- 1Institute of Edible Fungi, Shanghai Academy of Agricultural Sciences, Shanghai, China
- 2College of Food Science and Technology, Shanghai Ocean University, Shanghai, China
Hericium erinaceus has a high food and ornamental value and considerable benefits upon consumption and in research. The high polysaccharide content in H. erinaceus gives this fungus unique physiological functions and a smooth, palatable texture; notably, H. erinaceus is a superior source of polysaccharides compared with fruits and vegetables. This review focuses on polysaccharides derived from edible mushrooms, an area that has received limited attention in the literature, which has predominantly focused on plant-derived polysaccharides. The relevant literature on the extraction methods, purification processes, structural characterization, and biological functions of H. erinaceus polysaccharides (HEPs) has been systematically collated and summarized. H. erinaceus polysaccharides have immunomodulatory, lipid-lowering, antioxidant, antitumor, anti-inflammatory, hypoglycemic, and intestinal regulation effects. The aim of this study is to review the research progress pertaining to H. erinaceus polysaccharides, providing valuable insights and inspiration for future studies in related fields.
Highlights
• Methods of extraction and purification of Hericium erinaceus polysaccharides (HEPs).
• Different sources of HEPs exhibit distinct structural conformations and characteristics.
• Various mechanisms underlying biological activity and intestinal regulation of HEPs.
• Research challenges and future perspectives of HEPs.
1 Introduction
Hericium erinaceus, classified within the kingdom Fungi, phylum Ascomycetes, and family Boletaceae, is a notable edible and medicinal mushroom commonly referred to as the monkey head mushroom. Its name is derived from its appearance, which resembles that of a monkey's head. H. erinaceus is widely distributed worldwide, with significant prevalence in regions such as China, the United States, and New Zealand. Figure 1 shows the geographical distribution of H. erinaceus along with its various morphological forms. The distinct white color and unique shape of H. erinaceus resemble a cluster of white hairs that gather closely together, further reinforcing its nickname (1). Rich in essential nutrients—including proteins, polysaccharides, fats, vitamins, and minerals—H. erinaceus is considered a highly nutritious ingredient suitable for culinary applications. In addition to its use as a food ingredient, traditional pharmacology has attributed certain medicinal properties to H. erinaceus, as it has been utilized to promote human health (2). In recent years, advancements in extraction techniques, combined with an increasing interest in elucidating the mechanisms underlying the pharmacological actions of H. erinaceus have led to the isolation of various new compounds from both its fruiting bodies and mycelia; consequently, extensive research has been conducted to reveal the pharmacological activities associated with individual constituents. Given that H. erinaceus has long been employed across Southeast Asia for various purposes—particularly owing to the rising public awareness of health maintenance—H. erinaceus has attracted high interest worldwide, including in Europe and the United States (3, 4).

Figure 1. Geographical distribution of Hericium erinaceus. (a) World map illustrating the distribution of H. erinaceus. Images from various countries showcasing the different morphological forms of this species: (b) China, (c) France, (d) the United States of America, (e) the Netherlands, (f) the United Kingdom, and (g) Spain (Source https://www.gbif.org/species/2537743).
H. erinaceus polysaccharide is known for its diverse biological activities and nutritional benefits. This complex macromolecule is primarily composed of monosaccharides such as glucose, xylose, mannose, and galactose and features a sophisticated polysaccharide chain structure. Research indicates that H. erinaceus polysaccharides exhibit various biological functions, including lipid-lowering, antioxidant, antitumor, immunomodulatory, and anti-inflammatory effects, and these properties contribute positively to human health (5–7). Furthermore, the composition of H. erinaceus polysaccharides has been investigated for their influence on the intestinal flora and their potential applications in the regulation of intestinal health. Owing to their bioactivity and nutritional importance, H. erinaceus polysaccharides are widely applied in food products, nutraceuticals, and pharmaceuticals. Researchers have comprehensively studied these polysaccharides, including their extraction methods, purification processes, structural characteristics, biological activities, and potential application value. The exploration of H. erinaceus polysaccharides not only enhances our understanding of the nutritional profiles associated with this fungus but also provides a theoretical foundation for its applications within the domains of food science and medicine (8, 9). Thus, as crucial bioactive components derived from mushrooms, H. erinaceus polysaccharides are abundant in nutrients and exhibit a wide range of biological activities, presenting substantial prospects for utilization in food products, health supplements, and pharmaceuticals. This article discusses recent advancements in H. erinaceus polysaccharides worldwide from four perspectives—extraction methods, purification processes, structural characteristics, and biological functions—aiming to provide valuable references for ongoing research into H. erinaceus polysaccharides and the development of related products.
2 Polysaccharide extraction and purification
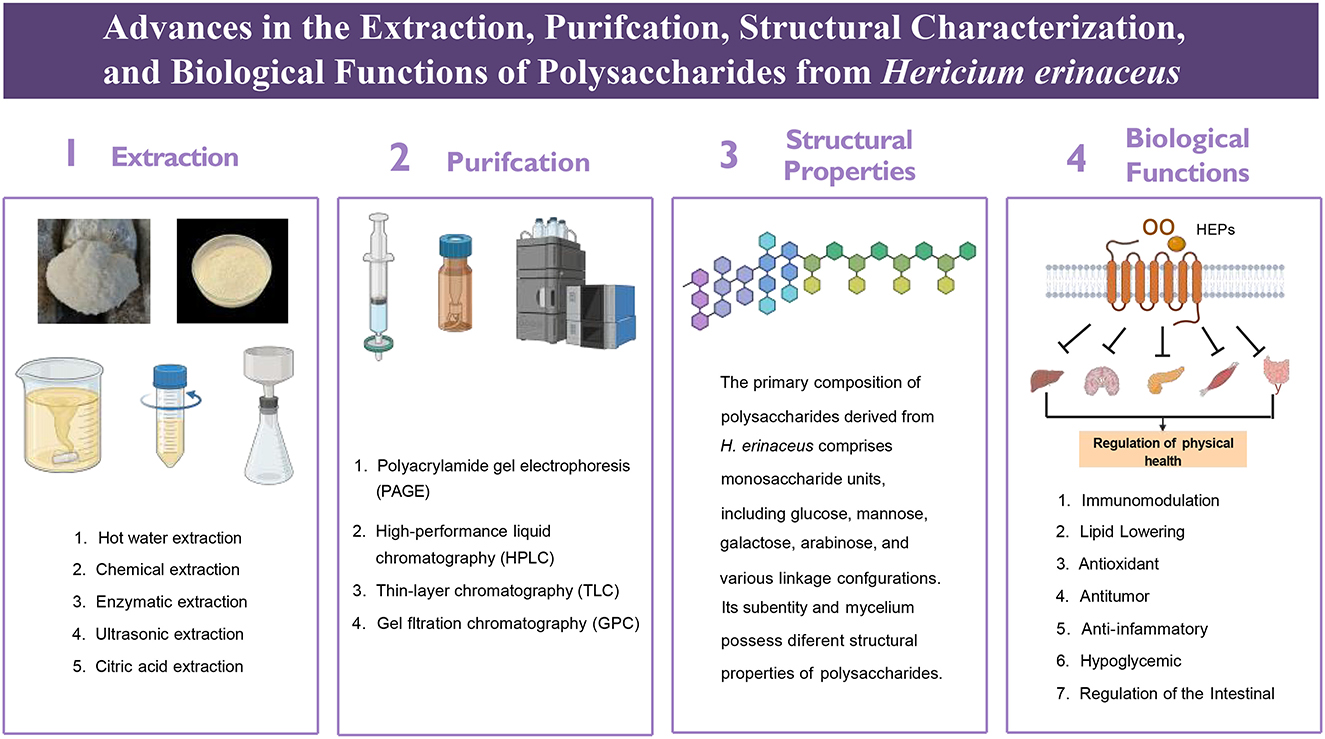
2.1 Extraction methods
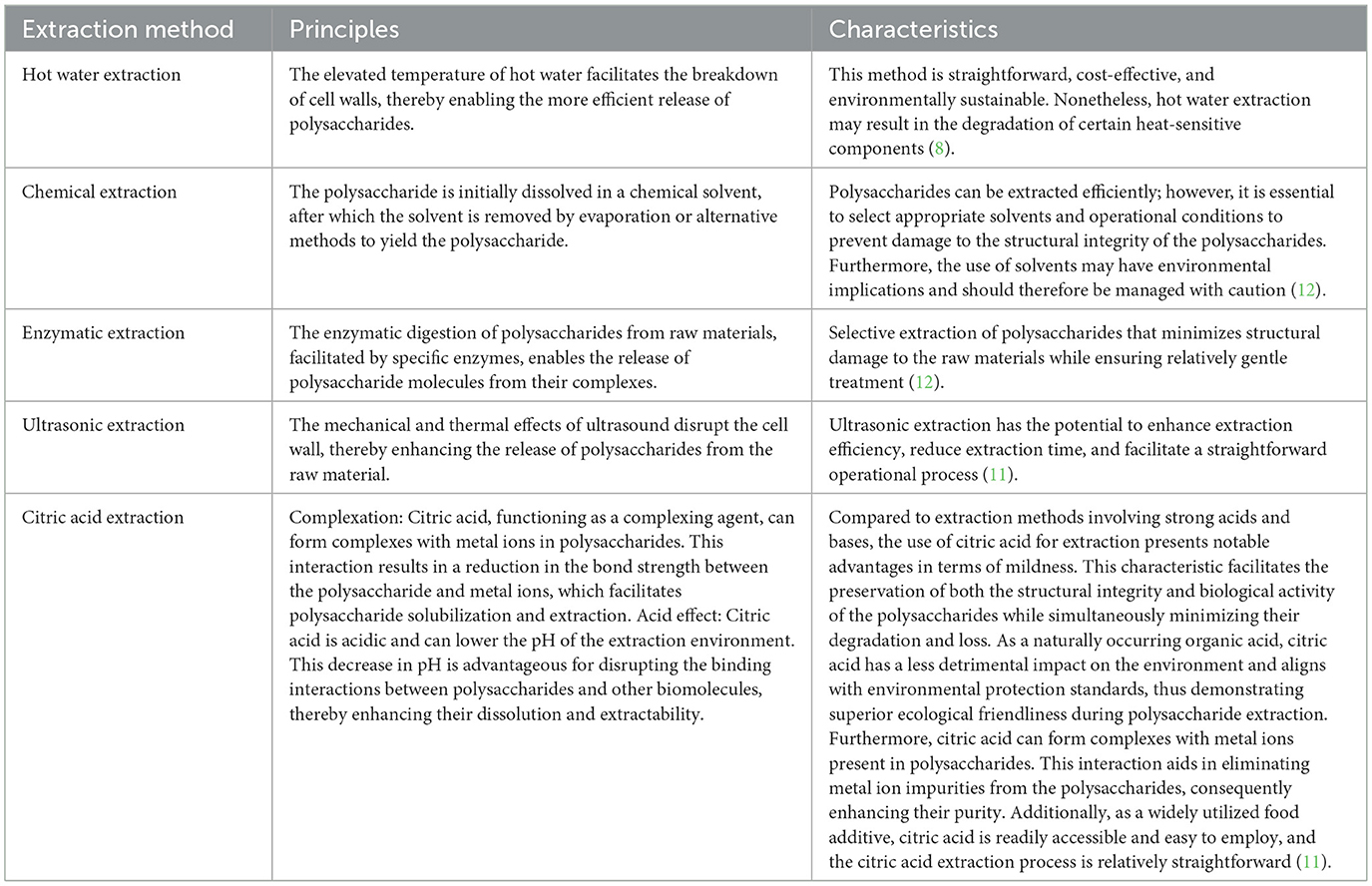
The extraction of polysaccharides from H. erinaceus serves as the foundation for investigations into their structure, properties, and pharmacological effects. Currently, various methods, such as hot water extraction, chemical extraction, enzymatic extraction, ultrasonic extraction, and citric acid extraction, have been employed to isolate polysaccharides from H. erinaceus, each with distinct characteristics (10). Traditional approaches typically utilize water or organic solvents for extraction; however, the intricate composition of the fungal cell wall complicates the extraction of H. erinaceus polysaccharides, potentially altering the biological activity of the extracted polysaccharides. Moreover, conventional techniques are often time-consuming and yield low rates of polysaccharide recovery while frequently necessitating multiple rounds of extraction. The residual liquid produced postextraction can also pose concerns regarding environmental pollution. To address the challenges associated with traditional polysaccharide isolation methods, researchers have turned to advanced techniques, including ultrasonic-assisted extraction, enzyme digestion protocols, and citric acid methods, which markedly increase both the polysaccharide recovery and the extraction yield while minimizing product degradation (11, 12).
The principles and characteristics of various polysaccharide extraction methods for H. erinaceus are presented in Table 1. Yang et al. (13) investigated the enzymatic extraction of polysaccharides from H. erinaceus substrates via response surface methodology and Box–Behnken design on the basis of one-way and orthogonal experiments to optimize the extraction conditions. The optimal parameters were as follows: pH 5.71, temperature 52.03 °C, and duration 33.79 min. Under these conditions, the polysaccharide yield peaked at 13.46 ± 0.37%, which represents a 67.72% increase compared to the yields obtained after hot water extraction. The extracted polysaccharides were characterized via Fourier transform infrared (FT-IR) spectroscopy, scanning electron microscopy (SEM), circular dichroism (CD), atomic force microscopy (AFM), and gas chromatography (GC) analyses, and the results indicated that the functional groups present in the polysaccharides derived from both the hot water extraction and the enzymatic methods were largely similar; however, notable conformational changes were observed. Furthermore, Wu et al. (14) examined the structural properties and in vitro immunological activities of cell wall polysaccharides from H. erinaceus, which were extracted continuously using hot water and sodium hydroxide solution. They revealed that the water-soluble cell wall polysaccharides were primarily composed of glucose and galactose, with molar ratios ranging from 3.4:1 to 14:1, but small amounts of glucuronic acid were also detectable within these extracts. The alkali-soluble cell wall polysaccharides consisted predominantly of glucans, which presented lower molecular weights than their water-soluble counterparts but demonstrated a higher macrophage activation activity in vitro. Zhang et al. (15) extracted polysaccharides from H. erinaceus by several methods and reported that the polysaccharides isolated via ultrasonic extraction exhibited superior performance, demonstrating the highest 1,1-diphenyl-2-dinitrophenylhydrazine DPPH) free radical scavenging activity and providing enhanced protection to L929 cells. Similarly, Yan et al. (16) employed various techniques to extract polysaccharides from H. erinaceus and concluded that the citric acid extraction method more effectively preserved the physicochemical properties and biological activities of the polysaccharides. These findings indicate that polysaccharides extracted with citric acid could be developed as bioactive ingredients for both food and medicinal applications.
2.2 Purification methods
Purifying H. erinaceus polysaccharides is fundamental for investigating their structure and biological activity. The purification process primarily encompasses steps such as deproteinization, decolorization, and graded precipitation. According to the available data, crude polysaccharides exhibit enhanced biological activity following deproteinization and purification (17). Therefore, to increase their activity while also providing purer material for further research, it is essential to deproteinize crude polysaccharides. Moreover, darker colored crude polysaccharides must undergo decolorization; otherwise, measurement errors may increase significantly. Deproteinization can be achieved by several methods, including the Sevag method, the trichloroacetic acid (TCA), and enzymatic methods. Currently, the Sevag method is the most widely employed; however, some studies have demonstrated that the TCA method is more effective, as it results in reduced loss of the polysaccharide content. Enzymatic methods are utilized less frequently because of their high selectivity and the challenges associated with identifying suitable enzymes. Nonetheless, highly efficient deproteinization using compatible enzymes has promising application prospects (18). Decolorization may be achieved using activated carbon, hydrogen peroxide (H2O2), or diethylaminoethyl (DEAE)-cellulose methods. Oligosaccharides, as small molecules, can be effectively removed by dialysis. Furthermore, polysaccharides can be purified through various methods, including ethanol-grade precipitation, electrophoresis, ultrafiltration, and column chromatography. Commonly employed columns for chromatography include DEAE-cellulose, Sephadex, and Sepharose columns, among others. To assess purity, techniques such as polyacrylamide gel electrophoresis (PAGE), high-performance liquid chromatography (HPLC), thin-layer chromatography (TLC), and gel filtration chromatography (GPC) are predominantly applied (19). Wang et al. (20) utilized hollow fiber ultrafiltration in addition to ion exchange chromatography to purify H. erinaceus polysaccharides while eliminating impurities; this approach laid the foundation for subsequent investigations into their polysaccharide activities. Xue et al. (21) extracted a novel polysaccharide from H. erinaceus and successfully purified it via DEAE Sepharose CL-6B and Sephadex G-200 column chromatographies for research related to the chelate preparation. Additionally, Qiao et al. (22) isolated an alkali-soluble β-glucan from H. erinaceus via hollow fiber ultrafiltration combined with anion-exchange DEAE cellulose column chromatography as well as Sepharose gel column chromatography for purification. Furthermore, Wang et al. (23) fractionated HP—a water-soluble crude polysaccharide derived from H. erinaceus—through DEAE-Sepharose CL-6B column chromatography, resulting in two distinct fractions, Hericium polysaccharide A (HPA) and Hericium polysaccharide B (HPB). HPA is composed of glucose, galactose, and fucose at a molar ratio of 1:2.110:0.423, whereas polysaccharide HPB consists solely of the monosaccharides galactose and glucose at a molar ratio of 1:11.529. Upon the application of methylation coupled with gas chromatography–mass spectrometry (GC–MS) analysis and periodate oxidation–Smith degradation and partial acid hydrolysis, the repeat units of HPA and HPB were elucidated, as illustrated in Figure 2; moreover, the HPB extraction and purification protocols are depicted in Figure 3. These methodologies serve as crucial techniques for obtaining high-purity samples of HPB, thereby establishing a solid foundation for subsequent structural characterization and bioactivity assessments.
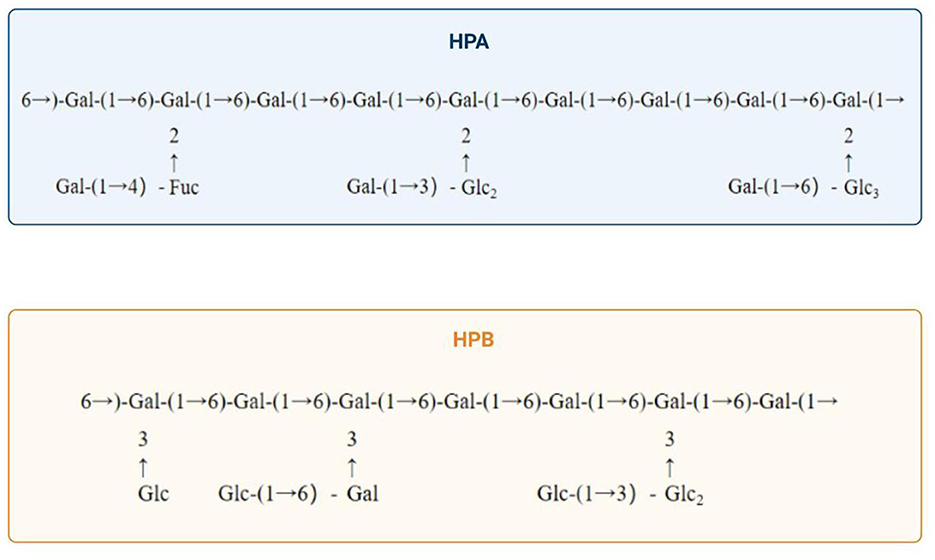
Figure 2. Composition of two Hericium erinaceus polysaccharides fractionated and purified via DEAE-Sepharose CL-6B column chromatography (23).
3 Structural properties of polysaccharides
3.1 Main structural properties
Through nuclear magnetic resonance (NMR), infrared (IR) spectroscopy, and mass spectrometry (MS) techniques, researchers have preliminarily analyzed the structural characteristics of polysaccharides derived from H. erinaceus. These analyses include the composition of the polysaccharide chains, molecular weights, and molecular structures (24). Investigating these structural properties can provide insights into the mechanisms underlying the biological activities of polysaccharides in H. erinaceus. Li et al. (25) predicted the principal structure of these polysaccharides, as illustrated in Figure 4. A variety of polysaccharides with different structures have been isolated and characterized from both artificially cultured and wild H. erinaceus substrates, mycelia, and fermentation culture broth. These polysaccharides typically exhibit large molecular weights, ranging from tens to thousands of kilodaltons. Such large molecular weights enhance the stability and biological activity of the polysaccharides in vivo; for instance, they play roles in receptor binding and regulating immune function. Additionally, higher molecular weights contribute to the resilience and prolonged effects of polysaccharides within biological systems (26). Polysaccharides derived from H. erinaceus are primarily comprised of monosaccharide units, including glucose, mannose, galactose, and arabinose, with various linkages. The specific composition and arrangement of these monosaccharides significantly influence the biological activities and physiological functions of the polysaccharides. For example, combining different monosaccharide units can impact the solubility, stability, and biomolecular interactions of the polysaccharide. Polysaccharides from H. erinaceus typically have complex structures consisting of multiple distinct chains that may be linear or branched. The intricacy of this chain architecture plays a crucial role in determining both the biological activity and bioavailability of these polysaccharides. In particular, branching within a structure may influence the solubility and stability, subsequently affecting the rates of degradation and absorption in biological systems. Moreover, the presence of branched architectures can modify how polysaccharides interact with other biomolecules—such as through receptor binding or crosslinking with other polysaccharide molecules (27).
3.2 Structural variations in polysaccharides derived from subentities and mycelium
Polysaccharides derived from the subentities and mycelia of H. erinaceus exhibit distinct structural characteristics. Polysaccharides isolated from H. erinaceus are predominantly heteropolysaccharides, comprising two or more monosaccharides, such as glucose, xylose, rhamnose, mannose, fucose, galactose, and arabinose, with molecular masses ranging from 13 to 1,000 kDa (28, 29). Liu et al. (30) reported the extraction of HEFP-2b, a specific polysaccharide from H. erinaceus sourced primarily from its subentity. This polysaccharide is mainly composed of fucose, galactose, glucose, and mannose and has a molecular mass of 32.52 kDa. It features a backbone consisting of ( → 6)-α-D-Glcp-(1 → and → 4)-β-D-Galp-(1 → bonds along with branched units (1 → ) and ( → 6)-β-D-Gap as well as (1 → ) and ( → 4)-α-D-Manp containing glucose and fucose as the residues on the non-reducing end. Moreover, scholars (31) identified a novel polysaccharide from H. erinaceus with a molecular mass of 12.714 kDa; this particular substance was predominantly composed of mannose (5.13%), glucose (43.02%), and galactose (51.85%), and its structure consists of various linkages, including (1 → )-Glc, (1 → 4)-Glc, (1 → 6)-Glc, (1 → 6)-Man, (1 → 3/6)-Man, and (1 → 6.-Gal). In contrast to the subentity-derived polysaccharides mentioned earlier, the purified fermented mycelium obtained from H. erinaceus yielded mainly heteropolysaccharides along with glycoproteins (32). The monosaccharides that constitute polysaccharides from the mycelia primarily include arabinose, xylose, mannose, galactose, and glucose. Cui et al. (33) isolated an acidic β-glycoprotein with a molecular mass of 14.4 kDa and a protein-to-polysaccharide ratio of 10:1 from H. erinaceus mycelium. This compound contains D-glucose, L-rhamnose, D-galactose, and D-mannose, and its main chain is composed of (1 → 4)-linked galactose and glucose residues. Shao et al. (34) identified a novel polysaccharide, EP-1, with a molecular mass of approximately 3.1 kDa that features an α-D-Glc-(1 → 3) backbone structure along with β-D-Glc-(1 → 3) branches at the C-4 position with α-D-Gal-(1 → 3) side chains; the terminal residue is α-D-Man.
4 Biological functions of polysaccharides
Studies have demonstrated that polysaccharides derived from H. erinaceus exhibit considerable antioxidant properties and can effectively scavenge free radicals. Furthermore, these compounds positively affect human health by reducing blood lipid and glucose levels, demonstrating antitumor effects, modulating the immune system, and regulating the intestinal microbiota (35). These bioactivities provide a crucial scientific foundation for the potential development of H. erinaceus polysaccharides as natural pharmaceuticals or health products.
4.1 Immunomodulatory effects
Immunity refers to the process by which the body identifies, combats, and eliminates foreign substances (e.g., pathogenic microorganisms, viruses, and bacteria) as well as abnormal cells (e.g., tumor cells and senescent cells) through the immune system. The primary function of immunity is to protect the body against infections, diseases, and foreign agents (36). Polysaccharides derived from H. erinaceus play crucial roles in modulating immune system function. These polysaccharides can activate various defense cells—including macrophages, T lymphocytes, B lymphocytes, cytotoxic T lymphocytes, and natural killer (NK) cells—while also facilitating the expression of biochemical compounds such as cytokines and chemokines that exhibit innate antiproliferative properties. Furthermore, these components can induce apoptosis in tumor cells and disturb the differentiation of tumor cells while promoting the secretion of reactive nitrogen species, oxygen intermediates, interleukins, and other bioactive substances. Several studies have demonstrated that H. erinaceus polysaccharides can stimulate splenic lymphocytes and macrophages while regulating and enhancing lymphocyte immune functions by modulating signaling pathways such as the nuclear factor kappa B (NF-κB), mitogen-activated protein kinase (MAPK), and phosphoinositide 3-kinase/Ak strain transforming (PI3K/Akt) pathways (35, 37). Immunofluorescence staining demonstrated that the enzymatic products of H. erinaceus polysaccharides significantly enhanced the phagocytic activity of nitric oxide (NO), cluster of differentiation 40 (CD40), and CD86 in macrophages, thereby augmenting their immunomodulatory functions in cyclophosphamide-induced immunosuppressed mice (38). Additionally, experiments on the mouse macrophage line RAW264.7 and the human intestinal epithelial cell line cancer coli-2 (Caco-2) revealed that H. erinaceus polysaccharides promoted the production of NO, interleukin-6 (IL-6), interleukin-10 (IL-10), and tumor necrosis factor-alpha (TNF-α), leading to increased immune activity (39). Furthermore, applying micro- and nanotechnology to H. erinaceus polysaccharides or their modification with specific molecules may enhance their immunomodulatory effects through targeted delivery and improved intestinal permeability. For example, encapsulating H. erinaceus polysaccharides in multiwalled carbon nanotubes (MWCNTs) effectively modulated the immune response in mice by significantly increasing immunoglobulin levels and promoting splenic lymphocyte activation (40). Similarly, selenide-encapsulated H. erinaceus polysaccharides formulated in poly(lactic acid-hydroxyacetic acid) copolymer nanoparticles not only enhanced the immunoreactivity of these polysaccharides but also significantly increased their phagocytosis by macrophages and upregulated CD40 and CD86 (41).
4.2 Lipid-lowering effects
Hyperlipidemia is characterized by abnormal or disturbed lipid metabolism, which increases the risk of cardiovascular diseases, central nervous system disorders, and other health issues. Consequently, the development of safe and effective lipid-lowering agents has a high social and economic implications and has emerged as a focal point of research worldwide. Although contemporary clinical lipid-lowering medications have substantially contributed to human health, they are also associated with adverse effects such as liver dysfunction, pancreatic impairment, and nervous system damage (42). As a result, interest in natural food-derived bioactive compounds for combating metabolic disorders is increasing.
Research has shown that polysaccharides derived from H. erinaceus can play a significant role in lowering blood lipids. These polysaccharides influence lipid metabolism through various pathways, as illustrated in Figure 5. This includes the regulation of hepatic cholesterol synthesis and the promotion of cholesterol excretion and metabolism, ultimately leading to reduced blood cholesterol levels. Such regulatory effects are crucial for the prevention and management of hypercholesterolemia. Furthermore, polysaccharides from H. erinaceus may offer benefits in terms of preventing conditions such as obesity and fatty liver by inhibiting adipocyte formation and reducing the activity of lipogenic enzymes, thus diminishing the synthesis and storage of fat within the body (42). Additionally, their antioxidant properties enable these polysaccharides to mitigate oxidative stress-induced damage to vascular endothelial cells, thereby helping to prevent the formation of atherosclerosis. Consequently, this contributes significantly to lowering blood lipid levels, particularly low-density lipoprotein cholesterol (LDL-C) levels. Moreover, H. erinaceus polysaccharides may enhance blood circulation by regulating vascular tone and promoting vasodilation, thereby assisting in reducing blood lipid levels while simultaneously decreasing the risk of atherosclerosis. Yang et al. (43) successfully isolated an extracellular polysaccharide with notable hypolipidemic effects from liquid cultures of H. erinaceus, which presented a protein content of 8.8%, a molecular weight of less than 40 kDa, and a sugar content of 91.2%. Feng et al. (44) investigated the mechanism by which β-glucan inhibits starch digestion in H. erinaceus. They analyzed how β-glucans with different molecular weights interact with starch to inhibit its digestion. The rates of starch digestion and glucose release decreased as the molecular weight of the H. erinaceus β-glucan increased. Notably, H. erinaceus β-glucan attenuated the leaching of straight-chain starch while simultaneously increasing the peak, minimum, and final viscosities. Furthermore, H. erinaceus β-glucan was shown to encapsulate starch particles via laser confocal scanning microscopy. H. erinaceus polysaccharides (HEPs) can promote bile acid secretion by regulating the intestinal microbiota. Bile acids effectively remove fats and help the liver convert excess cholesterol into bile acids for excretion, thereby reducing bad cholesterol (LDL-C) in the blood. Additionally, the β-glucan in HEP can coat starch particles in food, forming a protective layer around them, thereby slowing down their digestion into glucose and reducing fat synthesis (Figure 5). It both promotes fat excretion and reduces new fat production, and this dual action is key to its lipid-lowering effect. Therefore, it can be inferred that the regulation of glucose release and starch digestion by H. erinaceus β-glucans may be attributed to its capacity to increase system viscosity and enhance starch coating, which collectively contributes to a hypolipidemic effect.

Figure 5. Lipid-lowering effects of polysaccharides from Hericium erinaceus (HEP: H. erinaceus polysaccharide).
4.3 Antioxidant effects
Human cells derive energy through biological oxidation reactions to maintain the body's normal physiological functions. Concurrently, the formation of free radicals within cells can lead to structural and functional damage, resulting in various conditions, such as heart disease, cancer, diabetes, and immune system disorders. The excessive generation of reactive oxygen species (ROS) or insufficient antioxidant capacity within an organism triggers a cascade of lipid peroxidation reactions that compromise cell membranes, ultimately leading to cell death and accelerating aging. H. erinaceus polysaccharides exhibit considerable antioxidant activity and can scavenge free radicals, thereby reducing oxidative stress-related damage in the body. This contributes to maintaining intracellular homeostasis, mitigating the effects of aging, and providing protection against diseases associated with oxidative stress. At certain concentrations, H. erinaceus polysaccharides have various effects on scavenging DPPH free radicals, hydroxyl radicals, and superoxide anion radicals, and their scavenging effects are comparable to those of vitamin C (45, 46). Moreover, Tian et al. (7, 47) reported that H. erinaceus polysaccharides effectively increase the total antioxidant capacity (T-AOC) and the glutathione peroxidase (GSH-PX) and total superoxide dismutase (T-SOD) levels while reducing the malondialdehyde (MDA) content, thus increasing the antioxidant capacity in the hepatic tissues of mice. Xu et al. (48) investigated the pharmacological properties of H. erinaceus polysaccharides in aged rats and reported that these compounds significantly enhanced skin antioxidant enzyme activities as well as the levels of matrix metalloproteinase-1 (MMP-1), tissue inhibitor of metalloproteinases-1 (TIMP-1), and collagen in a dose-dependent manner. In conclusion, it can be inferred that H. erinaceus polysaccharides possess antiskin-aging activity.
4.4 Antitumor effects
The continuous deterioration of the environment and increasing pressures on daily life have made cancer one of the leading causes of mortality worldwide. Tumors arise from the abnormal differentiation and development of cells, which grow uncontrollably within an organism. Traditional surgical interventions and radiotherapy often damage healthy tissues; consequently, natural active antitumor substances are emerging as promising areas for research. Polysaccharides derived from H. erinaceus exhibit inhibitory effects on various tumor cell types; they can induce apoptosis, inhibit proliferation and metastasis, and exert regulatory effects on the tumor microenvironment. Thus, these polysaccharides hold high potential for application in both the prevention and treatment of tumors (49). The primary mechanisms underlying the antitumor effects of H. erinaceus polysaccharides are illustrated in Figure 6. The antitumor activity of HEPs is primarily achieved through two mechanisms: first, polysaccharides can activate the cell apoptosis program. Polysaccharides bind to the Dectin-1/Toll-like receptor (TLR4) receptors on the surface of tumor cells, initiating signal transduction, increasing the expression of pro-apoptotic proteins Bax/Bad, and simultaneously reducing the expression of antiapoptotic proteins Bcl2. This ultimately activates the “scissor protein” cysteine-aspartic acid protease 3 (Caspase-3), leading to the self-destruction of tumor cells. On the other hand, polysaccharides disrupt the tumor's energy supply. By inhibiting the PI3K/Akt pathway (similar to turning off the cell survival switch), polysaccharides block the energy supply required for tumor cell proliferation, trapping them in the S phase and preventing division. Kim et al. (50) demonstrated that H. erinaceus polysaccharides interact with the membrane of tumor cells to exert an inhibitory effect while also effectively inhibiting artificially induced lung metastases. Furthermore, Zan et al. (51) reported for the first time that HEG-5, a purified glycoprotein extracted from the fermented mycelium of H. erinaceus, significantly inhibits apoptosis and induces S-phase arrest in SGC-7901 cells by promoting their proliferation and colony formation. The results obtained from reverse transcription–polymerase chain reaction (RT–PCR) and protein blotting analyses indicate that glycoproteins significantly reduce the expression levels of Bcl2, PI3K, and AKT1 while concurrently increasing the expression of Caspase-8, Caspase-3, p53, CDK4, Bax, and Bad. These findings suggest that the molecular mechanisms underlying the apoptosis and cell cycle arrest induced by purified glycoproteins derived from fermented H. erinaceus mycelia are mediated through Caspase-8/-3-dependent pathways that are independent of p53, mitochondrial involvement, and the PI3K/Akt signaling pathway. Consequently, this study provides compelling evidence supporting the potential utility of purified glycoproteins from fermented H. erinaceus mycelia as promising drug candidates for gastric cancer treatment.
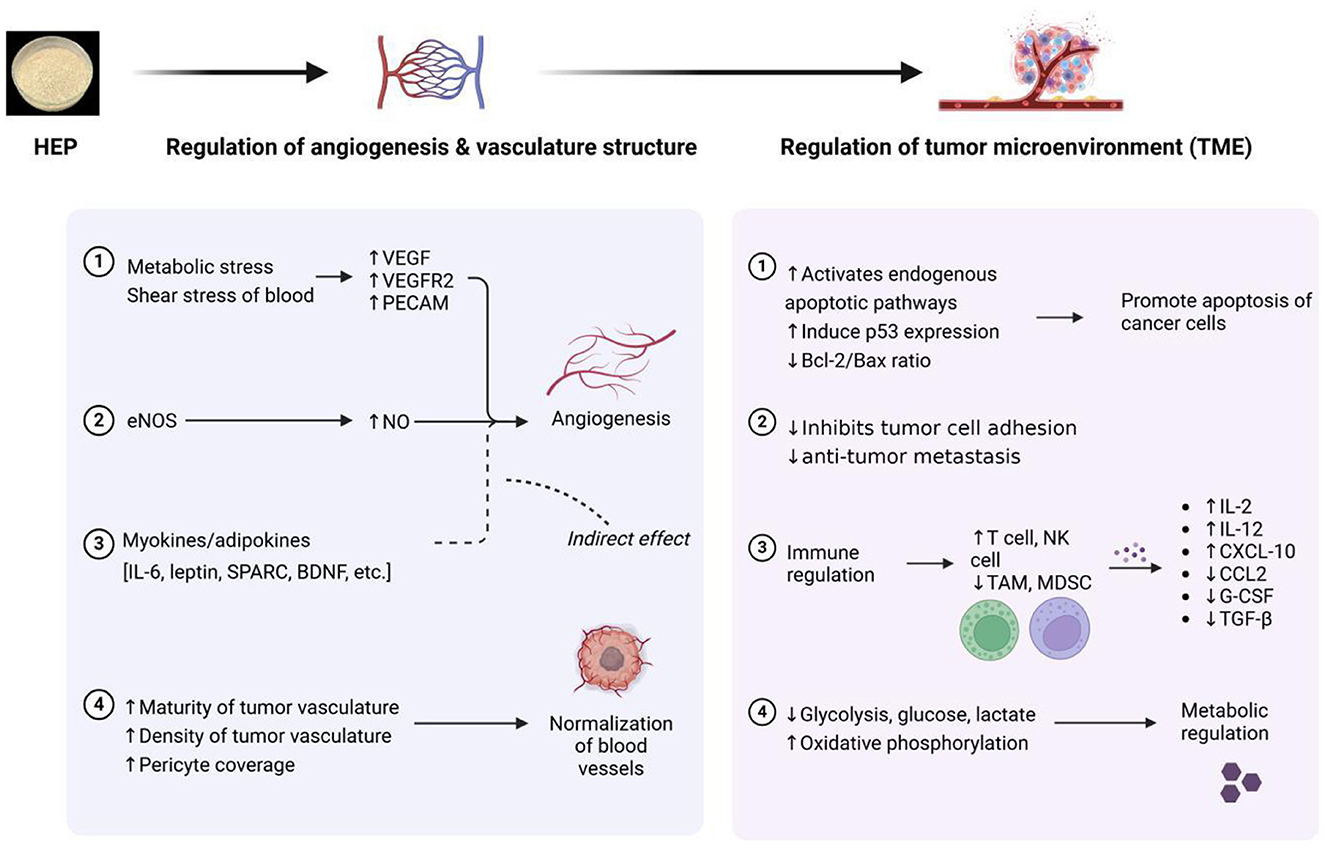
Figure 6. Primary mechanisms of the antitumor activity of polysaccharides from Hericium erinaceus (HEP: H. erinaceus polysaccharide).
4.5 Anti-inflammatory effects
Inflammation in an organism is characterized by fever, which is a normal physiological response of the immune system to external stimuli such as pathogenic microorganisms. This process is regulated by various inflammatory factors. Mild inflammation plays a crucial role in enhancing the body's immunity and inhibiting the proliferation of bacteria, viruses, and other microorganisms; however, excessive immune stimulation can lead to a significant release of inflammatory factors, resulting in organ inflammation or even organ failure (52). The polysaccharide derived from H. erinaceus has been shown to inhibit inflammatory reactions and alleviate symptoms associated with inflammation-related diseases. Its potential value for preventing and treating inflammatory disorders is notable, and its anti-inflammatory mechanisms are outlined in Figure 7 (53). When bacterial toxins (such as LPS) activate inflammatory signals, HEPs can directly block the progression of two pathways, namely, the NF-κB and JAK-STAT pathways. This reduces the production of inflammatory factors (such as TNF-α and IL-6). In neuroinflammatory models, HEPs also significantly reduce the levels of inflammatory factors (NO and ROS) in the brain, thereby protecting neurons from damage. Kushairi et al. (54) explored the anti-inflammatory properties of H. erinaceus polysaccharide and demonstrated its neuroprotective effects against hydrogen peroxide-induced neurotoxicity in mouse hippocampal neurons (HT22 cells). Additionally, this polysaccharide significantly reduced NO levels in lipopolysaccharide (LPS)-treated microglia (BV2 cells), suggesting that it has anti-inflammatory effects. Further research conducted by Zhang et al. (55) revealed that sulfated H. erinaceus polysaccharides exhibited strong anti-inflammatory effects in vitro by regulating the protein levels of NF-κB p65, STAT1, and TLR4 via the NF-κB and Janus kinases—signal transducers and activators of transcription 1 (JAK-STAT1) signaling pathways.
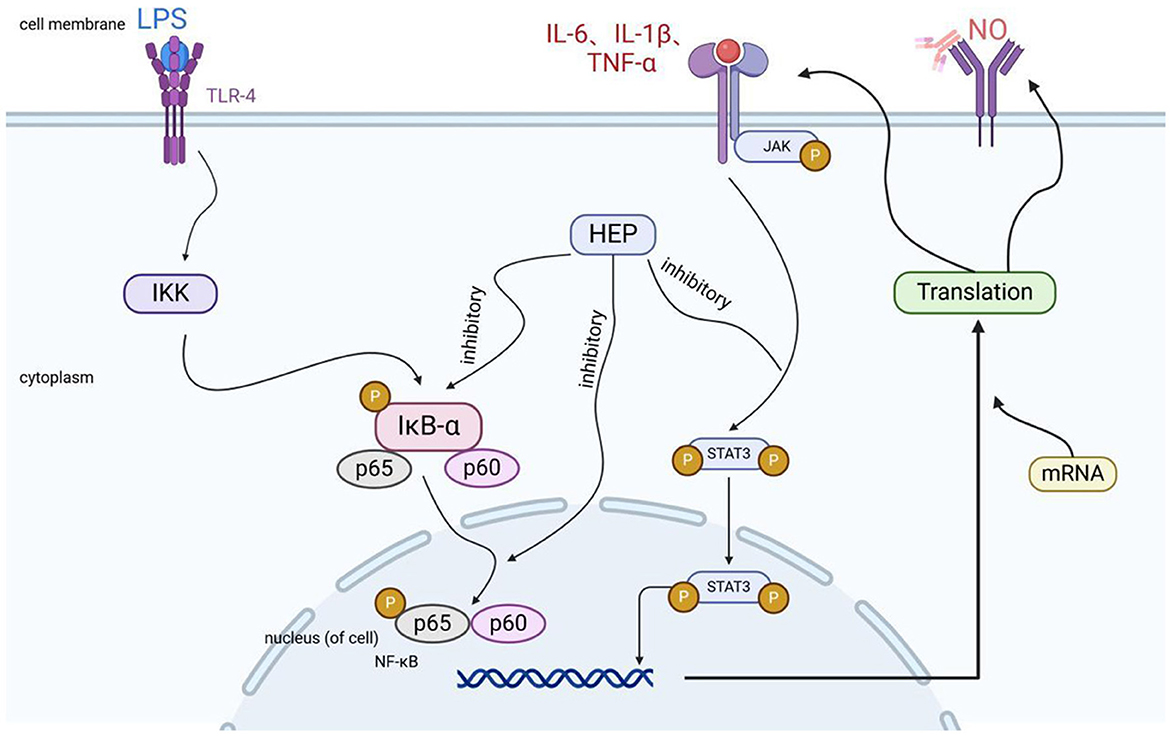
Figure 7. Mechanisms of the anti-inflammatory effects of Hericium erinaceus polysaccharide (HEP: H. erinaceus polysaccharide).
4.6 Hypoglycemic effects
Hyperglycemia is commonly recognized as a manifestation of diabetes, particularly type 2 diabetes, where blood glucose levels cannot be effectively regulated owing to insufficient insulin secretion or impaired cellular responses to insulin. Prolonged hyperglycemia not only contributes to the symptoms associated with diabetes but also leads to a range of serious complications. Consequently, the early detection and management of blood glucose levels are crucial for preventing the severe repercussions associated with elevated blood sugar (56, 57). Notably, the hypoglycemic potential of H. erinaceus polysaccharides has been investigated. These polysaccharides bind to specific receptors on cell membranes and convey information to mitochondria through cyclic adenosine monophosphate (cAMP). This mechanism enhances the activity of enzymatic systems involved in sugar metabolism, resulting in the accelerated oxidative breakdown of glucose and a subsequent reduction in blood sugar levels. Furthermore, H. erinaceus polysaccharides can improve both the morphology and functionality of insulin-producing cells while promoting insulin secretion. H. erinaceus polysaccharides have been shown to increase the utilization of glucose by peripheral tissues and target organs, including the liver and muscle (58). Together, these mechanisms enable H. erinaceus to significantly reduce blood glucose levels, as depicted in Figure 8. Cai et al. (59) isolated polysaccharides from the H. erinaceus subentity and evaluated their hypoglycemic effects, revealing that these polysaccharides effectively reduced body weight and fasting blood glucose levels, improved glucose tolerance, mitigated hepatic lesions in streptozotocin-induced diabetic rats, and positively influenced glycogen synthesis via activation of the phosphatidylinositol 3-kinase/protein kinase B signaling pathway. Furthermore, Cui et al. (60) explored the hypoglycemic mechanism of H. erinaceus polysaccharide. They found that H. erinaceus polysaccharide inhibited the progression of type 2 diabetes by inducing an imbalance in glucose metabolism, activating the adenosine monophosphate (AMP)-activated protein kinase (AMPK)/SREBP-1c signaling pathway, and increasing beneficial metabolites in the liver through the intestinal–hepatic axis. As novel dietary functional foods or therapeutic agents, H. erinaceus polysaccharides have considerable potential for preventing and treating diabetes as well as its complications.
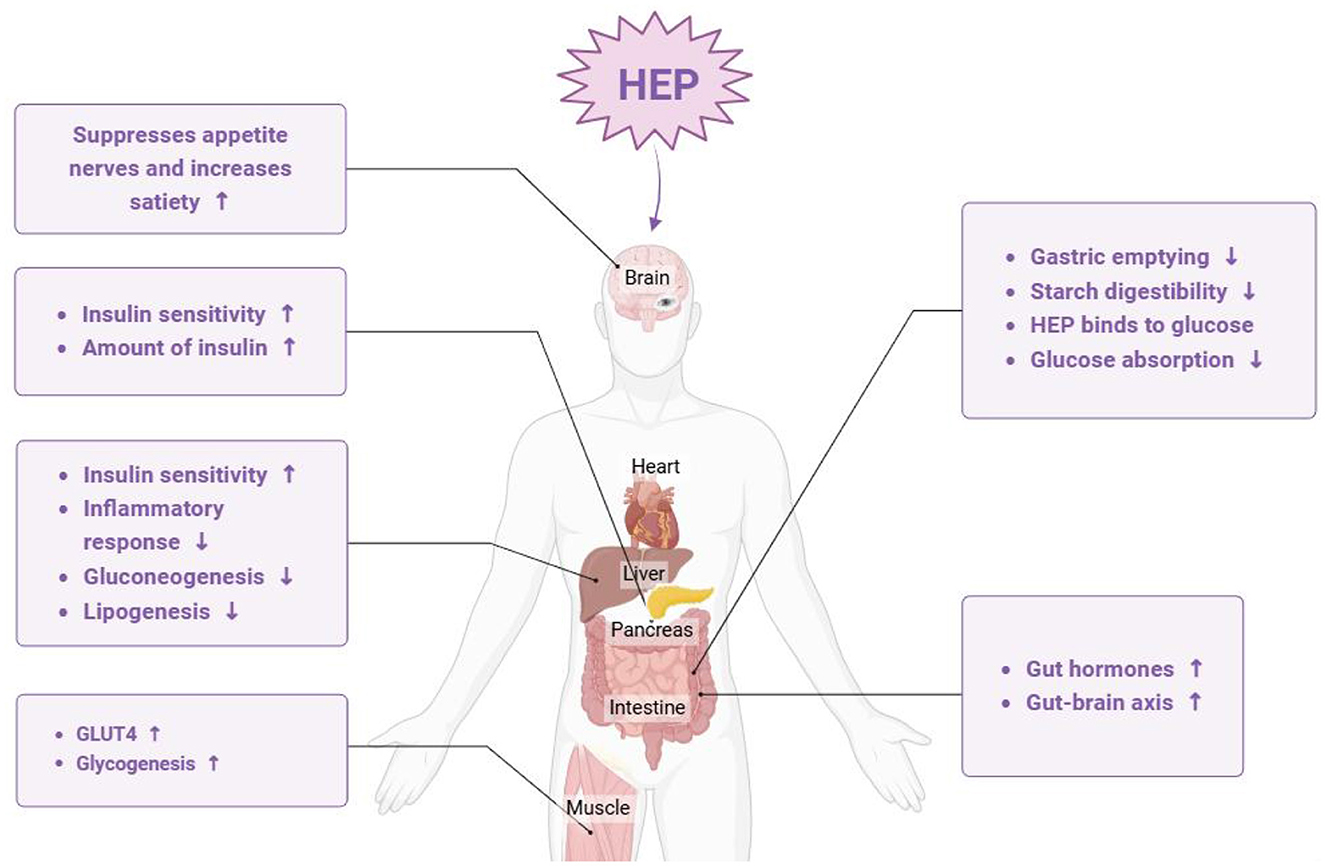
Figure 8. The main pathway by which Hericium erinaceus polysaccharide alleviates hypoglycemia (HEP: H. erinaceus polysaccharide).
4.7 Intestinal regulation
H. erinaceus polysaccharides exhibit a range of health-promoting effects by modulating the host intestinal flora composition and metabolism. The benefits of probiotics are associated with various functions, including immune regulation, anti-inflammatory bowel disease management, nervous system effects, antiobesity properties, cholesterol reduction, and inhibition of Helicobacter pylori. The specific effects and mechanisms underlying these activities are detailed in Table 2. Polysaccharides derived from H. erinaceus function by promoting the proliferation of intestinal probiotics, suppressing the growth of pathogenic bacteria, increasing short-chain fatty acid (SCFA) production, boosting intestinal immunity, and activating specific signaling pathways (61).
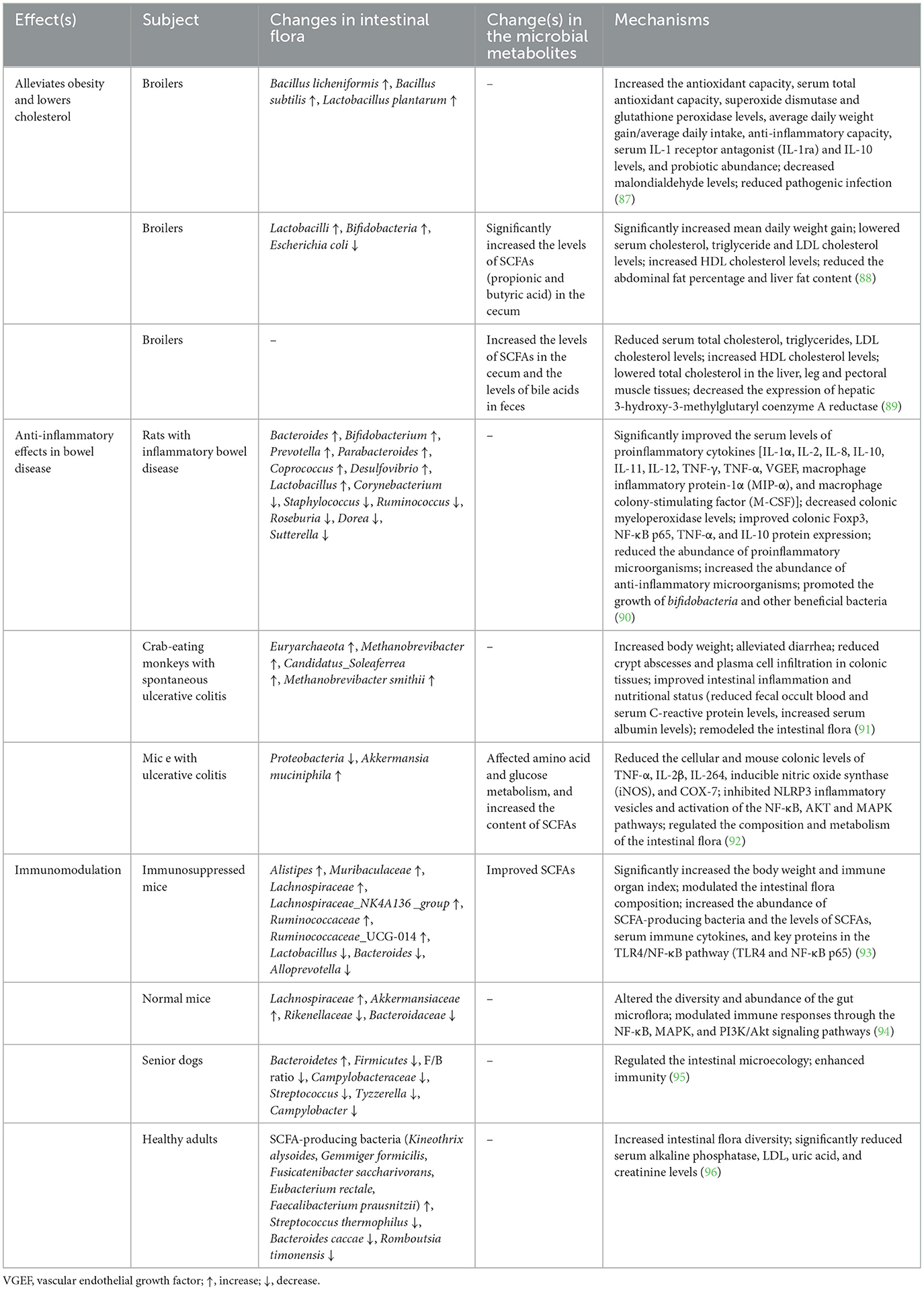
Table 2. Efficacy and potential mechanisms of Hericium erinaceus polysaccharides in regulating the intestinal flora.
SCFAs are the end products of dietary fiber fermentation by gut microorganisms and serve as crucial links between the gut flora and the host. SCFAs can enhance the intestinal mucosal barrier, stimulate the production of immunosuppressive cytokines, and function as signaling molecules that regulate and sustain the host immune system (61, 62). Investigations into the intestinal microbiota of mice, dogs, and humans have demonstrated that H. erinaceus polysaccharides significantly influence microbial diversity and abundance in the intestines while increasing the prevalence of SCFA-producing bacteria. Zhuang et al. (63) reported that both the water-soluble polysaccharide (HEP-W) and alkali-soluble polysaccharide (HEP-A) from H. erinaceus elevated the relative abundance of key butyric acid-producing bacterial genera during fermentation. Furthermore, these polysaccharides exhibited substantial regulation of the microbial community structure while markedly increasing gas production and SCFA yields in fermentation broth. These findings suggest that Hericium erinaceus polysaccharide W (HEP-W) and HEP-A hold promise as potential modulators of the intestinal microbiota with higher benefits for promoting intestinal health.
Disturbances in the intestinal flora can lead to disruptions of the host's intestinal mucosal barrier and an increase in the secretion of LPS. LPS enters the circulatory system through the mesenteric vein and acts upon its target organs and tissues, resulting in intestinal inflammation and potentially inducing cancer (64). Research using experimental animal models of intestinal inflammation and other diseases has demonstrated that polysaccharides from H. erinaceus can ameliorate disorders of the intestinal flora. These compounds significantly increase the abundance of anti-inflammatory bacteria within the intestine, such as Lactobacillus, Anaplasma, Bifidobacterium, and Prevotella, while simultaneously decreasing the levels of proinflammatory bacteria and pathogens, including Corynebacterium, Ruminal Streptococcus, Staphylococcus, Enterobacteriaceae, Campylobacter, and Shigella, among others. Furthermore, these polysaccharides help maintain the integrity of the intestinal barrier (65). Lactobacillus spp. alleviate colitis induced by dextran sodium sulfate by modulating the intestinal flora and stimulating NK cells, macrophages, and T lymphocytes (66). Additionally, Bifidobacterium shortum has been shown to improve symptoms of colitis by reducing the levels of TNF-α, IL-1β, and IL-6 while restoring balance within the intestinal flora (67).
The gut microbiota may serve as a potential target against H. pylori infection (68). H. pylori infection is known to alter colonic pH, leading to a decrease in the abundance of the phylum Anaplasma and an increase in the phyla of thick-walled bacteria and Aspergillus. These alterations induce changes in populations such as Vibrio desulfuricans, Prevotella, Haemophilus, Anaplasma, Serratia parapsilosis, and Enterobacteriaceae pubescens (69). Furthermore, H. pylori infection can modify gastrointestinal metabolism by influencing hormone secretion, thereby affecting the composition of the gut microbiome (70). Specifically, an increase in Lactobacillus salivarius coupled with a decrease in Lactobacillus gelatinosus within the infected gut correlates with reduced gastric acid secretion (71). Additionally, polysaccharides derived from H. erinaceus have been shown to strongly inhibit H. pylori (72). Zhu et al. (73) demonstrated that Bi3+ combined with H. erinaceus polysaccharides has anti-H. pylori activity comparable to that of bismuth potassium citrate and also effectively reduces the risks and adverse reactions, such as epilepsy and myoclonus, associated with bismuth toxicity. Currently, reports regarding the role of H. erinaceus polysaccharides in combating H. pylori infection through regulation of the intestinal flora composition and metabolism are limited; therefore, further studies are warranted.
Due to their complex structures, polysaccharides derived from H. erinaceus show significant variability in their regulatory effects on the intestinal flora. Research on how H. erinaceus polysaccharides affect the composition of the intestinal microbiota remains in its early stages, and a definitive understanding of the relationship between the structural characteristics of these polysaccharides and the resulting changes in the gut microbiota has yet to be established. Table 3 summarizes the reported structures of H. erinaceus polysaccharides and their corresponding effects on the intestinal flora. The ability of these polysaccharides to modulate the intestinal flora is closely associated with their molecular weight and monosaccharide composition; furthermore, this effect is also influenced to some extent by the degree of branching within the polysaccharide chain and the various functional groups attached to them. Notably, high molecular weight polysaccharides display weaker activity than their low molecular weight counterparts do, primarily because of their limited ability to penetrate cell membranes. Chen et al. (74) isolated two distinct polysaccharides with different molecular weights from the fruiting bodies of H. erinaceus and investigated their gastroprotective properties in a rat model of ethanol-induced gastric ulcers. These findings indicated that the higher molecular weight β-glucan H6PC20 (2,390 kDa) exhibited superior reparative and defensive effects, whereas the lower molecular weight α-heteropolysaccharide HPB-3 (15 kDa) demonstrated stronger anti-inflammatory effects. Additionally, the content of β-(1 → 3)-glucans present within H. erinaceus polysaccharides may significantly influence their biological activities. The structure of H. erinaceus polysaccharides, characterized by a (1 → 6)-glucopyranose main chain and (1 → 3)-glucopyranose side chains, is crucial for their antitumor and immunostimulatory effects. Compared with HIPS2, which lacks these functional groups, the hypoglycemic and pancreas, liver, and kidney protective effects of HIPS1, which contains functional groups such as –NH2, –COOH, and S=O, were superior in streptozotocin-induced diabetic mice (75). Furthermore, the polysaccharide zinc chelate (ZnHEP), formed by polysaccharide chelation of zinc ions, demonstrated significantly higher stability and antioxidant activity than H. erinaceus polysaccharides. The regulatory effects of H. erinaceus polysaccharides on the intestinal flora are intricately linked to various factors, including their molecular mass, monosaccharide composition, type of glycosidic bonds, degree of branching, presence of functional groups, and associated complexes. However, the specific structural characteristics of polysaccharides capable of stimulating particular intestinal bacteria—and their corresponding polysaccharide profiles following hydrolysis—remain poorly understood, and this gap poses a higher level of challenges for further research in this area. Thus, it is imperative to study the conformational relationships between H. erinaceus polysaccharides and their regulatory effects on the intestinal flora.
4.8 Neurological protection
Neurological protection indicates that certain substances or factors can protect neurons from injury or death. The primary objective of neuroprotection is to prevent the degeneration of nerve cells and preserve normal neurological function. In recent years, interest in the potential application of H. erinaceus polysaccharides for addressing various neurological and cognitive disorders has increased. Moreover, evidence suggests that these polysaccharides may exhibit neuroprotective effects, which have been attributed to their ability to support neuronal health and prevent cellular damage (76, 77). The principal mechanisms underlying the neuroprotective and trophic activities of H. erinaceus polysaccharides are illustrated in Figure 9. H. erinaceus polysaccharides promote the synthesis of nerve growth factor (NGF), inhibit β-amyloid (Aβ) cytotoxicity, and protect neuronal cells from apoptosis induced by oxidative stress or endoplasmic reticulum stress. Notable positive outcomes have been reported in the treatment of cognitive impairment, Alzheimer's disease (AD), ischemic stroke, Parkinson's disease, and age-related hearing loss (28, 78–80). Chiu et al. (81) investigated the effects of various concentrations of H. erinaceus polysaccharide (HEP) extracts on the toxicity to pheochromocytoma (PC12) cells induced by Aβ1–40. The accumulation of Aβ has been linked to the onset and progression of AD. Neurotoxic mechanisms associated with this condition include oxidative stress and mitochondrial dysfunction, which result in apoptosis and neuronal impairment. Researchers have reported that HEPs enhance the survival of PC12 cells under conditions of Aβ-induced toxicity. Furthermore, they noted that the ability of HEPs to scavenge free radicals and ROS increased. Consequently, HEPs protected against Aβ-induced apoptosis in PC12 cells. Hu et al. (82) demonstrated that polysaccharides isolated from the mycelium of H. erinaceus exhibited anti-AD activity; treatment with these polysaccharides significantly improved cognitive behaviors in AD mice while alleviating brain damage, reducing amyloid deposition, mitigating tau hyperphosphorylation, and decreasing oxidative stress within the brain. Additionally, aqueous extracts of mycelium-derived polysaccharides protected against L-glutamate-induced PC12 cell apoptosis, as did AlCl3 in D-galactose-induced mouse models of AD (83). Park et al. (84) reported that extracellular polysaccharides derived from H. erinaceus stimulated both the growth and axonal extension of adrenal nerve cells in a rat model. Moreover, ethanol extracts from H. erinaceus promoted NGF gene expression in human astrocytoma cell lines. Collectively, these neuroprotective effects suggest that polysaccharides extracted from H. erinaceus may represent a promising new approach for the treatment or prevention of neurodegenerative diseases (85, 86).
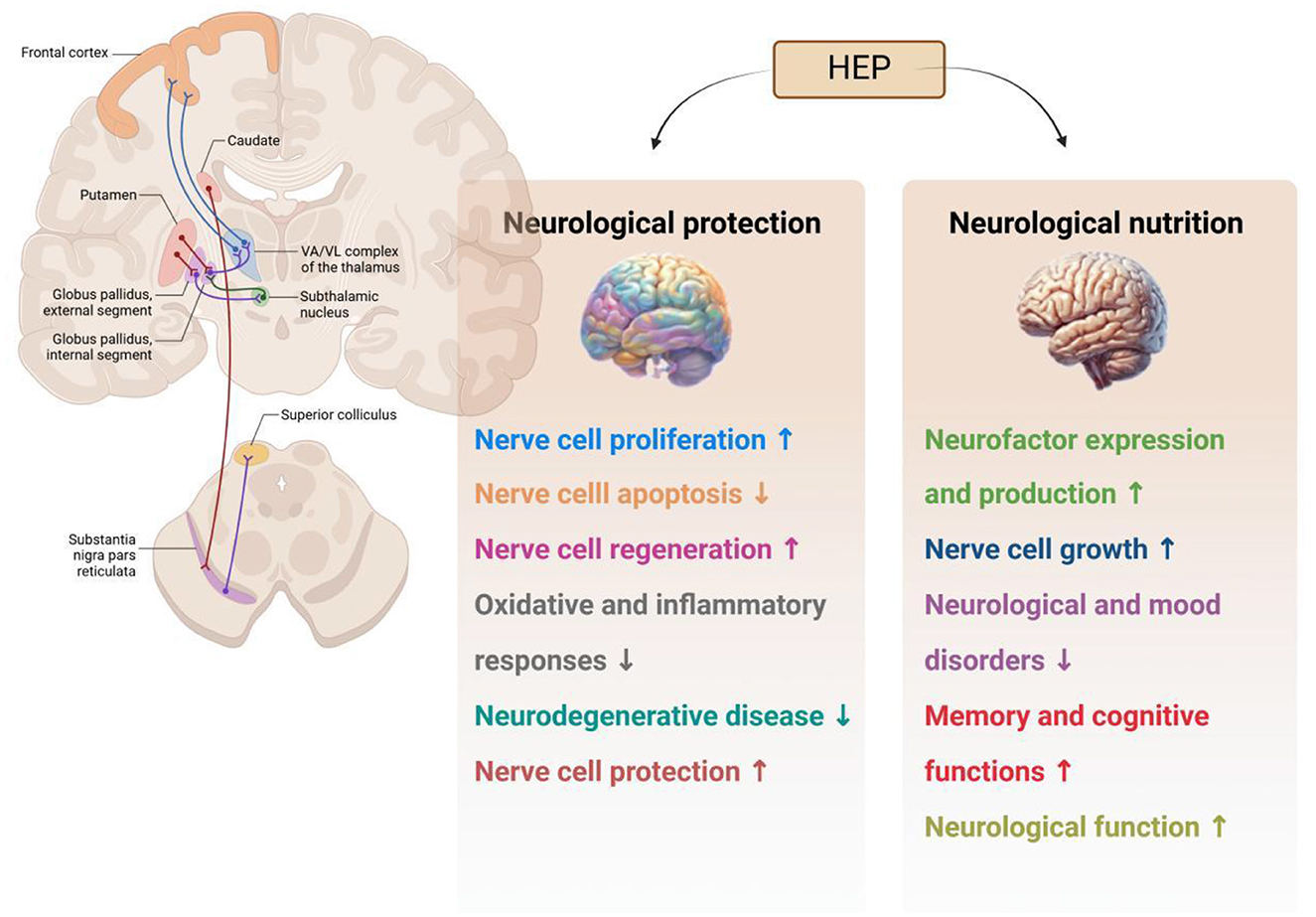
Figure 9. Neurological activities of polysaccharides from Hericium erinaceus (HEP: H. erinaceus polysaccharide).
5 Conclusion and future perspectives
Polysaccharides extracted from H. erinaceus are bioactive macromolecules derived from the fruiting bodies, mycelia, or fermentation culture broth of this edible and medicinal fungus. These polysaccharides exhibit a variety of biological activities and have garnered increasing attention in recent years. This article summarizes the extraction and purification processes, structural properties, and biological functions of H. erinaceus polysaccharides and reviews advancements in research regarding their mechanisms and health-promoting applications through modulation of the intestinal microbiota and metabolism in the host. In conclusion, H. erinaceus polysaccharides hold high therapeutic potential and promise for future applications. However, current research faces two fundamental limitations: (1) The studies of structure-activity relationship predominantly remain at the level of apparent bioactivity, lacking molecular-level mechanistic evidence; (2) Structural modification research has focused on simple derivatives without establishing systematic function-oriented design strategies. Nevertheless, further investigations into the structures, pharmacological effects, and mechanisms underlying H. erinaceus polysaccharides are essential. Specifically, research efforts can be directed toward two primary aspects. First, an in-depth examination of biological mechanisms is warranted. The existing mechanism research overly relies on animal models and microbiota sequencing correlation analyses, making it difficult to distinguish between direct and indirect effects. In the future, it is necessary to consider the integrating variables of host–microbiota interactions, and combine spatial metabolomics with single-cell transcriptomics to precisely locate polysaccharides targets in the intestinal tract, and achieve cellular-resolution mechanistic insights, thus providing a scientific foundation for their application within clinical settings and drug development. Second, emphasis should be placed on both the structural modification and functional enhancement of these polysaccharides. By employing computer-aided prediction integrated with biotechnology, a targeted delivery system is developed to construct an intelligent structural and functional optimization system, thereby enhancing the bioactivity, stability, and bioavailability of polysaccharides. Ultimately, H. erinaceus polysaccharides modification strategies must evolve from “empirical exploration” to “precision design” to advance medical and nutraceutical applications.
Author contributions
PY: Conceptualization, Validation, Formal analysis, Methodology, Writing – original draft, Investigation, Visualization. JM: Software, Formal analysis, Investigation, Writing – original draft. LY: Writing – review & editing. QD: Writing – review & editing. CY: Project administration, Writing – review & editing. LZ: Writing – review & editing, Software. BX: Writing – review & editing, Visualization. YZ: Resources, Funding acquisition, Writing – review & editing, Supervision, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported financially by National Key R&D Program of China (No. 2024YFD1200204), Shanghai Committee of Science and Technology (No. 19390743000), and the China Agriculture Research System (No. CARS20).
Acknowledgments
The authors acknowledge the resources and laboratory facilities provided by the Institute of Edible Fungi, Shanghai Academy of Agricultural Sciences.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen S, Zhang F, Liu L, Feng J, Zhang J, Yang Y, et al. Physicochemical properties of polysaccharides from Hericium erinaceus by steam explosion pretreatment and its effects on human gut microbiota. J Food Hydrocoll. (2024) 156:110365. doi: 10.1016/j.foodhyd.2024.110365
2. Liu J, Wang W, Hu Q, Wu X, Xu H, Su A, et al. Bioactivities and molecular mechanisms of polysaccharides from Hericium erinaceus. J Future Foods. (2022) 2:103–11. doi: 10.1016/j.jfutfo.2022.03.007
3. Lazur J, Kała K, Krakowska A, Ziaja KS, Szewczyk A, Piotrowska J, et al. Analysis of bioactive substances and essential elements of mycelia and fruiting bodies of Hericium spp. J Food Compos Anal. (2024) 127:105981. doi: 10.1016/j.jfca.2024.105981
4. Cui M, Ma Q, Zhang Z, Li W, Chen W, Liu P, et al. Semisolid enzymolysis enhanced the protective effects of fruiting body powders and polysaccharides of Herinaceus erinaceus on gastric mucosal injury. Int J Biol Macromol. (2023) 251:126388. doi: 10.1016/j.ijbiomac.2023.126388
5. Ren Z, Qin T, Liu X, Luo Y, Qiu F, Long Y, et al. Optimization of Hericium erinaceus polysaccharide-loaded Poly (lactic- co -glycolicacid) nanoparticles by RSM and its absorption in Caco-2 cell monolayers. Int J Biol Macromol. (2018) 118:932–7. doi: 10.1016/j.ijbiomac.2018.06.169
6. Shi XZ, Zhang XY, Wang YY, Zhao YM, Wang J. Polysaccharides from Hericium erinaceus and its immunomodulatory effects on RAW 264.7 macrophages. Int J Biol Macromol. (2024). 278:134947. doi: 10.1016/j.ijbiomac.2024.134947
7. Yue S, Hongxuan L, Ziyu H, Yu Z, Ling G, Meili S, et al. Research on degradation of polysaccharides during Hericium erinaceus fermentation. LWT. (2023) 173:114276. doi: 10.1016/j.lwt.2022.114276
8. Hiroyuki T, Kazuyoshi K, Hiraku O, LiTing S, Kento N, Junya K, et al. Hericium erinaceus ethanol extract and ergosterol exert anti-inflammatory activities by neutralizing lipopolysaccharide-induced pro-inflammatory cytokine production in human monocytes. J Biochem Biophys Res Commun. (2022) 636:1–9. doi: 10.1016/j.bbrc.2022.10.090
9. Li H, Feng J, Liu C, Hou S, Meng J, Liu JY, et al. Polysaccharides from an edible mushroom, Hericium erinaceus, alleviate ulcerative colitis in mice by inhibiting the NLRP3 inflammasomes and reestablish intestinal homeostasis. Int J Biol Macromol. (2024) 267:131251. doi: 10.1016/j.ijbiomac.2024.131251
10. Baltrusch KL, Torres MD, Domínguez H. Characterization, ultrafiltration, depolymerization and gel formulation of ulvans extracted via a novel ultrasound-enzyme assisted method. J Ultrason Sonochem. (2024) 111:107072. doi: 10.1016/j.ultsonch.2024.107072
11. Barbosa JR, Freitas MMdS, Martins LHdS, Carvalho RNd. Polysaccharides of mushroom Pleurotus spp.: New extraction techniques, biological activities and development of new technologies. J Carbohydr Polym. (2020) 229:115550. doi: 10.1016/j.carbpol.2019.115550
12. Wang XY, Zhang DD, Yin JY, Nie SP, Xie MY. Recent developments in Hericium erinaceus polysaccharides: extraction, purification, structural characteristics and biological activities. J Crit Rev Food Sci Nutr. (2019) 59:S96–115. doi: 10.1080/10408398.2018.1521370
13. Yang Z, Qian L, Guanghua M, Ye Z, Weiwei F, Daheng Z, et al. Optimization of enzyme-assisted extraction and characterization of polysaccharides from Hericium erinaceus. J Carbohydr Polym. (2014) 101:606–13. doi: 10.1016/j.carbpol.2013.09.099
14. Wu D, Yang S, Tang C, Liu Y, Li Q, Zhang H, et al. Structural properties and macrophage activation of cell wall polysaccharides from the fruiting bodies of Hericium erinaceus. J Polymers. (2018) 10:850. doi: 10.3390/polym10080850
15. Zhang ZF, Lv GY, Song TT, Xu ZW, Wang MY. Effects of different extraction methods on the structural and biological properties of Hericium coralloides polysaccharides. J Food Chem. (2024) 445:138752. doi: 10.1016/j.foodchem.2024.138752
16. Yan JK, Ding ZC, Gao X, Wang YY, Yang Y, Wu D, et al. Comparative study of physicochemical properties and bioactivity of Hericium erinaceus polysaccharides at different solvent extractions. J Carbohydr Polym. (2018) 193:373–82. doi: 10.1016/j.carbpol.2018.04.019
17. Gong M, Zhang H, Wu D, Zhang Z, Zhang J, Bao D, et al. Key metabolism pathways and regulatory mechanisms of high polysaccharide yielding in Hericium erinaceus. J BMC Genom. (2021) 22:160. doi: 10.1186/s12864-021-07480-x
18. Yang Y, Zhao C, Diao M, Zhong S, Sun M, Sun B, et al. The prebiotic activity of simulated gastric and intestinal digesta of polysaccharides from the Hericium erinaceus. J Mol. (2018) 23:3158. doi: 10.3390/molecules23123158
19. Seok LJ, Seok KJ, Pil WD, Hyun LJ, Eok LK, Young LS, et al. Study of macrophage activation and structural characteristics of purified polysaccharide from the fruiting body of Cordyceps militaris. J Microbiol Biotechnol. (2010) 20:1053–60. doi: 10.4014/jmb.0910.10022
20. Wang M, Gao Y, Xu D, Gao Q. A polysaccharide from cultured mycelium of Hericium erinaceus and its anti-chronic atrophic gastritis activity. Int J Biol Macromol. (2015) 81:656–61. doi: 10.1016/j.ijbiomac.2015.08.043
21. Xue Y, Ding X, Wu X, Xu Z, Zhang Z, He J, et al. Optimization of preparation process and antioxidant activity of the chelate of a Hericium erinaceus polysaccharide with zinc. J Food Measure Charact. (2021) 15:1–10. doi: 10.1007/s11694-020-00795-5
22. Qiao Z, Jia X, Wang Y, Wang Y, Zhou Y, Li F, et al. Structural analysis and antioxidant activity of alkaline-extracted glucans from Hericium erinaceus. J Foods. (2024) 13:2742. doi: 10.3390/foods13172742
23. Wang Z, Luo D, Liang Z. Structure of polysaccharides from the fruiting body of Hericium erinaceus Pers. J Carbohydr Polym. (2004) 57:241–7. doi: 10.1016/j.carbpol.2004.04.018
24. Guan Y, Wang C, Li L, Dai X, Liu Y, Hsiang T, et al. Structural characterization of Hericium coralloides polysaccharide and its neuroprotective function in Alzheimer's disease. Int J Biol Macromol. (2024) 277:133865. doi: 10.1016/j.ijbiomac.2024.133865
25. Li QZ, Wu D, Zhou S, Liu YF, Li ZP, Feng J, et al. Structure elucidation of a bioactive polysaccharide from fruiting bodies of Hericium erinaceus in different maturation stages. J Carbohydr Polym. (2016) 144:196–204. doi: 10.1016/j.carbpol.2016.02.051
26. Ren Y, Sun Q, Gao R, Sheng Y, Guan T, Li W, et al. Low weight polysaccharide of Hericium erinaceus ameliorates colitis via inhibiting the NLRP3 inflammasome activation in association with gut microbiota modulation. J Nutr. (2023) 15:739. doi: 10.3390/nu15030739
27. Tian B, Geng Y, Xu T, Zou X, Mao R, Pi X, et al. Digestive characteristics of Hericium erinaceus polysaccharides and their positive effects on fecal microbiota of male and female volunteers during in vitro fermentation. J Front Nutr. (2022) 9:858585. doi: 10.3389/fnut.2022.858585
28. He X, Wang X, Fang J, Chang Y, Ning N, Guo H, et al. Structures, biological activities, and industrial applications of the polysaccharides from Hericium erinaceus (Lion's Mane) mushroom: a review. Int J Biol Macromol. (2017) 97:228–37. doi: 10.1016/j.ijbiomac.2017.01.040
29. Chen SK, Li YH, Wang X, Guo YQ, Song XX, Nie SP, et al. Evaluation of the “relative ordered structure of Hericium erinaceus polysaccharide” from different origins: based on similarity and dissimilarity. J Agric Food Chem. 71:17886–98. doi: 10.1021/acs.jafc.3c04329
30. Liu JY, Hou XX, Li ZY, Shan SH, Chang MC, Feng CP, et al. Isolation and structural characterization of a novel polysaccharide from Hericium erinaceus fruiting bodies and its arrest of cell cycle at S-phage in colon cancer cells. Int J Biol Macromol. (2020) 157:288–95. doi: 10.1016/j.ijbiomac.2020.04.162
31. Liao BW, Zhou CH, Liu TT, Dai YY, Huang H. A novel Hericium erinaceus polysaccharide: structural characterization and prevention of H2O2-induced oxidative damage in GES-1 cells. Int J Biol Macromol. (2020) 154:1460–70. doi: 10.1016/j.ijbiomac.2019.11.027
32. Hou C, Liu L, Ren J, Huang M, Yuan E. Structural characterization of two Hericium erinaceus polysaccharides and their protective effects on the alcohol-induced gastric mucosal injury. J Food Chem. (2022) 375:131896. doi: 10.1016/j.foodchem.2021.131896
33. Cui FJ, Li YH, Zan XY, Yang Y, Sun WJ, Qian JY, et al. Purification and partial characterization of a novel hemagglutinating glycoprotein from the cultured mycelia of Hericium erinaceus. J Process Biochem. (2014) 49:1362–9. doi: 10.1016/j.procbio.2014.04.008
34. Shao S, Wang D, Zheng W, Li X, Zhang H, Zhao D, et al. A unique polysaccharide from Hericium erinaceus mycelium ameliorates acetic acid-induced ulcerative colitis rats by modulating the composition of the gut microbiota, short chain fatty acids levels and GPR41/43 respectors. J Int Immunopharmacol. (2019) 71:411–22. doi: 10.1016/j.intimp.2019.02.038
35. Yang Y, Li J, Hong Q, Zhang X, Liu Z, Zhang T, et al. Polysaccharides from Hericium erinaceus fruiting bodies: structural characterization, immunomodulatory activity and mechanism. J Nutr. (2022) 14:3721. doi: 10.3390/nu14183721
36. Rajoka MSR, Mehwish HM, Kitazawa H, Barba FJ, Berthelot L, Umair M, et al. Techno-functional properties and immunomodulatory potential of exopolysaccharide from Lactiplantibacillus plantarum MM89 isolated from human breast milk. J Food Chem. (2021) 377:131954. doi: 10.1016/j.foodchem.2021.131954
37. Kim H, Suh HJ, Kwon KH, Hwang JH, Yu KW. Immunostimulation activity of a polysaccharide from fermented ginseng with Hericium erinaceum mycelia in solid-state culture. J Food Sci Biotechnol. (2016) 25:311–8. doi: 10.1007/s10068-016-0044-4
38. Liu X, Ren Z, Yu R, Chen X, Zhang J, Xu Y, et al. Structural characterization of enzymatic modification of Hericium erinaceus polysaccharide and its immune-enhancement activity. Int J Biol Macromol. (2020) 166:1396–408. doi: 10.1016/j.ijbiomac.2020.11.019
39. Zhu L, Wu D, Zhang H, Li Q, Zhang Z, Liu Y, et al. Effects of Atmospheric and Room Temperature Plasma (ARTP) mutagenesis on physicochemical characteristics and immune activity in vitro of Hericium erinaceus polysaccharides. J Mol. (2019) 24:262. doi: 10.3390/molecules24020262
40. Ren Z, Luo Y, Meng Z, Zhang J, Yu R, Sun M, et al. Multi-walled carbon nanotube polysaccharide modified Hericium erinaceus polysaccharide as an adjuvant to extend immune responses. Int J Biol Macromol. (2021) 182:574–82. doi: 10.1016/j.ijbiomac.2021.03.180
41. Luo Y, Ren Z, Bo R, Liu X, Zhang J, Yu R, et al. Designing selenium polysaccharides-based nanoparticles to improve immune activity of Hericium erinaceus. Int J Biol Macromol. (2020) 143:393–400. doi: 10.1016/j.ijbiomac.2019.12.061
42. Yu P, Pan X, Chen M, Ma J, Xu B, Zhao Y, et al. Ultrasound-assisted enzymatic extraction of soluble dietary Fiber from Hericium erinaceus and its in vitro lipid-lowering effect. J Food Chem X. (2024) 23:101657. doi: 10.1016/j.fochx.2024.101657
43. Yang BK, Park JB, Song CH. Hypolipidemic effect of an exo-biopolymer produced from a submerged mycelial culture of Hericium erinaceus. J Biosci Biotechnol Biochem. (2003) 67:1292–8. doi: 10.1271/bbb.67.1292
44. Feng T, Shui M, Chen Z, Zhuang H, Wang W, Yang Y, et al. Hericium Erinaceus β-glucan modulates in vitro wheat starch digestibility. J Food Hydrocoll. (2019) 96:424–32. doi: 10.1016/j.foodhyd.2019.05.044
45. He H, Liu C, Shao C, Wu Y, Huang Q. Green synthesis of ultrasmall selenium nanoparticles (SeNPs) using Hericium erinaceus polysaccharide (HEP) as nanozymes for efficient intracellular antioxidation. J Mater Lett. 317:132079. doi: 10.1016/j.matlet.2022.132079
46. Tu JQ, Liu HP, Wen YH, Chen P, Liu ZT. A novel polysaccharide from Hericium erinaceus: preparation, structural characteristics, thermal stabilities, and antioxidant activities in vitro. J Food Biochem. (2021) 45:e13871. doi: 10.1111/jfbc.13871
47. Tian B, Wang P, Xu T, Cai M, Mao R, Huang L, et al. Ameliorating effects of Hericium erinaceus polysaccharides on intestinal barrier injury in immunocompromised mice induced by cyclophosphamide. J. Food Funct. (2023) 14:1011–24. doi: 10.1039/D2FO03769F
48. Xu H, Wu PR, Shen ZY, Chen XD. Chemical analysis of Hericium erinaceum polysaccharides and effect of the polysaccharides on derma antioxidant enzymes, MMP-1 and TIMP-1 activities. Int J Biol Macromol. (2010) 47:33–6. doi: 10.1016/j.ijbiomac.2010.03.024
49. Wang XY, Yin JY, Zhao MM, Liu SY, Nie SP, Xie MY, et al. Gastroprotective activity of polysaccharide from Hericium erinaceus against ethanol-induced gastric mucosal lesion and pylorus ligation-induced gastric ulcer, and its antioxidant activities. J Carbohydr Polym. (2018) 186:100–9. doi: 10.1016/j.carbpol.2018.01.004
50. Kim SP, Kang MY, Kim JH, Nam SH, Friedman M. Composition and mechanism of antitumor effects of Hericium erinaceus mushroom extracts in tumor-bearing mice. J Agric Food Chem. (2011) 59:9861–9. doi: 10.1021/jf201944n
51. Zan X, Cui F, Li Y, Yang Y, Wu D, Sun W, et al. Hericium erinaceus polysaccharide-protein HEG-5 inhibits SGC-7901 cell growth via cell cycle arrest and apoptosis. Int J Biol Macromol. (2015) 76:242–53. doi: 10.1016/j.ijbiomac.2015.01.060
52. Lima ATM, Santos MN, Souza LARd, Pinheiro TS, Paiva AAO, Dore CMPG, et al. Chemical characteristics of a heteropolysaccharide from Tylopilus ballouii mushroom and its antioxidant and anti-inflammatory activities. J Carbohydr Polym. (2016) 144:400–9. doi: 10.1016/j.carbpol.2016.02.050
53. Zhao C, Jiang Y, Yin H, Jin Z, Yuan J, Shang H, et al. Hericium caput-medusae (Bull.: Fr.) Pers. Fermentation concentrate polysaccharide ameliorate diarrhea in DSS-induced early colitis by modulating ion channel. J Funct Foods. (2023) 100:105390. doi: 10.1016/j.jff.2022.105390
54. Kushairi N, Phan CW, Sabaratnam V, David P, Naidu M. Lion's Mane Mushroom, Hericium erinaceus (Bull.: Fr.) Pers. Suppresses H2O2-induced oxidative damage and LPS-induced inflammation in HT22 hippocampal neurons and BV2 microglia. J Antioxidants. (2019) 8:261. doi: 10.3390/antiox8080261
55. Zhang Z, Ge M, Wu D, Li W, Chen W, Liu P, et al. Resveratrol-loaded sulfated Hericium erinaceus β-glucan-chitosan nanoparticles: preparation, characterization and synergistic anti-inflammatory effects. J Carbohydr Polym. (2024) 332:121916. doi: 10.1016/j.carbpol.2024.121916
56. Shen M, Fang Z, Chen Y, Chen Y, Xiao B, Guo L, et al. Hypoglycemic effect of the degraded polysaccharides from the wood ear medicinal mushroom Auricularia auricula-judae (Agaricomycetes). Int J Med Mushrooms. (2019) 21:1033–42. doi: 10.1615/IntJMedMushrooms.2019032353
57. Hu X, Hu J, Leng T, Liu S, Xie M. Rosa roxburghii-edible fungi fermentation broth attenuates hyperglycemia, hyperlipidemia and affects gut microbiota in mice with type 2 diabetes. J. Food Biosci. (2023) 52:102432. doi: 10.1016/j.fbio.2023.102432
58. Wang JC, Hu SH, Wang JT, Chen KS, Chia YC. Hypoglycemic effect of extract of Hericium erinaceus. J Sci Food Agric. (2005) 85:641–6. doi: 10.1002/jsfa.1928
59. Cai WD, Ding ZC, Wang YY, Yang Y, Zhang HN, Yan JK, et al. Hypoglycemic benefit and potential mechanism of a polysaccharide from Hericium erinaceus in streptozotoxin-induced diabetic rats. J Process Biochem. (2019) 88:180–8. doi: 10.1016/j.procbio.2019.09.035
60. Cui W, Song X, Li X, Jia L, Zhang C. Structural characterization of Hericium erinaceus polysaccharides and the mechanism of anti-T2DM by modulating the gut microbiota and metabolites. Int J Biol Macromol. (2023) 242:125165. doi: 10.1016/j.ijbiomac.2023.125165
61. Andrzej W, Marta Z, Martin S, Jakub F. Beneficial effects of probiotics, prebiotics, synbiotics, and psychobiotics in inflammatory bowel disease. J Inflamm Bowel Dis. (2015) 21:1674–82. doi: 10.1097/MIB.0000000000000364
62. Yin C, Noratto GD, Fan X, Chen Z, Yao F, Shi D, et al. The impact of mushroom polysaccharides on gut microbiota and its beneficial effects to host: a review. J Carbohydr Polym. (2020) 250:116942. doi: 10.1016/j.carbpol.2020.116942
63. Zhuang H, Dong H, Zhang X, Feng T. Antioxidant activities and prebiotic activities of water-soluble, alkali-soluble polysaccharides extracted from the fruiting bodies of the fungus Hericium erinaceus. J Polymers. (2023) 15:4165. doi: 10.3390/polym15204165
64. Marcello C, Laura F, Giulia P, Veronica O, Marcello C, Andrea P, et al. Interaction between Lipopolysaccharide and Gut Microbiota in Inflammatory Bowel Diseases. Int J Mol Sci. (2021) 22:6242. doi: 10.3390/ijms22126242
65. Wu L, Hu Z, Lv Y, Ge C, Luo X, Zhan S, et al. Hericium erinaceus polysaccharides ameliorate nonalcoholic fatty liver disease via gut microbiota and tryptophan metabolism regulation in an aged laying hen model. Int J Biol Macromol. (2024) 273:132735. doi: 10.1016/j.ijbiomac.2024.132735
66. Jin-Sil P, Won CJ, JooYeon J, Ye KJ, Bo-In L, Woo YC, et al. Lactobacillus acidophilus improves intestinal inflammation in an acute colitis mouse model by regulation of Th17 and Treg cell balance and fibrosis development. J Med Food. (2018) 21:215–24. doi: 10.1089/jmf.2017.3990
67. Chen Y, Jin Y, Stanton C, Ross RP, Zhao J, Zhang H, et al. Alleviation effects of Bifidobacterium breve on DSS-induced colitis depends on intestinal tract barrier maintenance and gut microbiota modulation. Eur J Nutr. (2020) 60:1–19. doi: 10.1007/s00394-020-02252-x
68. Chen X, Wang N, Wang N, Liao B, Cheng L, Ren B. The interactions between oral-gut axis microbiota and Helicobacter pylori. Front Cell Infect Microbiol. (2022) 12:914418. doi: 10.3389/fcimb.2022.914418
69. Farzaneh F, Behnoush A, Ali NR, Amir S, Neda S, Abbas Y, et al. The interplay between Helicobacter pylori and the gut microbiota: an emerging driver influencing the immune system homeostasis and gastric carcinogenesis. Front Cell Infect Microbiol. (2022) 12:953718. doi: 10.3389/fcimb.2022.953718
70. Fabian F, Tim K, Malte R, Corinna B, Andre F, Kathrin Z, et al. Helicobacter pylori infection associates with fecal microbiota composition and diversity. J Sci Rep. (2019) 9:20100. doi: 10.1038/s41598-019-56631-4
71. Chikara I, Tadashi S, Daisuke C, Tetsu A, Daisuke C, Shigeyuki N, et al. Infection of helicobacter pylori and atrophic gastritis influence lactobacillus in gut microbiota in a Japanese population. J Front Immunol. (2018) 9:712. doi: 10.3389/fimmu.2018.00712
72. Liu JH, Li L, Shang XD, Zhang JL, Tan Q. Anti-Helicobacter pylori activity of bioactive components isolated from Hericium erinaceus. J Ethnopharmacol. (2016) 183:54–8. doi: 10.1016/j.jep.2015.09.004
73. Zhu Y, Chen Y, Li Q, Zhao T, Zhang M, Feng W, et al. Preparation, characterization, and anti- Helicobacter pylori activity of Bi3+–Hericium erinaceus polysaccharide complex. J Carbohydr Polym. (2014) 110:231–7. doi: 10.1016/j.carbpol.2014.03.081
74. Chen W, Wu D, Jin Y, Li Q, Liu Y, Qiao X, et al. Pre-protective effect of polysaccharides purified from Hericium erinaceus against ethanol-induced gastric mucosal injury in rats. Int J Biol Macromol. (2020) 159:948–56. doi: 10.1016/j.ijbiomac.2020.05.163
75. Zhang C, Li J, Hu C, Wang J, Zhang J, Ren Z, et al. Antihyperglycaemic and organic protective effects on pancreas, liver and kidney by polysaccharides from Hericium erinaceus SG-02 in streptozotocin-induced diabetic mice. J Sci Rep. (2017) 7:10847. doi: 10.1038/s41598-017-11457-w
76. Kawagishi H, Zhuang C. Compounds for dementia from Hericium erinaceum. J Drugs Future. (2008) 33:149–55. doi: 10.1358/dof.2008.033.02.1173290
77. Koichiro M, Satoshi I, Kenzi O, Yoshihito A, Takashi T. Improving effects of the mushroom Yamabushitake (Hericium erinaceus) on mild cognitive impairment: a double-blind placebo-controlled clinical trial. J Phytother Res. (2009) 23:367–72. doi: 10.1002/ptr.2634
78. Lee KF, Tung SY, Teng CC, Shen CH, Hsieh MC, Huang CY, et al. Post-treatment with Erinacine A, a derived diterpenoid of H. erinaceus, attenuates neurotoxicity in MPTP model of Parkinson's disease. J Antioxid. (2020) 9:137. doi: 10.3390/antiox9020137
79. Mori K, Obara Y, Moriya T, Inatomi S, Nakahata N. Effects of Hericium erinaceus on amyloid β(25-35) peptide-induced learning and memory deficits in mice. J Biomed Res. (2011) 32:67–72. doi: 10.2220/biomedres.32.67
80. Saitsu Y, Nishide A, Kikushima K, Shimizu K, Ohnuki K. Improvement of cognitive functions by oral intake of Hericium erinaceus. J Biomed Res. (2019) 40:125–31. doi: 10.2220/biomedres.40.125
81. Chiu JH, Tsai CL, Lin YY, Li MS, Su SC. High molecular weight of polysaccharides from Hericium erinaceus against amyloid beta-induced neurotoxicity. J BMC Complement Altern Med. (2016) 16:170. doi: 10.1186/s12906-016-1154-5
82. Hu W, Shi M, Wang C, Guo Z, Liu Y, Wang D, et al. Structural characterization of polysaccharide purified from Hericium erinaceus fermented mycelium and its pharmacological basis for application in Alzheimer's disease: oxidative stress related calcium homeostasis. Int J Biol Macromol. (2021) 193:358–69. doi: 10.1016/j.ijbiomac.2021.10.117
83. Zhang J, An S, Hu W, Teng M, Wang X, Qu Y, et al. The Neuroprotective Properties of Hericium erinaceus in glutamate-damaged differentiated PC12 cells and an Alzheimer's disease mouse model. Int J Mol Sci. (2016) 17:1810. doi: 10.3390/ijms17111810
84. Shik PY, Soo LH, Ho WM, Ha LJ, Young LS, Yong LH, et al. Effect of an exo-polysaccharide from the culture broth of Hericium erinaceus on enhancement of growth and differentiation of rat adrenal nerve cells. J Cytotechnol. (2002) 39:155–62. doi: 10.1023/A:1023963509393
85. Mori K, Obara Y, Hirota M, Azumi Y, Kinugasa S, Inatomi S, et al. Nerve growth factor-inducing activity of Hericium erinaceus in 1321N1 human astrocytoma cells. Biol Pharm Bull. (2008) 31:1727–32. doi: 10.1248/bpb.31.1727
86. Lee SL, Hsu JY, Chen TC, Huang CC, Wu TY, Chin TY. Erinacine A prevents lipopolysaccharide-mediated glial cell activation to protect dopaminergic neurons against inflammatory factor-induced cell death in vitro and in vivo. Int J Mol Sci. (2022) 23:810. doi: 10.3390/ijms23020810
87. Zhang S, Wang C, Sun Y, Wang G, Chen H, Li D, et al. Xylanase and fermented polysaccharide of Hericium caputmedusae reduce pathogenic infection of broilers by improving antioxidant and anti-inflammatory properties. J Oxid Med Cell Longev. (2018) 2018:4296985. doi: 10.1155/2018/4296985
88. Shang HM, Song H, Shen SJ, Yao X, Wu B, Wang LN, et al. Effects of dietary polysaccharides from the submerged fermentation concentrate of Hericium caputme-dusae (Bull.:Fr.) Pers. on fat deposition in broilers. J Sci Food Agric. (2015) 95:267–74. doi: 10.1002/jsfa.6711
89. Shang HM, Zhao JC, Guo Y, Zhang HX, Song H. Effects of supplementing feed with fermentation concentrate of Hericium caput-medusae (Bull.:Fr.) Pers. on cholesterol deposition in broiler chickens. J Livestock Sci. (2020) 235:104009. doi: 10.1016/j.livsci.2020.104009
90. Chen D, Yang X, Zheng C, Yang J, Tang X, Chen J, et al. Extracts from Hericium erinaceus relieve inflammatory bowel disease by regulating immunity and gut microbiota. J Oncotarget. (2017) 8:85838–57. doi: 10.18632/oncotarget.20689
91. Ren Z, Xu Z, Amakye WK, Liu W, Zhao Z, Gao L, et al. Front cover: Hericium erinaceus mycelium-derived polysaccharide alleviates ulcerative colitis and modulates gut microbiota in cynomolgus monkeys. J Mol Nutr Food Res. (2023) 67:e202370006. doi: 10.1002/mnfr.202370006
92. Ookushi Y, Sakamoto M, Azuma J-i. Effects of microwave irradiation on water-soluble polysaccharides of the fruiting body of Hericium erinaceum. J Appl Glycosci. (2009) 56:153–7. doi: 10.5458/jag.56.153
93. Tian B, Liu R, Xu T, Cai M, Mao R, Huang L, et al. Modulating effects of Hericium erinaceus polysaccharides on the immune response by regulating gut microbiota in cyclophosphamide-treated mice. J Sci Food Agric. (2022) 103:3050–64. doi: 10.1002/jsfa.12404
94. Yang Y, Ye H, Zhao C, Ren L, Wang C, Georgiev MI, et al. Value added immunoregulatory polysaccharides of Hericium erinaceus and their effect on the gut microbiota. J Carbohydr Polym. (2021) 262:117668. doi: 10.1016/j.carbpol.2021.117668
95. HyunWoo C, Soyoung C, Kangmin S, Hyun KK, JungHwan J, Ho KC, et al. Gut microbiota profiling in aged dogs after feeding pet food contained Hericium erinaceus. J Animal Sci Technol. (2022) 64:937–49. doi: 10.5187/jast.2022.e66
96. Xie XQ, Geng Y, Guan QJ, Ren YL, Guo L, Lv QQ, et al. Influence of short-term consumption of Hericium erinaceus on serum biochemical markers and the changes of the gut microbiota: a pilot study. J Nutr. (2021) 13:1008. doi: 10.3390/nu13031008
97. Shang HM, Song H, Wang LN, Wu B, Ding GD, Jiang YY, et al. Effects of dietary polysaccharides from the submerged fermentation concentrate of Hericium caput-medusae (Bull.:Fr.) Pers. on performance, gut microflora, and cholesterol metabolism in broiler chickens. J Livestock Sci. (2014) 167:276–85. doi: 10.1016/j.livsci.2014.07.004
98. Zhao C, Sun C, Yuan J, Tsopmejio ISN, Li Y, Jiang Y, et al. Hericium caput-medusae (Bull.:Fr.) Pers. fermentation concentrate polysaccharides improves intestinal bacteria by activating chloride channels and mucus secretion. J Ethnopharmacol. (2022) 300:115721. doi: 10.1016/j.jep.2022.115721
99. Ren Y, Geng Y, Du Y, Wang L, Lu ZM, Xu HY, et al. Polysaccharide of Hericium erinaceus attenuates colitis in C57BL/6 mice via regulation of oxidative stress, inflammation-related signaling pathways and modulating the composition of the gut microbiota. J Nutr Biochem. (2018) 57:67–76. doi: 10.1016/j.jnutbio.2018.03.005
Keywords: Hericium erinaceus polysaccharides, extraction, purification, structural properties, biological functions
Citation: Yu P, Ma J, Yang L, Dong Q, Yu C, Zha L, Xu B and Zhao Y (2025) Advances in the extraction, purification, structural characterization, and elucidation of the biological functions of polysaccharides from Hericium erinaceus. Front. Nutr. 12:1634699. doi: 10.3389/fnut.2025.1634699
Received: 25 May 2025; Accepted: 11 August 2025;
Published: 29 August 2025.
Edited by:
Barbara E. Teixeira-Costa, Fluminense Federal University, BrazilReviewed by:
Wei Song, Peking Union Medical College Hospital (CAMS), ChinaAphichart Karnchanatat, Chulalongkorn University, Thailand
Copyright © 2025 Yu, Ma, Yang, Dong, Yu, Zha, Xu and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changxia Yu, eWN4NDE1MjlAMTYzLmNvbQ==; Yan Zhao, amlhbmRhbjI4OUAxMjYuY29t
 Panling Yu1,2
Panling Yu1,2 Lin Yang
Lin Yang Qin Dong
Qin Dong Changxia Yu
Changxia Yu Lei Zha
Lei Zha Yan Zhao
Yan Zhao