian, X, Zhao, J, et al. Multi-probiotics ameliorate major depressive disorder and accompanying gastrointestinal syndromes via serotonergic system regulation. J Adv Res. (2023) 45:117–25. doi: 10.1016/j.jare.2022.05.003
76. Badawy, AA-B. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int J Tryptophan Res. (2017) 10:1178646917691938. doi: 10.1177/1178646917691938
77. Cervenka, I, Agudelo, LZ, and Ruas, JL. Kynurenines: tryptophan’s metabolites in exercise, inflammation, and mental health. Science. (2017) 357:eaaf9794. doi: 10.1126/science.aaf9794
78. Desbonnet, L, Garrett, L, Clarke, G, Bienenstock, J, and Dinan, TG. The probiotic bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. (2008) 43:164–74. doi: 10.1016/j.jpsychires.2008.03.009
79. Agudelo, LZ, Femenía, T, Orhan, F, Porsmyr-Palmertz, M, Goiny, M, Martinez-Redondo, V, et al. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. (2014) 159:33–45. doi: 10.1016/j.cell.2014.07.051
80. Davidson, M, Rashidi, N, Nurgali, K, and Apostolopoulos, V. The role of tryptophan metabolites in neuropsychiatric disorders. Int J Mol Sci. (2022) 23:9968. doi: 10.3390/ijms23179968
81. Karnib, N, El-Ghandour, R, El Hayek, L, Nasrallah, P, Khalifeh, M, Barmo, N, et al. Lactate is an antidepressant that mediates resilience to stress by modulating the hippocampal levels and activity of histone deacetylases. Neuropsychopharmacology. (2019) 44:1152–62. doi: 10.1038/s41386-019-0313-z
82. Lach, G, Schellekens, H, Dinan, TG, and Cryan, JF. Anxiety, depression, and the microbiome: a role for gut peptides. Neurotherapeutics. (2018) 15:36–59. doi: 10.1007/s13311-017-0585-0
83. Zhang, Y, Liu, C, Zhu, Q, Wu, H, Liu, Z, and Zeng, L. Relationship between depression and epigallocatechin gallate from the perspective of gut microbiota: a systematic review. Nutrients. (2025) 17:259. doi: 10.3390/nu17020259
84. Koh, A, and Bäckhed, F. From association to causality: the role of the gut microbiota and its functional products on host metabolism. Mol Cell. (2020) 78:584–96. doi: 10.1016/j.molcel.2020.03.005
85. Gruenbaum, BF, Zlotnik, A, Frenkel, A, Fleidervish, I, and Boyko, M. Glutamate efflux across the blood-brain barrier: new perspectives on the relationship between depression and the glutamatergic system. Meta. (2022) 12:459. doi: 10.3390/metabo12050459
86. Onaolapo, AY, and Onaolapo, OJ. Glutamate and depression: reflecting a deepening knowledge of the gut and brain effects of a ubiquitous molecule. World J Psychiatry. (2021) 11:297–315. doi: 10.5498/wjp.v11.i7.297
87. Frank, D, Gruenbaum, BF, Shelef, I, Zvenigorodsky, V, Severynovska, O, Gal, R, et al. Blood glutamate scavenging with pyruvate as a novel preventative and therapeutic approach for depressive-like behavior following traumatic brain injury in a rat model. Front Neurosci. (2022) 16:832478. doi: 10.3389/fnins.2022.832478
88. Norris, GH, Milard, M, Michalski, M-C, and Blesso, CN. Protective properties of milk sphingomyelin against dysfunctional lipid metabolism, gut dysbiosis, and inflammation. J Nutr Biochem. (2019) 73:108224. doi: 10.1016/j.jnutbio.2019.108224
89. Leskanicova, A, Babincak, M, Mochnacky, F, Pipova Kokosova, N, Kukelova, D, Urbanska, N, et al. Sex-dependent differences in stress-induced depression in Wistar rats are accompanied predominantly by changes in phosphatidylcholines and sphingomyelins. J Physiol Pharmacol. (2021) 72:623–35. doi: 10.26402/jpp.2021.4.14
90. Norris, GH, Jiang, C, Ryan, J, Porter, CM, and Blesso, CN. Milk sphingomyelin improves lipid metabolism and alters gut microbiota in high fat diet-fed mice. J Nutr Biochem. (2016) 30:93–101. doi: 10.1016/j.jnutbio.2015.12.003
91. Duan, J, Sun, J, Ma, X, Du, P, Dong, P, Xue, J, et al. Association of escitalopram-induced shifts in gut microbiota and sphingolipid metabolism with depression-like behavior in Wistar-Kyoto rats. Transl Psychiatry. (2025) 15:54. doi: 10.1038/s41398-025-03277-8
92. Wang, X, Hui, R, Li, Q, Lu, Y, Wang, M, Shi, Y, et al. Gut microbiota-derived trimethylamine N-oxide involved in methamphetamine-induced depression-like behaviors of male mice. Neuropharmacology. (2025) 268:110339. doi: 10.1016/j.neuropharm.2025.110339
93. Janssen, AWF, van der Lugt, B, Duivenvoorde, LPM, Vos, AP, Bastiaan-Net, S, Tomassen, MMM, et al. Comparison of iPSC-derived human intestinal epithelial cells with Caco-2 cells and human in vivo data after exposure to Lactiplantibacillus plantarum WCFS1. Sci Rep. (2024) 14:26464. doi: 10.1038/s41598-024-74802-w
94. Nutt, DJ. Relationship of neurotransmitters to the symptoms of major depressive disorder. J Clin Psychiatry. (2008) 69:4–7.
95. Tizabi, Y, Bhatti, BH, Manaye, KF, Das, JR, and Akinfiresoye, L. Antidepressant-like effects of low ketamine dose is associated with increased hippocampal AMPA/NMDA receptor density ratio in female Wistar-Kyoto rats. Neuroscience. (2012) 213:72–80. doi: 10.1016/j.neuroscience.2012.03.052
96. Pan, J-X, Deng, F-L, Zeng, B-H, Zheng, P, Liang, W-W, Yin, B-M, et al. Absence of gut microbiota during early life affects anxiolytic behaviors and monoamine neurotransmitters system in the hippocampal of mice. J Neurol Sci. (2019) 400:160–8. doi: 10.1016/j.jns.2019.03.027
97. Moriguchi, S, Yamada, M, Takano, H, Nagashima, T, Takahata, K, Yokokawa, K, et al. Norepinephrine transporter in major depressive disorder: a PET study. Am J Psychiatry. (2017) 174:36–41. doi: 10.1176/appi.ajp.2016.15101334
98. Poe, GR, Foote, S, Eschenko, O, Johansen, JP, Bouret, S, Aston-Jones, G, et al. Locus coeruleus: a new look at the blue spot. Nat Rev Neurosci. (2020) 21:644–59. doi: 10.1038/s41583-020-0360-9
99. Klimek, V, Stockmeier, C, Overholser, J, Meltzer, HY, Kalka, S, Dilley, G, et al. Reduced levels of norepinephrine transporters in the locus coeruleus in major depression. J Neurosci. (1997) 17:8451–8. doi: 10.1523/JNEUROSCI.17-21-08451.1997
100. Hamon, M, and Blier, P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog Neuropsychopharmacol Biol Psychiatry. (2013) 45:54–63. doi: 10.1016/j.pnpbp.2013.04.009
101. Maletic, V, Eramo, A, Gwin, K, Offord, SJ, and Duffy, RA. The role of norepinephrine and its α-adrenergic receptors in the pathophysiology and treatment of major depressive disorder and schizophrenia: a systematic review. Front Psychiatry. (2017) 8:42. doi: 10.3389/fpsyt.2017.00042
102. Zhang, H, Cui, M, Cao, J-L, and Han, M-H. The role of beta-adrenergic receptors in depression and resilience. Biomedicines. (2022) 10:2378. doi: 10.3390/biomedicines10102378
103. Guiard, BP, El Mansari, M, Merali, Z, and Blier, P. Functional interactions between dopamine, serotonin and norepinephrine neurons: an in-vivo electrophysiological study in rats with monoaminergic lesions. Int J Neuropsychopharmacol. (2008) 11:625–39. doi: 10.1017/S1461145707008383
104. Blier, P. Crosstalk between the norepinephrine and serotonin systems and its role in the antidepressant response. J Psychiatry Neurosci. (2001) 26:S3–S10.
105. Sulser, F. Serotonin-norepinephrine receptor interactions in the brain: implications for the pharmacology and pathophysiology of affective disorders. J Clin Psychiatry. (1987) 48:12–8.
106. Lucki, I, and O’Leary, OF. Distinguishing roles for norepinephrine and serotonin in the behavioral effects of antidepressant drugs. J Clin Psychiatry. (2004) 65:11–24.
107. Blier, P, and Szabo, ST. Potential mechanisms of action of atypical antipsychotic medications in treatment-resistant depression and anxiety. J Clin Psychiatry. (2005) 66:30–40.
108. Gershon, MD, and Tack, J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. (2007) 132:397–414. doi: 10.1053/j.gastro.2006.11.002
109. Engevik, MA, Luck, B, Visuthranukul, C, Ihekweazu, FD, Engevik, AC, Shi, Z, et al. Human-derived Bifidobacterium dentium modulates the mammalian serotonergic system and gut-brain axis. Cell Mol Gastroenterol Hepatol. (2021) 11:221–48. doi: 10.1016/j.jcmgh.2020.08.002
110. Kong, Q, Wang, B, Tian, P, Li, X, Zhao, J, Zhang, H, et al. Daily intake of lactobacillus alleviates autistic-like behaviors by ameliorating the 5-hydroxytryptamine metabolic disorder in VPA-treated rats during weaning and sexual maturation. Food Funct. (2021) 12:2591–604. doi: 10.1039/d0fo02375b
111. de las Casas-Engel, M, Domínguez-Soto, A, Sierra-Filardi, E, Bragado, R, Nieto, C, Puig-Kroger, A, et al. Serotonin skews human macrophage polarization through HTR2B and HTR7. J Immunol. (2013) 190:2301–10. doi: 10.4049/jimmunol.1201133
112. Bot, M, Chan, MK, Jansen, R, Lamers, F, Vogelzangs, N, Steiner, J, et al. Serum proteomic profiling of major depressive disorder. Transl Psychiatry. (2015) 5:e599. doi: 10.1038/tp.2015.88
113. Phillips, C. Physical activity modulates common neuroplasticity substrates in major depressive and bipolar disorder. Neural Plast. (2017) 2017:7014146. doi: 10.1155/2017/7014146
114. Asano, Y, Hiramoto, T, Nishino, R, Aiba, Y, Kimura, T, Yoshihara, K, et al. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol. (2012) 303:G1288–95. doi: 10.1152/ajpgi.00341.2012
115. Maini Rekdal, V, Bess, EN, Bisanz, JE, Turnbaugh, PJ, and Balskus, EP. Discovery and inhibition of an interspecies gut bacterial pathway for levodopa metabolism. Science. (2019) 364:eaau6323. doi: 10.1126/science.aau6323
116. Chen, Z, Duan, S, Li, J, Su, J, and Lei, H. T-2 toxin triggers depression-like behaviors via upregulation of dopamine transporter in nucleus accumbens of male mice. Ecotoxicol Environ Saf. (2025) 289:117392. doi: 10.1016/j.ecoenv.2024.117392
117. Seaman, KL, Smith, CT, Juarez, EJ, Dang, LC, Castrellon, JJ, Burgess, LL, et al. Differential regional decline in dopamine receptor availability across adulthood: linear and nonlinear effects of age. Hum Brain Mapp. (2019) 40:3125–38. doi: 10.1002/hbm.24585
118. Wise, RA. Dopamine, learning and motivation. Nat Rev Neurosci. (2004) 5:483–94. doi: 10.1038/nrn1406
119. Alexopoulos, GS. Mechanisms and treatment of late-life depression. Transl Psychiatry. (2019) 9:188. doi: 10.1038/s41398-019-0514-6
120. Bown, AW, and Shelp, BJ. Does the GABA shunt regulate cytosolic GABA? Trends Plant Sci. (2020) 25:422–4. doi: 10.1016/j.tplants.2020.03.001
121. Kim, H, Kim, H, Suh, HJ, and Choi, H-S. Lactobacillus brevis-fermented gamma-aminobutyric acid ameliorates depression- and anxiety-like behaviors by activating the brain-derived neurotrophic factor-tropomyosin receptor kinase B signaling pathway in BALB/C mice. J Agric Food Chem. (2024) 72:2977–88. doi: 10.1021/acs.jafc.3c07260
122. Sokovic Bajic, S, Djokic, J, Dinic, M, Veljovic, K, Golic, N, Mihajlovic, S, et al. GABA-producing natural dairy isolate from artisanal Zlatar cheese attenuates gut inflammation and strengthens gut epithelial barrier in vitro. Front Microbiol. (2019) 10:527. doi: 10.3389/fmicb.2019.00527
123. Nakayama, Y, Hashimoto, K-I, Sawada, Y, Sokabe, M, Kawasaki, H, and Martinac, B. Corynebacterium glutamicum mechanosensitive channels: towards unpuzzling “glutamate efflux” for amino acid production. Biophys Rev. (2018) 10:1359–69. doi: 10.1007/s12551-018-0452-1
124. Pokusaeva, K, Johnson, C, Luk, B, Uribe, G, Fu, Y, Oezguen, N, et al. GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol Motil. (2017) 29:e12904. doi: 10.1111/nmo.12904
125. Chuang, C-Y, Shi, Y-C, You, H-P, Lo, Y-H, and Pan, T-M. Antidepressant effect of GABA-rich monascus-fermented product on forced swimming rat model. J Agric Food Chem. (2011) 59:3027–34. doi: 10.1021/jf104239m
126. Frost, G, Sleeth, ML, Sahuri-Arisoylu, M, Lizarbe, B, Cerdan, S, Brody, L, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. (2014) 5:3611. doi: 10.1038/ncomms4611
127. Kuwahara, A. Contributions of colonic short-chain fatty acid receptors in energy homeostasis. Front Endocrinol. (2014) 5:144. doi: 10.3389/fendo.2014.00144
128. Kamarehei, M, and Zahednasab, H. Neuroprotective effect of ac-SDKP peptide in SH-SY5Y cells and rat model of Parkinson’s disease against 6-OHDA-induced oxidative stress and ER stress. Neuropeptides. (2025) 112:102534. doi: 10.1016/j.npep.2025.102534
129. Mao, Q, Wang, J, Yang, Z, Ding, R, Lv, S, and Ji, X. The pathologic roles and therapeutic implications of ghrelin/GHSR system in mental disorders. Depress Anxiety. (2024) 2024:5537319. doi: 10.1155/2024/5537319
130. Lutter, M, Sakata, I, Osborne-Lawrence, S, Rovinsky, SA, Anderson, JG, Jung, S, et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. (2008) 11:752–3. doi: 10.1038/nn.2139
131. Sempach, L, JPK, D, Limbach, V, Marzetta, F, Schaub, AC, Schneider, E, et al. Examining immune-inflammatory mechanisms of probiotic supplementation in depression: secondary findings from a randomized clinical trial. Transl Psychiatry. (2024) 14:305. doi: 10.1038/s41398-024-03030-7
132. Blaga, TS, Dumitrascu, DL, Pop, AV, and Grad, S. Chapter 2—the interactions between gut and brain in gastrointestinal disorders In: C Stasi, editor. The complex interplay between gut-brain, gut-liver, and liver-brain axes. London: Academic Press (2021). 17–47.
133. Ma, L, Wang, H-B, and Hashimoto, K. The vagus nerve: an old but new player in brain-body communication. Brain Behav Immun. (2025) 124:28–39. doi: 10.1016/j.bbi.2024.11.023
134. Jameson, KG, Kazmi, SA, Son, C, Mazdeyasnan, D, Leshan, E, Vuong, HE, et al. (2023). Vagal interoception of microbial metabolites from the small intestinal lumen. bioRxiv. Available online at: https://doi.org/10.1101/2023.12.18.572257. [Epub ahead of preprint]
135. Wang, Y, Li, S-Y, Wang, D, Wu, M-Z, He, J-K, Zhang, J-L, et al. Transcutaneous auricular vagus nerve stimulation: from concept to application. Neurosci Bull. (2021) 37:853–62. doi: 10.1007/s12264-020-00619-y
136. Johnson, RL, and Wilson, CG. A review of vagus nerve stimulation as a therapeutic intervention. J Inflamm Res. (2018) 11:203–13. doi: 10.2147/JIR.S163248
137. Finisguerra, A, Crescentini, C, and Urgesi, C. Transcutaneous vagus nerve stimulation affects implicit spiritual self-representations. Neuroscience. (2019) 412:144–59. doi: 10.1016/j.neuroscience.2019.05.059
138. Elger, G, Hoppe, C, Falkai, P, Rush, AJ, and Elger, CE. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res. (2000) 42:203–10. doi: 10.1016/s0920-1211(00)00181-9
139. Fischer, R, Ventura-Bort, C, Hamm, A, and Weymar, M. Transcutaneous vagus nerve stimulation (tVNS) enhances conflict-triggered adjustment of cognitive control. Cogn Affect Behav Neurosci. (2018) 18:680–93. doi: 10.3758/s13415-018-0596-2
140. Fraga, A, Mesquita, B, Esteves-Sousa, D, Facucho-Oliveira, J, Albuquerque, M, Espada-Santos, P, et al. The role of vagus nerve stimulation in depression: what we know? Eur Psychiatry. (2022) 65:S566. doi: 10.1192/j.eurpsy.2022.1449
141. Bottari, SA, Rodriguez, A, and Williamson, JB. Influence of vagus nerve stimulation on mood and associated disorders In: MG Frasch and EC Porges, editors. Vagus nerve stimulation. New York, NY: Springer (2024). 131–55.
142. Liang, C, Wei, S, Ji, Y, Lin, J, Jiao, W, Li, Z, et al. The role of enteric nervous system and GDNF in depression: conversation between the brain and the gut. Neurosci Biobehav Rev. (2024) 167:105931. doi: 10.1016/j.neubiorev.2024.105931
143. Collins, J, Borojevic, R, Verdu, EF, Huizinga, JD, and Ratcliffe, EM. Intestinal microbiota influence the early postnatal development of the enteric nervous system. Neurogastroenterol Motil. (2014) 26:98–107. doi: 10.1111/nmo.12236
144. Vicentini, FA, Keenan, CM, Wallace, LE, Woods, C, Cavin, J-B, Flockton, AR, et al. Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome. (2021) 9:210. doi: 10.1186/s40168-021-01165-z
145. Allen, J, Romay-Tallon, R, Mitchell, MA, Brymer, KJ, Johnston, J, Sánchez-Lafuente, CL, et al. Reelin has antidepressant-like effects after repeated or singular peripheral injections. Neuropharmacology. (2022) 211:109043. doi: 10.1016/j.neuropharm.2022.109043
146. Carvajal, AE, Serrano-Morales, JM, Vázquez-Carretero, MD, García-Miranda, P, Calonge, ML, Peral, MJ, et al. Reelin protects from colon pathology by maintaining the intestinal barrier integrity and repressing tumorigenic genes. Biochim Biophys Acta Mol basis Dis. (2017) 1863:2126–34. doi: 10.1016/j.bbadis.2017.05.026
147. Schneider, KM, Blank, N, Alvarez, Y, Thum, K, Lundgren, P, Litichevskiy, L, et al. The enteric nervous system relays psychological stress to intestinal inflammation. Cell. (2023) 186:2823–2838.e20. doi: 10.1016/j.cell.2023.05.001
148. Vanderwinden, J-M, Timmermans, J-P, and Schiffmann, SN. Glial cells, but not interstitial cells, express P2X7, an ionotropic purinergic receptor, in rat gastrointestinal musculature. Cell Tissue Res. (2003) 312:149–54. doi: 10.1007/s00441-003-0716-2
149. Bohórquez, DV, Shahid, RA, Erdmann, A, Kreger, AM, Wang, Y, Calakos, N, et al. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest. (2015) 125:782–6. doi: 10.1172/JCI78361
150. Yu, Y, Yang, W, Li, Y, and Cong, Y. Enteroendocrine cells: sensing gut microbiota and regulating inflammatory bowel diseases. Inflamm Bowel Dis. (2020) 26:11–20. doi: 10.1093/ibd/izz217
151. Liu, L, Wang, H, Yu, Y, Zeng, B, Rao, X, Chen, J, et al. Microbial regulation of a lincRNA-miRNA-mRNA network in the mouse hippocampus. Epigenomics. (2020) 12:1377–87. doi: 10.2217/epi-2019-0307
152. Wang, H, Liu, L, Rao, X, Zeng, B, Yu, Y, Zhou, C, et al. Integrated phosphoproteomic and metabolomic profiling reveals perturbed pathways in the hippocampus of gut microbiota dysbiosis mice. Transl Psychiatry. (2020) 10:346. doi: 10.1038/s41398-020-01024-9
153. Liu, L, Wang, H, Rao, X, Yu, Y, Li, W, Zheng, P, et al. Comprehensive analysis of the lysine acetylome and succinylome in the hippocampus of gut microbiota-dysbiosis mice. J Adv Res. (2021) 30:27–38. doi: 10.1016/j.jare.2020.12.002
154. Chevalier, G, Siopi, E, Guenin-Macé, L, Pascal, M, Laval, T, Rifflet, A, et al. Effect of gut microbiota on depressive-like behaviors in mice is mediated by the endocannabinoid system. Nat Commun. (2020) 11:6363. doi: 10.1038/s41467-020-19931-2
155. Sittipo, P, Choi, J, Lee, S, and Lee, YK. The function of gut microbiota in immune-related neurological disorders: a review. J Neuroinflammation. (2022) 19:154. doi: 10.1186/s12974-022-02510-1
156. Hashikawa, N, Utaka, Y, Ogawa, T, Tanoue, R, Morita, Y, Yamamoto, S, et al. HSP105 prevents depression-like behavior by increasing hippocampal brain-derived neurotrophic factor levels in mice. Sci Adv. (2017) 3:e1603014. doi: 10.1126/sciadv.1603014
157. Sanmarco, LM, Wheeler, MA, Gutiérrez-Vázquez, C, Polonio, CM, Linnerbauer, M, Pinho-Ribeiro, FA, et al. Gut-licensed IFNγ+ NK cells drive LAMP1+ TRAIL+ anti-inflammatory astrocytes. Nature. (2021) 590:473–9. doi: 10.1038/s41586-020-03116-4
158. Fitzpatrick, Z, Frazer, G, Ferro, A, Clare, S, Bouladoux, N, Ferdinand, J, et al. Gut-educated IgA plasma cells defend the meningeal venous sinuses. Nature. (2020) 587:472–6. doi: 10.1038/s41586-020-2886-4
159. Luo, Y, Zeng, B, Zeng, L, Du, X, Li, B, Huo, R, et al. Gut microbiota regulates mouse behaviors through glucocorticoid receptor pathway genes in the hippocampus. Transl Psychiatry. (2018) 8:187. doi: 10.1038/s41398-018-0240-5
160. Birmann, PT, Casaril, AM, Pesarico, AP, Caballero, PS, Smaniotto, TÂ, Rodrigues, RR, et al. Komagataella pastoris KM71H modulates neuroimmune and oxidative stress parameters in animal models of depression: a proposal for a new probiotic with antidepressant-like effect. Pharmacol Res. (2021) 171:105740. doi: 10.1016/j.phrs.2021.105740
161. Kasarello, K, Cudnoch-Jedrzejewska, A, and Czarzasta, K. Communication of gut microbiota and brain via immune and neuroendocrine signaling. Front Microbiol. (2023) 14:1118529. doi: 10.3389/fmicb.2023.1118529
162. Demaude, J, Salvador-Cartier, C, Fioramonti, J, Ferrier, L, and Bueno, L. Phenotypic changes in colonocytes following acute stress or activation of mast cells in mice: implications for delayed epithelial barrier dysfunction. Gut. (2006) 55:655–61. doi: 10.1136/gut.2005.078675
163. Fromme, SE, Joergens, S, Schwarte, K, Hohoff, C, Dietrich, DE, and Baune, BT. The association between cytokines and cognitive function in patients with major depressive disorder and controls. J Affect Disord. (2025) 373:374–82. doi: 10.1016/j.jad.2024.12.097
164. Ghosh, SS, Wang, J, Yannie, PJ, and Ghosh, S. Intestinal barrier dysfunction, LPS translocation, and disease development. J Endocr Soc. (2020) 4:bvz039. doi: 10.1210/jendso/bvz039
165. Brown, GC. The endotoxin hypothesis of neurodegeneration. J Neuroinflammation. (2019) 16:180. doi: 10.1186/s12974-019-1564-7
166. Birmann, PT, Sinott, A, Zugno, GP, Rodrigues, RR, Conceição, FR, Sousa, FSS, et al. The antidepressant effect of Komagataella pastoris KM 71 H in maternal separation mice model mediated by the microbiota-gut-brain axis. Behav Brain Res. (2025) 476:115287. doi: 10.1016/j.bbr.2024.115287
167. Sanjeewa, KKA, Nagahawatta, DP, Yang, H-W, Oh, JY, Jayawardena, TU, Jeon, Y-J, et al. Octominin inhibits LPS-induced chemokine and pro-inflammatory cytokine secretion from RAW 264.7 macrophages via blocking TLRs/NF-κB signal transduction. Biomol Ther. (2020) 10:511. doi: 10.3390/biom10040511
168. Millischer, V, Heinzl, M, Faka, A, Resl, M, Trepci, A, Klammer, C, et al. Intravenous administration of LPS activates the kynurenine pathway in healthy male human subjects: a prospective placebo-controlled cross-over trial. J Neuroinflammation. (2021) 18:158. doi: 10.1186/s12974-021-02196-x
169. Singh, D. Astrocytic and microglial cells as the modulators of neuroinflammation in Alzheimer’s disease. J Neuroinflammation. (2022) 19:206. doi: 10.1186/s12974-022-02565-0
170. Kwon, HS, and Koh, S-H. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener. (2020) 9:42. doi: 10.1186/s40035-020-00221-2
171. Wang, H, He, Y, Sun, Z, Ren, S, Liu, M, Wang, G, et al. Microglia in depression: an overview of microglia in the pathogenesis and treatment of depression. J Neuroinflammation. (2022) 19:132. doi: 10.1186/s12974-022-02492-0
172. Patani, R, Hardingham, GE, and Liddelow, SA. Functional roles of reactive astrocytes in neuroinflammation and neurodegeneration. Nat Rev Neurol. (2023) 19:395–409. doi: 10.1038/s41582-023-00822-1
173. Wallen, ZD, Demirkan, A, Twa, G, Cohen, G, Dean, MN, Standaert, DG, et al. Metagenomics of Parkinson’s disease implicates the gut microbiome in multiple disease mechanisms. Nat Commun. (2022) 13:6958. doi: 10.1038/s41467-022-34667-x
174. Ye, T, Yuan, S, Kong, Y, Yang, H, Wei, H, Zhang, Y, et al. Effect of probiotic fungi against cognitive impairment in mice via regulation of the fungal microbiota-gut-brain axis. J Agric Food Chem. (2022) 70:9026–38. doi: 10.1021/acs.jafc.2c03142
175. Mayer, EA. The neurobiology of stress and gastrointestinal disease. Gut. (2000) 47:861–9. doi: 10.1136/gut.47.6.861
176. de Wied, D, Diamant, M, and Fodor, M. Central nervous system effects of the neurohypophyseal hormones and related peptides. Front Neuroendocrinol. (1993) 14:251–302. doi: 10.1006/frne.1993.1009
177. Sudo, N, Chida, Y, Aiba, Y, Sonoda, J, Oyama, N, Yu, X-N, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. (2004) 558:263–75. doi: 10.1113/jphysiol.2004.063388
178. Tofani, GSS, Leigh, S-J, Gheorghe, CE, Bastiaanssen, TFS, Wilmes, L, Sen, P, et al. Gut microbiota regulates stress responsivity via the circadian system. Cell Metab. (2025) 37:138–153.e5. doi: 10.1016/j.cmet.2024.10.003
179. Swaab, DF, Bao, A-M, and Lucassen, PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. (2005) 4:141–94. doi: 10.1016/j.arr.2005.03.003
180. Wang, Y, Chen, Y, Chen, H, Samsom, JN, Shu, M, Gao, X, et al. Exposure to “forever chemical” perfluorooctanoic acid induces depression-like behaviors in mice by activating the HPA axis. Environ Int. (2025) 202:109631. doi: 10.1016/j.envint.2025.109631
181. Georgiou, P, Farmer, CA, Medeiros, GC, Yuan, P, Johnston, J, Kadriu, B, et al. Associations between hypothalamic-pituitary-adrenal (HPA) axis hormone levels, major depression features and antidepressant effects of ketamine. J Affect Disord. (2025) 373:126–32. doi: 10.1016/j.jad.2024.12.036
182. Zajkowska, Z, Gullett, N, Walsh, A, Zonca, V, Pedersen, GA, Souza, L, et al. Cortisol and development of depression in adolescence and young adulthood—a systematic review and meta-analysis. Psychoneuroendocrinology. (2022) 136:105625. doi: 10.1016/j.psyneuen.2021.105625
183. Gordon, JL, Girdler, SS, Meltzer-Brody, SE, Stika, CS, Thurston, RC, Clark, CT, et al. Ovarian hormone fluctuation, neurosteroids, and HPA axis dysregulation in perimenopausal depression: a novel heuristic model. Am J Psychiatry. (2015) 172:227–36. doi: 10.1176/appi.ajp.2014.14070918
184. Keller, J, Gomez, R, Williams, G, Lembke, A, Lazzeroni, L, Murphy, GM, et al. HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatry. (2017) 22:527–36. doi: 10.1038/mp.2016.120
185. Shen, S-Y, Liang, L-F, Shi, T-L, Shen, Z-Q, Yin, S-Y, Zhang, J-R, et al. Microglia-derived interleukin-6 triggers astrocyte apoptosis in the hippocampus and mediates depression-like behavior. Adv Sci. (2025):e2412556. doi: 10.1002/advs.202412556
186. Mo, X, Cheng, R, Shen, L, Liu, N, Sun, Y, Lin, S, et al. Yeast β-glucan alleviates high-fat diet-induced Alzheimer’s disease-like pathologies in rats via the gut-brain axis. Int J Biol Macromol. (2024) 278:134939. doi: 10.1016/j.ijbiomac.2024.134939
187. Blackmer-Raynolds, LD, and Sampson, TR. The gut-brain axis goes viral. Cell Host Microbe. (2022) 30:283–5. doi: 10.1016/j.chom.2022.02.013
188. Coughlin, SS. Anxiety and depression: linkages with viral diseases. Public Health Rev. (2012) 34:7. doi: 10.1007/BF03391675
189. Liao, Y-T, Hsieh, M-H, Yang, Y-H, Wang, Y-C, Tsai, C-S, Chen, VC-H, et al. Association between depression and enterovirus infection: a nationwide population-based cohort study. Medicine. (2017) 96:e5983. doi: 10.1097/MD.0000000000005983
190. Wilson, PMJ, Kusumakar, V, McCartney, RA, and Bell, EJ. Features of coxsackie B virus (CBV) infection in children with prolonged physical and psychological morbidity. J Psychosom Res. (1989) 33:29–36. doi: 10.1016/0022-3999(89)90103-7
191. Bendinelli, M, Ruschi, A, Campa, A, and Toniolo, A. Depression of humoral and cell-mediated immune responses by coxsackieviruses in mice. Experientia. (1975) 31:1227–9. doi: 10.1007/BF02326807
192. Wright, K, Warren, F, Bucci, S, Dunn, BD, Jones, S, O’Mahen, H, et al. A study protocol for a randomized controlled feasibility trial of behavioural therapy for interepisode bipolar symptoms (STABILISE). Pilot Feasibility Stud. (2025) 11:97. doi: 10.1186/s40814-025-01678-6
193. Halverson, T, and Alagiakrishnan, K. Gut microbes in neurocognitive and mental health disorders. Ann Med. (2020) 52:423–43. doi: 10.1080/07853890.2020.1808239
194. Adedara, IA, Mohammed, KA, Canzian, J, Ajayi, BO, Farombi, EO, Emanuelli, T, et al. Utility of zebrafish-based models in understanding molecular mechanisms of neurotoxicity mediated by the gut-brain axis. Adv Neurotoxicol. (2024) 11:177–208. doi: 10.1016/bs.ant.2024.02.003
195. Evans, SJ, Bassis, CM, Hein, R, Assari, S, Flowers, SA, Kelly, MB, et al. The gut microbiome composition associates with bipolar disorder and illness severity. J Psychiatr Res. (2017) 87:23–9. doi: 10.1016/j.jpsychires.2016.12.007
196. Ye, Z, Gao, Y, Yuan, J, Chen, F, Xu, P, and Liu, W. The role of gut microbiota in modulating brain structure and psychiatric disorders: a Mendelian randomization study. NeuroImage. (2025) 315:121292. doi: 10.1016/j.neuroimage.2025.121292
197. Duncan, SH, Barcenilla, A, Stewart, CS, Pryde, SE, and Flint, HJ. Acetate utilization and butyryl coenzyme a (CoA):acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl Environ Microbiol. (2002) 68:5186–90. doi: 10.1128/aem.68.10.5186-5190.2002
198. Rudzki, L, Stone, TW, Maes, M, Misiak, B, Samochowiec, J, and Szulc, A. Gut microbiota-derived vitamins—underrated powers of a multipotent ally in psychiatric health and disease. Prog Neuropsychopharmacol Biol Psychiatry. (2021) 107:110240. doi: 10.1016/j.pnpbp.2020.110240
199. Lai, W, Zhao, J, Xu, S, Deng, W, Xu, D, Wang, M, et al. Shotgun metagenomics reveals both taxonomic and tryptophan pathway differences of gut microbiota in bipolar disorder with current major depressive episode patients. J Affect Disord. (2021) 278:311–9. doi: 10.1016/j.jad.2020.09.010
200. Atique-Ur-Rehman, H, and Neill, JC. Cognitive dysfunction in major depression: from assessment to novel therapies. Pharmacol Ther. (2019) 202:53–71. doi: 10.1016/j.pharmthera.2019.05.013
201. Akkasheh, G, Kashani-Poor, Z, Tajabadi-Ebrahimi, M, Jafari, P, Akbari, H, Taghizadeh, M, et al. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition. (2016) 32:315–20. doi: 10.1016/j.nut.2015.09.003
202. Dawkins, JJ, Allegretti, JR, Gibson, TE, McClure, E, Delaney, M, Bry, L, et al. Gut metabolites predict Clostridioides difficile recurrence. Microbiome. (2022) 10:87. doi: 10.1186/s40168-022-01284-1
203. Popescu, C, Munteanu, C, Anghelescu, A, Ciobanu, V, Spînu, A, Andone, I, et al. Novelties on neuroinflammation in Alzheimer’s disease-focus on gut and oral microbiota involvement. Int J Mol Sci. (2024) 25:11272. doi: 10.3390/ijms252011272
204. Guo, F, Jing, L, Xu, Y, Zhang, K, Li, Y, Sun, N, et al. Gut microbiota and inflammatory factor characteristics in major depressive disorder patients with anorexia. BMC Psychiatry. (2024) 24:334. doi: 10.1186/s12888-024-05778-0
205. Jayaraj, RL, Beiram, R, Azimullah, S, Mf, NM, Ojha, SK, Adem, A, et al. Valeric acid protects dopaminergic neurons by suppressing oxidative stress, neuroinflammation and modulating autophagy pathways. Int J Mol Sci. (2020) 21:7670. doi: 10.3390/ijms21207670
Keywords: gut microbiota, MGB axis, depression, metabolism, neurotransmitters, neuroinflammation
Citation: Zhou X, Wang S, Wang X, Chen X, Zhou P, Ma K and Zhang P (2025) Mechanisms of the effect of gut microbes on depression through the microbiota-gut-brain axis. Front. Nutr. 12:1634548. doi: 10.3389/fnut.2025.1634548
Edited by:
Gerard M. Moloney, University College Cork, IrelandReviewed by:
Qinxue Hu, The Affiliated Hospital of Southwest Medical University, ChinaPedro Borges De Souza, Federal University of Santa Catarina, Brazil
Copyright © 2025 Zhou, Wang, Wang, Chen, Zhou, Ma and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pei Zhou, emhvdTIwMjJAaHVzdC5lZHUuY24=; Kai Ma, bTIwMjQ3NjM4MkBodXN0LmVkdS5jbg==; Peng Zhang, emhhbmdwZW5nX3h0QGh1c3QuZWR1LmNu
†These authors have contributed equally to this work
 Xiangyan Zhou
Xiangyan Zhou Sixing Wang
Sixing Wang Xiaohui Wang4
Xiaohui Wang4